Abstract
The feasibility of isolating and manipulating mesenchymal stem cells (MSCs) from human patients provides hope for curing numerous diseases and disorders. Recent phenotypic analysis has shown heterogeneity of MSCs. Nestin progenitor cell is a subpopulation within MSCs which plays a role in pancreas regeneration during embryogenesis. This study aimed to separate nestin (+) cells from human bone marrow MSCs, and differentiate these cells into functional insulin producing cells (IPCs) compared with nestin (-) cells. Manual magnetic separation was performed to obtain nestin (+) cells from MSCs. Approximately 91±3.3% of nestin (+) cells were positive for anti-nestin antibody. Pluripotent genes were overexpressed in nestin (+) cells compared with nestin (-) cells as revealed by quantitative real time-PCR (qRT-PCR). Following in vitro differentiation, flow cytometric analysis showed that 2.7±0.5% of differentiated nestin (+) cells were positive for anti-insulin antibody in comparison with 0.08±0.02% of nestin (-) cells. QRT-PCR showed higher expression of insulin and other endocrine genes in comparison with nestin (-) cells. While immunofluorescence technique showed the presence of insulin and C-peptide granules in nestin (+) cells. Therefore, our results introduced nestin (+) cells as a pluripotent subpopulation within human MSCs which is capable to differentiate and produce functional IPCs.
Key Words: Human bone marrow derived mesenchymal stem cells, insulin producing cells, real time-quantitative PCR, mesenchymal stem cells, diabetes mellitus
Diabetes mellitus (DM) is a widespread devastating disease affecting millions of people worldwide. Developing countries are affected by DM as more than 80% of diabetes deaths occur in low-income nations. Maintaining good glycemic control with exogenous insulin imposes a burden on patients. For DM, maintaining an appropriate glycemic control using exogenous insulin is possible but represents a load on patients. Transplantation of an intact pancreas as well as isolated pancreatic islets is ideal alternative. However, the shortage of cadaveric organs and the need for immunosuppression are limiting factors (1).
The progress in regenerative therapy field provides the potential for the generation of surrogate β-cells from stem cells derived from various sources. Mesenchymal stem cells (MSCs) display a high capacity for self-replication, thereby providing a large number of autologous cells while avoiding the limitations of ethical issues, organ availability, and allogeneic rejection. MSCs derived from various tissues were utilized in an attempt to differentiate them into insulin producing cells (IPCs) (2). Bone marrow (3-5) and adipose tissue (6) are among the several other tissues that have also been used to generate IPCs. Although the use of MSCs as a source for surrogate 𝛽-cells is very attractive, the most successful differentiation protocols have produced only a modest number of functional IPCs (7).
Nestin, which is a marker of neural stem cells (8), has been reported to generate high yield of insulin producing cells in vitro from nestin (+) cells derived from mouse embryonic stem cells (ESCs) (9). The mechanism of nestin action in ESCs and adult pancreatic ductal stem (PDS) cells was investigated in regard to the neogenesis of insulin-secreting β cells. These data may indicate that nestin is a stem cell marker and constitutes a functional factor during stem cell differentiation (10). It was suggested that nestin has important roles in governing the process of cell differentiation into IPCs (11, 12). Previous novel study was first to show that rat bone marrow multipotent nestin (+) stem cells can be differentiated in vitro into pancreatic ductal and insulin-producing β-cells (13). In addition to a previous study which developed a 4-step protocol to obtain nestin (+) cells from human blood derivate stem cells incubated in a three-dimensional culture, these cells expressed nestin after eight days and they were able to differentiate into IPCs (14). Moreover, a short protocol for in vitro differentiation of rat bone marrow stem cells into insulin progenitor cells was developed by induction of nestin synthesis and this protocol was suggested to be followed on stem cells from different sources, to confirm the early response of nestin (+) cell differentiation into IPCs precursors (15). Herein, we isolated nestin (+) cells from human bone marrow MScs, and evaluated their efficiency to differentiate into IPCs comparing with nestin (-) cells.
Materials and methods
Retrieval of human MSCs
All consents from the volunteers of bone marrow aspirates were obtained, and the study was approved by the Ethical Committee of Mansoura University. Bone marrow aspirates were collected from the iliac crest of three type II insulin-requiring human at Mansoura University Hospital.
