Abstract
Vancomycin-resistant enterococci (VRE) are a leading cause of nosocomial infections due to the limited number of effective therapeutic options. In an effort to repurpose FDA-approved drugs against antibiotic-resistant bacteria, we identified auranofin as a potent drug against VRE. The present study determined that auranofin’s antibacterial activity was not affected when evaluated against a higher inoculum size of VRE (~107 CFU/mL), and auranofin successfully reduced the burden of stationary phase VRE cells via a time-kill assay. In addition, auranofin reduced VRE production of key virulence factors including proteases, lipase and hemagglutinin. The promising features of auranofin prompted us to evaluate its in vivo efficacy in a lethal mouse model of VRE septicemia. All mice receiving auranofin at 0.125 mg/kg orally, 0.125 mg/kg subcutaneously (S.C.), or 0.0625 mg/kg (S.C.) survived the lethal VRE challenge. Additionally, auranofin was superior to linezolid, the current drug of choice, in reducing VRE burden in the liver, kidneys and spleen of mice. Remarkably, auranofin successfully reduced VRE below the limit of detection in murine internal organs after only four days of oral or subcutaneous treatment. These results indicate that auranofin warrants further investigation as a new treatment for systemic VRE infections.
Keywords: Auranofin, septicemia, antivirulence, protease, lipase
1. Introduction
Multidrug-resistant enterococci, especially Enterococcus faecalis and E. faecium, have emerged as the leading cause of hospital-acquired infections since the 1980s [1]. Both species are associated with life-threatening infections including septicemia, endocarditis, surgical site infections and urinary tract infections [2]. Both E. faecalis and E. faecium pose a major challenge in healthcare settings due to their ability to acquire or develop resistance to multiple antibiotics [3]. Two main mechanisms are involved in enterococcal resistance to antibiotics: a) intrinsic resistance to several antibiotics such as β-lactams and aminoglycosides, and b) ability to acquire resistance to glycopeptides, fluoroquinolones, tetracyclines, macrolides and streptogramins via horizontal gene transfer through transposons and plasmids [4]. Vancomycin has been a mainstay of treatment for enterococcal infections; however, vancomycin-resistant enterococci (VRE) isolates have emerged causing infections that are difficult to treat, not least because they are often resistant to other classes of antibiotics. As a direct consequence, VRE, in particular vancomycin-resistant E. faecium, are listed as a high priority pathogen by the World Health Organization for which new antibiotics are urgently needed [5].
Repurposing FDA-approved drugs is a novel way to reduce both the time and cost associated with antimicrobial innovation [6–8]. Using this approach, we previously identified auranofin as a potent antimicrobial agent against VRE in vitro and in a mouse model of VRE decolonization [9]. Auranofin was initially approved as a treatment for rheumatoid arthritis and has a well - studied safety profile with rare, mild and self-limiting adverse effects reported. Currently auranofin is undergoing Phase II clinical trials for the treatment of amoebic dysentery, giardiasis (ClinicalTrials.gov Identifier: NCT02736968) and tuberculosis (ClinicalTrials.gov Identifier: NCT02968927) demonstrating its potential to be repurposed for other diseases.
The goal of this study was to evaluate the antimicrobial activity of auranofin against standard and high inocula of VRE, assess auranofin’s ability to inhibit production of proteases, lipase, and hemagglutination, and to investigate the efficacy of auranofin to enhance survival of mice in a VRE septicemia model. Results obtained further support the potential of auranofin to be repurposed as a novel antibacterial agent to treat VRE infections.
2. Materials and methods
2.1. Bacterial strains and reagents
Auranofin, linezolid (Chem-Impex International, Wood Dale, IL, USA) and vancomycin hydrochloride (Gold Biotechnology, St. Louis, MO, USA) were purchased from commercial vendors. Skim milk powder was purchased from Oxoid (Basingstoke, Hants, UK). Brain heart infusion (BHI), tryptic soy broth (TSB), Tryptic soy agar (TSA) and enterococcosel agar were purchased from Becton, Dickinson and Company (Cockeysville, MD, USA) and phosphate-buffered saline (PBS) was purchased from Corning (Manassas, VA, USA). Egg yolk emulsion was purchased from HIMEDIA Laboratories (Thane, MH, India). Defibrinated horse blood was obtained from Hemostat Laboratories (Dixon, CA, USA). Clinical isolates of VRE (Table S1) were obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI Resources) and the American Type Culture Collection (ATCC).
2.2. Effect of VRE inoculum size on the minimum inhibitory concentration of auranofin
The impact of VRE inoculum size on the minimum inhibitory concentration (MIC) of auranofin was determined using the broth microdilution assay following the guidelines of the Clinical and Laboratory Standards Institute [10]. Briefly, standard inoculum (SI: 5 × 105 CFU/mL) and high inoculum (HI: 5 × 107 CFU/mL) of each strain of VRE were prepared and tested against auranofin and linezolid.
2.3. Time-kill assay of auranofin against vancomycin-resistant E. faecium stationary phase cells
A time-kill assay was conducted for auranofin and linezolid against E. faecium NR-31909 stationary phase cells, as described previously [11]. Briefly, an overnight culture of E. faecium grown in TSB (~ 5 × 109 CFU/mL) was subsequently incubated with drugs (in triplicates) at 10 × MIC for 24 hours. After 12 and 24 hours, aliquots were diluted in PBS and plated onto BHI agar plates. Plates were incubated at 37°C for 18 hours before counting colonies to determine viable CFU/mL. Data are presented as average log10 (CFU/mL) of VRE stationary phase cells at the indicated time points. Data were analyzed via a two-way ANOVA with post-hoc Dunnet’s test (P < 0.05) for multiple comparisons.
