Abstract
Objective:
Among patients with incident, clinically diagnosed synucleinopathies, to compare survival by the presenting Parkinsonism symptoms at diagnosis.
Patients and Methods:
Using the Rochester Epidemiology Project medical records-linkage system, we identified all persons residing in Olmsted County, Minnesota, who received a diagnostic code of Parkinsonism from January 1, 1991, through December 31, 2010. A movement-disorder specialist reviewed the complete medical records of each individual to confirm the presence of Parkinsonism, determine the type of synucleinopathy, and identify the onset dates of each cardinal symptom (rest tremor, bradykinesia, rigidity, and impaired postural reflexes). We determined the median time from age at diagnosis to death or censoring (June 30, 2015) for each presenting symptoms, and the age- and sex-adjusted risk of death.
Results:
From 1991 through 2010, 433 individuals were diagnosed with a synucleinopathy (301 Parkinson’s disease [PD]; 68 Dementia with Lewy Bodies [DLB]; 52 PD dementia [PDD]; and 12 Multiple Systems Atrophy with Parkinsonism [MSA-p]). Overall, the risk of death in the tremor-predominant group was less than the bradykinesia/rigidity-only group (Hazard ratio [HR], 0.53 [95% CI; 0.36-0.78 P=.001]). Similarly, risk of death in the bradykinesia/rigidity-only group was significantly greater than the tremor-predominant group (HR= 1.75 CI 1.23-2.51 P=.002) and compared to tremor before bradykinesia (HR = 1.75 CI 1.24-2.47 P=.001).
Conclusions:
Patients with tremor as a presenting symptom have a longer survival. In contrast, the presence of bradykinesia/rigidity as a presenting symptom correlates with reduced survival across all types of synucleinopathies.
Introduction
Progression and survival are relevant questions in synucleinopathies (Parkinson’s disease [PD], Dementia with Lewy Bodies [DLB], Parkinson’s disease dementia [PDD], and Multiple System Atrophy [MSA]).1-4 A number of studies have examined factors associated with survival among the different synucleinopathies2-4; however, most of these studies primarily examined PD and MSA. Recently, we published a study on the survival and causes of death in clinically defined synucleinopathies in Olmsted County, MN, from 1991 to 2010.3 We reported that residents with MSA-p, DLB, and PDD were at increased risk of mortality compared to the general population, but the risk of mortality in PD was only moderately increased.
Few studies have assessed the role of the presenting clinical Parkinsonism phenotypes (tremor-predominant vs. akinetic-rigid) and the risk of mortality. Studies that examined presenting symptoms and mortality have primarily focused on PD,1,5 but there has been less examination of PDD, DLB, and MSA. It is crucial to understand the longterm outcomes of the different clinical phenotypes of synucleinopathies in order to provide individualized estimates to the patients affected and to the caregivers.
To address these gaps of knowledge, we utilized an incident study of synucleinopathy-associated clinical syndromes (PD, DLB, PDD, and MSA-p) from 1991 to 2010.2 We compared survival of incident patients by presenting clinical phenotypes (tremor-predominant vs akinetic-rigid).
Methods
Ascertainment of Patients with Synucleinopathy
Details about the ascertainment of Parkinsonism patients are reported elsewhere.2,6 Briefly, we used the medical records-linkage system of the Rochester Epidemiology Project (REP) to identify all individuals in Olmsted County, Minnesota, with clinically diagnosed synucleinopathy with Parkinsonism between 1991 and 2010. The REP infrastructure indexes and links essentially all medical information of the county population.7-10 All medical diagnoses, surgical interventions, and other procedures are abstracted and entered into computerized indexes using the Hospital Adaptation of the International Classification of Diseases(H-ICDA), Eighth Revision11 and the International Classification of Diseases Ninth Revision (ICD-9).12
We ascertained potential cases of Parkinsonism using a computerized-screening phase and a subsequent clinical-confirmation phase, as described in the original reports. The complete medical records of all persons receiving at least one screening diagnostic code for Parkinsonism were reviewed by a movement-disorders specialist (RS) using specifically designed abstracting forms and instruction manuals. The movement-disorders specialist established the onset year and type of Parkinsonism using specified diagnostic criteria.2,13-16 We included patients residing in Olmsted County at the time of the parkinsonian-symptom onset but excluded persons who denied permission to use their medical records for research.7 Further details about case-finding procedures are published elsewhere.3,13,17 The Institutional Review Boards of the Mayo Clinic and Olmsted Medical Center approved the study.
