Abstract
Polymers are the backbone of pharmaceutical drug delivery. There are several polymers with varying properties available today for use in different pharmaceutical applications. Alginate is widely used in biomedical research due to its attractive features such as biocompatibility, biodegradability, inertness, low cost, and ease of production and formulation. Encapsulation of therapeutic agents in alginate/alginate complex microspheres protects them from environmental stresses, including the acidic environment in the gastro-intestinal tract (GIT) and enzymatic degradation, and allows targeted and sustained delivery of the agents. Microencapsulation is playing an increasingly important role in drug delivery as evidenced by the recent surge in research articles on the use of alginate in the delivery of small molecules, cells, bacteria, proteins, vaccines, and for tissue engineering applications. Formulation of these alginate microspheres (AMS) are commonly achieved by conventional external gelation method using various instrumental manipulation such as vortexing, homogenization, ultrasonication or spray drying, and each method affects the overall particle characteristics. In this review, an inclusive summary of the currently available methods for the formulation of AMS, its recent use in the encapsulation and delivery of therapeutics, and future outlook will be discussed.
Keywords: alginate microspheres, delivery, drugs, cells, proteins, vaccines, cell culture
1. Introduction
Conventional methods of drug delivery involving use of tablets and capsules, and simple injections have been widely studied and applied in the biomedical arena for treatment of various human diseases due to the ease of formulation and administration. However, greater understanding of the complex nature of the pathogenesis in human diseases has led researchers and clinicians to explore the development of novel drug delivery systems for targeted delivery of therapeutics (e.g.: proteins, peptides, nucleic acids, cells and organelles) (Banskota et al., 2019, Cañibano-Hernández et al., 2019). In particular, complex dosage forms such as nanoparticles (liposomes, polymeric nanoparticles, metallic nanoparticles), microspheres, transdermal drug delivery systems, and mucoadhesive drug delivery systems are being studied to deliver therapeutic agents via different routes of administration. These formulations have the advantage of delivering sensitive molecules to the site of action via passive (enhanced permeation and retention effect (Dong et al., 2019)) and/ or active targeting (overexpressed receptors on the diseased cells (Ding et al., 2019)), while protecting the active molecule from degradation. For example, the encapsulated drug can be protected from the reticuloendothelial system by PEGylated liposomes (Yu et al., 2019), and from the acidic gastric environment by alginate - chitosan microspheres (Ling et al., 2019). Based on the intended application, various polymers can be used to design these complex formulations. Polymers can be classified as natural (lipids, alginate, cellulose, chitosan), semisynthetic (lipids, alginate derivatives, cellulose derivatives, chitosan derivatives) and chemically synthesized (poly esters, poly carbonates, lipids like 1,2-dipalmitoyl-sn-glycero- 3-phosphocholine).
Alginate polymer has several attractive properties including excellent biocompatibility, low cost, ease of gelation, inert nature, chemically compatible material, derivation from natural sources, ease of availability and feasible method of synthesis, which makes it a polymer of choice for most researchers developing platforms for drug delivery and tissue engineering (Becker et al., 2001). Further, alginate can be easily manipulated to develop different formulations by simple addition of crosslinkers like divalent calcium ions (Fig 1) (Lin et al., 2005).
Figure 1:
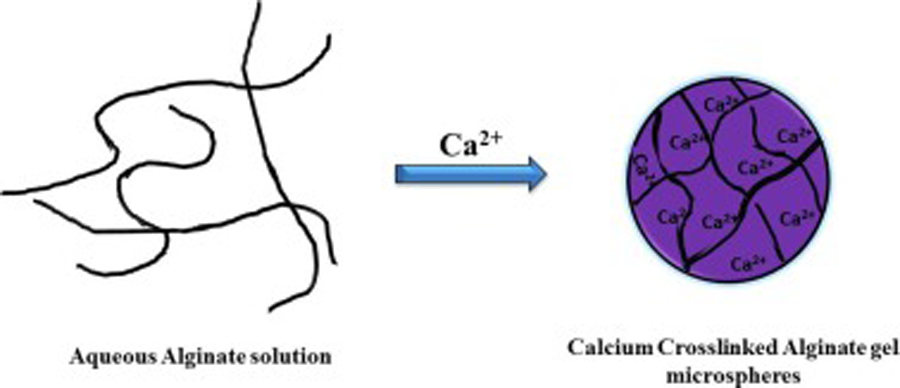
Representation of the standard calcium-alginate gel egg-box structure formation on addition of Ca2+ ions
Commercially available alginate is extracted from brown algae, which undergoes treatment with alkaline solutions, typically sodium hydroxide, and subsequently reacted and purified to yield sodium alginate (Pawar and Edgar, 2012). Bacterial (Azotobacter and Pseudomonas) synthesis of alginate provides superior chemical and physical properties as compared to alginate extracted from seaweed. Optimization of alginate synthesized by bacterial method is relatively simple and enables manufacture of alginate with the desired properties for easy modification and synthesis of derivatives (Rehm and Valla, 1997). Chemically, alginate is a polymer consisting of units of mannuronic acid (MA) and guluronic acid (GA) organized in an asymmetrical block design with varying proportions of MA and GA units (Augst et al., 2006). These microstructural open functional unit groups are a characteristic feature of alginates, which are derivatized for customized applications such as cell/microbe immobilization (Melvik and Dornish, 2004, Wang et al., 2003), drug delivery (Dalmoro et al., 2017, Mehta et al., 2015), and food industry (F. Liu et al., 2017).
Among the various available formulations of alginate, alginate microspheres (AMS) and alginate microcapsules (AMC) are the most widely explored due to their capacity to encapsulate small and large molecules with diverse chemical properties. Microcapsules consist of a central core containing the active therapeutic agent, and an exterior polymeric shell (Wong et al., 2018). The term ‘alginate microspheres’ is often used interchangeably in literature to refer to alginate microgels (Ching et al., 2017), alginate microcapsules (Chandy et al., 1999; Darrabie et al., 2005), alginate microparticles (Jain et al., 2015) or alginate microbeads (Mørch et al., 2006). Therefore, for the purposes of this review, all of the above formulations will be referred to as alginate microspheres (AMS). Most of these formulation methods are based on an external (ionic) gelation method in which an alginate solution turns to gel state in the presence of divalent calcium ion (Lin et al., 2005). Further, due to the presence of open functional MA and GA groups, alginates undergo reactions with other cationic polymers such as chitosan - another natural polymer, which can be used for encapsulation of therapeutics agents (Augst et al., 2006). With this background, the present review aims to discuss in detail the various methods for the development of alginate microparticles, and an update on its application in encapsulation of drugs, vaccines, bacteria, vaccines, cells etc. and in tissue engineering (Fig 2).
Figure 2:
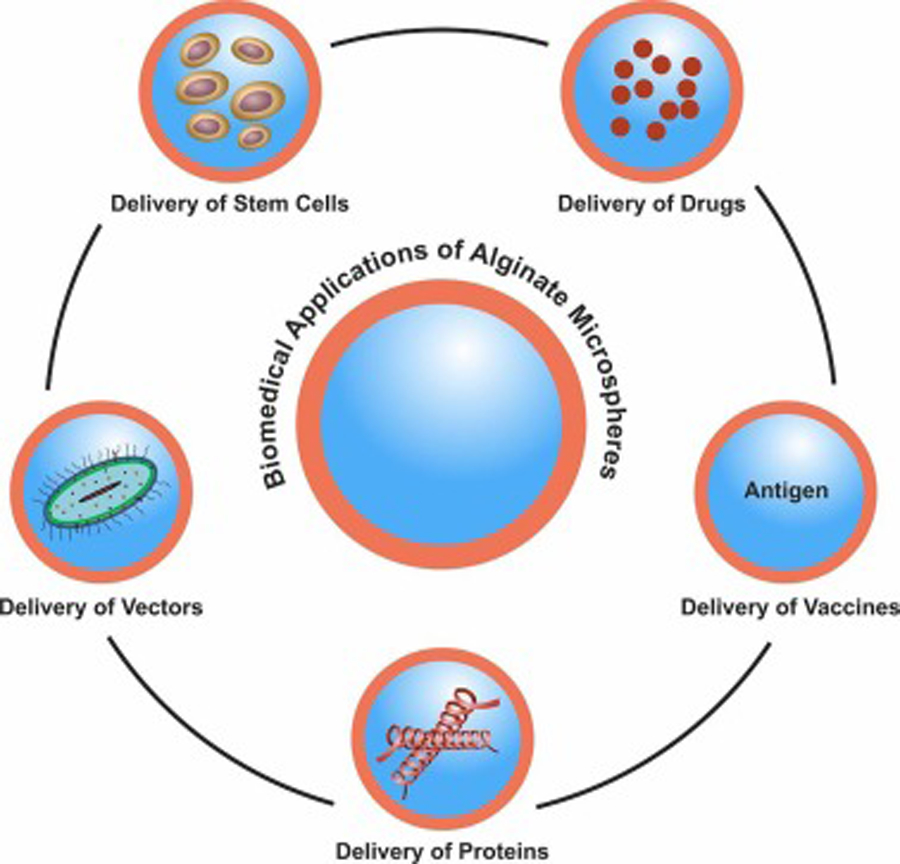
The various biomedical applications of alginate microspheres
2. Fabrication of alginate microspheres
AMS can be formulated through techniques such as single/double emulsion, extrusion, spray drying, or microfluidic methods. Conventionally, water in oil single emulsion is the most common method used for formulation of AMS (Fig 3). This method encapsulates the therapeutic agent in the aqueous sodium alginate polymer, which is then ionically cross-linked by agents such as divalent cations (Liao et al., 2017). Calcium chloride is most commonly used as a crosslinking agent for alginate microsphere formation. Barium chloride (Alizadeh Sardroud et al., 2017), strontium chloride (Lourenço et al., 2017) and zinc chloride (Taha et al., 2005) have also been used as crosslinking agents. Sodium alginate does not form a gel due to its rigid poly l-guluronic acids and solubility in water, but it easily undergoes sol-gel transformation when exposed to a divalent cation such as calcium or zinc (Baimark and Srisuwan, 2014). The alginate matrix specifically forms through the cross-linking and exchange of sodium ions from l-guluronic acid with the divalent ions. Gluconate blocks of alginate forms junctions with the other gluconate blocks of adjacent alginate chains, forming an egg-box model and the gel network results in the formation of sodium AMS (Fig 1). The size of the particles formed by this emulsion dispersion method can be controlled by making changes to the temperature during formulation, the viscosity of aqueous alginate solution, organic solvents or oil phase, the concentration of surfactants, vortexing/homogenization speed, and stir rates applied during the formulation (Patil and Sawant, 2009; Piornos et al., 2017). Emulsification technique is an economical technique and produces AMS with homogeneous morphology. However, this method has several disadvantages like poor industrial scale-up (Reis et al., 2006) and droplet fusion, which can change the characteristics of the particles and may cause random release profiles (Lopes et al., 2017) Also, the extra step of centrifugation done to purify the AMS from the residual oil and surfactants adds to the difficulty for scale-up (Sultana et al., 2000).
