Abstract
The histone H3K27M-mutant diffuse midline glioma is often seen in children and has a very poor prognosis regardless of its histological grade. Although it can occur in adults, few studies on adult cases have been reported. We examined adult midline glioma cases for their histological grade, presence of H3K27M mutation, and expression of related factors—enhancer of zeste homolog 2 (EZH2), H3K27me3, p16, and methylthioadenosine phosphorylase. These tumor characteristics were also evaluated for their prognostic value in adult midline glioma. High histological grade, H3K27M-mutant, high EZH2 expression, and high H3K27me3 expression was detected in 12/23 (53%), 11/23 (48%), 9/23 (39%), and 12/23 (52%) cases, respectively. Histological grade and prognosis were significantly correlated (P <0.01). The high expression of EZH2 and the low expression of H3K27me3 correlated with histological malignancy (P = 0.019 and 0.009) and prognosis (P = 0.048 and 0.047). To broaden the scope of our analysis, a review of cases reported in the literature (2014–2019) was performed. In the 171 cases, H3K27M-mutant showed poor prognosis in the young adult group (P = 0.001), whereas H3K27 status had no effect on prognosis in the older age group (P = 0.141). Histological grade was correlated with prognosis in both young adults and older groups (P <0.001, P = 0.003, respectively). We demonstrate differences in prognostic factors for diffuse gliomas in the midline region for children and adults. Importantly, the H3K27M mutation significantly influences prognosis in children, but not necessarily in adults. Contrarily, histological grading and immunostaining are important prognostic tools in adults.
Keywords: diffuse midline glioma, adult, H3K27M, H3K27me3, EZH2
Introduction
The World Health Organization (WHO) classification of brain tumors has been revised in 2016 and molecular diagnosis has become a major factor in pathological classification.1) H3K27M-mutant diffuse midline glioma (DMG) is a newly added pathological type in which molecular diagnosis plays a central role. It is a diffuse glioma that develops in the thalamus, brainstem, or spinal cord, and has a H3K27M mutation and a poor prognosis regardless of the histological grade.1) While this tumor is predominant in children, it can also occur in adults. The H3K27M mutation is a mutation that converts lysine to methionine at the 27th position from the N-terminus of the histone tail of core histone H3.
Epigenomic changes associated with H3K27 include expression of enhancer of zeste homolog 2 (EZH2), H3K27me3, and p16 protein. EZH2 is a subunit of polycomb repressive complex 2 (PRC2), a multiprotein complex responsible for the methylation of histone H3 at lysine 27 (H3K27me). EZH2 is amplified and/or overexpressed in a variety of solid tumors, including prostate, kidney, breast, and colorectal cancer, and elevated EZH2 activity in tumors is associated with poor prognosis.2,3) In mice, EZH2 is required for both the initiation of diffuse intrinsic pontine glioma tumorigenesis and for the sustained growth of these tumors in vivo.4) EZH2 is also associated with p16 protein down-regulation in cases without deletion of cyclin-dependent kinase inhibitor 2A (CDKN2A/p16); the loss of p16 protein expression correlates with high-grade gliomas.5) Recent studies demonstrated that EZH2 could contribute to glioma progression via more extensive mechanisms.6–8) EZH2 overexpression is a poor prognostic factor in glioma,9) with reported overexpression in children’s glioblastoma.10) Homozygous deletion of CDKN2A is generally confirmed using fluorescence in situ hybridization (FISH). However, correlation of the status of CDKN2A as detected by FISH with that of expression of the protein product of methylthioadenosine phosphorylase (MTAP) gene as detected by immunohistochemistry has also been reported.11) Indeed, when FISH cannot be used to detect the homozygous deletion of CDKN2A due to overfixation of the specimen by formalin, we have used MTAP immunostaining as a surrogate assay of FISH. On the other hand, EZH2 - methylated H3K27 (H3K27me3) acts as a transcriptional repressor. The H3K27M-mutant binds EZH2, suppresses PRC2 activity, and suppresses methylation of H3K27.12) As a result, in tumors with H3K27M mutation, levels of H3K27me3 have been observed to decrease.10)
Several studies characterizing genomic and epigenomic determinants of midline glioma have been reported, although there are few studies on adult cases.13,14) In this report, we examined the effect of H3K27M mutation, histological grading of glioma, and the expression status of EZH2, H3K27me3, p16, and MTAP on the prognosis of adult midline glioma.
Materials and Methods
Patients
We included cases of diffuse glioma that occurred in the thalamus, brain stem, or the spinal cord in patients >18 years, who were pathologically diagnosed at Fukuoka University Hospital between 1998 and 2017. Re-diagnosis and pathological classification was performed according to the 2016 WHO classification. Anonymous use of redundant tissues is part of the standard treatment agreement with patients at our hospital when no objection is expressed. The Fukuoka University Hospital Institutional Review Board (The Ethics Committee) approved the study protocol (approval number 2017M184).
Immunohistochemical analysis
Immunohistochemical staining was performed on the 4-μm-thick formalin-fixed paraffin-embedded (FFPE) tissue sections after epitope retrieval using Tris-EDTA buffer (pH 9.0) at 95°C for 20 min.
