Abstract
Objective
To investigate the genetic contribution to amyotrophic lateral sclerosis (ALS) and the phenotypic and genetic associations between ALS and psychiatric and cardiovascular disorders (CVD) we used the national registry data from Denmark linked to first-degree relatives to estimate heritability and cross-trait parameters.
Methods
ALS cases and 100 sex and birth-matched controls per case from the Danish Civil Registration System were linked to their records in the Danish National Patient Registry. Cases and controls were compared for (1) risk of ALS in first-degree relatives, used to estimate heritability, (2) comorbidity with psychiatric disorders and CVD, and (3) risk of psychiatric disorders and CVD in first-degree relatives.
Results
5,808 ALS cases and 580,800 controls were identified. Fifteen percent of cases and controls could be linked to both parents and full siblings, whereas 70% could be linked to children. (1) We estimated the heritability of ALS to be 0.43 (95% CI, 0.34–0.53). (2) We found increased rates of diagnosis of mental disorders (risk ratio = 1.18; 95% CI, 1.09–1.29) and CVD in those later diagnosed with ALS. (3) In first-degree relatives of those with ALS, we found increased rate of schizophrenia (1.17; 95% CI, 0.96–1.42), but no evidence for increased risk CVD.
Conclusions
Heritability of ALS is lower than commonly reported. There is likely a genetic relationship between ALS and schizophrenia, and a nongenetic relationship between ALS and CVD.
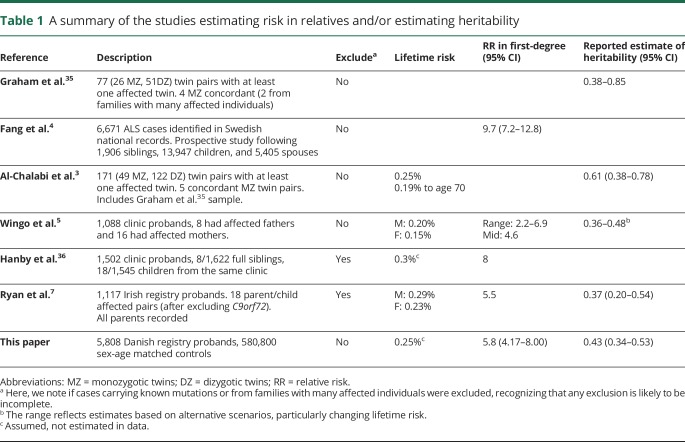
Multiple genes1 (e.g., SOD1 and C9orf72) can harbor causal mutations of amyotrophic lateral sclerosis (ALS) found in 5%–10% of those diagnosed,2 mostly those with many affected family members. Genetic factors likely contribute to ALS when these mutations are absent. Heritability of liability quantifies the relative genetic contribution to the disease, assuming a polygenic architecture. Estimates of heritability3–7 (table 1) are difficult to obtain for late-onset disorders because the essential information required is the increased risk of disease in relatives of those affected. This is particularly difficult for a disorder such as ALS where lifetime risk is ∼0.25%.8,9
Table 1.
A summary of the studies estimating risk in relatives and/or estimating heritability
More recently, genome-wide association study (GWAS) data have enabled estimation of the proportion of variation in liability associated with genome-wide single nucleotide polymorphisms (SNP-based heritability10). For ALS, SNP-based heritability has been estimated to be 8.2% (95% CI, 7.2–9.2),11 which provides a lower bound for heritability (because the estimate only captures variation associated with the GWAS common SNPs) and demonstrates evidence in support of a polygenic architecture. SNP-based heritability methods have bivariate extensions12,13 that allow estimation of genetic correlations using GWAS summary statistics from independently collected disease cohorts. Application of these methods to ALS gave a significant estimate for a genetic correlation of ALS with schizophrenia (0.14, 95% CI, 0.07–0.21).14 By contrast, the estimated genetic correlation with cardiovascular disease (CVD) was not different from zero,15 despite increased rates of CVD in those with ALS.16 Here, we apply genetic epidemiology approaches to population records of ALS from Denmark with the objective to provide independent evidence for genetic associations between ALS and other disorders.
