Abstract
Phosphodiesterases (PDEs) form a superfamily of enzymes that catalyze the hydrolysis of cyclic nucleotides adenosine 3′5′-cyclic monophosphate (cAMP) and guanosine 3′5′-cyclic monophosphate (cGMP) to their inactive 5′ monophosphates. cAMP plays a critical role as a second messenger in endocrine tissues, and activation of cAMP signaling has been reported in endocrine tumors. Germline variants in PDEs have been associated with benign cortisol-secreting adrenocortical adenomas and testicular germ cell cancer but not adrenocortical carcinoma. We performed whole genome sequencing (WGS) and whole exome sequencing (WES) of paired blood and tumor samples from 37 pediatric adrenocortical tumors (ACTs). Germline inactivating variants in PDEs were observed in 9 of 37 (24%) patients. Tumor DNA analysis revealed loss of heterozygosity, with maintenance of the mutated allele in all cases. Our results suggest that germline variants in PDEs and other regulators of the cAMP-signaling pathway may contribute to pediatric adrenocortical tumorigenesis, perhaps by cooperating with germline hypomorphic mutant TP53 alleles and uniparental disomy of chromosome 11p15 (Beckwith–Wiedemann syndrome).
Keywords: phosphodiesterase, cAMP pathway, adrenocortical tumor, TP53, 11p
1. Introduction
Cyclic nucleotide phosphodiesterases (PDEs) are members of a superfamily of enzymes involved in regulating the intracellular levels of the second messengers cyclic nucleotide AMP (cAMP) and guanosine 3′5′-cyclic monophosphate (cGMP) [1]. Intracellular cyclic nucleotide levels increase in response to extracellular stimulation by hormones, neurotransmitters, or growth factors and are downregulated through hydrolysis catalyzed by phosphodiesterases (PDEs) [2,3]. Thus, PDEs regulate a myriad of physiological processes and are implicated in genetic diseases, as well as associated with pathophysiology of the nervous and cardiovascular system, fertility, autoimmune diseases, and cancer [1,2,3,4].
Human PDEs are derived from 21 genes separated into 11 families (PDE1 to PDE11) and classified by amino acid sequences, regulatory properties, and catalytic characteristics, which are grouped by the homology of their conserved C-terminal catalytic domains [1,2,3]. The N-terminal portion of PDE molecules defines the specific properties of each member and variant of the PDE gene family [1,2,3]. Transcription from different initiation sites and differential splicing of their mRNAs results in multiple isoforms that are distinguished primarily by their substrate selectivity (cAMP versus cGMP) and modes of regulation. Each isoform is distinct due to its unique expression pattern at the level of the tissue or organ, cell type and subcellular compartment, and susceptibility to pharmacological inhibition, which has provided many possibilities for identifying increasingly selective therapeutic targets [3,4].
Cancer is driven by genetic and epigenetic changes, which lead to altered signaling pathways that control cell division, cell death, and cell motility, thereby fueling wider signaling networks that favor cancer progression. Regulation of cyclic nucleotide signaling is considered one of several components involved in biological processes, such as cell proliferation and energy homeostasis. Indeed, various alterations leading to activation or inactivation of key components of cAMP and cGMP signaling pathways occur in several pathophysiological conditions, including tumorigenesis [1]. Several studies have demonstrated that activation of cyclic nucleotide signaling through one of three mechanisms: Induction of cyclic nucleotide synthesis, inhibition of cyclic nucleotide degradation, or activation of cyclic nucleotide receptors is sufficient to inhibit proliferation and activate apoptosis in many types of cancer cells [5].
Many carcinomas and hematological malignancies have been associated with reduced levels of cAMP and/or cGMP secondary to an elevation in PDE activity [6,7,8,9,10]. Chronic lymphocytic leukemia cells exhibit increased PDE7B expression [11]; PDE5 is strongly expressed in glioblastoma multiforme [12] and colon cancer [13], and glioma cells overexpress one or more isoforms of PDE4 [14]. Furthermore, several PDE isoforms are present in granulosa cells as well as in oocytes in preovulatory follicles of the mammalian ovary regulating the meiotic cell cycle [15]. Additionally, many PDEs are expressed in cells of the spermatogenic pathway where they may regulate sperm motility [16], and PDE5 is expressed in the contractile tissues of the male excurrent duct and accessory glands where its increased activity contributes to erectile dysfunction [16].
Various cellular and molecular alterations of the cAMP-signaling pathway have been observed in endocrine diseases. Studies show that PDE2A, PDE8A, PDE8B, and PDE11A are the major PDEs expressed in the adrenal cortex and play a role in adrenal physiology [17]. Aberrant cAMP signaling has been linked to genetic forms of cortisol excess which can lead to Cushing’s syndrome and related adrenal hyperplasia [17]. Variants in PDE8B predispose to primary pigmented nodular adrenocortical disease (PPNAD), a bilateral form of micronodular adrenal hyperplasia that causes ACTH (adrenocorticotropic hormone)-independent Cushing’s syndrome [18]. A higher frequency of missense variants of PDE11A has been found in adult patients with macronodular adrenocortical hyperplasia and adrenocortical tumors (ACTs) than in control patients [19].
