Abstract
Background
Early hospital readmission for patients with cirrhosis continues to challenge the healthcare system. Risk stratification may help tailor resources, but existing models were designed using small, single-institution cohorts or had modest performance.
Aims
We leveraged a large clinical database from the Department of Veterans Affairs (VA) to design a readmission risk model for patients hospitalized with cirrhosis. Additionally, we analyzed potentially modifiable or unexplored readmission risk factors.
Methods
A national VA retrospective cohort of patients with a history of cirrhosis hospitalized for any reason from January 1, 2006 to November 30, 2013 was developed from 123 centers. Using 174 candidate variables within demographics, laboratory results, vital signs, medications, diagnoses and procedures, and healthcare utilization we built a 47 variable penalized logistic regression model with the outcome of all-cause 30-day readmission. We excluded patients who left against medical advice, transferred to a non-VA facility, or if the hospital length of stay was greater than thirty days. We evaluated calibration and discrimination across variable volume and compared the performance to recalibrated pre-existing risk models for readmission.
Results
We analyzed 67,749 patients and 179,298 index hospitalizations. The 30-day readmission rate was 23%. Ascites was the most common cirrhosis-related cause of index hospitalization and readmission. The AUC of the model was 0.670 compared to existing models (0.649, 0.566, 0.577). The Brier score of 0.165 showed good calibration.
Conclusions
Our model achieved better discrimination and calibration compared to existing models, even after local recalibration. Assessment of calibration by variable parsimony revealed performance improvements for increasing variable inclusion well beyond those detectable for discrimination.
Keywords: cirrhosis, hospital readmission, risk prediction, logistic regression, calibration
BACKGROUND
Cirrhosis carries significant morbidity and mortality due to decreased mental, physical, and biochemical function. The prevalence is estimated between 400,000 and 3,000,000 persons in the United States, and the disease causes 44,000 deaths annually.1–5 Liver disease costs the US over $2 billion annually in direct healthcare costs,1,6 and though the exact portion attributable to cirrhosis is challenging to calculate, a significant portion is likely due to the 150,000 hospitalizations and nearly 600,000 annual outpatient visits.7–9 Given that 69% of hospitalized cirrhotic patients will have at least one non-elective readmission,10 reducing readmission can be a significant cost saver. Additionally, early hospital readmission is seen as a marker of poor healthcare quality11,12 and is associated with increased mortality,13 increased psychosocial burden on the patient and their caregivers,14–16 and low patient satisfaction in other conditions.17
It has been estimated that 27.1% of readmissions may be avoidable.18 Though programs to reduce readmission have been met with mixed success, the more successful interventions are often a combination of multi-pronged approaches.19 In cirrhosis, interventions such as mandatory gastroenterology consultation,20 early outpatient follow-up,21 and specialized care delivery methods such as a “day hospital”22 may all improve readmission risk. These are resource intensive interventions and as such will require risk stratification to identify high-risk patients, an approach that has shown success for heart failure.23,24
Clinical decision support tools that risk stratify patients in support of targeted intervention require highly accurate, generalizable risk prediction models, lest misleading risk estimates lead to inappropriate treatment choices or wasting limited resources.25–28 Models trained on small samples often suffer from overfitting, resulting in reduced performance when applied to subsequent patients treated at the same institution or patients treated in other healthcare systems.29–31 The majority of studies in a recent systematic review assessing cirrhosis readmission risk were limited by either being smaller, single center cohorts or large administrative database analyses.32 The former lacks the power to analyze multiple risk factors and is limited by the possibility of uncounted readmissions to other centers, which may reduce generalizability. The latter studies lack the granular detail of institutional cohorts. Moreover, the majority of these papers primarily attempted to identify risk factors, as opposed to building a risk prediction model.32 Of the analyses that did create risk prediction models, they have been limited by modest performance, sample sizes, and number of candidate predictor variables (Refer to Table 1 for a summary).13,33–37
Table 1:
Summary of existing risk prediction models for hospital readmission among patients with cirrhosis.
| Model | Study Type & No. of Subjects | No. of Candidate Variables | Candidate Variable Domains | Discrimination | Calibration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | V | D | X | M | P | I | R | C | L | Y | U | T | S | ||||
| Berman (2011)33 | 447 | 836¥ | ∼ 27 | C=0.57¥ | n/a | ||||||||||||
| Bajaj (2016)35 | 1343 | ∼ 25 | C=0.64 | n/a | |||||||||||||
| Morales (2017)13 | 112 | ∼ 34 | C=0.76 | HL ns | |||||||||||||
| Singal (2013)34 | 629 | 209β | ∼ 30 | C=0.66 | HL ns | ||||||||||||
| Tapper (2015)37 | 489 | 245β | ∼ 22 | AUC=0.69 | n/a | ||||||||||||
| Volk (2012)36 | 402 | ∼ 22 | C=0.65 | n/a | |||||||||||||
| Our Model | 67,749 | 208 | |||||||||||||||
Note: Study Type = Validation (V) and Development (D). If a study assessed performance in a separate validation cohort, we have reported the performance in the validation cohort. The number of candidate variables had to be inferred based on the written methods and the cohort summary table as this number was not explicitly reported. Model discrimination reported as either C-statistic or area under the curve (AUC), which can be considered equivalent. Risk Variable Domains Coding: D: Demographics; X: Medical/Surgical Hx; M: Meds; P: Inpatient procedures; I: Physical impairment; R: Risk scores; C: Cirrhosis related complications; L: Labs; Y: Psychosocial; U: Healthcare Utilization; T: Transplant status; S: Discharge disposition.
Validation performed in separate study (Singal)34
Validation cohorts were created by random train-test split of the original cohort
HL: Hosmer-Lemeshow test for goodness-of-fit.
However, even models based on large datasets require continued validation and potential updating as model accuracy may decline over time in evolving clinical environments.38–40 Changes in the mix of patient risk factors, outcome prevalence, treatment patterns, or documentation practices may harm model calibration and lead to misaligned predicted-to-observed risk.38,41,42 Assessing, restoring, and maintaining model calibration are critical for risk prediction tools that provide clinical decision support.28,43,44 Only two of the studies in Table 1 attempted to assess calibration, and they used the Hosmer-Lemeshow test, which has faced criticism for being an incomplete assessment of calibration.45
Extensive clinical data warehouses generated by electronic health record systems provide new opportunities to develop risk prediction models based on larger datasets.25,46,47 The Department of Veterans Affairs (VA), with one of the largest clinical data warehouses encompassing over 20 million patients with granular data,48 faces an increasing burden of chronic liver disease due to substance use disorders, chronic viral hepatitis, and increasing numbers of patients with NAFLD.49 These dramatic increases in overall prevalence of cirrhosis at the VA necessitate targeted interventions and tailored care delivery pathways, for which risk stratification is essential. The aim of the current study was to leverage a large clinical database from the Department of Veterans Affairs to design a cirrhosis readmission risk prediction model.
