Abstract
The purpose of this study was to evaluate the probiotic characteristics and neuroprotective effects of bacteria isolated from Korean fermented foods. Three bacterial strains (Lactobacillus fermentum KU200060, Lactobacillus delbrueckii KU200171, and Lactobacillus buchneri KU200793) showed potential probiotic properties, such as high tolerance against artificial gastric juice and bile salts, sensitivity to antibiotics, nonproduction of carcinogenic enzymes, and high adhesion to intestinal cells. Heat-killed L. fermentum KU200060 and L. buchneri KU200793 showed higher antioxidant activity than heat-killed L. delbrueckii KU200171. The conditioned medium (CM) was used to evaluate the reaction between HT-29 cells and each heat-killed strain. All CMs protected SH-SY5Y cells from 1-methyl-4-phenylpyridinium (MPP+)-induced toxicity. The expression of brain-derived neurotropic factor (BDNF) mRNA in HT-29 cells treated with CM containing heat-killed L. buchneri KU200793 was the highest. The CM significantly reduced the Bax/Bcl-2 ratio and increased BDNF mRNA expression in SH-SY5Y cells treated with MPP+. These results indicate that L. buchneri KU200793 can be used as a prophylactic functional food, having probiotic potential and neuroprotective effects.
Keywords: probiotics, kimchi, Lactobacillus buchneri, gut-brain-axis, neuroprotective effect
1. Introduction
Probiotics are defined as living microorganisms administered in appropriate amounts that confer a beneficial effect on the host [1]. To be used as probiotics, microorganisms should have the capacity to withstand physical and chemical conditions in the human body and to colonize and adhere to the intestinal epithelial cells [2]. Some probiotics, known as brain probiotics, produce gamma-aminobutyric acid and serotonin [3,4]. Some heat-killed lactobacilli alter microbiota composition [5] and affect the host’s neurological and psychiatric functions [6,7].
The conceptual framework of the gut-brain-axis (GBA) has existed for decades [8]. Bidirectional communication between gut microbiota and the components of the GBA influences normal homeostasis and may contribute to neurodegenerative diseases [9]. A recent study showed that gut-brain communication aids in the modulation of immune activity. Chronic proinflammatory immune activity is increasingly recognized as the central element of neurodegenerative disorders, such as Parkinson’s disease (PD) inflammation in the intestine appears particularly related to their pathogenesis [10].
Kimchi is a Korean traditional fermented food, containing biologically active components including polyphenols, vitamins, flavonoids, and lactic acid bacteria (LAB) [11]. Various kinds of microorganisms, including Leuconostoc sp., Lactobacillus sp., and Lactococcus sp., are mainly found in kimchi. Some kimchi bacteria have been verified for antioxidant and immunostimulatory activities [12], antiallergic effects [13,14], and anticancer activity [15].
Some Lactobacillus buchneri have been reported for probiotic use [16,17,18]. L. buchneri P12 isolated from pickled juice showed cholesterol reduction and antimicrobial activity [17]. L. buchneri isolated from kimchi showed the production of high γ-aminobutyric acid (GABA), which as a neurotransmitter, is involved in brain development [16]. In addition, there was production of GABA and ornithine during cheese fermentation [18].
The purpose of this study was to identify L. fermentum KU200060, L. delbrueckii KU200171, and L. buchneri KU200793 isolated from kimchi and to evaluate its probiotic potential, including its tolerance to gastric acid and bile salt conditions, enzyme production, adhesion ability to intestine, and antibiotic susceptibility characteristics. Additionally, this study investigated the neuroprotective effect of heat-killed L. fermentum KU200060, L. delbrueckii KU200171, and L. buchneri KU200793 against MPP+- induced cytotoxicity in SH-SY5Y cells.
2. Results and Discussion
2.1. Tolerance to Artificial Gastric Conditions
The ingested lactobacilli can pass through the stomach, which has a low pH environment (pH 2.5–3.5), and then pass through the intestinal tract containing approximately 0.3% (w/v) bile acid [19,20]. The tolerance levels of the Lactobacillus strains to artificial gastric juice and bile salts are shown in Table 1. The survival rates of L. fermentum KU200060 (100.47% ± 0.07%) and L. buchneri KU200793 (102.12% ± 0.35%) in gastric acid (0.3% pepsin, pH 2.5) were higher than those of L. rhamnosus GG (99.68% ± 0.26%) and L. delbrueckii KU200171 (62.68% ± 0.12%). L. plantarum 9 showed a survival rate lower than 70% at pH 2.5 and a survival rate higher than 50% at pH 2 [21]. These findings indicate that these isolates have acceptable survival rates in the gastrointestinal environment.
Table 1.
Tolerance of Lactobacillus strains to artificial gastric conditions and its adhesion ability to the HT-29 cell.
| LAB Strains | Survival Rate (%) | Adhesion Ability to HT-29 Cells (%) | |
|---|---|---|---|
| Gastric Acid Tolerance (0.3% Pepsin, pH 2.5) | Bile Salts Tolerance (0.3% Oxgall) | ||
| L. rhamnosus GG | 99.68 ± 0.26 c (1) | 100.41 ± 0.15 b | 2.34 ± 0.15 b |
| L. fermentum KU200060 | 100.47 ± 0.07 b | 102.24 ± 0.70 a | 1.18 ± 0.04 c |
| L. delbrueckii KU200171 | 62.68 ± 0.12 d | 102.43 ± 0.50 a | 1.83 ± 0.01 bc |
| L. buchneri KU200793 | 102.12 ± 0.35 a | 91.47 ± 0.46 c | 14.10 ± 0.45 a |
(1) a–d Different superscript letters in the same row indicate significant differences in each characteristic (p < 0.05). All values are mean ± standard error of triplicate experiments.
