Abstract
The difficulty of early diagnosis and the development of drug resistance are two major barriers to the successful treatment of cancer. Autophagy plays a crucial role in several cellular functions, and its dysregulation is associated with both tumorigenesis and drug resistance. Unc-51-like kinase 1 (ULK1) is a serine/threonine kinase that participates in the initiation of autophagy. Many studies have indicated that compounds that directly or indirectly target ULK1 could be used for tumor therapy. However, reports of the therapeutic effects of these compounds have come to conflicting conclusions. In this work, we reviewed recent studies related to the effects of ULK1 on the regulation of autophagy and the development of drug resistance in cancers, with the aim of clarifying the mechanistic underpinnings of this therapeutic target.
Keywords: ULK1, autophagy, cancer, drug resistance
1. Introduction
Cancer is one of the leading causes of death in humans [1]. Although significant advances have been made in cancer research, the successful treatment of cancer still faces severe challenges [2]. Chemotherapy is a common form of cancer treatment [3] which is used as monotherapy or in combination with radiation therapy to treat cancers [4]. Chemotherapy drugs, whether they are commonly used cytotoxic drugs or small molecule targeted drugs, can encounter therapeutic barriers due to the development of drug resistance in tumor cells which is a common cause of tumor recurrence and metastasis [5]. Drugs targeting the pathways related to cancer development can improve the prognosis if used in the early treatment of some cancers. However, these targeted treatments can cause drug resistance in tumor cells resulting in the failure of therapies [6,7,8,9]. Tumor resistance can be divided into two categories, i.e., inherent and acquired. Inherent resistance, on the one hand, is defined as tumor drug resistance that occurs even without any drug treatment and is typically caused by genetic mutations. Acquired drug resistance, on the other hand, develops during cancer treatment as an adaptation to selective pressure from the therapeutics. These processes are related to the high expression of therapeutic targets and the activation of compensatory signaling pathways [10,11,12].
Autophagy is a system of self-degradation that is a prevalent element of drug resistance in tumors [13]. It can be either beneficial or harmful to the occurrence of drug resistance in tumor cells, either protecting tumor cells from the effects of chemotherapy drugs, or killing multidrug resistant cells [13,14]. Patients with poor prognoses typically have higher measured levels of autophagy relative to patients with good prognoses, suggesting that autophagy can lead to the development of MDR [15]. The effect of autophagy on tumor cells varies according to the tumor type and the stage of cancer development [16]. In the precancerous stage, autophagy eliminates obsolete cellular constituents such as misfolded proteins or damaged organelles from cells [17]. The inhibition of autophagy at this stage leads to the increase of intracellular reactive oxygen species, genomic dysfunction, and the accumulation of P62 protein. These changes collectively result in an increase of ER pressure and DNA damage, and thus promote the formation of tumors [18]. At this stage, autophagy is a tumor-suppressing factor [19]. However, under conditions of starvation or oxidative stress, autophagy can also provide nutrients and energy to established metastatic tumors. In this way, autophagy can paradoxically act as a cancer-promoting factor [20]. Tumor cells can reuse proteins and damaged organelles through autophagy, and therefore survive despite drug treatment. Indeed, here, inhibiting autophagy can promote the death of tumor cells [21].
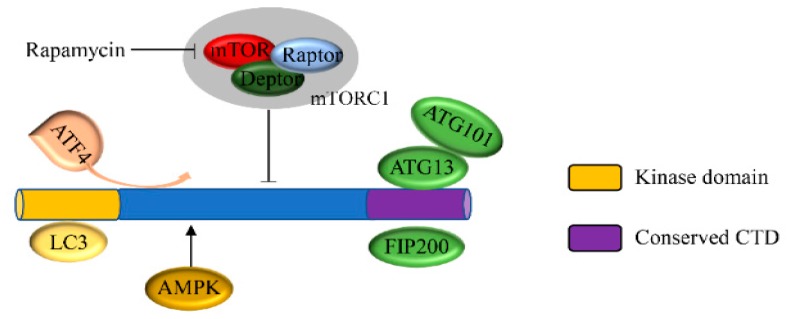
Unc-51-like kinase 1 (ULK1) is a cytoplasmic kinase that is important to the process of autophagy. It is a homologue of ATG1 gene in mammals, with a total similarity of 29% [22,23]. It has been shown that ULK1 can either promote or inhibit tumor growth through protein–protein interactions and post-translational modification-mediated autophagy in nutrient-deficient environments [24]. Thus, both the positive and negative regulation of ULK can situationally protect tumor cells from excessive autophagy [25]. ULK1 forms a protein complex together with the autophagy proteins mATG13, FIP200, and ATG101 to regulate the initiation of autophagy [26]. The induction of autophagy requires the activation of this ULK complex, and the ULK complex is directly regulated by mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) [27]. The observation of ULK1-mediated inhibition of the early autophagosome indicates that ULK1 not only participates in the initiation of autophagy, but also regulates the maturation of autophagosomes [28]. In this review, we summarized the biological function of ULK1 in tumors with respect to its potential as a target for tumor therapy and its impact on the occurrence of drug resistance by mediating autophagy in tumor cells.
2. Structure of ULK1 and Its Biological Functions in Tumors
The mammalian genome contains a total of five ATG1 homologues, ULK1, ULK2, ULK3, ULK4, and STK36 [29]. Among them, only ULK1 and ULK2 exhibit extensive sequence similarity over the entire protein length [30], whereas ULK3, ULK4, and STK36 only have sequence similarity to the Atg1 gene in the kinase structure domain [31]. The conserved domains of ULK1 in different model organisms have been confirmed (Table 1). Sequence analysis of ULK1 and ULK2 indicates that their kinase domains have 75% sequence identity [32,33]. All of these proteins have an N-terminal serine/threonine protein kinase domain (KD) and a C-terminal interacting domain (CTD). Between the kinase domain and CTD, there is a serine/proline-rich region that is the site of many post-translational modifications. However, the catalytic domain of ULK2 is a dimeric assembly that is different from the typical monomer of ULK1 [34,35]. The KD domain is responsible for the catalysis of kinase activity [36] and the interaction between ULK1 and LC3. The CTD domain contains two microtubules and a MIT domain structure that is a scaffold to enable the interaction of ULK1 with ATG13 and FIP200 [37,38]. ULK1 has a standard kinase fold that has several unique features, including a large loop between the N- and C-terminal lobes [33,39].
Table 1.
Identity of ULK1 in Homo sapiens as compared with its homologues in other model organisms [28,29,30,31,32,33,34,40].
| Homologues Name | Model Organisms | Open Reading Frame (ORF)/Amino Acids | N-Terminal Kinase Domain/Residue | Proline/Serine-Rich (PS)/Residue | C-Terminal Domains/Residue |
|---|---|---|---|---|---|
| ULK1 | Homo sapiens | 1050 | 16–278 | 279–832 | 833–1050 |
| ULK1 | Mus. musculus | 1051 | 26–278 | 279–828 | 829–1051 |
| ULK2 | Homo sapiens | 1036 | 9–271 | 272–811 | 812–1036 |
| ATG1 | S. cerevisiae | 897 | 24–325 | 326–740 | 741–897 |
| UNC-51 | C. elegans | 856 | 9–275 | 276–631 | 632–856 |
Studies have shown that knockdown of ULK inhibits autophagosome formation. Mice with defects in ULK1 and ULK2 die within 24 h after birth [41]. When ULK1 is expressed in mammalian cells, ULK2 is not necessary for autophagy. However, when ULK1 expression is inhibited, ULK2 can compensate for the function of ULK1 in regulating autophagy [42,43]. Recent evidence has revealed that ULK1 deficiency in mice does not affect survival or fertility. The cells still exhibit autophagy but with a delay in autophagic mitochondrial clearance in reticulocytes during erythrocyte development [44]. Knock out of ULK1 and ULK2 in mouse embryonic fibroblasts could destroy the autophagy induced by amino acid or glucose deficiency [45]. However, in varying cell environments, the roles of different ULK subtypes are diverse. It was found that siRNA-mediated silencing of ULK1 in HEK293 cells can completely inhibit autophagy [46]. It has also been reported that ULK1 can degrade rapidly in mouse embryonic stem cells when FIP200 is knocked out. In this case, the stability of ATG13 and ATG101 was also affected [46]. It has been shown that ATG101 in mammals has no homology with other ATG proteins and is a vital molecule participating in autophagy [32,47]. ATG101 regulates the phosphorylation of Atg13 by directly binding to Atg13 [48] and forming a stable complex with ULK1-Atg13-FIP200 [49].
The ULK complex in the phagophore membrane promotes the recruitment and activation of VPS34 in phosphatidylinositol-3 (PI3)-kinase complex I and forms PI3P in the phagophore membrane, a process crucial to the formation of the autophagosome [50,51]. In the state of amino acid deficiency, ULK1/2 are activated and transported to the ER to promote autophagosome nucleation [52,53]. Under conditions of severe hypoxia and ER stress, ATF4 (activating transcription factor 4) directly regulates transcription and expression of ULK1 [54,55]. The binding of ATG8 and ULK1 plays a vital role in the formation of the phagophore and autophagosome [56].
The mammalian target of rapamycin protein plays a fundamental role in sensing extracellular nutrition, regulating the rate of protein synthesis and dictating cell growth [57]. Under normal nutritional conditions, mTOR directly binds to ULK1 via the RAPTOR subunit to form a complex which inhibits the occurrence of autophagy by the phosphorylation of ULK1 (at serine 638 and 758) and ATG13 (at Ser258) [58]. When nutrients are sparse, mTOR activity is inhibited, the dephosphorylation of Atg13 is promoted, and the mTOR and ULK1 complex is separated. After separating from mTOR, ULK1 is activated and forms a complex with Atg13 and FIP200/Atg17, and thus promotes autophagy (Figure 1) [59,60,61]. In addition, in the absence of energy, AMPK is activated, and mTOR-induced autophagy is negatively regulated by the phosphorylation of ULK1 at s555 [62,63]. This complex process is summarized in Figure 1.
Figure 1.
Structure and biological function of ULK1. The domain of ULK1 contains three units including a kinase domain (KD), proline/serine-rich (PS) region, and C-terminal domain (CTD). AMPK and mTORC1 are upstream kinases that regulate ULK1. ATF4 is an activating transcription factor that directly regulates transcription of ULK1.
2.1. The Canonical Role of ULK1
The canonical functions of ULK are related to autophagy [64]. Autophagy is a highly evolutionarily conserved lysosomal degradation pathway in which intracellular misfolded proteins and damaged organelles are degraded [65]. Studies have suggested that maintenance of the correct level of autophagic activity is crucial to maintaining muscle integrity, with either reduced or excessive levels leading to specific myopathies. The ubiquitin ligase TRIM32 stimulates ULK1 activity via unanchored K63-linked polyubiquitin to regulate muscle autophagy in response to atrophic stimuli [66,67,68]. Autophagy includes the following four stages: autophagy initiation, autophagosome formation, autophagosome-lysosome fusion, and the degradation of autophagic substrates [69]. It has been shown that tumor of the central nervous system with the BRAFV600E mutation are autophagy-dependent, and late stage inhibition of autophagy improves the response to targeted BRAF inhibitors (BRAFi) in sensitive and resistant cells [70]. Autophagy is a rapid response to a variety of stress conditions that is carried out via the concerted action of evolutionarily conserved proteins, most of which are known as autophagy-related (ATG) proteins that function at different steps [71,72] (Table 2). Under normal physiological conditions, autophagy helps maintain cell homeostasis mainly through the control of proteins and organelles, which is called basic autophagy [73]. However, under stressful conditions, such as hypoxia or deficiencies of ATP or amino acids, intracellular autophagy is induced [49,74,75]. Upon sensing changes in cellular energy, the ULK complex acts as a scaffold to initiate the phagophore assembly site (PAS) and the recruitment of downstream ATG proteins that play an important role upstream of pathways of autophagy [76,77].
Table 2.
