Abstract
The consumption of functional foods and nutraceuticals is gaining more importance in modern society. The exploration of alternative sources and the utilization of by-products coming from the food industry are gaining more importance. The present study aimed to characterize the nutritional value and potential use of sea bass by-products as a source of high-added-value compounds for the development of supplements. The chemical composition (moisture, protein, fat, and ash contents) and profiles of amino acids (high-performance liquid chromatography coupled to a scanning fluorescence detector), fatty acids (gas chromatography coupled to a flame ionization detector), and minerals (inductively coupled plasma optical emission spectroscopy) were determined for sea bass fillet and its by-products (skin, guts, gills, liver, head, and fish bones). The chemical composition assays revealed that by-products were rich sources of proteins (skin; 25.27 g/100 g), fat (guts and liver; 53.12 and 37.25 g/100 g, respectively), and minerals (gills, head, and fish bones; 5.81, 10.11, and 7.51 g/100 g, respectively). Regarding the amino-acid profile, the skin and liver were the main sources of essential amino acids with an essential amino-acid index of 208.22 and 208.07, respectively. In the case of the fatty-acid profile, all by-products displayed high amounts of unsaturated fatty acids, particularly monounsaturated (from 43.46 to 49.33 g/100 g fatty acids) and omega-3 fatty acids (in the range 10.85–14.10 g/100 g fatty acids). Finally, the evaluation of mineral profile indicated high contents of calcium and phosphorus in gills (1382.62 and 742.60 mg/100 g, respectively), head (2507.15 and 1277.01 mg/100 g, respectively), and fish bone (2093.26 and 1166.36 mg/100 g, respectively). Therefore, the main sources of monounsaturated, unsaturated, and long-chain omega-3 fatty acids were guts and liver. The most relevant source of minerals, particularly calcium, phosphorus, and manganese, were head, fish bones, and gills. The most promising source of proteins and amino acids was the skin of sea bass.
Keywords: Dicentrarchus labrax, by-product, amino acids, fatty acids, omega-3, minerals, calcium, phosphorus, biological relevance
1. Introduction
The consumption of healthier and functional foods and nutraceuticals (foods or supplements that are associated with beneficial physiological effects) is an important part of modern society [1]. This important share of the food market is supported by consumers that are aware of the relationship between food composition and health. Essentially, the main motive among consumers to intake these products is the desire to reduce the risk associated with chronic diseases (main leading causes of death at a global scale) or to enhance health (improving the immune system or well-being) [2]. The biological effect obtained from functional foods and nutraceuticals is actually associated with a single or a group of compounds (present in their composition) that are naturally present or intentionally added to the product. Particularly for functional foods, these products can be classified as “fortified” (increased content of naturally occurring component) or “enriched” (adding an external component). In this sense, important dietary components were put in the spotlight, such as omega-3 (n-3) fatty acids, amino acids (such as arginine, leucine, and tyrosine), and minerals (calcium, phosphorus, and manganese, for instance) [3].
Aiming at the enrichment or fortification of food, functional ingredients can be obtained from natural sources, biotechnological generation, or chemical synthesis. For instance, omega-3 fatty acids can be found in fish oil (6–13 g C20:5n3 and 4–18 g C22:6n-3/100 g oil) and algae oil (10–95 g eicosapentaenoic acid—EPA/100 g oil and 20–45 g docosahexaenoic acid—DHA/100 g oil) [4,5]. At the commercial level, the most common n-3-rich products to produce functional food are fish and algae oil that can be found in liquid, microencapsulated, and emulsified forms [5,6]. In the case of amino acids, commercial production is carried out by fermentation of carbohydrates with specific bacterial strains such as Corynebacterium glutamicum and Escherichia coli, which can produce arginine and leucine [7]. Particularly for minerals (such as calcium, phosphorus, and manganese), the main current sources are organic and inorganic salts that are obtained from chemical synthesis [8]. Consequently, the application of functional ingredients in food was previously reported, for example, n-3 fatty acids were enriched in meat products [9], dairy products were fortified in calcium [10], and baked products were enriched in amino acids [11]. Furthermore, the development of dietary supplements such as n-3 fatty capsules is also widespread [12].
However, the increasing development of this sector created a demand for new/underutilized sources of these biologically relevant compounds [13]. Consequently, several researchers are re-evaluating the importance and potential use of food industry by-products [14,15], which can also reduce the environmental impact associated with this industrial sector. In this sense, the development and implementation of strategies to reuse food industry by-products is a necessary action to improve the efficiency of industrial operations, reduce the discard of wastes, and also recover high-added value compounds for further utilization in food products, which fulfill the concepts of sustainable circular economy [13]. Particularly for the fish industry, the by-products such as viscera, skin, fish bones, and head are the main by-products generated from industrialization processes [16]. Although these by-products are discarded as a low-value material, several studies reported the importance of exploring the by-products of fish processing as potential sources of functional ingredients. For instance, the fish skin is a rich source of amino acids [17], the fish liver is a relevant source of polyunsaturated fatty acids (PUFA), particularly long chain n-3 fatty acids [18], and the fish bone is rich in calcium [19].
This scenario supports the study of species largely consumed in key regions of the globe. With this line of thought, sea bass (Dicentrarchus labrax) can be seen as a relevant source of biologically relevant compounds. The sea bass is an edible fish largely found in the Mediterranean Sea and inshore waters of this region. This fish is also characterized by a silver-gray to a bluish color, small scales, and moderately forked caudal fin. The historical relevance of sea bass dates back to the 1960s when the culture of this fish was intensified, particularly in France and Italy. Moreover, the successful implementation of a culture system for sea bass larvae for mass products in the 1970s favored this species to became the first non-salmonid species to be widely produced in Europe and countries of the Mediterranean Sea [20]. The average per capita consumption of sea bass in the Mediterranean basin was around 200 g in 2016. Moreover, Greece, Portugal, and Cyprus were the leading countries in the consumption per capita of sea bass (796, 680, and 643 g), followed by Spain and Italy (545 and 513 g) [21].
Due to the scarce scientific information regarding the increasing demand for alternative, natural, and environmentally friendly sources of biologically relevant compounds, the present study aimed to characterize the chemical composition and profiles of amino acids, fatty acids, and minerals of sea bass by-products.
2. Materials and Methods
2.1. Samples
The present study was carried out with 10 sea bass fishes (full individuals) that were purchased in a local market in Ourense (Spain) and transported under refrigeration to the Centro Tecnológico de la Carne de Galicia (Ourense, Spain). The characteristics of animals are described as follows: average length of 35 cm (standard error of the mean (SEM) = 0.4); average weight of 512 g (SEM = 6); males around 36 months old; fishes were bought on five different occasions during October and November (2018). The fishes were filleted and dissected to obtain the following by-products: skin, guts, gills, liver, head, and fish bone. The fillets and each by-product were individually homogenized and kept at −80 °C until further analysis.
2.2. Proximate Composition
The evaluation of chemical composition was determined according to the International Organization for Standardization (ISO) recommended standards 1442:1997 [22] for moisture, 937:1978 [23] for protein content, and 936:1998 [24] for ash content. The lipid content was determined following the protocol established by the American Oil Chemists’ Society (AOCS) Official Procedure Am 5-04 [25] using the fat extractor Ankom XT10 (ANKOM Technology Corp., Macedon, NY, USA).
2.3. Amino-Acid Profile
The amino-acid profile was determined according to the protocol described by Domínguez et al. [26]. Briefly, 100 mg of samples were weighed in glass ampoules and 5 mL of 6 N hydrochloric acid solution were added. Afterward, the ampoule was sealed and kept at 110 °C for 24 h. Once the hydrolysis of proteins was completed, the hydrolysate was diluted with 200 mL of distilled water and filtered through a 0.45-µm filter (Filter Lab, Barcelona, Spain). Tryptophan was not determined due to its transformation into ammonium under acidic conditions. The derivatization of standards and samples was performed as follows: 10 µL of sample was buffered to pH 8.8 (AccQ-Fluor borate buffer) to yield a total volume of 100 µL. Then, 20 µL of AccQ-Fluor reagent (3 mg/mL in acetonitrile) was added in order to obtain a rapid derivatization of all primary and secondary amines. Excess reagent was hydrolyzed within 1 min. In order to complete the derivatization for tyrosine phenol modification, the vials were heated at 55 °C for 10 min.
