Abstract
Malaria drug resistance is a global public health concern. Though parasite mutations have been associated with resistance, other factors could influence the resistance. A robust surveillance system is required to monitor and help contain the resistance. This study established the role of travel and gender in dispersion of chloroquine resistant genotypes in malaria epidemic zones in Kenya. A total of 1,776 individuals presenting with uncomplicated malaria at hospitals selected from four malaria transmission zones in Kenya between 2008 and 2014 were enrolled in a prospective surveillance study assessing the epidemiology of malaria drug resistance patterns. Demographic and clinical information per individual was obtained using a structured questionnaire. Further, 2 mL of blood was collected for malaria diagnosis, parasitemia quantification and molecular analysis. DNA extracted from dried blood spots collected from each of the individuals was genotyped for polymorphisms in Plasmodium falciparum chloroquine transporter gene (Pfcrt 76), Plasmodium falciparum multidrug resistant gene 1 (Pfmdr1 86 and Pfmdr1 184) regions that are putative drug resistance genes using both conventional polymerase chain reaction (PCR) and real-time PCR. The molecular and demographic data was analyzed using Stata version 13 (College Station, TX: StataCorp LP) while mapping of cases at the selected geographic zones was done in QGIS version 2.18. Chloroquine resistant (CQR) genotypes across gender revealed an association with chloroquine resistance by both univariate model (p = 0.027) and by multivariate model (p = 0.025), female as reference group in both models. Prior treatment with antimalarial drugs within the last 6 weeks before enrollment was associated with carriage of CQR genotype by multivariate model (p = 0.034). Further, a significant relationship was observed between travel and CQR carriage both by univariate model (p = 0.001) and multivariate model (p = 0.002). These findings suggest that gender and travel are significantly associated with chloroquine resistance. From a gender perspective, males are more likely to harbor resistant strains than females hence involved in strain dispersion. On the other hand, travel underscores the role of transport network in introducing spread of resistant genotypes, bringing in to focus the need to monitor gene flow and establish strategies to minimize the introduction of resistance strains by controlling malaria among frequent transporters.
Keywords: Malaria, Drug resistance, Chloroquine, Gender, Travel, Plasmodium falciparum
Introduction
In 2006, the World Health organization (WHO) recommended artemisinin-based combination therapy (ACTs) use as first-line drug for treatment of uncomplicated malaria following widespread failure of sulfadoxine-pyrimethamine combination treatment. The strategy relied on the fast acting artemisinin derivatives rapidly to bring down the parasite biomass, relieves symptoms while the partner drug with longer half-life clears the residual parasites therefore cushioning the artemisinin derivative from emerging resistance (Nosten & White, 2007). The most widely used combinations globally include artemether-lumefantrine (AL, Coartem), dihydroartemisinin-piperaquine (DHAPPQ), artesunate-amodiaquine (ASAQ) and artesunate-mefloquine. These treatments alongside other intervention have contributed to about 37% decline in malaria cases and 60% reduction in mortality during the last fifteen years (Dhiman, 2019). However, the recent reports of resistance to artemisinin derivative of the ACTs in Southeast Asia (Woodrow & White, 2017) and its pattern of migration (Shetty et al., 2019) threaten this trajectory due to fears that the global dissemination of these strains would follow historical pattern of drug resistant strain dispersal. Africa parasite diversity studies show that these strains have not yet been detected in Africa (Kamau et al., 2015), but shows extant subpopulations of P. falciparum in sub-Saharan Africa (Amambua-Ngwa et al., 2019) suggestive of parallel emergence of ACT resistance in the region given the varying first-line drugs in across Africa. Notwithstanding the origin of the initial resistance, it is essential to understand patterns of the dispersal present-day characterized strains in natural infections at local level as a stop-gap for containment should ACT resistant strains emerge.
Non-synonymous mutations in the kelch propeller domain (K13-propeller) in Plasmodium falciparum have been associated with artemisinin resistance in samples from Southeast Asia (Witkowski et al., 2013). Further, the waning efficacy of ACTs has been partly associated with failure of the quinoline partners in the combination (Borrmann et al., 2008). Though most of these drugs have not been policy recommended, cross-resistance has been shown to occur owing to similarity in mechanisms of actions (Veiga et al., 2016). A case in point is the diminished mefloquine sensitivity in Kenya (Eyase et al., 2013) yet mefloquine had neither been first-line nor second-line treatment in the country. Single Nucleotide Polymorphisms (SNPs) in the P. falciparum multidrug resistance protein (Pfmdr1) and P. falciparum chloroquine resistance transporter (Pfcrt) genes confer resistance to a number of anti-malaria drugs. Chloroquine resistance has been associated to Pfcrt K76T SNP (Mohammed et al., 2013; Zhao et al., 2019). Pfmdr1 86Y and Y184 haplotypes have been shown to modulate chloroquine (CQ) resistance in the presence of Pfcrt 76T mutation (Venkatesan et al., 2014). Mefloquine (MQ) and lumefantrine (LU) sensitivities are linked to Pfmdr 1 86Y point mutation. Additionally, emerging Pfcrt K76 allele carrying parasites with Pfmdr 1 N86 and 184F in the last decade have been linked to declining susceptibility to LU, part of the AL treatment that is recommended in Kenya (Achieng et al., 2015; Eyase et al., 2013; Venkatesan et al., 2014), and a reciprocal rise in parasite susceptibility to CQ and amodiaquine (ADQ) (Achieng et al., 2015; Veiga et al., 2016). Conversely, our previous studies showed that the prevalence of Pfcrt K76 and N86 increased from 6.4% in 1995–1996 to 93.2% in 2014 and 0.0% in 2002-2003 to 92.4% in 2014 respectively (Achieng et al., 2015), a period when the first and second-line malaria drugs for Kenya are AL and DHAPPQ, respectively. A study done in Zanzibar reported that treatment with AL selects for chloroquine-susceptible Pfcrt K76 allele while re-infecting parasites harbor Pfmdr 1 N86 and 184F alleles (Sisowath et al., 2009).
Development of resistance is often attributed to acquisition of survival advantage of parasites against drugs (Hastings & Donnelly, 2005). The rate and pattern of spread of initial resistant strain is often dependent on multiple confounding factors that should be monitored alongside drug resistant surveillance programs concurrently (Mbugi et al., 2006). The confounding factors includes initial prevalence of mutations, population movement between transmission regions among others (Bloland, 2001). Distribution, control and prevention of malaria has been reported to be influenced by human population movement (HPM) from areas with high transmission rates to malaria-free areas (Cohen et al., 2012). Similarly, HPM has been shown to contribute to spread of drug resistant parasite strains elsewhere (Lynch & Roper, 2011). Other factors like age, gender, location, history of past malaria infection and previous malaria treatment have been shown to affect malaria prevention and control. These factors could consequently be associated with the dispersal and distribution of chloroquine resistance.
To combat drug resistance, the WHO recommends a robust surveillance system to monitor and detect emergence of resistant genotypes for timely containment replace the yellow highlighted (WHO, 2009). In areas with documented success and declining disease burden, there are calls for initiation of pre-elimination/elimination phase in malaria control and management (Whittaker, Dean & Chancellor, 2014; WHO, 2015). However, elimination process is facing numerous challenges. A study done in Nigeria reported research, political will/funding, attitude/behavior change, conflicts, terrorism/migration, climate change, insecticide resistance, drug resistance and treatment as challenges facing malaria elimination (Aribodor, Ugwuanyi & Aribodor, 2016), highlighting the importance of understanding the malaria drug resistance patterns alongside demographics/clinical factors and parasite characteristics among symptomatic malaria cases as a prerequisite for entry in pre-elimination phase.
As countries move towards the elimination phase, enhanced surveillance systems are required to ensure that every infection is detected, treated and reported (WHO, 2016a). However, there is lack of sufficient information to feed such a trend of reduced malaria burden in Kenya. Most studies done in Kenya often dwell on one site mostly endemic regions, with a smaller sample size and no detailed information on demographics. Therefore, this study aimed at determining the role of gender and travel in dispersion of chloroquine resistant genotypes among individuals with symptomatic malaria in epidemic zones, Kenya.
Materials & Methods
Ethics Statement, Study Protocol, Sites and Subjects
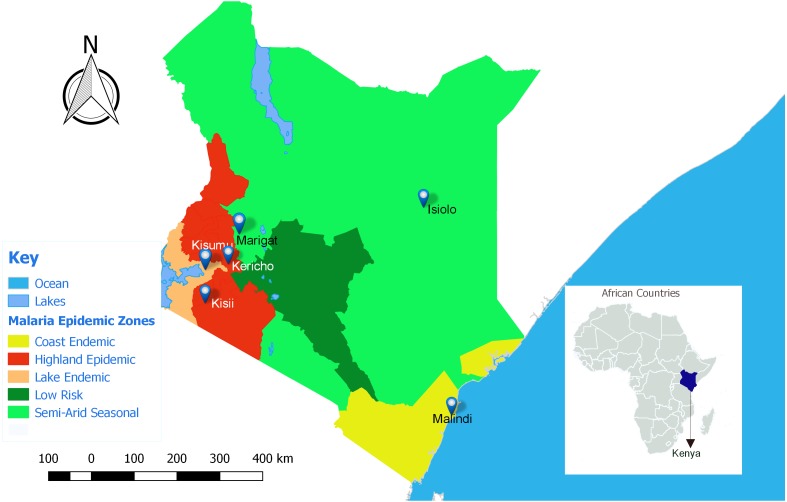
This study was approved by the Kenya Medical Research Institute (KEMRI), Walter Reed Army Institute of Research (WRAIR) and Human Research Protection office institutional review boards (protocol numbers: KEMRI #1330, WRAIR #1384 and HRPO Log #A-19306.3) respectively. Seven participating clinical centers were all Ministry of Health facilities located in various malaria epidemic regions in Kenya (Fig. 1); Lake endemic region (Kisumu East and Kisumu West/Kombewa District Hospitals) both situated in Kisumu County (0°14′60.00″N 34°54′59.99″E), Highland epidemic region had (Kericho District Hospitals and Kisii teaching/referral hospital) situated in Kericho County (0°22′2.3″S 35°16′52.72″E) and Kisii County (0°40′49.7352″S 34°46′37.4196″E) respectively, Coast endemic region (Malindi District Hospital) situated in Kilifi County (3°13′25″S 40°7′48″E) and Semiarid seasonal region (Isiolo District, Isiolo county (0°52′60.00″N 38°40′0.12″E) and Marigat Sub district Hospital, Baringo County (0°28′N 35°59′E). At each participating facility, training of medical staff, capacity building, and facility upgrades were provided by the Global Emerging Infections Surveillance (GEIS) Program, U.S. Department of Defense. Subjects attending outpatient clinics from 2008 to 2014, at least 6 months old and suspected of having non-complicated P. falciparum malaria were invited to participate. Written informed consent was obtained from adult subjects (≥18 years of age) or legal guardians for subjects <18 years of age. Infants weighing less than 5 kilograms were excluded.
