Abstract
The deleterious effects of chronically elevated free fatty acid (FFA) levels on glucose homeostasis are referred to as lipotoxicity, and the concurrent exposure to high glucose may cause synergistic glucolipotoxicity. Lipo- and glucolipotoxicity have been studied for over 25 years. Here, we review the current evidence supporting the role of pancreatic β-cell lipo- and glucolipotoxicity in type 2 diabetes, including lipid-based interventions in humans, prospective epidemiological studies and human genetic findings. In addition to total FFA quantity, the quality of FFAs (saturation and chain length) is a key determinant of lipotoxicity. We discuss in vitro and in vivo experimental models to investigate lipo- and glucolipotoxicity in β-cells and describe experimental pitfalls. Lipo- and glucolipotoxicity adversely affect many steps of the insulin production and secretion process. The molecular mechanisms underpinning lipo- and glucolipotoxic β-cell dysfunction and death comprise endoplasmic reticulum stress, oxidative stress and mitochondrial dysfunction, impaired autophagy and inflammation. Crosstalk between these stress pathways exists at multiple levels and may aggravate β-cell lipo- and glucolipotoxicity. Lipo- and glucolipotoxicity are therapeutic targets as several drugs impact the underlying stress responses in β-cells, potentially contributing to their glucose-lowering effects in type 2 diabetes.
Keywords: pancreatic β-cell, islet, insulin, lipotoxicity, palmitate
INTRODUCTION
It is well established that obesity is a critical risk factor for the development of type 2 diabetes (T2D). The worldwide prevalence of obesity has tripled since 1975 [1], driving in part the dramatic rise in T2D prevalence over the past few decades. Obesity is associated with elevated concentrations of circulating free fatty acids (FFAs), due to expanded adipose tissue mass and reduced FFA clearance [2]. The latter has been attributed to increased escape of FFAs from esterification in adipose tissue [3]. There is accumulating evidence that elevated FFAs may contribute to T2D pathogenesis and thus represent a mechanistic link between obesity and diabetes. A substantial body of data indicates that increased FFAs induce insulin resistance and pancreatic β-cell dysfunction, precipitating the two major defects underlying T2D pathophysiology. These deleterious effects of FFAs on glucose homeostasis are commonly referred to as lipotoxicity. The concurrent exposure to high glucose (glucotoxicity) after the development of impaired glucose tolerance is considered to exert synergistic toxic effects with FFAs, which led to the concept of glucolipotoxicity as first proposed by Prentki and Corkey [4]. In this review, we will focus on recent advances and current bottlenecks in the field of lipo- and glucolipotoxicity, taking a pancreatic β-cell perspective. The impact of FFAs on insulin action in insulin-sensitive tissues is outside the scope of this review and has been covered in several comprehensive reviews [5–7]. Here, we discuss current evidence supporting the concept of lipo- and glucolipotoxicity and experimental models utilized for its investigation in β-cells. In addition, we review the molecular mechanisms underpinning lipo- and glucolipotoxic β-cell dysfunction and death and the therapeutic modulation of glucolipotoxicity by currently used and potential future drugs.
EVIDENCE FOR LIPO- AND GLUCOLIPOTOXICITY IN HUMANS
The contribution of elevated FFA levels to β-cell dysfunction in human T2D remains debated [8]. An argument against the clinical relevance of lipo- and glucolipotoxicity is that insulin secretion is typically enhanced under conditions of elevated circulating FFA levels as seen in obese individuals, and that most studies showing deleterious effects of elevated FFAs on β-cell function were conducted in vitro. However, in this section we argue that several studies have clearly demonstrated an inhibitory effect of prolonged exposure to FFAs on insulin secretion, including in humans, at least in genetically predisposed individuals. In the following section, we review the experimental challenges of studying the impact of FFAs on the β-cell that explain some of the discrepancies reported in the literature.
Acute versus chronic effects of elevated FFAs on glucose homeostasis
In order to assess the effects of elevated FFAs on glucose metabolism in humans, most studies have used an intravenous lipid emulsion (Intralipid, a purified soybean oil emulsion, or Liposyn, a soybean and safflower oil emulsion) to experimentally raise FFA concentrations in participants. These commercially available lipid emulsions are composed mostly of polyunsaturated FFAs and they are usually administered together with heparin, to stimulate intravascular lipolysis. These studies demonstrated that acute and chronic elevations of FFAs by lipid infusion have differential effects on insulin secretion. Acute exposure enhances glucose-stimulated insulin secretion (GSIS), compensating for lipid-induced insulin resistance [9, 10]. In contrast, a more prolonged elevation of FFAs (24–48h) causes β-cell function to deteriorate, impairing the ability of β-cells to compensate for the prevailing insulin resistance [9, 11, 12]. Significantly, when lipids are co-infused with glucose, the FFA elevation inhibits the stimulatory effect of hyperglycemia on β-cell function [13].
These effects of elevated FFAs may be more pronounced in genetically predisposed individuals. Subjects with a genetic predisposition to develop T2D have increased susceptibility to β-cell dysfunction induced by lipid infusion, compared to subjects without a family history of T2D [14, 15]. In individuals with established T2D, however, lipid infusion does not further worsen β-cell function [16]. Prolonged FFA exposure may no longer detectably affect insulin secretion in the presence of overt β-cell failure. Reduction of FFA levels with short-term administration of acipimox, an inhibitor of lipolysis in adipose tissue, ameliorates the insulin response in normal glucose-tolerant individuals at high risk of T2D [17, 18]. Because long-term acipimox use can cause rebound FFA elevation, it cannot be used as a chronic treatment [18]. In a recent study combining oral glucose tolerance tests with isoglycemic intravenous glucose tolerance tests, FFA elevation was shown to impair the potentiation of insulin secretion by incretins, both in non-diabetic and T2D individuals [19]. This impaired incretin effect could play an important role in the pathophysiology of T2D. These observations in humans seem concordant with in vitro observations in β-cell models. In rodent [20] and human islets [21, 22], acute exposure to FFAs exerts a stimulatory effect on insulin secretion. In contrast, prolonged exposure to FFAs enhances basal secretion and impairs GSIS [20–22].
Is FFA quantity or quality critical in lipo- and glucolipotoxicity?
FFA levels are positively correlated to body mass index (BMI) and inversely correlated to insulin sensitivity, independently of age and BMI [23]. Genetic factors and the progression to a pre-diabetic state also impact FFA levels. In individuals genetically predisposed to T2D who have normal fasting triglyceride levels and normal glucose tolerance, postprandial FFAs are elevated compared to BMI-matched controls [24]. Fasting and steady-state FFAs increase in individuals with impaired fasting glucose and impaired glucose tolerance compared to individuals with normal glucose tolerance, even after correction for age, BMI and insulin sensitivity [23].
As outlined in the previous section, studies employing lipid infusions for up to 4 days in humans suggest that increased FFA levels worsen β-cell function. However, longitudinal studies that have evaluated the association between FFA levels and the evolution of β-cell function or incidence of T2D have yielded inconsistent results. Certain studies have found a positive association between total FFA levels and declining β-cell function [25, 26], whereas other studies did not and altogether questioned the concept of lipotoxicity in β-cells [23]. These discrepancies could, at least in part, be explained by the fact that FFA quality, and not only quantity, may impact β-cell function. Most intervention studies have used the same lipid infusion, containing predominantly polyunsaturated fat. A short-term study in healthy individuals showed that oral ingestion of fat with different degrees of saturation resulted in differential effects on insulin secretion and action: saturated palm oil caused insulin resistance without a compensatory increase in insulin secretion (pointing to β-cell dysfunction), polyunsaturated safflower oil reduced insulin secretion and monounsaturated olive oil was neutral [27].
Findings from prospective, epidemiological studies support the hypothesis that FFA composition rather than total levels mostly affects glucose homeostasis [28–33]. These studies evaluated fatty acid composition of plasma cholesterol esters and phospholipids at baseline as a predictor of incident T2D. Individuals who developed T2D had a higher baseline proportion of saturated palmitic acid [28–33], lower linoleic acid, the most abundant omega-6 polyunsaturated fatty acid [28, 29, 31], and a higher proportion of γ-linolenic acid [28, 29] (for FFA nomenclature and classification, see Fig. 1). The large-scale European EPIC-InterAct study identified a strong diabetes risk for a fatty acid combination of higher concentrations of saturated palmitic and myristic acid and lower concentrations of linoleic acid, very long-chain and odd-chain saturated FFAs. The top 20% of adults with this fatty acid profile had a 60% higher risk of T2D compared to the 20% with a fatty acid profile least like this combination [28]. These data were externally validated in the US-based NHANES cohort. This report [28] and other EPIC-InterAct analyses [33, 34] illustrate that even fatty acids belonging to the same subclass according to their degree of saturation (e.g. saturated or polyunsaturated) can be heterogeneous in their effects.
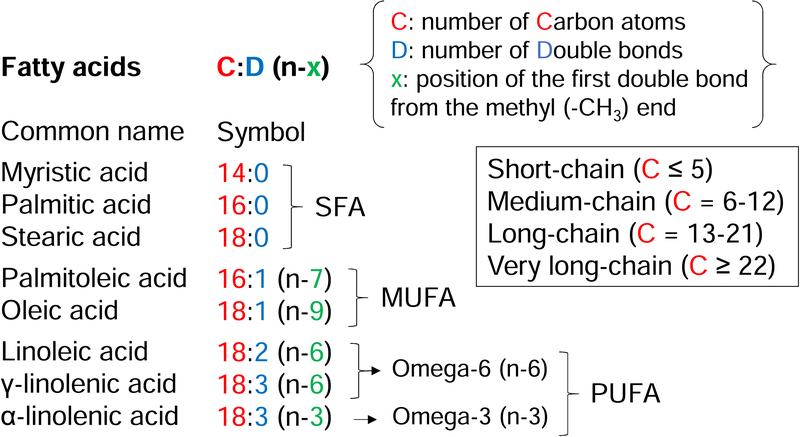
Figure 1: Types of FFAs and nomenclature.
FFAs can be categorized according to the length of the carbon chain (box), the presence of double bonds and the position of the first double bond. Depending on the presence of one or more double bonds, FFAs are classified into saturated (SFA), monounsaturated (MUFA) and polyunsaturated FFAs (PUFA). Depending on the position of the first double bond counting from the methyl end of the chain, FFAs are classified into omega-3, omega-6 etc. This figure does not provide an exhaustive list of common FFA names and symbols, but illustrates the FFAs mentioned in the text. Additional systems of FFA nomenclature exist.
Palmitic acid is the most prevalent saturated FFA in humans. The association between palmitic acid and T2D appears very consistent across epidemiological studies, and among individual saturated FFAs, palmitic acid has the higher hazard ratio for diabetes development [33]. This is in keeping with data from in vitro studies. Palmitate is more toxic than monounsaturated oleate and polyunsaturated linoleate in rodent and human β-cells [35]. Combining palmitate with oleate confers protection from palmitate-induced apoptosis [36, 37].
Evidence for lipotoxicity from human genetic studies
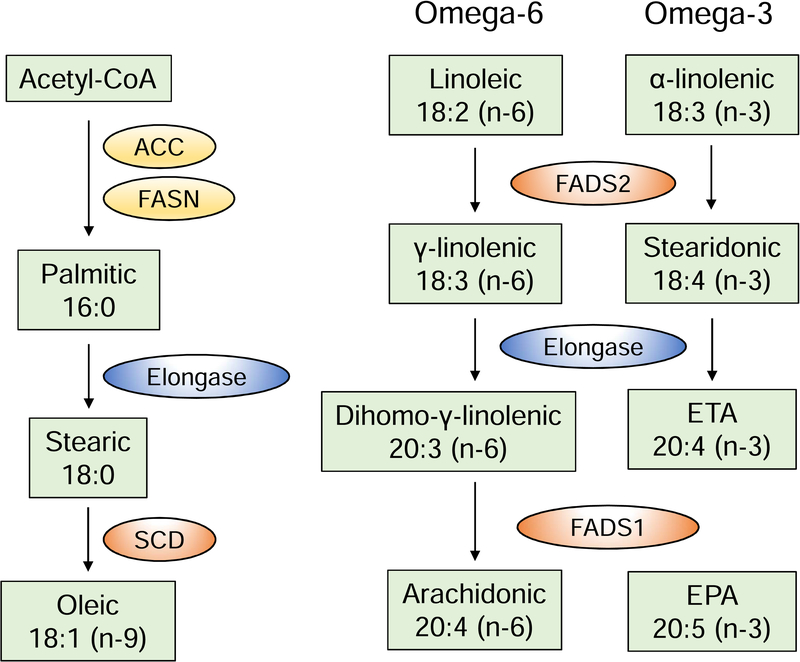
The plasma fatty acid profile does not only reflect dietary intake; it is largely influenced by endogenous FFA metabolism. Saturated FFAs can be synthesized from acetyl-CoA (de novo lipogenesis) and FFAs can be desaturated or elongated through the action of desaturases and elongases, respectively (Fig. 2). In particular, SCD (stearoyl-CoA desaturase) catalyzes the formation of monounsaturated from saturated FFAs, by inserting a double bond. Two SCD isoforms exist in humans, SCD1 and SCD5. Δ5- and Δ6-desaturase, encoded by FADS1 and FADS2 respectively, are required for the synthesis of long-chain omega-6 and omega-3 polyunsaturated FFAs.