2.4. Protease inhibition assay
The modified skimmed milk method was utilized to investigate the protease inhibitory activities of auranofin and linezolid, as described previously [12]. Initially, the ability of strain E. faecium NR-31909 to produce proteases was confirmed by inoculation onto TSA containing 3% skimmed milk and incubating at 37°C for 24 hours. The presence of a transparent zone around the colonies indicated the strain produced proteases [13]. In brief, an overnight culture of E. faecium NR-31909 incubated in TSB containing 0.25 × MIC of auranofin, linezolid or DMSO (negative control) (in triplicates) was centrifuged at 10,000 × g for 10 minutes. A total volume of 500 μL of culture supernatant was incubated with 1 mL skimmed milk (1.25%) at 37°C for 30 minutes. Afterwards, the OD600 was measured to indicate the degree of clearance of skim milk. Data are presented as percent protease production in the presence of each drug. DMSO (the solvent of the drugs) served as a control to determine the total protease production by the bacteria. TSB containing skimmed milk was used as a negative control to determine the OD values in the absence of protease production). Data were analyzed via unpaired Student t test.
2.5. Lipase inhibition activity
The ability of auranofin to inhibit E. faecium lipase production was detected as described previously [14], with some modifications. First, E. faecium NR-31909 was confirmed for the ability to produce lipase by culturing on TSA supplemented with egg yolk emulsion (10%) and incubated at 37°C for 24 to 48 hours. The formation of an opaque zone around colonies indicated lipolytic activity. An overnight culture of E. faecium NR-31909 grown in TSB containing 0.25 × MIC of auranofin, linezolid or DMSO (negative control) (in triplicates) was centrifuged at 10,000 × g for 10 minutes. An aliquot (500 μL) of supernatant was taken (in duplicates) and incubated with 1 mL of 10% egg yolk emulsion at 37°C for 30 minutes. The OD600 was measured to detect the lipolytic activity of E. faecium in the presence of drugs. The data are presented as percent lipase production relative to DMSO. DMSO served as a negative control to determine the total bacterial lipase production. Again, data were analyzed via unpaired Student t test.
2.6. Hemagglutination inhibition assay
To study the effects of auranofin and linezolid on the adhesive properties of hemagglutinins (involved in bacterial invasion) produced by enterococci, a hemagglutination inhibition assay was employed, as described before [15, 16]. E. faecium NR-31909 was first screened for the hemagglutination assay using horse blood. Briefly, red blood cells (RBCs) were collected by washing 5 mL of horse blood with 10 mL of PBS four times with subsequent centrifugation at 2,000 × g for 3 minutes. The supernatant was subsequently removed without disrupting the RBCs. Afterward, a 10% RBC suspension was prepared in PBS, diluted 10-fold, and a total volume of 50 µL was added to a round bottom 96-well plate. A total volume of 50 µL of the overnight bacterial supernatant (prepared as described in the protease assay above) was added to the RBCs suspension. Plates were then incubated at room temperature for one hour. Negative hemagglutination results will appear as dots in the center of the well. Positive hemagglutination results will form a uniform reddish color across the well.
After confirming E. faecium NR-31909 produced hemagglutinins, we investigated the ability of auranofin and linezolid to interfere with production of hemagglutinins. Briefly, an overnight culture of E. faecium NR-31909 grown in TSB containing subinhibitory concentrations of auranofin, linezolid or DMSO (negative control) (in triplicate) was centrifuged at 4700 RPM for 10 minutes. A total volume of 50 μL of culture supernatant was incubated with 50 μL of 1% RBCs suspension in PBS at room temperature for one hour. DMSO served as a negative control (i.e. positive for hemagglutination).
2.7. In vivo mouse peritonitis animal model
The study was reviewed, approved and performed under the guidelines of the Purdue University Animal Care and Use Committee and was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Each group of mice was housed in an individually ventilated cage and received food and water ad libitum throughout the duration of the experiment. The VRE mouse peritonitis lethal infection model was used, as described previously [17, 18], with some modifications. Eight-week-old female BALB/c mice (Jackson, ME, USA) were used for this study. E. faecium NR-31909 (a strain isolated from a human patient with bacteremia) was chosen for this study. Each group of mice (n = 5) was infected intraperitoneally with (LD50 ~2.5 × 107 CFU/mL) of E. faecium NR-31909 premixed with 20% sterile rat fecal extract (SRFE) in the ratio of 2:1. One hour post-infection, three groups were treated orally with auranofin (0.125 mg/kg, 0.25 mg/kg and 0.5 mg/kg), and three groups were treated subcutaneously (S.C.) with auranofin (0.0625 mg/kg, 0.125 mg/kg and 0.25 mg/kg). One group was treated orally with linezolid (20 mg/kg). Two groups were treated with the vehicle (10% DMSO in PBS orally and S.C.). Treatments were continued once daily for four days before the mice were humanely euthanized five days post-infection. Bacteria were recovered from the liver, kidneys and spleen of mice under aseptic conditions, serially diluted in PBS, and plated on enterococcosel agar supplemented with vancomycin (8 µg/mL). Plates were then incubated for 48 hours at 37°C to determine VRE burden in each organ. The data were analyzed via one way ANOVA with post hoc Dunnett’s test for multiple comparisons.
2.8. Statistical analyses
GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla CA) was used to conduct the statistical analyses presented in this study.
3. Results
3.1. The effect of inoculum size on the MIC of auranofin against VRE
VRE are known to colonize the gastrointestinal tract in large numbers. However, the standard broth microdilution assay typically evaluates antibacterial agents at a lower inoculum size (~105 CFU/mL). Consequently, we investigated the antibacterial activity of auranofin and linezolid against two different inoculum sizes of VRE: a standard inoculum (SI) (5 × 105) and high inoculum (HI) (5 × 107 CFU/mL). As depicted in Table 1, the MIC90 of auranofin did not change after increasing the VRE inoculum size from 5 × 105 CFU/mL to 5 × 107 CFU/mL. In contrast, the MIC90 of linezolid against VRE increased by one-fold in agreement with a previous report [19].
