Abstract
Background
Chronic inflammatory diseases in childhood and early adult life share aetiological factors operating from birth and onwards. In this study, we use data from the national Danish health registers to evaluate the risk of developing four common, immune-mediated hospital-diagnosed childhood chronic inflammatory diseases.
Methods
A national population-based registry study. Data from the Danish Medical Birth Registry and the Danish National Patient Registry from January 1973 to March 2016 were linked at a personal level to evaluate any potential associations between caesarean section and development of Inflammatory bowel diseases, rheumatoid arthritis, coeliac disease and diabetes mellitus among the offspring. A model adjusted for parental age at birth, decade of birth, gender of child, and parents’ chronic inflammatory disease status was used.
Results
This register-based national cohort study of 2672708 children with information on delivery mode found an increased risk of diabetes, arthritis, coeliac disease, and inflammatory bowel disease for both girls and boys after caesarean section compared with vaginal delivery. The higher risk was present at least 40 years after delivery. In a subgroup analysis, both acute and elective caesarean section was associated with an increased risk of developing a chronic inflammatory disease.
Conclusions
Being born by caesarean section leads to increased host susceptibility for chronic inflammatory diseases that last for decades. This finding should be further addressed in future studies with the aim to support the development of new strategies for prevention, treatment, and maybe even cure.
Keywords: caesarean delivery, population study, vaginal birth, chronic inflammatory disease, inflammatory bowel diseases, rheumatoid arthritis, coeliac disease
Key Messages
This study found an increased risk of four common, immune-mediated hospital-diagnosed childhood chronic inflammatory diseases (inflammatory bowel diseases, rheumatoid arthritis, coeliac disease, and diabetes mellitus) both as separate diseases and when combined as chronic inflammatory diseases, in children after delivery by caesarean section compared to vaginal delivery.
The increased risk was detectable in the offspring for at least 40 years of follow-up from birth for all four diseases.
The risk was increased for both girl and boys for all four diseases.
The risk of developing a chronic inflammatory disease was increased for both elective and acute caesarean section, as compared with vaginal delivery.
Introduction
Chronic inflammatory diseases (CIDs) include such disparate diseases as inflammatory bowel diseases (IBD, including Crohn’s disease1 and ulcerative colitis2), rheumatoid arthritis (RA),3 coeliac disease (CD)4 and diabetes mellitus (DM).5 CIDs are lifelong, frequently with onset early in life. CIDs affect the quality of life of patients and their families including aspects like participation in educational programs, family planning, and leisure activities. With a high lifetime risk of 40% at some locations in the world,6–8 CIDs impact society through work productivity losses and increased health system expenses caused by frequent visits, hospitalizations, and medications.9 This burden is expected to increase markedly in coming years due to a predicted rise in the number of CID patients.6, 10−13 Thus, there is an urgent need for further improvements in the treatment of CIDs and even for identifying ways to cure and prevent these diseases.
Since all CIDs involve immune-mediated aetiological mechanisms,14 including both common genetics15,16 and environmental susceptibility factors,17–20 the identification of shared and individual risk factors may improve the understanding of disease causation. Caesarean section (CS) has been associated with increased risk of CIDs of the offspring, possibly as a consequence of increased host susceptibility for CIDs mediated by alterations in the microbial exposure, and a delayed establishment of the gut microbiota that may impair the maturation of the mucosal immune system.21 In contrast to planned CS (elective CS), acute CS will often be performed after initiation of the labour, where an open birth canal might potentially give access to the vaginal microbial community. Studies have addressed the risk of developing IBD,22–27 DM,27–30 CD,31–33 and RA.34 CS, and in particular elective CS has been associated with risk of CIDs in some studies,22,24,27,32 but not all.26,28,29
The hypothesis for the present study is that CIDs share a common aetiological component associated with being born by CS and that the risk is higher for elective than planned CS. The aim of the present study was to evaluate the risk of developing CIDs (including four common hospital-diagnosed childhood CIDs) after being born by caesarean delivery, taking advantage of the National Danish registries completeness, size, and long follow-up.35–38
Materials and Methods
Study Cohort
The Danish Medical Birth Register (MBR), and the Danish National Patient Registry (NPR)36–38 was used to identify children born in Denmark (N=2701 408) from January 1973 to March 2016 from the MBR. The linkage between information from the various registries was performed using the unique individual personal identification number assigned to every Danish citizens.38 Data were specified by the authors and extracted by the Danish Health and Medicines Authority. After exclusion of children with missing birth date (N=1929) and children with missing information on parents in the Danish Civil Registration System (CRS), (N=26,771) the cohort comprised of 2672 708 children (Supplemental Figure 1).