Figure 2: FFA metabolic pathways.
Examples of FFA metabolic pathways, highlighting the action of elongases and desaturases, adapted from [204]. Elongases extend the chain of FFAs with two carbons. Stearoyl-coA desaturases (SCD) insert a double bond in saturated FFAs at position 9, counting from the carboxylic acid end (Δ9). Δ5- and Δ6-desaturases, designated as fatty acid desaturase 1 (FADS1) and 2 (FADS2) insert double bonds at positions Δ5 and Δ6, respectively. ACC: acetyl-CoA carboxylase; FASN: fatty acid synthase; ETA: eicosatetraenoic acid; EPA: eicosapentaenoic acid.
Recent genome-wide association studies (GWAS) have identified susceptibility loci for T2D related to desaturases. In a recent study aggregating data from 32 European GWAS, a T2D susceptibility signal was fine mapped to a coding variant of SCD5 [38, 39]. Another signal was linked to lipoprotein lipase (LPL), but the fine mapping was of poor resolution [38]. A large-scale GWAS meta-analysis of continuous diabetes-related traits identified a locus near FADS1 associated with fasting glucose [40]. A subsequent study using metabolic tests found that the FADS1 locus was associated with a lower insulinogenic index, a measure of GSIS, and had no effect on insulin sensitivity or proinsulin [41].
Mendelian randomization studies use genetic variants as an instrument to examine a causal link between an exposure (resulting from the genetic variants) and an outcome. As genotypes are distributed randomly, these studies are less susceptible to confounding or reverse causation than epidemiological studies. A Mendelian randomization study used a genetic variant associated with lower FADS1 and FADS2 activities to study the effect of desaturases on T2D risk. After adjustment for estimated enzymatic activities of FADS1 or FADS2, a genetically determined low FADS2 activity predicted lower T2D risk and low FADS1 activity tended to predict higher risk [42]. Low FADS1 activity would favor a low linoleic/γ-linolenic acid ratio. This appears to be in accordance with epidemiological studies showing that low concentrations of linoleic [28, 29, 31] and high concentrations of γ-linolenic acid [28, 29] are positively associated with T2D risk, as mentioned above. Taken together, these data provide further evidence for lipotoxicity as a contributor to T2D pathogenesis and highlight the importance of FFA composition.
EXPERIMENTAL APPROACHES TO STUDYING LIPO- AND GLUCOLIPOTOXICITY: CAVEATS AND LIMITATIONS
Since the early studies by the group of Grill in the 1990s [20, 43], a number of in vitro and in vivo systems have been used to study the mechanisms of lipo- and glucolipotoxicity, sometimes leading to conflicting results. Such discrepancies are at least in part related to methodological and experimental issues that are important to consider.
Methodological considerations for in vitro use of FFAs
Experiments aimed at investigating the impact of FFA exposure in isolated cells ex vivo attempt to mimic the concentrations present in the vicinity of the islets in situ. These concentrations are difficult to estimate as they depend not only on total circulating levels but also on factors influencing local delivery of FFAs, including LPL activity present in islets [44], as well as FFA release by islet cells [45].
FFAs are hydrophobic molecules with low solubility in aqueous solutions such as plasma or interstitial fluid, and are bound to albumin in the vascular and interstitial compartments [46, 47]. In healthy humans, on average 2 FFA molecules are bound to each albumin molecule in the circulation (range 1:1 to 3:1). The FFA/albumin molar ratio can rise to more than 5:1 in pathological conditions [48]. FFA transport and clearance from the circulation depend on FFA-albumin interactions. Thus, FFA accessibility for cellular uptake is a function of their unbound concentration at physiological concentrations of albumin [49] and investigating the effects of FFAs ex vivo in cultured cells requires previous complexation with albumin (usually bovine serum albumin, BSA) at a FFA:BSA molar ratio no greater than 5:1 [50, 51]. Because the biologically active fraction of FFAs depends on the FFA/BSA molar ratio, an increase in FFA concentration or decrease in BSA concentration will enhance their biological effects [50, 52]. Different methods exist to prepare FFA-BSA solutions. FFAs can be used with charcoal-absorbed BSA (to remove endogenous bovine FFAs) or FFA-free BSA, either directly or after precomplexing. FFAs used in the presence of 1% charcoal-absorbed BSA or 0.75% FFA-free BSA, or precomplexed FFAs used in the presence of 0.67% FFA-free BSA resulted in similar unbound FA concentrations [52], suggesting that the FFA-BSA precomplexing process results in lower FFA concentrations due to aggregation. Different FFA-free BSA preparations of variable purity are commercially available. As BSA contaminants can impact FFA binding, albumin used in in vitro experiments must be of high-grade purity. Albumin (2%) present in the fetal bovine serum that is often used in cell culture will also bind FFAs, thereby decreasing unbound FFA concentrations.
To complicate things further, the affinity of FFAs for albumin varies with the length of the carbon chain and the degree of saturation. For example, stearate has higher affinity for albumin than arachidonate, and therefore more stearate is needed than arachidonate to obtain the same of unbound fraction for the same total concentration [46]. This must be taken into account in experiments aimed at comparing the potencies of different FFAs. Although unbound FFA concentrations can be measured using a fluorescent probe (Acrylodan-Labeled Intestinal Fatty Acid Binding Protein) [51, 53, 54], this is not very practical and rarely reported in the literature.
In conclusion, given the number of experimental parameters that impact the biologically active, unbound fraction of FFAs and the possibility for non-specific detergent effects, it is essential that such parameters be precisely reported in manuscripts including total FFA concentration, type of BSA, molar ratio, and complexation protocol.
In vivo models for the study of lipo- and glucolipotoxicity
Pioneering studies by the Unger group in Zucker Diabetic Fatty (ZDF) rats established the concept of lipotoxicity [55]. ZDF rats carry a loss-of-function mutation (fa) in the leptin receptor leading to massive obesity, hyperlipidemia and insulin resistance. Male animals develop hyperglycemia due to β-cell dysfunction and a progressive loss of β-cell mass. β-Cell damage in this model is mediated by increased flux of the de novo ceramide biosynthesis pathway [56]. A pathogenic role for sphingolipids in β-cells was confirmed in other models, where ceramide induces β-cell apoptosis [57–59] and inhibition of insulin gene expression [60]. Because mutations in the leptin receptor gene profoundly perturb intracellular FFA metabolism and are extremely rare in humans [61], alternative rodent models have been developed that are more relevant to human physiopathology.
In an in vivo model of prolonged intravenous infusion of Intralipid with heparin in rats, GSIS was either unchanged [62], increased [63, 64] or decreased [43, 65–68]. These discrepancies may be explained by differences in strain, sex, age, duration/rate of lipid infusion and glucose levels. A 48-hour Intralipid infusion impaired GSIS in male Wistar rats [66], while a 96-hour infusion did not in male Sprague-Dawley rats [64]. Glucose and Intralipid infusion decreased insulin secretion in 6-but not in 2-month-old Wistar rats [69]. Genetic predisposition to diabetes also influences the rodent β-cell response to FFAs. Insulin secretion is impaired to a greater extent in lean heterozygous ZDF rats than in Wistar rats after Intralipid infusion [68].
Species differences exist between rodent and human β-cells with dissimilarities in islet architecture [70, 71] and β-cell metabolism [72], plasticity [73–77] and lifespan [72, 77–79]. Human islet transplantation into immunodeficient mice represents an attractive approach to study human β-cell pathophysiology in a long-term in vivo system. Gargani et al [80] transplanted human islets into immunodeficient mice that were subsequently placed on high-fat diet. Human islets from non-diabetic donors adapted to the high-fat obese environment with an increase in β-cell volume, although no increase in β-cell proliferation was detected. β-cell function (corrected for insulin sensitivity) declined in high fat-fed mice compared to chow-fed mice. Human islets from two T2D donors did not change in mass or function with high-fat feeding [80]. This transplantation model allows to study both the human islet graft and murine islets, thereby shedding light on differences between murine and human β-cells.
IMPACT OF LIPO- AND GLUCOLIPOTOXICITY ON β-CELL INSULIN PRODUCTION AND SECRETION
Insulin release by the β-cell results from a highly coordinated process that encompasses insulin gene transcription, proinsulin biosynthesis, and insulin secretion. Glucose, the major insulin secretagogue, positively regulates all these steps so as to ensure that adequate intracellular stores are available to meet the secretory demand. Lipo- and glucolipotoxic conditions have been shown to alter several key functions along the insulin factory (Fig. 3).
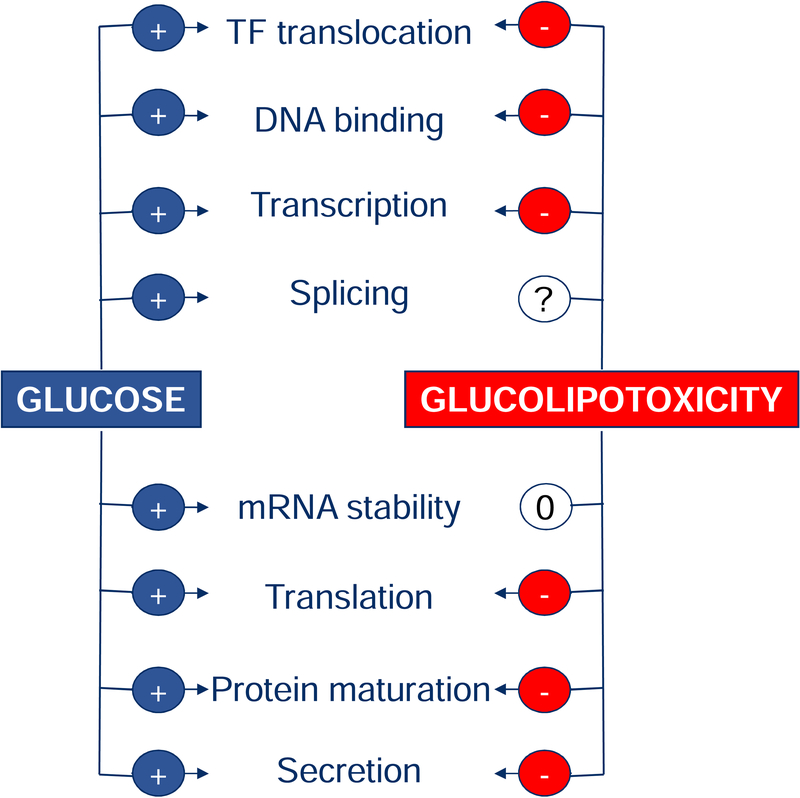
Figure 3: Impact of glucolipotoxicity on insulin production and secretion.
Glucose stimulates transcription of the insulin gene, pre-mRNA splicing, mRNA stability, proinsulin translation, protein maturation, and exocytosis. In contrast, lipo- and glucolipotoxic conditions have been shown to impair several of these steps. TF: transcription factor; +: stimulation; −: inhibition; 0: no effect; ?: not studied.
