Abstract
Background
Follow‐up examinations are commonly performed after primary treatment for women with breast cancer. They are used to detect recurrences at an early (asymptomatic) stage. This is an update of a Cochrane review first published in 2000.
Objectives
To assess the effectiveness of different policies of follow‐up for distant metastases on mortality, morbidity and quality of life in women treated for stage I, II or III breast cancer.
Search methods
For this 2014 review update, we searched the Cochrane Breast Cancer Group's Specialised Register (4 July 2014), MEDLINE (4 July 2014), Embase (4 July 2014), CENTRAL (2014, Issue 3), the World Health Organization (WHO) International Clinical Trials Registry Platform (4 July 2014) and ClinicalTrials.gov (4 July 2014). References from retrieved articles were also checked.
Selection criteria
All randomised controlled trials (RCTs) assessing the effectiveness of different policies of follow‐up after primary treatment were reviewed for inclusion.
Data collection and analysis
Two review authors independently assessed trials for eligibility for inclusion in the review and risk of bias. Data were pooled in an individual patient data meta‐analysis for the two RCTs testing the effectiveness of different follow‐up schemes. Subgroup analyses were conducted by age, tumour size and lymph node status.
Main results
Since 2000, one new trial has been published; the updated review now includes five RCTs involving 4023 women with breast cancer (clinical stage I, II or III).
Two trials involving 2563 women compared follow‐up based on clinical visits and mammography with a more intensive scheme including radiological and laboratory tests. After pooling the data, no significant differences in overall survival (hazard ratio (HR) 0.98, 95% confidence interval (CI) 0.84 to 1.15, two studies, 2563 participants, high‐quality evidence), or disease‐free survival (HR 0.84, 95% CI 0.71 to 1.00, two studies, 2563 participants, low‐quality evidence) emerged. No differences in overall survival and disease‐free survival emerged in subgroup analyses according to patient age, tumour size and lymph node status before primary treatment. In 1999, 10‐year follow‐up data became available for one trial of these trials, and no significant differences in overall survival were found. No difference was noted in quality of life measures (one study, 639 participants, high‐quality evidence).
The new included trial, together with a previously included trial involving 1264 women compared follow‐up performed by a hospital‐based specialist versus follow‐up performed by general practitioners. No significant differences were noted in overall survival (HR 1.07, 95% CI 0.64 to 1.78, one study, 968 participants, moderate‐quality evidence), time to detection of recurrence (HR 1.06, 95% CI 0.76 to 1.47, two studies, 1264 participants, moderate‐quality evidence), and quality of life (one study, 356 participants, high‐quality evidence). Patient satisfaction was greater among patients treated by general practitioners. One RCT involving 196 women compared regularly scheduled follow‐up visits versus less frequent visits restricted to the time of mammography. No significant differences emerged in interim use of telephone and frequency of general practitioners's consultations.
Authors' conclusions
This updated review of RCTs conducted almost 20 years ago suggests that follow‐up programs based on regular physical examinations and yearly mammography alone are as effective as more intensive approaches based on regular performance of laboratory and instrumental tests in terms of timeliness of recurrence detection, overall survival and quality of life.
In two RCTs, follow‐up care performed by trained and not trained general practitioners working in an organised practice setting had comparable effectiveness to that delivered by hospital‐based specialists in terms of overall survival, recurrence detection, and quality of life.
Plain language summary
Different follow‐up strategies for women after breast cancer treatment
Review question: Whether an intensive follow‐up decreases the number of recurrences or deaths and affects health‐related quality of life (HRQoL) compared with a less intensive follow‐up and whether a follow‐up offered by specialists is different from that performed by family physicians.
Background: Follow‐up after breast cancer is performed in order to check whether breast cancer has returned to the breast or other part of the body and to monitor side effects related to treatment. Follow‐up may be performed by specialists or family physicians, regularly or on demand, and may be based on routine clinical visits (physical examinations and yearly mammography), or on a more intensive surveillance (laboratory tests and imaging examinations). The first update of this Cochrane review published in 2004 has shown that having more tests does not improve the length or quality of life in breast cancer survivors and a comparable effectiveness of follow up by specialist to that by primary physician. Moreover, additional screening tests could increase anxiety related to false positive results, unnecessary radiation exposure and health‐related costs.
Study Characteristics: A literature search up to July 2014 found five trials (involving 4023 women with a median follow‐up variable from 16 to 120 months). Since the previous version of this Cochrane review in 2004, one new study has been published.
Key results: This review of trials found that follow‐up programs based on a regular physical examination and a yearly mammogram appear to be as effective as the more intensive approaches and to have similar impact on HRQoL. No significant differences were found between follow‐up performed by specialists or family physicians, regularly or on demand. These results should be interpreted with caution bearing in mind that these studies were conducted almost two decades ago; additional trials incorporating new biological knowledge and improved imaging technologies are needed.
Quality of the evidence: Allocation concealment was adequate in all but one trial; two trials were judged to be at low risk of selection bias; the blinding of the outcome assessor was not described in two trials. For one trial it was not possible to judge risk of bias because it reported no methodological information.
Summary of findings
Background
Description of the condition
Breast cancer is the second most common cancer in the world and the most frequent cancer among women with an estimated 1.67 million new cancer cases diagnosed in 2012 (25% of all cancers) (Globocan 2012). In Europe, the estimated age‐adjusted annual incidence of breast cancer was 94.2/100,000 and the mortality was 23.1/100,000 in 2012 (Ferlay 2013). In the USA, according to the SEER database (2006 to 2010), the age‐adjusted annual incidence of breast cancer was 124.6/100,000 and the mortality was 22.2/100,000. The median age at breast cancer diagnosis is 61 years. About 10% of breast cancers occur among women aged younger than 45 years, while 40% occur among women aged 65 years or older (SEER database 2014). Overall, 60% of breast cancers are diagnosed at a localised stage, 32% at a regional stage and 5% at an advanced stage. The five‐year relative survival rate for women diagnosed with localised breast cancer is 98.5%; survival declines to 84.6% for regional stage and 25% for distant stage. Due to both early detection through screening programs and the improvement in the available treatment strategies, the percentage of women surviving at least five years after diagnosis and treatment has shifted from 74.8% in the early 1970s to 90.3% in the late 1990s. In the last few years, the increase in the number of women diagnosed with breast cancer each year and the improvement in the survival rates, have led to a significant increase in the number of breast cancer survivors (SEER database 2014).
Description of the intervention
Follow‐up (care after primary treatment) of women with breast cancer includes terms such as "routine testing", "follow‐up" or "surveillance". These indicate the regular use of laboratory or instrumental tests in otherwise asymptomatic patients to detect distant metastases (that is, when the cancer has spread beyond the breast) earlier. The type of tests can vary by hospital or doctor or both and they typically include routine haematological tests, all biochemical first level test examinations, for example, liver function tests, kidney function test, C‐reactive protein, erythrocyte sedimentation rate, tumour markers, chest X‐rays, and bone and liver scans.
How the intervention might work
Follow‐up care should have several aims. These include the provision of physical and psychosocial rehabilitation, monitoring of treatment effectiveness including short‐ and long‐term toxicity, and detecting recurrence or new cancers.
Post‐treatment survivors are at risk for physical and psychosocial sequelae. Women with breast cancer may experience early and late treatment‐related side effects such as fatigue, arthralgia (joint pain), cardiotoxicity, reproductive system changes, osteoporosis and the development of second tumours. Other effects may include cognitive dysfunction and psychosocial issues such as anxiety and depression, disturbances in body image, and changes in sexual quality of life (Burstein 2000). So, the post‐treatment follow‐up is essential to ensure detection and management of late or long‐term effects and to provide psychological and supportive care.
In actual practice, however, follow‐up care is offered with the main objective of detecting local or distant recurrences earlier, so that treatment for any relapse can be started. Conceptually, follow‐up care can be considered as a screening program ‐ i.e. screening for early detection of metastases. As such, it is quite difficult to evaluate its efficacy retrospectively, because survival of asymptomatic patients who have relapses detected by these screening tests can only be compared with survival of symptomatic patients who have relapses. This kind of comparison can be severely biased by lead time (early detection simply increases the period during which a metastasis is observed), and length time (cases with a long pre‐clinical phase and, therefore, presumably less aggressive relapses are more likely to be detected by a screening program) (Duffy 2008). A randomised design is thus the only valid way to get an unconfounded estimate of the effectiveness of different follow‐up strategies. Despite the lack of convincing proof that this postoperative surveillance care improves outcomes in these patients, intensive follow‐up is quite common in clinical practice and represents a significant workload for radiotherapy, surgical and oncologic departments (Grunfeld 2010; Loprinzi 1994).
Why it is important to do this review
In the previous update of this review in 2004 of randomised clinical trials (RCTs) evaluating the different strategies of follow‐up for stage I, II or III breast cancer patients, we concluded that, according to the RCTs available at that time, a follow‐up based on routine clinical visits (physical examinations and yearly mammography) had the same effectiveness as a more intensive surveillance (laboratory tests and imaging examinations) in terms of overall survival and quality of life. Furthermore, a centralised approach (follow‐up performed by a specialist at a multidisciplinary breast clinic) showed the same effectiveness as the decentralised approach (surveillance performed by a general practitioner); finally, no differences were found among a regularly scheduled surveillance and a follow‐up strategy “on demand”.
Despite all the studies included in the systematic review concluding that new studies would have been necessary on this topic, only one new study has been published so far; moreover, to our knowledge, while cancer research is actively pushed in the field of anticancer treatments and drug development, no clinical trials evaluating the efficacy of the different follow‐up strategies are ongoing in ClinicalTrials.gov (Clinical trial 2014) or the WHO ICTRP search portal (WHO 2014).
Updating this systematic review about follow‐up strategies for women with breast cancer would allow us to understand better the state of the art on this clinically relevant topic, which could be the basis to focus future research in this field. In fact, due to the improvements in the understanding of the biology of breast cancer, the availability of advanced imaging technologies and the progress made in the treatment of breast cancer, new more personalised follow‐up approaches should be studied. In particular, positron emission tomography (PET)‐computed tomography (CT) showed to be more sensitive and specific in detecting distant metastatic lesion as compared to conventional imaging (Constantinidou 2011). Furthermore, new biomarkers such as circulating tumour cells are promising markers to detect microscopic residual disease after primary treatment (Lucci 2012). These new diagnostic tools should be studied for their ability to anticipate the clinical evidence of recurrence, and the subsequent impact on survival.