Isolation and expansion of MSCs
Isolation and expansion of MSCs were carried out as previously described (16). Low glucose Dulbecco’s modified Eagle’s medium (DMEM) (Sigma Aldrich, United States) was used to dilute the obtained bone marrow aspirates by the ratio 1:1. The mixture was then added drop-wise on Ficoll-Paque, 1.077 g/ml (Pharmacia, Uppsala, Sweden) by the ratio of 2:1 respectively in order to form 2 distinct layers. Then a centrifugation at 600 g for 10 min was applied. Thereafter, the layer of mononuclear cells was collected from the DMEM/Ficoll interface then, phosphate-buffered saline (PBS) was used to wash cells twice. After that, cells were resuspended in 10 ml low-glucose complete DMEM (provided with100U/ml penicillin, and 100U/ml streptomycin (Sigma Aldrich) in addition to 10% fetal bovine serum (HyClone, Logan, Utah, United States). The cells were then cultured in 25 cm2 tissue culture flasks at a density of 5×105 nucleated cells/ml, and incubated in a 5% CO2 incubator at 37 oC. Discarding the non-adherent cells was performed after 3 days of culture. Subculture using trypsin was performed for the adherent MSCs when they reached 80% confluence where the cells were resuspended in complete culture media (DMEM) and recultured at a ratio of 1:2 till reaching 80% confluence and these steps were performed till the end of expansion phase.
Charactarization of the isolated MSCs
Phenotyping
At passage three, 1×106 cells of MSCs were resuspended in 1 ml PBS. Aliquots of 100 𝜇l were incubated for 30 min in 20 µl of antibodies against CD14, CD45 (FITC) or CD73, CD34 phycoerythrin (PE) or in 5 µl of CD105 PE or CD90 (FITC) (BDbiosciences, USA), then washed with 1ml of stain buffer (BDPharmingen, USA), and re-suspended in 500 𝜇l of stain buffer. The labeled cells were analyzed using an argon ion laser at a wavelength of 488 nm by BD FACSCalibur (BDbiosciences, USA). 1×104 events were analyzed using CellQuest software (BDbiosciences, USA).
Multilineage differentiation potential
MSCs at passage 3 were induced to differentiate into adipocytes, chondrocytes and osteocytes using differentiation protocol as previously described (17). Adipocytes, chondrocytes and osteocytes were evaluated using Oil-Red solution, alcian blue and alizarin red, respectively.
Isolation and expansion of nestin cells
Nestin (+) cells were isolated by magnetic cell separation method (Easysep magnetic cell separator, Stemcell Technologies, Canada) using EasySep® “Do-It-Yourself” Selection Kit (Stem cell Technologies, Canada) and nestin monoclonal antibody (ThermoFisher Scientific, Waltham, Massachusetts, United States) (18). Cell suspension was prepared at a concentration of 1x107 cells/100 µl recommended medium (PBS with 2% FBS and 1 mM EDTA, Ca+2 and Mg+2 free), the cocktail assembled for positive selection was added to the cell suspension at 10 µl/100 µl cells, then the suspension was incubated for 15 min at room temperature (RT) after mixing well, then magnetic nanoparticles were added at 5 µl/100 µl cells and mixed well then incubated for 10 min at RT. After that, the cell suspension was completed to reach a total volume of 2.5 ml by adding recommended medium. The tube with the cell suspension was then placed into the magnet for 5 min, then the supernatant was poured off. The tube was then removed from the magnet and 2.5 ml of recommended medium were added, the cell suspension was mixed gently and the tube was then placed back in the magnet and for 5 min, the last 2 steps were repeated, for a total of three 5-min separations. The isolated nestin (+) and nestin (-) cells were then cultured with the same method as MSCs.
Differentiation of nestin cells into IPCs
In vitro differentiation was performed according to a protocol previously reported by Tayaramma and his team (19). At the first stage, a serum-free DMEM medium provided with trichostatin-A (TSA) (Sigma Aldrich, USA) at a concentration of 55 nanomoles was used for cell culture for 3 days. At the second stage, the cell culture lasted for another 7 days using high-glucose (25 millimoles) medium composed of DMEM:DMEM/F12 (Sigma Aldrich, USA) with a ratio of 1:1 which was provided with fetal bovine serum (10%) and 10 nanomoles of glucagon-like peptide-1 (GLP-1) (Sigma Aldrich, USA).
Immunofluorescence
Cell preparations were cultured on chamber slides (Nunc, ThermoFisher Scientific, Waltham, Massachusetts, United States). Then, the cells were fixed for 10 min at RT using paraformaldehyde (4%), permeabilized by chilled methanol (100%) for 10 min and blocked with normal goat serum (5%) at RT for 60 min, then incubated in the primary antibodies at 4o C overnight. These included mouse monoclonal anti-nestin (ThermoFisher Scientific, USA), mouse monoclonal anti-insulin, rabbit polyclonal anti-c-peptide (cell Signaling Technology, Danvers, Massachusetts, USA), Thereafter, a washing step using PBS was applied for the cells, followed by an incubation at RT for 2 h using the secondary antibodies (Alexa Fluor 555-conjugated antirabbit IgG (H + L) and Alexa Fluor 488-conjugated antimouse IgG (H + L)) (Cell Signaling Technology, USA). The nuclei of these cells were counterstained with DAPI (Invitrogen, UK). Performing negative controls was achieved by avoiding treatment with the primary antibody (21). Leica TCS SP8 microscope (Leica Microsystems, Mannheim, Germany) was used for capturing confocal images.
