Abstract
Background
Very high blood pressure during pregnancy poses a serious threat to women and their babies. The aim of antihypertensive therapy is to lower blood pressure quickly but safety, to avoid complications. Antihypertensive drugs lower blood pressure but their comparative effectiveness and safety, and impact on other substantive outcomes is uncertain.
Objectives
To compare different antihypertensive drugs for very high blood pressure during pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group Trials Register (9 January 2013).
Selection criteria
Studies were randomised trials. Participants were women with severe hypertension during pregnancy. Interventions were comparisons of one antihypertensive drug with another.
Data collection and analysis
Two review authors independently assessed trials for inclusion and assessed trial quality. Two review authors extracted data and checked them for accuracy.
Main results
Thirty‐five trials (3573 women) with 15 comparisons were included. Women allocated calcium channel blockers were less likely to have persistent high blood pressure compared to those allocated hydralazine (six trials, 313 women; 8% versus 22%; risk ratio (RR) 0.37, 95% confidence interval (CI) 0.21 to 0.66). Ketanserin was associated with more persistent high blood pressure than hydralazine (three trials, 180 women; 27% versus 6%; RR 4.79, 95% CI 1.95 to 11.73), but fewer side‐effects (three trials, 120 women; RR 0.32, 95% CI 0.19 to 0.53) and a lower risk of HELLP (haemolysis, elevated liver enzymes and lowered platelets) syndrome (one trial, 44 women; RR 0.20, 95% CI 0.05 to 0.81).
Labetalol was associated with a lower risk of hypotension compared to diazoxide (one trial 90 women; RR 0.06, 95% CI 0.00 to 0.99) and a lower risk of caesarean section (RR 0.43, 95% CI 0.18 to 1.02), although both were borderline for statistical significance.
Both nimodipine and magnesium sulphate were associated with a high incidence of persistent high blood pressure, but this risk was lower for nimodipine compared to magnesium sulphate (one trial, 1650 women; 47% versus 65%; RR 0.84, 95% CI 0.76 to 0.93). Nimodipine was associated with a lower risk of respiratory difficulties (RR 0.28, 95% CI 0.08 to 0.99), fewer side‐effects (RR 0.68, 95% CI 0.55 to 0.85) and less postpartum haemorrhage (RR 0.41, 95% CI 0.18 to 0.92) than magnesium sulphate. Stillbirths and neonatal deaths were not reported.
There are insufficient data for reliable conclusions about the comparative effects of any other drugs.
Authors' conclusions
Until better evidence is available the choice of antihypertensive should depend on the clinician's experience and familiarity with a particular drug; on what is known about adverse effects; and on women's preferences. Exceptions are nimodipine, magnesium sulphate (although this is indicated for women who require an anticonvulsant for prevention or treatment of eclampsia), diazoxide and ketanserin, which are probably best avoided.
Keywords: Female; Humans; Pregnancy; Antihypertensive Agents; Antihypertensive Agents/adverse effects; Antihypertensive Agents/therapeutic use; Calcium Channel Blockers; Calcium Channel Blockers/adverse effects; Calcium Channel Blockers/therapeutic use; Hypertension, Pregnancy‐Induced; Hypertension, Pregnancy‐Induced/drug therapy; Pre‐Eclampsia; Pre‐Eclampsia/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Drugs for treatment of very high blood pressure during pregnancy
Pregnant women with very high blood pressure (hypertension) can reduce their blood pressure with antihypertensive drugs, but the most effective antihypertensive drug during pregnancy is unknown. The aim of antihypertensive therapy is to lower blood pressure quickly but safely for both the mother and her baby, avoiding sudden drops in blood pressure that can cause dizziness or fetal distress.
During pregnancy, a woman's blood pressure falls in the first few weeks then rises again slowly from around the middle of pregnancy, reaching pre‐pregnancy levels at term. Pregnant women with very high blood pressure (systolic over 160 mmHg, diastolic 110 mmHg or more) are at risk of developing pre‐eclampsia with associated kidney failure and premature delivery, or of having a stroke. The review of 35 randomised controlled trials including 3573 women (in the mid to late stages of pregnancy, where stated) found that while antihypertensive drugs are effective in lowering blood pressure, there is not enough evidence to show which drug is the most effective. Fifteen different comparisons of antihypertensive treatments were included in these 35 trials, which meant that some comparisons were made by single trials. Only one trial had a large number of participants. This trial compared nimodipine with magnesium sulphate and showed that high blood pressure persisted in 47% and 65% of women, respectively. Calcium channel blockers were associated with less persistent hypertension than with hydralazine and possibly less side‐effects compared to labetalol. There is some evidence that diazoxide may result in a woman's blood pressure falling too quickly, and that ketanserin may not be as effective as hydralazine. Further research into the effects of antihypertensive drugs during pregnancy is needed.
Background
Description of the condition
During normal pregnancy there are considerable changes in blood pressure. Within the first weeks the woman's blood pressure falls, largely due to a general relaxation of muscles within the blood vessels (de Swiet 2002). Cardiac output also increases. From around the middle of pregnancy blood pressure slowly rises again until, at term, blood pressure is close to the level it was before pregnancy. Blood pressure during pregnancy can be influenced by many other factors including, time of day, physical activity, position and anxiety. Modest rises in blood pressure alone may have little effect on the outcome of pregnancy, but high blood pressure is often associated with other complications. Of these, the most common is pre‐eclampsia. This is a multisystem disorder of pregnancy which commonly presents with raised blood pressure and proteinuria (Roberts 2009), and occurs in between two to eight per cent of pregnancies (WHO 1988). Although the outcome for most of these pregnancies is good, women with pre‐eclampsia have an increased risk of developing serious problems, such as kidney failure, liver failure, abnormalities of the clotting system, stroke, premature delivery (birth before 37 completed weeks), stillbirth or death of the baby in the first few weeks of life (Tuffnell 2006).
In view of the many factors that can influence blood pressure, it is not surprising that there is often uncertainty about whether a specific abnormal measurement is potentially harmful for that woman. Once blood pressure rises above a certain level, however, there is a risk of direct damage to the blood vessel wall, regardless of what caused the rise. This risk is not specific to pregnancy, as it is similar for non‐pregnant people with very high blood pressure. The level at which this risk merits mandatory antihypertensive therapy is usually considered to be 170 mmHg systolic blood pressure or 110 mmHg diastolic (Tuffnell 2006). If the woman has signs and symptoms associated with severe pre‐eclampsia (such as hyperreflexia, severe headache, sudden onset of epigastric pain, or lowered platelets) a lower threshold for treatment may be recommended (CEMD‐UK 2011). The possible consequences of such high blood pressure for the mother include kidney failure, liver failure and cerebrovascular haemorrhage (stroke). In the UK, for example, stoke resulting from severe hypertension was the single most common cause of maternal death associated with pre‐eclampsia (CEMD‐UK 2011). For the baby, risks include fetal distress due to impaired blood supply across the placenta, and placental abruption (separation of the placenta from the wall of the womb before birth).
Description of the intervention
Once blood pressure reaches 170 mmHg systolic or 110 mmHg diastolic, the woman is at increased risk of harmful effects. There is therefore a general consensus that she should receive antihypertensive drugs, to lower her blood pressure, and that she should be in a hospital. The aim of treatment is to quickly bring about a smooth reduction in blood pressure to levels that are safe for both mother and baby, but avoiding any sudden drops that may in themselves cause problems such as dizziness or fetal distress.
A wide range of antihypertensive drugs have been compared for management of severe hypertension during pregnancy. The most commonly recommended drugs include hydralazine, labetalol and nifedipine (Lindheimer 2008; Lowe 2009; Magee 2008; NICE 2010; WHO 2011) and there is most experience with these.
In general, maternal side‐effects are not different from those in the non‐pregnant state, and are listed in pharmacological texts. All drugs used to treat hypertension in pregnancy cross the placenta, and so may affect the fetus directly by means of their action within the fetal circulation, or indirectly by their effect on uteroplacental perfusion.
The care of women with very high blood pressure during pregnancy is often complex.For women who have pre‐eclampsia, there is also the question of whether there is additional benefit from prophylactic anticonvulsant drugs, and this question is covered in the review 'Anticonvulsants for women with pre‐eclampsia' (Duley 2010). In addition, other Cochrane reviews relevant to the care of women with severe hypertension include plasma volume expansion (Duley 1999), and steroids for HELLP (haemolysis, elevated liver enzymes and lowered platelets) syndrome (Woudstra 2010). Once blood pressure is controlled, in many cases a decision will be made to deliver the baby fairly soon, particularly if the pregnancy is at or near to term. If the baby is very premature, the blood pressure responds well to initial treatment, and there are no other complicating factors, the pregnancy may be continued with the hope that this will improve outcome for the baby. This issue of timing of delivery for severe pre‐eclampsia before 34 weeks' gestation is covered by a separate review (Churchill 2002). Treatment of mild to moderate hypertension in pregnancy has been reviewed by Abalos 2007.
Why it is important to do this review
Very high blood pressure needs to be lowered to protect the woman. This needs to be done in a controlled manner, to avoid complications for the mother and baby, While all antihypertensive drugs lower blood pressure, their comparative benefits and adverse effects when used for very high blood pressure during pregnancy remain uncertain.
The aim of this review is to compare the different types of antihypertensive drugs used for women with severe hypertension during pregnancy to determine which agent has the greatest comparative benefit with the least risk.
Objectives
To compare the effects of different antihypertensive drugs when used to lower very high blood pressure during pregnancy on:
substantive maternal morbidity;
morbidity and mortality for the baby;
side‐effects for the woman.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials were included. Studies with clearly inadequate concealment of allocation were excluded, as were those with a quasi‐random or cross‐over design.
Cluster‐randomised studies designs are unlikely to be relevant to most interventions for treatment of women with high blood pressure, and are therefore unlikely to be identified. If such studies have been conducted, they will not be automatically excluded, rather, the relevant review authors will consider and justify whether or not it is appropriate to include them.
Studies presented only as abstract were considered for inclusion.
Types of participants
Women with severe hypertension (defined whenever possible as diastolic 105 mmHg or more and/or systolic 160 mmHg or more) during pregnancy, requiring immediate treatment. Women postpartum at trial entry were excluded, as the outcomes of interest for these women are substantially different.
Types of interventions
Any comparison of one antihypertensive drug with another regardless of dose, route of administration or duration of therapy. Comparisons of alternative regimens for the same drug and of alternatives within the same class of drug are not included, but may be considered for future updates.
Types of outcome measures
Primary outcomes
For the woman
Death: death during pregnancy or up to 42 days after end of pregnancy, or death more than 42 days after the end of pregnancy
Eclampsia (seizures superimposed on pre‐eclampsia), or recurrence of seizures
Stroke
Persistent high blood pressure: defined, if possible, as either the need for an antihypertensive drug other than the allocated treatment, or failure to control blood pressure on the allocated treatment
For the child
Death: stillbirths (death in utero at or after 20 weeks' gestation), perinatal deaths (stillbirths plus deaths in the first week of life), death before discharge from hospital, neonatal deaths (death in the first 28 days after birth), deaths after the first 28 days
Secondary outcomes
For the woman
Any serious morbidity: defined as at least one of stroke, kidney failure, liver failure, HELLP syndrome (haemolysis, elevated liver enzymes and low platelets syndrome), disseminated intravascular coagulation, pulmonary oedema (fluid in the lungs)
Kidney failure
Liver failure
HELLP syndrome
Disseminated intravascular coagulation
Pulmonary oedema (fluid in the lungs)
Hypotension (low blood pressure): defined if possible as low blood pressure causing clinical problems
Side‐effects of the drug
Abruption of the placenta or antepartum haemorrhage
Need for magnesium sulphate (added in the 2013 update)
Elective delivery: induction of labour or caesarean section
Caesarean section: emergency and elective
Postpartum haemorrhage: defined as blood loss of 500 mL or more
Use of hospital resources: visit to day care unit, antenatal hospital admission, intensive care (admission to intensive care unit, length of stay) ventilation, dialysis
Postnatal depression
Breastfeeding, at discharge and up to one year after the birth
Women's experiences and views of the interventions: childbirth experience, physical and psychological trauma, mother‐infant interaction and attachment
For the child
Preterm birth: defined as birth before 37 completed weeks' gestation, very preterm birth (before 32 to 34 completed weeks) and extremely preterm birth (before 26 to 28 completed weeks)
Death before discharge from hospital or in a special care nursery for more than seven days
Respiratory distress syndrome
Infection
Necrotising enterocolitis
Retinopathy of prematurity
Intraventricular haemorrhage
Apgar score at five minutes: low (less than seven) and very low (less than four) or lowest reported
Side‐effects associated with the drug
In a special care nursery for more than seven days
Use of hospital resources: admission to special care nursery, length of stay, endotracheal intubation, use of mechanical ventilation
Long‐term growth and development: blindness, deafness, seizures, poor growth, neurodevelopmental delay and cerebral palsy
Economic outcomes
Costs to health service resources: short term and long term for both mother and baby
Costs to the woman, her family, and society
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group Trials Register by contacting the Trials Search Co‐ordinator (9 January 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
For details of searching carried out in earlier versions of this review, please seeAppendix 1.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 2.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we will consulted a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third review author. We entered data into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
Studies with high risk of bias for allocation concealment were excluded.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies are at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We will assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed the methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups; ≦ 20% participants missing);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation; > 20% participants missing);
unclear risk of bias.
If it was not possible to enter data based on intention‐to‐treat or 20% or more participants were excluded from the analysis of that outcome, then the trial was excluded.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials and the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Although cluster‐randomised trials of interventions for treatment of very high blood pressure are unlikely, if identified in future updates and they meet all other eligibility criteria, they will be included along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were excluded.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
Data are presented by class of drug. In addition, the following subgroup analyses will be conducted when sufficient data become available:
treatment regimen within each class of drug;
whether severe hypertension alone, or severe hypertension plus proteinuria at trial entry.
The subgroup analysis will be restricted to the review’s primary outcomes.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2011). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
When appropriate, in future updates, we will carry out sensitivity analysis to explore the effect of trial quality based on concealment of allocation, by excluding studies with unclear allocation concealment.
Results
Description of studies
Results of the search
Thirty nine trial reports were identified from the updated search (2013). The review now includes: a total of 35 trials (Argentina 1990; Australia 1986; Australia 2007; Brazil 1992; Brazil 1994; Brazil 2011; England 1982; France 2010; Germany 1998; Germany 2006; India 2006; India 2011; Iran 2002; Iran 2011; Malaysia 2012; Mexico 1989; Mexico 1993; Mexico 1998; Netherlands 1999; Netherlands 2003; Nimodipine SG 2003; N Ireland 1991; Panama 2006; South Africa 1987; South Africa 1989; South Africa 1992; South Africa 1995; South Africa 1997; South Africa 1997a; South Africa 1997b; South Africa 2000; Switzerland 2012; Tunisia 2002; Turkey 1996; USA 1987); 65 trials are excluded (Adair 2009; Adair 2010; Anonymous 2006; Argentina 1986; Aslam 2007; Australia 2002; Bangladesh 2002; Belfort 2006; Brazil 1984; Brazil 1988; Brazil 1988a; China 2000; Devi 2012; Egerman 2008; Egypt 1988; Egypt 1989; Egypt 1992; Esmaoglu 2009; France 1986; Ghana 1995; Graves 2012; Gris 2011; Hladunewich 2006; Hopate 2008; India 1963; India 2001; Iran 1994; Israel 1991; Israel 1999; Italy 2004; Jamaica 1999; Japan 1999; Japan 2000; Japan 2002; Japan 2003; Johnston 2006; Lam 2008; Malaysia 1996; Manzur‐Verastegui 2008; Mexico 1967; Mexico 2000; Mexico 2004; Netherlands 2002; New Zealand 1986; New Zealand 1992; Philipines 2000; Pogue 2006; Roes 2006; Samangaya 2009; Schackis 2004; Scotland 1983; Singapore 1971; Smith 2005; South Africa 1982; South Africa 1984; South Africa 1993; South Africa 2002; Spain 1988; Steyn 2003; Sweden 1993; Unemori 2009; USA 1999; Venezuela 2001; Waheed 2005; Warren 2004); one trial is ongoing (Diemunsch 2008); and one trial (Mesquita 1995) is awaiting assessment.
Included studies
The review includes 35 trials into which 3573 women were recruited. All the trials were small, apart from one large study (1750 women) comparing nimodipine with magnesium sulphate (Nimodipine SG 2003) The women had very high blood pressure; almost all had diastolic blood pressure 110 mmHg or above at trial entry. Nine studies (2292 women) also stated that the women had either 'proteinuria' or 'pre‐eclampsia' as an inclusion criterion. Several trials specified a minimum gestational age for recruitment, and this ranged from 20 weeks to 36 weeks. Others stated that delivery was planned for soon after treatment. One small trial (30 women) (N Ireland 1991) had minimum entry criteria of a blood pressure of 140/90 mmHg but was included as most women were stated to have had labile blood pressure, proteinuria and symptoms. Another study included 150 women for whom first line therapy with methyldopa had not been successful (South Africa 2000).
The antihypertensive drugs evaluated in these trials were hydralazine, calcium channel blockers (nifedipine, nimodipine, nicardipine and isradipine), labetalol, atenolol, methyldopa, diazoxide, prostacyclin, ketanserin, urapidil, magnesium sulphate, prazosin and isosorbide. There are 15 comparisons in the review. Hydralazine was the most common comparator, being compared with another drug (labetalol, calcium channel blockers, prostacyclin, diazoxide, ketanserin or rapidil) in six comparisons. Most drugs were given either intravenously (IV) or intramuscularly (IM) except nifedipine, nimodipine, isosorbide and prazosin which were given orally. Dosage varied considerably between studies, in both amount and duration of therapy.
The primary hypothesis for the one large study (Nimodipine SG 2003) was to compare the effects on prevention of eclampsia, and this study is also included in the review of magnesium sulphate and other anticonvulsants for prevention of eclampsia (Duley 2010). It is also included here as it met the inclusion criteria for the review, and a secondary hypothesis in the trial was to compare the antihypertensive effects of these two drugs.
All but two studies were single comparisons comparing one type of antihypertensive drug with a different hypertensive drug. One study included three comparison groups (atenolol versus ketanserin versus methyldopa) (Argentina 1990). We undertook analysis for each single pair comparison, see Analyses 14, 15 and 16. One trial included four comparison groups (IV labetalol versus IV hydralazine versus oral nifedipine versus sublingual nifedipine) (Switzerland 2012). However, there were no outcome data that could be included in any analysis.
For further details seeCharacteristics of included studies table.
Excluded studies
Sixty‐five studies were excluded from the review. The reasons for exclusion are described in the Characteristics of excluded studies table. In summary, 15 studies were not a randomised trial, eight did not report clinical data, in 11 the women did not have very high blood pressure, in another 28 the intervention was not a comparison of two different antihypertensive drugs, two did not report outcome separately for women randomised before and after delivery, and in one more than 20% of women were excluded from the analysis.
Risk of bias in included studies
Most of the included trials were small. Only five studies recruited more than 100 women; Australia 2007 which recruited 124 women, Iran 2002 126 women, Nimodipine SG 2003 1750 women, South Africa 2000 150 and Panama 2006 200 women. As discussed above, a wide variety of agents were compared. Several trials were conducted in countries where English is not widely used, and it is possible that the search strategy may have missed other studies published in languages other than English.
SeeFigure 1; Figure 2 for summaries of 'Risk of bias' assessments in included trials.
1.
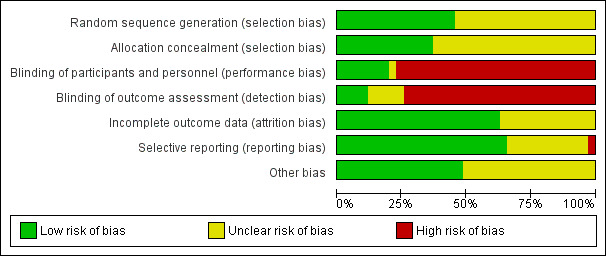
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sixteen trials had adequate methods for random sequence generation and 13 trials had adequate concealment of allocation. Most of the others did not give adequate information about how or whether the allocation to treatment group was concealed.
Blinding
For most trials the identity of the allocated drug could only be blinded after trial entry with use of a double placebo. This was stated to have been done in only two studies (100 women) (Brazil 1994; Malaysia 2012). In another four, the comparison was stated to have been blinded (Brazil 1992; South Africa 1995; South Africa 1997b; Turkey 1996). It was clearly stated in some trials that they were either "open" or not blinded (Germany 1998; Netherlands 1999; Netherlands 2003; South Africa 2000; Iran 2011; Panama 2006; Germany 2006).
In three trials, blinding of some outcome assessment was performed (Brazil 1992; Iran 2002; Malaysia 2012). In one trial, it was reported that it was not blinded, but that the primary outcome of eclampsia is a binary, objective outcome and therefore not subject to observer or measurement bias (Nimodipine SG 2003).
In the remaining trials, there was no mention of blinding of participants, personnel or outcome assessors and because of the nature of the different treatment regimens, performance and detection bias cannot be ruled out.
Incomplete outcome data
Only short‐term outcomes were reported in these trials, but losses to follow‐up for reported outcomes was low in the majority of studies (Australia 1986; Australia 2007; Brazil 1992; England 1982; France 2010; Germany 1998; Germany 2006; Iran 2002; Iran 2011; Malaysia 2012; Nimodipine SG 2003; N Ireland 1991; Panama 2006; South Africa 1987; South Africa 1992; South Africa 1995; South Africa 1997; South Africa 1997a; South Africa 2000; Switzerland 2012; Tunisia 2002; USA 1987) or information was lacking and so it was not possible to assess attrition bias (Argentina 1990; Brazil 1994; Brazil 2011; India 2006; India 2011; Mexico 1989; Mexico 1993; Mexico 1998; Netherlands 1999; Netherlands 2003; South Africa 1989; South Africa 1997b; Turkey 1996). There is no information about outcome after discharge from hospital for either mother or baby.
Selective reporting
In the majority of trial reports assessed, all expected outcomes appeared to have been reported fully within the results (Argentina 1990; Australia 1986; Australia 2007; Brazil 1992; England 1982; Germany 2006; Iran 2002; Iran 2011; Malaysia 2012; Netherlands 1999; Nimodipine SG 2003; N Ireland 1991; Panama 2006; South Africa 1987; South Africa 1989; South Africa 1992; South Africa 1995; South Africa 1997; South Africa 1997a; South Africa 1997b; South Africa 2000; Tunisia 2002; USA 1987). In other trial reports it was difficult to assess selective reporting, mainly due to trial reports being reported in abstract form with limited information (Brazil 1994; Brazil 2011; France 2010; India 2006; India 2011; Mexico 1989; Mexico 1993; Mexico 1998; Netherlands 2003; Switzerland 2012; Turkey 1996). In one trial, the results for fetal heart rate monitoring and ultrasound assessment of fetal growth appear to have been reported incompletely (Germany 1998).
Other potential sources of bias
Most trials appeared to be free of other problems that could put them at risk of bias, e.g. baseline characteristics were balanced (Argentina 1990; Australia 1986; Australia 2007; Brazil 1992; Germany 1998; Iran 2002; Iran 2011; Malaysia 2012; Netherlands 1999; N Ireland 1991; Panama 2006; South Africa 1987; South Africa 1992; South Africa 1997; South Africa 1997a; Tunisia 2002; USA 1987). In other trial reports, it was difficult to assess other potential sources of bias, again mainly due to trial reports being reported in abstract form with limited information (Brazil 1994; Brazil 2011; England 1982; France 2010; Germany 2006; India 2006; India 2011; Mexico 1989; Mexico 1993; Mexico 1998; Netherlands 2003; Nimodipine SG 2003; South Africa 1989; South Africa 1995; South Africa 1997b; South Africa 2000; Switzerland 2012; Turkey 1996).
Effects of interventions
This review includes 35 trials, into which 3573 women were recruited.
(1) Labetalol versus hydralazine
Four trials (269 women with outcome data) compared labetalol, with hydralazine. Two trials did not provide outcome data that could be included in an analysis (Brazil 2011; Switzerland 2012). Only two trials (220 women) reported data for persistent high blood pressure (risk ratio (RR) 1.57, 95% confidence interval (CI) 0.66 to 3.74), Analysis 1.3. Data were reported for all four trials only for fetal or neonatal death (RR 0.75, 95% CI 0.17 to 3.21), Analysis 1.4, caesarean section (average RR 0.85, 95% CI 0.58 to 1.26; Heterogeneity: Tau² = 0.08; Chi² = 6.75, df = 3 (P = 0.08); I² = 56%), Analysis 1.13, and fetal heart rate decelerations (average RR 0.80, 95% CI 0.13 to 4.95: Heterogeneity: Tau² = 1.42; Chi² = 4.25, df = 2 (P = 0.12); I² = 53%), Analysis 1.19. There are insufficient data for reliable conclusions about the comparative effects of these two agents.
1.3. Analysis.
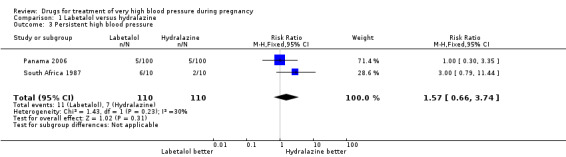
Comparison 1 Labetalol versus hydralazine, Outcome 3 Persistent high blood pressure.
1.4. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 4 Fetal or neonatal deaths.
1.13. Analysis.
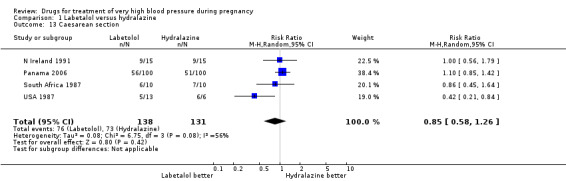
Comparison 1 Labetalol versus hydralazine, Outcome 13 Caesarean section.
1.19. Analysis.
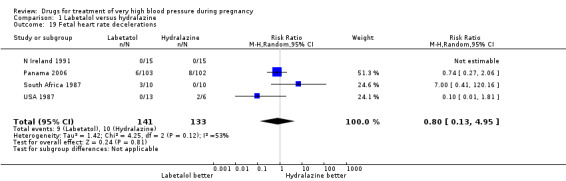
Comparison 1 Labetalol versus hydralazine, Outcome 19 Fetal heart rate decelerations.
(2) Calcium channel blockers versus hydralazine
Eight trials (404 women) compared calcium channel blockers (nifedipine and isradipine) with hydralazine. One trial (41 women) did not provide outcome data that could be included in an analysis (Switzerland 2012). Persistent high blood pressure was reported by six trials (313 women). Fewer women allocated calcium channel blockers rather than hydralazine had persistent high blood pressure (8%% versus 22%; RR 0.37, 95% CI 0.21 to 0.66), Analysis 2.1. For all other outcomes reported, CIs were wide and crossed the line of no difference in effect.
2.1. Analysis.
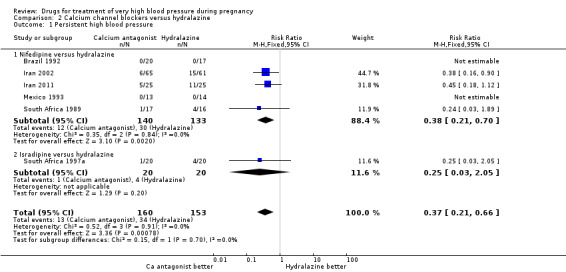
Comparison 2 Calcium channel blockers versus hydralazine, Outcome 1 Persistent high blood pressure.
(3) Prostacyclin versus hydralazine
One trial (47 women) compared prostacyclin with hydralazine. For all outcomes reported, CIs were wide and crossed the line of no difference in effect.
(4) Ketanserin versus hydralazine
Four trials (200 women) compared ketanserin with hydralazine. Ketanserin was associated with a substantially higher risk of persistent high blood pressure than hydralazine (27% versus 6%; three trials 180 women; RR 4.79, 95% CI 1.95 to 11.73), Analysis 4.3. However, side‐effects were less common with ketanserin than hydralazine (three trials 120 women; RR 0.32, 95% CI 0.19 to 0.53), Analysis 4.12. There was no clear evidence of a difference in the risk of hypotension (two trials 76 women; RR 0.26, 95% CI 0.07 to 1.03), Analysis 4.4. In the one small trial reporting HELLP syndrome, the risk of developing this complication of pre‐eclampsia was lower with ketanserin compared with hydralazine (44 women, RR 0.20, 95% CI 0.05 to 0.81), Analysis 4.6.
4.3. Analysis.