The survival rate of L. buchneri KU200793 (91.47% ± 0.46%) slightly decreased, whereas that of L. rhamnosus GG (100.41% ± 0.15%), L. fermentum 200060 (102.24% ± 0.70%), and L. delbrueckii KU200171 (102.43% ± 0.50%) increased under bile salt conditions (0.3% oxgall). Therefore, all the isolated strains pass through the gastrointestinal tract and persist in the intestinal tract. Previous studies suggested that, under acidic conditions, some LAB strains translocated the protons from the cytoplasm to the environment by an ATPase using ATP. So, they modify their cytoplasmic pH. Moreover, some LAB strains have bile salt hydrolase; they could grow with resistance to the bile salt condition [22,23]. According to research on similar strains, the probiotic strains L. buchneri 13-2-2 and L. buchneri 148-7-1 isolated from human fecal samples could live at pH 3.0 and 0.3% oxgall [24]. L. buchneri P2 could live at pH 3.0 for 3 h and in 0.3% oxgall for 14 h [17].
2.2. Enzyme Production of LAB Strains
To elicit probiotic properties, no harmful enzymes should be produced by the bacteria. β-glucuronidase is a typical harmful enzyme, which is associated with the induction of toxins, mutagens, and carcinogens [25]. None of the tested strains produced β-glucuronidase, measured using an API ZYM kit (Table 2). However, each strain of L. rhamnosus GG, L. fermentum KU200060, L. delbrueckii KU200171, and L. buchneri KU200793 showed the highest production of β-glucosidase, β-galactosidase, leucin arylamidase, and β-galactosidase, respectively. β-Glucosidase, β-galactosidase, and leucin arylamidase are known as beneficial enzymes for hydrolysis of the glycosidic bonds, lactase, and protease, respectively [12].
Table 2.
Enzyme activities of the Lactobacillus strains measured using the API ZYM kit.
| Enzyme | Enzyme Activity | |||
|---|---|---|---|---|
| L. rhamnosus GG | L. fermentum KU200060 | L. delbrueckii KU200171 | L. buchneri KU200793 | |
| Control | 0 (1) | 0 | 0 | 0 |
| Alkaline phosphate | 0 | 0 | 0 | 0 |
| Esterase | 2 | 1 | 0 | 0 |
| Esterase lipase | 1 | 1 | 0 | 0 |
| Lipase | 0 | 0 | 0 | 0 |
| Leucine arylamidase | 3 | 2 | 4 | 3 |
| Valine arylamidase | 3 | 0 | 3 | 2 |
| Cystine arylamidase | 0 | 0 | 0 | 0 |
| Trypsin | 0 | 0 | 0 | 0 |
| α-Chymotrypsin | 0 | 0 | 0 | 0 |
| Acid phosphatase | 1 | 1 | 1 | 0 |
| Naphthol-AS-BI-phosphohydrolase | 2 | 1 | 2 | 1 |
| α-Galactosidase | 0 | 4 | 0 | 0 |
| β-Galactosidase | 0 | 5 | 0 | 4 |
| β-Glucuronidase | 0 | 0 | 0 | 0 |
| α-Glucosidase | 3 | 1 | 0 | 0 |
| β-Glucosidase | 4 | 0 | 0 | 1 |
| N-Acetyl-β-glucosaminidase | 0 | 0 | 0 | 2 |
| α-Mannosidase | 0 | 0 | 0 | 0 |
| α-Fucosidase | 0 | 0 | 0 | 0 |
(1) 0, 0 nM; 1, 5 nM; 2, 10 nM; 3, 20 nM; 4, 30 nM; and 5, ≥40 nM.
2.3. Antibiotic Susceptibility of LAB Strains
Recent studies demonstrated that commensal bacteria including Lactobacillus species can deliver antibiotic resistance genes to another bacteria [26]. The main risk associated with these bacteria is that they can transfer resistance genes to pathogenic bacteria. Consequently, antibiotic susceptibility of probiotics should be tested to determine their safety. All tested LAB strains were resistant to gentamycin, kanamycin, streptomycin, and ciprofloxacin; however, they were susceptible to ampicillin, tetracycline, chloramphenicol, and doxycycline (Table 3). These results are acceptable based on the Clinical and Laboratory Standards Institute guideline [2,27].
Table 3.
Antibiotic resistances of the Lactobacillus strains.
| Antioxidant Activity | LAB Strains | |||
|---|---|---|---|---|
| L. rhamnosus GG | L. fermentum KU200060 | L. delbrueckii KU200171 | L. buchneri KU200793 | |
| Ampicillin | S (1) | S | S | S |
| Gentamicin | R | R | R | R |
| Kanamycin | R | R | R | R |
| Streptomycin | R | R | R | R |
| Tetracycline | S | S | S | S |
| Ciprofloxacin | R | R | R | R |
| Chloramphenicol | S | S | S | S |
| Doxycycline | S | S | S | S |
(1) S, susceptible; I, intermediate; and R, resistant.
2.4. Adhesion Ability of LAB Strains to HT-29 Intestinal Epithelial Cells
Adhesion ability of probiotic bacteria to epithelial cells is important for colonization and persistence in the intestinal tract. Moreover, probiotic bacteria that are superior in adhesion ability can competitively bind to the site of adhesion better, preventing the attachment of pathogenic bacteria [27].
To measure the adhesion level of LAB to human intestinal epithelial cells, its adhesion ability to HT-29 cells was analyzed (Table 1). L. buchneri KU200793 showed the best adhesion ability (14.10% ± 0.45%) compared to L. rhamnosus GG (2.34% ± 0.15%), L. fermentum KU200060 (1.18% ± 0.04%), and L. delbrueckii KU200171 (1.83% ± 0.01%). L. paraplantarum SC61 and Pediococcus pentosaceus SC28 have higher adhesion abilities to HT-29 cell (6.26% and 4.03%) than L. rhamnosus GG (2.74%) [27]. These results indicate that L. buchneri KU200793 had higher adhesion ability to HT-29 cell than other probiotic strains and could attach and colonize the human intestinal epithelial cells.