Induction factors, important autophagy-related proteins, and their relevance to different stages of autophagy in mammalian cells [73,87,89,91,92,93,94].
| Induction Factors | Autophagy Stages | Complex | Core Proteins of the Complex |
|---|---|---|---|
| Intrinsic cellular stress: Reactive oxygen species (ROS), damaged organelles, protein aggregates, infected pathogens, AMP/ATP, endoplasmic reticulum stress and hypoxia, etc. | Induction of autophagy | mTORC1 complex | AMPK mTOR, RAPTOR, mLST8, PRAS40, DEPTOR, CASTOR1, SLC38A9, GATOR1 |
| Nucleation and expansion of phagophore, Formation of autophagosomes |
ULK1-FIP200-ATG13complex Beclin-1-VPS15-VPS34-ATG14 l complex |
ULK1, ULK2, ATG13, ATG101, FIP200 ATG9, VPS34, VPS15, BECN1, ATG14L, AMBRA, ATG5, ATG12, ATG16 |
|
| Extrinsic cellular stress: Nutrients (amino acids, glucose, and oxygen), growth factors and drugs, etc. | Fusion of autophagosome-lysosome fusion | Lipidation complex | WIPI1/2, LC3, ATG3, ATG4, ATG7, ATG12, ATG16L, GABARAP |
| Degradation of autophagic substrates | syntaxin-17, SNARE, VAMP8, SNAP29, RAB7, EPG5, HOPS, PLEKHM1 |
It has been reported that the ULK complex can be recruited to two kinds of distinct membranes, the ER membrane and ATG9-positive autophagosome precursors [78]. After interaction between ULK complex, the PIS-enriched ER subdomain and ATG9A vesicles, the process of autophagy is initiated [27,79]. The mammalian target of rapamycin complex (mTORC1) and the adenylate-activated protein kinase (AMPK) directly participate in the regulation of ULK1 activity [49]. In both a mouse model and in primary hepatocytes, loss of AMPK or ULK1 resulted in aberrant accumulation of mitochondria and the autophagy adaptor p62. However, a study of ULK1-deficient cells found that the addition of a plasmid encoding a mutant ULK1 that could not be phosphorylated by AMPK revealed that phosphorylation is required for mitochondrial homeostasis and cell survival during starvation [79]. Under nutrient-rich conditions, autophagy is inhibited because AMPK is inhibited and the activity of ULK1 is inhibited by mTORC1 via the phosphorylation of ULK1 at Ser757 and Ser258 of ATG13 [80]. Under conditions when nutrient supply is limited, however, MTORC1 separates from the ULK complex and induces autophagy by ULK1 autophosphorylation and transphosphorylation of ATG13 and RB1CC1 [47]. Meanwhile, ULK1 causes phosphorylation of ATG9 at Tyr8 and Ser14 to promote the redistribution of ATG9 from the plasma membrane and Golgi region to the sites of autophagosome assembly [81]. ULK1 binds to ATG8 by partially recruiting its LIR motif to autophagosomes. Mutation of the LIR motif of ULK1 can cause the accumulation of early autophagic structures [82,83].
Once the active ULK complex is recruited to the initiation site, autophagy proceeds to the next stage, i.e., the formation of autophagosomes. It has been shown that the Beclin1-VPS34-Atg14L complex is critical to the formation of autophagosomes [84]. Activated ULK1 phosphorylates Beclin1 at Ser15 and ATG14 at Ser29. Beclin1 positively regulates Vps34 to increase the activity of the Beclin1-Vps34-Atg14L complex [85]. Matured autophagosomes are degraded by autolysosomes in a manner dependent on small GTPase RAB7, CCZ1-MON1, HOPS complex, syntaxin-17, and other SNARE proteins [86,87]. In mammalian cells, ULK1 binds to ATG8 at the interacting motif/LC3-interacting region and, then, is transferred to the lysosome for degradation [88,89,90]. The degradation of ULK1 not only involves lysosomes and proteasomes but also the transcription of inhibitory proteins. The inhibition of protein synthesis leads to a rapid decrease in ULK1 and limits ULK1-dependent autophagic degradation [91,92]. All of these functions are summarized in Table 2.
2.2. The Noncanonical Role of ULK1
In recent years, multiple reports have shown that ULK1 not only plays a canonical role in the occurrence and progress of autophagy, but also mediates or participates in many other important physiological activities [93]. For example, ULK1 plays a role in protein transportation, the promotion of apoptosis, and the regulation of the innate immune response [94]. Kemp et al. reported that UV radiation and chemical carcinogens enhance STING-dependent IRF3 activation, which exacerbates the symptoms of autoimmunity. Of note, ULK1 negatively regulates STING [95]. The natural immune response that is induced by ultraviolet light can be enhanced by downregulating the expression of ULK1 in keratinocytes [95,96]. The lack of ULK1 tends to result in impaired RBC macrocytosis and mitochondrial clearance [88], which leads to a shortened life of RBCs [97]. It has been reported that ULK1 has an important function in the activation of the spindle assembly checkpoint (SAC), which ensures the fidelity of chromosome segregation during mitosis. Of note, deletion of ULK1 increases chromosome instability and cytotoxicity following treatment with paclitaxel, resulting in significant impairment of cancer cell growth [98].
ULK1 is also reported to be important for neuronal development through mediating vesicular transport in axons [97,99]. In the absence of exogenous pressure, ULK/Atg1 regulates the transportation of specific macromolecules in the endoplasmic reticulum (ER)-Golgi apparatus to maintain cell homeostasis in Caenorhabditis elegans and mammalian cells [50,100,101]. In conditioned knockout mice, ULK1/2 results in neuronal degeneration, but there is no accumulation of P62, ubiquitinated protein, or abnormal membranous structures in nerve cells [102]. In mice, knockout of ULK1 improves the cognitive ability after traumatic brain injury (TBI) treatment and slows the histological changes in the hippocampus. Indeed, loss of ULK1 reduces hippocampus inflammation and apoptosis in TBI mice [103]. The mouse ULK1/2 proteins are localized to vesicular structures in the growth cones of mouse spinal sensory neurons. The Ulk1/2 proteins are necessary for efficient endocytosis of nerve growth factor (NGF) and can suppress excessive filopodia extension and axon branching in sensory neurons during NGF-induced axon outgrowth. Knockdown of ULK1 or ULK2 by RNAi results in impaired endocytosis of excessive axon arborization and severely stunted axon elongation [104,105,106].
According to the recent research reports, in the absence of energy metabolism, ULK1 can phosphorylate Rab9 at Ser179 to protect the heart from myocardial ischemia [107]. High-grade serous ovarian cancers (HGSOCs) grow in suspension and potentially escape from anoikis. However, knockdown of ULK1 significantly inhibits suspension growth of HGSOC cells, and analysis of the metabolic profile confirmed an important role of fatty acid metabolism in survival in suspension [108,109,110,111]. ULK1 regulates lipid metabolism to prevent heart dysfunction caused by obesity [112]. The differentiation of human bone marrow and the pro-osteogenic effect of galectin 3 are affected by the knockout of ULK1 [94]. ULK1 and EGFR levels in patients with normoalbuminuria are significantly higher than in microalbuminuria and macroalbuminuria [113]. ULK1 plays an important role in protecting hosts from infection by pathogens; inhibition of ULK1 expression prevents the death of host cells infected by Staphylococcus aureus [114].
3. ULK1 Mediates Signaling Pathways in Cancer Treatment
Autophagy is usually understood as a mechanism to protect normal cells from adverse conditions [115]. However, it is also known to inhibit the growth of tumors. Some studies have suggested that the role of autophagy can vary across stages of tumor development [116,117]. For example, in the early stage of tumor development, autophagy limits the proliferation of tumor cells by direct killing. However, after tumors have already formed, the response to stress can actually increase the survival, invasion, and metastasis of tumor cells [118]. In addition, autophagy has been found to influence the occurrence of tumors in cell- or tissue-specific ways. Therefore, autophagy plays a dual role in the development of tumors [72,119]. Compared with other cell functions, autophagy is relatively selectively druggable. During autophagy, only a few of enzymes are currently targeted by drugs, although we know of more than 36 autophagy-related proteins [120]. ULK1 plays a core role in the activation of the autophagy pathway. Therefore, it could be a feasible drug target. As a promoter of autophagy, ULK1 also plays different roles according to the types and stages of tumors. For example, it has been shown that low expression of ULK1 in operable breast cancer tissues is a marker of poor prognosis [121].
3.1. Inhibition of ULK1-Mediated Autophagy for Cancer Treatment
Autophagy provides building blocks and energy to tumor cells in response to metabolic stress and chemotherapeutic drug damage, thereby promoting the survival and development of tumor cells [122]. A growing number of studies have shown that the inhibition of autophagy an effective method for tumor therapy [123,124]. Many proteins are involved in this complex process. Thus, targeting these proteins can be one method to inhibit autophagy [125]. A strategy based around the inhibition of kinases has been successfully used in clinical application, making ULK1 an attractive candidate for inhibiting autophagy [126].
The crystal structure of ULK1 has been determined [127]. Petherick et al. reported two compounds, MRT67307 and MRT68921, that were effective in inhibiting ULK1 (IC50 values of 45.0 and 2.9 nM, respectively) and ULK2 activity IC50 (IC50 values of 38.0 and 1.1 nM, respectively) in an in vitro kinase assay and that also prevented autophagy in mouse embryonic fibroblasts (MEFs) cells [128]. In studies of a drug-resistant ULK1 mutant cell line, it was found that the compound inhibits the autophagy of tumor cells by specifically inhibiting the activity of ULK1 [128]. To increase the selectivity for ULK1, a novel inhibitor scaffold of ULK1 was explored, leading to the finding of several compounds that are potent inhibitors of ULK1 and ULK2 with good selectivity [127]. SBI-0206965 is a FAK inhibitor that has been demonstrated to be a highly selective inhibitor of ULK1 and ULK2 that acts by suppressing ULK1-mediated phosphorylation of Vps34 in tumor cells [129,130]. In clinical therapy, SBI-0206965 synergizes with mTOR inhibitors to kill cancer cells [131].
Martin et al. found two small-molecule compounds, ULK-100 and ULK-101, that inhibit the autophagy of tumor cells through efficiently and selectively inhibiting ULK1, thus making tumor cells more sensitive to nutrient deficiency. In in vitro tests, the EC50 of ULK-100 and ULK-101 were 83 nM and 390 nM, respectively. ULK-101 blocks LC3-II accumulation, and thus inhibits autophagy by stopping the formation of the early autophagy body. The inhibition of ULK-101 causes KARK-mutant tumor cells to be more sensitive as compared with wild cell in conditions of nutrient deficiency. However, the authors stated that further validation is still required in vivo and that the targeted therapeutic mechanisms of ULK1 are not suitable for all genetic or environmental contexts [132]. Liu J. et al. found that NVP-BEZ235, a dual PI3K/mTOR inhibitor, could induce autophagy through AMPK/ULK1 pathway in colon cancer cells. Autophagy was blocked by knocking down AMPK or ULK1 inhibiting cell proliferation and further promoting NVP-BEZ235 induced apoptosis [133]. Arsenic trioxide (ATO) and thalidomide (THAL) are used in the treatment of many types of hematologic malignancies. ATO prevents the proliferation of cells and induces apoptosis in some cancer cells. Moreover, THAL has immunomodulatory and antiangiogenic effects in malignant cells. Combined treatment with ATO and THAL inhibits proliferation and invasion of AML cells by downregulating ULK1 and BECLIN1 and upregulating PTEN and IL-6, and this effect was stronger than the effects of either ATO or THAL alone [134]. The IC50 for each inhibitor of ULK1 and ULK2 is listed in Table 3.
Table 3.
Small-molecule compounds targeting ULK1/ULK2-mediated autophagy in cancer.
| Function | Compound | In Vitro Kinase Assay (nM) | CellularEC50/IC50 (nM) | Cell Type | Reference | |
|---|---|---|---|---|---|---|
| ULK1 IC50 | ULK2 IC50 | |||||
| ULK1/ULK2 inhibitor |
Compound 1 | 5.3 | 13 | - | - | [127] |
| Compound2 | 67 | 200 | - | - | ||
| Compound 3 | 120 | 360 | - | - | ||
| BX-795 | 87 | 310 | - | - | ||
| Compound 6 | 8 | - | - | - | [33] | |
| MRT67307 | 45 | 38 | - | MEFs | [128] | |
| MRT68921 | 2.9 | 1.1 | - | |||
| SBI0206965 | 38 | 212 | EC50 = 2400 | U2OS cells | [135] | |
| ULK100 | 1.6 | 2.6 | EC50 = 83 | |||
| ULK101 | 8.3 | 30 | EC50 = 390 | |||
| NVP-BEZ235 | - | - | - | Colon cancer cells | [133] | |
| ATO -THAL | - | - | - | U93, KG-1 cells | [134] | |
| ULK1/ULK2 activator |
LYN-1604 | 18.94 | - | IC50 = 1.66 μM | MDA-MB-231 | [136] |
| AEC | - | - | 150 μg/mL | HT-29 cells | [137] | |
3.2. Activation of ULK1-Mediated Autophagy for Cancer Treatment
During the past decades, extensive research efforts have greatly improved our knowledge about autophagy. This process is now known to be widely implicated in pathophysiological processes including cancer, metabolic, and neuro degenerative disorders, making it an attractive target for drug discovery [138]. Recent publications have suggested that ULK1 is underexpressed in some tumor tissues, such as breast cancer [139]. This indicates that the activation of ULK1 to inhibit tumor growth has potential to be used as an effective treatment method for some tumors. Through in silico high-territorial screening and chemical synthesis, Ouyang et al. obtained a small molecule activator of ULK1, LYN1604, which regulated ULK1 and interacted with protein ATF3, RAD21, and CASP3/caspase3 to induce autophagy-associated cell death in triple-negative breast cancer cells [139]. LYN-1604 has been shown to be a potent ULK1 agonist with an EC50 of 18.94 nM in an in vitro kinase assay [136]. It has been suggested that fluoxetine could have significant therapeutic effects on triple negative breast cancer. It can induce autophagic cell death by activating the AMPK-mTOR-ULK axis in cells. In addition, fluoxetine can also induce apoptosis in TNBC cells by upregulating caspase3/8 and PARP expression [140].
A growing number of cell- and animal-based assays have shown that structurally different compounds from various natural products have the same chemotherapeutic effects on tumors [141]. It has been reported that an aqueous extract of clove can inhibit the growth of tumors by activating the AMPK/ULK1 pathway, thus modulating autophagy. AEC inhibits the growth of pancreatic ASPC-1 and colon HT-29 cancer cells in vitro at an IC50 of 150 μg/mL in colon HT-29 cells [137]. Although many natural products have health benefits and therapeutic effects on tumors, their poor water solubility, low bioavailability, and rapid elimination limit their practical application as drugs [142]. However, several combinations of natural products and nanotechnology have been tested to create natural product-based nanoformulations. The formed nanoparticles have better affinity, fewer side effects, and can target tumor cells to increase the therapeutic efficacy and improve prognoses for patients [143].
