Abstract
This is an extensive review on epiphytic plants that have been used traditionally as medicines. It provides information on 185 epiphytes and their traditional medicinal uses, regions where Indigenous people use the plants, parts of the plants used as medicines and their preparation, and their reported phytochemical properties and pharmacological properties aligned with their traditional uses. These epiphytic medicinal plants are able to produce a range of secondary metabolites, including alkaloids, and a total of 842 phytochemicals have been identified to date. As many as 71 epiphytic medicinal plants were studied for their biological activities, showing promising pharmacological activities, including as anti-inflammatory, antimicrobial, and anticancer agents. There are several species that were not investigated for their activities and are worthy of exploration. These epipythes have the potential to furnish drug lead compounds, especially for treating cancers, and thus warrant indepth investigations.
Keywords: epiphytes, medicinal plants, phytochemistry, pharmacology, drug leads
1. Introduction
Epiphytes are plants that grow on other plants and are often known as air plants. They are mostly found in moist tropical areas on canopy tree-tops, where they exploit the nutrients available from leaf and other organic debris. These plants exist within the plantae and fungi kingdom. The term epiphyte itself was first introduced in 1815 by Charles-François Brisseau de Mirbel in “Eléments de physiologie végétale et de botanique” [1]. Epiphytes can be categorized into vascular and non-vascular epiphytic plants; the latter includes the marchantiophyta (liverworts), anthocerotophyta (hornworts), and bryophyta (mosses). The common epiphytes are mosses, ferns, liverworts, lichens, and the orchids. Epiphytes fall under two major categories: As holo- and hemi-epiphytes. While orchids are a good example of holo-epiphytes, the strangler fig is a hemi-epiphyte. Although geological studies have proposed the existence of epiphytes since the pleistone epoch, an epiphyte was first depicted in “the Badianus Manuscript” by Martinus de la Cruz in 1552, which showed the Vanilla fragrans, a hemi-epiphytic orchid, being used by the tribal communities in latin America for fragrance and aroma, usually hung around their neck [1].
Epiphytes have been a source of food and medicine for thousands of years. Since they grow in a unique ecological environment, they produce interesting secondary metabolites that often show exciting biological activities. There are notable reviews on non-vascular epiphytes, bryophyta, regarding their phytochemical and pharmacological activities [2,3,4,5]. There are also extensive reviews on epiphytic lichens covering secondary metabolites and their pharmacological activities [6,7,8,9]. The only available review on vascular epiphytes related to medicinal uses was focused on Orchidaceae [10]. Therefore, to the best of our knowledge, there is no extensive database of vascular epiphytes regarding their medicinal contribution.
There are 27,614 recorded species of vascular epiphytes belonging to 73 families and 913 genera [11]. Vascular epiphyte species are commonly found in pteridophyta, gymnosperms, and angiosperms plant groups, which are mostly found in the moist tropical areas on canopy tree tops, where they exploits the nutrients available from leaf and other organic debris [12,13]. In this study, information on vascular epiphytic medicinal plant species was collected using search engines (Web of Science, Scifinder Scholar, prosea, prota, Google scholar), medicinal plant books (Plant Resources of South-East Asia: Medicinal and Poisonous Plants [14,15,16], Plant Resources of South-East Asia: Cryptogams: Ferns and Fern Allies [17], Mangrove Guide for South-East Asia [18], Medicinal Plants of the Asia-Pacific [19], Medicinal Plants of the Guiana [20], Indian Medicinal Plants [21,22], Medicinal Plants of Bhutan [23], Medicinal and aromatic plants of Indian Ocean islands: Madagascar, Comoros, Seychelles and Mascarenes [24]), and the Indonesian Medicinal Plants Database [25]. Scientific names of the epiphytic medicinal plant species were compared against the Plantlist database for accepted names to avoid redundancy [26]. The time-frame threshold for data coverage was from the earliest available data until early 2020. Nevertheless, empirical knowledge regarding traditional medicinal plants was passed through generations using verbal or written communication, with verbal communication highly practiced by remote tribes [27,28]. It is possible that some oral traditional medical knowledge may not be reported and therefore not captured in this review. In this current study, we collected and reviewed 185 epiphytic medicinal plants reported in the literature, covering ethnomedicinal uses of epiphytes, their phytochemical studies and the pharmacological activities. The data collection approach used is presented in Figure 1.
Figure 1.
Schematic data collection approach.
2. Ethnopharmacological Information of Vascular Epiphytic Medicinal Plants
2.1. Vascular Epiphytic Medicinal Plant Species Distribution within Plant Families
In this component of the study, we collated and analysed 185 of the medicinally used epiphytic plants species using ethnopharmacological information. This data (Table 1) includes the name of species, plant family, areas where the epiphytes are used in traditional medicines, part(s) of the plant being used in medication, how the medicine was prepared, and indications. Of the 185 medicinally used epiphytes, 53 species were ferns (mostly polipodiaceae), with 132 species belonging to the non-fern category. The Orchidaceae family contains the Dendrobium genus that contains the highest number of medicinal epiphytes, including 64 orchid species and 20 Dendrobium species. The Orchidaceae epiphytes were the majority of non-fern epiphytes. Cassytha filiformis L, Bulbophyllum odoratissimum (Sm.) Lindl. ex Wall., Cymbidium goeringii Rchb.f.) Rchb.f., Acrostichum aureum Limme, and Ficus natalensis Hochst. were the five most popular vascular epiphytic medicinal pants used (Figure 2).
Table 1.
Ethnopharmacological database of epiphytic medicinal plants.
| No | Epiphyte Species | Location | Part of Plants | Preparation and Route of Administration | Indication (traditional) | Pharmacological Testing (modern) |
|---|---|---|---|---|---|---|
| Fern species | ||||||
| Adiantaceae | ||||||
| 1 | Adiantum caudatum L. | India, Indonesia, Malaysia | LF | Decoction | Cough, heal wound, cold, tumors of spleen, liver and other viscera, skin diseases, bronchitis, and inflammatory diseases [40,49,50] | Antimicrobial (MeOH extract, gram +, -, fungi) [40] |
| Aspleanceae | ||||||
| 2 | Asplenium nidus L. | Tahiti, Malaysia, Philippines, Vanuatu, Indonesia | LF, WP | Ointment, decoction, eaten | Headache, hair loss (pounded leaves mixed with coconut oil), ease labor, fever (decoction), contraceptive, depurative, sedative agents. edible food (young leaves), ornament, anti-inflammation, promote blood circulation [51,52,53] | Antioxidative (MeOH extract, DPPH), tyrosinase inhibiting (MeOH extract, microtitre), antibacterial (MeOH extract) [44] |
| 3 | Asplenium macrophyllum Sw. | India | LF | Decoction | As laxative, emetic, diuretic, anthelmintic agent, to treat ophthalmia, jaundice, spleen diseases [52,54] | |
| 4 | Asplenium polydon G. Foster var bipinnatum (Sledge) | India | LF | Decoction, paste | Promote labor, tumor [55] | |
| 5 | Asplenium serratum L. | Columbia, Peru | na | Not mentioned | Liver problem, stomachache, ovary inflammation [52,56] | |
| Blechnaceae | ||||||
| 6 | Stenochlaena palustris (Burm. F.) Bedd. | Indonesia, India | LF, RZ | Eaten, decoction, poultice | Young reddish leaves are used as food, leaves are used to treat fever, skin diseases, throat, and gastric ulcer, as antibacterial, rhizome and leaves are used to treat burns and ulcers, as cooling agent [18,57] | |
| Davalliaceae | ||||||
| 7 | Davallia denticulata (Burm. f.) Mett. ex Kuhn | Malaysia, Indonesia | RT | Decoction | Gout, pain, as tonic [49,58] | |
| 8 | Araiostegia divaricata (Blume) M. Kato | China, Taiwan | WP | Not mentioned | Joint pain [59] | Anti-psoriasis [60], antioxidant (water extract, DPPH) [61] |
| 9 | Davallia parvula Wall. Ex Hook. & Grev. | na | Not mentioned | Not mentioned [18,62] | ||
| 10 | Davallia solida (G. Forst.) Sw. | Tahiti, Fiji, other Polynesian | WP | Decoction (external and internal) | Dysmennorrhea, luochorea, uterine hemorrhage, sore throat, asthma, constipation, fracture, fish sting, promote health pregnancy, as a bath for newborn, anti-microbial [53,63,64,65] | Antioxidant (extract, ABTS) [61], antioxidant (DPPH, all isolates) [66], anti-neurotoxicity (extract, (Neuro-2a cells, ATCC CCL-131) [67], C-terminal cytosolic domain of P-pg [68], anti-skin aging [69] |
| 11 | Leucostegia immersa Wall. ex C. Presl | Nepal | RZ | Decoction, paste | Boils (paste), constipation (decoction), as antibacterial (paste) [70] | |
| Gesneriaceae | ||||||
| 12 | Aeschynanthus radicans Jack | Malaysia | LF | Decoction | Headache [19] | |
| 13 | Cyrtandra sp | Indonesia | LF | Poultice | Skin ailments [71] | |
| Hymenophyllaceae | ||||||
| 14 | Hymenophyllum polyanthos Sw. | Suriname | WP | Burnt (smoke inhaling), decoction | Dizziness (insanity), pain, cramps [72] | |
| 15 | Hymenophyllum javanicum Spreng. | India | WP | Smoke together with garlic and onions | Headache [55] | |
| Lycopodiaceae | ||||||
| 16 | Huperzia carinata (Desv. ex Poir.) Trevis | South-East Asia | WP | Ointment | Stimulate hair growth [73] | Anti-acetylcholinesterase (74, 75, 76, colorimetric Ellman method) [74] |
| 17 | Huperzia phlegmaria (L.) Rothm | South-East Asia, India | WP | Ointment | Stimulate hair growth, skin diseases [75,76] | Cytotoxic activities against HuCCA-1, A-549, HepG2, and MOLT-3 cancer cell lines (81, 79, 77) [77] |
| 18 | Huperzia megastachya (Baker) Tardieu | Madagascar | LF | Decoction (infusion) | Tonic [78] | |
| 19 | Huperzia obtusifolia (Sw.) Rothm. | Madagascar | LF | Decoction (infusion) | Tonic [78] | |
| Nephrolepidaceae | ||||||
| 20 | Nephrolepis acutifolia (Desv.) Christ | Malaysia | WP | Boiled, eaten | Food [79] | |
| 21 | Nephrolepis biserrata (Sw.) Schott | Malaysia, Indonesia, Ivory Coast, New Guinea | LF, RZ, WP | Decoction, cooked | Leaves are used to treat boils, blister, abscesses, sores, and cough. Rhizomes are used as edible food [80,81] | Antibacterial (extract) [82] |
| Oleandraceae | ||||||
| 22 | Nephrolepis cordifolia (L.) C. Presl | India | RZ | Decoction (fresh leaves) | Cough, rheumatism, chest congestion, nose blockage, loss appetites, infection (antibacterial), pinnae is used to treat cough, wounds, jaundice, anti-fungal, styptic, anti-tussive [57] | Antibacterial, anti-fungal (extract fractions aerial part) [83] |
| 23 | Oleandra musifolia (Blume) C. Presl | Philippines, India | ST | Decoction | Anthelmintic, emmenagogue, antidote (snake bite) [70,84] | |
| Opioglossaceae | ||||||
| 24 | Botrychum lanuginosum Wall.ex Hook & Grev. | India | WP | Decoction, paste | Antibacterial, anti-dysentery agents [57] | |
| 25 | Ophioglossum pendulum L. | Indonesia, Philippines | LF | Ointment, decoction. | Hair treatment (crushed leaves), cough (decocotion), rid the first feces (spores), ornament [85] | Cell activator, skin whitening agent and antioxidant (patent, mixed with other Ophioglossum species) [86], anti-diarrhea (stipe MeOH extract, rabit jejenum) [86] |
| Polypodiaceae | ||||||
| 26 | Pyrrosia piloselloides (L.) M.G. Price | Indonesia, Malaysia, China, Philippines, Pacific islands | LF | Decoction (internal), chewed, poultice (external) | Smallpox, rashes, gonorrhea, dysentery, tuberculosis, urinary tract infection, headache, cough, gum inflammation, tooth sockets, eczema, coagulate blood [87,88,89,90] | Antibacterial, anti-fungal (extracts) [91] |
| 27 | Drynaria rigidula (Sw.) Bedd. | Indonesia, Philippines, Treasury Island | LF, RZ | Decoction, chewing | Gonorrhea, dysentery (rhizome, decoction), and seasickness (chewed) [21] | n-Hexane, dichloromethane and ethyl acetate fractions from both rhizome and leaves of Drynaria rigidula were screened for activity against Plasmodium falciparum, Mycobacterium tuberculosis, vero cells and herpes simplex virus which all extracts showed insignificant activities [92] |
| 28 | Drynaria sparsisora (Desv.) T. Moore | Indonesia, Philippines, Thailand | LF, RZ | External, decoction | Rhizome: headache, fever, diarrhea, gonorrhea, swollen limbs, fever. Leaves: anti-vomiting, snake bite, eye infection [21,71,93] | |
| 29 | Drynaria roosii Nakaike | China | WP | Decoction | Deficient kidney, invigorate blood, heal wound, stop bleeding [21] | Compound 230 was isolated and the biotesting showed the highest stimulation toward UMR 106 cells (osteoblast) by 42.6% at a concentration of 1 µM [94] |
| 30 | Drynaria propinqua (Wall. ex Mett.) Bedd | Bhutan, India and Nepal | ST | Pills | Antidote and detoxifier especially when suffering from meat poisoning and other human-made poisons (sbyar-dug) [95] | |
| 31 | Drynaria quercifolia (L.) J.Sm. | Malaysia, Philippines, Indonesia, India | LF, RZ | Decoction, poultice | Swelling, fever (poultice leaves), haemoptysis, typhoid fever, ulcers, dyspepsia, artharlgia, diarrhea (decocted rhizome), inflammation, anthelmitic, cough, fever, phthisis, poultice of rhizome mixed with Lannea coromandelica (Houtt.) Merr.) to treat headache, hepatoprotective agent [21,22,96] | Compound 200 from the ethyl acetate fraction to be responsible for good antimicrobial activity [97] |
| 32 | Lepisorus contortus (Christ) Ching | Bhutan, India, China | LF | Powder | Heals bone fracture, burns, wounds and kidney disorders [98] | |
| 33 | Loxogramme involuta (D. Don) C. Presl | Indonesia | LF, WP | Smoked | Smoked with tobacco [18] | |
| 34 | Loxogramme scolopendria (Bory) Presley | Indonesia | LF | Smoked | Cigarette paper [99] | |
| 35 | Microsorum fortunei (T. Moore) Ching | Indonesia | WP | Decoction | Diuretic, promote blood circulation [49,51] | |
| 36 | Microsorum punctatum (L.) Copel. | India | LF | Juice | Diuretic, purgative, wounds [70] | |
| 37 | Phlebodium aureum (L.) J.Sm | Mexico | RZ | Decoction | Cough, fever, sudorific agents [57] | |
| 38 | Phymatosorus scolopendria (Burm. f.) Pic. Serm. | South-East Asia, Madagascar | RZ | Fragrance (external), poultice, decoction | Fragrance, gecko bites, accelerate childbirthRespiratory disorder [18,47] | Bronchodilator (341, in-vivo) [47] |
| 39 | Platycerium coronarium (Mull.) Desv. | Indonesia | LF | Poultice (salt added) | Thyroid edema, scabies [18,100] | |
| 40 | Platycerium bifurcatum (Cav.) C. Chr. | Indonesia | LF | Poultice (salt added) | Thyroid edema, scabies, fever, swelling [100,101] | |
| 41 | Pleopeltis macrocarpa (Bory ex Willd.) Kaulf. | South-Africa, Mexico, Guatemala | LF, RZ | Decoction | Sore throat, itches, cough, febrifuge [70,102] | |
| 42 | Pyrrosia heterophylla (L.) M.G. Price | India | WP | Poultice | Swelling, sprain, pain (cooling agent) [103] | |
| 43 | Pyrrosia lanceolata (L.) Farw. | Malaysia, South-Africa, Mexico | LF, WP | Juice, poultice, decoction | Dysentery, headache, colds, sore throats, itch guard [55,87] | |
| 44 | Pyrrosia lingua (Thunb.) Farw. | Japan, China, Indonesia, Pacific Islands | LF, WP | Decoction | Diuretic, anti-inflammation, analgesic, cough, stomachache, urinary disorder (diuretic agent) [87,104,105,106] | Antioxidant [107], inhibition effects on virus-induced CPE when SARS-CoV strain BJ001 [108] |
| 45 | Pyrrosia longifolia (Burm. f.) C.V. Morton | Indonesia, Pacific Islands | LF | Poultice (cold water) | Ease pains in labor [18,87] | |
| 46 | Pyrrosia petiolosa (Christ) Ching | China | WP | Decoction | Urinary tract infections, as diuretic [109] | |
| 47 | Pyrrosia sheareri (Baker) Ching | China | LF | Decoction | Bacillary dysentery, rheumatism [87,110] | Antioxidant [110] |
| Psilotaceae | ||||||
| 48 | Psilotum nudum (L.) P. Beauv. | India | LF, SP | Fresh, decoction | Diarrhea (infants), antibacterial, purgative [55] | |
| Pteridaceae | ||||||
| 49 | Acrostichum aureum L. | South-East Asia, Bangladesh, Fiji, China, Panama | LF, RZ | Eaten, decoction | Wounds, peptic ulcers and boils, worm infections, asthma, constipation, elephantiasis, febrifuge, chest pain, emollients [18,35] | Anti-implantation (EtOH extract, albino rats) [111], Anti-tumour (hella cells, MTT assay) [112], Antioxidant (DPPH), tyrosine inhibition (96-well microtitre), antibacterial activity [44,113], anti-cancer ((gastric: AGS; colon: HT-29 and breast: MDA-MB-435S) using the MTT assay) [114] |
| 50 | Acrostichum speciosum Willd. | South-East Asia | Thatch [18] | |||
| 51 | Taenitis blechnoides (Willd.) Sw. | Malaysia | LF | Decoction | Postnatal protection [115] | |
| Selaginellaceae | ||||||
| 52 | Selaginella tamariscina (P.Beauv.) Spring | Nepal | WP, SP | Fresh (spore), decoction | Vermilion powder, prolapsed rectum, cough, bleeding piles, amenorrhea, antibacterial [57,116] | Anti-acne [117], thymus growth-stimulatory activity in adult mice (reversal of involution of thymus) and remarkable anti-lipid peroxidation activity [118] |
| Vittariaceae | ||||||
| 53 | Vittaria elongata Sw. | South-East Asia, Andaman | LF | Decoction | Rheumatism [57] | Cytotoxicity against two human cancer cell lines, lung carcinoma (NCI-H460) and central nervous system carcinoma (SF-268), antioxidant (DPPH) [119] |
| Non-Fern | ||||||
| Araceae | ||||||
| 54 | Philodendron fragrantissimum (Hook.) G.Don | Guyana, Suriname, Brazil | LF, RT | Decoction, external (leaves) | Inflammation, aphrodisiac, demulcent, diuretic [72] | |
| Aralliaceae | ||||||
| 56 | Schefflera caudata (Vidal) Merr. & Rolfe | Philippines | WP | Decoction | Tonic for women after birth [120] | |
| 57 | Schefflera elliptica (Blume) Harms. | South-East Asia, China, India | BK, LF, RT | Decoction, chewed, external | Bechic, vulnerary, toothache, aromatic bath, dropsy [120]. | Antibacterial [121] |
| 58 | Schefflera elliptifoliola Merr. | Philippines | LF | Decoction | Tonic for woman after birth [120] | |
| 59 | Schefflera oxyphylla (Miq.) R.Vig. | Thailand, Malaysia, Indonesia | RT | Decoction | Sedative for frightened child, externally to treat fevers [120] | |
| 60 | Schefflera simulans Craib | Thailand, Malaysia | LF, RT | Decoction | Stomach problem, protective medicine after birth [120] | |
| Asclepiadaceae | ||||||
| 61 | Asclopidae sp. | Indonesia | LF, RT | Decoction | Promote blood circulation [71] | |
| 62 | Dischidia acuminata Costantin | Vietnam | WP | Decoction | Blenorrhoea, promote urination [19] | |
| 63 | Dischidia bengalensis Colebr. | Thailand | LT, RT | Latex (external), decoction (tonic) | Anthemintic (ringworm), tonic [122] | |
| 64 | Dischidia imbricata (Blume) Steud. | Indonesia | LF | Poultice | Gonorrhea, burns and wounds [25,123] | |
| 65 | Dischidia major (Vahl) Merr. | India, Thailand, Philippines, Malaysia, Brunei | LF, RT, WP | Decoction, chrused (external), chewed with areca catechu | Peptic ulcer, liver dysfunction (decocted leaves mixed with Hoya kerii Craib leaves and Vanilla aphylla Blume stem), fever (root), goiter (crushed leaves mixed with salt), cough (root mixed betel quid), wound and injuries, stomache [19,124,125] | |
| 66 | Dischidia nummularia R.Br. | Thailand, Indonesia | LF, LT, WP | Decoction, latex (external) | Wound, gonorrhea, sprue in children, cirrhosis [126] | |
| 67 | Dischidia platyphylla Schltr | Philippines | LF | Decoction | Putrefaction [19] | |
| 68 | Dischidia purpurea Merr. | Philippines | LF | Crushed leaves mixed with coconut oil applied as external poultice | Eczema, herpes [19,127] | |
| 69 | Toxocarpus sp. | Indonesia | LF | Decoction | Headache, fever, nervous system problem [71] | |
| Balsaminaceae | ||||||
| 70 | Impatiens niamniamensis Gilg (semi epiphytic) | Congo | LF | Poultice | Wounds, sores, pain [128] | Anti-hyperglicemic (Rat) [129] |
| 71 | Convolvulaceace (parasite) | |||||
| 72 | Cassytha filiformis L | India, Taiwan, China, Vietnam, Malaysia, Philippines, Indonesia, Fiji, Africa, Central America. | WP, NT | Decoction | Cough, dysentery, diarrhea, intestinal problems, headache, malaria fever, nephritis, edema, hepatitis, sinusitis, gonorrhea, syphilis, skin ulcer, eczema, prevent haemoptysis. Parasite skin and scalp. Induce lactation (after still birth), promote hair growth, diuretic, vermifuge, laxative agent, saliva blood removal (childbirth) [19,130,131,132] | An α1-adrenoceptor antagonist (Rat thoracic aorta) [133], antiplatelet and vasorelaxing actions (Rabit platelet, aortic contraction) [134], anti-trypanosomal, citotoxicity [135], antioxidant [136] |
| 73 | Cuscuta australis R.Br. | Indonesia, Vietnam, China | WP, SD | Decoction, poultice | Whole plant: emollient, sedative, sudorific and tonic agents, urinary complaint. The seeds: sedative agent, diabetes, cornea opacity, acne, dandruff [137]. | Cytotoxicity, antioxidant activity, and inhibitory effects on tyrosinase activity and melanin biosynthesis were estd. by using melanoma Clone M-3 [138] |
| 74 | Cuscuta reflexa Roxb. | India | WP | Decoction, poultice | Mixed with the twigs of Vitex negundo L. applied as fomentation on the abdomen of kwarsiokor children, fever, itchy [139,140] | Anti-viral [141,142], anti-HIV [143], analgesic, relaxant (ether extract) [144], antisteroidogenic activity (MeOH extract) [141], antibacterial activity [145], hair growth activity in androgen-induced alopecia [146], anti-inflammatory (murine macrophage cell line RAW264.7), anti-cancer (Hep3B cells by MTT assay) [147], antioxidant (etOAc extract, DPPH), anti-obesity (EtOAc extract) [148] |
| Clusiaceae | ||||||
| 75 | Clusia grandiflora Splitg. (hemi epiphyte) | Guyana, Suriname | RT | Decoction | Aphrodisiac [72] | Antibacterial [149] |
| 76 | Clusia fockeana Miq. (hemi epiphyte) | Guyana, Suriname | ST(Exudate) | Poultice | Snake bites, ulcers [72] | |
| Gesneriaceae | ||||||
| 77 | Columnea nicaraguensis Oerst. | Panama | ST, LF, WP | Decoction, maceration | Fever [150] | |
| 78 | Columnea sanguinolenta (Klotzsch ex Oerst.) Hanst. | Panama | ST, LF | Decoction | Dysmenorrhea [150] | |
| 79 | Columnea tulae Urb. var. tomentulosa (C.V. Morton) B.D. Morley | Panama | ST | Decoction | Fever [150] | |
| 80 | Drymonia serrulata (Jacq.) Mart. | Amazon | na | Not mentioned | Eczema [151] | Analgesic, anti-inflammatory [152] |
| 81 | Drymonia coriacea (Oerst. ex Hanst.) Wiehler | Amazon | na | Not mentioned | Toothache [151] | |
| Loganiaceae | ||||||
| 82 | Fagraea auriculata Jack. (semi epiphyte) | Indonesia | ST | Stem for stick [25] | Anti-inflammatory [153] | |
| Loranthaceae (parasite) | ||||||
| 83 | Amyema bifurcata (Benth.) Tiegh. | Australia | ST, LF | Decoction | Colds, fever, sores [154] | |
| 84 | Amyema quandang (Lindl.) Tiegh. | Australia | LF | Decoction | Fever [155] | |
| 85 | Amyema maidenii (Blakely) Barlow | Australia | FT | Decoction | Inflammation in the genital regions [156] | |
| 86 | Dendrophthoe falcata (L.f.) Ettingsh | India | WP | Decoction | Pulmonary tuberculosis, asthma, menstrual disorders, swellings, wounds, ulcers, strangury, renal and vesical calculi, aphrodisiac, astringent, narcotic, diuretic [157]. | Wound healing activity was studied, antimicrobial activity and antioxidant activity [158] |
| 87 | Dendrophthoe frutescens L. | Indonesia | LF, WP | Drink (decoction) | Anti-inflammation, antibacterial [51] | |
| 88 | Dendrophthoe incarnata (Jack) Miq. | Malaysia | LF | Poultice | Mixed with Curcuma longa L and rice to make poultice to treat ringworm [159] | |
| 89 | Dendrophthoe pentandra (L.) Miq. | Indonesia, Malaysia, Thailand, Vietnam | LF, WP | Poultice, decoction | Sores, ulcers, other skins infections, protective medicine after childbirth, cough, hypertension, cancer, diabetes, tonsil problem [18,25,159,160] | Antioxidant (MeOH extract, DPPH), Tyrosinase activity [160] |
| 90 | Taxillus umbellifer (Schult. f.) Danser | Indonesia, Malaysia, Vietnam | RT, LF | Decoction drink, poultice | Fever, headache, wounds [159] | |
| 91 | Erianthemum dregei (Eckl. & Zeyh.) Tiegh. | Southern & Eastern Africa | BK | Mixed with milk | Powdered mixed with milk to treat stomach problems in children [161] | |
| 92 | Loranthus globosus Roxb | Malaysia, Indo-China | LF, ST, FT | Poultice (leaves), juice | Headache, expel afterbirth, cough [162] | Antimicrobial, cytotoxicity (brine shrimp) [163], toxicity (Evan’s rat) [164] |
| 93 | Loranthus spec div. | Indonesia | WP | Poultice, decoction | Ariola, varicella, diarrhea, ankylostomiasis, morbilli (gabag), cancer [25] | |
| 94 | Macrosolen robinsonii (Gamble) Danser | Vietnam | LF | Decoction | Enlarged abdomen (diuretic tea) [165] | |
| 95 | Macrosolen cochinchinensis (Lour.) Tiegh. | Malaysia, Indo-China | ST, LF | Decoction, juice, poultice | Expel after birth, headache, cough [165] | |
| 96 | Scurrula atropurpurea (Blume) Danser | Indonesia, Philippines | LF, ST, WP | Decoction | Mouthwash (gargled), cancer (breast, throat cancer), cowpox, chickenpox, diarrhea, hookworm, measles, hepatitis, and cancer [166,167,168] | Cancer cell invasion inhibitory effects [169,170] |
| 97 | Scurrula ferruginea (Jack) Danser | Malaysia | LF, WP | Decoction, poultice | Decocted whole plant (mixed with Millettia sericea (Vent.) Wight & Arnott) is used as bathing to relieve malaria, decocted leaves as protective medicine after childbirth, pounded leaves to treat wounds, snake bites [166] | Antiviral (HSV-1 and poliovirus) and cytotoxic activities on murine and human cancer lines (3LL, L1210, K562, U251, DU145, MCF-7) [171] |
