Abstract
Background
Usually, high-flow nasal cannula (HFNC) therapy is indicated for de novo acute hypoxemic respiratory failure (AHRF). Although only a few researches have examined the effectiveness of HFNC therapy for respiratory failure with hypercapnia, this therapy is often performed under such conditions for various reasons. We investigated the effectiveness of HFNC therapy for AHRF patients with hypercapnia compared to those without hypercapnia.
Methods
All consecutive patients receiving HFNC therapy between January 2012 and June 2018 at a university hospital were enrolled and classified into nonhypercapnic and hypercapnic groups. We compared the outcomes of both groups and adjusted the outcomes with propensity score matching.
Results
A total of 862 patients were enrolled, of which 202 were included in the hypercapnic group. HFNC weaning success rates were higher, and intensive care unit (ICU) and hospital mortality was lower in the hypercapnic group than in the nonhypercapnic group (all P < 0.05). However, no statistical differences in HFNC weaning success (adjusted P = 0.623, matched P = 0.593), ICU mortality (adjusted P = 0.463, matched P = 0.195), and hospital mortality (adjusted P = 0.602, matched P = 0.579) were noted from the propensity-adjusted and propensity-matched analyses. Additionally, in the propensity score-matched subgroup analysis (according to chronic lung diseases and causes of HFNC application), there was also no significant difference in outcomes between the two groups.
Conclusion
In AHRF with underlying conditions, HFNC therapy might be helpful for patients with hypercapnia. Large prospective and randomized controlled trials are required for firm conclusions.
Keywords: Oxygen Inhalation Therapy, Respiratory Insufficiency, Noninvasive Ventilation, Hypercapnia, Propensity Score
Graphical Abstract
INTRODUCTION
Oxygen administration is an essential supportive treatment for maintaining proper tissue oxygenation and alleviating breathlessness in patients with respiratory failure (RF). However, the choice of an appropriate form of oxygen therapy is unclear. The high-flow nasal cannula (HFNC) is a high-flow oxygen supply device that is increasingly used in the treatment of RF in intensive care unit (ICU) patients.1 HFNC therapy is usually used to treat de novo acute hypoxemic respiratory failure (AHRF) without hypercapnia and reduces the need for tracheal intubation compared to conventional oxygen therapies.2,3 In addition, HFNC therapy is also helpful for post extubation respiratory failure and invasive airway procedure such as intubation and bronchoscopy.4,5,6
In some patients with chronic lung disease (CLD), high-flow oxygen supply can aggravate hypercapnia,7,8 and noninvasive ventilation (NIV) is strongly recommended in patients with hypercapnic RF9; therefore, meticulous care is required in such cases when using an HFNC. However, in clinical practice, physicians have been using HFNC therapy frequently not only in patients with de novo AHRF but also in patients with CLD. Several studies have reported that HFNC therapy might also be helpful in patients with underlying CLD,10,11,12,13 while others reported no significant increase in carbon dioxide (CO2) levels and progression to NIV or mechanical ventilation in patients with hypercapnia compared to those without hypercapnia after HFNC therapy.14,15,16
We compared the effectiveness of HFNC therapy for patients with or without hypercapnia in AHRF using a risk stratification model that adjusts for potential differences between the two groups. The primary outcome was the HFNC weaning success rate, and the secondary outcomes were ICU mortality, hospital mortality, length of ICU stay, length of hospital stay, and complications during ICU stay. We also compared vital signs, pH and partial pressure of CO2 (PCO2) changes between the two groups to evaluate the short-term effects after HFNC application.
METHODS
Study design and subjects
We performed a retrospective observational study of critically ill patients older than 18 years who received HFNC therapy for a hypoxemic respiratory problem (ratio of partial pressure arterial oxygen [PO2] and fraction of inspired oxygen [FiO2] ≤ 200 mmHg) at the ICU of Ulsan University Hospital, Korea, between January 2012 and June 2018. Using the PCO2 in the arterial blood gas analysis (ABGA) just before HFNC therapy, we classified the study patients into nonhypercapnic and hypercapnic groups. We compared the outcomes of both groups and adjusted our model using propensity score analysis. Additionally, we performed further analyses of the outcomes in selected subgroups: patients without CLD, patients with CLD, patients who received HFNC therapy after extubation, and patients who received HFNC therapy for causes other than extubation.
HFNC device application
All study patients were treated with high-flow oxygen using an HFNC (Optiflow™ or AIRVO™ 2; Fisher and Paykel Healthcare, Auckland, New Zealand). Each patient was kept under close observation in the ICU and was continuously monitored until their conditions stabilized. We routinely determined HFNC application based on the attending physician’s assessment and consulted with the respiratory medicine specialists or critical care intensivists if necessary.
Definitions
We defined “hypercapnia” as PCO2 ≥ 45 mmHg based on pre-HFNC ABGA. “HFNC weaning” was defined as spontaneous breathing with an oxygen flow ≤ 6 L/min via a nasal cannula for > 48 hours after stopping HFNC therapy. “Use of immunosuppressive agents” was defined as treatment with steroids, immunosuppressive medications, and/or chemotherapeutic agents within 6 months of HFNC therapy. “After extubation” was defined as a state of HFNC application within 6 hours after extubation. Acute physiology and chronic health evaluation (APACHE) II and sequential organ failure assessment (SOFA) scores were calculated using the worst variable within 24 hours of HFNC application. The study participants were classified into four groups using the modified classification criteria according to the causes of RF necessitating the use of HFNC17: de novo acute RF, acute-on-CLD, septic shock for reasons other than respiratory infection, pulmonary edema, or after extubation. In cases of more than one cause of HFNC therapy in a patient, we included the major cause for HFNC indication. We assessed the short-term effectiveness of HFNC therapy by checking vital signs, pH and PCO2 values immediately before and after HFNC application within 2 hours.
Data collection
Clinical and laboratory findings were obtained from the clinical data warehouse appliance (uICE, Ulsan University Hospital Information of Clinical Ecosystem) in connection with the electronic medical records at the Ulsan University Hospital. One critical care intensivist (also specializing in respiratory medicine) reviewed all the patient records collected from the uICE and checked any faults by identifying the patient records directly.
Statistical analysis
The data are reported as means ± standard deviation, medians (interquartile range) or numbers (percentages). To investigate comparisons between nonhypercapnic and hypercapnic groups, the independent t-test was performed for continuous variables and the χ2 test was used for categorical variables.
