Abstract
Background
Nausea and vomiting remain a problem for children undergoing treatment for malignancies despite new antiemetic therapies. Optimising antiemetic regimens could improve quality of life by reducing nausea, vomiting, and associated clinical problems. This is an update of the original systematic review.
Objectives
To assess the effectiveness and adverse events of pharmacological interventions in controlling anticipatory, acute, and delayed nausea and vomiting in children and young people (aged less than 18 years) about to receive or receiving chemotherapy.
Search methods
Searches included the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS, PsycINFO, conference proceedings of the American Society of Clinical Oncology, International Society of Paediatric Oncology, Multinational Association of Supportive Care in Cancer, and ISI Science and Technology Proceedings Index from incept to December 16, 2014, and trial registries from their earliest records to December 2014. We examined references of systematic reviews and contacted trialists for information on further studies. We also screened the reference lists of included studies.
Selection criteria
Two review authors independently screened abstracts in order to identify randomised controlled trials (RCTs) that compared a pharmacological antiemetic, cannabinoid, or benzodiazepine with placebo or any alternative active intervention in children and young people (less than 18 years) with a diagnosis of cancer who were to receive chemotherapy.
Data collection and analysis
Two review authors independently extracted outcome and quality data from each RCT. When appropriate, we undertook meta‐analysis.
Main results
We included 34 studies that examined a range of different antiemetics, used different doses and comparators, and reported a variety of outcomes. The quality and quantity of included studies limited the exploration of heterogeneity to narrative approaches only.
The majority of quantitative data related to the complete control of acute vomiting (27 studies). Adverse events were reported in 29 studies and nausea outcomes in 16 studies.
Two studies assessed the addition of dexamethasone to 5‐HT3 antagonists for complete control of vomiting (pooled risk ratio (RR) 2.03; 95% confidence interval (CI) 1.35 to 3.04). Three studies compared granisetron 20 mcg/kg with 40 mcg/kg for complete control of vomiting (pooled RR 0.93; 95% CI 0.80 to 1.07). Three studies compared granisetron with ondansetron for complete control of acute nausea (pooled RR 1.05; 95% CI 0.94 to 1.17; 2 studies), acute vomiting (pooled RR 2.26; 95% CI 2.04 to 2.51; 3 studies), delayed nausea (pooled RR 1.13; 95% CI 0.93 to 1.38; 2 studies), and delayed vomiting (pooled RR 1.13; 95% CI 0.98 to 1.29; 2 studies). No other pooled analyses were possible.
Narrative synthesis suggests that 5‐HT3 antagonists are more effective than older antiemetic agents, even when these agents are combined with a steroid. Cannabinoids are probably effective but produce frequent side effects.
Authors' conclusions
Our overall knowledge of the most effective antiemetics to prevent chemotherapy‐induced nausea and vomiting in childhood is incomplete. Future research should be undertaken in consultation with children, young people, and families that have experienced chemotherapy and should make use of validated, age‐appropriate measures. This review suggests that 5‐HT3 antagonists are effective in patients who are to receive emetogenic chemotherapy, with granisetron or palonosetron possibly better than ondansetron. Adding dexamethasone improves control of vomiting, although the risk‐benefit profile of adjunctive steroid remains uncertain.
Keywords: Adolescent; Child; Humans; Antiemetics; Antiemetics/adverse effects; Antiemetics/therapeutic use; Antineoplastic Agents; Antineoplastic Agents/adverse effects; Dexamethasone; Dexamethasone/therapeutic use; Drug Therapy, Combination; Drug Therapy, Combination/adverse effects; Drug Therapy, Combination/methods; Nausea; Nausea/chemically induced; Nausea/drug therapy; Nausea/prevention & control; Neoplasms; Neoplasms/drug therapy; Randomized Controlled Trials as Topic; Serotonin Antagonists; Serotonin Antagonists/adverse effects; Serotonin Antagonists/therapeutic use; Vomiting; Vomiting/chemically induced; Vomiting/drug therapy; Vomiting/prevention & control
Plain language summary
Drugs to prevent nausea and vomiting in children and young people undergoing chemotherapy
Background
The use of chemotherapy to treat cancer in children and young people can produce nausea (a sensation that one might vomit) and vomiting. These extremely unpleasant sensations continue to be a problem despite better antiemetic (antisickness) drugs.
Review question
How effective are medications to prevent nausea and vomiting in children and young people undergoing chemotherapy?
Key results
We found only 34 properly randomised trials that had been undertaken in children, which examined 26 drug combinations. Trials tended to report vomiting rather than nausea, even though nausea is generally a more distressing experience. We could make no firm conclusions about which drugs are best, what dose of drug is most effective, or whether it is better to receive treatments orally (by mouth) or intravenously (injected). It seems that the 5‐HT3 antagonists (the 'trons', for example ondansetron, granisetron, or tropisetron) are better than older agents, and that adding dexamethasone to these drugs makes them even more effective. Further research should consider what patients and families deem to be important, use established measures of nausea and vomiting, and attempt to use even newer techniques in the undertaking of reviews in order to maximise the information available.
Background
Despite advances in antiemetic therapies, nausea and vomiting continue to be a problem for children undergoing treatment for malignancies (Holdsworth 2006), and are highly unpleasant (Dolgin 1989). The selection of an appropriate and effective antiemetic regimen has the potential to impact on quality of life by eradicating or reducing the symptom and its associated clinical problems. Due to limited studies in children, optimal paediatric dosing and scheduling of antiemetics remains uncertain (Antonarakis 2004a; Roila 2005). This results in inconsistencies and variation in prescribing, which is often underpinned by personal preference and experience, as opposed to research‐based evidence (Foot 1994). In contrast, international evidence‐based guidelines have been produced for adults (Kris 2005).
The development of 5‐HT3 antagonists, such as ondansetron, and the wider use of corticosteroids have greatly improved the control and reduction of chemotherapy‐associated nausea and vomiting (Culy 2001). However, the use of more intensive and emetogenic chemotherapeutic agents means that nausea and vomiting are still a major problem, and a significant number of children and young people continue to experience emesis (Holdsworth 2006). Efforts to reduce this side effect of treatment therefore must continue. In addition, these symptoms are frequently a feature of the palliative care phase and may have a detrimental effect on quality of life (Wolfe 2000).
Nausea and vomiting can have profound physical and psychological consequences. The physical consequences may include dehydration, electrolyte imbalance, anorexia, weight loss, weakness, increased susceptibility to infections, and disruption of normal childhood activities (Cotanch 1985). Chemotherapy‐induced nausea and vomiting are considered to be among the most aversive of side effects, causing much distress to the child and family (Zeltzer 1991). When asked to identify what factors were distressing when receiving chemotherapy, parents of children and young people themselves reported physical concerns such as nausea and vomiting as being particularly problematic (Hedstrom 2003). Interventions that affect the physiological and psychological dimensions of nausea and vomiting, as well as those that reduce the number of episodes of emesis, are required to provide effective and holistic management for these distressing and debilitating symptoms.
There appear to be distinct clinical phases of nausea and vomiting related to chemotherapy. These commence with anticipatory nausea and vomiting, symptoms that precede the administration of chemotherapy, often following a previous aversive chemotherapy experience. Estimates of the prevalence of anticipatory nausea and vomiting have ranged from 20% to 30% (Dolgin 1985). This experience responds poorly to pharmacological approaches to antiemesis (Richardson 2007). Symptoms following administration of chemotherapy, and within 24 hours, are defined as acute nausea and vomiting. The incidence of this varies according to the emetogenicity of the chemotherapy received, but without prophylaxis it can be upwards of 90% for some commonly used agents (for example cisplatin) (Holdsworth 2006). Symptoms beyond 24 hours are described as delayed nausea and vomiting, and may occur in up to half of patients, usually after receiving platinum compounds or alkylating agents (Holdsworth 2006).
Different antiemetic agents have different modes of action, Antonarakis 2004b, and differing effectiveness (Antonarakis 2004a; Holdsworth 2006). Within a class of agents, varying side effects may alter the overall utility of a drug (Sandoval 1999). Different dose schedules and routes of administration have been used with the same agent with uncertain results (Sandoval 1999; White 2000).
In order to clearly define the limits of our knowledge of antiemetic medications, we undertook a systematic review of pharmacological approaches to prevent or reduce anticipatory, acute, and delayed symptoms of nausea and vomiting in children and young people who have cancer. This is an update of the original review published in 2010 (Phillips 2010).
Objectives
Aim
To assess the effectiveness and adverse events of pharmacological interventions in preventing nausea and vomiting in children and young people (aged less than 18 years) about to receive or receiving chemotherapy.
Objectives
To assess the effectiveness of pharmacological interventions in controlling anticipatory nausea and vomiting in children and young people (aged less than 18 years) about to receive chemotherapy.
To assess the effectiveness of pharmacological interventions in controlling acute and delayed nausea and vomiting in children and young people (aged less than 18 years) receiving chemotherapy.
To determine the associated adverse events in participants receiving pharmacological antiemetics.
To assess the effect that pharmacological antiemetics have on the quality of life of treated participants.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), where a pharmacological antiemetic, cannabinoid, or benzodiazepine has been compared with either placebo or an alternative active intervention.
Types of participants
Children and young people (less than 18 years) with a diagnosis of cancer who have received chemotherapy and pharmacological antiemetics.
Types of interventions
Standard pharmacological antiemetics used in the treatment of chemotherapy‐induced nausea and vomiting. These include (but are not limited to):
5‐HT3 antagonists;
benzodiazepines;
cannabinoids;
corticosteroids;
cyclizine;
dopamine blockers; and
levomepromazine.
We excluded NK1 antagonists, which are the subject of another Cochrane review (Tremont‐Lukats 2007). We also excluded non‐pharmacological approaches from this review.
This review addressed the effectiveness of each agent in the prevention and control of acute and delayed chemotherapy‐induced nausea and vomiting compared to placebo or active comparators.
This review also sought to address the effectiveness of the following agents in the control of anticipatory nausea and vomiting compared to placebo or any active comparator:
cannabinoids;
benzodiazepines.
Types of outcome measures
Complete control of nausea (no nausea and no use of rescue medications) prior to chemotherapy delivery (anticipatory phase), in the acute phase (first 24 hours of treatment with chemotherapy), and in the delayed phase (after 24 hours of treatment with chemotherapy) of nausea and vomiting.
Complete control of vomiting (no vomiting and no use of rescue medications) prior to chemotherapy delivery (anticipatory phase), and in the acute and delayed phases of nausea and vomiting.
Adverse effects as defined by each trial found to be eligible for this review.
Quality‐of‐life measures.
Where data on the complete control of nausea and vomiting were absent, we estimated the effectiveness by analysis of the average difference using a continuous measure of vomiting (for example 'number of vomits per day') where available.
Search methods for identification of studies
Electronic searches
We undertook searches in the following databases in order to identify relevant studies. We have reported full details of the search strategies in Appendix 1.
The Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library ‐ http://www.thecochranelibrary.com/) from inception to 16 December 2014 (Issue 11, 2014).
Ovid MEDLINE and Ovid MEDLINE In Process and Other Non‐Indexed Citations (Ovid Online ‐ www.ovid.com) from 1966 to 16 December 2014.
EMBASE (Ovid Online ‐ www.ovid.com) from 1980 to 16 December 2014.
LILACS (Literatura Latino‐Americana e do Caribe em Ciências da Saúde) (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/) from inception to 17 December 2014.
PsycINFO (Ovid Online ‐ www.ovid.com) from 1806 to 16 December 2014.
We searched the following proceedings abstracts. We have reported full details of the search strategies in Appendix 2.
American Society of Clinical Oncology (http://www.asco.org) from 2008 to 2014.
International Society of Paediatric Oncology (SIOP) (http://www.siop.nl/) from 2008 to 2014.
Multinational Association of Supportive Care in Cancer (MASCC) (http://www.mascc.org/) from 2008 to 2014.
ISI Science and Technology Proceedings (http://wos.mimas.ac.uk/) from 2008 to 2014.
We also undertook searches for ongoing clinical trials using several Internet resources. We have reported full details of the search strategies in Appendix 3.
International Cancer Research Portfolio (ICRP) (http://www.cancerportfolio.org/) from inception to 16 December 2014.
National Cancer Institute Clinical Trials PDQ (http://www.cancer.gov/Search/SearchClinicalTrialsAdvanced.aspx) from inception to 16 December 2014.
National Cancer Research Institute (NCRI) (http://www.ncri.org.uk/) from inception to 16 December 2014.
Current Controlled Trials (mRCT Register) (http://www.isrctn.com/) from inception to 16 December 2014.
CinicalTrials.gov (http://clinicaltrials.gov/) from inception to 16 December 2014.
CenterWatch (http://www.centerwatch.com/) from inception to 16 December 2014.
Terminology
We identified the terms for the search strategies through discussion between an Information Specialist and the rest of the research team, by scanning the background literature, and by browsing the MEDLINE thesaurus (MeSH). The Cochrane Childhood Cancer Group also provided assistance. We searched all databases from their inception to the date of the search. Searches covered the inception of the database to 16 or 17 December 2014. We applied no language or other restrictions.
Cochrane filters
The Cochrane Childhood Cancer Group suggested several search filters for this review (Kremer 2014).
Study type: We used the sensitive trials filter developed by the Centre for Reviews and Dissemination in MEDLINE. A trials filter was developed for EMBASE based on the suggestions in the Cochrane Handbook for Systematic Reviews of Interventions. This was then adapted to run on PsycINFO. We did not use study type filters in the other databases.
Childhood: The Cochrane Childhood Cancer Group has a filter for children (age 0 to 18 years), which we used where appropriate (Kremer 2014).
Childhood cancer: Since we were using a filter for children, we adapted some aspects of the Cochrane Childhood Cancer Group filter to prevent duplication. For instance, 'childhood cancer' was replaced by 'cancer', since the concept of childhood was already expressed in the age facet.
Searching other resources
We screened the references of any identified systematic reviews and initiated personal communication with the authors of relevant trials to request further information on published, unpublished, or ongoing studies. We also screened the reference lists of included studies. We employed no language restrictions.
Data collection and analysis
Selection of studies
After employing the search strategy, two review authors independently screened each abstract to identify studies meeting the inclusion criteria. Discrepancies were resolved by consensus without the need for final resolution using a third‐party arbitrator. We obtained in full any study that seemed to meet the inclusion criteria on the grounds of the title, abstract, or both, for closer inspection and inclusion or exclusion.
Data extraction and management
Two review authors independently extracted outcome data from each included RCT in the following categories: participants, methods, interventions, and outcome measurements of interest. We recorded the outcome measurements as binary data (number of participants with total control of nausea and vomiting during the study period relative to the total number of participants evaluable for treatment) where possible. Discrepancies between review authors were resolved by consensus. We sought clarification from trial authors in cases of unclear or missing data.
Assessment of risk of bias in included studies
Two review authors independently extracted quality data from each included RCT according to the following criteria: concealment of treatment allocation, blinding of the care provider, blinding of the participants, blinding of the outcome assessor, random sequence generation, and incomplete outcome data. We partially assessed the potential for selective reporting of outcomes: we checked the reported outcomes against where the study methods stated which outcomes were collected. (In no cases did we check the trial reported against the previously or separately published trial protocol.) We also noted other potentials for bias. These were: publication bias; the funder of the study, and any explanation as to their role; and for cross‐over studies the drop‐out rates in each phase of the trial. For all quality items, we used the definitions as described in the module of the Cochrane Childhood Cancer Group (Kremer 2014). Discrepancies between review authors were resolved by consensus.
Data synthesis
When statistically appropriate, we combined the aggregate data to obtain a pooled effect size. We planned to assess for effects related to potential sources of bias, agent, dose, schedule, and route of administration.
We entered data into RevMan 5.3, RevMan 2014, and planned to undertake analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions for eight separate primary outcomes (Higgins 2011):
complete control of nausea before chemotherapy treatment (anticipatory nausea);
complete control of vomiting before chemotherapy treatment (anticipatory vomiting);
complete control of nausea up to 24 hours of chemotherapy treatment (acute nausea);
complete control of nausea after 24 hours of chemotherapy treatment (delayed nausea);
complete control of vomiting up to 24 hours of chemotherapy treatment (acute vomiting);
complete control of vomiting after 24 hours of chemotherapy treatment (delayed vomiting);
adverse event rate;
quality‐of‐life measures.
Where appropriate, we examined outcomes when studies were grouped by class of agent, dose, schedule, and route of administration. Where data on the complete control of nausea and vomiting were absent, we estimated the effectiveness by analysis of the average difference using a continuous measure of vomiting (for example 'number of vomits per day'). Where possible, we used an intention‐to‐treat analysis. Where data were missing, we undertook an available case analysis (using all cases with available data as the denominator). As this did not affect any pooled analysis, we did not undertake a sensitivity analysis. To maximise the data from cross‐over studies, we used paired data where available. Where these were not available, we analysed studies as if they had a traditional parallel design without accounting for paired data. We calculated risk ratios and combined data using methods described by Zou 2007.
We explored heterogeneity narratively, looking at alternative populations, doses, and comparators. We compared random‐effects and fixed‐effect models for pooled estimates when the calculated I² value was greater than 50%.
Results
Description of studies
Results of the search
The original search identified a total of 844 potentially useful individual articles (see Figure 1 for details). We identified 67 articles for detailed screening. We identified one ongoing study (NCT00429702 2007), and could not retrieve three studies (Gómez 1995; Xu 1997; Zeng 1995). We attempted to contact authors to clarify aspects of study design, data analysis, and to retrieve specific data on those participants under 18 years of age. We identified 27 articles reporting on 28 trials for inclusion in the review. These included 1719 participants (median 30, range 12 to 428) and 2226 episodes (median 50.5, range 20 to 428).
1.
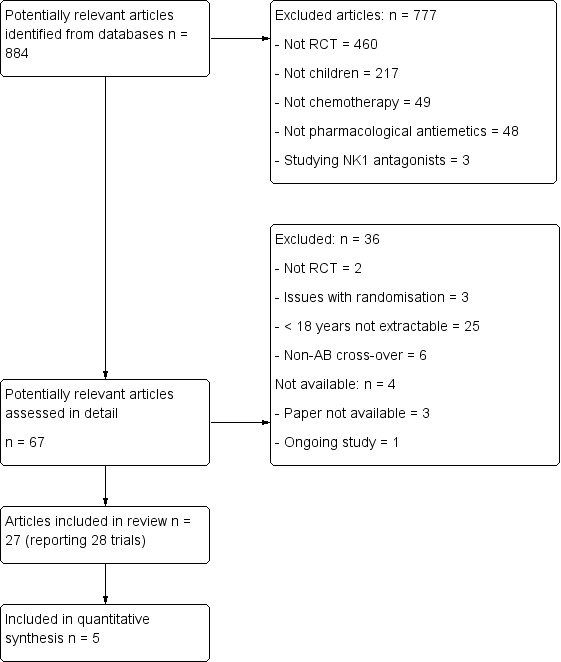
Flow diagram of study selection process original review (Phillips 2010).
The updated search in December 2014 found a further 344 potentially useful individual articles (see Figure 2 for details). We identified 12 articles for detailed screening. The previously identified ongoing study had been terminated without publication of results and was thus added to the Characteristics of excluded studies table. We identified six new articles for inclusion in the review, and identified that the ongoing study (NCT00429702 2007) had been closed through poor accrual. The new studies included a further 304 participants and 869 episodes.
2.
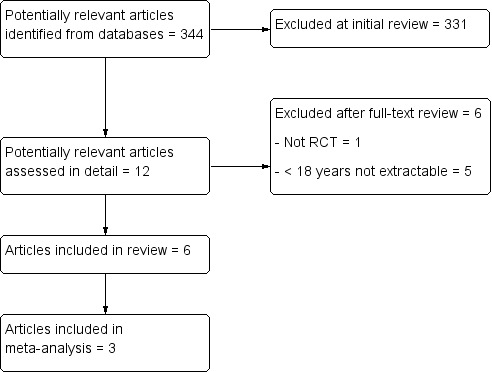
Flow diagram of study selection process for additional studies identified in 2014 update.
Included studies
The 34 included trials (28 from the original review and 6 from the update) examined a wide range of different pharmacological antiemetics, used different doses and comparators, and reported a variety of outcomes. With the exception of Parker 2001, who examined the use of ondansetron for intrathecal chemotherapy only, and Nagel 2008, who examined the use of ondansetron and fentanyl for intrathecal chemotherapy only, all trials looked at the effectiveness of treatments on systemic chemotherapy‐induced nausea and vomiting.
Of the eight outcome measures specified in this review, no data were available from any study on anticipatory nausea or vomiting, or for any validated quality‐of‐life measures. No trials compared different durations of antiemetic medication.
Data on any outcomes beyond the first 24 hours of chemotherapy were infrequently reported (Berrak 2007; Brock 1996; Emir 2013; Noguera 2001; Sepulveda‐Vildosola 2008; Siddique 2011).
Data on nausea were inconsistently reported using unvalidated measurement scales (see Characteristics of included studies for details). They were presented in thirteen trials (Brock 1996; Dalzell 1986; Ekert 1979; Ekert 1979a; Emir 2013; Mehta 1986; Nagel 2008; Noguera 2001; Orchard 1999; Sandoval 1999; Sepulveda‐Vildosola 2008; Siddique 2011; Suarez 1994), with a further four studies detailing a compound outcome of nausea, vomiting, or both (Dick 1995; Mabro 2000; Sandoval 1999; Tejedor 1999).
The majority of quantitative data related to the complete control of acute vomiting (27 trials) (Alvarez 1995; Basade 1996; Berrak 2007; Brock 1996; Chan 1987; Dick 1995; Ekert 1979; Ekert 1979a; Emir 2013; Graham‐Pole 1986; Hirota 1993; Jaing 2004; Komada 1999; Kurucu 2012; Mabro 2000; Marshall 1989; Mehta 1986; Nagel 2008; Noguera 2001; Orchard 1999; Parker 2001; Safonova 1999; Sandoval 1999; Sepulveda‐Vildosola 2008; Shi 2012; Siddique 2011; Suarez 1994). Adverse events were reported in all except five studies (Kurucu 2012; Mehta 1986; Parker 2001; Shi 2012; Tsuchida 1999), and not separately reported for children in one study (Orchard 1999).
For three groups of studies, we undertook a pooled analysis. These were for the addition of dexamethasone to 5‐HT3 antagonists (Analysis 1.1), for different doses of granisetron (Analysis 2.1), and for ondansetron versus granisetron (Analysis 3.1 to Analysis 3.4). No other pooled analyses were possible (data for individual studies are presented in Analysis 4.1 to Analysis 4.5). The quality and quantity of included studies limited the exploration of heterogeneity.
1.1. Analysis.
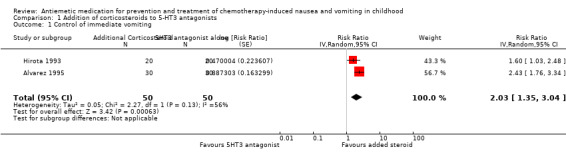
Comparison 1 Addition of corticosteroids to 5‐HT3 antagonists, Outcome 1 Control of immediate vomiting.
2.1. Analysis.
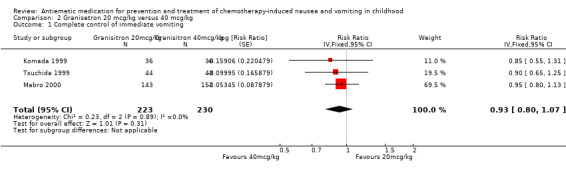
Comparison 2 Granisetron 20 mcg/kg versus 40 mcg/kg, Outcome 1 Complete control of immediate vomiting.
3.1. Analysis.
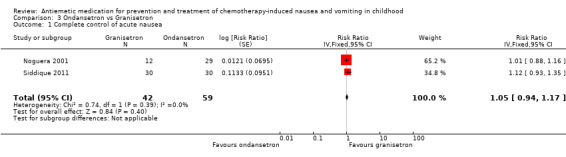
Comparison 3 Ondansetron vs Granisetron, Outcome 1 Complete control of acute nausea.
3.4. Analysis.
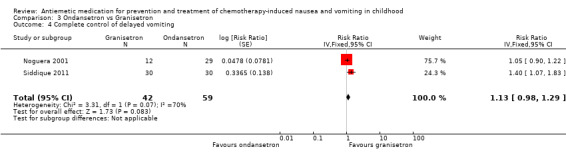
Comparison 3 Ondansetron vs Granisetron, Outcome 4 Complete control of delayed vomiting.
4.1. Analysis.
Comparison 4 Other antiemetic comparisons, Outcome 1 Complete control of acute nausea.
| Complete control of acute nausea | ||||||
|---|---|---|---|---|---|---|
| Study | Antiemetic 1 | Antiemetic 2 | Number receiving antiemetic 1 | Number receiving antiemetic 2 | Relative risk of complete control of acute nausea | (95% confidence interval) |
| Brock 1996 | Ondansetron 5 mg/m² IV over 15 minutes immediately prior to chemotherapy, then at +8 hours and +16. Ongoing ondansetron given orally < 1 m² 4 mg TDS, > 1 m² 8 mg TDS, continued for 3 days after last day of chemotherapy or 5 days if nausea and vomiting persisted | Ondansetron 10 mg/m² IV over 15 minutes immediately prior to chemotherapy, then 5 mg/m² IV at +8 hours and +16. Ongoing ondansetron given orally < 1 m² 4 mg TDS, > 1 m² 8 mg TDS, continued for 3 days after last day of chemotherapy or 5 days if nausea and vomiting persisted | 79 patients | 80 patients | 1.01 | 0.81 to 1.25 |
| Ekert 1979 | Tetrahydrocannabinol (THC) 10 mg/m², given at ‐2, 4, 8, 16 and 24 hours around chemotherapy administration | Metoclopramide 5 mg (for < 0.7 m² patients) or 10 mg (for > 0.7 m² patients) at ‐2, 8, 16 and 24 hours. Placebo given at +4 hours | 17 episodes | 25 episodes | 3.53 | 2.28 to 5.46 |
| Ekert 1979a | Tetrahydrocannabinol (THC) 10 mg/m2, given at ‐2, 4, 8, 16 and 24 hours around chemotherapy administration | Prochlorperazine ‐ tablets. Weight based schedule, using surface area (SA) 0.7 to 1.1 m² 20 mg/day divided, SA 1.1 to 1.4 m² 30 mg/day divided, SA > 1.1 m² 40 mg/day divided | 18 episodes | 18 episodes | 20.7 | 17.2 to 36.2 |
| Mehta 1986 | Methylprednisolone 4 mg/kg IV 30 minutes prior to chemo | Chlorpromazine 0.5 mg/kg IV 30 minutes prior to chemotherapy | 10 patients | 10 patients | 0.75 | 0.44 to 1.25 |
| Noguera 2001 | Ondansetron 0.45 mg/kg IV 15 minutes after chemotherapy | Tropisetron 0.20 mg/kg IV 15 minutes after chemotherapy | 29 episodes | 20 episodes | 0.97 | 0.54 to 1.69 |
| Sandoval 1999 | Ondansetron 0.6 mg/kg (max 32 mg) IV over 30 minutes before chemotherapy | Ondansetron 0.15 mg/kg (max 8 mg) IV over 30 minutes before chemotherapy then every 4 hours for a total of 4 doses | 16 patients | 15 patients | 1.25 | 0.70 to 2.22 |
| Suarez 1994 | Placebo, or tropisetron (0.05 mg/kg) given as single dose | Placebo, or tropisetron (0.1 to 0.4 mg/kg) given as single dose | 26 patients | 18 patients | 0.98 | 0.54 to 1.74 |
4.5. Analysis.
Comparison 4 Other antiemetic comparisons, Outcome 5 Other nausea &/or vomiting outcomes.
| Other nausea &/or vomiting outcomes | |||||||
|---|---|---|---|---|---|---|---|
| Study | Antiemetic 1 | Antiemetic 2 | Number receiving antiemetic 1 | Number receiving antiemetic 2 | Adverse outcome in patients/episodes receiving antiemetic 1 | Adverse outcome in patients/episodes receiving antiemetic 2 | Outcome details & notes |
| Dalzell 1986 | Nabilone 0.5 mg BD if < 18 kg, 1 mg BD if 18 to 36 kg, 1mg TDS if > 36 kg | Domperidone 5 mg TDS if < 18 kg, 10 mg TDS if 18 to 36 kg, 15 mg TDS if > 36 kg | 18 episodes | 18 episodes | 1.5 | 2.5 | Mean severity score (0 = nil, 3 = worst), P = 0.001 by Wilcoxon sign‐rank test |
| Graham‐Pole 1986 | Metoclopramide 0.5 mg/kg/dose IV | Chlorpromazine 0.5 mg/kg/dose IV | 24 patients | 26 patients | 3.5 (SD 4.1) | 1.8 (SD 2.3) | Mean number of vomits in first 24hrs |
| Hahlen 1995 | Granisetron 20 mcg/kg IV | Dexamethasone 2 mg/m²m2 IV plus Chlorpromazine 0.5 mg/kg IVI | 46 patients | 42 patients | 1.5 | 7 | Median number of vomits in first 24hrs, P = 0.001 by Wilcoxon sign‐rank test |
| Mehta 1986 | Methylprednisolone 4 mg/kg IV 30 minutes prior to chemotherapy | Chlorpromazine 0.5 mg/kg IV 30 minutes prior to chemotherapy | 10 patients | 10 patients | 10.9h (SD 3.3h) | 3.2h (SD 3.1h) | Mean duration of nausea |
| Nagel 2008 | Ondansetron (0.15 mg/kg) prior to anaethesia and intrathecal methotrexate administration | Placebo | 29 patients | 31 patients | 2 | 0.5 | Mean number of vomits (no SD given, p<0.001, graph extracted, in 24h post procedure) |
| Orchard 1999 | Granisetron: 10 mcg/kg/dose before start of chemotherapy/TBI then every 12 hours | Ondansetron: loading dose 0.15 mg/kg before start of chemotherapy/TBI then continuous infusion 0.03 mg/kg/h rounded to nearest 0.1 mg (until day of transplant ‐ day 0) | 23 patients | 28 patients | 0.82 (0.55 to 1.09) | 1.14 (0.90 to 1.38) | Mean nausea score (0 = nil, 5 = worst) (95% CI) |
| Orchard 1999 | Granisetron: 10 mcg/kg/dose before start of chemotherapy/TBI then every 12 hours | Ondansetron: loading dose 0.15 mg/kg before start of chemotherapy/TBI then continuous infusion 0.03 mg/kg/h rounded to nearest 0.1 mg (until day of transplant ‐ day 0) | 23 patients | 28 patients | 0.54 (0.27 to 0.81) | 0.87 (0.63 to 1.11) | Mean number of vomits in first 24 hours (95% CI) |
Excluded studies
We have included information on the 43 studies excluded at the detailed screening stage in the Characteristics of excluded studies table. The most common reason for exclusion was that information on participants less than 18 years old was unavailable. This was almost always a very small proportion of the study population.
