Abstract
The melanocortin receptors are stimulated by agonists (α-MSH, β-MSH, γ-MSH, and ACTH) processed from the proopiomelanocortin (POMC) gene transcript and possess a common His-Phe-Arg-Trp tetrapeptide sequence critical for receptor activation. Deficiency in POMC signaling in humans is associated with adrenal insufficiency, altered pigmentation, and rapid, early onset weight gain. Herein, 12 single nucleotide polymorphisms (SNPs) deposited into the Variation Viewer database within the His-Phe-Arg-Trp sequences of ACTH/α-MSH, β-MSH, and γ-MSH were substituted into tetrapeptide scaffolds to examine the in vitro signaling effects of these polymorphisms at the cloned melanocortin receptors. Every polymorphism decreased agonist potency and/or efficacy at the melanocortin receptors assayed, indicating that polymorphisms within the signaling sequence of POMC-derived agonists negatively impacts receptor activation. Future work to incorporate these substitutions into the full-length POMC agonists would confirm these findings, identifying new patient populations that might benefit from therapeutic regiments to treat POMC-deficient signaling.
Keywords: Melanocyte-stimulating hormones, single nucleotide polymorphisms, melanocortin receptors
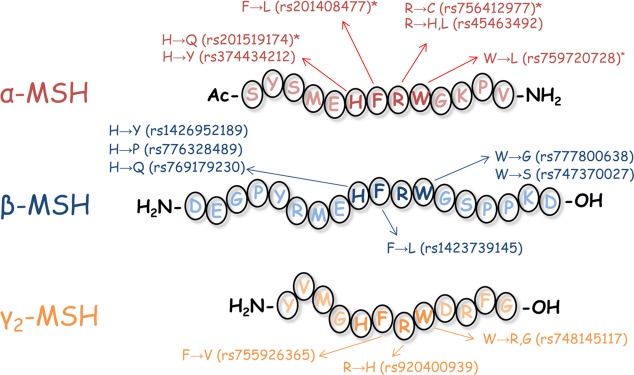
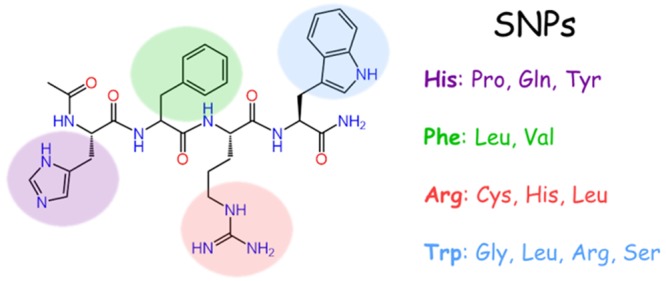
The five known melanocortin receptors (MC1–5R)1−8 are involved in numerous biological functions, including pigmentation,2,3 steroidogenesis,3 and energy homeostasis.4−6 These receptors are stimulated by agonists derived from the proopiomelanocortin (POMC) gene transcript,9 which is processed into α-MSH, β-MSH, γ-MSH, and ACTH among other peptide products. Common to these endogenous agonists is a His-Phe-Arg-Trp tetrapeptide sequence (Figure 1). Truncation studies of α-MSH using the frog (Rana pipiens)10 and lizard (Anolis carolinensis)11 skin bioassays indicated the minimal active sequence to be Ac-His-Phe-Arg-Trp-NH2, the common tetrapeptide sequence with the N-terminal acetylated and the C-terminal possessing an amide functionality. Due to the many physiological roles of the melanocortin receptors and one transcript producing the melanocortin agonists, individuals with insufficient POMC translation have many characteristic phenotypic traits, including adrenal insufficiency, early onset obesity, and often (though not always) hair and pigmentation effects (SI Table 1).
Figure 1.
Amino acid sequences of α-MSH, β-MSH, and γ-MSH. Missense SNPs deposited at the NIH within the His-Phe-Arg-Trp sequence are indicated for each POMC-derived agonist. An asterisk (*) indicates a polymorphism or substitution in α-MSH that has previously been characterized (His → Gln,12 Phe → Leu,13 Arg → Cys,14 and Trp → Leu25−28).
Additionally, single nucleotide polymorphisms (SNPs) have been reported for POMC-derived melanocortin agonists with phenotypic effects. For missense polymorphisms (changing the amino acid sequence) within the endogenous melanocortin agonists, three polymorphisms (His6Gln,12 Phe7Leu,13 and Arg8Cys;14Figure 1) in the His-Phe-Arg-Trp active sequence of α-MSH have been reported to decrease binding affinity, functional potency, and/or efficacy to the melanocortin receptors. In one patient that was homozygous for the Arg8Cys polymorphism, and in another patient that possessed Arg8Cys and null POMC alleles, adrenal insufficiency, red hair pigmentation, and obesity phenotypes were observed.14 In β-MSH, two polymorphisms (Tyr5Cys12,15 and Pro15Leu16) have been associated with early onset obesity. Thus, missense SNPs within the POMC-derived melanocortin agonists are sufficient to alter phenotypes.