Breast cancer is no longer considered a single disease, but is classified into at least four different intrinsic molecular subtypes based on the immunohistochemical classification (Luminal A‐like, Luminal B‐like, HER2 positive, and triple negative (Goldhirsch 2013)). Nowadays, it is well‐established that not only the stage at diagnosis, but also the biology of the disease influence the risk of breast cancer recurrence and death (Jatoi 2011). Moreover, a growing amount of evidence has shown that the different biological subtypes differ in terms of timing of disease recurrence (Jatoi 2011; Metzger‐Filho 2013) and pattern of metastatic spread (particularly, site of first recurrence) (Kennecke 2010). These observation could lead to the hypothesis that different schedules and different intensity of follow‐up strategies should vary according to the different breast cancer subtypes.
Moreover, thanks to the improvement of breast cancer management and the availability of new effective treatments, metastatic breast cancer, at least for a small percentage of women, should not be considered a fatal condition anymore (Hortobagyi 2002): particularly, women with oligometastatic disease treated with a multidisciplinary aggressive approach can still be cured (Kobayashi 2012). Based on this assumption, an early detection of metastatic disease could lead to the identification of patients with low burden of disease who can be still treated with curative intent.
Taking into account the improvement in the understanding of the biology of breast cancer, in the available imaging technologies and in the treatment of the disease, a better assessment of the state of the art about the follow‐up strategies should be considered the basis for the design of new clinical trials in this setting towards a more “personalised follow‐up approach”.
Objectives
To assess the effectiveness of different policies of routine follow‐up testing on morbidity, survival and quality of life in women with breast cancer after primary treatment. Specifically, the effectiveness of the following types of routine follow‐up policies were explored.
Follow‐up based on routine clinical visits plus yearly mammogram compared to a more intensive surveillance where radiological and laboratory tests are regularly added to routine visits.
Centralised compared to decentralised follow‐up (i.e. surveillance offered by a specialist at a multidisciplinary breast clinic compared to that delivered by a general practitioner).
Regular follow‐up compared to surveillance on demand.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) comparing different approaches to follow‐up after completion of primary treatment.
We extracted and reviewed additional information from prospective non‐randomised studies but the data were not used for quantitative pooling.
Types of participants
Women who have had primary surgical treatment for breast cancer (clinical stage I, II or III), with no evidence of recurrence.
Types of interventions
Follow‐up based on routine clinical visits plus yearly mammogram compared to a more intensive surveillance including radiological and laboratory tests.
Centralised versus decentralised follow‐up (i.e. surveillance offered by a specialist at a multidisciplinary breast clinic compared to that delivered by a general practitioner).
Regular follow‐up compared to surveillance on demand.
Types of outcome measures
Primary outcomes
Overall survival, as estimated from the date of randomisation to the date of last contact or death from any cause.
Health‐related quality of life (HRQoL), assessed using EORTC QLQ‐C30, SF‐36 and the "hospital anxiety and depression scale (HADS)"
Secondary outcomes
Disease‐free survival (expression of the time to detect a recurrence). It is used in this context to compare the power of different follow‐up strategies to detect recurrence earlier, possibly in an asymptomatic stage.
Occurrence of metastases detected in an asymptomatic status.
Search methods for identification of studies
Electronic searches
(a) The Cochrane Breast Cancer Group Specialised Register was searched on the 4 July 2014 (details of search strategies used by the group for the identification of studies and the procedure used to code references are outlined in the group's module at http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). Studies including the text words 'early breast cancer', 'locally advanced breast cancer', 'follow up', 'follow‐up studies', 'follow‐up care', 'centralised follow‐up', 'decentralised follow‐up', postoperative surveillance' and 'routine clinical visits' on the Specialised Register were retrieved for consideration. (b) MEDLINE (via OvidSP) from 1950 until 4 July 2014. Appendix 1. (c) Embase (via Embase.com) from 1966 until 4 July 2014. Appendix 2. (d) CENTRAL, 2014, Issue 3. See Appendix 3. (e) The WHO International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials on the 4 July 2014. See Appendix 4. (f) ClinicalTrials.gov ( http://clinicaltrials.gov/ct2/home ) was searched until 4 July 2014. See Appendix 5.
Searching other resources
No other sources were searched.
Data collection and analysis
Selection of studies
For this 2014 review update and the previous update two review authors (2014 update: Ivan Moschetti (IM), Michela Cinquini (MC); 2004 update: IM, Laura Coe (LC)) independently inspected the search hits by reading the titles and abstracts. The full‐text article of each potentially relevant study identified in the search was obtained and assessed for inclusion independently by the same two authors. No disagreements regarding eligibility occurred. Both review authors read and assessed 15 PDF documents. The other papers retrieved by the search strategy were not considered because they were not RCTs, or they clearly referred to follow‐up managed by nurses. For this reason a "Characteristics of excluded studies" table was not completed.
Data extraction and management
Two review authors independently extracted data (2014 update: IM, MC; 2004 update: IM, LC) using a standardised data collection form.
We extracted the following characteristics from each included study: participants, setting, interventions, comparators and outcomes, see "Characteristics of included studies" table. When a meta‐analysis was performed (i.e. for the GIVIO and Rosselli Del Turco trials), we used individual patient data. We did not contact any trial authors for the 2004 and 2014 updates.
For studies with more than one publication, we extracted data from all publications. However, we considered the final or updated version of each trial as the primary reference.
Assessment of risk of bias in included studies
Two review authors (2014 review update: IM, MC) independently assessed the risk of bias in each trial using Cochrane's 'Risk of bias' tool. Any differences were resolved in a consensus meeting.
We classified the generation of allocation sequence, allocation concealment, blinding of outcome assessor, completeness of outcome data, and selective outcome reporting as low risk of bias, high risk of bias, or ’unclear’ following the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011).
Given the nature of the interventions under investigation, only blinding of outcome assessor (avoidance of performance bias and detection bias) was considered for subjective outcomes (e.g. quality of life, disease‐free survival and occurrence of metastases).
No sensitivity analyses depending on 'Risk of bias' criteria were performed.
Measures of treatment effect
For each trial, the overall and disease‐free survival hazard ratio (HR) was calculated using the log rank "O‐E" and its variance (V). When the HR and the relative confidence interval (CI) were not reported in the article to permit a direct calculation of this two measures, we calculated them indirectly using the Mantel‐Haenszel estimate of the HR and its CI reported in the paper, or we used the total number of events and the number of patients at risk in each arm. The individual HR estimates were combined into an overall HR using the Peto odds ratio method that allows for fixed‐effect only.
We planned to report the occurrence of metastases detected in an asymptomatic state as risk ratio (RR) and its 95% CI.
Quality of life was considered as a continuous outcome and we planned to report the results as the mean difference (MD) and its standard error. However, none of the trials had information on health‐related quality of life (HRQoL) that could be extracted and meta‐analysed. These data have been described in a narrative way. For quality of life, we considered the "overall health perception", the "satisfaction of patients" and the "hospital anxiety and depression scale (HADS)".
Unit of analysis issues
The unit of analysis was the study participant.
Dealing with missing data
For this 2014 review update, we did not contact the authors of primary studies. All analyses were performed on an intention‐to‐treat basis.
Assessment of heterogeneity
A formal statistical test for heterogeneity was done using the Chi2 test and I2 statistic. A P value of 0.10 for heterogeneity was considered as statistically significant. We planned to use a fixed‐effect model or a random‐effects model, depending on the evidence of statistical heterogeneity.
Assessment of reporting biases
Publication bias was not evaluated given the type of intervention considered. We are confident that no relevant data on this type of intervention have been kept concealed.
Data synthesis
For time‐to‐event outcomes, we used a fixed‐effect analysis (O‐E and Variance). Because a certain degree of heterogeneity was not expected among trials for dichotomous outcomes, we used a fixed‐effect analysis (Mantel‐Haenszel). For continuous outcomes, we planned to use a fixed‐effect analysis (inverse‐variance method). RevMan 5.3 software was used for all the analyses.
The overall quality of the evidence for all the outcome was judged using the GRADE system (GRADE 2004), which takes into account the 'Risk of bias' assessment, imprecision and directness of results. The 'Summary of findings' tables present the main findings of the review.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses by age, tumour size and lymph node status before primary treatment were conducted based on the hypothesis that these may influence the biological behaviour of the disease and therefore lead to different benefits for the different follow‐up strategies (De Lena 1995; Greco 1998).
Sensitivity analysis
A sensitivity analysis was not carried out.
Results
Description of studies
Results of the search
In this 2014 review update, searching the Specialised Register, MEDLINE, Embase and CENTRAL yielded 1493 records while ClinicalTrials.gov and WHO ICTRP retrieved 669 ongoing trials. Two review authors (IM, MC) independently screened all records excluding 2102 of them by title and abstracts. For the remaining 15 records, after reading the full text, only one answered the review's question. No disagreement rose between review authors during the process of study selection.
This update includes information from one new report and provides the results of a study conducted in Ontario from 1997 to 2003 (Grunfeld 2006) comparing a follow‐up structured in the cancer centre according to usual practice and a follow‐up managed by family physicians (see Figure 1).
1.
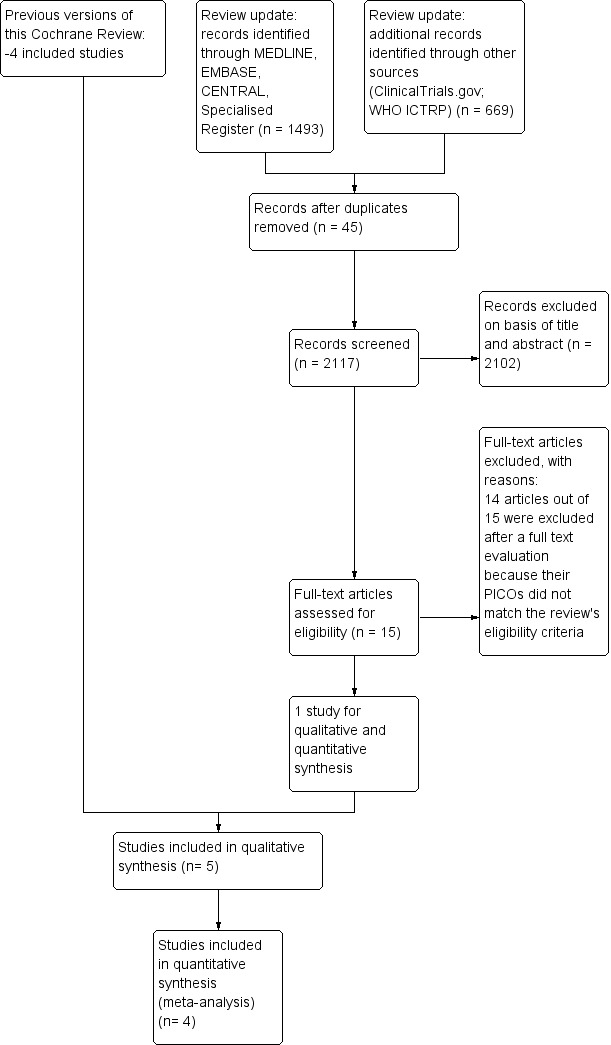
Study flow diagram.
