Abstract
Intravenous (i.v.) phenytoin/fosphenytoin is recommended as the second-line therapy of antiepileptic drugs in patients with status epilepticus (SE). i.v. Levetiracetam is regarded as an effective and safe equivalent with i.v. phenytoin/fosphenytoin. However, i.v. levetiracetam is not covered by public health insurance for SE in most countries. For this study, we performed the real-world practice pattern survey of antiepileptic drugs for status epilepticus using the nationwide inpatient database. We used the Japanese Diagnosis Procedure Combination inpatient database in Japan and identified all cases of emergency admission attributable to status epilepticus from March 2011 through March 2018. We described the patient characteristics and practice pattern of antiepileptic drugs. The analysis conducted for this study examined 31,472 cases. As the second-line therapy, the use of i.v. levetiracetam increased rapidly from 2016; 35% of cases received i.v. levetiracetam in 2017. By contrast, the use of i.v. phenytoin/fosphenytoin decreased from 2016. In-hospital mortality decreased year-by-year. No year-by-year change was observed for deaths within 24 h, length of hospital stay, drug-induced hepatitis, or drug-induced eruption. Although the use of levetiracetam for treatment of SE is not compensated by public health insurance in Japan, i.v. levetiracetam use is increasing dramatically as the second-line SE therapy. We propose that health insurance coverage be extended to include i.v. levetiracetam treatment for SE.
Keywords: epilepsy, seizure, phenytoin, fosphenytoin, levetiracetam
Introduction
Status epilepticus (SE) is an emergency condition in which convulsions persist, and in which recovery is not achieved. This life-threatening condition can cause irreversible cerebral damage.1) Therefore, it is necessary to stop convulsions promptly and certainly.2) Potent gamma amino-butyric acid agonists such as lorazepam or diazepam are recommended as first-line drugs.3,4) These drugs are short-acting. Therefore, another long-acting antiepileptic drugs (AEDs) are necessary as second-line therapy to stop convulsions definitely and to prevent recurrence.5)
Second-line drugs for adult SE are being chosen somewhat erratically. Phenytoin and fosphenytoin were recommended for many years.6) However, both phenytoin and fosphenytoin can induce adverse reactions such as allergic symptoms, arrhythmia, and reduction in blood pressure.7,8) Levetiracetam primarily binds to the synaptic vesicle protein 2A and regulates the release of neurotransmitters.9) Reportedly, the efficacy of intravenous (i.v.) levetiracetam for SE is similar to that of i.v. phenytoin/fosphenytoin. Adverse effects of i.v. levetiracetam are less than those of i.v. phenytoin/fosphenytoin.10–13) Given this background, the Committee of the American Epilepsy Society guideline introduced i.v. levetiracetam equivalently with i.v. phenytoin/fosphenytoin as the second-line therapy in the SE treatment algorithm.14) Levetiracetam was also recommended by expert consensus for use in emergency departments.15)
Nevertheless, i.v. levetiracetam is not included as a treatment for SE by public health insurance or health programs in most European nations. The European Federation of Neurological Societies’ guideline refers to levetiracetam by notice of the fact that it is not covered by insurance.16) Prepared i.v. levetiracetam was introduced into the Japanese market in December 2015. Nevertheless, it has never been covered by national health insurance for treatment of SE.
It is possible that i.v. levetiracetam can be used generally for SE in actual clinical practice. No report exists of nationwide descriptive research on which AEDs are used during the acute phase of SE and which AEDs are finally given for control of epilepsy after SE. For this study, we conducted a real-world practice pattern blot analysis of antiepileptic drugs for status epilepticus using a nationwide inpatient database.
Materials and Methods
The study design was a retrospective observational study using routinely collected data. The Institutional Review Board of The University of Tokyo approved the study. The board waived the requirement for informed consent because of the anonymous characteristics of the data because no information related to individual patients, hospitals, or treating physicians was obtained.
