Abstract
Background
Studies have shown that interferon-beta (IFN-β) treatment is associated with headaches in patients with multiple sclerosis (MS). Headaches can affect quality of life and overall function of patients with MS. We examined the frequency, relationships, patterns, and characteristics of headaches in response to IFN-β in patients with relapsing-remitting multiple sclerosis (RRMS).
Patients and Methods
This study was a prospective, longitudinal analysis with 1-year follow-up. The study comprised 796 patients with RRMS treated with IFN-β (mean age 30.84±8.98 years) at 5 tertiary referral center outpatient clinics in Egypt between January 2015 and December 2017. Headaches were diagnosed according to the International Classification of Headache Disorders ICHD-3 (beta version), and data were collected through an interviewer-administered Arabic-language-validated questionnaire with an addendum specifically designed to investigate the temporal relationship between commencement of interferon treatment, and headache onset and characteristics.
Results
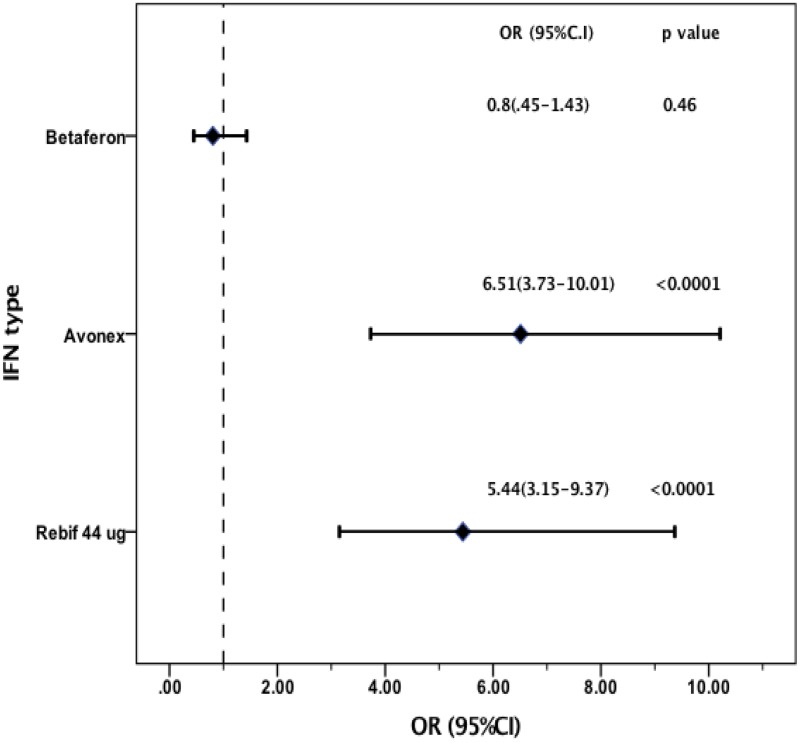
Two hundred seventy-six patients had pre-existing headaches, and 356 experienced de novo headaches. Of 122 patients who experienced headaches before IFN-β treatment, 55 reported headaches that worsened following onset of IFN-β treatment. In patients with post-IFN-β headaches, 329 had headaches that persisted for >3 months, 51 had chronic headaches, and 278 had episodic headaches, and 216 of these patients required preventive therapies. Univariate analysis showed a >6- and an approximately 5-fold increased risk of headache among those treated with intramuscular (IM) INF-β-1a (OR 6.51; 95% CI: 3.73–10.01; P-value <0.0001) and 44 μg of SC INF-β-1a (OR 5.44; 95% CI: 3.15–9.37; P-value <0.0001), respectively, compared with that in patients who received 22 μg of SC INF-β-1a.
Conclusion
Interferon-β therapy aggravated pre-existing headaches and caused primary headaches in patients with MS. Headache risk was greater following treatment with IM INF-β-1a and 44 μg SC INF-β-1a.
