Abstract
The major cat allergen Fel d 1 is a tetrameric glycoprotein of the secretoglobin superfamily. Structural aspects and allergenic properties of this protein have been investigated, but its physiological function remains unclear. Fel d 1 is assumed to bind lipids and steroids like the mouse androgen-binding protein, which is involved in chemical communication, either as a semiochemical carrier or a semiochemical itself. This study focused on the binding activity of a recombinant model of Fel d 1 (rFel d 1) towards semiochemical analogs, i.e., fatty acids and steroids, using both in silico calculations and fluorescence measurements. In silico analyses were first adopted to model the interactions of potential ligands, which were then tested in binding assays using the fluorescent reporter N-phenyl-1-naphthylamine. Good ligands were fatty acids, such as the lauric, oleic, linoleic, and myristic fatty acids, as well as steroids like androstenone, pregnenolone, and progesterone, that were predicted by in silico molecular models to bind into the central and surface cavities of rFel d 1, respectively. The lowest dissociation constants were shown by lauric acid (2.6 µM) and androstenone (2.4 µM). The specific affinity of rFel d 1 to semiochemicals supports a function of the protein in cat’s chemical communication, and highlights a putative role of secretoglobins in protein semiochemistry.
Keywords: secretoglobin, odorant-binding protein, chemical communication, pheromone, N-phenyl-1-naphthylamine, in silico docking, molecular modeling, protein–ligand interactions, 2D interaction maps, ligand-binding assays
1. Introduction
The major cat allergen Fel d 1 is a secreted globular protein belonging to the family of secretoglobins. It is produced in large amounts in various anatomical areas of cats, such as the salivary, lacrimal, and sebaceous glands from the facial area, skin, and anal sacs [1,2,3,4]. The secretion of Fel d 1 is under androgen control [5]. Fel d 1 is a 35–38 kDa tetrameric glycoprotein composed of two heterodimers with a dimerization interface. Each heterodimer consists of two polypeptide chains encoded by independent genes and linked by three disulfide bridges. Chain 1 is made of 70 residues, and chain 2 of 90 or 92 residues [4,6,7]. Chain 2 contains an N-linked oligosaccharide composed of triantennary glycans [8].
Structural and immunological Fel d 1 polymorphisms have long been described in samples from various origins [8,9,10]. Fel d 1 is a resistant protein easily airborne that is abundantly found in different indoor environments [11,12]. Despite its high abundance and the serious health issues associated with this protein, the biological function of Fel d 1 remains unclear [4].
For other members of the secretoglobin family, different biological roles have been suggested, mainly related to immunoregulation [13,14,15,16], but also in chemical signaling [17,18,19]. Also for Fel d 1, a role in intra-species chemical communication has been proposed based on the fact that the protein is produced in the same areas known to release the cat semiochemicals, including the facial area, the podial complex, and the perianal zone, which contain glands that secrete chemical cues involved in cat territorial marking and/or social communication [20,21]. Besides, Fel d 1 immunological features have been linked to cat sex and behavior [22]. From a structural perspective, Fel d 1 also displays interesting features regarding ligand binding capabilities due to the presence of two internal cavities [23]. Structural similarities between Fel d 1 and another secretoglobin involved in mice mate selection and communication, the mouse salivary androgen-binding protein (ABP) [18], have been previously described [24,25]. Binding of some steroids to members of the secretoglobin family was previously reported, involving interactions with their central hydrophobic cavity [19,23,26]. In particular, a recent paper extensively describes the evolutionary divergence, functional sites, and surface structural resemblance between Fel d 1 and ABP, suggesting that the first protein could be involved in semiochemical transport/processing in intra-species communication [25]. However, so far, no experimental evidence has been provided on the capability of Fel d 1 to bind semiochemicals.
Production of recombinant Fel d 1 (rFel d 1) has been challenging in the past since the two chains are encoded by two different genes, and attempts to refold them in a correct (i.e., with retained disulfide formations) and stable way failed [4,27]. Hence, some authors proposed a rFel d 1 construct made of chain 1 linked to chain 2 via a flexible peptide linker of the (GGGGS)n type [28], which minimizes the steric hindrance between the two fusion partners, since the small size of these amino acids provides flexibility, and allows for mobility of the connecting functional domains [29]. The rFel d 1 displayed similar biological and structural properties (notably the disulfide pairing) to its natural counterparts [8,28].
In the current study, we have investigated the binding properties of this recombinant form of Fel d 1 produced in a Pichia pastoris clone with the N-glycosylation site N103 mutated and commercially available (INDOOR Biotechnologies) [30]. As a first step to verify the hypothesis of a role of Fel d 1 in chemical communication, we focused on putative ligands that had already been described as semiochemicals in the domestic cat [21,31], i.e., some fatty acids and their derivatives found in the composition of the feline facial pheromone F3 and the maternal cat appeasing pheromone. The feline facial pheromone F3 has been shown to promote calmness and reduce stress with its related undesirable consequences in cats, such as urine spraying and marking behavior [32,33,34,35]. The maternal cat appeasing pheromone has been shown to have appeasing effects and to facilitate social interactions in cats [36,37]. We have also tested some steroids since several secretoglobins have been experimentally shown to bind steroid hormones, including pig pheromaxein, rabbit uteroglobin, mouse salivary ABP, and rat prostatein [19,38,39,40]. To determine the affinity of these putative ligands and structurally characterize their interactions with rFel d 1, we used a double approach combining in silico analysis (molecular docking) with in vitro fluorescence binding assays.
2. Results
2.1. In Silico Molecular Docking of Fel d 1 with Putative Ligands
As a first approach to evaluate the binding properties of rFel d 1, we performed docking simulations of flexible ligands into the binding pocket of a rigid binding protein, represented as a grid box [41]. The collected data are reported in Table 1.
Table 1.
In silico study of putative molecular residual interactions between recombinant Fel d 1 (rFel d 1) and the compounds and assessment of their capabilities to displace N-phenyl-1-naphthylamine (1-NPN).
