Abstract
Background and aims
Pancreatic ductal adenocarcinoma (PDAC) remains a deadly disease urgently requiring new treatments. Over-expression of the protein transporter exportin-1 (XPO1) leads to mislocalization of tumor suppressor proteins (TSPs) and their inactivation. Earlier, we showed that blocking XPO1 by CRISPR/Cas9 validated Selective Inhibitor of Nuclear Export (SINE) compounds (selinexor and analogs) restores the anti-tumor activity of multiple TSPs leading to suppression of PDAC in vitro and in orthotopic models.
Methods
We evaluate the synergy between SINE compounds and standard of care treatments in pre-clinical models and in a PDAC Phase Ib trial.
Results
SINE compounds synergize with gemcitabine (GEM) and nanoparticle albumin–bound (nab)-paclitaxel leading to suppression of PDAC cellular growth and cancer stem cell (CSC) spheroids disintegration. Label-Free quantitative proteome profiling with nuclear and cytoplasmic enrichment showed superior enhancement in nuclear protein fraction in combination treatment. Selinexor inhibited the growth of PDAC CSC and two patient derived (PDX) sub-cutaneous xenograft. Selinexor-GEM-nab-paclitaxel blocked PDX and orthotopic tumor growth. In a Phase 1b study ( NCT02178436), 9 patients were exposed to selinexor (60 mg oral) with GEM (1000 mg/m2 IV) and nab-paclitaxel (125 mg/m2 IV) on day 1, 8, 15 of 28-day cycle. Two patients showed partial response and 2 had stable disease. An outstanding, durable objective response was observed in one of the responders with progression free survival of 16 months and overall survival of 22 months.
Conclusion
Our pre-clinical and ongoing clinical study lend support to the use of selinexor-gemcitabine-nab-paclitaxel as an effective therapy for metastatic PDAC.
Keywords: Specific Inhibitor of Nuclear Export, SINE, Selinexor, Pancreatic Cancer, combination therapy, Phase II
Introduction
Pancreatic ductal adenocarcinoma (PDAC) has an abysmally low survival rate. This necessitates the development of new treatment approaches [1]. PDAC tumors carry aberrations in many different signaling mechanisms rendering therapy against a single pathway impractical [2]. To date there is no regimen that can simultaneously activate multiple tumor suppressors (TSPs) that are wild type and functional in PDAC. Therapies such as gemcitabine (GEM) plus nanoparticle albumin bound (nab)-paclitaxel, partially activate these TSPs. However, the over-expression of nuclear export protein exportin 1 (XPO1), leads to nuclear expulsion and functional inactivation of these TSPs [3, 4]. Coupled with low drug penetrance due to desmoplasia and immunosuppressive microenvironment, the inactivation of critical TSPs has been attributed for the lack of therapeutic response of different regimens in PDAC patients.
Nuclear transport is mediated by a set of proteins belonging to karyopherin family [5, 6]. XPO1 is member of the karyopherin family that carries out nuclear export of cargo proteins [7]. XPO1 is the main exporter of a majority of TSPs and growth regulatory proteins that carry conserved leucine rich nuclear export signal (NES). The transport to the cytosol occurs through an energy consuming process that involves Ran protein recycling (GTP-GDP) [8]. TSPs in particular require nuclear retention and DNA interaction to exert their tumor suppressor regulatory function [9]. Activation of TSPs (e.g. FOXO3a, p53, p27, Rb, Ikβ and Par-4) induced by chemotherapies (e.g. platinum based, GEM, or GEM-nab-paclitaxel) is not sufficient for their tumor suppressive function due in part to over-expression of XPO1 and mislocalization of these TSPs [10]. Supporting this, we and others have shown that over-expression of XPO1 has been associated with therapy resistance and poor survival in solid tumors [4]. Therefore, XPO1 inhibition and subsequent re-alignment of TSPs to the nucleus becomes an attractive anticancer strategy [11].
XPO1 expression has been shown to be increased in PDAC tissue and has been associated with tumor size, lymph node and liver metastases and suggested to be independent prognostic parameter for shorter progression-free and overall survival [12]. Therefore, targeting XPO1 using specific small molecule inhibitors could be a viable therapeutic strategy for PDAC [13]. Using consensus induced fit docking Selective Inhibitor of Nuclear Export (SINE) compounds have been developed for oncologic applications [14–16]. SINE compounds target the amino acid cysteine 528 in the XPO1 NES binding domain thereby blocking nuclear export function. This results in blocking TSPs in the nucleus, leading to selective apoptosis of cancer cells with damaged genomes while normal cells undergo reversible cell cycle arrest [17]. The clinical compound selinexor (KPT-330) and related analogs have broad activity against different tumor types (nanomolar IC50s) [18, 19]. In earlier studies we found that SINE can induce nuclear retention of several TSPs resulting in the suppression of PDAC growth and tumor growth retardation in mice [20]. Selinexor has been shown to be well tolerated in patients with advanced malignancies [21, 22]. Based on our result and numerous studies from other groups, selinexor was introduced into multiple phase I / II/III trials in different malignancies [23] and received FDA approval for penta-refractory multiple myeloma.
A large proportion of PDAC patients receive GEM-nab-paclitaxel based therapies [24]. We have recently demonstrated that SINE compounds synergizes with GEM [25]. In this study, we demonstrate that selinexor synergizes with GEM-nab-paclitaxel in PDAC cellular models, stem cells and patient derived xenografts. We also performed a Phase I study evaluating the safety and efficacy of selinexor in combination with GEM and nano-encapsulated nab-paclitaxel in patients with PDAC.
Materials and Methods
Cell lines and reagents
MiaPaCa-2, Panc-1 and AsPC-1 were purchased from ATCC. L3.6pl was a generous gift from Dr. Paul J. Chiao (MD Anderson Texas Cancer Center). CRISPR/Cas9 cell pair of HEK293 wild type and HEK XPO1C528S mutant were developed as previously described [26]. CSCs were developed according to previous publications[27]. Cell lines culture conditions and authentication are detailed in supplementary Methods.
MTT and colony formation assay
PDAC and HEK cells were treated with selinexor, GEM-nab-paclitaxel or combination for 72 hours (hrs). Conditions and procedure for MTT assay has been previously described [28]. Procedure for colony formation are detailed in Supplemental Methods.
Annexin V FITC apoptosis
PDAC, CSCs or HEK cell pair (50,000 cells/well) growing in 6 well plates were exposed to drugs (72 hrs). Cells were pelleted and processed for Annexin V FITC using standard protocol (Biovision, USA) and analyzed using a BD SLR II flow cytometer at Karmanos Cancer Institute flow cytometry core.
Spheroid disintegration assay
Spheroids growing in sphere formation culture media were seeded in ultra-low attachment plates (CostarR) and exposed to indicated doses of either selinexor alone, GEM-nab-paclitaxel or selinexor-GEM-nab-paclitaxel twice a week for two weeks. At the end of the treatment, spheroids were counted under an inverted microscope and photographed.
Immunofluorescence Assay
3,000 cells/well were grown in chambered slides and exposed to selinexor alone (1 μM) for 24 hrs followed by immunofluorescence assay as described previously [28]. In combination studies, cells were exposed to selinexor-GEM-nab-paclitaxel for 24 hrs at indicated concentrations followed by standard immunofluorescence analysis.
