Abstract
Bladder cancer (BlCa) is a common malignancy with significant morbidity and mortality. Current diagnostic methods are invasive and costly, showing the need for newer biomarkers. Although several epigenetic-based biomarkers have been proposed, their ability to discriminate BlCa from common benign conditions of the urinary tract, especially inflammatory diseases, has not been adequately explored. Herein, we sought to determine whether VIMme and miR663ame might accurately discriminate those two conditions, using a multiplex test. Performance of VIMme and miR663ame in tissue samples and urines in testing set confirmed previous results (96.3% sensitivity, 88.2% specificity, area under de curve (AUC) 0.98 and 92.6% sensitivity, 75% specificity, AUC 0.83, respectively). In the validation sets, VIMme-miR663ame multiplex test in urine discriminated BlCa patients from healthy donors or patients with inflammatory conditions, with 87% sensitivity, 86% specificity and 80% sensitivity, 75% specificity, respectively. Furthermore, positive likelihood ratio (LR) of 2.41 and negative LR of 0.21 were also disclosed. Compared to urinary cytology, VIMme-miR663ame multiplex panel correctly detected 87% of the analysed cases, whereas cytology only forecasted 41%. Furthermore, high miR663ame independently predicted worse clinical outcome, especially in patients with invasive BlCa. We concluded that the implementation of this panel might better stratify patients for confirmatory, invasive examinations, ultimately improving the cost-effectiveness of BlCa diagnosis and management. Moreover, miR663ame analysis might provide relevant information for patient monitoring, identifying patients at higher risk for cancer progression.
Keywords: bladder cancer, methylation, biomarkers
1. Introduction
Bladder cancer (BlCa) is one of the most incident cancers, ranking ninth in prevalence worldwide [1,2]. In men, which are more prone to develop BlCa, it represents the second most frequent urological malignancy after prostate cancer [1,2]. Moreover, it is expected that, by 2040, the number of estimated new cases and cancer-related deaths will almost double the 549,393 newly diagnosed cases and 199,922 deaths recorded in 2018 [1,2]. Most BlCa cases correspond to urothelial carcinoma, generally presenting as non-muscle invasive BlCa (NMIBC), accounting for 75–80% of all new cases, characterised by frequent recurrences and eventual progression to more aggressive, deeply invasive and metastatic disease, or muscle-invasive BlCa (MIBC), an aggressive, locally invading carcinoma, corresponding to 20–25% of all cases, with propensity for metastisation [3,4]. Haematuria is the most common clinical sign of BlCa, although it also occurs in several common benign disease such as urinary tract infections and non-infectious inflammatory conditions. Presently, BlCa diagnosis generally involves cytoscopic examination, an expensive and invasive procedure, complemented by urine cytology [5,6,7]. However, the latter has limited accuracy, particularly for identification of low-grade papillary tumours, and the invasive nature of cystoscopic examination entails patient discomfort and, in some cases, infection [5]. Moreover, because of the high incidence, recurrence and progression rate, active long follow-up is required, making BlCa the costliest malignancy [8]. Thus, early, accurate and non-invasive BlCa detection is the determinant to improve both patients and healthcare financial management.
Epigenetic changes, including DNA methylation, have been largely investigated for cancer detection [9]. Owing to chemical and biological stability, DNA methylation-based biomarkers have potential clinical applications in early cancer detection, diagnosis, follow-up and targeted therapies [10]. Previously, two independent DNA methylation-based biomarker panels have been reported as promising tests for accurate early detection of BlCa [11,12]. In 2010, a three-gene panel comprised GDF15, TMEFF2 and VIM methylation identified BlCa with 94% sensitivity and 100% specificity in urine samples from 51 BlCa patients [11]. More recently, a panel testing the promoter methylation of two microRNAs—miR129-2 and miR663a—identified urothelial carcinoma (from upper and lower urinary tracts) with a sensitivity of 87.8% and specificity of 82.7% in 49 urine samples from patients with urothelial carcinoma [12]. Furthermore, the same panels could discriminate BlCa from other common genitourinary cancers (i.e., from kidney and prostate). Nonetheless, both studies used a singleplex approach, and the ability of these tests to discriminate BlCa from common benign conditions of the urinary tract with overlapping manifestations, especially inflammatory diseases, has not been adequately explored, thus far. Indeed, inflammatory conditions of the urinary tract may negatively impact the specificity of urinary-based biomarkers for BlCa detection, increasing false positive results and entailing unnecessary complementary invasive tests [6,13,14].
Thus, we sought to assess whether the most promising markers in each published panel—miR-663a (miR663ame) and Vimentin (VIMme)—might accurately discriminate BlCa from inflammatory conditions in voided urine, allowing for the development of a multiplex test that could be used for early detection in clinical practice.
2. Experimental Section
2.1. Patients and Tumour Sample Collection
Ninety-four primary BlCa tissue samples were obtained from a consecutive series of patients diagnosed, treated with transurethral resection (TUR) or radical cystectomy, between 1994 and 2011, and followed at Portuguese Oncology Institute of Porto (IPO Porto), Portugal (Table 1). Briefly, tumour samples were obtained during surgery and immediately snap-frozen, stored at −80 °C and subsequently macrodissected for tumours’ cells enrichment and cut in cryostat for DNA extraction. Routine collection and processing of tissue samples allowed for pathological examination, classification, grading and staging [15]. For control purposes, an independent set of 19 normal bladder mucosae (NB) samples were also collected from BlCa-free individuals (prostate cancer patients submitted to radical prostatectomy) (Table 1).
Table 1.