Assessment of Familial Exposures
Parents of newborns were identified from the person-wise linkage of data from CRS and the health registries using the unique personal identification number assigned to each inhabitant of Denmark at birth or immigration.37 Cases of CIDs in the parents were ascertained by the first occurrence of relevant ICD diagnosis codes (Supplemental Table 1) from the NPR. Parental age at delivery of the child was derived from the CRS.
Exposures
A delivery was classified as a CS if the mother was registered with procedure codes corresponding with CS during a hospital admittance around the day of birth of the child, and as vaginal delivery, if no such procedure was registered. As data were not available from the NPR started prior to 1977, we used data on registered CS in the MBR37 for the period 1973–1976. For the years 1991–2016, information on acute versus elective CS was available from MBR, but no data were available to distinguish between acute and elective CS for the period 1973–1990.
Outcomes
Outcomes in the offspring were identified as the first occurrence of ICD diagnosis codes for the CIDs under study (Supplemental Table 1) in the NPR.36 Data on vital status and emigrations were obtained from the CRS.38
Statistical Analysis
We investigated the risk of developing each of the four CIDs separately, as well as the risk of developing at least one of the four diseases. The risk was calculated by using the Kaplan Meier method as well as Cox regression to compare vaginal delivery, acute CS and elective CS, respectively, for the period (1991–2016), while limiting the analysis to vaginal delivery and CS for the observation period 1973–2016. Censoring events included emigration and end-of-follow-up. We evaluated the proportional hazards assumption by estimating Schoenfeld residuals. We applied a univariate model containing the only mode of delivery as the exposure, a model adjusted for parental age at birth, decade of birth and gender of the child, and a model furthermore adjusted for the presence of CIDs in the parents. A sensitivity analysis was conducted testing interactions between exposures and sex of the child, as well as the decade of birth. Furthermore, we investigated if adjusting for year of birth in 1-year groups instead of decades changed the results. Moreover, we carried out subanalysis only including children born at gestational age at least 37 weeks to investigate the effect of premature birth.
Analyses were conducted using Stata version 15.0 (Stata Corp LP, College Station, TX).
Results
Among 2672 708 children liveborn in Denmark from January 1977 through March 2016, 2271 913 (85.0%) were born by vaginal delivery and 400 795 (15.0%) by CS. Characteristics of this cohort are shown in Table 1. For observations after 1990, it was possible to discriminate between children born by acute and elective CS. After excluding 5952 births with an unknown type of section from the analyses investigating elective and acute section separated 1598 834 births, 1312 522 (82.1%), 122 419 (7.7%), and 163 893 (10.3%) children were born by vaginal delivery, elective and acute CS, respectively. Characteristics of this subcohort are shown in Supplemental Table 2.
Table 1.
Counts and Proportions of Chronic Inflammatory Diseases by Mode of Delivery
| Full Period 1973–2016 (N=2,672,708 Births) | ||
|---|---|---|
| Vaginal Delivery | CS | |
| Total | 2,271,913 (85.0%) | 400,795 (15.0%) |
| DM | 20,936 (87.7%) | 2,932 (12.3%) |
| RA | 9,075 (86.7%) | 1,395 (13.3%) |
| CD | 6,085 (85.3%) | 1,047 (14.7%) |
| Crohn’s disease | 8,051 (87.9%) | 1,104 (12.1%) |
| Ulcerative colitis | 11,436 (88.9%) | 1,428 (11.1%) |
| IBD (Crohn’s disease + ulcerative colitis) | 17,293 (88.5%) | 2,240 (11.5%) |
| Combined (at least one) | 51,926 (87.5%) | 7,405 (12.5%) |
| DM & RA | 225 (89.6%) | 26 (10.4%) |
| DM & CD | 400 (83.2%) | 81 (16.8%) |
| DM & IBD | 383 (89.1%) | 47 (10.9%) |
| RA & CD | 58 (87.9%) | 8 (12.1%) |
| RA & IBD | 227 (91.5%) | 21 (8.5%) |
| CD & IBD | 202 (86.7%) | 31 (13.3%) |
| Dead | 25,067 (79.7%) | 6,392 (20.3%) |
| Emigrated | 73,661 (87.4%) | 10,661 (12.6%) |
Notes: The disease combinations do not sum up to the “Combined” count, as 35 persons in the full cohort experienced more than two of the diseases.