Data source
We used the Japanese Diagnosis Procedure Combination inpatient database, which includes discharge abstracts and administrative claims data for more than 1200 acute-care hospitals and which covers approximately 90% of all tertiary-care emergency hospitals in Japan. The database includes data related to age, sex, body weight, body height, level of consciousness at admission, diagnoses, procedures, prescriptions, and discharge status. The diagnoses are recorded using International Classification of Diseases Tenth Revision (ICD-10) codes and text in Japanese language. Therefore, the diagnostic records are linked to a payment system. For purposes of treatment cost reimbursement, attending physicians are obligated to report objective evidence for their diagnosis.17) An earlier study of these diagnostic and procedural records established validity of the database, with diagnostic specificity exceeding 96%, and sensitivity of 50–80%. Specificity and sensitivity of procedures each exceeded 90%.18)
Study population
We identified all patients with emergency admission due to SE in the database from March 1, 2011 to March 31, 2018. All hospitalized patients diagnosed with SE (ICD-10 code G41) at admission were included in the study.19) We did not include patients who developed SE after admission. We also excluded patients who were younger than 15 years of age or who were pregnant. When patients were admitted with a diagnosis of SE more than once during the study period, we regarded each admission as an independent case.
Study variables
We collected the following baseline patient characteristics: age, sex, body mass index at admission, Japan Coma Scale status on admission,20) type of SE,19) etiology of SE, and comorbidities of mental disorders.21) Body mass index data were categorized into <18.5, 18.5–24.9, 25.0–29.9, or ≥30.0 kg/m2, or missing. Japan Coma Scale status was categorized as alert consciousness, confusion, somnolence, and coma. Japan Coma Scale status has been shown to be well correlated with the Glasgow Coma Scale score.20) The type of SE was categorized as tonic–clonic SE (ICD-10 code: G410), epileptic absence status (G411), complex partial SE (G412), and others or unspecified (G418, G419).19) The SE etiology was defined using ICD-10 diagnosis code at admission, as below; subarachnoid or intracerebral hemorrhage: I60, I61, I690, I691, cerebral infarction: I63, I693, other cerebral vascular etiologies: G08, G46, I62, I64, I67, I68, I694, I698, Q282, Q283, metabolic etiologies: E035, E05, E100, E101, E110, E111, E120, E121, E130, E131, E140, E141, E15, E161, E162, E222, E232, E51, E52, E53, E61, E70, E71, E72, E73, E74, E75, E76, E77, E80, E83, E870, E871, intoxication: G92, T36, T37, T38, T39, T4, T5, T60, T61, T62, T63, T64, T65, T96, T97, traumatic brain injury: S06, T790, T791, T905, brain neoplasm: C70, C71, C793, D430, D431, D432, D439, D33, inflammation/immune etiologies: G03, G040, G041, G048, G049, G058, neurodegenerative etiologies: G10, G12, G13, G20, G21, G22, G23, G30, G31, G32, G35, G36, G37, brain infections: G00, G01, G02, G042, G050, G051, G052, G060, G062, G07, other etiologies: G09, G80, G91, G930, G931, G932, G933, G934, G935, G936, G937, G939, G94, I46, P20, P21, Q0, T670, T671, T672, T703, and undetermined: none of above. Comorbidities of mental disorders was categorized as organic, including symptomatic, mental disorders (F00–F09), schizophrenia (F20–F29), mood disorders (F30–F39), neurotic, stress-related and somatoform disorders (F40–F48), and other mental disorders (F10–F19, F50–F99).21)
We collected the following clinical outcomes: in-hospital mortality, death within 24 h, length of hospital stay, mechanical ventilation on the day of admission, vasopressor use on the day of admission, drug-induced hepatitis, and drug-induced eruption. ICD-10 codes K71 for complications after admission denoted drug-induced hepatitis. ICD-10 codes L270, L271, L51, or L530 for complications after admission denoted drug-induced eruption.
To investigate the real-world practice patterns of AEDs for SE, we collected the use of i.v. AEDs on the day of admission based on the three clinical phases: 50% glucose, vitamin B1, and diazepam as first-line therapy; phenytoin, fosphenytoin, levetiracetam, and phenobarbital as second-line therapy; and midazolam, propofol, thiopental, and thiamylal as third-line therapy.14) Because i.v. lorazepam has not been available in Japan, diazepam is often used as the first-line therapy. We also collected the prescriptions of per os (p.o.) AEDs at discharge.
Statistical Analysis
Categorical variables were presented as numbers and percentages. Continuous variables were presented as mean and standard deviation or median and interquartile range. We showed the clinical outcomes, i.v. AEDs on the day of admission, and p.o. AEDs at discharge for each year separated by March 1. Differences between categorical variables within each year were analyzed using the Cochrane–Armitage test for trend for proportions.22) Differences between continuous variables within each year were analyzed using the Jonckheere–Terpstra test for trend.23) All tests were two-tailed; results for which P <0.05 were inferred as statistically significant. All analyses were conducted using software (STATA/MP 16.0; StataCorp LP, College Station, TX, USA).