Figure 3:
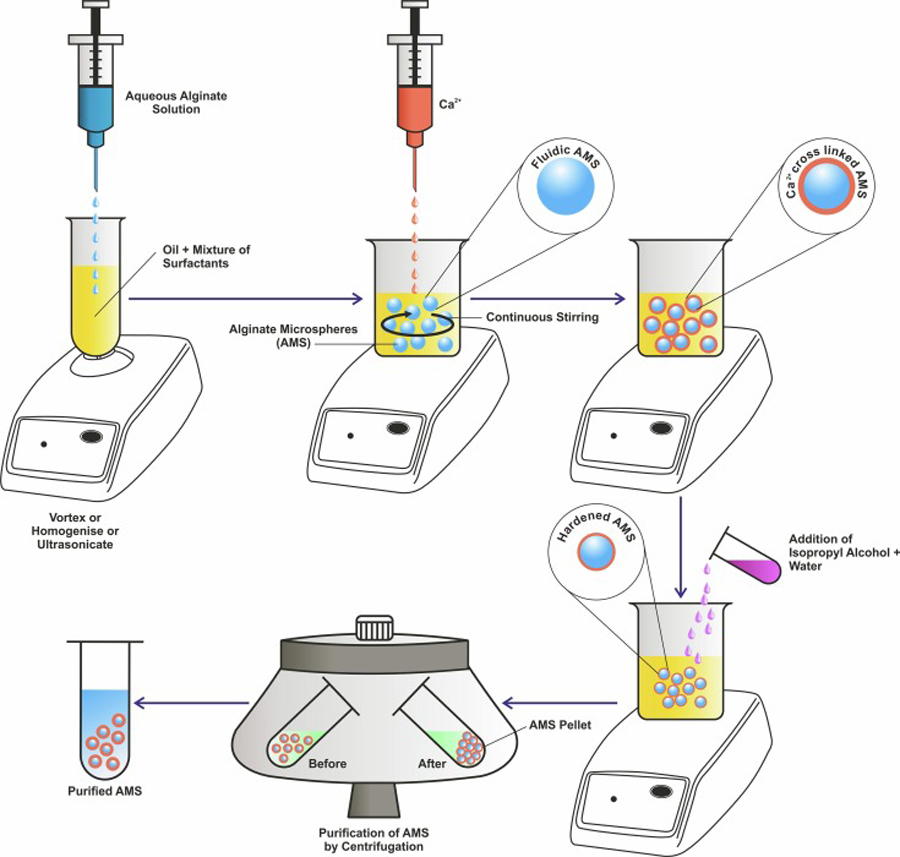
Schematic representation of the formulation of AMS by single emulsion technique
Another method used for the fabrication of AMS is extrusion (Ching et al., 2017), which generates AMS with uniform diameters. The extrusion techniques can control particle sizes using electric energy, mechanical energy or jet cutting mechanism. The power of the applied electrical field generates AMS in the range of 500–2000 µm in diameter with spherical morphology (Zhao et al., 2016) Mechanical vibration generates AMS with diameter in the range of 300 µm to 5 mm (Prüsse et al., 2008), while 200 µm to 5 mm diameter AMS with high uniformity is produced with jet cutting method (Koch et al., 2003). These extrusion techniques however have disadvantages such as requirement of gelling bath, complex nature of formulation, time consuming with poor scale up reproducibility (Ching et al., 2017). An extension of the extrusion method is the formulation of AMS using pneumatic nozzles air atomization method (Ahn and Kim, 2015). In this technique, pressurized sodium alginate solution is passed through a nozzle orifice using a pump. Just after passing through the orifice, the liquid jet of alginate solution is turned into droplets using pressurized gas, which is usually air, leading to atomization and formation of uniform droplets. These sodium alginate droplets (diameter range of 11–80 mm) are finally expelled into a bath/vessel containing crosslinking agent (e.g.: calcium chloride solution). Spray drying is an advanced version of this technique which ensure better uniformity and have an advantage of scalability.
Spray drying is commonly used for the encapsulation of food ingredients such as caffeine (Bagheri et al., 2014). It is also used in the encapsulation of tissue regeneration agents like dermatan sulfate (Martins et al., 2007; Wen et al., 2012), and in pharmaceutical industry to improve drug bioavailability (Ganesh et al., 2015). Spray drying (Fig 4) works on the principle that the drug-polymer dispersion can be transformed to a dry state by atomizing, and then exposed to hot gas to evaporate the solvent by a closed loop operation, to yield microspheres. Spray drying is advantageous for maintaining a stable drug shelf-life, for rapid, precise, scalable development of AMS, and for having high active material encapsulation efficiency (Agüero et al., 2017). Characteristics of AMS formulated by spray drying method are greatly influenced by the flow rate of the feeding solution, concentration of alginate, surfactants and crosslinking agent, atomization time, crosslinking exposure time and drying time (Dalmoro et al., 2014). However, the expensive apparatus and high inlet temperature operating conditions (approx. 100°C or higher), can make it difficult to use spray drying method for encapsulation of heat-sensitive agents (e.g.: proteins) (Anandharamakrishnan et al., 2007). Spray drying coupled with ultra-atomization is a promising technique which can be used for the fabrication of AMS with precise particle characteristics. Ultrasonic atomization can be achieved by high frequency ultrasonic energy or by ultrasonic vibrations. These ultrasonic energies rely on the use of high pressure and velocity motion to convert the fluid into small droplets unlike conventional spray drying atomization. This advanced technique, especially when coupled with ionotropical gelation, is known for its high reproducibility, robust nature, innovativeness, easy scale-up, low cost, faster process, consistent output and single step process. AMS prepared from this technology has high encapsulation efficiency and spherical morphology with uniform size distribution. (Dalmoro et al., 2012).
Figure 4:
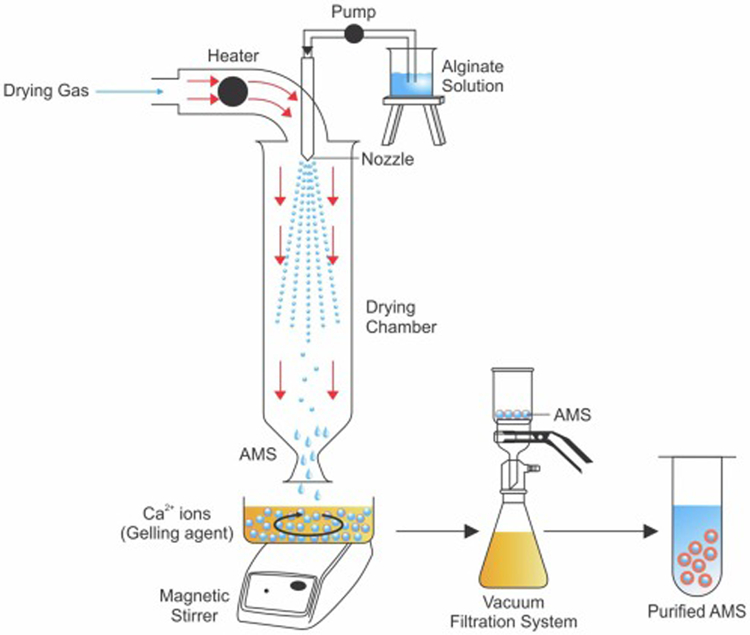
Schematic representation of the formulation of AMS by spray drying method
Another technique used to prepare calcium-linked AMS is the microfluidic method (Fig 5). It is used to produce alginate beads with precise diameters of less than 300 µm with minimal polydispersity. Sugiura et al. developed a new silicon micro-nozzle array-based microfluidic device for the formation of AMS with diameter in the range of 50–200 μm. The aqueous alginate was forced through a 30 x 30 μm construct and short 500 μm micro-nozzle followed by sheared flow of viscous oil to yield alginate droplets. The alginate droplets quickly reacted with calcium chloride to form AMS (Sugiura et al., 2005). Another study used a chip-based microfluidic system to produce 50–2000 μm uniform calcium alginate beads, and it can be programmed to create many ordered calcium-AMS (Huang et al., 2006).
Figure 5:
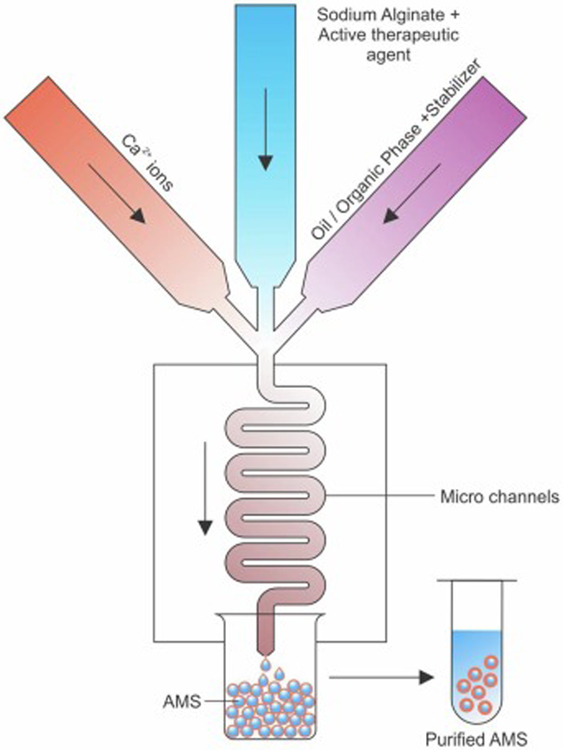
Schematic representation of the formulation of AMS by microfluidics method
3. Biomedical applications
3.1. Delivery of drugs
Microencapsulation is a well-known method used to deliver drugs and to control the rate of drug release. Drug-encapsulated microspheres can be used to improve bioavailability and stability of drugs, target the drugs to specific sites in the body, and provide prolonged or controlled drug release. Microencapsulation is particularly advantageous for delivery of low molecular weight or hydrophobic drugs and proteins, as well as to decrease dosing frequency, side effects, and to improve patient compliance.
Various drugs can be microencapsulated into alginate gels for oral administration. This reduces or eliminates the possibility of GI tract irritation. In the acidic gastric fluid, external layer of AMS is hydrated and transformed to an insoluble alginic acid form in which the alginate shrinks and consequently protects the encapsulated drugs. However, at higher intestinal pH, shrunken alginic acid turns to a soluble viscous layer ultimately leading to release of the payloads (Sosnik, 2014). For example, a well know a potent nonsteroidal anti-inflammatory drug (ibuprofen) was used to determine the effects of different parameters such as concentration of sodium alginate, calcium chloride and magnesium stearate on the release profile of ibuprofen encapsulated AMS (Nagpal et al., 2012). Outcomes of the studies showed that AMS were discrete and spherical in shape with a rough outer surface and the mean AMS particle size was 6.89 um when analyzed by scanning electron microscopy (SEM). Percent yields of the different batches was found to be between 66.6 and 96.6 %. Increase in polymer concentration contributed to greater sustained release whereas addition of magnesium stearate improved the drug encapsulation efficiency.