Keywords: headache, interferon-beta, multiple sclerosis
Introduction
Interferon-beta (IFN-β) is recommended as a first-line disease-modifying treatment (DMT) for relapsing-remitting multiple sclerosis (RRMS) in over 90 countries.1 Therapeutic options for MS have expanded, but clinical trials have generated a wealth of data on the long-term efficacy and safety of IFN-β for treatment of patients with MS (PwMS).2,3 Formulary restrictions by insurance systems, particularly in low- and middle-income countries, may result in exclusion of expensive drugs in favor of relatively less expensive options. In the largest Egyptian registry published in 2017, more than 50% of patients were treated with IFN-β.4
The most frequently reported adverse effects associated with IFN-β treatment worldwide are flu-like symptoms, injection site reactions, fatigue, and transient laboratory abnormalities.5,6 Although the original pivotal trials did not systemically investigate headache as a reported side effect of IFN-β-treatment,7–11 a significant relative risk for headaches with different types of IFN (beta 1b and 1a) treatments has been reported.12 In addition, previous studies showed a relationship between headaches and IFN-β, but without precise classification or characterization of headache type.13–16
The purpose of this study was to determine the frequency and characteristics of headaches in PwMS treated with IFN-β, and to classify common headache types experienced by these patients according to the International Headache Society (IHS) criteria.17
Methods
Study Design and Participant Characteristics
All treatment-naïve patients with RRMS who started IFN-β while attending the outpatient clinics of 5 tertiary referral centers in Cairo, Egypt in the period from November 2014 to December 2017 were invited to participate in this prospective, longitudinal study. A total of 977 consecutive patients with RRMS diagnosed according to McDonald 2010 criteria18 were screened for eligibility criteria and for compliance with 1-year follow-up visits. At the end of the study observation period, data from 796 patients were analyzed, of whom 283 were men and 513 were women, with ages ranging from 18 to 49 (30.84 ± 8.98) years. Patients were evaluated for the presence of headaches (before or after initiation of IFN-β) using a binary question. Subjects who responded “no” were asked to respond to the demographic and general medical questions only. Patients with reported headaches were asked to recall their headache characteristics and patterns using an interviewer-administered Arabic-language-structured validated questionnaire with an excellent intraclass correlation coefficient (ICC) of 0.903 (95% CI: 0.875–0.925) and overall Cronbach’s coefficient of 0.775 (95% CI: 0.682–0.837).19 An addendum was added to this validated questionnaire to determine the temporal relationship between commencement of interferon treatment and headache onset and characteristics. Patients were asked whether their headaches were pre-existing or appeared de novo, and had worsened or improved (in terms of frequency and intensity). In addition, the patients were asked to describe the outcomes of their headaches. Patients were excluded if they had secondary headaches (headache attributed to head and/or neck trauma, cranial and/or cervical vascular disorders, other non-vascular intracranial disorders, infections, headache or facial pain attributed to disorders of the cranium, facial or cervical structure, disorders of homoeostasis, or metabolic diseases or psychiatric disorders).
Patient Allocation and Follow-Up Protocol
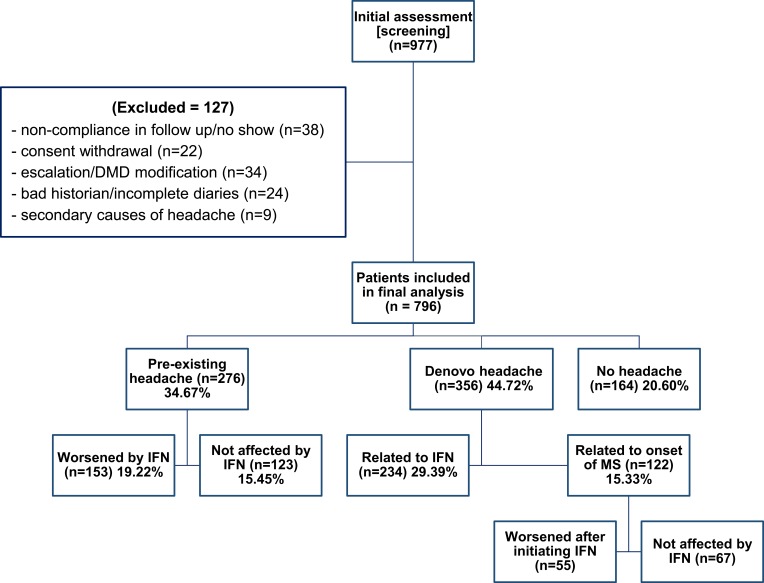
The patients were divided into 3 groups, including patients with pre-existing headaches, patients with de novo headaches, and patients without headaches (Figure 1). All patients who attended our centers prior to receiving IFN-β treatment were followed-up monthly to monitor treatment efficacy. The first interview regarding headaches was scheduled prior starting IFN-β treatment.
Figure 1.
Patient enrollment and disposition.
To obtain the most reliable data, patients with headaches were provided with headache diary recordings in which they were asked to map out the frequency, duration, and severity of headaches, the temporal relationship between headaches and each IFN-β injection, other triggers and response to treatments for headaches.
Patients with headaches who experienced relief from preventive therapies with sustained benefits were advised to continue these therapies. Patients who required initiation or alteration of prophylactic treatments for headaches were managed per the KAHDU (Kasr Al-Ainy Headache Disorders Unit) guidelines according to Bendtsen et al 20 and patient preference. Patients were also informed that flu-like symptoms could be managed using analgesics, if necessary.
Case Definitions and Diagnostic Criteria
Diagnoses of previous and present headaches were made according to the diagnostic criteria of the International Classification of Headache Disorders ICHD-3 (beta version).17 “De novo” headache was defined as a new headache occurring for the first time in close temporal relation to either the onset of MS and/or within 4 weeks of commencement of IFN-β treatment. The latter was coded as secondary headache attributed to IFN-β exposure. “Worsening pre-existing” headache was defined as a significant increase (>50%) in mean frequency or duration of headaches per month, and/or an increase in intensity of more than 3/10 on the Visual Analogue Scale (VAS).15 “De novo” and “worsening pre-existing” headaches with a close temporal relationship between headache onset and commencement of IFN-β were labelled as “post-IFN-β headaches.”
