Abstract
Background
Childhood cancer survivors have an increased risk of heart failure, ischemic heart disease, and stroke. They may benefit from prediction models that account for cardiotoxic cancer treatment exposures combined with information on traditional cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes.
Methods
Childhood Cancer Survivor Study participants (n = 22 643) were followed through age 50 years for incident heart failure, ischemic heart disease, and stroke. Siblings (n = 5056) served as a comparator. Participants were assessed longitudinally for hypertension, dyslipidemia, and diabetes based on self-reported prescription medication use. Half the cohort was used for discovery; the remainder for replication. Models for each outcome were created for survivors ages 20, 25, 30, and 35 years at the time of prediction (n = 12 models).
Results
For discovery, risk scores based on demographic, cancer treatment, hypertension, dyslipidemia, and diabetes information achieved areas under the receiver operating characteristic curve and concordance statistics 0.70 or greater in 9 and 10 of the 12 models, respectively. For replication, achieved areas under the receiver operating characteristic curve and concordance statistics 0.70 or greater were observed in 7 and 9 of the models, respectively. Across outcomes, the most influential exposures were anthracycline chemotherapy, radiotherapy, diabetes, and hypertension. Survivors were then assigned to statistically distinct risk groups corresponding to cumulative incidences at age 50 years of each target outcome of less than 3% (moderate-risk) or approximately 10% or greater (high-risk). Cumulative incidence of all outcomes was 1% or less among siblings.
Conclusions
Traditional cardiovascular risk factors remain important for predicting risk of cardiovascular disease among adult-age survivors of childhood cancer. These prediction models provide a framework on which to base future surveillance strategies and interventions.
More than 80% of children diagnosed with cancer now become long-term survivors, and there are approximately half a million survivors of childhood cancer living in the United States (1). Premature cardiovascular disease has become one of the most important causes of morbidity and mortality in this population (2–4). This increased risk for cardiovascular disease is due to cardiotoxic cancer treatments (eg, anthracycline chemotherapy, radiotherapy) (2), but also potentiated by the presence of traditional risk factors such as hypertension, dyslipidemia, and diabetes (5). Cancer therapy may also contribute to an increased burden of these traditional cardiovascular risk factors (6,7).
Cardiovascular disease risk prediction now plays a fundamental role in clinical decision making in the general adult population (8–11). However, these risk prediction models likely underestimate risk among young adult survivors of childhood cancer who also are exposed to cardiotoxic cancer treatments not accounted for in these risk calculators (2). Therefore, cancer survivors may benefit from prediction models that account both for prior cardiotoxic cancer treatment and traditional cardiovascular risk factors found in general population risk estimators (5,7). The development of such models may further personalize surveillance and management strategies now being disseminated by cancer survivor-specific long-term follow-up guidelines (12–14).
The Childhood Cancer Survivor Study (CCSS) is the largest continuously followed cohort of 5-year survivors of childhood cancer in the world, with nearly 30 000 participants (15). We have previously used the CCSS to develop models that predict heart failure, ischemic heart disease, and stroke among childhood cancer patients who have reached the 5-year survival milestone (16,17). These models did not incorporate data on traditional cardiovascular risk factors and do not apply to the vast majority of survivors who are farther out from treatment. To address these gaps in knowledge, herein we present results of models for long-term survivors that predict the risk of serious cardiovascular disease across a range of young adult ages based on readily accessible demographic characteristics, cancer treatment exposures, and the presence of predisposing cardiovascular risk factors.
Methods
Study Population
CCSS study methodology and subject accrual have been reported previously (15,18). The cohort consists of 24 734 individuals (diagnosis before age 21 years) treated for the most common types of childhood cancer at 27 institutions in the United States and Canada between 1970 and 1999, and who survived at least 5 years following diagnosis. Data newly available for this analysis, and not used in our prior cardiovascular disease prediction analyses, include information from 11 482 survivors who were diagnosed 1987 through 1999. For this analysis, we excluded those who did not provide any self-reported outcomes data (n = 1738) and those who experienced a major cardiovascular event (ischemic heart disease, stroke, heart failure, or any cardiovascular death) within 5 years of their initial cancer diagnosis (n = 353), leaving 22 643 (91.5%) of the overall cohort available. We randomly selected 50% of the cohort to serve as our discovery sample and reserved the other half for replication. A random sample of siblings of participating CCSS survivors served as a comparison population (n = 5056). The protocol was approved by the human subjects committee at each institution. Participants provided written informed consent.