METHODS
Study Cohort
We analyzed a retrospective cohort of cirrhotic patients hospitalized for any cause from among 123 medical centers in the Department of Veterans Affairs (VA) between January 1, 2006 and November 30, 2013. We included historical data from January 1, 2005 to allow for variable ascertainment and the last month in 2013 to assess for readmission from index hospitalizations during November 2013. We defined a history of cirrhosis as patients who had a cirrhosis diagnosis (based on a history of two outpatient or one inpatient) ICD-9 code (571.2 or 571.5) or an ICD-9 code for a cirrhosis complication (varices, hepatic encephalopathy, hepatorenal syndrome, or portal hypertension).50 The use of 1 inpatient or 2 outpatient codes for identification of disease is frequently used and has been validated for patients with cirrhosis.51
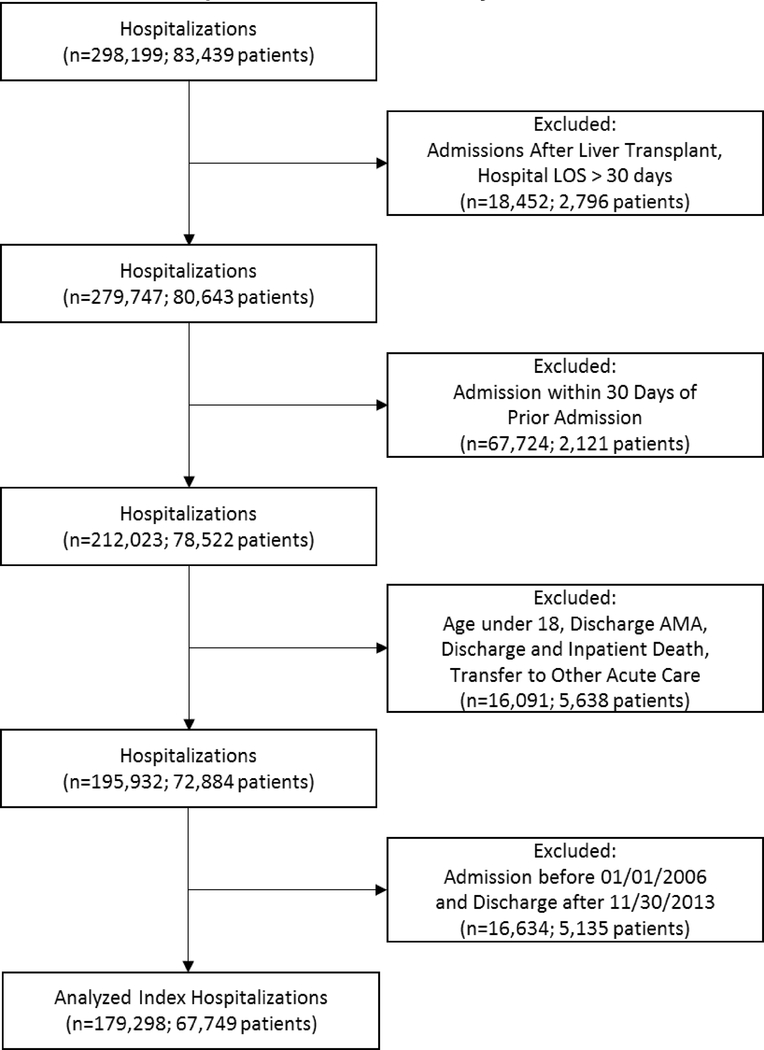
Because we included all-cause hospitalizations, they did not need to have a cirrhosis or cirrhosis complication administrative code during the index hospitalization. We excluded hospitalizations after liver transplant (including the transplant hospitalization itself), if the patient was discharged against medical advice, if the patients were transferred to a non-VA facility, if the patient’s age was less than eighteen, or if the hospital length of stay (LOS) was greater than thirty days. Patients with LOS greater than thirty days were excluded because they frequently represented issues with disposition rather than severity of illness, particularly at the VA where there are fewer patient-related financial incentives for rapid hospital discharge. We further limited index hospitalizations to not having another hospitalization within the 30 days prior to admission. Refer to Figure 1 for the cohort flow diagram.
Figure 1:
Flow of patients from total number of patients before exclusion criteria to total number of patients included in study.
Though we allowed for all-cause hospitalization, we identified cirrhosis related index admissions and readmissions similarly to other studies,52,53 as hospitalizations with discharge diagnoses identifying cirrhosis or cirrhosis related complications. Specifically, we used administrative codes to identify hepatorenal syndrome, hepatic encephalopathy, ascites, spontaneous bacterial peritonitis, hepatocellular carcinoma, portal hypertension, and “Other serious complications of chronic liver disease.” If a hospitalization had none of these codes, the hospitalization was deemed not cirrhosis related. Code sets used to determine these conditions are available in the appendix. The institutional review board and research and development committees of the Tennessee Valley Health Care System VA Medical Center, Nashville, TN approved this study.
Outcome Ascertainment
We generally followed Centers for Medicare & Medicaid Services (CMS) criteria for readmission measures.54 Our outcome was all-cause rehospitalization within a 30-day period from the date of discharge from an index admission. Per CMS criteria, planned admissions were excluded and not counted as readmission; refer to the Appendix Figure 1 for our definition of planned admissions. If a patient had multiple unplanned admissions within 30 days of the index hospitalization, only the first one was counted. However, if the first admission within 30 days of the index hospitalization was planned, then subsequent admissions within the 30-day timeframe of the index hospitalization were not treated as readmission for that index admission. We took this approach, consistent with CMS guidance, because the subsequent admissions could be a consequence of the intervening planned admission.
Data Collection
The VA is an integrated care network that includes acute inpatient hospitals, outpatient primary care and sub-specialist clinics, outpatient pharmacies, rehabilitation facilities, long-term care facilities and domicilliaries. All VA personnel use the same EHR, Veterans Information Systems and Technology Architecture/Computerized Patient Record System (ViSTa/CPRS), for documentation and administration of clinical care,20 and data from all sites is stored in the Corporate Data Warehouse and provisioned by the VA Informatics and Computing Infrastructure.48
Predictor Variables
We initially evaluated 208 variables and excluded 34 variables with > 60% missing or < 0.2% prevalence, leaving 174 candidate risk variables from demographics, labs, diagnoses/procedures, medications, vital signs, and healthcare utilization factors. We refer the reader to the appendix for a description of all candidate variables. Our final model contained 47 variables, determined by the variable selection procedure detailed below. Except for cirrhosis-related medications (e.g., lactulose), which were coded as separate variables, all medications were represented by their corresponding VA drug class code (e.g., “cephalosporin 3rd generation”). The VA drug class codes are available publicly through the VA National Drug File and the Unified Medical Language System.56 Most variables were assessed at the time of discharge. Laboratory values and vital signs were assessed as the last available value prior to the discharge date and time. Medications were represented as medication classes, and both home medications and medications administered during the index hospitalization were included. Variables representing risk scores (e.g., FIB-4) were calculated from discharge lab values. Missing values for laboratory tests and vital signs were imputed using a matrix completion approach based on non-negative matrix factorization57 using the R package NNLM.58
Model Development
In order to obtain a more parsimonious model, we used the 174 candidate risk variables noted above in a variable selection procedure. We performed a penalized logistic regression, using the L1 penalty (Least Absolute Shrinkage and Selection Operator — LASSO), to select a subset of the predictor variables.59 LASSO is a form of linear regression designed to both address overfitting and provide variable selection. The LASSO approach shrinks coefficients toward the null value of 0 based on a penalty parameter, lambda, which controls the sum of the model’s coefficients. This coefficient “shrinkage” reduces the risk of overfitting the model to the particulars of the training data. Variable selection is achieved when the coefficients of weakly predictive variables are reduced to 0 and the influence of these variables is eliminated from the model. Penalized regression with LASSO is frequently superior to other common variable selection methods such as forward/backward selection in many biomedical datasets.60 Refer to Figure 2 for a description of our overall model development workflow.
