Abstract
Despite the increasing popularity of zebrafish (Danio rerio) as an animal model, the environmental enrichment preferences of this species have been largely unexplored. We sought to determine the preferences of mature female zebrafish that were singly housed with or without access to one of 10 inanimate forms of enrichment. As a marker of preference, in-tank fish location was observed by video recording. All subjects showed a preference for the front of the tank when caretakers entered the room, demonstrating an effect of human presence on tank location. Among the 10 enrichment items tested, subjects showed the strongest preference for mirrored paper on the side of the tank when compared with the barren half of the tank. Fish also were observed interacting with PVC pipe, marbles, and tulle. Given the preference for enrichment imitating social interaction, we conducted a second study to assess the value of visual exposure of conspecifics in adjacent tanks. The experimental zebrafish were then provided one of 3 conditions—a singly housed neighbor fish, group-housed neighbor fish, or no neighbor fish. All zebrafish housed next to neighboring fish showed a preference to be on the side of the tank nearer to the other fish. Overall, our data indicate that singly housed zebrafish prefer enrichment items that resemble or promote social behaviors. Therefore items such as mirrored paper or housing next to conspecifics should be strongly considered as enrichment strategies for singly housed zebrafish.
Zebrafish (Danio rerio) are increasingly used as an animal model in scientific research, yet their social and environmental preferences in the laboratory need to be explored, given that few publications address the enrichment preferences of zebrafish. The Guide for the Care and Use of Laboratory Animals promotes environmental enrichment and social housing that are based on species-specific behaviors in natural settings, such as the use of group housing for shoaling fish and appropriate substrate for expression of environment-driven behaviors.6 Overall, environmental enrichment is intended to increase habitat complexity and allow expression of natural behaviors, thereby increasing physiologic and psychologic wellbeing. Although some institutions have made an effort to enrich the environment of their aquatic species, preferred enrichment strategies are not well established for group-housed zebrafish, and even less elucidated are enrichment strategies for singly housed zebrafish. Single housing can be a necessary requirement in the research setting, such as for genotyping, quarantine, or research paradigms. Zebrafish are a social species that live in structurally complex habitats that vary widely along a number of parameters.5 To reduce stress and continue to allow for species-specific behavior during a period of single housing in a barren tank, environmental enrichment could be used to simulate a more naturalistic setting.
Previous studies evaluating environmental enrichment for zebrafish have found that they prefer environments that resemble their natural environment and promote their preference for social grouping.4,13 Existing literature assessing enrichment for zebrafish often used group-housed zebrafish. These studies found that zebrafish, when given a choice, prefer enriched over barren conditions and prefer moving and dynamic stimuli, such as social conditions, over static and inanimate stimuli, such as plastic plants. These preferences were influenced by sex and social context (group- or pair-housed zebrafish).4,13 A previous study2 that evaluated the behavioral effects of single housing and environmental enrichment on zebrafish sought to determine whether singly housed zebrafish offered a choice of various tank compartments prefer inanimate enrichment over being near conspecifics. The investigators found that zebrafish preferred to be in the compartment containing conspecifics.4 When conspecifics are not available, zebrafish have been shown to prefer an environment containing inanimate enrichment items rather than a barren tank.13 Despite these studies, data regarding specific enrichment items and strategies preferred by zebrafish, particularly singly housed zebrafish, are sparse and require further exploration.
The development of appropriate and preferred enrichment strategies for singly housed zebrafish in the laboratory will be an important factor in optimizing animal welfare and wellbeing. Zebrafish have the ability to qualify their experiences subjectively because they have the anatomy and necessary processes to acquire, store, and act on information gathered from the environment.2 This concept is foundational to understanding the importance of environmental enrichment. With this in mind, we sought to determine enrichment preferences of mature female zebrafish that were singly housed with or without access to various forms of enrichment that recapitulated species-typical behaviors, including social interaction, foraging for food, and swimming close to structural complexities within the tank. We used place-preference testing, which allows an animal a choice in their environment,4 to evaluate the in-tank location preference of singly housed zebrafish with and without access to enrichment. We hypothesized that the position of fish in the tank would be altered by various enrichment strategies when compared with a barren tank and that the effect would be greater when enrichment imitated social contact.
Materials and Methods
Animals and housing.
All activities described were approved by the University of Michigan IACUC, and animals were housed in an AAALAC-accredited facility (University of Michigan, Ann Arbor, MI). Wild-type AB strain zebrafish (Danio rerio) were used in this experiment and were spawned from a colony maintained in our vivarium for more than 10 y. Zebrafish were fed 3 times daily during the week and twice daily during the weekend. The food was a blended homemade diet consisting of TetraMin Tropical Flakes (Tetra, Blacksburg, VA), spirulina (Pentair Aquatic Ecosystems, Cary, NC), and Golden Pearls (Kens Fish, Taunton, MA). The volume of feed was based on how much food a fish consumed in 2 to 3 min. Feeding was performed in the front half of the tank. Fish were group-housed on a 14:10-h light:dark cycle at a density of 5 fish per liter on a closed-loop recirculating system supplied with water purified by reverse osmosis (Pentair Aquatic Eco-systems, Cary, NC). The tank level light intensity was measured to be 150 to 250 lx by using a portable luminometer (UNI-T 5URG1, Grainger International, Lake Forest, IL). The system was maintained at 28 °C, pH 7, nitrates 0 to 30 mg/L, nitrites 0 ppm, ammonia 0 ppm, and conductivity of 550 µS. Water quality parameters assessed (YSI, Yellow Springs, OH) daily included temperature, pH, and conductivity. Weekly monitoring of nitrates, nitrites, ammonia, and pH was completed by using test kits for individual parameters (API, Mars Fishcare, Chalfont, PA).