CRISPR/Cas9 genome editing, siRNA transfection and silencing
Details of CRISPR/Cas9 protocol is available in our previous publication[26]. For siRNA studies, cells were transfected with csiRNA, XPO1 siRNA, using standard protocols [Santa Cruz Biotechnology Dallas, USA] and efficiency was verified by RT-PCR.
RNAi, RT-PCR and western blotting
Details of RNAi, RNA isolation procedure, RT-PCR and Western blotting are provided in Supplemental Methods section. Primer sequences used in this study are provided in Supplementary Table 1.
Proteomic analysis
MiaPaCa-2 cells were treated with 1 μM selinexor or 1 μM selinexor plus 300 nM GEM and 3 nM nab-paclitaxel for 24 hours. The cell pellets were collected and processed using Label-Free Quantitative (LFQ) Proteome Profiling with nuclear and cytoplasmic enrichment using Thermo Q-Exactive HF-X Orbitrap Mass Spectrometer at Bioproximity (Chantilly, VA). The intensity value of total 27528 proteins within 3617 protein sets in each samples was read.
Pre-clinical efficacy trial in subcutaneous xenograft of PDAC
In vivo studies were conducted under Wayne State University IACUC approved protocol in accordance with the approved guidelines. Experiments were approved by the institutes IACUC (Protocol IACUC-18-12-0887). Tumors were developed in female ICR-SCID mice (Taconic Biosciences). Briefly, CSCs growing as spheroids were injected subcutaneously. 1×106 CD44+/CD133+/EpCAM+ MiaPaCa-2 cells were implanted subcutaneously (unilaterally). After 2 weeks, mice were assigned into 2 groups (n=6): (i) vehicle control; (ii) selinexor at 15 mg/kg p.o every other day x3wks. On termination, the tumor tissue from 4 mice each from control or selinexor treated group were used for protein extraction, histopathologic examination and immunohistochemical analysis. In another set, mice bearing CSC tumors were divided into two groups (n=5). Group 1 received vehicle and group 2 received selinexor-GEM-nab-paclitaxel as indicated.
PDAC patient derived xenograft (PDX) development
Fresh pancreatic ductal adenocarcinoma tissue was obtained under an approved IRB protocol. All samples were obtained with patient consent and approval from our Institutional Ethics Committee (protocol # 1603014771). The PDAC origin was confirmed by pathologist on site and put on ice. Immediately (<20 minutes), the surgical material was subcutaneously transplanted into the flanks of 5 mice (NSG, JAX laboratory) and allowed to grow. Once tumor burden reached about 1500 mg, mice were euthanized, tumors collected and cut into 50 mg pieces and then serially propagated in additional mice. A portion of the tissue from first passage was snap frozen and the other portion was further characterized using IHC for PDAC marker cytokeratin-18 to rule out involvement of mice tissue, PCR for mouse vs human CHEK expression was performed. Details of IHC and PCR are provided in supplementary material.
PDAC orthotopic model
1×106 MiaPaCa2 viable cells were surgically injected in head of pancreas of 25 6–8 week old female ICR-SCID mice. On day 32 post implantation, 21 palpated mice were randomly assigned to different treatment cohorts as follows; untreated (n=5), single agent KPT-330-treated group (n=5 orally 10 mg/kg twice a week for 3 weeks), double combination (n=5 GEM + PAC 30mg/kg intravenously twice a week for 2 weeks) and triple combination group (n=6 dosing as above).
Phase Ib clinical study combining selinexor-GEM-nab-paclitaxel in metastatic pancreatic cancer
The detailed Phase Ib/II protocol is available as supplementary material. We obtained written informed consent from the patients, and the studies were conducted in accordance with recognized ethical guidelines and that the studies were approved by an institutional review board.
This phase Ib/II trial was initiated to identify the safety profile and efficiency of the regimen using gemcitabine, nab-paclitaxel and selinexor compared to historical controls in patients with metastatic pancreatic cancer.
Statistical analysis
Students t test was used to compare statistically significant differences. Wherever suitable the experiments were performed three times. The data were also subjected to unpaired two-tailed Student’s t-test wherever appropriate and p < 0.05 was considered statistically significant.
RESULTS
Selective Inhibitor of Nuclear Export (SINE) compound synergizes with GEM-nab-paclitaxel in PDAC cellular models
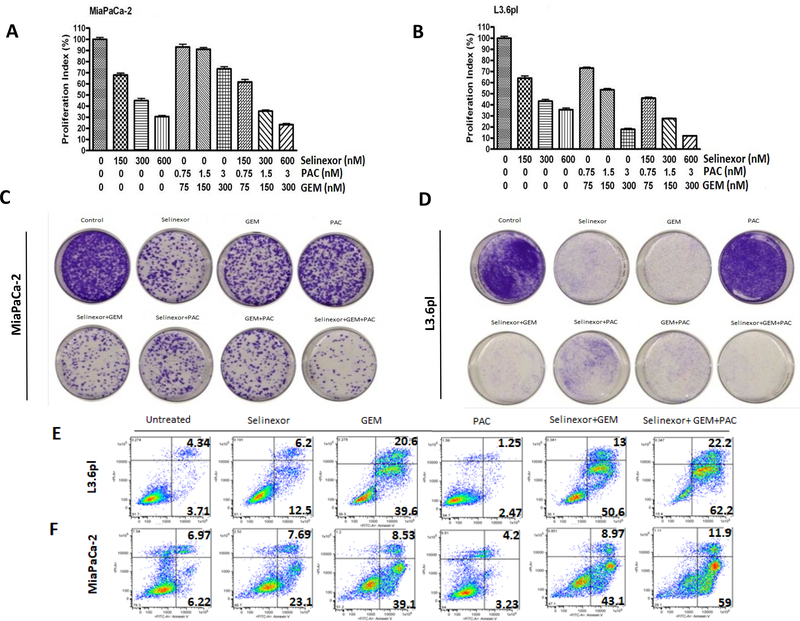
XPO1 is consistently activated in pancreatic ductal adenocarcinoma compared to normal tissue (supplementary Figure 1A). Earlier we demonstrated the single agent activity of SINE compounds [20] (structures given in supplementary Figure 1B) as well as synergy with GEM [25]. Building on these studies, we evaluated the impact of SINE compounds in combination with standard of care therapeutics in different cellular models of PDAC. In line with previous results, selinexor demonstrated synergy with GEM-nab-paclitaxel in various PDAC cell lines (Figure 1A and 1B and supplementary Figure 2A and 2B for isobologram analysis for MiaPaCa-2 and L3.6pl), as well as Panc-1 and AsPc-1 (see inset table of supplementary Figure 2). We further evaluated synergy using a colony formation assay. The combination of selinexor-GEM-nab-paclitaxel demonstrated superior inhibition of colonies compared to GEM-nab-paclitaxel, selinexor-GEM, selinexor-nab-paclitaxel or single agent selinexor in two PDAC cell lines (Figure 1C and 1D and supplementary Figure 2 C and 2D). Annexin V FITC apoptosis analysis mirrored MTT and colony formation results. Compared to single agent or dual combination treatments, selinexor-GEM-nab-paclitaxel showed enhancement in apoptosis in L3.6pl and MiaPaCa-2 cells (Figure 1E and 1F and supplementary Figure 2E). In order to assess whether the combination shows molecular synergy, a limited set of targets were probed. The combined treatment led to superior activation of the TSP, Bim (supplementary Figure 2F). These findings demonstrate the improvement of selinexor-GEM-nab-paclitaxel over standard of care GEM-nab-paclitaxel and support the use of this combination in patients with PDAC.
