Abstract
Carrageenans are thickening and gelling agents that may provide health benefits. Iota (ι)-carrageenan, a linear sulfated polysaccharide, is produced by the red seaweed, Sarconema filiforme. This study investigated the potential of this seaweed as a functional food for the reversal of metabolic syndrome and possible mechanisms. Male Wistar rats were divided into four groups in a 16-week protocol: corn starch diet-fed rats (C); C rats supplemented with 5% S. filiforme for the last 8 weeks (CSF); high-carbohydrate, high-fat diet-fed rats (H); and H rats supplemented with 5% S. filiforme for the last 8 weeks (HSF). S. filiforme was produced in tank-based aquaculture yielding 27 g dry weight/day/m2 of culture area. H rats developed obesity, hypertension, dyslipidaemia, glucose intolerance, fatty liver and increased left ventricular collagen deposition. S. filiforme supplementation decreased body weight, abdominal and liver fat, systolic blood pressure, plasma total cholesterol concentrations, and plasma activities of alanine transaminase and aspartate transaminase. S. filiforme supplementation modulated gut microbiota without changing the Firmicutes to Bacteroidetes ratio. S. filiforme improved symptoms of high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Possible mechanisms include a reduced infiltration of inflammatory cells into organs as well as prebiotic actions in the gastrointestinal tract.
Keywords: algae, Sarconema filiforme, sulfated polysaccharides, ι-carrageenan, prebiotics, gut microbiota, aquaculture, nutraceutical
1. Introduction
Carrageenans are a group of high molecular weight (>100 kDa) sulfated polygalactans isolated from red seaweeds (Rhodophyceae) that are “generally regarded as safe” for routine use as gelling and thickening agents in foods [1]. The three types of carrageenans, named kappa (κ), iota (ι) and lambda (λ), have one, two and three sulfate groups per disaccharide unit, respectively [1]. The major commercially cultivated warm-water species for carrageenans are Kappaphycus alvarezii and Eucheuma denticulatum [2], producing κ- and ι-carrageenans, respectively. These seaweeds are grown on a commercial scale primarily in Indonesia, the Philippines, Malaysia, Brazil and Tanzania [3]. Sarconema filiforme is a red seaweed containing ι-carrageenan, distributed throughout the tropical and subtropical Indo-Pacific, including along the eastern and western coasts of Australia [4,5].
The health benefits of red seaweeds include obesity reduction, decreased lipid absorption and modification of the binding of cholesterol in the gastrointestinal tract thereby reducing cardiovascular disease risk [1,6]. However, there have been no studies on Sarconema species or their major constituents in metabolic syndrome for reduction of obesity, hypertension, fatty liver, hyperlipidaemia or diabetes. Recently, 0.12% ι-carrageenan has been used in a nasal spray in patients against rhinitis, demonstrating safety and efficacy [7]. In our previous study, rats fed with κ-carrageenan-producing K. alvarezii showed normalised body weight and adiposity, lowered systolic blood pressure, improved heart and liver structure, and lowered plasma lipids, compared to diet-induced obese rats [8].
The aim of the present study was to determine changes in parameters defining cardiovascular and metabolic health following chronic consumption of S. filiforme, produced through intensive tank-based cultivation, as a potential commercial source of ι-carrageenan. Firstly, we evaluated the proximate and elemental composition of S. filiforme. Secondly, a validated diet-induced rat model of metabolic syndrome that closely mimics the symptoms of human metabolic syndrome was used to measure cardiovascular and metabolic parameters. We measured systolic blood pressure, diastolic stiffness, cardiac inflammatory cells and collagen deposition in the heart for cardiovascular effects; plasma liver enzyme activities, inflammatory cells and fat vacuoles in the liver for liver effects; and body weight, total cholesterol and triglyceride concentrations, and glucose and insulin tolerance tests for metabolic effects. Further, we characterised the microbial composition of faecal samples after seaweed treatment, since functional foods may reverse obesity-induced cardiometabolic changes through alterations in the gut microbiota [9]. We hypothesised that 5% S. filiforme supplementation for the last 8 weeks will reverse the changes induced by the high-carbohydrate, high-fat diet to a greater extent than K. alvarezii due to the additional sulfate group in the disaccharide units of ι-carrageenan. The mechanisms of these effects could include the actions of ι-carrageenan as a prebiotic in the colon and to prevent infiltration of inflammatory cells into organs such as the heart and liver.
2. Results
2.1. Preparation of S. filiforme Powder and Analysis
S. filiforme samples were taken from batches grown in the Austral summer during January, February and March 2018 with an overall average yield of 27 g dry weight/day/m2 (Figure 1A). This equates to weekly production of 189 g dry weight/m2 of available culture area for intensive land-based production of the seaweed. In the current study, high-carbohydrate, high-fat diet-fed rats treated with S. filiforme (HSF) consumed 5% S. filiforme for the last eight weeks (~1.05 g/day). Using the Reagan-Shaw calculation for rat-to-human scaling, this equates to approximately 6.3 g dry weight of seaweed/day for humans [10]. For perspective, based on the overall average yield and with 42 m2 of culture area, the University of the Sunshine Coast facility at Bribie Island could provide continuous seaweed at this dose for 179 people; at this overall yield, 5.7 km2 of culture area could provide continuous seaweed for the current Australian population. The seaweed powder contained (in % dry weight): 34.4% carbohydrates (comprising 21.7% total dietary fibre, including 9.6% insoluble fibre and 12.2% of soluble fibre, almost all as ι-carrageenan), 11.8% protein, 1.4% lipid, 50.8% ash and 1.6% moisture (Table 1). Elemental analysis showed 21.2% C, 3.3% H, 2.6% N, 4.2% S, 18.9% K and 2.7% Na (Table 1). The energy content was 8.7 kJ/g.
Figure 1.
(A) Biomass yields of Sarconema filiforme between January and March 2018. Data show means ± SEM, n = 20–27 weekly growth measurements from 1000 L outdoor tanks from each month. (B) Attenuated Total Reflectance-Fourier-Transform Infrared Spectroscopy (ATR-FTIR) transmittance from 950 to 675 cm−1 of ι-carrageenan (blue line), κ-carrageenan (red line), Kappaphycus alvarezii (green line) and Sarconema filiforme (orange line). Far left rectangle showing 900–905 cm−1, middle rectangle showing 820-850 cm−1 and far right rectange showing 800–805 cm−1.
Table 1.
Biochemical composition of Sarconema filiforme biomass.
| Proximate | % dry weight | Metals | mg/kg |
| Carbohydrate | 34.4 | ||
| Protein | 11.8 | Aluminium | 39.3 |
| Lipid | 1.4 | Antimony | 0.1 |
| Ash | 50.8 | Arsenic | 6.2 |
| Moisture | 1.6 | Barium | 0.5 |
| Energy (kJ/g) | 8.7 | Boron | 299 |
| Fibre | % dry weight | Cadmium | 0.1 |
| Total dietary fibre | 21.7 | Calcium | 2150 |
| Insoluble dietary fibre | 9.6 | Chromium | 0.3 |
| Soluble dietary fibre (by difference) | 12.2 | Cobalt | 0.6 |
| Ultimate | % dry weight | Copper | 1.6 |
| Carbon | 21.2 | Iron | 1875 |
| Nitrogen | 2.6 | Lead | 0.2 |
| Hydrogen | 3.3 | Magnesium | 3655 |
| Sulfur | 4.2 | Manganese | 20.3 |
| Amino acids | % dry weight | Mercury | 0.3 |
| Total amino acids | 11.8 | Molybdenum | 0.2 |
| Histidine | 0.2 | Nickel | 0.7 |
| Serine | 0.7 | Phosphorus | 2500 |
| Arginine | 1.0 | Potassium | 189,500 |
| Glycine | 0.7 | Selenium | 0.3 |
| Aspartic acid | 1.3 | Silver | 0.0 |
| Glutamic acid | 1.8 | Sodium | 27,000 |
| Threonine | 0.6 | Strontium | 31.3 |
| Alanine | 0.8 | Tin | 0.1 |
| Proline | 0.6 | Vanadium | 2.4 |
| Lysine | 0.6 | Zinc | 128 |
| Tyrosine | 0.3 | Fatty acids | % dry weight |
| Methionine | 0.2 | ||
| Valine | 0.8 | Total fatty acids | 1.1 |
| Isoleucine | 0.7 | C16:0 palmitic | 0.4 |
| Leucine | 1.0 | C20:4 ω-6 arachidonic | 0.5 |
| Phenylalanine | 0.6 | C20:5 ω-3 eicosapentaenoic | 0.1 |
Values are mean of two sub-samples of blended biomass.
2.2. ATR-FTIR of S. filiforme and K. alvarezii
The normalised transmission spectra of reference samples of ι-carrageenan and κ-carrageenan are given with those of S. filiforme and K. alvarezii (Figure 1B). The peak at approximately 800–805 cm−1 is most prominent in ι-carrageenan, which is indicative of 3,6-anhydro-d-galactose 2-sulfate and the bands between 820 and 850 cm−1 are indicative of galactose 2-sulfate, galactose 4-sulfate and galactose 2,6-disulfate, which are characteristic of carrageenans. The band at ~800–805 cm−1 is more prominent in ι-carrageenan and S. filiforme samples. The band at ~905–910 cm−1 is indicative of anhydro-galactose-2-sulfate which is consistent between ι-carrageenan and S. filiforme samples.
2.3. Physiological and Metabolic Responses
Food intake was higher in corn starch diet-fed rats (C) compared to high-carbohydrate, high-fat diet-fed rats (H). HSF had lower food intake than H rats (Table 2). The body weight of H rats was greater than C rats and that of HSF rats was lower than H rats (Figure 2A). Lean mass was unchanged in all groups. Fat mass measurements were consistent with body weight measurements (Table 2).
Table 2.