Comparison 4 Ketanserin versus hydralazine, Outcome 3 Persistent high blood pressure.
4.12. Analysis.
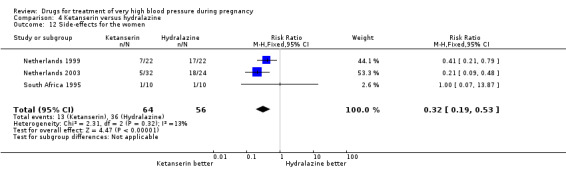
Comparison 4 Ketanserin versus hydralazine, Outcome 12 Side‐effects for the women.
4.4. Analysis.

Comparison 4 Ketanserin versus hydralazine, Outcome 4 Hypotension.
4.6. Analysis.
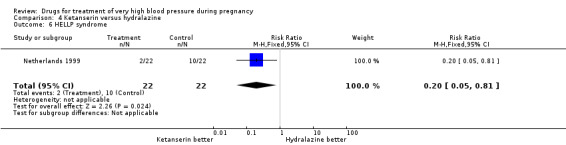
Comparison 4 Ketanserin versus hydralazine, Outcome 6 HELLP syndrome.
(5) Urapidil versus hydralazine
Three trials (101 women) compared urapidil with hydralazine. There were insufficient data for reliable conclusions about the comparative effects on side‐effects for woman allocated these two drugs (RR 0.32, 95% CI 0.09 to 1.19), Analysis 5.6. There was no clear evidence of a difference in the need for caesarean section between the groups (RR 0.83, 95% CI 0.66 to 1.04), Analysis 5.8. There are insufficient data for reliable conclusions about the comparative effects of these two agents on any other outcome reported.
5.6. Analysis.

Comparison 5 Urapidil versus hydralazine, Outcome 6 Side‐effects for the woman.
5.8. Analysis.
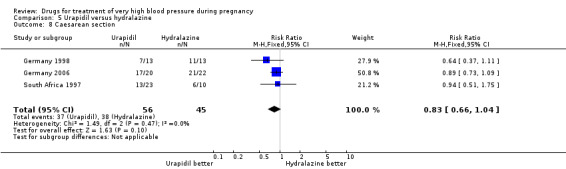
Comparison 5 Urapidil versus hydralazine, Outcome 8 Caesarean section.
(6) Labetalol versus calcium channel blockers
Five trials (171 women) compared labetalol with calcium channel blockers (nicardipine and nifedipine). Two trials did not provide outcome data that could be included in an analysis (India 2011; Switzerland 2012). Data provided from one trial (50 women) suggested that nifedipine was associated with fewer side‐effects for women than labetalol (RR 2.17, 95% CI 0.98 to 4.79), Analysis 6.5, which was borderline for statistical significance. There are insufficient data for reliable conclusions about the comparative effects of these two agents for other outcomes.
6.5. Analysis.
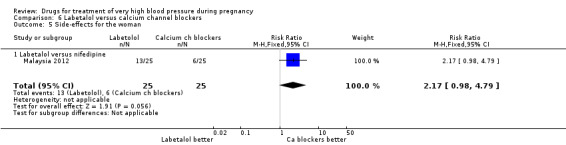
Comparison 6 Labetalol versus calcium channel blockers, Outcome 5 Side‐effects for the woman.
(7) Labetalol versus methyldopa
One trial (74 women) compared labetalol with methyl dopa. There are insufficient data for reliable conclusions about the comparative effects of these two agents.
(8) Labetalol versus diazoxide
One trial (90 women) compared labetalol with diazoxide. Labetalol was associated with less hypotension than diazoxide, although the CIs are wide and borderline for statistical significance (RR 0.06, 95% CI 0.00 to 0.99), Analysis 8.2. This was reflected in a similar comparative increase in the need for caesarean section in the diazoxide group, which was again borderline for statistical significance (RR 0.43, 95% CI 0.18 to 1.02), Analysis 8.3. Data were insufficient for any reliable conclusions about other outcomes reported.
8.2. Analysis.
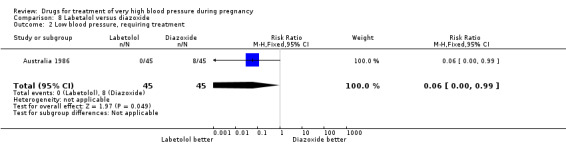
Comparison 8 Labetalol versus diazoxide, Outcome 2 Low blood pressure, requiring treatment.
8.3. Analysis.
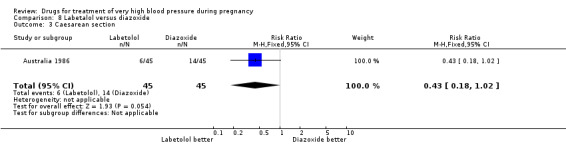
Comparison 8 Labetalol versus diazoxide, Outcome 3 Caesarean section.
(9) Nitrates versus magnesium sulphate
One trial (36 women) compared isosorbide with magnesium sulphate. Although there was no clear difference in persistent hypertension (RR 0.14, 95% CI 0.01 to 2.58), Analysis 9.2, isosorbide was associated with a lower risk of caesarean section than magnesium sulphate (RR 0.19, 95% CI 0.07 to 0.53), Analysis 9.3.
9.2. Analysis.
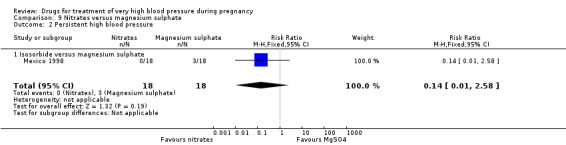
Comparison 9 Nitrates versus magnesium sulphate, Outcome 2 Persistent high blood pressure.
9.3. Analysis.
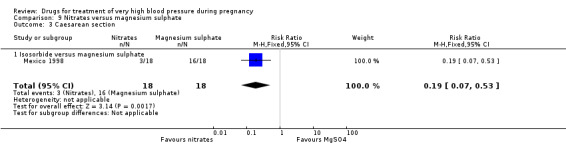
Comparison 9 Nitrates versus magnesium sulphate, Outcome 3 Caesarean section.
(10) Nimodipine versus magnesium sulphate
Two trials (1683 women) compared nimodipine with magnesium sulphate. Both drugs were associated with high levels of persistent high blood pressure (47% versus 65%), although the risk associated with nimodipine was lower than magnesium sulphate (RR 0.84, 95% CI 0.76 to 0.93), Analysis 10.3. The risk of eclampsia was higher with nimodipine compared with magnesium sulphate in one large well conducted study (Nimodipine SG 2003), but the pooled result, including results from a smaller trial (Turkey 1996), showed no clear difference and substantial heterogeneity (average RR 1.03, 95% CI 0.07 to 16.03; Heterogeneity: Tau² = 2.95; Chi² = 3.39, df = 1 (P = 0.07); I² = 70%), Analysis 10.1. Nimodipine was associated with a lower risk of respiratory difficulties for the woman (RR 0.28, 95% CI 0.08 to 0.99) although this was borderline for statistical significance, Analysis 10.6, fewer side‐effects (RR 0.68, 95% CI 0.55 to 0.85), Analysis 10.9, and a lower risk of postpartum haemorrhage (RR 0.41, 95% CI 0.18 to 0.92), Analysis 10.12. There were no clear differences in any other outcomes. Stillbirths and neonatal deaths were not reported.
10.3. Analysis.
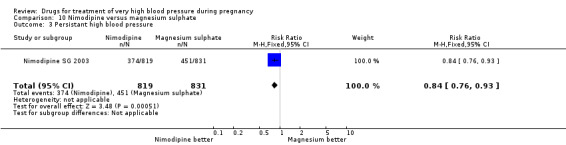
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 3 Persistant high blood pressure.
10.1. Analysis.
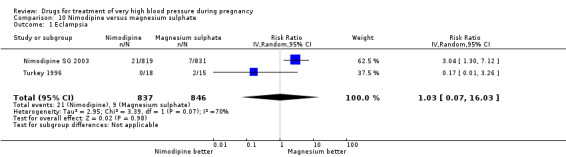
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 1 Eclampsia.
10.6. Analysis.

Comparison 10 Nimodipine versus magnesium sulphate, Outcome 6 Respiratory difficulty for the woman.
10.9. Analysis.
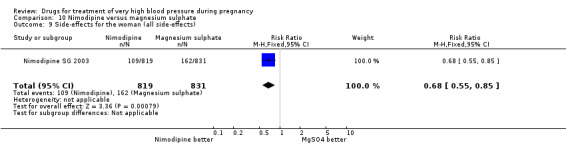
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 9 Side‐effects for the woman (all side‐effects).
10.12. Analysis.
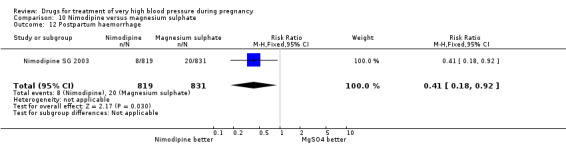
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 12 Postpartum haemorrhage.
(11) Nifedipine versus prazosin
One trial (130 women) compared nifedipine with prazosin. There are insufficient data for reliable conclusions about the comparative effects of these two agents.
(12) Nifedipine versus chlorpromazine
One small trial (60 women) compared nifedipine with chlorpromazine. There are insufficient data for reliable conclusions about the comparative effects of these two agents.
(13) Hydralazine versus diazoxide
One trial (97 women) compared hydralazine with diazoxide. There are insufficient data for reliable conclusions about the comparative effects of these two agents.
(14) Methyldopa versus atenolol
One three‐arm trial (90 women) compared ketanserin versus alpha methyldopa versus atenolol. We undertook analysis for the pair‐wise comparison methyldopa versus atenolol. For the comparison of methyldopa with atenolol, atenolol was associated with fewer side‐effects for women (somnolence), although the CI was very wide (RR 21.00, 95% CI 1.29 to 342.93), Analysis 14.3. There were no clear differences in any other outcomes.
14.3. Analysis.
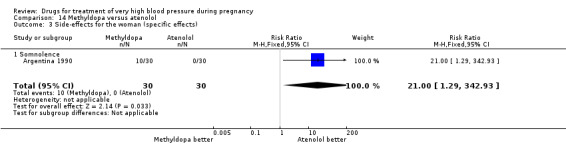
Comparison 14 Methyldopa versus atenolol, Outcome 3 Side‐effects for the woman (specific effects).
(15) Urapidil versus calcium channel blockers
One trial (18 women) compared urapidil versus calcium channel blockers (nicardipine). There was no difference between the two agents for side‐effects for the baby or women. No other outcomes were reported.
Side‐effects
Few trials provided data on the specific side‐effects related to the different agents. Reported side‐effects included:
for hydralazine: headache, flushing, light head, nausea and palpitations;
for labetalol: flushing, light head, palpitations and scalp tingling;
for nifedipine: flushing, nausea, vomiting;
for urapidil: nausea and tinnitus;
for magnesium sulphate: flushing;
for methyldopa: somnolence.
Discussion
Summary of main results
Most of the drugs included in this review reduce high blood pressure. This is not surprising, as there is no reason why drugs that are known to reduce blood pressure in people who are not pregnant should not also reduce blood pressure for women who are pregnant. Currently, for women with very high blood pressure during pregnancy there is insufficient evidence to conclude that any one antihypertensive drug is clearly better than another.
Probably the three most commonly recommended drugs for very high blood pressure during pregnancy are hydralazine, labetalol and the calcium channel blocker nifedipine. Data in this review do not suggest any significant differential effects, with the exception of for calcium channel blockers, which were associated with less persistent hypertension than hydralazine and possibly less side‐effects compared to labetalol.
Hydralazine was associated with a significant increase in the risk of HELLP syndrome when compared with ketanserin (46% versus 9%) however, such a high level of HELLP syndrome is difficult to explain with hydralazine use, and is in contrast to the low risk of HELLP syndrome in another study comparing hydralazine with labetalol where incidence of HELLP is 2% in both arms. There was insufficient evidence for any difference among these three drugs for other more substantive outcomes for the mother or baby.
From the data presented here it is clear that nimodipine, ketanserin, and high‐dose diazoxide have serious disadvantages, and so should not be used for women with very high blood pressure during pregnancy as better options are readily available. Nimodipine is generally no longer used to control high blood pressure in the non‐pregnant population, but instead, is used for improvement of neurological outcome after subarachnoid haemorrhage (Tomassoni 2008). Diazoxide given as repeated 75 mg bolus injections, seems to be associated with a greater risk of dropping the blood pressure so low that treatment is required to bring it back up again, with an associated increased risk of caesarean section, when compared with labetalol. Smaller doses may not have this disadvantage, as observed in a recent study in which 15 mg bolus injections were compared, with no ill effect on hypotension (Hennessy 2007). Ketanserin was far more likely to be associated with persistent hypertension than hydralazine.
In the one large trial that compared nimodipine with magnesium sulphate, 54% of women allocated magnesium sulphate had persistent hypertension. So, although it is clearly of value for seizure prophylaxis in women with pre‐eclampsia (Duley 2010), magnesium sulphate should not be used for control of very high blood pressure. Nearly half the women in the nimodipine arm also had persistently high blood pressure, as well as increased risk of eclampsia compared with magnesium sulphate
It would also seem sensible to avoid chlorpromazine. Although only one small trial has compared chlorpromazine with nifedipine, this antipsychotic drug has a complex mode of action and impacts on several organ systems. One well known side‐effect is convulsions, which is a serious disadvantage for women with hypertension during pregnancy. That this concern is real, rather than theoretical, is demonstrated by the review of magnesium sulphate versus lytic cocktail (which includes chlorpromazine) for women with eclampsia (Duley 2010a). This review shows a clear increase in the risk of further seizures associated with lytic cocktail compared to magnesium sulphate.
One trial did compare an antihypertensive, the nitrate isosorbide, with placebo for women with very high blood pressure (Mexico 2000). This study was excluded from the review, as our objective was to compare one antihypertensive drug with another. In this study, 60 women with diastolic blood pressure 110 mmHg or above after 20 minutes rest were randomised to either sublingual isosorbide or placebo. Both groups had an intravenous infusion of Hartmann solution. Outcome was assessed over one hour, during which time one woman allocated isosorbide had hypotension. At the end of the one‐hour study, mean blood pressure was substantially lower for women allocated isosorbide compared to placebo, there were no episodes of fetal distress or imminent eclampsia, and similar numbers of women in both groups complained of headache. Outcome after one hour is not reported. This study does show that isosorbide lowers blood pressure, but the clinically important question is not whether it is better than placebo, but whether it has any substantive advantages over other drugs in widespread clinical use.
Overall completeness and applicability of evidence
Any effect on a comparative improvement in control of blood pressure would be of far greater clinical importance if it was reflected in comparative improvements in other more substantive outcomes, such as stroke, serious maternal morbidity and perinatal death. With the exception of the large trial comparing nimodipine with magnesium sulphate, all the trials to date have been small, with few outcomes other than control of blood pressure reported.
During pregnancy, there are additional issues other than control of blood pressure, however, such as avoiding a precipitous drop in blood pressure that might cause problems for the unborn baby, side‐effects that are similar to symptoms of worsening pre‐eclampsia and so may delay recognition of the need to intervene, not lowering the blood pressure too far as this might also compromise blood supply across the placenta to the baby, and if the drug itself crosses the placenta not causing harm to the baby. There are relatively few data on the comparative effects of the alternative drugs on these other outcomes.
Surprisingly few studies have reported maternal side‐effects. Common side‐effects included severe headache and nausea, symptoms which are similar to those of imminent eclampsia and so may make clinical management more difficult. There has been concern that rapid‐release nifedipine capsules may increase the risk of hypotension, and in some countries these have been withdrawn from use. One small trial (64 women) compared nifedipine capsules with slower and longer‐acting nifedipine tablets (Australia 2002). Outcome was assessed after 90 minutes; similar proportions of women had persistent high blood pressure (11% allocated capsules versus 9% allocated tablets), and there was less hypotension amongst those allocated tablets although this did not achieve statistical significance (3/31 versus 1/33; risk ratio 3.19, 95% confidence interval 0.35 to 29.10).
There were insufficient data for the planned subgroup analysis by whether the severe hypertension was associated with proteinuria.
Quality of the evidence
The overall methodological quality of the trials contributing data to the review was low to moderate and has been summarised in Figure 1 and Figure 2. While none of the studies were assessed as being at high risk of bias for all domains, several trials did not provide clear information on methods. Fifteen of the 35 included trials failed to describe adequately the methods used for random sequence generation and allocation concealment and were assessed as unclear risk of bias. Lack of blinding was a problem in all of the included studies; blinding women and clinical staff to a randomised group is not feasible with this type of intervention. The impact of lack of blinding is difficult to judge. Knowledge of allocation could have affected other aspects of care and the assessment of many outcomes, particularly blood pressure. Loss to follow‐up was not always described, but did not appear to be a major source of bias in the majority of studies.
Potential biases in the review process
Problems with interpreting the data in this review include differences in the way persistent hypertension was defined for each study, and differences in the clinical characteristics of the women. For example, definitions for persistent hypertension included time taken to achieve target blood pressure, ability to achieve target blood pressure within a certain time period, and need for additional medication. These differences are reflected in the wide range of frequency of persistent high blood pressure across studies. For example, in the five categories with hydralazine as a comparator the frequency of persistent high blood pressure amongst women allocated hydralazine ranged from 0% to 20%, while amongst women allocated an alternative drug, it ranged from 0% to 60%. As few studies had blinding either of the intervention or the assessment of outcomes, there is considerable potential for bias in the assessment of blood pressure.
Agreements and disagreements with other studies or reviews
An alternative analysis of this topic concluded that the data do not support hydralazine as first line treatment for very high blood pressure in pregnancy (Magee 2003), and recommended future trials compare labetalol with nifedipine. However, that analysis included quasi‐randomised studies and women with very high blood pressure after delivery. Once the analysis is restricted to include only studies with less potential for bias and women with very high blood pressure during pregnancy or labour, as in our review, the data are insufficient to support the conclusion that labetalol is better than hydralazine.
Authors' conclusions
Implications for practice.
There is no clear evidence that one antihypertensive is preferable to the others for improving outcome for women with very high blood pressure during pregnancy, and their babies. Until better evidence is available, the best choice of drug for an individual woman probably depends on the experience and familiarity of her clinician with a particular drug, and on what is known about adverse maternal and fetal side‐effects. Probably best avoided are magnesium sulphate (although this is indicated for women who require an anticonvulsant for prevention or treatment of eclampsia), high‐dose diazoxide, ketanserin, nimodipine and chlorpromazine.
Implications for research.
Well designed large trials are needed to make reliable comparisons of the maternal, fetal and neonatal effects of antihypertensives in common clinical practice. Ideally, clinicians should compare an agent they are familiar with in their routine clinical practice with a promising alternative that is available locally, or would be likely to become available if shown to be preferable. Many hospitals around the world continue to use hydralazine, labetalol, or nifedipine as the first choice for women with very high blood pressure. The priority is therefore to compare these drugs with each other, or other more promising alternatives.
Future trials should measure outcomes that are important to women and their babies, rather than attempting to document relatively subtle differences in the effects on blood pressure. These outcomes should include persistent high blood pressure, need for additional antihypertensive drugs, further episodes of severe hypertension, low blood pressure, side‐effects, severe maternal morbidity (such as stroke, eclampsia, renal failure, and coagulopathy), need for magnesium sulphate, mode of delivery, length of stay in hospital, mortality for the baby, and admission and length of stay in a special/intensive care nursery. In order to reliably estimate differential effects on these substantive outcomes, high quality large studies will be required. There should also be long‐term follow‐up to assess possible effects on the woman's risk of cardiovascular problems after discharge from hospital, and on growth and development of the child. This is relevant not only because these drugs may cross the placenta, but also because too rapid lowering of blood pressure with a placenta that has marginal functional reserve could lead to ischaemic brain injury and long‐term neurodevelopment problems. Alongside data from randomised trials, mechanisms need to be developed to monitor possible rare adverse events related to in utero exposure to antihypertensive agents.
Interpretation of the results of future studies would be made easier and more clinically meaningful by the use of similar definitions for key outcomes, such as persistent high blood pressure, and hypotension. Studies that recruit women both before and after delivery should report outcome data separately for these two groups of women. Outcomes should also be reported separately for women with and without proteinuria at trial entry.
Once better information is available about the relative merits and hazards of agents already in widespread use, it will become possible to compare new drugs with the best of the traditional agents in well designed randomised trials.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2013 | New citation required but conclusions have not changed | Eleven new trials were included in this update. The review now includes a total of 35 trials into which 3573 women were recruited. |
| 9 January 2013 | New search has been performed | Search updated and 39 trial reports identified. Methods updated based on the PCG guidelines and the generic protocol. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 13 February 2012 | Amended | Search updated. Thirty‐seven trial reports added to Studies awaiting classification. |
| 2 September 2008 | Amended | Converted to new review format. |
| 31 March 2006 | New search has been performed | Search updated in February 2006. New included studies: Brazil 1992; Mexico 1998; Netherlands 2003; Tunisia 2002; South Africa 1997a. New excluded studies: Australia 2002; Bangladesh 2002; Brazil 1984; Brazil 1988; Brazil 1988a; China 2000; Egypt 1989; Egypt 1992; India 1963; India 2001; Italy 2004; Jamaica 1999; Japan 1999; Japan 2000; Japan 2003; Mexico 1967; Mexico 2004; Netherlands 2002; New Zealand 1986; Philipines 2000; South Africa 1984; Venezuela 2001. Study ID changed: South Africa 1994 changed to South Africa 1997b. New ongoing study: Warren 2004a, comparing labetolol with magnesium sulphate. Methods text expanded in line with the guidelines for the Cochrane Pregnancy and Childbirth Group. All text revised and expanded to reflect inclusion, and exclusion, of new studies. |
Acknowledgements
Many thanks to Antoinette Bolte for her generosity in supplying unpublished data from Netherlands 2003. Thanks also to Rory Collins who contributed to earlier versions of this review published within the Oxford Database of Perinatal Trials, later the Cochrane Pregnancy and Childbirth Database.
Many thanks to Therese Dowswell for her contribution in assessing studies and extracting data for the 2013 update.
The authors would like to acknowledge the enthusiastic contribution of David Henderson‐Smart to previous versions of this review. David Henderson‐Smart died in February 2013, and we would like to dedicate the review to him.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategy
In an earlier version of the review, we also searched MEDLINE (1966 to April 2002) using the MeSH terms 'pregnancy' and 'hypertension', limited to randomised controlled trials and the Cochrane Central Register of Controlled Trials (The Cochrane Library 2006, Issue 2) using the following strategy:
HYPERTENSION, PREGNANCY‐INDUCED:ME
PREECLAMP*
PRE‐ECLAMP*
(PRE next ECLAMP*)
ECLAMP*
(HYPERTENS* and PREGNAN*)
(((((#1 or #2) or #3) or #4) or #5) or #6)
((NIFEDIPINE or NIMODIPINE) or ISRADIPINE)
(HYDRALAZINE or DIHYDRALAZINE)
((LABETALOL or ATENOLOL) or PROPRANOLOL)
(GTN or (GLYCEROL and TRINITR*))
(URAPIDIL or PRAZOSIN)
((((#8 or #9) or #10) or #11) or #12)
(#7 and #13)
Appendix 2. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Australia 1986; Brazil 1992; Brazil 1994; England 1982; Germany 1998; Iran 2002; Mexico 1989; Mexico 1993; Mexico 1998; Netherlands 1999; Netherlands 2003; Nimodipine SG 2003; N Ireland 1991; South Africa 1987; South Africa 1989; South Africa 1992; South Africa 1995; South Africa 1997; South Africa 1997a; South Africa 1997b; South Africa 2000; Tunisia 2002; Turkey 1996; USA 1987; Argentina 1986; Australia 2002; Bangladesh 2002; Brazil 1984; Brazil 1988; Brazil 1988a; China 2000; Egypt 1988; Egypt 1989; Egypt 1992; France 1986; Ghana 1995; India 1963; India 2001; Iran 1994; Israel 1991; Israel 1999; Italy 2004; Jamaica 1999; Japan 1999; Japan 2000; Japan 2002; Japan 2003; Malaysia 1996; Mexico 1967; Mexico 2000; Mexico 2004; Netherlands 2002; New Zealand 1986; New Zealand 1992; Philipines 2000; Scotland 1983; Singapore 1971; South Africa 1982; South Africa 1984; South Africa 1993; South Africa 2002; Spain 1988; Sweden 1993; USA 1999; Venezuela 2001.
Selection of studies
Two authors independently evaluated studies to assess eligibility. Discrepancies were resolved by discussion. If there was no agreement, the third author was asked to independently assess the study for inclusion. If agreement was still not reached, the study was excluded until clarification could be obtained from the authors.
Assessment of methodological quality of included studies
Two authors independently extracted data on trial characteristics. Discrepancies were resolved by discussion. Quality of each included study was assessed using the criteria in the Cochrane Reviewers' Handbook (Clarke 2002).
(i) Selection bias (randomisation and allocation concealment)
Method for generating the randomisation sequence was described for each trial. Studies with a quasi‐random design were excluded. Concealment of allocation was assessed for each trial, with adequate concealment graded A, unclear B and clearly inadequate concealment C. Studies with clearly inadequate concealment of allocation were excluded. Where the method of allocation concealment was unclear, authors were contacted to provide further details.
(ii) Performance bias (blinding of participants, researchers and outcome assessment)
Quality scores for blinding of the assessment of outcome were assigned to each reported outcome using the following criteria (these scores are displayed in the methods column of the 'Characteristics of included studies' table):
(A) double blind, neither investigator nor participant knew or were likely to guess the allocated treatment; (B) single blind, either the investigator or the participant knew the allocation. Or the trial may be described as double blind, but side‐effects of one or other treatment mean that it is likely that for a significant proportion (more that 20 per cent) of participants the allocation could be correctly identified, or the method for blinding is not described; (C) no blinding, both investigator and participant knew (or were likely to guess) the allocated treatment, or blinding not mentioned.
(iii) Attrition bias (loss of participants, eg withdrawals, dropouts, protocol deviations)
For completeness of follow‐up, scores were assigned using the following criteria:
(A) less than three per cent of participants excluded from the analysis; (B) three per cent to 9.9 per cent of participants excluded from the analysis; (C) 10 per cent to 19.9 per cent of participants excluded from the analysis.
Excluded: If not possible to enter data based on intention to treat or 20% or more participants were excluded from the analysis of that outcome.
Data extraction and data entry
Two review authors extracted data on outcomes, and discrepancies were resolved through discussion. If agreement was not reached, that item was excluded until further clarification was available from the authors. Data were entered onto the Review Manager software (RevMan 2000) and checked for accuracy. There was no blinding of authorship or results.
Statistical analyses
Statistical analyses were carried out using Review Manager (RevMan 2000). Results were presented as summary relative risk with 95% confidence intervals and, if relevant, as risk difference and number needed to treat to benefit. The I2 statistic was used to assess heterogeneity between trials. In the absence of significant heterogeneity, results were pooled using a fixed‐effect model. If substantial heterogeneity was detected (I2 more than 50%), possible causes were explored and subgroup analyses for the main outcomes performed. Heterogeneity that was not explained by subgroup analyses was modelled using random‐effects analysis, where appropriate. Possible explanations for the variation, such as study quality and women's characteristics at trial entry, were explored.
Sensitivity analyses
When appropriate, in future updates, we will carry out sensitivity analysis to explore the effect of trial quality based on concealment of allocation, by excluding studies with unclear allocation concealment (rated B).
Subgroup analyses
Data are presented by class of drug. In addition, the following subgroup analyses will be conducted when sufficient data become available:
treatment regimen within each class of drug;
whether severe hypertension alone, or severe hypertension plus proteinuria at trial entry.
Data and analyses
Comparison 1. Labetalol versus hydralazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal deaths | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Eclampsia | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Persistent high blood pressure | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.66, 3.74] |
| 4 Fetal or neonatal deaths | 4 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.21] |
| 5 HELLP syndrome | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.96] |
| 6 Serious morbidity for woman: oliguria | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.67] |
| 7 Serious morbidity for woman: disseminated intravascular coagulation | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Serious morbidity for woman: acute renal insufficiency | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Serious morbidity for woman: pulmonary oedema | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 10 Hypotension | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.11] |
| 11 Side‐effects for the woman | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.49, 1.23] |
| 12 Placental abruption | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.43] |
| 13 Caesarean section | 4 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.58, 1.26] |
| 14 Respiratory distress syndrome | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.71] |
| 15 Necrotizinc enterocolitis | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.18, 21.50] |
| 16 Intraventricular haemorrhage | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.31, 28.09] |
| 17 Apgar < 7 at 1 minute | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.76, 2.64] |
| 18 Apgar < 7 at 5 minutes | 2 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.03, 10.36] |
| 19 Fetal heart rate decelerations | 4 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.13, 4.95] |
| 20 Neonatal hypoglycaemia | 2 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.19, 6.94] |
| 21 Admission to special care baby unit | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.66, 1.49] |
| 22 Neonate with complications (some neonates had more than one complication). | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
1.1. Analysis.
Comparison 1 Labetalol versus hydralazine, Outcome 1 Maternal deaths.
1.2. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 2 Eclampsia.
1.5. Analysis.
Comparison 1 Labetalol versus hydralazine, Outcome 5 HELLP syndrome.
1.6. Analysis.
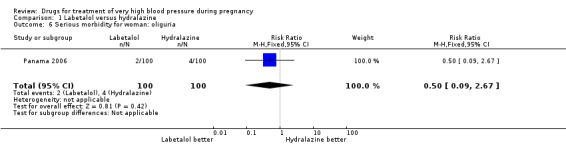
Comparison 1 Labetalol versus hydralazine, Outcome 6 Serious morbidity for woman: oliguria.
1.7. Analysis.
Comparison 1 Labetalol versus hydralazine, Outcome 7 Serious morbidity for woman: disseminated intravascular coagulation.
1.8. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 8 Serious morbidity for woman: acute renal insufficiency.
1.9. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 9 Serious morbidity for woman: pulmonary oedema.
1.10. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 10 Hypotension.
1.11. Analysis.
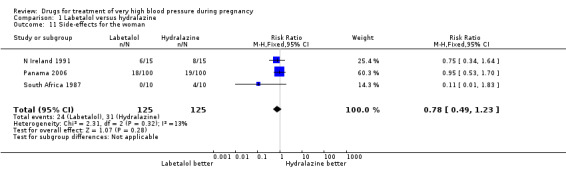
Comparison 1 Labetalol versus hydralazine, Outcome 11 Side‐effects for the woman.
1.12. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 12 Placental abruption.
1.14. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 14 Respiratory distress syndrome.
1.15. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 15 Necrotizinc enterocolitis.
1.16. Analysis.
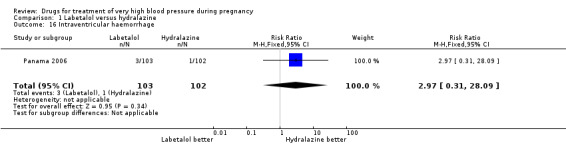
Comparison 1 Labetalol versus hydralazine, Outcome 16 Intraventricular haemorrhage.
1.17. Analysis.
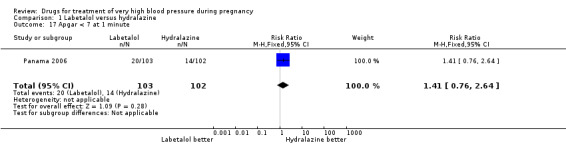
Comparison 1 Labetalol versus hydralazine, Outcome 17 Apgar < 7 at 1 minute.
1.18. Analysis.
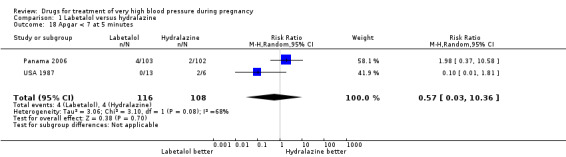
Comparison 1 Labetalol versus hydralazine, Outcome 18 Apgar < 7 at 5 minutes.
1.20. Analysis.

