Abstract
Background
Employees in contact with infectious tuberculosis (TB) patients in healthcare facilities of low-incidence countries are still at considerable risk of acquiring TB infections. However, formal precautions recommended on the protection of healthcare workers may not only vary from country to country but also within a single country. The objective of this study was to compare current guidelines with respect to hospital infection control of TB, focusing on common shared priorities and discrepancies between sets of recommendations.
Methods
Five types of procedures captured in guidelines of the World Health Organization, the United States of America, the United Kingdom and Germany are compared and the underlying evidence is discussed.
Results
Uncontroversially, personal protection by respirators in the TB ward and during aerosol-generating procedures is key to reducing Mycobacterium tuberculosis exposure. However, there is no consensus on the types of masks that should be worn in different situations. Closely connected to this, there is considerable uncertainty with respect to the optimal date of removing sputum smear-negative and multidrug-resistant TB patients from isolation. Indeed, the use of notable new tools for this purpose, such as the highly sensitive PCR tests recommended by the World Health Organization for detecting TB/multidrug-resistant TB, have yet to be sufficiently incorporated into TB guidelines. Perceptions differ, too, as to whether long-term control measures for M. tuberculosis infections in healthcare workers by serial testing for latent TB infection should be established and, if so, how testing results should be interpreted.
Conclusions
Although the current recommendations on protection of healthcare workers are otherwise homogeneous, there are considerable discrepancies that have important implications for daily practice.
Short abstract
Current @WHO, US, UK and German recommendations on protecting employees in healthcare facilities against M. tuberculosis transmission show considerable practical discrepancies. Harmonisation and practical amendments of such guidelines is most desirable. http://bit.ly/2EzGlBN
Introduction
As there were an estimated 1.2 million (range, 1.1–1.3 million) tuberculosis (TB) deaths among HIV-negative people in 2018 [1] TB can still be considered to be the top infectious killer worldwide. Although the epidemiological situation of TB in low-incidence countries has improved over the past few years, employees in unprotected contact with infectious TB patients in healthcare facilities are at considerable risk of Mycobacterium tuberculosis transmission [2]. Current guidelines suggest a bundle of complementary infection control measures for reducing such transmission. Recommendations for such control measures have been established by the World Health Organization (WHO) and authorities in the United States of America (USA), the United Kingdom (UK) and in Germany, the latter three serving as references for other low-incidence countries. How the positions and priorities set forth in these four sets of recommendations are shared or diverge has not yet been investigated.
The present review briefly summarises six important aspects of those recommendations and their implementation in practice for infection control in hospital facilities. The underlying evidence is discussed.
The four sets of recommendations each provide specifics for the six types of measures conventionally applied for the prevention of TB in healthcare settings:
Personal respiratory protection, such as the wearing of masks
There is consensus in all guidelines that hospital staff and those visiting infectious TB patients are to wear respiratory protection. For the USA, these must satisfy or exceed the N95 standards, providing a filtration efficiency of 95%, set by the Centers for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health; for the UK and Germany the nearly equivalent filtering face piece (FFP)2 standard with a filtration efficiency of at least 92% that is European Conformity (CE) certified [3–5] is required. The WHO recommends either standard [6, 7].
The recommendations differ on the use of respirators during high-risk aerosol-generating procedures (e.g. bronchoscopy, sputum induction, procedures or lung surgery with highspeed devices). For the UK, the often-followed guidance of Coia et al. [8], but not the UK National Institute for Health and Care Excellence (NICE) guidelines, suggests the use of FFP3 masks with a filtration efficiency of 98% when healthcare workers (HCWs) undertake bronchoscopy or other aerosol-generating procedures. The German recommendations either decline to go into any detail here [3] or recommend FFP3 masks, but only for protection against multidrug-resistant (MDR) TB patients [9]. In contrast, the CDC guidelines [4] generally suggest using a higher level of respiratory protection than N95 disposable respirators for aerosol-generating procedures.
Patients themselves should wear mouth-nose protectors (surgical masks) in the presence of others, especially of hospital staff, and whenever outside the isolation room. A conventional surgical mask is not a protective mask. It has a higher level of leakage and provides less protection against inhalation of infectious aerosols than does an FFP breath mask. It does, however, block the exit of the larger droplets otherwise destined to become droplet nuclei. As air filtration is not required, it thus appears to be sufficient for use by infectious patients as a barrier to catch their respiratory droplets. Nevertheless, the respiratory protection guidelines [9] of the German Central Committee against Tuberculosis (DZK) suggest that patients suffering from any case of drug-resistant TB should wear FFP2 masks. Given that drug-resistant M. tuberculosis strains are not any more contagious than susceptible strains, (see below) this constraint seems contradictory. Indeed, quantitative testing of the efficacy of surgical face masks when worn by patients with MDR-TB has demonstrated, in the meantime, that simple surgical masks, rather than the much more expensive N95 masks, may sufficiently reduce the extent to which MDR-TB patients emit infectious particles [10].
Fitting an FFP2 mask correctly requires training. As laboratory studies indicate that re-aerosolisation of viable mycobacteria from filter material is not probable under normal conditions [4], respirators can be used by HCWs until they have accumulated excessive moisture. Therefore, a disposable respirator can remain functional for weeks and months and be reused by the same HCW over a working shift for different patients. When deemed appropriate, it can be disposed of as normal hospital waste. To facilitate breathing, FFP masks are also offered with exhalation valves. These, however, may only be used by personnel and visitors for self-protection. Where a sterile filter is required in the course of surgical procedures, respirators with exhalation valves are not recommended because they do not protect a sterile field. Meanwhile, an FFP3 mask (3M 1883+) with two-way respiratory protection is available; it is also suitable for use in operating rooms. This novel device is not, however, mentioned in any of the current guidelines.
