Abstract
Background
Neutrophil gelatinase-associated lipocalin (NGAL), a bacteriostatic agent, is known to inhibit erythropoiesis leading to anemia. We aimed to investigate the associations of NGAL, anemia, and renal scarring in children with febrile urinary tract infections (UTIs).
Methods
We retrospectively reviewed the medical records of 261 children with febrile UTIs. The relationship between the presence of anemia and plasma NGAL levels was investigated. NGAL performance in comparison with serum C-reactive protein (CRP) at admission and after 72 hours of treatment was also evaluated for the prediction of renal scarring as well as acute pyelonephritis (APN) and vesicoureteral reflux (VUR).
Results
Plasma NGAL levels were elevated in patients with anemia compared with those without anemia. Multiple linear regression analysis showed an inverse relationship between NGAL levels and erythrocyte counts (standard β = −0.397, P < 0.001). Increased NGAL, but not CRP, was independently associated with the presence of anemia (odds ratio [OR], 2.37; 95% confidence interval [CI], 1.07–5.27; P < 0.05). Receiver operating curve analyses showed good diagnostic profiles of pre- and post-treatment NGAL for identifying APN, VUR, and renal scarring (all P < 0.05). For detecting renal scars, the area under the curve of post-treatment NGAL (0.730; 95% CI, 0.591–0.843) was higher than that of post-treatment CRP (0.520; 95% CI, 0.395–0.643; P < 0.05). The presence of anemia and elevated NGAL at admission (> 150 ng/mL) were independent risk factors for renal scarring in children with febrile UTIs. With anemia, NGAL levels increased consecutively in children with febrile UTI without renal involvement, with APN without scar, and with APN with renal scarring.
Conclusion
Increased plasma NGAL levels may be associated with the presence of anemia and renal scarring in children with febrile UTIs.
Keywords: Fibrosis, Hypoxia, Inflammation, Lipocalin-2
Graphical Abstract
INTRODUCTION
Neutrophil gelatinase-associated lipocalin (NGAL) is a glycoprotein belonging to a lipocalin superfamily.1 It is rapidly induced by various cell types including immune cells, hepatocytes, and renal tubular cells after cellular stresses such as inflammation and ischemia.1,2 Although recent studies have suggested the clinical utility of NGAL as a biomarker of acute and chronic kidney injury.3,4 NGAL was initially identified as an antibacterial factor of natural immunity and an acute-phase protein.5 It is therefore not surprising that various inflammatory conditions including those of urinary tracts are associated with significant increases of NGAL in tissues and circulating NGAL levels.6,7,8
Recent findings indicate that the anemia of inflammation is attributable to both iron sequestration and impaired erythropoiesis.9 Several systemic conditions causing secondary anemia are also accompanied by high local and systemic expression of NGAL.7 NGAL as a “stress protein” possibly activates iron dependent response pathways.10 NGAL binds with iron particles, the siderophores, and transports them into cells after interacting with specific membrane receptors.7 At sites of infection and inflammation, NGAL is released from neutrophils to sequester bacterial ferric siderophores, thus depriving bacteria of iron and leading to a failure of bacterial growth.11 Notably, NGAL is also known as a key factor in regulating erythrocyte growth.12 In vitro culture study has revealed that NGAL can inhibit erythropoiesis via induction of apoptosis and interruption of differentiation of erythroid progenitor cells. During acute anemia, NGAL expression can be downregulated in erythroid cells through a feedback system.7,12 In contrast, NGAL expression is upregulated by anemia/hypoxia in various animal models. Experimental induction of anemia caused by phlebotomy has shown that NGAL level is elevated during acute anemia, a state where iron consumption and mobilization from stores are increased. However, the expression of NGAL is not influenced by serum iron or liver iron levels, implying that the induction of NGAL might be regulated by hypoxia independent of body iron levels.13 The association between NGAL and anemia has also been reported in several human studies, including chronic kidney diseases (CKDs) and systemic inflammation.14,15 Hence, it can be assumed that during the course of various diseases that cause secondary anemia, NGAL may play a role in the beginning and worsening of anemia itself rather than being a simple concomitant phenomenon.7
Urinary tract infection (UTI) is one of the most common bacterial infections in children.16 Intensive evaluation and treatment are often required in children with UTIs since kidney scarring related to UTI has been linked with substantial long-term morbidity.17,18,19 Discovery of suitable biomarkers for the detection of renal scarring would be helpful for timely management and evaluation in children with UTIs.20 Simultaneously, a better understanding of the pathogenesis of renal scar formation after UTI may lead to the development of effective prophylactic and/or adjunct treatment strategies.17 Recent studies have shown the clinical utility of NGAL as a biomarker in pediatric UTI.21,22,23 Particularly, we have recently reported that plasma NGAL could be a reliable predictive biomarker for acute pyelonephritis (APN) in children with febrile UTIs.21,22 In the present study, we hypothesized that NGAL might be related with the presence of acute anemia in children with UTIs and that NGAL could be useful for predicting renal scarring in children with febrile UTIs. Because NGAL, a bacteriostatic agent, is known to induce the inhibition of erythropoiesis as well as the sequestration of iron, we intended to investigate the association of NGAL, anemia, and renal scarring in children with febrile UTIs.
