Abstract
Background
Fungal infections of the feet normally occur in the outermost layer of the skin (epidermis). The skin between the toes is a frequent site of infection which can cause pain and itchiness. Fungal infections of the nail (onychomycosis) can affect the entire nail plate.
Objectives
To assess the effects of topical treatments in successfully treating (rate of treatment failure) fungal infections of the skin of the feet and toenails and in preventing recurrence.
Search methods
We searched the Cochrane Skin Group Specialised Register (January 2005), the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 1, 2005), MEDLINE and EMBASE (from inception to January 2005). We screened the Science Citation Index, BIOSIS, CAB ‐ Health and Healthstar, CINAHL DARE, NHS Economic Evaluation Database and EconLit (March 2005). Bibliographies were searched.
Selection criteria
Randomised controlled trials (RCTs) using participants who had mycologically diagnosed fungal infections of the skin and nails of the foot.
Data collection and analysis
Two authors independently summarised the included trials and appraised their quality of reporting using a structured data extraction tool.
Main results
Of the 144 identified papers, 67 trials met the inclusion criteria. Placebo‐controlled trials yielded the following pooled risk ratios (RR) of treatment failure for skin infections: allylamines RR 0.33 (95% CI 0.24 to 0.44); azoles RR 0.30 (95% CI 0.20 to 0.45); ciclopiroxolamine RR 0.27 (95% CI 0.11 to 0.66); tolnaftate RR 0.19 (95% CI 0.08 to 0.44); butenafine RR 0.33 (95% CI 0.24 to 0.45); undecanoates RR 0.29 (95% CI 0.12 to 0.70). Meta‐analysis of 11 trials comparing allylamines and azoles showed a risk ratio of treatment failure RR 0.63 (95% CI 0.42 to 0.94) in favour of allylamines. Evidence for the management of topical treatments for infections of the toenails is sparser. There is some evidence that ciclopiroxolamine and butenafine are both effective but they both need to be applied daily for prolonged periods (at least one year). The six trials of nail infections provided evidence that topical ciclopiroxolamine has poor cure rates and that amorolfine might be substantially more effective but more research is required.
Authors' conclusions
Placebo‐controlled trials of allylamines and azoles for athlete's foot consistently produce much higher percentages of cure than placebo. Allylamines cure slightly more infections than azoles and are now available OTC. Further research into the effectiveness of antifungal agents for nail infections is required.
Plain language summary
Creams, lotions and gels (topical treatments) for fungal infections of the skin and nails of the foot
We found lots of evidence to show fungal skin infections of the skin of the feet (athlete's foot or tinea pedis) are effectively managed by over the counter topical antifungal creams, lotions and gels. The most effective topical agent was terbinafine. Other topical agents such as azoles, ciclopiroxolamine, butenafine, tolnaftate and undecanoate were also effective in curing athlete's foot.
Evidence for the management of topical treatments for management of dermatophyte infections of the toenails was sparser and the studies are small. There was some evidence that ciclopiroxolamine and butenafine are both effective but they both needed to be applied daily for prolonged periods (at least one year).
Background
Description of the condition
Dermatophyte is a collective term for the most common type of fungi which cause infection of the skin and nail. Dermatophytes have the ability to invade keratinised tissue (skin, hair and nails). Infection is normally restricted to the outermost layer of the skin (epidermis). The skin between the toes is a frequent site of fungal infection (athlete's foot or tinea pedis and this can cause pain and itchiness. The skin may become white and macerated and vesicles (small blisters) may form. These can erupt and spread to other areas of the foot especially the soles where the area becomes reddened and raw. Additionally, patches of hard thickened skin occur on the soles, heels and side of the feet. This can lead to splits (fissures) in the skin. Fungal infections of the nail (onychomycosis) can affect the entire nail plate, and one, several or all nails of the feet can be infected simultaneously. The nails often appear changed in colour; they may be thickened and changed in texture (Beaven & Brooks 1994). Fungal infections of the nail are often associated with a skin infection, in which case they can act as a source of reinfection if only the skin is treated (Petit 1983).
Epidemiology
Fungal infections of the skin and nails of the foot are common, reflecting the contagious nature of the organisms. They are thought to occur when individuals regularly use communal changing rooms and swimming pools. Some groups of workers, e.g. coal miners, have been found to have a prevalence of 80% (Roberts 1992). However, people living in institutions with shared bathing facilities such as boarding schools and long term care hospitals also show a higher than average prevalence of this condition (Roberts 1992). The prevalence of onychomycosis has been suggested to increase with age (Roberts 1992) and to be present at a rate of about 5% in people aged 55 years and older.
Description of the intervention
Clinicians faced with a public demand for effective treatment for these conditions face a difficult task as the conditions can be resistant to treatment (Brautigam et al 1995). Whilst these superficial infections are not life threatening, chronic fungal infections of the skin and nails carry a considerable morbidity.
There is wide variation in the methods of treating fungal infections of the skin and toe nails of the foot which reflects the uncertainty surrounding efficacy. Uncertainty also extends to the optimal period of treatment, appropriate dosage of drug and frequency of application. Topical preparations are much less costly than orally administered antifungal drugs and cause minimal adverse side effects. However, whilst they may be helpful in treating the symptoms in localised skin infections, uncertainty exists as to their effectiveness in the complete eradication of the infecting organisms. The ideal topical antifungal for the treatment of fungal infection should be fungicidal so that treatment can be of short duration, it should obtain high cure rates, minimise relapses, be conducive to participant compliance and have minimal adverse effects.
Why it is important to do this review
Fungal infections of the feet are treated by dermatologists, general practitioners and podiatrists. A systematic review of the various therapies used in the topical treatment of fungal infections affecting the skin and nails of the foot will help to inform the treatment approach of all these professionals.
Objectives
To identify and evaluate the evidence for topical treatments for fungal infections of the skin and nails of the foot. To establish the effectiveness of topical treatments used for fungal infections of the skin and nails compared with other treatments or untreated controls.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled studies of topical treatment for fungal infections of the skin and nails of the foot.
Types of participants
All men and women of any age who have a fungal infection of the skin or nails of human foot which has been identified by microscopy and growth of dermatophytes in culture.
Types of interventions
Any programme of treatments administered topically to treat fungal infections of the feet compared with other treatments, placebo or no treatments. All types of intervention were considered.
Types of outcome measures
Primary outcomes
1. For each trial we calculated the treatment failure rate at follow up from the reported mycological results (defined as negative results on microscopy and no growth of dermatophyte in culture). In the update of the review we have only included studies which subject all skin and nail samples to KOH (potassium hydroxide) and culture). 2. Quality of life as measured by the cosmetic acceptability of the end result to the participant, absence of itchiness, independence from medical treatment and advice with respect to the condition.
Secondary outcomes
1. Measurement of recurrence of the condition in: (a) skin ‐ maintenance of cure 12 weeks after initiation of intervention; (b) nail ‐ maintenance of cure 36 weeks after initiation of intervention. 2. Side effects as measured by the frequency of reported adverse events.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Skin Group Specialised Register (January 2005) search strategy in Appendix 1:
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2005) using the search strategy in Appendix 2
We searched MEDLINE (OVID) (from inception to January 2005) using the strategy in Appendix 3.
We searched EMBASE (from inception to January 2005) using the following keywords: athlete's foot, tinea pedis, topical treatment and onychomycosis.
Other databases
We searched the following databases in March 2005 using the term 'athlete's foot' in each: Science Citation Index and Social Science Citation Index within BIDS CAB‐Health and Healthstar The online versions of DARE NHS Economic Evaluation Database EconLit Online ARC version of CINAHL
Searching other resources
References from published studies
We searched the bibliographies of all the papers identified by these strategies.
Handsearching of journals
We handsearched podiatry journals which were not listed in the electronic databases, namely, The Foot, The Journal of British Podiatric Medicine and The Journal of the American Podiatry Association.
Unpublished literature
We searched for unpublished or unlisted studies by contacting all Schools of Podiatry in the UK and made a request for dissertation bibliographies. Where possible we established personal communication with corresponding trial authors of papers identified through the above strategy to enquire about other relevant studies. We contacted the pharmaceutical industry to request reports of further published and unpublished trials. We searched the Current Controlled Trials Register (on www.controlled‐trials.com) and www.clinicaltrials.gov in January 2005 for ongoing trials.
Language
We did not impose any language restrictions and sought translations where necessary.
Adverse Effects
We looked at the included RCTs for reports of adverse effects of the interventions. We have summarised our findings in the body of the review.
Data collection and analysis
Selection of studies
One author searched for trials (FC). Two authors checked titles and abstracts identified from the searches. One author (FC) obtained the full text of all studies of possible relevance for independent assessment. One author decided which trials fit the inclusion criteria and recorded their methodological quality using a structured data extraction tool. Any disagreement was resolved by discussion between the authors. Excluded studies and reasons for exclusion are stated.
Assessment of risk of bias in included studies
Assessment of methodological quality of included studies
Assessment of methodological quality was performed. The following areas were addressed, since these may be associated with biased estimates of treatment effect (Juni 2001): (a) the method of allocation; (b) the identity of study participants who were blind; (d) the loss to follow‐up and exclusions; (e) selective reporting; (f) other forms of bias as detailed below: (i) whether the aims were clearly defined; (ii) whether a prior sample size calculation was reported; (iii) whether the inclusion and exclusion criteria were defined (iv) whether the baseline comparability of groups was reported (based on age, sex, and duration of complaint); (v) whether interventions were defined; (vi) whether the compliance was assessed; (vii) were the infecting fungi identified; (viii) was the distribution of species between groups stated; (ix) were adverse events reported.
These items are reported in the Characteristics of included studies.
Measures of treatment effect
We reported results are reported as risk ratios (RR) of treatment failure with 95% confidence intervals (CI). To estimate differences between treatment regimens, we pooled trials that evaluated similar interventions. Since it was anticipated that there would be substantial heterogeneity between trials, we used random‐effects models when pooling.
Assessment of heterogeneity
Heterogeneity was assessed using I2. If substantial heterogeneity (I2 > 50%) existed between studies for the primary outcomes, reasons for heterogeneity, such as language, differences in health care systems, dosage and duration of treatment, were explored.
For major comparisons where overall pooling of the results was considered potentially appropriate, the results were also illustrated using L'Abbé plots (L'Abbe 1985). For each study, This plot showed the observed treatment failure rate in one group plotted against observed treatment failure rate in the other group. This was useful to illustrate the range of treatment failure rates among the trials, and the amount of heterogeneity between trials and sub groups. The diagonal line indicates no treatment effect (RR = 1). Points below this line correspond to trials where the treatment on the vertical axis has a lower treatment failure rate. The dashed line shows the pooled treatment effect. Each trial is plotted using a circle with size proportional to the standard error of the treatment effect so that trials providing more precise estimates are shown using larger circles.
We listed non‐randomised controlled studies but did not discuss them further.
Data synthesis
Statistical analysis
The primary outcome i.e. rate of treatment failure, was extracted for three time points, considered to reflect clinically important timings. Each time point is analysed separately with sub comparisons within each treatment comparison consistently numbered as below.
1. Short‐term (two weeks): the order of preference was two, one, three weeks. 2. Medium‐term (six weeks): the time point closest to six weeks follow‐up was used, provided that it was within four to eight weeks. If two time points equally close to six weeks were available then the longer follow‐up was used (e.g. eight rather than four weeks). Where a final follow‐up endpoint with more participants included was available with four to eight weeks, this was used preferentially. Only > 80% follow‐up included. A sensitivity analysis on the medium term data, restricted to data where there was clear documentation of at least 80% follow‐up of randomised participants (where follow‐up is given by group, at least 80% follow‐up was required in both groups). 3. Long‐term (12 weeks onwards): longest follow‐up of at least 12 weeks.
Sub comparisons were used to group together treatments by duration (note that for short‐term outcomes, grouping by duration was not necessary, since all durations were at least two weeks).
Sensitivity analysis
Sensitivity analyses were also conducted to examine the effects of excluding studies with poor quality.
Results
Description of studies
Results of the search
We considered all RCTs that evaluated topical treatments for fungal infections of the skin and nails of the foot. For skin infections we included only trials that used microscopy and culture to establish the presence of dermatophytes. For nail infections we included only trials that used culture to do so. We included duplicate trials only once. We excluded trials on fungal infections that contained data on infections at various body sites if foot‐specific data could not be extracted separately.
(a) Identified trials relating to skin of the foot
Included studies
We identified 144 papers reporting trials of topical treatments for fungal skin infections and included 67 (Ablon 1996; Akers 1989; Aly 2003; Bagatell 1986; Bagatell 1991a; Bagatell 1991b; Bergstresser 1993; Berman 1992; Bojanovsky 1985; Carter 1972; Chretien 1980; Coffey 1986; Del Palacio 1989; Dobson 1989; Elewski 1996; Ellis 1989; Evans 1991; Evans 1993a; Evans 1993b; Evans 1994; Friederich 1992; Fuerst 1980; Gentles 1974; Gomez 1986; Haas 1985; Hollmen 2002; Holti 1970; Ison 1990; Izuno 1986; Kagawa 1985; Klaschka 1984; Kligman 1985a; Kligman 1985b; Korting 1997; Kuhlwein 1990; Ledezma 2000; Leenutaphong 1999; Mandy 1974; Pereda 2003; Plotkin 1990; Qadripur 1979; Roberts 1985; Sanchez 1994; Satchell 2002; Savin 1990; Savin 1994; Savin 1997; Schachner 1990; Schopf 1999; Smith 1977; Smith 1986; Smith 1988a; Smith 1988b; Smith 1988c; Smith 1990a; Smith 1990b; Smith 1992; Spiekermann 1976a; Spiekermann 1976b; Sushka 2001; Syed 2000; Tong 1992; Tschen 1997; Vermeer 1996; Weller 1998; Woscoff 1986; Zaug 1992).
Comparisons
Twenty‐nine trials compared a single active treatment with placebo (Akers 1989; Aly 2003; Bagatell 1986; Bagatell 1991a; Bagatell 1991b; Berman 1992; Chretien 1980; Coffey 1986; Dobson 1989; Evans 1991; Gentles 1974; Gomez 1986; Ison 1990; Izuno 1986; Klaschka 1984; Kligman 1985a; Korting 2001; Mandy 1974; Savin 1990; Savin 1994; Savin 1997; Schachner 1990; Smith 1977; Smith 1986; Smith 1988a; Smith 1990a; Spiekermann 1976a; Spiekermann 1976b; Tschen 1997).
Twenty‐five trials compared two active treatment regimens (Bojanovsky 1985; Carter 1972; Del Palacio 1989; Evans 1993a; Evans 1993b; Friederich 1992; Haas 1985; Holti 1970; Kagawa 1985; Kligman 1985bKuhlwein 1990; Leenutaphong 1999; Plotkin 1990; Pereda 2003; Qadripur 1979; Roberts 1985; Sanchez 1994Schopf 1999Smith 1988b; Smith 1988c; Smith 1992; Sushka 2001; Vermeer 1996; Weller 1998; Woscoff 1986).
Thirteen trials compared more than two treatment regimens within the same trial (Ablon 1996; Bergstresser 1993; Elewski 1996; Ellis 1989; Evans 1994; Fuerst 1980; Korting 1997;Ledezma 2000; Satchell 2002; Smith 1990b; Syed 2000; Tong 1992; Zaug 1992).
Demographic information is presented for all studies in the Characteristics of included studies table.
Excluded studies
We excluded 77 trials and present the reasons for these exclusions in the Characteristics of excluded studies table.
Twelve studies evaluating topical treatments for skin infections which were included in the previous version of this review were excluded in this update (Daily 1985; Duncan 1975; Thomas 1976; Ortiz 1978; Tschen 1979; Smith 1977b; Fredriksson 1982; Privat 1982; Thomas 1986; Greer 1986; Tanenbaum 1982; Tsuboi 1996). These exclusions were made after a tightening of the review inclusion criteria to include only those studies that used both microscopy and culture to diagnose dermatophyte infections.
(b) Results of the search for trials relating to nails of the foot
Included studies
We identified 11 trials evaluating the efficacy of topical treatments for nails and included 6 in the review (Buck 1994; Gupta 2000a; Gupta 2000b; Mensing 1992; Montana 1994; Syed 1999). Please see Characteristics of included studies.
Excluded studies
The other five were excluded since they reported combined data from fingernails and toenails (Lauharanta 1992; Reinel 1992; Reinel 1992a; Ruping 1993; Terragni 1993). We also excluded four trials evaluating a combination of systemic and topical treatments for infected nails (Arenas 1991; Baran 2000; Barnetson 1998; Friedman 1997; Zaug 1995). Please see Characteristics of excluded studies.
Risk of bias in included studies
Allocation
The method of allocation was reported in only 17 trials (Ablon 1996; Akers 1989; Buck 1994; Chretien 1980; Evans 1993a; Evans 1993b; Evans 1994; Fuerst 1980; Gentles 1974; Holti 1970; Kligman 1985a; Kligman 1985b; Korting 1997; Mandy 1974; Plotkin 1990; Savin 1997; Smith 1977).
Blinding
Blinded outcome assessment was reported in only 12 trials (Ablon 1996; Buck 1994; Carter 1972; Evans 1993a; Evans 1994; Gentles 1974; Holti 1970; Korting 2001; Mensing 1992; Montana 1994; Savin 1990; Savin 1997). However only 12 did not report blinding of participants (Ablon 1996; Bojanovsky 1985; Friederich 1992; Leenutaphong 1999; Mensing 1992; Montana 1994; Roberts 1985; Sanchez 1994; Satchell 2002; Satchell 2002; Smith 1988b; Smith 1988c). One trial was reported to be single blind (Kagawa 1985). However only seven trials did not report blinding of subjects (Smith 1988a; Smith 1988b; Smith 1988c; Friederich 1992; Sanchez 1994; Roberts 1985; Friederich 1992).
Incomplete outcome data
Follow up and exclusions
A diagnosis of athlete's foot based on clinical signs and symptoms alone can be inaccurate because there are non‐fungal skin conditions which have a similar appearance, e.g. erythrasma. This review only included trials which reported the use of microscopy and culture tests to confirm the presence of fungi. The results from these lab‐based diagnostic tests can take up to several weeks to obtain, and often trial participants are randomised to an allocation and begin treatment before the test results are available. When the results show no fungi are present (negative test result) participants are then withdrawn from the study. In order to reduce bias from trials with high loss to follow up whilst recognising the practical constraints in which RCTs of athlete's foot generally take place, we performed a sensitivity analysis only including data which reported follow up data for at least 80% of the randomised sample.
Selective reporting
We found no evidence that selective reporting had occurred in any of the included trials.
Other potential sources of bias
Some trials were funded by industry however a sensitivity analysis of data from trials comparing allylamines versus azoles found no statistical differences between industry funded and non‐industry funded studies.
Effects of interventions
(a) Skin trials
(i) Placebo controlled trials
Allylamines Versus Placebo
Two different allylamines (naftifine 1% and terbinafine 1%) used for 1 to 4 weeks were evaluated in 11 placebo controlled randomised trials.
Short‐term outcome (two weeks)
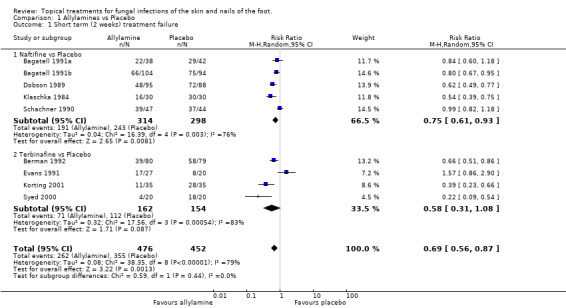
Naftifine Short‐term outcomes were available for all 5 trials using naftifine (n = 612) Klaschka 1984; Dobson 1989; Schachner 1990; Bagatell 1991a; Bagatell 1991b. Overall the observed relative reduction in risk of treatment failure was 25% (RR 0.75, 95% CI 0.60 to 0.93; Analysis 1.1), although there was substantial variation in the individual study results (I2 = 79%).
1.1. Analysis.
Comparison 1 Allylamines vs Placebo, Outcome 1 Short term (2 weeks) treatment failure.
Terbinafine For terbinafine, short‐term outcomes were available for 4 trials (n = 316, Berman 1992; Evans 1991; Korting 1997; Syed 2000). The results were inconsistent between studies, giving an overall relative reduction in treatment failure of 42% which was not statistically significant (RR 0.58, 95% CI 0.31 to 1.08; Analysis 1.1).
Across all 9 trials providing short‐term outcome of 1% allylamines for a period of 1 to 2 weeks compared with placebo, there was a pooled relative reduction in treatment failure at 2 weeks of 31% (RR 0.69, 95% CI 0.56 to 0.87; Analysis 1.1), with substantial variation in individual study results (I2 = 79%).
Medium‐term outcome (six weeks)
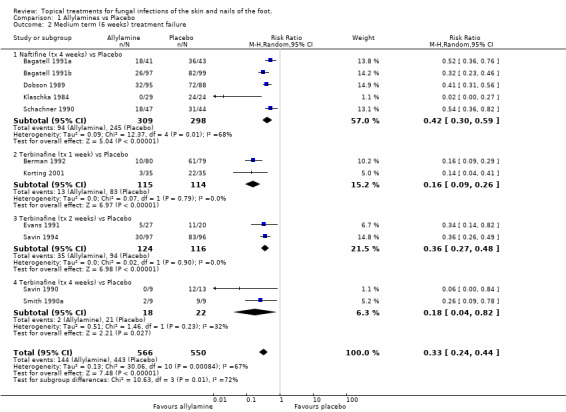
Naftifine Naftifine (1%) used for 4 weeks was evaluated in 5 trials (n = 607, Bagatell 1991a; Bagatell 1991b; Dobson 1989; Klaschka 1984; Schachner 1990), a 58% relative reduction in treatment failure was observed (RR 0.42 95% CI 0.30 to 0.59; Analysis 1.2, Naftifine (tx 4 weeks) versus Placebo), with substantial variation in individual study results (I2 = 68%).
1.2. Analysis.
Comparison 1 Allylamines vs Placebo, Outcome 2 Medium term (6 weeks) treatment failure.
Terbinafine Terbinafine (1%) was used for 1 week (2 trials, n = 229, Berman 1992; Korting 2001), 2 weeks (2 trials, n = 240, Evans 1991; Savin 1994) and 4 weeks (2 trials, n = 40, Savin 1990; Smith 1990a). A statistically significant reduction in risk of treatment failure was observed with each treatment duration (RR 0.16, 0.36, 0.18 respectively; Analysis 1.2). Pooling across all durations, a 77% relative reduction in treatment failure was observed (RR 0.23, 95% CI 0.15 to 0.38, pooled result not shown in the Forest plots (MetaView), with moderate variation in individual study results (I2 = 50%).
A meta‐analysis of data from all 11 trials (n = 1116) comparing 1% allylamines with placebo (treatment for a period of 1 to 4 weeks) provided an estimated relative reduction in the risk of treatment failure of 67% (RR 0.33, 95% CI 0.24 to 0.44; Analysis 1.2), with substantial variation in individual study results (I2 = 67%). All of the results were based on at least 80% follow up except those from the trials evaluating terbinafine used for 2 weeks. A sensitivity analysis based on the exclusion of data collected in these two trials (Evans 1991; Savin 1994) showed a similar overall estimate of effectiveness (RR of treatment failure 0.31, 95% CI 0.21 to 0.45; Analysis 1.3). The variation in individual study results remained substantial (I2 = 75%).
1.3. Analysis.
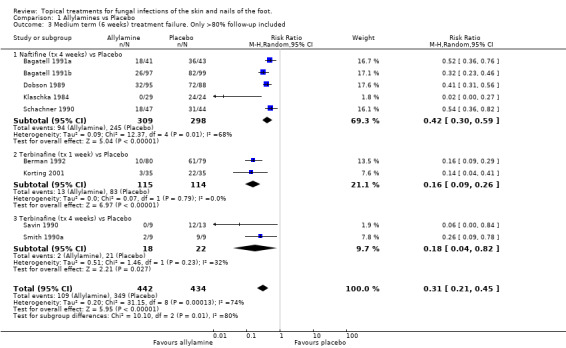
Comparison 1 Allylamines vs Placebo, Outcome 3 Medium term (6 weeks) treatment failure. Only >80% follow‐up included.
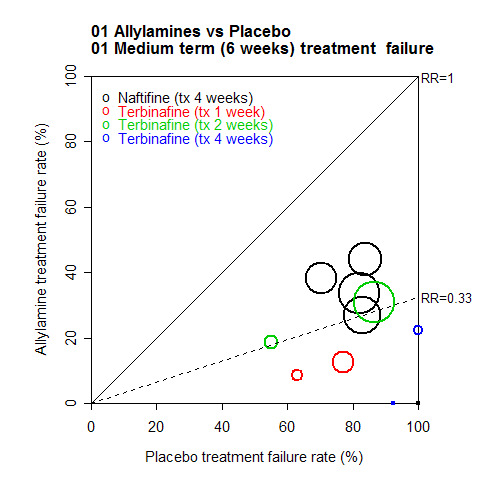
A L'Abbé plot of the outcomes at 6 weeks (Figure 1) demonstrates that the allylamines generally had treatment failure rates of around 30%, compared to around 85% for placebos, though there was considerable variation in individual trial results (see MeMethods, Assessment of heterogeneity for more details of L'Abbé plots and how to interpret them) .