| n° | Compound Names | Estimated Free Energy of Binding (kcal/mol) | Total Intermolecular Energy (kcal/mol) | Frequency | H-Bond Residue | Hydrophobic Residue (Alkyl/Pi-Alkyl/Pi-Sigma) | In Silico Screening a | 1-NPN Displacement Screening |
|---|---|---|---|---|---|---|---|---|
| Fatty Acids and Other Derivatives | ||||||||
| 1 | Isobutyric acid | −2.96 | −3.26 | 27% | A88, Y119, L129 | No | ND | |
| 2 | Capric Acid | −5.16 | −7.4 | 23% | L61, F80, V83, F84 | No | No | |
| 3 | Lauric Acid | −5.84 | −8.58 | 60% | Y119 | L61, F80, V83, F84 | Yes | Yes |
| 4 | Myristic Acid | −3.35 | −7.02 | 36% | F84 | F13, V133, M134, I137 | Yes | Yes |
| 5 | Palmitic Acid | −2.33 | −5.88 | 16% | F84 | V133, M134 | Yes | Yes |
| 6 | Oleic Acid | −2.82 | −7.05 | 50% | M134 | I64, F80, V83 | Yes | Yes |
| 7 | Linoleic Acid | −2.95 | −6.88 | 40% | P78 | A88, Y119, L129 | Yes | Yes |
| 8 | Dodecanal | −4.88 | −7.42 | 2% | F84, M134 | No | No | |
| 9 | Dodecanol | −3.93 | −7.02 | 2% | F84, V133, M134 | No | No | |
| 10 | Tetradecanol | −3.97 | −7.89 | 6% | P78, Y81 | No | No | |
| 11 | Ethyl Laurate | −4.7 | −8.02 | 12% | F84 | L61, I64, F80, V83, V133, M134, I137 | Yes | No |
| 12 | Methyl Palmitate | −2.53 | −6.67 | 20% | T76 | Yes | No | |
| 13 | Nonanamide | −4.53 | −6.51 | 4% | L61, I64, V83, F80 | No | ND | |
| 14 | Hexadecanamide | −2.84 | −6.3 | 18% | T135 | Y81 | Yes | ND |
| 15 | Octadecanamide | −2.81 | −6.96 | 6% | G131 | Y81, F85 | Yes | ND |
| Steroids | ||||||||
| 1 | Androstenone | −5.84 | −5.84 | 65% | S138 | P78, Y81, F85 | Yes | Yes |
| 2 | Androstenedione | −5.83 | −5.83 | 44% | Y81, F85 | Yes | Yes | |
| 3 | Androstenol | −5.06 | −5.36 | 22% | Y81, F85 | No | No | |
| 4 | Progesterone | −5.74 | −6.04 | 62% | T76 | Y81, F85 | Yes | Yes |
| 5 | Hydroxyprogesterone | −5.14 | −5.54 | 39% | Y81 | F85 | Yes | Yes |
| 6 | Pregnenolone | −5.59 | −6.17 | 58% | T76 | Y81, F85 | Yes | Yes |
| 7 | Estradiol | −4.94 | −5.54 | 26% | T76 | Y81, F85 | Yes | Yes |
| 8 | Testosterone | −5.6 | −5.9 | 35% | T76 | Y81, F85 | Yes | Yes |
| 9 | Dihydrotestosterone | −5.06 | −5.35 | 12% | Y81, F85 | No | No | |
| 10 | Estrone | −3.56 | −3.86 | 10% | D82, G131 | F85 | Yes | Yes |
| 11 | Dehydroepiandrosterone (DHEA) | −4.64 | −4.94 | 30% | Y81, F85 | Yes | Yes | |
| 12 | Corticosterone | −5.35 | −6.38 | 30% | T76, N89 | Y81, F85 | Yes | No |
| 13 | Deoxycorticosterone | −4.99 | −5.29 | 12% | Y81, F85 | No | No | |
| Fluorescent Probe | ||||||||
| 1 | 1-NPN (Central) | −6.7 | −7.41 | 50% | Y119 | L14, L61, M112 | Yes | / |
| 2 | 1-NPN (Surface) | −4.74 | −5.45 | 30% | Y81, F85 | Yes | / | |
ND: Not determined because of the fluorescence increase, probably due to non-specific hydrophobic interactions [42]. a The in silico screenings were considered to result in positive outcomes (“yes”) if the following were predicted: (1) minimum one H-bond interaction irrespective of the binding frequency or (2) ≥30% of binding frequency without H-bond. This threshold value of binding frequency (≥30%) was selected from the minimum binding frequency of the fluorescent probe (1-NPN) with rFel d 1.
Among the 15 fatty acids tested with rFel d 1, lauric, myristic, oleic, and linoleic acids were the best ligands based on their H-bond interactions, docking energy values, and binding frequency. In particular, lauric acid showed the highest frequency of binding with a free energy of −5.84 kcal/mol. The same compound also exhibited the lowest total intermolecular energy of −8.58 kcal/mol. Myristic, linoleic, and oleic acids were moderate ligands with free binding energies of −3.35, −2.95, and −2.82 kcal/mol, respectively. Furthermore, we observed non-bonded interactions (van der Waals and electrostatic), and pi-interactions with all the fatty acids tested.
The second series of chemicals tested includes several steroids. Among these, androstenone showed the maximum frequency of binding as well as the best free binding energy (−5.84 kcal/mol) with one H-bond interaction (S138) in rFel d 1. The behavior of androstenedione was very similar, with a binding energy of −5.83 kcal/mol, but this ligand exhibited a lower frequency of binding without H-bond interaction. On the other hand, progesterone and pregnenolone showed approximately 60% of the binding frequency, with binding energies comparable to those of androstenone and androstenedione. Pregnenolone and progesterone exhibited similar H-bond interactions (Thr76) but different from those of androstenone (S138). Furthermore, Tyr81 and Phe85 were often present as alkyl/pi-alkyl interactions in the steroid compounds.
Finally, our docking simulation predicted high binding activity of the fluorescent probe N-phenyl-1-naphthylamine (1-NPN) in the same range as those for fatty acids and steroids. Specifically, this compound has two potential binding localizations, i.e., in the central and in the surface binding cavities of rFel d 1. Conversely, some fatty acids and structurally related compounds (long-chain alcohols, aldehydes, ester, and amides), as well as few steroids, did not qualify as good ligands in docking simulations and fluorescent probe displacement (Table 1).
Overall, in silico screening indicated as the best potential ligands for the protein some fatty acids and steroids, which were further tested in fluorescence competitive binding assays.
2.2. Fluorescence Binding Studies
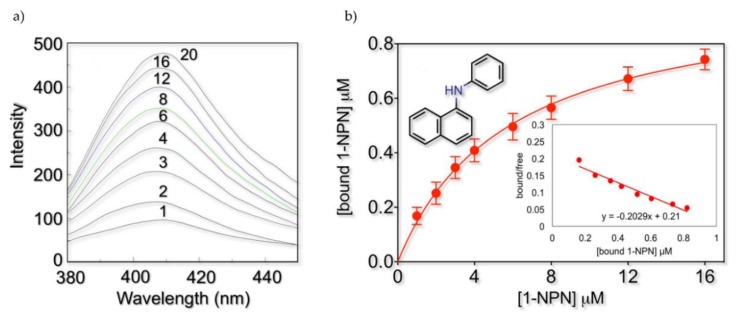
The rFel d 1 binds the fluorescent probe 1-NPN, producing a blueshift in the emission spectrum. Similarly to odorant-binding proteins (OBPs) and chemosensory proteins (CSPs) [42], the emission maximum occurs at 407 nm and is accompanied by a strong increase in fluorescence intensity. Figure 1 reports the actual emission spectra obtained with a rFel d 1 concentration of 1 µM and the relative binding curve obtained after processing the data with the GraphPad Software, Inc., giving a dissociation constant of 5.8 µM. Scatchard analysis confirmed the presence of a single binding site on the protein without any cooperativity effect and yielded a dissociation constant K1-NPN value of 4.8 µM. We also tested other fluorescent probes (2-NPN, 1-AMA (1-aminoanthracene), 1,8-ANS (8-anilinonaphtalene sulfonic acid), but none proved to perform better than 1-NPN (data not shown).
Figure 1.
1-NPN binding to rFel d 1. To a 1-µM solution of the protein in 50 mM Tris-HCl, pH 7.4, aliquots of 1 mM solution of 1-NPN in methanol were added to final concentrations of 1–20 µM. (a) The representative emission curves experimentally obtained. No significant fluorescence emission was recorded in the same conditions with the protein alone (not shown). (b) The saturation binding curve obtained from the average of three experiments. Data were analyzed with GraphPad software and gave a value of 5.8 µM for the binding constant (SD 0.62). The relative Scatchard plot (inset) shows a linear behavior, apparently indicating the presence of a single binding site without cooperativity effects.
