Abstract
Opisthorchiasis is a serious public health problem in East Asia and Europe. The pathology involves hepatobiliary abnormalities such as cholangitis, choledocholithiasis and tissue fibrosis that can develop into cholangiocarcinoma. Prevention of infection is difficult as multiple social and behavioral factors are involved, thus, progress on a prophylactic vaccine against opisthorchiasis is urgently needed. Opisthorchis viverrini tetraspanin-2 (Ov-TSP-2) was previously described as a potential vaccine candidate conferring partial protection against O. viverrini infections in hamsters. In this study, we generated a recombinant chimeric form of the large extracellular loop of Ov-TSP-2 and O. viverrini leucine aminopeptidase, designated rOv-TSP-2-LAP. Hamsters were vaccinated with 100 and 200 μg of rOv-TSP-2-LAP formulated with alum-CpG adjuvant via intraperitoneal injection and evaluated the level of protection against O. viverrini infection. Our results demonstrated that the number of worms recovered from hamsters vaccinated with either 100 or 200 μg of rOv-TSP-2-LAP were significantly reduced by 27% compared to the adjuvant control group. Furthermore, the average length of worms recovered from animals vaccinated with 200 μg of rOv-TSP-2-LAP was significantly shorter than those from the control adjuvant group. Immunized hamsters showed significantly increased serum levels of anti-rOv-TSP-2 IgG and IgG1 compared to adjuvant control group, suggesting that rOv-TSP-2-LAP vaccination induces a mixed Th1/Th2 immune response in hamsters. Therefore, the development of a suitable vaccine against opisthorchiasis requires further work involving new vaccine technologies to improve immunogenicity and protective efficacy.
Keywords: Opisthorchis viverrini, chimeric tetraspanin-leucine aminopeptidase, vaccination, Th1/Th2 response
Graphical Abstract
1. Introduction
Opisthorchiasis caused by Opisthorchis viverrini is endemic in the Lower Mekong sub-region including Thailand, Lao PDR, Cambodia, southern part of Vietnam and Myanmar (Sanpool et al., 2018; Sithithaworn et al., 2012). The infection rate remains high in areas where people consume undercooked cyprinid fish containing the infective metacercariae (Chavengkun et al., 2016). Adult flukes live for several years in the biliary tract of the definitive host (including humans) causing chronic inflammation of the bile duct epithelium that can develop into fibrosis and eventually cholangiocarcinoma (CCA) - a fatal bile-duct cancer (Khuntikeo et al., 2018; Sripa et al., 2012). Health education and mass drug administration are currently the main control strategies against O. viverrini infection (Khuntikeo et al., 2016), however, high infection rates are still maintained in many endemic areas due to rapid reinfection (Saengsawang et al., 2016). A protective vaccine against this liver fluke is not yet available, and a partially effective vaccine combined with other control measures may be useful to decrease infection rates.
Tetraspanins (TSPs) are transmembrane proteins containing four transmembrane domains and two extracellular domains, including a small extracellular loop (SEL) and a large extracellular loop (LEL). In trematodes, TSPs are distributed throughout the tegumental membranes, and have also been found in secreted extracellular vesicles (EVs) of many platyhelminthes, including O. viverrini (Chaiyadet et al., 2017; Chaiyadet et al., 2015; Cwiklinski et al., 2015; Nowacki et al., 2015; Piratae et al., 2012; Sotillo et al., 2016; Zhu et al., 2016). TSPs are also important immunogenic antigens that have been used for vaccine and diagnostic purposes (Chen et al., 2016; Tran et al., 2006; Wang et al., 2018). In this sense, the LEL sequence of a TSP from S. mansoni (Sm-TSP-2) is a promising candidate against schistosomiasis with an efficacy of more than 60% worm reduction in an experimental animal model (Tran et al., 2006), and Sm-TSP-2 has already completed a phase 1 clinical trial for schistosomiasis (Tebeje et al., 2016).
The leucine aminopeptidases (LAPs), members of the M17 family of Zn-metalloproteases, preferentially cleave leucine residues at the N-termini of proteins and short peptides (Rawlings et al., 2010). LAPs are involved in different functions such as migration, tissue invasion, and digestion of nutrients in helminth parasites (Maggioli et al., 2018). In addition, LAP has been detected in EVs of the liver fluke Fasciola hepatica (Cwiklinski et al., 2015) and has been proposed as a promising candidate for vaccination against fasciolosis with 85% worm reduction in sheep infected with F. hepatica (Maggioli et al., 2011) and 64% worm reduction in vaccinated mice challenged with Fasciola gigantica (Changklungmoa et al., 2013).
An interesting strategy to induce higher protection is the use of chimeric proteins that contain multiple antigenic epitopes from different proteins in a single polypeptide backbone (Dias et al., 2018). To date, several chimeric proteins have been used for vaccination to induce higher levels of protection against parasites, including a chimeric multi-antigen of the immunodominant B and T cell epitopes of Babesia bovis proteins against babesiosis (Jaramillo Ortiz et al., 2016), a chimeric protein composed of specific CD4+ and CD8+ T-cell epitopes of Leishmania infantum against visceral leishmaniasis (Martins et al., 2017) and a chimeric form of CSPVK210/VK247 proteins against Plasmodium vivax infection (Shabani et al., 2017). Chimeric proteins from S. mansoni have been expressed and tested as vaccines, including Sm-TSP-2 fused to Sm29 or Sm-TSP-2 fused to the immunodominant 5B region of the hookworm aspartic protease Na-APR-1, and both constructs induced higher protection levels against S. mansoni challenge infection in mice than immunization with Sm-TSP-2 or Sm29 alone (Pearson et al., 2012; Pinheiro et al., 2014). Furthermore, immunization with rOv-TSP-2 induced antibody responses in hamsters and partially protected against O. viverrini challenge infection (Chaiyadet et al., 2019). Thus, a combination of Ov-TSP-2 with other proteins could enhance vaccine potential and increase efficacy compared to single proteins.
In this study, we designed and produced the rOv-TSP-2-LAP chimeric protein composed of Ov-TSP-2 LEL and O. viverrini leucine aminopeptidase, and evaluated the immunogenicity and protective efficacy against O. viverrini challenge infection.
