Abstract
A joint is the point of connection between two bones in our body. Inflammation of the joint leads to several diseases, including osteoarthritis, which is the concern of this review. Osteoarthritis is a common chronic debilitating joint disease mainly affecting the elderly. Several studies showed that inflammation triggered by factors like biomechanical stress is involved in the development of osteoarthritis. This stimulates the release of early-stage inflammatory cytokines like interleukin-1 beta (IL-1β), which in turn induces the activation of signaling pathways, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), phosphoinositide 3-kinase/protein kinase B (PI3K/AKT), and mitogen-activated protein kinase (MAPK). These events, in turn, generate more inflammatory molecules. Subsequently, collagenase like matrix metalloproteinases-13 (MMP-13) will degrade the extracellular matrix. As a result, anatomical and physiological functions of the joint are altered. This review is aimed at summarizing the previous studies highlighting the involvement of inflammation in the pathogenesis of osteoarthritis.
1. Introduction
Osteoarthritis or degenerative arthritis is a public health issue in an aging society. It is a chronic musculoskeletal disorder of the movable joints, such as knee and hip joints [1]. Osteoarthritis affects around 250 million people around the world, the majority of which are the elderly [2]. Changes in the joint tissues during aging can contribute to the development of osteoarthritis. For instance, an increase in cells manifesting senescent secretory phenotype leads to enhanced production of cytokines and matrix metalloproteinases (MMPs) in the joint environment [3]. Furthermore, reduced growth factors and the responsiveness of chondrocytes will cause less matrix synthesis and repair [3]. Other than aging, environmental, biomechanical, and biochemical factors can also contribute to the initiation of osteoarthritis. Osteoarthritis affects the entire structures of the joints, including articular cartilage, subchondral bone, meniscus, synovial membrane, and infrapatellar fat pad (IFP). The common structural characteristics of osteoarthritis are cartilage degradation, subchondral bone remodeling, osteophyte formation, and changes in the synovium and joint capsule [4].
Patients with osteoarthritis have typical clinical symptoms, such as severe joint pain, stiffness, and significantly reduced mobility, leading to decreased productivity and quality of life among the patients, as well as increased socioeconomic burden to the patients and the society [5]. As the prevalence of osteoarthritis increases with age, the aging population worldwide makes this disease a nonnegligible issue [6]. Current therapies for osteoarthritis are limited to symptom-relieving drugs and total knee arthroplasty for severe cases. Drugs addressing the underlying biological causes of osteoarthritis are not available in the market currently [7].
The involvement of immune cells in the development and progression of osteoarthritis has been highlighted in recent studies [8]. Inflammatory components, such as cytokines and chemokines, are produced by chondrocytes and synoviocytes in the joints of patients with osteoarthritis. Synovial fibroblasts are also a source of proinflammatory cytokines and matrix-degrading enzymes under osteoarthritis condition [9]. Moreover, IFP has been shown to contain a significant amount of immune cells like macrophage and T cells. As a result, IFP acts as a site of inflammatory mediators in osteoarthritic knee [10]. These inflammatory mediators alter cell signaling pathways, gene expression, and behavior of joint tissue [11]. The changes in cellular signal transduction lead to enhanced activation of the inflammatory pathway. Thus, more inflammatory compounds and enzymes are released. As a result, anatomical and physiological functions of the joint are altered [12].
This review is aimed at summarizing the recent clinical and preclinical studies performed previously to investigate the relationship between joint inflammation and the pathogenesis of osteoarthritis. The mechanism by which inflammation contributes to the pathogenesis of osteoarthritis will also be discussed.
2. Literature Search
A literature search on original articles written in English and published between 2014 and 2019 was performed using Scopus and PubMed database with the string: (osteoarthritis OR chondrocytes) AND inflammation. Both preclinical and clinical studies were included in this review. The search showed studies on human, animal, and cell lines investigating the involvement of inflammation in osteoarthritis by determining the differential expressions of genes, inflammatory components, and enzymes. Samples collected in human studies include synovial fluid and articular cartilage, from patients who underwent total knee arthroplasty, as well as blood (Table 1). For animal studies, agents, such as interleukin-1 beta (IL-1β) and monosodium iodoacetate (MIA), were used to induce osteoarthritis in the animal models (Table 2). Samples like articular cartilage and synovial tissue were taken after the treatment period. Agents such as IL-1β and tumor necrosis factor-alpha (TNF-α) were applied on cell lines to trigger inflammation (Table 3).
Table 1.
Molecular changes in humans with osteoarthritis.
| Authors (year) | Subjects' characteristics | Sample | Molecules and cells involved | |||||
|---|---|---|---|---|---|---|---|---|
| Cytokines | Chemokines | Matrix metalloproteinases (MMPs) | Immune cells | Other proteins | miRNA | |||
| Hou et al. (2017) [9] | OA patients, n = 10 Non-OA patients, n = 8 |
Synovial tissue | ↑ CX3CL1 | ↑ MMP-3 | ||||
| Deligne et al. (2015) [14] | OA patients, n = 32, mean age 70.3 ± 9.8 years | Synovial tissues | ↑ IL-1β, IL-6, IL-8, IL-18, IL17, IL-22, and TGFβ1 | ↑ MMP-2 | ↑ Leukocyte (macrophages, T-lymphocytes, B-lymphocytes, neutrophils) infiltrates | ↑ MPO | ||
| Shan et al. (2017) [15] | OA patients, n = 40, median age 65 years Healthy control, n = 13, median age 61 years |
Fasting venous blood | ↑ IL-21, IFN-γ, and IL-17A | ↑ PD-1+CXCR5+CD4+ T cells, ICOS+CXCR5+CD4+ T cells, and IL-21+CXCR5+CD4+ T cells | ↑ CRP | |||
| Xia et al. (2017) [16] | OA patients, n = 16 Healthy control, n = 16 |
Blood Synovial fluid |
↑ IL-10 and TGF-β | ↑ LAG-3+ Treg cells | ||||
| Min et al. (2017) [20] | Knee OA patients, n = 148, mean age 68.0 years Healthy control, n = 101, mean age 57.7 years |
Fasting blood | ↑ TNF-α | ↑ Serum OPG ↓ DKK1 |
||||
| Chang et al. (2015) [21] | OA patients, n = 15, mean age 60.7 ± 4.4 years | Peripheral blood Articular synovial membrane |
↑ TNF-α and IL-1β | ↑ CCR3 and eotaxin-1(CCL11) | ↑ MMP-9 | |||
| Ni et al. (2015) [22] | OA patients, n = 58, median age 66 years Healthy control, n = 30, median age 60 years |
Blood Synovial fluid Synovial tissues |
↑ TNF-α, IL-1β, and IL-6 | ↑ FSTL1, p-p65, and p-IκBα ↓ p53 and p21 |
||||
| Ma et al. (2015) [23] | OA patients, n = 6, mean age 31.2 ± 2.91 years | Articular cartilages | ↑ IL-1α, IL-1β, and TNF-α | ↑ MMP-13 | ↑ p65 nuclear level ↓ IκBα degradation |
|||
| Ding et al. (2015) [24] | OA patients, n = 25, mean age 66.6 years | Synovial tissues | ↑ MMP-2 | ↑ Cadherin-11 | ||||
| Xu et al. (2015) [26] | OA patients, n = 5 Normal control, n = 3 |
Articular cartilage | ↑ Sam68 expression | |||||
| Jiang et al. (2017) [28] | OA patients, n = 20 | Articular cartilages | ↑ BRD4 | |||||
| Qu et al. (2018) [30] | Articular cartilage | ↑ IL-6 and TNF-α | ↑ MMP-13 | ↓ Ghrelin, ACAN, col2, sox-9, and GAG ↑ ADAMTS-5 and iNOS |
||||
| Xia et al. (2016) [31] | OA patients, n = 10 Normal control, n = 10 |
Blood Articular cartilage Synovial tissues |
↑ TNFα, IL-6, and IL8 | ↑ COX-2, iNOS, and p-p65 ↓ IkBα |
↑ miR-381a-3p | |||
| Zhang et al. (2016) [35] | OA patients, n = 20 Normal control, n = 20 |
Articular cartilages | ↑ IL-6, TNF-α | ↑ MMP-13 | ↓ ACAN, col2, and Ik-Bα expression ↑ TRAF2 and p-p65 expression |
↓ miR-502-5p | ||
| Snelling et al. (2017) [41] | OA patients, n = 152, mean age 73 ± 9 years | Blood Synovial fluid |
14 patients had detectable IL-17 in SF with ↑ IL-6 | ↑ CCL7 in the 14 patients | ↑ Leptin, resistin, and NGF | |||
| Monasterio et al. (2018) [42] | TMJ-OA patients, n = 4, mean age 53.6 ± 26.6 years Control: DDWR patients, n = 2, mean age 24.5 ± 2.1 years |
Synovial fluid | ↑ IL-17, IL-1β, IL-22, and RANKL | ↑ CCL5, CCL20, CCR5, and CCR7 | ||||