Figure 1. Map of Kenya showing the different malaria endemicity zones and locations of various surveillance hospitals.
Lake endemic region: Kisumu East and Kisumu West District Hospitals in Kisumu county, Semi-Arid, seasonal: Marigat District hospital in Baringo county and Isiolo District Hospital in Isiolo county. Coast endemic: Malindi District Hospital, Kilifi County. High land Epidemic: Kisii teaching and referral Hospital and Kericho District Hospital (QGIS version 2.18).
Sample Collection and Preparation
2 ml of blood was collected from eligible subjects who had tested positive by rapid diagnostic test (RDT; Parascreen H (Pan/Pf), Zephyr Biomedicals, Verna Goa, India) for P. falciparum malaria. Additionally, FTA filter paper (Whatman Inc., Bound Brook, New Jersey, USA) was used to collect three blood spots of about 100 µl each for P. falciparum DNA extraction and molecular analysis. Two blood films on glass slides were made for Giemsa staining at the laboratory for microscopic examination, to confirm RDT results and determine parasitemia. For discrepancies between RDT and microscopy, microscopy determined the final result. Collected specimens for molecular analysis were stored in −20 °C freezers at the respective hospitals temporarily, before being transported to the KEMRI/USAMRD-A Kenya research laboratory in Kisumu for long-term storage at −80 °C. The remainder ∼1.5 mL of blood was cryopreserved in liquid nitrogen for future culture and in vitro susceptibility testing.
Subjects’ demographic information, malaria history, symptoms for current infections and physical examination findings were recorded in case report forms (CRFs). Subjects were treated with oral artemether-lumefantrine (AL; Coartem) administered over three consecutive days, a standard of care for P. falciparum malaria in Kenya. The first dose was observed by the study team and remaining doses were self-administered at home.
Microscopy
Thick and thin smears were prepared for low and high parasitemia respectively. The smears were stained in fresh working Giemsa stain (diluted 1:10 in buffered water from stock) for 15–20 min and air dried. The parasite count was recorded as parasites per 200 white blood cells (WBCs) for thick smear and per 2,000 red blood cells for the thin smear. The two were standardized to parasites per microliter. An assumed WBCs count of 8,000/µl that has been accepted as reasonably accurate in estimating malaria parasite densities was used (Afrane et al., 2014; Gyasi et al., 2015). To convert parasite count per 2000 RBCs to parasites/µl an assumed RBCs count of 4.5 ×106/ µL was used (Desai et al., 2015).
DNA extraction
DNA was extracted from blood spotted onto FTA filter papers using the QIAamp DNA Mini Kit (QIAGEN sciences, Maryland, USA) in accordance with the manufacturer’s protocol for dried blood spots. The purified DNA was stored at −20 °C until required.
Pfcrt K76T SNP analysis
A fragment of Pfcrt gene spanning K76T SNP was amplified by conventional polymerase chain reaction (PCR) on an Applied Biosystems’ GenAmp PCR system 9700 (Foster City, CA, USA). The primer sequences used for the analysis are as described in Wang et al. (2005). The primers were purchased from Applied Biosystems (Foster City, CA, USA). The primary reaction was performed in a 15 µL reaction mixture which consisted of 0.5 µL AmpliTaq Gold (Applied Biosystems, Foster City, CA, USA), 2.5 µL of 10 µM forward primer and reverse primers, 2.5 µL of 10X PCR Buffer, 2.5 µL of 10 mM dNTPs, 2.0 µL of 25 Mm MgCl2, 1 µL of DNA sample, and 4 µL water. The primary amplification was performed at the following conditions: 94 °C, 3 min for initial denaturation, 94 °C, 30 s for secondary denaturation and 45 cycles consisting of 56 °C, 30 s annealing, 60 °C for 30 s elongation followed by 64 °C for 3 min of final elongation and held at 4 °C. The primary PCR amplicon was then used as a template in the secondary PCR. The Secondary reaction was performed in a 25 µL reaction mixture which consisted of 0.5 µL AmpliTaq Gold (Applied Biosystems, Foster City, CA, USA), 2.5 µL of 1 µM forward primer and reverse primers, 2.5 µL of 10X PCR Buffer, 2.5 µL of 10 mM dNTPs, 1.5 µL of 25 mM MgCl2, 3.7 µL of primary PCR product, and water 11.8 µL. The secondary amplification was performed at the following conditions: 94 °C, 3 min for initial denaturation, 94 °C, 30 s for secondary denaturation and 20 cycles consisting of 47 °C, 30 s annealing, 64 °C for 30 s elongation followed by 64 °C for 3 min of final elongation and held at 4 °C. The samples were run in duplicate with one set containing primers targeting the wild type allele (K76) while the second had primers specific for the mutant allele (76T). 5 µL of the amplicon was mixed with 5 µL of gel loading dye on a parafilm and run concurrently with reference DNA ladder on a 2% gel. The two products per sample from secondary PCR were run concurrently on agarose gel to discriminate between the wild type, mutant and mixed alleles. Ethidium bromide dye was used to stain the agarose gel for visualization under UV light. The electrophoresis was performed at 97 volts for 30 min for both primary and secondary products. The bands on the gels were visualized immediately under UV light system (Ultra Violet Products Inc. Transilluminator). DNA from W2 and D6 Plasmodium falciparum clones were used as positive controls for mutant and wild type respectively while distilled water was used as a negative control. The negative control was not expected to show any band on gel after electrophoresis while positive control and positive samples were expected to have sizes of 560 base pairs (bps) for primary product, 342 bps for both mutant and wild type of the secondary product.
Pfmdr1 N86Y and Pfmdr1 Y184F SNP analysis
A fragment of Pfmdr1 gene spanning N86Y and Y184F SNPs was amplified to determine mutations by real-time PCR machine, Applied Biosystems’ prism 7500 Fast real-time PCR system (Foster City, CA, USA). The primer and probe sequences used are described elsewhere (Purfield et al., 2004). The probes were labeled with 6FAM (for wild type) or VIC (for Mutant), at their 5′ ends and non-fluorescent quencher (TAMRA) covalently attached at the 3′ end for each. Each well on PCR plate contained both the wild type and mutant type primers and probes. The presence of either or both alleles was showed from the increase of the FAM or VIC fluorophores in distinguishing wild, mutant or mixed alleles. These primers and probes were purchased from Applied Biosystems (Foster City, CA, USA). The reactions were performed in a 25 µL reaction mixture made of 18.75 µL of the master mix and 6.25 µL of the Plasmodium DNA. The master mix consisted; 6.55 µL of water, 5 µL of 25 mM MgCl 2,20 µL of Amplitaq Gold, 2.5 µL of 10X PCR buffer, 2.5 µL of 100 Mm, 0.5 µL of 100 µM of Pfmdr1 (86 or 184,) forward primer, 0.5 µL of 100 µM of Pfmdr1 (86 or 184) reverse primer, 0.25 µL of 100 µM of Pfmdr1 (86 or 184) wild type probe and 0.25 µL of 100 µM of Pfmdr1 (86 or 184) mutant probe. Amplification was performed at the following conditions: 1 cycle at 50 °C for 1 min, 1 cycle at 95 °C for 10 min and 45 cycles consisting of 95 °C for 15 s followed by 58 °C for 1 min.
Quality control was done by running samples and controls in triplicates. Additionally, quality control amplification results were valid only when the positive control (clones W2-mutant and D6-wild type) generated the acceptable average cycle threshold (CT) value ≤ 30 while the negative control (distilled water) remained unamplified therefore undetected.
Data management and analysis
Numerical data were expressed as proportions and compared using Pearson’s Chi-square. Parasitemia expressed as parasites/microliter were log transformed to the natural log and presented as medians with inter quartile range (IQR) and range. Comparison of parasitemia across age and regions was determined by Kruskal–Wallis test by ranks or one way analysis of variance (ANOVA) on ranks. Dunn’s pairwise multiple comparison test was used for post hoc analysis. Univariate and multivariate Logistic regression was used to assess the association of demographic and clinical factors to chloroquine resistance molecular markers. Significance levels were set at 0.05 at 95% confidence interval. Statistical analyses were performed using Stata version 13 (Stata Corp LP, College Station, Texas) while mapping was done in QGIS version 2.18.
Results
A total of 1,776 subjects presenting with uncomplicated malaria from four malaria epidemic zones across Kenya between 2008 and 2014 were evaluated. The lake endemic zone enrolled the highest number of subjects owing to high disease endemicity followed by highland epidemic zone while Semi-arid seasonal had the least. The demographic and clinical presentation characteristics are summarized in Table 1. Both genders were equally represented with 48.8% (n = 867) male and 51.2% (n = 909) female. Majority 56.7% (n = 999) were children below five years followed by 6–15 years 21.9% (n = 386) and above 16 years 21.3% (n = 376).
Table 1. Demographic and clinical characteristics of subjects enrolled by resistance.
| Characteristics | Malaria Resistant Status | ||||
|---|---|---|---|---|---|
| Resistant n (%) n = 941 | Non-Resistant n (%) n = 835 | Total n (%) n = 1,776 | Chi Square | p - value | |
| Sex | |||||
| Male | 472(54.4%) | 395(45.6%) | 867(48.8%) | 1.44 | 0.23 |
| Female | 469(51.6%) | 440(48.4%) | 909(51.2%) | ||
| Age group, years | |||||
| ≤5 | 520(52.1%) | 479(47.9%) | 999(56.7%) | 0.4 | 0.82 |
| 6–15 | 206(53.4%) | 180(46.6%) | 386(21.9%) | ||
| ≥16 | 202(53.7%) | 174(46.3%) | 376(21.3%) | ||
| Mean body temperature ±SD | 38.1 ± 1.24 | 38.1 ± 1.18 | 38.1 ± 1.21 | ** | ** |
| Ever travelled in the last 2 months | |||||
| Yes | 368(55.3%) | 297(44.7%) | 665(37.5%) | 2.36 | 0.125 |
| No | 572(51.6%) | 537(48.4%) | 1,109(62.5%) | ||
| Ever had malaria | |||||
| Yes | 746(52.5%) | 676(47.5%) | 1,422(80.2%) | 0.79 | 0.372 |
| No | 194(55.1%) | 158(44.9%) | 352(19.8%) | ||
| Treated for malaria in the last 6 weeks | |||||
| Yes | 118(62.8%) | 70(37.8%) | 188(10.6%) | 8.07 | 0.004 |
| No | 822(51.8%) | 764(48.2%) | 1,586(89.4%) | ||
| Chief Complaint | |||||
| Fever | 611(52.5%) | 552(47.5%) | 1,163(66.2%) | 0.46 | 0.978 |
| Headache | 200(54.1%) | 170(45.9%) | 370(21.1%) | ||
| Coughing | 10(55.6%) | 8(44.4%) | 18(1.0%) | ||
| Joint pains | 20(50.0%) | 20(50.0%) | 40(2.3%) | ||
| Others | 87(52.4%) | 79(47.6%) | 166(9.4%) | ||
Notes.