Results
During the study period, 40,756 patients were admitted for SE. Of these, we excluded 7418 patients under 15 years old, 44 with pregnancy, and 1822 with planned admission. As a consequence, 31,472 patients were included in the analysis.
The baseline characteristics are shown in Table 1. Mean (standard deviation) age was 62 (21) years; 13,627 (43%) patients were 70 years old or older. The proportion of men was 57%. Consciousness on admission was deteriorated in most patients. Approximately 80% of SE was categorized into others or unspecified. Stroke was a major etiology of SE.
Table 1.
Baseline patient characteristics
| Total (n = 31,472) | |
|---|---|
| Age (years), mean (standard deviation) | 62 (21) |
| Age (years), n (%) | |
| 15–29 | 2983 (9.5) |
| 30–39 | 2311 (7.3) |
| 40–49 | 3197 (10) |
| 50–59 | 3686 (12) |
| 60–69 | 5668 (18) |
| 70–79 | 6365 (20) |
| 80+ | 7262 (23) |
| Male, n (%) | 17,860 (57) |
| Body mass index (kg/m2), n (%) | |
| <18.5 | 6199 (20) |
| 18.5–24.9 | 16,252 (52) |
| 25.0–29.9 | 3891 (12) |
| ≥30.0 | 1033 (3.3) |
| Missing | 4097 (13) |
| Japan Coma Scale on admission, n (%) | |
| Alert | 5172 (16) |
| Confusion | 10,323 (33) |
| Somnolence | 6019 (19) |
| Coma | 9958 (32) |
| Type of status epilepticus, n (%) | |
| Generalized tonic–clonic status epilepticus | 4106 (13) |
| Absence status epilepticus | 440 (1.4) |
| Complex partial status epilepticus | 1906 (6.1) |
| Other or unspecified | 25,020 (80) |
| Etiology of status epilepticus, n (%) | |
| Cerebral infarction | 4589 (15) |
| Subarachnoid or intracerebral hemorrhage | 2567 (8.2) |
| Other cerebral vascular etiologies | 759 (2.4) |
| Metabolic etiologies | 1246 (4.0) |
| Intoxication | 100 (0.3) |
| Traumatic brain injury | 1146 (3.6) |
| Brain neoplasm | 929 (3.0) |
| Inflammation/immune etiologies | 274 (0.9) |
| Neurodegenerative etiologies | 2042 (6.5) |
| Brain infections | 245 (0.8) |
| Other etiologies | 1399 (4.5) |
| Undetermined | 18,235 (58) |
| Comorbidities of mental disorder, n (%) | |
| Organic, including symptomatic, mental disorders | 1783 (5.7) |
| Schizophrenia | 1233 (3.9) |
| Mood disorders | 824 (2.6) |
| Neurotic, stress-related and somatoform disorders | 713 (2.3) |
| Other mental disorders | 1032 (3.3) |
Baseline characteristics of the status epilepticus included in this study are listed.
The use of i.v. AEDs on the day of admission and clinical outcomes based on the three clinical phases is shown in Table 2. No year-by-year change in the first-line therapy was found except for i.v. diazepam. Regarding the second-line therapy, i.v. fosphenytoin increased from January 2012; i.v. phenytoin decreased simultaneously. In 2015, i.v. phenytoin/fosphenytoin was used in almost 60% of SE. Subsequently, i.v. phenytoin/fosphenytoin use decreased after i.v. levetiracetam was used from 2016. The use of i.v. levetiracetam increased rapidly from 2016; 35% of patients received i.v. levetiracetam in 2017. The crude in-hospital mortality was 5.1% (n = 1590/31,472). In-hospital mortality decreased year-by-year (P <0.001 for trend). The year-by-year change was not observed in death within 24 h, length of hospital stay, drug-induced hepatitis, and drug-induced eruption.
Table 2.