The role of inactivating variants in PDEs in pediatric ACTs has not been investigated thoroughly—unlike in adrenocortical hyperplasia and in such tumors in adults. In the present investigation, we examined the frequency of germline and acquired PDEs variants in a cohort of pediatric patients. Our findings suggest the potential involvement of PDEs in pediatric adrenocortical tumorigenesis.
2. Results
2.1. Discovery Cohort of Pediatric ACT Patients Harboring PDE Variants
Whole genome sequencing (WGS) and whole exome sequencing (WES) data of 37 children with ACTs (“discovery cohort”) were retrieved for analyzing germline and acquired variants in PDE family genes (Figure 1) and other cAMP/cAMP-dependent kinase (PKA)-signaling pathway genes (PDE4DIP, CREB, GNAS and PRKACA). Demographics and clinical data for these patients are shown in Table 1.
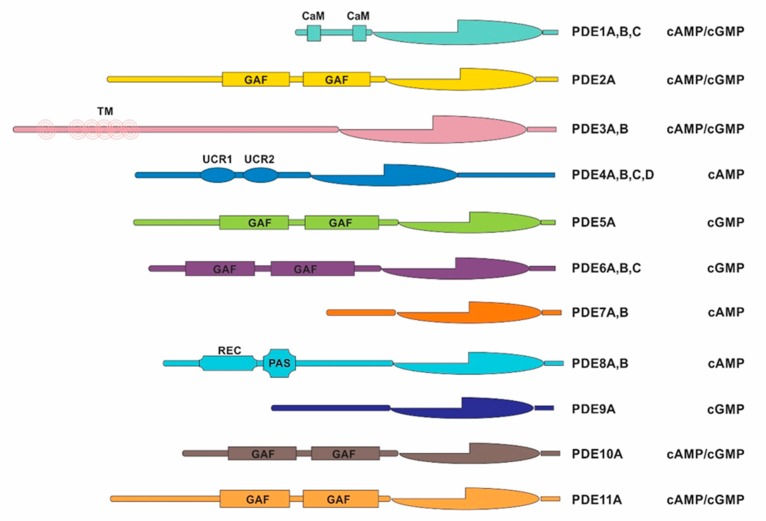
Figure 1.
Schematic representation of human phosphodiesterase genes. Phosphodiesterases (PDEs) are organized into 11 families with specific adenosine 3′5′-cyclic monophosphate (cAMP) and/or guanosine 3′5′-cyclic monophosphate (cGMP) substrates (identified on the right). CaM, calmodulin-binding domain; GAF, cGMP-binding PDEs Anabaena sp. adenylyl cyclase and Escherichia coli. FhlA; TM, transmembrane domain; REC, signal receiver domain; PAS, Per-Arnt-Sim domain; UCR, upstream conserved region.
Table 1.
Clinical data of pediatric adrenocortical tumor (ACT) patients with germline and acquired PDE and cAMP-signaling genes variants.
| Case | c-AMP Pathway/ Germline | c-AMP Pathway/ Somatic | TP53 status | Gender | Clinical Presentation | Histology | Age at diagnosis (months) | Tumor weight (g) | Stage | Survival Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | p.Q860*-PDE5A | WT | F | V | ACA | 59.8 | 20.5 | I | Alive | |
| 2 | p.Q860*-PDE5A | p.R337H | F | V | ACC | 38.0 | 6 | I | Alive | |
| 3 | p.R307*-PDE11A | p.S977I-PDE4DIP/p.R201H-GNAS | WT/UPD | F | A | Und | 140.6 | 388 | III | Alive |
| 4 | p.K20*-PDE11A | p.T125T | M | V | ACC | 103.0 | 500 | III | Alive | |
| 5 | p.R783*-PDE3B | WT | F | V | Und | 26.2 | 69 | I | Alive | |
| 6 | p.W1396*-PDE4DIP | p.R337H | M | V | ACC | 21.0 | Unk | I | Unk | |
| 7 | p.Q1968*-PDE4DIP | p.R337H | M | V+C | ACC | 24.4 | Unk | I | Died | |
| 8 | p.H341Qfs*23-PDE6B | p.R337H | F | V | ACC | 35.0 | 30 | I | Alive | |
| 9 | c.1953-4A>G -PDE8A | WT/UPD | F | C | ACA | 17.0 | 120 | III | Alive | |
| 10 | PDE4-ERRB4 | p.R273C | F | NF | ACC | 24.0 | Unk | IV | Died | |
| 11 | p.R201C-GNAS | WT | F | V | Und | 83.0 | 255.7 | II | Alive |
ACA, adrenocortical adenoma; ACC, adrenocortical carcinoma; Und, Undetermined, WT, wild-type; F, Female; M, Male; V, virilization; A, aldosterone producing tumor; C, Cushing; NF, non-functional; R, right; L, left; Unk, Unknown.