2.5. Antioxidant Effects of Heat-Killed LAB Strains
The results of in vitro antioxidant effects of heat-killed LAB strains are shown in Table 4. L. buchneri KU200793 showed DPPH scavenging activity of 23.04% at 109 CFU/mL, which was similar to that of L. fermentum KU200060 and L. rhamnosus GG. However, L. delbrueckii KU200171 showed lower antioxidant effects (17.20%). A stable ABTS radical, which has a blue-green color, is produced by the oxidation of ABTS with potassium persulfate. Radical scavenging activity is measured by the discoloration of ABTS [28]. ABTS radical scavenging activities of L. rhamnosus GG (90.92%), L. fermentum KU200060 (91.87%), and L. buchneri KU200793 (90.05%) were higher than the activity of L. delbrueckii KU200171 (68.33%). The inhibition rate of lipid peroxidation in LAB strains was determined by the β-carotene bleaching inhibition assay. L. buchneri KU200793 showed the highest inhibition rate of β-carotene and linoleic acid oxidation (38.42%). The inhibition rates were as follows: L. rhamnosus GG, 33.63%; L. fermentum KU200060, 28.49%; and L. delbrueckii KU200171, 16.09%.
Table 4.
Antioxidant activities of the Lactobacillus strains.
| Antioxidant Activity | LAB Strains | |||
|---|---|---|---|---|
| L. rhamnosus GG | L. fermentum KU200060 | L. delbrueckii KU200171 | L. buchneri KU200793 | |
| DPPH radical scavenging activity (%) | 23.76 ± 1.53 a (1) | 21.81 ± 1.62 a | 17.20 ± 0.48 b | 23.04 ± 0.88 a |
| ABTS radical scavenging activity (%) | 90.92 ± 1.91 a | 91.87 ± 3.27 a | 68.33 ± 1.91 b | 90.05 ± 3.27 a |
| Inhibition rate of β-carotene and linoleic acid oxidation (%) | 33.63 ± 2.60 ab | 28.49 ± 2.79 b | 16.09 ± 2.13 c | 38.42 ± 3.56 a |
(1) a–c Different superscript letters in the same row indicate significant differences in each characteristic (p < 0.05). All values are the mean ± standard error of triplicate experiments.
As a result, heat-killed L. fermentum KU200060 and L. buchneri KU200793 showed higher antioxidant activity than heat-killed L. delbrueckii KU200171. Heat-killed L. plantarum Ln 1 showed 17.60% DPPH radical scavenging activity, 70.18% ABTS radical scavenging activity, and 58.3% inhibition rate of lipid peroxidation at 107 CFU/mL [11]. L. acidophilus ATCC 4356 showed 20.8% of DPPH radical scavenging activity in intracellular extract [29]. Antioxidative properties of LAB have been considered as strain-specific depending on the cell wall component, antioxidant enzymes, and exopolysaccharides [20,30]. A recent study referred that oxidative damage could initiate alpha-synuclein, which is a protein involved in Parkinson’s disease [4]. Therefore, these antioxidant effects of heat-killed probiotics may underlie their neuroprotective effects.
2.6. Neuroprotective Effects of CM Against MPP+ -Induced Cell Death
The cell viability of the conditioned medium (CM) prepared with heat-killed LAB strains L. rhamnosus GG, L. fermentum KU200060, L. delbrueckii KU200171, and L. buchneri KU200793 were 107.2% ± 6.0%, 96.0% ± 2.4%, 107.7% ± 2.5%, and 108.6% ± 9.9%, respectively (data not shown). As a result, CM did not show any significant cytotoxicity to SH-SY5Y cells in all the strains.
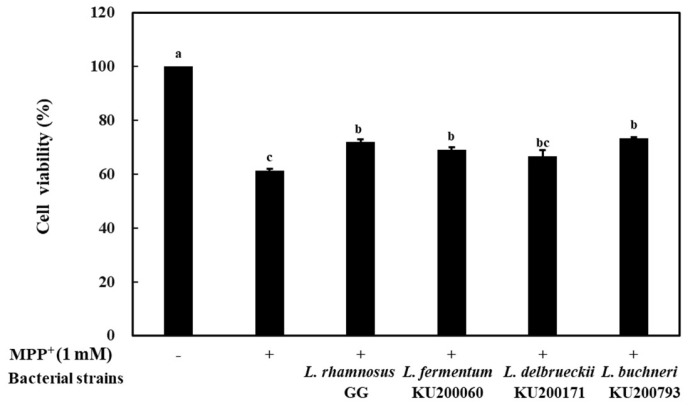
Treatment of neuroblastoma cells with MPP+ increases the levels of reactive oxygen species (ROS), leading to the death of dopaminergic neurons in the substantia nigra [31]. The neuroprotective effect of the bacterial strains was determined by assessing the viability of SH-SY5Y cells following MPP+ treatment (Figure 1). The viability of SH-SY5Y cells decreased to 61.3% with 1 mM MPP+ treatment. However, 4 h pretreatment with CM prepared using heat-killed LAB strains showed cell protective effects. The viability of SH-SY5Y cells following treatment with CM-containing L. rhamnosus GG, L. fermentum KU200060, L. delbrueckii KU200171, and L. buchneri KU200793 was 72.0% ± 1.0%, 69.2% ± 0.9%, 66.8% ± 2.2%, and 73.4% ± 0.4%, respectively. These results suggest that CM with LAB strains has potential neuroprotective effects against MPP+-treated SH-SY5Y cells. CM using mixed probiotics including Lactobacillus acidophilus, Bifidobacterium bifidum, Bifidobacterium animalis subsp. lactis, Lactobacillus salivarius, or Lactobacillus paracasei has been shown to have neuroprotective effects in SH-SY5Y cells treated with 2 μM MPP+ [32].
Figure 1.
Neuroprotective effect of conditioned medium (CM) on MPP+ (1 mM)-stressed SH-SY5Y cells. L. rhamnosus GG, CM of heat-killed L. rhamnosus GG; L. fermentum 200060, CM of heat-killed L. fermentum KU200060; L. delbrueckii 200171, CM of heat-killed L. delbrueckii KU200171; and L. buchneri 200793, CM of heat-killed L. buchneri KU200793. All values are expressed as mean ± standard error of triplicate experiments. Different superscript letters on each bar represent significant differences (p < 0.05).
2.7. mRNA Level of the Brain-Derived Neurotropic Factor (BDNF) in HT-29 Cells Treated with Heat-Killed LAB Strains
BDNF is a homodimeric protein with signaling actions mediated via the tyrosine kinase B (trkB) receptor, and it is the most abundant neurotrophic factor in the brain [33]. It has powerful synaptic effects, which promote synaptic transmission, synaptic plasticity, and synaptic growth [34].
