Abstract
Targeted delivery of chemotherapeutics aims to increase efficacy and lower toxicity by concentrating drugs at the site-of-action, a method embodied by the seven current FDA approved antibody-drug conjugates (ADCs). However, a variety of pharmacokinetic challenges result in relatively narrow therapeutic windows for these agents, hampering the development of new drugs. Here, we use a series of Prostate-Specific Membrane Antigen (PSMA)-binding single-domain (Humabody®) ADC constructs to demonstrate that tissue penetration of protein-drug conjugates plays a major role in therapeutic efficacy. Counterintuitively, a construct with lower in vitro potency resulted in higher in vivo efficacy than other protein-drug conjugates. Biodistribution data, tumor histology images, spheroid experiments, in vivo single-cell measurements, and computational results demonstrate that a smaller size and slower internalization rate enabled higher tissue penetration and more cell killing. The results also illustrate the benefits of linking an albumin binding domain to the single-domain ADCs. A construct lacking an albumin binding domain was rapidly cleared leading to lower tumor uptake (%ID/g) and decreased in vivo efficacy. In conclusion, these results provide evidence that reaching the maximum number of cells with a lethal payload dose correlates more strongly with in vivo efficacy than total tumor uptake or in vitro potency alone for these protein-drug conjugates. Computational modeling and protein engineering can be used to custom design an optimal framework for controlling internalization, clearance, and tissue penetration to maximize cell killing.
Introduction
Antibody-drug conjugates (ADCs) have opened a new field of targeted therapeutics based on ‘hybrid’ drugs combining desirable targeting properties of biologics with the potency of small molecule cytotoxic payloads. For ADCs, the protein carrier is typically a monoclonal antibody that specifically binds to a target antigen expressed on cancer cells, increasing the delivery of the small molecule payload to the site of action in vivo. To date, seven ADCs have been FDA approved1, 2, most recently enfortumab vedotin and trastuzumab deruxtecan, with a large pipeline of >70 in clinical trials. However, one drawback of antibodies is slow tumor penetration. The tumor uptake of antibodies is limited by their extravasation rate, and they tend to penetrate only a few cell layers outside of blood vessels due to their rapid antigen-binding rate relative to intratumoral diffusion3, 4. In the clinic, unconjugated antibodies are often well tolerated, such that they can be delivered at very high doses that saturate receptors on cell layers closer to the blood vessel, enabling the antibody to diffuse farther through the tumor. However, the payload toxicity of ADCs limits the dose and frequency of administration, restricting tumor penetration depths and allowing regrowth between doses (typically given every 3 weeks for current therapies)5. Therefore, devising design and treatment strategies that increase tumor penetration may yield greater efficacy and improve clinical success rates for ADCs and other protein-drug conjugates.
Many current ADCs in late-stage clinical trials are targeting receptors with high expression (e.g. TROP-2, HER2, folate receptor, and Nectin-4) where tissue penetration can play a significant role in efficacy. Previous work based on co-administration of trastuzumab with the ADC T-DM1 (ado-trastuzumab emtansine) demonstrated that higher tissue penetration could yield better efficacy in a mouse model of highly expressed HER2 positive cancer6. Based on a literature review of studies involving ADCs with a range of drug-to-antibody ratios (DAR)7, ADCs that delivered the same payload dose at a lower DAR (i.e. higher protein doses) generally yielded an increase in efficacy in animal models with moderate to high expression, likely as a result of better tissue penetration8. Although bystander effects can help mitigate transport challenges by allowing the payload to diffuse deeper into the tissue following release from the protein, analysis of literature data and predictive simulations indicated that higher tissue penetration is more efficient at improving efficacy even when using bystander payloads8. When the ADC is more uniformly distributed, the same total amount of cytotoxic payload delivered to the tumor is spread more homogeneously, so cells adjacent to vessels that receive an overdose of payload with heterogeneous ADC distribution now receive fewer payloads. Therefore, implicit in this approach is that the amount of payload delivered per cell exceeds the intrinsic potency of the payload, thereby maintaining a lethal cellular dose while increasing penetration to reach more cells. Similarly, lower target receptor expression can improve efficacy for extremely potent ADCs by enabling deeper tissue penetration. Lower receptor expression limits the amount of bound and internalized ADC for perivascular cells but increases tumor tissue penetration since penetration depth is inversely proportional to expression7, 9. Based on these and other results10, tumor tissue penetration is critical for the overall efficacy of ADC treatment and should be analyzed when optimizing protein-drug conjugates for maximum effectiveness.
Besides increasing the dose or co-administering the ADC with unconjugated antibody, there are protein engineering approaches to develop scaffolds for improved tissue penetration11–14. This may be particularly advantageous for highly expressed targets that would require very large antibody doses to saturate the tumor or for targeting metastases with varying expression levels. Reducing affinity is one method of increasing tissue penetration15. However, for large, bivalent IgGs, the intrinsic affinity of each Fab arm must be very low to penetrate tissue with highly expressed antigens (e.g. 270 nM Kd)16. Instead of modulating affinity, lower molecular weight proteins can penetrate tissue farther before being immobilized through target binding17 because of their faster diffusion coefficient (which is the dominant mode of transport in tumors due to elevated interstitial pressure18). Some smaller scaffolds are already being tested in the clinic, such as caplacizumab - a bivalent single-domain antibody (~30kDa) used to block platelet aggregation19, providing precedent for these formats. A major limitation for these smaller scaffolds is their rapid renal clearance11, 20, 21. However, fusion to albumin binding domains can extend plasma half-life and avoid glomerular filtration by increasing the effective molecular weight while still enabling better tissue penetration22–26.
In this work we investigated the tumor tissue pharmacokinetics and efficacy of 3 different Prostate-Specific Membrane Antigen (PSMA)-binding single-domain antibody (Humabody) drug conjugates and an IgG drug conjugate (referred to collectively as antibody drug conjugates or simply antibodies/antibody constructs if no drug is present). Humabodies are fully human, single heavy chain variable (VH) domains generated using a proprietary transgenic mouse platform27, 28 (Crescendo Biologics). The mice lack murine heavy chain, κ light chain, and λ light chain expression but contain human heavy chain genes. After in vivo maturation and development, multiple VH domain Humabodies can be constructed with a range of functionality to optimize target engagement and improve therapeutic benefit. We used this flexible platform to test the impact of two main design parameters with antibody drug conjugates: the cellular internalization rate and the plasma clearance rate. The first construct (VH2-VH1-HLE, MW = 44.7 kDa) is a fusion between three VH domains: two PSMA binding domains to different epitopes (VH1 and VH2) conjugated to an albumin-binding ‘half-life extender’ (HLE) VH domain to yield VH2-VH1-HLE. Targeting multiple epitopes results in antigen cross-linking and rapid internalization, while binding albumin can slow plasma clearance. Therefore, this construct was designed for rapid internalization and slow clearance. The second antibody construct, designed to isolate the impact of internalization (VH1-HLE, MW = 31.1 kDa), consists of a monovalent PSMA binding domain (VH1) connected via a linker to the albumin-binding domain resulting in the VH1-HLE construct. The lack of ability to cross-link receptors slows internalization. The third construct (VH2-VH1, MW = 29.7 kDa) is identical to VH2-VH1-HLE except without the HLE domain to compare the impact of different clearance rates. All Humabody constructs were site-specifically conjugated at the C-terminus with either a potent DNA-alkylating agent (DGN549, an indolinobenzodiazepine DNA-alkylating monoimine29) for potency/efficacy studies or a fluorescent dye for in vitro and in vivo kinetics and distribution experiments. The fluorescent antibody constructs (also referred to as fluorescent antibodies in this paper) were used to determine the overall tumor uptake (%ID/g), tumor tissue distribution, in vitro spheroid penetration, and in vivo single-cell pharmacokinetics for comparison with the in vitro and in vivo efficacy. Combined with a computational model, these results demonstrate that smaller monovalent protein scaffolds can increase tissue penetration enhancing in vivo efficacy in mouse models of prostate cancer.