Clinical and histopathological characteristics of patients with bladder carcinoma (BlCa), normal bladder mucosae (NB), healthy donors (HD) and inflammatory controls (IC).
| Tissues | Urines | ||||||
|---|---|---|---|---|---|---|---|
| Testing Set | Validation Sets | ||||||
| Clinicopaphological Features | Bladder UC | Normal Bladder Mucosae | Bladder UC | Healthy Donors | Bladder UC | Healthy Donors (#1) | Inflammatory Controls (#2) |
| Patients, n | 94 | 19 | 27 | 24 | 100 | 57 | 174 |
| Gender, n | |||||||
| Males | 78 | 19 | 20 | 13 | 79 | 16 | 132 |
| Females | 16 | 0 | 7 | 12 | 21 | 41 | 42 |
| Median age, yrs (range) | 69 (45–91) |
63 (48–75) |
69 (47–88) |
45 (39–61) |
68 (38–91) |
49 (41–64) |
64 (18–92) |
| Grade, n | |||||||
| Papillary, low-grade | 34 | n.a. | 13 | n.a. | 51 | n.a. | n.a. |
| Papillary, high-grade | 33 | n.a. | 8 | n.a. | 26 | n.a. | n.a. |
| Invasive, high-grade | 27 | n.a. | 6 | n.a. | 23 | n.a. | n.a. |
| Invasion of Muscular Layer, n | |||||||
| NMIBC | 67 | n.a. | 19 | n.a. | 77 | n.a. | n.a. |
| MIBC | 27 | n.a. | 8 | n.a. | 23 | n.a. | n.a. |
#1—Validation Set #1; #2—Validation Set #2; yrs—years; n.a.—non applicable; NMIBC—Non-Muscle Invasive Bladder Cancer; MIBC—Muscle Invasive Bladder Cancer, UC—Urothelial Carcinoma.
2.2. Urine Sample Collection and Processing
For the “Testing sets”, 27 voided urine samples (one per patient) were collected from BlCa patients, diagnosed and treated between 2006 and 2016 at IPO Porto, as well as a set of 24 voided urine samples from healthy donors (HD), also from IPO Porto, with no personal or familial history of cancer, used as controls (Table 1). The “Validation sets” comprised: (1) 100 urine samples from BlCa patients, diagnosed and treated between 2002 and 2016 at IPO Porto, and 57 urine samples from HD collected at IPO Porto, and (2) an independent set of control urine sediments (n = 174) from patients diagnosed with urinary tract inflammatory conditions (IC), diagnosed between 2008 and 2014 at the University Hospital of Cordoba (UHC). All BlCa patients’ urines were obtained before treatment. Moreover, all sets of samples were collected from different cohorts of patients. Informed consent was obtained from patients and controls after approval from the ethics committees of IPO Porto and UHC (CES-IPO 019/08, approval date: 16th January 2008). All urine samples were processed by immediate centrifugation at 4000 rpm for 10 min; the respective pellet was washed twice with phosphate-buffered saline (PBS) and stored at −80 °C.
2.3. Nucleic Acids Isolation, Bisulfite Modification and Multiplex qMSP Analysis
DNA was extracted from frozen BlCa and NB tissues, and all urine sample sets, using a standard phenol-chloroform protocol [16], and its concentration determined using a Qubit 3 Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). Bisulfite modification was performed through sodium bisulfite, using the EZ DNA Methylation-Gold™ Kit (Zymo Research, Irvine, CA, USA), according to manufacturer’s protocol. For this, 1000 ng and 50 ng of DNA were converted for tissues and urine sediments, respectively. Quantitative methylation levels were performed using Xpert Fast Probe Master Mix (GRiSP, Porto, Portugal), and multiplex reactions were run in triplicates in 96-well plates using an Applied Biosystems 7500 Sequence Detector (Perkin Elmer, Waltham, CA, USA), with Beta-Actin (ACTB) as internal reference gene for normalization. Primer and probe sequences were designed using Methyl Primer Express 1.0 and purchased from Sigma-Aldrich (St. Louis, MO, USA) (Supplementary Table S1). Additionally, six serial dilutions (dilution factor of 5×) of a fully methylated bisulphite modified universal DNA control were included in each plate to generate a standard curve. In each sample and for each gene, the relative DNA methylation levels were determined using the following formula: ((target gene/ACTB) ×1000). A run was considered valid when previously reported criteria were met [11].
2.4. Statistical Analysis
Differences in quantitative methylation values were assessed with the non-parametric Mann-Whitney U (MW) and Kruskal-Wallis (KW) tests. Associations between age, gender, grade, invasion of muscular layer and methylation levels were carried out using Spearman’s correlation, MW or KW tests, as appropriate. For multiple comparisons, Bonferroni’s correction was applied in pairwise comparisons.
Biomarker performance parameters, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy and positive and negative likelihood ratios (LR), were estimated [17]. Receiver operator characteristics (ROC) curves were constructed by plotting the true positive (sensitivity) against false positive (1-specificity) rate, and the area under the curve (AUC) was calculated. The higher value obtained from the sum of sensitivity and 1-specificity in each ROC-curve was used as cut-off to categorise samples as methylated or non-methylated. ROC curves were constructed using logistic regression model for DNA methylation panel. Disease-specific and disease-free survival curves (Kaplan-Meier with log rank test) were computed for standard variables and for categorised genes’ promoter methylation status. A Cox-regression model comprising all significant variables (univariable and multivariable model) was computed to assess the relative contribution of each variable to the follow-up status. All two-tailed p values were derived from statistical tests, using a computer-assisted program (SPSS Version 26.0, IBM, Armonk, NY, EUA) and the results were considered statistically significant at p < 0.05. Bonferroni’s correction for multiple comparisons was used when applicable.