2. Materials and methods
2.1. Construction, expression and purification of rOv -TSP-2-LAP
The chimeric Ov -tsp-2-lap sequence was designed by combination of the LEL sequence of Ov-tsp-2 (GenBank accession number JQ678707.1) with the 5’ end of the complete coding sequence (CDS) from O. viverrini leucine aminopeptidase (GenBank accession number KX187340.1). The fusion sequence was inserted into the pET15b vector (Novagen, USA) at Nde I and BamH I restriction enzyme sites to form the pET15b-Ov-tsp2-lap plasmid. This recombinant plasmid was constructed by gene synthesis (Genscript, NJ, USA).
The constructed plasmid was transformed into Escherichia coli (BL21 DE3 strain) to express the rOv-TSP-2-LAP chimeric protein. Briefly, a transformed single colony of E. coli BL21 was grown at 18°C for 12–14 h to an optical density of approximately 0.6–0.8 at 600 nm, and expression of the chimeric protein was induced by adding 0.4 mM IPTG and culturing at 18°C for 24 h. After induction, the bacterial cells were harvested by centrifugation at 15,000 g for 20 min at 4°C. The pellet was resuspended in resuspension buffer (25 mM HEPES, 10% (v/v) glycerol, 1.0 mM EDTA, 1% (v/v) Triton X-100) and submitted to three cycles of sonication lasting 30 s each and centrifuged at 15,000 g at 4°C for 10 min. The cell pellet was subsequently resuspended in lysis buffer (25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% TritonX-100, 1 mM EDTA, 5% glycerol) and high salt solution (500 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl, pH 8.0) and centrifuged as above to remove cell debris. The pellet was collected and solubilized in denaturing buffer (20 mM Tris, 6 M Guanidine-HCl, 2 mM β-ME, pH 8.0) with light stirring at room temperature overnight. The recombinant proteins in the supernatant was collected by centrifuging at 15,000 g at 4°C for 30 min.
The recombinant chimeric protein was purified under denaturing conditions using a Ni2+-NTA affinity column (Thermo Fisher Scientific, USA). The column was equilibrated with denaturing binding buffer (8 M urea, 20 mM NaH2PO4, 500 mM NaCl, pH 8.0). The recombinant protein under denaturing conditions was applied to the column and washed 2 times with denaturing wash buffer 1 (8 M urea, 20 mM NaH2PO4, 500 mM NaCl, pH 6) and subsequently with denaturing wash buffer 2 (8M urea, 20 mM NaH2PO4, 500 mM NaCl, pH 5.3). The recombinant protein was eluted with denaturing elution buffer (8 M urea, 20 mM NaH2PO4, 500 mM NaCl, pH 4). The denatured purified protein was refolded by stepwise dialysis to remove urea using D-tube dialyzer Mega, MWCO 3.5 kDa (Merck KGaA, Germany) at 4°C against 20 mM HEPES. The soluble chimeric protein was evaluated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS PAGE) and Western blot analyses.
2.2. Immunoblot analysis of the rOv-TSP-2-LAP chimeric protein
A total of 2.5 micrograms of rOv-TSP-2-LAP was separated on a 15% SDS–PAGE gel and transferred onto nitrocellulose membrane (Mini Trans-Blot Cell, Bio-Rad). The membrane was blocked with 5% skim milk in PBS containing 0.1% Tween-20 (PBST) for 2 hours at room temperature with shaking. Membranes were then washed 3 times with PBST and incubated with anti-6x-His Tag Monoclonal Antibody-HRP (Invitrogen, US) for 2 hours at room temperature or with hamster anti-Ov-LEL-TSP-2 antiserum as previously described (Chaiyadet et al., 2019) diluted 1:2,000 in PBST overnight at 4°C followed with secondary anti-hamster IgG-HRP antibody (Thermo Fisher scientific, USA) (diluted 1:3,000 in PBST) at room temperature for 2 hours. Membranes were then washed 3 times with PBST for 10 min. The colorimetric signal was developed using LuminataTM Forte Western HRP Substrate (Millipore, USA).
2.3. Preparation of O. viverrini metacercariae
O. viverrini metacercariae were prepared as previously described (Laha et al., 2007). Briefly, wild caught cyprinid fishes were mixed with 0.25% pepsin, 1.5% HCl in normal saline solution (NSS) and homogenized in an electric blender. The mixture was incubated in a shaking water bath at 37°C for 1 hour for digestion. The digested solution was filtered through a series of sieves (1,000, 300, and 106 μm meshes). The debris obtained by filtering on 106 μm mesh was washed and repeatedly sedimented with 0.85% NaCl in a sedimentation jar until the supernatant became clear. Sediments were examined for O. viverrini metacercariae under a stereomicroscope. O. viverrini metacercariae were collected and stored in sterile 0.85% NaCl at 4°C until used.
2.4. Experimental animals and vaccination protocol
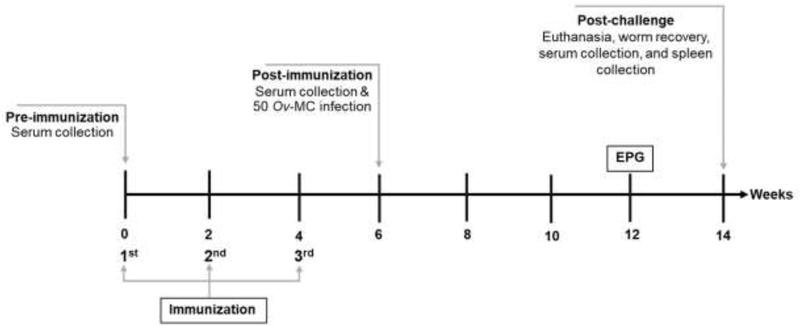
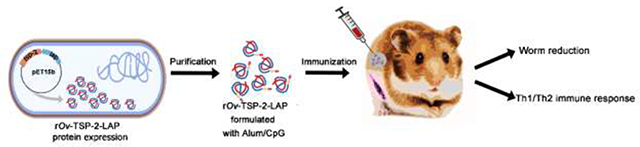
Male Syrian golden hamsters (8 weeks-old) were used for the vaccination experiment. Animal experiments were approved by the Animal Ethics Committee of Khon Kaen University (IACUC KKU 51/61). Hamsters were maintained in the animal house care unit at the Faculty of Medicine, Khon Kaen University. Thirty hamsters were divided into three groups (10 hamsters per group) as follows: 1) control hamsters immunized with a colloidal suspension of aluminium hydroxide gel (Invivogen, USA) and CpG ODN 1826 (10 μg) (Invivogen, USA) (Alum-CpG); 2) vaccinated hamsters immunized with 100μg of rOv-TSP-2-LAP and alum-CpG; and 3) vaccinated hamsters immunized with 200 μg of rOv-TSP-2-LAP and alum-CpG. Immunizations were performed three times at 2-week intervals by the intraperitoneal route. Two weeks after the third immunization, each hamster was orally infected with 50 O. viverrini metacercariae, and eight weeks later all hamsters were euthanized. Hamster livers were collected, dissected and number of adult worms counted. Blood samples were taken at day 0 (pre-immunization), at 6 weeks post-immunization via retrobulbar sinus and at necropsy (post-challenge) by cardiac puncture. A schematic representation of the hamster vaccination trial is shown in Fig. 1.