Included studies
Five studies met the inclusion criteria. All of the studies were multicentre randomised controlled trials (RCTs) comparing different types of follow‐up in women with breast cancer. Overall, these studies included 4023 women (the number of patients ranged from 196 to 1320) with breast cancer (clinical stages I, II or III) with no evidence of recurrence after their primary surgical treatment. The median follow‐up time available in the five trials varied from 16 to 120 months.
Data on overall and disease‐free survival were available for four trials (GIVIO; Grunfeld 1996; Grunfeld 2006; Rosselli Del Turco 1994). None of the trials had information on health‐related quality of life (HRQoL) that could be extracted and meta‐analysed. These data have been described in a narrative way. Only one trial (GIVIO) reported information about metastases.
The trials included in this review explored three different follow‐up strategies.
Two trials (GIVIO and Rosselli Del Turco 1994) compared follow‐up based on clinical visits and mammography alone, with a more intensive surveillance scheme including radiological and laboratory tests. Combined, they included 2563 women. Their outcomes were overall survival, disease‐free survival and, in one trial (GIVIO), HRQoL.
Two trials (Grunfeld 1996 and Grunfeld 2006) compared follow‐up offered by a specialist at the hospital with follow‐up offered by a general practitioner. The first trial included 296 women. Its outcomes were time to detection of recurrence and HRQoL. The second trial enrolled 968 women and evaluated overall survival, disease‐free survival and quality of life.
One trial (Gulliford 1997) compared conventionally scheduled follow‐up with follow‐up limited to the time of mammography, but with telephone and GP consultation available on demand. It included 196 women and was a pilot study to evaluate feasibility of women's acceptance of symptom‐driven follow‐up. Outcomes included acceptability of less frequent follow‐up, use of telephone and GP consultations and satisfaction with allocation to a particular follow‐up strategy.
Excluded studies
There are no excluded studies.
Risk of bias in included studies
An iconographic summary of the risk of bias is illustrated in Figure 2. The Gulliford 1997 trial was judged as having an unclear risk of bias on all domains because it reported no methodological information.
2.
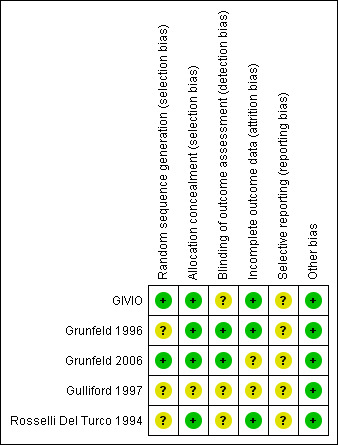
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
In the Gulliford 1997, Grunfeld 1996 and Rosselli Del Turco 1994 trials the method for generating the random sequence was not reported; in Grunfeld 1996, the trial authors stated only that "random allocation was in blocks of eight". GIVIO and Grunfeld 2006 had an adequate random sequence generation using a computer‐generated list.
Allocation concealment
Allocation concealment was adequate in all trials, except for Gulliford 1997.
Blinding
Double‐blinding was not considered as adequate for this kind of study, so only the blinding of the outcome assessor was judged.
Blinding of outcome assessors
In the GIVIO, Gulliford 1997 and Rosselli Del Turco 1994 trials, it was not described whether or not the outcome assessor was blinded. This can lead to a potential detection bias for disease‐free survival. In Grunfeld 1996, blinding of the outcome assessors was judged as adequate as they assessed recurrence independently even though the trial authors stated that "in some cases, the allocation group could have been deduced from the course of clinical events". A blinded committee adjudicated all events in Grunfeld 2006.
Incomplete outcome data
The GIVIO study lost 8% of its randomised patients, who could not be traced and were not included in the analyses. The overall loss to follow‐up in Rosselli Del Turco 1994 was 0.8%. In both trials (GIVIO and Rosselli Del Turco 1994), approximately 10% of the patients discontinued follow‐up care, with a similar distribution between intensive and clinical groups. Survival data were available for those lost to follow‐up and were included in the analyses (intention‐to‐treat analysis). In Grunfeld 1996, only one patient per group was lost to follow‐up. Grunfeld 2006 did not report any information.
Selective reporting
Grunfeld 1996 and Gulliford 1997 were not designed to assess overall survival. Their outcomes (quality of life, time to diagnosis of recurrence, interim use of telephone and GP consultations and patient satisfaction) were assessed to investigate differences within the first two years. For additional details see Characteristics of included studies.
As all the studies have been conducted before protocol registration was mandatory, we did not search for additional information.
Other potential sources of bias
No other sources of bias identified.
Effects of interventions
Summary of findings for the main comparison. Summary of findings: Non‐intensive versus intensive follow‐up for women treated for early breast cancer.
| Non‐intensive versus intensive follow‐up for women treated for early breast cancer | ||||||
| Patient or population: women treated for early breast cancer Settings: outpatients Intervention: non‐intensive follow‐up Comparison: intensive follow‐up | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Intensive | Non‐intensive | |||||
|
Overall survival Follow‐up: median 71 months |
Study population |
HR 0.98 (0.84 to 1.15) |
2563 (2 studies) | ⊕⊕⊕⊕ HIGH1 | ||
| 26 per 100 | 26 per 100 (23 to 30) | |||||
|
Disease‐free survival Follow‐up: median 71 months |
Study population |
HR 0.84 (0.71 to 1.00) |
2563 (2 studies) | ⊕⊕⊝⊝ LOW2,3 | ||
| 23 per 100 | 19 per 100 (17 to 23) | |||||
| Quality of life4 | See comment | See comment | Not estimable4 | 639 1 study | ⊕⊕⊕⊕ HIGH5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Heterogeneity was considered low (I2 = 28%) and we did not downgrade. 2Heterogeneity was considerable (I2 = 52%) and we decided to downgrade once for inconsistency of results. 3We downgraded once due to possible detection bias in both trials ‐ it was not described whether the outcome assessor was blinded. In Rosselli Del Turco 1994, the random sequence generation was not described but we did not downgrade for this because taking into account the period in which RCT was done, it was possible that the authors simply did not report the details of how the random sequence was generated. 4The study provided nominal information on quality of life and it was not possible to extract useful data. The trial authors concluded that there were no difference in quality of life between the two types of follow‐up. 5We did not downgrade the quality of evidence for this outcome (as suggested by the GRADE approach).
Summary of findings 2. Summary of findings: Decentralised versus centralised follow‐up for women treated for early breast cancer.
| Decentralised versus centralised follow‐up for women treated for early breast cancer | ||||||
| Patient or population: women treated for early breast cancer Settings: outpatients Intervention: decentralised follow‐up Comparison: centralised follow‐up | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Centralised follow‐up | Decentralised follow‐up | |||||
|
Overall survival Follow‐up: median 42 months |
Study population |
HR 1.07 (0.64 to 1.78) |
968 (1 study) | ⊕⊕⊕⊝ MODERATE1,2 | ||
| 6 per 100 | 7 per 100 (4 to 11) | |||||
| Disease‐free survival Follow‐up: median 42 months | Study population |
HR 1.06 (0.76 to 1.47) |
1264 (2 studies) | ⊕⊕⊕⊝ MODERATE1,2,3,4 | ||
| 13 per 100 | 13 per 100 (10 to 18) | |||||
| Quality of life5 | See comment | See comment | Not estimable | 356 (1 study) | ⊕⊕⊕⊕ HIGH | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; HR: Hazard Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The publication did not describe how many women were lost to follow‐up and how the random sequence was generated. We downgraded once for the first reason. 2There was a low event rate so we decided not to downgrade for imprecision. 3We downgraded once for this outcome because follow‐up schedules varied even within a study. 4General practitioners were assured that a "rapid re‐referral" would have been possible if any problem developed. This would only be feasible in a clinical trial setting or well organised health systems. 5Trial authors concluded that there were no difference in quality of life between the two types of follow‐up. As we were unable to judge the GRADE domains for this outcome due to a lack of information, we did not downgrade the quality of evidence for this outcome (as suggested by the GRADE approach).
Follow‐up based on routine clinical visits (experimental group) compared to a more intensive surveillance (i.e. with radiological/laboratory tests) (control group)
Overall survival
The meta‐analysis for overall survival of the GIVIO and Rosselli Del Turco 1994 trials found no significant survival advantage in the intensive surveillance group; hazard ratio (HR) 0.98 (95% confidence interval (CI) 0.84 to 1.15; two studies, 2563 participants; high‐quality evidence; Analysis 1.1). We found no significant differences in overall survival between the strategies in respect to the subgroup analyses by age (Analysis 1.2), tumour size (Analysis 1.3), nodal status (Analysis 1.4), or at five years (Analysis 1.5).
1.1. Analysis.
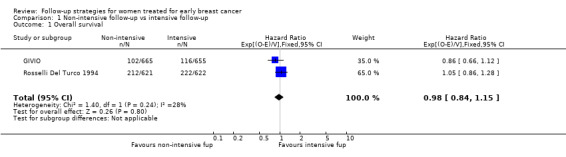
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 1 Overall survival.
1.2. Analysis.
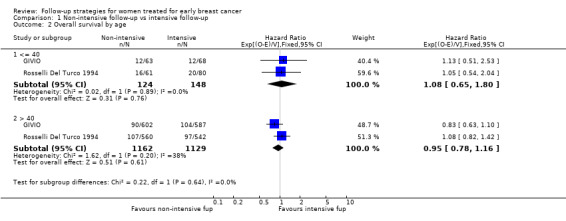
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 2 Overall survival by age.
1.3. Analysis.
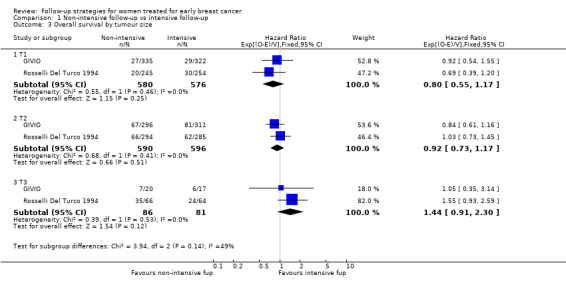
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 3 Overall survival by tumour size.