Methods
Study population
The Danish Civil Registration System (CRS) was established in 1968, registering all people alive and living in Denmark17 with a unique personal identifier (CRS number). All other national registries can be linked using the CRS number. Our study population included all persons alive in Denmark from January 1, 1968, or born between 1969 and January 1, 2017. The study was approved by the Danish Health Data Authority, the Danish Data Protection Agency, and Statistics Denmark and did not require ethical approval because its use serves a scientific cause and no reference is made to a single person.
ALS cases
ALS cases were identified using the Danish National Patient Registry18 (established January 1, 1977, accessing records until January 1, 2017). The patient registry records any individual with a hospital admission and covers information on all Danish somatic inpatient hospital contacts; outpatient contacts were included from January 1, 1994. We considered both main and auxiliary diagnoses for inpatient and outpatient contacts. Inclusion was based on persons who were living in Denmark at the time, were older than 16 years at the time of their first diagnosis, and had International Classifications of Disease (ICD) 8:348.0 or icd-10:G12.2 codes included in their records. These definitions have been used in previous studies of ALS using the Danish registry,19–22 and a very good agreement in diagnosis has been reported with death certificate data.19 A published validation study used these ICD codes for cases identified from the registry data and confirmed the ALS diagnosis in 160 of 173 ALS cases (92.5%) and found no difference in the predictive validity between ICD-8 and ICD-10 codes.22
Controls
For each case that was identified, 100 controls were extracted and matched for sex and date of birth (alive and living in Denmark at the time case diagnosis was first recorded). Particularly, for older cases, it was not possible to find 100 controls matched for an exact date of birth. Where necessary, the window was increased by ±1 day until the quota of controls was reached. The controls were sampled without replacement, and the cases could not be selected as controls.
Identification of family members
After the selection of ALS cases and controls, parents, siblings, or children were identified when possible. For people registered as children, a link to the CRS number of their legal parents is created. Since the registry was established in 1968, only those born after ∼1950 (aged 18 years or younger at the time when the registry was established) are expected to have parental CRS records. Full siblings could only be identified for those who had both parents linked to their records. Similarly, in identifying children of cases, only children born after 1950 and before 2017 would be identified. We note that the CRS system identifies legal parents; although these are expected to mostly reflect biological relationships, this will not always be true. We count parents, siblings, and children as first-degree relatives.
Psychiatric and CVD diagnoses
Information on individuals diagnosed with schizophrenia and other mental disorders was obtained from the Danish Psychiatric Central Research Register.23 From 1969, this Registry contained data on all admissions to Danish psychiatric inpatient facilities (secondary care treatment). From 1995, information on all contacts to outpatient psychiatric departments and visits to psychiatric emergency care units was included. For all cases and controls and their identified first-degree relatives, endorsements of records for psychiatric diagnoses were obtained24 (table e-1, links.lww.com/NXG/A224). The same hospital registry that was used to identify ALS cases, the Danish National Patient Registry, was also used to identify all persons with any diagnosis of heart disease. We defined heart disease under both a broad definition of CVD and a narrower definition of myocardial infarction (MI)20 (table e-1). The psychiatric or CVD diagnoses in both cases and their matched controls were restricted to those recorded before the first record of ALS for the case. We estimated risk of psychiatric disorders and CVD in ALS cases and controls and calculated risk ratios (RRs).
Quantitative genetic modeling
We estimated the increased risk of ALS in relatives of the cases compared with the controls for different types of relatives.
When families have 3 or more affected first-degree relatives, it is very likely that they have a mendelian type of ALS, given a lifetime risk for ALS of ∼0.25%. However, co-occurrence of ALS in a pair of relatives could be explained by shared genetic factors under a more polygenic model of disease (or even by shared environment). Many other diseases have mendelian and polygenic types (such as breast cancer, Parkinson disease, and Alzheimer disease). Under a polygenic genetic architecture, even when the total genetic contribution to disease etiology is high, most people affected present without a family history.25 Here, we assume that the observed increased risk in first-degree relatives is attributed to a polygenic contribution (the same assumptions as made in the studies listed in table 1) and estimate the heritability of ALS under the liability threshold model,26–28 assuming a lifetime risk of 0.25%. Recognizing that families harboring the known causal mutations cannot be identified from the registry data, we conducted sensitivity analyses and re-estimated heritability removing 20% of counts of ALS concordant relative pairs. Last, we estimated the risk of psychiatric disorders or CVD in relatives of ALS cases and controls. We used bivariate liability threshold methods to link risk in relatives to genetic correlation,27,28 assuming estimates of heritability for these traits made from Danish national registry data.28
Data availability
Access to individual-level Denmark data is governed by Danish authorities. These include the Danish Data Protection Agency, the Danish Health Data Authority, and Statistics Denmark. Each scientific project must be approved before initiation, and approval is granted to a specific Danish research institution. International researchers may gain data access through collaboration with a Danish research institution.
