Abstract
Natural killer cells (NK cells) play a major role in the immune response to cancer. An important element of NK target recognition is the binding of human leucocyte antigen (HLA) class I molecules by killer immunoglobulin-like receptors (KIRs). Colorectal carcinoma (CRC) is one of the most common types of inflammation-based cancer. The purpose of the present study was to investigate the presence of KIR genes and HLA class I and II alleles in 1074 CRC patients and 1272 controls. We imputed data from single-nucleotide polymorphism (SNP) Illumina OncoArray to identify associations at HLA (HLA–A, B, C, DPB1, DQA1, DQB1, and DRB1) and KIRs (HIBAG and KIR*IMP, respectively). For association analysis, we used PLINK (v1.9), the PyHLA software, and R version 3.4.0. Only three SNP markers showed suggestive associations (p < 10−3; rs16896742, rs28367832, and rs9277952). The frequency of KIR2DS3 was significantly increased in the CRC patients compared to healthy controls (p < 0.005). Our results suggest that the implication of NK cells in CRC may not act through allele combinations in KIR and HLA genes. Much larger studies in ethnically homogeneous populations are needed to rule out the possible role of allelic combinations in KIR and HLA genes in CRC risk.
Keywords: KIR, colorectal carcinoma, HLA, KIR2DS3, SNP, imputation
1. Introduction
Colorectal carcinoma (CRC) is a leading cancer by incidence and mortality, responsible for approximately 900,000 deaths per year worldwide [1]. CRC has long been appreciated to have a strong heritable basis, being a multifactorial entity since their pathogenesis involves both multiple genetic factors and diverse environmental factors. Large genome-wide analysis studies (GWAS) have increased our knowledge of the genetic risk factors of CRC [2,3], though much of the heritable risk of CRC remains unexplained and many rare and common variants have not yet been identified.
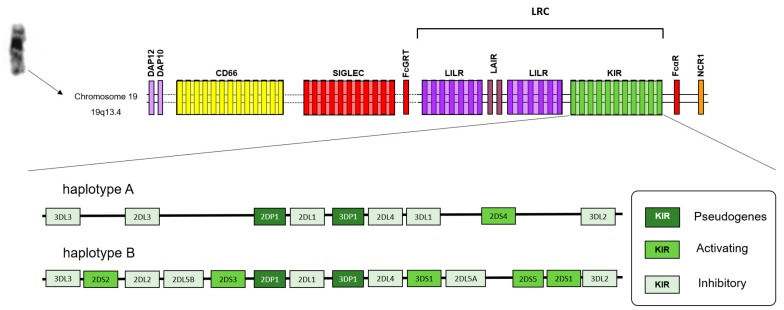
Natural killer cells (NK cells) play a major role in the immune response to cancer [4]. An important element of NK target recognition is the binding of human leucocyte antigen (HLA) class I molecules by killer immunoglobulin-like receptors (KIRs). The KIR gene cluster is located on human chromosome 19, and constitutes a multigene family with genomic diversity with regard to gene content and allelic polymorphism [5] (Figure 1). KIRs have two (2D) or three Ig-like domains (3D), possessing a short (S) or long (L) cytoplasmic tail (Figure 2). They have been classified into two types: activating (KIR2DS and KIR3DS), where the cytoplasmic tail has the capacity to interact with activating adaptor proteins such as DAP12, delivering activating signals through their immunoreceptor tyrosine-based activating (ITAM) motif [6], and inhibitory (KIR2DL and KIR3DL) KIRs, which have one or two immunoreceptor tyrosine-based inhibition (ITIM) motifs in their cytoplasmic tail [7]. KIR2DL4 is an exception, and some reports indicate that their presence enhances NK activity and induces interferon-γ secretion [8]. Additionally, the letter P denotes pseudogenes (KIR2DP1 and KIR3DP1). Based on the genes present in each person, two basic groups of haplotypes have been proposed (Figure 1): haplotype A, that contains only one activating KIR gene, KIR2DS4; and haplotype B, with various combinations of activating KIR genes (KIR2DS1, 2DS2, 2DS3, 2DS5, 3DS1, and 2DS4), exhibiting high levels of diversity, both in terms of gene content and allelic polymorphism.
Figure 1.
Schematic representations of the Leukocyte Receptor Complex (LRC) region and typical KIR A and B genotypes. Map of the Leukocyte Receptor Complex. The Leukocyte Receptor Complex (LRC) is formed by a cluster of genes that encode a family of proteins that contain immunoglobulin-like domains. These include the families “killer immunoglobulin-like receptors (KIR), “leukocyte immunoglobulin-like receptor” (LILR), and “leukocyte-associated immunoglobulin-like receptor” (LAIR). The “signaling lectins” (SIGLECs) and members of the family CD66 are found close to LCR.
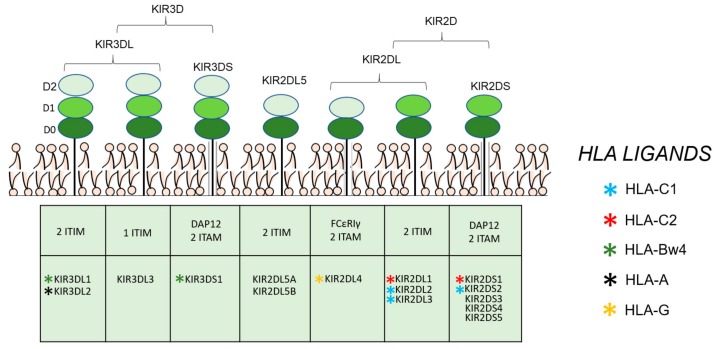
Figure 2.