Table 1.
Minimum inhibitory concentration (MIC, µg/mL) of auranofin and linezolid against clinical isolates of vancomycin-resistant E. faecium and E. faecalis at standard and high inocula.
| Strains | MIC (µg/mL) | |||
|---|---|---|---|---|
| Auranofin | Linezolid | |||
| SI | HI | SI | HI | |
| E. faecium NR-31916 | 0.5 | 0.5 | 1 | 1 |
| E. faecium ATCC 700221 | 0.5 | 0.5 | 0.5 | 1 |
| E. faecium NR-32054 | 0.5 | 0.5 | 1 | 2 |
| E. faecalis NR-31971 | 0.5 | 0.5 | 1 | 2 |
| E. faecium HM-952 | 1 | 1 | 1 | 2 |
| E. faecium NR-32065 | 0.5 | 0.5 | 1 | 2 |
| E. faecium NR-32094 | 1 | 1 | 1 | 2 |
| E. faecalis NR-31887 | 1 | 1 | 1 | 2 |
| E. faecalis HM-201 | 1 | 1 | 1 | 1 |
| E. faecalis HM-934 | 1 | 1 | 1 | 2 |
| E. faecalis NR-31970 | 1 | 1 | 1 | 2 |
| E. faecium HM-968 | 1 | 1 | 1 | 2 |
| E. faecalis HM-335 | 1 | 1 | 1 | 2 |
| E. faecium HM-965 | 1 | 1 | 1 | 2 |
| E. faecium NR-31909 | 1 | 1 | 1 | 1 |
| MIC90 | 1 | 1 | 1 | 2 |
SI, standard inoculum (~5 × 105 CFU/mL); HI, high inoculum (~5 × 107 CFU/mL); MIC90, the concentration of the test agent that inhibited the growth of 90% of the tested strains
3.2. Evaluation of the antibacterial activity of auranofin against vancomycin-resistant E. faecium stationary phase cells via a time-kill assay
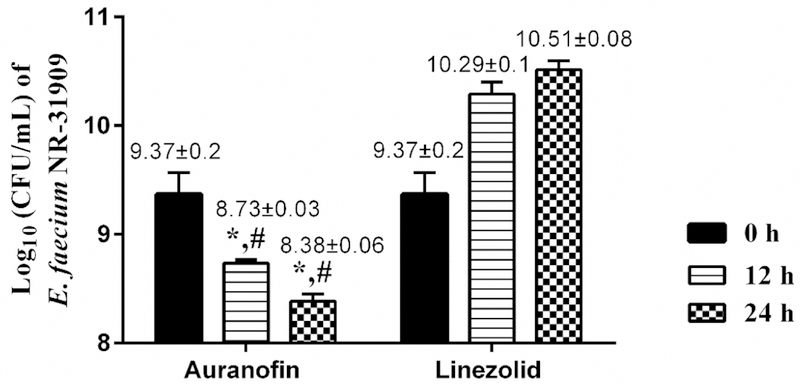
After confirming the antibacterial activity of auranofin remained consistent against a high inoculum size of VRE, we examined the drug’s activity against stationary phase VRE cells via a time-kill assay. These cells are highly-resistant to most antibiotics, in part because most antibiotics target metabolic processes expressed by cells actively dividing (i.e. in logarithmic stage of growth). Auranofin significantly reduced stationary phase VRE cells by 0.64-log10 after 12 hours and by 1-log10 after 24 hours (Fig. 1). In contrast, linezolid was ineffective against stationary phase cells as the bacterial CFU increased by 0.92-log10 after 12 hours and increased by 1.14-log10 after 24 hours. The result for linezolid is similar to what has been previously reported [20]. These results indicate that auranofin is superior to linezolid at reducing the burden of VRE stationary phase cells in vitro.
Fig. 1.
Time-kill kinetics assay of auranofin and linezolid against stationary phase vancomycin-resistant Enterococcus faecium NR-31909. Bacteria were incubated with test agents, and samples were collected at 0,12- and 24-h incubation period. The error bars represent standard deviation values obtained from triplicate samples used for each agent studied. (*) represents significant difference from 0 time. # represents significant difference from linezolid (*, # P < 0.05). Data were analyzed with two way ANOVA with post hoc Dunnet’s test.
3.3. Protease inhibition assay
Bacteria secrete proteases to invade and damage host epithelial cells. We studied the ability of auranofin and linezolid to inhibit protease production in E. faecium NR-31909. Auranofin, at 0.25 × MIC, significantly outperformed linezolid in inhibiting total protease production (P < 0.0001) (Fig. 2). Auranofin inhibited the total protease production of E. faecium NR-31909 by nearly 69.7%. Linezolid (which is known to inhibit protein synthesis) [21] inhibited approximately 55.3% of the total proteases produced by the bacterial strain.
Fig. 2.