| Alaaeddine et al. (2015) [46] | OA patients, n = 21, mean age 67 ± 19 years Non-OA donor, n = 8, mean age 30 ± 27 years |
Knee cartilage | ↑ IL-6 by CCL20 | ↑ CCL20 and CCR6 | ↑ MMP-1 and MMP-13 by CCL20 | ↑ PGE2 and proteoglycan by CCL20 ↑ ADAMTS-5 and col10 mRNA expression |
||
| Favero et al. (2018) [47] | Early-stage OA patients, n = 5, median age 34 years End-stage OA patients, n = 5, median age 62 years |
Synovial tissues | ↑ IL-6 and IL-8 | ↑ CCL2, CCL21, and CCL5 (RANTES) | ↑ MMP-3 and MMP-10 | ↑ TIMP-2, TIMP-4 ↑ GAG and ACAN CS846 epitope |
||
| Capsoni et al. (2015) [48] | Articular cartilage | ↑ IL-6 and IL-8 | ↑ MMP-3 and MMP-13 | ↑ NO, iNOS, TIMP-3, and TIMP-4 | ||||
| Zhang et al. (2017) [49] | OA patients, n = 11, average age 49 years Healthy control, n = 12, average age 42 years |
Knee articular cartilage Blood |
↑ IL-6, IL-8 | ↑ P2X7R | ↓ miR-373 | |||
| Böhm et al. (2016) [50] | OA patients, n = 22, mean age 66.7 years | Synovial tissue | ↑ IL-6 and IL-8 | |||||
| Wu et al. (2018) [51] | OA patients, n = 40, mean age 43 years Normal control, n = 10, mean age 39 years |
Synovial tissue | ↑ IL-6, IL-8 | ↑ MMP-3, MMP-13 | ↓ STC1 | ↑ miR-454 | ||
| van Geffen et al. (2016) [53] | OA patients, n = 8 | Articular cartilage | ↑ IL37, IL-6, and IL-8 | ↑ MMP-1, MMP-3, and MMP-13 | ↑ ADAMTS5 | |||
| Ding et al. (2017) [54] | OA patients, n = 72, mean age 63.89 ± 14.34 years, and disease duration 4.56 ± 3.9 years Healthy control, n = 40, mean age 62.32 ± 14.15 years |
Blood Synovial fluid Synovial tissues |
↑ IL-37, IL-1β, TNF-α, and IL-6 | |||||
| Mabey et al. (2016) [55] | OA patients, n = 32, mean age 64.6 ± 6.2 years Healthy control, n = 14, mean age 63.9 ± 6.4 years |
Blood samples Synovial fluid |
↑ IL-2, IL-4, IL-6, and IL-10 | |||||
| He et al. (2017) [56] | OA patients, n = 12, mean age 65.8 ± 3.5 years | Articular cartilage | ↑ IL-6, TNF-α, and IFN-γ ↓ IL-4 |
↑ SOCS1, caspase 9, Bax, Bcl-2, and iNOS | ||||
| Sun et al. (2015) [59] | Articular cartilage | ↑ IL-1R and TNF-α | ↑ MMP-1 and MMP-13 | ↑ PARP-1, iNOS, and p-p65 ↓ TIMP-1 |
||||
| Raghu et al. (2017) [63] | OA patients, n = 35 Normal control, n = 37 |
Synovial tissue Synovial fluid |
↑ CCL2 | |||||
| Chen et al. (2018) [64] | Facet joint OA patients, n = 48, mean age 64 ± 1.7 years Healthy control, n = 10, mean age 25 ± 1.2 years |
Facet joint tissues | ↑ CCL4 and CCL4L2 | ↑ DKK2 | ||||
| Belluzzi et al. (2018) [67] | OA patients, n = 5, median age 68 years | Cartilage Synovial membrane tissues Meniscus IFP |
↑ IL-6, IL-1β | ↑ CXCL8, CCL21 | ↑ MMP-10 | |||
| Huang et al. (2016) [68] | Synovial tissues | ↑ IL-6, IL-1β, and TNF-α | ↑ MCP-1 (CCL2) | ↑ NO, PGE2, iNOS, COX-2, VCAM-1, ICAM-1, ET-1, TF, and p-IkB | ||||
| Arkestål et al. (2018) [69] | OA patients, n = 7 Healthy control, n = 9 |
Peripheral blood Bone marrow |
↑ CCR2 ↓ CXCR3 |
|||||
| Chen et al. (2015) [70] | Synovial tissues | ↑ IL-6 | ↑ MMP-13 | ↑ COX-2, PGE2, VEGF, p-IKK α/β, p-IkBα, and p-p65 expression | ||||
| Zeng et al. (2019) [73] | OA patients, n = 12 Patients with other joint diseases, n = 12 |
Articular cartilage | ↑ TNF-α and IL-6 | ↑ MMP-3 and MMP-13 | ↑ FOXM1, iNOS, COX-2, NO, PGE2, and p-p65 | |||
| Xia et al. (2017) [74] | Cartilage tissues | ↑ IL-6 and TNF-α | ↑ MMP-13 | ↑ PRMT1, ADAMTS-5, NO, PGE2, iNOS, COX-2, SHH, Gli-1, and Patch 1 ↓ ACAN and COL2A1 |
||||
| Burguera et al. (2014) [75] | OA patients, n = 13, mean age 77.5 ± 10 years | Articular cartilages | ↑ IL-6 | ↑ MMP-13 | ↑ COX2, PGE-2, and nitrite | |||
| Peng et al. (2017) [76] | OA patients, n = 5, mean age 65.2 ± 3.2 years Normal control, n = 3, mean age 31.0 ± 5.9 years |
Synovial tissues | ↑ MMP-13 | ↓ DUSP1 ↑ p-p38, p-JNK, and COX-2 |
||||
| Ma et al. (2018) [77] | OA patients, n = 30 | Knee cartilage | ↑ IL-8 | ↑ MMP-13 | ↑ p-PKR, p-PKC, COX-2, ROS, p-ERK, and p65 activity ↓ SOD, catalase, and PPAR-γ |
|||
| Haneda et al. (2018) [78] | OA patients, n = 31, average age 76.4 years Normal control, n = 12, average age 85.1 years |
Cartilage tissues | ↑ MMP-3, MMP-13 | ↑ AQP1, ADAMTS-4, and ADAMTS-5 ↓ COL2A1 and ACAN expression |
||||
| Yang et al. (2017) [81] | OA patients, n = 8 Normal control, n = 12 |
Articular cartilage | ↑ MMP-13 | ↑ IRF-8 | ||||
| Fu et al. (2016) [82] | Normal donors | Articular cartilage | ↑ MMP-1, MMP-3, and MMP-9 | ↑ NO, PGE2, iNOS, COX-2, ADAMTS-4, ADAMTS-5, HMGB1, and TLR4 expression | ||||
| Chou et al. (2018) [83] | OA patients, n = 20 for cartilage samples, mean age 66.6 ± 9.9 years; n = 25 for synovial fluid samples, mean age 63.6 ± 15.7 years | Knee articular cartilage Synovial fluid |
↑ IL-6 | ↑ MMP-3 | ↑ TSG-6, TIMP1, and VEGF | |||
| Chen et al. (2018) [84] | OA patients, n = 20, mean age 62 ± 9.2 years Healthy donor, n = 20, mean age 55.2 ± 8.64 years |
Articular cartilage | ↑ IL-6 | ↑ MMP-3 | ↑ TAK1, nitrite, PGE2, and p-p65 ↓ IkBα |
↓ miR-149 | ||
| Alunno et al. (2017) [86] | OA patients, n = 24 | Synovial fluid FLS |
↑ MMP-2 | |||||
| Gui et al. (2017) [88] | OA patients, n = 10, mean age 63.4 years | Articular cartilages | ↑ SOCS3, NF-κB, and COX2 | |||||
| Terauchi et al. (2016) [95] | OA patients, n = 5 | Articular cartilage | ↑ Runx2 | |||||
| Tao et al. (2015) [96] | OA patients, n = 10, mean age 50 ± 10 years Healthy control, n = 10, mean age 55 ± 10 years |
Articular cartilage | ↑ KPNA2 | |||||
| Struglics et al. (2016) [100] | OA patients, n = 24, median age 64 years | Synovial fluid | ↑ IL-1β, IL-6, IL-8, and TNF-α | ↑ C4d, C3bBbP, and sTCC | ||||
| Daghestani et al. (2015) [101] | OA patients, n = 159, mean age 63.7 ± 11.8 years | Synovial fluid Blood |
↑ CD163 and CD14 | |||||
| Mao et al. (2017) [107] | OA patients, n = 8, mean age 65.8 ± 2.24 years Healthy donor, n = 8, mean age 64.4 ± 2.86 years Normal donors for bone marrow, n = 6, mean age 37 years |
Bone marrow Cartilage tissues |
↑ ADAMTS-4 and ADAMTS-5 ↓ ACAN |
↓ miR-92a-3p | ||||
Table 2.
Molecular changes in animal model of osteoarthritis.
| Authors (year) | Animal model | Osteoarthritis intervention | Samples | Findings |
|---|---|---|---|---|
| Schmidli et al. (2018) [18] | Dogs (n = 59): diseased group (n = 36) and control group (n = 23) | Diseased dogs with canine cruciate ligament disease used | Infrapatellar fat pad Subcutaneous adipose tissue Synovial fluid |
↑ IL-1β, IL-6, IL-10, MMP-1, MMP-3, MMP-13, and TNF-α ↑ T cells, macrophages |
| Xu et al. (2015) [26] | Male Sprague-Dawley (SD) rats (n = 20), aged 40–50 days: normal group (n = 10) and 8-week group (n = 10) | Meniscal/ligamentous injury (MLI) modeling in knee joint | Articular cartilage | ↑ Sam68 expression |
| Jiang et al. (2017) [28] | C57BL/6 mice (n = 20) | ACLT | Knee joints | ↑ BRD4, HMGB1, and p-p65 |
| Qu et al. (2018) [30] | Male C57BL/6 mice | Surgically induced destabilization of medial meniscus (DMM) | Knee joints | ↑ MMP-13, TNF-α, iNOS, ADAMTS-5, IL-6, COX-2, NF-κB2, and p-IκBα |
| Xia et al. (2016) [31] | SD rats | MIA injection (0.5 mg) for 4 weeks | Blood | ↑ miR-381a-3p, TNFα, COX-2, iNOS,IL-6, IL-8, and p-p65 ↓ IκBα |
| Ding et al. (2019) [32] | Male C57BL/6 mice (n = 36) | Medial meniscal tear surgery | Articular cartilages of medial tibial plateau Synovial fluid |
↓ miR-93 ↑ TNF-α, IL-1β, IL-6, TLR4, p-p65, and p-IκBα |
| Hu et al. (2016) [33] | Male Wistar rats (MIA group, n = 30; control, n = 15) | Intra-articular MIA injection (5 mg/kg) | Blood | ↑ P2X7R, TNF-α, IL-6, IL-1β, MMP-13, SP (substance P), and PGE2 ↑ IKKα, IKKβ, IκBα, p65, and all of their phosphorylated forms |
| Li et al. (2018) [34] | Female SD rats (n = 36) | Intra-articular MIA injection (0.2 mg/rat) for 10 days | Synovial fibroblasts Blood |
↑ TNF-α, IL-1β, IL-17a, IL-8, MMP-3, MMP-9, VEGF, ADAMTS-4, p-PI3K, and p-AKT |
| Raghu et al. (2017) [63] | C57BL/6J mice | Destabilization of medial meniscus (DMM) | Knee joints | ↑ CCL2, CCR2, MMP-13, MMP-6, and ADAMTS-4 |
| Adler et al. (2017) [72] | Mixed-breed dogs (n = 4) | TGF-1β (1 or 10 ng/ml) with or without IL-1β (10 ng/ml) | Stifle joints' cartilage | ↑ MMP-3, iNOS, NO, COX-2, and PGE ↓ TIMP-2 |
| Terauchi et al. (2016) [95] | Male STR/OrtCrlj mice (n = 24) | Articular cartilage with subchondral bone | ↑ Runx2 expression | |
| Blaney Davidson et al. (2015) [97] | Cow | TGF-β1 (1 and 10 ng/ml), IL-1β (1 or 10 ng/ml) | Metacarpal joint's cartilage | ↑ NGF |
| Alquraini et al. (2017) [110] | Male Prg4+/+ and Prg4-/- mice | rhPRG4 (100 μg/ml), CD44 Ab (1.25 μg/ml), or combination of rhPRG4 and CD44 Ab for 48 hours | Synovial tissues | ↑ NF-κB p50 and p65 in Prg4-/- compared to Prg4+/+ |
Table 3.