KIR protein structures and ligands. Individual KIR ligands are shown in small asterisks of different colors (KIR ligands for KIR3DL3, KIR2DL5, KIR2DS3, and KIR2DS5 are unknown).
KIRs bind to recognize normally expressed HLA class I molecules (Figure 2), controlling the activation of NK cells. HLA-C alleles can be divided into two allotypes defined serologically by the Ser77/Asn80 (C1) and Asn77/Lys80 (C2) amino acid positions. Some of KIR receptors bind to HLA-C molecules [9], KIR2DL2, KIR2DL3, and KIR2DS2 interact with HLA C1, whereas KIR2DL1 and KIR2DS1 recognize HLA-C2. KIR3DL1 binds the HLA-B α1 helix around residues 76–80, with specificity for all Bw4 alleles containing isoleucine at heavy chain residue 80 [10]. Because of the relevant homology of activating KIR3DS1 to inhibitory KIR3DL1, it has been speculated that they might share the same ligand, although this assumption has not yet been determined. In vitro studies have proposed that HLA-A3 and HLA-A11 are ligands of KIR3DL2, but these interactions appear to be weak, and peptide dependent [11].
Over the last years, KIR genes have been reported strongly associated with disease susceptibility, following a model in which KIRs synergize with HLAs generating genotypes that provide different levels of activation or inhibition [5]. Recognition of HLA by KIR modulates NK function, promoting the attack to cancer cells. Therefore, variation in KIR and HLA have been thought to affect in the risk of developing cancer [12] Expression of HLA class I molecules is downregulated in more than 70% of colorectal tumors [13], and the prognostic significance of this downregulation has been reported in a large cohort of CRC cases [14]. Thus, NK cells activation by the decreased expression of HLA class I molecules on tumor cells is a relevant issue. Moreover, previous studies have focused on the connection between CRC with the polymorphism of KIR genes, some of them analyzing HLA class I presence (mainly HLA-B and HLA-C). However, these investigations are limited by small populations in different ethnic groups, providing contradictory results. In the present study, we analyzed the presence of KIR genes and HLA class I and II alleles in CRC patients and healthy controls in a larger population that is ethnically homogeneous. To the best of our knowledge, this is the largest study of KIR genes and HLA ligands in CRC to date.
2. Materials and Methods
2.1. Patients and Samples
A total of 1074 patients with CRC and 1272 healthy controls were included in the work, combining data of two case-control studies from two different locations. The first one, performed in University Hospital of Bellvitge, L’Hospitalet, Barcelona, recruited a total of 693 CRC cases and 849 healthy controls. The second study was conducted in Hospital of Leon, Leon, recruiting a total of 381 CRC cases and 423 healthy controls. Subjects were interviewed in person to gather information on risk factors and biological samples for DNA extraction were collected. Peripheral blood (27 mL) was drawn from participants, which were aliquoted in whole blood for DNA extraction, and stored at −80 °C. Saliva was collected for subjects refusing to donate blood with the Oragene® DNA Kit and stored at room temperature until DNA extraction. Standardized basic clinical and pathological information on the diagnosis of tumors was collected from hospital records by using a predefined format. The protocol was approved by the Ethics committees of the participating institutions (registration number PR149/08 for the Bellvitge University Hospital; registration number ETICA-ULE-022/2017 for University of Leon). All participants were informed about the study objectives and signed an informed consent form. Confidentiality of data was secured by removing personal identifiers in the datasets.
2.2. Genotyping and Imputation
Genotyping was performed using the custom-designed 533,631 SNP Illumina OncoArray. This array was designed for cancer studies by the OncoArray Consortium, including fine-mapping of common cancer susceptibility loci with special emphasis on the HLA region, among others. Oncoarray genotyping and genotype quality controls procedure were done in the context of a large CRC genome-wide study using the OncoArray platform [2].
The classic HLA alleles at HLA–A, B, C, DPB1, DQA1, DQB1, and DRB1 were imputed using HLA Genotype Imputation with Attribute Bagging (HIBAG) and its corresponding European Ancestry reference panel [15]. SNP genotypes were phased using SHAPEIT2 [16], and the resulting phased haplotypes were uploaded to the KIR*IMP imputation server (http://imp.mcri.edu.au) [17], for KIR genes imputation.
2.3. Statistical Analysis
SNPs that met the quality criteria of a minor allele: a frequency of >0.01, missingness of <0.1, and/or a Hardy-Weinberg equilibrium (p > 0.001) were considered for inclusion in the association analyses. A total of 4874 SNPs was located on chromosome 6 (chr6: 28400339-33496193, hg19) (genotyping rate: 0.99761), whereas 791 SNPs were situated on chromosome 19 (chr19: 54500612-55599594, hg19) (genotyping rate: 0.997693). After quality control, 943 CRC cases and 1076 healthy controls were available for the association study. For single-variant association analysis (HLA and LRC regions, within chromosomes 6 and 19, respectively), we used PLINK (v1.9) [18] to perform logistic regression for binary phenotype (CRC and healthy controls). HLA association analysis was performed with the PyHLA software [19], using additive logistic regression models. The associations among KIR genes and KIR/HLA combinations with CRC were evaluated using an odds ratios (OR) estimated by logistic regression using R version 3.4.0. (https://cran.r-project.org/). For comparisons of the KIR genes frequencies, the Bonferroni correction was performed by multiplying the P value by the number of KIR genes tested (n = 18) to give the corrected P value (Pbonf). Age, sex, and the first two principal components of a PCA based on genetic data, were used as covariates in all tests.