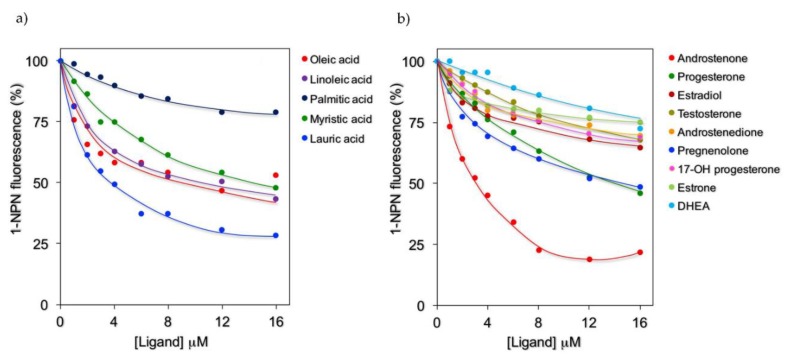
Among the 28 putative ligands, 5 fatty acids and 9 steroids were predicted to possibly interact with rFel d 1 based on the initial 1-NPN displacement screening (Table 1). These compounds were therefore tested in competitive binding experiments with 1-NPN and their displacement curves are reported in Figure 2. Table 2 lists the IC50 values for the best ligands, together with their dissociation constants. These were calculated using the value for 1-NPN (KD 5.8 µM; SD 0.62), obtained with GraphPad software, more reliable than that evaluated from the Scatchard plot. Among the fatty acids, lauric acid exhibited the best affinity to rFel d 1 (Kd = 2.6 µM), while oleic, linoleic, and myristic acids displayed only moderate to low affinities, and palmitic acid proved to be the weakest ligand. Among the steroids, the strongest ligand was androstenone (Kd = 2.4 µM), followed by progesterone and pregnenolone. These results are in agreement with the in silico docking predictions.
Figure 2.
Competitive binding of selected fatty acids (a) and steroids (b) to rFel d 1. Fluorescence emission spectra were recorded at 25 °C in the presence of 1 µM of rFel d 1 and 2 μM of 1-NPN; excitation and emission wavelengths were 337 and 407 nm, respectively. Fluorescence of probe-protein complexes in the absence of a competitor was normalized to 100%.
Table 2.
Affinities of different ligands to rFel d 1, evaluated in competitive binding assays.
| Ligand | (IC50) (µM) | Kd (µM) |
|---|---|---|
| Lauric acid | 3.3 | 2.6 |
| Oleic acid | 10.0 | 7.7 |
| Linoleic acid | 10.1 | 7.8 |
| Myristic acid | 14.4 | 11.1 |
| Androstenone | 3.1 | 2.4 |
| Pregnenolone | 13.1 | 10.1 |
| Progesterone | 13.6 | 10.5 |
2.3. Visualization of Molecular Interactions
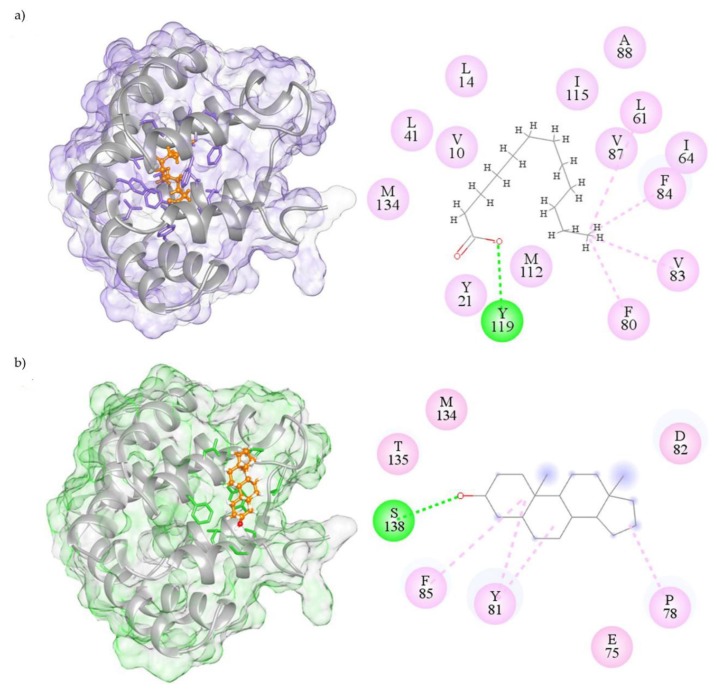
To visualize the possible binding modes of the best ligands to rFel d 1, molecular models and 2D molecular interaction maps were built and are shown in Figure 3. Lauric acid is predicted to bind in the central hydrophobic cavity of Fel d 1, where the strongest H-bond interaction occurs between the phenolic hydrogen of Tyr119 and the oxygen of lauric acid (Figure 3a). Androstenone, instead, is predicted to bind on the surface binding cavity of Fel d 1 and shows an H-bond between the Ser138 OH and the carbonyl group of the ligand (Figure 3b).
Figure 3.
Molecular residue interactions of Fel d 1 with the best ligands, lauric acid (a) and androstenone (b). The interactions are shown in molecular ligand binding view (surface mesh) with a 2D-interaction map of the selective best-fitting ligands to the central and surface binding cavities of Fel d 1. The 2D map reports H-bond interactions in green color and hydrophobic interactions (van der Waals and alkyl/pi-alkyl) in pink color. All the amino acid residue interactions within 4 Å from the ligand are shown.
3. Discussion
On the basis of ligand-binding experiments, using the displacement of a fluorescent probe, and in silico docking simulations, we have shown that a recombinant form of Fel d 1 binds with good affinities some fatty acids and steroids, the best ligands being lauric acid and androstenone (Kd = 2.6 and 2.4 µM, respectively). Lauric acid is a component of the mixture of fatty acids described as the cat appeasing pheromone having effects on cats’ social interactions [36,37], together with oleic, linoleic, and myristic acids, which also showed some affinity to rFel d 1. Androstenone is a volatile steroid pheromone found in high concentrations in the saliva of male pigs and triggers attraction/standing responses in estrous females [43]. Interestingly, some authors have also characterized the binding of isoforms from both native and recombinant pig OBP to fatty acids with appeasing effects and some steroids, indicating the biological relevance of these ligands in chemical communication [44,45]. Although the data here presented were obtained with a recombinant form of Fel d 1, they still support a role of this protein in the cat’s chemical communication, probably as a semiochemicals carrier, similar in its function to OBPs [46].
From a structural perspective, molecular docking suggests that, among good ligands, fatty acids, except for linoleic acid, bind in the internal/central cavity of rFel d 1, while steroids bind in the cavity at the surface of the protein. 1-NPN, however, is predicted to fit into both cavities. This last observation could explain how both fatty acids and steroids can displace the fluorescent probe. The same fact might also account for the observation that lauric acid and androstenone, the two best ligands, fail to completely quench 1-NPN fluorescence, showing asymptotic behavior at concentrations much higher than zero. The same phenomenon might occur with other ligands but would not be clearly visible due to their much lower affinities. The presence of two binding sites for 1-NPN might contrast with the linear Scatchard plot. However, if the two sites present similar affinities for 1-NPN and there is no cooperativity effect, the Scatchard analysis would still produce a linear behavior. Incidentally, it is worth noting that, to the best of our knowledge, this is the first report of using a hydrophobic fluorescent probe (1-NPN) to monitor the binding activity of a secretoglobin family member. This probe, therefore, represents a useful tool for monitoring ligand binding properties with other proteins of the family and investigating their putative involvement in chemical signaling [47].
Looking more closely at the residual interactions, the in silico predictions revealed that fatty acids would mainly interact with the hydrophobic residues Val10, Phe13, Leu14, Tyr21, Phe80, Phe84, Val87, Met112, Tyr119, Asp130, and Met134. In the same way, the amino acids Glu75, Thr76, Pro78, Tyr81, Asp82, Phe85, Gly131, Thr135, and Ser138 (all corresponding to only chain 2 residues of the natural Fel d 1 [48]) displayed predicted hydrophobic interactions with the steroids. The present results are in agreement with the few steroid interactions previously described in Fel d 1 [23]. In particular, Tyr21 was previously reported to be highly conserved in several secretoglobins [25] and possibly involved in ligand binding [49,50]. Phe6 was also predicted to interact with ligands [50]. These previous reports suggested that both these amino acids could be important for a function of the protein in chemical communication. Likewise, in the present study, we predicted that Tyr21, Phe84, and Tyr119 could interact with fatty acids, while Tyr81 and Phe85 could interact with steroids.
