Abstract
Historically, the gold standard for evaluation of cancer therapeutics, including medical devices, has been the randomized clinical trial. Although high-quality clinical data are essential for safe and judicious use of therapeutic oncology devices, class II devices require only preclinical data for US Food and Drug Administration approval and are often not rigorously evaluated prior to widespread uptake. Herein, we review master protocol design in medical oncology and its application to therapeutic oncology devices, using examples from radiation oncology. Unique challenges of clinical testing of radiation oncology devices (RODs) include patient and treatment heterogeneity, lack of funding for trials by industry and health-care payers, and operator dependence. To address these challenges, we propose the use of master protocols to optimize regulatory, financial, administrative, quality assurance, and statistical efficiency of trials evaluating RODs. These device-specific master protocols can be extrapolated to other devices and encompass multiple substudies with the same design, statistical considerations, logistics, and infrastructure. As a practical example, we outline our phase I and II master protocol trial of stereotactic magnetic resonance imaging–guided adaptive radiotherapy, which to the best of our knowledge is the first master protocol trial to test a ROD. Development of more efficient clinical trials is needed to promote thorough evaluation of therapeutic oncology devices, including RODs, in a resource-limited environment, allowing more practical and rapid identification of the most valuable advances in our field.
The gold standard for evaluating new therapeutic advances is a randomized clinical trial. Given their importance in medical investigation, frameworks for understanding cost-efficient and time-efficient trial design (1–3), ethical evaluation (4), and interpretation for regulatory and reimbursement considerations (5) have been established for these types of studies. However, in recent years, next-generation master protocol clinical trials in medical oncology have emerged as a potentially more efficient way to study new therapies (6). These study designs diverge from the traditional clinical trial design, with new sets of assumptions, multiple arms, and often the lack of a standard-of-care arm.
In contrast, although technological innovations are producing increasingly advanced and complex therapeutic oncology devices, including radiation oncology devices (RODs), these devices are often not evaluated rigorously until many years after their clinical uptake, if at all (7). The current environment of increased scrutiny of medical devices (8), limited health-care resources, and the new Centers for Medicare and Medicaid Services Innovation Center radiation oncology alternative payment model, which bundles reimbursement of radiotherapy services into a cancer-specific flat rate, necessitates identification of the most high-value oncology technologies (8–10). Master protocol trial design is an exciting avenue to more rapidly investigate emerging therapeutic oncology devices and their most clinically relevant uses. There are several evolving and emerging RODs, such as the magnetic resonance imaging–guided linear accelerator (MR-Linac) (11,12), proton and heavy ion therapy (13,14), ultra-high dose rate (FLASH) radiotherapy (15,16), positron emission–guided radiotherapy (17), and artificial intelligence–assisted radiation-planning software (18) that could be more efficiently and standardly evaluated using a similar master protocol trial design. Herein, we review existing master protocol trial design in medical oncology, discuss the unique challenges of clinical trials of RODs, and introduce master trial design as an avenue to efficiently and rigorously test new RODs in the clinic. As an example, we present our institution’s master protocol for the evaluation of stereotactic magnetic resonance imaging–guided adaptive radiotherapy using the MR-Linac, which is the first master protocol trial for RODs.
Lessons From Next-Generation Clinical Trial Design in Medical Oncology
Master Protocol Trials in Medical Oncology
Classically, chemotherapies have been evaluated in a cancer type–specific and stage-specific manner with sequential phase I, phase II, and phase III trials. However, the past two decades have brought a deeper understanding of tumor heterogeneity and molecular drivers of oncogenesis, followed by rapid development of new therapeutic drugs targeted to specific molecular alterations (19). Evaluating targeted agents in an unselected population, or in the absence of reliable biomarker assays, decreases the effect size and power of a trial. Although individual testing of single agents in their targeted phenotype ameliorates this issue, it is nonetheless resource intensive and has limited feasibility, especially in rare patient subpopulations (20). To address these issues, master protocol trials have been developed and implemented to study targeted agents in the face of increasing patient and tumor heterogeneity.
In medical oncology, a master protocol trial refers to a general protocol that evaluates several subtypes of cancer and/or several subtypes of their targeted therapies simultaneously (21). Several subprotocols exist under the master protocol, evaluating different hypotheses concurrently. Within oncology, master protocol trials can be either histology specific or histology agnostic and can test one or several targeted agents. After registration, patients are eligible only for specific substudies based on specific tumor characteristics, such as tumor type, histology, or molecular markers. Common master protocol trial designs are umbrella trials, basket trials, and platform trials. These designs have been previously reviewed in the literature (6,21,22); their key features and examples of trials employing these designs are shown in Table 1. Importantly, the US Food and Drug Administration (FDA) has released draft guidance for industry on its approach to evaluating cancer drug therapies in the context of master protocol trials, acknowledging the growing importance and relevance of these trials for drug development and approval (23).
Table 1.
Master protocols in medical oncology*
| Trial type | Definition | Disease | Example |
|---|---|---|---|
| Basket | Trial of a single targeted agent for multiple diseases (or disease subtypes) | Histology-agnostic, molecular marker–specific | |
| Umbrella | Trial of multiple targeted therapies for one disease | Histology-specific, molecular marker–specific | |
| Platform | Ongoing trial of multiple targeted therapies for one disease with no set stopping date, with removal and addition of therapies based on interim assessment during trial | Histology-specific, molecular marker–specific | I-SPY 2, Barker et al., 2009 (29) |
Adapted from (21).
Representative Basket, Umbrella, and Platform Trials in Medical Oncology
Representative Basket Trial—BRAF V600. BRAF V600 was an early phase II, histology-agnostic basket trial evaluating vemurafenib, the selective BRAF V600 inhibitor, in patients with BRAF V600 mutation-positive nonmelanoma cancers (24). This trial included eight cancer type–specific cohorts, an “all others” cohort, and no control arm. The goal of this study was to identify tumor cohorts with promising antitumor activity that could then be pursued with subsequent new studies or with amendment and increased enrollment on the BRAF V600 protocol. This study led the FDA to approve vemurafenib for the treatment of BRAF V600–mutant Erdheim-Chester disease, the first FDA approval of an indication based on results of a cancer type–agnostic, biomarker-specific basket trial.