| 98 | Scurrula parasitica L. | China, Vietnam | WP | Decoction | Swelling, back pains, numbness, soreness of limbs, hypertension, galactagogue, quieting uterus (no contraction), reducing lumbago, bone strengthening. [166] | Anti-cancer (flavonoids extract, Leukimia cell line HL-60) [172], NF-κB inhibition [173], recovery of cisplatin-induced nephrotoxicity [174], Antioxidant (extracts, DPPH) [175] anti-cancer (Polysacharide fraction, S180, K562 and HL-60 cell lines, MTT assay) [176], anti-obesity activity using porcine pancreatic lipase assay (EtOH extract, PPL; triacylglycerol lipase, EC 3.1.1.3)[177], neuroprotective activity (168, H2O2-induced oxidative damage in NG108-15 cells)[178], antibacterial (EtOH extract, MRSA) [179] |
| 99 | Viscum aethiopicum [sic] | Southern & Eastern Africa | LF | Decoction (tea) | Diarrhea [161] | |
| 100 | Viscum capense L.f. | Southern & Eastern Africa | ST, FT | Decoction, external | Wart, asthma, irregular menstruation, hemorrhage [161] | Antimicrobial activity (stems extract), Anticonvulsant activity (MeOH extract, albino mice) [180] |
| 101 | Viscum pauciflorum L.f. | Southern & Eastern Africa | WP | Decoction | Astringent [161] | |
| 102 | Viscum rotundifolium L.f. | Southern & Eastern Africa | WP | External | Wart [161] | Immunoassay (stem, aqueous extracts, T cell activity in ruminants) [181] |
| Melastomataceae | ||||||
| 103 | Medinilla radicans Blume | LF, RT | Leaves eaten to treat dysentery, adventitious roots applied as poultice to wound, young leaves to skin disorders | Dysentery, wound and skin disorders [123] | ||
| 104 | Pachycentria constricta (Bl) Blume | Indonesia | TB | Tubers are boiled and eaten | Hemorrhoids [18,71] | |
| Moraceae | ||||||
| 105 | Ficus annulata Blume | Indonesia | LF, RT | Leaves decoction to treat fever, the root to treat Hansen diseases | Fever and Hansen diseases [168] | |
| 106 | Ficus deltoidea Jack | Indonesia, Malaysia, Thailand | LF, RT, FT | Drink (decoction), oitment | Leucorrhea, headache, fever, diabetes, high blood pressure, skin infection, aphrodisiac agent, ornament [71,182,183,184] | Toxicity (aqueous extract, rats) [185], anti-nociceptive [186], antioxidant (leaves aqueous extracs, redn. power of iron (III), superoxide anion (O2-) scavenging, xanthine oxidase (XOD), nitric oxide (NO·) and lipid peroxidn) [187], anti-melanogenic effect (extract, B16F1 melanoma cells, MTT assay) [188], anti-cancer [189], hypoglycemic activity (extract, rodents) [45,188] antimicrobial activity (extract) [190], Anti-inflammatory [191] |
| 107 | Ficus lacor Buch.-Ham. | India | BK, LT, BD, SD | Decoction, poultice | Decocted stem bark to treat gastric and ulcer, latex to treat boils (external), typhoid and fever (internal), decocted bud to treat ulcer, leucorrhoea, Seed as tonic for stomach disorder [157,192,193,194] | The medicated liquor has effects of relaxing muscles and tendons, activating collateral flow, promoting blood circulation, dispelling blood stasis, expelling wind, removing dampness, and relieving pain [195] |
| 108 | Ficus natalensis Hochst. (semi epiphytic, secondary terrestrial) | Uganda, Tanzania, Senegal, West Africa, South Africa, | LF, LT, RT, BK | Decoction, poultice | Root was used to treat lumbago, headache, arthritis, cataract and cough, Leaves were used to treat snakes bite, malaria, dysentery, ulcers, wounds and used as septic ears [196] | Antibacterial, antimalarial, and/or antileishmania activities were obsd. in some crude extracts., and five of these exts. showed a significant cytotoxicity against human tumor cells [41] |
| 109 | Ficus parietalis Blume | Vietnam, Thailand, Malaysia, Indonesia | RT | Decoction | Stomach-ache [184] | |
| 110 | Ficus pumila L. | Vietnam | FT, LF, LT | Drink (decoction) | Diarrahea, hemaroid, rheumatic, anemia, haematura, dysentery, dropsy, galactoge, tonic for impotence, lumbago, anthelmintic agent, externally used to treat carbuncles [184] | Against T-cell leukemia [197], antimicrobial [198] |
| 111 | Poikilospermum suaveolens (Blume) Merr. | Indonesia, Thailand | BK | Decoction | Water from the stem for drink, aide the secretion of waste products from the vagina, pain, numbness, stomach ulcer [25,199,200] | Anti-viral (MeOH extract) [201] |
| Orchidaceae | ||||||
| 112 | Acampe carinata (Griff.) Panigrahi | Himalaya, Nepal | WP | Decoction | Rheumatism, sciatica, neuralgia, beneficial in secondary syphilis and uterine diseases [202] | |
| 113 | Acriopsis liliifolia (J.Koenig) Seidenf. | Malaysia | LF, RT | Decoction of the roots and leaves | Fever [203] | |
| 114 | Anoectochilus formosanus Hayata | Taiwan | WP | Decoction | Fever, anti-inflammatory agent, diabetes, liver disorder, chest and abdominal pain [204] | Anti-inflammatory (water extract, rat paw), hepatoprotective (water extract, rat, SGOT-OPT) [205], anti-hyperliposis (414, rat induced) [206], ameliorative effect (water extract, ovariectomised rat) [207], antioxidant (water extract, DPPH) [208], anti-hyperglycemic (water extract, diabetic rats induced by streptozotocin) [209], anti-cancer (extracts, breast cancer MCF-7 cell) [210], liver regeneration (extract, rat) [211,212], Hepatoprotective (414, CCl4 induced rat) anti-inflammatory (414, lps stimulate mice) [213,214], anti-cancer (polysaccharide water extract, protate cancer cell lin PC3) [215] |
| 115 | Anoectochilus roxburghii (Wall.) Lindl. | Taiwan, China, Japan | WP | Decoction | Fever, snake bite, lung and liver diseases, hypertension, child malnutrition [216] | Hypoglycemic effect (414, streptozotocin (STZ) diabetic rats) [217], hypoglycemic and antioxidant effects (water extract, alloxan-induced diabetic mice, DPPH) [218] |
| 116 | Ansellia africana Lindl. | Southern & Eastern Africa | PD, ST, ST, RT | Decoction | Pedi is used to treat cough, the stem is used as aphrodisiac, used as emetic agent [161] | |
| 117 | Bulbophyllum kwangtungense Schltr. | China, Japan | TB | Tonic | To treat pulmonary tuberculosis, promote body liquid production, reduce fever, hemostatic agent [219] | Anti-tumor activities (456, 457, 458, against HeLa and K562 human tumor cell line) [220] |
| 118 | Bulbophyllum odoratissimum (Sm.) Lindl. ex Wall. | China, Burma, Vietnam, Thailand, Laos, Nepal, Bhutan, India | WP | Decoction | To treat pulmonary tuberculosis, chronic inflammation and fracture [221] | Anti-tumor (bibenzyl, inhibiting NO microphage) [221,222], anti-cancer (225, 470, 471, 475, 476, 478, 479, 482, 484, human leukaemia cell lines K562 and HL-60, human lung adenocarcinoma A549, human hepatoma BEL-7402 and human stomach cancer SGC-790) [223], anti-cancer (human leukemia cell lines K562 and HL-60, human lung adenocarcinoma A549, human hepatoma BEL-7402 and human stomach cancer cell lines SGC-7901) Anti-cancer (473 and 474, human leukemia cell lines K562 and HL-60, human lung adenocarcinoma A549, human hepatoma BEL-7402 and human stomach cancer SGC-7901) [224] |
| 119 | Bulbophyllum vaginatum (Lindl.) Rchb.f. | Malaysia | WP | Juice | Juice of the plant is instilled in the ear to cure earache [130] | |
| 120 | Catasetum barbatum (Lindl.) Lindl. | Japan, Guiana, Paraguayan | WP | Decoction | Febrifuge, anti-inflammatory [46] | Anti-inflammatory (505, rat) [225] |
| 121 | Coelogyne sp | Indonesia | RT | Decoction | Headache, fever [71] | |
| 122 | Cymbidium aloifolium (L.) Sw. | Thailand, Vietnam | LF | Decoction (internal), juice from heated or crushed leaves. | Otitis media, colds, irregular periods, arthritis, sores, burns, tonic [226] | Antinociceptive, anti-inflammatory (EtOH extract, mice) [227] |
| 123 | Cymbidium canaliculatum R.Br | Australia | PdB | Chewed, poultice | Dysentery, boils, sores, wounds, itschy skin, fractured arms over the break [154,228] | |
| 124 | Cymbidium ensifolium (L.) Sw | Taiwan, Vietnam | LF, RT, FL, WP, RT | Decoction | Diuretic agent (leaves), pectoral agent (root), eye problem (flower), cough, lung, gastrointestinal problems and sedative [226] | |
| 125 | Cymbidium goeringii (Rchb.f.) Rchb.f. | Japan, China, Korea, Thailand, Vietnam, India | WP | Decoction | Hypertension, diuretic agent [229] | Anti-inflammatory (478, RAW 264.7 cells) [230], anti-hypertensive (515, rat), diuretic activity (515, rats) [229] |
| 126 | Cymbidium madidum Lindl. | Australia | PdB | Chewed | Dysentery [154] | |
| 127 | Dendrobium affine (Decne.) Steud. | Australia | PdB | Poultice, external | Chrushed pseudobulbs (sticky) is applied to itchy skins, boils, infected skin lesion, minor burns [154] | |
| 128 | Dendrobium aloifolium (Blume) Rchb.f. | South East Asia | LF | Poultice | Headache [18] | |
| 129 | Dendrobium amoenum Wall. ex Lindl. | China | LF | Dried and ground | Skin diseases [231] | Antioxidant (519, NBT), antibacterial (519, diffusion) [231] |
| 130 | Dendrobium chryseum Rolfe | Australia | LF | Decoction | Diabetes [232] | Antioxidant (526, 530, 532, DPPH) [233] |
| 131 | Dendrobium candidum Wall. ex Lindl. | China | LF | Decoction | Diabetes [234] | Inhibitory effect of atropine on salivary secretion (extracts, rabbit) [235], anti-hyperglicemic (extract, streptozotocin-induced diabetic (STZ-DM) rats) [234], antioxidant (polysaccharide, 10-phenanthroline-Fe2+-H2O2 systems and ammonium peroxydisulfate/N,N,N’,N’-tetra-methylethanediamine systems) [236] antioxidant (555, 556, DPPH) [237], antioxidant (558, 559, 560, DPPH) [238], anti-tumor (soluble polysacharride, human neuroblastoma (SH2SY5Y) induced by SPD was observed and analyzed by Hoechst stain method) [239] |
| 132 | Dendrobium canaliculatum var. foelschei (F.Muell.) Rupp & T.E.Hunt | Australia | PdB | Poultice, external | Chrushed pseudobulbs (sticky) is applied to infected skin and cuts [154] | |
| 133 | Dendrobium crumenatum Sw. | Malaysia, Indonesia | LF, PdTB | Leaves pounded, bulbs heated to produce juice and applied as external uses | Acne (leaves), infected ears (pseudo-tubers) [240,241] | Antimicrobial [242] |
| 134 | Dendrobium chrysanthum Wall. ex Lindl. | China | LF | Dried and ground | Skin diseases, immune regulator, anti-pyretic, improve eyesight [243,244] | Anti-inflammation (590, macrophages were harvested from 2-month-old male C57BL/6J mice) [244] |
| 135 | Dendrobium densiflorum Lindl. | China | LF | Tonic | Promote body fluid production [245] | |
| 136 | Dendrobium faciferum J.J.Sm | Indonesia | ST | Dried | For twist work (craft) [246] | |
| 137 | Dendrobium fimbriatum Hook. | Japan, China | LF | Decoction, paste | Promote body fluid production, set fractured bone (paste) [247] | Antioxidant (water-soluble crude polysaccharide (DFHP), DPPH) [248] |
| 138 | Dendrobium loddigesii Rolfe | China | LF | Decoction | Promote body fluid production, reduce fever, nourish the stomach., anti-cancer agent [249] | Inhibitors of Na+, K+-ATPase of rat kidney (607, 608) [250], antiplatelet aggregation activity (479, 523, 606, rabit platelet) [251], antioxidant (DPPH), anti NO production (activated macrophages-like cell line, RAW264.7) [252] |
| 139 | Dendrobium moniliforme (L.) Sw. | China, Taiwan | ST | Decocted dried stem | Anti-pyretic, analgesic, aphrodisiac, stomachic, tonic agents [253] | Anti-inflammatory (552, RAW 264.7 cells) [254], hypoglicemic (polisaccharide, mice) [255], antioxidant (polisacharide) [256] |
| 140 | Dendrobium moschatum (Buch.-Ham) S.w | Nepal | LF | Juice | Cure earache [257] | |
| 141 | Dendrobium nobile Lindl. | China, Indonesia | WP | Tonic | Fever, reduce mouth dryness, aphrodisiac, promote body fluid production, nourish stomach, anorexia, lumbago, impotence [240,258,259,260,261] | Immunomodulatory activity (656, 660, 661, 662, 663, lymphocyte proliferation test MTT test) [262,263], antioxidant (478, 523, 524, 528, 584, 641, 672, 673, 674, DPPH) anti-NO (478, 523, 524, 528, 584, 641, 672, 673, 674, murine macrophage-like cell line RAW 264.7) [264], antioxidant (water-soluble polysaccharide (DNP), DPPH) [265], antimicrobial (Extracts), antitumour (extracts, Dalton’s lymphoma ascites (DLA) cells w), induction of in vitro lipid peroxidation (extracts, TBARS) [266], NO inhibition (475, 523, 542, 632, 633, 634, 665–671, murine macrophage RAW 264.7 cells) [267], anti-tumor (polisachacaride extracts, sarcoma 180 in vivo and HL-60)[268] |
| 142 | Dendrobium pachyphyllum (Kuntze) Bakh.f. | Indonesia | WP | Decoction | Hydropsy [246] | |
| 143 | Dendrobium purpureum Roxb. | Indonesia, Malaysia | LF | Crushed and heated to make poultice | Nail fungal infection [240] | |
| 144 | Dendrobium salaccense (Blume) Lindl. | Indonesia | LF | Fragrance | Fragrance [246] | |
| 145 | Dendrobium teretifolium R.Br. | South-Pacific Island | LF | Decoction | Severe headache, other pains [269,270] | |
| 146 | Dendrobium catenatum Lindl. | China | LF | Decoction | Anxiety and panic [271] | |
| 147 | Dendrobium utile J.J.Sm. | Indonesia | ST | Dried | Twist work [246] | |
| 148 | Dichaea muricata (Sw.) Lindl. | Central, South American | LF | Decoction (wash) | Eye infection [260] | |
| 149 | Eulophia speciosa (R.Br.) Bolus | Indonesia | RT | Decoction | Analgesic [246] | |
| 150 | Epidendrum strobiliferum Rchb.f. | China, Korea | ST | Infusion, decoction | Analgesic [272] | Analgesic (676, 677 exhibited notable analgesic action at 3 mg/kg, causing 86 and 83% inhibition of abdominal constriction, respectively [272], antinociceptive effect (MeOH extract, methanolic ext. (ME) [273] |
| 151 | Epidendrum rigidum Jacq. | Mexico, North Sudamerica, Antilles | ST | Infusion, decoction | Replenish body fluid [274] | Phytotoxin (chloroform-methanol extract) [274] |
| 152 | Mycaranthes pannea (Lindl.) S.C.Chen & J.J.Wood | Vietnam, Malaysia | WP | External, medicinal bath | Medicinal bath to treat ague and malaria fever, fractures, bruises, skin complaints, dislocated joint to relieve severe pain, swelling, dislocation and fracture [123,275,276] | |
| 153 | Eriopsis biloba Lindl. | America | ST | Poultice | Sore gums and mouth membranes [260] | |
| 154 | Grammatophyllum scriptum (L.) Blume | Indonesia, Thailand | BL, SD, ST | Poultice | Pseudo bulb mixed with curcuma and salt applied to sores and abdomen to expel worms, to treat dropsy and aphthae, seeds mixed with food to treat dysentery, aphthae, crushed plant mixed with rice liquor to treat snake bite, scorpions’ and centipedes’ stings [246,277] | |
| 155 | Jumellea fragrans (Thouars) Schltr. | Madagascar | LF, ST | Decoction | Anti-spasmodic, anti-asthmatic agents, mixed leaves of Ziziphus mauritana, Mussaenda arcuate to treat eczema (deecotion), mixed with Eugenia uniflora to treat diarrhea [24] | |
| 156 | Liparis condylobulbon Rchb.f. | Indonesia | PdB, LF | Chewing, external | Intestinal complaints and constipation. (eastern Sulawesi, ambon), tormina, abscess [246,278] | |
| 157 | Liparis nervosa (Thunb.) Lindl. | China, Thailand, Malaysia | WP | Decoction, external | Stop internal/external bleeding, treat snake bites [278] | |
| 158 | Neottia ovata (L.) Bluff & Fingerh. | Spain | TB | Tincture | Stomach diseases [279] | Anti-viral (extract, SARS-CoV Frankfurt 1 strain [280] |
| 159 | Masdevallia uniflora Ruiz & Pav. | Mexico, south America | WP | Decoction | Facilitate urination (pregnant women), reduce bladder inflammation [260] | |
| 160 | Camaridium densum (Lindl.) M.A.Blanco | Mexico | WP | Decoction | Analgesic, relaxant agents [281] | Spasmolytic activity (667, 690, 693, 694, 695, Wistar rat) [37], antinociceptive activity (extract, mice) [281] |
| 161 | Nidema boothii (Lindl.) Schltr. | Malaysia | WP | Decoction | Relaxant agent [282] | Spasmolytic effects (471, 478, 488, 508, 671, 696, 697, 699, 700, 702, guinea ileum pig model) [282] |
| 162 | Oberonia lycopodioides (J.Koenig) Ormerod | Malaysia | LF | Poultice | Boils [123,283] | |
| 163 | Oberonia mucronata (D.Don) Ormerod & Seidenf. | China, Vietnam | WP | Decoction | Rheumatism, promote blood circulation, inflammation of the bladder/ureter, bruises and fractures, detoxicant, diuretic agent [284] | |
| 164 | Erycina pusilla (L.) N.H.Williams & M.W.Chase | Mali | WP | Decoction | Lacerations [260] | |
| 165 | Otochilus lancilabius Seidenf. | Bhutan, Nepal, India, China (Tibet), Laos and Vietnam | WP | Pills | Antiemetic, febrifuge for stomach inflammation (bad-tshad), and allays hyperdipsia and dehydration [23] | |
| 166 | Phragmipedium pearcei (Rchb.f.) Rauh & Senghas | South America | WP | Decoction | Stomachache [260] | |
| 167 | Pholidota articulata Lindl. | Himalaya, Nepal | WP | Whole plant: bone fractures [202] | ||
| 168 | Pholidota chinensis Lindl. | China, India | PdB | Tincture | Scrofula, toothache, stomachache, chronic bronchitis, duodenal ulcer [285] | Antioxidant (475, 539, 667, 670, 671, 711, 712, 717, 722, 723, 726, (DPPH), anti-inflammatory (475, 539, 667, 670, 671, 711, 712, 717, 722, 723, 726, inhibitory activity on NO production from activatedmacrophage-like cell line, RAW 264.7)[286], antioxidant (715, 741, 742, 746, 747, 749, 750, DPPH), anti-inflammatory (as above, inhibitory activity on NO production from activated macrophages-like cell line, RAW 264.7) [285] |
| 169 | Renanthera moluccana Blume | Indonesia | WP | Ornament | Ornament [246] | |
| 170 | Rhynchostylis retusa (L.) Blume | Himalaya, Nepal, India | LF | Rheumatic, hepaoprotective agent [96,202] | ||
| 171 | Scaphyglottis livida (Lindl.) Schltr. | Mexico | WP | Decoction | Analgesic, anti-inflammatory agents [281,287] | Spasmolytic (471, 475, 714, 754, 755, rat ileum rings) [288], antinociceptive (extracts, male mice ICR) [281], acute toxicity (extract, male mice ICR) [287] |
| 172 | Vanda tessellata (Roxb.) Hook. ex G.Don | India, Sri Lanka, Burma | LF, RT, FL | Leaves pounded to make juice, paste, extract (alcoholic) of the root and flower | Fever (as paste), otitis (dropped juice), the root to treat bronchitis, rheumatic, dyspepsia, sciatica, inflammation, otitis, nervous problem, fever and as aphrodisiac, laxative, tonic (for liver) agent [140,289,290,291] | Cholinergic activity (glycoside fraction), anti-arthritic (extract, albino rat) [292], anti-inflammatory (extract), antidiabetic (extract, rat) [291,293] |
| 173 | Papilionanthe teres (Roxb.) Schltr. | Indonesia | WP | Ornament | Ornamental [294] | Anti-aging (758, 759, HaCaT cytochrome C oxidase) [295] |
| 174 | Vanilla griffithii Rchb.f. | Indonesia | WP | Eaten | Edible [294] | |
| 175 | Vanilla planifolia Jacks. ex Andrews | Indonesia, Mexico | FT, STh | Decoction | Fever, rheumatism, hysteria, increase energy and muscular system [25,259,294] | Antimicrobial activity (extract) [296] |
| Piperaceae | ||||||
| 176 | Peperomia galioides Kunth | Peru | WP | Poultice (external), drink (internal) | Chrused plant is used to treat wounds, cuts, plant juice is used to treat gastric ulcers [297] | Antibacterial (oil) [298,299] |
| 177 | Piper retrofractum Vahl | Indonesia | FT, RT | Drink (decoction) | Anticonvulsion, antivomiting, diarrhea, dysentery, constipation, headache [300] | Anti-convulsan (776, mice) [301], cytotoxicity (extract, 779) [302], anti-platelet aggregation (extract) [303], anti-vector (extract, mosquito larvae) [304,305], antioxidant (228, 283, 334, 574, 771, 772, 782, 783, DPPH) [306], antileishmanial activity (extracts, leishmania donovani) [307], anti-obesity (776, 777, C57BL/6J mice) [308] |
| Rubiaceae | ||||||
| 178 | Hydnophytum formicarum Jack | Indonesia, Philippines, Thailand | TB | Poultice, decoction, powder | Poultice to treat swelling, headache, decoction to treat liver, intestinal complaints, powder as anthelmintic, heart tonic, antidiabetic agent and to treat skin, bone, knee, ankle, lung diseases [278] | Anti-tumor (extracts, against human tumor cell lines, HeLa and A549) [309], xanthine oxidase inhibitory (MeOH extract, assayed spectrophotometrically under aerobic conditions [310], antimicrobial, cytotoxicity (226, 786, 787, against HuCCA-1 and KB cell lines) [311], trigger cytochrome C release in treated MCF-7 cell (786, ELISA) [312], anti-cancer (786, the human breast carcinoma cell line MCF-7) [313] |
| 179 | Myrmecodia tuberosa Jack | Indonesia | PT | Drink (decocted) | Swelling, headache [18,71,314] | Immunomodulatory effect (EtOH fractions) [315] |
| 180 | Myrmecodia pendens Merr. & L.M.Perry | Papua | PT | Decoction | Rheumatism, headache, renal problems, tumor [316] | |
| Sterculiaceae | ||||||
| 181 | Scaphium macropodum (Miq.) Beumée ex K.Heyne (hemi-epiphyte) | Indonesia | RT | Drink (decoction) | Nervous system problem [71] | |
| Verbenaceae | ||||||
| 182 | Premna parasitica Blume | Indonesia | LF | Drink (decoction) | Fever [25] | |
| Viscaceae | ||||||
| 183 | Viscum articulatum Burm.f. | Cambodia, India, Taiwan, China | WP | Poultice, decoction | Decoction to treat bronchitis, skin tumour, neuralgia, arthritis and as tonic, sedative, febrifuge, crushed plant to treat cut [317] | Toxicity (extract, mice) [318], anti-tumor (820, MTT assay) [319], anti-inflammatory (1234718, superoxide inhibition) [320], cytotoxicity and anti-HIV-1 activity (shown by isolated compounds including 801, 804, 803, 813, 814, 815, 824, 828); MDAMB-435 and Hela cells, HIV-1ШB-infected C8166 cells) [321], anti-nephrotoxic (127, gentamicin-induced renal damage in Wistar rats) [322], antioxidant, anti-inflammatory (810, 811, 812, 822, 825, 829, 830, 831, 832, 833, 834, DPPH, NO production and cell viability assay. The murine macrophage cell line RAW264.7) [323], diuretic activity (MeOH extract, male rats) [324], antiepileptic activity (MeOH exctract, rat) [325], anti-hypertension (glucocorticoid-induced hypertension, Nω-nitro-l-arginine methyl in rats) [326,327], antioxidant (polisacharide fraction, DPPH) [328] |
| 184 | Viscum ovalifolium DC. | Cambodia, Malaysia | LF, WP | Poultice, external | Leaves (poultice) to treat neuralgia, as herbal bath to treat fever in children, ash mixed with sulphur, coconut oil to treat pustular itches [329] | |
| Zingiberaceae | ||||||
| 185 | Hedychium ongi cornotum Griff. | Indonesia | RZ, RT | Drink (decoction) | Rhizome is used to treat syphilis; root is used to treat worm [25] | |
Note: na: not mentioned; ST: stem, PT: pith; TB: tuber; SP: spore; BK: bark; LT: latex; NT: nutmeg; SD: seed; FT: fruit; BD: buds; PD: pedi; PdB: pseudobulbs; FL: flower; PdTB: pseudotuber; BL: bulbs: STh: sheath; WP: whole; LF: leaf; RT: root; RZ: rhizome.
Figure 2.
Five most popular medicinal epiphytes. (A) C. filiformis L. (B) B. odoratissimum (Sm.) Lindl. ex Wall. (C) C. goeringii (Rchb.f.) Rchb.f. (D) A. aureum Limme. (E) F. natalensis Hochst.
2.2. Distribution of Vascular Epiphytic Medicinal Plant Species by Country
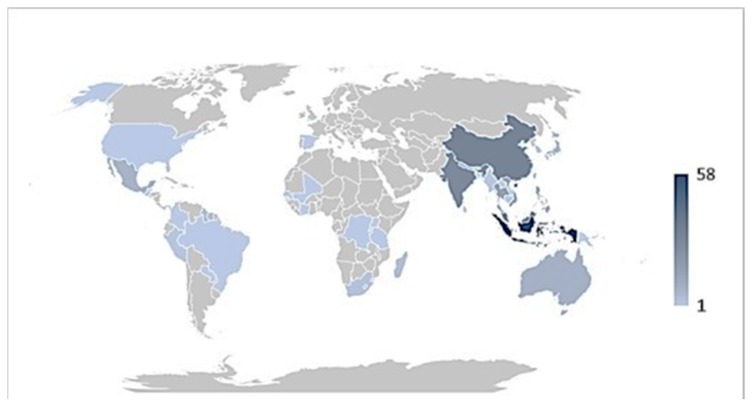
Based on the available records, the data curation and analysis revealed that the Indigenous Indonesians have used 58 diverse epiphytic medicinal plant species throughout the archipelago and have the highest record compared to other tropical countries (Figure 3). China is second and is well known for its traditional medicine, including the use of epiphytes in medicament preparation. This is followed by the Indigenous Indians, with the well-established Ayurveda as a formal record of Indian medicinal plants. The traditional medicinal plant knowledge of Indonesa has been heavily influenced by Indian culture and enriched by Chinese and Arabian traders since the kingdom era [27].
Figure 3.
Density map showing a number of epiphytic medicinal plant species used by different countries. The number of species used is proportional to colour intensity.
2.3. Parts of Vascular Epiphytic Medicinal Plant Species Used in Traditional Medicines
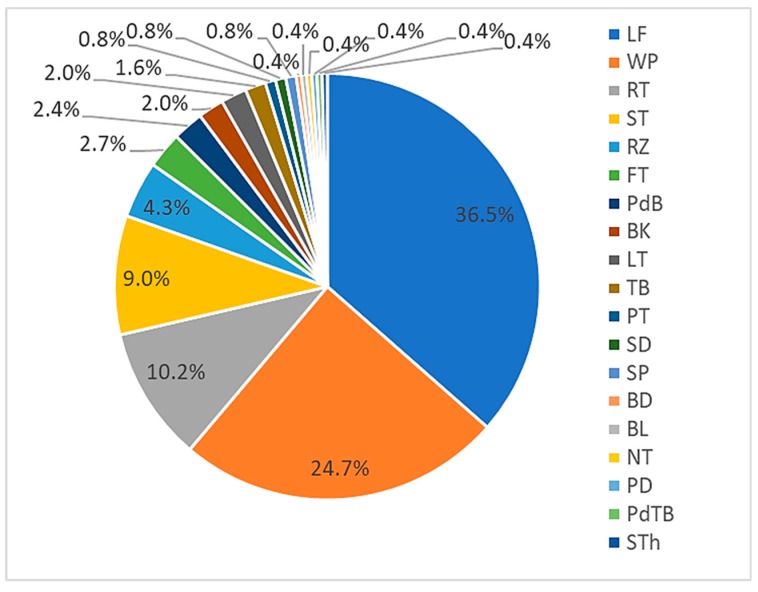
This review determined that leaves were the main plant components used in the traditional medicines (Figure 4). This was expected given they are more easily harvested (without excessive tools) and processed compared to other plant parts, e.g., the root and stem. As some epiphytes have a small biomass compared to higher trees, the whole plant is commonly harvested in medicament preparation. Interestingly, almost half of epiphytic medicinal plants were ferns, in which the stem-like stipe is prepared for medicine. Without haustoria (a specialised absorbing structure of a parasitic plant), the root and rhizome of epiphytic medicinal plants are easily harvested and prepared.
Figure 4.
Components of epiphytic plants used in medicinal preparations (represented in percentages). LF: leaf; WP: whole; RT: root; ST: stem, RZ: rhizome; FT: fruit; PdB: pseudobulbs; BK: bark; LT: latex; TB: tuber; PT: pith; SD: seed; SP: spore; BD: buds; BL: bulbs: NT: nutmeg; PD: pedi; PdTB: pseudotuber; STh: sheath.
2.4. Modes of Preparation and Dosage of Administration of Vascular Epiphytic Medicinal Plant Species in Traditional Medicines
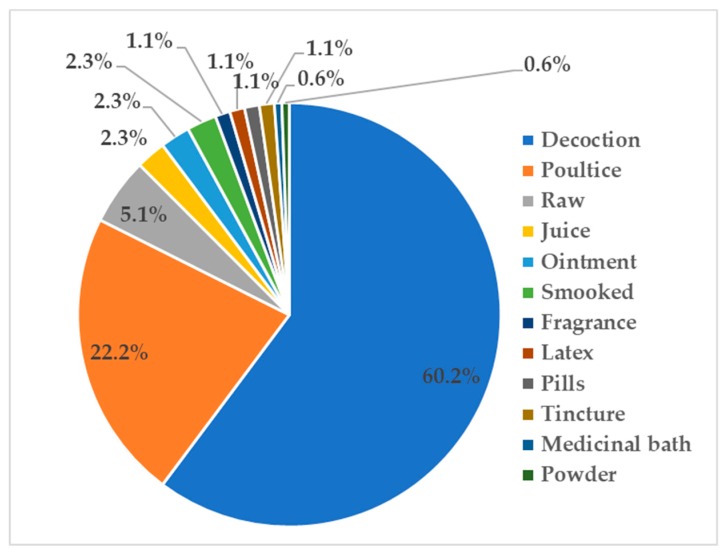
Generally, medicinally active secondary metabolites have a water solubility problem likely related to the lipophilic moieties in their structures [29]. Using boiling water, decoctions are able to increase the yield of secondary metabolites extracted from medicinal plants. Therefore, it is not surprising that decoctions are commonly used in traditional medicine preparations from plants (Figure 5). External applications are also commonly practiced in traditional medicinal therapies, including poultice (moist mass of material), raw, or less processed medicine. Poultices were commonly prepared for skin diseases while a decoction was ingested for internal infectious diseases (i.e., fever).
Figure 5.
Modes of preparation and administration of epiphytic medicinal plants (represented in percentages).
