Abstract
Background
Twin-to-twin transfusion syndrome (TTTS) is a serious complication of monochorionic twin pregnancies. It results from disproportionate blood supply to each fetus caused by abnormal vascular anastomosis within the placenta. Amniotic fluid (AF) is an indicator reflecting the various conditions of the fetus, and an imbalance in AF volume is essential for the antenatal diagnosis of TTTS by ultrasound. In this study, two different mass spectrometry quantitative approaches were performed to identify differentially expressed proteins (DEPs) within matched pairs of AF samples.
Methods
We characterized the AF proteome in pooled AF samples collected from donor and recipient twin pairs (n = 5 each) with TTTS by a global proteomics profiling approach and then preformed the statistical analysis to determine the DEPs between the two groups. Next, we carried out a targeted proteomic approach (multiple reaction monitoring) with DEPs to achieve high-confident TTTS-associated AF proteins.
Results
A total of 103 AF proteins that were significantly altered in their abundances between donor and recipient fetuses. The majority of upregulated proteins identified in the recipient twins (including carbonic anhydrase 1, fibrinogen alpha chain, aminopeptidase N, alpha-fetoprotein, fibrinogen gamma chain, and basement membrane-specific heparan sulfate proteoglycan core protein) have been associated with cardiac or dermatologic disease, which is often seen in recipient twins as a result of volume overload. In contrast, proteins significantly upregulated in AF collected from donor twins (including IgGFc-binding protein, apolipoprotein C-I, complement C1q subcomponent subunit B, apolipoprotein C-III, apolipoprotein A-II, decorin, alpha-2-macroglobulin, apolipoprotein A-I, and fibronectin) were those previously shown to be associated with inflammation, ischemic cardiovascular complications or renal disease.
Conclusion
In this study, we identified proteomic biomarkers in AF collected from donor and recipient twins in pregnancies complicated by TTTS that appear to reflect underlying functional and pathophysiological challenges faced by each of the fetuses.
Keywords: Twin-to-Twin Transfusion Syndrome, Amniotic Fluid, Proteomics
Graphical Abstract
INTRODUCTION
Twin-to-twin transfusion syndrome (TTTS) is a serious complication that occurs in 8%–10% of monochorionic diamniotic (MCDA) twin pregnancies.1 It results from progressive disproportionate blood supply to each fetus in a MCDA twin pregnancy caused by abnormal vascular anastomosis within the shared placenta, and is associated with significant neonatal morbidity and mortality. The primary problem in TTTS is the unidirectional flow of blood from one twin (the donor) to the other (the recipient) caused by abnormal vascular anastomoses within the shared placenta.2 This leads in turn to an imbalance in blood volume and perfusion between the fetuses, with volume depletion in the donor twin and volume overload in the recipient. All MCDA twin pregnancies have vascular anastomoses within their shared placenta, but most do not develop TTTS because the blood flow is balanced.3 It is not identified yet why TTTS develops in some cases, or what the associated biomarkers of TTTS are.
Amniotic fluid (AF) in the latter half of pregnancy is composed primarily of fetal urine and, as such, reflects fetal renal perfusion. An imbalance in AF volume (polyhydramnios/oligohydramnios sequence) is essential for the antenatal diagnosis of TTTS. While antenatal ultrasonography remains the primary tool to confirm the diagnosis of TTTS, discovering protein entities associated with pathophysiology of TTTS would aid in the development of diagnostic approach or treatment of this disorder. And it would possibly even lead to more effective antenatal treatments of TTTS. AF is a promising source of genomic and proteomic biomarkers for prenatal diagnosis of a wide range of fetal abnormalities. However, few biorepositories have stored AF from donor and recipient twins with TTTS, in part because amniocentesis of the donor twins is very difficult to perform given the low AF volume. Using our established AF biobank, we performed two different mass spectrometry quantitative approaches (global and targeted proteome profiling) to identify differentially expressed proteins (DEPs) within matched pairs of AF samples.
METHODS
Study population and AF samples
TTTS was diagnosed by antenatal ultrasound or differences of hemoglobin concentrations in the cord blood of fetuses at birth. There have been changes in the diagnostic criteria of TTTS. Previously, hemoglobin differences greater than 5 g/dL in monochorionic twins were used for diagnosis of TTTS, but in many cases, this finding appeared late and identification of hemoglobin levels was rather cumbersome in fetuses or could only be done postnatally. Currently, the ultrasound based diagnostic criteria of TTTS proposed by Quintero has been widely used in the antenatal diagnosis of TTTS.4 However, some cases with TTTS can present in advanced stages before the established sonographic criteria are met. In a recent study, these cases were referred to as “atypical TTTS”.5 Therefore, we embraced both criteria of TTTS. Twin pregnancies that met the following criteria were enrolled: 1) presence of a MCDA pregnancy; and 2) presence of oligohydramnios (defined as a maximal vertical pocket of < 2 cm) in one fetus, and of polyhydramnios (a maximal vertical pocket of > 8 cm) in the other fetus6 or differences of hemoglobin concentrations greater than 5 g/dL in cord blood between the donor and recipient twins; 3) collection of AF from the donor and recipient both in a pair of twin fetuses by transabdominal amniocentesis or amniocentesis during cesarean section. Transabdominal amniocentesis was performed when the procedure was clinically required for the evaluation of microbiologic conditions in patients with preterm labor or preterm rupture of membranes or the assessment of fetal lung maturity. Amniocentesis was executed under written informed consents from the patients. Obtained AF was centrifuged and stored in polypropylene tubes at −70°C until assay.