METHODS
Study population
We initially searched our hospital registry for pediatric patients undergoing plasma NGAL measurements between April 2013 and March 2017. Among them, all eligible individuals aged from 1 month to 15 years with their first febrile UTI were enrolled. Study subjects were included if they met the following inclusion criteria through reviewing electronic medical records retrospectively. A febrile UTI was defined as fever > 38°C related with positive urinalysis and urine culture (pure growth of > 50,000 organisms/mL).24 Urine specimens were obtained from suprapubic aspiration or urethral catheterization for nontoilet-trained infants and by a clean voided midstream for other children. Patients with previous UTI, acute kidney injury (AKI) based on pediatric risk, injury, failure, loss, and end-stage renal disease criteria,25 underlying CKD,26 and known anomalies of the kidney and urinary tract were excluded from this study. Anemia was defined as a reduction in hemoglobin concentration below the normal range: hemoglobin < 10.4 mg/dL for infants from 1 month of age to 23 months of age and < 11.4 mg/dL for children above 24 months of age.27
Protocol for assessment and management of a first febrile UTI
Patients with febrile UTIs were managed according to our department protocol. They were hospitalized and treated with empirical intravenous antibiotics (3rd generation cephalosporin) for 5 to 7 days. Antibiotic regimen was adjusted according to culture proven organism if needed. In admitted febrile UTI patients we performed blood and urine tests at the time of admission (pre-treatment) and after 72 hours of antibiotic treatment (post-treatment). Measurements of complete blood count, plasma NGAL, and serum C-reactive protein (CRP) were included. Plasma NGAL measurements were performed using the Triage NGAL assay (Alere, San Diego, CA, USA). Renal ultrasonography and 99mTc-demercaptosuccinic acid (DMSA) renal scintigraphy were performed upon UTI diagnosis. Voiding cystourethrogram (VCUG) was performed before discharge after confirming a negative urine culture. VCUG was selectively conducted in patients with abnormal findings on the ultrasound (hydronephrosis, dilatation of ureter, thickened wall of renal pelvis, signs of APN, and/or presence of renal duplication) and/or DMSA scan (renal cortical defects and/or loss of renal cortex volume). We defined hydronephrosis according to the classification of the Society for Fetal Urology.28 APN was defined when a DMSA renal scan showed focal, multifocal, or diffuse area of decreased uptake in the kidney without volume loss. Renal scar formation was evaluated on repeated DMSA scan at least 6 months later after UTIs among patients diagnosed with APN or vesicoureteral reflux (VUR). Renal scarring was defined according to the guidelines of the European Association of Nuclear Medicine.29 Patients were compared as subgroups between anemia and non-anemia and between renal scarring and no scarring, respectively.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (interquartile range). Categorical variables are described using frequencies and proportions. Student's t-test, Mann-Whitney U test, and χ2 test were performed to analyze data between two groups. Correlations between plasma NGAL or serum CRP levels and values of red blood cell (RBC) indices were calculated by Spearman's test. Stepwise multiple linear regression analysis was also used to identify the linear relationship between NGAL or CRP levels and values of RBC indices in the study population. Receiver operating characteristic (ROC) curves were constructed to determine the optimal cut-off values of NGAL and CRP for anemia, APN, VUR and renal scars. Area under the curves (AUCs), including 95% confidence intervals (CIs), were calculated and compared using a pairwise comparison method.30 Odds ratios (ORs) for the risk of anemia and renal scarring were determined by univariable and multivariable logistic regression analyses. To determine independent risk factors, variables that were found to be related to anemia and renal scar (P ≤ 0.20) in univariable analyses were entered into a multivariable logistic regression model.31 A Kruskal-Wallis test was also used to analyze differences in plasma NGAL level among groups with febrile UTI without renal involvement, with APN without renal scarring, and with APN with subsequent scar development. Data were analyzed using IBM SPSS software version 20.0 for Windows (SPSS Inc., Chicago, IL, USA). For all analyses, P values < 0.05 were considered significant.
Ethics statement
This study was approved by our Institutional Review Board (IRB) before initiation (Korea University Ansan Hospital, IRB No. 2018AS0140). The IRB waived the requirement to obtain informed consent since this study involved only a retrospective chart review of anonymous patient data that were routinely collected during clinical practice. This work was also carried out in accordance with the Declaration of Helsinki. Patients' data from our previous papers21,22 were partially included with follow-up extended to more than six months in our pediatric nephrology clinic.
RESULTS
Patient characteristics
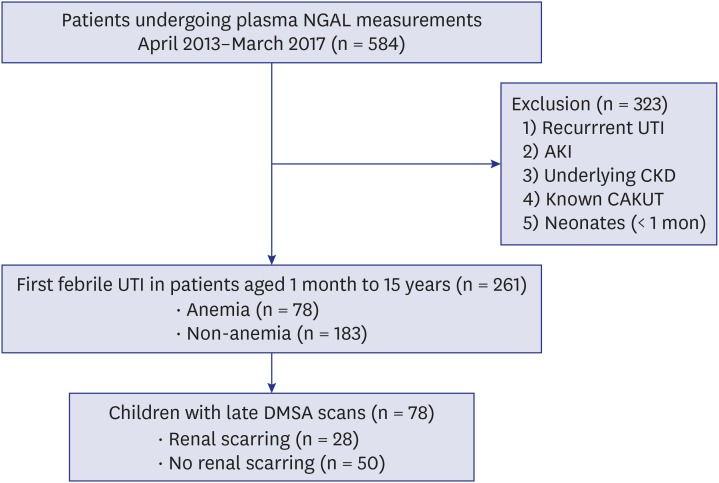
Among 584 children who underwent plasma NGAL measurements, 261 patients were enrolled in this study (Fig. 1). The mean age of patients with febrile UTIs was 19.9 ± 34.6 months (median, 6 months [3–16.5 months]). Male to female ratio was 146:115. Most of them were under the age of 24 months (n = 207, 79.3%). Among children with febrile UTIs, 100 children (38.3%) had hydronephrosis on renal ultrasonography and 124 patients (47.5%) were presented with APN on DMSA renal scan. VUR was found in 48 patients (20.8%) undergoing VCUG (n = 231). Twenty-three patients (49.9%) had bilateral VUR, and 37 patients (77%) had dilating VUR (grades 3–5). Mean duration of follow-up was 11.6 ± 13.1 months (median, 6 months [4–12 months]). Thirty-two children (17.2%) among 186 patients relapsed during the follow up period. Of 124 patients with APN, 78 children underwent late DMSA scan and renal scar formation was detected in 28 children (35.9%). Of 28 patients, 26 (96.4%) had preexistent renal scarring but only 1 (3.6%) manifested both new and preexistent renal scarring on follow-up DMSA scan.
Fig. 1. Enrollment of study patients.
NGAL = neutrophil gelatinase-associated lipocalin, UTI = urinary tract infection, AKI = acute kidney injury, CKD = chronic kidney disease, CAKUT = congenital anomaly of kidney and urinary tract, DMSA = demercaptosuccinic acid.
