At the first EMBO Workshop dedicated to caveolae held in Le Pouliguen, France, May 12–16 2019 (http://meetings.embo.org/event/19-caveolae), round-table discussions were used to address some of the long-standing issues in the field and to decide upon a consensus view regarding key aspects of caveola biology. Here we summarise some of the frequently asked questions (FAQs) posed by cell biologists about caveolae and provide some brief consensual answers based on these discussions.
FAQs
What are the protein components of caveolae?
In mammalian cells, integral membrane proteins termed caveolins (caveolin-1 in non-muscle cells and caveolin-3 in muscle) work together with cavins (particularly cavin-1) to generate caveolae. These proteins can be considered universal structural elements of caveolae and their knockout leads to loss of caveolae. In the absence of cavin-1, caveolin-1 can form functional non-caveolar domains called ‘scaffolds’ that have been characterized biochemically and, more recently, by single molecule super resolution microscopy. Other proteins work together with these components including caveolin-2, cavin-2, cavin-3, cavin-4, EHD (Eps-15 Homology Domain) 2, Pacsin2/Syndapin II and Pacsin3/Syndapin III, as well as ROR1 (Receptor tyrosine kinase-like Orphan Receptor 1) in specific cell types (for example cavin-4 in striated muscle, ROR1 in embryonic tissues and tumors). In addition to their plasma membrane association, it is also important to note that many of these components can have other cellular locations. For example, caveolins can also be detected in association with the Golgi complex, early endosomes, and lipid droplets.
Are caveolae morphologically similar to clathrin coated pits? How can these be distinguished?
Caveolae have a defined diameter of 60 to 80 nm and an omega or U shape depending on the EM technique used to visualize them. Clathrin coated pits usually have wider diameter (around 100 nm) and the coat is more evident. Using platinum-replica EM (PREM), the coat of clathrin coated pits is visualized as a network of hexagons, while the caveolar coat shows a striped structure (Figure 1), at times forming spirals. The organization of clathrin coated pits is relatively uniform, while caveolae can organize into clusters of different sizes (known as caveolar ‘rosettes’, or ‘clusters’) and can flatten and remain at the plasma membrane (see below).
Figure 1.
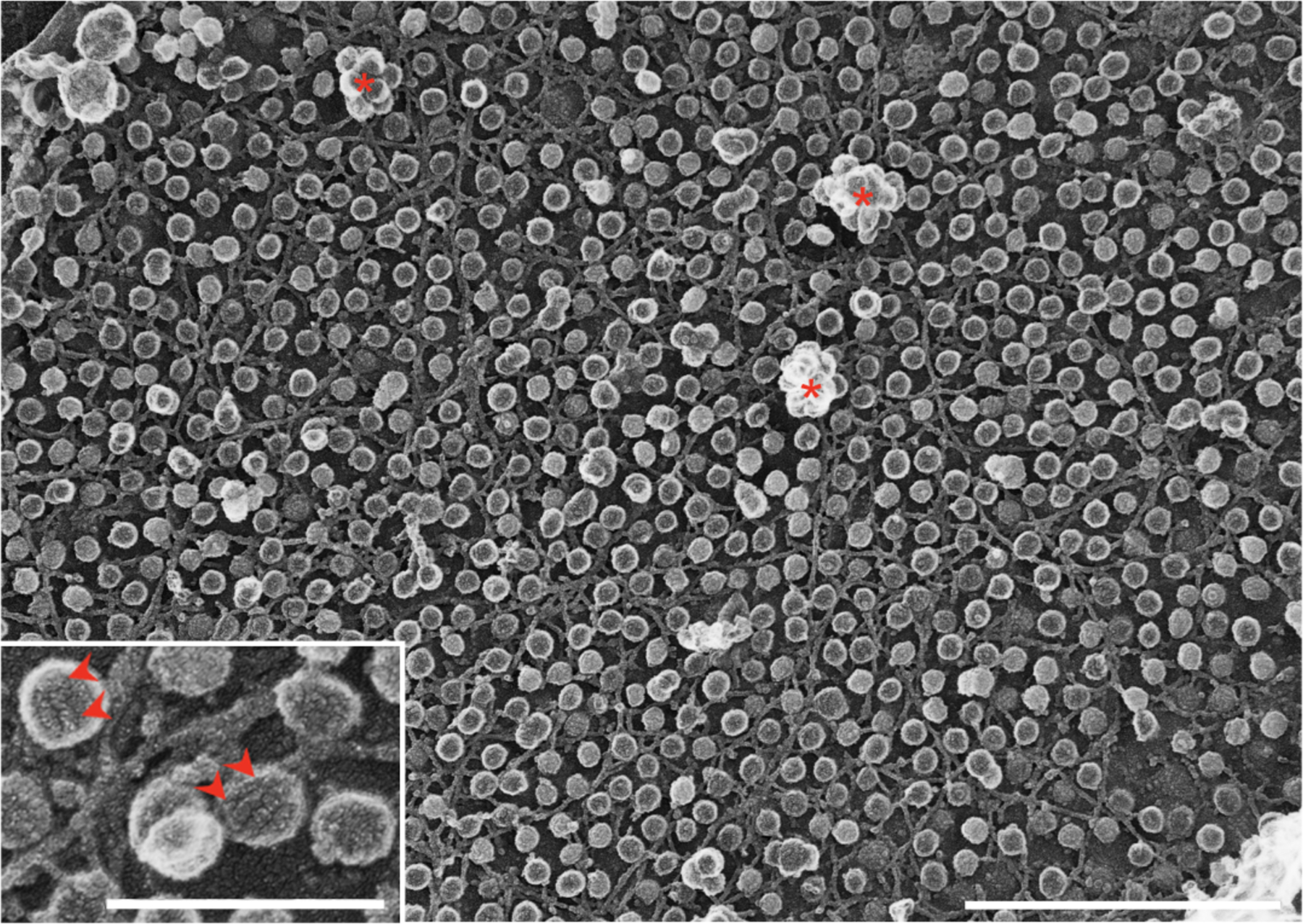
Platinum-replica EM of the plasma membrane of cultured myotubes showing abundant caveolae; both single caveolae and caveolar clusters or rosettes (asterisks) are evident. The characteristic striped coats of caveolae can be observed at higher magnification (inset; arrowheads). Bars, main figure 1μm; inset 0.25μm.
How do caveolae compare to clathrin coated pits in terms of abundance and density in different tissues?
Caveolae can occupy over 30% of the surface of some cell types (such as skeletal muscle, endothelial cells and adipocytes) but in other cell types, such as red blood cells, immune cells, and neurons, caveolae are not detectable. This contrasts with clathrin coated pits, which show a similar density in all nucleated mammalian cells. Caveolins, the major membrane proteins of caveolae, may have significant roles other than caveola formation in cells with very low levels of caveolins (for example in liver, neurons, and B and T lymphocytes).