Individuals with insufficient POMC production and SNPs within the POMC transcript were first identified from particular phenotypes (adrenal insufficiency, early onset obesity), and then subjected to genomic sequencing to identify and/or confirm the underlying genetic determinant. Another approach to identify potentially deleterious polymorphisms is to search genomic databanks for polymorphisms within a gene of interest, characterize the resulting transcribed product, and determine if the characterized product could result in an altered phenotype. Variation Viewer is one genomic database curated by the United States National Center for Biotechnology Information. Genomic variants from contributing laboratories can be deposited and freely accessed. These variants are not necessarily investigated by the laboratory who deposits them. However, if a gene of interest has previously been associated with a phenotype, these deposited polymorphisms provide a source of potential substitutions that can be investigated for a genetic basis of disease states.
As improper transcription and translation of the POMC gene transcript results in a characteristic triad of associated phenotypes, POMC polymorphisms within the common His-Phe-Arg-Trp melanocortin signaling tetrapeptides were identified in Variation Viewer (Figure 1, accessed in May 2018). Twelve missense SNPs were identified, nine of which have not been characterized, two characterized at the MC4R, and one characterized at the MC1R and MC4R. It was hypothesized these SNPs would decrease receptor signaling since this domain has previously been demonstrated to be critical for stimulating receptor activity. Polymorphisms at the His, Arg, and Trp positions were probed using the tetrapeptide Ac-His-dPhe-Arg-Trp-NH2 scaffold, as the d-isomer at the Phe position results in 20- to 100-fold increase in potency relative to the LPhe tetrapeptide.17 For polymorphisms at the Phe position, the all l-amino acid Ac-His-Phe-Arg-Trp-NH2 tetrapeptide served as the scaffold and lead ligand. The tetrapeptides were used to assess SNPs of all the POMC agonists in common scaffolds as a simplified initial screen. The resulting control and experimental tetrapeptide ligands were assayed for agonist activity at the MC1R, MC3R, MC4R, and MC5R.
Peptides were synthesized using standard Fmoc techniques. Following semipreparative RP-HPLC purification, peptides were assessed for purity (>95%) using analytical RP-HPLC using two solvent systems (acetonitrile and methanol), and the correct average molecular weight was confirmed through ESI–MS (SI Table 2; University of Minnesota Mass Spectrometry Laboratory). Compounds were assayed using the cAMP AlphaScreen assay in HEK293 cells stably expressing the mMC1R, mMC3R, mMC4R, and mMC5R as described by the manufacturer and our laboratory.18−20 The MC2R is only stimulated by ACTH21 and was therefore not examined. The positive controls α-MSH, [Nle,4dPhe7α-MSH (NDP–MSH),22 Ac-His-dPhe-Arg-Trp-NH2, and Ac-His-Phe-Arg-Trp-NH2 were included. Peptides were considered full agonists if they stimulated the receptors to at least 90% the maximal response of NDP–MSH, and were considered to not possess agonist activity at concentrations up to 100 μM if at least a 20% response (relative to the maximal NDP–MSH signal) was not observed. Compounds within a 3-fold potency range were considered equipotent. Both α-MSH and NDP–MSH ligands were nanomolar to subnanomolar potent agonists at the melanocortin receptors assayed. The lead tetrapeptide, Ac-His-dPhe-Arg-Trp-NH2 (MDE9–150), possessed 16, 100, 21, and 11 nM potencies at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively (Table 1), similar to previous reports of this ligand using the AlphaScreen cAMP assay.19,20,23
Table 1. Tetrapeptide Agonist Pharmacology at the Mouse Melanocortin Receptorsa.