To confirm the mRNA expression level of BDNF in HT-29 cells, RT-PCR analysis was performed (Figure 2). The expression levels of BDNF in HT-29 cells treated with heat-killed L. rhamnosus GG, L. fermentum KU200060, L. delbrueckii KU200171, and L. buchneri KU200793 were increased by 4.6-, 1.2-, 1.4-, and 5.8-fold compared to the levels in the negative control, respectively. Importantly, treatment with heat-killed L. buchneri KU200793 caused the highest increase in BDNF expression. Heat-killed Ruminococcus albus has been shown to increase BDNF levels in human intestinal epithelial cells by approximately 1.71-fold [35]. L. buchneri KU200793 showed better effects than that observed with R. albus treatment in human intestinal epithelial cells.
Figure 2.
mRNA expression levels of the brain-derived neurotropic factor (BDNF) gene in heat-killed lactic acid bacteria (LAB) treated HT-29 cells using RT-PCR. The fold change was calculated based on normalization with GAPDH gene expression. Control group was treated with PBS. L. rhamnosus GG, heat-killed L. rhamnosus GG; L. fermentum 200060, heat-killed L. fermentum KU200060; L. delbrueckii 200171, heat-killed L. delbrueckii KU200171; and L. buchneri 200793, heat-killed L. buchneri KU200793. All values are expressed as mean ± standard error of triplicate experiments. Different superscript letters on each bar represent significant differences (p < 0.05).
2.8. Bax/Bcl-2 and BDNF Expression Levels of CM in MPP+ Stressed SH-SY5Y Cells
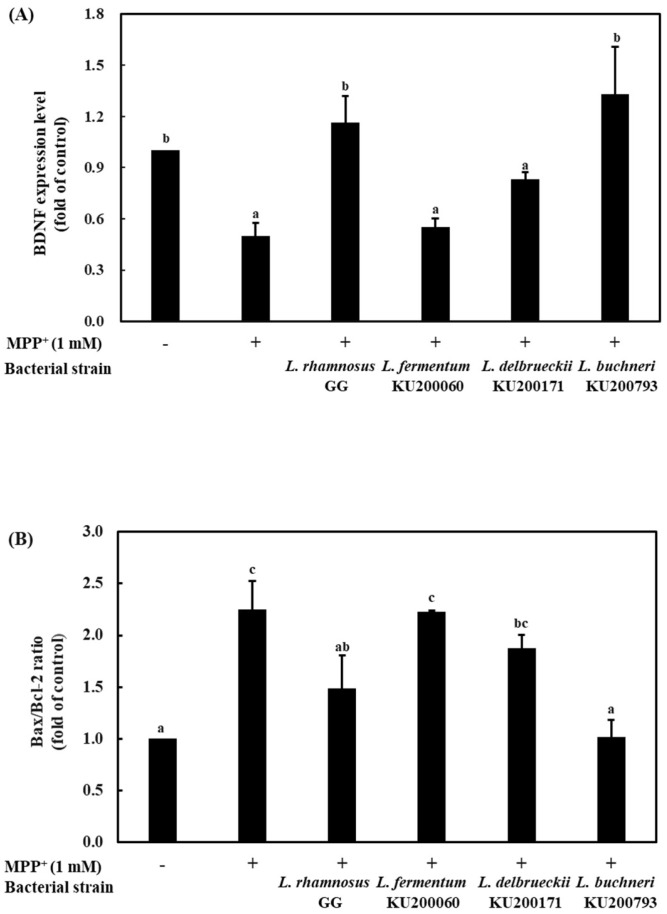
BDNF can enhance the survival of dopaminergic neurons and can protect them against the neurotoxic effects of MPP+ and mRNA expression of BDNF in the substantia nigra, which might affect the death of nigral dopaminergic neurons observed in Parkinson’s disease (PD) [36]. To determine the expression of BDNF in SH-SY5Y cells, RT-PCR was carried out (Figure 3A). Treatment with 1 mM MPP+ caused a 0.5-fold reduction in BDNF expression, whereas treatment with the CMs increased BDNF expression levels. L. rhamnosus GG and L. buchneri KU200793 caused a 1.2- and 1.3-fold increase in BDNF expression compared to that in the negative control, respectively. L. rhamnosus GG strain has been reported on neuroprotective potential using cell viability and BDNF mRNA expression in hippocampal neurons [37]. In this study, L. buchneri KU200793 has a similar BDNF expression as the L. rhamnosus GG strain. Therefore, L. buchneri KU200793 can protect MPP+-stressed SH-SY5Y cells more effectively.
Figure 3.
mRNA expression of (A) BDNF and (B) apoptosis-related genes (Bax/Bcl-2 ratio) in SH-SY5Y cells using RT-PCR. L. rhamnosus GG, CM of heat-killed L. rhamnosus GG; L. fermentum 200060, CM of heat-killed L. fermentum KU200060; L. delbrueckii 200171, CM of heat-killed L. delbrueckii KU200171; and L. buchneri 200793, CM of heat-killed L. buchneri KU200793. All values are mean ± standard error of triplicate experiments. Different superscript letters on each bar represent significant differences (p < 0.05).