Fig. 1. Diagramatic representation of the hamster vaccination-challenge trial to assess efficacy of immunization with rOv-TSP-2-LAP protein.
Ov-MC, O. viverrini metacercariae; EPG, worm eggs per gram of hamster faeces.
2.5. Worm recovery
The number of worms in hamster livers were counted and the percentage of worm reduction was calculated using the equation: % worm reduction = A−B/A × 100, where A represents the mean parasite recovery from the adjuvant control group, and B represents the mean parasite recovery from the 100 μg or 200 μg rOv-TSP-2-LAP immunized groups.
2.6. Measurement of worm length
Recovered worms from each experimental group were measured the body length as previously described (Chaiyadet et al., 2019). Briefly, worms were washed three times with sterile NSS. Worms were then dehydrated and fixed in pre-warmed 10% formalin. Images of the worms were captured, and worm length was measured using NIS-Element software (Nikon SMZ 745T, Japan).
2.7. Fecal egg counts
Feces from individual hamsters were collected weekly from weeks 4–7 post-challenge. Number of eggs per gram (EPG) of feces was determined using a modified formalin-ether acetate technique (Elkins et al., 1986). Briefly, stool samples were suspended in NSS then filtered through 2 layers of gauze. The flow-through was centrifuged at 4,000 g for 5 min, supernatants decanted, and 7 ml of 10% formalin and 3 ml of ethyl acetate were added to the pellet. Samples were shaken vigorously and centrifuged at 4,000 g for 5 min. The sediment was collected and resuspended in 10% formalin. To determine the EPG, the total number of drops of sediment were counted, and two drops were examined. EPG was calculated as follows: EPG = TV/vw, where T = the number of eggs counted, V = total volume of sediment in drops, v = the number of drops examined, w = the weight of the fecal sample.
2.8. Indirect ELISA for antibody responses
Specific total IgG and IgG isotypes (IgG1 and IgG2b) against rOv- TSP-2-LAP in the serum of hamsters were analyzed by indirect ELISA. Briefly, 96-well ELISA plates (NUNC-F96, Fisher Scientific, USA) were coated with 100 μl of rOv-TSP-2-LAP (1 μg/ml) in coating buffer (15 mM Na2CO3, 35mM NaHCO3, pH 9.6) and incubated at 4°C, overnight. Coated plates were washed three times with PBST, and non-specific binding was blocked with 200 μl/well of 5% skim milk in coating buffer for 2 h at 37°C. Plates were then washed three times with PBST. One hundred μl of hamster sera diluted in PBST with 2% of skim milk (1:1,000 dilution for total IgG; 1:100 for isotype IgG1; 1:50 for isotype IgG2b) were added and incubated at 37°C for 2 h. Plates were washed with PBST, probed with 100 μl of anti-hamster IgG-HRP, diluted 1:3,000 (Thermo Fisher scientific, USA) for total IgG detection; mouse anti-hamster IgG1-HRP (Southern Biotech), diluted 1:2000 for IgG1 detection and goat anti-mouse IgG2b-HRP (Thermo Fisher scientific, USA), diluted 1:2,000 for IgG2b detection, incubated for 2 h at 37°C and washed with PBST. Finally, 50 μl of TMB (Thermo Fisher Scientific, USA) was added to each well, plates were incubated for 20 minutes, and the reactions were stopped by addition of 50 μl/well of 0.5M H2SO4. Colorimetric reactions were read at a wavelength of 450 nm on a Spectra Max microplate reader (Molecular Devices, USA).
2.9. Statistical analysis
All data are presented as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA, USA). Data were analyzed by one- or two-way ANOVA and Tukey’s test to compare datasets. P-values < 0.05 were considered as statistically significant.
3. Results
3.1. Expression and purification of rOv- TSP-2-LAP protein
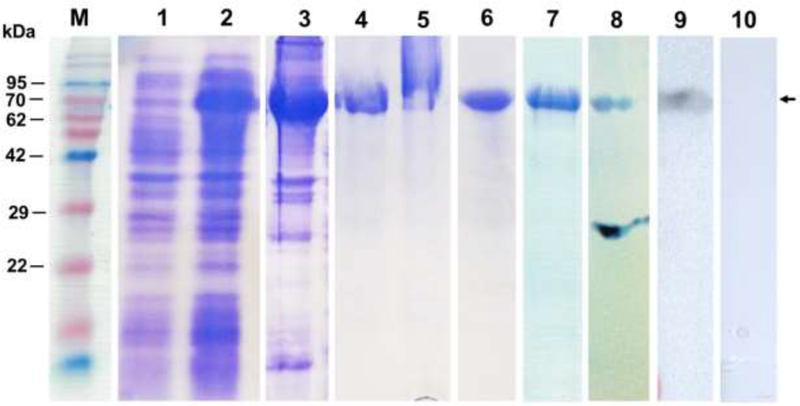
The total chimeric Ov-tsp-2-lap sequence was 1,929 base pairs including 234 base pairs from LEL of Ov-tsp-2 and 1,695 base pairs from Ov-lap, encoding for 643 amino acids (78 and 565 amino acids of Ov-TSP-2 and Ov-LAP, respectively) (Supplementary file 1). The theoretical molecular weight of the chimeric protein is 72.3 kDa, including 69.7 kDa of the chimeric Ov-TSP-2-LAP sequence fused to 2.6 kDa of vector-derived sequence from pET15b. The rOv-TSP-2-LAP was expressed as an insoluble protein and purified under denaturing conditions. The purified protein then was refolded to generate soluble material with a yield of 5 mg/liter of culture media. Western blot using anti-6×His tag and hamster anti-rOv-LEL-TSP-2 antibodies confirmed the identity of the protein (Fig. 2).
Fig. 2. SDS-PAGE and Western blot analysis of samples from expression, purification and refolding of insoluble rOv-TSP-2- LAP protein.