The primary antibodies used for immunohistochemical analysis were isocitrate dehydrogenase (IDH1) (Dianova, Hamburg, Germany, DIA-H09, clone H09 dilution 1:20), alpha thalassemia/mental retardation syndrome X-linked (ATRX) (Sigma-Aldrich, Missouri, USA, HPA001906, dilution 1:500), H3K27M-mutant (Millipore, Burlington, MA, USA, ABE419, dilution 1:1000), H3K27me3 (Cell Signaling Technology, Danvers, MA, USA, #9733, dilution 1:200), EZH2 (Cell Signaling Technology, 5246S, dilution 1:100), p16 (BD, New Jersey, USA, 551153, clone G175-405, dilution 1:50), and MTAP (Abnova, Taipei, Taiwan, H00004507-M01, clone 2G4, dilution 1:100). For immunopositive cases, the labeling index (LI) for EZH2, H3K27me3, p16, and MTAP was calculated as a percentage of positively stained nuclei using 1000 tumor cells counted under 400× magnification in the areas with highest density of positive nuclei. We divided the testing cohort low and high populations based on a cut-off point set at 50% of cells positively stained for EZH215) and MTAP,16) 70% of cells positively stained for H3K27me3,17) and 8.5% of cells positively stained for p16.11) The immunohistochemical analysis of EZH2 at a cut-off value of 50% has been shown to be useful in differentiating malignant and benign lesions with a reported specificity of 100%.15) Survival analysis revealed that high EZH2 expression was likely to be associated with decreased overall survival.15) A previous report described using the X-tile program for the assessment of the cut-off value of H3K27me3.17) With respect to MTAP and p16, the cut-off values for the differentiation of malignant pleural mesothelioma (MPM) from reactive mesothelial hyperplasia (RMH) were set using receiver operating characteristic analysis at 32.2% and 8.5%, respectively, based on the proportion of positively stained cells in each immunohistochemistry (IHC).11) However, the proportion of cells positive for MTAP IHC in MPM cases showed a bimodal distribution. Thus, we set the MTAP cut-off value at 50% as described previously.11,16) Representative photos of each positive example are presented in Fig. 1.
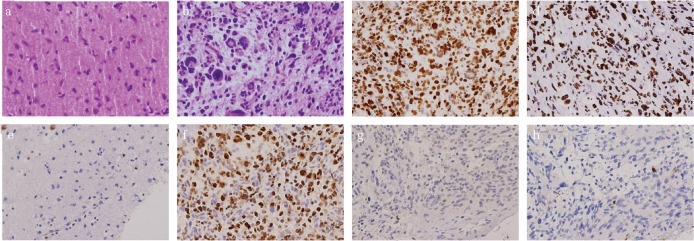
Fig. 1.
Hematoxylin–Eosin staining for diffuse glioma in low-grade (a) and high-grade (b), respectively. Immunohistochemical staining performed on FFPE tissue sections for H3K27M-mutant (c), H3K27me3 (d), ATRX (e), EZH2 (f), MTAP (g), and p16 (h). H3K27M, H3K27me3, and EZH2 are positive at nuclei. ATRX, MTAP, and p16 positivity are lost. Each original magnification is 100×. FFPE, formalin-fixed paraffin-embedded; ATRX, alpha thalassemia/mental retardation syndrome X-linked; EZH2, enhancer of zeste homolog 2; MTAP, methylthioadenosine phosphorylase.
DNA extraction
Two to three 10 μm thick sections from FFPE tissues were collected. DNA was extracted using spin column method according to the recommended protocol (RecoverAll Total Nucleic Acid Isolation Kit for FFPE, Cat. No. AM1975, Invitrogen, Carlsbad, CA, USA). Purity and concentration were measured with a NanoDrop Lite Spectrophotometer (Thermo Scientific, MA, USA) and amplification of β-globin was confirmed by PCR as a quality check for DNA. polymerase chain reaction (PCR) was carried out in a PC-812 thermal cycler system (Astec Co., Ltd., Kasuya, Fukuoka, Japan) in a total volume of 20 μL PCR mix containing 2 μL of DNA, 2× PCR buffer, 2 mM of each deoxynucleotide, 10 μM of forward and reverse primers, and 1.0 U of DNA polymerase (KOD FX Neo, Cat. No. KOD-201, TOYOBO Co., Ltd., Osaka, Osaka, Japan). PCR cycling conditions were as following: 94°C for 15 min followed by 35 cycles of 94°C for 10 s, 55°C for 30 s, and 68°C for 30 s.
Mutation analysis
Fully automated single nucleotide polymorphism (SNP) genotyping was performed to detect mutations for IDH1 (R132; exon 4), H3F3A (K27M, G34R, G34V; exon 2), and v-raf murine sarcoma viral oncogene homolog B1(BRAF) (V600E/K/D/R; exon 15) using quenching probe (QP) method on i-densy IS-5320 (Arkray Inc., Kyoto, Japan). HIST1H3B (K27M), IDH2 (R172; exon 4), and telomerase reverse transcriptase (TERT) promoter analysis was performed using PCR (KOD-Plus-Neo, Cat. No. KOD-401, TOYOBO Co., Ltd.). The obtained amplicon was purified (NucleoSpinGel and PCR Clean-up, MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany) and sanger sequencing was performed (FASMAC Co., Ltd., Atsugi, Kanagawa, Japan).
Statistical analysis
Fisher’s exact test or χ2-test was performed to compare categorical variables using SPSS (IBM statistics, version 21.0, Armonk, NY, USA). The overall survival (OS) was evaluated from the date of surgery on the tumor for any cause up to the date of death using Kaplan–Meier method and compared using log-rank test. A P-value <0.05 was used for statistical significance between two groups.