Experimental housing.
Female zebrafish (n = 40; age, 6 to 12 mo) were chosen randomly from the colony for participation and were singly housed in 3-L tanks within the recirculating housing system. Feeding during the experiment followed the same protocol as for colony fish and was completed 1 h prior to video recording. Tanks testing inanimate enrichment items (n = 10 fish) were divided across the midline of the tank's shorter axis by using an opaque partition, thus dividing the tank into a front half and back half. The partition contained an opening to allow the fish to pass through (Figure 1). The tanks testing animate enrichment with neighbor fish (n = 30) were divided lengthwise by using a single black line drawn on the tank exterior, thus dividing the tank visually into left and right compartments. The animals were allowed 7 d of acclimation to single housing prior to the start of the study.
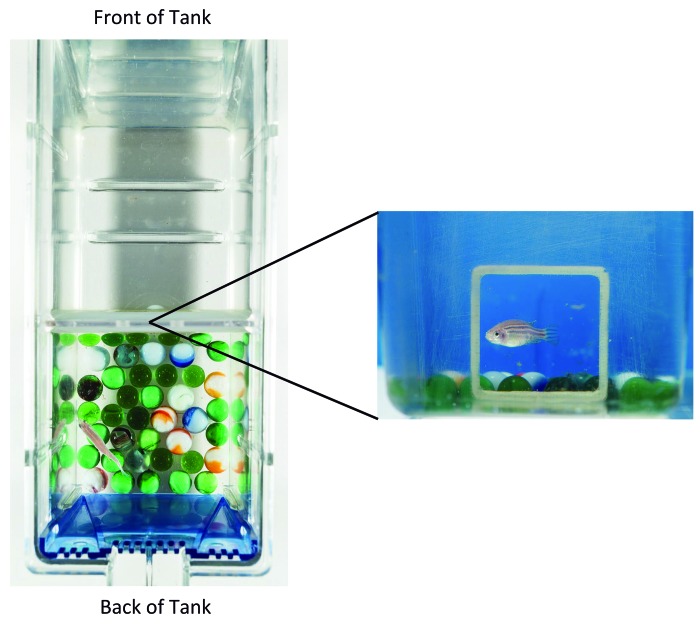
Figure 1.
Design of the inanimate enrichment experimental tanks. The tanks were divided in half into front and back compartments by using an opaque plastic divider that had a window at the bottom. Enrichment items were placed in the back half of the tank.
Inanimate environmental enrichment study.
To determine preferred environmental enrichment strategies, we identified for evaluation 10 enrichment items, including those reported in previous literature as well as items currently approved in our internal enrichment database.4,8,9,13,15 These items included: white PVC pipe; white tulle (very fine mesh material, cut into a strip and knotted); dark paper affixed to the bottom of the tank; multicolored marbles, green and brown plastic plant; image of the same multicolored marbles placed on the bottom of the tank; dark paper affixed to the side of the tank; image of multicolored plants on the side of the tank; image of zebrafish placed on the tank side; and mirrored paper affixed to the side of the tank (Figure 2). The images displayed to the fish of marbles and zebrafish were photographs of the same items placed in or on the tanks. The dark surface, plants, and mirrored paper were all commercially available and not of materials otherwise present in the tanks or room. The number of marbles used was enough to cover 50% of the available tank floor space in a single, even layer.
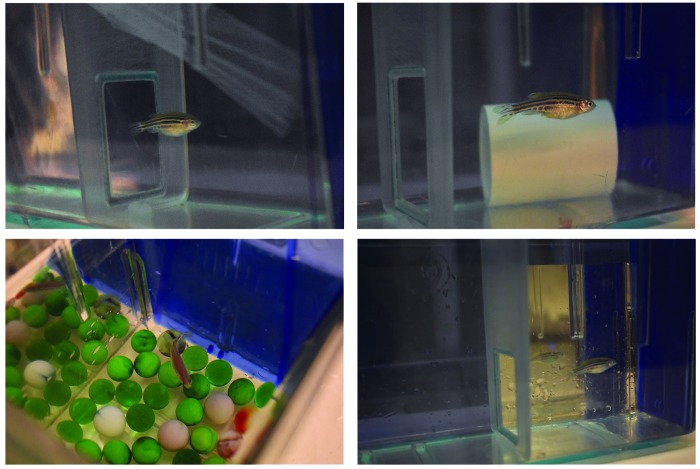
Figure 2.
