Abstract
Ovarian cancer has few known risk factors, hampering identification of high-risk women. We assessed the association of pre-diagnostic plasma metabolites (N=420) with risk of epithelial ovarian cancer, including both borderline and invasive tumors. 252 cases and 252 matched controls from the Nurses’ Health Studies were included. Multivariable logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (CI) comparing the 90th-10th percentile in metabolite levels, using the permutation-based Westfall and Young approach to account for testing multiple correlated hypotheses. Weighted gene co-expression network analysis (WGCNA) modules (n=10 metabolite modules) and metabolite set enrichment analysis (MSEA; n=23 metabolite classes) were also evaluated. An increase in pseudouridine levels from the 10th to the 90th percentile was associated with a 2.5-fold increased risk of overall ovarian cancer (OR=2.56, 95%CI=1.48-4.45; p=0.001/adjusted-p=0.15); a similar risk estimate was observed for serous/poorly-differentiated tumors (n=176 cases; comparable OR=2.38, 95%CI=1.33-4.32, p=0.004/adjusted-p=0.55. For non-serous tumors (n=34 cases), pseudouridine and C36:2 phosphatidylcholine (PC) plasmalogen had the strongest statistical associations (comparable OR=9.84, 95%CI=2.89-37.82; p<0.001/adjusted-p=0.07; and OR=0.11, 95%CI=0.03-0.35; p<0.001/adjusted-p=0.06, respectively). Five WGCNA modules and 9 classes were associated with risk overall at FDR≤0.20. Triacylglycerols (TAGs) showed heterogeneity by tumor aggressiveness (case-only heterogeneity-p<0.0001). The TAG association with risk overall and serous tumors differed by acyl carbon content and saturation. In summary, this study suggests that pseudouridine may be a novel risk factor for ovarian cancer and that TAGs may also be important, particularly for rapidly fatal tumors, with associations differing by structural features.
Keywords: ovarian cancer risk, circulating metabolomics, serous ovarian cancer risk, prospective analysis
Introduction
Ovarian cancer is the fifth leading cause of female cancer death in the U.S. (1). However, there are few known risk factors, such that current risk prediction models have a modest predictive capability, necessitating the identification of new risk factors to identify women at high risk.
Advances in technology have led to precise measures of small molecule metabolites that are critical for growth and maintenance of cells in biologic fluids (2). Several studies have identified metabolites as biomarkers of cancer risk. For example, branched chain amino acids were strongly associated with risk of pancreatic cancer (3) and lipid metabolites were inversely associated with risk of aggressive prostate cancer (4). Further, prediagnostic serum concentrations of metabolites related to alcohol, vitamin E, and animal fats were modestly associated with ER+ breast cancer risk (5), while BMI-related metabolites were strongly related to increased risk (6). These findings support metabolomics profiling as a valuable strategy for identifying new cancer risk biomarkers.
Therefore, we used metabolomics assays to quantify several classes of circulating metabolites in plasma samples collected three to twenty-three years prior to ovarian cancer diagnosis within a nested case-control study, and, in an agnostic analysis, assessed their potential as biomarkers of ovarian cancer risk.
Materials and Methods
Study population
We conducted nested case-control studies within the Nurses Health Studies (NHS (7), NHSII (8)). The NHS was established in 1976 among 121,700 US female nurses aged 30–55 years, and NHSII was established in 1989 among 116,429 female nurses aged 25–42 years. Participants have been followed biennially by questionnaire to update information on exposure status and disease diagnoses. Details are provided in the supplementary file.
Incident cases of epithelial ovarian cancer were identified through biennial questionnaires or linkage with the National Death Index, for whom we obtained related medical records and pathology reports or linked to the relevant cancer registry when medical records were unattainable. A gynecologic pathologist reviewed the records to confirm the diagnosis and abstract date of diagnosis, invasiveness, stage, and histotype (serous, poorly differentiated [PD], endometrioid, clear cell [CC], mucinous, other/unknown), which is highly concordant with centralized pathology review (9). Date of death was extracted from the death certificate.
Confirmed cases were diagnosed with ovarian cancer three years after blood collection until June 1, 2012 (NHS), or June 1, 2013 (NHSII): two hundred fifty-two cases of invasive and borderline epithelial ovarian cancer (212 in NHS and 40 in NHSII). We excluded cases diagnosed within three years of blood collection (N=46) as most ovarian cancer cases are diagnosed at a late stage, with evidence suggesting preclinical disease up to 3 years before diagnosis (10). Cases were matched to one control on: cohort (NHS, NHSII); menopausal status and hormone therapy use at blood draw (premenopausal, postmenopausal/ hormone therapy use, postmenopausal/ no hormone therapy use, missing/ unknown); menopausal status at diagnosis (premenopausal, postmenopausal, or unknown); age (±1 year), date of blood collection (±1 month); time of day of blood draw (±2 hours); and fasting status (>8 hours or ≤8 hours); women in NHSII who gave a luteal sample were matched on the luteal date (date of the next period minus date of blood draw, ±1 day).
Completion of the questionnaire was considered to imply informed consent when the study protocol was approved in 1976 (NHS) and 1989 (NHSII) by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. The studies were conducted in accordance with recognized ethical guidelines (Declaration of Helsinki).
Metabolite profiling
Plasma metabolites were profiled at the Broad Institute of MIT and Harvard (Cambridge, MA) using three complimentary liquid chromatography tandem mass spectrometry (LC-MS/MS) methods designed to measure polar metabolites and lipids as well as free fatty acids. Details are provided in the supplementary file.
In total, 608 known metabolites were measured. Metabolites with a coefficient of variation (CV) >25% or an intraclass correlation coefficient (ICC) <0.4 among blinded QC samples were excluded (N=132, Supplementary Table 1). Furthermore, metabolites with poor stability due to delayed processing (11) were excluded (N=56, Supplementary Table 1). Included metabolites (e.g., amino acids, amino acids derivatives, amines, lipids, fatty acids, bile acids; N=420 [69%]; Supplementary Table 1) exhibited good reproducibility within person over one year (11) and over 10 years. 197 metabolites had no missing values among participant samples.
Statistical analysis
Identification of individual metabolites associated with risk
Missing values for metabolites (N=211) with <10% missingness were imputed with 1/2 of the minimum value measured for that metabolite. We included a missing value indicator for metabolites (N=12) with more than 10% missingness. Continuous metabolite values were transformed to probit scores to reduce the influence of skewed distributions and heavy tails on the results and to scale the measured metabolite values to the same range. Conditional logistic regression was used to evaluate metabolite associations, modeled continuously (with an additional indicator if >10% missingness), with risk of overall ovarian cancer. We present the odds ratios (OR) and 95% confidence intervals (95% CI) for an increase from the 10th to 90th percentile in metabolite levels or the indicator variable.
We compared conditional logistic regression to unconditional logistic regression adjusting for the matching factors and found similar results (Supplementary Table 1.1). Thus, subsequent analyses by histotype, rapidly fatal status, time between blood collection and diagnosis, and sensitivity analyses were conducted using the latter approach, allowing the use of all controls.