Are caveolae endocytic? If so, what is the destination of budded caveolae and do they carry specific cargo?
Caveolae can bud from the plasma membrane and fuse with early endosomes. However, there appear to be few cargoes, if any, which are strictly dependent on caveolae for their uptake into cells. In fact, transmembrane proteins may be largely excluded from caveolae. The role of caveola budding may be to regulate the density of surface caveolae, rather than caveolae acting as an endocytic carrier analogous to a clathrin coated pit. An exception to this view is the proposed role of caveolae in endothelia where caveolae have been implicated in transendothelial transport.
What is the link between caveolae and the actin cytoskeleton?
Immunofluorescence images of caveolar markers, such as caveolin-1 or cavin-1 in tissue culture cells, frequently show a linear pattern that co-aligns with actin stress fibers. This pattern is not often observed with other membrane invaginations. The reasons for this co-alignment are still not completely clear, but a coordination between two tension-controlling systems has been proposed as an explanation. When visualized by PREM, caveolae are invariantly surrounded by cortical actin filaments although the exact function of these filaments on caveolae and their composition are still unknown. Actin cytoskeleton disruption leads to caveolar clustering and prevents their endocytosis. Caveolin-1 binds actin indirectly via filamin A and another, yet unknown, linker. Caveolin-1 regulates focal adhesions, stress fibers and acto-myosin contraction via Rho GTP loading through regulation of the localization of p190RhoGAP, an endogenous inhibitor of Rho. Cells devoid of caveolin-1 lose their intrinsic sense of directional cell migration and show a distorted actin cytoskeleton. Tyrosine phosphorylated caveolin-1 promotes actin cytoskeleton stabilization, focal adhesion tension, and tumor cell migration.
What is the evidence for the flattening and membrane buffering capabilities of caveolae?
Cells exposed to stimuli that increase plasma membrane tension, such as osmotic swelling, cell stretching, and excessive actin polymerization on stress fibers, cause a reduction of caveolae, which is a consequence of their flattening. This flattening reduces cortical tension, which acts as a buffer system to prevent plasma membrane rupture upon increased tension. This function could explain the abundance of caveolae in mechanically challenged tissues, such as muscle, the endothelia and adipose tissue. This plasticity has been observed in a variety of cell lines and at the organismal level in mice and zebrafish. By contrast, a reduction in tension at the plasma membrane favours the clustering of caveolae and, eventually, can give rise to internalization of caveolae.
What are the major functions of caveolae and how do these relate to the abundance of caveolae in different cell types?
