Abstract
Subclinical necrotic enteritis (SNE) caused by Clostridium perfringens (CP), is an important disease in chickens, which causes huge economic losses by damaging the intestinal mucosa, decreasing digestion and absorption of nutrients. Use of antibiotics at a sub-therapeutic level as antimicrobial growth promoters in poultry feed prevents the birds from SNE and improves growth. Due to the ban on the use of antibiotics in 2006 as antimicrobial growth promoters have led to the reemergence of the disease. Worldwide numerous studies have been carried out to investigate the alternatives to antibiotics for the prevention of SNE. Possible alternatives to control SNE include probiotics, prebiotics, bacteriophages, essential oils, organic acids, secondary metabolites and other microbial products. Currently, probiotics are most extensively used in poultry production as an alternative to antibiotics. This review summarizes recent insights and experimental evidence on the use of different microorganisms like Bacillus, Lactic acid bacteria, Bifidobacteria, Enterococcus, yeast, etc. as valuable probiotics for prevention of SNE and potential molecular mechanisms responsible for ameliorating effects of probiotics against SNE.
Keywords: Clostridium perfringens, Probiotics, Subclinical necrotic enteritis, Bacillus, Lactic acid bacteria, Antimicrobial growth promoters
Introduction
In broilers, subclinical necrotic enteritis (SNE) is caused by Clostridium perfringens (CP), which is also responsible for causing numerous diseases like enterotoxaemia and gangrenous dermatitis in animals. It is Gram-positive anaerobic bacteria, which produces spores and highly virulent toxins (Kay et al. 2019; Tian et al. 2016). This is commonly found in many environmental sources like soil, sewage, litter, feces of chicken as well as in the intestine of humans and animals (Songer 1996). This bacterium has zoonotic importance as it causes foodborne diseases in humans, but it also poses an important threat for animals (Grass et al. 2013; Wakabayashi et al. 2019). Outbreaks of necrotic enteritis (NE) usually peaks between 2 and 6 week of age (Hunter et al. 2019; Yang et al. 2016). Due to the subclinical nature of the disease, there is chronic damage to the intestinal mucosa of the chickens (Wu et al. 2019; Zhang et al. 2019). This leads to impaired absorption of nutrients, reduced weight gain and decreased overall performance (Antonissen et al. 2014; Skinner et al. 2010). Due to these factors, subclinical necrotic enteritis causes great economic loses to the poultry industry, which is estimated to be 2 billion dollars per year (Khalique et al. 2019; Timbermont et al. 2011).
CP is usually found in the intestines of healthy chicken less than 105 cfu/g intestinal content (Calik et al. 2019; Hernandez-Patlan et al. 2019). If CP counts are more, i.e. 106 cfu/g of digesta, in the small intestine of chickens, then birds become prone to NE (Kiu and Hall 2018; Yang et al. 2016). There are many predisposing factors, such as viscosity of digesta, the presence of non-digestible polysaccharides, the gastrointestinal tract transit time and the intestinal pH, which are responsible for the ability of bacteria to cause the disease (Moore 2016; Yang et al. 2019). One of the important factors is the coccidiosis caused by Eimeria species, which creates a suitable environment for the proliferation of CP (Si et al. 2007; Van Waeyenberghe et al. 2016). Due to coccidiosis, perforations are made in the intestinal epithelial lining, and plasma proteins accumulate in these holes. These proteins are utilized as a substrate by CP, and this leads to the development of disease (Van Immerseel et al. 2009). Any factor which induces stress in chickens disrupts the balance of microbial flora of intestine and suppresses immune system contributes to the risk of SNE (Tsiouris 2016).
Toxins are main virulence factors of CP, and there are 17 different types of toxins produced by CP, which include α, β, β2, ε, ι and the enterotoxin CPE (Gkiourtzidis et al. 2001; Kumar et al. 2019). Based on toxin production, isolates of CP are divided into five main groups (Uzal et al. 2014). Type A CP is mainly responsible for SNE in poultry by producing α toxin and the pore-forming toxin NetB. Mainly α toxin causes NE, but many reports have shown that NetB toxin alone can also cause the disease (Keyburn et al. 2010). The α toxin has 370 amino acid residues and is Zinc Metallo Phospholipase. Phosphatidylcholine and sphingomyelin in the eukaryotic cell membrane are the target of α toxin and lead to lysis of cells. Low concentrations of α toxin cause limited membrane damage and accumulation of diglycerol. This leads to activation of the arachidonic acid pathway and hence release of inflammatory mediators (Riaz et al. 2017). NetB toxin induces the formation of pores in eukaryotic cell membranes and thus causes cytotoxicity (M’Sadeq et al. 2015). The sequencing of genes encoding NetB toxin has shown that this coding sequence is highly conserved across all the strains of CP, which causes NE. Genomic analysis has shown that genes, in addition to NetB toxin, are also associated with NE causing by CP strains (Lacey et al. 2016). In addition to toxins, CP also produces bacteriocins, which play an important role in the virulence. These bacteriocins inhibit the growth of non-pathogenic microbes for the competition of nutrients that create an imbalance in intestinal microflora. Perfrin is one of the important bacteriocins produced by CP (Timbermont et al. 2014). The ability of pathogenic strains of CP to inhibit the proliferation of normal intestinal strains is one of the major reasons for the development of NE. Bacteriocins help the pathogenic strains of CP to replace the normal intestinal flora of chickens (Riaz et al. 2017). Hydrolytic and proteolytic enzymes produced by CP destroy basal lamina and lateral domains of intestinal cells. Due to increased activity of these enzymes, especially collagenolytic activity, there are morphological changes and mucosal damage, which play a very important role in development of NE (Olkowski et al. 2008).
Antibiotics had been most commonly used as growth promoters and for prophylaxis of SNE (Prescott et al. 2016). Residues of these antibiotics in the chicken meat have harmful effects on the health of human beings, i.e., cause of antibiotic resistance. There is a ban on the use of antibiotics as feed additives in Europe in 2006 under feed additives regulation 1831/2003/EC (Kemper 2008). To prevent the economic losses caused by SNE, there is a dire need for some alternatives to antibiotics. Probiotics, prebiotics, plants, molecules of plant origin, organic acids, enzymes, lysozyme or molecules of microbial origin, such as yeast extract and antimicrobial peptides are different substances, which can be used as alternatives to antibiotics (Caly et al. 2015). Whereas, probiotics have been used widely in the poultry industry as a potential alternative to antibiotics. This review summarizes the usefulness and effectiveness of different probiotic strains as alternatives to antibiotics for the prevention of SNE.