3. Results
Table 1 displays characteristics for the CRC patients that were used for the analysis. The mean age was 69, and 64.2% of the patients were men. Most of the CRC patients (505) were current or former smoker, whereas 413 had never smoked. The CRC group did not differ from the control group with regard to any of the sociodemographic or clinical parameters included in the study (data not shown). The Tumor-Node-Metastasis (TNM) stage at diagnosis was I-II in 418 CRC patients, III in 342, and IV in 131. The location of the tumor was mainly in the rectum in 311 CRC patients (33%), sigma in 168 (17.8%), and cecum in 98 (10.4%).
Table 1.
Baseline characteristics of the patient population.
| CRC Patients (n = 943) | Healthy Controls (n = 1076) | |
|---|---|---|
| Age (median, range) | 69 (23–91) | 64.15 (24–92) |
|
Gender Female Male |
338 (35.8%) 605 (64.2%) |
527 (48.98%) 549 (51.02%) |
| Smoke | ||
| Current smoker Former smoker Never smoker Unknown |
119 (12.6%) 386 (40.9%) 413 (43.8%) 26 (2.8%) |
180 (16.73%) 335 (31.13%) 553 (51.39%) 8 (0.74%) |
| TNM stage at diagnosis | ||
| I–II III IV Unknown |
418 (44.3%) 342 (36.3%) 131 (13.9%) 52 (5.5%) |
|
| Tumor location | ||
| Rectum Sigma Cecum Ascending colon Sigmoid colon Descending colon Others* Unknown |
311 (33%) 168 (17.8%) 98 (10.4%) 81 (8.6%) 70 (7.4%) 52 (5.5%) 152 (16.1%) 11 (1.2%) |
|
*colon unspecified, recto-sigma, rectosigmoid junction, sigmoid colon, hepatic flexure, splenic flexure and transvers colon.
We considered genotypic associations in the HLA and LRC regions based on SNP genotyping. Only three markers showed suggestive associations (p < 10−3), all of them located in intergenic regions: rs16896742 (p = 3.97 × 10−4), rs28367832 (p = 6.57 × 10−5), and rs9277952 (p = 4.01 × 10−5) (Table 2, Supplementary Files 1 and 2). Then, we proceeded to analyze associations based on HLA imputation. Table 3 shows the frequency distribution between CRC cases and controls of HLA alleles (top ten associations sorted by p-value). In the unadjusted regression model, some HLA alleles showed a significant association with CRC. Specifically, HLA-C*07:01 allele frequency was decreased in patients with CRC compared to healthy controls (p < 0.05, OR = 0.82, 95% CI 0.69–0.98), while HLA-C*12:03 and HLA DRB1*11:01 alleles was increased in CRC compared to controls (p < 0.05, OR = 1.30, 95% CI 1.01-1.67; and p < 0.05, OR = 1.32, 95% CI 1.06-1.65, respectively). However, when p-values were corrected and adjusted for false discovery rate (FDR) using the Benjamini-Hochberg test, no differences were observed in the distribution of the rest of HLA class I and II alleles among controls and CRC patients.
Table 2.
SNP association values (only p values < 10−3 are shown) based on the allele frequencies characterized for the CRC patients vs. healthy controls.
| Chr | SNP | Position | Associated Gene |
A1 | MAF CRC Cases | MAF Healthy Controls | p Value | OR |
|---|---|---|---|---|---|---|---|---|
| 6 | rs16896742 | 29922740 | Intergenic | G | 0.31 | 0.3669 | 3.97 × 10−4 | 0.79 |
| 6 | rs28367832 | 31305731 | Intergenic | A | 0.42 | 0.4845 | 6.57 × 10−5 | 0.77 |
| 6 | rs9277952 | 33204274 | Intergenic | A | 0.15 | 0.1097 | 4.01 × 10−5 | 1.47 |
A1: minor allele nucleotide; CRC: colorectal carcinoma; MAF: minor allele frequency; SNP: single nucleotide polymorphism; OR: odds ratio.
Table 3.
Frequency distribution of HLA alleles between CRC cases and controls.
| HLA Allele | AF CRC Cases | AF Healthy Controls | P value | OR | 95% CI | Padj |
|---|---|---|---|---|---|---|
| DRB1*11:01 | 0.1002 | 0.0785 | 0.0137 | 1.32 | 1.06–1.65 | 0.0961 |
| C*07:01 | 0.1304 | 0.1543 | 0.0321 | 0.82 | 0.69–0.98 | 0.1774 |
| C*12:03 | 0.0689 | 0.0534 | 0.0443 | 1.30 | 1.01–1.67 | 0.1774 |
| A*02:01 | 0.2264 | 0.2519 | 0.0576 | 0.87 | 0.75–1.00 | 0.3453 |
| A*29:02 | 0.0848 | 0.0730 | 0.1554 | 1.18 | 0.94–1.50 | 0.4662 |
| DRB1*04:01 | 0.0467 | 0.0562 | 0.1751 | 0.82 | 0.62–1.09 | 0.6128 |
| B*35:01 | 0.0583 | 0.0511 | 0.3096 | 1.15 | 0.88–1.52 | 0.8689 |
| C*16:01 | 0.0843 | 0.0757 | 0.3136 | 1.13 | 0.89–1.42 | 0.6060 |
| B*44:03 | 0.1071 | 0.0976 | 0.3240 | 1.11 | 0.90–1.35 | 0.8689 |
| C*07:02 | 0.0817 | 0.0897 | 0.3641 | 0.90 | 0.72–1.13 | 0.6060 |
AF: allele frequency; OR: odds ratio; CI: confidence interval; Padj: p-values adjusted and corrected for FDR using the Benjamini-Hochberg test.