Not applicable for chi-square test. P value < 0.05 was considered significant.
- SD
- standard deviation
All subjects enrolled by this study had a mean temperature of 38.1 ±1.21, mean ± standard deviation (SD). The chief complaint was fever followed by headache that is in line with classical malaria presentation. At least 62.4% (n = 1,109) subjects had not travelled from their residence within two months prior to enrolment to the study. 80.2% (n = 1,422) reported to have had at least one episode of malaria before date of enrollment. Of these, 10.6% (n = 188) had been treated for malaria in the last 6 weeks prior to enrollment.
Malaria parasite density
The log transformed parasitemia for the 1,776 samples assessed as number of parasites diagnosed by microscopy per microliter of whole blood was median 10.4, IQR 9.1–11.5 and range 3.7–14.2. Using Kruskal–Wallis test by ranks, there was a significant difference [F (3) = 282.4, p < 0.0001] in parasitemia across the seven study sites located in the four malaria endemicity zones of Kenya. The lake endemic region had the highest parasitemia median 10.8 IQR 9.9–11.6 range 3.7–14.2 followed by coast endemic region median 9.9 IQR 8.8–10.7 range 4.8–11.7 while highland epidemic had the least median 8.9, IQR 7.7–10.4, range 4.4–14.2 (Fig. 2). Post hoc analysis by Dunn’s test showed that the lake endemic region has a significantly higher parasitemia than the rest of the transmission regions (p < 0.05) as shown in Fig. 2.
Figure 2. Comparison of parasite density by malaria epidemic zones.
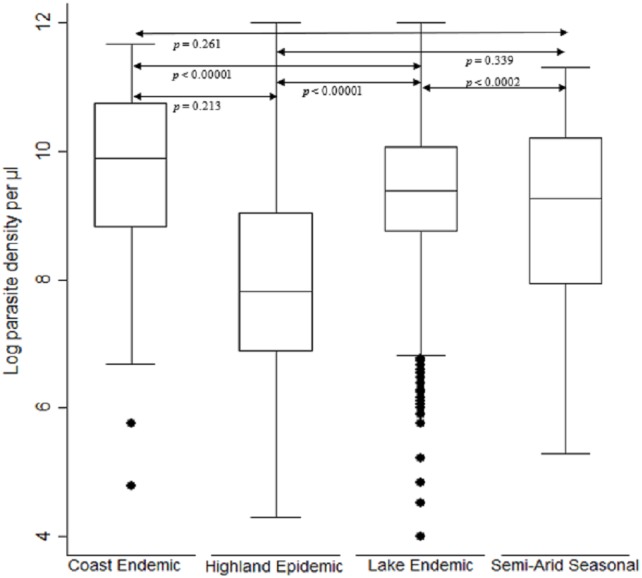
Parasite density (natural log transformation). Dots represent outliers. P value < 0.05 was considered significant.
When comparing parasite burden across age groups, individuals in age group <5 years had marginally higher parasitemia median 10.6, IQR 9.3–11.6 range 3.7–13.9 followed by that of age group 6–15 years, median 10.4, IQR 9.2–11.4 range 4.4–14.2. There was a significant difference [F (2) = 54.94, p < 0.0001] in parasite density among the age groups. Age group ≥ 16 years parasitemia differed significantly from those of both age groups of <5 years and 6–15 years (p < 0.05) (Fig. 3).
Figure 3. Comparison of parasite densities by age groups.
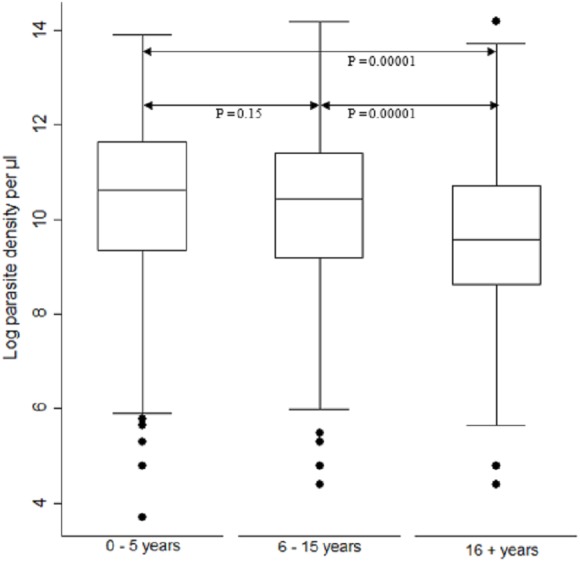
Parasite density (natural log transformation). Dots represent outliers. P value < 0.05 was considered significant.
Trends of Pfcrt 76, Pfmdr1 86 and Pfmdr1 184 polymorphisms
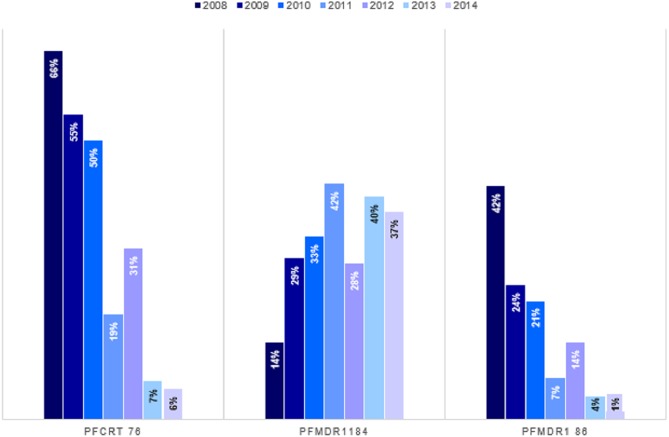
We established trends and distribution of chloroquine resistant genotypes; Pfcrt 76, Pfmdr 1 86 and Pfmdr 1 184 across Kenya between 2008 and 2014. The average frequency of the mutant genotype, Pfcrt 76T was 28.3% (n = 446), Wild-type Pfcrt K76 was 49.4% (n = 780) while mixed infection comprising both wild-type and mutant accounted for 22.3% (n = 353) infections countrywide (Table 2). Notably, there was a decline in frequency of Pfcrt 76T single nucleotide polymorphism (SNP) between 2008 and 2014 by 66% (n = 125) to 5.5% (n = 27) respectively (Fig. 4). There was a significant difference among the Pfcrt 76 polymorphisms across the years by Chi-square test [χ2(6) = 39.55, p <0.0001].
Table 2. Distribution of Pfcrt 76, Pfmdr1 86 and 184 single nucleotide polymorphisms by zone.
| Polymorphisms (n = 1,676) | Lake endemic | Highland epidemic | Semi-Arid seasonal | Coast endemic | Total | Chi square | p - value |
|---|---|---|---|---|---|---|---|
| Pfcrt−76 | |||||||
| Mutant | 302(29.6%) | 129(26.2%) | 3(11.1%) | 12(31.6%) | 446(28.3%) | 39.5 | 0.0001 |
| Wild-type | 467(45.7%) | 282(57.2%) | 21(77.8%) | 10(26.3%) | 780(49.4%) | ||
| Wild-type/mutant | 252(24.7%) | 82(16.6%) | 3(1.1%) | 16(42.1%) | 353(22.3%) | ||
| Pfmdr1-86 | |||||||
| Mutant | 155(14.4%) | 56(10.8%) | 3(12.5%) | 15(27.3%) | 229(13.7%) | 21.9 | 0.001 |
| Wild-type | 769(71.5%) | 415(79.7%) | 19(79.2%) | 35(63.6%) | 1,238(73.9%) | ||
| Wild-type/mutant | 152(14.1%) | 50(9.6%) | 2(8.3%) | 5(9.1%) | 209(12.5%) | ||
| Pfmdr1 184 | |||||||
| Mutant | 319(33.1%) | 168(32.3%) | 13(32.5%) | 7(13.5%) | 507(32.3%) | 17.1 | 0.009 |
| Wild-type | 434(45.0%) | 255(49.0%) | 13(32.5%) | 37(71.2%) | 739(47.1%) | ||
| Wild-type/mutant | 212(21.9%) | 97(18.7%) | 7(17.5%) | 8(15.4%) | 324(20.6%) |
Notes.
Samples with mutant, wild-type and a mixture of mutant/wild-type were identified for each epidemic zone. Undetermined samples contained neither mutant, Wild-type nor a mixture of mutant/Wild-type.
P < 0.05 was considered significant.
Figure 4. Trends of genotype frequencies of Pfcrt 76T, Pfmdr1 184F and Pfmdr1 86Y polymorphisms between 2008 and 2014.
There was a significant difference (p < 0.0001) for each of the Pfcrt 76T, Pfmdr1 184F and Pfmdr1 86Y SNPs with χ2 of 354, 780, 103 respectively from 2008 to 2014. Pfcrt 76 (n = 1,579), Pfmdr1 86 (n = 1,676) and Pfmdr1 184 (1570).
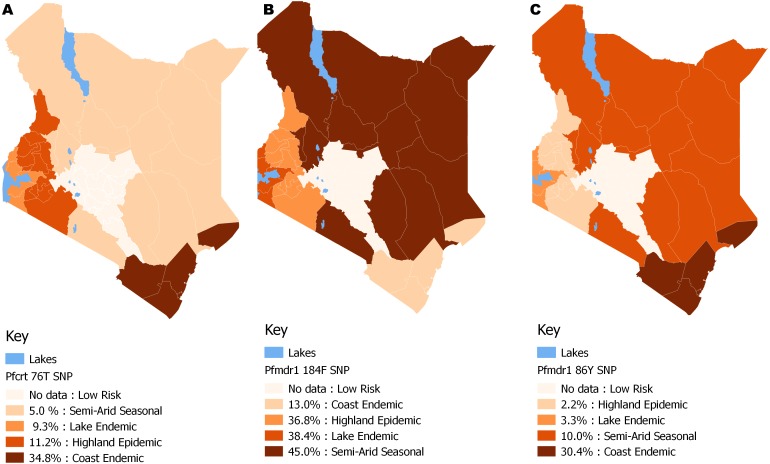
The distribution by the current years in the study (2012 to 2014) mutant genotype Pfcrt 76T by malaria endemicity zones showed that Coast endemic region had the highest 34.8% (n = 8) frequency as compared to highland epidemic 11.2% (n = 36), Lake endemic 26.2% (n = 39) and semi-arid seasonal with 5.0% (n = 3) as shown in Fig. 5. There was a significant difference in frequency of Pfcrt 76T polymorphism across the zones (p < 0.0001) over the study period. Distribution by age was 27.6% (n = 243), 26.9% (n = 94), 29.9% (n = 100) for ages ≤5, 6–15, ≥16 years respectively during the study period. There was no difference in Pfcrt 76T frequency across the age groups.