A limitation of the in silico study is that we used the docking protocol, which is a static or quasi-static method, to obtain the structure of the various Fel d 1-ligand complexes. Using a scoring function that is meant to reproduce the binding affinity in terms of free binding energy, these structures are ranked to reveal the best-fit ligands in a way comparable to the rank based on experimental data [51]. Although the molecular docking free energy differences estimations are fast, simple, and useful for the screening of ligands, they are not the most precise ones (compared to the free binding energies determined by molecular dynamics simulations for instance) due to the absence of mobility or the absence of an explicit solvation of the system [51]. Nevertheless, here, we also considered other computational factors like binding frequencies and residue interactions before concluding about the results of the in silico screening displayed in Table 1. Moreover, these results were further confirmed by in vitro experiments.
A limitation of the in vitro study is that we used a recombinant model of the native Fel d 1, in which a peptide segment was introduced as a linker between the two subunits in the place of disulfide bridges. However, the recombinant and native Fel d 1 secondary structures were found to be similar based on circular dichroism [52]. Most importantly, the disulfide pairing of recombinant Fel d 1 corresponds with that of the native Fel d 1 [8,52]. Therefore, the peptide link in rFel d 1 seems not to introduce major differences in the overall folding of the protein. Whereas the overall structures of native Fel d 1 and of its recombinant are reasonably similar, differences in the flexibility and residual conformations can still exist. Even minor changes may affect the binding activity of a protein: for instance, several authors have shown that post-translational modifications, such as phosphorylation and O-glycosylation, influence the binding profiles of pig OBP isoforms, and phosphorylation can even enhance the binding affinities for some compounds in native OBPs compared to their recombinant counterparts [45,53,54]. It was also hypothesized that the glycosylation pattern of Fel d 1 might affect its structural features, notably by reducing its cavity size, thus possibly altering/modulating its ligand-binding properties [23]. Therefore, we cannot exclude that differences between natural and recombinant forms of Fel d 1 may affect the binding properties of the protein. Confirming our results with the native Fel d 1 would be necessary to definitely assess its putative function as semiochemical carrier.
The proteins that participate in chemical communication have complex roles, such as solubilizing, transporting, serving as reservoirs, assisting in the controlled release of semiochemicals, or even acting themselves as chemical messages (e.g., MUPs) [55,56]. The binding and controlled release of volatile chemical cues via proteins are of particular interest for Felidae, which are mostly solitary carnivores and use scent marks to delimit their territories of variable sizes according to ecological resources [57]. Domestic cats vary greatly in spatial organization, from being solitary in well-dispersed populations at densities of a single individual per square km or lower to living in highly populated groups [58]. Whatever the cats’ social organization is, chemical communication mediated by scent marks is essential to assess social and territorial relationships [59]. The chemical composition of the marks can also provide physiological information in some cases, such as sex or sexual status [60]. Interestingly, other Felidae species also secrete proteins similar to Fel d 1 [61], which might as well have the function of extending the persistence of chemical cues in their environment. Because territory marking involves high energy costs [62], it is important to keep the chemical message as long as possible in general and specifically for Felidae [63].
In mammals, OBPs, sometimes referred to as pheromone-binding proteins (PBPs), are the main proteins that have been reported to mediate chemical communication. These proteins belong to the large family of lipocalins and bind semiochemicals and odorants representing various chemical classes [46,64]. The cat lipocalin Fel d 4 was shown to be involved in chemical communication as a kairomone by eliciting defensive behavior in mice [65]. The structure of secretoglobins (α-helix bundles assembled in a boomerang configuration, creating a central hydrophobic pocket), to which Fel d 1 belongs, is completely different from that of lipocalins (barrel of β-strands with a central apolar cavity) [64,66]. However, the binding data collected with a structural model of Fel d 1 suggest that a function of semiochemical carrier could be considered also for secretoglobins. More experimental evidence is needed, such as studying the expression of Fel d 1 in cat chemosensory organs, confirming its binding activity with the native protein, and perhaps identifying its natural ligands. We hope that our work can stimulate more research in the field of secretoglobins and confirm their putative role in mammalian chemical communication.
Unveiling the ligand-binding properties of Fel d 1 towards semiochemical compounds supports a function of this protein as a semiochemical carrier. As Fel d 1 is one of the most important aeroallergens [4], it is possible that lipid binding might also affect the allergenicity of this protein. Indeed, some authors have shown that another version of recombinant Fel d 1 was able to bind lipopolysaccharides (LPS), enhance lipid cellular signaling through Toll-like receptors, and potentiate the production of the pro-inflammatory cytokine TNF-α (Tumor Necrosis Factor-α), which could eventually influence the allergic sensitization process [67]. In this respect, ligand binding characteristics of Fel d 1 might help to understand the allergenic effects of the protein itself compared to that of its complexes with ligands [68]. Besides, the binding of a ligand to Fel d 1 might affect the allergen recognition by Immunoglobulin E (IgE) if the epitopes are altered through B-cell epitope conformational changes induced by the ligand or if the amino acid residues involved in IgE binding are obscured in the ligand-protein complex. Then, elucidating the ligand binding properties of Fel d 1 might provide valuable insights into this putative phenomenon of ligand-induced epitope masking. Along the same line, several approaches aiming at decreasing or controlling the cat production of immunologically active Fel d 1 have recently been investigated in order to alleviate the symptoms suffered by allergic cat owners [69]. In particular, the use of a diet supplemented with anti-Fel d 1 avian IgY [70] or the immunization of cats with a modified form of recombinant Fel d 1 to stimulate the production of neutralizing antibodies [71] have been proposed. However, as the results of this study suggest that Fel d 1 could play an important role in the cat’s chemical communication, our opinion is that any attempt to alter the production of Fel d 1 should consider possible consequences that might affect the cat’s biology.
4. Materials and Methods
4.1. System Configuration
All the computational analyses were carried out in a high-performance GPU workstation with Cent OS V.7.6 Linux and the Windows OS. The hardware specifications of the workstation (Model: LVX-1 × RTX-2080Ti) include a powerful Intel Core i9-9920X processor with 1GPU Nvidia RTX-2080Ti, 32GB RAM, running with a superfast boot-home 1 × M2-1TB NVME SSD and 2 × 8TB independent hard disks. The workstation has passed all the validation tests by the Linuxvixion GPU certified system.
4.2. Collection and Structure Conversion of Ligands
Molecular structures of the 28 putative ligands (15 fatty acids and their derivatives (FA) and 13 steroids) and N-phenyl-1-naphthylamine (1-NPN) (fluorescent probe) were collected from PubChem (https://pubchem.ncbi.nlm.nih.gov/). All the 2D structures of the ligands were converted into the corresponding three-dimensional (3D) coordinates (sdf to mol2 format) using OpenBabelGUI tools V.2.3.1 (http://openbabel.org). The selected compounds were used to obtain a drug-likeness score from the Lipinski rule of five (RO5) webserver (http://www.scfbio-iitd.res.in/software/drugdesign/lipinski.jsp) [72].
4.3. Physio-Chemical Properties Analysis
The physico-chemical properties of all putative ligands and 1-NPN were collected from various chemical databases such as PubChem and ChemSpider. The compound properties were classified as the chemical formula, molecular weight, H-bond donor, acceptor, topological polar surface area, and RO5 (Table 3).
Table 3.