Insulin gene expression and proinsulin biosynthesis
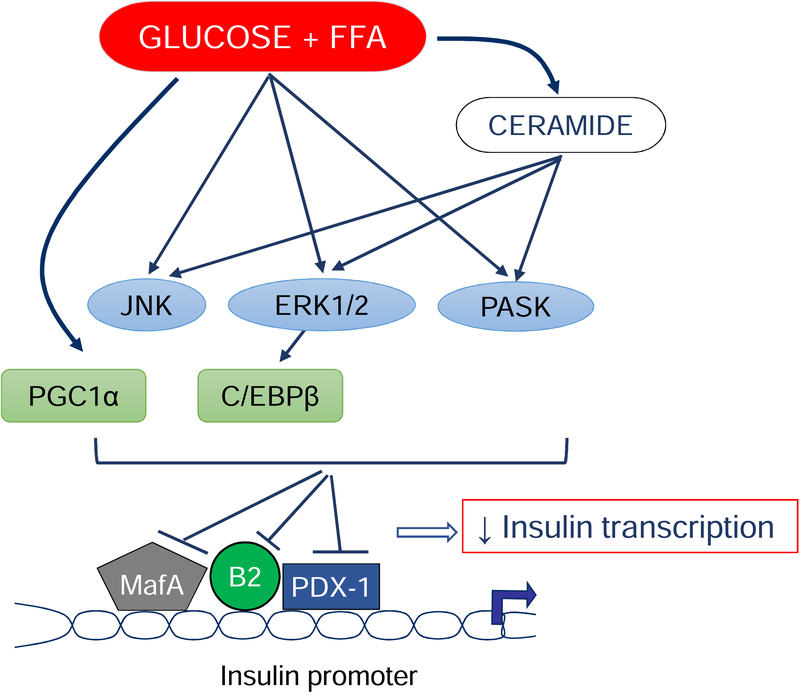
Studies by our group [60, 81–84] and others [85–89] have demonstrated a detrimental impact of elevated saturated FFAs on insulin gene transcription. In isolated islets, we found that combined exposure to high levels of glucose and palmitate impairs the expression of the transcription factor MafA and the nuclear translocation of the transcription factor PDX-1 [81], a finding that was confirmed in an in vivo model of glucolipotoxicity [62]. This results in diminished insulin promoter activity [60]. Kim et al observed that the increased expression of PGC1-α under glucolipotoxic conditions suppresses the activity of the transcription factor BETA2/NeuroD [89], while Plaisance et al [86] showed that palmitate-induced C/EBPβ expression reduces insulin promoter activity. These transcriptional changes are mediated, at least in part, via intracellular generation of ceramide [60, 90, 91] and activation of stress kinases including c-Jun N-terminal kinase (JNK) [88], extracellular-regulated kinases (ERK)1/2 and Per-Arnt-Sim (PAS) kinase [83] (Fig. 4). Interestingly, rare nonsynonymous mutations in the PAS kinase gene cause maturity-onset diabetes of the young [92].
Figure 4: Signaling leading to the glucolipotoxic impairment of insulin gene transcription.
High glucose and the FFA palmitate stimulate ceramide production and activate the stress kinases JNK, ERK1/2 and PASK. These suppress the binding of the transcriptional regulators MafA, PDX1 and BETA2/NeuroD (B2) to the insulin promoter, in part through the activity of the transcriptional factors PGC1α and C/EBPβ. This results in decreased insulin transcription.
The impairment of insulin gene transcription under lipo- and glucolipotoxic conditions is accompanied by a loss of glucose regulation of proinsulin biosynthesis both in vitro [93] and in vivo [69], thereby contributing to a dramatic decrease in intracellular insulin content.
Stimulus-secretion coupling
Glucose triggers insulin secretion through the generation of various coupling factors including a change in the ATP/ADP ratio; closure of ATP-sensitive potassium channels; membrane depolarization; and calcium influx which stimulates insulin exocytosis. Although mechanisms affecting β-cell function during chronic FFA exposure are not fully elucidated, different levels of the insulin secretion machinery are affected.
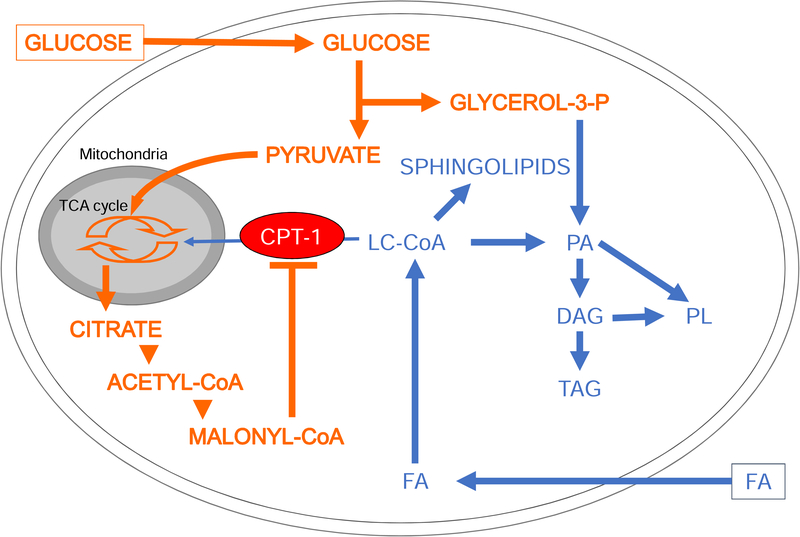
Prolonged exposure to FFAs, particularly palmitate, interferes with glucose metabolism at several levels. Intracellular FFAs are activated into long-chain CoAs and, at low or normal glucose levels, are readily oxidized by the β-cell. However, if glucose levels rise, FFA oxidation is blocked by the generation of malonyl-CoA, which inhibits carnitine-palmitoyl transferase 1, thereby diverting intracellular FFA metabolism towards esterification and the generation of complex lipids including sphingolipids (Fig. 5). In the “tricycling” model described by Prentki et al [45, 94, 95], the Krebs, pyruvate and glycerolipid/FFA cycles are interlinked. They generate essential metabolic coupling factors in the β-cell to amplify signals for insulin secretion, but become detrimental under fuel oversupply conditions.
Figure 5: Effects of glucose on intracellular lipid metabolism in the β-cell.
In the presence of simultaneously elevated levels of glucose and FFAs, the increase in cytosolic malonyl-CoA resulting from glucose metabolism inhibits carnitine-palmitoyl transferase 1 (CPT-1). Transport of long-chain acyl-CoA (LC-CoA) in the mitochondria is blocked, and FFA metabolism is diverted towards the synthesis of lipid-derived signaling molecules such as sphingolipids, diacylglycerols (DG), phosphatidic acid (PA), phospholipids (PL) and triacylglycerols (TG). Adapted from [51] with permission.
Lipo- and glucolipotoxicity are associated with mitochondrial dysfunction [96, 97] and disturbed calcium handling via upregulation of neprilysin, a plasma membrane protein that regulates Ca2+ influx in β-cells [98]. Given its classical function as a mitochondrial uncoupler, many studies have investigated the role of uncoupling protein-2 (UCP2) in insulin secretion and suggested a role in glucolipotoxicity [99]. UCP2 expression is increased in vitro by chronic high glucose and FFA exposure, and in some in vivo studies it was associated with impaired insulin secretion, hyperglycemia and T2D [100–105]. Acute inhibition of UCP2 by genipin increases GSIS in isolated islets [106] and, conversely, overexpression of UCP2 impairs insulin secretion [100, 102]. Whole-body UCP2 knockout mice were shown to have enhanced GSIS, higher ATP content and increased intracellular cytotoxic reactive oxygen species (ROS) levels in islets [107–109]. In a β-cell specific UCP2 knockout (βUCP2KO), Robson-Doucette et al observed that UCP2 does not act as a classical uncoupling protein in β-cells, with no changes in ATP levels or mitochondrial coupling in βUCP2KO islets, while ROS production and GSIS were increased [110]. This suggests that UCP2 negatively regulates GSIS by reducing mitochondrial ROS production and not through a defect in ATP production. The precise contribution of UCP2 in as a mediator of glucolipotoxicity is not fully understood.
Finally, FFAs have recently been shown to modulate post-translational modifications of intracellular proteins in the β-cell including acetylation [111], palmitoylation ([112], see below), and glycosylation [113].
Insulin exocytosis
In T2D patients, the first phase of insulin secretion is almost abolished, suggesting a defect in priming and/or fusion of insulin-containing granules with the β-cell plasma membrane. Andersson et al [114] observed a significant decrease in the expression of exocytotic genes and proteins in islets from T2D donors. Furthermore, in T2D β-cells insulin secretory granules docked at the plasma membrane do not cluster to voltage-gated Ca2+ channels [115]. This loss of proximity between the immediately releasable granule pool and high Ca2+ influx microdomains could contribute to the lost first-phase insulin secretion in diabetic patients. Similarly, exposure of mouse islets to palmitate does not decrease the number of release-competent granules but alters the tight complexes of Ca2+ channels with secretory granules [116, 117]. Loss of this organization leads to insufficient Ca2+ concentrations close to the secretory granule to produce exocytosis in response to brief depolarizations. Interestingly, in mouse and human islets treated with palmitate, the defect in insulin secretion is reversed by the K+ channel blocker tetraethylammonium that pharmacologically prolongs β-cell action potentials [116, 117]. In the same manner, clustering of Kv2.1 voltage-gated K+ channels with insulin secretory granules promotes exocytosis. Overexpression of these channels rescues insulin exocytosis from T2D β-cells [118]. Localized influx due to very close proximity of ion channel clusters and insulin-containing granules thus seems to be crucial for GSIS, and FFA-induced impairment in insulin secretion could be due to their structural disruption. Johnston et al recently identified a subset of specialized β-cells within islets, called hub cells, which coordinate calcium dynamics, electrical activity and insulin secretion, and the activity of which is disrupted under glucolipotoxic conditions [119].
Another link between FFA metabolism and the amplifying pathways of GSIS is the lipid-regulated protein kinase C (PKC) ε. Inhibition of PKCε increases insulin secretion and improves glucose homeostasis in rodent models of T2D [120]. PKCε knockout islets exposed to palmitate upregulate the amplification pathway of GSIS [121]. This enhanced GSIS, occurring selectively after lipid exposure, depends on glucose-induced hydrolysis of triglyceride stores. PKCε knockout islets have enhanced lipolysis rates and triglyceride lipase activity and pharmacological lipase inhibition abrogates GSIS.
MOLECULAR MECHANISMS OF LIPO- AND GLUCOLIPOTOXIC β-CELL DYSFUNCTION AND DEATH
Exposure of β-cells to palmitate recapitulates key features of β-cell failure in T2D, since it induces apoptosis, raises basal insulin secretion and inhibits GSIS. For this reason, it has been the most extensively used in vitro model of β-cell lipotoxicity. Mechanistic studies have revealed that palmitate with or without high glucose has pleiotropic effects on β-cell function and survival/death pathways. In vitro studies have provided good insight into the cell death process triggered in β-cells upon palmitate exposure. The detection of β-cell apoptosis in vivo is much more difficult as apoptotic cells are rapidly cleared. In this section, we summarize current knowledge on the molecular mechanisms underlying lipo- and glucolipotoxicity, focusing on the effects of palmitate (Fig. 6).
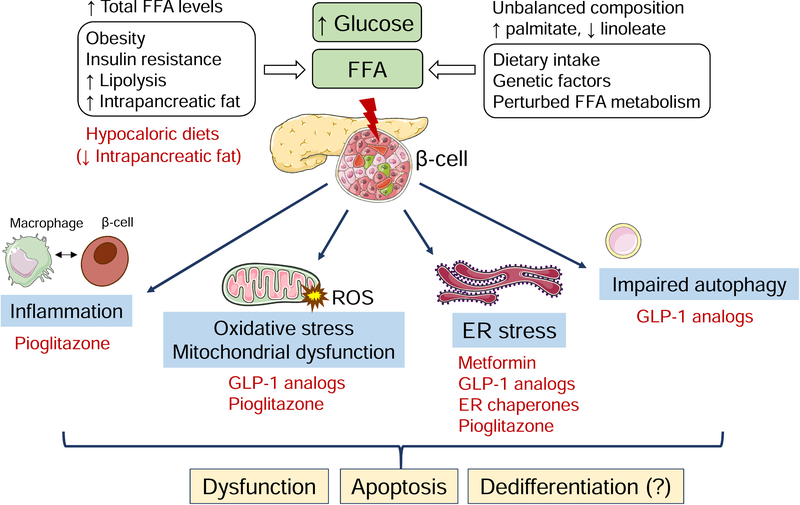
Figure 6: Molecular mechanisms of lipo- and glucolipotoxic β-cell demise and potential treatments.
A prolonged increased FFA supply and/or unbalanced FFA composition, alone or in combination with high glucose, elicits stress responses in pancreatic β-cells. These include ER stress, oxidative stress with excessive ROS production, mitochondrial dysfunction, inflammation and impaired autophagic flux. Crosstalk between these pathways may give rise to feed-forward mechanisms, aggravating glucolipotoxic stress. Collectively, these phenomena culminate in β-cell dysfunction, apoptosis and possibly dedifferentiation. In vitro data suggest that metformin, GLP-1 analogs, thiazolidinediones and ER chaperones mitigate lipo- and glucolipotoxicity. These therapies (shown in red) target distinct stress pathways. The graphic illustrations used in this figure are from Servier Medical art (https://smart.servier.com).
ER stress
The endoplasmic reticulum (ER) has a central role in lipid biosynthesis, Ca2+ storage and the synthesis and folding of secreted proteins. The accumulation of unfolded or misfolded proteins in the ER lumen is defined as ER stress and activates the ER stress response, also known as the unfolded protein response. This set of intracellular signaling pathways is initiated by three ER stress sensors and transducers, namely protein kinase RNA (PKR)-like ER kinase (PERK), activating transcription factor-6 (ATF6), and inositol-requiring enzyme-1 (IRE1). The signal transduction aims to restore ER homeostasis by attenuating global protein translation, upregulating ER folding capacity and degrading misfolded proteins. When this cannot be attained, apoptosis is triggered.