| EC50 (nM) |
|||||
|---|---|---|---|---|---|
| peptide | sequence | mMC1R | mMC3R | mMC4R | mMC5R |
| α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.04 ± 0.01 | 0.22 ± 0.06 | 3.7 ± 0.7 | 0.22 ± 0.03 |
| NDP–MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 0.012 ± 0.005 | 0.19 ± 0.09 | 1.1 ± 0.5 | 0.3 ± 0.1 |
| MDE9–150 | Ac-His-DPhe-Arg-Trp-NH2 | 16 ± 5 | 100 ± 50 | 21 ± 6 | 11 ± 4 |
| MDW1–22–1 | Ac-Pro-DPhe-Arg-Trp-NH2 | 2,100 ± 800 | 30% @ 100 μM | Partial Agonist 60% NDP–MSH (1,000 ± 300) | Partial Agonist 80% NDP–MSH (2,000 ± 700) |
| MDW1–22–3 | Ac-Gln-DPhe-Arg-Trp-NH2 | 1,600 ± 400 | Partial Agonist 80% NDP–MSH (1,300 ± 600) | Partial Agonist 80% NDP–MSH (270 ± 70) | 270 ± 90 |
| MDW1–22–2 | Ac-Tyr-DPhe-Arg-Trp-NH2 | 1,200 ± 400 | Partial Agonist 50% NDP–MSH (3,000 ± 1,000) | Partial Agonist 65% NDP–MSH (90 ± 30) | Partial Agonist 80% NDP–MSH (400 ± 100) |
| MDW1–52 | Ac-His-dPhe-Cys-Trp-NH2 | 400 ± 100 | Partial Agonist 85% NDP–MSH (6,000 ± 2,000) | 3,100 ± 300 | 1,700 ± 700 |
| MDW1–48 | Ac-His-dPhe-His-Trp-NH2 | 1,300 ± 300 | Partial Agonist 85% NDP–MSH (4,000 ± 700) | 500 ± 100 | 300 ± 100 |
| MDW1−44 | Ac-His-dPhe-Leu-Trp-NH2 | 90 ± 10 | 25% @ 100 μM | Partial Agonist 85% NDP–MSH (3,800 ± 300) | 2,000 ± 600 |
| MDW1–11 | Ac-His-DPhe-Arg-Gly-NH2 | 1,300 ± 400 | >100,000 | >100,000 | >100,000 |
| MDW1–15 | Ac-His-DPhe-Arg-Leu-NH2 | 1,600 ± 400 | 20% @ 100 μM | >100,000 | 40% @ 100 μM |
| MDW1–19 | Ac-His-DPhe-Arg-Arg-NH2 | 900 ± 200 | >100,000 | >100,000 | 20% @ 100 μM |
| MDW1–6 | Ac-His-DPhe-Arg-Ser-NH2 | 1,600 ± 600 | 50% @ 100 μM | >100,000 | 75% @ 100 μM |
| MDW1–88–3 | Ac-His-Phe-Arg-Trp-NH2 | 3,000 ± 1,000 | 1,500 ± 300 | 2,100 ± 500 | 220 ± 10 |
| MDW1–88–1 | Ac-His-Leu-Arg-Trp-NH2 | 25% @ 100 μM | >100,000 | >100,000 | 25% @ 100 μM |
| MDW1–88–2 | Ac-His-Val-Arg-Trp-NH2 | >100,000 | >100,000 | >100,000 | >100,000 |
The indicated errors represent the standard error of the mean determined from at least three independent experiments. >100,000 indicates that the compound lacked agonist activity at up to 100 μM concentrations. A percentage denotes the percent maximal stimulatory response observed at 100 μM concentrations, but not enough stimulation was observed to determine an EC50 value. Ligands that possessed partial agonist activity are indicated with the percent maximal NDP stimulation and apparent EC50 value (compounds showing >90% maximal NDP response were considered full agonists).
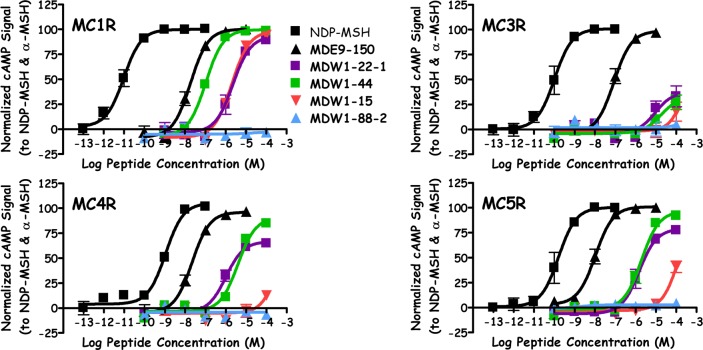
Three polymorphisms at the His position were examined. When Pro was substituted for His, resulting in the tetrapeptide MDW1–22–1, micromolar agonist potency (Figure 2) was observed at the MC1R, MC4R (60% NDP–MSH efficacy), and MC5R (80% NDP–MSH efficacy), while this ligand was only able to partially stimulate the MC3R at 100 μM concentrations (30% maximal NDP–MSH signal). These values are similar to a prior report for this tetrapeptide in a β-galactosidase cAMP assay, which reported EC50 values of 4000, 15000, 1500, and 680 nM at the mMC1R, mMC3R, mMC4R, and mMC5R, respectively.24 Substitution of His for Gln, MDW1–22–3, resulted in full agonist activity at the MC1R (1600 nM) and MC5R (270 nM), with 80% agonist efficacy at the MC3R (1300 nM) and MC4R (270 nM). The His to Tyr substitution (MDW1–22–2) was also a micromolar MC1R agonist (1200 nM) and possessed partial agonist efficacy at the MC3R (50%, EC50 = 3000 nM), MC4R (65%, EC50 = 90 nM), and MC5R (80%, EC50 = 400 nM).