Figure 1. Selinexor synergizes with GEM and nab-paclitaxel in PDAC cellular models.
MiaPaCa-2 [A] or L3.6pl [B] cells growing in 96 well plates (5,000 cells/well) were exposed to vehicle, selinexor, GEM-nab-paclitaxel or selinexor-GEM-nab-paclitaxel for 72 hrs and standard MTT assay was performed. Isobologram analysis was performed and combination index (CI) was calculated using GraphPad Prism software. Data is representative of 3 independent experiments. MiaPaCa-2 [C] or L3.6pl [D] cells growing in 6 well plates (50,000 cells/well) for 24 hrs and exposed to selinexor (300 nM), GEM (90 nM), nab-paclitaxel (1.5 nM), selinexor+GEM, selinexor+nab-paclitaxel, GEM+nab-paclitaxel or a triple combination of selinexor, GEM and nab-paclitexal for 72 hrs. The cells were trypsinized and collected. 1000 cells from each treatment group were reseeded in 100 mm petri dishes. Clonogenic assay was performed. The plates were photographed under a light microscope (Nikon). L3.6pl [E] or MiaPaCa-2 [F] cells were grown in 6 well plates in duplicate and exposed to different drugs (at doses mimicking clonogenic assay) for 72 hrs. At the end of the treatment period, cells were trypsinized and 10,000 cells were subjected to Annexin V FITC analysis (Biovision USA).
SINE compound-GEM-nab-paclitaxel abolishes PDAC CSC growth and molecularly reverses stemness
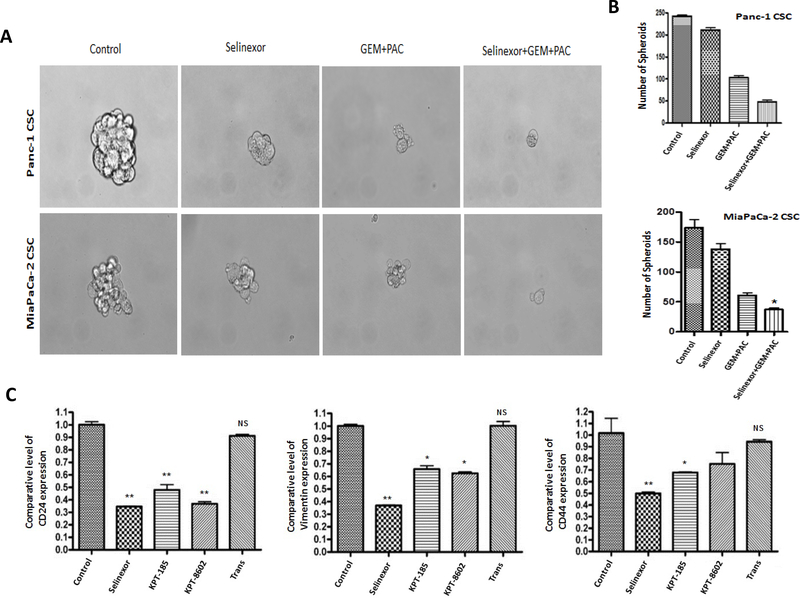
We next sought to evaluate the impact of selinexor-GEM-nab-paclitaxel on GEM-nab-paclitaxel resistant PDAC derived CSCs. PDAC CSCs (derived from Panc-1 or MiaPaCa-2) were exposed to either selinexor, or combinations of GEM-nab-paclitaxel or selinexor-GEM-nab-paclitaxel. Compared to single or dual combination, selinexor-GEM-nab-paclitaxel resulted in almost complete loss of the PDAC spheroids (Figure 2A and 2B). In order to confirm these studies in relevant stem cell models, we probed for a reduction of stemness markers using RT-PCR. As anticipated, SINE compounds selinexor, KPT-8602/eltanexor, or KPT-185, but not the inactive analog KPT-301 (a Trans isomer that does not bind to cys528 of XPO1) showed marked down-regulation in CSC promoters CD24, vimentin and CD44 (Figure 2C). Next, we examined the ability of this potent combination to induce apoptosis in the CSCs. In agreement with the spheroid data, selinexor-GEM-nab-paclitaxel treatment resulted in superior apoptosis compared to selinexor alone or GEM-nab-paclitaxel combination (Supplementary Figure 3A). Selinexor treatment enhanced E-cadherin while simultaneously suppressed cancer stemness markers snail and vimentin (Supplementary Figure 3B). We further investigated the impact of selinexor-GEM-nab-paclitaxel on EMT and apoptosis markers. Our results show that exposure of cells to either selinexor-GEM-nab-paclitaxel or eltanexor-GEM-nab-paclitaxel led to statistically significant reduction in CD44, CD133, EpCAM, Bcl-2 with simultaneous enhancement in FOXO3a and pro-apoptotic Bax (Supplementary Figure 3C). Taken together, these results demonstrate that selinexor can synergize with GEM-nab-paclitaxel and reverse stemness in therapy resistant stem cell like models of PDAC.
Figure 2. Selinexor synergizes with GEM-nab-paclitaxel in PDAC CSCs.
[A and B] Equal number of Panc-1 or MiaPaCa-2 CSC spheroids were seeded in ultra-low attachment plates for 1 week. Spheroids were exposed to KPT-330, GEM, nab-paclitaxel (100 nM) each or their combination for 1 week. Spheroids were counted and photographed under an inverted light microscope fitted with camera (at 100X magnification). [C] MiaPaCa-2 cells growing in 6 well plate (50,000 cells/well) in duplicate were exposed to different SINE analogs (100 nM each) for 72 hrs. RNA was isolated using standard methods. The RNA was subjected to RT-PCR using standard methods. The expression of CSC markers CD24, vimentin and CD44 was normalized to GAPDH (*p<0.05 and **p<0.001). Spheroid data is representative of two independent experiments.
Selinexor-GEM-nab-paclitaxel induced nuclear retention of TSPs in PDAC cellular models
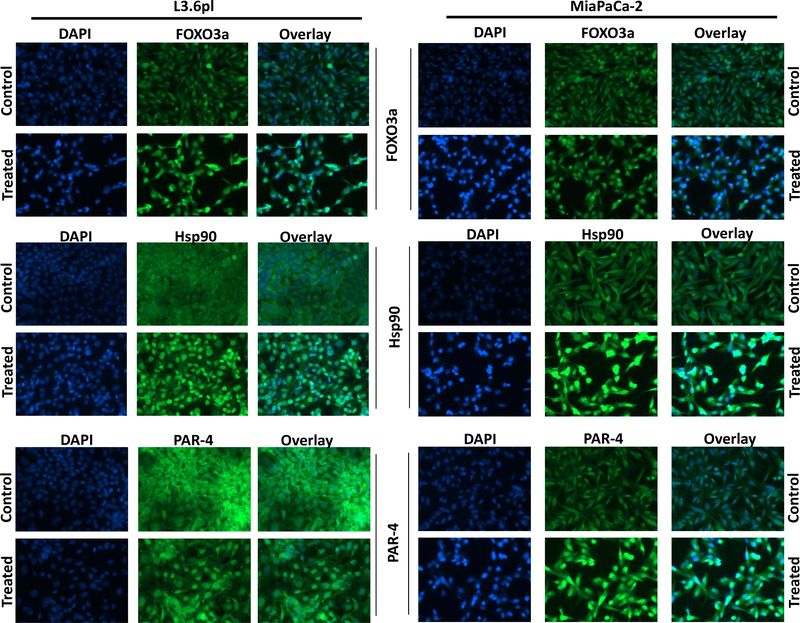
The primary mechanism of SINE compounds is nuclear retention of XPO1 cargo proteins. Therefore, we analyzed the impact of the combination treatment on nuclear retention of several TSPs. In line with the RNAi findings, selinexor-GEM-nab-paclitaxel treatment resulted in nuclear retention of several TSPs such as FOXO3a, HSP90 and PAR-4 in PDAC cell lines (Figure 3). These results clearly demonstrate that selinexor-GEM-nab-paclitaxel combination retains the ability to localize TSPs to the nucleus.