2.2. PDE Variants Identified in the Discovery Cohort
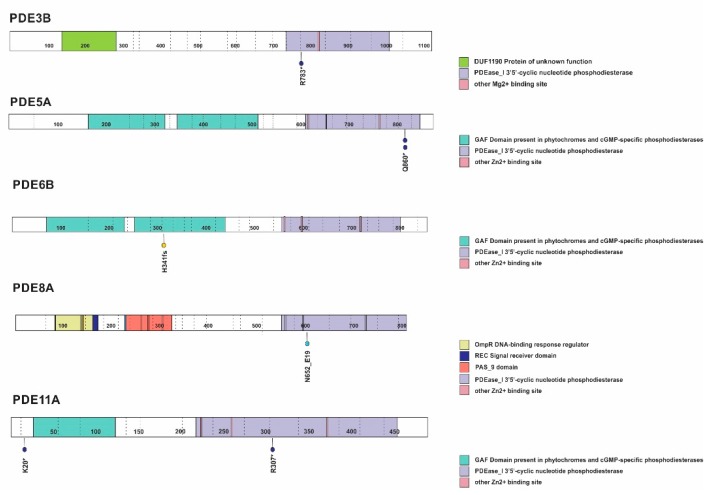
Sequencing analysis of genomic DNA from 37 pediatric ACT patients revealed the presence of germline-inactivating variants in PDEs and related genes in 9 (24%) patients (Figure 2 and Figure 3 and Table 1). Inactivating germline nonsense variants were documented in PDE3B (OMIM 602047, NM_000922.4) (p.R783*, c.2347C > T, rs150090666, gnomAD frequency, 0.06%), PDE5A (OMIM-603310, NM_001083.4) (2x p.Q860*, c.2578C > T, rs140289122, 0.17%) and PDE11A (OMIM-604961, NM_016953.4) (p.K20*, c.58A > T rs148183964, 0.06% and p.R307*, c.919C > T, rs76308115, 0.29%). Additional structural germline variants were observed in PDE6B (OMIM-180072, NM_000283.3 (p.H341Qfs*23) and PDE8A (OMIM-602972, NM_002605.3) (c.1953-4A > G splice region) (Figure 2). Excluding the p.K20* variant for PDE11A and p.H341Qfs*23 for PDE6B, all other variants were found in the PDE catalytic domain (Figure 2). Additional germline nonsense variants were verified in PDE4DIP (OMIM-608117, NM_001198834.3) (p.W1396*, c.4187G > A, rs782516582, 0.008% and p.Q1968*, c.5902C > T, new variant) (Figure 3). Notably, analysis of tumor DNA revealed loss of heterozygosity (LOH), with retention of the mutant allele in all cases. Additional rare germline variants maintained in tumor samples due to LOH and not reported in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) were also observed and included in Table S1. Acquired alterations in PDEs and other cAMP/PKA signaling pathway genes were observed in three cases. The GNAS (OMIM-139320, NM_000516.6) (p.R201H, c.602G > A) pathogenic variant in addition to the PDE4DIP p.S977I missense variant was observed in the tumor sample from patient #3. The GNAS p.R201C, c.601C > T variant was observed in the tumor sample of patient #11, and a gene fusion [chr5:58476419(-)::chr2:212615429(-)] showing PDE4D exon 5 fused to ERBB4 exon 5 was observed in the ACT from patient #10 (Table 1). No pathogenic or likely pathogenic variants were identified in CREB or PRKACA in this cohort.
Figure 2.
Phosphodiesterase variants in the discovery cohort. Germline inactivating variants in PDEs (dark blue, nonsense; light blue, splice-site; and yellow, frame-shift variants). Protein domains shown on right. Illustration based on PeCan Data Portal (https://pecan.stjude.cloud/home).
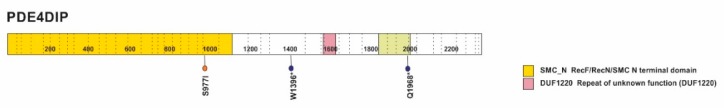
Figure 3.
PDE4DIP variants identified in the discovery cohort. Germline and acquired inactivating variants in PDE4DIP (dark blue, nonsense; and orange, missense variants). Protein domains shown on right. Illustration based on PeCan Data Portal (https://pecan.stjude.cloud/home).
Analysis of the TCGA whole exome sequence database representing 92 paired germline and adult ACC cases [20] revealed rare germline PDE variants that were retained in the tumor due to LOH and lacked representation of pathogenicity in Clinvar (Table S2). Of note, four somatic inactivating PDE variants (PDE2A, p.C935*; PE3A, p.E319*; PDE8A, c.1735-3C > A; and PDE8A, p.S386Hfs*4) were identified in this cohort. An acquired variant in PDE4C (p.A291G; COSV53206356) was also reported in an adrenocortical carcinoma among 41 adult cases analyzed by WES [21].
