Abstract
Traditionally, children presenting with appendicitis are referred for urgent appendectomy. Recent improvements in the quality and availability of diagnostic imaging allow for better pre-operative characterization of appendicitis, including severity of inflammation; size of the appendix; and presence of extra-luminal inflammation, phlegmon, or abscess. These imaging advances, in conjunction with the availability of broad spectrum oral antibiotics, allow for the identification of a subset of patients with uncomplicated appendicitis that can be successfully treated with antibiotics alone. Recent studies demonstrated that antibiotics alone are a safe and efficacious treatment alternative for patents with uncomplicated appendicitis.
The objective of this study is to perform a multi-institutional trial to examine the effectiveness of non-operative management of uncomplicated pediatric appendicitis across a group of large children’s hospitals. A prospective patient choice design was chosen to compare non-operative management to surgery in order to assess effectiveness in a broad population representative of clinical practice in which non-operative management is offered as an alternative to surgery. The risks and benefits of each treatment are very different and a “successful” treatment depends on which risks and benefits are most important to each patient and his/her family. The patient-choice design allows for alignment of preferences with treatment.
Patients meeting eligibility criteria are offered a choice of non-operative management or appendectomy. Primary outcomes include determining the success rate of non-operative management and comparing differences in disability days, and secondarily, complication rates, quality of life, and healthcare satisfaction, between patients choosing non-operative management and those choosing appendectomy.
Keywords: Appendicitis, Non-operative management, Antibiotics, Appendectomy, Patient choice trial
1. Introduction
Appendicitis is the most common indication for emergent abdominal surgery in both adults and children [1]. In the United States, over 180,000 adults and 70,000 children undergo appendectomy annually [2–4]. Although curative, appendectomy is a major intra-abdominal procedure requiring general anesthesia with associated peri-operative risks and post-operative pain and disability. Reported rates of peri-operative complications after appendectomy for uncomplicated appendicitis range between 5 and 15%, with serious complications (including need for readmission or reoperation) occurring in 1–7% of patients [5–13]. Recovery from an uncomplicated appendectomy is associated with a period of disability for both the child and caregiver [5,6,14,15].
Non-operative management has been shown to be safe and efficacious in several randomized controlled trials and meta-analyses comparing appendectomy to antibiotics alone in adults [5,6,15–24]. Data in children is more limited but multiple studies support that initial non-operative management is safe and efficacious [4,14,25–29]. In these studies, non-operative management has been associated with a 1 year success rate > 70%, decreased disability days, and lower costs.
With growing evidence for efficacy and safety, the effectiveness of offering non-operative management as a first-line treatment strategy for uncomplicated appendicitis across a larger population needs to be established to allow for widespread acceptance and adoption into clinical practice. This study will primarily establish the success rate of non-operative management and compare differences in treatment-related disability and secondly will compare health-related quality of life (HRQO)L, healthcare satisfaction, and treatment-associated complications between non-operative management and surgery in children with uncomplicated appendicitis across 10 children’s hospitals.
This trial employs a patient choice design that places an essential emphasis on incorporating the child-family perspective, shared decision making, and treatment preferences into trial design. Families engaged in shared decision making in clinical care experience improved medical outcomes [8,30–32]. In a trial evaluating an invasive intervention, such as surgery, patient and family preferences and beliefs can affect recruitment, the degree of engagement in the treatment course, attrition from the study, and outcomes [33–35]. To incorporate the child-family perspective, this trial was designed with significant input from a 44 member multi-disciplinary stakeholder group. Our stakeholder group reported that they considered appendectomy to be a major surgical procedure with surgical risks and a period of disability. This group was used to evaluate the potential impact of family preferences on treatment effects, recruitment, and reported outcomes in order to select the optimal trial design that respected the child-family perspective. Consensus confirmed that a patient choice trial that allowed for families to choose the most suitable intervention for their child would acknowledge the impact of family preferences, facilitate enrollment of a diverse patient population, and generate results that can be rapidly adopted by healthcare providers and systems into clinical practice [35–37].
2. Methods
2.1. Overview of study design and objectives
This is a prospective, non-randomized multi-institutional trial investigating a non-operative management strategy for children (7–17 years old) with uncomplicated appendicitis across 10 children’s hospitals. Patients and their families choose between two treatment options: non-operative management (non-operative group) with antibiotics alone or urgent appendectomy (surgery group).
We elected to perform a patient choice trial rather than a randomized clinical trial because we believe that the success of each treatment option depends on the circumstances of the patient and their family [38–42]. For example, although initial non-operative management of uncomplicated appendicitis may be safe for most patients, appendectomy may be a better treatment option for patients who live in remote areas or for families who are so fearful of a recurrence that they are likely to return to the ED every time their child develops abdominal pain. For these patients, the risk of post-operative complications may be perceived as minor compared to the benefit of a curative appendectomy. In contrast, for families who are averse to surgery, initial non-operative therapy may be the least stressful and most appealing choice because it may eliminate the need for an operation and its inherent risks while expediting return to activities.
Further, patient and family preferences can affect study recruitment, attrition from the study, and outcomes [33–35]. Recently published reports recognized that in surgical trials: 1) random assignment of interventions with different risk-benefit ratios may impede trial recruitment due to patient or surgeon preferences for one intervention over the other; 2) outcomes, especially patient reported outcomes, are susceptible to recall and reporting biases when treatment preferences exist; and 3) research evaluating surgical treatments in emergency and pediatric settings is particularly susceptible to preferences that preclude randomization [43–45]. This may be particularly relevant in our study of non-operative management of pediatric appendicitis in which patients and families may have strong preconceived biases towards needing an appendectomy. Patients with strong preferences may decline to participate in a RCT, thereby limiting external validity [46]. Moreover, patients who receive their preferred treatment may report better outcomes [46–49]. For these reasons, we opted to allow eligible patients and their families to choose between surgery and non-operative management rather than randomize patients to one of the two treatment groups.