Examples of the inanimate enrichment items evaluated. Clockwise from top left: tulle, PVC pipe, mirrored paper, and marbles.
The experiment used a crossover design, and each fish was exposed to each enrichment strategy in a randomized order. After the initial 3-d acclimation period to the divider, a 4-d baseline recording period established that approximately 70% of all fish displayed a preference for the front half of the tank in the absence of any enrichment items. Accordingly, enrichment items were randomly assigned and placed in the back half of the tank to overcome this natural preference for the front half of the tank and to assess how strongly the enrichment items are preferred.
The fish then were allowed a 3-d acclimation period to each enrichment item, followed by 4 d of video recording. Enrichment items were rotated after video recording was completed, until all 10 conditions had been tested. Additional baseline weeks were recorded halfway through and at the completion of the study to evaluate whether previous access to enrichment altered baseline in-tank location preference.
Animate environmental enrichment study.
To assess the value of visual exposure to conspecifics in adjacent tanks, subjects were exposed to either a singly housed neighbor fish (n = 10), group-housed neighbor fish (n = 10), or an empty neighbor tank (n = 10; Figure 3). The fish were allowed a 3-d acclimation period to their social condition, followed by 4 d of video recording for position in the tank relative to the enrichment. To eliminate tank side (left–right) bias, half of the tanks had the neighbor fish present on the left, and half on the right.
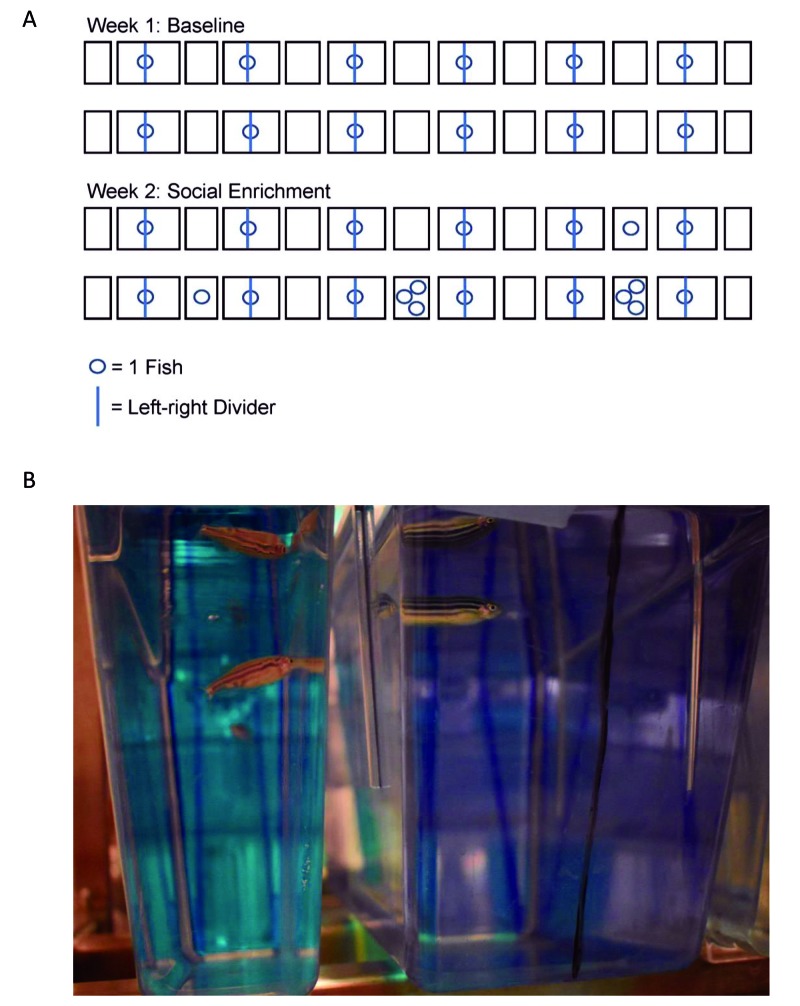
Figure 3.
(A) Schematic diagram of the tank set-up for the animate enrichment place-preference evaluation during the baseline and experimental weeks. During the baseline week, tanks adjacent to experimental tanks did not contain fish. During the experimental week, tanks adjacent to experimental tanks contained either no fish, a singly housed fish, or group-housed fish. (B) Design of the animate enrichment experimental tanks. The experimental tanks were larger and were divided into left and right sections by using a single black line drawn on the tank exterior.
Video recording and scoring of fish behavior.
Video recording (HD Everio, JVC, Long Beach, CA) was used to observe fish behavior twice daily (morning and afternoon) during an uninterrupted period of 1 h for each session, over the course of 4 d. Video was scored in a randomized order by a trained observer. One video camera was used to record 2 experimental fish tanks. By using a scan sampling method adapted from previously published literature,11 each video was broken into 6 clips (10 s each), for a total of 60 s of clips per hour of recording. This process resulted in 48 time points per week of recording for each tank. The clips were scored by using a fixed-interval 1–0 sampling method for fish position in the tank relative to the enrichment item. Each clip was scored as 0, indicating that fish spent the majority of the 10 s in the front half or on the nonsocial side of the tank; as 0.5, when fish spent equal amounts of time in or on each half of the tank; or as 1 when the majority of the fish's time was spent in the back half or on the social side of the tank. The counts were converted to percentages of time spent in the back half or on the social side of the tank.
