Abstract
Observation has been a mainstay in asymptomatic meningiomas, but it may increase the risk associated with treatment due to tumor enlargement and the aging of patients. Understanding the natural course of meningiomas is important to provide appropriate treatment. The majority of previous studies investigated factors related to their growth, but failed to demonstrate their relationship with symptomatic progression (sympP) because of its rarity. We reviewed and meta-analyzed 27 studies that investigated natural courses in asymptomatic or untreated meningiomas to find clinico-radiological factors predictive of radiological progression (radioP), growth speed, and sympP. In results of time-growth analysis, two-thirds of meningiomas showed radioP defined by a volume criterion and the rate approached a plateau at 4–5 years. In growth curve analyses, about half of incidental meningiomas presented decelerating or no growth, while less than one-quarter of them grew exponentially. RadioP, growth speed [annual volume change (AVC) or relative growth rate], and sympP each had different factors related to them. Younger age, non-calcification, and high intensity on T2-weighted image were related to radioP and rapid growth speed, but not to sympP. Tumors in males and those of larger size were likely to be symptomatic in the meta-analysis. AVC (≥2.1 cm3/year) was the strongest indicator of sympP. Apart from perifocal edema, radiological features at up-front imaging may not be useful for predicting sympP. This may be due to dynamic changes of those radiological markers in the long term. Quantified tumor size and growth speed, especially AVC, are important markers for deciding on treatment.
Keywords: incidental meningioma, natural history, growth rate, growth curve, volumetry
Introduction
Meningiomas are the most frequently diagnosed primary brain tumor, especially in the elderly. Earlier autopsy studies showed an incidence of meningiomas from 1.4% to 2.3% in Sweden and the United States, respectively.1,2) These tumors are usually asymptomatic and smaller than 2 cm in diameter. It is conceivable that the widespread use of radiological examination has led to the more frequent detection of asymptomatic meningiomas in the era of extended life expectancy. A recent investigation using magnetic resonance imaging (MRI) showed a frequency of meningioma of 2.5% as an incidental finding in a population-based neuroimaging study in the middle-aged and older.3) However, only a small proportion of the tumors appeared to become symptomatic. In patients with asymptomatic meningioma, a long follow-up for years is advised and treatments are recommended if the tumor grows considerably. This approach may reduce unnecessary invasive treatments, but it may increase the risk associated with intervention due to enlarged tumors and deteriorating conditions of patients with age. Repeated radiological examinations may also place a burden on patients. Therefore, the accurate prediction of individual tumor growth with symptomatic progression (sympP) is crucial for determining the most appropriate form of clinical management, including active observation and/or surgical resection, to avoid unnecessary radiological follow-up or risky intervention.
Many authors have investigated the growth of asymptomatic meningiomas to select patients suitable for treatment. Clinical parameters such as age, sex, and size of the tumors may be related to their radiological progression (radioP). Radiological findings such as the existence of calcification on a CT scan or signal intensity on T2-weighted imaging (T2WI) may also correlate with the growth rate. However, there are three problems in obtaining beneficial results from them. First, the different methodologies to describe the growth of tumors in each study make integrating the results difficult. Some researchers discriminated growing tumors among incidentally found meningiomas using various radioP criteria: volume criteria of an increase of anything up to 30%4–13) or diameter-based criteria.3,14–22) Because these criteria in radioP lack a timescale, other research groups attempted to overcome this problem by performing analysis based on tumor growth speed.6,7,13,23–27) Growth speed was defined in various ways, such as annual volume change (AVC), tumor doubling time (Td), relative growth rate (RGR), and annual diameter change (ADC). These growth indices have different meanings in the natural growth of tumors, but their differences have not been properly interpreted.
Second, clinico-radiological factors related to radioP criteria or various growth speed indices have not been demonstrated substantially to relate to sympP. It is unlikely that benign meningiomas maintain a constant rate of growth for a long period. Indeed, recent studies have revealed self-limiting growth in the majority of incidental meningiomas.4,26,28) Therefore, factors predictive of sympP may not be concordant with those of radioP.
Third, although the prediction of sympP is more important than the prediction of radioP, all previous studies presented only a small number of patients with sympP. It is thus necessary to integrate data from previous studies to draw definitive conclusions about predicting sympP to ensure reliable guides for the clinical management of incidental meningiomas.
We performed a review and meta-analysis to evaluate whether clinico-radiological factors related to various growth criteria and growth speed metrics can be integrated to predict the growth of meningiomas. For this purpose, we recalculated the growth speed and adjusted growth criteria from the data in the studies, whenever possible. We also evaluated whether factors associated with radioP may correspond to sympP.
Materials and Methods
Article selection
We searched for relevant articles using the keywords “meningioma,” combined with “human” and “incidental,” “asymptomatic,” “natural history,” or “untreated,” published from 1980 to December 2018 in PubMed. We excluded non-English literature and cases of tumors in the spine or in children. After discarding duplicates, 344 articles remained. We read their abstracts and excluded case reports and reports of small series containing fewer than 10 cases (53), extracranial meningiomas (17), non-meningiomas (57), treatment-related papers (83), radiological studies (37), those with descriptions of the pathology alone (13), and those on radiation-induced meningiomas (26). Among the remaining 58 papers, we selected those papers that had clinical data of untreated meningiomas with radiological examinations performed on at least two time-points, but excluded neurofibromatosis case series. Review articles were also excluded. Two studies that included only surgically treated patients were excluded. Three studies that analyzed only skull base meningiomas were also excluded. We searched articles on “meningioma” published after December 2018 until April 2019 to find recent ones relevant to this study. Twenty-seven papers3–10,12–30) were selected for data extraction. Among articles that included overlapping data previously reported from the same institute,8–10,14,16,22–24,30) we selected the papers that contained newer data or necessary data.
Quality of articles
Most articles presented are on retrospective observational studies. The rates of follow-up of incidental meningiomas varied because of different rates of early treatment without observation or loss to follow-up. In most of the studies, these rates were about 30–80% of the included cases. In one prospective population-based cohort study,3) the follow-up rate was 87.4%, although individual data were not available. In another prospective study, this rate was 95.3%.4)
Data extraction
From the selected papers, seven studies included individual data about age, sex, initial and last tumor volumes (or diameters), follow-up interval, and radiological findings.5,6,12,21,22,25,29) We were able to obtain individual data from two other series (3128) and 104 tumors26) of asymptomatic meningiomas). From another study27) data were obtained from a growth curve graph using the software WebPlotDigitizer.31) The error of the obtained data was less than 0.2% in terms of the mean initial volume and the mean follow-up interval described in the article. The data were used in analysis of a forest plot and radioP. We extracted data for forest plots in 18 studies,5,6,10,12,13,15–18,20–22,25–30) and the data on the mean follow-up period until radioP and sympP in 19 studies.3–5,7,9,12–14,17–20,22,25–30) When standard deviation (SD) was not available, it was estimated from the data on the range. Seven studies included symptomatic cases (such as those with seizures and mild neurological deficits) at a rate of more than 10% of the total cases.13,14,19,23,24,29,30) We included such studies for analyses of radioP but not for analyses of sympP.