1.
Azoles Versus Placebo
Six different azoles (bifonazole, clotrimazole, miconazole nitrate, oxiconazole nitrate, sulconazole nitrate and tioconazole) were evaluated in 13 placebo controlled randomised trials. The concentration of these drugs was generally 1%, but 2% for miconazole, they were used for 4 to 6 weeks.
Short‐term outcome (two weeks)
Bifonazole Short‐term outcome was reported only in the four trials of bifonazole, and one trial of oxiconazole. A meta‐analysis of the 4 trials evaluating bifonazole (n = 176, Bagatell 1986; Coffey 1986; Izuno 1986; Smith 1986) estimated the relative reduction in treatment failure at 2 weeks to be 48% (RR 0.52, 95% CI 0.37 to 0.73; Analysis 2.1), with good consistency between the individual trial results (I2 = 18%).
2.1. Analysis.
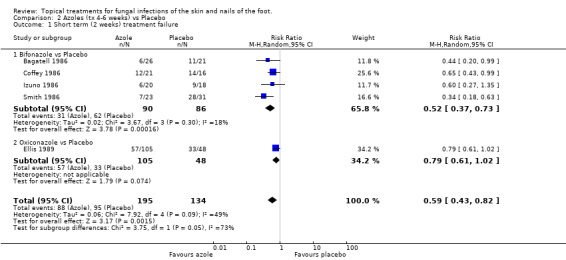
Comparison 2 Azoles (tx 4‐6 weeks) vs Placebo, Outcome 1 Short term (2 weeks) treatment failure.
Oxiconazole The trial of oxiconazole (n = 155, Ellis 1989) had an observed relative reduction in treatment failure at 2 weeks of 21% (RR 0.79, 95% CI 0.61 to 1.02; Analysis 2.1) which was not quite statistically significant. Overall the pooled estimated relative risk of treatment failure (n = 329, 5 trials) of 1% azoles (bifonazole or oxiconazole) versus placebo at 2 weeks was 41% (RR 0.59, 95% CI 0.43 to 0.82; Analysis 2.1), though there was considerable variation between the results for the 2 different azoles (overall I2 = 50%).
Medium‐term outcome (six weeks)
All azoles versus placebo There was statistically significant evidence of effectiveness for each of the azoles individually except miconazole nitrate, which was studied in only 54 people, providing inconclusive results (RR of treatment failure 0.41, 95% CI 0.14 to 1.14; Analysis 2.2) (Gentles 1974; Mandy 1974). The results were generally fairly consistent both between individual studies for each azole, and between different azoles. Overall, a meta‐analysis of data from 13 trials (n = 1235, Akers 1989; Bagatell 1986; Coffey 1986; Elewski 1996; Ellis 1989; Izuno 1986; Gentles 1974; Mandy 1974; Smith 1977; Smith 1986; Smith 1988a; Spiekermann 1976a; Spiekermann 1976b) comparing azoles with placebo estimated the pooled relative reduction in treatment failure as 60% (RR 0.40, 95% CI 0.35 to 0.46; Analysis 2.2).
2.2. Analysis.
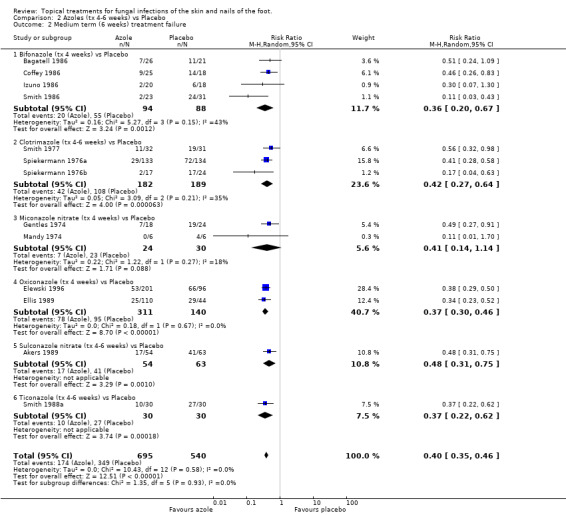
Comparison 2 Azoles (tx 4‐6 weeks) vs Placebo, Outcome 2 Medium term (6 weeks) treatment failure.
Sensitivity analysis
A sensitivity analysis based only on data collected in the 6 trials with at least 80% follow up at 6 weeks (n = 448, Akers 1989; Ellis 1989; Gentles 1974; Mandy 1974; Smith 1977; Smith 1988a) showed very similar results (RR of treatment failure 0.43 (95% CI 0.34 to 0.53; Analysis 2.3).
2.3. Analysis.
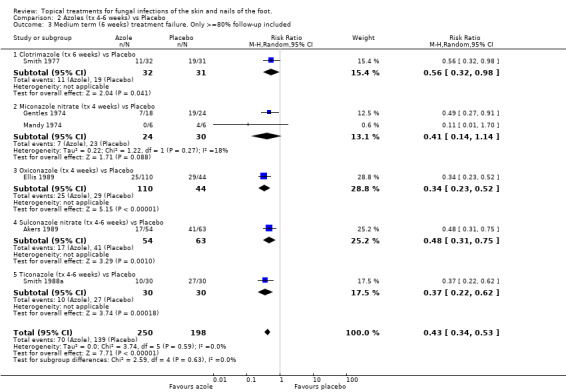
Comparison 2 Azoles (tx 4‐6 weeks) vs Placebo, Outcome 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included.
A L'Abbé plot of the outcomes at 6 weeks (Figure 2) demonstrates that the azoles generally had treatment failure rates of around 25%, compared to around 50 to 90% for placebos (see Methods, statistical analysis for more details of L'Abbé plots and how to interpret them).
2.
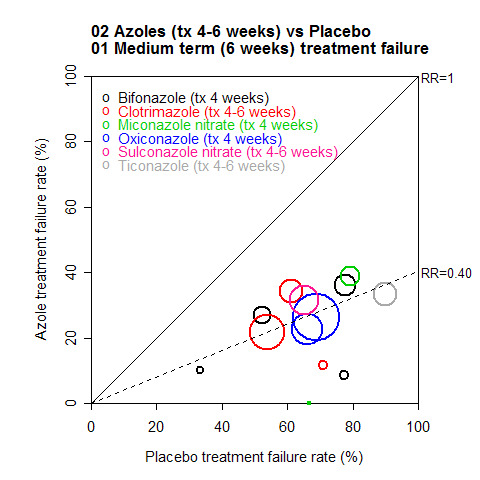
Other topical antifungal treatments versus placebo
Ten trials evaluated other topical antifungal creams versus placebo (Aly 2003;Chretien 1980; Fuerst 1980; Gomez 1986; Kligman 1985a; Satchell 2002; Savin 1997; Syed 2000; Tschen 1997; Tong 1992).
Short‐term outcomes (two weeks)
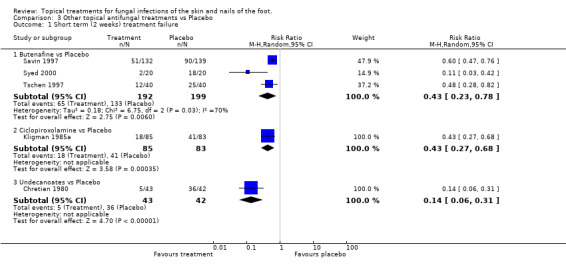
Butenafine Outcomes collected at two weeks in three placebo controlled trials of butenafine 1% used for 1 to 2 weeks (n = 271, Savin 1997; Tschen 1997; Syed 2000) showed a statistically significant relative reduction in treatment failure at 2 weeks of 57% (RR 0.43, 95% CI 0.23 to 0.78; Analysis 3.1).
3.1. Analysis.
Comparison 3 Other topical antifungal treatments vs Placebo, Outcome 1 Short term (2 weeks) treatment failure.
Ciclopiroxolamine A placebo controlled trial of 1% ciclopiroxolamine (n = 168, Kligman 1985a) shows a statistically significant relative reduction in treatment failure at 2 weeks of 57% (RR 0.43, 95% CI 0.27 to 0.68; Analysis 3.1).
Undecanoates A placebo controlled trial of undecanoates (n = 168, Chretien 1980) also showed a statistically significant effect with relative reduction in treatment failure at 2 weeks of 86% (RR 0.14, 95% CI 0.06 to 0.31; Analysis 3.1).
Medium‐term outcome (six weeks)
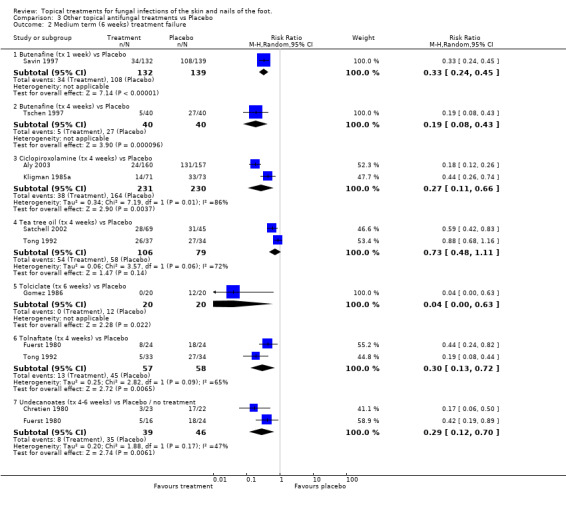
Butenafine A statistically significant effect of butenafine(1%) was observed when it was used for 1 week and for 4 weeks. Butenafine used for 1 week versus placebo was evaluated (n = 271) in one trial (Savin 1997). A statistically significant relative reduction in treatment failure of 67% was observed (RR 0.33, 95% CI 0.24 to 0.45; Analysis 3.2). Butenafine used for 4 weeks versus placebo was evaluated (n = 80) in another trial (Tschen 1997), giving a statistically significant relative reduction in treatment failure of 81% (RR 0.19, 95% CI 0.08 to 0.43; Analysis 3.2). Neither of these trials achieved at least 80% follow‐up.
3.2. Analysis.
Comparison 3 Other topical antifungal treatments vs Placebo, Outcome 2 Medium term (6 weeks) treatment failure.
Ciclopiroxolamine Ciclopiroxolamine (1% and 0.77%) used for 4 weeks was evaluated in 2 placebo controlled trials (n = 144, Kligman 1985a; n = 317, Aly 2003). A statistically significant relative reduction in treatment failure was observed (RR of treatment failure = 0.27, 95% CI 0.11 to 0.66; Analysis 3.2).
Tee tree oil Tea tree oil (10%) used for 4 weeks was evaluated in 2 placebo controlled trials (n = 185, Satchell 2002; Tong 1992). Although one of the individual trials showed a statistically significant effect (Satchell 2002), the results of the second trial were less favourable, and combining data from both trials did not show a statistically significant effect (RR of treatment failure 0.73, 95% CI 0.48 to 1.11;Analysis 3.2). Only the trial with the less favourable results (Tong 1992) had at least 80% follow‐up.
Tolciclate Tolciclate (1%) used for 6 weeks was evaluated in a small placebo controlled trial (n=40, Gomez 1986) and produced a relative reduction of treatment failure of 0.04 (95% CI 0.00 to 0.63; Analysis 3.2), a statistically significant effect, with at least 80% follow‐up.
Tolnaftate Tolnaftate (1%) used for 4 weeks was compared with placebo in 2 trials (n = 115, Fuerst 1980; Tong 1992) and a statistically significant relative reduction in treatment failure of 70% was found (RR 0.30, 95% CI 0.13 to 0.72; Analysis 3.2), with at least 80% follow‐up in both trials.
Undecanoates Undecanoates (Undecylenic acid, zinc undecylenic acid) were compared with placebo in two trials (n = 125, Chretien 1980; Fuerst 1980) and a statistically significant relative reduction in treatment failure of 71% was found (RR 0.29, 95% CI 0.12 to 0.70; Analysis 3.2), with at least 80% follow‐up in both trials.
(ii) Treatment versus treatment comparisons
Comparisons between Different Allylamines or Allylamine Regimens
Four trials compared the rate of treatment failure of different allylamines or allylamine regimens (all 1%, Ablon 1986; Bergstresser 1993; Evans 1994; Smith 1990b).
Short‐term outcome (two weeks)
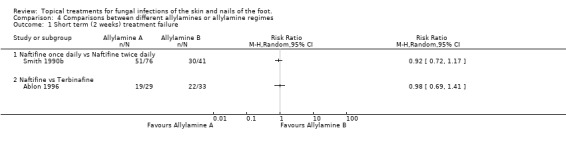
Naftifine twice daily In the trial by Smith 1990b (n = 117) no statistically significant difference was observed at 2 weeks between naftifine used once daily or twice daily (RR of treatment failure at 2 weeks 0.92, 95% CI 0.72 to 1.17; Analysis 4.1).
4.1. Analysis.
Comparison 4 Comparisons between different allylamines or allylamine regimes, Outcome 1 Short term (2 weeks) treatment failure.
Naftifine versus terbinafine In the evaluation of naftifine compared with terbinafine (n = 62, Ablon 1996) there were similar treatment failure rates at 2 weeks for each of the 2 treatments (RR of treatment failure 0.98, 95% CI 0.69 to 1.41; Analysis 4.1).
Medium‐term outcome (six weeks)
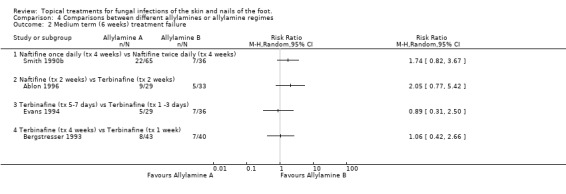
Naftifine twice daily versus naftifine once daily Smith 1990b compared 1% naftifine once daily to twice daily both for 4 weeks (n = 101) and found fewer treatment failures with twice daily, thought the difference was not statistically significant (RR of treatment failure = 1.74, 95% CI 0.82 to 3.67; Analysis 4.2).
4.2. Analysis.
Comparison 4 Comparisons between different allylamines or allylamine regimes, Outcome 2 Medium term (6 weeks) treatment failure.
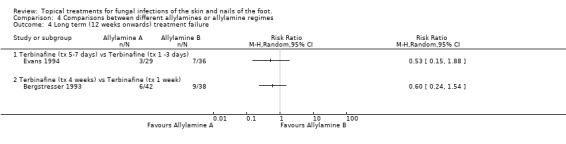
Naftifine versus Terbinafine Ablon 1996 compared naftifine with 1% terbinafine, both applied for 2 weeks (n = 62), the results favoured terbinafine but the difference was not statistically significant (RR of treatment failure 2.05, 95% CI 0.77 to 5.42; Analysis 4.2). Evans 1994 compared terbinafine applied for 5 to 7 days with terbinafine applied for 1 to 2 days (n = 65), no statistical difference was detected in the treatment failure rates (RR of treatment failure 0.89, 95% CI 0.31 to 2.50; Analysis 4.2).
Terbinafine four week versus terbinafine one week Bergstresser 1993 compared terbinafine used for 4 weeks with terbinafine used for 1 week (n = 83) but also did not detect any difference between the rate of treatment failure in the 2 groups (RR of treatment failure 1.06, 95% CI 0.42 to 2.66; Analysis 4.2). All of these results are based on at least 80% follow‐up except those of Bergstresser 1993.
Long‐term outcome (12 weeks)
Terbinafine one to three versus five to seven days Data collected in the trial of terbinafine used for 5 to 7 days versus terbinafine used for 1 to 3 days (n = 65, Evans 1994) found slightly less treatment failures with 5 to 7 days treatment, but the difference was not statistically significant (RR of treatment failure at 12 weeks 0.53, 95% CI 0.15 to 1.88).
Terbinafine four weeks versus terbinafine one week
A trial comparing outcomes from 1% terbinafine used for 4 weeks versus 1% terbinafine used for 1 week (n = 80, Bergstresser 1993) found less slightly treatment failures with 4 weeks treatment, but the difference was not statistically significant (RR of treatment failure at 12 weeks 0.60, 95% CI 0.24 to 1.54).
Comparisons between different azoles or azole regimens
Thirteen trials reported data collected in evaluations of one azole versus another (Bergstresser 1993; Elewski 1996; Ellis 1989; Friederich 1992; Korting 1997; Kuhlwein 1990; Pereda 2003; Qadripur 1979; Roberts 1985; Smith 1988b; Smith 1988c; Sushka 2001; Woscoff 1986).
Short‐term outcome (two weeks)
Clotrimazole versus econazole In 2 trials of 1% clotrimazole versus 1% econazole (n = 497, Korting 1997; Qadripur 1979) combined data did not show a statistically significant difference (RR of treatment failure at 2 weeks 1.13, 95% CI 0.92 to 1.39; Analysis 5.1).
5.1. Analysis.
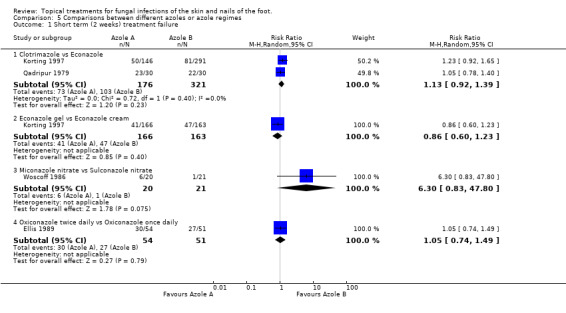
Comparison 5 Comparisons between different azoles or azole regimes, Outcome 1 Short term (2 weeks) treatment failure.
Econazole gel versus econazole cream In 2 arms of the trial by Korting 1997 (n = 229) 1% econazole gel was compared with gel 1% econazole cream, there was no statistically significant difference (RR of treatment failure 0.86, 95% CI 0.66 to 1.23; Analysis 5.1). Miconazole nitrate versus sulconazole nitrate Woscoff 1986 evaluated 2% miconazole nitrate versus 1% sulconazole nitrate in a small trial (n = 41), the results favoured sulconazole nitrate but were not statistically significant (RR of treatment failure at 2 weeks 6.30, 95% CI 0.83 to 47.80; Analysis 5.1). Oxiconazole once versus twice daily The trial of 1% oxiconazole used twice daily versus once daily (Ellis 1989) did not detect a statistically significant difference between the 2 regimens; RR of treatment failure at 2 weeks 1.05 (95% CI 0.74 to 1.49; Analysis 5.1).
Medium‐term outcome (six weeks)
Most trials achieved at least 80% follow‐up at 6 weeks, apart from Bergstresser 1993 and Smith 1988c.
Bifonazole Bifonazole versus croconazole One trial (Kuhlwein 1990) found 100% rate of treatment success in both arms of a small trial (n = 36) comparing 1% bifonazole for 3 weeks with 1% croconazole for 3 weeks.
Bifonazole used for three weeks versus bifonazole used for one hour for three weeks Another trial evaluated 1% bifonazole used for 3 weeks versus 1% bifonazole removed after one hour for 3 weeks (n = 73, Friederich 1992) but did not detect any difference between the rates of treatment failure (RR 4.34, 95% CI 0.51 to 37.01; Analysis 5.2).
5.2. Analysis.
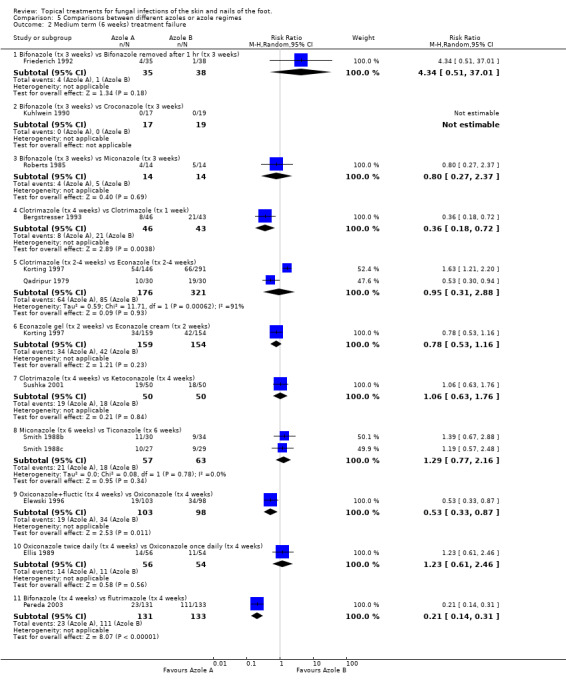
Comparison 5 Comparisons between different azoles or azole regimes, Outcome 2 Medium term (6 weeks) treatment failure.
Bifonazole versus miconazole A third trial, Roberts 1985, compared 1% bifonazole with 2% miconazole used for 3 weeks but did not observe a statistically significant difference between the 2 treatments (RR of treatment failure 0.80, 95% CI 0.27 to 2.37; Analysis 5.2).
Bifonazole versus flutrimazole A fourth trial Pereda 2003 (n = 264) compared bifonazole 1% powder with fluconazole 1% powder and found bifonazole to produce statistically significantly fewer treatment failures (0.21, 95% CI 0.14 to 0.31; Analysis 5.2).
Clotrimazole Clotrimazole for four weeks versus clotrimazole for one week Four trials evaluated 1% clotrimazole (Bergstresser 1993; Korting 1997; Qadripur 1979; Sushka 2001). Bergstresser 1993 compared 4 weeks of 1% clotrimazole with 1 week of 1% clotrimazole (n = 89) and found a statistically significant relative reduction in treatment failure of 64% in favour of 4 weeks treatment (RR of treatment failure = 0.36, 95% CI 0.18 to 0.72; Analysis 5.2), less than 80% follow‐up was achieved in this trial.
Clotrimazole versus econazole Two trials (n = 497, Qadripur 1979; Korting 1997) comparing 1% clotrimazole versus 1% econazole each individually had statistically significant results favouring each of the treatments, the pooled results did not show a statistically significant difference (RR of treatment failure = 0.95, 95% CI 0.31 to 2.88; Analysis 5.2).
Clotrimazole versus ketoconazole Sushka 2001 compared clotrimazole 1% used once daily with 2% ketoconazole 2% used twice daily but the results did not show a statistically significant difference between the 2 creams (RR of treatment failure = 1.06, 95% CI 0.63 to 1.76; Analysis 5.2).
Econazole gel versus econazole cream One trial (n = 313, Korting 1997) evaluated 1% econazole gel versus 1% econazole cream but did not show a statistically significant difference in rates of treatment failure (RR of treatment failure = 0.78, 5% CI 0.53 to 1.16; Analysis 5.2).
Miconazole versus ticonazole Combined data from 2 trials comparing 2% miconazole used for 6 weeks versus 1% tioconazole used for 6 weeks (n = 220, Smith 1988a; Smith 1988c) but did not show a statistically significant difference in the two treatments, RR of treatment failure 1.29 (95% CI 0.77 to 2.16; Analysis 5.2).
Oxiconazole + fluctic versus oxiconazole A trial of 1% oxiconazole + fluctic used for 4 weeks versus 1% oxiconazole used for 4 weeks (n = 201, Elewski 1996) produced a statistically significant relative reduction in treatment failure of 47% (RR 0.53, 95% CI 0.33 to 0.87; Analysis 5.2), favouring oxiconazole + fluctic.
Oxiconazole once per day versus oxiconazole twice per day Ellis 1989 evaluated oxiconazole once per day compared with oxiconazole twice per day (n = 110) but found no statistically significant difference in the treatment failure rates (RR 1.23, 95% CI 0.61 to 2.46; Analysis 5.2).
Long‐term outcome (12 weeks+)
Clotrimazole four weeks versus clotrimazole one week In a trial comparing 4 weeks of 1% clotrimazole with 1 week of 1% clotrimazole Bergstresser 1993 found a statistically significant relative reduction in treatment failure of 53% favouring the longer treatment time (RR at 12 weeks 0.47, 95% CI 0.28 to 0.78; Analysis 5.4).
5.4. Analysis.
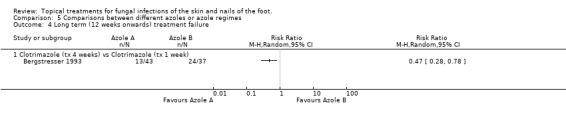
Comparison 5 Comparisons between different azoles or azole regimes, Outcome 4 Long term (12 weeks onwards) treatment failure.
Allylamines one to two weeks versus azoles one to two weeks
Short‐term outcome (two weeks)
Ten trials (n = 1519, Ablon 1996; Bojanovsky 1985; Evans 1993a; Evans 1993b; Haas 1985; Leenutaphong 1999; Sanchez 1994Schopf 1999; Smith 1990b; Smith 1992) comparing 1% allylamines (naftifine, terbinafine) with 1% azoles (bifonazole, clotrimazole, oxiconazole) used for 1 to 2 weeks showed a small, not statistically significant, difference in favour of allylamines; RR of treatment failure at 2 weeks 0.86 (95% CI 0.70 to 1.06; Analysis 6.1). There was considerable variation in the results of the individual trials (I2 = 60%).