The separation of derivatized amino acids was performed by high-performance liquid chromatography (Alliance 2695 model, Waters, Milford, MA, USA) using a scanning fluorescence detector (model 2475, Waters). The column used was a Waters AccQ-Tag column (3.9 × 150 mm, with a particle size of 3 µm). The chromatography conditions were as follows: flow rate of 1.0 mL/min at 37 °C. The wavelengths for excitation and emission were set at 250 and 395 nm, respectively. The mobile phase composition and the gradient were defined according to the validated and patented method of Waters Corporation (AccQ-Tag Amino acid analysis protocol). Briefly, the mobile phase was composed of three solvents: (A) AccQ Tag Eluent A concentrate for amino acids analysis (Waters, Milford, MA, USA), (B) acetonitrile (HPLC grade, Scharlab, Barcelona, Spain), and (C) ultra-pure water (Milli-Q system, Millipore, Darmstadt, Germany). The solvent gradient was set as follows: 0.0–17.0 min 99% A and 1% B; 17.0–22.0 min 96% A and 4% B; 22.0–24.0 min 95% A and 5% B; 24.0–31.5 min 91% A and 9% B; 31.5–36.0 min 83% A and 17% B; 36.0–42.0 min 83% A and 17% B; 42.0–44.0 min 60% B and 40% C; 42.0–45.0 min 99% A and 1% B.
The identification of amino acids was carried out by comparing the retention times. The quantification was performed using the external standard technique with an amino-acid standard (Amino Acid Standard H, Thermo Scientific, Rockford, IL, USA). The system operation and data acquisition were performed using the Empower 2 advanced software (Waters). The results were expressed as g/100 g of protein.
Once the amino-acid profile was determined, the sum of essential (EAA) and non-essential (NEAA) amino acids, as well as the EAA/NEAA ratio, was calculated. The EAA score for each EAA was determined according to the equation described by Domínguez et al. [26] considering the concentration of a given EAA in the sample (EAAt, g/100 g protein) and the pattern concentration (EAAp, g/100 g protein) according to World Health Organization/Food and Agriculture Organization/United Nations University (WHO/FAO/UNU) [27].
| (1) |
The EAA index for each section of sea bass was determined according to the equation described by Domíngez et al. [26], which takes into account EAAt and EAAp (according to WHO/FAO/UNU [27]) from i to n EAA quantified in the sample.
| (2) |
2.4. Fatty-Acid Profile
In order to determine the fatty-acid profile of sea bass fillets and by-products, the fatty acids were obtained using the protocol described by Bligh and Dyer [28] with modification proposed by Barros [29]. Once the fatty acids were extracted, these compounds were transesterified according to the method described by Domínguez et al. [30] with some modifications. Briefly, 1 mL of toluene was used to dissolve 20 mg of fat prior to mixing with 2 mL of 0.5 N sodium methoxide solution in a test tube. This mixture was vortexed for 10 s and rested for 15 min at room temperature. Afterward, 4 mL of 10% of H2SO4 methanolic solution was added to the mixture that was vortexed for a few sec. Then, the mixture was vortexed again for a few seconds after the addition of 2 mL of saturated sodium bicarbonate solution. Subsequently, the fatty acid methyl esters (FAMEs) were separated by adding 1 mL of hexane to the tube and vortexing for 10 s. Finally, the FAMEs were transferred to an appropriate GC vial.
The FAMEs were separated and quantified by gas chromatography (GC-Agilent 7890B, Agilent Technologies, Santa Clara, CA, USA) equipped with a PAL RTC-120 autosampler and a flame ionization detector (FID). The injection was carried out in split mode (1:50) with 1 μL, the injector was kept at 260 °C, and the total flow was set to 64.2 mL/min. The separation was carried in an SP-2560 fused silica capillary column (100 m, 0.25 mm inner diameter (i.d.), 0.25-μm film thickness; Supelco Inc., Bellefonte, PA, USA). The selected carrier gas was helium with a flow rate of 1.2 mL/min. The pressure in the head of column was set to 42.135 psi. The chromatography conditions were set as follows: initial oven temperature of 140 °C (held for 4 min), first ramp at 5 °C/min to 190 °C, second ramp at 2 °C/min to 210 °C (held for 4 min), third ramp at 1 °C/min to 220 °C and fourth ramp at 3 °C/min to a final temperature of 235 °C (held for 7 min). The operational in FID was set as follows: temperature of 260 °C, flow of H2 35 mL/min, air 350 mL/min, and make-up flow of 15 mL/min. The total time for chromatographic analysis was 50 min.
The software MassHunter GC/MS Acquisition B.07.05.2479 (Agilent Technologies, Santa Clara, CA, USA) was used to control the equipment and acquire the data. The data analysis was performed in the software MassHunter Quantitative Analysis B.07.01. Authenticated standards (FAME Mix, 37 components, docosapentaenoic acid, trans-vaccenic acid, cis-vaccenic acid, and CLA) were used to identify the FAMEs by comparing the retention times. The results were expressed as g/100 g of total identified fatty acids. After obtaining the fatty-acid data, the fractions of saturated (SFA), monounsaturated (MUFA), PUFA, n-3, and omega-6 (n-6) fatty-acids content were determined. The nutritional ratios PUFA/SFA and n-6/n-3 were calculated.
2.5. Mineral Profile
The mineral profile was determined using 5 g of ashes (obtained using the protocol ISO 936:1998 [24]) dissolved in 10 mL of 1 M HNO3. The assay was carried out using inductively coupled plasma optical emission spectroscopy (ICP-OES) in order to determine the concentration of Ca, K, Mg, Na, P, Fe, Mn, Zn, and Cu following the protocol defined by Lorenzo et al. [31]. The equipment used was the Thermo-Fisher ICAP 6000 plasma emission spectrometer (Thermo-Fisher, Cambridge, UK) with a radio frequency source set to 27.12 MHz, a peristaltic pump, a spraying chamber, and a concentric spray nebulizer, using 99.996% liquid argon plasma gas (Praxair, Madrid, Spain). The ICP software was used to control the system and acquire the data. The external standard procedure was used to determine the concentration of each mineral (average value of three determinations). The results were expressed as mg/100 g of meat.
2.6. Statistical Analysis
The statistical evaluation of data was carried out using the IBM SPSS Statistics 21 software (SPSS Inc., Armonk, NY, USA). Normal distribution and homogeneity of variance were previously tested. Significant differences were determined using either the analysis of variance (ANOVA; normal distribution of data and homogeneity of variance) or the Kruskal–Wallis test (non-normality and heterogeneity of variance). The Duncan test was applied for the data analyzed by ANOVA whereas the Dunn’s test was applied to data evaluated by Kruskal–Wallis test in the case of significant differences (p < 0.05). The values were presented as mean values and standard errors of the mean (SEMs).
3. Results
3.1. Chemical Composition
The evaluation of chemical composition of sea bass fillet and by-products is shown in Table 1. The evaluation of moisture content in the fillet and by-product of sea bass indicated that the higher moisture content (p < 0.05) was observed on the fillets. In the case of fat, gut contained more lipids (p < 0.05) than the fillets and other by-products. Regarding the protein content, the highest amount was obtained from the skin. Particularly for ash content, the head displayed the highest content.
Table 1.
Chemical composition of sea bass fillet and by-products.
| (g/100 g) | Fillet | Skin | Guts | Gills | Liver | Head | Fish Bone |
|---|---|---|---|---|---|---|---|
| Moisture | 72e | 54bc | 38a | 62de | 48ab | 59cd | 52b |
| SEM | 0.4 | 1 | 2 | 0.4 | 1 | 0.8 | 1 |
| Protein | 21ef | 25f | 8a | 16de | 12ab | 16cd | 15bc |
| SEM | 0.08 | 0.7 | 0.6 | 0.1 | 0.5 | 0.2 | 0.4 |
| Fat | 4a | 17bc | 53e | 13ab | 35de | 14ab | 19cd |
| SEM | 0.5 | 1 | 3 | 0.5 | 2 | 0.6 | 0.6 |
| Ash | 1.3bc | 3.0cd | 0.78a | 5.8de | 1.1ab | 10f | 7ef |
| SEM | 0.02 | 0.3 | 0.03 | 0.2 | 0.07 | 0.6 | 0.4 |
SEM: standard error of the mean; n.d.: not determined; n = 10. a–e Means in the same row with different letters differ significantly (p < 0.05; Dunn’s or Duncan’s test).