Preparation of AF tryptic digests for mass spectrometry
For global proteomic profiling, equal volumes (10 μL) of AF from each of the 5 donor and recipient twin fetuses with TTTS were pooled. In each group, 250 μg of isolated proteins were denatured in 6 M urea, reduced with 10 mM dithiothreitol at 37°C, and alkylated in 30 mM iodoacetamide at room temperature in the dark. The pooled sample was then diluted to 1 M urea with 50 mM ammonium bicarbonate, trypsin was added as 1:50 (trypsin:protein) ratio, and incubated overnight at 37°C. The digested peptide mixture was applied onto a Sep-pak C18 cartridge for desalting and lyophilized in a Centrivap concentrator (Labconco, Kansas City, MO, USA). To improve analytical dynamic range and protein coverage, high-pH reversed-phase HPLC peptide fractionation were performed with the POROS® R2 C18 spin column. The peptide mixtures were loaded on the spin column under basic condition (10 mM ammonium formate, pH 10) and eluted with a total of 10 different fractions of elution buffer (10 mM ammonium formate and 90% ACN, pH 10) from 5% to 100% and concatenated into 5 fractions. Eluted peptides were dried under vacuum and stored at −80°C until LC-MS/MS analysis.
Mass spectrometry analysis and database search
Peptides were reconstituted with 0.1% FA and separated by a linear gradient of solvent B (0.1% FA in ACN) using an EASY-nLC (Thermo Fisher Scientific, San Jose, CA, USA). MS data were analyzed on a Q-Exactive hybrid quadrupole-Orbitrap mass spectrometry (Thermo Fisher Scientific). MS data were searched against the Uniprot Human database (June 2014, 313,072 entries) with the SEQUEST (version 27; Thermo Fisher Scientific) program by Sorcerer™. Results were reported with a ProteinProphet probability ≥ 99% and a PeptideProphet probability ≥ 95%7 and MS/MS data were validated with Scaffold v.4.6.2 (Proteome Software, Portland, OR, USA). Label-free quantification was accomplished using the R program (version 2.15; R Foundation for Statistical Computing, Vienna, Austria) based power law global error model software (Bioconductor Software Packages, version 3.9) with statistically significant values (P value < 0.005 and signal-to-noise ratio ≥ ± 2).8
Determination of multiple reaction monitoring (MRM) targets
By global proteomics profiling based label-free quantification, a set of MRM target peptides for 103 DEPs were selected following the peptide transition selection criteria; amino acid length (5–30 amino acid), unique peptides, and charge state (doubly and triply charge) that could be detected within the m/z scan window (m/z ≤ 1,350). Peptides were reconstituted with 0.1% FA and separated on the Eclipse Plus column (C18, 1.8 μM, 2.1 mm × 50 mm) by a linear gradient of solvent B (0.1% FA in ACN) using a 1260 Infinity HPLC (Agilent Technologies, Santa Clara, CA, USA). MRM peptide transitions were determined using two scan modes; full MS/MS scan mode and unbiased Q3-ion monitoring mode.9 To screen the biological function of MRM target proteins related to TTTS, STRING database (version 10.5) and Cytoscape (version 3.6) software were used.
Quantitative MRM analysis
For relative quantification of MRM target proteins between donors and recipient twins (n = 5), an external standard peptide (GDFQFNISR, β-galactosidase) was spiked into each sample. The multiplexing MRM assay was conducted in technical triplicate with 111 MRM transitions of 37 MRM target peptides, including an external standard peptide. The peak area value of each MRM transition was generated from Mass Hunter Quantitative Analysis software (version B.6.0; Agilent Technologies).
Statistical analysis with MSstats
Quantitative MRM data were systematically validated with MSstats, which is an R package software for statistical relative quantification of proteins.10 Briefly, MSstats analysis was achieved by translating chromatographic peak areas of all transitions into log2 values, normalizing the quantity for target peptides by two external standard peptides across all MRM runs, and adjusting the bias between standards and endogenous MRM signals. The DEPs between donor and recipient groups were selected with the linear mixed-effects model implemented in MSstats. All proteins with a P value below 0.01 and a fold change (FC) above 1.5 were considered significant.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 1207-013-415) and the collection and use of obtained samples was also approved with informed consent (IRB No. 9712-038-002).
RESULTS
Global AF quantitative proteomic profiling of TTTS
For the global AF quantitative proteome profiling between the donor and recipient groups of TTTS, we used 5 AF samples from both pairs of twin fetuses with TTTS (Table 1). Clinical stages of all the AF samples were stages III/IV as classified by the Quintero staging system. Gestational age at amniocentesis ranged from 18 to 35 weeks. In two cases (3 and 5), the AF samples were collected at the time of cesarean delivery just before rupture of the membranes. In all cases, both fetuses survived, except case 1 where the gestational age was too early to allow survival. In case 5, immediate delivery was recommended given the advanced gestational age at the time of referral.