6.1. Analysis.
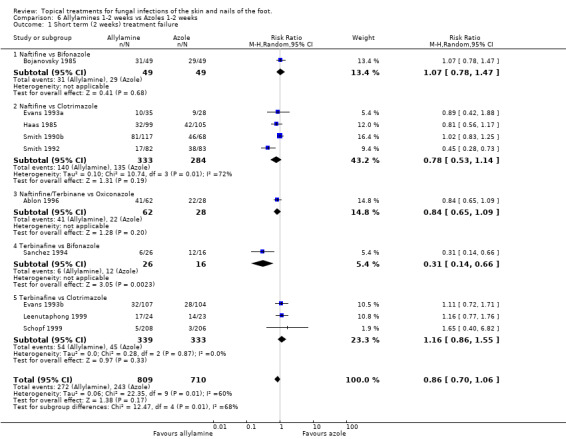
Comparison 6 Allylamines 1‐2 weeks vs Azoles 1‐2 weeks, Outcome 1 Short term (2 weeks) treatment failure.
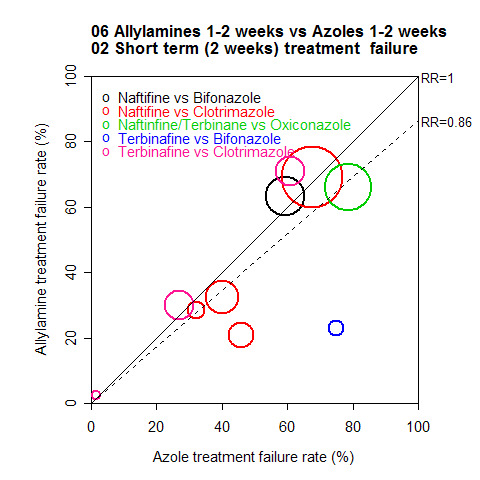
A L'Abbé plot of the outcomes at two weeks (Figure 3) demonstrates that the allylamines generally had similar treatment failure rates to the azoles, with a wide variation in failure rates.
3.
Medium‐term outcome (six weeks)
Data collected at 6 weeks from 2 trials (n = 1730, Ablon 1996; Bergstresser 1993) comparing 1% allylamines (naftifine, terbinafine) versus 1% azoles used for 1 to 2 weeks (oxiconazole, clotrimazole) produced a statistically significant relative reduction in treatment failure of 66% (RR 0.34, 95% CI 0.22 to 0.52; Analysis 6.2), favouring allylamines. Only one of these trials had follow up data of at least 80%of those originally randomised (Ablon 1996), but the results of both trials were very similar and these data provide a similar relative risk of treatment failure (0.33, 95% CI 0.20 to 0.56; Analysis 6.3).
6.2. Analysis.
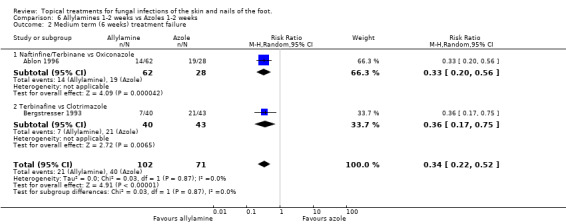
Comparison 6 Allylamines 1‐2 weeks vs Azoles 1‐2 weeks, Outcome 2 Medium term (6 weeks) treatment failure.
6.3. Analysis.
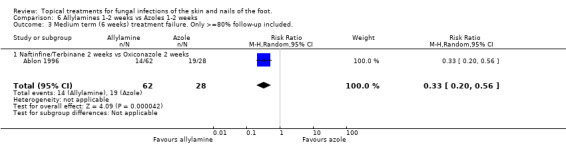
Comparison 6 Allylamines 1‐2 weeks vs Azoles 1‐2 weeks, Outcome 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included..
A L'Abbé plot of the outcomes at 6 weeks (Figure 4) shows the allylamines had treatment failure rates of around 15% to 20%, compared to 50% to 70% for the azoles (Methods, Assessment of heterogeneity for more details of L'Abbé plots and how to interpret them).
4.
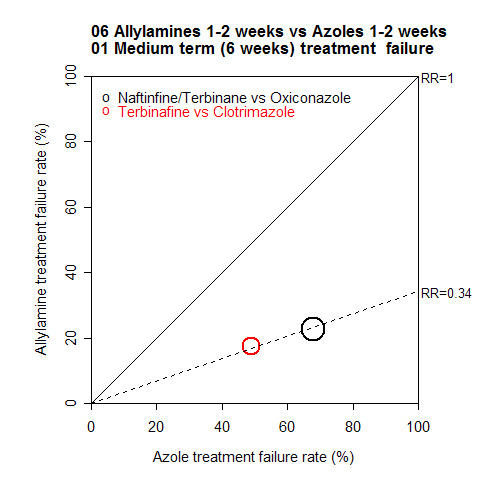
Long term outcome (12 weeks)
Data collected at 12 weeks from one trial (n = 75, Bergstresser 1993) comparing 1% allylamines (terbinafine) with 1% azoles (clotrimazole) used for 1 to 2 weeks produced a statistically significant relative reduction in treatment failure of 72% (RR 0.28, 95% CI 0.14 to 0.58; Analysis 6.4), favouring allylamines.
6.4. Analysis.
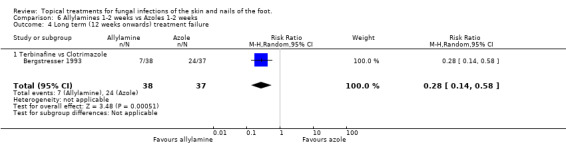
Comparison 6 Allylamines 1‐2 weeks vs Azoles 1‐2 weeks, Outcome 4 Long term (12 weeks onwards) treatment failure.
Allylamine one week versus azoles four weeks
Medium‐term outcome (six weeks)
Data collected at 6 weeks in 5 trials (n = 962, Bergstresser 1993; Evans 1993a; Leenutaphong 1999; Schopf 1999; Vermeer 1996) which compared a 1% allylamine (terbinafine) used for 1 week with an 1% azole used for 4 weeks (clotrimazole, miconazole) did not show a statistically significant difference in treatment failure (RR 0.75, 95% CI 0.33 to 1.72; Analysis 7.1). There was considerable variation in the results of the individual trials (I2 = 60%). Combining data from 3 of the trials which had at least 80% follow‐up (n = 685, Evans 1993b; Leenutaphong 1999; Schopf 1999) also did not show a statistically different difference in the treatment failure rates (RR 0.50, 95% CI 0.10 to 2.54; Analysis 7.2).
7.1. Analysis.
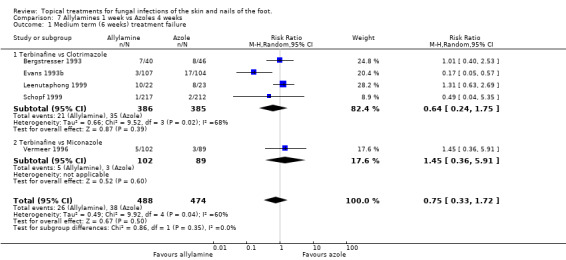
Comparison 7 Allylamines 1 week vs Azoles 4 weeks, Outcome 1 Medium term (6 weeks) treatment failure.
7.2. Analysis.
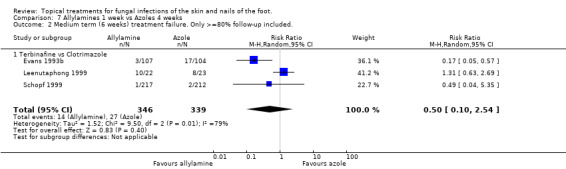
Comparison 7 Allylamines 1 week vs Azoles 4 weeks, Outcome 2 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included..
A L'Abbé plot of the outcomes at six weeks (Figure 5) demonstrates that the allylamines had broadly similar treatment failure rates to the azoles, with a wide variation in failure rates.
5.
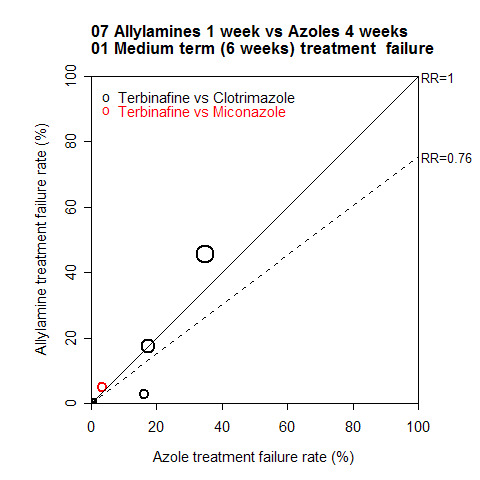
Allylamines versus azoles four to six weeks
Medium‐term outcome (six weeks)
A meta analysis of data collected in nine trials (n = 1003, Bergstresser 1993; Bojanovsky 1985; Evans 1993a; Haas 1985; Kagawa 1985; Plotkin 1990; Sanchez 1994; Smith 1990b; Smith 1992) found a statistically significant difference between 1% allylamines (naftifine, terbinafine) and 1% to 2% azoles (bifonazole, clotrimazole) used for 4 to 6 weeks, with a relative reduction in treatment failure of 37% favouring allylamines (RR 0.63, 95% CI 0.42 to 0.94; Analysis 8.1). There was considerable variation in the results of the individual trials (I2 = 68%) Six of those trials (Evans 1993a; Haas 1985; Kagawa 1985; Smith 1990a; Smith 1992) reported outcomes for at least 80% of those randomised (n = 896) and produced a relative risk of treatment failure of 0.55 (95% CI 0.34 to 0.89; Analysis 8.2) for the superiority of allylamines over azoles. The variation in the results of the individual trials was still substantial (I2 = 70%).
8.1. Analysis.
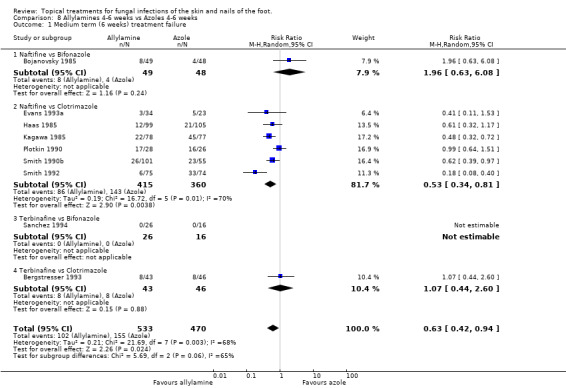
Comparison 8 Allylamines 4‐6 weeks vs Azoles 4‐6 weeks, Outcome 1 Medium term (6 weeks) treatment failure.
8.2. Analysis.
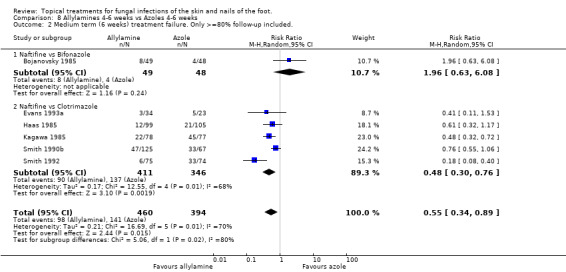
Comparison 8 Allylamines 4‐6 weeks vs Azoles 4‐6 weeks, Outcome 2 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included..
A L'Abbé plot of the outcomes at six weeks (Figure 6) demonstrates a wide variation in observed treatment failure rates.
6.
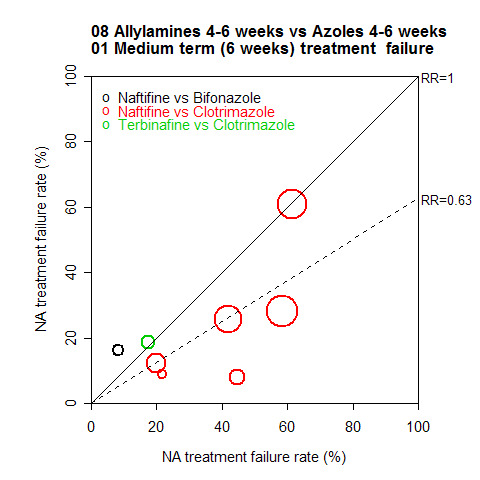
Long‐term outcome (12 + weeks)
Two trials (n = 141, Bergstresser 1993; Evans 1993a) collected long term outcomes from comparisons of allylamines versus azoles, the combined data produced a relative risk of treatment failure of 0.47 (95% CI 0.22 to 1.02; Analysis 8.3) which favoured allylamines, but did not quite reach statistical significance.
8.3. Analysis.
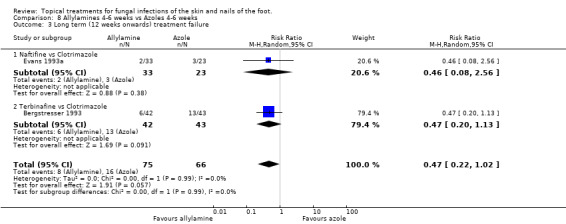
Comparison 8 Allylamines 4‐6 weeks vs Azoles 4‐6 weeks, Outcome 3 Long term (12 weeks onwards) treatment failure.
Allylamines versus other antifungal topical skin treatments
Two small trials compared an allylamine (terbinafine) with another topical skin treatment.
Short‐term outcome (two weeks)
Terbinafine versus butenafine One trial comparing 1% terbinafine versus 1% butenafine (Syed 2000 n = 40) found no statistically significant difference in treatment failure at 2 weeks (RR 2.00, 95% CI 0.41 to 9.71; Analysis 9.1)
9.1. Analysis.
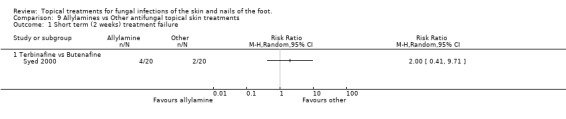
Comparison 9 Allylamines vs Other antifungal topical skin treatments, Outcome 1 Short term (2 weeks) treatment failure.
Medium‐term outcome (six weeks)
Terbinafine versus ajoene Ledezma 2000 compared terbinafine 1% for 1 week with ajoene 0.6% and 1.0% (n = 47). The treatment failure rate for terbinafine was between that for 0.6% and 1.0% ajoene, but neither concentration was significantly different to terbinafine.
Azoles versus other antifungal topical skin treatments
Medium‐term outcome (six weeks)
Bifonazole versus amorolfine In a very small trial comparing 1% bifonazole versus 0.5% amorolfine applied for 6 weeks (n = 9, Del Palacio 1989) all participants in each group were cured (no treatment failure) at 6 weeks; 6/6 in the bifonazole arm and 3/3 in the amorolfine arm.
Clotrimazole versus ciclopiroxolamine A trial comparing 1% clotrimazole versus 1% ciclopiroxolamine applied to the skin for 4 weeks (n = 87, Kligman 1985b) produced a relative risk of treatment failure of 1.41 (95% CI 0.67 to 2.95; Analysis 10.1) in outcomes taken at 2 weeks. Outcomes taken at 6 weeks found a relative risk of 1.78 (95% CI 0.59 to 5.38; Analysis 10.2), but less than 80% follow up was available at this time. At 4 weeks there was 100% follow‐up, and an RR of treatment failure of 1.71 (95% CI 0.54 to 5.42; Analysis 10.3).
10.1. Analysis.
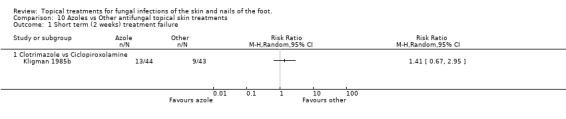
Comparison 10 Azoles vs Other antifungal topical skin treatments, Outcome 1 Short term (2 weeks) treatment failure.
10.2. Analysis.
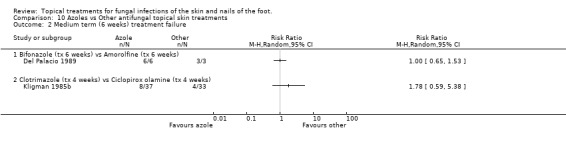
Comparison 10 Azoles vs Other antifungal topical skin treatments, Outcome 2 Medium term (6 weeks) treatment failure.
10.3. Analysis.
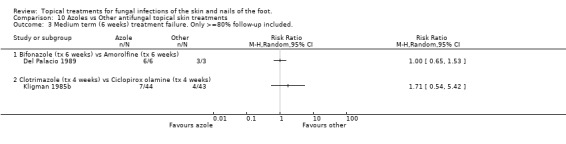
Comparison 10 Azoles vs Other antifungal topical skin treatments, Outcome 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included..
Comparison between other topical treatments
Short‐term outcome (two weeks)
Salicylic acid plus nitrate versus salicylic acid Weller 1998 reported outcomes collected at 2 weeks with statistically significantly fewer treatment failures when nitrite was added to salicylic acid, RR of treatment failure 0.09 (95% CI 0.01 to 0.62; Analysis 11.1).
11.1. Analysis.
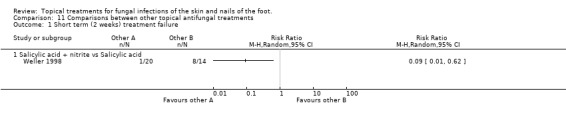
Comparison 11 Comparisons between other topical antifungal treatments, Outcome 1 Short term (2 weeks) treatment failure.
Medium‐term outcome (six weeks)
Ajoene 0.6% and 1.0% Ledezma 2000 compared ajoene 0.6% and 1.0% (n = 29), and found less treatment failures with 1.0% although the difference was not quite statistically significant (RR 0.07, 95% CI 0.00 to 1.17; Analysis 11.2). There was less than 80% follow‐up.
11.2. Analysis.
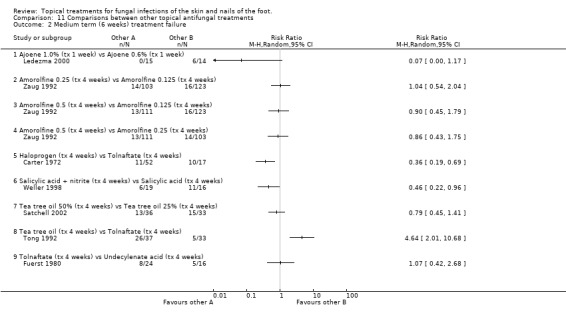
Comparison 11 Comparisons between other topical antifungal treatments, Outcome 2 Medium term (6 weeks) treatment failure.
Amorolfine 0.125, 0.25 and 0.5. Zaug 1992 compared amorolfine 0.125, 0.25 and 0.5 (n = 337), and found little difference in any of the treatment failure rates, follow‐up was at least 80%.
Halprogen versus tolnaftate In a trial of halprogen compared with 1% tolnaftate, both used for 4 weeks (n = 69), Carter 1972 reported outcomes at 6 weeks and found halprogen to provide a statistically significant relative reduction in treatment failure of 64% (RR 0.36, 95% CI 0.19 to 0.69; Analysis 11.4), follow‐up was at least 80%.
11.4. Analysis.
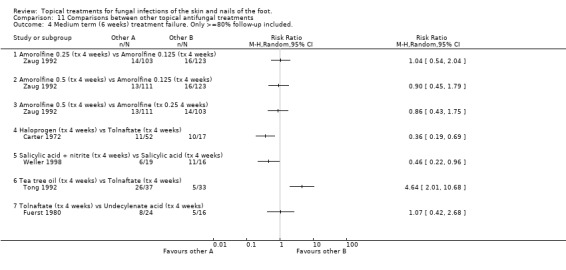
Comparison 11 Comparisons between other topical antifungal treatments, Outcome 4 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included..
Salicylic acid plus nitrate versus salicylic acid Weller 1998 evaluated salicylic acid plus nitrite versus salicylic acid both used for 4 weeks (n = 35) and found that the addition of nitrite provided a statistically significant relative reduction in treatment failure of 54% (RR 0.46, 95% CI 0.22 to 0.96; Analysis 11.4), follow‐up was at least 80%.
Tea tree oil versus tea tree oil A trial comparing 50% tea tree oil versus 25% tea tree oil (n = 69, Satchell 2002) did not show a statistically significant difference in treatment failures (RR 0.79, 95% 0.45 to 1.41; Analysis 11.2).
Tea tree oil versus tolnaftate In a comparison of 10% tea tree oil versus 1% tolnaftate (n = 70), Tong 1992 found tolnaftate to be associated with statistically significantly fewer treatment failures (RR of treatment failure = 4.64 (95% 2.01 to 10.68; Analysis 11.2), follow‐up was at least 80%.
Tolnaftate versus undecanoates In a comparison of 1% tolnaftate versus undecanoates (n = 40), Fuerst 1980 found no difference in treatment failure rates (RR 1.07, 95% 0.42 to 2.68; Analysis 11.2). Long‐term outcome (12+ weeks) Whitfield's ointment versus variotin A very small trial comparing Whitfield's ointment versus variotin applied for 8 weeks (n = 10, Holti 1970) did not detect a statistically significant difference between the 2 treatments (RR of treatment failure 1.33, 95% CI 0.17 to 10.25; Analysis 11.3).
11.3. Analysis.
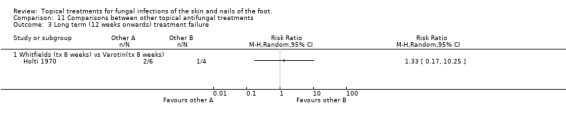
Comparison 11 Comparisons between other topical antifungal treatments, Outcome 3 Long term (12 weeks onwards) treatment failure.
Other outcome measures
Primary
No trials reported quality of life as measured by the cosmetic acceptability of the end result to the participant, absence of itchiness, independence from medical treatment and advice with respect to the condition.
Secondary
1. Measurement of recurrence of the condition in:
(a) skin ‐ maintenance of cure 12 weeks after initiation of intervention.
Clotrimazole four weeks versus clotrimazole one week
In a trial comparing 4 weeks of 1% clotrimazole with 1 week of 1% clotrimazole Bergstresser 1993 found a statistically significant relative reduction in treatment failure of 53% favouring the longer treatment time (RR at 12 weeks 0.47, 95% CI 0.28 to 0.78; Analysis 5.4).
Terbinafine one to three versus five to seven days
Data collected in the trial of terbinafine used for 5‐7 days versus terbinafine used for 1‐3 days (n = 65, Evans 1994) found slightly less treatment failures with 5‐7 days treatment, but the difference was not statistically significant (RR of treatment failure at 12 weeks 0.53, 95% CI 0.15 to 1.88; Analysis 4.4).
4.4. Analysis.
Comparison 4 Comparisons between different allylamines or allylamine regimes, Outcome 4 Long term (12 weeks onwards) treatment failure.
Terbinafine four weeks versus terbinafine one week
A trial comparing outcomes from 1% terbinafine used for 4 weeks versus 1% terbinafine used for 1 week (n = 80, Bergstresser 1993) found less slightly treatment failures with 4 weeks treatment, but the difference was not statistically significant (RR of treatment failure at 12 weeks 0.60, 95% CI 0.24 to 1.54; Analysis 4.4).
Allylamines versus azoles four to six weeks
Two trials (n = 141, Bergstresser 1993; Evans 1993a) collected long term outcomes from comparisons of allylamines versus azoles, the combined data produced a relative risk of treatment failure of 0.47 (95% CI 0.22 to 1.02; Analysis 8.3) which favoured allylamines, but did not quite reach statistical significance.
Long‐term outcome (12+ weeks)
Whitfield's ointment versus variotin
A very small trial comparing Whitfield's ointment versus variotin applied for 8 weeks (n = 10, Holti 1970) did not detect a statistically significant difference between the 2 treatments (RR of treatment failure 1.33, 95% CI 0.17 to 10.25; Analysis 11.3).
Adverse events
Fifty included trials mentioned adverse events in the report; Ablon 1996; Bagatell 1986; Bagatell 1991a; Bagatell 1991b; Bojanovsky 1985; Carter 1972; Del Palacio 1989; Dobson 1989; Elewski 1996; Ellis 1989; Evans 1991; Evans 1993a; Evans 1993b; Evans 1994; Fuerst 1980; Gomez 1986; Gupta 2000a; Gupta 2000b; Holti 1970; Ison 1990; Izuno 1986; Kagawa 1985; Klaschka 1984; Kligman 1985a; Kligman 1985b; Kuhlwein 1990; Ledezma 2000; Mandy 1974; Plotkin 1990; Qadripur 1979; Sanchez 1994; Savin 1990; Savin 1994; Savin 1997; Schachner 1990; Smith 1977; Smith 1986; Smith 1988a; Smith 1988b; Smith 1988c; Smith 1990a; Smith 1990b; Smith 1992; Spiekermann 1976a; Spiekermann 1976b; Syed 1999; Tong 1992; Tschen 1997; Weller 1998; Woscoff 1986; Zaug 1992. Few serious adverse events were reported with the exception of Savin 1997 which found an increase in liver enzymes in one person in the placebo arm of the trial and Smith 1990a which reported elevated liver enzymes in both terbinafine 1% cream and placebo arms of the trial and neutropaenia in one participant in the placebo arm. Most trials which gave detail of the adverse events experience by people participating in the trials reported burning, stinging, itching sensations. There are plans to extend the analysis of adverse events in future review updates.