Similarly, AMS were used in the encapsulation of isoniazid for oral sustained drug delivery (Rastogi et al., 2007). Isoniazid is a commonly used anti-mycobacterial agent for first line therapy in tuberculosis but, long term continuous therapy of isoniazid can cause hepatotoxicity and peripheral neuropathy, so it would therefore be advantageous to have a drug formulation with prolonged and controlled enteric release. AMS containing isoniazid were formulated by a single emulsification method in which the particles were found to be smooth and spherical and had a mean particle size of 3.72 µm. Release studies showed release of 26 % of the isoniazid in simulated gastric fluid of pH 1.2 in six hours, and complete release of remaining 71.25% in simulated intestinal fluid of pH 7.4 in 30 hours. Another study demonstrated the formulation of buccal nystatin AMS by the emulsification method for the treatment of oral candidiasis (Martín et al., 2015). The mucoadhesive characteristics of alginate aids in improved targeting to the absorptive sites and increased uptake of the drug. AMS can thus provide closer contact with larger mucosal surface area than traditional buccal tablets or lozenges. In this study, the mean diameter of AMS was found to be 89.03 μm for empty vehicles and 135.21 µm for drug loaded AMS. The microspheres had a clear inhibition effect on C. albicans growth. In vivo studies showed that the microspheres were retained in the mucosa, with no systemic absorption or tissue damage.
In another study, green tea polyphenol was encapsulated into AMS for the minimally invasive treatment of osteomyelitis (Chen et al., 2018). Osteomyelitis is an infection in the bone which is treated with regular high doses of systemic antibiotics. Polyphenols extracted from green tea are rich in anti-oxidative and antibacterial properties. In this study, green tea extracted polyphenols were encapsulated in porous silica nanospheres which were then encapsulated in AMS. The final formulation was then coated with pH-sensitive calcium carbonate to provide conducive environment for delivery of polyphenols at the site of action killing Staphylococcus aureus consequently promoting the proliferation of osteoblasts. Final microspheres were about 3 mm in diameter, had a drug loading efficacy of 92.96 weight percent, and were able to reduce the oxidative stress caused by osteomyelitis. Examples of other drugs delivered using AMS are shown in Table 1.
Table 1:
Examples of AMS used in drug delivery applications (arranged according for formulation method)
| Therapeutic agent | Formulation method | Materials used | Findings | Reference |
|---|---|---|---|---|
| Metformin | Emulsification | Metformin encapsulated chitosomes and niosomes loaded in AMS | Significant improvement of metformin hypoglycemic effect when administered orally as chitosomal-AMS. AMS-chitosan protected metformin from gastric acidic environment. | (Maestrelli et al., 2017) |
| Thyme essential oils | Emulsification ionic gelation | Calcium crosslinked AMS | AMS protected essentials oils from volatilization or oxidation. High encapsulation efficiency of 85% |
(Benavides et al., 2016) |
| Cocoa polyphenols | Emulsification/internal gelation | Calcium/citrate, alginate, spans | Optimized formulation showed 60% retention of cocoa extract encapsulated into AMS. | (Lupo et al., 2014) |
| Celecoxib | Co-grinding micro-mill for HPBCD and PVP composite with celecoxib and emulsion method for AMS | Hydroxypropyl-β-cyclodextrin (HPBCD) and PVP (polyvinylpyrroli done) Chitosan-Ca-AMS | HPBCD and PVP improved solubility of celecoxib, which significantly shortened pain alleviation onset in vivo | (Mennini et al., 2012) |
| Acyclovir | Simple emulsification phase separation technique | Alginate mucoadhesive microspheres | Entrapment efficiency = 51.42−80.46%. Optimized AMS possess good mucoadhesion and in vivo gastroretention for > 4h | (Md et al., 2011) |
| Doxorubicin | Emulsification | Samarium (Sm)/mesoporous bioactive glass (MBG)/ alginate composite microspheres | Sm/MBG/AMSs exhibit sustained doxorubicin delivery, and release by Fickian diffusion according the Higuchi model. The release is dependent on Sm doping and pH | (Zhang et al., 2016) |
| Azelastine | Ionotropic gelation | Chitosan, sodium alginate | Entrapment efficiency = 73.05%, 65% mucin binding efficiency, controlled release for 8h. | (Shinde et al., 2014) |
| Diclofenac sodium | Emulsification and/or gelation method | Thermosensitive hydrogels of chitosan and β-glycerophosphate combined with AMS | Sol-gel transition at 31.72 ± 0.42°C. Gel formation within 5 min following intraarticular injection. Sustained release for upto 5 days. | (Qi et al., 2016) |
| Doxorubicin + T1 and T2 MRI contrast agents | Spraying device | Barium crosslinked AMS loaded with temperature sensitive liposomes (TSL) | TSL incorporation had slight effect on temperature-triggered drug release. Particles detected by MRI before and after hyperthermia in vitro and in vivo | (van Elk et al., 2015) |
| Ropinirole hydrochloride | Spray drying | Sodium alginate | Spherical morphology (2.5 – 4.37 µm), yield = 70%, encapsulation efficiency = 100% when the inlet temperature of spray drying was 140 °C. | (Hussein et al., 2019) |
| Ranitidine | Spray drying | Sodium alginate | Smooth surface with narrow size distribution. Loading efficiency = 70.9 %, prolonged drug release observed | (Szekalska et al., 2015) |
| Caffeine | Spray drying | Antioxidant peptides, Sodium alginate | Peptidic nanoparticles encapsulating caffeine protected from gastric pH and caffeine leakage by spray drying and alginate encapsulation. 7.4 μm diameter, encapsulation efficiency = 85% | (Bagheri et al., 2014) |
| Rifampicin | Microfluidics | PLGA, Sodium alginate, dichloromethane, polyvinyl alcohol, toluene and span 80 | Monodispersed PLGA–alginate core–shell microspheres, controlled release profile | (Wu et al., 2013) |
3.2. Delivery of cells
Microencapsulation of cells involves immobilizing bioactive cells within microparticles. The cells are surrounded by a polymeric membrane to allow free movement of nutrients, oxygen and the therapeutic protein products into and out of membrane. The semi-permeable membrane impedes high molecular weight molecules (>150 kDa), antibodies as well as other immunologic substances from recognizing the encapsulated cells as foreign materials (Orive et al., 2003). Due to the advantageous mild environment of the alginate gel, the cells immobilized in the gel can retain strong viability in long-term culture. AMS can also act as a shield from physical stress and avoid immunologic reactions in host. Hence, there is a lot of research on encapsulation of cells with sodium alginate to form therapeutic products with the aim of treating different diseases.
In one work, the ability of human mesenchymal stem cells (hMSCs) encapsulated in Arginylglycylaspartic acid (RGD) peptide modified AMS to facilitate myocardial repair was studied (Yu et al., 2010). This in vitro study found that the RGD modified AMS improved cell attachment and growth, and increased angiogenic growth factor expression. Following intramyocardial delivery, the AMS encapsulated hMSCs were able to induce angiogenesis, which helped maintain left ventricular shape and prevent negative left ventricular remodeling after a myocardial infarction. Another study formed microspheres for hMSCs encapsulation using methacrylated alginate polymer in combination with microfluidic devices (Etter et al., 2018). It was observed that stem cell viability was maintained in the AMS after encapsulation. Grellier et al. encapsulated endothelial cells in alginate to study if these cells can regulate the osteogenic potential of osteoprogenitor cells in vitro and in vivo, for its use in a long bone defect (Grellier et al., 2009). When human osteoprogenitors in combination with human umbilical vein endothelial cells were encapsulated in RGD-grafted AMS, there was a significant increase in the mineralization of the bone, showing potential for a new injectable bone tissue engineering approach.
AMS are most often studied for the encapsulation of pancreatic islet cells for treatment of diabetes. Limitations of islet transplantation in diabetic patients include a severe shortage of human pancreas donors, and the necessity to use immunosuppressive drugs so as to prevent transplant rejection. Hence, one work developed a bio-artificial pancreas through microencapsulation of perm-selective coating of islets cells with biopolymers for graft immunoisolation, for the treatment of diabetic patients (Opara et al., 2013). Another study analyzed a multilayer microcapsule for encapsulation of pancreatic islets. Empty and islet containing AMS were coated with the concentric layers of polymers like polythelenemine, polyacrylacid or carboxymethylcellulose along with alginate. This encapsulation procedure and membrane forming step did not have any effect on the stimulatory response of the islet cells which showed improved glucose-induced stimulatory insulin response in three and five weeks of cell culture (Schneider et al., 2001). In another study, human pancreatic islets cells were encapsulated in Ca2+/Ba2+ crosslinked AMS and administered intraperitoneally into diabetic Balb/c mice (Qi et al., 2012). It was observed that mice were within normal glycemic levels from day one after transplantation and retained the normal glycemic levels with a significant improvement in the mean graft survival time. Animals transplanted with the same amount of non-encapsulated human islets cells rejected the cells about two to seven days after transplantation. The study concluded that the Ca2+/Ba2+ alginate microbeads could potentially be used to prevent human islets from xenogeneic rejection in immunocompetent mice without immunosuppression.
AMS can further play a role in regenerative medicine. Differentiation of stem cells into primordial germ cells have the potential to help treat patients with infertility and produce mature germ cells. One such study demonstrates the application of the mouse embryonic stem cells (ESCs) differentiation capacity to putative primordial germ cells encapsulated within alginate and alginate-collagen IV microspheres (Mansouri et al., 2017). Primordial germ cell gene and protein expression showed the differentiation potential of ESCs to putative primordial germ cell in alginate-collagen IV microspheres, which was significantly increased compared to the control groups. Encapsulation of cells with sodium alginate is being studied actively today to offer therapeutic solutions for a variety of diseases. Other examples of cells delivered using AMS are shown in Table 2
Table 2:
Examples of cells encapsulated AMS (arranged according for formulation method)
| Therapeutic agent | Formulation method | Materials used | Findings | Reference |
|---|---|---|---|---|
| Dental pulp stem cells (DPSC) | Emulsification | Calcium-AMS | Enhanced osteogenic potential of cells, maintained high cell viability for bone tissue regeneration | (Kanafi et al., 2014) |
| Enterococcus faecalis | Emulsification | Milk, alginate | Good tolerance to stimulated gastric fluid and bile salt solution (1.0 or 2.0%), improved storage stability of E. faecalis | (Shi et al., 2016) |
| Allogeneic pancreatic islet cells | Emulsification | Chemically modified alginate derivative Z1-Y15 | Three of the several chemically modified AMS elicited a reduced foreign body response, are now in clinical trials. | (Bochenek et al., 2018) |
| Mesenchyma l stem cells (MSC) | Microfluidics with calcium crosslinking | Sodium alginate, calcium chloride-ethylenediam ine tetra acetic acid. | MSC encapsulated within homogenous AMS, stable cell growth and proliferation for 15 days. | (Utech et al., 2015) |
| Modified human pancreatic islets cells (1.1B4) | Microfluidics | Alginatepoly-L-lysine-copolymer | Microspheres formulated as microcapsules in the static state and using microfluidics in dynamic state. Cell viability and glucose simulated insulin secretion better in microcapsules formulated by dynamic microfluidics method | (Acarregui et al., 2018) |
| MSC | Co-axial air-flow droplet generator | Alginate-polyethylene glycol microspheres | Encapsulated MSC displayed anti-fibrotic and anti-inflammatory effects on liver fibrosis in mice due to secretion of soluble factors | (Meier et al., 2015) |
3.3. Delivery of proteins
Research on encapsulation of proteins in AMS dates to more than 30 years ago. Systematic description of the encapsulation of proteins with examples is mentioned in a review done by Gombotz et al. (Gombotz and Wee, 1998) Recent reports on encapsulation of proteins using AMS system as the carrier is described here. Proteins such as enzymes, growth factors, hormones, and interleukins have several biomedical applications and are used in tissue regeneration or as therapeutic agents (Dimitrov, 2012). However, the structure of proteins can easily be denatured or altered in harsh environmental conditions, including changes in pH and temperature, and thus lose their therapeutic qualities (Dimitrov, 2012, Wells and Sheardown, 2007). Therefore, these proteins can be encapsulated in AMS to prevent degradation, and to provide controlled, site-specific release (Wells and Sheardown, 2007). AMS demonstrate improved protein loading compared to formulations prepared using other biomaterials.