Materials and Methods
Computational model – Krogh Cylinder
The Krogh cylinder model has been validated for tissue distribution of antibodies as well as smaller molecules by our group and others3, 9, 30–32. A 1-D cylinder model consisting of only radial gradients was used to represent the distribution of antibody constructs because they are permeability limited. The model tracked the concentration of free target as well as free, bound, and internalized antibody. Equations and parameters used for simulations are located in the Supplementary Material Table S1. The equations describe the rate of antibody extravasation from a blood vessel, the diffusion and subsequent binding to a PSMA receptor in the tissue, at which point the therapeutic is internalized and degraded. Modifications were made to parameters based on the size of the single-domain antibody tested. All parameters used in the Krogh cylinder were gathered from the literature or measured independently (i.e. not fit to tissue distribution data).
Single-Domain Antibody Constructs and Imaging Agents
Both fluorophore and drug (supplemental Methods) conjugation reactions occurred at the C-terminus of each single-domain antibody. Alexa Fluor 680 (AF680), Alexa Fluor 647 (AF647), or Alexa Fluor 488 (AF488) (ThermoFisher Scientific) were conjugated to each antibody through sortase labeling of the LPTGX motif present at the C-terminus. Briefly, NHS ester dyes were reacted with propargyl amine in aqueous solution buffered with 7.5% sodium bicarbonate for 30 minutes at room temperature, followed by purification on reverse-phase HPLC. The alkyne-dye was then reacted with a GGGX peptide (synthesized on a CEM Liberty Blue peptide synthesizer), where X is the non-natural amino acid azidohomoalanine, via copper-catalyzed click chemistry similar to previously published protocols33, 34 to generate GGGX-dye. The peptide-dye product was purified on reverse-phase HPLC and reacted with the antibody constructs (5:1 ratio) for 10 minutes with 2μM sortase and 5mM calcium chloride in aqueous HEPES buffer. The reaction mix was filtered with a Costar® spin-x centrifuge tube filter (ThermoFisher Scientific) containing 300μL of HisPur™ Ni-NTA Resin (ThermoFisher Scientific) to remove unreacted protein (containing a His-tag) and the His-tagged sortase and then purified via size exclusion chromatography/FPLC. The fluorescent constructs were concentrated with AMICON 10kDa molecular weight spin filters to 10–40μM with a degree-of-labeling (DoL) ~0.7 dyes/protein as confirmed with absorption readings from a NanoDrop 1000 spectrophotometer (ThermoFisher Scientific) (Fig S1). After purification, Native-PAGE gels were scanned on an Odyssey CLx to ensure all free dye was removed (Fig S2). Drug conjugates were purified and characterized via Q-ToF Mass spectrometry (Fig S3).
Cell Culture and Animals
The DU145 cell line (RRID: CVCL_0105, PSMA negative, certified by ATCC via STR analysis) and PSMA transfected DU145 cell line generated by Kampmeier et al. (DU145-PSMA)35 were received from Crescendo Biologics. The transfected cell line was confirmed for PSMA expression but the transfected cell line and CWR22Rv1 lines were not re-authenticated. Cells were cultured 2–3 times per week up to passage number of 50 and grown in RPMI 1640 supplemented with 10% (v/v) FBS, 50 U/mL penicillin, and 50 μg/mL streptomycin at 37°C with 5% CO2. Mycoplasma testing was performed annually using the Mycoalert Testing Kit (Thermo Fisher Scientific, NC9719283). All animal studies were approved and conducted in compliance with the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan and Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Pharmacokinetic and in vivo tumor distribution animal studies were conducted in 4–8 week old homozygous female nude (RRID: 2175030, Foxn1nu/nu, Jackson Laboratories) mice. For in vivo tumor distribution and growth studies, the nude mice were inoculated in both flanks with 5 × 106 cells in Matrigel (Fisher Scientific, CB40234A), one flank with DU145-PSMA and the other with DU145 cells. For tumor growth studies, tumor volume was measured with calipers 3x per week using the formula volume = 0.5*length*width2. Corrected sample size for each treatment group was calculated as 10 based on an a priori power test (details in supplemental).
Plasma Clearance
Plasma clearance was measured after tail-vein injection of 0.7 nmol of fluorescent antibody (or 0.067 nmol of J591 anti-PSMA IgG). Plasma samples were obtained through retro-orbital sampling 10 μL of whole blood, then mixed with 15 μL of PBS-EDTA (10 mM) and centrifuged at 3000g for 1 min. 18 μL of the resulting plasma was frozen at −80°C until further analysis. The antibody concentration was determined by scanning 15 μL of plasma in a 384-well black-walled plate (Corning) on the NIR Odyssey CLx Scanner (LI-COR) and comparing the signal intensity to a calibration curve of known antibody concentration to signal intensity at the same DoL and scan settings. The plasma concentration at each time point was normalized to the initial concentration, and then the clearance was fit to a biexponential decay using PRISM (GraphPad). Absolute plasma concentrations at 1 min were compared with theoretical initial concentrations calculated based on the dose and estimated plasma volume of the mouse.
Biodistribution
The biodistribution of fluorescent antibody was conducted as previously described7, 36, 37 with a single bolus dose delivered for each antibody. Briefly, 24 h after tail-vein injection of 0.7 nmol of fluorescent antibody (or 0.067 nmol of J591 anti-PSMA IgG), animals were euthanized, and organs were resected. Organs were then homogenized by mechanical disruption, incubated with 1:1 RIPA buffer (Fisher Scientific)/PBS solution supplemented with 5 mg/mL collagenase IV (Fisher Scientific) for 1.5 h, disrupted using a FB-120 Sonic Dismembrator, and incubated in 1:1 RIPA buffer/0.025% trypsin-EDTA solution for 1.5 h. After homogenization, organs were serially diluted and scanned on the Odyssey CLx scanner to ensure fluorescence detection was in the linear range. The signal intensity was compared to a calibration curve and normalized to organ weight and homogenate volume to calculate the percent injected-dose per gram (%ID/g).
Fluorescence Histology for Imaging Antibody Tumor Distribution
As previously described7, 32, 36, the tumor distribution of fluorescent antibody was analyzed using fluorescence microscopy 24 hours post-injection. Briefly, 0.7 nmol fluorescent antibody (or 0.067 nmol of J591 anti-PSMA IgG) was administered via tail-vein injection once the tumor volume was ~250mm3. The animal was imaged 24 hours post-injection. Hoechst 33342 (ThermoFisher Scientific) was administered 15 minutes before euthanasia via the tail-vein at 15 mg/kg to label functional vasculature in the tumor. Tumors were then resected, flash frozen in OCT using isopentane chilled on dry ice, and sectioned for histology on a cryostat (10-μm slices). Before imaging, tumor slices were stained for 30 min with anti-mouse CD31 (BioLegend, 102402) conjugated to Alexa Fluor 555. Microscopy was performed using an upright Olympus FV1200 confocal microscope equipped with a 20× objective and 405, 543, and 635 lasers. High resolution tumor images were obtained by stitching smaller images with the Olympus software. Images were exported and analyzed using ImageJ image analysis software as described previously7, 32, 36.