The frequency of each KIR gene in CRC patients and healthy controls is shown in Table 4. The frequency of KIR2DS3 was significantly increased in the CRC patients compared to healthy controls (39.24% vs. 32.53%, p < 0.005, Pbonf < 0.05, OR = 1.34, 95% CI 1.12-1.61). No other KIR gene showed different frequencies between cases with CRC compared with controls. Since KIR2DL3 and KIR2DL2 are considered alleles, as well as KIR3DL1 and KIR3DS1, we also tested for each gene pair the distribution of the homozygous and heterozygous genotypes of both alleles [20] (Table 5). Next, we analyzed the different interactions of KIR genes with and without their HLA ligands (Table 5). None of these analyses showed significant differences between any group of CRC patients and controls.
Table 4.
KIR gene and genotype frequencies in CRC patients and healthy controls.
| Healthy Controls | CRC Cases | ||||||
|---|---|---|---|---|---|---|---|
| KIR Gene | n | % | n | % | p-value * | Pbonf | OR (95% CI) |
| KIR2DL1 | 1075 | 99.91 | 942 | 99.89 | NS | NS | |
| KIR2DL2 | 490 | 45.54 | 418 | 44.33 | NS | NS | |
| KIR2DL3 | 925 | 85.97 | 818 | 86.74 | NS | NS | |
| KIR2DL4 | 1076 | 100 | 943 | 100 | NS | NS | |
| KIR2DL5 | 500 | 46.47 | 450 | 47.72 | NS | NS | |
| KIR3DL1ex4 | 1030 | 95.72 | 911 | 96.61 | NS | NS | |
| KIR3DL1ex9 | 1032 | 95.91 | 911 | 96.61 | NS | NS | |
| KIR3DL2 | 1076 | 100 | 943 | 100 | NS | NS | |
| KIR2DS1 | 386 | 35.87 | 346 | 36.69 | NS | NS | |
| KIR2DS2 | 530 | 49.26 | 480 | 50.9 | NS | NS | |
| KIR2DS3 | 350 | 32.53 | 370 | 39.24 | 0.002 | 0.036 | 1.34 (1.12–1.61) |
| KIR2DS4total | 1030 | 95.72 | 909 | 96.39 | NS | NS | |
| KIR2DS4wt | 366 | 34.01 | 306 | 32.45 | NS | NS | |
| KIR2DS4del | 905 | 84.11 | 801 | 84.94 | NS | NS | |
| KIR2DS5 | 286 | 26.58 | 257 | 27.25 | NS | NS | |
| KIR3DS1 | 337 | 31.32 | 319 | 33.83 | NS | NS | |
| KIR2DP1 | 1074 | 99.81 | 943 | 100 | NS | NS | |
| KIR3DP1 | 1076 | 100 | 943 | 100 | NS | NS | |
| KIR Genotype | n | % | n | % | p-value * | OR (95% CI) | |
| AA | 280 | 26.02 | 243 | 26.77 | NS | ||
| Bx | 796 | 73.98 | 700 | 74.23 | NS | ||
NS: not significant; OR: odds ratio; CI: confidence interval. Pbonf: P value using Bonferroni correction. * Two-tailed Fisher’s exact test.
Table 5.
Distribution of HLA ligands frequencies and KIR-HLA interactions in CRC cases and healthy controls.
| Healthy Controls | CRC Cases | |||||
|---|---|---|---|---|---|---|
| HLA Ligands | N | % | n | % | p-Value | OR (95% CI) |
| Bw4 | 726 | 67.47 | 650 | 68.93 | NS | |
| Bw6 | 885 | 82.25 | 766 | 81.23 | NS | |
| Bw4/Bw4 | 191 | 17.75 | 177 | 18.77 | NS | |
| Bw4/Bw6 | 535 | 49.72 | 473 | 50.16 | NS | |
| Bw6/Bw6 | 350 | 32.53 | 293 | 31.07 | NS | |
| Bw4I80 | 416 | 38.66 | 361 | 38.28 | NS | |
| Bw4T80 | 402 | 37.36 | 373 | 39.55 | NS | |
| HLA-C1 | 890 | 82.71 | 752 | 79.75 | NS | |
| HLA-C2 | 725 | 67.38 | 640 | 67.87 | NS | |
| HLA-C1C1 | 351 | 32.62 | 303 | 32.13 | NS | |
| HLA-C1C2 | 539 | 50.09 | 449 | 47.61 | NS | |
| HLA-C2C2 | 186 | 17.29 | 191 | 20.25 | NS | |
| KIR genotypes | N | % | n | % | p-value | OR (95% CI) |
| KIR3DL1/KIR3DL1 | 738 | 68.59 | 624 | 66.17 | NS | |
| KIR3DL1/KIR3DS1 | 294 | 27.32 | 287 | 30.43 | NS | |
| KIR3DS1/KIR3DS1 | 43 | 4.00 | 32 | 3.39 | NS | |
| KIR2DL2/KIR2DL2 | 149 | 13.85 | 125 | 13.26 | NS | |
| KIR2DL2/KIR2DL3 | 341 | 31.69 | 293 | 31.07 | NS | |
| KIR2DL3/KIR2DL3 | 584 | 54.28 | 525 | 55.67 | NS | |
| NS | ||||||
| KIR ligand associations | N | % | n | % | p-value | OR (95% CI) |