Prophylactic use of probiotics in poultry feed
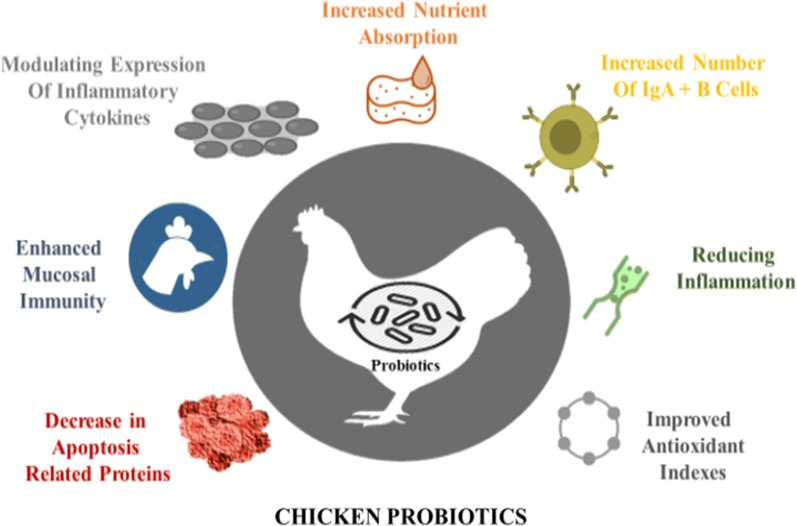
Probiotics are defined as live microbial supplements, which are used in the feed to improve the health of animals by balancing the intestinal microbes. Supplementation of single bacterial strain or mixture of different bacterial strains or yeast prevents the growth of pathogens in intestine. It not only decreases the incidence of intestinal diseases but also improves the overall performance of birds (Chaucheyras-Durand and Durand 2009). Probiotics reduce the risk of SNE by enhancing host immunity, improving the intestinal microflora balance and stimulating metabolism. These probiotics also produce antimicrobial substances, which inhibit the growth of pathogenic bacteria (Pan and Yu 2014). Probiotics also compete with pathogenic bacteria in the intestine of chickens for growth and nutrients, which is known as competitive exclusion (Caly et al. 2015). Possible mode of actions of probiotics includes competitive exclusion, increased digestive enzyme activity, production of substances that can inhibit the growth of pathogens or neutralize enterotoxins, modulation of the host’s immune development, and alteration of intestinal microbial activity (Sokale et al. 2019). Numerous mechanisms of action of bacterial and yeast probiotic have been proposed. These include the production of organic acids, bacteriocins and H2S, competition for nutrients, anti-inflammatory properties and inhibition of pathogen adhesion to the epithelium. Furthermore, these probiotics are also responsible for modulation of the immune system, interference on bacterial-induced signaling pathways, and actions on bacterial virulence factors (Vieira et al. 2013). Effects of probiotic on intestinal health of chicken are shown in (Fig. 1).
Fig. 1.
Effects of probiotics on intestinal health of chickens
Prevention of SNE by the composition of broiler intestinal flora
The composition of poultry intestinal microflora plays an important role in the development and prevention of SNE induced by CP. For example, butyrate-producing bacteria belonging to the Lachnospiraceae family inhibit the inflammation in the intestine of poultry and preserve the intestinal activity. Lactic acid bacteria promote the colonization of butyrate-producing bacteria. Similarly, Candidatus savagella stimulates Th17 cells and induces the formation of immunoglobulin A. Hence these Lactic acid bacteria and Candidatus spp. can be used as potential probiotics (Antonissen et al. 2016). Numerous studies have shown anti-CP activity of various microorganisms, i.e., genera Bacillus, Lactobacilli, Enterococci, Bifidobacteria, and yeasts (Table 1).
Table 1.
Different microorganisms used as probiotics for the prevention of subclinical necrotic enteritis (SNE)
| Bacteria | Species | References |
|---|---|---|
| Bacillus | Bacillus cereus 8A, Bacillus licheniformis, Bacillus pumilus, Bacillus subtilis, Bacillus coagulans | Bizani and Brandelli (2002), Barbosa et al. (2005), Klose et al. (2010), Jayaraman et al. (2013), Zhou et al. (2016), Khan et al. (2016) |
| Lactic acid bacteria | Lactobacillus acidophilus, Lactobacillus amylovorus, Lactobacillus animalis, Lactobacillus fermentum, Lactobacillus johnsonii FI9785, Lactobacillus johnsonii BS15, Lactobacillus mucosae, Lactobacillus plantarum, Lactobacillus reuteri, Lactobacillus salivarius | Cao et al. (2012), Caly et al. (2015), Allaart et al. (2011), Qing et al. (2017), Wang et al. (2018), Lin et al. (2008), Guo et al. (2017) |
| Enterococci | Enterococcus faecium, Enterococcus faecalis, Enterococcus durans | Klose et al. (2010), Fukata et al. (1991), Caly et al. (2015) |
| Bifidobacteria | Bifidobacteria animalis sub species lactis, Bifidobacteria thermoacidophilum | Schoster et al. (2013), Klose et al. (2010) |
| Yeast | Saccharomyces cerevisiae, Candidatus savagella | Layton et al. (2013), Eeckhaut et al. (2016) |
Bacillus species as probiotics for prevention against SNE
Several species of genus Bacillus have shown anti-CP activity. These species include Bacillus cereus 8A (Bizani and Brandelli 2002), Bacillus licheniformis and Bacillus pumilus (Barbosa et al. 2005) and Bacillus subtilis (Jayaraman et al. 2013; Klose et al. 2010). Bacillus spp. as probiotics promote the growth of broilers affected with NE by improving weight gain and feed efficiency (Lee et al. 2014). Bacillus spp. not only regulate the fatty acid synthesis and oxidation-related genes in the livers of CP affected chickens but also enhance antioxidant activity (Zhou et al. 2016). Bacillus spp. produce bacteriocins which inhibit the growth of CP. Bacillus subtilis strain SP6 has shown in vitro anti-CP activity. This activity was due to production of heat-stable protein substances. When Bacillus subtilis strain SP6 was used for the treatment of SNE infected chicken, it markedly reduced the mortality by improving the intestinal health of chickens (Teo and Tan 2005). Bacillus spores, used in the poultry feed, could increase the performance of chickens and reduce mortality in chickens affected with NE (Knap et al. 2010). Supplementation of Bacillus spp. in the CP affected chicken’s diet increased serum nitric oxide levels and decreased Coccidia by specific antibodies (Lee et al. 2014).