Figure 3. Selinexor-GEM-nab-paclitaxel retains TSPs in the nuclei of PDAC cellular models.
L3.6pl or MiaPaCa-2 cells grown on slides (3000 cells/well) and were exposed to vehicle or a combination of selinexor (300 nM), GEM (150 nM) and nab-paclitaxel (1.5 nM) for 24 hrs followed by IF analysis for nuclear TSPs (FOXO3a, Hsp90 and PAR-4).
Proteomic analysis of global protein changes by selinexor or selinexor-GEM-nab-paclitaxel
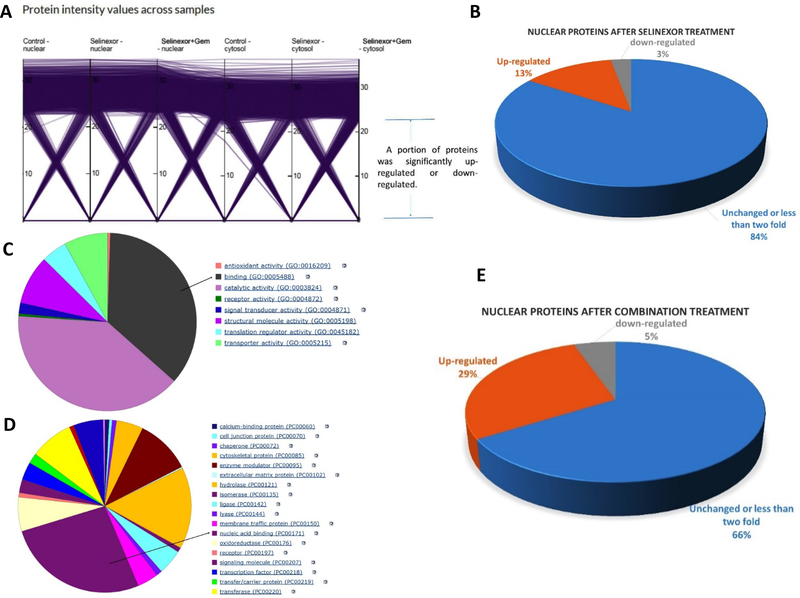
To systematically evaluate the proteins retained in the nucleus, MiaPaCa-2 cells were exposed to 1 μM selinexor alone or in combination with 300 nM gemcitabine and 3 nM nab-paclitaxel for 24 hours. Then the cell pellets were collected and the nuclear and cytoplasmic enrichment were analyzed by Label-Free Quantitative (LFQ) Proteome Profiling using Thermo Q-Exactive HF-X Orbitrap Mass Spectrometer (Bioproximity, Chantilly, VA). The intensity values of 27,528 proteins within 3,617 sets in each sample were analyzed. A specific set of proteins were found to be altered in the selinexor single agent treatment (Figure 4A). This indicates that selinexor treatment alters a limited number of proteins. Among the 27,528 proteins, the levels of 3,535 proteins (13%) were up-regulated more than two folds in nuclear compartment after selinexor treatment. Only 790 proteins (3%) were down-regulated more than two fold in nuclear compartment post single agent treatment (Figure 4B). This highlights the propensity for nuclear-enrichment of XPO1 cargo or associated proteins post selinexor treatment. Interestingly, in the cytoplasm, only 1,124 proteins (4%) were down-regulated more than two-fold after selinexor treatment, while 1,439 proteins (5%) were up-regulated more than two folds (supplementary Figure 4A). Annotation of the molecular function of the highest percentage of proteins includes binding (Figure 4C). Most of the nuclear enriched proteins were composed of nucleic acid binders (Figure 4D). These results support the hypothesis that nuclear retention of transcription factors are most affected by XPO1 inhibition. More intriguingly, comparison of selinexor alone to selinexor-GEM-nab-paclitaxel showed 29% (Figure 4E) of the proteins to be up-regulated in the nucleus compared to 13% by selinexor alone. Annotation of the nuclear target pathway indicated alterations in Wnt and cadherin signaling and p53 activation as well as other key pathways affected (Supplementary Figure 4B). These results suggest a potential biological synergy (in terms of protein nuclear retention) by selinexor-GEM-nab-paclitaxel. In addition, XPO1 was found to be significantly increased in the nucleus and decreased in the cytosol post-selinexor treatment (Supplementary Figure 5). The levels of p53 protein were enhanced in the nucleus post combination treatment (Supplementary Figure 6). Collectively, these results corroborate the synergy data and demonstrate that selinexor-GEM-nab-paclitaxel has the potential to increase the nuclear retention of proteins, compared to treatment with selinexor alone.
Figure 4. Proteomic profiling for nuclear retention of proteins.
MiaPaCa-2 cells were exposed to 1 μM selinexor, 1 μM selinexor plus 300 nM GEM and 3 nM nab-paclitaxel for 24 hours. The cell pellets were collected and analyzed by Label-Free Quantitative (LFQ) Proteome Profiling with nuclear and cytoplasmic enrichment using Thermo Q-Exactive HF-X Orbitrap Mass Spectrometer at Bioproximity. The intensity value of total 27528 proteins within 3617 protein sets in each samples was read. [A] Protein intensity value changes across samples. [B] Percentage of up-regulated and down regulated genes in nuclei of MiaPaCa-2 cells post selinexor single agent treatment. [C] Annotation of molecular function of proteins up-regulated in nucleus after single agent selinexor treatment. [D] Annotation of the protein class up-regulated in the nucleus post selinexor single agent treatment. [E] Percentage of nuclear proteins up-regulated post selinexor-GEM-nab-paclitaxel treatment.
