Abstract
Auxin contributes to almost every aspect of plant development and metabolism as well as the transport and signalling of auxin-shaped plant growth and morphogenesis in response to endo- and exogenous signals including stress conditions. Consistently with the common belief that auxin is a central trigger of developmental changes in plants, the auxin treatment of explants was reported to be an indispensable inducer of somatic embryogenesis (SE) in a large number of plant species. Treating in vitro-cultured tissue with auxins (primarily 2,4-dichlorophenoxyacetic acid, which is a synthetic auxin-like plant growth regulator) results in the extensive reprogramming of the somatic cell transcriptome, which involves the modulation of numerous SE-associated transcription factor genes (TFs). A number of SE-modulated TFs that control auxin metabolism and signalling have been identified, and conversely, the regulators of the auxin-signalling pathway seem to control the SE-involved TFs. In turn, the different expression of the genes encoding the core components of the auxin-signalling pathway, the AUXIN/INDOLE-3-ACETIC ACIDs (Aux/IAAs) and AUXIN RESPONSE FACTORs (ARFs), was demonstrated to accompany SE induction. Thus, the extensive crosstalk between the hormones, in particular, auxin and the TFs, was revealed to play a central role in the SE-regulatory network. Accordingly, LEAFY COTYLEDON (LEC1 and LEC2), BABY BOOM (BBM), AGAMOUS-LIKE15 (AGL15) and WUSCHEL (WUS) were found to constitute the central part of the complex regulatory network that directs the somatic plant cell towards embryogenic development in response to auxin. The revealing picture shows a high degree of complexity of the regulatory relationships between the TFs of the SE-regulatory network, which involve direct and indirect interactions and regulatory feedback loops. This review examines the recent advances in studies on the auxin-controlled genetic network, which is involved in the mechanism of SE induction and focuses on the complex regulatory relationships between the down- and up-stream targets of the SE-regulatory TFs. In particular, the outcomes from investigations on Arabidopsis, which became a model plant in research on genetic control of SE, are presented.
Keywords: auxin, Aux/IAA, auxin signalling, ARFs, LAFL group genes, somatic embryogenesis, transcription factors
1. Introduction
The process of somatic embryogenesis (SE) demonstrates the unique developmental capacity of plants for switching on the embryogenic programme of development in somatic cells that have already differentiated. As a result of the embryogenic transition, bipolar structures that resemble zygotic embryos, which are called somatic embryos, are formed. Somatic embryos are able to develop into complete plants and since 1960s, numerous protocols have been established that enable the efficient regeneration of dozens of plant species via somatic embryos that are induced under in vitro culture conditions (reviewed in [1]). In addition to its wide implementation in the mass micropropagation of plants for the market, the SE-based protocols are also commonly used to produce transgenic plants (reviewed in [2]). In addition to its practical significance in plant biotechnology, the SE process provides a valuable model system for studies on plant embryogenesis and the developmental plasticity of the plant somatic cells [3,4]. Identifying the exo- and endogenous factors that promote embryogenic transition in in vitro-cultured somatic cells leads to a better understanding of plant cell totipotency. In contrast to advanced knowledge on the tissue culture conditions that promote SE induction [5], the endogenous factors that determine the tissue capacity for an embryogenic response are much less recognised. However, distinct progress in deciphering the molecular mechanism that governs the embryogenic transition of plant somatic cells has been made in the last decade and the role of auxin in SE induction has been intensively studied at the molecular level.
2. Auxin, a Main Inducer of SE
Since the classical demonstration of SE induction in carrot callus [6], the impact of auxin on the embryogenic pathways that are induced in in vitro-cultured plant tissues has become evident and hundreds of protocols have been established to induce SE in a plethora of plant species [7]. Although there are some differences between the in vitro culture conditions that are applied to induce SE in different plants, treating explants with auxin and auxin-like substances seems to be commonly required for SE induction. In support of this statement, auxin treatment was applied in 74 of the 80 protocols reviewed in Table S1 and auxin was frequently combined with cytokinins (65%). The most effective and commonly used inducer of SE (78% of the protocols) is 2,4-dichlorophenoxyacetic acid (2,4-D), which is a synthetic plant growth regulator of auxin activity.
3. Auxin- and Stressor-Like Activity of 2,4-D in SE Induction
2,4-D was initially used in cereal agriculture as a systemic herbicide that selectively kills broadleaf weeds [8]. Treatment with 2,4-D causes a number of morphological changes in plants in a concentration-dependent manner. 2,4-D efficiently stimulates cell division, plant growth and in vitro-induced morphogenetic responses at low concentrations of 1–10 mg/L, while spraying the plants in the field with 2,4-D of a concentration of about one-thousand-fold higher (2 g/L, according to the weed control manuals) caused lethal symptoms that were similar to an auxin overdose [9]. Of note, in spite of the distinctly higher tolerance of the monocot vs. the dicot plants to the toxic effects of 2,4-D [10], similar concentrations of this substance are applied to induce SE in different plants, and relevantly, 1–2 mg/L of 2,4-D is recommended for SE induction in maize and wheat [11,12,13,14], while 1.1 mg/L of 2,4-D is effective for establishing an embryogenic culture in Arabidopsis [15].
2,4-D shares structural similarity with indole-3-acetic acid (IAA), which involves the presence of a carboxyl group and an aryl ring structure [10]. However, in contrast to natural auxin, IAA, which is rapidly inactivated via conjugation and degradation in plant cells [16], the effects of 2,4-D on plants are long-lasting because of its high level of stability in the plant cells [17]. Moreover, the polar cell-to-cell transport of 2,4-D and IAA differs in terms of the auxin carriers that are engaged. Accordingly, in contrast to IAA, which is transported polarly by both types of auxin carriers, i.e., the efflux PIN-FORMED (PIN) and P-GLYCOPROTEIN (PGP) and the influx AUXIN1/LIKE-AUX1 (AUX/LAX) proteins, the transport of 2,4-D by the PIN efflux carriers is limited, which results in an accumulation of 2,4-D in plant cells [18]. Moreover, 2,4-D has a weak binding affinity to the IAA receptor, AUXIN-BINDING PROTEIN1 (ABP1) [19]. However, similar to IAA, 2,4-D is able to promote the binding of AUXIN/INDOLE-3-ACETIC ACID (Aux/IAA) proteins to the TRANSPORT INHIBITOR RESPONSE1 (TIR1) F-box protein of the auxin-signalling pathway after the auxin responsive genes are activated by the AuxRE (AUXIN RESPONSIVE ELEMENT) motif in the promotors [20,21]. It is worth noting that the 2,4-D and IAA-induced transcriptomes tend to overlap only to some extent [20], which reflects the differences between IAA and synthetic auxin in their interactions with the signalling machinery [22].
In addition to its auxin-like activity, 2,4-D is believed to affect the plant developmental processes by inducing the stress responses. Accordingly, treatment with 2,4-D causes an accumulation of ROS (reactive oxygen species) and the stress hormones ethylene and abscisic acid (ABA) in plant tissue [23,24,25]. Relevant to the stress-related mechanism of 2,4-D activity, the central role of stress factors, in particular, oxidative stress, in promoting the developmental switches in plant cells including the induction of SE has been widely demonstrated (reviewed in [26,27]). In support of the stressor-like activity of 2,4-D, numerous stress-responsive genes were found to be differently expressed in 2,4-D-induced SE-transcriptomes [28,29,30,31,32,33].
However, a long-lasting debate on the auxin- vs. stress-like activity of 2,4-D seems to be groundless given that the complex relationships between auxin and stress responses in controlling plant development have been documented, and relevantly, different stress factors impact auxin signalling and biosynthesis [34] (reviewed in [35]). Moreover, 2,4-D treatment also activated the core regulators of the auxin-signalling AUXIN RESPONSE FACTORs (ARFs) and the TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1/YUCCA, TAA1/YUC-dependent auxin biosynthesis pathway in SE-induced explants of Arabidopsis [36,37]. Thus, both the auxin- and stressor-like intrinsic function of 2,4-D are closely related in promoting embryogenic development in plant cells.
4. Biosynthesis and Accumulation of Auxin During SE
The observation that the embryogenic explants of various plants contain a higher level of IAA than the non-embryogenic explants implies that there is a positive relationship between the auxin content and the capacity of tissue to induce SE [38,39,40,41,42,43,44,45]. However, in conifers, the SE-recalcitrant cultures had a higher level of IAA [46,47], which suggests that the auxin content that enables SE induction seems to be genotype and tissue specific. It seems that not the initial level of IAA in explant tissue but rather a dynamic increase of the endogenous auxin content in response to the SE-induction factors might provide an indispensable signal for the embryogenic transition. Relevantly, a significant increase in the IAA content was reported to accompany early SE induction in different plants including carrot [48], sunflower [49], robusta coffee [50,51,52], alfalfa [53], pineapple guava [54], Arabidopsis [36], cotton [55] and Norway spruce [56]. Besides in vitro culture conditions, transgenic plants of Arabidopsis that produced somatic embryos in planta as a result of the overexpression of the LEAFY COTYLEDON2 (LEC2) gene were also found to accumulate IAA [57]. Auxin accumulation in plant tissue in response to the SE-induction signal resembles the early stages of ZE. Accordingly, an increase in the endogenous auxin level was observed in the early-stage zygotic embryos of carrot [58] and other plants [54]. In the ZE of Arabidopsis, IAA is maternally biosynthesised de novo in the fertilised ovules and provides a source of auxin for the correct embryo development [59,60]. The auxin accumulation that accompanies the embryogenic development that is induced in both generative and somatic plant cells provides further support for a similarity in the molecular and hormonal signals that control embryogenic development in vivo and in vitro [3,61].
Two alternative metabolic pathways, the tryptophan-dependent and -independent control de novo IAA biosynthesis in plant development including zygotic embryo development [59,62,63]. In an SE culture of Arabidopsis, a TAA1/YUC pathway, which is controlled by the tryptophan aminotransferases TAA1 and TRYPTOPHAN AMINOTRANSFERASE RELATED1-4 (TAR) and YUCCA flavin-dependent monooxygenases, has been identified [36]. Six of eleven YUC genes, YUC1, YUC2, YUC4, YUC6, YUC10 and YUC11, were found to be expressed in different embryogenic cultures of Arabidopsis [36,64,65]. It is worth noting that the SE-associated YUCs were specifically expressed in the shoot part of a plant and that most of them were active in ZE [66,67]. The expression of the SE-involved YUCs was localised in the SE-involved explant part, i.e., in the cotyledons of the immature zygotic embryo, and that the relevant single and multiple yuc mutants, including yuc2, yuc4 and yuc1, yuc4, yuc10 and yuc11 had a severely reduced capacity for SE [36,65,68]. At the transcriptional level, auxin biosynthesis is regulated by the developmental and environmental signals that control the binding of specific TFs to the auxin biosynthesis-related genes including the YUCs [69]. Thus, the set of SE-involved YUC genes might differ among the plants, explants and treatment that is applied. Accordingly, in Arabidopsis, the 2,4-D treatment of immature zygotic embryos (IZE) explants activated YUC1, YUC4 and YUC10 [36], while only two of these genes were stimulated in the developing somatic embryos of the trichostatin A-induced IZE explants [68]. Ethylene was indicated to control the YUC expression and the negative impact of ethylene on SE induction in Arabidopsis was attributed to the inhibition of the YUCs, and subsequently, a disturbed auxin accumulation in the culture [65].