3.2. Amino-Acid Profile
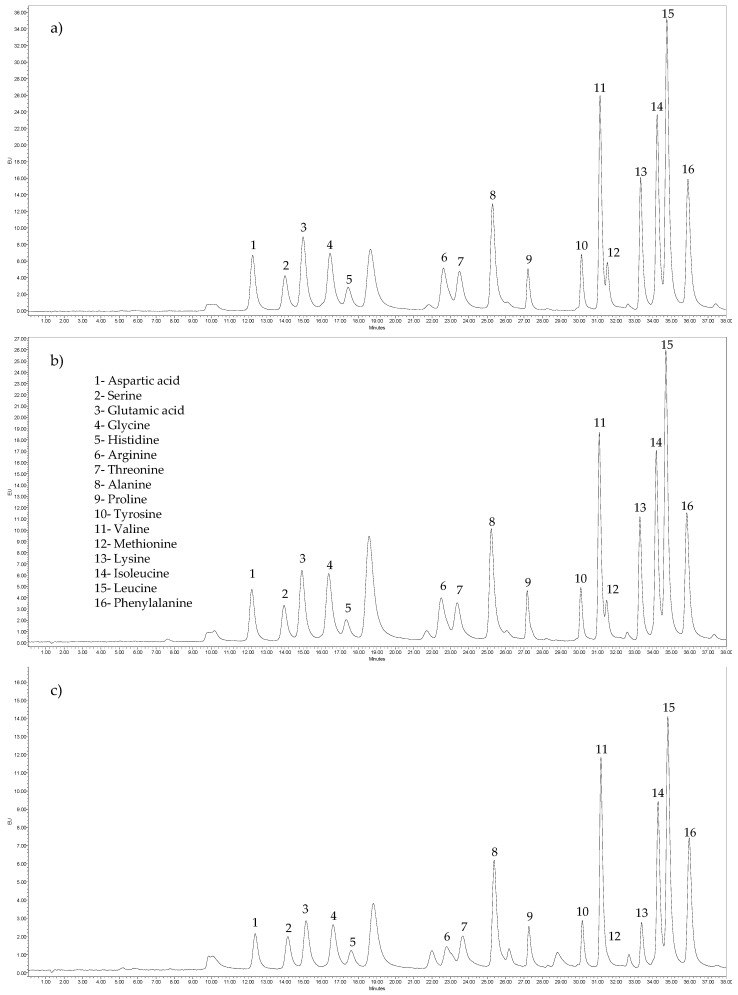
The evaluation of amino-acid profile indicated that fillet and skin were the main sources of both essential (8.4 and 7.9 g/100 g protein, respectively) and non-essential (8.4 and 10 g/100 g protein, respectively) amino acids (p < 0.05) in comparison to other samples (ranges of 0.9–6.6 and 1.3–7.8 g/100 g protein for total EAA and NEAA, respectively) (Table 2). Moreover, glutamic acid was the main amino acid among fillet, skin, guts, liver, head, and fish bone samples. It is relevant to mention that glycine was a major amino acid for skin, gills, head, and fish bone. Particularly for gills, the main amino acid was glycine, followed by glutamic acid and proline. Regarding the EAA/NEAA ratio, the highest ratio was obtained from fillet, followed by liver and fish bone. Taurine and cysteine were not determined. Figure 1 shows representative chromatograms from the amino-acid analysis.
Table 2.
Amino-acid profile of sea bass fillet and by-products.
| Amino Acid (mg/100 g Protein) |
Fillet | Skin | Guts | Gills | Liver | Head | Fish Bone |
|---|---|---|---|---|---|---|---|
| EAA | |||||||
| Histidine | 464c | 534c | n.d.a | 435c | 236 | 455c | 463c |
| SEM | 29 | 40 | - | 16 | 50 | 17 | 11 |
| Arginine | 1173bc | 1595c | 96.41a | 215a | 205a | 1189bc | 1195bc |
| SEM | 76 | 119 | 6 | 16 | 21 | 39 | 34 |
| Threonine | 798c | 850c | 107a | 808c | 286a | 637ab | 688bc |
| SEM | 46 | 71 | 5 | 23 | 80 | 22 | 26 |
| Valine | 922c | 897bc | 155a | 672a | 510a | 666a | 701ab |
| SEM | 49 | 80 | 4 | 57 | 97 | 27 | 25 |
| Methionine | 437d | 247c | n.d.a | n.d.a | 109b | 199bc | 197bc |
| SEM | 21 | 33 | - | - | 10 | 9 | 8 |
| Lysine | 1646d | 1532cd | 108a | 799ab | 407a | 1055ab | 1232bc |
| SEM | 99 | 168 | 4 | 48 | 147 | 54 | 58 |
| Isoleucine | 837c | 739bc | 112a | 451a | 365a | 547ab | 583ab |
| SEM | 42 | 75 | 4 | 39 | 74 | 25 | 24 |
| Leucine | 1350c | 1230bc | 169a | 7839ab | 609a | 899ab | 938ab |
| SEM | 68 | 118 | 15 | 61 | 128 | 41 | 39 |
| Phenylalanine | 799c | 771.87b | 124a | 554ab | 399a | 608ab | 601ab |
| SEM | 43 | 60 | 7 | 24 | 82 | 22 | 18 |
| Σ EAA | 8427d | 8397cd | 872a | 4718ab | 3126a | 6256ab | 6598bc |
| SEM | 465 | 723 | 126 | 293 | 656 | 231 | 222 |
| NEAA | |||||||
| Aspartic acid | 1638cd | 1695d | 225a | 1001ab | 520ab | 1258abc | 1350bcd |
| SEM | 91 | 160 | 1 | 63 | 68 | 53 | 50 |
| Serine | 686b | 841b | 129a | 624ab | 361ab | 692b | 679b |
| SEM | 45 | 64 | 11 | 29 | 74 | 23 | 19 |
| Glutamic acid | 2486c | 2546d | 340a | 1559ab | 913ab | 1887b | 1985bc |
| SEM | 142 | 236 | 25 | 94 | 89 | 79 | 72 |
| Glycine | 975b | 2086c | 252a | 1706c | 434ab | 1651c | 1469c |
| SEM | 68 | 225 | 56 | 12 | 141 | 87 | 71 |
| Alanine | 864ab | 1336c | 198a | 1048bc | 510a | 927bc | 954bc |
| SEM | 42 | 139 | 0.8 | 54 | 131 | 40 | 33 |
| Proline | 551bc | 1208e | 141a | 1097de | 402ab | 957de | 818cd |
| SEM | 32 | 145 | 27 | 52 | 56 | 60 | 35 |
| Tyrosine | 660c | 524bc | 40a | 299a | 203a | 413ab | 416ab |
| SEM | 40 | 50 | 0.5 | 16 | 36 | 20 | 18 |
| Σ NEAA | 7860b | 10237c | 1324a | 7334ab | 3344a | 7784b | 7671b |
| SEM | 451 | 844 | 160 | 315 | 385 | 271 | 207 |
| EAA/NEAA | 1.07d | 0.82abc | 0.66ab | 0.641a | 0.93cd | 0.80ab | 0.86bc |
| SEM | 0.006 | 0.03 | 0.01 | 0.004 | 0.05 | 0.02 | 0.02 |
SEM: standard error of the mean; EAA: essential amino acid; NEAA: non-essential amino acid; n.d.: not determined; n = 10. a–e Means in the same row with different letters differ significantly (p < 0.05; Dunn’s or Duncan’s test).
Figure 1.
Chromatograms for the amino acid analysis: (a) fillet; (b) skin; (c) liver.
The evaluation of amino-acid score (Table 3) indicated that the largest values for methionine, lysine, isoleucine, leucine, and valine were obtained from fillets (p < 0.05). In the case of histidine and threonine, the main sources were fish bone and gills, respectively (p < 0.05). Concerning the EAA index, the highest score was observed in the fillets, followed by skin and liver.
Table 3.
Amino-acid scores and index of sea bass fillet and by-products.
| EAA | Fillet | Skin | Guts | Gills | Liver | Head | Fish Bone |
|---|---|---|---|---|---|---|---|
| Histidine | 148bc | 147b | n.d.a | 174bcd | 154bc | 198cd | 217d |
| SEM | 9 | 12 | - | 19 | 5 | 8 | 6 |
| Threonine | 255de | 235cde | 81a | 323e | 186abc | 181ab | 211bcd |
| SEM | 14 | 23 | 2 | 36 | 4 | 7 | 8 |
| Valine | 295b | 249b | 118a | 269b | 331b | 111a | 127a |
| SEM | 15 | 26 | 6 | 40 | 15 | 5 | 5 |
| Methionine | 140c | 69.15b | n.d.a | n.d.a | 71b | 81b | 87b |
| SEM | 6 | 11 | - | - | 4 | 4 | 4 |
| Lysine | 526c | 426c | 82a | 322bc | 265bc | 153a | 193ab |
| SEM | 31 | 54 | 5 | 34 | 17 | 9 | 9 |
| Isoleucine | 267b | 205b | 85a | 180ab | 238b | 119a | 137a |
| SEM | 13 | 24 | 5 | 30 | 12 | 6 | 6 |
| Leucine | 431d | 341bc | 129abc | 314bcd | 397cd | 99.43a | 112ab |
| SEM | 21 | 39 | 15 | 31 | 21 | 5 | 5 |
| EAA index | 266c | 208bc | 26a | 115a | 208bc | 128a | 147ab |
| SEM | 14 | 23 | 1 | 9 | 14 | 6 | 5 |
SEM: Standard error of the mean; n.d.: not determined; n = 10. a–e Means in the same row with different letters differ significantly (p < 0.05; Dunn’s or Duncan’s test). Limiting amino acids are indicated in bold letters.