Methods for measuring tumor growth
Several methods were used to define a growing tumor or to describe the growth speed of meningiomas. To obtain comprehensive results, we attempted to standardize the method. RadioP of a tumor was redefined as tumor growth of 15% of the initial volume or more from individual data whenever possible.15,25,26,28,29) To adjust for the difference of follow-up interval in each study, the association between the percentage of radioP and the mean follow-up period was analyzed (Fig. 1A).
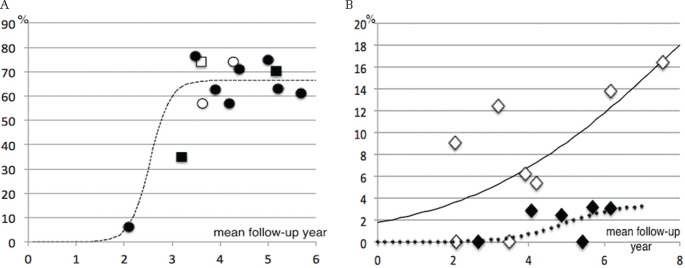
Fig. 1.
Percentage of growing tumors (A) and symptomatic progression (B) against mean follow-up in each study. (A) Fitting curve for percentage of growing tumors based on 15% volume criteria (circle) in each study depending on the mean follow-up period (R2 = 0.87). For reference, square indicates other volume criteria. Open circle or open square indicates a study containing symptomatic cases at a rate of more than 10%. (B) Studies with larger tumor series showed a higher clinical progression rate. Open circle, the mean tumor diameter was 2.19 cm or larger; solid circle, the mean diameter was less than 2.19 cm.
We calculated Td, RGR, and AVC from individual data when possible (for formulae, see the appendix). We used RGR and AVC for statistical analyses because Td can be converted to RGR (see Appendix). For individual case analyses, we converted maximum diameter to volume or vice versa using the following formula (1):
| (1) |
This equation was derived from analysis of 104 meningiomas26) (R2 = 0.93, P <0.0001).
Statistical methods
We used the statistical software EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan)32) for statistical analyses. In meta-analysis, effect size was measured using odds ratio or mean difference in forest plots to find the relationships among radioP, sympP, clinical factors (age, sex, initial size), and radiological findings (calcification, peritumoral edema, T2WI signal intensity). The analyses of radioP were performed separately in the diameter and volume criterion groups. A fixed-effect model was adopted except for the cases of high heterogeneity (I2 > 40%), in which a random-effect model was instead used. Publication bias could not be analyzed because of the small number of studies in the subgroups based on volume and diameter criteria (<10).
For individual data analyses, univariate analysis was conducted using Fisher’s exact test for categorical variables and the Mann–Whitney U-test or Kruskal–Wallis test for continuous variables. The Steel–Dwass or Bonferroni test was used as a post hoc test. Possible candidate predictors that correlated with tumor growth were factored into the multivariable regression analysis by a stepwise method. P-values <0.05 were considered statistically significant.
To define logistic regression curves between the mean follow-up period and radioP rates, non-linear regression analysis (nls function in R) was used to obtain the smallest residual sum of squares. Receiver operating characteristic (ROC) curves were analyzed to predict the clinical progression.
Results
Radiological progression
Radiological progression was analyzed based on the volume- and diameter-based growth criteria separately. We compared the percentages of tumor growth beyond the volume or diameter criteria in each study with their mean follow-up intervals. In individual volumetric studies (10 groups from nine studies, 502 cases), the relationship of percentage of radioP in the 15% criteria and the mean follow-up interval fitted a logistic curve (Fig. 1A) (R2 = 0.87). The fitting curve approached an asymptotic value of 66.7% after the mean follow-up interval of 4–5 years. The studies including symptomatic cases or those conducted with other growth volume criteria did not appear to influence the curve. In diameter-based studies (diameter increase >1–3 mm, nine studies, n = 695), the correlation was not as good as in volumetric studies (R2 = 0.48, asymptotic value = 45.8%, graph not shown).
Factors related to radiological progression
We analyzed clinical and radiological factors related to radioP by the different criteria based on diameter and volume separately (Table 1). Forest plots showed that young age and possibly maleness positively affected tumor growth. Radiologically, a high-intensity signal on T2WI was also related to tumor growth, whereas calcification and a low intensity on T2WI were related to no growth. Initial tumor size and perifocal edema did not show significant relationships.
Table 1.
