Abstract
Diffuse astrocytic and oligodendroglial tumors are frequently associated with symptomatic epilepsy, and predictive seizure control is important for the improvement of patient quality of life. To elucidate the factors related to drug resistance of brain tumor-associated epilepsy from a pathological perspective. From January 2012 to October 2017, 36 patients diagnosed with diffuse astrocytic or oligodendroglial tumors were included. Assessment for seizure control was performed according to the Engel classification of seizures. Patient clinical, radiological, and pathological data were stratified based on the following 16 variables: age, sex, location of tumor, existence of the preoperative seizure, extent of resection, administration of temozolomide, radiation therapy, recurrence, Karnofsky performance scale, isocitrate dehydrogenase 1, 1p/19q co-deletion, Olig2, platelet-derived growth factor receptor alpha, p53, ATRX, and Ki67. These factors were compared between the well-controlled group and drug-resistant seizure group. Twenty-seven patients experienced seizures; of these, 14 cases were well-controlled, and 13 cases were drug-resistant. Neither clinical nor radiological characteristics were significantly different between these two groups, though p53 immunodetection levels were significantly higher, and the frequency of 1p/19q co-deletion was significantly lower in the group with drug-resistant seizures than in the well-controlled group. In the multivariate analysis, only one item was selected according to stepwise methods, and a significant difference was observed for p53 (OR, 21.600; 95% CI, 2.135–218.579; P = 0.009). Upregulation of p53 may be a molecular mechanism underlying drug resistant epilepsy associated with diffuse astrocytic and oligodendroglial tumors.
Keywords: glioma, oligodendroglioma, diffuse astrocytoma, epilepsy
Introduction
Diffuse astrocytoma and oligodendroglioma are classified as low grade gliomas (LGG) in accordance with the 2016 revision of the World Health Organization (WHO) criteria for brain tumors. Diffuse astrocytoma and oligodendroglioma account for around 70% of LGG.1) The prevalence of seizures is 60–88% in patients with LGG, whereas the 5 years survival rate of these patients is around 70–90%.2–4) Therefore, it is important to achieve better seizure control to improve the patients’ quality of life.5,6) It has been reported that approximately 30% of the cases are uncontrolled seizures, regardless of appropriate treatment with antiepileptic drugs for epilepsy associated with LGG.7) Tumors causing drug-resistant seizures for more than 2 years are a cumbersome problem, and are defined as “long-term epilepsy-associated tumors”.8)
Recently, the risk factors for brain tumor-associated epilepsy were evaluated in LGG cases, especially seizure control.9) The efficacy of tumor treatment is important for seizure control,4,10–13) as maximal resection improves seizure control. Moreover, in 80% of patients with temporal lesion of Engel class I, seizure control was achieved with maximal resection.6,14) Radiotherapy of WHO grade II patients resulted in a reduction of seizure frequency by 50% in 76% of the patients at 3 months after therapy, while there were no apparent changes in tumor responses on magnetic resonance imaging (MRI).15,16) Nevertheless, seizure control is achieved upon chemotherapy regardless of the regimen.10) Temozolomide (TMZ), which is generally used in LGG and malignant gliomas, resulted in 50% reduction in seizure frequency in 48% of patients.17,18)
From a histopathological point of view, oligodendrogliomas are more often associated with the prevalence of seizures than are astrocytomas. It has been reported that isocitrate dehydrogenase 1 (IDH-1) mutation,19–22) 1p/19q co-deletion,23) and high expression levels of Ki67 are associated with seizure occurrence.7,23) Although these studies were focused on seizure occurrence, little is known about the factors associated with the degree of drug-resistant seizures. In this report, we analyzed the clinical and pathological factors associated with drug-resistance in brain tumor-associated epilepsy in patients with astrocytic and oligodendroglial tumors.
Materials and Methods
Description of patient population
We declare that this study has been approved by Institutional Ethics Committee, and have been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. The study protocol was approved by the Ethics Committee of Sapporo Medical University Hospital (No. 292-242). All patients provided informed consent before participating. From January 2012 to October 2017, 48 consecutive patients were diagnosed with diffuse astrocytic or oligodendroglial tumors (WHO grade II) either by analysis of specimens collected after brain tumor resection or through biopsy at our institute. Pathological diagnosis was based on the WHO guidelines, as revised in 2016.24) A minimum postoperative follow-up period of 12 months was required. Consequently, patients diagnosed with diffuse astrocytoma with IDH-1 mutation, oligodendroglioma with IDH-1 mutation and 1p/19q co-deletion, diffuse astrocytoma with wild-type IDH-1, and oligodendroglioma not-otherwise-specified (NOS) were included.