Ethical Clearance
This study was designed according to the recommendations of IHS and was approved by the Neurology Department Review Board of Cairo University. A written informed consent was obtained from each study participant for use of data and for publication prior to administration of questionnaires. All participants had the choice to refuse participation in the study.
Statistics
All statistical analyses were performed using IBM SPSS statistics software (Version 21.0 for Windows, SPSS Inc., NY, USA). The Shapiro–Wilk test was used to determine the normality of distributions. Baseline characteristics of the cohorts were summarized as frequencies (%) or medians with interquartile ranges (IQR) for non-normally distributed parameters, or as means ± SD for normally distributed variables. Analysis of variance (ANOVA) was used to determine differences in demographic and clinical characteristics among the IFN types. Comparison between the two independent groups (patients with post-IFN-β headaches, and those without post-IFN-β headaches) was performed using Student’s t-test. Univariate logistic regression analyses were conducted to calculate the odds ratio (OR) and 95% confidence intervals (CI) of the impacts of different IFN-β preparations on headache occurrence.
Results
Clinical Characteristics
The mean age of the patients who completed the study period (n=796) was 30.84 ± 8.98 years (range: 18–49 years) with a female:male ratio of 1.81:1. Among these patients, 223 (28.02%) were treated with IM-IFN-β-1a (Avonex®), 89 (11.18%) were treated with SC-IFNβ-1a (Rebif®, 22 μg), 259 (32.53%) were treated with SC-IFN-β-1a (Rebif®, 44 μg), and 225 (28.27%) were treated with IFNβ-1b (Betaferon®). The socio-demographic and major clinical characteristics of the participants did not significantly differ among the treatment groups (Table 1).
Table 1.
Demographic and Clinical Data of Patient in Different Treatment Groups
| Total | IFNβ1-a IM (n=223, 28.02%) | IFNβ1-a SC 22 µg (n=89, 11.18%) | IFNβ1-a SC 44 µg (n=259, 32.53%) | IFNβ1-b SC (n=225, 28.27%) | P value | ||
|---|---|---|---|---|---|---|---|
| Age at disease onset (range, mean±SD) | 16–47.5, 29.06±8.92 | 16–47.5, 29.23±8.90 | 16–47.5, 29.21±9.69 | 16–47.5, 29.21±8.99 | 16–47.5, 28.66±8.58 | 0.890 | |
| Sex (F) | 513 (64.45%) | 140 (62.78%) | 66 (74.16%) | 170 (65.64%) | 137 (60.89%) | 0.149 | |
| Occupation (n,%) | Professional | 103 (12.94%) | 29 (13.01%) | 15 (16.85%) | 38 (14.67%) | 21 (9.33%) | 0.171 |
| Managerial and technical | 85 (10.68%) | 23 (10.31%) | 9 (10.11%) | 27 (10.42%) | 26 (11.56%) | ||
| Skilled (manual and non-manual) | 138 (17.33%) | 39 (17.49%) | 16 (17.98%) | 43 (16.60%) | 40 (17.78%) | ||
| Unskilled | 172 (21.61%) | 47 (21.08%) | 18 (20.22%) | 53 (20.46%) | 54 (24%) | ||
| Student | 124 (15.58%) | 37 (16.59%) | 14 (15.73%) | 42 (16.21%) | 31 (13.78%) | ||
| Unemployed | 174 (21.86%) | 48 (21.52%) | 17 (19.10%) | 56 (21.62%) | 53 (23.56%) | ||
| Marriage (n,%) | Yes | 424 (53.27%) | 121 | 43 | 129 | 131 | 0.31 |
| No | 372 (46.73%) | 102 | 46 | 130 | 94 | ||
| Contraceptive pills/injection or HRT (n,%) (n=513) | 67/513 (13.06%) | 18/140 (12.86%) | 8/66 (12.12%) | 22/170 (12.94%) | 19/137 (13.87%) | 0.482 | |
| Disease duration at end of data collection (months) (Range, mean±SD) | 15–48 (21.36±7.21) | 15–48 (21.44±7.08) | 15–48 (21.57±8.05) | 15–48 (21.41±6.89) | 15–48 (21.23±7.22) | 0.982 | |
| Initial EDSS (Range, median, IQR) | 0.5–5.5, 2.5 (2–4) | 0.5–5.5, 3 (2–4) | 0.5–5.5, 2.5, (2–3.5) | 0.5–5.5, 3 (2.5–4) | 0.5–5.5, 2.5 (2–4) | 0.288 | |
| Time from disease onset to IFN-β commencement (months) | 4–36 (9.39±7.22) | 4–36 (9.44±7.08) | 4–36 (9.57±7.92) | 4–36 (9.41±6.98) | 4–36 (9.24±7.22) | 0.974 | |
Abbreviations: EDSS, expanded disability status scale; HRT, hormone replacement therapy; IM, intramuscular; IFN-β, interferon-beta; SC, subcutaneous; SD, Standard Deviation; IQR, interquartile range.