Abbreviations: CD, Coeliac disease; CS, Caesarean section; DM, Diabetes mellitus; IBD, Inflammatory bowel disease; RA, Rheumatoid arthritis.
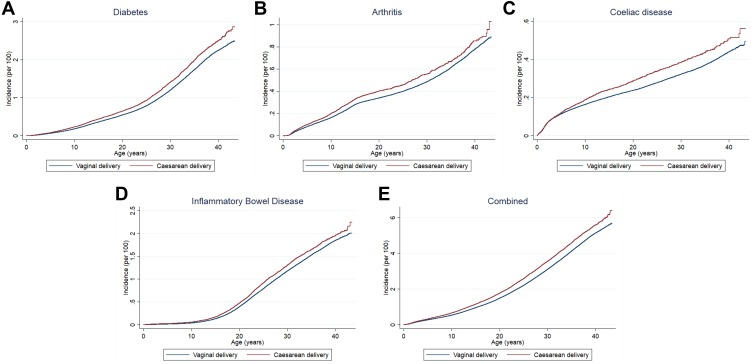
Kaplan-Meier curves for the incidence of the four outcome diseases by the delivery method are shown in Figure 1, indicating an increased incidence of all diseases in children born by CS with the difference notable from childhood but most clear in early adulthood.
Figure 1.
Kaplan Meier plots for age at diagnosis of outcome diseases by mode of delivery for (A) Diabetes, (B) Arthritis, (C) Coeliac Disease, (D) Inflammatory Bowel Disease, and (E) Combined.
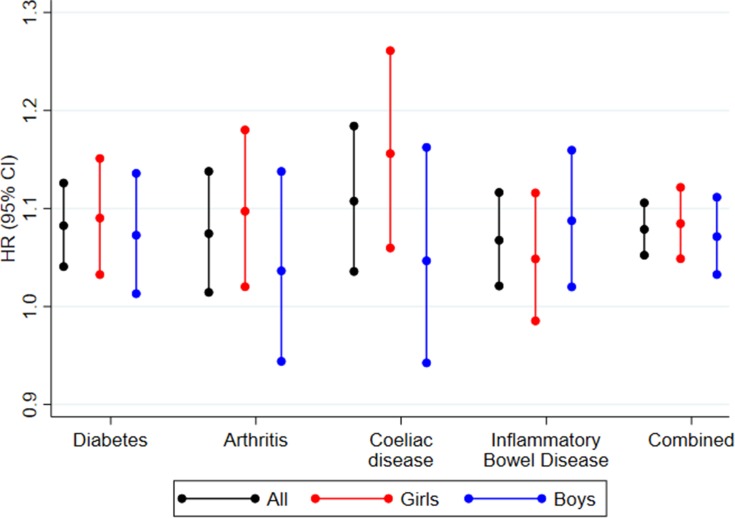
The hazard ratios (HRs) for the association between mode of delivery and disease development are shown in Figure 2 and Supplemental Tables 3–5 (for both genders combined, girls and boys, respectively). Figure 2 includes the fully adjusted estimates (adjusted for the decade of birth, child’s sex, mother’s and father’s age and mother’s and father’s DM, RA, DC, and IBD). Compared to being born vaginally, being born by CS was associated with development of DM (HR=1.08 (95% confidence interval (CI): 1.04, 1.12)), RA (HR=1.07 (95% CI: 1.02, 1.14)), CD (HR=1.10 (95% CI: 1.03, 1.18)), and IBD (HR=1.07 (95% CI: 1.02, 1.12)). Furthermore, we observed an association for the combined outcome (HR=1.08 (95% CI: 1.05, 1.10)) (Figure 2 and Supplemental Tables 3). Compared with vaginal delivery the risk estimates for elective as well as acute CS were elevated for DM with HRs of 1.14 (95% CI: 1.03, 1.25) and 1.05 (95% CI: 0.96, 1.14), respectively. The similar HRs for RA were 1.14 (95% CI: 1.02, 1.27) and 1.09 (95% CI: 0.99, 1.20), respectively, for CD 1.04 (95% CI: 0.92, 1.19) and 1.15 (95% CI: 1.03, 1.29), and for IBD 1.16 (95% CI: 1.03, 1.30) and 1.06 (95% CI: 0.95, 1.18). For the combined outcome we observed associations for both elective (HR=1.13 (95% CI: 1.07, 1.20)) and acute CS (HR=1.08 (95% CI: 1.03, 1.14)) (Supplemental Tables 6).