We evaluated each case for seizure control at least 12 months after its recurrence. The patients were classified into two groups based on the extent of seizure control. Assessment for seizure control was performed according to the Engel classification of seizures.25) Cases of Engel class I and II were classified as well-controlled, and cases of class III and IV were classified as drug-resistant in accordance with the study of Kwan et al.26)
Magnetic resonance imaging examination was performed using a 3T system (Signa Excite, version 11, GE Medical Systems, Waukesha, WI, USA) preoperatively, at 1 day and 1 month after operation. Subsequently, MRI was performed every 6 months. T1-weighted, gadolinium enhanced T1-weighted, T2-weighted, and fluid-attenuated inversion recovery sequences were used for evaluation.
Patient clinical and radiological data were stratified based on the following nine variables: (1) age; (2) sex; (3) tumor location (frontal, temporal, other); (4) presence of the preoperative seizure; (5) extent of tumor resection (≥95%; gross total resection and subtotal resection as a border, <95%; partial resection and biopsy); (6) TMZ administration; (7) radiation therapy; (8) decrease of tumor volume of <50% or ≥50% after treatment with operation, radiation, and chemotherapy; (9) recurrence; (10) neurological symptoms; (11) Karnofsky Performance Scale (KPS). The clinical and radiological factors were compared between the well-controlled group and drug-resistant seizure group.
Detection of 1p/19q co-deletion by fluorescence in situ hybridization
We performed fluorescence in situ hybridization (FISH) using Vysis LSI 1p36/LSI 1q25 and LSI 19q13/19p13 Dual-Color Probe (Abbott, Abbott Park, IL, USA) for 1p36 and 19q13 deletion. Each of the probes was labeled with red and green fluorescence in target and control loci, respectively. FISH was conducted on formalin-fixed, paraffin-embedded specimens sectioned into 4-μm slices on glass slides, as previously described.27–29) We counted 50 tumor nuclei and calculated the percentage of deletion signals. The deletion signals were classified as homozygous deletions, heterozygous deletions, and monosomy patterns. A homozygous deletion involved a complete deletion of the target locus in both alleles and exhibited only control green signal. A heterozygous deletion corresponded to the deletion of either of the target loci in one allele; the red signals for the target loci were detected in less nuclei than the green signals for control loci. Tumor cells with monosomy had one allele, and exhibited a pair of red and green signals. Monosomy was also considered as one of the patterns of heterozygous deletion. The results of FISH were confirmed by T.A., S.S., and T.H.
Pathological assessment by immunohistochemistry
We performed immunohistochemistry on formalin-fixed, paraffin-embedded specimens cut into 3 μm-thick sections. We used antibodies against the following antigens: IDH-1 (H09, Dianova, Hamburg, Germany), ATRX (ab97508, Abcam, Cambridge, MA, USA), p53 (DO-7, BioGenex, Fremont, CA, USA), Olig2 (polyclonal, IBL, Gunma, Japan), platelet-derived growth factor receptor alpha (PDGFR-α, 5241, Cell Signaling Technology, Danvers, MA, USA), and Ki67 (Ki67, Agilent Technologies, Santa Clara, CA, USA).
Figure 1 illustrates the scoring for each immunostaining. Scoring for Olig2 and PDGFR-α was done per specimen on a 4-point scale, from 0 to 3, at 200× magnification, defined as follows: 0 corresponded to no or rare staining and 1 corresponded to <10%, 2 corresponded to 10–49%, and 3 corresponded to ≥50% of positively stained cells. Scores for p53 and ATRX were analyzed per specimen at 200× magnification, using a scale of 0–1 [0 corresponded to low expression (<10%), and 1 corresponded to high expression (≥10%)]. Scoring for Ki67 was done on a 2-point scale, from 0 to 1 (0 corresponded to <5% and 1 to ≥5% of positively stained cells). Immunoreactivity was estimated by two neuro-oncologists (H.S. and T.M.) and one pathologist (S.S.). The pathological factors were compared between the group with seizures and the seizure-free group, and between the well-controlled and drug-resistant seizure groups.
Fig. 1.