3.3. Fatty-Acid Profile
The evaluation of fatty-acid profile indicated significant differences (p < 0.05) among samples regarding the fractions and individual fatty acids (Table 4). The highest PUFA content (p < 0.05) was observed in the head and fish bones, followed by fillets and skin. In the case of MUFA fraction, all the by-products displayed higher amounts (p < 0.05) than fillets. Moreover, the liver was the main source of MUFA. Regarding the SFA content, the lowest amounts (p < 0.05) were obtained from head and fish bones, followed by guts, fillets, and skin.
Table 4.
Fatty-acid profile of sea bass fillet and by-products.
| Fatty Acid (g/100 g Fatty Acids) |
Fillet | Skin | Guts | Gills | Liver | Head | Fish Bone |
|---|---|---|---|---|---|---|---|
| C14:0 | 3.2bc | 3.4cd | 3.9e | 3.5de | 2.2a | 2.4a | 2.47ab |
| SEM | 0.03 | 0.03 | 0.05 | 0.05 | 0.07 | 0.01 | 0.006 |
| C15:0 | 0.298b | 0.307b | 0.320bc | 0.342c | 0.224a | 0.257a | 0.250a |
| SEM | 0.003 | 0.004 | 0.007 | 0.006 | 0.01 | 0.001 | 0.0006 |
| C16:0 | 17cd | 17cd | 17bc | 19de | 20e | 16ab | 15a |
| SEM | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 | 0.04 | 0.03 |
| C16:1n-7 | 4.3bc | 4.6cd | 5.2de | 5.3e | 4.0ab | 4.0ab | 3.96a |
| SEM | 0.06 | 0.08 | 0.05 | 0.05 | 0.10 | 0.01 | 0.004 |
| C17:0 | 0.24a | 0.253ab | 0.240ab | 0.257b | 0.318c | 0.495d | 0.501d |
| SEM | 0.01 | 0.003 | 0.003 | 0.003 | 0.008 | 0.002 | 0.003 |
| C17:1n-7 | 0.192b | 0.204b | 0.211bc | 0.253cd | 0.248cd | 0.00a | 0.366d |
| SEM | 0.007 | 0.007 | 0.003 | 0.008 | 0.008 | 0.00 | 0.005 |
| C18:0 | 3.63d | 3.40cd | 3.14bc | 2.93ab | 5.23d | 2.82a | 3.09bc |
| SEM | 0.07 | 0.07 | 0.08 | 0.07 | 0.45 | 0.008 | 0.01 |
| C18:1n-9 | 29a | 31ab | 32b | 32b | 38d | 34c | 34c |
| SEM | 0.3 | 0.2 | 0.2 | 0.2 | 0.7 | 0.04 | 0.03 |
| C18:1n-7 | 2.8a | 3.0bc | 3.2c | 3.1c | 3.3d | 2.83ab | 2.7a |
| SEM | 0.04 | 0.01 | 0.03 | 0.03 | 0.2 | 0.003 | 0.02 |
| C18:2n-6 | 11ab | 12bc | 13cd | 11ab | 9a | 17de | 18e |
| SEM | 0.09 | 0.1 | 0.1 | 0.1 | 0.4 | 0.02 | 0.02 |
| C20:0 | 0.233bc | 0.243cd | 0.265d | 0.245cd | 0.186a | 0.199a | 0.211ab |
| SEM | 0.003 | 0.004 | 0.005 | 0.004 | 0.005 | 0.001 | 0.002 |
| C18:3n-6 | 0.210a | 0.223b | 0.224b | 0.208a | 0.272c | 0.289d | 0.265c |
| SEM | 0.002 | 0.002 | 0.002 | 0.002 | 0.009 | 0.003 | 0.002 |
| C20:1n-9 | 4.0bc | 4.3cd | 5d | 4.2bc | 3.0ab | 2.40a | 2.48a |
| SEM | 0.05 | 0.05 | 0.1 | 0.04 | 0.04 | 0.004 | 0.004 |
| C18:3n-3 | 3.0bc | 3.1c | 3.3cd | 2.7ab | 2a | 3.69de | 3.77e |
| SEM | 0.03 | 0.03 | 0.05 | 0.03 | 0.1 | 0.007 | 0.006 |
| C20:2n-6 | 0.67abc | 0.70bc | 0.73c | 0.63ab | 0.55a | 1.069d | 1.060d |
| SEM | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.004 | 0.003 |
| C22:1n-9 | 0.418bc | 0.450cd | 0.51de | 0.420bc | 0.29ab | 0.257a | 0.668e |
| SEM | 0.006 | 0.006 | 0.02 | 0.007 | 0.01 | 0.002 | 0.002 |
| C20:3n-3 | 0.213bc | 0.221c | 0.232c | 0.178ab | 0.148a | 0.289d | 0.277d |
| SEM | 0.007 | 0.004 | 0.005 | 0.002 | 0.006 | 0.001 | 0.002 |
| C20:4n-6 | 0.58c | 0.446ab | 0.359a | 0.469b | 0.31a | 0.548c | 0.484b |
| SEM | 0.02 | 0.006 | 0.003 | 0.009 | 0.02 | 0.001 | 0.001 |
| C20:5n-3 | 5.6e | 5.1de | 4.2bc | 4.4cd | 3a | 3.5ab | 3.37a |
| SEM | 0.07 | 0.06 | 0.08 | 0.07 | 0.2 | 0.01 | 0.005 |
| C24:1n-9 | 0.361bc | 0.38cd | 0.41d | 0.379cd | 0.27ab | 0.232a | 0.212a |
| SEM | 0.006 | 0.06 | 0.01 | 0.008 | 0.01 | 0.002 | 0.003 |
| C22:5n-3 | 1.2c | 1.2c | 1.1b | 0.96ab | 0.84a | 1.20c | 1.06ab |
| SEM | 0.02 | 0.02 | 0.02 | 0.02 | 0.05 | 0.007 | 0.004 |
| C22:6n-3 | 9e | 7de | 5.3ab | 6.5cd | 4.5ab | 5.5bc | 4.4a |
| SEM | 0.2 | 0.1 | 0.09 | 0.15 | 0.3 | 0.03 | 0.02 |
| SFA | 25bc | 25bc | 24b | 26cd | 29d | 22a | 22a |
| SEM | 0.1 | 0.2 | 0.3 | 0.2 | 0.65 | 0.06 | 0.04 |
| MUFA | 41a | 43b | 46d | 46cd | 49d | 43ab | 44bc |
| SEM | 0.3 | 0.2 | 0.1 | 0.2 | 0.5 | 0.04 | 0.04 |
| PUFA | 32cd | 30bc | 28ab | 27a | 22a | 34e | 33de |
| SEM | 0.3 | 0.3 | 0.3 | 0.3 | 1 | 0.06 | 0.03 |
| n-3 | 19d | 17cd | 14b | 15bc | 11a | 13a | 13a |
| SEM | 0.3 | 0.2 | 0.2 | 0.3 | 0.7 | 0.05 | 0.03 |
| n-6 | 13bc | 13bc | 14cd | 12ab | 11a | 19de | 20e |
| SEM | 0.09 | 0.1 | 0.2 | 0.1 | 0.4 | 0.02 | 0.02 |
| n-6/n-3 | 0.67a | 0.76ab | 1.0cd | 0.84bc | 0.99cd | 1.37de | 1.55e |
| SEM | 0.01 | 0.01 | 0.01 | 0.01 | 0.03 | 0.004 | 0.004 |
| Long chain n-3 | 15e | 14de | 11bc | 12cd | 8a | 10ab | 8.9a |
| SEM | 0.3 | 0.2 | 0.1 | 0.2 | 0.5 | 0.04 | 0.03 |
SEM: Standard error of the mean; SFA: saturated fatty acid; MUFA: monounsaturated fatty acid; PUFA: polyunsaturated fatty acid; n.d.: not determined; n = 10. a–e Means in the same row with different letters differ significantly (p < 0.05; Dunn’s or Duncan’s test).