Risk of bias in included studies
See the 'Risk of bias' section of the Characteristics of included studies table and Figure 3 for the exact scores and the support for the judgements made.
3.
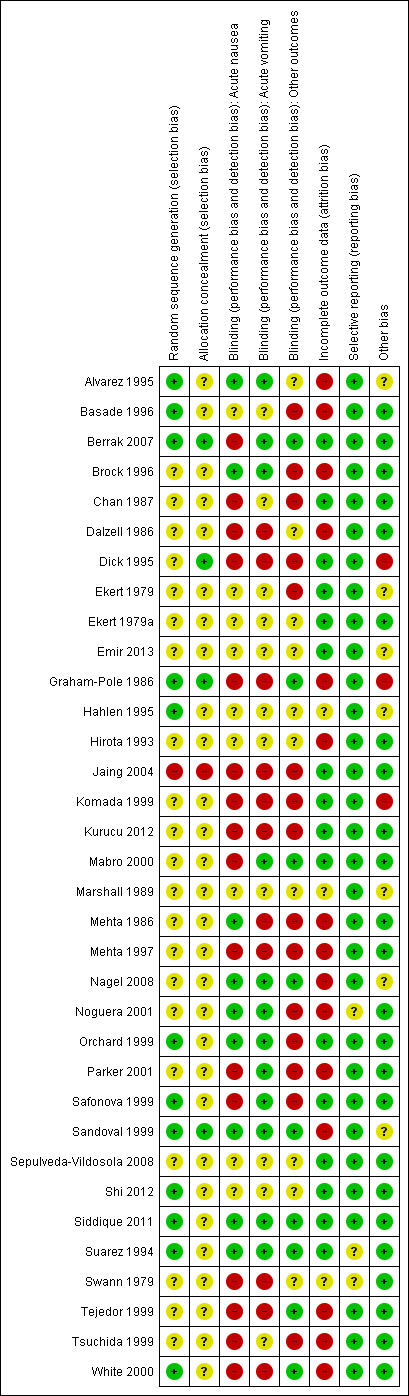
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was adequate, using computer‐generated number or random number tables, in 12 studies (Alvarez 1995; Basade 1996; Berrak 2007; Graham‐Pole 1986; Hahlen 1995; Orchard 1999; Safonova 1999; Sandoval 1999; Shi 2012; Siddique 2011; Suarez 1994; White 2000), inadequate in one (Jaing 2004), and not clearly reported in the remaining 21.
Allocation concealment was adequate in four studies (Berrak 2007; Dick 1995; Graham‐Pole 1986; Sandoval 1999), inadequate in one (Jaing 2004), and not clearly reported in the other 29.
Blinding
Blinding was reported to have been undertaken in 14 studies (Alvarez 1995; Berrak 2007; Brock 1996; Graham‐Pole 1986; Mabro 2000; Mehta 1986; Noguera 2001; Orchard 1999; Parker 2001; Safonova 1999; Sandoval 1999; Suarez 1994; Tejedor 1999; White 2000). It was unclear in 15 studies (Basade 1996; Chan 1987; Dalzell 1986; Ekert 1979; Ekert 1979a; Emir 2013; Hahlen 1995; Hirota 1993; Marshall 1989; Nagel 2008; Sepulveda‐Vildosola 2008; Shi 2012; Siddique 2011; Swann 1979; Tsuchida 1999), with four of these uncertain because of the obvious and frequent side effects of cannabinoids (Chan 1987; Dalzell 1986; Ekert 1979; Ekert 1979a). Five studies were reported as not blinded (Dick 1995; Jaing 2004; Komada 1999; Kurucu 2012; Mehta 1997). No study reported any assessment of the quality of blinding. We have provided details of the study roles masked to the intervention in each outcome of each study in the Characteristics of included studies table.
It was possible to attempt to assess the effect of blinding in Analysis 1.1, where studies with unclear blinding, as compared to studies where there was convincing blinding, were associated with a reduced estimate of the benefit of additional steroid to 5‐HT3 antagonists, in contrast to Analysis 2.1, where increasing certainty in blinding was associated with a smaller benefit of higher‐dose granisetron. These assessments were based on very small numbers of studies (Hirota 1993 and Alvarez 1995 in Analysis 1.1 and Komada 1999, Tsuchida 1999, and Mabro 2000 for Analysis 2.1) and alternative explanatory covariates, such as type of added steroid (in Analysis 1.1), the presence of paired data, and completeness of follow‐up should also be assessed. In Analysis 3.1 to Analysis 3.4, the only adequately blinded study, Noguera 2001, favoured granisetron for some outcomes and ondansetron for others.
Incomplete outcome data
In 16 studies (Berrak 2007; Chan 1987; Dick 1995; Ekert 1979; Ekert 1979a; Emir 2013; Jaing 2004; Komada 1999; Kurucu 2012; Mabro 2000; Orchard 1999; Safonova 1999; Sandoval 1999; Sepulveda‐Vildosola 2008; Shi 2012; Siddique 2011), there were no dropouts or failures to complete cross‐over. In three studies, uncertainty remained about the proportion of incomplete data (Hahlen 1995; Marshall 1989; Swann 1979). In the remaining 15 studies, the proportion of missing data ranged from 2.9%, in Basade 1996, to 50%, in Tejedor 1999.
No study obviously analysed participants according to the treatment they had received rather than that to which they had been randomised.
Selective reporting
We did not note selective reporting of particular outcomes within trials in this review, although in three studies there was inadequate detail to fully assess this (Noguera 2001; Suarez 1994; Swann 1979). It should be noted that this assessment was limited to the reporting of methods and results within the published paper, and in no cases referenced to a previously published protocol.
Other potential sources of bias
Two cross‐over studies provided paired data (Hirota 1993; Jaing 2004). The remaining cross‐over studies did not (Alvarez 1995; Basade 1996; Berrak 2007; Chan 1987; Dalzell 1986; Ekert 1979; Emir 2013; Mabro 2000; Marshall 1989; Nagel 2008; Parker 2001; Swann 1979; Tsuchida 1999), and were often unclear about when dropouts occurred, with the potential for unequal drop‐out influencing the results of these studies.
The approach of using cross‐over data in this review is supported by empirical evidence of a lack of 'cross‐over' effects from Tsuchida 1999 (treatment effect P = 0.18, period effect P = 0.76, carry‐over P = 0.38).
Given so few comparable studies (Peters 2008), we could not reasonably assess publication bias in this review. A relatively small number of as‐yet‐unidentified studies could alter the results of this review significantly. The lack of comparable studies also hampered any assessment of the effect of study funding source.
Effects of interventions
The detailed results of this review can be seen in the control of acute and delayed nausea (Analysis 4.1 and Analysis 4.2), acute and delayed emesis (Analysis 4.2 and Analysis 4.3), and combined outcomes (Analysis 4.4 and Analysis 4.5). As discussed previously, the majority of results in this review related to the control of acute emesis. Description of the effects of interventions separated by age group, tumour type, or chemotherapy received was not possible.
4.2. Analysis.
Comparison 4 Other antiemetic comparisons, Outcome 2 Complete control of acute vomiting.
| Complete control of acute vomiting | ||||||
|---|---|---|---|---|---|---|
| Study | Antiemetic 1 | Antiemetic 2 | Number assessed antiemetic 1 | Number assessed antiemetic 2 | Relative risk of complete control of acute vomiting | (95% confidence interval) |
| Alvarez 1995 | Ondansetron, IV, 0.15 mg/kg 30 minutes prior to chemotherapy, then BD on chemotherapy for 1 to 5 days plus dexamethasone either 4 mg/m² QDS or 8 mg/m² BD (depended on institution) | Ondansetron, IV, 0.15 mg/kg 30 minutes prior to chemotherapy, then BD on chemo for 1 to 5 days | 30 episodes | 30 episodes | 2.43 | 1.76 to 3.34 |
| Basade 1996 | Dexamethasone 8 mg/m² IV 15 minutes prior to chemotherapy | Metoclopramide 1.5 mg/kg IV 15 minutes prior to chemotherapy | 53 episodes | 52 episodes | 2.10 | 1.77 to 2.50 |
| Berrak 2007 | Granisetron 10 mcg/kg IV 30 minutes prior to Rx | Granisetron 40 mcg/kg IV 30 minutes prior to Rx | 104 episodes | 121 episodes | 0.88 | 0.70 to 1.10 |
| Brock 1996 | Ondansetron 5 mg/m² IV over 15 minutes immediately prior to chemotherapy, then at +8 hours and +16. Ongoing ondansetron given orally < 1 m² 4 mg TDS, > 1 m² 8 mg TDS, continued for 3 days after last day of chemotherapy or 5 days if nausea and vomiting persisted. | Ondansetron 10 mg/m² IV over 15 minutes immediately prior to chemotherapy, then 5 mg/m² IV at +8 hours and +16. Ongoing ondansetron given orally < 1 m² 4 mg TDS, > 1 m² 8 mg TDS, continued for 3 days after last day of chemotherapy or 5 days if nausea and vomiting persisted. | 79 patients | 79 patients | 0.89 | 0.72 to 1.10 |
| Chan 1987 | Nabilone orally (capsules) starting 8 to 12 hours prior to chemotherapy and repeated 2 or 3 times a day according to dosage schedule | Prochlorperazine orally (capsules) starting 8 to 12 hours prior to chemotherapy and repeated 2 or 3 times a day according to dosage schedule | 30 episodes | 30 episodes | 1.00 | 0.85 to 1.17 |
| Dick 1995 | Ondansetron 3 to 8 mg/m² given pre‐chemotherapy, then BD initially IV then orally for 3 days | Metoclopramide 10 mg/m² IV QDS for 3 days, with 2.5 mg procyclidine. Dexamethasone 4 mg/m² IV then 2 mg/m² TDS IV or PO | 15 patients | 15 patients | 3.67 | 2.25 to 5.98 |
| Ekert 1979 | Tetrahydrocannabinol (THC) 10 mg/m², given at ‐2, 4, 8, 16 and 24 hours around chemotherapy administration | Metoclopramide 5 mg (for < 0.7 m² patients) or 10 mg (for > 0.7 m² patients) at ‐2, 8, 16 and 24 hours. Placebo given at +4 hours | 17 episodes | 25 episodes | 3.53 | 2.28 to 5.46 |
| Ekert 1979a | Tetrahydrocannabinol (THC) 10 mg/m², given at ‐2, 4, 8, 16 and 24 hours around chemotherapy administration | Prochlorperazine ‐ tablets. Weight based schedule SA 0.7 to 1.1 m² 20 mg/day divided, SA 1.1 to 1.4 m² 30 mg/day divided, SA > 1.1 m² 40 mg/day divided | 18 episodes | 18 episodes | 19.00 | 13.71 to 26.33 |
| Hirota 1993 | Granisetron 40 mcg/kg IV 30 minutes prior to Rx plus methylprednisolone 10 mg/kg (max 500mg) IV | Granisetron 40 mcg/kg IV 30 minutes prior to Rx | 20 episodes | 20 episodes | 1.60 | 1.03 to 2.48 |
| Marshall 1989 | Cocktail. Metoclopramide 2 mg/kg/dose 0, 2, 6, 12 hours. Dex 0.7 mg/kg 0 hours. Benztropine 0.02 mg/kg/dose 0, 6 hours. Lorazepam 0.05 mg/kg/dose ‐1 hour and 12 hours (PO ‐ rest IV) | Chlorpromazine 0.825 mg/kg QDS IV for 4 doses | 26 episodes | 26 episodes | 2.40 | 1.76 to 3.27 |
| Mehta 1986 | Methylprednisolone 4 mg/kg IV 30 minutes prior to chemo | Chlorpromazine 0.5 mg/kg IV 30 minutes prior to chemotherapy | 10 patients | 10 patients | 1.00 | 0.54 to 1.86 |
| Parker 2001 | Placebo (saline) IV 15‐minute infusion 30 minutes prior to lumbar puncture | Low‐dose ondansetron 0.15 mg/kg IV 15‐minute infusion 30 minutes prior to lumbar puncture | 51 episodes | 47 episodes | 0.51 | 0.38 to 0.69 |
| Safonova 1999 | Ondansetron 8 mg PO BD plus 4 to 8 mg dexamethasone IV | Ondansetron 5 mg/m² IV BD plus 4 to 8 mg dexamethasone IV | 26 patients | 26 patients | 1.04 | 0.62 to 1.75 |
| Sandoval 1999 | Ondansetron 0.6 mg/kg (max 32 mg) IV over 30 minutes before chemotherapy. | Ondansetron 0.15 mg/kg (max 8 mg) IV over 30 minutes before chemotherapy then every 4 hours for a total of 4 doses | 16 patients | 15 patients | 0.94 | 0.49 to 1.79 |
| Suarez 1994 | Placebo, or tropisetron (0.05 mg/kg) given as single dose | Placebo, or tropisetron (0.1 to 0.4 mg/kg) given as single dose | 23 patients | 18 patients | 0.89 | 0.51 to 1.54 |
4.3. Analysis.
Comparison 4 Other antiemetic comparisons, Outcome 3 Complete control of delayed vomiting.
| Complete control of delayed vomiting | ||||||
|---|---|---|---|---|---|---|
| Study | Antiemetic 1 | Antiemetic 2 | Number receiving antiemetic 1 | Number receiving antiemetic 2 | Relative risk of complete control of delayed vomiting | (95% confidence interval) |
| Berrak 2007 | Granisetron 10 mcg/kg IV 30 minutes prior to Rx | Granisetron 40 mcg/kg IV 30 minutes prior to Rx | 18 episodes | 18 episodes | 1.07 | 0.94 to 1.21 |
4.4. Analysis.
Comparison 4 Other antiemetic comparisons, Outcome 4 Complete control of acute nausea and vomiting.
| Complete control of acute nausea and vomiting | ||||||
|---|---|---|---|---|---|---|
| Study | Antiemetic 1 | Antiemetic 2 | Number receiving antiemetic 1 | Number receiving antiemetic 2 | Relative risk of complete control of acute nausea & vomiting | (95% Confidence interval) |
| Dick 1995 | Ondansetron 3 to 8 mg/m² given pre‐chemotherapy, then BD initially IV then orally for 3 days | Metoclopramide 10 mg/m² IV QDS for 3 days, with 2.5 mg procyclidine Dexamethasone 4 mg/m² IV then 2 mg/m² TDS IV or PO | 15 patients | 15 patients | 3.67 | 2.25 to 5.98 |
| Mabro 2000 | 20 µg/kg oral granisetron (orange flavoured) given 1 hour before and again 6 to 12 hours | 40 µg/kg oral granisetron (orange flavoured) given 1 hour before and again 6 to 12 hours | 143 patients | 151 patients | 0.96 | 0.82 to 1.14 |
| Sandoval 1999 | Ondansetron 0.6 mg/kg (max 32 mg) IV over 30 minutes before chemotherapy. | Ondansetron 0.15 mg/kg (max 8 mg) IV over 30 minutes before chemotherapy then every 4 hours for a total of 4 doses | 16 patients | 15 patients | 1.25 | 0.70 to 2.23 |
| Tejedor 1999 | Tropisetron 0.2 mg/kg IV 30 minutes prior to infusion | Chlorpromazine 5 to 15 mg given over 2‐hour infusion and 2 further 6‐hourly plus dexamethasone 2 mg/m² IV bolus prior to and at12 hours | 44 episodes | 43 episodes | 1.03 | 0.79 to 1.35 |
Benzodiazepines
One study used a combination 'cocktail' of antiemetics that included benzodiazepines: lorazepam, dexamethasone, metoclopramide, and benztropine (Marshall 1989). A further study looked at another 'cocktail' including benzodiazepines: granisetron, dexamethasone, midazolam, and diphenhydramine (Emir 2013). These are reported in the 'Other agents' section below.
Cannabinoids
Four studies compared cannabinoids with alternative antiemetics (Chan 1987; Dalzell 1986; Ekert 1979; Ekert 1979a). They demonstrate markedly different results: the Ekert studies show benefit of tetrahydrocannabinol over prochlorperazine and metoclopramide in controlling nausea as well as vomiting. For example, for tetrahydrocannabinol versus prochlorperazine, complete control of acute nausea: risk ratio (RR) 20.7; 95% confidence interval (CI) 17.2 to 36.2, and for complete control of vomiting: RR 19.0; 95% CI 13.7 to 26.3. Dalzell 1986 showed an improvement with nabilone over domperidone in the reduction of nausea (nausea severity score 1.5 compared with 2.5, P = 0.01 (Wilcoxon signed‐rank) on scale of 0 (none) to 3 (worst)). Chan 1987, the largest and most recent study, demonstrated no benefit of tetrahydrocannabinol over prochlorperazine in the control of emesis (complete control of vomiting RR 1.0; 95% CI 0.85 to 1.17). The heterogeneity of studies meant no outcomes could be pooled.
Corticosteroids
Two studies examined steroids as a sole antiemetic agent. Basade 1996 looked at the comparative effectiveness of 8 mg/m² dexamethasone to 1.5 mg/kg metoclopramide, and found dexamethasone to be significantly better (complete control of vomiting: RR 2.10; 95% CI 1.77 to 2.50). Mehta 1986 compared 4 mg/kg of methylprednisolone to 0.5 mg/kg of chlorpromazine and found no evidence of a difference (complete control of vomiting: RR 1.0; 95% CI 0.54 to 1.86).
Two studies examined the use of additive steroids combined with 5‐HT3 antagonists (Alvarez 1995; Hirota 1993), which are pooled in Analysis 1.1 and demonstrate good benefit. There was moderate heterogeneity between these two studies (I² = 56%), with the methylprednisolone study, Hirota 1993, having a lower estimate of additional benefit compared with the added dexamethasone study, Alvarez 1995. The fixed‐effect and random‐effects models gave qualitatively similar results, with the expected increase in uncertainty with the random‐effects model and a relatively greater weighting given to the smaller study (fixed‐effect complete control of vomiting: RR 2.10; 95% CI 1.62 to 2.72, random‐effects complete control of vomiting: RR 2.03; 95% CI 1.35 to 3.04).
The use of steroids with non‐5‐HT3 antagonists compared with 5‐HT3 antagonists is reviewed below.
No trials directly compared different types of steroid, dosing, schedules, or routes of administration. Dexamethasone is the steroid most frequently studied.
5‐HT3 antagonists
Class
We found no direct comparisons of a single 5‐HT3 antagonists against single non‐5‐HT3 antagonists. Three studies examined the effectiveness of 5‐HT3 antagonists compared to concurrent dexamethasone and either metoclopramide, in Dick 1995, or chlorpromazine, in Hahlen 1995 and Tejedor 1999. Dick 1995 found ondansetron to be more effective than traditional antiemetics (complete control of nausea and vomiting: RR 3.67; 95% CI 2.25 to 5.98). Hahlen 1995 showed granisetron to be more effective (median number of vomiting episodes 1.5 on ondansetron, 7 on chlorpromazine/dexamethasone), but using a different outcome measure. Tejedor 1999 showed the chlorpromazine/dexamethasone combination to be equally effective (complete control of vomiting: RR 1.03; 95% CI 0.79 to 1.35). Nagel 2008 examined the use of ondansetron, fentanyl, or placebo (4‐way randomisation) after general anaesthesia and intrathecal methotrexate, and showed a reduction in the number of vomit/retch episodes with ondansetron (from 2 to 0.5 mean vomits in the 24 hours after the procedure, P < 0.001)
Drugs
Five studies undertook direct comparisons of different 5‐HT3 antagonists: Noguera 2001 examined ondansetron, granisetron, and tropisetron; Orchard 1999 compared granisetron and ondansetron (both with dexamethasone); Jaing 2004 compared different doses of granisetron and ondansetron (without dexamethasone); Siddique 2011 also compared ondansetron with granisetron; and Sepulveda‐Vildosola 2008 compared ondansetron with palonosetron.
The results of the three studies comparing ondansetron with granisetron are pooled in Analysis 3.1 to Analysis 3.4. We found no difference between the agents at preventing acute or delayed nausea or delayed vomiting, however granisetron was significantly better than ondansetron at preventing acute vomiting (RR 2.26; 95% CI 2.04 to 2.51). We could not include the results of Orchard 1999 due to marked differences in the way efficacy was reported.
Sepulveda‐Vildosola 2008 found a significant reduction in vomiting on days 1 to 3 (P = 0.010, 0.023, and 0.028, respectively) and nausea in days 1 to 4 (P = 0.001, 0.000, 0.000, and 0.002, respectively) in children given palonosetron rather than ondansetron.
For the effect of additive dexamethasone, see above.
Dose and schedule
Seven studies undertook dose comparisons of 5‐HT3 antagonists. Four studies compared granisetron doses. Berrak 2007 compared 10 mcg/kg with 40 mcg/kg. Their published results demonstrated no significant difference between the doses (complete control of acute vomiting: RR 0.88; 95% CI 0.70 to 1.10). The other three studies, Komada 1999, Mabro 2000, and Tsuchida 1999, compared granisetron 20 mcg/kg with 40 mcg/kg and were pooled in Analysis 2.1. This demonstrated no clear difference in the doses (complete control of vomiting: RR 0.93; 95% CI 0.80 to 1.07), with very little heterogeneity (I² = 0%).
Two studies compared ondansetron doses. Brock 1996 compared loading with 5 mg/m² and 10 mg/m², finding no advantage to the higher dose (complete control of vomiting: RR 0.89; 95% CI 0.72 to 1.10, complete control of nausea: RR 1.01; 95% CI 0.81 to 1.25). Sandoval 1999 examined the effect of giving a single high dose of 0.6 mg/kg ondansetron compared with dividing the dose into four 0.15 mg/kg aliquots over 16 hours and found no clear difference (complete control of vomiting: RR 0.94; 95% CI 0.49 to 1.79, complete control of nausea: RR 1.25; 95% CI 0.70 to 2.23).
Tropisetron doses are compared in a single dose‐finding study (Suarez 1994), which showed that doses of 0.1 mg/kg or greater were more effective than placebo in controlling acute nausea and vomiting.
Route
Only one uncompromised randomised study compared different routes of administration of 5‐HT3 antagonists. Safonova 1999 demonstrated that 8 mg oral ondansetron was equivalent to 5 mg/m² when combined with intravenous dexamethasone (complete control of vomiting: RR 1.04; 95% CI 0.62 to 1.75).
Other agents
Seven further studies examined the role of chlorpromazine (Graham‐Pole 1986; Marshall 1989), metoclopramide (Graham‐Pole 1986; Swann 1979), the combination 'cocktail' of lorazepam, dexamethasone, metoclopramide, and benztropine (LDMB) (Marshall 1989), the combination 'cocktail' of granisetron, dexamethasone, midazolam, and diphenhydramine (GDMD) (Emir 2013), hewei zhiou recipe (traditional Chinese herbal medicine) (Shi 2012), hydroxyzine (Kurucu 2012), and fentanyl (Nagel 2008).
Graham‐Pole 1986 demonstrated that chlorpromazine 0.5 mg/kg was more effective than a similar dose of metoclopramide (reported as mean number of vomits 1.8 (standard deviation (SD) 2.3) compared with 3.5 (SD 4.1) in 24 hours; duration of nausea reduced to 4.2 hours (SD 6.4 hours) compared with 9.0 hours (SD 9.7 hours). Swann 1979 showed domperidone (up to 1 mg/kg) was also more effective than metoclopramide 0.5 mg/kg (median number of vomits in 36 hours was 1, compared with 4; median duration of 0.5 hours versus 4.5 hours). Marshall 1989 showed an improvement for the LDMB cocktail over 0.325 mg/kg chlorpromazine (complete control of vomiting: RR 2.40; 95% CI 1.76 to 3.27). Emir 2013 showed slight superiority of the GDMD cocktail compared with ondansetron and dexamethasone in controlling acute vomiting, but this did not reach statistical significance. There was no difference between the GDMD cocktail compared with ondansetron and dexamethasone in controlling delayed vomiting. Shi 2012 showed less severe vomiting in participants given hewei zhiou recipe in addition to ondansetron compared to those given ondansetron alone (vomiting Z scores: ‐2.966, ‐3.256, ‐3.453, ‐4.870, ‐3.627 for treatment cycles 2 to 6, P < 0.01), although this effect was not seen in the first treatment cycle (vomiting Z score ‐0.470, P > 0.05). Kurucu 2012 found complete control of vomiting in 56% of participants given hydroxyzine in addition to ondansetron, compared to 22% of participants given ondansetron alone (P = 0.006). Nagel 2008 found no effect of adding fentanyl (0.1 mg/kg) to ondansetron (0.15 mg/kg) on nausea or vomiting after general anaesthesia and intrathecal methotrexate.
Adverse events
The reporting of adverse events varied markedly across different studies, making any pooling of the results inappropriate. The comparison of proportions across different studies and drugs as an indirect assessment of relative harms is unlikely to be valid, as different measures and methods of reporting were used. We have reported the results narratively below.
5‐HT3 antagonists
A wide range of adverse events were noted for 5‐HT3 antagonists. Those reported in more than one study included:
headache (10 studies; range from 2% in White 2000 to 53% in Mehta 1997);
sedation/somnolence (five studies; range from 2% in Mabro 2000 to 66% in Mehta 1997);
abdominal pain (six studies; range from 8% in White 2000 to 20% in Siddique 2011);
dizziness/vertigo (three studies; 1% in Brock 1996, 2% in Tejedor 1999, and 4% in Suarez 1994);
diarrhoea (four studies; 2% in White 2000, 4% in Suarez 1994, 17% in Alvarez 1995, and 20% in Siddique 2011);
constipation (four studies; proportion not noted in Jaing 2004, 5% in Tejedor 1999, 6% in Mabro 2000, 16.7% (ondansetron) and 13.3% (granisetron) in Siddique 2011);
fever (three studies; 3% in White 2000, 4% in Suarez 1994, and 17% in Hahlen 1995);
leg or muscle pains (two studies; 4% in Suarez 1994 and 6% in Dick 1995); and
hypertension (two studies; 2% in Tejedor 1999 and 4% in Suarez 1994).
Five studies reported that no adverse events occurred (Berrak 2007; Hirota 1993; Komada 1999; Safonova 1999; Sepulveda‐Vildosola 2008). No clear dose‐related or specific drug‐related effects were noted. For details see Table 1.