In line with the results on Arabidopsis, a RNA-seq analysis of the SE-transcriptome in cotton revealed the up-regulation of numerous genes that are involved in the tryptophan-dependent auxin biosynthesis, including the YUCs, TRP1 (TRYPTOPHAN BIOSYNTHESIS1), ASB1 (ANTHRANILATE SYNTHASE), TSB2 (TRYPTOPHAN SYNTHASE Β-SUBUNIT2), NIT4 (NITRILASE4) and CM1 (CHORISMATEMUTASE) [70,71]. An increase of the YUC1 and TAA1 transcripts that correlated with an increased level of endogenous IAA was also observed in the embryogenic explants of coffee [50]. Thus, the tryptophan-dependent TAA1-YUC pathway of IAA biosynthesis seems to commonly contribute to the auxin accumulation that is associated with the embryogenic transition in the somatic cells of plants. However, insights into the available RNA-seq data of an O. sativa embryogenic culture [72] indicated only a limited number of auxin biosynthesis-related transcripts and suggested a much less intensive activity of the TAA1-YUC IAA biosynthesis pathway in this plant than the one in Arabidopsis [73]. However, understanding the auxin biosynthesis pathways in O. sativa is still not well developed and further analyses are needed to reach a conclusion about the SE-associated auxin production in the model plants Arabidopsis vs. O. sativa.
5. The Core Regulatory Components of the Auxin-Signalling Pathway That Are Involved in SE
A key insight from the analyses of the SE-related transcriptomes in numerous plants, including wheat [74], palm [32], cotton [70,71,75], Arabidopsis [64,76], camphor tree [77], mangosteen [78], cork oak [79], Indian bean tree [80], coffee [81], maize [11] and lily [82], is that besides auxin biosynthesis other aspects of auxin action including auxin perception, polar transport and response/signalling are also involved in the mechanism of SE induction.
The core components of the auxin-signalling pathway that translate auxin sensing into transcriptional responses include the SKP/CULLIN/F-BOX-ubiquitin (SCFTIR1) complex, the Aux/IAA transcriptional co-regulators and the sequence-specific binding proteins, which are called ARFs. The SCFTIR1complex comprises TIR1, the AUXIN-SIGNALLING F-BOX1 (AFB1), AFB2 and AFB3 proteins, which are members of the clade of the auxin receptors family (TAARs). TAARs are a component of the SCF-ligase complexes, which direct the members of the AUX/IAA transcriptional repressor protein family to a proteasome-dependent degradation. The degradation of AUX/IAA releases the TOPLESS (TPL) transcriptional co-repressor and allows the ARF proteins to bind at the promoters of the primary auxin responsive genes in order to regulate their transcription [83]. Importantly for the role of auxin in establishing the SE-transcriptome in the explant cells, the specific expression pattern of the auxin-responsive genes is likely due to the specific cellular concentrations of several of the Aux/IAA proteins that seem to result from the auxin content in a cell. However, a quantitative relationship between cellular auxin concentration and gene activity has not yet been indicated, and whether there are separable gene sets that only respond to low or high auxin concentrations remains to be determined [69].
Auxin regulates the specific interactions between the Aux/IAAs and ARFs and almost all of the Aux/IAA proteins can interact with a subset of the ARFs with a long, Q-rich middle region (activating ARFs), whereas interactions with other ARFs are both limited and more specific [84]. In Arabidopsis, 29 and 23 members of the AUX/IAA and ARF gene families have been identified, respectively, and specific AUX/IAA-ARF interactions are assumed to control the auxin-dependent developmental processes including ZE [84,85]. Therefore, identifying the spatiotemporal and cell-specific sets of the AUX/IAA elements and the interacting ARFs that contribute to the embryogenic transition of somatic cells is required for deciphering auxin-mediated SE induction.
The transcripts encoding AUX/IAAs and ARFs are highly represented in the SE-transcriptomes of Arabidopsis [76,86] and other plants, including cyclamen [87], wheat [74], palm [32,88], cotton [71], camphor tree [77], mangosteen [78], cork oak [79], Indian bean tree [80], coffee [81], maize [11] and lily [82]. Insight into the SE-transcriptomes revealed that the expression profiles of the AUX/IAA and ARF genes are highly diverse and that the same gene might show both a significant up- and down-regulation depending on the embryogenic system (Table S2). The high degree of the diversity of the expression patterns of the ARF genes reflect the complexity of the transcriptional regulation of these genes, which are fine-tuned by numerous factors including the hormonal status of a tissue [84]. The direct regulation of the SE-expressed ARFs by auxin might be considered and an auxin-responsive AuxRE module was found in the majority (84%) of the SE-modulated ARFs in Arabidopsis, including ARF5, ARF6, ARF8, ARF10 and ARF16 [37].
In contrast to the numerous SE-transcriptomic data, functional analyses of the specific auxin-signalling genes that are engaged in SE are still rare and limited almost exclusively to Arabidopsis. A major drawback for such an analysis seems to be the high functional redundancy within members of the TAAR, AUX/IAA and ARF families and thus, the frequently unobvious and pleiotropic phenotype of the relevant mutant plants [84,85]. Analysis of plants with impaired TAAR receptors of auxin, the tir1-1 and afb2-3 mutants, indicated their hyposensitivity to 2,4-D treatment and a reduced embryogenic response in vitro and the TIR1 and AFB2 genes were also postulated to control SE induction via an miR393-mediated mechanism [89]. In addition to Arabidopsis, the down-regulation of TIR1 and AFB2 was also observed during SE in cotton but the regulatory pathway that controls their expression has not yet been identified [70,75,90]. Considering that the TAAR proteins have a different binding affinity to auxin herbicides [91], the high binding affinity of the SE-involved TIR1 and AFB2 to the 2,4-D that is used for SE induction in Arabidopsis might be expected [89].
The mutations in the IAA16, IAA29, IAA30 and IAA31 genes that resulted in an impaired embryogenic response of the Arabidopsis explants suggests the engagement of these genes in SE induction [76,86]. The expression of the SE-involved AUX/IAA genes seems to be regulated by the TFs that have a master function in the embryogenic transition, and relevantly, AGAMOUS-LIKE15 (AGL15) controls the expression of IAA16 and IAA30, while LEAFY COTYLEDON2 (LEC2), which interacts with AGL15 in a feedback regulatory loop (see next paragraph), directly targets IAA30 [86,92].
In accordance with SE-transcriptome data that suggest that the majority of the ARF genes seem to be engaged in controlling SE (Table S2), numerous arf mutants arf1-3, arf3, arf5-8, arf6, arf7, arf8 were found to be impaired in their embryogenic response [37]. It is noteworthy that the mutants in the ARF genes of the contrasting SE-expression profiles were found to display a reduced embryogenic response, and accordingly, the mutations in ARF5/ARF6 and ARF3 with an up- and down-regulated expression in SE, respectively, were indicated to negatively affect SE induction [37]. The lack of a direct relationship between the ARF gene expression profile and the phenotype of the relevant arf mutant implies the complexity of the factors that fine tune the ARF-mediated responses during SE induction. These factors include the distinct spatio-temporal and hormone-dependent regulation of ARF gene expression and the complex regulatory interactions within the ARF family members and between the ARFs and other components of the auxin-signalling pathway [37,84].
Several pieces of evidence suggest that similar to ZE (reviewed in [93]), ARF5, which encodes the MONOPTEROS (MP) protein, might have a fundamental role in regulating different auxin-controlled aspects of SE. Accordingly, ARF5 was observed to be the most highly up-regulated member of the ARF family during SE induction and the arf5 mutant had a significantly reduced capacity for an embryogenic response [37]. One of the functions of ARF5 in SE seems to be connected with regulating PHABULOSA (PHB), which is a positive regulator of LEC2 in ZE [94] and in support of this assumption, in contrast to the up-regulated expression of PHB and LEC2 in a WT embryogenic culture, an arf5 mutant culture displayed a significantly reduced LEC2 transcript level [37,95]. A regulatory ARF5-PHB connection was observed in vivo [96]. Thus, the ARF5 protein was postulated as controlling the TAA1-YUC-mediated pathway of auxin biosynthesis that operates in SE induction by regulating PHB and LEC2 [36]. In line with this hypothesis, ARF5 targets the components of this pathway (TAA1 and YUC1, YUC8) in order to transcriptionally initiate the embryonic ground tissue in an early zygotic embryo [97]. ARF5 might also contribute to the in vitro-induced embryogenic development by controlling the other TF genes that have a documented activity in SE, including HOMEOBOX GENE8 (ATHB8) and TARGET OF MONOPTEROS3 (TMO3), TMO5, TMO6 and TMO7 [64,76]. In support of this assumption, the regulatory relationships between these TFs and ARF5 were identified in planta [98,99]. Interestingly, the TMO7, which is a direct ARF5 target encoding a small TF, moves intercellularly to control the embryonic root specification [99]. Hence, it is tempting to question whether ARF5 also controls the mobility of TOM7 during SE induction and how the ARF5-TOM7 regulation could contribute to establishing the embryonic identity in the somatic cells of an explant.
Many members of the Aux/IAA family have been shown to interact with ARF5 in the development of Arabidopsis in planta [100] and among them is IAA30 of indicated involvement in SE [76,92]. The possible function of ARF5 may also involve regulating the PIN genes, which control the polar auxin transport during in vivo development [101,102] and in support of this statement, a pin1-7 mutant had a reduced embryogenic induction in vitro [103] and the 35S::ARF5 line, which phenocopies the pin1 mutant [104], was found to be incapable of inducing SE [37]. In order to stimulate the expression of PIN1 and to control early zygotic embryo patterning, ARF5 cooperates with the WUSCHEL-RELATED HOMEOBOX (WOX) TF gene family members, including WOX1, WOX2, WOX5, WOX8 and WOX9 [105,106,107]. Although the expression of the WOX2, WOX8 and WOX9 genes was reported in an embryogenic culture of several plants, including grapevine, spruce, Arabidopsis, cotton, barrelclover, larch and longan [64,75,108,109,110,111,112,113,114], the auxin-signalling regulators that control the WOX genes in SE induction have not been studied as yet. In particular, WOX2 seems to be an interesting ARF5 target candidate in regulating SE because of its role in balancing the cytokinin versus the auxin hormone pathways during the establishment of the shoot identity in the zygotic embryo [115]. Relevantly, ARF10, which has a positive regulatory impact on de novo shoot regeneration via the activation of the shoot meristem-specific genes, was highly expressed in the SE of Arabidopsis [37,116]. Therefore, ARF10 together with ARF16 and miR160 have been postulated to contribute to the LEC2-controlled auxin biosynthesis in SE-induced tissue [95]. Besides LEC2, other SE-related candidate targets of ARF10/ARF16 involve the PLETHORA (PLT) genes, which have an essential function in integrating the hormonal inputs during many developmental processes in plants including the auxin responses in the early embryogenic induction (reviewed in [117]). Because ARF10 and ARF16 act upstream of the PLTs to regulate the stem cell differentiation in Arabidopsis roots [118], the significant accumulation of ARF10 and ARF16 transcripts was accompanied by an increased expression of PLT1 and PLT2 in an embryogenic culture of Arabidopsis [37,64,76].