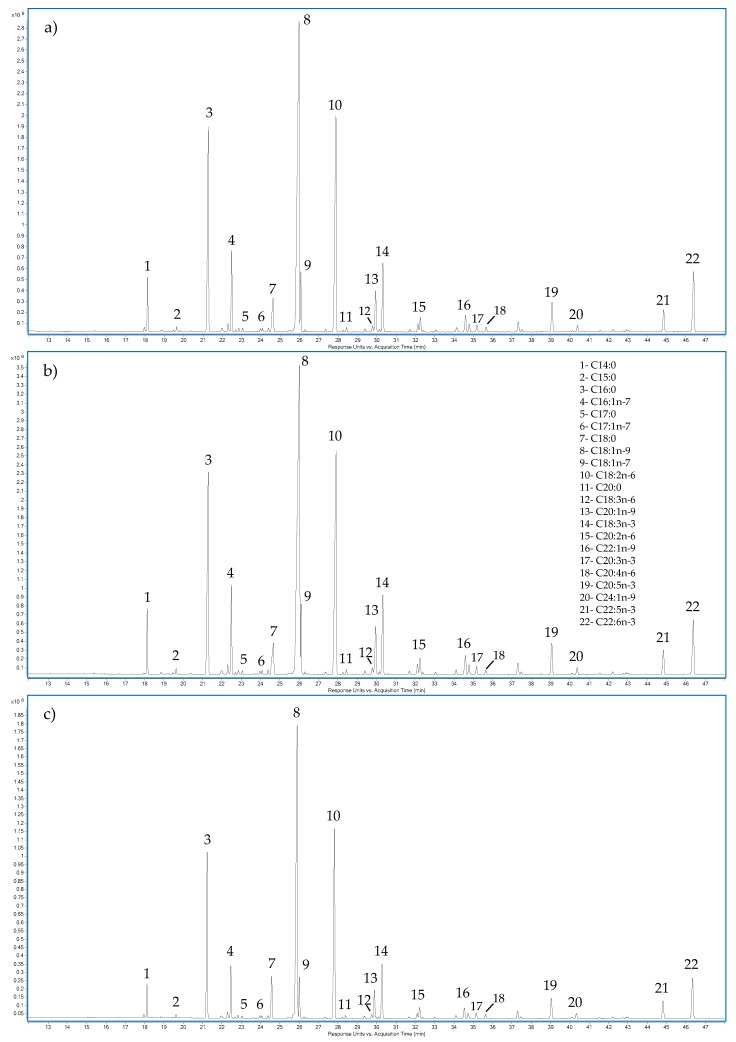
Concerning the individual fatty acids, a common outcome was observed for all samples; the main fatty acids were C18:1n-9 (oleic acid), C16:0 (palmitic acid), and C18:2n-6 (linoleic acid). In addition, the long chain n-3 fatty acids eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic acid (C22:6n-3, DHA) were present in all samples, particularly in the fillets. In the case of n-3 content, the highest amount was obtained from fillets, followed by skin. A different scenario was observed for the n-6 content, wherein the highest concentration of this group of fatty acids was obtained from the fish bone, followed by head and guts. In addition, significant differences (p < 0.05) in the n-6/n-3 ratio among samples were obtained. The lowest n-6/n-3 ratio was observed on fillet, followed by skin and gill samples. Figure 2 shows representative chromatograms from the fatty-acid analysis.
Figure 2.
Chromatogram for fatty-acid analysis: (a) fillet; (b) skin; (c) liver.
3.4. Mineral Profile
The mineral content of sea bass fillets and by-products is displayed in the Table 5. The highest concentrations of calcium and phosphorus were obtained from head samples, followed by fish bone and gills. In the case of potassium, fillet and fish bone were the samples with highest contents, followed by liver, head, skin, and gills. Regarding magnesium, the fillets, skin, guts, and gills had the highest concentrations. Particularly for sodium, the gills had the highest content. In the case of microminerals, the liver was the sample with the highest amount of copper, iron, and zinc, whereas the highest content of manganese was observed in the gills.
Table 5.
Mineral profile of sea bass fillet and by-products.
| Mineral | Fillet | Skin | Guts | Gills | Liver | Head | Fish Bone |
|---|---|---|---|---|---|---|---|
| Macro-minerals (mg/100 g) | |||||||
| Calcium | 32ab | 735bc | 26ab | 1382cd | 9a | 2507e | 2093de |
| SEM | 4 | 108 | 3 | 47 | 1 | 116 | 121 |
| Magnesium | 34c | 37c | 34c | 37c | 20a | 29bc | 25ab |
| SEM | 0.9 | 4 | 3 | 0.9 | 2 | 2 | 0.6 |
| Phosphorus | 206bc | 468cd | 113a | 743de | 175b | 1277e | 1166e |
| SEM | 3 | 53 | 4 | 23 | 8 | 58 | 60 |
| Potassium | 306c | 189b | 87a | 180b | 242b | 194b | 263c |
| SEM | 4 | 7 | 4 | 5 | 45 | 3 | 10 |
| Sodium | 139b | 161b | 144b | 251c | 163b | 163b | 96a |
| SEM | 4 | 8 | 7 | 5 | 13 | 6 | 3 |
| Micro-minerals (mg/100 g) | |||||||
| Copper | 0.112b | 0.15c | 0.42d | 0.094ab | 14e | 0.034a | 0.110b |
| SEM | 0.003 | 0.01 | 0.04 | 0.008 | 2 | 0.001 | 0.008 |
| Iron | 0.55b | 0.53b | 1.03c | 1.23c | 2d | 0.29a | 0.52ab |
| SEM | 0.05 | 0.03 | 0.07 | 0.06 | 0.2 | 0.04 | 0.03 |
| Zinc | 0.47a | 2cd | 1.2ab | 1.4bc | 4.13d | 2.1cd | 1.3b |
| SEM | 0.01 | 0.3 | 0.06 | 0.03 | 0.3 | 0.06 | 0.04 |
| Manganese (μg/100 g) | 20a | 184bc | 145b | 500d | 110ab | 267cd | 270cd |
| SEM | 1 | 36 | 17 | 52 | 9 | 18 | 7 |
SEM: Standard error of the mean; n.d.: not determined; n = 10. a–e Means in the same row with different letters differ significantly (p < 0.05; Dun or Duncan test).
4. Discussion
4.1. Chemical Composition
Scientific information about the chemical composition, fatty acid, protein, and mineral profile of fish by-products is scarce, reported for few species and using a different approach to obtain the fillets and dissect internal organs. Consequently, this scenario complicates the comparison across studies. Regarding the composition of sea bass fillets observed in the present study, these values were the ranges of values reported in a study about the effect of seasonal changes on sea bass fillets [32]. The animals fished during fall and winter displayed higher moisture values than samples obtained using spring and summer. Conversely, an opposite trend was observed for protein content between these two groups of seasons. It is also relevant to mention that the range for values obtained for moisture, protein, and fat (in the ranges of 62.9%–78.2%, 17.1%–20.8%, and 0.9%–16.4%, respectively) were in the range of values reported for sea bass reared in different systems (extensive, semi-extensive, intensive, and sea-cages) [33].
Concerning the composition of sea bass by-products, a similar pattern for the chemical components of dissected fish can be observed; viscera and/or carcass contain relevant amounts of protein and fats. This outcome is supported by the outcomes obtained with rainbow trout (Oncorhynchus mykiss) [34], blunt snout bream (Megalobrama amblycephala) [35], and olive flounder (Paralichthys olivaceus) [36] in comparison to the respective fillet or whole-fish composition. It is also relevant to mention that, to the best of our knowledge, the sea bass by-products evaluated in this study were collectively discarded without any separation into organs at industrial level.
4.2. Protein and Amino-Acid Profile
The amino-acid profile of sea bass indicated that the predominant amino acids were glutamic acid and glycine for most of the samples. Although the information regarding the amino-acid profile of sea bass is scarce and used the whole fish as a sample, the results obtained in the present study are in accordance with the experiment carried out by Özyurt and Polat [32]. These authors observed that glutamic acid was the predominant amino acid (from 7.69 to 9.61 g/100 g protein) in the composition of sea bass throughout the year. However, aspartic acid and lysine (in ranges of 8.25–9.14 and 7.43–9.49 g/100 g protein) were also indicated as main amino acids. Regarding the EAA/NEAA ratio, this index ranged from 0.75 to 0.77. The predominance of glutamic acid in sea bass was reported in another study that aimed to compare the differences between wild and farmed rearing systems (1475.00 vs. 2369.00 mg/100 g for wild and farmed sea bass, respectively) [37]. Interestingly, this study indicated lysine, aspartic acid, and leucine as the main amino acids in both farmed and wild sea bass. In relation to the EAA/NEAA ratio, the authors obtained values of 1.10 and 1.20 for farmed and wild fishes, respectively.
The analysis of EAA score is an important indicator to evaluate in depth the content of each one of these compounds. It is important to mention that only sea bass fillet displayed adequate values for all the amino acids (scores above 100; Table 3). This outcome is in agreement with the data obtained for Clupea harengus, Scomber scombrus, Trachurus trachurus, and Urophycis tenuis fresh fillets by achieving adequate scores for all EAA [38]. A similar outcome was reported for yellowfin (Thunnus albacares) and bigeye tuna (Thunnus obesus) fresh fillets in relation to the EAA scores [39]. However, the limiting EAA varies among fish species. These contrasting results were reported for the fillets Clarias anguillaris, Oreochromis niloticus, and Cynoglossus senegalensis [40]. In these three species, the limiting amino acid was threonine. Moreover, lower scores than those recommended by FAO/WHO were reported for isoleucine, leucine, lysine, methionine + cysteine, and valine by the authors.
In the case of sea bass by-products, methionine was the only limiting amino acid. Although the results obtained in the present study support the quality of EAA of sea bass by-products, the information regarding this aspect of fish by-products is limited and reported for other species. The study carried out with silver carp (Hypophthalmichthys molitrix) indicated histidine as the limiting EAA for the by-product portion (composed of bones, cutoffs, dark muscle, fins, frame, scales, skin, and viscera) [41].