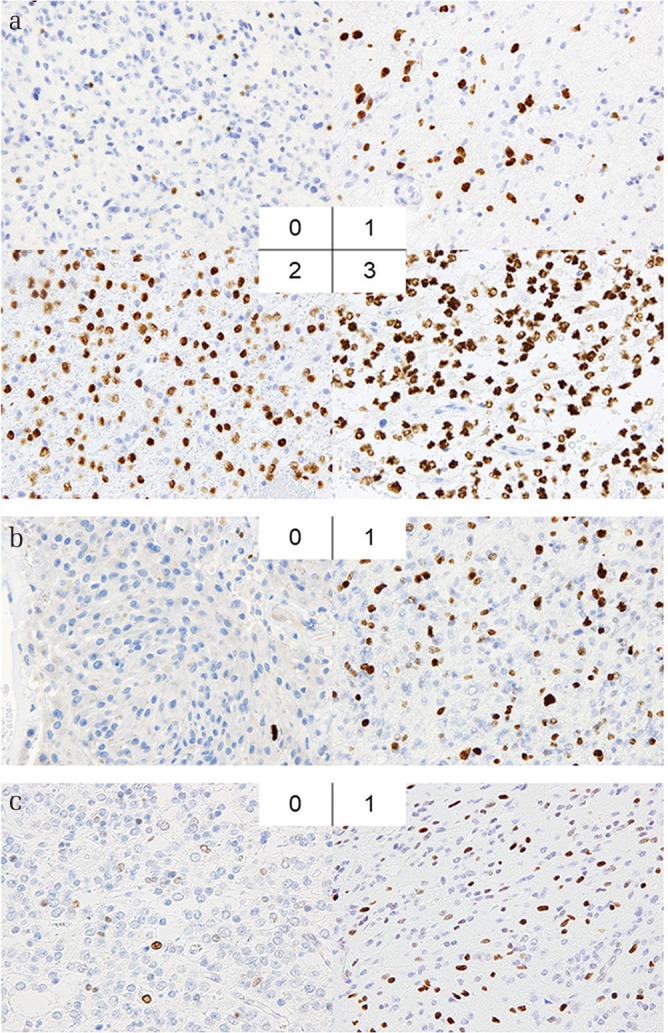
Microscopic images of tumor specimens stained by immunohistochemistry with different scores. (a) Scoring for Olig2 was defined follows: 0 corresponds to no or rare staining, 1 corresponds to <10% of positively stained cells, 2 to 10–49%, and 3 to ≥50% of positively stained cells, at 200× magnification. (b) Scoring for Ki67 was defined follows: 0 corresponds to <5%, and 1 to ≥5% of positively stained cells, at 200× magnification. (c) Scoring for p53 was defined as follows: 0 corresponds to low (<10%), and 1 to high expression (≥10%) at 200× magnification.
Statistical analysis
Data are expressed as median (interquartile range). Mann–Whitney U-test and Fisher’s exact probability tests were used for the comparison between the groups of patients with well-controlled seizures and patients with drug-resistant seizures. For tests that resulted in P <0.05, simple logistic regression was used in the univariate analyses. Odds ratios (ORs) were obtained through these models with 95% confidence intervals (CIs). Each item was then selected according to stepwise methods (model selection criterion, 0.10), and a multivariate analysis of all potential factors associated with drug-resistant seizure was performed. Kaplan–Meier estimates were used to assess the drug-resistance of seizures in patients with epilepsy. Endpoint was set at the day of recurrence of seizure. All statistical analyses were conducted using the SPSS software package (version 24.0, IBM Corp., Armonk, NY, USA), and P <0.05 was considered to be indicative of statistical significance.
Results
Patient data
Three patients with poor quality of specimens and nine patients with insufficient specimens for histopathological analysis were excluded from 48 patients. A total of 36 patients (19 men and 17 women) were enrolled and retrospectively analyzed with regards to the presence of drug-resistant seizures. In terms of pathological diagnosis, 26 cases carried an IDH-1 mutation and 10 cases did not. Of the 26 cases with the IDH-1 mutation, 13 cases carried the 1p/19q co-deletion and 13 cases did not. Pathological diagnosis according to the 2016 WHO guidelines was diffuse astrocytoma with IDH-1 mutation in 14 cases, diffuse astrocytoma with wild-type IDH in eight cases, oligodendroglioma with IDH-1 mutation and 1p/19q co-deletion in 13 cases, and oligodendroglioma with NOS in one case. The median patient age at the time of surgery was 35.5 years (interquartile range, 27.3–51.5; range, 4–82 years). The median follow-up period was 31.7 months (interquartile range, 24.7–65.9; range, 12.2–213.1 months). Mortality was observed at 1841 days, 2050 days, and 745 days after diagnosis in one case of diffuse astrocytoma with IDH-1 mutation and two cases of diffuse astrocytoma with wild-type IDH-1, respectively.
During the follow-up period, 27 out of 36 patients had seizures. Of these, 21 patients experienced seizures from disease onset, while six patients experienced seizures postoperatively. Mean duration from initial epileptic seizure to surgical intervention was 120 days in 21 cases; among these cases, focal to bilateral tonic–clonic seizures were presented in 12 patients, focal seizures with awareness in five patients, and focal seizures without awareness in four patients. Inducible seizures occurring only during the perioperative period were excluded. Seizures were classified based on the operational classification of seizure types by the International League Against Epilepsy,30) and the results were as follows: 14 patients presented with focal to bilateral tonic–clonic seizures, nine with focal seizures with awareness, and four with focal seizures without awareness.