Review of literature
Since the number of cases included in this study was limited, we also reviewed published literature to examine the relationship between H3K27M-mutant, histologic grade, and prognosis in adult midline glioma. PubMed database was queried with the search terms (“H3”, “K27M”, and “glioma”) to retrieve English language original articles reported between 2014 and 2019. Typical midline lesions such as those in the thalamus, brainstem, or spinal cord were included in the analysis, whereas those at other locations (cerebellum and corpus callosum) were excluded. We also excluded studies that lacked details of each case.
Results
We identified 30 midline region glioma patients in our analysis. Among them, there were five patients under the age of 18. One patient was excluded from the study due to lack of specimen volume sufficient for analysis. Another, a pilocytic astrocytoma of midbrain, was also excluded. Thus, we conducted retrospective data and tissue analysis of 23 adult midline gliomas. Demographic, clinical, pathologic, and radiographic features of these patient cases are summarized in Table 1. There were 13 males and 10 females. Twelve tumors were located in the thalamus, two in the areas from thalamus to midbrain, two in the midbrain, five in the pons, one in the medulla oblongata, and one in the cervical cord. Eleven of the tumors were histological grade II, 10 were grade III, and two were grade IV. We regarded grade II as histologically low-grade (11 cases) and grade III and IV as histologically high-grade (12 cases). The cases included nine diffuse astrocytoma (DA, H3K27-wild), three anaplastic astrocytoma (AA, H3K27-wild), and 11 H3K27M-mutant DMG. The mean ages and age ranges of different tumor subtypes were 48 years for DA (range: 20–85 years); 83 years for AA (range: 47–85 years); and 32 years for DMG (range: 19–67 years).
Table 1.
Clinicopathological characteristics of adult midline glioma
| Total (23) | |
| Male, N (%) | 13 (56.5) |
| Female | 10 (43.5) |
| Median age (years) | 47 |
| 18–19, N (%) | 1 (4.3) |
| 20–29 | 7 (30.4) |
| 30–39 | 2 (8.7) |
| 40–49 | 3 (13.0) |
| 50–59 | 2 (8.7) |
| 60–69 | 4 (17.4) |
| 70–79 | 0 (0) |
| 80–89 | 4 (17.4) |
| Midline glioma location, N (%) | |
| Thalamus | 12 (52.2) |
| Thalamus - Midbrain | 2 (8.7) |
| Midbrain | 2 (8.7) |
| Pons | 5 (21.7) |
| Medulla oblongata | 1 (4.3) |
| Cervical spinal cord | 1 (4.3) |
| Histological grade | |
| Grade II | 11 (47.8) |
| Grade III | 10 (43.5) |
| Grade IV | 2 (8.7) |
| Pathologic diagnosis | |
| Diffuse astrocytoma | 9 (39.1) |
| Anaplastic astrocytoma | 3 (13.0) |
| Diffuse midline glioma, H3K27M-mutant | 11 (47.8) |
| Median age at each diagnosis | |
| Diffuse astrocytoma | 48 |
| Anaplastic astrocytoma | 83 |
| Diffuse midline glioma, H3K27M-mutant | 32 |
Histological grade and immunohistochemical analysis
Among the high-grade cases, 9/12 (75%) were H3K27M-mutant, while among the low-grade cases, 2/11 (18%) were H3K27M-mutant (Table 2). Significant correlation between H3K27M mutation and histological grade was observed with a tendency for high-grade glioma in cases with H3K27M mutation (P = 0.009). High EZH2 expression was observed in 7/12 (58%) of high-grade cases and 1/11 (9%) of low-grade cases, with these differences being statistically significant (P = 0.019). High H3K27me3 expression was observed in 3/12 (25%) cases of high-grade and 9/11 (82%) cases of low-grade gliomas, with these differences being statistically significant (P = 0.009). Low MTAP and p16 expression was observed in 3/12 (25%) and 10/12 (83%) cases of high-grade, and 10/11 (90%) and 2/11 (18%) cases of low-grade gliomas, respectively, with these differences being statistically insignificant (P = 0.534 and 0.545, respectively).
Table 2.
Relationships between various tumor characteristics in adult midline glioma
| Histological grade | Total | P | |||
|---|---|---|---|---|---|
| High grade | Low grade | ||||
| H3K27M-mutant | (+) | 9 | 2 | 11 | 0.009 |
| (−) | 3 | 9 | 12 | ||
| EZH2 expression | High | 7 | 1 | 8 | 0.019 |
| Low | 5 | 10 | 15 | ||
| H3K27me3 | High | 3 | 9 | 12 | 0.009 |
| Low | 9 | 2 | 11 | ||
| p16 | High | 2 | 1 | 3 | 0.534 |
| Low | 10 | 10 | 20 | ||
| MTAP | High | 9 | 9 | 18 | 0.545 |
| Low | 3 | 2 | 5 | ||
| H3K27M-mutant | Total | ||||
|---|---|---|---|---|---|
| (+) | (−) | ||||
| EZH2 expression | High | 5 | 3 | 12 | 0.278 |
| Low | 6 | 9 | 11 | ||
| H3K27me3 expression | High | 3 | 9 | 12 | 0.030 |
| Low | 8 | 3 | 11 | ||
| p16 | High | 3 | 0 | 3 | 0.093 |
| Low | 8 | 12 | 20 | ||
| MTAP | High | 10 | 8 | 18 | 0.185 |
| Low | 1 | 4 | 5 | ||
| EZH2 expression | Total | ||||
|---|---|---|---|---|---|
| High | Low | ||||
| H3K27me3 expression | High | 2 | 10 | 12 | 0.071 |
| Low | 6 | 5 | 11 | ||
| p16 | High | 0 | 3 | 3 | 0.257 |
| Low | 8 | 12 | 20 | ||
| MTAP | High | 5 | 13 | 18 | 0.208 |
| Low | 3 | 2 | 5 | ||
| H3K27me3 expression | Total | ||||
|---|---|---|---|---|---|
| High | Low | ||||
| p16 | High | 2 | 1 | 3 | 0.534 |
| Low | 10 | 10 | 20 | ||
| MTAP | High | 10 | 8 | 18 | 0.455 |
| Low | 2 | 3 | 5 | ||
EZH2, enhancer of zeste homolog 2; MTAP, methylthioadenosine phosphorylase.
