Abstract
Decreased circulating levels of hydrogen sulfide (H2S) are associated with higher mortality following myocardial ischemia. This study aimed at determining the long-term dose-dependent effects of sodium hydrosulfide (NaSH) administration on myocardial ischemia-reperfusion (IR) injury. Male rats were divided into control and NaSH groups that were treated for 9 weeks with daily intraperitoneal injections of normal saline or NaSH (0.28, 0.56, 1.6, 2.8, and 5.6 mg/kg), respectively. At the end of the study, hearts from all rats were isolated and hemodynamic parameters were recorded during baseline and following IR. In isolated hearts, infarct size, oxidative stress indices as well as mRNA expression of H2S-, nitric oxide (NO)-producing enzymes, and inflammatory markers were measured. In heart tissue following IR, low doses of NaSH (0.28 and 0.56 mg/kg) had no effect, whereas an intermediate dose (1.6 mg/kg), improved recovery of hemodynamic parameters, decreased infarct size, and decreased oxidative stress. It also increased expression of cystathionine γ-lyase (CSE), Raf kinase inhibitor protein (RKIP), endothelial NO synthase (eNOS), and neuronal NOS (nNOS), as well as decreased expression of inducible NOS (iNOS) and nuclear factor kappa-B (NF-κB). At the high dose of 5.6 mg/kg, NaSH administration was associated with worse recovery of hemodynamic parameters and increased infarct size as well as increased oxidative stress. This dose also decreased expression of CSE, RKIP, and eNOS and increased expression of iNOS and NF-κB. In conclusion, chronic treatment with NaSH has a U-shaped concentration effect on IR injury in heart tissue. An intermediate dose was associated with higher CSE-derived H2S, lower iNOS-derived NO, lower oxidative stress, and inflammation in heart tissue following IR.
Keywords: hydrogen sulfide, nitric oxide, infarct size, ischemia–reperfusion injury, H2S-producing enzymes, NO-producing enzymes, RKIP, NF-κB, oxidative stress
1. Introduction
Myocardial ischemia (MI) is one of the leading causes of cardiovascular morbidity and mortality worldwide [1]. MI often occurs following a partial or complete occlusion of the coronary arteries, and while reperfusion rescues the ischemic heart from expected death, it is associated with ischemia-reperfusion (IR) injury [2]. Protective effects of ischemic pre-and post-conditioning against myocardial IR injury have been documented in animals [3]; clinical translation of these results has however not been very successful [4,5,6]. Another approach for protecting the heart against ischemia is using pharmacological agents, which appears to be a more realistic and feasible approach from the clinical perspective [7].
Hydrogen sulfide (H2S) is produced in the cardiomyocytes by at least three H2S-producing enzymes, i.e., cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST), of which CSE is the most important one [8]. In mice, CSE deficiency decreases tolerance to IR injury [9] and overexpression of the enzyme attenuates myocardial IR injury [10]. Protective effects of H2S against myocardial IR injury are nitric oxide (NO)-dependent [9,11,12]. In endothelial NO synthase (eNOS)-knockout mice, administration of H2S donors failed to protect the heart from IR injury [9]. eNOS-derived NO protected against myocardial IR injury [13,14]; while inducible NOS (iNOS)-derived NO contributed to IR injury [15,16]. Inhibition of CSE in mice exacerbated myocardial IR injury by decreasing eNOS-derived NO [9] and increasing iNOS-derived NO [17]. Administration of H2S donors increased tolerance to myocardial IR in rodents by decreasing iNOS expression [18,19] and increasing eNOS activation [9].
IR injury starts with oxidative stress and inflammation, followed by apoptosis and necrosis leading to irreversible cell death [20]. The Raf kinase inhibitory protein (RKIP) expression is associated with inflammation-induced diseases [21]. Loss of RKIP increases the nuclear factor kappa-B (NF-κB) transcription factor, whereas overexpression of RKIP reduces it [22]. Activation of NF-κB promotes inflammation in the setting of myocardial ischemia and exacerbates the heart’s response to IR injury [23]. Anti-inflammatory effects of sodium hydrosulfide (NaSH) have been reported after short-term administration before myocardial IR [24,25]; however, the effects of NaSH on RKIP and NF-κB have not been reported.
In fact, results of a recent meta-analysis in normal rats and mice have shown that exogenous H2S administration has a protective effect against myocardial IR injury [26]. The cardioprotective effects of H2S-releasing agents have been studied in vitro [26,27,28,29,30,31,32]. So far, the in vivo studies are mostly short-term where NaSH has been administrated 15 min [31], 6 days [32], or 7 days [28,29,30] before ischemia. To our knowledge, there is no long-term study assessing the dose-dependent effects of H2S on the cardiovascular system. In addition, a biphasic response to exposure to increasing H2S has been reported in brain; i.e., NaSH in the low-to-intermediate doses could protect the brain from IR injury, while at higher doses opposite effects were observed [33]. Therefore, the aim of this study was to determine dose-dependent, long-term in vivo effects of NaSH administration on myocardial IR injury in male rats and to evaluate and correlate RKIP, NF-κB, and oxidative stress responses under these conditions.
2. Results
2.1. Effect of NaSH Administration on Body and Heart Weights
The body weights of animals were similar in all assigned groups before starting the experiments (Table 1). NaSH administration for 9 weeks at 0.28–1.6 mg/kg had no significant effects on body weights; however, at 5.6 mg/kg, it significantly increased heart weight (p = 0.0002), and heart-to-body-weight ratio (p = 0.0004) as assessed at the end of study.
Table 1.
Effects of different doses of NaSH on body and heart weights in normal Wistar rats.
| Parameters | Groups | |||||
|---|---|---|---|---|---|---|
| Control | NaSH (mg/kg/day) | |||||
| 0.28 | 0.56 | 1.6 | 2.8 | 5.6 | ||
| Initial body weight (g) | 239.7 ± 2.8 | 238.0 ± 2.2 | 245.7 ± 2.5 | 241.7 ± 1.9 | 245.2 ± 1.6 | 242.8 ± 2.2 |
| Final body weight (g) | 291.3 ± 4.6 | 290.5 ± 3.3 | 290.2 ± 2.6 | 295.7 ± 2.2 | 299.8 ± 2.5 | 291.0 ± 2.7 |
| Body weight gain (g) | 51.67 ± 3.8 | 52.5 ± 5.2 | 44.5 ± 3.9 | 54.0 ± 3.6 | 54.7 ± 2.8 | 48.2 ± 2.3 |
| Heart weight (g) | 1.01 ± 0.05 | 1.01 ± 0.05 | 0.99 ± 0.40 | 1.10 ± 0.05 | 1.10 ± 0.04 | 1.37 ± 0.06 * |
| Heart weight/Body weight (%) | 0.35 ± 0.02 | 0.35 ± 0.02 | 0.34 ± 0.01 | 0.37 ± 0.02 | 0.37 ± 0.01 | 0.47 ± 0.02 * |
* Statistically significant difference compared to non-treated control rats. Values are mean ±SEM (n = 6/each group).
2.2. Effect of NaSH on Systolic Blood Pressure, Heart Rate, and Hemodynamic Parameters
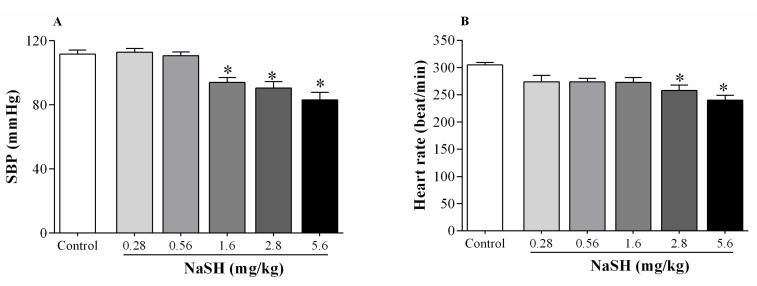
NaSH administration at 1.6, 2.8, and 5.6 mg/kg decreased systolic blood pressure (SBP) (Figure 1A); heart rate was decreased at 2.8 and 5.6 mg/kg (Figure 1B).
Figure 1.
Dose-dependent effects of NaSH on systolic blood pressure (SBP) (A) and heart rate (B). Values are mean ± SEM (n = 6/group); * p < 0.05 compared to non-treated control rats.