Bax (Bcl-2-associated X protein), which influences outer membrane permeability, promotes the releases of cytochrome C from the inter membrane of the mitochondria and, finally, induces apoptosis [38]. Bcl-2 (B-cell lymphoma) has antiapoptotic properties and stabilizes membrane permeability, thus preserving mitochondrial integrity, suppressing the release of cytochrome C, and inhibiting apoptosis [39]. Cell survival depends on the balance between pro- and antiapoptotic proteins of the Bcl-2 family. The Bax/Bcl-2 ratio is a better indicator of apoptosis than the absolute concentration of either Bax or Bcl-2 [40]. To investigate the neuroprotective effect of CM in SH-SY5Y cells, Bax and Bcl-2 gene expression levels were evaluated by RT-PCR analysis (Figure 3B). Bax/Bcl-2 mRNA expression ratio increased to 2.3-fold following 1 mM MPP+ treatment compared to that in the negative control. However, L. buchneri KU200793 significantly decreased the Bax/Bcl-2 ratio to almost normal levels (by 1.0-fold). L. rhamnosus GG, L. fermentum KU200060, and L. delbrueckii KU200171 decreased the ratio by 1.5-, 1.9-, and 2.2-fold, respectively. These results indicate that L. buchneri KU200793 was effective in protecting MPP+-induced cell apoptosis. The extract of the traditional herb Gastrodia elata blume has been shown to effectively reduce the Bax/Bcl-2 mRNA expression ratio in MPP+-treated cells. Importantly, pretreatment decreased the Bax/Bcl-2 mRNA expression ratio by 1.5-fold [31]. Compared with previous results, L. buchneri KU200793 effectively attenuated apoptosis.
3. Materials and Methods
3.1. Bacterial Strains Culture Condition
L. fermentum KU200060 and L. buchneri KU200793 were isolated from watery kimchi and cabbage kimchi, respectively, and L. delbrueckii KU200171 was isolated from soy-sauced based fermented crab. All samples (1 g) were serially diluted and plated with using Lactobacillus selective medium (BD Biosciences, Franklin Lakes, NJ, USA) and incubated at 37 °C for 24 h. A colony was inoculated and incubated in de Man, Rogosa and Sharpe (MRS; BD Biosciences) broth at 37 °C for 24 h. All strains were identified through 16S rRNA sequencing. L. rhamnosus GG was obtained from the Korean Collection for Type Cultures (KCTC; Daejeon, Korea) and was used as a reference probiotic strain. LAB strains were incubated in MRS broth at 37 °C for 24 h. To harvest cells that were intact, bacterial cultures were harvested using centrifuge at 14,240 × g at 4 °C for 5 min. The bacterial cells were washed three times and resuspended in phosphate-buffered saline (PBS; Gibco, Grand Island, NY, USA).
3.2. Cell Culture Condition
HT-29 (human colon adenocarcinoma; KCLB 30038) and SH-SY5Y (neuroblastoma; KCLB 22266) cells were used for intestine adherence and cytotoxicity experiments, respectively. Each cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) at Roswell Park Memorial Institute (RPMI) 1640 (Gibco) containing heat-inactivated 10% fetal bovine serum (FBS; Gibco) and penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C in a 5% CO2 incubator, respectively.
3.3. Tolerance to Artificial Gastric Conditions
To measure the tolerance of the strains for the gastric conditions, the experiment was performed according to a previous method [2]. The strains were incubated in MRS broth at 37 °C for overnight. MRS broth with artificial gastric juice (0.3% pepsin (Sigma-Aldrich), pH 2.5) and bile salts (0.3% oxgall (BD Biosciences)) was prepared, and then LAB strains were inoculated at a final concentration of 1 × 107 CFU/mL and incubated at 37 °C for 3 and 24 h, respectively. The viable cells were counted by dilution and plating on MRS agar after each treatment. Survival rate (%) was determined as follows:
| Survival rate (%) = Nt/Ni ×100 | (1) |
Ni and Nt represented the viable bacterial cell number before treatment (CFU/mL) and after treatment (CFU/mL), respectively.
3.4. Adhesion Ability to HT-29 Cells
The adhesion ability of LAB strains was evaluated using HT-29 cells. HT-29 cells were cultured in a 24-well plate (final concentration: 1 × 105 cells/well) and incubated at 37 °C for overnight. After incubation, LAB strains (1 × 108 CFU/well) were cultured with HT-29 cells for 37 °C for 2 h. Nonadherent bacterial cells were washed three times with PBS. Then, 1% Triton X-100 (Sigma-Aldrich) solution was treated to detach the adherent bacterial cells. The number of adherent bacterial cells to the HT-29 was evaluated by serial dilutions, spreading on MRS agar, and incubating at 37 °C for 24 h. Finally, there was counting of viable cells on MRS plates. Adhesion ability was also determined using Equation (1).
3.5. Enzyme Production Evaluated Using API ZYM Kit
Enzyme production was evaluated using API ZYM kit (bioMérieux, Marcy-l’Étoile, France). Bacterial samples (70 μL) were inoculated in each cupule and incubated at 37 °C for 4 h. After incubation, ZYM A and ZYM B reagents were added to each cupule in turn. Enzyme production was evaluated using color intensity scores from 0 (no activity) to 5 (≥40 nM).
3.6. Antibiotic Susceptibility
Antibiotic susceptibility of LAB strains was evaluated using the disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [27]. Susceptibility to the following eight antibiotics was evaluated: 0.2 mg/mL of ampicillin, gentamicin, and streptomycin; 0.6 mg/mL of kanamycin, tetracycline, chloramphenicol, and doxycycline; and 0.1 mg/mL of ciprofloxacin. One hundred-fifty microliters of each strain (1 × 106 CFU/mL) was spread on an MRS agar plate, and paper discs were set on the plate. Then, the antibiotics were loaded on the paper disc and incubated at 37 °C for overnight. After incubation, the inhibition zone (mm) was measured.
3.7. Preparation of Heat-Killed LAB
Bacteria cultures (1 × 109 CFU/mL) were harvested by centrifugation at 14,240 × g at 4 °C for 5 min, and the supernatant was removed. The bacterial cells were washed three times and were resuspended in phosphate-buffered saline. Then, the washed bacteria were heated at 121 °C for 15 min.