Molecular changes in cellular model of osteoarthritis.
| Authors (year) | Cell line | Treatment | Findings |
|---|---|---|---|
| Xu et al. (2015) [26] | SW1353 | 100 ng/ml human TNF-α for 0, 6, 12, 24, 36, and 48 h | ↑ Cleaved caspase-3, cleaved PARP, Sam68, MMP-13, ADAMTS-5, iNOS, and IL-6 ↓ IκBα, ↑ p-p65 |
| Yu et al. (2018) [27] | CHON-001 (human chondrocyte cell) | IL-1β (0.1 ng/ml, 2 ng/ml, 5 ng/ml, and 10 ng/ml) | ↑ miR-126, IL-6, IL-8, and TNF-α |
| Jiang et al. (2017) [28] | SW1353 | 10 ng/ml IL-1β | ↑ IL-6, IL-8, IL-10, TNF-α, MMP-2, MMP-3, MMP-9, MMP-13, BRD4, HMGB1, and nuclear p65 |
| Sun et al. (2017) [29] | ATDC5 (murine articular chondrocyte) | LPS (0, 1, 5, and 10 μg/ml) for 6 hrs | ↑ miR-146a, IL-1β, IL-6, IL-8, and TNF-α ↓ CXCR4 |
| Tao et al. (2015) [96] | SW1353 (human chondrosarcoma cells) | 10 ng/ml IL-1β for 0, 12, 24, 36, and 48 hrs respectively | ↑ KPNA2, MMP-13, and ADAMTS-5 ↑ Nuclear p65, p-p65 |
| Blaney Davidson et al. (2015) [97] | H4 (murine chondrocyte), G6 (human chondrocyte) | IL-1β (1 or 10 ng/ml) or TGF-β1 (0.1, 1, or 10 ng/ml) | ↑ NGF |
3. The Role of Immune Cells in Osteoarthritis
Immune cells like activated neutrophils and macrophages can secrete cytokines, such as IL-6 and IL-1β, which amplify the inflammatory process in osteoarthritis [13]. Increased infiltration of leukocytes (macrophages, T-lymphocytes, B-lymphocytes, and neutrophils) in the synovium, particularly within the subintimal layer, is a characteristic of osteoarthritis [14]. Shan et al. reported elevated PD1+CXCR5+CD4+ T cells, ICOS+CXCR5+CD4+ T cells, and IL 21+CXCR5+CD4+ T cells in peripheral blood of patients with osteoarthritis [15]. CD4+ T cell is the T helper cell (Th cell) which may induce inflammation in the early stage of osteoarthritis. C-X-C chemokine receptor type 5 (CXCR5), inducible costimulator (ICOS), and programmed cell death 1 (PD-1) are known to be expressed by the Th cell [15].
Lymphocyte activation gene-3 (LAG-3+) regulatory T cells (Treg cells) have also been shown to increase in osteoarthritis [16]. Treg cells act as an immunoregulator in many inflammatory diseases [17]. It regulates the secretion of anti-inflammatory cytokines and expression of cytokine receptors [17]. Evidence showed that the response of Treg cells decreased in osteoarthritis in concurrent with an increase of LAG-3 expression in osteoarthritis. It has been postulated that LAG-3 molecules can reduce Treg function and boost inflammation [16].
CD3+ T cells are revealed as the predominant immune cells in IFP of dogs with canine cruciate ligament disease, which is associated with osteoarthritis, followed by CD14+ macrophages [18]. Both cell types can produce various cytokines such as IL-1β and IL-6 when activated. Immune cells and inflammatory mediators secreted in IFP will interact with other joint tissues, which can promote the pathological process of osteoarthritis [10]. At the same time, cartilage and the synovium are also shown to modulate the IFP. IL-1β stimulates increased proinflammatory cytokine secretion by IFP [19], suggesting that a cross-talk happens between IFP and joint tissues.
4. The Role of Cytokines in Osteoarthritis
Cytokines secreted by the immune cells are the main players of any inflammatory conditions, including osteoarthritis [7]. Proinflammatory cytokines, such as IL-1β and TNF-α, are among the mediators secreted in early osteoarthritis [20–23]. IL-1β and TNF-α drive the inflammatory cascade independently or in collaboration with other cytokines [24]. They are produced by activated chondrocytes, synoviocytes, and mononuclear cells [25]. TNF-α and IL-1β have been used to trigger inflammation in chondrocyte and synoviocyte culture. Upon stimulation, the cells release IL-6 [26], IL-8 [27], IL-10 [28], IL-1β [29], and TNF-α [28]. Similar cytokine profile was increased in animal models of osteoarthritis [18, 30–34].
IL-1β is involved in a series of cellular activities, such as cell proliferation, differentiation, and apoptosis. It interferes with the production of essential structural proteins, including collagen type II and aggrecan, by influencing the activity of chondrocytes in the joint. Moreover, IL-1β affects MMPs' synthesis by chondrocytes, including MMP-1 and MMP-13, which, in turn, destroy the articular cartilage [35]. IL-1β was also shown to induce the production of reactive oxygen species, for example, nitric oxide (NO) [36]. Since IL-1β has been proven to play a significant role in the pathogenesis of osteoarthritis, it is commonly used to induce an in vitro osteoarthritis model in chondrocytes [37]. It stimulates expression of TNF-α and surface expression of TNF receptor (TNFR) in chondrocytes [38]. Binding of TNF-α to TNFR causes signal transduction and activates TNF receptor-associated factor 2 (TRAF2). TRAF2 will activate the nuclear factor kappa-light-chain-enhancer of activated B cell (NF-κB) signaling pathway involved in inflammatory diseases.
A study conducted among patients with osteoarthritis showed that IL-1β, IL-6, IL-8, IL-18, IL-17, IL-22, and transforming growth factor-beta 1 (TGFβ1) were increased in the inflamed synovium tissues compared to the noninflamed tissues [14]. IL-17 induces the release of IL-6, IL-8, and TNF-α by synovial fibroblasts and chondrocytes, leading to inflammation and cartilage breakdown [39]. It is secreted by T helper 17 cell (Th17), mast cell, and myeloid cell. Other than that, IL-17 promotes the recruitment and activation of neutrophils, which are the initial cell types recruited to the inflammation sites [40]. Activated neutrophils synthesize several inflammatory factors, which regulate the inflammation process in osteoarthritis. IL-17 has been shown to be present in the synovial fluid of a subset of patients with end-stage osteoarthritis [41]. Increased IL-17 and IL-22 levels are also detected in the synovial fluid of temporomandibular joint of patients with osteoarthritis [42]. The increase of these two cytokines is associated with the elevation of receptor activator of nuclear factor kappa-Β ligand (RANKL), which induces the differentiation of osteoclasts and resorption of subchondral bone, a layer of bone beneath the cartilage in joint [42]. IL-22 stimulates the proliferation of synovial cells and enhances the expression of MMPs in fibroblast-like synoviocytes (FLS) [43].
Interleukin 6 (IL-6) is known as a proinflammatory cytokine in chronic inflammatory diseases. In osteoarthritis, IL-6 released by joint tissue will bind to the soluble IL-6 receptor (IL-6R), leading to transsignaling [44]. As a consequence, the immune system is activated, whereby mononuclear cells like monocytes are recruited to the inflamed joint area [44]. IL-6 transsignaling will skew the differentiation of monocytes to macrophages through the upregulation of the macrophage colony-stimulating factor (M-CSF) receptor [45]. A significant increase of IL-6 and IL-8 has been observed in patients with osteoarthritis [46–51]. For instance, Favero et al. determined the inflammatory molecules produced from coculture of meniscus tissue and synovial membrane from patients with early-stage (n = 5) and end-stage (n = 5) osteoarthritis [47]. They demonstrated the presence of IL-6 and IL-8 in patients at both stages, but their levels were higher among the end-stage patients [47]. In osteoarthritis, IL-8 in the synovial fluid plays a role in recruiting neutrophils and activating them. The activated cells will secrete enzyme elastase to degrade type II collagen crosslinks and proteoglycan in the articular cartilage [52].
IL-37 is a member of the IL-1 family, and it is an anti-inflammatory cytokine [53]. Its level has been shown to elevate in osteoarthritis patients [53, 54]. IL-37 can reduce the synthesis of proinflammatory cytokines and catabolic enzymes by osteoarthritic chondrocytes and synoviocytes. Mabey et al. collected blood and synovial fluid samples from patients with osteoarthritis (n = 32) and healthy controls (n = 14) to determine inflammatory cytokine levels in patients with knee osteoarthritis. They demonstrated that IL-2, IL-4, IL-6, and IL-10 levels were higher in patients with osteoarthritis compared to the controls [55]. Besides, a study by Xia et al. also showed an increased IL-10 level in lymphocyte activation gene-3 negative (LAG-3−) regulatory T cells (Treg) from patients with knee osteoarthritis [16]. Conversely, He et al. demonstrated a decrease of IL-4 expression in articular cartilage from patients with osteoarthritis [56]. It may be due to the difference in the samples used between the signaling studies, whereby Mabey et al. used blood while He et al. used chondrocytes isolated from articular cartilage. The expression of IL-4 receptor had been shown to elevate in the serum of patients with osteoarthritis [57]. However, IL-4 was also shown to express at a decreased level in cartilage from patients with osteoarthritis compared to cartilage from healthy controls [58].