Cancer Therapy Exposures
Chemotherapy, surgery, and radiotherapy exposures within 5 years of initial cancer diagnosis were abstracted from medical records. Radiotherapy records were centrally reviewed and exposures to the brain, neck, chest, and abdomen were categorized as yes or no (yes/no; yes if at least part of the region was in the direct treatment field), and field-specific maximum total doses calculated for the brain, chest, and abdomen separately (19). Consistent with current Children’s Oncology Group (COG) guidelines, chest fields included any abdominal treatment that included the lower part of the chest (ie, above the diaphragm and affecting the base of the heart), as well as treatments directed at the thorax (eg, shoulders, ribs, and/or supraclavicular areas), even if the central chest was not a target. We previously have shown that the performance of models based on these fields was similar to those based on heart-specific dosimetry (16,17). In defining dose-specific exposures for each region, radiation scatter from adjacent fields also was noted, but these exposures were categorized as less than 5 Gy.
Outcomes and Cardiovascular Risk Factors
CCSS participants received serial questionnaires that cover demographic characteristics, health conditions, and health-related behaviors (available at http://ccss.stjude.org). Proxy responses from family members have been used for 5-year survivors who had subsequently died, were younger than 18 years, or were unable to complete the questionnaires. Linkage with the National Death Index ascertained deaths and causes of deaths from which we identified deaths due to heart failure (International Classification of Diseases, ninth revision [ICD-9] codes: 425–428, V42.1), ischemic heart diseases (410–411, 413–414, 427.5, 440), and stroke (430–434, 436, 437–438, 444); equivalent ICD-10 codes also were used. If an individual experienced more than one of these outcomes, we counted only the first event.
Using previously described methodology to define heart failure, ischemic heart disease, and stroke (5,20,21), questionnaire items related to these outcomes were classified and graded using the Common Terminology Criteria for Adverse Events (version 4.03) (22). Only those outcomes graded as severe (grade 3: cardiomyopathy and/or ischemic heart disease requiring medications), life-threatening (grade 4: heart failure requiring transplant; ischemic heart disease requiring therapeutic catheterization or surgery; any stroke/cerebrovascular accident), or fatal (grade 5) were included. Outcomes were limited to those occurring by age 50 years given the limited number of events beyond that age. Information on the age at onset of related cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes (all three limited to conditions requiring treatment with prescription medications) was also ascertained (5,7). Although information on other potential cardiovascular risk factors such as obesity, smoking, and physical activity are periodically collected by the CCSS, they are collected as prevalent exposures at each survey time point and could not be readily applied to prediction models requiring exposure status at predefined ages (eg, 20, 25, 30, and 35 years) (23–25).
Statistical Analysis
Predictions models for each outcome (heart failure, ischemic heart disease, stroke) were created for four prediction time points (age 20, 25, 30, and 35 years), resulting in 12 total models. Exposures selected a priori to be examined in our models included sex; age at diagnosis (5-year increments); alkylating agents (yes/no); anthracyclines (none, <100 mg/m2, 100–249 mg/m2, and ≥250 mg/m2); platinum agents (yes/no); vinca alkaloids (yes/no); and radiation to the head (none, <20, 20–29, 30–49, and ≥50 Gy), neck (yes/no), chest (none, <5, 5–14, 15–34, and ≥35 Gy), and abdomen (yes/no), along with hypertension, dyslipidemia, and diabetes treatment status. Anthracyclines included doxorubicin, daunorubicin, idarubicin, epirubicin, and mitoxantrone (an anthraquinone). For this analysis, anthracycline doses were converted to the following doxorubicin-equivalent dose: daunorubicin (0.5), idarubicin (3.0), epirubicin (0.67), and mitoxantrone (10.0) (26).
We used piecewise exponential models based on half the available cohort chosen at random, adjusted for current age, to predict each cardiovascular outcome by using the treatment variables selected from our previous models (16,17) with the cardiovascular risk factors (hypertension, dyslipidemia, and diabetes) included. Deaths from other causes were treated as competing risk events. To be consistent with our prior work, regression coefficient estimates of covariates that remained were then converted to integer risk scores for ease of summing in subsequent risk models (rate ratios [RR]= <1.3, 1.3–1.9, 2.0–2.9, 3.0–4.9, and ≥5.0, corresponding to risk scores 0, 1, 2, 3, and 4, respectively). Cox regression models estimated a model’s discriminatory and predictive power based on the area under the receiver operating characteristic curve (AUC) at age 50 y and the concordance (C) statistic (representing the weighted average AUC from study start through age 50 years) (27,28). Values 0.70 or greater are considered reasonable. AUC and C-statistics (at and through age 50 years, respectively) for the target cardiovascular outcomes were then estimated in the replication cohort. To improve the internal consistency of models, we allowed risk scores to be adjusted by one (and, rarely, two) point(s). To ensure that these changes did not alter the models’ discriminatory or predictive power, the AUC and C-statistics were re-estimated both in the discovery and replication cohorts using these revised scores.