2.3. Transcriptome Profiling of PDEs in the Discovery Cohort
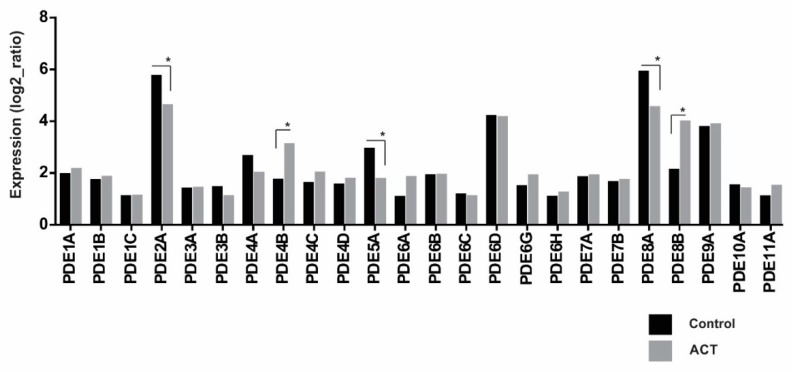
Transcriptome profiling of pediatric ACTs (n = 16) and normal adrenal cortex samples (n = 6) revealed that PDE2A, PDE6D, PDE8A, and PDE9A are highly expressed in both normal and adrenal tumor tissue, compared to other PDE family members. No significant differences in expression were observed when comparing tumor tissue and normal adrenal for most PDE genes. However, significant overexpression of PDE4B and PDE8B and downregulation of PDE2A, PDE5A, and PDE8A were observed in pediatric ACTs compared to normal adrenal (Figure 4).
Figure 4.
Transcriptome profiling of phosphodiesterase genes in pediatric adrenocortical tumors and normal adrenal. Significant overexpression of PDE4B and PDE8B and downregulation of PDE2A, PDE5A, and PDE8A were observed in adrenocortical tissues. (* p < 0.05; Bonferroni test—GraphPad Prism, v6, (GraphPad, San Diego, CA, USA)).
2.4. Germline PDE Variants Identified in Pediatric ACTs Associated with the Founder TP53 p.R337H Variant
Whole exome analysis of germline DNA from an independent cohort of 18 pediatric ACT patients harboring the TP53 p.R337H allele revealed additional inactivating variants in PDE6A (p.K827del) and PDE11A (p.K119Sfs*2). Nonsense variants were also observed in PDE4DIP (p.E515*, c.1543G > T, new variant / p.E1745*, c.5233G > T, new variant). Additional rare germline variants with no representation of clinical significance in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) observed in this cohort are included in Table S3.
3. Discussion
In this study, we identified nine germline inactivating variants in phosphodiesterases and related genes in a discovery cohort of 37 pediatric patients with ACT (24%). Inactivating variants were observed in PDE3B, PDE5A, PDE6B, PDE8A, and PDE11, and the phosphodiesterase interacting protein PDE4DIP. All observed variants, except for two, were in the catalytic domain, which suggests a loss of function of PDE [22]. Of these, only PDE11A p.R307* has been previously found in association with Cushing’s syndrome due to micronodular adrenocortical hyperplasia in a female carrier [19,23]. In our study, the female patient harboring the PDE11A p.R307* variant (Patient #3) developed an aldosterone-producing tumor at the age of 12 years. In addition to the germline PDE11A nonsense variant, the tumor acquired a pathogenic variant in GNAS. These findings agree with the role of PDE11A in genetic predisposition to the development of adrenal tumors [19,23], and that additional cooperating events leading to altered cAMP/PKA signaling [24] are required to drive adrenocortical tumorigenesis.
The most widely-used and valuable histopathological scoring criteria for predicting pediatric adrenocortical tumor malignancy is the Wieneke classification system [25], which relies on nine macroscopic and microscopic variables. Based on this system, tumors are classified as adenoma (ACA; 0–2 variables), undetermined malignant potential (Und; 3 variables), and carcinoma (ACC; 4 or more variables), which portends a poor clinical outcome [25]. However, lack of definitive and reliable histopathological criteria for malignancy is still a challenge for pediatric ACT. About 50% of pediatric ACTs are associated with germline TP53 variants that lead to more complex genomic landscapes [26]. Although discrete genomic changes were not independently associated with prognosis, complex genomic alterations tended to portend an unfavorable outcome [27]. Furthermore, TP53 variants observed in pediatric adrenocortical tumors did not correspond to the conventional hotspot variants associated with classic Li–Fraumeni syndrome (LFS), and most retain a wide range of functionality [28,29]. Consistent with these observations, among the five carriers of TP53 variants in our cohorts, only one (patient #4) harbored a predicted nonfunctional TP53 variant [28], and none were classified as LFS.