This study is being performed across 10 institutions participating in the Midwest Pediatric Surgery Consortium (MWPSC; www.mwpsc.org). The MWPSC is a research collaborative with the mission of advancing the practice of pediatric surgery through high-quality multi-institutional clinical studies. The member institutions represent freestanding and non-freestanding children’s hospitals of varying sizes (75 to 598 beds) and patient demography in 7 different states. Most of the hospitals are located within large urban areas and provide care for larger local minority populations. Each institution serves as both a local hospital for their immediate surrounding area and as a referral center for more remote and rural areas. The diversity of these institutions allows for enrollment of a diverse group of patients and for examination of variations in treatment choice and treatment effects by site and by various patient demographic, clinical and socioeconomic characteristics. IRB approval was obtained at each site participating in the study.
In addition, this trial was designed and is being performed with significant input from a multi-disciplinary stakeholder group that includes patients, caregivers, community-based pediatricians, emergency medicine physicians, surgeons, and nurses from across all 10 participating sites. Further, we have included patient educators, payers, and leaders of the American Academy of Pediatrics Section on Surgery (AAP) and the American Pediatric Surgical Association (APSA). We engaged our stakeholders to identify the most important outcomes and the necessary next steps to have non-operative management become a treatment option offered as part of routine clinical care for children with appendicitis. We engaged our stakeholder partners throughout the process of identifying the research question, the trial design, and outcomes to be assessed. The stakeholders were engaged through in-person individual interviews and stakeholder group meetings. Engagement has continued throughout the duration of the trial to focus on issues of recruitment and retention and currently on preparing for result interpretation and dissemination. The stakeholders identified treatment associated disability, the success rate of non-operative management, the risk of complicated appendicitis, and health related quality of life as the most important outcomes to assess in order to generate the information necessary to allow patients and families to make informed treatment decisions in clinical practice.
2.2. Study duration and enrollment
This study was initiated in 2015 and follow-up will be completed at the end of 2019. Study participants were recruited after surgical consultation was performed in the ED and were consented either in the ED or surgical inpatient units of each institution. Study enrollment is complete with continuing follow-up ongoing.
Children between the ages of 7–17 who were diagnosed with uncomplicated appendicitis were screened for eligibility. Patients were eligible if they had imaging confirmed uncomplicated appendicitis with the following criteria on ultrasound: hyperemia, ≤ 1.1 cm in diameter, compressible or non-compressible, no abscess, no fecalith, no phlegmon; or the following criteria on CT or MRI: hyperemia, fat stranding, ≤ 1.1 cm in diameter, no abscess, no fecalith, no phlegmon. Based on routine laboratory testing, patients with a WBC count > 5000/μL and ≤ 18,000/μL were eligible for inclusion. Lastly, patients were only eligible if they reported that their abdominal pain started less than or equal to 48 h prior to the start of antibiotics.
Patients were excluded from the study if they reported a history of chronic intermittent abdominal pain, were found to have diffuse peritonitis on physical exam by the surgical team, or had a positive urine pregnancy test at time of diagnosis. Additionally, patients were excluded if they were found to have a fecalith on imaging or evidence on imaging studies of evolving perforated appendicitis, including abscess or phlegmon. Due to the importance of patient reliability on reporting progression or resolution of symptoms, patients were also excluded if they were known to have communication difficulties (e.g. severe developmental delay).
3. Study procedures
3.1. Screening and enrollment
During initial contact with a potential subject, a physician-member of the surgical consult team assessed patient eligibility. If all eligibility criteria were met, a physician-member of the research team invited the child and legal guardian to enroll. A standardized script and one-page decision aid were used to perform recruitment and enrollment. The physician-member of the research team reviewed the written information about the study and answered any questions. Finally, the patient and family chose between non-operative management with antibiotics alone (non-operative group) and urgent appendectomy (surgery group).
3.2. Study procedures
3.2.1. Surgery group
Surgical management consists of hospital admission with initiation of intravenous (IV) antibiotics (piperacillin-tazobactam or ciprofloxacin/metronidazole if penicillin allergic) and urgent appendectomy performed within 12 h of admission. All appendectomies were expected to be performed laparoscopically. Postoperatively, antibiotics are discontinued; diet is advanced and patients are discharged when tolerating a liquid diet and their pain is controlled with oral pain medicines, usually within 24 h. Standardized discharge instructions include allowing for resumption of activities as tolerated with the exception of heavy lifting or contact sports which were allowed to be resumed at 2 weeks postoperatively.
3.2.2. Non-operative group
Non-operative management consists of hospital admission for observation with a minimum of 24 h of IV antibiotics (piperacillin-tazo-bactam or ciprofloxacin/metronidazole if penicillin allergic). Pain medication are administered as needed. Diet is advanced after a minimum of 12 h nil per os (NPO) and only when clinical improvement (decreased pain or tenderness) is recognized. Patients are switched to oral antibiotics (amoxicillin-clavulanate or ciprofloxacin/ metronidazole if penicillin allergic) when they tolerate a regular diet. At least one dose of oral antibiotics is administered in the hospital to ensure tolerance. Patients are discharged home with a prescription for oral antibiotics to complete a total antibiotic course of 7 days (inclusive of the IV antibiotics received) when tolerating a regular diet and oral antibiotics with minimal pain. Phone follow-up by a member of the research team is conducted at 2–5 days and 10–14 days to ensure resolution of symptoms and completion of antibiotic course. Standardized discharge instructions allow for resumption of activities as tolerated.
Failure of non-operative management and cross-over to appendectomy occurs in two situations during the initial admission: 1) Failure to improve after 24 h of IV antibiotics: Patients who do not exhibit clinical improvement (decreased tenderness, improvement in fever curve) or do not report symptomatic relief (decreased pain, resolution of nausea/ vomiting, advancement of diet) after receiving 24 h of intravenous antibiotics are recommended for appendectomy. If there is either objective or subjective improvement, then the child continues on IV antibiotics for up to another 24 h; 2) If clinical status worsens: If a patient’s symptoms worsen (increased abdominal pain) or there is evolving objective evidence of systemic signs of infection (increasing tachycardia, hypotension, persistent fever, or decreased mental status), then he/she undergoes appendectomy.
Any patient managed non-operatively who returns after discharge with abdominal pain and had a clinical evaluation consistent with appendicitis, is considered a failure. These patients undergo urgent appendectomy. Failure of non-operative management is defined in this way because our patient and family stakeholders reported that in order to make an informed decision between these two treatments, they needed to know the likelihood that a child treated non-operatively will eventually end up undergoing an appendectomy.