We conducted stratified analyses restricting to serous/poorly differentiated (PD) tumors (cases=176/controls=252), endometrioid/clear cell (CC) tumors (cases=34/controls=252), rapidly fatal invasive cases (death occurring <3 years after diagnosis; cases=86/controls=252), and less aggressive invasive tumors (all other cases; cases=138/controls=252), as well as premenopausal (cases=82/controls=82) and postmenopausal women (cases=137/controls=137) at blood collection and those diagnosed 3–11 years (cases=121/controls=252) and 12–23 years after blood collection (cases=131/controls=252 ). Models were adjusted for matching factors, duration of oral contraceptive use (none or <3 months, 3 months to 3 years, 3 to 5 years, >5 years), tubal ligation (yes/no) and parity (none, 1, 2, 3, 4+ children). We calculated heterogeneity by histotype, time to diagnosis and tumor aggressiveness using case-only analyses and by menopausal status at blood collection by introducing an interaction term between the metabolite and menopausal status.
We conducted sensitivity analyses excluding borderline tumors (N=25), known low-grade serous cases (N=4), samples processed >24 hours after collection (N=13 cases, N=6 controls), cases with a diagnosis of a prior cancer (N=17), or women with a diagnosis of another cancer after their matched case’s diagnosis (N=35 controls).
A permutation test (N=5000) was used to control the family-wise error rate (i.e. account for multiple testing) while accounting for the correlation structure of metabolites using the stepdown min P approach by Westfall and Young (12). Details are in the supplemental file. We report unadjusted and multiple comparison adjusted p-values and discuss individual metabolites associated with ovarian cancer risk at unadjusted p-values≤0.01 given the hypothesis generating nature of the study.
Identification of groups of metabolites associated with risk
Metabolite Set Enrichment Analysis (MSEA) (13) was used to identify groups of molecularly or biologically similar metabolites that were enriched among the metabolites associated with risk of overall ovarian cancer and histotypes and Weighted Gene Co-expression Network Analysis (WGCNA) (14) was used to identify metabolite modules and their association with ovarian cancer risk; details are in the supplemental file. We report nominal p-values and false discovery rates (FDR) (15) for all metabolite groups and modules, discussing those at FDR≤0.2. All analyses were performed using the statistical computing language R, version 3.5.0 (16).
Results
Study population
Of the 252 cases, 176 cases were diagnosed with serous/PD tumors, while 34 were classified as endometrioid/CC; 86 represented rapidly fatal tumors with death within 3 years of diagnosis (Table 1). Mean follow-up was 12.3 years. Distributions of ovarian cancer risk factors were generally in the expected directions.
Table 1:
Characteristics of overall, serous/poorly differentiated (PD) and endometrioid/clear cell (CC) ovarian cancer (OC) cases, rapidly fatal tumors, and all controls at time of blood collection.
| All Controls (N = 252) |
Overall OC (N = 252) |
Serous/PD OC (N = 176) |
Endometrioid/CC OC (N = 34) |
Other histotypes (N = 42) |
Rapidly fatal tumors (N=86) |
|
|---|---|---|---|---|---|---|
| Mean (SD) | ||||||
| Age at blood draw* | 55.6 (7.8) | 55.5 (7.9) | 55.3 (7.9) | 54.0 (8.1) | 57.8 (7.5) | 58.5 (6.8) |
| BMI at blood draw | 24.7 (4.1) | 25.0 (4.7) | 24.5 (4.2) | 26.9 (5.8) | 25.5 (5.4) | 25.3 (5.3) |
| Time to diagnosis (years) | - | 12.3 (5.2) | 12.8 (5.3) | 12.1 (5.1) | 10.9 (4.7) | 12.7 (5.2) |
| Age at diagnosis (years) | - | 69.7 (9.7) | 68.1 (9.8) | 66.0 (9.8) | 68.7 (9.2) | 71.2 (8.7) |
| N (Percent) | ||||||
| Tumor morphology | ||||||
| Invasive | - | 227 (90) | 163 (93) | 33 (97) | 31 (74) | 86 (100) |
| Borderline | - | 22 (9) | 13 (7) | 1 (3) | 8 (19) | 0 (0) |
| Unknown | - | 3 (1) | 0 (0) | 0 (0) | 3 (7) | 0 (0) |
| Menopausal status blood draw* | ||||||
| Premenopausal | 82 (33) | 82 (33) | 56 (32) | 16 (47) | 10 (24) | 14 (16) |
| Postmenopausal, No HT use | 71 (28) | 68 (27) | 47 (27) | 5 (15) | 16 (38) | 29 (34) |
| Postmenopausal, HT use | 66 (26) | 69 (27) | 48 (27) | 8 (24) | 13 (31) | 34 (40) |
| Unknown | 33 (13) | 33 (13) | 25 (14) | 5 (15) | 3 (7) | 9 (10) |
| Cohort* | ||||||
| NHS | 212 (84) | 212 (84) | 147 (84) | 27 (79) | 38 (90) | 79 (92) |
| Race | ||||||
| White | 251 (100) | 251 (100) | 175 (100) | 34 (100) | 42 (100) | 86 (100) |
| Oral contraceptive use duration | ||||||
| None or <3 months | 123 (49) | 118 (47) | 81 (46) | 18 (53) | 19 (45) | 48 (56) |
| 3 months to 3 years | 33 (13) | 32 (13) | 22 (12) | 3 (9) | 7 (17) | 10 (12) |
| 3 to 5 years | 45 (18) | 63 (25) | 46 (26) | 8 (24) | 9 (21) | 16 (19) |
| 5+ years | 51 (20) | 39 (15) | 27 (15) | 5 (15) | 7 (17) | 12 (14) |
| Parity | ||||||
| No children | 12 (5) | 24 (10) | 16 (9) | 5 (15) | 3 (7) | 7 (8) |
| 1 child | 11 (4) | 13 (5) | 8 (5) | 1 (3) | 4 (10) | 2 (2) |
| 2 children | 72 (29) | 89 (35) | 60 (34) | 15 (44) | 14 (33) | 23 (27) |
| 3 children | 77 (31) | 65 (26) | 45 (26) | 9 (26) | 11 (26) | 25 (29) |
| 4+ children | 80 (32) | 61 (24) | 47 (27) | 4 (12) | 10 (24) | 29 (34) |
| Tubal ligation | ||||||
| Yes | 43 (17) | 39 (15) | 30 (17) | 5 (15) | 34 (10) | 17 (20) |
matching factors; HT any type of hormone therapy
Measured metabolites and their association with ovarian cancer risk
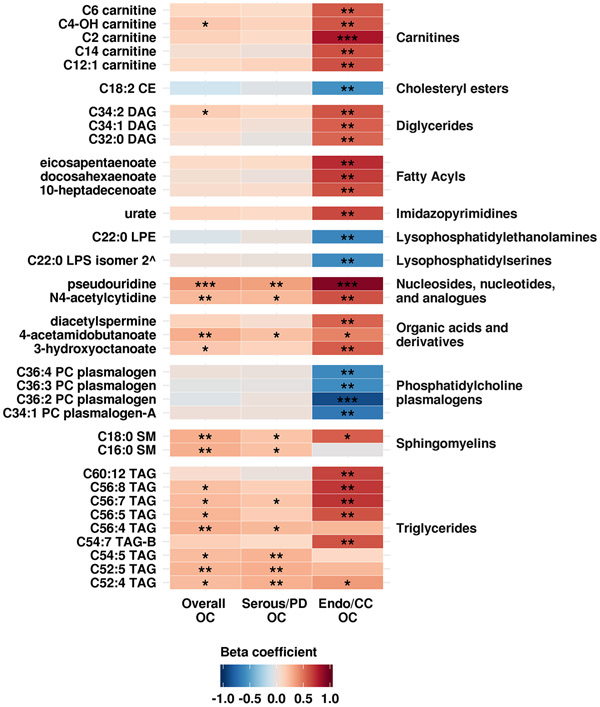
Of the 420 metabolites passing our QC filtering criteria, there were 159 lipids; 158 amino acids, amino acids derivatives amines and cationic metabolites; and 103 free fatty acids, bile acids and lipid mediators. Eight metabolites were associated with risk of overall ovarian cancer at a nominal p-value ≤0.01 (Table 2A, Figure 1 and Supplementary Table 1). Odds ratios for an increase from the 10th to the 90th percentile of levels ranged between 0.49 and 2.56. The top three metabolites associated with risk were pseudouridine (OR=2.56, 95% CI=1.48–4.45; p-value=0.001), C18:0 sphingomyelin (SM) (OR=2.10, 95% CI=1.26–3.49; p-value=0.004) and 4-acetamidobutanoate (OR=2.10, 95% CI=1.24–3.56; p-value=0.006). Pseudouridine had an adjusted-p=0.15 (accounting for all tested metabolites and their correlation structure); all other metabolites had adjusted-p>0.5. The test of the global null hypothesis that no metabolite was associated with risk had p=0.15. Results did not change in sensitivity analyses excluding specific case and control populations (Supplementary Table 1.1-1.5).