Diagnostic Criteria
Our diagnostic adjudication included two steps: the definition of Parkinsonism as a syndrome and the definition of different types of Parkinsonism within the syndrome. We defined Parkinsonism as the presence of at least two of four cardinal signs: rest tremor, bradykinesia, rigidity, and impaired postural reflexes. Among persons fulfilling the Parkinsonism criteria, we applied diagnostic criteria for specific types of Parkinsonism and grouped Parkinsonism patients into presumed synucleinopathies.2
PD was defined as Parkinsonism with all three of the following: no other cause (e.g., repeated stroke with stepwise progression, repeated head injury, history of encephalitis, neuroleptic treatment ≤6 months before onset, hydrocephalus, brain tumor); no documented unresponsiveness to levodopa at doses of at least 1 g/d in combination with carbidopa (applicable only to treated patients); and no prominent or early (<1 year of onset) signs of more extensive nervous system involvement (e.g., dysautonomia) not otherwise explained.13 We utilized previously published consensus criteria to diagnose DLB, PDD, and MSA.2,6,18,19 Of note, we diagnosed patients with DLB when dementia/memory decline occurred at the time of Parkinsonism diagnosis or within 1 year from the presence of cognitive decline, according to the Fourth DLB consensus.20 We did not explore the diagnosis of PDD when dementia occurred subsequent to the initial diagnosis of Parkinsonism.
Regarding MSA, we included only MSA-p because of the nature of our case-identification that is aimed toward parkinsonism 2; however, we estimated that we missed only 1 case of MSA without clear evidence of parkinsonism (data not published). For each case, we collected information regarding the presence and the dates of first occurrence of the cardinal symptoms of Parkinsonism (rest tremor, bradykinesia, rigidity, and impaired postural reflexes) as well as the presence of laterality at onset. Then, we categorized the cases of all the synucleinopathies (PD, DLB, PDD, and MSA-p) according to the time of tremor onset vs bradykinesia/rigidity: tremor before bradykinesia/rigidity, tremor and bradykinesia/rigidity simultaneous with onset, bradykinesia/rigidity before tremor, bradykinesia/rigidity only, and tremor-predominant.
Our case-finding procedures have been proven reliable.6 We also reviewed available autopsy reports of all patients who died during the study to validate our classification of presumed synucleinopathies and tauopathies and found 81.5% clinico-pathological agreement.2
Data Analysis
We followed patients from their date of diagnosis until death or end of the study (June 30, 2015), whichever occurred first. A rank sum test was used to determine whether the median age at diagnosis differed by sex. A chi-square test was used to determine whether the symptoms at diagnosis differed by sex. Cox proportional-hazards models, with age as the time scale, were used to calculate hazard ratios (HR) and 95% confidence interval (CI) for the risk of mortality by presenting symptom(s): tremor before bradykinesia/rigidity, tremor and bradykinesia/rigidity at the same time of onset, bradykinesia/rigidity before tremor, bradykinesia/rigidity only, and tremor-predominant. We first estimated a univariable model, followed by a multivariate model adjusting for age at diagnosis and sex. The proportional hazard assumption was examined using the Schoenfeld residuals test for each model. The test was non-significant (P > .05), indicating that the assumption was valid. We constructed Kaplan-Meier survival curves with death from any cause as the event of interest. All analyses were completed using Stata version 13.0 (Stata Corp, College Station, TX).