3. Results
3.1. Methylation Analysis and Performance of the Multiplex Panel in BlCa Tissue Series
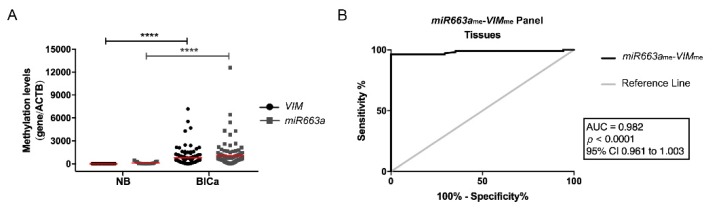
To confirm the previously published performance of miR663a and VIM promoter methylation as BlCa biomarkers, tissue samples were tested. As expected, both miR663a and VIM were found hypermethylated (76.6% and 94.4%, respectively) in most BlCa tissue samples, and methylation levels were significantly higher compared to NB (p < 0.0001 and p < 0.0001, respectively) (Figure 1A). The two genes independently performed well as BlCa detection biomarkers in tissues, with an AUC of 0.979 for VIMme (95% confidence interval (CI): 0.956–1.002, p < 0.0001), and of 0.897 for miR663ame (95% CI: 0.836–0.959, p < 0.0001). Moreover, in combination as multiplex panel, it accurately discriminated BlCa from NB with 96.3% sensitivity and 88.2% specificity, corresponding to an AUC of 0.982 (Figure 1B; Supplementary Table S2).
Figure 1.
(A) Distribution of VIMme and miR663ame levels in normal bladder mucosae (NB; n = 19) and bladder carcinoma (BlCa; n = 94) tissue samples. Mann-Whitney U test, **** p < 0.0001. Median is represented by the red line. (B) Receiver operator characteristic (ROC) curve evaluating the performance of the VIMme-miR663ame panel for the identification of BlCa in tissue samples. (AUC—Area under the curve; CI—Confidence interval; ACTB—Beta-Actin; VIM—Vimentin).
3.2. Methylation Analysis and Performance of Multiplex Panel in BlCa Testing Set
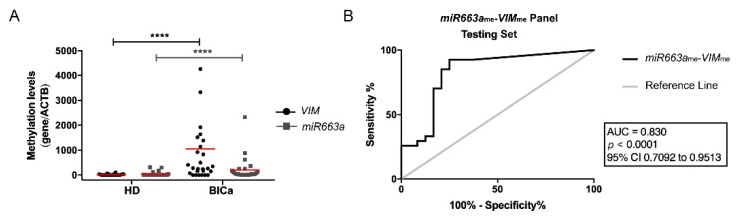
Paralleling the previous observations in tissues, miR663ame and VIMme levels were significantly higher in BlCa urine samples than in those of controls (p < 0.0001 and p < 0.0001, Figure 2A), and the multiplex panel discriminated BlCa from HD with 92.6% sensitivity and 90% NPV (Supplementary Table S2), corresponding to an AUC of 0.83 (Figure 2B).
Figure 2.
(A) Distribution of VIMme and miR663ame levels in the Testing Cohort, composed by healthy donors (HD; n = 24) and bladder carcinoma (BlCa; n = 27) urine samples. Mann-Whitney U test, **** p < 0.0001. Median is represented by the red line. (B) Receiver operator characteristic (ROC) curve evaluating the performance of the VIMme-miR663ame panel for the identification of BlCa in urine samples of the Testing Cohort. (AUC—Area under the curve; CI—Confidence interval; ACTB—Beta-Actin; VIM—Vimentin).
3.3. Methylation Analysis and Performance of VIMme and miR663me Multiplex Panel for BlCa vs. HD
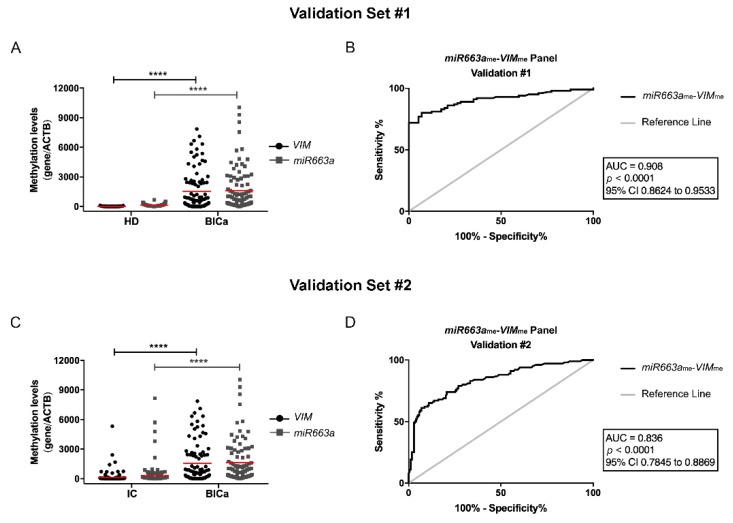
In line with the testing set results, a higher number of malignant samples disclosed significantly higher VIMme and miR663me levels than HDs (p < 0.0001 and p < 0.0001, respectively) in the validation sets (Figure 3A). ROC curve analysis confirmed a high discriminative ability of VIMme-miR663me panel, with an AUC of 0.91 (Figure 3B). Indeed, the multiplex panel discriminated BlCa from HD subjects with 87% sensitivity and 86% specificity (Table 2).
Figure 3.
(A) Distribution of VIMme and miR663ame levels in the Validation Cohort #1, composed by healthy donors (HD; n = 57) and bladder carcinoma (BlCa; n = 100) urine samples. Mann-Whitney U (MW) test, **** p < 0.0001. Median is represented by the red line. (B) Receiver operator characteristic (ROC) curve evaluating the performance of the VIMme-miR663ame panel for the identification of BlCa in urine samples of the Validation Cohort #1. (C) Distribution of VIMme and miR663ame levels in the Validation Cohort #2, composed by inflammatory controls (IC; n = 174) and bladder carcinoma (BlCa; n = 100) urine samples. MW test, **** p < 0.0001. (D) ROC curve evaluating the performance of the VIMme-miR663ame panel for the identification of BlCa in urine samples of the Validation Cohort #2. (AUC—Area under the curve; CI—Confidence interval; ACTB—Beta-Actin; VIM—Vimentin).
Table 2.
