Abstract
Agriculture is the source of food for both humans and animals. With the growing population demands, agricultural production needs to be scaled up where nanotechnology can play a significant role. The use of nanotechnology in agriculture can manage plant disease and growth for better and quality output. Therefore, this review focuses on the use of various nanoparticles for detection of nutrients and contaminants, nanosensors for monitoring the environmental stresses and crop conditions as well as the use of nanotechnology for plant pathogen detection and crop protection. In addition, the delivery of plant growth regulators and agrichemicals like nanopesticides and nanofertilizers to the plants along with the delivery of DNA for targeted genetic engineering and production of genetically modified (GM) crops are discussed briefly. Further, the future concerns regarding the use of nanoparticles and their possible toxicity, impact on the agriculture and ecosystem needs to be assessed along with the assessment of the nanoparticles and GM crops on the environment and human health.
Keywords: Nanotechnology, Disease detection, Nanopesticides, Nanofertilizers, Nanosensors, Plant health, Sustainable agriculture
Introduction
Nanotechnology has a great scope to address various issues prevalent in the agricultural sector. The management of energy and resources as well as problems related to overuse of pesticides and fertilizers and their impact can be resolved by the proper utilization of nanotechnology in the plant disease and growth management (Chen and Yada 2011; Ditta 2012; Parisi et al. 2015). The increasing demand for agricultural commodities and recent technologies for better and higher yields can be met by optimizing agricultural practices and the introduction of nanoscale materials in agriculture (Gogos et al. 2012; He et al. 2019). Nanotechnology may be utilized for the modernization of the agricultural sector for sustained and income-generating occupation. This can be achieved by maximizing the agricultural outputs for crop yields by minimizing the cost of the inputs of various fertilizers and monitoring environmental conditions (Servin et al. 2015; Fraceto et al. 2016). Recently, the emphasis on the use of nanomaterials has increased because of their potential for seed germination and growth. These particles can control the release of agrichemicals and minimize nutrient loss during fertilization. Besides this, their application in agriculture will boost the quality and production of food globally and in an environmentally friendly manner by protecting and solving soil and water problems (Biswal et al. 2012; Ditta 2012; Khot et al. 2012; Sonkaria et al. 2012; Prasad et al. 2014; Sekhon 2014).
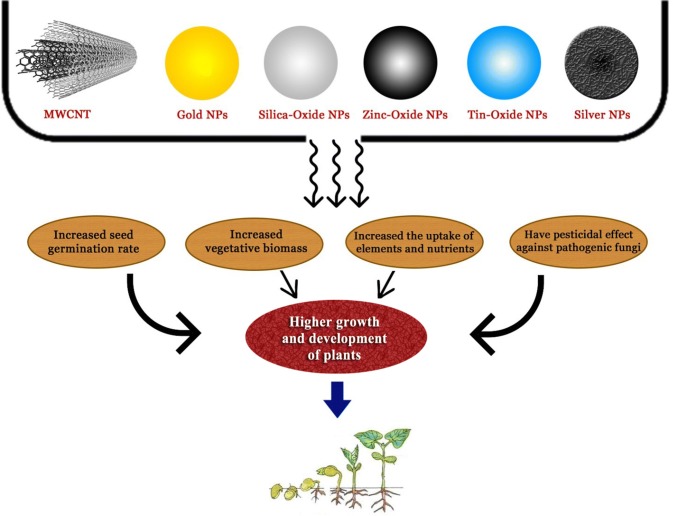
Nanomaterials (NMs) have become an integral part of nanoparticles (NPs) for monitoring and stimulating the plant growth because of their inherent unique chemical, electrical mechanical and thermal properties. The use of multi-walled carbon nanotubes (MWCNTs) has been observed to significantly increase the germination and vegetative biomass of tomato seeds (Khodakovskaya et al. 2009). For instance, the use of C-based nanotubes along with the NPs of other elements/compounds such as Au, SiO2, ZnO, and TiO2 has increased the uptake of elements and nutrients ultimately leading to better growth of plants (Khot et al. 2012). The utilization of nanopesticides reveals the concern for its possible toxicity. However, its use controls the release and delivery of herbicides as per the soil condition and thus reduces the required quantity of herbicides for weed removal (Gruère et al. 2011). Also, the nanoparticles may have a pesticidal side effect on pathogenic fungi. Further, it is observed that silver NPs inhibit the germination of conidia of Raffaelea and prevent the death of oak trees (Nair et al. 2010).
Therefore, the knowledge of nanoparticles and their potential applications in plant disease management is crucial for the researchers as well as the general public to address the problems in agriculture and increase the productivity to meet the increasing demands of the huge population. This review will briefly discuss different nanoparticles for the monitoring the plant growth, plant pathogen detection, delivery of agrichemicals, plant growth regulators and DNA for the betterment of crops and their productivity.
Nanotechnology for monitoring plant growth
Nanoparticles for detection of nutrients and contaminants
The use of various nanoparticles (NPs) can be employed for the regulation of plant disease and growth by various modes as depicted in Fig. 1. Besides this, the detection of contaminants like heavy metals is very crucial for monitoring proper plant growth. The separation of heavy metal from aqueous medium using C-based nanomaterials becomes difficult after the adsorption process. However, the magnetic metal/metal oxide nanocomposites’ use can simply the heavy metals separation by the application of a magnetic field. The nanocomposite of multi-walled carbon nanotubes (MWCNTs) coupled with ferrous ferric oxide (Fe3O4), i.e. MWCNT/nano-Fe3O4 has greater adsorption capacity for Cr3+ in comparison to pristine MWCNT or activated carbon in an aqueous solution (Gupta et al. 2011a). However, the amount of Cr3+ adsorbed is dependent on factors like pH, wet time, and the mixing rate of the solution. Besides this, the nanocomposite can also be used in packaging material of column-based filtration. Similarly, the nanocomposite of multi-walled carbon nanotubes with alumina (Al2O3), MWCNT/Al2O3, has an enhanced removal capability for Pb2+ (Gupta et al. 2011b). In addition, titanium dioxide (TiO2) is very efficient in nanocomposites for the detection and removal of heavy metals. The removal of several heavy metals by TiO2 involves the surface complexation mechanisms, while graphite oxide (GO) removes heavy metals through electrostatic interaction. The formation of TiO2-based flower-like structures on GO nanosheets further ease the process for removal of heavy metals like cadmium, lead and zinc (Lee and Yang 2012).
Fig. 1.
Application of various nanoparticles (NPs) for the regulation of plant disease and growth by increased seed germination, vegetative biomass, uptake of elements and nutrients along with the management of pathogenic fungi
As all toxic heavy metals do not exist in elemental forms (for example, As and Cr are present in oxidized anion forms), so the use of C-based nanomaterials is very effective in the removal of these toxic forms by sorption. The nanotubes and nanosheets containing iron oxide are found to be effective in removing arsenate and arsenite using positively charged iron particles for electrostatic interaction (Mishra and Ramaprabhu 2010; Veličković et al. 2012; Yu et al. 2015). Arsenite and arsenate have been removed by GO with iron oxide surface groups, respectively, up to 54.18 mg/g and 26.76 mg/g (Yu et al. 2015), with similar values of 53.15 and 39.08 mg/g for MWCNT coated with iron oxide nanoparticles (Mishra and Ramaprabhu 2010). The chromate (Cr(VI)) anions are removed using various nanomaterial adsorbents, however, their removal mechanism is unclear due to a variety of nanocomposites tested (Pillay et al. 2009; Dinda et al. 2013; Gu et al. 2013; Kumar and Rajesh 2013). In this light, pristine (non-functionalized MWCNT) can absorb most of Cr(VI) present in traces in an aqueous solution (Pillay et al. 2009; Gu et al. 2013). Furthermore, GO with liquid cation Aliquat-336 show effective sorption and total removal of toxic chromium from tannery effluent as well as wastewater (Kumar and Rajesh 2013).