Results
Cases and controls
We identified 5,808 ALS cases (3,227 men and 2,581 women), of which 62% had an ICD-10:G122 diagnosis, 27% ICD-8:34809, and 10% ICD-10:G122G. These statistics are in good agreement with the studies which previously accessed the ALS records from Danish registers.19–22 For more than 94% of cases, all their matched controls were born within 2 days (table e-2, links.lww.com/NXG/A224). The birth years of the cases ranged from 1886 to 2000, but given that ALS is typically a disease of late-onset, 94% of cases were born before 1960 (table e-3). We found that annual incidence was higher in later years (figure e-1), consistent with the previously reported increase in age-adjusted incidence of 1.6% annually after 1982.21 Because some people with ALS have no need for an inpatient hospitalization, the addition of outpatient records in 1994 may partially account for incidence increase. The median age at diagnosis for the ALS cohort (minimum age of diagnosis of 16 years) was 67.1 years (interquartile range 58.4–74.2 years) (figure e-2).
Relatives
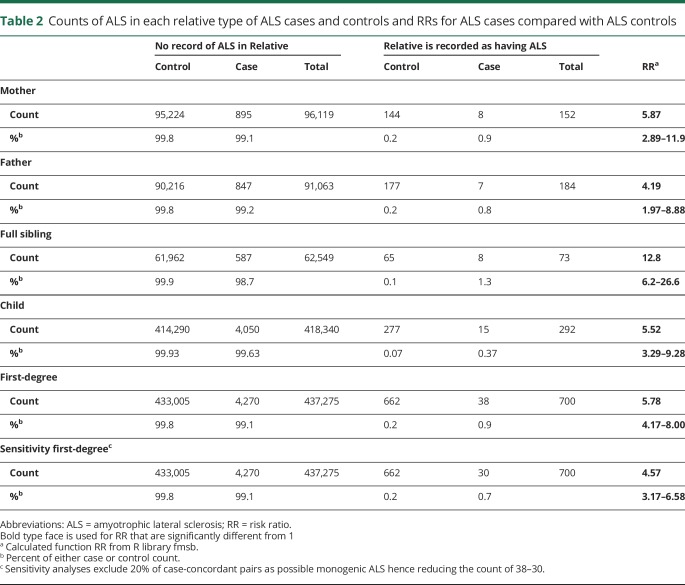
As a reflection of the timing of the registry establishment, the percentage of those with a link to a mother increased rapidly from 9.7% for those born in 1950 to 98.6% and 99.8% for those born in 1960 and 1970, respectively, retaining that level for all subsequent birth years. Similar findings were obtained regarding a link to a father. Given the age distribution for ALS diagnosis, only 16% of cases/controls could be linked to their mother, 15% could be linked to their father, and 15% could be linked to both parents, with no difference between the cases and controls (χ2 test, p = 0.12) (table e-4, links.lww.com/NXG/A224). Only those linked to their parents could have their siblings identified (table e-5). By contrast, children could be identified for 70% of people (table e-6). Overall, 74% of people had at least one first-degree relative identified in the registry data (table e-7). In these data, no ALS case or control had more than one affected sibling and the number of individuals with more than one affected child was less than 4 (concern about potential reidentification means that the exact number is nonreportable). The rate of ALS was 0.15% in mothers of controls and 0.20% in fathers of controls (among only the 15% controls with their parents identified) (table 2). Because ALS is a late-onset disorder, the parents of controls matched to the cases must be close to a full lifetime in age; hence, these rates provide a lower bound on lifetime risk.