Molecular structural properties of all putative semiochemical compounds and the fluorescent probe N-phenyl-1-naphthylamine (1-NPN).
| n° | Compounds | PubChem Compound ID (CID) | Chemical Formula | Molecular Weight (g/mol) | H-Bond Donor | H-Bond Acceptor | Topological Polar Surface Area (Ų) | Lipinski Rule of Five (RO5) |
|---|---|---|---|---|---|---|---|---|
| Fatty Acids and Their Derivatives | ||||||||
| 1 | Isobutyric acid | CID_6590 | C4H8O2 | 88.106 | 1 | 2 | 37.3 | 0 |
| 2 | Capric acid | CID_2969 | C10H20O2 | 172.268 | 1 | 2 | 37.3 | 0 |
| 3 | Lauric acid | CID_3893 | C12H24O2 | 200.322 | 1 | 2 | 37.3 | 0 |
| 4 | Myristic acid | CID_11005 | C14H28O2 | 228.376 | 1 | 2 | 37.3 | 0 |
| 5 | Palmitic acid | CID_985 | C16H32O2 | 256.43 | 1 | 2 | 37.3 | 1 |
| 6 | Oleic acid | CID_445639 | C18H34O2 | 282.468 | 1 | 2 | 37.3 | 1 |
| 7 | Linoleic acid | CID_5280450 | C18H32O2 | 280.442 | 1 | 2 | 37.3 | 1 |
| 8 | Dodecanal | CID_8194 | C12H24O | 184.323 | 0 | 1 | 17.1 | 0 |
| 9 | Dodecanol | CID_8193 | C12H26O | 186.339 | 1 | 1 | 20.2 | 0 |
| 10 | Tetradecanol | CID_8209 | C14H30O | 214.393 | 1 | 1 | 20.2 | 0 |
| 11 | Ethyl Laurate | CID_7800 | C14H28O2 | 228.376 | 0 | 2 | 26.3 | 0 |
| 12 | Methyl palmitate | CID_8181 | C17H34O2 | 270.457 | 0 | 2 | 26.3 | 1 |
| 13 | Nonanamide | CID_70709 | C9H19NO | 157.257 | 1 | 1 | 43.1 | 0 |
| 14 | Hexadecanamide | CID_69421 | C16H33NO | 255.446 | 1 | 1 | 43.1 | 0 |
| 15 | Octadecanamide | CID_31292 | C18H37NO | 283.5 | 1 | 1 | 43.1 | 1 |
| Steroids | ||||||||
| 1 | Androstenone | CID_6852393 | C19H28O | 272.432 | 0 | 1 | 17.1 | 1 |
| 2 | Androstenedione | CID_6128 | C19H26O2 | 286.415 | 0 | 2 | 34.1 | 0 |
| 3 | Androstenol | CID_101989 | C19H30O | 274.448 | 1 | 1 | 20.2 | 1 |
| 4 | Progesterone | CID_5994 | C21H30O2 | 314.469 | 0 | 2 | 34.1 | 0 |
| 5 | Hydroxyprogesterone | CID_6238 | C21H30O3 | 330.468 | 1 | 3 | 54.4 | 0 |
| 6 | Pregnenolone | CID_8955 | C21H32O2 | 316.485 | 1 | 2 | 37.3 | 0 |
| 7 | Estradiol | CID_5757 | C18H24O2 | 272.388 | 2 | 2 | 40.5 | 0 |
| 8 | Testosterone | CID_6013 | C19H28O2 | 288.431 | 1 | 2 | 37.3 | 0 |
| 9 | Dihydrotestosterone | CID_10635 | C19H30O2 | 290.447 | 1 | 2 | 37.3 | 0 |
| 10 | Estrone | CID_5870 | C18H22O2 | 270.372 | 1 | 2 | 37.3 | 0 |
| 11 | Dehydroepiandrosterone (DHEA) | CID_5881 | C19H28O2 | 288.431 | 1 | 2 | 37.3 | 0 |
| 12 | Corticosterone | CID_5753 | C21H30O4 | 346.467 | 2 | 4 | 74.6 | 0 |
| 13 | Deoxycorticosterone | CID_6166 | C21H30O3 | 330.468 | 1 | 3 | 54.4 | 0 |
| Fluorescent Probe | ||||||||
| 1 | N-phenyl-1-naphthylamine (1-NPN) | CID_7013 | C16H13N | 219.287 | 1 | 1 | 12 | 0 |
4.4. Molecular Docking Analysis
4.4.1. Ligand Optimization
The retrieved molecular structures of the putative ligands and 1-NPN (.mol) were energy minimized using the geometry optimization method (MMFF94 force field) with pH 7.0. The Gasteiger partial charge was added to the ligand atoms and the MMFF94 energies were found to differ between all the compounds. All the nonpolar atoms were merged, and rotatable bonds were defined.
4.4.2. Protein Grid Parameters
The 3D crystal structure of rFel d 1 (PDB ID: 2EJN) was retrieved from the Protein Data Bank (PDB) (https://www.rcsb.org/). The protein dimer and ligand dataset were uploaded to the DockingServer (https://www.dockingserver.com; Virtua Drug, Hungary), a web-based interface module consisting of Gasteiger and PM6 semiempirical quantum-mechanical partial charge calculations to enhance the accuracy of docking output utilizing the AutoDock 4 method [73]. The essential hydrogen atoms, Kollman united atom-type charges, and solvation parameters were added to the 3D structure of rFel d 1. The Gasteiger charge calculation method was selected for the protein clean step. The 3D dimensional grid box was constructed for permitting ligands to interact in the binding sites of Fel d 1. The affinity grid parameters (nx = 23; ny = 23; nz = 23 and cx = −0.48; cy = 0.81; cz = 0.22) and 0.375 Å spacing were generated using the Autogrid program [74]. The total Gasteiger charge of rFel d 1 was −6.959 kcal/mol. After completion of this step, the rFel d 1 structure was prepared for the docking simulation analysis.
4.4.3. Semi-Empirical Calculations
The docking simulation was performed using the Lamarckian genetic algorithm (LGA) and the Solis and Wets local search method to determine the optimum complex [75] in the AutoDock method. The AutoDock parameter set- and distance-dependent dielectric functions were used in the calculation of the van der Waals and the electrostatic terms, respectively. The initial position, orientation, and torsion of the ligand molecules were set randomly, and all rotatable torsions were released during docking. Each docking calculation was derived from 100 runs, which were set to terminate after a maximum of 2,500,000 energy calculations (540,000 for a generation with a population size of 150). A translational step of 0.2 Å, quaternion, and torsion steps of five were employed as parameters for the docking analyses. The AutoDock algorithms calculate the free binding energy to assess the orientation of a ligand binding pose to a protein while forming a stable complex. The protein–ligand complex was analyzed, and the molecular interaction poses of each compound were selected for the ranking of the best-fit ligands according to the docking score with several docking parameters. The estimation of the binding free energy was selected from the best- docked conformation of the protein–ligand complex using docking simulation.
4.4.4. Molecular Visualization
The protein–ligand interactions were visualized using Discovery studio visualizer DSV 4.5 (Accelrys, San Diego, CA, USA), USCF Chimera (https://www.cgl.ucsf.edu/chimera/) and the LigPlot V.4.5.3 program. The evaluation of semi-empirical docking values was computed regarding the score of lowest binding energy, hydrogen bonding (H-bonding), and polar and steric interactions.
4.5. Fluorescence Measurement and Binding Assays
N-Phenyl-1-naphthylamine (1-NPN) was used as a non-polar fluorescent probe in competitive binding experiments with the ligands (Sigma, France) to investigate binding efficiency of semiochemical analogs with pure rFel d 1 (INDOOR Biotechnologies, UK) [30]. The fluorescence experiments were performed on an FP-750 spectrofluorometer (JASCO, Japan) instrument at 25 °C in a right-angle configuration with a 1 cm light path fluorimeter quartz cuvette and 5-nm slits for both excitation and emission. The probe 1-NPN was excited at 337 nm and emission spectra were recorded between 380 and 450 nm, at 25 °C. The protein was dissolved in 50 mM Tris-HCl, pH 7.4, and ligands were added as 1 mM methanol solutions.