3.2.3. Surveys and follow-ups
Before discharge, the patient and caregiver complete a QOL survey (PEDSQL Quality of Life Inventory: Child and Parent Report) that asks about the child’s health, feelings, and social functioning in and out of school. As well, they answer questions about the reasons for their treatment choice. Phone, email/web-based, or in person follow-up is conducted at 30 days, 6 months and 1 year after discharge (see Table 1). Patient incentives are used to encourage continued study participation, and include a $25 VISA® gift card after completion of their 30 day and 6 month follow-ups and a $50 VISA® gift card after completion of the 1-year follow-up.
Table 1.
Outcomes and time points at which data is collected.
| Outcome | Time points |
|---|---|
| In all patients | |
| Disability Days of the child (Primary | 30 days, 1 year |
| Outcome) | 30 days, 1 year |
| Disability days of the caregiver | Index hospitalization |
| Length of Stay | 30 days, 1 year |
| Emergency Department Visits | 30 days, 1 year |
| Readmissions | 30 days, 1 year |
| Complicated Appendicitis | 30 days, 1 year |
| Post-treatment Related Complications | 30 days |
| Satisfaction with Health Care | Index hospitalization, 30 days, |
| Health Related Quality of Life | 1 year |
| Satisfaction with Decision | 30 days, 1 year |
| Additional surgical or interventional | 30 days, 1 year |
| procedures | 30 days, 1 year |
| Rate of imaging (CT, ultrasound) and total radiation exposure | |
| In Surgery group and non-operative patients | |
| that fail or recur | 30 days |
| Postoperative Infections | 30 days, 1 year |
| Re-operation | |
| In Non-operative group only | |
| Success rate (Primary Outcome) | 30 days,1 year |
| Need for appendectomy during initial | Index hospitalization |
| admission | 30 days,1 year |
| Recurrence | 30 days |
| Antibiotic complications |
3.3. Outcomes
The outcomes and time points at which they are assessed are listed in Table 1. Our stakeholder team identified disability days associated with the child’s treatment and the success rate of non-operative management as the most important outcomes to assess to allow for informed treatment decisions. Other endpoints that they believed to be important for informed treatment decisions included: the risk for developing worse appendicitis (complicated appendicitis), treatment associated complications, and HRQOL.
3.4. Primary outcomes
3.4.1. Disability days of the child (assessable in both groups and comparable)
Disability days are defined as the total number of days in which the child is not able to participate in all of his/her normal activities secondary to appendicitis-related care within one year of enrollment including: school, gym, recess, sports, and other after school activities. This comparison allows families to understand the expected disability of their child from each treatment option. The definition of disability days was developed to reflect all components of disability the stakeholders felt were important; these measures are reported by the patients and their caregivers during the 30 day, 6 month, and 1 year follow-ups.
3.4.2. Success rate of non-operative management at one year (assessable only in the non-operative group)
The success rate is defined as the percent of patients treated non-operatively who do not undergo an appendectomy within one year of enrollment.
3.5. Secondary outcomes
Secondary outcomes were chosen following review of previous study results and from feedback received from our stakeholders. The two treatment groups will be compared on:
3.5.1. Disability days of the caregiver
Disability days of the caregiver are reported as the total number of days without normal schedule for the caregiver, defined as the days the caregiver had not been able to perform his/her normal full schedule at work (including days off work) and/or home. This comparison will allow families to understand the expected disability of the caregiver from each treatment option. This definition was developed with the stakeholders to reflect all components of disability they felt were important to their daily lives. These measures will be directly reported by the caregivers. These measures are reported by the patients and their caregivers during the 30 day, 6 month, and 1 year follow-ups.
3.5.2. Healthcare related quality of life, healthcare satisfaction and satisfaction with decision making measures
These outcomes represent the patient reported outcomes (PROs) that patient, parent and physician stakeholders believed were necessary for informed decision making. We have identified specific validated tools that are appropriate to administer to study participants to assess these PROs in our study population including:
3.5.3. Health Related Quality of Life (HRQOL) [50–56]
HRQOL is assessed through the Pediatric Quality of Life Inventory™ (PedsQL™) scales. We chose the PedsQL Quality of Life Inventory™ because it is a group of questionnaires used to assess pediatric HRQOL in healthy and ill (acute or chronic) subjects that has been validated, is available in multiple languages and has been used to study pediatric patients with appendicitis [57,58]. Specifically, we will use PedsQL™4.0 Generic Core Scales - Child and Parent Report. The generic core scales module measures four dimensions (physical, emotional, social and school functioning) using 23 items and is designed to measure the HRQOL of healthy or ill (acute or chronic) children through self-reports and parent proxy reports. The generic core scales have been found to be valid and reliable with internal consistency values (Cronbach’s a) of 0.88 for the child report and 0.90 for the parent proxy report [52,55,59]. It has also been reported that these scales are interpreted similarly across pediatric race/ethnic groups, socioeconomic groups, and in various modes of administration [53,60,61]. This will be measured at discharge and at the 30-day and 1 year follow-up appointments.
3.5.4. Healthcare satisfaction [50,52–54,56,62]
Healthcare satisfaction is measured using the PedsQL™ 3.0 Healthcare Satisfaction Generic Module- Parent Report. This survey measures the caregiver’s satisfaction with the care his/her child received and measures six dimensions (information, inclusion of family, communication, technical skills, emotional needs, and overall satisfaction) using 24 items. Higher scores indicate higher satisfaction. The generic module was modified from the Healthcare Satisfaction Module-Parent Survey and the Pediatric Hematology/Oncology Parent Satisfaction survey whose Cronbach’s α = 0.92 and 0.96 respectively [62,63]. This is measured at the 30 day follow-up appointment.
3.5.5. Satisfaction with decision [64,65]
The parent’s satisfaction with the initial treatment decision is assessed using the Satisfaction with Decision Scale. This survey measures satisfaction with a health care decision and contains 6 items measured on a Likert scale from 1 (strongly agree) to 5 (strongly disagree). The internal consistency of the scale is good with a Cronbach’s α of 0.86. This will be measured at the 30 day and 1 year follow-up appointments.