When dealing with a person suspected of having or known to have MDR-TB, the guidelines for the USA, the UK and Germany consistently suggest the wearing of FFP3 masks [3–5]. In the WHO guidelines, however, the class of masks to be used is not defined [6, 7].
Surface disinfection
The daily disinfection of the surfaces in a TB patient´s isolation room requires mycobactericidal active agents. The German guidelines prescribe those listed by the German VAH-list [11]; in the US guidelines [4, 12], reference is made to disinfectants registered by the US Environmental Protection Agency. Once sedimented, mycobacteria do not return to the aerosol state (which is required for infection to take place). Hence, there is general consensus that the general cleaning procedures used throughout healthcare setting are appropriate, too, for TB isolation rooms. In the CDC guidelines for Disinfection and Sterilisation in Healthcare Facilities [12], it is pointed out that most products registered by the US Environmental Protection Agency for use against hepatitis B virus, HIV or M. tuberculosis specify a contact time of 10 min, but that significant reduction of the microbes is achieved with contact times of 30–60 s.
Notably, concrete instructions as to how disinfection should be implemented in functional areas under high time pressure (e.g. in endoscopy rooms), are completely lacking in all current guidelines.
According to the guidelines of the German Committee for Hospital Hygiene and Infection Control of the Robert Koch Institute [13] a routinely disinfected area (“non-targeted disinfection”) can be used again once the surface has dried post-application. This eliminates any requirement of delaying examination of subsequent patients, a practice often undertaken for fear of non-compliance with the exposure time required for the disinfectants recommended. Only in cases of visible contamination of a surface (e.g. by expectorated ichor), whereby in bronchoscopy relatively small areas are concerned, attention must be given to the disinfectant's exposure time as specified by the manufacturer (“targeted disinfection”). In cases of visible contamination, a rapid ready-to-use disinfectant solution for alcohol-resistant surfaces and medical instruments can be used, whereby exposure time between 15 s and 5 min is deemed sufficient.
Also, in Germany when discharging the patient with infectious TB from the hospital ward, a final anti-mycobacterial disinfection procedure of the room must be carried out. The German guidelines refer here to the VAH listing [11]. Special attention must be given to the disinfectant's exposure time as specified by the manufacturer, as higher concentrations of completely dried up expectorates are common and must be dissolved as part of the procedure.
Ventilation during isolation of the infectious TB patient
The principal goals of hospital isolation suites are to protect members of the hospital community from an infectious patient and to ensure sufficient air exchange with control of airflow direction. This is achieved by the dilution or elimination of infectious M. tuberculosis droplets, by means of sufficient ventilation, from the contaminated air, which prevents retransmission of the aerosols into other areas of the healthcare facility. Generally, the guidelines all recommend a negative pressure isolation room for sputum smear-positive patients, whereby the NICE guidelines [5] limit the recommendation as being required at least for patients suspected of having MDR-TB.
All pertinent guidelines stipulate that air should flow from corridors (cleaner areas) into the isolation rooms (contaminated areas). The air must then be directly discharged from the room to the outside of the building or passed through a high efficiency particulate air (HEPA) filter before being returned to circulation inside the facility. Negative pressure rooms are also known as “airborne infection isolation rooms” (AIIR) or “infectious isolation” facilities. If an AIIR has an anteroom, the anteroom should have either positive pressure compared to both the corridor and the patient´s room (to be achieved using filtered supply air), so preventing the escape of contaminants from the AIIR into the corridor, or negative pressure compared to both the corridor and the room. Ventilation rates are measured by air changes per hour (ACH). This is calculated by dividing room ventilation rate (m3·h−1) by the room volume (in m3). According to the CDC guidelines [4] and the WHO recommendations [6, 7], for existing isolation rooms, an air change rate to 12 ACH (e.g. equivalent to >80 L·s−1 for a 4×2×3 m3 room) is recommended where feasible, but a minimum of 6 ACH is considered acceptable (see table 1).
TABLE 1.
Air changes per hour (ACH) and removal efficiencies of Mycobacterium tuberculosis droplets
| ACH | Time required for removal efficiency min | |
| 99% | 99.9% | |
| 2 | 138 | 207 |
| 4 | 69 | 104 |
| 6 | 46 | 69 |
| 12 | 23 | 35 |
| 20 | 14 | 21 |
Reproduced and modified from [4].
As the installation of room ventilation systems is expensive, the German recommendations [3], as well as the WHO guidelines, explicitly offer as alternative the possibility of simply using natural window ventilation on the opposite sides of the room. In this case, fans can be used to achieve the intended flow of air but cannot replace frequent manual ventilation measures. In a Canadian study [14] including 17 hospitals, it was demonstrated that for non-isolation rooms, ventilation rates lower than 2 ACH were associated with grossly elevated (threefold) tuberculin skin test (TST) conversion rates among HCWs (adjusted hazard ratio, 3.4; 95% CI 2.1–5.8). Therefore, with natural ventilation, air turnover of two times per hour must be maintained. The door to the patient's room must always be kept closed to prevent spreading of infectious aerosols to surrounding areas.