Flow Cytometry
Resected tumors were digested into a single-cell suspension using a tumor dissociation kit (Miltenyi Biotech, 130–096-730) and passed through a 40 μm filter to remove clumped cells. The suspension was washed 2x with PBS and then analyzed using an Attune Acoustic Focusing Cytometer (Life Technologies). Quantitative fluorescent beads (Quantum™ Simply Cellular® anti-Mouse IgG) were used to convert fluorescent intensity units from flow experiments to number of proteins and later to number of payloads delivered based on DoL or DAR.
In vitro Toxicity Assay
Antibody constructs with a C-terminal cysteine residue were conjugated to a potent DNA-alkylating agent (DGN549) via maleimide chemistry similar to previous reports29, in a reaction buffer containing 25% N,N-Dimethylacetamide and 75% 50mM potassium phosphate-5mM EDTA, pH 6.0 for 3 hours at 25°C and then purified by Illustra NAP-25 columns (GE Healthcare) to achieve an average DAR of ~1.0. The free DGN549 payload has an IC50 = 20 pM +/− 15 pM in DU145 cells. The potency of the ADC constructs was then assessed by seeding DU145-PSMA cells at a density of 8,000 cells per well in 96-well plates for cell viability assays. Titrations of DGN549-conjugated antibodies were replaced daily for 6 days. At the endpoint, cells were washed twice with media and then incubated with PrestoBlue Cell Viability Reagent (ThermoFisher Scientific, A13261) for 25 minutes at 37°C in a 1:10 dilution in media. The fluorescence (560/590, Ex/Em) of each well was then measured using a Biotek Synergy plate reader to measure final cell viability. Background signal from wells without cells was subtracted from all samples, and then, viability was normalized to untreated cells.
In vivo Tumor Growth Curves
For the high expression DU145-PSMA xenografts, female homozygous Foxn1 nude mice (Jackson Laboratories, 002019) were injected in the left flank with 5 × 106 DU145-PSMA cells. Mice were assigned into six treatment groups: PBS vehicle control (n=9), non-binding control VH-HLE-DGN549 (n=6), VH1-HLE-DGN549 (n=9), VH2-VH1-HLE-DGN549 (n=10), VH2-VH1-DGN549 (n=9), and J591-DGN549 (n=9). Treatments were administered in a single dose as tumors reached an approximate volume of 250 mm3. Doses were matched to the amount of payload delivered for the maximum tolerated dose (90 μg/kg for Humabody drug conjugates, 30 μg/kg for J591). However, these doses all caused complete responses in CWRr22Rv1 tumors (Fig S4), so all the doses were decreased 3-fold to 30 μg/kg for Humabody drug conjugates, 10 μg/kg for J591. The J591 ADC was given at a 3-fold lower payload dose to match the toxicity between the agents as estimated from weight loss (Fig S5). In general, the Humabody drug conjugates were better tolerated, enabling a higher payload dose. Tumor sizes were monitored three times per week until the study endpoint.
Similarly, for the moderate expression CWR22Rv1 xenografts, male CB.17 SCID mice were implanted subcutaneously on one flank with 1 × 107 CWR22Rv1 human prostate carcinoma cells. After 20 days, animals with individual tumor xenograft volumes of 75 to 126 mm3 were sorted into groups (n =10) with a group mean tumor volume of 106 mm3 and dosing was initiated. Half-life extended (HLE) Humabody drug conjugates were administered in a single dose on day 1 of the study. Non-HLE Humabody drug conjugate was administered in three, 10 μg/kg doses, administered on day 1, 3, & 5 of the study (30 μg/kg cumulative dose). Tumor size in mm3 was monitored individually and a group median tumor volume calculated. Animals reaching the endpoint volume of 1000 mm3 or the end of the study were euthanized.
Tumor Spheroid Experiments
Tumor spheroids were cultured using custom 384-well plates described previously38. Briefly, 3000 cells suspended in 25μL of seeding media comprising 19.5μL complete RPMI culture media, 5μL of 1.2% (w/v) methocellulose (Dow Corning), and 0.5μL matrigel were added to alternate wells of the 384-well hanging drop plate. The edges of the 384-well plate were lined with sterile gauze soaked in sterile distilled water (Gibco) containing 0.1% penicillin/streptomycin. The 384-well plate was sandwiched in a 96-well clear well plate containing sterile water to minimize media evaporation from the hanging drops. Media changes were made every 2 days by removing 9μL media from the drop and adding in 10μL of fresh media (to adjust for evaporation), repeated twice for each drop. Spheroids were cultured for 7 days until they attained a diameter of 400–500μm.
To study distribution, the hanging drops were incubated in the 384-well plate with VH1-HLE-AF680, VH2-VH1-HLE-AF680, VH2-VH1-AF680, or J591-AF680 at a final concentration of 30nM, by replacing 10μL of the 25μL of the spheroid media with 10μL of 75nM (2.5X concentration) fluorescent antibody. The drug concentration in the media was assumed to be constant over the course of the incubation (i.e. no depletion effects). After 24 hours of incubation, the spheroids were individually extracted from the wells and washed 2X with PBS before being fixed with 4% formaldehyde, frozen in OCT, and stored at −80°C until further processing. Frozen OCT blocks were sectioned for histology on a cryostat (16-μm slices), and sections were stained ex-vivo with Hoechst 33342 (ThermoFisher Scientific) for 5min and J591-AF555 for 30min before imaging on the Olympus FV1200 confocal microscope. Multi-channel imaging of spheroid sections was performed with a 20X objective with 405 (Hoechst-33342), 543 (AF555), and 635 (AF680) lasers. Image analysis was performed to generate a Euclidean distance map (Fig S6).
Statistical Analysis
Plot values are shown as mean +/− standard deviation. Data were analyzed for statistical significance (p values <0.05) with an unpaired, two-tailed t-test with Welch’s correction using GraphPad Prism 8 for macOS.
Results
In vitro and in vivo efficacy of antibody drug conjugates
The in vitro toxicity of DU145-PSMA cells was measured after 6 days using a PrestoBlue viability assay (Fig S7). VH1-HLE-DGN549 had the lowest potency, followed by both VH2-VH1-HLE-DGN549 and VH2-VH1-DGN549. The J591-DGN549 ADC had the highest potency based on protein concentration, likely due to the multiple payloads per antibody. Potency based on payload concentration was similar for VH2-VH1-DGN549, VH2-VH1-HLE-DGN549, and J591-DGN549 (Fig S7B). These results are consistent with the internalization rates for these ADC constructs, with the biparatopic Humabodies (VH2-VH1-HLE-AF680 and VH2-VH1-AF680) capable of inducing surface-crosslinking and driving internalization39 (Fig S8), the J591 ADC inducing internalization40, and VH1-HLE having the slowest cellular uptake.