Risk scores were then summed to create moderate- and high-risk groups using data from both the discovery and replication cohorts for each cardiovascular outcome based on the absolute risks (cumulative incidence at age 50 years) and the rate ratios compared with siblings associated with individual risk scores (29,30). The risk groupings were designed so the high-risk group generally had an approximate 10% or greater cumulative incidence of the target outcome by age 50 years, and also so the high-risk group would be distinct from the moderate-risk group (two-sided Wald test P < .05). The resulting incidence rate of each cardiovascular outcome as predicted vs observed was then compared to determine model calibration. Finally, to improve ease of use, we created an online risk calculator in which exposure information can be entered and the appropriate risk group with cumulative incidence and risk ratios automatically displayed (available at ccss.stjude.org/cvcalc). Further methodologic details are provided in the Supplementary Methods (available online).
Results
The demographic and treatment characteristics of the discovery and replication cohorts were similar (Table 1). Those assessed at age 35 years generally had an older cancer diagnosis age and were more likely to have received an alkylator and/or radiation compared with those assessed at age 20 years. The characteristics of those with data available at age 25 and 30 years were intermediate to those age 20 and 35 years, respectively (data not shown). The prevalence of traditional risk factors (ie, diabetes, dyslipidemia, hypertension) was similar in the discovery and replication cohorts (Table 2). The prevalence tended to be limited at age 20 years (all <3%) but increased over time, with 8.8% to 9.0% of survivors reporting hypertension by age 35 years. Except for the age 35-years models, all the other age time point models in the discovery cohort had at least 50 serious cardiovascular events available for prediction.
Table 1.
Baseline demographic and cancer treatment characteristics of 5-year childhood cancer survivors available for prediction discovery and replication at age 20 and 35* years
| Characteristic | Discovery cohort, No. (%) |
Replication cohort, No. (%) |
||
|---|---|---|---|---|
| Age 20 y | Age 35 y | Age 20 y | Age 35 y | |
| (n = 7076) | (n = 2598) | (n = 7075) | (n = 2598) | |
| Female | 3311 (46.8) | 1222 (47.0) | 3390 (47.7) | 1221 (47.0) |
| Race/ethnicity | ||||
| White, non-Hispanic | 5920 (83.1) | 2304 (88.6) | 5873 (81.8) | 2311 (88.9) |
| Black, non-Hispanic | 386 (5.4) | 94 (3.6) | 405 (5.8) | 83 (3.2) |
| Hispanic | 363 (5.2) | 101 (3.9) | 372 (5.3) | 110 (4.2) |
| Other | 407 (6.3) | 99 (3.9) | 425 (7.0) | 94 (3.7) |
| Age at diagnosis, y | ||||
| <5 | 2804 (41.0) | 313 (12.0) | 2831 (41.0) | 256 (9.8) |
| 5–9 | 2042 (30.0) | 414 (15.8) | 2058 (30.6) | 419 (16.1) |
| 10–14 | 2230 (29.0) | 839 (32.4) | 2185 (28.3) | 879 (33.8) |
| ≥15 | 0 (–) | 1032 (39.8) | 1 (0.0) | 1044 (40.3) |
| Year of diagnosis | ||||
| 1970–1979 | 2077 (25.8) | 1495 (57.2) | 2098 (26.2) | 1504 (57.6) |
| 1980–1989 | 3014 (41.5) | 919 (35.5) | 3054 (42.2) | 931 (35.9) |
| 1990–1999 | 1985 (32.7) | 184 (7.3) | 1923 (31.6) | 163 (6.4) |