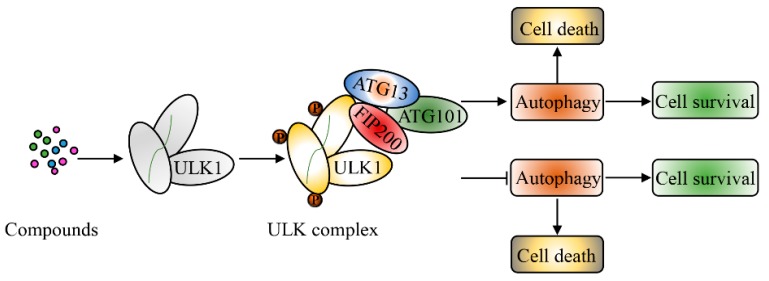
The roles of ULK1 in inducing autophagic cell death or cytoprotective autophagy in cancer are summarized in Figure 2.
Figure 2.
Summary of the relationships between ULK1, autophagy, and cancer.
4. ULK1 and Cancer Drug Resistance
With the rapid development of targeted drugs and cytotoxic drugs, most cancers are treatable, and some tumors can often even be completely controlled. However, some metastatic tumors are still incurable [144]. Drug therapy of tumors (including chemotherapy and targeted drugs) is currently the most widely used form of cancer treatment. However, unfortunately, it is usually only effective at the beginning of treatment. With the prolongation of the treatment cycle, the development of resistance to these drugs in tumor cells is often inevitable [145,146]. Multidrug resistance (MDR) is a leading cause of morbidity and mortality in cancer [147]. Drug resistance in tumor cells is caused by a variety of factors including genetic differences in tumor cells and microenvironment. It has been shown the tumor cells from the same tissue source are differently cytogenetic. The microenvironment around tumor cells is essential for the development of tumor resistance [148]. It has been reported that the extracellular milieu around tumors plays an important role in tumor development, metastasis, and the regulation of resistance in various treatments [149]. Although the drug resistance of tumor cells is inherent, acquired resistance is becoming more and more common [150,151,152].
4.1. The Pivotal Role of ULK in the Development of Drug Resistance in Human Cancers
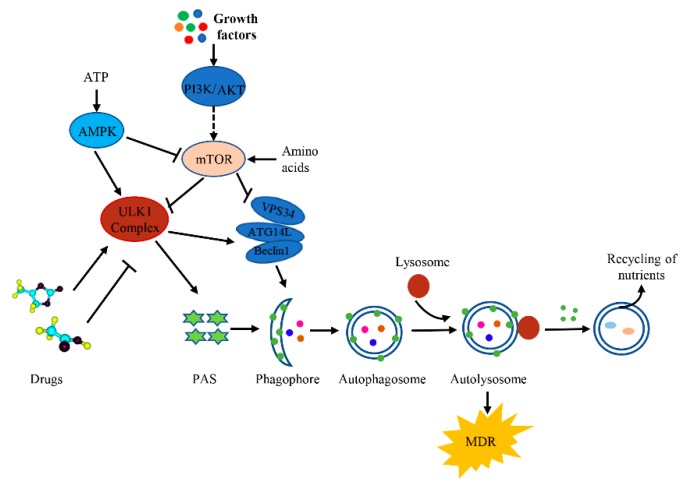
The purpose of precision medicine is to develop a personalized treatment in order to address the diversity of cancers. The targeted treatment of tumors is one such strategy and is one of the most effective cancer treatment methods [153]. However, tumor tissue is a multicell system that continues to evolve. Although some drugs and treatments have clinical significance, in the process of continuous treatment, some remaining cells continue to proliferate and, then, acquire drug resistance [154]. Autophagy which is essential to the efficacy of anticancer drugs, as well as drug resistance can have a prosurvival role in response to metabolic and therapeutic stresses [155]. Xia et al. demonstrated that the mitotic kinase NEK2 is involved in the development of MDR by regulating autophagy in multiple myeloma (MM). Autophagy is activated by the assembly of a complex of NEK2/USP7/Beclin-1 in MM cells. Indeed, treatment with a combination of the autophagy inhibitor chloroquine (CQ) and the chemotherapeutic bortezomib (BTZ) significantly prevents NEK2-induced drug resistance in MM cells [156]. The efficacy of fluorouracil (FU) in the treatment of colorectal cancer (CRC) is greatly limited by drug resistance. In this context, autophagy serves as a prosurvival mechanism during chemotherapy challenge [5]. The inhibition of autophagy has been shown to counteract the resistance to doxorubicin in breast cancer MCF-7 cells [157]. ULK1 is crucial in the regulation of autophagy in cancer cells; the phosphorylation of ULK1 alters the activity of autophagy [158]. TOPK (T-LAK cell-originated protein kinase) directly binds with and phosphorylates ULK1 at Ser469, Ser495, and Ser533. The phosphorylation of ULK1 decreases the activity and stability of ULK1, thus, inhibiting autophagy and promoting resistance against temozolomide in glioma cells [159]. The treatment of tumors by targeting ULK, however, has presented many challenges in the intrinsic and acquired drug resistance in cancer treatments [155] (Figure 3).
Figure 3.
A summary of how ULK1 is involved in the development of drug resistance in cancer.
4.2. Drug Resistance-Associated Mutations
Mutations in drug-targeted proteins lead to changes in protein conformation and insensitivity to anticancer drugs of tumor cells [160]. It has been reported that tyrosine kinase inhibitors are effective to treat chronic myeloid leukemia (CML) by targeting the BCR-ABL protein in CML cells [161,162]. However, the development of drug resistance in patients during treatment is mainly caused by a mutation in the BCR-ABL protein [163,164]. IIB02, the inhibitor of Hsp90, which has an inhibitory effect on a variety of tumor cells, could solve this problem by causing significant cytotoxicity to BCR-ABL cells regardless of their sensitivity to tyrosine kinase inhibitors [165,166,167]. This is due to the fact that BIIB021 can significantly induce autophagic cell death by the downregulation of the AKT-mTOR pathway and the activation of ULK1. Additionally, BIIB021 induces apoptosis by releasing of cytochrome c, which promotes consequential activation of caspase-9, -3, and PARP [167,168]. It has also been found that the mutation of BRAF protein in patients with colorectal cancer has an adverse prognosis, causing mortality rates to increase by 70% as compared with normal BRAF backgrounds [169,170]. Although BRAF inhibitors have been approved by the Food and Drug Administration, their therapeutic benefits for cancer patients with BRAF protein mutations are still not obvious [171]. The main reason why BRAFV600E-treated colorectal cancer (CRC) develops drug resistance to selective BRAF inhibitors is that BRAF inhibitors can induce autophagy through the AMPK-ULK1 pathway [172]. Inhibiting autophagy by pharmacological methods or siRNA, however, causes BRAFV600E CRC cells to be more sensitive to BRAF inhibitors to induce cell death and overcome the drug resistance-related mutations in tumor cells [167,173].
Findings from the literature suggest that about 50% of colon cancers have mutations in KRAS, a proto-oncogene that increases resistance to drugs [174]. Mutated KRAS regulates intracellular energy metabolism. When two mitochondrial-targeted compound 3-carboxyl proxyl nitroxide (Mito-CP) and mito-metformin were used to treat KRAS mutated cells, they not only decreased the ATP levels in the tumor cells but also inhibited ATP-related oxygen consumption in cells. However, no obvious effect was found in normal intestinal epithelial cells. Mitochondria-targeted drugs have the capability to activate an AMPK-ULK1 signaling cascade could overcome the resistance of mutant cells to drugs [175,176].
4.3. Immune Factors in the Tumor Microenvironment and Tumor Drug Resistance
The tumor microenvironment is the surrounding space composed of immune cells, stroma, and vasculature. The tumor microenvironment mediates drug resistance via several mechanisms, such as preventing immune clearance of tumor cells, hindering drug absorption, and stimulating paracrine growth factors to signal cancer cell growth [177,178]. Recently, a number of studies have shown that the tumor microenvironment could contribute to the regulation of tumor development, metastasis, and drug resistance against various therapeutic methods [179]. Until now, although significant advances have been made in chemotherapy and radiotherapy of tumors, the development of drug resistance during treatment reduces the effectiveness of drugs [180,181]. The tumor microenvironment is a dynamic network of tumor cells and extracellular matrix, usually lacking oxygen and nutrients and presenting a low pH [182]. Most tumor cells adapt to this harsh environment and make use of the limited resources in the environment to grow. Tumor cells can evade immune surveillance by inhibiting immune cell activity [183,184]. It has been reported that the natural product, rocaglamide (RocA) could enhance NK cell-mediated lethality, inhibit the growth of tumor cells, and shrink tumors in in vitro and in vivo tests. RocA not only improves the level of NK cell-derived GZMB and enhances the killing power of NK cells, but also targets ULK1, specifically inhibiting the translation of the ULK1 protein, and therefore inhibiting the progress of autophagy. The inhibition of autophagy increases the sensitivity of non-small cell lung cancer cells to NK cells. However, after inhibiting the activity of NK cells in mice with normal immune function, RocA’s inhibitory effect on tumor inoculation in mice was significantly weakened, indicating that the lack of NK cells could lead to the resistance of tumors to RocA [185,186].
4.4. MicroRNA and LncRNA Involved in Drug Resistance
The heterogeneity of tumor cell genes and the differences in transcription process limit the efficiency of many therapeutic methods [187]. MicroRNAs are a class of extremely conserved single-stranded noncoding RNAs of about 19 to 25 nucleotides that negatively regulate the expression of different genes at the transcriptional or post-transcriptional level [188,189,190]. MicroRNAs, as tumor suppressor genes or oncogenes, regulate the occurrence and development of tumors [191]. Increasing amounts of data have indicated that microRNAs play an important role in the development of drug resistance in tumors [192]. Three compounds, doxorubicin (DOX), tamoxifen, and cisplatin have been shown to be effective in the treatment of breast cancer [192]. However, due to inducing drug resistance in tumor cells, their wide application has been limited. They could increase autophagy in cells and induce protective autophagy, which ensures the survival of tumor cells in an adverse environment [193]. Mir-489 has been reported as a tumor suppressor in the treatment of breast cancer cells affecting the activity of multiple genes during autophagy [194,195]. When mir-489 was used to treat doxorubicin-, tamoxifen-, or cisplatin-resistant cell lines, it showed significant inhibition of cell proliferation [196]. It was found that mir-489 could directly target ULK1 and LAPTM4B genes, negatively regulate the expression of ULK1 and LAPTM4B, hinder the fusion of autophagosomes and lysosomes, inhibit autophagy, and thus overcome tumors’ drug resistance [197,198,199].
Long noncoding RNA (LncRNA) is a kind of RNA with no protein-coding capacity that is also over 200 nucleotides in length [200,201]. LncRNA affects protein regulation at transcriptional, post-transcriptional, and epigenetic levels [202]. A growing body of evidence suggests that abnormal expression of LncRNA contributes to tumor formation, metastasis, and drug resistance [203]. It has been reported in the literature that LncRNA plays an important role in the drug resistance of non-small cell lung cancer (NSCLC). The lncRNA small nucleolar RNA host gene 6 (SNHG6) enhances colorectal cancer cell resistance to 5-fluorouracil (5-FU), promotes autophagy, and inhibits 5-FU-induced apoptosis in vivo. Indeed, SNHG6 can promote chemoresistance through ULK1-induced autophagy by reducing the availability of miR-26a-5p in CRC cells. Cancer cells that had knocked down SNHG6 (by SNHG6-specific shRNAs), overexpression of miR-26a-5p, or that were treated with 3-MA were more sensitive to 5-FU [204,205]. Crizotinib showed a therapeutic effect on patients with NSCLC, but after several cycles of treatment, the patient’s resistance to crizotinib increased, leading to treatment failure [203]. HOTAIR (HOX transcript antisense intergenic RNA) is often highly expressed in NSCLC and promotes cisplatin resistance in NSCLC. The silencing of HOTAIR reduced the proliferation and induced apoptosis of NSCLC cells (A549). In addition, HOTAIR shRNA transfection inhibited the resistance of A549 cells to crizotinib, inhibited cell survival, and promoted apoptosis as compared with the HOTAIR scramble group. After HOTAIR was silenced, the number of LC3+ puncta and the expression of Beclin1, p-ULK1, and the ratio of LC3 II/I/in crizotinib-treated A549 cells decreased. Further studies have indicated that the main reason for HOTAIR silencing to reduce the resistance of NSCLC cells could be the inhibition of the phosphorylation of ULK1, thus inhibiting the autophagy of crizotinib-resistant cells [158].