(b) Nails trials
Placebo comparisons
Ciclopiroxolamine versus placebo
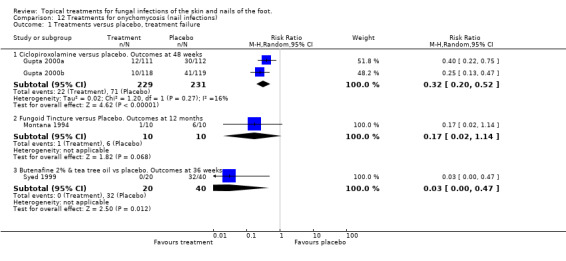
Ciclopiroxolamine lacquer (1%) was compared to placebo in 2 trials (Gupta 2000a; Gupta 2000b) relative risk 0.32; 95% CI 0.20 to 0.52 at 48 weeks (Analysis 12.1).
12.1. Analysis.
Comparison 12 Treatments for onychomycosis (nail infections), Outcome 1 Treatments versus placebo, treatment failure.
Fungoid tincture versus placebo
A comparison of fungoid tincture versus placebo found a relative risk 0.17; 95% CI 0.02 to 1.14 at 12 months; Analysis 12.1 (Montana 1994)
Butenafine + tea tree oil versus placebo
A comparison of 2% butenafine and 5% tea tree oil versus placebo produced a relative risk 0.03; 95% CI 0.00 to 0.47 at 36 weeks; Analysis 12.1, showing butenafine and tea tree oil to be statistically significantly more effective than placebo alone (Syed 1999).
Treatment versus treatment comparisons
Clotrimazole versus tea tree oil
A trial of 1% clotrimazole solution performed better than 100% tea tree oil, though not significantly (Buck 1994).
Amorolfine 5% + methylene versus amorolfine 5% + ethanol
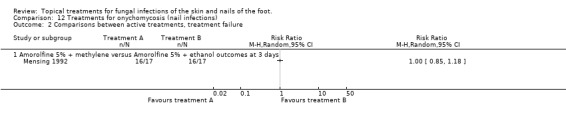
In 1 small trial 2 amorolfine 5% nail lacquer formulations with different vehicles used twice weekly for 4 weeks both achieved a relative risk of 1.00; 95% CI 0.85 to 1.18; Analysis 12.2, at both 3 and 14 days after the end of treatment. (Mensing 1992)
12.2. Analysis.
Comparison 12 Treatments for onychomycosis (nail infections), Outcome 2 Comparisons between active treatments, treatment failure.
Adverse events
Adverse events were reported in four trials evaluating topical nail treatments. In the trial of 2% butenafine with 5% tea tree oil 93.3% of participants had no drug‐related negative side effects. Four participants in the active cream group reported mild inflammation which did not lead to discontinuation or interruption of treatment (Syed 1999). In the trials of ciclopiroxolamine 8% nail lacquer adverse events considered by the investigator to be possibly related to the nail lacquer or vehicle were reported to be as follows: 16 participants in the ciclopiroxolamine group developed a rash compared to 3 participants in the vehicle group; 5 participants in the ciclopiroxolamine group developed nail disorders e.g. changes in the nail shape or colour compared to 5 participants in the vehicle group; 3 participants in the ciclopiroxolamine group showed an application site disorder compared to 3 participants in the vehicle group (Gupta 2000a; Gupta 2000b). In the placebo controlled trial of fungoid tincture minimal adverse effects were noted, mild peeling occurred in eight participants and erythema occurred in one. It is not clear from the report to which arm of the trial the affected participants belonged Montana 1994.
Discussion
Summary of main results
Superficial fungal infections affecting the foot are common and often the first line management strategy is the use of topical agents on both skin and nail infections. The review identified good evidence that allylamines, azoles, butenafine, ciclopiroxolamine, tolciclate and tolnaftate are all efficacious relative to placebo in the management of fungal infections of the skin. Allylamines produced evidence of greater effectiveness when used for longer and there is some evidence that the effect of allylamines increases over time. The observations were collected from a large number of participants (11 trials, n = 1116) providing strong evidence that allylamines are very much more effective than placebo in the management of athletes foot, with an estimated relative reduction in treatment failure at 6 weeks of 67% (RR 0.33, 95% CI 0.24 to 0.44; Analysis 1.1).
The effectiveness of azoles was also seen to improve over time; at outcomes collected six weeks after baseline greater effectiveness than outcomes taken earlier (two weeks after baseline). In common with the trials of allylamines versus placebo, azole creams are very much more effective than placebo, with an estimated relative reduction in treatment failure at 6 weeks of 60% (13 trials, n = 1235, RR 0.40, 95% CI 0.35 to 0.46; Analysis 2.2).
Given the strength of the evidence from a large number of trials and people, and the narrow confidence intervals around the estimates for both allylamines and azoles we would not recommend the use of placebo controls in future RCTs evaluating the use of topical treatments for athlete's foot.
Butenafine, ciclopiroxolamine, tolciclate and tolnaftate also showed greater effectiveness than placebo in the treatment of fungal skin conditions. These results are based on a limited number of trials including small numbers of people however and the evidence is consequently less strong than for allylamines and azoles.
Comparisons between different regimes of allylamines provided little evidence that any regime is more effective than another. Different types of allylamines or different doses were not found to have different treatment failure rates. This might be because there are genuinely no differences in the effectiveness of different types or regimes of topical allylamines, or it may because the trials included too few participants to detect differences between groups.
We did not detect any difference in treatment failure rates between any of the individual azoles, but there is some evidence that the length of treatment affects the success of azoles creams: clotrimazole used for four weeks instead of one week was shown to improve its effectiveness in one trial. Direct comparisons of allylamines versus azoles show allylamines to be generally more efficacious than azoles. Trials directly comparing the two compounds demonstrate the superiority of allylamines. There is little evidence of superiority at 2 weeks but this effect becomes detectable in outcomes taken 6 weeks after treatment begins and appears to remain at 12 weeks. The meta analysis of 8 trials and outcomes from 962 participants supports the finding that allylamines are more effective than azoles when applied for between 4 to 6 weeks. At 6 weeks, there was a relative reduction in treatment failure with allylamines compared to azoles of 37% (RR 0.63 95% CI 0.42 to 0.94; Analysis 8.1).
Meta analyses of randomised controlled trials of allylamines used for one week versus azoles for four weeks demonstrated fairly similar outcomes at six weeks, though there is insufficient evidence to claim that these regimes are equivalent.
Comparisons of other antifungal agents have found that halprogen is more effective than tolnaftate and that combining salicylic acid with nitrite produces greater effectiveness than that of salicylic acid alone. The small trial of Whitfield's ointment and variotin applied for eight weeks only included ten people with athlete's foot and it is therefore unsurprising that the trialists failed to detect differences between these two compounds. Unfortunately there is little evidence to assess tolnaftate against placebo or to compare butenafine, ciclopiroxolamine, tolciclate and tolnaftate with each other.
The review did not find any evidence to support the use of tea tree oil in the management of athlete's foot. Tea tree oil did not produce a greater benefit than placebo, and was significantly less effective than tolnaftate. In a trial of different concentrations of tea tree oil Satchell 2002 did not detect statistically significant differences between concentrations of 50% and 25%. All the randomised evaluations included in this systematic review of tea tree oil suggest that it is ineffective in the management of fungal skin infections.
Evidence about the efficacy of topical treatments for nail infections is sparse. Combining data from 2 trials of ciclopiroxolamine versus placebo found treatments failure rates of 61% and 64% for ciclopiroxolamine. These outcomes followed long treatment times (48 weeks) and this makes ciclopiroxolamine a poor choice for nail infections. Better results were observed with the use of amorolfine lacquer; 6% treatment failure rates were found after 1 month of treatment but these data were collected on a very small sample of people and these high rates of success might be unreliable. Butenafine 2% produced a treatment failure rate of 20%. There is limited evidence about the efficacy of tea tree oil for skin infections; it was evaluated in only one small trial however it was found to be ineffective for fungal nail conditions when compared with topical butenafine.
Quality of the evidence
The randomised controlled trials in this review were generally well reported, and follow up rates were reasonable for such a condition.
Since no trial reported the species obtained from participants who were resistant to treatment we cannot draw conclusions about susceptibility to individual compounds to help clinical decision‐making. Little data was available about the long term outcomes associated with the use of antifungal creams and there is uncertainty about rates of reinfection and relapse.
Authors' conclusions
Implications for practice.
All antifungal compounds demonstrated some success in curing athlete's foot. The best results were observed with the use of allylamines and there is a small amount of evidence that butenafine may be similarly good. Azoles are also very effective and participants should be advised that although all azoles appear to be similarly effective, using an azole cream for four weeks is likely to produce better results than using it for one week. Azoles may also be more efficacious than tolnaftate but they seem no more efficacious than undecanoic acid. There is limited evidence about the efficacy of tea tree oil for skin infections.
There is little evidence that topical anti‐fungals are effective in the management of onychomycosis or fungally infected toe nails. The majority of available data demonstrate low cure rates after long treatment times with ciclopiroxolamine. Amorolfine and butenafine may be much more effective than ciclopiroxolamine and tea tree oil but only a few observations are available.
Implications for research.
The estimates of effectiveness of allylamines and azoles relative to placebo have conclusively demonstrated these drugs to be of greater effectiveness and we recommend that no more placebo controlled trials of allylamines or azoles should be conducted.
More direct comparisons of undecanoic acid and tolnaftate with allylamines and azoles for athlete's foot are required. Large randomised controlled trials comparing the effectiveness of topical amorolfine and butenafine are needed to establish an alternative to oral treatments for toe nail infections.
Feedback
Feedback on section 'Effects of interventions' part (b) Nails trials, 8 January 2009
Summary
In the section 'Effects of interventions' part (b) Nails trials, the authors say "A comparison of 2% butenafine versus 5% tea tree oil produced a relative risk 0.03; 95% CI 0.00 to 0.47 at 36 weeks showing butenafine + tea tree oil to be statistically significantly more effective than tea tree oil alone (Syed 1999).
The study was placebo‐controlled. So, the review should say that butenafine + tea tree oil is more effective than placebo. The way you put it provides evidence that tea tree oil is ineffective. The real situation is that there no evidence one way or the other.
You need to check the graphs and tables for the same error.
NOTE: The submitter agrees with the default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
I wish to thank the person who has given us feedback about the mistake in the section 'Effects of interventions' part (b) Nails trials. I have referred to the original paper (Syed 1999) and can confirm that the criticism is valid; the comparisons were 2% Butenafine together with 5% melaleuca alternifolia versus placebo and I agree that the text should read "A comparison of 2% butenafine and 5% tea tree oil produced a relative risk 0.03; 95% CI 0.00 to 0.47 at 36 weeks showing butenafine and tea tree oil to be statistically significantly more effective than placebo alone”.
Fay Crawford (on behalf of all the authors of this review)
As a result of the above dialogue the editorial base made the appropriate changes to the text in the section 'Effects of interventions' part (b) Nails trials. The graph was moved from Analysis 12.2 'Comparisons between active treatments to Analysis12.1 'Treatments versus placebo'.
Contributors
Michael Power
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2016 | Amended | Edited the published note about the updating of the review. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 9 February 2016 | Amended | This review is going to be updated. We have written a published note to say that we decided to split the topic into topical and device‐based treatments for fungal infections of the toenails and topical treatments for athlete's foot, so a protocol and then a new review will be written. |
| 25 March 2009 | Feedback has been incorporated | Feedback in response to comments made by Micheal Power. |
| 25 March 2009 | Amended | As a result of the feedback received, Analysis 12.1 'Treatments versus placebo' was amended to include butenafine & tea tree oil versus placebo. The text in 'Effects of interventions' part (b) Nails trials was also amended to include this change. |
| 20 July 2008 | Amended | Converted to new review format. |
| 23 May 2007 | New citation required and conclusions have changed | Substantive amendment |
| 9 April 2007 | New search has been performed | Minor update |
| 23 May 2005 | Amended | Reformatted |
| 1 March 2005 | Amended | New studies found and included or excluded |
Notes
This review is being updated by way of a new protocol and then a review, as we decided to split the topic into topical and device‐based treatments for fungal infections of the toenails and topical treatments for athlete's foot. The citation for the new protocol is as follows: Gupta AK, Simpson F, Daigle D, Villanueva E, John D, Foley K. Topical and device‐based treatments for fungal infections of the toenails (Protocol). Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No.: CD012093. DOI: 10.1002/14651858.CD012093.
Acknowledgements
For help with the update of this review and the previous version we thank: Sally Bell‐Syer, Kath Cross, Zelda Di Blasi, Alison Eastwood, Annelise Emmans, Jill Ferrari, Nick Freemantle, Simon Gilbody, Mark Goodfield, Rod Hay, Mark Petticrew, Daphne Russell, Ian Russell, David Torgerson, Trevor Sheldon, Fujian Song, Wendy Tyrrell, Hywel Williams and the Cochrane Skin Group, and Philip Young. The first version of this review was funded by the Wales Office of Research and Development for Health and Social Care (WORD)
We would also like to thank Rachael Hart for helping with the first version of the review and some data extraction for this update.
Appendices
Appendix 1. Search strategy for Cochrane Skin Group specialised register
((FOOT OR FEET) OR TOE* OR NAIL*) AND ((FUNG* OR HYPH*) OR (YEAST OR SPORE*) OR RINGWORM OR (ATHLETE* AND FOOT) OR (TINEA AND PEDIS) OR DERMATOPHYT* OR DERMATOMYCOS* OR DERMATOS* OR MYCOCELI* OR MYCOS* OR MYCETE* OR ONCHYOMYCOS* OR (TINEA AND UNGIUM) OR PARONCHYIA OR (MICROSPORUM AND CANIS) OR TRICHOPHYTO*) AND ((MICONAZOLE OR DAKTARIN) OR (BENZOYL AND PEROXIDE) OR QUINOPED OR AMOROLFINE OR LOCERYL OR CLOTRIMAZOLE OR CANESTAN OR MASNODERM OR (ECONAZOLE AND NITRATE) OR ECOSTATIN OR PEVARYL OR TIOCONAZOLE OR TROSYL OR UNDECENOATE* OR MONPHYTOL OR MYCOTA OR KETOCONAZOL* OR (SALICYLIC AND ACID) OR PHYT* OR NYSTAT* OR TINADERM OR ASTEROL OR DERMONISTAT OR (BENZOIC AND ACID) OR (SULCANAZOLE AND NITRATE) OR EXELDERM OR MYCIL OR TINEAFAX OR TERBINAFINE OR LAMISIL OR ITRACONAZOLE OR SPORANOX)
Appendix 2. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
((foot or feet) or toe* or nail*) (fung* or hypha* or yeast* or spore*) (ringworm or (athlete* next foot) or (tinea next pedis)) FOOT DERMATOSES (dermatophyt* or dermatomycos*) ARTHRODERMATACEAE DERMATOMYCOSES (mycel* or mycete* or mycos*) MYCELIUM (epidermophyto* or microspor* or trichophyto*) EPIDERMOPHYTON MICROSPORUM TRICHOPHYTON (onychomycos* or (tinea next unguium) or paronychia) ONYCHOMYCOSIS PARONYCHIA (#1 or #4) (#2 or #3 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) (#17 and #18) (#14 or #15 or #16) (#19 or #20) (miconazole or daktarin or (benzoyl next peroxide) or amorolfine or loceryl or clotrimazole or canestin or masnoderm) ((econazole next nitrate) or ecostatin or pevaryl or tioconazole or trosyl or undecenoates or monphytol or mycota or ketoconazole or (salicylic next acid) or phytex or phytocil) (nystatin or nystaform or nystan or tinaderm or asterol or dermonistat or (benzoic next acid) or (sulcanazole next nitrate) or exelderm or mycil or tineafax) (#22 or #23 or #24) (#21 and #25)
Appendix 3. Search strategy for MEDLINE (OVID)
1. RANDOMIZED CONTROLLED TRIAL.pt. 2. CONTROLLED CLINICAL TRIAL.pt. 3. RANDOMIZED CONTROLLED TRIALS.sh. 4. RANDOM ALLOCATION.sh. 5. DOUBLE BLIND METHOD.sh. 6. SINGLE BLIND METHOD.sh. 7. OR / 1 ‐ 6 8. ANIMAL.sh. 9 HUMAN.sh. 10. 8 NOT ( 8 and 9 ) 11. 7 NOT 10 12. CLINICAL TRIAL.pt. 13. EXP CLINICAL TRIALS/ 14. ( CLIN$ ADJ 3 TRIAL$ ).ti,ab. 15. (( SINGL$ OR DOUBL$ OR TREB$ OR TRIPL$ ) ADJ3 (BLIND$ OR MASK$ )).ti,ab. 16. PLACEBOS.sh. 17. PLACEBO$.ti,ab. 18. RANDOM. ti,ab. 19. RESEARCH DESIGN.sh. 20. OR / 12 ‐ 19 21. 20 NOT 10 22. 21 NOT 11 23. COMPARATIVE STUDY.sh. 24. EXP EVALUATION STUDIES/ 25. FOLLOW‐UP STUDIES.sh. 26. PROSPECTIVE STUDIES.sh. 27. ( CONTROL$ OR PROSPECTIV$ OR VOLUNTEER$ ). ti,ab. 28. OR / 23 ‐ 27 29. 28 NOT 10 30. 29 NOT ( 11 OR 22 ) 31. ( FOOT or FEET ) ti,ab,sh. 32. ( TOE or TOES ) ti,ab,sh. 33. ( NAIL or NAILS ) ti,ab,sh. 34. OR / 31 ‐ 33 35. ( FUNGUS or FUNGAL or FUNGI or HYPHAE ) ti,ab,sh. 36. ( YEAST or SPORE or SPORES ) ti,ab,sh. 37. ( RINGWORM or ATHLETES FOOT or TINEA PEDIS ) ti,ab,sh. 38. ( DERMATOPHYT$ or DERMATOMYCOSES ) ti,ab,sh. 39. ( MYCELIUM or MYCOSIS or MYCOSES or MYCETES ) ti,ab,sh. 40. ( ONYCHOMYCOS$ or TINEA UNGIUM or PARONYCHIA ) ti,ab,sh. 41. OR / 35 ‐ 40 42. explode FOOT DERMATOSES/ 43. explode ONYCHOMYCOSIS/ 44. ( EPIDERMOPHYTON MICROSPORUM or MICROSPORUM CANIS or EPIDERMOPHYTON FLOCCOSUM or EPIDERMOPHYTOSIS ) ti,ab,sh. 45. ( TRICHOPHYTON RUBRUM or TRICHOPHYTON ERINACEI or TRICHOPHYTON TONSURANS ) ti,ab,sh. 46. ( TRICHOPHYTON MENTAGROPHYTES or TRICHOPHYTON INTERDIGITALE ) ti,ab,sh. 47. ( TRICHOPHYTON SOUDANESE or TRICHOPHYTON VIOLACEUM ) ti,ab,sh. 48. OR / 44 ‐ 47 49. ( MICONAZOLE or DAKTARIN ) ti,ab,sh. 50. ( BENZOYL PEROXIDE or QUINOPED ) ti,ab,sh. 51. ( AMOROLFINE or LOCERYL or CLOTRIMAZOLE or CANESTIN or MASNODERM ) ti,ab,sh. 52. ( ECONAZOLE NITRATE or ECOSTATIN or PEVARYL or TIOCONAZOLE or TROSYL ) ti,ab,sh. 53. ( UNDECENOATES or MONPHYTOL or MYCOTA ) ti,ab,sh. 54. ( KETOCONAZOLE or SALICYLIC ACID or PHYTEX or PHYTOCIL ) ti,ab,sh. 55. ( NYSTATIN or NYSTAFORM or NYSTAN or TINADERM or ASTEROL or DERMONISTAT ) ti,ab,sh. 56. ( BENZOIC ACID or SULCANAZOLE NITRATE or EXELDERM or MYCIL or TINEAFAX ) ti,ab,sh. 57. OR / 49 ‐ 56 58. 34 AND 41 59. 34 AND 48 60. 34 AND 57 61. 42 OR 43 OR 58 OR 59 OR 60 62. 61 AND ( 11 OR 22 OR 30 )
Data and analyses
Comparison 1. Allylamines vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 9 | 928 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.56, 0.87] |
| 1.1 Naftifine vs Placebo | 5 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.93] |
| 1.2 Terbinafine vs Placebo | 4 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.31, 1.08] |
| 2 Medium term (6 weeks) treatment failure | 11 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.24, 0.44] |
| 2.1 Naftifine (tx 4 weeks) vs Placebo | 5 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.30, 0.59] |
| 2.2 Terbinafine (tx 1 week) vs Placebo | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.09, 0.26] |
| 2.3 Terbinafine (tx 2 weeks) vs Placebo | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.27, 0.48] |
| 2.4 Terbinafine (tx 4 weeks) vs Placebo | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.82] |
| 3 Medium term (6 weeks) treatment failure. Only >80% follow‐up included | 9 | 876 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.21, 0.45] |
| 3.1 Naftifine (tx 4 weeks) vs Placebo | 5 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.30, 0.59] |
| 3.2 Terbinafine (tx 1 week) vs Placebo | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.16 [0.09, 0.26] |
| 3.3 Terbinafine (tx 4 weeks) vs Placebo | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.04, 0.82] |
Comparison 2. Azoles (tx 4‐6 weeks) vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 5 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.82] |
| 1.1 Bifonazole vs Placebo | 4 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.37, 0.73] |
| 1.2 Oxiconazole vs Placebo | 1 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.02] |
| 2 Medium term (6 weeks) treatment failure | 13 | 1235 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.35, 0.46] |
| 2.1 Bifonazole (tx 4 weeks) vs Placebo | 4 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.67] |
| 2.2 Clotrimazole (tx 4‐6 weeks) vs Placebo | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.27, 0.64] |
| 2.3 Miconazole nitrate (tx 4 weeks) vs Placebo | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.14] |
| 2.4 Oxiconazole (tx 4 weeks) vs Placebo | 2 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.30, 0.46] |
| 2.5 Sulconazole nitrate (tx 4‐6 weeks) vs Placebo | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.31, 0.75] |
| 2.6 Ticonazole (tx 4‐6 weeks) vs Placebo | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.22, 0.62] |
| 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included | 6 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.34, 0.53] |
| 3.1 Clotrimazole (tx 6 weeks) vs Placebo | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.98] |
| 3.2 Miconazole nitrate (tx 4 weeks) vs Placebo | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.14, 1.14] |
| 3.3 Oxiconazole (tx 4 weeks) vs Placebo | 1 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.23, 0.52] |
| 3.4 Sulconazole nitrate (tx 4‐6 weeks) vs Placebo | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.31, 0.75] |
| 3.5 Ticonazole (tx 4‐6 weeks) vs Placebo | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.22, 0.62] |
Comparison 3. Other topical antifungal treatments vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Butenafine vs Placebo | 3 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.23, 0.78] |
| 1.2 Ciclopiroxolamine vs Placebo | 1 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.27, 0.68] |
| 1.3 Undecanoates vs Placebo | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.06, 0.31] |
| 2 Medium term (6 weeks) treatment failure | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Butenafine (tx 1 week) vs Placebo | 1 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.24, 0.45] |
| 2.2 Butenafine (tx 4 weeks) vs Placebo | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.08, 0.43] |
| 2.3 Ciclopiroxolamine (tx 4 weeks) vs Placebo | 2 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.11, 0.66] |
| 2.4 Tea tree oil (tx 4 weeks) vs Placebo | 2 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.48, 1.11] |
| 2.5 Tolciclate (tx 6 weeks) vs Placebo | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.63] |
| 2.6 Tolnaftate (tx 4 weeks) vs Placebo | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.72] |
| 2.7 Undecanoates (tx 4‐6 weeks) vs Placebo / no treatment | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included. | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Ciclopiroxolamine (tx 4 weeks) vs Placebo | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.23, 0.66] |
| 3.2 Tea tree oil (tx 4 weeks) vs Placebo | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.68, 1.16] |
| 3.3 Tolciclate (tx 6 weeks) vs Placebo | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.63] |
| 3.4 Tolnaftate (tx 4 weeks) vs Placebo | 2 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.13, 0.72] |
| 3.5 Undecanoates (tx 4‐6 weeks) vs Placebo / no treatment | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.08, 0.74] |
3.3. Analysis.
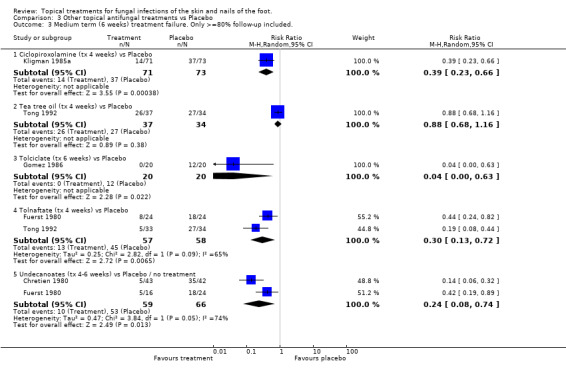
Comparison 3 Other topical antifungal treatments vs Placebo, Outcome 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included..