Comparison 1 Labetalol versus hydralazine, Outcome 20 Neonatal hypoglycaemia.
1.21. Analysis.
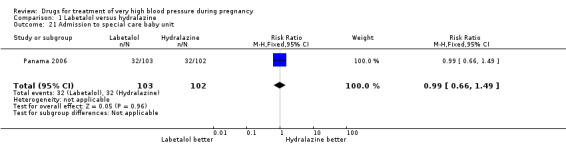
Comparison 1 Labetalol versus hydralazine, Outcome 21 Admission to special care baby unit.
1.22. Analysis.
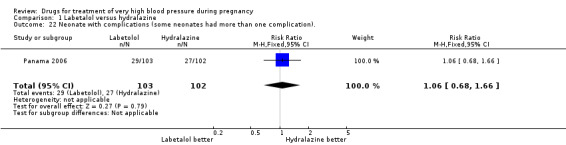
Comparison 1 Labetalol versus hydralazine, Outcome 22 Neonate with complications (some neonates had more than one complication)..
Comparison 2. Calcium channel blockers versus hydralazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent high blood pressure | 6 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.21, 0.66] |
| 1.1 Nifedipine versus hydralazine | 5 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.21, 0.70] |
| 1.2 Isradipine versus hydralazine | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.05] |
| 2 Hypotension | 4 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.32, 26.90] |
| 2.1 Nifedipine versus hydralazine | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.92 [0.32, 26.90] |
| 2.2 Isradapine versus hydralazine | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Further episode/s of very high blood pressure | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.11] |
| 3.1 Nifedipine versus hydralazine | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.65, 1.11] |
| 3.2 Isradipine versus hydralazine | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Side‐effects for the woman | 5 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.52, 1.25] |
| 4.1 Nifedipine versus hydralazine | 4 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.52, 1.25] |
| 4.2 Isradipine versus hydralazine | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Side‐effects for the woman (specific effects) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Palpatations | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.28, 1.39] |
| 5.2 Nausea and/or vomiting | 4 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.27, 10.81] |
| 5.3 Headache | 5 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.43, 3.02] |
| 5.4 Flushing | 4 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.15, 7.51] |
| 5.5 Dyspnoea | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.06, 12.59] |
| 6 Caesarean section | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.29] |
| 6.1 Nifedipine versus hydralazine | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.29] |
| 7 Fetal or neonatal death | 4 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.42, 4.41] |
| 7.1 Nifedipine versus hydralazine | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.40, 5.48] |
| 7.2 Isradapine versus hydralazine | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.06, 14.22] |
| 8 Apgar < 7 at 5 minutes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Fetal heart rate decelerations | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.31] |
| 9.1 Nifedipine versus hydralazine | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.99] |
| 9.2 Isradipine versus hydralazine | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.09, 1.83] |
2.2. Analysis.
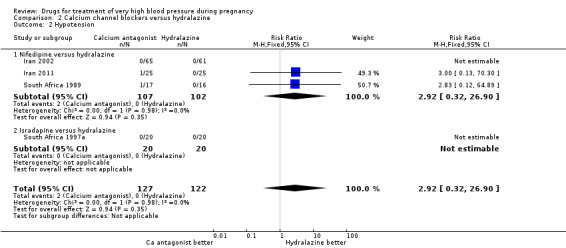
Comparison 2 Calcium channel blockers versus hydralazine, Outcome 2 Hypotension.
2.3. Analysis.

Comparison 2 Calcium channel blockers versus hydralazine, Outcome 3 Further episode/s of very high blood pressure.
2.4. Analysis.

Comparison 2 Calcium channel blockers versus hydralazine, Outcome 4 Side‐effects for the woman.
2.5. Analysis.
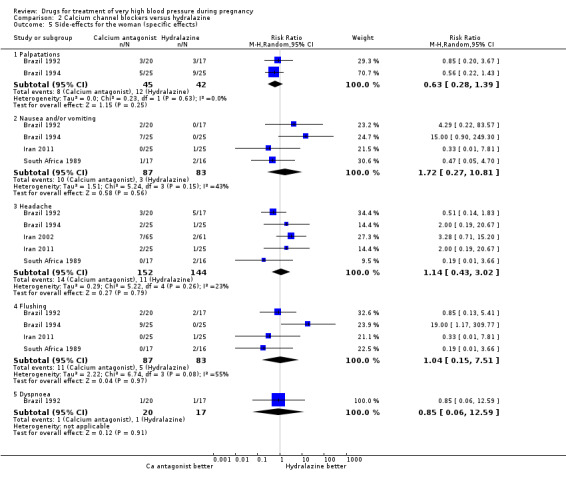
Comparison 2 Calcium channel blockers versus hydralazine, Outcome 5 Side‐effects for the woman (specific effects).
2.6. Analysis.

Comparison 2 Calcium channel blockers versus hydralazine, Outcome 6 Caesarean section.
2.7. Analysis.
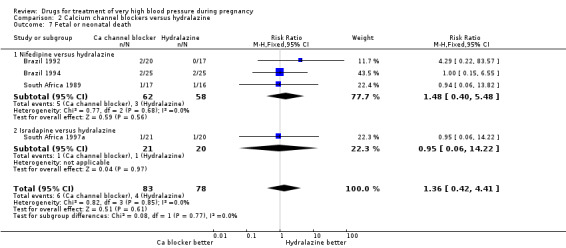
Comparison 2 Calcium channel blockers versus hydralazine, Outcome 7 Fetal or neonatal death.
2.8. Analysis.

Comparison 2 Calcium channel blockers versus hydralazine, Outcome 8 Apgar < 7 at 5 minutes.
2.9. Analysis.

Comparison 2 Calcium channel blockers versus hydralazine, Outcome 9 Fetal heart rate decelerations.
Comparison 3. Prostacyclin versus hydralazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent high blood pressure | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.47] |
| 2 Caesarean section | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.50, 1.10] |
| 3 Side‐effects for the woman | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.08, 17.11] |
| 4 Neonatal death | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.08, 17.11] |
| 5 Ventilation of the baby | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.08, 1.40] |
3.1. Analysis.

Comparison 3 Prostacyclin versus hydralazine, Outcome 1 Persistent high blood pressure.
3.2. Analysis.
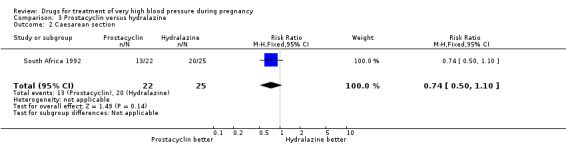
Comparison 3 Prostacyclin versus hydralazine, Outcome 2 Caesarean section.
3.3. Analysis.
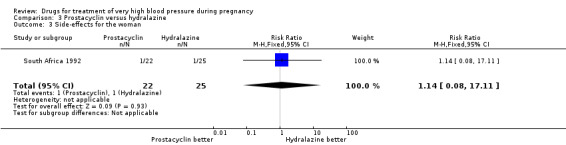
Comparison 3 Prostacyclin versus hydralazine, Outcome 3 Side‐effects for the woman.
3.4. Analysis.
Comparison 3 Prostacyclin versus hydralazine, Outcome 4 Neonatal death.
3.5. Analysis.
Comparison 3 Prostacyclin versus hydralazine, Outcome 5 Ventilation of the baby.
Comparison 4. Ketanserin versus hydralazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 2.96] |
| 2 Eclampsia | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.08, 4.24] |
| 3 Persistent high blood pressure | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.79 [1.95, 11.73] |
| 4 Hypotension | 2 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 1.03] |
| 5 Pulmonary oedema | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.95] |
| 6 HELLP syndrome | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.81] |
| 7 Disseminated intravascular coagulation | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.87] |
| 8 Severe maternal morbidity | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.09, 1.12] |
| 9 Delivery due to fetal distress | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.09, 2.33] |
| 10 Placental abruption | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.10] |
| 11 Caesarean section | 3 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.37, 1.58] |
| 12 Side‐effects for the women | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.19, 0.53] |
| 13 Perinatal death | 2 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.64] |
4.1. Analysis.
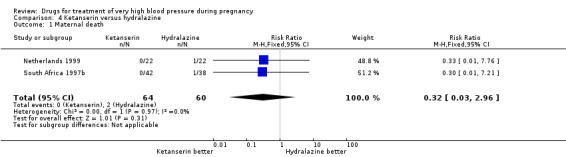
Comparison 4 Ketanserin versus hydralazine, Outcome 1 Maternal death.
4.2. Analysis.
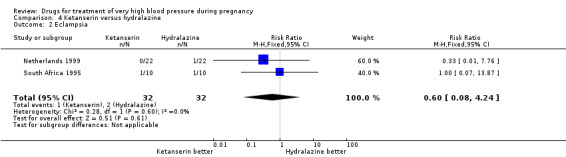
Comparison 4 Ketanserin versus hydralazine, Outcome 2 Eclampsia.
4.5. Analysis.
Comparison 4 Ketanserin versus hydralazine, Outcome 5 Pulmonary oedema.
4.7. Analysis.
Comparison 4 Ketanserin versus hydralazine, Outcome 7 Disseminated intravascular coagulation.
4.8. Analysis.
Comparison 4 Ketanserin versus hydralazine, Outcome 8 Severe maternal morbidity.
4.9. Analysis.
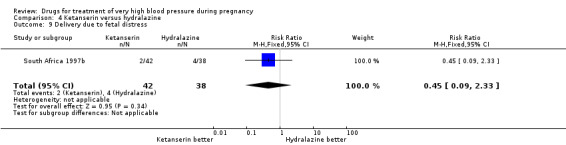
Comparison 4 Ketanserin versus hydralazine, Outcome 9 Delivery due to fetal distress.
4.10. Analysis.

Comparison 4 Ketanserin versus hydralazine, Outcome 10 Placental abruption.
4.11. Analysis.
Comparison 4 Ketanserin versus hydralazine, Outcome 11 Caesarean section.
4.13. Analysis.
Comparison 4 Ketanserin versus hydralazine, Outcome 13 Perinatal death.
Comparison 5. Urapidil versus hydralazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Persistent high blood pressure | 3 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.08, 5.66] |
| 3 Stillbirth | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Neonatal death | 3 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.10, 3.03] |
| 5 Hypotension | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.02, 2.13] |
| 6 Side‐effects for the woman | 3 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.09, 1.19] |
| 7 Placental abruption | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 3.46] |
| 8 Caesarean section | 3 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.66, 1.04] |
| 9 Respiratory distress syndrome | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.48] |
5.1. Analysis.
Comparison 5 Urapidil versus hydralazine, Outcome 1 Eclampsia.
5.2. Analysis.
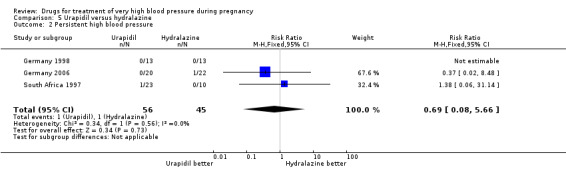
Comparison 5 Urapidil versus hydralazine, Outcome 2 Persistent high blood pressure.
5.3. Analysis.
Comparison 5 Urapidil versus hydralazine, Outcome 3 Stillbirth.
5.4. Analysis.

Comparison 5 Urapidil versus hydralazine, Outcome 4 Neonatal death.
5.5. Analysis.
Comparison 5 Urapidil versus hydralazine, Outcome 5 Hypotension.
5.7. Analysis.
Comparison 5 Urapidil versus hydralazine, Outcome 7 Placental abruption.
5.9. Analysis.

Comparison 5 Urapidil versus hydralazine, Outcome 9 Respiratory distress syndrome.
Comparison 6. Labetalol versus calcium channel blockers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.05, 10.26] |
| 1.1 Labetalol versus nifedipine | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.05, 10.26] |
| 2 Persistent high blood pressure | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.62, 2.09] |
| 2.1 Labetolol versus nicardopine | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.59, 2.51] |
| 2.2 Labetolol versus nifedipine | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.33, 3.03] |
| 3 Hypotension | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Labetolol versus nicardopine | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Labetalol versus nifedipine | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Side‐effects for the woman (specific effects) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea and or vomiting | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 4.2 Palpatations | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Moderate tachycardia | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 5 Side‐effects for the woman | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.98, 4.79] |
| 5.1 Labetalol versus nifedipine | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.98, 4.79] |
| 6 Elective delivery | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.65] |
| 6.1 Labetalol versus nifedipine | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.65] |
| 7 Caesarean section | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.31] |
| 7.1 Labetalol versus nifedipine | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.50, 1.31] |
| 8 Admission to intensive care | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 8.1 Labetalol versus nifedipine | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 9 Admission to special care baby unit | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.49] |
| 9.1 Labetalol versus nifedipine | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.22, 4.49] |
6.1. Analysis.
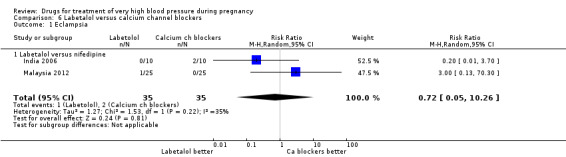
Comparison 6 Labetalol versus calcium channel blockers, Outcome 1 Eclampsia.
6.2. Analysis.
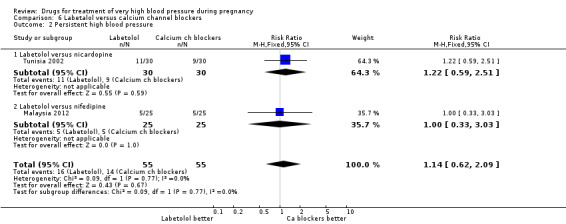
Comparison 6 Labetalol versus calcium channel blockers, Outcome 2 Persistent high blood pressure.
6.3. Analysis.
Comparison 6 Labetalol versus calcium channel blockers, Outcome 3 Hypotension.
6.4. Analysis.
Comparison 6 Labetalol versus calcium channel blockers, Outcome 4 Side‐effects for the woman (specific effects).
6.6. Analysis.
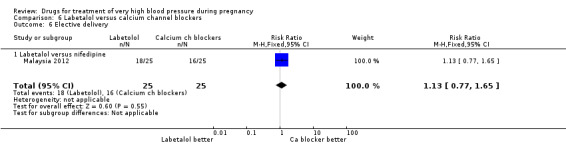
Comparison 6 Labetalol versus calcium channel blockers, Outcome 6 Elective delivery.
6.7. Analysis.