Responses to Sarconema filiforme.
| Variables | C | CSF | H | HSF | p Value | ||
|---|---|---|---|---|---|---|---|
| Diet | Treatment | Interaction | |||||
| Physiological variables | |||||||
| 0 week body weight, g | 338 ± 1 | 338 ± 1 | 339 ± 1 | 338 ± 1 | 0.66 | 0.66 | 0.66 |
| 8 week body weight, g | 371 ± 11b | 359 ± 5b | 451 ± 15a | 442 ± 10a | <0.0001 | 0.31 | 0.88 |
| 16 week body weight, g | 405 ± 4c | 398 ± 5c | 550 ± 15a | 498 ± 10b | <0.0001 | 0.004 | 0.025 |
| 16 week lean mass, g | 321 ± 9 | 324 ± 7 | 309 ± 10 | 338 ± 12 | 0.92 | 0.13 | 0.22 |
| 16 week fat mass, g | 59 ± 4c | 58 ± 6c | 251 ± 30a | 151 ± 16b | <0.0001 | 0.004 | 0.005 |
| 8 week lean/fat mass proportion | 4.4 ± 0.5b | 6.8 ± 0.8a | 2.2 ± 0.4c | 2.3 ±0.2c | <0.0001 | 0.056 | 0.08 |
| 16 week lean/fat mass proportion | 5.5 ± 0.4b | 6.0 ± 0.6a | 1.3 ± 0.1d | 2.5 ± 0.4c | <0.0001 | 0.047 | 0.93 |
| 16 week bone mineral content, g | 12.3 ± 0.8c | 12.1 ± 0.5c | 17.6 ± 0.7a | 14.6 ± 0.6b | <0.0001 | 0.021 | 0.041 |
| 16 week bone mineral density, g/cm2 | 0.180 ± 0.002 | 0.183 ± 0.004 | 0.184 ± 0.005 | 0.184 ± 0.002 | 0.53 | 0.70 | 0.70 |
| Food intake 0-8 weeks, g/day | 41.4 ± 0.9a | 39.0 ± 1.4a | 27.8 ± 0.9b | 26.5 ± 1.3b | <0.0001 | 0.21 | 0.71 |
| Food intake 9-16 weeks, g/day | 37.1 ± 0.4a | 37.0 ± 1.1a | 26.2 ± 0.7b | 20.9 ± 0.8c | <0.0001 | 0.012 | 0.015 |
| Water intake 0-8 weeks, g/day | 40.8 ± 4.4a | 32.7 ± 2.8b | 25.5 ± 0.8c | 29.3 ± 2.7b | 0.0061 | 0.51 | 0.07 |
| Water intake 9-16 weeks, g/day | 30.4 ± 3.8b | 42.3 ± 1.4a | 22.2 ± 0.6c | 41.8 ± 2.1a | 0.07 | <0.0001 | 0.11 |
| Energy intake 0-8 weeks, kJ/day | 468 ± 9b | 438 ±16b | 593 ± 13a | 597 ± 35a | <0.0001 | 0.65 | 0.55 |
| Energy intake 9-16 weeks, kJ/day | 415 ± 4b | 407 ± 13b | 558 ± 10a | 533 ± 17a | <0.0001 | 0.18 | 0.93 |
| 16 week abdominal circumference, cm | 20.1 ± 0.4c | 19.4 ± 0.1c | 23.8 ± 0.5a | 21.2 ± 0.3b | <0.0001 | <0.0001 | 0.005 |
| Body mass index, g/cm2 | 0.61 ± 0.02c | 0.65 ± 0.01c | 0.81 ± 0.03a | 0.74 ± 0.02b | <0.0001 | 0.46 | 0.011 |
| Retroperitoneal fat, mg/mm | 216 ± 37c | 196 ± 15c | 636 ± 67a | 423 ± 41b | <0.0001 | 0.007 | 0.025 |
| Epididymal fat, mg/mm | 78 ± 18c | 62 ± 9c | 191 ± 28a | 116 ± 12b | <0.0001 | 0.007 | 0.07 |
| Omental fat, mg/mm | 147 ± 21c | 142 ± 12c | 333 ± 29a | 245 ± 16b | <0.0001 | 0.019 | 0.035 |
| Visceral adiposity, % | 5.1 ± 0.6c | 4.8 ± 0.4c | 10.0 ± 0.6a | 7.8 ± 0.3b | <0.0001 | 0.011 | 0.048 |
| Liver wet weight, mg/mm | 218 ± 7b | 231 ± 7b | 365 ± 26a | 343 ± 16a | <0.0001 | 0.77 | 0.27 |
| Cardiovascular variables | |||||||
| 8 week systolic blood pressure, mmHg | 123 ± 4b | 122 ± 3b | 135 ± 3a | 138 ± 2a | 0.0001 | 0.76 | 0.54 |
| 16 week systolic blood pressure, mmHg | 128 ± 3b | 126 ± 4b | 145 ± 4a | 132 ± 3b | 0.007 | 0.07 | 0.18 |
| Left ventricle + septum wet weight, mg/mm | 22.6 ± 2.1 | 22.5 ± 1.0 | 21.2 ± 2.2 | 21.5 ± 0.6 | 0.38 | 0.94 | 0.88 |
| Right ventricle, mg/mm | 4.1 ± 0.3 | 3.5 ± 0.3 | 4.4 ± 0.1 | 4.3 ± 0.2 | 0.06 | 0.22 | 0.38 |
| Left ventricular diastolic stiffness (κ) | 21.2 ± 2.3c | 21.7 ± 1.7c | 29.6 ± 1.4a | 25.6 ± 1.9b | 0.022 | 0.36 | 0.24 |
| Left ventricle collagen area, % | 8.1 ± 2.4c | 8.3 ± 1.8c | 18.4 ± 1.9a | 12.1 ± 1.7b | 0.009 | 0.11 | 0.11 |
| Left ventricle inflammatory cells, cells/200µm2 | 7 ± 2c | 9 ± 2c | 25 ± 3a | 16 ± 3b | 0.0004 | 0.1949 | 0.0520 |
| Metabolic variables | |||||||
| Plasma total cholesterol, mmol/L | 1.59 ± 0.06b | 1.44 ± 0.06b | 1.73 ± 0.09a | 1.51 ± 0.08b | 0.18 | 0.021 | 0.65 |
| Plasma triglycerides, mmol/L | 0.50 ± 0.05b | 0.52 ± 0.08b | 1.15 ± 0.13a | 1.04 ± 0.21a | <0.0001 | 0.73 | 0.62 |
| Plasma non-esterified fatty acids, mmol/L | 0.68 ± 0.12b | 0.74 ± 0.24b | 2.71 ± 0.29a | 1.99 ± 0.59a | <0.0001 | 0.33 | 0.25 |
| Alanine transaminase, U/L | 39 ± 5ab | 33 ± 5ab | 50 ± 11ab | 31 ± 2b | 0.55 | 0.010 | 0.39 |
| Aspartate transaminase, U/L | 138 ± 20ab | 125 ± 20b | 174 ± 17a | 128 ± 13b | 0.29 | 0.011 | 0.37 |
| Liver inflammatory cells, cells/200µm2 | 11 ± 2 | 13 ± 2 | 30 ± 3a | 16 ± 3b | 0.0003 | 0.029 | 0.005 |
| Liver fat vacuoles area, µm2 | 12.4 ± 1.2 | 10.7 ± 1.8 | 90.6 ± 7.1a | 70.4 ± 2.7b | <0.0001 | 0.012 | 0.029 |
| Oral glucose tolerance test | |||||||
| 0 week basal blood glucose, mmol/L | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.5 ± 0.1 | 0.66 | 0.66 | 0.66 |
| 0 week area under the curve, mmol/L×min | 751 ± 26 | 650 ± 23 | 727 ± 23 | 689 ± 18 | 0.76 | 0.007 | 0.20 |
| 8 week basal blood glucose, mmol/L | 2.5 ± 0.1b | 2.8 ± 0.1b | 3.9 ± 0.4a | 3.4 ± 0.1a | <0.0001 | 0.60 | 0.044 |
| 8 week 120-min blood glucose, mmol/L | 3.6 ± 0.4b | 3.6 ± 0.1b | 5.2 ± 0.1a | 5.1 ± 0.1a | <0.0001 | 0.78 | 0.78 |
| 8 week area under the curve, mmol/L×min | 559 ± 34b | 538 ± 18b | 690 ± 17a | 645 ± 12a | <0.0001 | 0.11 | 0.56 |
| 16 week basal blood glucose, mmol/L | 2.6 ± 0.1b | 2.6 ± 0.2b | 3.5 ± 0.3a | 3.3 ± 0.2a | 0.002 | 0.67 | 0.67 |
| 16 week 120-min blood glucose, mmol/L | 3.6 ± 0.1b | 3.8 ± 0.2b | 5.8 ± 0.5a | 5.0 ± 0.1a | <0.0001 | 0.22 | 0.043 |
| 16 week area under the curve, mmol/L×min | 506 ± 22c | 547 ± 8b | 675 ± 20a | 647 ± 18a | <0.0001 | 0.71 | 0.056 |
| Insulin tolerance test | |||||||
| 8 week 120-min blood glucose, mmol/L | 2.1 ± 0.7b | 2.5 ± 0.5b | 3.8 ± 0.5a | 4.2 ± 0.4a | 0.004 | 0.47 | 1.00 |
| 8 week area under the curve, mmol/L×min | 204 ± 33b | 238 ± 36b | 349 ± 23a | 377 ± 24a | 0.0002 | 0.37 | 0.93 |
| 16 week 120-min blood glucose, mmol/L | 3.6 ± 0.4 | 3.3 ± 1.1 | 4.3 ± 1.1 | 3.4 ± 0.3 | 0.66 | 0.52 | 0.74 |
| 16 week area under the curve, mmol/L×min | 271 ± 64 | 225 ± 36 | 396 ± 56 | 341 ± 19 | 0.006 | 0.23 | 0.91 |
Values are presented as mean ± SEM, n = 10-12. Means in a row with unlike superscripts differ (a, b or c), p < 0.05. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
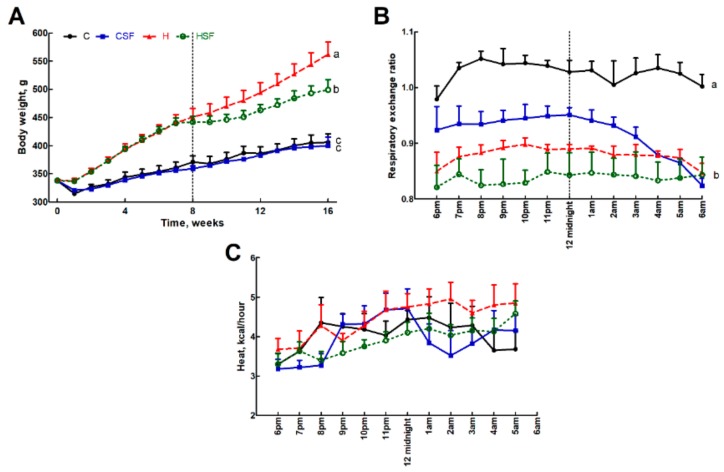
Figure 2.
(A) Body weight, (B) 12-hour indirect calorimeter data for respiratory exchange ratio and (C) heat production in corn starch diet-fed rats (C), corn starch diet-fed rats supplemented with Sarconema filiforme (CSF), high-carbohydrate, high-fat diet-fed rats (H) and high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme (HSF). End-point means with unlike superscripts differ (a, b or c), p < 0.05.
C rats had higher respiratory exchange ratio compared to H rats, while corn starch diet-fed rats treated with S. filiforme (CSF) and HSF were the same as H rats (Figure 2B). C rats had lower heat production than H rats, while CSF was higher than C and HSF was lower than H (Figure 2C). Total abdominal fat was highest in H rats followed by HSF, CSF and C rats.
After eight weeks, systolic blood pressures of H diet-fed groups (H and HSF) were higher than C diet-fed groups (C and CSF). Systolic blood pressures in H rats were higher at 16 weeks than in C rats. HSF rats had decreased systolic blood pressures compared to H control rats. Diastolic stiffness was higher in H rats compared to C rats. HSF rats showed normalised diastolic stiffness. Left ventricular with septum wet weights and right ventricular wet weights were unchanged across all groups. Left ventricles from H rats showed increased infiltration of inflammatory cells and collagen deposition, whereas these changes were not seen in left ventricles from C rats (Table 2). Infiltration of inflammatory cells and collagen deposition was normal in hearts from CSF rats and decreased in HSF rats (Figure 3 and Table 2).