1.4. Analysis.
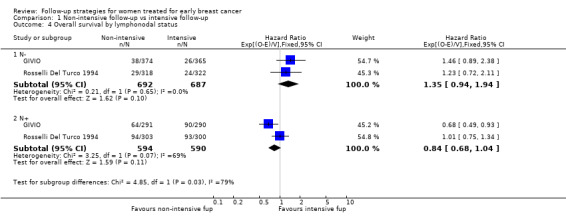
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 4 Overall survival by lymphonodal status.
1.5. Analysis.
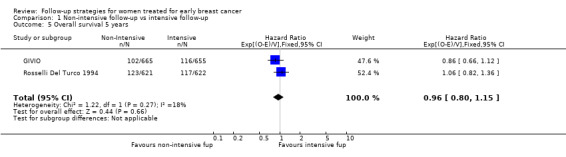
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 5 Overall survival 5 years.
Health‐related quality of life (HRQoL)
Data regarding quality of life were available just for the GIVIO trial. Questionnaires used were the Functional Living Index‐Cancer Scale, the Sickness Impact Profile, the Profile of Mood States, and the Cancer Inventory of Problem Situation. The HRQoL scores were assessed at baseline, six,12, 24, and 60 months, and administered four times between six and 60 months with an average response rate of 73.5%; overall, no significant difference was found between the two follow‐up strategies. The evidence was graded as high quality.
Disease‐free survival
The HR was 0.84 (95% CI 0.71 to 1.00; two studies, 2563 participants; low‐quality evidence; Analysis 1.6) for disease‐free survival after five years of follow‐up. For this outcome, the pooled effect did not confirm the statistically significant effect found in diagnostic anticipation in the Rosselli Del Turco 1994 trial. We did not observe any advantage either in the subgroup analysis for age (Analysis 1.7), or for tumour size (Analysis 1.8), or for lymphonodal status (Analysis 1.9).
1.6. Analysis.
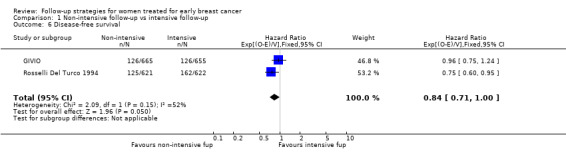
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 6 Disease‐free survival.
1.7. Analysis.
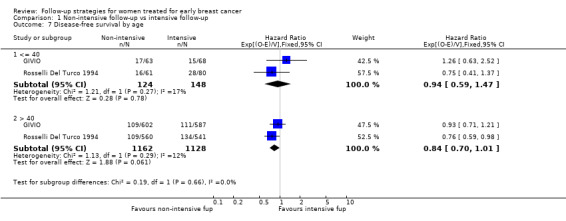
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 7 Disease‐free survival by age.
1.8. Analysis.
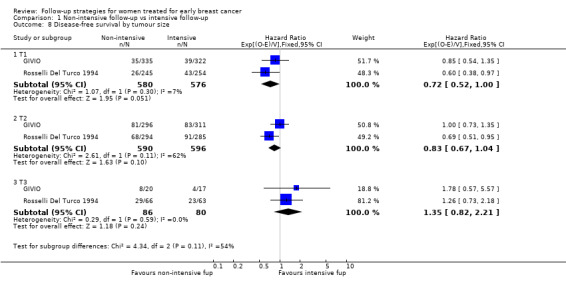
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 8 Disease‐free survival by tumour size.
1.9. Analysis.
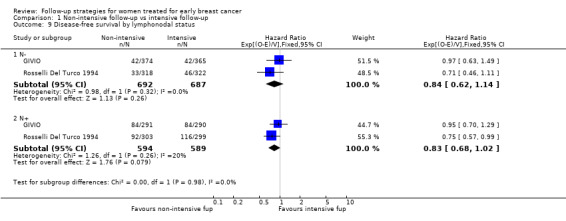
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 9 Disease‐free survival by lymphonodal status.
Occurrence of metastases detected in an asymptomatic status
Data regarding asymptomatic detection of metastases were available only from the GIVIO trial: 31% of cases of metastases in the intensive group and 21% in the clinical group were detected in an asymptomatic phase (Analysis 1.10). This information was not available in other studies where only the proportion of distant metastases has been reported. However, it is consistent with results of several prospective non‐randomised studies (Hannisdal 1993; Hietanen 1986; Logarer 1990; Mahoney 1986; Pandya 1983; Rutgers 1989; Vestergaard 1989; Wickerhan 1986).
1.10. Analysis.
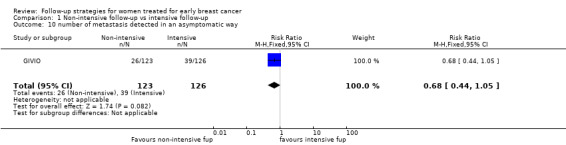
Comparison 1 Non‐intensive follow‐up vs intensive follow‐up, Outcome 10 number of metastasis detected in an asymptomatic way.
Centralised versus decentralised follow‐up (i.e. surveillance offered by a specialist at a multidisciplinary breast clinic compared to that delivered by a general practitioner)
Overall survival
Overall survival was available only for Grunfeld 2006: HR 1.07 (95% CI 0.64 to 1.78; one study, 968 participants; moderate‐quality evidence; Analysis 2.1).
2.1. Analysis.
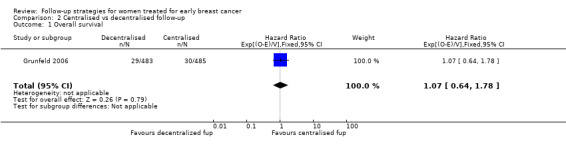
Comparison 2 Centralised vs decentralised follow‐up, Outcome 1 Overall survival.
Health‐related quality of life (HRQoL)
Quality of life shows an expected small deterioration for both groups during the Grunfeld 1996 trial (British version of the SF‐36; European Organisation for Research and Treatment of Cancer core quality of life questionnaire (EORTC QLQ‐C30); Hospital anxiety and depression scale. The HRQL scores were assessed at baseline, mid trial and at the end of the trial). The hospital group had a statistically significant increase in symptom scores for fatigue, dyspnoea and appetite loss. There was no difference in overall health, social and emotional functioning and levels of anxiety and depression.
This study also collected data on the patients who were asked about the trial but did not participate (149/445, 33.5%). These women were older than trial participants and had a lower education level, but there were no important differences in clinical characteristics or in baseline quality of life scores.
The Grunfeld 1996 data were used in a new publication that analysed patient satisfaction with care by general practitioners versus hospital specialists over an 18‐month period (see Grunfeld 1999). Questionnaires completed by 93% of patients indicated that they were more satisfied with service delivery, consultation and the continuity of care provided by their general practitioner than by a specialist.
As stated by the authors in Grunfeld 2006, no statistically significant differences were detected between groups over time for any of the scores (Medical Outcomes Study Short Form 36‐Item General Health Survey (SF‐36) 48 and Hospital Anxiety and Depression Scale (HADS) 49). The HRQL scores were assessed at baseline and during the subsequent seven follow‐ups at six, 12, 18, 24, 36, 48, and 60 months. Overall, SF‐36 PCS declined over time (0.4 units per year; P < 0.001), SF‐36 MCS increased slightly (0.2 units per year; P = 0.08), HADS anxiety declined (0.08 per year; P = 0.01), and HADS depression increased slightly (0.04 per year; P = 0.12). The evidence was graded as high quality.
Disease‐free survival
The two Grunfeld trials (Grunfeld 1996; Grunfeld 2006), comparing follow‐up offered by a hospital‐based specialist with follow‐up offered by a general practitioner, showed no differences in time to recurrence between groups. The HR was 1.06 (95% CI 0.76 to 1.47; two studies, 1264 participants; moderate‐quality evidence; Analysis 2.2).
2.2. Analysis.
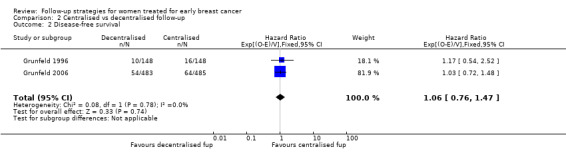
Comparison 2 Centralised vs decentralised follow‐up, Outcome 2 Disease‐free survival.
Occurrence of metastases detected in an asymptomatic status
No information about occurrence of metastases was available in Grunfeld 1996 and Grunfeld 2006.
Regular follow‐up versus surveillance on demand
The Gulliford 1997 trial comparing conventionally scheduled follow‐up and less frequent follow‐up (restricted to the time of mammography) showed that 7% of eligible patients refused to enter the study. The characteristics of these patients may suggest that younger women with more aggressive primary disease are not willing to reduce the frequency of follow‐up visits. Unfortunately, no assessments were available for these patients in relation to their quality of life. No significant differences were found between the groups with regards to the use of telephone and visits to general practitioners during the trial. Approximately one‐third of the patients in both groups expressed a preference for a less frequent schedule of follow‐up visits, but only 56 women answered this question on the questionnaire.
Overall survival
No information reported on this outcome.
Health‐related quality of life (HRQoL)
No information presented on this outcome.
Disease‐free survival
No information presented on this outcome.
Occurrence of metastases detected in an asymptomatic status
No information presented on this outcome.
Discussion
Summary of main results
It is important to remember that in the context of this review the terms "routine testing", "follow‐up" as well as "surveillance" refer to the regular use of laboratory or instrumental tests in otherwise asymptomatic patients. These are done with the aim of earlier detection of distant metastases. For this reason, this review does not explore other, important, aspects of a follow‐up program such as the provision of social and psychological support. Similarly, the review focuses on comprehensive follow‐up packages and does not consider individual components of follow‐up programs such as tumour markers or other diagnostic procedures. We chose to look only at this comparison (i.e. only clinical versus a package of tests) for pragmatic reasons as it would have been impossible to look at all possible contrasts among various types of intensive versus clinical follow‐up.
Concerning the first intervention assessed (follow‐up based on routine testing added to a regular visit and yearly mammogram compared to follow‐up based on visits and mammography alone), the results of this systematic review confirm that doing more tests in asymptomatic patients does not add a survival advantage nor anticipate diagnosis of recurrences. These data have been endorsed by major international practice guidelines (ASCO 2013; ESMO 2013; NCCN 2014): though with some variation in terms of frequency of visits and mammography, all endorse a less intensive clinical follow‐up (Table 3). Also, these last documents are similar to the previous in terms of frequency of visits and mammography and all three suggest a less intensive follow‐up regimen for women treated for early breast cancer.