2.5. Category of Diseases Treated by Vascular Epiphytic Medicinal Plant Species
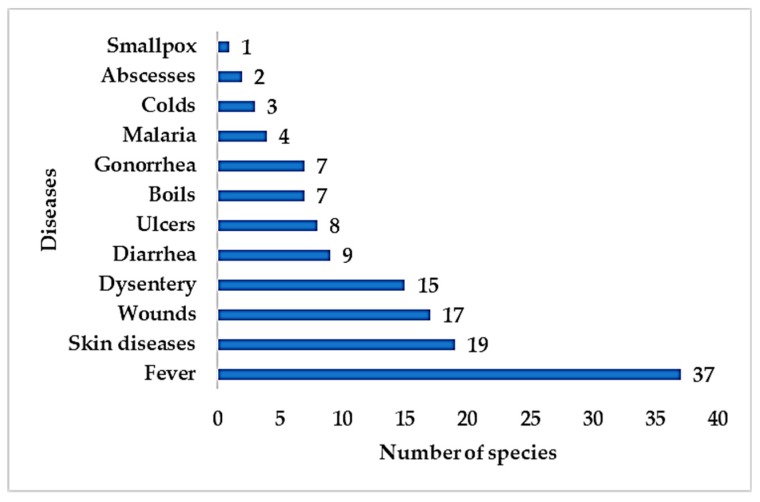
Interestingly, epiphytes have been used for treating various ailments, including both infectious and non-infectious diseases. Traditional communities described infectious diseases related to skin diseases (wounds, boils, ulcers, abscesses, smallpox) and non-skin diseases (fever, diarrhoea, ulcers, colds, worm infections, and malaria). A total of 54 epiphytic medicinal plant species were prescribed to treat skin diseases while 81 species to treat non-skin infectious diseases (Figure 6).
Figure 6.
Number of epiphytic medicinal plant species used traditionally to treat infectious diseases.
Hygiene has been a serious issue in traditional communities as it gives rise to infectious diseases. Fever is a common symptom of pathogenic infection and has been treated using medicinal plants, including epiphytes. Hygiene issues are also a common cause of skin disease, wounds, dysentery, and diarrhoea in traditional communities.
3. Phytochemical Composition of Vascular Epiphytic Medicinal Plants
Epiphytes belong to a distinctive plant class as they do not survive in soil and this influences the secondary metabolites present. Epiphytes are physically removed from the terrestrial soil nutrient pool and grow upon other plants in canopy habitats, shaping epiphyte morphologies by the method in which they acquire nutrients [30]. Nutrients, such as nitrogen and phosphorus, are obtained from different sources, including canopy debris (through fall) and host tree foliar leaching [30], the latter influencing canopy soil nutrient cycling [31,32]. In the conversion of sunlight into chemical energy, the epiphyte often uses a specific carbon fixation pathway (CAM: Crassulacean acid metabolism) as a result of harsh environmental conditions [33], making them unique and thus worthwhile for scientific studies.
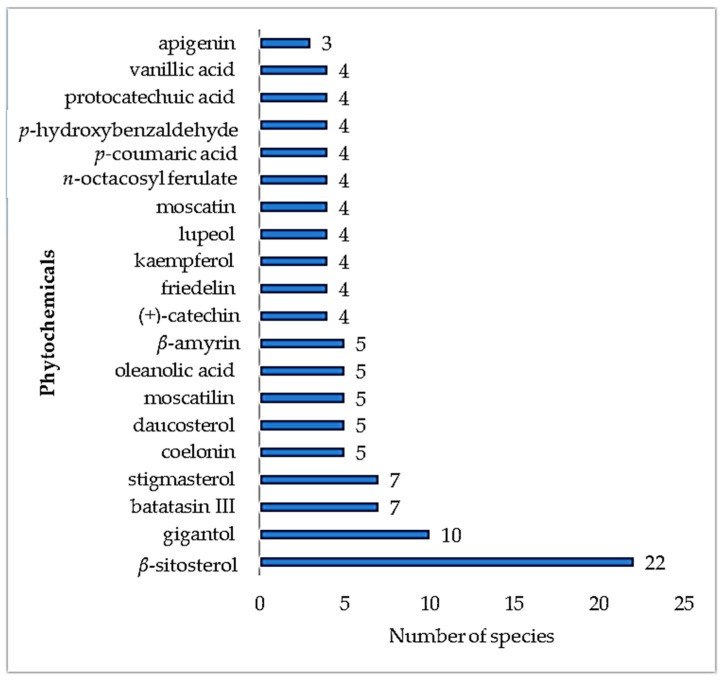
In the early 20th century, laboratory-based research on epiphytes studied the plant’s production of alkaloids, cyanogenetic, and organic sulfur compounds, with the plants producing limited quantities of these compounds [34]. Common plant steroids, e.g., β-sitosterol, have been shown to be present in 22 different epiphytic medicinal plants (Figure 7). This is possibly due to the function of the steroids as structural cell wall components, giving rise to a wide distribution across plant families and species. A further example of a common plant steroid present is stigmasterol.
Figure 7.
Number of epiphytic medicinal plant species producing the same secondary metabolites.
Table 2 lists the secondary metabolites identified in epiphytic medicinal plants and details the species, isolated compounds, and provides references. Currently, only 69 species have been phytochemically studied (23 fern and 46 non-fern epiphytes) and 842 molecules have been isolated from these epiphytic plants. Analysis of the literature showed epiphytes were able to produce a range of secondary metabolites, including terpenes and flavonoids, with no alkaloids being isolated from epiphytic fern medicinal plants thus far. β-Sitosterol, a common phytosterol in higher plants, was reported across fern genera. Interestingly, there is one unique terpene produced, hopane, which is commonly called fern sterol. Common flavonoids, such as kaempferol, quercetin, and flavan-3-ol derivatives (catechin), were also reported across the epiphytic ferns. Epiphytic pteridaceae, Acrostichum aureum Limme, is rich in quercetin [35]. Further analysis showed there were more secondary metabolites reported from non-fern epiphytic medicinal plants than from fern epiphytic medicinal plants, including terpene derivatives, flavonoids, and alkaloids. Included were flavanone, flavone, and flavonol derivatives but no flavan-3-ols were reported in these epiphytes so far. In the non-fern epiphytes, there were more phytochemical studies on orchid genera with additional classes of compounds reported, including penantrene derivatives (flavanthrinin, nudol, fimbriol B) [36,37] from the Bulbophyllum genus and the alkaloid dendrobine from the Dendrobium genus [38].
Table 2.
Phyctochemical constituents of epiphytic medicinal plants.
| No | Epiphyte Species | Constituents |
|---|---|---|
| Fern species | ||
| Adiantaceae | ||
| 1 | Adiantum caudatum L., Mant | 16-hentriacontanone 1, 19α-hydroxyferna-7,9(11)-diene 2, 29-norhopan-22-ol 3, 3α-hydroxy-4α-methoxyfilicane 4, 8α-hydroxyfernan-25,7β-olide 5, adiantone 6, filic-3-ene 7, hentriacontane 8, isoadiantone 9, quercetin-3-O-glucoside 10, β-sitosterol 11, β-sitosterol 11, β-sitosterol glucoside 12 [330,331,332] |
| Aspleanceae | ||
| 2 | Asplenium nidus L. | (-)-epiafzelechin 3-O-β-d-allopyranoside 13, homoserine 14 [333] |
| Blechnaceae | ||
| 3 | Stenochlaena palustris (Burm. F.) Bedd. | 1-O-β-D-glucopyranosyl-(2S*,3R*,4E,8Z)-2-N-[(2R)-hydroxytetracosanoyl]octadecasphinga 4,8-dienine 15, 3-formylindole 16, 3-oxo-4,5-dihydro-α-ionyl-β-d-lucopyranoside 17, kaempferol 3-O-β-d-glucopyranoside 18, kaempferol 3-O-(3′,6′-di-O-E-p-coumaroyl)-β-d-glucopyranoside 19, kaempferol 3-O-(3′-O-E-p-coumaroyl)-(6′-O-E-feruloyl)-β-d-glucopyranoside 20, kaempferol 3-O-(3′-O-E-p-coumaroyl)-β-d-glucopyranoside 21, kaempferol 3-O-(6′-O-E-p-coumaroyl)-β-d-glucopyranoside 22, lutein 23, stenopaluside 24, stenopalustrosides A–E 25–29, β-sitosterol-3-O-β-d-glucopyranoside 30 [334,335] |
| Davalliaceae | ||
| 4 | Araiostegia divaricata (Blume) M. Kato | (-)-epicatechin 3-O-β-d-(2”-O-vanillyl)allopyranoside 31, (-)-epicatechin 3-O-β-D-(2′-trans-cinnamoyl)allopyranoside 32, (-)-epicatechin 3-O-β-D-(3”-O-vanillvl)allopyranoside 33, (-)-epicatechin 3-O-β-d-(3′-trans-cinnamoyl)allopyranoside 34, (-)-epicatechin 3-O-β-d-allopyranoside 35, (-)-epicatechin 3-O-β-d-allopyranoside 35, (+)-catechin 3-O-β-allopyranoside 36, 24-norferna-4 (23) 37, 4β-carboxymethyl-(-)-epicatechin 38, 4β-carboxymethyl-(-)-epicatechin methyl ester 39, 4β-carboxymethyl-(-)-epicatechin potasium 40, 9(11)-diene 41, cyanin 42, davallic acid 43, epiafzelechin-(4β→8)-epicatechin 3-O-β-d-allopyranoside 44, epicatechin-(4β→6)-epicatechin-(4β→8)-epicatechin-(4β→6)-epicatechin-D-glucooctono-δ-lactone enediol 45, epicatechin-(4β→8)-4β-carboxymethylpicatechin 46, hop-21-ene 47, monardein 48, pelargonin 49, procyanidin B-2 3”-O-β-d-allopyranoside 50, sodium salts 51 [59,60,336,337,338,339,340] |
| 5 | Davallia solida (G. Forst.) Sw. | 18-diene 52, 18-diene 52, 19α-hydroxyfernenes 53, 19α-hydroxyfilic-3-ene 54, 2-C-β-d-glucopyranosyl-1,3,6,7-tetrahydroxyxanthone 55, 2-C-β-d-xylopyranosyl-1,3,6,7-tetrahydroxyxanthone 56, 2-C-β-d-xylopyranosyl-1,3,6,7-tetrahydroxyxanthone 56, 30-O-p-hydroxybenzoylmangiferin 57, 3-O-p-hydroxybenzoylmangiferin 58, 40-O-phydroxybenzoylmangiferin 59, 4-O-β-d-glucopyranosyl-2,6,4′-trihydroxybenzophenone 60, 4β-carboxymethyl-(-)-epicatechin 38, 4β-carboxymethyl-(-)-epicatechin methyl ester 39, 60-O-p-hydroxybenzoylmangiferin 61, eriodictyol 62, eriodictyol-8-C-β-d-glucopyranoside 63, fena-9(11) 64, fern-7-en-19α-ol 65, fern-9(11)-en-19α-ol 66, ferna-7 67, filic-3-en-19α-ol 68, filica-3,18,20-triene 69, filica-3,18-diene 70, icariside E3 71, icariside E5 72, mangiferin 73 [66,68,338,341,342] |
| Lycopodiaceae | ||
| 6 | Huperzia carinata (Desv. ex Poir.) Trevis | carinatumins A, B, and C 74, 75, 76 [74] |
| 7 | Huperzia phlegmaria (L.) Rothm | 14β,21α,29-trihydroxyserratan-3β-yl dihydrocaffeate (lycophlegmariol D) 77, 21α,24-dihydroxyserrat-14-en-3β-yl 4-hydroxycinnamate (lycophlegmariol C) 78, 21β,24,29-trihydroxyserrat-14-en-3β-yl dihydrocaffeate (lycophlegmariol B) 79, 21β,29-dihydroxyserrat-14-en-3α-yl dihydrocaffeate (lycophlegmariol A) 80, 21β-hydroxy-serrat-14-en-3α-ol 81, 21β-hydroxy-serrat-14-en-3α-yl acetate 82, 8,11,13-abietatriene-3β,12-dihydroxy-7-one (margocilin) 83, 8-deoxy-13-dehydroserratinine 84, 8-deoxyserratinidine 85, acrifoline 86, annotine 87, annotinine 88, dihydrolycopodine 89, epidihydrofawcettidine 90, fawcettidine 91, huperzine A 92, lycododine 93, lycoflexine 94, lycophlegmarin 95, lycophlegmarin 95, lycophlegmarine 96, lycophlegmine 97, lycopodine 98, malycorin A 99, malycorins B, C 100, 101, N,N′-dimethylphlegmarine 102, phlegmanol A–E 103–107, phlegmaric acid 108, α-obscurine 109, β-obscurine 110 [77,343,344,345,346,347,348] |
| 8 | Huperzia megastachya (Baker) Tardieu | 21-epi-serratenediol 111, 21-epi-serratenediol-3-acetate 112, lycoclavanol 113, megastachine 114, phlegmanol-D 115, serratenediol 116, serratenediol-3-acetate 117, serratenonediol diacetate 118, tohogenol diacetate 119 [349,350] |
| 9 | Nephrolepis biserrata (Sw.) Schott | 1β,11α-diacetoxy-11,12-epoxydrim-7-ene 120, 1β,3β,11α-triacetoxy-11,12-epoxydrim-7-ene 121, 1β,6α,11α-triacetoxy-11,12-epoxydrim-7-ene 122, sequoyitol 123 [339,351] |
| Oleandraceae | ||
| 10 | Nephrolepis cordifolia (L.) C. Presl | fern-9(11)-ene 124, hentriacontanoic acid 125, myristic acid octadecylester 126, oleanolic acid 127, sequoyitol (patent) 123, triacontanol 128, β-sitosterol 11 [352,353] |
| Opioglossaceae | ||
| 11 | Botrychum lanuginosum Wall.ex Hook & Grev. | (6′-O-palmitoyl)-sitosterol-3-O-β-d-glucoside 129, 1-O-β-D-glucopyranosyl-(2S,3R,4E,8Z)-2-[(2R-hydroxy hexadecanoyl) amino]-4,8-octadecadiene-1, 3-diol 130, 30-nor-21β-hopan-22-one 131, apigenin 132, β-sitosterol 133, daucosterol 134, luteolin 135, luteolin-7-O-glucoside 136, thunberginol A 137 [354] |
| Polypodiaceae | ||
| 12 | Drynaria roosii Nakaike | kaempferol 3-O-β-d-glucopyranoside-7-O-α-l-arabinoside 138, (2R)-naringin 139, (2S)-narigenin-7-O-β-d-glucoside 140, kaemperol 3-O-α-l-rhamnosyl-7-O-β-d-glucoside 141, luteolin-7-O-β-d-neohesperidoside 142, maltol glucoside 143, (-)-epicatechin 144, 12-O-caffeoyl-12-hydroxyldodecanoic acid 145, xanthogalenol 146, naringenin 147, kushennol F 148, sporaflavone G 149, kuraninone 150, leachianone A 151, 8-phenylkaempferol 152, kaempferol 153, chiratone 154, fern-9(11)-ene 155, hop-22(29)-ene 156, isoglaucanone 157, dryocrassol 158, dryocrassol acetate 159, (+)-afzelechin-3-O-β-allopyranoside 160, (+)-afzelechin-6-C-β-glucopyranoside 161, 4α-carboxymethyl-(+)-catechin methyl ester 162, (-)-epiafzelechin-(4β→8)-(-)-epiafzelechin-(4β→8)-4β-carboxymethyl-(-)-epiafzelechin methyl ester 163, (-)-epiafzelechin-(4β→8)-4β-carboxymethyl-(-)-epicatechin methyl ester 164, (-)-epiafzelechin-(4β→8)-4α-carboxymethy-(-)-epiafzelechin ethyl ester 165, (-)-epiafzelechin-3-O-β-d-allopyranoside 166, (-)-epicatechin-3-O-β-d-allopyranoside 167, (+)-catechin 168, 4β-carboxymethyl-(-)-epiafzelechin methyl ester 169, 4β-carboxymethyl-(-)-epiafzelechin 170, (-)-epiafzelechin-(4β→82→O→7)-epiafzelechin-(4β→8)-epiafzelechin 171, (-)-epiafzelechin 172, (-)-epiafzelechin-(4β→8)-4β-carboxymethyl-epiafzelechin methyl ester 173, epicatechin-(4β→8)-epicatechin 174, (+)-afzelechin 175, (+)-epicatechin-3-O-β-d-allopyranoside 176, (-)-epicatechin-8-C-β-d-gluclopyranoside 177, (-)-epiafzelechin-5-O-β-d-allopyranoside 178, drynachromoside A 179, drynachromoside B 180, fortunamide 181, curcumine 182, demethoxycurcumine 183, bisdemethoxycurcumine 184, bavachinine 185, isobavachalcone 186, (-)-epicatechin 144, liquiritine 187, bakuchiol 188, protocatechuic acid 189, (R)-5,7,3′,5′-tetrahydroxy-flavonone 7-O-neohesperidoside 190, (2S)-5,7,3′,5′-tetrahydroxyflavonone 7-O-β-d-glucopyranoside 191, 5,7,3′,5′-tetrahydroxflavanone 192, 3′-lavandulyl-4-methoxy-2,2′,4′,6′-tetrahydroxyylcalcone 193, 5,7-dihydroxychromone-7-O-β-d-glucopyranoside 194, 5,7-dyhidroxychromone-7-O-neohesperidosyl 195 [43,94,355,356,357,358] |
| 13 | Drynaria propinqua (Wall. ex Mett.) Bedd | (-)-epiafzelechin 3-O-β-d-allopyranoside 13 [359] |
| 14 | Drynaria quercifolia (L.) J.Sm. | friedelin 196, epifriedelinol 197, β-amyrin 198, β-sitosterol 11, 3-β-d-glucopyranoside 199, 3,4-dihydroxybenzoic acid 200, acetyllupeol 201 [97,360] |
| 15 | Drynaria rigidula (Sw.) Bedd. | fern-9(11)ene 202, hop-22(29)-ene 156, γ-sitosterol 203, 3,4-dihydroxybenzoic acid 200, 4-hydroxybenzoic acid 204, 4-hydroxyphenyl-1-(2-arabinopyranosyl)-tetrahydro-2H-pyran-3,4,5-triol 205, 4-hydroxyphenyl-1-tetrahydro-2H-pyran-3,4,5-triol 206, kaempferitrin 207, 3,5-dihydroxy-flavone-7-O-β-rhamnopyranosyl-4′-O-β-glucopyranoside 208 [92,361] |
| 16 | Phymatosorus scolopendria (Burm. f.) Pic. Serm. | 1,2-benzopyrone (coumarin) 209 [47] |
| 17 | Pyrrosia lingua (Thunb.) Farw. | diploptene 210, β-sitosterol 11, octanordammarane 211, dammara-18(28),21-diene 212, (18S)-18-hydroxydammar-21-en 213, (18R)-18-hydroxydammar-21-ene 214, (18S)-pyrrosialactone 215, (18R)-pyrrosialactone 216, (18S)-pyrrosialactol 217, 3-deoxyocotillol 218, dammara-18(28),21-diene 212, cyclohopenol 219, cyclohopanediol 220, hop-22(29)-en-28-al 221 [362,363,364] |
| 18 | Pyrrosia petiolosa (Christ) Ching | α-tocopherol 222, diploptene 210, 24-methylene-9,19-cyclolanost-3β-yl acetate 223, cycloeucalenol 224, β-sitosterol 11, daucosterol 134, vanillic acid 225, protocatechualdehyde 226, hydrocaffeic acid 227, caffeic acid 228, 7-O-[6-O-(α-l-arabinofuranosyl)-β-D-glucopyranosyl]gossypetin 229, kaempferol-3-O-β-d-glucopyranoside-7-O-α-l-arabinofuranoside 230 [365,366,367,368] |
| 19 | Pyrrosia sheareri (Baker) Ching | diploptene 210, β-sitosterol 11, vanillic acid 225, protocatechuic acid 189, mangiferin 73, fumaric acid 231, sucrose 232 [42] |
| Psilotaceae | ||
| 20 | Psilotum nudum (L.) P. Beauv | apigenin di-C-glycoside 233, 7,4′,4′-tri-O-β-d-glucopyranoside 234, 4′,4′-di-O-β-d-glucopyranoside 235, 7,4′-di-O-β-d-glucopyranoside 236, 3′-hydroxypsilotin (6-[4′-(β-D-glucopyranosyloxy)-3′-hydroxyphenyl]-5,6-dihydro-2-oxo-2H-pyran) 237, 24-methylene-5α-lanost-8-en-3β-ol 238, 24β-methyl-25-dehydrolophenol 239, codisterol 240, isofucosterol 241, 24-methylene-25-hydroxyphenol 242, avenasterol 243, psilotin 244 [368,369,370,371] |
| Pteridaceae | ||
| 21 | Acrostichum aureum L. | quercetin 3-O-β-d-glucoside 245, ponasterone A 246, lupeol 247, friedelin 196, β-sitosterol 11, stigmasterol 248, campesterol 249, tetracosanoic acid 250, ursolic acid 251, gallic acid 252, (2R,3S)-sulfated pterosin C 253, (2S,3S)-sulfated pterosin C 254, (2S,3S)-pterosin C 255, (2R)-pterosin P 256, patriscabratine 257, tetracosane 258, quercetin-3-O-β-d-glucoside 259, quercetin-3-O-β-d-glucosyl-(6→1)-α-l-rhamnoside 260, quercetin-3-O-α-l-rhamnoside 261, quercetin-3-O-α-l-rhamnosyl-7-O-β-d-glucoside 262, kaempferol 153 [35,372,373,374] |
| 22 | Selaginella involvens (P.Beauv.) Spring | hexadecanoic acid 263, stearic acid 264, β-sitosterol 11, stigmasterol 248, amentoflavone 265, β-d-glucopyranoside 266, (3β)-cholest-5-en-3yl 267, β-amyrin 198 [375] |
| Vittariaceae | ||
| 23 | Vittaria elongate Sw. | vittarin-A-F 268–273, 3-O-acetylniduloic acid 274, ethyl 3-O-acetylniduloate 275, methyl 4-O-coumaroylquinate 276, vittarilide-A, B 277, 278, vittariflavone 279, methyl 4-O-caffeoylquinate 280, ethyl 4-O-caffeoylquinate 281, methyl 5-O-caffeoylquinate 282, apigenin 132, vitexin 283, 5,7-dihydroxy-3′,4′,5′-trimethoxyflavone 284, amentoflavone 265, trans-p-coumaric acid 285, methyl trans-p-coumarate 286, methyl caffeate 287, ferulic acid 288, p-cresol 289, 4-hydroxybenzaldehyde 290, 4-hydroxybenzoic acid 204, methyl 4-hydroxybenzoate 291, protocatechualdehyde 226, protocatechuic acid 189, methyl protocatechuate 292, vanillin 293, vanillic acid 225 [119] |
| Non-Fern | ||
| Balsaminaceae | ||
| 24 | Impatiens niamniamensis Gilg (semi epiphytic) | α-N,N,N-trimethyltryptophan betaine 294 [129] |
| 25 | Convolvulaceace (parasite) | |
| 26 | Cassytha filiformis L. | N-(3,4-dimethoxyphenethyl)-4,5-methylenedioxy-2-nitrophenylacetamide 295, actinodaphnine 296, cassythine 297, isoboldine 298, cassameridine 299, cassamedine 300, lysicamine 301, cathafiline 302, cathaformine 303, actinodaphnine 304, N-methylactinodaphnine 305, cathafiline 306, cathaformine 307, predicentrine 308, ocoteine 309, filiformine 310, (+)-diasyringaresinol 311, cathafiline 312, cathaformine 313, actinodaphnine 314, N-methylactinodaphnine 315, predicentrine 308, ocoteine 316, neolitsine 317, dicentrine 318, cassythine (cassyfiline) 319, actinodaphnine 320, 4-O-methylbalanophonin 321, cassyformin 322, isofiliformine 323, cassythic acid 324, cassythic acid 324, cassythine 325, neolitsine 326, dicentrine 318, 1,2-methylenedioxy-3,10,11-trimethoxyaporphine 327, (-)-O-methylflavinatine 328, (-)-salutaridine 329, isohamnetin-3-O-β-glucoside 330, isohamnetin-3-O-rutinoside 331 [134,354,376,377,378,379,380] |
| 27 | Cuscuta australis R.Br. | 4-oic acid-7-oxo-kaurene-6α-O-β-d-glucoside 332, thymidine 333, caffeic acid 228, p-coumaric acid 334, caffeic-β-d-glucoside 335, kaempferol 153, quercetin 336, astragalin 337, hyperoside 338, astragalin 339, kaempferol 153, quercetin 336, β-sitosterol 11, β-sitosterol 3-O-β-D-xylopyranoside 340 [381,382,383] |
| 28 | Cuscuta reflexa Roxb. | coumarin 341, α-amyrin 342, β-amyrin 198, α-amyrin acetate 343, β-amyrin acetate 344, oleanolic acetate 345, oleanolic acid 127, stigmasterol 248, lupeol 247, stigmast-5-en-3-O-β-d-glucopyranoside tetraacetate 346, stigmast-5-en-3-O-β-d-glucopyranoside 347, stigmast-5-en-3-yl-acetate 348, β-sitosterol 11, 3,5,7,3′-pentahydroxyflavanone (taxifolin) 349, 3,5,7,4′-tetrahydroxyflavanone (aromadendrin) 350 [143,384,385] |
| Clusiaceae | ||
| 29 | Clusia grandiflora Splitg. (hemi epiphyte) | friedelin 196, β-amyrin 198, β-sitosterol 11, lupeol 247, chamone I 351, chamone II 352 [149,386] |
| Loganiaceae | ||
| 30 | Fagraea auriculata Jack. (semi epiphyte) | di-O-methylcrenatin 353, potalioside B 354, adoxosidic acid 355, adoxoside 356, (þ)-pinoresinol 357, salicifoliol 358 [153] |
| Loranthaceae (parasite) | ||
| 31 | Dendrophthoe falcata (L.f.)Ettingsh | 3β-acetoxy-1β-(2-hydroxy-2-propoxy)-11α-hydroxy-olean-12-ene 359, 3β-acetoxy-11α-ethoxy-1β-hydroxy-olean-12-ene 360, 3β-acetoxy-1β-hydroxy-11α-methoxy-olean-12-ene 361, 3β-acetoxy-1β,11α-dihydroxy-olean-12-ene 362, 3β-acetoxy-1β,11α-dihydroxy-urs-12-ene 363, 3β-acetoxy-urs-12-ene-11-one 364, 3β-acetoxy-lup-20(29)-ene 365, 30-nor-lup-3β-acetoxy-20-one 366, (20S)-3β-acetoxy-lupan-29-oic acid 367, kaempferol-3-O-α-l-rhamnopyranoside 368, quercetin-3-O-α-l-rhamnopyranoside 369, gallic acid 252 [387] |
| 32 | Loranthus globosus Roxb | (+)-catechin 168, 3,4-dimethoxycinnamyl alcohol 370, 3,4,5-trimethoxycinnamylalcohol 371 [163] |
| 33 | Macrosolen cochinchinensis (Lour.) Tiegh. | quercetin 336, gallic acid 252, orientin 372, rutin 373, quercetin-3-O-apiosyl(1→2)-[rhamnosyl(1→6)]-glucoside 374, vicenin 375 [388] |
| 34 | Scurrula atropurpurea (Blume) Danser | octadeca-8,10,12-triynoic acid 376, hexadec-8-ynoic acid 377, hexadec-10-ynoic acid 378, hexadeca-8,10-diynoic acid 379, hexadeca-6,8,10-triynoic acid 380, hexadeca-8,10,12-triynoic acid 381, (Z)-9-octadecenoic acid 382, (Z,Z)-octadeca-9,12-dienoic acid 383, (Z,Z,Z)-octadeca-9,12,15-trienoicacid 384, octadeca-8,10-diynoic acid 385, (Z)-octadec-12-ene-8,10-diynoic acid 386, octadeca-8,10,12-triynoic acid 376, theobromine 387, caffeine 388, quercitrin 389, rutin 373, icariside B2 390, aviculin 391, (+)-catechin 168, (-)-epicatechin 144, (-)-epicatechin-3-O-gallate 392, (-)-epigallocatechin-3-O-gallate 393 [169,170] |
| 35 | Scurrula ferruginea (Jack) Danser | glycoside 4′-O-acetyl-quercitrin 394 [389] |
| 36 | Scurrula parasitica L. | (+)-catechin 168 [178] |
| Moraceae | ||
| 37 | Ficus pumila L. | (1S,4S,5R,6R,7S,10S)-1,4,6-trihydroxyeudesmane 6-O-β-d-glucopyranoside 39, (1S,4S,5S,6R,7R,10S)-1,4-dihydroxymaaliane 1-O-β-d-glucopyranoside 396, (23Z)-3β-acetoxycycloart-23-en-25-ol 39, (23Z)-3β-acetoxyeupha-7,23-dien-25-ol 39, (24RS)-3β-acetoxycycloart-25-en-24-ol 39, (24S)-24-hydroxystigmast-4-en-3-one 400, (24S)-stigmast-5-ene-3β,24-diol 401, 10α,11-dihydroxycadin-4-ene 11-O-β-d-glucopyranoside 402, 3β-acetoxy-(20R,22E,24RS)-20,24-dimethoxydammaran-22-en-25-ol 403, 3β-acetoxy-(20S,22E,24RS)-20,24-dimethoxydammaran-22-en-25-ol 404, 3β-acetoxy-20,21,22,23,24,25,26,27-octanordammaran-17β-ol 405, 3β-acetoxy-22,23,24,25,26,27-hexanordammaran-20-one 406, cycloartane-type triterpenoids 407, triterpenoid 408 [390,391,392] |
| Orchidaceae | ||
| 38 | Anoectochilus formosanus Hayata | (6R,9S)-9-hydroxy-megastigma-4,7-dien-3-one-9-O-β-d-glucopyranoside 409, (R)-(+)-3,4-dihydroxybutanoic acid γ-lactone 410, 1-O-isopropyl-β-d-glucopyranoside 411, 2-(β-d-glucopyranosyloxymethyl)-5-hydroxymethylfuran 412, 3-(R)-3-β-d-glucopyranosyloxy-4-hydroxybutanoic acid 413, 3-(R)-3-β-d-glucopyranosyloxybutanolide (kinsenoside) 414, 4-(β-d-glucopyranosyloxy)benzyl alcohol 415, corchoionoside C 416 [393] |
| 39 | Anoectochilus roxburghii (Blume) | 24ξ-isopropenylcholesterol 417, 5-hydroxy-3′,4′,7-trimethoxyflavonol-3-O-β-D-rutinoside 418, 7-O-β-D-diglucoside 419, 8-C-β-hydroxybenzylquercetin 420, 8-p-hydroxybenzyl quercetin, 421, anoectosterol 422, campesterol 249, cirsilineol 423, daucosterol 134, ferulic acid 288, isorhamnetin 424, isorhamnetin-3 425, isorhamnetin-3, 4′-O-β-d-diglucoside 426, isorhamnetin-3-O-β-D-rutinoside 427, isorhamnetin-7-O-β-d-glucopyranoside 428, isorhamnetin-7-O-β-d-diglucoside 429, kaempferol-3-O-β-d-glucopyranoside 430, kaempferol-7-O-β-d-glucopyranoside 431, p-coumaric acid 334, p-hydroxybenzaldehyde 432, quercetin 336, quercetin 3′-O-β-d-glucopyranoside 433, quercetin 3-O-β-d-glucopyranoside 434, quercetin 3-O-β-d-rutinoside 435, quercetin 7-O-β-glucoside 436, quercetin-7-O-β-D-[6′-O-(trans-feruloyl)]-glucopyranoside 437, sitosterol 438, stigmasterol 248, succinic acid 439, 3′,4′,7-trimethoxy-3,5-dihydroxyflavone 440, 3-methoxyl-p-hydroxybenzaldehyde 441, daucosterol 134, daucosterol 134, ferulic acid 288, isorhamnetin-3-O-β-d-glucopyranoside 442, isorhamnetin-3-O-β-D-rutinoside 443, lanosterol 444, methy1 4-β-d-glucopyranosyl-butanoate 445, o-hydroxy phenol 446, oleanolic acid 127, palmitic acid 447, p-hydroxy benzaldehyde 448, p-hydroxy cinnamic acid 449, p-hydroxybenzaldehyde 432, rutin 373, sorghumol 3-O-E-p-coumarate 450, sorghumol 3-O-Z-p-coumarate 451, stearic acid 264, succinic acid 452, β-D-glucopyranosyl-(3R)-hydroxybutanolide 453, β-sitosterol 11 [394,395,396,397,398,399,400,401,402] |