Bacillus subtilis, when used as probiotics, improved the microbial balance in the intestine of chickens by immune stimulation and competitive exclusion (Khan et al. 2016). Bacillus subtilis having a broad activity against Clostridium spp. inhibits the pathogen and improves overall performance in broilers (Abudabos et al. 2017). Bacillus subtilis can support the reduction of C. perfringens in the ileum and caecum positively by altering the intestinal microbial population, and supporting the improvement of growth performance (Bortoluzzi et al. 2019; Whelan et al. 2018). Bacillus subtilis has the ability to improve NE-associated pathology and performance of broiler chickens (Jayaraman et al. 2013; Musa et al. 2019). The diet of broiler chickens, challenged with CP, was supplemented with Bacillus subtilis as probiotic, which showed improvement of serum biochemical profiles of chickens. Moreover, there was not only a decrease in the triglyceride and total cholesterol content in serum but also a significant increase in the number of lymphocytes. This study demonstrated the potential of Bacillus subtilis as probiotic for prevention of NE (Al-Baadani et al. 2018). Dietary supplementation of Bacillus subtilis DSM 32315 ameliorated the pathological conditions and performance detriments associated with NE. Birds supplemented with Bacillus subtilis DSM 32315 exhibited better body weight, lower mortality and intestinal NE lesion score as compared to the non-supplemented birds. Furthermore, histomorphometry of intestine showed shallower crypt depth and higher villus height to crypt depth ratio (Bortoluzzi et al. 2019).
The effects of Bacillus licheniformis as a dietary supplement were evaluated on growth performance, and the lipid metabolism genes in the chickens with CP induced NE. It was found that Bacillus licheniformis as probiotic in chickens with NE can enhance growth performance as well as alter the expression of the gene in fatty acid and lipid metabolism (Zhou et al. 2016). Chickens challenged with CP have a disruption in the microflora of caecum. Bacillus licheniformis supplementation in the diet of chickens improved the microbial balance by alleviating the disruption of caecal microbiota and improved the growth performance. This shows that Bacillus licheniformis can be used as probiotics for prevention of SNE (Lin et al. 2017). The diet of chickens infected with NE was supplemented with Bacillus licheniformis, which led to normalization of ileum microflora of chickens (Xu et al. 2018).
Bacillus coagulans promotes the growth performance of chickens and increases the feed digestibility by secreting enzymes such as protease, α-amylase, xylanase and lipidase. It also produces amino acids and vitamins (Hung et al. 2012). Bacillus coagulans, when used as probiotics, enhances the immunity of chickens and decreases gut inflammation (Fitzpatrick 2013). The influence of feeding Bacillus coagulans on the growth rate and intestinal health status of chickens with CP induced necrotic enteritis was investigated. CP challenged birds were fed diets supplemented with Bacillus coagulans exhibited lower gut lesion scores and decreased CP numbers in the caecum and liver. It also improved the growth performance and inhibited colonization and invasion of CP (Al-Baadani et al. 2018).
Lactic acid bacteria as probiotics for prevention against SNE
Lactic acid bacteria can be used as probiotics as an alternative to antibiotics due to their antimicrobial properties, as well as beneficial effects on the host. Probiotic potential of Lactic acid bacteria is attributed to the production of bacteriocins, lactate and hydrogen peroxide, and boosting the immunity of the host. These microbes increased the expression of cytokines in the host, thus helping to boost stronger immune response (Cao et al. 2012). As reported by Caly et al. (2015), that different species of the Lactic acid bacteria can be used as potential probiotics. These species include Lactobacillus acidophilus, Lactobacillus amylovorus, Lactobacillus animalis, Lactobacillus fermentum, Lactobacillus johnsonii FI9785, Lactobacillus mucosae, Lactobacillus plantarum, Lactobacillus reuteri and Lactobacillus salivarius. Lactobacillus fermentum inhibited the β2 toxin production by CP, and this phenomenon occurred at the transcriptome level (Allaart et al. 2011).
Lactobacillus johnsonii, when fed to 20-day old chickens, was able to persist in the intestine and after 15 days it inhibited the colonization of CP (La Ragione et al. 2004). Lactobacillus johnsonii BS15 was used as probiotic in broilers for prevention of SNE. This strain not only ameliorated the SNE induced average daily weight loss but also improved the liver abnormalities. Lactobacillus johnsonii BS15 ameliorated the lipid metabolism and improved the intestinal microflora of chickens. Thus, Lactobacillus johnsonii BS15 can be used as potential probiotic for prevention of SNE (Qing et al. 2017). Lactobacillus johnsonii BS15 improved the blood parameters related to immunity in chickens infected with SNE. The Lactobacillus johnsonii BS15 not only increased antioxidant capacities but also enhanced the serum levels of antibodies, interleukin-2 and interferon-gamma. This showed that Lactobacillus johnsonii BS15 could improve the immunity of birds infected with SNE (Wang et al. 2018). Hepatic transcriptome analysis showed that Lactobacillus johnsonii BS15 as a probiotic improves the lipid metabolism in broilers infected with SNE (Allaart et al. 2011). Lactobacillus johnsonii BS15 also decreased liver inflammation by regulating different inflammatory pathways and genes in the broiler chicken (Table 2) (Khalique et al. 2019).
Table 2.
Regulatory pathways and gene modifications by Lactobacillus johnsonii BS15 decreased liver inflammation
| Pathway | Genes |
|---|---|
| Cytokine-cytokine receptor interaction | CCR2; CX3CR1; IFNGR1; IL18; IL1B; IL2RA; IL2RG; TNFRSF1B; TNFRSF21; TNFSF11; XCL1; XCR1; CNTFR; GHR; IL12RB1; LIFR |
| Intestinal immune network for IgA production | CD80; HLA-DRA; ITGA4 |
| Toll-like receptor signaling pathway | CD80; IL1B; PIK3R5; TLR2A; TLR4; FOS |
| Phagosome | CTSS; HLA-DRA; MARCO; MRC2; TLR2A; TLR4, NCF2; TUBB6 |
| Influenza A | HLA-DRA; IFNGR1; IL18; IL1B; PIK3R5; TLR4; SOCS3 |
| TGF-beta signaling pathway | BMP5; BMP6; DCN; ZFYVE16 |
| Cytosolic DNA-sensing pathway | IL18; IL1B |
| NOD-like receptor signaling pathway | IL18; IL1B |
| MAPK signaling pathway | CACNA1D; FGF14; IL1B; NRK; PLA2G4A; PRKCB; PTPN5; RAC2, DUSP5; FOS; PLA2G4A, CACNA1D; CACNA2D1 |
| Herpes simplex infection | CD74; HLA-DRA; IFNGR1; IL1B; TLR2A; FOS; SOCS3 |
| Salmonella | FOS, PKN3 |
| Cell adhesion molecules (CAMs) | SIGLEC1, CD80; VCAM1 |
Lactobacillus acidophilus inhibits the growth of pathogenic bacteria in the gut of poultry and modulates the immune status of poultry birds (Lin et al. 2008). The impact of Lactobacillus acidophilus on growth performance and intestinal health of broiler chickens affected with CP was evaluated. The use of Lactobacillus acidophilus as probiotic increased the weight gain and decreased mortality. It was further concluded that it decreased the intestinal lesion of CP challenged birds, ileal populations of Escherichia coli and endotoxin quantity in the blood (Li et al. 2018). Lactobacillus fermentum and Lactobacillus acidophilus were investigated as potential probiotic for treatment of birds infected with CP. This Lactobacillus spp. inhibited the growth of CP, α toxin production and decreased the attachments of CP to cells (Guo et al. 2017).