| Cancer type | ||||
| Leukemia | 2377 (41.6) | 682 (26.7) | 2388 (41.6) | 668 (26.0) |
| Lymphoma | 1308 (16.3) | 977 (37.4) | 1282 (16.0) | 921 (35.3) |
| Central nervous system tumor | 1275 (15.8) | 315 (12.1) | 1318 (16.4) | 326 (12.5) |
| Wilms tumor | 681 (8.5) | 94 (3.6) | 742 (9.3) | 98 (3.8) |
| Neuroblastoma | 522 (6.5) | 57 (2.2) | 480 (6.0) | 42 (1.6) |
| Bone tumor | 530 (6.6) | 352 (13.5) | 485 (6.0) | 409 (15.7) |
| Rhabdomyosarcoma | 383 (4.8) | 121 (4.6) | 380 (4.7) | 134 (5.1) |
| Duration of follow-up, y | ||||
| 5–9 | 53 (0.7) | 0 (-) | 62 (0.8) | 0 (-) |
| 10–19 | 2271 (35.0) | 155 (6.0) | 2181 (34.0) | 130 (5.1) |
| 20–29 | 3681 (51.0) | 1378 (53.2) | 3761 (51.8) | 1438 (55.5) |
| ≥30 | 1071 (13.3) | 1065 (40.8) | 1071 (13.4) | 1030 (39.5) |
| Anthracycline dose, mg/m2 | ||||
| None | 3676 (48.0) | 1463 (56.0) | 3777 (49.7) | 1493 (57.3) |
| <100 | 581 (13.8) | 112 (4.5) | 573 (13.1) | 101 (4.0) |
| 100–249 | 1287 (18.0) | 338 (13.2) | 1280 (18.2) | 296 (11.5) |
| ≥250 | 1076 (14.2) | 474 (18.2) | 1026 (13.5) | 501 (19.3) |
| Unknown | 456 (6.0) | 211 (8.1) | 419 (5.5) | 207 (8.0) |
| Alkylator | ||||
| No | 4459 (66.7) | 1328 (51.4) | 4530 (67.5) | 1330 (51.3) |
| Yes | 2366 (30.2) | 1155 (44.2) | 2311 (29.6) | 1171 (44.9) |
| Unknown | 251 (3.1) | 115 (4.4) | 234 (3.0) | 97 (3.3) |
| Platinum-based agent | ||||
| No | 6586 (93.9) | 2406 (92.7) | 6604 (94.1) | 2415 (93.0) |
| Yes | 240 (3.0) | 75 (2.9) | 241 (3.0) | 85 (3.3) |
| Unknown | 250 (3.1) | 117 (4.5) | 230 (2.9) | 98 (3.8) |
| Vinca alkaloid | ||||
| No | 3511 (54.6) | 1006 (39.0) | 3481 (54.3) | 1012 (39.2) |
| Yes | 3314 (42.3) | 1476 (56.5) | 3361 (42.8) | 1489 (57.1) |
| Unknown | 251 (3.1) | 116 (4.4) | 233 (2.9) | 97 (3.7) |
| Cranial radiation dose, Gy | ||||
| None | 4425 (63.8) | 1624 (62.4) | 4447 (64.4) | 1642 (63.1) |
| <20 | 811 (2.4) | 248 (9.8) | 805 (12.3) | 276 (10.8) |
| 20–29 | 624 (8.5) | 319 (12.3) | 601 (7.9) | 303 (11.7) |
| 30–49 | 208 (2.6) | 82 (3.1) | 218 (2.8) | 72 (2.8) |
| ≥50 | 619 (7.7) | 165 (6.3) | 650 (8.1) | 159 (6.1) |
| Unknown | 389 (5.0) | 160 (6.1) | 354 (4.5) | 146 (5.6) |
| Neck radiation | ||||
| No | 5411 (78.5) | 1565 (60.4) | 5483 (79.5) | 1648 (63.5) |
| Yes | 1340 (17.4) | 884 (33.9) | 1292 (16.7) | 817 (31.4) |
| Unknown | 325 (4.0) | 149 (5.7) | 300 (3.7) | 133 (5.1) |
| Chest radiation dose, Gy | ||||
| None | 4599 (68.3) | 1307 (50.5) | 4667 (69.3) | 1372 (52.9) |
| <5 | 367 (4.7) | 159 (6.1) | 338 (4.2) | 147 (5.7) |
| 5–14 | 611 (7.7) | 233 (8.9) | 635 (8.0) | 203 (7.8) |
| 15–34 | 289 (4.0) | 66 (2.6) | 256 (3.6) | 58 (2.3) |
| ≥35 | 716 (9.2) | 339 (13.0) | 705 (8.9) | 334 (12.8) |
| Unknown | 494 (6.1) | 494 (18.9) | 474 (5.9) | 484 (18.6) |
| Abdominal radiotherapy | ||||
| No | 5171 (75.5) | 1702 (65.7) | 5287 (77.1) | 1725 (66.5) |
| Yes | 1579 (20.4) | 747 (28.6) | 1487 (19.1) | 740 (28.4) |
| Unknown | 326 (4.1) | 149 (5.7) | 301 (3.8) | 133 (5.1) |
Numbers of individuals available for analysis at ages 25 and 30 years were 6681 and 4490, respectively, for both the discovery and replication cohorts. Numbers and percentages may not completely match up because the percentages shown reflect sampling weights for acute lymphoblastic leukemia survivors.
Table 2.