In our study, patients were not randomly assigned to the nonhypercapnic and hypercapnic groups. To reduce the effect of selection bias based on hypercapnia and a potential confounding factor in the study, we performed careful adjustment for differences in baseline characteristics except ABGA using a propensity score analysis.18 Propensity scores were estimated by multiple logistic regression analysis, and all covariables previously described in Tables 1 except ABGA were included in the final model. To check multicollinearity, we also calculated the variance inflation factor using multiple linear regression analysis (Supplementary Table 1). The discrimination and calibration of the model were assessed by c-statistics (c = 0.701) and Hosmer-Lemeshow statistics (χ2 = 5.991, df = 8, P = 0.648), respectively. Additionally, we compared the standardized mean difference in both groups before and after propensity score matching. We also performed multiple logistic regression and multiple linear regression analyses to estimate the propensity scores and evaluate the variance inflation factors in each subgroup (Supplementary Tables 2–5).
Table 1. Baseline characteristics in study patients who received HFNC therapy.
| Baseline characteristics | Nonhypercapnic group (n = 660) | Hypercapnic group (n = 202) | Standardized mean difference | P-valuea | |
|---|---|---|---|---|---|
| Age, yr | 66.1 ± 13.9 | 65.6 ± 14.6 | 0.0310 | 0.700 | |
| Gender, men | 450/660 (68.2) | 145/202 (71.8) | 0.0946 | 0.333 | |
| Body mass index, kg/m2 | 23.8 ± 4.4 (n = 652) | 23.9 ± 4.9 | 0.0162 | 0.841 | |
| Smoker | 402/660 (60.9) | 128/201 (63.7) | 0.0651 | 0.479 | |
| Underlying diseases | |||||
| Diabetes mellitus | 256/660 (38.8) | 76/202 (37.6) | 0.0272 | 0.766 | |
| Hypertension | 373/660 (56.5) | 105/202 (52.0) | 0.1008 | 0.256 | |
| Solid malignancies | 182/660 (27.6) | 84/202 (41.6) | 0.3450 | < 0.001b | |
| Hematologic malignancies | 35/660 (5.3) | 4/202 (2.0) | 0.5621 | 0.047b | |
| Heart failure | 105/660 (15.9) | 30/202 (14.9) | 0.0448 | 0.717 | |
| Ischemic heart disease | 86/660 (13.0) | 23/202 (11.4) | 0.0847 | 0.538 | |
| Chronic kidney disease/dialysis | 57/660 (8.6) | 12/202 (5.9) | 0.2223 | 0.217 | |
| Liver cirrhosis | 49/660 (7.4) | 17/202 (8.4) | 0.0751 | 0.643 | |
| Use of immunosuppressive agents | 146/660 (22.1) | 49/202 (24.3) | 0.0662 | 0.525 | |
| Underlying chronic lung diseases | |||||
| Asthma | 19/660 (2.9) | 10/202 (5.0) | 0.3108 | 0.153 | |
| Chronic obstructive pulmonary disease | 135/660 (20.5) | 55/202 (27.2) | 0.2068 | 0.042b | |
| Pulmonary tuberculosis history | 84/660 (12.7) | 30/202 (14.9) | 0.0987 | 0.435 | |
| Bronchiectasis | 78/660 (11.8) | 48/202 (23.8) | 0.4653 | < 0.001b | |
| Lung cancer | 47/660 (7.1) | 43/202 (21.3) | 0.6950 | < 0.001b | |
| Interstitial lung disease | 23/660 (3.5) | 9/202 (4.5) | 0.1410 | 0.523 | |
| ICU type, medical ICU | 387/660 (58.6) | 91/202 (45.0) | 0.3019 | 0.001b | |
| HFNC initial setting | |||||
| FiO2, % | 54.9 ± 14.4 | 51.7 ± 12.8 | 0.2271 | < 0.001b | |
| Flow, L/min | 43.0 ± 8.4 | 41.9 ± 9.0 | 0.1291 | 0.110 | |
| PO2/FiO2 before HFNC application, mmHg | 124.5 ± 39.2 | 135.7 ± 38.8 | 0.2857 | < 0.001b | |
| APACHE II score | 20.9 ± 7.3 | 18.8 ± 7.9 | 0.2785 | 0.001b | |
| SOFA score | 8.2 ± 3.6 | 7.3 ± 3.5 | 0.2527 | 0.002b | |
| Time differences between ABGA and HFNC application, min | 42.0 (13.0–115) | 23.5 (6.8–76.0) | 0.0616 | 0.004b | |
| Causes of HFNC application | 0.4669 | < 0.001b | |||
| De novo acute respiratory failure | 168/660 (25.5) | 28/202 (13.9) | |||
| Acute-on-chronic lung disease | 143/660 (21.7) | 32/202 (15.8) | |||
| Septic shock for reasons other than respiratory infection | 52/660 (7.9) | 8/202 (4.0) | |||
| Pulmonary edema | 109/660 (16.5) | 29/202 (14.4) | |||
| After extubation | 188/660 (28.5) | 105/202 (52.0) | |||
| ABGA results before HFNC application | |||||
| pH | 7.44 ± 0.07 | 7.35 ± 0.09 | - | < 0.001b | |
| PCO2, mmHg | 35.2 ± 5.6 | 52.1 ± 8.5 | - | < 0.001b | |
| PO2, mmHg | 67.1 ± 17.5 | 71.3 ± 18.7 | - | 0.003b | |
| HCO3, mEq/L | 24.1 ± 5.6 | 29.0 ± 5.5 | - | < 0.001b | |
| SpO2, % | 91.5 ± 6.7 | 90.7 ± 7.8 | - | 0.182 | |
| Lactate, mmol/L | 1.6 (1.0–2.6) (n = 635) | 1.5 (0.9–2.5) (n = 196) | - | 0.115 | |
Data are presented as mean ± standard deviation or median (interquartile range) or number (%).
HFNC = high-flow nasal cannula, ICU = intensive care unit, FiO2 = fraction of inspired oxygen, PO2 = partial pressure of carbon dioxide, APACHE = acute physiology and chronic health evaluation, SOFA = sequential organ failure assessment, ABGA = arterial blood gas analysis, PCO2 = partial pressure of carbon dioxide, HCO3 = bicarbonate, SpO2 = oxygen saturation.
aStatistical comparisons of the data were performed by using the χ2 test for categorical variables and the independent t-test for continuous variables; bStatistically significant P values.
To compare outcomes between nonhypercapnic and hypercapnic groups, we performed a logistic regression analysis (HFNC weaning, ICU mortality, and hospital mortality) and a Cox proportional regression analysis (90-day mortality). To achieve the propensity score-adjusted outcome, the individual propensity score was incorporated into each outcome model as a covariable. We also performed propensity score matching. After we had completed all of the propensity score matches, we compared the baseline covariables between the two intervention groups using the paired t-test for continuous variables and the McNemar test or marginal homogeneity test for categorical variables. For a logit link that accounted for the clustering of propensity score-matched pairs, the risks of clinical end points were analyzed using a generalized estimating equation logistic regression model (HFNC weaning, ICU mortality, and hospital mortality). Cox proportional regression analysis (90-day mortality) that stratified the matched pairs was also performed for each of the two matched samples.