β-cells are susceptible to ER stress due to the high demand for insulin synthesis placed on the ER, particularly in the face of insulin resistance. There is now compelling evidence for a role of ER stress in diabetes pathogenesis. Islets from T2D patients show increased expression of ER stress markers and distended β-cell ER, an ultrastructural hallmark of ER stress [122]. Mutations affecting components of the ER stress response cause monogenic diabetes (reviewed in [123]).
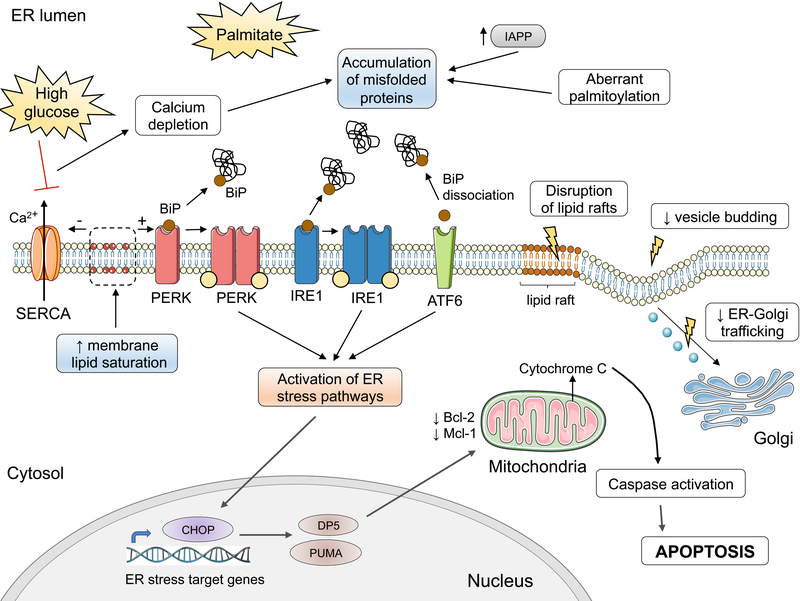
Palmitate triggers ER stress through perturbations that affect ER folding capacity and cause misfolded protein overload in the ER (Fig. 7). Palmitate depletes ER Ca2+ stores, compromising the folding capacity of Ca2+-dependent chaperones [36, 124]. Exposure to high glucose enhances palmitate-induced ER Ca2+ depletion. High glucose downregulates the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) that pumps Ca2+ into the ER [125]; Ca2+ depletion by FFAs is not mediated by transcriptional modulation of SERCA [36]. Palmitate may also affect protein folding by aberrant protein palmitoylation [112], a post-translational modification that occurs in cysteine residues of a protein. Palmitoylation increases protein hydrophobicity and this can alter protein activity, stability and trafficking [112]. Palmitate causes alteration of ER lipid species that affect the composition and distribution of ER lipid rafts [126]. These specialized membrane microdomains regulate the entry of certain types of proteins into the secretory pathway. The alteration of ER lipid rafts by palmitate causes reduced budding of ER-derived vesicles [126] and disrupts ER-to-Golgi trafficking [127, 128]. The perturbed protein trafficking promotes ER luminal protein overload, triggering ER stress. Furthermore, palmitate induces a rapid degradation of carboxypeptidase E [129], an enzyme required for insulin processing. This enhances ER stress possibly due to buildup of unprocessed proinsulin in the secretory pathway. Islet amyloid polypeptide (IAPP) is a peptide hormone co-secreted with insulin by β-cells. Accumulation of unprocessed IAPP can initiate the formation of amyloid deposits. FFAs induce IAPP expression in mouse and human β-cells and its aggregation contributes to ER stress and inflammation [130–132].
Figure 7: Activation of ER stress pathways by palmitate.
Exposure of β-cells to palmitate induces aberrant protein palmitoylation and Ca2+ depletion in the ER, affecting ER folding capacity. The depletion of Ca2+ stores is aggravated by the downregulation of the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) pump in high glucose conditions. In parallel, palmitate disrupts the export of cargo from the ER and trafficking to the Golgi, contributing to the buildup of unfolded or misfolded proteins. The misfolded proteins recruit the ER chaperone BiP, causing its dissociation from the luminal domain of the ER stress transducers PERK, IRE1 and ATF6. This, together with increased ER membrane lipid saturation, results in the activation of the ER stress transducers, eliciting downstream ER stress signaling. This in turn leads to the induction of the proapoptotic proteins CHOP, PUMA and DP5, the latter inhibiting anti-apoptotic members of the Bcl-2 family. These events culminate in mitochondrial permeabilization, cytochrome C release and mitochondrial apoptosis. Graphic elements used in this illustration come from Servier Medical art (https://smart.servier.com).
The accumulated unfolded proteins activate the ER sensors PERK and IRE1 through their luminal domains, either by engaging directly these domains [133] or by inducing their dissociation from the chaperone BiP [134]. Interestingly, the transmembrane domains of PERK and IRE1 can also sense increased ER membrane lipid saturation and directly activate the ER stress response, as mutant PERK and IRE1 proteins lacking the luminal stress-sensing domain remain responsive to palmitate [135].
Unresolved lipo- and glucolipotoxic ER stress leads to apoptosis, through the induction of the PERK-dependent pro-apoptotic transcription factor CHOP [36]. Lipotoxic ER stress induces the pro-apoptotic BH3-only proteins PUMA and DP5 and causes loss of the anti-apoptotic proteins Bcl-2 and Mcl-1 [136]. The activation of these effectors downstream of ER stress culminates in mitochondrial permeabilization and engagement of the mitochondrial pathway of apoptosis.
Oxidative stress and mitochondrial dysfunction
Physiological levels of ROS are a requisite for normal glucose sensing and GSIS [137]. Imbalance between ROS formation and cellular antioxidant defenses can lead to oxidative stress. FFA and excess glucose are potent inducers of ROS through different mechanisms. Long-chain FFAs (C>14), being poor substrates for mitochondrial β-oxidation, are shortened by peroxisomal β-oxidation and subsequently transported to mitochondria for further degradation. Peroxisomal β-oxidation of palmitate generates H2O2 [138]. Given that β-cells lack the H2O2-inactivating enzyme catalase in peroxisomes, peroxisomal H2O2 formation contributes to palmitate-induced oxidative stress [138].
Excess glucose generates ROS through the shunting of its metabolites into alternative metabolic pathways, such as dihydroxyacetone and diacylglycerol formation, glucosamine and hexosamine metabolism, and sorbitol metabolism [139]. Moreover, high glucose and palmitate lead to enhanced superoxide formation via activation of NADPH oxidase [140]. The latter is dependent on the activation of PKC, possibly through increases in diacylglycerol [140].
Oxidative stress impairs mitochondrial function and integrity. Mitochondrial DNA (mtDNA) carries 37 genes, encoding tRNAs, rRNAs and polypeptides of the electron transport chain complexes. mtDNA is more vulnerable than nuclear DNA to oxidative stress-related damage due to the lack of protective histones and low repair mechanisms [141]. Being at close proximity to the source of ROS generation, mitochondrial components are at high risk of oxidative injury [141]. FFAs cause a dose-dependent damage in mtDNA, contributing to apoptosis [142]. In addition to DNA damage, exposure to a combination of glucose and FFA disrupts mitochondrial dynamics. Under glucolipotoxic conditions, β-cell mitochondria lose their ability to undergo fusion and become fragmented [143].
Autophagy
Autophagy is a physiological process that regulates the balance between synthesis, degradation and recycling of cellular components. It serves as a quality control mechanism, removing damaged or redundant organelles, protein aggregates and lipids, and protects cellular homeostasis under conditions of nutrient deprivation. Autophagy starts with the sequestration of cytoplasmic constituents or organelles and the formation of a double-membrane structure, called the autophagosome. Autophagosomes fuse with lysosomes, in which the contents of the autophagosome are degraded by lysosomal enzymes. The significance of autophagy in β-cell physiology and metabolic adaptation has been highlighted by studies in transgenic mice deficient in autophagy. Mice with β-cell-specific ablation of Atg7, a gene essential for autophagosome formation, develop glucose intolerance due to decreased insulin secretion [144]. Furthermore, these mice exhibit a lack of compensatory increase in β-cell mass following high fat diet [144].
β-cells from T2D donors show signs of dysregulated autophagy, with more abundant autophagic vacuoles and autophagosomes and lower lysosomal associated membrane protein 2 and cathepsin expression compared to β-cells from non-diabetic donors [145]. Interestingly, these features could be induced by in vitro exposure of human islets to FFAs (oleate and palmitate) [145]. Autophagy is often measured by the conversion of LC3 I to its lipidated form (LC3 II), a necessary step for autophagosome biogenesis. Palmitate (and high glucose) increases the LC3 II to LC3 I ratio [132, 146–152] and inhibits expression of a number of genes involved in autophagy and lysosomal function [132]. Consistent with this gene expression signature in palmitate-treated human islets, studies using pH-sensitive LC3 expression vectors and measuring p62 degradation showed that palmitate (and high glucose) suppresses autophagic turnover, due to impaired fusion of autophagosomes with lysosomes and impaired lysosomal acidification [149, 151, 153]. Re-acidification of lysosomes by photoactivatable nanoparticles restored autophagic flux in (gluco-)lipotoxic conditions [152]. Treatment with carbamazepine or rapamycin (that both stimulate autophagic activity) confers protection against palmitate and glucose toxicity [132, 147, 149, 151, 154], while blocking autophagy exacerbates lipotoxicity [154]. These data support the concept that inhibition of autophagic flux in human β-cells by palmitate is a mediator of lipo- and glucolipotoxicity.
Inflammation
Saturated FFAs can trigger inflammatory pathways in β-cells, both via direct activation and secondarily to oxidative and ER stress [155]. Palmitate induces cytokine (IL-1β, IL-6 and IL-8) and chemokine (CCL2, CXCL1) production in β-cells, mediated by IL-1 receptor signaling [156, 157]. However, whether these pro-inflammatory changes per se induce β-cell glucolipotoxicity is controversial [155–157].
Interactions between β-cells and immune cells mediate inflammation-mediated lipo- and glucolipotoxicity. Palmitate induces the production of chemokines by β-cells via the Toll-like receptor 4 (TLR4), recruiting M1-type proinflammatory macrophages/monocytes to the islets [158]. Depletion of M1 macrophages/monocytes before ethyl palmitate infusion protected mice from β-cell dysfunction and preserved expression of key β-cell genes [158]. Palmitate and high glucose also synergistically trigger islet secretion of the S100 calcium-binding protein A8 (S100A8), a damage-associated pattern molecule that activates macrophages in a TLR4-dependent manner [159]. The subsequent cytokine production by macrophages elicits β-cell apoptosis [159].
Crosstalk between stress pathways
The above described stress pathways may operate simultaneously or synergistically, generating feed-forward mechanisms in lipo- and glucolipotoxicity. For example, oxidative stress caused by H2O2 induces ER Ca2+ depletion, leading to ER stress [125]. ER stress induces pro-inflammatory gene expression, through the activation of the transcription factor NF-κB and JNK [160]. Mild ER stress sensitizes β-cells to the cytokines IL-1β and TNF-α, amplifying the inflammatory response [161]. ER stress also stimulates autophagy, through the JNK pathway [146, 148, 153]. Crosstalk between stress pathways thus exists at multiple levels and may aggravate β-cell lipo- and glucolipotoxicity.
Differential effects of FFAs on stress pathways
As discussed above, FFAs are not homogeneous in their effects. In vitro β-cell studies of palmitate and oleate, the two best studied FFAs, have revealed significant differences. Palmitate activates more potently than oleate the PERK branch of the ER stress response, considered as the most pro-apoptotic branch, probably through more marked ER Ca2+ depletion [36]. Palmitate also preferentially activates the IRE1 branch, whereas ATF6 signaling is activated by both FFAs to an equal extent [36]. Contrary to palmitate, oleate does not induce peroxisomal H2O2 generation [162]. Oleate improved the fusion of autophagosomes to lysosomes and stimulated autophagic flux more effectively than palmitate [163]. Collectively, these differences may explain the mild to absent toxicity of oleate [35, 164]. In keeping with this, enhanced SCD expression in β-cells confers resistance against lipoapoptosis [165] and, conversely, SCD inhibition increases ER stress and apoptosis [166].