Disinfection of room air with ultraviolet light
Environmental controls are aimed at reducing the concentration of infectious droplet nuclei in the air. This may be achieved not only by using special ventilation systems to maximise airflow rates or filtration, but also by using germicidal ultraviolet air-cleaning technologies. Ultraviolet germicidal irradiation (UVGI) is a disinfection method that uses short-wavelength ultraviolet (UV-C) light to kill or inactivate airborne M. tuberculosis by destroying its DNA. Wavelengths of between about 200 nm and 300 nm are strongly absorbed by nucleic acids. Typically, a wavelength of 254 nm will be used in germicidal UV lamps. UVGI lamps can be placed in exhaust ducts, in upper-air irradiation systems, or in portable room air recirculation systems [4].
Upper-room germicidal ultraviolet (GUV) systems, as recommended by the WHO [6, 7] and the CDC [4], require an effective exchange of air between the upper and lower parts of a room (i.e. vertical air movement) and transport of the infectious microorganisms to the upper part of the room (achieved by using simple fans to facilitate air movement in a room). GUV effectiveness is highly variable, and falls sharply when relative humidity exceeds 50–60% [15].
Consequently, the implementation of GUV should be considered as part of a package than as a single intervention. This circumstance, however, has hampered the establishment of evidence as to the contribution of GUV systems, so very few retrospective studies are available, which is the reason why the German guidelines [3] do not support the WHO's recommendations. In the Cook County Hospital, an inner-city facility in Chicago, USA, 36 rooms throughout the hospital (19 different wards and areas) were retrofitted in 1992 with exhaust fans to create negative pressure and 6 or more ACH. A year later, for additional protection, UV lights were placed in the same rooms and in some hallways [16]. The number of TST conversions in the hospital employees decreased significantly from January 1994 through December 2002 (from 98 of 2221 to 6 of 2108, respectively, p<0.001).
In a tertiary hospital in Thailand [17], the TB laboratory unit was equipped with a class II safety cabinet equipped for GUV irradiation in 1995, but it remains unclear whether the routinely powered air-purifying respirators with HEPA filters worn by the 38 staff members or the GUV irradiation ultimately led to the decrease in TST conversions from 1995 to 1996 (from 28.1 to 13.1 per 100 person-years).
In a third study [18], UVGI boxes placed 8 feet above the floor in patients’ rooms in New York St. Clare's Hospital and Health Center as well as HEPA filter respirators were introduced between 1991 and 1993. The TST conversion rate among employees fell from 20.7% in the first 6 months of 1991 to 5.8% in the latter half of 1993. It remains unclear, as to which type of protection should get the credit for that impressive success.
Isolation and removal of isolation
M. tuberculosis is carried in airborne particles, called droplet nuclei, of 1–5 μm in diameter. The infectiousness of a TB patient is directly related to the number of droplet nuclei the patient expels into the air. Depending on the airflow in the environment, these tiny particles can remain suspended in the air for several hours until they are removed by natural or mechanical ventilation. Although theoretically one single droplet is sufficient to produce an M. tuberculosis infection, in praxi several thousands of mycobacteria, inhaled simultaneously (e.g. from the cough of a sputum smear-positive index case or accumulated over a longer time period), are required to establish a pulmonary infection [19].
A minimum of 2 weeks of isolation for smear-positive TB patients under effective first-line treatment is suggested in most guidelines. All refer to the historical publication by Rouillon et al. [20] which (based on the results of contact investigations) explicitly stated that hospital isolation to prevent transmission is usually not justified beyond 2 weeks. Furthermore, wearing of masks, according to Rouillon, is only required “for a few days at the beginning of treatment”, given that patients received an appropriate combination of anti-tuberculous drugs.
Indeed, early data from Jindani et al. [21] suggest that infectiousness appears to decline rapidly after the start of therapy. In his laboratory work, sputum counts from TB patients treated with isoniazid, a rifampin, and pyrazinamide fall by about 20-fold in the first 2 days and by a further 200-fold in the next 12 days. The counts of initially smear-positive patients were reduced to about 103 per mL of sputum at 2 weeks, a level below the estimates of 103–104 per mL, which are the limits indicating a change from smear-positive to smear-negative in culture-positive, untreated patients. However, in contrast to the former assumptions, fingerprinting studies have proven that untreated smear-negative patients, in whose sputum a number slightly below 103 mycobacteria per mL may be present, can also be infectious, though at a rate five times lower than that of smear-positive patients [22].
Sputum smear-positive TB patients
Thus, because microscopy results are not quite as black and white as they perhaps might appear, additional criteria are included in the guidelines for considering discontinuation of isolation.
According to CDC guidelines [4], an initially smear-positive TB patient should be considered infectious until the person has been treated for a minimum of 2 weeks and has had three negative sputum smear results obtained 8–24 h apart, with at least one being an early morning specimen, with a progressively decreasing quantity of mycobacteria on each smear result. Furthermore, the TB infection must not show resistance to one of the first-line drugs, the patient should have a substantial clinical response (i.e. reduction in cough or resolution of fever), and the final de-isolation decision must be made by a well-experienced physician.
According to NICE guidelines [5], sputum smear negativity after at least a 2-week treatment is the first criterion, followed by resolution of cough, absence of extensive pulmonary involvement, and only a low initial smear grade (2 or less). The German guidelines on infection prevention of the DZK [3] and those of the Committee of Hospital Hygiene and Infection Control [23], however, even go beyond the NICE guidelines and require treatment of at least 21 days, given clinical and radiographic response, but do not provide any evidence of the necessity for that prolonged period if isolation (see table 2).