Figure 2.
Illustration of NDP–MSH, MDE9–150, MDW1–22–1, MDW1–44, MDW1–15, and MDW1–88–2 agonist pharmacology at the mMC1R, mMC3R, mMC4R, and mMC5R.
Substitutions at the Arg position also decreased potency at the melanocortin receptors relative to the lead ligand MDE9–150. The tetrapeptide MDW1–52, possessing an Arg to Cys substitution, resulted in full agonist efficacy at the MC1R, MC4R, and MC5R with potencies of 400, 3100, and 1700 nM, respectively. This ligand resulted in 85% efficacy at the MC3R (EC50 = 6000 nM). Similar efficacies to MDW1–52 were observed with the Arg to His substituted MDW1–48, with potencies of 1300, 4000, 500, and 300 nM at the MC1R, MC3R, MC4R, and MC5R, respectively. Replacing the Arg with Leu, yielding tetrapeptide MDW1–44, resulted in a full agonist at the MC1R (90 nM) and MC5R (2000 nM), a partial agonist at the MC4R (85% efficacy, EC50 = 3800 nM), and only partially stimulated the MC3R (25%) at 100 μM concentrations (Figure 2).
The Trp position was found to be critical for agonist activity at the MC3R, MC4R, and MC5R, as the four tetrapeptides with amino acid substitutions at this position were unable to fully activate these receptors. Replacing Trp with Gly, MDW1–11 resulted in a tetrapeptide with agonist activity at the MC1R (1300 nM) and no agonist activity at concentrations up to 100 μM at the MC3R, MC4R, and MC5R. No agonist activity at the MC4R was also observed for the Trp to Leu substituted MDW1–15 (Figure 2), which partially activated the MC3R (20%) and MC5R (40%) at 100 μM concentrations and was a full agonist at the MC1R (EC50 = 1600 nM). The Trp to Arg tetrapeptide MDW1–19 was a full agonist at the MC1R (EC50 = 900 nM), was unable to stimulate the MC3R and MC4R at up to 100 μM concentrations, and resulted in partial MC5R activation at 100 μM concentrations (20%). The final Trp substitution, Ser (MDW1–6), resulted in a similar activity patter to MDW1–15, with full MC1R agonist activity (EC50 = 1600 nM), partial MC3R and MC5R activation at 100 μM concentrations (50% and 75%, respectively), and was unable to stimulate the MC4R.
While the Ac-His-dPhe-Arg-Trp-NH2 scaffold was used to examine single nucleotide polymorphisms at the His, Arg, and Trp positions due to the increased potency of using the dPhe compared to Phe, SNPs at the Phe position were examined as l isomers. As a control, the Ac-His-Phe-Arg-Trp-NH2 tetrapeptide (MDW1–88–3) was synthesized and characterized to possess full agonist efficacy at the MC1R, MC3R, MC4R, and MC5R with potencies of 3000, 1500, 2100, and 220 nM, respectively. The micromolar to submicromolar potencies of this all l-tetrapeptide are similar to the values of 2360, 960, 1210, and 70 nM previously reported using the AlphaScreen assay.20 Replacing the Phe with Leu, resulting in MDW1–88–1, yielded a tetrapeptide that partially stimulated the MC1R and MC5R at 100 μM concentrations (25% for both) and did not result in measurable agonist activity at concentrations up to 100 μM at the MC3R and MC4R. No agonist activity was observed when Phe was replaced with Val (MDW1–88–2) at any of the melanocortin receptors assayed (Figure 2).
All of the polymorphic tetrapeptides synthesized in the present study possessed decreased agonist potency at the melanocortin receptors relative to the control ligands. Since the control tetrapeptides were based on the common purported pharmacophore of the POMC-derived melanocortin agonists, and the all l-amino acid tetrapeptide represents the minimal active sequence in both the frog and lizard skin bioassays,10,11 modifications within this critical active domain were hypothesized to result in decreased agonist potency, supported by the results from this study. Incorporation of the amino acid alterations into the full-length agonists would confirm the biological relevance of these SNPs.