Results of forest plot analyses about radiological progression
| Factors | MD or OR (95% confidence interval) | Heterogeneity | References | |
|---|---|---|---|---|
| Age (n = 680): mean age, P <0.0001 | All | −4.67 (−6.21 to −3.12) | I2 = 0%, P = 0.44 | |
| Volume | −3.96 (−6.37 to −1.54) | I2 = 29%, P = 0.20 | 5, 6, 10, 25, 26, 28–30 | |
| Diameter | −5.16 (−7.18 to −3.15) | I2 = 0%, P = 0.82 | 13, 18, 20, 21 | |
| Sex (n = 835 ): women vs. men, P = 0.09 | All | 0.75 (0.52–1.07) | I2 = 20%, P = 0.24 | |
| Volume | 0.49 (0.26–0.91) | I2 = 0%, P = 0.47 | 12, 13, 15, 17, 20, 21 | |
| Diameter | 0.94 (0.60–1.48) | I2 = 30%, P = 0.21 | 7, 13, 28, 30, 33, 34 | |
| Initial size vol. n = 400, P = 0.35 Diam. n = 409, P = 0.18 |
All | NA | ||
| Volume | −1.03 (−3.22 to 1.16)* | I2 = 47%, P = 0.08 | 5, 6, 10, 25–29 | |
| Diameter | 2.46 (−1.10 to 6.02)* | I2 = 55%, P = 0.08 | 12, 13, 16, 18 | |
| Calcification (n = 774), P <0.0001 | All | 0.35 (0.25–0.50) | I2 = 0%, P = 0.70 | |
| Volume | 0.30 (0.16–0.54) | I2 = 0%, P = 0.76 | 6, 25, 26, 28, 30 | |
| Diameter | 0.39 (0.25–0.58) | I2 = 0%, P = 0.43 | 7, 13, 28, 30, 33, 37 | |
| Edema (n = 623 ): (+) vs. (−), P = 0.06 | All | 1.31 (0.50–3.42)* | I2 = 51%, P = 0.06 | |
| Volume | 0.83 (0.34–2.04) | I2 = 39%, P = 0.2 | 6, 26, 28, 29 | |
| Diameter | 1.85 (0.53–6.42)* | I2 = 50%, P = 0.11 | 12, 13, 15, 22 | |
| T2WI high (n = 566 ), P <0.0001 | All | 2.29 (1.54–3.40) | I2 = 0%, P = 0.57 | |
| Volume | 2.02 (1.07–3.79) | I2 = 0%, P = 0.41 | 6, 25, 26, 28 | |
| Diameter | 2.49 (1.50–4.15) | I2 = 0%, P = 0.49 | 12, 13, 22 | |
| T2WI low (n = 204 ), P <0.0001 | All | 0.22 (0.11–0.45) | I2 = 14%, P = 0.32 | |
| Volume | 0.22 (0.11–0.45) | I2 = 14%, P = 0.32 | 6, 12, 25, 26, 28 | |
| Diameter | NA | |||
Random effect model. Bold means statistically signify results. MD: mean difference, OD: odds ratio, NA: not available.
Tumor growth speed
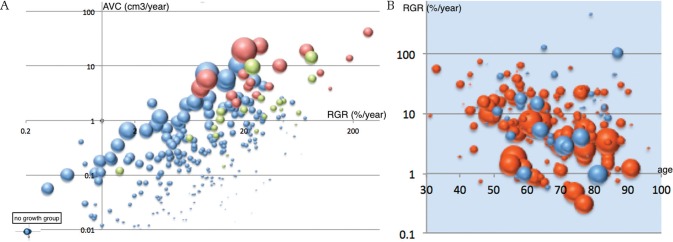
Figure 2A shows the relationship between AVC and RGR depending on initial tumor size from individual data (n = 298).5,6,12,22,25,26,28) The graph shows the apparent effect of initial tumor size on this relationship: smaller tumors tended to have a lower AVC/RGR ratio than larger ones. This is because RGR is an index of the proliferative potential of tumor cells, while AVC reflects both proliferative potential and tumor size.
Fig. 2.
(A) Relationship between relative growth rate (RGR) and annual volume change (AVC) based on initial tumor volume in a logarithmic scale.5,6,12,22,25,26,28) Size of markers indicating initial tumor volume. Brown, cases with symptomatic progression; light green, cases with early treatment after radiological progression; blue, asymptomatic tumors (n = 225). Four meningiomas showing no growth (inset). The graph lacks individual data of 94 non-growing tumors and 15 asymptomatic growing ones from three studies.6,12,22) Those non-growing tumors usually have AVC <0.18 and RGR <3.64 (75% range calculated from other series5,25,26,28)). (B) Relationship between relative growth rate and patient age in four studies.5,25,26,28) Size of markers indicating initial tumor volume. Red, tumors in females; blue, tumors in males. RGR in female tumors showed a negative correlation with age (R = −0.30, P <0.001), while RGR in male tumors did not.
From the individual data, factors related to RGR and AVC were separately evaluated (Table 2). Univariate analyses showed that younger age (≤60) (P = 0.0001), smaller initial volume (≤4 cm3) (P = 0.012), absence of calcification (P <0.00001), and high-intensity signal on T2WI (P <0.0001) were related to high RGR, but neither sex (P = 0.12) nor edema was (P = 0.20).
Table 2.
Factors related to growth speed in individual data analyses
| Relative growth rate (RGR) | Annual volume change (AVC) | |||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Age > 60 (n = 251) | Young > Old | P = 0.95 | Young > Old | P = 0.29 |
| P = 0.0001 | P = 0.006 | |||
| Sex (n = 220) | P = 0.12 | P = 0.61 | ||
| Initial size >4 cm3 or not (n = 229) | Large < Small | P = 0.08 | Large > Small | P = 0.28 |
| P = 0.012 | P <0.00001 | P <0.001* | ||
| Calcification (n = 180) | P <0.00001 | P <0.01 | P <0.0001 | P = 0.16 |
| P = 0.018* | ||||
| T2WI (n = 138) | P <0.00001 | P = 0.12 | P <0.002 | P = 0.02 |
| Low vs. iso, P = 0.006 | Low vs. iso, P = 0.03 | P = 0.014* | ||
| Low vs. high, P = 0.0001 | Low vs. high, P = 0.0002 | |||
| Iso vs. high, P = 0.32 | Iso vs. high, P = 0.09 | |||
| Edema (n = 159) | P = 0.20 | P = 0.000002 | P <0.000001 | |
Results when edema was excluded from multivariate analyses. Bold means statistically signify results.
Despite the lack of a difference of RGR between the sexes, RGR of female tumors showed a negative correlation with age (R = −0.30, P <0.001) (Fig. 2B), while RGR of male ones did not. Multivariate analysis showed only lack of calcification as being related to high RGR (P <0.01). On the other hand, younger age (P = 0.006), larger initial volume (>4 cm3, P <0.00001), lack of calcification (P <0.0001), high-intensity signal on T2WI (P <0.002), and perifocal edema (P = 0.000002) were related to high AVC, but sex was not (P = 0.61). Multivariate analysis showed that edema (P <0.00001) and T2 high-intensity signal (P = 0.02) showed significant relationships with high AVC. When we omitted perifocal edema from the multivariate analysis because only a few asymptomatic meningiomas presented edema (n = 15), larger initial volume (P <0.001), calcification (P = 0.018), and T2 signal intensity (P = 0.014) were found to be related to AVC.