Figure 2.
Forest plot of HR from Cox regressions (95% CIs) for associations between caesarean section and development of diabetes, arthritis, coeliac disease, inflammatory bowel disease, and combined for the combined cohort as well as by sex.
Among girls, the risk estimates for CS were elevated for DM (HR=1.09 (95% CI: 1.03, 1.15)), RA (HR=1.10 (95% CI: 1.02, 1.18)), CD (HR=1.15 (95% CI: 1.05, 1.25)), and IBD (HR=1.05 (95% CI: 0.99, 1.12)). Furthermore, we observed an association for the combined outcome (HR=1.08 (95% CI: 1.05, 1.12)) (Figure 2 and Supplemental Table 4). Furthermore, the risk estimates for both elective and acute CS were elevated for DM with HRs of 1.16 (95% CI: 1.02, 1.32) and 1.14 (95% CI: 1.01, 1.29), respectively. The similar HRs for RA were 1.12 (95% CI: 0.97, 1.30) and 1.13 (95% CI: 0.99, 1.29), for CD 1.07 (95% CI: 0.91, 1.27) and 1.26 (95% CI: 1.09, 1.45), and for IBD 1.25 (95% CI: 1.07, 1.46) and 0.97 (95% CI: 0.82, 1.14), respectively. For the combined outcome we observed associations for both elective (HR=1.15 (95% CI: 1.07, 1.24)) and acute CS (HR=1.13 (95% CI: 1.06, 1.22)) (Supplemental Tables 7).
Among boys, the risk estimates for CS were elevated for DM with a HR of 1.07 (95% CI: 1.01, 1.13)), RA with a HR of 1.04 (95% CI: 0.95, 1.14)), CD with a HR of 1.04 (95% CI: 0.94, 1.16)), and IBD with a HR of 1.09 (95% CI: 1.02, 1.16)). Furthermore, we observed an association for the combined outcome (HR=1.07 (95% CI: 1.03, 1.11)) (Figure 2 and Supplemental Table 5). In addition, the risk estimates for both elective and acute CS were elevated for DM with HRs of 1.11 (95% CI: 0.97, 1.27) and 0.97 (95% CI: 0.86, 1.10), respectively. The similar HRs for RA were 1.17 (95% CI: 0.98, 1.39) and 1.03 (95% CI: 0.89, 1.20), for CD 1.01 (95% CI: 0.81, 1.25) and 1.00 (95% CI: 0.83, 1.20), and for IBD 1.07 (95% CI: 0.90, 1.27) and 1.14 (95% CI: 0.99, 1.32), respectively. For the combined outcome we observed association for elective (HR=1.11 (95% CI: 1.02, 1.21)) but not for acute CS (HR=1.03 (95% CI: 0.96, 1.11)) (Supplemental Tables 8).
Adjusting the Cox models generally decreased the HR towards 1, indicating that this adjustment accounted for possible confounding. The sensitivity analyses investigating interactions between mode of delivery and decade of birth or sex of the child did not result in significant interactions (data not shown). The sensitivity analysis adjusting for a birth year in 1-year groups resulted in estimates very similar to the main analysis (data not shown). The subanalyses of children born at term resulted in similar estimates, although lower for CD (Supplemental Tables 9).
