Abstract
The social and economic impact of chronic inflammatory diseases, such as arthritis, explains the growing interest of the research in this field. The antioxidant and anti-inflammatory properties of the endogenous gasotransmitter hydrogen sulfide (H2S) were recently demonstrated in the context of different inflammatory diseases. In particular, H2S is able to suppress the production of pro-inflammatory mediations by lymphocytes and innate immunity cells. Considering these biological effects of H2S, a potential role in the treatment of inflammatory arthritis, such as rheumatoid arthritis (RA), can be postulated. However, despite the growing interest in H2S, more evidence is needed to understand the pathophysiology and the potential of H2S as a therapeutic agent. Within this review, we provide an overview on H2S biological effects, on its role in immune-mediated inflammatory diseases, on H2S releasing drugs, and on systems of tissue repair and regeneration that are currently under investigation for potential therapeutic applications in arthritic diseases.
Keywords: inflammation, arthritis, organosulfur compounds, oxidative stress, stem-cell therapy, H2S-releasing biomaterials, non-steroidal anti-inflammatory drugs (NSAIDs)
1. Introduction
Hydrogen sulfide is an endogenously produced biological agent belonging to the gasotransmitter family. The physiological role and the relevance of this molecule are rapidly expanding. Endogenous H2S plays pivotal roles in the biochemical pathways of the central nervous, respiratory, and cardiovascular systems. This gasotransmitter is physiologically present in the human body, and it is mainly produced endogenously by four enzymes: cystathionine beta-synthase (CBS EC 4.2.1.22), cystathionine gamma-lyase (CSE, EC 4.4.1.1), 3-mercaptopyruvate sulfotransferase (MST, EC 2.8.1.2), and cysteine aminotransferase (CAT) (reviewed in references [1,2,3,4,5]). However, other enzymes such as thiosulfate sulfurtransferase (TST) [6] and the more recently discovered selenium-binding protein 1 (SELENBP1) are able to catalyze H2S production [7].
Although there are limits of measurement techniques and the quantification of biologic H2S levels is debated, H2S physiological levels may range from 50–160 μM in the mammalian brain to 30 nM–100 μM in the peripheral blood and 25 μM in the synovial fluid of patients with non-inflammatory arthritis [8,9,10,11,12,13]. It is known that a relevant fraction of H2S is bound to proteins in several tissues, such as hemoglobin [14,15]. An endogenous source of H2S is also represented by the enterobacterial flora and by the non-enzymatic reduction of sulfurs [4].
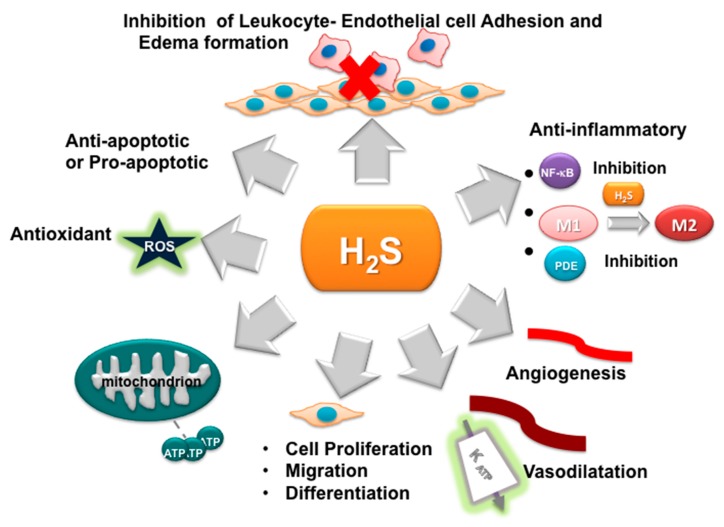
As a gasotransmitter, H2S can pass freely through cell membranes and does not require a specific receptor to mediate its effect. Only recently, H2S was considered an important signaling molecule. The biological effects of H2S are multiple and opposite, depending on its concentration. The first H2S biological effect was discovered in the vascular system, with the ability to induce the relaxation of vascular smooth muscle, causing vasodilation [16]. Despite the controversial role initially attributed to H2S, it is to date recognized that, at low concentrations, it exhibits anti-apoptotic, anti-nociceptive, cardio-protective, and blood pressure-lowering effects, while also improving angiogenesis, via the activation of KATP channels and extracellular signal-regulated kinases, such as Akt pathways [1,17,18,19,20,21,22]. Moreover, H2S shows neuroprotective and anti-inflammatory effects in general due to its antioxidant effects and inhibition of pro-inflammatory cytokines [23,24,25,26,27,28]. The potential effects of H2S were also discussed in several review articles [29,30,31,32] and are here summarized in Figure 1.
Figure 1.
Schematic description of the effects of H2S. The anti-inflammatory effect of H2S is due to its ability to inhibit some essential pro-inflammatory transcription factors and intracellular signaling, such as nuclear factor κB (NF-κB) and phosphodiesterases (PDEs), and to improve angiogenesis through KATP channel/mitogen-activated protein kinase (MAPK) pathway activation. It inhibits the production of inflammatory cytokines and avoids the adhesion of leukocytes and endothelial cells. Moreover, the gasotransmitter can have pro- or anti-apoptotic effects depending on the cell type and its concentration. At the appropriate concentration, it is also able to have an anti-apoptotic effect due to its antioxidant properties, as well as its ability to increase the mitochondrial activity and the expression of anti-apoptotic proteins [17]. However, exogenous H2S is also able to induce apoptosis in cancer cells. H2S can also act on the vascular smooth muscle producing vasodilation. M1, macrophages M1; M2, macrophages M2; KATP, ATP-dependent K -channels.
In the last few years, many studies demonstrated a relevant role of H2S to mediate the inflammation and the processes of tissues repair. Chronic inflammation is the key feature of inflammatory arthritis, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA). The impact of chronic inflammatory diseases on the quality of life and autonomy in the daily activities in a wide population with different ages and the consequent high economic costs explain the growing interest in this field. Recently, the connection between H2S and joint inflammation, in the context of arthritis, is growing, either as a pathogenic or potential therapeutic role. Therefore, in this review, we describe the effects of H2S on inflammatory arthritis and its potential therapeutic approach.
2. Oxidative Stress and Inflammation in Arthritis
RA and PsA represent the most common chronic inflammatory arthritis. They share common pathogenic features, both linked to chronic inflammation secondary to a dysregulation of the immune response; however, the clinical manifestations and outcomes are usually different. Despite the clinical and pathological differences, both diseases share some similarities in the inflammatory pathways. In the course of active arthritis, joint inflammation is overall characterized by increased vascularization, oxidative stress, and infiltration of immune cells in the synovium (Figure 2); these events induce fibroblast-like synoviocyte (FLS) hypertrophy which, ultimately, perpetuates the inflammation, generating a chronic loop. In the early stages of arthritis, the oxidative stress seems to have a key role in initiating the inflammatory process, as demonstrated in some studies [33,34]. Moreover, oxidative stress may account for post-translational modifications of proteins, potentially responsible for autoreactive antibody production [35], particular to RA. Synovial angiogenesis is also an early alteration in the arthritic joint and is characterized by endothelial swelling, cell infiltration, and tortuous vessels. The amplified expression in the synovium of pro-inflammatory cytokines and growth factors, particularly vascular endothelial growth factor (VEGF), contributes to increased vascularity [36]. However, the new vessels are mostly dysfunctional and, consequently, the RA/PsA synovial membrane results hypoxic. Unsurprisingly, the resulting synovial hypoxia was correlated with local joint inflammation [37]. In the inflamed synovium, altered mitochondrial function and oxidative damage were also observed, which are both possibly related to hypoxia [38,39]. The perpetuation of inflammation leads to damage of the cartilage, which allows the invasion of the subchondral bone by FLS, immune cells, and pro-inflammatory cytokines [40]. The exposure of the subchondral bone to the action of proteinase and activation of osteoclasts (OCs) leads to the characteristic bony erosions (Figure 2). Additionally, PsA is also characterized by inflammation of the enthesis associated with a peculiar osteoproductive phenomenon, which leads to calcification of tendons, ankylosis of joints, and consequently to impaired quality of life. This phenomenon is possibly related to impaired mechanisms of tissue repair. Despite the recent progress and advances, the pathogenic mechanisms behind RA and PsA onset are far from being completely understood. The pathogenesis of inflammatory arthritis is characterized by an immune dysregulation, which involves the activation of both innate and adaptive immunity. Immune-mediated pathogenesis was demonstrated by different evidence, such as the presence of auto-reactive T cells in the synovium [41], the association with major histocompatibility complex (MHC)-I, and the good response to immunosuppressive drugs [42]. An altered metabolic response of the immune cells may also contribute to the perpetuation of the inflammatory loop in the inflamed synovium. FLS and synovial macrophages are enhancers of joint inflammation, which ultimately leads to cartilage disruption [43]. In fact, FLS have a tumor-like behaviour in PsA joints. They are characterized by a few key features, i.e., the increased proliferation and invasiveness, the resistance to apoptosis, and the active production of matrix-degrading enzymes and pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), and interleukin 17 (IL-17) [44]. Regarding innate immunity, dendritic cells (DCs) are present in the synovium, synovial fluid, and ectopic lymph tissue of RA inflamed joints compared with osteoarthritis; in the context of inflammation, DCs have a role as antigen-presenting cells (APCs) and as a producer of pro-inflammatory cytokines, i.e., IL-23 and IL-12, which induce the differentiation of T helper 17 cells (Th17) and Th1 subsets, relevant in joint inflammation [45]. Moreover, defective regulatory T cells are associated with autoimmune disorders, such as RA [46]. Furthermore, macrophages play an active role in the pathogenesis of inflammatory arthritides because of the high expression of pro-inflammatory cytokines and matrix metalloproteinases, and they are APCs for T cells and B cells, representing a source of osteoclast precursors in an inflammatory context [47]. Lymphocytes infiltrate the synovium, where aggregates of T cells and B cells are found, and their absence is associated with remission [48,49]. Despite the progress in arthritis treatment over the last few decades, up to 40% of patients are still non-responders to the available treatments; for this reason, research toward a better understanding of the pathogenesis is continuously growing with a particular interest in new pathogenic mechanisms and possible therapeutic targets.
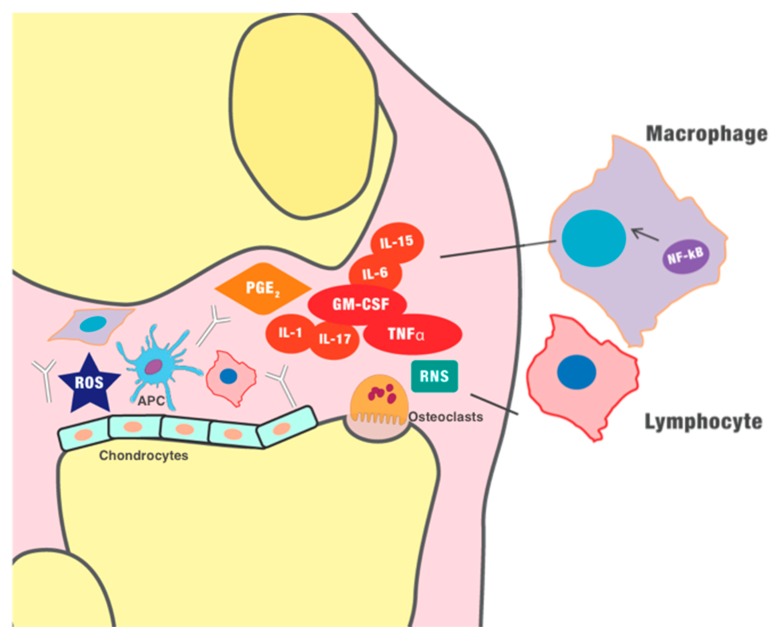
Figure 2.