1. Adverse events ‐ 5‐HT3 antagonists.
| Citation | Adverse events noted |
| Alvarez 1995 | Not shown separately by intervention. Overall mild‐moderate sedation 49%, restlessness 29%, headache 17%, diarrhoea 17%, and hiccups 2% |
| Berrak 2007 | None reported in either group (granisetron 10 mcg/kg or 40 mcg/kg) |
| Brock 1996 | Ondansetron 5 mg/m²: 2 almost certainly related: headache and dizziness. 2 probably related: headache and warm feeling Ondansetron 10 mg/m²: 1 probably related: headache |
| Dick 1995 | Ondansetron: leg pains (1) |
| Hahlen 1995 | Granisetron:
|
| Hirota 1993 | None reported |
| Jaing 2004 | Numbers not specified. Adverse effects same in each group: mild headache and constipation |
| Komada 1999 | No side effects related to the study medication |
| Mabro 2000 | 20 µg/kg granisetron: headache 8 (6%), constipation 9 (6%), deranged liver enzymes 8 (6%) 40 µg/kg granisetron: headache 14 (9%), constipation 6 (4%), deranged liver enzymes 7 (5%) |
| Mehta 1997 | Ondansetron: headaches (8), mild to moderate sedation (10) |
| Noguera 2001 | Numbers not given or specified by antiemetic ‐ "rare" and "low intensity" |
| Orchard 1999 | Not reported separately for children |
| Safonova 1999 | None recorded |
| Sandoval 1999 | "No patients suffered clinical or laboratory toxicity" |
| Sepulveda‐Vildosola 2008 | "None of the patients reported or presented any adverse effect" |
| Siddique 2011 | "Adverse effects like headache, constipation, abdominal pain and loose motion were common in both group of children but their number was much less in children who received granisetron" [compared with ondansetron] |
| Suarez 1994 | Placebo or tropisetron 0.05 mg/kg: anxiety (9), headache (5), abdominal pain (4), sweating (2), and 1 each of hypertension, diarrhoea, fever, and fall Tropisetron 0.10 to 0.50 mg/kg: headache (3), tremor (3), rash (3), muscle pain (2), 1 each of vertigo and fatigue |
| Tejedor 1999 | Tropisetron: hypertension (1), abdominal pain (5), constipation (2), headache (2), headache and dizziness (1) |
| White 2000 | Granisetron 20 mcg/kg. Most commonly reported: abdominal/gastrointestinal discomfort and pain 4%, fever/pyrexia 3%, diarrhoea and headaches 2% Granisetron 40 mcg/kg. Most commonly reported: abdominal/gastrointestinal discomfort and pain 3%, fever/pyrexia 3%, diarrhoea and headaches 2% |
Cannabinoids
Of the three studies that examined cannabinoids, the main side effects noted were:
drowsiness (three studies; 80% in Chan 1987, 67% in Dalzell 1986, and 28% in Ekert 1979);
dizziness (two studies; 60% in Chan 1987 and 44% in Dalzell 1986);
mood alteration (three studies; 17% in Chan 1987 and Dalzell 1986 and 5% in Ekert 1979); and
increased appetite (two studies; 3% in Chan 1987 and 5% in Dalzell 1986).
Isolated reports included ocular problems, orthostatic hypotension, muscle twitching, pruritis, vagueness, hallucinations, lightheadedness, and dry mouth. For details see Table 2.
2. Adverse events ‐ cannabinoids.
| Citation | Adverse events noted |
| Chan 1987 | Nabilone orally: dizziness (18), drowsiness (24), mood alteration (5), ocular swelling and irritation (4), orthostatic hypotension (3), muscle twitching (2), increased appetite (1) |
| Dalzell 1986 | Nabilone: hallucinations requiring withdrawal from trial (1), drowsiness (12), dizziness (8), mood changes (3), pruritis (1), dry mouth (1), vagueness (1), light‐headedness (1), increased appetite (1) |
| Ekert 1979 | Tetrahydrocannabinol: drowsiness (4) |
| Ekert 1979a | Tetrahydrocannabinol: drowsiness (6), mood alteration (2) |
As no study compared different cannabinoids, any differences in side effects noted may be due to the study design or rigour of data collection rather than different drugs or dosing schedules used.
Steroids
The adverse effects of steroids as an antiemetic are difficult to assess in the studies undertaken. Only Basade 1996 reported the adverse effects of dexamethasone; any adverse effects additional to those from ondansetron were not reported in the study by Alvarez 1995, and no adverse events at all were noted in the studies of Hirota 1993 and Safonova 1999. Further studies using steroids are contaminated by the co‐administration of chlorpromazine (Hahlen 1995; Tejedor 1999), metoclopramide (Dick 1995), or as part of the LDMB cocktail (Marshall 1989). For details see Table 1 and Table 3.
3. Adverse events ‐ other agents.
| Citation | Adverse events noted |
| Basade 1996 | Dexamethasone: insomnia (1), depression (1), anorexia (1), abdominal pain (1) Metoclopramide: dystonia (1), depression (3), anorexia (4), abdominal pain (2), headache (1) |
| Chan 1987 | Prochlorperazine orally: dizziness (1), drowsiness (6), mood alteration (4), ocular swelling and irritation (1), muscle twitching (1) |
| Dalzell 1986 | Domperidone: drowsiness (6), dizziness (1), mood changes (1), pruritis (1) |
| Dick 1995 | Metoclopramide and dexamethasone: stomach aches (2), agitation/behaviour (2), tiredness (1) |
| Ekert 1979 | Metoclopramide: drowsiness (2) |
| Ekert 1979a | Prochlorperazine: none recorded |
| Emir 2013 | Cocktail: granisetron, dexamethasone, midazolam, and diphenhydramine: constipation (2), sedation (4), hypotension (2) |
| Graham‐Pole 1986 | Metoclopramide: extrapyramidal side effects (5/24), somnolence (2/24) Chlorpromazine: extrapyramidal side effects (1/26), somnolence (14/26) |
| Hahlen 1995 | Dexamethasone:
|
| Kurucu 2012 | Hydroxyzine: side effects in 14 participants in control (ondansetron) and 10 participants in (ondansetron plus) hydroxyzine group, consisting of constipation, headache, and dry mouth |
| Marshall 1989 | Chlorpromazine: diarrhoea (3) Cocktail. Metoclopramide, dexamethasone, benzatropine, and lorazepam: diarrhoea (2), dystonia (1), akasthasia (2) |
| Mehta 1986 | Chlorpromazine: sedation (7) Methylprednisolone: sedation (3) |
| Mehta 1997 | Perphenazine plus diphenhydramine: dystonia (2), mild to moderate sedation (24) |
| Swann 1979 | Metoclopramide or domperidone. Followed up at 3‐weekly intervals: no adverse effects, no unanticipated change in blood count, no deaths, no clinical evidence of jaundice |
| Tejedor 1999 | Chlorpromazine plus dexamethasone: abdominal pain (2), somnolence (2), headaches (7) |
Metoclopramide
Four studies that reported adverse events assessed metoclopramide. These included:
dystonia/extrapyramidal effects (two studies; 20% in Graham‐Pole 1986 and 2% in Basade 1996);
drowsiness (two studies; approximately 8% in Ekert 1979 and Graham‐Pole 1986).
Other effects were only noted in one of the studies, and included depression, anorexia, abdominal pain, and headache. One study (Swann 1979) reported no adverse events at all. For details see Table 2 and Table 3.
Chlorpromazine
Two studies assessed chlorpromazine and found diarrhoea (12% in Marshall 1989), extrapyramidal effects (4%) and drowsiness (52%) (in Graham‐Pole 1986). For details see Table 3.
Other agents
One study assessed midazolam and diphenhydramine and found constipation (6.5%), sedation (12.9%), and hypotension (6.5%) (Emir 2013).
Discussion
This update of our systematic review of antiemetic medication for prophylaxis and treatment of chemotherapy‐induced nausea and vomiting in children and young adults found only 34 randomised controlled trials, examining 28 different pairs of combinations of antiemetic medication approaches. This adds just three studies to the original review of 2010. The quality of individual studies remains moderate, with relatively small numbers and wide confidence intervals limiting our ability to draw conclusions. The lack of adequate numbers of studies undertaking similar comparisons limits any interpretation of the threats to randomisation that were identified. The outcomes reported were largely related to emesis, rather than the more patient‐relevant and often more distressing experience of nausea. Where nausea was reported, it was done without the use of validated symptom scales. Nausea, assessed through self report, is particularly difficult and complex to assess. Children, certainly the very young, may not have the language skills to describe their experience, or understand what they are being asked to describe, and this may in part explain the focus on vomiting. Direct comparisons of different agents and classes of agents were generally lacking: granisetron may help with acute vomiting (but not nausea, or delayed vomiting or nausea) more than ondansetron. A single study suggests palonesotron may be better than ondansetron. When we sought information to assess the risk‐benefit balance, by addressing reporting of adverse events, this was still extremely variable. Our search strategy was extensive and we attempted through contact with authors to extract any useful data from studies with primarily adult participants. We sought, found, and included trials published in the Japanese, Latin American, and non‐English language European literature in order to reduce the possibility of language‐related publication bias (Egger 1997; Juni 2002; Moher 2003).
Four years on from our original review, our overall picture of which are the most effective antiemetics to prevent chemotherapy‐induced nausea and vomiting in childhood remains incomplete and imprecise.
What can we conclude from such sparse data? We continue to propose tentative clinical implications and restate our firm research questions.
The practical, clinical conclusions from these trials are that 5‐HT3 antagonists seem more effective than older antiemetic agents, even when those agents are combined with a steroid. Of the 5‐HT3 receptor antagonists, granisetron may be more effective at higher doses, and granisetron and palonosetron may be more effective than ondansetron, though the small quantity of evidence cautions against firm conclusions. The addition of dexamethasone to the 5‐HT3 antagonist of choice doubles the chance of complete control of acute vomiting. The use of steroids as an antiemetic, despite evidence of efficacy, remains controversial. The lowest effective dose is unclear. Pre‐clinical studies suggest that glucocorticoids reduce sensitivity of a wide range of cell lines to chemotherapy agents (Meyer 2006; Zhang 2006). However, no clinical studies have found an association between steroids as an antiemetic and worsened outcomes. Cannabinoids are probably effective, but produce high levels of side effects, which may be experienced as adverse by some patients, but not by others. We cannot clearly define a route, schedule, or dose of maximal efficiency of any antiemetic medication from this review.
A further issue is the duration of the antiemesis, particularly with respect to multi‐day/multi‐agent chemotherapy (Einhorn 2005; Roila 2005). Although the available evidence deals with antiemetic therapy given alongside chemotherapy, the optimal duration of antiemesis following the last dose of chemotherapy is unclear. In various studies, antiemesis is reported as being given during chemotherapy (for example Alvarez 1995), or for up to two (for example White 2000) to three (for example Brock 1996) days following the last administration of emetogenic chemotherapy. There appear to be no randomised trials that address this issue, and in this respect the duration of chemotherapy antiemesis is unclear and should be a subject of further investigation, particularly by way of randomised controlled trials.
The research questions that emerge concern the need for good primary research: the conduct of new paediatric symptom control studies and the use of basic pharmacological and pharmacogenetic studies to support our understanding of the drugs' use. They also raise questions about the methods used in Cochrane systematic reviews, namely the need to explore alternative approaches in order to incorporate data from adult studies and also maximise the use of data from existing studies.
It is acknowledged that studies in children for drugs that have a feasible use in this age group are an ethical imperative, WHO 2007, and increasingly have a financial benefit, Sammons 2009. Such studies should use appropriate doses as assisted by both adult and pharmacokinetic studies, and patient‐important valid outcomes. In this setting, the complete control of nausea has been shown to be most important, with young people distinguishing between nausea and vomiting when selecting the five most important symptoms to feature on an electronic questionnaire (Gibson 2007). Nausea is consistently mentioned as a frequent distressing problem by children and young people, with vomiting described as relatively less distressing (Hedstrom 2003; Williams 2006), and to some vomiting may be a relief from nausea. Despite the availability of validated instruments to measure nausea in children (Dupuis 2006; Linder 2005), these did not feature in the papers reviewed. Where nausea was assessed, see for example Berrak 2007, no validated instrument was used. Rather, participants between one and 23 years old recorded a combined assessment of nausea, retching, and vomiting in a diary, which failed to reveal the intensity of nausea as distinct. Future research would benefit from the use of validated instruments that capture both patient and caregiver perceptions if we are really to understand the cancer care experience (Dodd 2001; Hinds 2008).
This review has very few trials from which to assess the effects of publication bias, or make firm conclusions. As such, it is relatively 'unstable', as a few further trials addressing one specific issue may tip the clinical conclusion in an alternative direction. The interventions for which meta‐analysis was possible have weaknesses. The meta‐analysis assessing the effectiveness of additional steroid has only two studies and moderate heterogeneity. The use of a random‐effects model assumes a difference (heterogeneity) in the underlying populations or interventions, and undertaking such an analysis with few (less than five) studies has been questioned (Higgins 2009). The heterogeneity may come from the use of different steroids, and this would be supported by the findings of Basade 1996 and Mehta 1986, whose studies, if read simplistically, show that dexamethasone is a better antiemetic than methylprednisolone. However, their comparator agents (metoclopramide and chlorpromazine, respectively) are not shown to be equal in the study by Graham‐Pole 1986. Analysis with simple binary meta‐analysis cannot hope to address these problems. The studies also have a different reported quality of blinding. In the meta‐analysis of different doses of granisetron (Analysis 2.1), there are three different strengths of certainty about the blinding applied to outcome measurement in the studies, and the results follow the expected pattern of having greater observed benefit for the unblinded study. This may mean that the estimate is an exaggeration. The meta‐analytic comparison of granisetron and ondansetron for control of acute vomiting only included three studies, and was driven very strongly by the smallest study, which showed different results than the other two.
Alternative approaches to maximising data from existing studies using network meta‐analysis in order to develop robust indirect comparisons remain a developing field of inquiry (Sutton 2008). Such network meta‐analyses have provided useful answers to unsolved questions in adult cardiology (Caldwell 2005), neurology (Wilby 2005), and mental health (Cipriani 2009). An extension of this technique, using adult studies as an explicit starting point from which to assess the studies in children, is worth examining to answer this question and to provide a clear and potential methodological base from which to examine other symptom control questions in paediatric oncology, but it has not yet been taken up and developed.
Our conclusions remain fundamentally unchanged from 2010: the key research questions that require new evidence should be decided in consultation with children, young people, and families that have undergone chemotherapy and been exposed to the medications and their effects. It has been acknowledged there may be a mismatch between available research evidence and the research preferences of consumers (Glass 2002; Tallon 2000), and across a range of services and clinical care, there is an expectation of increased and more meaningful consumer participation and involvement, including children (Coyne 2006; Darbyshire 2005). As clinicians, we suggest that three key points may be: to clarify further whether there are any patient‐important differences in the 5‐HT3 antagonists; to understand the most beneficial dose and duration of dexamethasone; and to clarify the role of new agents (for example substance P antagonists) in the prevention of chemotherapy‐induced nausea.
The prevention and treatment of nausea, and to a lesser extent vomiting, caused by chemotherapy in children and young people remains an important issue for their quality of life. Continued research into improving our understanding of and refining our therapies is required. Until then, the results of this review suggest that 5‐HT3 antagonists with dexamethasone added are effective in patients who are to receive highly emetogenic chemotherapy, although the exact risk‐benefit profile of the addition of steroid remains uncertain.
Authors' conclusions
Implications for practice.
Our overall picture of which antiemetics are the most effective in preventing chemotherapy‐induced nausea and vomiting in childhood remains incomplete and imprecise. With this caveat, we suggest that 5‐HT3 antagonists with dexamethasone added are effective in patients who are to receive highly emetogenic chemotherapy, although it remains uncertain how the proven benefit of steroid in reducing emesis balances with the in vitro reduction in chemotherapy sensitivity.
Implications for research.
Children and young people rate therapy‐related symptoms as the overall most difficult aspect of cancer treatment (Moody 2006; Woodgate 2003; Woodgate 2005). Traditionally, symptom management has emphasised medical management with administration of pharmacologic agents. More recently, a holistic approach to symptom management has been recommended, which includes both pharmacological and non‐pharmacological interventions (Rheingans 2008). We would recommend this approach to care and hence advocate that the key questions that require new evidence should be decided in consultation with children, young people, and families that have undergone chemotherapy and been exposed to the medications and their effects. Symptom experiences are a family affair, as symptoms are multifaceted and reciprocal, impacting the whole family and its overall quality of life (Woodgate 2003). We would recommend that any new research questions should take into consideration multidimensional approaches to the symptom experience of children and young people.
As clinicians, we suggest that three key points are still likely to be to:
clarify potential patient‐important differences between the 5‐HT3 antagonists;
understand the most beneficial dose and duration of dexamethasone; and
clarify the role of new agents (e.g. substance P antagonists) in the prevention of chemotherapy‐induced nausea.
We would recommend that any future research make use of validated, age‐appropriate measures. Additionally, future research would benefit from the use of validated instruments that capture both patient and caregiver perceptions if we are really to understand the cancer care experience (Hinds 2008).
The role of newer techniques of meta‐analysis in paediatrics, which incorporate Bayesian approaches, is as yet uncertain and is a pressing area for further methodological research that may produce significant clinical benefits.
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2015 | New citation required but conclusions have not changed | Summary of most important changes in the update: The search for eligible studies was updated to December 2014; we identified six new studies. They included comparisons of different 5‐HT3 antagonists, and the addition of further agents to 5‐HT3 antagonist 'backbone' antiemetic strategies (including traditional Chinese medicine, anxiolytics, and other antiemetics). These new studies did not meaningfully alter the conclusions of the 2010 review. |
| 2 January 2015 | New search has been performed | The search for eligible studies was updated to December 2014. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 18 January 2011 | Amended | Contact details updated. |
Acknowledgements
This work is undertaken under the direction of the Children's Cancer and Leukaemia Group/Paediatric Oncology Nursing Forum Supportive Care Group, but is the responsibility of the authors.
The authors would like to acknowledge the contributions of the translators (Prof David Reeves, Dr Moyacr Nobre, Tiago Fonseca, and Koji Kawahara) for their time and help; the staff at the Centre for Reviews and Dissemination (University of York, UK) for their advice and support; and the Trials Search Co‐ordinator of the Cochrane Childhood Cancer Group and the peer reviewers whose comments have improved this paper. Kate Light was a coauthor of the protocol and the review, and we thank her for her valuable input. We also wish to acknowledge the particular assistance of Dr Aksoylar, Dr Berrak, Dr Jaing, and Dr Orchard for their provision of extra data from their studies.
RSP was funded by a Clinical Research Fellowship from Candlelighters: The Yorkshire Children's Cancer Charity. The editorial base of the Cochrane Childhood Cancer Group is funded by Kinderen Kankervrij (KIKA).
Appendices
Appendix 1. Search strategies for medical databases
Search used in CENTRAL/The Cochrane Library
#1 MeSH descriptor Benzodiazepines explode all trees
#2 (Alprazolam or Alprazolan or alprox or esparon or apo‐alpraz or apoalpraz or cassadan or d‐65mt or d65mt or kalma or novo‐alprazol or novoalprazol or nu‐alpraz or nualpraz or ralozam or u‐31,889 or u31,889 or xanax or tafil or trankimazin or Niravam)
#3 (Anthramycin or antramycin)
#4 (Bromazepam or anxyrex or apo‐bromazepam or bromalich or bromaz or bromazanil or bromazep or lexotan or lexomil or lexotanil or lexatin or ro 5‐3350 or ro 53350 or durazanil or gen‐bromazepam)
#5 (Clonazepam or antelepsin or rivotril or ro 5‐4023 or ro 54023 or klonopin)
#6 (Devazepide or mk‐329 or mk329)
#7 (Diazepam or apaurin or diazemuls or faustan or relanium or seduxen or sibazon or stesolid or valium or rimapam or tensium or dialar or valclair or diastat or dizac or q‐pam or valrelease)
#8 (Nordazepam or demethyldiazepam or desmethyldiazepam or deoxydemoxepam or nordiazepam or norprazepam or dealkylprazepam or calmday or nordaz or tranxilium n or vegesan)
#9 (Flumazenil or flumazepil or romazicon or anexate or lanexat or ro 15‐1788 or ro 151788 or Anexate)
#10 (Lorazepam or apo‐lorazepam or apolorazepam or ativan or orfidal or temesta or donix or duralozam or durazolam or idalprem or laubeel or lorazep von ct or novo‐lorazem or novolorazem or nu‐loraz or nuloraz or sedicepan or sinestron or somagerol or tolid or wy‐4036 or wy4036 or loraz)
#11 (Flunitrazepam or fluridrazepam or flunitrazepam‐teva or flunizep von ct or ro‐5‐4200 or ro54200 or flunimerck or flunitrazepam‐neuraxpharm or flunitrazepam‐ratiopharm or fluninoc or rohypnol or narcozep or rohipnol or flunibeta)
#12 (Flurazepam or dalmane or Dormodor or dalmadorm or staurodorm or apo‐flurazepam)
#13 (Nitrazepam or Nitrodiazepam or alodorm or dormalon or dormo‐puren or eatan or imadorm or imeson or mogadon or nitrazadon or nitrazep or novanox or radedorm or remnos or rhoxal‐nitrazepam or serenade or somnite)
#14 (Oxazepam or adumbran or serax or tazepam)
#15 (Pirenzepine or pirenzepin or pyrenzepine or ulcoprotect or ulgescum or gastrotsepin or piren‐basan or pirenzepin‐ratiopharm or gastrozepin)
#16 (Prazepam or centrax or demetrin or lysanxia or reapam)
#17 (Temazepam or 3‐hydroxydiazepam or hydroxydiazepam or methyloxazepam or oxydiazepam or pronervon t or remestan or restoril or ro‐5‐5345 or ro55345 or sah 47‐603 or sah 47603 or apo‐temazepam or euhypnos or planum or levanxol or pms‐temazepam or nu‐temazepam or novo‐temazepam or nortem or normitab or normison or nocturne or temtabs or gen‐temazepam or dasuen or signopam or temaze or temazep von ct or tenox or wy‐3917 or wy3917 or temaz)
#18 (Chlordiazepoxide or methaminodiazepoxide or chlozepid or elenium or librium or a‐poxide or chlordiazachel or librelease or libritabs or lygen)
#19 (Chlorazepate or tranxene or tranxilium)
#20 (Estazolam or nuctalon or prosom or tasedan)
#21 (Medazepam or nobrium or ro 5‐4556 or ro 54556 or rudotel or rusedal)
#22 (Midazolam or dormicum or ro 21‐3981 or ro 213981 or versed or hypnovel)
#23 (Triazolam or apo‐triazo or gen‐triazolam or halcyon or halcion or trilam)
#24 MeSH descriptor Cannabinoids explode all trees
#25 (cannabinoid* or canabinoid* or Tetrahydrocannabinol or thc or marinol or nabilone or cesamet)
#26 (#25 OR #26)
#27 MeSH descriptor Beclomethasone, this term only
#28 (Beclomethasone or beclometasone or qvar or aerobec forte or beclazone or ecobec or filair or aerobec or nasobec aqueous or prolair or respocort or ventolair or vancenase or vanceril or aldecin or viarin or apo‐beclomethasone or ascocortonyl or beclamet or beclocort or beclomet or beclorhinol or becloturmant or sanasthmax or beclovent or beconase or propaderm or sanasthmyl or becodisks or becotide or becloforte or bronchocort or junik or asmabec clickhaler or beclazone or clenil modulate)
#29 MeSH descriptor Betamethasone, this term only
#30 (Betamethasone or betadexamethasone or flubenisolone or celeston or celestona or celestone or cellestoderm or Betnelan or betnesol)
#31 MeSH descriptor Betamethasone 17‐Valerate, this term only
#32 (betamethasone 17‐valerate or flubenisolonvalerate or betnovate or Beta‐val or betaderm or betatrex or dermabet or luxiq or valisone or valnac or betacap or betamethasone valerate or bettamousse or diprosone)
#33 MeSH descriptor Budesonide, this term only
#34 (Budesonide or horacort or pulmicort or rhinocort or novolizer or entocort)
#35 MeSH descriptor Dexamethasone, this term only
#36 (Dexamethasone or hexadecadrol or methylfluorprednisolone or dexpak or maxidex or decaject or decameth or decaspray or dexasone or hexadrol or millicorten or oradexon or aeroseb‐dex or decaderm dexamethasone or decadron or decadron or mymethasone)
#37 MeSH descriptor Dexamethasone Isonicotinate, this term only
#38 (dexamethasone isonicotinate or auxison)
#39 MeSH descriptor Flumethasone, this term only
#40 (Flumethasone or fluorodexamethasone or locorten)
#41 MeSH descriptor Fluorometholone, this term only
#42 (Fluorometholone or cortisdin or flucon or fluoro‐ophtal or fml or pms‐fluorometholone or fluoropos or oxylone)
#43 MeSH descriptor Fluprednisolone, this term only
#44 (fluprednisolone or alphadrol)
#45 MeSH descriptor Flurandrenolone, this term only
#46 (Flurandrenolone or flurandrenolide or cordran or haelan or fludroxycortide)
#47 MeSH descriptor Melengestrol Acetate, this term only
#48 (melengestrol acetate or melengestrol)
#49 MeSH descriptor Methylprednisolone, this term only
#50 (Methylprednisolone or metipred or medrol or urbason or medrone or solu‐medrone or depo‐medrone)
#51 MeSH descriptor Methylprednisolone Hemisuccinate, this term only
#52 (a‐methapred or solu‐medrol or solumedrol or urbason‐soluble or urbasonsoluble)
#53 MeSH descriptor Prednisolone, this term only
#54 (Prednisolone or di‐adreson‐f or diadresonf or predate or predonine or cortalone or delta‐cortef or fernisolone‐p or meti‐derm or prelone or sterane)
#55 MeSH descriptor Prednisone, this term only
#56 (Prednisone or dehydrocortisone or delta‐cortisone or prednison galen or prednison hexal or pronisone or rectodelt or apo‐prednisone or cortancyl or panafcort or dacortin or deltasone or prednidib or predni tablinen or panasol or orasone or meticorten or liquid pred or kortancyl or enkortolon or encortone or encorton or prednison acsis or predniment or decortisyl or cutason or cortan or winpred or ultracorten or sone or sterapred or delta‐dome or fernisone or paracort or predincen‐m or servisone)
#57 MeSH descriptor Cyclizine, this term only
#58 (Cyclizine or marezine or valoid)
#59 (#59 OR #60)
#60 MeSH descriptor Chlorpromazine, this term only
#61 (Chlorpromazine or propaphenin or aminazine or chlordelazine or contomin or largactil or fenactil or chlorazine or thorazine or thorazine)
#62 MeSH descriptor Domperidone, this term only
#63 (Domperidone or domperidon or apo‐domperidone or domidon or domperidon‐teva or gastrocure or motilium or nauzelin or novo‐domperidone or nu‐domperidone or pms‐domperidone or peridys or ratio‐domperidone)
#64 MeSH descriptor Droperidol, this term only
#65 (Droperidol or dehydrobenzperidol or droleptan or dehidrobenzperidol or inapsine or inapsine)
#66 MeSH descriptor Haloperidol, this term only
#67 (Haloperidol or haldol or dozic or serenace)
#68 MeSH descriptor Methotrimeprazine, this term only
#69 (Methotrimeprazine or levomeprazin or levopromazine or levomepromazine or tisercin or tizertsin or tizercine or levoprome or nozinan)
#70 MeSH descriptor Metoclopramide, this term only
#71 (Metoclopramide or metaclopramide or metaclopromide or cerucal or maxolon or primperan or raglan or rimetin)
#72 MeSH descriptor Perphenazine, this term only
#73 (Perphenazine or chlorpiprazine or perfenazine or trilafon or fentazin)
#74 MeSH descriptor Prochlorperazine, this term only
#75 (Prochlorperazine or compazine or stemetil or buccastem or compro)
#76 MeSH descriptor Trifluoperazine, this term only
#77 (Trifluoperazine or trifluoroperazine or trifluperazine or stelazine or triftazin or apo‐trifluoperazine or apotrifluoperazine or eskazine or flupazine or terfluzine)
#78 MeSH descriptor Granisetron, this term only
#79 MeSH descriptor Ondansetron, this term only
#80 (granisetron):ti or (granisetron):ab
#81 (odansetron or ondansetron):ti or (odansetron or ondansetron):ab
#82 (tropisetron):ti or (tropisetron):ab
#83 (dolasetron):ti or (dolasetron):ab
#84 (anzemet):ti or (anzemet):ab
#85 (kytril or Eutrom or Kevatril or Taraz):ti or (kytril or Eutrom or Kevatril or Taraz):ab
#86 (zofran or Zofrene or Zophran or Zophren or Avessa or Ceramos):ti or (zofran or Zofrene or Zophran or Zophren or Avessa or Ceramos):ab
#87 (5‐HT3 antagonist*):ti or (5‐HT3 antagonist*):ab
#88 (5‐HT3 blocker*):ti or (5‐HT3 blocker*):ab
#89 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 or #85 or #86 or #87 or #88
#90 (infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy)
#91 MeSH descriptor Nausea, this term only
#92 (nausea)
#93 MeSH descriptor Vomiting, this term only
#94 MeSH descriptor Vomiting, Anticipatory, this term only
#95 (vomit$)
#96 (emesis)
#97 (sickness)
#98 (#91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97)
#99 MeSH descriptor Neoplasms explode all trees
#100 (cancer$)
#101 (neoplas$)
#102 (oncolog$)
#103 (malignan$)
#104 (tumor$ or tumour$)
#105 (carcinoma$)
#106 (adenocarcinoma$)
#107 (sarcoma$)
#108 (leukemia or leukaemia)
#109 (chemotherap$)
#110 #99 or #100 or #101 or #102 or #103 or #104 or #105 or #106 or #107 or #108 or #109
#111 #89 and #90 and #98 and #110
[$= zero or many characters; ti=title; ab=abstract]
All databases were searched
Search used in MEDLINE/OVID and MEDLINE In‐process/OVID
1. exp Benzodiazepines/
2. (Alprazolam or Alprazolan or alprox or esparon or apo‐alpraz or apoalpraz or cassadan or d‐65mt or d65mt or kalma or novo‐alprazol or novoalprazol or nu‐alpraz or nualpraz or ralozam or u‐31,889 or u31,889 or xanax or tafil or trankimazin or Niravam).ti,ab.