Relationship of anemia and plasma NGAL in children with febrile UTIs
Among 261 patients with febrile UTIs, 78 patients were assigned into the anemia group and 183 patients were allocated into the non-anemia group (Fig. 1). Mean age of the anemia group was younger than that of the non-anemia group (P < 0.001). Patients with anemia had higher incidence of hydronephrosis and APN compared to children without anemia (both P < 0.05). In the anemia group mean corpuscular volume (MCV) level increased and mean corpuscular hemoglobin concentration (MCHC) decreased (both P < 0.05). MCH was not different between the two groups. Children with anemia showed higher levels of plasma NGAL and serum CRP at admission (both P < 0.001) (Table 1).
Table 1. Clinical and laboratory data according to the presence of anemia.
| Variables | Anemia (n = 78) | Non-anemia (n = 183) | P value | |
|---|---|---|---|---|
| Age, mon | 9.42 ± 17.3 | 24.4 ± 38.9 | < 0.001a | |
| Female | 29 (37.2) | 86 (47.0) | 0.173b | |
| Hydronephrosis | 38/78 (48.7) | 62/183 (33.9) | 0.027b | |
| APN | 50/78 (64.1) | 74/183 (40.4) | 0.001b | |
| VUR | 21/75 (28.0) | 27/156 (17.3) | 0.082b | |
| Renal scarring | 16/34 (47.1) | 12/45 (26.7) | 0.096b | |
| Hemoglobin, g/dL | ||||
| < 24 mon | 9.73 ± 0.47 | 11.50 ± 0.75 | < 0.001a | |
| ≥ 24 mon | 10.70 ± 0.63 | 12.70 ± 0.90 | < 0.001a | |
| Hematocrit, % | 28.70 ± 1.62 | 33.70 ± 2.57 | < 0.001a | |
| Erythrocyte count, × 106/μL | 3.66 ± 0.49 | 4.37 ± 0.39 | < 0.001a | |
| MCV, fL | 79.30 ± 7.76 | 77.50 ± 4.57 | 0.009a | |
| MCH, pg | 27.20 ± 2.99 | 27.00 ± 1.77 | 0.239a | |
| MCHC, g/dL | 34.20 ± 0.94 | 34.90 ± 0.79 | < 0.001a | |
| White blood cells, Pre, /μL | 15,381 ± 6,125 | 15,052 ± 5,689 | 0.676a | |
| NGAL, ng/mL | 338.4 ± 372.7 | 153.3 ± 158.4 | < 0.001a | |
| CRP, mg/dL | 7.53 ± 7.16 | 3.76 ± 4.04 | < 0.001a | |
Data are presented as mean ± standard deviation or numbers (%).
APN = acute pyelonephritis, VUR = vesicoureteral reflux, MCV = mean corpuscular volume, MCH = mean corpuscular hemoglobin, MCHC = mean corpuscular hemoglobin concentration, NGAL = neutrophil gelatinase-associated lipocalin, CRP = C-reactive protein.
aMann-Whitney U test; bχ2 test.
Plasma NGAL was found to be negatively correlated with hemoglobin (r = −0.235), hematocrit (r = −0.233), and erythrocyte count (r = −0.330), and positively correlated with MCV (r = 0.220) and MCH (r = 0.165) in all participants (all P < 0.05). MCHC was not correlated with plasma NGAL level (r = −0.054). Concentration of CRP was also correlated with hemoglobin (r = −0.333), hematocrit (r = −0.322), erythrocyte count (r = −0.378), MCV (r = 0.156), and MCHC (r = −0.152) (all P < 0.05). MCH was not correlated with CRP level (r = 0.061). Multiple linear regression analysis revealed that erythrocyte counts were independently correlated with NGAL levels (standard β = −0.397, P < 0.001) or CRP concentrations (standard β = −0.320, P < 0.001), when age, sex, hemoglobin, hematocrit, MCV, MCH, and MCHC were included as independent variables (Table 2).
Table 2. Multiple linear regression analysis of the relationship between NGAL or CRP and RBC indices.
| Dependent variables | Independent variables | B | SE | β | t value | P value | VIF |
|---|---|---|---|---|---|---|---|
| NGAL | RBC count | −189.10 | 29.20 | −0.397 | −6.471 | < 0.001 | 1.00 |
| CRP | RBC count | −3.22 | 0.60 | −0.320 | −5.346 | < 0.001 | 1.00 |
Age, sex, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were included as independent variables.
NGAL = neutrophil gelatinase-associated lipocalin, CRP = C-reactive protein, RBC = red blood cell, SE = standard error, VIF = variance inflation factor.
In ROC analysis, optimal cut-off values of plasma NGAL and serum CRP for identifying anemia were 211 ng/mL (sensitivity 48.4%, specificity 81.0%) and 3.7 mg/dL (sensitivity 63.6%, specificity 62.2%), respectively. AUC difference between NGAL (0.641; 95% CI, 0.575–0.703) and CRP (0.668; 95% CI, 0.606–0.725) was not significant. Using these cut-off levels of plasma NGAL and serum CRP, univariate and multivariate logistic regression analyses were also carried out. Univariate analyses revealed that young age less than 24 months old, the presence of hydronephrosis and APN, and increased levels of plasma NGAL and serum CRP were associated with the presence of anemia in patients with febrile UTIs (all P < 0.05). However, in a multivariate analysis only elevated NGAL level and age less than 24 months were independently associated with the presence of anemia (both P < 0.05). Increased CRP was not related with the presence of anemia in patients with febrile UTIs after adjusting for age, sex, hydronephrosis, APN, VUR, and increased NGAL levels (Table 3).
Table 3. Univariate and multivariate logistic regression analyses for anemia.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, < 24 mon | 4.18 (1.70–10.20) | 0.002 | 3.76 (1.20–11.80) | 0.023 |
| Female sex | 0.68 (0.39–1.16) | 0.157 | 0.64 (0.33–1.27) | 0.205 |
| Hydronephrosis | 1.84 (1.07–3.15) | 0.027 | 1.27 (0.66–2.44) | 0.476 |
| APN | 2.67 (1.54–4.62) | < 0.001 | 1.02 (0.40–2.57) | 0.972 |
| VUR | 1.93 (1.00–3.72) | 0.050 | 1.87 (0.83–4.21) | 0.128 |
| White blood cell, /μL | 1.00 (1.00–1.00) | 0.706 | - | - |
| NGAL, > 211 ng/mL | 3.98 (2.12–7.48) | < 0.001 | 2.37 (1.07–5.27) | 0.035 |
| CRP, > 3.7 mg/dL | 2.88 (1.66–5.01) | < 0.001 | 1.53 (0.65–3.58) | 0.329 |
Variables with a P value of ≤ 0.20 in the univariable analyses were included in a multivariable logistic regression analysis, and the enter method was used to determine baseline risk factors.