Levetiracetam was used as the first-choice anticonvulsant in 22 cases; it was frequently used after it became available in our hospital. Other anticonvulsants included carbamazepine (6), lacosamide (3), phenobarbital (1), and sodium valproate (1). When seizures recurred, we weighted the drug or administered a different drug. The decision was made by the doctors based on guidelines.31) In 13 cases, a different drug was used because of drug-resistance: lacosamide (3), zonisamide (3), carbamazepine (2), lamotrigine (2), perampanel (2), and levetiracetam (1). There were eight cases receiving two kinds of anticonvulsants, three cases receiving three kinds of anticonvulsants, and two cases receiving four kinds of anticonvulsants. An additional anticonvulsant was not administered in any of these cases because of side effects of the previously administered anticonvulsant. TMZ Chemotherapy was performed on 25 patients with administration of 75 mg/m2 for 42 days (10) and 150 mg/m2 for 5 days (15). Radiation therapy was performed for 14 patients: 60 Gy/30 fr (8), 54 Gy/30 fr (4), 54 Gy/28 fr (1), and 46 Gy/23 fr (1).
The control of seizures in low grade glioma
Table 1 shows clinical and radiographical characteristics in the well-controlled group and the drug-resistant seizure group. The well-controlled group included 14 cases (51.9%) and the drug-resistant group included 13 cases (48.1%); in the well-controlled group, multiple anti-convulsant drugs were administered in one patient, and in the drug-resistant group, multiple drugs were administered in 12 patients. The median age was 43.5 years (range, 33.8–58.3) in the well-controlled group, and 32.0 years (range, 21.0–42.5) in the drug-resistant seizure group indicating that the younger patients exhibited unfavorable control of seizures (P = 0.038); neurological symptoms were observed in one case in the well-controlled group, and in six cases in the drug-resistant group (P = 0.021). There were no significant differences in sex, location of tumor, existence of the preoperative seizure, extent of tumor removal, prescription of TMZ, irradiation therapy, Tumor volume after treatment, and tumor recurrence between the two groups (P = 0.180, 0.486, 0.385, 1.000, 1.000, 0.440, 0.081, and 0.182, respectively). The median KPS was 90 (range, 90–100) in the well-controlled group, and 90, (range, 40–100), in the drug-resistant seizure group (P = 0.375). Therefore, the clinical characteristics of age and neurological symptoms were significantly associated with seizure control.
Table 1.
Clinical and pathological characteristics associated with seizure control in diffuse astrocytic and oligodendroglial tumors
| Well-controlled group (Engel Class I, II) | Drug-resistant group (Engel Class III, IV) | P-value | |
|---|---|---|---|
| Number | 14 | 13 | |
| Age | 43.5 (33.8–58.3) | 32.0 (21.0–42.5) | 0.038 |
| Sex (men/women) | 5/9 | 8/5 | 0.180 |
| Origin (frontal/temporal/other) | 7/6/1 | 6/4/3 | 0.486 |
| Existence of preoperative seizure (−/+) | 2/12 | 4/9 | 0.385 |
| Extent of tumor removal (<95% or ≥95%) | 4/10 | 4/9 | 1.000 |
| Temozolomide (−/+) | 5/9 | 4/9 | 1.000 |
| Radiation (−/+) | 10/4 | 7/6 | 0.440 |
| Tumor volume after treatment (<50% or ≥50%) | 11/3 | 6/7 | 0.081 |
| Recurrence (−/+) | 10/4 | 6/7 | 0.182 |
| Neurological symptoms (−/+) | 13/1 | 7/6 | 0.021 |
| Karnofsky Performance Scale | 90.0 (90.0–100.0) | 90.0 (40–100.0) | 0.375 |
| IDH-1 (−/+) | 2/12 | 5/8 | 0.209 |
| 1p/19q co-deletion (−/+) | 6/8 | 11/2 | 0.046 |
| Olig2 (−/+) | 6/8 | 10/3 | 0.072 |
| PDGFR-α (−/+) | 6/8 | 6/7 | 0.863 |
| p53 (−/+) | 9/5 | 1/12 | 0.004 |
| ATRX (−/+) | 8/6 | 8/5 | 0.816 |
| Ki67 (<5/≥5) | 8/6 | 7/6 | 0.863 |
P <0.05 was considered to be indicative of statistical significance.
From a pathological point of view, five cases (35.7%) in the well-controlled group and 12 cases (92.3%) in the drug-resistant seizure group were positive for p53 immunostaining and this difference was statistically significant (P = 0.004). Thus, the patients with p53-immunopositive tumors exhibited unfavorable seizure control. On the other hand, eight cases (57.1%) in well-controlled group and two cases (15.4%) in the drug-resistant seizure group had the 1p/19q co-deletion and this difference was statistically significant (P = 0.046), indicating that the patients without 1p/19q co-deletion mostly had drug-resistant seizures. There was no significant difference in immunodetection of IDH-1 between the well-controlled and drug-resistant seizure groups (P = 0.209). Moreover, immunostaining scores for Olig2, PDGFRα, and ATRX were not significantly different between the well-controlled and drug-resistant groups (P = 0.072, 0.863, 0.816, respectively). Additionally, high Ki67 expression (>5%) was detected in six cases (42.9%) in the well-controlled group and six cases (46.2%) in the drug-resistant group (P = 0.863). Consequently, the presence of p53 and 1p/19q co-deletion were identified to have a statistically significant association with seizure control.
