Abstract
Introduction
Currently there are no general guidelines for diagnosis or management of suspected drug-induced (DI) interstitial lung disease (ILD). The objective was to survey a sample of current European practice in the diagnosis and management of DI-ILD, in the context of the prescribing information approved by regulatory authorities for 28 licenced drugs with a recognised risk of DI-ILD.
Methods
Consultant physicians working in specialist ILD centres across Europe were emailed two surveys via a website link. Initially, opinion was sought regarding various diagnostic and management options based on seven clinical ILD case vignettes and five general questions regarding DI-ILD. The second survey involved 29 statements regarding the diagnosis and management of DI-ILD, derived from the results of the first survey. Consensus agreement was defined as 75% or greater.
Results
When making a diagnosis of DI-ILD, the favoured investigations used (other than computed tomography) included pulmonary function tests, bronchoscopy and blood tests. The preferred method used to decide when to stop treatment was a pulmonary function test. In the second survey, the majority of the statements were accepted by the 33 respondents, with only four of 29 statements not achieving consensus when the responses “agree” and “strongly agree” were combined as one answer.
Conclusion
The two surveys provide guidance for clinicians regarding an approach to the diagnosis and management of DI-ILD in which the current evidence base is severely lacking, as demonstrated by the limited information provided by the manufacturers of the drugs associated with a high risk of DI-ILD that we reviewed.
Short abstract
Two surveys illustrating current European practice in the diagnosis and management of drug-induced interstitial lung disease provide guidance for clinicians in a condition in which the present evidence base is lacking http://bit.ly/35A9YPk
Introduction
Although the exact number is unknown, at least 450 drugs have been reported to cause interstitial lung disease (ILD), and this number will likely continue to rise as new medications are developed [1]. The main categories of medications associated with drug-induced (DI) ILD include chemotherapeutic (e.g. bleomycin), biological (e.g. infliximab), anti-inflammatory (e.g. methotrexate), antimicrobial (e.g. nitrofurantoin), cardiovascular (e.g. amiodarone), and miscellaneous agents [2]. Importantly, checkpoint inhibitors as a class are associated with lung toxicity and use of these agents is increasing [3, 4]. A UK population-based study published in 2012 estimated that the incidence of drug/radiation-induced ILD between 1997 and 2008 was 4.1 per million person-years [5]. However, this is likely to be underestimated due to the increased use of biologics and checkpoint inhibitors over the past decade. The pathogenic mechanism responsible for the development of DI-ILD in humans is unclear [1]. Animal studies with drugs known to cause DI-ILD in humans use mainly just one agent, bleomycin [6].
Currently there are no general guidelines regarding the diagnostic and management approach for suspected DI-ILD. The diagnosis initially involves excluding infection and is particularly challenging due to the non-specific clinical, histological and radiological findings that can overlap with other ILD subtypes. Diagnosis of DI-ILD is supported by a temporal link between an exposure to the offending drug and the development of new respiratory symptoms, signs and/or radiological changes; however, DI-ILD may develop within the first few days or even several years after the drug was commenced [7]. Furthermore, drugs used to treat connective tissue diseases (CTDs) can themselves cause DI-ILD, making it difficult to determine whether the development of ILD is due to the underlying CTD or the drug in question. Likewise, checkpoint inhibitors may be used in patients with lung cancer who may already have respiratory symptoms.
When ILD is recognised as a potential adverse effect of a particular drug, regulatory authorities require this to be specified in the prescribing information. Recently introduced DI-ILD-associated drugs were generally approved following high-quality trials with exhaustive documentation of adverse events followed by rigorous regulatory scrutiny, and proportionate, evidence-based drug labelling. However other drugs were first introduced many decades ago (e.g. nitrofurantoin in 1953) when clinical trial design and regulatory science were less mature. These older agents are now generic, the evidence base for adverse effects is more limited, and the prescribing information is seldom updated to reflect the modern-day practice of respiratory medicine. If DI-ILD is suspected, it is important to consider discontinuation of the causative agent, and although good quality evidence is lacking, corticosteroids are often commenced. Improvement of symptoms and radiology usually occurs following discontinuation of the suspected drug. However, irreversible fibrosis may occur especially if the diagnosis of DI-ILD is delayed.