Performance of VIMme-miR663ame panel for the detection of bladder cancer in Validation Cohorts #1 and #2. (PPV—positive predictive value; NPV—negative predictive value).
| Samples | Biomarker Performance | miR663ame-VIMme (%) |
|---|---|---|
| Validation #1 | Sensitivity | 87.0 |
| Specificity | 86.0 | |
| PPV | 91.6 | |
| NPV | 79.0 | |
| Accuracy | 86.6 | |
| Validation #2 | Sensitivity | 80.0 |
| Specificity | 75.3 | |
| PPV | 65.0 | |
| NPV | 86.8 | |
| Accuracy | 77.0 |
PPV—Positive Predictive Value; NPV—Negative Predictive Value.
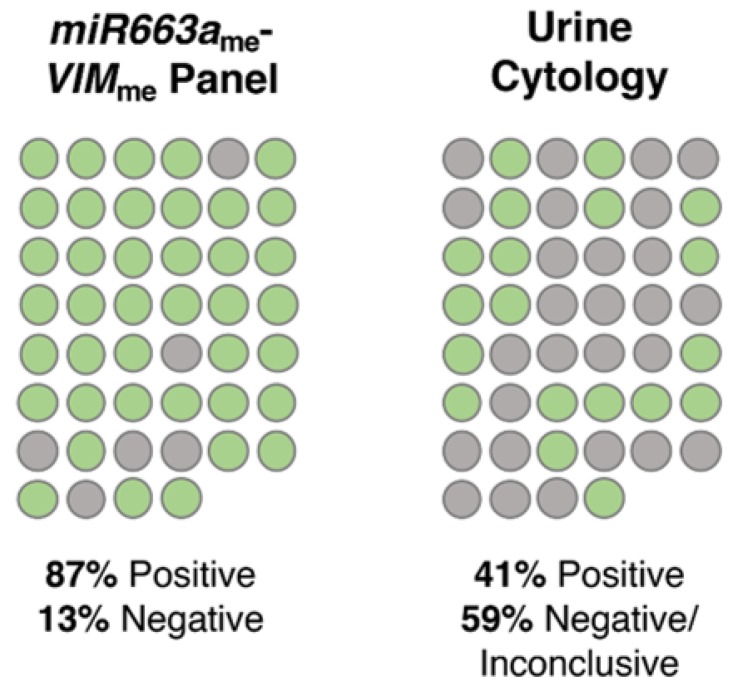
Remarkably, the proportion of true positive cases detected by the VIMme-miR663me multiplex panel was significantly higher than that of urine cytology (p < 0.001). Indeed, of 46 BlCa cases with valid urine cytology results, only 19 were classified as positive, 17 as negative and 10 as “inconclusive/suspicious”, corresponding to 41% sensitivity (Figure 4). Contrarily, the VIMme-miR663me multiplex panel correctly identified 40/46 cases as BlCa, corresponding to an overall sensitivity of 87% (Figure 4). Importantly, 12 of 14 low-grade papillary carcinomas were accurately identified by VIMme-miR663me multiplex panel, whereas cytology merely identified four cases.
Figure 4.
Representation of the percentage of bladder cancer (BlCa) cases correctly identified with the VIMme-miR663ame panel and a standard urine cytology analysis. Green circles represent positive cases, grey circles represent negative/inconclusive cases.
3.4. Methylation Analysis and Performance of VIMme and miR663me Multiplex Panel for BlCa vs. IC
In urine samples, VIMme-miR663me levels discriminated BlCa from IC patients (Figure 3C), with 80% sensitivity, 75.3% specificity and, importantly, 86.8% NPV (Table 2), corresponding to an AUC of 0.836 (Figure 3D). Remarkably, a 2.86 Positive LR and a Negative LR of 0.21 were also disclosed by VIMme-miR663me multiplex panel in this setting.
3.5. Clinicopathologic Correlations and Survival Analyses
High-grade papillary BlCa showed significantly higher miR663ame levels than low-grade papillary BlCa (p = 0.007), in tissue samples. The same was observed in urine samples from the validation set (p = 0.0072), a result which was extensive to VIMme (p = 0.0052) (Supplementary Figure S1). No additional associations were disclosed between VIMme and miR663ame levels and other standard clinical variables, including patients’ age and gender.
Follow-up data was available for 91 (out of 94) IPO Porto’s BlCa patients that provided tissue samples. The median follow-up time was 66 months (range: 1–203 months). At the last follow-up timepoint, 30 patients were alive with no evidence of cancer, 12 patients were alive with disease, 29 had deceased due to BlCa and 23 died from other causes. Univariable and multivariable Cox regression analysis were performed, including the variables grade, invasion of muscular layer, gender and age. As expected, a poor outcome was depicted for patients with higher grade and muscle invasive BlCa (p = 0.001 and p < 0.0001, respectively) (Table 3). In the multivariate model for disease-specific survival, miR663ame levels, higher grade and muscle invasion were independent predictors of outcome (p = 0.04, p = 0.035 and p = 0.031, respectively; Table 3). Moreover, after categorization into NMIBC vs. MIBC, tumours with higher miR663ame levels implied a 3.7-fold increased risk of cancer-related death among patients with MIBC (95% CI: 1.32–10.25, p = 0.013; Supplementary Figure S2). Contrarily, no associations were found for miR663ame or VIMme levels concerning disease-free survival.
Table 3.