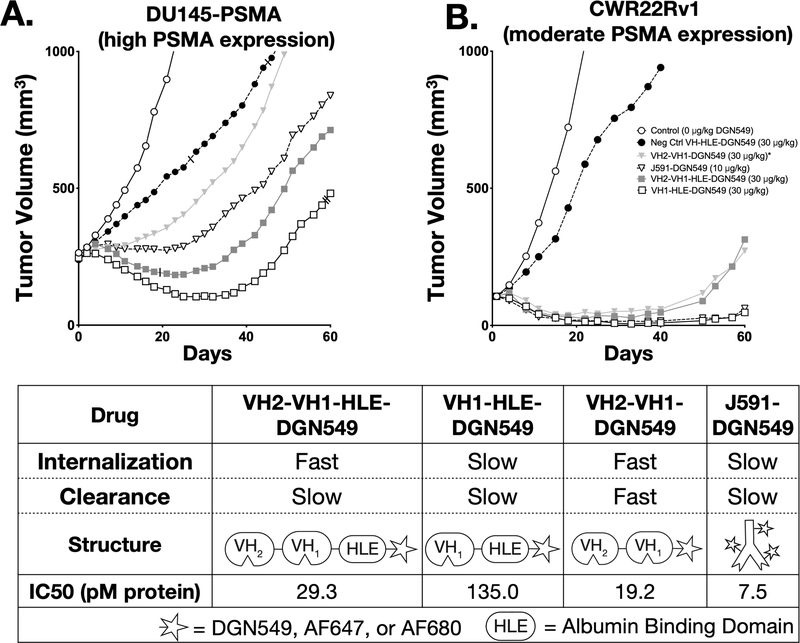
Tumor growth studies done in athymic nude or SCID mice with PSMA-expressing xenografts showed significant growth inhibition for all PSMA-targeted ADCs relative to untargeted controls. In the xenograft model with higher PSMA expression (DU145-PSMA), VH1-HLE-DGN549 shows the greatest efficacy among all experimental groups (p<0.05, Fig S9). VH2-VH1-HLE-DGN549 showed better efficacy than VH2-VH1-DGN549 given in a single bolus dose as a result of VH2-VH1-HLE’s prolonged circulation and tumor exposure. The albumin-binding VH domain (HLE) presumably reduced renal filtration to slow clearance (KD = 51.9 nM for mouse albumin with koff = 3.41×10−2/s and kon = 6.57×105/M*s as measured by surface plasmon resonance). Individual DU145-PSMA growth curves for all mice in each treatment group are displayed in Supplementary Fig S10. Overall, tumor growth inhibition was generally lower in the DU145-PSMA tumors compared to CWR22Rv1 xenografts, likely due a combination of the larger starting tumor size (250 vs. 100 mm3) which can reduce vascular density41 and higher PSMA expression resulting in greater heterogeneity in intratumoral distribution. In CWR22Rv1 tumors, VH1-HLE-DGN549 and J591-DGN549 were equally effective. Fractionated dosing of the rapidly cleared VH2-VH1-DGN549 in the CWR22Rv1 model to mitigate rapid renal clearance resulted in similar efficacy as the more slowly cleared VH2-VH1-HLE-DGN549, further highlighting the benefit of prolonged exposure. In contrast to the observed trend for in vitro potency of the Humabody drug conjugates, VH1-HLE-DGN549 had surprisingly higher efficacy than either VH2-VH1-HLE-DGN549 or VH2-VH1-DGN549 in both tumor models (Fig 1A&B). To better understand this effect, a detailed study of the distribution at the systemic, organ, tissue, and cellular level was initiated using near-infrared fluorescence to delineate the contribution of the multiple ADC delivery and processing steps in vivo towards efficacy.
Fig 1. In Vitro and In Vivo Efficacy of Antibody Drug Conjugates.
The ADCs were used in two separate tumor inhibition studies involving nude mice with either a high expression DU145-PSMA xenograft (A) or a moderate expressing CWR22Rv1 xenograft (B). The table displays relative internalization and clearance rates alongside ADC structures (all conjugations occurred at the C-terminus) and the in vitro potencies (C). The IC50 for monovalent and biparatopic antibody conjugates with and without an albumin binding ‘Half-Life Extension’ (HLE) were determined in DU145-PSMA cells. *VH2-VH1-DGN549 was dosed every other day for three total doses at a 10μg/kg DGN549 per dose for the CWR22Rv1 study and as a single bolus dose of 30 μg/kg DGN5459 in the DU145-PSMA study. Slashes mark mice removed early from the study (see Supplementary Methods).
VH1-HLE, VH2-VH1-HLE, and J591 have high tumor uptake relative to VH2-VH1
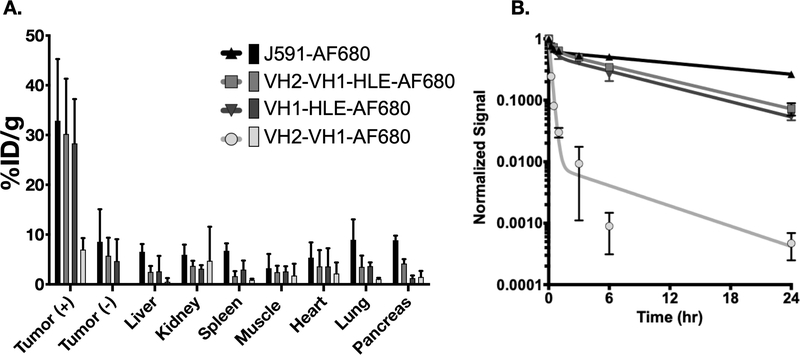
To study distribution, fluorescent antibodies were injected into nude mice bearing antigen positive DU145-PSMA cells in the left flank and DU145 (PSMA negative) cells in the right flank. (The clearance of the fluorescent antibodies was similar to non-fluorescent antibody (Fig S11) and ADCs fluorescently labeled via lysine side-chains (Fig S12). The slowly cleared VH1-HLE-AF680, VH2-VH1-HLE-AF680, and J591-AF680 antibody had similarly high tumor uptake in the antigen positive tumor as expected (~30%ID/g) with no statistically significant differences, while the more rapidly cleared VH2-VH1-AF680, had much lower uptake (~7% ID/g), Fig 2A. Rapid plasma clearance of VH2-VH1-AF680 also resulted in lower uptake in the antigen negative tumor and all other organs compared to VH1-HLE-AF680, VH2-VH1-HLE-AF680, and J591-AF680 (Fig 2B). Because the tumor uptake, plasma clearance, and in vitro toxicity did not sufficiently explain the trends in tumor growth inhibition, further studies on the intratumoral distribution of these agents were performed.
Fig 2. Biodistribution and Plasma Clearance of Alexa Fluor 680 Antibody Constructs.
The ADC biodistribution %ID/g (A) and Plasma clearance normalized signal (B) are displayed as a mean value with error bars for each conjugate representing standard deviation. Sample sizes were as follows: VH2-VH1-HLE-AF680 (n=5), VH1-HLE-AF680 (n=5), VH2-VH1-AF680 (n=3), and J591-AF680 (n=3).
Computational simulation and in vitro tumor spheroid distribution highlights improved penetration of VH1-HLE
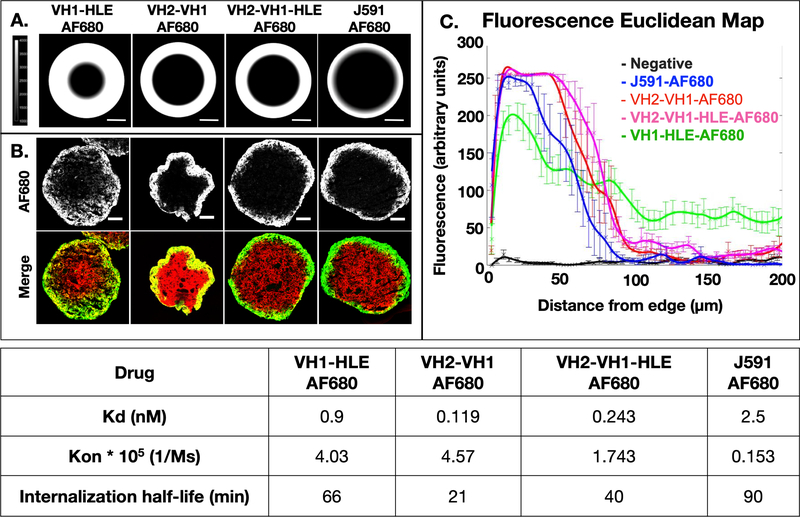
Previous work in the literature indicates that smaller protein scaffolds result in higher tissue penetration (e.g. 15, 17, 19, 42). We adapted the Krogh cylinder model for a spherical geometry and, after gathering parameters on binding kinetics, plasma clearance, and receptor expression, conducted simulations of tissue and spheroid distribution after 24 hours as depicted in Fig 3A. The computational model predicted the smaller VH1-HLE would exhibit the greatest tumor penetration, and that the rapidly internalizing VH2-VH1 and VH2-VH1-HLE would only target a few cell layers into the spheroid, similar to the J591 antibody. Notably, this model assumes that the specific, reversible albumin binding of HLE does not reduce the diffusion into tissue in contrast to non-specific albumin sticking from lipophilic agents43.