Use of i.v. antiepileptic drugs on the day of admission based and clinical outcomes on the three clinical phases
| Total (n = 31,472) | Year | P for trend | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2011 (n = 2603) | 2012 (n = 3461) | 2013 (n = 3715) | 2014 (n = 4613) | 2015 (n = 5075) | 2016 (n = 6080) | 2017 (n = 5925) | |||
| First-line therapy, n (%) | |||||||||
| 50% glucose | 631 (2.0) | 62 (2.4) | 62 (1.8) | 86 (2.3) | 91 (2.0) | 92 (1.8) | 119 (2.0) | 119 (2.0) | 0.29 |
| Vitamin B1 | 743 (2.4) | 70 (2.7) | 64 (1.8) | 93 (2.5) | 103 (2.2) | 131 (2.6) | 149 (2.5) | 133 (2.2) | 0.94 |
| Diazepam | 17262 (55) | 1592 (61) | 1947 (56) | 1972 (53) | 2530 (55) | 2789 (55) | 3228 (53) | 3204 (54) | <0.001 |
| Second-line therapy, n (%) | |||||||||
| Phenytoin | 8099 (26) | 1475 (57) | 1449 (42) | 1184 (32) | 1311 (28) | 1147 (23) | 948 (16) | 585 (9.9) | <0.001 |
| Fosphenytoin | 8554 (27) | 2 (0.1) | 528 (15) | 951 (26) | 1402 (30) | 1809 (36) | 1920 (32) | 1942 (33) | <0.001 |
| Levetiracetum | 3578 (11) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 99 (2.0) | 1432 (24) | 2047 (35) | <0.001 |
| Phenobarbital | 2678 (8.5) | 353 (14) | 450 (13) | 386 (10) | 390 (8.5) | 416 (8.2) | 401 (6.6) | 282 (4.8) | <0.001 |
| Third-line therapy, n (%) | |||||||||
| Midazoram | 4092 (13) | 319 (12) | 459 (13) | 460 (12) | 620 (13) | 702 (14) | 772 (13) | 760 (13) | 0.66 |
| Propofol | 2015 (6.4) | 182 (7.0) | 209 (6.0) | 242 (6.5) | 270 (5.9) | 332 (6.5) | 381 (6.3) | 399 (6.7) | 0.96 |
| Thiopental | 91 (0.3) | 15 (0.6) | 10 (0.3) | 15 (0.4) | 8 (0.2) | 9 (0.2) | 17 (0.3) | 17 (0.3) | 0.04 |
| Thiamylal | 122 (0.4) | 22 (0.8) | 23 (0.7) | 19 (0.5) | 15 (0.3) | 12 (0.2) | 17 (0.3) | 14 (0.3) | <0.001 |
| In-hospital mortality, n (%) | 1590 (5.1) | 185 (7.1) | 204 (5.9) | 187 (5.0) | 248 (5.4) | 243 (4.8) | 278 (4.6) | 245 (4.1) | <0.001 |
| Death within 24 h, n (%) | 141 (0.5) | 18 (0.7) | 10 (0.3) | 18 (0.5) | 26 (0.6) | 22 (0.4) | 23 (0.4) | 24 (0.4) | 0.20 |
| Length of hospital stay (days), median (IQR) | 12 (5, 26) | 12 (5, 29) | 11 (5, 26) | 12 (5, 28) | 11 (5, 26) | 11 (5, 26) | 12 (6, 27) | 12 (5, 25) | 0.97 |
| MV on the day of admission, n (%) | 3032 (9.6) | 295 (11) | 327 (9.4) | 380 (10) | 422 (9.1) | 461 (9.1) | 579 (9.5) | 568 (9.6) | 0.031 |
| Vasopressors on the day of admission, n (%) | 818 (2.6) | 97 (3.7) | 75 (2.2) | 110 (3.0) | 119 (2.6) | 121 (2.4) | 142 (2.3) | 154 (2.6) | 0.011 |
| Drug-induced hepatitis, n (%) | 46 (0.2) | 3 (0.1) | 6 (0.2) | 10 (0.3) | 10 (0.2) | 7 (0.1) | 5 (0.1) | 5 (0.1) | 0.068 |
| Drug-induced eruption, n (%) | 161 (0.5) | 11 (0.4) | 21 (0.6) | 18 (0.5) | 24 (0.5) | 27 (0.5) | 30 (0.5) | 30 (0.5) | 0.98 |
As the second-line therapy, i.v. fosphenytoin increased; i.v. phenytoin decreased simultaneously. Subsequently, i.v. phenytoin/fosphenytoin use decreased after i.v. levetiracetam was used. The use of i.v. levetiracetam increased rapidly. In-hospital mortality decreased year-by-year. MV: mechanical ventilation, IQR: interquartile range.