Table 2.
Counts of ALS in each relative type of ALS cases and controls and RRs for ALS cases compared with ALS controls
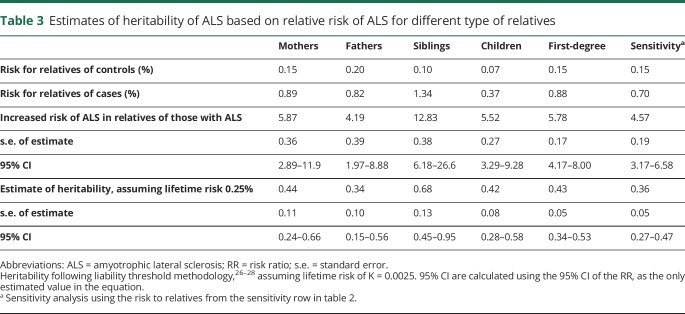
Estimate of heritability
The RR of ALS in first-degree relatives of ALS cases compared with those of the controls ranged from 4.2 in fathers to 12.8 in siblings (table 3). Despite this range, the 95% CI was overlapping. Across all first-degree relatives, there is a 5.78-fold (95% CI, 4.17–8.00) increase in the risk of ALS in relatives of the cases compared with in relatives of the controls. This generates an estimate of heritability of 0.43 (95% CI, 0.34–0.53), given a lifetime risk8,9 of 0.25% (table 3). Sensitivity analyses assumed lifetime risks of ALS in Denmark of 0.2% or 0.3%, which gave heritability estimates of 0.41 (95% CI, 0.32–0.50) and 0.45 (95% CI, 0.35–0.55), respectively, (tables e-8 and e-9, links.lww.com/NXG/A224). The methodology to estimate heritability assumes that the disease is polygenic, and hence, the estimate provided here is an upper limit of heritability on the liability scale because some of the first-degree relatives concordant for ALS may reflect families harboring mendelian mutations; as such, it is not possible to identify these families from the registry data. In a sensitivity analysis, with an exclusion of 20% of concordant pairs, the estimate of the relative risk reduced to 4.57 (95% CI, 3.17–6.58) and that of heritability reduced to 0.36 (95% CI, 0.27–0.47) (table 3). We note that no family included more than 2 affected individuals, and we were unable to find reports of rates of ALS mendelian mutations in Denmark. Given the intercountry variability, it is possible that the rates of ALS dominant mutations are not high. For example, in an international study,29 the most common form of mendelian ALS, associated with the hexanucleotide repeat in the gene C9orf72, was less frequent in the Netherlands and Sweden (albeit the latter from a small sample) than in other countries; Denmark did not contribute to the study.
Table 3.
Estimates of heritability of ALS based on relative risk of ALS for different type of relatives
Cross-trait analyses within individual and for relatives
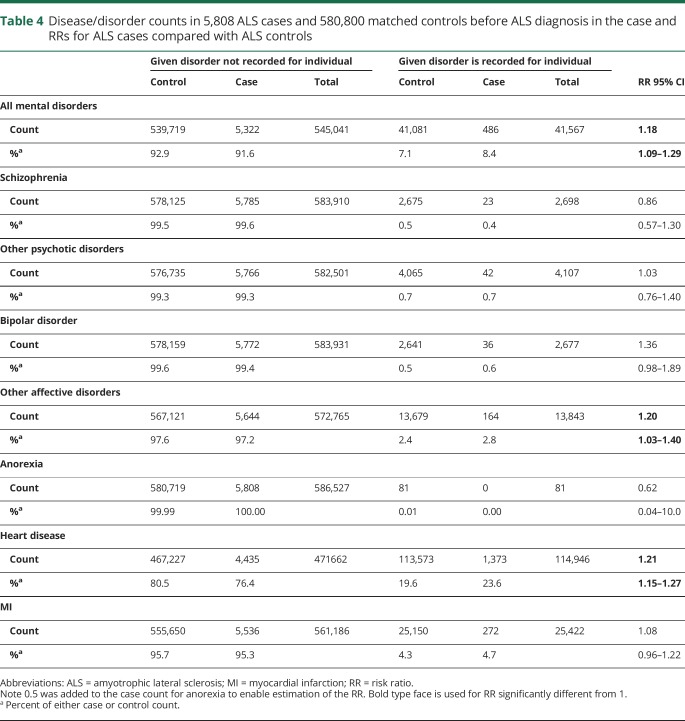