Using a previously validated ethogram for aggressive behaviors in zebrafish7,12,14 and the same video clips scored for in-tank location during the animate enrichment study, 2 trained observers evaluated the recordings for 5 behaviors— bite, chase, strike, freeze, and flee (Figure 4). This ethogram was not applied to the inanimate enrichment study to evaluate the behavior of zebrafish when the mirrored paper was present because the fish were obscured by the in-tank divider.
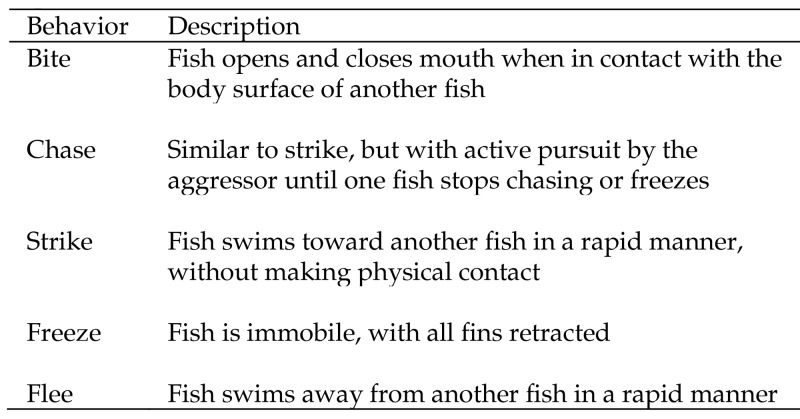
Figure 4.
Ethogram of aggressive behavior in zebrafish (adapted from reference 14).
Statistical analysis.
Visual and statistical analysis was performed by using R version 3.4.3 (CRAN). A P value less than 0.05 was used to define statistical significance. We used mixed-effect linear regression to determine the effect of time or enrichment items on tank location preference.3 Model fixed effects included the time point scored, form of enrichment, and week of study, to examine how each affected tank location preference. We also included nested model random effects for each tank to adjust for repeated sampling over time. Regression analysis was performed in R by using the lme4 package.1
For statistical comparison of the effect of neighbor status on tank location preference of singly housed fish, we used a paired Wilcoxon signed-rank test to compare each treatment in week 1 (the baseline week) with week 2 (the experimental week). We randomly assigned the social side of the tank prior to scoring video for fish that did not receive neighbors at any point in the study. To examine the effect of initial side preferences on response to neighbor housing, we ranked all fish according to their baseline preference, with the highest ranks representing fish with the greatest preference for a particular side. We then used Spearman correlation to assess the effect of initial side preference on the fold change in side preference after the experimental treatment (week 2) compared with baseline (week 1).
Results
Effect of inanimate environmental enrichment on location of zebrafish.
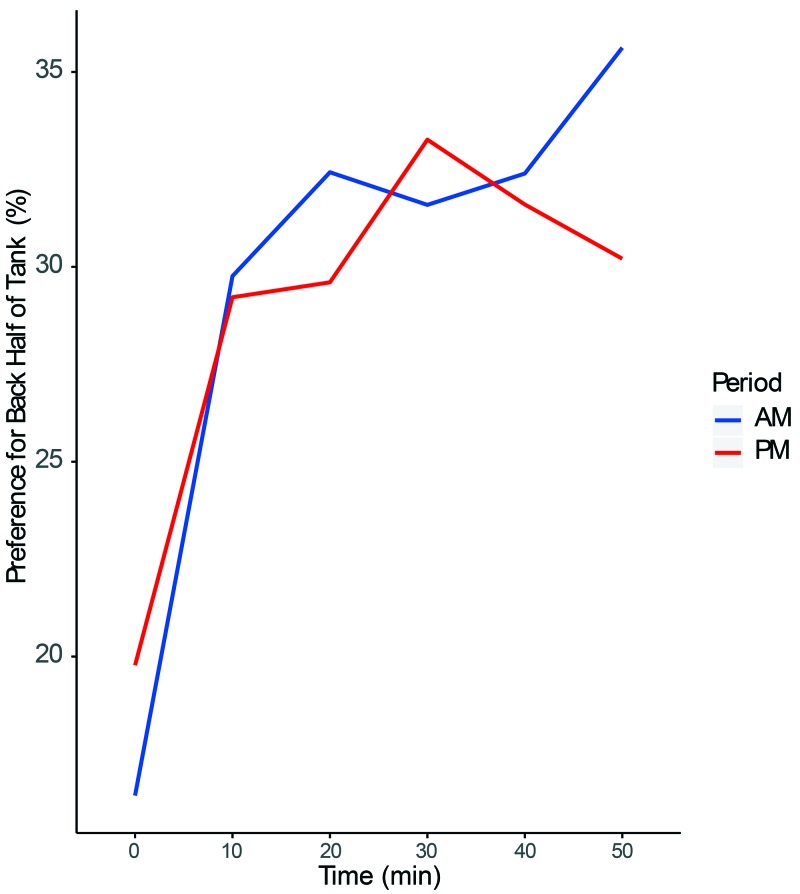
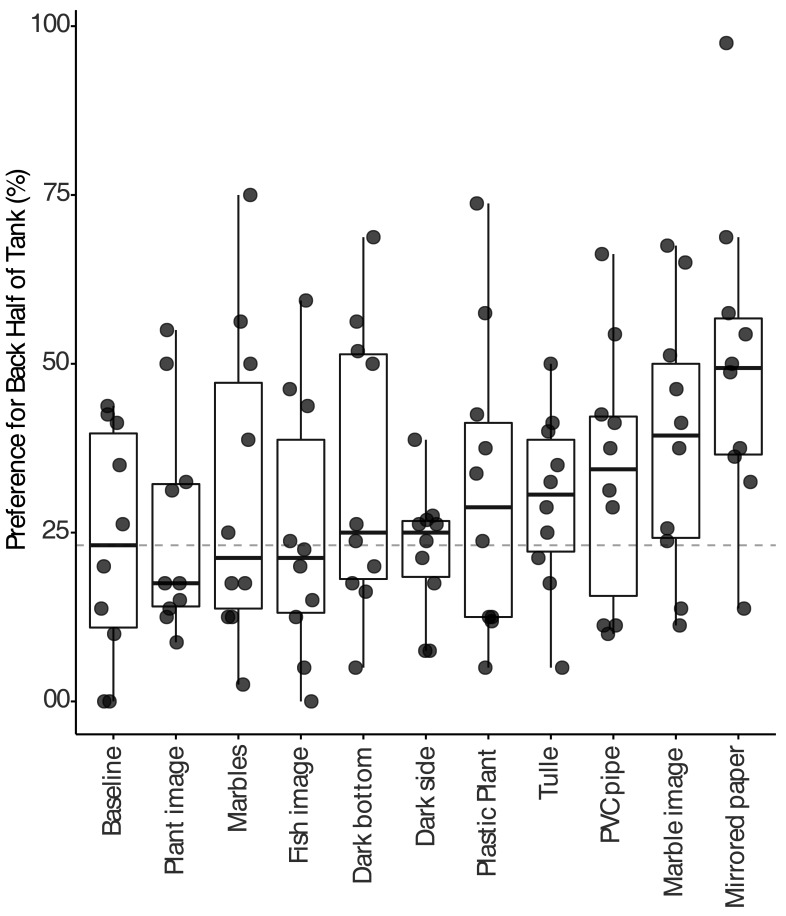