Protein marker (lane M), pre-induction cell lysate (lane 1), postinduction cell lysate (lane 2), insoluble protein in cell pellet post-sonication (lane 3), denatured and IMACpurified protein in 6M guanidine (lane 4), column flow-through (lane 5), insoluble eluted protein (lane 6), soluble purified protein (rOv-TSP-2-LAP) in 20 mM HEPES after refolding (lane 7), western blot of rOv-TSP-2-LAP probed with anti-6 his tag serum (lane 8), western blot of rOv-TSP-2-LAP probed with hamster anti-rOv-LEL-TSP-2 serum (lane 9), western blot of rOv-TSP-2-LAP probed with hamster naïve serum control (lane 10). Molecular weight of rOv-LEL-TSP-2 protein is approximately 75 kDa.
3.2. Vaccinated hamsters harbor reduced worm and egg burdens
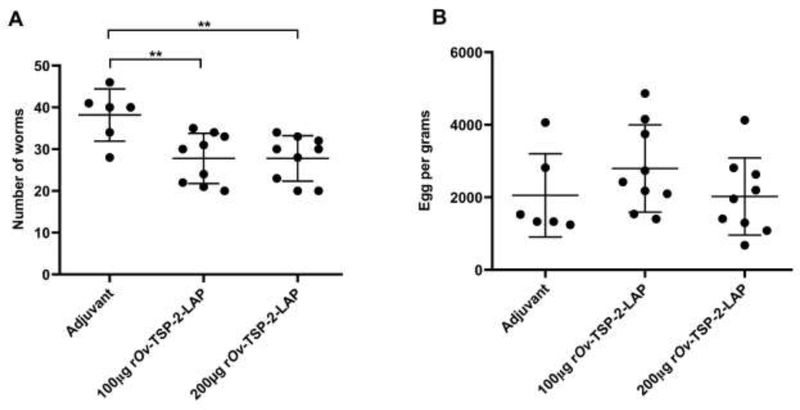
All adult flukes from individual hamster livers were collected and counted. The adjuvant control group had a mean worm recovery of 38.17 ± 6.27, while hamsters immunized with 100 μg and 200 μg of rOv -TSP-2-LAP had a significantly lower average worm burden of 27.8 ± 6 and 27.8 ± 5.5, respectively (P<0.01) (Fig. 3A). These equated to 27.2% reductions for both 100 μg and 200 μg rOv-TSP-2-LAP groups compared to the adjuvant control group. Fecal egg counts were not significantly different between vaccinated groups compared to the adjuvant control group (Fig. 3B).
Fig. 3. Worm recovery and egg per gram of feces (EPG).
(A) Number of worms recovered from the liver of each hamster group infected with 50 O. viverrini metacercariae after vaccination. (B) Eggs per gram of feces (EPG) from experimental hamsters. Each dot represents the total worm number or EPG of an individual hamster. Mean and SD bar is shown. * P < 0.01.
3.3. Vaccination dose affects size of recovered adult worms
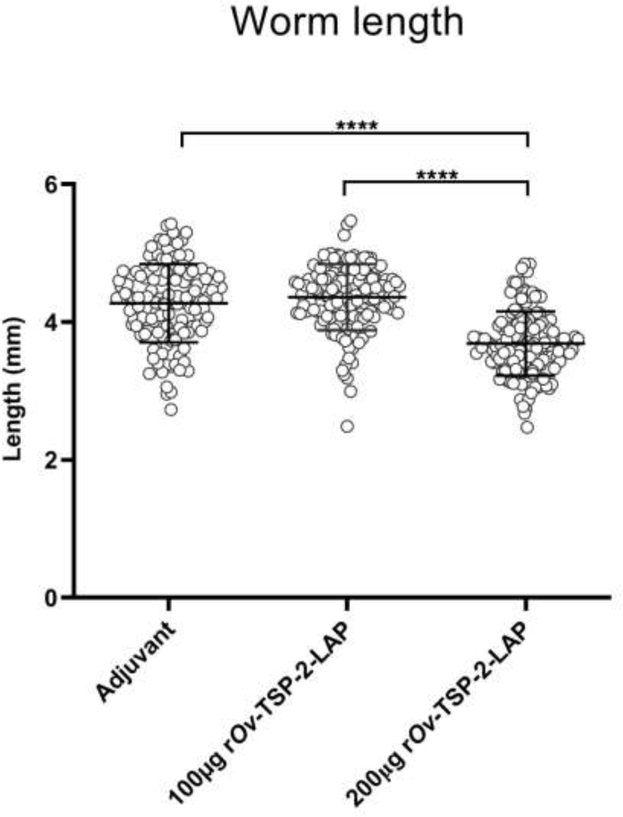
All intact worms from each animal group were fixed in 10% formalin to measure their length. Average worm length from the 200 μg rOv-TSP-2-LAP vaccinated group was significantly shorter compared to worms recovered from the 100 μg rOv-TSP-2-LAP and adjuvant control group (P<0.0001); there was no significant difference between the 100 μg rOv-TSP-2-LAP and adjuvant control groups. Mean ± SD of worm length in the adjuvant control group, 100 μg rOv-TSP-2-LAP group and 200 μg rOv-TSP-2-LAP group were 4.27 ± 0.57 mm, 4.37 ± 0.48 mm, 3.69 ± 0.46 mm, respectively (Fig. 4).
Fig. 4. Length of worms recovered from vaccinated hamsters.
Average length of all intact worms from each group was measured. Each dot represents the length of a single worm. **** P < 0.0001.