We estimated the risk of psychiatric disorders and CVD before ALS diagnosis date in those with ALS and their matched controls (table 4). We found an increased risk of CVD (RR) of (1.21; 95% CI, 1.15–1.27, p = 6 × 10−15) in those with ALS compared with those without (as previously reported16), but the increased risk was not significant for MI (RR = 1.08; 95% CI, 0.96–1.22, p = 0.19). We also found an increased risk of all psychiatric disorders (RR = 1.18; 95% CI, 1.09–1.29, p = 1.3 × 10−4) likely reflecting an increased risk in affective disorders excluding bipolar disorder (RR = 1.20; 95% CI, 1.03–1.40, p = 0.019). These affective disorders affect 2.4% of the controls (i.e., reflecting mostly hospitalized unipolar disorder), noting that the Danish Psychiatric Central Research Register does not include depression treated in primary care. There is no increased risk of severe psychiatric disorders such as schizophrenia, bipolar disorder, and anorexia.
Table 4.
Disease/disorder counts in 5,808 ALS cases and 580,800 matched controls before ALS diagnosis in the case and RRs for ALS cases compared with ALS controls
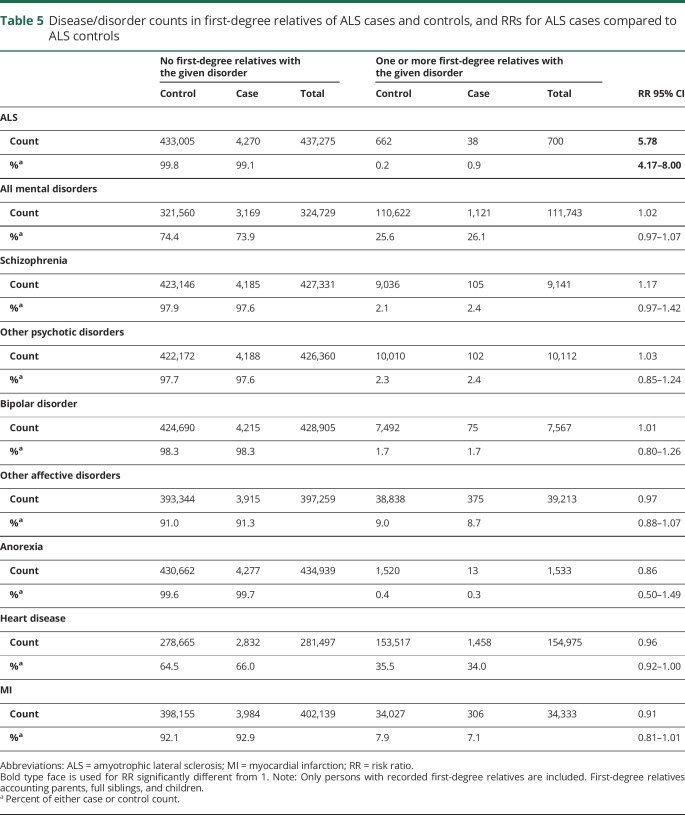
We estimated the risk of psychiatric and CVD disorders in first-degree relatives of those with and without ALS. When considering all first-degree relatives together, none of the risks to relatives were significant (table 5). The highest increased risk was 1.17 (95% CI, 0.97–1.42; p = 0.10) for schizophrenia (table 5). Despite being nonsignificant, the increased risk was in the direction expected from the genetic correlation estimates from GWAS summary data.14 Using lifetime risk and heritability estimates28 0.0112 and 0.67, respectively for schizophrenia, and of 0.0025 and 0.43, respectively for ALS, and assuming a genetic correlation of 0.14,14 we estimate the expected RR to be 1.32, which is within the 95% CI of our estimate. When considering different first-degree relatives, we found an increased risk of schizophrenia in mothers of ALS cases (RR = 2.21; 95% CI, 1.05–4.65, p = 0.033) and increased risk of all mental disorders in fathers of ALS case (RR = 1.29; 95% CI, 1.08–1.53, p = 0.0053) (table e-8, links.lww.com/NXG/A224).