Flow cytometric analysis of generated IPCs
Generated IPCs were fixed in 4% formaldehyde for 10 min at 37 °C, permeabilized by using chilled 90% methanol for 30 min and blocked in incubation buffer for 10 min at RT. Cells were then incubated with the conjugated antibody for 1 h at RT. The cells were washed with incubation buffer, then centrifuged and re-suspended in 0.5 ml PBS. The labeled cells were evaluated using a 15 mW argon ion laser at a wavelength of 488 nm by BD FACSCalibur flow cytometer. 1×104 cells were analyzed using CellQuest software (Becton, Dickinson). Mouse pancreatic islets served as a positive control.
Gene expression analysis by RT-qPCR
Total RNA was extracted from viable cells according to RNeasy Plus Mini Kit protocol (Qiagen, Germany). Then, the concentration and the purity of the extracted total RNA was measured by Nanodrop 2000 instrument (Thermo Fisher, USA). Conversion of three micrograms of total RNA into cDNA was performed using RT2 First Strand kit according to manufacturer's instruction (Qiagen Sciences, USA). Gene expression was evaluated for pluripotent genes (NANOG, SOX2, and OCT4), nestin and PDX1 genes at the end of expansion phase (Table 1). Custom gene arrays CAPH13024D were designed and supplied in 96-well plates for pancreatic endocrine genes (Qiagen, Germany) including; insulin (INS), glucagon (GCG), and somatostatin (SST), transcription factors (PDX1, RFX6, and Neurod-1), glucose transporter (GLUT2), and pancreatic enzyme (glucokin-ase). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene for mathematical calculation (20). Human islets were also included to serve as positive control. Each reaction of the RT-PCR was performed in 25 𝜇l total volume which contained 12.5 𝜇l of 2X SYBR Green Rox Master Mix (Qiagen Sciences, USA), 100 ng of cDNA template and 10 nmol of primers. This technique was performed by BioRad CFX96 thermal cycler (BioRad, USA) and the program was designed according to manufacturer’s instructions.
Table 1.
List of human gene-specific primers for qRT-PCR
| Gene | Forward primer | Reverse primer | Amplicon size (bp) | Accession number |
|---|---|---|---|---|
| SOX2 | GGATAAGTACACGCTGCCCG | CTGTCCATGCGCTGGTTCAC | 111 | NM_003106.3 |
| NANOG | GAAGGCCTCAGCACCTACCT | GGTTGCTCCACATTGGAAGGTT | 95 | NM_024865.3 |
| NES | GGGCCTACAGAGCCAGATCG | CAGGAGGGTCCTGTACGTGG | 103 | NM_006617.1 |
| OCT4 | TGCCAAGCTCCTGAAGCAGA | CGTTTGGCTGAATACCTTCCCAAA | 100 | NM_002701.5 |
| PDX1 | GCTGGCTGTCATGTTGAACT | CGCTTCTTGTCCTCCTCCTT | 93 | NM_000209.3 |
| GAPDH | TCTTTTGCGTCGCCAGCC | ACATGTAAACCATGTAGTTGAGGTC | 178 | NM_002046.5 |
SOX2: SRY-box2; NANOG: nanog homeobox; OCT4: POU class 5 homeobox 1, also known as POU5F1, OCT3, OCT4, OTF3, OTF-3; PDX1: pancreatic and duodenal homeobox 1; NES: nestin; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
Determenation of insulin and C-peptide release in response to increasing glucose concentrations
Insulin and C-peptide release of differentiated cells was performed according to the procedure described before (5). Three different sample (1×106) cells were collected from the same batch of each donor at the end of the differentiation period of nestin (+) and nestin (-) cells for measurement of released insulin and C-peptide. Cells were initially incubated for 3 h in glucose-free Krebs-Ringer bicarbonate buffer (KRB) (NACL 119 mM, Kcl 4.7 mM, CaCl2 2.5 mM, MgSO4 1.2 mM, KH2PO4 1.2 mM, NaHCO3 25 mM, Hepes 10 mM, and BSA 0.1%) . This was followed by incubation for 1 h in 3.0 ml of KRB containing 5.5, 12, or 25 mM glucose concentrations. At the end of incubation, the supernatant was collected and samples were assayed using an Elisa Kit (Diagnostic Automation /Cortez Diagnostics, Inc., USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were carried out using the program SPSS 16. Data from three donors are presented as mean and standard error (SE) and the error bar in the bar graph represents SE. Data were examined to determine whether they were normally distributed with the One- Sample Kolmogorov-Smirnov test and were found to be normally distributed, comparison of measurement data between the two groups was performed by independent sample t-test. Statistical tests were two-tailed and a p-value of less than 0.05 was considered statistically significant (22).