To compare the vital signs, pH and PCO2 changes before and after HFNC application between nonhypercapnic and hypercapnic groups, we conducted generalized estimating equations using linear regression to account for the clustering nature of matched pairs. In addition, survival curves for two groups were analyzed using the Kaplan-Meier method and compared by log rank test. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) Version 24.0 (IBM Corporation, Armonk, New York, USA), and differences with a P value < 0.05 was considered statistically significant.
Ethics statement
The present study was approved by the Institutional Review Board of Ulsan University Hospital (No. 2018-11-001), and the informed consent was waived because of the retrospective design of the study.
RESULTS
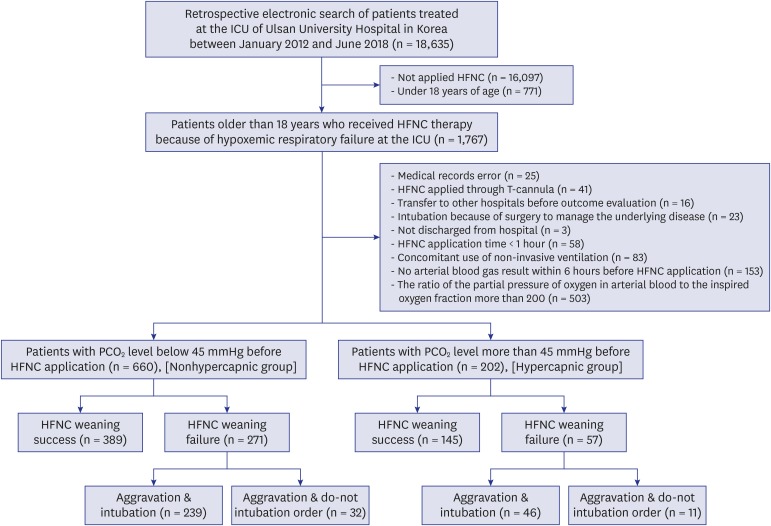
During the study period, there were 18,635 ICU admitted patients at Ulsan University Hospital. We excluded 16,868 patients who had not received HFNC therapy or were under 18 years of age. After the review of electronic medical records, we further excluded 905 patients who were not suitable for the evaluation of the effectiveness of HFNC therapy. Subsequently, the patients were classified into a nonhypercapnic group (n = 660) and a hypercapnic group (n = 202) based on their PCO2 values (Fig. 1).
Fig. 1. Distribution of the study patients according to the presence of hypercapnia before high-flow nasal cannula.
ICU = intensive care unit, HFNC = high-flow nasal cannula, PCO2 = partial pressure of carbon dioxide.
Patient characteristics
The baseline characteristics of the study population are presented in Table 1. There were more solid malignancies and CLDs (chronic obstructive pulmonary disease [COPD], bronchiectasis, and lung cancer) in the hypercapnic group than in the nonhypercapnic group. The nonhypercapnic group also had a significantly higher oxygen requirements (FiO2 and PO2/FiO2), and severity indexes (APACHE II and SOFA scores) than the hypercapnic group. The most common cause of HFNC application was “after extubation” in both groups. In the ABGA results, the hypercapnic group had lower pH and higher PCO2, PO2, and bicarbonate (HCO3) than the nonhypercapnic group.
Hospital outcomes
The hospital outcomes of the hypercapnic group were mostly better than those of the nonhypercapnic group (Table 2). The hypercapnic group was significantly more likely to have better HFNC weaning success, lower ICU mortality, lower hospital mortality and a shorter length of ICU stay than the nonhypercapnic group. During the ICU stay, there were also fewer complications, such as heart failure and cardiopulmonary resuscitation in the hypercapnic group.
Table 2. Hospital outcomes in study patients who received HFNC therapy.
| Hospital outcomes | Nonhypercapnic group (n = 660) | Hypercapnic group (n = 202) | P valuea | |
|---|---|---|---|---|
| HFNC weaning success rate | 389/660 (58.9) | 145/202 (71.8) | 0.001b | |
| ICU mortality | 130/660 (19.7) | 21/202 (10.4) | 0.002b | |
| Hospital mortality | 187/660 (28.3) | 38/202 (18.8) | 0.007b | |
| Length of ICU stay | 6.0 (3.0–12.0) | 4.0 (1.0–9.0) | 0.015b | |
| Length of hospital stay | 23.0 (14.0–39.0) | 20.0 (11.8–37.0) | 0.851 | |
| Complications during ICU stay | ||||
| Pneumonia | 155/660 (23.5) | 37/202 (18.3) | 0.122 | |
| Myocardial infarction | 15/660 (2.3) | 6/202 (3.0) | 0.603 | |
| Biliary infection | 2/660 (0.3) | 0/202 (0.0) | 0.999 | |
| Gastrointestinal infection | 17/660 (2.6) | 1/202 (0.5) | 0.090 | |
| Gastrointestinal bleeding (required endoscopy) | 14/660 (2.1) | 2/202 (1.0) | 0.385 | |
| Pulmonary thromboembolism | 2/660 (0.3) | 0/202 (0.0) | 0.999 | |
| Ischemic stroke | 6/660 (0.9) | 0/202 (0.0) | 0.345 | |
| Heart failure | 31/660 (4.7) | 3/202 (1.5) | 0.040b | |
| Pneumothorax | 18/660 (2.7) | 2/202 (1.0) | 0.188 | |
| Urinary tract infection | 8/660 (1.2) | 3/202 (1.5) | 0.726 | |
| Liver failure | 3/660 (0.5) | 2/202 (1.0) | 0.334 | |
| Catheter-related bloodstream infections | 19/660 (2.9) | 6/202 (3.0) | 0.946 | |
| Acute kidney injury (required continuous renal replacement therapy) | 119/660 (18.0) | 26/202 (12.9) | 0.086 | |
| Cardiopulmonary resuscitation | 48/660 (7.3) | 6/202 (3.0) | 0.027b | |
Data are presented as mean ± standard deviation or median (interquartile range) or number (%).
HFNC = high-flow nasal cannula, ICU = intensive care unit.
aStatistical comparisons of the data were performed by using the χ2 test for categorical variables and the independent t-test for continuous variables; bStatistically significant P values.