Table 5.
Disease/disorder counts in first-degree relatives of ALS cases and controls, and RRs for ALS cases compared to ALS controls
Discussion
Recorded in over 48 years of Danish national patient and outpatient registries were 5,808 ALS cases, who were matched with 580,800 controls. The increased risk of ALS in first-degree relatives of those with ALS compared with first-degree relatives of controls was 5.78 (95% CI, 4.17–8.00). The resulting estimate of heritability was 0.43 (95% CI, 0.34–0.53). This has a lower mean and smaller bounds than the published twin-based estimate3 (0.61%, 95% CI, 0.38–0.78%) but is in good agreement with later estimates (table 1).
The published estimate of the genetic correlation derived from ALS and schizophrenia GWAS data is 0.14 (95% CI, 0.07–0.21).14 Although psychosis has been reported in relatives of those with ALS in kindred segregating the C9orf72 hexanucleotide repeat expansion,2 increased rates of ALS in those with schizophrenia have not been reported, which would be expected if there was a shared genetic architecture between the disorders. Here, we found no evidence for increased rates of schizophrenia in those with ALS (RR 0.86, 95% CI, 0.57–1.30). However, the life expectancy is significantly reduced for those with schizophrenia: reported from survival analysis using the Danish registry data to be 18.7 years and 16.3 years shorter for men and women, respectively.30 The observed increased rate of schizophrenia in first-degree relatives of those with ALS was also not significant (1.17; 95% CI, 0.96–1.42; p = 0.10), but the RR expected from a genetic correlation of 0.14 is 1.32, which is within the confidence limits of our estimate. Hence, our results provide some support that the genetic relationship between ALS and schizophrenia is genetic. A Swedish national registry study, which identified 3,648 ALS cases and 364,800 matched controls found higher rates of psychiatric disorders in children of those with ALS.31 The overlap between ALS and schizophrenia is unlikely to be attributed to C9orf72 mutations because the hexanucleotide repeat is reported to have a frequency less than 0.1% in those with schizophrenia.32
We found increased rates of recorded cardiovascular disease (CVD) before diagnosis of ALS (RR = 1.21; 95% CI, 1.15–1.27), as previously reported.16 This association, however, was not detected in the narrow definition of heart disease; MI. By contrast, we found no increase in the rate of CVD among relatives of those with ALS, despite high rates in first-degree relatives of both the cases and controls (∼35%). This may suggest broad lifestyle factors, rather than a genetic relationship, and explain the observed increased rates of CVD in those with ALS,16 which would be consistent with the genetic correlation estimates from GWAS data between ALS and CVD that were not different from zero.15 Alternatively, the high rate of CVD recorded before ALS diagnosis may reflect the increased medical surveillance in those in the early stages of disease.
The key strength of the Danish registry data is its completeness so that ascertainment biases are minimized compared with other designs. Nonetheless, there are limitations. First, because ALS is a late-onset disorder and given the timing of the establishment of the registry system, we could only trace parents for those born after ∼1950. From the parents, we were able to trace the siblings, but only siblings born after 1950. To counter this, our design of 100 controls per case provides robustness to our estimates and any potential biases apply to both cases and controls. Second, although ∼70% of ALS cases had records of first-degree relatives in the registry system, the majority of such relatives were children, who were mostly below the expected age of ALS onset (median age diagnosis in these data of 67.1 years). The information for estimation of heritability was therefore mostly generated from ∼15% of the ALS cases that could be linked to both parents and hence also to siblings, which is still a sizeable data set (table 1). Third, in these registry data, we were unable to identify individuals and their family members carrying ALS causal mutations and inclusion of these individuals could inflate the estimate of heritability, which methodologically assumes a polygenic architecture. We did not attempt to access frontotemporal dementia diagnoses (relevant because it co-occurs in families carrying the C9orf72 hexanucleotide repeat33) because these diagnoses have not been validated in registry data.34 To address these limitations, we conducted sensitivity analyses in which we excluded from the analyses a proportion of concordant relative pairs, the proportion chosen as a likely upper limit that could be attributed to first-degree relative pairs with causal mutations. The resulting relative risk (RR = 4.57; 95% CI, 3.17–6.58) and heritability (RR = 0.36; 95% CI, 0.27–0.47) provide lower bounds of these estimates. Last, higher relative risks associated with maternal transmission have recently been reported in a prospective ALS study in Ireland,7 although results were not significant when families carrying the C9orf72 hexanucleotide repeat expansion were excluded. Here, we have not considered sex-specific risks because the number of concordant affected pairs is too few to generate meaningful results.