4.5. Small Molecule Drugs Targeting ULK Reverse Tumor Resistance
As noted herein, ULK1 plays an important role in the initiation of autophagy [206]. The induction of protective autophagy to inhibit apoptosis is one of the reasons for the development of drug resistance in tumor cells during therapy [207]. Some small molecule drugs targeting ULK1 show inhibitory effects on ULK1 expression and the activity of autophagy, and cause tumor cells to be more sensitive to chemotherapeutic drugs [136,208]. It has been reported that overexpression of ULK1 is inversely related to the prognosis of various tumors, such as colon cancer, breast cancer, lung cancer, nasopharyngeal cancer, and esophageal cancer [136]. The knockdown of ULK1 in NSCLC cells induces an increase in apoptosis and makes them more sensitive to cisplatin [209]. SBI0206965, a selective inhibitor of ULK1, can significantly reduce the cell survival of cisplatin-resistant NSCLC cells by decreasing the conversion of LC3 I to LC3 II, upregulating the expression of autophagy substrate P62, and inhibiting the progress of autophagy. A combination of SBI0206965 and cisplatin can block cisplatin-induced autophagy and promote cell death. However, inhibition of autophagy by SBI0206965 was one of the major reasons for overcoming the resistance of cisplatin to NSCLC cells [131,210]. In addition, more and more natural products are being developed to treat tumors. Most of them are multitargeted drugs, and the inhibition of tumors is achieved through multiple simultaneous effects. However, they can sometimes produce more adverse consequences, such as inhibiting the proliferation of healthy tissues and causing structural deformities, systemic toxicity, long-term side effects, drug resistance, and some psychological problems [140,211,212]. The relationship between ULK1 and cancer drug resistance is summarized in Table 4.
Table 4.
Molecular events of ULK1-related cancer drug resistance.
| Drug Resistance Cancer Cell Type | Event | Effects on Cancer Drug Resistance |
|---|---|---|
| Chronic myeloid leukemia (CML) resistance to tyrosine kinase inhibitors | A mutation in the BCR-ABL protein; IIB02 producing significant cytotoxicity to BCR-ABL mutation cells BIIB021 inducing autophagic cell death by the downregulation of AKT-mTOR pathway and the activation of ULK1 | Overcoming drug resistance |
| Colorectal cancer (CRC) resistance to selective BRAF inhibitors | A mutation in BRAF protein; BRAFV600E of colorectal cancer (CRC) develops drug resistance to selective BRAF inhibitors is that BRAF inhibitors induce cytoprotective autophagy through AMPK-ULK1 pathway | Inhibiting autophagy and overcoming drug resistance |
| Colorectal cancer cell resistance to 5-fluorouracil (5-FU) | SNHG6 enhances chemoresistance through ULK1-induced autophagy via reducing free miR-26a-5p | Inhibiting autophagy, SNHG6 knockdown or overexpression of miR-26a-5p, thus overcoming drug resistance |
| Colon cancers resistance to KRAS drugs | Two mitochondrial targeted compound 3-Carboxyl proxyl nitroxide (Mito-CP) and Mito-Metformin cause mitochondrial autophagy of KRAS cells by activating AMPK-ULK1 signaling cascade | Overcoming drug resistance of KRAS mutant cells |
| Non-small cell lung cancer (NSCLC) cells resistance to NK cell-mediated killing | The natural product rocaglamide (RocA) inhibited autophagy by targeting to ULK1 and restored the level of NK cell-derived GZMB (granzyme B) in NSCLC cells increasing their susceptibility to NK cell-mediated killing. | Overcoming resistance of NSCLC cells against NK cell-mediated killing |
| Breast cancer cell resistance to doxorubicin (DOX), tamoxifen and cisplatin | Mir-489 directly targeting ULK1 and LAPTM4B genes, negatively regulating the expression of ULK1 and LAPTM4B inhibiting autophagy | Overcoming resistance to doxorubicin (DOX), tamoxifen and cisplatin |
| NSCLC resistance to crizotinib | HOTAIR (HOX transcript antisense intergenic RNA) is a high expression in NSCLC and promotes cisplatin resistance in NSCLC | HOTAIR shRNA transfection overcoming the resistance of A549 cells to crizotinib by inhibiting autophagy activity decreasing the phosphorylation of ULK1 |
| Cisplatin-resistant NSCLC cells | SBI0206965, as a selective inhibitor of ULK1 blocks cisplatin-induced autophagy and promotes cell death | Overcoming NSCLC cells resistance to cisplatin |
5. Conclusions and Future Prospective
Drug resistance is a major problem in cancer therapy. The development of drug resistance in tumors counteracts the therapeutic effects of chemotherapeutic compounds, which leads to a more aggressive recurrence of tumors, and worse prognoses of cancer patients. So far, the solutions to tumor resistance have been mainly focused on selecting more sensitive drug targets, genetically modifying the target, changing the drug structure, using drugs in combination, inhibiting prosurvival pathways, etc. With an increasing number of anticancer drugs on the market, the improvement of preclinical models, and the development of technologies such as high-throughput screening, there are more opportunities to understand and overcome tumor resistance.
Mammalian Unc-51-like kinases 1 and 2 (ULK1 and ULK2) belong to the ULK/Atg1 family of ULK and are a promising therapeutic target for tumors because they are a direct target of energy- and nutrient-sensing kinases. ULK1 also mediates the adverse prognosis and drug resistance of tumors. Both the inhibition and activation of ULK1 have significant effects on tumor therapy. Regulation of ULK1 plays a fundamental role by influencing cell growth and the development of drug resistance in tumor cells. Although the use of ULK inhibitors as monotherapy have provided modest effects, combining them with other anticancer drugs could help overcome the development of drug resistance during tumor treatment.
Although the role of ULK in the progress of autophagy is clear, other functions of ULK beyond this are not well understood. Future studies are still needed to reveal the presence of further mechanisms. In addition, most studies, to date, have mainly focused on the expression of the ULK gene. With the advancing development of precision medicine, it is crucial to identify more potential predictive biomarkers for tumor treatments, such as ULK inhibitors. This would facilitate the effective design of clinical trials that would accelerate the introduction of these compounds to clinical practice in the most efficient and beneficial manner. Hence, cancer patients would benefit from a tailored therapy with various ULK inhibitors alone or in combination with other molecular targeted therapies.
Funding
This work was supported by the Key Research and Development Plan of Shaanxi Province (2017ZDXL-NY-0304, 2019ZDLNY01-02-02), the National Key R&D Program of China (2017YFE0105300), the China Agriculture Research System (CARS-29-jg-3), the Shaanxi Provincial Natural Science Foundation (no. 2018JQ3054).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chow L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020;382:60–72. doi: 10.1056/NEJMra1715715. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y., Shen Y., Chen C., Sui X., Yang J., Wang L., Zhou J. The crosstalk between autophagy and ferroptosis: what can we learn to target drug resistance in cancer? Cancer Biol. Med. 2019;16:630–646. doi: 10.20892/j.issn.2095-3941.2019.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasan N., Baselga J., Hyman D.M. A view on drug resistance in cancer. Nature. 2019;575:299–309. doi: 10.1038/s41586-019-1730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D., Wang C.Y. Targeting cancer stem cells in squamous cell carcinoma. Precis. Clin. Med. 2019;2:152–165. doi: 10.1093/pcmedi/pbz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ou J., Peng Y., Yang W., Zhang Y., Hao J., Li F., Chen Y., Zhao Y., Xie X., Wu S., et al. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat. Commun. 2019;10:1–14. doi: 10.1038/s41467-019-08902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fu Z.G., Wang Y., Wang S., Shao D., Tian L., Li Y.X., Jiang J.L., Chen Z.N., Wen N. Synthesis and Evaluation of a Novel Small-molecule Compound as an Anticancer Inhibitor of CD147. Biomed. Environ. Sci. 2019;32:673–686. doi: 10.3967/bes2019.086. [DOI] [PubMed] [Google Scholar]
- 7.Delou J.M.A., Souza A.S.O., Souza L.C.M., Borges H.L. Highlights in Resistance Mechanism Pathways for Combination Therapy. Cells. 2019;8:1013. doi: 10.3390/cells8091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voronkova M.A., Rojanasakul L.W., Kiratipaiboon C., Rojanasakul Y. SOX9-ALDH axis determines resistance to chemotherapy in non-small cell lung cancer. Mol. Cell. Biol. 2019;40:1–20. doi: 10.1128/MCB.00307-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H., Li J.M., Deng K., Zhou W., Wang C.X., Wang Q., Li K.H., Zhao H.Y., Huang S.W. Tumor acidity activated triphenylphosphonium-based mitochondrial targeting nanocarriers for overcoming drug resistance of cancer therapy. Theranostics. 2019;9:7033–7050. doi: 10.7150/thno.35748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An Y., Zhou L., Huang Z., Nice E.C., Zhang H., Huang C. Molecular insights into cancer drug resistance from a proteomics perspective. Expert Rev. Proteomics. 2019;16:413–429. doi: 10.1080/14789450.2019.1601561. [DOI] [PubMed] [Google Scholar]
- 11.Fu S., Liu X., Luo M., Xie K., Nice E.C., Zhang H., Huang C. Proteogenomic studies on cancer drug resistance: towards biomarker discovery and target identification. Expert Rev. Proteomics. 2017;14:351–362. doi: 10.1080/14789450.2017.1299006. [DOI] [PubMed] [Google Scholar]
- 12.Wang S.Y., Hu H.Z., Qing X.C., Zhang Z.C., Shao Z.W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer. 2020;11:69–82. doi: 10.7150/jca.36588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin T.Y., Chan H.H., Chen S.H., Sarvagalla S., Chen P.S., Coumar M.S., Cheng S.M., Chang Y.C., Lin C.H., Leung E., et al. BIRC5/Survivin is a novel ATG12-ATG5 conjugate interactor and an autophagy-induced DNA damage suppressor in human cancer and mouse embryonic fibroblast cells. Autophagy. 2019 doi: 10.1080/15548627.2019.1671643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.New J., Thomas S.M. Autophagy-dependent secretion: Mechanism, factors secreted, and disease implications. Autophagy. 2019;15:1682–1693. doi: 10.1080/15548627.2019.1596479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giuliano S., Cormerais Y., Dufies M., Grépin R., Colosetti P., Belaid A., Parola J., Martin A., Lacas-Gervais S., Mazure N.M., et al. Resistance to sunitinib in renal clear cell carcinoma results from sequestration in lysosomes and inhibition of the autophagic flux. Autophagy. 2015;11:1891–1904. doi: 10.1080/15548627.2015.1085742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang C.H., Bijian K., Wernic D., Su J., da Silva S.D., Yu H., Qiu D., Asslan M., Alaoui-Jamali M.A. A novel orally available seleno-purine molecule suppresses triple-negative breast cancer cell proliferation and progression to metastasis by inducing cytostatic autophagy. Autophagy. 2019;15:1376–1390. doi: 10.1080/15548627.2019.1582951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Qi Q., Zhou W., Feng Z., Huang B., Chen A., Zhang D., Li W., Zhang Q., Jiang Z., et al. Inhibition of glioma growth by flavokawain B is mediated through endoplasmic reticulum stress induced autophagy. Autophagy. 2018;14:2007–2022. doi: 10.1080/15548627.2018.1501133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng H., Yang F., Hu Q., Sun J., Peng C., Zhao Y., Huang C. The ubiquitin-specific protease USP8 directly deubiquitinates SQSTM1/p62 to suppress its autophagic activity. Autophagy. 2019 doi: 10.1080/15548627.2019.1635381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng J., Xiao J., Mi Y., Fang X., Sun Y., Li S., Qin Z., Li X., Liu T., Zhao S., et al. PCDHGA9 acts as a tumor suppressor to induce tumor cell apoptosis and autophagy and inhibit the EMT process in human gastric cancer. Cell Death Dis. 2018;9:1–27. doi: 10.1038/s41419-017-0189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P., Holowatyj A.N., Ulrich C.M., Edgar B.A. Tumor suppressive autophagy in intestinal stem cells controls gut homeostasis. Autophagy. 2019;15:1668–1670. doi: 10.1080/15548627.2019.1633863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dite T.A., Ling N.X.Y., Scott J.W., Hoque A., Galic S., Parker B.L., Ngoei K.R.W., Langendorf C.G., O’Brien M.T., Kundu M., et al. The autophagy initiator ULK1 sensitizes AMPK to allosteric drugs. Nat. Commun. 2017;8:571–588. doi: 10.1038/s41467-017-00628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stjepanovic G., Davies C.W., Stanley R.E., Ragusa M.J., Kim D.J., Hurley J.H. Assembly and dynamics of the autophagy-initiating Atg1 complex. Proc. Natl. Acad. Sci. USA. 2014;111:127–146. doi: 10.1073/pnas.1407214111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumsta C., Chang J.T., Lee R., Tan E.P., Yang Y., Loureiro R., Choy E.H., Lim S.H.Y., Saez I., Springhorn A., et al. The autophagy receptor p62/SQST-1 promotes proteostasis and longevity in C. elegans by inducing autophagy. Nat. Commun. 2019;10:1–21. doi: 10.1038/s41467-019-13540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechauve C., Keith J., Khandros E., Fowler S., Mayberry K., Freiwan A., Thom C.S., Delbini P., Romero E.B., Zhang J., et al. The autophagy-activating kinase ULK1 mediates clearance of free alpha-globin in beta-thalassemia. Sci. Transl. Med. 2019;11:1–16. doi: 10.1126/scitranslmed.aav4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quan Y., Lei H., Wahafu W., Liu Y., Ping H., Zhang X. Inhibition of autophagy enhances the anticancer effect of enzalutamide on bladder cancer. Biomed. Pharmacother. 2019;120:1–25. doi: 10.1016/j.biopha.2019.109490. [DOI] [PubMed] [Google Scholar]