Fig 3. Computational and Experimental Tissue Penetration of Fluorescent Antibody Constructs.
Simulations based on the molecular weight, binding kinetics, affinity, and internalization rate predict penetration depths for antibody constructs (A). Tumor spheroids incubated with the Alexa Fluor 680 antibody constructs represent experimental penetration depths for each construct in 50% mouse serum while ex vivo staining of PSMA (red) displays available antigen (B). These images were analyzed with a Euclidean map to semi-quantitatively depict penetration depths (C, n = 6 – 13 spheroids per group). The table displays binding affinity, on rates and the net internalization rate for these antibody constructs (which accounts for trafficking effects such as recycling and down-regulation).
These predictions were validated in vitro using spheroids made up of DU145-PSMA cells. Histology images of the spheroids are shown in Fig 3B. Ex vivo staining of the tissue slides indicated uniform PSMA expression throughout the spheroids (red), and incubation with antigen negative DU145 spheroids showed rapid and uniform distribution (which was only detectable at high concentrations due to a lack of binding, Fig S13). The absolute penetration distances were dependent on the cell packing, with spheroids seeded at lower cell density (2000 vs. 3000) and grown for longer periods of time (14 days vs. 7 days) resulting in lower penetration (Fig S14), likely due to higher cell density/lower void fraction, but the relative pattern of penetration remained consistent. These results were maintained across ~10 independent experiments (each done on separate days) containing ~25 spheroids per fluorescent antibody (Fig S15). Quantitative image analysis confirmed the higher penetration of VH1-HLE-AF680 relative to the other fluorescent antibodies (Fig 3C). The experimental data complements the computational predictions as VH1-HLE-AF680 was demonstrated to distribute farther than any of the other antibodies tested.
The spheroid experiments provided a high-throughput, well-controlled, and robust environment to quantify tissue penetration. To account for additional considerations in vivo, such as plasma clearance and extravasation, tumoral distribution was further analyzed in DU145-PSMA xenografts.
VH1-HLE demonstrates greater tissue penetration in vivo
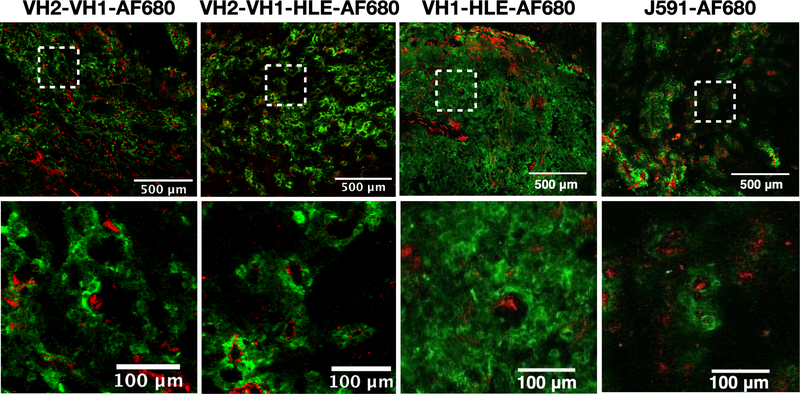
Tumor histology images and whole animal imaging (Fig S16) captured the overall fluorescent antibody distribution following intravenous delivery at the same doses as the efficacy study. Similar trends were seen for in vivo tumor distribution as those predicted by the computational model and demonstrated in the spheroid experiments. VH2-VH1-AF680 and VH2-VH1-HLE-AF680 exhibited limited penetration similar to that of the perivascular distributed J591-AF680 antibody, while the VH1-HLE-AF680 had greater penetration targeting more cells (Fig 4). The J591 antibody did not show any increase in penetration at later times (Fig S17 at 72 hrs), consistent with a pseudo-steady-state between vascular delivery and cellular degradation preventing deeper penetration over time. ADCs labeled non-site specifically with fluorophore showed a similar pattern as the antibodies lacking a payload, indicating the payload was not impacting the tumor distribution (Fig S18).
Fig 4. In Vivo Tissue Penetration of Fluorescent Antibodies.
24 hrs after tail vein administration of Alexa Fluor 680 antibody constructs dosed at the same level as the efficacy studies, DU145-PSMA xenografts were frozen in OCT and processed for histology. Blood vessels, shown in red, were ex vivo labeled with Alexa Fluor 555 anti-CD31 antibody while penetration of Alexa Fluor 680 antibody constructs is shown in green.
A Euclidean distance map of the histology images was compiled to provide a semi-quantitative look at the differences in tumor penetration between fluorescent antibodies (Fig S19) and was consistent with the in vivo computational predictions using the Krogh cylinder model (Fig S20). However, the images do not provide absolute quantification of payload delivery with single-cell resolution that would allow for precise correlations between efficacy and penetration.
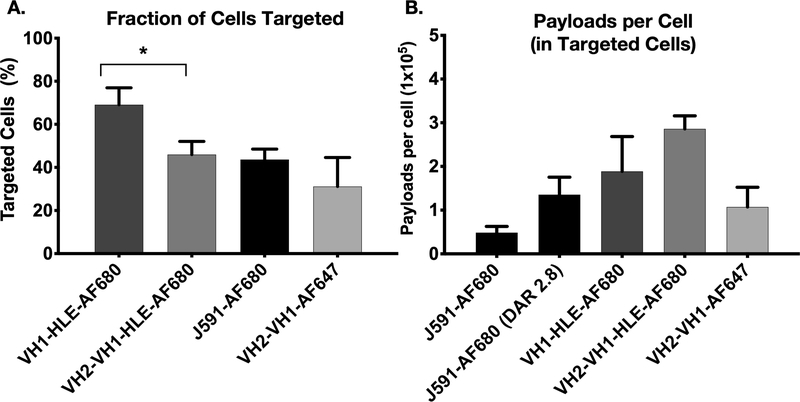
Single-cell data indicates VH1-HLE targets more cells at a lower payload concentration
Flow cytometry can provide absolute measurements of antibody uptake at the single cell level (e.g. Fig. S21, in vitro). The single-cell measurements enabled quantification of the fraction of cells targeted in the tumor and the number of payloads per cell for the targeted fraction (representative histograms Fig S22). Gating on the in vivo histograms, VH1-HLE-AF680 targeted more cells than the other antibodies (p < 0.05) (Fig 5A), consistent with the computational model and histology experiments. This finding is also consistent with in vivo efficacy in the same xenograft, where VH1-HLE-DGN549 showed the most tumor growth inhibition of the ADC constructs tested. While VH1-HLE-AF680 targeted the largest fraction of cells, the number of payloads per cell in these targeted cells was lower than VH2-VH1-HLE-AF680 (but not statistically significant due to high variability, Fig. 5B), which explains the similar tumor-averaged total uptake (%ID/g). Using an AF647 tag (and its corresponding calibration curve) for higher sensitivity due to lower total uptake, we found that VH2-VH1-AF647 reached a slightly lower fraction of targeted cells, and the number of payloads per cell was ~1/3 compared to VH2-VH1-HLE-AF680 ), resulting in the much lower overall %ID/g in Fig. 2. Note that the J591-AF680 antibody was administered at a lower payload dose, resulting in fewer payloads per cell even though it has a similar uptake efficiency (~30 %ID/g).