H3K27M-mutant
H3K27M mutation in H3F3A and HIST1H3B genes was detected by a combination of immunohistochemical and genetic analysis. Immunostaining findings show that H3K27M was positive in 11/23 (48%) cases. SNP genotyping detected H3K27M in 10/23 (43%) cases with all 10 cases showing mutation in H3F3A gene and none in HIST1H3B gene. In cases where H3K27M was identified by the genetic test, all cases were also positive by immunostaining whereas one case of H3K27M was detected only with immunostaining. Although the case was also subjected to sequencing of HIST1H3B, K27M mutation was not identified, and it is possible that HIST1H3C mutation may be possessed. In H3K27M-mutant cases, high EZH2 expression are observed in 6/11 (55%) cases. In H3K27-wild type cases, high EZH2 expression was observed in 9/12 (75%) cases (Table 2). There was no significant correlation between H3K27M mutation and EZH2 expression (P = 0.278). In H3K27M-mutant cases, high H3K27me3 expression was observed in 3/11 (27%) cases. In H3K27-wild type cases, high H3K27me3 expression was observed in 9/12 (75%) cases. There was a significant inverse correlation between H3K27M-mutant and H3K27me3 (P = 0.030). No significant correlation between H3K27M mutation and either p16 or MTAP immunoreactivity was observed (P = 0.093 and 0.185, respectively).
EZH2 expression
The patient cases were classified into eight high expression cases and 15 low expression cases based on EZH2 immunostaining. High H3K27me3 expression was observed in 2/8 (25%) of high EZH2 expression cases and in 10/15 (67%) of low EZH2 expression cases (Table 2). There was a nominally significant correlation between the expression of EZH2 and H3K27me3 (P = 0.071). There was no significant correlation between EZH2 expression and either p16 or MTAP immunoreactivity (P = 0.257 and 0.208, respectively).
H3K27me3 expression
The patient cases were classified into 12 high expression cases and 11 low expression cases based on H3K27me3 immunostaining (Table 2). There was no significant correlation between H3K27me3 and p16 or MTAP immunoreactivity (P = 0.534 and 0.455, respectively).
p16 and MTAP protein immunoexpression
Twenty cases showed decreased levels of p16 protein detected by immunostaining, whereas immunoreactivity was maintained in three cases. Five cases showed a decrease in MTAP immunostaining, whereas immunoreactivity was maintained in 18 cases.
IDH1 and ATRX expression
No evidence of IDH1 mutation was revealed by immunostaining in any of the cases. ATRX expression loss was seen in four cases with two cases each of H3K27-wild type and H3K27M-mutant.
DNA sequencing and PCR
Sequencing for HIST1H3B could only be performed in 12/23 (52%) cases. In the remaining cases, the amount of specimen was insufficient to conduct sequencing assays. Mutation of HIST1H3B was not detected in any of the cases that could be tested. With respect to IDH mutation, sequencing was performed for all cases. Mutation was not observed in either IDH1 or IDH2. TERT promoter mutation search could only be performed in 15/23 (65%) cases. The remaining eight cases could not be tested due to insufficient sample volume. TERT promoter mutation was confirmed in 2/15 (13%) cases. Similarly, BRAF V600E mutation could be tested in 21/23 (91%) cases with insufficient specimen preventing analysis in two cases. BRAF V600E mutation was not observed in the 21 cases tested.