Figure 3.
Heart inflammation (A–D) using haematoxylin and eosin stain; heart fibrosis (E–H) using picrosirius red stain; ileum (I–L) and colon (M–P) structure using haematoxylin and eosin stain in corn starch diet-fed rats (A,E,I,M), corn starch diet-fed rats supplemented with Sarconema filiforme (B,F,J,N), high-carbohydrate, high-fat diet-fed rats (C,G,K,O) and high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme (D,H,L,P). Fibrosis = fb; inflammation = in. Scale bar for images A-H is 200µm (20×) and for images I-P is 100µm (10×).
Plasma triglyceride concentrations were higher in H and HSF rats compared to C and CSF rats; plasma total cholesterol concentrations were highest in H rats and lowest in C, CSF and HSF rats; and plasma non-esterified fatty acids were unchanged across all groups (Table 2). C rats had lower basal blood glucose concentrations compared to H rats. Intervention with S. filiforme reduced basal blood glucose concentrations. The blood glucose area under the curve was not different between groups (Table 2).
H rats had higher plasma activities of ALT and AST compared to C rats. CSF and HSF rats were the same as C rats (Table 2). Livers from H rats showed increased fat deposition and infiltration of inflammatory cells compared to livers from C rats (Table 2 and Figure 4). HSF rats had reduced fat deposition compared to H rats (Figure 4).
Figure 4.
Fat vacuoles (A–D) and inflammation (E–H) using haematoxylin and eosin and liver fat using oil red O stain (I–L) in corn starch diet-fed rats (A,E,I), corn starch diet-fed rats supplemented with Sarconema filiforme (B,F,J), high-carbohydrate, high-fat diet-fed rats (C,G,K) and high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme (D,H,L). Fat vacuole containing cells = fc; inflammatory cells = in. Scale bar is 200µm (20×).
2.4. Gut Structure and Microbiota
Histology of ileum and colon did not show any structural abnormalities in the experimental groups with normal crypt depth, villi length and goblet cells, and less inflammatory cell infiltration (Figure 3).
The gut microbiota was characterised by a total of 739,069 quality-filtered sequences that were clustered into 1233 zero-radius operational taxonomic units (zOTUs), which are roughly equivalent to the taxonomic level of species or strains. The calculated rarefaction curves based on rarefied and unrarefied data as well as Good’s coverage of 99.67 ± 0.10% showed that the bacterial communities were almost fully recovered by the surveying effort.
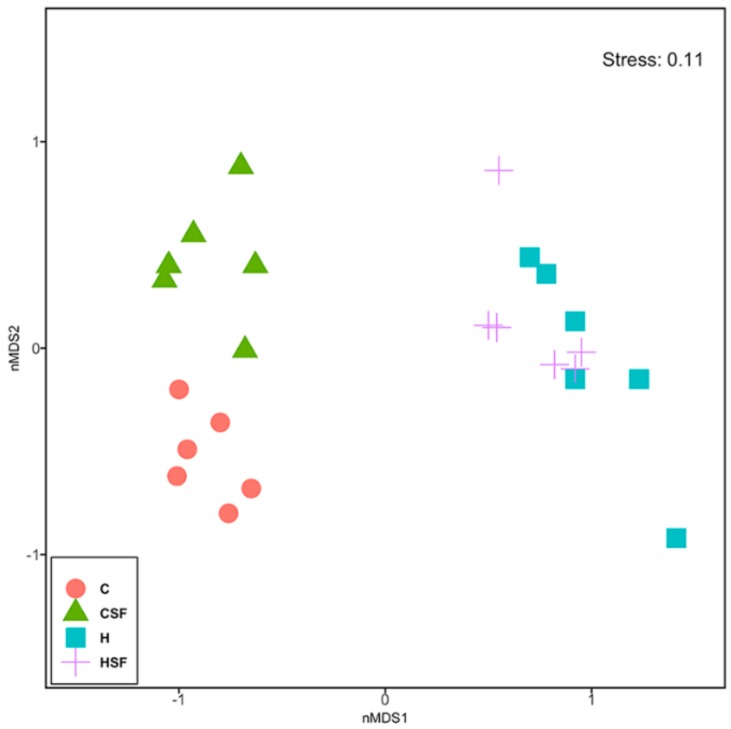
There was no difference in Shannon’s diversity or richness between the four groups (Figure 5). Diet and seaweed supplement both affected the overall bacterial community structure based on Bray-Curtis dissimilarity (Figure 6, Table 3; PERMANOVA, both p = 0.0001), and there was an interaction between the two factors (Figure 6, Table 3; PERMANOVA, p = 0.003). There were pairwise differences between the C and H groups indicating an effect of basal feed on the bacterial community structure (p = 0.0017). The addition of S. filiforme changed the bacterial communities (CSF, p = 0.0014; HSF, p = 0.0396). Bacterial communities in the CSF group were more variable compared to the C group (Figure 6, Table 3; PERMDISP; p = 0.022).
Figure 5.
(A) Shannon diversity and (B) richness of faecal samples. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
Figure 6.
Multi-disciplinary scaling (MDS) plot of bacterial community structure of faecal samples from different feeding regimes. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
Table 3.
PERMANOVAs based on Bray-Curtis similarity measure for square-root transformed abundances of all rat faecal samples.
| PERMANOVA | ||||||
| Source | df | SS | MS | Pseudo-F | P(perm) | Unique perms |
| Diet | 1 | 9306.2 | 9306.2 | 9.3671 | 0.0001 | 9912 |
| Treatment | 1 | 2477.6 | 2477.6 | 2.4938 | 0.0001 | 9860 |
| Diet × treatment | 1 | 1671.4 | 1671.4 | 1.6823 | 0.003 | 9836 |
| Res | 19 | 18876 | 993.5 | |||
| Total | 22 | 32369 | ||||
| PAIR-WISE TESTS | ||||||
| Source | t | P(perm) | Unique perms | |||
| C, CSF | 1.626 | 0.0014 | 462 | |||
| C, H | 2.4472 | 0.0017 | 462 | |||
| C, HSF | 2.544 | 0.0024 | 462 | |||
| CSF, H | 2.3327 | 0.0018 | 462 | |||
| CSF, HSF | 2.2125 | 0.0022 | 461 | |||
| H, HSF | 1.185 | 0.0396 | 462 | |||
| PERMDISP (PAIRWISE COMPARISONS) | ||||||
| Groups | t | P(perm) | ||||
| C, CSF | 1.7793 | 0.022 | ||||
| C, H | 0.77115 | 0.65 | ||||
| C, HSF | 0.80101 | 0.60 | ||||
| CSF, H | 0.01072 | 0.99 | ||||
| CSF, HSF | 1.6113 | 0.24 | ||||
| H, HSF | 1.139 | 0.35 | ||||
p values were calculated using 9,999 permutations under a residual model. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
C rats and CSF rats had lower ratios of Firmicutes to Bacteroidetes (F/B ratio) compared to H and HSF rats (Figure 7). There was no effect of S. filiforme supplementation on the F/B ratio under either diet.
Figure 7.
Effect of supplementation of diet (C or H) with Sarconema filiforme on the ratio of Firmicutes and Bacteroidetes abundances in rat faecal samples. Statistical analysis performed using ANOVA with Tukey’s post hoc test for multiple comparisons. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
2.5. Taxonomic Structure of the Bacterial Communities
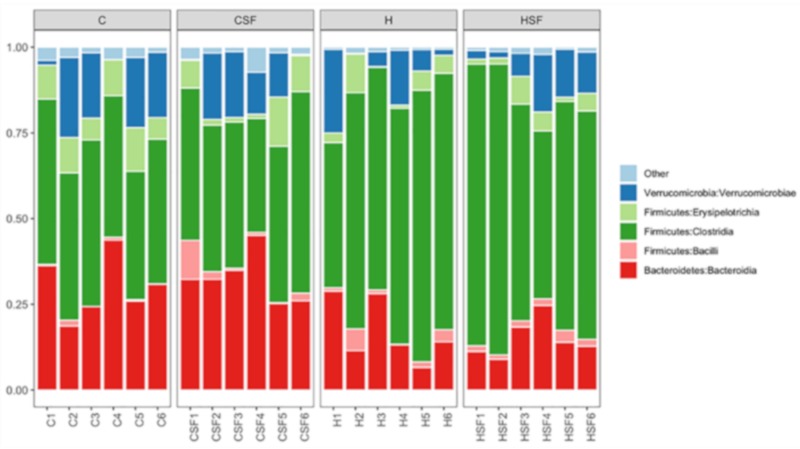
The most abundant bacterial classes found in the faecal samples for different treatment groups were Bacteroidia, Bacilli, Clostridia, Erysipelotrichia and Verrucomicrobia (Figure 8). Other bacterial classes, including Actinobacteria, Coriobacteriia, Melainabacteria, Deferribacteres, Saccharimonadia, Alphaproteobacteria, Deltaproteobacteria, Gammaproteobacteria and Mollicutes, were observed at lower abundance levels (<1%) in some (but not all) faecal samples.
Figure 8.
Taxonomic profiles of bacterial communities shown at the class level of all faecal samples. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; and HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
The relative abundance of bacteria from the class Bacteroidia was reduced in H rats (15.03% to 17.07%) compared to C rats (29.98% to 32.68%) (p < 0.001). An increase in the relative abundance of bacteria from class Bacilli was observed in CSF, H and HSF rats (2.01% to 2.89%) compared to the C rats (0.58%; p > 0.05). A higher abundance of bacteria from the class Clostridia was observed for H rats (66.51% to 68.76%) compared to C rats (43.47% to 44.59%) (p < 0.0001) (Figure 8). An increase in the relative abundance of bacteria from the class Verrucomicrobiae and Erysipelotrichia was observed in C rats (Verrucomicrobiae: 10.74% to 13.92%; Erysipelotrichia: 6.17% to 9.30%) (p > 0.05) compared to H rats (Verrucomicrobiae: 8.83% to 9%; Erysipelotrichia: 3.86% to 4.33%) (p > 0.05; Figure 8).
Analysis of the bacterial community structure at the family level showed that Bacteroidaceae (class Bacteriodia), Muribaculaceae (class Bacteriodia), Prevotellaceae (class Bacteroidia), Lactobacillaceae (class Bacilli), Clostridiaceae 1 (class Clostridia), Lachnospiraceae (class Clostridia), Peptostreptococcaceae (class Clostridia), Ruminococcaceae (class Clostridia), Erysipelotrichaceae (class Erysipelotrichia) and Akkermansiacaeae (class Verrucomicrobia) were most dominant in the faecal samples (Figure 9). The relative abundance of bacteria from the family Ruminococcaceae was reduced for H rats (8.83% to 9%) compared to C rats (10.74% to 13.92%) (p > 0.05). A high abundance of bacteria from the family Lachnospiraceae was detected in H rats and HSF rats (36.37% to 39.43%, p < 0.0001) compared to C rats (15.17% to 15.26%). In contrast, the abundance of bacteria from the family Muribaculaceae was reduced in H rats (9.16% to 10.28%) compared to C rats (21.27% to 22.77%, p < 0.05) (Figure 9). Moreover, lower abundance of bacteria from the family Lactobacillaceae was observed for C rats (0.16%) compared to CSF, H and HSF (1.56% to 2.49%) (p > 0.05).