Table 2: Odds ratio (OR) for an increase from the 10th to the 90th percentile of metabolite levels and 95% confidence intervals (CI) of associations with risk of overall, serous/poorly differentiated and endometrioid/clear cell ovarian cancer.
Results with p-values ≤0.01 are shown for overall and serous/poorly differentiated ovarian cancer. The top 10 (out of 30) metabolites with p-values ≤0.01 are shown for endometrioid/clear cell tumors. Complete results are available in Supplementary Tables 1-2.
| A Overall Ovarian Cancer (N = 252 cases and 252 controls) | ||||
|---|---|---|---|---|
| HMDB ID | Metabolite | OR (95% CI) | P-value | Adjusted P-value |
| HMDB00767 | pseudouridine | 2.56 (1.48-4.45) | 0.001 | 0.150 |
| HMDB01348 | C18:0 SM | 2.10 (1.26-3.49) | 0.004 | 0.570 |
| HMDB03681 | 4-acetamidobutanoate | 2.10 (1.24-3.56) | 0.006 | 0.672 |
| HMDB05398* | C56:4 TAG | 2.03 (1.21-3.39) | 0.007 | 0.722 |
| HMDB05380* | C52:5 TAG | 1.96 (1.20-3.20) | 0.007 | 0.733 |
| HMDB05923 | N4-acetylcytidine | 1.88 (1.18-3.02) | 0.008 | 0.772 |
| HMDB10169 | C16:0 SM1 | 2.06 (1.19-3.56) | 0.009 | 0.807 |
| -- | armillane2 | 0.49 (0.28-0.85) | 0.010 | 0.824 |
| B Serous/Poorly differentiated ovarian cancer (N = 176 cases and 252 controls) | ||||
| HMDB ID | Metabolite | OR (95% CI) | P-value | Adjusted P-value |
| HMDB00767 | pseudouridine | 2.38 (1.33-4.32) | 0.004 | 0.552 |
| HMDB05380* | C52:5 TAG | 2.09 (1.23-3.59) | 0.007 | 0.745 |
| HMDB05363* | C52:4 TAG | 2.03 (1.21-3.47) | 0.008 | 0.809 |
| HMDB05391* | C54:6 TAG-A | 1.99 (1.18-3.38) | 0.010 | 0.862 |
| HMDB05385* | C54:5 TAG | 1.99 (1.19-3.39) | 0.010 | 0.851 |
| C Endometrioid/Clear cell ovarian cancer (N = 34 cases and 252 controls) | ||||
| HMDB ID | Metabolite | OR (95% CI) | P-value | Adjusted P-value |
| HMDB11243* | C36:2 PC plasmalogen | 0.11 (0.03-0.35) | 0.0003 | 0.056 |
| HMDB00767 | pseudouridine | 9.84 (2.89-37.82) | 0.0004 | 0.072 |
| HMDB05462* | C56:7 TAG | 5.85 (2.04-18.02) | 0.001 | 0.236 |
| HMDB00201 | C2 carnitine | 7.4 (2.37-25.35) | 0.001 | 0.143 |
| HMDB05392* | C56:8 TAG | 5.75 (2.00-17.72) | 0.002 | 0.260 |
| HMDB01999 | eicosapentaenoate | 6.25 (2.05-20.65) | 0.002 | 0.286 |
| HMDB11208* | C34:1 PC plasmalogen-A | 0.18 (0.05-0.54) | 0.003 | 0.468 |
| HMDB07103* | C34:2 DAG | 4.46 (1.64-12.85) | 0.004 | 0.577 |
| HMDB11520 | C22:0 LPE | 0.21 (0.07-0.59) | 0.004 | 0.517 |
| HMDB02183 | docosahexaenoate | 5.49 (1.74-18.72) | 0.005 | 0.617 |
representative ID
HMBD ID not available
significantly associated with risk in our analysis of lipid-related metabolites and risk of ovarian cancer (manuscript in revision)
preliminary ID
Figure 1: Beta coefficients of the association between metabolites and overall OC, serous/poorly differentiated OC (Serous/PD OC) and endometrioid/clear cell OC (Endo/CC OC).
Coefficients with a p-value ≤0.01 in any of the analyses are shown. Shades of red represent positive coefficients while shades of blue indicate negative coefficients. Significance of the association (using unadjusted p-values) is overlaid on the heat map and marked as follows: * p-values≤0.1, ** p-values≤0.01, *** p-values≤0.001; all other p-values are >0.1. Based on the Westfall and Young stepdown min p approach for multiple comparisons, only pseudouridine for overall OC and endo/CC OC as well as C36:2 PC plasmalogen and C2 carnitine had an adjusted p-value<0.2. ^ preliminary ID
Five metabolites were associated with risk of serous/PD tumors at a nominal p-value ≤0.01 (Table 2B, Figure 1 and Supplementary Table 2). Odds ratios for an increase from the 10th to the 90th percentile of metabolites levels for these metabolites ranged between 1.99 and 2.38. The top three metabolites were pseudouridine (OR=2.38, 95% CI=1.33–4.32; p-value=0.004), C52:5 triacylglycerol (TAG) (OR=2.09, 95% CI=1.23–3.59; p-value=0.007) and C52:4 TAG (OR=2.03, 95% CI=1.21–3.47; p=0.008). However, none of the metabolites remained significant after accounting for multiple comparisons via permutation (adjusted p-value >0.55). The test of the global null hypothesis that no metabolite was associated with risk had p=0.55. Results did not change in sensitivity analyses in which we excluded low-grade serous cases (Supplementary Table 2.1).