The utilization of zero-valent iron nanoparticles (nZVI) with compost from organic wastes has been investigated for its potential for decontamination in soils (Fajardo et al. 2015). The metals treated with nZVI via immobilization can prevent its transport to the groundwater through different layers of soil (Crane and Scott 2012). However, the immobilization of metals by nZVI in soils has only been tested in “in vitro” conditions till date. The immobilization of Pb and Zn in nZVI-treated soils has been demonstrated through leachate analysis (Gil-Díaz et al. 2014b). Some positive results for mobilizing arsenic (Gil-Díaz et al. 2014a), antimony (Dorjee et al. 2014) besides other metals/metalloids in contaminated soils have been obtained. Though the long-term fate of nZVI in terms of environmental toxicity is a matter of concern, the nanoparticles can easily enter the food chain and cause bioaccumulation as well as spread other non-target pollutants in the soil (Masciangioli and Zhang 2003). Therefore, an in-depth understanding of the behaviour of nanomaterials is crucial to manage and control its toxicity.
Nanosensors for monitoring the environmental stresses and crop conditions
The idea of imaging sensors has been explored to monitor the plants under greenhouse during growth (Parsons et al. 2009). However, the process of assessing the ornamental quality is difficult, as only subjective references could be utilized. The feedforward artificial neural network (ANN) has been used to segregate the images, however, the prediction models in itself cannot achieve high accuracy because of low systematic improvement and robustness. The two important developments in the field of nanotechnology are biosensor and metal oxide-based semiconducting nanowires/nanotubes for plant monitoring. These assist in the exploration of nanoscale-based devices and sensors using different transducers (electric, optical, electrochemical and magnetic) as well as for in vivo detection (Liu 2008; Xia et al. 2010). In addition to this, wireless sensor technology (WST) in speciality crops has added new facets to the earlier communications system. The development of wireless sensor networking (WSN) technology has reduced the cost and power consumption and aided data processing using multifunctional sensor nodes. Besides, WSN helps in connecting to other sensors and access to data with external users. The applications of WST for monitoring crops along with some other applications are listed in Table 1.
Table 1.
Wireless sensing technology (WST) applications in speciality crops
| Sensor type | Applications | References |
|---|---|---|
| Wireless sensor networking (WSN) | WSN configurations for regulating vineyards | Burrell et al. (2004) |
| WSN | Management of heat up and potential frost damage in wine production | Beckwith et al. (2004) |
| WSN | Remote sensing network based on ZigBee | Morais et al. (2008) |
| WSN | Pest and phytophthora control in a potato field | Baggio (2005) |
| WSN | Management of distributed greenhouse | Gonda and Cugnasca (2006) |
| WSN | Regulation of environmental conditions in greenhouses with melon and cabbage | Yoo et al. (2007) |
| WSN | Real-time measurement of substrate water, temperature, electrical conductivity, photosynthetic radiation and leaf wetness | Lea-Cox et al. (2007) |
| WSN | ZigBee-based system for monitoring greenhouses | Zhou et al. (2007) |
| WSN | Temperature and humidity measurement of greenhouse horticulture | van Tuijl et al. (2007) |
| Radio-frequency identification (RFID) | Imaging and environmental sensing in greenhouses | Yang et al. (2008) |
| Wireless sensor node | Greenhouse monitoring | Wang et al. (2007) |
| Wireless sensor node | Measurement of soil parameters (electrical conductivity, humidity, salinity and temperature) in precision horticulture; | López et al. (2009) |
| WSN | Monitoring refrigerated storage chamber | Ruiz-Garcia et al. (2008) |
WSN is one of the best inventions of the twenty-first century. Unlike radio-frequency identification (RFID) system devices which lack cooperative capabilities, WSN allows communication among different network topologies and multihop. A multihop network is dynamically self-organized, where the nodes establish and maintain mesh connectivity among them. Further, motes (a small single unit of nodes) operate on various models to establish networks. These can also collect data on temperature and humidity (Ruiz-Garcia et al. 2009). RFID was initially developed for identification but later exploration in other facets led to the invention of various RFID-based wireless sensors. RFID is commercially active and semi-passive tags capable of collecting data information related to temperature (Amador et al. 2009; Jedermann et al. 2009). Besides this, some semi-passive tags coupled with sensors are under trial for monitoring moisture (Abad et al. 2009), vibration (Todd et al. 2009), light (Cho et al. 2005; Abad et al. 2009), pH (Steinberg and Steinberg 2009) as well as the amount of gases like acetaldehyde or ethylene (Vergara et al. 2007). These tags can also detect microbial contamination in food products and alert us with the food-borne pathogens in the supply chain (Wentworth 2003). These wireless sensor devices can be installed even in places without cable facility, such as in large fields (Morais et al. 2008) or can be embedded within the implements, that can match the readings to the true in situ crop properties (Ruiz-Garcia et al. 2009).
Nanotechnology for plant pathogen detection and crop protection
Pathogens infecting the plants are among the major factors responsible for reducing agricultural produce. Several emerging plant infectious diseases besides the old ones pose a threat to sustainable food supply (Roberts et al. 2006; Savary et al. 2012). Usually, the symptoms are visible on plant parts (leaves, stems and fruits) after infections (López et al. 2003; Al-Hiary et al. 2011). These symptoms help in the disease diagnosis, however, the early detection of pathogens is not possible as the plant infections are symptomless at the initial stages of infection. So, there is an immense need for prior detection of pathogens to control the proliferation of the diseases and monitor the plant health. This will also aid in the prevention of developing other new diseases, especially quarantine pathogens in case of cross-border issues (Vincelli and Tisserat 2008; Miller et al. 2009). For this purpose, several methods for diagnosing plant diseases like DNA-based methods and immunoassays (López et al. 2003), polymerase chain reaction (PCR)-assisted technique of DNA hybridization (Gutiérrez-Aguirre et al. 2009; Yvon et al. 2009), as well as other immunoassays namely enzyme-linked immunosorbent assay (ELISA), ELISA-based tissue print or direct dot-blot immunoassay (DTBIA) have been employed for the detection of pathogens (Nolasco et al. 2002). These molecular methods are robust but do have some limitations, especially when the few pathogens are present in plant parts or vectors during the early stages of infection. To overcome these limitations, biosensors based on antibodies and DNA play a significant role in the detection of plant pathogens (Sadanandom and Napier 2010; Singh et al. 2013). Some of the biosensors for detection of plant pathogens are shown in Table 2.
Table 2.
Biosensors for detection of the plant pathogen.