Headache History
Pre-existing headache was reported by 276 patients (34.67%), among whom 153 (55.43%) had worsening headache patterns within 4 weeks of initiation of IFN-β. Worsening headache included increased frequency in 85 patients (55.56%), increased intensity in 91 patients (59.48%), and increased duration in 53 patients (34.64%).
De novo headaches after onset of multiple sclerosis occurred in 356 patients (44.72%), among whom 122 (34.27%) suffered from headaches prior to IFN-β treatment and 234 (65.73%) experienced onset of headache within 4 weeks of treatment initiation. Among patients who experienced headache prior to IFN-β treatment (n=122), 55 (45.08%) reported worsening of headaches after initiation of IFN-β treatment. This worsening included increased duration (n=39) or intensity (n=26), and each of these patients experienced “tension-type characteristics”.
Pattern Identification
Post-interferon headaches included “worsened pre-existing” (n=153), “de novo headaches related to IFN-β” (n=234), and “de novo headaches related to MS onset that worsened after commencement of IFN-β” (n=55) (442 patients total; 55.53%). The remaining patients (n=354) were categorized as “without post-IFN-β headaches.” There were no statistically significant differences between the groups in terms of age, gender, disease duration, type of MS, or family history of headaches (Table 2).
Table 2.
Headache Patterns of Patient in Different Treatment Groups
| IFNβ1-a IM (n=223, 28.02%) | IFNβ1-a SC 22 µg (n=89, 11.18%) | IFNβ1-a SC 44 µg (n=259, 32.53%) | IFNβ1-b SC (n=225, 28.27%) | Total (n=796) | |||
|---|---|---|---|---|---|---|---|
| Pre-existing headache not worsened by IFN-β | Number | 13 | 11 | 25 | 74 | 123 (15.45%) | |
| Characters | Migraine | 9 | 7 | 17 | 51 | 84 | |
| Tension type | 3 | 4 | 6 | 20 | 33 | ||
| Others | 1 | 0 | 2 | 3 | 6 | ||
| Pre-existing headache worsened by IFN-β | Number | 55 | 14 | 61 | 23 | 153 (19.22%) | |
| Characters | Migraine | 17 | 5 | 19 | 12 | 53 | |
| Tension type | 36 | 8 | 40 | 9 | 93 | ||
| Others/unclassified | 2 | 1 | 2 | 2 | 7 | ||
| Denovo headache not related to IFN-β | 18 | 18 | 31 | 55 | 122 (15.33%) | ||
| Denovo headache related to IFN-β commencement | Number | 97 | 8 | 105 | 24 | 234 (29.39%) | |
| Character | Migraine | 21/97 | 1/8 | 18/105 | 6/24 | 46/234 | |
| Tension | 74/97 | 7/8 | 83/105 | 18/24 | 182/234 | ||
| Others/unclassified | 2/97 | 0 | 4/105 | 0 | 6/234 | ||
| No headache (n=165) | 40 | 38 | 37 | 49 | 164 (20.60%) | ||
Abbreviations: IM, intramuscular; IFN-β, interferon-beta; SC, subcutaneous.
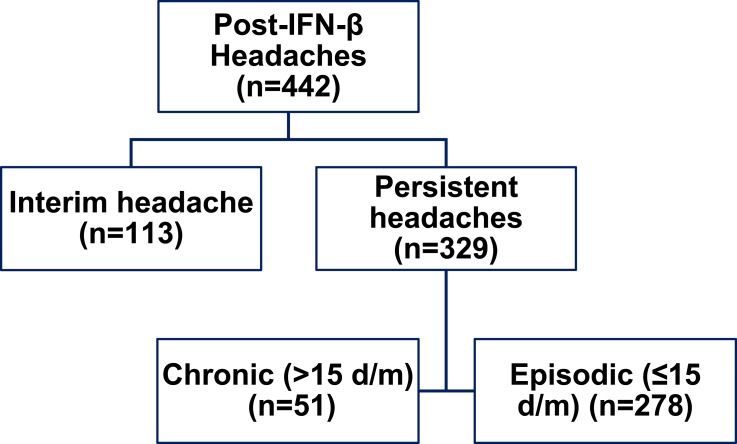
Two distinct headache categories were recognized. Headaches with remission (or regression to pre-IFN status) within ≤3 months were labelled as “interim headache” (n=113). Eighty-two of these patients had migraine headaches, and 6 had unclassified headaches. Headaches that persisted (or worsened) for >3 months (n=329) were categorized as “persistent headaches,” which were further subdivided into chronic or intermittent (episodic) patterns based on number of headache days/month (Figure 2). Of patients with “persistent headaches” (n=329), 216 (65.65%) required prophylactic headache medications. Medications administered included topiramate (n=56), valproate (n=43), BTX-A (n=9), venlafaxine (n=39), amitriptyline (n=62), mirtazapine (n=21), propranolol (n=49), and selective serotonin reuptake inhibitors (SSRIs) (n=91). Twenty-four patients were diagnosed with Medication overuse headache (MOH) (7.29%). These patients underwent an outpatient regimen for detox and management.