Thirty metabolites were associated with risk of endometrioid/CC tumors at a nominal p-value ≤0.01 (Table 2C, Figure 1 and Supplementary Table 2). Odds ratios for an increase from 10th to the 90th percentile of metabolites levels for these metabolites ranged between 0.11 and 0.24 for inverse associations, and between 3.85 and 9.84 for positive associations. The top three metabolites positively associated with risk were pseudouridine (OR=9.84, 95% CI=2.89–37.82; p=0.0003), C2 carnitine (OR=7.4, 95% CI=2.37–25.35; p=0.001) and C56:7 TAG (OR=5.85, 95% CI=2.04–18.02; p=0.001). The top three metabolites inversely associated with risk were C36:2 phosphatidylcholines (PC) plasmalogen (OR=0.11, 95% CI=0.03–0.35), p=0.0003), C34:1 PC plasmalogen-A (OR=0.18, 95% CI=0.05–0.54, p=0.003), C22:0 lysophosphatidylethanolamine (LPE) (OR=0.21, 95% CI=0.07–0.59; p=0.004). C36:2 PC plasmalogen and pseudouridine had an adjusted-p=0.06 and 0.07, respectively (accounting for all tested metabolites and their correlation structure). All other metabolites had adjusted-p≥0.14. The test of the global null hypothesis that no metabolite was associated with risk had p=0.06.
On the individual metabolite level, histograms and QQ-plots of the nominal p-values (Supplementary Figure 1) together with the results of the permutation-based approach to account for testing multiple correlated metabolites (Westfall and Young’s stepdown min p approach) suggest the existence of a metabolomic signal for overall ovarian cancer and non-serous tumors.
Metabolite groups associated with risk of ovarian cancer
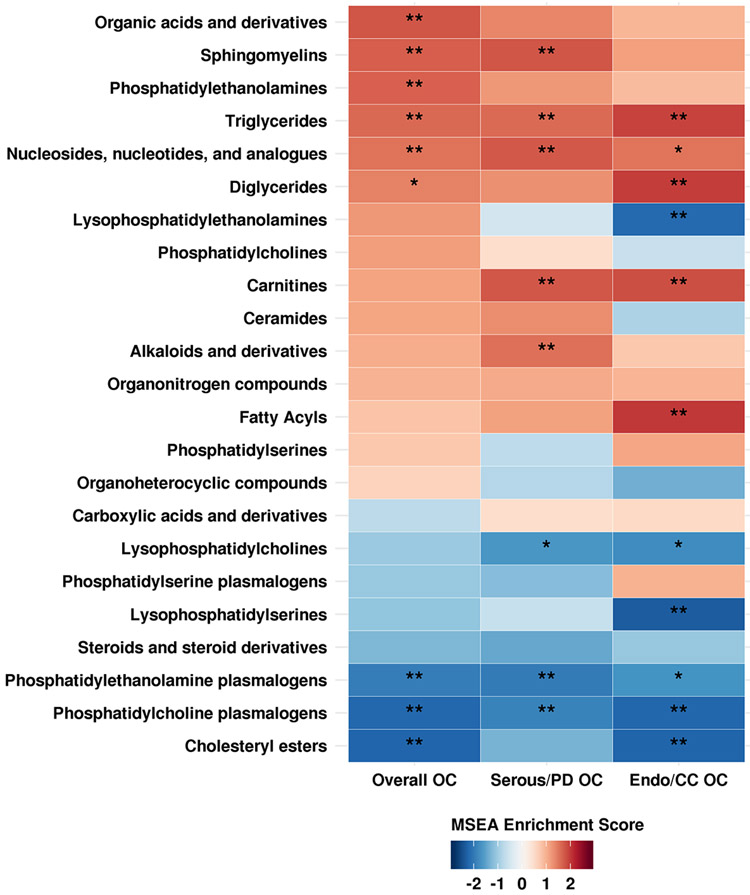
In the MSEA analysis, nine metabolite groups were enriched among metabolites associated with risk of ovarian cancer overall at an FDR ≤0.2 (Figure 2 and Supplementary Table 3). The top five groups were organic acids and derivatives; PE plasmalogens; TAGs; cholesteryl esters; and PC plasmalogens. Nine metabolite groups were associated with risk of serous/PD tumors with FDR ≤0.20 (Figure 2 and Supplementary Table 3). The top five were: nucleosides, nucleotides and analogues; TAGs; carnitines; sphingomyelins; and alkaloids and derivatives. Finally, eleven metabolite groups were associated with risk of endometrioid/CC tumors at FDR ≤0.20 (Figure 2 and Supplementary Table 3). The top five associated metabolite groups were TAGs, DAGs, fatty acyls, lysophosphatidylserines (LPS), and carnitines. TAGs were enriched in the above at FDR≤0.05. Notably, we observed differential associations by acyl carbon number and double bond content with risk of ovarian cancer overall (Supplementary Figure 2) and serous/PD tumors (Supplementary Figure 3, but not with endometrioid/CC tumors (Supplementary Figure 4). Specifically, TAGs with higher number of acyl carbon atoms and double bonds were associated with increased risk, while TAGs with lower number of acyl carbon atoms and double bonds were associated with decreased risk. We did not observe similar patterns for other lipid classes (Supplementary Figures 2-4).
Figure 2: MSEA results. Enriched metabolite groups associated with risk of overall OC, serous/poorly differentiated OC (Serous/PD OC) and endometrioid/clear cell OC (Endo/CC OC).
Significance of the association is overlaid on the heat map and marked as follows: * FDR ≤0.2, ** FDR ≤0.05; all other FDR >0.2.
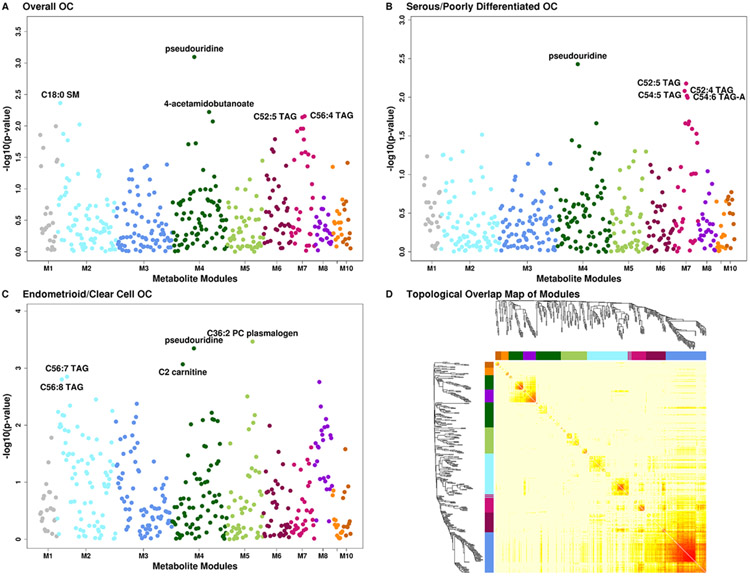
Metabolite modules associated with risk of ovarian cancer
WGCNA identified seven metabolite modules associated with risk of ovarian cancer with FDR ≤0.20 (Table 3 and Figure 3, panels A-D). Module 1 (M1, characterized by steroids and steroid derivatives, organic acids and derivatives, and organonitrogen compounds [Supplementary Figures 5-7, Supplementary Table 4]), M2 (characterized by TAGs, PCs, PE, LPCs, and LPEs), M6 (characterized by TAGs, LPEs and CEs), and M7 (characterized by TAGs, DAGs, ceramides and CEs) were associated with increased risk of ovarian cancer overall, OR, increase from 10th to 90th percentile=1.99 (p=0.013/FDR=0.072), 1.62 (p=0.093/FDR=0.186), 1.56 (p=0.081/FDR=0.186) and 1.8 (p=0.015/FDR=0.072), respectively. M4 (characterized by carnitines, pseudouridine [inversely weighted], and organic acids and derivatives) was associated with decreased risk (OR=0.5, p=0.022/FDR=0.072). M7 was associated with increased risk of serous/PD tumors (OR=1.97; p=0.012/FDR=0.117). Finally, four modules were associated with risk of endometrioid/CC tumors: M2 (OR=6.14; p=0.002/FDR=0.011), M4 (OR=0.17; p=0.003/FDR=0.011), M5 (PC and PE plasmalogens; OR=0.35; p=0.041/FDR=0.072), and M8 (fatty acyls, OR=0.22; p=0.007/FDR=0.017).