Discussion
This register-based national cohort study of 2672 708 children found an increased risk of DM, RA, CD, and IBD for girls as well as for boys after CS, compared with birth by vaginal delivery. The increased risk was detectable at least 40 years after delivery.
The main strength of this observational study is the inclusion of more than 2.5 million nationwide births with complete ascertainment and with an overall rate of CS of 15%. This, together with a follow-up of until 40 years of age and high data quality, has provided sufficient power to analyse the associations between delivery mode and disease risk. The chosen diseases, DM, RA, CD, and IBD, are common in childhood and they have immune-mediated etiologies. Furthermore, since they are managed at hospitals both diagnostic accuracy and ascertainment probability are assumed to be high. This is in contrast to eg astma and allergy that may also be treated at the general practitioner in Denmark to a large extent. As further strength, we have been able to adjust for known susceptibility factors including the presence of CIDs in the parents.
The main weakness is that the study, by means of the observational design, cannot infer causality from positive associations. Although we found, in general, a higher risk of CID by being born by elective CS compared with acute CS, we cannot from the data available provide insight into possible underlying mechanisms, for example the involvement of the vaginal or gut microbial environment. Furthermore, even though we have attempted to adjust for relevant confounders we cannot exclude the possibility of residual confounding in the analysis. We were, for example, not able to adjust for genetic susceptibility among the children. However, we did adjust for the presence of CIDs among the parents. Whereas it may be considered a strength to use already existing register data, produced for other purposes than that of the present study, the validity of the data may be affected by missing registrations and coding errors.39 Such errors could potentiate any residual confounding. Overall, although the results are very consistent, the estimated associations are small and we cannot exclude that they may be wholly or partly explained by residual confounding.
Large nation-wide populations based studies have evaluated whether the risk of CIDs was increased in CS compared to vaginal delivery.22,28,33,34 First, an increased risk of IBD was found among 2 mio. children in the Danish population (1973–2008 and 1977–2009, respectively).22,23 Next, Dydensborg et al found a non-statistically increased risk of Crohn’s Disease among 1 mio children in the Danish population (1.11 (95% CI: 0.96–1.29)) (1995–2010), though the risk was not found among 0.5 mio. children in the Norwegian population with a shorter follow-up (2004–2012) included in the same study. In contrast, CS was not associated with DM in a Danish study of 1.7 mio. children born between 1982–2010.28 In large Swedish case-control studies of 11,748 CD and 9376 DM case, respectively, no association with SC was found.29,32 Smaller studies resulted in conflicting findings.24,25,27,29–31 In contrast to the previous studies, the present study evaluated DM, RA, CD, and IBD simultaneously, and found children born by CS to have an increased risk of receiving a diagnosis of at least one of all the investigated CID, compared to vaginal delivery. Secondly, the present study has a long follow-up period of up to 40 years, enabling identification of CID cases beyond childhood and young adulthood. Our study found an increased risk for both genders in concordance with a previous IBD study.23 Previous studies have suggested that the risk of CID is higher for elective than for acute section.27,33 Whereas our study has confirmed an increased risk for both acute and elective section, our data is too limited to test for any possible differential risk in the two modes of CS, although the overall estimate is higher for elective CS.
The finding of an increased risk for CID by being born by SC aligns with the hypothesis that CIDs share a common aetiological component associated with being born by CS. Even though the association is statistically highly significant, the absolute increased disease risk by being born by caesarean section is low. Our results may generate hypotheses on shared disease mechanisms in CIDs which may be tested in future studies and thereby lead to improved understanding of biological mechanisms underlying CIDs. Future studies should in-depth explore the causal pathways underlying the disease risk conferred by CS since such knowledge may support the development of prevention and optimised treatment strategies in CIDs.