In the present study, fish preferred to be in the front half of the tank when inanimate environmental enrichment items were not present. This preference did not change throughout the study. When inanimate environment enrichment was present, fish preferred the front of the tank during the first time point, when the caretakers entered the room to turn on the video cameras (Figure 5, P = 2 × 10−16). This effect occurred during both the morning and afternoon recording sessions. Given this effect, we examined all later time points, which were not affected by room entry, to evaluate preferences for inanimate environmental enrichment strategies. Zebrafish showed the strongest (P < 0.0005) preference for the back of the tank when housed with mirrored paper on the side of the tank compared with the barren front half of the tank (Figure 6). Fish also were observed interacting with 3 additional items: PVC pipe, tulle, and marbles. We found no differences in the place-preference or behavior of zebrafish when they were housed with the remaining enrichment items. Previous access to enrichment did not significantly alter baseline in-tank location preference throughout the study.
Figure 5.
Average percentage of time spent by the zebrafish (n = 10) in the back half of the tank over the course of the recorded hour, controlled for all enrichments including baseline. The time scale indicates the 10-min intervals in which the first 10 s of each interval were scored during the hour-long video observation. Time interval during the hour of observation had a significant (P < 0.00001) effect on fish location in the tank. Significance was determined by using mixed-effect linear regression examining the effect of time on preference.
Figure 6.
Effect of inanimate environmental enrichment on preference (% of time) for the back half of the tank containing the various enrichment items. The dotted line represents the baseline preference value. Fish showed a significant (P < 0.0005) preference for the back half of the tank when the mirrored paper was the enrichment item. Significance was determined by using mixed effect linear regression examining the effect of enrichment on preference.
Effect of animate environmental enrichment on tank location and behavior.