Table 3:
P-values, FDR, odds ratio (OR) for an increase from the 10th to the 90th percentile of metabolite levels and 95% confidence intervals (CI) of WGCNA metabolite modules associated with risk of ovarian cancer overall and by histotype.
| Overall OC | Serous/PD OC | Endometrioid/CC OC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Module / number of metabolites |
OR (95% CI) |
P-value | FDR | explained variance [%] |
OR (95% CI) |
P-value | FDR | explained variance [%] |
OR (95% CI) |
P-value | FDR | explained variance [%] |
| M1 / 24 | 1.99 (1.15-3.42) | 0.013 | 0.072 | 14.51 | 1.64 (0.89-3.05) | 0.114 | 0.46 | 14.87 | 1.09 (0.38-3.11) | 0.875 | 0.613 | 14.62 |
| M2 / 79 | 1.62 (0.92-2.85) | 0.093 | 0.186 | 23.63 | 1.16 (0.65-2.07) | 0.624 | 0.701 | 23.35 | 6.14 (1.97-20.67) | 0.002 | 0.011 | 24.54 |
| M3 / 76 | 0.9 (0.56-1.46) | 0.678 | 0.682 | 65.22 | 1.06 (0.62-1.8) | 0.839 | 0.839 | 65.18 | 0.48 (0.17-1.28) | 0.151 | 0.212 | 66.23 |
| M4 / 74 | 0.5 (0.28-0.9) | 0.022 | 0.072 | 17.86 | 0.64 (0.35-1.15) | 0.138 | 0.46 | 18.13 | 0.17 (0.05-0.54) | 0.003 | 0.011 | 17.63 |
| M5 / 49 | 0.91 (0.56-1.46) | 0.682 | 0.682 | 24.23 | 1.2 (0.71-2.03) | 0.49 | 0.701 | 23.94 | 0.35 (0.12-0.94) | 0.041 | 0.072 | 21.61 |
| M6 / 37 | 1.56 (0.95-2.58) | 0.081 | 0.186 | 40.38 | 1.17 (0.68-2.01) | 0.575 | 0.701 | 40.32 | 1.4 (0.5-3.99) | 0.522 | 0.456 | 40.16 |
| M7 / 32 | 1.8 (1.12-2.88) | 0.015 | 0.072 | 32.45 | 1.97 (1.17-3.36) | 0.012 | 0.117 | 32 | 1.94 (0.71-5.5) | 0.203 | 0.237 | 32.69 |
| M8 / 24 | 0.82 (0.49-1.37) | 0.441 | 0.552 | 65.98 | 0.81 (0.47-1.4) | 0.451 | 0.701 | 65.34 | 0.22 (0.07-0.65) | 0.007 | 0.017 | 64.15 |
| M9 / 14 | 0.73 (0.45-1.19) | 0.205 | 0.341 | 37.33 | 0.88 (0.51-1.5) | 0.631 | 0.701 | 38.1 | 0.79 (0.29-2.21) | 0.651 | 0.506 | 36.26 |
| M10 / 11 | 0.75 (0.43-1.29) | 0.293 | 0.419 | 46.53 | 0.7 (0.38-1.29) | 0.257 | 0.644 | 45.92 | 1.88 (0.57-6.46) | 0.305 | 0.305 | 47.51 |
Figure 3: METhattan plots.
Manhattan plots of metabolites by metabolite groups, with each group being shown in a different color. A Overall ovarian cancer. B Serous/poorly differentiated ovarian cancer. C Endometrioid/clear cell ovarian cancer. D Topological Overlap Matrix (TOM). Metabolites in the rows and columns are sorted by the clustering tree. Light yellow shades represent low topological overlap (low similarity). Darker red shades represent higher overlap and similarity. Metabolite modules correspond to the squares along the diagonal.
Metabolites associated with ovarian cancer risk by menopausal status at blood collection
C22:0 LPS isomer was suggestively associated with increased risk among postmenopausal women (OR=1.83, 95%CI=0.92–3.63; p=0.085) and decreased risk among premenopausal women (OR=0.44, 95%CI=0.17–1.08; p=0.074), with a heterogeneity p=0.004 (Supplementary Table 5). C38:4 PC plasmalogen was suggestively associated with increased risk among postmenopausal women (OR=1.92, 95%CI=0.96–3.85; p=0.066) and decreased risk among premenopausal women (OR=0.16, 95%CI=0.05–0.51; p=0.002), with a heterogeneity p=0.005. Among premenopausal women, 14/22 (63%) metabolites associated with risk at p≤0.1 were inversely related, but among premenopausal women only 15/98 (15%) metabolites showed inverse associations. Pseudouridine did not show heterogeneity by menopausal status (heterogeneity p=0.32).
Metabolites associated with ovarian cancer risk by time between blood collection and diagnosis
Hydroxyvitamin D3 was associated with increased risk among participants with blood collection 12–23 years before diagnosis (OR=1.84, 95%CI=1.02–3.37; p=0.044) but not among participants with blood collection 3–11 years before diagnosis (OR=0.64, 95%CI=0.36–1.15; p=0.141), with a heterogeneity p=0.002 (Supplementary Table 6). C40:6 phosphatidylserine (PS) was associated with decreased risk among participants with blood collection 12–23 years before diagnosis (OR=0.55, 95%CI=0.3–0.99; p=0.049) but not among participants with blood collection 3–11 years before diagnosis (OR=1.41, 95%CI=0.79–2.51; p=0.245), with a heterogeneity p=0.008. Pseudouridine showed suggestively stronger associations (heterogeneity p=0.066) among women for whom sample collection was 3–11 years before diagnosis (OR=4.48, 95%CI=2.25–9.24; p≤0.001) compared to participants with samples collection 12–23 years before diagnosis (OR=2.00, 95%CI=1.06–3.85; p=0.035).
Metabolites associated with ovarian cancer risk by tumor aggressiveness
Fifty-three lipid-related metabolites (26 TAGs, 7 PCs, 6 LPEs, 3 PEs, 3 LPC, 4DAGs, 2 LPSs, and 2 PSs) showed differences by tumor aggressiveness at heterogeneity p≤0.01 (Supplementary Table 7). Seven metabolites (6 TAGs and 1 PS) were associated with increased risk of rapidly fatal disease with ORs ranging between 2.56 and 3.07 at p≤0.008, but not with less aggressive tumors (p>0.62), with heterogeneity p≤0.001. Several lipid-related metabolite classes (DAGs, LPCs, LPEs, PCs, PEs, PSs, and TAGs with high acyl carbon content and saturation) were overrepresented in rapidly fatal tumors versus controls, while carnitines were overrepresented in less aggressive tumors (Supplementary Figure 8, panels A and B). TAGs with lower acyl carbon content and saturation were inversely associated with less aggressive tumors. Pseudouridine did not show heterogeneity by tumor aggressiveness (heterogeneity p=0.13).