3. (Anthramycin or antramycin).ti,ab.
4. (Bromazepam or anxyrex or apo‐bromazepam or bromalich or bromaz or bromazanil or bromazep or lexotan or lexomil or lexotanil or lexatin or ro 5‐3350 or ro 53350 or durazanil or gen‐bromazepam).ti,ab.
5. (Clonazepam or antelepsin or rivotril or ro 5‐4023 or ro 54023 or klonopin).ti,ab.
6. (Devazepide or mk‐329 or mk329).ti,ab.
7. (Diazepam or apaurin or diazemuls or faustan or relanium or seduxen or sibazon or stesolid or valium or rimapam or tensium or dialar or valclair or diastat or dizac or q‐pam or valrelease).ti,ab.
8. (Nordazepam or demethyldiazepam or desmethyldiazepam or deoxydemoxepam or nordiazepam or norprazepam or dealkylprazepam or calmday or nordaz or tranxilium n or vegesan).ti,ab.
9. (Flumazenil or flumazepil or romazicon or anexate or lanexat or ro 15‐1788 or ro 151788 or Anexate).ti,ab.
10. (Lorazepam or apo‐lorazepam or apolorazepam or ativan or orfidal or temesta or donix or duralozam or durazolam or idalprem or laubeel or lorazep von ct or novo‐lorazem or novolorazem or nu‐loraz or nuloraz or sedicepan or sinestron or somagerol or tolid or wy‐4036 or wy4036 or loraz).ti,ab.
11. (Flunitrazepam or fluridrazepam or flunitrazepam‐teva or flunizep von ct or ro‐5‐4200 or ro54200 or flunimerck or flunitrazepam‐neuraxpharm or flunitrazepam‐ratiopharm or fluninoc or rohypnol or narcozep or rohipnol or flunibeta).ti,ab.
12. (Flurazepam or dalmane or Dormodor or dalmadorm or staurodorm or apo‐flurazepam).ti,ab.
13. (Nitrazepam or Nitrodiazepam or alodorm or dormalon or dormo‐puren or eatan or imadorm or imeson or mogadon or nitrazadon or nitrazep or novanox or radedorm or remnos or rhoxal‐nitrazepam or serenade or somnite).ti,ab.
14. (Oxazepam or adumbran or serax or tazepam).ti,ab.
15. (Pirenzepine or pirenzepin or pyrenzepine or ulcoprotect or ulgescum or gastrotsepin or piren‐basan or pirenzepin‐ratiopharm or gastrozepin).ti,ab.
16. (Prazepam or centrax or demetrin or lysanxia or reapam).ti,ab.
17. (Temazepam or 3‐hydroxydiazepam or hydroxydiazepam or methyloxazepam or oxydiazepam or pronervon t or remestan or restoril or ro‐5‐5345 or ro55345 or sah 47‐603 or sah 47603 or apo‐temazepam or euhypnos or planum or levanxol or pms‐temazepam or nu‐temazepam or novo‐temazepam or nortem or normitab or normison or nocturne or temtabs or gen‐temazepam or dasuen or signopam or temaze or temazep von ct or tenox or wy‐3917 or wy3917 or temaz).ti,ab.
18. (Chlordiazepoxide or methaminodiazepoxide or chlozepid or elenium or librium or a‐poxide or chlordiazachel or librelease or libritabs or lygen).ti,ab.
19. (Chlorazepate or tranxene or tranxilium).ti,ab.
20. (Estazolam or nuctalon or prosom or tasedan).ti,ab.
21. (Medazepam or nobrium or ro 5‐4556 or ro 54556 or rudotel or rusedal).ti,ab. (216)
22. (Midazolam or dormicum or ro 21‐3981 or ro 213981 or versed or hypnovel).ti,ab.
23. (Triazolam or apo‐triazo or gen‐triazolam or halcyon or halcion or trilam).ti,ab.
24. or/1‐23
25. exp Cannabinoids/
26. (cannabinoid$ or canabinoid$ or Tetrahydrocannabinol or thc or marinol or nabilone or cesamet).ti,ab.
27. 25 or 26
28. Beclomethasone/
29. (Beclomethasone or beclometasone or qvar or aerobec forte or beclazone or ecobec or filair or aerobec or nasobec aqueous or prolair or respocort or ventolair or vancenase or vanceril or aldecin or viarin or apo‐beclomethasone or ascocortonyl or beclamet or beclocort or beclomet or beclorhinol or becloturmant or sanasthmax or beclovent or beconase or propaderm or sanasthmyl or becodisks or becotide or becloforte or bronchocort or junik or asmabec clickhaler or beclazone or clenil modulate).ti,ab.
30. Betamethasone/
31. (Betamethasone or betadexamethasone or flubenisolone or celeston or celestona or celestone or cellestoderm or Betnelan or betnesol).ti,ab.
32. betamethasone 17‐valerate/
33. (betamethasone 17‐valerate or flubenisolonvalerate or betnovate or Beta‐val or betaderm or betatrex or dermabet or luxiq or valisone or valnac or betacap or betamethasone valerate or bettamousse or diprosone).ti,ab.
34. Budesonide/
35. (Budesonide or horacort or pulmicort or rhinocort or novolizer or entocort).ti,ab.
36. Dexamethasone/
37. (Dexamethasone or hexadecadrol or methylfluorprednisolone or dexpak or maxidex or decaject or decameth or decaspray or dexasone or hexadrol or millicorten or oradexon or aeroseb‐dex or decaderm dexamethasone or decadron or decadron or mymethasone).ti,ab.
38. dexamethasone isonicotinate/
39. (dexamethasone isonicotinate or auxison).ti,ab.
40. flumethasone/
41. (Flumethasone or fluorodexamethasone or locorten).ti,ab.
42. Fluorometholone/
43. (Fluorometholone or cortisdin or flucon or fluoro‐ophtal or fml or pms‐fluorometholone or fluoropos or oxylone).ti,ab.
44. fluprednisolone/
45. (fluprednisolone or alphadrol).ti,ab.
46. Flurandrenolone/
47. (Flurandrenolone or flurandrenolide or cordran or haelan or fludroxycortide).ti,ab.
48. melengestrol acetate/
49. (melengestrol acetate or melengestrol).ti,ab.
50. Methylprednisolone/
51. (Methylprednisolone or metipred or medrol or urbason or medrone or solu‐medrone or depo‐medrone).ti,ab.
52. methylprednisolone hemisuccinate/
53. (a‐methapred or solu‐medrol or solumedrol or urbason‐soluble or urbasonsoluble).ti,ab.
54. Prednisolone/
55. (Prednisolone or di‐adreson‐f or diadresonf or predate or predonine or cortalone or delta‐cortef or fernisolone‐p or meti‐derm or prelone or sterane).ti,ab.
56. Prednisone/
57. (Prednisone or dehydrocortisone or delta‐cortisone or prednison galen or prednison hexal or pronisone or rectodelt or apo‐prednisone or cortancyl or panafcort or dacortin or deltasone or prednidib or predni tablinen or panasol or orasone or meticorten or liquid pred or kortancyl or enkortolon or encortone or encorton or prednison acsis or predniment or decortisyl or cutason or cortan or winpred or ultracorten or sone or sterapred or delta‐dome or fernisone or paracort or predincen‐m or servisone).ti,ab.
58. or/28‐57
59. Cyclizine/
60. (Cyclizine or marezine or valoid).ti,ab.
61. 59 or 60
62. Chlorpromazine/
63. (Chlorpromazine or propaphenin or aminazine or chlordelazine or contomin or largactil or fenactil or chlorazine or thorazine or thorazine).ti,ab. (10015)
64. Domperidone/
65. (Domperidone or domperidon or apo‐domperidone or domidon or domperidon‐teva or gastrocure or motilium or nauzelin or novo‐domperidone or nu‐domperidone or pms‐domperidone or peridys or ratio‐domperidone).ti,ab.
66. Droperidol/
67. (Droperidol or dehydrobenzperidol or droleptan or dehidrobenzperidol or inapsine or inapsine).ti,ab.
68. Haloperidol/
69. (Haloperidol or haldol or dozic or serenace).ti,ab.
70. Methotrimeprazine/
71. (Methotrimeprazine or levomeprazin or levopromazine or levomepromazine or tisercin or tizertsin or tizercine or levoprome or nozinan).ti,ab.
72. Metoclopramide/
73. (Metoclopramide or metaclopramide or metaclopromide or cerucal or maxolon or primperan or raglan or rimetin).ti,ab.
74. Perphenazine/
75. (Perphenazine or chlorpiprazine or perfenazine or trilafon or fentazin).ti,ab.
76. Prochlorperazine/
77. (Prochlorperazine or compazine or stemetil or buccastem or compro).ti,ab.
78. Trifluoperazine/
79. (Trifluoperazine or trifluoroperazine or trifluperazine or stelazine or triftazin or apo‐trifluoperazine or apotrifluoperazine or eskazine or flupazine or terfluzine).ti,ab.
80. or/62‐79
81. granisetron/ or odansetron/
82. granisetron.ti,ab.
83. (odansetron or ondansetron).ti,ab.
84. tropisetron.ti,ab.
85. dolasetron.ti,ab.
86. anzemet.ti,ab.
87. kytril.ti,ab.
88. zofran.ti,ab.
89. 5‐HT3 antagonist$.ti,ab.
90. 5‐HT3 blocker$.ti,ab.
91. or/81‐90
92. 24 or 27 or 58 or 61 or 80 or 91
93. adolescent/ or child/ or child, preschool/ or infant/ or infant, newborn/
94. schools/ or schools, nursery/
95. (infant or infan$ or newborn or newborn$ or new‐born$ or baby or baby$ or babies or neonat$ or child or child$ or schoolchild$ or schoolchild or school child or school child$ or kid or kids or toddler$ or adolescent or adoles$ or teen$ or boy$ or girl$ or minors or minors$ or underag$ or under ag$ or juvenil$ or youth$ or kindergar$ or puberty or puber$ or pubescen$ or prepubescen$ or prepuberty$ or pediatrics or pediatric$ or paediatric$ or peadiatric$ or schools or nursery school$ or preschool$ or pre school$ or primary school$ or secondary school$ or elementary school$ or elementary school or high school$ or highschool$ or school age or schoolage or school age$ or schoolage$ or infancy or schools, nursery or infant, newborn).ti,ab.
96. 93 or 94 or 95
97. Nausea/
98. nausea.ti,ab.
99. vomiting/ or vomiting, anticipatory/
100. vomit$.ti,ab.
101. emesis.ti,ab.
102. sickness.ti,ab.
103. or/97‐102
104. clinical trial.pt.
105. randomized.ab.
106. placebo.ab.
107. randomly.ab.
108. trial.ab.
109. groups.ab.
110. 104 or 105 or 106 or 107 or 108 or 109
111. exp neoplasms/
112. cancer$.ti,ab.
113. neoplas$.ti,ab.
114. oncolog$.ti,ab.
115. malignan$.ti,ab.
116. tumo?r$.ti,ab.
117. carcinoma$.ti,ab.
118. adenocarcinoma$.ti,ab.
119. sarcoma$.ti,ab.
120. leuk?mia.ti,ab.
121. chemotherap$.ti,ab.
122. or/111‐121
123. 92 and 96 and 103 and 110 and 122
[$=zero or many characters; ti,ab=title or abstract; mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer or drug manufacturer name; /= MeSH term; ab=abstract]
Search used in EMBASE/OVID
1. Benzodiazepine/ or Alprazolam/ or Anthramycin/ or Bromazepam/ or Clonazepam/ or Devazepide/ or Diazepam/ or Nordazepam/ or Flumazenil/ or Lorazepam/ or Flunitrazepam/ or Flurazepam/ or Nitrazepam/ or Oxazepam/ or Pirenzepine/ or Prazepam/ or Temazepam/ or Chlordiazepoxide/ or Clorazepate/ or Estazolam/ or Medazepam/ or Midazolam/ or Triazolam/
2. (Alprazolam or Alprazolan or alprox or esparon or apo‐alpraz or apoalpraz or cassadan or d‐65mt or d65mt or kalma or novo‐alprazol or novoalprazol or nu‐alpraz or nualpraz or ralozam or u‐31,889 or u31,889 or xanax or tafil or trankimazin or Niravam).ti,ab.
3. (Anthramycin or antramycin).ti,ab.
4. (Bromazepam or anxyrex or apo‐bromazepam or bromalich or bromaz or bromazanil or bromazep or lexotan or lexomil or lexotanil or lexatin or ro 5‐3350 or ro 53350 or durazanil or gen‐bromazepam).ti,ab.
5. (Clonazepam or antelepsin or rivotril or ro 5‐4023 or ro 54023 or klonopin).ti,ab.
6. (Devazepide or mk‐329 or mk329).ti,ab.
7. (Diazepam or apaurin or diazemuls or faustan or relanium or seduxen or sibazon or stesolid or valium or rimapam or tensium or dialar or valclair or diastat or dizac or q‐pam or valrelease).ti,ab.
8. (Nordazepam or demethyldiazepam or desmethyldiazepam or deoxydemoxepam or nordiazepam or norprazepam or dealkylprazepam or calmday or nordaz or tranxilium n or vegesan).ti,ab.
9. (Flumazenil or flumazepil or romazicon or anexate or lanexat or ro 15‐1788 or ro 151788 or Anexate).ti,ab.
10. (Lorazepam or apo‐lorazepam or apolorazepam or ativan or orfidal or temesta or donix or duralozam or durazolam or idalprem or laubeel or lorazep von ct or novo‐lorazem or novolorazem or nu‐loraz or nuloraz or sedicepan or sinestron or somagerol or tolid or wy‐4036 or wy4036 or loraz).ti,ab.
11. (Flunitrazepam or fluridrazepam or flunitrazepam‐teva or flunizep von ct or ro‐5‐4200 or ro54200 or flunimerck or flunitrazepam‐neuraxpharm or flunitrazepam‐ratiopharm or fluninoc or rohypnol or narcozep or rohipnol or flunibeta).ti,ab.
12. (Flurazepam or dalmane or Dormodor or dalmadorm or staurodorm or apo‐flurazepam).ti,ab.
13. (Nitrazepam or Nitrodiazepam or alodorm or dormalon or dormo‐puren or eatan or imadorm or imeson or mogadon or nitrazadon or nitrazep or novanox or radedorm or remnos or rhoxal‐nitrazepam or serenade or somnite).ti,ab.
14. (Oxazepam or adumbran or serax or tazepam).ti,ab.
15. (Pirenzepine or pirenzepin or pyrenzepine or ulcoprotect or ulgescum or gastrotsepin or piren‐basan or pirenzepin‐ratiopharm or gastrozepin).ti,ab.
16. (Prazepam or centrax or demetrin or lysanxia or reapam).ti,ab.
17. (Temazepam or 3‐hydroxydiazepam or hydroxydiazepam or methyloxazepam or oxydiazepam or pronervon t or remestan or restoril or ro‐5‐5345 or ro55345 or sah 47‐603 or sah 47603 or apo‐temazepam or euhypnos or planum or levanxol or pms‐temazepam or nu‐temazepam or novo‐temazepam or nortem or normitab or normison or nocturne or temtabs or gen‐temazepam or dasuen or signopam or temaze or temazep von ct or tenox or wy‐3917 or wy3917 or temaz).ti,ab.
18. (Chlordiazepoxide or methaminodiazepoxide or chlozepid or elenium or librium or a‐poxide or chlordiazachel or librelease or libritabs or lygen).ti,ab.
19. (Chlorazepate or tranxene or tranxilium).ti,ab.
20. (Estazolam or nuctalon or prosom or tasedan).ti,ab.
21. (Medazepam or nobrium or ro 5‐4556 or ro 54556 or rudotel or rusedal).ti,ab.
22. (Midazolam or dormicum or ro 21‐3981 or ro 213981 or versed or hypnovel).ti,ab.
23. (Triazolam or apo‐triazo or gen‐triazolam or halcyon or halcion or trilam).ti,ab.
24. or/1‐23
25. Cannabinoid/ or Tetrahydrocannabinol/ or Cannabinol/ or Cannabidiol/
26. (cannabinoid$ or canabinoid$ or Tetrahydrocannabinol or thc or marinol or nabilone or cesamet).ti,ab.
27. 25 or 26
28. Beclometasone/
29. (Beclomethasone or beclometasone or qvar or aerobec forte or beclazone or ecobec or filair or aerobec or nasobec aqueous or prolair or respocort or ventolair or vancenase or vanceril or aldecin or viarin or apo‐beclomethasone or ascocortonyl or beclamet or beclocort or beclomet or beclorhinol or becloturmant or sanasthmax or beclovent or beconase or propaderm or sanasthmyl or becodisks or becotide or becloforte or bronchocort or junik or asmabec clickhaler or beclazone or clenil modulate).ti,ab.
30. Betamethasone/
31. (Betamethasone or betadexamethasone or flubenisolone or celeston or celestona or celestone or cellestoderm or Betnelan or betnesol).ti,ab.
32. Betamethasone Valerate/
33. (betamethasone 17‐valerate or flubenisolonvalerate or betnovate or Beta‐val or betaderm or betatrex or dermabet or luxiq or valisone or valnac or betacap or betamethasone valerate or bettamousse or diprosone).ti,ab.
34. Budesonide/
35. (Budesonide or horacort or pulmicort or rhinocort or novolizer or entocort).ti,ab.
36. Dexamethasone/
37. (Dexamethasone or hexadecadrol or methylfluorprednisolone or dexpak or maxidex or decaject or decameth or decaspray or dexasone or hexadrol or millicorten or oradexon or aeroseb‐dex or decaderm dexamethasone or decadron or decadron or mymethasone).ti,ab.
38. Dexamethasone Isonicotinate/
39. (dexamethasone isonicotinate or auxison).ti,ab.
40. Flumetasone/
41. (Flumethasone or fluorodexamethasone or locorten).ti,ab.
42. Fluorometholone/
43. (Fluorometholone or cortisdin or flucon or fluoro‐ophtal or fml or pms‐fluorometholone or fluoropos or oxylone).ti,ab.
44. fluprednisolone/
45. (fluprednisolone or alphadrol).ti,ab.
46. Fludroxycortide/
47. (Flurandrenolone or flurandrenolide or cordran or haelan or fludroxycortide).ti,ab.
48. melengestrol acetate/
49. (melengestrol acetate or melengestrol).ti,ab.
50. Methylprednisolone/
51. (Methylprednisolone or metipred or medrol or urbason or medrone or solu‐medrone or depo‐medrone).ti,ab.
52. Methylprednisolone Sodium Succinate/
53. (a‐methapred or solu‐medrol or solumedrol or urbason‐soluble or urbasonsoluble).ti,ab.
54. Prednisolone/
55. (Prednisolone or di‐adreson‐f or diadresonf or predate or predonine or cortalone or delta‐cortef or fernisolone‐p or meti‐derm or prelone or sterane).ti,ab.
56. Prednisone/
57. (Prednisone or dehydrocortisone or delta‐cortisone or prednison galen or prednison hexal or pronisone or rectodelt or apo‐prednisone or cortancyl or panafcort or dacortin or deltasone or prednidib or predni tablinen or panasol or orasone or meticorten or liquid pred or kortancyl or enkortolon or encortone or encorton or prednison acsis or predniment or decortisyl or cutason or cortan or winpred or ultracorten or sone or sterapred or delta‐dome or fernisone or paracort or predincen‐m or servisone).ti,ab.
58. or/28‐57
59. Cyclizine/
60. (Cyclizine or marezine or valoid).ti,ab.
61. 59 or 60
62. Chlorpromazine/
63. (Chlorpromazine or propaphenin or aminazine or chlordelazine or contomin or largactil or fenactil or chlorazine or thorazine or thorazine).ti,ab.
64. Domperidone/
65. (Domperidone or domperidon or apo‐domperidone or domidon or domperidon‐teva or gastrocure or motilium or nauzelin or novo‐domperidone or nu‐domperidone or pms‐domperidone or peridys or ratio‐domperidone).ti,ab.
66. Droperidol/
67. (Droperidol or dehydrobenzperidol or droleptan or dehidrobenzperidol or inapsine or inapsine).ti,ab.
68. Haloperidol/
69. (Haloperidol or haldol or dozic or serenace).ti,ab.
70. Levomepromazine/
71. (Methotrimeprazine or levomeprazin or levopromazine or levomepromazine or tisercin or tizertsin or tizercine or levoprome or nozinan).ti,ab.
72. Metoclopramide/
73. (Metoclopramide or metaclopramide or metaclopromide or cerucal or maxolon or primperan or raglan or rimetin).ti,ab.
74. Perphenazine/
75. (Perphenazine or chlorpiprazine or perfenazine or trilafon or fentazin).ti,ab.
76. Prochlorperazine/
77. (Prochlorperazine or compazine or stemetil or buccastem or compro).ti,ab.
78. Trifluoperazine/
79. (Trifluoperazine or trifluoroperazine or trifluperazine or stelazine or triftazin or apo‐trifluoperazine or apotrifluoperazine or eskazine or flupazine or terfluzine).ti,ab.
80. or/62‐79
81. granisetron/ or ondansetron/
82. granisetron.ti,ab.
83. (odansetron or ondansetron).ti,ab.
84. tropisetron.ti,ab.
85. dolasetron.ti,ab.
86. anzemet.ti,ab.
87. (kytril or Eutrom or Kevatril or Taraz).ti,ab.
88. (zofran or Zofrene or Zophran or Zophren or Avessa or Ceramos).ti,ab.
89. 5‐HT3 antagonist$.ti,ab.
90. 5‐HT3 blocker$.ti,ab.
91. or/81‐90
92. 24 or 27 or 58 or 61 or 80 or 91
93. NAUSEA/ or "ANTICIPATORY NAUSEA AND VOMITING"/ or "NAUSEA AND VOMITING"/
94. nausea.ti,ab.
95. Vomiting/ or Chemotherapy Induced Emesis/
96. vomit$.ti,ab.
97. emesis.ti,ab.
98. sickness.ti,ab.
99. or/93‐98
100. randomized.ab.
101. placebo.ab.
102. randomly.ab.
103. trial.ab.
104. groups.ab.
105. or/100‐104
106. exp neoplasms/
107. cancer$.ti,ab.
108. neoplas$.ti,ab.
109. oncolog$.ti,ab.
110. malignan$.ti,ab.
111. tumo?r$.ti,ab.
112. carcinoma$.ti,ab.
113. adenocarcinoma$.ti,ab.
114. sarcoma$.ti,ab.
115. leuk?emia.ti,ab.
116. chemotherap$.ti,ab.
117. or/106‐116
118. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/
119. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/
120. primary school/ or high school/ or kindergarten/ or nursery school/ or school/
121. (infant$ or newborn$ or new born$ or baby or baby$ or babies or neonate$).mp.
122. (child$ or school child$ or schoolchild$ or school age$ or schoolage$ or pre school$ or preschool$).mp.
123. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp.
124. (minors$ or under ag$ or underage$ or juvenil$ or youth$).mp.
125. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp.
126. (pediatric$ or paediatric$ or peadiatric$).mp.
127. (school or schools or high school$ or highschool$ or primary school$ or nursery school$ or elementary school or secondary school$ or kindergar$).mp.