3.4. Vaccination of hamsters induced anti-rOv-TSP-2-LAP antibody responses
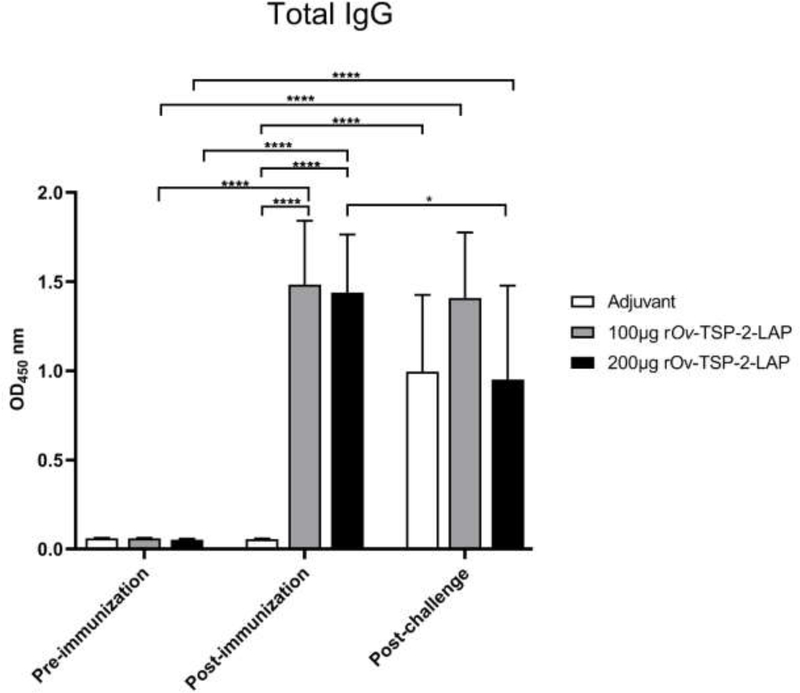
The specific total IgG levels against rOv-TSP-2-LAP in the sera of immunized hamsters from either 100 μg or 200 μg rOv-TSP-2-LAP groups significantly increased after the third immunization (P<0.0001), while there was no differences in the adjuvant control group. IgG levels in both vaccinated groups were significantly higher compared to the adjuvant control group post-immunization (P<0.0001). Furthermore, IgG levels in the adjuvant control group were significantly increased post-challenge compared to post-immunization (P<0.0001), whereas no significant differences were observed between pre- and post-challenge responses in the 100 μg (P=0.8999) group but were significantly decreased in the 200 μg rOv-TSP-2-LAP groups (P<0.05). In addition, serum IgG levels from the 100 μg and 200 μg rOv-TSP-2-LAP groups were significantly increased post-immunization and post-challenge compared to naïve animals (P<0.0001) (Fig. 5).
Fig. 5. Serum IgG levels in vaccinated hamsters.
Total serum IgG levels against rOv-TSP-2-LAP were determined by indirect ELISA at pre-immunization, post-immunization and post-challenge infection. Results represent the mean absorbance measured at 450 nm. Each bar represents mean ± SD of each group. * P < 0.05 and **** P < 0.0001.
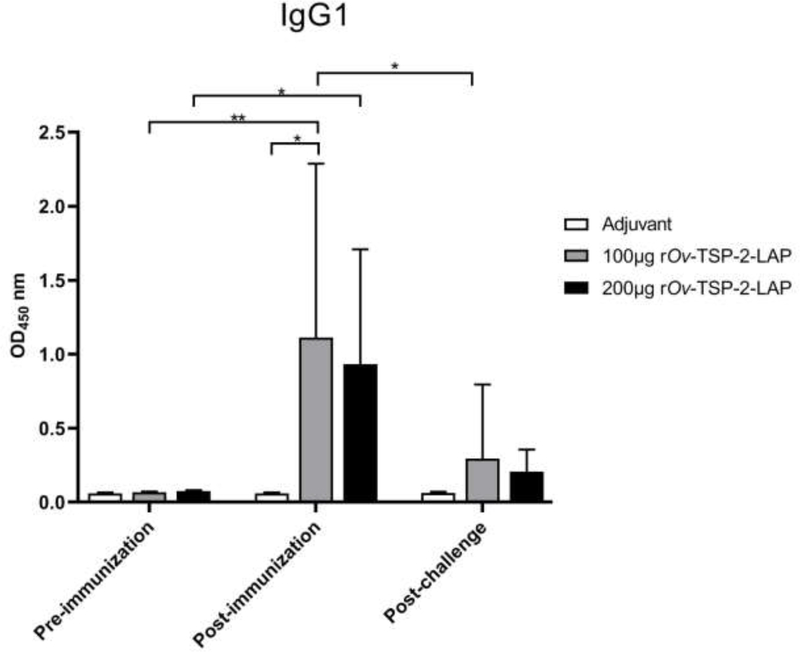
Specific IgG1 levels against rOv-TSP-2-LAP in the serum of hamsters immunized with either 100 μg or 200 μg rOv-TSP-2-LAP were significantly increased after the third immunization (P<0.01 and P<0.05, respectively) while IgG1 levels were not different in the adjuvant control group at any time. IgG1 levels in both vaccinated groups were significantly higher than in the adjuvant control group at post-immunization and post-challenge. Furthermore, IgG1 levels significantly decreased (P<0.05) post-challenge compared to post-immunization in the 200 μg rOv-TSP-2-LAP group (Fig. 6).
Fig. 6. Serum IgG1 levels in vaccinated hamsters.
Total serum IgG1 levels against rOv-TSP-2-LAP were determined by indirect ELISA at pre-immunization, post-immunization and post-challenge infection. Results represent the mean absorbance measured at 450 nm. Each bar represents mean ± SD of each group. * P < 0.05, ** P < 0.01.
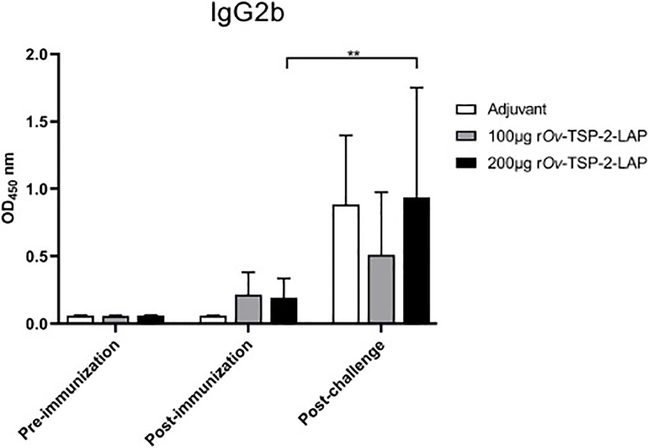
Specific serum IgG2b levels against rOv-TSP-2-LAP were non-significantly elevated post-immunization compared to pre-immunization in both 100 μg rOv-TSP-2-LAP and 200 μg rOv-TSP-2-LAP groups. IgG2b levels in both immunized groups trended towards being higher than in the adjuvant control group post-immunization but this did not reach statistical significance. Serum IgG2b level increased in the 200 μg rOv-TSP-2-LAP group post-challenge compared to post-immunization (P<0.01), while antibody levels in 100 μg rOv-TSP-2-LAP group were not significantly different between these two time points (Fig. 7).
Fig. 7. Serum IgG2b levels in vaccinated hamsters.