Fig 5. Single-Cell Payload Measurements.
A fraction of the tumor resected 24 hrs after antibody fluorophore conjugate administration was processed into a single cell suspension and used with flow cytometry to determine conjugate distribution and payload uptake. J591-AF680 antibody was given at a dose of ~0.07nmols (with a DAR of 2.8, so the equivalent payload uptake is shown) while Humabodies were dosed at ~0.7nmols. Targeted cells (A) and payloads per cell (B) are represented as median values with standard deviation error bars. Payload quantification involved the single cell analysis of three separately treated and dissociated tumors for each treatment. * = p < 0.05
Discussion
Following the first wave of ADC approvals, various protein-drug conjugates are being developed based on affinity, toxicity, and stability to achieve an optimal therapeutic effect. Although the heterogeneous, perivascular distribution of these agents is well documented9, 44–47, the impact on efficacy is not routinely isolated and investigated. Here, we demonstrated the improved penetration of a single variable heavy chain unit conjugated to an albumin binding domain (VH1-HLE-AF680) over other ADC constructs in a 3D spheroid cell culture system and in vivo. As a result, VH1-HLE-DGN549 exhibited higher in vivo efficacy than the other ADC constructs despite having lower potency in vitro.
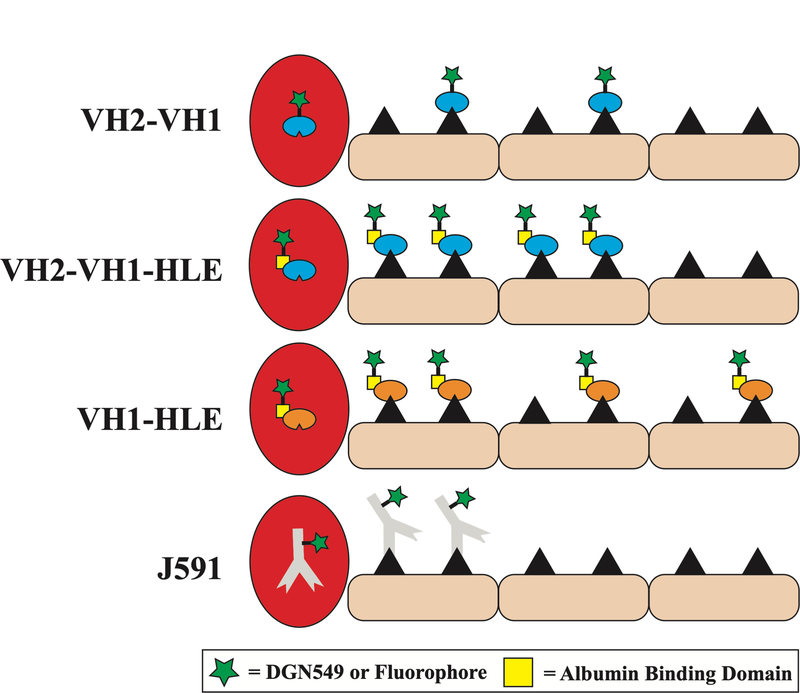
In vitro cellular toxicity assays indicated that the rapidly internalized biparatopic antibody conjugates (VH2-VH1-HLE, VH2-VH1) and J591 had the highest potency, followed by VH1-HLE (Fig S7.). The greater in vitro potency of the biparatopic ADC constructs is likely due to receptor surface clustering that causes rapid internalization of the drug39, 48, 49. In monolayer cell culture, there are no mass transport/drug delivery limitations, so cellular internalization/payload release is the rate-limiting step in payload uptake and the key determinant for cell killing. However, in vivo, the extravasation of ADC from the blood is the rate-limiting step and determines total tumor uptake (at sub-saturating doses, which is typical for highly expressed antigens). Therefore, the faster internalization rate (relative to binding kinetics and diffusion) did not deliver more total payload to the tumor, as seen by the similar tumor biodistribution for VH1-HLE-AF680 and VH2-VH1-HLE-AF680 (Fig. 2). The rapid internalization instead limited the penetration distance of VH2-VH1-AF680 and VH2-VH1-HLE-AF680 in tumor spheroids (Fig 3) and in vivo (Fig 4), which therefore reached fewer cells (Fig 5). Although the amount of VH1-HLE-AF680 and VH2-VH1-HLE-AF680 delivered to the tumor was the same, the slower internalizing VH1-HLE-AF680 was able to penetrate farther in the tumor to target and kill more cells than VH2-VH1-HLE-AF680 as illustrated by the diagram in Fig 6.
Fig 6.
Conceptual schematic of the distribution for the different single-domain and monoclonal antibodies.
One of the main disadvantages of using single-domain antibodies or other small protein scaffolds in place of antibodies is rapid renal clearance23. VH2-VH1-AF680 demonstrated poor tumor uptake caused by this rapid kidney filtration. Conjugating an albumin binding domain to the monovalent and biparatopic constructs slowed clearance nearly 100-fold, leading to increased tumor uptake, comparable to a monoclonal antibody (Fig 2).
Interestingly, some of the in vivo efficacy trends differed between the moderate and high expressing xenografts (Fig 1). In both models, the VH1-HLE-DGN549 demonstrated the greatest efficacy, but the relative efficacy of the other agents varied between the two tumor models. In the CWR22Rv1 tumor model with lower expression, VH1-HLE-DGN549 and the J591-DGN549 ADC showed almost identical tumor growth inhibition, as did VH2-VH1-HLE-DGN549 and VH2-VH1-DGN549. But in the DU145-PSMA tumor model, each protein-drug conjugate exhibited distinct efficacy. The most likely explanation for differences in efficacy between the DU145-PSMA and CWR22Rv1 tumor models is the level of PSMA expression: DU145 has relatively high expression (~106 receptors/cell), while CWR22Rv1 has moderate expression (< 105 receptors/cell). With more available targets for binding in a higher expression cell line, more ADC is required to saturate the first few cell layers, thus hindering diffusion to regions distal from vasculature. Cell death often correlates with intracellular concentration50, and VH1-HLE-DGN549 maintained efficacy (even exceeding VH2-VH1-HLE-DGN549 in vivo, Fig 1) with a lower (but still lethal) number of payloads per cell than VH2-VH1-HLE-DGN549 based on in vitro measurements (Fig 5, Fig S21). This is consistent with VH2-VH1-HLE-DGN549 delivering more of the potent DGN549 payloads than needed to kill targeted cells (i.e. overkill) in this model. Another contributing factor for the differences in degree of response between tumor models could be the starting tumor size. Treatments were generally less efficacious in the DU145-PSMA tumor model, where doses were administered at an average tumor size of 250 mm3, versus 100 mm3 in the CWR22Rv1 tumor model.
The slower clearance due to the presence of a HLE domain (i.e. higher plasma AUC) was expected to increase efficacy in the mouse model of prostate cancer based on greater drug exposure. Previous studies have demonstrated that exposure (i.e. plasma AUC) correlates with tumor uptake and response to ADCs (e.g. 51, 52). This is supported by the improved in vivo efficacy of VH2-VH1-HLE-DGN549 compared to VH2-VH1-DGN549 (Fig 1) in the DU145-PSMA tumor model (single dose each). The plasma clearance and biodistribution data confirmed that VH2-VH1-HLE-AF680 is cleared more slowly and has significantly higher tumor uptake than VH2-VH1-AF680 (Fig 2). According to single-cell measurements, VH2-VH1-HLE-AF680 targeted a higher number of cells than VH2-VH1-AF647 with more payloads per cell in each targeted cell (Fig 5). Alternatively, VH2-VH1-HLE-DGN549 and VH2-VH1-DGN549 showed almost identical efficacy in the CWR22Rv1 tumor model with lower PSMA expression where VH2-VH1-DGN549 was administered in three fractionated doses of 10 μg/kg. Fractionated dosing of the faster cleared VH2-VH1-DGN549 increased tumor exposure time compared to a single bolus dose. The similar efficacy of VH2-VH1-HLE-DGN549 and VH2-VH1-DGN549 in this system further highlights the significance of extended tumor exposure to in vivo efficacy.