We report the results of two online surveys with the aim of illustrating current European practice in the diagnosis and management of DI-ILD. We also summarise current prescribing information regarding the incidence of DI-ILD, guidance on respiratory monitoring and management of suspected or confirmed DI-ILD for 28 licenced drugs associated with a recognised risk of DI-ILD. This study was conducted in order to determine what specific prescribing guidance was available and to compare this to the results of expert consensus agreement from the surveys.
Methods
Consultant respiratory physicians working in Europe, with a sub-speciality interest in ILD were emailed two separate surveys, at different time points, via a website link (SurveyMonkey). The surveys were developed through an iterative process of review by a group of physicians with an interest in ILD. In the first survey, opinion was sought on 34 case-specific questions regarding seven clinical ILD case vignettes, exploring various diagnostic and management options. The cases were fictional and based on common clinical scenarios in ILD with an emphasis on DI-ILD. There were a further five general questions examining investigations used to diagnose DI-ILD, frequency of patient follow-up, weaning regimens for corticosteroids (prednisolone) and indications for discontinuation of treatment. The second survey involved 29 statements regarding diagnosis and management of DI-ILD. These statements were derived from responses to the first survey with the aim to obtain responses that were likely to achieve consensus and therefore allow for construction of expert-opinion-based guideline. For each statement, respondents had the option of choosing one of five choices (strongly agree, agree, neutral, disagree or strongly disagree). In both surveys, consensus agreement was defined as 75% or greater. Ethics committee approval was not required because there was no involvement of patients.
The prescribing information from the US Food and Drug Administration (FDA) for 28 licenced drugs previously identified as being associated with a significant risk of DI-ILD in a systematic review [2] were reviewed. The FDA is a long-established regulatory agency and publishes approved prescribing information in consistent formats, whereas prescribing information in European jurisdictions is more difficult to review, particularly for older drugs that predate the establishment of the European Medicines Agency (EMA). The most recent (2018) prescribing information posted at https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm was used. These 28 drugs were approved between 1953 and 2017 for a variety of indications. Prescribing information, therefore, reflects vast differences in clinical experience, patterns of clinical use and duration of post-marketing surveillance. Where possible FDA-approved text was cross-checked with EMA-approved advice.
Results
Survey 1
34 out of a total of 60 (57%) ILD physicians completed the first survey. Overall, 21 respondents were based in the UK and 13 were based in other European countries (1 Belgium, 1 Denmark, 2 France, 1 Germany, 4 Italy, 1 Netherlands, 1 Spain, 1 Switzerland and 1 Turkey). Five respondents were excluded because fewer than 50% of the questions were completed. The responses to the questions and a description of the clinical cases is in the supplementary material. Of the 34 case-specific questions, consensus was only achieved once (in case six, 91% of respondents chose RA-ILD as the diagnosis). Consensus was achieved in two of the five general questions pertaining to investigations and treatment discontinuation. When making a diagnosis of DI-ILD, the favoured investigations used (other than high resolution computed tomography (CT)) included pulmonary function tests (PFTs) (91% of respondents), bronchoscopy with bronchoalveolar lavage for differential cell count and/or microbiology (100% of respondents) and blood tests including auto-antibody screen (91% of respondents). The preferred methods used to decide when to stop treatment for DI-ILD included forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO) (87% of respondents). Although consensus was not reached, 70% of respondents used repeat CT and 65% used patients' symptoms to decide when to stop treatment.
Although no consensus was achieved regarding the management of DI-ILD in clinical cases, the results suggest some degree of shared practice. A total of 68% of respondents chose prednisolone 0.5 mg·kg−1 for 2–4 weeks reducing by 5 mg every 1–2 weeks as tolerated for the management of DI-ILD. There was no agreement regarding the optimal dose of intravenous (i.v.) methylprednisolone, with 26% of respondents choosing 500 mg once daily for 3 days and 26% of respondents choosing 1 g once daily for 3 days. Following i.v. methylprednisolone, the majority (39%) of respondents selected prednisolone 0.5 mg·kg−1 for 2–4 weeks, reducing by 5 mg every 1–2 weeks as tolerated as their preferred ongoing treatment strategy. Although no consensus was reached regarding the duration of prednisolone treatment before attempting dose reduction, the majority of respondents (44%) chose 4 weeks. When “second-line” immunosuppression was required the majority (40%) of respondents chose i.v. cyclophosphamide with the dose varying from 500 to 750 mg·m−2. Overall, 74% of respondents used both FVC and DLCO to decide when to attempt weaning the dose of prednisolone, with 13% of respondents using FVC alone. A total of 70% of respondents used patients' symptoms and 57% used repeat chest radiography, with 35% using repeat CT to decide the ideal time to reduce the prednisolone dose. In patients with a diagnosis of DI-ILD, the preferred frequency of follow-up was 6 weeks (48% of respondents).