- 26.Tamargo-Gomez I., Marino G. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int. J. Mol. Sci. 2018;19:3812. doi: 10.3390/ijms19123812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reggiori F., Ungermann C. A dimer to bridge early autophagosomal membranes. Cell. 2012;151:1403–1415. doi: 10.1016/j.cell.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Alers S., Loffler A.S., Paasch F., Dieterle A.M., Keppeler H., Lauber K., Campbell D.G., Fehrenbacher B., Schaller M., Wesselborg S., et al. Atg13 and FIP200 act independently of Ulk1 and Ulk2 in autophagy induction. Autophagy. 2011;7:1423–1433. doi: 10.4161/auto.7.12.18027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim B.W., Jin Y., Kim J., Kim J.H., Jung J., Kang S., Kim I.Y., Kim J., Cheong H., Song H.K. The C-terminal region of ATG101 bridges ULK1 and PtdIns3K complex in autophagy initiation. Autophagy. 2018;14:2104–2116. doi: 10.1080/15548627.2018.1504716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin M.G., Hurley J.H. Structure and function of the ULK1 complex in autophagy. Curr. Opin. Cell biol. 2016;39:61–80. doi: 10.1016/j.ceb.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagy P., Karpati M., Varga A., Pircs K., Venkei Z., Takats S., Varga K., Erdi B., Hegedus K., Juhasz G. Atg17/FIP200 localizes to perilysosomal Ref(2)P aggregates and promotes autophagy by activation of Atg1 in Drosophila. Autophagy. 2014;10:453–467. doi: 10.4161/auto.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stork B., Dengjel J. Study of ULK1 Catalytic Activity and Its Regulation. Methods Enzymol. 2017;587:391–404. doi: 10.1016/bs.mie.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus M.B., Novotny C.J., Shokat K.M. Structure of the human autophagy initiating kinase ULK1 in complex with potent inhibitors. ACS Chem. Biol. 2015;10:257–261. doi: 10.1021/cb500835z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puente C., Hendrickson R.C., Jiang X. Nutrient-regulated Phosphorylation of ATG13 Inhibits Starvation-induced Autophagy. J. Biol. Chem. 2016;291:6026–6035. doi: 10.1074/jbc.M115.689646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong P.M., Puente C., Ganley I.G., Jiang X. The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy. 2013;9:124–137. doi: 10.4161/auto.23323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young A.R., Narita M., Ferreira M., Kirschner K., Sadaie M., Darot J.F., Tavare S., Arakawa S., Shimizu S., Watt F.M., et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach M., Larance M., James D.E., Ramm G. The serine/threonine kinase ULK1 is a target of multiple phosphorylation events. Biochem. J. 2011;440:283–291. doi: 10.1042/BJ20101894. [DOI] [PubMed] [Google Scholar]
- 38.Chan E.Y., Longatti A., McKnight N.C., Tooze S.A. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol. Cell. Biol. 2009;29:157–171. doi: 10.1128/MCB.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egan D.F., Shackelford D.B., Mihaylova M.M., Gelino S., Kohnz R.A., Mair W., Vasquez D.S., Joshi A., Gwinn D.M., Taylor R., et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mack H.I., Zheng B., Asara J.M., Thomas S.M. AMPK-dependent phosphorylation of ULK1 regulates ATG9 localization. Autophagy. 2012;8:1197–1214. doi: 10.4161/auto.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheong H., Wu J., Gonzales L.K., Guttentag S.H., Thompson C.B., Lindsten T. Analysis of a lung defect in autophagy-deficient mouse strains. Autophagy. 2014;10:45–56. doi: 10.4161/auto.26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y., He J., Tian M., Zhang S.Y., Guo M.R., Kasimu R., Wang J.H., Ouyang L. UNC51-like kinase 1, autophagic regulator and cancer therapeutic target. Cell Prolif. 2014;47:494–505. doi: 10.1111/cpr.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L., Ouyang L., Guo Y., Zhang J., Liu B. UNC-51-like Kinase 1: From an Autophagic Initiator to Multifunctional Drug Target. J. Med. Chem. 2018;61:6491–6500. doi: 10.1021/acs.jmedchem.7b01684. [DOI] [PubMed] [Google Scholar]
- 44.Kundu M., Lindsten T., Yang C.Y., Wu J., Zhao F., Zhang J., Selak M.A., Ney P.A., Thompson C.B. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008;112:1493–1502. doi: 10.1182/blood-2008-02-137398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B., Maxwell B.A., Joo J.H., Gwon Y., Messing J., Mishra A., Shaw T.I., Ward A.L., Quan H., Sakurada S.M., et al. ULK1 and ULK2 Regulate Stress Granule Disassembly Through Phosphorylation and Activation of VCP/p97. Mol. Cell. 2019;74:742–757. doi: 10.1016/j.molcel.2019.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan E.Y., Kir S., Tooze S.A. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 2007;282:25464–25474. doi: 10.1074/jbc.M703663200. [DOI] [PubMed] [Google Scholar]
- 47.Guha P., Snyder S.H. Noncatalytic functions of IPMK are essential for activation of autophagy and liver regeneration. Autophagy. 2019;15:1473–1474. doi: 10.1080/15548627.2019.1615305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki H., Kaizuka T., Mizushima N., Noda N.N. Structure of the Atg101-Atg13 complex reveals essential roles of Atg101 in autophagy initiation. Nat. Struct. Mol. Biol. 2015;22:572–580. doi: 10.1038/nsmb.3036. [DOI] [PubMed] [Google Scholar]
- 49.Wauson E.M., Zaganjor E., Cobb M.H. Amino acid regulation of autophagy through the GPCR TAS1R1-TAS1R3. Autophagy. 2013;9:418–429. doi: 10.4161/auto.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mercer T.J., Gubas A., Tooze S.A. A molecular perspective of mammalian autophagosome biogenesis. J. Biol. Chem. 2018;293:5386–5395. doi: 10.1074/jbc.R117.810366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamb C.A., Yoshimori T., Tooze S.A. The autophagosome: origins unknown, biogenesis complex. Nat. Rev. Mol. Cell Biol. 2013;14:759–774. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 52.Kang S., Shin K.D., Kim J.H., Chung T. Autophagy-related (ATG) 11, ATG9 and the phosphatidylinositol 3-kinase control ATG2-mediated formation of autophagosomes in Arabidopsis. Plant Cell Rep. 2018;37:653–664. doi: 10.1007/s00299-018-2258-9. [DOI] [PubMed] [Google Scholar]
- 53.Russell R.C., Tian Y., Yuan H., Park H.W., Chang Y.Y., Kim J., Kim H., Neufeld T.P., Dillin A., Guan K.L. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 2013;15:741–750. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pike L.R., Singleton D.C., Buffa F., Abramczyk O., Phadwal K., Li J.L., Simon A.K., Murray J.T., Harris A.L. Transcriptional up-regulation of ULK1 by ATF4 contributes to cancer cell survival. Biochem. J. 2013;449:389–400. doi: 10.1042/BJ20120972. [DOI] [PubMed] [Google Scholar]
- 55.Luhr M., Torgersen M.L., Szalai P., Hashim A., Brech A., Staerk J., Engedal N. The kinase PERK and the transcription factor ATF4 play distinct and essential roles in autophagy resulting from tunicamycin-induced ER stress. J. Biol. Chem. 2019;294:8197–8217. doi: 10.1074/jbc.RA118.002829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grunwald D.S., Otto N.M., Park J.M., Song D., Kim D.H. GABARAPs and LC3s have opposite roles in regulating ULK1 for autophagy induction. Autophagy. 2019 doi: 10.1080/15548627.2019.1632620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki H., Kaizuka T., Mizushima N., Noda N.N. Open and closed HORMAs regulate autophagy initiation. Autophagy. 2015;11:2123–2124. doi: 10.1080/15548627.2015.1091144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shang L., Chen S., Du F., Li S., Zhao L., Wang X. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc. Natl. Acad. Sci. USA. 2011;108:4788–4793. doi: 10.1073/pnas.1100844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wirth M., Joachim J., Tooze S.A. Autophagosome formation--the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin. Cancer biol. 2013;23:301–309. doi: 10.1016/j.semcancer.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Chun Y., Kim J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells. 2018;7:278. doi: 10.3390/cells7120278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo M., Zhao X., Song Y., Cheng H., Zhou R. Nuclear autophagy: An evolutionarily conserved mechanism of nuclear degradation in the cytoplasm. Autophagy. 2016;12:1973–1983. doi: 10.1080/15548627.2016.1217381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xi G., Rosen C.J., Clemmons D.R. IGF-I and IGFBP-2 Stimulate AMPK Activation and Autophagy, Which Are Required for Osteoblast Differentiation. Endocrinology. 2016;157:268–281. doi: 10.1210/en.2015-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crawley O., Opperman K.J., Desbois M., Adrados I., Borgen M.A., Giles A.C., Duckett D.R., Grill B. Autophagy is inhibited by ubiquitin ligase activity in the nervous system. Nat. Commun. 2019;10:501–517. doi: 10.1038/s41467-019-12804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacquin E., Leclerc-Mercier S., Judon C., Blanchard E., Fraitag S., Florey O. Pharmacological modulators of autophagy activate a parallel noncanonical pathway driving unconventional LC3 lipidation. Autophagy. 2017;13:854–867. doi: 10.1080/15548627.2017.1287653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazzari E., Meroni G. TRIM32 ubiquitin E3 ligase, one enzyme for several pathologies: From muscular dystrophy to tumours. Int. J. Biochem. Cell Biol. 2016;79:469–477. doi: 10.1016/j.biocel.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 67.Kudryashova E., Struyk A., Mokhonova E., Cannon S.C., Spencer M.J. The common missense mutation D489N in TRIM32 causing limb girdle muscular dystrophy 2H leads to loss of the mutated protein in knock-in mice resulting in a Trim32-null phenotype. Hum. Mol. Genet. 2011;20:3925–3932. doi: 10.1093/hmg/ddr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Rienzo M., Antonioli M., Fusco C., Liu Y., Mari M., Orhon I., Refolo G., Germani F., Corazzari M., Romagnoli A., et al. Autophagy induction in atrophic muscle cells requires ULK1 activation by TRIM32 through unanchored K63-linked polyubiquitin chains. Sci. Adv. 2019;5:1–15. doi: 10.1126/sciadv.aau8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ivankovic D., Drew J., Lesept F., White I.J., Kittler J.T. Axonal autophagosome maturation defect through failure of ATG9A sorting underpins pathology in AP-4 deficiency syndrome. Autophagy. 2019;55:1–17. doi: 10.1080/15548627.2019.1615302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin S., Dudek-Peric A.M., Garg A.D., Roose H., Demirsoy S., Van Eygen S., Mertens F., Vangheluwe P., Vankelecom H., Agostinis P. An autophagy-driven pathway of ATP secretion supports the aggressive phenotype of BRAFV600E inhibitor-resistant metastatic melanoma cells. Autophagy. 2017;13:1512–1527. doi: 10.1080/15548627.2017.1332550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Levy J.M.M., Towers C.G., Thorburn A. Targeting autophagy in cancer. Nat. Rev. Cancer. 2017;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toit A.D., Loos B., Hofmeyr J.H.S. Supply and Demand Analysis of Autophagy. Methods Mol. Biol. 2020;2088:345–357. doi: 10.1007/978-1-0716-0159-4_16. [DOI] [PubMed] [Google Scholar]
- 73.Pohl C., Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366:818–822. doi: 10.1126/science.aax3769. [DOI] [PubMed] [Google Scholar]