| KIR3DS1-Bw4I80 | 131 | 12.17 | 114 | 12.09 | NS | |
| KIR3DS1-Bw4T80 | 125 | 11.62 | 123 | 13.04 | NS | |
| KIR3DS1-Bw4 | 228 | 21.19 | 206 | 21.85 | NS | |
| KIR3DS1-Bw6 | 285 | 26.49 | 264 | 27.99 | NS | |
| KIR3DL1-Bw4I80 | 402 | 37.36 | 349 | 37.01 | NS | |
| KIR3DL1-Bw4T80 | 386 | 35.87 | 356 | 37.75 | NS | |
| KIR3DL1-Bw4 | 697 | 64.78 | 626 | 66.38 | NS | |
| KIR3DL1-Bw6 | 847 | 78.72 | 742 | 78.69 | NS | |
| KIR2DL1/HLA-C1C1 | 351 | 32.62 | 302 | 32.03 | NS | |
| KIR2DL1/HLA-C1C2 | 538 | 50.00 | 449 | 47.61 | NS | |
| KIR2DL1/HLA-C2C2 | 186 | 17.29 | 191 | 20.25 | NS | |
| KIR2DL2/HLA-C1C1 | 149 | 13.85 | 125 | 13.26 | NS | |
| KIR2DL2/HLA-C1C2 | 189 | 17.57 | 153 | 16.22 | NS | |
| KIR2DL2/HLA-C2C2 | 152 | 14.13 | 140 | 14.85 | NS | |
| KIR2DL3/HLA-C1C1 | 339 | 31.51 | 297 | 31.50 | NS | |
| KIR2DL3/HLA-C1C2 | 403 | 37.45 | 337 | 35.74 | NS | |
| KIR2DL3/HLA-C2C2 | 183 | 17.01 | 184 | 19.51 | NS | |
| KIR2DS1/HLA-C1C1 | 115 | 10.69 | 102 | 10.82 | NS | |
| KIR2DS1/HLA-C1C2 | 194 | 18.03 | 175 | 18.56 | NS | |
| KIR2DS1/HLA-C2C2 | 77 | 7.16 | 69 | 7.32 | NS | |
NS: not significant; OR: odds ratio; CI: confidence interval.
4. Discussion
Different studies have shown conflicting results of specific KIR genes with HLA alleles and cancer susceptibility [12]. Regarding KIR/HLA and CRC, several genetic associations have been described, but not widely replicated (Table 6). Already in 2007, Middleton et al. reported no association of KIR with CRC in Europeans [21]. Analyzing the distribution of HLA-C1 and C2 groups, they found a significant difference in the CRC group. However, any theory was speculative because of the small number of samples taken into account. They only included 81 CRC patients and 100 healthy controls. Also, Al Omar et al. described a lack of association of KIR and CRC [22], although they showed a strong association of the presence of HLA-Bw4 in Caucasian population (128 CRC patients and 255 healthy controls). A study performed in Koreans, in 241 CRC patients and 159 healthy controls, showed that the frequency of KIR2DS5 was significantly increased in CRC patients [23]. They also reported that the frequencies of KIR3DL1, KIR2DS2 and KIR2DS4 were decreased in CRC patients. Moreover, KIR2DS2/HLA-C1 combination presented a protective effect in CRC susceptibility. In a preliminary study performed in the Saudi population [24] (52 CRC patients and 70 controls), was reported an increase of activating KIR2DS1, KIR2DS5 and KIR3DS1 in patients with CRC. A meta-analysis of four independent studies including a total of 470 individuals with CRC and 483 individuals in control group [25], indicated that CRC was affected by KIR2DS5. After, a report of 2016 performed in Brazilian CRC Caucasoid population [26], 154 CRC cases and 216 controls, showed no significant differences for HLA ligands and KIR genes between groups, but the Bx haplotypes (AB and BB) were more frequent in controls compared to in CRC patients. Recently, another study with an Iranian population examined a total of 165 patients with CRC as well as 165 healthy controls [27], showed that possessing more inhibitory KIR genes was a potential risk to CRC while genotypes with many activating KIR genes was associated with protection against it. Despite all bibliography, the role of KIR variability in cancer, CRC included, remains unclear, mainly due to the smaller number of studies involving large and well-characterized cohorts.
Table 6.
Basic research studies showing the associations between KIR and HLA in CRC patients.