| 40 | Bulbophyllum kwangtungense Schltr. | 10,11-dihydro-2,7-dimethoxy-3,4-methylenedioxydibenzo[b,f]oxepine 454, 5-(2,3-dimethoxyphenethyl)-6-methylbenzo[d][1,3]dioxole 455, 7,8-dihydro-3-hydroxy-12,13-methylenedioxy-11-methoxyldibenz[b,f]oxepin 456, 7,8-dihydro-4-hydroxy-12,13-methylenedioxy-11-methoxyldibenz[b,f]oxepin 457, 7,8-dihydro-5-hydroxy-12,13-methylenedioxy-11-methoxyldibenz [b,f]oxepin, 458, cumulatin 459, densiflorol A 460, plicatol B 461 [219,403] |
| 41 | Bulbophyllum odoratissimum (Sm.) Lindl. ex Wall. | (+)-lyoniresinol-3a-O-β-d-glucopyranoside 462, 3,5-dimethoxyphenethyl alcohol 463, 3,7-dihydroxy-2,4,6-trimethoxyphenanthren 464, 3-hydroxyphenethyl 4-O-(6′- O-β-apiofuranosyl)-β-d-glucopyranoside 465, 3-methoxy-4-hydroxycinnamic aldehyde 466, 3-methoxyphenethyl alc. 4-O-β-D-glucopynanoside 467, 4-hydroxy-3,5-dimethoxybenzaldehyde 468, 4-O-β-d-glucopynanoside 469, 7-hydroxy-2,3,4-trimethoxy-9,10-dihydrophenanthrene 470, batatasin III 471, Bulbophyllanthrone 472, bulbophythrins A, B 473, 474, Coelonin 475, densiflorol B 476, ethyl orsellinat 477, gigantol 478, moscatin 479, p-hydroxyphenylpropionic acid 480, p-hydroxyphenylpropionic methyl ester 481, syringaldehyde 482, syringin 483, tristin 484, vanillic acid 225 [223,224,404,405,406,407] |
| 42 | Bulbophyllum vaginatum (Lindl.) Rchb.f. | (±)-syringaresinol 485, (2R*,3S*)-3-hydroxymethyl-9-methoxy-2-(4′-hydroxy-3′,5′-dimethoxyphenyl)-2,3,6,7-tetrahydrophenanthro [4,3-b]furan-5,11-diol 486, 2,4-dimethoxyphenanthrene-3,7-diol 487, 3,4,6-trimethenanthrene-2,7-diol 488, 3,4,6-trimethoxy-9,10- dihydrophenanthrene-2,7-diol 489, 3,4′,5-trihydroxy-3′-methoxybibenzyl (tristin) 490, 3,4′-dihydroxy-5,5′-dimethoxybibenzyl 491, 3,4-dihydroxybenzoic acid 200, 3,4-dimethoxy-9,10- dihydrophenanthrene-2,7-diol (erianthridin) 492, 3,4-dimethoxyphenanthrene-2,7-diol (nudol) 493, 3,5-di- methoxy-9,10-dihydrophenanthrene-2,7-diol (6- methoxycoelonin) 494, 3,5-dimeth- oxyphenanthrene-2,7-diol 495, 3′-dihydroxy-5-methoxybibenzyl 496, 4,4′,6,6′-tetramethoxy-[1,1′-biphenanthrene]-2,2′,3,3′,7,7′-hexol 497, 4,6-dimethoxy-9,10-di- hydrophenanthrene-2,3,7-triol 498, 4,6-dimethoxyphenanthrene-2,3,7-triol 499, 4-methoxy-9,10- dihydrophenanthrene-2,7-diol (coelonin) 500, 4-methoxyphenan- threne-2,7-diol (flavanthrinin) 501, 4-methoxyphenanthrene- 2,3,5-triol (fimbriol B) 502, 9,10- dihydrophenanthrenes 503, dihydroferulic acid 504, Friedelin 196, p-coumaric acid, 334 [36,408,409] |
| 43 | Catasetum barbatum (Lindl.) Lindl. | 2,7-dihydroxy-3,4,8-trimethoxyphenanthrene 505 [225] |
| 44 | Cymbidium aloifolium (L.) Sw. | aloifol I 506, aloifol II 507, 6-O-methylcoelonin 508, batatasin III 471, coelonin 475, gigantol, 478, 1-(4′-hydroxy-3′,5′-dimethoxyphenyl)-2-(3″-hydroxyphenyl)ethane 509, 1-(4′-hydroxy-3′,5′-dimethoxyphenyl)-2-(4″-hydroxy-3″-methoxyphenyl)ethane 510, 2,7-dihydroxy-4,6-dimethoxy-9,10-dihydrophenanthrene 511, cymbinodin-A 512, cymbinodin B 513 [410,411,412] |
| 45 | Cymbidium goeringii (Rchb.f.) Rchb.f. | β-sitosterol 11, daucosterol 134, ergosterol 514, gigantol 478, cymbidine A 515 [229,230,413] |
| 46 | Dendrobium amoenum Wall. ex Lindl. | amotin 516, amoenin 517, amoenumin 518, amoenylin, isoamoenylin 519, 3,4′-dihydroxy-5-methoxybibenzyl, 520, 4,4′-dihydroxy-3,3′,5-trimethoxybibenzyl (moscatilin) 521 [414,415,416] |
| 47 | Dendrobium chryseum Rolfe | araxerol 522, coumarin 341, moscatilin 523, chrysotobibenzyl 524, chrysotoxin 525, gigantol 478, kaempferol 153, cis-melilotoside 526, defuscin 527, dendroflorin 528, dengibsin 529, dihydromelilotoside 530, naringenin 147, n-octacosyl ferulate 531, trans-melilotoside 532 [233,417] |
| 48 | Dendrobium candidum Wall. Ex Lindl. | (-)-loliolide 533, (-)-secoisolariciresinol 534, (-)syringaresinol 535, (+)-lyoniresinol-3a-O-β-d-glucopyranoside 462, (+)-syringaresinol-4-β-d-monoglucoside 536, (1′R)-1′-(4-hydroxy-3,5-dimethoxylphenyl) propan-1′-ol 4-O-β-d-glucopyranoside 537, (E)-p-Hydroxycinnamic acid 538, 2,4,7-trihydroxy-9,10-dihydrophenanthrene 539, 2-methoxyphenol-O-β-d-apiofuromosyl-(1→2)-β-d-glucopyranoside 540, 3,4-dihydroxy-5,4′-dimethoxybibenzyl 541, 3-O-methylgigantol 542, 4,4′-dihydroxy-3,5-dimethoxybibenzyl 543, 4′,5-dihydroxy-3,3′-dimethoxybibenzyl 544, 4-allyl-2,6-dimethoxyphenylglucoside 545, 4′-dihydroxy-5-methoxybibenzyl 546, 5-hydroxymethyl-furaldehyde 547, Adenosine 548, Aduncin 549, cis-feruloyl-p-hydroxybenzenethylamine 550, coniferyl alcohol 551, daucosterol 134, defuscin 527, denbinobin, 552, dendrocandin A 553, dendrocandin B 554, dendrocandin C 555, dendrocandin D 556, dendrocandin E 557, dendrocandins F—I 558–561, dendromoniliside E 562, dendrophenol 563, dihydroresveratrol 564, gigantol 478, guanosine 565, hentriacontane 8, heptadecanoic acid 566, hexadecanoic acid 263, icariol A 2-4-O-β-d-glucopyranoside 567, khaephuouside 568, leonuriside A 569, naringenin 147, n-octacosyl ferulate 531, N-trans-feruloyl tyramine 570, n-triacontyl cis-p-coumarate 571, p-hydroxy-phenylpropionic acid 480, sucrose 232, syringaresinol 572, syringaresinol-4,4′-O-bis-β-d-glucoside 573, trans-cinnamoyl-p-hydroxybenzenethylamine 574, uridine 575, vanillyl alcohol 576, β-sitosterol 11 [237,238,239,418,419,420] |
| 49 | Dendrobium chrysanthum Wall. ex Lindl. | (2S)-N-cis-cinnamoyl-2-oxopropyrrolidine 577, (2S)-N-trans-cinnamoyl-2-oxopropyrrolidine 578, (þ)-lyoniresinol 579, 2,5-dihydroxy-4,9-dimethoxylphenanthrene 580, 4,4′-dihydroxy-3,3′,5-trimethoxybibenzyl 581, 7,70-bis-(4-hydroxy-3,5-dimethoxyphenyl)-8,80-dihydroxymethyl-tetrahydrofuran-4-β-d-glucoside 582, chrysophanol 583, chrysotobibenzyl 524, chrysotobibenzyl 524, chrysotoxin 525, crepidatin 584, crepidatin 584, dehydrodiconiferyl alcohol-4-β-d-glucoside 585, denchrysans A, B 586, 587, denchryside A 588, denchryside B 589, dendrochrysanene 590, dendroflorin 528, dengibsin 529, dengibsin 529, emodin 591, gigantol 478, moscatilin 523, moscatilin 523, moscatin 479, physcion 592, β-sitosterol 11 [226,417,421,422,423,424] |
| 50 | Dendrobium fimbriatum Hook. | 2-hydroxyethyl caffeate 593, ayapin 594, chrysophanol 583, chrysotobibenzyl (I) 595, confusarin 596, crepidatin 584, defuscin 527, denhydroshizukanolide 597, fimbriatone 598, n-dotriacontanoic acid 599, n-octacosyl ferulate 531, n-triacontyl cis-p-coumarate 571, physcion 592, rhein 600, scopolin methyl ether 601, β-sitosterol 11 [425,426] |
| 51 | Dendrobium loddigesii Rolfe | dendrophenol (4,4′-dihydroxy-3,3′,5-trimethoxybibenzyl) 563, loddigesiinols A-D 602-605, moscatilin 523, moscatilin diacetate 606, moscatin 479, shihunidine 607, shihunine 608, stilbenes 609 [250,251,252] |
| 52 | Dendrobium moniliforme (L.) Sw. | heptacosane 610, 3,4-dihydroxy-4′,5-dimethoxy bibenzyl 611, 3,4-dihydroxy-5,4′-dimethoxy bibenzyl 612, 4-methoxybenzaldehyde 613, a known alkaloid 6-hydroxynobiline 614, alkyl 4′-hydroxy-cis-cinnamates 615, alkyl ferulates 616, daucosterol 134, denbinobin 552, denbinobin, alkyl 4′-hydroxy-trans-cinnamates 617, dendromoniliside E 562, ethyl linolenates 618, heptatriaconsanoic acid 619, linoleic acid 620, methyl linolenates 621, moniliformin 622, moniline 623, n-nonacosane 624, n-octacosyl ferulate 531, n-triacontyl p-hydroxy-cis-cinnamate 625, octacosanyl hexadecanoate 626, phytosterols 627, stigmast-4-en-3-one 628, vanillin 293, α-dihydropicrotoxinin 629, β-sitosterol 11 [255,427,428,429,430,431] |
| 53 | Dendrobium moschatum (Buch.-Ham) S.w | moscatin 479, moscatilin 523 [432,433] |
| 54 | Dendrobium nobile Lindl. | 10,12-dihydroxypicrotoxane 630, 10β,13,14-trihydroxyalloaromadendrane 631, 3,4,8-trimethoxyphenanthrene-2,5-diol 632, 3,4′-dihydroxy-5,5′-dimethoxydihydrostilbene 633, 3-O-methylgigantol 542, 5,7-dimethoxyphenanthrene-2,6-diol 634, 6-hydroxy-dendrobine (dendramine) 635, 6-hydroxy-dendroxine 636, 6α,10,12-trihydroxypicrotoxane 637, 7,12-dihydroxy-5-hydroxymethyl-11-isopropyl-6-methyl-9-oxatricyclo [6.2.1.02,6]undecan-10-one-15-O-β-d-glucopyranoside 638, batatasin III 471, bullatantirol 639, chrysotobibenzyl 524, coelonin 475, crepidatin 584, denbinobin 552, dendrobane A 640, dendrobin A,7 chrysotoxine 641, dendrobine 642, dendrobiumane 643, dendrodensiflorol, 644, dendroflorin 528, dendronobilin A-I 645–653, dendronobilin J 654, dendronobiline A 655, dendronobilosides A, B 656, 657, dendronophenol A-B 658, 659, dendroside A 660, dendroside E-G 661–663, dendroxineo 664, ephemeranthol A 665, epheneranthol C 666, erianthridin 667, fimbriol-B 668, flavanthridin 669, gigantol 478, hircinol 670, lusianthridin 671, moscatilin 523, moscatilin 523, moscatin, 479, gigantol 478, nobilin D-E 672, 673, nobilone 674, nobilonine 675, stigmasterol 248, β-sitosterol 11, β-sitosterol glucoside 12 [38,261,262,263,264,267,433,434,435,436,437,438] |
| 55 | Epidendrum strobiliferum Rchb.f. | 24-methylenecycloartanol 676, campesterol 249, pholidotin 677, stigmasterol 248, β-sitosterol 11 [272] |
| 56 | Epidendrum rigidum Jacq. | 2,3-dimethoxy-9,10-dihydrophenathrene-4,7-diol 678, 24-methyl-9,19-cyclolanostane-25-en-3β-ol 679, 3,4,9-trimethoxyphenanthrene-2,5-diol 680, apigenin 132, batatasin III 471, gigantol 478, isovitexin 681, stilbenoids I-IV 682–685, triterterpenoids 24,24-dimethyl-9,19-cyclolanostane-25-en-3β-ol 686, vitexin 283 [274] |
| 57 | Mycaranthes pannea (Lindl.) S.C.Chen & J.J.Wood | Acervatol 687, acervatone 688, flavanthridin 669, flavanthrinin 689 [276] |
| 58 | Camaridium densum (Lindl.) M.A.Blanco | 2,5-dihydroxy-3,4-dimethoxyphenanthrene 690, 2,5-dihydroxy-3,4-dimethoxyphenanthrene 690, 9,10-dihydro-2,5-dihydroxy-3,4-dimethoxyphenanthrene 691, 9,10-dihydro-2,7-dihydroxy-3,4-dimethoxyphenanthrene 692, erianthridin 667, fimbriol-A 693, gymnopusin 694, nudol 695 [37,439] |
| 59 | Nidema boothii (Lindl.) Schltr. | 1,5,7-trimethoxy-9,10-dihydrophenanthrene-2,6-diol, 696, 1,5,7-trimethoxyphenanthrene-2,6-diol 697, 2,4-dimethoxyphenanthrene-3,7-diol 488, 9,19-cyclolanosta-24,24-dimethyl-25-en-3β-yl trans-p-hydroxycinnamate 698, aloifol II 507, batatasin III 471, ephemeranthol B 699, ephemeranthoquinone 700, gigantol 478, lusianthridin 671, nidemin 701, nidemone 702 [282,440] |
| 60 | Pholidota articulata Lindl. | 2,7-dihydroxy-3,4,6-trimethoxyla 9, 10-dihydrophenanthrene flavidin 703, 2,7-dihydroxyll-methoxy-9,10-dihydrophenanthrene (coelonin) 704, 9, 10-dihydrophenanthrenes 705, coelogin 706, coeloginin 707, flavidin 708, flavidinin 709, oxoflavidinin 710 [441] |
| 61 | Pholidota chinensis Lindl. | (E)-2′,3,3′-trihydroxy-5-methoxystilbene (pholidotol C) 711, (Z)-3,3′-hydroxy-5-methoxystilbene (pholidotol D) 712, 2,4,7-trihydroxy-9,10-dihydrophenanthrene 539, 2,5-dimethoxy-3,4,3′,4′-bis(dimethylenedioxy)bibenzyl 713, 3,4′-dihydroxy-3′,5-dimethoxybibenzyl 714, 3,4-dihydroxy-4-methoxydihydrostilbene 715, 4,4′-dihydroxydiphenylmethane 716, 4,5-dihydroxy-2-methoxy-9,10-dihydrophenanthrene 717, 5,3′-dihydroxy-2,3-(methylenedioxy)bibenzyl 718, 9,10-dihydro-2,4-dihydroxy-7-methoxyphenanthrene 719, batatasin III 471, blestrianol A 720, blestrin A 721, bulbophylol B 722, cannabidihydrophenanthrene 723, coelonin 475, coelonin 475, cyclopholidone 724, cyclopholidone 724, cyclopholidonol 725, cyclopholidonol 725, erianthridin 667, eulophiol 726, flavanthrin 727, flavanthrin 727, gymconpin C 728, hircinol 670, lusianthridin 671, lusianthridin, 671, phochinenins A – F 729–734, phochinenins G-L 735–740, pholidotols A-B 741, 742, 3,4-dihydroxy-5-methoxydihydrostilbene 743, phoyunnanin D 744, p-hydroxybenzaldehyde 432, p-hydroxybenzyl alcohol 745, protocatechuic aldehyde 746, resveratrol 747, thunalbene 748, thunalbene 749, trans-3-3-dihydroxy-2,5-dimthoxystilbene 750, trans-3-hydroxy-2,3,5-trimthoxystilbene 751, β-daucosterol 752 [285,286,442,443,444,445] |
| 62 | Scaphyglottis livida (Lindl.) Schltr. | 24,24,dimethyl-9,19-cyclolanosta-9(11),25-dien-3-one (cyclobalanone) 753, 3,4′-dihydroxy-3′,4,5-trimetoxybibenzyl 754, 3,4′-dihydroxy-3′,5-dimethoxybibenzyl 714, 3,7-dihydroxy-2,4,8-trimethoxyphenanthrene 755, 3,7-dihydroxy-2,4-dimethoxyphenanthrene 756, 5α-lanosta-24,24-dimethyl-9(11),25-dien-3β-ol 757, batatasin III 471, coelonin 475, gigantol 478, nidemin 701 [287,288,440] |
| 63 | Papilionanthe teres (Roxb.) Schltr. | eucomic acid 758, vandaterosides I-III 759–761 [295] |
| 64 | Vanda tessellate (Roxb.) Hook. ex G. Don. | Oxotessallatin 762 [446] |
| Piperaceae | ||
| 65 | Peperomia galioides Kunth | (+)-epi-α-bisabolol 763, galopiperone 764, grifolic acid 765, grifolin 766, hydropiperone 767, piperogalin 768, piperogalone 769 [447,448,449] |
| 66 | Piper retrofractum Vahl | 28-methylnonacos-27-en-1-oic acid 770, 3-methyl-5-decanoylpyridine 771, caffeic acid 228, di-methyl 3,4-bis(4-hydroxyphenyl)-1,2-cyclobutanedicarboxylate 772, esculetin 773, methyl piperate 774, N-isobutyleicosa-2,4-dienamide 775, p-coumaric acid 334, pipereicosalidine 776, piperine 777, piperine 777, pipernonaline 778, piperoctadecalidine 779, retrofractamide-D 780, retrofractamides A, C 781, 782, uracil 783, uridine 575, vitexin 283, vitexin 2′-O-β-glucopyranoside 784, β-d-glucopyranoside 266, β-sitosterol 11 [301,306,450,451,452,453] |
| Rubiaceae | ||
| 67 | Hydnophytum formicarum Jack | 4-aminophenyl acetate 785, 7,3′,5′-trihydroxyflavone 786, butein 787, butin 788, Isoliquiritigenin 789, protocatechualdehyde 226, stigmast-4-en-3-one 628, stigmasterol 248, β-sitosterol 11 [313,361] |
| Viscaceae | ||
| 68 | Viscum articulatum Burm.f. | (2S)-5,3,4-trihydroxyflavanone 7-O-β-d-glucoside 790, (2S)-homoeriodictyol 791, (2S)-homoeriodictyol 7-O-β-d-glucoside 792, (2S)-naringenin 7-O-β-d-glucoside 793, (2S)-pinocembrin 7-O-[cinnamoyl(1→5)-β-d-apiosyl(1→2)]-β-d-glucoside 794, (2S)-pinocembrin 7-O-[β-d-apiosyl(1→2)]-β-d-glucoside (1) 795, (2S)-pinocembrin 7-O-β-d-glucoside 796, (4′-hydroxy-2′,3′,6′,3′′-tetramethoxy-1,3-diphenylpropane)-4′′-O-β-d-glucopyranoside 797, 1-O-benzyl-[5-O-benzoyl-β-Dapiofuranosyl(1→2)]-β-d-glucopyranoside 798, 2-deoxy-epi-inositol 799, 2-phenylethanol 800, 4-β-d-glucosyloxy-3-hydroxy-benzoic acid 801, 4′-hydroxy-7,3′-dimethoxyflavan-5-O-β-d-glucopyranoside 802, 4-O-cinnamoyl quinic acid 803, 5,3′,4′-trihydroxyflavanone-7-O-β-d-glucopyranoside 804, 5,4′-dihydroxyflavanone-7-O-β-d-lucopyranoside 805, 7-O-β-d-glucopyranoside 806, botulin 807, betulin 808, betulinic acid 809, cinnamic acid methyl ester 810, diphenylpropane glycoside 811, eriodictyol 7-O-β-d-glucopyranoside 812, homoeriodictyol 7-O-β-d-glucopyranoside 813, homoeriodictyol-7-O-β-d-glucopyranoside 814, homoeriodictyol-7-O-β-d-glucopyranoside-4′-O-β-d-(5′′′-cinnamoyl)apiofuranoside 815, homoeriodictyol-7-O-β-d-glucopyranoside-4′-O-β-d-apiofuranoside 816, lupenyl acetate 817, lupeol 247, lupeol acetate 818, lupeol palmitate 819, lupeol stearate 820, lycorin 821, methylparaben 822, naringenin 7-O-β-d-glucopyranoside 823, Oleanolic acid 127, p-hydroxybenzaldehyde 432, p-hydroxy-benzoic acid 824, pinocembrin 825, pinocembrin 7-O-β-d-glucopyranoside 826, pinocembrin-7-O-[cinnamoyl (1→5)-β-d-apiofuranosyl (1→2)]-β-d-glucopyranoside 827, pinocembrin-7-O-β-d-apio furanosyl(1→2)-β-d-glucopyranoside 828, pinocembrin-7-O-β-d-apiofuranosyl-(1→5)-β-d-apiofuranosyl-(1→2)-β-d-glucopyranoside 829, protocatechuic acid 189, vanillin 293, visartisides A-C 830, 831, 832, visartisides D-F (4–6) 833, 834, 835, viscumitol 836, α-amyrin 342, β-amyrin acetate 837, β-sitosterol 11 [319,320,321,322,323,454,455,456] |
| 69 | Viscum ovalifolium DC | 3-O-α-l-arabinopyranoyl-hederagenin-28-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside 838, gypsogenic acid 839, hederagenin 840, hederagenin-3-O-α-l-arabinopyranoside 841, hederagenin-3-O-α-l-arabinopyranoyl-(2→1)-O-β-d-glucopyranoside 842, lupeol acetate 818, lupeol palmitate 819, oleanolic acid 127, lupeol stearate 820, β-amyrin 198, β-amyrin acetate 344 [457,458] |
Therefore, while epiphytes may have limitations in accessing nutrients, adaptation has enabled them to successfully survive these environments. Studies on numerous medicinal epiphytes show that the unique environment does not constrain the plants from producing different types of secondary metabolites. These include terpenes, flavonoids, and alkaloids, especially the non-fern epiphytic medicinal plants.
4. Pharmacological Activities of Vascular Epiphytic Medicinal Plants
The pharmacological activities of medicinal epiphytes are summarised in Table 1, including the plant species, ethnopharmacological indication, and pharmacological test results. The ethnopharmacological uses of each plant are also present for a correlation and comparison with the pharmacological activities. There are a large number of phytochemical studies on the four fern-epiphytes (Stenochlaena palustris (Burm. F.) Bedd., Botrychum lanuginosum Wall.ex Hook & Grev., Pyrrosia petiolosa (Christ) Ching, Psilotum nudum (L.) P. Beauv) without any biological activity testing reported. This occurred to four non-fern epiphytes (Bulbophyllum vaginatum (Lindl.) Rchb.f, Mycaranthes pannea (Lindl.) S.C.Chen & J.J.Wood, Pholidota articulata Lindl., Viscum ovalifolium DC) and non-fern epiphytic medicinal plants. This lack of pharmacological testing limits scientific support for the traditional uses of these plants.
From the 191 collected records of epiphytic medicinal plants, around 71 species were subjected to bioactivity testing, with 25 of these species using crude extract samples. Although this testing represents almost 50% of the species examined, only a few of the pharmacological tests were related to ethnopharmacological claims. Here, we discuss selected species where the outcomes indicated a coherent relationship between bioactivities and traditional claims.
4.1. Infectious Disease Therapy
Research on epiphytes that have been used in infectious disease therapy include in wound healing, dysentery, and skin infections. A study on the methanol extract of Adiantum caudatum L., Mant showed anti-fungal activity against common fungi found in wounds (Aspergilus and Candida species) [39], including Aspergillus flavus, A. spinulosus, A. nidulans, and Candida albicans, with minimum inhibitory concentration (MIC) values of 15.6, 15.6, 31.2, and 3.9 µg/mL, respectively. Gallic acid was one of the bioactive constituents [40]. The methanol extract of Ficus natalensis Hochst (a semi-epiphytic plant) showed anti-malarial activity against Plasmodium falciparum, with an half maximal inhibitory concentration (IC50) value of 41.7 µg/mL, and weak bactericidal activity against Staphylococcus aureus, with an MIC value of 99 µg/mL [41]. These results became preliminary data for confirming its traditional uses as malarial fever therapy and wound healing. Phytochemical studies on Pyrrosia sheareri (Bak.) Ching successfully isolated several compounds and were subjected to anti-oxidant testing. While this was not in line with the plant’s ethnomedical uses for dysentery therapy [42], one of the isolated constituents was protocateuchic acid, which is known to possess anti-bacterial activity. It implies that the traditional uses of the epiphyte was for bacillary dysentery therapy.
4.2. Non-Infectious/Degenerative Disease-Related Therapy
An exploration on Drynaria species, highly prescribed in bone fracture therapy, successfully isolated flavonoid constituents that induce osteoblast proliferation [43]. Previous studies on Acrostichum aureum Limme failed to show its anti-bacterial activities [44] contrary to its traditional claims in wound management. However, patriscabratine 257 was isolated from the defatted methanol extract of whole plant of A. aureum, and subsequent testing showed it possessed anti-cancer activity in gastric cells and this supprted the traditional use of the plant in peptic ulcer therapy [35]. A decoction from the epiphyte Ficus deltoida has been used to treat diabetes. A study on the hot aqueous extract of this plant revealed anti-hyperglycemic activity by stimulating insulin secretion up to seven-fold. Furthermore, its activity mechanism was related to both the K+ATP-dependant and -non-dependant insulin secretion pathway [45]. However, further studies are required to identify the constituents responsible for the anti-hyperglycaemic activity.
The Indigenous people of Paraguay have used Catasetum barbatum Lindley to topically treat inflammation. Four bioactive compounds were isolated from this species and 2,7-dihydroxy-3,4,8-trimethoxyphenanthrene (confusarin) 595 showed the highest anti-inflammatory activity [46]. The study also revealed the compound to be a non-competitive inhibitor of the H1-receptor.
From the polypodiaceae family, the rhizome of Phymatodes scolopendria (burm.) Ching has been used to treat respiratory disorders. A bioassay-guided phytochemical study on Phymatodes scolopendria (Burm. f.) Pic. Serm. isolated 1,2-benzopyrone (coumarin) 209 as a bronchodilator [47].
5. Epiphytic Plant–Host Interactions on Secondary Metabolite Tapping
Secondary metabolite tapping has been an interesting study to reveal the molecular interactions between epiphytes and their host. This interaction was more visible when a physical channel between the two were developed. This channel (haustorium) made an epiphytic plant act as a parasite that enabled the plant to harvest molecular components from the host plant. A study on Scurulla oortiana (Korth.) Danser growth in three different host species (Citrus maxima, Persea Americana, and Camellia sinensis) identified three secondary metabolites (quercitrin, isoquercitrin, and rutin) in the S. oortiana (Korth.) Danser epiphyte growing on the three hosts [48]. Interestingly, extensive chromatographic and spectroscopic studies discovered that the flavonoids found in the S. oortiana (Korth.) Danser were independent of the host plants [48]. Secondary metabolite production in a host plant can also be triggered by the existence of a parasite, as discussed in a study on Tapirira guianensis infested by Phoradendron perrottetii, in which infested branches produced more tannin compare to non-infested branches, with infestation inducing a systemic response [48].