Miscellaneous microbes used as probiotic for prevention of subclinical necrotic enteritis
The use of Enterococci for prevention of SNE is attributed to their ability to produce acids and hydrogen peroxide (Klose et al. 2010). Enterococcus bacteria also release bacteriocins called enterocins, which have anti-CP activity. Enterocins type A and B produced by Enterococcus faecium, inhibit the growth of CP (Shin et al. 2008). Enterococci have the ability to inhibit toxin production by CP. Different Enterococci which have shown anti-CP activity include Enterococcus faecium (Klose et al. 2010), Enterococcus faecalis (Fukata et al. 1991) and Enterococcus durans (Caly et al. 2015). Administration of probiotic Enterococcus faecium NCIMB 1118 significantly alleviated body weight loss, intestinal lesions, histopathological inflammation and intestinal-cell apoptosis in NE challenged birds. Thus, Enterococcus faecium NCIMB 1118 reduced the intestinal barrier injury in broilers having NE (Wu et al. 2019). Butyricicoccus pullicaecorum strain 25-3T acts as potential probiotic by reducing the abundance of potentially important pathogens in the caeca and ileum (Eeckhaut et al. 2016). Schoster et al. (2013) demonstrated that Bifidobacteria animalis sub species lactis, which is a commercial strain, has an antagonistic activity for CP. It produces narrow spectrum molecules, which inhibit the growth of CP. Bifidobacteria thermoacidophilum isolated from the porcine intestine can also be used for prevention of SNE. Lactate and hydrogen peroxide production is the mechanism by which it prevents the growth of CP (Klose et al. 2010). Clostridium butyricum YH108 as a probiotic showed significant improvement in immune response and intestinal barrier function in chickens affected with necrotic enteritis (Huang et al. 2019).
Yeast as probiotics for prevention against SNE
Yeast products are important natural growth promoters. Saccharomyces cerevisiae was first reported yeast used as a growth promoter for animals. Probiotics that constitute various yeast products efficiently improve the production potential and immune indices of animal and even human beings by optimizing the microbial balance (Awais et al. 2019). Commercial yeast products specifically for animal feeds are used worldwide (Eckles and Williams 1925). The yeast cell wall consists of protein, glucans, and mannan. In poultry, yeast as a probiotic has more effect of improving the performance of birds. Saccharomyces cerevisiae, obtained from intestine of poultry, functions as an effective probiotic for prevention of NE (Layton et al. 2013). Priya and Babu (2013) showed that Saccharomyces cerevisiae supplementation had a positive effect on the morphology of intestine.
The diet of broiler chickens, challenged with CP, was supplemented with Saccharomyces cerevisiae as probiotic. The yeast not only had a positive effect on serum biochemical parameters in broilers but also boosted the immune system of chickens (Al-Baadani et al. 2018). Butyricicoccus pullicaecorum as probiotic was investigated on growth performance, the composition of intestinal microflora and resistance against CP. The strain not only decreased the number of necrotic lesions in chickens infected with necrotic enteritis but also improved the growth performance. It was concluded that Butyricicoccus pullicaecorum could be used as a potential valuable probiotic for prevention of necrotic enteritis (Eeckhaut et al. 2016). Saccharomyces boulardii, when used as a probiotic, antagonizes the growth of Candida albicans. It secretes capric acid, which inhibits colonization, adhesion and biofilm formation of Candida albicans (Krasowska et al. 2009; Murzyn et al. 2010). Saccharomyces boulardii was evaluated as probiotic to examine its antibacterial potential. It reduced caecal Salmonella colonization in broiler chicks. Interestingly, Saccharomyces boulardii did not inhibit Campylobacter colonization in the same group of broiler chickens (Line et al. 1998). Poultry birds when fed 2.5 g yeast culture/kg feed, modulated villus height to crypt depth ratios in the small intestine, improved digestibility of calcium and phosphate and soared the IgM and secretory IgA concentrations in the duodenum (Gao et al. 2008).
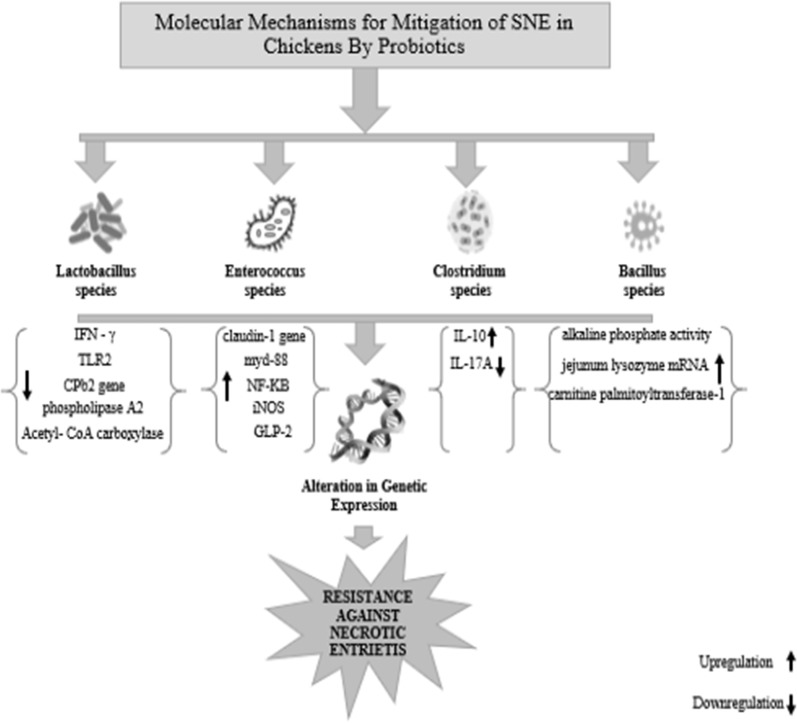
Molecular mechanisms explaining the positive effects of probiotics against SNE
Addition of Enterococcus faecium NCIMB 11181 to poultry feed as a probiotic upregulates the expression of the Claudin-1 gene encoding a tight-junction protein in necrotic enteritis affected chickens. Probiotic also leads to an increase in the expression of MyD88, NF-κB, iNOS, PI3K, GLP-2, IL-1β, IL-4, and HSP70 mRNA (Wu et al. 2019). Diets supplemented with Clostridium butyricum YH108 have a positive effect on the gut microbiota, immune response and intestinal barrier function in chickens affected with necrotic enteritis. Clostridium butyricum not only increased the expression of anti-inflammatory IL-10 in infected chickens but also inhibited the expression of IL-17A gene and reduction of Claudin-1 gene in infected chickens (Fig. 2) (Huang et al. 2019). Candidatus savagella induces the formation of antibodies in SNE affected chickens by stimulating T-helper cells (Sokale et al. 2019). Lactobacillus fermentum and Lactobacillus acidophilus decrease the expression of pro-inflammatory cytokines in birds affected with NE (Wang et al. 2018). Lactobacillus acidophilus was responsible for the decline in RNA expression of pro-inflammatory cytokines in birds affected with CP (Qing et al. 2017). By decreasing cpb2 mRNA, Lactobacillus fermentum prevented the β toxin production by CP (Allaart et al. 2011). By increasing IL-10 mRNA levels and decreasing the mRNA expression of IFN- γ and TLR2, Lactobacillus fermentum reduced the inflammatory damage caused by CP induced NE (Cao et al. 2012). Lactobacillus johnsonii BS15 increased the expressions of fatty acid-binding protein 2, acyl-CoA synthetase bubblegum family member 1, perilipin 1 and perilipin 2 and suppressed phospholipase