1. Comparison Guidelines on Selected Breast Cancer Follow‐up Components.
| Guidelines | Mammography | Clinical visit (history and physical exam) | Self‐breast examination | Intensive follow‐up | Others |
| (ASCO 2013) | Yearly. For women who have undergone breast‐conserving surgery, a post‐treatment mammogram should be obtained 1 year after the initial mammogram and at least 6 months after completion of radiation therapy. |
Every 3 to 6 months for the first 3 years, every 6 to 12 months for years 4 and 5, and annually thereafter. | Monthly | Not recommended | Gynecologic follow‐up for all women. |
| (ESMO 2013) | Every 1 to 2 years. | Every 3 to 4 months in the first 2 years, every 6 months from years 3 to 5, and annually thereafter. | Not specified | Not recommended | Annual gynaecological examination for women on tamoxifen; regular bone mineral density evaluation and lipid profile for women on aromatase inhibitors. |
| (NCCN 2014) version 2.2014 |
Yearly. | Every 4 to 6 months for 5 years, and annually thereafter. | Not specified | Not recommended | Annual gynaecological examination for women on tamoxifen; regular bone mineral density evaluation for women on aromatase inhibitors or who experienced premature ovarian failure. |
This review also allowed us to explore an organisational question, as well as the one about the intensity of follow‐up. Despite some limitations in the evidence from Grunfeld 1996, the results suggest that decentralised follow‐up (i.e. surveillance offered by a general practitioner) has the same effect on detection of recurrence as centralised follow‐up. This is the result of special training given to general practitioners and this should be taken into consideration when planning to either transfer this experience or further investigate this topic.
In this updated review we added a new trial (Grunfeld 2006), conducted in Canada including 968 women. This trial was similar to Grunfeld 1996 in that no difference was found between surveillance offered by a general practitioner versus surveillance done by specialist clinic about primary (rate of recurrence‐related serious clinical events) and secondary outcomes (HRQoL). Moreover, this trial was pragmatic because family physicians were provided with only one page of instruction based on published clinical guidelines without any specific training and using the usual procedures when referring patients with recurrence back to the cancer centre (no special procedures were put in place). In this trial, the 45% of approached patients chose not to participate. The authors gave no reason for this finding, but obviously this proportion could be change among different national/regional health systems.
Only one study compared the conventionally scheduled follow‐up with a surveillance on demand to evaluate the feasibility of women’s acceptance of symptom‐driven follow‐up. A reduced frequency of routine follow‐up showed to be popular among women at standard risk, but younger patients with aggressive primary disease seemed to refuse a less intensive approach. However, further data and a multicentre experience are needed to draw more solid conclusions about the effectiveness of a more personalised follow‐up with respect to disease outcomes.
Overall completeness and applicability of evidence
However, despite the evidence and consensus that intensive follow‐up schemes provided no benefit on survival, surveys throughout the late 1990s found this message had not been completely transferred into clinical practice (Tomiak 1998; Harries 1996, Stark 1996) and that women still seemed to prefer a frequent schedule of tests in order to be reassured about their health status. In this update, we searched for new information on current clinician behaviour but did not find any new studies on the topic. It would be worthwhile to evaluate whether a good strategy of sharing information between the doctor and the patient would help women to be equally reassured when a less intensive follow‐up is offered.
Compared to other areas in medicine, it is worth noting that three of the five RCTs in this review did include quality of life as an outcome. However, different quality of life indicators (stress, anxiety and depression) were mostly used to rule out differences and thus it may well be that small differences may have gone undetected. Besides, authors of these trials noted that choosing the best time and frame for the measurement of quality of life is problematic and far from being totally agreed upon.
Results coming from these different studies have been produced in a very specific socio‐cultural and geographical setting. Thus generalisability and direct application of the strategies here recommended should be carefully evaluated.
Finally, we did not find any eligible study that had evaluated the diagnostic value of using mammography as part of a follow‐up strategy to monitor ipsilateral recurrences and new cancers in the contralateral breast. We have only found two prospective studies (Carlotti 1993; Holli 1998) investigating the difficulties in the interpretation of mammograms on an irradiated breast after surgery.
Quality of the evidence
Overall, we are confident that all the included studies in the quantitative analyses have a low risk of selection bias (even though some information on the random sequence generation was missing ‐ see Grunfeld 1996 and Rosselli Del Turco 1994). Intention‐to‐treat analysis was performed in all trials and losses to follow‐up were absent or in a low percentage in all trials. It resulted at low risk for two trials out of five (GIVIO and Rosselli Del Turco 1994). Grunfeld 1996 was judged at low risk of selective reporting even if it did not take into account overall survival.
Potential biases in the review process
This review is based on RCTs that were initiated in the late 1980s. One must consider that now, more than two decades later, knowledge, technology and treatment for breast cancer have improved, which may justify new RCTs.
Agreements and disagreements with other studies or reviews
Since the first Cochrane review was published we have not found RCTs or reviews showing new evidence in disagreement with the direction of the results discussed in this update. Again, we highlight that the translation of the results in to practice “toute coure” should be done with caution particularly for the risk of indirectness due to old trials on which this review is based.
Authors' conclusions
Implications for practice.
In light of the evidence presented here, less intensive follow‐up strategies based on periodical clinical exam and annual mammography seem as effective as more intense surveillance schemes. Further laboratory and radiological examinations may add useful information where women are symptomatic or the clinical visit suggests the need for further investigations.
A general practitioner's participation in the delivery of follow‐up care appears feasible and appropriate but, to guarantee the directness of these results, the care should be organised in such a way that access to hospital care is easy when required.
Implications for research.
The evidence from randomised controlled trials (RCTs) summarised here must, however, be interpreted with caution bearing in mind that the studies were conducted almost two decades ago when a more limited understanding of breast cancer biology and less advanced imaging technologies were available. For this reason, the applicability of the results of these "old" trials to the current clinical practice is controversial. Moreover, among clinicians a substantial variation in adherence to guidelines is observed (Chopra 2014; Grandjean 2012). Many physicians perform a more intensive surveillance approach, under the assumption that early detection and treatment of recurrences results in better outcome (Margenthaler 2012;Puglisi 2014). For these reasons, additional trials, targeted at high‐risk patients and incorporating new biological knowledge and diagnostic modalities, are needed. These studies would provide new evidence of the impact of intensive follow‐up on diagnostic anticipation of distant metastases and survival in women with early‐stage breast cancer. A better understanding of this issue would lead to more personalised and cost‐effective follow‐up approaches in the case of positive results, and to the reduction of the actual non evidence‐based prescriptions of diagnostic examinations in the case of negative results.
Further investigation may be warranted on the effects of less frequent schedules of follow‐up and to identify the adequate frequency of mammography.
Further research should also focus on evaluating effects on long‐term outcomes such as overall survival and morbidity of follow‐up by a specialist compared to follow‐up in primary care.
In addition, it would be interesting to evaluate current physician behaviour compared to guideline recommendations to determine to what extent the evidence has been transferred into practice.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2016 | Review declared as stable | In the last decade, only one new study has been published and the review findings remain consistent. Therefore, the results of this review confined to trials of specialist‐ and physician‐led follow‐up programmes are unlikely to change and future updates of this review are not planned |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 4 July 2014 | New citation required but conclusions have not changed | One new study was included, involving 968 patients (Grunfeld 2006). Full 'Risk of bias' tables were added. Conclusions remain unchanged |
| 4 July 2014 | New search has been performed | Performed search for new studies on 4 July 2014 |
| 8 March 2012 | Amended | Additional table linked to text |
| 26 August 2008 | Amended | Converted to new review format. |
| 8 August 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank the Mario Negri Institute of Milan for the facilities and for paying Michela Cinquini's salary.
We wish to thank the Pontificia Universidad Catolica de Chile for supporting Dr. Rojas with a fellowship at the Italian Cochrane Centre.
The authors thank Laura Coe, Maria Paulina Rojas, Marco Del Turco Rosselli and Elena Telaro who contributed to the prior versions of the Cochrane meta‐analysis on “Follow‐up strategies for women treated for early breast cancer”. Laura Coe, Maria Rojas, Marco Del Turco Rosselli and Elena Telaro received no compensation for their support.