Results
We identified 433 patients with a diagnosis of Parkinsonism due to a presumed synucleinopathy and information on presenting symptoms from 1991 through 2010. Of these, 301 (69.5%) were diagnosed with PD, 68 (15.7%) with DLB, 52 with PDD (12.0%), and 12 (2.8%) with MSA-p.
The median age at diagnosis for all patients was 76.1 years (IQR: 68.8, 82.3 years) and 264 (61.0%) were men (Table 1). The median age at diagnosis did not differ by sex (P = .17, rank sum test). The median age at death (n=290) was 84.5 years (IQR: 79.3-89.8). Among all patients, the presenting symptom did not differ by sex (P = .29, X2(4) = 5.02).
Table 1.
Demographic characteristics of the patients included in the study with clinical diagnosis of synucleinopathy
| Men | Age at diagnosis | Age at Death | |||
|---|---|---|---|---|---|
| Diagnosis | Total N | N (%) | Median (IQR) |
Number Died* |
Median (IQR) |
| All | 433 | 264 (61.0%) | 76.1 (68.8, 82.3) | 290 | 84.5 (79.3, 89.8) |
| PD | 301 | 181 (60.1%) | 74.8 (66.9, 81.7) | 168 | 86.1 (79.6, 90.5) |
| PDD | 52 | 26 (50.0%) | 82.1 (75.9, 87.6) | 50 | 84.5 (79.7, 90.9) |
| DLB | 68 | 48 (70.6%) | 76.9 (72.4, 80.7) | 60 | 83.1 (78.8, 87.0) |
| MSA-p | 12 | 9 (75.0%) | 72.9 (64.3, 77.4) | 12 | 80.8 (67.1, 84.9) |
PD = Parkinson’s disease; PDD = Parkinson’s disease Dementia; DLB = Dementia with Lewy Bodies; MSA-p= Multiple Systems Atrophy with Parkinsonism
Died by end of the study timeframe (June 30, 2015)
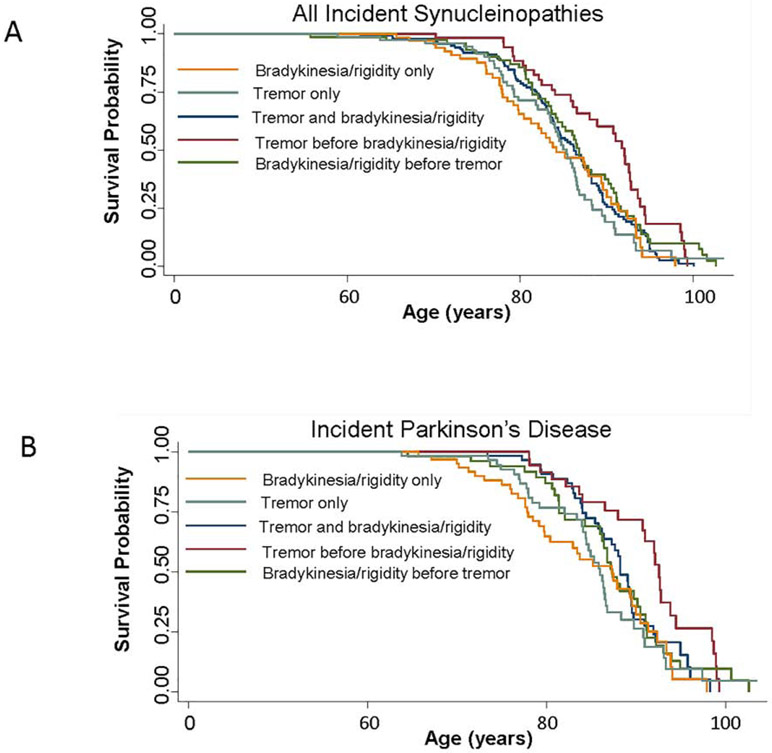
The risk of death by symptom at diagnosis for all synucleinopathies, and by clinical diagnosis, is shown in Table 2. Among all synucleinopathies and using bradykinesia/rigidity only as the reference group, patients with tremor only (HR = 0.45, 95% confidence interval [CI]: 0.31, 0.67), tremor before bradykinesia/rigidity (HR = 0.66, 95% CI: 0.46, 0.95), and bradykinesia/rigidity before tremor (HR = 0.61, 95% CI: 0.43, 0.86) all had a reduced risk of mortality in multivariate analyses adjusting for age at diagnosis and sex. Kaplan-Meier plots for all synucleinopathies are shown in Figure 1a. Among the PD only group (see Figure 1b), the patients with onset of tremor only had a reduced risk of mortality compared to the bradykinesia/rigidity only group in multivariate analyses (HR = 0.51, 95% CI: 0.30, 0.87). However, none of the other symptom presentations were associated with mortality compared to the bradykinesia/rigidity only group. Among the patients with DLB only or PDD only there were no associations between the presenting symptoms and risk of mortality, but the numbers with each presenting symptom were small. We also could not run the models for MSA-p because only 1 person each endorsed tremor and bradykinesia at the same time, tremor before bradykinesia/rigidity, or bradykinesia/rigidity before tremor.