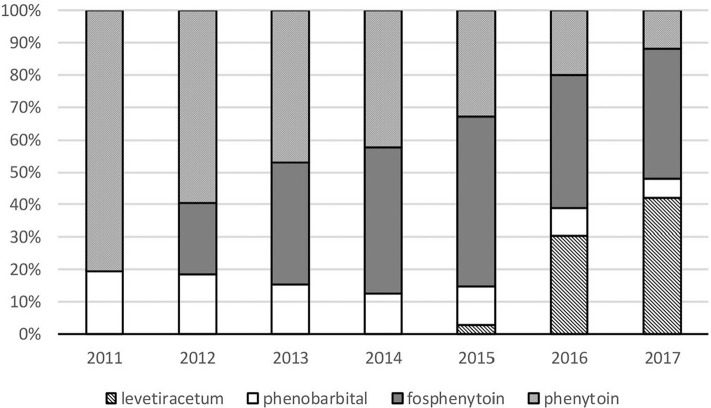
Figure 1 depicts the trajectory of the breakdown in second-line therapy drugs. Midazolam and propofol as third-line therapy showed no change year-by-year. Clinical outcomes in each second-line drug use on admission is shown in Table 3.
Fig. 1.
Breakdown trajectory of second-line therapy antiepileptic drug use for status epilepticus. Breakdown of second-line therapy for status epilepticus is described. Each box represents the total number of prescriptions of intravenous levetiracetam, phenobarbital, fosphenytoin, and phenytoin each year. As second-line therapy, intravenous fosphenytoin has been available from January 2012; intravenous levetiracetam has been available from December 2015 (national health insurance will not compensate the use of levetiracetam to treat status epilepticus). Valproate acid has not been available. From 2012, fosphenytoin use has increased; the use of phenytoin has decreased. The use of levetiracetam has increased rapidly since 2016. The use of levetiracetam exceeded that of fosphenytoin in 2017.
Table 3.
Clinical outcomes in each second-line anti-epileptic drug use on admission
| Phenytoin (n = 8099) | Fosphenytoin (n = 8554) | Levetiracetam (n = 3578) | Phenobarbital (n = 2678) | |
|---|---|---|---|---|
| In-hospital mortality, n (%) | 423 (5.2) | 357 (4.2) | 145 (4.1) | 132 (4.9) |
| Death within 24 h, n (%) | 33 (0.4) | 27 (0.3) | 7 (0.2) | 5 (0.2) |
| Length of hospital stay (days), median (IQR) | 12 (6, 27) | 12 (5, 25) | 13 (6, 26) | 11 (5, 26) |
| MV on the day of admission, n (%) | 937 (11.6) | 966 (11.3) | 387 (10.8) | 186 (6.9) |
| Vasopressors on the day of admission, n (%) | 243 (3.0) | 253 (3.0) | 83 (2.3) | 58 (2.2) |
| Drug-induced hepatitis, n (%) | 18 (0.2) | 10 (0.1) | 2 (0.1) | 5 (0.2) |
| Drug-induced eruption, n (%) | 45 (0.6) | 56 (0.7) | 22 (0.6) | 17 (0.6) |
Clinical outcomes in each anti-epileptic drug use as second-line therapy are shown. MV: mechanical ventilation, IQR: interquartile range.
Discharge prescriptions of p.o. AEDs and the numbers of p.o. AEDs at discharge among patients who received at least one p.o. AED at discharge are shown in Table 4. The proportion of prescription was the highest for p.o. levetiracetam (50%), followed by sodium valproate (29%) and carbamazepine (22%). The proportion of using p.o. levetiracetam increased year-by-year (P <0.001 for trend). Monotherapy increased year-by-year; combined therapy decreased year-by-year (P <0.001 for trend).
Table 4.