Source: Khater et al. (2017)
| Type of biosensor | Detection methods | Detecting plant pathogen |
|---|---|---|
| Antibody-based | Electrochemical/Enzyme label | Cucumber mosaic virus |
| Electrochemical/AuNPs tag | Pantoea stewartii sbusp. stewartii | |
| Electrochemical/label-free | Maize chlorotic mottle virus, Plum pox virus, Prunus necrotic ringspot virus | |
| Optical/AuNPs tag | Pantoea stewartii subsp. Stewartii, Potato virus x, | |
| Optical/fluorescent tag | Acidovorax avenae subsp., Citrulli, Chilli vein-banding mottle virus. Melon yellow spot virus, Pantoea stewartii sbusp., Stewartii, Watermelon silver mottle virus | |
| Optical/label free | Cymbidium mosaic virus, Odontoglossum ringspot virus | |
| DNA-based | Electrochemical/label-free | Plum pox virus, sugarcane white leaf disease, Trichoderma harzianum |
| Optical/AuNPs tags | Acidovorax avenae subsp. Citrulli, Banana bunchy top virus, Pseudomonas syringae | |
| Optical/magnetic tag | Botrytis cinereal | |
| Optical/fluorescent tag | Banana streak virus | |
| Optical/luminescent tag | Banana bunchy top virus |
Nanotechnology for delivery of agrichemicals
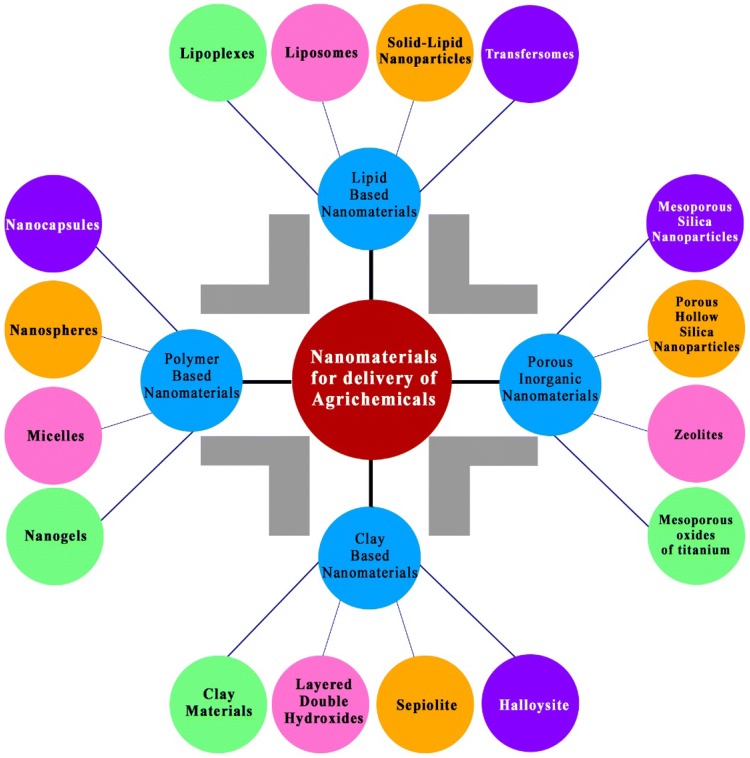
Nanotechnology has an immense potential to develop an agricultural sector in such a manner that its environmental impacts are minimal with the use of resources and proper management of the use of various agrichemicals (Chen and Yada 2011; Ditta 2012; Parisi et al. 2015). Figure 2 depicts the lipid, polymer, clay and porous inorganic-based nanomaterials for the delivery of agrichemicals (nanopesticides and nanofertilizers) to the plants through application in soil.
Fig. 2.
The lipid, polymer, clay and porous inorganic-based nanomaterials for the delivery of agrichemicals (nanopesticides and nanofertilizers) to the plants through application in soil
Nanopesticide
Nanotechnology is growing with promising “nanopesticides” for plant protection and management (Gogos et al. 2012; Khot et al. 2012; Kah et al. 2013). The “nanopesticide” consists of nanosized engineered structures having pesticidal properties (Bergeson 2010; Kookana et al. 2014). These nanosized particles are beneficial due to their unique properties like higher solubility, mobility and durability. In addition, they are designed for targeted release to avoid the harmful effects of chemicals and the development of resistance among pests (Sasson et al. 2007; Kah and Hofmann 2014). Nanocarriers are efficient in delivering agrichemicals including plant growth regulators (PGRs) to the specific target. The applications of nanomaterials for the detection of pesticide residues are enlisted in Table 3. Various mechanisms from the encapsulation of polymers to the use of bond interactions are utilized for long storage and controlled release of these nanoparticles (Pandey et al. 2003; Jordan et al. 2005; Kumar et al. 2014). Recently, Liang et al. (2017) reported bioinspired mussel avermectin NPs [P (St-MAA)-Av-Cat] which showed strong adhesion to plant leaves that can further reduce the loss and possible contamination of soils due to pesticide residues. It further exhibited excellent retention of avermectin with better storage stability and sustained release. In addition to this, Kumar et al. (2019) have developed a green, rapid, and cost-effective probe for detecting Mn(II) ions based on colorimetric measurements. For this, B-AgNPs were prepared by heating AgNPs with the extract from Bhilwa nuts. Then Mn(II) ions were used for the aggregation of B-AgNPs that leads to the colour change from yellowish-brown to dark red and also causes red shift (from 404 to 432 nm) in the surface plasmon resonance (SPR) peak. This colorimetric probe could be efficiently used for the detection of Mn(II) ions in water for field applications.
Table 3.
Application of nanomaterials for the detection of pesticide residues
| Pesticide/herbicide | Sensing material | Type of sensor | References |
|---|---|---|---|
| 2,4,5-Trichlorophenoxy acetic acid | Poly-O-toluidine zirconium (IV) phosphate nanocomposite | Electrochemical | Khan and Akhtar (2011) |
| Fenitrothion in water samples | Nano-TiO2/nafion composite | Electrochemical | Kumaravel and Chandrasekaran (2011) |
| Melamine in milk samples | 18-crown-6 ether functionalized Au nanoparticles | Optical | Kuang et al. (2011) |
| Organochlorine and organophosphorus pesticides in cabbage | Amino-functionalized nanocomposite with tetraethylenepentamine | Chromatography | Zhao et al. (2011) |
| Methyl parathion in water samples | Nano-ZrO2/graphite/paraffin | Electrochemical | Parham and Rahbar (2010) |
| Methyl parathion in vegetables (cabbage, spinach, lettuce) | Nano-Au/nafion composite | Electrochemical | Kang et al. (2010) |
| Methyl parathion, chlorpyrifos | Nano-sized polyaniline matrix with SWCNT, single-stranded DNA and enzyme | Electrochemical | Viswanathan et al. (2009) |
| Fenamithion and acetamiprid in water samples | Cd-tellurium quantum dots with p-sulfonatocalix(4)arene | Luminescence | Qu et al. (2009) |
| Parathion in water samples | Nano-ZrO2/Au composite | Electrochemical | Wang and Li (2008) |
| Dichlorvos and paraoxon in drinking water samples | Acetylcholinesterase and pyranine immobilized on nano-sized liposomes | Optical nanobiosensor | Vamvakaki and Chaniotakis (2007) |
| Parathion in vegetables | Nano-TiO2 on the glassy carbon electrode | Electrochemical | Li et al. (2006) |
| Pirimicarb in vegetables | Molecular imprinted nanopolymers | Piezoelectric | Sun and Fung (2006) |
The polymeric nanospheres and nanocapsules along with nanogels and nanofibers are developed for efficient and controlled release of pesticides (Anton et al. 2008; Bhagat et al. 2013; Brunel et al. 2013; Xiang et al. 2013). Furthermore, the complex nano-based formulations like solid lipid nanoparticles (NPs), inorganic NPs coupled with organic active ingredients and coated liposomes are more efficient for the delivery of pesticides. The examples of organic active ingredients are mesoporous silica and calcium carbonate used as carriers for the controlled release of pesticides. The TiO2-based nanoparticles (TiO2-NPs) are utilized to decrease the residual impact on plants and soil by photocatalyzing the released organic ingredients (Bang et al. 2009; Qian et al. 2011; Ao et al. 2012; Kang et al. 2012; Nguyen et al. 2012a, b; Song et al. 2012a, b). It is estimated that about 2 million tons of pesticides are used per year worldwide. Among this, most (around 45%) is used in Europe, 25% in the USA and the remaining 25% in other parts of the world (De et al. 2014). Therefore, proper management of pesticides is essential because they can lead to resistance among pests and pathogens, impact on soil biodiversity by killing useful microbes and can cause biomagnification of pesticides along with destroying natural pollinator like birds (Tilman et al. 2002). Furthermore, the use of nanoparticles assisted with a gene or DNA-based transfer techniques in plants for developing insects and pest-resistant varieties as well as the use of nanomaterial for developing various biosensors are trends that can modernize the agricultural sector (Rai and Ingle 2012).