Results
General characteristics of isolated MSCs
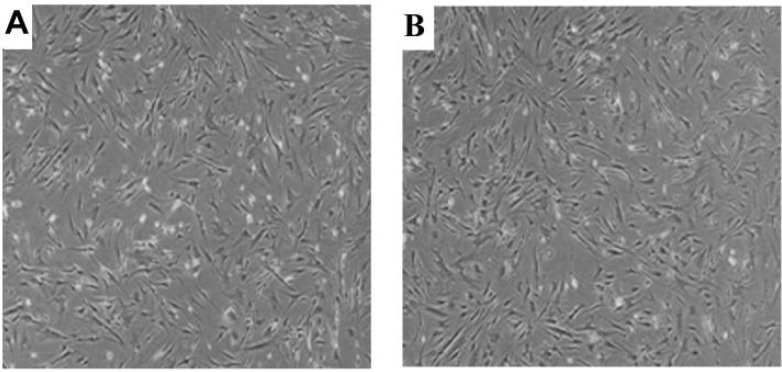
The cultured MSCs at the end of expansion phase became spindle-shaped, fibroblast-like cells arranged in monolayers. At passage 5, there was no difference in morphology between nestin (+) and nestin (-) cells (Fig. 1). Flow cytometric analysis of the isolated MSCs showed high positivity to mesenchymal surface markers (CD90, CD105 and CD73), while these cells were negligible for the expression of hematopoietic surface markers (CD14, CD34 and CD45) (Table. 2). Multili-neage differentiation potential was confirmed after staining the cells and investigation under an inverted microscope. Cells could be differentiated to form adipocytes, chondrocytes and osteocytes when the appropriate growth factors were added (Fig. 2).
Fig. 1.
Morphological features of nestin (+) and nestin (-) cells during expansion. A: cultured nestin (+) cells; B: cultured nestin (-) cells
Table 2.
Surface markers expression of the isolated human bone marrow-MSCs by flow cytometric analysis
| CD | CD105 | CD90 | CD73 | CD14 | CD34 | CD45 |
|---|---|---|---|---|---|---|
| Percentage (%) | 95.7 98.3 97.7 97.9 97.7 96.5 |
93.6 96.8 92.1 97.8 97.0 89.1 |
98.7 96.1 98.4 99.4 96.9 96.7 |
0.0 0.31 0.6 0.8 0.7 0.7 |
0.34 0.1 0.9 1.0 0.05 0.8 |
0.05 0.0 0.07 1.2 0.2 0.9 |
| Mean ± S.E. | 97.3±0.99 | 94.40±3.41 | 97.7±1.31 | 0.52±0.3 | 0.53±0.42 | 0.4±0.51 |
Fig. 2.