Numbers of individuals with traditional cardiovascular risk factors and grades 3–5 cardiovascular outcomes in the discovery and replication cohorts at each age time point*
| Age, y | Dataset | Cohort size | Prevalence of traditional risk factors (%) |
No. cardiovascular outcomes used for prediction (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Diabetes | Dyslipidemia | Hypertension | Heart failure | Ischemic heart disease | Stroke | |||
| 20 | Discovery | 7076 | 53 (0.8) | 46 (0.7) | 164 (2.4) | 100 (1.4) | 63 (0.9) | 83 (1.2) |
| Replication | 7075 | 31 (0.4) | 48 (0.7) | 176 (2.5) | 69 (1.0) | 36 (0.5) | 88 (1.2) | |
| 25 | Discovery | 6681 | 61 (1.1) | 79 (1.3) | 239 (3.6) | 86 (1.3) | 88 (1.3) | 75 (1.1) |
| Replication | 6681 | 58 (0.9) | 88 (1.4) | 228 (3.6) | 108 (1.6) | 91 (1.4) | 96 (1.4) | |
| 30 | Discovery | 4490 | 62 (1.4) | 115 (2.5) | 269 (6.0) | 68 (1.5) | 79 (1.8) | 65 (1.5) |
| Replication | 4490 | 67 (1.5) | 100 (2.2) | 238 (5.3) | 60 (1.3) | 80 (1.8) | 61 (1.4) | |
| 35 | Discovery | 2598 | 64 (2.5) | 129 (4.9) | 229 (8.8) | 37 (1.4) | 52 (2.0) | 36 (1.4) |
| Replication | 2598 | 49 (1.9) | 134 (5.2) | 233 (9.0) | 47 (1.8) | 68 (2.6) | 36 (1.4) | |
Numbers and percentages may not completely match up because the percentages shown reflect sampling weights for acute lymphoblastic leukemia survivors.
Influential predictors at each 5-year age increment for each cardiovascular outcome were identified (Supplementary Table 1, available online), from which corresponding integer-based scoring tables were created (Supplementary Table 2, available online). Whereas female sex was a predictor for cardiomyopathy (RRs = 1.42–2.04), men were at greater risk for ischemic heart disease (RRs = 1.57–1.92). For both heart failure and stroke, younger age at cancer treatment was associated with an increased risk. Among cancer treatment exposures, increased anthracycline dose was a strong predictor for heart failure across all models, with rate ratios greater than 4.00 associated with cumulative doses 250 mg/m2 or higher. Increased radiation dose was a strong predictor for all outcomes: chest doses 35 Gy or higher were associated with rate ratios greater than 5.00 both for heart failure and ischemic heart disease; cranial doses 50 Gy or higher were associated with rate ratios greater than 6.00 for stroke. Some treatment exposures were not found to be consistently predictive and were not included in the final models: vinca alkaloids, platinum-based agents, neck radiation, and abdominal radiation. The influence of diabetes, dyslipidemia, and hypertension varied across the models and outcomes but in general was found to be associated with the cardiovascular outcomes of interest in most situations, with rate ratios 1.30 or greater (ie, risk score value of at least 1) in two-thirds of models.
Overall, the resulting integer risk scores performed reasonably well both in the discovery and replication cohorts for predicting the occurrence of either heart failure or ischemic heart disease by age 50 years (Table 3). For discovery, AUCs and C-statistics were 0.70 or greater in 9 and 10 of the 12 models, respectively. For replication, AUCs and C-statistics were 0.70 or greater in 7 and 9 of the 12 models, respectively. In general, the AUC and C-statistic values in our models were similar, suggesting that estimates were stable at least through age 50 years.
Table 3.