CRISPR/Cas9 validation for the specificity of selinexor in enhancing GEM-nab-paclitaxel activity in PDAC
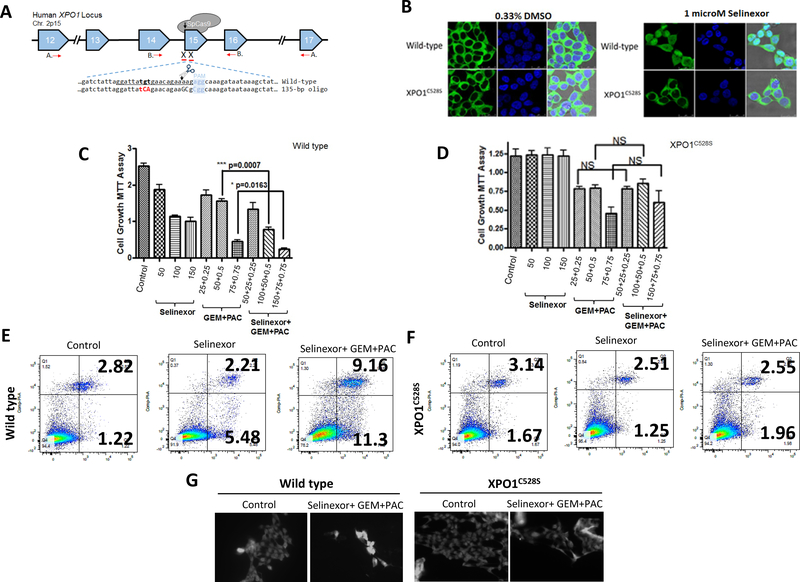
In a previous set of studies mutations in cysteine 528 to serine 528 by CRISPR/Cas9 editing results in complete abrogation of selinexor activity[26]. We used the HEK293 wild type and mutant HEK293 XPO1C528S pair of cell lines to evaluate the specificity of selinexor-GEM-nab-paclitaxel in PDAC cell (CRISPR/Cas9 scheme shown in Figure 5A). In the HEK293 wild type cell line (Figure 5B), exposure to selinexor causes nuclear retention of the XPO1 target RanBP1. However, in HEK293 XPO1C528S cells, we do not observe any nuclear retention of RanBP1 at similar doses of selinexor (Figure 5B, lower right panel). Selinexor alone or selinexor-GEM-nab-paclitaxel treatment effectively suppressed the growth of the HEK293 wild type cells (Figure 5C, **p<0.01). No statistically significant growth inhibition was observed in the HEK293 XPO1C528S cell line by selinexor alone (Figure 5D). Additionally, no statistically significant enhancement in growth inhibition in the combination treatment using selinexor-GEM-nab-paclitaxel when compared to GEM-nab-paclitaxel in the HEK293 XPO1C528S cells (Figure 5D). Annexin V FITC apoptosis analysis demonstrated enhancement of apoptosis in selinexor-GEM-nab-paclitaxel treated HEK293 wild type cells (Figure 5E). On the contrary, there was no statistically significant apoptosis induction in the HEK293 XPO1C528S cells treated with selinexor alone or with the triple combination (Figure 5F). We further evaluated the impact of CRISPR/Cas9 genome editing on nuclear retention of TSPs using immunofluorescence. As shown in Figure 5G left panels, the HEK293 wild type cells showed nuclear retention of HSP90 post selinexor-GEM-nab-paclitaxel treatment. However, there was no nuclear staining of HSP90 in HEK293 XPO1C528S cells (Figure 5G right panel). Collectively our results clearly show that selinexor plays a major and very specific role in enhancing the activity of GEM-nab-paclitaxel which is abrogated by mutations in NES biding pocket of XPO1.
Figure 5. XPO1 CRISPRCas9 genome editing abrogates the activity of selinexor-GEM-nab-paclitaxel.
[A] Schema of CRISPR/Cas9-induced homologous recombination of XPO1 (adopted from our previous publication (Chem and Biol 22 (1) 107–116, 2015). [B] HEK-293 wild-type or HEK-XPO1C528S mutant cells were grown at a density of 5,000 cells/well in chambered slides. The next day, cells were exposed to indicated concentrations of either vehicle (DMSO) or selinexor for 2 hrs. After the treatment was over, IF analysis was performed using RanBP1 antibody (Cell Signaling USA). [C] HEK-293 wild-type or [D] HEK-XPO1C528S mutant cells were grown at a density of 5,000 cells/well in 96 well plates. Cells were treated with selinexor or GEM-nab-paclitaxel or their combination for additional 72 hrs at indicated doses. MTT assay was performed using standard methods. [E] HEK-293 wild-type or [F] HEK-XPO1C528S mutant cells were grown at a density of 50,000 cells/well in six well plates and exposed to selinexor (150 nM), GEM (75 nM) and nab-paclitaxel (0.75 nM) for 72 hrs. Apoptosis analysis was performed using Annexin V FITC analysis. [G] HEK-293 wild-type or HEK-XPO1C528S mutant cells were grown in chambered slides (5,000 cells per well) in duplicate and were exposed to selinexor (150 nM), GEM (75 nM) and nab-paclitaxel (0.75 nM) for 24 hrs. IF assay was performed using Hsp90 antibody.
Selinexor-GEM-nab-paclitaxel pre-clinical efficacy trial in PDAC cell line, CSC and patient derived xenograft
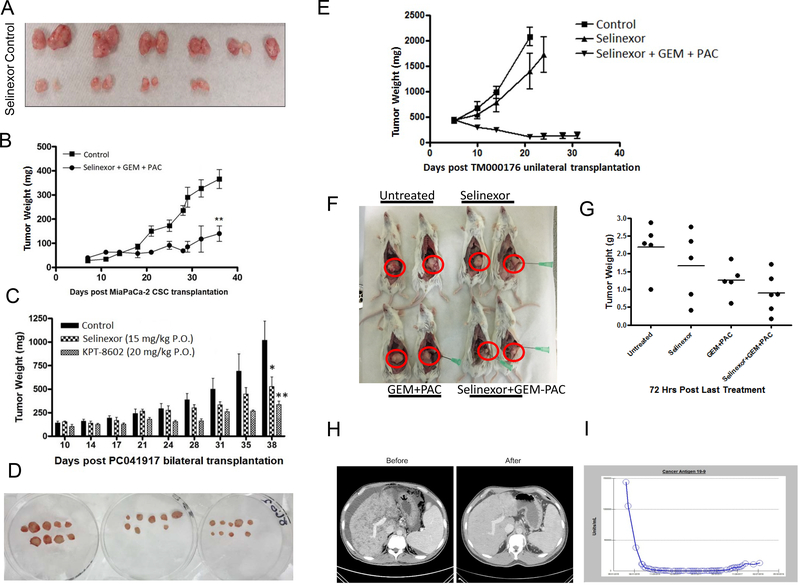
Selinexor either alone or with GEM has been extensively evaluated in sub-cutaneous and orthotopic PDAC animal tumor models [20, 25, 29]. Extending these studies, we first tested the impact of using selinexor as a single agent to treat PDAC derived CSCs. Selinexor given at 15 mg/kg every other day x 3weeks caused statistically significant reduction in the growth of PDAC CSC xenograft (Figure 6A and Supplementary Figure 7A, p<0.01). Molecular analysis of the residual tumor tissue demonstrated down-regulation of XPO1 (Supplementary Figure 7B). Having established single agent activity, we then tested the combination regimen of selinexor-GEM-nab-paclitaxel on CSC xenograft. We recently published data showing that the CSC xenograft demonstrate resistance to both GEM and nab-paclitaxel[27]. In line with our hypothesis, selinexor (at low dose of 5mg/kg) when combined with GEM-nab-paclitaxel led to reduction in tumor growth of PDAC CSC xenograft (Figure 6B). In view of the tumor suppressive activity of SINE compounds in tissue culture and CSC derived models, we evaluated their impact on PDAC patient derived xenografts (PDX). We conducted immunohistochemical staining to confirm the human origin of pancreatic PDX tumor using antibodies specifically against human leukocyte antigen (HLA) class I, human cytokeratin 19 and human Plectin-1 (Supplementary Figure 7C). Plectin-1 is the biomarker to identify primary and metastatic pancreatic ductal adenocarcinoma. The strong staining of human HLA, cytokeratin 19 and Plectin-1 in cancer cells demonstrated that the PDX was from human pancreatic ductal adenocarcinoma. This tissue also had increased XPO1 protein expression when compared to normal tissue (Supplementary Figure 7C). Human origin of PDX was additionally validated using RT-PCR for CHEK1 (Supplementary Figure 7D). The PCR results showed that the PDX tumor sample and MiaPaCa-2 cells had strong human CHEK1 amplification, suggesting their human origin. The weak mouse CHEK1 band observed in PDX sample could possibly come from mouse blood cells. After confirming the human tissue origin, we evaluated the impact of selinexor or KPT-8602 on PDX tumor growth in sub-cutaneous xenograft. Exposure to selinexor (15 mg/kg QoDx2) or KPT-8602 (20 mg/kg QDx5) resulted in statistically significant reduction in tumor growth of this pancreatic PDX [Figure 6C graph (*p=0.04 and **p=0.0098) and tumor photographs 6D]. The drugs are fairly well tolerated with very minimal loss in mice body weight (Supplementary Figure 7E). In combination studies, selinexor-GEM-nab-paclitaxel demonstrated much pronounced tumor growth inhibition compared to selinexor alone (Figure 6E). In a MiaPaCa-2 derived orthotopic model, administration of KPT-330 selinexor (at suboptimal doses of 10 mg/kg twice a week) enhanced the activity of GEM-Abraxane (give at 30 mg/kg i.v. twice a week) (Figure 6F tumor photographs, Figure 6G dot plot and tumor images in Supplementary Figure 7 F). These data suggest that selinexor can be used either alone or in combination with standard of care treatments for inhibition of pancreatic tumors.
