Abstract
The protein kinase B (Akt) exemplifies an important switch of cell death and survival within the PI3K/Akt signaling pathway, which renders Akt a valuable target in diseases such as cancer. Herein, we give a short overview of clinical applications involving Akt, outline promising and innovative approaches to investigate the role of this kinase in diseases, and highlight the current challenges that require thorough investigation to set the groundwork for successful therapeutic strategies.
Keywords: Cancer, covalent inhibitors, allosteric site, kinase, Akt, biomarker
Due to its importance in cell survival, antiapoptotic mechanisms, and migration, the PI3K/Akt signaling pathway has been investigated intensively over the last decades. Dysregulated proteins within this signaling cascade behave as oncogenic drivers in various cancer types. Genomic alterations, such as deletions, loss of function mutations in the tumor suppressor PTEN, activating mutations in PI3K, and amplifications of the genes encoding proteins involved result in an enhanced pathway activity.1 These genetic aberrations can lead to the hyperactivation of the protein kinase B (Akt), which functions as a major signaling node within the PI3K/Akt pathway. The complexity of this pathway is underlined by the presence of three isoforms of Akt (Akt1/Akt2/Akt3). Even though they share a high sequence homology, the isoforms differ in their intracellular functions and localizations, resulting in a highly complex signaling network with more than a hundred downstream substrates. Furthermore, activating mutations and overexpression of Akt are often related to resistance against traditional chemotherapy.2 Although mutations of Akt occur very rarely, the most common mutation in protein kinase B is the somatic Akt1E17K mutation, which has been reported recently in breast cancer (6–8%), colorectal cancer (2–6%), and meningioma (6%).3 In addition, AktE17K is linked to the Proteus Syndrome, a rare progressive overgrowth disease, that can affect any organ or tissue in the human body.4 These features make Akt a highly attractive target for the treatment of various diseases, motivating the development of targeted approaches.5
Three main categories of approaches have been used to address Akt (Figure 1). Fairly common in the field of kinases is targeting the active site with ATP-competitive molecules. However, in the case of Akt there is a unique allosteric binding pocket with obvious advantages regarding selectivity of developed ligands. Active site binders target the conserved ATP-binding pocket and stabilize the active conformation of the kinase, whereas allosteric inhibitors bind in an interdomain region between the kinase and the PH domain, resulting in the stabilization of the inactive “PH-in” conformation of Akt. Another competitive approach involves the PIP3-analogues, which bind to the PIP3-cavity within the PH-domain and disable the activation mechanism.1 However, PIP3-analogues harbor several disadvantages, such as poor drug-like properties as well as a lack of selectivity, due to over 300 structurally related PH domains in the human genome.6
Figure 1.
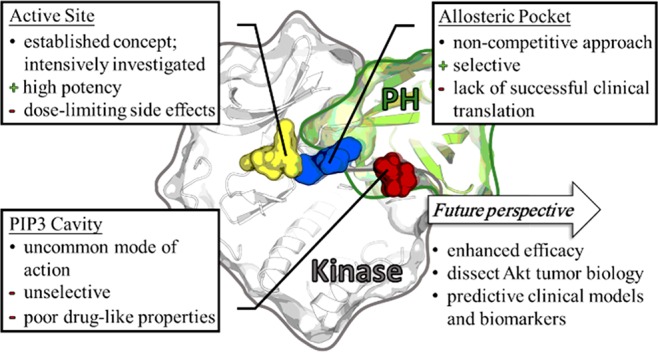
Drawbacks and future potentials in targeting Akt with small molecules in the clinic. Overview of protein kinase Akt in the inactive conformation and addressable binding cavities for inhibition: active site (yellow), PIP3-binding domain (red), and allosteric pocket (blue) (merged PDB-structures: 5KCV, 4GV1, 2UVM, and 6HHF).
Even though various small molecules have been shown to effectively inhibit Akt in vitro and in vivo, only a few have entered clinical evaluation.
Traditional ATP-competitive Akt inhibitors such as capivasertib (AZD5363) and ipatasertib (GDC-0068) are in phase I and phase II clinical trials for mono- or combination therapy with diverse indications. However, the ATP-binding pocket of Akt is highly conserved among kinases within the human cell, which limits selectivity. Dose-limiting side effects during treatment and the lack of efficacy resulting from the inevitable dose reduction occur.1 Efforts to identify Akt-specific and isoform-selective small molecules have resulted in the discovery and development of bioactive allosteric inhibitors. This alternative approach has led to ligands such as MK-2206 and miransertib (ARQ092), which offer greater specificity, reduced side-effects, and lower toxicity compared to the aforementioned targeted approaches.2 MK-2206 is an orally effective, highly potent and selective allosteric pan-Akt inhibitor and has been investigated in clinical studies, especially in breast cancer.7 However, the expected clinical success has not yet been achieved.