The univariate analysis associated with drug-resistant seizure in patients with diffuse astrocytic and oligodendroglial tumors are presented in Table 2. In the univariate analysis, the prevalence of drug resistant seizure was associated with neurological symptoms (OR, 11.143; 95% CI, 1.108–112.012; P = 0.041), 1p/19q co-deletion (OR, 0.136; 95% CI, 0.022–0.860; P = 0.034) and p53 (OR, 21.600; 95% CI, 2.135–218.579; P = 0.009). In the multivariate analysis, only one item was selected according to stepwise methods, and a significant difference was noted in the p53 (OR, 21.600; 95% CI, 2.135–218.579; P = 0.009).
Table 2.
The predictive factors associated with drug-resistant seizure in patients with diffuse astrocytic and oligodendroglial tumors
| Characteristics | Odds ratio (95% CI) | P-value |
|---|---|---|
| Age | 0.940 (0.884–1.000) | 0.050 |
| Sex | 2.880 (0.603–13.749) | 0.185 |
| Origin (fronto-temporal lobe) | 3.900 (0.351–43.364) | 0.268 |
| Existence of preoperative seizure (−/+) | 0.375 (0.056-2.519) | 0.313 |
| Extent of tumor removal (<95% or ≥95%) | 0.900 (0.172–4.699) | 0.901 |
| Temozolomide (−/+) | 1.250 (0.251–6.235) | 0.785 |
| Radiation (−/+) | 2.143 (0.436–10.526) | 0.348 |
| Tumor volume after treatment (<50% or ≥50%) | 4.278 (0.798–22.928) | 0.090 |
| Recurrence (−/+) | 2.917 (0.594–14.327) | 0.187 |
| Neurological symptoms (−/+) | 11.143 (1.108–112.012) | 0.041 |
| Karnofsky Performance Scale | 0.974 (0.939–1.010) | 0.161 |
| IDH-1 (−/+) | 0.267 (0.041–1.727) | 0.165 |
| 1p/19q co-deletion (−/+) | 0.136 (0.022–0.860) | 0.034 |
| Olig2 (−/+) | 0.225 (0.042–1.194) | 0.080 |
| PDGFR-α (−/+) | 0.875 (0.191–3.999) | 0.863 |
| p53 (−/+) | 21.600 (2.135–218.579) | 0.009 |
| ATRX (−/+) | 0.833 (0.179–3.884) | 0.816 |
| Ki67 (<5/≥5) | 1.143 (0.250–5.224) | 0.863 |
P <0.05 was considered to be indicative of statistical significance.
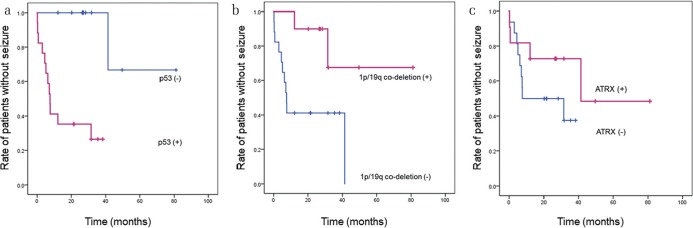
Twenty-seven patients with seizures were included in Kaplan–Meier estimation (Fig. 2a–c). The endpoint for the assessment of drug-resistance was defined as the day of recurrence seizure. The mean periods between seizure recurrence were 63.0 months (95% CI, 41.3–84.7) in the patients with the 1p/19q co-deletion versus 19.5 months (95% CI, 10.3–28.7) in the patients without 1p/19q co-deletion (P = 0.016). The mean periods for seizure recurrence were 16.1 months (95% CI, 8.7–23.5) in the patients with p53 upregulation versus 67.8 months (95% CI, 46.6–89.0) in the patients without p53 upregulation (P = 0.001). Moreover, the mean periods controlled by a single anticonvulsant were 50.5 months (95% CI, 27.7–73.3) in the patients with ATRX immunodetection versus 20.8 months (95% CI, 13.0–28.7) in the patients without ATRX staining (P = 0.181).
Fig. 2.
Kaplan–Meier estimates of period until the recurrence of seizure. The mean periods until the recurrence of seizure were significantly different between (a) cases with 1p/19q co-deletion and cases without the co-deletion (P = 0.016), and (b) cases with p53 upregulation and cases with normal p53 levels (P = 0.001). There was no significant difference in the mean periods controlled by a single anticonvulsant between (c) cases with tumors positive for ATRX staining and cases with tumors negative for ATRX (P = 0.181).