Cox regression models assessing the potential of clinical and VIMme and miR663ame levels in the prediction of disease-specific survival for bladder carcinoma (BlCa) patients.
| Disease-specific Survival | Variables | Hazard Ratio (HR) | 95% CI for OR | p |
|---|---|---|---|---|
| Univariate | Invasion of muscular layer | 6.15 | 2.76–13.72 | 0.0001 |
| Grade | ||||
| PLG vs. PHG | 15.59 | 2.03–119.94 | 0.008 | |
| PLG vs. IHG | 32.83 | 4.31–250.06 | 0.001 | |
| Age | 2.34 | 0.98–5.59 | 0.060 | |
| Gender | 1.02 | 0.39–2.70 | 0.970 | |
| miR663a methylation ≤ median | 1.61 | 0.75–3.48 | 0.225 | |
| VIM methylation ≤ median | 1.07 | 0.50–2.28 | 0.861 | |
| Multivariate | Invasion of muscular layer | 3.54 | 1.12–11.19 | 0.031 |
| Grade | ||||
| PLG vs. PHG | 8.03 | 0.97–66.32 | 0.053 | |
| PLG vs. IHG | 11.89 | 1.18–119.37 | 0.035 | |
| miR663a methylation ≤ median | 2.67 | 1.05–6.81 | 0.040 | |
| VIM methylation ≤ median | 1.12 | 0.51–2.42 | 0.783 |
CI—confidence interval; OR—odds ratio; PLG—papillary low-grade; PHG—papillary high-grade; IHG—invasive high-grade.
4. Discussion
Bladder cancer is a major health concern worldwide, with an expected significant increase in incidence and mortality within the next two decades [1,2]. Early detection is critical for adequate management, aiming to reduce disease-specific mortality, as well as the economic burden imposed by BlCa treatment and follow-up. Because currently available diagnostic tools require invasive examination [13,14], development of non-invasive and less costly tests for early detection and monitoring are likely to have a significant impact in clinical practice. Although several molecular biomarkers, including epigenetic-based, have been developed for that end, discrimination of BlCa from other urinary tract malignancies and, more importantly, from benign conditions causing haematuria, including inflammatory diseases, remains a challenge. Indeed, most control samples used in biomarker discovery studies, including our own, mostly comprise normal/healthy donors, disregarding the fact that a biomarker-based test would be offered to an “at-risk” population, including patients experiencing suspicious symptoms. Therefore, based on two previously published studies by our research team [11,12], we tested whether a miR663ame and VIMme multiplex panel could accurately discriminate BlCa from normal individuals and those afflicted with inflammatory conditions of the genitourinary tract.
Because both miR663ame and VIMme were previously assessed using two different “simplex” multi-gene biomarker panels, we firstly tested miR663ame and VIMme in multiplex in a consecutive series of primary BlCa tissue samples and normal urothelial mucosae to confirm those previous results. Indeed, employing a multiplex reaction allows for downscaling the initial tissue/body fluid sample requirements, but also the quantity of DNA required for each test [18]. Remarkably, as expected, the miR663ame-VIMme multiplex panel discriminated BlCa from NB tissues with high sensitivity and specificity (96.3% and 88.2%, respectively), confirming the previous observations for the two markers separately [11,12]. In urine samples from the testing set, although the performance of the multiplex panel was slightly inferior to that of tissues, 92.6% sensitivity and 90% NPV was reached. Indeed, it should be recalled that a relatively small number of cancer cells are exfoliated into urine, which are subsequently “diluted” among a larger population of normal-looking urothelial cells. Thus, the tumour DNA content in urine is actually minute [19] and sensitivity over 90% should be regarded as a very encouraging result. Furthermore, in the validation set, comprising a larger independent cohort, specificity of the miR663ame-VIMme multiplex panel increased to 86%, further increasing the potential usefulness of the test.
It should be emphasised, however, that the foremost aim of this study was to assess the multiplex panel ability to discriminate BlCa from IC, since this panel is envisaged to be tested in an “at-risk” population, including individuals complaining of haematuria, many of which will be found to harbour urinary tract inflammatory conditions. Although, in this setting, sensitivity and specificity were slightly reduced, NPV increased (86.8%), which is an important finding [20]. Indeed, it is expected that among tested individuals, most will not have a neoplastic condition and, thus, the higher the NPV, the larger the proportion of those subjects that will not be submitted to confirmatory, invasive, procedures, supporting the good performance of the test in discriminating patients negative for malignant condition. Importantly, an LR (+) of 2.86 and an LR (−) of 0.21 values were observed, indicating that a negative result decreases by 30% the probability of misdiagnosis [17].
Despite the fact that several studies suggest various genomic mutations and/or proteins’ expression deregulation as biomarkers for BlCa detection and prognostication [21], the search for novel epigenetic biomarkers, mostly DNA methylation-based, for BlCa detection has been attempted by several research teams, probably due to the stability of the markers and the possibility of high-throughput tests. Although some of those previous studies report an apparently superior performance to the panel reported herein, it should be recalled that in most cases the patients’ series were smaller, only healthy donors were included as controls or these were comprised of a mixed group of healthy donors and patients with diverse urological diseases, and/or did not use a multiplex approach, which might impact in sample availability, testing time length and cost [22,23,24,25,26,27,28]. Roperch et al. proposed a three gene multiplex methylation panel (HS3ST2, SEPTIN9 and SLIT2) combined with FGFR3 mutations assessment, age and smoking-status at time of diagnosis in a multivariate model, for diagnosis of NMIBC in urine samples, disclosing 97.6% sensitivity and 84.8% specificity, in a smaller control cohort [29]. Nonetheless, this strategy might be more difficult to implement in clinical practice, since it requires both mutation and methylation analyses, in which the multiplex is performed in two distinct gene duplex reactions. Similarly, Dahmcke et al. proposed a six gene methylation panel (SALL3, ONECUT2, CCNA1, BCL2, EOMES and VIM) combined with the mutational analysis of TERT and FGFR3, for early detection of BlCa, in urine samples, comparing BlCa patients and patients with gross haematuria [30]. Although this panel disclosed higher sensitivity (97%), specificity was similar (76.9%) [30], and, once again, our test uses a single technique in a single reaction, requiring less amount of sample, enabling shorter response time, reduced technical skills and lower cost.