- 74.Mokarram P., Albokashy M., Zarghooni M., Moosavi M.A., Sepehri Z., Chen Q.M., Hudecki A., Sargazi A., Alizadeh J., Moghadam A.R., et al. New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets. Autophagy. 2017;13:781–819. doi: 10.1080/15548627.2017.1290751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giatromanolaki A., Sivridis E., Mitrakas A., Kalamida D., Zois C.E., Haider S., Piperidou C., Pappa A., Gatter K.C., Harris A.L., et al. Autophagy and lysosomal related protein expression patterns in human glioblastoma. Cancer Biol. Ther. 2014;15:1468–1478. doi: 10.4161/15384047.2014.955719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawamata T., Kamada Y., Kabeya Y., Sekito T., Ohsumi Y. Organization of the pre-autophagosomal structure responsible for autophagosome formation. Mol. Biol. Cell. 2008;19:2039–2050. doi: 10.1091/mbc.e07-10-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheong H., Nair U., Geng J., Klionsky D.J. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. Mol. Biol. Cell. 2008;19:668–681. doi: 10.1091/mbc.e07-08-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ungermann C., Reggiori F. Atg9 proteins, not so different after all. Autophagy. 2018;14:1456–1459. doi: 10.1080/15548627.2018.1477382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nishimura T., Mizushima N. The ULK complex initiates autophagosome formation at phosphatidylinositol synthase-enriched ER subdomains. Autophagy. 2017;13:1795–1796. doi: 10.1080/15548627.2017.1358344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang H.W., Hu J.J., Fu R.Q., Liu X., Zhang Y.H., Li J., Liu L., Li Y.N., Deng Q., Luo Q.S., et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kgamma mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-29308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walker S.A., Ktistakis N.T. Autophagosome Biogenesis Machinery. J. Mol. Biol. 2019;6:1–27. doi: 10.1016/j.jmb.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 82.Joachim J., Tooze S.A. GABARAP activates ULK1 and traffics from the centrosome dependent on Golgi partners WAC and GOLGA2/GM130. Autophagy. 2016;12:892–903. doi: 10.1080/15548627.2016.1159368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nazio F., Cecconi F. Autophagy up and down by outsmarting the incredible ULK. Autophagy. 2017;13:967–968. doi: 10.1080/15548627.2017.1285473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin T., Ruan S., Huang D., Meng X., Li W., Wang B., Zou F. MeHg-induced autophagy via JNK/Vps34 complex pathway promotes autophagosome accumulation and neuronal cell death. Cell Death Dis. 2019;10:399–429. doi: 10.1038/s41419-019-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anwar T., Liu X., Suntio T., Marjamaki A., Biazik J., Chan E.Y.W., Varjosalo M., Eskelinen E.L. ER-Targeted Beclin 1 Supports Autophagosome Biogenesis in the Absence of ULK1 and ULK2 Kinases. Cells. 2019;8:475. doi: 10.3390/cells8050475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hara T., Mizushima N. Role of ULK-FIP200 complex in mammalian autophagy: FIP200, a counterpart of yeast Atg17? Autophagy. 2009;5:85–97. doi: 10.4161/auto.5.1.7180. [DOI] [PubMed] [Google Scholar]
- 87.Wang C., Wang H., Zhang D., Luo W., Liu R., Xu D., Diao L., Liao L., Liu Z. Phosphorylation of ULK1 affects autophagosome fusion and links chaperone-mediated autophagy to macroautophagy. Nat. Commun. 2018;9:34–49. doi: 10.1038/s41467-018-05449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kraft C., Kijanska M., Kalie E., Siergiejuk E., Lee S.S., Semplicio G., Stoffel I., Brezovich A., Verma M., Hansmann I., et al. Binding of the Atg1/ULK1 kinase to the ubiquitin-like protein Atg8 regulates autophagy. EMBO J. 2012;31:3691–3703. doi: 10.1038/emboj.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McAlpine F., Williamson L.E., Tooze S.A., Chan E.Y. Regulation of nutrient-sensitive autophagy by uncoordinated 51-like kinases 1 and 2. Autophagy. 2013;9:361–373. doi: 10.4161/auto.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allavena G., Boyd C., Oo K.S., Maellaro E., Zhivotovsky B., Kaminskyy V.O. Suppressed translation and ULK1 degradation as potential mechanisms of autophagy limitation under prolonged starvation. Autophagy. 2016;12:2085–2097. doi: 10.1080/15548627.2016.1226733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujioka Y., Suzuki S.W., Yamamoto H., Kondo-Kakuta C., Kimura Y., Hirano H., Akada R., Inagaki F., Ohsumi Y., Noda N.N. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat. Struct. Mol. Biol. 2014;21:513–521. doi: 10.1038/nsmb.2822. [DOI] [PubMed] [Google Scholar]
- 92.Yamamoto H., Fujioka Y., Suzuki S.W., Noshiro D., Suzuki H., Kondo-Kakuta C., Kimura Y., Hirano H., Ando T., Noda N.N., et al. The Intrinsically Disordered Protein Atg13 Mediates Supramolecular Assembly of Autophagy Initiation Complexes. Dev. Cell. 2016;38:86–99. doi: 10.1016/j.devcel.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 93.Joo J.H., Wang B., Frankel E., Ge L., Xu L., Iyengar R., Li-Harms X., Wright C., Shaw T.I., Lindsten T., et al. The Noncanonical Role of ULK/ATG1 in ER-to-Golgi Trafficking Is Essential for Cellular Homeostasis. Mol. Cell. 2016;62:982. doi: 10.1016/j.molcel.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 94.Wang B., Iyengar R., Li-Harms X., Joo J.H., Wright C., Lavado A., Horner L., Yang M., Guan J.L., Frase S., et al. The autophagy-inducing kinases, ULK1 and ULK2, regulate axon guidance in the developing mouse forebrain via a noncanonical pathway. Autophagy. 2018;14:796–811. doi: 10.1080/15548627.2017.1386820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balke D., Tatenhorst L., Dambeck V., Ribas V.T., Vahsen B.F., Michel U., Bahr M., Lingor P. AAV-Mediated Expression of Dominant-Negative ULK1 Increases Neuronal Survival and Enhances Motor Performance in the MPTP Mouse Model of Parkinson’s Disease. Mole. Neurobiol. 2019;3:1–25. doi: 10.1007/s12035-019-01744-0. [DOI] [PubMed] [Google Scholar]
- 96.Pla A., Pascual M., Guerri C. Autophagy Constitutes a Protective Mechanism against Ethanol Toxicity in Mouse Astrocytes and Neurons. PLoS ONE. 2016;11:3015–3037. doi: 10.1371/journal.pone.0153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sandoval H., Thiagarajan P., Dasgupta S.K., Schumacher A., Prchal J.T., Chen M., Wang J. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–245. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan F., Jin X., Li D., Song Y., Zhang N., Yang X., Wang L., Zhu W.G., Tian C., Zhao Y. ULK1 phosphorylates Mad1 to regulate spindle assembly checkpoint. Nucleic Acids Res. 2019;47:8096–8110. doi: 10.1093/nar/gkz602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meske V., Erz J., Priesnitz T., Ohm T.G. The autophagic defect in Niemann-Pick disease type C neurons differs from somatic cells and reduces neuronal viability. Neurobiol. Dis. 2014;64:88–97. doi: 10.1016/j.nbd.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 100.Joo J.H., Wang B., Frankel E., Ge L., Xu L., Iyengar R., Li-Harms X., Wright C., Shaw T.I., Lindsten T., et al. The Noncanonical Role of ULK/ATG1 in ER-to-Golgi Trafficking Is Essential for Cellular Homeostasis. Mol. Cell. 2016;62:491–506. doi: 10.1016/j.molcel.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gulbins A., Schumacher F., Becker K.A., Wilker B., Soddemann M., Boldrin F., Muller C.P., Edwards M.J., Goodman M., Caldwell C.C., et al. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Mol. Psychiatry. 2018;23:2324–2346. doi: 10.1038/s41380-018-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hawkins S.J., Crompton L.A., Sood A., Saunders M., Boyle N.T., Buckley A., Minogue A.M., McComish S.F., Jimenez-Moreno N., Cordero-Llana O., et al. Nanoparticle-induced neuronal toxicity across placental barriers is mediated by autophagy and dependent on astrocytes. Nat. Nanotechnol. 2018;13:427–433. doi: 10.1038/s41565-018-0085-3. [DOI] [PubMed] [Google Scholar]
- 103.Wei H.L., Ma S.Q., Li C.X. Deficiency of unc-51 like kinase 1 (Ulk1) protects against mice traumatic brain injury (TBI) by suppression of p38 and JNK pathway. Biochem. Biophys. Res. Commun. 2018;503:467–473. doi: 10.1016/j.bbrc.2018.04.154. [DOI] [PubMed] [Google Scholar]
- 104.Tsuboi D., Hikita T., Qadota H., Amano M., Kaibuchi K. Regulatory machinery of UNC-33 Ce-CRMP localization in neurites during neuronal development in Caenorhabditis elegans. J. Neurochem. 2005;95:1629–1641. doi: 10.1111/j.1471-4159.2005.03490.x. [DOI] [PubMed] [Google Scholar]
- 105.Fan Y., Wang N., Rocchi A., Zhang W., Vassar R., Zhou Y., He C. Identification of natural products with neuronal and metabolic benefits through autophagy induction. Autophagy. 2017;13:41–56. doi: 10.1080/15548627.2016.1240855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou X., Babu J.R., da Silva S., Shu Q., Graef I.A., Oliver T., Tomoda T., Tani T., Wooten M.W., Wang F. Unc-51-like kinase 1/2-mediated endocytic processes regulate filopodia extension and branching of sensory axons. Proc. Natl. Acad. Sci. USA. 2007;104:5842–5847. doi: 10.1073/pnas.0701402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dube K., Dhanabalan K., Salie R., Blignaut M., Huisamen B., Lochner A. Melatonin has profound effects on mitochondrial dynamics in myocardial ischaemia/reperfusion. Heliyon. 2019;5:642–659. doi: 10.1016/j.heliyon.2019.e02659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stefely J.A., Zhang Y., Freiberger E.C., Kwiecien N.W., Thomas H.E., Davis A.M., Lowry N.D., Vincent C.E., Shishkova E., Clark N.A., et al. Mass spectrometry proteomics reveals a function for mammalian CALCOCO1 in MTOR-regulated selective autophagy. Autophagy. 2020 doi: 10.1080/15548627.2020.1719746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dower C.M., Wills C.A., Frisch S.M., Wang H.-G. Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy. 2018;14:1110–1128. doi: 10.1080/15548627.2018.1450020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ashrafizadeh M., Ahmadi Z., Farkhondeh T., Samarghandian S. Autophagy as a molecular target of quercetin underlying its protective effects in human diseases. Arch. Physiol. Biochem. 2019 doi: 10.1080/13813455.2019.1671458. [DOI] [PubMed] [Google Scholar]
- 111.Michalcova K., Roychoudhury S., Halenar M., Tvrda E., Kovacikova E., Vasicek J., Chrenek P., Baldovska S., Sanislo L., Kren V., et al. In vitro response of human ovarian cancer cells to dietary bioflavonoid isoquercitrin. J. Environ. Sci. Health B. 2019;54:752–757. doi: 10.1080/03601234.2019.1633214. [DOI] [PubMed] [Google Scholar]
- 112.An M., Ryu D.R., Won Park J., Ha Choi J., Park E.M., Eun Lee K., Woo M., Kim M. ULK1 prevents cardiac dysfunction in obesity through autophagy-meditated regulation of lipid metabolism. Cardiovasc. Res. 2017;113:1137–1147. doi: 10.1093/cvr/cvx064. [DOI] [PubMed] [Google Scholar]
- 113.El-Shazly A.A.A., Sallam A.M., El-Hefnawy M.H., El-Mesallamy H.O. Epidermal growth factor receptor and podocin predict nephropathy progression in type 2 diabetic patients through interaction with the autophagy influencer ULK-1. J. Diabetes Complicat. 2019;33:128–133. doi: 10.1016/j.jdiacomp.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Radhi O.A., Davidson S., Scott F., Zeng R.X., Jones D.H., Tomkinson N.C.O., Yu J., Chan E.Y.W. Inhibition of the ULK1 protein complex suppresses Staphylococcus-induced autophagy and cell death. J. Biol. Chem. 2019;294:14289–14307. doi: 10.1074/jbc.RA119.008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Das C.K., Banerjee I., Mandal M. Pro-survival autophagy: An emerging candidate of tumor progression through maintaining hallmarks of cancer. Semin. Cancer Biol. 2019;82:1–25. doi: 10.1016/j.semcancer.2019.08.020. [DOI] [PubMed] [Google Scholar]
- 116.Newton P.T. New insights into niclosamide action: Autophagy activation in colorectal cancer. Biochem. J. 2019;476:779–781. doi: 10.1042/BCJ20190020. [DOI] [PubMed] [Google Scholar]
- 117.Amaravadi R., Kimmelman A.C., White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–1930. doi: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vera-Ramirez L., Vodnala S.K., Nini R., Hunter K.W., Green J.E. Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nat. Commun. 2018;9:19–44. doi: 10.1038/s41467-018-04070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hellmann M.D., Paz-Ares L., Bernabe Caro R., Zurawski B., Kim S.W., Carcereny Costa E., Park K., Alexandru A., Lupinacci L., de la Mora Jimenez E., et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 120.Hou J., Rao M., Zheng W., Fan J., Law B.Y.K. Advances on Cell Autophagy and Its Potential Regulatory Factors in Renal Ischemia-Reperfusion Injury. DNA Cell Biol. 2019;38:895–904. doi: 10.1089/dna.2019.4767. [DOI] [PubMed] [Google Scholar]