Figure 2.
Post-interferon headache patterns.
Impact of different IFN-β preparations on headache. Logistic regression was performed using SC-IFNβ-1a 22 ug as reference group. Univariate analysis showed more than 6-fold increased risk of headache for patients treated with IM-IFN-β-1a (OR 6.51; 95% CI 3.73–10.01; P-value <0.0001) and about 5-fold increased risk of headache among those treated with SC-IFNβ-1a 44ug (OR 5.44; 95% CI 3.15–9.37; P-value <0.0001) (Figure 3). ORs adjusted by sex and age were similar to those obtained by univariate analysis.
Figure 3.
Impact of different IFN-β preparations on headache occurrence.
Abbreviations: OR, odds ratio; CI, 95% confidence interval.
Discussion
In this prospective study, treatment with IFN-β significantly worsened pre-existing headaches and headaches that occurred concurrently with onset of MS in more than half of patients with histories of headaches that preceded commencement of IFN-β treatment. Treatment with IFN-β also triggered de novo headaches in about one-third of those who did not report having headaches prior to treatment. These three categories of patients accounted for more than half of our cohort (n=442/796, 55.53%). According to ICHD-3,17 these types were coded as “headache attributed to use of or exposure to a substance (code 8.1).” The presence of a causative substance and a close temporal relationship between onset of headache and onset of the presumed causative disorder was sufficient to establish causation in cases where the symptoms were not better explained by another ICHD-3 diagnosis.
In our study, 29.39% (234/796) of patients developed de novo headaches within 4 weeks of initiation of IFN-β treatment. Interferon-beta pivotal trials7–11 and post-market evidence21,22 underestimated the prevalence of headaches as an adverse effect. In these studies, headaches were either not included among adverse events,7,8 or may have been considered flu-like symptoms.13,15,22 However, headache is one of the most common adverse events associated with IFN-β treatment,14–16 with a reported prevalence from 13.1% to 58% (Table 3). Smith et al21 reported a more precise definition of headache incidence rates (IRs) per 100 person-years. This calculation incorporated headaches that occurred after initiation of therapy that were not present in the 90-day pre-index period, which resulted in a rate of 6.94 per 100 person-years (95% CI = 6.52–7.38). Moreover, headaches associated with IFN-β treatment were a significant reason for treatment withdrawal in a previous study.7 Furthermore, headaches were included as a flu-like symptom in other trials,22–24 but these studies did not specify which flu-like symptoms were responsible for withdrawal.
Table 3.
Comparative Analysis of Multiple Sclerosis Studies Potentially Addressing Headache in IFN-β Receivers
| Study | Type of IFN-β | No of Patients on IFN-ß | Study Duration | Study Design and IFN-ß Type | Headache Frequency |
|---|---|---|---|---|---|
| OWIMS 199912 | SC-IFNβ-1a 22 µg QW | 95 | 48 weeks | Randomized, double-blind study of IFN-ß-1a 22 µg, 44 µg, or placebo administered once weekly SC injection. | 46 (48%) |
| SC-IFNβ-1a 44 µg QW | 98 | 48 weeks | 49 (50%) | ||
| EVIDENCE 200223 | IFN-β-1a 44 µg SC t.i.w | 339 | 24 weeks | Randomized trial compared IFN-ß −1a 44 ug SC t.i.w, and IFN-ß −1a 30 µg IM QW | 143 (42%) |
| IFN-β-1a 30 µg QW | 337 | 165 (49%) | |||
| Herndon et al25 | IM IFN-β-1a QW | 279 | 8 years | Open-label extension study of the phase III trial of IM IFN- ß −1a | 58% |
| BENEFIT26 | 250 μg IFN-ß-1b | 292 | 24 months | Randomized, double-blind trial of IFN-ß-1b 250ug SC EOD vs placebo | 78 (26.7%) |
| REGARD27 | IFN-β-1a 44 µg SC t.i.w | 381 | 96 weeks | Open label, randomized trial of 44 ug SC t.i.w IFN-ß-1a vs daily SC 20 mg GA. | 74 (19.4%) |
| BEYOND28 | 500 μg IFN- β-1b | 887 | Filling of the triangle design (2–3.5 years) | Randomized trial assigned 2:2:1 to receive one of two doses of IFN- ß −1b (250 μg or 500 μg) SC, EOD vs daily SC 20 mg GA | 293 (33%) |
| 250 μg IFN-β-1b | 888 | 280 (32%) | |||
| RNF24 | IFN-β-1a (RNF) 44 µg SC t.i.w | 260 | 96 weeks | Single-arm, Phase IIIb, open-label study to evaluate RNF, 44 μg SC t.i.w. | 98 (37.7%) |
| 16-year LTF29 | IFN-β-1b | 69 (within past 2 years) | LTF visit (Past 2 years) | Multi-center, observational study through a structured questionnaire | 19 (27.5%) |
| ADVANCE30 | Peginterferon ß −1a 125 μg every 2 weeks | 512 | 2 years | Randomized, double-blind, placebo-controlled, phase III study to to receive placebo or SC peg-IFN-ß-1a 125 μg every 2 weeks or every 4 weeks | 224 (44%) |