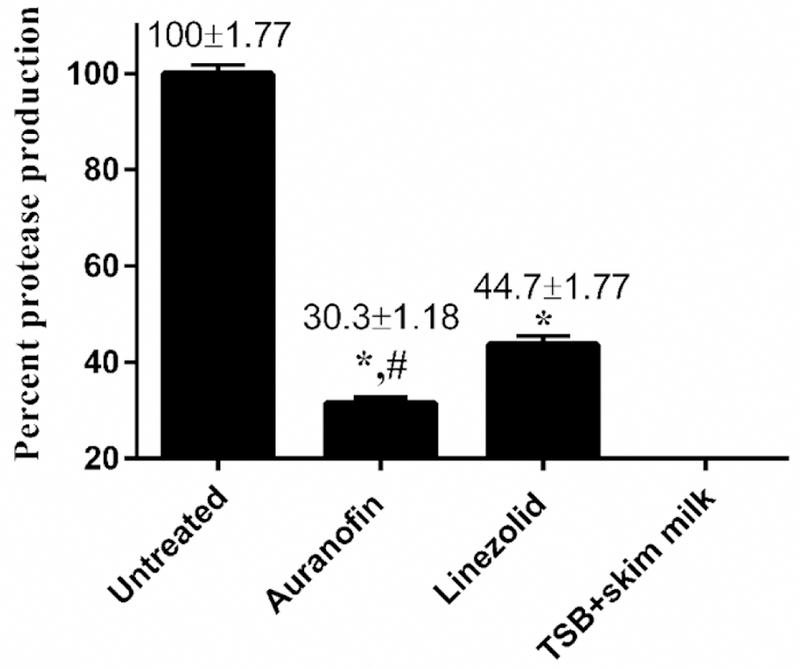
Total protease inhibition activity of auranofin and linezolid against vancomycin-resistant E. faecium NR-31909. Data are presented as percent protease production of each drug (tested in sexruplicate). TSB with skim milk served as a negative control. Data were analyzed via unpaired Student t test (p<0.05). Auranofin was compared to untreated (*) and to linezolid (#).
3.4. Lipase inhibition activity
In addition to the secretion of proteases, VRE produces lipase which damages the host’s epithelium and permits tissue invasion [13]. Given auranofin inhibited VRE protease production, we investigated the drug’s ability to also inhibit lipase produced by VRE. Auranofin was once again significantly superior to linezolid in reducing VRE lipase production (P value of 0.0006) (Fig. 3). At 0.25 × MIC, auranofin reduced lipase production by approximately 41.5%, while linezolid inhibited approximately 35.4% of lipase production by E. faecium NR-31909.
Fig. 3.
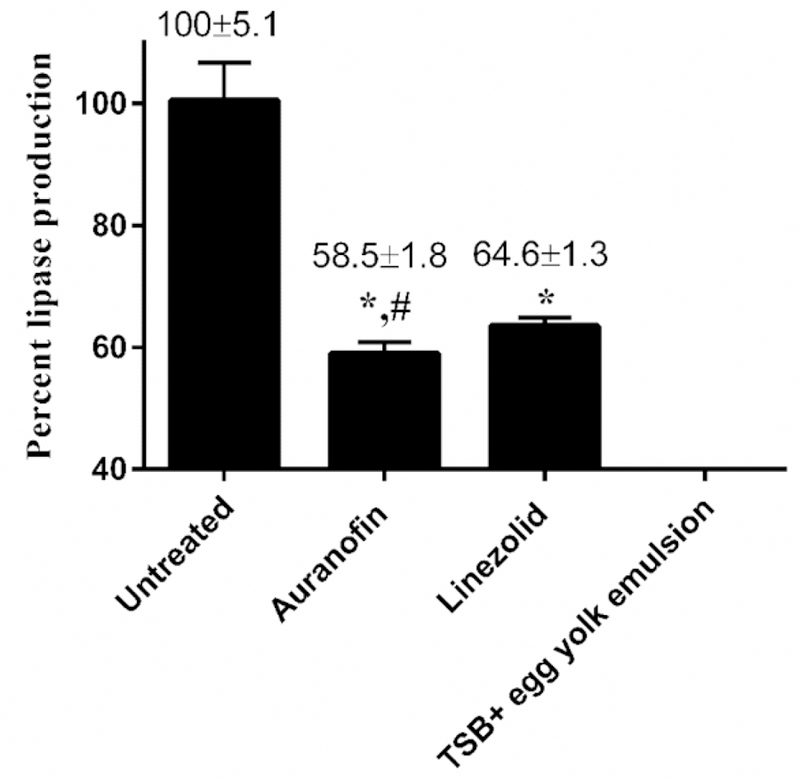
Lipase inhibition activity of auranofin and linezolid against vancomycin-resistant E. faecium NR-31909. Data are presented as percent lipase production in presence of each drug (tested in sexruplicate). TSB with egg yolk emulsion served as a negative control. Data were analyzed via unpaired Student t test (p<0.05). Auranofin was compared to untreated (*) and to linezolid (#).
3.5. Hemagglutination inhibition assay
Hemagglutination properties are highly common in different enterococcal species, especially VRE [22]. Due to their adhesive properties, hemagglutinins aid in bacterial invasion leading to damage of host epithelial tissue [13]. To evaluate auranofin’s ability to inhibit hemagglutination induced by VRE, a hemagglutination inhibition assay was performed. As depicted in Fig. 4, auranofin, at subinhibitory concentrations, was able to inhibit hemagglutination induced by E. faecium NR-31909. Linezolid was unable to achieve the same effect. We evaluated different subinhibitory concentrations of auranofin to determine the lowest concentration capable of inhibiting hemagglutination. Auranofin successfully inhibited VRE hemagglutination at a concentration as low as 0.0625 µg/mL. These results indicate auranofin has potent antivirulence activity and may be capable of interfering with VRE attachment to and invasion of host tissues.
Fig. 4.