Propensity score-adjusted and score-matched outcomes
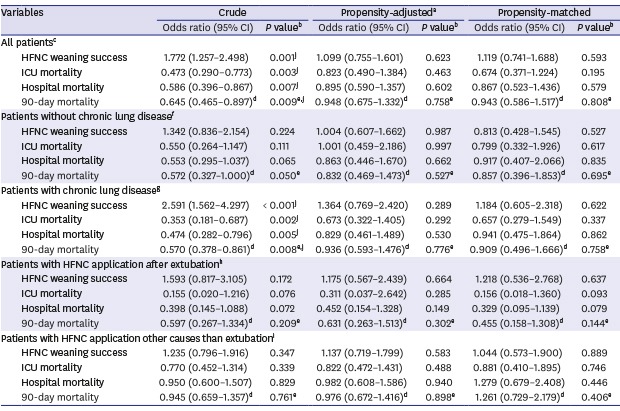
After propensity score matching, we selected 162 pairs of patients with similar baseline characteristics except pre-HFNC ABGA. From the ABGA results, the hypercapnic group had a lower pH and higher PCO2 and HCO3 than the nonhypercapnic group (Table 3). Unlike the results before the propensity score matching, there were no significant differences between both groups in terms of hospital outcomes and complications during ICU stay (Table 4). When we performed a propensity score-adjusted and score-matched analysis, no statistically significant differences were found in HFNC weaning success (adjusted P = 0.623, matched P = 0.593), ICU mortality (adjusted P = 0.463, matched P = 0.195), hospital mortality (adjusted P = 0.602, matched P = 0.579), and 90-day mortality (adjusted P = 0.758, matched P = 0.808) (Table 5).
Table 3. Baseline characteristics in study patients who received HFNC therapy after propensity matching.
| Baseline characteristics | Nonhypercapnic group (n = 162) | Hypercapnic group (n = 162) | Standardized mean difference | P valuea | |
|---|---|---|---|---|---|
| Age, yr | 64.7 ± 14.3 | 64.9 ± 15.4 | 0.0116 | 0.906 | |
| Gender, men | 116/162 (71.6) | 114/162 (70.4) | 0.0331 | 0.907 | |
| Body mass index, kg/m2 | 24.2 ± 4.7 | 24.3 ± 5.1 | 0.0091 | 0.930 | |
| Smoker | 96/162 (59.3) | 101/162 (62.3) | 0.0714 | 0.657 | |
| Underlying diseases | |||||
| Diabetes mellitus | 65/162 (40.1) | 63/162 (38.9) | 0.0285 | 0.909 | |
| Hypertension | 83/162 (51.2) | 91/162 (56.2) | 0.1096 | 0.440 | |
| Solid malignancies | 56/162 (34.6) | 52/162 (32.1) | 0.0613 | 0.703 | |
| Hematologic malignancies | 5/162 (3.1) | 4/162 (2.5) | 0.1265 | 0.999 | |
| Heart failure | 27/162 (16.7) | 28/162 (17.3) | 0.0241 | 0.999 | |
| Ischemic heart disease | 18/162 (11.1) | 20/162 (12.3) | 0.0658 | 0.868 | |
| Chronic kidney disease/dialysis | 9/162 (5.6) | 10/162 (6.2) | 0.0617 | 0.999 | |
| Liver cirrhosis | 16/162 (9.9) | 15/162 (9.3) | 0.0393 | 0.999 | |
| Use of immunosuppressive agents | 38/162 (23.5) | 38/162 (23.5) | 0.0000 | 0.999 | |
| Underlying chronic lung diseases | |||||
| Asthma | 6/162 (3.7) | 7/162 (4.3) | 0.0885 | 0.999 | |
| Chronic obstructive pulmonary disease | 31/162 (19.1) | 36/162 (22.2) | 0.1039 | 0.590 | |
| Pulmonary tuberculosis history | 20/162 (12.3) | 20/162 (12.3) | 0.0000 | 0.999 | |
| Bronchiectasis | 22/162 (13.6) | 27/162 (16.7) | 0.1330 | 0.511 | |
| Lung cancer | 15/162 (9.3) | 14/162 (8.6) | 0.0418 | 0.999 | |
| Interstitial lung disease | 5/162 (3.1) | 4/162 (2.5) | 0.1265 | 0.999 | |
| ICU type, medical ICU | 87/162 (53.7) | 83/162 (51.2) | 0.0546 | 0.720 | |
| HFNC initial setting | |||||
| FiO2, % | 52.7 ± 14.0 | 53.0 ± 13.6 | 0.0170 | 0.870 | |
| Flow, L/min | 42.5 ± 8.0 | 42.0 ± 9.6 | 0.0615 | 0.589 | |
| PO2/FiO2 before HFNC application, mmHg | 127.1 ± 38.9 | 129.7 ± 38.1 | 0.0684 | 0.509 | |
| APACHE II score | 19.4 ± 6.9 | 19.6 ± 8.2 | 0.0211 | 0.841 | |
| SOFA score | 7.4 ± 3.1 | 7.7 ± 3.5 | 0.1085 | 0.326 | |
| Time differences between ABGA and HFNC application, min | 34.5 (12.0–104) | 33.5 (9.8–104.3) | 0.0218 | 0.841 | |
| Causes of HFNC application | 0.0467 | 0.583b | |||
| De novo acute respiratory failure | 32/162 (19.8) | 27/162 (16.7) | |||
| Acute-on-chronic lung disease | 28/162 (17.3) | 30/162 (18.5) | |||
| Septic shock for reasons other than respiratory infection | 5/162 (3.1) | 8/162 (4.9) | |||
| Pulmonary edema | 33/162 (20.4) | 29/162 (17.9) | |||
| After extubation | 64/162 (39.5) | 68/162 (42.0) | |||
| ABGA results before HFNC application | |||||
| pH | 7.45 ± 0.07 | 7.36 ± 0.09 | - | < 0.001c | |
| PCO2, mmHg | 36.1 ± 5.7 | 51.5 ± 8.5 | - | < 0.001c | |
| PO2, mmHg | 66.4 ± 16.3 | 68.7 ± 17.8 | - | 0.218 | |
| HCO3, mEq/L | 25.6 ± 7.3 | 29.0 ± 5.7 | - | < 0.001c | |
| SpO2, % | 91.0 ± 9.5 | 90.0 ± 7.9 | - | 0.316 | |
| Lactate, mmol/L | 1.4 (0.9, 2.4) | 1.4 (0.9, 2.4) | - | 0.343 | |
Data are presented as mean ± standard deviation or median (interquartile range) or number (%).
HFNC = high-flow nasal cannula, ICU = intensive care unit, FiO2 = fraction of inspired oxygen, PO2 = partial pressure of carbon dioxide, APACHE = acute physiology and chronic health evaluation, SOFA = sequential organ failure assessment, ABGA = arterial blood gas analysis, PCO2 = partial pressure of carbon dioxide, HCO3 = bicarbonate, SpO2 = oxygen saturation.
aStatistical comparisons of the data were performed by using the McNemar test for categorical variables and the paired t-test for continuous variables; bStatistical comparison of the data was performed by using Marginal homogeneity test; cStatistically significant P values.