Multilineage differentiation of MSCs. A: adipocyte cells stained with oil red; B: chondrocyte cells stained with alcian blue; C: osteocyte cells stained with alizarin red
Immunofluorescence
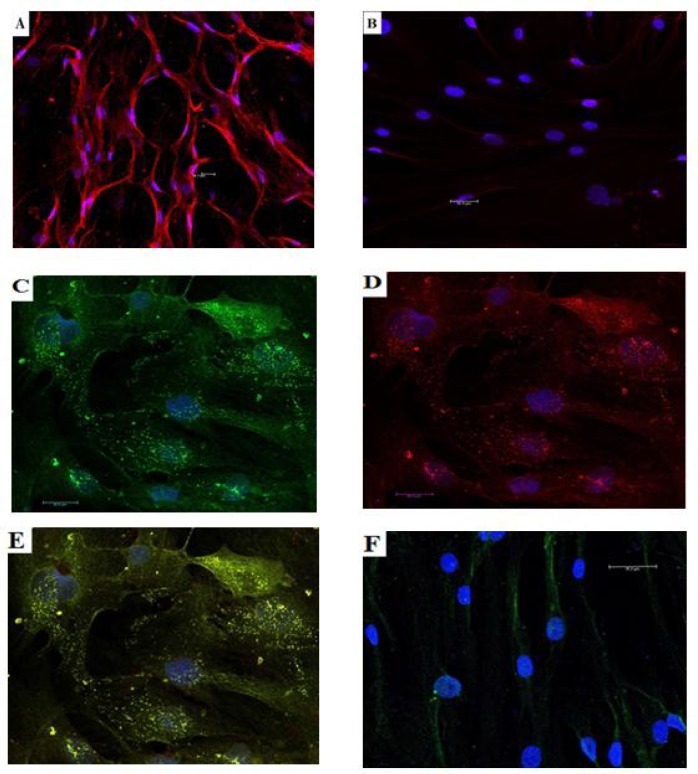
The expression of nestin surface marker of isolated cells were performed by staining the cells with monoclonal anti-nestin antibody after magnetic cell separation. Confocal microscope and cell count analysis was performed after cellular staining and the result showed that 91±3.3% of nestin (+) cell population was positive to anti-nestin expression, while only 1.2±0.26% of nestin (- ) cells were positive (P <0.01) (Fig. 3a-b). After in vitro differentiation protocol, the generated IPCs were stained with anti-insulin and anti C-peptide antibodies followed by confocal microscope and cell count detection. Approximately, 14.3±1.9% of differentiated nestin (+) cells were positive to insulin detection, while only 2.17±0.42% of nestin (-) cells were positive to insulin detection (P <0.01). Only in differentiated nestin (+) cells, immune staining of C-peptide was positive and the co-expression of insulin and C-peptide within the same cells was detected following electronic merging (Fig. 3c-f).
Fig. 3.
Immunofluorescence staining of nestin (+) and nestin (-) cells with counterstaning for DAPI (blue). A: nestin (+) cells stained with anti-nestin (red); B: nestin (-) cells stained with anti-nestin; C: IPCs derived from nestin (+) cells stained with anti-insulin (green); D: IPCs derived from nestin (+) cells stained with anti-C-peptide (red); E: co-expression of insulin and C-peptide by the same IPCs derived from nestin (+) cells by electron merge (yellow); F: nestin (-) cells stained with anti-insulin
Flow cytometric analysis of IPCs
Generated IPCs from nestin (+) and nestin (-) cells were intracellulary stained with anti-insulin. The results showed that 2.78±0.5% of differentiated nestin (+) cells were positive to anti-insulin, while 0.08±0.02% of nestin (-) cells were positive to anti-insulin (P <0.01) (Table. 3).
Table 3.
Flow cytometric analysis of generated IPCs
| Cells | Nestin (+) MSCs | Nestin (-) MSCs |
|---|---|---|
| Percentage of nestin positive cells (%) | 1.29 | 0.15 |
| 1.16 | 0.24 | |
| 1.33 | 0.14 | |
| 2.6 | 0.03 | |
| 2.43 | 0.01 | |
| 1.97 | 0.01 | |
| 4.73 | 0.07 | |
| 4.52 | 0.04 | |
| 5.03 | 0.04 | |
| Mean ± S.E. | 2.78±0.52 | 0.08±0.02 |
| P value | <0.01 | |
Gene expression evaluation by RT-PCR
The expression of nestin, NANOG, SOX2, PDX1 and OCT4 was higher in nestin (+) cells when compared to nestin (-) cells, and relatively to the expression of MSCs population by 3-fold, 1.5-fold, 1-fold, 1-fold and 1.5-fold, respectively (Fig. 4a). At the end of differentiation protocol, the relevant endocrine genes including INS, GCG and SST in addition to the transcription factor PDX1 were expressed in nestin (+) and nestin (-) cells. The results were calculated relative to the expression of human islet genes. Worthwhile, the expression of INS gene in nestin (+) cells was increased by 2.6 folds as well as GCG and SST by 2.24 and 2.83 folds, respectively. The transcription factor PDX1 was also upreulated in nestin (+) cells by 2.49 folds in comparison with nestin (-) cells (Fig. 4b).
Fig. 4.
QRT-PCR of nestin (+) and nestin (-) cells. A: relative gene expression of pluripotent markers, nestin and PDX1 of the undifferentiated nestin (+) and nestin (-) cells relative to MSCs; B: endocrine gene expression of IPCs derived from nestin (+) and nestin (-) cells relative to human islets gene expression
In vitro human insulin and C-peptide release in response to glucose challenge
The differentiated IPCs released increasing amounts of insulin and C-peptide in response to increasing glucose concentrations (P <0.01). The released insulin and C-peptide amounts at different concentrations of glucose were comparable between the two populations (Fig. 5).
Fig. 5.