Tumor growth curve patterns
Five studies investigated tumor growth curve patterns from serial volumetric analyses.4,7,19,26,28) (Table 3). Hashimoto et al.7) divided growth patterns of meningiomas (n = 113) to four groups as exponential (23.9%), linear (31.9%), no trend (7.1%) and no growth (37.2%). Two studies discriminated self-limiting growth approaching plateau (decelerating growth). Nakasu et al.26) (n = 61) and Behbahani et al.4) (n = 64) showed exponential growth in 14.8% and 25.9%, linear in 37.7% (including a part of decelerating cases) and 16.7%, and decelerating in 47.5% and 35.2% of their cases, respectively, while the latter further discriminated parabolic (16.7%) and continuous reduction (5.6%). Lee et al.23) presented the largest series (n = 232), and they divided meningiomas into slow (n = 173) and rapid-growth (n = 59) group. The latter showed exponential (22%), linear (67.8%) or decelerating growth (10.2%), while majority of the former exhibited linear, decelerating or no growth judged from their Fig. 1. Incorporating these results into one, we found that about a half of incidental meningiomas presented the decelerating (self-limiting) growth pattern or no growth while less than one-forth of them did exponential pattern. Few meningiomas were reported to decrease their volumes.4,33) Growth pattern might be modified by the length of follow-up time. In fact, the mean follow-up period for tumors displaying decelerating growth was longer than for tumors with linear or exponential growth.4) The study of the longest follow-up (mean 7.7 years) showed that growth of benign meningiomas simulated a Gomperzian curve with quasi-exponential growth followed by decelerating growth approaching plateau.28)
Table 3.
Growth curve patterns of meningiomas
| n | Mean follow period (years) | Expo. | Linear | No trend | No growth | Gompertzian growth | |
|---|---|---|---|---|---|---|---|
| Nakasu et al.28) | 52 | 7.5 | 8 (15.4) | 44 (84.6) | |||
| Hashimoto et al.7) | 113 | 3.9 | 27 (23.9) | 36 (31.9) | 8 (7.1) | 42 (37.2) | NA |
| n | Mean follow period (years) | Expo. | Linear | Self-limiting | No growth | |||
|---|---|---|---|---|---|---|---|---|
| Lee et al.23) | Rapid-growth group | 59 | 2.9 | 13 (22.0) | 40 (67.8) | 6 (10.2) | ||
| Slow-growth group | 173 | 4.0 | Almost absent | Most of the cases | ||||
| Nakasu et al.26) | 61 | 4.4 | 9 (14.8) | 23 (37.7) | 29* (47.5) | |||
| Behbahani et al.4) | 54 | 0.5–5 | 14 (25.9) | 9 (16.7) | 19 (35.2) | 12** (22.3) | ||
Including no growth.
Including parabolic and decrease growth: percentage in the parentheses. Expo.: exponential growth.
Symptomatic progression
The rate of sympP was evaluated in the same manner as for radioP (Fig. 1B). Seizures were included but non-specific symptoms were excluded from the analyses. Figure 1B shows that there was an apparent difference in sympP rates between the studies with a larger [diameter >2.2 cm (about 4 cm3 in volume)] mean tumor size and those with a smaller one. In the larger mean size group, the incidence of sympP reached up to 20%, but in the smaller size group, it was below 10%. Owing to the small number of subjects and data variation, significant logistic curves could not be produced. Overall, 46 of 989 patients (4.7%) developed neurological symptoms during the mean follow-up of 53 months, while 72 patients underwent treatment before symptom development.
Factors related to symptomatic progression
In weighted meta-analyses, forest plots showed that tumors in males and large initial maximum diameter (or initial volume) were significantly related to sympP (Table 4). We could not analyze radiological factors in forest plots because few studies assessed their relationships and each study included only a small number of patients with symptom development. Although Islim et al.34) presented radiological factors in their forest plots (online resource 6), raw data were not provided in the original papers.12,22,27)
Table 4.
Factors that affect symptomatic progression. Results of meta-analyses and individual data analyses
| Factors | Fixed effect model | Heterogeneity | References | Individual data analyses | |
|---|---|---|---|---|---|
| MD or OR (95% confidence interval) | SympP vs. asymptomatic | ||||
| Age | 4.17* (−1.79 to 10.12), P = 0.29 | I2 = 51%, P = 0.10 | 12, 15, 21, 22, 26, 27 (n = 331) | Med, 73 y.o. vs. Med, 69 y.o. | n = 268, P = 0.091 |
| Sex | 10.08 (3.02–33.65), P = 0.0002 | I2 = 0%, P = 0.96 | 5, 6, 12, 15, 21, 26 (n = 257) | M 8 W 2 vs. M 47 W 211 | n = 268, P <0.0001 |
| Initial size | Diam. 12.44 (8.21–16.68), P <0.0001 | I2 = 28%, P = 0.25 | 12, 15, 21, 22, 26 (n = 288) | Vol. 9.96 cm3 vs. 2.55 cm3 | n = 229, vol. P = 0.0032, diam. P = 0.0035 |
| Diam. 2.67 cm vs. 1.86 cm | |||||
| Calcification | N.A. | (+) 1 (−) 3 vs. (+) 83 (−) 71 | n = 158, P = 0.34 | ||
| T2WI | N.A. | high, 2; iso, 1; low, 0; high, 46; iso, 63; low, 35 | n = 147, P = 0.61 | ||
| Edema | N.A. | (+) 2 (−) 2 vs. (+) 7 (−) 118 | n = 129, P = 0.024 | ||
Random effect model. Bold means statistically signify results. MD: mean difference, OR: odds ratio, SympP: symptomatic progression, y.o.: year-old, M: men, N.A.: not available, W: women, Diam.: diameter, Vol.: volume.
Analyses of individual data showed that maleness (P <0.0001) and initial volume (median 2.55 vs. 9.96 cm3; P = 0.0032) or initial diameter (median 1.86 vs. 2.67 cm; P = 0.0035), but not age (P = 0.091), were related to sympP (Table 4). Perifocal edema showed a significant relationship to sympP (P = 0.024), but neither calcification (P = 0.34) nor intensity on T2WI (P = 0.61) did.
In the individual data, higher growth speed (AVC and RGR) was related to sympP even in relatively small tumors (Fig. 2A). ROC analyses showed that both AVC 2.1 cm3/year and RGR 15.4% were good indicators of sympP [AVC: area under the curve (AUC) 0.972, specificity 0.903, sensitivity 1.0; RGR: AUC 0.835, specificity 0.718, sensitivity 0.895]. Initial tumor size (maximum diameter 2.6 cm: AUC 0.773, specificity 0.759, sensitivity 0.800; or volume 5.6 cm3: AUC 0.775, specificity 0.717, sensitivity 0.800) may be reliable markers for sympP.
Kaplan–Meier curves showed a clear difference of time to sympP when tumors were categorized into rapid-growth (AVC ≥2.1 cm3/year) and slow-growth groups (AVC <2.1 cm3/year) (Fig. 3). The rapid-growth group had symptom-free rates of 69.3% (95% CI 44.6–84.6%) at 3 years and 55.4% (95% CI 23.8–78.4%) at 6 years.