Appendices
Appendix 1. MEDLINE
| # ▲ | Searches |
| 1 | randomised controlled trial.pt. |
| 2 | randomized controlled trial.pt. |
| 3 | controlled clinical trial.pt. |
| 4 | randomized.ab. |
| 5 | randomised.ab. |
| 6 | placebo.ab. |
| 7 | randomly.ab. |
| 8 | trial.ab. |
| 9 | groups.ab. |
| 10 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 |
| 11 | exp Breast Neoplasms/ |
| 12 | (milk or tender* or lactat* or feeding or fed).ti,ab. |
| 13 | exp Milk, Human/ |
| 14 | exp Breast Feeding/ |
| 15 | exp Lactation/ |
| 16 | 12 or 13 or 14 or 15 |
| 17 | 11 not 16 |
| 18 | exp Follow‐Up Studies/ |
| 19 | (follow‐up or follow‐up care or follow‐up stud* or centralised follow‐up or decentralised follow‐up or postoperative surveillance or routine).ti,ab. |
| 20 | 18 and 19 |
| 21 | 10 and 17 and 20 |
| 22 | limit 21 to humans |
Appendix 2. Embase
#38
#37 AND [humans]/lim AND [embase]/lim
#37
#9 AND #33 AND #36
#36
#34 AND #35
#35
'follow up'/exp OR 'follow up'
#34
'follow up':ab,ti OR 'follow‐up care':ab,ti OR 'follow‐up study':ab,ti OR 'centralised follow‐up':ab,ti OR 'decentralised follow‐up':ab,ti OR 'postoperative surveillance':ab,ti OR routine:ab,ti
#33
#27 NOT #32
#32
#28 OR #29 OR #30 OR #31
#31
'lactation'/exp OR 'lactation'
#30
'breast feeding'/exp OR 'breast feeding'
#29
'breast milk'/exp OR 'breast milk'
#28
milk:ab,ti OR tender*:ab,ti OR lactat*:ab,ti OR feeding:ab,ti OR fed:ab,ti
#27
#15 AND #26
#26
#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25
#25
'locally advanced breast tumor'
#24
'locally advanced breast tumour'
#23
'locally advanced breast carcinoma'
#22
'locally advanced breast neoplasm'
#21
'locally advanced breast cancer'
#20
'early breast tumor'
#19
'early breast tumour'
#18
'early breast carcinoma'
#17
'early breast neoplasm'
#16
'early breast cancer'
#15
#10 OR #11 OR #12 OR #13 OR #14
'breast tumour'
#13
'breast carcinoma'/exp
#12
'breast neoplasm'
#11
'breast cancer'/exp
#10
'breast tumor'/exp
#9
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#8
groups:ab
#7
trial:ab
#6
randomly:ab
#5
placebo:ab
#4
randomi*ed:ab
#3
controlled AND clinical AND trial
#2
randomized AND controlled AND trial
#1
randomised AND controlled AND trial
Appendix 3. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees
#2 early breast cancer* or early breast neoplas* or early breast tumour* or early breast tumor*:ti,ab,kw (Word variations have been searched)
#3 locally advanced breast cancer* or locally advanced breast neoplas* or locally advanced breast tumour* or locally advanced breast tumor*:ti,ab,kw (Word variations have been searched)
#4 #2 or #3
#5 #1 and #4
#6 MeSH descriptor: [Follow‐Up Studies] explode all trees
#7 follow‐up or follow‐up care or follow‐up stud* or centralised follow‐up or decentralised follow‐up or postoperative surveillance or routine:ti,ab,kw (Word variations have been searched)
#8 #6 and #7
#9 #5 and #8
Appendix 4. WHO ICTRP
Basic Searches:
1. Follow‐up strategies for women treated for early breast cancer
2. Breast cancer AND follow‐up
3. Breast cancer AND centralised follow‐up
4. Breast cancer AND decentralised follow‐up
5. Breast cancer AND postoperative surveillance
6. Breast cancer AND routine
Advanced Searches:
1. Title: Follow‐up strategies for women treated for early breast cancer
Recruitment: All
2. Condition: breast cancer
Intervention: follow‐up OR follow‐up care OR follow‐up studies
Recruitment: All
3. Condition: breast cancer
Intervention: centralised follow‐up OR decentralised follow‐up OR postoperative surveillance OR routine
Recruitment: All
Appendix 5. ClinicalTrials.gov
cancer
Basic Searches:
1. Follow‐up strategies for women treated for early breast cancer
2. Breast cancer AND follow‐up
3. Breast cancer AND centralised follow‐up
4. Breast cancer AND decentralised follow‐up
5. Breast cancer AND postoperative surveillance
6. Breast cancer AND routine
Advanced Searches:
1. Title: Follow‐up strategies for women treated for early breast cancer
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
2. Condition: breast cancer
Intervention: follow‐up OR follow‐up care OR follow‐up studies
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
3. Condition: breast cancer
Intervention: centralised follow‐up OR decentralised follow‐up OR postoperative surveillance OR routine
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All studies
Data and analyses
Comparison 1. Non‐intensive follow‐up vs intensive follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 2 | 2563 | Hazard Ratio (95% CI) | 0.98 [0.84, 1.15] |
| 2 Overall survival by age | 2 | Hazard Ratio (95% CI) | Subtotals only | |
| 2.1 <= 40 | 2 | 272 | Hazard Ratio (95% CI) | 1.08 [0.65, 1.80] |
| 2.2 > 40 | 2 | 2291 | Hazard Ratio (95% CI) | 0.95 [0.78, 1.16] |
| 3 Overall survival by tumour size | 2 | Hazard Ratio (95% CI) | Subtotals only | |
| 3.1 T1 | 2 | 1156 | Hazard Ratio (95% CI) | 0.80 [0.55, 1.17] |
| 3.2 T2 | 2 | 1186 | Hazard Ratio (95% CI) | 0.92 [0.73, 1.17] |
| 3.3 T3 | 2 | 167 | Hazard Ratio (95% CI) | 1.44 [0.91, 2.30] |
| 4 Overall survival by lymphonodal status | 2 | Hazard Ratio (95% CI) | Subtotals only | |
| 4.1 N‐ | 2 | 1379 | Hazard Ratio (95% CI) | 1.35 [0.94, 1.94] |
| 4.2 N+ | 2 | 1184 | Hazard Ratio (95% CI) | 0.84 [0.68, 1.04] |
| 5 Overall survival 5 years | 2 | 2563 | Hazard Ratio (95% CI) | 0.96 [0.80, 1.15] |
| 6 Disease‐free survival | 2 | 2563 | Hazard Ratio (95% CI) | 0.84 [0.71, 1.00] |
| 7 Disease‐free survival by age | 2 | Hazard Ratio (95% CI) | Subtotals only | |
| 7.1 <= 40 | 2 | 272 | Hazard Ratio (95% CI) | 0.94 [0.59, 1.47] |
| 7.2 > 40 | 2 | 2290 | Hazard Ratio (95% CI) | 0.84 [0.70, 1.01] |
| 8 Disease‐free survival by tumour size | 2 | Hazard Ratio (95% CI) | Subtotals only | |
| 8.1 T1 | 2 | 1156 | Hazard Ratio (95% CI) | 0.72 [0.52, 1.00] |
| 8.2 T2 | 2 | 1186 | Hazard Ratio (95% CI) | 0.83 [0.67, 1.04] |
| 8.3 T3 | 2 | 166 | Hazard Ratio (95% CI) | 1.35 [0.82, 2.21] |
| 9 Disease‐free survival by lymphonodal status | 2 | Hazard Ratio (95% CI) | Subtotals only | |
| 9.1 N‐ | 2 | 1379 | Hazard Ratio (95% CI) | 0.84 [0.62, 1.14] |
| 9.2 N+ | 2 | 1183 | Hazard Ratio (95% CI) | 0.83 [0.68, 1.02] |
| 10 number of metastasis detected in an asymptomatic way | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.44, 1.05] |
Comparison 2. Centralised vs decentralised follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | 968 | Hazard Ratio (95% CI) | 1.07 [0.64, 1.78] |
| 2 Disease‐free survival | 2 | 1264 | Hazard Ratio (95% CI) | 1.06 [0.76, 1.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
GIVIO.
| Methods | Multicentre randomised controlled trial.
26 general hospitals, Italy.
Randomisation by telephone, stratified by institution and pathological axillary nodal status.
Inclusion within 6 weeks of surgery.
Calculation of sample size reported.
Intention‐to‐treat analysis. Protocols for adjuvant therapy and treatment of metastatic disease. Median follow‐up of 71 months. |
|
| Participants | 1320 women younger than 70. Histologically‐confirmed, non‐inflammatory, unilateral, breast carcinoma. Stage T1 to T3 (any size tumour without direct extension to chest wall or skin), N0 to N1 (no regional lymphonodal metastases or metastases to movable ipsilateral axillary lymph nodes), and M0 (no distant metastases). | |
| Interventions | Intensive group (N = 655):
‐Physical exam every 3 months for 2 years and then every 6 months for 3 years.
‐Blood test every visit (alkaline phosphatase, gamma‐glutamyl transpeptidase)
‐Chest roentgenography every 6 months.
‐Annual radionuclide bone scan.
‐Annual liver ecography.
‐Annual contralateral mammography. Control group (N = 665): ‐Physical exam every 3 months for 2 years and then every 6 months for 3 years. ‐Annual contralateral mammography. |
|
| Outcomes | Overall survival. Disease‐free survival. HRQoL (quality of life perception, overall health perception, body image, emotional well‐being, social functioning, symptoms and satisfaction with care). Instruments used included the Functional Living Index‐Cancer Scale, the Sickness Impact Profile, the Profile of Mood States and the Cancer Inventory of Problem Situation. Time to detection of recurrence. Symptomatic status at diagnosis of metastases. | |
| Notes | Ipsilateral breast assessment only by physical examination. 123 patients (9.3%) discontinued or were lost before relapsing, and were included in the analysis (similar distribution between experimental and control group). Additional 8% of randomised patients lost to follow‐up not included in the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list, stratified by institution and pathologic axillary nodal status |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally via telephone |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not declared if assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall compliance was greater than 80% in both groups for every procedure. 91% of patients adhered to protocol recommendations. |
| Selective reporting (reporting bias) | Unclear risk | Study conducted before the registration of the protocol was mandatory |
| Other bias | Low risk | No other bias |
Grunfeld 1996.
| Methods | Randomised controlled trial. 2 district general hospitals, England. Eligible patients were invited by letter to participate. 296/445 agreed to participate. Randomisation by telephone in blocks of eight. Calculation of sample size reported, N = 300. Follow‐up 18 months Time to diagnosis assessed blinded by masking allocation information on clinical records. | |
| Participants | 296 women: ‐initial stage I, II or III breast cancer (no distant metastases), ‐primary treatment completed at least 3 months previously, ‐attending outpatient clinic for routine follow‐up, ‐no evidence of disease at last follow‐up visit. | |
| Interventions | Hospital group (N = 148):
Routine follow‐up with clinical visits and mammography, other exams only if clinically indicated.
Frequency of visits in one hospital was every 3 months for 1 year and every 6 months from second to fifth years: in the other hospital was every 3, 4 and 6 months for first, second and third years and every year thereafter. General Practice group (N = 148): Follow‐up with the same schedule of the reference hospital but made by the GP. GPs were sent a letter providing the patient's breast cancer history, a description of follow‐up routine recommended, and assuring that rapid referral to specialist care was possible. An educational handbook on breast cancer follow‐up care was provided. |
|
| Outcomes | Time to detection of recurrence. HRQoL assessed by 3 self‐administered instruments: ‐ British version of the SF‐36 ‐European Organisation for Research and Treatment of Cancer core quality of life questionnaire (EORTC QLQ‐C30) ‐Hospital anxiety and depression scale. | |
| Notes | Characteristics of non participants are included. Random allocation not stratified by clinical stage. Educative intervention with GPs. Overall loss to follow‐up 0.7%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ Authors reported :"random allocation was in blocks of eight" |
| Allocation concealment (selection bias) | Low risk | Follow‐up groups were assigned by a telephone call to the trial co‐ordination centre in Oxford |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All clinical information on patients with suspected recurrence (record of visit forms, hospital notes, test results, doctors' correspondence) was assessed independently by three of the authors and any discrepancies or difficulties discussed. The information was masked as far as possible with respect to allocation group so that “time to diagnosis” was assessed in a blinded fashion. In some cases, however, the allocation group could have been deduced from the course of clinical events. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One (0.7%) participant in each group moved out of the district and was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study conducted before the registration of the protocol was mandatory. |
| Other bias | Low risk | No other bias. |
Grunfeld 2006.
| Methods | Multicentre, randomised, controlled trial; Six of the nine regional cancer centres in Ontario, Canada. Tertiary‐care. The institutional review board of each participating institution approved the study protocol. All analyses were performed according to the intention‐to‐treat principle. Follow‐up (maximum of 5 years 31.9% patients). Median follow‐up was 3.5 years from randomisation (4.5 years from diagnosis) The calculated sample size of 1045 women was based on the assumption that the SCE proportion would be 4 % in both arms, with an upper level of tolerance of 5.5% in the FP arm (i.e. a non‐inferiority margin of 1.5%). 968 women were enrolled. 483 allocated to the family physician (FP) group and 485 to the usual practice (CC) group. |
|
| Participants | Routine follow‐up care after patients completed adjuvant therapy for early‐stage breast cancer.