Abbreviations
CI, Confidence intervals; CID, Chronic inflammatory disease; CD, Coeliac disease; CRS, The Danish Civil Registration System; CS, Caesarean section; HR, Hazard ratio; IBD, Inflammatory bowel disease; ICD10, International Classification of Diseases, 10th revision; MBR, The Danish Medical Birth Register; NPR, The Danish National Patient Register; OR, Odds ratio; RA, Rheumatoid arthritis; DM, Diabetes mellitus.
Transparency Statement
VA affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Ethics
Studies on data registries (that do not include biological material) should not be notified to the ethics committee in Denmark.
Data Sharing Statement
Data is stored at Open Patient data Explorative Network (OPEN). Bona fide researchers can apply to use the dataset by applying to open@rsyd.dk.
Author Contributions
Contributor and guarantor information: VA conceived the research and VA, AG, and SM wrote the first draft of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was funded by The Danish Rheumatism Association (Gigtforeningen) (R104-A2195-B760). The funders did not influence the conduct of the study, analysis, interpretation of the data, the writing of this report, or the decision to publish.
Disclosure
Professor Vibeke Andersen reports grants from The Danish Rheumatism Association (Gigtforeningen), during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9 [DOI] [PubMed] [Google Scholar]
- 2.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 4.Leonard MM, Sapone A, Catassi C, Fasano A. Celiac disease and nonceliac gluten sensitivity: a review. JAMA. 2017;318:647–656. doi: 10.1001/jama.2017.9730 [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KM, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabetes Endocrinol. 2014;2:867–874. doi: 10.1016/S2213-8587(14)70161-5 [DOI] [PubMed] [Google Scholar]
- 7.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 8.WHO. Available from: http://www.who.int/chp/topics/rheumatic/en/. Accessed February20, 2017.
- 9.Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369:754–762. doi: 10.1056/NEJMct1209614 [DOI] [PubMed] [Google Scholar]
- 10.Namatovu F, Sandstrom O, Olsson C, Lindkvist M, Ivarsson A. Celiac disease risk varies between birth cohorts, generating hypotheses about causality: evidence from 36 years of population-based follow-up. BMC Gastroenterol. 2014;14:59. doi: 10.1186/1471-230X-14-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. doi: 10.1038/nrgastro.2015.150 [DOI] [PubMed] [Google Scholar]
- 13.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(13)62154-6 [DOI] [PubMed] [Google Scholar]
- 14.Schultze JL, Rosenstiel P. Systems medicine in chronic inflammatory diseases. Immunity. 2018;48:608–613. doi: 10.1016/j.immuni.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 15.Lees CW, Barrett JC, Parkes M, Satsangi J. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739–1753. doi: 10.1136/gut.2009.199679 [DOI] [PubMed] [Google Scholar]
- 16.Lettre G, Rioux JD. Autoimmune diseases: insights from genome-wide association studies. Hum Mol Genet. 2008;17:R116–R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Sloot KWJ, Amini M, Peters V, Dijkstra G, Alizadeh BZ. Inflammatory bowel diseases: review of known environmental protective and risk factors involved. Inflamm Bowel Dis. 2017;23:1499–1509. doi: 10.1097/MIB.0000000000001217 [DOI] [PubMed] [Google Scholar]
- 18.Myleus A, Hernell O, Gothefors L, et al. Early infections are associated with increased risk for celiac disease: an incident case-referent study. BMC Pediatr. 2012;12:194. doi: 10.1186/1471-2431-12-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyerlein A, Wehweck F, Ziegler AG, Pflueger M. Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatr. 2013;167:800–807. doi: 10.1001/jamapediatrics.2013.158 [DOI] [PubMed] [Google Scholar]
- 20.Kemppainen KM, Vehik K, Lynch KF, et al. Association between early-life antibiotic use and the risk of islet or celiac disease autoimmunity. JAMA Pediatr. 2017;171:1217–1225. doi: 10.1001/jamapediatrics.2017.2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannattasio A, Guarino A. Caesarean-section and neonatal gut microbiome: short and long term effects and new targets for early prevention. Ann Nutr Metab. 2018;73(Suppl 3):1–3. doi: 10.1159/000491812 [DOI] [PubMed] [Google Scholar]