Data on ARF expression profiling in the SE of Arabidopsis and other plants also suggests that ARF6, ARF7, ARF8, ARF9 and ARF19 (Table S2) play a role in controlling SE induction; however, their specific functions and the SE-involved targets have not yet been identified.
6. Complex Interactions Between the TF Genes that Control Auxin-Induced SE
The transcripts of the TF genes that are related to hormone responses, in particular those that control auxin metabolism and signalling are overrepresented in the SE-transcriptomes of many plants (reviewed in [119]). In Arabidopsis, over 40% of the SE-modulated TF genes have been annotated to hormone-related pathways, primarily to the auxin metabolism and signalling pathways [76]. The best recognised TF-regulatory network that controls SE induction includes the LAFL group of genes (for LEC1/L1L, ABSCISIC ACID INSENSITIVE3 (ABI3), FUSCA3 (FUS3) and LEC2), which have a major and redundant regulatory function in controlling the identity and maturation of a zygotic embryo [120,121]. The overexpression of two of the LAFL genes, LEC1 and LEC2, in Arabidopsis seedlings resulted in somatic embryo formation, while the lec1 and lec2 mutations significantly inhibited the embryogenic response of the in vitro cultured explants [122,123,124]. LEC1 and LEC2 gene expression is co-localised with the SE-involved parts of the 2,4-D-treated explants [125,126,127]. Importantly, LEC1 and LEC2 seem to have a common SE-promoting function in plants and the overexpression of these TFs has been suggested as an efficient method for improving the embryogenic response in several crop species, including cassava, rapeseed, tobacco and cacao [128,129,130,131]. Taken together, these observations led to a conclusion on universal and essential function of LEC1/LEC2 in controlling both the somatic and zygotic embryonic development in higher plants (reviewed in [132]).
The diversity of the LEC1 and LEC2 targets implies that these proteins contribute to the regulation of various auxin-related processes, which involve the control of auxin biosynthesis (see the paragraph ‘Biosynthesis and Accumulation of Auxin During SE’) and relevantly, members of the Aux/IAA family were postulated to be under the control of LEC1 (IAA5, IAA16 and IAA19) and LEC2 (IAA1, IAA17, IAA30 and IAA31) during embryonic development [92,133,134]. The impact of LEC2 on auxin polar transport also cannot be ruled out because the upregulation of the auxin efflux facilitators, PIN1 and PIN2, was observed in the transgenic tobacco plants that over-expressed LEC2 [129]. Moreover, the auxin-responsive AuxRE motif, which is present in the LEC1 and LEC2 promotors, is evidence of the involvement of the ARF proteins in controlling the auxin-stimulated expression of these genes [37]. The candidate ARFs that control LEC1/LEC2 in the SE-induced explants might be searched for among the ARFs that have an up-regulated expression in embryogenic cultures (Table S2).
In addition to their auxin-related function, the LEC genes (LEC1 and LEC2 together with FUS3) seem to control the ability of explants to respond to SE via the control of the gibberellins (GA)/ abscisic acid (ABA) balance in tissue. Accordingly, AGL15, which is an activator of the GA2ox6 gene that encodes an enzyme that inactivates bioactive gibberellin, was identified among the direct targets of LEC2 [92,132,135]. AGL15 encodes a MADS box TF that increases the embryogenic competency of tissue when it is ectopically expressed [136]. The expression of AGL15 is autoregulated [137] and regulated by feedback interactions with other TFs, which involve the direct regulation of AGL15 by LEC2 and FUS3 and the control of LEC2 and FUS3 by AGL15 [86,92,138,139].
Insights into the AGL15-overexpression transcriptomes of Arabidopsis and soybean showed that AGL15 might enhance the embryogenic competency of the explants by promoting tissue dedifferentiation [140] and also revealed the complex interactions of AGL15 with the hormone pathways including auxin signalling [141]. Accordingly, AGL15 was demonstrated to negatively regulate the auxin-signalling components, including the direct repression of ARF6 and TIR1 ubiquitin ligase, the indirect repression of ARF8 and the direct up-regulation of IAA30 [86,141].
The SE-regulatory network of TFs also involves members of the AINTEGUMENTA-LIKE (AIL) family of the APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF) domain TFs [117]. The small AIL family comprises eight members, including the AINTEGUMENTA (ANT), AIL, BABY BOOM (BBM/PLT4) and five other PLT proteins that have overlapping functions in the dividing cells of meristematic and embryonic tissue [142,143]. Of all of the AIL TFs, a central position in regulating SE has been attributed to BBM/PLT4; however, other PLTs (PLT1, PLT2, PLT3, EMK/PLT5 and PLT7) also promote embryogenic induction in response to its overexpression [121,144,145]. Recently, PLT1/PLT2 was reported to control the LEC2 function in auxin-induced SE and the miR396–GRF module has been postulated to control the PLT2-LEC2 interaction [146].
To induce SE, LEC1 and LEC2 are positively regulated by BBM, which is another major TF protein that controls the identity and totipotency of the plant embryo, and the SE-promoting activity of BBM requires the LAFL/AGL15 proteins [147]. Knowledge about the other functionally characterised targets of BBM is limited. In search of direct BBM targets, a set of mostly uncharacterised genes that have assumed functions in transcription, cellular signalling and cell wall biosynthesis was identified including the ACTIN DEPOLYMERISING FACTOR9 (ADF9) gene, which encodes an ADF/cofilin protein [148]. Auxin-related genes were revealed within the BBM targets that are involved in SE in Arabidopsis including the genes that are associated with the biosynthesis (TAA1, YUC3 and YUC8), transport (PIN1 and PIN4) and signalling of auxin (ARF2, ARF6, ARF10, IAA2, IAA7 and IAA28) [121]. In addition, a possible role of PLTs in the auxin-related mechanism of SE induction implies the activation of the YUC genes (YUC1 and YUC4) by PLT3, EMK/PLT5 and PLT7 in order to stimulate auxin biosynthesis in phyllotaxis [149].
The activators of the LEC-mediated SE pathway also involve the class III HOMEODOMAIN LEUCINE ZIPPER (HD-ZIP III) TFs, PHB and PHAVOLUTA (PHV), which activate LEC2 in the vegetative development of Arabidopsis [94]. Similarly, PHB/PHV and LEC2 might interact in SE through a positive feedback loop, which, under the repression of miR166, was postulated to control the YUC-pathway of auxin biosynthesis in explants that were undergoing embryogenic induction [95].
Several TFs have been recognised to negatively control the LAFL genes. The PICKLE (PKL) protein of the CHD3-type ATP-dependent SWI/SNF chromatin-remodelling factors impacts the global H3K27me3 levels including LEC1 and LEC2 [150,151]. In addition, the VIVIPAROUS1/ABI3-LIKE (VAL)/HIGH-LEVEL (VAL1) and VAL2 proteins that like ABI3, FUS3 and LEC2 have a B3 domain were postulated to function as global regulators of the LEC1/B3 gene system, which controls embryo identity in seedlings [152]. The interactions of the VALs with the chromatin factors and the gene repression mechanism that involves the VAL-mediated recruitment of HDAC histone deacetylases and Polycomb group proteins to the target loci were recognised [153,154].
An SE-promoting capacity was also indicated for WUSCHEL (WUS), which is a member of the WOX superfamily of the genes encoding the plant-specific homeobox TFs that are involved in promoting cell division and/or preventing the premature cell differentiation of the stem cells in SAM (reviewed in [155,156]). The positive impact of WUS on the embryogenic potential of tissue was indicated given that the gain-of-function mutation in WUS led to the spontaneous formation of somatic embryos on Arabidopsis root explants [157] and an overexpression of WUS promoted or enhanced the embryogenic response in other plants [158,159,160]. Auxin treatment was found to be essential for correctly regulating the WUS expression and the WUS transcripts that were detected in a group of embryogenic callus cells were postulated to indicate an auxin accumulation in the SAM and the pro-embryo [103]. Although the ectopic expression of AtWUS in transgenic cotton callus was associated with the upregulation of the auxin biosynthesis-regulators, the LEC1 and LEC2 genes [160], the content of IAA was not modulated [161]. Hence, the contribution of WUS to the TF-regulatory network of SE seems to be unrelated to auxin biosynthesis and in support of this assumption, within the numerous auxin-related targets of WUS, the genes that are involved in auxin biosynthesis have not been identified [162]. In the mechanism of the WUS-mediated control of stem cells in SAM, WUS acts as an auxin rheostat that controls the target loci including numerous genes of the auxin-signalling pathway by regulating histone acetylation [162].
Insights into ZE regulation suggest that in addition to WUS, other members of the WOX genes family might also interact with the LEC-controlled pathway of SE induction. Accordingly, during stem cell initiation in early zygotic embryo, the WOX genes (WOX2 and its paralogs WOX1, WOX3 and WOX5) were shown to positively control the PHB/PHV TFs that activate the LEC genes in both zygotic and somatic embryogenesis [95,115]. Similar to ZE, the regulatory interaction between the WOXs and PHB/PHV might be expected during somatic embryo formation because the expression of both WOX5 and PHB/PHV was indicated in the embryogenic and callus cells of Arabidopsis [163]. Moreover, the WOX2, WOX8 and WOX9 transcripts accompany different stages of somatic embryo development in different plants [64,75,108,109,110,111,112,113,114]. However, the regulatory interactions between the WOX genes and the LEC-controlled SE pathway require experimental validation.