Regarding the EAA index, previous studies did not characterize this parameter for sea bass fillet and by-products. However, some studies reported the EAA index for other fish species. The EAA index was determined for Lates niloticus and Oreochromis niloticus fillets and whole-body composition of Rastrineobola argentea, Limnothrissa miodon, and Stolothrissa tanganicae [42]. According to the authors, the index varied between 92 (Limnothrissa miodon) and 102 (Oreochromis niloticus). In the experiment carried out by Wu and Mao [43], the EAA index of grass carp (Ctenopharyngodon idellus) fillets was 0.87 (or 87 using the equation of the present study). Thus, this scenario strengthens the biological importance of sea bass by-products as a source of EAA.
The characterization of the sea bass by-products also revealed an interesting scenario for their further use to elaborate functional ingredients and foods to prevent nutritional deficiencies and improve the supply of products with health claims. The nutritional and health claims from the European regulation [44] provide a relevant approach to evaluate the composition of sea bass by-products and indicate potential uses in the development of functional additives.
In relation to the protein content, the skin, gills, head, and fish bone of sea bass can be considered as the most promising “source of protein” or being labeled as “high in protein” among the by-products evaluated in this study (energy associated with protein contain superior to 12% and 20%, respectively). In this scenario, these fractions of sea bass by-products could be considered “high in protein”. It is also relevant to mention that the liver could be considered as a “source of protein”. Conversely, the guts did not achieve the limit to be considered a “source of protein”. In this sense, a potential application of this by-product could be the fortification of baked products [11].
4.3. Fat and Fatty Acids
The analysis of fatty-acid profile of sea bass fillets and by-products revealed a relevant proportion of MUFA and PUFA. Although other studies support the high proportion of unsaturated fatty acids (UFA) in the composition of sea bass [45,46,47], the proportion of each fraction varies across studies. This scenario can be observed in the study carried out by Mourente and Bell [47] who observed that MUFA was the main fraction of fatty acids, followed by PUFA in the muscles and liver of sea bass feed, with partial replacement of vegetables by fish oil. These two fractions of fatty acids accounted for more than 70% of the total fatty acids. In another experiment carried out to investigate the effect rearing system, Bhouri et al. [45] observed that PUFA was the main fraction of fatty acids, followed by MUFA in the dorsal and ventral muscles and liver of sea bass. Conversely, an experiment on the influence of iced storage on the fatty-acid profile of sea bass skin indicated that SFA was the main fraction, followed by MUFA and PUFA [46].
Regarding the individual fatty acids, the results obtained in this study are in agreement with other studies for fillets and by-products regarding the high content of oleic acid and palmitic acid (among MUFA and SFA, respectively) in the fillets, skin, and liver of sea bass [45,46,47]. However, some discrepancies can be noted regarding the main fatty acid among the PUFAs. In the present study, linoleic acid was the main fatty acid in this fraction, followed by DHA (Table 4). These results differ from those reported by other authors who indicated that DHA was the main fatty acid in the PUFA fraction in different portions of the sea bass [45,46,47,48]. A possible explanation could be related to the season of capture, since a previous study indicated that the proportion of both linoleic acid and DHA in the sea bass was influenced by the season [32]. According to the authors of that study, the concentration of DHA surpasses the concentration of linoleic acid during winter, while, during the other seasons, an opposite trend was observed.
Regarding the claims for fat composition, sea bass by-products contained relevant amounts of UFA, MUFA, and long chain n-3 fatty acids. These indicators are also present in European regulation [44] regarding nutritional and health claims. Regarding the UFA, this regulation determines the minimum limit of UFA (sum of MUFA and PUFA) as at least 70%, and more than 20% of energy must be derived from this combination of fractions for the use of a “high unsaturated fat” claim. In this sense, all by-products overcame both limits and could be considered as “high unsaturated fat” sources.
In the case of MUFA content, this regulation establishes that a “high monounsaturated fat” claim is attributed to products with MUFA proportion higher than 45% and with more than 20% of total energy provided by this fat fraction. With this line of thought, the sea bass by-products that overcame both limits for MUFA and percentual energy related to this fat fraction are guts, gills, liver, head, and fish bone, and they could be considered “high monounsaturated fat” sources. In the case of skin, the MUFA proportion did not surpass the minimum limit of 45% of MUFA in total fatty-acid content.
The same approach could also be applied for n-3 fatty acids, wherein the claim “source of n-3 fatty acids” is attributed to samples with at least 40 mg of EPA + DHA/100 g of product and per 100 kcal, and the “high n-3 fatty acid” content claim is attributed to samples with at least 80 mg of EPA + DHA/100 g of product and per 100 kcal [44]. In accordance with these limits, all by-products complied with the “high n-3 fatty acids” content claim. Following this line of thought, the incorporation of n-3 fatty acids in meat products can be seen as a promising strategy to develop healthier products [9].
4.4. Minerals
Only a few studies evaluated the mineral composition of sea bass wherein either fillet or whole fish was considered as a sample. Particularly for macro-minerals, the magnesium content obtained from the fillets in the present study is in agreement with the data reported by Zotos and Vouzanidou [49] for the edible portion of (fillet with skin) of sea bass. However, some differences in the content of macro-minerals can be observed in relation to studies that considered the whole fish as a sample. In the study carried out by Erkan and Özden [50], the main macro-minerals were potassium and phosphorus, followed by sodium and calcium (4597, 3736, 773, and 636 mg/kg, respectively). Particularly for by-products, a study carried out to characterize the composition of sea bass indicated that the liver of this fish was mainly composed of sodium and potassium, followed by calcium and magnesium (9580, 6350, 800, and 880 mg/kg, respectively) [45]. Significant differences between farmed and wild fishes were observed for all macro-minerals according to authors. Unlike that observed in the present study, the liver displayed a similar macro-mineral profile in comparison to fillet.
In relation to the micro-minerals, Yildiz [51] indicated that iron and zinc (in the ranges of 3.1–4.3 and 3.1–5.1 mg/kg, respectively) were among the main micro-minerals in the fillets of both farmed and wild sea bass, followed by manganese and copper (from 1.2 to 1.9 and from 0.1 to 0.3 mg/kg, respectively). Moreover, the author also observed that these minerals were predominant in fillets of sea bass regardless of changes in the micro-mineral of farmed and wild fish. Another similar outcome was reported by Zotos and Vouzanidou [49] who evaluated the effect of season on the mineral composition of the edible portion of sea bass. This study indicated that iron and zinc (in the ranges of 25–29 and 21–24 mg/kg, respectively) were the main micro-mineral components, similar to that observed in the present study. However, the authors did not observe significant differences across seasons. Unlike that observed in the present study, another study on the micro-mineral profile of sea bass liver indicated that iron and zinc were indicated as main components in this fraction of minerals (in the ranges of 134–581 and 97–132 mg/kg, respectively) [45].
It is relevant to mention that a similar outcome in relation to main micro-minerals can be observed in relation to authors that used the whole fish as a sample. A study that evaluated the differences between farmed and wild sea bass indicated iron and zinc as main micro-minerals, followed by manganese [52]. Conversely, only iron was indicated as main micro-mineral in the sea bass evaluated by Erkan and Özden [50], whereas zinc was present at low concentration (24.7 and 2.8 mg/kg, respectively).
In the case of claims for minerals, the classification of “source of” and “high content of” varies among minerals in accordance with daily recommended levels [44]; for calcium and phosphorus, the levels are 800 mg/100 g. In the case of sea bass head and fish bone, both can be considered as “sources of phosphorus” (content superior to 800 mg/100 g) and “high in calcium” (concentration higher than 1600 mg/100 g). Additionally, the gills can also be considered as a “source of Ca”. It would be interesting to explore the use of these sources of minerals in dairy products such as the use of calcium in yogurt [10].
The results obtained in this study can be used to support further experiments targeting both food applications (as indicated previously for amino acids, fatty acids, and minerals) and the development of nutraceuticals from each by-product of sea bass. A relevant example of application is the enrichment of meat products with n-3 fatty acids from sea bass liver and guts. This particular strategy combines the scientific evidence of n-3 fatty acids to promote health with the low natural n-3 fatty-acid content of meat products [9]. Alternatively, the consumption of n-3 fatty-acid capsules is an additional commercial application with health-related effects [12]. Another relevant approach is fortifying dairy products with calcium obtained from sea bass head, fish bones, and gills. In this case, calcium is a relevant component naturally found on yogurt, but increasing its content is of great value for consumers seeking to improve the daily ingestion of this mineral [10]. Likewise, these strategies can be applied for the other sea bass components evaluated in this study to develop healthier and functional food products or supplements. Furthermore, it is also relevant to mention that metals occur naturally in an aquatic system, but they can be considered as pollutants and they accumulate in the sea bass liver and other organs as a consequence of human activity (urban effluents and industrial activity, for instance). Other relevant pollutants (such as organochlorinated compounds) can also be detected in sea bass organs but at lower levels than those established by health organizations [53].