TABLE 2.
Comparison of guidelines on selected preventive measures for protection against Mycobacterium tuberculosis in hospitals separated by topics
| Guidelines/recommendations | WHO [6, 7] | USA [4, 12, 24, 26] | UK [5, 8, 25] | Germany [3, 9, 13, 23] |
| Spatial requirements | ||||
| NPI rooms | Yes | Yes | Yes | Yes |
| Normal ventilation room allowed | Yes | Yes, if air-cleaning technologies (e.g. a portable HEPA filtration system) are available | Yes, single rooms that are vented to the outside of the building | Yes, single rooms that are vented to the outside of the building |
| Frequency of air exchange in NPI rooms | Minimum 12 per h | Prefer ≥12 per h (minimum ≥6 per h) | “Adequate ventilation” | Optionally 12 per h |
| Frequency of air exchange in normal ventilation rooms | Not addressed | Minimum 2 per h | Not addressed | Not addressed |
| Personal protection | ||||
| Minimum standard of masks | N95 or FFP2 | N95 or FFP2 | FFP2 | FFP2 |
| Masks when encountering MDR-TB patients |
FFP3 not addressed | >N95 | FFP3 | FFP3 |
| Masks during aerosol-generating procedures |
FFP2 | >N95 | FFP2 [5], FFP3 [8] | FFP2 [3] or at least FFP 2 [23] |
| Removal from isolation | ||||
| Sputum smear-positive patients | Discussed, but no recommendation provided | Minimum 2-week treatment, progressively decreasing M. tuberculosis load, then 3 microscopy-negative sputum smear-results | Only after at least 2-week treatment, and given a low initial smear (grade 2 or less), then 3 negative sputum smear results | After 3-week treatment [23] or after 3-week treatment and 3 microscopy-negative sputum smear results [3], in each case dependent on clinical and radiographic improvement |
| Sputum smear-negative patients | Not addressed | After two negative results of the Xpert MTB/RIF test | No isolation | After 3 microscopy- negative sputum results, depending on further medical consideration [3] or after 3-week treatment and clinical and radiographic improvement [23] |
| MDR-TB patients | Not addressed (isolation only for culture-positive XDR-TB patients) | After at least 1 negative culture | After 3 microscopy-negative smears at weekly intervals and ideally 1 negative culture | After 3 microscopy-negative smears, possibly after 1 negative culture [3] or definitely after 1 negative culture [23] |
| Screening for LTBI | ||||
| Serial testing of healthcare workers | Not addressed | At the discretion of healthcare facilities; routine serial testing not recommended | Not addressed (BCG vaccination instead?) | Yes, required by law (Ordinance of Occupational Health Care) in risk-prone healthcare facilities |
| Disinfection | ||||
| Upper-air or in-duct UV disinfection (UVGI) |
Yes | Yes, but not in lieu of ventilation | Not addressed | Noted as general option, but not recommended |
WHO: World Health Organization; NPI: negative pressure isolation; HEPA: high efficiency particulate air; FFP: filtering face piece; MDR: multidrug-resistant; XDR: extensively drug-resistant; TB: tuberculosis; RIF: rifampicin; XDR: extensively drug resistant; LTBI: latent TB infection; BCG: bacille Calmette–Guérin; UVGI: ultraviolet germicidal irradiation.
Patients with initially negative sputum smear status
No direct information on the isolation of sputum smear-negative cases is provided in the NICE guidelines [5] nor in those of the CDC [4]. However, another CDC document [24] explicitly establishes the absence of acid-fast bacilli on sputum smear in microscopy as the indicator of a non-infectious state. Also, the previous British Thoracic Society (pre-NICE) recommendations, published in 2000, declare that TB patients with three negative sputum samples on separate days (or the absence of acid-fast bacilli in bronchoscopy and lavage) require no isolation [25].
In Germany, the recommendations are different. The recommendations of the Committee of Hospital Hygiene and Infection Control [23] do not differentiate between initially smear-positive and smear-negative TB patients, and require an isolation period of 3 weeks under appropriate treatment also for smear-negative TB cases. In contrast, according to the more detailed recommendations of the German Central Committee Against Tuberculosis [3], TB patients with initially three negative sputum smears in microscopy must not automatically be kept in long-term isolation, but are subject to further medical consideration. This includes the findings of imaging (presence of caverns), cough symptoms, existing secondary cases, the possible presence of MDR pathogens (especially in patients coming from countries of the former Soviet Union) and PCR results. As the limit of detection in sputum smear microscopy of 103–104 mycobacteria offers a false sense of security, PCR in particular is key with respect to the question of whether sputum smear-negative patients should be isolated or not [3].
In the USA, a recent consensus statement of the National TB Controllers Association and the Association of Public Health Laboratories suggests that patients with suspected pulmonary TB could be removed from airborne infection isolation units after two negative results of the Xpert MTB/RIF test, which simultaneously detects rifampicin resistance as a surrogate parameter of MDR-TB and has a reported limit of detection in sputum of 131 cell forming units per mL [26].
This guidance was based on an in-house clinical validation study that demonstrated negative predictive values of 99.7% for a single negative acid-fast bacilli smear and 100% for two consecutive negative Xpert MTB/RIF results [27].