- 121.Colecchia D., Dapporto F., Tronnolone S., Salvini L., Chiariello M. MAPK15 is part of the ULK complex and controls its activity to regulate early phases of the autophagic process. J. Biol. Chem. 2018;293:15962–15976. doi: 10.1074/jbc.RA118.002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ravanan P., Srikumar I.F., Talwar P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017;188:53–67. doi: 10.1016/j.lfs.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 123.Picca A., Lezza A.M.S., Leeuwenburgh C., Pesce V., Calvani R., Landi F., Bernabei R., Marzetti E. Fueling Inflamm-Aging through Mitochondrial Dysfunction: Mechanisms and Molecular Targets. Int. J. Mol. Sci. 2017;18:933. doi: 10.3390/ijms18050933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen P., Cescon M., Bonaldo P. Autophagy-mediated regulation of macrophages and its applications for cancer. Autophagy. 2014;10:192–200. doi: 10.4161/auto.26927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Antunes F., Erustes A.G., Costa A.J., Nascimento A.C., Bincoletto C., Ureshino R.P., Pereira G.J.S., Smaili S.S. Autophagy and intermittent fasting: the connection for cancer therapy? Clinics. 2018;73:181–196. doi: 10.6061/clinics/2018/e814s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Niekerk G., Hattingh S.M., Engelbrecht A.M. Enhanced Therapeutic Efficacy in Cancer Patients by Short-term Fasting: The Autophagy Connection. Front. Oncol. 2016;6:242–258. doi: 10.3389/fonc.2016.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lazarus M.B., Shokat K.M. Discovery and structure of a new inhibitor scaffold of the autophagy initiating kinase ULK1. Bioorg. Med. Chem. 2015;23:5483–5491. doi: 10.1016/j.bmc.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Petherick K.J., Conway O.J., Mpamhanga C., Osborne S.A., Kamal A., Saxty B., Ganley I.G. Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J. Biol. Chem. 2015;290:11376–11383. doi: 10.1074/jbc.C114.627778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qiu L., Zhou G., Cao S. Targeted inhibition of ULK1 enhances daunorubicin sensitivity in acute myeloid leukemia. Life Sci. 2019;243:117234. doi: 10.1016/j.lfs.2019.117234. [DOI] [PubMed] [Google Scholar]
- 130.Lu J., Zhu L., Zheng L.P., Cui Q., Zhu H.H., Zhao H., Shen Z.J., Dong H.Y., Chen S.S., Wu W.Z., et al. Overexpression of ULK1 Represents a Potential Diagnostic Marker for Clear Cell Renal Carcinoma and the Antitumor Effects of SBI-0206965. EBioMedicine. 2018;34:85–93. doi: 10.1016/j.ebiom.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tang F., Hu P., Yang Z., Xue C., Gong J., Sun S., Shi L., Zhang S., Li Z., Yang C., et al. SBI0206965, a novel inhibitor of Ulk1, suppresses non-small cell lung cancer cell growth by modulating both autophagy and apoptosis pathways. Oncol. Rep. 2017;37:3449–3458. doi: 10.3892/or.2017.5635. [DOI] [PubMed] [Google Scholar]
- 132.Martin K.R., Celano S.L., Solitro A.R., Gunaydin H., Scott M., O’Hagan R.C., Shumway S.D., Fuller P., MacKeigan J.P. A Potent and Selective ULK1 Inhibitor Suppresses Autophagy and Sensitizes Cancer Cells to Nutrient Stress. iScience. 2018;8:74–84. doi: 10.1016/j.isci.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J., Long S., Wang H., Liu N., Zhang C., Zhang L., Zhang Y. Blocking AMPK/ULK1-dependent autophagy promoted apoptosis and suppressed colon cancer growth. Cancer Cell Int. 2019;19:33–46. doi: 10.1186/s12935-019-1054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kian M.M., Mohammadi S., Tavallaei M., Chahardouli B., Nikbakht M. Inhibitory Effects of Arsenic Trioxide and Thalidomide on Angiogenesis and Vascular Endothelial Growth Factor Expression in Leukemia Cells. Asian Pac. J. Cancer Prev. 2018;19:1127–1134. doi: 10.22034/APJCP.2018.19.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Egan D.F., Chun M.G., Vamos M., Zou H., Rong J., Miller C.J., Lou H.J., Raveendra-Panickar D., Yang C.C., Sheffler D.J., et al. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol. Cell. 2015;59:285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang L., Fu L., Zhang S., Zhang J., Zhao Y., Zheng Y., He G., Yang S., Ouyang L., Liu B. Discovery of a small molecule targeting ULK1-modulated cell death of triple negative breast cancer in vitro and in vivo. Chem. Sci. 2017;8:2687–2701. doi: 10.1039/C6SC05368H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li C., Xu H., Chen X., Chen J., Li X., Qiao G., Tian Y., Yuan R., Su S., Liu X., et al. Aqueous extract of clove inhibits tumor growth by inducing autophagy through AMPK/ULK pathway. Phytother. Res. 2019;33:1794–1804. doi: 10.1002/ptr.6367. [DOI] [PubMed] [Google Scholar]
- 138.Pasquier B. Autophagy inhibitors. Cell. Mol. Life Sci. 2016;73:985–1001. doi: 10.1007/s00018-015-2104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ouyang L., Zhang L., Fu L., Liu B. A small-molecule activator induces ULK1-modulating autophagy-associated cell death in triple negative breast cancer. Autophagy. 2017;13:777–778. doi: 10.1080/15548627.2017.1283470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun D., Zhu L., Zhao Y., Jiang Y., Chen L., Yu Y., Ouyang L. Fluoxetine induces autophagic cell death via eEF2K-AMPK-mTOR-ULK complex axis in triple negative breast cancer. Cell Prolif. 2018;51:1–15. doi: 10.1111/cpr.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y., Zhong J., Bai J., Tong R., An F., Jiao P., He L., Zeng D., Long E., Yan J., et al. The Application of Natural Products in Cancer Therapy by Targeting Apoptosis Pathways. Curr. Drug Metab. 2018;19:739–749. doi: 10.2174/1389200219666180511154722. [DOI] [PubMed] [Google Scholar]
- 142.Byun W.S., Kim S., Shin Y.H., Kim W.K., Oh D.C., Lee S.K. Antitumor Activity of Ohmyungsamycin A through the Regulation of the Skp2-p27 Axis and MCM4 in Human Colorectal Cancer Cells. J. Nat. Prod. 2020;48:1–28. doi: 10.1021/acs.jnatprod.9b00918. [DOI] [PubMed] [Google Scholar]
- 143.Cheng G., Pi Z., Zheng Z., Liu S., Liu Z., Song F. Magnetic nanoparticles-based lactate dehydrogenase microreactor as a drug discovery tool for rapid screening inhibitors from natural products. Talanta. 2020;209:1205–1225. doi: 10.1016/j.talanta.2019.120554. [DOI] [PubMed] [Google Scholar]
- 144.Wei Q., Ye Z., Zhong X., Li L., Wang C., Myers R.E., Palazzo J.P., Fortuna D., Yan A., Waldman S.A., et al. Multiregion whole-exome sequencing of matched primary and metastatic tumors revealed genomic heterogeneity and suggested polyclonal seeding in colorectal cancer metastasis. Ann. Oncol. 2017;28:2135–2141. doi: 10.1093/annonc/mdx278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andrei L., Kasas S., Ochoa Garrido I., Stankovic T., Suarez Korsnes M., Vaclavikova R., Assaraf Y.G., Pesic M. Advanced technological tools to study multidrug resistance in cancer. Drug Resist. Update. 2019;48:1–20. doi: 10.1016/j.drup.2019.100658. [DOI] [PubMed] [Google Scholar]
- 146.Mirzaei S.A., Dinmohammadi F., Alizadeh A., Elahian F. Inflammatory pathway interactions and cancer multidrug resistance regulation. Life Sci. 2019;235:1168–1193. doi: 10.1016/j.lfs.2019.116825. [DOI] [PubMed] [Google Scholar]
- 147.Shehzad A., Ravinayagam V., AlRumaih H., Aljafary M., Almohazey D., Almofty S., Al-Rashid N.A., Al-Suhaimi E.A. Application of Three-dimensional (3D) Tumor Cell Culture Systems and Mechanism of Drug Resistance. Curr. Pharm. Des. 2019;25:3599–3607. doi: 10.2174/1381612825666191014163923. [DOI] [PubMed] [Google Scholar]
- 148.Arozarena I., Wellbrock C. Phenotype plasticity as enabler of melanoma progression and therapy resistance. Nat. Rev. Cancer. 2019;19:377–391. doi: 10.1038/s41568-019-0154-4. [DOI] [PubMed] [Google Scholar]
- 149.Freimund A.E., Beach J.A., Christie E.L., Bowtell D.D.L. Mechanisms of Drug Resistance in High-Grade Serous Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018;32:983–996. doi: 10.1016/j.hoc.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 150.Doebele R.C. Acquired Resistance Is Oncogene and Drug Agnostic. Cancer Cell. 2019;36:347–349. doi: 10.1016/j.ccell.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Boumahdi S., de Sauvage F.J. The great escape: Tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov. 2019;5:1–27. doi: 10.1038/s41573-019-0044-1. [DOI] [PubMed] [Google Scholar]
- 152.Kim I.S., Gao Y., Welte T., Wang H., Liu J., Janghorban M., Sheng K., Niu Y., Goldstein A., Zhao N., et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat. Cell Biol. 2019;21:1113–1126. doi: 10.1038/s41556-019-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Forrest S.J., Geoerger B., Janeway K.A. Precision medicine in pediatric oncology. Curr. Opin. Pediatr. 2018;30:17–24. doi: 10.1097/MOP.0000000000000570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fu J., Fernandez D., Ferrer M., Titus S.A., Buehler E., Lal-Nag M.A. RNAi High-Throughput Screening of Single- and Multi-Cell-Type Tumor Spheroids: A Comprehensive Analysis in Two and Three Dimensions. SLAS Discov. 2017;22:525–536. doi: 10.1177/2472555217696796. [DOI] [PubMed] [Google Scholar]
- 155.Al-Akra L., Bae D.H., Leck L.Y.W., Richardson D.R., Jansson P.J. The biochemical and molecular mechanisms involved in the role of tumor micro-environment stress in development of drug resistance. Biochim. Biophys. Acta Gen. Subj. 2019;1863:1390–1397. doi: 10.1016/j.bbagen.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 156.Xia J., He Y., Meng B., Chen S., Zhang J., Wu X., Zhu Y., Shen Y., Feng X., Guan Y., et al. NEK2 Induces Autophagy-mediated Bortezomib Resistance by Stabilizing Beclin-1 in Multiple Myeloma. Mol. Oncol. 2020;5:1–18. doi: 10.1002/1878-0261.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guo B., Tam A., Santi S.A., Parissenti A.M. Role of autophagy and lysosomal drug sequestration in acquired resistance to doxorubicin in MCF-7 cells. BMC Cancer. 2016;16:762–773. doi: 10.1186/s12885-016-2790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang Y., Jiang C., Yang Y., Guo L., Huang J., Liu X., Wu C., Zou J. Silencing of LncRNA-HOTAIR decreases drug resistance of Non-Small Cell Lung Cancer cells by inactivating autophagy via suppressing the phosphorylation of ULK1. Biochem. Biophys. Res. Commun. 2018;497:1003–1010. doi: 10.1016/j.bbrc.2018.02.141. [DOI] [PubMed] [Google Scholar]
- 159.Lu H., Xiao J., Ke C., Ni X., Xiu R., Tian Q., Pan H., Zou L., Wang F., Ma T., et al. TOPK inhibits autophagy by phosphorylating ULK1 and promotes glioma resistance to TMZ. Cell Death Dis. 2019;10:1–17. doi: 10.1038/s41419-019-1805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seifert M., Catanzaro D., Catanzaro A., Rodwell T.C. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review. PLoS ONE. 2015;10:119–128. doi: 10.1371/journal.pone.0119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou Z., Liu T., Zhang J. Morphine activates blast-phase chronic myeloid leukemia cells and alleviates the effects of tyrosine kinase inhibitors. Biochem. Biophys. Res. Commun. 2019;520:560–565. doi: 10.1016/j.bbrc.2019.10.067. [DOI] [PubMed] [Google Scholar]
- 162.Loscocco F., Visani G., Galimberti S., Curti A., Isidori A. BCR-ABL Independent Mechanisms of Resistance in Chronic Myeloid Leukemia. Front. Oncol. 2019;9:939–956. doi: 10.3389/fonc.2019.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Climent N., Plana M. Immunomodulatory Activity of Tyrosine Kinase Inhibitors to Elicit Cytotoxicity Against Cancer and Viral Infection. Front. Pharmacol. 2019;10:12–32. doi: 10.3389/fphar.2019.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.He W., Hu H. BIIB021, an Hsp90 inhibitor: A promising therapeutic strategy for blood malignancies (Review) Oncol. Rep. 2018;40:3–15. doi: 10.3892/or.2018.6422. [DOI] [PubMed] [Google Scholar]
- 165.Yan L., Zhang W., Zhang B., Xuan C., Wang D. BIIB021: A novel inhibitor to heat shock protein 90-addicted oncology. Tumour Biol. 2017;39:1–5. doi: 10.1177/1010428317698355. [DOI] [PubMed] [Google Scholar]
- 166.Ding Y., Adachi H., Katsuno M., Sahashi K., Kondo N., Iida M., Tohnai G., Nakatsuji H., Sobue G. BIIB021, a synthetic Hsp90 inhibitor, induces mutant ataxin-1 degradation through the activation of heat shock factor 1. Neuroscience. 2016;327:20–31. doi: 10.1016/j.neuroscience.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 167.He W., Ye X., Huang X., Lel W., You L., Wang L., Chen X., Qian W. Hsp90 inhibitor, BIIB021, induces apoptosis and autophagy by regulating mTOR-Ulk1 pathway in imatinib-sensitive and -resistant chronic myeloid leukemia cells. Int. J. Oncol. 2016;48:1710–1720. doi: 10.3892/ijo.2016.3382. [DOI] [PubMed] [Google Scholar]