The economic viability of exploring sea bass by-products to obtain functional ingredients and produce nutraceuticals depends on the final products. In the case of fatty acids, the existing industrial structure, machinery, and market [6] are favorable aspects for the insertion of sea bass by-products rich in omega-3 fatty acids into the current production chain of fish oil. In the context of the present study, it seems reasonable to consider that extracting omega-3 fatty acids from sea bass by-products would be possible in the current fish industry.
A different scenario is observed for the exploration of amino acids and minerals from sea bass by-products. Particularly for amino-acid extraction from sea bass by-products, it is necessary to break down fish proteins. Using microbial enzymes can be seen as a feasible approach from an economic point of view [54]. In the case of minerals, the preparation of mineral-rich products could be obtained by simple and low-cost operations such as treating the fish bones with weak solutions (alkali, acid, and hydrogen peroxide), followed by drying and grinding [55].
Further studies are necessary to strengthen these approaches. It is also important to highlight that the reuse of sea bass by-products (discarded material rich in biologically relevant compounds) is one of the necessary steps toward a more sustainable food industry [13].
5. Conclusions
The evaluation of sea bass fillet and by-products indicated a wide diversity of compounds associated with human health. Taking into account the amounts and the biological significance of fatty-acid, amino-acid, and mineral profiles analyzed in this study, it is possible to suggest the use of guts and liver to obtain MUFA, UFA, and long-chain n-3 fatty acids, as well as head, fish bones, and gills to recover minerals, particularly calcium, phosphorus, and manganese, and skin to obtain proteins and amino acids.
Acknowledgments
The authors thank GAIN (Axencia Galega de Innovación) for supporting this review (grant numberIN607A2019/01). Paulo E. S. Munekata acknowledges postdoctoral fellowship support from the Ministry of Economy and Competitiveness (MINECO, Spain) “Juan de la Cierva” program (FJCI-2016-29486). Jianjun Zhou was supported by a PhD fellowship from the China Scholarship Council (CSC) (No. 201908420246).
Author Contributions
Conceptualization, J.M.L.; formal analysis, P.E.S.M., M.P., and R.D.; data analysis, P.E.S.M., M.P., and R.D.; writing—original draft preparation and revision, P.E.S.M., M.P., R.D., J.Z., F.J.B., and J.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the EU Commission for the funds provided by the BBI-JU through the H2020 Project AQUABIOPRO-FIT “Aquaculture and agriculture biomass side stream proteins and bioactives for feed, fitness, and health promoting nutritional supplements” (Grant Agreement no. 790956).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Aronson J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017;83:8–19. doi: 10.1111/bcp.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan R.S., Grigor J., Winger R., Win A. Functional food product development - Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013;30:27–37. doi: 10.1016/j.tifs.2012.11.004. [DOI] [Google Scholar]
- 3.Siró I., Kápolna E., Kápolna B., Lugasi A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite. 2008;51:456–467. doi: 10.1016/j.appet.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 4.Saini R.K., Keum Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci. 2018;203:255–267. doi: 10.1016/j.lfs.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Martins D.A., Custódio L., Barreira L., Pereira H., Ben-Hamadou R., Varela J., Abu-Salah K.M. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs. 2013;11:2259–2281. doi: 10.3390/md11072259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobsen C. Enrichment of foods with omega-3 fatty acids: A multidisciplinary challenge. Ann. N. Y. Acad. Sci. 2010;1190:141–150. doi: 10.1111/j.1749-6632.2009.05263.x. [DOI] [PubMed] [Google Scholar]
- 7.Wendisch V.F., Jorge J.M.P., Pérez-García F., Sgobba E. Updates on industrial production of amino acids using Corynebacterium glutamicum. World J. Microbiol. Biotechnol. 2016;32:105. doi: 10.1007/s11274-016-2060-1. [DOI] [PubMed] [Google Scholar]
- 8.Ayala-Bribiesca E., Lussier M., Chabot D., Turgeon S.L., Britten M. Effect of calcium enrichment of Cheddar cheese on its structure, in vitro digestion and lipid bioaccessibility. Int. Dairy J. 2016;53:1–9. doi: 10.1016/j.idairyj.2015.09.002. [DOI] [Google Scholar]
- 9.Lorenzo J.M., Munekata P.E.S., Pateiro M., Campagnol P.C.B., Domínguez R. Healthy Spanish salchichón enriched with encapsulated n − 3 long chain fatty acids in konjac glucomannan matrix. Food Res. Int. 2016;89:289–295. doi: 10.1016/j.foodres.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Santillán-Urquiza E., Méndez-Rojas M.Á., Vélez-Ruiz J.F. Fortification of yogurt with nano and micro sized calcium, iron and zinc, effect on the physicochemical and rheological properties. LWT Food Sci. Technol. 2017;80:462–469. doi: 10.1016/j.lwt.2017.03.025. [DOI] [Google Scholar]
- 11.Oğur S. Evaluation of amino acid changes and crumb hardness of enriched bread with tench (Tinca tinca L., 1758) flesh in Turkey. J. Food Nutr. Res. 2014;2:985–992. doi: 10.12691/jfnr-2-12-20. [DOI] [Google Scholar]
- 12.Argo C.K., Patrie J.T., Lackner C., Henry T.D., De Lange E.E., Weltman A.L., Shah N.L., Al-Osaimi A.M., Pramoonjago P., Jayakumar S., et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: A double-blind, randomized, placebo-controlled trial. J. Hepatol. 2015;62:190–197. doi: 10.1016/j.jhep.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sibbel A. The sustainability of functional foods. Soc. Sci. Med. 2007;64:554–561. doi: 10.1016/j.socscimed.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S.K., Bansal S., Mangal M., Dixit A.K., Gupta R.K., Mangal A.K. Utilization of food processing by-products as dietary, functional, and novel fiber: A review. Crit. Rev. Food Sci. Nutr. 2016;56:1647–1661. doi: 10.1080/10408398.2013.794327. [DOI] [PubMed] [Google Scholar]
- 15.Jayathilakan K., Sultana K., Radhakrishna K., Bawa A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012;49:278–293. doi: 10.1007/s13197-011-0290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Khawli F., Pateiro M., Domínguez R., Lorenzo J.M., Gullón P., Kousoulaki K., Ferrer E., Berrada H., Barba F.J. Innovative green technologies of intensification for valorization of seafood and their by-products. Mar. Drugs. 2019;17:689. doi: 10.3390/md17120689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Guillén M.C., Lopez-Caallero M.E., Alemán A., López de Lacey A., Giménez B., Montero P. Antioxidant and antimicrobial peptide fractions from squid and tuna skin gelatin. Transw. Res. Netw. 2010;661:89–115. [Google Scholar]
- 18.Behera S.S. Dietary fish oil concentrates associated health benefits: A recent development of cardiovascular risk reduction. Curr. Pharm. Des. 2019;25:4053–4062. doi: 10.2174/1381612825666191112141320. [DOI] [PubMed] [Google Scholar]
- 19.Malde M.K., Graff I.E., Siljander-Rasi H., Venäläinen E., Julshamn K., Pedersen J.I., Valaja J. Fish bones—A highly available calcium source for growing pigs. J. Anim. Physiol. Anim. Nutr. 2010;94:e66–e76. doi: 10.1111/j.1439-0396.2009.00979.x. [DOI] [PubMed] [Google Scholar]
- 20.Food and Agriculture Organization of the United Nations Cultured Aquatic Species Information Programme—Dicentrarchus labrax (Linnaeus, 1758) [(accessed on 15 December 2019)]; Available online: http://www.fao.org/fishery/culturedspecies/Dicentrarchus_labrax/en.