Pathogenesis of inflammatory arthritides. Self-reactive T helper cells seem involved in the maintenance of inflammation, further sustained by B cells, especially in rheumatoid arthritis (RA), where it is possible to detect autoantibodies ( ), such as the rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Furthermore, innate immunity is involved in chronic inflammation. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the differentiation of the macrophages. Monocytes and macrophages, as well as dendritic cells, seem to play a vital role in the pathogenesis of inflammatory arthritis, as antigen-presenting cells (APCs), and they can express several pro-inflammatory cytokines. In early stages of inflammation, hypoxia and the production of ROS (reactive oxygen species) and RNS (reactive nitrogen species) seems to play a role in the initiation of the inflammatory process and induction of angiogenesis. The new blood vessels further maximize the recruitment of immune cells, amplifying the inflammatory process. The chronic inflammation finally perpetuates the production of pro-inflammatory cytokines (such as tumor necrosis factor-α (TNF-α)) and other mediators, such as prostaglandin E2, which ultimately generate vasodilation, infiltration of immune cells, and destruction of the cartilage.
), such as the rheumatoid factor and anti-cyclic citrullinated peptide (anti-CCP) antibodies. Furthermore, innate immunity is involved in chronic inflammation. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces the differentiation of the macrophages. Monocytes and macrophages, as well as dendritic cells, seem to play a vital role in the pathogenesis of inflammatory arthritis, as antigen-presenting cells (APCs), and they can express several pro-inflammatory cytokines. In early stages of inflammation, hypoxia and the production of ROS (reactive oxygen species) and RNS (reactive nitrogen species) seems to play a role in the initiation of the inflammatory process and induction of angiogenesis. The new blood vessels further maximize the recruitment of immune cells, amplifying the inflammatory process. The chronic inflammation finally perpetuates the production of pro-inflammatory cytokines (such as tumor necrosis factor-α (TNF-α)) and other mediators, such as prostaglandin E2, which ultimately generate vasodilation, infiltration of immune cells, and destruction of the cartilage.  , dendritic cell/APC;
, dendritic cell/APC;  , osteoclast;
, osteoclast;  , chondrocyte.
, chondrocyte.
3. H2S as Inhibitor of Oxidative Stress and Inflammation
H2S can be an endogenous mediator to limit free radical damage and inflammation [50]. A relevant role is played by H2S in balancing oxidative and reductive species, thus influencing the cell’s redox state. H2S is a strong reducing agent able to directly react with multiple oxidant stressors including superoxide radical anion [51], hydrogen peroxide [52], and peroxynitrite (ONOO−) [50] (see Figure 3). Furthermore, H2S is able to antagonize lipid peroxidation and oxidation of thiols, to reverse mitochondrial dysfunction [53], and to increase the activity of the most important enzymes involved in the cell’s antioxidant defense. One of these enzymes, the Cu/Zn superoxide dismutase (SOD) [54], is a target of H2S, which binds to the catalytic Cu center, thus increasing the ROS scavenging activity. Moreover, the persulfuration of Cys-111 of the human SOD1 stabilizes the enzyme against oxidation-induced aggregation without affecting its activity [55]. The activity of other enzymes, also implicated in the cell’s antioxidant response, such as catalase (CAT), glutathione reductase (GR), glutathione S-transferase (GST), quinone reductase (QR), and glutathione peroxidase (GPx), was likewise augmented in rat kidney upon treatment with diallyl sulfide (DAS), which is an H2S-releasing molecule [56].
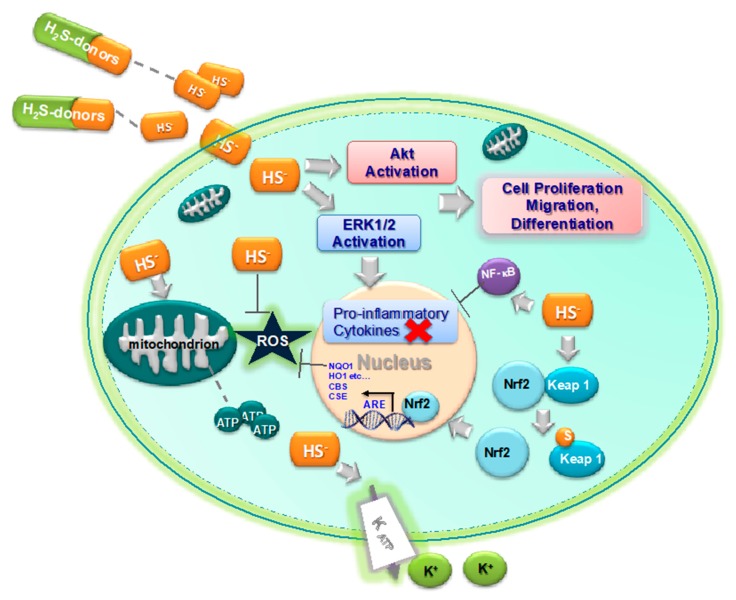
Figure 3.
Biological anti-inflammatory effects of H2S. H2S exerts an anti-inflammatory effect via different biologic effects: direct and indirect reducing action (Nrf2, ARE activation), a regulatory effect on the immune system via NF-kB interaction, interference with rolling and migration of circulating cells, inhibition of enzymes involved in the inflammatory signaling (protein tyrosine phosphatases (PTPs), PDEs). H2S induces separation between Nrf2 and Keap1, allowing Nrf2 to enter the nucleus and bind to the ARE gene; furthermore, it modulates in a dose-dependent manner the expression of many cytokine genes, while it obtains an anti-apoptotic effect through Akt activation. H2S, through action on ATP-sensitive potassium channels (KATP), inhibits the expression of adhesion molecules on the leukocytes (cluster of differentiation (CD)11/CD18) and endothelium (P-selectin, intracellular adhesion molecule 1 (ICAM1)).
H2S is also able to upregulate the antioxidant response elements (ARE) gene transcription [57] (Figure 3) and to produce glutathione persulfide (GSSH) in mitochondria [50,52], a more efficient H2O2-scavenging molecule than GSH. In more detail, H2S induces the dissociation between nuclear erythroid factor 2-related factor 2 (Nrf2) and Kelch-like ECH-associated protein 1 (Keap1) through the sulfhydration of Keap 1 at the Cys-151 residue and induction of a disulfide bond between Cys-288 and Cys-613 residues, thus allowing the Nrf2 nuclear translocation and binding to AREs (Figure 3) [58,59,60,61,62,63]. The antioxidant activity of this gasotransmitter is also related to the activation of KATP channels to reduce oxidative glutamate toxicity [64]. Moreover, the anti-oxidative effects of H2S are also related to the anti-inflammatory effect via an increase in the expression of anti-oxidant enzymes, such as indoleamine-pyrrole 2,3-dioxygenase 1 (IDO1) and heme oxygenase 1 (HO1), SOD, CAT, and GPx, which lead to the suppression of reactive oxygen species (ROS) production (Figure 3) [65,66]. Surely, the direct antioxidant effect as a free radical scavenger and as an inducer of antioxidant enzymes might have a potential anti-inflammatory effect in joint inflammation [67,68].
Interestingly, H2S is also an inflammation modulator that can have both pro- and anti-inflammatory effects on immune cells, depending on the concentration [68]. Commonly, a pro-inflammatory effect was observed at high H2S concentrations, whereas an anti-inflammatory effect was observed at physiological concentrations. Overall, the role of H2S in resolving ongoing inflammation and inducing tissue repair was suggested [26,31,69,70,71]. The exogenous administration of H2S at physiological levels enhances T-cell activation in T-cell lines and upregulates the expression of cluster of differentiation 69 (CD69), IL-2, and CD25 [72]; H2S-induced signaling plays a key role in T-cell activation. Moreover, H2S shows a regulatory interaction with IL-10. Furthermore, the upregulation of IL-10 expression was observed after the exogenous administration of H2S [73]. The ability of H2S donors to modulate the expression of genes for many pro-inflammatory cytokines, chemokines, and enzymes is largely linked to effects on NF-κB activity. In rodent models of colitis, treatment with H2S-donors significantly reduced tissue expression of IL-1β, interferon-γ, TNF-α, IL-12 p40, IL-2, regulated upon activation, normal T cell expressed and presumably secreted (RANTES), and inducible nitric oxide synthase (iNOS), without affecting the IL-10 expression [71,74,75]. Allyl disulfide treatment significantly inhibited NF-κB activation and production of TNF-α, as observed by biopsies from patients with ulcerative colitis. It was shown that H2S donors are able to reduce TNF-α release following lipopolysaccharide (LPS) exposure in RAW 264.7 cells, a murine monocyte/macrophage-like lineage [76]. Moreover, it was recently demonstrated that H2S can shift the macrophage phenotype from pro- to anti-inflammatory [70,77].
Additionally, H2S plays also a relevant role in orchestrating immune cell tissue recruitment and infiltration, which are vital in the generation of the inflammatory processes. Leukocyte recruitment and tissue infiltration at the site of inflammation are initial events in inflammation response that are linked to the increase of the production of vascular cell adhesion molecule 1 (VCAM1) and intracellular adhesion molecule 1 (ICAM1) in endothelial cells. H2S donors and non-selective inhibitors of CSE and CBS specifically inhibit the migration of leukocytes by directly reducing the adherence of circulating cells to the inflamed vascular walls (as shown in Figure 1); consequently, H2S reduces the infiltration of neutrophils and lymphocytes in tissue [78]. H2S downregulates ICAM expression in high-glucose-treated [79] and TNF-treated human umbilical vein endothelial cells [80]. Moreover, the upregulation of heme oxygenase 1 (HO1) and inhibition of the NF-κB pathway due to H2S-donors can induce the inhibition of VCAM1 expression [81,82]. In neutrophils, H2S may also induce the apoptosis, amplifying the anti-inflammatory effect [83].
Moreover, several enzymes involved in the inflammatory response can be inhibited by H2S. The majority of protein tyrosine phosphatases (PTPs) have a conserved catalytic domain that contains a cysteine residue, which is able to perform a nucleophilic attack on a substrate; this catalytic residue can be sulfhydrated, as well as in the case of PTP1B. PTP1B is ubiquitously expressed [84] and plays a regulatory role in the control of immune cell signaling in macrophages, monocytes, and granulocytes [85]. PTP1B is important in the release of inflammatory cytokines such as IL-4, IL-6, TNF-α, extracellular signal-regulated kinase (ERK), protein kinase B (PKB/Akt) (see Figure 3), human epidermal growth factor receptor 2 (HER2), and NF-κB [84,86,87]. Although PTP1B is a negative regulator of inflammation able to regulate inflammatory processes [88,89], it was also demonstrated that the administration of an inhibitor of PTP1B attenuates the LPS-induced neuroinflammation in mice [90]. PTP1B was the first phosphatase that was shown to be sulfhydrated [91]. The PTP1B sulfhydration occurs at the Cys-215 residue in the active site and leads to inhibition of the PTP activity at a second-order rate (22.4–1.8 M−1∙s−1) with a rate of H2S-mediated PTP1B inactivation of 10–1.4 M−1∙s−1. In a model of endoplasmic reticulum stress, the sulfhydration of PTP1B decreases its activity, increasing the phosphorylation and activation of eIF2a kinase protein kinase RNA-like endoplasmic reticulum kinase (PERK) Tyr-619, which is a direct PTP1B substrate and has an important role in the endoplasmic reticulum stress response.
Furthermore, H2S can have an inhibitory effect on some phosphodiesterase (PDE) enzymes and, consequently, a positive effect on inflammation [92,93]. PDE inhibitors can have beneficial effects in inflammation by increasing cAMP level, as well as inhibiting the production of ROS and cytokines such as TNF-α and IL-1. Furthermore, PDE5 inhibitors, which increase cGMP levels, can inhibit the production of IL-6 and TNF-α [94,95]. Both endogenous and exogenous H2S serves as an inhibitor of PDEs [96]. With a half maximal inhibitory concentration (IC50) of 1.6 µM, H2S can inhibit the activity of the cGMP-specific PDE5 widely distributed in the cardiovascular system [97,98] and of the mitochondrial PDE2A stimulating mitochondrial electron transport [99]. However, the H2S selectivity among different PDE isoforms should be deeply investigated. Notably, the inhibitor of PDE-4, apremilast, was approved for the treatment of PsA [100]; this further highlights the possible therapeutic role of H2S in inflammatory arthritis, due to its effect on PDE enzymes.