Thus, unless the other factors listed above indicate otherwise, sputum smear-negative TB suspects with 1–2 negative PCR results may immediately be removed from isolation. Although the Xpert MTB/RIF and its successor, the even more sensitive Xpert MTB/RIF Ultra have been recommended as diagnostic tools for detecting MTB especially in smear-negative, culture-positive specimens by the WHO [28], that option is presented only in the National TB Controllers Association consensus statement. Therefore, the future role of the Xpert and other highly sensitive, most recently WHO-endorsed PCR tests [29] for removing a patient from isolation must urgently be established in official country guidelines.
MDR-TB
MDR-TB, defined as tuberculosis disease caused by a M. tuberculosis strain that is resistant at least to the two most effective TB drugs, isoniazid and rifampin, is generally not more virulent or more infectious than other forms of TB. A recently published 3 year-prospective cohort (household follow-up) study in South Lima and Callao, Peru [30], compared the TB incidence in 1055 household contacts of 213 MDR-TB index cases and 2362 household contacts of 487 drug-susceptible index cases. It showed that MDR-TB was only about half as transmissible to household contacts as was susceptible TB, suggesting that the fitness of the resistant TB strain is even less than that of susceptible TB strains. In addition, although a recently published long-term fingerprinting study documented that recent transmission was found to be strongly associated with healthcare work in a low-TB-incidence metropole, MDR-TB was not transmitted more frequently than susceptible TB strains [2].
Objectively, transmission prevention of MDR-TB should involve the same isolation criteria as for drug-susceptible patients, but the consequences of acquiring MDR-TB are much more serious because of the complexities and duration of the required treatment regimens. Significantly, the current recommendations only slightly different are on paper, but in practice they are extremely different. The German guidelines of the Central Committee Against Tuberculosis [3] recommend airborne precautions until MDR-TB patients have produced three microscopy-negative smears, or until a negative culture has been documented. The CDC guidelines [4] call for a negative culture regardless of sputum smear results; the NICE guidelines [5] recommend isolation of MDR-TB patients until they have three negative smears at weekly intervals and ideally have a negative culture. The alternative German guidelines (i.e. those of Committee of Hospital Hygiene and Infection Control) [23], definitely call for a negative culture. The WHO guidelines do not cover this important issue.
Indeed, there is evidence that a decreasing bacterial load in MDR-TB patients cannot be used in the same way as is done for evaluation of a treatment of fully susceptible TB. In Fitzwater's study on prolonged infectiousness of TB patients [31] one third of MDR-TB patients (as identified by drug susceptibility testing later on) had turned to sputum smear negativity at day 60, although they had received an ineffective short course treatment for fully susceptible TB and were therefore culture-positive at this time point. Accordingly, the authors concluded that persistent smear positivity at day 60 is only a poor predictor of MDR-TB and not a good surrogate for drug susceptibility testing. Thus, waiting for at least one culture conversion from positive to negative before relaxing isolation appears to be an indispensable criterion for judging infectivity in MDR-TB treatment.
Periodic testing of HCWs for latent TB infection
Although most interventions aimed at preventing MTB transmission in healthcare facilities focus on known or suspected TB patients under effective treatment or on their close contacts, unsuspected and/or undiagnosed and therefore initially untreated patients are the primary source for transmissions to HCWs [4, 32]. Serial testing, annually or in perennial intervals, intends to detect such transmissions and has been considered the method of choice in occupational health programs for decades [33].
Historically, HCWs have been screened for latent TB infection (LTBI) with the TST, and preventive therapy has been provided for those scored positive. In the meantime, interferon-γ release assays (IGRAs) have been implemented as an alternative to the TST; the two currently commercially available assays are the QuantiFERON-TB Gold In-Tube (QFT, Qiagen, Hilden, Germany) and T-SPOT.TB (Oxford Immunotec, Oxfordshire, UK). IGRAs offers several advantages over the TST, in particular improved specificity and that only a single visit to draw blood for in vitro processing is required. A positive IGRA result, however, does not always indicate LTBI; the prevalence of LTBI in the tested collective must be carefully considered in test interpretation. As with TST, the interpretation of whether a positive IGRA result also represents an infection with M. tuberculosis depends on its positive predictive value (PPV), an a priori condition, is derived from test-specific properties (sensitivity and specificity of the IGRAs), and very importantly, on the proportion (prevalence, pre-test probability) of latent M. tuberculosis infection in the investigated collective. PPV is calculated as follows:
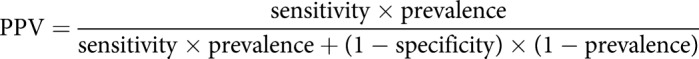 |
Therefore, chemoprevention based on a positive IGRA finding should be considered foremost in situations in which the likelihood of the test subject being infected is high enough to generate a high PPV. The recommendations of the DZK on environmental investigations [19] and those of the NICE guidelines [5] increase this probability through the careful selection of subjects for testing. Intense contact with the index patient (i.e. a cumulative contact of at least 8 h duration or during short episodes of unprotected, direct coughing by microscopically sputum smear-positive patients, or a cumulative contact duration of at least 40 h in microscopically negative, but culturally positive patients), is required before any contact testing is considered. The reliability of these time constraints was confirmed in an analysis of the results of the IGRA tests on 812 contact persons [34].