Total serum IgG2b levels against rOv-TSP-2-LAP were determined by indirect ELISA at pre-immunization, post-immunization and post-challenge infection. Results represent the mean absorbance measured at 450 nm. Each bar represents mean ± SD of each group. ** P < 0.01.
4. Discussion
Chimeric proteins have the advantage of containing several antigenic and immunogenic epitopes from at least two or more proteins from the parasite that induce protective effects against infection (Anugraha et al., 2015; Cabrera-Mora et al., 2016). In this study, a recombinant chimeric form of O. viverrini TSP-2 and LAP shows potential as a vaccine candidate against challenge infection. A previous study showed that a tetraspanin fused with the thioredoxin fusion tag of a plasmid vector stimulates protective immune responses against O. viverrini infection (Chaiyadet et al., 2019), and recombinant LAPs have been explored as promising vaccine candidates against F. hepatica and F. gigantica infections with reductions in worm burdens of up to 86.7% in sheep and 64% in mice, respectively (Changklungmoa et al., 2013; Maggioli et al., 2011). The chimeric recombinant protein of O. viverrini TSP-2 and LAP was successfully expressed and refolded to generate a soluble protein, and its immunogenicity and protective efficacy against O. viverrini challenge were comparable to previous studies using subunit vaccine antigens (Chaiyadet et al., 2019). The lack of increased protection of rOv-TSP-2-LAP compared to TSP-2 alone is most likely a result of the difficulty of expressing this protein in a soluble form. The chimeric protein was insoluble and subsequently refolded, which might have affected some of the most important epitopes of the protein since the refolded structure of rOv-TSP-2-LAP may not be completely similar to native OvTSP-2 and Ov-LAP. Therefore, the specific immune response in vaccinated animals was possibly targeted towards linear epitopes and not to the conformational epitopes present in rOv-TSP-2-LAP.
Immunization with rOv-TSP-2-LAP chimeric protein elicited a strong humoral immune response in all vaccinated hamsters, which had significantly increased total IgG levels after three immunizations compared to the control group. However, serum IgG levels were not further elevated post-challenge in any of the vaccinated hamster groups. This result was similar to that observed in previous studies when vaccinating with rOv-TSP-2 (Chaiyadet et al., 2019). A feasible explanation for this observation is that vaccination induced IgG-secreting memory B cells specific to chimeric rOv-TSP-2-LAP protein which acted upon newly excysted juvenile flukes as they migrate up the higher order bile ducts, interrupting EVhost cell communication (Chaiyadet et al., 2015) and retarding fluke development. This impacted the length of the flukes in vaccinated groups, which were significant shorter than flukes from the control animals. The process to produce specific antibodies from memory B cells occurs in germinal centers with high-affinity and high antigen-binding capacity (Siegrist, 2018). Therefore, the efficacy of the IgG antibodies to kill the worms in the vaccinated groups would be higher than in the control groups, where IgG antibody responses would not be detected until adult flukes had resided in the bile ducts and become less susceptible to antibody-mediated attack.
Since hamster and mouse immunoglobulins shared many structural features (McGuire et al., 1985) and there is a lack of commercially available anti-hamster secondary antibodies, we used goat anti-mouse IgG2b-HRP to detect IgG2b levels in the serum of hamsters in this study. The presence of IgG1 in hamsters is indicative of a Th2-like response, which has traditionally been described as most effective against extracellular pathogens (Conrad et al., 2017), while IgG2a, IgG2b and IgG3 are associated with a Th1 response and protection against intracellular pathogens (Germann et al., 1995). The vaccine used herein was formulated with alum and CpG oligodeoxynucleotide (CpG ODN) 1826 adjuvant, the latter of which stimulates antigen presenting cells via Toll-like receptor 9 (Hemmi et al., 2000), activates the innate and adaptive immune responses of the host, and the responses are strongly Th1-biased (Roman et al., 1997). Furthermore, previous studies showed that immunization of hamsters with O. viverrini crude antigen formulated with only CpG ODN 1826 adjuvant elicited a protective Th1-like response against O. viverrini infection (Kaewraemruaen et al., 2016). However, the levels of specific IgG1 to chimeric rOv-TSP-2-LAP were significantly increased after three immunizations compared to the adjuvant control, indicating that immunization with this protein adjuvanted with CpG ODN 1826 produced a mixed Th1/Th2 immune response. Moreover, increased levels of IgG2b were related to higher expression levels of TGF-β in spleen cells of vaccinated hamster compared to control animals (Supplementary Fig. 1.). In mice, TGF-β is a switch factor to promote differentiation to an IgG2b isotype in B cells (Deenick et al., 2005). Interestingly, IgG2 responses increased mostly after challenge, compared with IgG1 responses which peaked after vaccination and didn’t further increase after challenge. IgG2 responses dominate in the response to bacterial polysaccharide antigens and drive class switching, but lss is known about the role of this isotype in antibody responses to helminths compared to IgE and IgG4. Mixed Th1/Th2 immune responses have also been found in response to chimeric proteins such as rFhLAP-CL1 in sheep challenged with F. hepatica (Ortega-Vargas et al., 2019), SmTSP-2/Sm29 in mice challenged with S. mansoni (Pinheiro et al., 2014) and the chimeric epitope from multistage of filarial antigen against Brugia malayi in gerbil (Anugraha et al., 2015).
In conclusion, we have demonstrated that immunization of hamster with rOv-TSP-2-LAP chimeric protein adjuvanted with alum/CpG ODN 1826 induced partial protection against O. viverrini infection in terms of reduction in fluke burden. However, it does not enhance the protection levels achieved with rOv-TSP-2 alone. In addition, a marked humoral immune response was elicited, with production of both IgG1 and IgG2b specific antibodies against the recombinant chimeric protein, suggesting a mixed Th1/Th2 immune response associated with protection. The use of this protein as a vaccine candidate requires more research and use of new vaccine technologies to make it a valid candidate against opisthorchiasis.
Supplementary Material
Highlights.
Recombinant chimeric form of the large extracellular loop of Ov-TSP-2 and O. viverrini leucine aminopeptidase, designated rOv-TSP-2-LAP, was produced in bacteria.
Hamsters immunized with recombinant rOv-TSP-2-LAP produced humoral and cellular immune responses.
Hamsters vaccinated with rOv-TSP-2-LAP had significantly reduced fluke burdens compared to control animals that received adjuvant alone.
Acknowledgments
This research was supported by Faculty of Medicine, Khon Kaen University, a project grant from the National Health and Medical Research Council of Australia (NHMRC), grant identification number APP1085309, and the National Cancer Institute, National Institute of Health, grant number 2R01CA164719-06A1.