Other than the cytokines aforementioned, several other cytokines are involved in the pathogenesis of osteoarthritis. Some of the cytokines shown to increase in osteoarthritis are IL-18, TGFβ1 [14], IL-1 receptor (IL-1R) [59], and IL-1 alpha (IL-1α) [23]. Shan et al. showed enhanced serum IL-21, IL-17A, and IFN-γ levels in patients with osteoarthritis compared to controls [15]. IL-21 also upregulates RANKL expression, thereby stimulating bone marrow stem cells to differentiate into mature osteoclasts [60]. The interactions between pro- and anti-inflammatory cytokines were explored using macrophage conditioned medium (CM). Upregulation of proinflammatory cytokines (IL-1b, IL-6, MMP13, and ADAMTS5) and downregulation of cartilage matrix components (aggrecan, type II collagen) were observed in human osteoarthritic cartilage explants cultured with CM of proinflammatory macrophages expressing IFN-γ and TNF-α. However, CM of anti-inflammatory macrophages expressing IL-4 or IL-10 did not suppress the stimulation effects of conditioned media of proinflammatory cytokine-stimulated explants [61].
5. The Role of Chemokines in Osteoarthritis
Chemokine is a subfamily of cytokine with low molecular weight. It is classified into four families, namely, CXC, CC, C, and CX3C families, depending on the position of cysteine (C) residues [62]. Chemokine functions as a chemoattractant, which directs the migration of immune cells to damaged or infected sites [62]. A study by Monasterio et al. revealed increased C-C motif ligand 5 (CCL5), CCL20, C-C motif receptor 5 (CCR5), and CCR7 in patients with temporomandibular joint osteoarthritis (TMJ-OA) [42]. These chemokines play a role in the recruitment of T helper cell type 1 (Th1), T helper cell type 17 (Th17), and T helper cell type 22 (Th22) to the affected joint in the study [42]. As a consequence, proinflammatory cytokines like IL-1β, IL-17, and IL-22 will be released in the joint and trigger the inflammation process.
In laboratory studies, chemokine receptor CXCR4 was shown by Sun et al. to reduce in chondrocyte culture induced with inflammation [29]. It is a specific receptor for stromal-derived-factor-1 (SDF-1), also called CXCL12. Raghu et al. revealed that CCL2 and its receptor, CCR2, were increased in a mouse model of osteoarthritis [63]. The study suggested that CCL2 is secreted by injured chondrocytes and synovial fibroblasts and it recruits CCR2-expressing monocytes to the damaged tissues.
CCL7 is a monocyte chemoattractant, which has been shown to increase in the synovial fluid of patients with osteoarthritis [41]. Production of CCL7 from synoviocytes is enhanced by IL-17 [41]. Patients with facet joint osteoarthritis (n = 48) also demonstrated elevated CCL4 and C-C Motif Chemokine Ligand 4 Like 2 (CCL4L2) compared to healthy controls (n = 10) [64]. The study showed that CCL4 and CCL4L2 expressions were involved in the canonical NF-κB signaling pathway. Hou et al. discovered increased expression of CX3CL1 in synovial fibroblasts from patients with osteoarthritis [9]. CX3CL1 induced MMP-3 expression in a time-dependent and dose-dependent manner by reacting with its receptor, CX3CR1 [9]. Furthermore, Alaaeddine et al. showed enhanced expression of CCL20 and its receptor, CCR6, in cartilages compared to controls. This enhanced expression can further stimulate IL-6, MMP-1, and MMP-13 production [46].
Increased CCR3 and its ligand, CCL11, also known as eotaxin-1, have been detected in synovial cells from patients with osteoarthritis [21]. CCR3 is expressed by a few inflammatory cells such as T cells [65] and dendritic cells [66]. Besides, the study showed that CCL11 was able to stimulate the release of MMP-9 in synoviocytes from patients with osteoarthritis. Favero et al. observed the elevation of CCL21 and CCL5 in coculture of meniscus and synovial membrane from patients with osteoarthritis [47]. Conditioned media from osteoarthritis tissues like cartilage, IFP, meniscus, and synovium induced and enhanced production of CXCL8 and CCL21 in the synoviocyte cell line [67]. Several other studies showed an enhanced CCL2 level in patients with osteoarthritis [47, 63, 68]. CCL2 is also known as monocyte chemoattractant protein 1 (MCP-1). CCL2/CCR2 signaling will trigger monocyte trafficking into the inflamed joint area and, in turn, cause further inflammation. Arkestål et al. observed an increased CCR2 and a decreased CXCR3 expressions in the peripheral blood of patients with osteoarthritis compared to controls [69].
6. The Role of Matrix Metalloproteinases (MMPs) in Osteoarthritis
Matrix metalloproteinases (MMP) are a family of zinc-dependent enzymes well known for regulating the degradation of the extracellular matrix (ECM) through cleavage of peptide bond of the target proteins [70]. MMPs can be categorized into several groups, which are collagenases (MMP-1, MMP-13), gelatinases (MMP-2, MMP-9), stromelysins (MMP-3), metalloelastase (MMP-12), matrilysin (MMP-7), and membrane-type matrix metalloproteinases (MT-MMPs), according to its structure and substrates [71]. They can degrade ECM of the articular cartilage, which mainly consists of collagens and proteoglycans.
MMP-3, MMP-9, and MMP-13 levels increased in animals induced with osteoarthritis [18, 30, 33, 34, 63], but the TIMP-2 level was shown to decline [72]. Similarly, MMP-13, MMP-3, MMP-2, and MMP-9 increased in the cell line model of osteoarthritis [26, 28]. Furthermore, several studies have investigated the MMP-13 level in osteoarthritis patients, and consistent observation is obtained, whereby the MMP-13 level increased significantly in patients with osteoarthritis [30, 48, 59, 73–78]. MMP-13, as a collagenase, is responsible for the degradation of type II collagen [79], which is the main collagen type in articular cartilage [80]. The expression of MMP-13 increased through stimulation by CCL20 [46] and interferon regulatory factor-8 (IRF-8) in chondrocytes derived from patients with osteoarthritis [81]. Conversely, overexpression of IL-37 in chondrocytes decreased the MMP-13 level [53]. Two separate studies by Zhang et al. and Wu et al. showed that changes in microribonucleic acid (miRNA) expression in osteoarthritis could alter the MMP-13 level [35, 51]. They found that miR-502-5p and miR-454 enhanced the MMP-13 level. In addition, Ma et al. and Chen et al. demonstrated that advanced glycation end products (AGEs) remarkably induced MMP-13 expression in joint tissues from patients with osteoarthritis [23, 70].
MMP-1 is another known collagenase frequently found to be elevated in osteoarthritis [46, 53, 59, 82]. MMP-3, also known as stromelysin-1, cleaves type II collagen and aggrecan. Hou et al. showed that chemokine CX3CL1 induces MMP-3 production in a concentration-dependent and time-dependent manner using synovial fibroblasts from patients with osteoarthritis (OASFs) [9]. Other than that, several studies showed increased MMP-3 in osteoarthritis [47, 48, 53, 73, 78, 82, 83]. The induction of MMP-3 has been related to miR-149 and miR-454 expression in osteoarthritis [51, 84].
Expression of gelatinases such as MMP-2 (gelatinase A) and MMP-9 (gelatinase B) has been shown to associate with the pathogenesis of osteoarthritis. MMP-2 and MMP-9 are responsible to cleave ECM, cytokines, and chemokines, thus enhancing their activities [85]. MMP-2 level [14, 24, 86] and MMP-9 [21, 82] increase in inflamed synovial tissues compared to noninflamed tissues. MMP-10, named as stromelysin-2, also increases in early- and end-stage osteoarthritis. Favero et al. showed a significantly increased level of MMP-10 in the meniscus and synovial coculture compared to meniscus alone [47]. The synovium was collected from the suprapatellar pouch while meniscus was isolated from the inner superficial zone of osteoarthritis patients who underwent total knee replacement. The two tissues were cocultured using a transwell to separate it and allow interaction between the two tissues.
Tissue inhibitors of metalloproteinases (TIMPs) are MMP inhibitor. It is proposed that the imbalance between MMP and TIMP activities is linked to articular destruction in osteoarthritis [87]. TIMP-2, TIMP-3, and TIMP-4 are elevated in patients with osteoarthritis [47, 48], while TIMP-1 is decreased [59].
7. The Role of Signaling Pathways and Other Inflammatory Components in Osteoarthritis
The nuclear factor-kappa B (NF-κB) transcription factor plays a central role in the pathogenesis of osteoarthritis [88]. It is triggered by proinflammatory cytokines and ECM degradation products [89]. The activated NF-κB will modulate the expression of several cytokines, chemokines, and matrix-degrading enzymes, which explains its role in regulating catabolic events in osteoarthritis [26]. The NF-κB signaling pathway begins with the activation of IκB kinase (IKK), resulting in phosphorylation and degradation of IκBα by the proteasome. Subsequently, p65 protein is released, phosphorylated, and translocated from the cytoplasm to the nucleus. These events activate the expression of several genes, such as MMP-13 and IL-6 [89]. Therefore, the phosphorylated p65 (p-p65) level was found to increase in osteoarthritis whereas the IκBα level was decreased [22, 23, 31, 35, 84].
Enhanced p-p38, p-JNK, and p-ERK in osteoarthritis indicate the involvement of the mitogen-activated protein kinase (MAPK) signaling pathway [76]. MAPK is a mediator which regulates downstream expression of proinflammatory cytokines and MMPs [90]. It also acts as a pain mediator [90]. This pathway could be a potential avenue for new drug discovery to halt the progression of osteoarthritis. It begins when proinflammatory cytokines and growth factors bind to their respective receptors on the cell membrane. These act as upstream activators and cause intracellular MAP kinases (MKKs) to phosphorylate specific MAP kinases. MKK1 and 2 will activate ERK1 and 2 and MKK3 and 6 responsible for p38 phosphorylation while MKK4 and 7 phosphorylate JNK1 and 2. Activated MAP kinases in turn activate other protein kinases and transcriptional regulatory proteins which lead to the upregulation of certain inflammatory genes such as MMPs, IL-1, and TNF-α. These cytokines can then maintain JNK activation and cause more cytokine and MMP production [90].