Figure 5. Spatial distribution patterns of (A) Pfcrt 76T, (B) Pfmdr1 184F and (C) Pfmdr1 86Y SNPs by malaria epidemic zones using QGIS version 2.18.
There was a significant difference among the Pfcrt 76T, Pfmdr1 184F and Pfmdr1 86Y SNPs across the epidemic zones (χ2 (6) = 39.5, p < 0.0001), (χ2 (6) = 21.9, p < 0.001) and (χ2 (6) = 17.1, p < 0.009) respectively.
The average frequency of the mutant genotype, Pfmdr 1 184F was 32.3% (n = 507), wild type Pfmdr 1 Y184 was 47.1% (n = 739) while mixed infection accounted for 20.6% (n = 324) infections countrywide (Table 2). A three-fold increase in Pfmdr 1 184F, SNP was noted between 2008 and 2014 with frequency of 13.7% (n = 27) to 37.2% (n = 191) (Fig. 4). There was a significant difference among the Pfmdr 1 Y184F polymorphisms across the years [χ2(6) = 17.07, p <0.009].
The distribution by the current years in the study (2012 to 2014) of mutant genotype Pfmdr 1 184F by malaria transmission zones showed a marginally higher frequency in the Semi-arid seasonal 45.0% (n = 9) followed by Lake endemic 38.4% (n = 161), Highland epidemic 36.8% (n = 118) and Coast endemic 13.0% (n = 3) as shown in Fig. 5. There was a significant difference in frequency of Pfmdr 1 184F SNP among the zones (χ2 = 17.1, p <0.009), (Table 2) across the study period. The Pfmdr 1 184F, SNP was comparable across all age groups 31.4% (n = 280) during the study period, 34.3% (n = 118), 32.9% (n = 105) for ages ≤5, 6–15, ≥16 years respectively. There was no difference in Pfmdr1 184F genotype mutation across the age groups.
The average frequency of the mutant genotype, Pfmdr1 86Y was 13.7% (n = 229), wild type Pfmdr 1 N86 was 73.9% (n = 1,238) while mixed infection comprising both wild-type and mutant accounted for 12.5% (n = 209) infections countrywide (Table 2). Notably, there was a decline of the Pfmdr 1 86Y SNP from 41.8% (n = 81) to 1.4% (n = 7) between 2008 and 2014 countrywide (Fig. 4). There was a significant difference among the Pfmdr 1 N86Y polymorphism across the years [χ2(6) = 21.87, p < 0.001].
The distribution by current years in the study (2012 to 2014) of mutant genotype, Pfmdr 1 86Y by malaria transmission zones showed that the coastal endemic had the highest 30.4% (n = 7) frequency followed by Semi-arid seasonal 10% (n = 2), Lake endemic 3.3% (n = 14) and Highland epidemic 2.2% (n = 7) (Fig. 5). There was a significant difference in frequency of Pfmdr 1 86Y SNP (Table 2) across the study period and was comparable among all age groups 12.8% (n = 122), 13.4% (n = 49), 16.3% (n = 56) for ages ≤ 5, 6-15, ≥ 16 years respectively during the study period. There was no difference in Pfmdr 1 86 genotype mutation across the age groups.
Demographic and clinical factors associated with chloroquine resistance
To explore the association between demographic factors and chloroquine resistance (CQR) based on Pfcrt 76 allele, a CQR primary marker, univariate and multivariate logistic regression was used (Table 3). Demographic factors included sex, age group, and travel in the last 2 months, ever had malaria, treated for malaria within the last 6 weeks and the malaria transmission zones. Sex was significantly associated with CQR, p <0.027 by univariate model and p <0.025 by multivariate model. Males were more likely to have CQR, 1.28 times by univariate model and 1.29 times by multivariate model. On the other hand, age was not significantly associated with CQR, though it’s important to note that subjects above 6 years of age were about one more time likely to harbor resistant genotypes. There was a significant relationship between having travelled in the last two months and CQR both by univariate model (p < 0.001) and multivariate model (p < 0.002), with subjects who had travelled being one or more time likely to be chloroquine resistant than those who had not. Having had malaria infection prior to the present visit was not associated with CQR, but individuals who had suffered from malaria before were one more time likely to be CQR than those who had no malaria infection prior to study enrollment. On the contrary, treatment in the last 6 weeks before visit was significantly associated with CQR by multivariate model (p < 0.034). Individuals who had treatment six weeks prior to the visit were one more time likely to acquire CQR. No association between living in malaria epidemic zones and CQR was observed. However, individuals in Lake endemic region are more likely to be CQR by 1.52 times (univariate model) and 1.87 times (multivariate model). Lake endemic region was followed by highland epidemic while people living in semiarid seasonal were less likely to be CQR.
Table 3. Association of demographic and clinical factors with CQR.
| Parameter | Chloroquine | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| Resistant (n = 446) | Non-Resistant (n = 1,330) | Total (n = 1,776) | OR(95% CI) | p- value | OR(95% CI) | p- value | |
| Sex | |||||||
| Female | 208(22.8%) | 701(77.1%) | 909(51.2%) | ref | ref | ||
| Male | 238(27.4%) | 629(72.6%) | 867(48.8%) | 1.28(1.03–1.58) | 0.027 | 1.29(1.03–1.61) | 0.025 |
| Age group, years | |||||||
| ≤5 | 243(24.3%) | 756(75.7%) | 999(56.7%) | ref | ref | ||
| 6–15 | 94(24.4%) | 292(75.7%) | 386(21.9%) | 1.00(0.76–1.32) | 0.991 | 1.08(0.82–1.43) | 0.577 |
| ≤16 | 100(26.6%) | 276(73.4%) | 376(21.4%) | 1.13(0.86–1.48) | 0.386 | 1.20(0.90–1.59) | 0.207 |
| Ever travelled in the last 2 months | |||||||
| Yes | 198(29.7%) | 467(70.2%) | 665(37.5%) | 1.47(1.18–1.83) | 0.001 | 1.44(1.14–1.79) | 0.002 |
| No | 248(22.4%) | 861(77.6%) | 1109(62.5%) | ref | ref | ||
| Ever had malaria | |||||||
| Yes | 354(24.9%) | 1068(75.1%) | 1422(80.2%) | 0.94(0.72–1.22) | 0.631 | 0.81(0.60–1.08) | 0.155 |
| No | 92(26.1%) | 260(74.6%) | 352(19.8%) | ref | ref | ||
| Treated for malaria in the last 6 weeks | |||||||
| Yes | 58(30.9%) | 130(69.1%) | 188(10.6%) | 1.38(0.99–1.92) | 0.056 | 1.46(1.03–2.06 | 0.034 |
| No | 388(24.5%) | 1198(75.5%) | 1586(89.4%) | ref | ref | ||
| Epidemic Zones | |||||||
| Coast Endemic | 12(19.4%) | 52(80.7%) | 62(3.5%) | ref | ref | ||
| Highland Epidemic | 129(23.3%) | 424(76.7%) | 553(31.1%) | 1.27(0.65–2.45) | 0.027 | 1.44(0.72–2.9) | 0.302 |
| Lake Endemic | 302(26.8%) | 823(73.2%) | 1125(63.3%) | 1.52(0.8–2.91) | 0.184 | 1.87(0.93–3.74) | 0.077 |
| Semi-Arid Seasonal | 3(8.3%) | 33(91.7%) | 36(2.0%) | 0.38(0.09–1.45) | 0.124 | 0.38(.09–1.52) | 0.174 |
Notes.
- Ref
- Reference group
- OR
- Odds ratio
- CI
- confidence interval
P < 0.05 was considered significant.
Discussion
Antimalarial resistance has significantly hindered malaria control efforts. Since effective vaccines for malaria are still way off, and drugs are most relied upon, success of malaria control strategies are dependent on understanding factors that influence dispersion of malaria drug resistance genotypes. Most studies on CQR appear inconclusive on methods containing it’s spread due to insufficient demographic and clinical information (Akala et al., 2014; Angira et al., 2010; Zhong et al., 2008). This study showed that travel, gender and clinical factors were associated with chloroquine resistance among symptomatic malaria cases in four of the five malaria epidemic zones in Kenya. Additionally, this study established that while Pfcrt 76 and Pfmdr 1 86 SNPs had reduced, the Pfmdr1 184 SNP was on the rise.
Travel and gender were significantly associated with CQR among symptomatic malaria individuals by both univariate and multivariate model (Table 3). The association between gender and CQR implies that males are more likely to be chloroquine resistant than females, though literature indicates that gender norms exposes females and children to the risk of malaria (Ministry of Health K, 2015). On the contrary, recent studies in Yemen and Malaysia did not show association between CQR and gender (Atroosh et al., 2012; Bamaga, Mahdy & Lim, 2015) despite compelling evidence of potential role of gender in epidemiology of malaria elsewhere (Diiro et al., 2016; WHO, 2007). The contrary findings could be attributed to the social cultural values around gender. A study done on prevalence of asymptomatic and submicroscopic malaria infections on Islands in Lake Victoria, Kenya, reported to have a significant prevalence of malaria in males than in females (p < 0.005) (Idris et al., 2016). Globally, gender dimensions also influence access to treatment, care for malaria and use of preventive measures (WHO, 2007). Females play the primary role of care giving to other members in the household, including leading the majority of health care seeking role for the rest of the family members (Diiro et al., 2016; Roll back malaria partnership, 2016) though males still dominate decision making on health and economic issues in the households (Diiro et al., 2016). Malaria is “gender blind” as mosquitoes bite indiscriminately (Ministry of Health K, 2015). However, in endemic regions, males may delay seeking treatment and risk accumulating super infection. Research has shown that mutant strains that develop drug resistance enhance virulence (Shahinas, Folefoc & Pillai, 2013) wanton utilization of resources and therefore eliciting symptoms that distorts the host/parasite balance that would allow wild type parasites to remain asymptomatic (Akala et al., 2014; Deroost et al., 2016).
The current finding on association of travel and CQR is concurrent with similar studies reported. A study done in Ethiopia reported effects of migration on parasite gene flow promoting transmission of chloroquine resistance (Lo et al., 2017). At a smaller scale, another study in Congo, reported evidence of imported resistant P. falciparum strains into Guatemala from Congo given globalization and international travels. Travelers from endemic regions with malaria symptoms should be suspected of harboring chloroquine resistant strains (Patel et al., 2014). Human population movement has been shown to contribute to spread of drug resistant parasite strains elsewhere (Lynch & Roper, 2011).
It was also noteworthy that treatment in the last 6 weeks before enrollment was not associated with CQR by univariate model but was associated by multivariate model. This is an implication that other factors contribute to CQR. Literature shows that irrational use of drugs (Nsubuga et al., 2011) gender dimensions (WHO, 2007), travel (Juliao et al., 2013), number of parasites exposed to a drug, the drug concentration to which the parasites are exposed, and the simultaneous presence of other antimalarials in the blood to which the parasite is not resistant influence the emergence and spread of drug resistant malaria parasites (WHO, 2018). These multiple factors argue for deployment of integrated research for effective malaria control. Such a strategy would include systems thinking, out-come-oriented monitoring and evaluation approaches, collaboration - across disciplines, sectors and regions -, multi-stakeholder engagement, and sensitivity to social equity (Wiese, 2012).