Figure 6. Selinexor-GEM-nab-paclitaxel anti-tumor activity in PDAC CSC and patient derived xenograft.
MiaPaCa-2 cells were flow sorted for CSC markers (CD44, CD133, EpCAM). These CSCs form tumor from 20,000 cells [A] Twelve mice were injected with CSCs. When palpable tumors were formed, mice were divided into two groups of five mice each and exposed to selinexor as single agent (used at 15 mg/kg every other day x3 weeks). Tumor weight was recorded every three days. [B] 10 mice bearing CSCs were divided into two groups. Group I vehicle and group II selinexor at sub-MTD (5 mg/kg twice a weekx3weeks) in combination with GEM (50 mg/kg i.v.) and nab-paclitaxel (30 mg/kg i.v.) (**p<0.01). [C] Anti-tumor activity of selinexor or KPT-8602 (Eltanexor) in PDX sub-cutaneous xenograft. [D] Photograph of residual tumors post treatment. [E] Anti-tumor activity of selinexor in combination with GEM-nab-paclitaxel (triple combination) compared to selinexor (single agent) or vehicle control in PDAC Pdx. Selinexor was administered orally (5 mg/kg), while gemcitabine (GEM) and nab-paclitaxel (PAC) were given i.v. 30 mg/kg and 20 mg/kg respectively. [F] Photographs showing reduction in tumor size in the combination treatment on day 53 post MiaPACa-2 orthotopic implantation. [G] Dot Graph representing gross tumor weights 72 hours post last treatment. Evaluable response to selinexor-GEM-nab-paclitaxel based treatment in patient with metastatic PDAC. [H] Before and after treatment CT-Scan showing the changes in the liver metastatic disease. [I] Serial measurements of the tumor marker CA19-9 in the patient with PDAC treated with a selinexor based regimen. Serum CA19-9 showed a precipitous drop after the first few treatments and a lower, steady concentration thereafter.
Phase Ib Clinical Trial: Evaluable response in metastatic PDAC patients on selinexor-GEM-nab-paclitaxel therapy
Encouraged by our in vitro, in vivo and ex vivo studies, we obtained Intuitional Review Boards approval to proceed with Phase Ib/II clinical trial ( NCT02178436) to test the safety and efficiency of Selinexor 60mg in a combination with GEM 1000 mg/m2 and Nab-Paclitaxel 125 mg/m2 (day 1, 8, 15 of a 28-day cycle) as a first line for metastatic PDAC. The patients were eligible for this trial if they are >18 years old, has metastatic PDAC, did not receive prior systemic oncologic treatment or radiation therapy with adequate renal, hepatic and hematopoietic function. After obtaining the informed consents from the patients and in accordance with Declaration of Helsinki, we treated 9 patients with this combination. Two (40%) out of 5 evaluable patients had partial response and 2 patients (40%) had stable disease with only one patient had a progressive disease, per Response Evaluation Criteria In Solid Tumors (RECIST 1.1) (Supplementary Table 2). One of the patients with partial response demonstrated exceptionally durable response with a progressive free survival (PFS) of 16 months and an overall survival (OS) of 22 months using this regimen. This patient (66 years old, male) presented with abdominal, back pain, weight loss of 25 pounds, and grade 2 fatigue. Diabetes mellitus was diagnosed a year prior to his presentation. His overall performance status was 1 on Eastern Cooperative Oncology Group (ECOG) scale. Initial evaluation revealed a pancreatic tail mass, peritoneal nodules, and multiple liver lesions. Computed tomographic (CT)-guided biopsy of one of liver metastases revealed an adenocarcinoma compatible with a pancreatic primary. Serum CA19-9 was elevated at 1,444,000 units/mL (normal range is <36 units/mL). C-reactive protein (CRP) was 15.2 mg/L (normal range is < 9.10 mg/L). Treatment was started with gemcitabine 1,000 mg/m2, nab-paclitaxel 125 mg/m2, and selinexor 60 mg/m2 given on day 1, 8, 15 of a 28-day cycle. After three cycles, nab-paclitaxel was discontinued due to grade 3 neuropathy and peripheral edema. The patient continued treatment at a reduced dose of gemcitabine (750 mg/m2) combined with 60 mg/m2 dose of selinexor. CT scans were performed every 8 weeks with regular evaluations of serum CA19-9. He achieved a partial response based on RECIST 1.1 after two months of starting therapy. Continued shrinkage was seen in each of the subsequent restaging CT scans for at least eight months and was followed by disease stability (Figure 6H). Patient also experienced resolution of pain, improvement of performance status to 0, and weight gain. Serum CA19-9 level dropped precipitously to below 100 (Figure 6I). Although the patient remained only on selinexor with a reduced dose of gemcitabine for most of the treatment period, he had a PFS of 16 months and OS of 22 months. He maintained an excellent ECOG PS of 1 throughout the treatment. It is worth noting that molecular profiling of a pretreatment liver metastasis biopsy based on the CARIS 592 gene panel revealed mutations in three tumor suppressor genes (NF1 E18 c.2002–1G>T, SMAD4 E9 p.R361H, and TP53 E8 p.R282W) in addition to the expected KRAS-G12D oncogene mutation (Supplementary Tables 3-6).
Discussion
In this paper we show that the combination of nuclear export inhibitor selinexor with GEM and nab-paclitaxel can suppress PDAC proliferation and stemness potentially through nuclear retention of multiple tumor suppressor proteins. The activity was quite notable in PDAC stem cell and patient derived xenograft. In addition, a patient with stage IV PDAC treated with combination achieved a PR in our ongoing Phase Ib/II ( NCT02178436) clinical study.