Fig. 3.
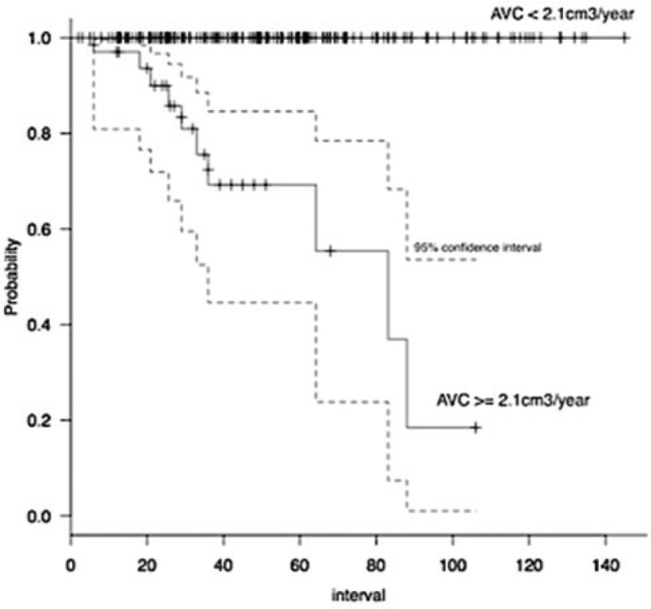
Kaplan–Meier analysis of symptomatic progression against annual volume change (AVC). No tumors with AVC <2.1 cm3/year became symptomatic, while tumors with AVC ≥2.1 cm3/year showed a 3-year clinical progression-free rate of 69.3% (P <0.000001).
Pathological diagnosis in the studies
Pathological diagnoses were obtained in 0–17.9% (mean 8.6%; SD 4.5%) of investigated series.3,5,6,9,12,15,17–23,25,26) Most of them were benign, whereas 11 out of 245 meningiomas had atypical or anaplastic features.5,6,9,15,21,25–27,35,36)
Discussion
This review revealed that two-thirds of incidental meningiomas exhibited radioP based on the criterion, increasing by 15% of their initial volume, which could be within the range of measurement error.6) We demonstrated that meningiomas with a high-intensity signal in T2WI without calcification in young males tended to exhibit radioP. However, only 4.7% of the patients became symptomatic, although the exact rate was difficult to determine because of early treatments being performed for asymptomatic growing tumors in the majority of the studies. Interestingly, significant predictive factors of sympP differed from those of radioP. Furthermore, significant factors of radioP were revealed to differ in terms of AVC and AGR. In previous studies on incidental meningiomas, insufficient attention was paid to those differences in prediction targets or assessment tools.
Radiological progression
In pioneering studies,5,14,15,18) asymptomatic meningiomas were considered to grow very slowly. Thereafter, individual researchers measured radioP using different definitions, which led to confusing interpretations. Moreover, the methods using maximum diameter or volume calculated from three perpendicular axes are insufficiently sensitive to detect subtle changes.29,37) Studies using volumetry based on image analysis software and a longer follow-up period showed higher growth rates of up to 70%.4,9,13) Our review showed that, in volumetric studies, the curve of percentage of growing tumor against mean follow-up time fitted well to a logistic curve that approached an asymptotic volume of 66.7% (Fig. 1A). This value was much higher than that of studies using diameter change, but comparable to 75% for a 15-year growth rate using life-table statistics in a study by Jadid et al.17) In the early phase of this curve, tumors grew slowly. After 2 years, the number of growing tumors increased, but it almost reached a plateau after 4–5 years. This curve pattern is concordant with the recently proposed concept that the growth of benign meningiomas is self-limiting.4,26,28) A tumor is reasonably considered to be indolent if it shows no growth for 5 years.
Zeng et al.38) showed a significant relationship between radiological tumor growth and lack of calcification and T2 signal intensity, but not sex, location, or edema. We obtained similar results; moreover, male sex tended to be related to radioP based on volumetric criteria (Table 1). However, these factors are merely indices for the identification of tumors with radioP but not with sympP. They would be useful for identifying tumors that will not grow and for decision-making about the duration of radiological follow-up.
Tumor growth speed
Tumor growth speed has been described by ADC, AVC, Td, and RGR. When a tumor maintains a constant growth rate, Td and RGR are indices of exponential growth, while AVC is that of linear growth, and ADC is that of power growth (third power). AVC is volume-dependent when RGR is constant, as shown in Fig. 2A, actually, from Equations (3) and (5) in Appendix,
Therefore, it is no wonder that almost all previous studies showed that larger tumors had higher AVC. It is no wonder that there are differences in clinical and radiological factors related to tumor growth speed between RGR and AVC. Actually, RGR was higher in smaller meningiomas, but AVC was opposite (Table 2) as reported previously.4,25,26) Previous studies often neglected the differences between RGR and AVC. Furthermore, the concept of the self-limiting growth of meningiomas was not incorporated into the majority of previous studies.
These two indices show no concordant changes during tumor growth. When a tumor maintains exponential growth, RGR is constant but AVC becomes larger (upward shift in Fig. 2A). In linearly growing tumors, RGR decreases but AVC remains constant (parallel shift in Fig. 2A). If a tumor shows self-limiting growth, both RGR and AVC decrease gradually, and the volume reaches a plateau. Therefore, smaller tumors in initial growth phase of meningioma have higher RGR’s but lower AVC’s than larger ones in their later growth phase except for in the last plateau phase.
In a review study, Sughrue et al.39) described that 51% of meningiomas ≤2.5 cm demonstrated no evidence of growth over a median follow-up of 4.6 years. In our review, however, tumor volume did not affect radioP (Table 1), while it had a conflicting relationship with tumor growth speed in the forms of RGR and AVC.
Univariate analyses showed that smaller tumors in younger patients with radiological findings of an absence of calcification and T2 high-intensity signal had high RGR. Calcification was the only factor related to low RGR in multivariate analysis. On the other hand, perifocal edema, initial large size, absence of calcification, and T2 high-intensity signal were related to AVC in univariate analyses. Multivariate analyses showed that edema was the factor most strongly associated with high AVC. Edema is known to be related to tumor size40) and possibly to tumor proliferation.41) As perifocal edema is rare in incidental meningiomas, we investigated factors other than edema in multivariate analysis. This showed that large initial size, lack of calcification, and high-intensity signal on T2WI were related to AVC. These results are similar but not concordant to the factors related to radioP. These results should be useful for decision-making for setting intervals in radiological follow-up.