Table 4. Hospital outcomes in study patients who received HFNC therapy after propensity matching.
| Hospital outcomes | Nonhypercapnic group (n = 162) | Hypercapnic group (n = 162) | P valuea | |
|---|---|---|---|---|
| HFNC weaning success rate | 107/162 (66.0) | 111/162 (68.5) | 0.598 | |
| ICU mortality | 28/162 (17.3) | 20/162 (12.3) | 0.688 | |
| Hospital mortality | 38/162 (23.5) | 34/162 (21) | 0.256 | |
| Length of ICU stay | 6.0 (3.0–12.0) | 5.0 (2.0–10.0) | 0.677 | |
| Length of hospital stay | 23.0 (13.0–40.5) | 21.0 (12.0–38.3) | 0.181 | |
| Complications during ICU stay | ||||
| Pneumonia | 31/162 (19.1) | 32/162 (19.8) | 0.999 | |
| Myocardial infarction | 4/162 (2.5) | 5/162 (3.1) | 0.999 | |
| Biliary infection | 0/162 (0.0) | 0/162 (0.0) | - | |
| Gastrointestinal infection | 4/162 (2.5) | 1/162 (0.6) | 0.375 | |
| Gastrointestinal bleeding (required endoscopy) | 2/162 (1.2) | 2/162 (1.2) | 0.999 | |
| Pulmonary thromboembolism | 1/162 (0.6) | 0/162 (0.0) | 0.999 | |
| Ischemic stroke | 1/162 (0.6) | 0/162 (0.0) | 0.999 | |
| Heart failure | 6/162 (3.7) | 3/162 (1.9) | 0.508 | |
| Pneumothorax | 6/162 (3.7) | 2/162 (1.2) | 0.289 | |
| Urinary tract infection | 0/162 (0.0) | 2/162 (1.2) | 0.500 | |
| Liver failure | 0/162 (0.0) | 2/162 (1.2) | 0.500 | |
| Catheter-related bloodstream infections | 6/162 (3.7) | 5/162 (3.1) | 0.999 | |
| Acute kidney injury (required continuous renal replacement therapy) | 23/162 (14.2) | 24/162 (14.8) | 0.999 | |
| Cardiopulmonary resuscitation | 12/162 (7.4) | 6/162 (3.7) | 0.210 | |
Data are presented as mean ± standard deviation or median (interquartile range) or number (%).
HFNC = high-flow nasal cannula, ICU = intensive care unit.
aStatistical comparisons of the data were performed by using the McNemar test for categorical variables and the paired t-test for continuous variables.
Table 5. Analysis of hospital outcomes in the hypercapnic group compared with the nonhypercapnic group (as reference).
| Variables | Crude | Propensity-adjusteda | Propensity-matched | ||||
|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P valueb | Odds ratio (95% CI) | P valueb | Odds ratio (95% CI) | P valueb | ||
| All patientsc | |||||||
| HFNC weaning success | 1.772 (1.257–2.498) | 0.001j | 1.099 (0.755–1.601) | 0.623 | 1.119 (0.741–1.688) | 0.593 | |
| ICU mortality | 0.473 (0.290–0.773) | 0.003j | 0.823 (0.490–1.384) | 0.463 | 0.674 (0.371–1.224) | 0.195 | |
| Hospital mortality | 0.586 (0.396–0.867) | 0.007j | 0.895 (0.590–1.357) | 0.602 | 0.867 (0.523–1.436) | 0.579 | |
| 90-day mortality | 0.645 (0.465–0.897)d | 0.009e,j | 0.948 (0.675–1.332)d | 0.758e | 0.943 (0.586–1.517)d | 0.808e | |
| Patients without chronic lung diseasef | |||||||
| HFNC weaning success | 1.342 (0.836–2.154) | 0.224 | 1.004 (0.607–1.662) | 0.987 | 0.813 (0.428–1.545) | 0.527 | |
| ICU mortality | 0.550 (0.264–1.147) | 0.111 | 1.001 (0.459–2.186) | 0.997 | 0.799 (0.332–1.926) | 0.617 | |
| Hospital mortality | 0.553 (0.295–1.037) | 0.065 | 0.863 (0.446–1.670) | 0.662 | 0.917 (0.407–2.066) | 0.835 | |
| 90-day mortality | 0.572 (0.327–1.000)d | 0.050e | 0.832 (0.469–1.473)d | 0.527e | 0.857 (0.396–1.853)d | 0.695e | |
| Patients with chronic lung diseaseg | |||||||
| HFNC weaning success | 2.591 (1.562–4.297) | < 0.001j | 1.364 (0.769–2.420) | 0.289 | 1.184 (0.605–2.318) | 0.622 | |
| ICU mortality | 0.353 (0.181–0.687) | 0.002j | 0.673 (0.322–1.405) | 0.292 | 0.657 (0.279–1.549) | 0.337 | |
| Hospital mortality | 0.474 (0.282–0.796) | 0.005j | 0.829 (0.461–1.489) | 0.530 | 0.941 (0.475–1.864) | 0.862 | |
| 90-day mortality | 0.570 (0.378–0.861)d | 0.008e,j | 0.936 (0.593–1.476)d | 0.776e | 0.909 (0.496–1.666)d | 0.758e | |
| Patients with HFNC application after extubationh | |||||||
| HFNC weaning success | 1.593 (0.817–3.105) | 0.172 | 1.175 (0.567–2.439) | 0.664 | 1.218 (0.536–2.768) | 0.637 | |
| ICU mortality | 0.155 (0.020–1.216) | 0.076 | 0.311 (0.037–2.642) | 0.285 | 0.156 (0.018–1.360) | 0.093 | |
| Hospital mortality | 0.398 (0.145–1.088) | 0.072 | 0.452 (0.154–1.328) | 0.149 | 0.329 (0.095–1.139) | 0.079 | |
| 90-day mortality | 0.597 (0.267–1.334)d | 0.209e | 0.631 (0.263–1.513)d | 0.302e | 0.455 (0.158–1.308)d | 0.144e | |
| Patients with HFNC application other causes than extubationi | |||||||
| HFNC weaning success | 1.235 (0.796–1.916) | 0.347 | 1.137 (0.719–1.799) | 0.583 | 1.044 (0.573–1.900) | 0.889 | |
| ICU mortality | 0.770 (0.452–1.314) | 0.339 | 0.822 (0.472–1.431) | 0.488 | 0.881 (0.410–1.895) | 0.746 | |
| Hospital mortality | 0.950 (0.600–1.507) | 0.829 | 0.982 (0.608–1.586) | 0.940 | 1.279 (0.679–2.408) | 0.446 | |
| 90-day mortality | 0.945 (0.659–1.357)d | 0.761e | 0.976 (0.672–1.416)d | 0.898e | 1.261 (0.729–2.179)d | 0.406e | |
CI = confidence interval, HFNC = high-flow nasal cannula, ICU = intensive care unit.
aThe individual propensity score was integrated into each outcome model as a co-variable; bStatistical comparisons of the data were performed using logistic regression analysis; cOf the 862 patients, 162 pairs were matched; dHazard ratios analyzed by Cox proportional regression model; eStatistical comparisons of the data were performed using Cox proportional regression analysis; fOf the 518 patients, 95 pairs were matched; gOf the 344 patients, 84 pairs were matched; hOf the 293 patients, 78 pairs were matched; iOf the 569 patients, 95 pairs were matched; jStatistically significant P values.