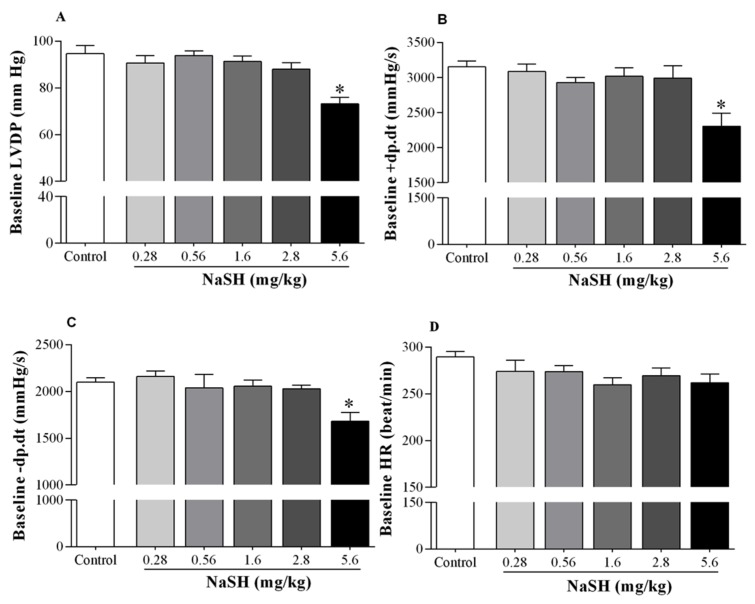
During the stabilization period, NaSH at 5.6 mg/kg decreased left ventricular developed pressure (LVDP) from 94.7 ± 3.5 to 73.2 ± 2.7 mmHg (p = 0.0001) (Figure 2A), peak rate of positive changes in left ventricular pressure (+dp/dt) from 3153 ± 84 to 2307 ± 185 (p = 0.0012) (Figure 2B), and peak rate of negative changes in left ventricular pressure (−dp/dt) from 2099 ± 50 to 1682 ± 93 (p = 0.0180) (Figure 2C). At all doses tested, NaSH had no effects on baseline heart rates (Figure 2D).
Figure 2.
Effects of different doses of NaSH on hemodynamic parameters during the stabilization period. Hemodynamic parameters included left ventricular developed pressure (LVDP, A); peak rate of positive changes in left ventricular pressure (+dp/dt, B); peak rate of negative changes in left ventricular pressure (−dp/dt, C); and heart rate (HR, D). Values are mean ± SEM (n = 6/group); * p < 0.05 compared to non-treated control rats.
Compared to controls, NaSH at 0.28 and 0.56 mg/kg had no effect on recoveries of LVDP (Figure 3A), +dp/dt (Figure 3B), and −dp/dt (Figure 3C) following ischemia; however, it significantly increased recoveries of LVDP, +dp/dt, and −dp/dt at 1.6 mg/kg and decreased these parameters at 2.8 and 5.6 mg/kg, Figure 3A–C. Heart rate recovery was not affected at any dose, Figure 3D.
Figure 3.
Effects of different doses of NaSH on hemodynamic parameters during the recovery period. Hemodynamic parameters included left ventricular developed pressure (LVDP, A); peak rate of positive changes in left ventricular pressure (+dp/dt, B); peak rate of negative changes in left ventricular pressure (−dp/dt, C) and heart rate (HR, D). Values are mean ± SEM (n = 6/group); * p < 0.05 compared to non-treated control rats.
2.3. Effect of NaSH on H2S and Nitrite + Nitrate (NOx) Levels in Heart Tissue
Following IR, H2S levels in the heart increased from 21.3 ± 2.7 to 33.4 ± 4.6 nmol/mg protein (p = 0.0274) when NaSH was administered at 1.6 mg/kg; at lower doses and at 2.8 mg/kg, there were no effects on H2S levels; however, at 5.6 mg/kg, H2S levels decreased to 11.2 ± 1.9 nmol/mg protein (p = 0.0920) Figure 4A.
Figure 4.
Effects of different doses of NaSH on heart hydrogen sulfide (H2S, A) and nitrite + nitrate (NOx, B) levels after the ischemia–reperfusion (IR) period. Values are mean ± SEM; (n = 6/group); * p < 0.05 compared to non-treated rats.
NOx levels decreased from 27.2 ± 2.7 to 14.2 ± 1.9 nmol/mg protein (p = 0.0906) at 1.6 mg/kg NaSH (Figure 5B), but NOx levels increased to 45.4 ± 6.9 nmol/mg protein (p = 0.0077) at 5.6 mg/kg NaSH, with no effect at any of the other doses, Figure 4B.
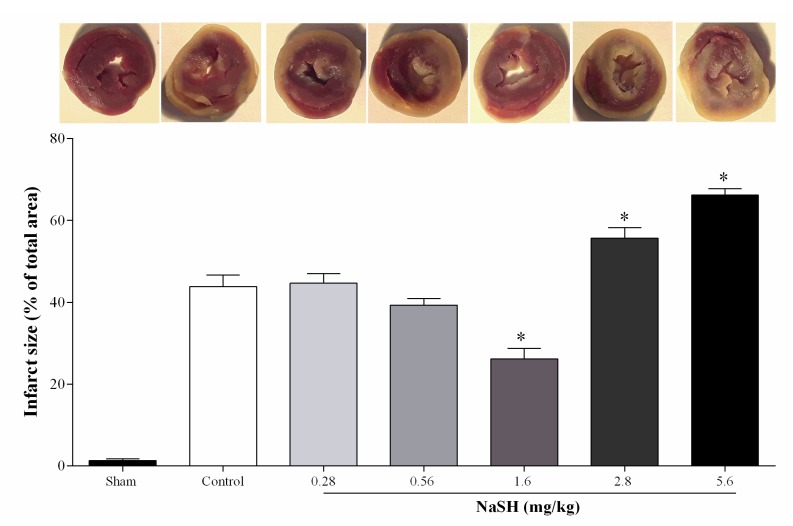
Figure 5.
Effects of different doses of NaSH on infarct sizes. Values are mean ± SEM; (n = 6/group); * p < 0.05 compared to non-treated control rats.
2.4. Effect of NaSH on Infarct Size
NaSH at 0.28 and 0.56 mg/kg had no effect on myocardial infarct size, while at 1.6 mg/kg this was decreased by 40% (p < 0.0001), and at 2.8 and 5.6 mg/kg this was increased by 27% (p = 0.0024) and 51%, (p < 0.0001), respectively (Figure 5).
2.5. Effect of NaSH on mRNA Expression of H2S- and NO-Producing Enzymes in the Heart
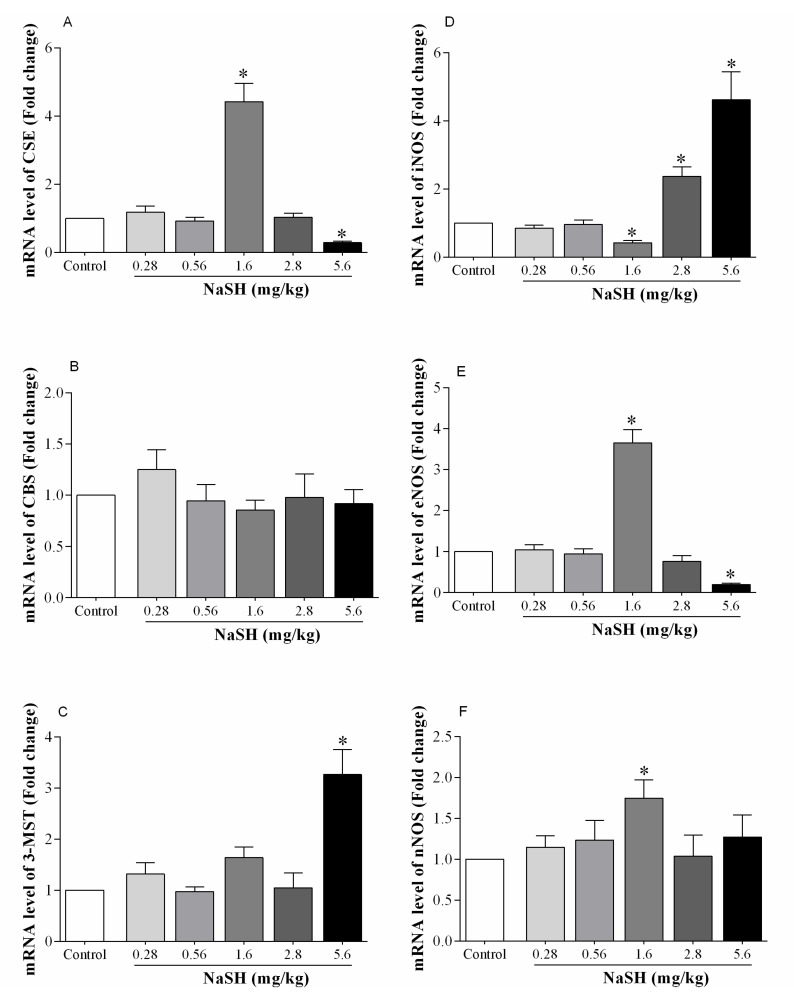
Following ischemia, mRNA expression for CSE increased by 342% at a NaSH dose of 1.6 mg/kg, while this was decreased by 71% at 5.6 mg/kg; at other doses, CSE expression was not significantly different from that of the controls (Figure 6A).
Figure 6.
Effect of NaSH on mRNA expression of H2S- and NO-producing enzymes in heart tissue. H2S-producing enzymes including cystathionine gamma-lyase (CSE, A), cystathionine-β-synthase (CBS, B), and mercaptopyruvate sulfurtransferase (3-MST, C) and NO-producing enzymes including inducible nitric oxide synthase (iNOS, D), endothelial nitric oxide synthase (eNOS, E), and neuronal nitric oxide synthase (nNOS, F). Values are mean ± SEM; (n = 6/group); * p < 0.05 compared to non-treated control rats.