| Peginterferon ß-1a 125 μg every 4 weeks | 500 | 204 (41%) | |||
| Smith et al21 | IFN-β-1a 44 µg SC t.i.w | 8,107 | 6 years (eligible subjects) | Observational, retrospective Post-marketing study of IFN-β-1a SC tiw | IRs/100 person-years 6.94 (6.52–7.38) |
| REFLEXION31 | SC IFN-β-1a tiw | 99 | 60 months | Extension trial of the phase III REFLEX study32 | 16(16.2%) |
| SC IFN-β-1a QW | 117 | 19(16.2%) | |||
| Delayed treatment | 84 | 11(13.1%) |
Abbreviations: ADVANCE, pegylated interferon ß-1a for relapsing-remitting multiple sclerosis; BENEFIT, Betaferon®/Betaseron® in Newly Emerging Multiple Sclerosis for Initial Treatment; BEYOND, Betaferon®/Betaseron® Efficacy Yielding Outcomes of a New Dose; EOD, every other day; EVIDENCE, Evidence of Interferon Dose-Response: European North American Comparative Efficacy; GA, Glatiramer acetate; IM, intramuscular; IFN-β, interferon-beta; IM, intramuscular; IRs, incidence rates; LTF, long term follow-up; OWIMS, The Once Weekly Interferon for MS Study Group; QW, once a week; REFLEX, REbif FLEXible dosing in early MS; REFLEXION, REbif FLEXible dosing in early MS extension; REGARD, the REbif vs Glatiramer Acetate in Relapsing MS Disease; RNF, Rebif ®New Formulation; SC, subcutaneous; t.i.w, three times a week.
Previous studies have rarely addressed worsening (aggravation) of pre-existing headaches following commencement of IFN-β treatment.13,15,16 La Mantia et al13 reported changes in pre-existing headache characteristics following initiation of IFN-β treatment that led to changes in headache diagnoses from tension-type headaches to migraines without aura. Previous studies have also evaluated the underlying mechanisms of headache aggravation, and attributed this phenomenon to neuronal hyperexcitability induced by IFN-β, or the effects of inflammatory mediators.13,33,34
In the current study, post-IFN-β headaches were pressing in character in 330/442 (~75%) patients, and persistent for the one-year study duration in 329/442 (~75%) patients. However, migraine characteristics were detected in only 99 patients (22.4%), most whom (82/99) experienced headache improvement after 3 months following initiation of IFN-β treatment. In contrast, the primary headaches in the study by La Mantia et al13 were migraine without aura, migraine not fulfilling all criteria, and tension-type headache without significant differences. This study included 150 patients, interferons were compared with other DMTs, and patients underwent a semi-structured interview to retrospectively recall their headache history. D’Amico et al35 reported that about half of patients with de novo headaches following commencement of IFN-β treatment-developed migraines, and the other half developed tension-type headaches (TTH). However, the mechanisms underlying tension-type headaches remain unclear, and the exact relationship between IFN-β and development of headaches has been a topic of debate. Another point of contention is whether there is a clear-cut separation of migraine as a vascular headache and TTH as a consequence of muscle contraction.36 It is possible that TTH and migraines share a common pathophysiology.37 Furthermore, the relationship among IFN-β, depression, and development of TTH has been demonstrated. However, in our study, about 85% of patients had episodic headaches (278/329) rather than the chronic pattern that is classically associated with higher levels of anxiety and depression.38,39 Few studies have directly addressed stress levels and TTH in MS.40
In our study, higher doses of SC-IFNβ-1a and IM-IFN-β-1a were more frequently associated with headaches than either SC-IFNβ-1b or lower doses of SC-IFNβ-1a, which agreed with findings in a study performed by Patti et al.16 Differences in the effects of IFNs on cytokine profiles (especially IL-4 and IL-10) could partially explain some of the differences in cephalalgia spectrum and headache profiles induced by the various forms of IFN.1,4,16 This study was the first to evaluate the relationship among various IFN-β preparations and headache occurrence, and represents the largest MS study aimed at evaluating the characteristics and patterns of post-IFN-β treatment and headache. However, our study was limited by lack of a control group comprised untreated patients with MS, and lack of other DMTs for comparison with IFN-β. Despite these limitations, we achieved our primary goal of characterization and identification of features of post-IFN-β headaches.