Table 1. Clinical characteristics of the 5 cases of TTTS.
| Cases | Gestational age at amniocentesis, wk | Stage of TTTSa | Treatment | Gestational age at delivery, wk | Neonatal outcome | Birth weight, donor/recipient, g | Cord blood hemoglobin, donor/recipient, g/dL |
|---|---|---|---|---|---|---|---|
| 1 | 22-0/7 | IV | None | 22-3/7 | Both died | 360/610 | NA |
| 2 | 23-0/7 | IV | Septostomy and amnioreduction | 31-2/7 | Both survived | 1,380/1,540 | 17.8/21 |
| 3 | 26-0/7 | III | Amnioreduction | 26-0/7 | Both survived | 440/880 | 11.2/13.9 |
| 4 | 18-1/7 | III | Fetoscopic laser photocoagulation | 31-4/7 | Both survived | 1,230/1,840 | 14.4/17 |
| 5 | 35-5/7 | IV | None | 35-5/7 | Both survived | 2,720/2,770 | 2.8/25.5 |
TTTS = twin-to-twin transfusion syndrome, NA = not available.
aStage of TTTS as classified by the Quintero staging system.
We initially performed a global TTTS AF biomarker discovery study using a label-free quantitative proteome profiling experiment in a set of pooled AF samples collected from donor and recipient twin fetuses. Of identified 569 proteins, 78 and 25 proteins (P value < 0.005) were upregulated in the donor and the recipient pooled AF samples, respectively (Table 2). The functional enrichment analysis for those 103 DEPs revealed that molecular functions involved in cell-to-cell signaling and interaction, carbohydrate metabolism, and cell death and survival were significantly enriched with 78 upregulated proteins in the donor group. On the other hand, the 25 upregulated proteins in the recipient group were mainly associated with developmental disorder, cellular compromise, and cardiovascular disease.
Table 2. Identification of the proteins that were differentially expressed in amniotic fluid between the donor and recipient groups.
| Uniprot ID | Protein | STNa | P value |
|---|---|---|---|
| P69891 | Hemoglobin subunit gamma-1 | 21.1 | < 0.001 |
| P69892 | Hemoglobin subunit gamma-2 | 19.4 | < 0.001 |
| P69905 | Hemoglobin subunit alpha | 17.8 | < 0.001 |
| P68871 | Hemoglobin subunit beta | 15.0 | < 0.001 |
| P02042 | Hemoglobin subunit delta | 13.3 | < 0.001 |
| P02008 | Hemoglobin subunit zeta | 10.7 | 0.001 |
| P00915 | Carbonic anhydrase 1 | 7.8 | 0.001 |
| P02675 | Fibrinogen beta chain | 7.6 | 0.001 |
| P02679 | Fibrinogen gamma chain | 5.7 | 0.001 |
| P32119 | Peroxiredoxin-2 | 5.4 | 0.001 |
| P02768 | Serum albumin | 4.8 | 0.001 |
| P04040 | Catalase | 4.2 | 0.002 |
| P02771 | Alpha-fetoprotein | 4.1 | 0.002 |
| P15144 | Aminopeptidase N | 3.3 | 0.002 |
| P20742 | Pregnancy zone protein | 3.2 | 0.002 |
| P00918 | Carbonic anhydrase 2 | 3.0 | 0.002 |
| P01023 | Alpha-2-macroglobulin | 3.0 | 0.002 |
| P35908 | Keratin, type II cytoskeletal 2 epidermal | 2.9 | 0.002 |
| P04264 | Keratin, type II cytoskeletal 1 | 2.9 | 0.003 |
| P02671 | Fibrinogen alpha chain | 2.6 | 0.003 |
| Q16820 | Meprin A subunit alpha | 2.4 | 0.003 |
| A8K7I4 | Calcium-activated chloride channel regulator 1 | 2.4 | 0.003 |
| Q13228 | Selenium-binding protein 1 | 2.3 | 0.004 |
| P14410 | Sucrase-isomaltase, intestinal | 2.3 | 0.004 |
| P35527 | Keratin, type I cytoskeletal 9 | 2.1 | 0.004 |
| P12111 | Collagen alpha-3 (VI) chain | 2.1 | 0.004 |
| Q9HC84 | Mucin-5B | −13.2 | 0.001 |
| A7Y9J9 | Mucin 5AC, oligomeric mucus/gel-forming | −8.4 | 0.001 |
| P19013 | Keratin, type II cytoskeletal 4 | −7.1 | 0.001 |
| P00738 | Haptoglobin | −5.7 | 0.001 |
| Q9Y6R7 | IgGFc-binding protein | −5.5 | 0.001 |
| P13646 | Keratin 13 | −5.5 | 0.001 |
| Q8IWL1 | Pulmonary surfactant-associated protein A2 | −5.2 | 0.001 |
| A8K2U0 | Alpha-2-macroglobulin-like protein 1 | −5.1 | 0.001 |
| P22105 | Tenascin-X | −4.9 | 0.001 |