128. or/118‐127
129. 92 and 99 and 105 and 117 and 128
[$=zero or many characters; ti,ab=title or abstract; mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer or drug manufacturer name; /= Emtree term; ab=abstract]
Search used in LILACS/ http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/
(Benzodiazepine$ or Alprazolam or Alprazolan or alprox or esparon or apo‐alpraz or apoalpraz or cassadan or kalma or novo‐alprazol or novoalprazol or nu‐alpraz or nualpraz or ralozam or xanax or tafil or trankimazin or Niravam or Anthramycin or antramycin or Bromazepam or anxyrex or apo‐bromazepam or bromalich or bromaz or bromazanil or bromazep or lexotan or lexomil or lexotanil or lexatin or durazanil or gen‐bromazepam or Clonazepam or antelepsin or rivotril or klonopin or Devazepide or Diazepam or apaurin or diazemuls or faustan or relanium or seduxen or sibazon or stesolid or valium or rimapam or tensium or dialar or valclair or diastat or dizac or q‐pam or valrelease or Nordazepam or demethyldiazepam or desmethyldiazepam or deoxydemoxepam or nordiazepam or norprazepam or dealkylprazepam or calmday or nordaz or tranxilium n or vegesan or Flumazenil or flumazepil or romazicon or anexate or lanexat or Anexate or Lorazepam or apo‐lorazepam or apolorazepam or ativan or orfidal or temesta or donix or duralozam or durazolam or idalprem or laubeel or lorazep von ct or novo‐lorazem or novolorazem or nu‐loraz or nuloraz or sedicepan or sinestron or somagerol or tolid or loraz or Flunitrazepam or fluridrazepam or flunitrazepam‐teva or flunizep or flunimerck or flunitrazepam‐neuraxpharm or flunitrazepam‐ratiopharm or fluninoc or rohypnol or narcozep or rohipnol or flunibeta or Flurazepam or dalmane or Dormodor or dalmadorm or staurodorm or apo‐flurazepam or Nitrazepam or Nitrodiazepam or alodorm or dormalon or dormo‐puren or eatan or imadorm or imeson or mogadon or nitrazadon or nitrazep or novanox or radedorm or remnos or rhoxal‐nitrazepam or serenade or somnite or Oxazepam or adumbran or serax or tazepam or Pirenzepine or pirenzepin or pyrenzepine or ulcoprotect or ulgescum or gastrotsepin or piren‐basan or pirenzepin‐ratiopharm or gastrozepin or Prazepam or centrax or demetrin or lysanxia or reapam or Temazepam or hydroxydiazepam or methyloxazepam or oxydiazepam or pronervon t or remestan or restoril or apo‐temazepam or euhypnos or planum or levanxol or pms‐temazepam or nu‐temazepam or novo‐temazepam or nortem or normitab or normison or nocturne or temtabs or gen‐temazepam or dasuen or signopam or temaze or temazep von ct or tenox or temaz or Chlordiazepoxide or methaminodiazepoxide or chlozepid or elenium or librium or a‐poxide or chlordiazachel or librelease or libritabs or lygen or Chlorazepate or tranxene or tranxilium or Estazolam or nuctalon or prosom or tasedan or Medazepam or nobrium or rudotel or rusedal or Midazolam or dormicum or versed or hypnovel or Triazolam or apo‐triazo or gen‐triazolam or halcyon or halcion or trilam or cannabinoid$ or canabinoid$ or Tetrahydrocannabinol or thc or marinol or nabilone or cesamet or Beclomethasone or beclometasone or qvar or aerobec forte or beclazone or ecobec or filair or aerobec or nasobec aqueous or prolair or respocort or ventolair or vancenase or vanceril or aldecin or viarin or apo‐beclomethasone or ascocortonyl or beclamet or beclocort or beclomet or beclorhinol or becloturmant or sanasthmax or beclovent or beconase or propaderm or sanasthmyl or becodisks or becotide or becloforte or bronchocort or junik or asmabec clickhaler or beclazone or clenil modulate or Betamethasone or betadexamethasone or flubenisolone or celeston or celestona or celestone or cellestoderm or Betnelan or betnesol or flubenisolonvalerate or betnovate or Beta‐val or betaderm or betatrex or dermabet or luxiq or valisone or valnac or betacap or betamethasone valerate or bettamousse or diprosone or Budesonide or horacort or pulmicort or rhinocort or novolizer or entocort or Dexamethasone or hexadecadrol or methylfluorprednisolone or dexpak or maxidex or decaject or decameth or decaspray or dexasone or hexadrol or millicorten or oradexon or aeroseb‐dex or decaderm dexamethasone or decadron or decadron or mymethasone or auxison or Flumethasone or fluorodexamethasone or locorten or Fluorometholone or cortisdin or flucon or fluoro‐ophtal or fml or pms‐fluorometholone or fluoropos or oxylone or fluprednisolone or alphadrol or Flurandrenolone or flurandrenolide or cordran or haelan or fludroxycortide or melengestrol acetate or melengestrol or Methylprednisolone or metipred or medrol or urbason or medrone or solu‐medrone or depo‐medrone or a‐methapred or solu‐medrol or solumedrol or urbason‐soluble or urbasonsoluble or Prednisolone or di‐adreson‐f or diadresonf or predate or predonine or cortalone or delta‐cortef or fernisolone‐p or meti‐derm or prelone or sterane or Prednisone or dehydrocortisone or delta‐cortisone or prednison galen or prednison hexal or pronisone or rectodelt or apo‐prednisone or cortancyl or panafcort or dacortin or deltasone or prednidib or predni tablinen or panasol or orasone or meticorten or liquid pred or kortancyl or enkortolon or encortone or encorton or prednison acsis or predniment or decortisyl or cutason or cortan or winpred or ultracorten or sone or sterapred or delta‐dome or fernisone or paracort or predincen‐m or servisone or Cyclizine or marezine or valoid or Chlorpromazine or propaphenin or aminazine or chlordelazine or contomin or largactil or fenactil or chlorazine or thorazine or thorazine or Domperidone or domperidon or apo‐domperidone or domidon or domperidon‐teva or gastrocure or motilium or nauzelin or novo‐domperidone or nu‐domperidone or pms‐domperidone or peridys or ratio‐domperidone or Droperidol or dehydrobenzperidol or droleptan or dehidrobenzperidol or inapsine or inapsine or Haloperidol or haldol or dozic or serenace or Methotrimeprazine or levomeprazin or levopromazine or levomepromazine or tisercin or tizertsin or tizercine or levoprome or nozinan or Metoclopramide or metaclopramide or metaclopromide or cerucal or maxolon or primperan or raglan or rimetin or Perphenazine or chlorpiprazine or perfenazine or trilafon or fentazin or Prochlorperazine or compazine or stemetil or buccastem or compro or Trifluoperazine or trifluoroperazine or trifluperazine or stelazine or triftazin or apo‐trifluoperazine or apotrifluoperazine or eskazine or flupazine or terfluzine or granisetron or odansetron or ondansetron or tropisetron or dolasetron or anzemet or kytril or Eutrom or Kevatril or Taraz or zofran or Zofrene or Zophran or Zophren or Avessa or Ceramos)
and
(cancer$ OR neoplas$ OR oncolog$ OR malignan$ OR tumor$ OR tumour$ OR carcinoma$ OR adenocarcinoma$ OR carcoma$ OR leukaemia OR leukemia OR chemotherap$)
and
(nausea OR vomit$ OR emesis OR sickness)
[$=zero or many characters]
Search used in PsycINFO/OVID
1. benzodiazepines/ or alprazolam/ or chlordiazepoxide/ or clonazepam/ or diazepam/ or flunitrazepam/ or flurazepam/ or lorazepam/ or midazolam/ or nitrazepam/ or oxazepam/ or triazolam/
2. (Alprazolam or Alprazolan or alprox or esparon or apo‐alpraz or apoalpraz or cassadan or d‐65mt or d65mt or kalma or novo‐alprazol or novoalprazol or nu‐alpraz or nualpraz or ralozam or u‐31,889 or u31,889 or xanax or tafil or trankimazin or Niravam).ti,ab.
3. (Anthramycin or antramycin).ti,ab.
4. (Bromazepam or anxyrex or apo‐bromazepam or bromalich or bromaz or bromazanil or bromazep or lexotan or lexomil or lexotanil or lexatin or ro 5‐3350 or ro 53350 or durazanil or gen‐bromazepam).ti,ab.
5. (Clonazepam or antelepsin or rivotril or ro 5‐4023 or ro 54023 or klonopin).ti,ab.
6. (Devazepide or mk‐329 or mk329).ti,ab.
7. (Diazepam or apaurin or diazemuls or faustan or relanium or seduxen or sibazon or stesolid or valium or rimapam or tensium or dialar or valclair or diastat or dizac or q‐pam or valrelease).ti,ab.
8. (Nordazepam or demethyldiazepam or desmethyldiazepam or deoxydemoxepam or nordiazepam or norprazepam or dealkylprazepam or calmday or nordaz or tranxilium n or vegesan).ti,ab.
9. (Flumazenil or flumazepil or romazicon or anexate or lanexat or ro 15‐1788 or ro 151788 or Anexate).ti,ab.
10. (Lorazepam or apo‐lorazepam or apolorazepam or ativan or orfidal or temesta or donix or duralozam or durazolam or idalprem or laubeel or lorazep von ct or novo‐lorazem or novolorazem or nu‐loraz or nuloraz or sedicepan or sinestron or somagerol or tolid or wy‐4036 or wy4036 or loraz).ti,ab.
11. (Flunitrazepam or fluridrazepam or flunitrazepam‐teva or flunizep von ct or ro‐5‐4200 or ro54200 or flunimerck or flunitrazepam‐neuraxpharm or flunitrazepam‐ratiopharm or fluninoc or rohypnol or narcozep or rohipnol or flunibeta).ti,ab.
12. (Flurazepam or dalmane or Dormodor or dalmadorm or staurodorm or apo‐flurazepam).ti,ab.
13. (Nitrazepam or Nitrodiazepam or alodorm or dormalon or dormo‐puren or eatan or imadorm or imeson or mogadon or nitrazadon or nitrazep or novanox or radedorm or remnos or rhoxal‐nitrazepam or serenade or somnite).ti,ab.
14. (Oxazepam or adumbran or serax or tazepam).ti,ab.
15. (Pirenzepine or pirenzepin or pyrenzepine or ulcoprotect or ulgescum or gastrotsepin or piren‐basan or pirenzepin‐ratiopharm or gastrozepin).ti,ab.
16. (Prazepam or centrax or demetrin or lysanxia or reapam).ti,ab.
17. (Temazepam or 3‐hydroxydiazepam or hydroxydiazepam or methyloxazepam or oxydiazepam or pronervon t or remestan or restoril or ro‐5‐5345 or ro55345 or sah 47‐603 or sah 47603 or apo‐temazepam or euhypnos or planum or levanxol or pms‐temazepam or nu‐temazepam or novo‐temazepam or nortem or normitab or normison or nocturne or temtabs or gen‐temazepam or dasuen or signopam or temaze or temazep von ct or tenox or wy‐3917 or wy3917 or temaz).ti,ab.
18. (Chlordiazepoxide or methaminodiazepoxide or chlozepid or elenium or librium or a‐poxide or chlordiazachel or librelease or libritabs or lygen).ti,ab.
19. (Chlorazepate or tranxene or tranxilium).ti,ab.
20. (Estazolam or nuctalon or prosom or tasedan).ti,ab.
21. (Medazepam or nobrium or ro 5‐4556 or ro 54556 or rudotel or rusedal).ti,ab.
22. (Midazolam or dormicum or ro 21‐3981 or ro 213981 or versed or hypnovel).ti,ab.
23. (Triazolam or apo‐triazo or gen‐triazolam or halcyon or halcion or trilam).ti,ab.
24. or/1‐23
25. cannabinoids/ or tetrahydrocannabinol/
26. (cannabinoid$ or canabinoid$ or Tetrahydrocannabinol or thc or marinol or nabilone or cesamet).ti,ab.
27. 25 or 26
28. (Beclomethasone or beclometasone or qvar or aerobec forte or beclazone or ecobec or filair or aerobec or nasobec aqueous or prolair or respocort or ventolair or vancenase or vanceril or aldecin or viarin or apo‐beclomethasone or ascocortonyl or beclamet or beclocort or beclomet or beclorhinol or becloturmant or sanasthmax or beclovent or beconase or propaderm or sanasthmyl or becodisks or becotide or becloforte or bronchocort or junik or asmabec clickhaler or beclazone or clenil modulate).ti,ab.
29. (Betamethasone or betadexamethasone or flubenisolone or celeston or celestona or celestone or cellestoderm or Betnelan or betnesol).ti,ab.
30. (betamethasone 17‐valerate or flubenisolonvalerate or betnovate or Beta‐val or betaderm or betatrex or dermabet or luxiq or valisone or valnac or betacap or betamethasone valerate or bettamousse or diprosone).ti,ab.
31. (Budesonide or horacort or pulmicort or rhinocort or novolizer or entocort).ti,ab.
32. Dexamethasone/
33. (Dexamethasone or hexadecadrol or methylfluorprednisolone or dexpak or maxidex or decaject or decameth or decaspray or dexasone or hexadrol or millicorten or oradexon or aeroseb‐dex or decaderm dexamethasone or decadron or decadron or mymethasone).ti,ab.
34. (dexamethasone isonicotinate or auxison).ti,ab.
35. (Flumethasone or fluorodexamethasone or locorten).ti,ab.
36. (Fluorometholone or cortisdin or flucon or fluoro‐ophtal or fml or pms‐fluorometholone or fluoropos or oxylone).ti,ab.
37. (fluprednisolone or alphadrol).ti,ab.
38. (Flurandrenolone or flurandrenolide or cordran or haelan or fludroxycortide).ti,ab.
39. (melengestrol acetate or melengestrol).ti,ab.
40. (Methylprednisolone or metipred or medrol or urbason or medrone or solu‐medrone or depo‐medrone).ti,ab.
41. (a‐methapred or solu‐medrol or solumedrol or urbason‐soluble or urbasonsoluble).ti,ab.
42. Prednisolone/
43. (Prednisolone or di‐adreson‐f or diadresonf or predate or predonine or cortalone or delta‐cortef or fernisolone‐p or meti‐derm or prelone or sterane).ti,ab.
44. (Prednisone or dehydrocortisone or delta‐cortisone or prednison galen or prednison hexal or pronisone or rectodelt or apo‐prednisone or cortancyl or panafcort or dacortin or deltasone or prednidib or predni tablinen or panasol or orasone or meticorten or liquid pred or kortancyl or enkortolon or encortone or encorton or prednison acsis or predniment or decortisyl or cutason or cortan or winpred or ultracorten or sone or sterapred or delta‐dome or fernisone or paracort or predincen‐m or servisone).ti,ab.
45. or/28‐44
46. (Cyclizine or marezine or valoid).ti,ab.
47. Chlorpromazine/
48. (Chlorpromazine or propaphenin or aminazine or chlordelazine or contomin or largactil or fenactil or chlorazine or thorazine or thorazine).ti,ab.
49. (Domperidone or domperidon or apo‐domperidone or domidon or domperidon‐teva or gastrocure or motilium or nauzelin or novo‐domperidone or nu‐domperidone or pms‐domperidone or peridys or ratio‐domperidone).ti,ab.
50. (Droperidol or dehydrobenzperidol or droleptan or dehidrobenzperidol or inapsine or inapsine).ti,ab.
51. Haloperidol/
52. (Haloperidol or haldol or dozic or serenace).ti,ab.
53. (Methotrimeprazine or levomeprazin or levopromazine or levomepromazine or tisercin or tizertsin or tizercine or levoprome or nozinan).ti,ab.
54. (Metoclopramide or metaclopramide or metaclopromide or cerucal or maxolon or primperan or raglan or rimetin).ti,ab.
55. Perphenazine/
56. (Perphenazine or chlorpiprazine or perfenazine or trilafon or fentazin).ti,ab.
57. Prochlorperazine/
58. (Prochlorperazine or compazine or stemetil or buccastem or compro).ti,ab.
59. Trifluoperazine/
60. (Trifluoperazine or trifluoroperazine or trifluperazine or stelazine or triftazin or apo‐trifluoperazine or apotrifluoperazine or eskazine or flupazine or terfluzine).ti,ab.
61. or/46‐60
62. granisetron.ti,ab.
63. (odansetron or ondansetron).ti,ab.
64. tropisetron.ti,ab.
65. dolasetron.ti,ab.
66. anzemet.ti,ab.
67. (kytril or Eutrom or Kevatril or Taraz).ti,ab.
68. (zofran or Zofrene or Zophran or Zophren or Avessa or Ceramos).ti,ab.
69. 5‐HT3 antagonist$.ti,ab.
70. 5‐HT3 blocker$.ti,ab.
71. or/62‐70
72. 24 or 27 or 45 or 61 or 71
73. (infant or infan$ or newborn or newborn$ or new‐born$ or baby or baby$ or babies or neonat$ or child or child$ or schoolchild$ or schoolchild or school child or school child$ or kid or kids or toddler$ or adolescent or adoles$ or teen$ or boy$ or girl$ or minors or minors$ or underag$ or under ag$ or juvenil$ or youth$ or kindergar$ or puberty or puber$ or pubescen$ or prepubescen$ or prepuberty$ or pediatrics or pediatric$ or paediatric$ or peadiatric$ or schools or nursery school$ or preschool$ or pre school$ or primary school$ or secondary school$ or elementary school$ or elementary school or high school$ or highschool$ or school age or schoolage or school age$ or schoolage$ or infancy or schools, nursery or infant, newborn).ti,ab.
74. Nausea/
75. nausea.ti,ab.
76. vomiting/
77. vomit$.ti,ab.
78. emesis.ti,ab.
79. sickness.ti,ab.
80. or/74‐79
81. randomized.ti,ab.
82. placebo.ti,ab.
83. random$.ti,ab.
84. trial$.ti,ab.
85. groups.ti,ab.
86. Clinical Trials/
87. or/81‐86
88. exp neoplasms/
89. cancer$.ti,ab.
90. neoplas$.ti,ab.
91. oncolog$.ti,ab.
92. malignan$.ti,ab.
93. tumo?r$.ti,ab.
94. carcinoma$.ti,ab.
95. adenocarcinoma$.ti,ab.
96. sarcoma$.ti,ab.
97. leuk?mia.ti,ab.
98. chemotherap$.ti,ab.
99. or/88‐98
100. 72 and 73 and 80 and 87 and 99
[$=zero or many characters; ?=one or many characters; ti,ab=title or abstract; mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer or drug manufacturer name; /= PsycInfor thesuarus term; ?= ]
Appendix 2. Search strategies for proceedings abstracts
Search used in American Society of Clinical Oncology
All meetings were searched except for the breast and prostate specific meetings (since these were expected to yield results that involved adults).
Separate searches were done for the following search terms:
nausea vomit emesis emetic antiemetic
The searches were limited to the title field and the results were deduplicated.
International Society of Paediatric Oncology (SIOP)
The site was browsed.
PDFs of the abstracts were downloaded and the text was searched for "vomiting"
Multinational Association of Supportive Care in Cancer (MASCC)
The site was browsed
ISI Proceedings: Science and Technology
#1 TS=( infan* or newborn* or (new same born*) or baby* or babies or neonat* or child* or schoolchild* or (school same child*) or kid or kids or toddler* or adoles* or teen* or boy* or girl* or minors* or underag* or juvenil* or youth* or kindergar* or puber* or pubescen* or prepubescen* or prepuber* or pediatric* or paediatric* or peadiatric* or schools or preschool* or highschool* or (school same age*) or schoolage*) #2 TS=(nausea or vomit*or emesis or sickness) #3 TS=(cancer* or neoplas* or oncolog* or malignan* or tumor* or tumour* or carcinoma* or adenocarcinoma* or sarcoma* or leukaemia or leukemia or chemotherap*) #1 and #2 and #3
[*=zero or many characters]
Appendix 3. Search strategies for trial registers
International Cancer Research Portfolio
The following selections were made:
Project Type=Clinical Trial
Type of Research=6.1 Cancer Control, Survivorship and Outcomes Research ‐ Patient Care and Survivorship Issues
There was a limit on the number of terms that could be entered in the search box, so the searching was done in the following batches:
(N.B. the default is to “OR” all terms)
Search one:
Benzodiazepine$ Alprazolam Alprazolan alprox esparon apo‐alpraz apoalpraz cassadan kalma novo‐alprazol novoalprazol nu‐alpraz nualpraz ralozam xanax tafil trankimazin Niravam Anthramycin antramycin Bromazepam anxyrex apo‐bromazepam bromalich bromaz bromazanil bromazep lexotan lexomil lexotanil lexatin durazanil gen‐bromazepam
Search two:
Clonazepam antelepsin rivotril klonopin Devazepide Diazepam apaurin diazemuls faustan relanium seduxen sibazon stesolid valium rimapam tensium dialar valclair diastat dizac q‐pam valrelease Nordazepam demethyldiazepam desmethyldiazepam deoxydemoxepam nordiazepam norprazepam dealkylprazepam
Search three:
calmday nordaz tranxilium Flumazenil flumazepil romazicon anexate lanexat Anexate Lorazepam apo‐lorazepam apolorazepam ativan orfidal temesta donix duralozam durazolam idalprem laubeel lorazep von ct novo‐lorazem novolorazem nu‐loraz nuloraz sedicepan sinestron
Search four:
somagerol tolid loraz Flunitrazepam fluridrazepam flunitrazepam‐teva flunizep flunimerck flunitrazepam‐neuraxpharm flunitrazepam‐ratiopharm fluninoc rohypnol narcozep rohipnol flunibeta Flurazepam dalmane Dormodor dalmadorm staurodorm apo‐flurazepam Nitrazepam Nitrodiazepam
Search five:
alodorm dormalon dormo‐puren eatan imadorm imeson mogadon nitrazadon nitrazep novanox radedorm remnos rhoxal‐nitrazepam serenade somnite Oxazepam adumbran serax tazepam Pirenzepine pirenzepin pyrenzepine ulcoprotect ulgescum gastrotsepin piren‐basan
Search six:
pirenzepin‐ratiopharm gastrozepin Prazepam centrax demetrin lysanxia reapam Temazepam hydroxydiazepam methyloxazepam oxydiazepam pronervon remestan restoril apo‐temazepam euhypnos planum levanxol pms‐temazepam nu‐temazepam novo‐temazepam nortem normitab
Search seven:
normison nocturne temtabs gen‐temazepam dasuen signopam temaze temazep tenox temaz Chlordiazepoxide methaminodiazepoxide chlozepid elenium librium a‐poxide chlordiazachel librelease libritabs lygen Chlorazepate tranxene tranxilium Estazolam nuctalon prosom tasedan
Search eight:
Medazepam nobrium rudotel rusedal Midazolam dormicum versed hypnovel Triazolam apo‐triazo gen‐triazolam halcyon halcion trilam cannabinoid$ canabinoid$ Tetrahydrocannabinol thc marinol nabilone cesamet Beclomethasone beclometasone qvar aerobec beclazone ecobec
Search nine:
filair aerobec nasobec prolair respocort ventolair vancenase vanceril aldecin viarin apo‐beclomethasone ascocortonyl beclamet beclocort beclomet beclorhinol becloturmant sanasthmax beclovent beconase propaderm sanasthmyl becodisks becotide becloforte
Search 10:
bronchocort junik asmabec beclazone clenil Betamethasone betadexamethasone flubenisolone celeston celestona celestone cellestoderm Betnelan betnesol flubenisolonvalerate betnovate Beta‐val betaderm betatrex dermabet luxiq valisone valnac betacap betamethasone
Search 11:
valerate bettamousse diprosone Budesonide horacort pulmicort rhinocort novolizer entocort Dexamethasone hexadecadrol methylfluorprednisolone dexpak maxidex decaject decameth decaspray dexasone hexadrol millicorten oradexon aeroseb‐dex decaderm dexamethasone decadron
Search 12:
decadron mymethasone auxison Flumethasone fluorodexamethasone locorten Fluorometholone cortisdin flucon fluoro‐ophtal fml pms‐fluorometholone fluoropos oxylone fluprednisolone alphadrol Flurandrenolone flurandrenolide cordran haelan fludroxycortide melengestrol acetate melengestrol
Search 13:
Methylprednisolone metipred medrol urbason medrone solu‐medrone depo‐medrone a‐methapred solu‐medrol solumedrol urbason‐soluble urbasonsoluble Prednisolone di‐adreson‐f diadresonf predate predonine cortalone delta‐cortef fernisolone‐p meti‐derm prelone sterane Prednisone
Search 14:
dehydrocortisone delta‐cortisone prednison pronisone rectodelt apo‐prednisone cortancyl panafcort dacortin deltasone prednidib predni panasol orasone meticorten kortancyl enkortolon encortone encorton predniment decortisyl cutason
Search 15:
levomepromazine tisercin tizertsin tizercine levoprome nozinan Metoclopramide metaclopramide metaclopromide cerucal maxolon primperan raglan rimetin Perphenazine chlorpiprazine perfenazine trilafon fentazin Prochlorperazine compazine stemetil buccastem compro Trifluoperazine
Search 16:
trifluoroperazine trifluperazine stelazine triftazin apo‐trifluoperazine apotrifluoperazine eskazine flupazine terfluzine
Search 17:
5‐HT3 antagonists 5‐HT3 antagonist 5‐HT3 blockers 5‐HT3 blocker
Search 18:
granisetron ondansetron ondansetron tropisetron dolasetron anzemet kytril Eutrom Kevatril Taraz zofran Zofrene Zophran Zophren Avessa Ceramos
[$=zero or more characters]
National Cancer Institute Clinical Trials PDQ
The interface uses menu selections there is no free text searching. The following selections were made:
Type of cancer:all
Type of trial:supportive care
Status of trial:active/closed (this was an either/or situation the same search was run twice once for active, once for closed)
Type of treatment:chemotherapy
Drug:alprazolam, lorazepam, midazolam, midazolam hydrochloride, cannabinol, tetrahydrocannabinol, beclomethasone dipropionate, budesonide, dexamethasone, Sk‐Dexamethasone, 6Alpha‐Methylprednisolone, 9alpha‐Fluoro‐16alpha‐ methylprednisolone, 9Alpha‐fluoro‐16alpha‐methylprednisolone, methylprednisolone, Methylprednisolone Acetate, Methylprednisolone Succinate, prednisolone, prednisone, Sk‐Prednisone, chlorpromazine, domperidone, droperidol, haloperidol, metoclopramide hydrochloride, prochlorperazine, trifluoperazine hydrochloride
Drug combination search:no
Phase of trial:all
Sponsor of trial:all
Special category: all
National Cancer Research Institute (NCRI)
The site was browsed.
Current Controlled Trials (mRCT Register)
(cancer% OR neoplas% OR oncolog% OR malignan% OR tumor% OR tumour% OR carcinoma% OR adenocarcinoma% OR carcoma% OR leukaemia OR leukemia OR chemotherap%) and (nausea OR vomit% OR emesis OR sickness)
[%=zero or many characters]
Search used in Clinical Trials.gov
(Cancer OR carcinoma OR leukaemia OR leukemia) and (nausea OR vomit)
Centerwatch
The interface uses a combination of menu selections and free text searching. The following selection was made:
Disease or condition: Cancer/Chemotherapy
All other boxes were left blank.
Keywords used were: vomit or nausea or emesis or sickness
Data and analyses
Comparison 1. Addition of corticosteroids to 5‐HT3 antagonists.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Control of immediate vomiting | 2 | 100 | Risk Ratio (Random, 95% CI) | 2.03 [1.35, 3.04] |
Comparison 2. Granisetron 20 mcg/kg versus 40 mcg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete control of immediate vomiting | 3 | 453 | Risk Ratio (Fixed, 95% CI) | 0.93 [0.80, 1.07] |
Comparison 3. Ondansetron vs Granisetron.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete control of acute nausea | 2 | 101 | Risk Ratio (Fixed, 95% CI) | 1.05 [0.94, 1.17] |
| 2 Complete control of acute vomiting | 3 | 167 | Risk Ratio (Fixed, 95% CI) | 2.26 [2.04, 2.51] |
| 3 Complete control of delayed nausea | 2 | 101 | Risk Ratio (Fixed, 95% CI) | 1.13 [0.93, 1.38] |
| 4 Complete control of delayed vomiting | 2 | 101 | Risk Ratio (Fixed, 95% CI) | 1.13 [0.98, 1.29] |
3.2. Analysis.
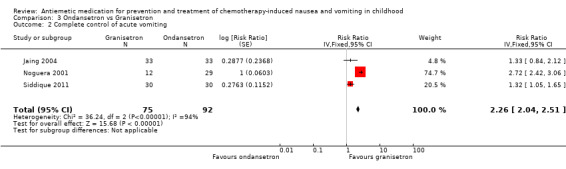
Comparison 3 Ondansetron vs Granisetron, Outcome 2 Complete control of acute vomiting.
3.3. Analysis.
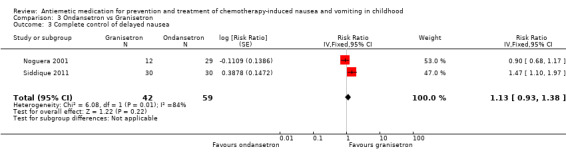
Comparison 3 Ondansetron vs Granisetron, Outcome 3 Complete control of delayed nausea.