PI3K/AKT signaling is known to be activated by cytokines like IL-1β when it binds to its cell surface receptor. Upon stimulation, membrane protein PI3K induces phosphorylation of AKT which has shown to have a synergistic effect on NF-κB signaling. Activation of the PI3K/AKT pathway will enhance production of MMPs by cells, for example, chondrocytes [91].
The nitric oxide (inducible nitric oxide synthase (iNOS), nitric oxide (NO)) and prostacyclin pathway (cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2)) are also integral to the pathogenesis of osteoarthritis. IL-1β upregulates both iNOS and COX-2 in osteoarthritis, leading to increased production of NO [92] and PGE2 [93], respectively. Elevated NO will inhibit the synthesis of collagen type II (Col2) and proteoglycan [92]. Besides, enhanced PGE2 inhibits chondrocyte proliferation and reduces ECM synthesis [93]. In addition, IL-1β also stimulates A Disintegrin and Metalloproteinase with Thrombospondin motif (ADAMTS-5) production, an aggrecanase which causes aggrecan degradation [94]. iNOS, NO, COX-2, PGE2, ADAMTS-5, ADAMTS-4, and VEGF were found to increase in animals with osteoarthritis, leading to enhanced inflammatory factor production and ECM degradation [30, 34].
There are other factors influencing the progression of osteoarthritis. The expression of runt-related transcription factor 2 (Runx2), an osteogenic transcriptional activator, is enhanced in osteoarthritis [95]. Terauchi et al. found elevated Runx2 in STR/OrtCrlj mouse osteoarthritis model [95]. Runx2 was shown to promote MMP-13 expression [95]. The expression of karyopherin alpha 2 (KPNA2), which regulates delivery of p65 to the nucleus, also increased in osteoarthritis [96]. Bromodomain-containing protein 4 (BRD4) played a role in the NF-κB signaling pathway [28]. Inhibition of BRD4 will suppress IL-1β-induced expression of proinflammatory cytokines and phosphorylation of p65. The BRD4 level was increased in a C57BL/6 mouse model of osteoarthritis [28]. Nerve growth factor (NGF) had been shown by Blaney Davidson et al. to increase in bovine chondrocytes treated with TGF-β1 and IL-1β [97]. TGF-β1-induced NGF expression was found to be dependent on an activin receptor-like kinase 5-Smad2/3 (ALK5-Smad2/3) signaling pathway as blockage of this pathway can prevent the expression. ALK5 is a type of transmembrane receptor of TGF-β. Once it activates, it will trigger downstream signaling cascades via the Smad-dependent pathway [98]. In a recent study using cartilage explants cultured with osteoarthritic synovium-CM or IL-1β, the TGF-β/Smad2/3P pathway that is protective against mechanical loading was diminished [99], which could lead to further destruction of the cartilage. Moreover, the complement system components [100] and soluble macrophage biomarkers (CD163 and CD14) [101] are also shown to be upregulated in osteoarthritis.
Overall, the integrated regulation of cytokines, chemokines, MMPs, and the signaling pathway is summarized in Figure 1.
Figure 1.
The signaling pathways (brown, grey, and purple box) involved and interactions between cytokines (pink box), chemokines (beige box), matrix metalloproteinases (green box), and other proteins in osteoarthritis. IL-1β and TNF-α (pink box) produced by cells; for example, activated chondrocytes, synoviocytes, and mononuclear cells are among the early mediators in the inflammatory cascades. Continuous lines indicate stimulation while dotted lines indicate inhibition of the downstream molecule or activity. The outcomes of the molecule interactions are shown in yellow boxes.
8. The Role of miRNA in Osteoarthritis
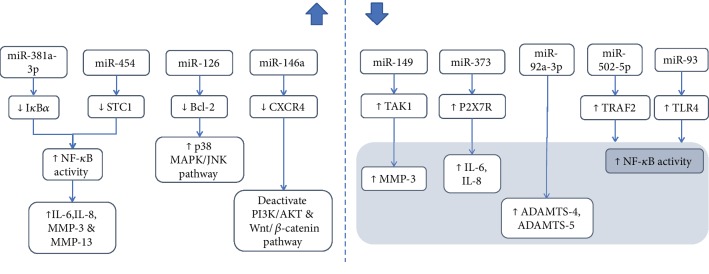
MicroRNAs (miRNAs) are short, endogenous noncoding RNAs functioning to regulate posttranscriptional gene expression by binding to the 3′untranslated region (3′UTR) of target genes [31, 35]. Once bound to target genes, they can block the translation process or decrease the stability of the messenger RNAs (mRNAs) [102]. Several microarray and database analyses have shown the involvement of miRNAs in osteoarthritis (Figure 2) [103].
Figure 2.
Alteration of miRNA levels in osteoarthritis and its downstream influences.
Laboratory studies showed that miR-146a [29] and miR-126 [27] were upregulated in chondrocytes induced with inflammation with lipopolysaccharide (LPS) and IL-1β, respectively. miR-146a targets CXCR4 and downregulated its expression. Besides, upregulation of miR-126 in IL-1β-treated chondrocytes downregulated B-cell lymphoma 2 (Bcl-2) expression. miR-381a-3p was found to be elevated, and miR-93 was decreased in a rodent model of osteoarthritis [31, 32].
Recent human studies highlighted that miR-381a-3p [31] and miR-454 [51] were upregulated in osteoarthritis. Xia et al. discovered that nuclear factor of kappa light polypeptide gene enhancer in B-cell inhibitor alpha (IκBα) is a target gene of miR-381a-3p [31]. Hence, upregulation of miR-381a-3p will decrease IκBα expression and further enhance the activation of NF-κB. Wu et al. showed that miR-454 overexpression in osteoarthritis promoted the proliferation of synovial fibroblasts and increased inflammatory factors [51].
On the other hand, Chen et al. showed that miR-149 bound to transforming growth factor- (TGF-) 1-activating kinase 1 (TAK1) 3′UTR to regulate its expression [84]. TAK1 expression increased when miR-149 decreased in osteoarthritis. NF-κB is the downstream signaling pathway of TAK1; thus, upregulated TAK1 causes increased activation of NF-κB in osteoarthritis [104]. Zhang et al. demonstrated that miR-373 decreased in osteoarthritis and it targeted P2X7 receptor (P2X7R) [49]. P2X7R expression was enhanced in osteoarthritis; this led to increased chondrocyte proliferation and release of inflammatory factors such as IL-6 and IL-8. In addition, P2X7R is adenosine triphosphate- (ATP-) gated plasma membrane ion channel which is involved in IL-1β maturation and its release from activated immune cells [105]. Damaged cells released ATP which led to activation of P2X7R and further release of inflammatory cytokines [106]. Mao et al. revealed that miR92a-3p directly targeted 3′UTR of ADAMTS-4 and ADAMTS-5 mRNAs [107]. Zhang et al. showed that miR-502-5p targeted 3′UTR of TNF receptor-associated factor 2 (TRAF2) to inhibit its expression [35]. Since these miRNAs target mRNAs which contribute to the development of osteoarthritis, they could be potential drug targets to treat osteoarthritis.
8.1. Future Research Area
The understanding of mechanisms involved in the pathogenesis of osteoarthritis is very important. It is the basis of rational drug development as currently, most pharmacotherapies for osteoarthritis are symptomatic. For example, the integral role of IL-1β in the pathogenesis of osteoarthritis can be targeted to prevent further degradation of the joint tissue. IL-1 receptor antagonist has been developed, and it showed promising results in animal studies. However, its effects on humans require further studies [108]. Besides, IL-6 is well known for its contribution to progression of osteoarthritis. IL-6 or its receptor such as soluble IL-6 receptor can be a target for drug development. The recently discovered functional role of miRNAs in regulating joint homeostasis should also be explored, and they are another avenue for intervention. Circular RNAs (circRNAs), a single-stranded, noncoding regulatory RNA, can function as miRNA sponges and inhibit miRNA activity. It provides a possibility to be targeted as a therapeutic strategy.
The use of inflammatory markers to predict disease progression and treatment efficacy should also be explored. Currently, the evaluation of patients' conditions and improvements is based on subjective instruments like Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and radiographic findings. Future studies should attempt to validate the correlation of these instruments with inflammatory markers to provide a more objective measurement of disease progression. Established biomarkers of osteoarthritis progression such as urinary type II collagen degradation (uCTX-II) are shown to associate with incidence and progression of radiographic osteoarthritis [109].
9. Conclusion
Inflammation plays an integral role in the pathogenesis of osteoarthritis. Various molecules released by the chondrocytes and synoviocytes and infiltrating immune cells, such as cytokines, chemokines, and MMPs, are involved in regulating the joint anabolism and catabolism process. Their expressions are in turn governed by NFκB, MAPK, PI3K/AKT, prostacyclin, and nitric oxide pathways. The involvement of these molecules and signaling pathways is well-established in cellular, animal, and human studies. However, more studies are required to link and explore the connection between all these molecules involved. This review article collates the latest evidence on the relationship between inflammation and osteoarthritis to provide a better understanding on the pathogenesis of osteoarthritis. These pathways should be exploited as potential targets for drug intervention as currently pharmacotherapies targeting the underlying mechanism of osteoarthritis are still lacking in the market.
Acknowledgments
The authors thank the Ministry of Education, Malaysia, for Fundamental Research Grant Scheme (FRGS/1/2018/SKK10/UKM/03/1) and Universiti Kebangsaan Malaysia for Fundamental Research Grant (FF-2018-405).