Survey 2
33 out of a total of 60 (55%) ILD physicians completed the second survey, with a 100% answer rate. 22 respondents were based in the UK and 11 were based in other European countries (1 Belgium, 1 Czech Republic, 1 Denmark, 1 France, 1 Germany, 4 Italy, 1 Netherlands, 1 Turkey). 21 of the 33 respondents also completed the first survey. Consensus (≥75% of respondents choosing “strongly agree”) was achieved in five of the 29 statements pertaining to statements regarding clinical suspicion, diagnosis and management of DI-ILD (table 1). Of the remaining 24 statements, when the responses “agree” and “strongly agree” were combined, consensus was achieved on 20 occasions (table 2). Therefore, when the responses “agree” and “strongly agree” were combined only four of the 29 statements did not achieve consensus (table 3).
TABLE 1.
Statements achieving consensus (≥75% of respondents choosing strongly agree)
| Statement | Responses: strongly agree |
| DI-ILD should be considered when patients present with respiratory symptoms while receiving treatment with a drug known to be associated with DI-ILD | 30 (91%) |
| DI-ILD should be suspected if there are radiological and physiological abnormalities emerging in a patient taking a drug known to cause DI-ILD | 28 (85%) |
| DI-ILD should be strongly suspected if there is a temporal relationship between commencing a drug known to cause DI-ILD and symptom onset | 28 (85%) |
| When a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, and should initial investigations and treatment not resolve the clinical scenario, investigations should include pulmonary function tests (spirometry and transfer factor) | 28 (85%) |
| When a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, and should initial investigations and treatment not resolve the clinical scenario, investigations should include chest radiography and HRCT scan | 28 (85%) |
DI-ILD: drug-induced interstitial lung disease; HRCT: high-resolution computed tomography.
TABLE 2.
Statements achieving consensus when agree and strongly agree is combined as one answer (excludes the five statements in table 1)
| Statement | Responses: agree or strongly agree |
| DI-ILD should not be discounted if there is no clear temporal relationship between drug commencement and symptom onset | 27 (82%) |
| DI-ILD should be considered if an alternative cause of symptoms, abnormal physiology and radiological changes cannot be identified, or if the patient fails to respond to treatment for the alternative cause | 25 (76%) |
| A diagnosis of DI-ILD should not be made without the involvement of a specialist ILD MDT | 28 (85%) |
| When a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, and should initial investigations and treatment not resolve the clinical scenario, investigations should include bronchoalveolar lavage with samples sent to exclude infection (typical/atypical), should the patient be deemed able to undertake the procedure | 28 (85%) |
| When a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, and should initial investigations and treatment not resolve the clinical scenario, investigations should include bronchoalveolar lavage with samples examined for differential cell count, should the patient be deemed able to undertake the procedure | 28 (85%) |
| When a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, and should initial investigations and treatment not resolve the clinical scenario, investigations should include blood tests including tests for infection, to assess underlying comorbid disease activity and to assess the inflammatory response | 28 (85%) |
| When a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, and should initial investigations and treatment not resolve the clinical scenario, investigations should not routinely include transbronchial lung biopsy | 29 (88%) |