NaSH had no effect on mRNA expression of CBS at any of the doses employed (Figure 6B). The level of 3-MST mRNA was increased by 220% at 5.6 mg/kg NaSH with no effects at any of the other doses (Figure 6C).
Following IR, NaSH at 1.6 mg/kg decreased mRNA expression of iNOS by 58% (p < 0.0001), that of eNOS and neuronal NOS (nNOS) were increased by 265% (p < 0.0001) and 75% (p = 0.0225), respectively, Figure 6D–F. NaSH at 2.8 mg/kg increased iNOS expression by 204% (p < 0.0001), and at 5.6 mg/kg by 362% (p < 0.0001). NaSH at 2.8 mg/kg did not have an effect on eNOS expression; however, at 5.6 mg/kg it decreased it by 81% (p < 0.0001). NaSH at 0.28 and 0.56 mg/kg had no effect on mRNA expression of the NO-producing enzymes.
2.6. Effect of NaSH on mRNA Expression of Inflammation-Related Markers in the Heart
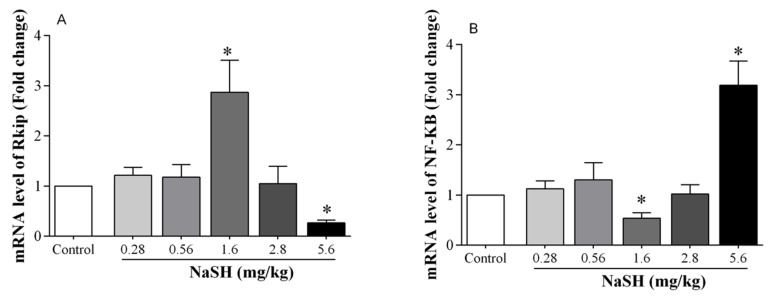
RKIP expression was increased by 187% (p = 0.0260) at a NaSH dose of 1.6 mg/kg, and it was decreased by 73% (p < 0.0001) at a dose of 5.6 mg/kg, Figure 7A. NaSH at a dose of 1.6 mg/kg decreased mRNA expression of NF-κB by 46% (p = 0.0018), whereas at 5.6 mg/kg it increased it by 219% (p < 0.0001), Figure 7B. Other doses of NaSH had no effect on RKIP or NF-κB expression.
Figure 7.
Effect of NaSH on mRNA expression of Raf kinase inhibitor protein (RKIP, A) and nuclear factor kappa-B (NF-κB, B) in heart tissue. Values are mean ± SEM; (n = 6/group); * p < 0.05 compared to non-treated control rats.
2.7. Effect of NaSH on Oxidative Stress Indices in Heart Tissue
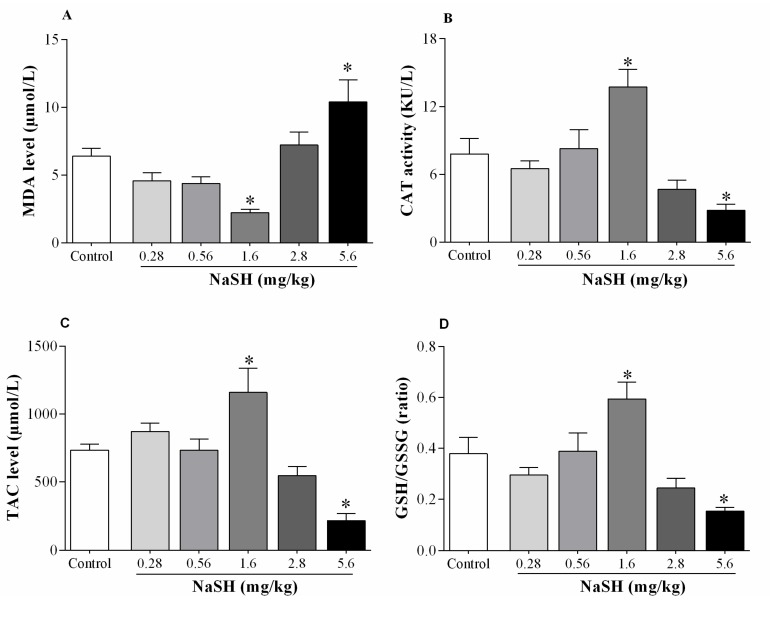
Following IR, compared to the non-treated rats, NaSH at 1.6 mg/kg decreased malondialdehyde (MDA) levels by 65% (p = 0.0303), Figure 8A; increased catalase (CAT) activity by 76% (p = 0.0228), Figure 8B; increased total antioxidant capacity (TAC) concentration by 58% (p = 0.0408), Figure 8C; and increased reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio by 57% (p = 0.0948), Figure 8D.
Figure 8.
Effects of NaSH administration on oxidative stress indices of heart tissue. Oxidative stress indices including malondialdehyde (MDA, A), catalase activity (CAT, B), total antioxidant capacity (TAC, C), and reduced glutathione-to-oxidized-glutathione ratio (GSH/GSSG, D). Values are mean ± SEM; (n = 6/group); * p < 0.05 compared to non-treated control rats.
NaSH at 5.6 mg/kg increased MDA levels by 62% (p = 0.0437), Figure 8A; decreased CAT activity by 64% (p = 0.0938), Figure 8B; decreased TAC concentration by 71% (p = 0.0064), Figure 8C; and decreased GSH/GSSG ratio by 59% (p = 0.0690), Figure 8D. Other doses of NaSH had no effects on any of these parameters.
3. Discussion
Our results showed a biphasic effect of NaSH on myocardial IR injury in normal rats. At an intermediate dose (1.6 mg/kg), NaSH had a protective effect against IR; at low doses (0.28 and 0.56 mg/kg), it had no effect; and at a high dose (5.6 mg/kg), it exacerbated myocardial IR injury. Favorable effects of NaSH at the intermediate dose of 1.6 mg/kg on cardiac function were, at least in part, associated with increased CSE expression, which is in line with the higher measured cardiac H2S levels, higher eNOS expression, and lower iNOS expression, which is also in line with the lower levels of cardiac NO and attenuated IR-induced oxidative stress and inflammation. NaSH at the highest dose tested, 5.6 mg/kg, had the opposite effects.
In this study, NaSH at doses of ≥1.6 mg/kg decreased SBP, and, at a dose ≥2.8 mg/kg, it decreased heart rate in the whole intact animal. What should be emphasized is that under physiological conditions, a decrease in blood pressure is accompanied with an increase in heart rate, this phenomenon is dampened with NaSH administration. Thus, in the long run, H2S may lead to cardiac remodeling, which may prove to be detrimental to the overall cardiac function. At what dose or dose-range, this remodeling may occur is not currently apparent and needs further long-term studies. In line with our results, a reduction in SBP and heart rate was observed in rats when NaSH was administered at a dose of 5.6 mg/kg for 28 days [34]. The underlying mechanism(s) for the reduction in SBP and heart rate in response to H2S-releasing agents is not clear; however, these are most likely not mediated through the activation of the parasympathetic nervous system, ATP-sensitive K+ and/or L-type voltage-sensitive Ca2+ channels [35,36]. Reductions in heart rate could be due to the inhibitory effects of H2S on the metabolism [37]; we recently reported that long-term administration of NaSH to rats perturbed carbohydrate metabolism [38].
NaSH at a dose of 5.6 mg/kg caused an increase in the weight of the hearts. This increase may be associated with lower cardiac contractility and is congruent with decreased baseline LVDP and ±dp/dt that was observed in our study. In addition, increased heart weight can be attributed to the vasodilatory effects of NaSH, since vasodilators at high doses can increase heart weight in normal mice [39].
In this study, NaSH at a dose of 1.6 mg/kg decreased infarct size and increased recoveries of LVDP and ±dp/dt, thus exhibiting a protective effect. Dose-dependent effects of short-term NaSH administration against myocardial IR injury in normal rats have been assessed both in vivo [40] and in vitro [41]; results indicate that NaSH at an intermediate dose (1.6 mg/kg) has protective effects whereas lower doses and higher doses have no effects on myocardial IR injury. Similarly, Kang et al. [28] reported that NaSH at 1.6 mg/kg increases tolerance against myocardial IR injury in normal rats. Our results regarding the protective effects of NaSH administration for 63 days extend previous short-term studies of NaSH administration that were for 5 days [42], 6 days [32], and 7 days [29,30] before inducing ischemia. In these studies, NaSH was administered at a dose of 0.78 mg/kg, which showed decreases in infarct size following myocardial IR injury.
Regarding the relatively higher doses of NaSH (2.8 and 5.6 mg/kg), in our study, NaSH decreased recoveries of hemodynamic parameters following myocardial IR injury and increased infract size; This is in contrast to some reported studies where NaSH at 3 mg/kg, 15 min before ischemia and at 5.6 and 16.8 mg/kg, 10 min before ischemia, had a protective effect [24,31] or no effect [40] against myocardial IR injury in normal rats. This apparent discrepancy may be due to the duration of NaSH administration, which in our study was 63 days, as toxic effects of H2S are dependent on both dose and duration of exposure [43].