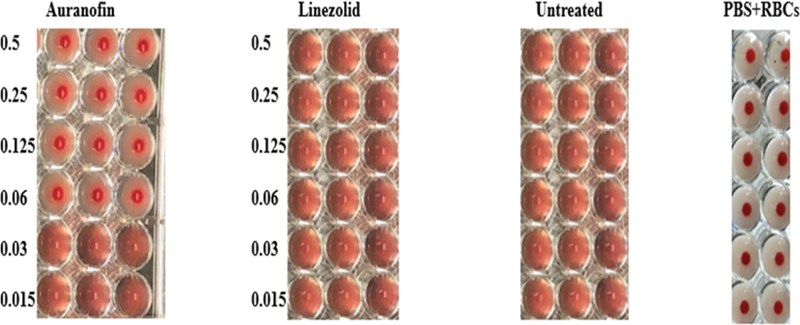
Hemagglutination inhibitory activities of auranofin and linezolid against E. faecalis NR-31909. Sub-inhibitory concentrations (starting from 0.5 µg/mL to 0.015 µg/mL) of the drugs were added with bacterial supernatant and RBCs, and then incubated for 1 hour at 37 °C. Positive hemagglutination inhibition results appear as dots in the center of round-bottomed plates. Negative hemagglutination inhibition results appear as a uniform reddish color across the well.
3.6. In vivo murine peritonitis model
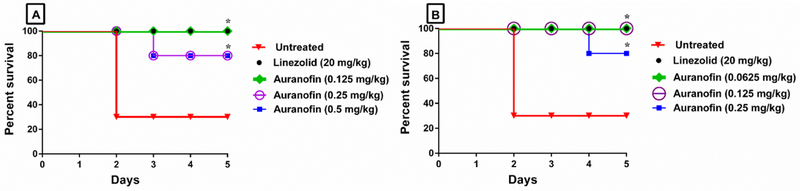
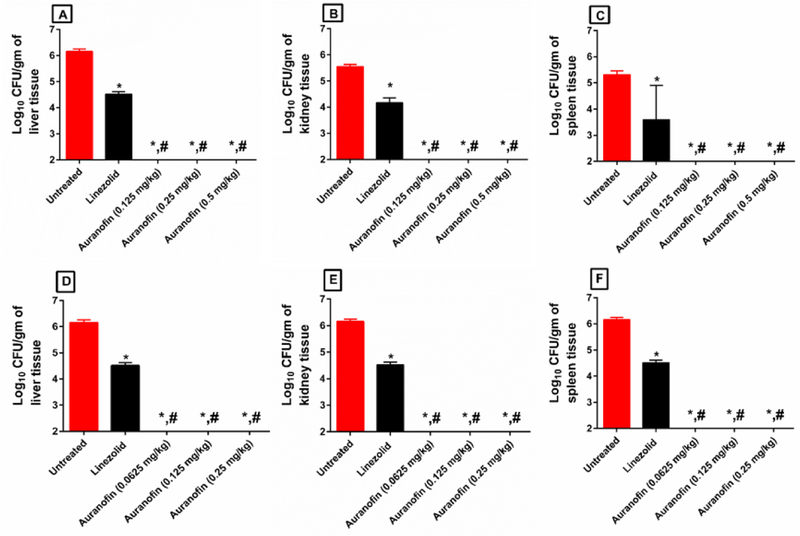
The promising in vitro antibacterial and antivirulence results led us to evaluate the ability of auranofin to treat septicemia induced by vancomycin-resistant E. faecium in a murine peritonitis model [17, 18]. As presented in Fig. 5, auranofin, at the same dose administered to human patients for treatment of rheumatoid arthritis (0.125 mg/kg orally), significantly protected 100% of the mice from a lethal challenge of VRE. Interestingly, higher oral doses of auranofin (0.25 mg/kg and 0.5 mg/kg) significantly protected only 80% of the mice. The same pattern was also observed with auranofin groups treated S.C. Auranofin administered at 0.25 mg/kg S.C. protected only 80% of the mice, while lower doses (0.0625 mg/kg and 0.125 mg/kg) of auranofin protected 100% of the mice. Linezolid (20 mg/kg) successfully protected 100% of the mice. Auranofin was significantly superior to linezolid in decreasing the burden of VRE in internal organs (liver, kidneys and spleen) of infected mice (Fig. 6). Remarkably, auranofin reduced VRE burden below the limit of detection (100 CFU/gm) from the liver, kidneys, and spleen of all mice after only four days of oral or S.C. treatment. Linezolid, in contrast, reduced VRE burden by approximately 1.64-log10, 1.38-log10 and 1.72-log10 in the liver, kidneys and spleen of mice, respectively.
Fig. 5.
In vivo antibacterial activity of auranofin against E. faecium NR-31909 in the murine septicemia model when administered (A) Orally at 0.125 mg/kg, 0.25 mg/kg and 0.5 mg/kg; and (B) Subcutaneously (S.C.) at 0.0625 mg/kg, 0.125 mg/kg and 0.25 mg/kg compared to the vehicle control and the standard antibiotic linezolid given orally at 20 mg/kg. Mice survival was monitored for 5 days. Results were analyzed for statistical difference utilizing graphpad prism. (*) Denotes significant difference between each treated group and the untreated group (P < 0.05).
Fig. 6.
VRE counts in (A) liver, (B) kidney, and (C) spleen of the infected mice (5/group) orally treated with auranofin, and (D) liver, (E) kidney, and (F) spleen of the infected mice (5/group) treated S.C. after 4 days. Auranofin was compared to untreated (*) and to linezolid (#). (P < 0.05).
4. Discussion
Enterococci are normal inhabitants of the gastrointestinal tract, and VRE colonization is often followed by translocation across human epithelial cells leading to systemic infections including sepsis, urinary tract infections, endocarditis and surgical site infections [9, 23]. The U.S. Centers for Disease Control and Prevention (CDC) has identified VRE as a serious public health threat because it is responsible for more than 20,000 infections annually leading to more than 5% of all deaths attributed to an antibiotic-resistant infection in the U.S. [24].VRE are a leading cause of nosocomial infections and limited therapeutic options are available [1].