In cells with abundant caveolae, such as skeletal muscle, adipocytes and endothelial cells, caveolae mediate mechanoprotection and control of lipid homeostasis. These roles are intimately coupled to the properties and components of the caveolae and further transduced via signalling and transcriptional regulation. For example, caveolar disassembly is associated with changes in caveolin phosphorylation and release of cavin and EHD2 proteins from the plasma membrane; EHD2 translocates to the nucleus, where it can regulate gene transcription. Caveolae regulate fatty acid transport, lipid metabolism and, in the case of adipocytes, lipid storage. Consistent with this, caveolin or cavin dysfunction can lead to lipoatrophy in which adipocyte lipid stores are depleted. In endothelial cells, caveolae have been linked to transcellular transport and are crucial regulators of nitric oxide synthase (eNOS/NOS3).
What diseases are associated with caveolar dysfunction?
Patients lacking caveolin-1 show a severe lipodystrophy while mutations in caveolin-1 are associated with pulmonary arterial hypertension. Mutations in muscle-specific caveolin-3 cause a number of muscle diseases including Limb Girdle Muscular Dystrophies and Rippling Muscle disease. Loss of cavin-1 is associated with lipodystrophy and muscle disease whereas mutations in muscle-specific cavin-4 are associated with cardiomyopathies. In addition to these specific effects of loss or mutations of key caveolar proteins, there are numerous studies linking caveolins and cavins to cancer. This literature is currently difficult to summarise because striking examples exist of caveolar proteins linked to tumor promoting or tumor suppressive roles depending on the tumor type. Caveolin-1 expression in the tumor stroma also modifies tumor cell growth and invasion capabilities. Undoubtedly, the role of caveola proteins in cancer is highly complex and it is likely that caveolae will have tumor type-specific roles at multiple stages of cancer progression.
What is the evolutionary conservation of caveolae and caveolar components?
Caveolae are well-characterized in vertebrate cells, where they show the classical bulb-shaped architecture and are generated by caveolins and cavins. Cavins are exclusively found in vertebrates but caveolins are expressed in some invertebrates. These include Caenorhabditis elegans and the honey bee Apis mellifera but not Drosophila melanogaster or yeast.
If caveolae are so abundant why does a loss of caveolae not cause embryonic lethality? Is there compensation?
Mice lacking caveolin-1 or cavin-1 can survive to adulthood but fertility and lifespan are reduced and they are more prone to develop tumors and other conditions. These mice lack caveolae in either a tissue-specific manner (caveolin-1 or caveolin-3) or in all tissues (cavin-1). Undoubtedly, compensatory mechanisms are involved in coping without this abundant surface feature but interestingly, this occurs without formation of recognisable pits with similar morphology. The consequence of caveolar dysfunction can be late onset human diseases, such as muscle disease conditions associated with caveolin-3 mutations. Loss of caveolae can have prominent effects in specific tissues as shown by the lipodystrophy associated with loss of caveolin-1 or cavin-1, or only revealed in response to specific stresses, such as defective liver regeneration after damage. Caveolar dysfunction has been also linked to age-related diseases with caveolin-1 being an important regulator of cellular senescence and caveolin-1 loss linked to neurodegeneration.
Outlook
The number of disease conditions associated with dysfunction of caveolae emphasizes the need to understand this enigmatic structure. This article hopefully represents the current view of the majority of researchers in the field, but clearly the consensus view can evolve as the scientific process runs its course. We hope that this article will stimulate further work to test these ideas, particularly to test some of the key unresolved questions (Box 1).
Box 1. Key questions.
What is the structural architecture of the caveolar complex formed by caveolins, cavins, and membrane lipids?
What happens to caveolar components upon caveolar disassembly?
What is the structural state of these components after release/during reassembly?
How does caveola disassembly/reassembly regulate or impact cellular responses?
What are the dynamics of caveolae (including caveolar formation, trafficking and flattening) in different tissues in vivo?
What is the role of non-caveolar caveolin and what fraction of total cellular caveolin exists outside caveolae?
What is the function of lipid droplet-associated caveolin?
What lipid species are required for caveola formation/function and how does caveolar disassembly impact lipids?
How is fission of caveolae from the plasma membrane achieved?
Are there subpopulations of caveolae that differ in composition and dynamics?
What is the role of caveolin Tyr-phosphorylation in vivo?
What is the role of the “caveolin scaffolding domain” in vivo?
What is the relationship with other endocytic pathways, particularly the CLIC/GEEC pathway, in both trafficking and mechanoadaption?
How is the expression of caveolar components and the dynamics of caveolae regulated in disease?
What role do caveolae play in integrin endocytosis and recycling?
What is the relationship between caveolae and exosomes/extracellular vesicles?
Synopsis.