Comparison 4. Other antiemetic comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete control of acute nausea | Other data | No numeric data | ||
| 2 Complete control of acute vomiting | Other data | No numeric data | ||
| 3 Complete control of delayed vomiting | Other data | No numeric data | ||
| 4 Complete control of acute nausea and vomiting | Other data | No numeric data | ||
| 5 Other nausea &/or vomiting outcomes | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alvarez 1995.
| Methods | RCT Cross‐over |
|
| Participants | Participants aged 9 years median (range 3 to 18 years) with solid tumours receiving "emetogenic" chemotherapy. 19/33 participants were male. Cross‐over on an identical course. Chemotherapy consisted of 4‐ to 5‐day ifosfamide 1.8 g/m² to 2.5 g/m² plus doxorubicin 25 mg/m² or etoposide 100 mg/m²; 4‐ to 5‐day cisplatin 20 to 120 mg/m² plus etoposide, doxorubicin, cyclophosphamide, or ifosfamide; 1‐day cyclophosphamide 1.2 g to 2.2 g plus doxorubicin and actinomycin; 1‐day carboplatin 700 mg/m², or day 1 of ABVD. | |
| Interventions | Ondansetron, IV, 0.15 mg/kg 30 minutes prior to chemo, then BD on chemotherapy for 1 to 5 days Ondansetron, IV, 0.15 mg/kg 30 minutes prior to chemo, then BD on chemotherapy Dexamethasone either 4 mg/m² QDS or 8 mg/m² BD (depended on institution) |
|
| Outcomes | Emetic episode defined as vomiting that produced liquid or any retches within a 5‐minute period. A complete response was achieved if no emetic episode occurred. 1 or 2 emetic episodes constituted a "major response". 3 to 5 episodes constituted a "minor response". More than 5 episodes, the need for rescue medication, or participant withdrawal constituted a "failure". Unvalidated nausea assessment of "none, a little, some, a lot" |
|
| Notes | Data reported by episode; no paired analysis possible. 2 institutions and dexamethasone dose varied (see above) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated randomised |
| Allocation concealment (selection bias) | Unclear risk | Randomisation list given to institutional pharmacy |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes (as above, and nursing staff) |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes (as above, and nursing staff) |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 33 had 1 course, 25 had 2 courses (= 58 episodes). Loss after course 1 = 1 death, 3 PD, and 4 "failures" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Note a variety of chemotherapies used (with different emetogenicity) |
Basade 1996.
| Methods | RCT Cross‐over |
|
| Participants | 27 children with any paediatric malignancy, receiving chemotherapy that included cyclophosphamide > 600 mg/m². Median 7 years (range 3 to 14 years) | |
| Interventions | Dexamethasone 8 mg/m² IV 15 minutes prior to chemotherapy Metoclopramide 1.5 mg/kg IV 15 minutes prior to chemotherapy |
|
| Outcomes | Emetic episodes recorded by doctor, participant, or nurse. Unvalidated vomiting assessment, where participant report or clinicians opinion of the participant's nausea was recorded as "None, moderate, severe". | |
| Notes | Paired data not presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear Stated "single blind" but no further information |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear Stated "single blind" but no further information |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 participant (2.9%) failed to complete cross‐over; no reason given |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other risks of bias noted |
Berrak 2007.
| Methods | RCT Cross‐over |
|
| Participants | 18 participants with optic pathway glioma receiving carboplatin. 7.7 years median age (1 to 23 years) | |
| Interventions | Granisetron, OD, IV, 10 mcg/kg Granisetron, OD, IV, 40 mcg/kg |
|
| Outcomes | Diary collected vomiting assessment | |
| Notes | Investigator helpfully supplied data to extract paired, first cross‐over analysis for immediate and delayed vomiting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The dose of granisetron was randomly ordered by two physicians (ET and BB)." |
| Allocation concealment (selection bias) | Low risk | "The study blind was maintained using a double dummy technique ... two physicians (ET and BB) who were not involved in either the collection or evaluation of self‐report diary cards or safety assessments." |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | "granisetron was prepared accordingly by the pharmacy in similar syringes labelled simply 'granisetron'" Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes (as above, and nursing staff) |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Combined nausea and vomiting "granisetron was prepared accordingly by the pharmacy in similar syringes labelled simply 'granisetron'" Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes (as above, and nursing staff) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data available |
| Selective reporting (reporting bias) | Low risk | All outcome data available |
| Other bias | Low risk | No other risk of bias noted |
Brock 1996.
| Methods | RCT | |
| Participants | All participants scheduled to receive highly emetogenic chemotherapy except brain tumours, cerebral metastases, or meningeal leukaemia, where highly emetogenic chemotherapy was defined as:
Actinomycin > 15 mcg/kg or > 0.45 mg/m²
Carboplatin > 400 mg/m²
Cisplatin > 20 mg/m²
Cyclophosphamide > 500 mg/m²
Cytosine > 500 mg/m²
Dacarbazine > 250 mg/m²
Daunorubicin > 40 mg/m²
Doxorubicin > 40 mg/m²
Ifosfamide > 1 g/m²
Methotrexate > 5 g/m²
Mitoxantrone > 8 mg/m²
Nitrogen mustard > 6 mg/m²
Epirubicin > 45 mg/m² Mean age 8.5 years (range 1.9 to 16.7 years), 102/160 participants were male |
|
| Interventions | Ondansetron 5 mg/m² IV over 15 minutes immediately prior to chemotherapy, then 8 hours and 16 hours after initial dose. From day 2 ondansetron given orally < 1 m² 4 mg TDS, > 1 m² 8 mg TDS, continued for 3 days after last day of chemotherapy or 5 days if nausea and vomiting persisted. Ondansetron 10 mg/m² IV over 15 minutes immediately prior to chemotherapy, then 5 mg/m² IV 8 hours and 16 hours after initial dose. From day 2 ondansetron given orally < 1 m² 4 mg TDS, > 1 m² 8 mg TDS, continued for 3 days after last day of chemotherapy or 5 days if nausea and vomiting persisted |
|
| Outcomes | Emesis recorded as timing and number of episodes of vomiting or retching. Unvalidated nausea assessment of "none, mild or severe" on diary cards | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | "The anti‐emetic loading dose of ondansetron was blinded to the clinicians, the patients, the parents and the nurses." Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | "The anti‐emetic loading dose of ondansetron was blinded to the clinicians, the patients, the parents and the nurses." Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 participants (15%) of 5 mg/m² lost to follow‐up, and 13 participants (14%) of 10 mg/m² lost to follow‐up. Data for these participants not included |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other risk of bias noted |
Chan 1987.
| Methods | RCT Cross‐over |
|
| Participants | Any paediatric malignancy, but patients in whom a chemotherapy cycle was previously shown to cause moderate to severe drug‐induced nausea and vomiting No participants received cisplatin. Participants had not previously been treated with either nabilone or prochlorperazine. 30 participants, median age 11.8 years (3.5 to 17.8 years), gender not specified |
|
| Interventions | Nabilone orally (1 mg capsules) starting 8 to 12 hours prior to chemotherapy and repeated 2 or 3 times a day according to dosage schedule Original schedule: 18 to 27 kg 1 mg BD 27.1 to 36 kg 1 mg TDS > 36 kg 2 mg BD Modified schedule: < 18 kg 0.5 mg BD 18 to 30 kg 1 mg BD > 30 kg 1 mg TDS Prochlorperazine orally (capsules) starting 8 to 12 hours prior to chemotherapy and repeated 2 or 3 times a day according to dosage schedule Original schedule: 18 to 27 kg 5 mg BD 27.1 to 36 kg 5 mg TDS > 36 kg 10 mg BD Modified schedule: < 18 kg 2.5 mg BD 18 to 30 kg 5 mg BD > 30 kg 5 mg TDS |
|
| Outcomes | Vomiting was recorded as the total number of episodes of vomiting or retching | |
| Notes | Study supported by a grant from Eli Lilly (company supplying nabilone) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | Medical staff and participants/parents not aware of drug allocation, but nabilone has frequent, significant, and immediately identifiable side effects Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 withdrawals, all included in toxicity assessments. 4 change of chemotherapy after cycle 1; 2 unable to cope with diagnosis and treatment; 2 received other antiemetics during study period; 2 cycle 2 of chemotherapy deferred because of severe dizziness/drowsiness after a single 2 mg dose of nabilone prior to treatment |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other risk of bias noted |
Dalzell 1986.
| Methods | RCT Cross‐over |
|
| Participants | Any paediatric malignancy, as long as 2+ identical courses of chemotherapy scheduled. Only 1 with cisplatin, mainly had high‐dose cyclophosphamide. Mean age 8.6 years (range 0.8 to 17 years), 18/23 participants were male | |
| Interventions | Nabilone 0.5 mg BD if < 18 kg, 1 mg BD if 18 to 36 kg, 1 mg TDS if > 36 kg Domperidone 5 mg TDS if < 18 kg, 10 mg TDS if 18 to 36 kg, 15 mg TDS if > 36 kg |
|
| Outcomes | Vomiting episodes recorded. Nausea assessed as "0, 1, 2, 3", but unclear if as a visual analogue scale or integers to circle/tick. No indication of the performance of the tool (e.g. is "3" three times as bad as "1"?) | |
| Notes | Only data on completers were used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Medical staff and participants/parents not aware of drug allocation, but nabilone has frequent, significant, and immediately identifiable side effects Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 18/23 participants (78%) completed cross‐over; 2 lost from nabilone arm, 3 from metoclopramide arm |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other risk of bias noted |
Dick 1995.
| Methods | RCT | |
| Participants | 30 participants with ALL undergoing MRC Intensification (daunorubicin, etoposide, and high‐dose cytarabine). Age and gender of participants not recorded | |
| Interventions | 15 participants received ondansetron 3 to 8 mg/m² given pre‐chemotherapy, then BD initially IV then orally for 3 days (dose < 0.6 m² ‐ load 3 mg/m², maint 3 mg/m² or 2 mg; 0.6 to 1.2 m² ‐ load 3 mg/m², maint 3 mg/m² or 4 mg; > 1.2 m² ‐ load 8 mg, maint 8 mg) 15 participants received metoclopramide 10 mg/m² IV QDS for 3 days, with 2.5 mg procyclidine. Dexamethasone 4 mg/m² IV then 2 mg/m² TDS IV or PO |
|
| Outcomes | Vomiting numbers recorded on diary cards. Unvalidated nausea assessment of "not feeling very sick at all, feeling sick, feeling very sick" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "block balanced" but no further detail given |
| Allocation concealment (selection bias) | Low risk | "coding held in the pharmacy." |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Combined nausea and vomiting Blinding of care provider: no Blinding of participant: no Blinding of outcome assessors: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | 10 of 15 metoclopramide patients were switched to ondansetron during D1, so 'contaminated' any ostensibly randomised comparisons of later efficacy |
Ekert 1979.
| Methods | RCT Multiple randomisations per participant, each independent |
|
| Participants | Any paediatric malignancy. Age and gender not recorded. Any regimen of chemotherapy, with mixed emetogenicity. No platinums. Courses included: high‐dose methotrexate (7.5 g/m²) = 6, lower‐dose vincristine (.625 g/m²) = 5, doxorubicin (60) = 2, vincristine‐doxorubicin‐dacarbazine = 7, vincristine‐prednisolone‐cyclophosphamide‐doxorubicin = 4, cytosine‐cyclophosphamide‐asparaginase = 6, cytarabine/6Thioguanine = 3, 5FU‐doxorubicin‐actinomycinD = 2, lomustine‐vincristine = 7 |
|
| Interventions | Tetrahydrocannabinol 10 mg/m², given at ‐2, 4, 8, 16, and 24 hours around chemotherapy administration Metoclopramide 5 mg (for < 0.7 m² participants) or 10 mg (for > 0.7 m² participants) at ‐2, 8, 16, and 24 hours. Placebo given at +4 hours |
|
| Outcomes | Nurse recorded outcomes (as inpatient) or parent/child recorded diary. Vomiting episodes and nausea (present/absent) reported | |
| Notes | First of 2 randomisations, second reported as Ekert 1979a | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | Stated "double blinded", but cannabinoids have frequent, significant, and immediately identifiable side effects Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | Stated "double blinded", but cannabinoids have frequent, significant, and immediately identifiable side effects Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full report of data |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Unclear risk | Stopped early because of failure of efficacy of metoclopramide (7 courses of tetrahydrocannabinol "missed") |
Ekert 1979a.
| Methods | RCT Multiple randomisation per participant, each independent |
|
| Participants | Any paediatric malignancy. Age and gender not recorded. Any regimen of chemotherapy, with mixed emetogenicity. No platinums. Courses included: high‐dose methotrexate (7.5 g/m²) = 4, doxorubicin (60) = 3, vinc‐procarbazine‐pred‐lomustine = 12, vinc‐pred‐cyclo‐doxorubicin = 2, cytosine‐cyclo‐asparaginase = 2, 5FU‐dox‐actinoD = 2, lomustine‐vinc = 2, vinc‐actinomycinD = 4, vinc‐6MP‐dox‐pred = 3, cytosines‐daunorubicin/doxorubicin = 2 Age and sex not reported |
|
| Interventions | Tetrahydrocannabinol 10 mg/m², given at ‐2, 4, 8, 16, and 24 hours around chemotherapy administration Prochlorperazine ‐ tablets. Complex schedule SA 0.7 to 1.1 m² = 5 mg at ‐2, 8, 16, 24 hours SA 1.1 to 1.4 m² = 10 mg at ‐2, 8 hours and 5 mg at 16, 24 hours SA > 1.1 m² = 10 mg at ‐2, 8, 16, 24 hours Placebo given at +4 hours |
|
| Outcomes | Nurse recorded outcomes (as inpatient) or parent/child recorded diary. Vomiting episodes and nausea (present/absent) reported | |
| Notes | Second randomisation in paper (see Ekert 1979) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | Stated "double blinded", but cannabinoids have frequent, significant, and immediately identifiable side effects Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | Stated "double blinded", but cannabinoids have frequent, significant, and immediately identifiable side effects Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full report of data |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Emir 2013.
| Methods | RCT Cross‐over |
|
| Participants | Paediatric patients (age 1 to 16 years, median 7 years; 13/23 male) receiving cisplatin‐containing chemotherapy regimens | |
| Interventions | Granisetron 0.04 mg/kg plus dexamethasone 0.2 mg/kg Granisetron 0.04 mg/kg, dexamethasone 0.2 mg/kg, midazolam 0.04 mg/kg, and diphenhydramine 2.5 mg/kg |
|
| Outcomes | Number of vomits Severity of nausea (no definition of how this was defined given in paper) Use of rescue therapy Adverse events Complete response was defined as no nausea or vomiting, partial response as 1 or 2 vomits but no need for rescue therapy, no response as more than 3 emetic episodes or need for rescue therapy |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in results |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Paired data not provided |
Graham‐Pole 1986.
| Methods | RCT | |
| Participants | 50 participants with any paediatric malignancy (included osteogenic sarcoma, AML, Ewing's sarcoma, and lymphoma). Chemotherapy included aggressive regimens, e.g. cisplatin 100 to 120 mg/m², methotrexate > 7.5 g/m², cyclophosphamide > 900 mg/m², and anthracyclines > 60 mg/m². 12 participants were receiving conditioning for BMT with cytosine 3 g/m²/dose or melphalan 60 mg/m²/dose 40/50 participants were younger than 10 years, and 34/50 male |
|
| Interventions | 24 participants received metoclopramide 0.5 mg/kg/dose IV infusion over 15 minutes beginning 30 minutes before chemotherapy and repeated every 3 hours for 5 doses 26 participants received chlorpromazine 0.5 mg/kg/dose IV infusion over 15 minutes starting 30 minutes before chemotherapy and repeated every 3 hours for 5 doses |
|
| Outcomes | Unvalidated nausea assessment with duration in hours recorded. Number and volume of vomits recorded. Unclear who recorded the information | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants assigned with a random numbers table |
| Allocation concealment (selection bias) | Low risk | Drugs prepared in inpatient pharmacy. Both supplied to ward in 50 ml normal saline and labelled with "Reglan Study" and participant's name. Participants and clinicians did not know which antiemetic was being used |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Not clear who collected data, but "only pharmacy were aware of drug allocation" Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants who experienced an extrapyramidal reaction had no further data recorded |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | High risk | Discrepancy on numbers of participants who had received prior chemotherapy 19/24 participants in metoclopramide group had prior chemotherapy 10/26 participants in chlorpromazine group had prior chemotherapy |
Hahlen 1995.
| Methods | RCT | |
| Participants | 88 children with any paediatric malignancy, excluding primary or secondary brain tumour. Majority of children had soft tissue sarcomas. Chemotherapy consisted of high‐dose ifosfamide treatment (> = 3 g/m²) for 2 or 3 consecutive days. Other cytostatic agents, e.g. dactinomycin, doxorubicin, and vincristine, were permitted to be given concurrently (as in most cases) or after completion of ifosfamide administration. Mean age was 9.5 years, and around half the children were boys |
|
| Interventions | 46 children received granisetron 20 mcg/kg in 20 ml saline by IVI over 5 minutes, then ifosfamide infusion started
Up to 2 further doses granisetron 20 mcg/kg per dose within each 24‐hour period if moderate or severe nausea or any vomiting (to a maximum of 60 mcg/kg in 24 hours) 42 children received dexamethasone 2 mg/m² by IVI 30 minutes before ifosfamide infusion started, then again at 8 and 16 hours, plus chlorpromazine 0.5 mg/kg by IVI 25 minutes before start of ifosfamide and at 4‐ to 6‐hourly intervals (reduced to 0.3 mg/kg loading dose and 0.3 to 0.5 mg/kg 4‐ to 6‐hourly after reports of unacceptable levels of sedation in some children) |
|
| Outcomes | Assessment of severity of nausea and frequency of vomiting or retching episodes during 6 hourly periods by hospital staff
At end of treatment period, subjective assessment of overall response to antiemetic therapy (very good, good, average, poor, very poor) made by clinician Unvalidated nausea assessment of none, mild, moderate, severe. Vomiting episodes recorded |
|
| Notes | Stopped early due to emerging evidence of effectiveness and tolerability of granisetron over control therapy (88 out of 100 planned participants) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code. Randomisation stratified to ensure that 2‐ and 3‐day ifosfamide treatments were evenly represented in both groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | "Children were assigned on a single blind basis". Blinding of care provider: unclear Blinding of participant: yes Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | "Children were assigned on a single blind basis". Blinding of care provider: unclear Blinding of participant: yes Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | "Children were assigned on a single blind basis". Blinding of care provider: unclear Blinding of participant: yes Blinding of outcome assessors: unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "... all children were included in efficacy and safety analysis", however unclear if any missing outcomes at each time point |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Unclear risk | Stopped early due to emerging evidence of effectiveness and tolerability of granisetron over control therapy (88 out of 100 planned participants) |
Hirota 1993.
| Methods | RCT Cross‐over |
|
| Participants | Any paediatric malignancy: osteosarcoma (2), NHL (3), "brain tumour" (2), ALL (2), other sarcoma (3) Any chemotherapy protocol that was moderate or highly emetogenic, with 2 courses required (for cross‐over) Mean age 10.8 years (4 to 18 years), 8/12 were male |
|
| Interventions | Granisetron 40 mcg/kg IV 30 minutes prior to Rx Granisetron 40 mcg/kg IV 30 minutes prior to Rx plus methylprednisolone 10 mg/kg (max 500 mg) IV |
|
| Outcomes | Parents reported vomiting episodes | |
| Notes | Paired data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants did not complete cross‐over. Data not given |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Jaing 2004.
| Methods | RCT Open‐label, 2‐period cross‐over study |
|
| Participants | ALL with no CNS involvement who were receiving cyclophosphamide 300 mg/m² plus etoposide 300 mg/m² or cyclophosphamide 1000 mg/m² alone Mean 88 months old with 21/33 males |
|
| Interventions | Oral granisetron single dose 1 hour before administration of chemotherapy
25 to 50 kg 0.5 mg
> 50 kg 1 mg Ondansetron 0.15 mg/kg IV 1 hour before chemotherapy and 4 hours after the first dose. Oral dose given 8 hours after the first dose |
|
| Outcomes | Parent reported number of vomiting episodes | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocated on the basis of hospital number |
| Allocation concealment (selection bias) | High risk | Sequence generated by hospital number |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Blinding of care provider: no Blinding of participant: no Blinding of outcome assessors: no |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Blinding of care provider: no Blinding of participant: no Blinding of outcome assessors: no |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Blinding of care provider: no Blinding of participant: no Blinding of outcome assessors: no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Komada 1999.
| Methods | RCT Cross‐over |
|
| Participants | Participants with ALL receiving either high‐dose methotrexate (3 g/m²) or high‐dose cytarabine plus dexamethasone (3 g/m²). Participants aged 6.3 years (range 1 year to 14 years), 21/49 male | |
| Interventions | Granisetron 20 mcg/kg given immediately prior to chemotherapy at 30 min IV infusion Granisetron 40 mcg/kg given immediately prior to chemotherapy at 30 min IV infusion |
|
| Outcomes | Episodes of vomiting recorded | |
| Notes | 13 participants receiving Ara‐C had their treatment 'contaminated' by the concurrent use of dexamethasone as an antiemetic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Blinding of care provider: no Blinding of participant: no Blinding of outcome assessors: no |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts in 24‐hour data, all completed cross‐over |
| Selective reporting (reporting bias) | Low risk | Unclear if the HD‐MTX and high‐dose Ara‐C were identified a priori |
| Other bias | High risk | Participants receiving Ara‐C had their treatment 'contaminated' by the concurrent use of dexamethasone |
Kurucu 2012.
| Methods | RCT | |
| Participants | 18 children (aged 35 to 207 months) receiving chemotherapy including at least 1 highly emetogenic drug. A total of 70 courses were studied | |
| Interventions | 36 courses ‐ participants received ondansetron 5 mg/m2 34 courses ‐ participants received ondansetron 5 mg/m2 plus hydroxyzine 1 mg/kg |
|
| Outcomes | Control of emesis using Skoup‐Smith criteria (Skoup 1990; Smith 1990) Patient performance using Lansky Play‐Performance Scale (Lansky 1987) Degree of symptoms using Symptom Distress Scale (McCorcle 1998) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Placebo not used; hydroxyzine given regularly (orally) throughout chemotherapy course; participants, caregivers, and observers would be aware of additional drug being given |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Placebo not used; hydroxyzine given regularly (orally) throughout chemotherapy course; participants, caregivers, and observers would be aware of additional drug being given |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Placebo not used; hydroxyzine given regularly (orally) throughout chemotherapy course; participants, caregivers, and observers would be aware of additional drug being given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other risk of bias noted |
Mabro 2000.
| Methods | RCT | |
| Participants | Any patients with paediatric malignancy excluding cerebral tumours associated with vomiting Receiving moderately emetogenic chemotherapy:
OR highly emetogenic chemotherapy:
Patients were excluded if: received more than 1 course of chemotherapy in preceding year, radiation therapy in preceding 7 days or during course of study, emetogenic chemotherapy in preceding 7 days, persistent nausea or vomiting in preceding 48 hours, a food intolerance in preceding 4 days, intestinal obstruction, corticosteroids outside of chemotherapy treatment, other antiemetic treatments, cerebral tumours associated with vomiting, liver enzymes outside of specified range Mean age 7.8 years (range 1 year to 16 years). 177/294 participants were male |
|
| Interventions | 143 participants received 20 µg/kg oral granisetron (orange flavoured) ‐ diluted to 0.2 mg/ml and given 1 hour before and again 6 to 12 hours after the start of chemotherapy on each day of chemotherapy for 1 to 5 days (depending on chemotherapy regimen) 151 participants received 40 µg/kg oral granisetron (orange flavoured) ‐ diluted to 0.2 mg/ml and given 1 hour before and again 6 to 12 hours after the start of chemotherapy on each day of chemotherapy for 1 to 5 days (depending on chemotherapy regimen) |
|
| Outcomes | Number of vomits recorded every 6 hours for each 24‐hour period. Nausea assessed by unvalidated self/parent report using a scale of "none, mild, moderate, severe" | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | States "double blind" Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | States "double blind" Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis reported by day |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | Randomisation stratified by emetogenic level of chemotherapy |
Marshall 1989.
| Methods | RCT Cross‐over |
|
| Participants | Any child with a paediatric malignancy on any chemotherapy protocol including BMT conditioning in 6 participants, who had a second course planned. Median age 7 years (4 to 15 years), with 17/26 male | |
| Interventions | Chlorpromazine 0.825 mg/kg QDS IV for 4 doses Cocktail. Metoclopramide (IV) 2 mg/kg/dose 0, 2, 6, 12 hours. Dexamethasone (IV) 0.7 mg/kg 0 hours. Benzatropine (IV) 0.02 mg/kg/dose 0, 6 hours. Lorazepam (PO) 0.05 mg/kg/dose, 1 hour and 12 hours |
|
| Outcomes | Recorded number and duration of vomiting using ?structured interview ‐ unclear | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | "Double blinded" but unclear how effective the placebos would be Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2/26 did not complete the cross‐over |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Unclear risk | 6/26 on conditioning regimens given after 24 hours ‐ could there be a carry‐over effect? |
Mehta 1986.
| Methods | RCT | |
| Participants | 20 children with paediatric malignancy who had any chemotherapy containing adriamycin > 30 mg/m², cisplat > 100 mg/m², MTX > 300 mg/m², actinoD > 0.5 mg/m², cyclo > 1 g/m² or lomustine > 6 mg/m², and had experienced significant toxicity on a previous course. Gender not specified | |
| Interventions | 10 participants received chlorpromazine 0.5 mg/kg IV 30 minutes prior to chemotherapy 10 participants received methylprednisolone 4 mg/kg IV 30 minutes prior to chemotherapy |
|
| Outcomes | Unvalidated assessment of nausea by clinicians for the first 2 to 8 hours, then parental/participant report. Duration of nausea and number of vomiting episodes recorded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | "Double blinded" Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | "Double blinded" Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | High risk | "Double blinded" Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 not evaluated, unclear from which arm or even if excluded prior to randomisation |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Mehta 1997.
| Methods | RCT | |
| Participants | 28 participants with need for stem cell (bone marrow) transplantation (allograft or autologous), including those receiving TBI. Median age 8 years (range 4 to 12 years), 9/15 male in ondansetron group. Median age 9.5 years (range 4 to 17), 9/13 male in perphenazine group. | |
| Interventions | 15 participants received ondansetron loading 0.15 mg/kg then 0.45 mg/kg as continuous infusion 13 participants received perphenazine 0.06 mg/kg loading and 0.4 mg/kg per 24 hours continuous infusion), plus diphenhydramine 1 mg/kg QDS |
|
| Outcomes | "Continuous" recording of vomiting and retching (events within 5 minutes considered as a single episode). Probably nursing staff recording outcomes | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Blinding of care provider: no Blinding of participant: no Blinding of outcome assessors: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 not evaluated, unclear from which arm or even if excluded prior to randomisation |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Nagel 2008.
| Methods | RCT cross‐over trial | |
| Participants | 25 participants aged 4 to 17 years with newly diagnosed ALL | |
| Interventions | Placebo + placebo Fentanyl 1 mg/kg + placebo Placebo + ondansetron 0.15 mg/kg Fentanyl 1 mg/kg + ondansetron 0.15 mg/kg |
|
| Outcomes | Number of vomits Severity of nausea (reported by parents based on disruption of daily activity) Severity of pain (reported using Wong‐Baker FACES) Use of antiemetics Use of analgesia |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but details not provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | Double‐blind, placebo used |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | Double‐blind, placebo used |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Double‐blind, placebo used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11 of 24 participants did not complete all 4 trial arms |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Paired data not provided |
Noguera 2001.
| Methods | RCT Randomised per course, but multiple episodes per participant |
|
| Participants | Participants with 6 different neoplasms: ALL, Wilms, brain, rhabdomyosarcoma, ovarian germ cell, and retinoblastoma, who received highly or very highly emetogenic chemotherapy (cisplatin, high‐dose carboplatin, high‐dose cyclophosphamide, high‐dose methotrexate, or daunorubicin). Median age 6 years (3 months to 14 years), 6/12 participants were male | |
| Interventions | Ondansetron 0.45 mg/kg IV 15 minutes after chemotherapy Tropisetron 0.20 mg/kg IV 15 minutes after chemotherapy Granisetron 20 µg/kg IV 15 minutes after chemotherapy |
|
| Outcomes | Number of vomiting episodes recorded by nurses in charge of care | |
| Notes | Translation and extraction by Moacyr Nobre, InCor HCFMUSP, São Paulo, Brazil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | Stated "double blinded" but no further detail Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | Stated "double blinded" but no further detail Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11 episodes in total (˜15%) missing data ‐ not clear out of which groups |
| Selective reporting (reporting bias) | Unclear risk | Could not be determined |
| Other bias | Low risk | No other risk of bias noted |
Orchard 1999.
| Methods | RCT | |
| Participants | 187 paediatric patients (not all had malignancy) receiving any of the standard conditioning regimens (chemotherapy alone or with TBI) for autologous or allogeneic BMT. Age ranged from 2 to 16 years. Gender split not given | |
| Interventions | 90 participants received granisetron: 10 mcg/kg/dose before start of chemo/TBI then every 12 hours, and placebo: continuous infusion 5% dextrose (until day of transplant ‐ day 0) 97 participants received ondansetron: loading dose 0.15 mg/kg before start of chemo/TBI then continuous infusion 0.03 mg/kg/hour rounded to nearest 0.1 mg (until day of transplant ‐ day 0), and placebo: intermittent 5% dextrose administered every 12 hours All participants: dexamethasone 10 mg/m²/day (max 10 mg). For breakthrough nausea/vomiting, lorazepam 0.05 mg/kg IV or promethazine 0.25 to 1.0 mg/kg IV every 4 to 6 hours. Other antiemetic support was considered as needed |
|
| Outcomes | Child recorded nausea using an unvalidated 'faces' approach (nausea VAS: smiling/frowning faces) Nurse recorded episodes of vomiting |
|
| Notes | 73% of participants had TBI in addition to conditioning chemotherapy Authors provided additional graphical data showing the changes in nausea scores and episodes of vomiting in children over time |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States randomised, but no further details given |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | States double‐blind. Placebo used. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | States double‐blind. Placebo used. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals/dropouts confirmed by author |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | Potentially: 73% of participants had TBI in addition to conditioning chemotherapy |
Parker 2001.
| Methods | RCT Cross‐over | |
| Participants | Participants with ALL receiving intrathecal chemotherapy +/‐ maintenance chemotherapy. Median age 6 years (range 2 to 17 years). 12/26 were male | |
| Interventions | High‐dose ondansetron 0.45 mg/kg IV 15‐minute infusion 30 minutes prior to lumbar puncture Low‐dose ondansetron 0.15 mg/kg IV 15‐minute infusion 30 minutes prior to lumbar puncture Placebo (saline) IV 15‐minute infusion 30 minutes prior to lumbar puncture |
|
| Outcomes | Parental report of number of vomiting episodes recorded | |
| Notes | Given the low potential of chemotherapy itself, seems more like a trial of post‐anaesthetic antiemetics than of chemotherapy‐induced emesis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts between cross‐overs (1 before placebo, 2 before ondansetron 0.45 mg/kg) |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Safonova 1999.
| Methods | RCT | |
| Participants | Any child with a paediatric malignancy whose body surface area was < 1.6 m², who had no pre‐existing nausea and vomiting problems, and had no previous Rx with antiemetics within 24 hours or systematic Rx with steroids or benzodiazepines Chemotherapy was of moderate or high emetogenicity containing dactinomycin > 15 mg/kg, carboplatin > 300 mg/m², cisplatin > 20 mg/m², cyclophosphamide > 500 mg/m², dacarbazine > 200 mg/m², etoposide > 200 mg/m², ifosfamide > 1000 mg/m², methotrexate > 3000 mg/m², cytarabine > 100 mg/m² Age not given. 23/52 participants were male |
|
| Interventions | Ondansetron 8 mg PO BD plus 4 to 8 mg dexamethasone IV Ondansetron 5 mg/m² IV BD plus 4 to 8 mg dexamethasone IV |
|
| Outcomes | Unvalidated parent/participant‐reported nausea outcome, graded from 0, none to 3, strong. Number of episodes of vomiting also recorded | |
| Notes | Translation by Prof David Reeves | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated randomised |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | States double blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Sandoval 1999.