Three of the α-MSH SNPs synthesized in the tetrapeptide scaffold in the current study have previously been characterized. The His6Gln substitution, identified in a United Kingdom proband, was characterized at the MC4R to decrease binding affinity approximately 100-fold and to reduce the agonist efficacy approximately 2-fold relative to wildtype α-MSH, without modulating potency.12 The equivalent tetrapeptide, MDW1–22–3, also resulted in decreased efficacy at the MC4R, while also possessing 10-fold decreased potency at the MC4R relative to the Ac-His-dPhe-Arg-Trp-NH2 control ligand. The Phe7Leu substitution, identified in an obese French children cohort, decreased binding affinity and functional potency >100-fold at the MC4R compared to α-MSH.13 The corresponding tetrapeptide, MDW1–88–1, also decreased agonist potency, with no stimulation of the MC4R at concentrations up to 100 μM. The Arg8Cys polymorphism, reported in two French-Canadian families, decreased affinity 10-fold and 100-fold at the MC1R and MC4R, respectively, and functional potency >100-fold at both the MC1R and MC4R.14 The tetrapeptide MDW1–52, possessing the equivalent substitution, decreased potency 25- and 140-fold at the MC1R and MC4R, respectively. Thus, a decrease in melanocortin activity was previously observed for three SNPs in α-MSH. Similar decreases were observed when the SNPs were incorporated into tetrapeptide scaffolds in the present study, supporting the approach of screening melanocortin SNPs in a simplified tetrapeptide scaffold as a preliminary measure of receptor activity.
In addition to the published SNPs explored in α-MSH, the Trp to Leu substitution was also utilized in various bioassays. The [Leu9]α-MSH peptide was found to possess 4-, 50-, 500-, and 100-fold decreased potency compared to α-MSH in the Rana pipiens, Anolis carolinensis, Xenopus laevis, and Cloudman S-91 melanoma cell line bioassays.25,26 Similar large increases in the Xenopus laevis (>150,000-fold)27 have also been reported. This Leu substitution has also been demonstrated to alter protein phosphorylation patterns in S-91 cells28 and the grooming behavior when centrally administered in rats.26 The equivalent tetrapeptide, MDW1–15, decreased agonist potency 100-fold at the MC1R, similar to the ranges previously described. This further supports the use of the model tetrapeptide scaffold as a screening tool for examining SNPs in the hypothesized pharmacophore the POMC-derived agonists.
Different melanocortin receptors were differentially affected by SNP substitutions in the tetrapeptide scaffolds. In tetrapeptides possessing a dPhe in the second position, compounds possessed full agonist efficacy, with micromolar to submicromolar agonist potencies, at the MC1R. The majority of these compounds were able to result in at least a partial activation of the MC5R (MDW1–11 was inactive at the MC5R). Two compounds were full agonists at the MC4R (MDW1–52 and MDW1–48), while no SNP tetrapeptide was a full agonist at the MC3R. The position of the substitution also impacted the observed activities. Substitutions at the Arg position resulted in full or >80% partial agonist efficacy at the melanocortin receptors except for the Arg to Leu substitution (MDW1–44) at the MC3R that resulted in partial receptor activation at 100 μM concentrations. Previous work substituting a Nle residue at this position resulted in a compound with full agonist efficacy at the MC1R and a potency of 50 nM, similar to that observed for the Leu substitution in the present study, though the Nle was reported to be a full agonist at the MC3R and possess partial agonist efficacy at the MC4R and MC5R.29 Substitutions at the His position resulted in full agonist efficacy at the MC1R and greater than 80% efficacy at the MC5R, but decreased efficacy at the MC3R and MC4R. Replacement of the Trp retained MC1R full agonist efficacy but resulted in partial to no activation of the MC3R and MC5R, and none of these substitutions resulted in activity at concentrations up to 100 μM at the MC4R. Replacing the dPhe with Phe, resulted in a scaffold that was 10- to 100-fold less potent (comparing MDE9–150 to MDW1–88–3). Substitution of the Phe with Leu or Val resulted in no more than 25% receptor activation at 100 μM, demonstrating the importance of the aromatic group at this position.
The decreased potency and efficacy following incorporation of the SNP residues into the tetrapeptide scaffolds indicates that individuals with these polymorphisms may experience decreased melanocortin receptor signaling. Patients deficient in melanocortin signaling demonstrate several altered physiological conditions, including adrenal insufficiency, rapid and early onset weight gain, and potential pigmentation effects. Individuals possessing the SNPs included in the current Letter may therefore benefit from the therapeutic regimes for patients with insufficient POMC signaling. As an example, setmelanotide (an MC4R agonist) decreased the body weight in POMC deficient patients,30,31 without evidence of cardiovascular effects presented from previous clinically trials with MC4R agonists.32 This indicates one therapeutic avenue that mitigates a consequence of insufficient POMC signaling. The present work suggests a new population that may have deficiencies in POMC signaling and may benefit from pharmaceutical intervention.