Other factors such as age, ever had malaria and location by epidemic zone were not associated with CQR. However, age has been shown to be a risk factor of malaria with a vast majority of malaria cases occurring under age of 5 years (WHO, 2016b). Though children who live in endemic areas may have parasites with drug resistance to Plasmodium falciparum, they often recover after chemotherapy. This is an indication that acquired immunity works in synergy with antimalarials (Enevold et al., 2007). On the other hand, the history of malaria is important in determining recurrent malaria, recrudescence of malaria, relapse and infection (Velho et al., 2014). Recurrence has been reported to be due to one or many of the following: therapeutic failure resulting from non-adherence to treatment, resistance of the parasite to the drug-use, poor quality of the medication, or sub-therapeutic doses of the drugs; (b) reactivation of hypnozoites; and (c) exposure to new infection by the mosquito vector (Hedt, Laufer & Cohen, 2011; White, 2011). Additionally, though location by epidemic zones was also not associated with CQR, determining location of origin of patients who present with malaria is key for successful intervention strategies (Pindolia et al., 2013).
High parasitemia was noted for samples from across all sites (Fig. 2) and age groups (Fig. 3) in Kenya regardless of the difference in disease transmission burden. Studies suggest that residents of holoendemic regions are often immune to malaria (Rolfes et al., 2012). They therefore can harbor parasitemia for long prior to showing symptoms than those in non-immune individuals residing in highland epidemic and semi-arid seasonal transmission zones of Kenya. It is however worth noting that microscopy that is the Kenya ministry of health’s guidelines recommended diagnosis method is less sensitive for detection of low parasitemia (Lo et al., 2015), a factor that could have excluded individuals with low parasitemia. Additionally, a study done in Tanzania reported a two-fold difference in parasite density when samples were collected at two time points (−2 and 0 h) prior to treatment (Carlsson et al., 2011), It is therefore possible that parasitemia for low transmission region would be higher than that reported here if all sub-microscopic parasitemia’s were depicted using sensitive methods and if two samples were collected at different time points. Further, the study design that included only symptomatic individuals with detectable malaria could have been biased towards individuals with higher load across all sites though similar studies in Southeast Asia have reported significantly lower parasitemia (Chaorattanakawee et al., 2013).
Analysis of trends and patterns of distribution of chloroquine resistant genotypes revealed a notable decline by 56.7% in the Pfcrt 76T and 38.8% for the Pfmdr 1 86Y mutant genotypes between 2008 and 2014 (Fig. 4). This presents a possibility for future introduction of chloroquine use for malaria treatment as a combination therapy. On the other hand, this observation indicates an increased risk of reinfection after treatment with AL. A study done to determine effects of Pfcrt and Pfmdr 1 gene polymorphisms on therapeutic responses to artesunate-amodiaquine (ASAQ) and AL reported that parasites with the Pfmdr 1 N86, and Pfcrt K76 alleles re-infected patients earlier after AL treatment (Venkatesanal et al., 2014), due to selection of less susceptible parasites (Sisowath et al., 2009). Another study done on genetically engineered P. falciparum lines reported that Pfmdr1 N86Y mutation increased parasite susceptibility to a wide range of first line antimalarials including Lumefantrine and Dihydroartemisinin (DHA) while decreasing parasite susceptibility to CQ. Pfmdr1 N86Y mutation was reported to alter parasite response DHA when compared to N86 parasites (Veiga et al., 2016). The mixed genotype Pfcrt K76T mutant and wild type allele is directly linked to both in vitro and clinical resistance. Therefore, it can still be used as a biomarker for CQ resistance (Wellems & Plowe, 2001).
On the other hand, there was a reciprocal increase for the Pfmdr 1 184F mutant genotype from 13.7% to 37.2% representing a 23.5% rise between 2008 and 2014 (Fig. 4). The implication of Pfmdr 1 Y184F mutation has been reported to be minimal on antimalarial drug susceptibilities (Veiga et al., 2016). This observation was contrary to a study reported in Grande Comore Island, Comoros between 2006–2014 where Pfmdr 1 184F mutations dropped from 52.2 to 30.0% (p < 0.01) (Huang et al., 2016). Another study done in Guinea-Bissau between 2003 and 2012 reported that there was no significant change in the Pfmdr 1 184F mutant allele (Jovel et al., 2015). A study done in Yemen, Tehama region reported a high prevalence of Pfmdr 1 184F mutant allele (99%) (Atroosh et al., 2016)and an increase from 19.6 to 22.7% in 2010–2012 in Maputo after introduction of ACT (Lobo et al., 2014). In Kenya, a significant association between polymorphisms at the Pfmdr 1 184 and lumefantrine have been reported (Achieng et al., 2015). However, a previous study from Odisha has reported the high prevalence of Pfmdr 1 86Y mutation and low prevalence of Pfmdr 1 184F mutation (Antony et al., 2016). Additionally, increased Pfmdr1 copy number has been associated with treatment failure after short 4-dose AL therapy (Sisowath et al., 2009). The increased Pfmdr1 copy numbers has been reported to be a rare observation in African population due to little exposure to MQ (Venkatesan et al., 2014).
Significant variation in genotype distribution was noted; the Coast and Lake endemic regions had the highest frequency of 31.6% and 29.6% for the Pfcrt 76T mutant allele respectively (Table 2). Genotype distribution for year 2014 (Fig. 5) by spatial analysis also showed varied distribution (Fig. 5). This observation could be partly due to inadequate enforcement on the ban of CQ and that CQ is still in use after its official withdrawal in Kenya (Rebelo et al., 2015). The coastal region had the highest Pfmdr 1 86Y mutant allele with 23.4%. CQ selects for parasites with Pfmdr 1 86Y mutation (Li et al., 2014). Semi-Arid seasonal had the highest Pfmdr 1 184F mutant allele with 32.5% while the coastal endemic region had the highest Pfmdr 1 Y184 allele at 57.8% suggesting that the population in the Semi-Arid seasonal zone have a higher risk to parasite tolerance to some antimalarials. It has been reported that polymorphism in the Pfmdr 1 gene is associated with an increase in parasite tolerance/resistance to some anti-malarials (Atroosh et al., 2016). This would also imply increased use of lumefantrine in the region. It has been reported that existence of Pfmdr 1 184F mutant allele increases with the use of lumefantrine (Malmberg et al., 2013; Sisowath et al., 2007). An increase of 18.4% to 23.5% access to AL from within 48 h of fever on set had been reported in 2015 (Wasunna et al., 2015), after implementation of a new drug policy in Kenya 2004 (Gitonga et al., 2008; Watsierah et al., 2012). The high parasitemia means that individuals living in the Lake endemic region will consequently have high rates of transmission of CQR genotypes as compared to other regions.
Previous studies show increasing chloroquine sensitivity (Eyase et al., 2013; Kiarie et al., 2015; Mohammed et al., 2013) after it was replaced by sulfadoxine-pyrimethamine in 1998 (Eyase et al., 2013). A study done in Tanzania reported >90% recovery of CQ susceptibility ten years after its withdrawal (Mohammed et al., 2013). Two studies done in Western Kenya reported a significant difference to CQ sensitivity in the Pfcrt 76 codon between 2008 and 2011 p < 0.001 (Eyase et al., 2013), and a decline from 76% to 6% of Pfcrt 76T prevalence from 2003 to 2015 (Vardo-Zalik et al., 2018). Another study done in Msabweni Kenya reported a 41% (n-99) in the Pfcrt 76T mutant allele in the year 2013 representing a significant decline in frequency compared to 2006 (p ≤ 0.05) (Kiarie et al., 2015). Similarly, this study shows increasing frequency of Pfcrt K76 and Pfmdr1 N86 genotypes that are suggestive of increasing CQ sensitivity in the four out of five malaria endemicity zones of Kenya heralding possible reintroduction of CQ based combination treatment. On the other hand, the prevalence Pfmdr 1 184F genotype increased during the 2008-14 period. The Pfmdr 1 184F mutation has been associated with AL resistance (Antony et al., 2016), and poses a threat to the sensitivity of lumefantrine and MQ (Ministry of Health, 2010). Lumefantrine in combination with artemether has been the recommended first-line treatment for uncomplicated malaria in Kenya since 2006 (Gitonga et al., 2008; Lucchi et al., 2015; Ministry of Health, 2010), while MQ is used as a chemoprophylaxis in Kenya (Ministry of Health, 2010).
Studies have shown that understanding the patterns of parasite dispersal from local hotspots of transmission can aid the design of additional targeted control by identifying both the regions where imported infections originate and where they may contribute substantially to transmission (Wesolowski et al., 2012). It will therefore be of great importance to establish gene flow patterns of Plasmodium falciparum resistant genotypes within the Kenyan population. This will be crucial in designing strategies towards control and prevention of malaria per epidemiological zone.
The limitation to this study was using a convenience sample. This implies that the sample size used in some regions could not be a representative of the whole population. Univariate and multivariate regression was used to analyze data that was stratified by age, gender and location to ensure that the analysis outcome is not due to confounding factors, bias or effect modifiers.
Conclusions
According to this study gender and travel have contributed to the dispersion of CQR genotypes in Kenya. The coast and lake endemic region bears the brunt of the burden among other regions. Additionally, children under the age of 5 years remain to be at a higher risk of malaria burden. Decline in CQR based on Pfcrt 76T SNP, is an indication that CQ can be re-considered for treatment of Pf malaria in future. An increase in Pfmdr 1 N86 wild type implies tolerance to lumefantrine. On the other hand, increased Pfmdr 1 184F mutant genotype frequency indicates a reduced sensitivity to lumefantrine. This study recommends continuous monitoring of CQR with wholesome information. Further studies should be done to determine migration patterns of Pf CQ resistant genotypes and gender factors associated with CQR in the malaria epidemic zones in Kenya.
Supplemental Information
Acknowledgments
We thank all GEIS clinical staff serving at the sentinel sites for their assistance and the study subjects and their parents/guardians who participated in the study and contributed their samples for study.
Funding Statement
This work was supported by the U.S. Department of Defense Global Emerging Infections System, Silver Spring, Maryland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Moureen Maraka, Email: maraka204@gmail.com.
Ben Andagalu, Email: Ben.Andagalu@usamru-k.org.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Moureen Maraka conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Hoseah M. Akala, Asito S. Amolo conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Dennis Juma, Duke Omariba, Agnes Cheruiyot, Benjamin Opot, Charles Okello Okudo, Edwin Mwakio, Gladys Chemwor, Jackline A. Juma, Raphael Okoth, Redemptah Yeda performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Ben Andagalu conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by the Kenya Medical Research Institute (KEMRI) and Walter Reed Army Institute of Research (WRAIR) institutional review boards (protocol numbers: KEMRI 1330, WRAIR 1384, and HRPO Log #A-19306.3) respectively.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.