The effects on the gastrointestinal tract are representative of the behaviour of this gasotransmitter in inflammation. In detail, H2S is able to promote healing of experimentally induced stomach ulcers in rats, while treatment with dl-propargylglycine (PAG), which is a CSE inhibitor, has the opposite effect [18,101]. Moreover, ABT-346, which is H2S-releasing naproxen, showed significantly less or even the absence of gastrointestinal toxicity compared to naproxen in rats [102].
In the last few years, the molecular mechanism of the H2S effect on inflammation was widely investigated. IκB/NF-κB (inhibitor of κB /Nuclear factor κ B) is an important molecular pathway that is a key target of H2S. NaHS inhibits IκB-α degradation and, therefore, reduces NF-κΒ translocation into the nucleus [103,104]. The first evidence was that LPS-induced NF-κΒ activation in cultured mouse macrophages was inhibited by H2S [105]. Subsequently, other studies showed evidence that, in different cell lines, H2S regulates the transcription, via NF-κΒ downregulation, of a plethora of pro-inflammatory mediators such as TNF-α, IL-1β, IL-6, IL-8, IL-18, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and some adhesion molecules. Additionally, slow-releasing H2S donors such as GYY4137 (morpholin-4-ium 4 methoxyphenyl (morpholino) phosphinodithioate) S-diclofenac, and S-propargyl-cysteine (SPRC) have similar effects [106,107,108,109]. Furthermore, H2S can also promote cell survival via sulfhydration of the p65 subunit of NF-κB at Cys-38 leading to an anti-apoptotic effect of NF-κB in response to pro-inflammatory agents such as TNF-α and LPS in macrophages [110]. Although the effect of H2S on NF-kB is relevant in the inflammation, other transduction mechanisms such as Akt/PKB and MAPK pathways, signal transducer and activator of transcription 3 (STAT-3), and Nrf2 cannot be ruled out as mediator of the inflammatory response to this gasotransmitter [56,60,106,111]. Additionally, it was recently demonstrated that H2S can induce the activation of forkhead box P3 (FOXP3) and, consequently, the differentiation of T regulatory cells. The functional enhancement of T regulatory cells by H2S highlights the role that this T-cell population may have in the H2S regulatory network of autoimmune inflammation, i.e., inflammatory arthritis [112].
Interestingly, the stage of inflammation is also relevant for the effect of H2S-releasing donors that can be either pro- or anti-inflammatory.
In addition, lower plasma/serum H2S concentrations were also related to vascular inflammation associated with childhood disorder Kawasaki disease (autoimmune blood vessel inflammation) [113] and skin inflammation associated with psoriasis [114].
More studies are necessary to not only fully understand the complex role of H2S in inflammation, but also to investigate further opportunities for the treatment of existing inflammatory diseases.
4. H2S and Arthritic Diseases
The effect of H2S on joint inflammation was investigated in the last few years due to the anti-inflammatory effect of the gasotransmitter. The beneficial effect of H2S seems to be dose-dependent; in fact, different studies demonstrated opposite results. At low concentration, H2S exerts an anti-inflammatory effect on tissue and cells, suggesting a potentially positive effect on arthritis. The effect of H2S on the monocyte/macrophages compartment [69,76] seems particularly relevant due to the central role that macrophages play in the pathogenesis of inflammatory arthritis. Pro-inflammatory macrophages are vital APCs and can differentiate into osteoclasts, cells responsible for irreversible bony erosions; furthermore, macrophages are one of the major sources of TNF-α.
In murine macrophages stimulated with LPS, H2S at low concentration inhibits the activation and the synthesis of several pro-inflammatory mediators, i.e., NO, NF-κB, IL-6, and IL-1β; however, at higher concentration, H2S stimulates the production of pro-inflammatory molecules by macrophages [107]. This dual effect was also demonstrated on RA FLS by Kloesch et al. [115] in two different experimental stings, showing that short-term exposure to H2S induces IL-6 expression, while long-term exposure has the opposite effect [115,116]. Interestingly, in RA synovial fluids, levels of H2S were found to be higher than in patients with osteoarthritis (OA); furthermore, the levels detected were positively correlated with disease activity and inflammation. In addition, H2S concentration in the bloodstream was increased in patients with RA, and it was also associated with high numbers of circulating leukocytes [13]. As further support of a possible pathogenic role in inflammation, d-penicillamine, used in the past for the treatment of RA, resulted as a direct inhibitor of the synthesis of H2S and as a direct inhibitor of CSE activity [117]. In particular circumstances, the pro-inflammatory effect of H2S seems to be mediated by the induction of the expression of the intracellular adhesion molecule (ICAM)1, which may increase cell migration into the inflamed tissues [118].
However, H2S was found elevated in inflammatory models, and it can represent an attempt at increasing synthesis, to reduce the local inflammation. Various studies demonstrated numerous anti-inflammatory properties of H2S mainly explained by the reduced expression of pro-inflammatory mediators and adhesion molecules. In animal models, the administration of H2S donors reduced the leukocyte adherence and infiltration and repressed carrageenan-induced paw edema.
The inhibition of endogenous H2S synthesis had the opposite effect, enhancing leukocyte migration [118]. Notably, chondrocytes and mesenchymal stem cells which differentiate in chondrocytes express CBS and CSE, responsible for the synthesis of H2S, most probably used as a preserving mechanism [119]. H2S showed a protective effect on cartilage damage in OA patients in different studies [120,121]. This effect seems mediated by the inhibition of matrix metalloproteinases and the production of extracellular matrix proteins induced by H2S-releasing compounds, which reverted the catabolic effect of IL-1β [122,123]. In addition, H2S was demonstrated to reduce the IL-1β-induced expression of IL-6, IL-8, MMP-2, and MMP-14 in OA FLS [124]. Remarkably, H2S can also inhibit TNF-α activity, an essential cytokine in inflammatory arthritis pathogenesis, by binding the zinc-proteinase TNF-α-converting enzyme (TACE), as demonstrated by Li et al. [121]. This inhibitory activity may account for the potential anti-inflammatory effect of H2S in inflammatory arthritis. Moreover, the NF-κB pathway was inhibited by H2S in different experimental studies [117,125]. Hence, the anti-inflammatory role is highly probably secondary to the inhibition of the transcription factor NF-κB, which has a key role in the pathogenesis of inflammatory arthritis as described above (see Figure 2 and Figure 3) [126,127]; furthermore, it can induce the synthesis of the anti-inflammatory cytokine IL-10 [106]. The inhibition of NF-κB activity by H2S is, therefore, able to reduce the production of the pro-inflammatory cytokines, mediating the anti-inflammatory effect of the gas in an animal model of sepsis [128], however it may also play a role in joint inflammation. Moreover, NF-κB is a key factor also for the differentiation and maturation of osteoclasts, responsible for bone erosions in arthritis [119]. Interestingly, H2S conjugated to the non-steroidal anti-inflammatory drug (NSAID) diclofenac inhibited mature osteoclasts and osteoclastogenesis, thus preventing osteolysis in an animal model of breast cancer metastasis; the inhibitory effect was demonstrated to be dependent on IκB kinase (IKK)/NF-κB [129,130]. This result is particularly interesting, because it may represent a relevant added value to drugs currently used for inflammatory arthritis, not only because H2S has a pleiotropic anti-inflammatory profile, but also because it may potentially act on bone erosion, the main long-term target in the treatment of arthritis. Moreover, as described above, H2S can directly inhibit the migration and adhesion of leukocytes to endothelial cells (see Figure 1) and the infiltration of neutrophils and lymphocytes [78]. Therefore, defective H2S compensatory production can contribute to the pathogenesis of joint inflammation in immune-mediated arthritis.
5. H2S-Donors as Potential Anti-Arthritis Drugs
H2S-donors acquired great therapeutic potential for widely diffused pathologies, such as cardiovascular [131,132], neurodegenerative [133,134,135,136], and gastrointestinal diseases [137,138]. Their H2S release can also be prolonged and potentiated by biological thiols that are normally present in biological systems such as protein thiol groups, cysteine, and glutathione (GSH). Furthermore, one of the speculated mechanisms is that H2S donors can induce the synthesis of glutathione by increasing the metabolic pathways and enzymes leading to its production [139].
As discussed above, recent evidence of the anti-inflammatory properties and the tissue repair effects of H2S increased the interest in its therapeutic potential in arthritis. The research on human species was developed mainly for OA patients, but that on inflammatory arthritis is also promising. Several non-steroidal anti-inflammatory drugs (NSAIDs) were conjugated with H2S (Table 1), allowing a slow release of the gasotransmitter into the target tissues. The molecular structures of H2S donors that are under study for clinical applications are shown in Figure 4. In animal models, NSAIDs conjugated to H2S, i.e., naproxen and celecoxib, demonstrated a strong protective effect on gastrointestinal epithelium compared to the toxic effect of the parent drug [102]. For example, the H2S-releasing naproxen, called ATB-346, which releases H2S via a hydrolytic mechanism [102], was demonstrated to have a greater anti-inflammatory and chondro-protective effect on osteoarthritic joints in animal models, reducing leukocyte migration and reducing TNF-α and NF-κB expression, and less gastrointestinal toxicity [102,140,141]. Recently, a phase II clinical trial investigating 244 healthy subjects demonstrated a drastic reduction of gastric ulcer investigated with endoscopy when treated with ATB-346 (42.2% vs. 2.5% ulcer development with naproxen and ATB-346, respectively). This effect was associated with an increased suppression of COX activity [141]. The efficacy of ATB-346 was recently evaluated in patients with OA, demonstrating that ATB-346 can reduce joint pain, possibly to a greater extent than other standard NSAIDs, such as naproxen or celecoxib [142]. Another derivative drug-releasing H2S is S-mesalamine (ATB-429) (Figure 4), used for the treatment of inflammatory colitis. ATB-429 exerted a protective role to the gastrointestinal mucosa and higher anti-inflammatory properties than the parent drug [75]. Therefore, ATB-429 could be a good candidate for reducing inflammation in arthritis. Moreover, the synthesis of other compounds able to release both NO and H2S recently led to the development of new potential drugs for the treatment of inflammatory arthritis, such as NOSH (nitric oxide and hydrogen sulfide)- sulindac (AVT-18A) and NOSH-aspirin (NBS-1120) (see Table 1). NOSH-sulindac was similar to sulindac in inhibiting the inflammatory response and demonstrating safety in the carrageenan-induced arthritis animal model, but with a larger effect on the reduction of circulating TNF-α level [143]. Similarly, NBS-1120 had a better safety profile in an animal model of systemic and local inflammation when compared to aspirin [144].
Table 1.
H2S-releasing drugs as potential anti-inflammatory drugs in arthritis.
| H2S-Derivative Drug | Drug | Company | Clinical Phase | Clinical Applications | References |
|---|---|---|---|---|---|
| AVT-18A | Sulindac | Sulfidris | Preclinical | Cancer, inflammation | [143] |
| NBS-1120 | Aspirin | Preclinical | Cancer, inflammation | [144] | |
| ACS-14 | Aspirin | CTG Ph. | Preclinical | Inflammation, cardiac injury, Arthritis |
[149] |
| ACS-21 | Aspirin | CTG Ph. | Preclinical | Inflammation, cardiac injury, Osteoarthritis |
[149] |
| ACS-6 | Ketorolac | CTG Ph. | Preclinical | Arthritis Antioxidant |
[149,150] |
| ATB-337/ACS-15 | Diclofenac | Antibe T. | Preclinical | Arthritis, inflammation | [149] |
| ATB-343 | Naproxen | Antibe T. | Preclinical | Inflammatory diseases, Alzheimer’s disease | [149] |
| ATB-346 | Naproxen | Antibe T. | Phase II | Osteoarthritis, inflammation | [102,141,149] |
| ATB-345 | Naproxen | Antibe T. | Preclinical | Inflammatory diseases | [102] |
| ATB-429 | Meselamine | Antibe T. | Preclinical | Cancer, inflammatory diseases, colitis | [75] |
| GYY4137 DAS/DADS |
National Uni. of Singapore | Preclinical Preclinical |
Inflammatory diseases, cancer, hypertension, arthritis Cancer, arthritis |
[82,107,122,151] [147,148] |
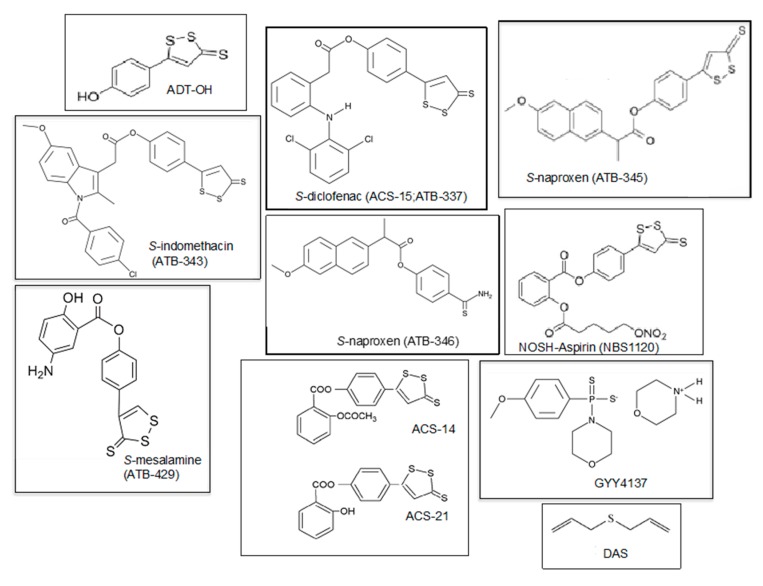
Figure 4.