Unlike for most Gram-positive pathogens, cell-wall inhibiting antibiotics, such as ampicillin or vancomycin, exhibit bacteriostatic activity against enterococci. Bactericidal activity requires a combination of a cell wall inhibitor and an aminoglycoside. However, many enterococci are resistant to β-lactams and aminoglycosides [25], and VRE are also resistant to glycopeptides. Furthermore, enterococci, in general, and VRE specifically, have an uncanny ability to acquire or develop resistance to multiple antibiotics [3]. Most seriously, VRE strains have been isolated that exhibit resistance to last-resort antibiotics, such as linezolid, quinupristin-dalfopristin, daptomycin, tigecycline and oritavancin [20]. Given the dearth of effective therapeutic options, there is an urgent need to develop novel antimicrobial agents to treat VRE infections.
In an intensive search for new drugs to combat VRE infections, our group previously discovered niclosamide, auranofin and ebselen as effective VRE decolonizing agents [9, 26, 27]. Building upon our previous work, this study investigated auranofin’s ability to inhibit production of key virulence factors utilized by VRE to promote infection. Furthermore, we evaluated auranofin’s ability to protect mice in vivo in a lethal VRE septicemia model.
One of the major challenges associated with VRE infection is that they overgrow and colonize the human intestine in large numbers upon the disturbance of the normal microbiota [26, 27]. VRE typically colonize the gastrointestinal tract of humans in a larger population than the inoculum size used for standard antibacterial susceptibility assays. This higher inoculum size has a negative effect on the antibacterial activity of certain antibiotics. For example, increasing the inoculum size of enterococci resulted in increased MIC values for several antibiotics, in previous reports, such as piperacillin and piperacillin-tazobactam [28], vancomycin and teicoplanin, [29] and daptomycin, linezolid, gentamicin and rifampin [19]. Consequently, higher doses of these antibiotics are needed to successfully suppress VRE growth. Thus, we first confirmed auranofin was capable of inhibiting a high inoculum of VRE. Inoculum effect testing is typically performed using an inoculum 100-fold greater than the CLSI-recommended inoculum, as discussed previously [30]. In our study, increasing the inoculum size of VRE from 105 CFU/mL to 107 CFU/mL did not affect the MIC values of auranofin. In contrast, the MIC values for linezolid increased when evaluated against a higher inoculum size of VRE. This characteristic of auranofin is highly advantageous for treatment of severe VRE infections such as sepsis.
Next, a time-kill assay was utilized to study the killing kinetics of auranofin against stationary phase enterococcal cells. Stationary phase cells are well-known to be highly-resistant to most antibacterial drugs. These cells contain a high percentage of persisters that are considered as the reason behind recurring infections and a major cause of drug resistance [31, 32]. Against stationary phase cells, auranofin was superior to linezolid in reducing the burden of VRE in the time-kill assay after 12 and 24 hours. Auranofin (at 10 × MIC) reduced VRE burden by 0.64-log10 and 1-log10 after 12 and 24 hours of incubation. In contrast, linezolid (10 × MIC), in agreement with previous report [20], was ineffective at reducing VRE burden.
After confirming that auranofin’s antibacterial activity was not affected by a high inoculum of VRE or VRE in stationary phase, we investigated auranofin’s ability to inhibit key virulence factors that promote infections. VRE produces multiple virulence factors, such as proteases, gelatinase, lipase, hemolysin, hemagglutinin, and DNase, which lead to cell damage and progression of infection [13]. Proteases are considered one of the most important virulence factors produced by bacteria. Proteases have been shown to damage immunoglobulins, which protect mucous membranes, as well as disrupt tight junctions between host epithelial cells, leading to tissue invasion and damage [33]. Proteases can also cleave secreted toxins to regulate the abundance of virulence factors depending on the specific niche encountered within the host [34]. Additionally, it has been reported that gelatinase (a protease produced by enterococci) played an essential role in the progression and severity of infection in an E. faecalis endocarditis animal model [35]. Another study found a strong correlation between protease secretion and virulence in a murine model of enterococcal catheter-associated urinary tract infection [36]. In addition to proteases, lipases play an essential role in microbial nutrient acquisition through digesting lipids, especially when grown in carbohydrate-restricted environments [37]. In addition, lipases promote adhesion by degrading host surface molecules; the released free fatty acids are postulated to increase unspecific hydrophobic interactions with the host receptors [38].
Auranofin was previously reported to inhibit protein synthesis and virulence factors (namely toxin production) expressed by Staphylococcus aureus [39]. Consequently, we hypothesized that auranofin, at subinhibitory concentrations, would be able to inhibit virulence factors expressed by VRE. To test this hypothesis, the inhibitory activities of auranofin against VRE protease and lipase production was evaluated. Auranofin (at 0.25 × MIC) significantly outperformed linezolid in reducing the VRE protease and lipase production. Furthermore, auranofin (at 0.25 × MIC) reduced VRE lipase production by 41%. These results collectively indicate that auranofin can reduce production of both proteases and lipase in VRE which may curb the pathogen’s ability to attach and invade host tissues.
In addition to the secretion of lipase and proteases, hemagglutination of RBCs is another important virulence factor utilized by VRE. Bacterial binding to host tissues is dependent on microbial surface adhesins that recognize specific receptors on the host cell surface. Upon binding to the host, these bacterial adhesins cause agglutination of RBCs [40]. It has been reported that enterococci possess characteristic thermostable proteinaceous agglutinins capable of agglutinating rabbit, human and sheep RBCs, indicating the diversity in the surface structures involved in enterococcal adhesion [13]. The protein synthesis-inhibiting activities of auranofin and its adherence-inhibition activity (protease and lipase inhibitions) prompted us to evaluate the drug’s hemagglutination-inhibition activity. As expected, auranofin inhibited the hemagglutination induced by E. faecium NR-31909 at a concentration as low as 0.0625 µg/mL. These results indicate auranofin has potent antivirulence activity and may be capable of interfering with VRE attachment to and invasion of host tissues.