Potential role of (gluco-)lipotoxicity in β-cell dedifferentiation
There is mounting evidence that β-cells can revert to progenitor-like cells (β-cell dedifferentiation) or transdifferentiate to other endocrine cell types. This process may contribute to the loss of functional β-cell mass, in addition to apoptosis [167–169]. As described in the section on insulin gene expression, glucotoxicity decreases insulin gene expression, due to reduced expression and binding of key transcription factors to the insulin promoter. Glucotoxicity downregulates other genes critical for the maintenance of the differentiated β-cell phenotype. These include transcription factors, such as NKX6.1, HNF1α and HNF4α, glucose sensing and metabolism genes, and secretory pathway genes (for detailed description, see [170]).
Conversely, hyperglycemia results in the upregulation of genes with absent or very low expression in mature β-cells under physiological conditions. Those ‘forbidden’ genes are involved in metabolic pathways, transcriptional regulation and stress responses in β-cells [170]. In animal models of diabetes, hyperglycemia has been associated with the expression of transcription factors typically expressed in progenitor cells at the embryonic stage, such as neurogenin 3 (NGN3) and OCT4 [170]. High glucose [171], as well as FFA exposure [172], upregulate the inhibitor of differentiation 1 (ID1), another transcriptional regulator. ID1 upregulation has been shown to contribute to the suppression of insulin secretion and the development of glucose intolerance under high-fat diet [172].
Foxo1, a transcription factor integrating nutrient and hormone-driven signals [167], has been shown to play a role in β-cell dedifferentiation. Palmitate treatment in vitro induces Foxo1 activation by nuclear translocation [173]. Foxo1 activation is initially an adaptive response to maintain the balance of mitochondrial glucose versus lipid utilization [167]. However, prolonged Foxo1 activation leads to its degradation [167]. Foxo1 depletion has been associated with preferential oxidation of lipids rather than glucose for energy generation and with suppressed expression of β-cell identity transcription factors [174]. Using immunostaining for Foxo1 and ALDH1A3, a β-cell dedifferentiation marker [169], a recent report found that islets from mice chronically exposed to a high-fat diet showed signs of β-cell dedifferentiation (suppression of Foxo1 and ALDH1A3 expression) [175]. No such signs were observed in islets from low-fat fed mice. The above data raise the hypothesis that glucolipotoxicity may act as a driver for β-cell dedifferentiation.
LIPO- AND GLUCOLIPOTOXICITY: THERAPEUTIC TARGETS?
Metformin
Metformin is recommended as first-line medication for T2D treatment. Besides acting as an insulin sensitizer, it has beneficial effects in β-cells under (gluco-)lipotoxic conditions. Metformin prevents the impairment in glucose-stimulated secretion induced by FFAs and high glucose in rat [176] and human islets [177]. This protective effect is, at least in part, mediated by the restoration of glucose utilization and oxidation by metformin [176, 177]. Metformin further prevents lipotoxic β-cell apoptosis, through attenuation of the ER stress response with decreased pro-apoptotic PERK/CHOP signaling [178, 179].
Glucagon-like peptide 1 analogs
Glucagon-like peptide 1 (GLP-1) receptor agonists are more recently developed glucose-lowering medications. GLP-1 engages a G-coupled protein receptor, which activates adenylate cyclase and augments GSIS, through cAMP-induced activation of protein kinase A. The GLP-1 analog exendin-4 protects against lipotoxic β-cell dysfunction/apoptosis via a number of mechanisms. Exendin-4 protects β-cells from palmitate by increasing cellular defenses via the induction of the ER chaperone BiP and the anti-apoptotic protein JunB [180]. Exendin also abrogates the palmitate-induced activation of the pro-apoptotic stress kinases JNK and p38 MAPK [181]. Both protective mechanisms are cAMP/protein kinase A-dependent [180, 181]. In addition, exendin restores lysosomal function and prevents the inhibition of autophagic flux by glucolipotoxicity [153].
With regard to function, exendin corrects the palmitate-induced impairment in insulin release in mouse β-cells [182]. In high fat-fed mice treated or not with exendin, islets isolated from exendin-treated mice preserved glucose responsiveness, in contrast to islets from non-treated mice. In this study, islets were isolated after the disappearance of exendin from the circulation, suggesting durable effects of GLP-1 receptor agonists on insulin secretion [183]. Liraglutide, another GLP-1 analog, abolished the downregulation by palmitate of the transcription factors Pdx-1, MafA and NeuroD, through activation of the PI3K/Akt pathway [184]. The preservation of these key β-cell transcription factors may contribute to the improvement of β-cell function under lipotoxic conditions by GLP-1 receptor agonists.
Thiazolidinediones
Thiazolidinediones (or glitazones) are ligands of peroxisome proliferator-activated receptor γ (PPARγ), a transcription factor regulating lipid storage and metabolism. Use of these antidiabetic drugs has declined over the past years, after controversy sparked by studies suggesting an association of rosiglitazone use with cardiovascular events and of pioglitazone with increased risk of bladder cancer. Nonetheless, in vitro studies have demonstrated protective effects of the two drugs against β-cell glucolipotoxicity. Rosiglitazone prevented FFA-induced downregulation of PPARγ and impairment of insulin release in human islets [185]. Pioglitazone attenuates β-cell oxidative stress [186], inflammation and ER stress in FFA-treated β-cells [125, 187], through the repression of NF-κB activation [187] and restoration of SERCA pump expression [188]. However, β-cell-specific overexpression of PPARγ in a mouse model led to worse glucose tolerance and β-cell loss upon high fat diet [189]. Thus, the potential of PPARγ agonism as anti-lipotoxic therapy remains uncertain.
Chaperones
Chaperones are low molecular weight compounds known to stabilize protein conformation and improve ER folding capacity. The two chaperones most extensively studied are 4-phenyl butyric acid (PBA) and the taurine-congugated derivative of ursodeoxycholic acid TUDCA. Both chaperones have good safety profiles [190]. In rat β-cells, PBA [191] and TUDCA [192] decreased palmitate-induced ER stress and ameliorated GSIS [191]. In a small study of obese or overweight non-diabetic individuals, participants were pretreated for 2 weeks with PBA before a lipid infusion over 48h. PBA was found to partially prevent the decrease in disposition index induced by the lipid infusion, pointing to a protective effect of PBA against β-cell lipotoxicity [190]. It is unknown whether this was mediated by alleviation of ER stress or by ER stress-independent mechanisms.
Caloric restriction and exercise to reduce pancreatic fat
The DiRECT trial showed that T2D was reversible in almost half of the patients that received a very low-calorie diet over 3–5 months [193]. In a smaller, earlier study of acute weight loss with a very low-calorie diet, recovery of the first-phase insulin secretion was associated with a decrease in pancreatic fat [194]. A further study evaluating normal glucose tolerant and T2D patients undergoing bariatric surgery found that the decrease in pancreatic fat was specific to T2D patients and did not occur in non-diabetic individuals, despite a similar weight loss [195]. This suggested that pancreatic triacylglycerol accumulation is not a mere reflection of obesity and may be associated with T2D-related metabolic abnormalities. However, in a subgroup of DiRECT study participants who underwent detailed metabolic testing, the decrease in intrapacreatic fat was similar in patients who had diabetes remission and in non-responders [196]. Fat accumulation in human pancreas mostly occurs in intrapancreatic adipocytes that can occupy up to 20% of pancreas area [197]. Local lipolysis could induce β-cell lipotoxicity in a paracrine manner. Whether a causal relationship exists between pancreatic fat deposition and declining β-cell function remains an open question (reviewed in [198]).
Two weeks of exercise training decreased pancreatic fat in non-diabetic, prediabetic (impaired fasting glucose and/or impaired glucose tolerance) and T2D subjects, especially in those with fatty pancreas, with some improvement in measures of β-cell function [199].
Novel therapies
In search for new therapeutic targets against glucolipotoxicity, a high-throughput screening study was performed on rat β-cells exposed to palmitate. Applying computational methods to the initial hits pointed to mitogen-activated protein kinase 4 (MAP4K4) inhibition as a β-cell protective mechanism. Genetic variants in MAP4K4 have been associated with prediabetes. Pharmacological and siRNA-mediated inhibition of MAP4K4 partially reverted lipotoxic cell death in human islets [200]. MAP4K4 inhibition has been shown to protect β-cells against TNF-α-induced suppression of insulin secretion in vitro [201]. In adipocytes, MAP4K4 is a negative regulator of PPARγ [202]. Whether the protection from lipotoxicity by MAP4K4 inhibition is secondary to anti-inflammatory effects or other mechanisms is unclear.
PERSPECTIVES.
In conclusion, there is substantial evidence (including human studies) that lipo- and glucolipotoxicity contribute to β-cell demise in T2D. In experimental models, lipo- and glucolipotoxicity adversely affect many steps of the insulin production and secretion machinery (Figs. 3 & 4). The underlying mechanisms are still unclear but likely involve the generation of intracellular lipid metabolites (Fig. 5) and several interrelated and stress-induced pathways (Fig. 6). While much progress has been made in identifying the molecular basis of lipo- and glucolipotoxicity in experimental systems, whether or not this phenomenon actually plays an important role in human T2D remains debated. There are several reasons for this controversy. First, as discussed in this review, mimicking in vivo FFA levels in in vitro systems is technically challenging. Second, the changes brought about by lipo- and glucolipotoxic conditions are, by definition, progressive and chronic, which precludes their direct assessment in short-term clinical studies. Third, the difficulty to accurately quantify β-cell function and our inability to quantify β-cell mass in humans represent major limitations for glucolipotoxicity studies. With the development of in vivo β-cell imaging methods [203], it will hopefully become possible to longitudinally assess β-cell mass in the future and therefore assess the impact of fuel surfeit on human β-cells.
Highlights.
Prolonged exposure to elevated FFA and glucose levels contributes to β-cell demise
Human and genetic studies support the relevance of lipotoxicity for T2D
Mimicking prevailing in situ islet FFA levels in in vitro experiments is challenging
Glucolipotoxicity impairs key steps in insulin biosynthesis and release
FFAs elicit ER and oxidative stress and impair autophagy, causing β-cell apoptosis
ACKNOWLEDGMENTS
The authors’ work was funded by the European Union’s Horizon 2020 research and innovation programme, project T2DSystems, under grant agreement No 667191, the Innovative Medicines Initiative 2 Joint Undertaking Rhapsody, under grant agreement No 115881, supported by the European Union’s Horizon 2020 research and innovation programme, EFPIA and the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number 16.0097, the Fonds National de la Recherche Scientifique (FNRS), the Brussels Region Innoviris project DiaType to MC; the Fonds Erasme for Medical Research to ML and MC; the National Institutes of Health (grant R01-DK-58096 to VP); and the Canadian Institutes of Health Research (grant MOP 77686 to VP). VP holds the Canada Research Chair in Diabetes and Pancreatic Beta Cell Function. ALC was supported by the Association pour la Recherche sur le Diabète and the Société Française d’Endocrinologie et Diabétologie Pédiatrique.
Abbreviations
- ACC
acetyl-coA carboxylase
- BMI
body mass index
- BSA
bovine serum albumin
- ER
endoplasmic reticulum
- FFA
free fatty acid
- GSIS
glucose-stimulated insulin secretion
- GWAS
genome-wide association study
- JNK
c-Jun N-terminal kinase
- LPL
lipoprotein lipase
- PBA
4-phenyl butyric acid
- PKC
protein kinase C
- PPARγ
peroxisome proliferator-activated receptor γ
- ROS
reactive oxygen species
- T2D
type 2 diabetes
- UCP2
uncoupling protein-2
- ZDF
Zucker Diabetic Fatty
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Organization WH. Obesity and overweight. 2018; February 16.