3.8. Antioxidant Activity of Heat-Killed LAB Strains
The antioxidant activity of the heat-killed LAB strains was measured by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The assay was evaluated according to a previous method with some modifications [12]. DPPH solution of 400 μM was prepared using ethanol, and 500 μL of heat-killed LAB samples or PBS (control) was mixed with 500 μL of this solution. The mixture reacted in dark conditions at 37 °C for 30 min. The absorbance of the mixture was measured at 517 nm and calculated using Equation (2).
| Radical scavenging activity (%) = (1-Asample/Acontrol) × 100 | (2) |
The radical scavenging activity of 2,2′-azinobis (2-ethylbenzothiazoline-6-sulfonate) (ABTS) was measured as described by a previous method with some modifications [2]. To prepare ABTS solution, 7 mM ABTS and 5 mM potassium persulfate was mixed with 20 mM sodium phosphate buffer (pH 7.4), and they were allowed to react at room temperature in the dark for 16 h. Using 20 mM sodium phosphate buffer, ABTS+ solution was diluted until the final absorbance changed to 0.7 ± 0.02 at 734 nm. Thereafter, 100 μL of heat-killed bacterial samples and PBS (control) were mixed with 900 μL of ABTS+ solution and reacted at 37 °C for 10 min in the dark condition. The percentage scavenging of ABTS radicals was measured at 734 nm and was calculated using Equation (2).
The β-carotene bleaching inhibition assay was measured as described by a previous method with some modifications [20]. β-carotene solution was prepared using 3 mg of β-carotene, 66 μL of linoleic acid, 300 μL of Tween 80, and 10 mL of chloroform. The solution was evaporated to remove chloroform at 40 °C under vacuum using a rotary evaporator and were diluted with 75 mL of distilled water. Heat-killed bacterial samples (200 μL) and control (PBS, 200 μL) were mixed immediately with 4 mL of the emulsion and were reacted in a water bath at 50 °C for 2 h. Finally, the absorbance of reacted solution was measured at 470 nm for 0 and 2 h. Inhibition of β-carotene and linoleic acid oxidation was calculated as the following formula:
Inhibition of β-carotene and linoleic acid oxidation (%) = (AS,2 h -AC,2 h) / (AC,0 h -AC,2 h) × 100
AC and AS represent the absorbance of control and sample with each time, respectively.
3.9. Preparation of LAB-Conditioned Medium (CM)
HT-29 cells were plated in 6-well plates at a concentration of 5 × 105 cells/mL and were incubated to form a monolayer. Next, heat-killed LAB strains and PBS (for control) treated the cell and incubated 24 h. After 24 h, the cell supernatant was centrifugated at 14,000 × g for 5 min. Subsequently, the supernatant was filtered with a 0.45 μm pore size syringe filter and was stored at −80 °C.
3.10. Cytotoxicity Measurement
3.10.1. Cytotoxicity Effect of CM on SH-SY5Y Cell
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to measure the effect of CM on the survival rates of SH-SY5Y cells. SH-SY5Y cells were plated in 96-well plates at a concentration of 1 × 105 cells/well and incubated 24 h. After overnight, CM was applied in the SH-SY5Y cells. After then, MTT dissolved in PBS solution (5 mg/mL) was added to the cells, and the plates were incubated at 37 °C for 1 h. The solution was removed, and 100 μL of DMSO was added to each well. After 15 min, the absorbance was measured at 540 nm using an ELISA reader (Molecular Devises, Sunnyvale, CA, USA), and viability was calculated as in Equation (3).
| Cell viability (%) = As/Ac × 100 | (3) |
As and Ac represent the absorbance of sample treatment and control without sample, respectively.
3.10.2. Neuroprotective Effect of CM on MPP+-stressed SH-SY5Y Cells.
Cell cytotoxicity was induced using 1-methyl-4-phenylpyridinium (MPP+) (Sigma-Aldrich, St. Louis, MO, USA) to determine the protective effect of CM on SH-SY5Y cells. Cells (1 × 105 cells/well) were seeded into 96-well plates. Then, cells were treated with CM for 4 h, and subsequently, MPP+ (1 mM) was used in treating the cells for 20 h.
The media were removed, and MTT solution (5 mg/mL) was added and incubated for 1 h. Next, the solution was removed, and 100 μL DMSO was added to each well. Absorbance was measured at 540 nm using an ELISA reader. Then, cell viability (%) was calculated using Equation (3).
3.11. Relative Quantification of Gene Expression by RT-PCR
HT-29 and SH-SY5Y cells were plated in 6-well plates at 1 × 106 cells/well and were incubated for 7 days and 48 h, respectively. BDNF expression in HT-29 cells was induced by inoculation of 1 × 108 CFU/well of heat-killed cells and incubated for 24 h. To measure the neuroprotective effect of CM, SH-SY5Y cells were applied for 4 h using CM, and subsequently, 1 mM MPP+ was used in treating the cells for 20 h.
Total RNA was using RNeasy® Mini Kit (QIAGEN, Hilden, Germany) and cDNA was synthesized using SensiFAST™ cDNA Synthesis Kit (Bioline, London, UK). The expression levels of cytokines related to cell apoptosis and the neurotrophic factor were determined using SYBR Green PCR Master mix with real-time PCR (PikoReal 96, Scientific Pierce, Waltham, MA, USA). The primers are listed in Table 5. Real-time PCR was performed at 95 °C for 2 min for initial denaturation, and subsequently, 40 cycles were performed as the following conditions: denaturation at 95 °C for 5 s and 60 °C for 15 s for annealing/extension. The results were analyzed using the delta-delta Cq method. The melting curve was used for analyses to measure reaction specificity.
Table 5.
Primer sequences related to the neuroprotective effect used in real-time PCR.
| Primer (1) | Sequence (5′-3′) | Reference |
|---|---|---|
| BDNF | (sense) CAAACATCCGAGGACAAGGTGG | [35] |
| (antisense) CTCATGGACATGTTTGCAGCATCT | ||
| Bax | (sense) GTGGTTGCCCTCTTCTACTTTGC | |
| (antisense) GAGGACTCCAGCCACAAAGATG | ||
| Bcl-2 | (sense) CGGCTGAAGTCTCCATTAGC | |
| (antisense) CCAGGGAAGTTCTGGTGTGT | ||
| GAPDH | (sense) GAGTCAACGGATTTGGTCGT | |
| (antisense) GACAAGCTTCCCGTTCTCAG |
(1) BDNF, brain-derived neurotrophic factor; Bax, bcl-2-associated X protein; Bcl-2, B-cell lymphoma; and GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
3.12. Statistical Analysis
All experiments were repeated in triplicates, and one-way analysis of variance (ANOVA) and Duncan’s multiple range tests were conducted using SPSS software (Version 18; SPSS Inc., Chicago, IL, USA) to measure significant differences (p < 0.05). Data are presented as mean ± standard error.