Discussion
The role of p53 in the control of seizures in patients with LGG
The results of our study indicated that the control of seizures in diffuse astrocytoma and oligodendroglioma is associated with p53 immunopositivity. This is the first study reporting that p53 is related to the drug-resistance of seizures. p53 is a major tumor suppressor gene that is located on human chromosome 17p13 and plays an important role in cellular homeostasis by DNA repair, control of apoptosis, and regulation of the cell cycle.32) Many types of cancer present abnormalities in p53 such as loss and mutation. In gliomas, immunodetection of p53 upregulation is indicative of the presence of p53 abnormalities and poor prognosis.33–35) So far, the association between p53 in diffuse astrocytoma and oligodendroglioma and seizure control has not been investigated. In preclinical studies on rats, normal p53 levels had an inhibitory effect on epilepsy. Engel et al. reported that lacking normal p53 results in prolongation of electrographic seizures and aggravation of the epileptic phenotype.36) p53 is associated with neuronal death. Neuronal impairment induced by excessive neuronal excitotoxity and prolonged seizures results in upregulation of p53, induction of DNA repair, and apoptosis. Indeed, in human temporal epilepsy and animal temporal epilepsy models, the levels of normal p53 were elevated in the hippocampus, and the absence of p53 was related to worse seizure outcomes because of seizure-induced neuronal death.37–39) Duan et al. reported that epilepsy nerve cell apoptosis is regulated by the genes such as p53, Bcl, and Bax, and they exhibit a neuroprotective effect.40) Therefore, p53 abnormalities might facilitate further intractability of seizures when epileptogenesis is established.
Our results demonstrated that the good control of seizures was also associated with 1p/19q co-deletion. This result is consistent with a previous report by You et al.23) With regard to the histopathology of the tumor, reciprocal characteristics between p53 mutation and 1p/19q co-deletion seem to exist, which can be adapted for the design of seizure control treatments. Only p53 was selected in the multivariate analysis, which revealed that p53 is a strong factor for seizure control. In addition, Olig2, a marker of mature oligodendrocytes, and PDGFRα, a marker of immature oligodendrocytes, were not significantly associated with seizure control. Therefore, the outcome on seizure control could not be explained by the pathological identity of oligodendrogliomas. Furthermore, it is reported that 80% of ATRX immunopositivity was detected in p53-positive diffuse astrocytoma and oligodendroglioma41) and, in our study ATRX immunodetection was not significantly related to the drug-resistance of seizures. Consequently, our results suggest that the drug-resistance of seizures is caused by p53 signaling in diffuse astrocytoma and oligodendroglioma, but via a pathway different than p53-ATRX.
With regard to clinical characteristics, neurological symptoms such as hemiparesis and aphasia are related to the anatomical and functional location of the tumors and risk factors of epileptic seizure.42,43) Moreover, the recurrence and malignant progression were related to epileptic seizure status and there were no significant differences in existence of preoperative seizure. It was considered that these patients with preoperative seizures were treated surgically before acquisition of drug-resistance.42) However, in the multivariate analysis performed in our study, we excluded these factors and only included p53.
Limitation
This study had several limitations. First, pathological factors presented in this study were estimated by immunostaining procedures. Since the WHO brain tumor classification was revised in 2016, a molecular evaluation and assessment would be necessary for brain tumor diagnosis. The second problem is the validity of the evaluation and assessment for seizures. The Engel classification, which was used for the assessment of seizure control, bears some discrepancies between the original use for idiopathic epilepsy and the adaptation for brain tumor-associated epilepsy.7,16,44,45) Moreover, the follow-up period was shorter than that used in idiopathic epilepsy. This is the most important discrepancy between the conventional approach for brain tumor-related epilepsy and the original approach for idiopathic epilepsy. Finally, this study involved a relatively small number of participants and use of individualized treatment approach. Therefore, statistical analysis was performed using a univariate nonparametric test for all comparisons. In addition, the influence on epileptic seizure by each treatment result was not assessed. Studies with larger sample sizes are needed in the future for the confirmation of our observations.
Conclusion
In diffuse astrocytoma and oligodendroglioma, the upregulation of p53 was associated with drug-resistance. In seizure control, there was a reciprocal association between p53 mutation and 1p/19q co-deletion as evidenced by the pathological characteristics of the tumors. In cases of diffuse astrocytoma and oligodendroglioma, long-life prognosis and disease-free survival are anticipated. Therefore, the assessment and prognosis of brain tumor-associated epilepsy are important for the management of diffuse astrocytoma and oligodendroglioma.
Acknowledgment
This investigation was supported by the JSPS KAKENHI (18K08999).
Footnotes
Ethical Approval
We declare that this study has been approved by the Ethics Committee of Sapporo Medical University Hospital (No. 292-242), and have been performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
All patients provided informed consent before participating.
Conflict of Interest Disclosure
The authors declare that they have no conflicts of interest.