Miransertib and its next generation inhibitor Arq751, both orally available highly selective pan-AKT inhibitors, are currently undergoing clinical investigations. Positive results with miransertib were observed in cells harboring the biomarker AktE17K. Currently both inhibitors are under study as a potential therapy for the Proteus Syndrome.4
Despite the multitude of clinical studies for the treatment of cancer, none of the targeted Akt inhibitors have reached phase III in clinical trials, indicating the enormous complexity of Akt-signal dependent malfunctions. In diseases such as the Proteus Syndrome, for which the AktE17K mutation is reported to be a relevant biomarker, promising response rates in monotherapy were achieved.3,8 In cancer, however, Akt monotherapy often relies on PI3K/Akt pathway alterations upstream of Akt and is associated with unsatisfactory results, indicating that the classic model of oncogene addiction in precision medicine reaches its limits for these kinds of tumors. Therefore, a greater understanding of the Akt-dependent biology in the context of diseases is an important goal.
The limitations of previously developed targeted Akt inhibitors in clinical trials emphasize the need for innovative approaches to clarify the role of Akt in disease. The approach of covalent-allosteric Akt inhibitors (CAAIs), described by Weisner and colleagues, combines the selectivity of allosteric inhibitors with the irreversible covalent modification of two noncatalytic cysteines in the activation loop of Akt (Figure 2). Thus, CAAIs show prolonged target residence time only limited by the kinases turnover in the cell, and the lead compound borussertib demonstrated enhanced inhibitory efficacy compared to clinically relevant Akt inhibitors.9 Furthermore, borussertib exhibits strong antiproliferative effects and showed target engagement in cancer cell lines harboring alterations in the PI3K/AKT signaling pathway. In addition, the in vivo efficacy of borussertib was proven in treatment combination studies with the MEK-inhibitor trametinib in KRAS mutant patient-derived xenograft (PDX) models leading to a partial response.10
Figure 2.
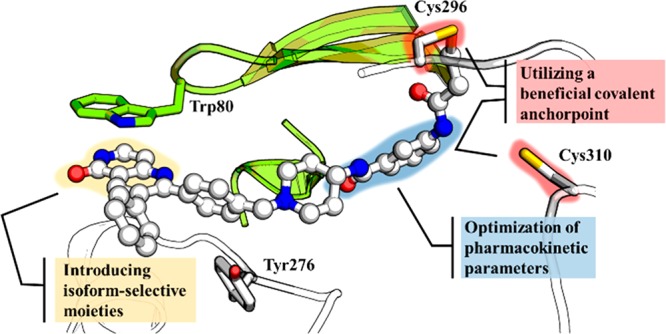
CAAIs as innovative and versatile tools to perturb the allosteric site of Akt. Binding mode of covalent-allosteric ligands, key interactions to the protein, and the auspicious space examined for functional alteration, e.g., optimization of pharmacokinetic characteristics or increase of isoform-selectivity (PDB: 6HHI).
Despite limitations in its pharmacokinetic properties, borussertib showed promising results in preclinical studies underlining the potential of CAAIs for targeting Akt in relevant diseases. However, the pharmacokinetic properties of borussertib must be refined by structure-based design before per oral applications can be attained.11 Structural insights into the allosteric site of the different isoforms of Akt recently guided the development of specific CAAIs, enabling future investigations of isoform-specific functions through the use of selective probes.12
CAAIs present a powerful approach to examine key questions about the regulation and impact of Akt in various diseases. By addressing these questions, predictive statements for the clinical efficacy of Akt inhibitors can be made.
Currently, diagnosis, prediction of response to treatment, and success of targeted therapy rely on biomarker profiling as a core technique for personalized medicine.13 In the case of Akt, mainly pathway predictive markers have been identified so far. Overexpression or different phosphorylation states of proteins involved in the pathway (either Akt itself or downstream targets) indicate dysregulation of the signaling cascade in addition to genomic modifications in the PTEN or PIK3CA genes. Growing evidence also suggests that the AktE17K mutation is an oncogenic hotspot.3 The reliable prediction of specific biomarkers remains a significant problem for Akt since the markers lack a direct link to treatment success. This is different in other cancer types, e.g., lesions identified in the therapy of non-small cell lung cancer (NCSLC).13 More extensive profiling studies are needed to define genetic profiles in various types of cancer, their models, and specific tissues. Results of deep-sequencing may help to identify more predictive biomarkers, such as RNAs regulating gene expression or proteins, that are somehow involved in Akt-regulation.