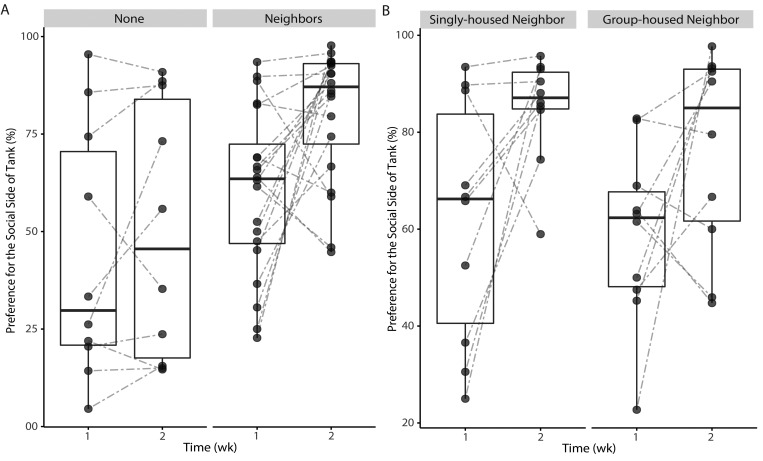
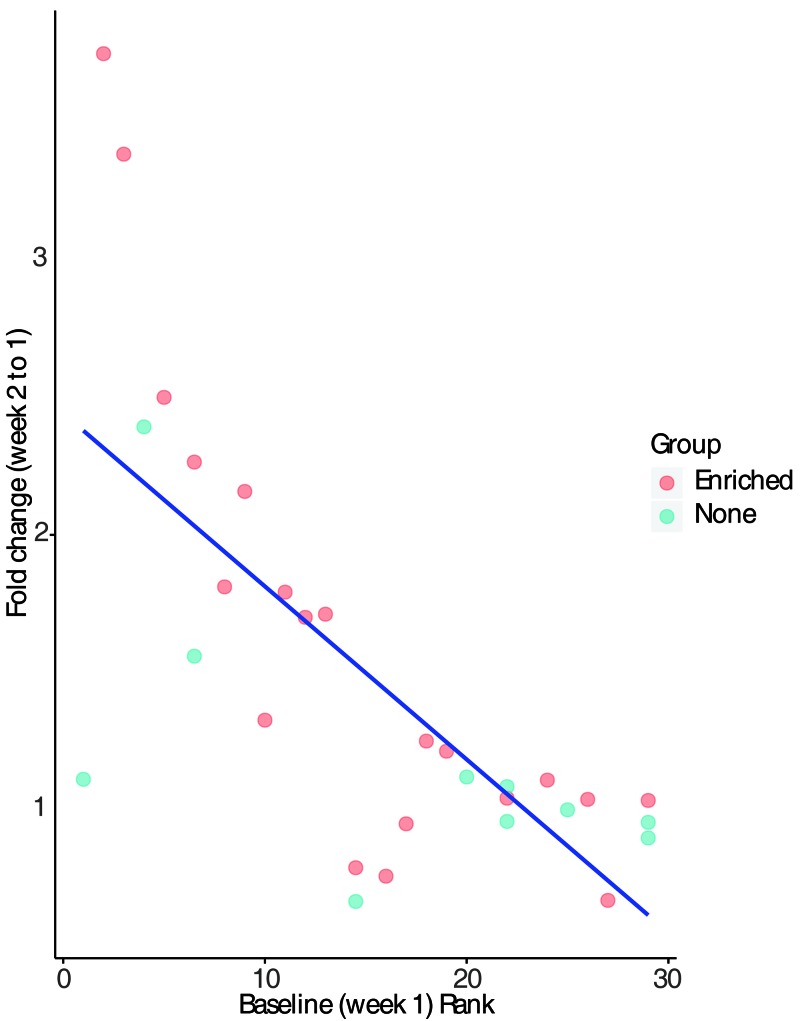
Zebrafish preferred to be on the social side of the tank (Figure 7 A, P = 0.004) when neighbors, either singly or group-housed, were present. This preference shift did not occur when neighbors were not present (Figure 7 A, P = 0.678). This preference was significant when the neighbor fish was singly housed (Figure 7 B, P = 0.025) as compared with group-housed (Figure 7 B, P < 0.092). In addition, there was no significant preference related to the side on which the tank housing the adjacent fish was placed during the animate enrichment portion of the study. Fish that were housed without neighbors did not demonstrate a preference for a particular side. Despite a baseline difference between the enriched and nonenriched groups, no significant factors were identified. Fish were ranked according to their initial side preference during the baseline week (week 1), with higher rank indicating a higher preference for the assigned ‘social’ side. This rank was then compared with the fold change in side preference after the experimental week (week 2, enriched) relative to the baseline week. We found an inverse correlation between week 1 rank (initial side preference) and the fold change in side preference due to the presence of neighbors (Figure 8, Spearman r = –0.733, P = 4.06 × 10−06).
Figure 7.
(A) Effect of animate environmental enrichment on preference (% of time) for the social side of the tank during the baseline and experimental weeks. The lines connecting data points indicate the change in side preference between weeks for individual fish. The box-and-whisker plot for each group for each week represents 5 values: median with interquartile range, minimum, and maximum. The time spent on the social side of the tank differed significantly (P < 0.005) when neighbors were present. Significance was determined by using the paired Wilcoxon signed rank test to compare tank location preference between weeks 1 and 2. (B) Effect of singly housed or group-housed neighbors on preference (% of time) for the social side of the tank during the baseline and experimental weeks. The lines connecting data points indicate the change in side preference between weeks for individual fish. Fish showed a significant (P < 0.05) preference for the social side of the tank when singly housed neighbors were present. Significance was determined by using the paired Wilcoxon signed-rank test to compare tank location preference in week 1 with that in week 2.