Protein-containing AMS are often used in tissue engineering, especially for bone tissue generation, or osteogenesis. In a study done by Quinlan et al, poly lactic-co-glycolic acid (PLGA)-AMS encapsulated recombinant human bone morphogenic protein-2 (rhBMP-2), a pro-osteogenic factor into collagen-hydroxyapatite scaffolds, served as a temporary platform for improving cell growth (Quinlan et al., 2015). AMS (size range: 1–10 μm) showed sustained release of rhBMP-2 and therefore has huge potential for therapeutic delivery not only for bone regeneration but also to other tissues by regulating the chemical composition of the scaffold frame and the incorporated growth factor. In another study, BMP-2 was encapsulated by AMS (around 30 µm) and delivered at the site of action by using thermosensitive chitosan/dextran-polylactide/glycerophosphate hydrogel for osteogenesis (Zhu et al., 2016). Similarly, carboxymethyl chitosan/poly (vinyl alcohol) gel base was used as a carrier for bovine serum albumin (BSA) loaded AMS (average diameter - 408.6 ± 9.4 µm) which was intended to aid in regeneration after transplantation of components such as bio-interactive implants and artificial skin (Liu et al., 2015). Another study investigated the application of combination of TGF-β3 encapsulated AMS and human mesenchymal stem cells in hyaluronic acid hydrogels base for the fabrication of implantable constructs for cartilage repair (Bian et al., 2011). The TGF-β3 encapsulated AMS were around 38.9 ± 9.2 μm in diameter with high encapsulation efficiency and high biocompatibility, and demonstrated controlled drug release profile (Bian et al., 2011). On similar lines, nanofibrous gelatin scaffolds were also used alongside AMS to encapsulate nerve growth factor for brain tissue engineering (Büyüköz et al., 2018).
Certain proteins will typically have difficulty being absorbed in the body due to their innate properties such as isoelectric points and hydrophilicity (Wells and Sheardown, 2007; Zhai et al., 2015). One study had lysozyme and chymotrypsin encapsulated to test for the rapid loading and sustained released of proteins with high isoelectric points (Wells and Sheardown, 2007). Crosslinking of alginate with the loaded high isoelectric point proteins lead to the sustained release behavior of the system. In another study, PLGA-AMS (around 3 µm) loaded with hydrophilic proteins were used for protein delivery (Zhai et al., 2015). BSA or rabbit anti-laminin antibody protein were used for encapsulation. Higher protein encapsulation efficiency and more controlled protein release rate was observed from the PLGA-AMS compared to PLGA alone, due to the slower degradation rate of the composite microspheres.
AMS have also been used to encapsulate horseradish peroxidase (HRP) and tetramethyl rhodamine conjugated BSA for applications in protein drug delivery and as a biosensor. These AMS (average diameter 700 ± 80 μm) were coated with silk fibroin for improved stability and drug delivery. The silk coating acted as a barrier to help slow the release of the encapsulated agents and limit the amount of degradation of microspheres in comparison to microspheres without a coating. Additionally, the system allowed the attachment of the ligands to the coating to allow for more specific targeting (Wang et al., 2007). Delivery of therapeutic proteins using AMS is being explored in other areas of tissue engineering and regenerative medicine. Some examples of proteins delivered using AMS are shown in Table 3.
Table 3:
Examples of proteins, microbes and vaccines encapsulated AMS (arranged according for formulation method)
| Category | Therapeutic agent | Formulation method | Materials used | Findings | Reference |
|---|---|---|---|---|---|
| Proteins | Bone morphogenetic protein 2 (BMP- 2) | Emulsification | Calcium phosph ate cement with PLGA microsph eres incorporated in AMS | BMP-2 release from AMS led to the programmed delive ry to cell cultures representing fracture healing | (Bayer et al., 2017) |
| Basic fibroblast growth factor (bFGF) | Emulsification | bFGF alginate, carboxymethyl chitosan (CMCS), poly (vinyl alcohol) (PVA) | bFGF encapsulated AMS were formulated and delivered using CMCS-PVA hydrogel base for wound healing in burns. | (Q. Liu et al., 2017) | |
| Diamine oxidase enzyme | Ionotropic gelation | Carboxymethyl starch (CMS)/alginate by complexation with Ca2+ | CMS and alginate were used as a novel matrix system microsphere for the encapsulation of diamine oxidase for protection against gastrointestinal degradation. | (Blemur et al., 2016) | |
| Basic fibroblast growth factor (bFGF) | Electrostatic droplet generation technique followed by a layer-by-layer self-assembly technique | Microsphere, heparin-conjugated alginate | Polyelectrolyte multilayer-coated heparin-conjugated AMS demonstrated controlled and sustained release of heparin-binding growth factors. | (Zuo et al., 2015) | |
| Vascular Endothelial Growth Factor (VEGF) | high-pressure sterilizer, dried and preserved | Calcium AMS | VEGF-loaded calcium AMS significantly promoted the neovascularization of adipose graft, improved survival rate of adipocytes. | (Ding et al., 2015) | |
| Vaccines | Bordetella pertussis | Emulsification | Alginate microparticles | The microparticles showed mean size of 151.1 μm, loading efficiency of 89.6%, and constant release | (Dounighi et al., 2017) |
| Omp16-based vaccine | Emulsion internal gelation method | Alginate-chitosan microspheres | Vaccines provided significant protection against Haemophilus parasuis in mice | (Zheng et al., 2017) | |
| Hepatitis B surface antigen | Modified spray-solidification technique | Antigen-loaded DDAB/PLA (didodecyldimet hylammonium bromide/poly (lactic acid)) nanoparticles (NPs)-alginate composite microcapsules | Single-shot microcapsule vaccine formulation induced high enough antigen-specific IgG antibody responses and cytokine secretion levels | (Yang et al., 2018) | |
| Tumor vaccine | Spray drying | AMS (named PaLtTcAdMIP3 α) that encapsulated tumor lysates, live tumor cells engineering with a recombinant MIP-3α adenovirus and BCG | AMS expressed and excreted macrophage inflammatory protein 3α, which effectively attracted dendritic cells ex vivo and in vivo | (Huang et al., 2015) | |
| Vectors and microbes | Trichoderma viride spores | Emulsification / cross-linking | Calcium AMS | Trichoderma viride germination inside microsphere lowered the amount of released calcium ions and slowed release kinetics than microspheres without Tricoderma viride | (Jurić et al., 2019) |
| Recombinant human adenovirus type 5 (HAd5) | Emulsification | Alginate | Had5 encapsulation efficiently avoided vector-specific immune response. | (Sailaja et al., 2002) | |
| Macroalgal spores of a green alga (Ulva intestinalis) and brown algae (Undaria pinnatifida and Ecklonia cava) | Emulsification | Alginate | Encapsulated spores of the three algal species tended to grow faster than non-encapsulated spores | (Jung et al., 2018) | |
| Arbuscular Mycorrhizal Fungi Organic Fertilizer Pellets | Emulsification | Calcium-Alginate Complex | Pellets were fully degraded within 30 days | (Pitaktamrong et al., 2018) | |
| Ganoderma lucidum spores (GLS) | Electrospraying | GLS–Alginate (GLS/A) micro beads | No release of GLS encapsulated beads in the simulated gastric fluid (pH of 1.8), a rapid, size dependent release was found in the simulated intestinal solution (pH of 7.5) | (Zhao et al., 2016) |
3.4. Delivery of vaccines
Vaccines are products that aid in stimulating the production of antibodies in the immune system, either for preventative or therapeutic measures. In this way, the body is able to amass an effective adaptive immune response against foreign pathogens or antigens, and can therefore develop an immunity to a particular disease (Kim et al., 2002). Vaccines carry a huge commercial value because of their well-known use in contagious diseases such as mumps, rubella, and measles. In most of the cases, vaccines are administered via the parenteral route, and non-parenteral route is used rarely (Giudice and Campbell, 2006). However, scientists are now recognizing the importance of immunization by oral or intranasal route. Effective delivery of vaccines through non-parenteral routes can be successfully achieved by using AMS due to its excellent mucoadhesive property and the ability to protect the vaccine from the GIT acidic environment (Gombotz and Wee, 1998).
Previous reports indicate that AMS allows sustained release of the loaded vaccines and helps maintain the integrity of the encapsulated agents within the microspheres. They have also been tested for long term storage by freeze-drying the AMS loaded vaccines (Hariyadi et al., 2014). For examples, BSA (a model antigen) was encapsulated within AMS protein carriers and freeze-dried for oral delivery (Hariyadi et al., 2014). In this study, Maltodextrin was used as a cryoprotectant and the formulated AMS were approximately 22–65 µm in diameter after lyophilization. Release study experiments revealed that less than 7% of BSA was released from the freeze-dried formulations in simulated gastric fluid in 2 h, whereas 90 % of the BSA was released in simulated intestinal fluid in 10 h. In another study, Tafaghodi et al, had developed AMS loaded with autoclaved Leishmania major (ALM) as antigen and CpG-ODN as immunoadjuvant (Tafaghodi et al., 2011). L. major is a parasitic species associated with the cutaneous disease leishmaniasis, which is transmissible to humans from an animal vector. CpG-ODN acts as an adjuvant due to its ability to enhance the humoral immune response. AMS loaded with ALM and CpG-ODN had diameter of less than 5 µm and with sustained release profile desirable for vaccine delivery. Similarly, AMS encapsulating the rotavirus promoted an immune response after oral and enteric immunizations in domestic animals (Kim et al., 2002). In another study, AMS were loaded with recombinant adenovirus to protect against any possible vector-specific responses (Sailaja et al., 2002). The most successful routes of delivery for this vaccine were intranasal and intraperitoneal. Similarly, lipopolysaccharide-antigenic fractions from Klebsiella pneumoniae were encapsulated within AMS for intratracheal delivery to treat nosocomial infections (Jain et al., 2015). The size of the AMS was less than 5 µm for effective nasal and lung delivery. AMS successfully protected its loaded contents and induced strong systemic and mucosal immune responses within Swiss albino mice (Tafaghodi et al., 2006).