| When a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, and should initial investigations and treatment not resolve the clinical scenario, investigations should not routinely include open lung biopsy | 28 (85%) |
| Prior to making any modification to drug therapy, discussion with colleagues that initiated therapy should occur to ensure the safety of drug cessation and to consider alternative options | 30 (91%) |
| In patients without significant hypoxia (oxygen saturation on room air ≥94%), initial management should be to stop the offending drug | 28 (85%) |
| In patients with significant hypoxia (oxygen saturation on room air <94%), initial management should include drug cessation and commencement of oral corticosteroid | 28 (85%) |
| Recommended dosage of first line oral corticosteroid is prednisolone 0.5–1 mg·kg−1 | 28 (85%) |
| In patients with life threatening hypoxia due to presumptive DI-ILD, treatment should initially be with i.v. methylprednisolone | 31 (94%) |
| Recommended doses of i.v. methylprednisolone are 500–1000 mg once daily for 3 successive days | 30 (91%) |
| Long term monitoring of response should include assessment of symptoms, pulmonary physiology (spirometry/transfer factor), blood tests (if appropriate) and radiology (chest radiography, HRCT; dependent on presence or absence of abnormalities on chest radiography that can be reliably monitored) | 33 (100%) |
| In patients showing response to therapy, consideration of weaning of medication to lowest dose that controls disease activity (which may include no treatment) should occur at 2–4 weekly intervals | 28 (85%) |
| In patients that respond to initial corticosteroid therapy, and following a weaning protocol cannot be reduced to levels of oral prednisolone (or equivalent) of <20 mg once daily, a steroid sparing agent should be considered | 27 (82%) |
| Steroid sparing agents may include mycophenolate mofetil (preferred), azathioprine, methotrexate or cyclophosphamide | 25 (76%) |
| If a drug has been proven, or is highly suspected, to have caused DI-ILD, unless no alternative agent is available and treatment is absolutely required, the offending drug should not be re-used | 31 (94%) |
| If a patient has experienced a DI-ILD in the past, careful consideration of the likelihood or possibility of further DI-ILD from future therapeutic interventions should be considered. If possible, the use of agents not known to be associated with DI-ILD should be selected. | 26 (79%) |
DI-ILD: drug-induced interstitial lung disease; ILD: interstitial lung disease; MDT: multidisciplinary team; HRCT: high-resolution computed tomography.
TABLE 3.
Statements not achieving consensus when agree and strongly agree is combined as one answer
| Statement | Responses: agree or strongly agree |
| When a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, initial investigations and management should focus around more common causes of symptoms | 16 (49%) |
| Pulsed i.v. cyclophosphamide should be considered in those patients not responding to i.v. methylprednisolone or oral prednisolone | 14 (42%) |
| In patients responding to therapy, follow-up should occur within 6 weeks of initial therapy | 24 (73%) |
| If a drug has been proven, or is highly suspected, to have caused DI-ILD, if no alternative agent is available and treatment is absolutely required, the offending drug should be cautiously reintroduced ideally at reduced dosage and with frequent monitoring (1–2 weekly) | 19 (58%) |
DI-ILD: drug-induced interstitial lung disease.
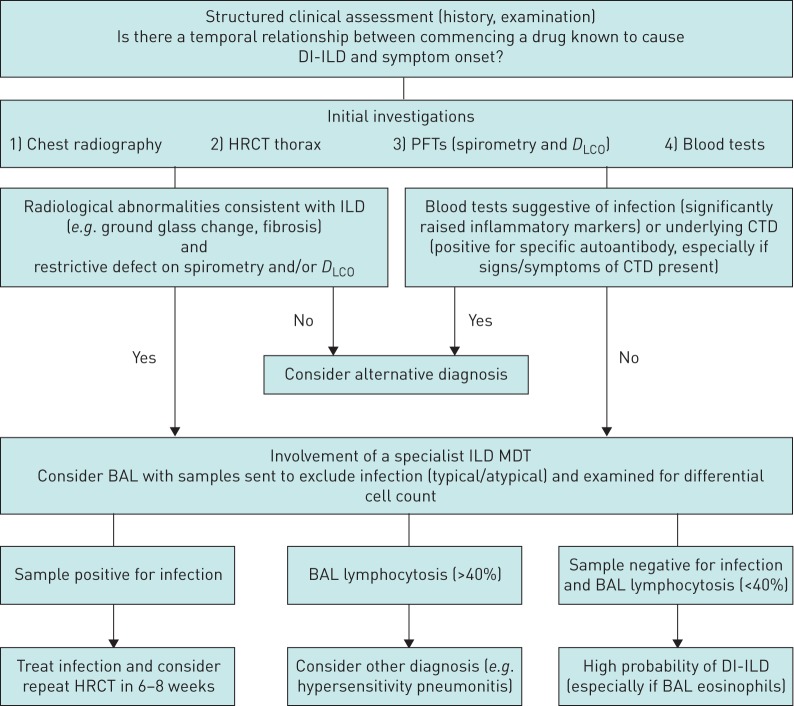
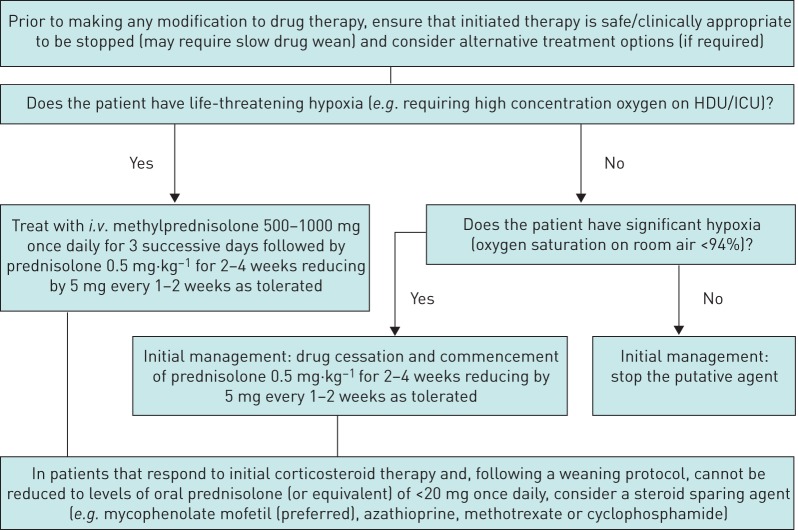
Figures 1 and 2 illustrate a proposed DI-ILD diagnostic and treatment algorithm respectively, based on the consensus statements from the two surveys.