| Reference | Type of Experiment/Objective | Conclusions |
|---|---|---|
| [19] | 109 CRC patients (70 bladder and 34 laryngeal) and 100 controls. HLA and KIR genotyping. | No differences in KIR/HLA frequencies was observed between patients and controls. |
| [20] | 128 CRC patients and 255 controls. KIR and HLA genotyping. | The data showed no significant differences between KIR gene frequencies in CRC patients versus controls. |
| [21] | 241 CRC patients and 159 controls from Korean populations. KIR and HLA-C genotyping | The activating KIR2DS5 was more frequent in Korean CRC patients, showing a risk for the disease. The frequencies of KIR3DL1, KIR2DS2 and KIR2DS4 were lower in the rectal cancer subgroup, and they could have a protective effect against CRC. Also, the lower frequency of KIR2DS2 in patients with HLA-C1 homozygote, may be a protective effect too. |
| [22] | 52 CRC patients and 70 controls from Saudi population. KIR and HLA-C genotyping. | Activating KIRs (2DS1, 2DS2, 2DS3, 2DS5 and 3DS1) was more frequent in CRC patients, suggesting their presence a risk for disease. |
| [23] | 470 CRC patients and 483 controls. KIR genotyping. | The presence of KIR2DS5 was associated with CRC like as a non-protective gene. This result explains the inflammatory basis of this cancer. |
| [24] | 154 CRC patients and 216 controls from Caucasian Brazilian population. KIR and HLA genotyping. | No associations between KIRs and HLA in CRC was observed. However, the Bx haplotype was more frequent in controls than in patients, being a possible mechanism of protection to CRC. |
| [25] | 165 colorectal adenocarcinoma patients and 165 controls. KIR genotyping. | The presence of activating KIRs (≥ 4) and KIR3DL1, 3DS1, 2DS1 and 2DS4, were associated with protection against metastasis in CRC patients. |
| [26] | 29 CRC recurrent patients (in 5 years) vs. 58 CRC non-recurrent patients (in 5 years) after surgery and 154 controls. KIR and HLA-class I genotyping. | The increment of activating KIRs (in particularly 2DS2 and 2DS3) and the lack of inhibitory KIRs (in particularly 2DL1) was associated with long term disease-free survival and this was independent of tumor localization or stage. Also, HLA-A-Bw4 was associated with recurrent disease. |
In light of such discrepancies and considering the need for larger sample size studies, this study was conducted with a large and ethnically homogeneous population for CRC patients and healthy controls. Only KIR2DS3 exhibited suggestive association, showing that their frequency was significantly increased in patients with CRC compared with control subjects. The associations of the remaining KIR genes included in the study were not significant. In a previous study published by Beksac et al. [28] KIR2DS3 showed a similar distribution between CRC cases and control (39.1% vs. 33.8%, respectively), which was not significant because of the sample size. Moreover, the study showed that KIR2DS3 was associated with protection from recurrence. We could not assess this, since we have not yet performed a follow-up of the CRC patients to know their evolution. KIR2DS3 is an NK cell associated gene, that functions, for example, as host risk factor predicting failure to spontaneously clear Hepatitis C virus (HCV) virus by immune mediated mechanisms [29]. Also, KIR2DS3 has been associated with fatal outcome in Ebola virus infection [30]. However, there is currently no known function for KIR2DS3, and evidence suggests that it is not expressed at the cell surface [31]. We think that KIR2DS3 could be a genetic marker for a closely related linked gene that could be responsible of the biological effect. Obviously, further research is required to confirm this assertion.
We tested the effects of KIR genes in combination with their natural ligand, specifically: KIR3DL1 and HLA-Bw4I80 (also KIR3DS1); and combinations of KIR2DL1, 2DL2, 2DL3, and 2DS1, with HLA-C groups (C1 and C2). However, despite a large number of comparisons performed, none of these tests showed significant changes between CRC and healthy controls. The activation profile of KIRs is genetically determined in each individual and leads to diverse levels of functionality in NK cells. Thus, many works describe associations of combinations KIR/HLA and disease, proposing that compound genotypes provide different levels of activation or inhibition for NK and T cells. We rule out this possibility in CRC, at least in Caucasoid populations.
The HLA region plays a crucial role in numerous pathologies, as it accounts for 25% of known associations from the GWAS catalog (https://www.ebi.ac.uk/gwas/), especially with immune-related diseases. However, the link between HLA and CRC is unknown. There are data showing that the expression of HLA class II molecules on CRC cells is involved in its pathogenesis [32]. Specifically, presence of particular DRB1 alleles influence the susceptibility to ulcerative colitis-associated CRC [33]. Aureli et al. described that the DRB1*13:01 and DRB1*11:01 alleles were associated with an increased and reduced risk to develop CRC [34], respectively. Although the number of CRC patients analyzed is limited for this study (n = 53), it may provide a starting point. However, we observed an inverse relationship between the allele frequency of DRB1*11:01 and CRC, increased in cases compared to controls. Moreover, this association was not confirmed when p-value was corrected. We cannot exclude the association, as DRB1*11:01 allele has been associated with a variety of malignancies, including breast cancer and hairy cell leukaemia [35,36], but at least their implication is not due to the participation of any KIR receptor.
Several limitations in our design should be kept in mind. The imputation of HLA alleles may be controversial due to the complexity of the HLA region. Although SNP association studies importantly expanded in the last decade, direct HLA allele association has been hindered by the complexity of typing. HLA imputation offers a statistical alternative to current HLA typing, cutting costs, and time alike. HLA imputation will be important in the context of association studies [37]. Moreover, although most current results are negative, we believe that our data may add significantly to the available literature in the field. We described the possible implication of KIR2DS3 in the susceptibility to CRC, at least involving a genetic marker linked to a potential causal variant. We also showed that the implication of NK cells in CRC could not be related to specific associations between KIR/HLA combinations. It may be a mistake to believe that single KIR receptor/HLA ligand can lead or influence the function of NK cells in this regard. In summary, we show the need to conduct studies in larger and ethnically homogeneous populations in order to reach robust conclusions.
Acknowledgments
Sample collection of this work was supported by the Xarxa de Bancs de Tumors de Catalunya sponsored by Pla Director d’Oncología de Catalunya (XBTC)”, Plataforma Biobancos PT17/0015/002 and ICOBIOBANC, sponsored by the Catalan Institute of Oncology. We thank CERCA Program, Generalitat de Catalunya for institutional support.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/9/2/514/s1, Supplementary File 1: SNP association values based on the allele frequencies characterized for the CRC patients vs. healthy controls (HLA region); Supplementary File 2: SNP association values based on the allele frequencies characterized for the CRC patients vs. healthy controls (LRC region).
Author Contributions
Conceptualization, R.D.-P; methodology, P.M.-M., R.D.-P.; software, R.D.-P.; writing—original draft preparation, P.M.-M. and R.D.-P.; writing—review and editing, R.D.-P.; formal analysis, R.D.-P., A.J.M.d.l.T. and R.S.-P., investigation, A.J.M.d.l.T. and R.S.-P., resources, V.M. (Vicente Martín) and V.M. (Víctor Moreno), data curation, A.J.M.d.l.T. and R.S.-P., visualization, A.J.M.d.l.T. and R.S.-P., supervision, R.D.-P. and V.M. (Víctor Moreno), project administration, V.M. (Vicente Martín) and V.M. (Víctor Moreno), funding acquisition, V.M. (Vicente Martín) and V.M. (Víctor Moreno) All authors have read and agreed to the published version of the manuscript.