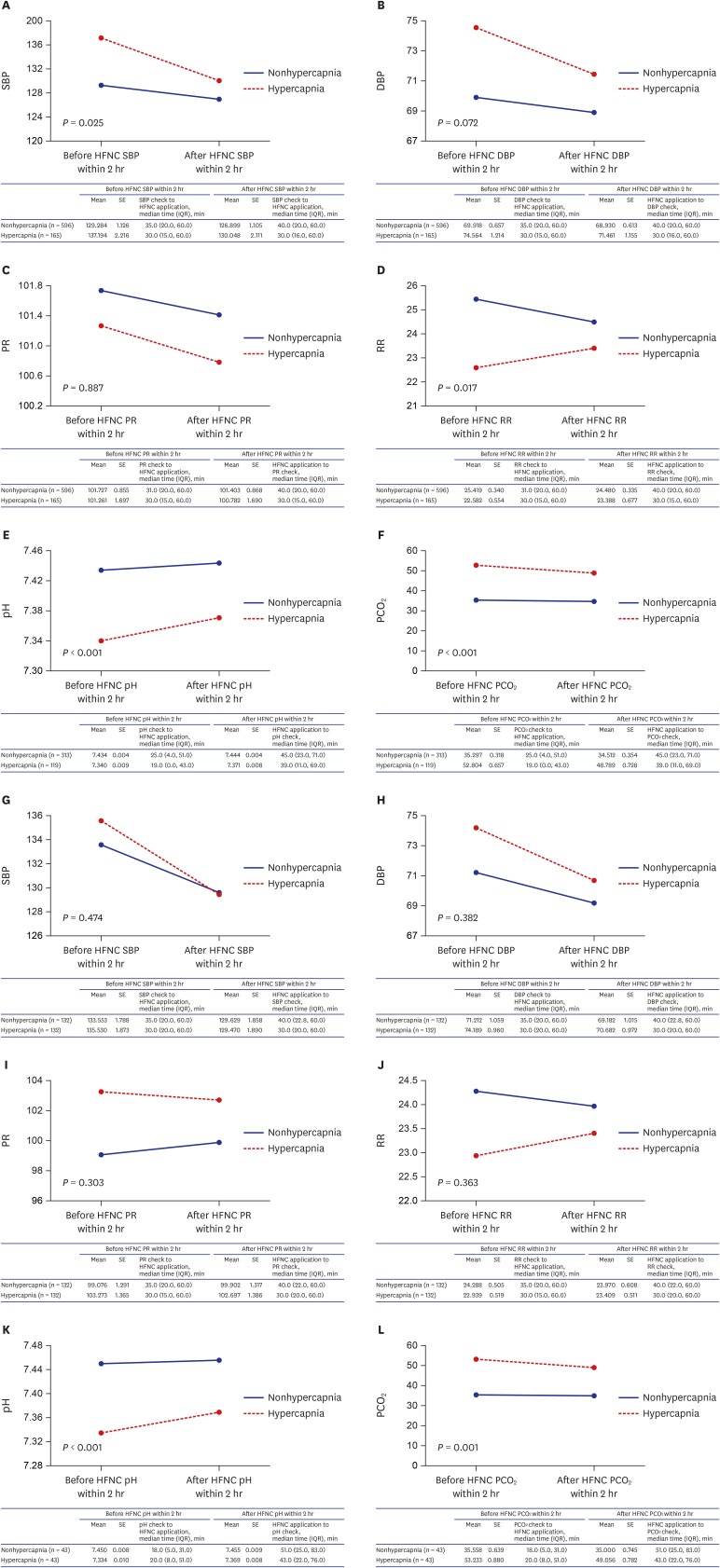
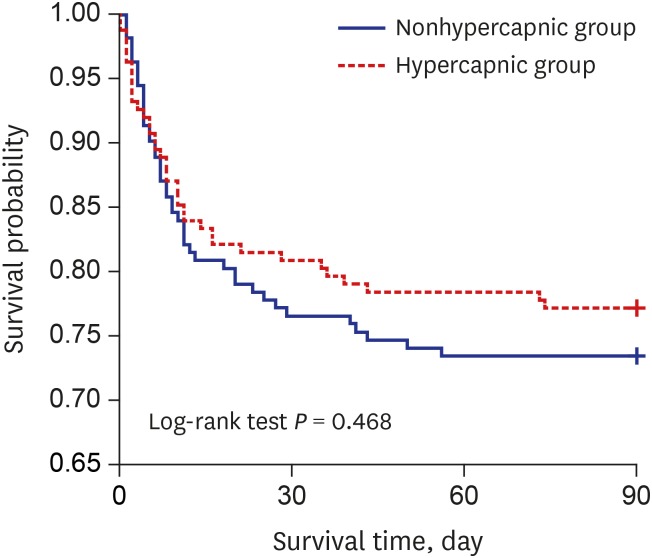
Fig. 2 presented the short-term effectiveness of HFNC therapy by using HFNC application immediately before and after vital signs, pH and PCO2 values in before and after propensity score matching cohorts. There were no interactions associated with vital sign changes according to HFNC therapy between nonhypercapnic and hypercapnic groups in after propensity score matching cohort. However, there were interactions associated with pH (before propensity score matching P < 0.001, after propensity score matching P < 0.001) and PCO2 (before propensity score matching P < 0.001, after propensity score matching P = 0.001) values according to HFNC therapy between the two groups. Fig. 3 shows the survival curve of the matched groups stratified by the presence of hypercapnia, and there was no significant difference for the 90-day survival between the two groups (P = 0.468).
Fig. 2. Changes of vital signs and arterial blood gas analysis results between nonhypercapnic and hypercapnic groups after initiation of high-flow nasal cannula therapy. In before propensity score matching cohort: (A) SBP change after HFNC application, (B) DBP change after HFNC application, (C) PR change after HFNC application, (D) RR change after HFNC application, (E) pH change after HFNC application, (F) PCO2 change after HFNC application, And in after propensity score matching cohort: (G) SBP change after HFNC application, (H) DBP change after HFNC application, (I) PR change after HFNC application, (J) RR change after HFNC application, (K) pH change after HFNC application, (L) PCO2 change after HFNC application.
HFNC = high-flow nasal cannula, SBP = systolic blood pressure, SE = standard error, IQR = interquartile range, DBP = diastolic blood pressure, PR = pulse rate, RR = respiration rate, PCO2 = partial pressure of carbon dioxide.