References
- Achieng et al. (2015).Achieng AO, Muiruri P, Ingasia LA, Opot BH, Juma DW, Yeda R, Ngalah B, Ogutu B, Andagalu B, Akala H, Kamau E. Temporal trends in prevalence of Plasmodium falciparum molecular markers selected for by artemether- lumefantrine treatment in pre- ACT and post-ACT parasites in western Kenya. International Journal for Parasitology: Drugs and Drug Resistance. 2015;5(3):92–99. doi: 10.1016/j.ijpddr.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrane et al. (2014).Afrane Y, Zhou G, Githeko A, Yan G. Clinical malaria case definition and malaria attributable fraction in the highlands of western Kenya. Malaria Journal. 2014;13:405. doi: 10.1186/1475-2875-13-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akala et al. (2014).Akala HM, Achieng AO, Eyase FL, Juma DW, Ingasia L, Cheruiyot AC, Okello C, Omariba D, Owiti EA, Muriuki C, Yeda R, Andagalu B, Johnson JD, Kamau E. Five-year tracking of plasmodium falciparum allele frequencies in a holoendemic area with indistinct seasonal transitions. Journal of Multidisciplinary Healthcare. 2014;7:515–523. doi: 10.2147/JMDH.S67252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amambua-Ngwa et al. (2019).Amambua-Ngwa A, Amenga-Etego L, Kamau E, Amato R, Ghansah A, Golassa L, Randrianarivelojosia M, Ishengoma D, Apinjoh T, Maïga-Ascofaré O, Andagalu B, Yavo W, Bouyou-Akotet M, Kolapo O, Mane K, Worwui A, Jeffries D, Simpson V, D’Alessandro U, Kwiatkowski D, Djimde AA. Major subpopulations of Plasmodium falciparum in sub-Saharan Africa. Science. 2019;365(6455):813–816. doi: 10.1126/science.aav5427. [DOI] [PubMed] [Google Scholar]
- Angira et al. (2010).Angira CHO, Otieno OA, Muga RO, Abong’o BO. Factors contributing to antimalarial drug resistance in Rachuonyo district, Kenya. East African Journal of Public Health. 2010;7(1):11–15. doi: 10.4314/eajph.v7i1.64670. [DOI] [PubMed] [Google Scholar]
- Antony et al. (2016).Antony HA, Das S, Parija SC, Padhi S. Sequence analysis of pfcrt and pfmdr1 genes and its association with chloroquine resistance in Southeast Indian Plasmodium falciparum isolates. Genomics Data. 2016;8(2016):85–90. doi: 10.1016/j.gdata.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aribodor, Ugwuanyi & Aribodor (2016).Aribodor DN, Ugwuanyi IK, Aribodor OB. Challenges to achieving malaria elimination in Nigeria. American Journal of Public Health Research. 2016;4(1):38–41. doi: 10.12691/ajphr-4-1-6. [DOI] [Google Scholar]
- Atroosh et al. (2016).Atroosh WM, Al-Mekhlafi HM, Al-Jasari A, Sady H, Dawaki SS, Elyana FN, Al-Areeqi MA, Nasr NA, Abdulsalam AM, Subramaniam LR, Azzani M, Ithoi I, Lau YL. Different patterns of pfcrt and pfmdr1 polymorphism in Plasmodium falciparum isolates from Tehama region, Yemen. PeerJ. 2016;4:e2191. doi: 10.7717/peerj.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atroosh et al. (2012).Atroosh WM, Al-Mekhlafi HM, Mahdy MA, Surin J. The detection of pfcrt and pfmdr1 point mutations as molecular markers of chloroquine drug resistance, Pahang, Malaysia. Malaria Journal. 2012;11(1):251. doi: 10.1186/1475-2875-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamaga, Mahdy & Lim (2015).Bamaga OAA, Mahdy MA, Lim YA. Survey of chloroquine-resistant mutations in the Plasmodium falciparum pfcrt and pfmdr-1 genes in Hadhramout, Yemen. Acta Tropica. 2015;149:59–63. doi: 10.1016/J.ACTATROPICA.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Bloland (2001).Bloland PB. WHO/CDS/CSR/DRS/2001.4 Drug resistance in malaria. 2001. http://www.who.int/emc http://www.who.int/emc
- Borrmann et al. (2008).Borrmann S, Matsiegui PB, Missinou MA, Kremsner PG. Effects of Plasmodium falciparum parasite population size and patient age on early and late parasitological outcomes of antimalarial treatment in children. Antimicrobial Agents and Chemotherapy. 2008;52(5):1799–1805. doi: 10.1128/AAC.00755-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson et al. (2011).Carlsson AM, Ngasala BE, Dahlström S, Membi C, Veiga IM, Rombo L, Abdulla S, Premji Z, Gil JP, Björkman A, Mårtensson A. Plasmodium falciparum population dynamics during the early phase of anti-malarial drug treatment in Tanzanian children with acute uncomplicated malaria. Malaria Journal. 2011;10:380. doi: 10.1186/1475-2875-10-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaorattanakawee et al. (2013).Chaorattanakawee S, Tyner SD, Lon C, Yingyuen K, Ruttvisutinunt W, Sundrakes S, Sai-gnam P, Johnson JD, Walsh DS, Saunders DL, Lanteri CA. Direct comparison of the histidine-rich protein-2 enzyme-linked immunosorbent assay (HRP-2 ELISA) and malaria SYBR green i fluorescence (MSF) drug sensitivity tests in Plasmodium falciparum reference clones and fresh ex vivo field isolates from Cambodia. Malaria Journal. 2013;12(1):239. doi: 10.1186/1475-2875-12-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen et al. (2012).Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B. Malaria resurgence: a systematic review and assessment of its causes. Malaria Journal. 2012;11(1):122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroost et al. (2016).Deroost K, Pham TT, Opdenakker G, Van den Steen PE. The immunological balance between host and parasite in malaria. FEMS Microbiology Reviews. 2016;40(2):208–257. doi: 10.1093/femsre/fuv046. [DOI] [PubMed] [Google Scholar]
- Desai et al. (2015).Desai M, Gutman J, L’lanziva A, Otieno K, Juma E, Kariuki S, Ouma P, Were V, Laserson K, Katana A, Williamson J, Ter Kuile FO. Intermittent screening and treatment (IST) or 4 intermittent preventive treatment (IPT) with dihydroartemisinin-piperaquine versus IPT with 5 sulphadoxine-pyrimethamine for the control of malaria in pregnancy in western Kenya: a 6 randomized controlled su. Lancet Global Health. 2015;3(15):712–723. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiman (2019).Dhiman S. Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infectious Diseases of Poverty. 2019;8:14. doi: 10.1186/s40249-019-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diiro et al. (2016).Diiro GM, Affognon HD, Muriithi BW, Wanja SK, Mbogo C, Mutero C, Amexo M, Tolhurst R, Barnish G, Bates I, Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, Kioko UM, Mwabu G, Kimuyu P, Asenso K, Okyere F, Asante A, Tarekegn J, Andam KS, Russell S, Mutero CM, Kabutha C, Kimani V, Kabuage L, Gitau G, Ssennyonga J, Pell C, Strausx L, Andrew EV, Meñaca A, Pool R, Sena LD, Deressa WA, Ali AA, Mutulei ACN, Belay M, Deressa W, Ankomah A, Adebayo SB, Arogundade ED, Anyanti J, Nwokolo E, Ladipo O, Atieli HE, Zhou G, Afrane Y, Lee M-C, Mwanzo I, Githeko AK, Dike N, Onwujekwe O, Ojukwu J, Ikeme A, Uzochukwu B, Shu E, Deressa W, Fentie G, Girma S, Reithinger R, Ahmed SM, Zerihun A, Auta A, Rashed S, Johnson H, Dongier P, Moreau R, Lee C, Crepeau R, Macintyre K, Keating J, Sosler S, Kibe L, Mbogo CM, Githeko AK, Roy AD, Hanemann WM, Solís D, Bravo-Ureta BE, Quiroga RE, Kiiza BA, Pederson GD, Aliber M. The role of gender on malaria preventive behaviour among rural households in Kenya. Malaria Journal. 2016;15(1):14. doi: 10.1186/s12936-015-1039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enevold et al. (2007).Enevold A, Nkya WM, Theisen M, Vestergaard LS, Jensen AT, Staalsoe T, Theander TG, Bygbjerg IbC, Alifrangis M, Rapuoda B, Ali A, Bakyaita N, Mutabingwa TK, Mwita A, Rwagacondo C, Mugisha V, Staedke S, Snow RW, Watkins WM, Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR, Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LMB, Sidhu ABS, Naude B, Deitsch KW, Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WMWM, Winstanley PA, EstradaFranco JG, Mollinedo RE, Avila JC, Cespedes JL, Basco LK, Tahar R, Keundjian A, Ringwald P, Nzila AM, Nduati E, Mberu EK, Sibley CH, Monks SA, Winstanley PA, Watkins WM, Kublin JG, Dzinjalamala FK, Kamwendo DD, Malkin EM, Cortese JF, Martino LM, Mukadam RAG, Rogerson SJ, Lescano AG, Molyneux ME, Kyabayinze D, Cattamanchi A, Kamya MR, Rosenthal PJ, Dorsey G, Wellems TE, Plowe CV, Ochong EO, Van den Broek IVF, Keus K, Nzila A, Wernsdorfer WH, Noedl H, Dorsey G, Kamya MR, Singh A, Rosenthal PJ, Omar SA, Adagu IS, Warhurst DC, Djimde AA, Doumbo OK, Traore O, Guindo AB, Kayentao K, Diourte Y, Niare-Doumbo S, Coulibaly D, Kone AK, Cissoko Y, Khalil IF, Alifrangis M, Tarimo DS, Staalso T, Satti GMH, Theander TG, Ronn AM, Bygbjerg IC, Francis D, Nsobya SL, Talisuna A, Yeka A, Kamya MR, Machekano R, Dokomajilar C, Rosenthal PJ, Dorsey G, Targett GAT, Terlouw DJ, Aidoo MA, Udhayakumar V, Kolczak MS, Oloo AJ, Kager PA, Lal AA, Nahlen BL, Kuile FO ter, Robert V, Roeffen W, Brasseur P, Aribot G, Verhave JP, Roussilhon C, Mayxay M, Chotivanich K, Pukrittayakamee S, Newton P, Looareesuwan S, White NJ, Mawili-Mboumba DP, Borrmann S, Cavanagh DR, McBride JS, Matsiegui PB, Missinou MA, Kremsner PG, Ntoumi F, Pinder M, Sutherland CJ, Sisay-Joof F, Ismaili J, Mccall MBB, Ord R, Hallett R, Holder AA, Milligan P, Aubouy A, Migot-Nabias F, Deleron P, Pearce RJ, Drakeley C, Chandramohan D, Mosha F, Roper C, Snounou G, Zhu XP, Siripoon N, Jarra W, Thaithong S, Brown KN, Viriyakosol S, Alifrangis M, Enosse S, Pearce R, Drakeley C, Roper C, Khalil IF, Nkya WMMM, Ronn AM, Theander TG, Bygbjerg IC, Enevold A, Vestergaard LS, Lusingu J, Drakeley CJ, Lemnge MM, Theander TG, Bygbjerg IC, Alifrangis M, Liu YT, Old JM, Miles K, Fisher CA, Weatherall DJ, Clegg JB, Carvalho LJM, Oliveira SG, Theisen M, Alves FA, Andrade MCR, Zanini GM, Brigido MCO, Oeuvray C, Povoa MM, Muniz JAPC, Theisen M, Vuust J, et al. Potential impact of host immunity on malaria treatment outcome in Tanzanian children infected with Plasmodium falciparum. Malaria Journal. 