- 22.Bager P, Simonsen J, Nielsen NM, Frisch M. Cesarean section and offspring’s risk of inflammatory bowel disease: a national cohort study. Inflamm Bowel Dis. 2012;18:857–862. doi: 10.1002/ibd.21805 [DOI] [PubMed] [Google Scholar]
- 23.Andersen V, Erichsen R, Froslev T, Sorensen HT, Ehrenstein V. Differential risk of ulcerative colitis and Crohn’s Disease among boys and girls after cesarean delivery. Inflamm Bowel Dis. 2013;19:E8–E10. doi: 10.1002/ibd.22841 [DOI] [PubMed] [Google Scholar]
- 24.Roberts SE, Wotton CJ, Williams JG, Griffith M, Goldacre MJ. Perinatal and early life risk factors for inflammatory bowel disease. World J Gastroenterol. 2011;17:743–749. doi: 10.3748/wjg.v17.i6.743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein CN, Banerjee A, Targownik LE, et al. Cesarean section delivery is not a risk factor for development of inflammatory bowel disease: a population-based analysis. Clin Gastroenterol Hepatol. 2016;14:50–57. doi: 10.1016/j.cgh.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 26.Bruce A, Black M, Bhattacharya S. Mode of delivery and risk of inflammatory bowel disease in the offspring: systematic review and meta-analysis of observational studies. Inflamm Bowel Dis. 2014;20:1217–1226. doi: 10.1097/MIB.0000000000000075 [DOI] [PubMed] [Google Scholar]
- 27.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA. 2015;314:2271–2279. doi: 10.1001/jama.2015.16176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clausen TD, Bergholt T, Eriksson F, Rasmussen S, Keiding N, Lokkegaard EC. Prelabor cesarean section and risk of childhood Type 1 diabetes: a Nationwide Register-based Cohort Study. Epidemiology. 2016;27:547–555. doi: 10.1097/EDE.0000000000000488 [DOI] [PubMed] [Google Scholar]
- 29.Samuelsson U, Lindell N, Bladh M, Akesson K, Carlsson A, Josefsson A. Caesarean section per se does not increase the risk of offspring developing type 1 diabetes: a Swedish population-based study. Diabetologia. 2015;58:2517–2524. doi: 10.1007/s00125-015-3716-3 [DOI] [PubMed] [Google Scholar]
- 30.Phillips J, Gill N, Sikdar K, Penney S, Newhook LA. History of cesarean section associated with childhood onset of T1DM in Newfoundland and Labrador, Canada. J Environ Public Health. 2012;2012:635097. doi: 10.1155/2012/635097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decker E, Engelmann G, Findeisen A, et al. Cesarean delivery is associated with celiac disease but not inflammatory bowel disease in children. Pediatrics. 2010;125:e1433–40. doi: 10.1542/peds.2009-2260 [DOI] [PubMed] [Google Scholar]
- 32.Marild K, Stephansson O, Montgomery S, Murray JA, Ludvigsson JF. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142:39–45.e3. doi: 10.1053/j.gastro.2011.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dydensborg Sander S, Hansen AV, Stordal K, Andersen AN, Murray JA, Husby S. Mode of delivery is not associated with celiac disease. Clin Epidemiol. 2018;10:323–332. doi: 10.2147/CLEP.S152168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristensen K, Henriksen L. Cesarean section and disease associated with immune function. J Allergy Clin Immunol. 2016;137:587–590. doi: 10.1016/j.jaci.2015.07.040 [DOI] [PubMed] [Google Scholar]
- 35.Andersen JS, Olivarius Nde F, Krasnik A. The Danish National Health Service Register. Scand J Public Health. 2011;39:34–37. doi: 10.1177/1403494810394718 [DOI] [PubMed] [Google Scholar]
- 36.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39:30–33. doi: 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- 37.Bliddal M, Broe A, Pottegard A, Olsen J, Langhoff-Roos J. The Danish Medical Birth Register. Eur J Epidemiol. 2018;33:27–36. doi: 10.1007/s10654-018-0356-1 [DOI] [PubMed] [Google Scholar]
- 38.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- 39.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO. Available from: http://www.who.int/chp/topics/rheumatic/en/. Accessed February20, 2017.