Fig. 3. Kaplan-Meier survival curves stratified by the presence of hypercapnia before high-flow nasal cannula application.
Subgroup analysis
We performed subgroup analysis according to the presence of CLD and causes of HFNC application. Although several baseline characteristics of each subgroup were different between the nonhypercapnic and hypercapnic groups, the hypercapnic group had similar or better HFNC weaning success, similar or lower mortality, and similar or fewer complications in all subgroups (Supplementary Tables 6–9). After propensity score matching for each subgroup, there was no significant difference in HFNC weaning success and mortality between the two groups (Supplementary Tables 10–13). When the propensity score-adjusted and score-matched analysis were performed in each subgroup, both groups also did not differ significantly in terms of HFNC weaning success, ICU mortality, hospital mortality and 90-day mortality (Table 5).
DISCUSSION
Our current study findings indicated that the effectiveness of HFNC therapy in AHRF patients with hypercapnia was not inferior to the effectiveness of the therapy in those without hypercapnia under various conditions. In other words, AHRF patients could be treated with HFNC therapy regardless of the presence of hypercapnia before HFNC application. This finding was in accordance with the results from previous studies that reported the effectiveness of HFNC therapy in patients with acute RF,14,15,19 acute exacerbation of COPD,20,21 and after extubation4,22 when accompanied with hypercapnia. However, these studies involved small sample sizes, and there has been no prospective randomized controlled study for the evaluation of HFNC weaning success and mortality. However, the current study was well constructed and confirmed these results using a cohort of relatively large sample sizes.
The choice of the appropriate form of oxygen device in AHRF patients with hypercapnia is unclear. Conventional oxygen devices have limited efficacy in RF because of the significant decrease in the delivered FiO2. Invasive mechanical ventilation is a lifesaving technique, but it is also associated with various complications. NIV is recommended for use only in patients with hypercapnia who have an acidic pH but not in those who do not have an acidic pH.9 Additionally, NIV is not recommended in patients with altered mental states, unstable hemodynamic conditions, or an inability to protect the airway.23 Considering these reasons and our study results, HFNC therapy might overcome the limitations of the other devices and be a good alternative for patients in AHRF with hypercapnia.
Derangements in gas exchange may be developed in patients with CLD and cause hypercapnia.24 These results were consistent with the fact that the hypercapnia group had a greater number of underlying CLDs in our study. The hospital outcomes and complications were worse in the nonhypercapnic group than in the hypercapnic group before propensity matching. This was presumed to be because of the severity of the nonhypercapnic group being higher than that of the hypercapnic group in most of the baseline characteristics except for underlying CLD. There were more solid malignancies in the hypercapnic group. However, considering the number of lung cancer patients, nonlung cancerous solid malignancies were similar between the nonhypercapnic and hypercapnic groups (20.5% vs. 20.3%). Although these differences between both groups were compensated for after propensity matching, the outcomes of the hypercapnic group were not significantly different from those of the nonhypercapnic group in our study. The subgroup results were also similar to these results. Our findings indicate that HFNC therapy might be beneficial for AHRF patients with hypercapnia in various conditions.
Although HFNC therapy is safe and useful in different clinical situations, including RF,25 patients with hypercapnia were mostly excluded in the previous large-scale HFNC studies.2,26,27,28,29 Therefore, the efficacy of HFNC in patients with hypercapnia has not been sufficiently evaluated. One study has even asserted that hypercapnia has potentially deleterious effects in patients with CLD.24 Our study findings showed the effectiveness of HFNC therapy in AHRF patients with hypercapnia using AHRF patients without hypercapnia as a control group under various conditions. However, one thing to note is that the effectiveness of HFNC was evaluated in AHRF patients with hypercapnia only and not in patients with hypercapnic RF. Although it was reported that HFNC therapy was used to successfully manage hypercapnic RF,30 special attention in the treatment of hypercapnic RF using HFNC therapy and a large prospective randomized controlled study are required. We hope the findings from our study contribute to establishing an appropriate indication for HFNC therapy.
Our study had several limitations. First, it was a retrospective observational study. However, we used propensity score matching and evaluated the effectiveness of HFNC treatment in AHRF patients with or without hypercapnia in a group of carefully selected patients to identify a possible link between hypercapnia and hospital outcomes. Second, our study was conducted at a single center. Therefore, selection bias cannot be excluded, and the results must be interpreted with caution. Large-scale multicenter and randomized controlled studies are required to obtain more accurate and reliable results. Third, there are possible differences in the actual FiO2 supplied to the patients and the FiO2 setting of the HFNC device. Data, including FiO2, should be carefully interpreted because the delivered FiO2 cannot be measured.
Because hypercapnia might be associated with poor prognosis in RF, HFNC therapy was used very cautiously under this condition. Our study findings showed that the effectiveness of HFNC therapy in AHRF patients with hypercapnia was not significantly different from those without hypercapnia in terms of HFNC weaning success and mortality. HFNC therapy might be effective in AHRF patients with hypercapnia. We believe that our study can contribute to the establishment of appropriate indications for HFNC therapy. Further large-scale, prospective, and randomized controlled trials are warranted.
ACKNOWLEDGMENTS
This work was supported by the Medical Information Center of Ulsan University Hospital in the data extraction.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kang BJ, Ahn J.
- Data curation: Bae SH, Kim C, Lee H, Kim JH.
- Formal analysis: Han M, Kang BJ.
- Investigation: Kim JH, Lee H.
- Methodology: Kim C, Ahn J.
- Writing - original draft: Bae SH.
- Writing - review & editing: Han M, Kim C, Lee H, Ahn J, Kim JH, Kang BJ.