2007;6(1):153. doi: 10.1186/1475-2875-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyase et al. (2013).Eyase FL, Akala HM, Ingasia L, Cheruiyot A, Omondi A, Okudo C, Juma D, Yeda R, Andagalu B, Wanja E, Kamau E, Schnabel D, Bulimo W, Waters NC, Walsh DS, Johnson JD. The role of Pfmdr1 and Pfcrt in changing Chloroquine, Amodiaquine, Mefloquine and Lumefantrine susceptibility in Western-Kenya P. falciparum Samples during 2008–2011. PLOS ONE. 2013;8(5):e64299. doi: 10.1371/journal.pone.0064299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitonga et al. (2008).Gitonga CW, Amin AA, Ajanga A, Kangwana BB, Noor AM, Snow RW. The use of artemether-lumefantrine by febrile children following national implementation of a revised drug policy in Kenya. Tropical Medicine & International Health. 2008;13(4):487–494. doi: 10.1111/j.1365-3156.2008.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyasi et al. (2015).Gyasi A, Asante K, Newton S, Amoako S, Dosoo D, Ankrah L, Adjei G, Amenga-Etego S, Owusu-Agyei S. Malaria parasite density estimated with white blood cells count reference value agrees with density estimated with absolute in children less than 5 years in central Ghana. Malaria Research and Treatment. 2015;1155(10):923674. doi: 10.1155/2015/923674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings & Donnelly (2005).Hastings IM, Donnelly MJ. The impact of antimalarial drug resistance mutations on parasite fitness, and its implications for the evolution of resistance. Drug Resistance Updates. 2005;8(1–2):43–50. doi: 10.1016/j.drup.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Ministry of Health K (2015).Ministry of Health K Gender and malaria in Kenya. Nairobi: Malaria Control Unithttps://www.measureevaluation.org/pima/malaria/gender-and-malaria-in-kenya. [1 August 2018];2015
- Ministry of Health (2010).Ministry of Health http://www.thehealthcompass.org/sites/default/files/project_examples/Kenya_Malaria_Tx_Guideline_2010.pdf National guidelines for the diagnosis, treatment and prevention of malaria in Kenya. 2010
- Hedt, Laufer & Cohen (2011).Hedt BL, Laufer MK, Cohen T. Drug resistance surveillance in resource-poor settings: current methods and considerations for TB, HIV, and malaria. American Journal of Tropical Medicine and Hygiene. 2011;84(2):192–199. doi: 10.4269/ajtmh.2011.10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2016).Huang B, Wang Q, Deng C, Wang J, Yang T, Huang S, Su X-z, Liu Y, Pan L, Li G, Li D, Zhang H, Bacar A, Abdallah KS, Attoumane R, Mliva AMSA, Zheng S, Xu Q, Lu F, Guan Y, Song J. Prevalence of crt and mdr-1 mutations in Plasmodium falciparum isolates from Grande Comore island after withdrawal of chloroquine. Malaria Journal. 2016;15(1):414. doi: 10.1186/s12936-016-1474-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris et al. (2016).Idris Z, Chan CW, Kongere J, Gitaka J, Logedi J, Omar A, Obonyo C, Machini BK, Isozumi R, Teramoto I, Kimura M, Kaneko A. High and heterogeneous prevalence of asymptomatic and sub-microscopic malaria infections on Islands in Lake Victoria, Kenya. Scientific Reports. 2016;6(1):36958. doi: 10.1038/srep36958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovel et al. (2015).Jovel IT, Kofoed PE, Rombo L, Rodrigues A, Ursing J. Temporal and seasonal changes of genetic polymorphisms associated with altered drug susceptibility to chloroquine, lumefantrine, and quinine in Guinea-Bissau between 2003 and 2012. Antimicrobial Agents and Chemotherapy. 2015;59(2):872–879. doi: 10.1128/AAC.03554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliao et al. (2013).Juliao PC, Sosa S, Gonzalez LD, Padilla N, Ortiz L, Goldman I, Udhayakumar V, Lindblade KA. Importation of chloroquine-resistant Plasmodium falciparum by Guatemalan peacekeepers returning from the Democratic Republic of the Congo. Malaria Journal. 2013;12:344. doi: 10.1186/1475-2875-12-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamau et al. (2015).Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, Mumba D, Kekre M, Yavo W, Mead D, Bouyou-Akotet M, Apinjoh T, Golassa L, Randrianarivelojosia M, Andagalu B, Maiga-Ascofare O, Amambua-Ngwa A, Tindana P, Ghansah A, Macinnis B, Kwiatkowski D, Djimde AA. K13-propeller polymorphisms in plasmodium falciparum parasites from Sub-Saharan Africa. Journal of Infectious Diseases. 2015;211(8):1352–1355. doi: 10.1093/infdis/jiu608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiarie et al. (2015).Kiarie WC, Wangai L, Agola E, Kimani FT, Hungu C. Chloroquine sensitivity: diminished prevalence of chloroquine-resistant gene marker pfcrt-76 13 years after cessation of chloroquine use in Msambweni, Kenya. Malaria Journal. 2015;14:328. doi: 10.1186/s12936-015-0850-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2014).Li J, Chen J, Xie D, Urbano J, Eyi M, Matesa RA, Miko M, Obono O, Ehapo CS, Yang L, Lu D, Yang H, Yang H-T, Lin M. High prevalence of pfmdr1 N86Y and Y184F mutations in Plasmodium falciparum isolates from Bioko island, equatorial Guinea. Pathogens and Global Health. 2014;108(7):339–343. doi: 10.1179/2047773214Y.0000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo et al. (2017).Lo E, Hemming-Schroeder E, Yewhalaw D, Nguyen J, Kebede E, Zemene E, Getachew S, Tushune K, Zhong D, Zhou G, Petros B, Yan G. Transmission dynamics of co-endemic Plasmodium vivax and P. falciparum in Ethiopia and prevalence of antimalarial resistant genotypes. PLOS Neglected Tropical Diseases. 2017;11(7):e0005806. doi: 10.1371/journal.pntd.0005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo et al. (2015).Lo E, Zhou G, Oo W, Afrane Y, Githeko A, Yan G. Low parasitemia in submicroscopic infections significantly impacts malaria diagnostic sensitivity in the highlands of Western Kenya. PLOS ONE. 2015;10(3):1–15. doi: 10.1371/journal.pone.0121763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo et al. (2014).Lobo E, De Sousa B, Rosa S, Figueiredo P, Lobo L, Pateira S, Fernandes N, Nogueira F. Prevalence of pfmdr1 alleles associated with artemether-lumefantrine tolerance/resistance in Maputo before and after the implementation of artemisinin-based combination therapy. Malaria Journal. 2014;13(1):300. doi: 10.1186/1475-2875-13-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchi et al. (2015).Lucchi NW, Komino F, Okoth SA, Goldman I, Onyona P, Wiegand RE, Juma E, Shi YP, Barnwell JW, Udhayakumar V, Kariuki S. In vitro and molecular surveillance for antimalarial drug resistance in plasmodium falciparum parasites in Western Kenya reveals sustained artemisinin sensitivity and increased chloroquine sensitivity. Antimicrobial Agents and Chemotherapy. 2015;59(12):7540–7547. doi: 10.1128/AAC.01894-15.Address. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch & Roper (2011).Lynch C, Roper C. The transit phase of migration: circulation of malaria and its multidrug-resistant forms in Africa. PLOS Medicine. 2011;8(5):e1001040. doi: 10.1371/journal.pmed.1001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg et al. (2013).Malmberg M, Ferreira PE, Tarning J, Ursing J, Ngasala B, Björkman A, Märtensson A, Gil JP. Plasmodium falciparum drug resistance phenotype as assessed by patient antimalarial drug levels and its association with pfmdr1 polymorphisms. Journal of Infectious Diseases. 2013;207(5):842–847. doi: 10.1093/infdis/jis747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbugi et al. (2006).Mbugi EV, Mutayoba BM, Malisa AL, Balthazary ST, Nyambo TB, Mshinda H. Drug resistance to sulphadoxine-pyrimethamine in Plasmodium falciparum malaria in Mlimba, Tanzania. Malaria Journal. 2006;5(1):94. doi: 10.1186/1475-2875-5-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed et al. (2013).Mohammed A, Ndaro A, Kalinga A, Manjurano A, Mosha JF, Mosha DF, Van Zwetselaar M, Koenderink JB, Mosha FW, Alifrangis M, Reyburn H, Roper C, Kavishe RA. Trends in chloroquine resistance marker, Pfcrt-K76T mutation ten years after chloroquine withdrawal in Tanzania. Malaria Journal. 2013;12(1):415. doi: 10.1186/1475-2875-12-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten & White (2007).Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. The American Society of Tropical Medicine and Hygiene. 2007;77(Suppl 6):181–192. doi: 10.4269/ajtmh.2007.77.181. [DOI] [PubMed] [Google Scholar]
- Nsubuga et al. (2011).Nsubuga P, Johnson K, Tetteh C, Oundo J, Weathers A, Vaughan J, Elbon S, Tshimanga M, Ndugulile F, Ohuabunwo C, Evering-Watley M, Mosha F, Oleribe O, Nguku P, Davis L, Preacely N, Luce R, Antara S, Imara H, Ndjakani Y, Doyle T, Espinosa Y, Kazambu D, Delissaint D, Ngulefac J, Njenga K. Field epidemiology and laboratory training programs in sub-saharan Africa from 2004 to 2010: need, the process, and prospects. Pan African Medical Journal. 2011;10(November):0–12. doi: 10.4314/pamj.v10i0.72235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll back malaria partnership (2016).Roll back malaria partnership https://endmalaria.org/sites/default/files/RBM_Gender_Fact_Sheet_170915.pdf Gender and Malaria Website. 2016