APN = acute pyelonephritis, VUR = vesicoureteral reflux, NGAL = neutrophil gelatinase-associated lipocalin, CRP = C-reactive protein, OR = odds ratio, CI = confidence interval.
Anemia, plasma NGAL, and renal scarring in children with febrile UTIs
We further investigated the association of anemia, plasma NGAL, and renal scarring in children with febrile UTIs. Among 78 patients undergoing late DMSA scan, renal scarring occurred in 28 (35.9%) patients with febrile UTIs (Fig. 1). Children with renal scarring showed a higher incidence of APN and VUR, elevated serum WBC and CRP concentration, and increased plasma NGAL levels before and after antibiotic treatment as compared to patients without renal scarring (all P < 0.05) (Table 4).
Table 4. Differences of clinical and laboratory data according to the presence of renal scarring.
| Variables | Renal scarring (n = 28) | No renal scarring (n = 50) | P value |
|---|---|---|---|
| Age, mon | 20.5 ± 31.6 | 12.8 ± 22.8 | 0.114a |
| Female | 8 (28.6) | 26 (51.0) | 0.092b |
| Hydronephrosis | 17 (60.7) | 19 (37.3) | 0.077b |
| APN | 27 (96.4) | 38 (74.5) | 0.033b |
| VUR | 20 (71.4) | 14 (27.5) | < 0.001b |
| White blood cells, /μL | 19,480 ± 6,754 (n = 28) | 16,833 ± 6,353 (n = 50) | 0.031a |
| Hemoglobin, g/dL | 10.60 ± 1.18 (n = 28) | 11.10 ± 1.15 (n = 50) | 0.119a |
| Pre-NGAL, ng/mL | 497.5 ± 399.6 (n = 28) | 255.3 ± 265.5 (n = 50) | 0.002a |
| Pre-CRP, mg/dL | 11.20 ± 7.18 (n = 28) | 6.36 ± 5.30 (n = 50) | 0.003a |
| Post-NGAL, ng/mL | 144.9 ± 90.1 (n = 22) | 93.4 ± 35.6 (n = 31) | 0.005a |
| Post-CRP, mg/dL | 1.14 ± 1.13 (n = 27) | 0.94 ± 0.80 (n = 41) | 0.783a |
| Follow-up duration, mon | 16.7 ± 14.5 | 14.8 ± 13.8 | 0.518a |
| Relapse | 12 (42.9) | 11 (22.0) | 0.093b |
Data are presented as mean ± standard deviation or numbers (%).
APN = acute pyelonephritis, VUR = vesicoureteral reflux, Pre- = pre-treatment, Post- = post-treatment, NGAL = neutrophil gelatinase-associated lipocalin, CRP = C-reactive protein.
aMann-Whitney U test; bχ2 test.
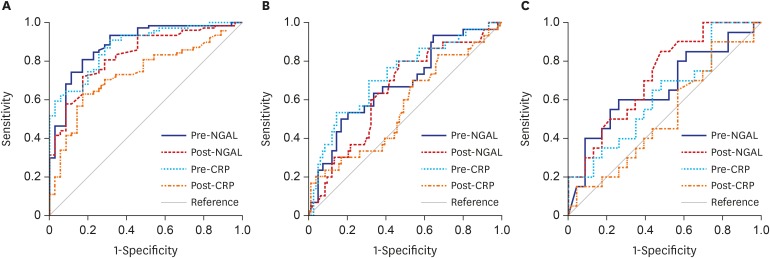
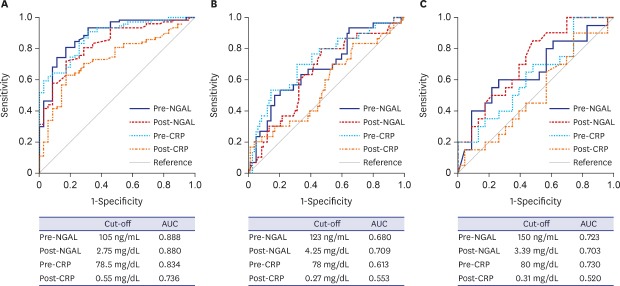
ROC analyses were performed to determine the best cut-off values of NGAL and CRP for identifying renal scars. The AUC values of NGAL and CRP to predict APN and VUR were also examined to confirm our previous findings.18,19 For detecting APN, VUR, and renal scarring, the AUCs of pre- and post-treatment NGAL were all significantly high (all P < 0.05). However, the AUCs of pre- and post-treatment CRP were considerably elevated only for detecting APN (both P < 0.05). For identifying VUR, the AUC value of pre-treatment CRP was greater than that of post-treatment CRP (P < 0.05). For detecting renal scarring, the AUC value of post-treatment NGAL was higher than that of post-treatment CRP (P < 0.05) (Table 5 and Fig. 2).
Table 5. The best cut-off values, AUC, sensitivity, and specificity of NGAL and CRP for predicting APN, VUR, and renal scarring.