FIGURE 1.
Diagnostic algorithm for drug-induced interstitial lung disease (DI-ILD). HRCT: high-resolution computed tomography; PFT: pulmonary function test; DLCO: diffusing capacity of the lung for carbon monoxide; ILD: interstitial lung disease; CTD: connective tissue disease; MDT: multidisciplinary team; BAL: bronchoalveolar lavage.
FIGURE 2.
Treatment algorithm for drug-induced interstitial lung disease. HDU: high-dependency unit; ICU: intensive care unit.
Prescribing information
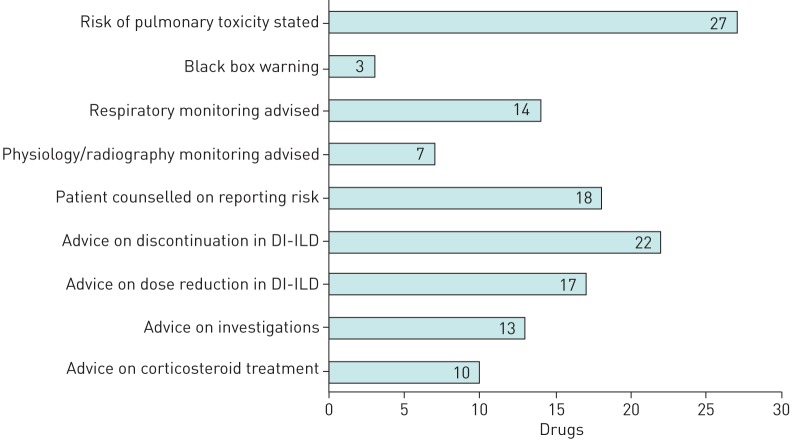
The results of the prescribing information reviewed are summarised in figure 3 (full details can be found in table 1 of supplementary material). The classes of drugs described include chemotherapeutic (including checkpoint inhibitors), biological, anti-inflammatory, antimicrobial (nitrofurantoin) and cardiovascular (amiodarone). For 27 of 28 drugs, the prescribing information warns of the risk of DI pulmonary toxicity, variously described as ILD, lung infiltration, pneumonitis, hypersensitivity pneumonitis, interstitial pneumonitis, alveolar pneumonitis, immune-mediated pneumonitis, non-infective pneumonitis, eosinophilic pneumonia, bronchiolitis obliterans, organising pneumonia, or pulmonary fibrosis. For three of 27 drugs (amiodarone, bleomycin and methotrexate) DI pulmonary toxicity features as a “boxed warning”. In 14 of 27 cases, respiratory monitoring (e.g. symptoms/signs, physiology, radiography) is advised, and in 7 of 14 cases, physicians are specifically advised to use PFTs or radiography in their monitoring. Regular chest radiography every 1–2 weeks is advised during bleomycin treatment; whereas, chest radiography monitoring is advised every 3–6 months following the initiation of amiodarone. There was no specific advice regarding the frequency of PFTs or radiography in the monitoring of the other five drugs. For 18 of 27 drugs, the patient information leaflet specifically counselled patients to report any change in respiratory symptoms to their healthcare provider. In 22 of 27 cases, the prescribing information discussed discontinuation in cases of suspected or confirmed DI-ILD, and of these, 17 of 22 also discussed dose reduction or temporary discontinuation. 13 of 27 discussed the use of diagnostic imaging tests, and 10 of 27 advised on corticosteroid use, of which 6 provided specific recommendations for dosage of corticosteroids.