Comparison 6 Labetalol versus calcium channel blockers, Outcome 7 Caesarean section.
6.8. Analysis.
Comparison 6 Labetalol versus calcium channel blockers, Outcome 8 Admission to intensive care.
6.9. Analysis.

Comparison 6 Labetalol versus calcium channel blockers, Outcome 9 Admission to special care baby unit.
Comparison 7. Labetalol versus methyldopa.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent high blood pressure | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.74, 1.94] |
| 2 Changed drugs due to side‐effects | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.08 [0.45, 144.73] |
| 3 Caesarean section | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.30] |
| 4 Fetal or neonatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Stillbirth | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Neonatal death | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [0.22, 90.30] |
| 4.3 Total stillbirths and neonatal deaths | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.49 [0.22, 90.30] |
| 5 Small‐for‐gestational age | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.43, 1.39] |
| 6 Admission to special care baby unit | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.71] |
7.1. Analysis.

Comparison 7 Labetalol versus methyldopa, Outcome 1 Persistent high blood pressure.
7.2. Analysis.

Comparison 7 Labetalol versus methyldopa, Outcome 2 Changed drugs due to side‐effects.
7.3. Analysis.
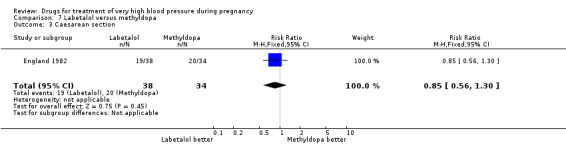
Comparison 7 Labetalol versus methyldopa, Outcome 3 Caesarean section.
7.4. Analysis.
Comparison 7 Labetalol versus methyldopa, Outcome 4 Fetal or neonatal death.
7.5. Analysis.

Comparison 7 Labetalol versus methyldopa, Outcome 5 Small‐for‐gestational age.
7.6. Analysis.
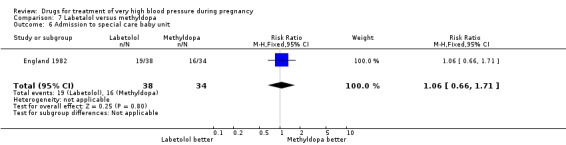
Comparison 7 Labetalol versus methyldopa, Outcome 6 Admission to special care baby unit.
Comparison 8. Labetalol versus diazoxide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent high blood pressure | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 1.88] |
| 2 Low blood pressure, requiring treatment | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.99] |
| 3 Caesarean section | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.18, 1.02] |
| 4 Perinatal deaths | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.69] |
8.1. Analysis.
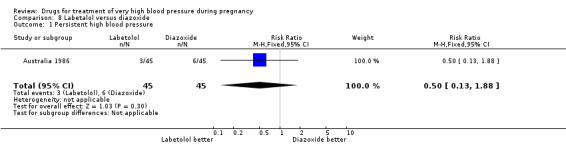
Comparison 8 Labetalol versus diazoxide, Outcome 1 Persistent high blood pressure.
8.4. Analysis.
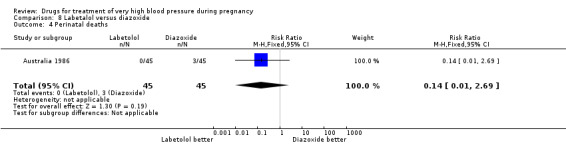
Comparison 8 Labetalol versus diazoxide, Outcome 4 Perinatal deaths.
Comparison 9. Nitrates versus magnesium sulphate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 Isosorbide versus magnesium sulphate | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Persistent high blood pressure | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.58] |
| 2.1 Isosorbide versus magnesium sulphate | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.58] |
| 3 Caesarean section | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.53] |
| 3.1 Isosorbide versus magnesium sulphate | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.07, 0.53] |
9.1. Analysis.
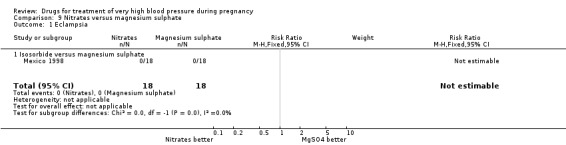
Comparison 9 Nitrates versus magnesium sulphate, Outcome 1 Eclampsia.
Comparison 10. Nimodipine versus magnesium sulphate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 2 | 1683 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.07, 16.03] |
| 2 Stroke | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Persistant high blood pressure | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.93] |
| 4 Hypotension | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.23, 2.27] |
| 5 Coagulopathy for the woman | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.41, 7.05] |
| 6 Respiratory difficulty for the woman | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.99] |
| 7 Placental abruption | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.27, 2.18] |
| 8 Side‐effects for the woman (specific effects) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Headache | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.71, 1.58] |
| 8.2 Flushing | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.12, 0.40] |
| 8.3 Nausea and/or vomiting | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.24] |
| 9 Side‐effects for the woman (all side‐effects) | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.55, 0.85] |
| 10 Oliguria | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.26] |
| 11 Caesarean section | 2 | 1683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.06] |
| 12 Postpartum haemorrhage | 1 | 1650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.92] |
| 13 Baby intubated at delivery | 1 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.09] |
| 14 Respiratory distress syndrome | 1 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.55, 1.20] |
| 15 Low blood pressure for the baby | 1 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.63, 15.40] |
| 16 Hypotonia for the baby | 1 | 1564 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.29, 1.10] |
10.2. Analysis.

Comparison 10 Nimodipine versus magnesium sulphate, Outcome 2 Stroke.
10.4. Analysis.

Comparison 10 Nimodipine versus magnesium sulphate, Outcome 4 Hypotension.
10.5. Analysis.
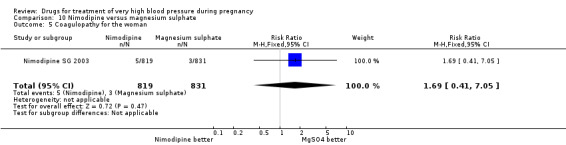
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 5 Coagulopathy for the woman.
10.7. Analysis.
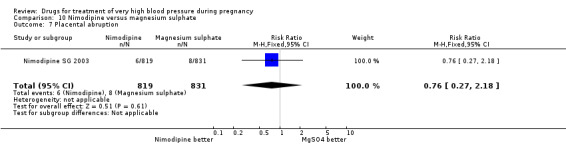
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 7 Placental abruption.
10.8. Analysis.

Comparison 10 Nimodipine versus magnesium sulphate, Outcome 8 Side‐effects for the woman (specific effects).
10.10. Analysis.
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 10 Oliguria.
10.11. Analysis.

Comparison 10 Nimodipine versus magnesium sulphate, Outcome 11 Caesarean section.
10.13. Analysis.
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 13 Baby intubated at delivery.
10.14. Analysis.
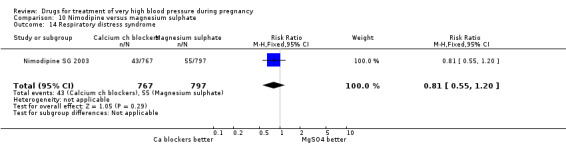
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 14 Respiratory distress syndrome.
10.15. Analysis.
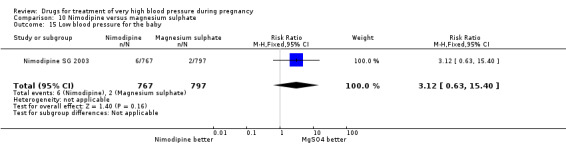
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 15 Low blood pressure for the baby.
10.16. Analysis.
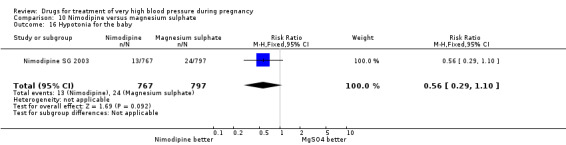
Comparison 10 Nimodipine versus magnesium sulphate, Outcome 16 Hypotonia for the baby.
Comparison 11. Nifedipine versus prazosin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.73] |
| 2 Eclampsia | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 HELLP syndrome | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.37, 3.60] |
| 4 Renal failure | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.17] |
| 5 Pulmonary oedema | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.60] |
| 6 Admission to intensive care | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.73] |
| 7 Magnesium sulphate prophylaxis | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.17, 3.10] |
| 8 Placental abruption | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.40, 2.28] |
| 9 Caesarean section | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 10 Stillbirth | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.18, 1.13] |
| 11 Admission to special care baby unit | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.49, 1.23] |
| 12 Severe respiratory distress syndrome | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.52, 2.82] |
11.1. Analysis.
Comparison 11 Nifedipine versus prazosin, Outcome 1 Maternal death.
11.2. Analysis.
Comparison 11 Nifedipine versus prazosin, Outcome 2 Eclampsia.
11.3. Analysis.

Comparison 11 Nifedipine versus prazosin, Outcome 3 HELLP syndrome.
11.4. Analysis.
Comparison 11 Nifedipine versus prazosin, Outcome 4 Renal failure.
11.5. Analysis.
Comparison 11 Nifedipine versus prazosin, Outcome 5 Pulmonary oedema.
11.6. Analysis.
Comparison 11 Nifedipine versus prazosin, Outcome 6 Admission to intensive care.
11.7. Analysis.

Comparison 11 Nifedipine versus prazosin, Outcome 7 Magnesium sulphate prophylaxis.
11.8. Analysis.

Comparison 11 Nifedipine versus prazosin, Outcome 8 Placental abruption.
11.9. Analysis.

Comparison 11 Nifedipine versus prazosin, Outcome 9 Caesarean section.
11.10. Analysis.
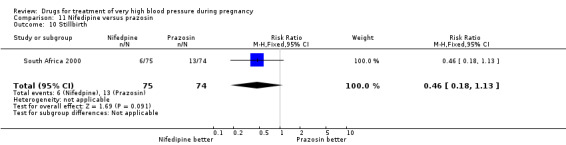
Comparison 11 Nifedipine versus prazosin, Outcome 10 Stillbirth.
11.11. Analysis.
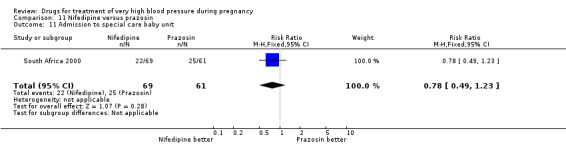
Comparison 11 Nifedipine versus prazosin, Outcome 11 Admission to special care baby unit.
11.12. Analysis.

Comparison 11 Nifedipine versus prazosin, Outcome 12 Severe respiratory distress syndrome.
Comparison 12. Nifedipine versus chlorpromazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eclampsia | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [0.11, 59.18] |
| 2 Persistent high blood pressure | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.57] |
| 3 Caesarean section | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.60, 1.05] |
12.1. Analysis.
Comparison 12 Nifedipine versus chlorpromazine, Outcome 1 Eclampsia.
12.2. Analysis.
Comparison 12 Nifedipine versus chlorpromazine, Outcome 2 Persistent high blood pressure.
12.3. Analysis.
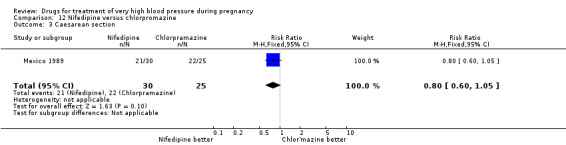
Comparison 12 Nifedipine versus chlorpromazine, Outcome 3 Caesarean section.
Comparison 13. Hydralazine versus diazoxide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.42 [0.39, 140.06] |
| 2 Stillbirth | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.3 [0.26, 107.70] |
| 3 Neonatal death | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 4 Death in first 7 days | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.13, 76.25] |
| 5 Caesarean section | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.72, 1.18] |
| 6 Respiratory distress syndrome | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.52, 1.88] |
| 7 Necrotising enterocolitis | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.47] |
| 8 Apgar score < 7 at 5 minutes | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.28, 4.01] |
| 9 Hypoglycaemia of the baby | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.29, 2.71] |
| 10 Ventilation of the baby | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.16] |
13.1. Analysis.
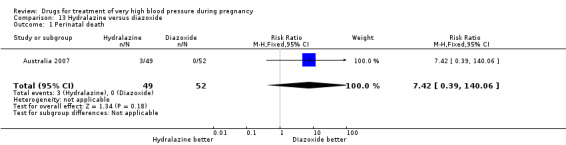
Comparison 13 Hydralazine versus diazoxide, Outcome 1 Perinatal death.
13.2. Analysis.
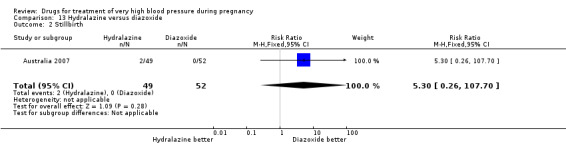
Comparison 13 Hydralazine versus diazoxide, Outcome 2 Stillbirth.
13.3. Analysis.

Comparison 13 Hydralazine versus diazoxide, Outcome 3 Neonatal death.
13.4. Analysis.

Comparison 13 Hydralazine versus diazoxide, Outcome 4 Death in first 7 days.
13.5. Analysis.
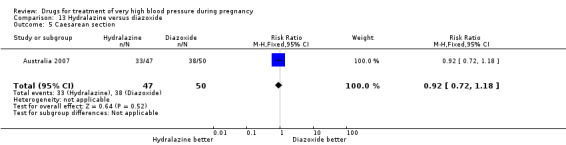
Comparison 13 Hydralazine versus diazoxide, Outcome 5 Caesarean section.
13.6. Analysis.

Comparison 13 Hydralazine versus diazoxide, Outcome 6 Respiratory distress syndrome.
13.7. Analysis.
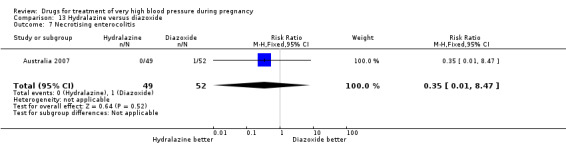
Comparison 13 Hydralazine versus diazoxide, Outcome 7 Necrotising enterocolitis.
13.8. Analysis.
Comparison 13 Hydralazine versus diazoxide, Outcome 8 Apgar score < 7 at 5 minutes.
13.9. Analysis.

Comparison 13 Hydralazine versus diazoxide, Outcome 9 Hypoglycaemia of the baby.
13.10. Analysis.
Comparison 13 Hydralazine versus diazoxide, Outcome 10 Ventilation of the baby.
Comparison 14. Methyldopa versus atenolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stillbirth | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 2 Neonatal death | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 3 Side‐effects for the woman (specific effects) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 21.0 [1.29, 342.93] |
| 3.1 Somnolence | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 21.0 [1.29, 342.93] |
| 4 Respiratory distress syndrome | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.87] |
| 5 Apgar score < 7 at 5 minutes | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.17, 1.48] |
| 6 Side‐effects for the baby | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.
Comparison 14 Methyldopa versus atenolol, Outcome 1 Stillbirth.
14.2. Analysis.
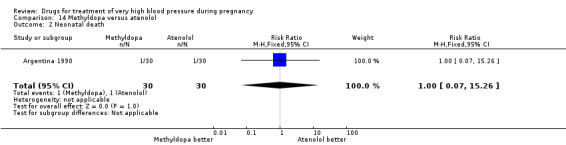
Comparison 14 Methyldopa versus atenolol, Outcome 2 Neonatal death.
14.4. Analysis.
Comparison 14 Methyldopa versus atenolol, Outcome 4 Respiratory distress syndrome.
14.5. Analysis.
Comparison 14 Methyldopa versus atenolol, Outcome 5 Apgar score < 7 at 5 minutes.
14.6. Analysis.
Comparison 14 Methyldopa versus atenolol, Outcome 6 Side‐effects for the baby.
Comparison 15. Urapidil versus calcium channel blockers.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Side‐effects for the woman | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.12] |
| 2 Side‐effects for the baby | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
15.1. Analysis.

Comparison 15 Urapidil versus calcium channel blockers, Outcome 1 Side‐effects for the woman.
15.2. Analysis.