Figure 9.
Taxonomic profiles of bacterial communities shown at the family level of all faecal samples. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
Analysis of the bacterial community structure at the genus level showed that Bacteroides (family Bacteroidaceae), unclassified Muribaculaeceae, Clostridium sensu stricto 1 (family Clostridiaceae), Lachnospiraceae NK4A136 group (family Lachnospiraceae), Roseburia (family Lachnospiraceae), Ruminococcus 1 (family Ruminococcaceae), unclassified Ruminococcaceae, Turicibacter (family Erysipelotrichaceae) and Akkermansia (family Akkermansiaceae) were most dominant in the faecal samples (Figure 10).
Figure 10.
Taxonomic profiles of bacterial communities shown at the genus level of all faecal samples. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
2.6. Multivariate Analysis of Physiological Data
A total of 23 physiological parameters were assessed and included in the analysis below (body weight, fat mass, lean mass, water intake, food intake, energy intake, feed efficiency, left ventricle with septum wet weight, right ventricle wet weight, retroperitoneal fat, omental fat, epididymal fat, total abdominal fat, liver wet weight, kidney wet weight, spleen wet weight, plasma non-esterified fatty acids, plasma triglycerides, systolic blood pressure, oral glucose tolerance area under the curve, oral glucose tolerance 120 min blood glucose concentrations, plasma aspartate transaminase activity and plasma alanine transaminase activity) for rats fed with the C and H diets and supplemented with S. filiforme (Table 4).
Table 4.
PERMANOVAs based on Euclidean distance matrix for physiological data of all rat faecal samples.
| PERMANOVA | ||||||
| Source | df | SS | MS | Pseudo-F | P(perm) | Unique perms |
| Diet | 1 | 4081700 | 4081700 | 53.366 | 0.0001 | 9924 |
| Treatment | 1 | 435050 | 435050 | 5.6882 | 0.0154 | 9942 |
| Diet × treatment | 1 | 408770 | 408770 | 5.3446 | 0.0187 | 9939 |
| Res | 20 | 1529700 | 76484 | |||
| Total | 23 | 6455200 | ||||
| PAIR-WISE TESTS | ||||||
| Source | t | P(perm) | Unique perms | |||
| C, CSF | 0.6848 | 0.82 | 462 | |||
| C, H | 5.6852 | 0.0018 | 461 | |||
| C, HSF | 4.4192 | 0.0024 | 462 | |||
| CSF, H | 5.8892 | 0.0021 | 462 | |||
| CSF, HSF | 4.7191 | 0.0023 | 462 | |||
| H, HSF | 2.5161 | 0.0268 | 462 | |||
| PERMDISP (PAIRWISE COMPARISONS) | ||||||
| Groups | t | P(perm) | ||||
| C, CSF | 1.5351 | 0.23 | ||||
| C, H | 1.9906 | 0.11 | ||||
| C, HSF | 1.4159 | 0.20 | ||||
| CSF, H | 2.4448 | 0.0398 | ||||
| CSF, HSF | 2.2429 | 0.0139 | ||||
| H, HSF | 1.0507 | 0.29 | ||||
p values were calculated using 9999 permutations under a residual model. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
Distance-based multivariate analysis showed that treatments have distinct responses on the physiological parameters. Diet and supplement affected the rats’ physiological variables (Figure 11, Table 4; PERMANOVA; p = 0.0001 and p = 0.0154, respectively) and there was an interaction between the two factors (Figure 11, Table 4; PERMANOVA, p = 0.0187).
Figure 11.
Non-metric multi-disciplinary scaling (nMDS) plot of physiological data from all physiological parameters measured after 16 weeks of feeding. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
There was statistical support for differences between C and H rats without S. filiforme (p = 0.0018, Table 4) indicating an effect of basal diet on the overall physiological variables. There was also an effect for the addition of S. filiforme to the H diet (p = 0.0268; Figure 11, Table 4), however supplementation had no effect for C diet. Rat physiological variables in H and HSF rats were more variable between replicates compared to the CSF rats (Figure 11, Table 4; PERMDISP; p = 0.0398, p = 0.0139, respectively).
2.7. Differentially Abundant zOTUs under Different Feeding Treatments
Multivariate analysis of individual zOTUs using the R package Mvabund revealed that diet and supplement, as well as the interaction between diet and supplement had a significant effect on the bacterial community structure in the faecal samples (Table 5). At the zOTU level, diet had a stronger effect than supplementation with S. filiforme on the bacterial community structure by affecting the abundance of 77 zOTUs (6.24% of total zOTUs) (Table 5).
Table 5.
Summary of statistical tests on differential zOTU abundance.
| Global Test (GLMs) by Mvabund | ||
| Diet: p < 0.0001 | ||
| Treatment: p = 0.003 | ||
| Diet × Treatment: p < 0.007 | ||
| Univariate Analysis by Mvabund (p < 0.05) | ||
| Factor | Number of differentially abundant OTUs | % of total number of OTUs |
| Diet | 77 | 6.24% |
| Treatment | 35 | 0.32% |
| Total (unique zOTUs affected by one or more factors) | 81 | 6.57% |
zOTUs belonging to the phylum Firmicutes (families: Lachnospiraceae, Peptococcaceae and Ruminococcaceae; genus: Acetatifactor, Anaerostipes, Blautia, GCA-900066575, Lachnoclostridium, Lachnospiraceae FCS020 group, Lachnospiraceae NK4A136 group, Lachnospiraceae UCG-006, Lachnospiraceae UCG-008, Roseburia, unclassified Lachnospiraceae, unclassified Peptococcaceae, Butyricicoccus, Ruminiclostridium, Ruminiclostridium 9 and unclassified Ruminococcaceae) were reduced in abundance or absent in C and CSF rats compared to H and HSF rats, respectively, while zOTUs belonging to the family Ruminococcaceae (genus: Ruminococcaceae NK4A214 group) were enriched in C and CSF rats compared to H and HSF rats (Table 6). Bacteria belonging to the phylum Actinobacteria (families: Bifidobacteriaceae and Eggerthellaceae; genus: Bifidobacterium and Enterorhabdus) and the phylum Bacteroidetes (families: Bacteroidaceae, Muribaculaceae and Prevotellaceae; genus: Bacteroides, unclassified Muribaculaceae and Prevotellaceae UCG-001) were either reduced or absent in H and HSF rats (Table 6).
Table 6.
Relative abundance of zOTUs affected by diet (ANOVA with p adjusted <0.05) between C, CSF, H and HSF rats.
| OTU_ID | C (%) | CSF (%) | H (%) | HSF (%) | Phylum | Family | Genus |
|---|---|---|---|---|---|---|---|
| Zotu42 | 0.62 | 0.31 | 0.00 | 0.02 | Actinobacteria | Bifidobacteriaceae | Bifidobacterium |
| Zotu80 | 0.29 | 0.22 | 0.00 | 0.00 | Actinobacteria | Bifidobacteriaceae | Bifidobacterium |
| Zotu168 | 0.12 | 0.09 | 0.01 | 0.01 | Actinobacteria | Eggerthellaceae | Enterorhabdus |
| Zotu20 | 0.95 | 0.90 | 0.02 | 0.08 | Bacteroidetes | Bacteroidaceae | Bacteroides |
| Zotu21 | 1.11 | 0.73 | 0.16 | 0.09 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu27 | 1.05 | 0.37 | 0.05 | 0.06 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu79 | 0.40 | 0.13 | 0.02 | 0.01 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu541 | 0.02 | 0.05 | 0.00 | 0.00 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu857 | 0.08 | 0.04 | 0.00 | 0.00 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu978 | 0.12 | 0.11 | 0.01 | 0.03 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu1036 | 0.05 | 0.02 | 0.00 | 0.00 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu1144 | 0.12 | 0.04 | 0.00 | 0.01 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu10 | 2.06 | 2.28 | 0.28 | 0.19 | Bacteroidetes | Prevotellaceae | Prevotellaceae UCG-001 |
| Zotu916 | 0.00 | 0.00 | 0.02 | 0.02 | Firmicutes | Lachnospiraceae | Acetatifactor |
| Zotu77 | 0.04 | 0.05 | 0.30 | 0.54 | Firmicutes | Lachnospiraceae | Anaerostipes |
| Zotu244 | 0.01 | 0.00 | 0.07 | 0.11 | Firmicutes | Lachnospiraceae | Blautia |
| Zotu49 | 0.01 | 0.02 | 0.81 | 0.43 | Firmicutes | Lachnospiraceae | GCA-900066575 |
| Zotu76 | 0.03 | 0.03 | 0.64 | 0.20 | Firmicutes | Lachnospiraceae | Lachnoclostridium |
| Zotu856 | 0.00 | 0.00 | 0.03 | 0.02 | Firmicutes | Lachnospiraceae | Lachnospiraceae FCS020 group |
| Zotu1061 | 0.00 | 0.00 | 0.03 | 0.03 | Firmicutes | Lachnospiraceae | Lachnospiraceae FCS020 group |