Funding
Agency for Management of University and Research Grants (AGAUR) of the Catalan Government grant 2017SGR723; Instituto de Salud Carlos III, co-funded by FEDER funds –a way to build Europe– grants PI14-00613, PI17-00092; Spanish Association Against Cancer (AECC) Scientific Foundation. Agency for Management of University and Research Grants (AGAUR) of the Catalan Government grant 2017SGR723; Junta de Castilla y León, co-funded by FEDER funds–a way to build Europe– grant LE071P17, Instituto de Salud Carlos III, co-funded by FEDER funds –a way to build Europe– grants PI12/01270, PI14-00613, PI17-00092; Spanish Association Against Cancer (AECC) Scientific Foundation.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet (Lond. Engl.) 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 2.Schmit S.L., Edlund C.K., Schumacher F.R., Gong J., Harrison T.A., Huyghe J.R., Qu C., Melas M., Van Den Berg D.J., Wang H., et al. Novel Common Genetic Susceptibility Loci for Colorectal Cancer. J. Natl. Cancer Inst. 2019;111:146–157. doi: 10.1093/jnci/djy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law P.J., Timofeeva M., Fernandez-Rozadilla C., Broderick P., Studd J., Fernandez-Tajes J., Farrington S., Svinti V., Palles C., Orlando G., et al. Association analyses identify 31 new risk loci for colorectal cancer susceptibility. Nat. Commun. 2019;10:2154. doi: 10.1038/s41467-019-09775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillerey C., Huntington N.D., Smyth M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- 5.Parham P., Guethlein L.A. Genetics of Natural Killer Cells in Human Health, Disease, and Survival. Annu. Rev. Immunol. 2018;36 doi: 10.1146/annurev-immunol-042617-053149. [DOI] [PubMed] [Google Scholar]
- 6.Lanier L.L., Cortiss B.C., Wu J., Leong C., Phillips J.H. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 7.Olcese L., Cambiaggi A., Semenzato G., Bottino C., Moretta A., Vivier E. Human killer cell activatory receptors for MHC class I molecules are included in a multimeric complex expressed by natural killer cells. J. Immunol. 1997;158:5083–5086. [PubMed] [Google Scholar]
- 8.Shahjahan Miah S.M., Hughes T.L., Campbell K.S. KIR2DL4 Differentially Signals Downstream Functions in Human NK Cells through Distinct Structural Modules. J. Immunol. 2008;180:2922–2932. doi: 10.4049/jimmunol.180.5.2922. [DOI] [PubMed] [Google Scholar]
- 9.Winter C.C., Gumperz J.E., Parham P., Long E.O., Wagtmann N. Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J. Immunol. 1998;161:571–577. [PubMed] [Google Scholar]
- 10.Cella M., Longo A., Ferrara G.B., Strominger J.L., Colonna M. NK3-specific natural killer cells are selectively inhibited by Bw4-positive HLA alleles with isoleucine 80. J. Exp. Med. 1994;180:1235–1242. doi: 10.1084/jem.180.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansasuta P., Dong T., Thananchai H., Weekes M., Willberg C., Aldemir H., Rowland-Jones S., Braud V.M. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur. J. Immunol. 2004 doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- 12.Augusto D.G. The impact of KIR polymorphism on the risk of developing cancer: Not as strong as imagined? Front. Genet. 2016;7:121. doi: 10.3389/fgene.2016.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon A.G., Morreau H., Tollenaar R.A.E.M., Alphenaar E., Van Puijenbroek M., Putter H., Janssen-Van Rhijn C.M., Van De Velde C.J.H., Fleuren G.J., Kuppen P.J.K. Down-regulation of HLA-A expression correlates with a better prognosis in colorectal cancer patients. Lab. Investig. A J. Tech. Methods Pathol. 2002;82:1725–1733. doi: 10.1097/01.LAB.0000043124.75633.ED. [DOI] [PubMed] [Google Scholar]
- 14.Watson N.F.S., Ramage J.M., Madjd Z., Spendlove I., Ellis I.O., Scholefield J.H., Durrant L.G. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int. J. Cancer. 2006;118:6–10. doi: 10.1002/ijc.21303. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X., Shen J., Cox C., Wakefield J.C., Ehm M.G., Nelson M.R., Weir B.S. HIBAG—HLA genotype imputation with attribute bagging. Pharm. J. 2014;14:192–200. doi: 10.1038/tpj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell J., Gurdasani D., Delaneau O., Pirastu N., Ulivi S., Cocca M., Traglia M., Huang J., Huffman J.E., Rudan I., et al. A general approach for haplotype phasing across the full spectrum of relatedness. PLoS Genet. 2014;10:e1004234. doi: 10.1371/journal.pgen.1004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vukcevic D., Traherne J.A., Næss S., Ellinghaus E., Kamatani Y., Dilthey A., Lathrop M., Karlsen T.H., Franke A., Moffatt M., et al. Imputation of KIR Types from SNP Variation Data. Am. J. Hum. Genet. 2015;97:593–607. doi: 10.1016/j.ajhg.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan Y., Song Y.-Q. PyHLA: Tests for the association between HLA alleles and diseases. BMC Bioinform. 2017;18:90. doi: 10.1186/s12859-017-1496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middleton D., Gonzelez F. The extensive polymorphism of KIR genes. Immunology. 2010;129:8–19. doi: 10.1111/j.1365-2567.2009.