Comparison 15 Urapidil versus calcium channel blockers, Outcome 2 Side‐effects for the baby.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Argentina 1990.
| Methods | Described as a “Prospective, randomized, comparative study”. | |
| Participants | 90 women with severe chronic hypertension during pregnancy or severe pregnancy‐induced hypertension, with or without proteinuria. Severe hypertension defined as BP ≥ 160/100 mmHg. Initial readings of BP were 24 hrs apart and follow‐up was weekly. No drugs were administered during the 1st 24 hrs after hospitalisation. Women with hypertensive emergencies were excluded as well as women requiring more than 1 drug to control their BP. |
|
| Interventions | Atenolol, 50‐200 mg daily (n = 30). Ketanserin, 80‐120 mg daily (n = 30). Alpha methyldopa, 500‐2000 mg daily (n = 30). |
|
| Outcomes | BP at onset of treatment, weekly for 3 weeks, and at the end of pregnancy; adverse effects from drugs; preeclamptic clinical signs and symptoms; creatinine, haematocrit, proteinuria and uric acid levels; fetal vitality (through weekly non‐stress tests and ultrasound studies). Perinatal outcomes: gestational age at delivery; birthweight; 1‐min Apgar score; fetal and neonatal mortality. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different drug regimens would mean blinding women and staff was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some blinding of outcome assessment apparent, “All the patients were hospitalized and their preeclamptic clinical signs and symptoms, as well as the adverse effects from the drugs, were weekly evaluated by residents who ignored the drug administered to the patients, and who simply elicited from them, by means of a questionnaire, if they presented or not with those symptoms.” This is not likely to be a successful method of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not clear how many women were excluded after randomisation (e.g. women whose BP increased and became an emergency). It appears that full data were available for the 90 included women. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported within the results. |
| Other bias | Low risk | Baseline characteristics similar, although 19/30 in the ketanserin group had PI hypertension compared with 13/30 in the atenolol and 9/30 in the alpha methyldopa groups. |
Australia 1986.
| Methods | Randomly allocated, no further information. CFU ‐ A, blinding ‐ C. | |
| Participants | 90 women with DBP > 105 mmHg after sedation with either phenobarbitone 200 mg or diazepam 10 mg 6‐hourly. Delivery planned for soon after treatment. | |
| Interventions | Labetalol: 200 mg in 200 mL 5% dextrose IV at 0.5 mg/kg/hr to a maximum of 3 mg/kg/hr, to keep DBP at 85‐90 mmHg. Continued until 24 hrs after delivery. Diazoxide: 75 mg IV, repeated every 30 min until BP controlled. Continued until 24 hrs after delivery. | |
| Outcomes | Woman: persistent high BP, low BP requiring treatment, caesarean section. Baby: death, RDS, hypoglycaemia, hypothermia. | |
| Notes | No data on which women received phenobarbitone and which received diazepam. Funding: Glaxo (makers of labetalol). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No mention of blinding, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised included within results (45 in each group: Tables 3 – 6). |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported upon. |
| Other bias | Low risk | Groups appear balanced for baseline characteristics. |
Australia 2007.
| Methods | Randomised controlled trial. | |
| Participants | Antenatal and postnatal women with severe hypertension (some data for antenatal women presented separately). | |
| Interventions | IV Hydralazine – 5 mg boluses every 20 min for up to 3 doses, with a maximum dose of 15 mg (n = 47 antenatal, 49 babies). Mini‐bolus Diazoxide – 15 mg boluses every 3 mins until the BP reached target or until 300 mg was given (20 x 15 mg mini‐bolus doses) within a 1‐hr period (n = 50, 52 babies). The treatment was concurrent with MgSO4 infusion (4 g bolus IV over 15 min then 2 g per hr infusion for 24 h) at the commencement of treatment). |
|
| Outcomes | Caesarean section rate; perinatal deaths; Apgar < 7 at 5 min; RDS; neonatal hypoglycaemia; neonatal ventilation. | |
| Notes | Antenatal and postnatal women with severe hypertension were included, but we have included the outcome data for the antenatal group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | “Patients were randomised by sequential selection of numbered opaque envelopes containing a randomised allocation.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Different regimens. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis by intention‐to‐treat. Protocol violations described. Study flow diagram clearly documented. No‐one lost to follow‐up or excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | None apparent. Baseline characteristics similar. |
Brazil 1992.
| Methods | 'Randomly assigned' by drawing an envelope from a box, each containing active treatment and placebo. CFU ‐ A, blinding ‐ A. | |
| Participants | 37 primigravid women over 28 weeks' gestation with DBP 110 mmHg or more after 60 min rest, and proteinuria > 300 mg in 24 hrs. Singleton pregnancy and a live fetus. Excluded: antihypertensive drug before trial entry, medical surgical or obstetric problem. | |
| Interventions | Nifedipine: 10 mg orally. Hydralazine: 5 mg IV. | |
| Outcomes | Woman: need for additional treatment. Baby: stillbirth. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling of envelopes “ nurse draw an envelope from a jumbled box”. |
| Allocation concealment (selection bias) | Unclear risk | Not enough detail reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Patients were blindly allocated”. Described as “double‐blind” and “The treating physicians were blinded to whether the drug being administered was hydralazine or nifedipine”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Some blinding of outcome assessment described: “All fetal heart rate tracings were examined by a single obstetrician, who was blinded to the drug regimen utilized..” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women are accounted for results tables 2‐7. |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported upon. |
| Other bias | Low risk | Groups appear balanced for baseline characteristics. |
Brazil 1994.
| Methods | Sealed envelopes. | |
| Participants | 50 women with DBP > 110 mmHg after 60 min rest and > 28 weeks' gestation. | |
| Interventions | Nifedipine: 10 mg sl and IV placebo. Hydralazine: 20 mg IV and sl placebo. | |
| Outcomes | Woman: time to lower BP, side‐effects (flushing, nausea, palpitations). Baby: stillbirth, neonatal death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough detail, just states "draw of sealed envelopes". |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “neither the patient nor the author knew about the drug used until the end of the protocol”, also “the placebo was obtained from the combination of natural mint essence and orange colourant, maintaining the characteristics of colour and taste.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear ‐ cannot tell from translation of paper or reports in abstract. |
| Selective reporting (reporting bias) | Unclear risk | Unclear ‐ cannot tell from translation of paper or reports in abstract. |
| Other bias | Unclear risk | Unclear ‐ cannot tell from translation of paper or reports in abstract. |
Brazil 2011.
| Methods | 16 pregnant women with gestational age between 20 and 32 weeks in acute severe hypertension were 'randomly allocated' to receive either hydralazine or labetalol. | |
| Participants | Pregnant women in acute severe hypertension with gestational age between 20 and 32 weeks and body mass index ≤ 40 kg/m2. Acute severe hypertension was defined according to the guidelines of the National High Blood Pressure Education Program (NHBPEP): sustained high BP: ≥160 mm Hg systolic, ≥ 105 mm Hg diastolic or both. | |
| Interventions | Labetalol: 20 mg IV bolus dose followed by 40 mg if not effective within 10 min; then 80 mg every 10 min until BP lower than 150/100 mmHg or maximum total dose of 220 mg (n = 8). Hydralazine: 5‐10 mg doses intravenously every 15‐20 min until BP lower than 150/100 mm Hg (n = 8). |
|
| Outcomes | BP and Doppler parameters from maternal uterine arteries and fetal middle cerebral and umbilical arteries observed during acute severe hypertension: SBP (mm Hg); DBP (mm Hg); umbilical artery PI; umbilical artery RI; middle cerebral artery PI; middle cerebral artery RI; uterine artery PI; uterine artery RI. | |
| Notes | A total of 17 women agreed to participate were randomly assigned to receive either labetalol or hydralazine but 1 was excluded from the study because both drugs were necessary to control BP. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported. |
| Allocation concealment (selection bias) | Unclear risk | No details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Likely to be unblinded as regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Likely to be unblinded as regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1/17 excluded post randomisation as both treatments were required. It was not clear what group she had been assigned to. |
| Selective reporting (reporting bias) | Unclear risk | No details reported. |
| Other bias | Unclear risk | No details reported. |
England 1982.
| Methods | 'Randomised', no further information. Interim report on ongoing study. 2 women not delivered at time of reporting. CFU ‐ A, blinding ‐ C. | |
| Participants | 74 women with BP 170/110 mmHg, or above, and < 36 weeks' gestation. Excluded: multiple pregnancy, diabetes, rhesus isoimmunisation. | |
| Interventions | Labetalol: 100 mg x 4/day. Methyldopa: 250 mg x 4/day. Oral or IV hydralazine in both groups if BP not controlled. | |
| Outcomes | Woman: need for other drugs, side‐effects, caesarean section. Baby: stillbirth, neonatal death, SCBU. | |
| Notes | Interim analysis of an ongoing trial. Final report not published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 74 patients entered the trial, and 72 have delivered. All results available for 72 women who had delivered. |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported upon. |
| Other bias | Unclear risk | Unclear – no baseline characteristics table. |
France 2010.
| Methods | Described as a “Preliminary randomized controlled trial”. France. | |
| Participants | 18 women with severe PE without previous antihypertensive treatment. The therapeutic goal was control BP to a mean BP of between 105 and 125 mmgHg. | |
| Interventions | Urapidil 6.25 mg boluses every 5 mins until the DBP dropped below 105 mmHg followed by a 4 mg/hr infusion as needed (n = 9). Nicardipine 1 µ/kg/min infusion until a 15% reduction in mean BP, followed by a 0.75 µ/kg/min infusion adjusted as needed (n = 9). |
|
| Outcomes | Achievement of BP goal in 2 hrs or less; number of episodes of hypotension (MBP below 100 mmHg); maternal and neonatal side‐effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details, available as abstract only. |
| Allocation concealment (selection bias) | Unclear risk | No details, available as abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Different regimens. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All main outcomes reported for all women, but there was 1 protocol deviation. |
| Selective reporting (reporting bias) | Unclear risk | No details, available as abstract only. |
| Other bias | Unclear risk | No details, available as abstract only. |
Germany 1998.
| Methods | Computer‐generated randomisation list. CFU ‐ A, blinding C. | |
| Participants | 26 women with BP 160/110 mmHg after 3 hr bed rest, 1+ of proteinuria, oedema or hyperreflexia. Gestation 26‐38 weeks. No IV antihypertensive before entry. | |
| Interventions | Urapidil: 6.25 mg IV repeated after 5 min if BP not decreased. Then 2‐4 mg/hr until delivery. Hydralazine: IV, mean 0.13 mg/kg/4 hrs. | |
| Outcomes | Woman: eclampsia, side‐effects, caesarean section. Baby: stillbirth, neonatal death. | |
| Notes | Both groups of women also received IV magnesium ascorbate (4 g load and 1‐2 g/hr maintenance. 31 women reported to have been recruited in 1 German paper, no clinical data in that report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomly assigned to the urapidil or dihydralazine group according to a computer generated randomization.” |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States “Treatment was not blinded”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | States “Treatment was not blinded”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Initially 26 subjects met the criteria for enrolment in the study. None of the patients dropped out during the study.” |
| Selective reporting (reporting bias) | High risk | FHR monitoring 3 times daily and weekly ultrasound assessment of fetal growth ‐ reported incompletely. |
| Other bias | Low risk | Groups appear balanced for baseline characteristics. |
Germany 2006.
| Methods | Prospective randomised multicentre study. 6 centres, Germany. | |
| Participants | 42 women with pregnancy‐induced hypertension and PE. Most women had severe hypertension according to mean values for baseline characteristics. | |
| Interventions | IV Urapidil at initial dose of 12.5‐25 mg (n = 20). IV Dihydralazine at a uniform initial dose of 5 mg (n = 22). |
|
| Outcomes | BP and HR; method of delivery; adverse events; persistent hypertension; hypotensive periods; neonatal deaths; RDS. | |
| Notes | Numbers of women randomised to each group not actually reported (only report total number randomised n = 42) ‐ calculated from data on caesarean sections in table 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list used, “Subjects were randomly assigned to the urapidil or dihydralazine group. For this purpose, a random list was generated with the help of the SAS procedure PROC SAS.” |
| Allocation concealment (selection bias) | Low risk | Each centre received a set of sealed, opaque envelopes – the envelopes were consecutively numbered and were opened in that consecutive order. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not done, “Blinding was not feasible”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not done, “Blinding was not feasible”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Do not report actual numbers randomised to each group, but no mention of loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | Baseline characteristics similar, although 5 women in the dihydralazine group had previous PE compared with only 1 in the urapidil group. |
India 2006.
| Methods | Described as "randomized prospective study" ‐ no further details given. | |
| Participants | 20 pregnant women admitted with severe hypertension in 2nd and 3rd trimester. | |
| Interventions | Labetalol versus nifedipine. Treatment was titrated to achieve 20% lowering of BP. | |
| Outcomes | Maternal BP; maternal heart rate; fetal heart rate; success rate; length of time needed to achieve therapeutic goal; maternal adverse effects (eclampsia; hypotension; moderate tachycardia); fetal adverse effects. | |
| Notes | Available as abstract only, so results limited and difficult to assess method of randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details, available as abstract only. |
| Allocation concealment (selection bias) | Unclear risk | No details, available as abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned for women, it would be clear to staff as regimens are different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It would be clear to assessors as regimens are different. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details, available as abstract only. |
| Selective reporting (reporting bias) | Unclear risk | No details, available as abstract only. |
| Other bias | Unclear risk | No details, available as abstract only. |
India 2011.
| Methods | Described as a "randomized control trial" ‐ but no further details. Available as abstract only. | |
| Participants | Women with SBP of more than 160 mm hg or more and DBP of 110 or more were included ‐ hypertensive emergencies of pregnancy. | |
| Interventions | IV labetalol Oral nifedipine Both agents were repeated at sequentially escalating dosages every 20 mins until a therapeutic goal was reached. |
|
| Outcomes | Time to achieve therapeutic goal. Therapeutic goal: SBP of < 150 mm hg and diastolic of < 100 mm hg; adverse effects and perinatal outcome. | |
| Notes | No details of number of women randomised given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details, available as abstract only. |
| Allocation concealment (selection bias) | Unclear risk | No details, available as abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not mentioned, but different regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not mentioned, but different regimens. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details, available as abstract only. Numbers randomised not stated. |
| Selective reporting (reporting bias) | Unclear risk | No details, available as abstract only. |
| Other bias | Unclear risk | No details, available as abstract only. |
Iran 2002.
| Methods | Consecutively numbered sealed envelopes. Randomised in blocks of 4. | |
| Participants | 126 women with BP at least 160/110 mmHg, and criteria for severe PE as defined by American College of Obstetricians and Gynecologists. | |
| Interventions | Nifedipine: 8 mg sl, repeated until DBP 90‐100 mmHg.
Hydralazine: 5‐10 mg IV, repeated until DBP 90‐100 mmHg. Both: MgSO4, 4 g bolus IV, then 1‐2 g/hr for 24 hr. |
|
| Outcomes | Woman: persistent high BP (not controlled after 20 mins), further hypertensive crises, adverse effects. Baby: Apgar scores. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The block‐randomized technique was used and each block had four cases” – but no details on whether computer generated or other methods. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. “Women were allocated consecutive, numbered, opaque, sealed envelopes indicating their medication.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States single blind – only outcome assessment blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Some blinding of outcome assessment, states “The observer who measured BP was blind to the type of treatment”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to have been accounted for in the analyses. No mention of drop outs or loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported upon. |
| Other bias | Low risk | Groups appear balanced for baseline characteristics. |
Iran 2011.
| Methods | Randomised controlled trial. Women’s Hospital, Tehran, Iran. | |
| Participants | 50 pregnant women admitted for labour diagnosed with severe PE or chronic hypertension superimposed by PE, of at least 24 weeks' gestation. Hypertensive emergency was defined as measured sustained SBP ≥ 170 mmHg or DBP ≥ 105 mmHg. Exclusion criteria: women diagnosed with heart disease or severe renal impairment or cerebrovascular accident. |
|
| Interventions | Oral nifedipine 10 mg capsules, administered initially at a dose of 10 mg, then 20 mg, with intervals of 20 min up to a maximum of 5 doses or when desired BP (150/90‐100) achieved (n = 25). IV hydralazine 5 mg, administered initially at 5 mg and repeated in 10 mg doses, up to maximum of 5 injections in intervals of 20 min. IV hydration were all set at rate of 125 mg/h (n = 25). |
|
| Outcomes | Primary: time and frequency of doses to achieve target BP. Secondary: urinary output; maternal (headache; hypotension; flushing; nausea) and neonatal side‐effects (fetal heart rate abnormalities; neonatal Apgar score). |
|
| Notes | All women received prophylactic infusion of MgSO4 continually to avoid convulsion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Referred to a random number table “We dispensed either nifedipine or hydralazine according to a random number table”. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, “It was not possible for us to blind the study, because there was no placebo group due to ethical considerations”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, “It was not possible for us to blind the study, because there was no placebo group due to ethical considerations.”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes fully reported upon. |
| Other bias | Low risk | None apparent. Baseline characteristics of 2 groups similar. |
Malaysia 2012.
| Methods | A double‐blind randomised trial. A university hospital in Malaysia. | |
| Participants | 50 pregnant women with severe gestational hypertension ≥ 160/110 mmgH who required immediate treatment. | |
| Interventions | Nifedipine 10 mg tablet, orally, up to 5 doses and IV placebo saline injection until target BP of ≤ 150/100 mmHg achieved (N = 25). IV labetalol injection (in an escalating dose regimen of 20, 40, 80, 80 and 80 mg) and a placebo tablet every 15 mins until target BP of ≤ 150/100 mmHg achieved (n = 25). Cross‐over treatment was effected if the initial treatment regimen was unsuccessful. |
|
| Outcomes | Outcomes: time taken to achieve target BP (SBP ≤ 150 mmHg and DBP ≤ 100 mmHg); total antihypertensive doses to achieve target BP; systolic and DBP and maternal heart rate profile; CTG abnormality; maternal hypotension (BP < 90/60 mmHg); induction of labour/caesarean section; mode of delivery; birthweight; cord arterial pH; cord arterial blood base excess; maternal intensive care admission; neonatal intensive care admission; reported side‐effects (nausea; vomiting; dizziness; palpitations; headache; chest pain; shortness of breath). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation sequence was computer generated in blocks of four or eight..” |
| Allocation concealment (selection bias) | Low risk | “The randomisation sequence was computer generated in blocks of four or eight and placed in numbered sealed envelopes with the allocated drugs” “These envelopes were opened by a research nurse or investigator sequentially to allocate treatment..” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | IV drug and placebo prepared by the research nurse or investigator as a fluid drawn into a 60‐mL syringe labelled as A and given to care provider for administration together with the 5 tablets. Oral nifedipine and placebo tablets were identical in appearance. “Both provider and participant were blinded to the treatment given.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Care provider taking BP readings was blinded to treatment – unless treatment goal not achieved after randomised treatment A and then cross‐over treatment B– then open‐label treatment carried out according to preference of the provider. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women accounted for – 1 did not adhere to study protocol for labetalol and there was cross‐over to the other treatment in 5 women from nifedipine group and 4 women in the labetalol group – but analysis based on an intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | All expected outcome results reported. |
| Other bias | Low risk | Baseline characteristics similar – apart from slight difference in DBP between the groups – 110 mmHg in nifedipine group compared to 108 mmHg for labetalol group (P = 0.012) – small but absolute difference. |
Mexico 1989.
| Methods | 'Randomised', no further information. 5 women excluded from chlorpromazine group because they received another antihypertensive. CFU ‐ B, blinding C. | |
| Participants | 60 women with severe PE or eclampsia. Excluded if cardiopathy, diabetes, isoimmunisation, twin pregnancy, or antihypertensive in 48 hr before trial entry. | |
| Interventions | Chlorpromazine: 12.5 mg IV and 12.5 mg IM. 12.5 mg IV repeated every 30 min, to a total of 50 mg, until BP controlled or an additional antihypertensive. Nifedipine: 10 mg sl, repeated every 30 min to a max of 4 doses until BP controlled or an additional antihypertensive. | |
| Outcomes | Woman: eclampsia, additional antihypertensive, caesarean section. Baby: gestation at delivery (mean). | |
| Notes | All women received phenytoin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomised assignment took place using permutation blocks and random number tables.” |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. States “...the scheme for each patient in a sealed envelope identified with a number” ‐ but no information whether envelopes were sequentially numbered or opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 60 women were randomised, but 5 women in the chlorpromazine group were excluded from the analysis as they received other medications, reducing this group to 25. Don't appear to present any data on these 5 women ‐ though this was from a translation. |
| Selective reporting (reporting bias) | Unclear risk | Unclear, article in Spanish and can not tell from translation. |
| Other bias | Unclear risk | Unclear, article in Spanish and can not tell from translation. |
Mexico 1993.
| Methods | Consecutively numbered sealed opaque envelopes. | |
| Participants | 27 women at 28‐42 weeks with severe PE (BP 150 mmHg or more, 2/3+ protein), and 1 or more of epigastric pain, convulsions, headache. No chronic hypertension, or renal or cardiac disease. | |
| Interventions | Hydralazine: 5 mg IV. Repeated every 20 min if DBP 110 mmHg or more, max x 3. If BP not controlled, chlorpromazine 12.5 mg IV plus 12.5 mg IM x 2. Nifedipine: 10 mg sl. Repeated every 20 min if DBP 110 mmHg or more, max x 3. If BP not controlled, chlorpromazine 12.5 mg IV plus 12.5 mg IM x 2. | |
| Outcomes | Woman: control of BP, days in hospital (mean). Baby: Apgar at 1 and 5 min (mean). | |
| Notes | All women had a diazepam infusion for 24 hr after delivery. Data not included in analysis. Mean hospital stay (days): for nifedipine n = 13, 5.5 SD [2.1] and for hydralazine n = 14, 6.0 [2.2]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The process of randomisation was carried out using numbered permutation blocks of 6; using a table of random numbers ....” |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. “..the blocks were selected and the allocation sealed in opaque envelopes and numbered progressive order.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear, article in Spanish and can not tell from translation. |
| Selective reporting (reporting bias) | Unclear risk | Unclear, article in Spanish and can not tell from translation. |
| Other bias | Unclear risk | Unclear, article in Spanish and can not tell from translation. |
Mexico 1998.
| Methods | Randomised, no further information. | |
| Participants | 36 women > 36 weeks' gestation with severe PE (DBP > 110 mmHg + proteinuria). Excluded: diabetes, essential hypertension, history of drug or alcohol abuse, antihypertensive drugs in the last week. | |
| Interventions | Isosorbide: 1.25 mg by sl aerosol. If BP dropped by < 15%, second dose 10 min later. MgSO4: infusion of 4 g in 1 hr, then 1 g/hr for 5 hrs. | |
| Outcomes | Woman: need for additional antihypertensive, caesarean section, eclampsia. Baby: none. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details on how randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. No allocation concealment methods described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop outs or withdrawals reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear, article in Spanish and can not tell from translation. |
| Other bias | Unclear risk | Unclear, article in Spanish and can not tell from translation. |
N Ireland 1991.
| Methods | Sequentially numbered sealed envelopes. CFU ‐ A, blinding ‐ C. | |
| Participants | 30 women with singleton pregnancy before labour, no previous antihypertensive. BP 140/90 or above, clinical decision to treat ‐ usually because of labile BP, proteinuria and symptoms. | |
| Interventions | Labetalol: 100 mg IV. Hydralazine: 10 mg IV. | |
| Outcomes | Woman: side‐effects (flushing, light head, nausea, scalp tingling). Baby: death. | |
| Notes | Long study to delivery interval (range 0.1‐11 weeks). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. “Randomization was by sequentially numbered sealed envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts/loss to follow‐up reported. All 30 patients appear to have contributed data to analyses (Fig 1, 2, 3). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Groups appear balanced for baseline characteristics. |
Netherlands 1999.
| Methods | Open randomised multicentre trial with 4 centres, randomisation by telephone call to answering service. CFU ‐ A, blinding ‐ C. | |
| Participants | 44 women at 26‐32 weeks' gestation, DBP 110 mmHg or above. All women given plasma volume expansion at trial entry, 27 out of 44 monitored with a pulmonary artery catheter (12 ketanserin, 15 hydralazine). MgSO4 for women with impending eclampsia (8 ketanserin, 11 hydralazine). |
|
| Interventions | Ketanserin: 5 mg IV bolus then 4 mg/hr. Increased every 20 min until target BP. Max 10 mg/hr. Further 5 mg with every 2 mg/hr increment.
Hydralazine: 1 mg/hr IV, hourly increments of 1 mg/hr until target BP. Max 10 mg/hr. Both groups, if BP not controlled given other study drug. |
|
| Outcomes | Woman: death, eclampsia, pulmonary oedema, HELLP, DIC, abruption, additional drugs (cross‐over, given other study drug), caesarean section. Baby: death (babies > 28 weeks' gestation only). | |