A2 group IVA in arachidonic acid metabolism (Qing et al. 2018). Lactobacillus johnsonii BS15 prevented subclinical necrotic enteritis by reducing expression of genes expression of acetyl-CoA carboxylase, fatty acid synthase and sterol regulatory element-binding protein-1c. It also enhanced gene expression of peroxisome proliferator-activated receptor α and carnitine palmitoyltransferase-1 while helped in mitigating subclinical necrotic enteritis (Qing et al. 2017). Lactobacillus johnsonii BS15 significantly increased in the CD3+CD4+ T-lymphocyte percentage in peripheral blood of birds affected with SNE. It also increased the serum levels of immunoglobulins and cytokines that were affected by SNE (Cao et al. 2012). Lactobacillus johnsonii BS15 improved intestinal immunity in broilers against subclinical necrotic enteritis by positively altering mRNA expression levels of apoptosis-related proteins (Wang et al. 2017). Bacillus coagulans when fed as probiotic to SNE affected birds led to increase in the IgA levels, alkaline phosphatase (IAP) activity in the jejunum and the expression of jejunum lysozyme mRNA (Wu et al. 2018).
Fig. 2.
Molecular mechanisms explaining the positive effects of probiotics against SNE
Bacillus licheniformis helped to normalize the ileum microbiota of chickens infected with SNE by positively correlating a tumour suppressor gene, p53 (Xu et al. 2018). Bacillus licheniformis significantly upregulated catabolism-related genes, i.e. peroxisome proliferator-activated receptor-α and carnitine palmitoyltransferase-1 in livers in SNE affected birds. It also altered the expression of lipid-anabolism genes (Zhou et al. 2016).
Conclusion
SNE caused by CP causes gigantic economic loses in terms of growth and production performance in poultry. Due to the ban of antibiotics in poultry feed, probiotics are best available alternatives to limit the intestinal inflammation caused by CP. Different microorganisms like Bacillus, Lactic acid bacteria, Bifidobacteria, Enterococcus, and yeast have been investigated as potential probiotics. The beneficial effects of many of the developed probiotics have been well demonstrated in the prevention of SNE in chickens. The general consensus is that the outcome and results of these probiotics may vary under farm conditions. Furthermore, the mode of action of these probiotics needs to be better understood. To achieve the ultimate goal of reducing or preventing antibiotic use in the poultry feed as a growth promoter. Therefore, it is an urgent need to develop new probiotics which are practically more feasible under farm conditions.
Acknowledgements
A. Khalique wants to thank Dr. Muhammad Ali Raza and Dr. Meki Gul over for their continuous support, encouragement and cooperation.
Authors’ contributions
AK, DZ and XN finished the writeup, MS reviewed/proofread this article and HW, XQ, KP, and DSR compiled the literature. All authors read and approved the final manuscript.
Funding
The present study was supported by International Cooperative Project of Science and Technology Bureau of Sichuan Province (2018HH0103) and National Natural Science Foundation of China (31672318).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abdul Khalique, Dong Zeng and Muhammad Shoaib are joint first authors
References
- Abudabos AM, Alyemni AH, Dafalla YM, Khan RU. Effect of organic acid blend and Bacillus subtilis alone or in combination on growth traits, blood biochemical and antioxidant status in broilers exposed to Salmonella typhimurium challenge during the starter phase. J Appl Anim Res. 2017;45(1):538–542. doi: 10.1080/09712119.2016.1219665. [DOI] [Google Scholar]
- Al-Baadani HH, Abudabos AM, Al-Mufarrej SI, Al-Baadani AA, Alhidary IA. Dietary supplementation of Bacillus subtilis, Saccharomyces cerevisiae and their symbiotic effect on serum biochemical parameters in broilers challenged with Clostridium perfringens. J Appl Anim Res. 2018;46(1):1064–1072. doi: 10.1080/09712119.2018.1454325. [DOI] [Google Scholar]
- Allaart JG, van Asten AJ, Vernooij JC, Gröne A. The effect of Lactobacillus fermentum on beta2 toxin production by Clostridium perfringens. Appl Environ Microbiol: AEM; 2011. p. 03002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonissen G, Van Immerseel F, Pasmans F, Ducatelle R, Haesebrouck F, Timbermont L, Verlinden M, Janssens GPJ, Eeckhaut V, Eeckhout M. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS ONE. 2014;9(9):e108775. doi: 10.1371/journal.pone.0108775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonissen G, Eeckhaut V, Van Driessche K, Onrust L, Haesebrouck F, Ducatelle R, Moore RJ, Van Immerseel F. Microbial shifts associated with necrotic enteritis. Avian Pathol. 2016;45(3):308–312. doi: 10.1080/03079457.2016.1152625. [DOI] [PubMed] [Google Scholar]
- Awais MM, Jamal MA, Akhtar M, Hameed MR, Anwar MI, Ullah MI. Immunomodulatory and ameliorative effects of Lactobacillus and Saccharomyces based probiotics on pathological effects of eimeriasis in broilers. Microb Pathog. 2019;126:101–108. doi: 10.1016/j.micpath.2018.10.038. [DOI] [PubMed] [Google Scholar]
- Barbosa TM, Serra CR, La Ragione RM, Woodward MJ, Henriques AO. Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol. 2005;71(2):968–978. doi: 10.1128/AEM.71.2.968-978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizani D, Brandelli A. Characterization of a bacteriocin produced by a newly isolated Bacillus sp. Strain 8 A. J Appl Microbiol. 2002;93(3):512–519. doi: 10.1046/j.1365-2672.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi C, Serpa Vieira B, de Paula Dorigam JC, Menconi A, Sokale A, Doranalli K, Applegate TJ. Bacillus subtilis DSM 32315 supplementation attenuates the effects of Clostridium perfringens challenge on the growth performance and intestinal microbiota of broiler chickens. Microorganisms. 2019;7(3):71. doi: 10.3390/microorganisms7030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik A, Omara II, White MB, Evans NP, Karnezos TP, Dalloul RA. Dietary non-drug feed additive as an alternative for antibiotic growth promoters for broilers during a necrotic enteritis challenge. Microorganisms. 2019;7(8):257. doi: 10.3390/microorganisms7080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly DL, D’Inca R, Auclair E, Drider D. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist’s perspective. Front Microbiol. 2015;6:1336. doi: 10.3389/fmicb.2015.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Yang X, Li Z, Sun F, Wu X, Yao J. Reduced lesions in chickens with Clostridium perfringens-induced necrotic enteritis by Lactobacillus fermentum 1.2029. Poult Sci. 2012;91(12):3065–3071. doi: 10.3382/ps.2012-02548. [DOI] [PubMed] [Google Scholar]