Survival outcome
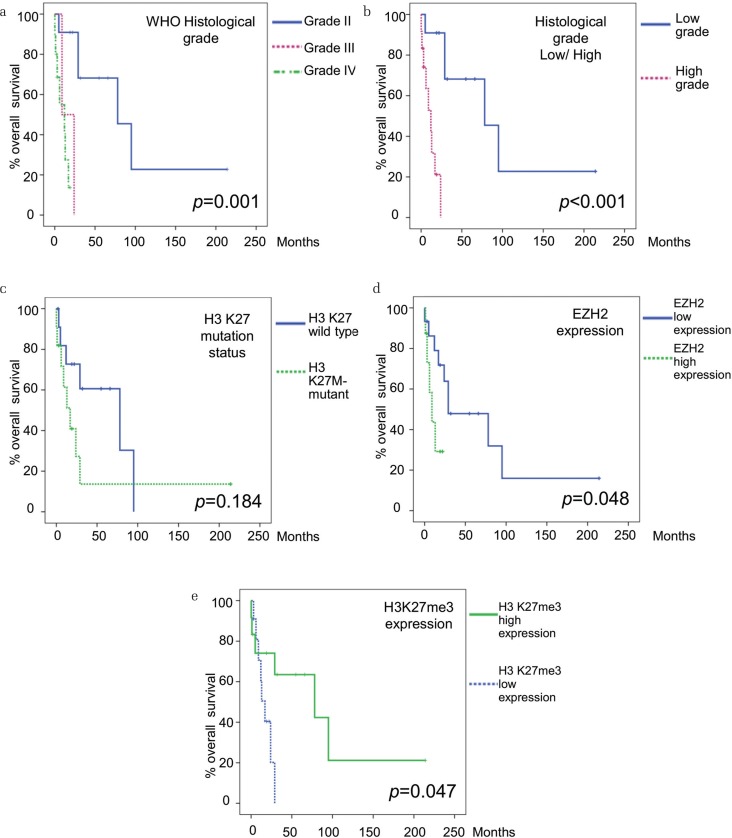
Survival outcomes are outlined in Table 3 and displayed in Fig. 2. The median OS was 58.2 months for all patients. The median OS was 78 months for DA (H3K27-wild), 3 months for AA (H3K27-wild), and 17 months for DMG (H3K27M-mutant). The median OS was 78 months for grade II cases, 12 months for grade III, and 9 months for grade IV. Histologically, the cases with high-grade gliomas had a significantly worse prognosis than the cases with low-grade gliomas (Figs. 2a and 2b) (P <0.001). When classified by H3K27M-mutant or H3K27-wild type, the median OS was 17 months for H3K27M-mutant and 78 months for H3K27-wild type. However, there was no statistically significant difference (Fig. 2c) (P = 0.184). The median OS based on EZH2 expression was 9 months for high expression and 29 months for low expression (Fig. 2d) (P = 0.048). The median OS based on H3K27me3 expression was 78 months for high expression and 18 months for low expression (Fig. 2e) (P = 0.047). Thus, the difference in survival was significant for EZH2 and H3K27me3 expressions. The median OS based on p16 expression was 17 months for high expression and 29 months for low expression (P = 0.820). The median OS based on MTAP expression was 29 months for high expression and 24 months for low expression (P = 0.828). The immunostaining results indicate that expression of p16 and MTAP was not correlated with prognosis.
Table 3.
Survival outcomes for adult midline glioma
| Entire cohort, N = 23, median OS (months) | 58.2 |
| Median OS by WHO classification 2016 (months) | |
| Diffuse astrocytoma, H3K27-wild type | 78* |
| Anaplastic astrocytoma, H3K27-wild type | 3 |
| Diffuse midline glioma, H3K27M-mutant | 17 |
| Median OS by histological grade (months) | |
| Grade II | 78** |
| Grade III | 12 |
| Grade IV | 9 |
| Median OS by H3K27M (months) | |
| H3K27M-mutant | 17*** |
| H3K27-wild type | 78 |
| Median OS by EZH2 expression (months) | |
| EZH2 low expression | 29# |
| EZH2 high expression | 9 |
| Median OS by H3K27me3 expression (months) | |
| H3K27me3 low expression | 17+ |
| H3K27me3 high expression | 78 |
P = 0.037, log-rank test for trend, comparing overall survival (OS) for diffuse astrocytoma, anaplastic astrocytoma, and diffuse midline glioma.
P = 0.001, log-rank test for trend, comparing histological grade.
P = 0.187, log-rank test for trend, comparing with or without H3 K27M mutation.
P = 0.048, log-rank test for trend, comparing high or low EZH2 expression.
P = 0.047, log-rank test for trend, comparing high or low H3K27me3 expression. WHO, World Health Organization; EZH2, enhancer of zeste homolog 2.
Fig. 2.
Relationship between adult midline glioma characteristics and overall survival. (a) and (b), Relationship between histological grade and overall survival. Cases exhibiting grade II histology tended to have a better prognosis than those exhibiting grade III and IV. (c) Overall survival based on presence or absence of H3K27M-mutant. There was no significant difference between the two groups. (d) Overall survival based on expression of EZH2. High EZH2 expression cases had significantly poor survival. (e) Overall survival based on expression of H3K27me3. High H3K27me3 expression cases had significantly better survival. EZH2, enhancer of zeste homolog 2.
Review of literature: H3K27M-mutant and histological grade
We reviewed eight articles and 187 cases (including the 23 cases in this study) of adult diffuse midline glioma.13,18–24) The prognosis was examined by the presence or absence of K27M or by histological grade in 171 or 164 cases, respectively. When the prognosis was examined based on the presence or absence of K27M mutation, cases that did not determine K27M mutation status were excluded and we reviewed 171 cases. When the prognosis was examined by histological grade, cases that did not determine K27M mutation status were included and we reviewed 164 cases.