In conclusion, the current study showed that aggravation of pre-existing headaches, or onset of de novo primary headaches, should be considered common IFN-β adverse effects, as headaches can significantly affect quality of life and function of PwMS. Prospective monitoring of IFN-β-related headaches might facilitate development of appropriate treatment strategies to enhance IFN-β therapy adherence.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen C, Wu N, Watson C. Multiple sclerosis patients who are stable on interferon therapy show better outcomes when staying on same therapy than patients who switch to another interferon. Clinicoecon Outcomes Res. 2018;10:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reder AT, Oger JF, Kappos L, O’Connor P, Rametta M. Short-term and long-term safety and tolerability of interferon beta-1b in multiple sclerosis. Mult Scler Relat Disord. 2014;3(3):294–302. doi: 10.1016/j.msard.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Ziemssen T, De Stefano N, Sormani MP, Van Wijmeersch B, Wiendl H, Kieseier BC. Optimizing therapy early in multiple sclerosis: an evidence-based view. Mult Scler Relat Disord. 2015;4(5):460–469. doi: 10.1016/j.msard.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 4.Hamdy SM, Abdel-naseer M, Shalaby NM, et al. Characteristics and predictors of progression in an Egyptian multiple sclerosis cohort: a multicenter registry study. Neuropsychiatr Dis Treat. 2017;13:1895–1903. doi: 10.2147/NDT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neilley LK, Goodin DS, Goodkin DE, Hauser SL. Side effect profile of interferon beta-1b in MS: results of an open label trial. Neurology. 1996;46(2):552–554. doi: 10.1212/WNL.46.2.552 [DOI] [PubMed] [Google Scholar]
- 6.Walther EU, Hohlfeld R. Multiple sclerosis: side effects of interferon beta therapy and their management. Neurology. 1999;53(8):1622–1627. doi: 10.1212/WNL.53.8.1622 [DOI] [PubMed] [Google Scholar]
- 7.The IFN-B Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655–661. doi: 10.1212/WNL.43.4.655 [DOI] [PubMed] [Google Scholar]
- 8.The IFN-B Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45(7):1277–1285. doi: 10.1212/WNL.45.7.1277 [DOI] [PubMed] [Google Scholar]
- 9.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis: the Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol. 1996;39:285–294. [DOI] [PubMed] [Google Scholar]
- 10.Ebers GC. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–1504. doi: 10.1016/S0140-6736(98)03334-0 [DOI] [PubMed] [Google Scholar]
- 11.The Once Weekly Interferon for MS Study Group. Evidence of interferon beta-1a dose response in relapsing-remitting MS: the OWIMS Study. Neurology. 1999;53(4):679–686. doi: 10.1212/WNL.53.4.679 [DOI] [PubMed] [Google Scholar]
- 12.Filippini G, Munari L, Incorvaia B, et al. Interferons in relapsing remitting multiple sclerosis: a systematic review. Lancet. 2003;361(9357):545–552. doi: 10.1016/S0140-6736(03)12512-3 [DOI] [PubMed] [Google Scholar]
- 13.La Mantia L, D’Amico D, Rigamonti A, Mascoli N, Bussone G, Milanese C. Interferon treatment may trigger primary headaches in multiple sclerosis patients. Mult Scler. 2006;12(4):476–480. doi: 10.1191/1352458506ms1298oa [DOI] [PubMed] [Google Scholar]
- 14.Khromov A, Segal M, Nissinoff J, Fast A. Migraines linked to interferon-beta treatment of multiple sclerosis. Am J Phys Med Rehabil. 2005;84(8):644–647. doi: 10.1097/01.phm.0000171012.86932.10 [DOI] [PubMed] [Google Scholar]
- 15.Pollmann W, Erasmus LP, Feneberg W, Then Bergh F, Straube A. Interferon beta but not glatiramer acetate therapy aggravates headaches in MS. Neurology. 2002;59(4):636–639. doi: 10.1212/WNL.59.4.636 [DOI] [PubMed] [Google Scholar]
- 16.Patti F, Nicoletti A, Pappalardo A, et al. Frequency and severity of headache is worsened by Interferon-beta therapy in patients with multiple sclerosis. Acta Neurol Scand. 2012;125(2):91–95. doi: 10.1111/j.1600-0404.2011.01532.x [DOI] [PubMed] [Google Scholar]
- 17.Olesen J, Bes A, Kunkel R, et al. The international classification of headache disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658 [DOI] [PubMed] [Google Scholar]