The rFel d 1 intrinsic fluorescence was expected to be negligible since no tryptophan is present in the sequences of both Fel d 1 chains [48], yet it was verified. The binding of 1-NPN to rFel d 1 was tested at two protein concentrations (1 µM and 2 µM) by titrating the protein solution with aliquots of a 1-mM solution of 1-NPN in methanol to final concentrations of 1–20 µM. The bound ligand was evaluated from the values of fluorescence intensity assuming that the protein was 100% active, with a stoichiometry of 1:1 protein: ligand. Dose–responses curves were performed in triplicate and linearized using Scatchard plots to calculate the 1-NPN dissociation constant (Kd 1-NPN).
Semiochemicals were first screened for their capabilities to bind rFel d 1 using 1 µM of rFel d 1, 1 μM of 1-NPN, and 2 µM of a competitive ligand. Active compounds were then used to measure their affinity to the protein, using a concentration range of 0–16 μM. The dissociation constants of the competitor ligands (Kd) were calculated from the respective IC50 values (IC50: competitor’s concentration halving the initial fluorescence), using the equation:
| Kd = [IC50]/(1 + [1 − NPN]/K1 − NPN) |
where [1-NPN] is the free concentration of 1-NPN and K1-NPN is the dissociation constant of the complex rFel d 1/1-NPN. IC50 was graphically determined from the dose–response curve of each competitor ligand.
Acknowledgments
The authors thank the American Journal Expert for the English editing of this manuscript.
Author Contributions
C.B.-F. and P.P. (Patrick Pageat) conceived the study; C.B.-F. and P.P. (Paolo Pelosi) conceived, designed, and performed the in vitro experiments and analyzed the data; R.D. conceived, designed, and performed the in silico experiments and analyzed the data; P.P. (Patrick Pageat) and P.P. (Paolo Pelosi) contributed reagents/materials/analysis tools; C.B.-F. and P.P. (Patrick Pageat) supervised and administered the project; C.B.-F. and R.D. wrote the original draft preparation; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Patrick Pageat is the inventor of the patent “Properties of cat’s facial pheromone” n° WO1996023414A1 and the patent “Cat Appeasing Pheromone” n° WO2015140631A1.
References
- 1.Van Milligen F.J., Vroom T.M., Aalberse R.C. Presence of Felis domesticus Allergen I in the Cat’s Salivary and Lacrimal Glands. Int. Arch. Allergy Appl. Immunol. 1990;92:375–378. doi: 10.1159/000235168. [DOI] [PubMed] [Google Scholar]
- 2.Charpin C., Mata P., Charpin D., Lavaut M.N., Allasia C., Vervloet D. Fel d I allergen distribution in cat fur and skin. J. Allergy Clin. Immunol. 1991;88:77–82. doi: 10.1016/0091-6749(91)90303-6. [DOI] [PubMed] [Google Scholar]
- 3.De Andrade A.D., Birnbaum J., Magalon C., Magnol J.P., Lanteaume A., Charpin D., Vervloet D. Fel d I levels in cat anal glands. Clin. Exp. Allergy. 1996;26:178–180. doi: 10.1111/j.1365-2222.1996.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet B., Messaoudi K., Jacomet F., Michaud E., Fauquert J.L., Caillaud D., Evrard B. An update on molecular cat allergens: Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen. Allergy Asthma Clin. Immunol. 2018;14:1–9. doi: 10.1186/s13223-018-0239-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zielonka T.M., Charpin D., Berbis P., Lucciani P., Casanova D., Vervloet D. Effects of castration and testosterone on Fel dI production by sebaceous glands of male cats: I--Immunological assessment. Clin. Exp. Allergy. 1994;24:1169–1173. doi: 10.1111/j.1365-2222.1994.tb03324.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaiser L., Grönlund H., Sandalova T., Ljunggren H.G., van Hage-Hamsten M., Achour A., Schneider G. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J. Biol. Chem. 2003;278:37730–37735. doi: 10.1074/jbc.M304740200. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser L., Velickovic T.C., Badia-Martinez D., Adedoyin J., Thunberg S., Hallen D., Berndt K., Grönlund H., Gafvelin G., Van Hage M., et al. Structural characterization of the tetrameric form of the major cat allergen Fel d 1. J. Mol. Biol. 2007;370:714–727. doi: 10.1016/j.jmb.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 8.Kristensen A.K., Schou C., Roepstorff P. Determination of isoforms, N-linked glycan structure and disulfide bond linkages of the major cat allergen Fel d1 by a mass spectrometric approach. Biol. Chem. 1997;378:899–908. doi: 10.1515/bchm.1997.378.8.899. [DOI] [PubMed] [Google Scholar]
- 9.Bienboire-Frosini C., Lebrun R., Vervloet D., Pageat P., Ronin C. Distribution of core fragments from the major cat allergen Fel d 1 is maintained among the main anatomical sites of production. Int. Arch. Allergy Immunol. 2010;152:197–206. doi: 10.1159/000283024. [DOI] [PubMed] [Google Scholar]
- 10.Bienboire-Frosini C., Lebrun R., Vervloet D., Pageat P., Ronin C. Variable content of Fel d 1 variants in house dust and cat extracts may have an impact on allergen measurement. J. Investig. Allergol. Clin. Immunol. 2012;22:270–279. [PubMed] [Google Scholar]
- 11.Liccardi G., D’Amato G., Russo M., Canonica G.W., D’Amato L., De Martino M., Passalacqua G. Focus on cat allergen (Fel d 1): Immunological and aerodynamic characteristics, modality of airway sensitization and avoidance strategies. Int. Arch. Allergy Immunol. 2003;132:1–12. doi: 10.1159/000073259. [DOI] [PubMed] [Google Scholar]
- 12.Zahradnik E., Raulf M. Animal allergens and their presence in the environment. Front. Immunol. 2014;5:76. doi: 10.3389/fimmu.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee A.B., Zhang Z., Chilton B.S. Uteroglobin: A steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr. Rev. 2007;28:707–725. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama S., Cai Y., Murata M., Tomita T., Yoneda M., Xu L., Pilon A.L., Cachau R.E., Kimura S. A novel pathway of LPS uptake through syndecan-1 leading to pyroptotic cell death. Elife. 2018;7:1–25. doi: 10.7554/eLife.37854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiba Y., Kurotani R., Kusakabe T., Miura T., Link B.W., Misawa M., Kimura S. Uteroglobin-related protein 1 expression suppresses allergic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2006;173:958–964. doi: 10.1164/rccm.200503-456OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maccioni M., Riera C.M., Rivero V.E. Identification of rat prostatic steroid binding protein (PSBP) as an immunosuppressive factor. J. Reprod. Immunol. 2001;50:133–149. doi: 10.1016/S0165-0378(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 17.Karn R.C. Evolution of Rodent Pheromones: A Review of the ABPs with Comparison to the ESPs and the MUPs. Int. J. Biochem. Res. Rev. 2013;3:328–363. doi: 10.9734/IJBCRR/2013/5763. [DOI] [Google Scholar]
- 18.Chung A.G., Belone P.M., Bímová B.V., Karn R.C., Laukaitis C.M. Studies of an Androgen-Binding Protein Knockout Corroborate a Role for Salivary ABP in Mouse Communication. Genetics. 2017;205:1517–1527. doi: 10.1534/genetics.116.194571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin C.J., Emberson L., Nicholls P. Purification and characterization of pheromaxein, the porcine steroid-binding protein. A member of the secretoglobin superfamily. Eur. J. Biochem. 2004;271:2593–2606. doi: 10.1111/j.1432-1033.2004.04188.x. [DOI] [PubMed] [Google Scholar]