- [2].Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008;37:635–46, viii-ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29. [DOI] [PubMed] [Google Scholar]
- [4].Prentki M, Corkey BE. Are the beta-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes. 1996;45:273–83. [DOI] [PubMed] [Google Scholar]
- [5].Chavez JA, Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim Biophys Acta. 2010;1801:252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53:1270–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weir GC. Glucolipotoxicity, beta-Cells, and Diabetes: The Emperor Has No Clothes. Diabetes. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carpentier A, Mittelman SD, Lamarche B, Bergman RN, Giacca A, Lewis GF. Acute enhancement of insulin secretion by FFA in humans is lost with prolonged FFA elevation. Am J Physiol. 1999;276:E1055–66. [DOI] [PubMed] [Google Scholar]
- [10].Paolisso G, Gambardella A, Amato L, Tortoriello R, D’Amore A, Varricchio M, et al. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia. 1995;38:1295–9. [DOI] [PubMed] [Google Scholar]
- [11].Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF. Lipid-induced pancreatic beta-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab. 2011;300:E255–62. [DOI] [PubMed] [Google Scholar]
- [12].Carpentier AC, Bourbonnais A, Frisch F, Giacca A, Lewis GF. Plasma nonesterified Fatty Acid intolerance and hyperglycemia are associated with intravenous lipid-induced impairment of insulin sensitivity and disposition index. J Clin Endocrinol Metab. 2010;95:1256–64. [DOI] [PubMed] [Google Scholar]
- [13].Leung N, Sakaue T, Carpentier A, Uffelman K, Giacca A, Lewis GF. Prolonged increase of plasma non-esterified fatty acids fully abolishes the stimulatory effect of 24 hours of moderate hyperglycaemia on insulin sensitivity and pancreatic beta-cell function in obese men. Diabetologia. 2004;47:204–13. [DOI] [PubMed] [Google Scholar]
- [14].Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52:2461–74. [DOI] [PubMed] [Google Scholar]
- [15].Storgaard H, Jensen Cb, Vaag AA, Volund A, Madsbad S. Insulin secretion after short- and long-term low-grade free fatty acid infusion in men with increased risk of developing type 2 diabetes. Metabolism. 2003;52:885–94. [DOI] [PubMed] [Google Scholar]
- [16].Carpentier A, Mittelman SD, Bergman RN, Giacca A, Lewis GF. Prolonged elevation of plasma free fatty acids impairs pancreatic beta-cell function in obese nondiabetic humans but not in individuals with type 2 diabetes. Diabetes. 2000;49:399–408. [DOI] [PubMed] [Google Scholar]
- [17].Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E1775–81. [DOI] [PubMed] [Google Scholar]
- [18].Paolisso G, Tagliamonte MR, Rizzo MR, Gualdiero P, Saccomanno F, Gambardella A, et al. Lowering fatty acids potentiates acute insulin response in first degree relatives of people with type II diabetes. Diabetologia. 1998;41:1127–32. [DOI] [PubMed] [Google Scholar]
- [19].Astiarraga B, Chueire VB, Souza AL, Pereira-Moreira R, Monte Alegre S, Natali A, et al. Effects of acute NEFA manipulation on incretin-induced insulin secretion in participants with and without type 2 diabetes. Diabetologia. 2018;61:1829–37. [DOI] [PubMed] [Google Scholar]
- [20].Zhou YP, Grill VE. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. The Journal of Clinical Investigation. 1994;93:870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Roomp K, Kristinsson H, Schvartz D, Ubhayasekera K, Sargsyan E, Manukyan L, et al. Combined lipidomic and proteomic analysis of isolated human islets exposed to palmitate reveals time-dependent changes in insulin secretion and lipid metabolism. PLoS One. 2017;12:e0176391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou YP, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. 1995;80:1584–90. [DOI] [PubMed] [Google Scholar]
- [23].Rebelos E, Seghieri M, Natali A, Balkau B, Golay A, Piatti PM, et al. Influence of endogenous NEFA on beta cell function in humans. Diabetologia. 2015;58:2344–51. [DOI] [PubMed] [Google Scholar]
- [24].Axelsen M, Smith U, Eriksson JW, Taskinen MR, Jansson PA. Postprandial hypertriglyceridemia and insulin resistance in normoglycemic first-degree relatives of patients with type 2 diabetes. Ann Intern Med. 1999;131:27–31. [DOI] [PubMed] [Google Scholar]
- [25].Johnston LW, Harris SB, Retnakaran R, Giacca A, Liu Z, Bazinet RP, et al. Association of NEFA composition with insulin sensitivity and beta cell function in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort. Diabetologia. 2018;61:821–30. [DOI] [PubMed] [Google Scholar]
- [26].Paolisso G, Tataranni PA, Foley Je, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38:1213–7. [DOI] [PubMed] [Google Scholar]
- [27].Xiao C, Giacca A, Carpentier A, Lewis GF. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia. 2006;49:1371–9. [DOI] [PubMed] [Google Scholar]
- [28].Imamura F, Sharp SJ, Koulman A, Schulze MB, Kroger J, Griffin JL, et al. A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study. PLoS Med. 2017;14:e1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes. 1994;43:1353–7. [DOI] [PubMed] [Google Scholar]
- [30].Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH, Investigators AS. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:91–8. [DOI] [PubMed] [Google Scholar]
- [31].Laaksonen DE, Lakka TA, Lakka HM, Nyyssonen K, Rissanen T, Niskanen LK, et al. Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabet Med. 2002;19:456–64. [DOI] [PubMed] [Google Scholar]
- [32].Ma W, Wu JH, Wang Q, Lemaitre RN, Mukamal KJ, Djousse L, et al. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. The lancet Diabetes & endocrinology. 2014;2:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Forouhi NG, Imamura F, Sharp SJ, Koulman A, Schulze MB, Zheng J, et al. Association of Plasma Phospholipid n-3 and n-6 Polyunsaturated Fatty Acids with Type 2 Diabetes: The EPIC-InterAct Case-Cohort Study. PLoS Med. 2016;13:e1002094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–63. [DOI] [PubMed] [Google Scholar]
- [36].Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008;121:2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sargsyan E, Artemenko K, Manukyan L, Bergquist J, Bergsten P. Oleate protects beta-cells from the toxic effect of palmitate by activating pro-survival pathways of the ER stress response. Biochim Biophys Acta. 2016;1861:1151–60. [DOI] [PubMed] [Google Scholar]
- [38].Mahajan A, Wessel J, Willems SM, Zhao W, Robertson NR, Chu AY, et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet. 2018;50:559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50:1505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ingelsson E, Langenberg C, Hivert MF, Prokopenko I, Lyssenko V, Dupuis J, et al. Detailed physiologic characterization reveals diverse mechanisms for novel genetic Loci regulating glucose and insulin metabolism in humans. Diabetes. 2010;59:1266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2011;93:127–42. [DOI] [PubMed] [Google Scholar]
- [43].Sako Y, Grill VE. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990;127:1580–9. [DOI] [PubMed] [Google Scholar]
- [44].Cruz WS, Kwon G, Marshall CA, McDaniel ML, Semenkovich CF. Glucose and insulin stimulate heparin-releasable lipoprotein lipase activity in mouse islets and INS-1 cells. A potential link between insulin resistance and beta-cell dysfunction. J Biol Chem. 2001;276:12162–8. [DOI] [PubMed] [Google Scholar]
- [45].Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes. 2006;55 Suppl 2:S16–23. [DOI] [PubMed] [Google Scholar]
- [46].Spector AA. Fatty acid binding to plasma albumin. J Lipid Res. 1975;16:165–79. [PubMed] [Google Scholar]
- [47].van der Vusse GJ. Albumin as fatty acid transporter. Drug Metab Pharmacokinet. 2009;24:300–7. [DOI] [PubMed] [Google Scholar]
- [48].Kleinfeld AM, Prothro D, Brown DL, Davis RC, Richieri GV, DeMaria A. Increases in serum unbound free fatty acid levels following coronary angioplasty. Am J Cardiol. 1996;78:1350–4. [DOI] [PubMed] [Google Scholar]
- [49].Fujiwara S, Amisaki T. Fatty acid binding to serum albumin: molecular simulation approaches. Biochim Biophys Acta. 2013;1830:5427–34. [DOI] [PubMed] [Google Scholar]
- [50].Alsabeeh N, Chausse B, Kakimoto PA, Kowaltowski AJ, Shirihai O. Cell culture models of fatty acid overload: Problems and solutions. Biochimica et biophysica acta Molecular and cell biology of lipids. 2018;1863:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontes G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Oliveira AF, Cunha DA, Ladriere L, Igoillo-Esteve M, Bugliani M, Marchetti P, et al. In vitro use of free fatty acids bound to albumin: A comparison of protocols. Biotechniques. 2015;58:228–33. [DOI] [PubMed] [Google Scholar]
- [53].Richieri GV, Anel A, Kleinfeld AM. Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32:7574–80. [DOI] [PubMed] [Google Scholar]
- [54].Simard JR, Kamp F, Hamilton JA. Acrylodan-labeled intestinal fatty acid-binding protein to measure concentrations of unbound fatty acids. Methods Mol Biol. 2007;400:27–43. [DOI] [PubMed] [Google Scholar]
- [55].Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–70. [DOI] [PubMed] [Google Scholar]
- [56].Shimabukuro M, Higa M, Zhou YT, Wang MY, Newgard CB, Unger RH. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–90. [DOI] [PubMed] [Google Scholar]
- [57].Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–79. [DOI] [PubMed] [Google Scholar]
- [58].Boslem E, Meikle PJ, Biden TJ. Roles of ceramide and sphingolipids in pancreatic beta-cell function and dysfunction. Islets. 2012;4:177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Veret J, Bellini L, Giussani P, Ng C, Magnan C, Le Stunff H. Roles of Sphingolipid Metabolism in Pancreatic beta Cell Dysfunction Induced by Lipotoxicity. Journal of clinical medicine. 2014;3:646–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, Poitout V. Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem. 2003;278:30015–21. [DOI] [PubMed] [Google Scholar]
- [61].Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. [DOI] [PubMed] [Google Scholar]
- [62].Hagman DK, Latour MG, Chakrabarti SK, Fontes G, Amyot J, Tremblay C, et al. Cyclical and alternating infusions of glucose and intralipid in rats inhibit insulin gene expression and Pdx-1 binding in islets. Diabetes. 2008;57:424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Magnan C, Collins S, Berthault MF, Kassis N, Vincent M, Gilbert M, et al. Lipid infusion lowers sympathetic nervous activity and leads to increased beta-cell responsiveness to glucose. J Clin Invest. 1999;103:413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Steil GM, Trivedi N, Jonas JC, Hasenkamp WM, Sharma A, Bonner-Weir S, et al. Adaptation of beta-cell mass to substrate oversupply: enhanced function with normal gene expression. Am J Physiol Endocrinol Metab. 2001;280:E788–96. [DOI] [PubMed] [Google Scholar]
- [65].Ivovic A, Oprescu AI, Koulajian K, Mori Y, Eversley JA, Zhang L, et al. IKKbeta inhibition prevents fat-induced beta cell dysfunction in vitro and in vivo in rodents. Diabetologia. 2017;60:2021–32. [DOI] [PubMed] [Google Scholar]
- [66].Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, Lewis GF, et al. Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes. 1999;48:524–30. [DOI] [PubMed] [Google Scholar]
- [67].Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, et al. Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes. 2007;56:2927–37. [DOI] [PubMed] [Google Scholar]
- [68].Goh TT, Mason TM, Gupta N, So A, Lam TK, Lam L, et al. Lipid-induced beta-cell dysfunction in vivo in models of progressive beta-cell failure. Am J Physiol Endocrinol Metab. 2007;292:E549–60. [DOI] [PubMed] [Google Scholar]
- [69].Fontes G, Zarrouki B, Hagman DK, Latour MG, Semache M, Roskens V, et al. Glucolipotoxicity age-dependently impairs beta cell function in rats despite a marked increase in beta cell mass. Diabetologia. 2010;53:2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–97. [DOI] [PubMed] [Google Scholar]
- [71].Steiner DJ, Kim A, Miller K, Hara M. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, Brown LJ, et al. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, atp citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem. 2011;286:18383–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–6. [DOI] [PubMed] [Google Scholar]
- [74].Parnaud G, Bosco D, Berney T, Pattou F, Kerr-Conte J, Donath MY, et al. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51:91–100. [DOI] [PubMed] [Google Scholar]
- [75].Peshavaria M, Larmie BL, Lausier J, Satish B, Habibovic A, Roskens V, et al. Regulation of pancreatic beta-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes. 2006;55:3289–98. [DOI] [PubMed] [Google Scholar]
- [76].Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. [DOI] [PubMed] [Google Scholar]
- [77].Cnop M, Igoillo-Esteve M, Hughes SJ, Walker JN, Cnop I, Clark A. Longevity of human islet α- and β-cells. Diabetes Obes Metab. 2011;13 Suppl 1:39–46. [DOI] [PubMed] [Google Scholar]