| P11047 | Laminin subunit gamma-1 | −4.8 | 0.001 |
| O43707 | Alpha-actinin-4 | −4.7 | 0.001 |
| P26038 | Moesin | −4.5 | 0.001 |
| P14618 | Pyruvate kinase PKM | −4.5 | 0.002 |
| P07942 | Laminin subunit beta-1 | −4.5 | 0.002 |
| P15311 | Ezrin | −4.4 | 0.002 |
| P06733 | Alpha-enolase | −4.4 | 0.002 |
| P0DMV8 | Heat shock 70 kDa protein 1A | −4.3 | 0.002 |
| P0C0L4 | Complement C4-A | −4.3 | 0.002 |
| P98088 | Mucin-5AC | −4.3 | 0.002 |
| P24043 | Laminin subunit alpha-2 | −4.3 | 0.002 |
| P04075 | Fructose-bisphosphate aldolase A | −4.2 | 0.002 |
| P07355 | Annexin A2 | −4.1 | 0.002 |
| Q9UGM3 | Deleted in malignant brain tumors 1 protein | −4.1 | 0.002 |
| Q8WXI7 | Mucin-16 | −4.1 | 0.002 |
| P02751 | Fibronectin | −3.7 | 0.002 |
| P08727 | Keratin, type I cytoskeletal 19 | −3.7 | 0.002 |
| P35555 | Fibrillin-1 | −3.6 | 0.002 |
| O00299 | Chloride intracellular channel protein 1 | −3.6 | 0.002 |
| P07585 | Decorin | −3.5 | 0.002 |
| P04406 | Glyceraldehyde-3-phosphate dehydrogenase | −3.4 | 0.002 |
| P04083 | Annexin A1 | −3.2 | 0.002 |
| P00558 | Phosphoglycerate kinase 1 | −3.2 | 0.002 |
| P08758 | Annexin A5 | −3.1 | 0.002 |
| O60437 | Periplakin | −3.0 | 0.003 |
| Q13219 | Pappalysin-1 | −2.9 | 0.003 |
| P02746 | Complement C1q subcomponent subunit B | −2.9 | 0.003 |
| P19801 | Amiloride-sensitive amine oxidase [copper-containing] | −2.9 | 0.003 |
| P02656 | Apolipoprotein C-III | −2.9 | 0.003 |
| P63261 | Actin, cytoplasmic 2 | −2.8 | 0.003 |
| Q16787 | Laminin subunit alpha-3 | −2.8 | 0.003 |
| P60903 | Protein S100-A10 | −2.8 | 0.003 |
| P98160 | Basement membrane-specific heparan sulfate proteoglycan core protein | −2.8 | 0.003 |
| Q9UBG3 | Cornulin | −2.8 | 0.003 |
| Q13751 | Laminin subunit beta-3 | −2.7 | 0.003 |
| P20930 | Filaggrin | −2.7 | 0.003 |
| P17948 | Vascular endothelial growth factor receptor 1 | −2.6 | 0.003 |
| P24821 | Tenascin | −2.6 | 0.003 |
| P13611 | Versican core protein | −2.5 | 0.003 |
| P60174 | Triosephosphate isomerase | −2.5 | 0.003 |
| P02533 | Keratin, type I cytoskeletal 14 | −2.5 | 0.003 |
| P31947 | 14-3-3 protein sigma | −2.5 | 0.003 |
| S6B291 | IgG H chain | −2.5 | 0.003 |
| Q6N089 | Uncharacterized protein | −2.5 | 0.003 |
| Q99715 | Collagen alpha-1(XII) chain | −2.5 | 0.003 |
| Q01469 | Fatty acid-binding protein, epidermal | −2.5 | 0.003 |
| P29401 | Transketolase | −2.4 | 0.003 |
| P02654 | Apolipoprotein C-I | −2.4 | 0.003 |
| P02649 | Apolipoprotein E | −2.4 | 0.003 |
| P02647 | Apolipoprotein A-I | −2.4 | 0.003 |
| P11166 | Solute carrier family 2, facilitated glucose transporter member 1 | −2.4 | 0.003 |
| Q6UVK1 | Chondroitin sulfate proteoglycan 4 | −2.4 | 0.003 |
| A8K008 | Uncharacterized protein | −2.4 | 0.003 |
| Q13938 | Calcyphosin | −2.3 | 0.004 |
| P02538 | Keratin, type II cytoskeletal 6A | −2.3 | 0.004 |
| P02545 | Prelamin-A/C | −2.3 | 0.004 |
| P29508 | Serpin B3 | −2.3 | 0.004 |
| Q8N1N4 | Keratin, type II cytoskeletal 78 | −2.3 | 0.004 |
| P13727 | Bone marrow proteoglycan | −2.2 | 0.004 |
| Q08380 | Lectin galactoside-binding soluble 3 binding protein isoform 1 | −2.2 | 0.004 |
| O75369 | Filamin-B | −2.2 | 0.004 |
| P10915 | Hyaluronan and proteoglycan link protein 1 | −2.2 | 0.004 |
| P68363 | Tubulin alpha-1B chain | −2.2 | 0.004 |
| P08174 | Complement decay-accelerating factor | −2.1 | 0.004 |
| O00339 | Matrilin-2 | −2.0 | 0.005 |
| Q6UWP8 | Suprabasin | −2.0 | 0.005 |
| P35542 | Serum amyloid A-4 protein | −2.0 | 0.005 |
| P02652 | Apolipoprotein A-II | −2.0 | 0.005 |
aSTN: signal-to-noise ratio generated by PLGEM analysis. The positive and negative values indicate upregulation in recipent and donor groups, respectively.