Human insulin and C-peptide release by IPCs derived from nestin (+) and nestin (-) cells by ELISA technique in response to glucose concentration challenge. A: insulin release; B: C-peptide release
Discussion
Stem cells have tracked the attention as a prospective cure for DM (23-25). Bone marrow has been known to be a rich and accessible source for adult stem cells. It was reported that MSCs derived from bone marrow are better than adipose tissue MSCs in terms of differentiation into IPCs (26). Other studies had provided data indicating that human MSCs can express insulin in addition to key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or micro environmental manipulation in vitro (27, 28) and even from other subpopulation within MSCs such as Muse cells (29).
The intermediate filament protein “nestin” has been detected in several cellular phenotypes during embryonic and adult life. Nestin expression would indicate multipotent and regenerative character of cells (30). In pancreas, the expression of nestin has been reported to be a marker for pancreatic stem cells and for islet progenitor cells (31). Previous literature has reported that insulin-producing cells are generated by the isolation of nestin expressing mouse (32) and human (33) ESCs by the selection of progenitor cells expressing nestin (9, 34).
Nowadays, there are two different techniques to isolate cellular subpopulations; they are fluorescence-activated cell sorting (FACS) and magnetically activated cell sorting (MACS). It has been shown that FACS separation represents a physical stress on cells and can affect proliferation capacity of the cells while this can be avoided by using MACS because of biodegradability of the magnetic microbeads, hence the last technique was used for the isolation of the two populations (35).
Isolated MSCs showed the full characteristics of multipotent MSCs after applying the minimal criteria by Dominici et al. (36). Immuno-fluorescence by confocal microscope was used to evaluate nestin (+) cell percentage in the two isolated subpopulations. The highly significant difference between nestin (+) and nestin (-) cells indicated that they were successfully isolated from the whole bone marrow MSCs population.
Moreover, the evaluation of undifferentiated cells of the two subpopulations by gene expression showed that nestin expression was significantly higher in nestin (+) than nestin (-) cells. While nestin (+) cells expressed pluripotent and PDX1 genes in higher amount compared with nestin (-) cells.
Various protocols have been applied for inducing MSCs derived from bone marrow to differentiate into IPCs in vitro (3, 37, 38). In the current study, we have utilized TSA in a two-step protocol to induce the expression of PDX1. TSA is a natural product isolated from the metabolites of strains of Streptomyces hygroscopicus with antifungal and antibiotic activities (39).
Evidence was provided that TSA has the potential of chromatin remodeling and can permit bone marrow stem cells to differentiate into IPCs under appropriated culture conditions in the presence of high glucose concentrations and GLP-1 (19). Glucose is well known as a growth factor for 𝛽 cells (40). It promotes 𝛽 cells replication in vitro as well as in vivo at concentrations of 20–30 mM (41). The conversion of intestinal epithelial cells into functional IPCs can be achieved by the incretin hormone GLP-1 (42).
At the end of differentiation, all the relevant endocrine genes, particularly INS, GCG, and SST, were expressed. Their relative values for nestin (+) cells were significantly higher than those for nestin (-) cells.
In the present study, at the end of in vitro differentiation, flow cytometric analysis indicated that the proportion of insulin-positive cells was significantly higher in nestin (+) cells with a mean percentage of 2.78% than in nestin (-) cells with a mean percentage of 0.08%. In addition, immunofluorescence by confocal microscope was used to determine the insulin percentage of the differentiated nestin (+) cell population, which was 14.3% while it was 2.17% for nestin (-) cell population with a significant difference.
Several investigators had argued that the insulin present in differentiated cells does not indicate intrinsic insulin production as insulin may be absorbed from the culture media and sequestrated in these cells (43-45). In this study, immunofluorescence staining for the differentiated nestin (+) cells was positive for insulin and C-peptide with coexpression of insulin and C-peptide within the same cells confirming that proinsulin synthesis occurred in the cells, and insulin was not derived from any insulin in the culture media.
The poor insulin release in response to glucose challenge was also reported by several investigators (45, 46). The use of different sources of cells and measurement units for reference, causes a difficulty in comparing different data (47). The results of insulin and C-peptide release in this study indicated their increase with increasing glucose concentration which was significantly higher for nestin (+) MSCs than nestin (-) MSCs. These data indicate that for a similar number of cells, insulin release at an equivalent glucose concentration for nestin (+) was 0.018 ng/µg/h. Accordingly, this amount corresponds to roughly 3% of that released by human islets, this ratio is supported by the previous finding (4).