| Variables | Cut-off levels | AUC (95% CI) | Sensitivity, % | Specificity, % | P value | |
|---|---|---|---|---|---|---|
| APN | ||||||
| Pre-NGAL | 105 ng/mL | 0.888 (0.822–0.954) | 89.8 | 82.5 | < 0.001 | |
| Pre-CRP | 2.75 mg/dL | 0.880 (0.818–0.943) | 91.1 | 68.6 | < 0.001 | |
| Post-NGAL | 78.5 ng/mL | 0.834 (0.757–0.911) | 72.2 | 82.9 | < 0.001 | |
| Post-CRP | 0.56 mg/dL | 0.736 (0.641–0.831) | 63.3 | 82.9 | < 0.001 | |
| VUR | ||||||
| Pre-NGAL | 123 ng/mL | 0.680 (0.611–0.743) | 76.7 | 56.6 | < 0.001 | |
| Pre-CRP | 4.25 mg/dL | 0.709 (0.645–0.767)a | 70.8 | 64.1 | < 0.001 | |
| Post-NGAL | 78 ng/mL | 0.613 (0.526–0.695) | 74.3 | 52.4 | 0.037 | |
| Post-CRP | 0.27 mg/dL | 0.553 (0.477–0.627) | 84.1 | 33.6 | 0.307 | |
| Renal scarring | ||||||
| Pre-NGAL | 150 ng/mL | 0.723 (0.602–0.824) | 92.3 | 46.5 | < 0.001 | |
| Pre-CRP | 3.39 mg/dL | 0.703 (0.590–0.801) | 100.0 | 35.3 | 0.001 | |
| Post-NGAL | 80 ng/mL | 0.730 (0.591–0.843)b | 100.0 | 38.7 | 0.001 | |
| Post-CRP | 0.31 mg/dL | 0.520 (0.395–0.643) | 92.6 | 24.4 | 0.784 | |
AUC = area under the curve, NGAL = neutrophil gelatinase-associated lipocalin, CRP = C-reactive protein, APN = acute pyelonephritis, VUR = vesicoureteral reflux, Pre- = pre-treatment, Post- = post-treatment, CI = confidence interval.
aPairwise comparison test for VUR, Pre-CRP vs. Post-CRP, P = 0.03; bPairwise comparison test for renal scarring, Post-NGAL vs. Post-CRP, P = 0.035.
Fig. 2. ROC curve analysis in children with febrile urinary tract infections. (A) ROC curves of plasma NGAL and serum CRP for predicting APN. (B) ROC curves of plasma NGAL and serum CRP for predicting VUR. (C) ROC curves of plasma NGAL and serum CRP for predicting renal scarring. While the area under the curve values of Pre- and Post-NGAL were significant to predict APN, VUR, and renal scarring, those of Pre- and Post-CRP were significant to detect APN only (all P < 0.05).
ROC = receiver operating characteristic, APN = acute pyelonephritis, VUR = vesicoureteral reflux, Pre- = pre-treatment, Post- = post-treatment, NGAL = neutrophil gelatinase-associated lipocalin, CRP = C-reactive protein.
In univariable logistic regression analyses, the presence of hydronephrosis, APN, and VUR and increased plasma NGAL levels at admission were associated with renal scarring (all P < 0.05). Elevated CRP concentration before treatment was not related with renal scarring. In a multivariable analysis, the presence of hydronephrosis and VUR, anemia, increased NGAL level before treatment, and relapse of UTI were independently associated with the development of renal scarring (all P < 0.05). The presence of anemia and plasma NGAL elevation (> 150 ng/mL, cut-off level) at admission were useful laboratory findings for identifying individuals with renal scarring in children with febrile UTIs (Table 6).
Table 6. Univariate and multivariate logistic regression analyses for renal scarring.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age, < 24 mon | 0.73 (0.21–2.56) | 0.625 | - | - |
| Female sex | 0.39 (0.14–1.03) | 0.058 | 0.27 (0.06–1.28) | 0.099 |
| Hydronephrosis | 2.60 (1.01–6.71) | 0.048 | 10.50 (1.51–72.5) | 0.017 |
| APN | 9.24 (1.14–74.9) | 0.037 | 4.22 (0.14–123.8) | 0.404 |
| VUR | 6.43 (2.30–17.9) | < 0.001 | 7.68 (1.43–41.1) | 0.017 |
| Anemia | 2.44 (0.95–6.28) | 0.063 | 8.29 (1.07–64.1) | 0.043 |
| White blood cells, /μL | 1.00 (1.00–1.00) | 0.098 | 1.00 (1.00–1.00) | 0.061 |
| Pre-NGAL, > 150 ng/mL | 10.40 (2.19–49.8) | 0.003 | 14.50 (1.34–157.1) | 0.028 |
| Pre-CRP, > 3.39 mg/dL | 1.37 × 109 (0.000–) | 0.998 | - | - |
| Relapse | 2.66 (0.97–7.26) | 0.056 | 19.00 (2.05–175.0) | 0.009 |
| Follow up duration | 1.01 (0.98–1.04) | 0.568 | - | - |
Variables with a P value of ≤ 0.20 in the univariate analysis were included in a multivariate logistic regression analysis, and the enter method was used to determine baseline risk factors.
APN = acute pyelonephritis, VUR = vesicoureteral reflux, Pre- = pre-treatment, NGAL = neutrophil gelatinase-associated lipocalin, CRP = C-reactive protein, OR = odds ratio, CI = confidence interval.
Comparison of plasma NGAL levels with and without anemia
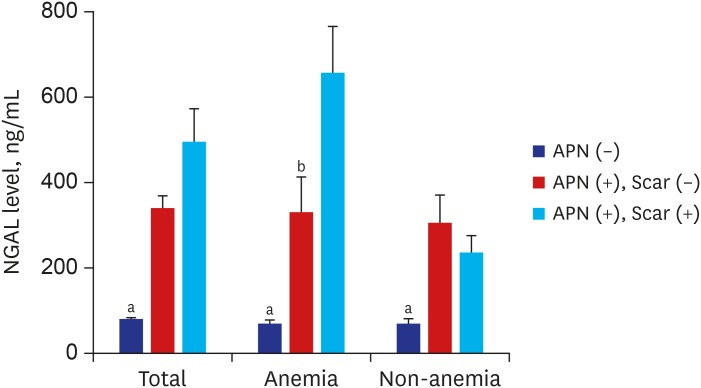
With anemia, NGAL levels increased consecutively in children with febrile UTI without APN, with APN without scar, and with APN with renal scarring (P < 0.05). Without anemia, the difference in NGAL level between patients with APN without scar and those with APN with renal scarring was not significant, while plasma NGAL levels were lower in patients with febrile UTI without APN than those with APN without scar and those with APN with renal scarring (both P < 0.05) (Fig. 3).