7. Conclusions and Future Prospects
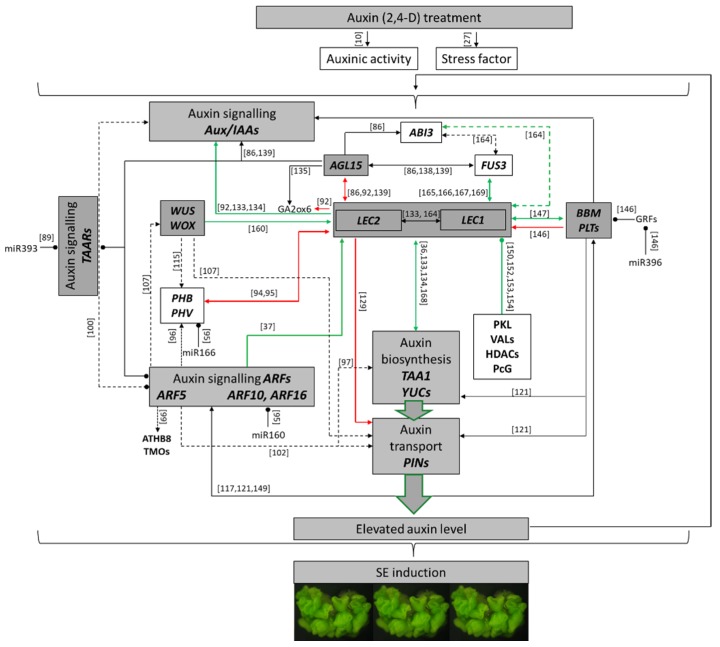
In the past ten years, omics data analysis together with the candidate gene approach have significantly contributed to identifying the regulatory interactions between the TFs that have a key function in SE and the phytohormones, mainly auxin. A revealing picture of the SE-regulatory gene network reveals a high level of the complexity of the extensive crosstalk between the SE-TFs and auxin during the auxin- inducted embryogenic development in somatic plant tissue (Figure 1).
Figure 1.
An overview of the regulatory interactions that control SE induction. Treating explants with auxin (mostly 2,4-D) activates a number of the TF genes that have a regulatory function in embryonic development, including the LEC1 and LEC2 of the LAFL group, BBM (PLTs), AGL15 and WUS/WOX genes. The down-stream targets include the genes of the TAA1/YUC of the auxin biosynthesis pathway, the PINs that control auxin transport and the core regulators of auxin signalling (AUX/IAAs and ARFs). Other regulatory elements of the SE-network also involve miRNAs and the epigenetic regulators (PKL, VALs, HDACs). The arrows and circle-shaped ends indicate the activation or repression of gene expression, respectively. The solid and dashed lines indicate the experimentally validated and suggested regulatory interactions, respectively. The red and green lines indicate the regulatory interaction between LEC2 and LECs, respectively [164,165,166,167,168,169].
Significant progress in establishing the genetic network that controls SE has been observed and both the down-stream targets and up-stream regulators of the SE-involved TFs have been identified and their functions in relation to auxin have been revealed. However, knowledge about the role of auxin in the regulatory network that controls SE induction is still fragmentary since in addition to gene transcription, auxin regulates a plethora of other cellular processes, including the epigenetic state of chromatin, protein stability/protein-protein interactions and metabolic pathways. Moreover, the interactions of auxin with other hormone pathways, in particular cytokinins, need to be explored in cells that are undergoing SE induction. Since only a small subset of explant cells is capable of embryogenic transition, the methods that enable a single-cell resolution analysis would provide new insights into the auxin-mediated regulatory network that governs the unique developmental plasticity that is demonstrated by somatic plant cells.
The ongoing debate on the SE regulatory network also concerns the degree of the molecular similarity of the embryogenic development that is induced in vitro in somatic tissue to the ZE process. In accordance with the common belief of a close similarity of the regulatory pathways that control both types of plant embryogenesis, several master regulators of ZE, including LEC, PLT and WOX TFs, were found to play a decisive role in the embryogenic reprogramming of plant somatic cells. Moreover, the auxin-related processes including auxin biosynthesis and signalling was placed in centre of SE and ZE regulation. However, recent RNAseq data of an embryogenic culture of Arabidopsis and Pinus pinaster showed that the SE-transcriptome seems to be distinctly different from the transcriptome of a zygotic embryo [170,171]. This unexpected finding suggests that the molecular events that trigger SE induction might differ from the ones that operate during ZE and points to the epigenetic processes that orchestrate the transcriptomes of in vitro cultured somatic cells in response to plethora of endo- and exogenous factors, thus making them different from those found in ZE. Therefore, identifying the epigenetic processes that contribute to the embryogenic transition in somatic plant cells in response to auxin would lead to further progress in revealing the SE regulatory network.
Abbreviations
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| AFB | AUXIN F-BOX PROTEIN |
| AGL15 | AGAMOUS-LIKE15 |
| ARF | AUXIN RESPONSE FACTOR |
| Aux/IAA | AUXIN/INDOLE-3-ACETIC ACID |
| AuxRE | AUXIN RESPONSIVE ELEMENT |
| BBM | BABYBOOM |
| HDAC | HISTONE DEACETYLASE |
| IAA | indole-3-acetic acid |
| IZE | immature zygotic embryos |
| LAFL | LEC1, ABI3, FUS3, LEC2 |
| LEC | LEAFY COTYLEDON |
| miRNA | microRNA |
| PIN | PIN-FORMED |
| PHB | PHABULOSA |
| PHV | PHAVOLUTA |
| PLT | PLETHORA |
| SAM | shoot apical meristem |
| SE | somatic embryogenesis |
| TAA1 | TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 |
| TAAR | TIR1/AFBs clade of Auxin Receptors |
| TAR | TRYPTOPHAN AMINOTRANSFERASE RELATED |
| TF | transcription factor |
| TIR1 | TRANSPORT INHIBITOR1 |
| YUC | YUCCA |
| WOX | WUSCHEL-RELATED HOMEOBOX |
| WUS | WUSCHEL |
| ZE | zygotic embryogenesis |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/4/1333/s1.
Author Contributions
All of the authors listed have made a substantial, direct and intellectual contribution to the work and approved it for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant from the National Science Centre in Poland (OPUS13 2017/25/B/NZ1/01615).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Monja-Mio K.M., Herrera-Alamillo M.Á., Robert M.L. Somatic embryogenesis in temporary immersion bioreactors. In: Loyola-Vargas V., Ochoa-Alejo N., editors. Somatic Embryogenesis: Fundamental Aspects and Applications. 1st ed. Springer International Publishing; Cham, Switzerland: 2016. pp. 435–454. [Google Scholar]
- 2.Ochoa-Alejo N. The uses of somatic embryogenesis for genetic transformation. In: Loyola-Vargas V., Ochoa-Alejo N., editors. Somatic Embryogenesis: Fundamental Aspects and Applications. 1st ed. Springer International Publishing; Cham, Switzerland: 2016. pp. 415–434. [Google Scholar]
- 3.Ikeda M., Kamada H. Comparison of molecular mechanisms of somatic and zygotic embryogenesis. In: Mujib A., Samaj J., editors. Somatic Embryogenesis. 1st ed. Springer; Berlin, Heidelberg: 2006. pp. 51–68. [Google Scholar]
- 4.Wójcikowska B., Gaj M.D. Somatic embryogenesis in Arabidopsis. In: Loyola-Vargas V., Ochoa-Alejo N., editors. Somatic Embryogenesis: Fundamental Aspects and Applications. 1st ed. Springer International Publishing; Cham, Switzerland: 2016. pp. 185–199. [Google Scholar]
- 5.Gaj M.D. Factors influencing somatic embryogenesis induction and plant regeneration with particular reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regul. 2004;43:27–47. doi: 10.1023/B:GROW.0000038275.29262.fb. [DOI] [Google Scholar]
- 6.Reinert J. Morphogenese und ihre Kontrolle an Gewebekulturen aus Carotten. Naturwissenschaften. 1958;45:344–345. doi: 10.1007/BF00640240. [DOI] [Google Scholar]
- 7.Mujib A. Somatic Embryogenesis in Ornamentals and Its Applications. Springer; New Delhi, India: 2016. pp. 67–86. [Google Scholar]
- 8.Serbent M.P., Rebelo A.M., Pinheiro A., Giongo A., Tavares L.B.B. Biological agents for 2,4-dichlorophenoxyacetic acid herbicide degradation. Appl. Microbiol. Biotechnol. 2019;103:5065–5078. doi: 10.1007/s00253-019-09838-4. [DOI] [PubMed] [Google Scholar]
- 9.Teixeira M.C., Duque P., Sá-Correia I. Environmental genomics: Mechanistic insights into toxicity of and resistance to the herbicide 2,4-D. Trends Biotechnol. 2007;25:363–370. doi: 10.1016/j.tibtech.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Song Y. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J. Integr. Plant Biol. 2014;56:106–113. doi: 10.1111/jipb.12131. [DOI] [PubMed] [Google Scholar]
- 11.Juárez-González V.T., López-Ruiz B.A., Baldrich P., Luján-Soto E., Meyers B.C., Dinkova T.D. The explant developmental stage profoundly impacts small RNA-mediated regulation at the dedifferentiation step of maize somatic embryogenesis. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delporte F., Muhovski Y., Pretova A., Watillon B. Analysis of expression profiles of selected genes associated with the regenerative property and the receptivity to gene transfer during somatic embryogenesis in Triticum aestivum L. Mol. Biol. Rep. 2013;40:5883–5906. doi: 10.1007/s11033-013-2696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.She M., Yin G., Li J., Li X., Du L., Ma W., Ye X. Efficient regeneration potential is closely related to auxin exposure time and catalase metabolism during the somatic embryogenesis of immature embryos in Triticum aestivum L. Mol. Biotechnol. 2013;54:451–460. doi: 10.1007/s12033-012-9583-y. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadpour R., Zare N., Asghari-zakarta R., Sheikhzadeh P. Efficient in vitro somatic embryogenesis and plant regeneration from mature and immature embryos of wheat (Triticum aestivum L.) Braz. Arch. Biol. Technol. 2016;59:1–12. doi: 10.1590/1678-4324-2016160288. [DOI] [Google Scholar]
- 15.Gaj M.D. Direct somatic embryogenesis as a rapid and efficient system for in vitro regeneration of Arabidopsis thaliana. Plant Cell. Tissue Organ Cult. 2001;64:39–46. doi: 10.1023/A:1010679614721. [DOI] [Google Scholar]
- 16.Grossmann K. Auxin herbicides: Current status of mechanism and mode of action. Pest. Manag. Sci. 2010;66:113–120. doi: 10.1002/ps.1860. [DOI] [PubMed] [Google Scholar]
- 17.Eyer L., Vain T., Pařízková B., Oklestkova J., Barbez E., Kozubíková H. 2,4-D and IAA amino acid conjugates show distinct metabolism in Arabidopsis. PLoS ONE. 2016;11:e0159269. doi: 10.1371/journal.pone.0159269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rybel B., Audenaert D., Beeckman T., Kepinski S. The past, present, and future of chemical biology in auxin research. Acs Chem. Biol. 2009;4:987–998. doi: 10.1021/cb9001624. [DOI] [PubMed] [Google Scholar]
- 19.Teale W.D., Paponov I.A., Palme K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 20.Pufky J., Qiu Y., Rao M.V., Hurban P., Jones A.M. The auxin-induced transcriptome for etiolated Arabidopsis seedlings using a structure/function approach. Funct. Integr. Genom. 2003;3:135–143. doi: 10.1007/s10142-003-0093-7. [DOI] [PubMed] [Google Scholar]