Nanofertilizer
In the same manner, as nanopesticides, the nanofertilizers have a significant role to enhance the agricultural produce by supplying sufficient nutrients to the plants for their optimum growth and also modifying the conventional fertilization system (Liu and Lal 2015). The nanocoatings applied on the surface of fertilizers can adhere to plant parts more efficiently because of increased surface tension (Ghormade et al. 2011; Yang et al. 2012). Therefore, nanomaterial-based fertilizers, e.g. nutrient-augmented zeolites (Malekian et al. 2011; Zwingmann et al. 2011) enhances the efficiency of plants for the uptake of nutrients and thereby reducing the adverse effects of conventional fertilization system (Liu and Lal 2015). Khalifa and Hasaneen (2018) used chitosan (CS)-based fertilizer for the slow release. The CS–PMAA–NPK NP complex was from CS nanoparticles by polymerizing methacrylic acid (PMAA) for the entrapment of nitrogen, phosphorus and potassium (NPK) nanoparticles (NP). The impact of CS–PMAA–NPK NP complex was evaluated on the garden pea at different concentrations. The results showed reduced root elongation with the accumulation of starch at its tip in a dose-dependent manner. The mitotic cell division was also induced along with upregulation of some proteins like convicilin, vicilin and legumin β. However, the genotoxic effect was observed on the DNA at all the concentrations used, therefore, negative effects could be observed on plants and environment by this carrier with its accumulation in the agricultural fields.
Nanotechnology for delivery of plant growth regulators (PGRs)
Plant growth regulators (PGRs) are plant hormones that play an essential role in agriculture for increasing productivity and quality of crops (E1-Otmani et al. 2000; Rademacher 2015). PGRs can be either natural or synthetic compounds and mainly include auxin, gibberellins, cytokinins, and ethylene (Pereira et al. 2017). In 2014, the global market for agricultural inputs accounted for around 2.5% from these compounds (Rademacher 2015). Chitosan nanoparticles-based release system has been used for the controlled release of nitric oxide (Oliveira et al. 2016), whereas micro-particulates were used for the controlled release of brassinosteroid hormones (Quiñones et al. 2010) and naphthalene acetic acid (Tao et al. 2012). However, the major obstacles in the use of PGRs are their degradation when exposed to environmental factors like heat and light. To overcome these difficulties, the nanoscale materials are employed to increase the stability as well as to reduce their adverse environmental impacts (Campos et al. 2014; Rossi et al. 2014; Grillo et al. 2016). Various nanocarrier systems based on chitosan (CS) have been used for crop protection as well as for gene delivery (Kashyap et al. 2015).
Nanotechnology for delivery of DNA (targeted genetic engineering)
Nanotechnology has a significant role in producing transgenic plants through genetic engineering that have been proven to be an immense asset for the agriculture sector as well as for the pharmaceutical industry (Cunningham et al. 2018; Fachel et al. 2019). Most of the herbicide-tolerant plants, e.g. soy and cotton plants in the US are produced by modifying the gene using genetic engineering (Fernandez-Cornejo and Caswell 2006). The major aspect of transgenic plants is their potential to produce recombinant and therapeutic proteins of pharmaceutical importance, as they are safer medium compared to animal-based (yeast or bacterial) expression systems (Daniell 2006; Verma and Daniell 2007). The use of a suitable binary vector with Agrobacterium is an appropriate method to produce transgenic plants. Arabidopsis thaliana and Nicotiana tabacum are model systems for the genetic transformation of the nucleus and chloroplast of cells, as they are efficient for delivery of DNA and recovery of transformants. Furthermore, flowers immersed in liquid Agrobacterium culture by the floral dip method obtain a stable nuclear transformation of Arabidopsis germline cells (Clough and Bent 1998).
Salinity or salt stress poses a major concern for crop productivity. It is stress due to the combined effect of elevated osmotic and ionic stress in the soil (Flowers et al. 2014; Song and Wang 2014; Zhang et al. 2014). Plants have inbuilt mechanisms to counter these abiotic stresses through the accumulation of compatible solutes (Chen and Murata 2002). Compatible solutes are highly soluble in water and non-toxic low-molecular-weight compounds. Their concentration increases significantly in the cytosol in response to the saline environment, thereby decreasing the detrimental effects of salinity (Koyro et al. 2013; Zhang et al. 2014). One of the compatible solutes found in plants and animals (especially in marine invertebrates, bacteria and mammals) is glycine betaine (GB) (Chen and Murata 2011). The use of gene technology to transfer GB or genes related to GB has developed tolerance against various environmental stresses (Sakamoto and Murata 2000; Chen and Murata 2008). For example, GB has been applied in the case of non-GB synthesizing plants, tomato (Wyn Jones and Storey 1981). Potassium, on the other hand, balances membrane potential, activates enzymes and regulates the osmotic pressure and controls the stomal movement of cells (Chérel 2004). Besides this, it also acts as an activator of many biochemical processes like photosynthesis and signalling agent for linking plant adaptive responses to the environment (Römheld and Kirkby 2010; Anschütz et al. 2014). Therefore, using targeted gene delivery, genetically modified (GM) crops with specific stress resistance can be developed to withstand the adverse environmental conditions and thus enhance agricultural production. The GM crops are among the fastest adopted commodities in the agricultural biotechnology industry. However, there is a need for precise and sensitive detection methods of genetically modified organisms (GMOs) in GM crops before they are released commercially. Furthermore, an appropriate regulatory framework should be set up to assess the environmental and health-related risks associated with the use of GM crops (Kamle et al. 2017).
Conclusion and future perspectives
The application of nanomaterials in agriculture raises various socio-economic concerns due to their potential to contaminate food, cause phytotoxicity and ultimately impact the ecosystem (Chaudhry and Castle 2011). The impact of nanomaterials in agriculture and their health outcomes have been reviewed by Bouwmeester et al. (2009) and Kahru and Dubourguier (2010). However, the “toxicokinetics and toxicodynamics” of nanomaterials still needs in-depth research (Bouwmeester et al. 2009; Bergeson 2010). Nair et al. (2010) pointed out the effects of the nanomaterials on various agricultural produce as well as the alternative methods of cropping systems if nanomaterials are not degraded quickly. However, in a nutshell, future concern still lies in better understanding of their toxicity mechanisms in biological systems, exposure outcomes and proper control measures. Therefore, future research should be focused on understanding nanomaterials’ toxicity, their impact on the ecosystem and suitable methods for sustainable agricultural production along with the proper assessment of the impact of GM crops on the environment and human health.
Acknowledgements
Authors are highly grateful to the authority of the respective departments and institutions for their support in doing this research. The author PK would like to thank DST-SERB (file no ECR/2017/001143) for financial support.
Author contributions
PK conceived and designed the manuscript. DKM, MK and PK wrote the manuscript. SD and AKM helped in the editing of the manuscript. VT and RS drew the figures. PK critically reviewed the manuscript and did the required editing.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Madhu Kamle and Dipendra Kumar Mahato equally contributed.
Contributor Information
Awdhesh Kumar Mishra, Email: awadhesh.biotech07@gmail.com.
Pradeep Kumar, Email: pkbiotech@gmail.com.