Abbreviations
- ACAN:
Aggrecan
- ACLT:
Anterior cruciate ligament transection
- ADAMTS:
A Disintegrin and Metalloproteinase with Thrombospondin motifs
- AGEs:
Advanced glycation end products
- AKT:
Protein kinase B
- ALK5:
Activin receptor-like kinase 5
- AQP1:
Aquaporin 1
- ATP:
Adenosine triphosphate
- BCL-2:
B-cell lymphoma 2
- BRD4:
Bromodomain-containing protein 4
- CCL:
C-C motif ligand
- CCR:
C-C motif receptor
- CRP:
C reactive protein
- CXCR:
C-X-C chemokine receptor
- col10:
Collagen type X
- col2:
Collagen type II
- COX-2:
Cyclooxygenase 2
- DDWR:
Disk displacement with reduction
- DKK:
Dickkopf WNT signaling pathway inhibitor
- DMM:
Destabilization of medial meniscus
- DUSP1:
Dual specificity protein phosphatase 1
- ECM:
Extracellular matrix
- ERK:
Extracellular signal-regulated kinase
- ET-1:
Endothelin-1
- FLS:
Fibroblast-like synoviocytes
- FOXM1:
Forkhead box M1
- FSTL1:
Follistatin-like protein 1
- GAG:
Glycosaminoglycan
- HMGB1:
High mobility group box 1
- ICAM-1:
Intracellular adhesion molecule-1
- ICOS:
Inducible costimulator
- IFP:
Infrapatellar fat pad
- IFN:
Interferon
- IKK:
IκB kinase
- IL:
Interleukin
- iNOS:
Inducible nitric oxide synthase
- IRF-8:
Interferon regulatory factor-8
- JNK:
c-Jun N-terminal kinase
- KPNA2:
Karyopherin alpha 2
- LAG:
Lymphocyte activation gene
- MAPK:
Mitogen-activated protein kinase
- MCP-1:
Monocyte chemoattractant protein 1
- M-CSF:
Macrophage colony-stimulating factor
- MIA:
Monosodium iodoacetate
- MKKs:
MAP kinase kinases
- MMPs:
Matrix metalloproteinases
- MPO:
Myeloperoxidase
- mRNA:
Messenger ribonucleic acid
- miRNA:
Microribonucleic acid
- NF-κB:
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NGF:
Nerve growth factor
- NO:
Nitric oxide
- OA:
Osteoarthritis
- OPG:
Osteoprotegerin
- PARP-1:
Poly [ADP-ribose] polymerase 1
- PD-1:
Programmed cell death 1
- PGE2:
Prostaglandin E2
- PI3K:
Phosphoinositide 3-kinase
- PKC:
Protein kinase C
- PKR:
Protein kinase R
- PPAR-γ:
Peroxisome proliferator-activated receptor gamma
- PRMT1:
Protein arginine N-methyltransferase 1
- RANKL:
Receptor activator of nuclear factor kappa-Β ligand
- ROS:
Reactive oxygen species
- Runx2:
Runt-related transcription factor 2
- SD:
Sprague-Dawley
- SOCS1:
Suppressor of cytokine signaling 1
- SOD:
Superoxide dismutase
- STC1:
Stanniocalcin-1
- sTCC:
Soluble terminal complement complex
- TAK1:
Transforming growth factor-1-activating kinase 1
- TF:
Tissue factor
- TGF:
Transforming growth factor
- Th:
T helper cell
- TIMP:
Tissue inhibitor of metalloproteinase
- TLR4:
Toll-like receptor 4
- TMJ:
Temporomandibular joint
- TNF:
Tumor necrosis factor
- TRAF2:
TNF receptor-associated factor 2
- Treg:
Regulatory T cells
- TSG-6:
TNF-stimulated gene-6
- uCTX-II:
Urinary type II collagen degradation
- UTR:
Untranslated region
- VCAM-1:
Vascular cell adhesion molecule-1
- VEGF:
Vascular endothelial growth factor
- WOMAC:
Western Ontario and McMaster Universities Osteoarthritis Index.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Kraus V. B., Blanco F. J., Englund M., Karsdal M. A., Lohmander L. S. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthritis and Cartilage. 2015;23(8):1233–1241. doi: 10.1016/j.joca.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotti M., Duffell L. D., Faisal A. A., McGregor A. H. The complexity of human walking: a knee osteoarthritis study. PloS One. 2014;9(9, article e107325) doi: 10.1371/journal.pone.0107325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shane Anderson A., Loeser R. F. Why is osteoarthritis an age-related disease? Best Practice & Research. Clinical Rheumatology. 2010;24(1):15–26. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldring M. B., Goldring S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Annals of the New York Academy of Sciences. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 5.Litwic A., Edwards M. H., Dennison E. M., Cooper C. Epidemiology and burden of osteoarthritis. British Medical Bulletin. 2013;105(1):185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy S. L., Robinson-Lane S. G., Niemiec S. L. S. Knee and hip osteoarthritis management: a review of current and emerging non-pharmacological approaches. Current Treatment Options in Rheumatology. 2016;2(4):296–311. doi: 10.1007/s40674-016-0054-7. [DOI] [Google Scholar]
- 7.Sokolove J., Lepus C. M. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Therapeutic Advances in Musculoskeletal Disease. 2013;5(2):77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scanzello C. R., Goldring S. R. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou S. M., Hou C. H., Liu J. F. CX3CL1 promotes MMP-3 production via the CX3CR1, c-Raf, MEK, ERK, and NF-κB signaling pathway in osteoarthritis synovial fibroblasts. Arthritis Research and Therapy. 2017;19(1):p. 282. doi: 10.1186/s13075-017-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ioan-Facsinay A., Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Research & Therapy. 2013;15(6):p. 225. doi: 10.1186/ar4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojdasiewicz P., Poniatowski L. A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of Inflammation. 2014;2014:19. doi: 10.1155/2014/561459.561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldring M. B., Otero M. Inflammation in osteoarthritis. Current Opinion in Rheumatology. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tecchio C., Micheletti A., Cassatella M. A. Neutrophil-derived cytokines: facts beyond expression. Frontiers in Immunology. 2014;5:p. 508. doi: 10.3389/fimmu.2014.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deligne C., Casulli S., Pigenet A., et al. Differential expression of interleukin-17 and interleukin-22 in inflamed and non-inflamed synovium from osteoarthritis patients. Osteoarthritis and Cartilage. 2015;23(11):1843–1852. doi: 10.1016/j.joca.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Shan Y., Qi C., Liu Y., Gao H., Zhao D., Jiang Y. Increased frequency of peripheral blood follicular helper T cells and elevated serum IL-21 levels in patients with knee osteoarthritis. Molecular Medicine Reports. 2017;15(3):1095–1102. doi: 10.3892/mmr.2017.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia J., Ni Z., Wang J., Zhu S., Ye H. Overexpression of lymphocyte activation gene-3 inhibits regulatory T cell responses in osteoarthritis. DNA and Cell Biology. 2017;36(10):862–869. doi: 10.1089/dna.2017.3771. [DOI] [PubMed] [Google Scholar]
- 17.Li Y.-s., Luo W., Zhu S. A., Lei G. H. T cells in osteoarthritis: alterations and beyond. Frontiers in Immunology. 2017;8:356–356. doi: 10.3389/fimmu.2017.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidli M. R., Fuhrer B., Kurt N., et al. Inflammatory pattern of the infrapatellar fat pad in dogs with canine cruciate ligament disease. BMC Veterinary Research. 2018;14(1):p. 161. doi: 10.1186/s12917-018-1488-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clockaerts S., Bastiaansen-Jenniskens Y. M., Feijt C., et al. Cytokine production by infrapatellar fat pad can be stimulated by interleukin 1β and inhibited by peroxisome proliferator activated receptor α agonist. Annals of the Rheumatic Diseases. 2012;71(6):1012–1018. doi: 10.1136/annrheumdis-2011-200688. [DOI] [PubMed] [Google Scholar]
- 20.Min S., Wang C., Lu W., et al. Serum levels of the bone turnover markers dickkopf-1, osteoprotegerin, and TNF-α in knee osteoarthritis patients. Clinical Rheumatology. 2017;36(10):2351–2358. doi: 10.1007/s10067-017-3690-x. [DOI] [PubMed] [Google Scholar]
- 21.Chang X., Shen J., Yang H., et al. Upregulated expression of CCR3 in osteoarthritis and CCR3 mediated activation of fibroblast-like synoviocytes. Cytokine. 2016;77:211–219. doi: 10.1016/j.cyto.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Ni S., Miao K., Zhou X., et al. The involvement of follistatin-like protein 1 in osteoarthritis by elevating NF-κB-mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Research and Therapy. 2015;17(1):p. 91. doi: 10.1186/s13075-015-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma C., Zhang Y., Li Y. Q., Chen C., Cai W., Zeng Y. L. The role of PPARγ in advanced glycation end products-induced inflammatory response in human chondrocytes. PloS One. 2015;10(5, article e0125776) doi: 10.1371/journal.pone.0125776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding X., Zhang Y., Huang Y., Liu S., Lu H., Sun T. Cadherin-11 involves in synovitis and increases the migratory and invasive capacity of fibroblast-like synoviocytes of osteoarthritis. International Immunopharmacology. 2015;26(1):153–161. doi: 10.1016/j.intimp.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Sellam J., Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nature Reviews Rheumatology. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 26.Xu L., Sun C., Zhang S., et al. Sam68 promotes NF-κB activation and apoptosis signaling in articular chondrocytes during osteoarthritis. Inflammation Research. 2015;64(11):895–902. doi: 10.1007/s00011-015-0872-3. [DOI] [PubMed] [Google Scholar]
- 27.Yu C. D., Miao W. H., Zhang Y. Y., Zou M. J., Yan X. F. Inhibition of miR-126 protects chondrocytes from IL-1β induced inflammation via upregulation of Bcl-2. Bone & Joint Research. 2018;7(6):414–421. doi: 10.1302/2046-3758.76.BJR-2017-0138.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Y., Zhu L., Zhang T., et al. BRD4 has dual effects on the HMGB1 and NF-κB signalling pathways and is a potential therapeutic target for osteoarthritis. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2017;1863(12):3001–3015. doi: 10.1016/j.bbadis.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Sun T., Li X., Song H., et al. miR-146a aggravates LPS-induced inflammatory injury by targeting CXCR4 in the articular chondrocytes. Cellular Physiology and Biochemistry. 2017;44(4):1282–1294. doi: 10.1159/000485488. [DOI] [PubMed] [Google Scholar]