Clinically, maximum tumor diameter is a readily available index for measuring tumor size. However, it is associated with two problems. The first is that tumors do not always extend in the direction of their maximum diameter. The second is that ADC value is also affected by tumor size. Therefore, a large growing tumor can be misinterpreted as a non-growing one upon radiological examination. On the other hand, when tumors have the same RGR, larger tumors have a larger ADC. Figure 4 shows the relationship between maximum tumor diameter and ADC when the volume increases by 2.1 cm3/year, calculated using Equation 1. As shown in this figure, a tumor of 1 cm in diameter needs to gain nearly 1 cm in diameter to grow 2.1 cm3/year in volume, while a large tumor of 5 cm needs to gain only 1 mm to achieve the same increase in volume.
Fig. 4.
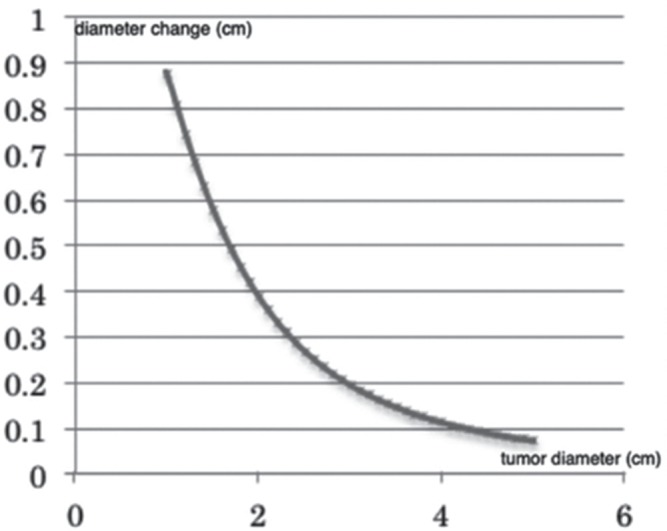
Relationship between annual diameter change and maximum tumor diameter when annual volume change is set at 2.1 cm3. Diameter changes to a small degree when a tumor is large.
Symptomatic progression
The rate of sympP in asymptomatic meningiomas was difficult to define from previous studies. In their review, Islim et al.34) described that 66 out of 608 patients with an incidental meningioma developed symptoms during the mean follow-up of about 40 months; this incidence is higher than our result (4.7%; 46 of 989 patients, mean follow-up of 53 months). This difference is considered to be due to the different inclusion criteria. Their review included two series consisting of only surgically treated asymptomatic meningiomas.35,36) We excluded them because of their high radioP and sympP rates. In a large retrospective study, Kim et al.9) reported a high sympP rate (40 of 201 patients, median follow-up of 62 months). However, they included patients who developed non-specific symptoms such as headache, dizziness, and scalp paresthesia. Neurological deficits were observed in only five patients. Considering the relatively small median initial volume (1.0 cm3) of their cases, we chose “five” for analyses.
The rate (4.7%) of sympP in this review is supposed to be lower than the true incidence because of strategic early treatments for tumors showing any growth before sympP in the majority of studies. For instance, a prospective study showed no patients with sympP, but 32 out of 64 patients underwent treatment at the time of radioP.4) Tumors in patients that underwent early treatments in representative studies are shown as light green balloons (n = 22) in Fig. 2A. About 64% of these tumors had comparable RGR (>15.4%), smaller AVC, and smaller initial size than the tumors that progressed symptomatically (in brown, n = 20). This means that, if treatment had been withheld for those tumors for several years, the majority of them would have enlarged to become symptomatic. We suspected that nearly 10% of all asymptomatic meningiomas might have grown to become symptomatic if no patients had undergone early treatment in Fig. 2A.
In one autopsy study (during 1950–1982),1) the ratio of symptomatic to asymptomatic meningiomas was 1:6. Another study showed 172 meningiomas found in 11,973 autopsies (during 1957–1966), while 29 meningiomas were surgically treated in the same period.2) Therefore, this ratio was supposed to have been about 1:6 before the era of the expanding use of CT scans.
In this review, tumors in males were likely to grow symptomatically. However, no sex difference was detected in growth speed. This might be explained by the result that growth speed of the tumors in females was more likely to decelerate with age (Fig. 2B). Kim et al.9) reported that young age, absence of calcification, perifocal edema, and high-intensity signal on T2WI were related to clinical progression. However, as described above, their definition of clinical progression included radiological progression and unspecific symptomatic progression.
Larger tumors were likely to become symptomatic, in both meta-analysis and individual data analysis. In other reviews, the importance of the initial size of a tumor for symptomatic progression was also emphasized. Sughrue et al.39) concluded that most meningiomas ≤2.5 cm do not cause symptoms in the following 5 years, while our study showed that a tumor diameter >2.6 cm was one of the risk factors for sympP.
In this review, radiological factors did not reach significance as for sympP, except for edema, although they were related to tumor growth. This may be due to the fact that radiological features such as calcification or T2 intensity can change during the course of tumor growth, as well as growth speed while tumor size was much more important for sympP. Actually, tumors were able to become symptomatic without significant growth when enough large.19,36)
Annual volume change ≥2.1 cm3/year was the most important indicator for sympP. AVC is more useful for the clinical assessment of sympP than RGR because AVC is a function of tumor size, which is a crucial factor for symptomatic growth as well as tumor growth speed. This value is similar to that in the study by Lee et al.,23) who defined the rapid-growth group as those with AVC ≥2.0 cm3 and asserted that membership of this group was predictive of symptom aggravation. We excluded their study from this analysis because it included symptomatic cases at a rate of more than 10%. Therefore, these results of AVC as an indicator of sympP were based on the two different populations.
Limitations of the study
The location of meningioma could also be an important factor of sympP. Tumors close to the cranial nerves would become symptomatic even in smaller size than convexity tumors. However, this was not analyzed here because of the small number of events in the reviewed studies.
In the majority of cases included in this study, the diagnosis of meningioma was made solely based on imaging features. Although most of the pathological diagnoses at surgery were benign meningioma, a few of them included atypical or anaplastic ones. On the other hand, in a prospective study by Behabahani et al.,4) two non-meningioma cases were excluded from 70 enrolled cases. Jadid et al.17) and Kim et al.9) also reported radiological misdiagnosis: three in 88 presumed meningioma cases and four in 327 cases, respectively. As only some of the tumors described in these three articles were surgically treated, a larger number of misdiagnosed tumors could possibly have been included. Therefore, we have to keep in mind that radiologically presumed meningioma includes other diseases as well as aggressive forms of meningioma.