References
- Abad E, Palacio F, Nuin M, De Zarate AG, Juarros A, Gómez JM, Marco S. RFID smart tag for traceability and cold chain monitoring of foods: demonstration in an intercontinental fresh fish logistic chain. J Food Eng. 2009;93(4):394–399. [Google Scholar]
- Al-Hiary H, Bani-Ahmad S, Reyalat M, Braik M, ALRahamneh Z. Fast and accurate detection and classification of plant diseases. Int J Comput Appl. 2011;17(1):31–38. [Google Scholar]
- Amador C, Emond J-P, do Nascimento Nunes MC. Application of RFID technologies in the temperature mapping of the pineapple supply chain. Sens Instrum Food Qual Saf. 2009;3(1):26–33. [Google Scholar]
- Anschütz U, Becker D, Shabala S. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J Plant Physiol. 2014;171(9):670–687. doi: 10.1016/j.jplph.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Anton N, Benoit J-P, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—a review. J Control Release. 2008;128(3):185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Ao M, Zhu Y, He S, Li D, Li P, Li J, Cao Y. Preparation and characterization of 1-naphthylacetic acid–silica conjugated nanospheres for enhancement of controlled-release performance. Nanotechnology. 2012;24(3):035601. doi: 10.1088/0957-4484/24/3/035601. [DOI] [PubMed] [Google Scholar]
- Baggio A (2005) Wireless sensor networks in precision agriculture. In: ACM workshop on real-world wireless sensor networks (REALWSN 2005), Stockholm, Sweden, 2005. Citeseer, pp 1567–1576
- Bang SH, Yu YM, Hwang IC, Park HJ. Formation of size-controlled nano carrier systems by self-assembly. J Microencapsul. 2009;26(8):722–733. doi: 10.3109/02652040902726994. [DOI] [PubMed] [Google Scholar]
- Beckwith R, Teibel D, Bowen P (2004) Report from the field: results from an agricultural wireless sensor network. In: 29th annual IEEE international conference on local computer networks. IEEE, pp 471–478
- Bergeson LL. Nanosilver: US EPA's pesticide office considers how best to proceed. Environ Qual Manag. 2010;19(3):79–85. [Google Scholar]
- Bhagat D, Samanta SK, Bhattacharya S. Efficient management of fruit pests by pheromone nanogels. Sci Rep. 2013;3:1294. doi: 10.1038/srep01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal SK, Nayak AK, Parida UK, Nayak PL. Applications of nanotechnology in agriculture and food sciences. Int J Sci Innov Discov. 2012;2(1):21–36. [Google Scholar]
- Bouwmeester H, Dekkers S, Noordam MY, Hagens WI, Bulder AS, De Heer C, Ten Voorde SECG, Wijnhoven SWP, Marvin HJP, Sips AJAM. Review of health safety aspects of nanotechnologies in food production. Regul Toxicol Pharmacol. 2009;53(1):52–62. doi: 10.1016/j.yrtph.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Brunel F, El Gueddari NE, Moerschbacher BM. Complexation of copper (II) with chitosan nanogels: toward control of microbial growth. Carbohyd Polym. 2013;92(2):1348–1356. doi: 10.1016/j.carbpol.2012.10.025. [DOI] [PubMed] [Google Scholar]
- Burrell J, Brooke T, Beckwith R. Vineyard computing: sensor networks in agricultural production. IEEE Pervas Comput. 2004;1:38–45. [Google Scholar]
- Campos EVR, de Oliveira JL, Fraceto LF. Applications of controlled release systems for fungicides, herbicides, acaricides, nutrients, and plant growth hormones: a review. Adv Sci Eng Med. 2014;6(4):373–387. [Google Scholar]
- Chaudhry Q, Castle L. Food applications of nanotechnologies: an overview of opportunities and challenges for developing countries. Trends Food Sci Technol. 2011;22(11):595–603. [Google Scholar]
- Chen TH, Murata N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr Opin Plant Biol. 2002;5(3):250–257. doi: 10.1016/s1369-5266(02)00255-8. [DOI] [PubMed] [Google Scholar]
- Chen THH, Murata N. Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2008;13(9):499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Chen THH, Murata N. Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ. 2011;34(1):1–20. doi: 10.1111/j.1365-3040.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Yada R. Nanotechnologies in agriculture: new tools for sustainable development. Trends in Food Sci Technol. 2011;22(11):585–594. [Google Scholar]
- Chérel I. Regulation of K+ channel activities in plants: from physiological to molecular aspects. J Exp Bot. 2004;55(396):337–351. doi: 10.1093/jxb/erh028. [DOI] [PubMed] [Google Scholar]
- Cho N, Song S-J, Kim S, Kim S, Yoo H-J (2005) A 5.1-μW UHF RFID tag chip integrated with sensors for wireless environmental monitoring. In: Esscirc 2005: proceedings of the 31st European solid-state circuits conference, pp 279–282
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Crane RA, Scott TB. Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater. 2012;211:112–125. doi: 10.1016/j.jhazmat.2011.11.073. [DOI] [PubMed] [Google Scholar]
- Cunningham FJ, Goh NS, Demirer GS, Matos JL, Landry MP. Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 2018;36(9):882–897. doi: 10.1016/j.tibtech.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H. Production of biopharmaceuticals and vaccines in plants via the chloroplast genome. Biotechnol J Healthc Nutr Technol. 2006;1(10):1071–1079. doi: 10.1002/biot.200600145. [DOI] [PubMed] [Google Scholar]
- De A, Bose R, Kumar A, Mozumdar S. Targeted delivery of pesticides using biodegradable polymeric nanoparticles. Berlin: Springer; 2014. [Google Scholar]
- Dinda D, Gupta A, Saha SK. Removal of toxic Cr (VI) by UV-active functionalized graphene oxide for water purification. J Mater Chem A. 2013;1(37):11221–11228. [Google Scholar]
- Ditta A. How helpful is nanotechnology in agriculture? Adv Nat Sci Nanosci Nanotechnol. 2012;3(3):033002. [Google Scholar]
- Dorjee P, Amarasiriwardena D, Xing B. Antimony adsorption by zero-valent iron nanoparticles (nZVI): Ion chromatography—inductively coupled plasma mass spectrometry (IC–ICP-MS) study. Microchem J. 2014;116:15–23. [Google Scholar]
- El-Otmani M, Coggins CW, Jr, Agustí M, Lovatt CJ. Plant growth regulators in citriculture: world current uses. Crit Rev Plant Sci. 2000;19(5):395–447. [Google Scholar]
- Fachel FNS, Schuh RS, Veras KS, Bassani VL, Koester LS, Henriques AT, Braganhol E, Teixeira HF. An overview of the neuroprotective potential of rosmarinic acid and its association with nanotechnology-based delivery systems: a novel approach to treating neurodegenerative disorders. Neurochem Int. 2019;122:47–58. doi: 10.1016/j.neuint.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Fajardo C, Gil-Díaz M, Costa G, Alonso J, Guerrero AM, Nande M, Lobo MC, Martín M. Residual impact of aged nZVI on heavy metal-polluted soils. Sci Total Environ. 2015;535:79–84. doi: 10.1016/j.scitotenv.2015.03.067. [DOI] [PubMed] [Google Scholar]
- Fernandez-Cornejo J, Caswell MF. The first decade of genetically engineered crops in the United States. USDA-ERS Econ Info Bull. 2006;11:20. [Google Scholar]
- Flowers TJ, Munns R, Colmer TD. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot. 2014;115(3):419–431. doi: 10.1093/aob/mcu217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraceto LF, Grillo R, de Medeiros GA, Scognamiglio V, Rea G, Bartolucci C. Nanotechnology in agriculture: which innovation potential does it have? Front Environ Sci. 2016;4:20. [Google Scholar]
- Ghormade V, Deshpande MV, Paknikar KM. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv. 2011;29(6):792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Gil-Díaz M, Alonso J, Rodríguez-Valdés E, Pinilla P, Lobo MC. Reducing the mobility of arsenic in brownfield soil using stabilised zero-valent iron nanoparticles. J Environ Sci Health A. 2014;49(12):1361–1369. doi: 10.1080/10934529.2014.928248. [DOI] [PubMed] [Google Scholar]