Representative Umbrella Trial—Lung-MAP. Lung-MAP is an ongoing phase II and III histology-specific umbrella trial evaluating multiple targeted agents with their corresponding molecular markers in squamous cell lung cancer (33). In this trial, patients’ tumor samples undergo centralized biomarker screening prior to randomization and then are assigned to a biomarker-specific substudy randomizing to the investigational targeted agent or standard of care. If screening reveals no targeted biomarkers with an available investigational agent, patients are enrolled into a “nonmatch” substudy. All substudies share the same protocol design and underlying statistical assumptions. Substudies can be added to the protocol when there is adequate rationale for a new agent-biomarker pairing, as determined by a multidisciplinary committee. Based on results of futility analysis of the phase II portion, substudies can be rapidly closed or seamlessly moved on to a confirmatory phase III study.
Representative Platform Trial—I-SPY 2. I-SPY-2 is a phase II, multicenter histology specific and biomarker-specific adaptive platform trial evaluating multiple investigational neoadjuvant therapies for high-risk, locally advanced breast cancer (29). In contrast to an umbrella trial such as Lung-MAP, platform trials integrate seamless removal and addition of therapies based on planned interim analyses, often using Bayesian decision rules (30,31). In I-SPY 2, patients undergo biomarker testing to assess eligibility and to determine their investigational molecular signature subtype. Based on these results, patients are stratified by HER2 status and randomly assigned to standard of care with or without one of five investigational agents. I-SPY 2 uses pathological complete response as the primary outcome measure. This trial uses Bayesian methods of adaptive randomization so that promising agents for a molecular signature are preferentially assigned to patients with that signature and are progressed more rapidly (30). Drugs are graduated or dropped for futility when the Bayesian predictive probability of success in a phase III trial drops sufficiently high or low, respectively (30). If an investigational agent graduates, the Bayesian predictive probability for each agent-signature pair is shared with the drug company to inform rational development of confirmatory phase III trials (32). As in Lung-MAP, new investigational drugs can be added when other drugs are dropped or graduated. Investigational agents are selected by an independent expert committee based on clinical and preclinical data.
Implications of Master Protocol Trial Design for Trial Efficiency and Regulation
It has been argued that master protocol trials may enhance clinical trial efficiency (20,21,31,34–36). By decreasing patient heterogeneity within a substudy, patients are more likely to be treated with the most rationally targeted agent based on their disease’s biology. Furthermore, substudy design, statistical analysis, and trial infrastructure are shared between arms, decreasing the regulatory, statistical, and administrative burden of running large clinical trials, ultimately reducing trial time and cost (21,31,35). Protocols that allow for the addition or removal of targeted agents and/or biomarkers based on interim evaluation of efficacy can decrease regulatory and administrative delays down the line in the study. Additionally, aggregation of data from substudies with similar results may allow conclusions to be reached more quickly and with fewer patients (34,36). An important feature of many of these studies is an emphasis on up-front regulatory considerations and discussions with regulatory agencies, with a goal to streamline protocol adjustments and, ultimately, US FDA approval of the investigational agents (31,35). Overall, these trial designs may allow more rapid identification, selection, and approval of effective tumor-drug combinations.
Standards and Shortcomings of ROD Evaluation
Just as for oncology drugs, phase I, II, and III trials have been the nominal standard for evaluating novel RODs. However, in contrast to oncology drugs, many RODs are adopted in the absence of prospective, level I evidence (37,38). This is largely because oncology drugs and RODs have different FDA approval pathways, driving differences in clinical implementation and standards of evaluation. RODs are classified as medium-risk, or class II, devices and as such are approved via the premarket notification (510[k]) pathway based on a finding of “substantial equivalence” to predicate devices and require only preclinical supporting data (39). Although this designation facilitates rapid development and dissemination of novel technology, the lack of careful clinical evaluation has come at some cost to our patients and society. Although classified as medium risk, RODs have the capacity to inflict substantial harm in the event of an error leading to undertreatment or toxicity. There is a need for more thorough clinical evaluation of new ROD technologies to demonstrate the value of radiation treatments and to minimize patient harm.
As a field, we have often assumed the superiority of a novel ROD based on technical and physical parameters without clinical evidence. For example, intensity modulated radiotherapy (IMRT) was widely adopted for the treatment of head and neck squamous cell carcinoma prior to prospective studies showing a clinical benefit (40). Prospective studies following this adoption have subsequently provided evidence for improved toxicity reduction with IMRT, supporting this assumption (41–44). In contrast, other ROD trials have produced surprising results. For example, the first prospective randomized trial comparing proton therapy vs IMRT was recently reported. This study evaluated radiation modality in locally advanced non–small cell lung cancer and showed lower low-dose but higher high-dose lung exposure on the proton arm, with no differences in clinical endpoints (45). There is a concern that comparison of dose plans between different RODs may compromise clinical equipoise, complicating the ethical considerations of these studies (37). However, as illustrated in this trial, our understanding of the true physical and clinical properties of new RODs is often based on inaccurate assumptions drawn from older technology. Premature widespread implementation of these high-cost therapies potentially leads to excess health-resource use without adding meaningful clinical value (46,47).
A careful examination of ROD adverse event and recall rates provides further evidence that more careful ROD evaluation is needed (48,49). An analysis of the FDA’s postmarket surveillance database from 1991 to 2015 revealed 4234 ROD adverse event reports, about half of which involved external beam therapy devices (48). There were 103 individual deaths reported because of these errors, 22 of which were clearly due to a ROD. Concerningly, the rate of adverse events has increased over time (48). Additionally, between 2003 and 2012, linear accelerators were the most commonly recalled medical devices, and the rate of recalls has increased over time (49,50).
Two high-profile cases of radiotherapy errors leading to patient mortality, both largely attributed to software errors leading to inappropriate treatment delivery, have increased public scrutiny of our field and underline the need for systematic evaluation of RODs as ever more complexity is introduced into our devices (51). In addition to the imperative to provide high-quality care to our patients, these incidents have been widely reported by the lay media and may contribute to a deterioration of trust in radiation oncology (51).
These challenges are not limited to RODs (52). For example, liver tumor radiofrequency ablation achieved FDA approval after its ability to destroy liver and tumor tissue was demonstrated, and it was clinically adopted in advance of clinical data assessing efficacy (53,54). Recently, robotically assisted surgical devices, also classified as class II devices by the FDA, were rapidly adopted for several oncologic applications prior to efficacy assessment, including gynecologic cancers, genitourinary cancers, gastrointestinal cancers, and breast cancers. FDA evaluation of these devices in general was based on surgical complication rates at 30 days, and not oncologic endpoints. A noninferiority trial comparing open radical hysterectomy to robotically assisted radical hysterectomy in women with early-stage cervical cancer showed lower disease-free and overall survival in the robotically assisted surgery arm (55). In response, the FDA published a safety communication recommending caution when using robotically assisted surgical devices for mastectomy and any cancer-related therapy (56). Although evaluation of each therapeutic oncology device has unique efficacy and safety concerns, many of the regulatory, trial funding, and user-dependency challenges are similar. Thus, experiences from ROD evaluation can inform optimal evaluation of therapeutic oncology devices in general.