In conclusion, the present study examined single nucleotide polymorphisms within the His-Phe-Arg-Trp tetrapeptide sequences of α-MSH/ACTH, β-MSH, and γ-MSH for functional effects on the melanocortin signaling pathway. The His, Arg, and Trp positions were examined in the Ac-His-dPhe-Arg-Trp-NH2 tetrapeptide scaffold. Two polymorphisms at the Phe position were examined in the Ac-His-Phe-Arg-Trp-NH2 tetrapeptide scaffold and were found to minimally activate or have no activity at up to 100 μM concentrations. Every SNP decreased potency and/or efficacy compared to the parent tetrapeptides, indicating these polymorphisms may decrease melanocortin signaling in the full-length POMC-derived agonists. Testing of these SNPs in the full-length molecules will confirm the biologically relevance of these SNPs, indicating a patient population that may benefit from intervention to address the deficiency in POMC-based receptor activation.
Acknowledgments
This work has been supported by NIH Grants R01DK091906, R01DK108893, and R01DK097838, and a 2017 Wallin Neuroscience Discovery Fund Award through the University of Minnesota (to C.H.-L.). M.D.E. was a recipient of an NIH F32 Postdoctoral Fellowship (F32DK108402).
Glossary
Abbreviations
- POMC
proopiomelanocortin
- SNP
single nucleotide polymorphism
- MCR
melanocortin receptor
- RP-HPLC
reverse-phase high pressure chromatography
- NDP–MSH
[Nle4,dPhe7]α-MSH
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.9b00198.
Protocols for peptide synthesis, biological assays, data analysis, summary of POMC-deficient signaling in humans, and peptide characterization (PDF)
Author Contributions
† M.D.W. and M.D.E. contributed equally to this work. M.D.E. and C.H.L. designed the research, M.D.W. synthesized the compounds with the assistance of M.D.E., K.T.F. performed the biologically assays, K.T.F. and M.D.E. analyzed the data, and M.D.W. and M.D.E. wrote the manuscript with the assistance of C.H.L.
The authors declare no competing financial interest.
Supplementary Material
References
- Chhajlani V.; Muceniece R.; Wikberg J. E. Molecular cloning of a novel human melanocortin receptor. Biochem. Biophys. Res. Commun. 1993, 195 (2), 866–873. 10.1006/bbrc.1993.2125. [DOI] [PubMed] [Google Scholar]
- Chhajlani V.; Wikberg J. E. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992, 309 (3), 417–420. 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- Mountjoy K. G.; Robbins L. S.; Mortrud M. T.; Cone R. D. The cloning of a family of genes that encode the melanocortin receptors. Science 1992, 257 (5074), 1248–1251. 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Gantz I.; Konda Y.; Tashiro T.; Shimoto Y.; Miwa H.; Munzert G.; Watson S. J.; DelValle J.; Yamada T. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 1993, 268 (11), 8246–8250. [PubMed] [Google Scholar]
- Gantz I.; Miwa H.; Konda Y.; Shimoto Y.; Tashiro T.; Watson S. J.; DelValle J.; Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993, 268 (20), 15174–15179. [PubMed] [Google Scholar]
- Roselli-Rehfuss L.; Mountjoy K. G.; Robbins L. S.; Mortrud M. T.; Low M. J.; Tatro J. B.; Entwistle M. L.; Simerly R. B.; Cone R. D. Identification of a receptor for γ melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc. Natl. Acad. Sci. U. S. A. 1993, 90 (19), 8856–8860. 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz I.; Shimoto Y.; Konda Y.; Miwa H.; Dickinson C. J.; Yamada T. Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200 (3), 1214–1220. 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- Griffon N.; Mignon V.; Facchinetti P.; Diaz J.; Schwartz J. C.; Sokoloff P. Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem. Biophys. Res. Commun. 1994, 200 (2), 1007–1014. 10.1006/bbrc.1994.1550. [DOI] [PubMed] [Google Scholar]