FIGURE 3.
Drugs with information on pulmonary toxicity included in the prescribing information (n=28). DI-ILD: drug-induced interstitial lung disease.
Discussion
Our first survey revealed significant variation amongst ILD experts in the diagnosis and management of seven different ILD cases. We do not feel that this is surprising given the poor inter-multidisciplinary meeting agreement in the diagnosis of non-specific interstitial pneumonia and hypersensitivity pneumonitis reported by Walsh et al. [8], this being indicative of significant interobserver variation within the field of ILD generally. In our first survey, consensus was achieved in the diagnosis of only one of the cases and no consensus was reached in the specific management of any case. There appeared to be some agreement regarding the initial dose and weaning regimen of prednisolone, with the majority of respondents choosing 0.5 mg·kg−1 for 2–4 weeks reducing by 5 mg every 1–2 weeks as tolerated. The same prednisolone dose and weaning regimen was preferred following treatment with i.v. methylprednisolone and the favoured second-line immunosuppressant was i.v. cyclophosphamide. There was consensus regarding the investigations used to diagnose DI-ILD and when to stop treatment. FVC and DLCO were the preferred investigations used to decide when to stop DI-ILD treatment and the investigations favoured by physicians in the diagnosis of DI-ILD included PFTs, bronchoscopy with bronchoalveolar lavage for differential cell count and/or microbiology and bloods (including auto-antibody screen). Although no consensus was reached, the preferred duration of prednisolone treatment before attempting dose reduction was 4 weeks, the majority of respondents favoured the use of both FVC and DLCO to decide when to attempt weaning the dose of prednisolone. The most common frequency of follow-up for DI-ILD was 6 weeks.
Our second survey focused on multiple statements regarding particular aspects of the diagnosis and management of DI-ILD, with a view to generating an expert-opinion-defined clinical guideline. The statements were derived from responses to the initial survey. Five out of the 29 statements achieved consensus in terms of a response of “strongly agree” (table 1). All five statements relate to the diagnosis of DI-ILD, suggesting that there is greater agreement between clinicians in the diagnosis of DI-ILD than the management of DI-ILD. This reflects the lack of evidence-based guidelines on the management of DI-ILD with ILD clinicians tending to extrapolate from treatment of other ILD subtypes. Consensus regarding the management of DI-ILD could also be influenced by the heterogeneity of DI-ILD severity and disease course. The majority of the statements were accepted by the 33 respondents, with only four out of the 29 statements not achieving consensus when the responses “agree” and “strongly agree” were combined as one answer (table 3). Of the four statements that did not reach consensus, two had less than a 50% response as either “agree” or “strongly agree”. Seven (21%) respondents were neutral and 10 (30%) either “disagreed” or “strongly disagreed” that when a patient presents with new respiratory symptoms while using a medication known to cause DI-ILD, initial investigations and management should focus around more common causes of symptoms. 12 (36%) respondents were neutral and seven (21%) either “disagreed” or “strongly disagreed” that pulsed i.v. cyclophosphamide should be considered in those patients not responding to i.v. methylprednisolone or oral prednisolone.
Of the 28 drugs associated with a significant risk of DI-ILD that were reviewed, only 10 advised on the use of corticosteroids to treat suspected or confirmed cases of DI-ILD. The manufacturers of six of these medications advise treatment with initial doses of 1–2 mg·kg−1·day−1 prednisolone which is at least double the initial dose that the physicians in our surveys favoured. The manufacturers of one of the 10 drugs advise treatment with initial doses of 40–60 mg·day−1 prednisolone which is more in keeping with the results of our surveys. The three remaining manufacturers did not comment on a specific dose of corticosteroid. The ability to determine the role of corticosteroids in the treatment of DI-ILD in a recent systematic review was felt to be limited by a lack of randomised studies, as well as the problem of incomplete data regarding the dose and duration of treatment and variation in both the eligibility criteria for patient selection and corticosteroid dose [2]. The authors in this systematic review concluded that there is currently insufficient evidence on which to base recommendations for the use of corticosteroids in DI-ILD and that when corticosteroids are used, the dosing regimens used should be at the discretion of the physician treating the patient. The consensus gained from our surveys significantly adds to the literature, albeit ILD physicians' opinion rather than being a prospective study.