Unique Challenges in Clinical Evaluation of RODs
RODs have several key differences from oncology drugs that complicate their prospective clinical evaluation. First, oncology drugs are often initially evaluated in the metastatic setting and then tested in the curative setting once the drug is shown to be safe and effective. However, this logic is not applicable to evaluating RODs because of the heterogeneity of treatment paradigms and techniques in the curative vs palliative and/or metastatic settings. Also, whereas tumor heterogeneity is a key concern in oncology drug trials, patient-specific and cancer type–specific heterogeneity is perhaps a larger concern in radiation oncology because each plan is individualized for each patient’s disease, anatomy, and comorbidities. Further, given the rapid pace of development and the frequently long latency period for toxicities and oncologic endpoints, technologies are often obsolete by the time a long-term study is complete, limiting its interpretability and utility (57).
A consequence of the premarket notification pathway is that ROD manufacturers are disincentivized to sponsor clinical studies of new RODs. Indeed, radiotherapy trials are less likely to be sponsored by industry than other oncology trials (58). Without investment in clinical trials to evaluate these advances, our field often lacks prospective data demonstrating value, often leading to insurers denying payment for novel technologies. Because novel RODs on trial are often not paid for by the participant's insurance company, a vicious cycle is created by which lack of insurance coverage leads to poor accrual to clinical trials and an inability to produce timely, high-quality evidence for that device (59,60). For example, trials evaluating proton therapy have been severely limited by this challenge. In a large phase III trial comparing proton therapy to IMRT in localized prostate cancer, approximately 30% of eligible patients were unable to participate because of restrictive insurance coverage (61). A consequence of this flaw in ROD trial funding is that study populations may be biased toward wealthier patients and older patients with Medicare, limiting generalizability and potentially contributing to health-care disparities (61). As efforts to reduce health-care costs escalate, clinicians and manufacturers will need to demonstrate the value of a novel ROD with efficient, rationally designed trials.
Operator differences can introduce heterogeneity and limit interpretation, especially for multi-institutional trials. Because ROD use is operator dependent, safe and effective delivery requires adequate staff training and expertise. This is highlighted by the fact that between 1991 and 2015, 20% of ROD FDA adverse events were due to user error (48). Quality assurance is a critical challenge, which should be addressed with robust user training and prospective cross-site quality assurance in future trials (62). Additionally, simulation studies, such as phantom studies, dosimetric studies, and healthy volunteer “dry-run” studies, are essential to the early evaluation of any new ROD to understand both how to safely use the ROD and in which types of patients it may provide the most benefit (63).
A structural barrier to the evaluation of RODs is the immense institutional investment in infrastructure, equipment, and personnel required for many new RODs, particularly treatment-delivery and treatment-planning machines (59). This limits availability of the device, hampering trial accrual. Further, once these RODs are in place, institutions understandably wish to use these devices to their full potential. Consequently, investigators and institutions may be disincentivized to carry out trials comparing new RODs to older devices. Outcomes of novel RODs, therefore, are often compared with historical controls or outcomes from another institution with a different standard technique, introducing bias into the evaluation. In an age of ever-growing patient-level electronic health records, a practical avenue to address these biases is the use of real-world and prior clinical trial data as a comparator (64–67). With this approach, it would be reasonable to pool data from patients treated with any ROD that is standard of care at the time of a trial as a comparator for the experimental ROD, maximizing generalizability and providing a more pragmatic understanding of the benefit of the new technology.
Prioritizing reimbursement over early clinical evaluation comes at a long-term cost when insurance companies become reluctant to reimburse these RODs without clinical evidence of their value, as is currently occurring with proton therapy (68,69), making it difficult to use these devices for patients who may benefit most. Policy and pricing reforms, such as reference pricing for proton therapy, have been proposed to mitigate these issues (70). Perhaps most important, the new radiation oncology alternative payment model value-based bundled payment model rewards cost reduction via hypofractionation and delivery of more effective, less toxic treatments, which may be better achieved with emerging RODs, but which can be clearly demonstrated only via clinical investigation (9,10). A commitment to trials that establish the value and indications for cutting-edge RODs is needed for the field’s continued advancement.
The above challenges limit the implementation and interpretability of large clinical trials of RODs. Given the clinical importance of quality radiotherapy and the potential harm of unsafe RODs, more efficient means to rapidly identify high-value innovations in radiation oncology are needed. With pragmatic and careful clinical evaluation of RODs, we can provide our patients high-quality evidence-based care, accurately demonstrate the value of high-cost RODs to health-care payers, rationally distribute RODs to the patients who stand to benefit most from them, and determine the most promising avenues for future investigation and advancement.
Criteria for Prospective Evaluation of RODs
The definition of a new ROD is not clearly defined by the current 510(k) clearance process. As a result, predicate creep, or incremental changes in devices that are each deemed substantially equivalent to the preceding device but ultimately lead to a very different device from the one that initially received FDA approval, can occur without requiring a new 510(k) approval (71). A clearer definition is needed to guide clinical evaluation and regulatory standards. We believe that any ROD that has the potential to affect clinical outcome or safety; results in substantial alteration in standard-of-care processes and/or procedures; results in substantial cost escalation; and/or lacks clinical data to suggest improved efficacy, reduced toxicity, increased efficiency, or improved safety compared with standard-of-care merit prospective clinical evaluation. Such RODs may include hardware such as simulation scanners and linear accelerators and software such as treatment-planning systems and newer artificial intelligence–based systems that automate existing workflow.
Novel Clinical Trial Designs in Radiation Oncology
Several frameworks to more rigorously evaluate RODs in the clinical trial setting have been proposed. The model-based approach was initially proposed for evaluation of proton therapy but can be used to evaluate any new ROD (37,72). In this method, radiation plans using both the standard modality and the new RODs are calculated, and expected toxicities are calculated and compared using the normal tissue complication probability model (72). Although this paradigm allows rapid introduction of new ROD into clinic, there is no prospective comparative component and so selection bias presents limitations. Further, this approach evaluates only toxicity, not clinical effectiveness. Finally, normal tissue complication probability models are not clinically validated, and the model-based system assumes that all potential toxicity and safety concerns of the novel RODs are known a priori.