Figure 2: Description of overall model development workflow.
Our variable selection pipeline considers 208 distinct variables in our EHR and eliminates variables with very low prevalence or excessive missingness (Step 1, highlighted in figure). Subsequently we used penalized logistic regression (via LASSO) to perform variable selection (Step 2). LASSO creates separate models with increasing numbers of variables starting with the most important to least important. We selected the LASSO model that sufficiently reduced classification error while still producing a parsimonious model (Step 4). The literature models (Bajaj, Singal, and Berman) were then tailored to the VA data via logistic re-calibration (Step 5) and we compared the VAtailored version of those models with ours (Step 6).
We considered two LASSO models, one generating the lowest error and a second more heavily regularized (i.e., fewer number of variables) model yielding deviance one standard error from the former model. Since the latter afforded a more parsimonious model (174 vs. 47 variables, respectively) with similar predictive performance (AUC difference of 0.01), we selected the second model for risk prediction. This model contained 47 variables and included: healthcare utilization (6 variables), history of cirrhosis complications (4), comorbidities (4), laboratory tests (9), inpatient administered (13) and home (5) medications, and miscellaneous variables (6). A summary of the selected variables with their percent missingness is available in the Appendix Table 5. We subsequently used the 47 variables identified by the LASSO procedure in an unpenalized logistic regression model with 30-day readmission as the outcome.
Model Evaluation
We assessed overall discrimination using the Area Under the receiver operator characteristic Curve (AUC) 61 and assessed model accuracy using the Brier score, for which 0 implies perfect accuracy.61 We estimated optimism using 200 bootstrap samples and calculated confidence intervals for performance metrics.62 Additionally, we graphically analyzed calibration using smoothed calibration belts, which highlight the relationship between observed and predicted probabilities across the range of predictions.63,64
Variable selection requires a tradeoff between performance and parsimony. In order to assess this, we evaluated the discrimination and calibration performance of our model across a spectrum of regularization/shrinkage under varying values of the penalty parameter, lambda, within the LASSO. For models with each variable set, we assessed model performance as a function of model complexity (as measured by the number of model variables) based on the AUC and the proportion of observations falling within calibrated ranges of the calibration belts.
Model Comparison
We compared our model against VA-data updated versions of models published by Singal,34 Berman,33 and Bajaj.35 Re-calibrating a risk prediction model tailors the model to a new cohort, attempting to resolve loss of model performance due to changes in event rates and case-mix.42 We tailored the three published models to VA data by constructing new, separate logistic regression models using the original model components. We report the AUC and Brier scores for these VA-tailored versions of the literature models using similar procedures as discussed above. As Bajaj offers a model for both the admission and discharge timeframe, we chose to evaluate the discharge timeframe model. We defined statistical significance as non-crossing of the 95% bootstrapped confidence intervals.
Additionally, we used the outcome-specific Net Reclassification Index (NRI) to analyze our model’s improvement over existing models for two use cases: (a) identifying patients at very low risk of readmission, < 10%; and (b) finding very high risk patients, > 40% risk of readmission.65,66 The NRI compares our primary model against each established model with values > 0 indicating improved prediction performance. The outcome specific NRI can be interpreted as the change in true positive rate for predicting readmission, or conversely the true negative rate for predicting no-readmission. All statistical analyses were performed using the R statistical programming suite, version 3.3.2.
RESULTS
Study Population
After applying inclusion and exclusion criteria, 67,749 patients were included in the study with a total of 179,298 all-cause index hospitalizations. There were 41,134 readmissions within 30 days for an unadjusted readmission rate of 23%. Males represented 97.6% of the total admissions, with an age of 60.6 ± 9.0 (mean ± SD). Caucasian patients accounted for the majority of hospital admissions (73.6%). The etiology of cirrhosis was mainly alcoholic (29.4%), viral hepatitis (11.8%), or alcoholic and viral (33.2%). In the remaining patients, the causes of cirrhosis were NAFLD (24,066, 13.4%), primary biliary cirrhosis (649, 0.4%), autoimmune hepatitis (222, 0.1%), hemochromatosis (733, 0.4%) and other/cryptogenic (20,302, 11.3%). The average MELD score at index hospitalization was 13.3 ± 5.4 with 30,907 hospitalizations (17.2%) having a MELD score ≥ 18. Refer to Table 2 for a description of the cohort.
Table 2:
Demographic, clinical and laboratory variables of included patients at first admission.
| Variables | All index admissions (n=179,298 admission, 67,749 patients) |
|---|---|
| Age, mean (SD) | 60.6 (9.0) |
| Gender (male), n (%) | 175,068 (97.6) |
| Race, n (%) | |
| White | 132,015 (73.6) |
| Black | 32,919 (18.4) |
| Asian-Hawaiian-Pacific Islander | 2,885 (1.6) |
| American Indian-Alaskan Native | 2,885 (1.6) |
| Unknown | 8,594 (4.8) |
| Cirrhosis Etiology, n (%) | |
| Alcoholic | 52,736 (29.4) |
| Viral (Hep B and C) | 21,144 (11.8) |
| Alcoholic and Viral | 259,446 (33.2) |
| NAFLD | 24,066 (13.4) |
| Hemochromatosis | 733 (0.4) |
| Autoimmune hepatitis | 222 (0.1) |
| Biliary Cirrhosis | 649 (0.4) |
| Other/Cryptogenic | 20,302 (11.3) |
| Healthcare Utilization (past 1 year), median (IQR) | |
| ER Visits | 2 (0, 4) |
| Inpatient Hospitalizations | 2 (1,4) |
| Outpatient Visits | 31 (16, 55) |
| Non-face-to-face Communication | 4 (1, 10) |
| Congestive Heart Failure, n (%) | 43,382 (24) |
| Diabetes Mellitus, n (%) | 76.8 (42.8) |
| h/o Cirrhosis Complications, n (%) | |
| Hepatic Encephalopathy | 41,511 (23.1) |
| Varices | 39,653 (22.1) |
| SBP | 10,019 (5.6) |
| Ascites | 63,941 (35.7) |
| Hepatocellular Carcinoma | 18,940 (10.6) |
| Hepatorenal Syndrome | 3,897 (2.1) |
| Other Sequelae of Chronic Liver Disease | 17,599 (9.8) |
| Vitals, mean (SD) | |
| Systolic Blood Pressure | 124.1 (19.5) |
| Diastolic Blood Pressure | 72.3 (12.2) |
| Labs, median (IQR) | |
| Creatinine | 0.9 (0.8, 1.2) |
| Blood Urea Nitrogen | 15.0 (10.0, 21.6) |
| Sodium | 137.0 (134.0, 139.0) |
| Potassium | 4.0 (3.7, 4.3) |
| Total Bilirubin | 1.3 (0.7, 2.3) |
| Albumin | 2.9 (2.5, 3.4) |
| INR | 1.3 (1.1, 1.5) |
| White Blood Cell | 6.1 (4.4, 8.1) |
| Platelets | 125.0 (79.0, 190.0) |
| Alanine aminotransferase (ALT) | 33.0 (19.0, 60.0) |
| Aspartate aminotransferase (AST) | 45.0 (26.0, 83.0) |
| Risk Scores | |
| MELD, mean (SD) | 13.3 (5.4) |
| MELD < 12, n (%) | 87,912 (49.0) |
| MELD >= 12 and < 18, n (%) | 60,479 (33.7) |
| MELD >= 18, n (%) | 30,907 (17.2) |
| Disposition, n (%) | |
| Home | 164,646 (91.8) |
| Hospice | 123 (0.1) |
| Hospital | 1,839 (1.0) |
| Nursing Home | 11,778 (6.6) |
| Other House | 129 (0.1) |
| Unknown | 776 (0.4) |
SBP: Spontaneous Bacterial Peritonitis
Details of Index Admission and Readmissions
Approximately 62% and 60% of index hospitalizations and readmissions were cirrhosis related, respectively (Refer to Table 3). Ascites was the most common cause of cirrhosis related index hospitalization (32686, 18.23%) and readmission (9822, 23.88%). Hepatic Encephalopathy was associated with 10% of index hospitalizations and 15% of readmissions. Both hepatic encephalopathy and ascites had a significant increase as causes of readmission as opposed to index hospitalization. Relationships between index hospitalization diagnoses and readmission diagnoses are displayed in the flow model (Refer to Figure 3).