- Chaucheyras-Durand F, Durand H. Probiotics in animal nutrition and health. Benef Microbes. 2009;1(1):3–9. doi: 10.3920/BM2008.1002. [DOI] [PubMed] [Google Scholar]
- Eckles C, Williams V. Yeast as a supplementary feed for lactating cows. J Dairy Sci. 1925;8(2):89–93. doi: 10.3168/jds.S0022-0302(25)93944-6. [DOI] [Google Scholar]
- Eeckhaut V, Wang J, Van Parys A, Haesebrouck F, Joossens M, Falony G, Raes J, Ducatelle R, Van Immerseel F. The probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front Microbiol. 2016;7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LR. Probiotics for the treatment of Clostridium difficile associated disease. World J Gastrointest Pathophysiol. 2013;4(3):47. doi: 10.4291/wjgp.v4.i3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata T, Hadate Y, Baba E, Arakawa A. Influence of bacteria on Clostridium perfringens infections in young chickens. Avian Dis. 1991;35:224–227. doi: 10.2307/1591319. [DOI] [PubMed] [Google Scholar]
- Gao F, Jiang Y, Zhou G, Han Z. The effects of xylanase supplementation on performance, characteristics of the gastrointestinal tract, blood parameters and gut microflora in broilers fed on wheat-based diets. Anim Feed Sci Technol. 2008;142(1–2):173–184. doi: 10.1016/j.anifeedsci.2007.07.008. [DOI] [Google Scholar]
- Gkiourtzidis K, Frey J, Bourtzi-Hatzopoulou E, Iliadis N, Sarris K. PCR detection and prevalence of α-, β-, β2-, ε-, ι-and enterotoxin genes in Clostridium perfringens isolated from lambs with clostridial dysentery. Vet Microbiol. 2001;82(1):39–43. doi: 10.1016/S0378-1135(01)00327-3. [DOI] [PubMed] [Google Scholar]
- Grass JE, Gould LH, Mahon BE. Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998–2010. Foodborne Pathog Dis. 2013;10(2):131–136. doi: 10.1089/fpd.2012.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Liu D, Zhang B, Li Z, Li Y, Ding B, Guo Y. Two Lactobacillus species inhibit the growth and α-toxin production of Clostridium perfringens and induced proinflammatory factors in chicken intestinal epithelial cells in vitro. Front Microbiol. 2017;8:2081. doi: 10.3389/fmicb.2017.02081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Patlan D, Solis-Cruz B, Pontin KP, Hernandez X, Merino-Guzman R, Adhikari B, López-Arellano R, Kwon YM, Hargis BM, Arreguin-Nava M. Impact of a Bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions and ileal microbiota in broiler chickens using a laboratory challenge model. Front Vet Sci. 2019;6:108. doi: 10.3389/fvets.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Peng X-Y, Gao B, Wei Q-L, Xiang R, Yuan M-G, Xu Z-H. The effect of Clostridium butyricum on the gut microbiota, immune response and intestinal barrier function during the development of necrotic enteritis in chickens. Front Microbiol. 2019;10:2309. doi: 10.3389/fmicb.2019.02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AT, Lin S-Y, Yang T-Y, Chou C-K, Liu H-C, Lu J-J, Wang B, Chen S-Y, Lien T-F. Effects of Bacillus coagulans ATCC 7050 on growth performance, intestinal morphology, and microflora composition in broiler chickens. Anim Prod Sci. 2012;52(9):874–879. doi: 10.1071/AN11332. [DOI] [Google Scholar]
- Hunter JG, Wilde S, Tafoya AM, Horsman J, Yousif M, Diamos AG, Roland KL, Mason HS. Evaluation of a toxoid fusion protein vaccine produced in plants to protect poultry against necrotic enteritis. PeerJ. 2019;7:e6600. doi: 10.7717/peerj.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S, Thangavel G, Kurian H, Mani R, Mukkalil R, Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult Sci. 2013;92(2):370–374. doi: 10.3382/ps.2012-02528. [DOI] [PubMed] [Google Scholar]
- Kay S, Edwards J, Brown J, Dixon R. Galleria mellonella infection model identifies both high and low lethality of Clostridium perfringens toxigenic strains and their response to antimicrobials. Front Microbiol. 2019;10:1281. doi: 10.3389/fmicb.2019.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Ind. 2008;8(1):1–13. doi: 10.1016/j.ecolind.2007.06.002. [DOI] [Google Scholar]
- Keyburn AL, Yan X-X, Bannam TL, Van Immerseel F, Rood JI, Moore RJ. Association between avian necrotic enteritis and Clostridium perfringens strains expressing NetB toxin. Vet Res. 2010;41(2):1–8. doi: 10.1051/vetres/2009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalique A, Zeng D, Wang H, Qing X, Zhou Y, Xin J, Zeng Y, Pan K, Shu G, Jing B. Transcriptome analysis revealed ameliorative effect of probiotic Lactobacillus johnsonii BS15 against subclinical necrotic enteritis induced hepatic inflammation in broilers. Microb Pathog. 2019;132:201–207. doi: 10.1016/j.micpath.2019.05.011. [DOI] [PubMed] [Google Scholar]
- Khan RU, Naz S, Dhama K, Kathrik K, Tiwari R, Abdelrahman MM, Alhidary IA, Zahoor A. Direct-fed microbial: beneficial applications, modes of action and prospects as a safe tool for enhancing ruminant production and safeguarding health. Int J Pharmacol. 2016;12(3):220–231. doi: 10.3923/ijp.2016.220.231. [DOI] [Google Scholar]