Caveolae are an abundant, but enigmatic, plasma membrane feature of vertebrate cells. In this brief commentary, the authors attempt to answer some key questions related to the formation and function of caveolae based on round-table discussions at the first EMBO Workshop on Caveolae held in France in May 2019.
Acknowledgments
The authors are grateful to all the participants of the EMBO Workshop on ‘Caveolae and Nanodomains’ for their input as well as Dr. Asier Echarri for comments. RGP is supported by the National Health and Medical Research Council of Australia (grants APP1140064 and APP1150083 and fellowship APP1156489) and the Australian Research Council (ARC) Centre of Excellence in Convergent Bio-Nano Science and Technology. CL is supported by institutional grants from the Curie Institute, INSERM and CNRS, and by grants from Association Française contre les Myopathies (CAV-STRESS-MUS n°14293), Agence Nationale de la Recherche (MOTICAV ANR-17-CE13-0020-01), the Fondation ARC pour la Recherche sur le Cancer (Programme Labellisé PGA1-RF20170205456), and programme ECOS n° C17S03. MAdP is supported by MICINN (Spain Ministry of Science, Innovation and Universities) through grants SAF2017-83130-R and the Severo Ochoa Center of Excellence scheme (SEV-2015-0505, grant No 007-2016-IGP-SO), the Marató de TV3 Foundation (674/C/2013), the Worldwide Cancer Research Foundation (AICR 15-0404), the EU Horizon 2020 programme (Marie Sklodowska-Curie grant No 641639), and the Pro CNIC Foundation. AC and SLL are supported by Région des Pays de la Loire « RFI BIOREGATE » Caveodisc project, INSERM, Nantes University and Angers University. AKK is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL144131 and the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM106720. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Further Reading
- 1.Le Lay S, Kurzchalia TV. Getting rid of caveolins: phenotypes of caveolin-deficient animals. Biochim Biophys Acta. December 30 2005;1746(3):322–333. [DOI] [PubMed] [Google Scholar]
- 2.Le Lay S, Blouin CM, Hajduch E, Dugail I. Filling up adipocytes with lipids. Lessons from caveolin-1 deficiency. Biochim Biophys Acta. June 2009;1791(6):514–518. [DOI] [PubMed] [Google Scholar]
- 3.Mercier I, Jasmin JF, Pavlides S, et al. Clinical and translational implications of the caveolin gene family: lessons from mouse models and human genetic disorders. Lab Invest. June 2009;89(6):614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chidlow JH, Jr., Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. May 1 2010;86(2):219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. April 2010;20(4):177–186. [DOI] [PubMed] [Google Scholar]
- 6.Pilch PF, Liu L. Fat caves: caveolae, lipid trafficking and lipid metabolism in adipocytes. Trends Endocrinol Metab. August 2011;22(8):318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou H, Stoppani E, Volonte D, Galbiati F. Caveolin-1, cellular senescence and age-related diseases. Mech Ageing Dev. Nov-Dec 2011;132(11–12):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. February 2013;14(2):98–112. [DOI] [PubMed] [Google Scholar]
- 9.Echarri A, Del Pozo MA. Caveolae - mechanosensitive membrane invaginations linked to actin filaments. J Cell Sci. August 1 2015;128(15):2747–2758. [DOI] [PubMed] [Google Scholar]
- 10.Kovtun O, Tillu VA, Ariotti N, Parton RG, Collins BM. Cavin family proteins and the assembly of caveolae. J Cell Sci. April 1 2015;128(7):1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng JP, Nichols BJ. Caveolae: One Function or Many? Trends Cell Biol. March 2016;26(3):177–189. [DOI] [PubMed] [Google Scholar]
- 12.Sohn J, Brick RM, Tuan RS. From embryonic development to human diseases: The functional role of caveolae/caveolin. Birth Defects Res C Embryo Today. March 2016;108(1):45–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busija AR, Patel HH, Insel PA. Caveolins and cavins in the trafficking, maturation, and degradation of caveolae: implications for cell physiology. Am J Physiol Cell Physiol. April 1 2017;312(4):C459–C477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamaze C, Tardif N, Dewulf M, Vassilopoulos S, Blouin CM. The caveolae dress code: structure and signaling. Curr Opin Cell Biol. June 19 2017;47:117–125. [DOI] [PubMed] [Google Scholar]
- 15.Fiala GJ, Minguet S. Caveolin-1: The Unnoticed Player in TCR and BCR Signaling. Adv Immunol. 2018;137:83–133. [DOI] [PubMed] [Google Scholar]
- 16.Parton RG. Caveolae: Structure, Function, and Relationship to Disease. Annu Rev Cell Dev Biol. October 6 2018;34:111–136. [DOI] [PubMed] [Google Scholar]