Loss of heterozygosity was observed for all inactivating phosphodiesterase/phosphodiesterase-related variants. One can hypothesize that a complete inactivation of those proteins, due to an inactivating variant in one allele and the loss of the second wild-type allele, in the right environment could favor the development of tumors. The “right” environment could be formed by the presence of a TP53 hypomorphic variant, that predisposes the cells to form tumors. A previous study demonstrated that PDEs can act as phenotype modifiers, leading to adrenal tumors in Carney complex patients carrying PRKAR1A variant [30].
A high frequency of PDE variants was observed in patients with prostate cancer [31]. The pCREB:CREB ratio (phosphorylated cAMP response element-binding protein: cAMP response element-binding protein) showed an imbalance in the cAMP availability, probably due to downregulation of some of the PDE molecules [31]. In the present work, when the transcriptome analysis was accessed, a decrease of PDE2A, PDE5A, and PDE8A expression and a significant overexpression of PDE4B and PDE8B was observed in adrenocortical tumors, compared to normal adrenal tissue. PDEs have been found to play critical roles in modulating multiple signaling pathways. The presence of consistent and significative differences in the expression of PDEs throughout patients’ tumors as compared with that in controls could indicate an imbalance of the cAMP pathway. Corroborating this hypothesis, a knockout mouse for the Prkar1a gene in the pancreas leads to neuroendocrine tumorigenesis, probably due to the dysregulation of the (cAMP)-dependent kinase (PKA) pathway [32].
Of interest, we have documented germline nonsense variants in PDE4DIP, a protein that interacts with the cyclic nucleotide phosphodiesterase PDE4D to anchor this protein to the Golgi/centrosome region of the cell. This gene has been associated with myeloproliferative disorders, as shown by its fusions with platelet-derived growth factor receptor beta gene (PDGFRB) [33]. In addition, PDE4DIP variants are one of the most frequent in metastatic adrenocortical carcinomas in adults [34]. Of note, all four of our patients with an inactivating variant in PDE4DIP also harbor the hypomorphic widespread TP53 p.R337H founder allele [35,36,37]. These findings suggest that PDE4DIP variants (and perhaps others c-AMP signaling pathway genes) predispose TP53 p.R337H carriers to adrenocortical tumorigenesis.
Genome-wide associations studies demonstrate that PDE2A, PDE8B, and PDE11A modulate steroidogenesis [17] and are associated with adrenal Cushing’s syndrome and/or bilateral adrenal hyperplasia [17]. In contrast, our findings identified germline inactivating PDE variants in patients who developed adrenocortical carcinomas, including cases with virilization, and aldosterone-producing tumors. The observed concomitant PDE variants and hypomorphic TP53 alleles or chromosome 11p uniparental disomy in the germline of pediatric ACT cases support the combined additive effect of multiple genetic variants in cancer susceptibility [38].
In this study, we added PDE variants as a candidate causative gene for pediatric adrenocortical lesions. Altogether, sequencing analysis and transcriptome profiling support the importance of alterations in the cAMP signaling pathway in adrenocortical tumors. As we learn more about the functional roles and molecular interactions of each PDE, as well as how the variants operate in adrenocortical tumorigenesis, we will better understand the full potential of PDEs as therapeutic targets.
4. Materials and Methods
4.1. Phosphodiesterase Variants in the Discovery Cohort
We retrieved data from primary ACTs and matched peripheral blood DNA analyzed by WGS (n = 19) at an average 41.9x coverage or by WES (n = 18) at an average 84.8x coverage. Of the nine cases with germline variants in PDEs, six were disclosed by WGS and three by WES [26]. WGS and WES were performed as previously described [26]. Sequencing data for variants in the PDE family of genes (PDE1 to PDE11, Figure 1), and PDE4DIP as well as cAMP/PKA-signaling pathway genes GNAS, PRKACA, and CREB were analyzed. Detected germline variants were evaluated in tumor tissue to determine heterozygosity status.
4.2. Transcriptome Profiling
Transcriptome profiling was performed by using total RNA extracted from pediatric ACTs (n = 16) in the discovery cohort. Six samples of normal adrenocortical tissue obtained during nephrectomy for Wilms tumor were used as controls in gene expression studies. Library construction and sequencing was performed as previously described [26]. The RNA expression level of PDE genes was measured as fragments per kilobase of transcript per million fragments mapped [26].
Sequencing and transcriptome data were retrieved from the European Genome-phenome Archive (EGA) under accession code EGAS00001000192.
4.3. Whole Exome Sequencing of an Independent Cohort of Pediatric ACTs Harboring the Germline TP53 p.R337H Variant
Peripheral blood DNA was isolated from 18 children with ACT and 36 cancer-free parents and analyzed by WES. All patients with ACTs tested positive for the germline TP53 p.R337H variant. Written informed consent was obtained from all participants. This research was approved by the Pequeno Príncipe Hospital Ethics Committees (Curitiba, PR, Brazil, under ethic codes CAA: 0023.0.208.000-05 (2005), and CAAE 0612.0.015.000-08 (2009 and 2012).