Table 3:
Cirrhosis related index hospitalizations and readmissions.
| Condition | Index Hospitalization (N = 179,298) | Readmission (N = 41,134) |
|---|---|---|
| Ascites, n (%) | 32,686 (18.23) | 9,822 (23.88) |
| HCC, n (%) | 12,751 (7.11) | 2,589 (6.29) |
| HE, n (%) | 17,895 (9.98) | 6,305 (15.33) |
| HRS, n (%) | 1,466 (0.82) | 917 (2.23) |
| Portal Hypertension, n (%) | 18,462 (10.30) | 3,959 (9.62) |
| SBP, n (%) | 3,874 (2.16) | 1,226 (2.98) |
| Varices, n (%) | 12,194 (6.80) | 2,838 (6.90) |
| Other sequelae of chronic liver disease, n(%) | 5,479 (3.06) | 2,037 (4.95) |
| Possibly Cirrhosis Related,¥ n (%) | 42,327 (23.61) | 6,904 (16.78) |
| Total | ||
| Cirrhosis Related Including “Cirrhosis” ICD code, n (%) | 111,053 (61.94) | 24,850 (60.41) |
| Cirrhosis Related Excluding “Cirrhosis” ICD code, n (%) | 68,726 (38.33) | 17,946 (43.62) |
Note: Conditions were identified using ICD codes (Refer to Appendix for relevant definitions). A single hospitalization could include multiple conditions among the discharge diagnoses.
This refers to the ICD codes for Cirrhosis itself. Because the codes for cirrhosis itself, i.e. 571.2 and 571.5, may be less specific, we have identified possibly cirrhosis related admissions as hospitalizations that included “Cirrhosis” in the discharge diagnoses but no codes for the specific complications. Additionally, we calculated the total cirrhosis related admissions with and without these “Cirrhosis” codes.
Figure 3: Index hospitalization reason and readmission hospitalization reason.
For purposes of clarity, diagnoses of “Portal Hypertension” and “Other sequelae of chronic liver disease” were combined into “Other Complications.” “Possibly Cirrhosis Related” hospitalizations refer to hospitalizations using the ICD code for cirrhosis only, which may be non-specific. HE: Hepatic Encephalopathy; HCC: Hepatocellular Carcinoma; SBP: Spontaneous Bacterial Peritonitis; HRS: Hepatorenal Syndrome.
Model for 30-Day Readmission
The model’s discrimination produced an AUC of 0.670 (95% CI: 0.666 – 0.674). The calibration for our model as assessed by the Brier score was excellent 0.165 (95% CI: 0.163 – 0.166). Table 4 lists the odds ratios from the logistic regression model. In general variables reflecting poor psychosocial status, increased healthcare utilization, higher disease severity, and decompensation all were associated with higher risk of readmission. Psychosocial risk factors identified included Medicaid insurance (OR: 1.21, 95% CI: 1.09 – 1.34), drug abuse (OR: 1.07, 95% CI: 1.04 – 1.10), and psychiatric disturbance (OR: 1.11, 95% CI: 1.08 – 1.15). Among healthcare utilization predictors, number of inpatient visits, outpatient visits, and ER visits, primary care provider visits all were associated with increased risk of readmission. If the patient had surgical procedure during admission, the patient has significant lower chance of readmission (OR: 0.77, 95% CI: 0.73 – 0.80). Laboratory values suggesting worse liver disease were associated with higher risk of readmission, e.g. every 1 g/dl increase in serum albumin decreased readmission risk by 9% (OR: 0.91, 95% CI: 0.89 – 0.93).
Table 4:
Odds ratios from the logistic regression model predicting 30-day readmission for patients hospitalized with cirrhosis.
| Beta (SE) | OR (95% CI) | p value | |
|---|---|---|---|
| Healthcare Utilization | |||
| Cumulative LOS | 0.002 (0.000) | 1.00 (1.00, 1.00) | < 0.001 |
| Total Inpatient Visits | 0.132 (0.003) | 1.14 (1.13, 1.15) | < 0.001 |
| Total Outpatient Visits | 0.001 (0.000) | 1.00 (1.00, 1.00) | < 0.001 |
| Total ER Visits | 0.006 (0.001) | 1.01 (1.00, 1.01) | < 0.001 |
| PCP Visits | 0.000 (0.000) | 1.00 (1.00, 1.00) | 0.631 |
| # of Types of PCP Visits | 0.002 (0.001) | 1.00 (1.00, 1.00) | 0.024 |
| h/o Cirrhosis Complications | |||
| Ascites | 0.027 (0.017) | 1.03 (0.99, 1.06) | 0.104 |
| Hepatic Encephalopathy | 0.024 (0.016) | 1.02 (0.99, 1.06) | 0.146 |
| HCC | −0.161 (0.020) | 0.85 (0.82, 0.89) | < 0.001 |
| Paracentesis | 0.185 (0.019) | 1.20 (1.16, 1.25) | < 0.001 |
| Comorbidities¥ | |||
| Hypothyroidism | 0.057 (0.018) | 1.06 (1.02, 1.10) | 0.002 |
| Fluid/Electrolyte Disorder | 0.031 (0.013) | 1.03 (1.01, 1.06) | 0.017 |
| Drug Abuse | 0.069 (0.014) | 1.07 (1.04, 1.10) | < 0.001 |
| Psychotic Disorders | 0.106 (0.016) | 1.11 (1.08, 1.15) | < 0.001 |
| Labs | |||
| Albumin | −0.096 (0.011) | 0.91 (0.89, 0.93) | < 0.001 |
| Alanine Aminotransferase | 0.000 (0.000) | 1.00 (1.00, 1.00) | 0.125 |
| Blood Urea Nitrogen | 0.002 (0.000) | 1.00 (1.00, 1.00) | < 0.001 |
| Calcium | −0.082 (0.011) | 0.92 (0.90, 0.94) | < 0.001 |
| Hematocrit | −0.007 (0.001) | 0.99 (0.99, 1.00) | < 0.001 |
| Mean Corpuscular Volume | 0.003 (0.001) | 1.00 (1.00, 1.00) | < 0.001 |
| Sodium | −0.018 (0.002) | 0.98 (0.98, 0.99) | < 0.001 |
| White Blood Cell Count | 0.007 (0.002) | 1.01 (1.00, 1.01) | < 0.001 |
| Potassium | 0.053 (0.013) | 1.05 (1.03, 1.08) | < 0.001 |
| Inpatient Medications | |||
| Proton Pump Inhibitor | 0.046 (0.013) | 1.05 (1.02, 1.07) | < 0.001 |
| Vancomycin | −0.100 (0.022) | 0.90 (0.87, 0.94) | < 0.001 |
| Lactulose | 0.051 (0.017) | 1.05 (1.02, 1.09) | 0.002 |
| Midodrine | −0.244 (0.055) | 0.78 (0.70, 0.87) | < 0.001 |
| Albumin | 0.237 (0.022) | 1.27 (1.21, 1.32) | < 0.001 |
| NSAIDs | 0.095 (0.021) | 1.10 (1.05, 1.15) | < 0.001 |
| Aminoglycosides | 0.160 (0.044) | 1.17 (1.08, 1.28) | < 0.001 |
| 1st Gen Cephalosporin | −0.213 (0.030) | 0.81 (0.76, 0.86) | < 0.001 |
| Glucocorticoids | 0.068 (0.023) | 1.07 (1.02, 1.12) | 0.003 |
| Lincomycin Antibiotics | −0.156 (0.047) | 0.86 (0.78, 0.94) | < 0.001 |
| Extended Spectrum Penicillins | −0.082 (0.024) | 0.92 (0.88, 0.97) | < 0.001 |