- 168.Kim S.H., Kang J.G., Kim C.S., Ihm S.H., Choi M.G., Yoo H.J., Lee S.J. Synergistic cytotoxicity of BIIB021 with triptolide through suppression of PI3K/Akt/mTOR and NF-kappaB signal pathways in thyroid carcinoma cells. Biomed. Pharmacother. 2016;83:22–32. doi: 10.1016/j.biopha.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 169.Sanz-Garcia E., Argiles G., Elez E., Tabernero J. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann. Oncol. 2017;28:2648–2657. doi: 10.1093/annonc/mdx401. [DOI] [PubMed] [Google Scholar]
- 170.Cicenas J., Tamosaitis L., Kvederaviciute K., Tarvydas R., Staniute G., Kalyan K., Meskinyte-Kausiliene E., Stankevicius V., Valius M. KRAS, NRAS and BRAF mutations in colorectal cancer and melanoma. Med. Oncol. 2017;34:1–26. doi: 10.1007/s12032-016-0879-9. [DOI] [PubMed] [Google Scholar]
- 171.Kakadia S., Yarlagadda N., Awad R., Kundranda M., Niu J., Naraev B., Mina L., Dragovich T., Gimbel M., Mahmoud F. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. OncoTargets Ther. 2018;11:7095–7107. doi: 10.2147/OTT.S182721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sueda T., Sakai D., Kawamoto K., Konno M., Nishida N., Koseki J., Colvin H., Takahashi H., Haraguchi N., Nishimura J., et al. BRAF V600E inhibition stimulates AMP-activated protein kinase-mediated autophagy in colorectal cancer cells. Sci. Rep. 2016;6:1–18. doi: 10.1038/srep18949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Feng D., Qin B., Pal K., Sun L., Dutta S., Dong H., Liu X., Mukhopadhyay D., Huang S., Sinicrope F.A. BRAF(V600E)-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene. 2019;38:6752–6766. doi: 10.1038/s41388-019-0919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rawla P., Barsouk A., Hadjinicolaou A.V., Barsouk A. Immunotherapies and Targeted Therapies in the Treatment of Metastatic Colorectal Cancer. Med. Sci. 2019;7:83. doi: 10.3390/medsci7080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kawada K., Toda K., Sakai Y. Targeting metabolic reprogramming in KRAS-driven cancers. Int. J. Clin. Oncol. 2017;22:651–659. doi: 10.1007/s10147-017-1156-4. [DOI] [PubMed] [Google Scholar]
- 176.Boyle K.A., Van Wickle J., Hill R.B., Marchese A., Kalyanaraman B., Dwinell M.B. Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation. J. Biol. Chem. 2018;293:14891–14904. doi: 10.1074/jbc.RA117.001469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Russo M., Crisafulli G., Sogari A., Reilly N.M., Arena S., Lamba S., Bartolini A., Amodio V., Magri A., Novara L., et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science. 2019;366:1473–1480. doi: 10.1126/science.aav4474. [DOI] [PubMed] [Google Scholar]
- 178.Sharifi M., Jamshidi A., Sarvestani N.N. An Adaptive Robust Control Strategy in a Cancer Tumor-Immune System under Uncertainties. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018 doi: 10.1109/TCBB.2018.2803175. [DOI] [PubMed] [Google Scholar]
- 179.Pinard C., Debled M., Ben Rejeb H., Velasco V., Tunon de Lara C., Hoppe S., Richard E., Brouste V., Bonnefoi H., MacGrogan G. Residual cancer burden index and tumor-infiltrating lymphocyte subtypes in triple-negative breast cancer after neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2019 doi: 10.1007/s10549-019-05437-z. [DOI] [PubMed] [Google Scholar]
- 180.Lin C.M., Yu C.F., Huang H.Y., Chen F.H., Hong J.H., Chiang C.S. Distinct Tumor Microenvironment at Tumor Edge as a Result of Astrocyte Activation Is Associated with Therapeutic Resistance for Brain Tumor. Front. Oncol. 2019;9:307–322. doi: 10.3389/fonc.2019.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Seo J.S., Kim A., Shin J.Y., Kim Y.T. Comprehensive analysis of the tumor immune micro-environment in non-small cell lung cancer for efficacy of checkpoint inhibitor. Sci. Rep. 2018;8:145–176. doi: 10.1038/s41598-018-32855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Crayton S., Tsourkas A. pH-titratable superparamagnetic iron oxide for improved nanoparticle accumulation in acidic tumor microenvironments. ACS Nano. 2011;5:9592–9601. doi: 10.1021/nn202863x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Inaguma S., Lasota J., Felisiak-Golabek A., Kowalik A., Wang Z., Zieba S., Kalisz J., Ikeda H., Miettinen M. Histopathological and genotypic characterization of metastatic colorectal carcinoma with PD-L1 (CD274)-expression: Possible roles of tumour micro environmental factors for CD274 expression. J. Pathol. Clin. Res. 2017;3:268–278. doi: 10.1002/cjp2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bailey K.M., Airik M., Krook M.A., Pedersen E.A., Lawlor E.R. Micro-Environmental Stress Induces Src-Dependent Activation of Invadopodia and Cell Migration in Ewing Sarcoma. Neoplasia. 2016;18:480–498. doi: 10.1016/j.neo.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jain I., Danger J.L., Burgess C., Uppal T., Sumby P. The group A Streptococcus accessory protein RocA: regulatory activity, interacting partners and influence on disease potential. Mol. Microbiol. 2019;68:1–18. doi: 10.1111/mmi.14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yao C., Ni Z., Gong C., Zhu X., Wang L., Xu Z., Zhou C., Li S., Zhou W., Zou C., et al. Rocaglamide enhances NK cell-mediated killing of non-small cell lung cancer cells by inhibiting autophagy. Autophagy. 2018;14:1831–1844. doi: 10.1080/15548627.2018.1489946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wu H., Yu J., Kong D., Xu Y., Zhang Z., Shui J., Li Z., Luo H., Wang K. Population and singlecell transcriptome analyses reveal diverse transcriptional changes associated with radioresistance in esophageal squamous cell carcinoma. Int. J. Oncol. 2019;55:1237–1248. doi: 10.3892/ijo.2019.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dehghanzadeh R., Jadidi-Niaragh F., Gharibi T., Yousefi M. MicroRNA-induced drug resistance in gastric cancer. Biomed. Pharmacother. 2015;74:191–209. doi: 10.1016/j.biopha.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 189.Aghdam A.M., Amiri A., Salarinia R., Masoudifar A., Ghasemi F., Mirzaei H. MicroRNAs as Diagnostic, Prognostic, and Therapeutic Biomarkers in Prostate Cancer. Crit. Rev. Eukaryot. Gene Expr. 2019;29:127–139. doi: 10.1615/CritRevEukaryotGeneExpr.2019025273. [DOI] [PubMed] [Google Scholar]
- 190.Qian K., Li Q., Deng W., Xiang X. Multiple-Scales Integrative Analysis of MicroRNAs Unveils Biomarkers and Key Regulatory Connections for Hepatocellular Carcinoma. Crit. Rev. Eukaryot. Gene Expr. 2019;29:189–241. doi: 10.1615/CritRevEukaryotGeneExpr.2019025931. [DOI] [PubMed] [Google Scholar]
- 191.Donzelli S., Mori F., Biagioni F., Bellissimo T., Pulito C., Muti P., Strano S., Blandino G. MicroRNAs: short non-coding players in cancer chemoresistance. Mol. Cell. Ther. 2014;2:1–16. doi: 10.1186/2052-8426-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Duan F.G., Wang M.F., Cao Y.B., Dan L., Li R.Z., Fan X.X., Khan I., Lai H.L., Zhang Y.Z., Hsiao W.W., et al. MicroRNA-421 confers paclitaxel resistance by binding to the KEAP1 3’UTR and predicts poor survival in non-small cell lung cancer. Cell Death Dis. 2019;10:821–841. doi: 10.1038/s41419-019-2031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ruggeri C., Gioffre S., Achilli F., Colombo G.I., D’Alessandra Y. Role of microRNAs in doxorubicin-induced cardiotoxicity: an overview of preclinical models and cancer patients. Heart Fail. Rev. 2018;23:109–122. doi: 10.1007/s10741-017-9653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Soni M., Patel Y., Markoutsa E., Jie C., Liu S., Xu P., Chen H. Autophagy, Cell Viability, and Chemoresistance Are Regulated By miR-489 in Breast Cancer. Mol. Cancer Res. 2018;16:1348–1360. doi: 10.1158/1541-7786.MCR-17-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Patel Y., Soni M., Awgulewitsch A., Kern M.J., Liu S., Shah N., Singh U.P., Chen H. Overexpression of miR-489 derails mammary hierarchy structure and inhibits HER2/neu-induced tumorigenesis. Oncogene. 2019;38:445–453. doi: 10.1038/s41388-018-0439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jiang L., He D., Yang D., Chen Z., Pan Q., Mao A., Cai Y., Li X., Xing H., Shi M., et al. MiR-489 regulates chemoresistance in breast cancer via epithelial mesenchymal transition pathway. FEBS Lett. 2014;588:2009–2015. doi: 10.1016/j.febslet.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 197.Zhang B., Fang S., Cheng Y., Zhou C., Deng F. The long non-coding RNA, urothelial carcinoma associated 1, promotes cell growth, invasion, migration, and chemo-resistance in glioma through Wnt/beta-catenin signaling pathway. Aging. 2019;11:8239–8253. doi: 10.18632/aging.102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lin Y., Liu J., Huang Y., Liu D., Zhang G., Kan H. microRNA-489 Plays an Anti-Metastatic Role in Human Hepatocellular Carcinoma by Targeting Matrix Metalloproteinase-7. Transl. Oncol. 2017;10:211–220. doi: 10.1016/j.tranon.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McGeary S.E., Lin K.S., Shi C.Y., Pham T.M., Bisaria N., Kelley G.M., Bartel D.P. The biochemical basis of microRNA targeting efficacy. Science. 2019;366:1–15. doi: 10.1126/science.aav1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li J., Qu W., Jiang Y., Sun Y., Cheng Y., Zou T., Du S. miR-489 Suppresses Proliferation and Invasion of Human Bladder Cancer Cells. Oncol. Res. 2016;24:391–398. doi: 10.3727/096504016X14666990347518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hu X., Jiang J., Xu Q., Ni C., Yang L., Huang D. A Systematic Review of Long Noncoding RNAs in Hepatocellular Carcinoma: Molecular Mechanism and Clinical Implications. BioMed Res. Int. 2018;2018:8126–8148. doi: 10.1155/2018/8126208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Deng G., Sui G. Noncoding RNA in oncogenesis: A new era of identifying key players. Int. J. Mol. Sci. 2013;14:18319–18349. doi: 10.3390/ijms140918319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rui L., Guodong X., Meng W., Xiang L., Yuan L., ZengQian H., Xin S., Sida Q., Boxiang Z., Ning D. SNHG6 functions as a competing endogenous RNA to regulate E2F7 expression by sponging miR-26a-5p in lung adenocarcinoma. Biomed. Pharmacother. 2018;107:1434–1446. doi: 10.1016/j.biopha.2018.08.099. [DOI] [PubMed] [Google Scholar]
- 205.Wang X., He J., Lai Q., Yao X., Li Q., Liu Y., Lai H., Gu C., Yan Q., Fang Y., et al. LncRNA SNHG6 promotes chemoresistance through ULK1-induced autophagy by sponging miR-26a-5p in colorectal cancer cells. Cancer Cell Int. 2019;19:999–1012. doi: 10.1186/s12935-019-0951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang F., Cao M., Fan M., Wu H., Huang W., Zhang Y., Hu Z., Jin X. AMPK-mTOR-ULK1 axis activation-dependent autophagy promotes hydroxycamptothecin-induced apoptosis in human bladder cancer cells. J. Cell. Physiol. 2019;235:4302–4315. doi: 10.1002/jcp.29307. [DOI] [PubMed] [Google Scholar]
- 207.Li X., Zhou Y., Li Y., Yang L., Ma Y., Peng X., Yang S., Liu J., Li H. Autophagy: A novel mechanism of chemoresistance in cancers. Biomed. Pharmacother. 2019;119:1094–1115. doi: 10.1016/j.biopha.2019.109415. [DOI] [PubMed] [Google Scholar]
- 208.Ouyang L., Zhang L., Zhang S., Yao D., Zhao Y., Wang G., Fu L., Lei P., Liu B. Small-Molecule Activator of UNC-51-Like Kinase 1 (ULK1) That Induces Cytoprotective Autophagy for Parkinson’s Disease Treatment. J. Med. Chem. 2018;61:2776–2792. doi: 10.1021/acs.jmedchem.7b01575. [DOI] [PubMed] [Google Scholar]
- 209.Zhao Z., Li J., Jiang Y., Xu W., Li X., Jing W. CLDN1 Increases Drug Resistance of Non-Small Cell Lung Cancer by Activating Autophagy via Up-Regulation of ULK1 Phosphorylation. Med. Sci. Monit. 2017;23:2906–2916. doi: 10.12659/MSM.904177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Qian S., Tong S., Wu J., Tian L., Qi Z., Chen B., Zhu D., Zhang Y. Paris saponin VII extracted from Trillium tschonoskii induces autophagy and apoptosis in NSCLC cells. J. Ethnopharmacol. 2020;248:1–25. doi: 10.1016/j.jep.2019.112304. [DOI] [PubMed] [Google Scholar]
- 211.Jang E.J., Kim S.C., Lee J.H., Lee J.R., Kim I.K., Baek S.Y., Kim Y.W. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress. BMC Complement. Altern. Med. 2018;18:97–116. doi: 10.1186/s12906-018-2164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Emanuele S., Notaro A., Palumbo Piccionello A., Maggio A., Lauricella M., D’Anneo A., Cernigliaro C., Calvaruso G., Giuliano M. Sicilian Litchi Fruit Extracts Induce Autophagy versus Apoptosis Switch in Human Colon Cancer Cells. Nutrients. 2018;10:1490. doi: 10.3390/nu10101490. [DOI] [PMC free article] [PubMed] [Google Scholar]