- [78].Cnop M, Hughes SJ, Igoillo-Esteve M, Hoppa MB, Sayyed F, van de Laar L, et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53:321–30. [DOI] [PubMed] [Google Scholar]
- [79].Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab. 2010;95:E234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gargani S, Thevenet J, Yuan JE, Lefebvre B, Delalleau N, Gmyr V, et al. Adaptive changes of human islets to an obesogenic environment in the mouse. Diabetologia. 2013;56:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hagman DK, Hays LB, Parazzoli SD, Poitout V. Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem. 2005;280:32413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V. Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes. 2001;50:315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fontes G, Semache M, Hagman DK, Tremblay C, Shah R, Rhodes CJ, et al. Involvement of Per-Arnt-Sim Kinase and extracellular-regulated kinases-1/2 in palmitate inhibition of insulin gene expression in pancreatic beta-cells. Diabetes. 2009;58:2048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jacqueminet S, Briaud I, Rouault C, Reach G, Poitout V. Inhibition of insulin gene expression by long-term exposure of pancreatic beta cells to palmitate is dependent on the presence of a stimulatory glucose concentration. Metabolism. 2000;49:532–6. [DOI] [PubMed] [Google Scholar]
- [85].Gremlich S, Bonny C, Waeber G, Thorens B. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem. 1997;272:30261–9. [DOI] [PubMed] [Google Scholar]
- [86].Plaisance V, Perret V, Favre D, Abderrahmani A, Yang JY, Widmann C, et al. Role of the transcriptional factor C/EBPbeta in free fatty acid-elicited beta-cell failure. Mol Cell Endocrinol. 2009;305:47–55. [DOI] [PubMed] [Google Scholar]
- [87].Ritz-Laser B, Meda P, Constant I, Klages N, Charollais A, Morales A, et al. Glucose-induced preproinsulin gene expression is inhibited by the free fatty acid palmitate. Endocrinology. 1999;140:4005–14. [DOI] [PubMed] [Google Scholar]
- [88].Solinas G, Naugler W, Galimi F, Lee MS, Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc Natl Acad Sci U S A. 2006;103:16454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kim JW, You YH, Ham DS, Cho JH, Ko SH, Song KH, et al. Suppression of peroxisome proliferator-activated receptor gamma-coactivator-1alpha normalizes the glucolipotoxicity-induced decreased BETA2/NeuroD gene transcription and improved glucose tolerance in diabetic rats. Endocrinology. 2009;150:4074–83. [DOI] [PubMed] [Google Scholar]
- [90].Ng MC, Shriner D, Chen BH, Li J, Chen WM, Guo X, et al. Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS genetics. 2014;10:e1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Manukyan L, Ubhayasekera SJ, Bergquist J, Sargsyan E, Bergsten P. Palmitate-induced impairments of beta-cell function are linked with generation of specific ceramide species via acylation of sphingosine. Endocrinology. 2015;156:802–12. [DOI] [PubMed] [Google Scholar]
- [92].Semplici F, Vaxillaire M, Fogarty S, Semache M, Bonnefond A, Fontes G, et al. Human mutation within Per-Arnt-Sim (PAS) domain-containing protein kinase (PASK) causes basal insulin hypersecretion. J Biol Chem. 2011;286:44005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest. 1998;101:1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Prentki M, Madiraju SR. Glycerolipid/free fatty acid cycle and islet beta-cell function in health, obesity and diabetes. Mol Cell Endocrinol. 2012;353:88–100. [DOI] [PubMed] [Google Scholar]
- [95].Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 2013;18:162–85. [DOI] [PubMed] [Google Scholar]
- [96].Barlow J, Affourtit C. Novel insights into pancreatic beta-cell glucolipotoxicity from real-time functional analysis of mitochondrial energy metabolism in INS-1E insulinoma cells. Biochem J. 2013;456:417–26. [DOI] [PubMed] [Google Scholar]
- [97].Somesh BP, Verma MK, Sadasivuni MK, Mammen-Oommen A, Biswas S, Shilpa PC, et al. Chronic glucolipotoxic conditions in pancreatic islets impair insulin secretion due to dysregulated calcium dynamics, glucose responsiveness and mitochondrial activity. BMC Cell Biol. 2013;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Zraika S, Koh DS, Barrow BM, Lu B, Kahn SE, Andrikopoulos S. Neprilysin deficiency protects against fat-induced insulin secretory dysfunction by maintaining calcium influx. Diabetes. 2013;62:1593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diabetes Obes Metab. 2010;12 Suppl 2:141–8. [DOI] [PubMed] [Google Scholar]
- [100].Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, et al. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302–10. [DOI] [PubMed] [Google Scholar]
- [101].Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105:745–55. [DOI] [PubMed] [Google Scholar]
- [102].Chan CB, MacDonald PE, Saleh MC, Johns DC, Marban E, Wheeler MB. Overexpression of uncoupling protein 2 inhibits glucose-stimulated insulin secretion from rat islets. Diabetes. 1999;48:1482–6. [DOI] [PubMed] [Google Scholar]
- [103].Joseph JW, Koshkin V, Zhang CY, Wang J, Lowell BB, Chan CB, et al. Uncoupling protein 2 knockout mice have enhanced insulin secretory capacity after a high-fat diet. Diabetes. 2002;51:3211–9. [DOI] [PubMed] [Google Scholar]
- [104].Lameloise N, Muzzin P, Prentki M, Assimacopoulos-Jeannet F. Uncoupling protein 2: a possible link between fatty acid excess and impaired glucose-induced insulin secretion? Diabetes. 2001;50:803–9. [DOI] [PubMed] [Google Scholar]
- [105].Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes. 2002;51:2749–56. [DOI] [PubMed] [Google Scholar]
- [106].Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, Lin CT, et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–27. [DOI] [PubMed] [Google Scholar]
- [107].Joseph JW, Koshkin V, Saleh MC, Sivitz WI, Zhang CY, Lowell BB, et al. Free fatty acid-induced beta-cell defects are dependent on uncoupling protein 2 expression. J Biol Chem. 2004;279:51049–56. [DOI] [PubMed] [Google Scholar]
- [108].Lee SC, Robson-Doucette CA, Wheeler MB. Uncoupling protein 2 regulates reactive oxygen species formation in islets and influences susceptibility to diabetogenic action of streptozotocin. J Endocrinol. 2009;203:33–43. [DOI] [PubMed] [Google Scholar]
- [109].Pi J, Bai Y, Daniel KW, Liu D, Lyght O, Edelstein D, et al. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology. 2009;150:3040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Robson-Doucette CA, Sultan S, Allister EM, Wikstrom JD, Koshkin V, Bhattacharjee A, et al. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes. 2011;60:2710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ciregia F, Bugliani M, Ronci M, Giusti L, Boldrini C, Mazzoni MR, et al. Palmitate-induced lipotoxicity alters acetylation of multiple proteins in clonal p cells and human pancreatic islets. Sci Rep. 2017;7:13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Baldwin AC, Green CD, Olson LK, Moxley MA, Corbett JA. A role for aberrant protein palmitoylation in FFA-induced ER stress and beta-cell death. Am J Physiol Endocrinol Metab. 2012;302:E1390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ohtsubo K, Chen MZ, Olefsky JM, Marth JD. Pathway to diabetes through attenuation of pancreatic beta cell glycosylation and glucose transport. Nat Med. 2011;17:1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Andersson SA, Olsson AH, Esguerra JL, Heimann E, Ladenvall C, Edlund A, et al. Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes. Mol Cell Endocrinol. 2012;364:36–45. [DOI] [PubMed] [Google Scholar]
- [115].Gandasi NR, Yin P, Riz M, Chibalina MV, Cortese G, Lund PE, et al. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J Clin Invest. 2017;127:2353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hoppa MB, Collins S, Ramracheya R, Hodson L, Amisten S, Zhang Q, et al. Chronic Palmitate Exposure Inhibits Insulin Secretion by Dissociation of Ca(2+) Channels from Secretory Granules. Cell Metab. 2011;13:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Olofsson CS, Collins S, Bengtsson M, Eliasson L, Salehi A, Shimomura K, et al. Long-term exposure to glucose and lipids inhibits glucose-induced insulin secretion downstream of granule fusion with plasma membrane. Diabetes. 2007;56:1888–97. [DOI] [PubMed] [Google Scholar]
- [118].Fu J, Dai X, Plummer G, Suzuki K, Bautista A, Githaka JM, et al. Kv2.1 Clustering Contributes to Insulin Exocytosis and Rescues Human beta-Cell Dysfunction. Diabetes. 2017;66:1890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Johnston NR, Mitchell RK, Haythorne E, Pessoa MP, Semplici F, Ferrer J, et al. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab. 2016;24:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Schmitz-Peiffer C, Laybutt DR, Burchfield JG, Gurisik E, Narasimhan S, Mitchell CJ, et al. Inhibition of PKCepsilon improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab. 2007;6:320–8. [DOI] [PubMed] [Google Scholar]
- [121].Cantley J, Burchfield JG, Pearson GL, Schmitz-Peiffer C, Leitges M, Biden TJ. Deletion of PKCepsilon selectively enhances the amplifying pathways of glucose-stimulated insulin secretion via increased lipolysis in mouse beta-cells. Diabetes. 2009;58:1826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486–94. [DOI] [PubMed] [Google Scholar]
- [123].Cnop M, Toivonen S, Igoillo-Esteve M, Salpea P. Endoplasmic reticulum stress and eIF2alpha phosphorylation: The Achilles heel of pancreatic beta cells. Molecular metabolism. 2017;6:1024–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E690–701. [DOI] [PubMed] [Google Scholar]
- [125].Hara T, Mahadevan J, Kanekura K, Hara M, Lu S, Urano F. Calcium efflux from the endoplasmic reticulum leads to beta-cell death. Endocrinology. 2014;155:758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Boslem E, Weir JM, MacIntosh G, Sue N, Cantley J, Meikle PJ, et al. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic beta-cells. J Biol Chem. 2013;288:26569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Petremand J, Puyal J, Chatton JY, Duprez J, Allagnat F, Frias M, et al. HDLs protect pancreatic beta-cells against ER stress by restoring protein folding and trafficking. Diabetes. 2012;61:1100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Preston AM, Gurisik E, Bartley C, Laybutt DR, Biden TJ. Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia. 2009;52:2369–73. [DOI] [PubMed] [Google Scholar]
- [129].Jeffrey KD, Alejandro EU, Luciani DS, Kalynyak TB, Hu X, Li H, et al. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci U S A. 2008;105:8452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Krizhanovskii C, Fred RG, Oskarsson ME, Westermark GT, Welsh N. Addition of exogenous sodium palmitate increases the IAPP/insulin mRNA ratio via GPR40 in human EndoC-betaH1 cells. Ups J Med Sci. 2017;122:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Qi D, Cai K, Wang O, Li Z, Chen J, Deng B, et al. Fatty acids induce amylin expression and secretion by pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2010;298:E99–e107. [DOI] [PubMed] [Google Scholar]
- [132].Cnop M, Abdulkarim B, Bottu G, Cunha DA, Igoillo-Esteve M, Masini M, et al. RNA sequencing identifies dysregulation of the human pancreatic islet transcriptome by the saturated fatty acid palmitate. Diabetes. 2014;63:1978–93. [DOI] [PubMed] [Google Scholar]
- [133].Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. [DOI] [PubMed] [Google Scholar]
- [135].Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A. 2013;110:4628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Cunha DA, Igoillo-Esteve M, Gurzov EN, Germano CM, Naamane N, Marhfour I, et al. Death protein 5 and p53-upregulated modulator of apoptosis mediate the endoplasmic reticulum stress-mitochondrial dialog triggering lipotoxic rodent and human beta-cell apoptosis. Diabetes. 2012;61:2763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Leloup C, Tourrel-Cuzin C, Magnan C, Karaca M, Castel J, Carneiro L, et al. Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes. 2009;58:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Elsner M, Gehrmann W, Lenzen S. Peroxisome-generated hydrogen peroxide as important mediator of lipotoxicity in insulin-producing cells. Diabetes. 2011;60:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Morgan D, Oliveira-Emilio HR, Keane D, Hirata AE, Santos da Rocha M, Bordin S, et al. Glucose, palmitate and pro-inflammatory cytokines modulate production and activity of a phagocyte-like NADPH oxidase in rat pancreatic islets and a clonal beta cell line. Diabetologia. 2007;50:359–69. [DOI] [PubMed] [Google Scholar]
- [141].Li N, Frigerio F, Maechler P. The sensitivity of pancreatic beta-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem Soc Trans. 2008;36:930–4. [DOI] [PubMed] [Google Scholar]
- [142].Grishko V, Rachek L, Musiyenko S, Ledoux SP, Wilson GL. Involvement of mtDNA damage in free fatty acid-induced apoptosis. Free Radic Biol Med. 2005;38:755–62. [DOI] [PubMed] [Google Scholar]