| Methods | RCT | |
| Participants | Any newly diagnosed patient with malignancy undergoing moderately or highly emetogenic chemotherapy Moderately: anthracyclines 25 to 60 mg/m², carboplatin 175 mg/m², cyclophosphamide 1200 to 1500 mg/m², and etoposide 100 mg/m² Highly: cisplatin 100 mg/m², cyclophosphamide > 1500 mg/m², dacarbazine 375 mg/m², and actinomycin 1.35 mg/m² Median age 4 years (range 0.25 to 18 years) with 16/31 participants male |
|
| Interventions | 16 participants received ondansetron 0.6 mg/kg (max 32 mg) IV over 30 minutes before chemotherapy then 3 normal saline doses given every 4 hours starting 4 hours after ondansetron 15 participants received ondansetron 0.15 mg/kg (max 8 mg) IV over 30 minutes before chemotherapy then every 4 hours for a total of 4 doses |
|
| Outcomes | Unvalidated nausea assessment tool, unclear who recorded nausea. Scaled: 1. No nausea or emesis 2. Nauseous but able to eat 3. Nauseous and unable to eat 4. Emesis Number of vomiting episodes and time‐to‐first‐vomit were recorded |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacist not involved in participant care using a random number table |
| Allocation concealment (selection bias) | Low risk | Allocation by pharmacist not involved with participant care |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No loss of participants |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Unclear risk | Multi‐dose group had 7/15 (47%) highly emetogenic chemotherapy regimens compared with 5/16 (31%) in single‐dose group |
Sepulveda‐Vildosola 2008.
| Methods | RCT | |
| Participants | Participants under 16 years old with solid tumours undergoing a total of 100 courses of highly emetogenic chemotherapy (number of individual participants not stated, therefore it is unclear whether some participants had more than 1 course included in the study) | |
| Interventions | In 50 courses, participants received ondansetron 8 mg/m2 IV every 8 hours In 50 courses, participants received palonosetron 0.25 mg IV single dose |
|
| Outcomes | Number of emetic events Severity of nausea (reported by parents/guardians as impact on oral intake) Side effects (though not reported which were assessed) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | Double blind. Difference in dose frequency may have meant participants were not actually blinded |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | Double blind. Difference in dose frequency may have meant participants were not actually blinded |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Double blind. Difference in dose frequency may have meant participants were not actually blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other risk of bias noted |
Shi 2012.
| Methods | RCT | |
| Participants | 80 children with solid tumours | |
| Interventions | 40 children received ondansetron 4 mg IV 40 children received ondansetron 4 mg IV plus hewei zhiou recipe (traditional Chinese medicine) oral |
|
| Outcomes | Self reported vomiting scores (number of vomits and intensity of vomiting) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random digit table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) Acute nausea | Unclear risk | No details |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | No details |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for in results |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other risk of bias noted |
Siddique 2011.
| Methods | RCT | |
| Participants | 60 children aged 4 to 11 scheduled to receive high‐dose methotrexate | |
| Interventions | 30 received ondansetron 4 mg 30 received granisetron 1 mg |
|
| Outcomes | Modified MANE scale (self reported occurrence of nausea, retching, or vomiting) Requirement of additional dose of antiemetic Side effects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified using random sampling |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | Double blind |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | Double blind |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other risk of bias noted |
Suarez 1994.
| Methods | RCT | |
| Participants | Participants with malignant solid tumours receiving low to moderately emetogenic agents only, i.e. single agent or combination therapy with actinomycin, vincristine, methotrexate, cytarabine, etoposide, doxorubicin, daunorubicin, hydroxycarbamide, asparaginase, bleomycin, and/or vinblastine (n = 23) or highly emetogenic agents, i.e. high doses of ifosfamide or cyclophosphamide (n = 21) Excluded if: severe hepatic, renal, or cardiac insufficiency; uncontrolled infection; hypersensitivity or drug allergy; or other current or previous medical condition that might confound findings or put person at risk. Administration during study of antiemetic agents other than study drug, neuroleptics, drugs known to induce hepatic enzyme synthesis, and azole antifungal agents were prohibited. Mean age ˜10 years, 37/44 participants male |
|
| Interventions | (Randomised to 1 of 6 strata: placebo, or tropisetron (0.05, 0.10, 0.20, 0.33, or 0.50 mg/kg) given as single dose by IV infusion (diluted in normal saline to give volume of 1 ml/kg body weight, administered over 15 minutes immediately before start of chemotherapy) or, on discharge, orally in small quantity of orange juice for 5, 6, or 7 days when receiving 1 to 3, 4, or 5 days chemotherapy, respectively | |
| Outcomes | Daily record cards used by participant/family to record nausea and vomiting (unclear exactly how) | |
| Notes | Tropisetron and matching placebo ampoules were provided by Sandoz Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States 2:1 unequal randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Acute vomiting | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis stated; withdrawals detailed and included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Grouping of arms apparently post‐hoc and driven by the trial data |
| Other bias | Low risk | No other risk of bias noted |
Swann 1979.
| Methods | RCT Cross‐over trial but unclear if multiple cross‐overs |
|
| Participants | Any paediatric patient receiving chemotherapy for a malignancy. Age range 2 to 13 years with 6/18 participants male | |
| Interventions | IV metoclopramide (0.5 mg/kg) IV domperidone (1 mg/kg) |
|
| Outcomes | Parents or nurses, or both reported nausea and vomiting episodes and duration. Unvalidated assessment, unclear how undertaken | |
| Notes | Data used as though parallel trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomised crossover design" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not enough detail to assess |
| Other bias | Low risk | No other risk of bias noted |
Tejedor 1999.
| Methods | RCT Serial cross‐over |
|
| Participants | Participants with solid tumour receiving their first (and subsequent) courses of highly emetogenic (ifos or cyclo > 1 g/m², MTX > 1 g/m², dacarbazine > 100 mg/m², cisplat > 30 mg/m², dox > 45 mg/m², actinoD > 0.3 mg/m², carbo > 150 mg/m²) plus another agent Overall mean age 11.5 years (range 2 to 17 years), with 16/30 participants male |
|
| Interventions | Tropisetron 0.2 mg/kg IV 30 minutes prior to infusion Chlorpromazine 5 to 15 mg given over 2‐hour infusion and 2 further 6‐hourly plus dexamethasone 2 mg/m² IV bolus prior to and at 12 hours |
|
| Outcomes | Unvalidated 'verbal report' of nausea given by parent/child, and a numerical assessment of vomiting made | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Cross‐over with only 50% second‐agent completed |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Tsuchida 1999.
| Methods | RCT Cross‐over |
|
| Participants | Participants with solid tumour receiving 2 identical courses of cyclo > 1.2 mg/m² or cisplatin > 90 mg/m². Mean age was 5 years (0.85 to 13 years) with 23/44 episodes in males | |
| Interventions | Granisetron 20 mcg/kg IV prior to chemotherapy Granisetron 40 mcg/kg IV prior to chemotherapy |
|
| Outcomes | Unclear who undertook assessment of number of vomiting episodes | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | Unclear risk | Not stated Blinding of care provider: unclear Blinding of participant: unclear Blinding of outcome assessors: unclear |
| Blinding (performance bias and detection bias) Other outcomes | High risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 dropouts in higher‐dose ondansetron arm ‐ unclear how accounted for; nil in lower‐dose arm |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
White 2000.
| Methods | RCT | |
| Participants | Included people with a variety of malignancies excluding primary and secondary tumours of the CNS, who were to receive moderately to highly emetogenic chemotherapy agents* given for 1 to 8 days (or interspersed with 1 or 2 single days of no or low emetogenic chemotherapy ‐ vincristine, thioguanine). 16% had cisplatin. Excluded if: primary and secondary tumours of the CNS; BSA > 1.6 m², severe concurrent illness, illness associated with nausea and vomiting, emesis or severe nausea in 24 hours preceding chemotherapy, receiving antiemetics other than those under investigation (or in preceding 24 hours), pregnancy or contraindications to ondansetron or dexamethasone Mean age 8 years (range 2 to 17 years), 58% of 250 participants male *These were: cyclophosphamide, doxorubicin or doxorubicin HCL, etoposide, cytarabine, methotrexate, ifosfamide, cisplatin, actinomycin D. Doses not reported. (Participants receiving low emetogenic agents alone were excluded from the analysis.) |
|
| Interventions | 215 participants were given a loading dose IVI ondansetron 5 mg/m² + oral dexamethasone 2 to 4 mg + placebo syrup (20 minutes pre‐chemotherapy) then 6 to 8 hours post‐loading dose, oral dexamethasone 2 mg BD if BSA <= 0.6 m², or 4 mg BD if BSA > 0.6 m². 2 days post‐cessation of chemotherapy, oral ondansetron 4 mg syrup BD. 223 participants were given a loading dose ondansetron syrup 8 mg + oral dexamethasone 2 to 4 mg + placebo by IVI (20 minutes pre‐chemotherapy) then 6 to 8 hours post‐loading dose, oral dexamethasone 2 mg BD if BSA <= 0.6 m², or 4 mg BD if BSA > 0.6 m². 2 days post‐cessation of chemotherapy, oral ondansetron 4 mg syrup BD. |
|
| Outcomes | Unvalidated nausea and vomiting diary cards completed by parent/guardian, child, or healthcare staff. Graded nausea as: none, little bit, or very to reflect: none, mild, moderate, severe Graded appetite as: none, less than usual, same, more than usual. Episodes of vomiting recorded | |
| Notes | Placebos identical to the oral and IV ondansetron preparations were provided by Glaxo Wellcome R&D Ltd. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) Acute nausea | High risk | Not reported |
| Blinding (performance bias and detection bias) Acute vomiting | High risk | Not reported |
| Blinding (performance bias and detection bias) Other outcomes | Low risk | States double‐blind. No further details. Blinding of care provider: yes Blinding of participant: yes Blinding of outcome assessors: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 9/250 participants were excluded from analysis, and study groups were very similar in numbers |
| Selective reporting (reporting bias) | Low risk | — |
| Other bias | Low risk | No other risk of bias noted |
Dosing abbreviations:
BD = twice a day IV = intravenous IVI = intravenous infusion OD = once a day PO = orally QDS = four times a day TDS = three times a day
Chemotherapy abbreviations:
5FU = 5‐fluorouracil 6MP = 6‐mercaptopurine ABVD = adriamycin, bleomycin, vincristine, dacarbazine actinoD = actinomycin D Ara‐C = cytarabine carbo = carboplatin cisplat = cisplatin cyclo = cyclophosphamide dox = doxorubicin dauno = daunorubicin HD‐MTX = high‐dose methotrexate ifos = ifosfamide pred = prednisolone vinc = vincristine
Other abbreviations:
ALL = acute lymphoblastic leukaemia AML = acute myeloid leukaemia BMT = bone marrow transplant BSA = body surface area CNS = central nervous system MANE = Morrow Assessment of Nausea and Emesis MRC = Medical Research Council (UK) NHL = non‐Hodgkin's lymphoma PD = progressive disease RCT = randomised controlled trial Rx = treatment SA = surface area TBI = total body irradiation THC = tetrahydrocannabinol VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aksoylar 2001 | 'Day' randomised rather than participant or episode. Results reported per participant |
| Alavi 1985 | < 18‐year‐olds not extractable |
| Basch 2011 | Not randomised |
| Bauduer 1999 | < 18‐year‐olds not extractable |
| Bonaventura 1999 | < 18‐year‐olds not extractable |
| Brice 1989 | < 18‐year‐olds not extractable |
| Corapcioglu 2005 | Randomisation not adhered to |
| Dana 1987 | < 18‐year‐olds not extractable |
| Delgado 1983 | < 18‐year‐olds not extractable |
| Fauser 1998 | < 18‐year‐olds not extractable |
| Feng 2000 | < 18‐year‐olds not extractable |
| Feng 2002 | < 18‐year‐olds not extractable |
| Forni 2000 | < 18‐year‐olds not extractable |
| Friedman 2000 | < 18‐year‐olds not extractable |
| Gagen 1984 | < 18‐year‐olds not extractable |
| Gez 1989 | < 18‐year‐olds not extractable |
| Gorena 1996 | Not randomised |
| Hatae 1989 | Not randomised |
| Herrstedt 2007 | < 18‐year‐olds not extractable |
| Hirota 1993a | Multiple cross‐overs, uncertainly about how randomised the data are, no data to extract parallel or AB/BA results |
| Jara 1999 | < 18‐year‐olds not extractable |
| Joss 1994 | < 18‐year‐olds not extractable |
| Kearsley 1989 | < 18‐year‐olds not extractable |
| Keyhanian 2009 | Participants not < 18 |
| Koseoglu 1998 | Multiple cross‐overs, uncertainly about how randomised the data are, no data to extract parallel or AB/BA results |
| Luisi 2006 | Multiple cross‐overs, uncertainly about how randomised the data are, no data to extract parallel or AB/BA results |
| Matsuoka 2003 | < 18‐year‐olds not extractable (only 2 participants, 1 in each arm) |
| Nathan 2006 | n‐of‐1 randomisation |
| NCT00429702 2007 | Terminated without publication of results |
| Needles 1999 | < 18‐year‐olds not extractable |
| Onat 1995 | < 18‐year‐olds not extractable |
| Pillai 2011 | < 18‐year‐olds not extractable |
| Relling 1993 | Multiple courses per randomised participant but without any data to enable cluster or first course assessment |
| Roila 1995 | < 18‐year‐olds not extractable |
| Roila 1996 | < 18‐year‐olds not extractable |
| Roila 1998 | < 18‐year‐olds not extractable |
| Roila 2000 | < 18‐year‐olds not extractable |
| Stiakaki 1999 | Multiple courses per randomised participant but without any data to enable cluster or first course assessment |
| Sumer 1988 | Multiple courses per randomised participant but without any data to enable cluster or first course assessment |
| Tian 2011 | Participants not < 18 |
| Tsukuda 2009 | Participants not < 18 |
| Yalcin 1999 | < 18‐year‐olds not extractable |
| Yonemura 2009 | Participants not < 18 |
AB/BA = alternating cross‐over trial with each participant receiving either one treatment (A) followed by a second (B), or in the opposite sequence (B then A)
Characteristics of studies awaiting assessment [ordered by study ID]
Gómez 1995.
| Methods | "un estudio comparativo", "fueron asignados en forma aleatoria" (Comparative study, random assignment) |
| Participants | "31 pacientes con enfermedad neoplásica recibiendo por primera vez quimioterapia en base a cisplatino (CDDP) 100 mg/m²" (31 participants receiving chemotherapy for the first time based on cisplatin) "la edad media fue de 43 años (15‐69)" (mean 43 years old, range 15 to 69) |
| Interventions | "tropisetron (TROP) 5 mg IV (grupo 1), TROP 5 mg con dexametasona (DEX) 20 mg IV (grupo 2) 10 mg con DEX 20 mg IV (grupo 3)" |
| Outcomes | "control total del vómito" y "respuesta completa" (Total control of vomiting and the "complete answer") |
| Notes | Not available via British Library. No web source |
Xu 1997.
| Methods | "a multicenter cooperative study" |
| Participants | 773 participants, no ages given, receiving cisplatin chemotherapy |
| Interventions | "i.v. OND 8 mg once or twice a day" "i.v. OND 8 mg plus dexamethasone (DXM) 10 mg once a day" |
| Outcomes | Acute and delayed nausea and vomiting |
| Notes | Not available via British Library. No web source |
Zeng 1995.
| Methods | "multiple centre, randomized cross‐over trial" |
| Participants | "patients receiving non‐cisplatin chemotherapy (containing CTX and/or ADM)" 155 participants, no ages given |
| Interventions | Ondansetron (Qilu), metoclopramide or Zofran (Glaxo) |
| Outcomes | Control of acute vomiting, frequency of vomits |
| Notes | Not available via British Library. No web source |
Any help in the discovery, translation, and appraisal of these studies would be greatly appreciated.
Differences between protocol and review
The objectives were clarified; the overarching aim was supplemented with specific objectives.
We explicitly excluded non‐pharmacological therapies in the text of this review, instead of implying it in the text of the protocol.
We used marginally different search filters. After discussion within the team, it was felt that the Centre for Reviews and Dissemination (CRD) search filters, which are used to undertake many Health Technology Appraisal reports from the CRD, were the most appropriate filters to apply. Sampling 200 random citations 'missed' with the CRD filter did not demonstrate any additional relevant studies.
During data extraction we also collected data on "potential for selective reporting of outcomes" and "other potential sources of bias" (particularly in respect to cross‐over studies). This was advised following the initial protocol.
The explicit analysis of outcomes to assess for the effect of potential sources of bias, including publication bias, was undertaken but not explicitly stated in the original protocol.
We stated that we would not assess any outcome where more than 50% of participants did not have that outcome. In no trial was there a drop‐out rate that high, and so we did not drop any assessments for this reason.
We did not specify the type of analysis of paired/cross‐over data in the protocol.
Contributions of authors
RSP took part in protocol development, search formulation, initial screening, data extraction and data synthesis, and drafted the report.
AJF undertook screening and data extraction of the 2014 update, and drafted the updated report.
FG took part in protocol development and drafted the report.
EH undertook data extraction and data synthesis.
SG undertook screening and data extraction, and drafted the report.
JVC took part in protocol development and data extraction.
BP took part in protocol development, undertook data extraction and synthesis, and drafted the report.
All review authors have contributed to the final report.
Sources of support
Internal sources
-
Candlelighters: The Yorkshire Children's Cancer Charity, UK.
Financial support for RSP and the conduct of the original review
External sources
No sources of support supplied
Declarations of interest
RSP: no financial conflicts of interest
AJF: no financial conflicts of interest
FG: no financial conflicts of interest
EH: no financial conflicts of interest
SG: no financial conflicts of interest
JVC: no financial conflicts of interest
BP: no financial conflicts of interest
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Alvarez 1995 {published data only}
- Alvarez O, Freeman A, Bedros A, Call SK, Volsch J, Kalbermatter O, et al. Randomized double‐blind crossover ondansetron‐dexamethasone versus ondansetron‐placebo study for the treatment of chemotherapy‐induced nausea and vomiting in pediatric patients with malignancies. Journal of Pediatric Hematology/Oncology 1995;17(2):145‐50. [DOI] [PubMed] [Google Scholar]
Basade 1996 {published data only}
- Basade M, Kulkarni SS, Dhar AK, Sastry PS, Saikia B, Advani SH. Comparison of dexamethasone and metoclopramide as antiemetics in children receiving cancer chemotherapy. Indian Pediatrics 1996;33(4):321‐3. [PubMed] [Google Scholar]
Berrak 2007 {published data only (unpublished sought but not used)}
- Berrak SG, Ozdemir N, Bakirci N, Turkkan E, Canpolat C, Beker B, et al. A double‐blind, crossover, randomized dose‐comparison trial of granisetron for the prevention of acute and delayed nausea and emesis in children receiving moderately emetogenic carboplatin‐based chemotherapy. Supportive Care in Cancer 2007;15(10):1163‐8. [DOI] [PubMed] [Google Scholar]
Brock 1996 {published data only}
- Brock P, Brichard B, Rechnitzer C, Langeveld NE, Lanning M, Soderhall S, et al. An increased loading dose of ondansetron: a north European, double‐blind randomised study in children, comparing 5 mg/m2 with 10 mg/m2. European Journal of Cancer 1996;32A(10):1744‐8. [DOI] [PubMed] [Google Scholar]
Chan 1987 {published data only}
- Chan HS, Correia JA, MacLeod SM. Nabilone versus prochlorperazine for control of cancer chemotherapy‐induced emesis in children: a double‐blind, crossover trial. Pediatrics 1987;79(6):946‐52. [PubMed] [Google Scholar]
Dalzell 1986 {published data only}
- Dalzell AM, Bartlett H, Lilleyman JS. Nabilone: an alternative antiemetic for cancer chemotherapy. Archives of Disease in Childhood 1986;61(5):502‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dick 1995 {published data only}
- Dick GS, Meller ST, Pinkerton CR. Randomised comparison of ondansetron and metoclopramide plus dexamethasone for chemotherapy induced emesis. Archives of Disease in Childhood 1995;73(3):243‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekert 1979 {published data only}
- Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy‐induced nausea and vomiting by delta‐9‐tetrahydrocannabinol. Medical Journal of Australia 1979;2:657‐9. [PubMed] [Google Scholar]
Ekert 1979a {published data only}
- Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy‐induced nausea and vomiting by delta‐9‐tetrahydrocannabinol. Medical Journal of Australia 1979;2(12):657‐9. [PubMed] [Google Scholar]
Emir 2013 {published data only}
- Emir S, Erturgut P, Vidinlisan S. Comparison of granisetron plus dexamethasone versus an antiemetic cocktail containing midazolam and diphenhydramine for chemotherapy induced nausea and vomiting in children. Indian Journal of Medical and Paediatric Oncology 2013;34(4):270‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham‐Pole 1986 {published data only}
- Graham‐Pole J, Weare J, Engel S, Gardner R, Mehta P, Gross S. Antiemetics in children receiving cancer chemotherapy: a double‐blind prospective randomized study comparing metoclopramide with chlorpromazine. Journal of Clinical Oncology 1986;4(7):1110‐3. [DOI] [PubMed] [Google Scholar]
Hahlen 1995 {published data only}
- Hahlen K, Quintana E, Pinkerton CR, Cedar E. A randomized comparison of intravenously administered granisetron versus chlorpromazine plus dexamethasone in the prevention of ifosfamide‐induced emesis in children. Journal of Pediatrics 1995;126(2):309‐13. [DOI] [PubMed] [Google Scholar]
Hirota 1993 {published data only}
- Hirota T, Honjo T, Kuroda R, Saeki K, Katano N, Sakakibara Y, et al. Antiemetic efficacy of granisetron in pediatric cancer treatment ‐ (2). Comparison of granisetron and granisetron plus methylprednisolone as antiemetic prophylaxis. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1993;20(15):2369‐73. [PubMed] [Google Scholar]
Jaing 2004 {published and unpublished data}
- Jaing T‐H, Tsay P‐K, Hung I‐J, Yang C‐P, Hu W‐Y. Single‐dose oral granisetron versus multidose intravenous ondansetron for moderately emetogenic cyclophosphamide‐based chemotherapy in pediatric outpatients with acute lymphoblastic leukemia. Pediatric Hematology & Oncology 2004;21(3):227‐35. [DOI] [PubMed] [Google Scholar]
Komada 1999 {published data only}
- Komada Y, Matsuyama T, Takao A, Hongo T, Nishimura Y, Horibe K, et al. A randomised dose‐comparison trial of granisetron in preventing emesis in children with leukaemia receiving emetogenic chemotherapy. European Journal of Cancer 1999;35(7):1095‐101. [DOI] [PubMed] [Google Scholar]
Kurucu 2012 {published data only}
- Kurucu N, Durmaz M. Effect of hydroxyzine in controlling acute chemotherapy‐induced vomiting in children: a randomised trial. UHOD ‐ Uluslararasi Hematoloji‐Onkoloji Dergisi 2012;22(2):99‐106. [Google Scholar]
Mabro 2000 {published data only}
- Mabro M, Cohn R, Zanesco L, Madon E, Hahlen K, Margueritte G, et al. Oral granisetron solution as prophylaxis for chemotherapy‐induced emesis in children: double‐blind study of 2 doses. Bulletin du Cancer 2000;87(3):259‐64. [PubMed] [Google Scholar]
Marshall 1989 {published data only}
- Marshall G, Kerr S, Vowels M, O'Gorman‐Hughes D, White L. Antiemetic therapy for chemotherapy‐induced vomiting: metoclopramide, benztropine, dexamethasone, and lorazepam regimen compared with chlorpromazine alone. Journal of Pediatrics 1989;115(1):156‐60. [DOI] [PubMed] [Google Scholar]
Mehta 1986 {published data only}
- Mehta P, Gross S, Graham‐Pole J, Gardner R. Methylprednisolone for chemotherapy‐induced emesis: a double‐blind randomized trial in children. Journal of Pediatrics 1986;108(5 Pt 1):774‐6. [DOI] [PubMed] [Google Scholar]
Mehta 1997 {published data only}
- Mehta NH, Reed CM, Kuhlman C, Weinstein HJ, Parsons SK. Controlling conditioning‐related emesis in children undergoing bone marrow transplantation. Oncology Nursing Forum 1997;24(9):1539‐44. [PubMed] [Google Scholar]
Nagel 2008 {published data only}
- Nagel K, Willan AR, Lappan J, Korz L, Buckley N, Barr RD. Pediatric Oncology Sedation Trial (POST): a double‐blind randomized study. Pediatric Blood and Cancer 2008;51:634‐8. [DOI] [PubMed] [Google Scholar]
Noguera 2001 {published data only}
- Noguera D, Gómez R, Marcano A. 5‐hydroxytriptamine type 3 receptor antagonists in the prevention of vomiting by cytostatics drugs in children [Antagonistas de receptores 5‐hidroxitriptamina tipo 3 en la prevención de emesis por citostáticos en niños]. Salus Militiae 2001;26(1):71‐6. [Google Scholar]
Orchard 1999 {published data only (unpublished sought but not used)}
- Orchard PJ, Rogosheske J, Burns L, Rydholm N, Larson H, DeFor TE, et al. A prospective randomized trial of the anti‐emetic efficacy of ondansetron and granisetron during bone marrow transplantation. Biology of Blood & Marrow Transplantation 1999;5(6):386‐93. [DOI] [PubMed] [Google Scholar]
Parker 2001 {published data only}
- Parker RI, Prakash D, Mahan RA, Giugliano DM, Atlas MP. Randomized, double‐blind, crossover, placebo‐controlled trial of intravenous ondansetron for the prevention of intrathecal chemotherapy‐induced vomiting in children. Journal of Pediatric Hematology/Oncology 2001;23(9):578‐81. [DOI] [PubMed] [Google Scholar]
Safonova 1999 {published data only}
- Safonova SA, Gershanovich ML, Punanov IuA, Kolygin BA. Evaluation of the anti‐emetic effectiveness of two drug formulations of ondansetron in combined chemotherapy for children with malignant tumors. Voprosy Onkologii 1999;45(4):424‐8. [PubMed] [Google Scholar]
Sandoval 1999 {published data only}
- Sandoval C, Corbi D, Strobino B, Ozkaynak MF, Tugal O, Jayabose S. Randomized double‐blind comparison of single high‐dose ondansetron and multiple standard‐dose ondansetron in chemotherapy‐naive pediatric oncology patients. Cancer Investigation 1999;17(5):309‐13. [DOI] [PubMed] [Google Scholar]
Sepulveda‐Vildosola 2008 {published data only}
- Sepulveda‐Vildosola AC, Betanzos‐Cabrera Y, Lastiri GG, Rivera‐Marquez H, Villasis‐Keever MA, Angel VW, et al. Palonosetron hydrochloride is an effective and safe option to prevent chemotherapy‐induced nausea and vomiting in children. Archives of Medical Research 2008;39:601‐6. [DOI] [PubMed] [Google Scholar]
Shi 2012 {published data only}
- Shi X, Liu ZM, Zhu XD. Treatment of vomiting in children patients with solid tumor by hewei zhiou recipe combined ondansetron hydrochloride. Chinese Journal of Integrated Traditional and Western Medicine 2012;32(4):468‐70. [PubMed] [Google Scholar]
Siddique 2011 {published data only}
- Siddique R, Hafiz MG, Rokeya B, Jamal CY, Islam A. Ondansetron versus granisetron in the prevention of chemotherapy induced nausea and vomiting in children with acute lymphoblastic leukaemia. Mymensingh Medical Journal 2011;20(4):680‐8. [PubMed] [Google Scholar]
Suarez 1994 {published data only}
- Suarez A, Stettler ER, Rey E, Pons G, Simonetta‐Chateauneuf C, Bruijn KM, et al. Safety, tolerability, efficacy and plasma concentrations of tropisetron after administration at five dose levels to children receiving cancer chemotherapy. European Journal of Cancer 1994;30A(10):1436‐41. [DOI] [PubMed] [Google Scholar]
Swann 1979 {published data only}
- Swann IL, Thompson EN, Qureshi K. Domperidone or metoclopramide in preventing chemotherapeutically induced nausea and vomiting. British Medical Journal 1979;2(6199):1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tejedor 1999 {published data only}
- Tejedor I, Idoate A, Jimenez M, Sierrasesumaga L, Giraldez J. Cost‐effectiveness analysis of tropisetron vs. chlorpromazine‐dexamethasone in the control of acute emesis induced by highly emetogenic chemotherapy in children. Pharmacy World & Science 1999;21(2):60‐8. [DOI] [PubMed] [Google Scholar]
Tsuchida 1999 {published data only}
- Tsuchida Y, Hayashi Y, Asami K, Yamamoto K, Yokoyama J, Mugishima H, et al. Effects of granisetron in children undergoing high‐dose chemotherapy: a multi‐institutional, cross‐over study. International Journal of Oncology 1999;14(4):673‐9. [DOI] [PubMed] [Google Scholar]
White 2000 {published data only}
- White L, Daly SA, McKenna CJ, Zhestkova N, Leal C, Breatnach F, et al. A comparison of oral ondansetron syrup or intravenous ondansetron loading dose regimens given in combination with dexamethasone for the prevention of nausea and emesis in pediatric and adolescent patients receiving moderately/highly emetogenic chemotherapy. Pediatric Hematology & Oncology 2000;17(6):445‐55. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aksoylar 2001 {published and unpublished data}
- Aksoylar S, Akman SA, Ozgenc F, Kansoy S. Comparison of tropisetron and granisetron in the control of nausea and vomiting in children receiving combined cancer chemotherapy. Pediatric Hematology & Oncology 2001;18(6):397‐406. [DOI] [PubMed] [Google Scholar]
Alavi 1985 {published data only}
- Alavi JB, Torri S, Glover D, Hurwitz S, Glick JH. High‐dose oral metoclopramide. An effective antiemetic agent. American Journal of Clinical Oncology 1985;8(3):260‐5. [DOI] [PubMed] [Google Scholar]
Basch 2011 {published data only}
- Basch E, Hesketh PJ, Kris MG, Prestrud AA, Temin S, Lyman GH. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. Journal of Oncology Practice 2011;7(6):395‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bauduer 1999 {published data only}
- Bauduer F, Coiffier B, Desablens B. Granisetron plus or minus alprazolam for emesis prevention in chemotherapy of lymphomas: a randomized multicenter trial. Granisetron Trialists Group. Leukemia & Lymphoma 1999;34(3‐4):341‐7. [DOI] [PubMed] [Google Scholar]
Bonaventura 1999 {published data only}
- Bonaventura A, Freeman S, Stewart J, Ackland S, Hall K, Gebski V. Control of acute and delayed vomiting with lorazepam, dexamethasone and metochlopramide (LDM) versus dexamethasone and ondansetron (DO) following highly emetogenic chemotherapy (meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1999:2299.