6. Conclusions
Epiphytes are the most beautiful vascular plants and contain interesting phytochemicals and possess exciting pharmacological activities. An analysis of the literature revealed 185 epiphytes that are used in traditional medicine, in which phytochemical studies identified a total of 842 secondary metabolites. Only 71 epiphytic medicinal plants were studied for their pharmacological activities and showed promising pharmacological activities, including anti-inflammatory, antimicrobial, and anticancer. Several species were not investigated for their activities and are worthy of exploration, including epiphytes from the Araceae (P. fragantissimum), Aralliaceae (S. caudata, S. elliptica, S. elliptifoliola, S. oxyphylla, S. simulans), and Asclepidaceae (Asclopidae sp., D. acuminate, D. benghalensis, D. imbricate, D. major, D. nummularia, D. platyphylla, D. purpurea, Toxocarpus sp) families, in which no phytochemical and pharmacological studies had been reported. These species have been used by Indigenous populations to treat both degenerative and nondegenerative diseases. It is known that there are examples of Indigenous populations living in protected forest reserves (e.g., in Indonesia) where epiphytes are used in their medicine, e.g., some species of Dischidia are used to treat fever, eczema, herpes etc.; these plants have not yet been studied. Therefore, the possibility of responsible bioprospecting exists (in compliance with the Nagoya protocol), which would be invaluable in biodiscovery knowledge as well as in mutual benefit sharing agreements.
Acknowledgments
ASN thanks to University of Jember and University of Wollongong for research support. Authors thank to Frank Zich (Australian Tropical Herbarium & National Research Collections Australia) for providing taxonomy consultation.
Author Contributions
Conceptualization, A.S.N., P.W., P.A.K.; data curation and analysis, A.S.N.; making and editing of the figures, A.S.N.; writing—original draft preparation, A.S.N., P.W., P.A.K.; writing—review and editing, A.S.N., B.T., P.W., P.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Benzing D.H. Vascular Epiphytes: General Biology and Related Biota. Cambridge University Press; Cambridge, UK: 1990. [Google Scholar]
- 2.Asakawa Y., Ludwiczuk A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2018;81:641–660. doi: 10.1021/acs.jnatprod.6b01046. [DOI] [PubMed] [Google Scholar]
- 3.Asakawa Y., Ludwiczuk A., Nagashima F. Phytochemical and biological studies of bryophytes. Phytochemistry. 2013;91:52–80. doi: 10.1016/j.phytochem.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 4.Ludwiczuk A., Asakawa Y. Bryophytes as a source of bioactive volatile terpenoids—A review. Food Chem. Toxicol. 2019;132:110649. doi: 10.1016/j.fct.2019.110649. [DOI] [PubMed] [Google Scholar]
- 5.Sabovljevic M.S., Sabovljevic A.D., Ikram N.K.K., Peramuna A., Bae H., Simonsen H.T. Bryophytes—An emerging source for herbal remedies and chemical production. Plant Genet. Resour. 2016;14:314–327. doi: 10.1017/S1479262116000320. [DOI] [Google Scholar]
- 6.Basnet B.B., Liu H., Liu L., Suleimen Y.M. Diversity of anticancer and antimicrobial compounds from lichens and lichen-derived fungi: A systematic review (1985–2017) Curr. Org. Chem. 2018;22:2487–2500. doi: 10.2174/1385272822666181109110813. [DOI] [Google Scholar]
- 7.Kekuda T.R.P., Lavanya D., Rao P. Lichens as promising resources of enzyme inhibitors: A review. J. Drug Deliv. Ther. 2019;9:665–676. doi: 10.22270/jddt.v9i2-s.2546. [DOI] [Google Scholar]
- 8.Shrestha G., Clair L.L. Lichens: A promising source of antibiotic and anticancer drugs. Phytochem. Rev. 2013;12:229–244. doi: 10.1007/s11101-013-9283-7. [DOI] [Google Scholar]
- 9.Solárová Z., Liskova A., Samec M., Kubatka P., Büsselberg D., Solár P. Anticancer Potential of Lichens’ Secondary Metabolites. Biomolecules. 2020;10:87. doi: 10.3390/biom10010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sut S., Maggi F., Dall’Acqua S. Bioactive Secondary Metabolites from Orchids (Orchidaceae) Chem. Biodivers. 2017;14 doi: 10.1002/cbdv.201700172. [DOI] [PubMed] [Google Scholar]
- 11.Zotz G. The systematic distribution of vascular epiphytes—A critical update. Bot. J. Linn. Soc. 2013;171:453–481. doi: 10.1111/boj.12010. [DOI] [Google Scholar]
- 12.Köster N., Nieder J., Barthlott W. Effect of host tree traits on epiphyte diversity in natural and anthropogenic habitats in ecuador. Biotropica. 2011;43:685–694. doi: 10.1111/j.1744-7429.2011.00759.x. [DOI] [Google Scholar]
- 13.Zotz G., Hietz P. The physiological ecology of vascular epiphytes: Current knowledge, open questions. J. Exp. Bot. 2001;52:2067–2078. doi: 10.1093/jexbot/52.364.2067. [DOI] [PubMed] [Google Scholar]
- 14.De Padua L.S., Bunyapraphatsō̜n N., Lemmens R.H.M.J., Foundation P. Plant Resources of South-East Asia: Medicinal and Poisonous Plants 1. Backhuys Publishers; Leiden, The Netherlands: 1999. [Google Scholar]
- 15.van Valkenburg J.L.C.H., De Padua L.S., Bunyapraphatsara N., Lemmens R.H.M.J., Foundation P. Plant Resources of South-East Asia: Medicinal and Poisonous Plants 2. Backhuys Publishers; Leiden, The Netherlands: 2001. [Google Scholar]
- 16.Bunyapraphatsō̜n N., Lemmens R.H.M.J., Foundation P. Plant Resources of South-East Asia: Medicinal and Poisonous Plants 3. Backhuys Publishers; Leiden, The Netherlands: 2003. [Google Scholar]
- 17.De Winter W.P. Plant Resources of South-East Asia: Cryptogams: Ferns and Fern Allies. Backhuys Publishers; Kerkwerve, The Netherlands: 2003. [Google Scholar]
- 18.Giesen W., Wulffraat S., Zieren M., Scholten L. Mangrove Guidebook for Southeast Asia. FAO and Wetlands International; Bangkok, Thailand: 2007. [Google Scholar]
- 19.Wiart C. Medicinal Plants of the Asia-Pacific: Drugs for the Future. World Scientific; Singapore: 2006. [Google Scholar]
- 20.DeFilipps R.A., Crepin J., Maina S.L. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana) National Museum of Natural History, Smithsonian Institution; Washington, DC, USA: 2004. [Google Scholar]
- 21.Praptosuwiryo T.N. Drynaria (Bory) J. Smith. In: De Winter W.P., Amoroso V.B., editors. Plant Resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 101–104. [Google Scholar]
- 22.Warrier P.K., Nambiar V.P.K., Raman-Kutty C. Indian Medicinal Plants. Orient Longman Ltd.; Hyderabad, India: 1996. [Google Scholar]
- 23.Wangchuk P., Yeshi K., Jamphel K. Pharmacological, ethnopharmacological, and botanical evaluation of subtropical medicinal plants of Lower Kheng region in Bhutan. Integr. Med. Res. 2017;6:372–387. doi: 10.1016/j.imr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurib-Fakim A., Brendler T. Medicinal and Aromatic Plants of Indian Ocean islands: Madagascar, Comoros, Seychelles and Mascarenes. Medpharm Scientific Publisher; Stuttgart, Germany: 2004. [Google Scholar]
- 25.Anonim . Medicinal Herb Index in Indonesia. PT Eisai Indonesia; Jakarta, Indonesia: 1986. [Google Scholar]
- 26.The Plant List. [(accessed on 3 January 2020)]; Available online: http://www.theplantlist.org/
- 27.Nugraha A.S., Keller P.A. Revealing indigenous Indonesian traditional medicine: Anti-infective agents. Nat. Prod. Commun. 2011;6:1953–1966. doi: 10.1177/1934578X1100601240. [DOI] [PubMed] [Google Scholar]
- 28.Roosita K., Kusharto Clara M., Sekiyama M., Fachrurozi Y., Ohtsuka R. Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J. Ethnopharmacol. 2008;115:72–81. doi: 10.1016/j.jep.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Leeson P.D., Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 2007;6:881–890. doi: 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]
- 30.Cardelu’s C.L., Mack M.C. The nutrient status of epiphytes and their host trees along an elevational gradient in Costa Rica. Plant Ecol. 2010;207:25–37. doi: 10.1007/s11258-009-9651-y. [DOI] [Google Scholar]
- 31.Benner J.W., Conroy S., Lunch C., Toyoda N. Phosphorus fertilization increases the abudance and nitrogenase activity of the cyanolichen Pseudocyphellaria crocata in Hawaian Montane Forest. Biotropica. 2007;39:400–405. doi: 10.1111/j.1744-7429.2007.00267.x. [DOI] [Google Scholar]
- 32.Cardelu’s C.L., Mack M.C., Woods C.L., DeMarco J., Treseder K.K. Nutrient cycling in canopy and terrestrial soils at lowland rainforest site, Costa Rica. Plant Soil. 2009;318:47–61. doi: 10.1007/s11104-008-9816-9. [DOI] [Google Scholar]
- 33.Reinert F. Epiphytes: Photosynthesis, water balance and nutrients. Oecologia Bras. 1998;4:5. doi: 10.4257/oeco.1998.0401.05. [DOI] [Google Scholar]
- 34.McNair J.B. Epiphytes, parasites and geophytes and the production of alkaloids, cyanogenetic and organic sulfur compounds. Am. J. Bot. 1941;28:733–737. doi: 10.1002/j.1537-2197.1941.tb11001.x. [DOI] [Google Scholar]
- 35.Uddin S.J., Grice D., Tiralongo E. Evaluation of cytotoxic activity of patriscabratine, tetracosane and various flavonoids isolated from the Bangladeshi medicinal plant Acrostichum aureum. Pharm. Biol. 2012;50:1276–1280. doi: 10.3109/13880209.2012.673628. [DOI] [PubMed] [Google Scholar]
- 36.Leong Y.W., Kang C.C., Harrison L.J., Powell A.D. Phenanthrenes, dihydrophenanthrenes and bibenzyls from the orchid Bulbophyllum Vaginatum. Phytochem. 1996;44:157–165. doi: 10.1016/S0031-9422(96)00387-1. [DOI] [Google Scholar]
- 37.Estrada S., López-Guerrero J.J., Villalobos-Molina R., Mata R. Spasmolytic stilbenoids from Maxillaria densa. Fitoterapia. 2004;75:690–695. doi: 10.1016/j.fitote.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto T., Natsume M., Onaka T., Uchimaru F., Shimizu M. Alkaloidal constituents of Dendrobium nobile (Orchidaceae). Structure determination of 4-hydroxydendroxine and nobilomethylene. Chem. Pharm. Bull. 1972;20:418–421. doi: 10.1248/cpb.20.418. [DOI] [Google Scholar]
- 39.Chellan G., Shivaprakash S., Karimassery Ramaiyar S., Varma A.K., Varma N., Thekkeparambil Sukumaran M., Rohinivilasam Vasukutty J., Bal A., Kumar H. Spectrum and prevalence of fungi infecting deep tissues of lower-limb wounds in patients with type 2 diabetes. J. Clin. Microbiol. 2010;48:2097–2102. doi: 10.1128/JCM.02035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh M., Singh N., Khare P.B., Rawat A.K.S. Antimicrobial activity of some important Adiantum species used traditionally in indigenous systems of medicine. J. Ethnopharmacol. 2008;115:327–329. doi: 10.1016/j.jep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Krief S., Huffman M.A., Sevenet T., Hladik C.M., Grellier P., Loiseau P.M., Wrangham R.W. Bioactive properties of plant species ingested by chimpanzees (Pan troglodytes schweinfurthii) in the Kibale National Park, Uganda. Am. J. Primatol. 2006;68:51–71. doi: 10.1002/ajp.20206. [DOI] [PubMed] [Google Scholar]
- 42.Han G., Wang M. Chemical constituents of Pyrrosia sheareri (Bak.) Ching. Nanjing Yaoxueyuan Xuebao. 1984;15:40–44. [Google Scholar]
- 43.Wang X.L., Wang N.L., Gao H., Zhang G., Qin L., Wong M.S., Yao X.S. Phenylpropanoid and flavonoids from osteoprotective fraction of Drynaria fortunei. Nat. Prod. Res. 2010;24:1206–1213. doi: 10.1080/14786410902991860. [DOI] [PubMed] [Google Scholar]
- 44.Lai H.Y., Lim Y.Y., Tan S.P. Antioxidative, tyrosinase inhibiting and antibacterial activities of leaf extracts from medicinal ferns. Biosci. Biotechnol. Biochem. 2009;73:1362–1366. doi: 10.1271/bbb.90018. [DOI] [PubMed] [Google Scholar]
- 45.Adam Z., Khamis S., Ismail A., Hamid M. Ficus deltoidea: A potential alternative medicine for diabetes mellitus. Evid. Based Complement. Alternat. Med. 2012;2012:632763. doi: 10.1155/2012/632763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu M., Shogawa H., Hayashi T., Arisawa M., Suzuki S., Yoshizaki M., Morita N., Ferro E., Basualdo I., Berganza L.H. Antiinflammatory constituents of topically applied crude drugs. III. Constituents and anti-inflammatory effect of Paraguayan crude drug “Tamandá cuná” (Catasetum barbatum LINDLE) Chem. Pharm. Bull. 1988;36:4447–4452. doi: 10.1248/cpb.36.4447. [DOI] [PubMed] [Google Scholar]
- 47.Ramanitrahasimbola D., Rakotondramanana D.A., Rasoanaivo P., Randriantsoa A., Ratsimamanga S., Palazzino G., Galeffi C., Nicoletti M. Bronchodilator activity of Phymatodes scolopendria (Burm.) Ching and its bioactive constituent. J. Ethnopharmacol. 2005;102:400–407. doi: 10.1016/j.jep.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 48.Kirana C. Master’s thesis. University of Adelaide; Adelaide, Australia: 1996. Bio-active Compounds Isolated from Mistletoe (Scurulla oortiana (Korth.) Danser) Parasitizing Tea Plant (Camellia sinensis L.) [Google Scholar]
- 49.Anonim . Jenis Paku Indonesia. Bali Pustaka; Jakarta, Indonesia: 1979. [Google Scholar]
- 50.Burkill I. A dictionary of the Economic Products of the Malay Peninsula. Government of Malaysia and Singapore; Kuala Lumpur, Malaysia: 1996. [Google Scholar]
- 51.Djumidi H. Inventaris Tanaman Obat Indonesia V. Balai Penelitian Tanaman Obat; Tawangmangu, Indonesia: 2006. [Google Scholar]
- 52.Rusea G. Asplenium L. In: De Winter W.P., Amoroso V.B., editors. Plant Resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 61–62. [Google Scholar]
- 53.Baltrushes N. Honors Thesis. University of California; Berkeley, CA, USA: 2006. Medical Ethnobotany, Phytochemistry, and Bioactivity of the Ferns of Moorea, French Polynesia. Senior. [Google Scholar]
- 54.Mannan M.M., Maridass M., Victor B. A review on the potential uses of ferns. Ethnobot. Leafl. 2008;2:281–285. [Google Scholar]
- 55.Manickam V.S., Irudayaraj V. Pteridophytes Flora of the Western Ghats of South India. BI Publications Pvt Ltd.; New Dehli, India: 1992. [Google Scholar]
- 56.Luziatelli G., Sorensen M., Theilade I., Molgaard P. Ashaninka medicinal plants: A case study from the native community of Bajo Quimiriki, Junin, Peru. J. Ethnobiol. Ethnomed. 2010;6:21. doi: 10.1186/1746-4269-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh H.B. Potential medicinal pteridophytes of India and their chemical constituents. J. Econ. Tax. Bot. 1999;23:63–78. [Google Scholar]
- 58.Ahmad F.B., Holdsworth D.K. Medicinal plants of Sarawak, Malaysia, part I. The Kedayans. Pharm. Biol. 1994;32:384–387. doi: 10.3109/13880209409083020. [DOI] [Google Scholar]
- 59.Hwang T.H., Kashiwada Y., Nonaka G.I., Nishioka I. Flavan-3-ol and proanthocyanidin allosides from Davallia divaricata. Phytochemistry. 1989;28:891–896. doi: 10.1016/0031-9422(89)80138-4. [DOI] [Google Scholar]
- 60.Vargas Gonzalez J.F., Yesares Ferrer M. Extraction of α-D-glucooctono-δ-lactone enediol from ferns, as a drug for the treatment of psoriasis. 2012734. Spain Patent. 1990 Apr 1;
- 61.Chang H.C., Huang G.J., Agrawal D.C., Kuo C.L., Wu C.R., Tsay H.S. Antioxidant activities and polyphenol contents of six folk medicinal ferns used as “Gusuibu”. Bot. Stud. 2007;48:397–406. [Google Scholar]
- 62.Praptosuwiryo T.N., Jansen P.C.M. Davallia parvula Wall. Ex Hook. & Grev. In: de Winter W.P.D., Amoroso V.B., editors. Plant resources of South-East Asia 15 (2). Cryptograms: Ferns and Fern Allies. Prosea Foundation by Backhuys Publishes; Leiden, The Netherlands: 2003. p. 92. [Google Scholar]
- 63.Praptosuwiryo T.N., Jansen P.C.M. Davalia J.E. Smith. In: De Winter W.P., Amoroso V.B., editors. Plant Resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 89–90. [Google Scholar]
- 64.Grepin F., Grepin M. La Medicine Tahitienne traditionnelle, Raau Tahiti. Societe Nouvelle des Editions du Pacifique.; Papeete, Tahiti: 1984. [Google Scholar]
- 65.Petard P. Raau Tahiti: The Use of Polynesia Medicinal Plants in Tahitian Medicine. South Pacific Commission; Noumea, New Caledonia: 1972. [Google Scholar]
- 66.Chen Y.H., Chang F.R., Lin Y.J., Hsieh P.W., Wu M.J., Wu Y.C. Identification of antioxidants from rhizome of Davallia solida. Food Chem. 2008;107:684–691. doi: 10.1016/j.foodchem.2007.08.066. [DOI] [Google Scholar]
- 67.Boydron-Le Garrec R., Benoit E., Sauviat M.P., Lewis R.J., Molgó J., Laurent D. Ability of some plant extracts, traditionally used to treat ciguatera fish poisoning, to prevent the in vitro neurotoxicity produced by sodium channel activators. Toxicon. 2005;46:625–634. doi: 10.1016/j.toxicon.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Rancon S., Chaboud A., Darbour N., Comte G., Bayet C., Simon P.N., Raynaud J., Di P.A., Cabalion P., Barron D. Natural and synthetic benzophenones: Interaction with the cytosolic binding domain of P-glycoprotein. Phytochemistry. 2001;57:553–557. doi: 10.1016/S0031-9422(01)00120-0. [DOI] [PubMed] [Google Scholar]
- 69.Renimel I., Olivier M., Andre P. Use of Davallia Plant Extract in Cosmetic and Pharmaceutical Compositions for the Treatment of Skin Aging. 2757395A1. France Patent. 1998 Jun 26;
- 70.Benjamin A., Manickam V.S. Medicinal pteridophytes from Western Ghats. Indian J. Tradit. Knowl. 2007;6:611–618. [Google Scholar]
- 71.Caniago I., Siebert S.F. Medicinal plant ecology, knowledge and conservation in Kalimantan, Indonesia (FN1) Econ. Bot. 1998;52:229–250. doi: 10.1007/BF02862141. [DOI] [Google Scholar]
- 72.Lachman-White D.A., Adams C.D., Trotz U.O.D. A Guide to the Medicinal Plants of Coastal Guyana. Commonwealth Science Council; London, UK: 1992. [Google Scholar]
- 73.Boonkerd T. Huperzia carinata (desv. ex Poir.) Trevis. In: De Winter W.P., Amoroso V.B., editors. Plant resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 112–113. [Google Scholar]
- 74.Choo C.Y., Hirasawa Y., Karimata C., Koyama K., Sekiguchi M., Kobayashi J.i., Morita H. Carinatumins A–C, new alkaloids from Lycopodium carinatum inhibiting acetylcholinesterase. Bioorganic Med. Chem. 2007;15:1703–1707. doi: 10.1016/j.bmc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Amoroso V.B. Huperzia phlegmaria (L) Rothm. In: De Winter W.P., Amoroso V.B., editors. Plant resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 113–115. [Google Scholar]
- 76.Ragupathy S., Steven N., Maruthakkutti M., Velusamy B., Ul-Huda M. Consensus of the ‘Malasars’ traditional aboriginal knowledge of medicinal plants in the Velliangiri holy hills, India. J. Ethnobiol. Ethnomed. 2008;4:8. doi: 10.1186/1746-4269-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wittayalai S., Sathalalai S., Thorroad S., Worawittayanon P., Ruchirawat S., Thasana N. Lycophlegmariols A-D: Cytotoxic serratene triterpenoids from the club moss Lycopodium phlegmaria L. Phytochemistry. 2012;76:117–123. doi: 10.1016/j.phytochem.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Zimudzi C., Bosch C.H. Lycopodium. In: Schmelzer G.H., editor. Volume 11 of Plant Resources of Tropical Africa: Medicinal Plants 1. PROTA; Leiden, Netherland: 2008. pp. 366–369. [Google Scholar]
- 79.Noweg T., Abdullah A.R., Nidang D. Forest plants as vegetables for communities bordering the crocker range national park. ARBEC. 2003;1-3:1–18. [Google Scholar]
- 80.Darnaedi D., Praptosuwiryo T.N. Nephrolepsis Schott. In: De Winter W.P., Amoroso V.B., editors. Plant resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 141–145. [Google Scholar]
- 81.Christensen H. Uses of Ferns in Two Indigenous Communities in Sarawak, Malaysia. In: Johns R.J., editor. Holttum Memorial Volume. Royal Botanic Gardens; Kew, UK: 1997. pp. 177–192. [Google Scholar]
- 82.Ojo O.O., Ajayi A.O., Anibijuwon I.I. Antibacterial potency of methanol extracts of lower plants. J. Zhejiang Univ. Sci. B. 2007;8:189–191. doi: 10.1631/jzus.2007.B0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rani D., Khare P.B., Dantu P.K. In vitro antibacterial and antifungal properties of aqueous and non-aqueous frond extracts of Psilotum nudum, Nephrolepis biserrata and Nephrolepis cordifolia. Indian J. Pharm. Sci. 2010;72:818–822. doi: 10.4103/0250-474X.84606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumari P., Otaghvari A.M., Govindapyari H., Bahuguna Y.M., Uniyal P.L. Some ethno-medicinally important Pterodophytes of India. In. J. Med. Arom. Plants. 2011;1:18–22. [Google Scholar]
- 85.Ong H.C., Aguilar N.O. Ophioglossum pendulum L. In: De Winter W.P., Amoroso V.B., editors. Plant Resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 151–153. [Google Scholar]
- 86.Hatani A., Okumura Y., Maeda H. Cell Activator, Skin Whitening Agent and Antioxidant Containing Plant Extract of Ophioglossum of Ophioglossaceae. 2005089375. Japan Patent. 2005 Apr 7;
- 87.Hovenkamp P.H. Pyrrosia Mirbel. In: De Winter W.P., Amoroso V.B., editors. Plant resources of South-East Asia No 15(2): Ferns and Fern Alies. Backhuys; Leiden, The Netherlands: 2003. pp. 170–174. [Google Scholar]
- 88.Anonim . Materia Medika Indonesia. Volume V Departemen Kesehatan Republik Indonesia; Jakarta, Indonesia: 1989. [Google Scholar]
- 89.Abdul R.M.D. Pengenalan dan Penggunaan Herba Ubatan. Orient Press Sdn. Bhd.; Kuala Lumpur, Malaysia: 1996. [Google Scholar]
- 90.Dalimartha S. Atlas Tumbuhan Obat Indonesia. PT. Pustaka Pembangunan; Jakarta, Indonesia: 2008. p. 89. [Google Scholar]
- 91.Somchit M.N., Hassan H., Zuraini A., Chong L.C., Mohamed Z., Zakaria Z.A. In vitro anti-fungal and anti-bacterial activity of Drymoglossum piloselloides L. Presl. against several fungi responsible for Athlete’s foot and common pathogenic bacteria. Afr. J. Microbiol. Res. 2011;5:3537–3541. doi: 10.5897/AJMR11.719. [DOI] [Google Scholar]
- 92.Nugraha A.S., Haritakun R., Keller P.A. Constituents of the Indonesian epiphytic medicinal plant Drynaria rigidula. Nat. Prod. Commun. 2013;8 doi: 10.1177/1934578X1300800606. [DOI] [Google Scholar]
- 93.Neamsuvan O., Singdam P., Yingcharoen K., Sengnon N. A survey of medicinal plants in mangrove and beach forests from sating Phra Peninsula, Songkhla Province, Thailand. J. Med. Plants Res. 2012;6:2421–2437. doi: 10.5897/JMPR11.1395. [DOI] [Google Scholar]
- 94.Wang X.L., Wang N.L., Zhang Y., Gao H., Pang W.Y., Wong M.S., Zhang G., Qin L., Yao X.S. Effects of eleven flavonoids from the osteoprotective fraction of Drynaria fortunei (KUNZE) J. SM. on osteoblastic proliferation using an osteoblast-like cell line. Chem. Pharm. Bull. 2008;56:46–51. doi: 10.1248/cpb.56.46. [DOI] [PubMed] [Google Scholar]
- 95.Wangchuk P., Pyne S.G., Keller P.A. Ethnobotanical authentication and identification of Khrog-sman (Lower Elevation Medicinal Plants) of Bhutan. J. Ethnopharmacol. 2011;134:813–823. doi: 10.1016/j.jep.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 96.Majumdar H.C., Shyam J.M., Chowdhury U., Koch D., Roy N. Traditional hepatoprotective herbal medicine of Koch tribe in the South-West Garo hills district, Meghalaya. Indian J. Tradit. Knowl. 2019;18:312–317. [Google Scholar]
- 97.Khan A., Haque E., Mukhlesur R.M., Mosaddik A., Rahman M., Sultana N. Isolation of antibacterial constituent from rhizome of Drynaria quercifolia and its sub-acute toxicological studies. Daru J. Fac. Pharm. Tehran Univ. Med Sci. 2007;15:205–211. [Google Scholar]
- 98.Wangchuk P., Namgay K., Gayleg K., Dorji Y. Medicinal plants of Dagala region in Bhutan: Their diversity, distribution, uses and economic potential. J. Ethnobiol. Ethnomed. 2016;12:28. doi: 10.1186/s13002-016-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boonkerd T., de Winter W.P. Loxogramme scolopendrina (Bory) C. Presl. In: De Winter W.P., Amoroso V.B., editors. Plant resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 120–121. [Google Scholar]
- 100.Syamsuhidayat S.S., Hutapea J.R. Inventaris Tanaman Obat Indonesia. Volume I Badan Penelitian dan Pengembangan Kesehatan Departemen Kesehatan Republik Indonesia; Jakarta, Indonesia: 1991. [Google Scholar]
- 101.Darnaedi D., Praptosuwiryo T.N. Platycerium bifucartum C. Chr. In: De Winter W.P., Amoroso V.B., editors. Plant resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 157–159. [Google Scholar]
- 102.May L. The economic uses and associated folklore of ferns and fern allies. Bot. Rev. 1978;44:491–528. doi: 10.1007/BF02860848. [DOI] [Google Scholar]
- 103.Nair B.K. Medicinal fern of India. Bull. Nat. Bot. Gard. 1959;29:1–36. [Google Scholar]
- 104.Suryana Keanekaragaman jenos tumbuhan paku terestrial dan epifit di Kawasan PLTP Kamojang Kab. Garut Jawa Barat. J. Biot. 2009;7:20–26. [Google Scholar]
- 105.Namba T. Coloured illustration of Wakan-Yaku. Hoikusha; Osaka, Japan: 1980. [Google Scholar]
- 106.Masuda K., Yamashita H., Shiojima K., Itoh T., Ageta H. Fern constituents: Triterpenoids isolated from rhizomes of Pyrrosia lingua L. Chem. Pharm. Bull. 1997;45:590–594. doi: 10.1248/cpb.45.590. [DOI] [Google Scholar]