In conclusion, nestin (+) and nestin (-) cells showed the ability to generate functional IPCs in vitro with modest percentage. Till now the answer to “which sub-population within MSCs generates IPCs” question is not provided. Furthermore, our study aimed to investigate whether nestin (+) cells are responsible only for generating IPCs or not, and the results showed that nestin (-) cells generated IPCs too but in lower percentage in comparison with nestin (+) cells. Improving and identifying culture conditions and differentiation protocol may help to understand the mystery of MSCs heterogeneity.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Hirshberg B, Rother KI, Digon BJ, 3rd , et al. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care. 2003;26:3288–95. doi: 10.2337/diacare.26.12.3288. [DOI] [PubMed] [Google Scholar]
- 2.Liu M, Han ZC. Mesenchymal stem cells: biology and clinical potential in type 1 diabetes therapy. J Cell Mol Med. 2008;12:1155–68. doi: 10.1111/j.1582-4934.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y, Chen L, Hou XG, et al. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl) 2007;120:771–6. [PubMed] [Google Scholar]
- 4.Karnieli O, Izhar-Prato Y, Bulvik S, et al. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–44. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 5.Gabr MM, Zakaria MM, Refaie AF, et al. Insulin-producing cells from adult human bone marrow mesenchymal stem cells control streptozotocin-induced diabetes in nude mice. Cell Transplant. 2013;22:133–45. doi: 10.3727/096368912X647162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Timper K, Seboek D, Eberhardt M, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135–40. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 7.Evans-Molina C, Vestermark GL, Mirmira RG. Development of insulin-producing cells from primitive biologic precursors. Curr Opin Organ Transplant. 2009;14:56–63. doi: 10.1097/MOT.0b013e3283186fc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuoka M, Osada T, Yoshida-Matsuoka J, et al. A comparative immunocytochemical study of development and regeneration of chemosensory neurons in the rat vomeronasal system. Brain Res. 2002;946:52–63. doi: 10.1016/s0006-8993(02)02823-8. [DOI] [PubMed] [Google Scholar]
- 9.Blyszczuk P, Czyz J, Kania G, et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SY, Lee S, Hong SW, et al. Nestin action during insulin-secreting cell differentiation. J Histochem Cytochem. 2010;58:567–76. doi: 10.1369/jhc.2010.955682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maria-Engler SS, Correa-Giannella ML, Labriola L, et al. Co-localization of nestin and insulin and expression of islet cell markers in long-term human pancreatic nestin-positive cell cultures. J Endocrinol. 2004;183:455–67. doi: 10.1677/joe.1.05703. [DOI] [PubMed] [Google Scholar]
- 12.Zulewski H, Abraham EJ, Gerlach MJ, et al. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes. 2001;50:521–33. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 13.Milanesi A, Lee JW, Xu Q, et al. Differentiation of nestin-positive cells derived from bone marrow into pancreatic endocrine and ductal cells in vitro. J Endocrinol. 2011;209:193–201. doi: 10.1530/JOE-10-0344. [DOI] [PubMed] [Google Scholar]
- 14.Hur J, Yang JM, Choi JI, et al. New method to differentiate human peripheral blood monocytes into insulin producing cells: Human hematosphere culture. Biochem Biophys Res Commun. 2012;418:765–9. doi: 10.1016/j.bbrc.2012.01.096. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Gamboa M, Cruz-Vega DE, Moreno-Cuevas J, et al. Induction of Nestin Early Expression as a Hallmark for Mesenchymal Stem Cells Expression of PDX-1 as a Pre-disposing Factor for Their Conversion into Insulin Producing Cells. Int J Stem Cells. 2017;10:76–82. doi: 10.15283/ijsc16040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabr MM, Zakaria MM, Refaie AF, et al. Generation of insulin-producing cells from human bone marrow-derived mesenchymal stem cells: comparison of three differentiation protocols. Biomed Res Int. 2014;2014:832736. doi: 10.1155/2014/832736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 18.Dobbin ZC, Landen CN. Isolation and characterization of potential cancer stem cells from solid human tumors—potential applications. Curr Protoc Pharmacol. 2013;63:Unit 14 28. doi: 10.1002/0471141755.ph1428s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thatava T, Ma B, Rohde M, et al. Chromatin-remodeling factors allow differentiation of bone marrow cells into insulin-producing cells. Stem Cells. 2006;24:2858–67. doi: 10.1634/stemcells.2006-0109. [DOI] [PubMed] [Google Scholar]