- 20.Carayol N., Birnbaum J., Magnan A., Ramadour M., Lanteaume A., Vervloet D., Tessier Y., Pageat P. Fel d 1 production in the cat skin varies according to anatomical sites. Allergy. 2000;55:570–573. doi: 10.1034/j.1398-9995.2000.00588.x. [DOI] [PubMed] [Google Scholar]
- 21.Pageat P., Gaultier E. Current research in canine and feline pheromones. Vet. Clin. N. Am. Small Anim. Pract. 2003;33:187–211. doi: 10.1016/S0195-5616(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 22.Bienboire-Frosini C., Cozzi A., Lafont-Lecuelle C., Vervloet D., Ronin C., Pageat P. Immunological differences in the global release of the major cat allergen Fel d 1 are influenced by sex and behaviour. Vet. J. 2012;193:162–167. doi: 10.1016/j.tvjl.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Ligabue-Braun R., Sachett L.G., Pol-Fachin L., Verli H. The Calcium Goes Meow: Effects of Ions and Glycosylation on Fel d 1, the Major Cat Allergen. PLoS ONE. 2015;10:e0132311. doi: 10.1371/journal.pone.0132311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karn R.C. The mouse salivary androgen-binding protein (ABP) alpha subunit closely resembles chain 1 of the cat allergen Fel dI. Biochem. Genet. 1994;32:271–277. doi: 10.1007/BF00555830. [DOI] [PubMed] [Google Scholar]
- 25.Durairaj R., Pageat P., Bienboire-Frosini C. Another cat and mouse game: Deciphering the evolution of the SCGB superfamily and exploring the molecular similarity of major cat allergen Fel d 1 and mouse ABP using computational approaches. PLoS ONE. 2018;13:e0197618. doi: 10.1371/journal.pone.0197618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karn R.C., Clements M.A. A comparison of the structures of the alpha:beta and alpha:gamma dimers of mouse salivary androgen-binding protein (ABP) and their differential steroid binding. Biochem. Genet. 1999;37:187–199. doi: 10.1023/A:1018786622052. [DOI] [PubMed] [Google Scholar]
- 27.Chapman M.D., Smith A.M., Vailes L.D., Arruda L.K., Dhanaraj V., Pomés A. Recombinant allergens for diagnosis and therapy of allergic disease. J. Allergy Clin. Immunol. 2000;106:409–418. doi: 10.1067/mai.2000.109832. [DOI] [PubMed] [Google Scholar]
- 28.Vailes L.D., Sun A.W., Ichikawa K., Wu Z., Sulahian T.H., Chapman M.D., Guyre P.M. High-level expression of immunoreactive recombinant cat allergen (Fel d 1): Targeting to antigen-presenting cells. J. Allergy Clin. Immunol. 2002;110:757–762. doi: 10.1067/mai.2002.129035. [DOI] [PubMed] [Google Scholar]
- 29.Chen X., Zaro J.L., Shen W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013;65:1357–1369. doi: 10.1016/j.addr.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wuenschmann S., Vailes L.D., King E., Aalberse R.C., Chapman M.D. Expression of a Deglycosylated Recombinant Fel d 1 in Pichia pastoris. J. Allergy Clin. Immunol. 2008;121:S214. doi: 10.1016/j.jaci.2007.12.798. [DOI] [Google Scholar]
- 31.Vitale Shreve K.R., Udell M.A.R. Stress, security, and scent: The influence of chemical signals on the social lives of domestic cats and implications for applied settings. Appl. Anim. Behav. Sci. 2017;187:69–76. doi: 10.1016/j.applanim.2016.11.011. [DOI] [Google Scholar]
- 32.Mills D.S., White J.C. Long-term follow up of the effect of a pheromone therapy on feline spraying behaviour. Vet. Rec. 2000;147:746–747. [PubMed] [Google Scholar]
- 33.Mills D.S., Mills C. Evaluation of a novel method for delivering a synthetic analogue of feline facial pheromone to control urine spraying by cats. Vet. Rec. 2001;149:197–199. doi: 10.1136/vr.149.7.197. [DOI] [PubMed] [Google Scholar]
- 34.Kronen P.W., Ludders J.W., Erb H.N., Moon P.F., Gleed R.D., Koski S. A synthetic fraction of feline facial pheromones calms but does not reduce struggling in cats before venous catheterization. Vet. Anaesth. Analg. 2006;33:258–265. doi: 10.1111/j.1467-2995.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 35.Pereira J.S., Fragoso S., Beck A., Lavigne S., Varejão A.S., da Graça Pereira G. Improving the feline veterinary consultation: The usefulness of Feliway spray in reducing cats’ stress. J. Feline Med. Surg. 2016;18:959–964. doi: 10.1177/1098612X15599420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cozzi A., Monneret P., Lafont-Lecuelle C., Bougrat L., Gaultier E., Pageat P. The maternal cat appeasing pheromone: Exploratory study of the effects on aggressive and affiliative interactions in cats. J. Vet. Behav. Clin. Appl. Res. 2010;5:37–38. doi: 10.1016/j.jveb.2009.10.014. [DOI] [Google Scholar]
- 37.DePorter T.L., Bledsoe D.L., Beck A., Ollivier E. Evaluation of the efficacy of an appeasing pheromone diffuser product vs placebo for management of feline aggression in multi-cat households: A pilot study. J. Feline Med. Surg. 2019;21:293–305. doi: 10.1177/1098612X18774437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridlansky F., Milgrom E. Interaction of uteroglobin with progesterone, 5αpregnane-3, 20-dione and estrogens. Endocrinology. 1976;99:1244–1251. doi: 10.1210/endo-99-5-1244. [DOI] [PubMed] [Google Scholar]
- 39.Karn R.C. Steroid binding by mouse salivary proteins. Biochem. Genet. 1998;36:105–117. doi: 10.1023/A:1018708404789. [DOI] [PubMed] [Google Scholar]
- 40.Chen C., Schilling K., Hiipakka R.A., Huang I.Y., Liao S. Prostate α-protein. Isolation and characterization of the polypeptide components and cholesterol binding. J. Biol. Chem. 1982;257:116–121. [PubMed] [Google Scholar]
- 41.Taylor R.D., Jewsbury P.J., Essex J.W. A review of protein-small molecule docking methods. J. Comput. Aided Mol. Des. 2002;16:151–166. doi: 10.1023/A:1020155510718. [DOI] [PubMed] [Google Scholar]
- 42.Pelosi P., Zhu J., Knoll W. From radioactive ligands to biosensors: Binding methods with olfactory proteins. Appl. Microbiol. Biotechnol. 2018;102:8213–8227. doi: 10.1007/s00253-018-9253-5. [DOI] [PubMed] [Google Scholar]
- 43.Dorries K.M., Adkins-Regan E., Halpern B.P. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav. Evol. 1997;49:53–62. doi: 10.1159/000112981. [DOI] [PubMed] [Google Scholar]
- 44.Guiraudie-Capraz G., Pageat P., Cain A.H., Madec I., Nagnan-Le Meillour P. Functional characterization of olfactory binding proteins for appeasing compounds and molecular cloning in the vomeronasal organ of pre-pubertal pigs. Chem. Senses. 2003;28:609–619. doi: 10.1093/chemse/bjg052. [DOI] [PubMed] [Google Scholar]