Comparison 4. Comparisons between different allylamines or allylamine regimes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Naftifine once daily vs Naftifine twice daily | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Naftifine vs Terbinafine | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Medium term (6 weeks) treatment failure | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Naftifine once daily (tx 4 weeks) vs Naftifine twice daily (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Naftifine (tx 2 weeks) vs Terbinafine (tx 2 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Terbinafine (tx 5‐7 days) vs Terbinafine (tx 1 ‐3 days) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Terbinafine (tx 4 weeks) vs Terbinafine (tx 1 week) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included. | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Naftifine once daily (tx 4 weeks) vs Naftifine twice daily (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Naftifine (tx 2 weeks) vs Terbinafine (tx 2 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Terbinafine (tx 5‐7 days) vs Terbinafine (tx 1‐3 days) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Long term (12 weeks onwards) treatment failure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Terbinafine (tx 5‐7 days) vs Terbinafine (tx 1 ‐3 days) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Terbinafine (tx 4 weeks) vs Terbinafine (tx 1 week) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.3. Analysis.
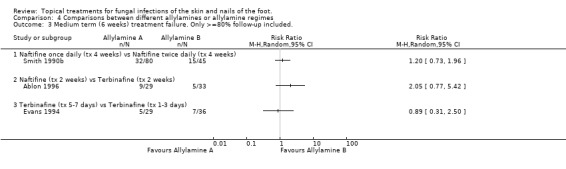
Comparison 4 Comparisons between different allylamines or allylamine regimes, Outcome 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included..
Comparison 5. Comparisons between different azoles or azole regimes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Clotrimazole vs Econazole | 2 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.39] |
| 1.2 Econazole gel vs Econazole cream | 1 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.60, 1.23] |
| 1.3 Miconazole nitrate vs Sulconazole nitrate | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 6.30 [0.83, 47.80] |
| 1.4 Oxiconazole twice daily vs Oxiconazole once daily | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.74, 1.49] |
| 2 Medium term (6 weeks) treatment failure | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Bifonazole (tx 3 weeks) vs Bifonazole removed after 1 hr (tx 3 weeks) | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 4.34 [0.51, 37.01] |
| 2.2 Bifonazole (tx 3 weeks) vs Croconazole (tx 3 weeks) | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Bifonazole (tx 3 weeks) vs Miconazole (tx 3 weeks) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.27, 2.37] |
| 2.4 Clotrimazole (tx 4 weeks) vs Clotrimazole (tx 1 week) | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.18, 0.72] |
| 2.5 Clotrimazole (tx 2‐4 weeks) vs Econazole (tx 2‐4 weeks) | 2 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.31, 2.88] |
| 2.6 Econazole gel (tx 2 weeks) vs Econazole cream (tx 2 weeks) | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.53, 1.16] |
| 2.7 Clotrimazole (tx 4 weeks) vs Ketoconazole (tx 4 weeks) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.63, 1.76] |
| 2.8 Miconazole (tx 6 weeks) vs Ticonazole (tx 6 weeks) | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.77, 2.16] |
| 2.9 Oxiconazole+fluctic (tx 4 weeks) vs Oxiconazole (tx 4 weeks) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.87] |
| 2.10 Oxiconazole twice daily (tx 4 weeks) vs Oxiconazole once daily (tx 4 weeks) | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.61, 2.46] |
| 2.11 Bifonazole (tx 4 weeks) vs flutrimazole (tx 4 weeks) | 1 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.14, 0.31] |
| 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included. | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Bifonazole (tx 3 weeks) vs Bifonazole removed after 1 hr (tx 3 weeks) | 1 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 4.34 [0.51, 37.01] |
| 3.2 Bifonazole (tx 3 weeks) vs Croconazole (tx 3 weeks) | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Bifonazole (tx 3 weeks) vs Miconazole (tx 3 weeks) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.27, 2.37] |
| 3.4 Clotrimazole (tx 2‐4 weeks) vs Econazole (tx 2‐4 weeks) | 2 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.31, 2.88] |
| 3.5 Econazole gel (tx 2 weeks) vs Econazole cream (tx 2 weeks) | 1 | 313 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.53, 1.16] |
| 3.6 Miconazole (tx 6 weeks) vs Ticonazole (tx 6 weeks) | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.77, 2.16] |
| 3.7 Oxiconazole+fluctic (tx 4 weeks) vs Oxiconazole (tx 4 weeks) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.87] |
| 3.8 Oxiconazole twice daily (tx 4 weeks) vs Oxiconazole once daily (tx 4 weeks) | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.61, 2.46] |
| 4 Long term (12 weeks onwards) treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Clotrimazole (tx 4 weeks) vs Clotrimazole (tx 1 week) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.3. Analysis.
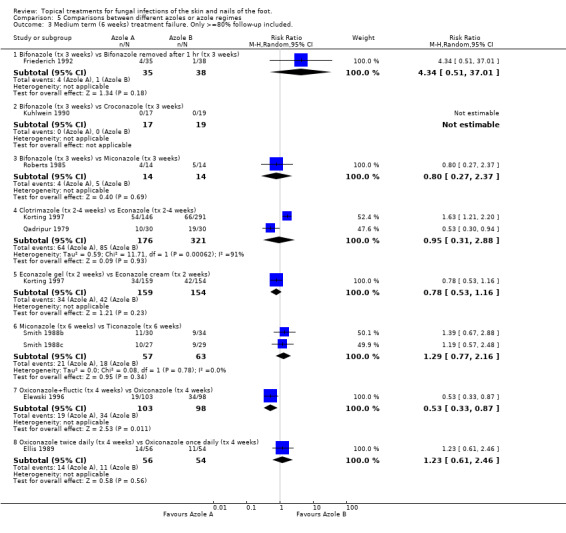
Comparison 5 Comparisons between different azoles or azole regimes, Outcome 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included..
Comparison 6. Allylamines 1‐2 weeks vs Azoles 1‐2 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 10 | 1519 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.06] |
| 1.1 Naftifine vs Bifonazole | 1 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.78, 1.47] |
| 1.2 Naftifine vs Clotrimazole | 4 | 617 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.53, 1.14] |
| 1.3 Naftinfine/Terbinane vs Oxiconazole | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.65, 1.09] |
| 1.4 Terbinafine vs Bifonazole | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.14, 0.66] |
| 1.5 Terbinafine vs Clotrimazole | 3 | 672 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.86, 1.55] |
| 2 Medium term (6 weeks) treatment failure | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.22, 0.52] |
| 2.1 Naftinfine/Terbinane vs Oxiconazole | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.20, 0.56] |
| 2.2 Terbinafine vs Clotrimazole | 1 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.17, 0.75] |
| 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included. | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.20, 0.56] |
| 3.1 Naftinfine/Terbinane 2 weeks vs Oxiconazole 2 weeks | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.20, 0.56] |
| 4 Long term (12 weeks onwards) treatment failure | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.58] |
| 4.1 Terbinafine vs Clotrimazole | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.14, 0.58] |
Comparison 7. Allylamines 1 week vs Azoles 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Medium term (6 weeks) treatment failure | 5 | 962 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.33, 1.72] |
| 1.1 Terbinafine vs Clotrimazole | 4 | 771 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.24, 1.75] |
| 1.2 Terbinafine vs Miconazole | 1 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.36, 5.91] |
| 2 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included. | 3 | 685 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.10, 2.54] |
| 2.1 Terbinafine vs Clotrimazole | 3 | 685 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.10, 2.54] |
Comparison 8. Allylamines 4‐6 weeks vs Azoles 4‐6 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Medium term (6 weeks) treatment failure | 9 | 1003 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.42, 0.94] |
| 1.1 Naftifine vs Bifonazole | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.63, 6.08] |
| 1.2 Naftifine vs Clotrimazole | 6 | 775 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.81] |
| 1.3 Terbinafine vs Bifonazole | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Terbinafine vs Clotrimazole | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.44, 2.60] |
| 2 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included. | 6 | 854 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.34, 0.89] |
| 2.1 Naftifine vs Bifonazole | 1 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.63, 6.08] |
| 2.2 Naftifine vs Clotrimazole | 5 | 757 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.30, 0.76] |
| 3 Long term (12 weeks onwards) treatment failure | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.22, 1.02] |
| 3.1 Naftifine vs Clotrimazole | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.08, 2.56] |
| 3.2 Terbinafine vs Clotrimazole | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.20, 1.13] |
Comparison 9. Allylamines vs Other antifungal topical skin treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Terbinafine vs Butenafine | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Medium term (6 weeks) treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Terbinafine 1% (tx 1 week) vs Ajoene 0.6% (tx 1 week) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Terbinafine 1% (tx 1 week) vs Ajoene 1% (tx 1 week) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
9.2. Analysis.
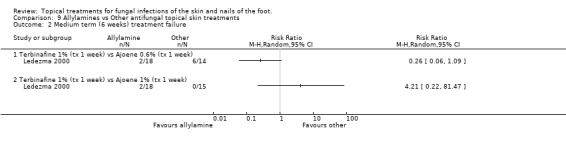
Comparison 9 Allylamines vs Other antifungal topical skin treatments, Outcome 2 Medium term (6 weeks) treatment failure.
Comparison 10. Azoles vs Other antifungal topical skin treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Clotrimazole vs Ciclopiroxolamine | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Medium term (6 weeks) treatment failure | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Bifonazole (tx 6 weeks) vs Amorolfine (tx 6 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Clotrimazole (tx 4 weeks) vs Ciclopirox olamine (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included. | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Bifonazole (tx 6 weeks) vs Amorolfine (tx 6 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Clotrimazole (tx 4 weeks) vs Ciclopirox olamine (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Comparisons between other topical antifungal treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short term (2 weeks) treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Salicylic acid + nitrite vs Salicylic acid | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Medium term (6 weeks) treatment failure | 7 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Ajoene 1.0% (tx 1 week) vs Ajoene 0.6% (tx 1 week) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Amorolfine 0.25 (tx 4 weeks) vs Amorolfine 0.125 (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Amorolfine 0.5 (tx 4 weeks) vs Amorolfine 0.125 (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Amorolfine 0.5 (tx 4 weeks) vs Amorolfine 0.25 (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Haloprogen (tx 4 weeks) vs Tolnaftate (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Salicylic acid + nitrite (tx 4 weeks) vs Salicylic acid (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Tea tree oil 50% (tx 4 weeks) vs Tea tree oil 25% (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 Tea tree oil (tx 4 weeks) vs Tolnaftate (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.9 Tolnaftate (tx 4 weeks) vs Undecylenate acid (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Long term (12 weeks onwards) treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Whitfields (tx 8 weeks) vs Varotin(tx 8 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Medium term (6 weeks) treatment failure. Only >=80% follow‐up included. | 5 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Amorolfine 0.25 (tx 4 weeks) vs Amorolfine 0.125 (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Amorolfine 0.5 (tx 4 weeks) vs Amorolfine 0.125 (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Amorolfine 0.5 (tx 4 weeks) vs Amorolfine (tx 0.25 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Haloprogen (tx 4 weeks) vs Tolnaftate (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Salicylic acid + nitrite (tx 4 weeks) vs Salicylic acid (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Tea tree oil (tx 4 weeks) vs Tolnaftate (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Tolnaftate (tx 4 weeks) vs Undecylenate acid (tx 4 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Treatments for onychomycosis (nail infections).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatments versus placebo, treatment failure | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Ciclopiroxolamine versus placebo. Outcomes at 48 weeks | 2 | 460 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.20, 0.52] |
| 1.2 Fungoid Tincture versus Placebo. Outcomes at 12 months | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.14] |
| 1.3 Butenafine 2% & tea tree oil vs placebo. Outcomes at 36 weeks | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.47] |
| 2 Comparisons between active treatments, treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Amorolfine 5% + methylene versus Amorolfine 5% + ethanol outcomes at 3 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ablon 1996.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: not stated Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: 11 | |
| Participants | Total evaluable sample size: 90 Inclusion criteria: male Exclusion criteria: onychomychosis Treatment setting: Ambulatory care dermatology clinic | |
| Interventions | a. Naftifine cream Duration: two weeks Frequency: applied once daily b. Terbinafine cream Duration: two weeks Frequency: applied once daily c. Oxiconazole lotion Duration: two weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, scaling, fissuring, exudation, pruritus, maceration, vesiculation, burning Adverse events: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Akers 1989.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age:yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: no Intention to treat analysis: no A Priori sample size calculation: no Fungi identified: yes Distribution of species between groups: not stated Adverse events reported: yes Number of drop outs stated: 30 | |
| Participants | Total evaluable sample size: 229 | |
| Interventions | a. Sulconazole nitrate 1% cream Duration: four weeks Frequency: applied twice daily b. Placebo cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: Cure Secondary Outcomes: Signs and symptoms ‐ itching, erythems, scaling, fissuring, exudation, pustules, maceration, vesiculation | |
| Notes | Chronic moccasin type tinea pedis. Data extracted for T. rubrum only (92% of those with + ve cultures) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Aly 2003.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: no Intention to treat analysis: no A Priori sample size calculation: no Fungi identified: yes Distribution of species between groups: not stated Adverse events reported: yes Number of drop outs stated: 57 | |
| Participants | Total evaluable sample size: 317 Setting: Multicentre study in USA. Exclusion criteria treatment with any medication that could affect the course of the disease. Fungal infection else where on the body. Topical antifungals had a 14 day wash‐out period and systemic a 28 day washout period. |
|
| Interventions | a. Ciclopirox Gel 0.77% Duration X 2 daily for 28 days b. Vehicle Duration X 2 daily for 28 days | |
| Outcomes | Primary outcome: treatment success defined as mycological cure and > % 75% clinical improvement | |
| Notes | Interdigital tinea pedis Adverse effects; 14 ciclopirox and 13 vehicle subjects reported burning sensations of the skin. Four ciclopirox and one vehicle subjects reported pruritus. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Bagatell 1986.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint:yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 47 Inclusion criteria: tinea pedis interdigitalis Exclusion criteria: use of topical/systemic antifungal therapy within one week prior to study, use of Griseofulvin four weeks prior to study Treatment setting: out‐patient department | |
| Interventions | a. Bifonazole 1% solution Duration: four weeks Frequency: applied once daily b. Placebo solution Duration: four weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms Adverse events: a. two burning sensations b. one(three) burning sensations | |
| Notes | Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bagatell 1991a.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: no Intention to treat analysis: no A Priori sample size calculation: no Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: 13 | |
| Participants | Total evaluable sample size: 88 Treatment setting: Four different sites | |
| Interventions | a. Naftifine 1% gel Duration: four weeks Frequency: applied twice daily b. Placebo gel Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: Cure Secondary Outcomes: Signs and symptoms ‐ fissuring, pruritus,vesiculation Global improvement Adverse events: a. 8 b. 18 burning, stinging, itching | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bagatell 1991b.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: no Intention to treat analysis: no A Priori sample size calculation: no Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: 13 | |
| Participants | Total evaluable sample size: 88 Treatment setting: Four different sites | |
| Interventions | a. Naftifine 1% gel Duration: four weeks Frequency: applied twice daily b. Placebo gel Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: Cure Secondary Outcomes: Signs and symptoms ‐ fissuring, pruritus,vesiculation Global improvement Adverse events: a. 8 b. 18 burning, stinging, itching | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bergstresser 1993.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not stated Adverse events reported: not stated Number of drop outs stated: not stated | |
| Participants | Total evaluable sample size: 193 Inclusion criteria: baseline total score for the target lesion of six or more, including a score of at least two for erythema or a score of two or more for each of the two other signs Treatment setting: eight multicentres | |
| Interventions | a. Terbinafine Duration: one week Frequency: applied twice daily b. Terbinafine Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, scaling, fissuring, exudation, pruritus, maceration, vesiculation, burning and stinging | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Berman 1992.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: no Intention to treat analysis: yes A Priori sample size calculation: no Fungi identified: yes Distribution of species between groups: yes Adverse events reported: no Number of drop outs stated: 18 | |
| Participants | Total evaluable sample size: 159 Inclusion criteria: total score for target lesion of six or more including a score of two or more for erythema or two for at least two other signs Exclusion criteria: Non‐interdigital lesions (i.e. Moccasin type), onychomychosis, systemic fungal disease Treatment setting: multicentre | |
| Interventions | a. Terbinafine Duration: one week Frequency: applied twice daily b. Placebo Duration: one week Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, desquamation, fissuring, maceration, vesiculation, exudation, pruritus, burning/stinging | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bojanovsky 1985.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: no Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 99 Exclusion criteria: onychomycosis | |
| Interventions | a. Bifonazol cream Duration: five weeks Frequency: applied once a day b. Naftifine cream Duration: five weeks Frequency: applied once a day | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: a. dermatitis,dry skin, burning sensation (5) | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Buck 1994.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: not stated A Priori sample size calculation: yes Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size:117 Exclusion criteria:immune suppressant therapy within the previous 6 months. Had used a topical agent on the toenails in the previous two weeks, a history of HIV or psoriasis |
|
| Interventions | a. 1% clotrimazole soln b. Tea tree oil | |
| Outcomes | Primary outcome; cure
Secondary outcomes;
a. measuring the distance between the cuticle and the most proximal onychomycotic border b. recording the percentage of involvement in 25% increments |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Carter 1972.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not given Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 81 Exclusion criteria: any therapeutic or prophylactic medication taken within three weeks prior to study Treatment setting: prison | |
| Interventions | a. Haloprogin 1% foam, solution or cream Duration: four weeks Frequency: applied twice daily b. Tolnaftate 1% solution Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: lesion scores Adverse events: none in either group | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Chretien 1980.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: not stated Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Reoccurrence status: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: not stated Number of drop outs stated: not stated | |
| Participants | Total evaluable sample size: 85 | |
| Interventions | A. 2% undecylenic acid and 20% zinc acid powder Duration: four weeks Frequency: applied twice daily b. Placebo powder Duration: four weeks Frequency: applied twice daily |
|
| Outcomes | Primary outcome: cure Secondary outcomes:signs and symptoms ‐ erthythema, scaling, fissuring, hyperkeratosis | |
| Notes | Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Coffey 1986.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: not stated Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Reoccurrence status: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: not stated Number of drop outs stated: not stated | |
| Participants | Total evaluable sample size: 43 Exclusion criteria: Topical and systemic antifungal treatments used within one week prior to study Treatment setting: Dermatology medical clinic | |
| Interventions | a. Bifonazole 1% cream Duration: four weeks Frequency: applied once daily b. Placebo cream Duration: four weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythems, scaling, fissuring, exudation, pustules, maceration, vesiculation | |
| Notes | Tinea pedis interdigital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Del Palacio 1989.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Reoccurrence status: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: nine Exclusion criteria: use of antifungal therapy within two weeks prior to study | |
| Interventions | a. Bifonazole 1% cream Duration: max six weeks Frequency: once daily b. Amorolfine 0.5% cream Duration: max six weeks Frequency: once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, burning, redness, weeping, scaling, pustulation, incrustation Adverse events: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dobson 1989.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Reoccurrence status: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: 41 | |
| Participants | Total evaluable sample size: 183 Inclusion criteria: 12 yrs and older Exclusion criteria: Hyperkeratotic plantar type tinea pedis Treatment setting: eight different sites | |
| Interventions | a. Naftifine 1% cream Duration: four weeks Frequency: applied once daily b. Placebo cream Duration: four weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: Cure Secondary Outcomes: Signs and symptoms ‐ erythems, maceration, pruritus Global improvement Adverse events: a. 2 b. 5 soreness, burning, eczema, exacerbation of folliculitis, itching | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Elewski 1996.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not given Adverse events reported: yes Number of drop outs stated: three | |
| Participants | Total evaluable sample size: 321 Inclusion criteria: minimum severity score of six at least 1.5 for erythema and 2 for pruritus Treatment setting: 17 centres U.S.A | |
| Interventions | a. Oxiconazole nitrate 1% and fluticazole 0.05% combination cream Duration: one week of above, 3 weeks of oxiconazole nitrate 1% Frequency: applied twice daily b. Oxiconazole nitrate 1% cream Duration: four weeks Frequency: applied twice daily c. Placebo cream Duration: four weeks Frequency: Twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: a. 5.3% b. 7.5% c. 6% burning and pruritus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ellis 1989.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified:yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: 108 | |
| Participants | Total evaluable sample size: 163 Exclusion criteria: use of topical antifungal therapy within 7 days prior to study use of systemic antifungal therapy within 30 days prior to study Treatment setting: twelve centres | |
| Interventions | a. Oxiconazole nitrate cream Duration: four weeks Frequency: once daily plus once daily Placebo b. oxiconazole nitrate cream Duration: four weeks Frequency: twice daily c. Placebo Duration: four weeks Frequency: twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, scaling, fissuring, pustules, maceration, burning, crusting Global response Adverse events: burning, irritation, pruritus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Evans 1991.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not given Adverse events reported: yes Number of drop outs stated: 38 | |
| Participants | Total evaluable sample size: 48 Exclusion criteria: use of systemic antifungal therapy within two weeks prior to study | |
| Interventions | a. Terbinafine 1% cream Duration: two weeks Frequency: applied once daily b. Placebo cream Duration: two weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: a. 2 b. 1 erythematous rash | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Evans 1993a.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Reoccurrence status: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes | |
| Participants | Total evaluable sample size: 63 Inclusion criteria: symptom/sign score greater than three Treatment setting: multicentre | |
| Interventions | a. Naftifine 1% cream Duration: four weeks Frequency: applied twice daily b. 1% Clotrimazole and 1% hydrocortisone cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, scaling, exudation, pustules, vesiculation, pruritus and crusting Adverse events | |
| Notes | Data extracted only for patients who were microscopy and culture positive at baseline only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Evans 1993b.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: yes A Priori sample size calculation: yes Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: 44 | |
| Participants | Total evaluable sample size: 211 Exclusion criteria: use of topical antifungal within seven days prior to study use of systemic antifungal within six weeks prior to study Treatment setting: General practice and hospital | |
| Interventions | a. Terbinafine 1% cream Duration: one week plus three weeks Placebo Frequency: applied twice daily b. Clotrimazole 1% cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: a. 4 stinging, cracks, itching, eye irritation, erythema, swelling b. 4 erythema, soreness, rash, cellulitis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Evans 1994.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not given Adverse events reported: yes Number of drop outs stated: 13 | |
| Participants | Total evaluable sample size: 65 Exclusion criteria: use of topical antifungal within seven days of study use of systemic antifungal within six weeks prior to study use of oral Terbinafine within three months prior to study Treatment setting: general practice | |
| Interventions | a. Terbinafine 1% cream Duration: one day plus six days Placebo Frequency: applied once daily b. Terbinafine 1% cream Duration: three days plus four days Placebo Frequency: once daily c. Terbinafine 1% cream Duration: five days plus two days Placebo Frequency: once daily d. Terbinafine 1% cream Duration: seven days Frequency: once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: c. 1 mild pruritus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Friederich 1992.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: not stated Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: no Adverse events reported: no Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 73 Inclusion criteria: patients consent to remain in hospital for 21 days Exclusion criteria: use of antimycotic treatment four weeks prior to study | |
| Interventions | a. Bifonazol cream Duration: three weeks Frequency: applied once daily b. Bifonazol cream Duration: three weeks Frequency: applied once daily, washed off after one hour | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation | |
| Notes | Translated from German Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fuerst 1980.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: not stated Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 103 Exclusion criteria: use of antifungal therapy within two weeks prior to study, use of Griseofulvin within six weeks prior to study Treatment setting: Prison rehabilitation centre | |
| Interventions | a. Undecylenic acid ointment Duration: four weeks Frequency: applied twice daily b. Tolnaftate cream Duration: four weeks Frequency: applied twice daily c. Placebo Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: b. 1 worsening of symptoms | |
| Notes | Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Gentles 1974.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: no Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 45 | |
| Interventions | a. Miconazole cream and powder Duration: four weeks Frequency: cream ‐ pm powder ‐ am b. Placebo cream and powder Duration: four weeks Frequency: cream ‐ pm powder ‐ am | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms | |
| Notes | Data extracted for positive culture at baseline only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Gomez 1986.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study:yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: no A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 40 Exclusion criteria: use of any topical/systemic antifungal treatment within two weeks prior to study Treatment setting: University of California | |
| Interventions | a. Tolciclate 1% solution Duration: six weeks Frequency: applied twice daily b. Placebo solution Duration: six weeks Frequency: applied twice daily: | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, scaling, fissuring, maceration, suppuration Adverse events: none in either group | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Gupta 2000a.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study; yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 211 Exclusion criteria: white superficial or proximal subungual onychomycosis. Nail dystrophy. Infection which led to epinychium, antifungal therapy 24 weeks prior to study. Treatment setting; Multicentre studies USA | |
| Interventions | a. Ciclopirox nail lacquer 8% solution Duration: 48 weeks Frequency: applied once daily b. Placebo solution Duration: 48 weeks Frequency: applied once daily: | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Planimetric measurements % affected nail, Global evaluation score 0=cured, 1=excellent improvement, 2=moderate improvement; 3=slight improvement; 4= no change. Adverse events: 10 (9%) group a, 7 (6%) group b. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Gupta 2000b.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study; yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 237 Exclusion criteria: white superficial or proximal subungual onychomycosis. Nail dystrophy. Infection which led to epinychium, antifungal therapy 24 weeks prior to study. Treatment setting; Multicentre studies USA | |
| Interventions | a. Ciclopirox nail lacquer 8% solution Duration: 48 weeks Frequency: applied once daily b. Placebo solution Duration: 48 weeks Frequency: applied once daily: | |
| Outcomes | Primary outcome: cure
Secondary Outcomes: Planimetric measurements % affected nail, Global evaluation score 0=cured, 1=excellent improvement, 2=moderate improvement; 3=slight improvement; 4= no change.