More recently, Verkooijen et al. (63) have proposed the R-IDEAL (Radiotherapy — Idea, Development, Exploration, Assessment, and Long-term evaluation) framework as a practical system to rigorously investigate RODs. R-IDEAL was adapted from the surgical IDEAL system for evaluation of surgical innovations (73) and altered to address specific concerns regarding RODs. In a key departure from the traditional phase I, II, and III models, randomization is preferably moved to the exploration phase to better control for patient and tumor heterogeneity and the challenges of comparing with historical controls. The authors describe five stages of assessment: stage 0 (radiation predicate studies), preclinical studies to determine how and in what stetting the ROD may be used; stage I (idea), structured case reports for proof of concept; stage 2a (development), prospective, small case series for ROD refinement, feasibility, and safety assessment; stage 2 b (exploration), prospective studies with a preferably randomized component to determine early effectiveness; stage 3 (assessment), randomized, controlled trials to determine effectiveness; and stage 4 (long-term evaluation), prospective registries to assess long-term outcomes. This approach is very comprehensive; however, practical financial, patient number, and time constraints may limit its ability to be implemented rapidly in a broad range of technologies.
Master Protocol Trial Design in Radiation Oncology
Master protocol trials in radiation oncology refer to single cancer type–agnostic protocols encompassing multiple cancer type–specific substudies with the same design, statistical considerations, logistics, and infrastructure. Because the technical considerations of implementing a ROD is a substantial challenge, but often largely independent of the clinical indication, master protocols may be particularly well suited to efficient ROD evaluation. The general eligibility, treatment techniques, quality assurance, and clinical assessment are specified by the master protocol. More specific eligibility criteria, treatment specifications, and disease-specific assessment will be detailed separately for each substudy. Our hope is that these protocols will be multi-institutional, hastening accrual and maximizing generalizability. The ability to pool select endpoints across different cancer types that share similar locations and thus radiation technique and toxicity considerations (eg, all tumors in the upper abdomen, with similar abdominal organ motion due to respiration and gastrointestinal motility and nearby dose-limiting structures) can allow more rapid assessment of feasibility and safety of treating tumors with these properties as elaborated below.
In radiation oncology, the pertinent clinical question is often not whether the new technology is better, but to what extent, in which indications, and whether it reduces toxicity (57). Addressing this question has previously been limited by heterogeneity of treatment era, patient selection, and radiation delivery and quality within and across trials. Master protocol trials testing several clinical indications in a standardized fashion may allow for more rapid and direct comparisons of different technologies, allowing us to better understand the true value of the technology. Master protocols should be written to allow for efficient addition or removal of substudies as knowledge about the ROD is developed or new clinical questions emerge, enabling more rapid identification of the best indications for a new ROD as more is learned about the technology.
A well-designed master protocol should address, and has the potential to improve, the key concern of quality assurance and user competency in RODs. A critical difference between trials of drug therapies and RODs is the importance of operator competency and aptitude with the ROD. In the past, inadequate user training and quality assurance has compromised outcomes and confounded interpretation, limiting our ability to directly compare technologies (74,75). A master protocol should both serve as a teaching document and include prospective quality assurance to standardize techniques and ensure high-skill implementation of the new ROD. Credentialing programs to ensure consistent, quality treatment delivery on trial have been shown to be an effective and important avenue for educating providers about high-quality application of a new ROD indication (62). Incorporating training and quality assurance procedures into the master protocol minimizes the resources needed per substudy compared with traditional trial design and maximizes standardization. This is particularly valuable for studies of rare indications for which intensive credentialing programs may be most important but not economically realistic with traditional study design. Umbrella training and credentialing also simplifies the addition of new substudies across multiple study sites, further adding to the efficiency of completing these trials.
Although not used in the below example, a randomized phase II trial could be a particularly efficient design for rapid identification of the most promising therapies while avoiding the challenges of comparison to a historical control. An appealing approach that takes full advantage of the efficiencies of master protocols is randomization to one of several new technologies, with a “pick-the-winner” approach to identify and prioritize the most promising new RODs for investigation in a phase III trial. Unequal randomization to the experimental ROD arm(s) vs standard-of-care technology would maximize experience and the understanding of the new technology. The efficiency of a 2:1 randomization is approximately 50% (ie, the 2:1 randomization design does not require a much larger sample size compared with a 1:1 randomization) (76,77). These strategies may mitigate the practical concern that patients often seek novel technology and may choose to not enroll in studies in which they could be randomly assigned to standard of care. Of note, although randomization to one of several novel RODs would be an exciting avenue to rapidly compare many new devices simultaneously, this may be limited by availability of each of these new devices within and across institutions, potentially hampering patient enrollment. In multisite studies, standardized and thorough quality assurance will be particularly critical (62). An alternative solution is to design an externally controlled trial in which real-world data and/or data from prior clinical trials are used as a comparator arm (67). Efforts to aggregate real-world data on MR-Linac are ongoing and will be a valuable resource in the future (78).
Although the current standard of prospectively evaluated RODs after widespread clinical implementation hastens introduction of novel technology, history has shown that our assumptions of a value of a new technology may not always translate into clinical benefit and can sometimes cause harm. Further, limitations in evaluation of these devices hamper our ability to accurately determine their safety and effectiveness. In the long run, this approach can both clinically and financially disincentivize the use of a novel ROD because of the lack of data demonstrating value. The goal of master protocol trials in radiation oncology is not to slow innovation, but rather to allow more efficient and reliable evaluation and identification of high-value innovations for the most appropriate clinical indications. We believe that this will ultimately push our field not just forward but also in the right direction to optimize patient care and value for the health-care system.
Ethical Considerations and Imperatives in Master Protocol Trials of RODs
Although we acknowledge the above-stated ethical concern of lack of equipoise when comparing dosimetric plans deliverable by two RODs when prescription dose is held constant (37), history has shown that our assumptions do not always translate into clinically perceptible differences (45,79). Additionally, the history of safety issues with rapid implementation of new technologies raises the concern of maleficence and underscores the ethical importance of careful clinical evaluation to ensure the risks of a therapy do not outweigh the benefits (51). Particularly when considering high-cost therapies, justice must also be considered because health resources are limited and should be rationally distributed (80). In light of these concerns, we believe that in most settings, high-quality clinical evaluation of ROD is ethically necessary. Master protocol trials can address the above ethical considerations by minimizing patient burden via decreasing the number of patients needed to assess for safety and by allowing for faster, more resource-efficient evaluation and identification of the optimal uses for new RODs.