SUPPLEMENTARY MATERIALS
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of study patients
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of patients without chronic lung disease
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of patients with chronic lung disease
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of patients with HFNC application after extubation
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of patients with HFNC application for causes other than extubation
Baseline characteristics and hospital outcomes in patients without chronic lung disease who received HFNC therapy
Baseline characteristics and hospital outcomes in patients with chronic lung disease who received HFNC therapy
Baseline characteristics and hospital outcomes in study patients who received HFNC therapy after extubation
Baseline characteristics and hospital outcomes in study patients who received HFNC therapy for causes other than extubation
Baseline characteristics and hospital outcomes in study patients without chronic lung disease who received HFNC therapy after propensity score matching
Baseline characteristics and hospital outcomes in study patients with chronic lung disease who received HFNC therapy after matching on propensity score
Baseline characteristics and hospital outcomes in study patients who received HFNC therapy after extubation after propensity score matching
Baseline characteristics and hospital outcomes in study patients who received HFNC therapy for causes other than extubation after propensity score matching
References
- 1.Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ. 2017;189(7):E260–E267. doi: 10.1503/cmaj.160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 3.Rochwerg B, Granton D, Wang DX, Helviz Y, Einav S, Frat JP, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 4.Yoo JW, Synn A, Huh JW, Hong SB, Koh Y, Lim CM. Clinical efficacy of high-flow nasal cannula compared to noninvasive ventilation in patients with post-extubation respiratory failure. Korean J Intern Med. 2016;31(1):82–88. doi: 10.3904/kjim.2016.31.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HJ, Asai T. High-flow nasal oxygenation for anesthetic management. Korean J Anesthesiol. 2019;72(6):527–547. doi: 10.4097/kja.19174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung SM, Choi JW, Lee YS, Choi JH, Oh JY, Min KH, et al. Clinical effectiveness of high-flow nasal cannula in hypoxaemic patients during bronchoscopic procedures. Tuberc Respir Dis. 2019;82(1):81–85. doi: 10.4046/trd.2017.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdo WF, Heunks LM. Oxygen-induced hypercapnia in COPD: myths and facts. Crit Care. 2012;16(5):323. doi: 10.1186/cc11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. doi: 10.1136/bmj.c5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 10.Pisani L, Vega ML. Use of nasal high flow in stable COPD: rationale and physiology. COPD. 2017;14(3):346–350. doi: 10.1080/15412555.2017.1315715. [DOI] [PubMed] [Google Scholar]
- 11.Bräunlich J, Beyer D, Mai D, Hammerschmidt S, Seyfarth HJ, Wirtz H. Effects of nasal high flow on ventilation in volunteers, COPD and idiopathic pulmonary fibrosis patients. Respiration. 2013;85(4):319–325. doi: 10.1159/000342027. [DOI] [PubMed] [Google Scholar]
- 12.Rea H, McAuley S, Jayaram L, Garrett J, Hockey H, Storey L, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med. 2010;104(4):525–533. doi: 10.1016/j.rmed.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Seo KW, Ahn JJ, Jegal Y, Ra SW, Bae S, Kim JH, et al. High-flow nasal cannula oxygen therapy for acute hypoxemic respiratory failure in patients with chronic lung disease in terms of hospital outcomes. Intensive Care Med. 2018;44(3):387–388. doi: 10.1007/s00134-017-5018-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim ES, Lee H, Kim SJ, Park J, Lee YJ, Park JS, et al. Effectiveness of high-flow nasal cannula oxygen therapy for acute respiratory failure with hypercapnia. J Thorac Dis. 2018;10(2):882–888. doi: 10.21037/jtd.2018.01.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong JH, Kim DH, Kim SC, Kang C, Lee SH, Kang TS, et al. Changes in arterial blood gases after use of high-flow nasal cannula therapy in the ED. Am J Emerg Med. 2015;33(10):1344–1349. doi: 10.1016/j.ajem.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 16.Onodera Y, Akimoto R, Suzuki H, Nakane M, Kawamae K. A high-flow nasal cannula system set at relatively low flow effectively washes out CO2 from the anatomical dead space of a respiratory-system model. Korean J Anesthesiol. 2017;70(1):105–106. doi: 10.4097/kjae.2017.70.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demoule A, Girou E, Richard JC, Taillé S, Brochard L. Increased use of noninvasive ventilation in French intensive care units. Intensive Care Med. 2006;32(11):1747–1755. doi: 10.1007/s00134-006-0229-z. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 19.Lee HW, Choi SM, Lee J, Park YS, Lee CH, Yoo CG, et al. Reduction of PaCO2 by high-flow nasal cannula in acute hypercapnic respiratory failure patients receiving conventional oxygen therapy. Acute Crit Care. 2019;34(3):202–211. doi: 10.4266/acc.2019.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim SH, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046–2056. doi: 10.1111/crj.12772. [DOI] [PubMed] [Google Scholar]
- 21.Bräunlich J, Wirtz H. Nasal high-flow in acute hypercapnic exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3895–3897. doi: 10.2147/COPD.S185001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing G, Li J, Hao D, Wang T, Sun Y, Tian H, et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: a pilot randomized controlled trial. Res Nurs Health. 2019;42(3):217–225. doi: 10.1002/nur.21942. [DOI] [PubMed] [Google Scholar]
- 23.Bello G, De Pascale G, Antonelli M. Noninvasive ventilation: practical advice. Curr Opin Crit Care. 2013;19(1):1–8. doi: 10.1097/MCC.0b013e32835c34a5. [DOI] [PubMed] [Google Scholar]
- 24.Vadász I, Hubmayr RD, Nin N, Sporn PH, Sznajder JI. Hypercapnia: a nonpermissive environment for the lung. Am J Respir Cell Mol Biol. 2012;46(4):417–421. doi: 10.1165/rcmb.2011-0395PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roca O, Hernández G, Díaz-Lobato S, Carratalá JM, Gutiérrez RM, Masclans JR. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with respiratory failure. Crit Care. 2016;20(1):109. doi: 10.1186/s13054-016-1263-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azoulay E, Lemiale V, Mokart D, Nseir S, Argaud L, Pène F, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320(20):2099–2107. doi: 10.1001/jama.2018.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez R, Subira C, Frutos-Vivar F, Rialp G, Laborda C, Masclans JR, et al. High-flow nasal cannula to prevent postextubation respiratory failure in high-risk non-hypercapnic patients: a randomized multicenter trial. Ann Intensive Care. 2017;7(1):47. doi: 10.1186/s13613-017-0270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández G, Vaquero C, González P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354–1361. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 29.Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 30.Millar J, Lutton S, O'Connor P. The use of high-flow nasal oxygen therapy in the management of hypercarbic respiratory failure. Ther Adv Respir Dis. 2014;8(2):63–64. doi: 10.1177/1753465814521890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of study patients
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of patients without chronic lung disease
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of patients with chronic lung disease
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of patients with HFNC application after extubation
Results of multiple logistic regression analysis to estimate the propensity score and multiple linear regression analysis to evaluate the variance inflation factor for baseline characteristics of patients with HFNC application for causes other than extubation
Baseline characteristics and hospital outcomes in patients without chronic lung disease who received HFNC therapy
Baseline characteristics and hospital outcomes in patients with chronic lung disease who received HFNC therapy
Baseline characteristics and hospital outcomes in study patients who received HFNC therapy after extubation
Baseline characteristics and hospital outcomes in study patients who received HFNC therapy for causes other than extubation
Baseline characteristics and hospital outcomes in study patients without chronic lung disease who received HFNC therapy after propensity score matching
Baseline characteristics and hospital outcomes in study patients with chronic lung disease who received HFNC therapy after matching on propensity score
Baseline characteristics and hospital outcomes in study patients who received HFNC therapy after extubation after propensity score matching
Baseline characteristics and hospital outcomes in study patients who received HFNC therapy for causes other than extubation after propensity score matching