| Loop Diuretics | 0.042 (0.014) | 1.04 (1.01, 1.07) | 0.003 |
| Benzodiazepines | 0.105 (0.014) | 1.11 (1.08, 1.14) | < 0.001 |
| Home Medications | |||
| Lactulose | 0.109 (0.020) | 1.12 (1.07, 1.16) | < 0.001 |
| Glucocorticoids | 0.132 (0.034) | 1.14 (1.07, 1.22) | < 0.001 |
| Loop Diuretics | 0.043 (0.016) | 1.04 (1.01, 1.08) | 0.008 |
| K Sparing Diuretic | 0.046 (0.017) | 1.05 (1.01, 1.08) | 0.006 |
| Fluoroquinolones | 0.093 (0.028) | 1.10 (1.04, 1.16) | < 0.001 |
| Miscellaneous | |||
| Heart Rate | 0.004 (0.000) | 1.00 (1.00, 1.00) | < 0.001 |
| Medicaid Insurance | 0.190 (0.053) | 1.21 (1.09, 1.34) | < 0.001 |
| Surgery During Admission | −0.268 (0.023) | 0.77 (0.73, 0.80) | < 0.001 |
| Risk Scores | |||
| Fibrosis-4 score | 0.000 (0.000) | 1.00 (1.00, 1.00) | 0.037 |
| LACE Score | 0.015 (0.002) | 1.01 (1.01, 1.02) | < 0.001 |
| MELD | 0.012 (0.001) | 1.01 (1.01, 1.01) | < 0.001 |
Note: LACE: Length-of-Stay, Admission acuity, Charlson co-morbidity index, Emergency Department visits in prior 6 months; MELD: Model for End-Stage Liver Disease
Comorbidities were defined using the Elixhauser definitions.88
Patients with hepatocellular carcinoma (HCC) or who had surgery within 24 hours of admission were associated with decreased risk of readmission. The HCC patients in our cohort had decreased prevalence of comorbid conditions including heart failure (12.6% vs. 25.6% in HCC vs. non-HCC, p < 0.001) and chronic kidney disease (14.0% vs. 21.0%, p < 0.001). Similarly, patients receiving surgery had fewer comorbidities and had experienced fewer complications of cirrhosis, including hepatic encephalopathy (12.0% vs. 24.4% in surgical vs. non-surgical patients, p < 0.001), ascites (25.4% vs. 36.8%, p < 0.001), and varices (18.2% vs. 22.6%, p < 0.001).
Effect of Variable Number
When analyzing model performance as a function of the number of variables, we identified a plateau effect for discrimination with the AUC asymptotically approaching 0.68. The calibration also showed a shoulder effect after ~ 40 variables (Figure 4). We note that for underfit models with very few variables, calibration appears superior however the model is unable to discriminate between cases and controls due to a lack of variability in predicted values. Even after recalibrating the Bajaj, Berman, and Singal models to VA data, their calibration was particularly poor as measured by the percentage of observations that were calibrated (41.0%, 28.1%, and 35.3%, respectively).
Figure 4: Discrimination and calibration performance of 30-day readmission models as a function of the number of included variables.
The variables used at each stage depend on the “solution path” of the LASSO. Confidence intervals obtained through 5-fold cross-validation. Panel A: Change in discrimination (as measured by AUC); Panel B: Change in calibration. We measured calibration performance as the number of observations that were well calibrated. We defined well calibrated as the observed probability matching the predicted probability (within a 20% margin of error). The dotted line represents the performance of our final model (47 variables). The performance of the VA-data tailored literature models (Bajaj, Berman, and Singal) are also provided.
Comparison to Existing Models
Our model’s AUC was statistically significantly better than the Singal, Berman, and Bajaj models: 0.649 (95% CI: 0.645 – 0.653), 0.565 (95% CI: 0.562 – 0.570), and 0.577 (95% CI: 0.573 – 0.581), respectively (Refer to Table 5). The NRI demonstrated that our model, when compared to the three extant models, achieved 3 – 16 percent improvement in predicting readmission accurately for high risk patients (Figure 5). For low-risk patients, the Singal, Berman, and Singal models were poorly calibrated and did not predict that any of the patients had a low risk of readmission. Refer to the Appendix for details.
Table 5:
Discrimination and calibration results for our model versus the three comparator, literature models.
Figure 5: Improvement in prediction accuracy for identifying low and high probability of 30-day readmission measured by the Net Reclassification Index.
The Net Reclassification Index compares our primary model against each established model with values > 0 indicating improved prediction performance. Performance is shown for two use cases: (a) identifying patients at low risk of readmission, < 10%; and (b) finding very high risk patients, > 40% risk of readmission. Our model shows improved overall performance and outcome specific performance (readmission versus no readmission). The outcome specific NRI can be interpreted as the change in true positive rate for predicting readmission (or conversely improvement in the true negative rate for predicting no readmission).
The Brier scores for the Singal, Berman, and Bajaj models were 0.167 (95% CI: 0.165 – 0.168), 0.175 (95% CI: 0.174 – 0.177), and 0.175 (95% CI: 0.173 – 0.176), respectively. Though calibration appears similar when assessed numerically, the smoothed calibration belts (Figure 6) demonstrate differences in ranges and uncertainty in calibration. The Bajaj and Berman models appear well calibrated for admissions predicted to have a probability of readmission between 0.2 and 0.4; however, closer inspection reveals that this is primarily due to a very narrow range of predicted probabilities. Both our model and the Singal model exhibit better calibration across a wider range of predictions. Yet in comparison to the Singal model, our model’s calibration curve is either closer or similarly close to ideal calibration across the range of predictions and displays less uncertainty in this performance, particularly among higher risk observations.