| Notes | 19 women in each group had antenatal steroids. Funding: Janssen‐Cilag (manufacture ketanserin). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Block randomisation was carried out using centres as strata...” |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. “...randomisation was carried out using centres as strata; after dialling a central telephone number, an answering service communicated with medication allocated.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open randomised prospective multicentre trial – so no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open randomised prospective multicentre trial – so no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts/loss to follow‐up reported. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Groups appear balanced for baseline characteristics. |
Netherlands 2003.
| Methods | 'Randomised' no further information. Published as an abstract only. | |
| Participants | 56 women beyond 32 weeks' gestation with DBP 110 mmHg or above. | |
| Interventions | Ketanserin: no information about dose. Hydralazine: no information about dose. | |
| Outcomes | Woman: vaginal delivery, composite outcome of maternal morbidity (eclampsia, renal failure, pulmonary oedema, and/or HELLP). Baby: none reported. | |
| Notes | Unpublished data provided by the authors: hypotension (defined as DBP < 75 mmHg), failure to reach target BP (DBP 85‐105 mmHg). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Limited information – trial report is in abstract form. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Limited information – trial report is in abstract form. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as “An open randomized prospective trial” – so no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Described as “An open randomized prospective trial” – so no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Limited information – trial report is in abstract form. |
| Selective reporting (reporting bias) | Unclear risk | Limited information – trial report is in abstract form. |
| Other bias | Unclear risk | Limited information – trial report is in abstract form. |
Nimodipine SG 2003.
| Methods | Randomisation stratified by centre, blocks of 6. Sealed opaque envelopes. Recruitment 1995‐2000. 100 women (6%) excluded from analysis: 99 did not get allocated treatment, 1 withdrawn. Recruitment stopped early following interim analysis. CFU ‐ B, blinding ‐ C. | |
| Participants | 1750 women with PE, planned delivery and no previous MgSO4. BP >/= 140/90 and 1+ proteinuria plus 1 of: headache, clonus, visual disturbance, epigastric pain, oliguria, pulmonary oedema, raised liver enzymes, haemolysis, oligohydramnios, IUGR. | |
| Interventions | Nimodipine: 60 mg 4‐hourly, orally MgSO4: according to local protocol. Either 4 g IV then 1 g/hr, or 6 g IV then 2 g/hr. All continued either for 24 hr total, or until 24 hr after delivery. Serum monitoring not required. | |
| Outcomes | Woman: eclampsia, stroke, coagulopathy, respiratory problems, cardiac failure, antihypertensive drugs, side‐effects, abruption, caesarean section, PPH. Baby: RDS, hypotonia, intubation, hypotension. | |
| Notes | Recruitment at 14 hospitals in 8 countries. Data for stillbirths and neonatal deaths not reported. These data were requested from the investigators, but have been lost. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were randomly assigned according to center (Epistat Services) in blocks of six...” does not refer to random number table or use of a computer number generator. |
| Allocation concealment (selection bias) | Low risk | “Patients were randomly assigned according to center (Epistat Services) in blocks of six, with the use of sealed opaque envelopes...” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as “The study was not blinded, because of logistic and economic constraints. The primary outcome measure (eclampsia) was binary, objective, and not subject to observer or measurement bias”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as “The study was not blinded, because of logistic and economic constraints. The primary outcome measure (eclampsia) was binary, objective, and not subject to observer or measurement bias”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were available for 1650 of 1750 patients (94.3%) – so minimal loss. 99 patients did not receive the study drug mainly because they gave birth before the drug could be administered or because of logistic issues and 1 patient in the MgSO4 group was withdrawn because induction of labour was stopped and conservative management instituted. However, no baseline details for these 100 patients – so do not know how similar they were the sample as a whole. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | Groups appear well balanced for baseline characteristics, apart from SBP. Study was stopped early because a planned interim analysis showed a significantly higher rate of seizure in the nimodipine group. Initially planned 1000 patients per group. |
Panama 2006.
| Methods | Randomised clinical trial. | |
| Participants | 200 women randomised. Inclusion criteria: severe hypertension (SBP ≥ 160 mmHg and/or DBP ≥110 mmHg) in pregnancy; ≥ 24 weeks' gestation; no concurrent antihypertensive therapy or absolute contraindications for labetalol or hydralazine. | |
| Interventions | Hydralazine (5 mg as a slow bolus dose given intravenously, and repeated every 20 min up to a maximum of 5 doses) (n = 100). Labetalol (20 mg IV bolus dose followed by 40 mg if not effective within 20 min, followed by 80 mg every 20 min up to a maximum dose of 300 mg) (n = 100). |
|
| Outcomes | Maternal: maternal death; side‐effects (palpitations; headache; nausea or vomiting; flushing; epigastric pain; visual symptoms; dizziness); hypotension; successful lowering of BP; 1‐2 doses for effective BP control; 3‐4 doses for effective BP control; persistent severe hypertension; hypertensive encephalopathy; caesarean section; placental abruption; pulmonary edema; HELLP syndrome; Eclampsia; DIC; oliguria; acute renal insufficiency. Perinatal outcomes: gestational age, birthweight; fetal growth restriction; 1‐ and 5‐min Apgar scores; heart rate; blood glucose; neonatal death; hypotension; admission to NICU; RDS; necrotising enterocolitis; intraventricular haemorrhage grades III/IV. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Refer to a computer‐generated list, “Randomization was performed according to a computer‐generated list by means of sequentially numbered, opaque, sealed envelopes indicating their medication”. |
| Allocation concealment (selection bias) | Low risk | “Randomization was performed according to a computer‐generated list by means of sequentially numbered, opaque, sealed envelopes indicating their medication.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Randomization was performed according to a computer‐generated list by means of sequentially numbered, opaque, sealed envelopes indicating their medication.” No blinding, “The study was not blind, because of logistic and economic constraints”. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “Randomization was performed according to a computer‐generated list by means of sequentially numbered, opaque, sealed envelopes indicating their medication.” No blinding, “The study was not blind, because of logistic and economic constraints.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 200 women randomised – 100 to each treatment group; 1 woman in hydralazine group did not receive medication due to medication error; 2 women in labetalol group did not receive medication (1 medication error; 1 refusal); however all patients randomised appear to have been analysed – 100 in each group (see Fig. 1, flow diagram). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes fully reported upon. |
| Other bias | Low risk | None apparent. Baseline characteristics of 2 groups similar. |
South Africa 1987.
| Methods | Randomly allocated, no other information. CFU ‐ A, blinding ‐ C. | |
| Participants | 20 women with DBP 110 mmHg or above, not settled after 2 hrs bed rest and 200 mg phenobarbitone. At least 32 weeks' gestation, no previous hypotensive therapy, not in labour and no imminent eclampsia. No PMH of asthma, diabetes or heart disease. | |
| Interventions | Labetalol: 200 mg in 200 mL 5% dextrose at 20 mg/hr. Increased every 20 min by 20 mg/hr until DBP 90‐100 mmHg, or maximum dose of 160 mg/hr. Then continued for 1 hr. Hydralazine: 25 mg in 200 mL saline at 3.7 mg/hr. Increased every 20 min by 3.7 mg/hr until DBP 90‐100 mmHg, or maximum dose of 15 mg/hr. Then continued for 1 hr. | |
| Outcomes | Woman: failure of BP control, eclampsia, caesarean section. Baby: death, hypoglycaemia, mean Apgar scores. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 patients randomised to each group and all appear to have been included in analyses. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Groups appear well balanced for baseline characteristics. |
South Africa 1989.
| Methods | Random number table, no further information. CFU ‐ A, blinding ‐ C. | |
| Participants | 33 primigravid women; no hypertension, renal disease, or other medical problems; no antihypertensive therapy; DBP 110 mmHg or more for 2 hrs; and at least 28 weeks' gestation. Not needing immediate delivery and no fetal distress. | |
| Interventions | Nifedipine: 10 mg oral. Repeated after 30 mins if no response. Hydralazine: 6.25 mg in 10 mL water IV over 5‐10 mins. Repeated after 30 mins if no response. | |
| Outcomes | Woman: need for second dose, low BP causing fetal distress, side‐effects (headache, flushing nausea, retrosternal pain). Baby: death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...they were allocated to one of two groups using a random number table.” |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | Unclear. |
South Africa 1992.
| Methods | Random number tables, no further information. CFU ‐ A, blinding ‐ C. | |
| Participants | 47 women admitted to labour ward with DBP > 110 mmHg, which did not settle after phenobarbitone and bed rest. At least 1+ proteinurea, and above 33 weeks' gestation. Excluded if imminent eclampsia or requiring immediate delivery. All had a central venous line. | |
| Interventions | Prostacyclin: 0.5 ng/kg/min IV increased at increments of 1.5 ng/kg/min to maximum of 10 ng/kg/min. Continued for 24 hr after delivery. Hydralazine: 0.5 mg/kg/min IV increased every 15 min to a maximum of 1.5 mg/kg/min. Continued for 24 hr after delivery. | |
| Outcomes | Woman: caesarean section, need for additional antihypertensive, side‐effects (headache, nausea and vomiting). Baby: death, ventilation. | |
| Notes | Funding: Wellcome, MRC South Africa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...they were allocated to one of two groups using a random number tables.” |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised appear to be included within results (47 randomised: 25 in 1 group; 22 in the other group). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | Groups appear balanced for baseline characteristics “There were no significant differences between the two treatment groups in respect of clinical or laboratory variables.” |
South Africa 1995.
| Methods | Sealed envelopes, no other information. Drug solutions prepared by someone not involved in clinical care, and blinded. CFU ‐ A, blinding ‐ A. | |
| Participants | 20 women at > 28 weeks' gestation; DBP > 110 mmHg after 5 mins rest, or, 100 mmHg or above on 2 occasions 30 mins apart. Excluded if fetal distress, antihypertensive therapy during previous 12 hrs, or epidural anaesthesia. | |
| Interventions | Hydralazine: 5 mg in 2 mL IV over 2 min. Repeated after 20 min if BP not below 100 mmHg. Ketanserin: 10 mg in 2 mL IV over 2 min. Repeated after 20 min if BP not below 100 mmHg. | |
| Outcomes | Woman: need for more than 1 dose of drug, low BP causing fetal distress, caesarean section, eclampsia. Baby: none reported. | |
| Notes | All women reached target BP. In the hydralazine group this one achieved with a single dose for all women, 6 women in the ketanserin group needed additional doses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Sealed envelopes, no other information, “Patients were randomized by means of sealed envelopes...” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as “Double blind” and also says “A person not involved in the clinical management of the patient prepared the drugs for injection.” Both drugs were administered in 2 ml solutions via a syringe and it states, “Therefore, it was impossible for the clinician to know which drug was being used.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 patients received each study drug, but it was reported that “Doppler results were available in 18 patients of whom 9 received hydralazine and 9 received ketanserin.” So data missing for 2 women. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | “The two groups of patients were comparable in respect of age, gravidity, duration of pregnancy and body mass.” However, more patients in the hydralazine group had severe proteinuria and this is the group that developed severe complications. |
South Africa 1997.
| Methods | Sealed sequentially numbered envelopes. 2:1 randomisation. 4 women excluded, but data on most clinical outcomes reported. CFU ‐ A, blinding ‐ C. | |
| Participants | 33 women with MAP > 125 mmHg x 3 at least 5 min apart in 30 min period. Excluded if antihypertensive other than single dose of methyl dopa or 1.25 mg hydralazine. | |
| Interventions | Urapidil: 12.5 mg IV repeated every 3 min in bolus of 25 mg if MAP > 120 mmHg. Max dose of 400 mg. Hydralazine: 6.25 IV over 15 min, repeated every 30 min to maintain MAP > 120 mmHg. | |
| Outcomes | Woman: hypotension, side‐effects (headache, palpitations, nausea, tinnitus), caesarean section, treatment failure. Baby: death, Apgar (mean), cord pH (mean). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was computer generated, and trial medication allocation was kept in sealed, sequentially numbered opaque envelopes until after a patient qualified for the trial.” |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. “Randomization was computer generated, and trial medication allocation was kept in sealed, sequentially numbered opaque envelopes until after a patient qualified for the trial.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as “Single blind” but no other detail given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as “Single blind” but no other detail given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 33 patients entered the trial – 3 patients were excluded (in 2 patients not all haemodynamic assessments were recorded due to equipment failure; 1 did not fulfil entry criteria; and 1 patient in urapidil required in excess of 400 mg to control her MAP during trial and was considered a treatment failure). 29 protocol correct patients were analysed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | “The groups were similar at trial entry.” |
South Africa 1997a.
| Methods | Women randomly allocated using a computer‐generated randomisation sheet. No information about concealment of allocation. CFU ‐ A, blinding ‐ C. | |
| Participants | 40 primigravid women with severe hypertension (DBP 110 mmHg or more) and no signs or symptoms of imminent eclampsia. All had 200 mg phenobarbitone 2 hrs before trial entry. | |
| Interventions | Isradipine: IV infusion of 0.15 mcg/kg/min, increased by 0.0025 mcg/kg every 15 min until DBP < 95 mmHg. Hydralazine: 6.25 mg IV over 10 min, repeated once if DBP still > 95 mmHg. | |
| Outcomes | Woman: persistent high BP, hypotension. Baby: fetal heart rate deceleration, stillbirth, neonatal death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Using a computer‐generated randomization sheet, patients were randomly allocated to receive either isradipine or dihydralazine.” |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but different regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but different regimens. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 20 patients were randomised to each group and all are included in the analysis – “An intention to treat analysis was used.” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | “The two groups were comparable with respect to age, parity and blood pressure.” |
South Africa 1997b.
| Methods | Sealed, numbered, opaque envelopes. Nursing sister not involved in clinical care then made up the allocated solution (4 mL). 8 women excluded (9%) as delivered without receiving antihypertensive therapy. CFU ‐ B, blinding ‐ B. | |
| Participants | 88 women at least 28 weeks' gestation, DBP > 110 mmHg or DBP > 100 mmHg for 30 mins. | |
| Interventions | Ketanserin: 500 mL crystalloid IV over 15 min, then bolus 10 mg ketanserin in 4 mL IV. Bolus repeated every 20 min, until DBP 90 mmHg, to a maximum of 4 doses. Hydralazine: 500 mL crystalloid IV over 15 min, then bolus 5 mg hydralazine in 4 mL IV. Bolus repeated every 20 min, until DBP 90 mmHg, to a maximum of 4 doses. | |
| Outcomes | Woman: death, persistent high BP (DBP > 90 mmHg after 4 bolus injections), delivery for fetal distress, caesarean section. Baby: death. | |
| Notes | Trial stopped by 'monitoring committee', reason not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were then assigned to receive either 5 mg dihydralazine or 10 mg of ketanserin according to random numbers which had been previously generated by computer.” |
| Allocation concealment (selection bias) | Low risk | “Successively numbered sealed, opaque envelopes contained the instructions for the preparation of each new patient’s medication. A nursing sister not involved in the management of the particular prepared patient.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant/clinician appeared to be blinded, “In either case, the managing physician was given a syringe with four millilitre of clear fluid.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study was stopped early on the advice of the monitoring committee “the study stopped after the analysis of 88 consecutive patients who qualified for the study.” 8 of these were not included in the analysis – 6 patients who qualified for the study were not randomised because their BP was lower than 90 mm Hg after the fluid overload and 2 patients did not receive the medication after randomisation – in both the fetal heart rate pattern deteriorated to such a degree that emergency caesarean sections were performed. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | “The groups were comparable regarding maternal age, gravidity and gestation age” However, study stopped early ‐ reasons not described and no baseline characteristics for 8 patients who were not included in the analysis. |
South Africa 2000.
| Methods | Consecutive numbered sealed opaque envelopes. 5 women excluded; 2 postpartum, 1 delivered before treatment started, 1 randomised twice, 1 wrongly identified. CFU ‐ B, blinding ‐ C. | |
| Participants | 150 women with severe early onset PE, and BP not controlled by methyldopa 2 g/day. Excluded: planned termination of pregnancy, onset of PE after 34 weeks, postpartum, already on either agent. | |
| Interventions | Prazosin: 1 mg x 3/day, to max 21 mg/day.
Nifedipine: 10 mg x3/day, to max 60 mg/day. If BP still not controlled, cross‐over. |
|
| Outcomes | Woman: death, eclampsia, HELLP, renal failure, pulmonary oedema, ICU admission, abruption, MgSO4 prophylaxis, caesarean section. Baby: stillbirth, hyaline membrane disease, septicaemia, SCBU admission. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “An epidemiologist who was not involved in the clinical management performed randomization using balanced blocks of 50 computer‐generated random numbers.” |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. “Women were allocated consecutive, numbered, opaque sealed envelopes indicating their medication.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “The clinicians were not blind to the allocated medication.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 150 women were entered into the trial: 5 randomised women were excluded from the analysis (2 were postpartum, the pregnancy of 1 woman was terminated before administration of medication, once woman was incorrectly identified, and 1 woman was randomised twice) – so minimal loss. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Unclear risk | No baseline characteristics provided. |
Switzerland 2012.
| Methods | Pilot prospective randomised study. Obstetrics Department, Geneva, Switzerland. | |
| Participants | 41 pregnant women with a gestational age > 24 weeks and admitted with severe hypertension (SBP ≥ 165 mmHg; DBP ≥ 105 mmHg). | |
| Interventions | Women were randomised into 4 groups: 20 mg IV labetalol (9 women); 5 mg IV hydralazine (9 women); 10 mg oral nifedipine tablets (11 women); 10 mg sl nifedipine (12 women). Treatment repeated every 20 min until target SBP/DBP reached (150/95 mmHg). |
|
| Outcomes | Time needed to achieve effective BP control; treatment failure – inability to reach the target BP within 1 hr; hypotension ‐ but SBP < 120. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details, available as abstract only. |
| Allocation concealment (selection bias) | Unclear risk | No details, available as abstract only. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned, different regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not mentioned, different regimens. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women seem to be accounted for. |
| Selective reporting (reporting bias) | Unclear risk | No details, available as abstract only. |
| Other bias | Unclear risk | No details, available as abstract only. |
Tunisia 2002.
| Methods | Computer‐generated randomisation. Allocation concealment in sealed sequentially numbered opaque envelopes. CFU ‐ A, blinding ‐ C. | |
| Participants | 60 women aged > 18 years with severe hypertension (SBP 170 mmHg or more, or DBP 110 mmHg or more x 2 30 min apart) after 24 weeks' gestation. All women had MgSO4 for seizure prophylaxis before trial entry. Excluded: contraindication to beta blockers or calcium channel blockers, or either study drug given in the last 4 hrs. | |
| Interventions | Nicardipine: 10 mg over 5 min, then if needed 12.5 mg at 5 min intervals. When 20% reduction in BP, infusion at 1‐3 mg/hr for 1 hr.
Labetalol: 1 mg/kg over 1 min, then 1.5 mg/kg after 5 min if BP not lowered. If BP not reduced by 20% in next 5 min, treatment failure. If BP does drop by 20%, infusion of 100‐150 mg over next hr. At end of study period ‐ treatment at discretion of clinicians for both groups. |
|
| Outcomes | Woman (assessed only after 1 hr): control of BP, hypotension, side‐effects. Baby: none. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was computer generated.” |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. “Allocation to one of the trial medications was kept in sealed sequentially numbered opaque envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was described as “single blinded” but no details given of what they meant by this, i.e. which group (participants/clinicians/outcome assessors) were blinded. The study drugs were administered following different infusion modalities – so difficult to blind participants and clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was described as “single blinded” but no details given of what they meant by this, i.e. which group (participants/clinicians/outcome assessors) were blinded. The study drugs were administered following different infusion modalities – so difficult to blind participants and clinicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 60 women randomised and all analysed for primary and secondary outcomes. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | There was no difference in the clinical characteristics of the 2 treatment groups (Table 1) – demographic data. |
Turkey 1996.
| Methods | Randomised, no further information. Drugs identically packaged and infusion rates identical. CFU ‐ A, blinding ‐ A. | |
| Participants | 33 women with severe PE. | |
| Interventions | Nimodipine: 100 mL crystalloid, then infusion of 30 mg/kg/hr. MgSO4: 6 g IV in 100 mL crystalloid, then infusion of 2 g/hr. | |
| Outcomes | Woman: eclampsia (during therapy only), caesarean section. Baby: none. | |
| Notes | Available as abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial reported as an abstract, so limited information. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Trial reported as an abstract, so limited information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as “a double blind, randomized controlled clinical trial” and also states that “All bolus solutions and drugs were packaged similarly and infusion rates were identical for both groups.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Trial reported as an abstract, so limited information. |
| Selective reporting (reporting bias) | Unclear risk | Trial reported as an abstract, so limited information. |
| Other bias | Unclear risk | Trial reported as an abstract, so limited information. |
USA 1987.
| Methods | Random numbers, 2:1 allocation. No information about concealment of allocation. CFU ‐ A, blinding ‐ C. | |
| Participants | 19 women with hypertension during pregnancy. Also, 41 women with postpartum hypertension, but these are excluded from this review. | |
| Interventions | Labetalol: either, 20 mg IV then 10‐50 mg every 10 min until DBP 100 mmHg or less, or 20 mg I then repeat doses of 20 mg, 40 mg, 80 mg, 80 mg every 10 min to a maximum of 300 mg or until DBP 100 mgHg or less. Hydralazine: 5 mg IV every 10 min until DBP 100 mmHg or less. | |
| Outcomes | Woman: caesarean section, no others reported separately from the postpartum women. Baby: Apgar scores, RDS, hypoglycaemia, hypothermia. | |
| Notes | Women with postpartum hypertension excluded from this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported, but regimens different. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported, but regimens different. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data appears to be complete for some of the outcomes, e.g. Figure 1 included all randomised patient data. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | “There were no differences in the clinical characteristics of the two treatment groups, as shown in Table 1.” |
BP: blood pressure CFU: completeness of follow‐up CTG: cardiotocography DBP: diastolic blood pressure DIC: disseminated intravascular coagulation FHR: fetal heart rate HELLP: haemolysis, elevated liver enzymes, lowered platelets HR: heart rate hr: hours ICU: intensive care unit IM: intramuscular IUGR: intrauterine growth restriction IV: intravenous MAP: mean arterial pressure MRC: Medical Research Council MgSO4: magnesium sulphate min: minutes PE: pre‐eclampsia PPH: postpartum haemorrhage PMH: past medical history RDS: respiratory distress syndrome SCBU: special care baby unit SD: standard deviation SBP: systolic blood pressure sl: sublingual
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adair 2009 | Comparison with placebo and patients already on antihypertensive drugs or received other antihypertensives as needed based on clinical decision. |
| Adair 2010 | Comparison with placebo and patients already on antihypertensive drugs or received other antihypertensives as needed based on clinical decision. |
| Anonymous 2006 | Ongoing study, but not women with severe pre‐eclampsia and comparison with placebo. |
| Argentina 1986 | No data on clinical outcomes. Available as abstract only. Study design: "randomly divided". Participants: 60 women. Interventions: comparison of atenolol with methyl dopa. |
| Aslam 2007 | Not an randomised controlled trial or quasi‐randomised controlled trial and compared the same drug – alpha methyldopa versus combination of alpha methyldopa with long‐acting nifedipine or amlodipine. |
| Australia 2002 | Comparison of different ways of giving nifedipine. Study design: 'randomised' double blind. Capsules marked 'A' and 'B'. Participants: 64 women over 20 weeks' gestation, with SBP 170 mmHg or above and/or DBP 110 mmHg or above. Interventions: rapid release capsules nifedipine versus slow release tablets. |
| Bangladesh 2002 | Dosage comparison. Probably not a randomised trial. Study design: 'divided' no further information. Participants: 77 women with eclampsia and severe hypertension. Interventions: 5 mg hydralazine IV followed by 2 mg at 15‐min intervals versus infusion of 20 mg hydralazine in 200 mL saline at 10 drops/min, increasing at 5 drops/min at 15‐min intervals. Outcomes: time to BP control, hypertensive crisis, total dose of hydralazine. |
| Belfort 2006 | Not women with severe hypertension. |
| Brazil 1984 | Not women with very high BP. Study design: 'randomly' divided into 2 halves. Participants: 100 women with severe chronic hypertension, with or without super imposed PE. Interventions: comparison of pindolol with no antihypertensive drug. |
| Brazil 1988 | No data on clinical outcomes. Study design: double‐blind comparison. Participants: 13 women. Intervention: single dose of oral nifedipine versus single bolus iv hydralazine. |
| Brazil 1988a | No data on clinical outcomes. Study design: random number tables. Participants: 16 women with DBP above 120 mmHg after 120 mins rest. Interventions: single dose hydralazine 5‐10 mg IV versus single dose oral nifedipine 5‐10 mg. |
| China 2000 | Intervention to reduce postpartum blood loss. Study design: 'randomly divided'. Participants: 64 women with pregnancy‐induced hypertension. Interventions: comparison of nifedipine with placebo during labour. Outcomes: postpartum blood loss. |