4. Conclusions
L. fermentum KU200060, L. delbrueckii KU200171, and L. buchneri KU200793 isolated from Korean fermented foods have probiotic potential. These bacteria showed high gastric acid and bile salt tolerance, safe enzyme activity, acceptable antibiotic susceptibility, and a high HT-29 adhesion ability. Among the three strains, heat-killed L. buchneri KU200793 showed relatively good antioxidant activity. Cells treated with CM containing heat-killed L. buchneri KU200793 had the highest levels of BDNF and survival rates. L. buchneri KU200793 can be used as a functional food ingredient to prevent Parkinson’s disease. However, further in vivo studies are needed to verify that L. buchneri KU200793 is effective as a neuroprotective agent.
Acknowledgments
This paper was supported by Konkuk University, Seoul, Korea in 2019.
Abbreviations
| MPP+ | 1-methyl-4-phenylpyridinium |
| GBA | gut-brain-axis |
| PD | Parkinson’s disease |
| LAB | lactic acid bacteria |
| BDNF | brain-derived neurotropic factor |
| GABA | γ-aminobutyric acid |
| CM | conditioned medium |
| ROS | reactive oxygen species |
| TrkB | tyrosine kinase B |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azinobis (2-ethylbenzothiazoline-6-sulfonate) |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
Author Contributions
M.-J.C. was involved in the experimental design, conducting the experiments, and writing the manuscript. S.-M.L. was involved in the experiments design. N.-K.L. was involved in the writing, review, and editing of the manuscript. H.-D.P. was involved in the administration of the project. All authors reviewed and agree to the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mary E.S. Probiotics: Considerations for human health. Nutr. Rev. 2003;61:91–99. doi: 10.1301/nr.2003.marr.91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S.J., Lee J.E., Lim S.M., Kim Y.J., Lee N.K., Paik H.D. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci. Biotechnol. 2019;28:491–499. doi: 10.1007/s10068-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinan T.G., Stanton C., Cryan J.F. Psychobiotics: A Novel Class of Psychotropic. Biol. Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Pardo P., Kliest T., Dodiya H.B., Broersen L.M., Garssen J., Keshavarzian A., Kraneveld A.D. The gut-brain axis in Parkinson’s disease: Possibilities for food-based therapies. Eur. J. Pharmacol. 2017;817:86–95. doi: 10.1016/j.ejphar.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Warda A.K., Rea K., Fitzgerald P., Hueston C., Gonzalez-Tortuero E., Dinan T.G., Hill C. Heat-killed lactobacilli alter both microbiota composition and behavior. Behav. Brain Res. 2019;362:213–223. doi: 10.1016/j.bbr.2018.12.047. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa R., Fukushima H., Nakakita Y., Kado H., Kida S. Dietary heat-killed Lactobacillus brevis SBC8803 (SBL88™) improves hippocampus-dependent memory performance and adult hippocampal neurogenesis. Neuropsychopharmacol. Rep. 2019;39:140–145. doi: 10.1002/npr2.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maehata H., Kobayashi Y., Mitsuyama E., Kawase T., Kuhara T., Xiao J.Z., Tsukahara T., Toyoda A. Heat-killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci. Biotechnol. Biochem. 2019;83:1239–1247. doi: 10.1080/09168451.2019.1591263. [DOI] [PubMed] [Google Scholar]
- 8.Sherman M.P., Zaghouani H., Niklas V. Gut microbiota, the immune system, and diet influence the neonatal gut-brain axis. Pediatr. Res. 2014;77:127–135. doi: 10.1038/pr.2014.161. [DOI] [PubMed] [Google Scholar]
- 9.Foster J., McVey Neufeld K.A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Houser M.C., Tansey M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis. 2017;3:3. doi: 10.1038/s41531-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang H.J., Song M.W., Lee N.K., Paik H.D. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. J. Food Sci. Technol. 2018;55:3174–3180. doi: 10.1007/s13197-018-3245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Son S.H., Yang S.J., Jeon H.L., Yu H.S., Lee N.K., Park Y.S., Paik H.D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb. Pathog. 2018;125:486–492. doi: 10.1016/j.micpath.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Lee N.K., Kim S.Y., Han K.J., Eom S.J., Paik H.D. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT Food Sci. Technol. 2014;58:130–134. doi: 10.1016/j.lwt.2014.02.028. [DOI] [Google Scholar]
- 14.Lee N.K., Han K.J., Son S.H., Eom S.J., Lee S.K., Paik H.D. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT Food Sci. Technol. 2015;64:1036–1041. doi: 10.1016/j.lwt.2015.07.019. [DOI] [Google Scholar]
- 15.Kim B.K., Song J.L., Ju J.H., Kang S.A., Park K.Y. Anticancer effects of kimchi fermented for different times and with added ingredients in human HT-29 colon cancer cells. Food Sci. Biotechnol. 2015;24:629–633. doi: 10.1007/s10068-015-0082-3. [DOI] [Google Scholar]
- 16.Cho Y.R., Chang J.Y., Chang H.C. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechn. 2007;17:104–109. [PubMed] [Google Scholar]
- 17.Zeng X.Q., Pan D.D., Guo Y.X. The probiotic properties of Lactobacillus buchneri P2. J. Appl. Microbiol. 2009;108:2059–2066. doi: 10.1111/j.1365-2672.2009.04608.x. [DOI] [PubMed] [Google Scholar]
- 18.Fröhlich-Wyder M.T., Guggisberg D., Badertscher R., Wechsler D., Wittwer A., Irmler S. The effect of Lactobacillus buchneri and Lactobacillus parabuchneri on the eye formation of semi-hard cheese. Int. Dairy J. 2013;33:120–128. doi: 10.1016/j.idairyj.2013.03.004. [DOI] [Google Scholar]