The potent antibacterial and antivirulence activity of auranofin against VRE in vitro prompted us to evaluate its effectiveness in a murine VRE septicemia model. VRE bloodstream infections, in most cases, lead to infective endocarditis that can be fatal in up to 46% of cases [41]. Different doses of auranofin administered either orally or subcutaneously (S.C.) were evaluated to determine the most effective dose and route of administration. Interestingly, lower doses of auranofin (0.0625 mg/kg S.C. and 0.125 mg/kg oral and S.C.) protected 100% of the mice, while higher doses (0.25 mg/kg S.C. and oral and 0.5 mg/kg oral) protected 80% of the mice (considered a non-significant difference relative to mice treated with linezolid). Most importantly, auranofin was superior to linezolid in decreasing the burden of VRE in the internal organs (liver, kidneys and spleen) of infected mice. Remarkably, auranofin reduced VRE below the limit of detection from all three internal organs evaluated, after only four days of oral or S.C. treatment. In contrast, linezolid (20 mg/kg) did not efficiently clear VRE in internal organs and reduced the VRE burden by 1.64-log10, 1.38-log10, and 1.72-log10 in the livers, kidneys and spleens, respectively. This pattern is postulated to be due to its bacteriostatic activity that could be the reason behind its lower efficacy in reducing VRE burden in internal organs, in agreement with previous studies [9, 26, 27]. It is worth noting that the oral dose of auranofin that provided 100% protection to mice is clinically achievable in humans. Auranofin has been used clinically since 1985 to treat rheumatoid arthritis in humans and has an excellent safety profile. The toxicity profile of auranofin has been well characterized in humans and has been found to be safe in 5,000 patients taken auranofin and monitored for longer than 7 years [42, 43]. This duration is a much greater interval than would be expected for VRE therapy.
In conclusion, the current study highlights that auranofin has potent in vitro antivirulence activity and in vivo activity against VRE and warrants further investigation as a novel treatment option for systemic VRE infections.
Supplementary Material
Highlights.
Auranofin is a potent inhibitor of vancomycin-resistant enterococci (VRE).
Auranofin, not linezolid, inhibited the growth of stationary-phase VRE cells.
Auranofin possessed a potent antivirulent activity against VRE.
Auranofin protected mice against lethal VRE challenge.
Auranofin cleared VRE in the internal organs of infected mice.
Acknowledgement
We would like to thank Marwa Alhashimi and Ahmed Elkashif for their kind help in this work. We are also grateful to Dr. Haroon Mohammad for editing the manuscript.
Funding: This work was supported by the National Institutes of Health (Grant No. R01AI130186).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: None declared.
Ethical Approval: All animal housing and experiments were reviewed, approved and performed under the guidelines of the Purdue University Animal Care and Use Committee (1704001567) and carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
References
- [1].Lebreton F, van Schaik W, McGuire AM, Godfrey P, Griggs A, Mazumdar V, et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio. 2013;4:e00534–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cetinkaya Y, Falk P, Mayhall CG. Vancomycin-resistant enterococci. Clinical microbiology reviews. 2000;13:686–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gilmore MS, Lebreton F, van Schaik W. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Current opinion in microbiology. 2013;16:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mundy L, Sahm D, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clinical microbiology reviews. 2000;13:513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tacconelli E, Magrini N, Kahlmeter G, Singh N. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization. 2017;27.
- [6].Younis W, AbdelKhalek A, S Mayhoub A, N Seleem M. In Vitro Screening of an FDA-Approved Library Against ESKAPE Pathogens. Current pharmaceutical design. 2017;23:2147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thangamani S, Mohammad H, Abushahba MF, Sobreira TJ, Seleem MN. Repurposing auranofin for the treatment of cutaneous staphylococcal infections. Int J Antimicrob Agents. 2016;47:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thangamani S, Younis W, Seleem MN. Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol. 2015;6:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].AbdelKhalek A, Abutaleb NS, Elmagarmid KA, Seleem MN. Repurposing auranofin as an intestinal decolonizing agent for vancomycin-resistant enterococci. Sci Rep. 2018;8:8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].CLSI calsi. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. (9th ed), Approved Standard M07-A9, CLSI, Wayne, PA: 2012; 32 No. 2. [Google Scholar]
- [11].Mohamed MF, Brezden A, Mohammad H, Chmielewski J, Seleem MN. Targeting biofilms and persisters of ESKAPE pathogens with P14KanS, a kanamycin peptide conjugate. Bba-Gen Subjects. 2017;1861:848–59. [DOI] [PubMed] [Google Scholar]
- [12].Skindersoe ME, Alhede M, Phipps R, Yang L, Jensen PO, Rasmussen TB, et al. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrobial agents and chemotherapy. 2008;52:3648–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Biswas PP, Dey S, Adhikari L, Sen A. Virulence markers of vancomycin resistant enterococci isolated from infected and colonized patients. Journal of global infectious diseases. 2014;6:157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Owens J. The egg yolk reaction produced by several species of bacteria. Journal of Applied Bacteriology. 1974;37:137–48. [DOI] [PubMed] [Google Scholar]
- [15].Neter E, Gorzynski EA, Zalewski NJ, Rachman R, Gino RM. Studies on bacterial hemagglutination. American journal of public health and the nation’s health. 1954;44:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wu Y, Cho M, Shore D, Song M, Choi J, Jiang T, et al. Hemagglutination Inhibition (HI) Assay of Influenza Viruses with Monoclonal Antibodies. Bio-protocol. 2016;6:e1828. [Google Scholar]