It is particularly notable that despite the recent advances in quantitative CT and magnetic resonance imaging-based imaging biomarkers [9], that in the prescribing information the use of imaging for monitoring is generally vague (often specifying only chest radiography) and non-quantitative. Imaging biomarkers, with defined evidence-based cut-offs, already play a pivotal role in managing drug-induced cardiotoxicity [10] and there seems to be a clear opportunity to similarly advance imaging biomarkers for the management of DI-ILD.
We appreciate that there are limitations in our study. The number of physicians participating in the surveys was relatively small and five of the 34 respondents in the first survey completed fewer than 50% of the questions. The majority of the respondents in both surveys were based in the UK and therefore it could be argued that the diagnostic and management approach to DI-ILD in the UK is over-represented in the surveys. The information provided in the cases was limited and although descriptions of the CT scans were included there were no actual CT images available to view. However, both surveys provided expert consensus guidance that will help clinicians in the diagnosis and management of DI-ILD in which the current evidence base is severely lacking. This is demonstrated by the limited information provided by the manufacturers of the 28 drugs associated with a high risk of DI-ILD that we reviewed.
Conclusion
Our initial survey illustrates some significant differences in opinion between experienced ILD physicians regarding the diagnosis and management of seven different ILD clinical scenarios. This may reflect the lack of evidence or guidelines in DI-ILD and highlights the need for further research in this area to help guide physicians. The results of the two surveys provide guidance for clinicians regarding an approach for the diagnosis and management of DI-ILD. However, we recognise that treatment and follow-up of DI-ILD needs to be individualised, including the consideration of the severity of disease and other comorbidities.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00286-2019.supplement (244.3KB, pdf)
Survey 00286-2019.supp_survey (188.7KB, pdf)
Acknowledgements
We are grateful to the following physicians who responded to one or both of our surveys. Abdul Ashish; Wrightington, Wigan and Leigh NHS Foundation Trust, UK. Elisabeth Bendstrup; Aarhus University Hospital, Denmark. Vincent Cottin; Louis Pradel Hospital, University of Lyon, France. Bruno Crestani; Bichat Hospital, France. Ahmed Fahim; The Royal Wolverhampton NHS Trust, UK. Sophie Fletcher; University Hospital Southampton NHS Foundation Trust, UK. Manuela Funke-Chambour; University of Bern, Switzerland. Peter George; Royal Brompton and Harefield NHS Foundation Trust, UK. Michael Gibbons; Royal Devon and Exeter NHS Foundation Trust, UK. Jan Grutters; St Antonius Hospital Nieuwegein, the Netherlands. Simon Hart; Hull University Teaching Hospitals NHS Trust, UK. Karol Henry; South Eastern Health and Social Care Trust, UK. Rachel Hoyles; Oxford University Hospitals NHS Foundation Trust, UK. Gisli Jenkins; Nottingham University Hospitals NHS Trust, UK. Eoin Judge; Aintree University Hospital NHS Foundation Trust, UK. Vasileios Kouranos; Royal Brompton and Harefield NHS Foundation Trust, UK. Michael Kreuter; Thoraxklinik, University of Heidelberg, Germany. Toby Maher; Royal Brompton and Harefield NHS Foundation Trust, UK. Nesrin Mogulkoc; Ege University Hospital, Turkey. Maria Molina; Bellvitge University Hospital, Spain. Philip Molyneaux; Royal Brompton and Harefield NHS Foundation Trust, UK. Eoin Murtagh; Northern Health and Social Care Trust, UK. Venerino Poletti; G.B. Morgagni Hospital, Italy. Joanna Porter; University College London Hospitals NHS Foundation Trust, UK. Claudia Ravaglia; G.B. Morgagni Hospital, Italy. Elizabeth Renzoni; Royal Brompton and Harefield NHS Foundation Trust, UK. Luca Richeldi; University Hospital A. Gemelli, Italy. Pilar Rivera-Ortega; University Hospital of South Manchester NHS Foundation Trust, UK. Peter Sherwood Burge; Heart of England NHS Foundation Trust, UK. John Simpson; The Newcastle upon Tyne Hospitals NHS Foundation Trust, UK. Paolo Spagnolo; University Hospital of Padova, Italy. Lisa Spencer; Aintree University Hospital NHS Foundation Trust, UK. Tim Sutherland; The Leeds Teaching Hospitals NHS Trust, UK. David Thickett; University Hospitals Birmingham NHS Foundation Trust, UK. Martina Vasakova; Thomayer Hospital, Czech Republic. Athol Wells; Royal Brompton and Harefield NHS Foundation Trust, UK. Alex West; Guy's and St Thomas’ NHS Foundation Trust, UK. Felix Woodhead; University Hospitals of Leicester NHS Trust, UK. Wim Wuyts; University Hospitals Leuven, Belgium.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Author contributions: J.A. Eaden and S.M. Bianchi conceived and designed the study. J.A. Eaden and N. Chaudhuri distributed the survey. J.A. Eaden analysed the survey results. J.C. Waterton and S. Skeoch collected and analysed the prescribing information. J.A. Eaden, J.C. Waterton and S.M. Bianchi drafted the manuscript for intellectual content. All authors edited the manuscript.