- 21.European Market Observatory for Fisheries and Aquaculture Products . Case Study in the EU Seabass. European Commission; Luxembourg: 2019. [Google Scholar]
- 22.ISO 1442 . Meat and Meat Products—Determination of Moisture Content (Reference Method) 2nd ed. International Organization for Standarization; Geneva, Switzerland: 1997. [Google Scholar]
- 23.ISO 937 . Meat and Meat Products—Determination of Nitrogen Content (Reference Method) 1st ed. International Organization for Standarization; Geneva, Switzerland: 1978. [Google Scholar]
- 24.ISO 936 . Meat and Meat Products—Determination of Total Ash. 2nd ed. International Organization for Standarization; Geneva, Switzerland: 1998. [Google Scholar]
- 25.AOCS Official Procedure Am5-04 . Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction. American Oil Chemists Society; Urbana, IL, USA: 2005. [Google Scholar]
- 26.Domínguez R., Borrajo P., Lorenzo J.M. The effect of cooking methods on nutritional value of foal meat. J. Food Compos. Anal. 2015;43:61–67. doi: 10.1016/j.jfca.2015.04.007. [DOI] [Google Scholar]
- 27.World Health Organization. Food and Agriculture Organization. United Nations University . Protein and Amino Acid Requirements in Human Nutrition. World Health Organization; Geneva, Switzerland: 2002. [Google Scholar]
- 28.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 29.Barros J.C., Munekata P.E.S., de Carvalho F.A.L., Pateiro M., Barba F.J., Domínguez R., Trindade M.A., Lorenzo J.M. Use of tiger nut (Cyperus esculentus L.) oil emulsion as animal fat replacement in beef burgers. Foods. 2020;9:1–15. doi: 10.3390/foods9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Domínguez R., Crecente S., Borrajo P., Agregán R., Lorenzo J.M. Effect of slaughter age on foal carcass traits and meat quality. Animal. 2015;9:1713–1720. doi: 10.1017/S1751731115000671. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzo J.M., Bermúdez R., Domínguez R., Guiotto A., Franco D., Purriños L. Physicochemical and microbial changes during the manufacturing process of dry-cured lacón salted with potassium, calcium and magnesium chloride as a partial replacement for sodium chloride. Food Control. 2015;50:763–769. doi: 10.1016/j.foodcont.2014.10.019. [DOI] [Google Scholar]
- 32.Özyurt G., Polat A. Amino acid and fatty acid composition of wild sea bass (Dicentrarchus labrax): A seasonal differentiation. Eur. Food Res. Technol. 2006;222:316–320. doi: 10.1007/s00217-005-0040-z. [DOI] [Google Scholar]
- 33.Xiccato G., Trocino A., Tulli F., Tibaldi E. Prediction of chemical composition and origin identification of european sea bass (Dicentrarchus labrax L.) by near infrared reflectance spectroscopy (NIRS) Food Chem. 2004;86:275–281. doi: 10.1016/j.foodchem.2003.09.026. [DOI] [Google Scholar]
- 34.Jobling M., Koskela J., Savolainen R. Influence of dietary fat level and increased adiposity on growth and fat deposition in rainbow trout, Oncorhynchus mykiss (Walbaum) Aquac. Res. 1998;29:601–607. doi: 10.1046/j.1365-2109.1998.00251.x. [DOI] [Google Scholar]
- 35.Li X.-F., Liu W.-B., Jiang Y.-Y., Zhu H., Ge X.-P. Effects of dietary protein and lipid levels in practical diets on growth performance and body composition of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquaculture. 2010;303:65–70. doi: 10.1016/j.aquaculture.2010.03.014. [DOI] [Google Scholar]
- 36.Kim K.D., Kang Y.J., Lee H.Y.M., Kim K.W., Jang M.S., Choi S.M., Lee S.M., Cho S.H. Effects of dietary protein and lipid levels on growth and body composition of subadult olive flounder, Paralichthys olivaceus, at a suboptimal water temperature. J. World Aquac. Soc. 2010;41:263–269. doi: 10.1111/j.1749-7345.2010.00366.x. [DOI] [Google Scholar]
- 37.Baki B., Gönener S., Kaya D. Comparison of food, amino acid and fatty acid compositions of wild and cultivated Sea bass (Dicentrarchus labrax L., 1758) Turkish J. Fish. Aquat. Sci. 2015;15:175–179. doi: 10.4194/1303-2712-v15_1_19. [DOI] [Google Scholar]
- 38.Oluwaniyi O.O., Dosumu O.O., Awolola G.V. Effect of local processing methods (boiling, frying and roasting) on the amino acid composition of four marine fishes commonly consumed in Nigeria. Food Chem. 2010;123:1000–1006. doi: 10.1016/j.foodchem.2010.05.051. [DOI] [Google Scholar]
- 39.Peng S., Chen C., Shi Z., Wang L. Amino acid and fatty acid composition of the muscle tissue of yellowfin tuna (Thunnus Albacares) and bigeye tuna (Thunnus Obesus) J. Food Nutr. Res. 2013;1:42–45. doi: 10.12691/jfnr-1-4-2. [DOI] [Google Scholar]
- 40.Adeyeye E.I. Amino acid composition of three species of Nigerian fish: Clarias anguillaris, Oreochromis niloticus and Cynoglossus senegalensis. Food Chem. 2009;113:43–46. doi: 10.1016/j.foodchem.2008.07.007. [DOI] [Google Scholar]
- 41.Zhong S., Liu S., Cao J., Chen S., Wang W., Qin X. Fish protein isolates recovered from silver carp (Hypophthalmichthys molitrix) by-products using alkaline pH solubilization and precipitation. J. Aquat. Food Prod. Technol. 2016;25:400–413. doi: 10.1080/10498850.2013.865282. [DOI] [Google Scholar]
- 42.Babangida A., Bwathondi P.O.J., Suleiman M., Ringim A.S. A study on the amino acid profiles of five fresh water fishes commonly consumed in Tanzania. J. Zool. Biosci. Res. 2017;42:1–6. doi: 10.24896/jzbr.2017421. [DOI] [Google Scholar]
- 43.Wu T., Mao L. Influences of hot air drying and microwave drying on nutritional and odorous properties of grass carp (Ctenopharyngodon idellus) fillets. Food Chem. 2008;110:647–653. doi: 10.1016/j.foodchem.2008.02.058. [DOI] [Google Scholar]
- 44.European Parliament Regulation (EU) No 1924/2006. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union L. 2006 Dec 20;404:9–25. [Google Scholar]
- 45.Bhouri A.M., Bouhlel I., Chouba L., Hammami M., El Cafsi M., Chaouch A. Total lipid content, fatty acid and mineral compositions of muscles and liver in wild and farmed sea bass (Dicentrarchus labrax) African J. Food Sci. 2010;4:522–530. [Google Scholar]
- 46.Sae-leaw T., Benjakul S. Fatty acid composition, lipid oxidation, and fishy odour development in seabass (Lates calcarifer) skin during iced storage. Eur. J. Lipid Sci. Technol. 2014;116:885–894. doi: 10.1002/ejlt.201300381. [DOI] [Google Scholar]
- 47.Mourente G., Bell J.G. Partial replacement of dietary fish oil with blends of vegetable oils (rapeseed, linseed and palm oils) in diets for European sea bass (Dicentrarchus labrax L.) over a long term growth study: Effects on muscle and liver fatty acid composition and effectiv. Comp. Biochem. Physiol. - B Biochem. Mol. Biol. 2006;145:389–399. doi: 10.1016/j.cbpb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Torrecillas S., Mompel D., Caballero M.J., Montero D., Merrifield D., Rodiles A., Robaina L., Zamorano M.J., Karalazos V., Kaushik S., et al. Effect of fishmeal and fish oil replacement by vegetable meals and oils on gut health of European sea bass (Dicentrarchus labrax) Aquaculture. 2017;468:386–398. doi: 10.1016/j.aquaculture.2016.11.005. [DOI] [Google Scholar]
- 49.Zotos A., Vouzanidou M. Seasonal changes in composition, fatty acid, cholesterol and mineral content of six highly commercial fish species of Greece. Food Sci. Technol. Int. 2012;18:139–149. doi: 10.1177/1082013211414785. [DOI] [PubMed] [Google Scholar]
- 50.Erkan N., Özden Ö. Proximate composition and mineral contents in aqua cultured sea bass (Dicentrarchus labrax), sea bream (Sparus aurata) analyzed by ICP-MS. Food Chem. 2007;102:721–725. doi: 10.1016/j.foodchem.2006.06.004. [DOI] [Google Scholar]
- 51.Yildiz M. Mineral composition in fillets of sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata): A comparison of cultured and wild fish. J. Appl. Ichthyol. 2008;24:589–594. doi: 10.1111/j.1439-0426.2008.01097.x. [DOI] [Google Scholar]
- 52.Alasalvar C., Taylor K.D.A., Zubcov E., Shahidi F., Alexis M. Differentiation of cultured and wild sea bass (Dicentrarchus labrax): Total lipid content, fatty acid and trace mineral composition. Food Chem. 2002;79:145–150. doi: 10.1016/S0308-8146(02)00122-X. [DOI] [Google Scholar]
- 53.Fernandes D., Bebianno M.J., Porte C. Assessing pollutant exposure in cultured and wild sea bass (Dicentrarchus labrax) from the Iberian Peninsula. Ecotoxicology. 2009;18:1043–1050. doi: 10.1007/s10646-009-0368-4. [DOI] [PubMed] [Google Scholar]
- 54.Herpandi N.H., Rosma A., Wan Nadiah W.A. The tuna fishing industry: A new outlook on fish protein hydrolysates. Compr. Rev. Food Sci. Food Saf. 2011;10:195–207. doi: 10.1111/j.1541-4337.2011.00155.x. [DOI] [Google Scholar]
- 55.Bubel F., Dobrzański Z., Bykowski P.J., Chojnacka K., Opaliński S., Trziszka T. Production of calcium preparations by technology of saltwater fish by product processing. Open Chem. 2015;13:1333–1340. doi: 10.1515/chem-2015-0146. [DOI] [Google Scholar]