Integer risk scores for survivors across 5-year age categories associated with cardiovascular outcomes and corresponding model discrimination and predictive power at and through age 50 years*
| Characteristic | Heart failure |
Ischemic heart disease |
Stroke |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age 20 y | Age 25 y | Age 30 y | Age 35 y | Age 20 y | Age 25 y | Age 30 y | Age 35 y | Age 20 y | Age 25 y | Age 30 y | Age 35 y | |
| Sex | ||||||||||||
| Male | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0† | 0 | 1 |
| Female | 1 | 1† | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age at diagnosis, y | ||||||||||||
| <5 | 1† | 2† | 2† | 2 | N/A | N/A | N/A | N/A | 1 | 1† | 1 | 1† |
| 5–9 | 1 | 1† | 0 | 0 | N/A | N/A | N/A | N/A | 1† | 1† | 1 | 1 |
| 10–14 | 0 | 0† | 0 | 0 | N/A | N/A | N/A | N/A | 0 | 0 | 0 | 0 |
| ≥15 | 0 | 0 | 0 | 0 | N/A | N/A | N/A | N/A | 0 | 0 | 0 | 0 |
| Alkylator | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | 1† | 1† | 1 |
| Anthracycline, mg/m2 | ||||||||||||
| None | 0 | 0 | 0 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| <100 | 0 | 0† | 0 | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| 100–249 | 3 | 3 | 2† | 2 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| ≥250 | 4 | 4 | 4† | 4 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Cranial radiation‡, Gy | ||||||||||||
| None | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | 0 | 0 | 0 |
| <20 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | 0 | 0 | 0 |
| 20–29 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | 1 | 1 | 3 |
| 30–49 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 4 | 4 | 4† | 4 |
| ≥50 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 4 | 4 | 4 | 4 |
| Chest radiation, Gy | ||||||||||||
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| <5 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5–14 | 1 | 1† | 1 | 0 | 1 | 2 | 3 | 4 | 0 | 0 | 0 | 0 |
| 15–34 | 2 | 2† | 2 | 2† | 2† | 3 | 4 | 4 | 0 | 0 | 0 | 0 |
| ≥35 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 1 | 1 | 1 | 1 |
| Cardiovascular risk factors | ||||||||||||
| Diabetes | 3 | 2 | 2† | 2† | 3 | 3† | 2 | 1 | 0 | 0 | 1† | 3 |
| Dyslipidemia | 2 | 1† | 0 | 0 | 0 | 1 | 1† | 1 | 1 | 1 | 2† | 2 |
| Hypertension | 3† | 1† | 1† | 1 | 2† | 2 | 2† | 2† | 1 | 1† | 2† | 2† |
| Discovery cohort | ||||||||||||
| AUC, age 50 y | 0.76 | 0.73 | 0.67 | 0.70 | 0.73 | 0.73 | 0.72 | 0.75 | 0.74 | 0.72 | 0.64 | 0.64 |