Clinical implications
We showed in this study that about 30% of incidental meningiomas did not grow. Those can be discriminated during the first 4–5 years and further follow-up would not be necessary in such cases. Factors related to radioP may be useful for decision-making on the duration of radiological follow-up.
Although tumor with calcification is a good marker of no or slow growth of meningiomas, it may not be useful for predicting non-sympP, especially when tumors are already large. In this context, it is necessary to be aware that radiological findings can change over time.26) For example, non-calcified tumor can become calcified in the long term and T2 signal intensity may change accordingly. Moreover, perifocal edema may arise in association with tumor growth. Therefore, common upfront radiological features for growth are not always valuable in the long term. Because growing tumors may decelerate their growth to reach plateau before being symptomatic, these radiological features should be assessed in combination with changes in tumor volume. Actually, the progression of calcification may be a marker of growth deceleration.26)
We demonstrated that AVC ≥2.1 cm3/year is an important index for sympP. However, it takes time to determine AVC for individual patients, with at least two radiological assessments for 1–2 years. Tumor size may be useful if combined with other radiological markers, as reported by Lee et al.23)
On the other hand, even small tumors have a chance of being symptomatic in cases of high RGR. If tumors continue to grow exponentially, small tumors become larger and their AVCs exceed 2.1 cm3/year. However, it is difficult to predict whether tumors will continue to grow at the same speed. Such tumors should be watched carefully via regular radiological examinations.
Other important factors for treatment selection are patients’ age and general condition. In elderly patients, the risk of surgical intervention might increase annually. Our study showed that tumor size [initial maximum diameter 2.6 cm (AUC 0.773, specificity 0.759, sensitivity 0.800)] is a risk factor for sympP. When a tumor has already grown beyond a suitable size for radiosurgery, a growing tumor (AVC ≥2.1 cm3/year) should be treated surgically before sympP considering the general risk.
Although prospective investigation of the growth of asymptomatic meningiomas has been proposed, it is difficult to follow many patients for longer than 5 years. Moreover, given the data accumulated in many retrospective studies, only a few new findings about tumor growth kinetics would be obtained from a prospective study. However, the most important issues remain unresolved: who becomes symptomatic, how we can predict them, and whether early intervention benefits the patients. To resolve these issues, we need to randomize patients to either an intervention or an observation arm when their tumors grow in a prospective study.
Conclusion
Despite increased detection of asymptomatic meningiomas, a lack of prospective studies had led to inconclusive results about determination of the timing of treatment. However, reports from long-term observational studies have accumulated and supplied important data. In this study, we showed that about 30% of incidental meningiomas did not grow radiologically. Factors predictive of no growth are tumor calcification, T2 low-intensity signal, and being elderly and female. Growth speed analysis is important to analyze complex growth patterns influenced by various factors. RGR and AVC represent different characteristics of tumor growth, and would not be constant Calcification is related to RGR, whereas initial size and edema are related to AVC.
The prediction of neurological sympP is more important than that of radioP. Only a small proportion of meningiomas would become symptomatic, but this has been underestimated because of early treatment indicated at the time of radioP in the majority of studies. We showed that a tumor diameter >2.6 cm is a risk factor for sympP. We also revealed that AVC (≥2.1 cm3/year) is a strong indicator of sympP, reflecting crucial factors of intracranial tumors, namely, both tumor volume and proliferative potential.
Acknowledgment
We are grateful to Dr. Akifumi Notsu (Clinical Research Center, Shizuoka Cancer Center) for his statistical consultation.
Appendix
Formulas used in this review:
| (2) |
| (3) |
| (4) |
| (5) |
From Equations (4) and (5), we get
V0, volume at time 0; Vt, volume at time t; D0, diameter at time 0; Dt, diameter at time t.
Footnotes
Ethical Approval and Informed Consent
This review did not involve direct studies on humans, so no informed consent was required.
Conflicts of Interest Disclosure
The authors declare that they have no conflicts of interest.
References
- 1).Nakasu S, Hirano A, Shimura T, Llena JF: Incidental meningiomas in autopsy study. Surg Neurol 27: 319–322, 1987 [DOI] [PubMed] [Google Scholar]
- 2).Rausing A, Ybo W, Stenflo J: Intracranial meningioma—a population study of ten years. Acta Neurol Scand 46: 102–110, 1970 [DOI] [PubMed] [Google Scholar]
- 3).Bos D, Poels MM, Adams HH, et al. : Prevalence, clinical management, and natural course of incidental findings on brain MR images: the population-based Rotterdam scan study. Radiology 281: 507–515, 2016 [DOI] [PubMed] [Google Scholar]
- 4).Behbahani M, Skeie GO, Eide GE, Hausken A, Lund-Johansen M, Skeie BS: A prospective study of the natural history of incidental meningioma—Hold your horses! Neuro-Oncol Pract 6: 438–450, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Firsching RP, Fischer A, Peters R, Thun F, Klug N: Growth rate of incidental meningiomas. J Neurosurg 73: 545–547, 1990 [DOI] [PubMed] [Google Scholar]
- 6).Hashiba T, Hashimoto N, Izumoto S, et al. : Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. J Neurosurg 110: 675–684, 2009 [DOI] [PubMed] [Google Scholar]
- 7).Hashimoto N, Rabo CS, Okita Y, et al. : Slower growth of skull base meningiomas compared with non-skull base meningiomas based on volumetric and biological studies. J Neurosurg 116: 574–580, 2012 [DOI] [PubMed] [Google Scholar]