Discussion
We conducted the first large-scale agnostic analysis of metabolomics and risk of ovarian cancer. We identified a potential novel risk factor, plasma pseudouridine, which was associated with an increased risk of ovarian cancer overall and non-serous tumors and suggestively for serous/PD disease. Stronger associations for pseudouridine were observed among cases diagnosed within 3–11 years after blood collection. We identified several metabolite groups and metabolite modules associated with risk of ovarian cancer risk, as well as multiple subtype-specific associations, that open up new opportunities for assessing novel metabolite pathways involved in ovarian cancer development.
Pseudouridine
Pseudouridine is the most abundant post-transcriptionally modified nucleoside, and is an isomer of uridine. It is produced by pseudouridine synthase by isomerizing uridines from transfer RNA, which is involved in in protein translation, or spliceosomal snRNA, which plays a role in pre-mRNA splicing. Pseudouridine was nominally associated with risk overall and for both histotypes, with no significant heterogeneity (p=0.16) by histotype. This suggests that pseudouridine may represent a common etiologic mechanism underlying different histotypes of ovarian cancer, which has been observed for other risk factors, such as aspirin and CRP. In retrospective studies, pseudouridine was elevated in urine (17) and plasma (18) from epithelial ovarian cancer patients versus healthy controls. This, in combination with our finding that pseudouridine had a stronger association when assessed 3–11 years before diagnosis, suggests that this modified nucleotide may be important in progression of preclinical lesions to fully overt invasive disease, which for high-grade serous ovarian cancer appears to be about 7–9 years (19). Increasing evidence suggests that pseudouridylation plays a role in cancer-associated splicing distributions, which are more variable than in normal tissues. Notably, tissue-specific alternative splicing reverts to a default cancer pattern that directly contributes to cellular transformation and cancer progression (20). This has been observed in serous carcinomas, which have highly dysregulated splicing compared to normal tissue (21). Further, aberrant pseudouridylation may lead to altered and reduced translational fidelity of p53 (22), which is mutated in nearly all high-grade serous tumors (23). Another potential mechanism is via circular RNA activity, which is altered due to isomerization of uridine to pseudouridine, and has been shown to be dysregulated in ovarian cancer (24). Interestingly, pseudouridine is associated with the estimated glomerular filtration rate, a marker of kidney function (25). While kidney function alters the immune response, potentially contributing to the development of cancer (26), associations with cancer incidence are mixed, although a recent study observed that thiazide diuretics, which can affect kidney function, are associated with a higher risk of ovarian cancer (27). Additional research should explore the potential role of pseudouridine in precursor lesions to ovarian cancer, the relation between circulating pseudouridine to ovarian and fallopian tube tissue levels, and if kidney function plays a role in the initiation or development of ovarian cancer.
Triacylglycerides
Notably, several individual TAGs were nominally related to risk and showed significantly stronger associations with rapidly fatal tumors. Evidence suggests that established ovarian cancer risk factors vary by tumor aggressiveness (28). As high grade serous ovarian cancer was the predominant histotype among rapidly fatal as well as less aggressive tumors, our data suggest that there are potential differences between the metabolic profiles of these two groups of tumors independent of histotype. TAGs as a group were enriched in the MSEA analysis, and 3 of 7 WCGNA modules related to risk were characterized by TAGs. Long chain fatty acids, a main source of energy in the human body, are stored and transported from the small intestine and liver to peripheral cells as TAGs (29). Lipid synthesis and metabolism that releases free fatty acids from TAGs are dysregulated in ovarian tumors, increasing cell migration and invasive potential (30). Further, several human studies reported suggestive associations of ovarian cancer risk with total cholesterol (31) (positive) or HDL (inverse) (32). Additionally, ovarian cancer metastasizes preferentially to the adipose-rich omentum (33). Omental fat possesses a distinct lipidomic signature with several lipid groups, including TAGs, DAGs, and SMs, showing differences when compared to subcutaneous fat (34). Finally, plasma TAGs represent known risk factors for cardiovascular disease and coronary heart disease. A recent study identified that TAGs at the extremes of carbon atoms and saturation had differential associations with diabetes risk (35). We also observed differential associations by TAG fatty acids length and saturation, with higher number of carbon atoms and double bonds related to an increased risk and lower number of carbon atoms and double bonds related to decreased risk, particularly for serous/PD tumors. A similar pattern was observed in a retrospective study of serum samples from high-grade serous ovarian cancer cases and controls (36). Together with our results, these findings suggest that circulating TAG levels may be a risk biomarker for ovarian cancer, particularly for rapidly fatal tumors. Additional prospective studies are needed to validate these associations in different populations and assess the potential differential role of various TAG species in ovarian carcinogenesis.
Other metabolite groups
A number of metabolite groups and classes were associated with ovarian cancer risk, including organic acids and derivatives, and SMs, the latter of which was hypothesized a priori as a potential risk biomarker and is discussed elsewhere (37). A metabolite module driven by carnitines, organic acids and derivatives, carboxylic acids and derivatives, which included pseudouridine (highly negatively weighted), was associated with decreased risk of overall ovarian cancer and non-serous tumors. This module includes asymmetric dimethylarginine (ADMA), which has been related to risk of cardiovascular disease (38), and inhibits nitric oxide synthesis and may have antiproliferative properties (39) including in ovarian tumors (40). LPEs were also represented in WCGNA modules associated with increased risk of overall and endometrioid/CC ovarian cancers. LPEs have been shown to increase migration in response to chemotherapy as well as have invasive potential in ovarian cancer cell lines (41). In MSEA analyses, several metabolite classes had a significant negative enrichment score, including PE plasmalogens, PC plasmalogens and cholesteryl esters, independent of subtype.
Little work has examined these markers in ovarian cancer development or etiology. Sphingolipids ([SL]; including SMs, PCs, PEs, LPCs, LPEs, cholesteryl esters, acylcarnitines) are associated with a series of conditions that may be related to ovarian cancer, including thrombosis in a mouse study (42), myocardial infraction among symptomatic coronary artery disease patients (43), type 1 and type 2 diabetes in human studies (44), diabetic kidney disease in mice (45, 46) and airway inflammation and asthma in mouse and human studies (47-49). Notably, patients with ovarian cancer have the highest incidence of venous thromboembolism (VTE) of all solid tumor types (50), which is significantly related to higher mortality (51). Coagulation activation by tumors promotes development of VTE which in turn favors cancer progression through tumor growth, angiogenesis, invasion, immune evasion, and metastasis (52). Further, cholesterol-lowering statins have anti-inflammatory, anti-proliferative, apoptotic and anti-invasive qualities (53-57), and can lower SMs (58). Data on ovarian cancer risk reduction by statins have been mixed. A meta-analysis of existing studies suggested a lower risk for ovarian cancer associated with statin use (59) while post-diagnostic statin use was inversely associated with overall survival and ovarian cancer specific mortality (60-62). Additional work should evaluate whether these conditions and medications associated with the identified metabolites represent novel risk factors for ovarian cancer, preferably using large consortia to ensure power.