Molecular structures of slow H2S-releasing agents with potential anti-inflammatory properties for the treatment of arthritis. ADT-OH (5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione) and ATB-429 ([4-(5-sulfanylidenedithiol-3-yl) phenyl] 5-amino-2-hydroxybenzoate) are H2S-releasing derivatives of mesalamine. ATB-337, ATB- 343, and ATB-345 are respectively diclofenac, indomethacin, and naproxen linked to a hydrogen sulfide-releasing moiety. ATB-346 is naproxen covalently linked to 4-hydroxythiobenzamide (TBZ). ACS-14 is aspirin linked to H2S donors, ACS-21 is deacetylated aspirin linked to H2S donors, and NBS1120 is a NO H2S-releasing derivative of aspirin. GYY4137—morpholin-4-ium 4 methoxyphenyl phosphinodithioate; DAS—diallyl sulfide.
Another potential candidate drug for clinical trials in inflammatory diseases is the slow-releasing H2S donor, GYY4137, as discussed above. This compound was demonstrated to directly inhibit joint inflammation in a mouse animal model by reducing the production of pro-inflammatory cytokines in macrophages [107]. In particular, GYY4137 inhibits several inflammatory molecules such as IL-1β, IL-6, and TNF-α, in LPS-challenged macrophages in culture [107]; moreover, GYY4137 is able to reduce LPS-evoked septic shock [106] and knee-joint edema in response to intra-articular injection of Freund’s adjuvant [122]. The administration of GYY4137 leads to an anti-inflammatory effect on knee-joint swelling and might be used for clinical applications [82].
Moreover, the endogenous metabolism of natural organosulfur compounds (OSCs) derived from garlic can also lead to the slow-releasing production of H2S [126,145] and, consequently, to an antioxidant and anti-inflammatory action [146] with organ/tissue protection. Therefore, OSCs can be considered as potential natural drugs for arthritis. The effects of diallyl sulfide (DAS) on arthritis were investigated in a model of crystal-induced arthritis, human synoviocytes and chondrocytes. DAS was able to inhibit the inflammatory response both ex vivo and in rat with induced joint inflammation [147]. In a recent study, the efficacy of diallyl disulfide (DADS) in controlling joint inflammation was evaluated in an animal model of Freund’s adjuvant-induced arthritis. DADS was demonstrated to be effective in reducing paw edema and joint and cartilage destruction [148]. These studies highlight the therapeutic potential of natural H2S donors for the treatment of inflammatory arthritis; however, more in vivo studies are needed to confirm the efficacy of these natural H2S donors, their safety profile, and their potential application in inflammatory arthritis.
6. Tissue Regeneration as a Therapeutic Approach in Arthritis
The chronic inflammatory process of arthritis leads to the disruption of the cartilaginous tissue, which ultimately contributes to subchondral bone erosions, articular deformities, and impaired quality of life. Physiologically, the cartilage tissue is avascular, aneural, and hypocellular; therefore, there is no self-reparation. A potential therapeutic approach for arthritis is to facilitate its regeneration and/or repair. Linked to the above-cited properties of H2S, H2S donors could be used as biochemical factors able to induce cartilage and bone repair in the context of arthritis. Therefore, the possibility of fabricating systems conditioned with H2S-releasing agents able to improve the repair/regeneration of cartilage and bone in degenerative diseases, as well as RA, is currently being investigated.
Two main approaches able to improve tissue repair and regeneration were investigated: the injection of stem cells (scaffold-less approach) and of three-dimensional (3D) scaffolds. In the scaffold-less approach, mesenchymal stem cells (MSCs) are the most used kind of cells showing a multipotent property [152,153]. MSCs, under appropriate differentiation stimuli, are able to express chondrogenic potential and improve the repair of cartilage [152,154,155].
Intriguingly, the MSC-based approach has the potential to solve some symptoms related to osteoarticular loss; however, it presents various problems such as cell senescence, de-differentiation, and expression of the hypertrophic phenotype. Some of these problems could be solved using the pre-conditioning of MSCs with H2S donors. Recently, it was demonstrated that the pretreatment of MSCs with H2S provided protection of MSCs upon exposure to hypoxia–ischemic insult whether in vitro or in vivo [156].
The lack of mechanical support in joint repair strategies led to relatively poor results in the clinical application of the scaffold-less approach. Consequently, substances and polymeric supports (both natural or synthetic) were designed with the aim of providing a scaffolding structure and, finally, promoting tissue regeneration more efficiently.
Many synthetic polymers with chondrogenic properties were studied, such as polylactic acid (PLA), polyethylene glycol (PEG), and polycaprolactone (PCL), but only some of them are now commercially available for clinical use [152,157,158,159].
One of these is a poly (l-lactide) (PLLA) scaffold with fibrin gel [157], available in trade under the name PLA-based, is made of a porous microstructure with fibrin which results in an excellent combination of mechanical stability, resistance to mechanical stress, and retention of the cellular component. The high level of hydration of the gel is an important requirement for having a homogeneous cellular distribution and excellent values of cell viability.
Injectable gels, based on alginate and hyaluronic acid (HA–MA) were produced to induce cartilage repair [160,161]. Alginate hydrogel showed a highly organized structure with uniform pores, which demonstrated the stimulation of a gradual increase in the cell population and an increase in type II collagen production, which is the principal ECM component of articular cartilage, even if a downregulation of collagen X was observed [160]. The polymerization of this material was obtained directly in situ using an argon laser at 512 nm, and its mechanical properties were easily modulated by the chemical composition.
Another PEG-based hydrogel scaffold with three-layer composition was also synthetized [158], showing significant effects on cartilage repair. In this scaffold, chondroitin sulfate (CS) and matrix metalloproteinase-sensitive peptides (MMP-pep) were in the upper layer, while CS was in the middle layer, and hyaluronic acid was in the lower layer. This multi-layer composition mimicked the structure of native articular cartilage with mechanical and biochemical properties that change in space. Furthermore, it was seen that this structural variability can stimulate tissue regeneration, leading to an increased production of both collagen II and X.
Another cell-free product that had excellent results for cartilage repair is Gelrin C, a hydrogel scaffold made of PEG–fibrinogen [162,163]. It was demonstrated to effectively induce cartilage repair measured with the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score (84.4 out of 100 MOCART score after 24 months). At the moment, Gelrin C is in clinical phase II for cartilage repair.
All these biomaterials can be functionalized with H2S-donors in order to improve their potential in tissue repair and regeneration. PEG–fibrinogen was, in fact, functionalized by embedding albumin microbubbles able to catalyze the production of H2S [164]. This functionalization improved the proliferation of human cardiac progenitor stem cells, promoting their spindled morphology and suggesting a potential application in repair for other biological tissues, such as human cartilage. H2S-releasing biomaterials can have several protective actions, including antioxidant and anti-inflammatory effects, angiogenesis, and vasodilation, thus improving the regenerative capacity of polymers. At the moment, there are few in vitro and in vivo studies on scaffolds able to release H2S. Scaffolds based on PCL and PLA were produced using an electrospinning technique, and they were respectively functionalized with N-benzoyl thio-benzamide (NSHD1) and phosphonamidothioate templates that generate H2S in a pH-controlled manner, as well as slow-releasing H2S donors extracted from garlic (GaOS and DADS) [159,165,166]. These three scaffolds showed protective effects on the oxidative damage of ROS and the ability to improve cell viability. A sponge H2S-releasing silk fibroin (SF) was also doped with GYY4137 [167], resulting in a scaffold with the same mechanical properties, which was able to induce a significant increase in differentiation to mature osteoblasts (OBs) and expression of osteogenic genes after three weeks of growth in a 3D culture of human MSCs. Table 2 summarizes scaffolds and H2S-releasing scaffolds that are currently under investigation for tissue repair. The H2S-releasing scaffolds could have a potential therapeutic application in the degenerative stages of inflammatory arthritis.
Table 2.
Scaffolds and H2S-releasing scaffolds for potential applications in the therapy of arthritis.
| Scaffold | Characteristics and Effects | Type of Cells | Commercial Product | Phase of Study | References |
|---|---|---|---|---|---|
|
PLLA/fibrin
1PLLA/chondrocyte/atelocollagen |
Improved cell proliferation and expression of type I and type II collagen | Chondrocytes | PLA-based BioSeedR-C (BioTissue, AG, Zurich, Switzerland) |
In vitro | [157] |
|
PEG dyacrylate systems
PEG/chitosan PEG/albumin |
In situ photopolymerization and potential modulation of its mechanical properties, increasing of the expression of type I and II collagen and the amount of sulfated GAG | MSCs | In vitro | [158] | |
| Alginate | Increase in chondrocyte viability | Chondrocytes | In vivo (SCID mice) | [160] | |
|
Hyaluronic acid/fibrin
Hyaluronic acid/collagen type I |
In situ photopolymerization, potential modulation of its mechanical properties, stimulation of ECM production and proteoglycan synthesis, and improved chondrocyte growth | Chondrocytes | Hyaluronic-based HyalograftR C autograft (Anika Therapeutics, Inc., Bedford, MA, USA) |
In vivo (human) | [161] |
| PEG-DA/denatured human fibrinogen (DHF) | In situ photopolymerization, potential modulation of its mechanical properties, gradual resorption by the body being replaced by new cartilage tissue | Cell free | GelrinC (Regentis, Haifa, Israel) |
Phase II | [163,164] |
| H2S-releasing scaffolds | |||||
| PCL/NSHD1 | Significant decrease in apoptosis in a model of tissue transplantation, protection from ROS damage, and increase in expression of collagen type I and type III | 3T3 | In vivo | [154] | |
| PFM/GaOS or DADS | Improved MSC proliferation and anti-microbial activity and protective effect against oxidative damage | hMSCs | In vitro | [166] | |
| TSTMBs-PFHy | In situ photopolymerization, potential modulation of its mechanical properties, induced spindled morphology of cells and cell proliferation | HFFs hCPCs |
In vitro | [164] | |
| ALG-CHO/2-aminopyridine-5-thiocarboxamide/tetraaniline | Increase in ejection fraction value, reduction of the myocardial infarct size in rats | ADSCs | In vivo | [168] | |
| SATO/CaCl2 | Decrease in intimal hyperplasia in human veins | Endothelial cells | In vivo | [169] | |
| SF/GYY4137 | Significant increase in osteogenic differentiation of stem cells, upregulation of osteogenic and angiogenic genes and integrins | OBs, hMSC | In vitro | [167] | |
| HA or PCL/JK1 | H2S release in pH-dependent manner, improved cell proliferation. tissue regeneration, re-epithelialization, collagen deposition, angiogenesis | Raw 264.7 | In vivo (mouse Male C57BL) | [165] | |
1PLLA—poly (l-lactide); PEG—polyethylene glycol; PCL—polycaprolactone; NSHD1—N-benzoyl thio-benzamide; GaOS—garlic oil soluble extracts; DADS—diallyl disulfide; SF—silk fibroin; HA—hyaluronic acid; ECM—extracellular matrix; MSC—mesenchymal stem cell; HFF—human foreskin fibroblasts; CPC—cardiac progenitor cell; ADSC—adipose-derived stem cell; OB—osteoblast. TSTMBs-PFHy— fibrinogen hydrogel incorporating albumin microbubbles functionalized with thiosulfate:cyanide sulfurtransferase; ALG-CHO—Partially Oxidized Alginate; PFM poly(lactic) acid fibrous; SATO aromatic peptide amphiphile and the H2S moiety, S-aroylthiooxime; PEG-DA—polyethylene glycol diacrylate; JK1—H2S donor synthesized from phenylphosphonothioic dichloride.