Genomic DNA was isolated from blood samples using the ReliaPrep™ Blood gDNA Miniprep System (Promega, Madison, WI, USA) and quantified with the Qubit® 3.0 Fluorometer and Qubit dsDNA HS assay kit (Thermo Fisher Scientific, Grand Island, NY, USA). WES was performed by using the SureSelect Human All Exon V5 (Agilent Technologies, Santa Clara, CA, USA) for sequence capture and Illumina HiSeq2500 (2 × 125 bp paired-end) for sequencing (Illumina, San Diego, CA, USA). Read alignment, variant calling, prioritization, and filtering were performed on the Sirius online platform (Integragen, Evry, France). A custom Python script was used to compute filtered variant data into tables.
5. Conclusions
We reported recurrent inactivating germline PDE variants in association with pediatric adrenocortical tumors. In each case, the wild-type allele was selected against by LOH, suggesting an imbalance of the cAMP signaling pathway contributes to tumor progression.
Acknowledgments
We especially thank the patients and their family members for participating in this study. We thank the Hartwell Center and Tissue Resources Core Facility at St. Jude Children’s Research Hospital, the Speer Charitable Trust and Vani Shanker for scientific editorial assistance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/2/506/s1, Table S1: Additional variants observed in the discovery cohort of pediatric adrenocortical tumors, Table S2: Acquired and constitutive PDEs variants in TCGA database, Table S3: Additional variants observed in an independent cohort of carriers of the germline TP53 p.R337H variant.
Author Contributions
Conceptualization, E.M.P.; methodology and data analysis, E.M.P., G.W and E.L.; writing—original draft preparation, E.M.P.; writing—review and editing, E.M.P., F.R.F., L.Z.P., G.W., E.S.F., J.B., C.A.S., E.L., R.C.R., C.R.-G., B.C.F., G.P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the American Lebanese Syrian Associated Charities (ALSAC).
Conflicts of Interest
C.A.S. holds patent on the PRKAR1A, PDE11A and GPR101 genes and/or their function and his laboratory has received research funding from Pfizer Inc. The remaining authors declare no conflict of interest.
References
- 1.Azevedo M.F., Faucz F.R., Bimpaki E., Horvath A., Levy I., de Alexandre R.B., Ahmad F., Manganiello V., Stratakis C.A. Clinical and molecular genetics of the phosphodiesterases (PDEs) Endocr. Rev. 2014;35:195–233. doi: 10.1210/er.2013-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong M.L., Whelan F., Deloukas P., Whittaker P., Delgado M., Cantor R.M., McCann S.M., Licino J. Phosphodiesterase genes are associated with susceptibility to major depression and antidepressant treatment response. Proc. Natl. Acad. Sci. USA. 2006;103:15124–15129. doi: 10.1073/pnas.0602795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis S.H., Blount M.A., Corbin J.D. Mammalian cyclic nucleotide phosphodiesterases: Molecular mechanisms and physiological functions. Physiol. Rev. 2011;91:651–690. doi: 10.1152/physrev.00030.2010. [DOI] [PubMed] [Google Scholar]
- 4.Baillie G.S., Tejeda G.S., Kelly M.P. Therapeutic targeting of 3’,5’-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Rev. Drug Discov. 2019;18:770–796. doi: 10.1038/s41573-019-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fajardo A.M., Piazza G.A., Tinsley H.N. The Role of Cyclic Nucleotide Signaling Pathways in Cancer: Targets for Prevention and Treatment. Cancers. 2014;6:436–458. doi: 10.3390/cancers6010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerner A., Epstein P.M. Cyclic nucleotide phosphodiesterases as targets for treatment of haematological malignancies. Biochem. J. 2006;393:21–41. doi: 10.1042/BJ20051368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chawla R.K., Shlaer S.M., Lawson D.H., Murray T.G., Schmidt F., Shoji M., Nixon D.W., Richmond A., Rudman D. Elevated plasma and urinary guanosine 3’:5’-monophosphate and increased production rate in patients with neoplastic diseases. Cancer Res. 1980;40:3915–3920. [PubMed] [Google Scholar]
- 8.Pertuit M., Barlier A., Enjalbert A., Gérard C. Signalling pathway alterations in pituitary adenomas: Involvement of Gsalpha, cAMP and mitogen-activated protein kinases. J. Neuroendocrinol. 2009;21:869–877. doi: 10.1111/j.1365-2826.2009.01910.x. [DOI] [PubMed] [Google Scholar]
- 9.DeRubertis F.R., Craven P.A. Sequential alterations in the hepatic content and metabolism of cyclic AMP and cyclic GMP induced by DL-ethionine: Evidence for malignant transformation of liver with a sustained increase in cyclic AMP. Metabolism. 1976;25:1611–1625. doi: 10.1016/0026-0495(76)90114-1. [DOI] [PubMed] [Google Scholar]