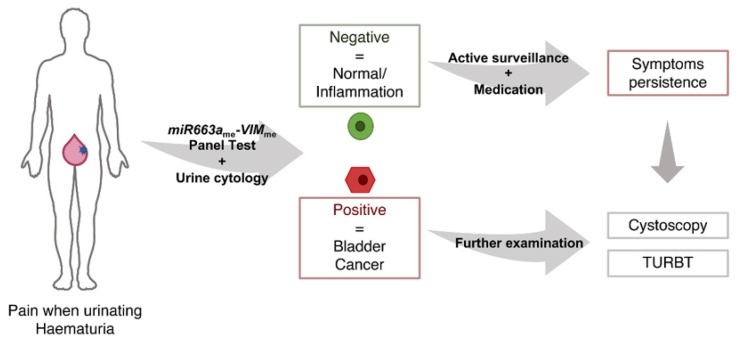
Although urine cytology and UroVysionTM fluorescence in situ hybridization (FISH) assay are the two most commonly used urine-based tests in daily practice, they present important limitations. On one hand, UroVysionTM presents a not-negligible rate of false positive results; on the other hand, urine cytology has limited accuracy, especially in low grade tumours detection [6,31,32]. Although no direct comparison can be done with UroVysionTM, the 91.6% PPV obtained for the multiplex panel clearly demonstrates higher accuracy in identifying true positive BlCa cases. In the present study, urine cytology reached 41% sensitivity, which was easily surpassed by the 86% displayed by miR663ame-VIMme multiplex panel. Notwithstanding, urine cytology remains an easy-to-perform and informative test, as it allows pathologists to have the first look at exfoliated neoplastic cells in urine. Having that in mind, we propose an algorithm where a urine cytology and the miR663ame-VIMme multiplex panel could be combined as first-line diagnostic tests in patients with common urinary complaints, with the ultimate goal of reducing the number of unnecessary cystoscopies, which are invasive, uncomfortable and costly procedures (Figure 5).
Figure 5.
Proposed algorithm for the combination of urine cytology and VIMme-miR663ame panel as a first-line diagnostic tests in patients with common urinary complaints. (TURBT—Transurethral Resection of Bladder Tumour).
In this work, we further explored the prognostic ability of the gene methylation markers, aiming to strengthen its clinical potential. Interestingly, survival analysis revealed that high miR663ame levels independently predicted poor disease-specific survival in BlCa patients, especially those with MIBC. Thus, the miR663ame-VIMme multiplex panel not only conveys diagnostic, but also prognostic information.
Taking into account the promising results obtained, unveiling the putative biological relevance of miR663a and VIM promoter methylation in bladder carcinogenesis may provide new important insights. VIM encodes for vimentin, an intermediate filament characteristic of cells with mesenchymal phenotype, not expressed in most normal epithelia (including urothelium), nor in most carcinomas [33]. VIM de-novo expression or overexpression has been reported in various epithelial cancers, including those of prostate [34], breast [35] and lung [36], associating with increased tumour growth and invasion. In these instances, vimentin expression has been associated with epithelial to mesenchymal transition (EMT), a biological process associated with tumour invasiveness [33]. Although VIM promoter methylation has been proposed as a detection and/or prognostic marker for other malignancies, biological functions are yet to be truly explored. Moreover, microRNAs have been extensively implicated in urological malignancies [37]. Interestingly, a dual role has already been described for miR663a, having a tumour suppressive activity in thyroid carcinoma [38] and glioblastoma [39], whereas an oncogenic function was reported in prostate cancer [40] and osteosarcoma [41]. Additionally, miR663a’s downregulation fostered cell proliferation by JunD overexpression in small-cell lung carcinoma [42], and HMGA2 in hepatocellular carcinoma [43], while Transforming Growth Factor-1 (TGF-β1) [44] overexpression was linked with invasion in the tumour type. Nevertheless, it should be recalled that not all biomarkers require to have a relevant biological role in tumorigenesis.
Importantly, to assure accuracy and validity of the proposed methylation multiplex test, additional validation by others, with larger sets of samples from prospectively collected data (from both BlCa and inflammatory conditions) is warrant.
5. Conclusions
In summary, we demonstrated that a miR663ame-VIMme multiplex panel accurately identifies BlCa, allowing for precise identification of this common neoplasm in urine samples. Importantly, it also discriminates BlCa patients from those with urinary tract inflammatory conditions, although with inferior performance comparatively to healthy subjects. Thus, the implementation of this panel might assist clinicians in better stratifying patients for confirmatory, invasive examinations, ultimately improving the cost-effectiveness of BlCa diagnosis and management. Moreover, in the same analysis, miR663ame analysis would identify patients at higher risk for cancer progression, further highlighting the promise of this panel for patient monitoring.
Acknowledgments
The authors are grateful to the patients that volunteered to provide samples and to all the personnel of the Departments of Pathology (section of Cytopathology) and of Urology of Portuguese Oncology Institute of Porto that kindly collaborated in this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/2/605/s1, Figure S1: Distribution of miR663ame and VIMme levels in bladder carcinoma tissue samples categorised by grade; Figure S2: Kaplan-Meyer curves representing disease-specific survival according to miR663ame status; Table S1: Sequences of the primers and probes used in the quantitative methylation-specific PCR experiments; Table S2: Performance of VIMme, miR663ame and VIMme-miR663ame panel for the detection of bladder cancer in Tissues and Testing Set.
Author Contributions
Conceptualization, S.M.-R., R.H. and C.J.; methodology, S.M.-R., A.B., J.T.-M., I.C., D.M., P.M. and J.O.; formal analysis, S.M.-R. and L.A.; writing—original draft preparation, S.M.-R.; review and editing, R.H., C.J. and A.L.-B.; supervision, R.H. and C.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Center of Portuguese Institute of Porto (CI-IPOP-27-2016). S.M-R. was supported by the FCT—Fundação para a Ciência e Tecnologia Grant (SFRH/BD/112673/2015).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Tomorrow. [(accessed on 26 October 2019)]; Available online: https://gco.iarc.fr/tomorrow/home.