As summarized in Table 2 [18,24,28,29,30,31,32,40,42,44,45,46], we did not find any studies that addressed the long-term in vivo effects of an H2S-releasing agent(s) on cardiac function; all studies reported were for up to 1 week of treatment. In addition, there are only a handful of studies that have addressed the dose-dependent in vivo effects of NaSH on cardiac tolerance against IR injury. Cardioprotective effects of NaSH have mostly been reported for short-term (up to 7 days) daily injections of NaSH, or for a single dose shortly before ischemia. In general, H2S-releasing agents have shown biphasic effects on IR injury using perfused hearts [10,40,47] with a very narrow therapeutic window [26,48]. Favorable effects of H2S are observed at low-to-intermediate concentrations, while detrimental effects are observed at high concentrations [43]. Our data are in line with studies that have suggested protective effects at low-to-intermediate concentrations of H2S (<10 μM) in the heart, while higher concentrations (>10 μM) have deleterious effects [43]. Thus, determining the appropriate dose of H2S is a critical issue for the development of H2S-based therapeutics [48].
Table 2.
Summary of studies indicating protective in vivo effect of NaSH administration on myocardial IR injury in normal rats *.
| Study | Year | Rat strain | Dose (μmol/kg) # | Duration ** | Mechanism | Administration Route | Ref. |
|---|---|---|---|---|---|---|---|
| Geng et al. | 2004 | Wistar | 2.8 and 14 | 5 days | Inhibition of oxidative stress | Daily IP injection | [42] |
| Sivarajah et al. | 2006 | Wistar | 50 | 15 min | Opening of mitochondrial KATP channels | Single IV injection | [31] |
| Zhu et al. | 2007 | Wistar | 14 | 7 day | Elevation of H2S concentrations | Daily IP injection | [30] |
| Zhu et al. | 2008 | Sprague–Dawley | 2.8 and 14 | 20 min | Inhibition of apoptosis | Single IV injection | [44] |
| Zhuo et al. | 2009 | Wistar | 14 | 6 days | Inhibition of apoptosis | Daily IP injection | [32] |
| Sivarajah et al. | 2009 | Wistar | 50 | 15 min | Inhibition of apoptosis and inflammation | Single IV injection | [24] |
| Pan et al. | 2009 | Sprague–Dawley | 0.1, 1, 3, 10 and 30 † | 1 day | Activation of protein kinase C | Single IP injection | [45] |
| Yao et al. | 2010 | Sprague–Dawley | 1, 10, 30, 100, and 300 ‡ | 10 min | Inhibition of apoptosis | Single IV injection | [40] |
| Yao et al. | 2012 | Wistar | 14 | 7 days | Inhibition of apoptosis | Daily IP injection | [29] |
| Issa et al. | 2013 | Wistar | 3.57 | 10 min | Inhibition of inflammation and iNOS expression and activation of Akt/eNOS pathway | Single IV injection | [18] |
| Li et al. | 2015 | Sprague–Dawley | 1.4, 2.8, and 14 | 10 min | Inhibition of endo/sarcoplasmic reticulum stress | Single IV injection | [46] |
| Kang et al. | 2017 | Sprague–Dawley | 30 | 30 min | Inhibition of apoptosis | Single IP injection | [28] |
* All studies have been conducted in male rats; ** duration of administration before ischemia; † protective effects have been observed for 0.1, 1, 3, and 10 μmol/kg but not for 30 μmol/kg; ‡ protective effect has been observed only at 30 μmol/kg; # to convert from μmol/kg to mg/kg multiply by 0.056; IP, intraperitoneal; IV, intravenous.
In this study, we showed that NaSH at 1.6 mg/kg increased CSE expression, while at 5.6 mg/kg it decreased the same, these correlated well with the measured H2S levels. CSE is the most important H2S-producing enzyme in the cardiovascular system [49], and cardiac H2S levels decrease ~80% in CSE knockout mice [50]. Here, administration of NaSH at 5.6 mg/kg lowered mRNA expression of cardiac CSE by ~70%, which may explain in part the lower cardiac H2S levels of ~50% that were observed in our study. We are not aware of any studies addressing the chronic in vivo effects of NaSH administration on H2S-producing enzymes in the heart after IR; however, lower CSE expression in cardiomyocytes has been reported in vitro with several H2S-releasing agents at high doses [51]. Most often data obtained from in vitro studies cannot be directly applied to predict the response of a whole organism [52,53] since in vitro studies do not repeat or represent the whole animal physiology [54]. In our study, tolerance to IR injury correlated well with CSE expression in the heart tissue. In support of these data, CSE knockout [9] or pharmacologic inhibition of CSE [55,56] decreased tolerance to IR injury in rats. In addition, CSE overexpression in mice increases tolerance to myocardial IR injury [10]. In our study, NaSH at 5.6 mg/kg increased 3-MST expression, we propose that this may be in compensation for the observed decreased CSE expression as has been previously reported for heart tissue after IR in mice [57].
In this study, NaSH at 1.6 mg/kg increased eNOS and nNOS expressions and it decreased iNOS expression following myocardial IR; while at 5.6 mg/kg it increased iNOS and decreased eNOS expressions with effectively no changes in nNOS. Thus, the increased cardiac NOx levels observed in our study after IR, could be ascribed to an increase in iNOS activity. In our study, enzyme activity or expression was not measured, but an elevated mRNA expression for iNOS was found. Higher NOx levels in the heart tissue are an important factor for increasing IR injury [58] as it increases lipid peroxidation [59] and nitrosative stress [60]. In our study, decreased and increased iNOS/eNOS ratios were associated with higher and lower tolerance against myocardial IR, respectively. In this regard, it has been reported that eNOS- and nNOS-derived NO [13,16,61] has protective roles against myocardial IR injury; however iNOS-derived NO contributes to myocardial IR injury [15,62] and is also accompanied by cardiac hypertrophy [63] and oxidative stress [64]. Both detrimental effects were observed in our study following NaSH administration at the high dose. In line with our results, it has been reported that low-to-intermediate doses of NaSH [18] and diallyl trisulfide present in garlic [19], prior to reperfusion provide tolerance against myocardial IR by decreasing iNOS expression in rats and mice. It has also been reported that CSE-derived H2S modulates NOS activity [65,66], e.g., inhibition of CSE in mice decreases eNOS-derived NO [9] and increases iNOS-derived NO [17].
In this study, NaSH at 1.6 mg/kg decreased markers of oxidative stress in the heart tissue after IR, while at 5.6 mg/kg it increased them. In line with our results, decreased MDA levels in heart tissues have been reported following short-term in vivo NaSH administration at low dose (0.78 mg/kg/day for 5 days before IR) [42]. Low dose of NaSH has been suggested to inhibit oxidative stress by increasing SOD activity [67], decreasing ROS levels [68], increasing expression or activity of eNOS [69] and CSE [70], while at high doses it increases oxidative stress by increasing ROS as well as decreasing GSH levels [71,72].
Finally, we showed that NaSH at 1.6 mg/kg increased mRNA expression of RKIP and decreased the mRNA expression of NF-κB in the heart tissue following IR, while at 5.6 mg/kg, it had the opposite effects. The effect of NaSH on mRNA level of RKIP in a setting of IR injury has not been previously reported; however the positive effect of NaSH on protein kinase C (PKC) activation [73] (upstream pathway of RKIP) and negative effects on mitogen-activated protein kinase (MAPK) activation and NF-κB translocation [24] (downstream pathways of RKIP) following myocardial ischemia have been reported. PKC and MAPK have protective and detrimental effects against myocardial IR injury, respectively [24,73]. Anti-inflammatory effects of NaSH have been reported following short-term administration (i.e., 15 min and 7 days) at doses of 3 mg/kg [24] and 0.78 mg/kg [25] before myocardial IR. In addition, the biphasic effects of H2S on inflammatory signaling have also been observed in LPS-treated murine macrophages; NaSH at low doses decreases NF-κB activity, but at high doses, it increases the synthesis of proinflammatory mediators and NF-κB activity [74]. These biphasic or U-shaped effects of chronic NaSH administration on oxidative stress and inflammation indices provide further evidence of a protective effect of intermediate dose and detrimental effect of high dose of H2S-releasing agent(s).
As a strength, we evaluated multiple long-term doses of NaSH administered in an in vivo setting on tolerance against myocardial IR injury. The dose–response design has been reported to be one of the most important criteria that would increase the chance of an animal study to be translated from the bench to the bedside [75]. In addition, given that a living day in a rat is equivalent to 26 days in a human [76], 9 weeks of NaSH administration in our rat studies could be considered as a long-term intervention in a human. However, further studies are needed to determine time-dependent effects of H2S donors on myocardial IR injury.
As a limitation, H2S concentrations were measured by the methylene blue method, which measures all sulfur species rather than only free H2S. We did not evaluate the effects of our NaSH intervention on the various parameters by Western blots, due to lack of resources. However, at least for our measurements of H2S and NO levels, our mRNA data correlate well with these measured values, and we may infer that these would also be in line with protein expressions as well.