- 21.Tan X., Calderon-Villalobos L.I.A., Sharon M., Zheng C., Robinson C.V., Estelle M., Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 22.Woodward A.W., Bartel B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen H., Grossmann K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 2000;124:1437–1448. doi: 10.1104/pp.124.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arteca R.N., Arteca J.M. Effects of brassinosteroid, auxin, and cytokinin on ethylene production in Arabidopsis thaliana plants. J. Exp. Bot. 2008;59:3019–3026. doi: 10.1093/jxb/ern159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pazmino D.M., Rodriguez-Serrano M., Romero-Puertas M.C., Archilla-Ruiz A., Del Rio L.A., Sandalio L.M. Differential response of young and adult leaves to herbicide 2,4-dichlorophenoxyacetic acid in pea plants: Role of reactive oxygen species. Plant Cell Environ. 2011;34:1874–1889. doi: 10.1111/j.1365-3040.2011.02383.x. [DOI] [PubMed] [Google Scholar]
- 26.Feher A., Pasternak T.P., Dudits D. Transition of somatic plant cells to an embryogenic state. Plant Cell. Tissue Organ Cult. 2003;74:201–228. doi: 10.1023/A:1024033216561. [DOI] [Google Scholar]
- 27.Zavattieri M.A., Frederico A.M., Lima M., Sabino R., Arnholdt-Schmitt B. Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron. J. Biotechnol. 2010;13:12–13. doi: 10.2225/vol13-issue1-fulltext-4. [DOI] [Google Scholar]
- 28.Thibaud-Nissen F., Shealy R.T., Khanna A., Vodkin L.O. Clustering of microarray data reveals transcript patterns associated with somatic embryogenesis in soybean. Plant Physiol. 2003;132:118–136. doi: 10.1104/pp.103.019968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantiri F.R., Kurdyukov S., Lohar D.P., Sharopova N., Saeed N.A., Wang X.-D., VandenBosch K.A., Rose R.J. The Transcription Factor MtSERF1 of the ERF Subfamily Identified by Transcriptional Profiling Is Required for Somatic Embryogenesis Induced by Auxin Plus Cytokinin in Medicago truncatula1[W][OA] Plant Physiol. 2008;146:1622–1636. doi: 10.1104/pp.107.110379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma S.K., Millam S., Hedley P.E., McNicol J., Bryan G.J. Molecular regulation of somatic embryogenesis in potato: An auxin led perspective. Plant Mol. Biol. 2008;68:185–201. doi: 10.1007/s11103-008-9360-2. [DOI] [PubMed] [Google Scholar]
- 31.Imin N., Goffard N., Nizamidin M., Rolfe B.G. Genome-wide transcriptional analysis of super-embryogenic Medicago truncatula explant cultures. BMC Plant Biol. 2008;8:110. doi: 10.1186/1471-2229-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H.C., Morcillo F., Dussert S., Tranchant-Dubreuil C., Tregear J.W., Tranbarger T.J. Transcriptome analysis during somatic embryogenesis of the tropical monocot Elaeis guineensis: Evidence for conserved gene functions in early development. Plant Mol. Biol. 2009;70:173–192. doi: 10.1007/s11103-009-9464-3. [DOI] [PubMed] [Google Scholar]
- 33.Sun L., Wu Y., Su S., Liu H., Yang G., Li S. Differential gene expression during somatic embryogenesis in the maize (Zea mays L.) inbred line H99. Plant Cell. Tissue Organ Cult. 2012;109:271–286. doi: 10.1007/s11240-011-0093-6. [DOI] [Google Scholar]
- 34.Shani E., Salehin M., Zhang Y., Sanchez S.E., Doherty C., Wang R., Mangado C.C., Song L., Tal I., Pisanty O., et al. Plant Stress Tolerance Requires Auxin-Sensitive Aux/IAA Transcriptional Repressors. Curr. Biol. 2017;27:437–444. doi: 10.1016/j.cub.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blakeslee J.J., Spatola Rossi T., Kriechbaumer V. Auxin biosynthesis: Spatial regulation and adaptation to stress. J. Exp. Bot. 2019;70:5041–5049. doi: 10.1093/jxb/erz283. [DOI] [PubMed] [Google Scholar]
- 36.Wójcikowska B., Jaskóła K., Gąsiorek P., Meus M., Nowak K., Gaj M.D. LEAFY COTYLEDON2 (LEC2) promotes embryogenic induction in somatic tissues of Arabidopsis, via YUCCA-mediated auxin biosynthesis. Planta. 2013;238:425–440. doi: 10.1007/s00425-013-1892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wójcikowska B., Gaj M.D. Expression profiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep. 2017;36:843–858. doi: 10.1007/s00299-017-2114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanova A., Velcheva M., Denchev P., Atanassov A., Van Onckelen H.A. Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol. Plant. 1994;92:85–89. doi: 10.1111/j.1399-3054.1994.tb06658.x. [DOI] [Google Scholar]
- 39.Guiderdoni E., Mérot B., Eksomtramage T., Paulet F., Feldmann P., Glaszmann J.C. Somatic embryogenesis in sugarcane (Saccharum Species) In: Bajaj Y.P.S., editor. Somatic Embryogenesis and Synthetic Seed II. 1st ed. Vol. 31. Springer; Berlin, Heidelberg: 1995. pp. 92–113. [Google Scholar]
- 40.Michalczuk L., Druart P. Indole-3-acetic acid metabolism in hormone-autotrophic, embryogenic callus of Inmil® cherry rootstock (Prunus incisa x serrula ’GM 9′) and in hormone-dependent, nonembryogenic calli of Prunus incisa x serrula and Prunus domestica. Physiol. Plant. 1999;107:426–432. doi: 10.1034/j.1399-3054.1999.100408.x. [DOI] [Google Scholar]
- 41.Jiménez V.M., Bangerth F. Endogenous hormone levels in explants and in embryogenic and non-embryogenic cultures of carrot. Physiol. Plant. 2001;111:389–395. doi: 10.1034/j.1399-3054.2001.1110317.x. [DOI] [PubMed] [Google Scholar]
- 42.Jiménez V.M., Bangerth F. Endogenous hormone concentrations and embryogenic callus development in wheat. Plant Cell. Tissue Organ Cult. 2001;67:37–46. doi: 10.1023/A:1011671310451. [DOI] [Google Scholar]
- 43.Jiménez V.M., Bangerth F. Hormonal status of maize initial explants and of the embryogenic and non-embryogenic callus cultures derived from them as related to morphogenesis in vitro. Plant Sci. 2001;160:247–257. doi: 10.1016/S0168-9452(00)00382-4. [DOI] [PubMed] [Google Scholar]
- 44.Grzyb M., Kalandyk A., Waligórski P., Mikuła A. The content of endogenous hormones and sugars in the process of early somatic embryogenesis in the tree fern Cyathea delgadii Sternb. Plant Cell. Tissue Organ Cult. 2017;129:387–397. doi: 10.1007/s11240-017-1185-8. [DOI] [Google Scholar]
- 45.Li Q., Deng C., Xia Y., Kong L., Zhang H., Zhang S., Wang J. Identification of novel miRNAs and miRNA expression profiling in embryogenic tissues of Picea balfouriana treated by 6-benzylaminopurine. PLoS ONE. 2017;12:1–17. doi: 10.1371/journal.pone.0176112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liao Y.K., Liao C.K., Ho Y.L. Maturation of somatic embryos in two embryogenic cultures of Picea morrisonicola Hayata as affected by alternation of endogenous IAA content. Plant Cell. Tissue Organ Cult. 2008;93:257–268. doi: 10.1007/s11240-008-9371-3. [DOI] [Google Scholar]
- 47.Zhou X., Zheng R., Liu G., Xu Y., Zhou Y., Laux T., Zhen Y., Harding S.A., Shi J., Chen J. Desiccation treatment and endogenous IAA levels are key factors influencing high frequency somatic embryogenesis in Cunninghamia lanceolata (Lamb.) Hook. Front. Plant Sci. 2017;8:2054. doi: 10.3389/fpls.2017.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michalczuk L., Cooke T.J., Cohen J.D. Auxin levels at different stages of carrot somatic embryogenesis. Phytochemistry. 1992;31:1097–1103. doi: 10.1016/0031-9422(92)80241-6. [DOI] [Google Scholar]
- 49.Thomas C., Bronner R., Molinier J., Prinsen E., Van Onckelen H., Hahne G. Immuno-cytochemical localization of indole-3-acetic acid during induction of somatic embryogenesis in cultured sunflower embryos. Planta. 2002;215:577–583. doi: 10.1007/s00425-002-0791-8. [DOI] [PubMed] [Google Scholar]
- 50.Ayil-Gutiérrez B., Galaz-Avalos R.M., Peña-Cabrera E., Loyola-Vargas V.M. Dynamics of the concentration of IAA and some of its conjugates during the induction of somatic embryogenesis in Coffea canephora. Plant Signal. Behav. 2013;8:e26998. doi: 10.4161/psb.26998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Márquez-López R.E., Pérez-Hernández C., Ku-González Á., Galaz-Ávalos R.M., Loyola-Vargas V.M. Localization and transport of indole-3-acetic acid during somatic embryogenesis in Coffea canephora. Protoplasma. 2018;255:695–708. doi: 10.1007/s00709-017-1181-1. [DOI] [PubMed] [Google Scholar]
- 52.Awada R., Campa C., Gibault E., Déchamp E., Georget F., Lepelley M., Abdallah C., Erban A., Seidel M.-, Kopka J., et al. Unravelling the Metabolic and Hormonal Machinery During Key Steps of Somatic Embryogenesis: A Case Study in Coffee. Int. J. Mol. Sci. 2019;20:4665. doi: 10.3390/ijms20194665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasternak T.P., Prinsen E., Ayaydin F., Miskolczi P., Potters G., Asard H., Van Onckelen H.A., Dudits D., Fehér A. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiol. 2002;129:1807–1819. doi: 10.1104/pp.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pescador R., Kerbauy G.B., De Ferreira W.M., Purgatto E., Suzuki R.M., Guerra M.P. A hormonal misunderstanding in Acca sellowiana embryogenesis: Levels of zygotic embryogenesis do not match those of somatic embryogenesis. Plant Growth Regul. 2012;68:67–76. doi: 10.1007/s10725-012-9694-2. [DOI] [Google Scholar]
- 55.Cheng W.H., Zhu H.G., Tian W.G., Zhu S.H., Xiong X.P., Sun Y.Q., Zhu Q.H., Sun J. De novo transcriptome analysis reveals insights into dynamic homeostasis regulation of somatic embryogenesis in upland cotton (G. hirsutum L.) Plant Mol. Biol. 2016;92:279–292. doi: 10.1007/s11103-016-0511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vondrakova Z., Dobrev P.I., Pesek B., Fischerova L., Vagner M., Motyka V. Profiles of endogenous phytohormones over the course of Norway spruce somatic embryogenesis. Front. Plant Sci. 2018;9:1283. doi: 10.3389/fpls.2018.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wójcikowska B., Gaj M.D. LEAFY COTYLEDON2-mediated control of the endogenous hormone content: Implications for the induction of somatic embryogenesis in Arabidopsis. Plant Cell. Tissue Organ Cult. 2015;121:255–258. doi: 10.1007/s11240-014-0689-8. [DOI] [Google Scholar]