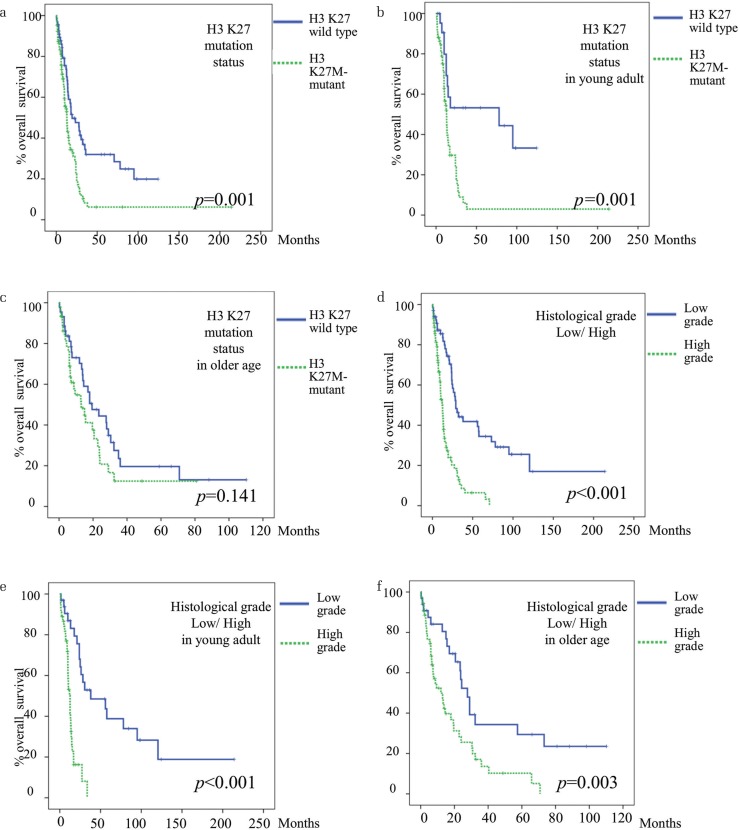
There were 104 H3K27M-mutant cases and 67 H3K27-wild type cases. The median OS of H3K27M-mutant cases and H3K27-wild type cases was 13 months and 19.4 months, respectively (Fig. 3a) (P = 0.001). In this study, we further investigated the relationship between H3K27M mutation and prognosis by age group. This patient group was divided into a young adult group (18–39 years) and an older group (≥40 years), and the prognosis was examined based on the presence or absence of H3K27M mutation. In the young adult group, the median OS was 13 months in H3K27M-mutant cases and 78 months in H3K27-wild type cases (Fig. 3b) (P = 0.001). In the older age group, the median OS of H3K27M-mutant cases was 13 months, and that of H3K27-wild type cases was 19.4 months (Fig. 3c) (P = 0.141). Histologically, there were 66 low-grade cases and 98 high-grade cases. The median OS of high-grade and low-grade cases was 12.6 months and 29 months, respectively (Fig. 3d) (P <0.001). Furthermore, we examined the effect of histological grades on survival time in the two different age groups. In the young adult group, the median OS in high-grade cases was 12.6 months and in low-grade was 38 months (Fig. 3e) (P <0.001). In the older age group, the median OS in high-grade cases was 12 months and in low-grade cases was 27.7 months (Fig. 3f) (P = 0.003).
Fig. 3.
Relationship between H3K27 status, overall survival, and histological grade in a meta-analysis of adult midline glioma. Findings from cases in the present study were included in the meta-analysis. (a) Overall survival based on H3K27 status in all adult cases. H3K27M-mutant cases had significantly poor prognosis. (b) and (c) All cases were divided into young adult (18–39 years) and older age (≥40 years) groups and overall survival was assessed based on H3K27 status. In the older age group, overall survival did not correlate with H3K27 status. (d–f), Overall survival based on histological grade. High-grade cases had significantly poor survival. Similar survival trends were seen in the young adult group and the older age group.
Discussion
We examined 23 diffuse gliomas of adults which developed in the midline region. H3K27M-mutant was confirmed by immunostaining and SNP genotyping in about half of the cases (11/23, 48%). In addition, the median age of H3K27M-mutant DMG patients was younger than wild type cases. In pediatric cases, H3K27M-mutant is understood to have poor prognosis regardless of histological grade.25) However, in our adult patient study, H3K27M-mutant does not necessarily correlate with prognosis; rather, histological grade more strongly correlates with prognosis. EZH2 overexpression was frequently observed in high-grade gliomas of the midline region in adults and correlated with prognosis. Correlation with prognosis was also observed for high expression of H3K27me3 in histologically low-grade gliomas. Overall, our results show that in adult midline glioma, histology is correlated with prognosis, and supplementary of EZH2 and H3K27me3 expression can also be useful for estimating prognosis.
Analysis of the findings from the present study in addition to those in the published literature revealed that H3K27M mutation was associated with prognosis in the young adult group, whereas there was no statistically significant effect on prognosis in the older age group. In addition, the correlation between histological grade and prognosis was significant in both the young adult group and the older age group. In pediatric cases, H3K27M-mutant is associated with poor prognosis, regardless of histological grade. H3K27M-mutant did not strongly correlate with prognosis in older patients (≥40 years), although it was correlated with prognosis in young adults (18–39 years). Previously, it has been reported that H3K27M-mutant does not correlate with prognosis in adults.26) Our findings suggest that there is an age-dependent effect of H3K27M mutation on prognosis in adults. H3K27M mutation was a significant prognostic factor even in young adults (18–39 years), while it was not in older adults (≥40 years). Older age was associated with poor prognosis, even in the H3K27-wild type cases. The poor prognosis of H3K27-wild type cases in the older age group explains the observation of no significant difference in prognosis between H3K27M-mutant and H3K27-wild type in this age group. With increasing age, the prognosis of H3K27-wild type also worsens, suggesting that the determination of H3K27M-mutant in the older age group is not as important as that in children and young adults.
Analysis based on histological grade revealed that the prognosis was significantly worse in high-grade cases in all age groups of adults (P <0.001). Even when examined separately in the young adult group and the older age group, the strong correlation between histological grade and prognosis was conserved for both groups (P <0.001, P = 0.003, respectively). There is evidence to suggest that H3K27M-mutant has a stronger association with prognosis than histological grade in the midline glioma of children. Our findings show that in adult midline glioma, the histological grade is an important prognostic factor regardless of patient age.