Our heritability estimate of 0.43 withstands sensitivity analyses and supports a genetic contribution to the disease. If anything, our estimate might be upwardly biased, yet the estimate is lower than the twin estimate of heritability of ALS that has been most often cited. The weight of evidence is now such that a lower estimate must be seen as more plausible (table 1), we suggest ∼0.40. We find evidence for previous risk of psychiatric disorders and CVD in individuals with ALS, plus tentative evidence that this relationship has a genetic contribution for schizophrenia and other psychiatric disorders but not for CVD.
Acknowledgment
N.R.W. and F.C.G. acknowledge funding from the Australian National Health and Medical Research Council (1078901, 1113400, 1087889). B.B.T., P.B.M., and E.A. acknowledge financial support from The Stanley Medical Research Institute and The Lundbeck Foundation Initiative for Integrative Psychiatric Research, iPSYCH (grant no. R102-A9118 and R155-2014-1724).
Glossary
- ALS
amyotrophic lateral sclerosis
- CRS
Civil Registration System
- CVD
cardiovascular disease/cardiovascular disorder
- GWAS
genome-wide association study
- ICD
International Classifications of Disease
- MI
myocardial infarction
- RR
risk ratio
Appendix. Authors
Study funding
The Australian National Health and Medical Research Council (1078901, 1113400, 1087889) to N.R.W. and F.G. The Stanley Medical Research Institute and The Lundbeck Foundation Initiative for Integrative Psychiatric Research, iPSYCH (grant no. R102-A9118 and R155-2014-1724) to B.B.T., P.B.M., and E.A.
Disclosure
B.B. Trabjerg, F.C. Garton, W. van Rheenen, F. Fang, R.D. Henderson, P.B. Mortensen, E. Agerbo, and N.R. Wray report no disclosures. Go to Neurology.org/NG for full disclosures.
References
- 1.Taylor JP, Brown RH Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature 2016;539:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne S, Walsh C, Lynch C, et al. Rate of familial amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2011;82:623–627. [DOI] [PubMed] [Google Scholar]
- 3.Al-Chalabi A, Fang F, Hanby MF, et al. An estimate of amyotrophic lateral sclerosis heritability using twin data. J Neurol Neurosurg Psychiatry 2010;81:1324–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang F, Kamel F, Lichtenstein P, et al. Familial aggregation of amyotrophic lateral sclerosis. Ann Neurol 2009;66:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wingo TS, Cutler DJ, Yarab N, Kelly CM, Glass JD. The heritability of amyotrophic lateral sclerosis in a clinically ascertained United States research registry. PLoS One 2011;6:e27985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanby MF, Scott KM, Scotton W, et al. The risk to relatives of patients with sporadic amyotrophic lateral sclerosis. Brain 2011;134:3454–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan M, Heverin M, McLaughlin RL, Hardiman O. Lifetime risk and heritability of amyotrophic lateral sclerosis. JAMA Neurol 2019;76:1367–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso A, Logroscino G, Jick SS, Hernan MA. Incidence and lifetime risk of motor neuron disease in the United Kingdom: a population-based study. Eur J Neurol 2009;16:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet 2011;377:942–955. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Zeng J, Goddard ME, Wray NR, Visscher PM. Concepts, estimation and interpretation of SNP-based heritability. Nat Genet 2017;49:1304. [DOI] [PubMed] [Google Scholar]
- 11.van Rheenen W, Shatunov A, Dekker AM, et al. Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat Genet 2016;48:1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, Yang J, Goddard ME, Visscher PM, Wray NR. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics 2012;28:2540–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulik-Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet 2015;47:1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin RL, Schijven D, van Rheenen W, et al. Genetic correlation between amyotrophic lateral sclerosis and schizophrenia. Nat Commun 2017;8:14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandres-Ciga S, Noyce AJ, Hemani G, et al. Shared polygenic risk and causal inferences in amyotrophic lateral sclerosis. Ann Neurol 2019;85:470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kioumourtzoglou MA, Seals RM, Gredal O, Mittleman MA, Hansen J, Weisskopf MG. Cardiovascular disease and diagnosis of amyotrophic lateral sclerosis: a population based study. Amyotroph Lateral Scler Frontotemporal Degeneration 2016;17:548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen CB. The Danish Civil Registration system. Scand J Public Health 2011;39:22–25. [DOI] [PubMed] [Google Scholar]