4. Materials and Methods
4.1. Animals
Male Wistar rats (190–210 g) were housed under controlled conditions (23 ± 2 °C, 12/12 h light–dark cycle, relative humidity of 50% ± 6%) with food and water ad libitum. All experimental procedures employed, as well as caring and handling of the rats, were approved and performed in accordance with guidelines provided by the local ethics committee of the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical Sciences (IR.SBMU.ENDOCRINE.REC.1398.036, 6 August, 2019).
4.2. Experimental Design
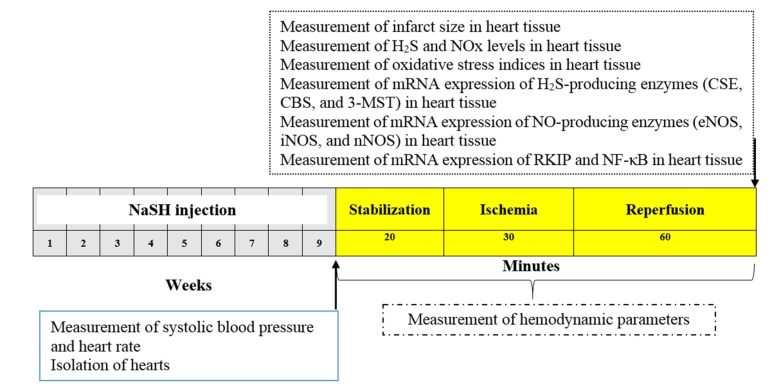
The experimental protocol is shown in Figure 9. Male rats were divided into control group (n = 6) and NaSH (0.28, 0.56, 1.6, 2.8, and 5.6 mg/kg/day) groups (n = 6/group). The control group received intraperitoneal (IP) injections of normal saline and the NaSH groups received IP injection of 0.28, 0.56, 1.6, 2.8, and 5.6 mg/kg/day of NaSH, freshly prepared each day, for 9 weeks. Body weights (using Tefal Scale; sensitivity 1 g) were recorded at the start and end of the interventions. At the end of study, systolic blood pressure and heart rate were measured in rats using a noninvasive tail-cuff method (AD Instruments, MLT125R, New South Wales, Australia). Systolic blood pressure and heart rate values were averaged from three consecutive recordings obtained from each rat. At week 9, hearts from all rats were isolated and connected to a Langendorff apparatus and hemodynamic parameters’ (LVDP, +dP/dt and −dp/dt) change in left ventricular pressure were recorded both at baseline (stabilization period) and also during IR. In addition, the weights of the hearts, levels of H2S, NOx, MDA, TAC, GSH, total glutathione (GSH + GSSG), CAT activity, and infarct size were measured in all groups after the IR period. We also measured the mRNA expression levels of CSE, CBS, 3-MST, eNOS, iNOS, nNOS, RKIP, and NF-κB in the heart tissue following IR.
Figure 9.
Experimental protocol and the timeline of the study. NaSH at 0.28, 0.56, 1.6, 2.8, and 5.6 mg/kg/day was administrated for a period of 9 weeks as detailed in Section 2.2. NaSH, sodium hydrosulfide; H2S, hydrogen sulfide; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; 3-MST, 3-mercaptopyruvate sulfurtransferase; NOS, nitric oxide synthase; nNOS, neuronal NOS; eNOS, endothelial NOS; iNOS, inducible NOS; RKIP, Raf kinase inhibitor protein; NF-κB, nuclear factor kappa-B.
4.3. Measurement of Hemodynamic Parameters
Details for the measurements of the heart rate, LVDP, and ±dp/dt by the Langendorff apparatus were previously described [77]. In brief, at the end of the interventions, all rats were anesthetized with an IP injection of ketamine/xylazine (50/10 mg/kg) and the hearts were quickly removed. Isolated hearts were immersed in ice-cold perfusion buffer, the aortae were rapidly cannulated and connected to the Langendorff apparatus. A retrograde perfusion was performed with Krebs–Henseleit solution (KHS). Composition of KHS in mM was: 118.6 NaCl; 4.7 KCl; 2.5 CaCl2; 1.6 MgSO4; 1.2 KH2PO4; 25 NaHCO3; 11.1 glucose (all from Merck, Darmstadt, Germany), equilibrated with 95% O2:5% CO2, (pH 7.4). For measurement of the heart rates, LVDP, ±dp/dt, and LVEDP, a latex balloon was inserted into the left ventricle and LVEDP was adjusted at 5–10 mmHg in all hearts by filling the latex balloon with water. Isolated hearts were subjected to 20 min of stabilization, 30 min of global ischemia, and 60 min of reperfusion, respectively. LVEDP, LVDP, and ±dp/dt were digitalized by a data acquisition system (Power Lab, AD instrument, Australia). At the end of the reperfusion phase, the isolated hearts were separated from the Langendorff apparatus, weighed, and stored at −80 °C for later analyses.
4.4. Measurements of H2S and NOx Levels in Heart Tissues
At the end of study, tissue samples from the hearts were homogenized in phosphate-buffered saline (100 mM, pH 7.4, 1:5 w/v) and then were centrifuged at 4 °C for 10 min 10,000× g; the supernatants were then used for measuring H2S and NOx levels. The methylene blue method was used for measuring H2S [78]; details can be found elsewhere [38]. This method overestimates H2S levels as it measures free H2S, HS− (hydrosulfide anion), and S2− (sulfide) [79,80]. Therefore, our results presented here indicate the sum total of these species. In addition, NOx concentrations in the heart tissue were measured by the Griess method [81] using a commercial kit (Pazhoheshkave Kav Afagh, Tehran, Iran). Intra-assay coefficient of variation for H2S and NOx in the hearts were 3.2% and 2.9%, respectively. In addition, concentrations of total protein in isolated hearts were measured using the Bradford method [82], and results for H2S and NOx are reported based on nmol/mg protein.
4.5. Measurement of Infarct Size
At the end of the reperfusion period, infarct size was measured as previously described [83]. In brief, the frozen heart samples were cut into thin slices and incubated in 2, 3, 5-triphenyltetrazolium chloride (1% in phosphate buffer solution, 20 mM, pH 7.4) at 37 °C for 10 min. The slices were immersed in 10% formalin for 24 h to identify viable myocardium (red color) from necrotic tissue (gray color). The infarct size for each heart was analyzed by Photoshop CS6 software and expressed as percentage of the total area.
4.6. Measurement of mRNA Expression
Details of RNA extraction, cDNA synthase, and qRT-PCR have been previously reported [84]. Total RNA was extracted from 10 mg of rat heart tissue with the RNX-Plus solution kit (Cinagen Co., Tehran, Iran). cDNA synthesis was performed using Thermo Scientific RevertAid Reverse Transcriptase in accordance with the manufacturers’ instructions. Primers were designed using primer3 and Gene Runner; primer sequences employed are shown in Table 3. Amplifications were performed in a Rotor Gene 6000 real-time PCR machine (Corbett, Life science, Sydney, Australia). Target genes were normalized with ß-actin as reference. Fold changes in mRNA expression for CSE, CBS, 3-MST, eNOS, iNOS, nNOS, RKIP, and NF-κB genes were calculated by the method.
Table 3.
Primers used for real-time PCR analysis.
| Primer Name | Gene bank Accession No. | Primer Sequence (5′→3’) |
|---|---|---|
| CSE | NM_017074.1 | Forward: TTGTATACAGCCGCTCTGGA Reverse: CGAGCGAAGGTCAAACAGTG |
| CBS | NM_012522.2 | Forward: TGGTGACTCTCGGGAACATG Reverse: AGGTGGATCGGCTTGAACTG |
| 3-MST | NM_138843.1 | Forward: GGCATCGAACCTGGACACATC Reverse: ACTGGCGTTGGATCTCCTCTG |
| iNOS | NM_012611 | Forward: ACCATGGAGCATCCCAAGTA Reverse: CAGCGCATACCACTTCAGC |
| eNOS | NM_021838.2 | Forward: TGACCCTCACCGATACAACA Reverse: CGGGTGTCTAGATCCATGC |
| nNOS | NM_052799.1 | Forward: AATCTCAGGTCGGCCATCAC Reverse: ATCCCCCAAGGTAGAGCCAT |
| RKIP | NM_017236.1 | Forward: ACTTCCTGGTGGTCAACATGAA Reverse: TCCGGAGCCCACGTATTC |
| NF-κB p50 | NM_001276711.1 | Forward: AGAGGATGTGGGGTTTCAGG Reverse: GCTGAGCATGAAGGTGGATG |
| ß-actin | NM_031144.3 | Forward: GCGTCCACCCGCGAGTACAAC Reverse: CGACGACGAGCGCAGCGATA |
CSE, cystathionine γ-lyase; CBS, cystathionine β-synthase; 3-MST, 3-mercaptopyruvate sulfurtransferase; NOS, nitric oxide synthase; iNOS, inducible NOS; eNOS, endothelial NOS; nNOS, neuronal NOS; RKIP, Raf kinase inhibitor protein; NF-κB, nuclear factor kappa-B.