With a five year survival rate of ~7%, PDAC is considered to be among the most lethal malignancies [30]. This dismal picture is due to both the lack of early diagnosis and the absence of effective treatments for patients with advanced disease. Therapy resistance has been linked to low drug delivery, low tumor vascularity and complex immune suppressive tumor microenvironment (TME) [31]. Additionally, PDAC tumors have been shown to harbor a resistant population of cells termed stem like cells (CSCs) [32]. Combination of all of these factors causes PDAC to have high intrinsic resistance to therapeutic treatments [33]. Despite this knowledge, attempts to overcome drug resistance by re-programming the stromal microenvironment to re-vascularize tumors and enhance drug penetrance have resulted in only marginal benefits in the clinic. This could be due to the fact that most of the studies so far have focused on targeting individual or few set of molecules in the pathways supporting CSCs and DR. Multiple meaningful perturbations are required to impact the TME. Holistic strategies need to be designed that can simultaneously target the pathways that sustain CSCs and the DR. Based on the studies presented here and supporting evidence from other laboratories, we postulate that XPO1 is one potential target of multiple pathways that maintain PDAC. We provide evidence that targeted inhibition of XPO1 via SINE compounds can induce meaningful perturbations in multiple survival and therapy resistant signaling pathways in PDAC.
SINE block nuclear export by covalently binding to cysteine 528 in the XPO1 NES cargo-binding domain in a slowly reversible manner. This results in blocking TSPs in the nucleus which leads to selective apoptosis of cancer cells [17]. The clinical SINE compound selinexor and related analogs have a broad activity against different tumor types [18, 19]. These compounds have been independently studied for their impact on tumor stromal component, angiogenesis, and the immune system as well as synergy with several chemo and targeted therapies. Selinexor has been shown to impair cellular cross talk in the tumor microenvironment by blocking osteoclastogenesis in multiple myeloma [34]. The drug can suppress pro-angiogenic and pro-osteolytic cytokines and reduced osteoclastogenesis in prostate cancer models [35]. In studies more relevant to PDAC, selinexor has been shown to exert enhanced anti-cancer activity when combined with T-Cell checkpoint inhibitors in solid tumor models [36]. Furthermore, SINE compounds can effectively overcome therapy resistance in several solid tumor cell lines [10]. Our studies on CSCs are supported by additional findings in AML tumor models showing activity of selinexor against blasts and leukemia-initiating cells both in vitro and in vivo [37].
We used a systematic proteomic analyses approach to better understand selinexor synergy. Compared to single agent activity, we observed an increase in nuclear retention of TSPs in the selinexor-GEM-nab-paclitaxel combination treatment group. This data suggests that the combination may be selective as opposed to being more cytotoxic. Rather, it shows that selinexor-GEM-nab-paclitaxel combination may exert a more selective synergy through enhanced nuclear retention and activation of tumor suppressors. Annotation of these nuclear targets suggests increased activation of DNA binding proteins. These results perhaps indicate that selinexor and the combination with GEM-nab-paclitaxel work through nuclear reactivation of TSP signaling. Collectively, the evidence we present here indicate that XPO1 inhibition by selinexor can be a viable therapeutic strategy. Additionally, XPO1 inhibition can induce meaningful perturbations on the components critical for supporting the PDAC tumor microenvironment to overcome therapy resistance.
Leptomycin B (LMB), first isolated by researchers as a novel antibiotic from Streptomyces, was found to non-reversibly target Cyc528 in XPO1 [38]. LMB (or elactocin) was used in a single Phase I study but was discontinued due to severe toxicity that included anorexia and malaise with a narrow therapeutic window [39]. Similarly, selinexor alone or combinations with GEM-nab-paclitaxel may have undesirable side effects resulting from nuclear retention of unwanted oncogenes in cancer cell nucleus or toxicity to normal cells. However, data from several early and later stage trials show that selinexor is well tolerated in cancer patients [22, 40]. Selinexor is currently being evaluated in >60 Phase I-III studies. More than 2,700 patients have been exposed to selinexor alone or in combination. Objective responses were observed in patients with solid or hematological malignancies. In these studies, the reported drug-related adverse events (AEs) are mostly anorexia, nausea, vomiting and fatigue (Grade 1 and 2) and are well managed with dosing holidays and supportive care. Although the most frequently reported grade 3 or 4 AEs were anemia, thrombocytopenia, fatigue, leukopenia and lymphopenia, major clinically significant organ or cumulative toxicities were not observed. The Phase I data has also validated changes in XPO1 mRNA expression in patient blood samples as well as FOXO nuclear retention as two pharmacodynamic response markers in paired tissue biopsies. In the first study involving 189 patients, evaluable XPO1 expression changes were observed using RT-PCR on leukocytes along with FOXO3a retention in paired patient tissue biopsies using IHC [41]. In another study selinexor plus fludarabine and cytarabine was used to treatment refractory or relapsed leukemia or myelodysplastic syndrome (MDS). Fifteen out to 16 patients were confirmed to have at-least two-fold induction of XPO1 mRNA expression in the blood and FOXO3a nuclear retention in the tumors [42]. Our pre-clinical work in PDAC also confirmed FOXO3a nuclear retention in PDAC cellular models as well as XPO1 protein down-regulation in mice xenografts.
In the Phase Ib study presented in this report, the interim analysis shows that dose limiting toxicity was not achieved when selinexor was administered at a dose up to 60 mg/m2 with GEM-nab-paclitaxel. Among the responding evaluable patients, we had 80 % disease control rate. One can argue that the exceptional response that was observed in one of the patients on this trial who was maintained only on selinexor with reduced dose GEM after only 3 cycles of initial Nab-Paclitaxel is also observed historically with GEM-nab-paclitaxel combination. However, we hypothesize that this effect could be primarily due to selinexor and its synergy with GEM in this patient as nab-Paclitaxel was omitted earlier in the course of the treatment due to severe peripheral sensory neuropathy. The complete Phase I trial is part of our forthcoming publication and is beyond the scope of this manuscript. Collectively, these in vitro, ex vivo and early Phase clinical data support the expansion phase of SINE compounds for the treatment of PDAC.
Supplementary Material
Statement of translational relevance.
Pancreatic ductal adenocarcinoma (PDAC) is a deadly disease in urgent of newer treatments. In this paper we demonstrate that the nuclear exporter protein exportin 1 (XPO1) is critical for PDAC tumor growth and sustenance. Targeting XPO1 with specific inhibitor of nuclear export compounds (selinexor and analogs) either alone or in combination with standard of care chemotherapeutics demonstrates anti-tumor activity in vitro, in PDAC stem cell derived models and multiple patient derived models. More significantly, in a Phase I clinical study we show remarkable response in a patient with metastatic PDAC tripling the survival on a regimen of selinexor-gemcitabine-nab-paclitaxel. Our studies bring forward a new and clinically effective therapy for PDAC.
Acknowledgements
NIH R01 grant 1R37CA215427 to Asfar S. Azmi is acknowledged. The authors thank the SKY Foundation Inc. for pancreatic cancer for supporting part of this study.
Funding: Work in the lab of Azmi AS is supported by NIH R37 grant 1R37CA215427. The authors thank the SKY Foundation, and Perri Family Foundation for supporting part of this study.
Footnotes
Conflict of interest statement: None to declare.