- 58.Ribnicky D.M., Cohen J.D., Hu W.S., Cooke T.J. An auxin surge following fertilization in carrots: A mechanism for regulating plant totipotency. Planta. 2002;214:505–509. doi: 10.1007/s004250100639. [DOI] [PubMed] [Google Scholar]
- 59.Robert H., Park C., Gutièrrez C.L., Wójcikowska B., Pěnčík A., Novák O., Chen J., Grunewald W., Dresselhaus T., Friml J., et al. Maternal auxin supply contributes to early embryo patterning in Arabidopsis. Nat. Plants. 2018;4:548–553. doi: 10.1038/s41477-018-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robert H.S. Molecular communication for coordinated seed and fruit development: What can we learn from auxin and sugars? Int. J. Mol. Sci. 2019;20:936. doi: 10.3390/ijms20040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winkelmann T. Somatic versus zygotic embryogenesis: Learning from seeds. In: Germana M.A., Lambardi M., editors. In Vitro Embryogenesis in Higher Plants. 1st ed. Springer Science+Business Media LLC; New York, NY, USA: 2016. pp. 25–46. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang B., Chu J., Yu T., Xu Q., Sun X., Yuan J., Xiong G., Wang G., Wang Y., Li J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2015;112:4821–4826. doi: 10.1073/pnas.1503998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wickramasuriya A.M., Dunwell J.M. Global scale transcriptome analysis of Arabidopsis embryogenesis in vitro. Bmc Genom. 2015;16:301. doi: 10.1186/s12864-015-1504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bai B., Su Y.H., Yuan J., Zhang X.S. Induction of somatic embryos in Arabidopsis requires local YUCCA expression mediated by the down-regulation of ethylene biosynthesis. Mol. Plant. 2013;6:1247–1260. doi: 10.1093/mp/sss154. [DOI] [PubMed] [Google Scholar]
- 66.Robert H.S., Crhak Khaitova L., Mroue S., Benková E. The importance of localized auxin production for morphogenesis of reproductive organs and embryos in Arabidopsis. J. Exp. Bot. 2015;66:5029–5042. doi: 10.1093/jxb/erv256. [DOI] [PubMed] [Google Scholar]
- 67.Radoeva T., Lokerse A.S., Llavata-Peris C.I., Wendrich J.R., Xiang D., Liao C.Y., Vlaar L., Boekschoten M., Hooiveld G., Datla R., et al. A robust auxin response network controls embryo and suspensor development through a basic helix loop helix transcriptional module. Plant Cell. 2019;31:52–67. doi: 10.1105/tpc.18.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wójcikowska B., Botor M., Morończyk J., Wójcik A.M., Nodzyński T., Karcz J., Gaj M.D. Trichostatin A triggers an embryogenic transition in Arabidopsis explants via an auxin-related pathway. Front. Plant Sci. 2018;9:1353. doi: 10.3389/fpls.2018.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weijers D., Wagner D. Transcriptional Responses to the Auxin Hormone. Annu. Rev. Plant Biol. 2016;67:539–574. doi: 10.1146/annurev-arplant-043015-112122. [DOI] [PubMed] [Google Scholar]
- 70.Yang X., Zhang X., Yuan D., Jin F., Zhang Y., Xu J. Transcript profiling reveals complex auxin signalling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton. Bmc Plant Biol. 2012;12:110. doi: 10.1186/1471-2229-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu Z., Zhang C., Zhang X., Liu C., Wu Z., Yang Z., Zhou K., Yang X., Li F. Transcriptome profiling reveals auxin and cytokinin regulating somatic embryogenesis in different sister lines of cotton cultivar CCRI24. J. Integr. Plant Biol. 2013;55:631–642. doi: 10.1111/jipb.12073. [DOI] [PubMed] [Google Scholar]
- 72.Indoliya Y., Tiwari P., Chauhan A.S., Goel R., Shri M., Bag S.K., Chakrabarty D. Decoding regulatory landscape of somatic embryogenesis reveals differential regulatory networks between japonica and indica rice subspecies. Sci. Rep. 2016;6:23050. doi: 10.1038/srep23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saptari R.T., Susila H. Data mining study of hormone biosynthesis gene expression reveals new aspects of somatic embryogenesis regulation. Vitr. Cell. Dev. Biol.-Plant. 2019;55:139–152. doi: 10.1007/s11627-018-9947-5. [DOI] [Google Scholar]
- 74.Singla B., Tyagi A.K., Khurana J.P., Khurana P. Analysis of expression profile of selected genes expressed during auxin-induced somatic embryogenesis in leaf base system of wheat (Triticum aestivum) and their possible interactions. Plant Mol. Biol. 2007;65:677–692. doi: 10.1007/s11103-007-9234-z. [DOI] [PubMed] [Google Scholar]
- 75.Cao A., Zheng Y., Yu Y., Wang X., Shao D., Sun J., Cui B. Comparative transcriptome analysis of SE initial dedifferentiation in cotton of different SE capability. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-08763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gliwicka M., Nowak K., Balazadeh S., Mueller-Roeber B., Gaj M.D. Extensive Modulation of the transcription factor transcriptome during somatic embryogenesis in Arabidopsis thaliana. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0069261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi X., Zhang C., Liu Q., Zhang Z., Zheng B., Bao M. De novo comparative transcriptome analysis provides new insights into sucrose induced somatic embryogenesis in camphor tree (Cinnamomum camphora L.) Bmc Genom. 2016;17:26. doi: 10.1186/s12864-015-2357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fadryin N., Rohani E.R., Muhammed-Hussein Z.A., Noor N.M. Somatic embryogenesis-related gene expression and functional genomics in mangosteen. Plant Gene. 2018;15:51–66. doi: 10.1016/j.plgene.2018.07.002. [DOI] [Google Scholar]
- 79.Capote T., Usié A., Barbosa P., Ramos M., Morais-Cecílio L., Gonçalves S. Transcriptome dynamics of cork oak (Quercus suber) somatic embryogenesis reveals active gene players in transcription regulation and phytohormone homeostasis of embryo development. Tree Genet. Genomes. 2019;15:52. doi: 10.1007/s11295-019-1353-6. [DOI] [Google Scholar]
- 80.Liu W., Wang C., Shen X., Liang H., Wang Y., He Z., Zhang D., Chen F. Comparative transcriptome analysis highlights the hormone effects on somatic embryogenesis in Catalpa bungei. Plant Reprod. 2019;32:141–151. doi: 10.1007/s00497-018-0349-y. [DOI] [PubMed] [Google Scholar]
- 81.Quintana-Escobar A.O., Nic-Can G.I., Avalos R.M.G., Loyola-Vargas V.M., Gongora-Castillo E. Transcriptome analysis of the induction of somatic embryogenesis in Coffea canephora and the participation of ARF and Aux/IAA genes. PeerJ. 2019;7:e7752. doi: 10.7717/peerj.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen M., Zhang J., Zhou Y., Li S., Fan X., Yang L., Guan Y., Zhang Y. Transcriptome analysis of Lilium Oriental × Trumpet hybrid roots reveals auxin-related genes and stress-related genes involved in picloram-induced somatic embryogenesis induction. J. Hortic. Sci. Biotechnol. 2019;94:317–330. doi: 10.1080/14620316.2018.1500086. [DOI] [Google Scholar]
- 83.Leyser O. Auxin signaling. Plant Physiol. 2018;176:465–479. doi: 10.1104/pp.17.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li S.B., Xie Z.Z., Hu C.G., Zhang J.Z. A review of AUXIN RESPONSE FACTORS (ARFs) in plants. Front. Plant Sci. 2016;7:47. doi: 10.3389/fpls.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luo J., Zhou J.J., Zhang J.Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018;19:259. doi: 10.3390/ijms19010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zheng Y., Ren N., Wang H., Stromberg A.J., Perry S.E. Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-like15. Plant Cell. 2009;21:2563–2577. doi: 10.1105/tpc.109.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rensing S.A., Lang D., Schumann E., Reski R., Hohe A. EST sequencing from embryogenic Cyclamen persicum cell cultures identifies a high proportion of transcripts homologous to plant genes involved in somatic embryogenesis. J. Plant Growth Regul. 2005;24:102–115. doi: 10.1007/s00344-005-0033-y. [DOI] [Google Scholar]
- 88.Ooi S.E., Choo C.N., Ishak Z., Ong-Abdullah M. A candidate auxin-responsive expression marker gene, EgIAA9, for somatic embryogenesis in oil palm (Elaeis guineensis Jacq.) Plant Cell. Tissue Organ Cult. 2012;110:201–212. doi: 10.1007/s11240-012-0143-8. [DOI] [Google Scholar]
- 89.Wójcik A.M., Gaj M.D. miR393 contributes to the embryogenic transition induced in vitro in Arabidopsis via the modification of the tissue sensitivity to auxin treatment. Planta. 2016;244:231–243. doi: 10.1007/s00425-016-2505-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang X., Wang L., Yuan D., Lindsey K., Zhang X. Small RNA and degradome sequencing reveal complex miRNA regulation during cotton somatic embryogenesis. J. Exp. Bot. 2013;64:1521–1536. doi: 10.1093/jxb/ert013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Villalobos L.I.A.C., Lee S., De Oliveira C., Ivetac A., Brandt W., Armitage L., Sheard L.B., Tan X., Parry G., Mao H., et al. A combinatorial TIR1/AFB–Aux/IAA co-receptor system for differential sensing of auxin. Nat. Chem. Biol. 2012;8:477. doi: 10.1038/nchembio.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braybrook S.A., Stone S.L., Park S., Bui A.Q., Le B.H., Fischer R.L., Goldberg R.B., Harada J.J. Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc. Natl. Acad. Sci. USA. 2006;103:3468–3473. doi: 10.1073/pnas.0511331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tvorogova V.E., Lutova L.A. Genetic regulation of zygotic embryogenesis in angiosperm plants. Russ. J. Plant Physiol. 2018;65:1–14. doi: 10.1134/S1021443718010107. [DOI] [Google Scholar]