3.5.6. Rates of complicated appendicitis
The proportion of patients with complicated appendicitis, as identified during appendectomy with either visualization of a hole in the appendix, extramural appendicolith, frank pus in the abdomen, or pathologic findings of transmural inflammation with perforation through the wall of the appendix, will be compared between the non-operative group (identified during appendectomy in those that fail non-operative management) and in the surgery group. This outcome compares the risk of a patient having more severe or worse appendicitis if they choose non-operative management vs. surgery.
3.5.7. Rates of post-treatment related complications
Post-treatment related complications includes: episodes of symptoms (e.g. pain, nausea/emesis) requiring unplanned physician or Emergency Department visits or hospital admission and additional surgical or interventional procedures. The proportion of these complications will be compared between treatment groups. This outcome assesses differences in medical morbidity between the treatment groups which is necessary to provide patients and families with a complete risk-benefit assessment of each treatment option.
3.5.8. Other outcomes
Other secondary outcomes include hospital length of stay, antibiotic associated side effects (e.g. nausea) and complications (e.g. Clostridium difficile infection), and rates of imaging (both CT and ultrasound together and separately) and total appendicitis-related radiation exposure (effective dose) before enrollment and at 1 year follow-up.
Consistent with the previous trials of non-operative management of appendicitis, the primary and secondary outcomes for this study are based on 1 year follow-up. In these trials, the median time to recurrence was 6 months with almost no recurrences and minimal post-surgical morbidity reported after 1 year.
3.6. Statistical methods
3.6.1. Sample size and power
The sample size needed to assess the primary outcome of the 1-year success rate of non-operative management is based on preliminary data, which expected the success rate to be between 76 and 78%. Based on previous studies and input from our stakeholders and participating surgeons, the lowest acceptable success rate of non-operative management required for it to be considered as part of routine clinical practice was determined to be 70% (p0) at 1 year follow-up. It was expected that the study success rate (p1) would be higher, ranging from 76 to 78%. Under a group sequential design, with one interim and one final analysis, overall type I error (two-sided) of 5% (adjusted for the 2 primary endpoints), the required sample sizes required to achieve 80% power are listed in Table 2 [66,67].
Table 2.
Estimated required sample sizes of the non-operative group.
| Success rate | Power | Sample size Non-operative groupa |
|---|---|---|
| 0.78 | 80% | 250 |
| 0.77 | 80% | 330 |
Accounts for 1 interim analysis to determine futility with overall two-sided type I error rate of 5%. Calculated in EAST 6.0 Base.
Given these estimates, the expected rates of patient choice of non-operative management (40%) or surgery (60%), and an approximately 10% expected rate of loss to follow up, we aimed initially to enroll 908 patients in order to obtain the minimum number of non-operative participants (n = 364). Throughout the study period, the rate of patients choosing non-operative management was lower than expected (approximately 35%). The total enrollment of the trial was increased to allow enrollment of the target non-operative sample size of 364 patients. Enrollment closed on 11/½018. A total of 1076 patients were enrolled across all sites, 373 (35%) patients choose non-operative management.
The sample size required to have adequate power for the first primary outcome, the success rate, provided more than adequate power to assess the other primary endpoint of disability days. Based on previous data, we expected children who underwent non-operative management to have at least five fewer disability days in the year following treatment (assuming 10 days (sd = 9.8) on average) than children who had initial surgery (assuming 15 (sd = 7.7) on average). With the proposed sample sizes in each group, we expected over 90% power to detect these mean differences, assuming an adjusted overall type I error of 5% (adjusted to account for the two primary endpoints).
3.6.2. Analysis plan
Analysis of the primary endpoints, disability days of the child and the success rate, will employ propensity score methods as a means to quantify differences in baseline characteristic between groups and to balance them in final analysis [68–70]. Balance in all pre-treatment characteristics between groups will be measured through the standardized difference of each covariate. All of these covariates (including demographics, race, ethnicity, socioeconomic variables, clinical and imaging characteristics, laboratory values and patient recruitment site) will be collected from the patient, caregiver and medical record at the time of enrollment; this will allow robust data capture with minimal missing data. All measured pre-treatment covariates considered to potentially confound the relationship between treatment and outcome or those considered to be highly associated with outcome were included in the development of the propensity score/probability of treatment model. We utilize inverse probability of treatment weighting (IPTW) by the estimated propensity score. The distribution of the propensity scores (and inverse probability weights) will be described and graphically displayed. Stabilized inverse probability weights will be considered to mitigate the influence of very small estimated probabilities from the propensity score model [71]. Regression models were utilized to make inference, taking into account the estimated inverse probability of treatment weight and imbalanced covariates, and will estimate standard errors either as robust standard errors or through bootstrap procedures [72]. Inverse probability weighting was chosen instead of propensity score matching because it enables us to estimate the average treatment effect (ATE), rather than the average treatment effect in the treated (ATT), on all outcomes, including success of non-operative management. Where possible, we will estimate effects stratified by institution and will examine the sensitivity of results to varying methods of analysis. Heterogeneity of effects due to three main factors of interest: age group (≤ 10 vs. > 10 yrs), household income (< $50,000, ≥$50,000), transfer status, will be explored by evaluating these factors as potential effect modifiers by including each in regression models. Treatment effects will be estimated for each level of factor and compared across these groups.