| Zotu37 | 0.01 | 0.01 | 0.81 | 1.19 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu100 | 0.36 | 0.14 | 0.00 | 0.02 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu123 | 0.00 | 0.00 | 0.20 | 0.40 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu182 | 0.00 | 0.00 | 0.18 | 0.23 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu201 | 0.01 | 0.01 | 0.18 | 0.13 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu544 | 0.00 | 0.00 | 0.13 | 0.09 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu561 | 0.00 | 0.00 | 0.06 | 0.03 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu658 | 0.00 | 0.00 | 0.48 | 0.54 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu762 | 0.00 | 0.00 | 0.02 | 0.04 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu847 | 0.00 | 0.00 | 0.03 | 0.02 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu966 | 0.00 | 0.00 | 0.08 | 0.10 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu1157 | 0.00 | 0.01 | 0.12 | 0.35 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group |
| Zotu26 | 0.00 | 0.00 | 1.65 | 1.27 | Firmicutes | Lachnospiraceae | Lachnospiraceae UGC-006 |
| Zotu110 | 0.01 | 0.02 | 0.49 | 0.25 | Firmicutes | Lachnospiraceae | Lachnospiraceae UGC-008 |
| Zotu35 | 0.01 | 0.01 | 1.69 | 0.32 | Firmicutes | Lachnospiraceae | Roseburia |
| Zotu556 | 0.00 | 0.00 | 0.02 | 0.04 | Firmicutes | Lachnospiraceae | Roseburia |
| Zotu582 | 0.00 | 0.00 | 0.02 | 0.05 | Firmicutes | Lachnospiraceae | Roseburia |
| Zotu604 | 0.00 | 0.00 | 0.01 | 0.05 | Firmicutes | Lachnospiraceae | Roseburia |
| Zotu625 | 0.00 | 0.00 | 0.01 | 0.03 | Firmicutes | Lachnospiraceae | Roseburia |
| Zotu634 | 0.00 | 0.00 | 0.01 | 0.05 | Firmicutes | Lachnospiraceae | Roseburia |
| Zotu25 | 0.00 | 0.00 | 1.10 | 0.83 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu52 | 0.09 | 0.04 | 0.48 | 0.48 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu73 | 0.02 | 0.00 | 0.24 | 0.44 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu101 | 0.00 | 0.00 | 0.28 | 0.24 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu174 | 0.00 | 0.00 | 0.13 | 0.32 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu197 | 0.00 | 0.00 | 0.46 | 0.05 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu198 | 0.02 | 0.02 | 0.27 | 0.32 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu221 | 0.00 | 0.00 | 0.14 | 0.09 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu226 | 0.00 | 0.00 | 0.15 | 0.22 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu277 | 0.00 | 0.00 | 0.20 | 0.04 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu293 | 0.00 | 0.00 | 0.04 | 0.18 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu516 | 0.00 | 0.00 | 0.07 | 0.06 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu528 | 0.00 | 0.00 | 0.05 | 0.03 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu530 | 0.00 | 0.00 | 0.06 | 0.05 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu590 | 0.00 | 0.00 | 0.05 | 0.04 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu757 | 0.00 | 0.00 | 0.01 | 0.04 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu891 | 0.00 | 0.00 | 0.02 | 0.02 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu937 | 0.00 | 0.00 | 0.07 | 0.26 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu988 | 0.00 | 0.00 | 0.01 | 0.01 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu1043 | 0.01 | 0.02 | 0.23 | 0.17 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu1161 | 0.00 | 0.00 | 0.09 | 0.04 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu1200 | 0.00 | 0.01 | 0.33 | 0.16 | Firmicutes | Lachnospiraceae | unclassified |
| Zotu247 | 0.00 | 0.00 | 0.13 | 0.04 | Firmicutes | Peptococcaceae | unclassified |
| Zotu398 | 0.05 | 0.04 | 0.00 | 0.00 | Firmicutes | Peptococcaceae | unclassified |
| Zotu384 | 0.00 | 0.00 | 0.10 | 0.02 | Firmicutes | Ruminococcaceae | Butyricicoccus |
| Zotu279 | 0.00 | 0.00 | 0.07 | 0.08 | Firmicutes | Ruminococcaceae | Ruminiclostridium |
| Zotu614 | 0.00 | 0.00 | 0.02 | 0.04 | Firmicutes | Ruminococcaceae | Ruminiclostridium |
| Zotu958 | 0.00 | 0.00 | 0.01 | 0.01 | Firmicutes | Ruminococcaceae | Ruminiclostridium |
| Zotu63 | 0.00 | 0.00 | 0.51 | 0.30 | Firmicutes | Ruminococcaceae | Ruminiclostridium 9 |
| Zotu133 | 0.01 | 0.00 | 0.15 | 0.23 | Firmicutes | Ruminococcaceae | Ruminiclostridium 9 |
| Zotu135 | 0.00 | 0.00 | 0.17 | 0.21 | Firmicutes | Ruminococcaceae | Ruminiclostridium 9 |
| Zotu643 | 0.01 | 0.04 | 0.00 | 0.00 | Firmicutes | Ruminococcaceae | Ruminiclostridium 9 |
| Zotu38 | 0.93 | 0.21 | 0.00 | 0.00 | Firmicutes | Ruminococcaceae | Ruminiclostridium NK4A214 group |
| Zotu218 | 0.08 | 0.07 | 0.01 | 0.01 | Firmicutes | Ruminococcaceae | Ruminiclostridium NK4A214 group |
| Zotu50 | 0.01 | 0.04 | 0.50 | 0.38 | Firmicutes | Ruminococcaceae | unclassified |
| Zotu62 | 0.02 | 0.00 | 0.46 | 0.43 | Firmicutes | Ruminococcaceae | unclassified |
| Zotu275 | 0.00 | 0.00 | 0.06 | 0.12 | Firmicutes | Ruminococcaceae | unclassified |
Differential abundance analysis was performed using Mvabund. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
A total of four zOTUs (0.32% of total zOTUs) belonging mostly to phylum Firmicutes were significantly affected by S. filiforme supplementation (Table 7). One zOTU belonging to the phylum Bacteroidetes was enriched in C and H rats, while bacteria belonging to the phylum Firmicutes and family Ruminococcaceae (genus: Ruminococcaceae UCG-014) and the phylum Proteobacteria and family Desulfovibrionaceae (genus: Bilophila) were absent in C and H rats (Table 7).
Table 7.
Relative abundance of zOTUs affected by treatment (ANOVA with p adjusted <0.05) between C, CSF, H and HSF rats.
| OTU_ID | C (%) | CSF (%) | H (%) | HSF (%) | Phylum | Family | Genus |
|---|---|---|---|---|---|---|---|
| Zotu15 | 0.72 | 0.00 | 3.02 | 0.00 | Bacteroidetes | Muribaculaceae | unclassified |
| Zotu232 | 0.00 | 0.06 | 0.00 | 0.13 | Firmicutes | Ruminococcaceae | Ruminococcaceae UCG-014 |
| Zotu595 | 0.00 | 0.04 | 0.00 | 0.01 | Firmicutes | Ruminococcaceae | Ruminococcaceae UCG-014 |
| Zotu40 | 0.00 | 0.70 | 0.00 | 0.43 | Proteobacteria | Desulfovibrionaceae | Bilophila |
Differential abundance analysis was performed using Mvabund. C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
2.8. Correlation of Microbiota and Physiological Parameters
Combined analysis of bacterial community structure and physiological parameters was performed. The Mantel test revealed that overall the bacterial community structure and the physiological data are correlated (Mantel statistic r = 0.2177; p = 0.0071). Table 8 and Figure 12 show how individual physiological parameters contribute to the differences in bacterial community structure between treatments (function envfit – vegan R package).
Table 8.
Correlation between bacterial community structure and physiological parameters (p < 0.05).
| Physiological Variables | R2 | p-Value |
|---|---|---|
| Epididymal fat | 0.59 | 0.001 |
| Water intake | 0.58 | 0.002 |
| Total abdominal fat | 0.56 | 0.001 |
| Retroperitoneal fat | 0.54 | 0.002 |
| Systolic blood pressure | 0.53 | 0.001 |
| Left ventricle and septum wet weight | 0.47 | 0.001 |
| Fat mass | 0.46 | 0.001 |
| Kidneys wet weight | 0.44 | 0.002 |
| Omental fat | 0.40 | 0.007 |
| Body weight | 0.39 | 0.004 |
| Liver wet weight | 0.37 | 0.011 |
| Right ventricle wet weight | 0.33 | 0.003 |
| Oral glucose tolerance area under the curve | 0.33 | 0.025 |
| Oral glucose tolerance 120-minute blood glucose | 0.29 | 0.027 |
| Food intake | 0.25 | 0.033 |
Figure 12.
Correlation between bacterial community structure (points) and environmental variables (arrows). C, corn starch diet-fed rats; CSF, corn starch diet-fed rats supplemented with Sarconema filiforme; H, high-carbohydrate, high-fat diet-fed rats; HSF, high-carbohydrate, high-fat diet-fed rats supplemented with Sarconema filiforme.
Physiological variables were further correlated with individual zOTUs (Table 9). A total of 44 zOTUs were found to be statistically correlated with at least one of the physiological parameters (p < 0.05). Of the zOTUs, 33 out of 44 belonged to the phylum Firmicutes, nine zOTUs to the phylum Bacteroidetes, one zOTU to the phylum Proteobacteria and one zOTU to the phylum Actinobacteria. Food intake (7 of 44 zOTUs or 15.91%) was inversely correlated with the relative abundance of the selected zOTU. In contrast, water intake (16 of 44 zOTUs or 36.36%), epididymal fat (4 of 44 zOTUs or 9.1%), left ventricle and septum weight (3 of 44 zOTUs or 6.82%), oral glucose tolerance (3 of 44 zOTUs or 6.82%), systolic blood pressure (3 of 44 zOTUs or 6.82%), liver wet weight (3 of 44 zOTUs or 6.82%) and total abdominal fat (2 of 44 zOTUs or 4.55%) were positively correlated with the selected zOTUs (Table 9).
Table 9.
Taxonomic assignments of the zOTUs strongly correlated with physiological parameters.