Figure 6: Calibration curves for our model versus the three comparator, literature models.
Note: Perfect calibration is represented by the diagonal black line. Curves are limited to the range of predicted probabilities provided by each model. GLM: “General Linear Model,” i.e. our model.
DISCUSSION
Existing research has identified targeted interventions that help reduce readmissions amongst patients with cirrhosis. However, for these interventions to be cost effective and successful, healthcare systems must identify patients at high readmission risk. In this work, we constructed a model to predict 30-day readmission amongst patients with cirrhosis. To the best of our knowledge, this was the largest dataset with granular, clinical data used for this endeavor (67,749 patients with 179,298 admissions). Our model was significantly better at predicting readmission compared to three published models.33–35
There is an ongoing tension in risk prediction modeling used in clinical settings between model variable parsimony and performance. Perhaps this work’s most critical finding is that sustained, adequate levels of model calibration require retaining many more variables than would be required to show only discrimination. Furthermore, recalibrating external models with fewer variables does not provide the same level of performance in a local environment. This will be especially important for identifying high risk patients, for whom the most expensive interventions would be targeted. Misleading patient-level risk estimates may lead to overconfidence, inappropriately alter treatment choices, or misappropriate limited resources.25,28,67 The re-calibrated Bajaj, Berman, and Singal models had less than 50% of observations well calibrated.
Overall, there were several risk factors identified that were significantly associated with readmission that may guide decision making, and in some cases, may be modifiable. Unsurprisingly, increased disease severity was associated with higher risk of readmission. These findings have been corroborated in other studies, including ascites68,69 and HE.70,71 We also identified PPI use associated with increased risk of readmission, similar to Bajaj et al.35 Our finding that patients with HCC were associated with a lower risk of readmission was also identified by Brown et al.72 Higher mortality among these patients and discharge to hospice or palliative care status may account for these findings.
It is essential to include modifiable risk factors whenever possible, and we favored allowing more variables in our model over further parsimony. We identified electrolyte disturbance and diuretic use to be a strongly associated with readmission. Electrolyte disturbances including hyponatremia73 and hypokalemia70 have been associated with readmission in other studies. More investigation needs to be done in how to dial in the right diuretic dose for cirrhosis as outpatients, as this is a common problem after hospitalization as diuretics are often changed during hospitalization. For example, there is exciting work in passive sensor technologies such as wearable electrolyte sensors that can measure various serum electrolyte levels measured through sweat.74
Our study further highlights the importance of psychosocial risk factors mediating readmission risk. Drug abuse, severe psychiatric disorders, and Medicaid insurance (a surrogate for income and disability) were all strongly correlated with readmission. The VA’s patients face a higher burden of substance abuse, particularly among Iraq and Afghanistan veterans where the prevalence has been estimated at 11%.75 Patients with cirrhosis face a high burden of depression, unemployment (44%), and financial risk.76 Mental health comorbidities and substance abuse was a common predictor in other models of readmission risk in other diseases.77
Historically, risk models relied on relatively few predictor variables;33–35 however, the power of the EHR has allowed risk prediction models to be built using significantly larger cohorts with a larger candidate predictor variable pool. Routine revalidation and possible recalibration allows prediction models to be tailored to the local clinical setting.41,78–80 All three tested models declined in discrimination and calibration performance in this cohort, despite following best practices and updating the models for VA data. Our data suggest increased accuracy using a large national dataset; however, our analysis also suggests that there might be a ceiling effect for predictive accuracy using all relevant EHR variables within a system, at least for patients with cirrhosis.
The EHR allows automated risk calculation and integration of predictive analytics into the clinical workflow for decision support. Several studies have shown improved outcomes embedding more complicated prediction models, automatically calculated by the EHR, within routine care. 81–83 Numerous studies have described interventions to reduce hospital readmission, though effective interventions tend to be complex and involve patients in self-care.84 Medicare initiatives targeting multifactorial improvements in post-discharge transitions of care have been shown to be effective in community hospital settings.85 Examples of post discharge interventions include more intensive medical monitoring or telemonitoring, early post-discharge visits and phone calls, and team-based approaches.86 Tapper et al. have shown that using checklists and standard protocols for postdischarge treatment of patients with encephalopathy or subacute bacterial peritonitis effectively reduced readmission rates in decompensated cirrhotics.87 Careful consideration of patient risk and appropriate interventions to prevent readmissions continues to remain a challenge.
Our analysis has limitations. First, our cohort is largely comprised of male patients and may not generalize to a population with a greater proportion of female patients, particularly as female patients may face specific psychosocial risk factors that impact readmission risk. In terms of applicability, the current model can be applied within the VA EHR system at any facility. Regardless, none of the variables we used in our model are specific to the Department of Veterans Affairs. Validating model performance in other healthcare systems would be essential future work for testing the concept that this comprehensive group of variables would provide greater predictive accuracy.
Second, the transition to ICD-10 in the US (and its use in the rest of the world) will require revalidation for codes without a one-to-one mapping. Third, the VA clinical data warehouse captures information regarding all inpatient admissions to the VA and all admissions outside the VA system for which the VA pays. However, there will be a minority of patients who seek inpatient care outside the VA system, which would lead to underestimation of readmission. Fourth, the structured data only identified 123 hospitalizations where the patient was discharged to hospice care, which is likely heavily underestimated. As discussed previously, this ascertainment bias may have led to the association of reduced readmission for HCC patients. Finally, the utility of the model will require prospective validation at multiple sites within the VA system, first for corresponding accuracy and second to see if it can be used to target appropriate interventions to help reduce short term readmissions.
Conclusion
In summary, this study identified a high 30-day readmission rate in patients with advanced liver disease. To our knowledge, this is the largest study building a readmission risk model using granular clinical data for patients with cirrhosis. Our evaluation of published readmission risk models indicated all would require recalibration to be used in a new healthcare setting and some may require fully rebuilding the model. Predicting readmission for patients with cirrhosis is challenging. The current model is a step towards personalized medicine and advancing high performing predictive analytics deployed within routine care and describes the use of a robust modeling method that brings us closer to the promise of big data.
Supplementary Material
Acknowledgement of Grant Support
JK was supported by the Department of Veterans Affairs, Office of Academic Affiliations, Advanced Fellowship Program in Medical Informatics, and the Department of Biomedical Informatics, Vanderbilt University, Nashville, TN. GC was supported by the NIH Precision Medicine Initiative Cohort Program Data and Research Support Center (1U2COD023196). MEM, GC, and SBH were supported by Veterans Health Administration Health Services Research & Development (HSR&D) Investigator Initiated Research (IIR 13-052).
Conflict of Interest: We wish to confirm for all authors that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Kim WR, Brown RS, Terrault NA, et al. Burden of liver disease in the United States: Summary of a workshop. Hepatology 2002;36:227–242. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2011;9:524–530.e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 3.ASRANI SK, LARSON JJ, YAWN B, et al. Underestimation of Liver-Related Mortality in the United States. Gastroenterology 2013;145:375–82.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuppan D, Afdhal NH. Liver Cirrhosis. Lancet 2008;371:838–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everhart JE, Ruhl CE. Burden of Digestive Diseases in the United States Part III: Liver, Biliary Tract, and Pancreas. Gastroenterology 2009;136:1134–1144. [DOI] [PubMed] [Google Scholar]
- 6.HCUPnet. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. https://hcupnet.ahrq.gov. Published 2014 Accessed December 5, 2018.