- 45.Nagnan-Le Meillour P., Joly A., Le Danvic C., Marie A., Zirah S., Cornard J.P. Binding specificity of native odorant-binding protein isoforms is driven by phosphorylation and O-N-acetylglucosaminylation in the pig Sus scrofa. Front. Endocrinol. 2019;9:816. doi: 10.3389/fendo.2018.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelosi P. The role of perireceptor events in vertebrate olfaction. Cell. Mol. Life Sci. 2001;58:503–509. doi: 10.1007/PL00000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stopkova R., Klempt P., Kuntova B., Stopka P. On the tear proteome of the house mouse (Mus musculus musculus) in relation to chemical signalling. PeerJ. 2017;5:e3541. doi: 10.7717/peerj.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgenstern J.P., Griffith I.J., Brauer A.W., Rogers B.L., Bond J.F., Chapman M.D., Kuo M.-C. Amino acid sequence of Fel dI, the major allergen of the domestic cat: Protein sequence analysis and cDNA cloning. Proc. Natl. Acad. Sci. USA. 1991;88:9690–9694. doi: 10.1073/pnas.88.21.9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emes R.D., Riley M.C., Laukaitis C.M., Goodstadt L., Karn R.C., Ponting C.P. Comparative evolutionary genomics of androgen-binding protein genes. Genome Res. 2004;14:1516–1529. doi: 10.1101/gr.2540304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson B.C., Thompson D.C., Wright M.W., McAndrews M., Bernard A., Nebert D.W., Vasiliou V. Update of the human secretoglobin (SCGB) gene superfamily and an example of “evolutionary bloom” of androgen-binding protein genes within the mouse Scgb gene superfamily. Hum. Genomics. 2011;5:691–702. doi: 10.1186/1479-7364-5-6-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Golebiowski J., Topin J., Charlier L., Briand L. Interaction between odorants and proteins involved in the perception of smell: The case of odorant-binding proteins probed by molecular modelling and biophysical data. Flavour Fragr. J. 2012;27:445–453. doi: 10.1002/ffj.3121. [DOI] [Google Scholar]
- 52.Grönlund H., Bergman T., Sandström K., Alvelius G., Reininger R., Verdino P., Hauswirth A., Liderot K., Valent P., Spitzauer S., et al. Formation of disulfide bonds and homodimers of the major cat allergen Fel d 1 equivalent to the natural allergen by expression in Escherichia coli. J. Biol. Chem. 2003;278:40144–40151. doi: 10.1074/jbc.M301416200. [DOI] [PubMed] [Google Scholar]
- 53.Le Danvic C., Guiraudie-Capraz G., Abderrahmani D., Zanetta J.P., Nagnan-Le Meillour P. Natural ligands of porcine olfactory binding proteins. J. Chem. Ecol. 2009;35:741–751. doi: 10.1007/s10886-009-9645-1. [DOI] [PubMed] [Google Scholar]
- 54.Brimau F., Cornard J.P., Le Danvic C., Lagant P., Vergoten G., Grebert D., Pajot E., Nagnan-Le Meillour P. Binding specificity of recombinant odorant-binding protein isoforms is driven by phosphorylation. J. Chem. Ecol. 2010;36:801–813. doi: 10.1007/s10886-010-9820-4. [DOI] [PubMed] [Google Scholar]
- 55.Burger B.V. The Chemistry of Pheromones and Other Semiochemicals II. Springer; Berlin/Heidelberg, Germany: 2005. Mammalian semiochemicals; pp. 231–278. [Google Scholar]
- 56.Beynon R.J., Armstrong S.D., Gómez-Baena G., Lee V., Simpson D., Unsworth J., Hurst J.L. The complexity of protein semiochemistry in mammals. Biochem. Soc. Trans. 2014;42:837–845. doi: 10.1042/BST20140133. [DOI] [PubMed] [Google Scholar]
- 57.Bradshaw J.W.S., Cameron-Beaumont C. The signalling repertoire of the domestic cat and its undomesticated relatives. In: Turner D.C., Bateson P., editors. The Domestic Cat, The Biology of Its Behaviour. Cambridge University Press; Cambridge, UK: 2000. pp. 68–93. [Google Scholar]
- 58.Natoli E., De Vito E. Agonistic behaviour, dominance rank and copulatory success in a large multi-male feral cat, Felis catus L., colony in central Rome. Anim. Behav. 1991;42:227–241. doi: 10.1016/S0003-3472(05)80554-8. [DOI] [Google Scholar]
- 59.Natoli E. Behavioural Responses of Urban Feral Cats to Different Types of Urine Marks. Behaviour. 1985;94:234–243. doi: 10.1163/156853985X00208. [DOI] [Google Scholar]
- 60.Smith J.L.D., McDougal C., Miquelle D. Scent marking in free-ranging tigers, Panthera tigris. Anim. Behav. 1989;37:1–10. doi: 10.1016/0003-3472(89)90001-8. [DOI] [Google Scholar]
- 61.De Groot H., van Swieten P., Aalberse R.C. Evidence for a Fel d I-like molecule in the “big cats” (Felidae species) J. Allergy Clin. Immunol. 1990;86:107–116. doi: 10.1016/S0091-6749(05)80130-7. [DOI] [PubMed] [Google Scholar]
- 62.Burgos T., Virgós E., Valero E.S., Arenas-Rojas R., Rodríguez-Siles J., Recio M.R. Prey density determines the faecal-marking behaviour of a solitary predator, the Iberian lynx (Lynx pardinus) Ethol. Ecol. Evol. 2019;31:219–230. doi: 10.1080/03949370.2018.1544594. [DOI] [Google Scholar]
- 63.Darden S.K., Steffensen L.K., Dabelsteen T. Information transfer among widely spaced individuals: Latrines as a basis for communication networks in the swift fox? Anim. Behav. 2008;75:425–432. doi: 10.1016/j.anbehav.2007.05.007. [DOI] [Google Scholar]
- 64.Tegoni M., Pelosi P., Vincent F., Spinelli S., Grolli S., Ramoni R., Cambillau C. Mammalian odorant binding proteins. Biochim. Biophys. Acta. 2000;1482:229–240. doi: 10.1016/S0167-4838(00)00167-9. [DOI] [PubMed] [Google Scholar]
- 65.Papes F., Logan D.W., Stowers L. The Vomeronasal Organ Mediates Interspecies Defensive Behaviors through Detection of Protein Pheromone Homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Callebaut I., Poupon A., Bally R., Demaret J.P., Housset D., Delettre J., Hossenlopp P., Mornon J.P. The uteroglobin fold. Ann. N. Y. Acad. Sci. 2000;923:90–112. doi: 10.1111/j.1749-6632.2000.tb05522.x. [DOI] [PubMed] [Google Scholar]
- 67.Herre J., Grönlund H., Brooks H., Hopkins L., Waggoner L., Murton B., Gangloff M., Opaleye O., Chilvers E.R., Fitzgerald K., et al. Allergens as immunomodulatory proteins: The cat dander protein Fel d 1 enhances TLR activation by lipid ligands. J. Immunol. 2013;191:1529–1535. doi: 10.4049/jimmunol.1300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bublin M., Eiwegger T., Breiteneder H. Do lipids influence the allergic sensitization process? J. Allergy Clin. Immunol. 2014;134:521–529. doi: 10.1016/j.jaci.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satyaraj E., Wedner H.J., Bousquet J. Keep the cat, change the care pathway: A transformational approach to managing Fel d 1, the major cat allergen. Allergy Eur. J. Allergy Clin. Immunol. 2019;74:5–17. doi: 10.1111/all.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Satyaraj E., Gardner C., Filipi I., Cramer K., Sherrill S. Reduction of active Fel d1 from cats using an antiFel d1 egg IgY antibody. Immun. Inflamm. Dis. 2019;7:68–73. doi: 10.1002/iid3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thoms F., Jennings G.T., Maudrich M., Vogel M., Haas S., Zeltins A., Hofmann-Lehmann R., Riond B., Grossmann J., Hunziker P., et al. Immunization of cats to induce neutralizing antibodies against Fel d 1, the major feline allergen in human subjects. J. Allergy Clin. Immunol. 2019;144:193–203. doi: 10.1016/j.jaci.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 72.Jayaram B., Singh T., Mukherjee G., Mathur A., Shekhar S., Shekhar V. Sanjeevini: A freely accessible web-server for target directed lead molecule discovery. BMC Bioinform. 2012;13:S7. doi: 10.1186/1471-2105-13-S17-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bikadi Z., Hazai E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminform. 2009;1:15. doi: 10.1186/1758-2946-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solis F.J., Wets R.J.-B. Minimization by Random Search Techniques. Math. Oper. Res. 1981;6:19–30. doi: 10.1287/moor.6.1.19. [DOI] [Google Scholar]