Figure 8.
Effect of baseline (week 1) side preference on social side preference during week 2. Each fish was ranked (0 to 30) from least to greatest preference for the assigned social side of the tank during week 1, prior to exposure to neighbor tanks. A higher rank indicates greater side preference. Fold change in preference for the social side during the experimental week (week 2) relative to the baseline week (week 1) was calculated by dividing the value for week 2 by that for week 1. Spearman correlation between baseline rank and fold change demonstrated an inverse correlation (Spearman r = –0.733, P = 4.06 × 10−06).
To evaluate whether housing next to neighbors, in the absence of physical contact, represented a stressor for the fish rather than an enrichment, their behavior was evaluated for indicators of aggression and stress. Zebrafish did not display aggressive behaviors (Figure 4) during the baseline week or when neighbors, either singly or group-housed, were present.
Discussion
Zebrafish may require individual housing for research purposes, yet information regarding enrichment strategies for these fish is sparse. The goal of our current study was to evaluate enrichment preferences of mature singly housed female zebrafish. Previous work established that pair- or group-housed zebrafish prefer or demonstrate positively altered behavioral and physiologic states in enriched compared with barren environments.4,8,9,13,15 We compared 10 enrichment strategies with the no-enrichment condition to determine which were preferred housing conditions. The enrichment items evaluated were intended to add complexity to the tank environment and elicit species-specific behaviors.
When we evaluated the data regarding inanimate enrichment items, a particularly interesting finding was the preference for the front half of the tank during the baseline weeks (when the fish were without enrichment) and during the first time point of each recording (when enrichment was present and animal caretakers entered the room to turn on the cameras). One possible explanation for this preference is that all zebrafish were routinely fed in the front of the tank. Because we placed the enrichment in the back half of the tank, a shift in location preference was a clear signal that fish were outranked their preference for food to be near the enrichment item. This finding also indicates that zebrafish can visually assess the environment outside of the tank.
Given that zebrafish are a shoaling species5 and that previous literature4 has established their preference for cohousing with conspecifics, we hypothesized that enrichment recapitulating social interaction would be a preferred housing condition. The mirrored paper was found to significantly shift their tank location preference. Because zebrafish have not been documented to display visual self-recognition, their moving reflection on mirrored paper likely is recognized as another fish. The importance of simulated movement is suggested, because the static image of zebrafish on the side of the tank, scaled to size, did not elicit a similar response. Behaviors displayed when the fish was near the mirrored paper were obscured by the divider and thus unable to be assessed.
Although our study did not find significant preferences regarding the majority of the inanimate enrichment items, we did observe the zebrafish interacting with 3 of the items— marbles, tulle, and PVC pipe—in addition to mirrored paper. Chosen for practicality within the laboratory, these items were also able to elicit species-typical behaviors, such as foraging for food and swimming among vegetation.
Our most robust preference finding was in regard to neighbor fish. The findings from mirrored paper demonstrated that a visual image of other fish had a greater effect than other forms of inanimate enrichment. To further investigate this effect, zebrafish were exposed to either other singly housed fish or groups of fish in a neighboring tank, as a form of visual enrichment. Zebrafish housed next to a tank containing other zebrafish preferred to be on the side of the tank closer to the neighbor fish; this preference was statistically significant when the neighbor tank contained singly housed fish. A possible explanation for this difference may be that having a neighbor when both fish are singly housed promotes a stronger drive and has a larger effect than when the neighbor tank is group-housed. This result may also have been influenced by the previous social housing experience of the fish. We were concerned that housing next to neighbor fish that could be visually observed and sensed by olfactory cues but that could not be contacted physically might be stressful rather than beneficial and thus lead to behavioral indicators of aggression and distress. However, behavioral analysis revealed no abnormal behaviors in our singly housed fish. Perhaps these behaviors were displayed during the 3-d acclimation period that took place prior to video recording or the behaviors were not displayed at a sufficiently high enough to allow detection during the evaluated video clips. This aspect may require further exploration, with the possible development of a modified ethogram that addresses the particular zebrafish in our study. We noted several aggressive interactions, albeit within normal limits, between fish in the group-housed neighbor tank. On the basis of the information that we have gathered, housing single fish next to other singly housed fish should be considered as an enrichment strategy, which may improve animal welfare for zebrafish during periods of single housing.