Precision medicine seems more challenging in targeted therapies that address cell death signaling pathways with negative regulators, where resistances occur, and the escape from the drug-induced inhibition relies on adopting alternative signaling circuitry. Downstream alterations, cross-talk in signaling, and activation of complex cellular feedback loops as a response of targeting Akt redefines the classification of an oncogenic addiction for that pathway.14 Compensatory actions through activation of proliferative signaling cascades may need to be addressed with combination therapies, as recently demonstrated in our lab by tackling the Akt and MEK pathways simultaneously.10
The necessary degree of target occupancy and total pathway knockdown required to mute the signaling in a sufficiently antiproliferative manner remains to be defined. Conformational dependence for Akt signaling has been described, which may contribute to the success and failure of ATP binders vs allosteric inhibitors by stabilizing distinct conformations of Akt and thereby alter important interaction platforms in the cell.15 Additionally, the relevance of Akt isoform specificity in solid tumors is not fully understood, and different studies report conflicting results, underlining the complexity of addressing this drug target.12
In summary, we have presented a compact overview of current drawbacks concerning the role of Akt as a therapeutic target, as well as innovative approaches with high potential, and current challenges yet to be overcome. More extensive studies are needed to dissect the complex signaling network that Akt is involved in, as well as a thorough investigation of the regulatory mechanisms of Akt within diseases. Selective small molecules, such as CAAIs, could serve as powerful tools to probe those systems and unravel new biomarkers, which could lead to improved outcomes of therapeutic perturbation.
Glossary
Abbreviations
- Akt
protein kinase B
- CAAIs
covalent allosteric Akt inhibitors
- MEK
mitogen-activated protein kinase kinase
- NSCLC
non-small cell lung cancer
- PH domain
pleckstrin homology domain
- PI3K
phosphatidylinositol 3-kinase
- PTEN
phosphatase and tensin homolog
Author Contributions
‡ I.L., L.Q., and L.D. contributed equally. The manuscript was written through contributions of all authors.
L.Q. is grateful for a scholarship by the German Academic Scholarship Foundation.
Views expressed in this editorial are those of the authors and not necessarily the views of the ACS.
The authors declare no competing financial interest.
References
- Mundi P. S.; Sachdev J.; McCourt C.; Kalinsky K. AKT in cancer: new molecular insights and advances in drug development. Br. J. Clin. Pharmacol. 2016, 82 (4), 943–56. 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitulescu G. M.; Van De Venter M.; Nitulescu G.; Ungurianu A.; Juzenas P.; Peng Q.; Olaru O. T.; Gradinaru D.; Tsatsakis A.; Tsoukalas D.; Spandidos D. A.; Margina D. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53 (6), 2319–2331. 10.3892/ijo.2018.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman D. M.; Smyth L. M.; Donoghue M. T. A.; Westin S. N.; Bedard P. L.; Dean E. J.; Bando H.; El-Khoueiry A. B.; Perez-Fidalgo J. A.; Mita A.; Schellens J. H. M.; Chang M. T.; Reichel J. B.; Bouvier N.; Selcuklu S. D.; Soumerai T. E.; Torrisi J.; Erinjeri J. P.; Ambrose H.; Barrett J. C.; Dougherty B.; Foxley A.; Lindemann J. P. O.; McEwen R.; Pass M.; Schiavon G.; Berger M. F.; Chandarlapaty S.; Solit D. B.; Banerji U.; Baselga J.; Taylor B. S. AKT Inhibition in Solid Tumors With AKT1Mutations. J. Clin. Oncol. 2017, 35 (20), 2251–2259. 10.1200/JCO.2017.73.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Savage R. E.; Eathiraj S.; Meade J.; Wick M. J.; Hall T.; Abbadessa G.; Schwartz B. Targeting AKT1-E17K and the PI3K/AKT Pathway with an Allosteric AKT Inhibitor, ARQ 092. PLoS One 2015, 10 (10), e0140479 10.1371/journal.pone.0140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers I.; Vincent E. E.; Tavare J. M. Akt signalling in health and disease. Cell. Signalling 2011, 23 (10), 1515–27. 