Patients were enrolled between January 1997 and June 2001, and were observed until June 2003. Women who had completed adjuvant chemotherapy, radiotherapy, or both at least 3 months previously; who were disease‐free; and who were between 9 and 15 months after diagnosis were targeted. Patients may have continued receiving adjuvant hormonal therapy. |
|
| Interventions | Patients were allocated in a 1:1 ratio to receive follow‐up either in the cancer centre according to usual practice (CC group) or from their own family physician (FP group). Family physicians were provided with a one‐page guideline on follow‐up that recommended the following: physical examination and medical history every 3 to 6 months for 3 years, every 6 months for 2 years, and then yearly indefinitely; mammograms yearly; diagnostic tests to investigate signs or symptoms suggestive of recurrent or new primary cancer, but such tests were not to be performed routinely. For women taking tamoxifen, the guideline recommended that a history of vaginal bleeding be taken at each visit and a pelvic examination be performed annually. Family physicians were instructed to refer patients back to the cancer centre if a recurrence or new primary breast cancer developed. For patients in the FP group, if a surgeon had been involved in the patient’s follow‐up care, that follow‐up was also transferred to the FP. |
|
| Outcomes |
Primary outcome: recurrence‐related serious clinical event (SCE) defined as any one of spinal cord compression, pathologic fracture, hypercalcaemia, uncontrolled local recurrence, brachial plexopathy, or poor functional status (Karnofsky performance score [KPS]70)47 at the time of diagnosis of recurrence. Secondary outcome: HRQoL: assessed using the Medical Outcomes Study Short Form 36‐Item General Health Survey (SF‐36) 48 and the Hospital Anxiety and Depression Scale (HADS) 49 while patients were recurrence‐free. The SF‐36 is scored to yield two summary scales: the Physical Component Summary (PCS) scale measuring physical health and the Mental Component Summary (MCS) scale measuring mental health. In all cases, SF‐36 scales yield a score from 0 to 100, with a higher score indicating better quality of life. The HADS contains two subscales measuring anxiety and depression, with scores ranging from 0 to 21.49 Higher scores indicate greater levels of anxiety or depression. The HRQoL standardised scores assessed at baseline and during the subsequent seven follow‐ups at 6, 12, 18, 24, 36, 48, and 60 months were summarised as means and change scores from baseline within each group. Recurrence, death. |
|
| Notes | Patients were excluded if they had persistent complications of primary treatment; were unable to comply with the study protocol including completing specialist follow‐up; or were actively observed at a cancer centre for another primary cancer. KPS</=70 is one of the SCEs because patients usually have high functional status when recurrence is first diagnosed. Low functional status could suggest the potential for poor management of recurrence‐related symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using a computer‐generated centre‐specific schedule. |
| Allocation concealment (selection bias) | Low risk | By a telephone call to the trial co‐ordinating centre of the Ontario Clinical Oncology Group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A committee that was blinded to treatment allocation adjudicated all events. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Study conducted before the registration of the protocol was mandatory. |
| Other bias | Low risk | The sponsors of the research had no role in any aspect of the development, conduct, analysis, or reporting of the study. |
Gulliford 1997.
| Methods | Randomised controlled trial. 1 Breast clinic, England 211 eligible patients, 196 accepted randomisation. No information about randomisation method. Median follow‐up: 16 months 13 excluded after randomisation | |
| Participants | 196 women with: ‐history of breast cancer proved by biopsy. ‐no recurrence of the disease. ‐no symptoms suggesting recurrence. ‐only tamoxifen like adjuvant treatment. ‐home telephone. ‐English speaker. | |
| Interventions | Conventional group (N = 96):
1. Breast self examination monthly.
2. Immediate telephone access if symptoms or doubts were developed.
3. Mammography scheduled depending on primary surgery (every year for 5 years and every 2 years thereafter if lumpectomy, every 2 years since second year if mastectomy).
4. Clinical visits scheduled depending on time from diagnosis (every 3 months the first year, every 4 months the second year, every 6 months from years 3 to 5 and annually thereafter) Mammography only group (N = 97): 1, 2 and 3 are the same. 4. Clinical visits scheduled only with mammography. |
|
| Outcomes | Acceptability of randomised allocation. Use of telephone and GP. Satisfaction with allocation to follow‐up. | |
| Notes | Calculation of sample size not reported. Small sample size and short duration of follow‐up ‐16 months‐. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Study conducted before the registration of the protocol was mandatory. |
| Other bias | Low risk | No other bias. |
Rosselli Del Turco 1994.
| Methods | Multicentre randomised controlled trial. 12 breast clinics in Italy (oncologic centres). Randomisation by telephone, stratified by institution. Inclusion within 6 months of surgery. Follow‐up at 5 and10 years. Adjuvant therapy and treatment of recurrence according to national guidelines. Intention‐to‐treat analysis. | |
| Participants | 1243 women younger than 70. Histologically‐confirmed, unilateral invasive carcinoma of the breast with no evidence of metastases. | |
| Interventions | Intensive group (N = 622): ‐Physical exam every 3 months for 2 years and then every 6 months for 3 years. ‐Two‐view chest roentgenography every 6 months. ‐Radionuclide bone scan every 6 months. ‐Annual mammography. Control group (N = 621): ‐Physical exam every 3 months for 2 years and then every 6 months for 3 years. ‐Annual mammography. | |
| Outcomes | Overall survival. Disease‐free survival. | |
| Notes | Calculation of sample size not reported. 161 patients (12.9%) were lost to follow‐up at some point during the study and were included in the analysis (similar distribution between experimental and control group). Vital status information available for all except 10 patients (.8%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Randomisation was centrally performed, individual case of allocation was communicated by telephone. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 161 (12.9%) patients were lost to clinical follow‐up at some point. 86 patients intensive vs 75 patients non intensive follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study conducted before the registration of the protocol was mandatory |
| Other bias | Low risk | No other bias |
CC: usual practice FP: family physician HRQoL: health‐related quality of life SCE: serious clinical event M: distant metastases N: regional lymph nodes T: primary tumour
Differences between protocol and review
In the 2014 review update:
outcomes have now been differentiated into primary and secondary, and
additional text has been added to the background section of the review for completeness.
Contributions of authors
For the 2014 review update: Ivan Moschetti and Michela Cinquini identified, selected and critically appraised the studies and completed the 'Risk of bias' table of all included studies. Both authors wrote the previous and current version of this review including updated analyses and text according to the new findings. Alessia Levaggi and Matteo Lambertini reviewed the manuscript from a clinical point of view updating the introduction and the discussion, writing the implications for research section and updating the guidelines table.
For the previous versions of the review: Paulina Rojas and Elena Telaro identified, selected and critically appraised the studies to be included in the review, and wrote the first draft. Roldano Fossati designed the review, supervised the analysis of the data and reviewed the earlier drafts of the manuscript. Antonio Russo analysed the data and reviewed the earlier drafts of the manuscript. Domenico Palli and Marco Rosselli del Turco contributed to the development of the protocol and reviewed the manuscript of the final review. Alessandro Liberati designed the review, supervised the assessment of the studies and reviewed the manuscript. Ivan Moschetti and Laura Coe analysed the search results for inclusion of new studies, updated analyses and text according to new findings for the first update of this review.
Sources of support
Internal sources
No specific funding was available for the original conduct and update, Other.
-
Ivan Moschetti and Michela Cinquini: Mario Negri Institute for Pharmacological Research IRCCS, Italy.
The form of support is related to facilities and salary provided by Mario Negri Institute
External sources
No resources of external support were provided, Other.
Declarations of interest
Roldano Fossati (RF) and Alessandro Liberati (AL) were members of the Steering Group of the GIVIO study included in this review and wrote several review articles of the effect of follow‐up care. The GIVIO trial (of which RF and AL were authors) was originally partially supported by a educational grant from Astra Zeneca Italia. No specific funding was available for the original conduct and update of this review. Ivan Moschetti, Michela Cinquini, Alessia Levaggi and Laura Coe do not have any conflict of interests.
'Deceased'
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
GIVIO {published and unpublished data}
- The GIVIO Investigators. Impact of follow‐up testing on survival and health‐related quality of life in breast cancer patients. A multicenter randomized controlled trial. JAMA 1994;271(20):1587‐92. [DOI] [PubMed] [Google Scholar]
Grunfeld 1996 {published data only}
- Grunfeld E, Fitzpatrick R, Mant D, Yudkin P, Adewuyi‐Dalton R, Stewart J, et al. Comparison of breast cancer patient satisfaction with follow‐up in primary care vs specialist care: results from a randomized controlled trial. British Journal of Clinical Practice 1999;49(446):705‐10. [PMC free article] [PubMed] [Google Scholar]
- Grunfeld E, Mant D, Yudkin P, Adewuyi‐Dalton R, Cole D, Stewart J, et al. Routine follow up of breast cancer in primary care: randomised trial. BMJ 1996;313(7058):665‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grunfeld 2006 {published data only}
- Grunfeld E, Levine MN, Julian JA, Coyle D, Szechtman B, Mirsky D, et al. Randomized trial of long‐term follow‐up for early‐stage breast cancer: a comparison of family physician versus specialist care. Journal of Clinical Oncology 2006;24(6):848‐55. [DOI] [PubMed] [Google Scholar]
Gulliford 1997 {published data only}
- Gulliford T, Opomo M, Wilson E, Hanham I, Epstein R. Popularity of less frequent follow‐up for breast cancer in randomised study: initial findings from the hotline study. BMJ 1997;314(7075):171‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosselli Del Turco 1994 {published and unpublished data}
- Palli D, Russo A, Saieva C, Ciatto S, Rosselli Del Turco M, Distante V, et al: for the National Research Council Project on Breast Cancer Follow‐up. Intensive vs clinical follow‐up after treatment of primary breast cancer: 10‐year update of a randomized trial. JAMA 1999;281(17):1586. [DOI] [PubMed] [Google Scholar]
- Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic follow‐up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow‐up. JAMA 1994;271(20):1593‐7. [DOI] [PubMed] [Google Scholar]
Additional references
ASCO 2013
- Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, et al. Breast cancer follow‐up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology 2013;31(7):961–5. [DOI] [PubMed] [Google Scholar]
Burstein 2000
- Burstein HJ, Winer EP. Primary care for survivors of breast cancer. New England Journal of Medicine 2000;343(15):1086‐94. [DOI] [PubMed] [Google Scholar]
Carlotti 1993
- Carlotti A, Siragusa A, Grillo Ruggeri F, Vitali ML, Grimaldi A, Barone D. The mammographic images of the irradiated breast after conservative therapy for carcinoma [Quadri mammografici della mammela irradiata dopo terapia conservativa per carcinoma]. La Radiologia Medica 1993;86:101‐5. [PubMed] [Google Scholar]
Chopra 2014
- Chopra I, Chopra A. Follow‐up care for breast cancer survivors: improving patient outcomes. Patient Related Outcome Measures 2014;30(5):71‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clinical trial 2014
- Home ‐ ClinicalTrials.gov [Internet]. [cited 2014 Mar 29]. Available from: http://www.clinicaltrials.gov/.