MRM assay of selected proteins
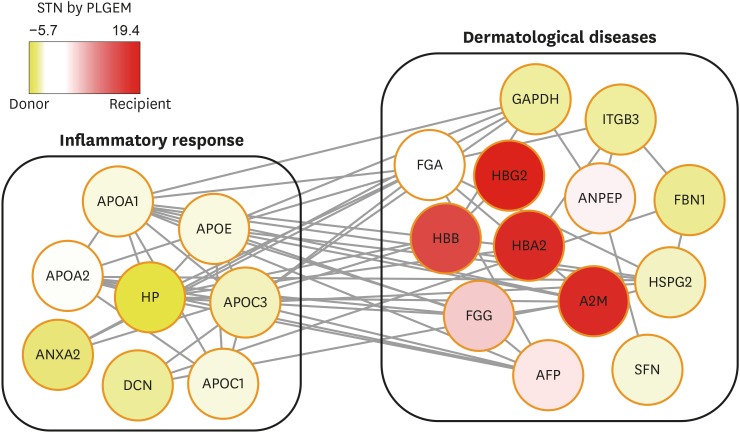
To further validate the expression levels of DEPs identified from the global proteome analysis, we performed MRM analysis for selected DEPs using individual (unpooled) AF samples from the donor and recipient groups. A set of MRM target peptides for 103 DEPs were selected following the peptide transition selection criteria, such as amino acid length, unique peptides, and charge state that could be detected within the m/z scan window (m/z ≤ 1,350). With the selected MRM target peptide transitions, we conducted the preliminary MRM assay to ensure their detectibility using the unbiased Q3 ion monitoring method.9 Among the 103 DEPs, 36 peptides originated from 28 proteins were determined for the final MRM targets following the selection criteria; co-eluted 3 transitions per each target peptide showing the S/N ratio above 3 (Supplementary Table 1). Functional enrichment of these final MRM target proteins showed that they were related to inflammatory response in the donor group and dermatological diseases in both groups (Fig. 1).
Fig. 1. Interactive network analysis of the final 28 MRM target proteins. An enriched functional network of the final 28 MRM target proteins is shown. Yellow and red colors are used to identify proteins that are upregulated in the donor and recipient amniotic fluid, respectively.
MRM = multiple reaction monitoring, APOA1 = apolipoprotein A-I, APOA2 = apolipoprotein A-II, APOE = apolipoprotein E, HP = haptoglobin, APOC3 = apolipoprotein C-III, ANXA2 = annexin A2, DCN = decorin, APOC1 = apolipoprotein C-I, FGA = fibrinogen alpha chain, GAPDH = glyceraldehyde 3-phosphate dehydrogenase, ITGB3 = integrin beta-3, HBG2 = hemoglobin subunit gamma-2, ANPEP = aminopeptidase N, FBN1 = fibrillin 1, HSPG2 = basement membrane-specific heparan sulfate proteoglycan core protein, SFN = stratifin, AFP = alpha-fetoprotein, FGG = fibrinogen gamma chain, A2M = alpha-2-macroglobulin, HBB = beta globin, HBA2 = hemoglobin A2, STN = signal-to-noise ratio.
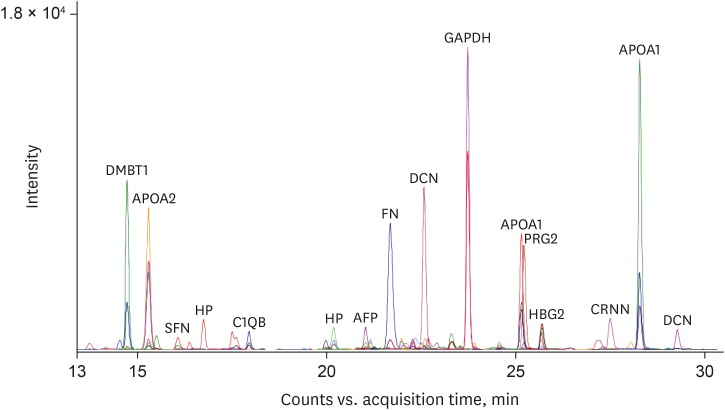
To estimate the expression levels of the 28 final MRM target proteins in the donor and recipient groups, equal amount of an external standard peptide (GDFQFNISR, beta-galactosidase peptide, m/z, 542.3) were spiked into each AF sample for subsequent normalization of the MRM data. Fig. 2 is a representative chromatographic trace with 108 MRM transitions of 36 MRM target peptides in the donor AF sample showing the quantitative dynamics of the peptide transitions. Triplicate MRM datasets of each sample were statistically analyzed for the quantification of peptide transitions using MSstats/Skyline. We identified that carbonic anhydrase 1 (CA1), fibrinogen alpha chain (FGA), aminopeptidase N (ANPEP), alpha-fetoprotein (AFP), fibrinogen gamma chain (FGG), and basement membrane-specific heparan sulfate proteoglycan core protein (HSPG2) were significantly upregulated (log2FC = 1.33, 0.84, 0.62, 0.49, 0.48, and 0.36 in CA1, FGA, ANPEP, AFP, FGG, and HSPG2, respectively) in the recipient group, while IgGFc-binding protein (FCGBP), apolipoprotein C-I (APOC1), complement C1q subcomponent subunit B (C1QB), apolipoprotein C-III (APOC3), apolipoprotein A-II (APOA2), decorin (DCN), haptoglobin (HP), alpha-2-macroglobulin (A2M), apolipoprotein A-I (APOA1), and fibronectin (FN) 1 were up-regulated (log2FC = 1.64, 1.16, 0.99, 0.83, 0.81, 0.78, 0.71, 0.71, 0.53, and 0.50 in FCGBP, APOC1, C1QB, APOC3, APOA2, DCN, HP, A2M, APOA1, and FN1, respectively) in the donor group (Table 3).