Statistical Considerations in Master Protocol Trials of RODs
There are multiple possibilities for efficient yet flexible statistical design in master protocol arrangements, with the complexity varying depending on the overarching goals of the trial. To provide illustration and also contrast, consider master protocols aimed at early feasibility vs those targeting efficacy testing as their primary goal. In an early-feasibility design, especially of the type consistent with ROD research, the master protocol could provide omnibus null and alternative hypotheses that apply to each of the subprotocols. For example, a master protocol aimed at establishing feasibility of treatment delivery in at least 90% of patients could easily apply across diseases and would require an efficiently small number of patients within each subprotocol (10 to 12, for example). The advantages to this design are commonality of statistical approach, simplicity in data collection and study calendars, and simple statistical decision making, allowing the protocol to easily scale to other diseases added at a later date through straightforward amendments. Another important advantage is the ability to pool participants across the subprotocols for other, perhaps secondary, endpoints. For example, assessing the precision around estimating adverse events benefits from pooling across subprotocols. In a given subprotocol with 10 patients, the 90% confidence interval on a binary adverse event has maximal width of 55.5%, which is fairly broad but pooling across four subprotocols (n = 40) reduces that width to half: 27.8%. Thus, from a statistical perspective, borrowing strength across the subprotocols can have real advantages and can enable identification of serious adverse events that would not have been identified in smaller, single-arm studies before widespread adoption of the ROD.
Contrast the feasibility design with that of establishing efficacy in a phase II context. Imagine a ROD design for which local disease control is being considered as the overarching endpoint of the master protocol. The statistical designs necessary for subprotocols are not so straightforward in this case. Statistical complications arise because the appropriate standard-of-care null hypotheses may vary considerably by disease location, that is, timing of established standard cancer control rates could be variable. Other nontrivial statistical factors come into play as well: variable rates of distant metastasis development across diseases, subsequent and/or concurrent systemic therapy and so forth, all lead to complexity and individuality within each substudy, not the least of which are the thornier statistical issues of competing risks that likely will vary by disease. The result for this sort of master protocol will be that the statistical designs in the efficacy testing realm likely will lead to separate within-subprotocol requirements for sample sizes, study calendars, data collection, and even primary statistical analyses. For example, early interim evaluations might be practical in some diseases but not in others. Complexity and variability in statistical design of efficacy subprotocols also would be expected to limit the practicality of pooling participants for secondary endpoints, again in contrast to the feasibility and toxicity examples discussed earlier.
Example of Master Protocol Trial Design for RODs: Evaluation of Stereotactic MR-Guided Adaptive Radiotherapy
An emerging ROD is the MR-Linac, a novel radiotherapy-delivery system that merits prospective evaluation based on its potential to affect safety and outcomes, its high cost, and its considerable differences from standard treatment practices and procedures. The MR-Linac platforms include both a technological leap in hardware and advances in software that disrupt this historical paradigm by allowing real-time, online adaptation of the radiation treatment plan (11,12). The improved soft-tissue contrast of MR imaging may allow for more accurate radiation planning and delivery and hence minimize undertreatment and overtreatment, potentially translating to better disease control and fewer toxicities (81,82). There is clinical interest in using the MR-Linac in many disease sites; however, given the novelty of the device, its safety and value is not yet well understood (81–83). Master protocol trials are, therefore, ideally suited for rapidly evaluating this ROD. At our institution, we are evaluating the use of stereotactic magnetic resonance imaging–guided adaptive radiotherapy delivered via MR-Linac in multiple disease sites using a phase I and II master protocol. To the best of our knowledge, this is the first master protocol trial design used to evaluate RODs. We outline our trial design and highlight the strengths of the approach below.
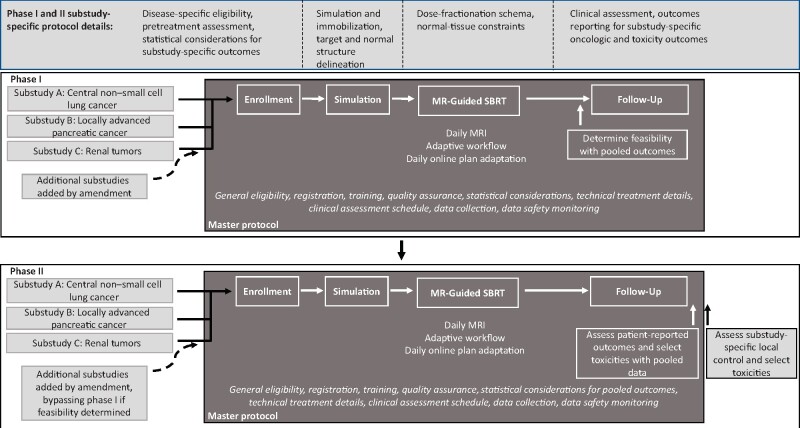
Prior to treating patients on trial, our study is preceded by simulation studies, including phantom studies, in vitro dosimetric planning studies, and healthy volunteer studies to evaluate the onboard imaging. These studies serve to train the study staff and providers on safe use of the device and to investigate which patient groups may stand to gain the most from the device. Figure 1 shows the schema of the phase I and phase II adaptive stereotactic body radiotherapy (SBRT) study designs. The phase I study evaluates the feasibility of delivering MR-guided SBRT in patients with cancer. The phase II study evaluates safety and efficacy of this treatment with specific reference to local control and improvement in patient-reported outcome measures. Substudies will evaluate SBRT in non–small cell lung cancer, locally advanced pancreatic cancer, and renal tumors. Subprotocols will be added as amendments specific to each cancer treated.
Figure 1.
Schema for phase I and II master protocol evaluating magnetic resonance imaging–guided linear accelerator (MR-Linac) for online-adaptive stereotactic body radiotherapy (SBRT) in several disease sites. Feasibility outcomes are pooled for the phase I study, allowing more rapid determination of this outcome. For the phase II study, cancer type–agnostic outcomes will be pooled across disease sites that share similar technical and toxicity consideration (eg, fatigue among all substudies and acute gastrointestinal toxicity among the pancreatic cancer and renal tumor SBRT substudies). The master protocol is written to allow seamless addition and removal of substudies via amendment. MRI = magnetic resonance imaging.
The general treatment workflow is specified in the master document, including the technical procedures for imaging parameters, daily setup, target and normal-structure contour adjustments, radiation plan reoptimization, and treatment-delivery techniques. The subprotocols specify disease-specific technical elements such as simulation and immobilization techniques, cancer-specific target and normal-structure contouring, dose-fractionation schema, and normal-tissue dose constraints. Because many of the radiation planning and delivery techniques are disease-site agnostic, a shared protocol dramatically decreases the work needed to create duplicative, detailed, and robust policies and procedures, including training and quality assurance programs, compared with individual protocols. Additionally, having multiple substudies with similar workflows within a single department maintains participant volume and thus operator competency with the technology.