- 2.Antoni S., Ferlay J., Soerjomataram I., Znaor A., Jemal A., Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Sanli O., Dobruch J., Knowles M.A., Burger M., Alemozaffar M., Nielsen M.E., Lotan Y. Bladder cancer. Nat. Rev. Dis. Primers. 2017;3:17022. doi: 10.1038/nrdp.2017.22. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Cancer Research . In: WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. Moch H., Ulbright T., Humphrey P., Reuter V., editors. IARC; Lyon, France: 2016. [Google Scholar]
- 5.Kaufman D.S., Shipley W.U., Feldman A.S. Bladder cancer. Lancet (London, England) 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 6.Babjuk M., Burger M., Comperat E.M., Gontero P., Mostafid A.H., Palou J., van Rhijn B.W.G., Roupret M., Shariat S.F., Sylvester R., et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Alfred Witjes J., Lebret T., Comperat E.M., Cowan N.C., De Santis M., Bruins H.M., Hernandez V., Espinos E.L., Dunn J., Rouanne M., et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017;71:462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Leal J., Luengo-Fernandez R., Sullivan R., Witjes J.A. Economic Burden of Bladder Cancer Across the European Union. Eur. Urol. 2016;69:438–447. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Esteller M. Epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 10.Costa-Pinheiro P., Montezuma D., Henrique R., Jeronimo C. Diagnostic and prognostic epigenetic biomarkers in cancer. Epigenomics. 2015;7:1003–1015. doi: 10.2217/epi.15.56. [DOI] [PubMed] [Google Scholar]
- 11.Costa V.L., Henrique R., Danielsen S.A., Duarte-Pereira S., Eknaes M., Skotheim R.I., Rodrigues A., Magalhaes J.S., Oliveira J., Lothe R.A., et al. Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clin. Cancer Res. 2010;16:5842–5851. doi: 10.1158/1078-0432.CCR-10-1312. [DOI] [PubMed] [Google Scholar]
- 12.Padrao N.A., Monteiro-Reis S., Torres-Ferreira J., Antunes L., Leca L., Montezuma D., Ramalho-Carvalho J., Dias P.C., Monteiro P., Oliveira J., et al. MicroRNA promoter methylation: A new tool for accurate detection of urothelial carcinoma. Br. J. Cancer. 2017;116:634–639. doi: 10.1038/bjc.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller M.T., Tublin M.E. In search of a consensus: Evaluation of the patient with hematuria in an era of cost containment. Am. J. Roentgenol. 2014;202:1179–1186. doi: 10.2214/AJR.13.12266. [DOI] [PubMed] [Google Scholar]
- 14.Grover S., Srivastava A., Lee R., Tewari A.K., Te A.E. Role of inflammation in bladder function and interstitial cystitis. Ther. Adv. Urol. 2011;3:19–33. doi: 10.1177/1756287211398255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphrey P.A., Moch H., Cubilla A.L., Ulbright T.M., Reuter V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016;70:106–119. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Pearson H., Stirling D. DNA extraction from tissue. In: Bartlett J.M.S., Stirling D., editors. PCR Protocols. 2nd ed. Humana Press; Totowa, NJ, USA: 2003. [Google Scholar]
- 17.McGee S. Simplifying likelihood ratios. J. Gen. Intern. Med. 2002;17:646–649. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guest P.C. Multiplex Biomarker Approaches to Enable Point-of-Care Testing and Personalized Medicine. Methods Mol. Biol. 2017;1546:311–315. doi: 10.1007/978-1-4939-6730-8_28. [DOI] [PubMed] [Google Scholar]
- 19.Larsen L.K., Lind G.E., Guldberg P., Dahl C. DNA-Methylation-Based Detection of Urological Cancer in Urine: Overview of Biomarkers and Considerations on Biomarker Design, Source of DNA, and Detection Technologies. Int. J. Mol. Sci. 2019;20:2657. doi: 10.3390/ijms20112657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anna K Füzéry D.W.C. Cancer Biomarker Assays: Performance Standards. In: Srivastava S., editor. Biomarkers in Cancer Screening and Early Detection. 1st ed. John Wiley & Sons; Hoboken, NJ, USA: 2017. pp. 267–276. [Google Scholar]
- 21.Tan W.S., Tan W.P., Tan M.Y., Khetrapal P., Dong L., de Winter P., Feber A., Kelly J.D. Novel urinary biomarkers for the detection of bladder cancer: A systematic review. Cancer Treat. Rev. 2018;69:39–52. doi: 10.1016/j.ctrv.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Chihara Y., Kanai Y., Fujimoto H., Sugano K., Kawashima K., Liang G., Jones P.A., Fujimoto K., Kuniyasu H., Hirao Y. Diagnostic markers of urothelial cancer based on DNA methylation analysis. BMC Cancer. 2013;13:275. doi: 10.1186/1471-2407-13-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Yu Y., Ye R., Zhang D., Li Q., An D., Fang L., Lin Y., Hou Y., Xu A., et al. An epigenetic biomarker combination of PCDH17 and POU4F2 detects bladder cancer accurately by methylation analyses of urine sediment DNA in Han Chinese. Oncotarget. 2016;7:2754–2764. doi: 10.18632/oncotarget.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yegin Z., Gunes S., Buyukalpelli R. Hypermethylation of TWIST1 and NID2 in tumor tissues and voided urine in urinary bladder cancer patients. DNA Cell Biol. 2013;32:386–392. doi: 10.1089/dna.2013.2030. [DOI] [PubMed] [Google Scholar]