Fig. 3. Plasma NGAL levels at admission in febrile UTI without renal involvement (blue bar), APN without renal scarring (red bar), and APN with scar development (sky blue bar). Only in the presence of anemia, plasma NGAL concentrations were increased in consecutive order in febrile UTI without APN (n = 27), APN without scar (n = 34), and APN with scarring (n = 17) (70.6 ± 42.1 ng/mL vs. 330.8 ± 286.2 ng/mL vs. 661.1 ± 426.1 ng/mL). Without anemia, the difference in NGAL level between patients with APN without scar (n = 62) and those with APN with renal scarring (n = 11) was not significant, although plasma NGAL levels were lower in patients with febrile UTI without APN (n = 110) (83.3 ± 53.8 ng/mL) than those in patients with APN without scar (307.8 ± 287.2 ng/mL) and those with APN with renal scarring (235.9 ± 131.9 ng/mL).
NGAL = neutrophil gelatinase-associated lipocalin, APN = acute pyelonephritis, UTI = urinary tract infection.
aP < 0.05, Kruskal-Wallis test; bP < 0.05, APN without scar vs. APN with scar, Mann-Whitney test.
DISCUSSION
In this study, we investigated the association of plasma NGAL with anemia and the usefulness of plasma NGAL as a biomarker of renal scar development in pediatric patients with febrile UTIs. While both plasma NGAL and serum CRP were correlated with several indices of anemia in febrile UTI, increased NGAL, but not CRP, was independently associated with anemia during febrile UTI. We also found that elevated NGAL level and the presence of anemia were independent risk factors for renal scarring in children with febrile UTIs. Plasma levels of NGAL before and after antibiotic treatment showed good diagnostic accuracy for identifying renal scarring as well as APN and VUR. Only in the presence of anemia, NGAL levels consecutively increased in febrile UTI without renal involvement, APN without scar, and APN with subsequent renal scarring. These findings suggest plasma NGAL could be closely associated with anemia and renal scarring in children with febrile UTIs.
NGAL has emerged as a critical factor in iron homeostasis and erythrocyte growth regulation that can cause anemia when it is persistently elevated.10,14,32 Because the limitation of iron supply to erythropoiesis is a main factor in anemia of inflammation,33 we initially hypothesized that increased NGAL might be associated with the presence of anemia through modulation of iron metabolism in patients with febrile UTIs. However, levels of MCV and MCH as surrogate markers for predicting iron deficiency were not reduced in children with anemia. It is currently unclear whether hypoferremia is associated with the development of anemia during febrile UTIs since we did not check direct serum iron levels. Nevertheless, the negative correlation between plasma NGAL and erythrocyte count was distinct, suggesting that increased circulating NGAL would have an adverse effect on red cell homeostasis of the bone marrow. It might be linked to its involvement in iron balance. NGAL could contribute to regulating erythrocyte growth and iron homeostasis as well as inhibiting bacterial growth in this clinical setting. As the interaction of various specific proteins as well as the interplay between iron absorption and recycling are involved in systemic regulation of iron metabolism,34 the pathophysiology of iron homeostasis during inflammatory states would be more complex than expected. On the contrary, NGAL could be controlled by acute anemia and resulting renal hypoxia regardless of body iron levels.13 Several studies have shown that NGAL decreases renal hypoxia/reoxygenation-induced tubular cell damage via adjusting cell apoptosis or autophagy.35,36 NGAL knockdown significantly reduced the level of autophagy and the addition of exogenous NGAL diminished endotoxin-induced renal tubular cell damage, indicating the protective role of NGAL on renal tubules against septic AKI.37 Therefore, a cause-and-effect relationship between NGAL and anemia needs further research to confirm. Although the inverse relationship between CRP levels and erythrocyte counts was also revealed, the association between CRP and anemia disappeared after adjusting for age, sex, hydronephrosis, APN, VUR and high NGAL level. In patients with febrile UTIs, independent risk factors for anemia were young age less than 24 months and elevated NGAL concentration before treatment (> 211 ng/mL, cut-off value for anemia). Consistent with this observation, Shrestha et al.14 have shown that plasma NGAL levels are closely associated with erythrocytes, hemoglobin, and red cell distribution width related to inflammation in patients with chronic systolic heart failure. Choi et al.15 have also reported that high plasma NGAL concentration (> 156 ng/mL) can lead to 1.3-fold of increase in the risk of anemia in comparison with a low NGAL level (≤ 156 ng/mL) in patients with systemic inflammation including UTI. The association between NGAL levels and anemia remained significant after adjusting for confounding factors. These findings suggest elevated circulating NGAL may contribute to the development or worsening of anemia in patients with systemic inflammation. Therefore, NGAL may play a role in the presence of anemia in children with febrile UTIs. Still, more research is needed to define the pathomechanisms that lead to anemia under infectious or inflammatory conditions including UTI.
We further analyzed the usefulness of plasma NGAL as a predictor of renal scar and the association of NGAL, anemia, and renal scarring in patients with febrile UTIs. NGAL is well known as a diagnostic and prognostic marker of AKI as it is produced by injured renal tubular epithelial cells.3 It has also emerged as a promising biomarker for early identification of CKD and prediction of CKD progression.38 Upregulation of NGAL during a few days after renal insult is a possible protective mechanism to limit renal injury.11 However, persistent immune system activation and inflammation late after AKI could contribute to the pathogenesis of CKD and continued elevation of NGAL might predict sustained renal damage after AKI.39 We have previously reported that both plasma and urine NGAL levels would have good diagnostic performances for the presence of APN and/or VUR in children with UTIs without AKI.18,19,20,21 Higher plasma NGAL concentrations were independently related with an increased risk of APN in pediatric patients with febrile UTIs, suggesting that NGAL might serve as a marker of acute inflammatory condition even without AKI. In these studies, there was no relationship between NGAL and renal scarring possibly due to a small number of patients.21,22 In the present study, more patients were involved with an extended follow-up duration. No patients with AKI and underlying CKD were included. According to ROC curve analyses, plasma NGAL before and after antibiotic therapy was found to be helpful for identifying renal scarring as well as APN and VUR. The best cut-off values of pre-treatment NGAL to detect APN, VUR, and renal scarring were elevated sequentially (105 ng/mL, 123 ng/mL, and 150 ng/mL, respectively), and those of post-treatment NGAL for APN, VUR, and renal scarring were similar (78.5 ng/mL, 78 ng/mL, and 80 ng/mL, respectively). Even though the AUCs of pre-treatment CRP were sufficiently high for detecting APN, VUR, and renal scarring, those of post-treatment CRP were not helpful to predict VUR and scars. Especially for renal scarring, post-treatment NGAL revealed a higher AUC than that of post-treatment CRP. More importantly, anemia and elevated NGAL level (> 150 ng/mL, cut-off value for renal scarring) at admission were independently associated with the presence of renal scarring, in addition to the existence of hydronephrosis, VUR, and recurrence of UTI. Only in the presence of anemia, NGAL concentrations increased consecutively in febrile UTI without APN, APN without scar, and APN with subsequent renal scarring. Therefore, the presence of anemia and plasma NGAL elevation in acute phase might be relevant to future renal scar formation in children with febrile UTIs. Enhanced NGAL production may be associated with the development or worsening of anemia, acute renal parenchymal injury, and late renal scarring in patients with febrile UTIs.