Several studies have also revealed the use of AMS in delivery of vaccine in animals, especially fish. For example, an AMS loaded with plasmid DNA that codes for fish lymphocystis disease virus was used for the treatment of Japanese flounder (Tian et al., 2008). The study showed that the plasmid DNA maintained high transfection efficiencies, implying that AMS effectively preserved the integrity of the plasmid DNA. Another study had AMS encapsulating a DNA vaccine for infectious hematopoietic necrosis virus (pIRF1A-G vaccine), which was then re-suspended in sodium citrate at room temperature for oral delivery (Ballesteros et al., 2015). Results demonstrated that AMS containing DNA vaccine significantly improves fish immune responses and resistance to infectious hematopoietic necrosis virus after the oral delivery. AMS was also found to protect DNA vaccine from the acidic and degradative conditions of the gastrointestinal tract (Ballesteros et al., 2015). Research is ongoing into the development of AMS encapsulating antigens and antibodies for immunization in humans. Some examples of vaccines delivered using AMS are shown in Table 3.
3.5. Delivery of microbes and vectors
Microbes play a crucial role especially in the GIT of the body (Ma et al., 2008). Microbiota in the lumen of the GIT helps to maintain optimal GIT conditions and outcompete other harmful pathogens and bacteria that could otherwise colonize and infect the GIT. Microbes such as bacteriophages and probiotics can be administered for therapeutic purposes, such as to prevent diarrhea and balance intestinal microbiota. AMS containing bacteriophages and vectors allow for better acid stability and protection of the encapsulated contents from GIT acid degradation as well as controlled, sustained release of the contents. In GIT conditions, alginate acts as a pH buffering agent and prevents excessive temperature changes as the viscosity of the alginates is increased at lower pH (around pH 3) which protects the therapeutic agent from the GI acidic environment. At lower pH carboxylate group of alginates are protonated and form hydrogen bond leading to increase in the viscosity (Lee and Mooney, 2012). AMS also act to prevent encapsulated microbes from being broken down by enzymes and bile in the GIT. A study reports the designing of chitosan – AMS loaded bacteriophage Felix O1 for oral administration (Ma et al., 2008). The free phage is sensitive to acidic environments and could not be detected at pH below 3.7 following a 5-min exposure. Microspheres containing Felix O1, on the other hand, were found to be stable after lyophilization and significantly improved phage survival. Release studies suggested that encapsulated phage was released at the simulated intestinal pH of 6.8 in 6 h.
One study done by Lamas et al, had AMS encapsulating fungal spores and viable cells of Bacillus subtilis (Lamas et al., 2001). The results indicated that both types of encapsulated contents were protected from the media due to the biocompatible nature of alginate. Encapsulated spores and cells had a significantly longer germination time than the control (free spores and cells). The growth of encapsulated cells was like the cell growth of free cells, even after the lag-time from encapsulation.
In certain cases, alginate is paired with other biopolymer such as chitosan for enhancement of the protective properties of the microspheres. Complexes of chitosan-AMS were used to protect the bacteriophages from highly acidic conditions in the GIT (Ma et al., 2012). In another study involving chitosan-AMS, the microspheres encapsulated both Staphylococcus aureus Phage K with calcium carbonate microparticles for the reduction of pathogen colonization in the intestine (Ma et al., 2012). Calcium carbonate (alkaline compound) was incorporated in the formulation to protect Phage K by gastric fluid. The complex of chitosan-AMS (average diameter 900 ± 28 µm) preserved Phage K and its viability from bile and acidic gastric fluid (Ma et al., 2012).
Based on the requirement and the end application, alginates can be manipulated and derivatized to form modified polymers. In one such study, a complex of protein-AMS was used to encapsulate Lactobacillus bulgaricus which served as a wall carrier for probiotic encapsulation (Chen et al., 2014). Efficacy of the formulation was found to be highly dependent on the diameter of AMS. Alginate-milk microspheres were used in another study to encapsulate probiotics (Lactobaccilus bulgaricus) to test for the microspheres ability to protect against simulated GIT conditions (Shi et al., 2013). This complex proved successful at protecting L. bulgaricus from highly acidic environments.
In these studies, the microspheres effectively improved the capability of the bacteriophages to provide their beneficial effects to the system. Additionally, AMS allowed for high encapsulation rates of the various payloads. AMS, especially when combined with chitosan, are suitable vectors for probiotic and microbe delivery. Some examples of microbes and vectors delivered using AMS are shown in Table 3
3.6. Applications in cell culture
AMS have been exponentially utilized in cell and tissue engineering. Alginates were initially not widely used for cellular growth due to their hydrophilic surface, which results in poor protein adsorption (Machida-Sano et al., 2014). Alginate also lacks the ability to support cell adhesion due to the absence of cell binding sites (Lee et al., 2016). However, with the increase in demand for cell culture substrates that simulate the natural environment, and technological advances, alginates were successfully coupled with synthetic peptides in a synchronized manner to design/modify the surface to make it conducive for cell adhesion. For example, alginates complexed with peptides such as arginine, glycine and aspartic acid have been shown to augment proliferation and adhesion of osteoblasts (Chen et al., 2015), myoblasts (Sandvig et al., 2015), and bone marrow cells (Guo et al., 2017). Chemical functionalization with such peptides makes alginate an ideal biocompatible scaffold for cellular proliferation in vitro and can consequently be explored for translational research in regenerative medicine and tissue engineering.
Although, three-dimensional (3D) tissue systems using alginate hydrogels are not new to the bioengineering field, studies investigating the use of AMS for this application has been scant. Absence of endogenous alginase enzyme in mammals is one of the key reasons that the use of alginate in the clinic has been limited in the past, as alginates are not effectively resorbed and excreted/eliminated from the body. To overcome such limitations, recently researchers have designed alginate hydrogels loaded with alginate lyase-encapsulated PLGA microspheres and the results have demonstrated that these hydrogels were digested in a sustained and tunable fashion to deliver therapeutic stem cells (Ashton et al., 2007).
In another study, alginate core-shell microbeads were incorporated to provide bio-mechanical structural stability to MCF-7 human breast cancer cell lines leading to formation of 3D spheroids model for upto 14 days. These well-structured shells not only resisted the growth of cells as a monolayer but provided long-term protection to cells to facilitate cellular proliferation for formation of multicellular aggregates within the core shell layer. Further, cell encapsulation was done by using one-layer microfluidics method, which generates microspheres with narrow polydispersity and less batch to batch variations (Yu et al., 2015).
4. Conclusions and future perspectives
AMS are used in a wide range of therapeutic applications including encapsulation, therapeutics delivery, tissue engineering, regenerative medicine and in vitro cell culture. Some of the striking characteristics of alginate are their biocompatible nature, easy gelation and availability of open functional groups with ease of manipulation to synthesize alginate derivatives with advanced desirable properties (Augst et al., 2006). In the present review, we have summarized different methods used for formulation of AMS and discussed the process parameters that controls the characteristics of the microspheres. Further, the key applications of AMS for delivery of therapeutics such as drugs, vaccines, cells, bacteria and proteins, and its use in cell culture techniques are addressed. Encapsulation within AMS is attractive for protection of therapeutic molecules from GIT environment, high drug entrapment and loading, and for drug delivery via a biocompatible and mucoadhesive carrier (Wong et al., 2018). However, AMS can be limited by immediate release of the therapeutic agents and poor long-term stability (Aguirre Calvo and Santagapita, 2016 Halder et al., 2005). Porous nature of alginate polymer matrix is one of the key reasons for this immediate release/ leakage of drugs (Mallikarjuna Setty et al., 2005). Blending alginate with other polymers such as chitosan, dextran sulphate, poly-L-lysine, acrylic polymers or pectin (Silva et al., 2006) not only improves the encapsulation efficiency but also helps control the release profile. For example –the positive charge on chitosan/pectin interacts with the negative charge on alginate to form an intact membrane, having control over the release of encapsulated agents (Jaya et al., 2009). On the other hand, negatively charged dextran sulphate is known to have higher encapsulation efficiency of proteins when used along with alginate (Martins et al., 2007). Further, combination of chitosan and dextran sulphate showed synergistic effect on encapsulation of insulin with better control over the release profile (Martins et al., 2007). Secondary encapsulation of the alginate polymer blends within a scaffold or hydrogel as appropriate for the application, can further modulate the drug release kinetics. In a study by Aguilar et al., poly-caprolactone (PCL)-alginate composite microspheres were placed within a bioscaffold to modulate the release profile of a representative biomolecule. The PCL-alginate composite microspheres demonstrated slower release than AMS alone, and this release could be further modulated by secondary encapsulation within the bioscaffold (Aguilar et al., 2019). However, care needs to be taken during derivatization of alginates to synthesize semisynthetic alginates, as this may lead to decrease in biocompatibility and inertness of the original polymer. Hence an efficient purification method also needs to be developed to ensure complete removal of by-products and unreacted chemicals. Additionally, it is critical to assess the cytotoxicity, blood compatibility, response from immune system and bio-degradation behavior of the synthesized polymer and particles in their potential applications (Sobol et al., 2013)
It is worth noting that AMS are excellent formulations for the microencapsulation and delivery of islet cells, due to their ability to evade antigen reaction, thereby protecting the cells from the host immune response. Due to this property of AMS, advanced clinical trials are now in progress in which the porcine islet cells encapsulated alginate microcapsules were tested in humans for diabetes type I without administration of an immunosuppressive agent. In this clinical trial, the microcapsules (DIABECELL®) were intraperitonially implanted in humans and the outcomes have demonstrated hypoglycemic effect for a long time (U.S. National Library of Medicine, 2017). This clearly demonstrates that the alginate-based microspheres have a huge commercial potential as cell carriers. The increase in research publications on alginate-based cell microencapsulation techniques in recent years indicate that we can expect to see significant strides in this research area using AMS, in the future.
There has also been considerable advancement in the technology used to develop AMS. Spray drying and microfluidics are available for the large-scale production of monodispersed AMS with improved chemical and mechanical stability. Various strategies like use of other cations (example - Ca2+, Mg2+, K+, Na+) for gelation, varying instrumental parameters, chemical modification with other robust polymers, and manipulation of the chains of MA and GA in terms of molecular weight are being considered to design stable and biocompatible AMS for controllable and efficient therapeutics delivery and release. Extensive characterization and optimization studies are required to manipulate and generate new groups of alginate derivatives and polymers with customized and controlled physico-chemical properties for the specific biomedical application which could transform and encourage the use of alginates as a potential carrier for various payloads. Given the various applications that AMS are already being used for due to their attractive physicochemical properties, these new classes of alginates will be significant contributors towards revolutionizing therapeutic research in the future.