- 25.Renard I., Joniau S., van Cleynenbreugel B., Collette C., Naome C., Vlassenbroeck I., Nicolas H., de Leval J., Straub J., Van Criekinge W., et al. Identification and validation of the methylated TWIST1 and NID2 genes through real-time methylation-specific polymerase chain reaction assays for the noninvasive detection of primary bladder cancer in urine samples. Eur. Urol. 2010;58:96–104. doi: 10.1016/j.eururo.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 26.Yu J., Zhu T., Wang Z., Zhang H., Qian Z., Xu H., Gao B., Wang W., Gu L., Meng J., et al. A novel set of DNA methylation markers in urine sediments for sensitive/specific detection of bladder cancer. Clin. Cancer Res. 2007;13:7296–7304. doi: 10.1158/1078-0432.CCR-07-0861. [DOI] [PubMed] [Google Scholar]
- 27.Sun J., Chen Z., Zhu T., Yu J., Ma K., Zhang H., He Y., Luo X., Zhu J. Hypermethylated SFRP1, but none of other nine genes “informative” for western countries, is valuable for bladder cancer detection in Mainland China. J. Cancer Res. Clin. Oncol. 2009;135:1717–1727. doi: 10.1007/s00432-009-0619-z. [DOI] [PubMed] [Google Scholar]
- 28.Chan M.W., Chan L.W., Tang N.L., Tong J.H., Lo K.W., Lee T.L., Cheung H.Y., Wong W.S., Chan P.S., Lai F.M., et al. Hypermethylation of multiple genes in tumor tissues and voided urine in urinary bladder cancer patients. Clin. Cancer Res. 2002;8:464–470. [PubMed] [Google Scholar]
- 29.Roperch J.P., Grandchamp B., Desgrandchamps F., Mongiat-Artus P., Ravery V., Ouzaid I., Roupret M., Phe V., Ciofu C., Tubach F., et al. Promoter hypermethylation of HS3ST2, SEPTIN9 and SLIT2 combined with FGFR3 mutations as a sensitive/specific urinary assay for diagnosis and surveillance in patients with low or high-risk non-muscle-invasive bladder cancer. BMC Cancer. 2016;16:704. doi: 10.1186/s12885-016-2748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahmcke C.M., Steven K.E., Larsen L.K., Poulsen A.L., Abdul-Al A., Dahl C., Guldberg P. A Prospective Blinded Evaluation of Urine-DNA Testing for Detection of Urothelial Bladder Carcinoma in Patients with Gross Hematuria. Eur. Urol. 2016;70:916–919. doi: 10.1016/j.eururo.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Brimo F., Vollmer R.T., Case B., Aprikian A., Kassouf W., Auger M. Accuracy of urine cytology and the significance of an atypical category. Am. J. Clin. Pathol. 2009;132:785–793. doi: 10.1309/AJCPPRZLG9KT9AXL. [DOI] [PubMed] [Google Scholar]
- 32.Lavery H.J., Zaharieva B., McFaddin A., Heerema N., Pohar K.S. A prospective comparison of UroVysion FISH and urine cytology in bladder cancer detection. BMC Cancer. 2017;17:247. doi: 10.1186/s12885-017-3227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satelli A., Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011;68:3033. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh S., Sadacharan S., Su S., Belldegrun A., Persad S., Singh G. Overexpression of vimentin: Role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003;63:2306–2311. [PubMed] [Google Scholar]
- 35.Kokkinos M.I., Wafai R., Wong M.K., Newgreen D.F., Thompson E.W., Waltham M. Vimentin and Epithelial-Mesenchymal Transition in Human Breast Cancer—Observations in vitro and in vivo. Cells Tissues Organs. 2007;185:191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 36.Al-Saad S., Al-Shibli K., Donnem T., Persson M., Bremnes R.M., Busund L.T. The prognostic impact of NF-kappaB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br. J. Cancer. 2008;99:1476–1483. doi: 10.1038/sj.bjc.6604713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jerónimo C., Henrique R. Epigenetic biomarkers in urological tumors: A systematic review. Cancer Lett. 2014;342:264–274. doi: 10.1016/j.canlet.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z., Zhang H., Zhang P., Dong W., He L. MicroRNA-663 suppresses cell invasion and migration by targeting transforming growth factor beta 1 in papillary thyroid carcinoma. Tumour Biol. 2015;37:7633–7644. doi: 10.1007/s13277-015-4653-y. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y., Chen C., Yu S., Liu Q., Rao J., Zhang H.R., Xiao H.L., Fu T.W., Long H., He Z., et al. MiR-663 suppresses oncogenic function of CXCR4 in glioblastoma. Clin. Cancer Res. 2015;21:4004–4013. doi: 10.1158/1078-0432.CCR-14-2807. [DOI] [PubMed] [Google Scholar]
- 40.Jiao L., Deng Z., Xu C., Yu Y., Li Y., Yang C., Chen J., Liu Z., Huang G., Li L.C., et al. MiR-663 induces castration-resistant prostate cancer transformation and predicts clinical recurrence. J. Cell Physiol. 2014;229:834–844. doi: 10.1002/jcp.24510. [DOI] [PubMed] [Google Scholar]
- 41.Huang C., Sun Y., Ma S., Vadamootoo A.S., Wang L., Jin C. Identification of circulating miR-663a as a potential biomarker for diagnosing osteosarcoma. Pathol. Res. Pract. 2019;215:152411. doi: 10.1016/j.prp.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Xu X., Zhang M., Wang X., Bai X., Li H., Kan L., Zhou Y., Niu H., He P. MicroRNA-663a is downregulated in non-small cell lung cancer and inhibits proliferation and invasion by targeting JunD. BMC Cancer. 2016;16:315. doi: 10.1186/s12885-016-2350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang W., Li J., Guo X., Zhao Y., Yuan X. MiR-663a inhibits hepatocellular carcinoma cell proliferation and invasion by targeting HMGA2. Biomed. Pharmacother. 2016;81:431–438. doi: 10.1016/j.biopha.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 44.Zhang C., Chen B., Jiao A., Li F., Sun N., Zhang G., Zhang J. MiR-663a inhibits tumor growth and invasion by regulating TGF-β1 in hepatocellular carcinoma. BMC Cancer. 2018;18:1179. doi: 10.1186/s12885-018-5016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.