The presence of APN increases the risk of renal scar formation and the potential for chronic renal insufficiency.17 Improved knowledge of the pathogenesis of APN and renal scarring will lead to effective strategies to reduce recurrent UTIs and renal fibrosis.40 Predicting high risk patients and selecting appropriate diagnostic methods are also important in the treatment of children with febrile UTIs. If there is a non-invasive marker that can reflect the severity of inflammation as well as the degree of renal damage, imaging studies are expected to be more selectively performed according to the optimally tailored strategy for individual patients. Previously, we identified plasma NGAL can be useful for identifying APN in children with febrile UTIs.21,22 In this study, we also found that NGAL, a bacteriostatic agent, could be associated with anemia and renal scarring in patients with febrile UTIs. It is presumed that more severe infection may induce higher systemic NGAL expression (an acute phase reactant), leading to an acute anemia of inflammation and later to the development of renal scarring. Although we could not determine a cause-and-effect relationship among NGAL, anemia, and renal scarring, it will be insightful to determine whether induction of NGAL might be regulated by anemia/hypoxia in the kidney during APN, and whether manipulation of this interplay could alter renal pathology and prevent progression to renal scarring in APN. Collectively, measurement of plasma NGAL can be useful in choosing children with UTIs who may need early intervention or long-term follow-up. An individualized approach based on NGAL measurements could assist pediatricians on UTI management decisions. However, further data are required for evidence-based practice guidelines.
This study has some limitations. First, physiologic anemia of infancy should be considered. Hemoglobin levels also vary depending on the age of child. To minimize these confounding factors, we excluded patients under 1 month of age and defined anemia strictly according to pediatric textbook.27 Secondly, the presence of iron deficiency or iron supplementation before UTI and iron status during the course of infection were not determined. Considering the antimicrobial property of NGAL, additional studies are necessary to address the role of NGAL in iron homeostasis and inflammation in renal damage.11 A cause-and-effect relationship between NGAL and anemia should be determined in a diverse research study as well. Lastly, this is a small retrospective study conducted in a single center with a relatively short duration of the follow-up. Patients aged from 1 month to 15 years with their first febrile UTIs were enrolled. Our results may not be generalizable to all patients with febrile UTIs since we included only hospitalized children in this study. While several imaging studies during or after a febrile UTI had been conducted according to the guidelines and recommendations of management UTI in children,24,41 selection bias should be taken into account. A follow-up DMSA scan for evaluating renal scar was performed in only 78 of 124 (62.9%) patients with APN. Larger, multicenter, prospective studies are needed in the future to better assess whether plasma NGAL could be used as a reliable marker for renal scarring in pediatric patients with febrile UTIs. In conclusion, our results suggest that plasma NGAL can be associated with the presence of anemia and that serial measurement of plasma NGAL may be useful for predicting renal scarring as well as APN and VUR in children with febrile UTIs. Understanding the biology of NGAL under these conditions is necessary for implementation strategies of its use in clinical practice. Future investigations are needed to determine the role of NGAL related to anemia/hypoxia, renal damage, and disturbance of iron homeostasis in pediatric UTI.
ACKNOWLEDGMENTS
We would like to express our great appreciation to Dr. Jaehyung Cha, Research Professor, Medical Science Research Center, Korea University Ansan Hospital, for his valuable advice in the statistical analysis of this research work.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee JH, Yim HE.
- Data curation: Lee JH, Yim HE.
- Formal analysis: Lee JH, Yim HE, Yoo KH.
- Writing - original draft: Lee JH, Yim HE.
- Writing - review & editing: Yim HE, Yoo KH.
References
- 1.Kjeldsen L, Johnsen AH, Sengeløv H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268(14):10425–10432. [PubMed] [Google Scholar]
- 2.Abella V, Scotece M, Conde J, Gómez R, Lois A, Pino J, et al. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers. 2015;20(8):565–571. doi: 10.3109/1354750X.2015.1123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerico A, Galli C, Fortunato A, Ronco C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: a review of the laboratory characteristics and clinical evidences. Clin Chem Lab Med. 2012;50(9):1505–1517. doi: 10.1515/cclm-2011-0814. [DOI] [PubMed] [Google Scholar]
- 4.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(2):337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432(7019):917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 6.Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000;1482(1-2):298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 7.Bolignano D, Coppolino G, Donato V, Lacquaniti A, Bono C, Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL): a new piece of the anemia puzzle? Med Sci Monit. 2010;16(6):RA131–5. [PubMed] [Google Scholar]
- 8.Forster CS, Devarajan P. Neutrophil gelatinase-associated lipocalin: utility in urologic conditions. Pediatr Nephrol. 2017;32(3):377–381. doi: 10.1007/s00467-016-3540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraenkel PG. Anemia of Inflammation: a review. Med Clin North Am. 2017;101(2):285–296. doi: 10.1016/j.mcna.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 11.Malyszko J, Tesar V, Macdougall IC. Neutrophil gelatinase-associated lipocalin and hepcidin: what do they have in common and is there a potential interaction? Kidney Blood Press Res. 2010;33(2):157–165. doi: 10.1159/000315436. [DOI] [PubMed] [Google Scholar]