- Gil-Díaz M, Ortiz L, Costa G, Alonso J, Rodríguez-Membibre ML, Sánchez-Fortún S, Pérez-Sanz A, Martín M, Lobo MC. Immobilization and leaching of Pb and Zn in an acidic soil treated with zerovalent iron nanoparticles (nZVI): physicochemical and toxicological analysis of leachates. Water Air Soil Pollut. 2014;225(6):1990. [Google Scholar]
- Gogos A, Knauer K, Bucheli TD. Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem. 2012;60(39):9781–9792. doi: 10.1021/jf302154y. [DOI] [PubMed] [Google Scholar]
- Gonda L, Cugnasca CE (2006) A proposal of greenhouse control using wireless sensor networks. In: Computers in agriculture and natural resources, 23–25 July 2006, Orlando Florida, 2006. American Society of Agricultural and Biological Engineers, p 229
- Grillo R, Abhilash PC, Fraceto LF. Nanotechnology applied to bio-encapsulation of pesticides. J Nanosci Nanotechnol. 2016;16(1):1231–1234. doi: 10.1166/jnn.2016.12332. [DOI] [PubMed] [Google Scholar]
- Gruère G, Narrod C, Abbott L. Agricultural, food, and water nanotechnologies for the poor. Washington, DC: International Food Policy Research Institute; 2011. [Google Scholar]
- Gu H, Rapole SB, Huang Y, Cao D, Luo Z, Wei S, Guo Z. Synergistic interactions between multi-walled carbon nanotubes and toxic hexavalent chromium. J Mater Chem A. 2013;1(6):2011–2021. [Google Scholar]
- Gupta VK, Agarwal S, Saleh TA. Chromium removal by combining the magnetic properties of iron oxide with adsorption properties of carbon nanotubes. Water Res. 2011;45(6):2207–2212. doi: 10.1016/j.watres.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Agarwal S, Saleh TA. Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater. 2011;185(1):17–23. doi: 10.1016/j.jhazmat.2010.08.053. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Aguirre I, Mehle N, Delić D, Gruden K, Mumford R, Ravnikar M. Real-time quantitative PCR based sensitive detection and genotype discrimination of Pepino mosaic virus. J Virol Methods. 2009;162(1–2):46–55. doi: 10.1016/j.jviromet.2009.07.008. [DOI] [PubMed] [Google Scholar]
- He X, Deng H, Hwang H-m. The current application of nanotechnology in food and agriculture. J Food Drug Anal. 2019;27(1):1–21. doi: 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedermann R, Ruiz-Garcia L, Lang W. Spatial temperature profiling by semi-passive RFID loggers for perishable food transportation. Comput Electron Agric. 2009;65(2):145–154. [Google Scholar]
- Jordan J, Jacob KI, Tannenbaum R, Sharaf MA, Jasiuk I. Experimental trends in polymer nanocomposites—a review. Mater Sci Eng A. 2005;393(1–2):1–11. [Google Scholar]
- Kah M, Hofmann T. Nanopesticide research: current trends and future priorities. Environ Int. 2014;63:224–235. doi: 10.1016/j.envint.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Kah M, Beulke S, Tiede K, Hofmann T. Nanopesticides: state of knowledge, environmental fate, and exposure modeling. Crit Rev Environ Sci Technol. 2013;43(16):1823–1867. [Google Scholar]
- Kahru A, Dubourguier H-C. From ecotoxicology to nanoecotoxicology. Toxicology. 2010;269(2–3):105–119. doi: 10.1016/j.tox.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Kamle M, Kumar P, Patra JK, Bajpai VK. Current perspectives on genetically modified crops and detection methods. 3 Biotech. 2017;7(3):219. doi: 10.1007/s13205-017-0809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang T-F, Wang F, Lu L-P, Zhang Y, Liu T-S. Methyl parathion sensors based on gold nanoparticles and Nafion film modified glassy carbon electrodes. Sens Actuator B-Chem. 2010;145(1):104–109. [Google Scholar]
- Kang MA, Seo MJ, Hwang IC, Jang C, Park HJ, Yu YM, Youn YN. Insecticidal activity and feeding behavior of the green peach aphid, Myzus persicae, after treatment with nano types of pyrifluquinazon. J Asia Pac Entomol. 2012;15(4):533–541. [Google Scholar]
- Kashyap PL, Xiang X, Heiden P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int J Biol Macromol. 2015;77:36–51. doi: 10.1016/j.ijbiomac.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Khalifa NS, Hasaneen MN. The effect of chitosan–PMAA–NPK nanofertilizer on Pisum sativum plants. 3 Biotech. 2018;8(4):193. doi: 10.1007/s13205-018-1221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Akhtar T. Adsorption thermodynamics studies of 2, 4, 5-trichlorophenoxy acetic acid on poly-o-toluidine Zr (IV) phosphate, a nano-composite used as pesticide sensitive membrane electrode. Desalination. 2011;272(1–3):259–264. [Google Scholar]
- Khater M, de la Escosura-Muñiz A, Merkoçi A. Biosensors for plant pathogen detection. Biosens Bioelectron. 2017;93:72–86. doi: 10.1016/j.bios.2016.09.091. [DOI] [PubMed] [Google Scholar]
- Khodakovskaya M, Dervishi E, Mahmood M, Xu Y, Li Z, Watanabe F, Biris AS. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano. 2009;3(10):3221–3227. doi: 10.1021/nn900887m. [DOI] [PubMed] [Google Scholar]
- Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW. Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot. 2012;35:64–70. [Google Scholar]
- Kookana RS, Boxall ABA, Reeves PT, Ashauer R, Beulke S, Chaudhry Q, Cornelis G, Fernandes TF, Gan J, Kah M. Nanopesticides: guiding principles for regulatory evaluation of environmental risks. J Agric Food Chem. 2014;62(19):4227–4240. doi: 10.1021/jf500232f. [DOI] [PubMed] [Google Scholar]
- Koyro H-W, Zörb C, Debez A, Huchzermeyer B. The effect of hyper-osmotic salinity on protein pattern and enzyme activities of halophytes. Funct Plant Biol. 2013;40(9):787–804. doi: 10.1071/FP12387. [DOI] [PubMed] [Google Scholar]
- Kuang H, Chen W, Yan W, Xu L, Zhu Y, Liu L, Chu H, Peng C, Wang L, Kotov NA. Crown ether assembly of gold nanoparticles: melamine sensor. Biosens Bioelectron. 2011;26(5):2032–2037. doi: 10.1016/j.bios.2010.08.081. [DOI] [PubMed] [Google Scholar]
- Kumar ASK, Rajesh N. Exploring the interesting interaction between graphene oxide, Aliquat-336 (a room temperature ionic liquid) and chromium (VI) for wastewater treatment. RSC Adv. 2013;3(8):2697–2709. [Google Scholar]
- Kumar A, Khan S, Dhawan A. Comprehensive molecular analysis of the responses induced by titanium dioxide nanoparticles in human keratinocyte cells. J Transl Toxicol. 2014;1(1):28–39. [Google Scholar]
- Kumar D, Nair M, Painuli R. Highly responsive bioinspired AgNPs probe for the precise colorimetric detection of the Mn (II) in aqueous systems. Plasmonics. 2019;14(2):303–311. [Google Scholar]
- Kumaravel A, Chandrasekaran M. A biocompatible nano TiO2/nafion composite modified glassy carbon electrode for the detection of fenitrothion. J Electroanal Chem. 2011;650(2):163–170. [Google Scholar]
- Lea-Cox JD, Kantor G, Anhalt J, Ristvey A, Ross DS (2007) A wireless sensor network for the nursery and greenhouse industry. In: Southern nursery association research conference
- Lee Y-C, Yang J-W. Self-assembled flower-like TiO2 on exfoliated graphite oxide for heavy metal removal. J Ind Eng Chem. 2012;18(3):1178–1185. [Google Scholar]
- Li C, Wang C, Wang C, Hu S. Development of a parathion sensor based on molecularly imprinted nano-TiO2 self-assembled film electrode. Sens Actuator B-Chem. 2006;117(1):166–171. [Google Scholar]