- 30.Qu R., Chen X., Wang W., et al. Ghrelin protects against osteoarthritis through interplay with Akt and NF-κB signaling pathways. FASEB Journal. 2018;32(2):1044–1058. doi: 10.1096/fj.201700265R. [DOI] [PubMed] [Google Scholar]
- 31.Xia S., Yan K., Wang Y. Increased miR-381a-3p contributes to osteoarthritis by targeting IkBα. Annals of Clinical and Laboratory Science. 2016;46(3):247–253. [PubMed] [Google Scholar]
- 32.Ding Y., Wang L., Zhao Q., Wu Z., Kong L. MicroRNA-93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NF-κB signaling pathway. International Journal of Molecular Medicine. 2019;43(2):779–790. doi: 10.3892/ijmm.2018.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu H., Yang B., Li Y., Zhang S., Li Z. Blocking of the P2X7 receptor inhibits the activation of the MMP-13 and NF-κB pathways in the cartilage tissue of rats with osteoarthritis. International Journal of Molecular Medicine. 2016;38(6):1922–1932. doi: 10.3892/ijmm.2016.2770. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Xie S., Qi Y., Li H., Zhang R., Lian Y. TNF-α increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the PI3K/AKT pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Experimental and Therapeutic Medicine. 2018;16(6):4737–4744. doi: 10.3892/etm.2018.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang G., Sun Y., Wang Y., Liu R., Bao Y., Li Q. miR-502-5p inhibits IL-1β-induced chondrocyte injury by targeting TRAF2. Cellular Immunology. 2016;302:50–57. doi: 10.1016/j.cellimm.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Jayasuriya C. T., Chen Q. Role of inflammation in osteoarthritis. Rheumatology. Current Research. 2013;3(2) doi: 10.4172/2161-1149.1000121. [DOI] [Google Scholar]
- 37.Fernandes J. C., Martel-Pelletier J., Pelletier J. P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1-2):237–246. [PubMed] [Google Scholar]
- 38.Saperstein S., Chen L., Oakes D., Pryhuber G., Finkelstein J. IL-1beta augments TNF-alpha-mediated inflammatory responses from lung epithelial cells. Journal of Interferon & Cytokine Research. 2009;29(5):273–284. doi: 10.1089/jir.2008.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honorati M. C., Neri S., Cattini L., Facchini A. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis and Cartilage. 2006;14(4):345–352. doi: 10.1016/j.joca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Frontiers in Physiology. 2018;9:113–113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snelling S. J. B., Bas S., Puskas G. J., et al. Presence of IL-17 in synovial fluid identifies a potential inflammatory osteoarthritic phenotype. PloS One. 2017;12(4, article e0175109) doi: 10.1371/journal.pone.0175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monasterio G., Castillo F., Rojas L., et al. Th1/Th17/Th22 immune response and their association with joint pain, imagenological bone loss, RANKL expression and osteoclast activity in temporomandibular joint osteoarthritis: a preliminary report. Journal of Oral Rehabilitation. 2018;45(8):589–597. doi: 10.1111/joor.12649. [DOI] [PubMed] [Google Scholar]
- 43.Carrión M., Juarranz Y., Martínez C., et al. IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology. 2013;52(12):2177–2186. doi: 10.1093/rheumatology/ket315. [DOI] [PubMed] [Google Scholar]
- 44.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 45.Chomarat P., Banchereau J., Davoust J., Palucka A. K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nature Immunology. 2000;1(6):510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 46.Alaaeddine N., Antoniou J., Moussa M., et al. The chemokine CCL20 induces proinflammatory and matrix degradative responses in cartilage. Inflammation Research. 2015;64(9):721–731. doi: 10.1007/s00011-015-0854-5. [DOI] [PubMed] [Google Scholar]
- 47.Favero M., Belluzzi E., Trisolino G., et al. Inflammatory molecules produced by meniscus and synovium in early and end-stage osteoarthritis: a coculture study. Journal of Cellular Physiology. 2019;234(7):11176–11187. doi: 10.1002/jcp.27766. [DOI] [PubMed] [Google Scholar]
- 48.Capsoni F., Ongari A. M., Lonati C., Accetta R., Gatti S., Catania A. α-Melanocyte-stimulating-hormone (α-MSH) modulates human chondrocyte activation induced by proinflammatory cytokines. BMC Musculoskeletal Disorders. 2015;16(1):p. 154. doi: 10.1186/s12891-015-0615-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang W., Zhong B., Zhang C., Luo C., Zhan Y. miR-373 regulates inflammatory cytokine-mediated chondrocyte proliferation in osteoarthritis by targeting the P2X7 receptor. FEBS Open Bio. 2018;8(3):325–331. doi: 10.1002/2211-5463.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Böhm M., Apel M., Lowin T., et al. α-MSH modulates cell adhesion and inflammatory responses of synovial fibroblasts from osteoarthritis patients. Biochemical Pharmacology. 2016;116:89–99. doi: 10.1016/j.bcp.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Wu Y., Li Z., Jia W., Li M., Tang M. Upregulation of stanniocalcin-1 inhibits the development of osteoarthritis by inhibiting survival and inflammation of fibroblast-like synovial cells. Journal of Cellular Biochemistry. 2019;120(6):9768–9780. doi: 10.1002/jcb.28257. [DOI] [PubMed] [Google Scholar]
- 52.Elford P. R., Cooper P. H. Induction of neutrophil-mediated cartilage degradation by interleukin-8. Arthritis and Rheumatism. 1991;34(3):325–332. doi: 10.1002/art.1780340310. [DOI] [PubMed] [Google Scholar]
- 53.van Geffen E. W., van Caam A. P. M., van Beuningen H. M., et al. IL37 dampens the IL1β-induced catabolic status of human OA chondrocytes. Rheumatology. 2016;56(3):351–361. doi: 10.1093/rheumatology/kew411. [DOI] [PubMed] [Google Scholar]
- 54.Ding L., Hong X., Sun B., et al. IL-37 is associated with osteoarthritis disease activity and suppresses proinflammatory cytokines production in synovial cells. Scientific Reports. 2017;7(1, article 11601) doi: 10.1038/s41598-017-11397-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mabey T., Honsawek S., Tanavalee A., Yuktanandana P., Wilairatana V., Poovorawan Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers. 2016;21(7):639–644. doi: 10.3109/1354750X.2016.1171907. [DOI] [PubMed] [Google Scholar]
- 56.He Q., Sun C., Lei W., Ma J. SOCS1 regulates apoptosis and inflammation by inhibiting IL-4 signaling in IL-1β-stimulated human osteoarthritic chondrocytes. BioMed Research International. 2017;2017:9. doi: 10.1155/2017/4601959.4601959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silvestri T., Pulsatelli L., Dolzani P., Facchini A., Meliconi R. Elevated serum levels of soluble interleukin-4 receptor in osteoarthritis. Osteoarthritis and Cartilage. 2006;14(7):717–719. doi: 10.1016/j.joca.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Assirelli E., Pulsatelli L., Dolzani P., et al. Human osteoarthritic cartilage shows reduced in vivo expression of IL-4, a chondroprotective cytokine that differentially modulates IL-1β-Stimulated production of chemokines and matrix-degrading enzymes in vitro. PloS One. 2014;9(5, article e96925) doi: 10.1371/journal.pone.0096925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y., Zhou L., Lv D., Liu H., He T., Wang X. Poly(ADP-ribose) polymerase 1 inhibition prevents interleukin-1β-induced inflammation in human osteoarthritic chondrocytes. Acta Biochimica et Biophysica Sinica. 2015;47(6):422–430. doi: 10.1093/abbs/gmv033. [DOI] [PubMed] [Google Scholar]
- 60.Kwok S. K., Cho M. L., Park M. K., et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis and Rheumatism. 2012;64(3):740–751. doi: 10.1002/art.33390. [DOI] [PubMed] [Google Scholar]
- 61.Utomo L., Bastiaansen-Jenniskens Y. M., Verhaar J. A. N., van Osch G. J. V. M. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthritis and Cartilage. 2016;24(12):2162–2170. doi: 10.1016/j.joca.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 62.Rollins B. J. Chemokines. Blood. 1997;90(3):909–928. [PubMed] [Google Scholar]
- 63.Raghu H., Lepus C. M., Wang Q., et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Annals of the Rheumatic Diseases. 2017;76(5):914–922. doi: 10.1136/annrheumdis-2016-210426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen C., Bao G. F., Xu G., Sun Y., Cui Z. M. Altered Wnt and NF-κB signaling in facet joint osteoarthritis: insights from RNA deep sequencing. The Tohoku Journal of Experimental Medicine. 2018;245(1):69–77. doi: 10.1620/tjem.245.69. [DOI] [PubMed] [Google Scholar]
- 65.Rubbert A., Combadiere C., Ostrowski M., et al. Dendritic cells express multiple chemokine receptors used as coreceptors for HIV entry. Journal of Immunology. 1998;160(8):3933–3941. [PubMed] [Google Scholar]
- 66.Gerber B. O., Zanni M. P., Uguccioni M., et al. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Current Biology. 1997;7(11):836–843. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- 67.Belluzzi E., Olivotto E., Toso G., et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connective Tissue Research. 2019;60(2):136–145. doi: 10.1080/03008207.2018.1470167. [DOI] [PubMed] [Google Scholar]
- 68.Huang D., Zhao Q., Liu H., Guo Y., Xu H. PPAR-α agonist WY-14643 inhibits LPS-induced inflammation in synovial fibroblasts via NF-kB pathway. Journal of Molecular Neuroscience. 2016;59(4):544–553. doi: 10.1007/s12031-016-0775-y. [DOI] [PubMed] [Google Scholar]
- 69.Arkestål K., Mints M., Enocson A., et al. CCR2 upregulated on peripheral T cells in osteoarthritis but not in bone marrow. Scandinavian Journal of Immunology. 2018;88(6):p. e12722. doi: 10.1111/sji.12722. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y. J., Chan D. C., Chiang C. K., et al. Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-κB pathway activation. Journal of Orthopaedic Research. 2016;34(5):791–800. doi: 10.1002/jor.23083. [DOI] [PubMed] [Google Scholar]
- 71.Sapolsky A. I., Howell D. S. Further characterization of a neutral metalloprotease isolated from human articular cartilage. Arthritis and Rheumatism. 1982;25(8):981–988. doi: 10.1002/art.1780250811. [DOI] [PubMed] [Google Scholar]