Fig. 2. Multiplexed-MRM analysis of AF. Multiplexed-MRM analysis of pooled AF samples was performed using 36 peptides of 28 target proteins. The extracted ion chromatograms are shown representing the observed 111 MRM transitions. An external standard peptide is included as a positive control.
MRM = multiple reaction monitoring, AF = amniotic fluid, DMBT1 = deleted in malignant brain tumors 1, APOA2 = apolipoprotein A-II, SFN = stratifin, HP = haptoglobin, C1QB = complement C1q subcomponent subunit B, AFP = alpha-fetoprotein, FN = fibronectin, DCN = decorin, GAPDH = glyceraldehyde 3-phosphate dehydrogenase, APOA1 = apolipoprotein A-I, PRG2 = proteoglycan 2, HBG2 = hemoglobin subunit gamma-2, CRNN = cornulin.
Table 3. The 16 DEPs that were significantly different between the donor and recipient groups as identified by MSstats.
| Gene | Protein | Upregulated group | log2FC | P value |
|---|---|---|---|---|
| FCGBP | IgGFc-binding protein | Donor | 1.64 | 0.000 |
| APOC1 | Apolipoprotein C-I | Donor | 1.16 | 0.000 |
| C1QB | Complement C1q subcomponent subunit B | Donor | 0.99 | 0.000 |
| APOC3 | Apolipoprotein C-III | Donor | 0.83 | < 0.001 |
| APOA2 | Apolipoprotein A-II | Donor | 0.81 | 0.000 |
| DCN | Decorin | Donor | 0.78 | < 0.001 |
| HP | Haptoglobin | Donor | 0.71 | < 0.001 |
| A2M | Alpha-2-macroglobulin | Donor | 0.71 | 0.000 |
| APOA1 | Apolipoprotein A-I | Donor | 0.53 | 0.000 |
| FN | Fibronectin | Donor | 0.50 | < 0.001 |
| CA1 | Carbonic anhydrase 1 | Recipient | 1.33 | < 0.001 |
| FGA | Fibrinogen alpha chain | Recipient | 0.84 | 0.000 |
| ANPEP | Aminopeptidase N | Recipient | 0.62 | < 0.001 |
| AFP | Alpha-fetoprotein | Recipient | 0.49 | 0.048 |
| FGG | Fibrinogen gamma chain | Recipient | 0.48 | 0.000 |
| HSPG2 | Basement membrane-specific heparan sulfate proteoglycan core protein | Recipient | 0.36 | 0.000 |
DISCUSSION
The current study was performed using a small number of AF pairs, because matching AF samples from both fetuses in a MCDA twin pregnancy with TTTS is hard to come by. Although it is relatively easy to obtain large amounts of AF from the sac of recipient fetuses at the time of fetoscopic laser photocoagulation or amnioreduction for antenatal treatment of TTTS, obtaining AF from the donor twin is far more difficult. Based on these limitations, we utilized both global and targted quantitative proteomic approaches to achieve high-confident identification of potential TTTS biomarkers. While the global quantitative proteomic approach is capable of providing unbiased identification of DEPs between the donor and recipient groups, the targeted quantitative proteomic approach leads to higher sensitivity and better quantitative accuracy for the biomarker candidates identified from the global approach.11,12,13 Combining the two quantitative proteomics approaches leads to the identification of high-confident protein factors that are related to inflammatory response and dermatological diseases according to the functional network analysis in which several proteins associated with the inflammatory response are upregulated in the donor twins. There is a paucity of publications about the relationship between inflammation and TTTS. However, Pierce et al.14 reported that placental hypoperfusion results in an increased production of inflammatory cytokines. Donor fetuses in TTTS are characterised by placental hypoperfusion, because the abnormal vascular communications within the shared placenta results in reduced blood supply. Therefore, as identified by our interactive network analysis, upregulation of proteins associated with the inflammatory reponse in donor twins is likely due to placental hypoperfusion. Recipient fetuses in TTTS are edematous, because of increased circulating blood volume leading to abnormal accumulation of fluid in the interstitium, the skin, or third spaces within various body cavities. As the disease worsens, the edema may become more exaggerated. Hydrops fetalis is diagnosed by the presence of an abnormal accumulation of fluid in at least two fetal body compartments. If present, this confirms the diagnosis of stage III TTTS. Proteins upregulated in the recipient group are located in the category of dermatological diseases. It is possible that this may be associated with edematous changes in the skin of recipient twins, because all cases in this study were Quintero stage III or IV.
In donor twins, the volume depletion that results from the reduced placental perfusion leads to reduced renal perfusion and oliguria. Some of the upregulated proteins in the AF of the donor twins have been previously reported to be associated with ischemic cardiovascular complications and renal diseases. APOC1 and APOC3 belong to the apolipoprotein family and are known to be related to the development of atherosclerosis and ischemic stroke.15 Elevated plasma concentrations of APOC3 have been previously documented in patients with type 2 diabetes mellitus,16,17 and pregnant women who subsequently developed gestational diabetes had significantly higher levels of APOC3 than those that did not.18 Increased levels of APOC1, APOC3, and APOA2 have also been associated with an increased risk of cardiovascular disease.19 Similarly, C1QB has been associated with advanced atherosclerotic disease and coronary complications in patients with familial hypercholesterolemia,20 HP binds to plasma hemoglobin, facilitates hepatic recycling of heme iron, and prevents renal damage in patients on hemolysis. And urinary HP has been identified as a biomarker for the early diagnosis of acute allograft rejection following kidney transplantation.21 FN is known to be associated with the development of glomerulopathy.22 Taken together, these proteins that are elevated in the AF of donor twins appear to be associated with ischemic cardiovascular and renal diseases, which may provide insight into the pathophysiology of donor fetuses characterized by volume depletion.