Footnotes
Conflict of interest
The authors confirm that they have no conflicts of interest in relation to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anugraha G, Madhumathi J, Prince PR, Prita PJ, Khatri VK, Amdare NP, Reddy MV, Kaliraj P, 2015. Chimeric Epitope Vaccine from Multistage Antigens for Lymphatic Filariasis. Scandinavian journal of immunology 82, 380–389. [DOI] [PubMed] [Google Scholar]
- Cabrera-Mora M, Fonseca JA, Singh B, Zhao C, Makarova N, Dmitriev I, Curiel DT, Blackwell J, Moreno A, 2016. A Recombinant Chimeric Ad5/3 Vector Expressing a Multistage Plasmodium Antigen Induces Protective Immunity in Mice Using Heterologous Prime-Boost Immunization Regimens. J. Immunol 197, 2748–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyadet S, Krueajampa W, Hipkaeo W, Plosan Y, Piratae S, Sotillo J, Smout M, Sripa B, Brindley PJ, Loukas A, Laha T, 2017. Suppression of mRNAs encoding CD63 family tetraspanins from the carcinogenic liver fluke Opisthorchis viverrini results in distinct tegument phenotypes. Sci. Rep 7, 14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyadet S, Sotillo J, Krueajampa W, Thongsen S, Brindley PJ, Sripa B, Loukas A, Laha T, 2019. Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesiclederived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLoS neglected tropical diseases 13, e0007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyadet S, Sotillo J, Smout M, Cantacessi C, Jones MK, Johnson MS, Turnbull L, Whitchurch CB, Potriquet J, Laohaviroj M, Mulvenna J, Brindley PJ, Bethony JM, Laha T, Sripa B, Loukas A, 2015. Carcinogenic Liver Fluke Secretes Extracellular Vesicles That Promote Cholangiocytes to Adopt a Tumorigenic Phenotype. J. Infect. Dis 212, 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changklungmoa N, Kueakhai P, Riengrojpitak S, Chaithirayanon K, Chaichanasak P, Preyavichyapugdee N, Chantree P, Sansri V, Itagaki T, Sobhon P, 2013. Immunization with recombinant leucine aminopeptidase showed protection against Fasciola gigantica in mice. Parasitology research 112, 3653–3659. [DOI] [PubMed] [Google Scholar]
- Chavengkun W, Kompor P, Norkaew J, Kujapun J, Pothipim M, Ponphimai S, Kaewpitoon SJ, Padchasuwan N, Kaewpitoon N, 2016. Raw Fish Consuming Behavior Related to Liver Fluke Infection among Populations at Risk of Cholangiocarcinoma in Nakhon Ratchasima Province, Thailand. Asian Pacific journal of cancer prevention : APJCP. 17, 2761–2765. [PubMed] [Google Scholar]
- Chen L, Chen Y, Zhang D, Hou M, Yang B, Zhang F, Zhang W, Luo X, Ji M, Wu G, 2016. Protection and immunological study on two tetraspanin-derived vaccine candidates against schistosomiasis japonicum. Parasite immunology 38, 589–598. [DOI] [PubMed] [Google Scholar]
- Conrad NL, Cruz McBride FW, Souza JD, Silveira MM, Felix S, Mendonca KS, Santos CS, Athanazio DA, Medeiros MA, Reis MG, Dellagostin OA, McBride AJ, 2017. LigB subunit vaccine confers sterile immunity against challenge in the hamster model of leptospirosis. PLoS neglected tropical diseases 11, e0005441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwiklinski K, de la Torre-Escudero E, Trelis M, Bernal D, Dufresne PJ, Brennan GP, O’Neill S, Tort J, Paterson S, Marcilla A, Dalton JP, Robinson MW, 2015. The Extracellular Vesicles of the Helminth Pathogen, Fasciola hepatica: Biogenesis Pathways and Cargo Molecules Involved in Parasite Pathogenesis. Mol. Cell Proteomics 14, 3258–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick EK, Hasbold J, Hodgkin PD, 2005. Decision criteria for resolving isotype switching conflicts by B cells. European journal of immunology 35, 2949–2955. [DOI] [PubMed] [Google Scholar]
- Dias DS, Ribeiro PAF, Martins VT, Lage DP, Costa LE, Chavez-Fumagalli MA, Ramos FF, Santos TTO, Ludolf F, Oliveira JS, Mendes TAO, Silva ES, Galdino AS, Duarte MC, Roatt BM, Menezes-Souza D, Teixeira AL, Coelho EAF, 2018. Vaccination with a CD4(+) and CD8(+) T-cell epitopes-based recombinant chimeric protein derived from Leishmania infantum proteins confers protective immunity against visceral leishmaniasis. Translational research : the journal of laboratory and clinical medicine 200, 18–34. [DOI] [PubMed] [Google Scholar]
- Elkins DB, Haswell-Elkins M, Anderson RM, 1986. The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India. I. Study design and pre- and posttreatment observations on Ascaris lumbricoides infection. Trans. R. Soc. Trop. Med. Hyg 80, 774–792. [DOI] [PubMed] [Google Scholar]
- Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kolsch E, Podlaski FJ, Gately MK, Rude E, 1995. Interleukin-12 profoundly up-regulates the synthesis of antigenspecific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. European journal of immunology 25, 823–829. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S, 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745. [DOI] [PubMed] [Google Scholar]
- Jaramillo Ortiz JM, Molinari MP, Gravisaco MJ, Paoletta MS, Montenegro VN, Wilkowsky SE, 2016. Evaluation of different heterologous prime-boost immunization strategies against Babesia bovis using viral vectored and protein-adjuvant vaccines based on a chimeric multi-antigen. Vaccine 34, 3913–3919. [DOI] [PubMed] [Google Scholar]
- Kaewraemruaen C, Sermswan RW, Wongratanacheewin S, 2016. CpG oligodeoxynucleotides with crude parasite antigens reduce worm recovery in Opisthorchis viverrini infected hamsters. Acta tropica 164, 395–401. [DOI] [PubMed] [Google Scholar]
- Khuntikeo N, Sithithaworn P, Loilom W, Namwat N, Yongvanit P, Thinkhamrop B, Kiatsopit N, Andrews RH, Petney TN, 2016. Changing patterns of prevalence in Opisthorchis viverrini sensu lato infection in children and adolescents in northeast Thailand. Acta tropica 164, 469–472. [DOI] [PubMed] [Google Scholar]