4.7. Measurement of Oxidative Stress Indices in the Heart Tissue
Measurement of MDA concentration, CAT activity, TAC concentration, as well as GSH and GSSG + GSH concentration were done by the method of Satoh [85], the method of Hadwan [86], ferric reducing/antioxidant power (FRAP) assay [87], and the method of Sedlak and Lindsay [88], respectively; details of measurement have been previously reported [38]. Intra-assay coefficients of variation for MDA, CAT, TAC, and GSH were 3.6%, 2.5%, 0.77%, and 1.7%, respectively.
4.8. Statistical Analyses
Data were analyzed using GraphPad Prism software (Version 6, La Jolla, San Diego CA, USA), values are expressed as mean ± SEM. To compare the body weights at the start and the end of study, body weight gain, heart weight, heart weight/body weight, systolic blood pressure, H2S level, NOx level, oxidative stress indices, baseline hemodynamic parameters (LVDP, heart rate, and ±dp/dt), and infarct size between groups, one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test was used. For analyzing the data for heart rate, LVDP and ±dp/dt during the IR period between groups, two-way mixed (between-within) ANOVA, followed by the Bonferroni post hoc test was used. The Mann-Whitney U test was used for comparing fold changes in mRNA expression of CSE, CBS, 3-MST, eNOS, iNOS, nNOS, RKIP, and NF-κB genes between groups. Two-sided p-values < 0.05 were considered statistically significant.
5. Conclusions
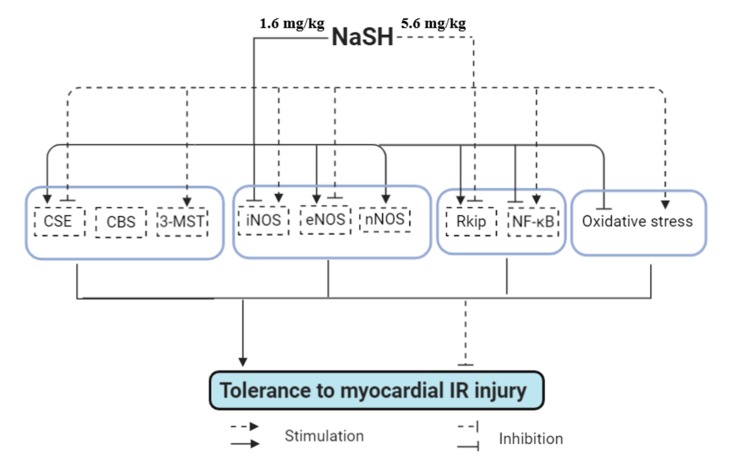
As illustrated in Figure 10, NaSH exhibited biphasic effects in our study, i.e., low dose had no effect, intermediate dose had a protective effect, whereas a high dose exacerbated myocardial IR injury. Higher tolerance to IR injury in hearts isolated from rats treated with intermediate dose of NaSH, at least in part, was associated with higher CSE-derived H2S and lower iNOS-derived NO as well as lower oxidative stress in the heart tissue after IR. In addition, the beneficial effects of H2S was accompanied with a decrease in NF-κB expression and increase in RKIP expression. NaSH at a high dose (5.6 mg/kg) had the opposite effects.
Figure 10.
Proposed mechanism for the protective and detrimental effects of NaSH against myocardial IR injury in normal rat. Solid and dashed arrows indicate effect of NaSH administration at 1.6 mg/kg and 5.6 mg/kg, respectively. Standard arrows indicate upregulation while flat-headed arrows indicate downregulation. CSE, cystathionine γ-lyase; CBS, cystathionine β-synthase; 3-MST, 3-mercaptopyruvate sulfurtransferase; NOS, nitric oxide synthase; nNOS, neuronal NOS; eNOS, endothelial NOS; iNOS, inducible NOS; RKIP, Raf kinase inhibitor protein; NF-κB, nuclear factor kappa-B.
Acknowledgments
We are indebted to the ethics committee of RIES for approving the proposal for this study.
Author Contributions
Conceptualization, S.J., S.G., A.G., M.C., and K.K.; methodology, S.J. and S.G.; validation, A.G., M.C., and K.K.; formal analysis, S.J., S.G., A.G., M.C., and K.K.; investigation, S.J., S.G., A.G., M.C., and K.K.; resources, A.G. and K.K.; data curation, S.J. and S.G.; writing-original draft preparation, S.J. and S.G.; writing-review and editing, A.G., M.C., and K.K.; supervision, A.G.; project administration, A.G; funding acquisition, A.G. All authors agreed on the final approval of the version to be published.
Funding
This research was funded by a grant (No. 98070) from the Research Institute for Endocrine Science (RIES), Shahid Beheshti University of Medical Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dalen J.E., Alpert J.S., Goldberg R.J., Weinstein R.S. The epidemic of the 20th century: Coronary heart disease. Am. J. Med. 2014;127:807–812. doi: 10.1016/j.amjmed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri P., di Russo C., Rigattieri S., Fedele S., Todaro D., Ferraiuolo G., Altamura G., Loschiavo P. MicroRNAs and ischemic heart disease: Towards a better comprehension of pathogenesis, new diagnostic tools and new therapeutic targets. Recent Pat. Cardiovasc. Drug Discov. 2009;4:109–118. doi: 10.2174/157489009788452977. [DOI] [PubMed] [Google Scholar]
- 3.Ovize M., Baxter G.F., di Lisa F., Ferdinandy P., Garcia-Dorado D., Hausenloy D.J., Heusch G., Vinten-Johansen J., Yellon D.M., Schulz R. Postconditioning and protection from reperfusion injury: Where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 4.Heusch G. Cardioprotection: Chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 5.Sluijter J.P., Condorelli G., Davidson S.M., Engel F.B., Ferdinandy P., Hausenloy D.J., Lecour S., Madonna R., Ovize M., Ruiz-Meana M., et al. Novel therapeutic strategies for cardioprotection. Pharmacol. Ther. 2014;144:60–70. doi: 10.1016/j.pharmthera.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Ferdinandy P., Schulz R., Baxter G.F. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol. Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 7.Granfeldt A., Lefer D.J., Vinten-Johansen J. Protective ischaemia in patients: Preconditioning and postconditioning. Cardiovasc. Res. 2009;83:234–246. doi: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan L.L., Liu X.H., Gong Q.H., Yang H.B., Zhu Y.Z. Role of cystathionine γ-lyase/hydrogen sulfide pathway in cardiovascular disease: A novel therapeutic strategy? Antioxid. Redox Signal. 2012;17:106–118. doi: 10.1089/ars.2011.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King A.L., Polhemus D.J., Bhushan S., Otsuka H., Kondo K., Nicholson C.K., Bradley J.M., Islam K.N., Calvert J.W., Tao Y.-X., et al. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. USA. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elrod J.W., Calvert J.W., Morrison J., Doeller J.E., Kraus D.W., Tao L., Jiao X., Scalia R., Kiss L., Szabo C., et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolluru G.K., Shen X., Kevil C.G. A tale of two gases: NO and H2S, foes or friends for life? Redox Biol. 2013;1:313–318. doi: 10.1016/j.redox.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu D., Hu Q., Zhu D. An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System. Oxidative Med. Cell. Longev. 2018;2018:4579140. doi: 10.1155/2018/4579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger D.E., Lu X., Lei M., Xiang F.L., Hammoud L., Jiang M., Wang H., Jones D.L., Sims S.M., Feng Q. Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation. 2009;120:1345–1354. doi: 10.1161/CIRCULATIONAHA.108.846402. [DOI] [PubMed] [Google Scholar]
- 14.Xia R., Zhao B., Wu Y., Hou J.B., Zhang L., Xu J.J., Xia Z.Y. Ginsenoside Rb1 preconditioning enhances eNOS expression and attenuates myocardial ischemia/reperfusion injury in diabetic rats. J. BioMed. Biotechnol. 2011;2011:767930. doi: 10.1155/2011/767930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parlakpinar H., Ozer M.K., Acet A. Effect of aminoguanidine on ischemia-reperfusion induced myocardial injury in rats. Mol. Cell. Biochem. 2005;277:137–142. doi: 10.1007/s11010-005-5779-9. [DOI] [PubMed] [Google Scholar]
- 16.Fan Q., Yang X.C., Liu Y., Wang L.F., Liu S.H., Ge Y.G., Chen M.L., Wang W., Zhang L.K., Irwin M.G., et al. Postconditioning attenuates myocardial injury by reducing nitro-oxidative stress in vivo in rats and in humans. Clin. Sci. 2011;120:251–261. doi: 10.1042/CS20100369. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z., Yan Y., Wang Y., Tong F. The interaction between CSE/H2S and the iNOS/NO-mediated resveratrol/poly(ethylene glycol)-poly(phenylalanine) complex alleviates intestinal ischemia/reperfusion injuries in diabetic rats. BioMed. Pharmacother. 2019;112:108736. doi: 10.1016/j.biopha.2019.108736. [DOI] [PubMed] [Google Scholar]