7. Conclusions
Currently, the interest in the pathological and therapeutic role of H2S in inflammatory diseases is growing. Recently, the evidence of the anti-inflammatory properties of H2S at physiological concentrations increased. As a gasotransmitter, H2S is involved in the regulation of production and release of several cytokines, as well as in the differentiation of adaptative and innate immune cells. Moreover, H2S plays a role as a radical scavenger in hypoxic conditions, frequently associated with inflammatory arthritis, and as a promoter of tissue repair.
Due to the growing evidence, several therapeutic approaches were investigated in different inflammatory diseases. In the context of inflammatory arthritis, the most interesting approaches result in the conjugation of anti-inflammatory compounds with H2S and the induction of tissue repair.
The first therapeutic approach aims to target the early stages of the inflammatory process or active inflammation. Despite the recent advances in the approach to inflammatory joint diseases, a significant number of patients need multi-therapeutic strategies with not always successfully outcomes. In this context, the use of H2S-conjugated drugs may be a potential add-on treatment. NSAIDs conjugated with H2S were already demonstrated to be effective in managing pain in OA patients with significantly reduced toxicity.
The modern treatments for inflammatory arthritis are able to target the active inflammation; however, strategies to treat established bone and cartilage damage are currently lacking. Scaffolds and H2S-functionalized scaffolds, with or without cell deliveries, are opening a completely new therapeutic approach in the arthritis field. Currently, a surgical approach is the most used in advanced cases; however, it can have its limitations in elderly patients and with contraindications, and this treatment is not always indicated in patients with mild joint damage. The possibility to induce tissue repair in a damaged joint with the associated anti-inflammatory effect of H2S represents a very promising new potential approach. H2S-functionalized scaffolds are still in early clinical studies as most of the studies applied them in vitro or in animal models. It will be promising to combine injectable hydrogel scaffolds that stimulate cartilage repair, such as Gelrin C (Regentis, Haifa, Israel), which is in a phase II clinical trial for early OA, with H2S-donors and MSCs for further potential clinical applications in both OA and inflammatory arthritis.
Clearly, more work is needed to improve the sensitivity and specificity of H2S assays, as well as to improve the patient selection in studies assessing the efficacy of this new promising treatment strategy.
Abbreviations
| ADSCs | adipose-derived stem cells |
| ADT-OH | 5-(4-hydroxyphenyl)-3H-1:2-dithiole-3-thione |
| ATB-429 | 4-(5-sulfanylidenedithiol-3-yl) phenyl 5-amino-2-hydroxybenzoate |
| ALG-CHO | partially oxidized alginate |
| anti CCP | anti-cyclic citrullinated peptide |
| ARE | antioxidant response element |
| APCs | antigen-presenting cells |
| bFGF | basic fibroblast growth factor |
| cAMP | cyclic adenosine monophosphate |
| CAT | cysteine aminotransferase |
| CAT | catalase |
| CD | cluster of differentiation |
| cGMP | cyclic guanosine monophosphate |
| COX 2 | cyclooxygenase 2 |
| CS | chondroitin sulfate |
| CBS | cystathionine beta-synthase |
| CSE | cystathionine gamma lyase |
| DAS | diallyl sulfide |
| DCs | dendritic cells |
| EC | endothelial cells |
| ECM | extracellular matrix |
| eIF2a | eukaryotic translation initiation factor 2 |
| ErK | extracellular signal-regulated kinase |
| FLS | fibroblast like synoviocytes |
| FOXP3 | forkhead box P3 |
| GaOS | garlic oil soluble extracts |
| GM-CSF | granulocyte macrophage colony stimulating factor |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | glutathione |
| GSSH | glutathione persulfide |
| GST | glutathione-S-transferase |
| HA | hyaluronic acid |
| hCPCs | human cardiac progenitor cells |
| HER 2 | human epidermal growth factor receptor 2 |
| HFFs | human foreskin fibroblasts |
| HO1 | heme oxygenase 1 |
| ICAM 1 | intercellular Adhesion Molecule 1 |
| IDO 1 | indoleamine-pyrrole 2:3- dioxygenase 1 |
| IGF 1 | insulin-like growth factor 1 |
| IκB/NF-κB | inhibitor of κB /Nuclear factor κ B |
| IKK | IκB kinase |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| JK1 | H2S donors synthesized from phenylphosphonothioic dichloride |
| Keap 1 | Kelch-like ECH-associated protein 1 |
| LPS | lipopolysaccharides |
| M1 | macrophages M1 |
| M2 | macrophages M2 |
| MAPK | mitogen-activated protein kinase |
| MBs-PFHy | fibrinogen hydrogel incorporating albumin microbubbles |
| MHC-I | major histocompatibility complex |
| MMP | matrix metalloproteinase |
| MMP | pepmatrix metalloproteinase-sensitive peptides |
| MOCART | magnetic resonance observation of cartilage repair tissue |
| MSCs | mesenchymal stem cells |
| MST | 3-mercaptopyruvate sulfotransferase |
| NFκB | nuclear factor kappa beta |
| NOSH | nitric oxide and hydrogen sulfide |
| Nrf2 | nuclear erythroid factor 2-related factor 2 |
| NSHD 1 | N-benzoyl thio-benzamide |
| OA | osteoarthritis |
| OBs | osteoblast cell |
| OCs | osteoclasts cell |
| OSC | organosulfur compounds |
| PAG | propargylglycine |
| PCL | polycaprolactone |
| PDE | phosphodiesterases |
| PEG | polyethylene glycol |
| PERK | protein kinase RNA-like endoplasmic reticulum kinase |
| PFM | poly(lactic) acid fibrous |
| PKB | protein kinase B |
| PLA | polylactic acid |
| PLLA | poly (l-lactide) |
| PsA | psoriatic arthritis |
| PTPs | protein tyrosine phosphatases |
| QR | quinone reductase |
| RA | rheumatoid arthritis |
| RANTES | regulated upon activation, normal T Cell expressed and presumably secreted |
| RAW 264.7 | murine monocyte/macrophage-like lineage |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SATO | S-aroylthiooxime |
| SELENBP1 | selenium-binding protein 1 |
| SF | silk fibroin |
| SOD | superoxide dismutase |
| SpA | spondyloarthritis |
| STAT 3 | signal transducer and activator of transcription 3 |
| TBZ | 4-hydroxythiobenzamide |
| TGF-β1 | transforming growth factor beta 1 |
| Th | T helper cells |
| TNF α | tumor necrosis factor-α |
| TST | thiosulfate sulfurtransferase |
| VCAM 1 | vascular cell adhesion molecule 1 |
Author Contributions
Conceptualization, S.M. and F.S.; data curation, S.M. and F.S.; writing—original draft, S.M. and F.S.; writing—review and editing, S.M., F.S., S.D.S., and M.S.C.; supervision, S.M. and M.S.C.; project, S.M. and M.S.C.; administration, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 3.Li L., Moore P.K. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol. Sci. 2008;29:84–90. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 5.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 6.Nagahara N., Koike S., Nirasawa T., Kimura H., Ogasawara Y. Alternative pathway of H2S and polysulfides production from sulfurated catalytic-cysteine of reaction intermediates of 3-mercaptopyruvate sulfurtransferase. Biochem. Biophys. Res. Commun. 2018;496:648–653. doi: 10.1016/j.bbrc.2018.01.056. [DOI] [PubMed] [Google Scholar]
- 7.Pol A., Renkema G.H., Tangerman A., Winkel E.G., Engelke U.F., de Brouwer A.P.M., Lloyd K.C., Araiza R.S., van den Heuvel L., Omran H., et al. Mutations in SELENBP1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat. Genet. 2018;50:120–129. doi: 10.1038/s41588-017-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuto J.M., Collins M.D. Interactive endogenous small molecule (gaseous) signaling: Implications for teratogenesis. Curr. Pharm. Des. 2007;13:2952–2978. doi: 10.2174/138161207782110525. [DOI] [PubMed] [Google Scholar]
- 9.Cao J.T., Zhang W.S., Fu X.L., Wang H., Ma S.H., Liu Y.M. Copper ion modified graphitic C3N4 nanosheets enhanced luminol-H2O2 chemiluminescence system: Toward highly selective and sensitive bioassay of H2S in human plasma. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020;230:118040. doi: 10.1016/j.saa.2020.118040. [DOI] [PubMed] [Google Scholar]
- 10.Furne J., Saeed A., Levitt M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 11.Olson K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Biophys. Acta. 2009;1787:856–863. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Nagashima K.F.K., Kamaya M. Determination of trace amounts of sulfide in human serum by high-performance liquid chromatography with fluorometric detection after derivatization with 1-amino-5-n, n-diethylaminotoluene and iron (III) J. Liq. Chrom. Relat. Technol. 1995;18:515. doi: 10.1080/10826079508009253. [DOI] [Google Scholar]
- 13.Whiteman M., Haigh R., Tarr J.M., Gooding K.M., Shore A.C., Winyard P.G. Detection of hydrogen sulfide in plasma and knee-joint synovial fluid from rheumatoid arthritis patients: Relation to clinical and laboratory measures of inflammation. Ann. N. Y. Acad. Sci. 2010;1203:146–150. doi: 10.1111/j.1749-6632.2010.05556.x. [DOI] [PubMed] [Google Scholar]
- 14.Kabil O., Motl N., Banerjee R. H2S and its role in redox signaling. Biochim. Biophys. Acta. 2014;1844:1355–1366. doi: 10.1016/j.bbapap.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Searcy D.G., Lee S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998;282:310–322. doi: 10.1002/(SICI)1097-010X(19981015)282:3<310::AID-JEZ4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W., Wang R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H474–H480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- 17.Jha S., Calvert J.W., Duranski M.R., Ramachandran A., Lefer D.J. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: Role of antioxidant and antiapoptotic signaling. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace J.L. Physiological and pathophysiological roles of hydrogen sulfide in the gastrointestinal tract. Antioxid. Redox Signal. 2010;12:1125–1133. doi: 10.1089/ars.2009.2900. [DOI] [PubMed] [Google Scholar]
- 19.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 20.Kanagy N.L., Szabo C., Papapetropoulos A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell Physiol. 2017;312:C537–C549. doi: 10.1152/ajpcell.00329.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papapetropoulos A., Pyriochou A., Altaany Z., Yang G., Marazioti A., Zhou Z., Jeschke M.G., Branski L.K., Herndon D.N., Wang R., et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J.F., Li Y., Song J.N., Pang H.G. Role of hydrogen sulfide in secondary neuronal injury. Neurochem. Int. 2014;64:37–47. doi: 10.1016/j.neuint.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Kida K., Ichinose F. Hydrogen Sulfide and Neuroinflammation. Handb. Exp. Pharmacol. 2015;230:181–189. doi: 10.1007/978-3-319-18144-8_9. [DOI] [PubMed] [Google Scholar]
- 24.Wallace J.L., Ianaro A., Flannigan K.L., Cirino G. Gaseous mediators in resolution of inflammation. Semin. Immunol. 2015;27:227–233. doi: 10.1016/j.smim.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Sen N. Functional and Molecular Insights of Hydrogen Sulfide Signaling and Protein Sulfhydration. J. Mol. Biol. 2017;429:543–561. doi: 10.1016/j.jmb.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia M. H2S and Inflammation: An Overview. Handb. Exp. Pharmacol. 2015;230:165–180. doi: 10.1007/978-3-319-18144-8_8. [DOI] [PubMed] [Google Scholar]
- 27.Coletta C., Szabo C. Potential role of hydrogen sulfide in the pathogenesis of vascular dysfunction in septic shock. Curr. Vasc. Pharmacol. 2013;11:208–221. [PubMed] [Google Scholar]