The standardized procedures included in the master protocol include general eligibility and exclusion criteria, registration and randomization procedures, statistical considerations, criteria for taking patients off protocol, follow-up schedules, disease evaluation and response duration definitions, outcome and adverse event reporting, and data safety monitoring. Disease-site specifics, such as disease-specific inclusion and exclusion criteria, pretreatment assessment, expected toxicities, and details on clinical assessment, are specified in the substudies. Inclusion of as many study details in the master protocol as possible centralizes and simplifies the administrative burden of running this prospective trial evaluating multiple disease sites and facilitates our planned outcome pooling.
Our protocol takes advantage of the statistical efficiencies offered by master protocols discussed above. For the phase I portion, feasibility is defined as enrolling patients and delivering MR-guided adaptive SBRT; assessing tumors using MR-guidance before, during, and after treatment; and generating adaptive plans. Because these factors are independent of tumor type, this is a pooled endpoint, requiring fewer patients to determine feasibility and facilitate rapid completion of this portion of the trial. If feasibility is determined, new substudies can bypass phase I and start at phase II. In the phase II study, the patient-reported outcomes and select toxicity endpoints, particularly for tumors treated in similar anatomic locations and treated with similar dose-fractionation schedules, can be pooled to more rapidly identify more well-tolerated treatments and unexpected serious adverse events. Cancer type–agnostic outcomes will be pooled selectively, such as general patient-reported outcomes and select toxicities (eg, fatigue among all substudies and acute gastrointestinal toxicity among substudies involving upper abdominal tumors such as pancreatic cancer and renal cancer). Table 2 demonstrates how pooling endpoints across substudies improves the lowest detectable event probability, enhancing efficiency. Conversely, the efficacy endpoints of the phase II trial do not lend themselves well to pooling given its disease-specific nature, therefore, statistical consideration will be substudy specific.
Table 2.
Statistical efficiency of endpoint pooling
| Phase I endpoints | Relevant subprotocol sites* | Pooled No. | Lowest detectable event probability with 80% power† |
|---|---|---|---|
| Feasibility | NSCLC, LAPC, renal | 30 | 0.053 |
| Safety endpoints | |||
| Lung toxicity | NSCLC | 10 | 0.15 |
| GI toxicity | LAPC, renal | 20 | 0.078 |
| Skin toxicity | NSCLC, LAPC, renal | 30 | 0.053 |
n = 10 for each phase I subprotocol. GI = gastrointestinal; LAPC = locally advanced pancreatic cancer; NSCLC = non–small cell lung cancer.
Chance of observing one or more events with probability at least 0.80.
Our hope is that the master protocol design for a potentially high-risk, high-reward novel ROD will allow rapid determination of safety and identification of the most promising applications for this new tool. This will facilitate rational selection of the best candidates for larger, high-cost phase III trials of the device. By optimizing financial, administrative, quality assurance, and statistical efficiency, master protocols facilitate careful prospective evaluation of RODs in multiple disease sites, ultimately aimed at the challenging goal of demonstrating the value of the novel ROD.
Master protocol trials have the potential to enhance the quality and efficiency of prospective evaluation of novel RODs and therapeutic oncology devices in general. By minimizing the resources needed to run trials, oncologists can more practically and rapidly identify the most valuable advances and push the field in the most promising directions. With the emergence of novel clinical trial paradigms, investigators should partner with device manufacturers and the FDA to ensure that new therapeutic oncology devices, including RODs, are safely and judiciously evaluated and implemented in the clinic.
Notes
Daniel N. Cagney is a recipient of research funding from NH Theraguix. Lisa L. Singer is a recipient of research funding from the Brigham Research Institute. Paul J. Catalano sits on a data and safety monitoring board for Eli Lilly and Company, for which he is compensated less than $10,000. Paul L. Nguyen has consulted for Janssen, Bayer, Ferring Pharmaceuticals, Nanobiotix, Augmentix, Boston Scientific, Cota, Dendreon, Astellas Pharma, Medivation, GenomeDx, and Blue Earth. He has received research funding from Janssen and Astellas Pharma. Raymond H. Mak is on the scientific advisory board of AstraZeneca and has received a lecture honorarium at NewRT Medical Systems. The Department of Radiation Oncology at Dana-Farber Cancer Institute/Brigham and Women’s Hospital will be receiving research grants from ViewRay, the manufacturer of the department’s MR-Linac, to fund studies on the MR-Linac.
References
- 1.Duley L, Antman K, Arena J, et al. Specific barriers to the conduct of randomized trials. Clin Trials. 2008;5(1):40–48. [DOI] [PubMed] [Google Scholar]
- 2.Lauer MS, Gordon D, Wei G, et al. Efficient design of clinical trials and epidemiological research: is it possible? Nat Rev Cardiol. 2017;14(8):493–501. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux PJ, Yusuf S.. When it comes to trials, do we get what we pay for? N Engl J Med. 2013;369(20):1962–1963. [DOI] [PubMed] [Google Scholar]
- 4.Freedman B.Equipoise and the ethics of clinical research. N Engl J Med. 1987;317(3):141–145. [DOI] [PubMed] [Google Scholar]
- 5.Polsky D, Glick H.. Costing and cost analysis in randomized controlled trials: caveat emptor. Pharmacoeconomics. 2009;27(3):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catenacci DV.Next-generation clinical trials: novel strategies to address the challenge of tumor molecular heterogeneity. Mol Oncol. 2015;9(5):967–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chetty IJ, Martel MK, Jaffray DA, et al. Technology for innovation in radiation oncology. Int J Radiat Oncol Biol Phys. 2015;93(3):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolata G.Popular prostate cancer therapy is short, intense, and unproven. New York Times. March 20, 2017. [Google Scholar]
- 9.Centers for Medicare & Medicaid Services, Department of Health and Human Services. 42 CFR Part 512 [CMS-5527-P]. 2019. https://www.hhs.gov/sites/default/files/CMS-5527-P.pdf. Accessed July 26, 2019.