- Nakanishi S.; Inoue A.; Kita T.; Nakamura M.; Chang A. C.; Cohen S. N.; Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature 1979, 278 (5703), 423–427. 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Hruby V. J.; Wilkes B. C.; Hadley M. E.; Al-Obeidi F.; Sawyer T. K.; Staples D. J.; Devaux A. E.; Dym O.; Castrucci A. M. D.; Hintz M. F.; Riehm J. P.; Rao K. R. α-Melanotropin: The minimal active sequence in the frog-skin bioassay. J. Med. Chem. 1987, 30 (11), 2126–2130. 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- Castrucci A. M.; Hadley M. E.; Sawyer T. K.; Wilkes B. C.; Al-Obeidi F.; Staples D. J.; de Vaux A. E.; Dym O.; Hintz M. F.; Riehm J. P.; Rao K. R.; Hruby V. α-Melanotropin: The minimal active sequence in the lizard skin bioassay. Gen. Comp. Endocrinol. 1989, 73 (1), 157–163. 10.1016/0016-6480(89)90066-X. [DOI] [PubMed] [Google Scholar]
- Lee Y. S.; Challis B. G.; Thompson D. A.; Yeo G. S.; Keogh J. M.; Madonna M. E.; Wraight V.; Sims M.; Vatin V.; Meyre D.; Shield J.; Burren C.; Ibrahim Z.; Cheetham T.; Swift P.; Blackwood A.; Hung C. C.; Wareham N. J.; Froguel P.; Millhauser G. L.; O’Rahilly S.; Farooqi I. S. A POMC variant implicates β-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006, 3 (2), 135–40. 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dubern B.; Lubrano-Berthelier C.; Mencarelli M.; Ersoy B.; Frelut M. L.; Bougle D.; Costes B.; Simon C.; Tounian P.; Vaisse C.; Clement K. Mutational analysis of the pro-opiomelanocortin gene in french obese children led to the identification of a novel deleterious heterozygous mutation located in the alpha-melanocyte stimulating hormone domain. Pediatr. Res. 2008, 63 (2), 211–216. 10.1203/PDR.0b013e31815ed62b. [DOI] [PubMed] [Google Scholar]
- Samuels M. E.; Gallo-Payet N.; Pinard S.; Hasselmann C.; Magne F.; Patry L.; Chouinard L.; Schwartzentruber J.; Rene P.; Sawyer N.; Bouvier M.; Djemli A.; Delvin E.; Huot C.; Eugene D.; Deal C. L.; Van Vliet G.; Majewski J.; Deladoey J.; Consortium F. C. Bioinactive ACTH causing glucocorticoid deficiency. J. Clin. Endocrinol. Metab. 2013, 98 (2), 736–742. 10.1210/jc.2012-3199. [DOI] [PubMed] [Google Scholar]
- Biebermann H.; Castaneda T. R.; van Landeghem F.; von Deimling A.; Escher F.; Brabant G.; Hebebrand J.; Hinney A.; Tschop M. H.; Gruters A.; Krude H. A role for β-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 2006, 3 (2), 141–146. 10.1016/j.cmet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Buono P.; Pasanisi F.; Nardelli C.; Ieno L.; Capone S.; Liguori R.; Finelli C.; Oriani G.; Contaldo F.; Sacchetti L. Six novel mutations in the proopiomelanocortin and melanocortin receptor 4 genes in severely obese adults living in Southern Italy. Clin. Chem. 2005, 51 (8), 1358–1364. 10.1373/clinchem.2005.047886. [DOI] [PubMed] [Google Scholar]
- Haskell-Luevano C.; Holder J. R.; Monck E. K.; Bauzo R. M. Characterization of melanocortin NDP–MSH agonist peptide fragments at the mouse central and peripheral melanocortin receptors. J. Med. Chem. 2001, 44 (13), 2247–52. 10.1021/jm010061n. [DOI] [PubMed] [Google Scholar]
- Ericson M. D.; Schnell S. M.; Freeman K. T.; Haskell-Luevano C. A fragment of the Escherichia coli ClpB heat-shock protein is a micromolar melanocortin 1 receptor agonist. Bioorg. Med. Chem. Lett. 2015, 25 (22), 5306–5308. 10.1016/j.bmcl.2015.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lensing C. J.; Freeman K. T.; Schnell S. M.; Adank D. N.; Speth R. C.; Haskell-Luevano C. An in vitro and in vivo investigation of bivalent ligands that display preferential binding and functional activity for different melanocortin receptor homodimers. J. Med. Chem. 2016, 59 (7), 3112–3128. 10.1021/acs.jmedchem.5b01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Tala S. R.; Flores V.; Freeman K.; Haskell-Luevano C. Synthesis and pharmacology of α/β3-peptides based on the melanocortin agonist Ac-His-dPhe-Arg-Trp-NH2 sequence. ACS Med. Chem. Lett. 2015, 6 (5), 568–572. 10.1021/acsmedchemlett.5b00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schioth H. B.; Chhajlani V.; Muceniece R.; Klusa V.; Wikberg J. E. Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sci. 1996, 59 (10), 797–801. 10.1016/0024-3205(96)00370-0. [DOI] [PubMed] [Google Scholar]