In contrast, recipient fetuses of TTTS develop complications related to volume overload with the development of congestive heart failure as TTTS worsens. Carbonic anhydrases, a protein upregulated in the AF of recipient fetuses in this study, are involved in various physiological and pathological processes such as pH regulation, ion transport, or biosynthesis. Elevated expression of carbonic anhydrases are known to be associated with heart failure and cardiomyopathy.23,24 Some diuretic drugs for treatment of congestive heart failure are based on the inhibition of carbonic anhydrase.25 AFP was also identified as one of the upregulated proteins in the recipient group. In addition to its known association with open neural tube and abdominal wall defects, previous reports have demonstrated that elevated AF AFP concentrations are also correlated to an elevated pulsatility index in the fetal ductus venosus,26 which suggests underlying congestive heart failure. Whether there is a direct relationship between the level of AF AFP and the severity of congestive heart failure of the recipient twin in TTTS is not known.
In conclusion, proteomic analysis is a powerful tool for analyzing disease biomarkers and requires small amounts of fluids from any biological compartment. In this study, we identified differentially expressed AF proteins between donor and recipient twin fetuses in pregnancies complicated by TTTS by integrating the results from two different proteomic approaches, global and targeted proteome analysis. Several of these DEPs are known to be associated with cardiovascular diseases, nephropathy, inflammation, or dermatological disease, which may provide insight into the pathophysiology and complications of TTTS. If present, such differentially regulated proteins may prove useful for the prediction, prevention, or monitoring of disease progression in the setting of TTTS.
Footnotes
Funding: This study was supported by a grant of the Korea Health R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI12C00240300) and by a grant of the Seoul National University Hospital Research Fund (03-2012-0160) and by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning of Korea (2016M3A9B6902061) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03029883).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim SM, Park JS.
- Data curation: Kim SM, Cho BK, Kang MJ, Yi EC.
- Formal analysis: Kim SM, Cho BK, Lee HY, Kang MJ, Yi EC.
- Funding acquisition: Kim SM, Park JS.
- Investigation: Kim SM, Cho BK, Kim BJ, Lee HY, Norwitz ER, Kang MJ, Lee SM, Park CW, Jun JK, Yi EC.
- Methodology: Kim SM.
- Supervision: Kim BJ, Norwitz ER, Lee SM, Park CW, Jun JK, Yi EC, Park JS.
- Validation: Cho BK, Kim BJ, Kang MJ, Yi EC, Park JS.
- Visualization: Cho BK, Lee HY, Yi EC.
- Writing - original draft: Kim SM.
SUPPLEMENTARY MATERIAL
Multiple reaction monitoring peptide transitions of the 28 target proteins
References
- 1.Lewi L, Jani J, Blickstein I, Huber A, Gucciardo L, Van Mieghem T, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol. 2008;199(5):514.e1–514.e8. doi: 10.1016/j.ajog.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 2.Nikkels PG, Hack KE, van Gemert MJ. Pathology of twin placentas with special attention to monochorionic twin placentas. J Clin Pathol. 2008;61(12):1247–1253. doi: 10.1136/jcp.2008.055210. [DOI] [PubMed] [Google Scholar]
- 3.Bajoria R, Wigglesworth J, Fisk NM. Angioarchitecture of monochorionic placentas in relation to the twin-twin transfusion syndrome. Am J Obstet Gynecol. 1995;172(3):856–863. doi: 10.1016/0002-9378(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 4.Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin-twin transfusion syndrome. J Perinatol. 1999;19(8 Pt 1):550–555. doi: 10.1038/sj.jp.7200292. [DOI] [PubMed] [Google Scholar]
- 5.Paek B, Dorn M, Walker M. Atypical twin-to-twin transfusion syndrome: prevalence in a population undergoing fetoscopic laser ablation of communicating placental vessels. Am J Obstet Gynecol. 2016;215(1):115.e1–115.e5. doi: 10.1016/j.ajog.2016.01.169. [DOI] [PubMed] [Google Scholar]
- 6.Johnson A. Diagnosis and management of twin-twin transfusion syndrome. Clin Obstet Gynecol. 2015;58(3):611–631. doi: 10.1097/GRF.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 7.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75(17):4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 8.Pavelka N, Pelizzola M, Vizzardelli C, Capozzoli M, Splendiani A, Granucci F, et al. A power law global error model for the identification of differentially expressed genes in microarray data. BMC Bioinformatics. 2004;5(1):203. doi: 10.1186/1471-2105-5-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho BK, Koo YD, Kim K, Kang MJ, Lee YY, Kim Y, et al. Determination of selected reaction monitoring peptide transitions via multiplexed product-ion scan modes. Rapid Commun Mass Spectrom. 2014;28(7):773–780. doi: 10.1002/rcm.6837. [DOI] [PubMed] [Google Scholar]