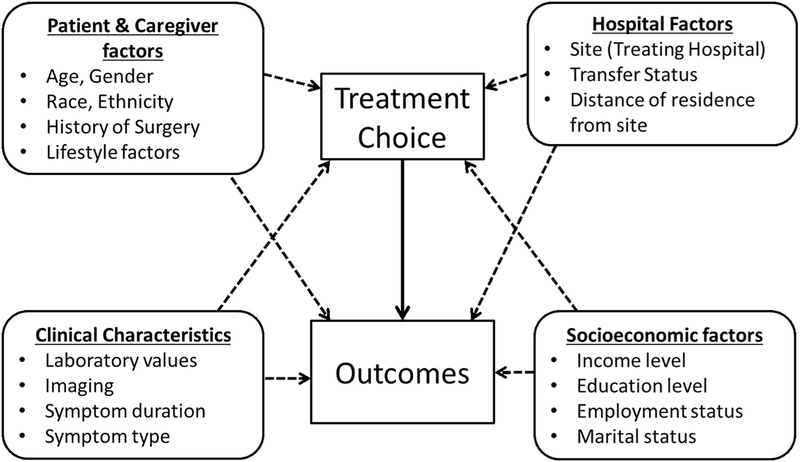
Analysis of the success rate of non-operative management will be estimated and reported along with the 95% confidence interval. This rate will be examined overall and by treatment institution. The standardized success rate (to the overall population) will also be estimated and reported (along with the associated 95% CI). Success rate of non-operative management at 30 days and the rate of complicated appendicitis at 1 year (both secondary endpoints) will be estimated along with their 95% CI. Secondary outcomes that involve the comparison of treatment groups will employ propensity score methods, as a means to quantify differences in baseline characteristic between groups and to balance them in final analysis [68–70]. Analysis will mimic that of the primary endpoint comparison above for disability days, including inverse probability weighting by the estimated propensity score and will consider further adjustment in regression models for any covariate that does not appear to be adequately balanced following estimation of the propensity score. Stratification by institution will be pursued to examine both primary and secondary endpoints and will be detailed descriptively (Fig. 1: Conceptual Model).
Fig. 1.
Conceptual model.
3.7. Interim analyses
An interim analysis was planned when a quarter of the expected total number of non-operative patients had been accrued and followed for 1 year. The interim analyses were interested in assessing futility of the primary endpoints. The trial monitored the primary outcomes of disability days and the success rate of therapy (proportion estimate), and utilized the error spending function approach described by Lan and Demets [67]. Throughout the trial, these endpoints were monitored for safety and regularly reported to the Data and Safety Monitoring Committee (DSMC). The interim analyses were completed and reviewed by the DSMC. No safety concerns were identified and no stopping rules were met; therefore, the trial was allowed to enroll to completion.
4. Discussion
Meta-analyses and systematic reviews have demonstrated the efficacy and safety of non-operative management across multiple studies. These reviews have identified the need for large multi-institutional studies to determine the effectiveness of non-operative management across diverse institutions and in specific patient populations including extremes of age [5,6,21,73–75]. These types of studies will help define specific criteria for selecting patients for non-operative management that can be used in clinical practice [ 6,74,76]. The results from previous studies indicate that non-operative management is a reasonable first-line treatment strategy for uncomplicated appendicitis [4–6,14–29]. Additional studies are needed to focus on outcomes important to informing treatment choice for patients and families Furthermore, additional studies are necessary which resemble, as closely as possible, usual clinical care and which are inclusive of the vast majority of patients and families who are treated for uncomplicated acute appendicitis. The current study addresses the evidence gaps by determining the effectiveness of non-operative management and surgery across a broad pediatric patient population using a pragmatic approach with a patient choice design.
Published panel reports suggest that high-quality parallel observational patient choice studies are a valuable alternative when randomized trials are not feasible due to strong preferences by the participants and when only a small proportion will accept randomization [34,44,45,77]. As compared to a RCT, potential benefits of a patient choice design include allowing for broad enrollment with greater representation of minority groups (both race and ethnicity), minimizing the effects of patient/parent preferences on outcomes by aligning their preferences with the treatment, and allowing for rapid adoption of the results [78–80]. Although this design will need to account for treatment selection bias, we took several steps to minimize this including: a standardized enrollment script and decision aid, clearly specified inclusion and exclusion criteria, standardized treatment protocols and algorithms, and by achieving buy-in from all participating surgeons prior to beginning the trial. Furthermore, treatment decision making in clinical practice occurs within the biases of patients, families, and surgeons. Therefore, results from this trial should be reflective of the effectiveness of treatments in clinical practice. The patient choice design allowed for enrollment of a diverse patient population and will generate results that can be rapidly adopted by healthcare providers and systems into clinical practice.
This multi-institutional trial is the first study to determine the effectiveness of non-operative management as an alternative first line therapy for children with uncomplicated appendicitis. Regardless of their choice of therapy, patient and family stakeholders on our research team identified the ability to avoid surgery as an important and meaningful improvement in care. In addition, these stakeholders ranked post-treatment disability and health-related quality of life as two of the most meaningful outcomes to them as patients and families. This study is focused on helping children and their families choose which treatment for appendicitis is best for them. Specifically, it is designed to allow patients and their families to make an informed treatment choice based on characteristics of their child and family and their preferences for one of two treatment options with different risks and benefits, and evaluate two acceptable treatment options for uncomplicated appendicitis with clear delineation of the specific risk-benefit profiles associated with each.
Furthermore, the size of the study will allow us to investigate the effectiveness of non-operative management across subgroups based on age, race, ethnicity, socioeconomic status, and rural and urban demography. If the effectiveness of non-operative management in the proposed trial is consistent with previous results, then over 75% of patients treated non-operatively can successfully avoid surgery and its associated disability and risks for complications. [4–6,14–29] By performing this trial in 10 children’s hospitals across 7 states, we will be able to provide generalizable estimates of the effects of non-operative management on disability, health-related quality of life, medical/surgical complications, and healthcare satisfaction. Furthermore, the patient choice design incorporates inherent biases and preferences of the patient and family that occur as part of clinical care. The risks and benefits of non-operative management with antibiotics alone and surgery are very different; therefore, a “successful” treatment depends on which risks and benefits are most important to each patient and their family. Using an informed shared decision making process.to determine the appendicitis treatment should allow for results that are reflective of effectiveness in broad clinical practice.
Acknowledgments
Funding
This study is funded by the Patient-Centered Outcomes Research Institute (PCORI) contract # CER-1507–31325. PCORI is an independent, nonprofit organization authorized by U.S. Congress in 2010. Its mission is to fund research that will provide patients, their caregivers and clinicians with the evidence-based information needed to make better-informed healthcare decisions. For more information about PCORI’s funding, visit www.pcori.org. This project is also supported by Award Number UL1TR001070 from the National Center for Advancing Translational Sciences. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of PCORI, the National Center for Advancing Translational Sciences or the National Institutes of Health.