- 19.Han Q., Kong B., Chen Q., Sun F., Zhang H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Foods. 2017;32:391–400. doi: 10.1016/j.jff.2017.03.020. [DOI] [Google Scholar]
- 20.Jang H.J., Lee N.K., Paik H.D. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci. Biotechnol. 2019;28:1521–1528. doi: 10.1007/s10068-019-00576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gharbi Y., Fhoula I., Ruas-Madiedo P., Afef N., Boudabous A., Gueimonde M., Ouzari H.I. In-vitro characterization of potentially probiotic Lactobacillus strains isolated from human microbiota: Interaction with pathogenic bacteria and the enteric cell line HT-29. Ann. Microbiol. 2019;69:61–72. doi: 10.1007/s13213-018-1396-1. [DOI] [Google Scholar]
- 22.Charalampopoulos D., Pandiella S.S., Webb C. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int. J. Food Microbiol. 2003;82:133–141. doi: 10.1016/S0168-1605(02)00248-9. [DOI] [PubMed] [Google Scholar]
- 23.Patel A.K., Singhania R.R., Pandey A., Chincholkar S.B. Probiotic bile salt hydrolase: Current developments and perspectives. Appl. Biochem. Biotechnol. 2009;162:166–180. doi: 10.1007/s12010-009-8738-1. [DOI] [PubMed] [Google Scholar]
- 24.Kõll P., Mändar R., Smidt I., Hütt P., Truusalu K., Mikelsaar R.H., Shchepetova J., Krogh-Andersen K., Marcotte H., Hammarström L., et al. Screening and evaluation of human intestinal lactobacilli for the development of novel gastrointestinal probiotics. Curr. Microbiol. 2010;61:560–566. doi: 10.1007/s00284-010-9653-y. [DOI] [PubMed] [Google Scholar]
- 25.Shin H.J., Choi H.J., Kim D.W., Ahn C.S., Lee Y.G., Jeong Y.K., Joo W.H. Probiotic potential of Pediococcus pentosaceus BCNU 9070. J. Life Sci. 2012;22:1194–1200. doi: 10.5352/JLS.2012.22.9.1194. [DOI] [Google Scholar]
- 26.Mathur S., Singh R. Antibiotic resistance in food lactic acid bacteria-a review. Int. J. Food Microbiol. 2005;105:281–295. doi: 10.1016/j.ijfoodmicro.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Son S.H., Jeon H.L., Jeon E.B., Lee N.K., Park Y.S., Kang D.K., Paik H.D. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT Food Sci. Technol. 2017;85:181–186. doi: 10.1016/j.lwt.2017.07.018. [DOI] [Google Scholar]
- 28.Morales G., Paredes A. Antioxidant activities of Lampaya medicinalis extracts and their main chemical constituents. BMC Complement. Altern. Med. 2014;14:1–12. doi: 10.1186/1472-6882-14-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin M.Y., Chang F.J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig. Dis. Sci. 2000;45:1617–1622. doi: 10.1023/A:1005577330695. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Hu P., Fan M., Liao Q. Study on effect elements of exopolysaccharide production of Lactobacillus kimchi SR8 and DPPH radical scavenging activity. J. Food Nutr. Res. 2017;5:928–934. doi: 10.12691/jfnr-5-12-8. [DOI] [Google Scholar]
- 31.An H., Kim I.S., Koppula S., Kim B.W., Park P.J., Lim B.O., Choi W.S., Lee K.H., Choi D.K. Protective effects of Gastrodia elata Blume on MPP+-induced cytotoxicity in human dopaminergic SH-SY5Y cells. J. Ethnopharmacol. 2010;130:290–298. doi: 10.1016/j.jep.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Michael D.R., Davies T.S., Loxley K.E., Allen M.D., Good M.A., Hughes T.R., Plummer S.F. In vitro neuroprotective activities of two distinct probiotic consortia. Benef. Microbes. 2019;10:437–447. doi: 10.3920/BM2018.0105. [DOI] [PubMed] [Google Scholar]
- 33.Szapacs M.E., Mathews T.A., Tessarollo L., Ernest Lyons W., Mamounase L.A., Andrews A.M. Exploring the relationship between serotonin and brain-derived neurotrophic factor: Analysis of BDNF protein and extra neuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J. Neurosci. Methods. 2004;140:81–92. doi: 10.1016/j.jneumeth.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Lu B., Nagappan G., Guan X., Nathan J.P., Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 35.Park J.E., Lee J.Y., Yeom Z., Heo D.H., Lim Y.H. Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci. Rep. 2017;7:14520. doi: 10.1038/s41598-017-15163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howells D.W., Porritt M.J., Wong J.Y., Batchelor P.E., Kalnins R., Hughes A.J., Donnan G.A. Reduced BDNF mRNA expression in the Parkinson’s disease substantia nigra. Exp. Neurol. 2000;166:127–135. doi: 10.1006/exnr.2000.7483. [DOI] [PubMed] [Google Scholar]
- 37.Cheng R., Xu T., Zhang Y., Wang F., Zhao L., Jiang Y., He F. Lactobacillus rhamnosus GG and Bifidobacterium bifidum TMC3115 Can Affect Development of Hippocampal Neurons Cultured In Vitro in a Strain-Dependent Manner. Probiotics Antimicrob. Proteins. 2019:1–11. doi: 10.1007/s12602-019-09571-4. [DOI] [PubMed] [Google Scholar]
- 38.Crompton M. Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr. Opin. Cell Biol. 2000;12:414–419. doi: 10.1016/S0955-0674(00)00110-1. [DOI] [PubMed] [Google Scholar]
- 39.Jung H.W., Jin G.Z., Kim S.Y., Kim Y.S., Park Y.K. Neuroprotective effect of methanol extract of Phellodendri cortex against 1-methyl-4-phenylpyridinium (MPP+)-induced apoptosis in PC-12 cells. Cell Biol. Int. 2009;33:957–963. doi: 10.1016/j.cellbi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Jung T.W., Lee J.Y., Shim W.S., Kang E.S., Kim J.S., Ahn C.W., Lee H.C., Cha B.S. Adiponectin protects human neuroblastoma SH-SY5Y cells against MPP+-induced cytotoxicity. Biochem. Biophys. Res. Commun. 2006;343:564–570. doi: 10.1016/j.bbrc.2006.02.186. [DOI] [PubMed] [Google Scholar]