- 107.Ding Z.T., Fang Y.S., Tai Z.G., Yang M.H., Xu Y.Q., Li F., Cao Q.E. Phenolic content and radical scavenging capacity of 31 species of ferns. Fitoterapia. 2008;79:581–583. doi: 10.1016/j.fitote.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 108.Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., Zhang X., Hua S.N., Yu J., Xiao P.G., et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antivir. Res. 2005;67:18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsu C.Y. Antioxidant activity of Pyrrosia petiolosa. Fitoterapia. 2008;79:64–66. doi: 10.1016/j.fitote.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 110.Gan R.Y., Kuang L., Xu X.R., Zhang Y., Xia E.Q., Song F.L., Li H.B. Screening of natural antioxidants from traditional Chinese medicinal plants associated with treatment of rheumatic disease. Molecules. 2010;15:5988–5997. doi: 10.3390/molecules15095988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Prakash A.O., Saxena V., Shukla S., Tewari R.K., Mathur S., Gupta A., Sharma S., Mathur R. Anti-implantation activity of some indigenous plants in rats. Acta Eur. Fertil. 1985;16:441–448. [PubMed] [Google Scholar]
- 112.Dai H., Mei W., Hong K., Zeng Y., Zhuang L. Screening of the tumor cytotoxic activity of sixteen species of mangrove plants in Hainan. Zhongguo Haiyang Yaowu. 2005;24:44–46. [Google Scholar]
- 113.Thomas T. In vitro evaluation of antibacterial activity of Acrostichum aureum Linn. Indian J. Nat. Prod. Resour. 2012;3:135–138. [Google Scholar]
- 114.Uddin S.J., Grice I.D., Tiralongo E. Cytotoxic effects of bangladeshi medicinal plant extracts. Evid. Based Complement. Alternat. Med. 2011;2011:578092. doi: 10.1093/ecam/nep111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schneider H., Tawan C.S. Taenitis blechnoides (Willd.) Swartz. In: De Winter W.P., Amoroso V.B., editors. Plant Resources of South-East Asia No 15(2): Ferns and Fern Allies. Backhuys; Leiden, The Netherlands: 2003. pp. 188–190. [Google Scholar]
- 116.Manandhar P.N. Ethnobotanical observations on ferns and ferns allies of Nepals. J. Econ. Taxon. Bot. 1996;12:414–422. [Google Scholar]
- 117.Joo S.S., Jang S.K., Kim S.G., Choi J.S., Hwang K.W., Lee D.I. Anti-acne activity of Selaginella involvens extract and its non-antibiotic antimicrobial potential on Propionibacterium acnes. Phytother. Res. PTR. 2008;22:335–339. doi: 10.1002/ptr.2319. [DOI] [PubMed] [Google Scholar]
- 118.Gayathri V., Asha V.V., John J.A., Subramoniam A. Protection of immunocompromised mice from fungal infection with a thymus growth-stimulatory component from Selaginella involvens, a fern. Immunopharmacol. Immunotoxicol. 2011;33:351–359. doi: 10.3109/08923973.2010.518617. [DOI] [PubMed] [Google Scholar]
- 119.Wu P.L., Hsu Y.L., Zao C.W., Damu A.G., Wu T.S. Constituents of Vittaria anguste-elongata and their biological activities. J. Nat. Prod. 2005;68:1180–1184. doi: 10.1021/np050060o. [DOI] [PubMed] [Google Scholar]
- 120.Tap N., Sosef M.S.M. Schefflera J.R. Foster & J.G. Foster. In: de Padua L.S., Bunyapraphatsara N., Lemmens R.H.M.J., editors. Plant Resources of South-East Asia No 12(1): Medicinal and Poisonous Plants 1. Backhuys; Leiden, The Netherlands: 1999. pp. 433–438. [Google Scholar]
- 121.Oshima R., Soda M. Antibacterial Agent/Highly Safe Antibacterial Agent Obtained from Plants. 2000136141A. Japan Patent. 2000 May 16;
- 122.Chuakul W., Soonthornchareonnon N., Ruangsomboon O. Dischidia bengalensis Colebr. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. p. 172. [Google Scholar]
- 123.Lemmens R.H.M.J., Bunyapraphatsara N. Plat Resources of Sout-East Asia 12 (3): Medicinal and Poisonous Plants. Prosea Foundation by Backhuys Publishers; Leiden, The Netherlands: 2003. [Google Scholar]
- 124.Chuakul W., Soonthornchareonnon N., Ruangsomboon O. Dischidia major (Vahl) Merr. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. p. 172. [Google Scholar]
- 125.Hynniewta S.R., Kumar Y. Herbal remidies among the Khasi traditional healers and village folks in Meghalaya. Indian J. Tradit. Knowl. 2008;7:581–586. [Google Scholar]
- 126.Chuakul W., Soonthornchareonnon N., Ruangsomboon O. Dischidia nummularia R.Br. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. p. 173. [Google Scholar]
- 127.Chuakul W., Soonthornchareonnon N., Ruangsomboon O. Dischidia purpurea Merr. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. p. 173. [Google Scholar]
- 128.Bosch C.H. Impatiens niamniamensis Gilg. In: Grubben G.J.H., Denton O.A., editors. PROTA (Plant Resources of Tropical Africa/Ressources Végétales de l’Afrique Tropicale) PROTA; Wageningen, The Netherlands: 2004. [Google Scholar]
- 129.Chand K., Rahuja N., Mishra D.P., Srivastava A.K., Maurya R. Major alkaloidal constituent from Impatiens niamniamensis seeds as antihyperglycemic agent. Med. Chem. Res. 2011;20:1505–1508. doi: 10.1007/s00044-010-9401-7. [DOI] [Google Scholar]
- 130.Wiart C. Ethnopharmacology of Medicinal Plants: Asia and the Pacific. Humana Press Inc.; Totowa, NJ, USA: 2006. [Google Scholar]
- 131.Hariana H.A. Tumbuhan Obat & Khasiatnya 3. Niaga Swadaya; Depok, Indonesia: 2008. [Google Scholar]
- 132.Wardini T.H. Cassytha filiformis L. In: van Valkenburg J.L.C.H., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(2): Medicinal and Poisonous Plants 2. Backhuys; Leiden, The Netherlands: 2001. pp. 142–144. [Google Scholar]
- 133.Chang C.W., Ko F.N., Su M.J., Wu Y.C., Teng C.M. Pharmacological evaluation of ocoteine, isolated from Cassytha filiformis, as an α1-adrenoceptor antagonist in rat thoracic aorta. Jpn. J. Pharmacol. 1997;73:207–214. doi: 10.1254/jjp.73.207. [DOI] [PubMed] [Google Scholar]
- 134.Wu Y.C., Chang F.R., Chao Y.C., Teng C.M. Antiplatelet and vasorelaxing actions of aporphinoids from Cassytha filiformis. Phytother. Res. 1998;12:S39–S41. doi: 10.1002/(SICI)1099-1573(1998)12:1+<S39::AID-PTR244>3.0.CO;2-O. [DOI] [Google Scholar]
- 135.Hoet S., Stevigny C., Block S., Opperdoes F., Colson P., Baldeyrou B., Lansiaux A., Bailly C., Quetin-Leclercq J. Alkaloids from Cassytha filiformis and related aporphines: Antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. Planta Med. 2004;70:407–413. doi: 10.1055/s-2004-818967. [DOI] [PubMed] [Google Scholar]
- 136.Sharma S., Hullatti K.K., Kumar S., Tiwari K.B. Comparative antioxidant activity of Cuscuta reflexa and Cassytha filiformis. J. Pharm. Res. 2012;5:441–443. [Google Scholar]
- 137.Hoesen D.D.H. Cuscuta asutralis R.Br. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 144–145. [Google Scholar]
- 138.Chang S.J., Suk K.D. Inhibitory effects on melanin biosynthesis and tyrosinase activity, cytotoxicity in clone M-3 and antioxidant activity by Cuscuta japonica, C. australis, and C. chinensis extracts. Yakhak Hoechi. 2006;50:421–428. [Google Scholar]
- 139.Gaur R.D., Tiwari J.K. Indigenous medicinal plants of Garhwal Himalaya (India): An ethnobotanical study; Proceedings of Medicinal and Poisonous Plants of the Tropics: Proceedings of Symposium 5-35 of the 14th International Botanical Congress (Compiler); Berlin, UK. 24 July–1 August 1987. [Google Scholar]
- 140.Chopra R.N., Nayar S.L., Chopra I.C., Asolkar L.V., Kakkar K.K., Chakre O.J., Varma B.S., Council S., Industrial R. Glossary of Indian Medicinal Plants. Council of Scientific & Industrial Research; New Delhi, India: 1956. [Google Scholar]
- 141.Gupta M., Mazumder U.K., Pal D.K., Bhattacharya S. Anti-steroidogenic activity of methanolic extract of Cuscuta reflexa roxb. stem and Corchorus olitorius Linn. seed in mouse ovary. Indian J. Exp. Biol. 2003;41:641–644. [PubMed] [Google Scholar]
- 142.Awasthi L.P. The purification and nature of an antiviral protein from Cuscuta reflexa plants. Arch. Virol. 1981;70:215–223. doi: 10.1007/BF01315128. [DOI] [PubMed] [Google Scholar]
- 143.Mahmood N., Pacente S., Burke A., Khan A., Pizaa C. Constituents of Cuscuta reflexa are anti-HIV agents. Antivir. Chem. Chemother. 1997;8:70–74. doi: 10.1177/095632029700800108. [DOI] [Google Scholar]
- 144.Pal D., Panda C., Sinhababu S., Dutta A., Bhattacharya S. Evaluation of psychopharmacological effects of petroleum ether extract of Cuscuta reflexa Roxb. stem in mice. Acta Pol. Pharm. 2003;60:481–486. [PubMed] [Google Scholar]
- 145.Pal D.K., Mandal M., Senthilkumar G.P., Padhiari A. Antibacterial activity of Cuscuta reflexa stem and Corchorus olitorius seed. Fitoterapia. 2006;77:589–591. doi: 10.1016/j.fitote.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 146.Pandit S., Chauhan N.S., Dixit V.K. Effect of Cuscuta reflexa Roxb on androgen-induced alopecia. J. Cosmet. Dermatol. 2008;7:199–204. doi: 10.1111/j.1473-2165.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 147.Suresh V., Sruthi V., Padmaja B., Asha V.V. In vitro anti-inflammatory and anti-cancer activities of Cuscuta reflexa Roxb. J. Ethnopharmacol. 2011;134:872–877. doi: 10.1016/j.jep.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 148.Poudel A., Kim S.G., Kim D.K., Kim Y.K., Lee Y.S., Lee G.W., Min B.S., Jung H.J. Antioxidative and antiobesity activity of nepalese wild herbs. Nat. Prod. Sci. 2011;17:123–129. [Google Scholar]
- 149.Lokvam J., Braddock J.F., Reichardt P.B., Clausen T.P. Two polyisoprenylated benzophenones from the trunk latex of Clusia grandiflora (Clusiaceae) Phytochemistry. 2000;55:29–34. doi: 10.1016/S0031-9422(00)00193-X. [DOI] [PubMed] [Google Scholar]
- 150.Gupta M.P., Solís P.N., Calderón A.I., Guinneau-Sinclair F., Correa M., Galdames C., Guerra C., Espinosa A., Alvenda G.I., Robles G., et al. Medical ethnobotany of the Teribes of Bocas del Toro, Panama. J. Ethnopharmacol. 2005;96:389–401. doi: 10.1016/j.jep.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 151.Kubitzki K., Kadereit J.W. Lamiales (Except Acanthaceae Including Avicenniaceae) Springer; Heidelberg, Germany: 2004. The Families and Genera of Vascular Plants: Flowering Plants, Dicotyledons. [Google Scholar]
- 152.Esposito Avella M., Gupta M.P., Calderon A., Zamora V.O., Buitrago de Tello R. The analgesic and anti-inflammatory effects of Drymonia serrulata (Jacq.) Mart. Rev. Med. Panama. 1993;18:211–216. [PubMed] [Google Scholar]
- 153.Suciati S., Lambert L.K., Ross B.P., Deseo M.A., Garson M.J. Phytochemical study of Fagraea spp. uncovers a new terpene alkaloid with anti-Inflammatory properties. Aust. J. Chem. 2011;64:489–494. doi: 10.1071/CH10421. [DOI] [Google Scholar]
- 154.Territory A.C.O.T.N. Traditional Aboriginal Medicines in the Northern Territory of Australia. Conservation Commission of the Northern Territory of Australia; Darwin, Australia: 1993. [Google Scholar]
- 155.Roth W.E. Superstition, magic, and medicine. North Qld. Ethnogr. Bull. 1903;5:1–42. [Google Scholar]
- 156.Cleland J.B., Johnston T.H. Aboriginal names and uses of plants in the Northern Flinders Ranges. T. Roy. Soc. South Aust. 1939;63:172–179. [Google Scholar]
- 157.Warrier P.K., Nambiar V.P.K., Ramankutty C., Nair R.V. Indian Medicinal Plants: A Compendium of 500 Species. Orient Longman; Chennai, India: 1993. [Google Scholar]
- 158.Pattanayak S.P., Sunita P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (L.f) Ettingsh. J. Ethnopharmacol. 2008;120:241–247. doi: 10.1016/j.jep.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 159.Chuakul W., Soonthornchareonnon N., Ruangsomboon O. Dendrophthoe Mart. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 157–159. [Google Scholar]
- 160.Arung E.T., Kusuma I.W., Christy E.O., Shimizu K., Kondo R. Evaluation of medicinal plants from Central Kalimantan for antimelanogenesis. J. Nat. Med. 2009;63:473–480. doi: 10.1007/s11418-009-0351-7. [DOI] [PubMed] [Google Scholar]
- 161.Watt J.M., Breyer-Brandwijk M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa: Being an Account of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal. E. & S. Livingstone; Edinburgh, UK: 1962. [Google Scholar]
- 162.Rahayu S.S.B. Loranthus globosus Roxb. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 284–285. [Google Scholar]
- 163.Sadik G., Islam R., Rahman M.M., Khondkar P., Rashid M.A., Sarker S.D. Antimicrobial and cytotoxic constituents of Loranthus globosus. Fitoterapia. 2003;74:308–311. doi: 10.1016/S0367-326X(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 164.Islam R., Alam A.H.M.K., Rahman B.M., Salam K.A., Hossain A., Baki A., Sadik G. Toxicological studies of two compounds isolated from Loranthus globosus Roxb. Pak. J. Biol. Sci. 2007;10:2073–2077. doi: 10.3923/pjbs.2007.2073.2077. [DOI] [PubMed] [Google Scholar]
- 165.Rahayu S.S.B. Macrosolen Blume. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 284–285. [Google Scholar]
- 166.Cardenas L.B. Scurrula L. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 370–373. [Google Scholar]
- 167.Ikawati M., Wibowo A.E., Octa N.S., Adelina R. The Utilization of Parasite as Anticancer Agent. Faculty of Pharmacy-Gadjah Mada University; Yogyakarta, Indonesia: 2000. [Google Scholar]
- 168.Djumidi H. Inventaris Tanaman Obat Indonesia. Volume IV Badan Litbangkes Depkes RI; Jakarta, Indonesia: 1997. [Google Scholar]
- 169.Ohashi K., Winarno H., Mukai M., Shibuya H. Preparation and cancer cell invasion inhibitory effects of C16-alkynic fatty acids. Chem. Pharm. Bull. 2003;51:463–466. doi: 10.1248/cpb.51.463. [DOI] [PubMed] [Google Scholar]
- 170.Ohashi K., Winarno H., Mukai M., Inoue M., Prana M.S., Simanjuntak P., Shibuya H. Indonesian medicinal plants. XXV. Cancer cell invasion inhibitory effects of chemical constituents in the parasitic plant Scurrula atropurpurea (loranthaceae) Chem. Pharm. Bull. 2003;51:343–345. doi: 10.1248/cpb.51.343. [DOI] [PubMed] [Google Scholar]
- 171.Lohezic-Le Devehat F., Bakhtiar A., Bezivin C., Amoros M., Boustie J. Antiviral and cytotoxic activities of some Indonesian plants. Fitoterapia. 2002;73:400–405. doi: 10.1016/S0367-326X(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 172.Xiao Y.J., Chen Y.Z., Chen B.H., Chen J.H., Lin Z.X., Fan Y.L. Study on cytotoxic activities on human leukemia cell line HL-60 by flavonoids extracts of Scurrula parasitica from four different host trees. Zhongguo Zhong Yao Za Zhi. 2008;33:427–432. [PubMed] [Google Scholar]
- 173.Chen Y., Xiao Y., Xu J., Wu Y. Uses of Extracts of Loranthaceae Plants as NF-κB Inhibitor for Treating Diseases Associated with Abnormal Activation of NF-κB. 101548995A. China Patent. 2009 Oct 7;
- 174.Sohn S.H., Lee H., Nam J.-y., Kim S.H., Jung H.J., Kim Y., Shin M., Hong M., Bae H. Screening of herbal medicines for the recovery of cisplatin-induced nephrotoxicity. Environ. Toxicol. Pharmacol. 2009;28:206–212. doi: 10.1016/j.etap.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 175.Chen B.H., Lai J.J., Zheng Q., Li J., Xiao Y.J. Effects of different extraction solvents on the antioxidant activities of leaves extracts of Scurrula parasitica. Fujian Shifan Daxue Xuebao Ziran Kexueban. 2010;26:86–90. [Google Scholar]
- 176.Xiao Y., Fan Y., Chen B., Zhang Q., Zeng H. Polysaccharides from Scurrula parasitica L. inhibit sarcoma S180 growth in mice. Zhongguo Zhong Yao Za Zhi. 2010;35:381–384. doi: 10.4268/cjcmm20100328. [DOI] [PubMed] [Google Scholar]
- 177.Roh C., Jung U. Screening of crude plant extracts with anti-obesity activity. Int. J. Mol. Sci. 2012;13:1710–1719. doi: 10.3390/ijms13021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wong D.Z.H., Abdul K.H., Ling S.K. Bioassay-guided isolation of neuroprotective compounds from Loranthus parasiticus against H2O2-induced oxidative damage in NG108-15 cells. J. Ethnopharmacol. 2012;139:256–264. doi: 10.1016/j.jep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 179.Zuo G.Y., Zhang X.J., Yang C.X., Han J., Wang G.C., Bian Z.Q. Evaluation of traditional Chinese medicinal plants for anti-MRSA activity with reference to the treatment record of infectious diseases. Molecules. 2012;17:2955–2967. doi: 10.3390/molecules17032955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Amabeoku G.J., Leng M.J., Syce J.A. Antimicrobial and anticonvulsant activities of Viscum capense. J. Ethnopharmacol. 1998;61:237–241. doi: 10.1016/S0378-8741(98)00054-3. [DOI] [PubMed] [Google Scholar]
- 181.Tibe O., Pernthaner A., Sutherland I., Lesperance L., Harding D.R.K. Condensed tannins from Botswanan forage plants are effective priming agents of γδ T cells in ruminants. Vet. Immunol. Immunopathol. 2012;146:237–244. doi: 10.1016/j.vetimm.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 182.Nurdin H., Dachriyanus, Nordin M. Profil fitokimia dan aktifitas antiacetylcholinesterase dari daun Tabat barito (Ficus deltoidea Jack) J. Ris. Kim. 2009;2:169–173. [Google Scholar]
- 183.Adam H., Ismail A., Khamis S., Mokhtar M.H.M., Hamid M. Antihyperglycemic activity of F. deltoidea ethanolic extract in normal rats. Sains Malays. 2011;40:489–495. [Google Scholar]
- 184.Rojo J.P., Pitargue F.C., Sosef M.S.M. Ficus L. In: de Padua L.S., Bunyapraphatsara N., Lemmens R.H.M.J., editors. Plant Resources of South-East Asia No 12(1): Medicinal and Poisonous Plants 1. Backhuys; Leiden, The Netherlands: 1999. pp. 277–289. [Google Scholar]
- 185.Fazliana M.S., Muhajir H., Hazilawati H., Shafii K., Mazleha M. Effects of Ficus deltoidea aqueous extract on hematological and biochemical parameters in rats. Med. J. Malays. 2008;63:103–104. [PubMed] [Google Scholar]
- 186.Sulaiman M.R., Hussain M.K., Zakaria Z.A., Somchit M.N., Moin S., Mohamad A.S., Israf D.A. Evaluation of the antinociceptive activity of Ficus deltoidea aqueous extract. Fitoterapia. 2008;79:557–561. doi: 10.1016/j.fitote.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 187.Zunoliza A., Khalid H., Zhari I., Rasadah M.A., Mazura P., Fadzureena J., Rohana S. Evaluation of extracts of leaf of three Ficus deltoidea varieties for antioxidant activities and secondary metabolites. Pharmacogn. Res. 2009;1:216–223. [Google Scholar]
- 188.Ilyanie Y., Wong T.W., Choo C.Y. Evaluation of hypoglycemic activity and toxicity profiles of the leaves of Ficus deltoidea in rodents. J. Complement. Integr. Med. 2011;8 doi: 10.2202/1553-3840.1469. [DOI] [PubMed] [Google Scholar]
- 189.Oh M.J., Hamid Mariani A., Ngadiran S., Seo Y.K., Sarmidi Mohamad R., Park Chang S. Ficus deltoidea (Mas cotek) extract exerted anti-melanogenic activity by preventing tyrosinase activity in vitro and by suppressing tyrosinase gene expression in B16F1 melanoma cells. Arch Dermatol. Res. 2011;303:161–170. doi: 10.1007/s00403-010-1089-5. [DOI] [PubMed] [Google Scholar]
- 190.Abdsamah O., Zaidi N.T.A., Sule A.B. Antimicrobial activity of Ficus deltoidea Jack (Mas Cotek) Pak. J. Pharm. Sci. 2012;25:675–678. [PubMed] [Google Scholar]
- 191.Zakaria Z.A., Hussain M.K., Mohamad A.S., Abdullah F.C., Sulaiman M.R. Anti-inflammatory activity of the aqueous extract of Ficus deltoidea. Biol. Res. Nurs. 2012;14:90–97. doi: 10.1177/1099800410395378. [DOI] [PubMed] [Google Scholar]
- 192.Bhatt D.D. Natural History and Economic Botany of Nepal. Dept. of Information, His Majesty’s Govt. of Nepal; Kathmandu, Nepal: 1970. [Google Scholar]
- 193.Bajracharya D., Rana S.J.B., Shrestha A.K. A general survey and biochemical analysis of fodder plants found in Nagarjun hill forest of Kathmandu valley. J. Nat. Hist. Mus. 1978;2:105–116. [Google Scholar]
- 194.Rai S.K., Subedi S., Mishra S. Utilization pattern of medicinal plants in Thumpakhar, Sindhupalchok, Nepal. Bot. Orient. 2004;4:75–78. [Google Scholar]
- 195.Lan Z. Oral Medicated Liquor Comprising Caulis et Folium Piperis, Radix Celastri Angulati and Ficus Lacor Buch-Ham with Effects of Eliminating Dampness Relieving Pain. 1814035. China Patent. 2006 Aug 9;
- 196.Oyen L.P.A. Ficus natalensis Hochst. In: Brink M., Achigan-Dako E.G., editors. PROTA (Plant Resources of Tropical Africa/Ressources Végétales de l’Afrique Tropicale) PROTA; Wageningen, The Netherlands: 2011. [Google Scholar]
- 197.Nakano D., Ishitsuka K., Hatsuse T., Tsuchihashi R., Okawa M., Okabe H., Tamura K., Kinjo J. Screening of promising chemotherapeutic candidates against human adult T-cell leukemia/lymphoma from plants: Active principles from Physalis pruinosa and structure-activity relationships with withanolides. J. Nat. Med. 2011;65:559–567. doi: 10.1007/s11418-011-0543-9. [DOI] [PubMed] [Google Scholar]
- 198.Ragasa C.Y., Juan E., Rideout J.A. A triterpene from Ficus pumila. J. Asian Nat. Prod. Res. 1999;1:269–275. doi: 10.1080/10286029908039875. [DOI] [PubMed] [Google Scholar]
- 199.Panyaphu K., On T.V., Sirisa-ard P., Srisa-nga P., ChansaKaow S., Nathakarnkitkul S. Medicinal plants of the Mien (Yao) in Northern Thailand and their potential value in the primary healthcare of postpartum women. J. Ethnopharmacol. 2011;135:226–237. doi: 10.1016/j.jep.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 200.Chua S. Bachelor’s Thesis. Universiti Malaya; Kuala Lumpur: 1996. Kajian Etnobotani ke Atas Komuniti Temuan di Semenyih, Selangor. [Google Scholar]
- 201.Nardiah R.J., Nazlina I., Mohd R.A.R., Siti N.A.Z., Ling C.Y., Shariffah M.S.A., Farina A.H., Yaacob W.A., Ahmad I.B., Din L.B. A survey on phytochemical and bioactivity of plant extracts from Malaysian forest reserves. J. Med. Plants Res. 2010;4:203–210. [Google Scholar]
- 202.Jalal J.S., Kumar P., Pangtey Y.P.S. Ethnomedicinal orchids of Uttarakhand, western Himalaya. Ethnobot. Leafl. 2008;12:1227–1230. [Google Scholar]
- 203.Sulistiarini D. Acriopsis javanica Reinw. ex Blume. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 33–34. [Google Scholar]
- 204.Satish M.N., Abhay P.S., Chen-Yue L., Chao-Lin K., Hsin-Sheng T. Studies on tissue culture of Chinese medicinal plant resources in Taiwan and their sustainable utilization. Bot. Bull. Acad. Sin. 2003;44 [Google Scholar]
- 205.Lin J.M., Lin C.C., Chiu H.F., Yang J.J., Lee S.G. Evaluation of the anti-inflammatory and liver-protective effects of Anoectochilus formosanus, Ganoderma lucidum and Gynostemma pentaphyllum in Rats. Am. J. Chin. Med. 1993;21:59–69. doi: 10.1142/S0192415X9300008X. [DOI] [PubMed] [Google Scholar]
- 206.Du X.M., Sun N.Y., Tamura T., Mohri A., Sugiura M., Yoshizawa T., Irino N., Hayashi J., Shoyama Y. Higher yielding isolation of kinsenoside in Anoectochilus and its anti-hyperliposis Effect. Biol. Pharm. Bull. 2001;24:65–69. doi: 10.1248/bpb.24.65. [DOI] [PubMed] [Google Scholar]
- 207.Shih C.C., Wu Y.W., Lin W.C. Ameliorative effects of Anoectochilus formosanus extract on osteopenia in ovariectomized rats. J. Ethnopharmacol. 2001;77:233–238. doi: 10.1016/S0378-8741(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 208.Wang S.Y., Kuo Y.H., Chang H.N., Kang P.L., Tsay H.S., Lin K.F., Yang N.S., Shyur L.F. Profiling and characterization antioxidant activities in Anoectochilus formosanus Hayata. J. Agric. Food. Chem. 2002;50:1859–1865. doi: 10.1021/jf0113575. [DOI] [PubMed] [Google Scholar]
- 209.Shih C.C., Wu Y.W., Lin W.C. Antihyperglycaemic and anti-oxidant properties of Anoectochilus Formosanus in diabetic rats. Clin. Exp. Pharmacol. Physiol. 2002;29:684–688. doi: 10.1046/j.1440-1681.2002.03717.x. [DOI] [PubMed] [Google Scholar]
- 210.Shyur L.F., Chen C.H., Lo C.P., Wang S.Y., Kang P.L., Sun S.J., Chang C.A., Tzeng C.M., Yang N.S. Induction of apoptosis in MCF-7 human breast cancer cells by phytochemicals from Anoectochilus formosanus. J. Biomed. Sci. 2004;11:928–939. doi: 10.1159/000081840. [DOI] [PubMed] [Google Scholar]
- 211.Shih C.C., Wu Y.W., Hsieh C.C., Lin W.C. Effect of Anoectochilus formosanus on fibrosis and regeneration of the liver in rats. Clin. Exp. Pharmacol. Physiol. 2004;31:620–625. doi: 10.1111/j.1440-1681.2004.04062.x. [DOI] [PubMed] [Google Scholar]
- 212.Shih C.C., Wu Y.W., Lin W.C. Aqueous extract of Anoectochilus formosanus attenuate hepatic fibrosis induced by carbon tetrachloride in rats. Phytomedicine. 2005;12:453–460. doi: 10.1016/j.phymed.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 213.Hsiao H.B., Wu J.B., Lin H., Lin W.C. Kinsenoside isolated from Anoectochilus formosanus suppresses LPS-stimulated inflammatory reactions in macrophages and endotoxin shock in mice. Shock. 2011;35:184–190. doi: 10.1097/SHK.0b013e3181f0e7a3. [DOI] [PubMed] [Google Scholar]
- 214.Hsieh W.T., Tsai C.T., Wu J.B., Hsiao H.B., Yang L.C., Lin W.C. Kinsenoside, a high yielding constituent from Anoectochilus formosanus, inhibits carbon tetrachloride induced Kupffer cells mediated liver damage. J. Ethnopharmacol. 2011;135:440–449. doi: 10.1016/j.jep.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 215.Lin W.C., Hsieh C.C., Lu T.J., Tsay H.S., Yang L.C., Lin C.C., Wang C.H. Anoectochilus spp. Polysaccharide Extracts for Stimulating Growth of Advantageous Bacteria, Stimuating Release of Granulocyte Colony-Stimulating Factor, Modulating T Helper Cell Type I, and/or Modulating T Helper Cell Type II and Uses of the Sa. 20110082103. U.S. Patent. 2011 Apr 7;
- 216.Ye S., Shao Q., Zhang A. Anoectochilus roxburghii: A review of its phytochemistry, pharmacology, and clinical applications. J. Ethnopharmacol. 2017;209:184–202. doi: 10.1016/j.jep.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 217.Zhang Y., Cai J., Ruan H., Pi H., Wu J. Antihyperglycemic activity of kinsenoside, a high yielding constituent from Anoectochilus roxburghii in streptozotocin diabetic rats. J. Ethnopharmacol. 2007;114:141–145. doi: 10.1016/j.jep.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 218.Cui S.C., Yu J., Zhang X.H., Cheng M.Z., Yang L.W., Xu J.Y. Antihyperglycemic and antioxidant activity of water extract from Anoectochilus roxburghii in experimental diabetes. Exp. Toxicol. Pathol. 2012 doi: 10.1016/j.etp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 219.Wu B., He S., Pan Y.J. New dihydrodibenzoxepins from Bulbophyllum kwangtungense. Planta Med. 2006;72:1244–1247. doi: 10.1055/s-2006-947200. [DOI] [PubMed] [Google Scholar]