Table 2.
Risk of death by symptom(s) at diagnosis for all synucleinopathies and by clinical diagnosis.
| All (N=433) | ||||
|---|---|---|---|---|
| Univariate Analyses | Adjusting for Age at diagnosis and sex |
|||
| Group | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Bradykinesia/rigidity only | 1.00 (ref) | 1.00 (ref) | ||
| Tremor only | 0.59 (0.40, 0.87) | .007 | 0.45 (0.31, 0.67) | <.001 |
| Tremor and bradykinesia/rigidity | 0.81 (0.58, 1.13) | .21 | 0.72 (0.52, 1.01) | .05 |
| Tremor before Bradykinesia/rigidity | 1.14 (0.80, 1.62) | .46 | 0.66 (0.46, 0.95) | .02 |
| Bradykinesia/rigidity before tremor | 1.15 (0.82, 1.62) | .41 | 0.61 (0.43, 0.86) | .006 |
| PD only (N=301) | ||||
| Univariate Analyses | Adjusting for Age at diagnosis and sex |
|||
| Group | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Bradykinesia/rigidity only | 1.00 (ref) | 1.00 (ref) | ||
| Tremor only | 0.60 (0.36, 1.02) | .06 | 0.51 (0.30, 0.87) | .01 |
| Tremor and bradykinesia/rigidity | 1.02 (0.64, 1.62) | .95 | 0.92 (0.57, 1.48) | .74 |
| Tremor before Bradykinesia/rigidity | 1.41 (0.90, 2.22) | .14 | 0.94 (0.59, 1.49) | .79 |
| Bradykinesia/rigidity before tremor | 1.31 (0.82, 2.07) | .26 | 0.78 (0.49, 1.25) | .30 |
| DLB only (N=68) | ||||
| Univariate Analyses | Adjusting for Age at diagnosis and sex |
|||
| Group | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Bradykinesia/rigidity only | 1.00 (ref) | 1.00 (ref) | ||
| Tremor only | 0.74 (0.34, 1.63) | .46 | 0.66 (0.28, 1.53) | .33 |
| Tremor and bradykinesia/rigidity | 0.76 (0.36, 1.62) | .48 | 1.06 (0.48, 2.33) | .89 |
| Tremor before Bradykinesia/rigidity | 1.32 (0.46, 3.79) | .61 | 1.96 (0.65, 5.95) | .23 |
| Bradykinesia/rigidity before tremor | 2.13 (0.94, 4.81) | .07 | 1.15 (0.49, 2.70) | .75 |
| PDD only (N=52) | ||||
| Univariate Analyses | Adjusting for Age at diagnosis and sex |
|||
| Group | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Bradykinesia/rigidity only | 1.00 (ref) | 1.00 (ref) | ||
| Tremor only | 1.04 (0.36, 3.00) | .94 | 2.18 (0.42, 11.30) | .35 |
| Tremor and bradykinesia/rigidity | 0.69 (0.33, 1.47) | .34 | 0.34 (0.09, 1.30) | .12 |
| Tremor before Bradykinesia/rigidity | 1.91 (0.44, 8.32) | .39 | 1.67 (0.69, 4.00) | .25 |
| Bradykinesia/rigidity before tremor | 1.53 (0.57, 4.07) | .40 | 1.07 (0.29, 3.90) | .92 |
HR = hazard ratio; PD = Parkinson’s disease; PDD = Parkinson’s disease Dementia; DLB = Dementia with Lewy Bodies
Figure 1.