Prescriptions of p.o. antiepileptic drugs at discharge
| Total (n = 17,743) | Year | P for trend | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2011 (n = 1488) | 2012 (n = 1993) | 2013 (n = 2090) | 2014 (n = 2579) | 2015 (n = 2888) | 2016 (n = 3399) | 2017 (n = 3306) | |||
| Type of antiepileptic drugs at discharge | |||||||||
| Levetiracetum, n (%) | 8917 (50) | 250 (17) | 683 (34) | 785 (38) | 1031 (40) | 1656 (57) | 2212 (65) | 2300 (70) | <0.001 |
| Carbamazepine, n (%) | 3825 (22) | 348 (23) | 495 (25) | 580 (28) | 692 (27) | 587 (20) | 584 (17) | 529 (16) | <0.001 |
| Sodium valproate, n (%) | 5226 (29) | 600 (40) | 797 (40) | 806 (39) | 914 (35) | 747 (26) | 761 (22) | 601 (18) | <0.001 |
| Phenytoin, n (%) | 2085 (12) | 320 (22) | 356 (18) | 345 (17) | 350 (14) | 313 (11) | 257 (7.6) | 144 (4.4) | <0.001 |
| Lamotrigine, n (%) | 1204 (6.8) | 95 (6.4) | 107 (5.4) | 133 (6.4) | 247 (9.6) | 185 (6.4) | 233 (6.9) | 204 (6.2) | 0.60 |
| Phenobarbital, n (%) | 809 (4.6) | 102 (6.9) | 140 (7.0) | 119 (5.7) | 135 (5.2) | 115 (4.0) | 97 (2.9) | 101 (3.1) | <0.001 |
| Clonazepam, n (%) | 854 (4.8) | 80 (5.4) | 94 (4.7) | 130 (6.2) | 162 (6.3) | 109 (3.8) | 134 (3.9) | 145 (4.4) | <0.001 |
| Others, n (%) | 6970 (39) | 695 (47) | 885 (44) | 971 (47) | 1139 (44) | 1013 (35) | 1094 (32) | 1173 (36) | <0.001 |
| Number of drugs | |||||||||
| 1 | 9420 (53) | 758 (51) | 922 (46) | 876 (42) | 1100 (43) | 1660 (58) | 2079 (61) | 2025 (61) | <0.001 |
| 2 | 5095 (29) | 481 (32) | 652 (33) | 763 (37) | 982 (38) | 727 (25) | 753 (22) | 737 (22) | <0.001 |
| 3 | 1985 (11) | 165 (11) | 257 (13) | 275 (13) | 315 (12) | 298 (10) | 356 (11) | 319 (9.6) | <0.001 |
| ≥4 | 1243 (7.0) | 84 (5.6) | 162 (8.1) | 176 (8.4) | 182 (7.1) | 203 (7.0) | 211 (6.2) | 225 (6.8) | 0.13 |
Prescriptions of p.o. antiepileptic drugs at discharge for patients prescribed antiepileptic drugs at discharge are described. The proportion of prescription was the highest for p.o. levetiracetam and it increased year by year. Monotherapy increased year by year. Patients who received an antiepileptic prescription at discharge were 17,743.
Discharge prescriptions following i.v. levetiracetam on the day of admission and i.v. phenytoin/fosphenytoin on the day of admission from 2015 through 2017 are shown in Table 5. Results show that p.o. levetiracetam at discharge following i.v. levetiracetam on the day of admission was prescribed for over 80% of the patients. By contrast, p.o. phenytoin at discharge following i.v. phenytoin/fosphenytoin on the day of admission was only prescribed for 5.4–6.5%; p.o. levetiracetam at discharge following i.v. phenytoin/fosphenytoin on the day of admission was prescribed for 58.5–65.5%.
Table 5.
Prescriptions at discharge following i.v. levetiracetam on the day of admission and i.v. phenytoin/fosphenytoin on the day of admission
| Prescriptions at discharge | i.v. LEV on the day of admission | i.v. PHT/FPHT on the day of admission | ||||
|---|---|---|---|---|---|---|
| 2015 (n = 61) | 2016 (n = 886) | 2017 (n = 1271) | 2015 (n = 1751) | 2016 (n = 1678) | 2017 (n = 1451) | |
| Levetiracetum, n (%) | 52 (85) | 722 (82) | 1036 (82) | 1024 (59) | 1065 (64) | 951 (66) |
| Carbamazepine, n (%) | 5 (8.2) | 118 (13) | 159 (13) | 340 (19) | 299 (18) | 248 (17) |
| Sodium valproate, n (%) | 6 (9.8) | 136 (15) | 178 (10) | 435 (25) | 387 (23) | 285 (20) |
| Phenytoin, n (%) | 2 (3.3) | 40 (4.5) | 61 (4.8) | 215 (12) | 152 (9.1) | 85 (5.9) |
| Lamotrigine, n (%) | 2 (3.3) | 40 (4.5) | 61 (4.8) | 114 (6.5) | 107 (6.4) | 79 (5.4) |
| Phenobarbital, n (%) | 4 (6.6) | 14 (1.6) | 30 (2.4) | 59 (3.4) | 49 (2.9) | 32 (2.2) |
| Clonazepam, n (%) | 1 (1.6) | 21 (2.4) | 43 (3.4) | 54 (3.1) | 73 (4.4) | 63 (4.3) |
| Others, n (%) | 8 (13) | 226 (26) | 346 (27) | 584 (33) | 557 (33) | 561 (39) |
Prescriptions at discharge following i.v. levetiracetam or phenytoin/fosphenytoin on the day of admission are described. In both groups, per os levetiracetam was widely prescribed after status epilepticus management. i.v.: intravenous, LEV: levetiracetum, PHT: phenytoin, FPHT: fosphenytoin.