- 10.Aleksijevic A., Lugnier C., Giron C., Mayer S., Stoclet J.C., Lang J.M. Cyclic AMP and cyclic GMP phosphodiesterase activities in Hodgkin’s disease lymphocytes. Int. J. Immunopharmacol. 1987;9:525–531. doi: 10.1016/0192-0561(87)90119-6. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L., Murray F., Zahno A., Kanter J.R., Chou D., Suda R., Fenlon M., Rassenti L., Cottam H., Kipps T.J., et al. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2008;105:19532–19537. doi: 10.1073/pnas.0806152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesarini V., Martini M., Vitiani L.R., Gravina G.L., DiAgostino S., Graziana G., D’Aessandris Q.G., Pallini R., Larocca L.M., Rossi P., et al. Type 5 phosphodiesterase regulates glioblastoma multiforme aggressiveness and clinical outcome. Oncotarget. 2017;8:13223–13239. doi: 10.18632/oncotarget.14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwan D.G., Brunton V.G., Baillie G.S., Leslie N.R., Houslay M.D., Frame M.C. Chemoresistant KM12C colon cancer cells are addicted to low cyclic AMP levels in a phosphodiesterase 4-regulated compartment via effects on phosphoinositide 3-kinase. Cancer Res. 2007;67:5248–5257. doi: 10.1158/0008-5472.CAN-07-0097. [DOI] [PubMed] [Google Scholar]
- 14.Goldhoff P., Warrington N.M., Limbrick DDJr Hope A., Woerner B.M., Jackson E., Perry A., Piwnica-Worms D., Rubin J.B. Targeted inhibition of cyclic AMP phosphodiesterase-4 promotes brain tumor regression. Clin. Cancer Res. 2008;14:7717–7725. doi: 10.1158/1078-0432.CCR-08-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vigone G., Shuhaibar L.C., Egbert J.R., Uliasz T.F., Movsesian M.A., Jaffe L.A. Multiple cAMP Phosphodiesterases Act Together to Prevent Premature Oocyte Meiosis and Ovulation. Endocrinology. 2018;159:2142–2152. doi: 10.1210/en.2018-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drobnis E.Z., Nangia A.K. Phosphodiesterase Inhibitors (PDE Inhibitors) and Male Reproduction. Adv. Exp. Med. Biol. 2017;1034:29–38. doi: 10.1007/978-3-319-69535-8_5. [DOI] [PubMed] [Google Scholar]
- 17.Szarek E., Stratakis C.A. Phosphodiesterases and adrenal Cushing in mice and humans. Horm. Metab. Res. 2014;46:863–868. doi: 10.1055/s-0034-1389916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothenbuhler A., Horvath A., Libé R., Faucz F.R., Fratticci A., Raffin Sanson M.L., Vezzosi D., Azevedo M., Levy I., Almeida M.Q., et al. Identification of novel genetic variants in phosphodiesterase 8B (PDE8B), a cAMP-specific phosphodiesterase highly expressed in the adrenal cortex, in a cohort of patients with adrenal tumours. Clin. Endocrinol. 2012;77:195–199. doi: 10.1111/j.1365-2265.2012.04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Libé R., Fratticci A., Coste J., Tissier F., Horvath A., Ragazzon B., Rene-Corail F., Groussin L., Bertagna X., Raffin-Sanson M.L., et al. Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin. Cancer Res. 2008;14:4016–4024. doi: 10.1158/1078-0432.CCR-08-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng S., Cherniack A.D., Dewal N., Moffitt R.A., Danilova L., Murray B.A., Lerario A.M., Else T., Knijnenburg T.A., Ciriello G., et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell. 2016;29:723–736. doi: 10.1016/j.ccell.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juhlin C.C., Goh G., Healy J.M., Fonseca A.L., Scholl U.I., Stenman A., Kunstman J.W., Brown T.C., Overton J.D., Mane S.M., et al. Whole-exome Sequencing Characterizes the Landscape of Somatic Mutations and Copy Number Alterations in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2015;100:E493–E502. doi: 10.1210/jc.2014-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butt E., Beltman J., Becker D.E., Jensen G.S., Rybalkin S.D., Jastorff B., Beavo J.A. Characterization of cyclic nucleotide phosphodiesterases with cyclic AMP analogs: Topology of the catalytic sites and comparison with other cyclic AMP-binding proteins. Mol. Pharmacol. 1995;47:340–347. [PubMed] [Google Scholar]
- 23.Horvath A., Boikis S., Giatzakis C., Robinson-White A., Groussin L., Griffin K.J., Stein E., Levine E., Delimpasi G., Hsiao H.P., et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat. Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 24.Sato Y., Maekawa S., Ishii R., Sanada M., Morikawa T., Shiraishi Y., Yoshida K., Nagata Y., Sato-Otsubo A., Yoshizato T., et al. Recurrent somatic mutations underlie corticotropin-independent Cushing’s syndrome. Science. 2014;344:917–920. doi: 10.1126/science.1252328. [DOI] [PubMed] [Google Scholar]