- 18.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health 2011;39:30–33. [DOI] [PubMed] [Google Scholar]
- 19.Kioumourtzoglou MA, Seals RM, Himmerslev L, Gredal O, Hansen J, Weisskopf MG. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Frontotemporal Degener 2015;16:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laursen TM, Munk-Olsen T, Agerbo E, Gasse C, Mortensen PB. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry 2009;66:713–720. [DOI] [PubMed] [Google Scholar]
- 21.Seals RM, Hansen J, Gredal O, Weisskopf MG. Age-period-cohort analysis of trends in amyotrophic lateral sclerosis in Denmark, 1970–2009. Am J Epidemiol 2013;178:1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seals RM, Kioumourtzoglou MA, Hansen J, Gredal O, Weisskopf MG. Amyotrophic lateral sclerosis and the Military: a population-based study in the Danish Registries. Epidemiology 2016;27:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mors O, Perto GP, Mortensen PB. The Danish psychiatric Central research register. Scand J Public Health 2011;39:54–57. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen CB, Mors O, Bertelsen A, et al. A Comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry 2014;71:573–581. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Visscher PM, Wray NR. Sporadic cases are the norm for complex disease. Eur J Hum Genet 2010;18:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reich T, Morris CA, James JW. Use of multiple thresholds in determining mode of transmission of semi-continuous traits. Ann Hum Genet 1972;36:163–184. [DOI] [PubMed] [Google Scholar]
- 27.Falconer DS. Inheritance of liability to certain diseases estimated from incidence among relatives. Ann Hum Genet 1965;29:51-76. [Google Scholar]
- 28.Wray NR, Gottesman II. Using summary data from the Danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front Genet 2012;3:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majounie E, Renton AE, Mok K, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol 2012;11:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res 2011;131:101–104. [DOI] [PubMed] [Google Scholar]
- 31.Longinetti E, Mariosa D, Larsson H, et al. Neurodegenerative and psychiatric diseases among families with amyotrophic lateral sclerosis. Neurology 2017;89:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducharme S, Bajestan S, Dickerson BC, Voon V. Psychiatric Presentations of C9orf72 mutation: what are the diagnostic implications for Clinicians? J Neuropsychiatry Clin Neurosci 2017;29:195–205. [DOI] [PubMed] [Google Scholar]
- 33.Rohrer JD, Isaacs AM, Mizielinska S, et al. C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol 2015;14:291–301. [DOI] [PubMed] [Google Scholar]
- 34.Phung TK, Andersen BB, Hogh P, Kessing LV, Mortensen PB, Waldemar G. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord 2007;24:220–228. [DOI] [PubMed] [Google Scholar]
- 35.Graham AJ, Macdonald AM, Hawkes CH. British motor neuron disease twin study. J Neurol Neurosurg Psychiatry 1997;62:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanby MF, Scott KM, Scotton W, et al. The risk to relatives of patients with sporadic amyotrophic lateral sclerosis. Brain 2011;134:3454–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to individual-level Denmark data is governed by Danish authorities. These include the Danish Data Protection Agency, the Danish Health Data Authority, and Statistics Denmark. Each scientific project must be approved before initiation, and approval is granted to a specific Danish research institution. International researchers may gain data access through collaboration with a Danish research institution.