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Middleton D., Vilchez J.R., Cabrera T., Meenagh A., Williams F., Halfpenny I., Maleno I., Ruiz-Cabello F., Lopez-Nevot M.A., Garrido F. Analysis of KIR gene frequencies in HLA class I characterised bladder, colorectal and laryngeal tumours. Tissue Antigens. 2007;69:220–226. doi: 10.1111/j.1399-0039.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- 22.Al Omar S., Middleton D., Marshall E., Porter D., Xinarianos G., Raji O., Field J.K., Christmas S.E. Associations between genes for killer immunoglobulin-like receptors and their ligands in patients with solid tumors. Hum. Immunol. 2010;71:976–981. doi: 10.1016/j.humimm.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Kim H.J., Choi H.B., Jang J.P., Baek I.C., Choi E.J., Park M., Kim T.G., Oh S.T. HLA-Cw polypmorphism and killer cell immunoglobulin-like receptor (KIR) gene analysis in Korean colorectal cancer patients. Int. J. Surg. 2014;12:815–820. doi: 10.1016/j.ijsu.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Al Omar S.Y., Mansour L., Dar J.A., Alwasel S., Alkhuriji A., Arafah M., Al Obeed O., Christmas S. The Relationship between Killer Cell Immunoglobulin-Like Receptors and HLA-C Polymorphisms in Colorectal Cancer in a Saudi Population. Genet. Test. Mol. Biomark. 2015;19:617–622. doi: 10.1089/gtmb.2015.0105. [DOI] [PubMed] [Google Scholar]
- 25.Ghanadi K., Shayanrad B., Ahmadi S.A.Y., Shahsavar F., Eliasy H. Colorectal cancer and the KIR genes in the human genome: A meta-analysis. Genom. Data. 2016;10:118–126. doi: 10.1016/j.gdata.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portela P., Merzoni J., Lindenau J.D., Damin D.C., Wilson T.J., Roesler R., Schwartsmann G., Jobim L.F., Jobim M. KIR genes and HLA class I ligands in a Caucasian Brazilian population with colorectal cancer. Hum. Immunol. 2017;78:263–268. doi: 10.1016/j.humimm.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Barani S., Hosseini S.V., Ghaderi A. Activating and inhibitory killer cell immunoglobulin like receptors (KIR) genes are involved in an increased susceptibility to colorectal adenocarcinoma and protection against invasion and metastasis. Immunobiology. 2019;224:681–686. doi: 10.1016/j.imbio.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Beksac K., Beksac M., Dalva K., Karaagaoglu E., Tirnaksiz M.B. Impact of “Killer Immunoglobulin-Like Receptor /Ligand” Genotypes on Outcome following Surgery among Patients with Colorectal Cancer: Activating KIRs Are Associated with Long-Term Disease Free Survival. PLoS One. 2015;10:e0132526. doi: 10.1371/journal.pone.0132526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dring M.M., Morrison M.H., McSharry B.P., Guinan K.J., Hagan R., O’Farrelly C., Gardiner C.M. Innate immune genes synergize to predict increased risk of chronic disease in hepatitis C virus infection. Proc. Natl. Acad. Sci. United States Am. 2011;108:5736–5741. doi: 10.1073/pnas.1016358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wauquier N., Padilla C., Becquart P., Leroy E., Vieillard V. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics. 2010;62:767–771. doi: 10.1007/s00251-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VandenBussche C.J., Mulrooney T.J., Frazier W.R., Dakshanamurthy S., Hurley C.K. Dramatically reduced surface expression of NK cell receptor KIR2DS3 is attributed to multiple residues throughout the molecule. Genes Immun. 2009;10:162–173. doi: 10.1038/gene.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sconocchia G., Eppenberger-Castori S., Zlobec I., Karamitopoulou E., Arriga R., Coppola A., Caratelli S., Spagnoli G.C., Lauro D., Lugli A., et al. HLA class II antigen expression in colorectal carcinoma tumors as a favorable prognostic marker. Neoplasia (United States) 2014;16:31–42. doi: 10.1593/neo.131568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrity-Park M.M., Loftus E.V., Sandborn W.J., Bryant S.C., Smyrk T.C. MHC class II alleles in ulcerative colitis-associated colorectal cancer. Gut. 2009;58:1226–1233. doi: 10.1136/gut.2008.166686. [DOI] [PubMed] [Google Scholar]
- 34.Aureli A., Canossi A., Del Beato T., Franceschilli L., Buonomo O., Papola F., De Sanctis F., Lanzilli G., Sileri P., Coppola A., et al. HLA-DRB1∗13:01 allele in the genetic susceptibility to colorectal carcinoma. Int. J. Cancer. 2015;136:2464–2468. doi: 10.1002/ijc.29285. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhuri S., Cariappa A., Tang M., Bell D., Haber D.A., Isselbacher K.J., Finkelstein D., Forcione D., Pillai S. Genetic susceptibility to breast cancer: HLA DQB*03032 and HLA DRB1*11 may represent protective alleles. Proc. Natl. Acad. Sci. United States Am. 2000;97:11451–11454. doi: 10.1073/pnas.97.21.11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arons E., Adams S., Venzon D.J., Pastan I., Kreitman R.J. Class II human leucocyte antigen DRB1*11 in hairy cell leukaemia patients with and without haemolytic uraemic syndrome. Br. J. Haematol. 2014;166:729–738. doi: 10.1111/bjh.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer D., Vitor V.R., Bitarello B.D., Débora D.Y., Nunes K. A genomic perspective on HLA evolution. Immunogenetics. 2018;70:5–27. doi: 10.1007/s00251-017-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.