| OTU_ID | Phylum | Family | Genus | Correlation with Physiological Parameters |
|---|---|---|---|---|
| Zotu42 | Actinobacteria | Bifidobacteriaceae | Bifidobacterium | Water intake (−) |
| Zotu20 | Bacteroidetes | Bacteroidaceae | Bacteroides | Left ventricle and septum wet weight (+), water intake (−) |
| Zotu1036 | Bacteroidetes | Muribaculaceae | unclassified | Water intake (−) |
| Zotu1144 | Bacteroidetes | Muribaculaceae | unclassified | Water intake (−) |
| Zotu21 | Bacteroidetes | Muribaculaceae | unclassified | Water intake (−) |
| Zotu27 | Bacteroidetes | Muribaculaceae | unclassified | Water intake (−) |
| Zotu541 | Bacteroidetes | Muribaculaceae | unclassified | Epididymal fat (+) |
| Zotu79 | Bacteroidetes | Muribaculaceae | unclassified | Water intake (−) |
| Zotu857 | Bacteroidetes | Muribaculaceae | unclassified | Water intake (−) |
| Zotu10 | Bacteroidetes | Prevotellaceae | Prevotellaceae UCG-001 | Left ventricle and septum wet weight (+), water intake (−) |
| Zotu77 | Firmicutes | Lachnospiraceae | Anaerostipes | Food intake (−), water intake (+) |
| Zotu244 | Firmicutes | Lachnospiraceae | Blautia | Water intake (+) |
| Zotu856 | Firmicutes | Lachnospiraceae | Lachnospiraceae FCS020 group | Water intake (+) |
| Zotu100 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group | Water intake (−) |
| Zotu37 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group | Left ventricle and septum wet weight (−) |
| Zotu762 | Firmicutes | Lachnospiraceae | Lachnospiraceae NK4A136 group | Water intake (+) |
| Zotu556 | Firmicutes | Lachnospiraceae | Roseburia | Food intake (−), water intake (+) |
| Zotu582 | Firmicutes | Lachnospiraceae | Roseburia | Food intake (−) |
| Zotu604 | Firmicutes | Lachnospiraceae | Roseburia | Food intake (−) |
| Zotu625 | Firmicutes | Lachnospiraceae | Roseburia | Water intake (+) |
| Zotu634 | Firmicutes | Lachnospiraceae | Roseburia | Food intake (−) |
| Zotu101 | Firmicutes | Lachnospiraceae | unclassified | Water intake (−) |
| Zotu174 | Firmicutes | Lachnospiraceae | unclassified | Food intake (−) |
| Zotu198 | Firmicutes | Lachnospiraceae | unclassified | Left ventricle and septum wet weight (−) |
| Zotu25 | Firmicutes | Lachnospiraceae | unclassified | Water intake (+) |
| Zotu516 | Firmicutes | Lachnospiraceae | unclassified | Water intake (+) |
| Zotu52 | Firmicutes | Lachnospiraceae | unclassified | Left ventricle and septum wet weight (−) |
| Zotu530 | Firmicutes | Lachnospiraceae | unclassified | Water intake (+) |
| Zotu590 | Firmicutes | Lachnospiraceae | unclassified | Left ventricle and septum wet weight (−) |
| Zotu73 | Firmicutes | Lachnospiraceae | unclassified | Water intake (+) |
| Zotu757 | Firmicutes | Lachnospiraceae | unclassified | Food intake (−) |
| Zotu891 | Firmicutes | Lachnospiraceae | unclassified | Water intake (+) |
| Zotu988 | Firmicutes | Lachnospiraceae | unclassified | Water intake (+) |
| Zotu398 | Firmicutes | Peptococcaceae | unclassified | Water intake (−) |
| Zotu279 | Firmicutes | Ruminococcaceae | Ruminiclostridium | Left ventricle and septum wet weight (−), water intake (+) |
| Zotu614 | Firmicutes | Ruminococcaceae | Ruminiclostridium | Left ventricle and septum wet weight (−) |
| Zotu643 | Firmicutes | Ruminococcaceae | Ruminiclostridium 9 | Body weight (+), retroperitoneal fat (+), epididymal fat (+), omental fat (+), Total abdominal fat (+), fat mass (+), Liver wet weight (+), Left ventricle and septum wet weight (+), oral glucose tolerance 120-minute blood glucose (+), systolic blood pressure (+), water intake (−) |
| Zotu133 | Firmicutes | Ruminococcaceae | Ruminiclostridium 9 | Water intake (+) |
| Zotu135 | Firmicutes | Ruminococcaceae | Ruminiclostridium 9 | Water intake (+) |
| Zotu63 | Firmicutes | Ruminococcaceae | Ruminiclostridium 9 | Left ventricle and septum wet weight (−) |
| Zotu595 | Firmicutes | Ruminococcaceae | Ruminococcaceae UCG-014 | Epididymal fat (+), retroperitoneal fat (+), right ventricle wet weight (+), systolic blood pressure (+) |
| Zotu232 | Firmicutes | Ruminococcaceae | Ruminococcaceae UCG-014 | Liver wet weight (+) |
| Zotu62 | Firmicutes | Ruminococcaceae | unclassified | Left ventricle and septum wet weight (−), water intake (+) |
| Zotu40 | Proteobacteria | Desulfovibrionaceae | Bilophila | Epididymal fat (+), omental fat (+), retroperitoneal fat (+), total abdominal fat (+), kidney wet weight (+), liver wet weight (+), oral glucose tolerance 120-minute blood glucose (+), oral glucose tolerance area under the curve (+), systolic blood pressure (+) |
Differential abundance analysis was performed using Mvabund. This table includes the physiological parameters strongly correlated (P < 0.05) with the bacterial community and incorporates zOTUs that interact with at least 1 of these parameters. Plus sign (+) indicates positive correlations, while minus sign (−) indicates negative correlations.
The relative abundances of zOTUs belonging to the phylum Firmicutes and the families Lachnospiraceae (genus: Anaerostipes, Blautia, Lachnospiraceae FCS020 group, Lachnospiraceae NK4A136 group, Roseburia, unclassified Lachnospiraceae: zOTU77, zOTU244, zOTU856, zOTU762, zOTU37, zOTU556, zOTU582, 174, zOTU25, zOTU198, zOTU52 and zOTU590) and Ruminococcaceae (genus: Rumiclostridium and Rumiclostridium 9: zOTU279, zOTU614, zOTU133, zOTU135 and zOTU63) were inversely correlated with food intake and left ventricle and septum weight and positively correlated with water intake. While bacteria belonging to the phylum Firmicutes and family Peptococcaceae (zOTU398) were negatively correlated to water intake, bacteria belonging to the family Ruminococcaceae UCG-014 (zOTU595, zOTU232) were positively correlated to several physiological parameters including epididymal fat, retroperitoneal fat, right ventricle weight, systolic blood pressure and liver wet weight.
The relative abundances of bacteria belonging to the phylum Actinobacteria and family Bifidobacteriaceae; the phylum Bacteroidetes and families Bacteroidaceae, Muribaculaceae and Prevotellaceae (for example: zOTU42, zOTU1036, zOTU1144, zOTU21, zOTU27, zOTU79 and zOTU857) were negatively correlated to water intake. Several zOTUs belonging to the phylum Bacteroidetes and families Bacteroidaceae and Prevotellaceae were also positively correlated with left ventricle and septum weight (zOTU20 and zOTU10). The relative abundance of bacteria belonging to the phylum Proteobacteria and family Desulfovibrionaceae was positively correlated to epididymal fat, kidney weight, liver weight, oral glucose tolerance 120-minute blood glucose, oral glucose tolerance area under the curve, omental fat, retroperitoneal fat, systolic blood pressure and total abdominal fat (zOTU40).
3. Discussion
This project demonstrates that local Australian cultivation of S. filiforme produced significant and reliable yields of biomass in intensive tank-based culture, which can therefore potentially be a source of commercial quantities of ι-carrageenan. Australia has unique and untapped seaweed resources [11]; however, as of 2014, the Australian seaweed industry was small and only based on the harvest of stormcast kelp for alginate and fertiliser and on introduced species of Undaria for the extraction of bioactive compounds [12]. However, indigenous species such as S. filiforme could supply high-value fresh and dried foods as well as compounds for the nutraceutical and pharmaceutical markets. Consuming locally grown foods is considered important, because it reduces transport to markets and environmental impact to decrease CO2 emissions. In addition, local production supports the local economy as evidenced by the successful commercial cultivation of the red seaweed, K. alvarezii, in countries such as the Philippines and Indonesia as a main source of carrageenan for the food industry for more than 40 years [13].
Further, we show that whole dried S. filiforme may be useful in reversing metabolic syndrome. Metabolic syndrome including abdominal obesity, hypertension, hyperglycaemia, fatty liver and inflammation increases the risk of cardiovascular disease and diabetes; this syndrome is mimicked by a diet high in simple sugars, saturated and trans fats in rats [14]. This validated dietary model of human metabolic syndrome has been previously reported to show reversal of changes by interventions with seaweeds [15,16]. The major findings from the current reversal study were that S. filiforme decreased metabolic, cardiovascular and liver changes by 9-40% in obese rats fed a high-carbohydrate, high-fat diet, including some variables that were effectively reversed including liver enzymes and systolic blood pressure. These results are consistent with our previous study where K. alvarezii containing κ-carrageenan was used in a prevention protocol [8]. The soluble fibre content, which also approximates the carrageenan content, was ~35% in K. alvarezii and ~12% in S. filiforme, giving a soluble fibre content in the rat diets of around 1.7% and 0.5%, respectively. This was markedly less than the upper limit of 5% to avoid any safety risks, as recommended by the Scientific Committee on Food of the European Commission, for carrageenans below a molecular weight limit of 50 kDa when used as a food additive [17]. Although carrageenans have been used in subcutaneous injections to induce rat paw oedema as a model of inflammation [18], there have been dietary studies with no adverse effects using 5% ι-carrageenan prepared from E. denticulatum (previously E. spinosum) [19,20], although systemic administration of carrageenan increased biliary antibody titre, which is suggestive of bacterial intrusion [20,21]. In the literature, there are conflicting reports on the effects of carrageenans on the gastrointestinal tract, although the confusion may be in part due to inconsistent nomenclature. Degraded carrageenans have been incorrectly referred to as poligeenans, which are not produced biologically. Poligeenans are produced in the laboratory or commercially by subjecting carrageenan to very low pH at 0.9–1.3 and non-physiological temperatures of >80°C for several hours [22]. As carrageenans are not absorbed from the gastrointestinal tract after oral administration, studies using systemic administration are not appropriate for a risk assessment of carrageenans when used as food products in subjects without gastrointestinal pathology [23]. However, oral studies can determine local gastrointestinal risk; the current study shows no histopathological impact on the ileum or colon in H rats compared to HSF rats, consistent with the previous study on oral administration of K. alvarezii containing κ-carrageenan [8].
Traditionally, people in East Asian countries, such as Korea, Japan and China, consume more seaweeds as food and ingredients of traditional medicine than other populations [24]. The average intake of seaweeds in Japan was 14.3 g (wet weight)/day [25]. In the current study, the dose in rats equates to approximately 6.3 g of seaweed/day for humans [10]. Therefore, it is realistic that this quantity can be consumed as a single seaweed such as S. filiforme in the usual diet to translate its health benefits. Further, production of therapeutic amounts of this seaweed requires a small area, as the 42 m2 research facility of the University of the Sunshine Coast could provide enough seaweed for 179 people to be treated continuously, based on measured seaweed growth rates. This is markedly more efficient usage of space than with other sources of dietary fibre such as cereals; as an example, the average cereal yield in the USA was 828 g/m2/year in 2017 [26] compared to the average yield of seaweed at the University of the Sunshine Coast of around 189 g/m2/week (or around 10 kg/m2/year). Scaling up to produce commercial amounts of seaweed is therefore realistic.
Dietary fibre is classified as either soluble (non-cellulosic, polysaccharides, oligosaccharides, pectins, β-glucans and gums) or insoluble (cellulose, hemicellulose and lignin). The major physiological effects of soluble fibre are delayed gastric emptying, regulation of blood glucose levels and lowered serum cholesterol concentrations, due to increasing gut content viscosity and colonic fermentation. In contrast, the major effects of insoluble fibre are shortened gut transit time and improved laxation, both due to faecal bulking capacity and support for the growth of intestinal bacteria due to colonic fermentation [27]. S. filiforme contains about 22% fibre, which equates to 1.3 g of fibre per day if the human dose is 6.3 g/day, based on the Reagan-Shaw rat to human scaling equation [10]. The American Dietetic Association recommended a daily dietary fibre intake of 25 g for adult females and 38 g for adult males [28]. Therefore, Sarconema at this dose would increase fibre consumption, but alone would not provide sufficient fibre to meet this recommendation; consequently, other fibre-rich foods such as fruits, vegetables, beans and grains would be necessary components of the diet. Epidemiological and clinical studies have demonstrated that dietary fibre intake is inversely related to obesity [29], type 2 diabetes [30] and cardiovascular disease [31]. The consumption of dietary fibre likely reduces body fat accumulation due to several factors such as fermentation of the fibre in the colon, stimulating production of glucagon-like-peptide-1 and peptide YY [32]. These gut hormones increase satiety, which may lead to decreased meal frequency and size. Consumption of insoluble fibre reduced body weight and body fat and also normalised fasting glucose and insulin concentrations in overweight and obese adults [33].