Adverse events: 10 (9%) group a,
7 (6%) group b. Data not presented |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Haas 1985.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 204 Exclusion criteria: onychomychosis, use of systemic/topical antifungal treatment within four weeks prior to study | |
| Interventions | a. Naftifine cream Duration: four weeks Frequency: applied twice daily b. Clotrimazole cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: ten patients in each group complained of either irritation, burning or dryness | |
| Notes | multicenter trial Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Hollmen 2002.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study; yes Participants comparable at baseline for age: yes Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: yes Fungi identified: not stated Distribution of species between groups: not stated Adverse events reported: not stated Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 70 Exclusion criteria: less than 12 years of age. Inclusion criteria; tinea pedis. Treatment setting Six centres (three Belgium, three Finland) | |
| Interventions | a. Terbinafine 1% gel Duration: Seven days Frequency: applied once daily b. Placebo Duration: seven days Frequency: applied once daily | |
| Outcomes | Primary outcome; Mycological cure
Minimal signs of clinical disease erythema, desquamation, pruritis < 2 with no individual score > 1 and a severity score for pustulation, encrustation, and vesiculation of 0 Clinical cure; 6 symptoms assessed; 0= absent, 3= severe |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Holti 1970.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: not stated | |
| Participants | Total evaluable sample size: 12 Treatment setting: multicentre | |
| Interventions | a. Whitfields ointment Duration: eight weeks Frequency: once daily b. Varotin ointment Duration: eight weeks Frequency: once daily | |
| Outcomes | Primary outcome: cure Adverse events: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ison 1990.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: 13 | |
| Participants | Total evaluable sample size: 111 Exclusion criteria: onychomycosis use of systemic antifungal therapy within two weeks prior to study topical treatment stopped at entry | |
| Interventions | a. Econazole nitrate 1% cream Duration: four weeks Frequency: applied once daily b. Placebo cream Duration: four weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Adverse events: six patients in each group complained of either upper respiratory tract infections, back pain, tooth ache, headache, pleurisy or swollen ankles | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Izuno 1986.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size:38 Exclusion criteria: use of topical/systemic antifungal therapy one week prior to study, use of Griseofulvin four weeks prior to study | |
| Interventions | a. Bifonazole 1% cream Duration: four weeks Frequency: applied once daily b. Placebo Duration: four weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms Adverse events: none | |
| Notes | Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kagawa 1985.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Single blind study Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: 55 | |
| Participants | Total evaluable sample size: 379 Exclusion criteria: Hyperkeratotic tinea pedis use of systemic antifungals one month prior to study use of topical antifungals one week prior to study | |
| Interventions | a. Naftifine Duration: five weeks Frequency: applied twice daily b. Clotrimazole Duration: five weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, exudation, papules, vesiculation, erosion Global efficacy Adverse events: slight | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Klaschka 1984.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study:yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported:yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 60 Exclusion criteria: use of other systemic/topical antifungal therapies | |
| Interventions | a. Naftifine gel Duration: four weeks Frequency: applied twice daily b. Placebo gel Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: a. 11 b. 15 slight burning sensation, dryness of skin | |
| Notes | Translated from German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kligman 1985a.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: 12 | |
| Participants | Total evaluable sample size: 168 Exclusion criteria: use of antifungal therapy one week prior to study Treatment setting: Multicentre | |
| Interventions | a. Ciclopiroxolamine 1% cream Duration: four weeks Frequency: applied twice daily b. Placebo cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: one person in each group had worsening of symptoms | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kligman 1985b.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 87 Exclusion criteria: use of antifungal therapy within one week prior to study Treatment setting: multicenter | |
| Interventions | a. Ciclopiroxolamine 1% cream Duration: four weeks Frequency: applied twice daily b. Clotrimazole 1% cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Clinical cure Adverse events: none | |
| Notes | Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Korting 1997.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: yes A Priori sample size calculation: yes Fungi identified: no Distribution of species between groups: no Adverse events reported: no Number of drop outs stated: 111 | |
| Participants | Total evaluable sample size: 424 | |
| Interventions | a. Econazole liposome 1% gel Duration: two weeks Frequency: once daily b. Branded econazole 1% cream Duration: two weeks Frequency: applied once daily c. Generic Clotrimazole 1% cream Duration: two weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ pruritus, erythema, scaling, fissuring, maceration Tolerability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Korting 2001.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated. Fungi identified: no Distribution of species between groups: no Adverse events reported: reported none occurred Number of drop outs stated: 30 | |
| Participants | Total evaluable sample size: 70 Exclusion criteria;concomitant toenail/finger nail onychomycosis, use of cytotoxic immunosuppressants. | |
| Interventions | a. Terbinafine cream 1% 7 days once daily. b. Placebo (vehicle cream) seven days once daily. |
|
| Outcomes | Primary outcome: cure Secondary outcomes: Total clinical signs and symptoms and clinical response. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Kuhlwein 1990.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: not stated Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 36 Exclusion criteria: use of other systemic/topical antimycotic therapy | |
| Interventions | a. Croconazole 1% cream Duration: three weeks Frequency: applied once daily b. Bifonazole cream Duration: three weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Adverse events: none in either group | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ledezma 2000.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study; yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Adverse events reported: no Number of drop outs stated: yes | |
| Participants | Total evaluable sample; 47/70 Participants; Venezuelan army soldiers Exclusion criteria; use of topical antifungals within 30 days of commencement of study. | |
| Interventions | a. 0.6% ajone (alcoholic extract of garlic) b. 1% ajone 1% Terbinafine all applied twice daily for one week |
|
| Outcomes | Cure = negative culture Effective treatment = cured and signs and symptoms less than 2 on the following scale; 0=absent 1=light 2=moderate 3=intense |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Leenutaphong 1999.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: not stated Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 48 Exclusion criteria: not stated | |
| Interventions | a. Terbinafine cream 1% 1x daily for 1 week (3 weeks Placebo) Miconazole cream 2% 2 x daily for 4 weeks. |
|
| Outcomes | Primary outcomes: Mycological cure Secondary cure: Clinical efficacy, erythema, scaling, pustules and pruritis. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mandy 1974.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 12 Treatment setting: USAF medical centre‐ Keesler air force base | |
| Interventions | a. Miconazole nitrate 2% cream Duration: four weeks Frequency: applied twice daily b. Placebo cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, scaling, fissuring, exudation, maceration, erosions, cellulitis Adverse events: none | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Mensing 1992.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: no Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: no Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not stated Adverse events reported: no Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 34 Treatment setting: not clear Exclusion criteria:pregnancy, patients with concomitant disease predisposing to onychomycosis, abnormal baseline laboratory values,treatment with topical or systemic antifungal during the 4 weeks preceding treatment with amorolfine, treatment with another topical or systemic antifungal during the course of the study. |
|
| Interventions | a. 5% amorolfine in a methylene chloride vehicle b. 5% amorolfine in an ethanol vehicle |
|
| Outcomes | Primary outcome: cure Secondary outcome: Inhibition zones |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Montana 1994.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: no Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: no Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not stated Adverse events reported: no Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 20 Treatment setting: not clear, Exclusion criteria: psoriasis, lichen planus, any other disease which affects the nails. Hypersensitivity, use of topical antifungal medication within one month, use of a systematic antifungal medication with in three months. |
|
| Interventions | a. Fungoid tincture b. Placebo | |
| Outcomes | Primary outcome: cure Secondary outcome: Global assessment. Measuring the distance between the cuticle and the most proximal onychomycotic border. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Pereda 2003.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: no Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: no Intention to treat analysis: no A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: ten | |
| Participants | Total evaluable sample size:264 Treatment setting: 11 Dermatology outpatient clinics in Spain Inclusion criteria: + ve KOH and culture systemic antimycotic within four weeks of trial entry, topical antifungals within seven days. Concurrent therapy with steroids or other antifungal agent. |
|
| Interventions | a. Bifonazole 1% powder b. Flutrimazole 1% powder Both applied 2 X daily for 4 weeks. |
|
| Outcomes | Primary outcome:Clinical cure = total signs and symptoms score of < 2. Global cure clinical cure plus negative microscopy |
|
| Notes | Adverse events;non serious. Dishydrotic eczema by one in bifonazole group. Itching by one in bifonazole group and one in fluconazole group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Plotkin 1990.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: not stated Distribution of species between groups: not stated Adverse events reported: yes Number of drop outs stated: 23 | |
| Participants | Total evaluable sample size: 57 Exclusion criteria: Systemic fungal infection use of topical antifungals within four weeks prior to study Treatment setting: Colleges of Podiatric Medicine | |
| Interventions | a. Naftifine 1% cream Duration: four to six weeks Frequency: applied twice daily b. Clotrimazole 1% cream Duration: four to six weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, scaling, fissuring, maceration, pruritus Adverse events: a. erythema and itching b. tingling | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Qadripur 1979.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study:yes Participants comparable at baseline for age: yes Sex : yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 60 | |
| Interventions | a. Clotrimazole cream Duration: four weeks Frequency: b. Econazole cream Duration: four weeks Frequency: | |
| Outcomes | Primary outcome: cure Adverse events: a. 0 b. 4 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Roberts 1985.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: not stated Participants comparable at baseline for age: not stated Sex: not staed Duration of complaint: not stated Inclusion and exclusion criteria specified: no Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: no Adverse events reported: no Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 31 Treatment setting: Dermatology out‐patient department | |
| Interventions | a. Bifonazole 15 cream Duration: three weeks Frequency: applied twice daily b. Miconazole 2% cream Duration: three weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ weeping, itching, fissuring,maceration, burning, peeling | |
| Notes | Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sanchez 1994.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: not stated Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified:yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 43 Exclusion criteria: Bacterial/ yeast infection, use of topical antifungal therapy within two weeks prior to study, use of systemic griseofulvin or ketoconazole within four weeks prior to study Treatment setting: Dermatological departments | |
| Interventions | a. Terbinafine 1% cream Duration: four weeks Frequency: applied once daily b. Bifonazole 1% cream Duration: four weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling Adverse events: none in either group | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Satchell 2002.
| Methods | RCT. Study aim clearly described: yes Assessor blind to treatment allocation; not stated Blinding of subjects: not stated Study groups comparable at baseline for age; yes, Sex: yes, duration; not stated Were the inclusion/exclusion criteria specified; yes Were the interventions welldescribed; no Was there an assessment of compliance; not stated Was data included from subjects who withdrew after randomisation;no Was an A priori sample size calculation performed;yes |
|
| Participants | Total evaluable sample size: 137 Inclusion criteria: aged 14 +, intertriginious tinea pedis Exclusion criteria: use of antifungal treatment within seven days (topical) or 6 months (systemic) prior to study | |
| Interventions | a. Placebo (20% ethanol) b. 25% Tea tree oil c. 50% Tea tree oil | |
| Outcomes | Primary outcome; cure Secondary outcomes clinical cure (burnng, itching) graded as absent, mild, moderate, severe, or very severe. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Savin 1990.
| Methods | RCT Study aim clearly defined: yes Blinded assesor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Reoccurrence status: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: five | |
| Participants | Total evaluable sample size: 22 Inclusion criteria: Men Exclusion criteria: Use of sysytemic antifungals within four weeks prior to study Use of topical antifungals within two weeks prior to study | |
| Interventions | a. Terbinafine 1% cream Duration: four weeks Frequency: applied twice daily b. Placebo cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: none reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Savin 1994.
| Methods | RCT Study aim clearly defined: yes Blinded assesor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Reoccurrence status: yes Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: not stated | |
| Participants | Total evaluable sample size: 193 Inclusion criteria: baseline score of four or more (signs and symptoms) Exclusion criteria: more than 30% nail involvement Treatment setting: five different sites | |
| Interventions | a. Terbinafine 1% cream Duration: two weeks Frequency: applied twice daily b. Placebo cream Duration: two weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Patients own assessment Overall assessment by physicians Adverse events: a. four b. three | |
| Notes | Moccasin type tinea pedis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Savin 1997.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 271 Inclusion criteria: Erythema and at least scaling or pruritus Exclusion criteria: concomittant fungal infections diffuse moccasin type tinea pedis use of topical antifungals within two weeks prior to study use of systemic antifungals within two months of study Treatment setting: ten study sites | |
| Interventions | a. Butenafine 1% cream Duration: one week Frequency: applied twice daily b. Placebo cream Duration: one week Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: a. burning and stinging b. burning, tingling, elevated AST and ALT levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Schachner 1990.
| Methods | RCT Study aim clearly defined: yes Blinded assesor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: nine | |
| Participants | Total evaluable sample size: 91 | |
| Interventions | a. Naftifine 1% cream Duration: four weeks Frequency: applied twice daily b. Placebo cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: a. pruritus, burning, erythema b. pruritus, burning, drying | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Schopf 1999.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: 9 | |
| Participants | Total evaluable sample size: 429
Inclusion criteria: radiation, systematic immunosuppresants, topical antifungal drugs within two weeks, oral antifungals in six weeks. 35 centres in Germany. |
|
| Interventions | a. Terbinafine 1% solution 2 x daily for 1 week. b. Clotrimazole 1% solution 2 x daily for 2 weeks |
|
| Outcomes | Primary outcomes; | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Smith 1977.
| Methods | RCT Study aim clearly defined: yes Blinded assesor of primary outcome: yes Double blind study: yes Participants comparable at baseline for age: yes Sex: not stated Duration of complaint: yes Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not stated Adverse events reported: yes Number of drop outs stated: nine | |
| Participants | Total evaluable sample size: 104 Treatment setting: Health Center | |
| Interventions | a. 20% zinc undecylenate and 2% undecylenic acid ‐ new commercial powder Duration: six weeks Frequency: applied once daily b. 20% Undecylenate and 2% undecylenic acid ‐ over the counter powder Duration: six weeks Frequency: applied once daily c. Placebo powder Duration: six weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Adverse events: a. two patients with pruritus | |
| Notes | Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Smith 1986.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 54 Inclusion criteria: interdigital type tinea pedis Exclusion criteria: use of anti‐infective medications within one week prior to study, use of Griseofulvin within four weeks prior to study | |
| Interventions | a. Bifonazole 15 solution Duration: four weeks Frequency: applied once daily b. Placebo solution Duration: four weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation Adverse events: a. 3 mild burning b. 2 mild burning, tingling | |
| Notes | Culture results only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Smith 1988a.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yess Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described:yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: yes Fungi identified: yes Distribution of species between groups: not given Adverse events reported: yes Number of drop outs stated: not stated | |
| Participants | Total evaluable sample size: 60 Exclusion criteria: recent use of antifungal agents | |
| Interventions | a. Ticonazole 1% cream Duration: six weeks Frequency: applied twice daily b. Placebo cream Duration: six weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms Adverse events: both groups encountered transient stinging or burning after application two in a. and seven in b. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Smith 1988b.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not given Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 64 | |
| Interventions | a. Ticonazole 1% cream Duration: six weeks Frequency: applied twice daily b. Miconazole 2% cream Duration: six weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Adverse events: Three patients in each group complained of stinging | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Smith 1988c.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: no Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: yes Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 59 | |
| Interventions | a. Tioconazole 1% cream Duration: six weeks Frequency: applied twice daily b. Miconazole 2% cream Duration: six weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms Adverse events: Five patients in group 1 and eight in group 2 complained of stinging | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Smith 1990a.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: no Sex: yes Duration of complaint: no Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: yes A Priori sample size calculation: no Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 20 Exclusion criteria: use of systemic antifungals within four weeks prior to study, use of topical antifungals within two weeks prior to study | |
| Interventions | a. Terbinafine 1% cream Duration: four weeks Frequency: applied twice daily b. Placebo cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms Overall efficacy‐ complete mycologic cure with total or substatial remission of signs and symptoms Adverse events: a: elevated AST b: mild itching, neutropenia, elevated ALT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Smith 1990b.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Doublr blind study: not stated Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not given Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 207 | |
| Interventions | a. Naftifine Duration: four weeks Frequency: applied once daily plus Placebo once daily b. Naftifine Duration: four weeks Frequency: applied twice daily c. Clotrimazole Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, fissuring, pruritus Adverse events: a: four b: four c: three | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Smith 1992.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: not stated Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: not given Adverse events reported: yes Number of drop outs stated: four | |
| Participants | Total evaluable sample size: 172 | |
| Interventions | a. Naftifine cream Duration: four weeks Frequency: applied twice daily b. Clotrimazole betamethasone dipropionate cream Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ erythema, scaling, papules, pruritus Adverse reactions: two patients in group 1 complained of erythema, eczema, burning and five patients in group 2 complained of fissuring, erythema, burning, edema, pruritus,vesicular infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Spiekermann 1976a.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study:yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 267 Exclusion criteria: use of topical/systemic anti infective or anti‐inflammatory agents within two weeks prior to study | |
| Interventions | a. Clotrimazole 1% solution Duration: six weeks Frequency: applied twice daily b. Placebo solution Duration: six weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation, inflammation, edema Adverse events: a: irritation, stinging, urticaria | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Spiekermann 1976b.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study:yes Participants comparable at baseline for age: not stated Sex: not stated Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 41 Exclusion criteria: use of topical/systemic anti infective or anti‐inflammatory agents within two weeks prior to study | |
| Interventions | a. Clotrimazole 1% cream Duration: six weeks Frequency: applied twice daily b. Placebo cream Duration: six weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation, inflammation, edema Adverse events: a. irritation, stinging, burning | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sushka 2001.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: no Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: no Intention to treat analysis: no A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: eight | |
| Participants | Total evaluable sample size; 100 Setting:not stated Exclusion criteria: less that 18 years of age, ‐ ve KOH, tinea pedis of the maccasin type or concomitant onychomycosissystemic corticosteroids or antibiotics |
|
| Interventions | a. Clotrimazole 1% once daily b. Ketoconazole 2% twice daily |
|
| Outcomes | Primary outcome: cure Secondary outcomes;Signs and symptoms; fissuring, erythema, masceration, vesiculation, desquamation, exsudation, pruritis, and burning stinging. |
|
| Notes | Interdigital tinea pedis Adverse events; burning, scaling, redness and scaling of the skin |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Syed 1999.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study:yes Participants;yes comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: no drop outs A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 60 Exclusion criteria: use of topical/systemic anti fungals within two weeks or 3 months prior to study | |
| Interventions | a. Butenafine 2% + 5% melaleuca alternafolia b. Placebo Duration: eight weeks Frequency: applied three times weekly |
|
| Outcomes | Primary outcome: cure Secondary Outcomes: clinical success resolution of all clincal symptoms Signs and symptoms ‐ itching, erythema, scaling, fissuring, exudation, pustules, maceration, vesiculation, inflammation, edema Adverse events: a. irritation, stinging, burning | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Syed 2000.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study; yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 40 Exclusion criteria: women. Inclusion criteria: men. Moccasin and ID type tinea pedis and | |
| Interventions | a. Butenafine 1% 1x daily for 2 weeks b. Terbinafine 1% 1 x daily for 2 weeks. c. Placebo 1 x daily for 2 weeks. |
|
| Outcomes | Primary outcome; cure Secondary outcomes; erythema, pustules, encrustation, pruritis. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tong 1992.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study:yes Participants comparable at baseline for age: yes Sex: no Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: yes Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 104 Exclusion criteria: use of systemic antifungals within six months prior to study, use of topical antifungals within one week prior to study | |
| Interventions | a. Tolnaftate 1% cream Duration: four weeks Frequency: applied twice dialy b. Tea Tree Oil 10% in cream Duration: four weeks Frequency: applied twice daily c. Placebo Duration: four weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, scaling, inflammation and burning Adverse events: a: mild erythema | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tschen 1997.
| Methods | RCT Study aim clearly defined: yes Blinded assesor of primary outcome: not stated Double blind study Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: | |
| Participants | Total evaluable sample size: 80 Inclusion criteria: minimum erythema score of two, minimum score of two for pruritus or scaling Exclusion criteria: recently used antifungals | |
| Interventions | a. Butenafine 1% cream Duration: four weeks Frequency: applied once daily b. Placebo cream Duration: four weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms Investigator's and patient's assessment of treatment Adverse events: a: mild burning sensation (1) b: burning, itching, stinging (4) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Vermeer 1996.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study:yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: no Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 191 Treatment setting: Department of Dermatology (hospital) | |
| Interventions | a. Terbinafine cream Duration: one week plus one week Placebo Frequency: b. Miconazole cream Duration: four weeks Frequency: | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, vesiculation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Weller 1998.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study; yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: yes Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: no Number of drop outs stated: no | |
| Participants | Total evaluable sample size; 35 Exclusion criteria; psoriasis, treatment with antifungals, and steroids in previous weeks. Topical antifungals within 1 week or oral drugs within weeks. |
|
| Interventions | a. Salicylic acid 3% + Nitrite acid 3% 2 x daily for 4 weeks Salicylic acid 3% 2 x daily for 4 weeks. |
|
| Outcomes | Primary outcomes; cure Secondary outcomes; clinical signs; scaling, pruritis, masceration, erythema, blistering, crusting, fissures and burning |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Woscoff 1986.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: no Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: Assessment of compliance: not stated Intention to treat analysis: not stated A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: no Adverse events reported: yes Number of drop outs stated: yes | |
| Participants | Total evaluable sample size: 43 Exclusion criteria: Extensive chronic tinea pedis of more than six months, antifungal therapy used within one week prior to study | |
| Interventions | a. Sulconazole nitrate 1% cream Duration: three weeks Frequency: applied twice daily b. Miconazole nitrate 2% cream Duration: three weeks Frequency: applied twice daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms ‐ itching, erythema, scaling, maceration Adverse events: b: severe fissuring ansd desquamation, severe burning, erythema and pruritus | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zaug 1992.
| Methods | RCT Study aim clearly defined: yes Blinded assessor of primary outcome: not stated Double blind study: yes Participants comparable at baseline for age: yes Sex: yes Duration of complaint: not stated Inclusion and exclusion criteria specified: yes Interventions well described: yes Assessment of compliance: not stated Intention to treat analysis: yes A Priori sample size calculation: not stated Fungi identified: yes Distribution of species between groups: yes Adverse events reported: yes Number of drop outs stated: no | |
| Participants | Total evaluable sample size: 337 Exclusion criteria: onychomycosis, trichomycosis. Use of antifungal within two weeks prior to study Treatment setting: 24 centers in Europe and Latin America | |
| Interventions | a. Amorolfine 0.125% cream Duration: two ‐ six weeks Frequency: applied once daily b. Amorolfine 0.25% cream Duration: two ‐ six weeks Frequency: applied once daily c. Amorolfine 0.5% cream Duration: two ‐ six weeks Frequency: applied once daily | |
| Outcomes | Primary outcome: cure Secondary Outcomes: Signs and symptoms Adverse events: a. 5 b. 4 c. 0 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albanese 1992 | Looks at preventative effects rather than curative |
| Alexander 1972 | Combined data |
| Aly 1994 | Nine patients with yeast only at baseline |
| Arenas 1991 | Oral versus topical |
| Athow Frost 1986 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Avlia 1985 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Baran 2000 | Compares oral versus topical treatments |
| Barnetson 1998 | Oral versus topical treatments |
| Bjornberg 1986 | Cannot separate mycological results for tinea pedis from tinea cruris |
| Clayton 1982 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Cuce 1980 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Cuce 1989 | No results for culture are given, only microscopy |
| Daily 1985 | Positive culture and or KOH at baseline |
| Del Palacio 1989 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Del Palacio 1991 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Del Palacio 1992 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Del Palacio 1999 | Combined for candida and dermatophyte infections and sites. |
| Del Palacio 2000 | Combined data for candida and dermatophyte infections |
| Del Palacio A 1992 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Duncan 1975 | Only half participants had positive culture at entry |
| Effendy 1987 | Cannot separate mycological results for dermatophytes from those related to yeasts and moulds |
| Evans E 1994 | Duplicate study |
| Fredriksson 1972 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Fredriksson 1974 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body and those infected with candida infection |
| Fredriksson 1977 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Fredriksson 1982 | Microscopy was not performed at baseline |
| Friedman 1997 | Oral versus topical treatments. Combined data for finger and toe nails |
| Fulton 1975 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Galimberti 1984 | Looks at preventative effects rather than curative |
| Gip 1983 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Greer 1986 | Duplicate study |
| Greer 1987 | +ve KOH and or culture |
| Grigoriu 1983 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Grooten 1992 | Combined data |
| Kashin 1985 | There is no definate end of treatment time from which data can be extracted |
| Katayama 1987 | No culture assessment is made |
| Kates 1990 | Looks at the effects on bacterial infection, not fungal infection |
| Katz 1972 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Koca 2001 | Experimental agent is an antiperspirant (aluminium hyroxychloride). |
| Lassus 1983 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Lassus 1988 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Lauharanta 1992 | Results not clear |
| Lebwohl 2001 | Definitions of population unclear |
| Lestienne 1982 | The written report of the study is too confusing to be able to extract the appropriate data |
| Li 2001 | Combined data for hands and feet infections |
| Lison 1985 | No mycological assessment was carried out |
| Lynfield 1974 | Compares systemic treatment with topical treatment |
| Maibach 1978 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| McVie 1986 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Meinicke 1985 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Mertens 1976 | Study looks at bacterial infection as well as fungal infection and does not separate the results |
| Nolting 1992 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Nolting 1993 | No clear treatment time given Mycology results are not clear Only treatment groups one and two are double blind, not group three |
| Ortiz 1978 | Incomplete data for diagnosis at entry |
| Oyeka 1991 | Cannot separate mycological results for tinea pedis of the skin from infection of the nail |
| Pariser 1994 | Detailed numerical reults are not given |
| Patel 1999 | One hundred and four (104/217) only had culture confirmed at baseline. No microscopy for any participant |
| Privat 1982 | No microscopy |
| Qadripur 1984 | Cannot separate mycological results for tinea pedis from those related to fungal infection elsewhere on the body |
| Reinel 1992 | Combined finger and toenail data |
| Reinel 1992a | Duplicate report of Reinel 1992 |
| Ruping 1993 | Combined finger and toenail data |
| Saple 2001 | Data combined for three sites; tinea pedis, tinea cruris, tinea corporis |
| Shellow 1982 | Culture is used to identify pathogens at baseline but not to assess outcome |
| Smith 1974 | Results for mycological cure do not separate out those for tinea pedis from tinea cruris |
| Smith 1977b | Data extracted for negative culture only |
| Stettendorf 1983 | Cannot separate tinea pedis results from those of tinea corporis and inguilalis |
| Suschka 2001 | Only 56% in Clotrimazole group and 60% in ketoconazole group had + ve test results for dermatophytes. |
| Tanenbaum 1982 | Combined data for dermatophytes and candida |
| Tanenbaum 1983 | Studies effect of treatment on candidiasis, not dermatophytes |
| Tanuma 2001 | Fungi demnstrated by direct microscopic exam OR culture |
| Terragni 1993 | Combined finger and toenail data |
| Thomas 1976 | Unit of analysis sites not patients unclear as to the numbers of units in study |
| Thomas 1986 | Unit of analysis site not patient |
| Tschen 1979 | Only 63/90 had + culture at baseline |
| Tsuboi 1996 | Diagnosis only established by KOH |
| Weil 1996 | Study only looks at culture results and not mycology. The data combines that of dermatophytes and yeasts. |
| Zaug 1995 | Oral versus topical |
Contributions of authors
FC ran the searches, extracted data and applied the QA tool to the additional studies. Rachel Hart acted as co‐reviewer and her contribution is acknowledged. SH undertook all the statistical analyses associated with this version of the review.