- 28.Akter F. The role of hydrogen sulfide in burns. Burns. 2016;42:519–525. doi: 10.1016/j.burns.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Predmore B.L., Lefer D.J., Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad A., Sattar M.A., Rathore H.A., Khan S.A., Lazhari M.I., Afzal S., Hashmi F., Abdullah N.A., Johns E.J. A critical review of pharmacological significance of Hydrogen Sulfide in hypertension. Indian J. Pharmacol. 2015;47:243–247. doi: 10.4103/0253-7613.157106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner F., Asfar P., Calzia E., Radermacher P., Szabo C. Bench-to-bedside review: Hydrogen sulfide-the third gaseous transmitter: Applications for critical care. Crit. Care. 2009;13:213. doi: 10.1186/cc7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R., Szabo C., Ichinose F., Ahmed A., Whiteman M., Papapetropoulos A. The role of H2S bioavailability in endothelial dysfunction. Trends Pharmacol. Sci. 2015;36:568–578. doi: 10.1016/j.tips.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coaccioli S., Panaccione A., Biondi R., Sabatini C., Landucci P., Del Giorno R., Fantera M., Monno Mondo A., Di Cato L., Paladini A., et al. Evaluation of oxidative stress in rheumatoid and psoriatic arthritis and psoriasis. Clin. Ter. 2009;160:467–472. [PubMed] [Google Scholar]
- 34.Phillips D.C., Dias H.K., Kitas G.D., Griffiths H.R. Aberrant reactive oxygen and nitrogen species generation in rheumatoid arthritis (RA): Causes and consequences for immune function, cell survival, and therapeutic intervention. Antioxid. Redox Signal. 2010;12:743–785. doi: 10.1089/ars.2009.2607. [DOI] [PubMed] [Google Scholar]
- 35.Kurien B.T., Scofield R.H. Autoimmunity and oxidatively modified autoantigens. Autoimmun. Rev. 2008;7:567–573. doi: 10.1016/j.autrev.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fearon U., Reece R., Smith J., Emery P., Veale D.J. Synovial cytokine and growth factor regulation of MMPs/TIMPs: Implications for erosions and angiogenesis in early rheumatoid and psoriatic arthritis patients. Ann. N. Y. Acad. Sci. 1999;878:619–621. doi: 10.1111/j.1749-6632.1999.tb07743.x. [DOI] [PubMed] [Google Scholar]
- 37.Ng C.T., Biniecka M., Kennedy A., McCormick J., Fitzgerald O., Bresnihan B., Buggy D., Taylor C.T., O’Sullivan J., Fearon U., et al. Synovial tissue hypoxia and inflammation in vivo. Ann. Rheum. Dis. 2010;69:1389–1395. doi: 10.1136/ard.2009.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biniecka M., Kennedy A., Fearon U., Ng C.T., Veale D.J., O’Sullivan J.N. Oxidative damage in synovial tissue is associated with in vivo hypoxic status in the arthritic joint. Ann. Rheum. Dis. 2010;69:1172–1178. doi: 10.1136/ard.2009.111211. [DOI] [PubMed] [Google Scholar]
- 39.Harty L.C., Biniecka M., O’Sullivan J., Fox E., Mulhall K., Veale D.J., Fearon U. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann. Rheum. Dis. 2012;71:582–588. doi: 10.1136/annrheumdis-2011-200245. [DOI] [PubMed] [Google Scholar]
- 40.Kruithof E., Baeten D., De Rycke L., Vandooren B., Foell D., Roth J., Canete J.D., Boots A.M., Veys E.M., De Keyser F. Synovial histopathology of psoriatic arthritis, both oligo- and polyarticular, resembles spondyloarthropathy more than it does rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R569–R580. doi: 10.1186/ar1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costello P.J., Winchester R.J., Curran S.A., Peterson K.S., Kane D.J., Bresnihan B., FitzGerald O.M. Psoriatic arthritis joint fluids are characterized by CD8 and CD4 T cell clonal expansions appear antigen driven. J. Immunol. 2001;166:2878–2886. doi: 10.4049/jimmunol.166.4.2878. [DOI] [PubMed] [Google Scholar]
- 42.Olivieri I., D’Angelo S., Palazzi C., Padula A. Advances in the management of psoriatic arthritis. Nat. Rev. Rheumatol. 2014;10:531–542. doi: 10.1038/nrrheum.2014.106. [DOI] [PubMed] [Google Scholar]
- 43.Ospelt C. Synovial fibroblasts in 2017. RMD Open. 2017;3:e000471. doi: 10.1136/rmdopen-2017-000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colucci S., Brunetti G., Cantatore F.P., Oranger A., Mori G., Quarta L., Cirulli N., Mancini L., Corrado A., Grassi F.R., et al. Lymphocytes and synovial fluid fibroblasts support osteoclastogenesis through RANKL, TNFalpha, and IL-7 in an in vitro model derived from human psoriatic arthritis. J. Pathol. 2007;212:47–55. doi: 10.1002/path.2153. [DOI] [PubMed] [Google Scholar]
- 45.Qu N., Xu M., Mizoguchi I., Furusawa J., Kaneko K., Watanabe K., Mizuguchi J., Itoh M., Kawakami Y., Yoshimoto T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013;2013:968549. doi: 10.1155/2013/968549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehrenstein M.R., Evans J.G., Singh A., Moore S., Warnes G., Isenberg D.A., Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurowska-Stolarska M., Alivernini S. Synovial tissue macrophages: Friend or foe? RMD Open. 2017;3:e000527. doi: 10.1136/rmdopen-2017-000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baeten D., Kruithof E., De Rycke L., Boots A.M., Mielants H., Veys E.M., De Keyser F. Infiltration of the synovial membrane with macrophage subsets and polymorphonuclear cells reflects global disease activity in spondyloarthropathy. Arthritis Res. Ther. 2005;7:R359–R369. doi: 10.1186/ar1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canete J.D., Santiago B., Cantaert T., Sanmarti R., Palacin A., Celis R., Graell E., Gil-Torregrosa B., Baeten D., Pablos J.L. Ectopic lymphoid neogenesis in psoriatic arthritis. Ann. Rheum. Dis. 2007;66:720–726. doi: 10.1136/ard.2006.062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiteman M., Armstrong J.S., Chu S.H., Jia-Ling S., Wong B.S., Cheung N.S., Halliwell B., Moore P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 51.Mitsuhashi H., Yamashita S., Ikeuchi H., Kuroiwa T., Kaneko Y., Hiromura K., Ueki K., Nojima Y. Oxidative stress-dependent conversion of hydrogen sulfide to sulfite by activated neutrophils. Shock. 2005;24:529–534. doi: 10.1097/01.shk.0000183393.83272.de. [DOI] [PubMed] [Google Scholar]
- 52.Geng B., Chang L., Pan C., Qi Y., Zhao J., Pang Y., Du J., Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 53.Olas B. Hydrogen sulfide in signaling pathways. Clin. Chim. Acta. 2015;439:212–218. doi: 10.1016/j.cca.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 54.Sun W.H., Liu F., Chen Y., Zhu Y.C. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem. Biophys. Res. Commun. 2012;421:164–169. doi: 10.1016/j.bbrc.2012.03.121. [DOI] [PubMed] [Google Scholar]
- 55.De Beus M.D., Chung J., Colon W. Modification of cysteine 111 in Cu/Zn superoxide dismutase results in altered spectroscopic and biophysical properties. Protein Sci. 2004;13:1347–1355. doi: 10.1110/ps.03576904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalayarasan S., Prabhu P.N., Sriram N., Manikandan R., Arumugam M., Sudhandiran G. Diallyl sulfide enhances antioxidants and inhibits inflammation through the activation of Nrf2 against gentamicin-induced nephrotoxicity in Wistar rats. Eur. J. Pharmacol. 2009;606:162–171. doi: 10.1016/j.ejphar.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 57.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 58.Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., Khaper N., Wu L., Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 59.Xie L., Gu Y., Wen M., Zhao S., Wang W., Ma Y., Meng G., Han Y., Wang Y., Liu G., et al. Hydrogen Sulfide Induces Keap1 S-sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes. 2016;65:3171–3184. doi: 10.2337/db16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calvert J.W., Jha S., Gundewar S., Elrod J.W., Ramachandran A., Pattillo C.B., Kevil C.G., Lefer D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim S., Lee H.G., Park S.A., Kundu J.K., Keum Y.S., Cha Y.N., Na H.K., Surh Y.J. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS ONE. 2014;9:e85984. doi: 10.1371/journal.pone.0085984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu W.J., Jia W.W., Liu X.H., Pan L.L., Zhang Q.Y., Yang D., Shen X.Y., Liu L., Zhu Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016;10:157–167. doi: 10.1016/j.redox.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hourihan J.M., Kenna J.G., Hayes J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid. Redox Signal. 2013;19:465–481. doi: 10.1089/ars.2012.4944. [DOI] [PubMed] [Google Scholar]
- 64.Xie Z.Z., Liu Y., Bian J.S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid. Med. Cell. Longev. 2016;2016:6043038. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimura Y., Dargusch R., Schubert D., Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid. Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 66.Hine C., Harputlugil E., Zhang Y., Ruckenstuhl C., Lee B.C., Brace L., Longchamp A., Trevino-Villarreal J.H., Mejia P., Ozaki C.K., et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biesalski H.K., Shakibaei M., Henrotin Y. Antioxidants and Osteoarthritis. Syst. Biol. Free Radic. Antioxid. 2014:2997–3026. doi: 10.1007/978-3-642-30018-9_130. [DOI] [Google Scholar]
- 68.Whiteman M., Winyard P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 69.Dufton N., Natividad J., Verdu E.F., Wallace J.L. Hydrogen sulfide and resolution of acute inflammation: A comparative study utilizing a novel fluorescent probe. Sci. Rep. 2012;2:499. doi: 10.1038/srep00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallace J.L., Ferraz J.G., Muscara M.N. Hydrogen sulfide: An endogenous mediator of resolution of inflammation and injury. Antioxid. Redox Signal. 2012;17:58–67. doi: 10.1089/ars.2011.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallace J.L., Vong L., McKnight W., Dicay M., Martin G.R. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–578. doi: 10.1053/j.gastro.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Miller T.W., Wang E.A., Gould S., Stein E.V., Kaur S., Lim L., Amarnath S., Fowler D.H., Roberts D.D. Hydrogen sulfide is an endogenous potentiator of T cell activation. J. Biol. Chem. 2012;287:4211–4221. doi: 10.1074/jbc.M111.307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gemici B., Wallace J.L. Anti-inflammatory and cytoprotective properties of hydrogen sulfide. Methods Enzymol. 2015;555:169–193. doi: 10.1016/bs.mie.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 74.Flannigan K.L., Agbor T.A., Blackler R.W., Kim J.J., Khan W.I., Verdu E.F., Ferraz J.G., Wallace J.L. Impaired hydrogen sulfide synthesis and IL-10 signaling underlie hyperhomocysteinemia-associated exacerbation of colitis. Proc. Natl. Acad. Sci. USA. 2014;111:13559–13564. doi: 10.1073/pnas.1413390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fiorucci S., Orlandi S., Mencarelli A., Caliendo G., Santagada V., Distrutti E., Santucci L., Cirino G., Wallace J.L. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang C.W., Feng W., Peh M.T., Peh K., Dymock B.W., Moore P.K. A novel slow-releasing hydrogen sulfide donor, FW1256, exerts anti-inflammatory effects in mouse macrophages and in vivo. Pharmacol. Res. 2016;113:533–546. doi: 10.1016/j.phrs.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 77.Du C., Jin M., Hong Y., Li Q., Wang X.H., Xu J.M., Wang F., Zhang Y., Jia J., Liu C.F., et al. Downregulation of cystathionine beta-synthase/hydrogen sulfide contributes to rotenone-induced microglia polarization toward M1 type. Biochem. Biophys. Res. Commun. 2014;451:239–245. doi: 10.1016/j.bbrc.2014.07.107. [DOI] [PubMed] [Google Scholar]