- 220.Chen Y., Xu J., Yut H., Qin C.W., Zhangt Y., Liu Y., Wang J. Bulbophyllum Odoratissimum 3,7- Dihydroxy- 2,4,6-trimethoxyphenanthrene. J. Korean Chem. Soc. 2007;51:352. [Google Scholar]
- 221.Yao X., Wang N., Bei Z., Liu D. Bulbophyllispiradienone Compound and its Derivatives as Antitumor Agent and Inhibiting NO Release from Macrophage. 1594311. China Patent. 2005 Mar 16;
- 222.Yao X., Wang N., Bei Z., Liu D., Zhang J. New Dibenzyl Compounds as Antitumor Agent and Inhibiting Macrophage from Releasing NO. 1594309. China Patent. 2005 Mar 16;
- 223.Chen Y., Xu J., Yu H., Chen Q., Zhang Y., Wang L., Liu Y., Wang J. Cytotoxic phenolics from Bulbophyllum odoratissimum. Food Chem. 2007;107:169–173. doi: 10.1016/j.foodchem.2007.07.077. [DOI] [Google Scholar]
- 224.Xu J., Yu H., Qing C., Zhang Y., Liu Y., Chen Y. Two new biphenanthrenes with cytotoxic activity from Bulbophyllum odoratissimum. Fitoterapia. 2009;80:381–384. doi: 10.1016/j.fitote.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 225.Shimizu M., Shogawa H., Hayashi T., Arisawa M., Suzuki S., Yoshizaki M., Morita N., Ferro E., Basualdo I., Berganza L.H. Chemical and pharmaceutical studies on medicinal plants in Paraguay. Anti-inflammatory constituents of topically applied crude drugs. III. Constituents and anti-inflammatory effect of Paraguayan crude drug “Tamanda cuna” (Catasetum barbatum Lindle) Chem. Pharm. Bull. 1988;36:4447–4452. doi: 10.1248/cpb.36.4447. [DOI] [PubMed] [Google Scholar]
- 226.Huyen D.D. Cymbidium aloifolium (L.) Sw. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 147–148. [Google Scholar]
- 227.Howlader M.A., Alam M., Ahmed K.T., Khatun F., Apu A.S. Antinociceptive and anti-inflammatory activity of the ethanolic extract of Cymbidium aloifolium (L.) Pak. J. Biol. Sci. 2011;14:909–911. doi: 10.3923/pjbs.2011.909.911. [DOI] [PubMed] [Google Scholar]
- 228.Webb L.J. Queensland. Proc. Roy. Soc. 1959;71:103. [Google Scholar]
- 229.Watanabe K., Tanaka R., Sakurai H., Iguchi K., Yamada Y., Hsu C.S., Sakuma C., Kikuchi H., Shibayama H., Kawai T. Structure of cymbidine A, a monomeric peptidoglycan-related compound with hypotensive and diuretic activities, isolated from a higher plant, Cymbidium goeringii (Orchidaceae) Chem. Pharm. Bull. 2007;55:780–783. doi: 10.1248/cpb.55.780. [DOI] [PubMed] [Google Scholar]
- 230.Won J.H., Kim J.Y., Yun K.J., Lee J.H., Back N.I., Chung H.G., Chung S.A., Jeong T.S., Choi M.S., Lee K.T. Gigantol isolated from the whole plants of Cymbidium goeringii inhibits the LPS-induced iNOS and COX-2 expression via NF-κB inactivation in RAW 264.7 macrophages cells. Planta Med. 2006;72:1181–1187. doi: 10.1055/s-2006-947201. [DOI] [PubMed] [Google Scholar]
- 231.Venkateswarlu S., Raju M.S., Subbaraju G.V. Synthesis and biological activity of isoamoenylin, a metabolite of Dendrobium amoenum. Biosci. Biotechnol. Biochem. 2002;66:2236–2238. doi: 10.1271/bbb.66.2236. [DOI] [PubMed] [Google Scholar]
- 232.Yang L., Wang Z., Xu L. Simultaneous determination of phenols (Bibenzyl, phenanthrene, and fluorene) in Dendrobium species by high-performance liquid chromatography with diode array detection. J. Chromatogr. A. 2006;1104:230–237. doi: 10.1016/j.chroma.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 233.Yang L., Han H., Nakamura N., Hattori M., Wang Z., Xu L. Bio-guided isolation of antioxidants from the stems of Dendrobium aurantiacum var. denneanum. Phytother. Res. 2007;21:696–698. doi: 10.1002/ptr.2133. [DOI] [PubMed] [Google Scholar]
- 234.Wu H.S., Xu J.H., Chen L.Z., Sun J.J. Studies on anti-hyperglycemic effect and its mechanism of Dendrobium candidum. Zhongguo Zhong Yao Za Zhi. 2004;29:160–163. [PubMed] [Google Scholar]
- 235.Xu J., Chen L., Li L. Effects of white dendrobium (Denbrobium candidum) and American ginseng (Panax quinquefolium) on nourishing the Yin and promoting glandular secretion in mice and rabbits. Zhongcaoyao. 1995;26:79–80. [Google Scholar]
- 236.He T.G., Yang L.T., Li Y.R., Wan C.Q. Antioxidant activity of crude and purified polysaccharide from suspension-cultured protocorms of Dendrobium candidum in vitro. Zhongchengyao. 2007;29:1265–1269. [Google Scholar]
- 237.Li Y., Wang C.L., Wang Y.J., Guo S.X., Yang J.S., Chen X.M., Xiao P.G. Three New Bibenzyl Derivatives from Dendrobium candidum. Chem. Pharm. Bull. 2009;57:218–219. doi: 10.1248/cpb.57.218. [DOI] [PubMed] [Google Scholar]
- 238.Li Y., Wang C.L., Wang Y.J., Wang F.F., Guo S.X., Yang J.S., Xiao P.G. Four new bibenzyl derivatives from Dendrobium candidum. Chem. Pharm. Bull. 2009;57:997–999. doi: 10.1248/cpb.57.997. [DOI] [PubMed] [Google Scholar]
- 239.Guan H., Zhang X., Tu F., Yao X. Chemical components of Dendrobium candidum. Zhongcaoyao. 2009;40:1873–1876. [Google Scholar]
- 240.Sulistiarini D. Dendrobium crumenatum Sw. In: van Valkenburg J.L.C.H., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(2): Medicinal and Poisonous Plants 2. Backhuys; Leiden, The Netherlands: 2001. p. 216. [Google Scholar]
- 241.Mardisiswojo S., Rajakmangunsudarso H. Cabe Puyang, Warisan Nenek Moyang. Balai Pustaka; Jakarta, Indonesia: 1985. [Google Scholar]
- 242.Sandrasagaran U.M., Ramanathan S., Subramnaniam S., Mansor S.M., Murugaiyah V. Antimicrobial activity of Dendrobium crumenatum (Pigeon Orchid) Malays. J. Pharm. Sci. 2010;1:111–112. [Google Scholar]
- 243.Li Y.M., Wang H.Y., Liu G.Q. Erianin induces apoptosis in human leukemia HL-60 cells. Acta Pharmacol. Sin. 2001;22:1018–1022. [PubMed] [Google Scholar]
- 244.Yang L., Qin L.H., Bligh S.W., Bashall A., Zhang C.F., Zhang M., Wang Z.T., Xu L.S. A new phenanthrene with a spirolactone from Dendrobium chrysanthum and its anti-inflammatory activities. Bioorganic Med. Chem. 2006;14:3496–3501. doi: 10.1016/j.bmc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 245.Fan C., Wang W., Wang Y., Qin G., Zhao W. Chemical constituents from Dendrobium densiflorum. Phytochemistry. 2001;57:1255–1258. doi: 10.1016/S0031-9422(01)00168-6. [DOI] [PubMed] [Google Scholar]
- 246.Heyne K. De Nuttige Planten Van Indonesie. N.V.Uitgeverij W. van Hoeve; ‘s-Gravenhage, The Netherlands: 1950. [Google Scholar]
- 247.Bi Z.M., Wang Z.T., Xu L.S., Xu G.J. Studies on the chemical constituents of Dendrobium fimbriatum. Yao Xue Xue Bao. 2003;38:526–529. [PubMed] [Google Scholar]
- 248.Luo A., Fan Y. In vitro antioxidant of a water-soluble polysaccharide from Dendrobium fimbriatum Hook.var.oculatum Hook. Int. J. Mol. Sci. 2011;12:4068–4079. doi: 10.3390/ijms12064068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ho C.K., Chen C.C. Moscatilin from the orchid Dendrobrium loddigesii is a potential anticancer agent. Cancer Investig. 2003;21:729–736. doi: 10.1081/CNV-120023771. [DOI] [PubMed] [Google Scholar]
- 250.LI M.F., Hirata Y., Xu G.J., Niwa M., Wu H.M. Studies on the chemical constituents of Dendrobium loddigesii rolfe. Yao Xue Xue Bao. 1991;26:307–310. [PubMed] [Google Scholar]
- 251.Chen C.C., Wu L.G., Ko F.N., Teng C.M. Antiplatelet aggregation principles of Dendrobium loddigesii. J. Nat. Prod. 1994;57:1271–1274. doi: 10.1021/np50111a014. [DOI] [PubMed] [Google Scholar]
- 252.Ito M., Matsuzaki K., Wang J., Daikonya A., Wang N.L., Yao X.S., Kitanaka S. New Phenanthrenes and Stilbenes from Dendrobium loddigesii. Chem. Pharm. Bull. 2010;58:628–633. doi: 10.1248/cpb.58.628. [DOI] [PubMed] [Google Scholar]
- 253.Chen K.K., Chen A.L. The alkaloid of Chin-Shih-Hu. J. Biol. Chem. 1935:653–658. [Google Scholar]
- 254.Lin T.H., Chang S.J., Chen C.C., Wang J.P., Tsao L.T. Two phenanthraquinones from Dendrobium moniliforme. J. Nat. Prod. 2001;64:1084–1086. doi: 10.1021/np010016i. [DOI] [PubMed] [Google Scholar]
- 255.Chen Y.L., He G.Q., Zhang M., Li H.J. Hypoglycemic effect of the polysaccharide from Dendrobium moniliforme. Zhejiang Daxue Xuebao Lixueban. 2003;30:693–696. [Google Scholar]
- 256.Wang S., Wei F.J., Cai Y.P., Lin Y. Anti-oxidation activity in vitro of polysaccharides of Dendrobium huoshanense and Dendrobium moniliforme. Agric. Sci. Technol. 2009;10:121–124. [Google Scholar]
- 257.Malla B., Gauchan D.P., Chhetri R.B. An ethnobotanical study of medicinal plants used by ethnic people in Parbat district of western Nepal. J. Ethnopharmacol. 2015;165:103–117. doi: 10.1016/j.jep.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 258.van Valkenburg J.L.C.H., Bunyaprapphatsara N. Plant resources of South-East Asia 12 (2). Medicinal and poisonous plants 2. Back-huys Publisher; Leiden, The Netherlands: 2001. [Google Scholar]
- 259.Gutiérrez R.M.P. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants Res. 2010;4:592–638. [Google Scholar]
- 260.Kong J.M., Goh N.K., Chia L.S., Chia T.F. Recent advances in traditional plant drugs and orchids. Acta Pharmacol. Sin. 2003;24:7–21. [PubMed] [Google Scholar]
- 261.Liu Q.F., Zhao W. A new dedonbrine-type alkaloid from Dendrobium nobile. Chin. Chem. Lett. 2003;14:278–279. [Google Scholar]
- 262.Zhao W., Ye Q., Tan X., Jiang H., Li X., Chen K., Kinghorn A.D. Three new sesquiterpene glycosides from Dendrobium nobile with immunomodulatory activity. J. Nat. Prod. 2001;64:1196–1200. doi: 10.1021/np0102612. [DOI] [PubMed] [Google Scholar]
- 263.Ye Q., Qin G., Zhao W. Immunomodulatory sesquiterpene glycosides from Dendrobium nobile. Phytochemistry. 2002;61:885–890. doi: 10.1016/S0031-9422(02)00484-3. [DOI] [PubMed] [Google Scholar]
- 264.Zhang X., Xu J.K., Wang J., Wang N.L., Kurihara H., Kitanaka S., Yao X.S. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J. Nat. Prod. 2006;70:24–28. doi: 10.1021/np060449r. [DOI] [PubMed] [Google Scholar]
- 265.Luo A., He X., Zhou S., Fan Y., He T., Chun Z. In vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. extracts. Int. J. Biol. Macromol. 2009;45:359–363. doi: 10.1016/j.ijbiomac.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 266.Uma D. Antitumor and antimicrobial activities and inhibition of in-vitro lipid peroxidation by Dendrobium nobile. Afr. J. Biotechnol. 2009;8:2289. [Google Scholar]
- 267.Hwang J.S., Lee S.A., Hong S.S., Han X.H., Lee C., Kang S.J., Lee D., Kim Y., Hong J.T., Lee M.K., et al. Phenanthrenes from Dendrobium nobile and their inhibition of the LPS-induced production of nitric oxide in macrophage RAW 264.7 cells. Bioorganic Med. Chem. Lett. 2010;20:3785–3787. doi: 10.1016/j.bmcl.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 268.Wang J.H., Luo J.P., Zha X.Q., Feng B.J. Comparison of antitumor activities of different polysaccharide fractions from the stems of Dendrobium nobile Lindl. Carbohydr. Polym. 2010;79:114–118. doi: 10.1016/j.carbpol.2009.07.032. [DOI] [Google Scholar]
- 269.Lassak E.V., McCarthy T. Australian Medicinal Plants: A Complete Guide to Identification and Usage. New Holland; Chatswood, Australia: 2011. [Google Scholar]
- 270.Maiden J.H. Indigenous vegetable drugs. Part II. Agric. Gaz. N.S.W. 1899;10:131–141. [Google Scholar]
- 271.Lo S.F., Mulabagal V., Chen C.L., Kuo C.L., Tsay H.S. Bioguided fractionation and isolation of free radical scavenging components from in vitro propagated chinese medicinal plants Dendrobium tosaense Makino and Dendrobium moniliforme SW. J. Agric. Food Chem. 2004;52:6916–6919. doi: 10.1021/jf040017r. [DOI] [PubMed] [Google Scholar]
- 272.Floriani A.E., Ferreira J., Santos A.R., Delle-Monache F., Yunes R.A., Cechinel-Filho V. Analgesic compounds from Epidendrum mosenii stems. Pharmazie. 1998;53:426–427. [PubMed] [Google Scholar]
- 273.Ferreira J., Floriani A.E.O., Cechinel F.V., Delle M.F., Yunes R.A., Calixto J.B., Santos A.R.S. Antinociceptive properties of the methanolic extract and two triterpenes isolated from Epidendrum mosenii stems (Orchidaceae) Life Sci. 2000;66:791–802. doi: 10.1016/S0024-3205(99)00652-9. [DOI] [PubMed] [Google Scholar]
- 274.Hernández-Romero Y., Acevedo L., Sánchez M.L., Shier W.T., Abbas H.K., Mata R. Phytotoxic activity of bibenzyl derivatives from the orchid Epidendrum rigidum. J. Agric. Food Chem. 2005;53:6276–6280. doi: 10.1021/jf0508044. [DOI] [PubMed] [Google Scholar]
- 275.Huyen D.D. Eria pannea Lindley. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. p. 192. [Google Scholar]
- 276.Namsa N.D., Tag H., Mandal M., Kalita P., Das A.K. An ethnobotanical study of traditional anti-inflammatory plants used by the Lohit community of Arunachal Pradesh, India. J. Ethnopharmacol. 2009;125:234–245. doi: 10.1016/j.jep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 277.Sulistiarini D. Grammatophyllum scriptum Bl. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. p. 222. [Google Scholar]
- 278.Herman M.J. Liparis treubii J.J. Smith. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 273–274. [Google Scholar]
- 279.Olof T.C. Survival and flowering of some perennial herbs II. The behavior of some orchids on permanent plots. Oikos. 1972;23:23–28. [Google Scholar]
- 280.Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Balzarini J., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007;75:179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Déciga-Campos M., Palacios-Espinosa J.F., Reyes-Ramírez A., Mata R. Antinociceptive and anti-inflammatory effects of compounds isolated from Scaphyglottis livida and Maxillaria densa. J. Ethnopharmacol. 2007;114:161–168. doi: 10.1016/j.jep.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 282.Hernández-Romero Y., Rojas J.I., Castillo R., Rojas A., Mata R. Spasmolytic effects, mode of action, and structure-activity relationships of stilbenoids from Nidema boothii. J. Nat. Prod. 2004;67:160–167. doi: 10.1021/np030303h. [DOI] [PubMed] [Google Scholar]
- 283.Huyen D.D. Oberonia anceps Lindley. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. p. 319. [Google Scholar]
- 284.Huyen D.D. Oberobia denticulate Wight. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. p. 319. [Google Scholar]
- 285.Wang J., Matsuzaki K., Kitanaka S. Stilbene derivatives from Pholidota chinensis and their anti-inflammatory activity. Chem. Pharm. Bull. 2006;54:1216–1218. doi: 10.1248/cpb.54.1216. [DOI] [PubMed] [Google Scholar]
- 286.Wang J., Wang L., Kitanaka S. Stilbene and dihydrophenanthrene derivatives from Pholidota chinensis and their nitric oxide inhibitory and radical-scavenging activities. J. Nat. Med. 2007;61:381–386. doi: 10.1007/s11418-007-0162-7. [DOI] [Google Scholar]
- 287.Déciga-Campos M., Rivero-Cruz I., Arriaga-Alba M., Castañeda-Corral G., Angeles-López G.E., Navarrete A., Mata R. Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. J. Ethnopharmacol. 2007;110:334–342. doi: 10.1016/j.jep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 288.Estrada S., Rojas A., Mathison Y., Israel A., Mata R. Nitric oxide/cGMP mediates the spasmolytic action of 3,4′-dihydroxy-5,5′-dimethoxybibenzyl from Scaphyglottis livida. Planta Med. 1999;65:109–114. doi: 10.1055/s-1999-14056. [DOI] [PubMed] [Google Scholar]
- 289.Basu K.D., Gupta B., Bhattacharya S.K., Lal R., Das P.K. Antiinflammatory principles of Vanda roxburghii. Curr. Sci. 1971;40:40–86. [Google Scholar]
- 290.Suresh P.K., Subramoniam A., Pushpangadan P. Aphodisiac activity of Vanda tessellata. Indian J. Pharmacol. 2000;32:300–304. [Google Scholar]
- 291.Chawla A.S., Sharma A.K., Handa S.S., Dhar K.L. Chemical studies and anti-inflammatory activity of Vanda roxburghii roots. Indian J. Pharm. Sci. 1992;54:159–161. [Google Scholar]
- 292.Prasad D.N., Achari G. A study of anti-arthritic action of Vanda roxburghii in albino rats. J. Indian Med. Assoc. 1966;46:234–237. [PubMed] [Google Scholar]
- 293.Arya A., Abdullah M.A., Haerian B.S., Mohd M.A. Screening for hypoglycemic activity on the leaf extracts of nine medicinal plants: In-Vivo evaluation. J. Chem. 2012;9 doi: 10.1155/2012/103760. [DOI] [Google Scholar]
- 294.Corner E.J.H., Watanabe K. Illustrated Guide to Tropical Plants. Hirokawa Publishing Co.; Tokyo, Japan: 1969. [Google Scholar]
- 295.Simmler C., Antheaume C., Andreé P., Bonteé F.d.R., Lobstein A. Glucosyloxybenzyl eucomate derivatives from Vanda teres stimulate HaCaT cytochrome c oxidase. J. Nat. Prod. 2011;74:949–955. doi: 10.1021/np1006636. [DOI] [PubMed] [Google Scholar]
- 296.Shanmugavalli N., Umashankar V., Raheem S. Anitmicrobial activity of Vanilla planifolia. Indian J. Sci. Technol. 2009;2:37–40. [Google Scholar]
- 297.Hammond G.B., Ferna’ndez I.D., Villegas L.F., Vaisberg A.J. A survey of traditional medicinal plants from the Callejo’n de Huaylas, Department of Ancash, Peru’. J. Ethnopharmacol. 1998;61:17–30. doi: 10.1016/S0378-8741(98)00009-9. [DOI] [PubMed] [Google Scholar]
- 298.De Feo V., Belaunde A.J., Sandoval J.G., Senatore F., Formisano C. Antibacterial activity and composition of the essential oil of Peperomia galioides HBK (Piperaceae) from Peru. Nat. Prod. Commun. 2008;3:933–936. doi: 10.1177/1934578X0800300622. [DOI] [Google Scholar]
- 299.Langfield R.D., Scarano F.J., Heitzman M.E., Kondo M., Hammond G.B., Neto C.C. Use of a modified microplate bioassay method to investigate antibacterial activity in the Peruvian medicinal plant Peperomia galioides. J. Ethnopharmacol. 2004;94:279–281. doi: 10.1016/j.jep.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 300.Samsali O. Bachelor’s Thesis. Universitas Sebelas Maret; Surakarta, Indoensia: 2008. Tumbuhan Epifit Berkhasiat Obat di Sepanjang Jalur Pendakian Cemara Sewu Gunung Lawu. [Google Scholar]
- 301.Shin K.H., Yun H.S., Woo W.S., Lee C.K. Pharmacologically active principle of Piper retrofractum. Soul Taehakkyo Saengyak Yonguso Opjukjip. 1979;18:87–89. [Google Scholar]
- 302.Masuda T., Oyama Y., Yamamoto N., Umebayashi C., Nakao H., Toi Y., Takeda Y., Nakamoto K., Kuninaga H., Nishizato Y., et al. Cytotoxic screening of medicinal and edible plants in Okinawa, Japan, and identification of the main toxic constituent of Rohdea japonica (Omoto) Biosci. Biotechnol. Biochem. 2003;67:1401–1404. doi: 10.1271/bbb.67.1401. [DOI] [PubMed] [Google Scholar]
- 303.Huh T.R., Lee S.E., Park B.S. Alkaloids Having Potent Inhibiting Activity of Platelet Aggregation. 2004009637. Korea Patent. 2004 Jan 31;
- 304.Chansang U. Mosquito larvicidal activity of aqueous extracts of long pepper (Piper retrofractum vahl) from Thailand. J. Vector Ecol. 2005;30:195–200. [PubMed] [Google Scholar]
- 305.Komalamisra N., Trongtokit Y., Palakul K., Prummongkol S., Samung Y., Apiwathnasorn C., Phanpoowong T., Asavanich A., Leemingsawat S. Insecticide susceptibility of mosquitoes invading tsunami-affected areas of Thailand. Southeast Asian J. Trop. Med. Public Health. 2006;37:118–122. [PubMed] [Google Scholar]
- 306.Kametani S., Kikuzaki H., Honzawa M., Nakatani N. Chemical constituents of Piper retrofractum vahl and their antioxidant and radical scavenging activities. ITE Lett. Batter. New Technol. Med. 2005;6:566–573. [Google Scholar]
- 307.Bodiwala H., Singh G., Singh R., Dey C., Sharma S., Bhutani K., Singh I. Antileishmanial amides and lignans from Piper cubeba and Piper retrofractum. J. Nat. Med. 2007;61:418–421. doi: 10.1007/s11418-007-0159-2. [DOI] [Google Scholar]
- 308.Kim K.J., Lee M.S., Jo K., Hwang J.K. Piperidine alkaloids from Piper retrofractum Vahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase. Biochem. Biophys. Res Commun. 2011;411:219–225. doi: 10.1016/j.bbrc.2011.06.153. [DOI] [PubMed] [Google Scholar]
- 309.Ueda J.Y., Tezuka Y., Banskota A.H., Tran Q.L., Tran Q.K., Harimaya Y., Saiki I., Kadota S. Antiproliferative activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2002;25:753–760. doi: 10.1248/bpb.25.753. [DOI] [PubMed] [Google Scholar]
- 310.Nguyen M.T.T., Awale S., Tezuka Y., Tran Q.L., Watanabe H., Kadota S. Xanthine oxidase Iinhibitory activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2004;27:1414–1421. doi: 10.1248/bpb.27.1414. [DOI] [PubMed] [Google Scholar]
- 311.Prachayasittikul S., Buraparuangsang P., Worachartcheewan A., Isarankura-Na-Ayudhya C., Ruchirawat S., Prachayasittikul V. Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules. 2008;13:904–921. doi: 10.3390/molecules13040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Hasmah Release of cytochrome c in MCF-7 cells treated with 7,3′,5′-trihydroxyflavanone of Hydnophytum formicarium. Biomed. Pharmacol. J. 2009;2:1–6. [Google Scholar]
- 313.Abdullah H., Pihie A.H.L., Hohmann J., Molnar J. A natural compound from Hydnophytum formicarum induces apoptosis of MCF-7 cells via up-regulation of Bax. Cancer Cell Int. 2010;10 doi: 10.1186/1475-2867-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lemmens R.H.M.J. Myrmecodia tuberosa Jack. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 314–315. [Google Scholar]
- 315.Hertiani T., Sasmito E., Ulfah M. Preliminary study on immunomodulatory effect of Sarang-Semut tubers Myrmecodia tuberosa and Myrmecodia pendens. Online J. Biol. Sci. 2010;10:136–141. doi: 10.3844/ojbsci.2010.136.141. [DOI] [Google Scholar]
- 316.Syahrawi N.F. Bachelor’s Thesis. Istitut Pertanian Bogor; Bogor, Indonesia: 2008. Studi Pemanfaatan Sarang Semut (Myrmecodia pendans Merr. & Perry) oleh Masyarakat Sekitar Taman Nasional Wasur. [Google Scholar]
- 317.van Valkenburg J.L.C.H. Viscum articulatum Burm.f. In: Lemmens R.H.M.J., Bunyapraphatsara N., editors. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3. Backhuys; Leiden, The Netherlands: 2003. pp. 417–418. [Google Scholar]
- 318.Samuelsson G. Screening of plants of the family Loranthaceae for toxic proteins. Acta Pharm. Suec. 1966;3:353–362. [PubMed] [Google Scholar]
- 319.Yui S., Mikami M., Kitahara M., Yamazaki M. The inhibitory effect of lycorine on tumor cell apoptosis induced by polymorphonuclear leukocyte-derived calprotectin. Immunopharmacol. 1998;40:151–162. doi: 10.1016/S0162-3109(98)00040-X. [DOI] [PubMed] [Google Scholar]
- 320.Leu Y.L., Kuo S.M., Hwang T.L., Chiu S.T. The Inhibition of superoxide anion generation by neutrophils from Viscum articulactum. Chem. Pharm. Bull. 2004;52:858–860. doi: 10.1248/cpb.52.858. [DOI] [PubMed] [Google Scholar]
- 321.Li Y., Zhao Y.L., Huang N., Zheng Y.T., Yang Y.P., Li X.L. Two new phenolic glycosides from Viscum articulatum. Molecules. 2008;13:2500–2508. doi: 10.3390/molecules13102500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Patil C.R., Jadhav R.B., Singh P.K., Mundada S., Patil P.R. Protective effect of oleanolic acid on gentamicin induced nephrotoxicity in rats. Phytother. Res. 2010;24:33–37. doi: 10.1002/ptr.2861. [DOI] [PubMed] [Google Scholar]
- 323.Kuo Y.J., Yang Y.C., Zhang L.J., Wu M.D., Kuo L.M.Y., Kuo Y.C., Hwang S.Y., Chou C.J., Lee K.H., Ho H.O., et al. Flavanone and diphenylpropane glycosides and glycosidic acyl esters from Viscum articulatum. J. Nat. Prod. 2010;73:109–114. doi: 10.1021/np9004294. [DOI] [PubMed] [Google Scholar]
- 324.Jadhav R.B. Diuretic and natriuretic activity of two mistletoe species in rats. Pharmacogn. Res. 2010;2:50. doi: 10.4103/0974-8490.60576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Geetha K.M., Bhaskara Gopal P.V.V.S., Murugan V. Antiepileptic activity of aerial parts of Viscum articulatum (Viscaceae) in rats. J. Pharm. Res. 2010;3:2886–2887. [Google Scholar]
- 326.Bachhav S.S., Patil S.D., Bhutada M.S., Surana S.J. Oleanolic acid prevents glucocorticoid-induced hypertension in rats. Phytother. Res. 2011;25:1435–1439. doi: 10.1002/ptr.3431. [DOI] [PubMed] [Google Scholar]
- 327.Bachhav S.S., Bhutada M.S., Patil S.D., Baser B., Chaudhari K.B. Effect of Viscum articulatum Burm. (Loranthaceae) in Nω-nitro-l-arginine methyl ester induced hypertension and renal dysfunction. J. Ethnopharmacol. 2012;142:467–473. doi: 10.1016/j.jep.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 328.Zhong W., Peng W., Yu Z., Chen Y. In vitro antioxidant activity of polysaccharides from Viscum articulatum. Shipin Kexue. 2011;32:25–28. [Google Scholar]
- 329.van Valkenburg J.L.C.H. Plant Resources of South-East Asia No 12(3): Medicinal and Poisonous Plants 3, Lemmens, R.H.M.J., Bunyapraphatsara, N., Eds. Backhuys; Leiden, The Netherlands: 2003. Viscum ovalifolium DC; pp. 417–418. [Google Scholar]
- 330.Singh J., Rao M.N.A., Hardikar S.G. Chemical constituents of Adiantum caudatum. Indian J. Pharm. 1975;37:64–65. [Google Scholar]
- 331.Gupta M., Bagchi A., Roy S.K., Ray A.B. Chemical constituents of a member of Adiantum caudatum complex. J. Indian Chem. Soc. 1990;67:86–88. [Google Scholar]
- 332.Tsuzuki K., Ohashi A., Arai Y., Masuda K., Takano A., Shiojima K., Ageta H., Cai S.Q. Triterpenoids from Adiantum caudatum. Phytochemistry. 2001;58:363–367. doi: 10.1016/S0031-9422(01)00198-4. [DOI] [PubMed] [Google Scholar]