Kaplan-Meier survival curves by presenting symptom for (A) patients with any synucleinopathy and (B) Parkinson’s disease only.
Discussion
Our study suggests a reduced risk of mortality in patients with synucleinopathies that present with tremor, especially when tremor is the principal symptom of the parkinsonian phenotype. We also observed this association among the PD only group, but not the DLB or PDD groups. Although there were a limited number of MSA-p patients (n=12), the majority had bradykinesia/rigidity as their prominent symptoms. Thus, overall, patients with synucleinopathies and bradykinesia/rigidity died earlier than patients with mostly tremor or tremor during the course of the disease.
Our findings are consistent with observations from a previous report in the Olmsted County population examining the association between the clinical characteristics of PD patients from 1976 to 1995 and survival.1 Patients with PD that had rest tremor or pronounced asymmetry had a longer survival than the patients without it. We avoided the overlap of the two studies by removing patients with PD included in the 1976–1995 period from the period spanning 1991 to 2010. Thus, our study confirms the previous findings and expands it toward the entire spectrum of alpha-synucleinopathies.
Our study is also consistent with the pivotal study reporting a less favorable prognosis in cases with early-onset postural instability and gait difficulty (PIGD) compared to tremor-onset cases.5 However, in contrast with our findings, this study reported that PIGD did not have a synuclein-related pathology whereas tremor-predominant did.5 Our results are also consistent with a recent large clinical series that reported patients with akinetic-rigid PD had faster progression in gait difficulty compared to tremor-predominant PD.21 In addition, a number of studies in the past have explored clinical phenotypes of symptoms using cluster analyses approach. Indeed, a number of studies identified motor phenotypes such as “tremor dominant” and a “bradykinesia/rigidity and PIGD dominant” cluster profile of symptoms of PD.22 One study reported that non-tremor-dominant PD is associated with worsening cognitive, neuropsychiatric symptoms, and hallucinations.23 A systematic review reported that the most common symptoms that are identified by cluster analyses are “old age at onset and rapid disease progression” and “young age at onset and slow disease progression” 24; the same study identify that the tremor-predominant PD has a slower disease progression than the other cluster identified.24 A meta-analysis on 27 studies reported that non-tremor predominant PD had more neuropsychiatric symptoms than tremor-predominant PD. Our data are supportive of these findings.