Our study has several strengths and limitations. Importantly, this is a prospective study of ovarian cancer risk with coverage of multiple different metabolite classes. Additional strengths include the long follow-up time and detailed covariate information. Our cohort consisted of registered nurses, a group that are not representative of the general population (e.g. social economic status), although established risk factor associations in these cohorts are similar to those in other more representative studies (63). While we had over 250 ovarian cancer cases and controls, we had more limited sample sizes for specific histotypes, which have been shown to have different associations for known risk factors (64). We used medical records and pathology reports to confirm diagnosis and extract histotype and cannot rule out the possibility of histotype misclassification, although we previously showed high concordance to centralized slide review (9). To maximize power, borderline and tumors of unknown morphology were included. We did not include information on family history of ovarian cancer. However, only 2 cases were diagnosed before age 45 suggesting that early onset disease, likely due to high risk mutations, does not play a role in this study. We also applied stringent QC criteria to limit identification of spurious associations. Another limitation is that we only analyzed blood samples collected at one point in time; however, we demonstrate that the majority of the measured metabolites have a high within person stability over time (11). Further, we do not have an independent validation dataset. As this type of data becomes more common, further population studies are needed to validate the results discussed here, while experimental studies are required to understand the biological mechanisms underlying these associations.
In summary, circulating levels of plasma pseudouridine were associated with higher risk of ovarian cancer 3–23 years before diagnosis, with stronger associations among participants with samples collected closer to diagnosis. Additionally, several metabolite groups and metabolite modules were associated with risk of disease overall and by subtype. While independent prospective studies are needed for validation, our results highlight some potentially important novel metabolites that may play a role in the etiology of ovarian cancer. Potential experimental studies to understand the biological mechanisms of these risk biomarkers could examine their role in carcinogenic tendencies in ovarian and fallopian tube cancer cell lines (with and without p53 mutations), mouse models of early and late ovarian lesions leading to ovarian cancer as well as xenograft mouse models in which pseudouridine production has been impaired or enhanced. Adding these new risk biomarkers to current risk prediction models may help the identification of high-risk women.
Supplementary Material
Statement of significance: Pseudouridine represents a potential novel risk factor for ovarian cancer and triglycerides may be important particularly in rapidly fatal ovarian tumors.
Acknowledgements
This study was funded by the National Cancer Institute (P01 CA087969, U01 CA176726, UM1 CA186107) and the Department of Defense (W81XWH-12–1-0561). We would like to thank the participants and staff of the Nurses Health Studies and Nurses Health Studies II for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2018. Atlanta: American Cancer Society; 2018. [Google Scholar]
- 2.Krumsiek J, Bartel J, Theis FJ. Computational approaches for systems metabolomics. Curr Opin Biotechnol. 2016;39:198–206. [DOI] [PubMed] [Google Scholar]
- 3.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20(10):1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mondul AM, Moore SC, Weinstein SJ, Karoly ED, Sampson JN, Albanes D. Metabolomic analysis of prostate cancer risk in a prospective cohort: The alpha‐tocopherol, beta‐carotene cancer prevention (ATBC) study. Int J Cancer. 2015;137(9):2124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Playdon MC, Ziegler RG, Sampson JN, Stolzenberg-Solomon R, Thompson HJ, Irwin ML, et al. Nutritional metabolomics and breast cancer risk in a prospective study. The American journal of clinical nutrition. 2017;106(2):637–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore SC, Playdon MC, Sampson JN, Hoover RN, Trabert B, Matthews CE, et al. A metabolomics analysis of body mass index and postmenopausal breast cancer risk. JNCI: Journal of the National Cancer Institute. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hankinson SE, Willett WC, Michaud DS, Manson JE, Colditz GA, Longcope C, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(7):629–34. [DOI] [PubMed] [Google Scholar]
- 8.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66(4):2476–82. [DOI] [PubMed] [Google Scholar]
- 9.Barnard ME, Pyden A, Rice MS, Linares M, Tworoger SS, Howitt BE, et al. Inter-pathologist and pathology report agreement for ovarian tumor characteristics in the Nurses’ Health Studies. Gynecol Oncol. 2018;150(3):521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terry KL, Schock H, Fortner RT, Hüsing A, Fichorova RN, Yamamoto HS, et al. A prospective evaluation of early detection biomarkers for ovarian cancer in the European EPIC cohort. Clinical Cancer Research. 2016;22(18):4664–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clinical chemistry. 2013;59(11):1657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westfall PH, Young SS. Resampling-based multiple testing: Examples and methods for p-value adjustment: John Wiley & Sons; 1993. [Google Scholar]
- 13.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences. 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9(1):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93(3):491–507. [Google Scholar]
- 16.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2013. 2014. [Google Scholar]
- 17.Zhang T, Wu X, Ke C, Yin M, Li Z, Fan L, et al. Identification of potential biomarkers for ovarian cancer by urinary metabolomic profiling. J Proteome Res. 2012;12(1):505–12. [DOI] [PubMed] [Google Scholar]
- 18.Ke C, Hou Y, Zhang H, Fan L, Ge T, Guo B, et al. Large‐scale profiling of metabolic dysregulation in ovarian cancer. Int J Cancer. 2015;136(3):516–26. [DOI] [PubMed] [Google Scholar]
- 19.Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nature communications. 2017;8(1):1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Marabti E, Younis I. The Cancer Spliceome: reprograming of alternative splicing in cancer. Frontiers in molecular biosciences. 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinck R, Bramard A, Inkel L, Dufresne-Martin G, Gervais-Bird J, Madden R, et al. Multiple alternative splicing markers for ovarian cancer. Cancer research. 2008;68(3):657–63. [DOI] [PubMed] [Google Scholar]
- 22.Penzo M, Guerrieri A, Zacchini F, Treré D, Montanaro L. RNA pseudouridylation in physiology and medicine: for better and for worse. Genes. 2017;8(11):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanchi KL, Johnson KJ, Lu C, McLellan MD, Leiserson MD, Wendl MC, et al. Integrated analysis of germline and somatic variants in ovarian cancer. Nature communications. 2014;5:3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen L, Hansen T, Venø M, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37(5):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekula P, Goek O-N, Quaye L, Barrios C, Levey AS, Römisch-Margl W, et al. A metabolome-wide association study of kidney function and disease in the general population. Journal of the American Society of Nephrology. 2016;27(4):1175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–71. [DOI] [PubMed] [Google Scholar]