| C-statistic, age 50 y | 0.79 | 0.75 | 0.69 | 0.74 | 0.72 | 0.74 | 0.74 | 0.78 | 0.76 | 0.75 | 0.67 | 0.70 |
| Replication cohort | ||||||||||||
| AUC, age 50 y | 0.77 | 0.67 | 0.69 | 0.67 | 0.70 | 0.70 | 0.72 | 0.71 | 0.73 | 0.63 | 0.64 | 0.70 |
| C-statistic, age 50 y | 0.78 | 0.71 | 0.75 | 0.69 | 0.69 | 0.72 | 0.74 | 0.73 | 0.75 | 0.66 | 0.70 | 0.72 |
Risk scores 0, 1, 2, 3, and 4 correspond to rate ratios <1.3, 1.3 to 1.9, 2.0 to 2.9, 3.0 to 4.9, and ≥5.0, respectively. AUC = area under the receiver operating characteristic curve; C = concordance; N/A = characteristic not applicable for a given model.
Risk scores that were adjusted to improve their internal consistency, either across age time points and/or by characteristic categories.
Cranial radiation exposure was considered only for the stroke outcome models.
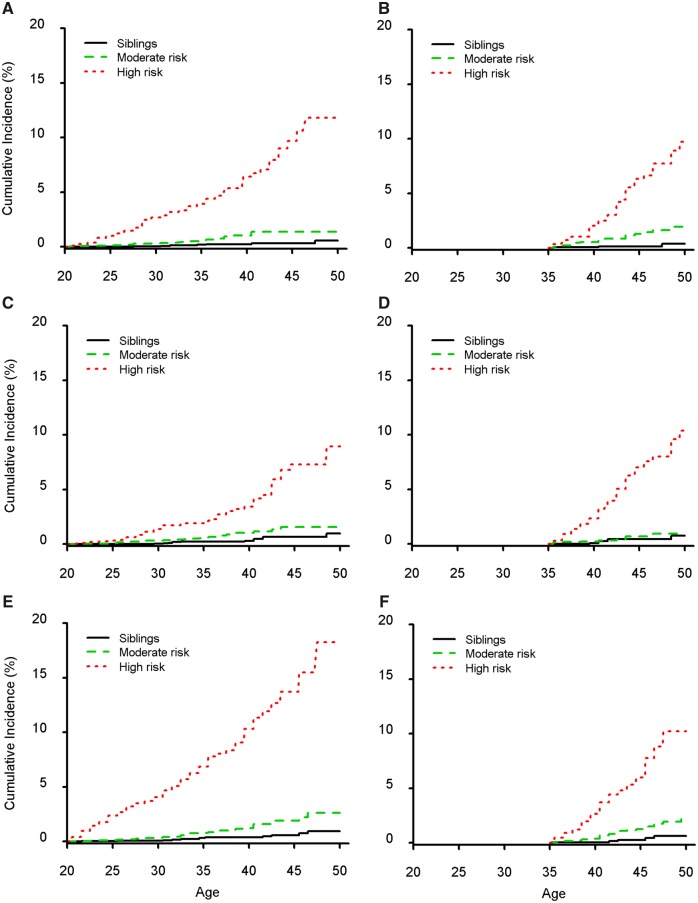
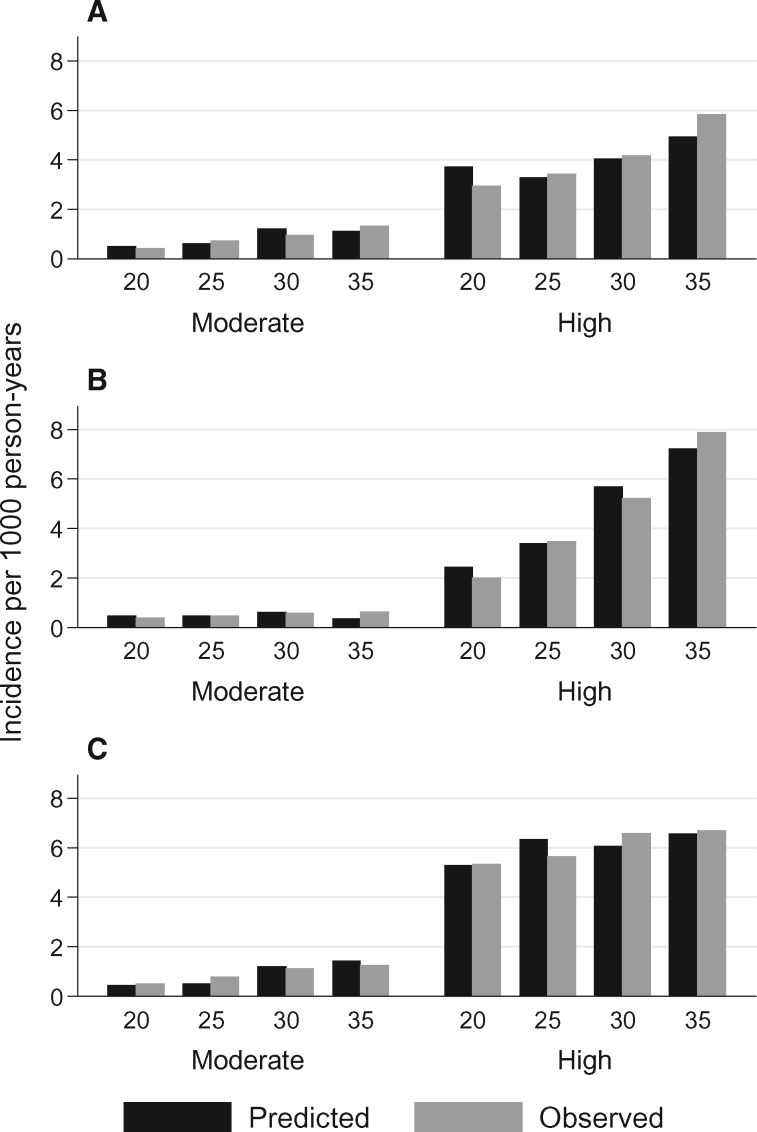
When risk scores were then summed and converted to risk groups, we were able to create high-risk groups with age 50-years cumulative incidences of approximately 10% or greater (Figure 1;Supplementary Figure 1, available online) and with statistically significantly greater rate ratios compared with moderate-risk survivors (Table 4; P < .001 for all comparisons). Except for ischemic heart disease at the age 30- and 35-year-prediction time points, the moderate-risk groups also were at statistically significantly increased risk vs siblings (P < .05), with age 50-years cumulative incidences between 1% and 3%, compared with no more than 1% for siblings. Ten-year risk estimates also were provided for all groups. Overall, the calibration of the models for all three outcomes and across all 5-year age time points appeared reasonable, with the difference between predicted and observed incidence rates within 0.3% for moderate-risk survivors and within 0.9% for high-risk survivors per 1000 person-years (Figure 2).
Figure 1.
Cumulative incidence of cardiovascular outcomes stratified by prediction risk status. Incidence curves for heart failure (A, B), ischemic heart disease (C, D), and stroke (E, F) participants at age 20 years (left column; 14 151 survivors and 4521 siblings) and age 35 years (right column; 5196 survivors and 2077 siblings). Supplementary Figure 1 (available online) shows the incidence of events for the age 25- and 30-year prediction time points.
Table 4.