Discussion
For this study, we investigated the real-world practice patterns of AEDs for SE using a nationwide inpatient database in Japan. Every time new AEDs (such as i.v. fosphenytoin and i.v. levetiracetam) emerged, the second-line therapy for SE changed greatly. Both i.v. and p.o. levetiracetam increased rapidly and were predominantly prescribed in preference to other AEDs in Japan.
To date, i.v. levetiracetam has never been covered by public health insurance or tax in most countries. This study revealed that i.v. levetiracetam was actually used more than i.v. fosphenytoin recently in Japan for patients with SE.
Three randomized controlled trials which compared i.v. levetiracetam and i.v. phenytoin in SE concluded that the efficacy was similar and severe adverse events occurred only in patients with i.v. phenytoin and not observed in patients with i.v. levetiracetam.10–12) Side effects of levetiracetam were psychiatric disorders of agitation or somnolence and were not severe even if high-dose levetiracetam was administered.24) Because of safety, p.o. levetiracetam has been used most frequently as a routine epilepsy treatment in the United States.25) Our real-world data showed similar results to those obtained from those earlier studies; clinical outcomes and side effects did not worsen year-by-year. Therefore, we propose that public health insurance coverage of i.v. levetiracetam be extended to treatment of SE.
In this study, p.o. levetiracetam at discharge following i.v. levetiracetam on the day of admission was prescribed more than 80% of cases. However, p.o. phenytoin at discharge following i.v. phenytoin/fosphenytoin on the day of admission was prescribed in only 5.4–6.5% of cases. Same-drug switching was able to facilitate the continuous, stable control of epileptic seizures. This point should be investigated in a future study.
This study has some limitations. First, the diagnosis of SE based on diagnostic codes has not been well validated. Second, the results of trend analyses did not directly demonstrate the association between clinical outcomes and changes of AEDs year-by-year. Third, because the use of i.v. levetiracetam is not compensated by health insurance, some hospitals might not declare the costs of levetiracetam. It is therefore possible that this study has underestimated the use of i.v. levetiracetam.
Conclusion
Although levetiracetam is not recognized as a treatment for SE by the public health insurance system in Japan, i.v. levetiracetam use is increasing dramatically as second-line therapy. We propose that public health insurance coverage of i.v. levetiracetam should be extended to treatment of SE.
Footnotes
Availability of Data and Materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ Contributions
KN contributed in conception and conduction of the study, drafting the manuscript. HO contributed in data analysis and interpretation, drafting the manuscript. HM contributed in data analysis. YT, AM, YI contributed in interpretation and contribution to the manuscript. KF contributed in revisions of the manuscript. HY contributed in revisions of the manuscript and supervised the study. All authors have read and approved the manuscript.
Ethics Approval and Consent to Participate
The Institutional Review Board of The University of Tokyo approved the study.
Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (19AA2007 and H30-Policy-Designated-004) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (17H04141).
Role of the Funding Source
The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the study data and had final responsibility for the decision to submit the manuscript for publication.
Conflicts of Interest Disclosure
The authors state that they have no conflict of interest related to this paper or the study it describes.