This study was approved by the Institutional Review Board at NCH. Information about the study is presented to the patients and legal guardians of patients < 18 in both written and oral form. Written informed consent is obtained from the legal guardian, and assent is obtained from patients > 9 years. Participation in the study is voluntary and patients can withdraw from the study at any time.
Footnotes
Declarations of interest
None.
Ethics approval and consent to participate
Competing interests
The authors declare that they have no competing interests.
References
- [1].McCaig LF, Burt CW, National Hospital Ambulatory Medical Care Survey: 1999 emergency department summary, Adv. Data 320 (2001) 1–34. [PubMed] [Google Scholar]
- [2].Coran AG, Adzick NS, Caldamone AA, Krummel TM, Laberge JM, Shamberger RC, Pediatric Surgery, 7th edition, Elsevier Inc, Philadelphia, 2012. [Google Scholar]
- [3].Owings MFKL, Ambulatory and Inpatient Procedures in the United States, 1996, Hyattsville, National Center for Health Statistics, 1998. [PubMed] [Google Scholar]
- [4].Abes M, Petik B, Kazil S, Nonoperative treatment of acute appendicitis in children, J. Pediatr. Surg 42 (8) (2007) 1439–1442. [DOI] [PubMed] [Google Scholar]
- [5].Wilms IM, de Hoog DE, de Visser DC, Janzing HM, Appendectomy versus antibiotic treatment for acute appendicitis, Cochrane Database Syst. Rev 11 (2011) CD008359. [DOI] [PubMed] [Google Scholar]
- [6].Varadhan KK, Neal KR, Lobo DN, Safety and efficacy of antibiotics compared with appendicectomy for treatment of uncomplicated acute appendicitis: meta-analysis of randomised controlled trials, Bmj. 344 (2012) e2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Emil S, Laberge JM, Mikhail P, et al. , Appendicitis in children: a ten-year update of therapeutic recommendations, J. Pediatr. Surg 38 (2) (2003) 236–242. [DOI] [PubMed] [Google Scholar]
- [8].Cash CL, Frazee RC, Abernathy SW, et al. , A prospective treatment protocol for outpatient laparoscopic appendectomy for acute appendicitis, J. Am. Coll. Surg 215 (1) (2012) 101–105 (discussion 105–106). [DOI] [PubMed] [Google Scholar]
- [9].Ikeda H, Ishimaru Y, Takayasu H, Okamura K, Kisaki Y, Fujino J, Laparoscopic versus open appendectomy in children with uncomplicated and complicated appendicitis, J. Pediatr. Surg 39 (11) (2004) 1680–1685. [DOI] [PubMed] [Google Scholar]
- [10].Malagon AM, Arteaga-Gonzalez I, Rodriguez-Ballester L, Outcomes after laparoscopic treatment of complicated versus uncomplicated acute appendicitis: a prospective, comparative trial, J. Laparoendoscopic Adv. Surg. Techniq 19 (6) (2009) 721–725. [DOI] [PubMed] [Google Scholar]
- [11].Tiwari MM, Reynoso JF, Tsang AW, Oleynikov D, Comparison of outcomes of laparoscopic and open appendectomy in management of uncomplicated and complicated appendicitis, Ann. Surg 254 (6) (2011) 927–932. [DOI] [PubMed] [Google Scholar]
- [12].Kocatas A, Gonenc M, Bozkurt MA, Karabulut M, Gemici E, Alis H, Comparison of open and laparoscopic appendectomy in uncomplicated appendicitis: a prospective randomized clinical trial, TJTES 19 (3) (2013) 200–204. [DOI] [PubMed] [Google Scholar]
- [13].Armstrong J, Merritt N, Jones S, Scott L, Butter A, Non-operative management of early, acute appendicitis in children: is it safe and effective? J. Pediatr. Surg 49 (5) (2014) 782–785. [DOI] [PubMed] [Google Scholar]
- [14].Minneci PC, Sulkowski JP, Nacion KM, et al. , Feasibility of a nonoperative management strategy for uncomplicated acute appendicitis in children, J. Am. Coll. Surg 219 (2) (2014) 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Salminen P, Paajanen H, Rautio T, et al. , Antibiotic therapy vs appendectomy for treatment of uncomplicated acute appendicitis: the APPAC randomized clinical trial, JAMA. 313 (23) (2015) 2340–2348. [DOI] [PubMed] [Google Scholar]
- [16].Eriksson S, Granstrom L, Randomized controlled trial of appendicectomy versus antibiotic therapy for acute appendicitis, Br. J. Surg 82 (2) (1995) 166–169. [DOI] [PubMed] [Google Scholar]
- [17].Hansson J, Korner U, Khorram-Manesh A, Solberg A, Lundholm K, Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients (vol 96, pg 473, 2009), Br. J. Surg 96 (5) (2009) 473–481. [DOI] [PubMed] [Google Scholar]
- [18].Hansson J, Korner U, Ludwigs K, Johnsson E, Jonsson C, Lundholm K, Antibiotics as first-line therapy for acute appendicitis: evidence for a change in clinical practice, World J. Surg 36 (9) (2012) 2028–2036. [DOI] [PubMed] [Google Scholar]
- [19].Styrud J, Eriksson S, Nilsson I, et al. , Appendectomy versus antibiotic treatment in acute appendicitis. A prospective multicenter randomized controlled trial, World J. Surg 30 (6) (2006) 1033–1037. [DOI] [PubMed] [Google Scholar]
- [20].Vons C, Barry C, Maitre S, et al. , Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis: an open-label, non-inferiority, randomised controlled trial, Lancet. 377 (9777) (2011) 1573–1579. [DOI] [PubMed] [Google Scholar]
- [21].Fitzmaurice GJ, McWilliams B, Hurreiz H, Epanomeritakis E, Antibiotics versus appendectomy in the management of acute appendicitis: a review of the current evidence, Can. J. Surg 54 (5) (2011) 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Malik AA, Bari SU, Conservative management of acute appendicitis, J. Gastrointest. Surg 13 (5) (2009) 966–970. [DOI] [PubMed] [Google Scholar]
- [23].Shindoh J, Niwa H, Kawai K, et al. , Predictive factors for negative outcomes in initial non-operative management of suspected appendicitis, J. Gastrointest. Surg 14 (2) (2010) 309–314. [DOI] [PubMed] [Google Scholar]
- [24].Turhan AN, Kapan S, Kutukcu E, Yigitbas H, Hatipoglu S, Aygun E, Comparison of operative and non operative management of acute appendicitis, TJTES 15 (5) (2009) 459–462. [PubMed] [Google Scholar]