Few studies have examined the association between onset symptoms and survival across the synucleinopathies. The biological reasons to explain the differences across the parkinsonian-symptom phenotypes of synucleinopathies are still unknown. However, a number of explanations are possible. First, there may be differences in the localization of the alpha-synuclein deposition in the striatum causing a different downstream effect that has consequences regarding disease progression. It is possible that individuals with bradykinesia/rigidity have more prominent involvement of the matrix of the striatum, whereas the striasomes are involved in patients with tremor-predominant disease.25 In addition, the evidence for “tremor-neurons” in the striatum26 and in the direct/indirect pathway is observed during the intraoperative monitoring period of deep brain-stimulation surgery.27 Second, the deposit of alpha-synuclein in different parts of the striatum may cause differential damage to the tremor neurons of the striasome,25 leading to a more redundant circuitry of response with less downstream effect on the progression and cell-to-cell spreading of the disease.28 It is also possible that the striasome has more closed self-sufficient physiological activity with a better defensive system toward neurodegenerative diseases. Third, patients with mostly tremor may have better ability to move and exercise, given that the bradykinesia/rigidity is less present, and can take advantage of the protective effect of exercise on the progression of neurodegenerative diseases.29
Our study has several strengths. First, we included all incident cases of synucleinopathy from a defined population over a defined time window and age- and sex-matched referent subjects derived from the same population and time window. Thus, referral bias should be minimal.30 Second, previous studies primarily evaluated survival from the study enrollment date rather than from the date of diagnosis, a design that may overestimate relative mortality. Third, our study explored synucleinopathies as a group and also as separate types including PD, PDD, DLB, and MSA-p. Therefore, we established mortality risks of the different clinical synucleinopathy subtypes which is in contrast to previous studies that focused only on PD.1 Fourth, a movement-disorders specialist adjudicated all patients and dated all the symptoms at the time of abstraction reducing the diagnostic-criteria differences.
Our study also has limitations. First, assessing the precise chronology of symptoms, time of onset of clinical features, severity of parkinsonian symptoms, and treatment history from an historical review of medical records can be difficult. Second, our methods did not identify cases of DLB presenting without Parkinsonism. However, given that Parkinsonism is present in the vast majority of patients with DLB, especially after some years into the disease process, we most likely did not overlook a large number of patients; for this purpose, we conducted an exploratory analyses in a different cohort of DLB (a research case series) observing that ~3% of patients affected by DLB did not develop Parkinsonism over the disease course (data not published). Third, our study population was relatively small, which limits the stability of estimates, particularly for less common diseases such as MSA.
Conclusions
Among incident synucleinopathy patients, we found a reduced risk of mortality for those presenting with tremor compared to bradykinesia/rigidity. Our findings provide important evidence about the natural history and prognosis of individuals affected by synucleinopathies of various types. Our results may guide medical providers and caregivers in prognostication and may serve as a guide toward more personalized management of the disease.
ACKNOWLEDGMENT SECTION
Financial Support and Conflict of Interest Disclosures: This study was supported by award R01 AG034676, from the National Institute on Aging of the National Institutes of Health and by the Mayo Foundation for Medical Education and Research. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Disclosures: Dr. Savica receives funding from the National Institute on Aging, the National Institute of Neurological Disorders and Stroke, and the Parkinson ’s Disease Foundation, Inc. and unrestricted research grants from Acadia Pharmaceuticals, Inc. Dr. Bower receives funding from the Parkinson’s Disease Foundation, Inc. Dr. Ahlskog receives royalties from Oxford University Press for three recently published books. Dr. Mielke receives funding from the National Institutes of Health, and unrestricted research grants from Biogen and Lundbeck. She has consulted for Lysosomal Therapeutics, Inc, and Eli Lilly. Dr. Turcano has no disclosures.