Reference List
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Kitano H Cancer robustness: tumour tactics. Nature 2003;426:125. [DOI] [PubMed] [Google Scholar]
- [3].Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol 2012;83:1021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Turner JG, Sullivan DM. CRM1-mediated nuclear export of proteins and drug resistance in cancer. Curr Med Chem 2008;15:2648–55. [DOI] [PubMed] [Google Scholar]
- [5].Moroianu J Distinct nuclear import and export pathways mediated by members of the karyopherin beta family. J Cell Biochem 1998;70:231–9. [PubMed] [Google Scholar]
- [6].Azmi AS. The evolving role of nuclear transporters in cancer. Semin Cancer Biol 2014. [DOI] [PubMed] [Google Scholar]
- [7].Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol 2007;17:193–201. [DOI] [PubMed] [Google Scholar]
- [8].Fukuda M, Asano S, Nakamura T, et al. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature 1997;390:308–11. [DOI] [PubMed] [Google Scholar]
- [9].Shaulsky G, Goldfinger N, Tosky MS, Levine AJ, Rotter V. Nuclear localization is essential for the activity of p53 protein. Oncogene 1991;6:2055–65. [PubMed] [Google Scholar]
- [10].Turner JG, Dawson J, Cubitt CL, Baz R, Sullivan DM. Inhibition of CRM1-dependent nuclear export sensitizes malignant cells to cytotoxic and targeted agents. Semin Cancer Biol 2014;27:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Azmi AS, Mohammad RM. Targeting Cancer at the Nuclear Pore. J Clin Oncol 2016;34:4180–2. [DOI] [PubMed] [Google Scholar]
- [12].Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med 2009;32:E315. [PubMed] [Google Scholar]
- [13].Yashiroda Y, Yoshida M. Nucleo-cytoplasmic transport of proteins as a target for therapeutic drugs. Curr Med Chem 2003;10:741–8. [DOI] [PubMed] [Google Scholar]
- [14].Lapalombella R, Sun Q, Williams K, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood 2012;120:4621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Etchin J, Sun Q, Kentsis A, et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia 2013;27:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ranganathan P, Yu X, Na C, et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood 2012;120:1765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kalid O, Toledo WD, Shechter S, Sherman W, Shacham S. Consensus Induced Fit Docking (cIFD): methodology, validation, and application to the discovery of novel Crm1 inhibitors. J Comput Aided Mol Des 2012;26:1217–28. [DOI] [PubMed] [Google Scholar]
- [18].Inoue H, Kauffman M, Shacham S, et al. CRM1 Blockade by Selective Inhibitors of Nuclear Export (SINE) attenuates Kidney Cancer Growth. J Urol 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang K, Wang M, Tamayo AT, et al. Novel selective inhibitors of nuclear export CRM1 antagonists for therapy in mantle cell lymphoma. Exp Hematol 2013;41:67–78. [DOI] [PubMed] [Google Scholar]
- [20].Azmi AS, Aboukameel A, Bao B, et al. Selective Inhibitors of Nuclear Export Block Pancreatic Cancer Cell Proliferation and Reduce Tumor Growth in Mice. Gastroenterology 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tan DS, Bedard PL, Kuruvilla J, Siu LL, Razak AR. Promising SINEs for Embargoing Nuclear-Cytoplasmic Export as an Anticancer Strategy. Cancer Discov 2014;4:527–37. [DOI] [PubMed] [Google Scholar]
- [22].Abdul Razak AR, Mau-Soerensen M, Gabrail NY, et al. First-in-Class, First-in-Human Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Patients With Advanced Solid Tumors. J Clin Oncol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Senapedis WT, Baloglu E, Landesman Y. Clinical translation of nuclear export inhibitors in cancer. Semin Cancer Biol 2014. [DOI] [PubMed] [Google Scholar]
- [24].Von Hoff DD, Goldstein D, Renschler MF. Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med 2014;370:479–80. [DOI] [PubMed] [Google Scholar]
- [25].Kazim S, Malafa MP, Coppola D, et al. Selective Nuclear Export Inhibitor KPT-330 Enhances the Antitumor Activity of Gemcitabine in Human Pancreatic Cancer. Mol Cancer Ther 2015;14:1570–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Neggers JE, Vercruysse T, Jacquemyn M, et al. Identifying drug-target selectivity of small-molecule CRM1/XPO1 inhibitors by CRISPR/Cas9 genome editing. Chem Biol 2015;22:107–16. [DOI] [PubMed] [Google Scholar]
- [27].Aboukameel A, Muqbil I, Senapedis W, et al. Novel p21-Activated Kinase 4 (PAK4) Allosteric Modulators Overcome Drug Resistance and Stemness in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther 2017;16:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Azmi AS, Muqbil I, Wu J, et al. Targeting the Nuclear Export Protein XPO1/CRM1 Reverses Epithelial to Mesenchymal Transition. Sci Rep 2015;5:16077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gao J, Azmi AS, Aboukameel A, et al. Nuclear retention of Fbw7 by specific inhibitors of nuclear export leads to Notch1 degradation in pancreatic cancer. Oncotarget 2014;5:3444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mettu NB, Abbruzzese JL. Clinical Insights Into the Biology and Treatment of Pancreatic Cancer. J Oncol Pract 2016;12:17–23. [DOI] [PubMed] [Google Scholar]
- [31].Rasheed ZA, Matsui W, Maitra A. Pathology of pancreatic stroma in PDAC. 2012. [PubMed]
- [32].ngi-Garimella S, Krantz SB, Shields MA, Grippo PJ, Munshi HG. Epithelial-mesenchymal transition and pancreatic cancer progression. 2012. [PubMed]
- [33].Sheikh R, Walsh N, Clynes M, O’Connor R, McDermott R. Challenges of drug resistance in the management of pancreatic cancer. Expert Rev Anticancer Ther 2010;10:1647–61. [DOI] [PubMed] [Google Scholar]
- [34].Gravina GL, Tortoreto M, Mancini A, et al. XPO1/CRM1-selective inhibitors of nuclear export (SINE) reduce tumor spreading and improve overall survival in preclinical models of prostate cancer (PCa). J Hematol Oncol 2014;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tai YT, Landesman Y, Acharya C, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 2014;28:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farren MR, Hennessey RC, Shakya R, et al. The Exportin-1 Inhibitor Selinexor Exerts Superior Antitumor Activity when Combined with T-Cell Checkpoint Inhibitors. Mol Cancer Ther 2017;16:417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Etchin J, Montero J, Berezovskaya A, et al. Activity of a selective inhibitor of nuclear export, selinexor (KPT-330), against AML-initiating cells engrafted into immunosuppressed NSG mice. Leukemia 2016;30:190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hamamoto T, Gunji S, Tsuji H, Beppu T. Leptomycins A and B, new antifungal antibiotics. I. Taxonomy of the producing strain and their fermentation, purification and characterization. J Antibiot (Tokyo) 1983;36:639–45. [DOI] [PubMed] [Google Scholar]
- [39].Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. Br J Cancer 1996;74:648–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shafique M, Ismail-Khan R, Extermann M, et al. A Phase II Trial of Selinexor (KPT-330) for Metastatic Triple-Negative Breast Cancer. Oncologist 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gounder MM, Zer A, Tap WD, et al. Phase IB Study of Selinexor, a First-in-Class Inhibitor of Nuclear Export, in Patients With Advanced Refractory Bone or Soft Tissue Sarcoma. J Clin Oncol 2016;34:3166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Alexander TB, Lacayo NJ, Choi JK, Ribeiro RC, Pui CH, Rubnitz JE. Phase I Study of Selinexor, a Selective Inhibitor of Nuclear Export, in Combination With Fludarabine and Cytarabine, in Pediatric Relapsed or Refractory Acute Leukemia. J Clin Oncol 2016;34:4094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.