- Patel et al. (2014).Patel JC, Taylor SM, Juliao PC, Parobek CM, Janko M, Gonzalez LD, Meshnick SR, et al. Genetic evidence of importation of falciparum to guatemala from the democratic Republic of the Congo. Emerging Infectious Diseases. 2014;20(6):932–940. doi: 10.3201/eid2006.131204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindolia et al. (2013).Pindolia DK, Garcia AJ, Huang Z, Smith DL, Alegana VA, Noor AM, Snow RW, Tatem AJ. The demographics of human and malaria movement and migration patterns in East Africa. Malaria Journal. 2013;12:397. doi: 10.1186/1475-2875-12-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purfield et al. (2004).Purfield A, Nelson A, Laoboonchai A, Congpuong K, McDaniel P, Miller RS, Welch K, Wongsrichanalai C, Meshnick SR, Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR, Plowe CV, Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF, Wernsdorfer WH, Noedl H, Talisuna AO, Bloland P, D’Alessandro U, Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, Zalewski C, Kawamoto F, Miller RS, Meshnick SR, Price RN, Cassar C, Brockman A, Duraisingh M, Van Vugt M, White NJ, Nosten F, Krishna S, Takechi M, Matsuo M, Ziba C, MacHeso A, Butao D, Zungu IL, Chakanika I, Bustos MD, Ringwald P, Basco LK, Segurado AA, Di Santi SM, Shiroma M, Dua VK, Kar PK, Gupta NC, Kar I, Sharma VP, Trager W, Jensen JB, Livak KJ, Afonina I, Zivarts M, Kutyavin I, Lukhtanov E, Gamper H, Meyer RB, Holland PM, Abramson RD, Watson R, Gelfand DH, De Monbrison F, Raynaud D, Latour-Fondanaiche C, Staal A, Favre S, Kaiser K, Peyron F, Picot S, Viriyakosol S, Siripoon N, Zhu XP, Jarra W, Seugorn A, Brown KN, Snounou G. A new method for detection of pfmdr1 mutations in Plasmodium falciparum DNA using real-time PCR. Malaria Journal. 2004;3(1):9. doi: 10.1186/1475-2875-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebelo et al. (2015).Rebelo M, Tempera C, Fernandes JF, Grobusch MP, Hänscheid T. Assessing anti-malarial drug effects ex vivo using the haemozoin detection assay. Malaria Journal. 2015;14(1):140. doi: 10.1186/s12936-015-0657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfes et al. (2012).Rolfes MA, McCarra M, Magak NG, Ernst KC, Dent AE, Lindblade KA, John CC. Development of clinical immunity to malaria in highland areas of low and unstable transmission. American Journal of Tropical Medicine and Hygiene. 2012;87(5):806–812. doi: 10.4269/ajtmh.2012.11-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahinas, Folefoc & Pillai (2013).Shahinas D, Folefoc A, Pillai DR. Targeting Plasmodium falciparum Hsp90: towards reversing antimalarial resistance. Pathogens. 2013;2(1):33–54. doi: 10.3390/pathogens2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty et al. (2019).Shetty AC, Jacob CG, Huang F, Li Y, Agrawal S, Saunders DL, Lon C, Fukuda MM, Ringwald P, Ashley EA, Han KT, Hlaing TM, Nyunt MM, Silva JC, Stewart KE, Plowe CV, O’Connor TD, Takala-Harrison S, Onyamboko MA, Artemisinin Resistance Confirmation, Characterization, and Containment (ARC3) Artemisinin Resistance Containment and Elimination (ARCE) Tracking Resistance to Artemisinin Collaboration (TRAC) Genomic structure and diversity of Plasmodium falciparum in Southeast Asia reveal recent parasite migration patterns. Nature Communications. 2019;10(1):2665. doi: 10.1038/s41467-019-10121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisowath et al. (2007).Sisowath C, Ferreira PE, Bustamante LY, Dahlström S, Mårtensson A, Björkman A, Krishna S, Gil JP. The role of pfmdr1 in Plasmodium falciparum tolerance to artemether-lumefantrine in Africa. Tropical Medicine and International Health. 2007;12(6):736–742. doi: 10.1111/j.1365-3156.2007.01843.x. [DOI] [PubMed] [Google Scholar]
- Sisowath et al. (2009).Sisowath C, Petersen I, Veiga MI, Mårtensson A, Premji Z, Björkman A, Fidock DA, Gil JP. In vivo selection of plasmodium falciparum parasites carrying the chloroquine-susceptible pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. Journal of Infectious Diseases. 2009;199(5):750–757. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardo-Zalik et al. (2018).Vardo-Zalik A, Dixit A, Zhong D, Atieli H, Umukoro E, Hemming-Schroeder E, Fung B, Lo E, Githeko A, Yan G, Zhou G, Tomás-Domingo P. Impacts of antimalarial drugs on plasmodium falciparum drug resistance markers. Western Kenya. 2018;2003–2015:tpmd170763. doi: 10.4269/ajtmh.17-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga et al. (2016).Veiga MI, Dhingra SK, Henrich PP, Straimer J, Gnädig N, Uhlemann A-C, Martin RE, Lehane AM, Fidock DA. Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nature Communications. 2016;7:11553. doi: 10.1038/ncomms11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho et al. (2014).Velho DP, Alves-Jr ER, Ribatski-silva D, Gomes LT. Fatores associados às recidivas de malária causada por Plasmodium vivax no Município de Porto Velho, Rondônia, Brasil, 2009. Cad. Saúde Pública, Rio de Janeiroa. 2014;30(7):1–15. doi: 10.1590/S0074-02762007005000051. [DOI] [PubMed] [Google Scholar]
- Venkatesan et al. (2014).Venkatesan M, Gadalla NB, Stepniewska K, Dahal P, Nsanzabana C, Moriera C, Price RN, Martensson A, Rosenthal PJ, Dorsey G, Sutherland CJ, Guérin P, Davis TME, Ménard D, Adam I, Ademowo G, Arze C, Baliraine FN, Berens-Riha N, Björkman A, Borrmann S, Checchi F, Desai M, Dhorda M, Djimdé AA, El-Sayed BB, Eshetu T, Eyase F, Falade C, Faucher JF, Fröberg G, Grivoyannis A, Hamour S, Houzé S, Johnson J, Kamugisha E, Kariuki S, Kiechel JR, Kironde F, Kofoed PE, LeBras J, Malmberg M, Mwai L, Ngasala B, Nosten F, Nsobya SL, Nzila A, Oguike M, Otienoburu SD, Ogutu B, Ouédraogo JB, Piola P, Rombo L, Schramm B, Somé AF, Thwing J, Ursing J, Wong RPM, Zeynudin A, Zongo I, Plowe CV, Sibley CH. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. The American Journal of Tropical Medicine and Hygiene. 2014;91(4):833–843. doi: 10.4269/ajtmh.14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2005).Wang X, Mu J, Li G, Chen P, Guo X, Fu L, Chen L, Su X, Wellems TE. Decreased prevalence of the Plasmodium falciparum chloroquine resistance transporter 76T marker associated with cessation of chloroquine use against P. falciparum malaria in Hainan, People’s Republic of China. American Journal of Tropical Medicine and Hygiene. 2005;72(4):410–414. doi: 10.4269/ajtmh.2005.72.410. [DOI] [PubMed] [Google Scholar]
- Wasunna et al. (2015).Wasunna B, Okiro EA, Webster J, Todd J, Snow RW, Jones C. The impact of a community awareness strategy on caregiver treatment seeking behaviour and use of artemether-lumefantrine for febrile children in rural Kenya. PLOS ONE. 2015;10(7):1–19. doi: 10.1371/journal.pone.0130305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watsierah et al. (2012).Watsierah CA, Onyango RO, Ombaka JH, Abong’o BO, Ouma C. Provider knowledge of treatment policy and dosing regimen with artemether-lumefantrine and quinine in malaria-endemic areas of western Kenya. Malaria Journal. 2012;11:436. doi: 10.1186/1475-2875-11-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems & Plowe (2001).Wellems TE, Plowe CV. Chloroquine-resistant malaria. Journal of Infectious Diseases. 2001;184(6):770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- Wesolowski et al. (2012).Wesolowski A, Eagle N, Tatem AJ, Smith DL, Noor AM, Snow RW, Buckee CO. Quantifying the impact of human mobility on malaria. Science. 2012;338(6104):267–270. doi: 10.1126/science.1223467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White (2011).White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria determinants of relapse periodicity in Plasmodium vivax malaria. Malaria Journal. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker, Dean & Chancellor (2014).Whittaker MA, Dean AJ, Chancellor A. Advocating for malaria elimination—learning from the successes of other infectious disease elimination programmes. Malaria Journal. 2014;13(1):221. doi: 10.1186/1475-2875-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2007).WHO Gender, health and malaria. 2007. http://www.who.int/gender-equity-rights/knowledge/gender-health-malaria.pdf http://www.who.int/gender-equity-rights/knowledge/gender-health-malaria.pdf
- WHO (2009).WHO . Methods for surveillance of antimalarial drug efficacy. WHO; 2009. [30 September 2019]. [Google Scholar]
- WHO (2015).WHO Malaria in pregnant women. 2015. https://www.who.int/malaria/areas/high_risk_groups/pregnancy/en/ [19 January 2017]. https://www.who.int/malaria/areas/high_risk_groups/pregnancy/en/
- WHO (2016a).WHO Malaria. 2016a. http://www.who.int/mediacentre/factsheets/fs094/en/ [16 November 2016]. http://www.who.int/mediacentre/factsheets/fs094/en/
- WHO (2016b).WHO Malaria. 2016b. http://www.who.int/mediacentre/factsheets/fs094/en/ [09 November 2016]. http://www.who.int/mediacentre/factsheets/fs094/en/
- WHO (2018).WHO . World malaria report. 2018. [Google Scholar]
- Wiese (2012).Wiese M. Integrated approaches to malaria control—addressing new challenges to malaria research. Malaria Journal. 2012;11:P104. doi: 10.1186/1475-2875-11-s1-p104. [DOI] [Google Scholar]
- Witkowski et al. (2013).Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale JC, Mercereau-Puijalon O, Menard D. Reduced artemisinin susceptibility of plasmodium falciparum ring stages in western cambodia. Antimicrobial Agents and Chemotherapy. 2013;57(2):914–923. doi: 10.1128/AAC.01868-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodrow & White (2017).Woodrow CJ, White NJ. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiology Reviews. 2017;41:34–48. doi: 10.1093/femsre/fuw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2019).Zhao Y, Liu Z, Soe MT, Wang L, Soe TN, Wei H, han A, Aung PL, Li Y, Zhang X, Hu Y, Wei H, Zhang Y, Burgess J, Siddiqui FA, Menezes L, Wang Q, Kyaw MP, Cao Y, Cui L. Genetic variations associated with drug resistance markers in asymptomatic plasmodium falciparum infections in Myanmar. Gene. 2019;10(9):692. doi: 10.3390/genes10090692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong et al. (2008).Zhong D, Afrane Y, Githeko A, Cui L, Menge DM, Yan G. Molecular epidemiology of drug-resistant malaria in western Kenya highlandshighlands. BMC Infectious Diseases. 2008;8:105. doi: 10.1186/1471-2334-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.