In fact, it is conceivable that there are H3K27M adult DMG cases where the survival time is remarkably long. Similar to pediatric cases, H3K27M-mutant cases were often high-grade gliomas. However, we also encountered two H3K27M cases with low grade histology. Long-term survival was observed in one of them. Thus, consequently no significant differences in survival time were observed in H3K27M-mutant or H3K27-wild type gliomas in our single institutional study. Previous reports suggest that while H3K27M-mutant may be a poor prognostic factor in brain stem lesions in adults, this observation was not necessarily true for thalamic lesions.14) In our study, 7/11 (64%) H3K27M cases were thalamic occurrences. The observation of lack of prognostic value for the H3K27M-mutant could be attributed to the fact that thalamic lesions comprise more than half of the H3K27M-mutant group. Furthermore, no significant difference in survival was seen comparing brain stem or thalamic lesions between H3K27M-mutant and H3K27-wild type cases in our single institutional study (thalamus P = 0.238, brainstem P = 0.208). This may be due to the small sample size of the study. Collectively, the data shows that H3K27M mutation is predictive in determining the malignancy even in young adults as in children.
Findings from our study show that EZH2 over-expression was associated with histology and prognosis, and H3K27me3 was associated with H3K27M mutation, histology, and prognosis. Previous reports examining gliomas not restricted to the midline region showed correlation between EZH2 and histologically high-grade gliomas, and our study supports those observations.5) Indeed, correlation of EZH2 with prognosis and histological grade is also applicable to glioma in the midline region. In H3K27M-mutant cases, H3K27M acts on the SET point of EZH2 to suppress its methylation activity. While, H3K27M suppresses the activity of EZH2, it does not have a significant influence on EZH2 protein expression. Accordingly, there was no correlation between H3K27M-mutant and EZH2 expression. There was a correlation between H3K27M-mutant and H3K27me3, with the expression of H3K27me3 being significantly decreased in H3K27M-mutant samples. Decreased levels of H3K27me3 in pediatric glioblastoma multiforme (GBM) cases with H3K27M-mutant have been reported.10) In our adult midline glioma, H3K27me3 expression was decreased in cases showing high-grade histology and in those with H3K27M mutation; a clear association with poor prognosis was observed. The correlation between H3K27me3 and H3K27M was conserved regardless of age or tumor location. H3K27me3 plays a role in repression of lineage-regulatory genes during pluripotency in embryonic stem cells.27) Further, H3K27me3 is also known to affect DNA methylation.28) Global loss of H3K27me3 may contribute to pathogenesis in H3F3A K27M-mutant pediatric GBM by affecting differentiation pathways.10) Our study in adult midline gliomas suggests a similar role for H3K27me3 in pathogenesis and the utility of immunohistochemistry of H3K27me3 in estimating prognosis.
Methylthioadenosine phosphorylase immunostaining detects homozygous deletion of CDKN2A in malignant pleural mesothelioma with a sensitivity of 74.1% and a specificity of 100%.11) Previous reports have shown that the expression of EZH2 is elevated in 80% of the high-grade gliomas with loss of p16 protein expression and conserved CDKN2A.5) In our study, p16 and MTAP had no significant correlation with histological grade, EZH2, and H3K27me3. It is unclear whether this lack of correlation observed for p16 and MTAP is unique to midline gliomas or does it apply to gliomas in other regions as well. While it is possible that p16 and MTAP immunostaining accuracy issues could explain this lack of correlation, it should be noted that low expression of MTAP was observed only in the case that also had a loss of p16, although, the correlation between the two was not significant (P = 0.350).
All the specimens tested in our study were wild type for IDH1 and IDH2. The presence or absence of IDH mutation is known to correlate with prognosis in diffuse gliomas. However, the frequency of IDH mutation is variable dependent upon the location of glioma.25) The frequency of IDH mutation in the midline region has previously been reported to be low.29) Wild type IDH is associated with variable tumor prognosis, and other useful markers of prognosis are necessary. While, histological grade and IDH status are of course important in judging the malignancy of diffuse gliomas, in midline glioma, IDH is likely to exhibit wild type status and is of limited prognostic value.
In this study, H3K27M-mutant was seen in approximately half of midline gliomas in patients over 18 years of age. Compared with other gliomas, the midline gliomas are more prevalent in younger patients. In older age group, there was no significant difference in prognosis with the presence or absence of H3K27M-mutant, unlike that seen in children. Our findings validate the value of histopathology in prognosis related to adult midline glioma. Additionally, we show that the expression analysis of EZH2 and H3K27me3 is useful adjunctively in the determination of tumor prognosis.
A limitation of this study is the small sample size. In addition, in many cases genetic testing could not be performed due to insufficient specimen volume. Furthermore, the changes in treatment approach over time may have led to differences in survival period for each case.
Acknowledgments
The authors thank Mr. S. Matsumoto, Ms. H. Fukagawa, and M. Onitsuka, Department of Pathology, Fukuoka University School of Medicine and Hospital for their excellent technical assistance. This work was supported in part by grants from the Research Center for Advanced Molecular Medicine, Fukuoka University.
Footnotes
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the Institutional Review Board (The Ethics Committee) of Fukuoka University (No. 2017M184).
Conflicts of Interest Disclosure
The authors declare that they have no conflicts of interest.