- 20.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Huang Y, Guo Q. Differentiation of IPCss into insulin-producing cells via adenoviral transfection of PDX-1, NeuroD1 and MafA. Diabetes Res Clin Pract. 2014;104:383–92. doi: 10.1016/j.diabres.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Chinna K, Karuthan K, Choo WY. Statistical Analysis Using SPSS. Pearson Malaysia; 2012. [Google Scholar]
- 23.Gabr MM, Sobh MM, Zakaria MM, et al. Transplantation of insulin-producing clusters derived from adult bone marrow stem cells to treat diabetes in rats. Exp Clin Transplant. 2008;6:236–43. [PubMed] [Google Scholar]
- 24.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Ye L, Liu H, et al. Reprogramming of bone marrow-derived mesenchymal stem cells into functional insulin-producing cells by chemical regimen. Am J Stem Cells. 2012;1:128–37. [PMC free article] [PubMed] [Google Scholar]
- 26.Marappagounder D, Somasundaram I, Dorairaj S, et al. Differentiation of mesenchymal stem cells derived from human bone marrow and subcutaneous adipose tissue into pancreatic islet-like clusters in vitro. Cell Mol Biol Lett. 2013;18:75–88. doi: 10.2478/s11658-012-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 28.Moriscot C, de Fraipont F, Richard MJ, et al. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23:594–603. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 29.Fouad AM, Gabr MM, Abdelhady EK. In vitro differentiation of human multilineage differentiating stress-enduring (Muse) cells into insulin producing cells. Journal of Genetic Engineering and Biotechnology (IJGEB) 2018;16:433–40. doi: 10.1016/j.jgeb.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiese C, Rolletschek A, Kania G, et al. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–22. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SY, Lee SH, Kim BM, et al. Activation of nestin-positive duct stem (NPDS) cells in pancreas upon neogenic motivation and possible cytodifferentiation into insulin-secreting cells from NPDS cells. Dev Dyn. 2004;230:1–11. doi: 10.1002/dvdy.20012. [DOI] [PubMed] [Google Scholar]
- 32.Soria B, Roche E, Berna G, et al. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–62. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 33.Assady S, Maor G, Amit M, et al. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–7. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 34.Lumelsky N, Blondel O, Laeng P, et al. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–94. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 35.Kerenyi F, Tarapcsak S, Hrubi E, et al. [Comparison of sorting of fluorescently and magnetically labelled dental pulp stem cells] Fogorv Sz. 2016;109:29–33. [PubMed] [Google Scholar]
- 36.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 37.Choi KS, Shin JS, Lee JJ, et al. In vitro trans-differentiation of rat mesenchymal cells into insulin-producing cells by rat pancreatic extract. Biochem Biophys Res Commun. 2005;330:1299–305. doi: 10.1016/j.bbrc.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 38.Jahr H, Bretzel RG. Insulin-positive cells in vitro generated from rat bone marrow stromal cells. Transplant Proc. 2003;35:2140–1. doi: 10.1016/s0041-1345(03)00747-4. [DOI] [PubMed] [Google Scholar]
- 39.Otoguro K, Oiwa R, Iwai Y, et al. Screening for new antitrichomonal substances of microbial origin and antitrichomonal activity of trichostatin A. J Antibiot (Tokyo) 1988;41:461–8. doi: 10.7164/antibiotics.41.461. [DOI] [PubMed] [Google Scholar]
- 40.Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205–19. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- 41.Bonner-Weir S, Deery D, Leahy JL, et al. Compensatory growth of pancreatic beta-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. doi: 10.2337/diab.38.1.49. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:5034–9. doi: 10.1073/pnas.0936260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansson M, Tonning A, Frandsen U, et al. Artifactual insulin release from differentiated embryonic stem cells. Diabetes. 2004;53:2603–9. doi: 10.2337/diabetes.53.10.2603. [DOI] [PubMed] [Google Scholar]
- 44.Mc Kiernan E, Barron NW, O'Sullivan F, et al. Detecting de novo insulin synthesis in embryonic stem cell-derived populations. Exp Cell Res. 2007;313:1405–14. doi: 10.1016/j.yexcr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Rajagopal J, Anderson WJ, Kume S, et al. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. doi: 10.1126/science.1077838. [DOI] [PubMed] [Google Scholar]
- 46.Boyd AS, Wu DC, Higashi Y, et al. A comparison of protocols used to generate insulin-producing cell clusters from mouse embryonic stem cells. Stem Cells. 2008;26:1128–37. doi: 10.1634/stemcells.2007-0762. [DOI] [PubMed] [Google Scholar]
- 47.Kim SJ, Choi YS, Ko ES, et al. Glucose-stimulated insulin secretion of various mesenchymal stem cells after insulin-producing cell differentiation. J Biosci Bioeng. 2012;113:771–7. doi: 10.1016/j.jbiosc.2012.02.007. [DOI] [PubMed] [Google Scholar]