- Khuntikeo N, Titapun A, Loilome W, Yongvanit P, Thinkhamrop B, Chamadol N, Boonmars T, Nethanomsak T, Andrews RH, Petney TN, Sithithaworn P, 2018. Current Perspectives on Opisthorchiasis Control and Cholangiocarcinoma Detection in Southeast Asia. Front. Med. (Lausanne) 5, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha T, Pinlaor P, Mulvenna J, Sripa B, Sripa M, Smout MJ, Gasser RB, Brindley PJ, Loukas A, 2007. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics 8, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggioli G, Acosta D, Silveira F, Rossi S, Giacaman S, Basika T, Gayo V, Rosadilla D, Roche L, Tort J, Carmona C, 2011. The recombinant gut-associated M17 leucine aminopeptidase in combination with different adjuvants confers a high level of protection against Fasciola hepatica infection in sheep. Vaccine 29, 9057–9063. [DOI] [PubMed] [Google Scholar]
- Maggioli G, Rinaldi G, Giaudrone I, Berasain P, Tort JF, Brindley PJ, Carmona C, 2018. Expression, purification and characterization of two leucine aminopeptidases of the blood fluke, Schistosoma mansoni. Molecular and biochemical parasitology 219, 17–23. [DOI] [PubMed] [Google Scholar]
- Martins VT, Duarte MC, Lage DP, Costa LE, Carvalho AM, Mendes TA, Roatt BM, Menezes-Souza D, Soto M, Coelho EA, 2017. A recombinant chimeric protein composed of human and mice-specific CD4(+) and CD8(+) T-cell epitopes protects against visceral leishmaniasis. Parasite immunology 39. [DOI] [PubMed] [Google Scholar]
- McGuire KL, Duncan WR, Tucker PW, 1985. Phylogenetic conservation of immunoglobulin heavy chains: direct comparison of hamster and mouse Cmu genes. Nucleic acids research 13, 5611–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowacki FC, Swain MT, Klychnikov OI, Niazi U, Ivens A, Quintana JF, Hensbergen PJ, Hokke CH, Buck AH, Hoffmann KF, 2015. Protein and small non-coding RNA-enriched extracellular vesicles are released by the pathogenic blood fluke Schistosoma mansoni. J. Extracell. Vesicles 4, 28665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Vargas S, Espitia C, Sahagun-Ruiz A, Parada C, Balderas-Loaeza A, Villa-Mancera A, Quiroz-Romero H, 2019. Moderate protection is induced by a chimeric protein composed of leucine aminopeptidase and cathepsin L1 against Fasciola hepatica challenge in sheep. Vaccine 37, 3234–3240. [DOI] [PubMed] [Google Scholar]
- Pearson MS, Pickering DA, McSorley HJ, Bethony JM, Tribolet L, Dougall AM, Hotez PJ, Loukas A, 2012. Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS neglected tropical diseases 6, e1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro CS, Ribeiro AP, Cardoso FC, Martins VP, Figueiredo BC, Assis NR, Morais SB, Caliari MV, Loukas A, Oliveira SC, 2014. A multivalent chimeric vaccine composed of Schistosoma mansoni SmTSP-2 and Sm29 was able to induce protection against infection in mice. Parasite immunology 36, 303–312. [DOI] [PubMed] [Google Scholar]
- Piratae S, Tesana S, Jones MK, Brindley PJ, Loukas A, Lovas E, Eursitthichai V, Sripa B, Thanasuwan S, Laha T, 2012. Molecular characterization of a tetraspanin from the human liver fluke, Opisthorchis viverrini. PLoS neglected tropical diseases 6, e1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A, 2010. MEROPS: the peptidase database. Nucleic Acids Res. 38, D227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A, Kornbluth RS, Richman DD, Carson DA, Raz E, 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nature medicine 3, 849–854. [DOI] [PubMed] [Google Scholar]
- Saengsawang P, Promthet S, Bradshaw P, 2016. Reinfection by Opisthorchis viverrini after treatment with praziquantel. Asian Pac. J. Cancer Prev. 17, 857–862. [DOI] [PubMed] [Google Scholar]
- Sanpool O, Aung WPP, Rodpai R, Maleewong W, Intapan PM, 2018. Human liver fluke Opisthorchis viverrini (Trematoda, Opisthorchiidae) in Central Myanmar: New records of adults and metacercariae identified by morphology and molecular analysis. Acta tropica 185, 149–155. [DOI] [PubMed] [Google Scholar]
- Shabani SH, Zakeri S, Salmanian AH, Amani J, Mehrizi AA, Snounou G, Nosten F, Andolina C, Mourtazavi Y, Djadid ND, 2017. Biological, immunological and functional properties of two novel multi-variant chimeric recombinant proteins of CSP antigens for vaccine development against Plasmodium vivax infection. Molecular immunology 90, 158–171. [DOI] [PubMed] [Google Scholar]
- Siegrist C-A, 2018. Vaccine Immunology, in: Plotkin SA, Orenstein WA, Offit PA, Edwards KM (Eds.), Plotkin’s Vaccines (Seventh Edition). Elsevier, pp. 16–34.e17. [Google Scholar]
- Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, Nawa Y, Liang S, Brindley PJ, Sripa B, 2012. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol. Int. 61, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo J, Pearson M, Potriquet J, Becker L, Pickering D, Mulvenna J, Loukas A, 2016. Extracellular vesicles secreted by Schistosoma mansoni contain protein vaccine candidates. Int. J. Parasitol. 46, 1–5. [DOI] [PubMed] [Google Scholar]
- Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A, 2012. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 28, 395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebeje BM, Harvie M, You H, Loukas A, McManus DP, 2016. Schistosomiasis vaccines: where do we stand? Parasit Vectors 9, 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, Don TA, McManus DP, Correa-Oliveira R, Loukas A, 2006. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat. Med 12, 835–840. [DOI] [PubMed] [Google Scholar]
- Wang L, Giri BR, Chen Y, Xia T, Liu J, Li H, Li J, Cheng G, 2018. Molecular characterization, expression profile, and preliminary evaluation of diagnostic potential of CD63 in Schistosoma japonicum. Parasitology research 117, 3625–3631. [DOI] [PubMed] [Google Scholar]
- Zhu L, Liu J, Dao J, Lu K, Li H, Gu H, Liu J, Feng X, Cheng G, 2016. Molecular characterization of S. japonicum exosome-like vesicles reveals their regulatory roles in parasitehost interactions. Sci. Rep 6, 25885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.