- 94.Tang X., Bian S., Tang M., Lu Q., Li S., Liu X., Tian G., Nguyen V., Tsang E.W.T., Wang A., et al. MicroRNA-mediated repression of the seed maturation program during vegetative development in Arabidopsis. Plos Genet. 2012;8:20–22. doi: 10.1371/journal.pgen.1003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wójcik A.M., Nodine M.D., Gaj M.D. miR160 and miR166/165 contribute to the LEC2-mediated auxin response involved in the somatic embryogenesis induction in Arabidopsis. Front. Plant Sci. 2017;8:2024. doi: 10.3389/fpls.2017.02024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Müller C.J., Valdés A.E., Wang G., Ramachandran P., Beste L., Uddenberg D., Carlsbecker A. PHABULOSA mediates an auxin signaling loop to regulate vascular patterning in Arabidopsis. Plant Physiol. 2016;170:956–970. doi: 10.1104/pp.15.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Möller B.K., Ten Hove C.A., Xiang D., Williams N., López L.G., Yoshida S., Smit M., Datla R., Weijers D. Auxin response cell-autonomously controls ground tissue initiation in the early Arabidopsis embryo. Proc. Natl. Acad. Sci. USA. 2017;114:E2533–E2539. doi: 10.1073/pnas.1616493114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Donner T.J., Sherr I., Scarpella E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development. 2009;136:3235–3246. doi: 10.1242/dev.037028. [DOI] [PubMed] [Google Scholar]
- 99.Schlereth A., Möller B., Liu W., Kientz M., Flipse J., Rademacher E.H., Schmid M., Jürgens G., Weijers D. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature. 2010;464:913–916. doi: 10.1038/nature08836. [DOI] [PubMed] [Google Scholar]
- 100.Krogan N.T., Yin X., Ckurshumova W., Berleth T. Distinct subclades of Aux/IAA genes are direct targets of ARF5/MP transcriptional regulation. New Phytol. 2014;204:474–483. doi: 10.1111/nph.12994. [DOI] [PubMed] [Google Scholar]
- 101.Wenzel C.L., Schuetz M., Yu Q., Mattsson J. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J. 2007;49:387–398. doi: 10.1111/j.1365-313X.2006.02977.x. [DOI] [PubMed] [Google Scholar]
- 102.Krogan N.T., Marcos D., Weiner A.I., Berleth T. The auxin response factor MONOPTEROS controls meristem function and organogenesis in both the shoot and root through the direct regulation of PIN genes. New Phytol. 2016;212:42–50. doi: 10.1111/nph.14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Su Y.H., Zhao X.Y., Liu Y.B., Zhang C.L., O’Neill S.D., Zhang X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009;59:448–460. doi: 10.1111/j.1365-313X.2009.03880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hardtke C.S., Ckurshumova W., Vidaurre D.P., Singh S.A., Stamatiou G., Tiwari S.B., Hagen G., Gilfoyle T.J., Berleth T. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development. 2004;131:1089–1100. doi: 10.1242/dev.00925. [DOI] [PubMed] [Google Scholar]
- 105.Breuninger H., Rikirsch E., Hermann M., Ueda M., Laux T. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev. Cell. 2008;14:867–876. doi: 10.1016/j.devcel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Möller B., Weijers D. Auxin control of embryo patterning. Cold Spring Harb. Perspect. Biol. 2009;1:1–13. doi: 10.1101/cshperspect.a001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guan C., Wu B., Yu T., Wang Q., Krogan N.T., Liu X., Jiao Y. Spatial auxin signaling controls leaf flattening in Arabidopsis. Curr. Biol. 2017;27:2940–2950. doi: 10.1016/j.cub.2017.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gambino G., Minuto M., Boccacci P., Perrone I., Vallania R., Gribaudo I. Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in Vitis vinifera. J. Exp. Bot. 2010;62:1089–1101. doi: 10.1093/jxb/erq349. [DOI] [PubMed] [Google Scholar]
- 109.Palovaara J., Hallberg H., Stasolla C., Hakman I. Comparative expression pattern analysis of WUSCHEL-related homeobox 2 (WOX2) and WOX8/9 in developing seeds and somatic embryos of the gymnosperm Picea abies. New Phytol. 2010;188:122–135. doi: 10.1111/j.1469-8137.2010.03336.x. [DOI] [PubMed] [Google Scholar]
- 110.Klimaszewska K., Pelletier G., Overton C., Stewart D., Rutledge R.G. Hormonally regulated overexpression of Arabidopsis WUS and conifer LEC1 (CHAP3A) in transgenic white spruce: Implications for somatic embryo development and somatic seedling growth. Plant Cell Rep. 2010;29:723–734. doi: 10.1007/s00299-010-0859-z. [DOI] [PubMed] [Google Scholar]
- 111.Rupps A., Raschke J., Rümmler M., Linke B., Zoglauer K. Identification of putative homologs of Larix decidua to BABY BOOM (BBM), LEAFY COTYLEDON1 (LEC1), WUSCHEL-related HOMEOBOX2 (WOX2) and SOMATIC EMBRYOGENESIS RECEPTOR-like KINASE (SERK) during somatic embryogenesis. Planta. 2016;243:473–488. doi: 10.1007/s00425-015-2409-y. [DOI] [PubMed] [Google Scholar]
- 112.Tvorogova V.E., Lebedeva M.A., Lutova L.A. Expression of WOX and PIN genes during somatic and zygotic embryogenesis in Medicago truncatula. Russ. J. Genet. 2015;51:1189–1198. doi: 10.1134/S1022795415120121. [DOI] [PubMed] [Google Scholar]
- 113.Tvorogova V.E., Fedorova Y.A., Potsenkovskaya E.A., Kudriashov A.A., Efremova E.P., Kvitkovskaya V.A., Wolabu T.W., Zhang F., Tadege M., Lutova L.A. The WUSCHEL-related homeobox transcription factor MtWOX9-1 stimulates somatic embryogenesis in Medicago truncatula. Plant Cell. Tissue Organ Cult. 2019;138:517–527. doi: 10.1007/s11240-019-01648-w. [DOI] [Google Scholar]
- 114.Chen Y., Xu X., Liu Z., Zhang Z., XuHan X., Lin Y., Lai Z. Global scale transcriptome analysis reveals differentially expressed genes involve in early somatic embryogenesis in Dimocarpus longan Lour. Bmc Genom. 2020;21:1–22. doi: 10.1186/s12864-019-6393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z., Tucker E., Hermann M., Laux T. A molecular framework for the embryonic initiation of shoot meristem stem cells. Dev. Cell. 2017;40:264–277. doi: 10.1016/j.devcel.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Qiao M., Zhao Z., Song Y., Liu Z., Cao L., Yu Y., Li S., Xiang F. Proper regeneration from in vitro cultured Arabidopsis thaliana requires the microRNA-directed action of an auxin response factor. Plant J. 2012;71:14–22. doi: 10.1111/j.1365-313X.2012.04944.x. [DOI] [PubMed] [Google Scholar]
- 117.Horstman A., Willemsen V., Boutilier K., Heidstra R. AINTEGUMENTA-LIKE proteins: Hubs in a plethora of networks. Trends Plant Sci. 2014;19:146–157. doi: 10.1016/j.tplants.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 118.Ding Z., Friml J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl. Acad. Sci. USA. 2010;107:12046–12051. doi: 10.1073/pnas.1000672107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nowak K., Gaj M.D. Transcription factors in regulations of somatic embryogenesis. In: Loyola-Vargas V., Ochoa-Alejo N., editors. Somatic Embryogenesis: Fundamental Aspects and Applications. 1st ed. Springer International Publishing; Cham, Switzerland: 2016. pp. 455–469. [Google Scholar]
- 120.Jia H., Suzuki M., Mccarty D.R. Regulation of the seed to seedling developmental phase transition by the LAFL and VAL transcription factor networks. Wires Dev. Biol. 2014;3:135–145. doi: 10.1002/wdev.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Horstman A., Li M., Heidmann I., Weemen M., Chen B., Muino J.M., Angenent G.C., Boutiliera K. The BABY BOOM transcription factor activates the LEC1-ABI3-FUS3-LEC2 network to induce somatic embryogenesis. Plant Physiol. 2017;175:848–857. doi: 10.1104/pp.17.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lotan T., Ohto M.A., Matsudaira Yee K., West M.A.L., Lo R., Kwong R.W., Yamagishi K., Fischer R.L., Goldberg R.B., Harada J.J. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell. 1998;93:1195–1205. doi: 10.1016/S0092-8674(00)81463-4. [DOI] [PubMed] [Google Scholar]
- 123.Stone S.L., Kwong L.W., Yee K.M., Pelletier J., Lepiniec L., Fischer R.L., Goldberg R.B., Harada J.J. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl. Acad. Sci. USA. 2001;98:11806–11811. doi: 10.1073/pnas.201413498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gaj M.D., Zhang S., Harada J.J., Lemaux P.G. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta. 2005;222:977–988. doi: 10.1007/s00425-005-0041-y. [DOI] [PubMed] [Google Scholar]
- 125.Kurczyńska E.U., Gaj M.D., Ujczak A., Mazur E. Histological analysis of direct somatic embryogenesis in Arabidopsis thaliana (L.) Heynh. Planta. 2007;226:619–628. doi: 10.1007/s00425-007-0510-6. [DOI] [PubMed] [Google Scholar]
- 126.Ledwoń A., Gaj M.D. LEAFY COTYLEDON2 gene expression and auxin treatment in relation to embryogenic capacity of Arabidopsis somatic cells. Plant Cell Rep. 2009;28:1677. doi: 10.1007/s00299-009-0767-2. [DOI] [PubMed] [Google Scholar]
- 127.Ledwoń A., Gaj M.D. LEAFY COTYLEDON1, FUSCA3 expression and auxin treatment in relation to somatic embryogenesis induction in Arabidopsis. Plant Growth Regul. 2011;65:157–167. doi: 10.1007/s10725-011-9585-y. [DOI] [Google Scholar]
- 128.Belide S., Zhou X.R., Kennedy Y., Lester G., Shrestha P., Petrie J.R., Singh S.P. Rapid expression and validation of seed-specific constructs in transgenic LEC2 induced somatic embryos of Brassica napus. Plant Cell. Tissue Organ Cult. 2013;113:543–553. doi: 10.1007/s11240-013-0295-1. [DOI] [Google Scholar]
- 129.Guo F., Liu C., Xia H., Bi Y., Zhao C., Zhao S., Hou L., Li F., Wang X. Induced expression of AtLEC1 and AtLEC2 differentially promotes somatic embryogenesis in transgenic tobacco plants. PLoS ONE. 2013;8:e71714. doi: 10.1371/journal.pone.0071714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fister A.S., Landherr L., Perryman M., Zhang Y., Guiltinan M.J., Maximova S.N. Glucocorticoid receptor-regulated TcLEC2 expression triggers somatic embryogenesis in Theobroma cacao leaf tissue. PLoS ONE. 2018;13:1–19. doi: 10.1371/journal.pone.0207666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brand A., Quimbaya M., Tohme J., Chavarriaga-Aguirre P. Arabidopsis LEC1 and LEC2 orthologous genes are key regulators of somatic embryogenesis in cassava. Front. Plant Sci. 2019;10:673. doi: 10.3389/fpls.2019.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Braybrook S., Harada J. LECs go crazy in embryo development. Trends Plant Sci. 2008;13:624–630. doi: 10.1016/j.tplants.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 133.Stone S.L., Braybrook S.A., Paula S.L., Kwong L.W., Meuser J., Pelletier J., Hsieh T.F., Fischer R.L., Goldberg R.B., Harada J.J. Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: Implications for somatic embryogenesis. Proc. Natl. Acad. Sci. USA. 2008;105:3151–3156. doi: 10.1073/pnas.0712364105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Junker A., Mönke G., Rutten T., Keilwagen J., Seifert M., Thi T.M.N., Renou J.P., Balzergue S., Viehöver P., Hähnel U., et al. Elongation-related functions of LEAFY COTYLEDON1 during the development of Arabidopsis thaliana. Plant J. 2012;71:427–442. doi: 10.1111/j.1365-313X.2012.04999.x. [DOI] [PubMed] [Google Scholar]