- 12.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Lipocalin 2 functions as a negative regulator of red blood cell production in an autocrine fashion. FASEB J. 2005;19(13):1881–1883. doi: 10.1096/fj.05-3809fje. [DOI] [PubMed] [Google Scholar]
- 13.Jiang W, Constante M, Santos MM. Anemia upregulates lipocalin 2 in the liver and serum. Blood Cells Mol Dis. 2008;41(2):169–174. doi: 10.1016/j.bcmd.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrestha K, Borowski AG, Troughton RW, Klein AL, Tang WH. Association between systemic neutrophil gelatinase-associated lipocalin and anemia, relative hypochromia, and inflammation in chronic systolic heart failure. Congest Heart Fail. 2012;18(5):239–244. doi: 10.1111/j.1751-7133.2012.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JW, Fujii T, Fujii N. Elevated plasma neutrophil gelatinase-associated lipocalin level as a risk factor for anemia in patients with systemic inflammation. BioMed Res Int. 2016;2016:9195219. doi: 10.1155/2016/9195219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morello W, La Scola C, Alberici I, Montini G. Acute pyelonephritis in children. Pediatr Nephrol. 2016;31(8):1253–1265. doi: 10.1007/s00467-015-3168-5. [DOI] [PubMed] [Google Scholar]
- 17.Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011;365(3):239–250. doi: 10.1056/NEJMra1007755. [DOI] [PubMed] [Google Scholar]
- 18.Bae HJ, Park YH, Cho JH, Jang KM. Comparison of 99mTc-DMSA renal scan and power doppler ultrasonography for the detection of acute pyelonephritis and vesicoureteral reflux. Child Kidney Dis. 2018;22(2):47–51. [Google Scholar]
- 19.Lee J, Woo BW, Kim HS. Prognostic factors of renal scarring on follow-up DMSA scan in children with acute pyelonephritis. Child Kidney Dis. 2016;20(2):74–78. [Google Scholar]
- 20.Yim HE. Neutrophil gelatinase-associated lipocalin and kidney diseases. Child Kidney Dis. 2015;19(2):79–88. [Google Scholar]
- 21.Kim BK, Yim HE, Yoo KH. Plasma neutrophil gelatinase-associated lipocalin: a marker of acute pyelonephritis in children. Pediatr Nephrol. 2017;32(3):477–484. doi: 10.1007/s00467-016-3518-y. [DOI] [PubMed] [Google Scholar]
- 22.Sim JH, Yim HE, Choi BM, Lee JH, Yoo KH. Plasma neutrophil gelatinase-associated lipocalin predicts acute pyelonephritis in children with urinary tract infections. Pediatr Res. 2015;78(1):48–55. doi: 10.1038/pr.2015.59. [DOI] [PubMed] [Google Scholar]
- 23.Valdimarsson S, Jodal U, Barregård L, Hansson S. Urine neutrophil gelatinase-associated lipocalin and other biomarkers in infants with urinary tract infection and in febrile controls. Pediatr Nephrol. 2017;32(11):2079–2087. doi: 10.1007/s00467-017-3709-1. [DOI] [PubMed] [Google Scholar]
- 24.Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management. Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 25.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 26.Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 27.Lerner NB. The anemias. In: Kliegman RM, Stanton BF, St. Gemo JW, Schor NF, editors. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016. pp. 2309–2312. [Google Scholar]
- 28.Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993;23(6):478–480. doi: 10.1007/BF02012459. [DOI] [PubMed] [Google Scholar]
- 29.Piepsz A, Colarinha P, Gordon I, Hahn K, Olivier P, Roca I, et al. Guidelines for 99mTc-DMSA scintigraphy in children. Eur J Nucl Med. 2001;28(3):BP37–41. [PubMed] [Google Scholar]
- 30.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 31.Ranganathan P, Pramesh CS, Aggarwal R. Common pitfalls in statistical analysis: Logistic regression. Perspect Clin Res. 2017;8(3):148–151. doi: 10.4103/picr.PICR_87_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emans ME, Braam B, Diepenbroek A, van der Putten K, Cramer MJ, Wielders JP, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in chronic cardiorenal failure is correlated with endogenous erythropoietin levels and decreases in response to low-dose erythropoietin treatment. Kidney Blood Press Res. 2012;36(1):344–354. doi: 10.1159/000343392. [DOI] [PubMed] [Google Scholar]
- 33.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46(4):387–393. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papanikolaou G, Pantopoulos K. Systemic iron homeostasis and erythropoiesis. IUBMB Life. 2017;69(6):399–413. doi: 10.1002/iub.1629. [DOI] [PubMed] [Google Scholar]
- 35.Yan C, Yuanjie T, Zhengqun X, Jiayan C, Kongdan L. Neutrophil Gelatinase-Associated Lipocalin attenuates ischemia/reperfusion injury in an in vitro model via autophagy activation. Med Sci Monit. 2018;24:479–485. doi: 10.12659/MSM.908158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui LY, Yang S, Zhang J. Protective effects of neutrophil gelatinase-associated lipocalin on hypoxia/reoxygenation injury of HK-2 cells. Transplant Proc. 2011;43(10):3622–3627. doi: 10.1016/j.transproceed.2011.08.090. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Chen Y, Deng F, Zhao S. Protective effect and mechanisms of exogenous neutrophil gelatinase-associated lipocalin on lipopolysaccharide-induced injury of renal tubular epithelial cell. Biochem Biophys Res Commun. 2019;515(1):104–111. doi: 10.1016/j.bbrc.2019.05.102. [DOI] [PubMed] [Google Scholar]
- 38.Moriya H, Mochida Y, Ishioka K, Oka M, Maesato K, Hidaka S, et al. Plasma neutrophil gelatinase-associated lipocalin (NGAL) is an indicator of interstitial damage and a predictor of kidney function worsening of chronic kidney disease in the early stage: a pilot study. Clin Exp Nephrol. 2017;21(6):1053–1059. doi: 10.1007/s10157-017-1402-0. [DOI] [PubMed] [Google Scholar]
- 39.Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol. 2010;298(6):F1472–83. doi: 10.1152/ajprenal.00619.2009. [DOI] [PubMed] [Google Scholar]
- 40.Li B, Haridas B, Jackson AR, Cortado H, Mayne N, Kohnken R, et al. Inflammation drives renal scarring in experimental pyelonephritis. Am J Physiol Renal Physiol. 2017;312(1):F43–53. doi: 10.1152/ajprenal.00471.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein R, Dogan HS, Hoebeke P, Kočvara R, Nijman RJ, Radmayr C, et al. Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol. 2015;67(3):546–558. doi: 10.1016/j.eururo.2014.11.007. [DOI] [PubMed] [Google Scholar]