Abbreviations
- CI
Confidence interval
- DLB
Dementia with Lewy bodies
- HR
Hazard ratio
- IQR
Interquartile Range
- MSA
Multiple system atrophy
- MSA-p
Multiple system atrophy with Parkinsonism
- PD
Parkinson’s disease
- PDD
Parkinson’s disease with dementia
- PIGD
Postural instability and gait difficulty
- REP
Rochester Epidemiology Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elbaz A, Bower JH, Peterson BJ, et al. Survival study of Parkinson disease in Olmsted County, Minnesota. Arch Neurol. 2003;60:91–96. [DOI] [PubMed] [Google Scholar]
- 2.Savica R, Grossardt BR, Bower JH, Ahlskog JE, and Rocca WA Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol. 2013;70:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savica R, Grossardt BR, Bower JH, et al. Survival and Causes of Death Among People With Clinically Diagnosed Synucleinopathies With Parkinsonism: A Population-Based Study. JAMA Neurol. 2017;74:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Figueroa JJ, Singer W, Parsaik A, et al. Multiple system atrophy: prognostic indicators of survival. Mov Disord. 2014;29:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajput AH, Pahwa R, Pahwa P, and Rajput A Prognostic significance of the onset mode in parkinsonism. Neurology. 1993;43:829–830. [DOI] [PubMed] [Google Scholar]
- 6.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, and Rocca WA Incidence of Dementia With Lewy Bodies and Parkinson Disease Dementia. JAMA Neurol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, and Rocca WA Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, and Rocca WA Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, and Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commission on Professional and Hospital Activities: H-ICDA, Hospital Adaptation of ICDA. 2nd ed. Ann Arbor, MI: National Center for Health Statistics; 1973. [Google Scholar]
- 12.Manual of the International Classification of Diseases, Injuries, and Causes of Death, based on the Recommendations of the Ninth Revision Conference, 1975, and Adopted by the Twenty-ninth World Health Assembly Geneva, Switzerland: World Health Organization; 1977. [Google Scholar]
- 13.Bower JH, Maraganore DM, McDonnell SK, and Rocca WA Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976-1990. Neurology. 1999;52:1214–1220. [DOI] [PubMed] [Google Scholar]
- 14.Collins SJ, Ahlskog JE, Parisi JE, and Maraganore DM Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry. 1995;58:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilman S, Low P, Quinn N, et al. Consensus statement on the diagnosis of multiple system atrophy. American Autonomic Society and American Academy of Neurology. Clin Auton Res. 1998;8:359–362. [DOI] [PubMed] [Google Scholar]
- 16.McKeith IG Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–423. [DOI] [PubMed] [Google Scholar]
- 17.Savica R, Grossardt BR, Bower JH, Ahlskog JE, and Rocca WA Risk factors for Parkinson's disease may differ in men and women: an exploratory study. Horm Behav. 2013;63:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 19.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konno T, Deutschlander A, Heckman MG, et al. Comparison of clinical features among Parkinson's disease subtypes: A large retrospective study in a single center. J Neurol Sci. 2018;386:39–45. [DOI] [PubMed] [Google Scholar]
- 22.Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, and Barker RA Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005;76:343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reijnders JS, Ehrt U, Lousberg R, Aarsland D, and Leentjens AF The association between motor subtypes and psychopathology in Parkinson's disease. Parkinsonism Relat Disord. 2009;15:379–382. [DOI] [PubMed] [Google Scholar]
- 24.van Rooden SM, Heiser WJ, Kok JN, Verbaan D, van Hilten JJ, and Marinus J The identification of Parkinson's disease subtypes using cluster analysis: a systematic review. Mov Disord. 2010;25:969–978. [DOI] [PubMed] [Google Scholar]
- 25.Brimblecombe KR, and Cragg SJ The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem Neurosci. 2017;8:235–242. [DOI] [PubMed] [Google Scholar]
- 26.Kiss ZH, Mooney DM, Renaud L, and Hu B Neuronal response to local electrical stimulation in rat thalamus: physiological implications for mechanisms of deep brain stimulation. Neuroscience. 2002;113:137–143. [DOI] [PubMed] [Google Scholar]
- 27.Deep-Brain Stimulation for Parkinson's Disease Study, G., Obeso JA, Olanow CW, et al. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001;345:956–963. [DOI] [PubMed] [Google Scholar]
- 28.Chu Y, and Kordower JH The prion hypothesis of Parkinson's disease. Curr Neurol Neurosci Rep. 2015;15:28. [DOI] [PubMed] [Google Scholar]
- 29.Schenkman M, Moore CG, Kohrt WM, et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol. 2018;75:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackett DL Bias in analytic research. J Chronic Dis. 1979;32:51–63. [DOI] [PubMed] [Google Scholar]