FC and SH jointly wrote the text of the review and are both guarantors of the work.
Sources of support
Internal sources
No sources of support supplied
External sources
Fay Crawford is funded by the Chief Scientist Office, The Scottish Executive, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Ablon 1996 {published data only}
- Ablon G, Rosen T, Spedale J. Comparative efficacy of naftifine oxiconazole and terbinafine in short term treatment of tinea pedis. International Journal of Dermatology 1996;35(8):591‐3. [DOI] [PubMed] [Google Scholar]
Akers 1989 {published data only}
- Akers WA, Lane A, Lynfield Y, Greenberg J, Hall J, Mangan C, et al. Sulconazole nitrate 1% cream in the treatment of chronic moccasin type tinea pedis caused by trichophyton rubrum. Journal the of American Academy of Dermatology 1989;21(4):686‐9. [DOI] [PubMed] [Google Scholar]
Aly 2003 {published data only}
- Aly R, Fisher G, Katz HI, Levine N, Lookingbill DP, Lowe N, et al. Ciclopirox gel in the treatment of patients with interdigital tinea pedis. International Journal of Dermatology 2003;42(Suppl 1):29‐35. [DOI] [PubMed] [Google Scholar]
Bagatell 1986 {published data only}
- Bagatell FK. Elimination of dermatophytes causing tinea pedis interdigitalis with once daily application of bifonazole 1% solution. Advances in Therapeutics 1986;3:265‐71. [Google Scholar]
Bagatell 1991a {published data only}
- Bagatell FK, Bart BJ, Cole GW, Drake LA, Ellis CN, Arbor A, et al. Naftifine Gel in the treatment of tinea pedis: Two double blind multi‐centre studies. Cutis 1991;48:85‐8. [PubMed] [Google Scholar]
Bagatell 1991b {published data only}
- Bagatell FK, Bart BJ, Cole GW, Drake LA, Ellis CN, Arbor A, et al. Naftifine Gel in the treatment of tinea pedis: Two double blind multi‐centre studies. Cutis 1991;48:85‐8. [PubMed] [Google Scholar]
Bergstresser 1993 {published data only}
- Bergstresser PR, Elewski B, Hanifin J, Lesher J, Savin R, Shupack J, et al. Topical terbinafine and clotrimazole in interdigital tinea pedis: a multicentre comparison of cure and relapse rates with 1 and 4 week treatment regimens. Journal of the American Academy of Dermatology 1993;28:648‐51. [DOI] [PubMed] [Google Scholar]
Berman 1992 {published data only}
- Berman B, Ellis C, Leyden J, Lowe N, Savin R, Shupack J, et al. Efficacy of a 1‐week, twice‐daily regimen of terbinafine 1% cream in the treatment of interdigital tinea pedis. Journal of the American Academy of Dermatology 1992;26:956‐60. [DOI] [PubMed] [Google Scholar]
Bojanovsky 1985 {published data only}
- Bojanovsky A, Haas P. Antimycotic efficacy of naftifine ‐ double blind comparative study with bifonazole in tinea pedis. Fortschritte der Medizin 1985;103:677‐9. [PubMed] [Google Scholar]
Buck 1994 {published data only}
- Buck DS, Nidorf DM, Addino JG. Comparison of topical preparations for the treatment of onychomycosis: Melanlenca alternifolia (tea tree oil) and clotrimazole. Journal of Family Practice 1994;38:601‐605. [PubMed] [Google Scholar]
Carter 1972 {published data only}
- Carter VH. A controlled study of haloprogen and tolnaftate in tinea pedis. Current Therapeutic Research, Clinical and Experimental 1972;14:307‐10. [PubMed] [Google Scholar]
Chretien 1980 {published data only}
- Chretien JH, Esswein JG, Sharpe LM, Kiely JJ, Lyddon FE. Efficacy of unclynic acid ‐zinc undeclynate powder in culture positive tinea pedis. International Journal of Dermatology 1980;19:51‐4. [DOI] [PubMed] [Google Scholar]
Coffey 1986 {published data only}
- Coffey W. Management of tinea pedis interdigitalis with bifonazole 1% cream: double blind study. Advances in Therapy 1986;3:301‐7. [Google Scholar]
Del Palacio 1989 {published data only}
- Palacio Hernanz A, Lopez‐Gomez S, Moreno‐Palancar P, Gonzales‐Lastra F. A clinical double blind trial comparing amorolfine cream (0.5%) with bifonazole cream 1% in the treatment of dermatomycoses. Clinical and Experimental Dermatology 1989;14:141‐4. [DOI] [PubMed] [Google Scholar]
Dobson 1989 {published data only}
- Dobson RL, Binder R, Hickman JG, Katz HI, Landow RK, Rex IH, et al. Once daily naftifine cream 1% (Naftin) in the treatment of tinea pedis. Clin Trials J 1989;26:418‐23. [Google Scholar]
Elewski 1996 {published data only}
- Elewski B, Jones T, Zaias N. Comparison of an antifungal agent used alone with an antifungal used with a topical steroid in inflammatory tinea pedis. Cutis 1996;58:305‐7. [PubMed] [Google Scholar]
Ellis 1989 {published data only}
- Ellis CN, Gammon WR, Goldfarb MT, Griffin TB, Jegasothy BV, Kamm AR, Katz HI, Kornberg R, et al. A placebo controlled evaluation of once daily vs twice daily oxiconazole nitrate (1%) cream in the treatment of tinea pedis. Current Therapeutic Research, Clinical and Experimental 1989;46:269‐76. [Google Scholar]
Evans 1991 {published data only}
- Evans EGV, James IGV, Joshipura RC. Two week treatment of tinea pedis with terbinafine: a placebo controlled study. Journal of Dermatological Treatment 1991;2:95‐7. [Google Scholar]
Evans 1993a {published data only}
- Evans EGV, James IGV, Seaman RAJ, Richardson MD. Does naftifine have anti‐inflammatory properties? A double‐blind comparative study with 1% clotrimazole/ 1% hydrcortisone in clinically diagnosed fungal infection of the skin. British Journal of Dermatology 1993;129:437‐42. [DOI] [PubMed] [Google Scholar]
Evans 1993b {published data only}
- Evans EGV, Dodman B, Williamson DM, Brown GJ, Bowen RG. Comparison of terbinafine and clotrimazole in treating tinea pedis. BMJ 1993;307:645‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1994 {published data only}
- Evans EGV, Seaman RAJ, James IGV. Short duration therapy with terbinafine 1% cream in dermatophyte skin infections. British Journal of Dermatology 1994;130:83‐84. [DOI] [PubMed] [Google Scholar]
Friederich 1992 {published data only}
- Friederich h, Hagedorn M, Lachnit H. Wirksamkeit von mycosspor creme bei fubmykosen. Z Allgmein Med 1992;68:325‐9. [Google Scholar]
Fuerst 1980 {published data only}
- Fuerst JF, Cox GF, Weaver SM, Duncan WC. Comparison between undeclynic acid and tolnaftate in the treatment of tinea pedis. Cutis 1980;25:544‐9. [PubMed] [Google Scholar]
Gentles 1974 {published data only}
- Gentles JC, Jones GR, Roberts DT. Efficacy of miconazole in the topical treatment of tinea pedis in sportsmen. British Journal of Dermatology 1975;93:79‐84. [DOI] [PubMed] [Google Scholar]
Gomez 1986 {published data only}
- Gomez EC, Huntley, Isseroff R, Tanji J. Efficacy of tolciclate solution in patients with tinea pedis. Clinical Therapeutics 1986;8:694‐9. [PubMed] [Google Scholar]
Gupta 2000a {published data only}
- Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution in the treatment of toenail onychomycosis. Journal of the American Academy of Dermatology 2000;43(4):S70‐S80. [DOI] [PubMed] [Google Scholar]
Gupta 2000b {published data only}
- Gupta AK, Fleckman P, Baran R. 2000. Ciclopirox nail lacquer topical solution in the treatment of toenail onychomycosis. Journal of the American Academy of Dermatology 2000;43(4):S70‐S80. [DOI] [PubMed] [Google Scholar]
Haas 1985 {published data only}
- Haas PJ, Tronnier H, Weidinger G. Naftifin in foot mycoses. Double blind therapeutic comparison with clotrimazole. Mykosen 1985;28:33‐40. [PubMed] [Google Scholar]
Hollmen 2002 {published data only}
- Hollmen KA, Kinnunen T, Kilstala U, Vaananen A, Salarlainen IO, De Cuyper, Decroix J, Broeckx W, Karvonen J. Efficacy and tolerability of terbinafine 1% emulsion gel in patients with tinea pedis. European Academy of Dermatology and Venereology 2002;16:81‐94. [DOI] [PubMed] [Google Scholar]
Holti 1970 {published data only}
- Holti G. A double blind controlled trial of Whitfield's ointment and Variotin in ringworm infections with a two year 'follow‐up. Acta Dermato‐Venereol 1970;50:229‐31. [PubMed] [Google Scholar]
Ison 1990 {published data only}
- Ison AE, Lufrano L, Thorne EG. Once daily application of econazole nitrate in the treatment of tinea pedis. Advances in Therapy 1990;7:119‐23. [Google Scholar]
Izuno 1986 {published data only}
- Izuno GT. A double blind study of bifonazole 1% cream applied once daily in the treatment of tinea (pityriasis) veriscolor and tinea pedis interdigitalis. Advances in Therapy 1986;3:308‐33. [Google Scholar]
Kagawa 1985 {published data only}
- Kagawa S. Comparative clinical trial of Naftifine and Clotrimazole in Tinea pedum, Tinea cruris and Tinea corporis. Mykosen 1985;28:82‐8. [Google Scholar]
Klaschka 1984 {published data only}
- Klaschka F, Gartmann H, Weidinger G. Antimycotic naftifine. Placebo controlled comparison in tinea pedum. Z‐Hauktr 1984;59:1218‐25. [PubMed] [Google Scholar]
Kligman 1985a {published data only}
- Kligman AM, Bogaert H, Cordero C, et al. Evaluation of ciclopirox olamine cream for the treatment of tinea pedis: Multicentre double blind comparative studies. Clinical Therapeutics 1985;7:409‐17. [PubMed] [Google Scholar]
Kligman 1985b {published data only}
- Kligman AM, Bogaert H, Cordero C, et al. Evaluation of ciclopirox olamine cream for the treatment of tinea pedis: Multicentre double blind comparative studies. Clinical Therapeutics 1985;7:409‐17. [PubMed] [Google Scholar]
Korting 1997 {published data only}
- Korting HC, Klovekorn W, Klovekorn G, Orfanos C, Heilgemeir E, et al. Comparatice efficacy and tolerability of econazole loiposomal gel 1%, branded econazole conventional cream 1% and generic clotrimazole cream 1% in tinea pedis. Clinical drug investigation 1997;14:246‐93. [Google Scholar]
Korting 2001 {published data only}
- Korting HC, Tietz HJ, Brautigam M, Mayser P, Rapatz G, Paul C. One week terbinafine 1% cream (Lamisil) once daily is effective in the treatment of interdigital tinea pedis: a vehicle controlled study. LAS‐INT‐06 Study Group. Med Mycol 2001;39(4):335. [DOI] [PubMed] [Google Scholar]
Kuhlwein 1990 {published data only}
- Kuhlwein A, Busch T. Tinea inguinalis und Tinea Pedis. Therapiewoche 1990;40:1643‐5. [Google Scholar]
Ledezma 2000 {published data only}
- Ledezema E, Marcano K, Jorquera A, Sousa L, Padila, Pulgar M, Apitz‐Castro R. Efficacy of ajoene in the treatment of tinea pedis: A double blind and comparative study with terbinafine. Journal of the American Academy 2000;43:829‐32. [DOI] [PubMed] [Google Scholar]
Leenutaphong 1999 {published data only}
- Leenutaphong V, Niumpradit N, Tangwiwat S, Sritaveesuwan R, Muanprasat C. Double‐blind study of the efficacy of 1 week topical terbinafine cream compared to 4 weeks miconazole cream in patients with tinea pedis. Journal of the Medical Association of Thailand = Chotmaihet thangphaet 1999;82(10):1006‐10. [PubMed] [Google Scholar]
Mandy 1974 {published data only}
- Mandy SH, Garrott TC. Miconazole treatment for severe dermatomycoses. JAMA 1974;230:725. [PubMed] [Google Scholar]
Mensing 1992 {published data only}
- Mensing H, Polak‐Wyss A, Splanemann V. Determination of the subungual antifungal activity of almorolfine after 1 months treatment in patients with onychomycosis. Clinical and Experimental Dermatology 1992;17 (suppl):29‐32. [DOI] [PubMed] [Google Scholar]
Montana 1994 {published data only}
- Montana JB, Scher RK. A double‐blind vehicle controlled study of the safety and efficacy of fungoid tincture in patients with distal subungual onychomycosis of the toes. Cutis 1994;53:313‐6. [PubMed] [Google Scholar]
Pereda 2003 {published data only}
- Pereda J, Noguera X, Boncompte E, Alguero M, Izquierdo I. Efficacy of flutrimazole 1% powder in the treatment of tinea pedis. Mycoses 2003;46:126‐131. [DOI] [PubMed] [Google Scholar]
Plotkin 1990 {published data only}
- Plotkin E, Machtinger MA, Vlahos M, Lipkin L, Pascente RW, et al. Naftifine cream 1% versus Clotrimazole cream 1% in the treatment of tinea pedis. Journal of the American Podiatric Medical Association 1990;80:314‐8. [DOI] [PubMed] [Google Scholar]
Qadripur 1979 {published data only}
- Qadripur SA, Krause U. Doppelblindversuch mit Clotrimazol und Econazol bei Tinea Pedis. Mykosen 1979;22:28‐34. [PubMed] [Google Scholar]
Roberts 1985 {published data only}
- Roberts DT, Adriiaans B, Gentles JC. A comparative study of once daily bifonazole cream vs twice daily miconazole cream in the treatment of tinea pedis. Mykosen 1985;28:550‐2. [PubMed] [Google Scholar]
Sanchez 1994 {published data only}
- Sanchez‐Carazo JL, Fuente C, Oliver V, Umbert P. A comparative study of terbinafine. Actas dermo‐sifiliográficas 1994;85:388‐94. [Google Scholar]
Satchell 2002 {published data only}
- Satchell AC, Saurajen A, Bell C, Barnetson RStC. Treatment of interdigital tinea pedis with 25% and 50% tea tree oil solution: A randomised placebo‐controlled, blinded study. Australasian Journal of Dermatology 2000;45:175‐178. [DOI] [PubMed] [Google Scholar]
Savin 1990 {published data only}
- Savin RC. Treatment of chronic tinea pedis (athletes foot type) with topical terbinafine. Journal of American Academy of Dermatology 1990;23:786‐9. [DOI] [PubMed] [Google Scholar]
Savin 1994 {published data only}
- Savin R, Atton AV, Bergstrasser PR, Elewski B, Jones HE, Levine L, et al. Efficacy of terbinafine 1% cream in the treatment of moccasin type tinea pedis: Results of placebo controlled multicentre trials. Journal of the American Academy of Dermatology 1994;30:663‐7. [DOI] [PubMed] [Google Scholar]
Savin 1997 {published data only}
- Savin R, DeVillez RL, Elewski B, Hong S, Jones T, Lowe N, Lucky A, Reyes B, Stewart D, Willis I. One‐week therapy with twice‐daily butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicentre double‐blind trial. Journal of the American Academy of Dermatology 1997;36:S15‐9. [DOI] [PubMed] [Google Scholar]
Schachner 1990 {published data only}
- Schachner LA, Bagatell FK, Whittington CV, Willis I, Sefton J. The saftey and efficacy of Naftifine cream 1% in the treatment of tinea pedis. Advances in Therapy 1990;7:148‐52. [Google Scholar]
Schopf 1999 {published data only}
- Schopf R, Hettler O, Brautigam M, Weidinger G, Kaben U, Mayser P, Resl V. Efficacy and tolerability of terbinafine 1% topical solution used for 1 week compared with 4 weeks clotrimazole 1% topical solution in the treatment of interdigital tinea pedis: a randomized, double‐blind, multi‐centre, 8‐week clinical trial [Wirksamkeit und Vertäglichkeit einer einwöchenigen Therapie mit 1%iger Terbinafin‐Lösung im Vergleich zu einer vierwochigen Therapie mit 1%iger Clotrimazol‐Lösung in der Behandlung der interdigitalen Tinea pedis: Eine randomisierte, dopple‐blinde, multizentrische, achtwöchige klinische Studie]. Mycoses 1999;42(5‐6):415‐420. [DOI] [PubMed] [Google Scholar]
Smith 1977 {published data only}
- Smith EB, Graham JL, Ulrich JA. Topical clotrimazole in tinea pedis. Southern Medical Journal 1977;70:47‐8.. [DOI] [PubMed] [Google Scholar]
Smith 1986 {published data only}
- Smith EB, Tschen E. Treatment of interdigital tinea pedis and tinea (pityriasis) versicolour with bifonazole 1% solution applied once daily. Advances in Therapy 1986;3:250‐6. [Google Scholar]
Smith 1988a {published data only}
- Smith EB, Becker LE, Tschen EH, Way B, Way A. Topical tioconazole in tinea pedis. Advances in Therapy 1988;5:313‐8. [Google Scholar]
Smith 1988b {published data only}
- Smith EB, Becker LE, Tschen EH, Way B, Way A. Topical tioconazole in tinea pedis. Advances in Therapy 1988;5:313‐8. [Google Scholar]
Smith 1988c {published data only}
- Smith EB, Becker LE, Tschen EH, Way B, Way A. Topical tioconazole in tinea pedis. Advances in Therapy 1988;5:313‐8. [Google Scholar]
Smith 1990a {published data only}
- Smith EB, Noppakun N, Newton RC. A clinical trial of topical terbinafine (a new allylamine antifungal) in the treatment of tinea pedis. Journal of the American Academy of Dermatology 1990;23:790‐4. [DOI] [PubMed] [Google Scholar]
Smith 1990b {published data only}
- Smith EB, Wiss K, Hanifin JM, Jordon RE, Rapini RP, Lasser AE, Kirschenbaum MB, Millikan LE, Parish LC, et al. Comparison of once and twice daily Naftifine cream regimens with twice daily Clotrimazole in the treatment of tinea pedis. Journal of the American Academy of Dermatology 1990;22:1116‐7. [DOI] [PubMed] [Google Scholar]
Smith 1992 {published data only}
- Smith EB, Breneman DL, Griffith RF, Hebet AA, Hickman JG, Maloney JM, Millikan LE, Sulica VI, Dromgoole SH, Sefton J, et al. Double blind comparison of Naftifine cream and Clotrimazole beta methasone depropionate cream in the treatment of tinea pedis. Journal of the American Academy of Dermatology 1992;26:125‐7. [DOI] [PubMed] [Google Scholar]
Spiekermann 1976a {published data only}
- Speikerman PH, Young MD. Clinical evaluation of clotrimazole; a broad spectrum antifungal agent. Archives of Dermatology 1976;112:350‐2. [PubMed] [Google Scholar]
Spiekermann 1976b {published data only}
- Speikerman PH, Young MD. Clinical evaluation of clotrimazole; a broad spectrum antifungal agent. Archives of Dermatology 1976;112:350‐2. [PubMed] [Google Scholar]
Sushka 2001 {published data only}
- Suschka S, Fladung B, Merk H. Clinical comparison of the efficacy and tolerability of once daily canestin with twicw daily nizoral (clotrimazole cream vs ketoconazole 2% cream) during a 28 day topical treatment of interdigital tinea pedis. Mycoses 2001;45:91‐96. [DOI] [PubMed] [Google Scholar]
Syed 1999 {published data only}
- Syed TA, Qureshi ZA, Ali SM, Ahmad SA. Treatment of toenail onychomycosis with 2% butenafine and 5% Melaleuca alternifolia (tea tree) oil in cream. Tropical Medicine and International Health 1999;4:284‐287. [DOI] [PubMed] [Google Scholar]
Syed 2000 {published data only}
- Syed TA, Hadi SM, Qureshi ZA, Ali SM, Ahmad SA. Butenafine 1% versus terbinafine 1% in cream for the treatment of tinea pedis ‐ A placebo‐controlled, double‐blind, comparative study. Clinical Drug Investigation 2000;19(6):393‐7. [Google Scholar]
Tong 1992 {published data only}
- Tong MM, Altman PM, Barnetson RStC. Tea tree oil in the treatment of tinea pedis. Australasian Journal of Dermatology 1992;33:145‐9. [DOI] [PubMed] [Google Scholar]
Tschen 1997 {published data only}
- Tschen E, Elewski B, Gorsulowsky DC, Pariser DM. Treatment of interdigital tinea pedis with a 4 week once‐daily regimen of butenafine hydrochloride 1% cream. Journal of the American Academy of Dermatology 1997;36:9‐14. [DOI] [PubMed] [Google Scholar]
Vermeer 1996 {published data only}
- Vermeer BJ, Staats CCG, Houwelingen JC. Terbinafine versus miconazol bij patienten met tinea pedis. Nederlands tijdschrift voor geneeskunde 1996;140:1605‐8. [PubMed] [Google Scholar]
Weller 1998 {published data only}
- Weller R, Ormerod AD, Hobson RP, Benjamin NJ. A randomised trial of acidified nitrite cream in the treatment of tinea pedis. Journal of the American Academy of Dermatology 1998;38:559‐63. [DOI] [PubMed] [Google Scholar]
Woscoff 1986 {published data only}
- Woscoff A, Carabeli S. Treatment of tinea pedis with sulconazole nitrate 1% cream or miconazole nitrate 2% cream. Current Therapeutic Research, Clinical and Experimental 1986;39:753‐7. [Google Scholar]
Zaug 1992 {published data only}
- Zaug M, Bergstraesser M. Amorolfine in the treatment of onychomycosis and dermatomycosis: an overview. Clinical and Experimental Dermatology 1992;17(suppl):61‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Albanese 1992 {published data only}
- Albanese G, Cintio R, Giorgetti P, Galbiati G, Ciampini Ml. Recurrent tinea pedis: a double blind study on the prophylactic use of fenticonazole powder. Mycoses 1992;35:5‐6. [DOI] [PubMed] [Google Scholar]
Alexander 1972 {published data only}
- Alexander JOD, Grant PW. The treatment of skin infections: a double blind trial of fentiderm. Clinical Trials Journal 1972;9:9‐12. [Google Scholar]
Aly 1994 {published data only}
- Aly R, Bayles CI, Oakes RA, Bibel DJ, Maibach HI. Topical griseofulvin in the treatment of dermatophytoses. Clin Exp Dermatol 1994;19:43‐6. [DOI] [PubMed] [Google Scholar]
- Li Q, Li M, Jin L, Qin L‐M, Gu J, Liu WD, Guo Z‐L. Clinical efficacy of compound econazole cream in the treatment of tinea corporis, tinea inguinalis, tinea manus, tinea pedis and its safety. Pharmaceutical care and research (Yaoxue Fuwu Yu Yanjin) 2001;4(1):52‐4. [Google Scholar]
Arenas 1991 {published data only}