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Nitulescu G. M.; Margina D.; Juzenas P.; Peng Q.; Olaru O. T.; Saloustros E.; Fenga C.; Spandidos D.; Libra M.; Tsatsakis A. M. Akt inhibitors in cancer treatment: The long journey from drug discovery to clinical use (Review). Int. J. Oncol. 2016, 48 (3), 869–85. 10.3892/ijo.2015.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y.; Lin N. U.; Maurer M. A.; Chen H.; Mahvash A.; Sahin A.; Akcakanat A.; Li Y.; Abramson V.; Litton J.; Chavez-MacGregor M.; Valero V.; Piha-Paul S. A.; Hong D.; Do K. A.; Tarco E.; Riall D.; Eterovic A. K.; Wulf G. M.; Cantley L. C.; Mills G. B.; Doyle L. A.; Winer E.; Hortobagyi G. N.; Gonzalez-Angulo A. M.; Meric-Bernstam F. Phase II trial of AKT inhibitor MK-2206 in patients with advanced breast cancer who have tumors with PIK3CA or AKT mutations, and/or PTEN loss/PTEN mutation. Breast Cancer Res. 2019, 21 (1), 78. 10.1186/s13058-019-1154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni C.; Gullo G.; Resta N.; Fagotti A.; Onesimo R.; Schwartz B.; Kazakin J.; Abbadessa G.; Crown J.; Collins C. D.; Ranieri C.; Scambia G.; Zampino G. First evidence of a therapeutic effect of miransertib in a teenager with Proteus syndrome and ovarian carcinoma. Am. J. Med. Genet., Part A 2019, 179 (7), 1319–1324. 10.1002/ajmg.a.61160. [DOI] [PubMed] [Google Scholar]
- Weisner J.; Gontla R.; van der Westhuizen L.; Oeck S.; Ketzer J.; Janning P.; Richters A.; Mühlenberg T.; Fang Z.; Taher A.; Jendrossek V.; Pelly S. C.; Bauer S.; van Otterlo W. A.; Rauh D. Covalent-Allosteric Kinase Inhibitors. Angew. Chem., Int. Ed. 2015, 54 (35), 10313–6. 10.1002/anie.201502142. [DOI] [PubMed] [Google Scholar]
- Weisner J.; Landel I.; Reintjes C.; Uhlenbrock N.; Trajkovic-Arsic M.; Dienstbier N.; Hardick J.; Ladigan S.; Lindemann M.; Smith S.; Quambusch L.; Scheinpflug R.; Depta L.; Gontla R.; Unger A.; Müller H.; Baumann M.; Schultz-Fademrecht C.; Gunther G.; Maghnouj A.; Müller M. P.; Pohl M.; Teschendorf C.; Wolters H.; Viebahn R.; Tannapfel A.; Uhl W.; Hengstler J. G.; Hahn S. A.; Siveke J. T.; Rauh D. Preclinical Efficacy of Covalent-Allosteric AKT Inhibitor Borussertib in Combination with Trametinib in KRAS-Mutant Pancreatic and Colorectal Cancer. Cancer Res. 2019, 79 (9), 2367–2378. 10.1158/0008-5472.CAN-18-2861. [DOI] [PubMed] [Google Scholar]
- Uhlenbrock N.; Smith S.; Weisner J.; Landel I.; Lindemann M.; Le T. A.; Hardick J.; Gontla R.; Scheinpflug R.; Czodrowski P.; Janning P.; Depta L.; Quambusch L.; Müller M. P.; Engels B.; Rauh D. Structural and chemical insights into the covalent-allosteric inhibition of the protein kinase Akt. Chemical Science 2019, 10 (12), 3573–3585. 10.1039/C8SC05212C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quambusch L.; Landel I.; Depta L.; Weisner J.; Uhlenbrock N.; Müller M. P.; Glanemann F.; Althoff K.; Siveke J. T.; Rauh D. Covalent-Allosteric Inhibitors to Achieve Akt Isoform-Selectivity. Angew. Chem., Int. Ed. 2019, 58 (52), 18823–18829. 10.1002/anie.201909857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway L. A.; Jänne P. A. Circumventing Cancer Drug Resistance in the Era of Personalized Medicine. Cancer Discovery 2012, 2 (3), 214–226. 10.1158/2159-8290.CD-12-0012. [DOI] [PubMed] [Google Scholar]
- Saura C.; Roda D.; Rosello S.; Oliveira M.; Macarulla T.; Perez-Fidalgo J. A.; Morales-Barrera R.; Sanchis-Garcia J. M.; Musib L.; Budha N.; Zhu J.; Nannini M.; Chan W. Y.; Sanabria Bohorquez S. M.; Meng R. D.; Lin K.; Yan Y.; Patel P.; Baselga J.; Tabernero J.; Cervantes A. A First-in-Human Phase I Study of the ATP-Competitive AKT Inhibitor Ipatasertib Demonstrates Robust and Safe Targeting of AKT in Patients with Solid Tumors. Cancer Discovery 2017, 7 (1), 102–113. 10.1158/2159-8290.CD-16-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanco I.; Chen Z. C.; Tanos B.; Oldrini B.; Hsieh W. Y.; Yannuzzi N.; Campos C.; Mellinghoff I. K.. A kinase-independent function of AKT promotes cancer cell survival. eLife 2014, 3, 10.7554/eLife.03751 [DOI] [PMC free article] [PubMed] [Google Scholar]