- 78.Zanardo R.C., Brancaleone V., Distrutti E., Fiorucci S., Cirino G., Wallace J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 79.Guan Q., Wang X., Gao L., Chen J., Liu Y., Yu C., Zhang N., Zhang X., Zhao J. Hydrogen sulfide suppresses high glucose-induced expression of intercellular adhesion molecule-1 in endothelial cells. J. Cardiovasc. Pharmacol. 2013;62:278–284. doi: 10.1097/FJC.0b013e31829875ef. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y., Zhao X., Jin H., Wei H., Li W., Bu D., Tang X., Ren Y., Tang C., Du J. Role of hydrogen sulfide in the development of atherosclerotic lesions in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009;29:173–179. doi: 10.1161/ATVBAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 81.Pan L.L., Liu X.H., Gong Q.H., Wu D., Zhu Y.Z. Hydrogen sulfide attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in vascular endothelial cells. PLoS ONE. 2011;6:e19766. doi: 10.1371/journal.pone.0019766. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Pan L.L., Liu X.H., Zheng H.M., Yang H.B., Gong Q.H., Zhu Y.Z. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuated tumor necrosis factor-alpha-induced inflammatory signaling and dysfunction in endothelial cells. Int. J. Cardiol. 2012;155:327–332. doi: 10.1016/j.ijcard.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 83.Mariggio M.A., Minunno V., Riccardi S., Santacroce R., De Rinaldis P., Fumarulo R. Sulfide enhancement of PMN apoptosis. Immunopharmacol. Immunotoxicol. 1998;20:399–408. doi: 10.3109/08923979809034822. [DOI] [PubMed] [Google Scholar]
- 84.Traves P.G., Pardo V., Pimentel-Santillana M., Gonzalez-Rodriguez A., Mojena M., Rico D., Montenegro Y., Cales C., Martin-Sanz P., Valverde A.M., et al. Pivotal role of protein tyrosine phosphatase 1B (PTP1B) in the macrophage response to pro-inflammatory and anti-inflammatory challenge. Cell Death Dis. 2014;5:e1125. doi: 10.1038/cddis.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pao L.I., Badour K., Siminovitch K.A., Neel B.G. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu. Rev. Immunol. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- 86.Camer D., Huang X.F. The endothelin pathway: A protective or detrimental target of bardoxolone methyl on cardiac function in patients with advanced chronic kidney disease? Am. J. Nephrol. 2014;40:288–290. doi: 10.1159/000368563. [DOI] [PubMed] [Google Scholar]
- 87.Rivera Franco M.M., Leon Rodriguez E., Martinez Benitez B., Villanueva Rodriguez L.G., de la Luz Sevilla Gonzalez M., Armengol Alonso A. Association of PTP1B with Outcomes of Breast Cancer Patients Who Underwent Neoadjuvant Chemotherapy. Breast Cancer (Auckl.) 2016;10:177–184. doi: 10.4137/BCBCR.S40934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berdnikovs S., Pavlov V.I., Abdala-Valencia H., McCary C.A., Klumpp D.J., Tremblay M.L., Cook-Mills J.M. PTP1B deficiency exacerbates inflammation and accelerates leukocyte trafficking in vivo. J. Immunol. 2012;188:874–884. doi: 10.4049/jimmunol.1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bleidorn J., Alamzad-Krabbe H., Gerhards B., Kraus T., Brand P., Krabbe J., Martin C. The pro-inflammatory stimulus of zinc- and copper-containing welding fumes in whole blood assay via protein tyrosine phosphatase 1B inhibition. Sci. Rep. 2019;9:1315. doi: 10.1038/s41598-018-37803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Song G.J., Jung M., Kim J.H., Park H., Rahman M.H., Zhang S., Zhang Z.Y., Park D.H., Kook H., Lee I.K., et al. A novel role for protein tyrosine phosphatase 1B as a positive regulator of neuroinflammation. J. Neuroinflamm. 2016;13:86. doi: 10.1186/s12974-016-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krishnan N., Fu C., Pappin D.J., Tonks N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andruski B., McCafferty D.M., Ignacy T., Millen B., McDougall J.J. Leukocyte trafficking and pain behavioral responses to a hydrogen sulfide donor in acute monoarthritis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R814–R820. doi: 10.1152/ajpregu.90524.2008. [DOI] [PubMed] [Google Scholar]
- 93.Heneberg P. Reactive nitrogen species and hydrogen sulfide as regulators of protein tyrosine phosphatase activity. Antioxid. Redox Signal. 2014;20:2191–2209. doi: 10.1089/ars.2013.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Houslay M.D., Sullivan M., Bolger G.B. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: Intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. Adv. Pharmacol. 1998;44:225–342. doi: 10.1016/s1054-3589(08)60128-3. [DOI] [PubMed] [Google Scholar]
- 95.Haddad J.J., Land S.C., Tarnow-Mordi W.O., Zembala M., Kowalczyk D., Lauterbach R. Immunopharmacological potential of selective phosphodiesterase inhibition. I. Differential regulation of lipopolysaccharide-mediated proinflammatory cytokine (interleukin-6 and tumor necrosis factor-alpha) biosynthesis in alveolar epithelial cells. J. Pharmacol. Exp. Ther. 2002;300:559–566. doi: 10.1124/jpet.300.2.559. [DOI] [PubMed] [Google Scholar]
- 96.Bucci M., Papapetropoulos A., Vellecco V., Zhou Z., Pyriochou A., Roussos C., Roviezzo F., Brancaleone V., Cirino G. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler. Thromb. Vasc. Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 97.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Modis K., Panopoulos P., Asimakopoulou A., Gero D., Sharina I., Martin E., et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Panagiotis P., Yang G., Asimakopoulou A., Topouzis T., Wang R., Szabo C., Papapetropoulos A. Selectivity of hydrogen sulfide toward cyclic nucleotide phosphodiesterases. Nitric Oxide. 2015;47:S39. [Google Scholar]
- 99.Modis K., Panopoulos P., Coletta C., Papapetropoulos A., Szabo C. Hydrogen sulfide-mediated stimulation of mitochondrial electron transport involves inhibition of the mitochondrial phosphodiesterase 2A, elevation of cAMP and activation of protein kinase A. Biochem. Pharmacol. 2013;86:1311–1319. doi: 10.1016/j.bcp.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 100.Wells A.F., Edwards C.J., Kivitz A.J., Bird P., Nguyen D., Paris M., Teng L., Aelion J.A. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: Results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology. 2018;57:1253–1263. doi: 10.1093/rheumatology/key032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wallace J.L., Dicay M., McKnight W., Martin G.R. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 102.Wallace J.L., Caliendo G., Santagada V., Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346) Br. J. Pharmacol. 2010;159:1236–1246. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Q., Liu H.R., Mu Q., Rose P., Zhu Y.Z. S-propargyl-cysteine protects both adult rat hearts and neonatal cardiomyocytes from ischemia/hypoxia injury: The contribution of the hydrogen sulfide-mediated pathway. J. Cardiovasc. Pharmacol. 2009;54:139–146. doi: 10.1097/FJC.0b013e3181ac8e12. [DOI] [PubMed] [Google Scholar]
- 104.Tamizhselvi R., Sun J., Koh Y.H., Bhatia M. Effect of hydrogen sulfide on the phosphatidylinositol 3-kinase-protein kinase B pathway and on caerulein-induced cytokine production in isolated mouse pancreatic acinar cells. J. Pharmacol. Exp. Ther. 2009;329:1166–1177. doi: 10.1124/jpet.109.150532. [DOI] [PubMed] [Google Scholar]
- 105.Oh G.S., Pae H.O., Lee B.S., Kim B.N., Kim J.M., Kim H.R., Jeon S.B., Jeon W.K., Chae H.J., Chung H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 106.Li L., Salto-Tellez M., Tan C.H., Whiteman M., Moore P.K. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic. Biol. Med. 2009;47:103–113. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 107.Whiteman M., Li L., Rose P., Tan C.H., Parkinson D.B., Moore P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li L., Rossoni G., Sparatore A., Lee L.C., Del Soldato P., Moore P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 109.Gong Q.H., Shi X.R., Hong Z.Y., Pan L.L., Liu X.H., Zhu Y.Z. A new hope for neurodegeneration: Possible role of hydrogen sulfide. J. Alzheimer’s Dis. 2011;24(Suppl. S2):173–182. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- 110.Sen N., Paul B.D., Gadalla M.M., Mustafa A.K., Sen T., Xu R., Kim S., Snyder S.H. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol. Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shao J.L., Wan X.H., Chen Y., Bi C., Chen H.M., Zhong Y., Heng X.H., Qian J.Q. H2S protects hippocampal neurons from anoxia-reoxygenation through cAMP-mediated PI3K/Akt/p70S6K cell-survival signaling pathways. J. Mol. Neurosci. 2011;43:453–460. doi: 10.1007/s12031-010-9464-4. [DOI] [PubMed] [Google Scholar]
- 112.Yang R., Qu C., Zhou Y., Konkel J.E., Shi S., Liu Y., Chen C., Liu S., Liu D., Chen Y., et al. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity. 2015;43:251–263. doi: 10.1016/j.immuni.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li X.H., Zhang C.Y., Wu J.X., Zhang T. Changes in plasma hydrogen sulfide and nitric oxide levels and their clinical significance in children with Kawasaki disease. Chin. Med. J. 2011;124:3445–3449. [PubMed] [Google Scholar]
- 114.Alshorafa A.K., Guo Q., Zeng F., Chen M., Tan G., Tang Z., Yin R. Psoriasis is associated with low serum levels of hydrogen sulfide, a potential anti-inflammatory molecule. Tohoku J. Exp. Med. 2012;228:325–332. doi: 10.1620/tjem.228.325. [DOI] [PubMed] [Google Scholar]
- 115.Kloesch B., Liszt M., Broell J. H2S transiently blocks IL-6 expression in rheumatoid arthritic fibroblast-like synoviocytes and deactivates p44/42 mitogen-activated protein kinase. Cell Biol. Int. 2010;34:477–484. doi: 10.1042/CBI20090436. [DOI] [PubMed] [Google Scholar]
- 116.Kloesch B., Liszt M., Krehan D., Broell J., Kiener H., Steiner G. High concentrations of hydrogen sulphide elevate the expression of a series of pro-inflammatory genes in fibroblast-like synoviocytes derived from rheumatoid and osteoarthritis patients. Immunol. Lett. 2012;141:197–203. doi: 10.1016/j.imlet.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 117.Brancaleone V., Esposito I., Gargiulo A., Vellecco V., Asimakopoulou A., Citi V., Calderone V., Gobbetti T., Perretti M., Papapetropoulos A., et al. D-Penicillamine modulates hydrogen sulfide (H2S) pathway through selective inhibition of cystathionine-gamma-lyase. Br. J. Pharmacol. 2016;173:1556–1565. doi: 10.1111/bph.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tamizhselvi R., Koh Y.H., Sun J., Zhang H., Bhatia M. Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-kappaB and Src-family kinases pathway. Exp. Cell Res. 2010;316:1625–1636. doi: 10.1016/j.yexcr.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 119.Fox B., Schantz J.T., Haigh R., Wood M.E., Moore P.K., Viner N., Spencer J.P., Winyard P.G., Whiteman M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: Is H2S a novel cytoprotective mediator in the inflamed joint? J. Cell. Mol. Med. 2012;16:896–910. doi: 10.1111/j.1582-4934.2011.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ha C., Tian S., Sun K., Wang D., Lv J., Wang Y. Hydrogen sulfide attenuates IL-1beta-induced inflammatory signaling and dysfunction of osteoarthritic chondrocytes. Int. J. Mol. Med. 2015;35:1657–1666. doi: 10.3892/ijmm.2015.2183. [DOI] [PubMed] [Google Scholar]