With a sensitivity and specificity of 84.5% and 99%, respectively, for the IGRA QFT [35], a prevalence of infection of 20%, which is realistic as an infection probability in close contacts of infectious TB patients, results in a high PPV of 95.5%. That means that only about 4.5% of the contact persons testing positive actually have no LTBI. However, with a prevalence reduced to 5%, the PPV falls to 81.6% and therefore will falsely classify nearly 20% of the persons examined as being infected.
Where active TB is rare, there is not a high risk of M. tuberculosis infection in general for all healthcare activities. It is then not advisable to regularly check all HCWs for LTBI because of the generally low PPV of the tests in such low-risks situations. In Germany, it can be assumed that those who start a new activity in the healthcare sector are very seldom infected, so that in absence of individual risk factors for LTBI there is no indication for assessing a “zero value”. In a recent study, QFT was rarely positive for nursing students (2%) and those who were positive had risk factors for LTBI (family TB, migration from a high prevalence country) [36].
Further, serial testing of the same employee over a longer period of time during their hospital career may lead to a variability of the test results (conversions and reversions of the respective previous negative or positive result) with both screening methods, TST and IGRAs. Using a higher cut-off for scoring a TST or IGRA result as positive, or alternatively confirming positive results with a second (positive) test and consider the initially positive test falsely-positive if the second test shows a negative result (assuming a negligible correlation between those tests) may reduce the number of false-positive results, but at the consequence of detecting fewer people with true infection.
Given the uncertainty of how to deal with such variabilities, current guidelines each address the topic of HCW screening very differently:
The WHO guidelines on managing LTBI [37] initially consider systematic testing (and treatment) of HCWs for LTBI in countries with a low TB incidence. In its final recommendation, however, HCWs are not identified as specific group at risk for progression to active TB.
To evaluate the outcome of serial testing in the USA, a working group of the US National Tuberculosis Advisory Committee and CDC conducted a systematic review of literature published from January 2006 through November 2017. It reported prevalence rates for LTBI, rates of conversion or reversion of TB test results and TB transmission rates among healthcare workers in high-income countries, in which the incidence of TB was low. The pooled data coming from the 39 included studies (the majority of which came from the USA), demonstrated that approximately 3% of US HCWs scored positive for M. tuberculosis at baseline when tested with TST and 5% scored positive when tested with an IGRA. Over time, negative baseline TST results converted from negative to positive on serial testing in less than 1% of this population, whilst with the IGRA the conversion rate was 4%. Surprisingly, with the TST approximately 62% of those who were scored positive at baseline subsequently tested negative on serial testing, whilst with an IGRA the reversion rate was only 48%. Furthermore, no HCWs in the studies developed active TB.
As a consequence of that review the CDC very recently updated its guidelines. While in the 2005 CDC guidelines [4] serial testing was still recommended for HCWs with a “medium” risk (e.g. in TB clinics), American HCWs testing negative for LTBI in an entry screening are no longer recommended to undergo routine serial TB screening or testing at any interval after baseline. Indeed, under the latest guidelines, healthcare facilities themselves should define groups who may be at increased occupational risk for TB exposure (e.g. pulmonologists, respiratory therapists, or employees of emergency departments where previous annual testing has revealed ongoing transmission) and consider serial TB screening in the same manner as before either using TST or IGRAs, as deemed appropriate [38].
In the UK, where there has been a year-on-year decline in the number and incidence of TB cases between 2011 and 2015, down to an incidence of 9.6 per 100 000 (6240 cases) [39], serial testing for HCWs exposed to TB patients is not foreseen at all. Instead, employees new to the UK National Health Service who will be working with patients or clinical specimens may not start work until they have completed a TB screen or health check. Here, according to current cost–benefit models, HCWs or laboratory worker who are previously unvaccinated, and have no known LTBI (TST- or IGRA-negative) and are not new entrants from high-incidence countries, a two-step test (TST positive, followed by an IGRA, if positive), should be offered. If the interferon-γ release assay is positive, active TB has to be excluded and, if this assessment is negative, treatment for LTBI should be performed. One-step IGRA testing is preferred where a new HCW may have had contact with patients in settings where TB is highly prevalent.
Importantly, bacille Calmette–Guérin (BCG) vaccination will be offered to all new and previously unvaccinated and TST- (or IGRA)-negative healthcare or laboratory workers at occupational risk through direct clinical contact with patient diagnosed with TB, or those in contact with infectious TB materials. It is explicitly stated that if the person still declines BCG vaccination, he or she should not work where there is a risk of exposure to TB.
In Germany, according to the Ordinance on Occupational Health Prevention (ArbMedVV [40]), serial testing is mandatory for HCWs in TB wards or other hospital departments where regular contact with TB patients can be expected, and for laboratory employees working with infectious samples. Based on the results of a recently published study on conversions and reversions in German HCWs [41], the Statutory Accident Insurance and Prevention in the Health and Welfare Services recommends, a “grey area” for the QFT test (i.e. of 0.4 to <0.7 IU·mL−1) instead of a dichotomic yes or no result at 0.35 IU·mL−1. This grey area would be helpful in avoiding unnecessary radiography and preventive chemotherapy by repeating the IGRA test in IGRA-positive employees whose values fall in that range. In the case of a reversion in the second test, no further procedures would be required. However, multicentric long-term studies would be helpful in order to evaluate the impact of such a “grey area” for LTBI management.