- 333.Berg A.M., Kari S., Alfthan M., Virtanen A.I. Homoserine and α-aminoadipic acid in green plants. Acta Chem. Scand. 1954;8:358. doi: 10.3891/acta.chem.scand.08-0358. [DOI] [Google Scholar]
- 334.Liu H., Orjala J., Rali T., Sticher O. Glycosides from Stenochlaena palustris. Phytochemistry. 1998;49:2403–2408. doi: 10.1016/S0031-9422(98)00352-5. [DOI] [Google Scholar]
- 335.Liu H., Orjala J., Sticher O., Rali T. Acylated flavonol glycosides from leaves of Stenochlaena palustris. J. Nat. Prod. 1999;62:70–75. doi: 10.1021/np980179f. [DOI] [PubMed] [Google Scholar]
- 336.Lin Y.Y., Kakisawa H., Shiobara Y., Nakanishi K. Structure of davallic acid. Chem. Pharm. Bull. 1965;13:986–995. doi: 10.1248/cpb.13.986. [DOI] [PubMed] [Google Scholar]
- 337.Harborne J.B. Comparative biochemistry of flavonoids. II. 3-Deoxyanthocyanins and their systematic distribution in ferns and gesnerads. Phytochemistry. 1966;5:589–600. doi: 10.1016/S0031-9422(00)83637-7. [DOI] [Google Scholar]
- 338.Tanaka Y., Tohara K., Terasawa K., Sawada M., Ageta H. Pharmacognostical studies on Ku-tsui-po. II. Shoyakugaku Zasshi. 1978;32:260–266. [Google Scholar]
- 339.Murakami T., Wada H., Tanaka N., Kuraishi T., Saiki Y., Chen C.M. Chemical and chemotaxonomical studies of Filices. 56. Constituents of the davalliaceous ferns. 1. Yakugaku Zasshi. 1985;105:649–654. doi: 10.1248/yakushi1947.105.7_649. [DOI] [Google Scholar]
- 340.Hwang T.H., Kashiwada Y., Nonaka G., Nishioka I. Tannins and related compounds. Part 89. 4-Carboxymethyl flavan-3-ols and procyanidins from Davallia divaricata. Phytochemistry. 1990;29:279–282. doi: 10.1016/0031-9422(90)89050-J. [DOI] [Google Scholar]
- 341.Tanaka Y., Kitajima J.I., Ageta H. Pharmacognostical studies on “Ku-tui-po”. III. Constituents of the rhizomes of Davallia solida. Nat. Med. 1998;52:409–413. [Google Scholar]
- 342.Rancon S., Chaboud A., Darbour N., Comte G., Barron D., Raynaud J., Cabalion P. A new C-glycosyl xanthone isolated from Davallia solida. Phytochemistry. 1999;52:1677–1679. doi: 10.1016/S0031-9422(99)00190-9. [DOI] [Google Scholar]
- 343.Rouffiac R. Alkaloids in Lycopodium phlegmaria. Compt. Rend. 1961;253:2612–2613. [PubMed] [Google Scholar]
- 344.Rouffiac R. Alkaloids of lycopods, particularly of Lycopodium phlegmaria. Ann. Pharm. Fr. 1963;21:685–698. [PubMed] [Google Scholar]
- 345.Inubushi Y., Hibino T., Hasegawa T., Somanathan R. Isolation and structure of phlegmanol F. Chem. Pharm. Bull. 1971;19:2640–2642. doi: 10.1248/cpb.19.2640. [DOI] [Google Scholar]
- 346.Shi H., Li Z.Y., Guo Y.W. A new serratane-type triterpene from Lycopodium phlegmaria. Nat. Prod. Res. 2005;19:777–781. doi: 10.1080/14786410500044906. [DOI] [PubMed] [Google Scholar]
- 347.Hirasawa Y., Tanaka T., Kobayashi J.i., Kawahara N., Goda Y., Morita H. Malycorins A-C, new lycopodium alkaloids from Lycopodium phlegmaria. Chem. Pharm. Bull. 2008;56:1473–1476. doi: 10.1248/cpb.56.1473. [DOI] [PubMed] [Google Scholar]
- 348.Inubushi Y., Harayama T. Alkaloid constituents of Lycopodium phlegmaria L. Yakugaku Zasshi. 1982;102:434–439. doi: 10.1248/yakushi1947.102.5_434. [DOI] [Google Scholar]
- 349.Miller N., Hootele C., Braekman J.C. Triterpenoids of Lycopodium megastachyum. Phytochemistry. 1973;12:1759–1761. doi: 10.1016/0031-9422(73)80398-X. [DOI] [Google Scholar]
- 350.Braekman J.C., Hootele C., Miller N., Declercq J.P., Germain G., Van Meerssche M. Megastachine, a new alkaloid from Lycopodium megastachyum. Can. J. Chem. 1979;57:1691–1693. doi: 10.1139/v79-271. [DOI] [Google Scholar]
- 351.Siems K., Weigt F., Wollenweber E. Drimanes from the epicuticular wax of the fern Nephrolepis biserrata. Phytochemistry. 1996;41:1119–1121. doi: 10.1016/0031-9422(95)00753-9. [DOI] [Google Scholar]
- 352.Sun M., Wang T. Traditional Chinese Herbal Extractscontaining Sequoyitol for Preventing and Treating Diabetes and Complications. 1957992. China Patent. 2007 May 9;
- 353.Liang Z. Chemical constituents of Nephrolepis cordifolia. Guangxi Zhiwu. 2008;28:420. [Google Scholar]
- 354.Tsai T.H., Wang G.J., Lin L.C. Vasorelaxing alkaloids and flavonoids from Cassytha filiformis. J. Nat. Prod. 2008;71:289–291. doi: 10.1021/np070564h. [DOI] [PubMed] [Google Scholar]
- 355.Liang Y.H., Wang W., Yu S.W., Ye M., He X.H., Gong N.B., Lu Y., Khan I.A., Guo D.A. A new chiratane type triterpenoid from the rhizomes of Drynaria fortunei. Fitoterapia. 2010;81:988–991. doi: 10.1016/j.fitote.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 356.Liang Y.H., Ye M., Yang W.Z., Qiao X., Wang Q., Yang H.J., Wang X.L., Guo D.A. Flavan-3-ols from the rhizomes of Drynaria fortunei. Phytochem. 2011;72:1876–1882. doi: 10.1016/j.phytochem.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 357.Shang Z.P., Meng J.J., Zhao Q.C., Su M.Z., Luo Z., Yang L., Tan J.J. Two new chromone glycosides from Drynaria fortunei. Fitoterapia. 2013;84:130–134. doi: 10.1016/j.fitote.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 358.Trinh P.T.N., Hao N.C., Thao P.T., Dung L.T. Chemical components of the rhizomes of Drynaria fortunei (KUNZE) J. Sm. (polypodiaceae) in Vietnam. Collect. Czech. Chem. Commun. 2011;76:1133–1139. doi: 10.1135/cccc2010098. [DOI] [Google Scholar]
- 359.Liu S., Xiao Z., Feng R. A flavanol glycoside from Drynaria propinqua. Phytochemistry. 1994;35:1595–1596. doi: 10.1016/S0031-9422(00)86903-4. [DOI] [Google Scholar]
- 360.Ramesh N., Viswanathan M.B., Saraswathy A., Balakrishna K., Brindha P., Lakshmanaperumalsamy P. Phytochemical and antimicrobial studies on Drynaria quercifolia. Fitoterapia. 2001;72:934–936. doi: 10.1016/S0367-326X(01)00342-2. [DOI] [PubMed] [Google Scholar]
- 361.Nugraha A.S., Wangchuk T., Willis A.C., Haritakun R., Sujadmiko H., Keller P.A. Phytochemical and pharmacological studies on four Indonesian epiphytic medicinal plants: Drynaria rigidula, Hydnophytum formicarum, Usnea misaminensis, and Calymperes schmidtii. Nat. Prod. Commun. 2019;14 doi: 10.1177/1934578X19856792. [DOI] [Google Scholar]
- 362.Hikin H., Meguro K., Takemot T. Isolation of diploptene from Pyrrosia lingua. Chem. Pharm. Bull. 1963;11:409–410. doi: 10.1248/cpb.11.409. [DOI] [Google Scholar]
- 363.Yamashita H., Masuda K., Kobayashi T., Ageta H., Shiojima K. Dammarane triterpenoids from rhizomes of Pyrrosia lingua. Phytochemistry. 1998;49:2461–2466. doi: 10.1016/S0031-9422(98)00303-3. [DOI] [Google Scholar]
- 364.Yamashita H., Masuda K., Ageta H., Shiojima K. Fern constituents: Cyclohopenol and cyclohopanediol, novel skeletal triterpenoids from rhizomes of Pyrrosia lingua. Chem. Pharm. Bull. 1998;46:730–732. doi: 10.1248/cpb.46.730. [DOI] [Google Scholar]
- 365.Yang C., Shi J.G., Mo S.Y., Yang Y.C. Chemical constituents of Pyrrosia petiolosa. J. Asian Nat. Prod. Res. 2003;5:143–150. doi: 10.1080/1028602031000066843. [DOI] [PubMed] [Google Scholar]
- 366.Yang Y.C., Yang C., Mo S.Y., Shi J.G. A new flavonol diglycoside from Pyrrosia petiolosa. Chin. Chem. Lett. 2003;14:920–922. [Google Scholar]
- 367.Wang N., Wang J.H., Li X., Ling J.H., Li N. Flavonoids from Pyrrosia petiolosa (Christ) Ching. J. Asian Nat. Prod. Res. 2006;8:753–756. doi: 10.1080/10286020500246550. [DOI] [PubMed] [Google Scholar]
- 368.Markham K.R. The structures of amentoflavone glycosides isolated from Psilotum nudum. Phytochemistry. 1984;23:2053–2056. doi: 10.1016/S0031-9422(00)84969-9. [DOI] [Google Scholar]
- 369.Balza F., Muir A.D., Towers G.H.N. 3′-Hydroxypsilotin, a minor phenolic glycoside from Psilotum nudum. Phytochemistry. 1985;24:529–531. doi: 10.1016/S0031-9422(00)80761-X. [DOI] [Google Scholar]
- 370.Akihisa T., Kawashima T., Takahashi S., Sahashi N., Okamoto T., Niiya I., Tamura T. Sterols and fatty acids of a whisk fern Psilotum nudum. J. Am. Oil Chem. Soc. 1992;69:1232–1235. doi: 10.1007/BF02637687. [DOI] [Google Scholar]
- 371.Zheng L. Psilotin with Antitumor Effect. 1028278. China Patent. 2007 Sep 5;
- 372.Tanaka N., Murakami T., Saiki Y., Chen C.M., Gomez P.L.D. Chemical and chemotaxonomical studies of ferns. XXXVII. Chemical studies on the constituents of Costa Rican ferns. 2. Chem. Pharm. Bull. 1981;29:3455–3463. doi: 10.1248/cpb.29.3455. [DOI] [Google Scholar]
- 373.Sultana S., Ilyas M., Shaida W.A. Chemical investigation of Acrostichum aureum Linn. J. Indian Chem. Soc. 1986;63:1074–1075. [Google Scholar]
- 374.Uddin S.J., Jason T.L.H., Beattie K.D., Grice I.D., Tiralongo E. (2S,3S)-Sulfated Pterosin C, a cytotoxic sesquiterpene from the Bangladeshi,angrove fern Acrostichum aureum. J. Nat. Prod. 2011;74:2010–2013. doi: 10.1021/np2004598. [DOI] [PubMed] [Google Scholar]
- 375.Lu M., Huang K., Shi S., Zhang H. Study on the chemical constituents of Selaginella involvens Spring and in vitro antibacterial activities of partial chemical constituents. Tianran Chanwu Yanjiu Yu Kaifa. 2009;21:973–975. [Google Scholar]
- 376.Merchant J.R., Desai H.K. Isolation of nantenine from Cassytha filiformis and its synthesis. Indian J. Chem. 1973;11:342–344. [Google Scholar]
- 377.Wu Y.C., Chao Y.C., Chang F.R., Chen Y.Y. Alkaloids from Cassytha filiformis. Phytochemistry. 1997;46:181–184. [Google Scholar]
- 378.Chang F.R., Chao Y.C., Teng C.M., Wu Y.C. Chemical constituents from Cassytha filiformis II. J. Nat. Prod. 1998;61:863–866. doi: 10.1021/np970348g. [DOI] [PubMed] [Google Scholar]
- 379.Stevigny C., Block S., De Pauw-Gillet M.C., De Hoffmann E., Llabres G., Adjakidje V., Quetin-Leclercq J. Cytotoxic aporphine alkaloids from Cassytha filiformis. Planta Med. 2002;68:1042–1044. doi: 10.1055/s-2002-35651. [DOI] [PubMed] [Google Scholar]
- 380.Ho J.C., Chen C.M., Row L.C. Neolignans from the parasitic plants. Part 2. Cassytha filiformis. J. Chin. Chem. Soc. 2004;51:221–223. doi: 10.1002/jccs.200400034. [DOI] [Google Scholar]
- 381.Li G., Chen Y. Study on the chemical constituents of Cuscuta australis R.Br. Zhongguo Zhongyao Zazhi. 1997;22:548–550. [PubMed] [Google Scholar]
- 382.Guo C., Han G., Su Z. Chemical constituents from the seeds of Cuscuta australis. Zhongguo Yaoxue Zazhi. 1997;32:8–11. [Google Scholar]
- 383.Guo H., Li J. Study on constituents of the seed from Cuscuta australis. Beijing Zhongyiyao Daxue Xuebao. 2000;23:20–23. [Google Scholar]
- 384.Anis E., Mustafa G., Ullah N., Malik A., Afza N., Badar Y. Phytochemical studies on Cuscuta reflexa. Pak. J. Sci. Ind. Res. 1999;42:170–172. [Google Scholar]
- 385.Anis E., Mustafa G., Ahmed S., Malik A., Afza N., Badar Y. Sterols and sterol glycosides from Cuscuta reflexa. Nat. Prod. Sci. 1999;5:124–126. [Google Scholar]
- 386.Gonzalez J., Arias T., Moreno B., Arias B. Terpenes isolated from the fruits of Clusia ssp. Rev. Colomb. Quim. 1988;17:89–91. [Google Scholar]
- 387.Mallavadhani U.V., Narasimhan K., Sudhakar A.V.S., Mahapatra A., Li W., van Breemen R.B. Three new pentacyclic triterpenes and some flavonoids from the fruits of an Indian ayurvedic plant Dendrophthoe falcata and their estrogen receptor binding activity. Chem. Pharm. Bull. 2006;54:740–744. doi: 10.1248/cpb.54.740. [DOI] [PubMed] [Google Scholar]
- 388.Wang Q., Li L., Li M. Studies on the chemical constituents of qiaohuajisheng (Macrosolen cochinchinensis) Zhongcaoyao. 1996;27:518–521. [Google Scholar]
- 389.Lohezic-Le Devehat F., Tomasi S., Fontanel D., Boustie J. Flavonols from Scurrula ferruginea Danser (Loranthaceae) Z. Fuer Nat. C J. Biosci. 2002;57:1092–1095. doi: 10.1515/znc-2002-11-1224. [DOI] [PubMed] [Google Scholar]
- 390.Kitajima J., Kimizuka K., Tanaka Y. New sterols and triterpenoids of Ficus pumila fruit. Chem. Pharm. Bull. 1998;46:1408–1411. doi: 10.1248/cpb.46.1408. [DOI] [PubMed] [Google Scholar]
- 391.Kitajima J., Kimizuka K., Tanaka Y. New dammarane-type acetylated triterpenoids and their related compounds of Ficus pumila fruit. Chem. Pharm. Bull. 1999;47:1138–1140. doi: 10.1248/cpb.47.1138. [DOI] [Google Scholar]
- 392.Kitajima J., Kimizuka K., Tanaka Y. Three new sesquiterpenoid glucosides of Ficus pumila fruit. Chem. Pharm. Bull. 2000;48:77–80. doi: 10.1248/cpb.48.77. [DOI] [PubMed] [Google Scholar]
- 393.Du X.M., Sun N.Y., Irino N., Shoyama Y. Glycosidic constituents from in Vitro Anoectochilus formosanus. Chem. Pharm. Bull. 2000;48:1803–1804. doi: 10.1248/cpb.48.1803. [DOI] [PubMed] [Google Scholar]
- 394.Markham K.R., Ternai B., Stanley R., Geiger H., Mabry T.J. Carbon-13 NMR studies of flavonoids-III: Naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron. 1978;34:1389–1397. doi: 10.1016/0040-4020(78)88336-7. [DOI] [Google Scholar]
- 395.He C., Wang C., Guo S., Yang J., Xiao P. Study on chemical constituents of Anoectochilus roxburghii (wall.): From the n-hexane soluble fraction of the ethanol extracts of Anoectochilus roxburghii, sorghumol (1), friedelin (2), palmitic acid (3), and a mixture of sterols were isolated from the plant for the first time. Tianran Chanwu Yanjiu Yu Kaifa. 2005;17:259–262. [Google Scholar]
- 396.Wang L.F., Lin C.M., Shih C.M., Chen H.J., Su B., Tseng C.C., Gau B.B., Cheng K.T. Prevention of cellular oxidative damage by an aqueous extract of Anoectochilus formosanus. Ann. N. Y. Acad. Sci. 2005;1042:379–386. doi: 10.1196/annals.1338.060. [DOI] [PubMed] [Google Scholar]
- 397.He C., Wang C., Guo S., Yang J., Xiao P. Study on chemical constituents in herbs of Anoectochilus roxburghii II. Zhongguo Zhongyao Zazhi. 2005;30:761–763. [PubMed] [Google Scholar]
- 398.Guan J., Wang C., Guo S. Isolation and structural elucidation of flavonoids from Ancecotochilus roxburghii. Zhongcaoyao. 2005;36:1450–1453. [Google Scholar]
- 399.He C.N., Wang C.L., Guo S.X., Yang J.S., Xiao P.G. A novel flavonoid glucoside from Anoectochilus roxburghii (Wall.) Lindl. J. Integr. Plant Biol. 2006;48:359–363. doi: 10.1111/j.1744-7909.2006.00179.x. [DOI] [Google Scholar]
- 400.Yang X., Han M., Jin Y. Chemical constituents from herba anoectochili. Zhongyaocai. 2007;30:797–800. [PubMed] [Google Scholar]
- 401.Han M.H., Yang X.W., Jin Y.P. Novel triterpenoid acyl esters and alkaloids from Anoectochilus roxburghii. Phytochem. Anal. 2008;19:438–443. doi: 10.1002/pca.1070. [DOI] [PubMed] [Google Scholar]
- 402.Cai J., Gong L., Zhang Y., Ruan H., Pi H., Wu J. Chemical constituents from Anoectochilus roxburghii. Zhongyaocai. 2008;31:370–372. [PubMed] [Google Scholar]
- 403.Wu B., Chen J.B., He S., Pan Y.J. Oxepine and bibenzyl compounds from Bulbophyllum kwangtungense. Gaodeng Xuexiao Huaxue Xuebao. 2008;29:305–308. [Google Scholar]
- 404.Majumder P.L., Sen R.C. Bulbophyllanthrone, a phenanthraquinone from Bulbophyllum odoratissimum. Phytochemistry. 1991;30:2092–2094. doi: 10.1016/0031-9422(91)85078-E. [DOI] [Google Scholar]
- 405.Liu D., Pang F., Zhang J., Wang N., Yao X. Studies on the chemical constituents of Bulbophyllum odoratissimum Lindl. Zhongguo Yaowu Huaxue Zazhi. 2005;15:103–107. [Google Scholar]
- 406.Liu D., Pang F., Zhang X., Gao H., Wang N., Yao X. Water-soluble phenolic glycosides from the whole plant of Bulbophyllum odoratissimum. Yaoxue Xuebao. 2006;41:738–741. [PubMed] [Google Scholar]
- 407.Chen Y.G., Xu J.J., Yu H., Qing C., Zhang Y.L., Liu Y., Wang J.H. 3,7-dihydroxy-2,4,6-trimethoxyphenanthrene, a new phenanthrene from Bulbophyllum odoratissimum. J. Korean Chem. Soc. 2007;51:352–355. [Google Scholar]
- 408.Leong Y.W., Harrison L.J., Powell A.D. Phenanthrene and other aromatic constituents of Bulbophyllum vaginatum. Phytochemistry. 1999;50:1237–1241. doi: 10.1016/S0031-9422(98)00687-6. [DOI] [Google Scholar]
- 409.Leong Y.W., Harrison L.J. A Biphenanthrene and a Phenanthro[4,3-b]furan from the orchid Bulbophyllum vaginatum. J. Nat. Prod. 2004;67:1601–1603. doi: 10.1021/np049909b. [DOI] [PubMed] [Google Scholar]
- 410.Juneja R.K., Sharma S.C., Tandon J.S. Two substituted bibenzyls and a dihydrophenanthrene from Cymbidium aloifolium. Phytochemistry. 1987;26:1123–1125. doi: 10.1016/S0031-9422(00)82362-6. [DOI] [Google Scholar]
- 411.Barua A.K., Ghosh B.B., Ray S., Patra A. Cymbinodin A, a phenanthraquinone from Cymbidium aloifolium. Phytochem. 1990;29:3046–3047. doi: 10.1016/0031-9422(90)87138-K. [DOI] [Google Scholar]
- 412.Ghosh B.B., Ray S., Bhattacharyya P., Datta P.K., Mukherjee B.B., Patra A., Banerjee A.K., Barua A.K. Cymbinodin B, a phenanthraquinone from Cymbidium aloifolium. Indian J. Chem. Sect. B. 1992;31:557–558. [Google Scholar]
- 413.Lee J.H., Kim D.H., Bang M.H., Yang H.J., Bang S.H., Chung I.S., Kwon B.M., Kim S.H., Kim D.K., Park M.H., et al. Isolation of sterols from the methanol extracts of Cymbidium goeringii REICHB. fil. Han’guk Eungyong Sangmyong Hwahakhoeji. 2005;48:263–266. [Google Scholar]
- 414.Dahmen J., Leander K. Amotin and amoenin, two sesquiterpenes of the picrotoxane group from Dendrobium amoenum. Phytochemistry. 1978;17:1949–1952. doi: 10.1016/S0031-9422(00)88740-3. [DOI] [Google Scholar]
- 415.Veerraju P., Rao N.S.P., Rao L.J., Rao K.V.J., Rao P.R.M. Amoenumin, a 9,10-dihydro-5H-phenanthro-(4,5-b,c,d)-pyran from Dendrobium amoenum. Phytochemistry. 1989;28:950–951. doi: 10.1016/0031-9422(89)80154-2. [DOI] [Google Scholar]
- 416.Majumder P.L., Guha S., Sen S. Bibenzyl derivatives from the orchid Dendrobium amoenum. Phytochemistry. 1999;52:1365–1369. doi: 10.1016/S0031-9422(99)00370-2. [DOI] [Google Scholar]
- 417.Yang L., Wang Z., Xu L. Phenol and a triterpene from Dendrobium aurantiacum var. denneanum (Orchidaceae) Biochem. Syst. Ecol. 2006;34:658–660. doi: 10.1016/j.bse.2006.03.003. [DOI] [Google Scholar]
- 418.Li Y., Wang C.L., Guo S.X., Yang J.S., Xiao P.G. Two new compounds from Dendrobium candidum. Chem. Pharm. Bull. 2008;56:1477–1479. doi: 10.1248/cpb.56.1477. [DOI] [PubMed] [Google Scholar]
- 419.Yan L.I. Chemical constituents of Dendrobium candidum. Zhongguo Zhongyao Zazhi. 2010;35:1715. doi: 10.4268/cjcmm20101314. [DOI] [PubMed] [Google Scholar]
- 420.Wang F., Li Y., Dong H., Guo S., Wang C., Yang J. A new compound from Dendrobium candidum. Zhongguo Yaoxue Zazhi. 2010;45:898–902. [Google Scholar]
- 421.Min Z.D., Tanaka T., Iinuma M., Mizuno M. A new dihydrostilbene in Dendrobium chrysanthum. J. Nat. Prod. 1987;50:1189. doi: 10.1021/np50054a042. [DOI] [Google Scholar]
- 422.Yang L. Studies on chemical constituents of Dendrobium chrysanthum. Zhongguo Tian Ran Yao Wu. 2004;2:280. [Google Scholar]
- 423.Ye Q.H., Zhao W.M., Qin G.W. Lignans from Dendrobium chrysanthum. J. Asian Nat. Prod. Res. 2004;6:39–43. doi: 10.1080/1028602031000119808. [DOI] [PubMed] [Google Scholar]
- 424.Yang L., Zhang C., Yang H., Zhang M., Wang Z., Xu L. Two new alkaloids from Dendrobium chrysanthum. Heterocycles. 2005;65:633–636. [Google Scholar]
- 425.Bi Z. Chemical constituents of Dendrobium fimbriatum Hook. (I) Zhongguo Yaoke Daxue Xuebao. 2001;32:200. [Google Scholar]
- 426.Qing L.H., Rui L., Xing W.T., Yuan L.G. Isolation and purification of two constitutes from Dendrobium fimbriatum Hook by high-speed counter-current chromatography using stepwise elution. Sep. Sci. Technol. 2009;44:1218–1227. doi: 10.1080/01496390902728850. [DOI] [Google Scholar]
- 427.Lin T.H. Constituents from the stems of Dendrobium moniliforme. Chin. Pharm. J. 2000;52:251. [Google Scholar]
- 428.Bi Z.M., Yang L., Wang Z.T., Xu L.S., Xu G.J. A new bibenzyl derivative from Dendrobium moniliforme. Chin. Chem. Lett. 2002;13:535–536. [Google Scholar]
- 429.Zhao C.S., Zhao W.M. A new bibenzyl glycoside from Dendrobium moniliforme. Chin. Chem. Lett. 2003;14:276–277. [Google Scholar]
- 430.Bi Z., Wang Z., Xu L. Chemical constituents of Dendrobium moniliforme. Acta Bot. Sin. 2004;46:124–126. [Google Scholar]
- 431.Liu W.H. Moniline, a new alkaloid from Dendrobium moniliforme. J. Chem. Res. 2007;2007:317–318. doi: 10.3184/030823407X218048. [DOI] [Google Scholar]
- 432.Majumder P.L., Sen R.C. Structure of moscatin-A new phenanthrene derivative from the orchid Dendrobium moscatum. Indian J. Chem. Sect. B. 1987;26:18–20. [Google Scholar]
- 433.Talapatra Denbinobin, a new phenanthraquinone and other constituents from Dendrobium nobile Lindl (Orchidaceae) Int. Conf. Chem. Biotechnol. Biol. Act. Nat. Prod. 1981;3:215. [Google Scholar]
- 434.Talapatra B., Mukhopadhyay P., Chaudhury P., Talapatra S.K. Denbinobin, a new phenanthraquinone from Dendrobium nobile Lindl (Orchidaceae) Indian J. Chem. Sect. B. 1982;21:386–387. [Google Scholar]
- 435.Shu Y., Zhang D.M., Guo S.X. A new sesquiterpene glycoside from Dendrobium nobile Lindl. J. Asian Nat. Prod. Res. 2004;6:311–314. doi: 10.1080/10286020310001595971. [DOI] [PubMed] [Google Scholar]
- 436.Zhang X., Gao H., Han H., Liu H., Wang N., Yao X., Wang Z. Sesquiterpenes from Dendrobium nobile. Zhongcaoyao. 2007;38:1771–1774. [Google Scholar]
- 437.Liu Q.F., Chen W.L., Tang J., Zhao W.M. Novel bis(bibenzyl) and (propylphenyl)bibenzyl derivatives from Dendrobium nobile. Helv. Chim. Acta. 2007;90:1745–1750. doi: 10.1002/hlca.200790183. [DOI] [Google Scholar]
- 438.Li Y. Studies on chemical constituents from Dendrobium nobile Lindl. Shizhen Guoyi Guoyao. 2010;21:39. [Google Scholar]
- 439.Estrada S., Toscano R.A., Mata R. New phenanthrene derivatives from Maxillaria densa. J. Nat. Prod. 1999;62:1175–1178. doi: 10.1021/np990061e. [DOI] [PubMed] [Google Scholar]
- 440.Estrada S., Acevedo L., Rodriguez M., Toscano R.A., Mata R. New triterpenoids from the orchids Scaphyglottis livida and Nidema boothii. Nat. Prod. Lett. 2002;16:81–86. doi: 10.1080/10575630290019967. [DOI] [PubMed] [Google Scholar]
- 441.Majumder P., Sarkar A.K., Chakraborti J. Isoflavidinin and iso-oxoflavidinin, two 9,10-dihydrophenanthrenes from the orchids Pholidota articulata, Otochilus porecta and Otochilus fusca. Phytochemistry. 1982;21:2713–2716. doi: 10.1016/0031-9422(82)83104-X. [DOI] [Google Scholar]
- 442.Lin W., Chen W., Xue Z., Liang X. New triterpenoids of Pholidota chinensis. Planta Med. 1986;52:4–6. [Google Scholar]
- 443.Yao S., Tang C.P., Ye Y., Kurtán T., Kiss-Szikszai A., Antus S., Pescitelli G., Salvadori P., Krohn K. Stereochemistry of atropisomeric 9,10-dihydrophenanthrene dimers from Pholidota chinensis. Tetrahedron Asymmetry. 2008;19:2007–2014. doi: 10.1016/j.tetasy.2008.08.013. [DOI] [Google Scholar]
- 444.Yao S., Tang C.P., Li X.Q., Ye Y. Phochinenins A – F, dimeric 9,10-dihydrophenanthrene derivatives, from Pholidota chinensis. Helv. Chim. Acta. 2008;91:2122–2129. doi: 10.1002/hlca.200890228. [DOI] [Google Scholar]
- 445.Wu B., Qu H., Cheng Y. Cytotoxicity of new stilbenoids from Pholidota chinensis and their spin-labeled derivatives. Chem. Biodiv. 2008;5:1803–1810. doi: 10.1002/cbdv.200890169. [DOI] [PubMed] [Google Scholar]
- 446.Lin L., Zhang Y., Wu C., Wang Y. Chemical constituents of Pholidota chinensis Lindl. Shizhen Guoyi Guoyao. 2009;20:922–923. [Google Scholar]
- 447.Anuradha V., Rao M.V.B., Aswar A.S. Oxo-tessallatin, a novel phenanthrapyrone isolated from Vanda tessalata. Orient. J. Chem. 2008;24:1119–1122. [Google Scholar]
- 448.Villegas L.F., Fernandez I.D., Maldonado H., Torres R., Zavaleta A., Vaisberg A.J., Hammond G.B. Evaluation of the wound-healing activity of selected traditional medicinal plants from Peru. J. Ethnopharmacol. 1997;55:193–200. doi: 10.1016/S0378-8741(96)01500-0. [DOI] [PubMed] [Google Scholar]
- 449.Mahiou V., Roblot F., Hocquemiller R., Cave A., Barrios A.A., Founet A., Ducrot P.H. Piperogalin, a new prenylated diphenol from Peperomia galioides. J. Nat. Prod. 1995;58:324–328. doi: 10.1021/np50116a031. [DOI] [PubMed] [Google Scholar]
- 450.Banerji A., Bandyopadhyay D., Sarkar M., Siddhanta A.K., Pal S.C., Ghosh S., Abraham K., Shoolery J.N. Structural and synthetic studies on the retrofractamides—Amide constituents of Piper retrofractum. Phytochemistry. 1985;24:279–284. doi: 10.1016/S0031-9422(00)83537-2. [DOI] [Google Scholar]
- 451.Ahn J.W., Ahn M.J., Zee O.P., Kim E.J., Lee S.G., Kim H.J., Kubo I. Piperidine alkaloids from Piper retrofractum fruits. Phytochemistry. 1992;31:3609–3612. [Google Scholar]
- 452.Pande A., Shukla Y.N., Srivastava R., Verma M. 3-Methyl-5-decanoylpyridine and amides from Piper retrofractum. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1997;36:377–379. [Google Scholar]
- 453.Banerji A., Sarkar M., Datta R., Sengupta P., Abraham K. Amides from Piper brachystachyum and Piper retrofractum. Phytochemistry. 2002;59:897–901. doi: 10.1016/S0031-9422(01)00364-8. [DOI] [PubMed] [Google Scholar]
- 454.Ray S., Thakur T.N., Ghosh A., Barua A.K. Chemical investigation of Viscum articulatum. J. Indian Chem. Soc. 1984;61:727–728. [Google Scholar]
- 455.Richter A. Viscumitol, a dimethyl-ether of muco-inositol from Viscum album. Phytochemistry. 1992;31:3925–3927. doi: 10.1016/S0031-9422(00)97555-1. [DOI] [Google Scholar]
- 456.Wang X., Li L., Li M. Chemical constituents of Viscum articulatum Burm. F. (III) Huaxi Yaoxue Zazhi. 1995;10:1–3. [Google Scholar]
- 457.Yang Y. Determination of chemical constituents in Viscum ovalifolium DC. Guangzhou Zhongyiyao Daxue Xuebao. 2005;22:144. [Google Scholar]
- 458.Yang Y., Sha C., Chen M. Constituents of Viscum ovalifolium DC(II) Zhongguo Yaoxue Zazhi. 2011;46:11–13. [Google Scholar]