- 7.Peery AF, Dellon ES, Lund J, et al. Burden of Gastrointestinal Disease in the United States: 2012 Update. Gastroenterology 2012;143:1179–1187.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peery AF, Crockett SD, Barritt AS, et al. Burden of Gastrointestinal, Liver, and Pancreatic Diseases in the United States. Gastroenterology 2015;149:1731–1741.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peery AF, Crockett SD, Murphy CC, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019;156:254–272.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volk ML. Hospital readmissions for decompensated cirrhosis. Clin Liver Dis 2014;4:138–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benbassat J, Taragin M. Hospital Readmissions as a Measure of Quality of Health Care: Advantages and Limitations. Arch Intern Med 2000;160:1074–1081. [DOI] [PubMed] [Google Scholar]
- 12.Halfon P, Eggli Y, Pêtre-Rohrbach I, et al. Validation of the Potentially Avoidable Hospital Readmission Rate as a Routine Indicator of the Quality of Hospital Care. Med Care 2006;44:972–981. [DOI] [PubMed] [Google Scholar]
- 13.Morales BPP. Early hospital readmission in decompensated cirrhosis: Incidence, impact on mortality, and predictive factors. Dig Liver Dis 2017;49:903–909. [DOI] [PubMed] [Google Scholar]
- 14.Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology 2001;120:170–178. [DOI] [PubMed] [Google Scholar]
- 15.Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Dig Dis Sci 2003;48:1622–1626. [DOI] [PubMed] [Google Scholar]
- 16.Rakoski MO, McCammon RJ, Piette JD, et al. Burden of cirrhosis on older Americans and their families: Analysis of the health and retirement study. Hepatology 2012;55:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourne RB, Chesworth BM, Davis AM, et al. Patient Satisfaction after Total Knee Arthroplasty: Who is Satisfied and Who is Not? Clin Orthop Relat Res 2010;468:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Walraven C, Bennett C, Jennings A, et al. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011;183:E391–E402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen LO, Young RS, Hinami K, et al. Interventions to Reduce 30-Day Rehospitalization: A Systematic Review. Ann Intern Med 2011;155:520. [DOI] [PubMed] [Google Scholar]
- 20.Ghaoui RF. Outcomes associated with a mandatory gastroenterology consultation to improve the quality of care of patients hospitalized with decompensated cirrhosis. J Hosp Med Online 2015;10:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanwal FA. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology 2016;64:569–581. [DOI] [PubMed] [Google Scholar]
- 22.Morando FM. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol 2013;59:257–264. [DOI] [PubMed] [Google Scholar]
- 23.Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol 2002;39:471–480. [DOI] [PubMed] [Google Scholar]
- 24.Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med 2009;4:211–218. [DOI] [PubMed] [Google Scholar]
- 25.Amarasingham R, Patzer RE, Huesch M, et al. Implementing Electronic Health Care Predictive Analytics: Considerations And Challenges. Health Aff (Millwood) 2014;33:1148–1154. [DOI] [PubMed] [Google Scholar]
- 26.Ohno-Machado L, Resnic FS, Matheny ME. Prognosis in Critical Care. Annu Rev Biomed Eng 2006;8:567–599. [DOI] [PubMed] [Google Scholar]
- 27.Moons KGM, Altman DG, Vergouwe Y, et al. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ 2009;338:b606. [DOI] [PubMed] [Google Scholar]
- 28.Van Calster B, Vickers AJ. Calibration of Risk Prediction Models: Impact on Decision-Analytic Performance. Med Decis Making 2015;35:162–169. [DOI] [PubMed] [Google Scholar]
- 29.Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996;49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 30.van der Ploeg T, Austin PC, Steyerberg EW. Modern modelling techniques are data hungry: a simulation study for predicting dichotomous endpoints. BMC Med Res Methodol 2014;14:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steyerberg EW, Borsboom GJJM, van Houwelingen HC, et al. Validation and updating of predictive logistic regression models: a study on sample size and shrinkage. Stat Med 2004;23:2567–2586. [DOI] [PubMed] [Google Scholar]
- 32.Orman ES, Ghabril M, Emmett TW, et al. Hospital Readmissions in Patients with Cirrhosis: A Systematic Review. J Hosp Med April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berman KT. Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease.[Erratum appears in Clin Gastroenterol Hepatol. 2011 Jul;9(7):625 Note: Vuppalanch, Raj [corrected to Vuppalanchi, Raj]]. Clin Gastroenterol Hepatol 2011;9:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singal AGR. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol 2013;11:1335–1341.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajaj JSR. The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology 2016;64:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volk ML, Tocco RS, Bazick J, et al. Hospital Re-Admissions among Patients with Decompensated Cirrhosis. Am J Gastroenterol 2012;107:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tapper EBF. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology 2015;62:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickey GL, Grant SW, Murphy GJ, et al. Dynamic trends in cardiac surgery: why the logistic EuroSCORE is no longer suitable for contemporary cardiac surgery and implications for future risk models. Eur J Cardiothorac Surg 2013;43:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minne L, Eslami S, de Keizer N, et al. Effect of changes over time in the performance of a customized SAPS-II model on the quality of care assessment. Intensive Care Med 2012;38:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis SE, Lasko TA, Chen G, et al. Calibration drift in regression and machine learning models for acute kidney injury. J Am Med Inform Assoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toll DB, Janssen KJM, Vergouwe Y, et al. Validation, updating and impact of clinical prediction rules: A review. J Clin Epidemiol 2008;61:1085–1094. [DOI] [PubMed] [Google Scholar]
- 42.Moons KGM, Kengne AP, Grobbee DE, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012;98:691–698. [DOI] [PubMed] [Google Scholar]
- 43.Van Calster B, Nieboer D, Vergouwe Y, et al. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol 2016;74:167–176. [DOI] [PubMed] [Google Scholar]
- 44.Van Hoorde K, Van Huffel S, Timmerman D, et al. A spline-based tool to assess and visualize the calibration of multiclass risk predictions. J Biomed Inform 2015;54:283–293. [DOI] [PubMed] [Google Scholar]
- 45.Nezic D, Borzanovic M, Spasic T, et al. Calibration of the EuroSCORE II risk stratification model: is the Hosmer–Lemeshow test acceptable any more? Eur J Cardiothorac Surg 2013;43:206–206. [DOI] [PubMed] [Google Scholar]
- 46.Pencina MJ, Peterson ED. Moving From Clinical Trials to Precision Medicine: The Role for Predictive Modeling. JAMA 2016;315:1713–1714. [DOI] [PubMed] [Google Scholar]
- 47.Parikh RB, Kakad M, Bates DW. Integrating Predictive Analytics Into High-Value Care: The Dawn of Precision Delivery. JAMA 2016;315:651. [DOI] [PubMed] [Google Scholar]
- 48.Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff Proj Hope 2014;33:1203–1211. [DOI] [PubMed] [Google Scholar]
- 49.Beste LA, Leipertz SL, Green PK, et al. Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001–2013. Gastroenterology 2015;149:1471–1482.e5. [DOI] [PubMed] [Google Scholar]
- 50.Nehra MS, Ma Y, Clark C, et al. Use of Administrative Claims Data for Identifying Patients with Cirrhosis. J Clin Gastroenterol 2013;47:e50–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Re VL, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf 2011;20:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanwal F, Kramer JR, Buchanan P, et al. The Quality of Care Provided to Patients With Cirrhosis and Ascites in the Department of Veterans Affairs. Gastroenterology 2012;143:70–77. [DOI] [PubMed] [Google Scholar]
- 53.Vincent P, Larochelle H, Lajoie I, et al. Stacked Denoising Autoencoders: Learning Useful Representations in a Deep Network with a Local Denoising Criterion. J Mach Learn Res 2010;11:3371–3408. [Google Scholar]
- 54.Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation (YNHHSC/CORE). 2014 Measures Updates and Specifications Report Hospital-Level 30-Day Risk-Standardized Readmission Measures., 2014:61. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html. Accessed December 19, 2018.