- [25].Ein SH, Langer JC, Daneman A, Nonoperative management of pediatric ruptured appendix with inflammatory mass or abscess: presence of an appendicolith predicts recurrent appendicitis, J. Pediatr. Surg 40 (10) (2005) 1612–1615. [DOI] [PubMed] [Google Scholar]
- [26].Ein SH, Shandling B, Is interval appendectomy necessary after rupture of an appendiceal mass? J. Pediatr. Surg 31 (6) (1996) 849–850. [DOI] [PubMed] [Google Scholar]
- [27].Svensson JF, Patkova B, Almstrom M, et al. , Nonoperative treatment with anti biotics versus surgery for acute nonperforated appendicitis in children: a pilot randomized controlled trial, Ann. Surg 261 (1) (2015) 67–71. [DOI] [PubMed] [Google Scholar]
- [28].Tanaka Y, Uchida H, Kawashima H, et al. , Long-term outcomes of operative versus nonoperative treatment for uncomplicated appendicitis, J. Pediatr. Surg 50 (11) 1893–1897. [DOI] [PubMed] [Google Scholar]
- [29].Minneci PC, Mahida JB, Lodwick DL, et al. , The effectiveness of patient choice in non-operative versus surgical management of pediatric uncomplicated acute appendictiis, JAMA Surg 151 (5) (2016) 408–15. [DOI] [PubMed] [Google Scholar]
- [30].Brinkman WB, Epstein JN, Treatment planning for children with attention-def-icit/hyperactivity disorder: treatment utilization and family preferences, Patient Preference Adher. 5 (2011) 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bussing R, Gary FA, Mills TL, Garvan CW, Parental explanatory models of ADHD: gender and cultural variations, Soc. Psychiatry Psychiatr. Epidemiol 38 (10) (2003) 563–575. [DOI] [PubMed] [Google Scholar]
- [32].Liu C, Robin AL, Brenner S, Eastman J, Social acceptability of methylphenidate and behavior modification for treating attention deficit hyperactivity disorder, Pediatrics. 88 (3) (1991) 560–565. [PubMed] [Google Scholar]
- [33].Corrigan PW, Salzer MS, The conflict between random assignment and treatment preference: implications for internal validity, Eval. Prog. Plan 26 (2) (2003) 109–121. [DOI] [PubMed] [Google Scholar]
- [34].King M, Nazareth I, Lampe F, et al. , Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials, Health Technol. Assess 9 (35) (2005) 1–186 (iii-iv). [DOI] [PubMed] [Google Scholar]
- [35].Patsopoulos NA, A pragmatic view on pragmatic trials, Dialogues Clin. Neurosci 13 (2) (2011) 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bradley C, Clinical trials-time for a paradigm shift? Diabetic Med. 5 (2) (1988) 107–109. [DOI] [PubMed] [Google Scholar]
- [37].Brewin CR, Bradley C, Patient preferences and randomised clinical trials, Bmj. 299 (6694) (1989) 313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Coyne I, Gallagher P, Participation in communication and decision-making: children and young people’s experiences in a hospital setting, J. Clin. Nurs 20 (15–16) (2011) 2334–2343. [DOI] [PubMed] [Google Scholar]
- [39].Lewis CC, Pantell RH, Sharp L, Increasing patient knowledge, satisfaction, and involvement: randomized trial of a communication intervention, Pediatrics. 88 (2) (1991) 351–358. [PubMed] [Google Scholar]
- [40].Lipstein EA, Brinkman WB, Britto MT, What is known about parents’ treatment decisions? A narrative review of pediatric decision making, Med. Decis. Making 32 (2012) 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pennarola BW, Rodday AM, Mayer DK, et al. , Factors associated with parental activation in pediatric hematopoietic stem cell transplant, Med. Care Res. Rev 69 (2012) 194–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Post DM, Cegala DJ, Miser WF, The other half of the whole: teaching patients to communicate with physicians, Fam. Med 34 (5) (2002) 344–352. [PubMed] [Google Scholar]
- [43].Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM. Evaluation and stages of surgical innovations. Lancet.374(9695):1089–1096. [DOI] [PubMed] [Google Scholar]
- [44].Ergina PL, Cook JA, Blazeby JM, et al. Challenges in evaluating surgical innovation. Lancet.374(9695):1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet.374(9695):1105–1112. [DOI] [PubMed] [Google Scholar]
- [46].Preference Collaborative Review Group, Patients’ preferences within randomised trials: systematic review and patient level meta-analysis, Bmj. 337 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bradley C, Gamsu DS, Knight G, Boulton AJ, Ward JD, Predicting risk of diabetic ketoacidosis in patients using continuous subcutaneous insulin infusion, Br. Med. J 293 (6541) (1986) 242–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fiks AG, Mayne S, Debartolo E, Power TJ, Guevara JP, Parental preferences and goals regarding ADHD treatment, Pediatrics. 132 (4) (2013) 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kwan BM, Dimidjian S, Rizvi SL, Treatment preference, engagement, and clinical improvement in pharmacotherapy versus psychotherapy for depression, Behav. Res. Ther 48 (8) (2010) 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chan KS, Mangione-Smith R, Burwinkle TM, Rosen M, Varni JW, The PedsQL: reliability and validity of the short-form generic core scales and Asthma Module, Med. Care 43 (3) (2005) 256–265. [DOI] [PubMed] [Google Scholar]
- [51].Varni JW, Burwinkle TM, Dickinson P, et al. , Evaluation of the built environment at a children’s convalescent hospital: development of the Pediatric Quality of Life Inventory parent and staff satisfaction measures for pediatric health care facilities, J. Dev. Behav. Pediatr 25 (1) (2004) 10–20. [DOI] [PubMed] [Google Scholar]
- [52].Varni JW, Burwinkle TM, Seid M, Skarr D, The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity, Ambul. Pediatr 3 (6) 329–341. [DOI] [PubMed] [Google Scholar]