Constantinidou 2011
- Constantinidou A, Martin A, Sharma B, Johnston SRD. Positron emission tomography/computed tomography in the management of recurrent/metastatic breast cancer: a large retrospective study from the Royal Marsden Hospital. Annals of Oncology 2011;22(2):307–14. [DOI] [PubMed] [Google Scholar]
De Lena 1995
- Lena M, Ferguson J, Liberati A. Consensus Conference on Follow‐up in Breast Cancer. Selected Papers. Annals of Oncology 1995;6 Suppl 2:1‐70. [PubMed] [Google Scholar]
Duffy 2008
- Duffy SW, Nagtegaal ID, Wallims M, Cafferty FH, Houssami N, Warwich J, et al. Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. American Journal of Epidemiology 2008;168(1):98‐104. [DOI] [PubMed] [Google Scholar]
ESMO 2013
- Senkus E, Kyriakides S, Penault‐Llorca F, Poortmans P, Thompson A, Zackrisson S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2013;Suppl 6:vi7–23. [DOI] [PubMed] [Google Scholar]
Ferlay 2013
- Ferlay J, Steliarova‐Foucher E, Lortet‐Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimate for 40 countries in 2012. European Journal of Cancer 2013;49(6):1374‐403. [DOI] [PubMed] [Google Scholar]
Globocan 2012
- International Agency for Research on Cancer, World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012.. Available from: http://globocan.iarc.fr/Default.aspx (accessed 15 May 2015) 2012.
Goldhirsch 2013
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart‐Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Annals of Oncology 2013;24(9):2206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2004
- The GRADE working group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grandjean 2012
- Grandjean I, Kwast AB, Vries H, Klaase J, Schoevers WJ, Siesling S. Evaluation of the adherence to follow‐up care guidelines for women with breast cancer. European Journal of Oncology Nursing 2012;16(3):281‐5. [DOI] [PubMed] [Google Scholar]
Greco 1998
- Greco M, Agresti R, Giovanazzi R. Impact of the diagnostic methods on the therapeutics strategies. The Quarterly Journal of Nuclear Medicine and Molecular Imaging 1998;42:66‐80. [PubMed] [Google Scholar]
Grunfeld 1999
- Grunfeld E, Fitzpatrick R, Mant D, Yudkin P, Adewuyi‐Dalton R, Stewart J, et al. Comparison of breast cancer patient satisfaction with follow‐up in primary care vs specialist care: results from a randomized controlled trial. British Journal of Clinical Practice 1999;49(446):705‐10. [PMC free article] [PubMed] [Google Scholar]
Grunfeld 2010
- Grunfeld E, Hodgson DC, Giudice ME, Moineddin R. Population‐based longitudinal study of follow‐up care for breast cancer survivors. Journal of Oncology Practice 2010;6(4):174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hannisdal 1993
- Hannisdal E, Gundersen S, Kvaloy S, Lindegaard, Aas M, Finnanger AM, et al. Follow‐up of breast cancer patients stage I‐II: A baseline strategy. European Journal of Cancer 1993;29A(7):992‐7. [DOI] [PubMed] [Google Scholar]
Harries 1996
- Harries SA, Lawrence RN, Scrivener R, Friedman NR, Kissin MW. A survey of the management of breast cancer in England and Wales. Annals of the Royal College of Surgeons of England 1996;78(3):197‐202. [PMC free article] [PubMed] [Google Scholar]
Hietanen 1986
- Hietanen P. Chest radiography in the follow‐up of breast cancer. Acta Radiologica Oncology 1986;25:15‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holli 1998
- Holli K, Saaristo R, Isola J, Hyoti M, Hakama M. Effect of radiotherapy on the interpretation of routine follow‐up mammography after conservative breast surgery: a randomised study. British Journal of Cancer 1998;78(4):542‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hortobagyi 2002
- Hortobagyi GN. Can we cure limited metastatic breast cancer?. Journal of Clinical Oncology 2002;20(3):620‐3. [DOI] [PubMed] [Google Scholar]
Jatoi 2011
- Jatoi I, Anderson WF, Jeong J‐H, Redmond CK. Breast cancer adjuvant therapy: time to consider its time‐dependent effects. Journal of Clinical Oncology 2011;29(17):2301‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennecke 2010
- Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. Journal of Clinical Oncology 2010;28(20):3271‐7. [DOI] [PubMed] [Google Scholar]
Kobayashi 2012
- Kobayashi T, Ichiba T, Sakuyama T, Arakawa Y, Nagasaki E, Aiba K, et al. Possible clinical cure of metastatic breast cancer: lessons from our 30‐year experience with oligometastatic breast cancer patients and literature review. Breast Cancer 2012;19(3):218–37. [DOI] [PubMed] [Google Scholar]
Logarer 1990
- Logarer VB, Vestergaard A, Herrstedt J, Thomsen HS, Zedeler K, Dombernowsky. The limited value of routine chest X‐ray in the follow‐up of stage II breast cancer. European Journal of Cancer 1990;26(5):553‐5. [DOI] [PubMed] [Google Scholar]
Loprinzi 1994
- Loprinzi CL. It is now the age to define the appropriate follow‐up of primary breast cancer patients [editorial]. Journal of Clinical Oncology 1994;12(5):881‐3. [DOI] [PubMed] [Google Scholar]
Lucci 2012
- Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, et al. Circulating tumour cells in non‐metastatic breast cancer: a prospective study. Lancet Oncology 2012;13(7):688–95. [DOI] [PubMed] [Google Scholar]
Mahoney 1986
- Mahoney L. Methods for detecting locally recurrent and controlateral second primary breast cancer. Canadian Journal of Surgery 1986;29(5):372‐3. [PubMed] [Google Scholar]
Margenthaler 2012
- Margenthaler JA, Allam E, Chen L, Virgo KS, Kulkarni UM, Patel AP, et al. Surveillance of patients with breast cancer after curative‐intent primary treatment: current practice patterns. Journal of Oncology Practice 2012;8(2):79‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Metzger‐Filho 2013
- Metzger‐Filho O, Sun Z, Viale G, Price KN, Crivellari D, Snyder RD, et al. Patterns of Recurrence and outcome according to breast cancer subtypes in lymph node‐negative disease: results from international breast cancer study group trials VIII and IX. Journal of Clinical Oncology 2013;31(25):3083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCN 2014
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer ‐ breast.pdf [Internet]. [cited 2014 Mar 29]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Pandya 1983
- Pandya KJ, McFadden ET, Kalish LA, Carbone PP. Frequency and patterns of early disease recurrence and method of detection in operable breast cancer with pathologically positive axillary lymph nodes on Eastern Cooperative Oncology Group Adjuvant studies: A preliminary report. Cancer Treatment Symposium 1983;2:117‐22. [Google Scholar]
Puglisi 2014
- Puglisi F, Fontanella C, Numico G, Sini V, Evangelista L, Monetti F, et al. Follow‐up of patients with early breast cancer: is it time to rewrite the story?. Critical Reviews in Oncology/Hematology 2014;91(2):130‐41. [DOI] [PubMed] [Google Scholar]
RevMan [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutgers 1989
- Rutgers EJ, Slooten EA, Kluck HM. Follow‐up after treatment of primary breast cancer. British Journal of Surgery 1989;76:187‐90. [DOI] [PubMed] [Google Scholar]
SEER database 2014
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. SEER Stat Fact Sheets: Female Breast Cancer. Available from: http://seer.cancer.gov (accessed 15 May 2015) 2014.
Stark 1996
- Stark ME, Crowe JP Jr. Breast cancer evaluation and follow‐up: a survey of The Ohio Chapter of The American College of Surgeons. American Surgeon 1996 Jun;62(6):458‐60. [PubMed] [Google Scholar]
Tomiak 1998
- Tomiak EM, Diverty B, Verma S, Evans WK, Petit C, Will P, et al. Follow‐up practices for patients with early stage breast cancer: a survey of Canadian oncologists. Cancer Prevention & Control 1998;2(2):63‐71. [PubMed] [Google Scholar]
Vestergaard 1989
- Vestergaard A, Herrstedt J, Thomsen HS, Dombernowsky P, Zedeler K. The value of yearly chest x‐ray in patients with stage I breast cancer. European Journal of Cancer and Clinical Oncology 1989;25(4):687‐9. [DOI] [PubMed] [Google Scholar]
WHO 2014
- WHO | Welcome to the WHO ICTRP [Internet]. [cited 2014 Mar 29]. Available from: http://www.who.int/ictrp/en/.
Wickerhan 1986
- Wickerham L, Fisher B, Cronin W and Members of the NSABP Committee for treatment failure Criteria. The efficacy of bone scanning in the follow‐up of patients with operable breast cancer. Breast Cancer Research and Treatment 1984;4:303‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rojas 2000
- Rojas MP, Telaro E, Russo A, Fossati R, Palli D, Rosselli TM, et al. Follow‐up strategies for women treated for early breast cancer. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001768] [DOI] [PubMed] [Google Scholar]
Rojas 2005
- Rojas MPMP, Telaro E, Moschetti I, Coe L, Fossati R, Liberati A, et al. Follow‐up strategies for women treated for early breast cancer. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD001768.pub2] [DOI] [PubMed] [Google Scholar]