Conclusions
In conclusion, the current recommendations of the WHO, USA, UK and Germany on protecting HCWs from M. tuberculosis transmission in healthcare facilities show considerable practical discrepancies (see also the summary overview in table 2). To the extent it is feasible, harmonisation and practical amendments of such guidelines is most desirable, as such discrepancies across low-incidence countries may provoke uncertainty in daily practice and ultimately impede the implementation of the guidelines. First of all, consensus should be achieved on the types of masks that should be worn in different situations. The importance of UVGI has still to be evaluated in better, well-designed studies. Rules for removing sputum smear-negative and/or MDR pulmonary TB patients from isolation rooms should be standardised, with particular attention to the future role of the Xpert MTB/RIF Ultra and/or other new diagnostic tests. Finally, the impact of establishing a “grey zone” for scoring an IGRA result as positive in German serial testing of HCWs may be evaluated in long-term studies. With harmonised, more evidence-based international guidelines at hand, the credibility of local guidelines will be easier to establish and success in implementation of these more likely.
Footnotes
Conflict of interest: R. Diel reports personal fees for lectures and consulting from Cepheid outside the submitted work.
Conflict of interest: A. Nienhaus has nothing to disclose.
Conflict of interest: P. Witte has nothing to disclose.
Conflict of interest: R. Ziegler has nothing to disclose.
References
- 1.World Health Organization. Global TB Report 2018. Geneva, WHO, 2019. [Google Scholar]
- 2.Diel R, Niemann S, Nienhaus A. Risk of tuberculosis transmission among healthcare workers. ERJ Open Res 2018; 4: 00161-2017. doi: 10.1183/23120541.00161-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler R, Just HM, Castell S, et al. Tuberculosis infection control - recommendations of the DZK. Gesundheitswesen 2012; 74: 337–350. doi: 10.1055/s-0032-1306680 [DOI] [PubMed] [Google Scholar]
- 4.Jensen PA, Lambert LA, Iademarco MF, et al. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 2005; 54: 1–141. [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Tuberculosis: Prevention, Diagnosis, Management and Service Organisation (NICE Guideline 33). www.nice.org.uk/guidance/ng33 Date last accessed: August 25, 2019. Date last updated: 2016. [PubMed]
- 6.World Health Organization. WHO policy on TB infection controlling health-care facilities, congregate settings and households. Geneva, WHO, 2009. [PubMed] [Google Scholar]
- 7.World Health Organization. WHO guidelines on tuberculosis infection prevention and control, 2019 update. Geneva, WHO, 2019. [PubMed] [Google Scholar]
- 8.Coia JE, Ritchie L, Adisesh A, et al. Healthcare Infection Society Working Group on Respiratory and Facial Protection. Guidance on the use of respiratory and facial protection equipment. J Hosp Infect 2013; 85: 170–182. doi: 10.1016/j.jhin.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaberg T, Hauer B, Loddenkemper R, et al. German Central Committee for the control of tuberculosis. Recommendations for personal respiratory protection in tuberculosis. Pneumologie 2004; 58: 92–102. doi: 10.1055/s-2003-812527 [DOI] [PubMed] [Google Scholar]
- 10.Dharmadhikari AS, Mphahlele M, Stoltz A, et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward. Am J Respir Crit Care Med 2012; 185: 1104–1109. doi: 10.1164/rccm.201107-1190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desinfektionsmittel-Kommission im Verbund für Angewandte Hygiene [Disinfectants Commission in the Association for Applied Hygiene] e.V. (VAH). Desinfektionsmittel-Liste des VAH. Wiesbaden: Mhp-Verlag GmbH; 2011 https://vah-online.de/en/vah-list Date last accessed: September 3, 2019.
- 12.Guideline for Disinfection and Sterilization in Healthcare Facilities (2008). Edits and Changes (February 2017) www.cdc.gov/infectioncontrol/guidelines/disinfection/updates.html Date last accessed: August 25, 2019.
- 13.[Responsibilities of public health in cleaning and disinfection of surfaces. Recommendation by the Committee of Hospital Hygiene and Infection Control by the Robert Koch Institute]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2004; 47: 51–61. doi: 10.1007/s00103-003-0752-9 [DOI] [PubMed] [Google Scholar]
- 14.Menzies D, Fanning A, Yuan L, et al. Hospital ventilation and risk for tuberculous infection in Canadian healthcare workers. Canadian Collaborative Group in Nosocomial Transmission of TB. Ann Intern Med 2000; 133: 779–789. doi: 10.7326/0003-4819-133-10-200011210-00010 [DOI] [PubMed] [Google Scholar]
- 15.Xu P, Kujundzic E, Peccia J, et al. Impact of environmental factors on efficacy of upper-room air ultraviolet germicidal irradiation for inactivating airborne mycobacteria. Environ Sci Tech 2005; 39: 9656–9664. doi: 10.1021/es0504892 [DOI] [PubMed] [Google Scholar]
- 16.Welbel SF, French AL, Bush P, et al. Protecting health care workers from tuberculosis: a 10-year experience. Am J Infect Control 2009; 37: 668–673. doi: 10.1016/j.ajic.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 17.Yanai H, Limpakarnjanarat K, Uthaivoravit W, et al. Risk of Mycobacterium tuberculosis infection and disease among health care workers, Chiang Rai, Thailand. Int J Tuberc Lung Dis 2003; 7: 36–45. [PubMed] [Google Scholar]