- 10.Kavanagh B.Radiation oncology APM: why us? Why now? Int J Radiat Biol. 2019;105(1):22–24. [DOI] [PubMed] [Google Scholar]
- 11.Choudhury A, Budgell G, MacKay R, et al. The future of image-guided radiotherapy. Clin Oncol (R Coll Radiol). 2017;29(10):662–666. [DOI] [PubMed] [Google Scholar]
- 12.Pollard JM, Wen Z, Sadagopan R, et al. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 2017;90(1073):20160667.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. 2015;16(2):e93–e100. [DOI] [PubMed] [Google Scholar]
- 14.Durante M, Orecchia R, Loeffler JS.. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol. 2017;14(8):483–495. [DOI] [PubMed] [Google Scholar]
- 15.Maxim PG, Tantawi SG, Loo BW Jr.. PHASER: a platform for clinical translation of FLASH cancer radiotherapy. Radiother Oncol. 2019;139:28–33. doi:10.1016/j.radonc.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Vozenin MC, De Fornel P, Petersson K, et al. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res. 2019;25(1):35–42. [DOI] [PubMed] [Google Scholar]
- 17.Mazin SR, Nanduri AS.. Emission-guided radiation therapy: biologic targeting and adaptive treatment. J Am Coll Radiol. 2010;7(12):989–990. [DOI] [PubMed] [Google Scholar]
- 18.Mak RH, Endres MG, Paik JH, et al. Use of crowd innovation to develop an artificial intelligence-based solution for radiation therapy targeting. JAMA Oncol. 2019;5(5):654. 10.1001/jamaoncol.2019.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sleijfer S, Bogaerts J, Siu LL.. Designing transformative clinical trials in the cancer genome era. J Clin Oncol. 2013;31(15):1834–1841. [DOI] [PubMed] [Google Scholar]
- 20.Cunanan KM, Iasonos A, Shen R, et al. An efficient basket trial design. Stat Med. 2017;36(10):1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodcock J, LaVange LM.. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. [DOI] [PubMed] [Google Scholar]
- 22.Hirakawa A, Asano J, Sato H, et al. Master protocol trials in oncology: review and new trial designs. Contemp Clin Trials Commun. 2018;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, US Food and Drug Administration. Master protocols: Efficient clinical trial design strategies to expedite development of oncology drugs and biologics. 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and. Accessed February 26, 2019.
- 24.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute. NCI-MATCH trial (Molecular Analysis for Therapy Choice). https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match. Accessed August 21, 2018.
- 26.Steuer CE, Papadimitrakopoulou V, Herbst RS, et al. Innovative clinical trials: the LUNG-MAP study. Clin Pharmacol Ther. 2015;97(5):488–491. [DOI] [PubMed] [Google Scholar]
- 27.Kim ES, Herbst RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadimitrakopoulou V, Lee JJ, Wistuba II, et al. The BATTLE-2 study: a biomarker-integrated targeted therapy study in previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol. 2016;34(30):3638–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker AD, Sigman CC, Kelloff GJ, et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86(1):97–100. [DOI] [PubMed] [Google Scholar]
- 30.Berry DA.Bayesian clinical trials. Nat Rev Drug Discov. 2006;5(1):27–36. [DOI] [PubMed] [Google Scholar]
- 31.Renfro LA, Sargent DJ.. Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples. Ann Oncol. 2017;28(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JW, Liu MC, Yee D, et al. Adaptive randomization of neratinib in early breast cancer. N Engl J Med. 2016;375(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrarotto R, Redman MW, Gandara DR, et al. Lung-MAP–framework, overview, and design principles. Chin Clin Oncol. 2015;4(3):36.. [DOI] [PubMed] [Google Scholar]
- 34.Cunanan KM, Gonen M, Shen R, et al. Basket trials in oncology: a trade-off between complexity and efficiency. J Clin Oncol. 2017;35(3):271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redman MW, Allegra CJ.. The master protocol concept. Semin Oncol. 2015;42(5):724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobbs BP, Barata PC, Kanjanapan Y, et al. Seamless designs: current practice and considerations for early-phase drug development in oncology. J Natl Cancer Inst. 2019;111(2):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pignol JP, Janus C.. The evaluation of innovation in radiation oncology—what can we do and what should we do? Acta Oncol. 2015;54(9):1251–1253. [DOI] [PubMed] [Google Scholar]
- 38.Bentzen SM, Wasserman TH.. Balancing on a knife’s edge: evidence-based medicine and the marketing of health technology. Int J Radiat Oncol Biol Phys. 2008;72(1):12–14; discussion 14–18. [DOI] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration. Premarket Notification (510k). 2010. https://www.fda.gov/medical-devices/premarket-submissions/premarket-notification-510k. Accessed January 30, 2019. [Google Scholar]
- 40.Razfar A, Mundi J, Grogan T, et al. IMRT for head and neck cancer: cost implications. Am J Otolaryngol. 2016;37(6):479–483. [DOI] [PubMed] [Google Scholar]
- 41.Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol. 2012;104(3):343–348. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh-Laskar S, Yathiraj PH, Dutta D, et al. Prospective randomized controlled trial to compare 3-dimensional conformal radiotherapy to intensity-modulated radiotherapy in head and neck squamous cell carcinoma: long-term results. Head Neck. 2016;38(suppl 1):E1481–E1487. [DOI] [PubMed] [Google Scholar]
- 43.Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. [DOI] [PubMed] [Google Scholar]
- 44.Bourhis J, Auperin A, Alfonsi M, et al. Dose escalation of radiotherapy (RT) for locally advanced head and neck carcinomas treated with concomitant chemotherapy (CT) and RT: results of the GORTEC 2004-01 randomized trial. J Clin Oncol. 2017;35(suppl 15):6015. [Google Scholar]
- 45.Liao Z, Lee JJ, Komaki R, et al. Bayesian adaptive randomization trial of passive scattering proton therapy and intensity-modulated photon radiotherapy for locally advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(18):1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sher DJ, Tishler RB, Pham NL, et al. Cost-effectiveness analysis of intensity modulated radiation therapy versus proton therapy for oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2018;101(4):875–882. [DOI] [PubMed] [Google Scholar]
- 47.Verma V, Mishra MV, Mehta MP.. A systematic review of the cost and cost-effectiveness studies of proton radiotherapy. Cancer. 2016;122(10):1483–1501. [DOI] [PubMed] [Google Scholar]
- 48.Connor MJ, Marshall DC, Moiseenko V, et al. Adverse events involving radiation oncology medical devices: comprehensive analysis of US Food and Drug Administration data, 1991 to 2015. Int J Radiat Oncol Biol Phys. 2017;97(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connor MJ, Tringale K, Moiseenko V, et al. Medical device recalls in radiation oncology: analysis of US Food and Drug Administration data, 2002-2015. Int J Radiat Oncol Biol Phys. 2017;98(2):438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferriter A. Center for Devices and Radiologic Health, US Food and Drug Administration. Medical device recall report FY 2003 - FY 2012. 2014. http://fmdic.org/wp-content/uploads/2014/04/Medical-Device-Recall-Report-amf-2.pdf. Accessed February 26, 2019.