Acknowledgements
We acknowledge funding support in part by the Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103430, and the University of Rhode Island Council for Research award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The author reports no conflicts of interest in this work.
References
- Acarregui A, Ciriza J, Saenz del Burgo L, Gurruchaga Iribar H, Yeste J, Illa X, Orive G, Hernández RM, Villa R, Pedraz JL, 2018. Characterization of an encapsulated insulin secreting human pancreatic beta cell line in a modular microfluidic device. J. Drug Target 26, 36–44. [DOI] [PubMed] [Google Scholar]
- Agüero L, Zaldivar-Silva D, Pena L, Dias ML, 2017. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym 168, 32–43. [DOI] [PubMed] [Google Scholar]
- Aguilar LMC, Kapsa RM, O’Connell CD, McArthur SL, Stoddart PR, Moulton SE, 2019. Controlled release from PCL–alginate microspheres via secondary encapsulation using GelMA/HAMA hydrogel scaffolds. Soft Matter 15, 3779–3787. [DOI] [PubMed] [Google Scholar]
- Aguirre Calvo T, Santagapita P, 2016. Physicochemical characterization of alginate beads containing sugars and biopolymers. J. Qual. Reliab. Eng 2016. [Google Scholar]
- Ahn S, Kim G, 2015. Cell-encapsulating alginate microsized beads using an air-assisted atomization process to obtain a cell-laden hybrid scaffold. J. Mater. Chem. B 3, 9132–9139. [DOI] [PubMed] [Google Scholar]
- Alizadeh Sardroud H, Nemati S, Baradar Khoshfetrat A, Nabavinia M, Beygi Khosrowshahi Y, 2017. Barium-cross-linked alginate-gelatine microcapsule as a potential platform for stem cell production and modular tissue formation. J. Microencapsul 34, 488–497. [DOI] [PubMed] [Google Scholar]
- Anandharamakrishnan C, Rielly CD, Stapley AGF, 2007. Effects of process variables on the denaturation of whey proteins during spray drying. Dry. Technol 25, 799–807. [Google Scholar]
- Ashton RS, Banerjee A, Punyani S, Schaffer DV, Kane RS, 2007. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials 28, 5518–5525. [DOI] [PubMed] [Google Scholar]
- Augst AD, Kong HJ, Mooney DJ, 2006. Alginate hydrogels as biomaterials. Macromol. Biosci 6, 623–633. [DOI] [PubMed] [Google Scholar]
- Bagheri L, Madadlou A, Yarmand M, Mousavi ME, 2014. Spray-dried alginate microparticles carrying caffeine-loaded and potentially bioactive nanoparticles. Food Res. Int 62, 1113–1119. [Google Scholar]
- Baimark Y, Srisuwan Y, 2014. Preparation of alginate microspheres by water-in-oil emulsion method for drug delivery: Effect of Ca2+ post-cross-linking. Adv. Powder Technol 25, 1541–1546. [Google Scholar]
- Ballesteros NA, Alonso M, Saint-Jean SR, Perez-Prieto SI, 2015. An oral DNA vaccine against infectious haematopoietic necrosis virus (IHNV) encapsulated in alginate microspheres induces dose-dependent immune responses and significant protection in rainbow trout (Oncorrhynchus mykiss). Fish Shellfish Immunol 45, 877–888. [DOI] [PubMed] [Google Scholar]
- Banskota S, Yousefpour P, Kirmani N, Li X, Chilkoti A, 2019. Long circulating genetically encoded intrinsically disordered zwitterionic polypeptides for drug delivery. Biomaterials 192, 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer EA, Jordan J, Roy A, Gottardi R, Fedorchak MV, Kumta PN, Little SR, 2017. Programmed Platelet-Derived Growth Factor-BB and Bone Morphogenetic Protein-2 Delivery from a Hybrid Calcium Phosphate/Alginate Scaffold. Tissue Eng. Part A 23, 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TA, Kipke DR, Brandon T, 2001. Calcium alginate gel: a biocompatible and mechanically stable polymer for endovascular embolization. J. Biomed. Mater. Res. An Off. J. Soc. Biomater. Japanese Soc. Biomater 54, 76–86. [DOI] [PubMed] [Google Scholar]
- Benavides S, Cortés P, Parada J, Franco W, 2016. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem 204, 77–83. [DOI] [PubMed] [Google Scholar]
- Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA, 2011. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32, 6425–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blemur L, Le TC, Marcocci L, Pietrangeli P, Mateescu MA, 2016. Carboxymethyl starch/alginate microspheres containing diamine oxidase for intestinal targeting. Biotechnol. Appl. Biochem 63, 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, 2018. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng 2, 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büyüköz M, Erdal E, Alsoy Altinkaya S, 2018. Nanofibrous gelatine scaffolds integrated with nerve growth factor- loaded alginate microspheres for brain tissue engineering. J. Tissue Eng. Regen. Med 12, e707–e719. [DOI] [PubMed] [Google Scholar]
- Cañibano-Hernández A, del Burgo LS, Espona-Noguera A, Orive G, Hernández RM, Ciriza J, Pedraz JL, 2019. Hyaluronic acid enhances cell survival of encapsulated insulin-producing cells in alginate-based microcapsules. Int. J. Pharm 557, 192–198. [DOI] [PubMed] [Google Scholar]
- Chandy T, Mooradian DL, Rao GHR, 1999. Evaluation of modified alginate- chitosan- polyethylene glycol microcapsules for cell encapsulation. Artif. Organs 23, 894–903. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Ke C-J, Yen K-C, Hsieh H-C, Sun J-S, Lin F-H, 2015. 3D porous calcium-alginate scaffolds cell culture system improved human osteoblast cell clusters for cell therapy. Theranostics 5, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-Y, Zheng W, Dong Q-Y, Li Z-H, Shi L-E, Tang Z-X, 2014. Activity of encapsulated Lactobacillus bulgaricus in alginate-whey protein microspheres. Brazilian Arch. Biol. Technol 57, 736–741. [Google Scholar]
- Chen Z, Lv X, Zhao M, Zhang P, Ren X, Mei X, 2018. Encapsulation of green tea polyphenol by pH responsive, antibacterial, alginate microgels used for minimally invasive treatment of bone infection. Colloids Surfaces B Biointerfaces 170, 648–655. [DOI] [PubMed] [Google Scholar]
- Ching SH, Bansal N, Bhandari B, 2017. Alginate gel particles–A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr 57, 1133–1152. [DOI] [PubMed] [Google Scholar]
- Dalmoro A, Barba AA, d’Amore M, Lamberti G, 2014. Single-pot semicontinuous bench scale apparatus to produce microparticles. Ind. Eng. Chem. Res 53, 2771–2780. [Google Scholar]
- Dalmoro A, Barba AA, Lamberti G, d’Amore M, 2012. Intensifying the microencapsulation process: Ultrasonic atomization as an innovative approach. Eur. J. Pharm. Biopharm 80, 471–477. [DOI] [PubMed] [Google Scholar]
- Dalmoro A, Sitenkov AY, Cascone S, Lamberti G, Barba AA, Moustafine RI, 2017. Hydrophilic drug encapsulation in shell-core microcarriers by two stage polyelectrolyte complexation method. Int. J. Pharm 518, 50–58. [DOI] [PubMed] [Google Scholar]
- Darrabie MD, Kendall WF Jr, Opara EC, 2005. Characteristics of poly-L-ornithine-coated alginate microcapsules. Biomaterials 26, 6846–6852. [DOI] [PubMed] [Google Scholar]
- Dimitrov DS, 2012. Therapeutic proteins, in: Therapeutic Proteins Springer, pp. 1–26. [Google Scholar]
- Ding D, Zhang Y, Sykes EA, Chen L, Chen Z, Tan W, 2019. The influence of physiological environment on the targeting effect of aptamer-guided gold nanoparticles. Nano Res 12, 129–135. 10.1007/s12274-018-2191-9 [DOI] [Google Scholar]
- Ding S-L, Zhang M-Y, Tang S-J, Yang H, Tan W-Q, 2015. Effect of calcium alginate microsphere loaded with vascular endothelial growth factor on adipose tissue transplantation. Ann. Plast. Surg 75, 644–651. [DOI] [PubMed] [Google Scholar]
- Dong X, Liu H-J, Feng H-Y, Yang S-C, Liu X-L, Lai X, Lu Q, Lovell JF, Chen H-Z, Fang C, 2019. Enhanced Drug Delivery by Nanoscale Integration of a Nitric Oxide Donor to Induce Tumor Collagen Depletion. Nano Lett [DOI] [PubMed]
- Dounighi N, Shahcheraghi F, Razzaghi-Abyaneh M, Nofeli M, Zolfagharian H, 2017. A New Vaccine Delivery Vehicle and Adjuvant Candidate: Bordetella pertussis Inactivated Whole Cells Entrapped in Alginate Microspheres. Curr. Pharm. Des 23, 2665–2672. [DOI] [PubMed] [Google Scholar]
- Etter JN, Karasinski M, Ware J, Oldinski RA, 2018. Dual-crosslinked homogeneous alginate microspheres for mesenchymal stem cell encapsulation. J. Mater. Sci. Mater. Med 29, 143. [DOI] [PubMed] [Google Scholar]
- Ganesh M, Jeon UJ, Ubaidulla U, Hemalatha P, Saravanakumar A, Peng MM, Jang HT, 2015. Chitosan cocrystals embedded alginate beads for enhancing the solubility and bioavailability of aceclofenac. Int. J. Biol. Macromol 74, 310–317. [DOI] [PubMed] [Google Scholar]
- Giudice EL, Campbell JD, 2006. Needle-free vaccine delivery. Adv. Drug Deliv. Rev 58, 68–89. [DOI] [PubMed] [Google Scholar]
- Gombotz WR, Wee S, 1998. Protein release from alginate matrices. Adv. Drug Deliv. Rev 31, 267–285. [DOI] [PubMed] [Google Scholar]
- Grellier M, Granja PL, Fricain J-C, Bidarra SJ, Renard M, Bareille R, Bourget C, Amédée J, Barbosa MA, 2009. The effect of the co-immobilization of human osteoprogenitors and endothelial cells within alginate microspheres on mineralization in a bone defect. Biomaterials 30, 3271–3278. [DOI] [PubMed] [Google Scholar]
- Guo Y-H, Zhao S, Du Y-X, Xing Q-J, Chen B-L, Yu C-Q, 2017. Effects of ginsenoside Rg1-loaded alginate-chitosan microspheres on human bone marrow stromal cells. Biosci. Rep 37, BSR20160566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder A, Maiti S, Sa B, 2005. Entrapment efficiency and release characteristics of polyethyleneimine-treated or-untreated calcium alginate beads loaded with propranolol–resin complex. Int. J. Pharm 302, 84–94. [DOI] [PubMed] [Google Scholar]
- Hariyadi DM, Ma Y, Wang Y, Bostrom T, Malouf J, Turner MS, Bhandari B, Coombes AGA, 2014. The potential for production of freeze-dried oral vaccines using alginate hydrogel microspheres as protein carriers. J. Drug Deliv. Sci. Technol 24, 178–184. [Google Scholar]