- 18.Fella P, Rivera P, Hale M, et al. Dramatic decrease in tuberculin skin test conversion rate among employees at a hospital in New York City. Am J Infect Control 1995; 23: 352–356. doi: 10.1016/0196-6553(95)90265-1 [DOI] [PubMed] [Google Scholar]
- 19.Diel R, Loytved G, Nienhaus A, et al. [New recommendations for contact tracing in tuberculosis. German Central Committee Against Tuberculosis]. Pneumologie 2011; 65: 359–378. doi: 10.1055/s-0030-1256439 [DOI] [PubMed] [Google Scholar]
- 20.Rouillon A, Perdrizet S, Parrot R. Transmission of tubercle bacilli: The effects of chemotherapy. Tubercle 1976; 57: 275–299. doi: 10.1016/S0041-3879(76)80006-2 [DOI] [PubMed] [Google Scholar]
- 21.Jindani A, Aber VR, Edwards EA, et al. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 1980; 121: 939–949. [DOI] [PubMed] [Google Scholar]
- 22.Behr MA, Hopewell PC, Paz EA, et al. Predictive value of contact investigation for identifying recent transmission of Mycobacterium tuberculosis. Am J Respir Crit Care Med 1998; 158: 465–469. doi: 10.1164/ajrccm.158.2.9801062 [DOI] [PubMed] [Google Scholar]
- 23.Ruscher C. Infektionsprävention im Rahmen der Pflege und Behandlung von Patienten mit übertragbaren Krankheiten. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO) beim Robert Koch-Institut. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2015; 58: 1151–1170. doi: 10.1007/s00103-015-2234-2 [DOI] [PubMed] [Google Scholar]
- 24.CDC. Chapter 7 Tuberculosis Infection Control https://www.cdc.gov/tb/education/corecurr/pdf/chapter7.pdf Date last accessed: August 25, 2019.
- 25.Control and prevention of tuberculosis in the United Kingdom: code of practice 2000. Joint Tuberculosis Committee of the British Thoracic Society. Thorax 2000; 55: 887–901. doi: 10.1136/thorax.55.11.887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NTCA. NTCA/APHL Consensus Statement on the Use of Cepheid Xpert MTB/RIF Assay in Making Decisions to Discontinue Airborne Infection Isolation in Healthcare Settings www.tbcontrollers.org/docs/resources/NTCA_APHL_GeneXpert_Consensus_Statement_Final.pdf Date last accessed: August 25, 2019.
- 27.Rice JP, Seifert M, Moser KS, et al. Performance of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis and rifampin resistance in a low-incidence, high resource setting. PLoS ONE 2017; 12: e0186139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Word Health Organization. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. Geneva, WHO, 2017. [Google Scholar]
- 29.Word Health Organization . Meeting report of a technical expert consultation: accuracy of centralized assays for TB detection and detection of resistance to rifampicin and isoniazid. Geneva, WHO, 2019. [Google Scholar]
- 30.Grandjean L, Gilman RH, Martin L, et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a prospective cohort study. PLoS Med 2015; 12: e1001843. doi: 10.1371/journal.pmed.1001843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzwater SP, Caviedes L, Gilman RH, et al. Prolonged infectiousness of tuberculosis patients in a directly observed therapy short-course program with standardized therapy. Clin Infect Dis 2010; 51: 371–378. doi: 10.1086/655127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seidler A, Nienhaus A, Diel R. Review of epidemiological studies on the occupational risk of tuberculosis in low-incidence areas. Respiration 2005; 72: 431–446. doi: 10.1159/000086261 [DOI] [PubMed] [Google Scholar]
- 33.Loddenkemper R, Diel R, Nienhaus A. To repeat or not to repeat-that is the question! Serial testing of health-care workers for TB infection. Chest 2012; 142: 10–11. doi: 10.1378/chest.12-0045 [DOI] [PubMed] [Google Scholar]
- 34.Diel R, Loddenkemper R, Meywald-Walter K, et al. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube assay, and T-Spot.TB test in contact investigations for tuberculosis. Chest 2009; 135: 1010–1018. doi: 10.1378/chest.08-2048 [DOI] [PubMed] [Google Scholar]
- 35.Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a meta-analysis. Chest 2010; 137: 952–968. doi: 10.1378/chest.09-2350 [DOI] [PubMed] [Google Scholar]
- 36.Schablon A, Peters C, Diel R, et al. Serial IGRA testing of trainees in the healthcare sector in a country with low incidence for tuberculosis - a prospective cohort study. GMS Hyg Infect Control 2013; 8: Doc17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organisation. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva, WHO, 2018. [PubMed] [Google Scholar]
- 38.Sosa LE, Njie GJ, Lobato MN, et al. Tuberculosis screening, testing, and treatment of US health care personnel: recommendations from the National Tuberculosis Controllers Association and CDC, 2019. MMWR Morb Mortal Wkly Rep 2019; 68: 439–443. doi: 10.15585/mmwr.mm6819a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Public Health England. Tuberculosis: The Green Book, Chapter 32 www.gov.uk/government/publications/tuberculosis-the-green-book-chapter-32 Date last accessed: August 22, 2019.
- 40.German Federal Ministry of Labour and Social Affairs. Verordnung zur arbeitsmedizinischen Vorsorge (ArbMedVV) vom 18. Dezember 2008 (BGBl.I S.2768), zuletzt geändert durch Art.1 V v. 12.7.2019 I 1082.
- 41.Schablon A, Nienhaus A. Tuberkulose bei Beschäftigten im Gesundheitswesen. Arbeitsmed Sozialmed Umweltmed 2017; 52: 38–40. [Google Scholar]