References
- 1).Louis DN, Ohgaki H, Wiestler OD, Cavenee WK: WHO Classification of Tumours of the Central Nervous System, ed 4 International Agency for Research on Cancer (IARC), Lyon, 2016, pp. 57–59 [Google Scholar]
- 2).Varambally S, Dhanasekaran SM, Zhou M, et al. : The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419: 624–629, 2002 [DOI] [PubMed] [Google Scholar]
- 3).Helin K, Dhanak D: Chromatin proteins and modifications as drug targets. Nature 502: 480–488, 2013 [DOI] [PubMed] [Google Scholar]
- 4).Mohammad F, Weissmann S, Leblanc B, et al. : EZH2 is a potential therapeutic target for H3K27M-mutant pediatric gliomas. Nat Med 23: 483–492, 2017 [DOI] [PubMed] [Google Scholar]
- 5).Purkait S, Sharma V, Jha P, et al. : EZH2 expression in gliomas: Correlation with CDKN2A gene deletion/p16 loss and MIB-1 proliferation index. Neuropathology 35: 421–431, 2015 [DOI] [PubMed] [Google Scholar]
- 6).Kim E, Kim M, Woo DH, et al. : Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell 23: 839–852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Pang B, Zheng XR, Tian JX, et al. : EZH2 promotes metabolic reprogramming in glioblastomas through epigenetic repression of EAF2-HIF1α signaling. Oncotarget 7: 45134–45143, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Zhang K, Sun X, Zhou X, et al. : Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget 6: 537–546, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Zhang Y, Yu X, Chen L, Zhang Z, Feng S: EZH2 overexpression is associated with poor prognosis in patients with glioma. Oncotarget 8: 565–573, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Venneti S, Garimella MT, Sullivan LM, et al. : Evaluation of histone 3 lysine 27 trimethylation (H3K27me3) and enhancer of Zest 2 (EZH2) in pediatric glial and glioneuronal tumors shows decreased H3K27me3 in H3F3A K27M mutant glioblastomas. Brain Pathol 23: 558–564, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Hida T, Hamasaki M, Matsumoto S, et al. : Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer 104: 98–105, 2017 [DOI] [PubMed] [Google Scholar]
- 12).Lewis PW, Müller MM, Koletsky MS, et al. : Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science 340: 857–861, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Aihara K, Mukasa A, Gotoh K, et al. : H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro-oncology 16: 140–146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Feng J, Hao S, Pan C, et al. : The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum Pathol 46: 1626–1632, 2015 [DOI] [PubMed] [Google Scholar]
- 15).Shinozaki-Ushiku A, Ushiku T, Morita S, Anraku M, Nakajima J, Fukayama M: Diagnostic utility of BAP1 and EZH2 expression in malignant mesothelioma. Histopathology 70: 722–733, 2017 [DOI] [PubMed] [Google Scholar]
- 16).Kinoshita Y, Hida T, Hamasaki M, et al. : A combination of MTAP and BAP1 immunohistochemistry in pleural effusion cytology for the diagnosis of mesothelioma. Cancer Cytopathol 126: 54–63, 2018 [DOI] [PubMed] [Google Scholar]
- 17).Cai MY, Hou JH, Rao HL, et al. : High expression of H3K27me3 in human hepatocellular carcinomas correlates closely with vascular invasion and predicts worse prognosis in patients. Mol Med 17: 12–20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Reyes-Botero G, Giry M, Mokhtari K, et al. : Molecular analysis of diffuse intrinsic brainstem gliomas in adults. J Neurooncol 116: 405–411, 2014 [DOI] [PubMed] [Google Scholar]
- 19).Meyronet D, Esteban-Mader M, Bonnet C, et al. : Characteristics of H3 K27M-mutant gliomas in adults. Neuro-oncology 19: 1127–1134, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Daoud EV, Rajaram V, Cai C, et al. : Adult brain-stem gliomas with H3K27M mutation: radiology, pathology, and prognosis. J Neuropathol Exp Neurol 77: 302–311, 2018 [DOI] [PubMed] [Google Scholar]
- 21).Kleinschmidt-DeMasters BK, Mulcahy Levy JM: H3 K27M-mutant gliomas in adults vs. Children share similar histological features and adverse prognosis. Clin Neuropathol 37: 53–63, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Wang L, Li Z, Zhang M, et al. : H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol 78: 89–96, 2018 [DOI] [PubMed] [Google Scholar]
- 23).Karlowee V, Amatya VJ, Takayasu T, et al. : Immunostaining of increased expression of enhancer of zeste homolog 2 (EZH2) in diffuse midline glioma H3K27M-mutant patients with poor survival. Pathobiology 86: 152–161, 2019 [DOI] [PubMed] [Google Scholar]
- 24).Liu Y, Zhang Y, Hua W, Li Z, Wu B, Liu W: Clinical and molecular characteristics of thalamic gliomas: retrospective report of 26 cases. World Neurosurg 126: e1169–e1182, 2019 [DOI] [PubMed] [Google Scholar]
- 25).Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C: Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol 128: 573–581, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Ebrahimi A, Skardelly M, Schuhmann MU, et al. : High frequency of H3 K27M mutations in adult midline gliomas. J Cancer Res Clin Oncol 145: 839–850, 2019 [DOI] [PubMed] [Google Scholar]
- 27).Vastenhouw NL, Schier AF: Bivalent histone modifications in early embryogenesis. Curr Opin Cell Biol 24: 374–386, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Hagarman JA, Motley MP, Kristjansdottir K, Soloway PD: Coordinate regulation of DNA methylation and H3K27me3 in mouse embryonic stem cells. PLoS ONE 8: e53880, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Sturm D, Witt H, Hovestadt V, et al. : Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22: 425–437, 2012 [DOI] [PubMed] [Google Scholar]