- Huang Feng-ying, Huang Feng-ru, Chen B, Liu Q, Wang H, Zhou S, Zhao H, Huang Y, Lin Y, Tan G, 2015. Microencapsulation of tumor lysates and live cell engineering with MIP-3α as an effective vaccine. Biomaterials 53, 554–565. [DOI] [PubMed] [Google Scholar]
- Huang K-S, Lai T-H, Lin Y-C, 2006. Manipulating the generation of Ca-alginate microspheres using microfluidic channels as a carrier of gold nanoparticles. Lab Chip 6, 954–957. [DOI] [PubMed] [Google Scholar]
- Hussein N, Omer H, Ismael A, Albed Alhnan M, Elhissi A, Ahmed W, 2019. Spray-Dried Alginate Microparticles for Potential Intranasal Delivery of Ropinirole Hydrochloride: Development, Characterization and Histopathological Evaluation. Pharm. Dev. Technol 1–36. [DOI] [PubMed]
- Jain RR, Mehta MR, Bannalikar AR, Menon MD, 2015. Alginate microparticles loaded with lipopolysaccharide subunit antigen for mucosal vaccination against Klebsiella pneumoniae. Biologicals 43, 195–201. [DOI] [PubMed] [Google Scholar]
- Jaya S, Durance TD, Wang R, 2009. Effect of alginate-pectin composition on drug release characteristics of microcapsules. J. Microencapsul 26, 143–153. 10.1080/02652040802211345 [DOI] [PubMed] [Google Scholar]
- Jung SM, Lee JH, Lee HJ, Jeon JY, Park TH, Yoon JH, Shin HW, 2018. The growth of alginate-encapsulated macroalgal spores. Aquaculture 491, 333–337. [Google Scholar]
- Jurić S, Đermić E, Topolovec-Pintarić S, Bedek M, Vinceković M, 2019. Physicochemical properties and release characteristics of calcium alginate microspheres loaded with Trichoderma viride spores. J. Integr. Agric
- Kanafi MM, Ramesh A, Gupta PK, Bhonde RR, 2014. Dental pulp stem cells immobilized in alginate microspheres for applications in bone tissue engineering. Int. Endod. J 47, 687–697. [DOI] [PubMed] [Google Scholar]
- Kim B, Bowersock T, Griebel P, Kidane A, Babiuk LA, Sanchez M, Attah-Poku S, Kaushik RS, Mutwiri GK, 2002. Mucosal immune responses following oral immunization with rotavirus antigens encapsulated in alginate microspheres. J. Control. release 85, 191–202. [DOI] [PubMed] [Google Scholar]
- Koch S, Schwinger C, Kressler J, Heinzen CH, Rainov NG, 2003. Alginate encapsulation of genetically engineered mammalian cells: comparison of production devices, methods and microcapsule characteristics. J. Microencapsul 20, 303–316. [DOI] [PubMed] [Google Scholar]
- Lamas MC, Bregni C, D’Aquino M, Degrossi J, Firenstein R, 2001. Calcium alginate microspheres of Bacillus subtilis. Drug Dev. Ind. Pharm 27, 825–829. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kim H, Lee KY, 2016. Effect of spacer arm length between adhesion ligand and alginate hydrogel on stem cell differentiation. Carbohydr. Polym 139, 82–89. [DOI] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ, 2012. Alginate: properties and biomedical applications. Prog. Polym. Sci 37, 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Wang B, Huang Y, Qu Y, Peng J, Qian Z, 2017. Injectable alginate hydrogel cross-linked by calcium gluconate-loaded porous microspheres for cartilage tissue engineering. ACS omega 2, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-H, Liang H-F, Chung C-K, Chen M-C, Sung H-W, 2005. Physically crosslinked alginate/N, O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials 26, 2105–2113. [DOI] [PubMed] [Google Scholar]
- Ling K, Wu H, Neish AS, Champion JA, 2019. Alginate/chitosan microparticles for gastric passage and intestinal release of therapeutic protein nanoparticles. J. Control. Release 295, 174–186. [DOI] [PubMed] [Google Scholar]
- Liu F, Ma C, Gao Y, McClements DJ, 2017. Food- Grade Covalent Complexes and Their Application as Nutraceutical Delivery Systems: A Review. Compr. Rev. Food Sci. Food Saf 16, 76–95. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Lan Y, Zuo Q, Li C, Zhang Y, Guo R, Xue W, 2017. Acceleration of skin regeneration in full- thickness burns by incorporation of bFGF- loaded alginate microspheres into a CMCS–PVA hydrogel. J. Tissue Eng. Regen. Med 11, 1562–1573. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zuo Q, Guo R, Hong A, Li C, Zhang Y, He L, Xue W, 2015. Fabrication and characterization of carboxymethyl chitosan/poly (vinyl alcohol) hydrogels containing alginate microspheres for protein delivery. J. Bioact. Compat. Polym 30, 397–411. [Google Scholar]
- Lopes M, Abrahim B, Veiga F, Seiça R, Cabral LM, Arnaud P, Andrade JC, Ribeiro AJ, 2017. Preparation methods and applications behind alginate-based particles. Expert Opin. Drug Deliv 14, 769–782. 10.1080/17425247.2016.1214564 [DOI] [PubMed] [Google Scholar]
- Lourenço AH, Neves N, Ribeiro-Machado C, Sousa SR, Lamghari M, Barrias CC, Cabral AT, Barbosa MA, Ribeiro CC, 2017. Injectable hybrid system for strontium local delivery promotes bone regeneration in a rat critical-sized defect model. Sci. Rep 7, 5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo B, Maestro A, Porras M, Gutiérrez JM, González C, 2014. Preparation of alginate microspheres by emulsification/internal gelation to encapsulate cocoa polyphenols. Food Hydrocoll 38, 56–65. [Google Scholar]
- Ma Y, Pacan JC, Wang Q, Sabour PM, Huang X, Xu Y, 2012. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll 26, 434–440. [Google Scholar]
- Ma Y, Pacan JC, Wang Q, Xu Y, Huang X, Korenevsky A, Sabour PM, 2008. Microencapsulation of bacteriophage Felix O1 into chitosan-alginate microspheres for oral delivery. Appl. Environ. Microbiol 74, 4799–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida-Sano I, Hirakawa M, Matsumoto H, Kamada M, Ogawa S, Satoh N, Namiki H, 2014. Surface characteristics determining the cell compatibility of ionically cross-linked alginate gels. Biomed. Mater 9, 25007. [DOI] [PubMed] [Google Scholar]
- Maestrelli F, Mura P, González-Rodríguez ML, Cózar-Bernal MJ, Rabasco AM, Mannelli LDC, Ghelardini C, 2017. Calcium alginate microspheres containing metformin hydrochloride niosomes and chitosomes aimed for oral therapy of type 2 diabetes mellitus. Int. J. Pharm 530, 430–439. [DOI] [PubMed] [Google Scholar]
- Mallikarjuna Setty C, Sahoo SS, Sa B, 2005. Alginate-coated alginate-polyethyleneimine beads for prolonged release of furosemide in simulated intestinal fluid. Drug Dev. Ind. Pharm 31, 435–446. [DOI] [PubMed] [Google Scholar]
- Mansouri V, Salehi M, davood Omrani M, Niknam Z, Ardeshirylajimi A, 2017. Collagen-alginate microspheres as a 3D culture system for mouse embryonic stem cells differentiation to primordial germ cells. Biologicals 48, 114–120. [DOI] [PubMed] [Google Scholar]
- Martín MJ, Calpena AC, Fernández F, Mallandrich M, Gálvez P, Clares B, 2015. Development of alginate microspheres as nystatin carriers for oral mucosa drug delivery. Carbohydr. Polym 117, 140–149. [DOI] [PubMed] [Google Scholar]
- Martins S, Sarmento B, Souto EB, Ferreira DC, 2007. Insulin-loaded alginate microspheres for oral delivery–effect of polysaccharide reinforcement on physicochemical properties and release profile. Carbohydr. Polym 69, 725–731. [Google Scholar]
- Md S, Ahuja A, Khar RK, Baboota S, Chuttani K, Mishra AK, Ali J, 2011. Gastroretentive drug delivery system of acyclovir-loaded alginate mucoadhesive microspheres: formulation and evaluation. Drug Deliv 18, 255–264. [DOI] [PubMed] [Google Scholar]
- Mehta AS, Singh BK, Singh N, Archana D, Snigdha K, Harniman R, Rahatekar SS, Tewari RP, Dutta PK, 2015. Chitosan silk-based three-dimensional scaffolds containing gentamicin-encapsulated calcium alginate beads for drug administration and blood compatibility. J. Biomater. Appl 29, 1314–1325. [DOI] [PubMed] [Google Scholar]
- Meier RPH, Mahou R, Morel P, Meyer J, Montanari E, Muller YD, Christofilopoulos P, Wandrey C, Gonelle-Gispert C, Bühler LH, 2015. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol 62, 634–641. [DOI] [PubMed] [Google Scholar]
- Melvik JE, Dornish M, 2004. Alginate as a carrier for cell immobilisation, in: Fundamentals of Cell Immobilisation Biotechnology Springer, pp. 33–51. [Google Scholar]
- Mennini N, Furlanetto S, Cirri M, Mura P, 2012. Quality by design approach for developing chitosan-Ca-alginate microspheres for colon delivery of celecoxib-hydroxypropyl-β-cyclodextrin-PVP complex. Eur. J. Pharm. Biopharm 80, 67–75. [DOI] [PubMed] [Google Scholar]
- Mørch ÝA, Donati I, Strand BL, Skjåk-Bræk G, 2006. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 7, 1471–1480. [DOI] [PubMed] [Google Scholar]
- Nagpal M, Maheshwari DK, Rakha P, Dureja H, Goyal S, Dhingra G, 2012. Formulation development and evaluation of alginate microspheres of ibuprofen. J. Young Pharm 4, 13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opara EC, McQuilling JP, Farney AC, 2013. Microencapsulation of pancreatic islets for use in a bioartificial pancreas, in: Organ Regeneration Springer, pp. 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G, Hernández RM, Gascón AR, Calafiore R, Chang TMS, De Vos P, Hortelano G, Hunkeler D, Lacík I, Shapiro AMJ, 2003. Cell encapsulation: promise and progress. Nat. Med 9, 104. [DOI] [PubMed] [Google Scholar]
- Patil SB, Sawant KK, 2009. Development, optimization and in vitro evaluation of alginate mucoadhesive microspheres of carvedilol for nasal delivery. J. Microencapsul 26, 432–443. [DOI] [PubMed] [Google Scholar]
- Pawar SN, Edgar KJ, 2012. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 33, 3279–3305. [DOI] [PubMed] [Google Scholar]
- Piornos JA, Burgos-Díaz C, Morales E, Rubilar M, Acevedo F, 2017. Highly efficient encapsulation of linseed oil into alginate/lupin protein beads: Optimization of the emulsion formulation. Food Hydrocoll 63, 139–148. [Google Scholar]