References
- 1).Chapman MG, Smith M, Hirsch NP: Status epilepticus. Anaesthesia 56: 648–659, 2001 [DOI] [PubMed] [Google Scholar]
- 2).Trinka E, Cock H, Hesdorffer D, et al. : A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 56: 1515–1523, 2015 [DOI] [PubMed] [Google Scholar]
- 3).Kapur J, Macdonald RL: Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci 17: 7532–7540, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Treiman DM, Meyers PD, Walton NY, et al. : A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 339: 792–798, 1998 [DOI] [PubMed] [Google Scholar]
- 5).Brophy GM, Bell R, Claassen J, et al. : Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 17: 3–23, 2012 [DOI] [PubMed] [Google Scholar]
- 6).Chang CW, Bleck TP: Status epilepticus. Neurol Clin 13: 529–548, 1995 [PubMed] [Google Scholar]
- 7).Coplin WM, Rhoney DH, Rebuck JA, Clements EA, Cochran MS, O’Neil BJ: Randomized evaluation of adverse events and length-of-stay with routine emergency department use of phenytoin or fosphenytoin. Neurol Res 24: 842–848, 2002 [DOI] [PubMed] [Google Scholar]
- 8).Swadron SP, Rudis MI, Azimian K, Beringer P, Fort D, Orlinsky M: A comparison of phenytoin-loading techniques in the emergency department. Acad Emerg Med 11: 244–252, 2004 [DOI] [PubMed] [Google Scholar]
- 9).Meehan AL, Yang X, McAdams BD, Yuan L, Rothman SM: A new mechanism for antiepileptic drug action: vesicular entry may mediate the effects of levetiracetam. J Neurophysiol 106: 1227–1239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Mundlamuri RC, Sinha S, Subbakrishna DK, et al. : Management of generalised convulsive status epilepticus (SE): a prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam—Pilot study. Epilepsy Res 114: 52–58, 2015 [DOI] [PubMed] [Google Scholar]
- 11).Gujjar AR, Nandhagopal R, Jacob PC, et al. : Intravenous levetiracetam vs phenytoin for status epilepticus and cluster seizures: a prospective, randomized study. Seizure 49: 8–12, 2017 [DOI] [PubMed] [Google Scholar]
- 12).Chakravarthi S, Goyal MK, Modi M, Bhalla A, Singh P: Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci 22: 959–963, 2015 [DOI] [PubMed] [Google Scholar]
- 13).Nakamura K, Inokuchi R, Daidoji H, et al. : Efficacy of levetiracetam versus fosphenytoin for the recurrence of seizures after status epilepticus. Medicine (Baltimore) 96: e7206, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Glauser T, Shinnar S, Gloss D, et al. : Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr 16: 48–61, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Shih JJ, Whitlock JB, Chimato N, Vargas E, Karceski SC, Frank RD: Epilepsy treatment in adults and adolescents: expert opinion, 2016. Epilepsy Behav 69: 186–222, 2017 [DOI] [PubMed] [Google Scholar]
- 16).Meierkord H, Boon P, Engelsen B, et al. : EFNS guideline on the management of status epilepticus in adults. Eur J Neurol 17: 348–355, 2010 [DOI] [PubMed] [Google Scholar]
- 17).Matsuda S: Development of case mix based evaluation system in Japan. Jpn Hosp 35–44, 2016 [PubMed] [Google Scholar]
- 18).Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H: Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol 27: 476–482, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Jette N, Beghi E, Hesdorffer D, et al. : ICD coding for epilepsy: past, present, and future—a report by the International League Against Epilepsy Task Force on ICD codes in epilepsy. Epilepsia 56: 348–355, 2015 [DOI] [PubMed] [Google Scholar]
- 20).Shigematsu K, Nakano H, Watanabe Y: The eye response test alone is sufficient to predict stroke outcome—reintroduction of Japan Coma Scale: a cohort study. BMJ Open 3: pii: e002736, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).World Health Organization The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. World Health Organization. [Google Scholar]
- 22).Royston P: PTREND: Stata module for trend analysis for proportions. Statistical Software Components S426101, 2014.
- 23).Coveney J: JONTER: Stata module to perform Jonckheere–Terpstra test. Statistical Software Components S423601, 2002.
- 24).Ramael S, Daoust A, Otoul C, et al. : Levetiracetam intravenous infusion: a randomized, placebo-controlled safety and pharmacokinetic study. Epilepsia 47: 1128–1135, 2006 [DOI] [PubMed] [Google Scholar]
- 25).Faught E, Helmers S, Thurman D, Kim H, Kalilani L: Patient characteristics and treatment patterns in patients with newly diagnosed epilepsy: a US database analysis. Epilepsy Behav 85: 37–44, 2018 [DOI] [PubMed] [Google Scholar]