References
- 1).Brain Tumor Registry of Japan (2005–2008). Neurol Med Chir (Tokyo) 57: 9–102, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Danfors T, Ribom D, Berntsson SG, Smits A: Epileptic seizures and survival in early disease of grade 2 gliomas. Eur J Neurol 16: 823–831, 2009 [DOI] [PubMed] [Google Scholar]
- 3).Rudà R, Trevisan E, Soffietti R: Epilepsy and brain tumors. Curr Opin Oncol 22: 611–620, 2010 [DOI] [PubMed] [Google Scholar]
- 4).Avila EK, Chamberlain M, Schiff D, et al. : Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro-oncology 19: 12–21, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Mulligan L, Ryan E, O’Brien M, et al. : Genetic features of oligodendrogliomas and presence of seizures. The relationship of seizures and genetics in LGOs. Clin Neuropathol 33: 292–298, 2014 [DOI] [PubMed] [Google Scholar]
- 6).Chang EF, Potts MB, Keles GE, et al. : Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg 108: 227–235, 2008 [DOI] [PubMed] [Google Scholar]
- 7).You G, Sha ZY, Yan W, et al. : Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro-oncology 14: 230–241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Radhakrishnan A, Abraham M, Vilanilam G, et al. : Surgery for “Long-term epilepsy associated tumors (LEATs)”: Seizure outcome and its predictors. Clin Neurol Neurosurg 141: 98–105, 2016 [DOI] [PubMed] [Google Scholar]
- 9).Huang C, Chi XS, Hu X, et al. : Predictors and mechanisms of epilepsy occurrence in cerebral gliomas: What to look for in clinicopathology. Exp Mol Pathol 102: 115–122, 2017 [DOI] [PubMed] [Google Scholar]
- 10).Koekkoek JA, Kerkhof M, Dirven L, Heimans JJ, Reijneveld JC, Taphoorn MJ: Seizure outcome after radiotherapy and chemotherapy in low-grade glioma patients: a systematic review. Neuro-oncology 17: 924–934, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Takada S, Iwasaki M, Suzuki H, Nakasato N, Kumabe T, Tominaga T: Angiocentric glioma and surrounding cortical dysplasia manifesting as intractable frontal lobe epilepsy—case report. Neurol Med Chir (Tokyo) 51: 522–526, 2011 [DOI] [PubMed] [Google Scholar]
- 12).Mikuni N, Ikeda A, Takahashi JA, et al. : A step-by-step resection guided by electrocorticography for nonmalignant brain tumors associated with long-term intractable epilepsy. Epilepsy Behav 8: 560–564, 2006 [DOI] [PubMed] [Google Scholar]
- 13).Dobran M, Nasi D, Chiriatti S, et al. : Prognostic factors in glioblastoma: is there a role for epilepsy? Neurol Med Chir (Tokyo) 58: 110–115, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Englot DJ, Berger MS, Barbaro NM, Chang EF: Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg 115: 240–244, 2011 [DOI] [PubMed] [Google Scholar]
- 15).Rudà R, Magliola U, Bertero L, et al. : Seizure control following radiotherapy in patients with diffuse gliomas: a retrospective study. Neuro-oncology 15: 1739–1749, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Kahlenberg CA, Fadul CE, Roberts DW, et al. : Seizure prognosis of patients with low-grade tumors. Seizure 21: 540–545, 2012 [DOI] [PubMed] [Google Scholar]
- 17).Pace A, Vidiri A, Galiè E, et al. : Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol 14: 1722–1726, 2003 [DOI] [PubMed] [Google Scholar]
- 18).Sherman JH, Moldovan K, Yeoh HK, et al. : Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg 114: 1617–1621, 2011 [DOI] [PubMed] [Google Scholar]
- 19).Chen H, Judkins J, Thomas C, et al. : Mutant IDH1 and seizures in patients with glioma. Neurology 88: 1805–1813, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Neal A, Kwan P, O’Brien TJ, Buckland ME, Gonzales M, Morokoff A: IDH1 and IDH2 mutations in postoperative diffuse glioma-associated epilepsy. Epilepsy Behav 78: 30–36, 2018 [DOI] [PubMed] [Google Scholar]
- 21).Stockhammer F, Misch M, Helms HJ, et al. : IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure 21: 194–197, 2012 [DOI] [PubMed] [Google Scholar]
- 22).Snijders TJ, Berendsen S, Seute T, Robe PA: Glioma-associated epilepsy: toward mechanism-based treatment. Transl Cancer Res 6: S337–S341, 2017 [Google Scholar]
- 23).You G, Huang L, Yang P, et al. : Clinical and molecular genetic factors affecting postoperative seizure control of 183 Chinese adult patients with low-grade gliomas. Eur J Neurol 19: 298–306, 2012 [DOI] [PubMed] [Google Scholar]
- 24).Weller M, van den Bent M, Tonn JC, et al. : European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 18: e315–e329, 2017 [DOI] [PubMed] [Google Scholar]