- Pitaktamrong P, Kingkaew J, Yooyongwech S, Cha-um S, Phisalaphong M, 2018. Development of Arbuscular Mycorrhizal Fungi-Organic Fertilizer Pellets Encapsulated with Alginate Film. Eng. J 22, 65–79. [Google Scholar]
- Prüsse U, Bilancetti L, Bučko M, Bugarski B, Bukowski J, Gemeiner P, Lewińska D, Manojlovic V, Massart B, Nastruzzi C, 2008. Comparison of different technologies for alginate beads production. Chem. Pap 62, 364–374. [Google Scholar]
- Qi M, Mørch Y, Lacík I, Formo K, Marchese E, Wang Y, Danielson KK, Kinzer K, Wang S, Barbaro B, 2012. Survival of human islets in microbeads containing high guluronic acid alginate crosslinked with Ca2+ and Ba2+. Xenotransplantation 19, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Qin X, Yang R, Qin J, Li W, Luan K, Wu Z, Song L, 2016. Intra-articular Administration of Chitosan Thermosensitive In Situ Hydrogels Combined With Diclofenac Sodium–Loaded Alginate Microspheres. J. Pharm. Sci 105, 122–130. [DOI] [PubMed] [Google Scholar]
- Quinlan E, López-Noriega A, Thompson E, Kelly HM, Cryan SA, O’brien FJ, 2015. Development of collagen–hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J. Control. Release 198, 71–79. [DOI] [PubMed] [Google Scholar]
- Rastogi R, Sultana Y, Aqil M, Ali A, Kumar S, Chuttani K, Mishra AK, 2007. Alginate microspheres of isoniazid for oral sustained drug delivery. Int. J. Pharm 334, 71–77. [DOI] [PubMed] [Google Scholar]
- Rehm BHA, Valla S, 1997. Bacterial alginates: biosynthesis and applications. Appl. Microbiol. Biotechnol 48, 281–288. [DOI] [PubMed] [Google Scholar]
- Reis CP, Neufeld RJ, Vilela S, Ribeiro AJ, Veiga F, 2006. Review and current status of emulsion/dispersion technology using an internal gelation process for the design of alginate particles. J. Microencapsul 23, 245–257. [DOI] [PubMed] [Google Scholar]
- Sailaja G, HogenEsch H, North A, Hays J, Mittal SK, 2002. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther 9, 1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig I, Karstensen K, Rokstad AM, Aachmann FL, Formo K, Sandvig A, Skjåk-Bræk G, Strand BL, 2015. RGD- peptide modified alginate by a chemoenzymatic strategy for tissue engineering applications. J. Biomed. Mater. Res. Part A 103, 896–906. [DOI] [PubMed] [Google Scholar]
- Schneider S, Feilen PJ, Slotty V, Kampfner D, Preuss S, Berger S, Beyer J, Pommersheim R, 2001. Multilayer capsules: a promising microencapsulation system for transplantation of pancreatic islets. Biomaterials 22, 1961–1970. [DOI] [PubMed] [Google Scholar]
- Shi L-E, Li Z-H, Li D-T, Xu M, Chen H-Y, Zhang Z-L, Tang Z-X, 2013. Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng 117, 99–104. [Google Scholar]
- Shi L-E, Zheng W, Zhang Y, Tang Z-X, 2016. Milk-alginate microspheres: Protection and delivery of Enterococcus faecalis HZNU P2. LWT-Food Sci. Technol 65, 840–844. [Google Scholar]
- Shinde UA, Shete JN, Nair HA, Singh KH, 2014. Design and characterization of chitosan-alginate microspheres for ocular delivery of azelastine. Pharm. Dev. Technol 19, 813–823. [DOI] [PubMed] [Google Scholar]
- Silva CM, Ribeiro AJ, Ferreira D, Veiga F, 2006. Insulin encapsulation in reinforced alginate microspheres prepared by internal gelation. Eur. J. Pharm. Sci 29, 148–159. [DOI] [PubMed] [Google Scholar]
- Sobol M, Bartkowiak A, de Haan B, de Vos P, 2013. Cytotoxicity study of novel water- soluble chitosan derivatives applied as membrane material of alginate microcapsules. J. Biomed. Mater. Res. Part A 101, 1907–1914. [DOI] [PubMed] [Google Scholar]
- Sosnik A, 2014. Alginate particles as platform for drug delivery by the oral route: state-of-the-art. ISRN Pharm 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura S, Oda T, Izumida Y, Aoyagi Y, Satake M, Ochiai A, Ohkohchi N, Nakajima M, 2005. Size control of calcium alginate beads containing living cells using micro-nozzle array. Biomaterials 26, 3327–3331. [DOI] [PubMed] [Google Scholar]
- Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K, 2000. Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol 62, 47–55. [DOI] [PubMed] [Google Scholar]
- Szekalska M, Amelian A, Winnicka K, 2015. Alginate microspheres obtained by the spray drying technique as mucoadhesive carriers of ranitidine. Acta Pharm 65, 15–27. [DOI] [PubMed] [Google Scholar]
- Tafaghodi M, Eskandari M, Khamesipour A, Jaafari MR, 2011. Alginate microspheres encapsulated with autoclaved Leishmania major (ALM) and CpG-ODN induced partial protection and enhanced immune response against murine model of leishmaniasis. Exp. Parasitol 129, 107–114. [DOI] [PubMed] [Google Scholar]
- Tafaghodi M, Tabassi SAS, Jaafari MR, 2006. Induction of systemic and mucosal immune responses by intranasal administration of alginate microspheres encapsulated with tetanus toxoid and CpG-ODN. Int. J. Pharm 319, 37–43. [DOI] [PubMed] [Google Scholar]
- Taha MO, Aiedeh KM, Al-Hiari Y, Al-Khatib H, 2005. Synthesis of zinc-crosslinked thiolated alginic acid beads and their in vitro evaluation as potential enteric delivery system with folic acid as model drug. Die Pharm. Int. J. Pharm. Sci 60, 736–742. [PubMed] [Google Scholar]
- Tian J-Y, Sun X-Q, Chen X-G, 2008. Formation and oral administration of alginate microspheres loaded with pDNA coding for lymphocystis disease virus (LCDV) to Japanese flounder. Fish Shellfish Immunol 24, 592–599. [DOI] [PubMed] [Google Scholar]
- U.S. National Library of Medicine, 2017. Open-label Investigation of the Safety and Effectiveness of DIABECELL(R) in Patients With Type I Diabetes Mellitus [WWW Document] URL https://clinicaltrials.gov/ct2/show/NCT00940173
- Utech S, Prodanovic R, Mao AS, Ostafe R, Mooney DJ, Weitz DA, 2015. Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture. Adv. Healthc. Mater 4, 1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elk M, Ozbakir B, Barten-Rijbroek AD, Storm G, Nijsen F, Hennink WE, Vermonden T, Deckers R, 2015. Alginate microspheres containing temperature sensitive liposomes (TSL) for MR-guided embolization and triggered release of doxorubicin. PLoS One 10, e0141626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Shelton RM, Cooper PR, Lawson M, Triffitt JT, Barralet JE, 2003. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 24, 3475–3481. [DOI] [PubMed] [Google Scholar]
- Wang Xiaoqin, Wenk E, Hu X, Castro GR, Meinel L, Wang Xianyan, Li C, Merkle H, Kaplan DL, 2007. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials 28, 4161–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells LA, Sheardown H, 2007. Extended release of high pI proteins from alginate microspheres via a novel encapsulation technique. Eur. J. Pharm. Biopharm 65, 329–335. [DOI] [PubMed] [Google Scholar]
- Wen Y, Grøndahl L, Gallego MR, Jorgensen L, Møller EH, Nielsen HM, 2012. Delivery of dermatan sulfate from polyelectrolyte complex-containing alginate composite microspheres for tissue regeneration. Biomacromolecules 13, 905–917. [DOI] [PubMed] [Google Scholar]
- Wong CY, Al-Salami H, Dass CR, 2018. Microparticles, microcapsules and microspheres: a review of recent developments and prospects for oral delivery of insulin. Int. J. Pharm 537, 223–244. [DOI] [PubMed] [Google Scholar]
- Wu J, Kong T, Yeung KWK, Shum HC, Cheung KMC, Wang L, To MKT, 2013. Fabrication and characterization of monodisperse PLGA–alginate core–shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater 9, 7410–7419. [DOI] [PubMed] [Google Scholar]
- Yang M, Yang T, Jia J, Lu T, Wang H, Yan X, Wang L, Yu L, Zhao Y, 2018. Fabrication and characterization of DDAB/PLA-alginate composite microcapsules as single-shot vaccine. RSC Adv 8, 13612–13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chen H, Jiang L, Wang Jianhong, Dai J, Wang Jie, 2019. Codelivery of Adriamycin and P-gp Inhibitor Quercetin Using PEGylated Liposomes to Overcome Cancer Drug Resistance. J. Pharm. Sci [DOI] [PubMed]
- Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, Wu JC, Lee RJ, 2010. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 31, 7012–7020. [DOI] [PubMed] [Google Scholar]
- Yu L, Ni C, Grist SM, Bayly C, Cheung KC, 2015. Alginate core-shell beads for simplified three-dimensional tumor spheroid culture and drug screening. Biomed. Microdevices 17, 33. [DOI] [PubMed] [Google Scholar]
- Zhai P, Chen XB, Schreyer DJ, 2015. PLGA/alginate composite microspheres for hydrophilic protein delivery. Mater. Sci. Eng C 56, 251–259. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Su Y, Chen D, Zhong W, 2016. A doxorubicin delivery system: samarium/mesoporous bioactive glass/alginate composite microspheres. Mater. Sci. Eng. C 67, 205–213. [DOI] [PubMed] [Google Scholar]
- Zhao D, Li J-S, Suen W, Chang M-W, Huang J, 2016. Preparation and characterization of Ganoderma lucidum spores-loaded alginate microspheres by electrospraying. Mater. Sci. Eng. C 62, 835–842. [DOI] [PubMed] [Google Scholar]
- Zheng X, Yang X, Li X, Qiu G-H, Dai A, Huang Q, Huang C, Guo X, 2017. Omp16-based vaccine encapsulated by alginate-chitosan microspheres provides significant protection against Haemophilus parasuis in mice. Vaccine 35, 1417–1423. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang J, Wu J, Zhang J, Wan Y, Wu H, 2016. Injectable hydrogels embedded with alginate microspheres for controlled delivery of bone morphogenetic protein-2. Biomed. Mater 11, 25010. [DOI] [PubMed] [Google Scholar]
- Zuo Q, Guo R, Liu Q, Hong A, Shi Y, Kong Q, Huang Y, He L, Xue W, 2015. Heparin-conjugated alginate multilayered microspheres for controlled release of bFGF. Biomed. Mater 10, 35008. [DOI] [PubMed] [Google Scholar]