- 135.Wang H., Caruso L.V., Downie A.B., Perry S.E. The embryo MADS domain protein AGAMOUS-LIKE 15 directly regulates expression of a gene encoding an enzyme involved in gibberellin metabolism. Plant Cell. 2004;16:1206–1219. doi: 10.1105/tpc.021261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Harding E.W., Tang W., Nichols K.W., Fernandez D.E., Perry S.E. Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiol. 2003;133:653–663. doi: 10.1104/pp.103.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu C., Perry S.E. Control of expression and autoregulation of AGL15, a member of the MADS-box family. Plant J. 2005;41:583–594. doi: 10.1111/j.1365-313X.2004.02320.x. [DOI] [PubMed] [Google Scholar]
- 138.Wang F., Perry S.E. Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development. Plant Physiol. 2013;161:1251–1264. doi: 10.1104/pp.112.212282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zheng Q., Perry S.E. Alterations in the transcriptome of soybean in response to enhanced somatic embryogenesis promoted by orthologs of AGAMOUS-Like15 and AGAMOUS-Like18. Plant Physiol. 2014;164:1365–1377. doi: 10.1104/pp.113.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Perry S.E., Zheng Q., Zheng Y. Transcriptome analysis indicates that GmAGAMOUS-Like 15 may enhance somatic embryogenesis by promoting a dedifferentiated state. Plant Signal. Behav. 2016;11:1–7. doi: 10.1080/15592324.2016.1197463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zheng Q., Zheng Y., Ji H., Burnie W., Perry S.E. Gene regulation by the AGL15 transcription factor reveals hormone interactions in somatic embryogenesis. Plant Physiol. 2016;172:2374–2387. doi: 10.1104/pp.16.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aida M., Beis D., Heidstra R., Willemsen V., Blilou I., Galinha C., Nussaume L., Noh Y.S., Amasino R., Scheres B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 143.Billou I., Xu J., Wildwater M., Willemsen V., Paponov I., Frimi J., Heldstra R., Aida M., Palme K., Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 144.Boutilier K., Offringa R., Sharma V.K., Kieft H., Ouellet T., Zhang L. Ectopic Expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14:1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tsuwamoto R., Yokoi S., Takahata Y. Arabidopsis EMBRYOMAKER encoding an AP2 domain transcription factor plays a key role in developmental change from vegetative to embryonic phase. Plant Mol. Biol. 2010;73:481–492. doi: 10.1007/s11103-010-9634-3. [DOI] [PubMed] [Google Scholar]
- 146.Szczygieł-Sommer A., Gaj M.D. The miR396–GRF regulatory module controls the embryogenic response in Arabidopsis via an auxin-related pathway. Int. J. Mol. Sci. 2019;20:5221. doi: 10.3390/ijms20205221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Horstman A., Bemer M., Boutilier K. A transcriptional view on somatic embryogenesis. Regeneration. 2017;4:201–216. doi: 10.1002/reg2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Passarinho P., Ketelaar T., Xing M., Van Arkel J., Maliepaard C., Hendriks M.W., Joosen R., Lammers M., Herdies L., Den Boer B., et al. BABY BOOM target genes provide diverse entry points into cell proliferation and cell growth pathways. Plant Mol. Biol. 2008;68:225–237. doi: 10.1007/s11103-008-9364-y. [DOI] [PubMed] [Google Scholar]
- 149.Pinon V., Prasad K., Grigg S.P., Sanchez-Perez G.F., Scheres B. Local auxin biosynthesis regulation by PLETHORA transcription factors controls phyllotaxis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2013;110:1107–1112. doi: 10.1073/pnas.1213497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ogas J., Kaufmann S., Henderson J., Somerville C. PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1999;96:13839–13844. doi: 10.1073/pnas.96.24.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang H., Rider S.D., Henderson J.T., Fountain M., Chuang K., Kandachar V., Simons A., Edenberg H.J., Romero-Severson J., Muir W.M., et al. The CHD3 remodeler PICKLE promotes trimethylation of histone H3 lysine 27. J. Biol. Chem. 2008;283:22637–22648. doi: 10.1074/jbc.M802129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suzuki M., Wang H.H.Y., McCarty D.R. Repression of the Leafy Cotyledon 1/B3 regulatory network in plant embryo development by VP1/Abscisic Acid Insensitive 3-Like B3 genes. Plant Physiol. 2007;143:902–911. doi: 10.1104/pp.106.092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou Y., Tan B., Luo M., Li Y., Liu C., Chen C., Yu C.W., Yang S., Dong S., Ruan J., et al. HISTONE DEACETYLASE19 interacts with HSL1 and participates in the repression of seed maturation genes in Arabidopsis seedlings. Plant Cell. 2013;25:134–148. doi: 10.1105/tpc.112.096313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chhun T., Chong S.Y., Park B.S., Wong E.C.C., Yin J.L., Kim M., Chua N.H. HSI2 repressor recruits MED13 and HDA6 to down-regulate seed maturation gene expression directly during arabidopsis early seedling growth. Plant Cell Physiol. 2016;57:1689–1706. doi: 10.1093/pcp/pcw095. [DOI] [PubMed] [Google Scholar]
- 155.Van Der Graaff E., Laux T., Rensing S.A. Protein family review The WUS homeobox-containing (WOX) protein family. Genome Biol. 2009;10:248. doi: 10.1186/gb-2009-10-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dolzblasz A., Nardmann J., Clerici E., Causier B., Van der Graaff E., Chen J., Davies B., Werr W., Laux T. Stem cell regulation by Arabidopsis WOX genes. Mol. Plant. 2016;9:1028–1039. doi: 10.1016/j.molp.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 157.Zuo J., Niu Q.W., Frugis G., Chua N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002;30:349–359. doi: 10.1046/j.1365-313X.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 158.Solís-Ramos L.Y., González-Estrada T., Nahuath-Dzib S., Zapata-Rodriguez L.C., Castaño E. Overexpression of WUSCHEL in C. chinense causes ectopic morphogenesis. Plant Cell. Tissue Organ Cult. 2009;96:279–287. doi: 10.1007/s11240-008-9485-7. [DOI] [Google Scholar]
- 159.Arroyo-Herrera A., Ku Gonzalez A., Canche Moo R., Quiroz-Figueroa F.R., Loyola-Vargas V.M., Rodriguez-Zapata L.C., Burgeff D’Hondt C., Suárez-Solís V.M., Castaño E. Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell. Tissue Organ Cult. 2008;94:171–180. doi: 10.1007/s11240-008-9401-1. [DOI] [Google Scholar]
- 160.Zheng W., Zhang X., Yang Z., Wu J., Li F., Duan L., Liu C., Lu L., Zhang C., Li F. AtWuschel promotes formation of the embryogenic callus in Gossypium hirsutum. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0087502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bouchabké-Coussa O., Obellianne M., Linderme D., Montes E., Maia-Grondard A., Vilaine F., Pannetier C. WUSCHEL overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutum L.) tissues cultured in vitro. Plant Cell Rep. 2013;32:675–686. doi: 10.1007/s00299-013-1402-9. [DOI] [PubMed] [Google Scholar]
- 162.Ma Y., Miotk A., Šutiković Z., Ermakova O., Wenzl C., Medzihradszky A., Gaillochet C., Forner J., Utan G., Brackmann K., et al. WUSCHEL acts as an auxin response rheostat to maintain apical stem cells in Arabidopsis. Nat. Commun. 2019;10:1–11. doi: 10.1038/s41467-019-13074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Magnani E., Jiménez-Gómez J.M., Soubigou-Taconnat L., Lepiniec L., Fiume E. Profiling the onset of somatic embryogenesis in Arabidopsis. Bmc Genom. 2017;18:1–12. doi: 10.1186/s12864-017-4391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.To A., Valon C., Savino G., Guilleminot J., Devic M., Giraudat J., Parcy F.A. network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell. 2006;18:1642–1651. doi: 10.1105/tpc.105.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kagaya Y., Toyoshima R., Okuda R., Usui H., Yamamoto A., Hattori T. LEAFY COTYLEDON1 controls seed storage protein genes through its regulation of FUSCA3 and ABSCISIC ACID INSENSITIVE3. Plant Cell Physiol. 2005;46:399–406. doi: 10.1093/pcp/pci048. [DOI] [PubMed] [Google Scholar]
- 166.Yamamoto A., Kagaya Y., Usui H., Hobo T., Takeda S., Hattori T. Diverse roles and mechanisms of gene regulation by the Arabidopsis seed maturation master regulator FUS3 revealed by microarray analysis. Plant Cell Physiol. 2010;51:2031–2046. doi: 10.1093/pcp/pcq162. [DOI] [PubMed] [Google Scholar]
- 167.Gazzarrini S., Tsuchiya Y., Lumba S., Okamoto M., McCourt P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev. Cell. 2004;7:373–385. doi: 10.1016/j.devcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 168.Hu Y., Zhou L., Huang M., He X., Yang Y., Liu X., Li Y., Hou X. Gibberellins play an essential role in late embryogenesis of Arabidopsis. Nat. Plants. 2018;4:289–298. doi: 10.1038/s41477-018-0143-8. [DOI] [PubMed] [Google Scholar]
- 169.Mu J., Tan H., Hong S., Liang Y., Zuo J. Arabidopsis transcription factor genes NF-YA1, 5, 6, and 9 play redundant roles in male gametogenesis, embryogenesis, and seed development. Mol. Plant. 2013;6:188–201. doi: 10.1093/mp/sss061. [DOI] [PubMed] [Google Scholar]
- 170.Hofmann F., Schon M.A., Nodine M.D. The embryonic transcriptome of Arabidopsis thaliana. Plant Reprod. 2019;32:77–91. doi: 10.1007/s00497-018-00357-2. [DOI] [PubMed] [Google Scholar]
- 171.Rodrigues A.S., Chaves I., Costa B.V., Lin Y.C., Lopes S., Milhinhos A., Van de Peer Y., Miguel C.M. Small RNA profiling in Pinus pinaster reveals the transcriptome of developing seeds and highlights differences between zygotic and somatic embryos. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-47789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.