- 25.Wieneke J.A., Thompson L.D.R., Heffess C.S. Adrenal Cortical Neoplasms in the Pediatric Population: A Clinicopathologic and Immunophenotypic Analysis of 83 Patients. Am. J. Surg. Pathol. 2003;27:867–881. doi: 10.1097/00000478-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Pinto E.M., Chen X., Easton J., Finkelstein D., Liu Z., Pounds S., Rodriguez-Galindo C., Lund T.C., Mardis E.R., Wilson R.K., et al. Genomic landscape of paediatric adrenocortical tumours. Nat. Commun. 2015 doi: 10.1038/ncomms7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto E.M., Rodriguez-Galindo C., Pounds S.B., Wang L., Clay M.R., Neale G., Garfinkle E.A.R., Lam C.G., Levy C.F., Pappo A.S., et al. Identification of Clinical and Biologic Correlates Associated With Outcome in Children With Adrenocortical Tumors Without Germline TP53 Mutations: A St Jude Adrenocortical Tumor Registry and Children’s Oncology Group Study. J. Clin. Oncol. 2017;35:3956–3963. doi: 10.1200/JCO.2017.74.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasserman J.D., Novokmet A., Eichler-Jonsson C., Ribeiro R.C., Rodriguez-Galingo C., Zambetti G.P., Malkin D. Prevalence andFunctional Consequence of TP53 Mutations in Pediatric Adrenocortical Carcinoma: A Children’s Oncology Group Study. J. Clin. Oncol. 2015;33:602–609. doi: 10.1200/JCO.2013.52.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zambetti G.P. The p53 Mutation “Gradient Effect” and Its Clinical Implications. J. Cell Physiol. 2007;213:370–373. doi: 10.1002/jcp.21217. [DOI] [PubMed] [Google Scholar]
- 30.Libe R., Horvath A., Vezzosi D., Fratticci A., Coste J., Perlemoine K., Ragazzon B., Guillaud-Bataille M., Groussin L., Clauser E., et al. Frequend Phosphodiesterase 11A Gene (PDE11A) Defects in Patients With Carney Complex (CNC) Caused by PRKAR1A Mutations: PDE11A May Contribute to Adrenal and Testicular Tumors in CNC as a Modifier of the Phenotype. J. Clin. Endocrinol. Metab. 2011;96:E208–E214. doi: 10.1210/jc.2010-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Alexandre R.B., Horvath A.D., Szarek E., Manning A.D., Leal L.F., Kardauke F., Epstein J.A., Carraro D.M., Soares F.A., Apanasovich T.V., et al. Phosphodiesterase sequence variants may predispose to prostate cancer. Endocr. Relat. Cancer. 2015;22:519–530. doi: 10.1530/ERC-15-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saloustros E., Salpea P., Starost M., Liu S., Faucz F.R., London E., Szarek E., Song W.J., Hussain M., Stratakis C.A. Prkar1a Gene Knockout in the Pancreas Leads to Neuroendocrine Tumorigenesis. Endocr. Relat. Cancer. 2017;24:31–40. doi: 10.1530/ERC-16-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson K., Velloso E.R.P., Lopes L.F., Lee C., Aster J.C., Shipp M.A., Aguiar R.C.T. Cloning of the t(1;5)(q23;q33) in a myeloproliferative disorder associated with eosinophilia: Involvement of PDGFRB and response to imatinib. Blood. 2003;102:4187–4190. doi: 10.1182/blood-2003-04-1150. [DOI] [PubMed] [Google Scholar]
- 34.Gara S.K., Lack J., Zhang L., Harris E., Cam M., Electron K. Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors. Nat. Commun. 2018 doi: 10.1038/s41467-018-06366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribeiro R.C., Sandrini F., Figueiredo B., Zambetti G.P., Michalkiewicz E., Lafferty A.R., DeLacerda L., Rabin M., Cadwell C., Sampaio G., et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc. Natl. Acad. Sci. USA. 2001;98:9330–9335. doi: 10.1073/pnas.161479898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latronico A.C., Pinto E.M., Domenice S., Fragoso M.C., Martin R.M., Zerbini M.C., Lucon A.M., Mendonca B.B. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 2001;86:4970–4973. doi: 10.1210/jcem.86.10.7957. [DOI] [PubMed] [Google Scholar]
- 37.Pinto E.M., Billerbeck A.E., Villares M.C., Domenice S., Mendonca B.B., Latronico A.C. Founder effect for the highly prevalent R337H mutation of tumor suppressor p53 in Brazilian patients with adrenocortical tumors. Arq. Bras. Endocrinol. Metabol. 2004;48:647–650. doi: 10.1590/S0004-27302004000500009. [DOI] [PubMed] [Google Scholar]
- 38.Yang Q., Khoury M.J., Friedman J.M., Little J., Flanders W.D. How many genes underlie the occurrence of common complex diseases in the population? Int. J. Epidemiol. 2005;34:1129–1137. doi: 10.1093/ije/dyi130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.