- Kiu R, Hall LJ. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg Microbes Infect. 2018;7(1):1–15. doi: 10.1038/s41426-018-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose V, Bayer K, Bruckbeck R, Schatzmayr G, Loibner A-P. In vitro antagonistic activities of animal intestinal strains against swine-associated pathogens. Vet Microbiol. 2010;144(3–4):515–521. doi: 10.1016/j.vetmic.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Knap I, Lund B, Kehlet A, Hofacre C, Mathis G. Bacillus licheniformis prevents necrotic enteritis in broiler chickens. Avian Dis. 2010;54(2):931–935. doi: 10.1637/9106-101509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Krasowska A, Murzyn A, Dyjankiewicz A, Łukaszewicz M, Dziadkowiec D. The antagonistic effect of Saccharomyces boulardii on Candida albicans filamentation, adhesion and biofilm formation. FEMS Yeast Res. 2009;9(8):1312–1321. doi: 10.1111/j.1567-1364.2009.00559.x. [DOI] [PubMed] [Google Scholar]
- Kumar NP, Kumar NV, Karthik A. Molecular detection and characterization of Clostridium perfringens toxin genes causing necrotic enteritis in broiler chickens. Trop Anim Health Prod. 2019;51(6):1559–1569. doi: 10.1007/s11250-019-01847-9. [DOI] [PubMed] [Google Scholar]
- La Ragione R, Narbad A, Gasson M, Woodward MJ. In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett Appl Microbiol. 2004;38(3):197–205. doi: 10.1111/j.1472-765X.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- Lacey JA, Johanesen PA, Lyras D, Moore RJ. Genomic diversity of necrotic enteritis-associated strains of Clostridium perfringens: a review. Avian Pathol. 2016;45(3):302–307. doi: 10.1080/03079457.2016.1153799. [DOI] [PubMed] [Google Scholar]
- Layton SL, Hernandez-Velasco X, Chaitanya S, Xavier J, Menconi A, Latorre JD, Kallapura G, Kuttappan VA, Wolfenden RE, Andreatti Filho RL. The effect of a Lactobacillus-based probiotic for the control of necrotic enteritis in broilers. Food Nutr Sci. 2013;04(11):1–7. doi: 10.4236/fns.2013.411A001. [DOI] [Google Scholar]
- Lee K-W, Lillehoj HS, Jang SI, Lee S-H. Effects of salinomycin and Bacillus subtilis on growth performance and immune responses in broiler chickens. Res Vet Sci. 2014;97(2):304–308. doi: 10.1016/j.rvsc.2014.07.021. [DOI] [PubMed] [Google Scholar]
- Li Z, Wang W, Liu D, Guo Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J Anim Sci Biotechnol. 2018;9(1):25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-K, Tsai H-C, Lin P-P, Tsen H-Y, Tsai C-C. Lactobacillus acidophilus LAP5 able to inhibit the Salmonella choleraesuis invasion to the human Caco-2 epithelial cell. Anaerobe. 2008;14(5):251–255. doi: 10.1016/j.anaerobe.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Lin Y, Xu S, Zeng D, Ni X, Zhou M, Zeng Y, Wang H, Zhou Y, Zhu H, Pan K. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS ONE. 2017;12(8):e0182426. doi: 10.1371/journal.pone.0182426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line JE, Bailey JS, Cox NA, Stern NJ, Tompkins T. Effect of yeast-supplemented feed on Salmonella and Campylobacter populations in broilers. Poult Sci. 1998;77(3):405–410. doi: 10.1093/ps/77.3.405. [DOI] [PubMed] [Google Scholar]
- Moore RJ. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016;45(3):275–281. doi: 10.1080/03079457.2016.1150587. [DOI] [PubMed] [Google Scholar]
- M’Sadeq SA, Wu S, Swick RA, Choct M. Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim Nutr. 2015;1(1):1–11. doi: 10.1016/j.aninu.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzyn A, Krasowska A, Stefanowicz P, Dziadkowiec D, Łukaszewicz M. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS ONE. 2010;5(8):e12050. doi: 10.1371/journal.pone.0012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa BB, Duan Y, Khawar H, Sun Q, Ren Z, Elsiddig Mohamed MA, Abbasi IHR, Yang X. Bacillus subtilis B21 and Bacillus licheniformis B26 improve intestinal health and performance of broiler chickens with Clostridium perfringens‐induced necrotic enteritis. J Anim Physiol Anim Nutr. 2019;103(4):1039–1049. doi: 10.1111/jpn.13082. [DOI] [PubMed] [Google Scholar]
- Olkowski A, Wojnarowicz C, Chirino-Trejo M, Laarveld B, Sawicki G. Sub-clinical necrotic enteritis in broiler chickens: novel etiological consideration based on ultra-structural and molecular changes in the intestinal tissue. Res Vet Sci. 2008;85(3):543–553. doi: 10.1016/j.rvsc.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Pan D, Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5(1):108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott JF, Smyth JA, Shojadoost B, Vince A. Experimental reproduction of necrotic enteritis in chickens: a review. Avian Pathol. 2016;45(3):317–322. doi: 10.1080/03079457.2016.1141345. [DOI] [PubMed] [Google Scholar]
- Priya BS, Babu SS. Effect of different levels of supplemental probiotics (Saccharomyces cerevisiae) on performance, haematology, biochemistry, microbiology, histopathology, storage stability and carcass yield of broiler chicken. Int J Pharm Biol Arch. 2013;4(1):201–207. [Google Scholar]
- Qing X, Zeng D, Wang H, Ni X, Liu L, Lai J, Khalique A, Pan K, Jing B. Preventing subclinical necrotic enteritis through Lactobacillus johnsonii BS15 by ameliorating lipid metabolism and intestinal microflora in broiler chickens. AMB Express. 2017;7(1):139. doi: 10.1186/s13568-017-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing X, Zeng D, Wang H, Ni X, Lai J, Liu L, Khalique A, Pan K, Jing B. Analysis of hepatic transcriptome demonstrates altered lipid metabolism following Lactobacillus johnsonii BS15 prevention in chickens with subclinical necrotic enteritis. Lipids Health Dis. 2018;17(1):93. doi: 10.1186/s12944-018-0741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz A, Umar S, Munir MT, Tariq M. Replacements of antibiotics in the control of necrotic enteritis: a review. Sci Lett. 2017;5(3):208–216. [Google Scholar]
- Schoster A, Kokotovic B, Permin A, Pedersen P, Dal Bello F, Guardabassi L. Inávitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Anaerobe. 2013;20:36–41. doi: 10.1016/j.anaerobe.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Shin M, Han S, Ji A, Kim K, Lee W. Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J Appl Microbiol. 2008;105(6):2203–2212. doi: 10.1111/j.1365-2672.2008.03935.x. [DOI] [PubMed] [Google Scholar]