Carrageenans undergo minimal digestion in the stomach and are then fermented by colonic bacteria [34], hence meeting the definition of prebiotics [35]. Health benefits of prebiotics include decreased blood pressure and body weight [27,36] similar to the responses from the current study. Using the same rat model of diet-induced obesity, a prebiotic mixture of inulin and oligofructose was reported as an effective dietary fibre, reducing body weight, plasma concentrations of free fatty acids and triglycerides, and systolic blood pressure and attenuating inflammatory cell infiltration in the heart and liver [37]. Red seaweeds show responses that suggest the biological actions of fibre as prebiotics. Subjects supplemented with the red seaweed Chondrus crispus showed improved gut health and immune modulation [38]. Another red seaweed, Mastocarpus stellatus, with 31.7% dietary fibre reduced triglycerides and total cholesterol concentrations, however, there was no effect on body weight in healthy Wistar rats [39]. Complex polysaccharides exert their action through a wide range of mechanisms including selective fermentation, lowering the gut pH, faecal bulking, preventing gut colonisation by pathogens, controlling putrefactive bacteria, and therefore reducing the host’s exposure to toxic metabolites [40]. These effects are likely due to dietary fibre increasing short-chain fatty acid production as these are used as an energy source by selected gut microbiota. Short-chain fatty acids decrease the luminal pH, improve calcium and magnesium absorption, reduce potential pathogenic bacteria and act as an energy source for epithelial cells [41].
4. Materials and Methods
4.1. Sarconema filiforme Source and Analysis
S. filiforme was cultured at the University of the Sunshine Coast seaweed aquaculture facility at the Bribie Island Research Centre, Woorim, QLD, Australia (27°04′10″ S; 153°12′15″ E) from January to March 2018. Production (yield of dry weight/m2/day) was measured through weekly harvests from up to five 1000 L fibreglass tanks over 13 weeks from cultures initially stocked each week at, on average, 7 g fresh weight per 1000 L of seawater. Cultures received flow-through seawater with weekly addition of nutrients (MAF, Manutech, Cavan, SA, Australia). Fresh samples were harvested each week, dehydrated and a total of 3 kg dried and milled S. filiforme was sub-sampled across the three months of production and stored in vacuum-sealed bags containing silica desiccant. Compositional analysis of the seaweed was performed as detailed previously [42,43].
Attenuated Total Reflectance-Fourier-Transform Infrared Spectroscopy (ATR-FTIR) was performed at Southern Cross University, Lismore, NSW, Australia to determine carrageenan subtypes in S. filiforme and K. alvarezii as a control and commercial grade ι-carrageenan and κ-carrageenan (Sigma-Aldrich Australia, Castle Hill, NSW, Australia) as standards. The transmittance spectra were recorded from 2000 to 675 cm−1 [44].
4.2. Rats and Diets
All experimental protocols were approved by the Animal Ethics Committee of the University of Southern Queensland under the guidelines of the National Health and Medical Research Council of Australia. Male Wistar rats (8–9 weeks old; 338 ± 1 g, n = 48) were obtained from the Animal Resource Centre, Murdoch, WA, Australia. Rats were individually housed in a temperature-controlled (21 ± 2˚C), 12-hour light/dark conditions with free access to food and water. Rats were randomly distributed into four groups, each of 12 rats. Two groups were fed either corn starch or high-carbohydrate, high-fat diets (C and H, respectively) [14] for a full 16 weeks. The other two groups received C and H diets for the first eight weeks and then received 5% S. filiforme in the diet for the last eight weeks (CSF and HSF, respectively). The C diet contained 570 g of cornstarch, 155 g of powdered rat food (Specialty Feeds, Glen Forest, WA, Australia), 25 g of Hubble, Mendel and Wakeman salt mixture (MP Biomedicals, Seven Hills, NSW, Australia), and 250 g of water per kilogram of diet. The H diet contained 175 g of fructose, 395 g of sweetened condensed milk, 200 g of beef tallow, 155 g of powdered rat food (all obtained from local food suppliers and supermarkets), 25 g of Hubble, Mendel and Wakeman salt mixture and 50 g of water per kilogram of diet. In addition, the drinking water for the H and HSF groups was supplemented with 25% fructose [14].
4.3. Rat Measurements
Dual-energy X-ray absorptiometry, non-invasive systolic blood pressure, abdominal circumference, oral glucose and insulin tolerance tests and indirect calorimetry were performed as described [14]. Euthanasia followed by heparin injection, blood collection, centrifugation, storage and then isolated Langendorff heart preparation and measurements, plasma measurements, organ weights, organ bath study and histological analyses were performed as described [14].
4.4. Gut Microbiota Analysis
Immediately after euthanasia and organ removal, two or three faecal pellets were collected from the colon of rats and stored at −80 °C in nuclease-free tubes. Total microbial community DNA was extracted from faecal samples using the DNeasy Powersoil Kit (Qiagen Australia, Chadstone, VIC, Australia) following the manufacturer’s instructions [45]. The bacterial gut microbiota was then characterised by amplifying and sequencing the 16S rRNA gene. The primers, 341F (TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG) and 785R (GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC) were used to amplify the V3-V4 regions of the 16S rRNA gene, which was then sequenced on an Illumina MiSeq platform. Sequencing reads were processed to form zOTUs, which were taxonomically classified against the SILVA database.
The reaction mixture (50 μL total volume per sample) to amplify the 16S rRNA gene consisted of Econotaq® PLUS GREEN 2× Master Mix (Astral Scientific, Gymea, NSW, Australia) (25 uL), Ambion® nuclease-free water (17 μL), the primer pair 341F and 785R (1.5 μL of each; 10 μM) and DNA template (5 μL). The PCR program consisted of an initial denaturation at 94 °C (2 min), followed by 35 cycles of denaturation at 94oC (30 s), annealing at 55oC (30 s), extension at 72 °C (40 s) and a final extension at 72 °C (7 min). PCR products were then quantified using gel electrophoresis. Paired-end sequencing (2 × 300 bp) of the resulting 16S rRNA gene amplicons was performed at the Ramaciotti Centre for Genomics, University of New South Wales on an Illumina MiSeq platform following the MiSeq System User Guide [46]. For 16S rRNA gene sequencing analysis, sequence data were initially quality-filtered and trimmed using Trimmomatic version 0.36 truncating reads if the quality dropped below 20 in a sliding window of 4 bp [47]. USEARCH version 11.0.667 [48] was used for further processing [49] to merge and quality-filter sequencing reads, excluding reads with < 250 or > 550 nucleotides, in addition to reads with more than one ambiguous base or an expected error of more than 1. Filtered sequences were denoised and clustered into unique sequences (zero-radius operational taxonomic units; zOTUs) using the UNOISE algorithm [50] implemented in USEARCH. zOTUs represent unique bacterial entities and roughly are equivalent to species or strains. Chimeric sequences were removed de novo during clustering and subsequently in reference mode using UCHIME [51] with the SILVA database (https://www.arb-silva.de/browser/) (SILVA SSURef 132 NR) as a reference [52]. zOTUs were then taxonomically classified (i.e., assigned a likely taxonomic name) by BLASTN [53] against the SILVA database. All non-bacterial zOTUs were removed along with non-BLAST aligned and singleton zOTUs. Finally, processed sequences were mapped on zOTU sequences to calculate the distribution and counts of each zOTU in every sample. Only zOTUs occurring in more than two samples were considered for further statistical analysis.
4.5. Statistical Analysis
Physiological and metabolic data are presented as mean ± standard error of the mean (SEM). These results were tested for variance using Bartlett’s test and variables that were not normally distributed were transformed using log10 function prior to statistical analyses. Data from the four groups were tested by two-way analysis of variance. When the interactions and/or the main effects were significant, means were compared using the Newman-Keuls multiple comparison post hoc test. Where transformations did not result in normality or constant variance, a Kruskal-Wallis non-parametric test was performed. A p value of < 0.05 was considered as statistically significant. All statistical analyses were performed using Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA).
For microbiota results, rarefaction curves were generated using the rarecurve function in vegan [54] and used to determine if a complete representation of the sample’s microbiota had been achieved given the sequencing effort. Prior to further analysis, the numbers of sequences were standardised across samples to account for different sequencing depths by randomly subsampling each sample to the lowest number of sequences counts obtained for any given sample (i.e., 19,706 counts). Bacterial alpha-diversities (zOTU richness and Shannon’s diversity) were calculated in R (version 3.5.3) using the rrarefy function in the vegan package [55]. A one-way analysis of variance test in GraphPad Prism 8.0.2 (San Diego, CA, USA) followed by Tukey’s pairwise comparisons test was used to determine the significance between the different groups and a p value of <0.05 was considered to be significant.
For multivariate analysis of bacterial communities, zOTU tables were imported into PRIMER [55] to compare the community structure (i.e. relative abundance data). Bray-Curtis similarity coefficients were calculated using square-root transformed zOTU abundances and the resulting similarity matrix was visualised using non-metric, multi-dimensional scaling (nMDS). Permutational multivariate analysis of variance (PERMANOVA) [56] with 9,999 random permutations was used to test the effect of treatment on bacterial communities in rat faecal samples.
5. Conclusions
Rats fed a high-carbohydrate, high-fat diet supplemented with S. filiforme as a source of ι-carrageenan decreased body weight, systolic blood pressure, abdominal and liver fat and plasma total cholesterol concentrations compared to H controls. These results are comparable to our previous study with K. alvarezii as a source of κ-carrageenan, providing evidence that red seaweeds contain compounds such as sulfated polysaccharides (carrageenans) which attenuate symptoms of diet-induced metabolic syndrome in rats. The correlations between changes in the gut microbiota and physiological changes following administration of S. filiforme suggests that the mechanism is likely through carrageenans acting as prebiotics as well as systemic anti-inflammatory responses in organs such as heart and liver. Further studies with mechanistic analyses will be valuable to determine the actions of carrageenans in vivo that are responsible for their health benefits.
Acknowledgments
The authors thank Bryan Bynon, School of Veterinary Sciences, The University of Queensland, Gatton, QLD for plasma biochemical analyses, Ana Wegner from the University of the Sunshine Coast for seaweed cultivation and the University of Southern Queensland for postgraduate research scholarship for R.d.P.
Author Contributions
Conceptualisation, S.K.P., L.B., N.P.; methodology, R.d.P., S.K.P., P.M., M.E.M.; formal analysis, R.d.P., P.M., M.E.M., S.K.P., T.T.; investigation, R.d.P., S.K.P., M.E.M.; resources, S.K.P., L.B.; writing—original draft preparation, R.d.P., S.K.P.; writing—review and editing, L.B.; supervision, S.K.P., L.B.; project administration, S.K.P.; funding acquisition, S.K.P., L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of Southern Queensland Research and Innovation Division.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Necas J., Bartosikova L. Carrageenan: A review. Vet. Med. 2013;58:187–205. doi: 10.17221/6758-VETMED. [DOI] [Google Scholar]
- 2.Phang S.-M., Yeong H.-Y., Lim P.-E., Nor A.R.M., Gan K.T. Commercial varieties of Kappaphycus and Eucheuma in Malaysia. Malays. J. Sci. 2010;29:214–224. doi: 10.22452/mjs.vol29no3.4. [DOI] [Google Scholar]
- 3.Van De Velde F., Lourenço N.D., Pinheiro H.M., Bakker M. Carrageenan: A food-grade and biocompatible support for immobilisation techniques. Adv. Synth. Catal. 2002;344:815–835. doi: 10.1002/1615-4169(200209)344:8<815::AID-ADSC815>3.0.CO;2-H. [DOI] [Google Scholar]
- 4.Womersley H. The Marine Benthic Flora of Southern Australia: Rhodophyta. ABRS; Canberra, Australia: 1994. Part IIIA. [Google Scholar]
- 5.Western Australian Flora Department of Biodiversity Home Page. [(accessed on 7 June 2019)]; Available online: https://florabase.dpaw.wa.gov.au.