- 10.Choi M, Chang CY, Clough T, Broudy D, Killeen T, MacLean B, et al. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics. 2014;30(17):2524–2526. doi: 10.1093/bioinformatics/btu305. [DOI] [PubMed] [Google Scholar]
- 11.Morrissey B, O'Shea C, Armstrong J, Rooney C, Staunton L, Sheehan M, et al. Development of a label-free LC-MS/MS strategy to approach the identification of candidate protein biomarkers of disease recurrence in prostate cancer patients in a clinical trial of combined hormone and radiation therapy. Proteomics Clin Appl. 2013;7(5-6):316–326. doi: 10.1002/prca.201300004. [DOI] [PubMed] [Google Scholar]
- 12.Heywood WE, Galimberti D, Bliss E, Sirka E, Paterson RW, Magdalinou NK, et al. Identification of novel CSF biomarkers for neurodegeneration and their validation by a high-throughput multiplexed targeted proteomic assay. Mol Neurodegener. 2015;10(1):64. doi: 10.1186/s13024-015-0059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addona TA, Shi X, Keshishian H, Mani DR, Burgess M, Gillette MA, et al. A pipeline that integrates the discovery and verification of plasma protein biomarkers reveals candidate markers for cardiovascular disease. Nat Biotechnol. 2011;29(7):635–643. doi: 10.1038/nbt.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce BT, Pierce LM, Wagner RK, Apodaca CC, Hume RF, Jr, Nielsen PE, et al. Hypoperfusion causes increased production of interleukin 6 and tumor necrosis factor α in the isolated, dually perfused placental cotyledon. Am J Obstet Gynecol. 2000;183(4):863–867. doi: 10.1067/mob.2000.108887. [DOI] [PubMed] [Google Scholar]
- 15.Allard L, Lescuyer P, Burgess J, Leung KY, Ward M, Walter N, et al. ApoC-I and ApoC-III as potential plasmatic markers to distinguish between ischemic and hemorrhagic stroke. Proteomics. 2004;4(8):2242–2251. doi: 10.1002/pmic.200300809. [DOI] [PubMed] [Google Scholar]
- 16.Florez H, Mendez A, Casanova-Romero P, Larreal-Urdaneta C, Castillo-Florez S, Lee D, et al. Increased apolipoprotein C-III levels associated with insulin resistance contribute to dyslipidemia in normoglycemic and diabetic subjects from a triethnic population. Atherosclerosis. 2006;188(1):134–141. doi: 10.1016/j.atherosclerosis.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Béliard S, Nogueira JP, Maraninchi M, Lairon D, Nicolay A, Giral P, et al. Parallel increase of plasma apoproteins C-II and C-III in type 2 diabetic patients. Diabet Med. 2009;26(7):736–739. doi: 10.1111/j.1464-5491.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim SM, Park JS, Norwitz ER, Lee SM, Kim BJ, Park CW, et al. Identification of proteomic biomarkers in maternal plasma in the early second trimester that predict the subsequent development of gestational diabetes. Reprod Sci. 2012;19(2):202–209. doi: 10.1177/1933719111417889. [DOI] [PubMed] [Google Scholar]
- 19.Brewer HB., Jr Hypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular disease. Am J Cardiol. 1999;83(9B):3F–12F. doi: 10.1016/s0002-9149(99)00308-2. [DOI] [PubMed] [Google Scholar]
- 20.Bos S, Phillips M, Watts GF, Verhoeven AJ, Sijbrands EJ, Ward NC. Novel protein biomarkers associated with coronary artery disease in statin-treated patients with familial hypercholesterolemia. J Clin Lipidol. 2017;11(3):682–693. doi: 10.1016/j.jacl.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Stubendorff B, Finke S, Walter M, Kniemeyer O, von Eggeling F, Gruschwitz T, et al. Urine protein profiling identified alpha-1-microglobulin and haptoglobin as biomarkers for early diagnosis of acute allograft rejection following kidney transplantation. World J Urol. 2014;32(6):1619–1624. doi: 10.1007/s00345-014-1263-z. [DOI] [PubMed] [Google Scholar]
- 22.Ishimoto I, Sohara E, Ito E, Okado T, Rai T, Uchida S. Fibronectin glomerulopathy. Clin Kidney J. 2013;6(5):513–515. doi: 10.1093/ckj/sft097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alvarez BV, Quon AL, Mullen J, Casey JR. Quantification of carbonic anhydrase gene expression in ventricle of hypertrophic and failing human heart. BMC Cardiovasc Disord. 2013;13(1):2. doi: 10.1186/1471-2261-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torella D, Ellison GM, Torella M, Vicinanza C, Aquila I, Iaconetti C, et al. Carbonic anhydrase activation is associated with worsened pathological remodeling in human ischemic diabetic cardiomyopathy. J Am Heart Assoc. 2014;3(2):e000434. doi: 10.1161/JAHA.113.000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iqbal S, Saleem M, Azim MK, Taha M, Salar U, Khan KM, et al. Carbohydrazones as new class of carbonic anhydrase inhibitors: synthesis, kinetics, and ligand docking studies. Bioorg Chem. 2017;72:89–101. doi: 10.1016/j.bioorg.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Huber A, Diehl W, Zikulnig L, Held KR, Bregenzer T, Hackelöer BJ, et al. Amniotic fluid and maternal blood characteristics in severe mid-trimester twin-twin transfusion syndrome. Fetal Diagn Ther. 2004;19(6):504–509. doi: 10.1159/000080163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple reaction monitoring peptide transitions of the 28 target proteins