Taken together, the differences seen with the in vivo efficacy studies cannot be explained solely by total tumor uptake or in vitro potency, given that VH1-HLE outperformed VH2-VH1-HLE and J591 in vivo with similar tumor uptake (Fig 2) and lower in vitro potency (Fig 1). The best correlate for efficacy was the delivery of a lethal cellular dose of payload to the maximum fraction of cells within the tumor. The in vitro spheroid and in vivo histology images agreed with a predictive computational model based on the fundamental binding kinetics, tumor physiology, target specific expression/internalization, and biophysical properties (e.g. diffusion) of the ADCs.
These results can be used to help design more effective ADCs for efficient payload delivery and cell killing. Importantly, while this work demonstrates that the VH1-HLE-DGN549 is the most effective in this system, it does not indicate that monovalent binding agents are always the most efficacious agents in vivo. Rather, the work highlights the need to customize the construct based on the target properties (expression, internalization), payload (with or without bystander effects, maximum tolerated dose), and properly scale these results to the clinic (i.e. clearance rates). Likewise, the PSMA expression in these animal models was moderate to high, and the payload used is extremely potent. Under these conditions, the constitutive internalization rate of PSMA was sufficient for tumor cell killing in these animal models without the need to drive more rapid internalization through biparatopic clustering. Many current ADCs demonstrating therapeutic response in late-stage clinical trials have high expression levels (sacituzumab govitecan, trastuzumab deruxtecan, mirvetuximab soravtansine, and enfortumab vedotin)53 and may benefit from methods that improve penetration to reach more cells at a lower, yet lethal payload dose. However, driving faster internalization could be very useful under circumstances with lower expression or slower constitutive turnover. In fact, it raises the intriguing possibility of driving internalization of targets that are internalized too slowly for efficient payload delivery, which are generally considered ‘non-internalizing’. Although rapid internalization was not beneficial for this PSMA model, biparatopic clustering in theory could also be used to overcome resistance mechanisms due to poor cellular internalization/trafficking49. These data could be compared with truly ‘non-internalizing’ ADCs that release their payload outside the cell54, 55.
In summary, single-domain antibodies provide a promising platform for controlling the internalization kinetics, binding affinity, size, and plasma clearance of these ADC constructs. While the use of a variable heavy chain binding domain provides improved transport characteristics (e.g. blood vessel permeability, diffusion), rapid clearance from the blood through the kidneys results in short plasma half-lives that limit therapeutic potential. The addition of a small albumin binding domain improved plasma half-lives without significantly impacting the extravasation and tissue penetration of the agents, allowing ‘antibody-like’ targeting efficiencies. The investigation of four ADC constructs (VH1-HLE, VH2-VH1, VH2-VH1-HLE, and J591) showed markedly different in vitro potencies, tissue penetration, plasma clearance, and efficacy. Interestingly, neither plasma clearance/AUC nor in vitro potency correlated with tumor response. The systemic, organ, tissue, and single-cell pharmacokinetic data indicated that the ability to reach a maximum number of cells with a lethal payload dose was the driving factor in efficacy. These results were corroborated by a computational Krogh cylinder model. This combination of computational modeling and experimental quantitative pharmacology can be used to scale these results to other targeting systems and the clinic for more efficient design of highly efficacious agents.
Supplementary Material
Statement of Significance.
A mechanistic study of protein-drug conjugates demonstrates that a lower potency compound is more effective in vivo than other agents with equal tumor uptake due to improved tissue penetration and cellular distribution.
Acknowledgements
We would like to thank Dr. Shuichi Takayama and lab members for assistance with setting up the tumor spheroids experiments and Dr. Geeta Mehta and lab members for discussion around tumor spheroids processing and quantification. DGN549 was provided by ImmunoGen, Waltham, MA. Funding was provided in part by Takeda Pharmaceutics, Crescendo Biologics, NSF GRFP (IN), and NIH Grant R35 GM128819 (GMT).
Footnotes
Conflict of Interest
SV, TS, JL, LT, and NG were employed by Crescendo, QQ and TAK were employed by Immunogen, and AA was employed by Takeda during the study. GMT sits on the Scientific Advisory Board of Advanced Proteome Therapeutics.
References
- 1.Polakis P. Antibody Drug Conjugates for Cancer Therapy. Pharmacological Reviews 68, 3–19 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Diamantis N. & Banerji U. Antibody-drug conjugates-an emerging class of cancer treatment. British Journal of Cancer 114, 362–367 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasalou C, Helmlinger G. & Gomes B. A Mechanistic Tumor Penetration Model to Guide Antibody Drug Conjugate Design. Plos One 10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter LT & Jain RK TRANSPORT OF FLUID AND MACROMOLECULES IN TUMORS .4. A MICROSCOPIC MODEL OF THE PERIVASCULAR DISTRIBUTION. Microvascular Research 41, 252–272 (1991). [DOI] [PubMed] [Google Scholar]
- 5.Lambert JM & Chari RVJ Ado-trastuzumab Emtansine (T-DM1): An Antibody-Drug Conjugate (ADC) for HER2-Positive Breast Cancer. Journal of Medicinal Chemistry 57, 6949–6964 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Cilliers C, Menezes B, Nessler I, Linderman J. & Thurber GM Improved Tumor Penetration and Single-Cell Targeting of Antibody-Drug Conjugates Increases Anticancer Efficacy and Host Survival. Cancer Research 78, 758–768 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cilliers C, Guo H, Liao JS, Christodolu N. & Thurber GM Multiscale Modeling of Antibody-Drug Conjugates: Connecting Tissue and Cellular Distribution to Whole Animal Pharmacokinetics and Potential Implications for Efficacy. Aaps J. 18, 1117–1130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khera E, Cilliers C, Bhatnagar S. & Thurber GM Computational transport analysis of antibody-drug conjugate bystander effects and payload tumoral distribution: implications for therapy. Molecular Systems Design & Engineering 3, 73–88 (2018). [Google Scholar]
- 9.Thurber GM, Schmidt MM & Wittrup KD Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev 60, 1421–1434 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsumura R. et al. Influence of the dissociation rate constant on the intra-tumor distribution of antibody-drug conjugate against tissue factor. Journal of Controlled Release 284, 49–56 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Wu AM & Senter PD Arming antibodies: prospects and challenges for immunoconjugates. Nature Biotechnology 23, 1137–1146 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Stern LA, Case BA & Hackel BJ Alternative non-antibody protein scaffolds for molecular imaging of cancer. Current Opinion in Chemical Engineering 2, 425–432 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidle UH, Auer J, Brinkmann U, Georges G. & Tiefenthaler G. The Emerging Role of New Protein Scaffold-based Agents for Treatment of Cancer. Cancer Genomics & Proteomics 10, 155–168 (2013). [PubMed] [Google Scholar]
- 14.Simon M, Frey R, Zangemeister-Wittke U. & Pluckthun A. Orthogonal Assembly of a Designed Ankyrin Repeat Protein-Cytotoxin Conjugate with a Clickable Serum Albumin Module for Half-Life Extension. Bioconjugate Chemistry 24, 1955–1966 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Thurber GM & Wittrup KD Quantitative spatiotemporal analysis of antibody fragment diffusion and endocytic consumption in tumor spheroids. Cancer Research 68, 3334–3341 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudnick SI et al. Influence of Affinity and Antigen Internalization on the Uptake and Penetration of Anti-HER2 Antibodies in Solid Tumors. Cancer Research 71, 2250–2259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokota T, Milenic D, Whitlow M. & Schlom J. Rapid Tumor Penetration of a Single-Chain Fv and Comparison with Other Immunoglobulin Forms. Cancer Research 52, 3402–3408 (1992). [PubMed] [Google Scholar]