- 18.Issa K., Kimmoun A., Collin S., Ganster F., Fremont-Orlowski S., Asfar P., Mertes P.M., Levy B. Compared effects of inhibition and exogenous administration of hydrogen sulphide in ischaemia-reperfusion injury. Crit. Care. 2013;17:R129. doi: 10.1186/cc12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Predmore B.L., Kondo K., Bhushan S., Zlatopolsky M.A., King A.L., Aragon J.P., Grinsfelder D.B., Condit M.E., Lefer D.J. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability, American journal of physiology. Heart Circ. Physiol. 2012;302:H2410–H2418. doi: 10.1152/ajpheart.00044.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Salam S., Hashmi S. Myocardial Ischemia Reperfusion Injury: Apoptotic, Inflammatory and Oxidative Stress Role of Galectin-3. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;50:1123–1139. doi: 10.1159/000494539. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz K., Rosner M.R., Brand T., Schmitt J.P. Raf kinase inhibitor protein: Lessons of a better way for beta-adrenergic receptor activation in the heart. J. Physiol. 2017;595:4073–4087. doi: 10.1113/JP274064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung K.C., Rose D.W., Dhillon A.S., Yaros D., Gustafsson M., Chatterjee D., McFerran B., Wyche J., Kolch W., Sedivy J.M. Raf kinase inhibitor protein interacts with NF-kappaB-inducing kinase and TAK1 and inhibits NF-kappaB activation. Mol. Cell. Biol. 2001;21:7207–7217. doi: 10.1128/MCB.21.21.7207-7217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valen G., Yan Z.-Q., Hansson G.K. Nuclear factor kappa-B and the heart. J. Am. Coll. Cardiol. 2001;38:307–314. doi: 10.1016/S0735-1097(01)01377-8. [DOI] [PubMed] [Google Scholar]
- 24.Sivarajah A., Collino M., Yasin M., Benetti E., Gallicchio M., Mazzon E., Cuzzocrea S., Fantozzi R., Thiemermann C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock. 2009;31:267–274. doi: 10.1097/SHK.0b013e318180ff89. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y., Yao X., Zhang Y., Li W., Kang K., Sun L., Sun X. The protective role of hydrogen sulfide in myocardial ischemia-reperfusion-induced injury in diabetic rats. Int. J. Cardiol. 2011;152:177–183. doi: 10.1016/j.ijcard.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Karwi Q.G., Bice J.S., Baxter G.F. Pre- and postconditioning the heart with hydrogen sulfide (H2S) against ischemia/reperfusion injury in vivo: A systematic review and meta-analysis. Basic Res. Cardiol. 2018;113:6. doi: 10.1007/s00395-017-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvert J.W., Jha S., Gundewar S., Elrod J.W., Ramachandran A., Pattillo C.B., Kevil C.G., Lefer D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang B., Li W., Xi W., Yi Y., Ciren Y., Shen H., Zhang Y., Jiang H., Xiao J., Wang Z. Hydrogen Sulfide Protects Cardiomyocytes against Apoptosis in Ischemia/Reperfusion through MiR-1-Regulated Histone Deacetylase 4 Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017;41:10–21. doi: 10.1159/000455816. [DOI] [PubMed] [Google Scholar]
- 29.Yao X., Tan G., He C., Gao Y., Pan S., Jiang H., Zhang Y., Sun X. Hydrogen sulfide protects cardiomyocytes from myocardial ischemia-reperfusion injury by enhancing phosphorylation of apoptosis repressor with caspase recruitment domain. Tohoku J. Exp. Med. 2012;226:275–285. doi: 10.1620/tjem.226.275. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y.Z., Wang Z.J., Ho P., Loke Y.Y., Zhu Y.C., Huang S.H., Tan C.S., Whiteman M., Lu J., Moore P.K. Hydrogen sulfide and its possible roles in myocardial ischemia in experimental rats. J. Appl. Physiol. 2007;102:261–268. doi: 10.1152/japplphysiol.00096.2006. [DOI] [PubMed] [Google Scholar]
- 31.Sivarajah A., McDonald M.C., Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock. 2006;26:154–161. doi: 10.1097/01.shk.0000225722.56681.64. [DOI] [PubMed] [Google Scholar]
- 32.Zhuo Y., Chen P.F., Zhang A.Z., Zhong H., Chen C.Q., Zhu Y.Z. Cardioprotective effect of hydrogen sulfide in ischemic reperfusion experimental rats and its influence on expression of survivin gene. Biol. Pharm. Bull. 2009;32:1406–1410. doi: 10.1248/bpb.32.1406. [DOI] [PubMed] [Google Scholar]
- 33.Yin J., Tu C., Zhao J., Ou D., Chen G., Liu Y., Xiao X. Exogenous hydrogen sulfide protects against global cerebral ischemia/reperfusion injury via its anti-oxidative, anti-inflammatory and anti-apoptotic effects in rats. Brain Res. 2013;1491:188–196. doi: 10.1016/j.brainres.2012.10.046. [DOI] [PubMed] [Google Scholar]
- 34.Gomez C.B., de la Cruz S.H., Medina-Terol G.J., Beltran-Ornelas J.H., Sánchez-López A., Silva-Velasco D.L., Centurión D. Chronic administration of NaHS and L-Cysteine restores cardiovascular changes induced by high-fat diet in rats. Eur. J. Pharmacol. 2019;863:172707. doi: 10.1016/j.ejphar.2019.172707. [DOI] [PubMed] [Google Scholar]
- 35.Swan K.W., Song B.M., Chen A.L., Chen T.J., Chan R.A., Guidry B.T., Katakam P.V.G., Kerut E.K., Giles T.D., Kadowitz P.J. Analysis of decreases in systemic arterial pressure and heart rate in response to the hydrogen sulfide donor sodium sulfide, American journal of physiology. Heart Circ. Physiol. 2017;313:H732–H743. doi: 10.1152/ajpheart.00729.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo D., Jupiter R.C., Pankey E.A., Reddy V.G., Edward J.A., Swan K.W., Peak T.C., Mostany R., Kadowitz P.J. Analysis of cardiovascular responses to the H2S donors Na2S and NaHS in the rat, American journal of physiology. Heart Circ. Physiol. 2015;309:H605–H614. doi: 10.1152/ajpheart.00171.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpato G.P., Searles R., Yu B., Scherrer-Crosbie M., Bloch K.D., Ichinose F., Zapol W.M. Inhaled hydrogen sulfide: A rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology. 2008;108:659–668. doi: 10.1097/ALN.0b013e318167af0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gheibi S., Jeddi S., Kashfi K., Ghasemi A. Effects of Hydrogen Sulfide on Carbohydrate Metabolism in Obese Type 2 Diabetic Rats. Molecules. 2019;24:190. doi: 10.3390/molecules24010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Saito Y., Kuwahara K., Rong X., Kishimoto I., Harada M., Horiuchi M., Murray M., Nakao K. Vasodilator therapy with hydralazine induces angiotensin AT receptor-mediated cardiomyocyte growth in mice lacking guanylyl cyclase-A. Br. J. Pharmacol. 2010;159:1133–1142. doi: 10.1111/j.1476-5381.2009.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao L.L., Huang X.W., Wang Y.G., Cao Y.X., Zhang C.C., Zhu Y.C. Hydrogen sulfide protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis by preventing GSK-3beta-dependent opening of Mptp. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H1310–H1319. doi: 10.1152/ajpheart.00339.2009. [DOI] [PubMed] [Google Scholar]
- 41.Sun X., Wang W., Dai J., Jin S., Huang J., Guo C., Wang C., Pang L., Wang Y. A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury. Sci. Rep. 2017;7:3541. doi: 10.1038/s41598-017-03941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geng B., Chang L., Pan C., Qi Y., Zhao J., Pang Y., Du J., Tang C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 43.Szabo C., Ransy C., Modis K., Andriamihaja M., Murghes B., Coletta C., Olah G., Yanagi K., Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br. J. Pharmacol. 2014;171:2099–2122. doi: 10.1111/bph.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu X.Y., Yan X.H., Chen S.J. H(2)S protects myocardium against ischemia/reperfusion injury and its effect on c-Fos protein expression in rats. Sheng Li Xue Bao (Acta Physiol. Sin.) 2008;60:221–227. [PubMed] [Google Scholar]
- 45.Pan T.T., Chen Y.Q., Bian J.S. All in the timing: A comparison between the cardioprotection induced by H2S preconditioning and post-infarction treatment. Eur. J. Pharmacol. 2009;616:160–165. doi: 10.1016/j.ejphar.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Li C., Hu M., Wang Y., Lu H., Deng J., Yan X. Hydrogen sulfide preconditioning protects against myocardial ischemia/reperfusion injury in rats through inhibition of endo/sarcoplasmic reticulum stress. Int. J. Clin. Exp. Pathol. 2015;8:7740–7751. [PMC free article] [PubMed] [Google Scholar]
- 47.Johansen D., Ytrehus K., Baxter G.F. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia–reperfusion injury. Basic Res. Cardiol. 2006;101:53–60. doi: 10.1007/s00395-005-0569-9. [DOI] [PubMed] [Google Scholar]
- 48.Zheng Y., Yu B., de la Cruz L.K., Choudhury M.R., Anifowose A., Wang B. Toward Hydrogen Sulfide Based Therapeutics: Critical Drug Delivery and Developability Issues. Med. Res. Rev. 2018;38:57–100. doi: 10.1002/med.21433. [DOI] [PubMed] [Google Scholar]