- Arenas R, Fernadez G, Dominguez L. Onychomycosis treated with itraconazole or griseofulvin alone with and without a topical antimycotic or keratolytic agent. International Journal of Dermatology 1991;30(8):586‐589. [DOI] [PubMed] [Google Scholar]
Athow Frost 1986 {published data only}
- Athow‐Frost TAM, Freeman K, Mann TAN, Marks R, Vollum D, Warin AP. Clinical evaluation of fenticonazole cream in cutaneous fungal infections: a comparison with miconazole cream. Current Medical Research and Opinion 1986;10:107‐16. [DOI] [PubMed] [Google Scholar]
Avlia 1985 {published data only}
- Avila JM. Treatment of dermatomycoses with sulconazole 1% nitrate cream or miconazole nitrate 2% cream: a double‐blind comparative study. Current Therapeutic Research, Clinical and Experimental 1985;38:328‐33. [Google Scholar]
Baran 2000 {published data only}
- Baran R, Feuilhade M, Datry A, Goettmann S, Pierini P, Vigue C, Badillet G, Larner C, Czernielewski J. A randomised trial of amorolfine 5% solution nail lacquer combined with oral terbinafine compared with terbinafine alone in the treatment of dermatophytic nail toenail onychomycosis. British Journal of Dermatology 2000;142:1177‐1183. [DOI] [PubMed] [Google Scholar]
Barnetson 1998 {published data only}
- Barnetson RS, Marley J, Bullen M, Brookman S, Cowen P, Ellis D, Williams T. Comparison of one week of oral terbinafine (250 mg/day) with four weeks of treatment with clotrimazole 1% cream in interdigital tinea pedis. British Journal of Dermatology 1998;139(4):675‐8. [DOI] [PubMed] [Google Scholar]
Bjornberg 1986 {published data only}
- Bjornberg A, Tegner E. Treatment of tinea with miconazole and miconazole‐hydrocortisone. Current Therapeutic Research, Clinical and Experimental 1986;40:471‐4. [Google Scholar]
Clayton 1982 {published data only}
- Clayton YM, Hay RJ, McGibbon DH, Pye RJ. Double‐blind comparison of the efficacy of tioconazole and miconazole for the treatment of fungal infection of the skin. Clinical and Experimental Dermatology 1982;7:543‐51. [DOI] [PubMed] [Google Scholar]
Cuce 1980 {published data only}
- Cuce LC, Contijo Assuncao BF, Medawar LGA, Salibian A, Groppi W. Tolciclate versus miconazole: a double‐blind trial in patients with dermatomycosis. The Journal of international medical research 1980;8:144‐7. [DOI] [PubMed] [Google Scholar]
Cuce 1989 {published data only}
- Cuce LC. Sulcanazole nitrate 1% cream versus clotrimazole 1% cream in the treatment of tinea pedis. Current Therapeutic Research, Clinical and Experimental 1989;45:421‐7. [Google Scholar]
Daily 1985 {published data only}
- Daily AD, Kramer KJ, Rex IH, Thorne EG. Econazole nitrate (spectazole) cream 1%: a topical agent for the treatment of tinea pedis. Cutis 1985;35:278‐80. [PubMed] [Google Scholar]
Del Palacio 1989 {published data only}
- Palacio A, Gip L, Bergstraesser M, Zaug M. Dose‐finding study of amorolfine cream (0.125%, 0.25% and 0.5%) in the treatment of dermatomycoses. Clinical and Experimental Dermatology 1992;17(suppl 1):50‐5. [DOI] [PubMed] [Google Scholar]
Del Palacio 1991 {published data only}
- Palacio A, Lopez S, Gimeno C, Garcia‐Lacalle C, Cuetara S, Garcia‐Bravo M, et al. Randomized comparative study: amorolfine cream (0.125%, 0.25% and 0.5%) in dermatomycoses. Journal of Dermatological Treatment 1991;1:299‐303. [Google Scholar]
Del Palacio 1992 {published data only}
- Palacio A, Lopez‐Gomez S, Cuetara S, Iglesias‐Diez L, Rodriguez‐Noriega A. Experience with amorolfine in the treatment of dermatomycoses. Dermatology 1992;184(suppl 1):25‐9. [DOI] [PubMed] [Google Scholar]
Del Palacio 1999 {published data only}
- Palacio A, Cuetara S, Perez A, Garau M, Calvo T, Sanchez‐Alor G. Topical treatment of dermatophytosis and cutaneous candidosis with flutrimazole 1% cream: double‐blind, randomised comparative trial with ketoconazole cream. Mycoses 1999;42:649‐55. [DOI] [PubMed] [Google Scholar]
Del Palacio 2000 {published data only}
- Palacio A, Oritz FJ, Perez A, Pazos C, Garan M, Font E. A double‐blind randomised comparative trial: eberconazole 1%cream versus clotrimazole 1% cream twice daily in candida and dermatophyte skin infections. Mycosis 2000;44:173‐80. [DOI] [PubMed] [Google Scholar]
Del Palacio A 1992 {published data only}
- Palacio A, Gip L, Bergstraesser M, Zaug M. Dose‐finding study of amorolfine cream (0.125%, 0.25% and 0.5%) in the treatment of dermatomycoses. Clinical and Experimental Dermatology 1992;17(suppl 1):50‐5. [DOI] [PubMed] [Google Scholar]
Duncan 1975 {published data only}
- Duncan WC. Tinea pedis: treatment with topical miconazole. Cutis 1975;16:647‐9. [Google Scholar]
Effendy 1987 {published data only}
- Effendy I, Friederich HC. Double‐blind randomised comparative trial of naftifine solution (once daily) and clotrimazole solution (twice daily) in the treatment of dermatomycoses. Mykosen 1987;30:104‐11. [Google Scholar]
Evans E 1994 {published data only}
- Evans EGV. A comparison of terbinafine (Lamisil) 1% cream given for one week with clotrimazole (Canesten) 1% cream given for four weeks in the treatment of tinea pedis. British Journal of Dermatology 1994;130:12‐4. [DOI] [PubMed] [Google Scholar]
Fredriksson 1972 {published data only}
- Fredriksson T. Topical treatment with BAY b 5097: a new broad‐spectrum antimycotic agent. Br J Dermatol 1972;86:628‐30. [DOI] [PubMed] [Google Scholar]
Fredriksson 1974 {published data only}
- Fredriksson T. Topical treatment of superficial mycoses with clotrimazole. Post Graduate Medical Journal 1974;50:62‐4. [PubMed] [Google Scholar]
Fredriksson 1977 {published data only}
- Fredriksson T. Treatment of dermatomycosis with topical miconazole (Daktar). Opusc Med 1977;22:80‐1. [Google Scholar]
Fredriksson 1982 {published data only}
- Fredriksson T. Treatment of dermatomycosis with topical tioconazole and miconazole. Dermatologica 1983;166:14‐19. [DOI] [PubMed] [Google Scholar]
- Fredriksson T, Faergemann J. Comparative study of the therapeutic effect of bifonazole and econazole in the treatment of dermatomycoses. Proceedings of the International Antifungal Symposium: Bifonazole 1982. 1983:120‐5.
Friedman 1997 {published data only}
- Friedman‐Birnbaum R, Cohen A, Shemer A, Bitterman O, Bergman R, Stettendorf S. Treatment of onychomycosis: a randomised double blind comparison study with topical bifonazole‐urea ointment alone and in combination with short duration oral griseofulvin. International Journal of Dermatology 1997;36(1):67‐69. [DOI] [PubMed] [Google Scholar]
Fulton 1975 {published data only}
- Fulton JEJ. Miconazole therapy for endemic fungal disease. Arch Dermatol 1975;111:596‐8. [PubMed] [Google Scholar]
Galimberti 1984 {published data only}
- Galimberti RL, Belli L, Negroni R, Castro JM, Rohwedder R, Tuculet MA. Prophylaxis of tinea pedis interdigitalis with bifonazole 1% powder. Dermatologica 1984;169(suppl 1):111‐6. [DOI] [PubMed] [Google Scholar]
Gip 1983 {published data only}
- Gip L, Forsstrom S. A double‐blind parallel study of sulconazole nitrate 1% cream compared with miconazole nitrate 2% cream in dermatophytoses. Mykosen 1983;26:231‐41. [PubMed] [Google Scholar]
Greer 1986 {published data only}
- Greer DL. Topical treatment for moccasin‐type tinea pedis. Journal of the American Academy of Dermatology 1987;16:554‐8. [DOI] [PubMed] [Google Scholar]
Greer 1987 {published data only}
- Greer D, Jolly HW. Comparative trial of a two‐dosage schedule of ketoconazole 2% cream for the treatment of tinea pedis. Journal of the American Academy of Dermatology 1987;17:53‐6. [DOI] [PubMed] [Google Scholar]
Grigoriu 1983 {published data only}
- Grigoriou D, Grigoriou A. A double‐blind comparison of the efficacy, toleration and safety of tioconazole base 1% and econazole nitrate 1% creams in the treatment of patients with fungal infections of the skin or erythasma. Dermatologica 1983;166(suppl 1):8‐13. [DOI] [PubMed] [Google Scholar]
Grooten 1992 {published data only}
- Grooten A, Meijman FJ. The treatment of the dermatomycoses of toes, feet and inguinal region: comparative study on the efficacy of miconazole and Whitfield's ointment. Huisarts Wet 1992;35:26‐8. [Google Scholar]
Kashin 1985 {published data only}
- Kashin P, Ohyfferoen MC, Gibbs DL. A comparative study of once‐ versus twice‐daily treatment of superficial dermatophyte and yeast infections with tioconazole 1% cream. The Journal of international medical research 1985;13:85‐95. [DOI] [PubMed] [Google Scholar]
Katayama 1987 {published data only}
- Katayama H. The treatment of tinea with topically applied leukotriene B4. Prostaglandins 1987;34:797‐804. [DOI] [PubMed] [Google Scholar]
Kates 1990 {published data only}
- Kates SG, Myung KB, McGinley KJ, Leyden JJ. The antibacterial efficacy of econazole nitrate in interdigital toe web infections. Journal of the American Academy of Dermatology 1990;22:583‐6. [DOI] [PubMed] [Google Scholar]
Katz 1972 {published data only}
- Katz R. Haloprogen therapy for dermatophyte infections. Archives of Dermatology 1972;106:837‐8. [PubMed] [Google Scholar]
Koca 2001 {published data only}
- Koca R, Canturk T, Senturk N, Turanli AY. Comparison of combined clotrimazole and aluminium hydroxychloride with clotrimazole therapy in interdigital type tinea pedis. Ondokutz Mayis Universitesi Tip Dergisi 2001;18:192‐197. [Google Scholar]
Lassus 1983 {published data only}
- Lassus A, Forstrom S, Salo O. A double‐blind comparison of sulconazole nitrate 1% cream with clotrimazole 1% cream in the treatment of dermatophytoses. British Journal of Dermatology 1983;108:195‐8. [DOI] [PubMed] [Google Scholar]
Lassus 1988 {published data only}
- Lassus A, Nolting KS, Savopoulos C. Comparison of ciclopiroxolamine 1% cream with ciclopirox 1%‐hydrocortisone acetate 1% cream in the treatment of inflamed superficial mycoses. Clinical Therapeutics 1988;10:594‐9. [PubMed] [Google Scholar]
Lauharanta 1992 {published data only}
- Lauharanta J. Comparative efficacy and safety of almorolfine lacquer 2% versus 5% once weekly. Clinical and Experimental Dermatology 1992;17((suppl 1 Sept)):41‐43. [DOI] [PubMed] [Google Scholar]
Lebwohl 2001 {published data only}
- Lebwohl M, Elewski B, Eisen D, Savin RC. Efficacy and safety of terbinafine 1% solution in the treatment of interdigital tinea pedis and tinea corporis or tinea cruris. Cutis 2001;67(3):261‐6. [PubMed] [Google Scholar]
Lestienne 1982 {published data only}
- Lestienne MC, Chemali R, Tennstedt D, Vanaele R, Lachapelle JM, Lapiere CM. Double‐blind comparison of econazole spray powder and placebo as therapy and prophylaxis of athlete's foot. Mykosen 1982;25:335‐40. [DOI] [PubMed] [Google Scholar]
Li 2001 {published data only}
- Li Q, Li M, Qin L‐M, Gu J, Liu WD, Guo ZL. Clinical efficacy of compound econazole cream in the treatment of tinea corporis, tinea inguinalis, and tinea manus, tinea pedis and its safety. Parmaceutical care and research (Yaoxue fuwu yu yanjiu) 2001;4(1):52‐4. [Google Scholar]
Lison 1985 {published data only}
- Lison EM, Pearson BG, Goodfellow RC. Athlete's foot: a step forward. Occupational Health 1985;37:66‐70. [PubMed] [Google Scholar]
Lynfield 1974 {published data only}
- Lynfield YL, Littman ML, Feingold LE. Treatment of tinea pedis with micronized griseofulvin and tolnaftate. Cutis 1974;13:460‐2. [Google Scholar]
Maibach 1978 {published data only}
- Maibach HI. Iodochlorhydroxyquin‐hydrocortisone treatment of fungal infections. Archives of Dermatology 1978;114:1773‐5. [PubMed] [Google Scholar]
McVie 1986 {published data only}
- McVie DH, Littlewood S, Alle BR, Pollock AC, Wood P, Milne LJR. Sulconazole versus clotrimazole in the treatment of dermatophytosis. Clinical and Experimental Dermatology 1986;11:613‐8. [DOI] [PubMed] [Google Scholar]
Meinicke 1985 {published data only}
- Meinicke K, Streigel C, Weidinger G. Treatment of dermatomycoses with naftifine: therapeutic efficacy on application once daily and twice daily. Mykosen 1987;30:98‐103. [PubMed] [Google Scholar]
Mertens 1976 {published data only}
- Mertens RLJ, Morias J, Verhanmme G. A double‐blind study comparing Daktacort, miconazole and hydrocortisone in inflammatory skin infections. Dermatologica 1976;153:228‐35. [DOI] [PubMed] [Google Scholar]
Nolting 1992 {published data only}
- Nolting S, Semig G, Fredrich HK, Dietz M, Reckers‐Czaschka R, Bergstraesser M, et al. Double‐blind comparison of amorolfine and bifonazole in the treatment of dermatomycoses. Clinical and Experimental Dermatology 1992;17(suppl 1):56‐60. [DOI] [PubMed] [Google Scholar]
Nolting 1993 {published data only}
- Nolting S, Reinel D, Semig G, Reckers‐Czaschka R, Bergstraesser M. Amorolfine spray in the treatment of foot mycoses (dose finding study). British Journal of Dermatology 1993;129:170‐4. [DOI] [PubMed] [Google Scholar]
Ortiz 1978 {published data only}
- Ortiz LG, PapaPR. Topical miconazole nitrate therapy in tinea pedis and tinea versicolor. Clinical Therapeutics 1978;1:444‐50. [Google Scholar]
Oyeka 1991 {published data only}
- Oyeka CA, Gugnani HC. Isoconazole nitrate versus clotrimazole in foot and nail infections due to hendersonula toruloidea, scytalidium hyalinum and dermatophytes. Mycoses 1992;35:357‐61. [DOI] [PubMed] [Google Scholar]
Pariser 1994 {published data only}
- Pariser DM, Pariser RJ. Oxiconazole nitrate lotion 1% ‐‐ an effective treatment for tinea pedis. Cutis 1994;54:43‐4. [PubMed] [Google Scholar]
Patel 1999 {published data only}
- Patel A, Brookman SD, Bullen MU, Marley J, Ellis DH, Williams T, Barnetson RS. Topical treatment of interdigital tinea pedis: terbinafine compared with clotrimazole. Australasian Journal of Dermatology 1999;40(4):197‐200. [DOI] [PubMed] [Google Scholar]
Privat 1982 {published data only}
- Privat Y, Konopka CA. A comparative controlled clinical trial of bifonazole vs econazole in superficial mycoses. In: Urabe H, editor. Proceedings of the International Antifungal Symposium: Bifonazole 1982. 1982:136‐40.
Qadripur 1984 {published data only}
- Qadripur SA. Double‐blind parallel comparison of sulconazole nitrate 1% cream and powder with econazole 1% cream and powder in the treatment of cutaneous dermophytoses. Current Therapeutic Research, Clinical and Experimental 1984;35:753‐8. [Google Scholar]
Reinel 1992 {published data only}
- Reinel D. Topical treatment of onychomycosis with Almorolfine 5% nail laquer: comparative efficacy and tolerability of once weekly use. Dermatology 1992;184:21‐24. [DOI] [PubMed] [Google Scholar]
Reinel 1992a {published data only}
- Reinel D, Clark C. Comparative efficacy and safety of amorolfine nail laquer 5% in onychomycosis.. Clinical and experimental dermatology 1992;17 (suppl):44‐49. [DOI] [PubMed] [Google Scholar]
Ruping 1993 {published data only}
- Ruping KH, Haas PJ. Treatment of onychomycosis ‐ bifonazole nail set in comparison with urea. Zeitschritfur Artzliche Fortbildung 1993;87 (5):425‐429. [PubMed] [Google Scholar]
Saple 2001 {published data only}
- Saple DG, Amar AKJ, Ravichandran G, Korde KM, Desai A. Efficacy and safety of butenafine in superficial dermatophytosis. Journal of the Indian Medical Association 2001;99(5):274‐5. [PubMed] [Google Scholar]
Shellow 1982 {published data only}
- Shellow WVR. 2% miconazole nitrate powder in aerosol spray form: its efficacy in treating tinea pedis. The Journal of international medical research 1982;10:28‐31. [DOI] [PubMed] [Google Scholar]
Smith 1974 {published data only}
- Smith E, David LM, Knox JM. Topical clotrimazole in dermatophytosis in a prison environment. Post Graduate Medical Journal 1974;50(suppl 1):64‐6. [PubMed] [Google Scholar]
Smith 1977b {published data only}
- Smith E, Powell RF, Graham JL, Ulrich JA. Topical undecylenic acid in tinea pedis: a new look. International Journal of Dermatology 1977;16:52‐6. [DOI] [PubMed] [Google Scholar]
Stettendorf 1983 {published data only}
- Stettendorf S. Tolerability and efficacy of bifonazole in dermatomycoses.. Arzneimittel Forschung 1983;33:750‐4. [PubMed] [Google Scholar]
Suschka 2001 {published data only}
- Suschka S, Fladung B, Merk HF. Clinical comparison of the efficacy and tolerability of once daily canesten with twice daily Nizoral (Clotrimazole 1% cream vs Ketoconazole 2% cream) during a 28 day topical treatment of interdigital tinea pedis. Mycoses 2001;45:91‐6. [DOI] [PubMed] [Google Scholar]
Tanenbaum 1982 {published data only}
- Tanenbaum L, Anderson C, Rosenberg MJ, Howard W, McDaniel W, Neimanis A, Ryan ME, Perez R. Sulconazole nitrate 1% cream. A comparison with miconazole in the treatemnt of tinea pedis and tinea cruris corporis. Therapeutics for the Clinician 1982;30:105‐7. [PubMed] [Google Scholar]
Tanenbaum 1983 {published data only}
- Tanenbaum L, Anderson C, Rosenberg M, Dorr A. A new treatment for cutaneous candidiasis ‐‐ sulconazole nitrate cream 1%. International Journal of Dermatology 1983;22:318‐20. [DOI] [PubMed] [Google Scholar]
Tanuma 2001 {published data only}
- Tanuma H, Tanuma M, Abe M, Kume H. Usefulness of lanoconazole (Astat) cream in the treatment of hyperkeratotic type tinea pedis. Comparative study of monotherapy and combination therapy with 10% urea ointment (Pastaron) [Stellenwert von Lanoconazol (Astat®) ‐ Creme bei der Behandlung der hyperkeratotischen Form der Tinea pedis: Vergleichende Studie über Monotherapie und Kombinationstherapie mit 10% Harnstoffsalbe (Pastaron®)]. Mycoses 2001;44(5):181‐190. [DOI] [PubMed] [Google Scholar]
Terragni 1993 {published data only}
- Terragni l, Caputo R. Efficacy and tolerability of of kevis nails associated with antimycotic product in the treatment of onychomycosis. G‐Ital ‐ Dermatol‐Venereologica 1993;128(4):XLI‐XLIII. [Google Scholar]
Thomas 1976 {published data only}
- Thomas DJ. A study in industry of clotrimazole cream in tinea pedis and tinea cruris. Curr Med Res 1976;3:630‐3. [Google Scholar]
Thomas 1986 {published data only}
- Thomas DJ, Evans A. A study in Industry of Bifonazole (1%gel) and sulconazole cream in tinea pedis and tinea cruris. In: Hay R editor(s). Advances in Topical Antifungal Therapy. Berlin: Springer‐Verlag, 1986:68‐75. [Google Scholar]
Tschen 1979 {published data only}
- Tschen EH, Becker LE, Ulrich JA, Hoge WH, Smith EB. Comparison of over the counter agents for tinea pedis. Cutis 1979;23:696‐8. [PubMed] [Google Scholar]
Tsuboi 1996 {published data only}
- Tsuboi R, Matsumoto T, Ogawa H, et al. Hyperkeratotic chronic tinea pedis treated with neticonazole cream. Int J Dermatol 1996;35:371‐3. [DOI] [PubMed] [Google Scholar]
Weil 1996 {published data only}
- Weil M, Elewski B. Topical econazole versus terbinafine in the treatment of toe web space infections: a comparison. Advances in Therapy 1996;13:355‐64. [Google Scholar]
Zaug 1995 {published data only}
- Zaug M. Amorolfine nail lacquer: clinical experiance in onychomycosis. Journal of European Academy of Dermatology and venereology 1995;4(1):S23‐30. [Google Scholar]
References to studies awaiting assessment
Herrera‐Arellano {published data only}
- Herrera‐Arellano A, Rodriguez‐Soberanes A, Angeles Martinez Riviera M, Martinez Cruz E, Zamilpa A, Alvarez L, Tortoriello J. Effectiveness and tolerability of a standard phytodrugderived from solanum chrysitrichum on tinea pedis: a randomised clinical trial. Planta medica 2003;69(5):390‐5. [DOI] [PubMed] [Google Scholar]
Sugiura {published data only}
- Sugiura M, Hata Y, Fukuda T, Ishizaki S, Hanyaku H, Naka W, Harada T, Nishikawa T. One‐week application of terbinafine cream compared with four‐week application in treatment of tinea pedis. Japanese Journal of Medical Mycology 2001;42(4):223‐228. [DOI] [PubMed] [Google Scholar]
Additional references
Beaven & Brooks 1994
- Beaven DW, Brooks SE. The nail in clinical diagnosis. Mosby‐Wolfe, 1994. [Google Scholar]
Brautigam et al 1995
- Brautigam M, Nolting S, Schopf G, Weidinger G. Randomised double blind comparison of terbinafine and itraconazole for the treatment of toenail tinea infection. BMJ 1995;311:919‐921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbe 1985
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐233. [DOI] [PubMed] [Google Scholar]
Petit 1983
- Pettit J. Manual of practical dermatology. Edinburgh: Churchill Livingstone, 1983. [Google Scholar]
Roberts 1992
- Roberts DT. Prevalence of dermatophyte onychomycosis in the UK: results of an omnibus survey. British Journal of Dermatology 1992;126 suppl 39:23‐7. [DOI] [PubMed] [Google Scholar]