- [53].Varni JW, Limbers CA, The pediatric quality of life inventory: measuring pediatric health-related quality of life from the perspective of children and their parents, Pediatr. Clin. N. Am 56 (4) (2009) 843–863. [DOI] [PubMed] [Google Scholar]
- [54].Varni JW, Seid M, Knight TS, Uzark K, Szer IS, The PedsQL 4.0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making, J. Behav. Med 25 (2) (2002) 175–193. [DOI] [PubMed] [Google Scholar]
- [55].Varni JW, Seid M, Kurtin PS, PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations, Med. Care 39 (8) (2001) 800–812. [DOI] [PubMed] [Google Scholar]
- [56].Varni JW, Seid M, Rode CA, The PedsQL: measurement model for the pediatric quality of life inventory, Med. Care 37 (2) (1999) 126–139. [DOI] [PubMed] [Google Scholar]
- [57].Varni JW. Pediatric Quality ofLife Inventory (PedsQL™). 2012; Accessed August 15, 2012. [Google Scholar]
- [58].Schurman JV, Cushing CC, Garey CL, Laituri CA, St Peter SD, Quality of life assessment between laparoscopic appendectomy at presentation and interval appendectomy for perforated appendicitis with abscess: analysis of a prospective randomized trial, J. Pediatr. Surg 46 (6) (2011) 1121–1125. [DOI] [PubMed] [Google Scholar]
- [59].Varni JW, Limbers CA, Burwinkle TM, Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL 4.0 Generic Core Scales, Health Qual. Life Outcomes 5 (2007) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Limbers CA, Newman DA, Varni JW, Factorial invariance of child self-report across socioeconomic status groups: a multigroup confirmatory factor analysis utilizing the PedsQL 4.0 Generic Core Scales, J. Behav. Med 31 (5) (2008) 401–411. [DOI] [PubMed] [Google Scholar]
- [61].Varni JW, Limbers CA, The PedsQL 4.0 Generic Core Scales Young Adult Version: feasibility, reliability and validity in a university student population, J. Health Psychol 14 (4) (2009) 611–622. [DOI] [PubMed] [Google Scholar]
- [62].Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P, The PedsQL Family Impact Module: preliminary reliability and validity, Health Qual. Life Outcomes 2 (2004) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Varni JW, Quiggins DJL, Ayala GX, Development of the Pediatric Hematology/ Oncology Parent Satisfaction Survey, Childrens Health Care. 29 (4) (2000) 243–255. [Google Scholar]
- [64].Holmes-Rovner M, Kroll J, Schmitt N, et al. , Patient satisfaction with health care decisions: the satisfaction with decision scale, Med. Decis. Making 16 (1) (1996) 58–64. [DOI] [PubMed] [Google Scholar]
- [65].Wills CE, Holmes-Rovner M, Preliminary validation of the Satisfaction With Decision scale with depressed primary care patients, Health Expect. 6 (2) (2003) 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jennison C, Turnbull BW, Group Sequential Methods with Applications to Clinical Trials, Boca Raton, USA, Chapman & Hall/ CRC, 2000. [Google Scholar]
- [67].Lan KKG, Demets DL, Discrete sequential boundaries for clinical-trials, Biometrika. 70 (3) (1983) 659–663. [Google Scholar]
- [68].Lunceford JK, Davidian M, Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study, Stat. Med 23 (19) (2003)2937–2960. [DOI] [PubMed] [Google Scholar]
- [69].Robins JM, Hernan MA, Brumback B, Marginal structural models and causal inference in epidemiology, Epidemiology. 11 (5) (2000) 550–560. [DOI] [PubMed] [Google Scholar]
- [70].Rosenbaum PR, Rubin DB, The central role of the propensity score in observational studies for causal effects, Biometrika. 70 (1) (1983) 41–55. [Google Scholar]
- [71].Cole SR, Hernan MA, Constructing inverse probability weights for marginal structural models, Am. J. Epidemiol 168 (6) (2008) 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hernan MA, Brumback B, Robins JM, Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men, Epidemiology. 11 (2000) 561–570. [DOI] [PubMed] [Google Scholar]
- [73].Mason RJ, Moazzez A, Sohn H, Katkhouda N, Meta-analysis of randomized trials comparing antibiotic therapy with appendectomy for acute uncomplicated (no abscess or phlegmon) appendicitis, Surg. Infect 13 (2) (2012) 74–84. [DOI] [PubMed] [Google Scholar]
- [74].McCutcheon BA, Chang DC, Marcus LP, et al. , Long-term outcomes of patients with nonsurgically managed uncomplicated appendicitis, J. Am. Coll. Surg 218 (5) (2014) 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Liu K, Fogg L, Use of antibiotics alone for treatment of uncomplicated acute appendicitis: a systematic review and meta-analysis, Surgery. 150 (4) (2011) 673–683. [DOI] [PubMed] [Google Scholar]
- [76].Di Saverio S, Sibilio A, Giorgini E, et al. , The NOTA Study (Non Operative Treatment for Acute Appendicitis): prospective study on the efficacy and safety of antibiotics (amoxicillin and clavulanic acid) for treating patients with right lower quadrant abdominal pain and long-term follow-up of conservatively treated suspected appendicitis, Ann. Surg 260 (1) (2014) 109–117.24646528 [Google Scholar]
- [77].Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C, Choosing between randomised and non-randomised studies: a systematic review, Health Technol. Assess 2 (13) (1998) 1–124. [PubMed] [Google Scholar]
- [78].Hussain-Gambles M, Atkin K, Leese B, Why ethnic minority groups are under-represented in clinical trials: a review of the literature, Health Soc. Care Commun 12 (5) (2004) 382–388. [DOI] [PubMed] [Google Scholar]
- [79].Murthy VH, Krumholz HM, Gross CP, Participation in cancer clinical trials: race-, sex-, and age-based disparities, JAMA. 291 (22) (2004) 2720–2726. [DOI] [PubMed] [Google Scholar]
- [80].Somerson JS, Bhandari M, Vaughan CT, Smith CS, Zelle BA, Lack of diversity in orthopaedic trials conducted in the United States, J. Bone Joint Surg. Am 96 (7) (2014) 00531. [DOI] [PubMed] [Google Scholar]