- 27.Huang T, Poole EM, Eliassen AH, Okereke OI, Kubzansky LD, Sood AK, et al. Hypertension, use of antihypertensive medications, and risk of epithelial ovarian cancer. Int J Cancer. 2016;139(2):291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole EM, Merritt MA, Jordan SJ, Yang HP, Hankinson SE, Park Y, et al. Hormonal and reproductive risk factors for epithelial ovarian cancer by tumor aggressiveness. Cancer Epidemiology and Prevention Biomarkers. 2013;22(3):429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin MA. Plasma triglyceride as a risk factor for coronary heart disease. The epidemiologic evidence and beyond. Am J Epidemiol. 1989;129(2):249–59. [DOI] [PubMed] [Google Scholar]
- 30.Tania M, Khan M, Song Y. Association of lipid metabolism with ovarian cancer. Current oncology. 2010;17(5):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helzlsouer KJ, Alberg AJ, Norkus EP, Morris JS, Hoffman SC, Comstock GW. Prospective study of serum micronutrients and ovarian cancer. J Natl Cancer Inst. 1996;88(1):32–7. [DOI] [PubMed] [Google Scholar]
- 32.Melvin JC, Seth D, Holmberg L, Garmo H, Hammar N, Jungner I, et al. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1381–4. [DOI] [PubMed] [Google Scholar]
- 33.Motohara T, Masuda K, Morotti M, Zheng Y, El-Sahhar S, Chong KY, et al. An evolving story of the metastatic voyage of ovarian cancer cells: cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene. 2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jové M, Moreno-Navarrete JM, Pamplona R, Ricart W, Portero-Otín M, Fernández-Real JM. Human omental and subcutaneous adipose tissue exhibit specific lipidomic signatures. The FASEB Journal. 2014;28(3):1071–81. [DOI] [PubMed] [Google Scholar]
- 35.Al-Sulaiti H, Diboun I, Banu S, Al-Emadi M, Amani P, Harvey TM, et al. Triglyceride profiling in adipose tissues from obese insulin sensitive, insulin resistant and type 2 diabetes mellitus individuals. J Transl Med. 2018;16(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braicu EI, Darb-Esfahani S, Schmitt WD, Koistinen KM, Heiskanen L, Pöhö P, et al. High-grade ovarian serous carcinoma patients exhibit profound alterations in lipid metabolism. Oncotarget. 2017;8(61):102912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeleznik OA, Clish CB, Kraft P, Avila-Pancheco J, Eliassen AH, Tworoger SS. Circulating Lysophosphatidylcholines, Phosphatidylcholines, Ceramides, and Sphingomyelins and Ovarian Cancer Risk: a 23-year Prospective Study. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krzyzanowska K, Mittermayer F, Wolzt M, Schernthaner G. ADMA, cardiovascular disease and diabetes. diabetes research and clinical practice. 2008;82:S122–S6. [DOI] [PubMed] [Google Scholar]
- 39.Lüneburg N, Harbaum L, Hennigs JK. The endothelial ADMA/NO pathway in hypoxia-related chronic respiratory diseases. BioMed research international. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Sehemy A, Postovit L-M, Fu Y. Nitric oxide signaling in human ovarian cancer: a potential therapeutic target. Nitric Oxide. 2016;54:30–7. [DOI] [PubMed] [Google Scholar]
- 41.Park KS, Lee HY, Lee SY, Kim M-K, Kim SD, Kim JM, et al. Lysophosphatidylethanolamine stimulates chemotactic migration and cellular invasion in SK‐OV3 human ovarian cancer cells: Involvement of pertussis toxin‐sensitive G‐protein coupled receptor. FEBS letters. 2007;581(23):4411–6. [DOI] [PubMed] [Google Scholar]
- 42.Dang VT, Huang A, Zhong LH, Shi Y, Werstuck GH. Comprehensive plasma metabolomic analyses of atherosclerotic progression reveal alterations in glycerophospholipid and sphingolipid metabolism in apolipoprotein E-deficient mice. Scientific reports. 2016;6:35037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee M, Rath D, Schlotterbeck J, Rheinlaender J, Walker-Allgaier B, Alnaggar N, et al. Regulation of oxidized platelet lipidome: implications for coronary artery disease. European heart journal. 2017;38(25):1993–2005. [DOI] [PubMed] [Google Scholar]
- 44.Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, et al. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes. 2004;53(5):1215–21. [DOI] [PubMed] [Google Scholar]
- 45.Kang HM, Ahn SH, Choi P, Ko Y-A, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nature medicine. 2015;21(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huwiler A, Pfeilschifter J. Sphingolipid signaling in renal fibrosis. Matrix biology. 2018;68:230–47. [DOI] [PubMed] [Google Scholar]
- 47.Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, et al. Inhalation of sphingosine kinase inhibitor attenuates airway inflammation in asthmatic mouse model. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;294(6):L1085–L93. [DOI] [PubMed] [Google Scholar]
- 48.Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. The FASEB journal. 2001;15(7):1212–4. [DOI] [PubMed] [Google Scholar]
- 49.Trinh HKT, Kim S-C, Cho K, Kim S-J, Ban G-Y, Yoo H-J, et al. Exploration of the sphingolipid metabolite, sphingosine-1-phosphate and sphingosine, as novel biomarkers for aspirin-exacerbated respiratory disease. Scientific reports. 2016;6:36599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levitan N, Dowlati A, Remick S, Tahsildar H, Sivinski L, Beyth R, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using Medicare claims data Medicine (Baltimore). 1999;78(5):285–91. [DOI] [PubMed] [Google Scholar]
- 51.Stålberg K, Svensson T, Lönn S, Kieler H. The influence of comorbidity on mortality in ovarian cancer patients. Gynecologic oncology. 2014;133(2):298–303. [DOI] [PubMed] [Google Scholar]
- 52.Swier N, Versteeg HH. Reciprocal links between venous thromboembolism, coagulation factors and ovarian cancer progression. Thrombosis research. 2017;150:8–18. [DOI] [PubMed] [Google Scholar]
- 53.Cho SJ, Kim JS, Kim JM, Lee JY, Jung HC, Song IS. Simvastatin induces apoptosis in human colon cancer cells and in tumor xenografts, and attenuates colitis‐associated colon cancer in mice. Int J Cancer. 2008;123(4):951–7. [DOI] [PubMed] [Google Scholar]
- 54.Spampanato C, De Maria S, Sarnataro M, Giordano E, Zanfardino M, Baiano S, et al. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int J Oncol. 2012;40(4):935–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wächtershäuser A, Akoglu B, Stein J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis. 2001;22(7):1061–7. [DOI] [PubMed] [Google Scholar]
- 56.Denoyelle C, Vasse M, Körner M, Mishal Z, Ganné F, Vannier J-P, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis. 2001;22(8):1139–48. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Liang S-L, Kumar S, Weyman CM, Liu W, Zhou A. Statins induce apoptosis in ovarian cancer cells through activation of JNK and enhancement of Bim expression. Cancer Chemother Pharmacol. 2009;63(6):997. [DOI] [PubMed] [Google Scholar]
- 58.Schlitt A, Blankenberg S, Yan D, von Gizycki H, Buerke M, Werdan K, et al. Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutr Metab (Lond). 2006;3(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Qin A, Li T, Qin X, Li S. Effect of statin on risk of gynecologic cancers: a meta-analysis of observational studies and randomized controlled trials. Gynecologic oncology. 2014;133(3):647–55. [DOI] [PubMed] [Google Scholar]
- 60.Lavie O, Pinchev M, Rennert HS, Segev Y, Rennert G. The effect of statins on risk and survival of gynecological malignancies. Gynecologic oncology. 2013;130(3):615–9. [DOI] [PubMed] [Google Scholar]
- 61.Elmore RG, Ioffe Y, Scoles DR, Karlan BY, Li AJ. Impact of statin therapy on survival in epithelial ovarian cancer. Gynecol Oncol. 2008;111(1):102–5. [DOI] [PubMed] [Google Scholar]
- 62.Vogel TJ, Goodman MT, Li AJ, Jeon CY. Statin treatment is associated with survival in a nationally representative population of elderly women with epithelial ovarian cancer. Gynecologic oncology. 2017;146(2):340–5. [DOI] [PubMed] [Google Scholar]
- 63.Birmann BM, Barnard ME, Bertrand KA, Bao Y, Crous-Bou M, Wolpin BM, et al. Nurses’ Health Study contributions on the epidemiology of less common cancers: endometrial, ovarian, pancreatic, and hematologic. Am J Public Health. 2016;106(9):1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.