- 8).Jo KW, Kim CH, Kong DS, et al. : Treatment modalities and outcomes for asymptomatic meningiomas. Acta Neurochir (Wien) 153: 62–67; discussion 67, 2011 [DOI] [PubMed] [Google Scholar]
- 9).Kim KH, Kang SJ, Choi JW, et al. : Clinical and radiological outcomes of proactive Gamma Knife surgery for asymptomatic meningiomas compared with the natural course without intervention. J Neurosurg 130: 1740–1749, 2018 [DOI] [PubMed] [Google Scholar]
- 10).Kuratsu J, Kochi M, Ushio Y: Incidence and clinical features of asymptomatic meningiomas. J Neurosurg 92: 766–770, 2000 [DOI] [PubMed] [Google Scholar]
- 11).Nakasu S, Fukami T, Nakajima M, Watanabe K, Ichikawa M, Matsuda M: Growth pattern changes of meningiomas: long-term analysis. Neurosurgery 56: 946–955, 2005 [PubMed] [Google Scholar]
- 12).Niiro M, Yatsushiro K, Nakamura K, Kawahara Y, Kuratsu J: Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry 68: 25–28, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Oya S, Kim SH, Sade B, Lee JH: The natural history of intracranial meningiomas. J Neurosurg 114: 1250–1256, 2011 [DOI] [PubMed] [Google Scholar]
- 14).Braunstein JB, Vick NA: Meningiomas: the decision not to operate. Neurology 48: 1459–1462, 1997 [DOI] [PubMed] [Google Scholar]
- 15).Go RS, Taylor BV, Kimmel DW: The natural history of asymptomatic meningiomas in Olmsted County, Minnesota. Neurology 51: 1718–1720, 1998 [DOI] [PubMed] [Google Scholar]
- 16).Herscovici Z, Rappaport Z, Sulkes J, Danaila L, Rubin G: Natural history of conservatively treated meningiomas. Neurology 63: 1133–1134, 2004 [DOI] [PubMed] [Google Scholar]
- 17).Jadid KD, Feychting M, Höijer J, Hylin S, Kihlström L, Mathiesen T: Long-term follow-up of incidentally discovered meningiomas. Acta Neurochir (Wien) 157: 225–230; discussion 230, 2015 [DOI] [PubMed] [Google Scholar]
- 18).Olivero WC, Lister JR, Elwood PW: The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg 83: 222–224, 1995 [DOI] [PubMed] [Google Scholar]
- 19).Romani R, Ryan G, Benner C, Pollock J: Non-operative meningiomas: long-term follow-up of 136 patients. Acta Neurochir (Wien) 160: 1547–1553, 2018 [DOI] [PubMed] [Google Scholar]
- 20).Rubin G, Herscovici Z, Laviv Y, Jackson S, Rappaport ZH: Outcome of untreated meningiomas. Isr Med Assoc J 13: 157–160, 2011 [PubMed] [Google Scholar]
- 21).Sonoda Y, Sakurada K, Saino M, Kondo R, Sato S, Kayama T: Multimodal strategy for managing meningiomas in the elderly. Acta Neurochir (Wien) 147: 131–136; discussion 136, 2005 [DOI] [PubMed] [Google Scholar]
- 22).Yano S, Kuratsu J: Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg 105: 538–543, 2006 [DOI] [PubMed] [Google Scholar]
- 23).Lee EJ, Kim JH, Park ES, et al. : A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg 127: 971–980, 2017 [DOI] [PubMed] [Google Scholar]
- 24).Lee EJ, Park JH, Park ES, Kim JH: “Wait-and-See” strategies for newly diagnosed intracranial meningiomas based on the risk of future observation failure. World Neurosurgery 107: 604–611, 2017 [DOI] [PubMed] [Google Scholar]
- 25).Nakamura M, Roser F, Michel J, Jacobs C, Samii M: The natural history of incidental meningiomas. Neurosurgery 53: 62–70; discussion 70–71, 2003 [DOI] [PubMed] [Google Scholar]
- 26).Nakasu S, Onishi T, Kitahara S, Oowaki H, Matsumura KI: CT Hounsfield unit is a good predictor of growth in meningiomas. Neurol Med Chir (Tokyo) 59: 54–62, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Yoneoka Y, Fujii Y, Tanaka R: Growth of incidental meningiomas. Acta Neurochir (Wien) 142: 507–511, 2000 [DOI] [PubMed] [Google Scholar]
- 28).Nakasu S, Nakasu Y, Fukami T, Jito J, Nozaki K: Growth curve analysis of asymptomatic and symptomatic meningiomas. J Neurooncol 102: 303–310, 2011 [DOI] [PubMed] [Google Scholar]
- 29).Chang V, Narang J, Schultz L, et al. : Computer-aided volumetric analysis as a sensitive tool for the management of incidental meningiomas. Acta Neurochir (Wien) 154: 589–597; discussion 597, 2012 [DOI] [PubMed] [Google Scholar]
- 30).Zeidman LA, Ankenbrandt WJ, Du H, Paleologos N, Vick NA: Growth rate of non-operated meningiomas. J Neurol 255: 891–895, 2008 [DOI] [PubMed] [Google Scholar]
- 31).Webplotdigitizer : https://automeris.io/WebPlotDigitizer/ (Accessed on 2019 Mar. 28)
- 32).Kanda Y: Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: 452–458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Hirota K, Fujita T, Akagawa H, Onda H, Kasuya H: Spontaneous regression together with increased calcification of incidental meningioma. Surg Neurol Int 5: 73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Islim AI, Mohan M, Moon RDC, et al. : Incidental intracranial meningiomas: a systematic review and meta-analysis of prognostic factors and outcomes. J Neurooncol 142: 211–221, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Kasuya H, Kubo O, Kato K, Krischek B: Histological characteristics of incidentally-found growing meningiomas. J Med Invest 59: 241–245, 2012 [DOI] [PubMed] [Google Scholar]
- 36).Liu Y, Li F, Wang C: Clinical features and surgical treatment of asymptomatic meningiomas. Turk Neurosurg 25: 121–125, 2015 [DOI] [PubMed] [Google Scholar]
- 37).Ishi Y, Terasaka S, Yamaguchi S, et al. : Reliability of the size evaluation method for meningiomas: maximum diameter, ABC/2 formula, and planimetry method. World Neurosurg 94: 80–88, 2016 [DOI] [PubMed] [Google Scholar]
- 38).Zeng L, Liang P, Jiao J, Chen J, Lei T: Will an asymptomatic meningioma grow or not grow? A meta-analysis. J Neurol Surg A Cent Eur Neurosurg 76: 341–347, 2015 [DOI] [PubMed] [Google Scholar]
- 39).Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT: Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg 113: 1036–1042, 2010 [DOI] [PubMed] [Google Scholar]
- 40).Berhouma M, Jacquesson T, Jouanneau E, Cotton F: Pathogenesis of peri-tumoral edema in intracranial meningiomas. Neurosurg Rev 42: 59–71, 2019 [DOI] [PubMed] [Google Scholar]
- 41).Nakasu S, Nakajima M, Matsumura K, Nakasu Y, Handa J: Meningioma: proliferating potential and clinicoradiological features. Neurosurgery 37: 1049–1055, 1995 [DOI] [PubMed] [Google Scholar]