- 6.Wells M.L., Potin P., Craigie J.S., Raven J.A., Merchant S.S., Helliwell K.E., Smith A.G., Camire M.E., Brawley S.H. Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 2017;29:949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eccles R., Winther B., Johnston S., Robinson P., Trampisch M., Koelsch S. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: the ICICC trial. Respir. Res. 2015;16:121. doi: 10.1186/s12931-015-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanyonyi S., Du Preez R., Brown L., Paul N.A., Panchal S.K. Kappaphycus alvarezii as a food supplement prevents diet-induced metabolic syndrome in rats. Nutrients. 2017;9:1261. doi: 10.3390/nu9111261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyu M., Wang Y.-F., Fan G.-W., Wang X.-Y., Xu S.-Y., Zhu Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front. Microbiol. 2017;8:2146. doi: 10.3389/fmicb.2017.02146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 11.Winberg P.C., Ghosh D., Tapsell L. Seaweed culture in integrated multi-trophic aquaculture-Nutritional benefits and systems for Australia. University of Wollongong Australia Web site. [(accessed on 30 June 2019)]; Available online: https://ro.uow.edu.au/smfc/5/
- 12.AgriFutures Australia Home page. Cultivated seaweed. [(accessed on 30 June 2019)]; Available online: https://www.agrifutures.com.au/farm-diversity/cultivated-seaweed/
- 13.Bindu M., Levine I.A. The commercial red seaweed Kappaphycus alvarezii-an overview on farming and environment. J. Appl. Phycol. 2011;23:789–796. doi: 10.1007/s10811-010-9570-2. [DOI] [Google Scholar]
- 14.Panchal S.K., Poudyal H., Iyer A., Nazer R., Alam A., Diwan V., Kauter K., Sernia C., Campbell F., Ward L., et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011;57:611–624. doi: 10.1097/FJC.0b013e3181feb90a. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S.A., Magnusson M., Ward L.C., Paul N.A., Brown L. Seaweed supplements normalise metabolic, cardiovascular and liver responses in high-carbohydrate, high-fat fed rats. Mar. Drugs. 2015;13:788–805. doi: 10.3390/md13020788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S.A., Magnusson M., Ward L.C., Paul N.A., Brown L. A Green algae mixture of Scenedesmus and Schroederiella attenuates obesity-linked metabolic syndrome in rats. Nutrients. 2015;7:2771–2787. doi: 10.3390/nu7042771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scientific Committee on Food . Opinion of the Scientific Committee on Food on Carrageenan (SCF/CS/ADD/EMU/199 Final) Scientific Committee on Food; Brussels, Belgium: 2003. [Google Scholar]
- 18.David S., Levi C.S., Fahoum L., Ungar Y., Meyron-Holtz E.G., Shpigelman A., Lesmes U. Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018;9:1344–1352. doi: 10.1039/C7FO01721A. [DOI] [PubMed] [Google Scholar]
- 19.Dewar E.T., Maddy M.L. Faecal excretion of degraded and native carrageenan by the young rat. J. Pharm. Pharmacol. 1970;22:791–793. doi: 10.1111/j.2042-7158.1970.tb08437.x. [DOI] [PubMed] [Google Scholar]
- 20.Weiner M.L. Food additive carrageenan: Part II: A critical review of carrageenanin in vivo safety studies. Crit. Rev. Toxicol. 2014;44:244–269. doi: 10.3109/10408444.2013.861798. [DOI] [PubMed] [Google Scholar]
- 21.Mallett A.K., Rowland I.R., Bearne C.A., Nicklin S. Influence of dietary carrageenans on microbial biotransformation activities in the cecum of rodents and on gastrointestinal immune status in the rat. Toxicol. Appl. Pharmacol. 1985;78:377–385. doi: 10.1016/0041-008X(85)90243-1. [DOI] [PubMed] [Google Scholar]
- 22.McKim J.M., Willoughby J.A., Sr., Blakemore W.R., Weiner M.L. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: A review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Crit. Rev. Food Sci. Nutr. 2019;59:3054–3073. doi: 10.1080/10408398.2018.1481822. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S.M., Ito N. A critical review of the toxicological effects of carrageenan and processed Eucheuma seaweed on the gastrointestinal tract. Crit. Rev. Toxicol. 2002;32:413–444. doi: 10.1080/20024091064282. [DOI] [PubMed] [Google Scholar]
- 24.Sanjeewa K.K.A., Lee W., Jeon Y.-J. Nutrients and bioactive potentials of edible green and red seaweed in Korea. Fish. Aquat. Sci. 2018;21:19. doi: 10.1186/s41240-018-0095-y. [DOI] [Google Scholar]
- 25.Fukuda S., Saito H., Nakaji S., Yamada M., Ebine N., Tsushima E., Oka E., Kumeta K., Tsukamoto T., Tokunaga S. Pattern of dietary fiber intake among the Japanese general population. Eur. J. Clin. Nutr. 2007;61:99–103. doi: 10.1038/sj.ejcn.1602505. [DOI] [PubMed] [Google Scholar]
- 26.Buse K. The World Bank. Heal. Policy Plan. 1994;9 doi: 10.1093/heapol/9.1.95. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.O., Komarek A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017;1:47–59. doi: 10.1093/fqs/fyx007. [DOI] [Google Scholar]
- 28.Slavin J.L. Position of the American Dietetic Association: health implications of dietary fiber. J. Am. Diet. Assoc. 2008;108:1716–1731. doi: 10.1016/j.jada.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Tucker L.A., Thomas K.S. Increasing total fiber intake reduces risk of weight and fat gains in women. J. Nutr. 2009;139:576–581. doi: 10.3945/jn.108.096685. [DOI] [PubMed] [Google Scholar]
- 30.Meyer K.A., Kushi L.H., Jacobs D.R., Jr., Slavin J., Sellers T.A., Folsom A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000;71:921–930. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 31.Streppel M.T., Ocké M.C., Boshuizen H.C., Kok F.J., Kromhout D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: the Zutphen Study. Am. J. Clin. Nutr. 2008;88:1119–1125. doi: 10.1093/ajcn/88.4.1119. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J., Martin R.J., Raggio A.M., Shen L., McCutcheon K., Keenan M.J. The importance of GLP-1 and PYY in resistant starch’s effect on body fat in mice. Mol. Nutr. Food Res. 2015;59:1000–1003. doi: 10.1002/mnfr.201400904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson S.V., Hannon B.A., An R., Holscher H.D. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017;106:1514–1528. doi: 10.3945/ajcn.117.163246. [DOI] [PubMed] [Google Scholar]
- 34.Tobacman J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Heal. Perspect. 2001;109:983–994. doi: 10.1289/ehp.01109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson G.R., Scott K.P., Rastall R.A., Tuohy K.M., Hotchkiss A., Dubert-Ferrandon A., Gareau M., Murphy E.F., Saulnier D., Loh G., et al. Dietary prebiotics: current status and new definition. Food Sci. Technol. Bull. Funct. Foods. 2010;7:1–19. doi: 10.1616/1476-2137.15880. [DOI] [Google Scholar]
- 36.Lattimer J.M., Haub M.D. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2:1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S.A., Ward L.C., Brown L. Inulin oligofructose attenuates metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Br. J. Nutr. 2016;116:1502–1511. doi: 10.1017/S0007114516003627. [DOI] [PubMed] [Google Scholar]
- 38.Liu J., Kandasamy S., Zhang J., Kirby C.W., Karakach T., Hafting J., Critchley A.T., Evans F., Prithiviraj B. Prebiotic effects of diet supplemented with the cultivated red seaweed Chondrus crispus or with fructo-oligo-saccharide on host immunity, colonic microbiota and gut microbial metabolites. BMC Complement. Altern. Med. 2015;15:279. doi: 10.1186/s12906-015-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez-Ordóñez E., Jiménez-Escrig A., Rupérez P. Effect of the red seaweed Mastocarpus stellatus intake on lipid metabolism and antioxidant status in healthy Wistar rats. Food Chem. 2012;135:806–811. doi: 10.1016/j.foodchem.2012.04.138. [DOI] [PubMed] [Google Scholar]
- 40.de Jesus Raposo M.F., de Morais A.M., de Morais R.M. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs. 2016;14:27. doi: 10.3390/md14020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lordan C., Thapa D., Ross R.P., Cotter P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes. 2019;11:1–20. doi: 10.1080/19490976.2019.1613124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Channiwala S., Parikh P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel. 2002;81:1051–1063. doi: 10.1016/S0016-2361(01)00131-4. [DOI] [Google Scholar]
- 43.Gosch B.J., Magnusson M., Paul N.A., De Nys R. Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy. 2012;4:919–930. doi: 10.1111/j.1757-1707.2012.01175.x. [DOI] [Google Scholar]
- 44.Gómez-Ordóñez E., Rupérez P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011;25:1514–1520. doi: 10.1016/j.foodhyd.2011.02.009. [DOI] [Google Scholar]
- 45.Zhou J., A Bruns M., Tiedje J.M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 1996;62:316–322. doi: 10.1128/AEM.62.2.316-322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Illumina MiSeq system user guide. In Illumina Inc., FU Berlin. [(accessed on 30 June 2019)]; Available online: https://en.novogene.com/technology/illumina-systems.
- 47.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinform. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 49.Wemheuer B., Wemheuer F. Assessing bacterial and fungal diversity in the plant endosphere. Methods Mol. Biol. 2017;1539:75–84. doi: 10.1007/978-1-4939-6691-2_6. [DOI] [PubMed] [Google Scholar]
- 50.Ozkan J., Willcox M., Wemheuer B., Wilcsek G., Coroneo M., Thomas T. Biogeography of the human ocular microbiota. Ocul. Surf. 2019;17:111–118. doi: 10.1016/j.jtos.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glockner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: Architecture and applications. BMC Bioinformat. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oksanen J., Kindt R., Legendre P., O’Hara B., Simpson G.L., Solymos P., Stevens M.H.H., Wagner H. Vegan: Community Ecology Package. R Project Institute for Statistics and Mathematics, WU Wirtschaftsuniversität Wien; Wien, Austria: 2010. [(accessed on 15 August 2019)]. R package version: 1.15-4. Available online: http://CRAN.R-project.org/package=vegan. [Google Scholar]
- 55.Clarke K., Gorley R. PRIMER v6. [(accessed on 30 March 2019)];2006 Available online: https://www.researchgate.net/publication/235425881_Primer_v6_User_ManualTutorial.
- 56.Anderson M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]