- 51.Bogdanich W. Radiation offers new cures, and ways to do harm. New York Times. January 23, 2010.
- 52.Ergina PL, Cook JA, Blazeby JM, et al. Challenges in evaluating surgical innovation. Lancet. 2009;374(9695):1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mutsaerts EL, Van Coevorden F, Krause R, et al. Initial experience with radiofrequency ablation for hepatic tumours in the Netherlands. Eur J Surg Oncol. 2003;29(9):731–734. [DOI] [PubMed] [Google Scholar]
- 54.Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018;68(4):783–797. [DOI] [PubMed] [Google Scholar]
- 55.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379(20):1895–1904. [DOI] [PubMed] [Google Scholar]
- 56.US Food and Drug Administration. Caution when using robotically-assisted surgical devices in women’s health including mastectomy and other cancer-related surgeries: FDA safety communication. 2019. Available from: https://www.fda.gov/medical-devices/safety-communications/caution-when-using-robotically-assisted-surgical-devices-womens-health-including-mastectomy-and. Accessed March 1, 2019.
- 57.Fraass BA, Moran JM.. Quality, technology and outcomes: evolution and evaluation of new treatments and/or new technology. Semin Radiat Oncol. 2012;22(1):3–10. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Zhang Y, Tang LL, et al. Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years. JAMA Oncol. 2018;4(8):1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bortfeld TR, Loeffler JS.. Three ways to make proton therapy affordable. Nature. 2017;549(7673):451–453. [DOI] [PubMed] [Google Scholar]
- 60.Smith GL, Ganz PA, Bekelman JE, et al. Promoting the appropriate use of advanced radiation technologies in oncology: summary of a National Cancer Policy Forum workshop. Int J Radiat Oncol Biol Phys. 2017;97(3):450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah A, Ricci KI, Efstathiou JA.. Beyond a moonshot: insurance coverage for proton therapy. Lancet Oncol. 2016;17(5):559–561. [DOI] [PubMed] [Google Scholar]
- 62.Kron T, Chesson B, Hardcastle N, et al. Credentialing of radiotherapy centres in Australasia for TROG 09.02 (Chisel), a phase III clinical trial on stereotactic ablative body radiotherapy of early stage lung cancer. Br J Radiol. 2018;91(1085):20170737.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verkooijen HM, Kerkmeijer LGW, Fuller CD, et al. R-IDEAL: a framework for systematic clinical evaluation of technical innovations in radiation oncology. Front Oncol. 2017;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwala V, Khozin S, Singal G, et al. Real-world evidence in support of precision medicine: clinico-genomic cancer data as a case study. Health Aff (Millwood). 2018;37(5):765–772. [DOI] [PubMed] [Google Scholar]
- 65.Corrigan-Curay J, Sacks L, Woodcock J.. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320(9):867–868. [DOI] [PubMed] [Google Scholar]
- 66.Khozin S, Blumenthal GM, Pazdur R.. Real-world data for clinical evidence generation in oncology. J Natl Cancer Inst. 2017;109(11):djx187. [DOI] [PubMed] [Google Scholar]
- 67.Ventz S, Lai A, Cloughesy TF, et al. Design and evaluation of an external control arm using prior clinical trials and real-world data. Clin Cancer Res. 2019;25(16):4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hancock J, For cancer centers, proton therapy’s promise is undercut by lagging demand. New York Times. April 27, 2018.
- 69.Dayal McCluskey P. An insurer denied her $95,000 cancer treatment. She’s fighting back. Boston Globe. May 4, 2019.
- 70.Bekelman JE, Hahn SM.. Reference pricing with evidence development: a way forward for proton therapy. J Clin Oncol. 2014;32(15):1540–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Institute of Medicine. Medical devices and the public's health: The FDA 510(k) clearance process at 35 years. 2011. https://www.nap.edu/catalog/13150/medical-devices-and-the-publics-health-the-fda-510k-clearance. Accessed January 30, 2019.
- 72.Langendijk JA, Lambin P, De Ruysscher D, et al. Selection of patients for radiotherapy with protons aiming at reduction of side effects: the model-based approach. Radiother Oncol. 2013;107(3):267–273. [DOI] [PubMed] [Google Scholar]
- 73.Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374(9695):1089–1096. [DOI] [PubMed] [Google Scholar]
- 74.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters LJ, O’Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. J Clin Oncol. 2010;28(18):2996–3001. [DOI] [PubMed] [Google Scholar]
- 76.Dumville JC, Hahn S, Miles JN, et al. The use of unequal randomisation ratios in clinical trials: a review. Contemp Clin Trials. 2006;27(1):1–12. [DOI] [PubMed] [Google Scholar]
- 77.Lachin JM.Statistical properties of randomization in clinical trials. Control Clin Trials. 1988;9(4):289–311. [DOI] [PubMed] [Google Scholar]
- 78.Applied RadiationOncology.MR-linac Consortium launches MOMENTUM study to guide MR/RT, continue Elekta Unity progress. 2019. https://appliedradiationoncology.com/articles/mr-linac-consortium-launches-the-momentum-study-to-guide-mr-rt-and-continue-development-of-elekta-s-unity. Accessed July 26, 2019.
- 79.Veldeman L, Madani I, Hulstaert F, et al. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol. 2008;9(4):367–375. [DOI] [PubMed] [Google Scholar]
- 80.Jagsi R, DeLaney TF, Donelan K, et al. Real-time rationing of scarce resources: the Northeast Proton Therapy Center experience. J Clin Oncol. 2004;22(11):2246–2250. [DOI] [PubMed] [Google Scholar]
- 81.Palacios MA, Bohoudi O, Bruynzeel AME, et al. Role of daily plan adaptation in MR-guided stereotactic ablative radiotherapy for adrenal metastases. Int J Radiat Oncol Biol Phys. 2018;102(2):426–433. [DOI] [PubMed] [Google Scholar]
- 82.Henke L, Kashani R, Yang D, et al. Simulated online adaptive magnetic resonance-guided stereotactic body radiation therapy for the treatment of oligometastatic disease of the abdomen and central thorax: characterization of potential advantages. Int J Radiat Oncol Biol Phys. 2016;96(5):1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Acharya S, Fischer-Valuck BW, Mazur TR, et al. Magnetic resonance image guided radiation therapy for external beam accelerated partial-breast irradiation: evaluation of delivered dose and intrafractional cavity motion. Int J Radiat Oncol Biol Phys. 2016;96(4):785–792. [DOI] [PubMed] [Google Scholar]