Brice 1989 {published data only}
- Brice P, Fiere D, Gastaut JL, Simon‐Lejeune C, Carcassonne Y, Tredaniel J, et al. Comparison of the antiemetic effectiveness of high‐dose corticosteroids with synacthene in nausea induced by chemotherapy: results of a randomized study. Bulletin du Cancer 1989;76(6):637‐42. [PubMed] [Google Scholar]
Corapcioglu 2005 {published data only}
- Corapcioglu F, Sarper N. A prospective randomized trial of the antiemetic efficacy and cost‐effectiveness of intravenous and orally disintegrating tablet of ondansetron in children with cancer. Pediatric Hematology and Oncology 2005;22(2):103‐14. [DOI] [PubMed] [Google Scholar]
Dana 1987 {published data only}
- Dana BW, McDermott M, Everts E, Abdulhay G. A randomized trial of high‐dose bolus metoclopramide versus low‐dose continuous infusion metoclopramide in the prevention of cisplatin‐induced emesis. American Journal of Clinical Oncology 1987;10(3):253‐6. [DOI] [PubMed] [Google Scholar]
Delgado 1983 {published data only}
- Delgado G, Stefanuto W, Novo NF. Preconous toxicity syndrome due to cancer chemotherapy: therapeutic effects of metoclopramide and corticosteroids compared with placebo [Síndrome da toxidade precoce induzida pela quimioterapia antineoplásica: efeito terapêutico de metoclopramida e corticosteróide versus placebo e proposta de um modelo patogênico]. Acta Oncologica (Brasil) 1983;3(1/3):19‐29. [Google Scholar]
Fauser 1998 {published data only}
- Fauser A, Pizzocaro G, Schuller G, Khayat D, Wilkinson P. A double‐blind parallel study of intravenous (IV) dolasetron + dexamethasone vs. iv dolasetron alone in preventing fractionated cisplatin emesis (meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1998:abstract 223.
Feng 2000 {published data only}
- Feng FY, Zhang P, He YJ, Li YH, Zhou MZ, Cheng G, et al. Comparison of the selective serotonin antagonists ramosetron and granisetron in treating acute chemotherapy‐induced emesis, nausea, and anorexia: a single‐blind, randomized, crossover study. Current Therapeutic Research ‐ Clinical and Experimental 2000;61(12):901‐9. [Google Scholar]
Feng 2002 {published data only}
- Feng FY, Zhang P, He YJ, Li YH, Zhou MZ, Cheng G, et al. Oral formulations of the selective serotonin antagonists ramosetron (intraoral disintegrator formulation) and granisetron hydrochloride (standard tablet) in treating acute chemotherapy‐induced emesis, nausea, and anorexia: a multicenter, randomized, single‐blind, crossover, comparison study. Current Therapeutic Research ‐ Clinical and Experimental 2002;63(11):725‐35. [Google Scholar]
Forni 2000 {published data only}
- Forni C, Ferrari S, Loro L, Mazzei T, Beghelli C, Biolchini A, et al. Granisetron, tropisetron, and ondansetron in the prevention of acute emesis induced by a combination of cisplatin‐adriamycin and by high‐dose ifosfamide delivered in multiple‐day continuous infusions. Supportive Care in Cancer 2000;8(2):131‐3. [DOI] [PubMed] [Google Scholar]
Friedman 2000 {published data only}
- Friedman CJ, Burris HA, Yocom K, Blackburn LM, Gruben D. Oral granisetron for the prevention of acute late onset nausea and vomiting in patients treated with moderately emetogenic chemotherapy. Oncologist 2000;5(2):136‐43. [DOI] [PubMed] [Google Scholar]
Gagen 1984 {published data only}
- Gagen M, Gochnour D, Young D, Gaginella T, Neidhart J. A randomized trial of metoclopramide and a combination of dexamethasone and lorazepam for prevention of chemotherapy‐induced vomiting. Journal of Clinical Oncology 1984;2(6):696‐701. [DOI] [PubMed] [Google Scholar]
Gez 1989 {published data only}
- Gez E, Ben‐Yosef R, Catane R, Brufman G, Biran S. Chlorpromazine and dexamethasone versus high‐dose metoclopramide and dexamethasone in patients receiving cancer chemotherapy, particularly cis‐platinum: a prospective randomized crossover study. Oncology 1989;46(3):150‐4. [DOI] [PubMed] [Google Scholar]
Gorena 1996 {published data only}
- Gorena M, Giacaman A, Pastor P. Antiemetic therapy in testis cancer chemotherapy: comparison in cross‐over study of ondansetron and metoclopramide [Terapia antiemética en quimioterapia por cáncer testicular: comparación en estudio cross‐over de ondansetron y metoclopramida]. Revista Chile Urologica 1996;61(1):72‐4. [Google Scholar]
Hatae 1989 {published data only}
- Hatae Y, Takeda T, Nakadate H, Hatayama Y, Kishino T, Ogawa Y. The antiemetic effect and clinical evaluation of metoclopramide alone and combined with betamethasone in children with malignant tumor. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1989;16(11):3639‐42. [PubMed] [Google Scholar]
Herrstedt 2007 {published data only}
- Herrstedt J, Sigsgaard TC, Nielsen HA, Handberg J, Langer SW, Ottesen S, et al. Randomized, double‐blind trial comparing the antiemetic effect of tropisetron plus metopimazine with tropisetron plus placebo in patients receiving multiple cycles of multiple‐day cisplatin‐based chemotherapy. Supportive Care in Cancer 2007;15(4):417‐26. [DOI] [PubMed] [Google Scholar]
Hirota 1993a {published data only}
- Hirota T, Honjo T, Kuroda R, Saeki K, Katano N, Sakakibara Y, et al. Antiemetic efficacy of granisetron in the treatment of pediatric cancer ‐ (1). Clinical evaluation of granisetron at a dose of 40 micrograms/kg. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1993;20(14):2201‐5. [PubMed] [Google Scholar]
Jara 1999 {published data only}
- Jara C, Hernandez M, Alonso C, Fernandez A, Gamez‐Aldarava JL, Mayordomo JI. Dexamethasone with oral granisetron or metoclopramide in preventing chemotherapy‐induced delayed emesis. A randomized, double‐blind trial (meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1999:abstract 2289.
Joss 1994 {published data only}
- Joss RA, Bacchi M, Buser K, Kirchner V, Neuenschwander H, Orth B, et al. Ondansetron plus dexamethasone is superior to ondansetron alone in the prevention of emesis in chemotherapy‐naive and previously treated patients. Swiss Group for Clinical Cancer Research (SAKK). Annals of Oncology 1994;5(3):253‐8. [DOI] [PubMed] [Google Scholar]
Kearsley 1989 {published data only}
- Kearsley JH, Williams AM, Fiumara AM. Antiemetic superiority of lorazepam over oxazepam and methylprednisolone as premedicants for patients receiving cisplatin‐containing chemotherapy. Cancer 1989;64(8):1595‐9. [DOI] [PubMed] [Google Scholar]
Keyhanian 2009 {published data only}
- Keyhanian S, Taziki O, Saravi MM, Fotokian Z. A randomized comparison of granisetron plus dexamethason with granisetron alone for the control of acute chemotherapy‐induced emesis and nausea. International Journal of Hematology‐Oncology and Stem Cell Research 2009;3(2):27‐30. [Google Scholar]
Koseoglu 1998 {published data only}
- Koseoglu V, Kurekci AE, Sarici U, Atay AA, Ozcan O. Comparison of the efficacy and side‐effects of ondansetron and metoclopramide‐diphenhydramine administered to control nausea and vomiting in children treated with antineoplastic chemotherapy: a prospective randomized study. European Journal of Pediatrics 1998;157(10):806‐10. [DOI] [PubMed] [Google Scholar]
Luisi 2006 {published data only}
- Luisi FAV, Petrilli AS, Tanaka C, Caran EMM. Contribution to the treatment of nausea and emesis induced by chemotherapy in children and adolescents with osteosarcoma. Sao Paulo Medical Journal 2006;124(2):61‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuoka 2003 {published data only}
- Matsuoka S, Okamoto S, Watanabe R, Mori T, Nagayama H, Hamano Y, et al. Granisetron plus dexamethasone versus granisetron alone in the prevention of vomiting induced by conditioning for stem cell transplantation: a prospective randomized study. International Journal of Hematology 2003;77(1):86‐90. [DOI] [PubMed] [Google Scholar]
Nathan 2006 {published data only}
- Nathan PC, Tomlinson G, Dupuis LL, Greenberg ML, Ota S, Bartels U, et al. A pilot study of ondansetron plus metopimazine vs. ondansetron monotherapy in children receiving highly emetogenic chemotherapy: a Bayesian randomized serial N‐of‐1 trials design. Supportive Care in Cancer 2006;14(3):268‐76. [DOI] [PubMed] [Google Scholar]
NCT00429702 2007 {published data only}
- NCT00429702. Diphenhydramine, lorazepam, and dexamethasone in treating nausea and vomiting caused by chemotherapy in young patients with newly diagnosed cancer. clinicaltrials.gov/show/NCT00429702 (accessed 15 May 2010).
Needles 1999 {published data only}
- Needles B, Miranda E, Garcia Rodriguez FM, Diaz LB, Spector J, Craig J, et al. A multicenter, double‐blind, randomized comparison of oral ondansetron 8 mg b.i.d., 24 mg q.d., and 32 mg q.d. in the prevention of nausea and vomiting associated with highly emetogenic chemotherapy. S3AA3012 Study Group. Supportive Care in Cancer 1999;7(5):347‐53. [DOI] [PubMed] [Google Scholar]
Onat 1995 {published data only}
- Onat H, Inanc SE, Saip P, Topuz E. Tropisetron (TRP) in the prevention of emesis associated with 5‐day cisplatin‐based chemotherapy (CT) in patients (pts) with germ cell tumors (Meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1995:abstract 1760.
Pillai 2011 {published data only}
- Pillai AK, Sharma KK, Gupta YK, Bakshi S. Anti‐emetic effect of ginger powder versus placebo as an add‐on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatric Blood and Cancer 2011;56:234‐8. [DOI] [PubMed] [Google Scholar]
Relling 1993 {published data only}
- Relling MV, Mulhern RK, Fairclough D, Baker D, Pui CH. Chlorpromazine with and without lorazepam as antiemetic therapy in children receiving uniform chemotherapy. Journal of Pediatrics 1993;123(5):811‐6. [DOI] [PubMed] [Google Scholar]
Roila 1995 {published data only}
- Roila F, Angelis V, Cognetti F, Ricci S, Contu A, Nuzzo A, et al. Ondansetron vs granisetron, both combined with dexamethasone in the prevention of cisplatin‐induced emesis (Meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1995:abstract 1721.
Roila 1996 {published data only}
- Roila F, Angelis V, Contu A, Scagliotti G, Tateo S, Massidda B, et al. Ondansetron (OND) vs metoclopramide (MTC) both combined with dexamethasone (DEX) in the prevention of cisplatin (CDDP)‐induced delayed emesis (Meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1996:abstract 1705.
Roila 1998 {published data only}
- Roila F, Ballatori E, Contu A, Tateo S, Ricci S, Ionta MT, et al. Optimal dose of intravenous dexamethasone in the prevention of cisplatin‐induced acute emesis: a double blind randomized study. (Meeting abstract). ASCO Conference Abstracts. Chicago: ASCO, 1998:abstract 195.
Roila 2000 {published data only}
- Roila F, Ballatori E, Bosnjak S, Fava S, Nuzzo A, Contu A, et al. Dexamethasone (DEX) alone or combined with ondansetron (OND) in the prevention of delayed emesis induced by moderately emetogenic chemotherapy (MEC). Proceedings of the American Society of Clinical Oncology 2000;19:(abstr 2358). [Google Scholar]
Stiakaki 1999 {published data only}
- Stiakaki E, Savvas S, Lydaki E, Bolonaki I, Kouvidi E, Dimitriou H, et al. Ondansetron and tropisetron in the control of nausea and vomiting in children receiving combined cancer chemotherapy. Pediatric Hematology & Oncology 1999;16(2):101‐8. [DOI] [PubMed] [Google Scholar]
Sumer 1988 {published data only}
- Sumer T, Abu‐Melha A, Maqbool G, Al‐Mulhim I. Dexamethasone as an antiemetic in children receiving cis‐platinum. American Journal of Pediatric Hematology/Oncology 1988;10(2):126‐8. [Google Scholar]
Tian 2011 {published data only}
- Tian W, Wang Z, Zhou J, Zhang S, Wang J, Chen Q, et al. Randomized, double‐blind, crossover study of palonosetron compared with granisetron for the prevention of chemotherapy‐induced nausea and vomiting in a Chinese population. Medical Oncology 2011;28:71‐8. [DOI] [PubMed] [Google Scholar]
Tsukuda 2009 {published data only}
- Tsukuda M, Ishitoya J, Mikami Y, Matsuda H, Katori H, Horiuchi C, et al. Antiemetic effects of granisetron and dexamethasone combination therapy during cisplatin‐containing chemotherapy for head and neck cancer: dexamethasone dosage verification trial. International Journal of Clinical Oncology 2009;14:337‐43. [DOI] [PubMed] [Google Scholar]
Yalcin 1999 {published data only}
- Yalcin S, Tekuzman G, Baltali E, Ozisik Y, Barista I. Serotonin receptor antagonists in prophylaxis of acute and delayed emesis induced by moderately emetogenic, single‐day chemotherapy: a randomized study. American Journal of Clinical Oncology: Cancer Clinical Trials 1999;22(1):94‐6. [DOI] [PubMed] [Google Scholar]
Yonemura 2009 {published data only}
- Yonemura M, Katsumata N, Hashimoto H, Satake S, Kaneko M, Kobayashi Y, et al. Randomized controlled study comparing two doses of intravenous granisetron (1 and 3 mg) for acute chemotherapy‐induced nausea and vomiting in cancer patients: a non‐inferiority trial. Japanese Journal of Clinical Oncology 2009;39(7):443‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gómez 1995 {published data only}
- Gómez MH, Mas LL, Vallejos SC. Tropisetron with dexamethasone in the control of nausea and vomiting in emetogenic chemotherapy [Tropisetron mas dexametasona en el control de náuseas y vómitos en pacientes con quimioterapia emetizante]. Acta Cancerologica 1995;25(2):55‐60. [Google Scholar]
Xu 1997 {published data only}
- Xu B, Zhou J, Zhou A. Phase III clinical studies with ondansetron (Qilu) in the prophylaxis of nausea and vomiting induced by cisplatin. Chung‐Hua Chung Liu Tsa Chih [Chinese Journal of Oncology] 1997;19(5):358‐61. [PubMed] [Google Scholar]
Zeng 1995 {published data only}
- Zeng W, Zhou J, Zhang P. The role of ondansetron (Qilu) in the prevention of non‐cisplatin‐induced vomiting ‐ a randomized clinical trial. Chung‐Hua Chung Liu Tsa Chih [Chinese Journal of Oncology] 1995;17(4):294‐7. [PubMed] [Google Scholar]
Additional references
Antonarakis 2004a
- Antonarakis ES, Evans JL, Heard GF, Noonan LM, Pizer BL, Hain RDW. Prophylaxis of acute chemotherapy‐induced nausea and vomiting in children with cancer: what is the evidence?. Pediatric Blood and Cancer 2004;43(6):651‐8. [DOI] [PubMed] [Google Scholar]
Antonarakis 2004b
- Antonarakis ES, Hain RDW. Nausea and vomiting associated with cancer chemotherapy: drug management in theory and in practice. Archives of Disease in Childhood 2004;89(9):877‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331(7521):897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2009
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JPT, Churchill R, et al. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. The Lancet 2009;373(9665):746‐58. [DOI] [PubMed] [Google Scholar]
Cotanch 1985
- Cotanch P, Hockenberry M, Herman S. Self‐hypnosis as antiemetic therapy in children receiving chemotherapy. Oncology Nursing Forum 1985;12(4):41‐6. [PubMed] [Google Scholar]
Coyne 2006
- Coyne I. Children's experiences of hospitalization. Journal of Child Health Care: For Professionals Working with Children in the Hospital and Community 2006;10(4):326‐36. [DOI] [PubMed] [Google Scholar]
Culy 2001
- Culy CR, Bhana N, Plosker GL. Ondansetron: a review of its use as an antiemetic in children. Paediatric Drugs 2001;3(6):441‐79. [DOI] [PubMed] [Google Scholar]
Darbyshire 2005
- Darbyshire P, MacDougall C, Schiller W. Multiple methods in qualitative research with children: more insight or just more?. Qualitative Research 2005;5(4):417‐36. [Google Scholar]
Dodd 2001
- Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, et al. Advancing the science of symptom management. Journal of Advanced Nursing 2001;33(5):668‐76. [DOI] [PubMed] [Google Scholar]
Dolgin 1985
- Dolgin MJ, Katz ER, McGinty K, Siegel SE. Anticipatory nausea and vomiting in pediatric cancer patients. Pediatrics 1985;75(3):547‐52. [PubMed] [Google Scholar]
Dolgin 1989
- Dolgin MJ, Katz ER, Zeltzer LK, Landsverk J. Behavioral distress in pediatric patients with cancer receiving chemotherapy. Pediatrics 1989;84(1):103‐10. [PubMed] [Google Scholar]
Dupuis 2006
- Dupuis LL, Taddio A, Kerr EN, Kelly A, MacKeigan L. Development and validation of the pediatric nausea assessment tool for use in children receiving antineoplastic agents. Pharmacotherapy 2006;26(9):1221‐31. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Zellweger‐Zohner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. The Lancet 1997;350(9074):326‐9. [DOI] [PubMed] [Google Scholar]
Einhorn 2005
- Einhorn LH, Rapoport B, Koeller J, Grunberg SM, Feyer P, Rittenberg C, et al. Antiemetic therapy for multiple‐day chemotherapy and high‐dose chemotherapy with stem cell transplant: review and consensus statement. Supportive Care in Cancer 2005;13(2):112‐6. [DOI] [PubMed] [Google Scholar]
Foot 1994
- Foot AB, Hayes C. Audit of guidelines for effective control of chemotherapy and radiotherapy induced emesis. Archives of Disease in Childhood 1994;71(5):475‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2007
- Gibson F, Aldiss S, Kearney N, Miller M, Maguire R, McCann L, et al. An evaluation of an advanced symptom management system (ASyMS(C)) to monitor and manage chemotherapy related toxicity with young people. Centre for Research Nursing and Allied Health Professions, Great Ormond Street Hospital for Children and Cancer Care. London, 2007.
Glass 2002
- Glass N. UK charity to involve public in decision making for cancer research priorities. The Lancet 2002;360(9344):1487. [Google Scholar]
Hedstrom 2003
- Hedstrom M, Haglund K, Skolin I, Essen L. Distressing events for children and adolescents with cancer: child, parent, and nurse perceptions. Journal of Pediatric Oncology Nursing 2003;20(3):120‐32. [DOI] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinds 2008
- Hinds PS. Patient‐reported outcomes: a desirable specialty standard for oncology or an incomplete measurement approach?. Cancer Nursing 2008;31(4):259‐60. [DOI] [PubMed] [Google Scholar]
Holdsworth 2006
- Holdsworth MT, Raisch DW, Frost J. Acute and delayed nausea and emesis control in pediatric oncology patients. Cancer 2006;106(4):931‐40. [DOI] [PubMed] [Google Scholar]
Juni 2002
- Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta‐analyses of controlled trials: empirical study. International Journal of Epidemiology 2002;31(1):115‐23. [DOI] [PubMed] [Google Scholar]
Kremer 2014
- Kremer LCM, Leclercq E, Dalen EC. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2014, issue 8. Art. No.: CHILDCA.
Kris 2005
- Kris MG, Hesketh PJ, Herrstedt J, Rittenberg C, Einhorn LH, Grunberg SM, et al. Consensus proposals for the prevention of acute and delayed vomiting and nausea following high‐emetic‐risk chemotherapy. Supportive Care in Cancer 2005;13(2):85‐96. [DOI] [PubMed] [Google Scholar]
Lansky 1987
- Lansky SB, List MA, Lansky LL, Ritter‐Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer 1987;60:1651‐6. [DOI] [PubMed] [Google Scholar]
Linder 2005
- Linder LA. Measuring physical symptoms in children and adolescents with cancer. Cancer Nursing 2005;28(1):16‐26. [DOI] [PubMed] [Google Scholar]
McCorcle 1998
- McCorcle R, Cooley M, Shea JA. A User's Manual for the Symptom Distress Scale. Philadelphia: University of Pennsylvania ‐ National Institute of Nursing Research, 1998. [Google Scholar]
Meyer 2006
- Meyer S, Eden T, Kalirai H. Dexamethasone protects against cisplatin‐induced activation of the mitochondrial apoptotic pathway in human osteosarcoma cells. Cancer Biology & Therapy 2006;5(8):915‐20. [DOI] [PubMed] [Google Scholar]
Moher 2003
- Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technology Assessment (Winchester, England) 2003;7(41):1‐90. [DOI] [PubMed] [Google Scholar]
Moody 2006
- Moody K, Meyer M, Mancuso CA, Charlson M, Robbins L. Exploring concerns of children with cancer. Supportive Care in Cancer 2006;14(9):960‐6. [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2014.
Rheingans 2008
- Rheingans JI. Pediatric oncology nurses' management of patients' symptoms. Journal of Pediatric Oncology Nursing 2008;25(6):303‐11. [DOI] [PubMed] [Google Scholar]
Richardson 2007
- Richardson J, Smith JE, McCall G, Richardson A, Pilkington K, Kirsch I. Hypnosis for nausea and vomiting in cancer chemotherapy: a systematic review of the research evidence. European Journal of Cancer Care 2007;16(5):402‐12. [DOI] [PubMed] [Google Scholar]
Roila 2005
- Roila F, Feyer P, Maranzano E, Olver I, Clark‐Snow R, Warr D, et al. Antiemetics in children receiving chemotherapy. Supportive Care in Cancer 2005;13(2):129‐31. [DOI] [PubMed] [Google Scholar]
Sammons 2009
- Sammons H. Ethical issues of clinical trials in children: a European perspective. Archives of Disease in Childhood 2009;94(6):474‐7. [DOI] [PubMed] [Google Scholar]
Skoup 1990
- Skoup M. A comparison of two dose levels of granisetron in patients receiving high dose cisplatin. European Journal of Cancer 1990;26(Suppl 1):15‐9. [PubMed] [Google Scholar]
Smith 1990
- Smith IE. A comparison of two dose levels of granisetron in patients receiving moderately emetogenic cytostatic chemotherapy. European Journal of Cancer 1990;26(Suppl 1):19‐23. [PubMed] [Google Scholar]
Sutton 2008
- Sutton AJ, Higgins JPT. Recent developments in meta‐analysis. Statistics in Medicine 2008;27(5):625‐50. [DOI] [PubMed] [Google Scholar]
Tallon 2000
- Tallon D, Chard J, Dieppe P. Relation between agendas of the research community and the research consumer. The Lancet 2000;355(9220):2037‐40. [DOI] [PubMed] [Google Scholar]
Tremont‐Lukats 2007
- Tremont‐Lukats IW, Bruera E, González‐Barboteo J. Neurokinin‐1 receptor antagonists for prevention of chemotherapy‐related nausea and vomiting in adults. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006844] [DOI] [Google Scholar]
WHO 2007
- WHO. Make medicines child size. http://www.who.int/childmedicines/en/index.html 6 Dec 2007 (accessed October 2009).
Wilby 2005
- Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al. Clinical effectiveness, tolerability and cost‐effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technology Assessment (Winchester, England) 2005;9(15):1‐157, iii‐iv‐1‐157, iii‐iv. [DOI] [PubMed] [Google Scholar]
Williams 2006
- Williams PD, Schmideskamp J, Ridder EL, Williams AR. Symptom monitoring and dependent care during cancer treatment in children: pilot study. Cancer Nursing 2006;29(3):188‐97. [DOI] [PubMed] [Google Scholar]
Wolfe 2000
- Wolfe J, Grier HE, Klar N, Levin SB, Ellenbogen JM, Salem‐Schatz S, et al. Symptoms and suffering at the end of life in children with cancer. The New England Journal of Medicine 2000;342(5):326‐33. [DOI] [PubMed] [Google Scholar]
Woodgate 2003
- Woodgate RL, Degner LF, Yanofsky R. A different perspective to approaching cancer symptoms in children. Journal of Pain and Symptom Management 2003;26(3):800‐17. [DOI] [PubMed] [Google Scholar]
Woodgate 2005
- Woodgate RL. A different way of being: adolescents' experiences with cancer. Cancer Nursing 2005;28(1):8‐15. [DOI] [PubMed] [Google Scholar]
Zeltzer 1991
- Zeltzer LK, Dolgin MJ, LeBaron S, LeBaron C. A randomized, controlled study of behavioral intervention for chemotherapy distress in children with cancer. Pediatrics 1991;88(1):34‐42. [PubMed] [Google Scholar]
Zhang 2006
- Zhang C, Beckermann B, Kallifatidis G, Liu Z, Rittgen W, Edler L, et al. Corticosteroids induce chemotherapy resistance in the majority of tumour cells from bone, brain, breast, cervix, melanoma and neuroblastoma. International Journal of Oncology 2006;29(5):1295‐301. [PubMed] [Google Scholar]
Zou 2007
- Zou GY. One relative risk versus two odds ratios: implications for meta‐analyses involving paired and unpaired binary data. Clinical Trials 2007;4(1):25‐31. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Phillips 2009
- Phillips RS, Gibson F, Gopaul S, Light K, Craig JV, Pizer B. Antiemetic medication for prevention and treatment of chemotherapy induced nausea and vomiting in childhood (Protocol). Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007786] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2010
- Phillips RS, Gopaul S, Gibson F, Houghton E, Craig JV, Light K, Pizer B. Antiemetic medication for prevention and treatment of chemotherapy induced nausea and vomiting in childhood. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007786.pub2] [DOI] [PubMed] [Google Scholar]