- [17].Singh KV, Qin X, Weinstock GM, Murray BE. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. The Journal of infectious diseases. 1998;178:1416–20. [DOI] [PubMed] [Google Scholar]
- [18].Gollapudi S, Gupta A, Thadepalli H, Perez A. Use of lymphokines in treatment of experimental intra-abdominal abscess caused by Bacteroides fragilis. Infection and immunity. 1988;56:2369–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Luther MK, Arvanitis M, Mylonakis E, LaPlante KL. Activity of Daptomycin or Linezolid in Combination with Rifampin or Gentamicin against Biofilm-Forming Enterococcus faecalis or E. faecium in an In Vitro Pharmacodynamic Model Using Simulated Endocardial Vegetations and an In Vivo Survival Assay Using Galleria mellonella Larvae. Antimicrobial agents and chemotherapy. 2014;58:4612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gandt AB, Griffith EC, Lister IM, Billings LL, Han A, Tangallapally R, et al. In vivo and in vitro effects of a ClpP-activating antibiotic against vancomycin-resistant enterococci. Antimicrobial agents and chemotherapy. 2018;62:e00424–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Swaney SM, Aoki H, Ganoza MC, Shinabarger DL. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrobial agents and chemotherapy. 1998;42:3251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Maria da Glória SC, Teixeira LM. Hemagglutination properties of Enterococcus. Current microbiology. 1995;30:265–8. [DOI] [PubMed] [Google Scholar]
- [23].Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. The Journal of clinical investigation. 2010;120:4332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Control CfD, Prevention. Antibiotic resistance threats in the United States, 2013: Centres for Disease Control and Prevention, US Department of Health; and …; 2013. [Google Scholar]
- [25].Arias CA, Contreras GA, Murray BE. Management of multidrug-resistant enterococcal infections. Clinical microbiology and infection. 2010;16:555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mohammad H, AbdelKhalek A, Abutaleb NS, Seleem MN. Repurposing niclosamide for intestinal decolonization of vancomycin-resistant enterococci. International journal of antimicrobial agents. 2018;51:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].AbdelKhalek A, Abutaleb NS, Mohammad H, Seleem MN. Repurposing ebselen for decolonization of vancomycin-resistant enterococci (VRE). PloS one. 2018;13:e0199710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Okhuysen PC, Singh KV, Murray BE. Susceptibility of beta-lactamase-producing enterococci to piperacillin with tazobactam. Diagnostic microbiology and infectious disease. 1993;17:219–24. [DOI] [PubMed] [Google Scholar]
- [29].RafaelCantón N, Sánchez Miguel, Baquero Fernando. MIC distribution and inoculum effect of LY333328: a study of vancomycin-susceptible and VanA-type and VanC-type enterococci obtained from intensive care unit patient surveillance cultures. Clinical microbiology and infection. 1999;5:554–9. [DOI] [PubMed] [Google Scholar]
- [30].Coppi G, Borella F, Gatti MT, Comini A, Dall’Asta L. Synthesis, antiinflammatory and antiarthritic properties of a new tiopronine gold derivative. Bollettino chimico farmaceutico. 1989;128:22–4. [PubMed] [Google Scholar]
- [31].Conlon BP, Nakayasu ES, Fleck LE, LaFleur MD, Isabella VM, Coleman K, et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jaishankar J, Srivastava P. Molecular Basis of Stationary Phase Survival and Applications. Front Microbiol. 2017;8. [DOI] [PMC free article] [PubMed]
- [33].Heras B, Scanlon MJ, Martin JL. Targeting virulence not viability in the search for future antibacterials. British journal of clinical pharmacology. 2015;79:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lindsay JA, Foster SJ. Interactive regulatory pathways control virulence determinant production and stability in response to environmental conditions in Staphylococcus aureus. Mol Gen Genet. 1999;262:323–31. [DOI] [PubMed] [Google Scholar]
- [35].Gutschik E, MØller S, Christensen N. Experimental Endocarditis in Rabbits: 3. Significance of the Proteolytic Capacity of the Infecting Strains of Streptococcus Faecalis. Acta Pathologica Microbiologica Scandinavica Section B Microbiology. 1979;87:353–62. [PubMed] [Google Scholar]
- [36].Wei Xu ALF-M, Cusumano Zachary T., Takagi Enzo, Hultgren Scott J. and Caparon Michael G. Host and bacterial proteases influence biofilm formation and virulence in a murine model of enterococcal catheterassociated urinary tract infection. nature partner journals. 2017;28. [DOI] [PMC free article] [PubMed]
- [37].Longshaw CM, Farrell AM, Wright JD, Holland KT. Identification of a second lipase gene, gehD, in Staphylococcus epidermidis: comparison of sequence with those of other staphylococcal lipases. Microbiol-Uk. 2000;146:1419–27. [DOI] [PubMed] [Google Scholar]
- [38].Stehr F, Kretschmar M, Kroger C, Hube B, Schafer W. Microbial lipases as virulence factors. J Mol Catal B-Enzym. 2003;22:347–55. [Google Scholar]
- [39].Thangamani S, Mohammad H, Abushahba MF, Sobreira TJ, Hedrick VE, Paul LN, et al. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci Rep. 2016;6:22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shareef HA, Abdulla ET. Hemagglutination properties of some intestinal bacterial pathogens isolated from clinical samples. Tikrit Journal of Pure Science. 2010;15:5–10. [Google Scholar]
- [41].Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. The New England journal of medicine. 2014;370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marcolongo R, Mathieu A, Pala R, Giordano N, Fioravanti A, Panzarasa R. The efficacy and safety of auranofin in the treatment of juvenile rheumatoid arthritis. A long-term open study. Arthritis Rheum. 1988;31:979–83. [DOI] [PubMed] [Google Scholar]
- [43].Blodgett RC Jr., Pietrusko RG. Long-term efficacy and safety of auranofin: a review of clinical experience. Scand J Rheumatol Suppl. 1986;63:67–78. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.