- 18.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurology. 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-sherbiny NA, Shehata HS, Amer H, et al. Development and validation of an Arabic-language headache questionnaire for population-based surveys. J Pain Res. 2017;10:1289–1295. doi: 10.2147/JPR.S137795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendtsen L, Birk S, Kasch H, et al. Reference programme: diagnosis and treatment of headache disorders and facial pain. Danish Headache Society, 2nd Edition, 2012. J Headache Pain. 2012;13 Suppl(1):S1–S29. doi: 10.1007/s10194-011-0402-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MY, Sabidó-Espin M, Trochanov A, et al. Post-marketing safety profile of subcutaneous interferon beta-1a given 3 times weekly: a retrospective administrative claims analysis. J Manag Care Spec Pharm. 2015;21(8):650–660. doi: 10.18553/jmcp.2015.21.8.650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabidó-espin M, Munschauer R. Reasons for discontinuation of subcutaneous interferon β-1a three times a week among patients with multiple sclerosis: a real-world cohort study. BMC Neurol. 2017;17(1):57. doi: 10.1186/s12883-017-0831-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE trial. Neurology. 2002;59(10):1496–1506. doi: 10.1212/01.WNL.0000034080.43681.DA [DOI] [PubMed] [Google Scholar]
- 24.Giovannoni G, Barbarash O, Casset-semanaz F, et al. Safety and immunogenicity of a new formulation of interferon beta-1a (Rebif New Formulation) in a Phase IIIb study in patients with relapsing multiple sclerosis: 96-week results. Mult Scler. 2009;15(2):219–228. doi: 10.1177/1352458508097299 [DOI] [PubMed] [Google Scholar]
- 25.Herndon RM, Rudick RA, Munschauer FE III, et al. Eight-year immunogenicity and safety of interferon beta-1a-Avonex treatment in patients with multiple sclerosis. Mult Scler. 2005;11(4):409–419. doi: 10.1191/1352458505ms1209oa [DOI] [PubMed] [Google Scholar]
- 26.Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d [DOI] [PubMed] [Google Scholar]
- 27.Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7(10):903–914. doi: 10.1016/S1474-4422(08)70200-X [DOI] [PubMed] [Google Scholar]
- 28.O’connor P, Filippi M, Arnason B, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8(10):889–897. doi: 10.1016/S1474-4422(09)70226-1 [DOI] [PubMed] [Google Scholar]
- 29.Reder AT, Ebers GC, Traboulsee A, et al. Cross-sectional study assessing long-term safety of interferon-beta-1b for relapsing-remitting MS. Neurology. 2010;74(23):1877–1885. doi: 10.1212/WNL.0b013e3181e240d0 [DOI] [PubMed] [Google Scholar]
- 30.Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, Phase 3, double-blind study. Lancet Neurol. 2014;13(7):657–665. doi: 10.1016/S1474-4422(14)70068-7 [DOI] [PubMed] [Google Scholar]
- 31.Comi G, De Stefano N, Freedman MS, et al. Subcutaneous interferon beta-1a in the treatment of clinically isolated syndromes: 3-year and 5-year results of the Phase III dosing frequency-blind multicentre REFLEXION study. J Neurol Neurosurg Psychiatry. 2017;88(4):285–294. [DOI] [PubMed] [Google Scholar]
- 32.Comi G, De Stefano N, Freedman MS, et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol. 2012;11(1):33–41. doi: 10.1016/S1474-4422(11)70262-9 [DOI] [PubMed] [Google Scholar]
- 33.Hadjilambreva G, Mix E, Rolfs A, Muller J, Strauss U. Neuromodulation by a cytokine: interferon-beta differentially augments neocortical neuronal activity and excitability. J Neurophysiol. 2005;93(2):843–852. doi: 10.1152/jn.01224.2003 [DOI] [PubMed] [Google Scholar]
- 34.Munno I, Marinaro M, Bassi A, Cassiano MA, Causarano V, Centonze V. Immunological aspects in migraine: increase of IL-10 plasma levels during attack. Headache. 2001;41(8):764–767. doi: 10.1046/j.1526-4610.2001.01140.x [DOI] [PubMed] [Google Scholar]
- 35.D’amico D, La Mantia L, Rigamonti A, et al. Prevalence of primary headaches in people with multiple sclerosis. Cephalalgia. 2004;24(11):980–984. doi: 10.1111/j.1468-2982.2004.00790.x [DOI] [PubMed] [Google Scholar]
- 36.Krusz JC. Tension-type headaches: what they are and how to treat them. Prim Care. 2004;31(2):293–311. doi: 10.1016/j.pop.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 37.Riley TL. Muscle-contraction headache. Neurol Clin. 1983;1(2):489–500. doi: 10.1016/S0733-8619(18)31159-9 [DOI] [Google Scholar]
- 38.Zebenholzer K, Lechner A, Broessner G, et al. Impact of depression and anxiety on burden and management of episodic and chronic headaches - a cross-sectional multicentre study in eight Austrian headache centres. J Headache Pain. 2016;17:15. doi: 10.1186/s10194-016-0603-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zampieri MA, Tognola WA, Galego JC. Patients with chronic headache tend to have more psychological symptoms than those with sporadic episodes of pain. Arq Neuropsiquiatr. 2014;72(8):598–602. doi: 10.1590/0004-282X20140084 [DOI] [PubMed] [Google Scholar]
- 40.Kister I, Caminero AB, Herbert J, Lipton RB. Tension-type headache and migraine in multiple sclerosis. Curr Pain Headache Rep. 2010;14(6):441–448. doi: 10.1007/s11916-010-0143-5 [DOI] [PubMed] [Google Scholar]