Our study had several limitations. All zebrafish used in the study had previous exposure to conspecifics, thus potentially influencing the amount of time they spent near enrichment imitating social housing. A previous study found that the common groupings of zebrafish (pair, group, or single) in research altered behavioral preferences.4 Furthermore, given that zebrafish are scototaxic,10 the use of light-colored enrichment items such as white PVC pipes may have deterred their use. In addition, when exposed to a novel environment, such as one enhanced by enrichment, zebrafish tend to explore vertically;4 consequently, enrichment strategies that included items placed in the tank or on the tank bottom, such as plants and marbles, may have decreased their use, although these strategies did allow for foraging behavior. Items with a darker color or within the upper half of the tank may provide different results and should be considered for future studies. Another study4 tested enrichment preferences in a novel tank, which alone can alter behavior. In an attempt to eliminate this confounder, we determined in-tank location preference within the individual fish's home tank. In the spirit of the 3Rs and to reduce animal numbers, we partnered with a fish laboratory on campus to use fish that were already part of their program; consequently, options regarding animal sex and number were limited. Differences due to sex, age, and strain in conjunction with larger sample sizes should be a topic in future studies.
In conclusion, our data support our hypothesis that the position of zebrafish in their tank would be altered by various enrichment strategies when compared with a barren tank and that the effect would be greater when enrichment imitated social contact. Thus, as enrichment strategies, we recommend the use of mirrored paper on a side of the tank or housing single zebrafish next to tanks containing other fish. Our recommendations are consistent with previous work that indicated a preference to be in an enriched environment, particularly when the enrichment was social housing. In addition, enrichment strategies that increase tank complexity and allow zebrafish to express natural behaviors such as foraging for food should be considered when neighbors or mirrored paper are unavailable. These alternative items include marbles, PVC pipes, and tulle.
Acknowledgments
We thank the Goldman Lab for their animals and technical support, Amy Puffenberger for her contribution of photographs, and the animal care technicians of the University of Michigan, Unit for Laboratory Animal Medicine. This research was supported by the University of Michigan Office of Research's Quality Improvement Funds.
References
- 1.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. [Google Scholar]
- 2.Brown C. 2015. Fish intelligence, sentience and ethics. Anim Cogn 18:1–17. 10.1007/s10071-014-0761-0. [DOI] [PubMed] [Google Scholar]
- 3.Cnaan A, Laird NM, Slasor P. 1997. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med 16:2349–2380. . [DOI] [PubMed] [Google Scholar]
- 4.Collymore C, Tolwani RJ, Rasmussen S. 2015. The behavioral effects of single housing and environmental enrichment on adult zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 54:280–285. [PMC free article] [PubMed] [Google Scholar]
- 5.Harper C, Lawrence C. 2011. The laboratory zebrafish. Boca Raton (FL): CRC Press. [Google Scholar]
- 6.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 7.Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS, Craddock C, Kyzar EJ, Roth A, Landsman S, Gaikwad S, Robinson K, Baatrup E, Tierney K, Shamchuk A, Norton W, Miller N, Nicolson T, Braubach O, Gilman CP, Pittman J, Rosemberg DB, Gerlai R, Echevarria D, Lamb E, Neuhauss SC, Weng W, Bally-Cuif L, Schneider H; Zebrafish Neuroscience Research Consortium. 2013. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10:70–86. 10.1089/zeb.2012.0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keck VA, Edgerton DS, Hajizadeh S, Swift LL, Dupont WD, Lawrence C, Boyd KL. 2015. Effects of habitat complexity on pair-housed zebrafish. J Am Assoc Lab Anim Sci 54:378–383. [PMC free article] [PubMed] [Google Scholar]
- 9.Manuel R, Gorissen M, Stokkermans M, Zethof J, Ebbesson LO, van de Vis H, van den Bos R. 2015. The effects of environmental enrichment and age-related differences on inhibitory avoidance in zebrafish (Danio rerio Hamilton). Zebrafish 12:152–165. 10.1089/zeb.2014.1045. [DOI] [PubMed] [Google Scholar]
- 10.Maximino C, Marques de Brito T, Dias CA, Gouveia A, Jr, Morato S. 2010. Scototaxis as anxiety-like behavior in fish. Nat Protoc 5:209–216. 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- 11.Oliver VL, Athavale S, Simon KE, Kendall LV, Nemzek JA, Lofgren JL. 2017. Evaluation of pain assessment techniques and analgesia efficacy in a female guinea pig (Cavia porcellus) model of surgical pain. J Am Assoc Lab Anim Sci 56:425–435. [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira RF, Silva JF, Simoes JM. 2011. Fighting zebrafish: characterization of aggressive behavior and winner-loser effects. Zebrafish 8:73–81. 10.1089/zeb.2011.0690. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder P, Jones S, Young IS, Sneddon LU. 2014. What do zebrafish want? Impact of social grouping, dominance and gender on preference for enrichment. Lab Anim 48:328–337. 10.1177/0023677214538239. [DOI] [PubMed] [Google Scholar]
- 14.Teles MC, Oliveira RF. 2016. Quantifying aggressive behavior in zebrafish. Methods Mol Biol 1451:293–305. 10.1007/978-1-4939-3771-4_20. [DOI] [PubMed] [Google Scholar]
- 15.Wafer LN, Jensen VB, Whitney JC, Gomez TH, Flores R, Goodwin BS. 2016. Effects of Environmental enrichment on the fertility and fecundity of zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 55:291–294. [PMC free article] [PubMed] [Google Scholar]