- 25).Engel J, International League Against Epilepsy : A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 42: 796–803, 2001 [DOI] [PubMed] [Google Scholar]
- 26).Kwan P, Arzimanoglou A, Berg AT, et al. : Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51: 1069–1077, 2010 [DOI] [PubMed] [Google Scholar]
- 27).Miura Y, Keira Y, Ogino J, et al. : Detection of specific genetic abnormalities by fluorescence in situ hybridization in soft tissue tumors. Pathol Int 62: 16–27, 2012 [DOI] [PubMed] [Google Scholar]
- 28).Sugita S, Aoyama T, Kondo K, et al. : Diagnostic utility of NCOA2 fluorescence in situ hybridization and Stat6 immunohistochemistry staining for soft tissue angiofibroma and morphologically similar fibrovascular tumors. Hum Pathol 45: 1588–1596, 2014 [DOI] [PubMed] [Google Scholar]
- 29).Sugita S, Asanuma H, Hasegawa T: Diagnostic use of fluorescence in situ hybridization in expert review in a phase 2 study of trabectedin monotherapy in patients with advanced, translocation-related sarcoma. Diagn Pathol 11: 37, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Fisher RS, Cross JH, French JA, et al. : Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58: 522–530, 2017 [DOI] [PubMed] [Google Scholar]
- 31).Nunes VD, Sawyer L, Neilson J, Sarri G, Cross JH: Diagnosis and management of the epilepsies in adults and children: summary of updated NICE guidance. BMJ 344: e281, 2012 [DOI] [PubMed] [Google Scholar]
- 32).Case AJ, Domann FE: Absence of manganese superoxide dismutase delays p53-induced tumor formation. Redox Biol 2: 220–223, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Ogura R, Tsukamoto Y, Natsumeda M, et al. : Immunohistochemical profiles of IDH1, MGMT and P53: practical significance for prognostication of patients with diffuse gliomas. Neuropathology 35: 324–335, 2015 [DOI] [PubMed] [Google Scholar]
- 34).Nieder C, Petersen S, Petersen C, Thames HD: The challenge of p53 as prognostic and predictive factor in gliomas. Cancer Treat Rev 26: 67–73, 2000 [DOI] [PubMed] [Google Scholar]
- 35).Jin Y, Xiao W, Song T, Feng G, Dai Z: Expression and prognostic significance of p53 in glioma patients: a meta-analysis. Neurochem Res 41: 1723–1731, 2016 [DOI] [PubMed] [Google Scholar]
- 36).Engel T, Tanaka K, Jimenez-Mateos EM, Caballero-Caballero A, Prehn JH, Henshall DC: Loss of p53 results in protracted electrographic seizures and development of an aggravated epileptic phenotype following status epilepticus. Cell Death Dis 1: e79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Ding DX, Tian FF, Guo JL, et al. : Dynamic expression patterns of ATF3 and p53 in the hippocampus of a pentylenetetrazole-induced kindling model. Mol Med Rep 10: 645–651, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Engel T, Murphy BM, Schindler CK, Henshall DC: Elevated p53 and lower MDM2 expression in hippocampus from patients with intractable temporal lobe epilepsy. Epilepsy Res 77: 151–156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Engel T, Murphy BM, Hatazaki S, et al. : Reduced hippocampal damage and epileptic seizures after status epilepticus in mice lacking proapoptotic Puma. FASEB J 24: 853–861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Duan W, Chen Y, Wang XR: MicroRNA-155 contributes to the occurrence of epilepsy through the PI3K/Akt/mTOR signaling pathway. Int J Mol Med 42: 1577–1584, 2018 [DOI] [PubMed] [Google Scholar]
- 41).Jiao Y, Killela PJ, Reitman ZJ, et al. : Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3: 709–722, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Pallud J, Audureau E, Blonski M, et al. : Epileptic seizures in diffuse low-grade gliomas in adults. Brain 137: 449–462, 2014 [DOI] [PubMed] [Google Scholar]
- 43).Berger MS, Ghatan S, Haglund MM, Dobbins J, Ojemann GA: Low-grade gliomas associated with intractable epilepsy: seizure outcome utilizing electrocorticography during tumor resection. J Neurosurg 79: 62–69, 1993 [DOI] [PubMed] [Google Scholar]
- 44).Chaichana KL, Parker SL, Olivi A, Quiñones-Hinojosa A: Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg 111: 282–292, 2009 [DOI] [PubMed] [Google Scholar]
- 45).Khan RB, Boop FA, Onar A, Sanford RA: Seizures in children with low-grade tumors: outcome after tumor resection and risk factors for uncontrolled seizures. J Neurosurg 104: 377–382, 2006 [DOI] [PubMed] [Google Scholar]