- Si W, Gong J, Han Y, Yu H, Brennan J, Zhou H, Chen S. Quantification of cell proliferation and alpha-toxin gene expression of Clostridium perfringens in the development of necrotic enteritis in broiler chickens. Appl Environ Microbiol. 2007;73(21):7110–7113. doi: 10.1128/AEM.01108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JT, Bauer S, Young V, Pauling G, Wilson J. An economic analysis of the impact of subclinical (mild) necrotic enteritis in broiler chickens. Avian Dis. 2010;54(4):1237–1240. doi: 10.1637/9399-052110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Sokale A, Menconi A, Mathis G, Lumpkins B, Sims M, Whelan R, Doranalli K. Effect of Bacillus subtilis DSM 32315 on the intestinal structural integrity and growth performance of broiler chickens under necrotic enteritis challenge. Poult Sci. 2019;98(11):5392–5400. doi: 10.3382/ps/pez368. [DOI] [PubMed] [Google Scholar]
- Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9(2):216. doi: 10.1128/CMR.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo AY-L, Tan H-M. Inhibition of Clostridium perfringens by a novel strain of Bacillus subtilis isolated from the gastrointestinal tracts of healthy chickens. Appl Environ Microbiol. 2005;71(8):4185–4190. doi: 10.1128/AEM.71.8.4185-4190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Shao Y, Wang Z, Guo Y. Effects of dietary yeast β-glucans supplementation on growth performance, gut morphology, intestinal Clostridium perfringens population and immune response of broiler chickens challenged with necrotic enteritis. Anim Feed Sci Technol. 2016;215:144–155. doi: 10.1016/j.anifeedsci.2016.03.009. [DOI] [Google Scholar]
- Timbermont L, Haesebrouck F, Ducatelle R, Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40(4):341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Timbermont L, De Smet L, Van Nieuwerburgh F, Parreira VR, Van Driessche G, Haesebrouck F, Ducatelle R, Prescott J, Deforce D, Devreese B. Perfrin, a novel bacteriocin associated with netB positive Clostridium perfringens strains from broilers with necrotic enteritis. Vet Res. 2014;45(1):40. doi: 10.1186/1297-9716-45-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiouris V. Poultry management: a useful tool for the control of necrotic enteritis in poultry. Avian Pathol. 2016;45(3):323–325. doi: 10.1080/03079457.2016.1154502. [DOI] [PubMed] [Google Scholar]
- Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, Adams V, Moore RJ, Rood JI, McClane BA. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol. 2014;9(3):361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F, Rood JI, Moore RJ, Titball RW. Rethinking our understanding of the pathogenesis of necrotic enteritis in chickens. Trends Microbiol. 2009;17(1):32–36. doi: 10.1016/j.tim.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Van Waeyenberghe L, De Gussem M, Verbeke J, Dewaele I, De Gussem J. Timing of predisposing factors is important in necrotic enteritis models. Avian Pathol. 2016;45(3):370–375. doi: 10.1080/03079457.2016.1156647. [DOI] [PubMed] [Google Scholar]
- Vieira AT, Teixeira MM, Martins FdS. The role of probiotics and prebiotics in inducing gut immunity. Front Immunol. 2013;4:445. doi: 10.3389/fimmu.2013.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Nariya H, Yasugi M, Kuwahara T, Sarker MR, Miyake M. An enhanced green fluorescence protein (EGFP)-based reporter assay for quantitative detection of sporulation in Clostridium perfringens SM101. Int J Food Microbiol. 2019;291:144–150. doi: 10.1016/j.ijfoodmicro.2018.11.015. [DOI] [PubMed] [Google Scholar]
- Wang H, Ni X, Qing X, Zeng D, Luo M, Liu L, Li G, Pan K, Jing B. Live probiotic Lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers. Front Microbiol. 2017;8:1073. doi: 10.3389/fmicb.2017.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ni X, Qing X, Liu L, Xin J, Luo M, Khalique A, Dan Y, Pan K, Jing B. Probiotic Lactobacillus johnsonii BS15 improves blood parameters related to immunity in broilers experimentally infected with subclinical necrotic enteritis. Front Microbiol. 2018;9:49. doi: 10.3389/fmicb.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan RA, Doranalli K, Rinttilä T, Vienola K, Jurgens G, Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult Sci. 2018;98(9):3450–3463. doi: 10.3382/ps/pey500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Shao Y, Song B, Zhen W, Wang Z, Guo Y, Shahid MS, Nie W. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J Anim Sci Biotechnol. 2018;9(1):9. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhen W, Geng Y, Wang Z, Guo Y. Pretreatment with probiotic Enterococcus faecium NCIMB 11181 ameliorates necrotic enteritis-induced intestinal barrier injury in broiler chickens. Sci Rep. 2019;9(1):10256. doi: 10.1038/s41598-019-46578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Lin Y, Zeng D, Zhou M, Zeng Y, Wang H, Zhou Y, Zhu H, Pan K, Jing B. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci Rep. 2018;8(1):1744. doi: 10.1038/s41598-018-20059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang Q, Diarra MS, Yu H, Hua Y, Gong J. Functional assessment of encapsulated citral for controlling necrotic enteritis in broiler chickens. Poult Sci. 2016;95(4):780–789. doi: 10.3382/ps/pev375. [DOI] [PubMed] [Google Scholar]
- Yang W-Y, Lee Y-J, Lu H-Y, Branton SL, Chou C-H, Wang C. The netB-positive Clostridium perfringens in the experimental induction of necrotic enteritis with or without predisposing factors. Poult Sci. 2019;98(11):5297–5306. doi: 10.3382/ps/pez311. [DOI] [PubMed] [Google Scholar]
- Zhang B, Gan L, Shahid MS, Lv Z, Fan H, Liu D, Guo Y. In vivo and in vitro protective effect of arginine against intestinal inflammatory response induced by Clostridium perfringens in broiler chickens. J Anim Sci Biotechnol. 2019;10(1):1–14. doi: 10.1186/s40104-018-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Zeng D, Ni X, Tu T, Yin Z, Pan K, Jing B. Effects of Bacillus licheniformis on the growth performance and expression of lipid metabolism-related genes in broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Lipids Health Dis. 2016;15(1):48. doi: 10.1186/s12944-016-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.