- 121.Li L., Fox B., Keeble J., Salto-Tellez M., Winyard P.G., Wood M.E., Moore P.K., Whiteman M. The complex effects of the slow-releasing hydrogen sulfide donor GYY4137 in a model of acute joint inflammation and in human cartilage cells. J. Cell. Mol. Med. 2013;17:365–376. doi: 10.1111/jcmm.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vela-Anero A., Hermida-Gomez T., Gato-Calvo L., Vaamonde-Garcia C., Diaz-Prado S., Meijide-Failde R., Blanco F.J., Burguera E.F. Long-term effects of hydrogen sulfide on the anabolic-catabolic balance of articular cartilage in vitro. Nitric Oxide. 2017;70:42–50. doi: 10.1016/j.niox.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 123.Pan L.P., Cao Y.P., Wen L.C., Chai W.B., Du J.B., Jin H.F., Liu J., Yang X., Meng Z.C., Liu H., et al. Hydrogen sulfide in cartilage and its inhibitory effect on matrix metalloproteinase 13 expression in chondrocytes induced by interlukin-1beta. Beijing Da Xue Xue Bao Yi Xue Ban. 2016;48:194–202. [PubMed] [Google Scholar]
- 124.Sieghart D., Liszt M., Wanivenhaus A., Broll H., Kiener H., Klosch B., Steiner G. Hydrogen sulphide decreases IL-1beta-induced activation of fibroblast-like synoviocytes from patients with osteoarthritis. J. Cell. Mol. Med. 2015;19:187–197. doi: 10.1111/jcmm.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee H.S., Lee C.H., Tsai H.C., Salter D.M. Inhibition of cyclooxygenase 2 expression by diallyl sulfide on joint inflammation induced by urate crystal and IL-1beta. Osteoarthr. Cartil. 2009;17:91–99. doi: 10.1016/j.joca.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 126.Maniatis D.T. Transcriptional Regulation of Endothelial Cell Adhesion Molecules: Nf-Kappa B And Cytokine-Inducible Enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 127.Perry M.M., Hui C.K., Whiteman M., Wood M.E., Adcock I., Kirkham P., Michaeloudes C., Chung K.F. Hydrogen sulfide inhibits proliferation and release of IL-8 from human airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2011;45:746–752. doi: 10.1165/rcmb.2010-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang H., Zhi L., Moochhala S., Moore P.K., Bhatia M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L960–L971. doi: 10.1152/ajplung.00388.2006. [DOI] [PubMed] [Google Scholar]
- 129.Braun T., Zwerina J. Positive regulators of osteoclastogenesis and bone resorption in rheumatoid arthritis. Arthritis Res. Ther. 2011;13:235. doi: 10.1186/ar3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frantzias J., Logan J.G., Mollat P., Sparatore A., Del Soldato P., Ralston S.H., Idris A.I. Hydrogen sulphide-releasing diclofenac derivatives inhibit breast cancer-induced osteoclastogenesis in vitro and prevent osteolysis ex vivo. Br. J. Pharmacol. 2012;165:1914–1925. doi: 10.1111/j.1476-5381.2011.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang G., Wu L., Liang W., Wang R. Direct stimulation of K(ATP) channels by exogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. Mol. Pharmacol. 2005;68:1757–1764. doi: 10.1124/mol.105.017467. [DOI] [PubMed] [Google Scholar]
- 132.Sen U., Mishra P.K., Tyagi N., Tyagi S.C. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem. Biophys. 2010;57:49–58. doi: 10.1007/s12013-010-9079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem. Int. 2013;63:492–497. doi: 10.1016/j.neuint.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 134.Dawe G.S., Han S.P., Bian J.S., Moore P.K. Hydrogen sulphide in the hypothalamus causes an ATP-sensitive K+ channel-dependent decrease in blood pressure in freely moving rats. Neuroscience. 2008;152:169–177. doi: 10.1016/j.neuroscience.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 135.Eto K., Asada T., Arima K., Makifuchi T., Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 136.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 137.Mard S.A., Neisi N., Solgi G., Hassanpour M., Darbor M., Maleki M. Gastroprotective effect of NaHS against mucosal lesions induced by ischemia-reperfusion injury in rat. Dig. Dis. Sci. 2012;57:1496–1503. doi: 10.1007/s10620-012-2051-5. [DOI] [PubMed] [Google Scholar]
- 138.Ju Y., Zhang W., Pei Y., Yang G. H(2)S signaling in redox regulation of cellular functions. Can. J. Physiol. Pharmacol. 2013;91:8–14. doi: 10.1139/cjpp-2012-0293. [DOI] [PubMed] [Google Scholar]
- 139.Dief A.E., Mostafa D.K., Sharara G.M., Zeitoun T.H. Hydrogen sulfide releasing naproxen offers better anti-inflammatory and chondroprotective effect relative to naproxen in a rat model of zymosan induced arthritis. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1537–1546. [PubMed] [Google Scholar]
- 140.Ekundi-Valentim E., Mesquita F.P., Santos K.T., de Paula M.A., Florenzano J., Zanoni C.I., Rodrigues L., de Nucci G., Teixeira S.A., Ferreira H.H., et al. A comparative study on the anti-inflammatory effects of single oral doses of naproxen and its hydrogen sulfide (H2S)-releasing derivative ATB-346 in rats with carrageenan-induced synovitis. Med. Gas Res. 2013;3:24. doi: 10.1186/2045-9912-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wallace J.L., Nagy P., Feener T.D., Allain T., Ditroi T., Vaughan D.J., Muscara M.N., de Nucci G., Buret A.G. A proof-of-concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wallace J.L., Vaughan D., Dicay M., MacNaughton W.K., de Nucci G. Hydrogen Sulfide-Releasing Therapeutics: Translation to the Clinic. Antioxid. Redox Signal. 2018;28:1533–1540. doi: 10.1089/ars.2017.7068. [DOI] [PubMed] [Google Scholar]
- 143.Kashfi K., Chattopadhyay M., Kodela R. NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Redox Biol. 2015;6:287–296. doi: 10.1016/j.redox.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kodela R., Chattopadhyay M., Velazquez-Martinez C.A., Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 2015;98:564–572. doi: 10.1016/j.bcp.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Benavides G.A., Squadrito G.L., Mills R.W., Patel H.D., Isbell T.S., Patel R.P., Darley-Usmar V.M., Doeller J.E., Kraus D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martelli A., Testai L., Breschi M.C., Blandizzi C., Virdis A., Taddei S., Calderone V. Hydrogen sulphide: Novel opportunity for drug discovery. Med. Res. Rev. 2012;32:1093–1130. doi: 10.1002/med.20234. [DOI] [PubMed] [Google Scholar]
- 147.B Baluchnejadmojarad T., Kiasalari Z., Afshin-Majd S., Ghasemi Z., Roghani M. S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur. J. Pharmacol. 2017;794:69–76. doi: 10.1016/j.ejphar.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 148.Chen Y., Xue R., Jin X., Tan X. Antiarthritic Activity of Diallyl Disulfide against Freund’s Adjuvant-Induced Arthritic Rat Model. J. Environ. Pathol. Toxicol. Oncol. 2018;37:291–303. doi: 10.1615/JEnvironPatholToxicolOncol.2018027078. [DOI] [PubMed] [Google Scholar]
- 149.Fiorucci S., Santucci L. Hydrogen sulfide-based therapies: Focus on H2S releasing NSAIDs. Inflamm. Allergy Drug Targets. 2011;10:133–140. doi: 10.2174/187152811794776213. [DOI] [PubMed] [Google Scholar]
- 150.Muzaffar S., Jeremy J.Y., Sparatore A., Del Soldato P., Angelini G.D., Shukla N. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: Role of protein kinases A and G. Br. J. Pharmacol. 2008;155:984–994. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu D., Hu Q., Zhu Y. Therapeutic application of hydrogen sulfide donors: The potential and challenges. Front. Med. 2016;10:18–27. doi: 10.1007/s11684-015-0427-6. [DOI] [PubMed] [Google Scholar]
- 152.Szychlinska M.A., D’Amora U., Ravalli S., Ambrosio L., Di Rosa M., Musumeci G. Functional Biomolecule Delivery Systems and Bioengineering in Cartilage Regeneration. Curr. Pharm. Biotechnol. 2019;20:32–46. doi: 10.2174/1389201020666190206202048. [DOI] [PubMed] [Google Scholar]
- 153.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 154.Mahmoudifar N., Doran P.M. Chondrogenesis and cartilage tissue engineering: The longer road to technology development. Trends Biotechnol. 2012;30:166–176. doi: 10.1016/j.tibtech.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 155.Vater C., Kasten P., Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011;7:463–477. doi: 10.1016/j.actbio.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 156.Zhang Q., Liu S., Li T., Yuan L., Liu H., Wang X., Wang F., Wang S., Hao A., Liu D., et al. Preconditioning of bone marrow mesenchymal stem cells with hydrogen sulfide improves their therapeutic potential. Oncotarget. 2016;7:58089–58104. doi: 10.18632/oncotarget.11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao H., Ma L., Gong Y., Gao C., Shen J. A polylactide/fibrin gel composite scaffold for cartilage tissue engineering: Fabrication and an in vitro evaluation. J. Mater. Sci. Mater. Med. 2009;20:135–143. doi: 10.1007/s10856-008-3543-x. [DOI] [PubMed] [Google Scholar]
- 158.Nguyen L.H., Kudva A.K., Saxena N.S., Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32:6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 159.Feng S., Zhao Y., Xian M., Wang Q. Biological thiols-triggered hydrogen sulfide releasing microfibers for tissue engineering applications. Acta Biomater. 2015;27:205–213. doi: 10.1016/j.actbio.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang C.C., Yang K.C., Lin K.H., Liu Y.L., Liu H.C., Lin F.H. Cartilage regeneration in SCID mice using a highly organized three-dimensional alginate scaffold. Biomaterials. 2012;33:120–127. doi: 10.1016/j.biomaterials.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 161.Nettles D.L., Vail T.P., Morgan M.T., Grinstaff M.W., Setton L.A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann. Biomed. Eng. 2004;32:391–397. doi: 10.1023/B:ABME.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 162.Almany L., Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials. 2005;26:2467–2477. doi: 10.1016/j.biomaterials.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 163.Trattnig S., Ohel K., Mlynarik V., Juras V., Zbyn S., Korner A. Morphological and compositional monitoring of a new cell-free cartilage repair hydrogel technology-GelrinC by MR using semi-quantitative MOCART scoring and quantitative T2 index and new zonal T2 index calculation. Osteoarthr. Cartil. 2015;23:2224–2232. doi: 10.1016/j.joca.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 164.Mauretti A., Neri A., Kossover O., Seliktar D., Di Nardo P., Melino S. Design of a Novel Composite H2 S-Releasing Hydrogel for Cardiac Tissue Repair. Macromol. Biosci. 2016;16:847–858. doi: 10.1002/mabi.201500430. [DOI] [PubMed] [Google Scholar]
- 165.Wu J., Li Y., He C., Kang J., Ye J., Xiao Z., Zhu J., Chen A., Feng S., Li X., et al. Novel H2S Releasing Nanofibrous Coating for In Vivo Dermal Wound Regeneration. ACS Appl. Mater. Interfaces. 2016;8:27474–27481. doi: 10.1021/acsami.6b06466. [DOI] [PubMed] [Google Scholar]
- 166.Cacciotti I., Ciocci M., Di Giovanni E., Nanni F., Melino S. Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress. Int. J. Mol. Sci. 2018;19:2368. doi: 10.3390/ijms19082368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gambari L., Amore E., Raggio R., Bonani W., Barone M., Lisignoli G., Grigolo B., Motta A., Grassi F. Hydrogen sulfide-releasing silk fibroin scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:471–482. doi: 10.1016/j.msec.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 168.Liang W., Chen J., Li L., Li M., Wei X., Tan B., Shang Y., Fan G., Wang W., Liu W. Conductive Hydrogen Sulfide-Releasing Hydrogel Encapsulating ADSCs for Myocardial Infarction Treatment. ACS Appl. Mater. Interfaces. 2019;11:14619–14629. doi: 10.1021/acsami.9b01886. [DOI] [PubMed] [Google Scholar]
- 169.Carter J.M., Qian Y., Foster J.C., Matson J.B. Peptide-based hydrogen sulphide-releasing gels. Chem. Commun. 2015;51:13131–13134. doi: 10.1039/C5CC04883D. [DOI] [PubMed] [Google Scholar]