- [143].Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A, et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab. 2008;8:325–32. [DOI] [PubMed] [Google Scholar]
- [145].Masini M, Bugliani M, Lupi R, del Guerra S, Boggi U, Filipponi F, et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083–6. [DOI] [PubMed] [Google Scholar]
- [146].Chen YY, Sun LQ, Wang BA, Zou XM, Mu YM, Lu JM. Palmitate induces autophagy in pancreatic beta-cells via endoplasmic reticulum stress and its downstream JNK pathway. Int J Mol Med. 2013;32:1401–6. [DOI] [PubMed] [Google Scholar]
- [147].Choi SE, Lee SM, Lee YJ, Li LJ, Lee SJ, Lee JH, et al. Protective role of autophagy in palmitate-induced INS-1 beta-cell death. Endocrinology. 2009;150:126–34. [DOI] [PubMed] [Google Scholar]
- [148].Komiya K, Uchida T, Ueno T, Koike M, Abe H, Hirose T, et al. Free fatty acids stimulate autophagy in pancreatic beta-cells via JNK pathway. Biochem Biophys Res Commun. 2010;401:561–7. [DOI] [PubMed] [Google Scholar]
- [149].Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in beta-cells. J Biol Chem. 2011;286:42534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, et al. Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS One. 2012;7:e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Mir SU, George NM, Zahoor L, Harms R, Guinn Z, Sarvetnick NE. Inhibition of autophagic turnover in beta-cells by fatty acids and glucose leads to apoptotic cell death. J Biol Chem. 2015;290:6071–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Trudeau KM, Colby AH, Zeng J, Las G, Feng JH, Grinstaff MW, et al. Lysosome acidification by photoactivated nanoparticles restores autophagy under lipotoxicity. J Cell Biol. 2016;214:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Zummo FP, Cullen KS, Honkanen-Scott M, Shaw JAM, Lovat PE, Arden C. Glucagon-Like Peptide 1 Protects Pancreatic beta-Cells From Death by Increasing Autophagic Flux and Restoring Lysosomal Function. Diabetes. 2017;66:1272–85. [DOI] [PubMed] [Google Scholar]
- [154].Bugliani M, Mossuto S, Grano F, Suleiman M, Marselli L, Boggi U, et al. Modulation of Autophagy Influences the Function and Survival of Human Pancreatic Beta Cells Under Endoplasmic Reticulum Stress Conditions and in Type 2 Diabetes. Front Endocrinol (Lausanne). 2019;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Lytrivi M, Igoillo-Esteve M, Cnop M. Inflammatory stress in islet beta-cells: therapeutic implications for type 2 diabetes? Curr Opin Pharmacol. 2018;43:40–5. [DOI] [PubMed] [Google Scholar]
- [156].Igoillo-Esteve M, Marselli L, Cunha DA, Ladriere L, Ortis F, Grieco FA, et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia. 2010;53:1395–405. [DOI] [PubMed] [Google Scholar]
- [157].Boni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, et al. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150:5218–29. [DOI] [PubMed] [Google Scholar]
- [158].Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–33. [DOI] [PubMed] [Google Scholar]
- [159].Inoue H, Shirakawa J, Togashi Y, Tajima K, Okuyama T, Kyohara M, et al. Signaling between pancreatic beta cells and macrophages via S100 calcium-binding protein A8 exacerbates beta-cell apoptosis and islet inflammation. J Biol Chem. 2018;293:5934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Meyerovich K, Ortis F, Allagnat F, Cardozo AK. Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. J Mol Endocrinol. 2016;57:R1–R17. [DOI] [PubMed] [Google Scholar]
- [161].Miani M, Colli ML, Ladriere L, Cnop M, Eizirik DL. Mild endoplasmic reticulum stress augments the proinflammatory effect of IL-1beta in pancreatic rat beta-cells via the IRE1alpha/XBP1s pathway. Endocrinology. 2012;153:3017–28. [DOI] [PubMed] [Google Scholar]
- [162].Gehrmann W, Wurdemann W, Plotz T, Jorns A, Lenzen S, Elsner M. Antagonism Between Saturated and Unsaturated Fatty Acids in ROS Mediated Lipotoxicity in Rat Insulin-Producing Cells. Cell Physiol Biochem. 2015;36:852–65. [DOI] [PubMed] [Google Scholar]
- [163].Chu KY, O’Reilly L, Mellet N, Meikle PJ, Bartley C, Biden TJ. Oleate disrupts cAMP signaling, contributing to potent stimulation of pancreatic beta-cell autophagy. J Biol Chem. 2019;294:1218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–407. [DOI] [PubMed] [Google Scholar]
- [165].Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, et al. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic beta-cells from lipoapoptosis. Diabetes. 2005;54:2917–24. [DOI] [PubMed] [Google Scholar]
- [166].Thorn K, Hovsepyan M, Bergsten P. Reduced levels of SCD1 accentuate palmitate-induced stress in insulin-producing beta-cells. Lipids Health Dis. 2010;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Accili D, Talchai SC, Kim-Muller JY, Cinti F, Ishida E, Ordelheide AM, et al. When beta-cells fail: lessons from dedifferentiation. Diabetes Obes Metab. 2016;18 Suppl 1:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150:1223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Cinti F, Bouchi R, Kim-Muller JY, Ohmura Y, Sandoval PR, Masini M, et al. Evidence of beta-Cell Dedifferentiation in Human Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Bensellam M, Jonas JC, Laybutt DR. Mechanisms of beta-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol. 2018;236:R109–r43. [DOI] [PubMed] [Google Scholar]
- [171].Wice BM, Bernal-Mizrachi E, Permutt MA. Glucose and other insulin secretagogues induce, rather than inhibit, expression of Id-1 and Id-3 in pancreatic islet beta cells. Diabetologia. 2001;44:453–63. [DOI] [PubMed] [Google Scholar]
- [172].Akerfeldt MC, Laybutt DR. Inhibition of Id1 augments insulin secretion and protects against high-fat diet-induced glucose intolerance. Diabetes. 2011;60:2506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–59. [DOI] [PubMed] [Google Scholar]
- [174].Kim-Muller JY, Kim YJ, Fan J, Zhao S, Banks AS, Prentki M, et al. FoxO1 Deacetylation Decreases Fatty Acid Oxidation in beta-Cells and Sustains Insulin Secretion in Diabetes. J Biol Chem. 2016;291:10162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Tersey SA, Levasseur EM, Syed F, Farb TB, Orr KS, Nelson JB, et al. Episodic beta-cell death and dedifferentiation during diet-induced obesity and dysglycemia in male mice. FASEB J. 2018:fj201800150RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Patane G, Piro S, Rabuazzo AM, Anello M, Vigneri R, Purrello F. Metformin restores insulin secretion altered by chronic exposure to free fatty acids or high glucose: a direct metformin effect on pancreatic beta-cells. Diabetes. 2000;49:735–40. [DOI] [PubMed] [Google Scholar]
- [177].Lupi R, Del Guerra S, Fierabracci V, Marselli L, Novelli M, Patane G, et al. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes. 2002;51 Suppl 1:S134–7. [DOI] [PubMed] [Google Scholar]
- [178].Simon-Szabo L, Kokas M, Mandl J, Keri G, Csala M. Metformin attenuates palmitate-induced endoplasmic reticulum stress, serine phosphorylation of IRS-1 and apoptosis in rat insulinoma cells. PLoS One. 2014;9:e97868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Cen J, Sargsyan E, Forslund A, Bergsten P. Mechanisms of beneficial effects of metformin on fatty acid-treated human islets. J Mol Endocrinol. 2018;61:91–9. [DOI] [PubMed] [Google Scholar]
- [180].Cunha DA, Ladriere L, Ortis F, Igoillo-Esteve M, Gurzov EN, Lupi R, et al. Glucagon-like peptide-1 agonists protect pancreatic beta-cells from lipotoxic endoplasmic reticulum stress through upregulation of BiP and JunB. Diabetes. 2009;58:2851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Natalicchio A, Labarbuta R, Tortosa F, Biondi G, Marrano N, Peschechera A, et al. Exendin-4 protects pancreatic beta cells from palmitate-induced apoptosis by interfering with GPR40 and the MKK4/7 stress kinase signalling pathway. Diabetologia. 2013;56:2456–66. [DOI] [PubMed] [Google Scholar]
- [182].Chowdhury AI, Bergsten P. GLP-1 analogue recovers impaired insulin secretion from human islets treated with palmitate via down-regulation of SOCS2. Mol Cell Endocrinol. 2017;439:194–202. [DOI] [PubMed] [Google Scholar]
- [183].Winzell MS, Ahren B. Durable islet effects on insulin secretion and protein kinase A expression following exendin-4 treatment of high-fat diet-fed mice. J Mol Endocrinol. 2008;40:93–100. [DOI] [PubMed] [Google Scholar]
- [184].Shao S, Nie M, Chen C, Chen X, Zhang M, Yuan G, et al. Protective action of liraglutide in beta cells under lipotoxic stress via PI3K/Akt/FoxO1 pathway. J Cell Biochem. 2014;115:1166–75. [DOI] [PubMed] [Google Scholar]
- [185].Lupi R, Del Guerra S, Marselli L, Bugliani M, Boggi U, Mosca F, et al. Rosiglitazone prevents the impairment of human islet function induced by fatty acids: evidence for a role of PPARgamma2 in the modulation of insulin secretion. Am J Physiol Endocrinol Metab. 2004;286:E560–7. [DOI] [PubMed] [Google Scholar]
- [186].Saitoh Y, Chun-ping C, Noma K, Ueno H, Mizuta M, Nakazato M. Pioglitazone attenuates fatty acid-induced oxidative stress and apoptosis in pancreatic beta-cells. Diabetes Obes Metab. 2008;10:564–73. [DOI] [PubMed] [Google Scholar]
- [187].Hong SW, Lee J, Cho JH, Kwon H, Park SE, Rhee EJ, et al. Pioglitazone Attenuates Palmitate-Induced Inflammation and Endoplasmic Reticulum Stress in Pancreatic beta-Cells. Endocrinology and metabolism (Seoul, Korea). 2018;33:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Kono T, Ahn G, Moss DR, Gann L, Zarain-Herzberg A, Nishiki Y, et al. PPAR-gamma activation restores pancreatic islet SERCA2 levels and prevents beta-cell dysfunction under conditions of hyperglycemic and cytokine stress. Mol Endocrinol. 2012;26:257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Hogh KL, Craig MN, Uy CE, Nygren H, Asadi A, Speck M, et al. Overexpression of PPARgamma specifically in pancreatic beta-cells exacerbates obesity-induced glucose intolerance, reduces beta-cell mass, and alters islet lipid metabolism in male mice. Endocrinology. 2014;155:3843–52. [DOI] [PubMed] [Google Scholar]
- [190].Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Choi SE, Lee YJ, Jang HJ, Lee KW, Kim YS, Jun HS, et al. A chemical chaperone 4-PBA ameliorates palmitate-induced inhibition of glucose-stimulated insulin secretion (GSIS). Arch Biochem Biophys. 2008;475:109–14. [DOI] [PubMed] [Google Scholar]
- [192].Zhu Q, Zhong JJ, Jin JF, Yin XM, Miao H. Tauroursodeoxycholate, a chemical chaperone, prevents palmitate-induced apoptosis in pancreatic beta-cells by reducing ER stress. Exp Clin Endocrinol Diabetes. 2013;121:43–7. [DOI] [PubMed] [Google Scholar]
- [193].Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541–51. [DOI] [PubMed] [Google Scholar]
- [194].Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Steven S, Hollingsworth KG, Small PK, Woodcock SA, Pucci A, Aribisala B, et al. Weight Loss Decreases Excess Pancreatic Triacylglycerol Specifically in Type 2 Diabetes. Diabetes Care. 2016;39:158–65. [DOI] [PubMed] [Google Scholar]
- [196].Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS, et al. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for beta Cell Recovery. Cell Metab. 2018;28:547–56.e3. [DOI] [PubMed] [Google Scholar]
- [197].Pinnick KE, Collins SC, Londos C, Gauguier D, Clark A, Fielding BA. Pancreatic ectopic fat is characterized by adipocyte infiltration and altered lipid composition. Obesity (Silver Spring, Md). 2008;16:522–30. [DOI] [PubMed] [Google Scholar]
- [198].Saisho Y Pancreas Volume and Fat Deposition in Diabetes and Normal Physiology: Consideration of the Interplay Between Endocrine and Exocrine Pancreas. Rev Diabet Stud. 2016;13:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Heiskanen MA, Motiani KK, Mari A, Saunavaara V, Eskelinen JJ, Virtanen KA, et al. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: a randomised controlled trial. Diabetologia. 2018;61:1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Lee SH, Cunha D, Piermarocchi C, Paternostro G, Pinkerton A, Ladriere L, et al. High-throughput screening and bioinformatic analysis to ascertain compounds that prevent saturated fatty acid-induced beta-cell apoptosis. Biochem Pharmacol. 2017;138:140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Bouzakri K, Ribaux P, Halban PA. Silencing mitogen-activated protein 4 kinase 4 (MAP4K4) protects beta cells from tumor necrosis factor-alpha-induced decrease of IRS-2 and inhibition of glucose-stimulated insulin secretion. J Biol Chem. 2009;284:27892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Tang X, Guilherme A, Chakladar A, Powelka AM, Konda S, Virbasius JV, et al. An RNA interference-based screen identifies MAP4K4/NIK as a negative regulator of PPARgamma, adipogenesis, and insulin-responsive hexose transport. Proc Natl Acad Sci U S A. 2006;103:2087–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Gotthardt M, Eizirik DL, Cnop M, Brom M. Beta cell imaging - a key tool in optimized diabetes prevention and treatment. Trends Endocrinol Metab. 2014;25:375–7. [DOI] [PubMed] [Google Scholar]
- [204].Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–76. [DOI] [PubMed] [Google Scholar]