| Devi 2012 | Not a randomised controlled trial – consecutively allocated to groups (quasi‐RCT). |
| Egerman 2008 | All women admitted with severe PE for expectant management and randomised to relaxin or placebo – so not comparing different types of anti‐hypertensive drugs. |
| Egypt 1989 | Intervention was aimed at cervical ripening. Study design: 'allocated at random', no further information. Participants: 27 women at 34‐40 weeks' gestation with severe PE (BP > 160/110 mmHg with proteinuria) who were receiving prostaglandin A1 infusion. Interventions: 3‐arm comparison of different timings of prostaglandin E2 gel in the cervical canal. |
| Egypt 1988 | Not women with very high BP. Available as abstract only. Study design: randomly allocated, no further information. Participants: 50 primigravid women with PE and 20 multigravid women with chronic hypertension. Interventions: 3‐arm comparison of bromocriptine with methyl dopa with placebo. |
| Egypt 1992 | Intervention not an antihypertensive drug. Study design: 'randomly allocated', no further information. Participants: 30 women with severe PE. Interventions: comparison of prostaglandin A1 infusion with placebo. |
| Esmaoglu 2009 | Interventions being compared were sedatives – and women were postpartum. All eclamptic women – not severe hypertensive. |
| France 1986 | No data on clinical outcomes. Available as abstract only. Study design: 'randomised', no further information. Participants: 35 women with DBP > 105 mmHg after 20 weeks' gestation, and in hospital. Interventions: comparison of clonidine and labetalol. |
| Ghana 1995 | Quasi‐random study, allocation by alternate odd and even numbers. Participants: 104 women. Interventions: comparison of nifedipine with hydralazine. |
| Graves 2012 | Comparison of digoxin‐binding fab immunoglobulin with placebo. Secondary analysis of original study data. |
| Gris 2011 | Intervention being investigated was heparin, not antihypertensive. |
| Hladunewich 2006 | Intervention being investigated was L‐arginine, not antihypertensive and was being compared with placebo. Women did not have severe hypertension. |
| Hopate 2008 | Intervention being investigated was digoxin immune antibody fragments, not antihypertensive and was being compared with placebo. Women had severe PE, not severe hypertension. |
| India 1963 | Quasi‐random study, alternate allocation. Study included women without very high BP. Participants: women with 'mild to severe toxaemia'. Interventions: comparison of guanethidine with placebo. |
| India 2001 | Unlikely to be a randomised trial. Study design: 'cases grouped as A and B', no further information. Participants: 120 women with eclampsia. Interventions: comparison of nifedipine plus magnesium sulphate with sedation plus magnesium sulphate. Outcomes: maternal death, mode of delivery, stillbirth. |
| Iran 1994 | Available as abstract only. No clinical outcomes reported. Participants: 30 women. Interventions: comparison of nifedipine with hydralazine. |
| Israel 1991 | Not a randomised trial, women allocated to treatment group according to week of the month. Participants: 54 women. Interventions: comparison of nifedipine with hydralazine. |
| Israel 1999 | Not women with very high BP, and no clinically useful outcomes reported. Study design: randomised trial. Participants: women with DBP 90 mmHg. |
| Italy 2004 | Intervention not an antihypertensive drug. Study design: randomly allocated, using a computer‐generated randomisation list in blocks of 8. Participants: 23 women at 24‐33 weeks' gestation with PE. Interventions: comparison of single antithrombin infusion with antithrombin infusion plus 5 days maintenance. |
| Jamaica 1999 | Quasi‐random study. Study design: "selecting numbers blindly from an envelope by assigning odd numbers to hydralazine and even to isradipine". Participants: 39 women with severe PE. Interventions: comparison of isradipine with hydralazine. |
| Japan 1999 | Not a randomised trial ‐ 'patients divided according to doctors choice'. Participants: 20 women with severe PE. Interventions: comparison of long‐term epidural with bed rest plus diet plus antihypertensive drugs. Outcomes: caesarean section, days to delivery. |
| Japan 2000 | Intervention not an antihypertensive drug. Study design: telephone randomisation, using minimisation. Participants: 133 women with severe PE at 24‐35 weeks' gestation. Interventions: comparison of antithrombin with placebo. |
| Japan 2002 | Not a randomised trial. Study design: women grouped according to length of treatment with nicardipine. Participants: 50 women with severe PE. |
| Japan 2003 | Interventions were not antihypertensive drugs. Study design: telephone randomisation, with recruitment 1988‐1990. Participants: women with PE at 24‐36 weeks' gestation. Interventions: comparison of antithrombin concentrate plus heparin with heparin alone. Outcomes: caesarean section, blood loss > 500 mL, mean gestation at birth, baby death, bleeding disorder for the neonate. |
| Johnston 2006 | Intervention being investigated was digoxin immune antibody fragments and was being compared with placebo. Intervention in addition to other antihypertensives, not for control of acute severe hypertension. |
| Lam 2008 | Intervention being investigated was digoxin immune antibody fragments and was being compared with placebo. Women with severe PE not severe hypertension and intervention not being used for the treatment of acute severe hypertension. |
| Malaysia 1996 | Quasi‐random study. Study design: treatment allocation by odd and even numbers on identity cards. Participants: 200 women with DBP above 120 mmHg and over 28 weeks' gestation. Interventions: comparison of nifedipine and hydralazine. |
| Manzur‐Verastegui 2008 | All women with severe PE – unclear whether they all had severe hypertension. Nitroglycerine versus nifedipine. |
| Mexico 1967 | Not clearly a randomised trial ‐ 'test made in two groups with a comparable degree of toxaemia'. Abstract only available. Participants: women with toxaemia. Interventions: comparison of frusemide with chlorothiazide plus sedation plus potassium. Outcomes: mean glomerular filtration rate. |
| Mexico 2000 | Not a comparison of 1 antihypertensive drug with another. Study design: "assigned randomly". Participants: women with severe PE after 28 weeks with DBP 110 mmHg or more after 20 min rest. Interventions: comparison of isosorbide with placebo. Normal clinical care after 1 hour. |
| Mexico 2004 | Comparison of antihypertensive drugs with epidural. Study design: randomised, no further information. Participants: 24 women at > 29 weeks' gestation with PE, platelets above 70,000 and no other contraindication to an epidural. Interventions: comparison of usual care (plasma volume expansion, hydralazine, phenytoin, dexamethasone, dypiridamol) with epidural plus plasma volume expansion. Outcomes: haemodynamic measures. |
| Netherlands 2002 | Intervention was not an antihypertensive drug. Study design: randomised, double blind, no further information. Participants: 38 women with early onset severe PE. Interventions: comparison of N‐acetylcysteine with placebo. Outcomes: eclampsia. |
| New Zealand 1986 | Clinical data not reported for > 20% of participants. Abstract only available. Study design: 'randomised' no further information. Participants: 117 women with severe hypertension, with or without proteinuria. Interventions: comparison of atenolol with pindolol. |
| New Zealand 1992 | No clinical outcomes reported or available from authors. Participants: 24 women. Interventions: comparison of nifedipine with hydralazine. |
| Philipines 2000 | Not women with very high BP. Abstract only available. Study design: 'randomly assigned', no further information. Participants: 16 women with PE. Interventions: comparison of nitroglycerin patches with placebo. Outcomes: no clinical outcomes reported. |
| Pogue 2006 | Not comparing different types of antihypertensive drugs. Conventional treatment for preeclampsia – but not defined versus continuous haemodiafiltration. |
| Roes 2006 | Not antihypertensive drugs. Oral N‐acetylcysteine versus placebo. |
| Samangaya 2009 | Comparison with placebo, not another antihypertensive drug. Sildenafil citrate versus placebo. |
| Schackis 2004 | Comparison with placebo, not another antihypertensive drug; not severe hypertension. Probenecid 250 mg twice daily versus placebo twice daily. |
| Scotland 1983 | No clinical outcomes reported. Participants: 21 women. Interventions: comparison of labetalol with hydralazine. |
| Singapore 1971 | Quasi‐random study. Data for a case series of treatment with dihydrzinophthalazine included, not possible to separate. Study design: women allocated "in strict rotation". Participants: 285 women with BP 180/110 mmHg or above, or 160/100 mmHg and above with proteinuria. Interventions: comparison of protoveratrine with guanethidine with dihydrzinophthalazine. |
| Smith 2005 | Not women with severe hypertension, women with severe PE. |
| South Africa 1982 | Women with antepartum (6 women) and postpartum (6 women) hypertension not reported separately. Participants: 12 women with hypertension, either before delivery or immediately postpartum. Intervention: comparison of labetalol with hydralazine. |
| South Africa 1984 | Dose comparisons. Probably not a randomised trial. Study design: women 'divided' into 2 groups. Participants: 21 women > 29 weeks' gestation with DBP 110 mmHg or more after 2 hours rest. Interventions: comparison of 60 mg IV diazoxide every 10 min with 150 mg IV every 10 min. Outcomes: total dose of diazoxide, hypotension. |
| South Africa 1993 | 40 women randomised. Numerators and denominators only reported for a subset of 34 women for whom an analysis of arrhythmias is reported. Denominators are not given for the clinical outcomes, and unclear whether they refer to the full 40 women or the subset of 34. Authors contacted, no further data available. Study design: 'randomly allocated' no further information. Intervention: comparison of labetalol with hydralazine. |
| South Africa 2002 | Dose finding study. Some women did not meet eligibility criteria. Study design: randomised by consecutively numbered sealed envelopes. Computer‐generated random numbers in blocks of 20. Participants: 30 women with DBP 105 mmHg or more, x 2 10 min apart, or 100 mmHg or more for 30 min. Intervention: comparison of 10 mg ketanserin every 10 min with every 20 min. |
| Spain 1988 | Available as abstract only. No clinical data. Study design: described as "double blind controlled trial", no other information about concealment of allocation. Numbers allocated to each intervention not reported. Interventions: comparison of hydralazine plus methyl dopa with labetalol. |
| Steyn 2003 | Comparing alternative regimens of the same drug: nifedipine 6‐hourly verus nifedipine 8‐hourly. |
| Sweden 1993 | 2 studies, both quasi‐random and allocated according to year of birth and both comparing labetalol with hydralazine. (a) 97 women, but outcome only reported for 22 women; (b) 20 women, 3 of whom were also in study (a). |
| Unemori 2009 | Ongoing trial comparing 3 different doses of relaxin with placebo, not comparing different antihypertensives. |
| USA 1999 | Data not presented separately for women randomised before and after delivery. Participants: 50 women with severe PE, or with chronic hypertension and superimposed PE. Interventions: comparison of nifedipine with labetalol. |
| Venezuela 2001 | Women did not have very high BP. Available as abstract only. Study design: randomly assigned, no further information. Participants: 30 women with PE. Interventions: comparison of nitroglycerin patches with placebo. |
| Waheed 2005 | Comparison of alternative regimens of the same drug hydralazine. |
| Warren 2004 | LAMPET trial. Women do not all have severe hypertension. The primary aim of this study is to prevent seizures rather than control hypertension. |
BP: blood pressure DBP: diastolic blood pressure IV: intravenous min: minutes PE: pre‐eclampsia RCT: randomised controlled trial SBP: systolic blood pressure
Characteristics of studies awaiting assessment [ordered by study ID]
Mesquita 1995.
| Methods | Randomised double‐blind study. Comparing hypertensive emergencies during pregnancy. |
| Participants | 50 pregnant women with DBP ≧ 110 mm Hg. |
| Interventions | 5 mg hydralazine IV and placebo. Oral nifedipine and placebo. |
| Outcomes | BP levels and fetal vitality during cardiotocography; side‐effects. |
| Notes | Report in Portugese ‐ similar to trial 1994, not clear whether a duplicate report, though drug amounts different. Awaiting translation. |
BP: blood pressure DBP: diastolic blood pressure IV: intravenous
Characteristics of ongoing studies [ordered by study ID]
Diemunsch 2008.
| Trial name or title | Treatment of severe hypertension during pre‐eclampsia. A preliminary equivalence study between urapidil and nicardipine |
| Methods | Randomised, open‐label, parallel assignment, safety/efficacy study. |
| Participants | Women with severe hypertension during pre‐eclampsia; 18 to 51 years. |
| Interventions | Urapidil versus nicardipine. |
| Outcomes | Primary: systolic, diastolic, mean blood pressure. Secondary: maternal and fetal ultrasonography; biological and clinical assessment; type of delivery; postpartum bleeding; neonatal evaluation by neonatologist during the first 24 hours of life. |
| Starting date | December 2006. Estimated enrolment: 72. |
| Contact information | Pierre Auguste Diemunsch, Service d'Anesthesie et de Reanimation Medicale, Hopital de Hautepierre, Hopitaux Universitaires, Strasbourg, France. Pierre.Diemunsch@chru‐str |
| Notes |
Differences between protocol and review
This review was updated in January 2011, and the methods revised according to the generic protocol (Duley 2009). The methods were revised according to Cochrane Pregnancy and Childbith Group current standards for the 2013 update. Also in the 2013 update, 'need for magnesium sulphate' was added as an outcome.
Contributions of authors
Methods for the review were developed by Lelia Duley and David Henderson‐Smart. Lelia Duley wrote the initial text of the review, with discussion and comments from David Henderson‐Smart. Data for the initial review and first update were extracted by Lelia Duley and David Henderson‐Smart and then entered by Lelia Duley.
For the 2005 update, the search strategy was updated by Shireen Meher. Lelia Duley and Shireen Meher selected studies for inclusion and exclusion. All three authors extracted and checked data, which were entered by Lelia Duley. Lelia Duley revised the text of the review, in consultation with David Henderson‐Smart and Shireen Meher.
For the 2013 update, Leanne Jones, Shireen Meher and Therese Dowswell selected studies for inclusion and exclusion. Leanne Jones and Therese Dowswell extracted and checked data, which was entered by Leanne Jones. Leanne Jones revised the text of the review, in consultation with Lelia Duley and Shireen Meher. Shireen Meher and Lelia Duley revised the text of the review.
Sources of support
Internal sources
Medical Research Council, UK.
Resource Centre for Randomised Trials, Oxford, UK.
External sources
-
National Institute for Health Research, UK.
NIHR Programme of centrally‐managed pregnancy and childbirth systematic reviews of priority to the NHS and users of the NHS:10/4001/02
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Argentina 1990 {published data only}
- Voto LS, Quiroga CA, Lapidus AM, Catuzzi P, Uranga IF, Margulies M. Effectiveness of antihypertensive drugs in the treatment of hypertension in pregnancy. Clinical and Experimental Hypertension ‐ Part B Hypertension in Pregnancy 1990;9(3):339‐48. [Google Scholar]
Australia 1986 {published data only}
- Michael CA. Intravenous labetalol and intravenous diazoxide in severe hypertension complicating pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 1986;26:26‐9. [DOI] [PubMed] [Google Scholar]
Australia 2007 {published data only}
- Hennessy A, Thornton C, Makris A, Ogle R, Henderson‐Smart D, Gillin A, et al. Parenteral intravenous optimal therapy trial ‐ A RCT of hydralazine versus mini‐bolus diazoxide for hypertensive crises in the obstetric setting [abstract]. Hypertension in Pregnancy 2006;25(Suppl 1):22. [Google Scholar]
- Hennessy A, Thornton CE, Makris A, Ogle RF, Henderson‐Smart DJ, Gillin AG, et al. A randomised comparison of hydralazine and mini‐bolus diazoxide for hypertensive emergencies in pregnancy: the pivot trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(4):279‐85. [DOI] [PubMed] [Google Scholar]
- Hennessy AM. Diazoxide versus hydralazine for acute treatment of very high blood pressure in pregnancy. Personal communication.
Brazil 1992 {published data only}
- Martins‐Costa S, Ramos JG, Barros E, Bruno RM, Costa CA. Randomized, controlled trial of hydralazine versus nifedpine in preeclamptic women with acute hypertension. Clinical and Experimental Hypertension 1992;B11(1):25‐44. [Google Scholar]
Brazil 1994 {published data only}
- Mesquita MR, Attalah AN, Camano L, Bertini AM. The use of hydralazine and nifedipine as treatment of hypertension emergency during pregnancy. Proceedings of 2nd World Congress of Perinatal Medicine; 1993 September 19‐24; Rome, Italy. 1993:41.
- Souza MR, Nagib A, Bertini AM. Use of hydralazine and nifedipine in hypertensive emergency in pregnancy [Empleo de la hidralazina y de la nifedipina en las emergencias hipertensivas en la gestacion]. Progresos de Obstetricia y Ginecologia 1994;37:90‐6. [Google Scholar]
- Souza Mesquita MR, Atallah AN, Bertini AM. The use of hydralazine and nifedipine as treatment for hypertension emergency during pregnancy. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:163.
Brazil 2011 {published data only}
- Baggio MR, Martins WP, Calderon AC, Berezowski AT, Marcolin AC, Duarte G, et al. Changes in fetal and maternal Doppler parameters observed during acute severe hypertension treatment with hydralazine or labetalol: a randomized controlled trial. Ultrasound in Medicine & Biology 2011;37(1):53‐8. [DOI] [PubMed] [Google Scholar]
England 1982 {published data only}
- Moore MP, Redman CWG. The treatment of hypertension in pregnancy. Current Medical Research and Opinion 1982;8:S39‐S46. [Google Scholar]
France 2010 {published data only}
- Vizitiu R, Krauss‐Grignard M, Garcia V, Valentin L, Samain E, Diemunsch P. Urapidyl for hypertension control in severe pre‐eclampsia: a comparative study with nicardipine. Critical Care 2010;14 Suppl 1:S48. [Google Scholar]
Germany 1998 {published data only}
- Schulz M, Wacker J, Bastert G. Effect of urapidil in antihypertensive therapy of preeclampsia on newborns. Zentralblatt fur Gynakologie 2001;123(9):529‐33. [DOI] [PubMed] [Google Scholar]
- Wacker J, Christ M, Grischke EM, Bastert G. Treatment of patients with pre‐eclampsia with urapidil. International Journal of Gynecology & Obstetrics 1994;46:121. [Google Scholar]
- Wacker J, Christ M, Muller J, Grischke EM, Bastert G. Treatment of patients with pre‐eclampsia with urapidil. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:186.
- Wacker J, Piel P, Bastert G. The treatment of pre‐eclampsia with urapidil. Proceedings of 10th World Congress, International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, USA. 1996:185.
- Wacker J, Schulz M, Werner P, Bastert G. Fetal outcome after treatment of hypertension in patients with preeclampsia with urapidil [abstract]. 12th World Congress of the International Society for the Study of Hypertension in Pregnancy; 2000 July 9‐15; Paris, France. 2000:1.
- Wacker J, Werner P, Walter‐Sack I, Bastert G. Treatment of hypertension in patients with pre‐eclampsia: a prospective parallel‐group study comparing dihydralazine with urapidil. Nephrology, Dialysis, Transplantation 1998;13:318‐25. [DOI] [PubMed] [Google Scholar]
Germany 2006 {published data only}
- Wacker JR, Wagner BK, Briese V, Schauf B, Heilmann L, Bartz C, et al. Antihypertensive therapy in patients with pre‐eclampsia: a prospective randomised multicentre study comparing dihydralazine with urapidil. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;127(2):160‐5. [DOI] [PubMed] [Google Scholar]
India 2006 {published data only}
- Aswathkumar R, Gilvaz S. Management of severe hypertension in pregnancy: prospective comparison of labetalol vs nifedipine [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 Jan 6‐9; Cochin, Kerala State, India. 2006:38.
India 2011 {published data only}
- Desai BB, Swamy MK, Patil KP. A randomized controlled trial of oral nifedipine vs intravenous labetalol in acute control of blood pressure in hypertensive emergencies of pregnancy. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:179.
Iran 2002 {published data only}
- Aali B, Nejad SS. Nifedipine or hydralazine as a first‐line agent to control hypertension in severe pre‐eclampsia. Acta Obstetricia et Gynecologica Scandinavica 2002;81:25‐30. [DOI] [PubMed] [Google Scholar]
Iran 2011 {published data only}
- Rezaei Z, Sharbaf FR, Pourmojieb M, Youefzadeh‐Fard Y, Motevalian M, Khazaeipour Z, et al. Comparison of the efficacy of nifedipine and hydralazine in hypertensive crisis in pregnancy. Acta Medica Iranica 2011;49(11):701‐6. [PubMed] [Google Scholar]
Malaysia 2012 {published data only}
- Raheem IA, Saaid R, Omar SZ, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2012;119(1):78‐85. [DOI] [PubMed] [Google Scholar]
Mexico 1989 {published data only}
- Rodriguez RJW, Amaya LAH. Severe preeclamsia. Nifedipine versus chlorpromazine in the management of the acute hypertensive state [Pre‐eclampsia severa. Nifedipina versus Clorpromazina en el manejo del estado hipertensivo agudo]. Revista Medica Instituto Mexicano del Seguro Social 1989;27:359‐63. [Google Scholar]
Mexico 1993 {published data only}
- Walss Rodriguez RJ, Flores Padilla LM. Management of severe pre‐eclampsia/eclampsia. Comparison between nifedipine and hydralazine as antihypertensive drugs. Ginecologia y Obstetricia de Mexico 1993;61:76‐9. [PubMed] [Google Scholar]
Mexico 1998 {published data only}
- Vargas AG, Salmeron PI, Sanchez GAR, Limenez AAL, Rubio GAF. Efficacy of isosorbide in aerosol form in the management of hypertensive crisis in severe preeclampsia. Ginecologia y Obstetricia de Mexico 1998;66:316‐9. [PubMed] [Google Scholar]
Netherlands 1999 {published data only}
- Bolte AC, Dekker GA, Eyck J, Bruinse HW, Kanhai HH, Vries A. Comparison of the effectivity and safety of ketanserin versus dihydralazine in the treatment of severe early onset pre‐eclampsia. American Journal of Obstetrics and Gynecology 1995;172(1 Pt 2):384. [Google Scholar]
- Bolte AC, Dekker GA, Eyck J, Bruinse HW, Kanhai HH, Vries A. Ketanserin versus dihydralazine in the treatment of early onset pre‐eclampsia. Proceedings of 10th International Congress, International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, USA. 1996:148.
- Bolte AC, Dekker GA, Eyck J, Bruinse HW, Kanhai HH, Vries A, et al. Comparison of the effectivity and safety of ketanserin versus dihydralazine in the treatment of severe early onset preeclampsia. Proceedings of the International Society for the Study of Hypertension in Pregnancy, European Branch; 1995 July 20‐22; Leuven Belgium. 1995:18.
- Bolte AC, Dekker GA, Eyck J, Strack van Schijndel RJM, Vries A, Geijn HP. Comparison of the effectivity and safety of ketanserin vs dihydralazine in the treatment of severe pre‐eclampsia. Proceedings of 9th International Congress, International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:42.
- Bolte AC, Eyck J, Kanhai H, Bruinse HW, Geijn HP, Dekker GA. Ketanserin versus dihydralazine in the management of severe early onset preeclampsia: maternal outcome. American Journal of Obstetrics and Gynecology 1999;180:371‐7. [DOI] [PubMed] [Google Scholar]
- Bolte AC, Eyck J, Strack van Schijndel RJ, Geijn HP, Dekker GA. The haemodynamic effects of ketanserin versus dihydralazine in severe early onset hypertension in pregnancy. British Journal of Obstetrics and Gynaecology 1998;105:723‐31. [DOI] [PubMed] [Google Scholar]
Netherlands 2003 {published data only}
- Bolte A, Geijn H, Dekker G. Use of ketanserin in hypertensive disorders of pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S171. [Google Scholar]
- Bolte AC, Geijin HP, Bekedam DJ, Dekker GA. Ketanserin, a serotonin2 receptor blocker, for hypertension in pregnancy. Hypertension in Pregnancy 2002;21(Suppl 1):9. [Google Scholar]
- Bolte BC, Geijn HPV, Bekedam DJ, Dekker GA. Ketanserin for hypertension in pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43:179. [Google Scholar]
Nimodipine SG 2003 {published data only}
- Belfort M, Anthony J, Saade G, Nimodipine Study Group. Interim report of the nimodipine vs. magnesium sulfate for seizure prophylaxis in severe preeclampsia study: an international, randomized controlled trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S7. [Google Scholar]
- Belfort M, Saade G, Yared M, Abedejos P, Dorman K. Change in estimated cerebral perfusion pressure following nimodipine or magnesium sulfate in patients with severe preeclampsia. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S114. [DOI] [PubMed] [Google Scholar]
- Belfort MA, Anthony J, Saade GR, Allen JC, Nimodipine Study Group. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. New England Journal of Medicine 2003;348:304‐11. [DOI] [PubMed] [Google Scholar]
- Belfort MA, Saade GR, Yared M, Grunewald C, Herd JA, Varner MA, et al. Change in estimated cerebral perfusion pressure after treatment with nimodipine or magnesium sulfate in patients with preeclampsia. American Journal of Obstetrics and Gynecology 1999;181:402‐7. [DOI] [PubMed] [Google Scholar]
- Hollenberg NK. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. Current Hypertension Reports 2003;5(4):288‐9. [PubMed] [Google Scholar]
N Ireland 1991 {published data only}
- Harper A, Murnaghan GA. Maternal and fetal haemodynamics in hypertensive pregnancies during maternal treatment with intravenous hydralazine or labetalol. British Journal of Obstetrics and Gynaecology 1991;98:453‐9. [DOI] [PubMed] [Google Scholar]
Panama 2006 {published data only}
- Vigil‐De Gracia P, Lasso M, Ruiz E, Vega‐Malek JC, Mena FT, Lopez JC, et al. Severe hypertension in pregnancy: hydralazine or labetalol. A randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;128:157‐62. [DOI] [PubMed] [Google Scholar]
South Africa 1987 {published data only}
- Ashe RG, Moodley J, Richards AM, Philpott RH. Comparison of labetalol and dihydralazine in hypertensive emergencies of pregnancy. South African Medical Journal 1987;71:354‐6. [PubMed] [Google Scholar]
South Africa 1989 {published data only}
- Moodley J. The use of nifedipine in acute hypertensive emergencies of pregnancy. Proceedings of 6th International Congress, International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Canada. 1988:141.
- Seabe SJ, Moodley J, Becker P. Nifedipine in acute hypertensive emergencies in pregnancy. South African Medical Journal 1989;76:248‐50. [PubMed] [Google Scholar]
South Africa 1992 {published data only}
- Moodley J, Gouws E. A comparative study of the use of epoprostenol and dihydralazine in severe hypertension in pregnancy. British Journal of Obstetrics and Gynaecology 1992;99:727‐30. [DOI] [PubMed] [Google Scholar]
South Africa 1995 {published data only}
- Rossouw HJ, Howarth G, Odendaal HJ. Ketanserin and hydralazine in hypertension in pregnancy ‐ a randomised double‐blind trial. South African Medical Journal 1995;85:525‐8. [PubMed] [Google Scholar]
South Africa 1997 {published data only}
- Howarth GR, Seris A, Venter C, Pattinson RC. A randomized controlled pilot study comparing urapidil to dihydralazine in the management of severe hypertension in pregnancy. Hypertension in Pregnancy 1997;16:213‐21. [Google Scholar]
- Pattinson RC, Seris A, Venter CP, Howarth G. Urapidil versus dihydralazine for control of severe hypertension in pregnancy: a pilot study. Proceedings of the 12th Conference on Priorities in Perinatal Care; 1993; South Africa. 1993:140‐3.
South Africa 1997a {published data only}
- Maharaj B, Khedun SM, Moodley J, Byl K, Rapiti N. A comparative study of intravenous isradipine and dihydralazine in the treatment of severe hypertension of pregnancy in black patients. Hypertension in Pregnancy 1997;16:1‐9. [Google Scholar]
- Maharaj B, Moodley J, Khedun SM, Rapiti N, Byl K. Intravenous isradipine in the management of severe hypertension in pregnancy. Proceedings of 10th International Congress, International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, USA. 1996:131.
- Maharaj B, Moodley J, Khedun SM, Rapiti N, Byl K. Intravenous isradipine in the management of severe hypertension in pregnancy. Proceedings of 9th International Congress, International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:158.
South Africa 1997b {published data only}
- Steyn DW, Odendaal HJ. Dihydralazine or ketanserin for severe hypertension in pregnancy?. Proceedings of 9th International Congress, International Society for the Study of Hypertension in Pregnancy; 1994 March 15‐18; Sydney, Australia. 1994:152.
- Steyn DW, Odendaal HJ. Dihydralazine or ketanserin for severe hypertension in pregnancy? Preliminary results. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;75:155‐9. [DOI] [PubMed] [Google Scholar]
South Africa 2000 {published data only}
- Hall D, Odendaal H, Steyn D, Smith M, Carstens E. Prazosin or nifedipine as a second agent to control early severe hypertension in pregnancy ‐ a randomized controlled trial. 29th Congress of the South African Society of Obstetricians and Gynaecologists; 1998 March 8‐12; South Africa. 1998.