- 18.Less JR et al. INTERSTITIAL HYPERTENSION IN HUMAN TUMORS .4. INTERSTITIAL HYPERTENSION IN HUMAN BREAST AND COLORECTAL TUMORS. Cancer Research 52, 6371–6374 (1992). [PubMed] [Google Scholar]
- 19.Bannas P, Hambach J. & Koch-Nolte F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics. Frontiers in Immunology 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurber GM, Zajic SC & Wittrup KD Theoretic criteria for antibody penetration into solid tumors and micrometastases. Journal of Nuclear Medicine 48, 995–999 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Schmidt MM & Wittrup KD A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Molecular Cancer Therapeutics 8, 2861–2871 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoefman S, Ottevaere I, Baumeister J & Sargentini-Maier ML. Pre-Clinical Intravenous Serum Pharmacokinetics of Albumin Binding and Non-Half-Life Extended Nanobodies (R). Antibodies 4, 141–156 (2015). [Google Scholar]
- 23.Tijink BM et al. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: taking advantage of modular Nanobody technology. Molecular Cancer Therapeutics 7, 2288–2297 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Dennis MS et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Research 67, 254–261 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Dennis MS et al. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. Journal of Biological Chemistry 277, 35035–35043 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Steiner D. et al. Half-life extension using serum albumin-binding DARPin (R) domains. Protein Eng. Des. Sel 30, 583–591 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Spiess C, Zhai QT & Carter PJ Alternative molecular formats and therapeutic applications for bispecific antibodies. Molecular Immunology 67, 95–106 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Teng Y. et al. Diverse human VH antibody fragments with bio-therapeutic properties from the Crescendo Mouse. N Biotechnol 55, 65–76 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Miller ML et al. A DNA-Interacting Payload Designed to Eliminate Cross-Linking Improves the Therapeutic Index of Antibody-Drug Conjugates (ADCs). Mol Cancer Ther 17, 650–660 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Shah DK, Haddish-Berhane N. & Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. Journal of Pharmacokinetics and Pharmacodynamics 39, 643–659 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Fujimori K, Covell DG, Fletcher JE & Weinstein JN A MODELING ANALYSIS OF MONOCLONAL-ANTIBODY PERCOLATION THROUGH TUMORS - A BINDING-SITE BARRIER. Journal of Nuclear Medicine 31, 1191–1198 (1990). [PubMed] [Google Scholar]
- 32.Bhatnagar S, Deschenes E, Liao J, Cilliers C. & Thurber GM Multichannel Imaging to Quantify Four Classes of Pharmacokinetic Distribution in Tumors. Journal of Pharmaceutical Sciences 103, 3276–3286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Navaratna T, Liao JS & Thurber GM Dual-Purpose Linker for Alpha Helix Stabilization and Imaging Agent Conjugation to Glucagon-Like Peptide-1 Receptor Ligands. Bioconjugate Chemistry 26, 329–337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Navaratna T. & Thurber GM A Helix-Stabilizing Linker Improves Subcutaneous Bioavailability of a Helical Peptide Independent of Linker Lipophilicity. Bioconjugate Chemistry 27, 1663–1672 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kampmeier F, Williams JD, Maher J, Mullen GE & Blower PJ Design and preclinical evaluation of a Tc-99m-labelled diabody of mAb J591 for SPECT imaging of prostate-specific membrane antigen (PSMA). Ejnmmi Research 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cilliers C, Nessler I, Christodolu N. & Thurber GM Tracking Antibody Distribution with Near-Infrared Fluorescent Dyes: Impact of Dye Structure and Degree of Labeling on Plasma Clearance. Molecular Pharmaceutics 14, 1623–1633 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L. & Thurber GM Quantitative Impact of Plasma Clearance and Down-regulation on GLP-1 Receptor Molecular Imaging. Molecular Imaging and Biology 18, 79–89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tung YC et al. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 136, 473–478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayor S, Rothberg K. & Maxfield F. Sequestration of GPI-Anchored Proteins in Caveolae Triggered by Cross-Linking. Science 264, 1948–1951 (1994). [DOI] [PubMed] [Google Scholar]
- 40.Liu H. et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res 58, 4055–4060 (1998). [PubMed] [Google Scholar]
- 41.Hilmas DE & Gillette EL Morphometric analyses of the microvasculature of tumors during growth and after x-irradiation. Cancer 33, 103–110 (1974). [DOI] [PubMed] [Google Scholar]
- 42.Dennis MS et al. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. 67, 254–261 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Khera E. et al. Blocking Glucagon Like Peptide-1 Receptors in the Exocrine Pancreas Improves Specificity for Beta Cells in a Mouse Model of Type 1 Diabetes. Journal of nuclear medicine : official publication, Society of Nuclear Medicine (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eary JF et al. SUCCESSFUL IMAGING OF MALIGNANT-MELANOMA WITH TECHNETIUM-99M-LABELED MONOCLONAL-ANTIBODIES. Journal of Nuclear Medicine 30, 25–32 (1989). [PubMed] [Google Scholar]
- 45.Oldham RK et al. MONOCLONAL-ANTIBODY THERAPY OF MALIGNANT-MELANOMA - INVIVO LOCALIZATION IN CUTANEOUS METASTASIS AFTER INTRAVENOUS ADMINISTRATION. Journal of Clinical Oncology 2, 1235–1244 (1984). [DOI] [PubMed] [Google Scholar]
- 46.Oosterwijk E. et al. ANTIBODY LOCALIZATION IN HUMAN RENAL-CELL CARCINOMA - A PHASE-I STUDY OF MONOCLONAL ANTIBODY-G250. Journal of Clinical Oncology 11, 738–750 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Ong G. & Mattes MJ Penetration and Binding of Antibodies in Experimental Human Solid Tumors Grown in Mice. Cancer Research 49, 4264–4273 (1989). [PubMed] [Google Scholar]
- 48.Sancey L. et al. Clustering and internalization of integrin alphavbeta3 with a tetrameric RGD-synthetic peptide. Molecular therapy : the journal of the American Society of Gene Therapy 17, 837–843 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li JY et al. ABiparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell 29, 117–129 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Li F. et al. Intracellular Released Payload Influences Potency and Bystander-Killing Effects of Antibody-Drug Conjugates in Preclinical Models. Cancer Research 76, 2710–2719 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Hamblett KJ et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clinical cancer research : an official journal of the American Association for Cancer Research 10, 7063–7070 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Hinrichs MJM et al. Fractionated Dosing Improves Preclinical Therapeutic Index of Pyrrolobenzodiazepine-Containing Antibody Drug Conjugates. Clinical cancer research : an official journal of the American Association for Cancer Research 23, 5858–5868 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Nejadmoghaddam MR et al. Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna J Med Biotechnol 11, 3–23 (2019). [PMC free article] [PubMed] [Google Scholar]
- 54.Dal Corso A, Gebleux R, Murer P, Soltermann A. & Neri D. A non-internalizing antibody-drug conjugate based on an anthracycline payload displays potent therapeutic activity in vivo. Journal of Controlled Release 264, 211–218 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gebleux R, Stringhini M, Casanova R, Soltermann A. & Neri D. Non-internalizing antibody-drug conjugates display potent anti-cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix. Int. J. Cancer 140, 1670–1679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.