- Sawyer T. K.; Sanfilippo P. J.; Hruby V. J.; Engel M. H.; Heward C. B.; Burnett J. B.; Hadley M. E. 4-Norleucine, 7-D-phenylalanine-α-melanocyte-stimulating hormone - a highly potent α-melanotropin with ultralong biological-activity. Proc. Natl. Acad. Sci. U. S. A. 1980, 77 (10), 5754–5758. 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering S. R.; Freeman K. T.; Schnell S. M.; Haslach E. M.; Dirain M.; Debevec G.; Geer P.; Santos R. G.; Giulianotti M. A.; Pinilla C.; Appel J. R.; Speth R. C.; Houghten R. A.; Haskell-Luevano C. Discovery of mixed pharmacology melanocortin-3 agonists and melanocortin-4 receptor tetrapeptide antagonist compounds (TACOs) based on the sequence Ac-Xaa(1)-Arg-(pI)dPhe-Xaa(4)-NH2. J. Med. Chem. 2017, 60 (10), 4342–4357. 10.1021/acs.jmedchem.7b00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. R.; Bauzo R. M.; Xiang Z. M.; Haskell-Luevano C. Structure-activity relationships of the melanocortin tetrapeptide Ac-His-dPhe-Arg-Trp-NH2 at the mouse melanocortin receptors. 1. Modifications at the His position. J. Med. Chem. 2002, 45 (13), 2801–2810. 10.1021/jm0104872. [DOI] [PubMed] [Google Scholar]
- Eberle A.; Schwyzer R. Divergent melanophore-dispersing and tyrosinase-stimulating activity of synthetic leucine9-α-melanotropin. Helv. Chim. Acta 1979, 62 (7), 2452–9. 10.1002/hlca.19790620740. [DOI] [Google Scholar]
- Spruijt B. M.; De Graan P. N. E.; Eberle A. N.; Gispen W. H. Comparison of structural requirements of α-MSH and ACTH for inducing excessive grooming and pigment dispersion. Peptides 1985, 6 (6), 1185–9. 10.1016/0196-9781(85)90448-6. [DOI] [PubMed] [Google Scholar]
- De Graan P. N. E.; Molenaar R.; Van de Veerdonk F. C. G. A new in vitro melanophore bioassay for MSH using tail-fins of Xenopus tadpoles. Mol. Cell. Endocrinol. 1983, 32 (2–3), 271–84. 10.1016/0303-7207(83)90088-6. [DOI] [PubMed] [Google Scholar]
- Eberle A. N.; De Graan P. N. E.; Brussaard A. B.; Gamboni G.; Siegrist W.; Girard J. Phosphorylation changes of a 34 kDa membrane protein of melanoma cells by α-melanocyte-stimulating hormone and structural analogs. Biochem. Soc. Trans. 1986, 14 (6), 1106. 10.1042/bst0141106. [DOI] [Google Scholar]
- Mowlazadeh Haghighi S.; Zhou Y.; Dai J.; Sawyer J. R.; Hruby V. J.; Cai M. Replacement of Arg with Nle and modified D-Phe in the core sequence of MSHs, Ac-His-D-Phe-Arg-Trp-NH2, leads to hMC1R selectivity and pigmentation. Eur. J. Med. Chem. 2018, 151, 815–823. 10.1016/j.ejmech.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnen P.; Clement K.; Wiegand S.; Blankenstein O.; Gottesdiener K.; Martini L. L.; Mai K.; Blume-Peytavi U.; Gruters A.; Krude H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 2016, 375 (3), 240–246. 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- Clement K.; Biebermann H.; Farooqi I. S.; Van der Ploeg L.; Wolters B.; Poitou C.; Puder L.; Fiedorek F.; Gottesdiener K.; Kleinau G.; Heyder N.; Scheerer P.; Blume-Peytavi U.; Jahnke I.; Sharma S.; Mokrosinski J.; Wiegand S.; Muller A.; Weiss K.; Mai K.; Spranger J.; Gruters A.; Blankenstein O.; Krude H.; Kuhnen P. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018, 24, 551–555. 10.1038/s41591-018-0015-9. [DOI] [PubMed] [Google Scholar]
- Greenfield J. R.; Miller J. W.; Keogh J. M.; Henning E.; Satterwhite J. H.; Cameron G. S.; Astruc B.; Mayer J. P.; Brage S.; See T. C.; Lomas D. J.; O’Rahilly S.; Farooqi I. S. Modulation of blood pressure by central melanocortinergic pathways. N. Engl. J. Med. 2009, 360 (1), 44–52. 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.