- Hall DR, Odendaal HJ, Steyn DW, Smith M. Nifedipine or prazosin as a second agent to control early severe hypertension in pregnancy: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2000;107:759‐65. [DOI] [PubMed] [Google Scholar]
- Hall DR, Odendaal HJ, Steyn DW, Smith M. Nifedipine or prazosin as a second agent to control early severe hypertension in pregnancy: a randomised controlled trial. Hypertension in Pregnancy 2000;19(Suppl 1):12. [DOI] [PubMed] [Google Scholar]
- Hall DR, Odendaal HJ, Steyn DW, Smith M. Nifedipine or prazosin as a second agent to control early severe hypertension in pregnancy: a randomised controlled trial. Women's Health ‐ into the new millenium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. RCOG, 1999:49.
Switzerland 2012 {published data only}
- Saudan P, Billieux MH, Pechere A, Irion O, Savoldelli G, Boulvain M. Which first‐line drug to control severe hypertension in pregnancy? A pilot study. Pregnancy Hypertension 2012;2(3):182. [DOI] [PubMed] [Google Scholar]
Tunisia 2002 {published data only}
- Elatrous S, Nouira S, Ouanes Besbes L, Marghli S, Boussarssar M, Sakkouhi M, et al. Short‐term treatment of severe hypertension of pregnancy: prospective comparison of nicardipine and labetalol. Intensive Care Medicine 2002;28(9):1281‐6. [DOI] [PubMed] [Google Scholar]
Turkey 1996 {published data only}
- Belfort M, Taskin O, Buhur A, Saade G, Yalcinoglu A. Intravenous nimodipine in the management of severe preeclampsia: a double blind randomised controlled clinical trial. Proceedings of 10th International Congress, International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Seattle, USA. 1996:124.
USA 1987 {published data only}
- Mabie WC, Gonzalez AR, Amon E, Sibai BM. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Proceedings of 6th Annual Meeting of the Society of Perinatal Obstetricians; 1986; San Antonio, USA. 1986:221. [PubMed]
- Mabie WC, Gonzalez AR, Sibai BM, Amon E. A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstetrics & Gynecology 1987;70:328‐33. [PubMed] [Google Scholar]
- Mabie WC, Gonzalez‐Ruiz A, Amon E, Sibai BM. A comparative trial of labetolol and hydralazine for acute management of severe hypertension complicating pregnancy. Proceedings of 5th International Congress, International Society for the Study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, UK. 1987:91.
References to studies excluded from this review
Adair 2009 {published data only}
- Adair CD, Luper A, Rose JC, Russell G, Veille JC, Buckalew VM. The hemodynamic effects of intravenous digoxin‐binding fab immunoglobulin in severe preeclampsia: a double‐blind, randomized, clinical trial. Journal of Perinatology 2009;29(4):284‐9. [DOI] [PubMed] [Google Scholar]
Adair 2010 {published data only}
- Adair CD, Buckalew V, Graves SW, Chauhan N, Lam G, DEEP studygroup. Digoxin Immune Fab treatment for severe preeclampsia; relationship between response and baseline endogenous digitalis‐like factor. Pregnancy Hypertension 2010;1 Suppl 1:S21. [Google Scholar]
- Adair CD, Buckalew VM, Graves SW, Lam GK, Johnson DD, Saade G, et al. Digoxin immune fab treatment for severe preeclampsia. American Journal of Perinatology 2010;27(8):655‐62. [DOI] [PubMed] [Google Scholar]
Anonymous 2006 {published data only}
- Anonymous. Sildenafil citrate for the treatment of established pre‐eclampsia (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
Argentina 1986 {published data only}
- Voto L, Lapidus A, Neira J, Margulies M. Atenolol versus alpha methyl dopa in the treatment of hypertension in pregnancy. Proceedings of the 5th International Congress, International Society for the Study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, UK, 1986. 1986:138.
Aslam 2007 {published data only}
- Aslam A, Talat W. Pregnancy induced hypertension; anti‐hypertensive therapy in a study using single drug versus multiple drugs. Professional Medical Journal 2007;14(1):30‐2. [Google Scholar]
Australia 2002 {published data only}
- Brown MA, Buddle ML, Farrell T, Davis GK. Efficacy and safety of nifedipine tablets for the acute treatment of severe hypertension in pregnancy. American Journal of Obstetrics and Gynecology 2002;187:1046‐50. [DOI] [PubMed] [Google Scholar]
- Buddle ML, Brown MA, Farrell T. Rapid treatment of severe hypertension in pregnancy. 37th Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology; 2001 September 5‐7; Darwin, Australia. 2001:118.
Bangladesh 2002 {published data only}
- Begum MR, Quadir E, Begum A, Akhter S, Rahman K. Management of hypertensive emergencies of pregnancy by hydralazine bolus injection vs continuous drip‐‐a comparative study. Medscape Womens Health eJournal 2002;7(5):1‐6. [PubMed] [Google Scholar]
Belfort 2006 {published data only}
- Belfort MA. Labetalol versus MgSO4 for the prevention of eclampsia trial (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
Brazil 1984 {published data only}
- Kahhale S, Carrara W, Barros ACSD, Zugaib M, Neme B. A comparative study between treated (beta‐blocker pindolol) and untreated chronic hypertension. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1984:56.
- Kahhale S, Zugaib M, Carrara W, Jota de Paula F, Sabbaga E, Neme B. Comparative study of chronic hypertensive pregnant women treated and non‐treated with pindolol. Ginecologia e Obstetricia Brasileiras 1985;8(2):85‐9. [Google Scholar]
Brazil 1988 {published data only}
- Bruno RM, Germany L, Behle I, Barros E. Nifedipine versus hydralazine: randomized, placebo‐controlled and double blind trial in severe hypertension complicating pregnancy [Nifedipina versus hidralazina: estudo randomizado e duplo cego no tratemento agudo da hipertensao arterial severa na gravidez]. Revista do Hospital de Clinicas de Porto Alegre 1988;8:75‐8. [Google Scholar]
Brazil 1988a {published data only}
- Atallah A, Delascio D, Santos J, Mesquita G, Kenj G. Double blinded randomized controlled study using hydralazine or nifedipine for hypertensives crisis in pregnancy. World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:181.
- Atallah AN, Souza Mesquita MR, dos Santos JFK, Bertini AM, Gebara M, Camano L, et al. A randomized controlled study of hydralazine and nifedipine in hypertensive crisis during pregnancy. Revista Brasileira de Ginecologia y Obstetricia 1990;12:10‐4. [Google Scholar]
China 2000 {published data only}
- Yang X, Liu Y. The effect of nifedipine on postpartum blood loss in patients with pregnancy induced hypertension [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2000;35(3):151‐2. [PubMed] [Google Scholar]
Devi 2012 {published data only}
- Devi R, Anjali T. Intravenous labetalol versus oral nifedipine in the treatment of severe hypertension in pregnancy. Kuwait Medical Journal 2012;44(4):287‐90. [Google Scholar]
Egerman 2008 {published data only}
- Egerman R. Evaluation of the safety of relaxin in severe preeclampsia. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Egypt 1988 {published data only}
- Salem H, Ghanemah S, Seleem S, Sayed E, Abdel‐Latif A, Chard T. Bromocriptine therapy in pre‐eclamptic toxaemia of pregnancy (PET). World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil, 1988. 1988:184.
Egypt 1989 {published data only}
- Toppozada M, Barakat T, Shaala S, Ismail AAA. Management of severe pre‐eclampsia with prostaglandin A1: a useful therapeutic approach. Journal of Obstetrics and Gynaecology 1989;9:184‐8. [Google Scholar]
Egypt 1992 {published data only}
- Toppozada M, Medhat I, Sallam H, Ismail AAA, El‐Badawy ES, Rabbo SA. Improving placental blood flow in pre‐eclampsia with prostaglandin A1. Acta Obstetricia et Gynecologica Scandinavica 1992;71:22‐7. [DOI] [PubMed] [Google Scholar]
Esmaoglu 2009 {published data only}
- Esmaoglu A, Ulgey A, Akin A, Boyaci A. Comparison between dexmedetomidine and midazolam for sedation of eclampsia patients in the intensive care unit. Journal of Critical Care 2009;24(4):551‐5. [DOI] [PubMed] [Google Scholar]
France 1986 {published data only}
- Fievet P, Esper N, Gueroult J, Gueroult J, Fournier A. Comparative study of clonidine and labetalol in severe hypertension induced by pregnancy. 5th International Congress for the International Society for the study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, England. 1986:136.
Ghana 1995 {published data only}
- Kwawukume EY, Ghosh TS. Oral nifedipine therapy in the management of severe preeclampsia. International Journal of Gynecology & Obstetrics 1995;49:265‐9. [DOI] [PubMed] [Google Scholar]
Graves 2012 {published data only}
- Graves SW, Hopoate‐Sitake M, Johnston A, Buckalew V, Lam G, Mason L, et al. Deep trial secondary analysis: Digoxin immune fab fragment treatment has additional benefits in endogenous digitalis‐like factor positive preeclamptic women. Pregnancy Hypertension 2012;2(3):287‐8. [DOI] [PubMed] [Google Scholar]
Gris 2011 {published data only}
- Gris JC, Chauleur C, Molinari N, Mares P, Fabbro‐Peray P, Quere I, et al. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre‐eclampsia: The pilot randomised controlled NOH‐PE trial. Thrombosis and Haemostasis 2011;106(6):1053‐61. [DOI] [PubMed] [Google Scholar]
Hladunewich 2006 {published data only}
- Hladunewich MA, Derby GC, Lafayette RA, Blouch KL, Druzin ML, Myers BD. Effect of L‐Arginine therapy on the glomerular injury of preeclampsia: a randomized controlled trial. Obstetrics & Gynecology 2006;107(4):886‐95. [DOI] [PubMed] [Google Scholar]
Hopate 2008 {published data only}
- Hopate M, Graves S, Adair CD, Lam G, Johnson D, Saade G, et al. In‐vivo reversal of functional sodium pump inhibition with Digibind treatment. Hypertension in Pregnancy 2008;27(4):460. [Google Scholar]
India 1963 {published data only}
- Daftary SN, Desa Souza JM, Kumar A, Mandrekar SS, Lotlikar KD, Sheth UK. A controlled clinical trial of guanethidine in toxemia of pregnancy. Indian Journal of Medical Sciences 1963;17:812‐8. [PubMed] [Google Scholar]
India 2001 {published data only}
- Samal S, Gupta U, Agarwal P. Management of eclampsia with magnesium sulphate and nifedipine. Journal of Obstetrics and Gynecology of India 2001;51(3):71‐4. [Google Scholar]
Iran 1994 {published data only}
- Ghahiri A, Salehpour S. The effect of nifedipin on the BP of the patients with severe preeclampsia. International Journal of Gynecology & Obstetrics 1994;46:121. [Google Scholar]
Israel 1991 {published data only}
- Fenakel K, Fenakel G, Appelman Z, Lurie S, Katz Z, Shoham Z. Nifedipine in the treatment of severe preeclampsia. Obstetrics & Gynecology 1991;77:331‐7. [PubMed] [Google Scholar]
Israel 1999 {published data only}
- Thaler I, Amit A, Kamil D, Itskovitz‐Eldor J. The effect of isosorbide dinitate on placental blood flow and maternal blood pressure in women with pregnancy induced hypertension. American Journal of Hypertension 1999;12:341‐7. [DOI] [PubMed] [Google Scholar]
Italy 2004 {published data only}
- Paternoster DM, Fantinato S, Manganelli F, Milani M, Nicolini U, Girolami A. Efficacy of AT in pre‐eclampsia: a case control prospective trial. Thrombosis and Haemostasis 2004;91(2):283‐9. [DOI] [PubMed] [Google Scholar]
Jamaica 1999 {published data only}
- Fletcher H, Roberts G, Mullings A, Forrester T. An open trial comparing isradipine with hydralazine and methyl dopa in the treatment of patients with severe pre‐eclampsia. Journal of Obstetrics and Gynaecology 1999;19:235‐8. [DOI] [PubMed] [Google Scholar]
- Fletcher H, Roberts G, Mullings A, Simeon DT, Forrester TE. An open trial comparing usual care (hydralazine) with injectable isradipine in severe pre‐eclampsia [abstract]. West Indian Medical Journal 1996;45(2 Suppl):27. [Google Scholar]
Japan 1999 {published data only}
- Kanayama N, Belayet HM, Khatun S, Tokunaga N, Sugimura M, Kobayashi T, et al. A new treatment of severe pre‐eclampsia by long term epidural anaesthesia. Journal of Human Hypertension 1999;13:167‐71. [DOI] [PubMed] [Google Scholar]
Japan 2000 {published data only}
- Maki M, Kobayashi T, Terao T, Ikenoue T, Satoh K, Nakabayashi M, et al. Antithrombin therapy for severe preeclampsia: results of a double‐blind, randomized, placebo‐controlled trial. Bi51.017 Study group. Thrombosis and Haemostasis 2000;84(4):583‐90. [PubMed] [Google Scholar]
Japan 2002 {published data only}
- Seki H, Takeda S, Kinoshita K. Long‐term treatment with nicardipine for severe pre‐eclampsia. International Journal of Gynecology & Obstetrics 2002;76:135‐41. [DOI] [PubMed] [Google Scholar]
Japan 2003 {published data only}
- Kobayashi T, Terao T, Ikenoue T, Sameshima H, Nakabayashi M, Kajiwara Y, et al. Treatment of severe preeclampsia with antithrombin concentrate: results of a prospective feasibility study. Seminars in Thrombosis and Hemostasis 2003;29(6):645‐52. [DOI] [PubMed] [Google Scholar]
Johnston 2006 {published data only}
- Johnston A. Efficacy study of digibind for treatment of severe preeclampsia (ongoing trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006).
Lam 2008 {published data only}
- Lam G, Johnson D, Robinson C, Saade G, Lewis D, Porter K, et al. Antepartum administration of a digoxin immune fab (Digibind) improves renal function in patients with severe preeclampsia. Hypertension in Pregnancy 2008;27(4):422. [Google Scholar]
Malaysia 1996 {published data only}
- Jegasothy R, Paranthaman S. Sublingual nifedipine compared with intravenous hydrallazine in the acute treatment of severe hypertension in pregnancy: potential for use in rural practice. Journal of Obstetrics and Gynaecology Research 1996;22:21‐4. [DOI] [PubMed] [Google Scholar]
Manzur‐Verastegui 2008 {published data only}
- Manzur‐Verastegui S, Mandeville PB, Gordillo‐Moscoso A, Hernandez‐Sierra JF, Rodriguez‐Martinez M. Efficacy of nitroglycerine infusion versus sublingual nifedipine in severe pre‐eclampsia: a randomized, triple‐blind, controlled trial. Clinical and Experimental Pharmacology and Physiology 2008;35(5‐6):580‐5. [DOI] [PubMed] [Google Scholar]
Mexico 1967 {published data only}
- Sandoval JB, Perez FR. Study of glomerular filtration in toxemia of pregnancy. Modifications with the use of furosemid (lasix) [abstract]. 5th World Congress of Gynecology and Obstetrics; 1967; Sydney, Australia. 1967:891.
Mexico 2000 {published data only}
- Martinez‐Abundis E, Gonzalez‐Ortiz M, Hernandez‐Salazar F, Huerta‐J‐Lucas MT. Sublingual isosorbide dinitrate in the acute control of hypertension in patients with severe preeclampsia. Gynecologic and Obstetric Investigation 2000;50:39‐42. [DOI] [PubMed] [Google Scholar]
Mexico 2004 {published data only}
- Pardo‐Morales RV, Romero‐Figueroa S, Vazquez‐de Anda GF, Briones‐Garduno JC, Herrera‐Villalobos JE, Gonzalez‐Vargas A. New therapeutics alternative in severe preeclampsia. Cirugia y Cirujanos 2004;72(3):203‐7. [PubMed] [Google Scholar]
Netherlands 2002 {published data only}
- Roes EM, Raijmakers MTM, Zusterzeel PLM, Boo T, Merkus JMWM, Peters WHM, et al. Oral n‐acetylcysteine supplementation does not prolong pregnancy in women with severe preeclampsia: a randomised, placebo‐controlled trial [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):47. [Google Scholar]
New Zealand 1986 {published data only}
- Lubbe W. Maternal and fetal responses to b‐blockers with and without ISA in hypertensive pregnancy. 5th International Congress for the International Society for the study of Hypertension in Pregnancy; 1986 July 7‐10; Nottingham, England. 1986:89.
New Zealand 1992 {published and unpublished data}
- Duggan PM, McCowan LME, Stewart AW. Antihypertensive drug effects on placental flow velocity waveforms in pregnant women with severe hypertension. Australian and New Zealand Journal of Obstetrics and Gynaecology 1992;32:335‐8. [DOI] [PubMed] [Google Scholar]
Philipines 2000 {published data only}
- Decano MB, Cabrera LT. The effects of transdermal nitroglycerin (nitrol patch) on the uterine and umbilical artery blood flow in preeclampsia: a randomized double blind placebo controlled study [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 1). 2000:26.
Pogue 2006 {published data only}
- Pogue V, Ticas R, Sandoval X. Removal of agonistic autoantibodies against the angiotensin AT receptor in patients with preeclampsia [abstract]. Journal of the American Society of Nephrology 2006;17:658A. [Google Scholar]
Roes 2006 {published data only}
- Roes EM, Raijmakers MT, Boo TM, Zusterzeel PL, Merkus HM, Peters WH, et al. Oral n‐acetylcysteine administration does not stabilise the process of established severe preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;127(1):61‐7. [DOI] [PubMed] [Google Scholar]
Samangaya 2009 {published data only}
- Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double‐blinded, placebo‐controlled trial of the phosphodiesterase type 5 inhibitor sildenafil in the treatment of preeclampsia. Hypertension in Pregnancy 2009;28:369‐82. [DOI] [PubMed] [Google Scholar]
- Samangaya RA, Wareing M, Skillern L, Baker PN. Phosphodiesterase inhibitor effect on small artery function in preeclampsia. Hypertension in Pregnancy 2011;30(2):144‐52. [DOI] [PubMed] [Google Scholar]
Schackis 2004 {published data only}
- Schackis RC. Hyperuricaemia and preeclampsia: is there a pathogenic link?. Medical Hypotheses 2004;63(2):239‐44. [DOI] [PubMed] [Google Scholar]
Scotland 1983 {published data only}
- Walker JJ, Greer I, Calder AA. Treatment of acute pregnancy‐related hypertension: labetalol and hydralazine compared. Postgraduate Medical Journal 1983;59:168‐70. [PubMed] [Google Scholar]
Singapore 1971 {published data only}
- Ratnam SS, Lean TH, Sivasamboo R. A comparison of hypotensive drugs in patients with hypertensive disorders in late pregnancy. Australian and New Zealand Journal of Obstetrics and Gynaecology 1971;11:78‐84. [DOI] [PubMed] [Google Scholar]
Smith 2005 {published data only}
- Smith D, Warren J, Saade G, Clark S, Belfort M. Oral labetalol given to treated non hypertensive patients with preeclampsia is no more likely to cause hypotension than magnesium sulfate [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S78. [Google Scholar]
South Africa 1982 {published data only}
- Garden A, Davey DA, Dommisse J. Intravenous labetalol and intravenous dihydralazine in severe hypertension in pregnancy. Clinical and Experimental Hypertension 1982;B1:371‐83. [DOI] [PubMed] [Google Scholar]
South Africa 1984 {published data only}
- Sankar D, Moodley J. Low‐dose diazoxide in the emergency management of severe hypertension in pregnancy. South African Medical Journal 1984;65:279‐80. [PubMed] [Google Scholar]
South Africa 1993 {published and unpublished data}
- Bhorat IE, Datshana P, Naidoo P, Rout CC, Moodley J. Malignant ventricular arrhythmias in eclampsia: a comparison of labetalol with dihydralazine. American Journal of Obstetrics and Gynecology 1993;168:1292‐6. [DOI] [PubMed] [Google Scholar]
- Bhorat IE, Naidoo DP, Rout CC, Moodley J. Malignant ventricular arrhythmias in eclampsia: a comparison of labetalol with dihydralazine. Proceedings of 9th International Congress, International Society for the Study of Hypertension in Pregnancy; 1994 march 15‐18; Sydney, Australia. 1994:162.
South Africa 2002 {published data only}
- Schie D, Jeu R, Steyn D, Odendaal H, van GH. The optimal dosage of ketanserin for pateints with severe hypertension in pregnancy. European Journal Obstetrics & Gynecology and Reproductive Biology 2002;102:161‐6. [DOI] [PubMed] [Google Scholar]
Spain 1988 {published data only}
- Cararach V, Torres Pons PJ, Roca M, Codina C, Cobo E, Gonzalez‐Merlo J. Treatment of severe hypertension in pregnancy. Double blind controlled trial a treatment pattern (TP) with hydralazine + methyldopa a single TP with labetolol. Proceedings of 6th International Congress, International Society for the Study of Hypertension in Pregnancy; 1988 May 22‐26; Montreal, Canada. 1988:101.
Steyn 2003 {published data only}
- Steyn DW, Hall DR, Odendaal H. The optimal dosage of nifedipine in patients with early onset severe pre‐eclampsia ‐ a randomised controlled trial. 22nd Conference on Priorities in Perinatal Care in South Africa; 2003 March 11‐14; Free State, South Africa. 2003.
Sweden 1993 {published and unpublished data}
- Hjertberg R, Faxelius G, Belfrage P. Comparison of outcome of labetalol or hydralazine therapy during hypertension in pregnancy in very low birth weight infants. Acta Obstetricia et Gynecologica Scandinavica 1993;72:611‐5. [DOI] [PubMed] [Google Scholar]
- Hjertberg R, Faxelius G, Lagercrantz H. Neonatal adaptation in hypertensive pregnancy ‐ a study of labetalol vs hydralazine treatment. Journal of Perinatal Medicine 1993;21:69‐75. [DOI] [PubMed] [Google Scholar]
- Hjertberg R, Faxelius G, Lagercrantz H. Neonatal adaptation in hypertensive pregnancy ‐ a study of labetalol vs hydralazine treatment. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:18. [DOI] [PubMed]
Unemori 2009 {published data only}
- Unemori E, Sibai B, Teichmana SL. Scientific rationale and design of a phase I safety study of relaxin in women with severe preeclampsia. Annals of the New York Academy of Sciences 2009;1160:381‐4. [DOI] [PubMed] [Google Scholar]
USA 1999 {published data only}
- Scardo JA, Vermillion ST, Newman RB, Chauhan SP, Brost B. Randomized double blinded hemodynamic study of oral nifedipine and IV labetolol in hypertensive urgencies of pregnancy. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S18. [DOI] [PubMed] [Google Scholar]
- Scardo JA, Vermillion ST, Newman RB, Chauhan SP, Hogg BB. A randomized double blind hemodynamic evaluation of nifedipine and labetolol in preeclamptic hypertensive emergencies. American Journal of Obstetrics and Gynecology 1999;181:862‐6. [DOI] [PubMed] [Google Scholar]
- Vermillion S, Scardo J, Newman R, Chauhan S. A prospective randomized double blind trial of oral nifedipine and intravenous labetolol in hypertensive emergencies. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S14. [DOI] [PubMed] [Google Scholar]
- Vermillion ST, Scardo JA, Newman RB, Chauhan SP. A randomized double blind trial of oral nifedipine and intravenous labetolol in hypertensive emergencies of pregnancy. American Journal of Obstetrics and Gynecology 1999;181:858‐61. [DOI] [PubMed] [Google Scholar]
Venezuela 2001 {published data only}
- Reyna‐Villasmil E, Prieto‐Franchi M, Guerra‐Velazquez M, Torres‐Montilla M. Effect of transdermal nitroglycerin on umbilical artery blood flow in preeclampsia [abstract]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):486. [Google Scholar]
Waheed 2005 {published data only}
- Waheed F, Chohan A. Comparison of intravenous hydralazine‐bolus dose versus continuous infusion drip in eclampsia. Annals of King Edward Medical College 2005;11(4):521‐3. [Google Scholar]
Warren 2004 {published data only}
- Chandran JR, Devi U, Devi S, Khadeeja M, Vinayachandran S, Jacob KJ, et al. LAMPET Trial (labetalol vs magnesium sulfate in prevention of eclampsia trial). 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:55.
- Warren J, Lacoursiere Y, Varner M, Silver R, Anthony J, Belfort M. First interim report on the labetalol versus magnesium sulfate for the prevention of eclampsia trial (LAMPET) [abstract]. Hypertension in Pregnancy 2004;23(Suppl 1):9. [Google Scholar]
References to studies awaiting assessment
Mesquita 1995 {published data only}
- Mesquita MRDS, Atallah AN, Rocha NDSC, Camano L, Bertini AM. The use of hydralazine and nifedipine in hypertensive emergencies in pregnancy [Emprego da hidralazina e da nifedipina nas emergencias hipertensivas na gestacao]. Revista Brasileira de Ginecologia e Obstetricia 1995;17(2):103‐11. [Google Scholar]
References to ongoing studies
Diemunsch 2008 {published data only}
- Diemunsch PA. Treatment of severe hypertension during pre‐eclampsia. A preliminary equivalence study between urapidil and nicardipine. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Additional references
Abalos 2007
- Abalos E, Duley L, Steyn DW, Henderson‐Smart David J. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002252.pub2] [DOI] [PubMed] [Google Scholar]
CEMD‐UK 2011
- Cantwell R, Clutton‐Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006‐2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG: an international journal of obstetrics and gynaecology 2011;118(Suppl. 1):1‐203. [DOI] [PubMed] [Google Scholar]
Churchill 2002
- Churchill D, Duley L. Interventionist versus expectant care for severe pre‐eclampsia before term. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003106] [DOI] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD, editors. Cochrane Reviewers’ Handbook 4.1.5 [updated April 2002]. In: The Cochrane Library, Issue 3, 2002. Oxford: Update Software. Updated quarterly.
de Swiet 2002
- Redman CWG. Hypertension. In: Swiet M editor(s). Medical Disorders in Obstetric Practice. 4th Edition. Blackwell Scientific Publications, 2002:159. [Google Scholar]
Duley 1999
- Duley L, Williams J, Henderson‐Smart DJ. Plasma volume expansion for treatment of pre‐eclampsia. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2009
- Duley L, Henderson‐Smart DJ, Walker GJA. Interventions for treating pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007756] [DOI] [Google Scholar]
Duley 2010
- Duley L, Gülmezoglu AM, Henderson‐Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre‐eclampsia. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD000025.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duley 2010a
- Duley L, Gülmezoglu AM, Chou D. Magnesium sulphate versus lytic cocktail for eclampsia. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD002960.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hennessy 2007
- Hennessy A, Thornton CE, Makris A, Ogle RF, Henderson‐Smart DJ, Gillin AG, et al. A randomised comparison of hydralazine and mini‐bolus diazoxide for hypertensive emergencies in pregnancy: the PIVOT trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(4):279‐85. [PUBMED: 17627681] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lindheimer 2008
- Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. Journal of the American Society of Hypertension 2008;2(6):484‐94. [DOI] [PubMed] [Google Scholar]
Lowe 2009
- Lowe SA, Brown MA, Dekker GA, Gatt S, McLintock CK, McMahon LP. Guidelines for the management of hypertensive disorders of pregnancy. Australia and New Zealand Journal of Obstetrics and Gynaecology 2009;49(3):242‐6. [DOI] [PubMed] [Google Scholar]
Magee 2003
- Magee LA, Cham C, Waterman EJ, Ohlsson A, Dadelszen P. Hydralazine for treatment of severe hypertension in pregnancy: meta‐analysis. BMJ 2003;327:955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magee 2008
- Magee L, Helewa, ME, Moutquin, JM, von Dadelszen, P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Journal of Obstetrics and Gynaecology Canada 2008;30(Supp 1):S1‐S48. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute of Clinical Excellence. NICE clinical guideline No. 107. Hypertension in Prenancy. Management of Hypertensive Disorders in Pregnancy. http://www.nice.org.uk/nicemedia/live/13098/50418/50418.pdf 2010.
RevMan 2000 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.1 for Windows. Oxford, England: The Cochrane Collaboration, 2000.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roberts 2009
- Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta 2009;30(Suppl 1):32‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomassoni 2008
- Tomassoni D, Lanari A, Silvestrelli G, Traini E, Amenta F. Nimodipine and its use in cerebrovascular disease: evidence from recent preclinical and controlled clinical studies. Clinical and Experimental Hypertension 2008;30(8):744‐66. [DOI] [PubMed] [Google Scholar]
Tuffnell 2006
- Tuffnell D, Shennan AH, Waugh JJS, Walker JJ. The management of severe pre‐eclampsia/eclampsia. RCOG guideline number 10(A). Royal College of Obstetricians and Gynaecologists, 2006. [Google Scholar]
WHO 1988
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. American Journal of Obstetrics and Gynecology 1988;158:80‐3. [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. WHO Recommendations for Prevention and Treatment of Pre‐eclampsia and Eclampsia. http://whqlibdoc.who.int/publications/2011/9789241548335_eng.pdf (accessed 2013) 2011. [PubMed]
Woudstra 2010
- Woudstra DM, Chandra S, Hofmeyr GJ, Dowswell T. Corticosteroids for HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome in pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008148.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Duley 1995a
- Duley L. IV labetalol vs iv diazoxide in severe pre‐eclampsia. In: Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database Issue 2, Oxford: Update Software 1995.
Duley 1995b
- Duley L. Labetalol vs hydralazine in severe pregnancy‐induced hypertension. In: Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database Issue 2, Oxford: Update Software 1995.
Duley 1995c
- Duley L. Nifedipine vs hydralazine in severe pregnancy‐induced hypertension. In: Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database Issue 2, Oxford: Update Software 1995.
Duley 1995d
- Duley L. Prostacyclin vs dihydralazine in severe hypertension. In: Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database Issue 2, Oxford: Update Software 1995.
Duley 1999a
- Duley L, Henderson‐Smart DJ. Drugs for rapid treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001449] [DOI] [PubMed] [Google Scholar]
Duley 2002c
- Duley L, Henderson‐Smart DJ. Drugs for rapid treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001449] [DOI] [PubMed] [Google Scholar]
Duley 2006
- Duley L, Henderson‐Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001449.pub2] [DOI] [PubMed] [Google Scholar]