- 49.Kabil O., Vitvitsky V., Xie P., Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011;15:36–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nandi S.S., Mishra P.K. H(2)S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep. 2017;7:3639. doi: 10.1038/s41598-017-03776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain A.K., Singh D., Dubey K., Maurya R., Mittal S., Pandey A.K. Chapter 3—Models and Methods for In Vitro Toxicity. In: Dhawan A., Kwon S., editors. In Vitro Toxicology. Academic Press; Cambridge, MA, USA: 2018. pp. 45–65. [Google Scholar]
- 53.Cox L.A., Popken D.A., Kaplan A.M., Plunkett L.M., Becker R.A. How well can in vitro data predict in vivo effects of chemicals? Rodent carcinogenicity as a case study. Regul. Toxicol. Pharmacol. 2016;77:54–64. doi: 10.1016/j.yrtph.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Chen T., Vunjak-Novakovic G. In vitro Models of Ischemia-Reperfusion Injury. Regen. Eng. Transl. Med. 2018;4:142–153. doi: 10.1007/s40883-018-0056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y.-E., Tang Z.-H., Xie W., Shen X.-T., Liu M.-H., Peng X.-P., Zhao Z.-Z., Nie D.E.B., Liu L.-S., Jiang Z.-S. Endogenous hydrogen sulfide mediates the cardioprotection induced by ischemic postconditioning in the early reperfusion phase. Exp. Ther. Med. 2012;4:1117–1123. doi: 10.3892/etm.2012.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bliksøen M., Kaljusto M.-L., Vaage J., Stensløkken K.-O. Effects of hydrogen sulphide on ischaemia–reperfusion injury and ischaemic preconditioning in the isolated, perfused rat heart. Eur. J. Cardio-Thorac. Surg. 2008;34:344–349. doi: 10.1016/j.ejcts.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Li N., Wang M.J., Jin S., Bai Y.D., Hou C.L., Ma F.F., Li X.H., Zhu Y.C. The H2S Donor NaHS Changes the Expression Pattern of H2S-Producing Enzymes after Myocardial Infarction. Oxid. Med. Cell. Longev. 2016;2016:6492469. doi: 10.1155/2016/6492469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Venditti P., de Rosa R., Cigliano L., Agnisola C., di Meo S. Role of nitric oxide in the functional response to ischemia-reperfusion of heart mitochondria from hyperthyroid rats. Cell. Mol. Life Sci. 2004;61:2244–2252. doi: 10.1007/s00018-004-4125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masullo P., Venditti P., Agnisola C., di Meo S. Role of nitric oxide in the reperfusion induced injury in hyperthyroid rat hearts. Free Radic. Res. 2000;32:411–421. doi: 10.1080/10715760000300411. [DOI] [PubMed] [Google Scholar]
- 60.Ferdinandy P., Schulz R. Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu L., Wang J., Zhu H., Wu X., Zhou L., Song Y., Zhu S., Hao M., Liu C., Fan Y., et al. Ischemic postconditioning protects the heart against ischemia-reperfusion injury via neuronal nitric oxide synthase in the sarcoplasmic reticulum and mitochondria. Cell Death Dis. 2016;7:e2222. doi: 10.1038/cddis.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li D., Qu Y., Tao L., Liu H., Hu A., Gao F., Sharifi-Azad S., Grunwald Z., Ma X.L., Sun J.Z. Inhibition of iNOS protects the aging heart against beta-adrenergic receptor stimulation-induced cardiac dysfunction and myocardial ischemic injury. J. Surg. Res. 2006;131:64–72. doi: 10.1016/j.jss.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 63.Mungrue I.N., Gros R., You X., Pirani A., Azad A., Csont T., Schulz R., Butany J., Stewart D.J., Husain M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J. Clin. Investig. 2002;109:735–743. doi: 10.1172/JCI0213265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun Y., Carretero O.A., Xu J., Rhaleb N.-E., Wang F., Lin C., Yang J.J., Pagano P.J., Yang X.-P. Lack of inducible NO synthase reduces oxidative stress and enhances cardiac response to isoproterenol in mice with deoxycorticosterone acetate-salt hypertension. Hypertension. 2005;46:1355–1361. doi: 10.1161/01.HYP.0000192651.06674.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagpure B.V., Bian J.S. Interaction of Hydrogen Sulfide with Nitric Oxide in the Cardiovascular System. Oxid. Med. Cell. Longev. 2016;2016:6904327. doi: 10.1155/2016/6904327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kazakov A., Hall R.A., Werner C., Meier T., Trouvain A., Rodionycheva S., Nickel A., Lammert F., Maack C., Bohm M., et al. Raf kinase inhibitor protein mediates myocardial fibrosis under conditions of enhanced myocardial oxidative stress. Basic Res. Cardiol. 2018;113:42. doi: 10.1007/s00395-018-0700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun W.-H., Liu F., Chen Y., Zhu Y.-C. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem. Biophys. Res. Commun. 2012;421:164–169. doi: 10.1016/j.bbrc.2012.03.121. [DOI] [PubMed] [Google Scholar]
- 68.Bai Y.-D., Yang Y.-R., Mu X.-P., Lin G., Wang Y.-P., Jin S., Chen Y., Wang M.-J., Zhu Y.-C. Hydrogen Sulfide Alleviates Acute Myocardial Ischemia Injury by Modulating Autophagy and Inflammation Response under Oxidative Stress. Oxidative Med. and Cell. Longev. 2018;2018:3402809. doi: 10.1155/2018/3402809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kondo K., Bhushan S., King A.L., Prabhu S.D., Hamid T., Koenig S., Murohara T., Predmore B.L., Gojon G., Sr., Gojon G., Jr., et al. H₂S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation. 2013;127:1116–1127. doi: 10.1161/CIRCULATIONAHA.112.000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kimura H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox Signal. 2015;22:362–376. doi: 10.1089/ars.2014.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 72.Wang R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 73.Pan T.T., Neo K.L., Hu L.F., Yong Q.C., Bian J.S. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am. J. Physiol.-Cell Physiol. 2008;294:C169–C177. doi: 10.1152/ajpcell.00282.2007. [DOI] [PubMed] [Google Scholar]
- 74.Whiteman M., Li L., Rose P., Tan C.-H., Parkinson D.B., Moore P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hackam D.G., Redelmeier D.A. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. doi: 10.1001/jama.296.14.1731. [DOI] [PubMed] [Google Scholar]
- 76.Quinn R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 77.Ghanbari M., Jeddi S., Bagheripuor F., Ghasemi A. The effect of maternal hypothyroidism on cardiac function and tolerance to ischemia-reperfusion injury in offspring male and female rats. J. Endocrinol. Investig. 2015;38:915–922. doi: 10.1007/s40618-015-0267-x. [DOI] [PubMed] [Google Scholar]
- 78.Shen X., Pattillo C.B., Pardue S., Bir S.C., Wang R., Kevil C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee Z.W., Zhou J., Chen C.S., Zhao Y., Tan C.H., Li L., Moore P.K., Deng L.W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kodela R., Chattopadhyay M., Kashfi K. Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential. MedChemComm. 2013;4:1472–1481. doi: 10.1039/c3md00185g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghasemi A., Zahediasl S. Preanalytical and analytical considerations for measuring nitric oxide metabolites in serum or plasma using the Griess method. Clin. Lab. 2012;58:615–624. [PubMed] [Google Scholar]
- 82.Kruger N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- 83.Zaman J., Jeddi S., Daneshpour M.S., Zarkesh M., Daneshian Z., Ghasemi A. Ischemic postconditioning provides cardioprotective and antiapoptotic effects against ischemia-reperfusion injury through iNOS inhibition in hyperthyroid rats. Gene. 2015;570:185–190. doi: 10.1016/j.gene.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Gholami H., Jeddi S., Zadeh-Vakili A., Farrokhfall K., Rouhollah F., Zarkesh M., Ghanbari M., Ghasemi A. Transient Congenital Hypothyroidism Alters Gene Expression of Glucose Transporters and Impairs Glucose Sensing Apparatus in Young and Aged Offspring Rats. Cell. Physiol. Biochem. 2017;43:2338–2352. doi: 10.1159/000484386. [DOI] [PubMed] [Google Scholar]
- 85.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 86.Hadwan M.H. New Method for Assessment of Serum Catalase Activity. Indian J. Sci. Technol. 2016;9:1–5. doi: 10.17485/ijst/2016/v9i4/80499. [DOI] [Google Scholar]
- 87.Benzie I.F.F., Strain J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999;299:15–27. doi: 10.1016/s0076-6879(99)99005-5. [DOI] [PubMed] [Google Scholar]
- 88.Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]