Conflict of interest: J.A. Eaden reports grants from Innovative Medicines Initiatives 2 Joint Undertaking under grant agreement number 116106. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA.
Conflict of interest: S. Skeoch reports an Innovative Medicines Initiatives 2 Joint Undertaking under grant agreement number 116106. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA.
Conflict of interest: J.C. Waterton reports an Innovative Medicines Initiatives 2 Joint Undertaking under grant agreement number 116106. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA. He also declares personal fees from Bioxydyn Ltd and Antaros Ltd, outside the submitted work.
Conflict of interest: N. Chaudhuri reports an Innovative Medicines Initiatives 2 Joint Undertaking under grant agreement number 116106. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA. She also reports grants, support for conference attendance and speaker fees at meetings from Boehringer Ingelheim and Roche Ltd, outside the submitted work.
Conflict of interest: S.M. Bianchi reports an Innovative Medicines Initiatives 2 Joint Undertaking under grant agreement number 116106. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA.
Support statement: this study was an Innovative Medicines Initiatives 2 Joint Undertaking. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA (grant 116106).
References
- 1.Schwaiblmair M, Behr W, Haeckel T, et al. . Drug-induced interstitial lung disease. Open Respir Med J 2012; 6: 63–74. doi: 10.2174/1874306401206010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skeoch S, Weatherley N, Swift AJ, et al. . Drug-induced interstitial lung disease: a systematic review. J Clin Med 2018; 7: E356. doi: 10.3390/jcm7100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaunay M, Cadranel J, Lusque A, et al. . Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 2017; 50: 1700050. doi: 10.1183/13993003.00050-2017 [DOI] [PubMed] [Google Scholar]
- 4.Sears CR, Peikert T, Possick JD, et al. . Knowledge gaps and research priorities in immune checkpoint inhibitor-related pneumonitis. An official American Thoracic Society research statement. Am J Respir Crit Care Med 2019; 200: e31–e43. doi: 10.1164/rccm.201906-1202ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amar RK, Jick SS, Rosenberg D, et al. . Drug-/radiation-induced interstitial lung disease in the United Kingdom general population: incidence, all-cause mortality and characteristics at diagnosis. Respirology 2012; 17: 861–868. doi: 10.1111/j.1440-1843.2012.02187.x [DOI] [PubMed] [Google Scholar]
- 6.Mahmutovic Persson I, Von Wachenfeldt K, Pindoria K, et al. . Systematic review: in vivo imaging in animal models relevant to drug-induced interstitial lung disease (DIILD). Eur Respir J 2018; 52: Suppl. 62, PA2956. [Google Scholar]
- 7.Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 2012; 13: 39. doi: 10.1186/1465-9921-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh SLF, Wells AU, Desai SR, et al. . Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med 2016; 4: 557–565. doi: 10.1016/S2213-2600(16)30033-9 [DOI] [PubMed] [Google Scholar]
- 9.Weatherley ND, Eaden JA, Stewart NJ, et al. . Experimental and quantitative imaging techniques in interstitial lung disease. Thorax 2019; 74: 611–619. doi: 10.1136/thoraxjnl-2018-211779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simoni LJC, Brandao SCS. New imaging methods for detection of drug-induced cardiotoxicity in cancer patients. Curr Cardiovasc Imaging Rep 2017; 10: 18. doi: 10.1007/s12410-017-9415-3 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00286-2019.supplement (244.3KB, pdf)
Survey 00286-2019.supp_survey (188.7KB, pdf)