- 55.Brown SH, Lincoln MJ, Groen PJ, et al. VistA—U.S. Department of Veterans Affairs national-scale HIS. Int J Med Inf 2003;69:135–156. [DOI] [PubMed] [Google Scholar]
- 56.VA National Drug File - Data.gov. https://catalog.data.gov/dataset/va-national-drug-file-may-2015. Accessed June 13, 2017.
- 57.Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature 1999;401:788–791. [DOI] [PubMed] [Google Scholar]
- 58.Lin X, Boutros PC. NNLM: Fast and Versatile Non-Negative Matrix Factorization., 2016. https://cran.r-project.org/web/packages/NNLM/index.html. Accessed April 25, 2017.
- 59.Tibshirani R Regression Shrinkage and Selection Via the Lasso. J R Stat Soc Ser B 1994;58:267–288. [Google Scholar]
- 60.Steyerberg EW, Eijkemans MJC, Harrell FE, et al. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med 2000;19:1059–1079. [DOI] [PubMed] [Google Scholar]
- 61.Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Springer Science & Business Media, 2008. [Google Scholar]
- 62.Harrell FE, Lee KL, Mark DB. Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. Stat Med 1996;15:361–387. [DOI] [PubMed] [Google Scholar]
- 63.Finazzi S, Poole D, Luciani D, et al. Calibration Belt for Quality-of-Care Assessment Based on Dichotomous Outcomes. PLOS ONE 2011;6:e16110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nattino G, Finazzi S, Bertolini G. A new calibration test and a reappraisal of the calibration belt for the assessment of prediction models based on dichotomous outcomes. Stat Med 2014;33:2390–2407. [DOI] [PubMed] [Google Scholar]
- 65.Pencina MJ, D’ Agostino RB, D’ Agostino RB, et al. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 66.Kerr KF, Wang Z, Janes H, et al. Net Reclassification Indices for Evaluating Risk-Prediction Instruments: A Critical Review. Epidemiol Camb Mass 2014;25:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang X, Osl M, Kim J, et al. Calibrating predictive model estimates to support personalized medicine. J Am Med Inform Assoc 2012;19:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Le SS. Could Adherence to Quality of Care Indicators for Hospitalized Patients With Cirrhosis-Related Ascites Improve Clinical Outcomes? Am J Gastroenterol 2016;111:87–92. [DOI] [PubMed] [Google Scholar]
- 69.Yang Y-YL. Identification of diuretic non-responders with poor long-term clinical outcomes: a 1-year follow-up of 176 non-azotaemic cirrhotic patients with moderate ascites. Clin Sci 2011;121:509–521. [DOI] [PubMed] [Google Scholar]
- 70.Gaduputi VC. Prognostic significance of hypokalemia in hepatic encephalopathy. Hepatogastroenterology 2014;61:1170–1174. [PubMed] [Google Scholar]
- 71.Rassameehiran SM. Predictor of 90-Day Readmission Rate for Hepatic Encephalopathy. South Med J 2016;109:365–369. [DOI] [PubMed] [Google Scholar]
- 72.Brown CL, Hammill BG, Qualls LG, et al. Significant Morbidity and Mortality Among Hospitalized End-Stage Liver Disease Patients in Medicare. J Pain Symptom Manage 2016;52:412–419.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deitelzweig SA. Hyponatremia-associated healthcare burden among US patients hospitalized for cirrhosis. Adv Ther 2013;30:71–80. [DOI] [PubMed] [Google Scholar]
- 74.Bariya M, Nyein HYY, Javey A. Wearable sweat sensors. Nat Electron 2018;1:160. [Google Scholar]
- 75.Seal KH, Cohen G, Waldrop A, et al. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001–2010: Implications for screening, diagnosis and treatment. Drug Alcohol Depend 2011;116:93–101. [DOI] [PubMed] [Google Scholar]
- 76.Bajaj JS, Wade JB, Gibson DP, et al. The Multi-Dimensional Burden of Cirrhosis and Hepatic Encephalopathy on Patients and Caregivers. Am J Gastroenterol 2011;106:1646–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. Jama 2011;306:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janssen KJM, Moons KGM, Kalkman CJ, et al. Updating methods improved the performance of a clinical prediction model in new patients. J Clin Epidemiol 2008;61:76–86. [DOI] [PubMed] [Google Scholar]
- 79.Kappen TH, Vergouwe Y, van Klei WA, et al. Adaptation of Clinical Prediction Models for Application in Local Settings. Med Decis Making 2012;32:E1–E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vergouwe Y, Nieboer D, Oostenbrink R, et al. A closed testing procedure to select an appropriate method for updating prediction models. Stat Med 2017;36:4529–4539. [DOI] [PubMed] [Google Scholar]
- 81.Kuzniewicz MW, Puopolo KM, Fischer A, et al. A Quantitative, Risk-Based Approach to the Management of Neonatal Early-Onset Sepsis. JAMA Pediatr 2017;171:365–371. [DOI] [PubMed] [Google Scholar]
- 82.Amarasingham R, Patel PC, Toto K, et al. Allocating scarce resources in real-time to reduce heart failure readmissions: a prospective, controlled study. BMJ Qual Saf 2013;22:998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cronin PR, Greenwald JL, Crevensten GC, et al. Development and Implementation of a Real-Time 30-Day Readmission Predictive Model. AMIA Annu Symp Proc 2014;2014:424–431. [PMC free article] [PubMed] [Google Scholar]
- 84.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-Day Hospital Readmissions: A Systematic Review and Meta-analysis of Randomized Trials. JAMA Intern Med 2014;174:1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brock J, Mitchell J, Irby K, et al. Association Between Quality Improvement for Care Transitions in Communities and Rehospitalizations Among Medicare Beneficiaries. JAMA 2013;309:381–391. [DOI] [PubMed] [Google Scholar]
- 86.Gheorghiade M, Vaduganathan M, Fonarow GC, et al. Rehospitalization for Heart Failure: Problems and Perspectives. J Am Coll Cardiol 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 87.Tapper EBF. A Quality Improvement Initiative Reduces 30-Day Rate of Readmission for Patients With Cirrhosis. Clin Gastroenterol Hepatol 2016;14:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Quan H, Sundararajan V, Halfon P, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.