- Liang J, Yu M, Guo L, Cui B, Zhao X, Sun C, Wang Y, Liu G, Cui H, Zeng Z. Bioinspired development of P (St–MAA)-avermectin nanoparticles with high affinity for foliage to enhance folia retention. J Agric Food Chem. 2017;66(26):6578–6584. doi: 10.1021/acs.jafc.7b01998. [DOI] [PubMed] [Google Scholar]
- Liu A. Towards development of chemosensors and biosensors with metal-oxide-based nanowires or nanotubes. Biosens Bioelectron. 2008;24(2):167–177. doi: 10.1016/j.bios.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Liu R, Lal R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci Total Environ. 2015;514:131–139. doi: 10.1016/j.scitotenv.2015.01.104. [DOI] [PubMed] [Google Scholar]
- López MM, Bertolini E, Olmos A, Caruso P, Gorris MT, Llop P, Penyalver R, Cambra M. Innovative tools for detection of plant pathogenic viruses and bacteria. Int Microbiol. 2003;6(4):233–243. doi: 10.1007/s10123-003-0143-y. [DOI] [PubMed] [Google Scholar]
- López J, Soto F, Sánchez P, Iborra A, Suardiaz J, Vera J. Development of a sensor node for precision horticulture. Sensors. 2009;9(5):3240–3255. doi: 10.3390/s90503240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekian R, Abedi-Koupai J, Eslamian SS. Influences of clinoptilolite and surfactant-modified clinoptilolite zeolite on nitrate leaching and plant growth. J Hazard Mater. 2011;185(2–3):970–976. doi: 10.1016/j.jhazmat.2010.09.114. [DOI] [PubMed] [Google Scholar]
- Masciangioli T, Zhang W-X. Peer reviewed: environmental technologies at the nanoscale. Washington, DC: ACS Publications; 2003. [DOI] [PubMed] [Google Scholar]
- Miller SA, Beed FD, Harmon CL. Plant disease diagnostic capabilities and networks. Annu Rev Phytopathol. 2009;47:15–38. doi: 10.1146/annurev-phyto-080508-081743. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Ramaprabhu S. Magnetite decorated multiwalled carbon nanotube based supercapacitor for arsenic removal and desalination of seawater. J Phys Chem C. 2010;114(6):2583–2590. [Google Scholar]
- Morais R, Fernandes MA, Matos SG, Serôdio C, Ferreira PJSG, Reis MJCS. A ZigBee multi-powered wireless acquisition device for remote sensing applications in precision viticulture. Comput Electron Agric. 2008;62(2):94–106. [Google Scholar]
- Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS. Nanoparticulate material delivery to plants. Plant Sci. 2010;179(3):154–163. [Google Scholar]
- Nguyen HM, Hwang IC, Park JW, Park HJ. Enhanced payload and photo-protection for pesticides using nanostructured lipid carriers with corn oil as liquid lipid. J Microencapsul. 2012;29(6):596–604. doi: 10.3109/02652048.2012.668960. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Hwang IC, Park JW, Park HJ. Photoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticles. Pest Manag Sci. 2012;68(7):1062–1068. doi: 10.1002/ps.3268. [DOI] [PubMed] [Google Scholar]
- Nolasco G, Sequeira Z, Soares C, Mansinho A, Bailey AM, Niblett CL. Asymmetric PCR ELISA: increased sensitivity and reduced costs for the detection of plant viruses. Eur J Plant Pathol. 2002;108(4):293–298. [Google Scholar]
- Oliveira HC, Gomes BCR, Pelegrino MT, Seabra AB. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide. 2016;61:10–19. doi: 10.1016/j.niox.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Pandey R, Zahoor A, Sharma S, Khuller GK. Nanoparticle encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. Tuberculosis. 2003;83(6):373–378. doi: 10.1016/j.tube.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Parham H, Rahbar N. Square wave voltammetric determination of methyl parathion using ZrO2-nanoparticles modified carbon paste electrode. J Hazard Mater. 2010;177(1–3):1077–1084. doi: 10.1016/j.jhazmat.2010.01.031. [DOI] [PubMed] [Google Scholar]
- Parisi C, Vigani M, Rodríguez-Cerezo E. Agricultural nanotechnologies: what are the current possibilities? Nano Today. 2015;10(2):124–127. [Google Scholar]
- Parsons NR, Edmondson RN, Song Y. Image analysis and statistical modelling for measurement and quality assessment of ornamental horticulture crops in glasshouses. Biosyst Eng. 2009;104(2):161–168. [Google Scholar]
- Pereira AES, Sandoval-Herrera IE, Zavala-Betancourt SA, Oliveira HC, Ledezma-Pérez AS, Romero J, Fraceto LF. γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: characterization and evaluation of biological activity. Carbohyd Polym. 2017;157:1862–1873. doi: 10.1016/j.carbpol.2016.11.073. [DOI] [PubMed] [Google Scholar]
- Pillay K, Cukrowska EM, Coville NJ. Multi-walled carbon nanotubes as adsorbents for the removal of parts per billion levels of hexavalent chromium from aqueous solution. J Hazard Mater. 2009;166(2–3):1067–1075. doi: 10.1016/j.jhazmat.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Prasad R, Kumar V, Prasad KS. Nanotechnology in sustainable agriculture: present concerns and future aspects. Afr J Biotechnol. 2014;13(6):705–713. [Google Scholar]
- Qian K, Shi T, Tang T, Zhang S, Liu X, Cao Y. Preparation and characterization of nano-sized calcium carbonate as controlled release pesticide carrier for validamycin against Rhizoctonia solani. Mikrochim Acta. 2011;173(1–2):51–57. [Google Scholar]
- Qu F, Zhou X, Xu J, Li H, Xie G. Luminescence switching of CdTe quantum dots in presence of p-sulfonatocalix [4] arene to detect pesticides in aqueous solution. Talanta. 2009;78(4–5):1359–1363. doi: 10.1016/j.talanta.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Quiñones JP, García YC, Curiel H, Covas CP. Microspheres of chitosan for controlled delivery of brassinosteroids with biological activity as agrochemicals. Carbohyd Polym. 2010;80(3):915–921. [Google Scholar]
- Rademacher W. Plant growth regulators: backgrounds and uses in plant production. J Plant Growth Regul. 2015;34(4):845–872. [Google Scholar]
- Rai M, Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl Microbiol Biotechnol. 2012;94(2):287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- Roberts MJ, Schimmelpfennig DE, Ashley E, Livingston MJ, Ash MS, Vasavada U (2006) The value of plant disease early-warning systems: a case study of USDA's soybean rust coordinated framework
- Römheld V, Kirkby EA. Research on potassium in agriculture: needs and prospects. Plant Soil. 2010;335(1–2):155–180. [Google Scholar]
- Rossi M, Cubadda F, Dini L, Terranova ML, Aureli F, Sorbo A, Passeri D. Scientific basis of nanotechnology, implications for the food sector and future trends. Trends Food Sci Technol. 2014;40(2):127–148. [Google Scholar]
- Ruiz-Garcia L, Barreiro P, Robla JI. Performance of ZigBee-based wireless sensor nodes for real-time monitoring of fruit logistics. J Food Eng. 2008;87(3):405–415. [Google Scholar]
- Ruiz-Garcia L, Lunadei L, Barreiro P, Robla I. A review of wireless sensor technologies and applications in agriculture and food industry: state of the art and current trends. Sensors. 2009;9(6):4728–4750. doi: 10.3390/s90604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandom A, Napier RM. Biosensors in plants. Curr Opin Plant Biol. 2010;13(6):736–743. doi: 10.1016/j.pbi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Sakamoto A, Murata N. Genetic engineering of glycinebetaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot. 2000;51(342):81–88. [PubMed] [Google Scholar]
- Sasson Y, Levy-Ruso G, Toledano O, Ishaaya I. Insecticides design using advanced technologies. Berlin: Springer; 2007. Nanosuspensions: emerging novel agrochemical formulations; pp. 1–39. [Google Scholar]
- Savary S, Ficke A, Aubertot J-N, Hollier C. Crop losses due to diseases and their implications for global food production losses and food security. Berlin: Springer; 2012. [Google Scholar]