- 72.Adler N., Schoeniger A., Fuhrmann H. Effects of transforming growth factor-β and interleukin-1β on inflammatory markers of osteoarthritis in cultured canine chondrocytes. American Journal of Veterinary Research. 2017;78(11):1264–1272. doi: 10.2460/ajvr.78.11.1264. [DOI] [PubMed] [Google Scholar]
- 73.Zeng R. M., Lu X. H., Lin J., et al. Knockdown of FOXM1 attenuates inflammatory response in human osteoarthritis chondrocytes. International Immunopharmacology. 2019;68:74–80. doi: 10.1016/j.intimp.2018.12.057. [DOI] [PubMed] [Google Scholar]
- 74.Xia L., Zhang H. X., Xing M. L., et al. Knockdown of PRMT1 suppresses IL-1β-induced cartilage degradation and inflammatory responses in human chondrocytes through Gli1-mediated Hedgehog signaling pathway. Molecular and Cellular Biochemistry. 2018;438(1-2):17–24. doi: 10.1007/s11010-017-3109-7. [DOI] [PubMed] [Google Scholar]
- 75.Burguera E. F., Vela-Anero A., Magalhaes J., Meijide-Failde R., Blanco F. J. Effect of hydrogen sulfide sources on inflammation and catabolic markers on interleukin 1β-stimulated human articular chondrocytes. Osteoarthritis and Cartilage. 2014;22(7):1026–1035. doi: 10.1016/j.joca.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 76.Peng H. Z., Yun Z., Wang W., Ma B. A. Dual specificity phosphatase 1 has a protective role in osteoarthritis fibroblast-like synoviocytes via inhibition of the MAPK signaling pathway. Molecular Medicine Reports. 2017;16(6):8441–8447. doi: 10.3892/mmr.2017.7617. [DOI] [PubMed] [Google Scholar]
- 77.Ma C. H., Wu C. H., Jou I. M., et al. PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes. Redox Biology. 2018;14:72–81. doi: 10.1016/j.redox.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haneda M., Hayashi S., Matsumoto T., et al. Depletion of aquaporin 1 decreased ADAMTS-4 expression in human chondrocytes. Molecular Medicine Reports. 2018;17(4):4874–4882. doi: 10.3892/mmr.2018.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Griffin F. M., Math K., Scuderi G. R., Insall J. N., Poilvache P. L. Anatomy of the epicondyles of the distal femur: MRI analysis of normal knees. The Journal of Arthroplasty. 2000;15(3):354–359. doi: 10.1016/s0883-5403(00)90739-3. [DOI] [PubMed] [Google Scholar]
- 80.Zeng G. Q., Chen A. B., Li W., Song J. H., Gao C. Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genetics and Molecular Research. 2015;14(4):14811–14822. doi: 10.4238/2015.November.18.46. [DOI] [PubMed] [Google Scholar]
- 81.Yang Q., Ding W., Cao Y., et al. Interferonregulatoryfactor-8(IRF-8) regulates the expression of matrix metalloproteinase-13 (MMP-13) in chondrocytes. Cell Stress and Chaperones. 2018;23(3):393–398. doi: 10.1007/s12192-017-0849-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fu Y., Lei J., Zhuang Y., Zhang K., Lu D. Overexpression of HMGB1 A-box reduced IL-1β-induced MMP expression and the production of inflammatory mediators in human chondrocytes. Experimental Cell Research. 2016;349(1):184–190. doi: 10.1016/j.yexcr.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 83.Chou C. H., Attarian D. E., Wisniewski H. G., Band P. A., Kraus V. B. TSG-6 – a double-edged sword for osteoarthritis (OA) Osteoarthritis and Cartilage. 2018;26(2):245–254. doi: 10.1016/j.joca.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Q., Wu S., Wu Y., Chen L., Pang Q. miR-149 suppresses the inflammatory response of chondrocytes in osteoarthritis by down-regulating the activation of TAK1/NF-κB. Biomedicine and Pharmacotherapy. 2018;101:763–768. doi: 10.1016/j.biopha.2018.02.133. [DOI] [PubMed] [Google Scholar]
- 85.Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2012;1825(1):29–36. doi: 10.1016/j.bbcan.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 86.Alunno A., Falcinelli E., Luccioli F., et al. Platelets contribute to the accumulation of matrix metalloproteinase type 2 in synovial fluid in osteoarthritis. Thrombosis and Haemostasis. 2017;117(11):2116–2124. doi: 10.1160/TH17-06-0379. [DOI] [PubMed] [Google Scholar]
- 87.Burrage P. S., Mix K. S., Brinckerhoff C. E. Matrix metalloproteinases: role in arthritis. Frontiers in Bioscience. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 88.Gui T., He B., Gan Q., Yang C. Enhanced SOCS3 in osteoarthiritis may limit both proliferation and inflammation. Biotechnic and Histochemistry. 2017;92(2):107–114. doi: 10.1080/10520295.2017.1278792. [DOI] [PubMed] [Google Scholar]
- 89.Marcu K. B., Otero M., Olivotto E., Borzi R. M., Goldring M. B. NF-κB signaling: multiple angles to target OA. Current Drug Targets. 2010;11(5):599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Loeser R. F., Erickson E. A., Long D. L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Current Opinion in Rheumatology. 2008;20(5):581–586. doi: 10.1097/BOR.0b013e3283090463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie L., Xie H., Chen C., Tao Z., Zhang C., Cai L. Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin for attenuating the development of osteoarthritis: in vitro and in vivo studies. Food & Function. 2019;10(4):2161–2175. doi: 10.1039/c8fo01786g. [DOI] [PubMed] [Google Scholar]
- 92.Sasaki K., Hattori T., Fujisawa T., Takahashi K., Inoue H., Takigawa M. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. Journal of Biochemistry. 1998;123(3):431–439. doi: 10.1093/oxfordjournals.jbchem.a021955. [DOI] [PubMed] [Google Scholar]
- 93.Goggs R., Carter S. D., Schulze-Tanzil G., Shakibaei M., Mobasheri A. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Veterinary Journal. 2003;166(2):140–158. doi: 10.1016/S1090-0233(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 94.Daheshia M., Yao J. Q. The interleukin 1β pathway in the pathogenesis of osteoarthritis. The Journal of Rheumatology. 2008;35(12):2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- 95.Terauchi K., Kobayashi H., Yatabe K., et al. The NAD-dependent deacetylase sirtuin-1 regulates the expression of osteogenic transcriptional activator runt-related transcription factor 2 (Runx2) and production of matrix metalloproteinase (MMP)-13 in chondrocytes in osteoarthritis. International Journal of Molecular Sciences. 2016;17(7, article 1019) doi: 10.3390/ijms17071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tao R., Xu X., Sun C., et al. KPNA2 interacts with P65 to modulate catabolic events in osteoarthritis. Experimental and Molecular Pathology. 2015;99(2):245–252. doi: 10.1016/j.yexmp.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 97.Blaney Davidson E. N., van Caam A. P. M., Vitters E. L., et al. TGF-β is a potent inducer of Nerve Growth Factor in articular cartilage via the ALK5-Smad2/3 pathway. Potential role in OA related pain? Osteoarthritis and Cartilage. 2015;23(3):478–486. doi: 10.1016/j.joca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 98.Matsunobu T., Torigoe K., Ishikawa M., et al. Critical roles of the TGF-β type I receptor ALK5 in perichondrial formation and function, cartilage integrity, and osteoblast differentiation during growth plate development. Developmental Biology. 2009;332(2):325–338. doi: 10.1016/j.ydbio.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Madej W., Buma P., van der Kraan P. Inflammatory conditions partly impair the mechanically mediated activation of Smad2/3 signaling in articular cartilage. Arthritis Research & Therapy. 2016;18(1):p. 146. doi: 10.1186/s13075-016-1038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Struglics A., Okroj M., Swärd P., et al. The complement system is activated in synovial fluid from subjects with knee injury and from patients with osteoarthritis. Arthritis Research & Therapy. 2016;18(1):p. 223. doi: 10.1186/s13075-016-1123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daghestani H. N., Pieper C. F., Kraus V. B. Soluble macrophage biomarkers indicate inflammatory phenotypes in patients with knee osteoarthritis. Arthritis & Rheumatology. 2015;67(4):956–965. doi: 10.1002/art.39006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bartel D. P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsezou A. Osteoarthritis year in review 2014: genetics and genomics. Osteoarthritis and Cartilage. 2014;22(12):2017–2024. doi: 10.1016/j.joca.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 104.Gao D., Wang R., Li B., Yang Y., Zhai Z., Chen D.-Y. WDR34 is a novel TAK1-associated suppressor of the IL-1R/TLR3/TLR4-induced NF-κB activation pathway. Cellular and Molecular Life Sciences. 2009;66(15):2573–2584. doi: 10.1007/s00018-009-0059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giuliani A. L., Sarti A. C., Falzoni S., Di Virgilio F. The P2X7 Receptor-Interleukin-1 Liaison. Frontiers in Pharmacology. 2017;8(123) doi: 10.3389/fphar.2017.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burnstock G., Knight G. E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signalling. 2018;14(1):1–18. doi: 10.1007/s11302-017-9593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mao G., Wu P., Zhang Z., et al. MicroRNA-92a-3p regulates aggrecanase-1 and aggrecanase-2 expression in chondrogenesis and IL-1β-induced catabolism in human articular chondrocytes. Cellular Physiology and Biochemistry. 2017;44(1):38–52. doi: 10.1159/000484579. [DOI] [PubMed] [Google Scholar]
- 108.Wu Y., Goh E. L., Wang D., Ma S. Novel treatments for osteoarthritis: an update. Open access Rheumatology : Research and Reviews. 2018;10:135–140. doi: 10.2147/OARRR.S176666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saberi Hosnijeh F., Siebuhr A. S., Uitterlinden A. G., et al. Association between biomarkers of tissue inflammation and progression of osteoarthritis: evidence from the Rotterdam study cohort. Arthritis Research & Therapy. 2016;18(1):p. 81. doi: 10.1186/s13075-016-0976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alquraini A., Jamal M., Zhang L., Schmidt T., Jay G. D., Elsaid K. A. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Research and Therapy. 2017;19(1):p. 89. doi: 10.1186/s13075-017-1301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]