- Sekhon BS. Nanotechnology in agri-food production: an overview. Nanotechnol Sci Appl. 2014;7:31. doi: 10.2147/NSA.S39406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servin A, Elmer W, Mukherjee A, De la Torre-Roche R, Hamdi H, White JC, Bindraban P, Dimkpa C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J Nanopart Res. 2015;17(2):92. [Google Scholar]
- Singh A, Poshtiban S, Evoy S. Recent advances in bacteriophage based biosensors for food-borne pathogen detection. Sensors. 2013;13(2):1763–1786. doi: 10.3390/s130201763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Wang B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann Bot. 2014;115(3):541–553. doi: 10.1093/aob/mcu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Gao Y, Wu H, Hou W, Zhang C, Ma H. Physiological effect of anatase TiO2 nanoparticles on Lemna minor. Environ Toxicol Chem. 2012;31(9):2147–2152. doi: 10.1002/etc.1933. [DOI] [PubMed] [Google Scholar]
- Song M-R, Cui S-M, Gao F, Liu Y-R, Fan C-L, Lei T-Q, Liu D-C. Dispersible silica nanoparticles as carrier for enhanced bioactivity of chlorfenapyr. J Pestic Sci. 2012;20:D12–027. [Google Scholar]
- Sonkaria S, Ahn S-H, Khare V. Nanotechnology and its impact on food and nutrition: a review. Recent Patents Food Nutr Agric. 2012;4(1):8–18. doi: 10.2174/2212798411204010008. [DOI] [PubMed] [Google Scholar]
- Steinberg IM, Steinberg MD. Radio-frequency tag with optoelectronic interface for distributed wireless chemical and biological sensor applications. Sens Actuator B-Chem. 2009;138(1):120–125. [Google Scholar]
- Sun H, Fung Y. Piezoelectric quartz crystal sensor for rapid analysis of pirimicarb residues using molecularly imprinted polymers as recognition elements. Anal Chim Acta. 2006;576(1):67–76. doi: 10.1016/j.aca.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Tao S, Pang R, Chen C, Ren X, Hu S. Synthesis, characterization and slow release properties of O-naphthylacetyl chitosan. Carbohyd Polym. 2012;88(4):1189–1194. [Google Scholar]
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418(6898):671. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Todd B, Phillips M, Schultz SM, Hawkins AR, Jensen BD. Low-cost RFID threshold shock sensors. IEEE Sens J. 2009;9(4):464–469. [Google Scholar]
- van Tuijl B, van Os E, van Henten E (2007) Wireless sensor networks: state of the art and future perspective. In: International symposium on high technology for greenhouse system management: greensys, vol 801, pp 547–554
- Vamvakaki V, Chaniotakis NA. Pesticide detection with a liposome-based nano-biosensor. Biosens Bioelectron. 2007;22(12):2848–2853. doi: 10.1016/j.bios.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Veličković Z, Vuković GD, Marinković AD, Moldovan M-S, Perić-Grujić AA, Uskoković PS, Ristić MĐ. Adsorption of arsenate on iron (III) oxide coated ethylenediamine functionalized multiwall carbon nanotubes. Chem Eng J. 2012;181:174–181. [Google Scholar]
- Vergara A, Llobet E, Ramírez JL, Ivanov P, Fonseca L, Zampolli S, Scorzoni A, Becker T, Marco S, Wöllenstein J. An RFID reader with onboard sensing capability for monitoring fruit quality. Sens Actuator B-Chem. 2007;127(1):143–149. [Google Scholar]
- Verma D, Daniell H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007;145(4):1129–1143. doi: 10.1104/pp.107.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincelli P, Tisserat N. Nucleic acid-based pathogen detection in applied plant pathology. Plant Dis. 2008;92(5):660–669. doi: 10.1094/PDIS-92-5-0660. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Radecka H, Radecki J. Electrochemical biosensor for pesticides based on acetylcholinesterase immobilized on polyaniline deposited on vertically assembled carbon nanotubes wrapped with ssDNA. Biosens Bioelectron. 2009;24(9):2772–2777. doi: 10.1016/j.bios.2009.01.044. [DOI] [PubMed] [Google Scholar]
- Wang M, Li Z. Nano-composite ZrO2/Au film electrode for voltammetric detection of parathion. Sens Actuator B-Chem. 2008;133(2):607–612. [Google Scholar]
- Wang C, Zhao C, Qiao X, Zhang X, Zhang Y (2007) The design of wireless sensor networks node for measuring the greenhouse's environment parameters. In: International conference on computer and computing technologies in agriculture. Springer, pp 1037–1046
- Wentworth SM (2003) Microbial sensor tags. In: IFT (The Institute of Food Engineering) annual meeting book of abstracts, Chicago, Illinois, USA, July 2003
- Wyn Jones RG, Storey R. Betaines. In: Paleg LG, Aspinall D, editors. The physiology and biochemistry of drought resistance in plants. Sydney: Academic Press; 1981. pp. 171–204. [Google Scholar]
- Xia L, Wei Z, Wan M. Conducting polymer nanostructures and their application in biosensors. J Colloid Interface Sci. 2010;341(1):1–11. doi: 10.1016/j.jcis.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Xiang C, Taylor AG, Hinestroza JP, Frey MW. Controlled release of nonionic compounds from poly (lactic acid)/cellulose nanocrystal nanocomposite fibers. J Appl Polym Sci. 2013;127(1):79–86. [Google Scholar]
- Yang I-C, Chen S, Huang Y-I, Hsieh K-W, Chen C-T, Lu H-C, Chang C-L, Lin H-M, Chen Y-L, Chen C-C (2008) RFID-integrated multi-functional remote sensing system for seedling production management. In: Food processing automation conference proceedings, 28–29 June 2008, Providence, Rhode Island. American Society of Agricultural and Biological Engineers, p 15
- Yang Y, Hong-tao Z, Jian W, Meng X, Yang L, Yu-long Z. Preparation and properties of modified polyvinyl alcohol film for encapsulation of fertilizer. Plant Nutr Fert Sci. 2012;20:5. [Google Scholar]
- Yoo S-e, Kim J-e, Kim T, Ahn S, Sung J, Kim D A (2007) 2 S: automated agriculture system based on WSN. In: 2007 IEEE international symposium on consumer electronics. IEEE, pp 1–5
- Yu F, Sun S, Ma J, Han S. Enhanced removal performance of arsenate and arsenite by magnetic graphene oxide with high iron oxide loading. Phys Chem Chem Phys. 2015;17(6):4388–4397. doi: 10.1039/c4cp04835k. [DOI] [PubMed] [Google Scholar]
- Yvon M, Thébaud G, Alary R, Labonne G. Specific detection and quantification of the phytopathogenic agent ‘Candidatus Phytoplasma prunorum’. Mol Cell Probe. 2009;23(5):227–234. doi: 10.1016/j.mcp.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang L, Song L, Shao H, Shao C, Li M, Liu M, Brestic M, Xu G. Spatio-temporal variation of rhizosphere soil microbial abundance and enzyme activities under different vegetation types in the coastal zone, Shandong. China Plant Biosyst. 2014;148(3):403–409. [Google Scholar]
- Zhao Y-G, Shen H-Y, Shi J-W, Chen X-H, Jin M-C. Preparation and characterization of amino functionalized nano-composite material and its application for multi-residue analysis of pesticides in cabbage by gas chromatography–triple quadrupole mass spectrometry. J Chromatogr A. 2011;1218(33):5568–5580. doi: 10.1016/j.chroma.2011.06.090. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yang X, Guo X, Zhou M, Wang L (2007) A design of greenhouse monitoring and control system based on ZigBee wireless sensor network. In: 2007 international conference on wireless communications, networking and mobile computing. IEEE, pp 2563–2567
- Zwingmann N, Mackinnon IDR, Gilkes RJ. Use of a zeolite synthesised from alkali treated kaolin as a K fertiliser: glasshouse experiments on leaching and uptake of K by wheat plants in sandy soil. Appl Clay Sci. 2011;53(4):684–690. [Google Scholar]