Classification of cardiovascular event groups within the entire cancer survivor cohort based on summed risk scores across 5-year age prediction time points
| Outcome and model | Risk score | Risk group | No. of events | No. at risk* | Cumulative incidences, % (SD) |
vs siblings |
vs group above† |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| 10 y | Age 50 y | Rate ratio (95% CI) | P ‡ | Rate ratio (95% CI) | P ‡ | |||||
| Heart failure | ||||||||||
| Age 20 y | — | Sibling | 8 | 4521 | 0.03 (0.03) | 0.6 (0.3) | 1.00 (Referent) | — | — | — |
| <5 | Moderate | 41 | 10 819 | 0.4 (0.1) | 1.4 (0.3) | 4.41 (2.07 to 9.41) | <.001 | 1.00 (Referent) | — | |
| ≥5 | High | 110 | 3996 | 2.7 (0.3) | 11.8 (2.1) | 30.89 (14.99 to 63.64) | <.001 | 7.00 (4.89 to 10.04) | <.001 | |
| Age 25 y | — | Sibling | 8 | 3796 | 0.2 (0.1) | 0.6 (0.3) | 1.00 (Referent) | — | — | — |
| <5 | Moderate | 54 | 8873 | 0.5 (0.1) | 2.4 (0.5) | 4.62 (2.19 to 9.73) | <.001 | 1.00 (Referent) | — | |
| ≥5 | High | 116 | 4050 | 2.8 (0.3) | 11.2 (1.6) | 21.75 (10.60 to 44.63) | <.001 | 4.71 (3.41 to 6.50) | <.001 | |
| Age 30 y | — | Sibling | 7 | 2923 | 0.2 (0.1) | 0.5 (0.3) | 1.00 (Referent) | — | — | — |
| <5 | Moderate | 41 | 6129 | 0.8 (0.2) | 2.2 (0.5) | 4.19 (1.87 to 9.40) | <.001 | 1.00 (Referent) | — | |
| ≥5 | High | 69 | 2087 | 3.2 (0.5) | 10.2 (1.5) | 17.74 (8.12 to 38.75) | <.001 | 4.24 (2.88 to 6.23) | <.001 | |
| Age 35 y | — | Sibling | 3 | 2077 | 0.1 (0.1) | 0.4 (0.3) | 1.00 (Referent) | — | — | — |
| <5 | Moderate | 26 | 3427 | 1.3 (0.3) | 1.9 (0.5) | 7.09 (2.15 to 23.42) | <.001 | 1.00 (Referent) | — | |
| ≥5 | High | 46 | 1229 | 6.3 (1.0) | 9.7 (1.7) | 31.12 (9.67 to 100.16) | <.001 | 4.39 (2.72 to 7.08) | <.001 | |
| Ischemic heart disease | ||||||||||
| Age 20 y | — | Sibling | 12 | 4521 | 0.03 (0.03) | 1.0 (0.4) | 1.00 (Referent) | — | — | — |
| <3 | Moderate | 47 | 13 286 | 0.3 (0.06) | 1.6 (0.4) | 3.09 (1.63 to 5.85) | <.001 | 1.00 (Referent) | — | |
| ≥3 | High | 49 | 2067 | 1.4 (0.3) | 8.9 (2.1) | 13.39 (7.09 to 25.28) | <.001 | 4.34 (2.89 to 6.52) | <.001 | |
| Age 25 y | — | Sibling | 12 | 3796 | 0.2 (0.1) | 1.0 (0.4) | 1.00 (Referent) | — | — | — |
| <3 | Moderate | 38 | 9891 | 0.4 (0.1) | 1.4 (0.4) | 2.13 (1.11 to 4.10) | .02 | 1.00 (Referent) | — | |
| ≥3 | High | 122 | 3588 | 2.0 (0.3) | 10.5 (1.2) | 12.97 (7.17 to 23.47) | <.001 | 6.09 (4.20 to 8.85) | <.001 | |
| Age 30 y | — | Sibling | 11 | 2923 | 0.3 (0.1) | 0.9 (0.4) | 1.00 (Referent) | — | — | — |
| <4 | Moderate | 23 | 5975 | 0.5 (0.1) | 1.2 (0.3) | 1.68 (0.82 to 3.46) | .16 | 1.00 (Referent) | — | |
| ≥4 | High | 111 | 2649 | 3.4 (0.4) | 11.1 (1.2) | 12.46 (6.70 to 23.15) | <.001 | 7.40 (4.74 to 11.56)‡ | <.001 | |
| Age 35 y | — | Sibling | 6 | 2077 | 0.5 (0.2) | 0.8 (0.4) | 1.00 (Referent) | — | — | — |
| <4 | Moderate | 11 | 3091 | 0.7 (0.3) | 0.9 (0.4) | 1.77 (0.66 to 4.76) | .26 | 1.00 (Referent) | — | |
| ≥4 | High | 85 | 1821 | 7.0 (0.8) | 10.4 (1.3) | 17.79 (7.80 to 40.48) | <.001 | 10.06 (5.38 to 18.80) | <.001 | |
| Stroke | ||||||||||
| Age 20 y | — | Sibling | 14 | 4521 | 0.1 (0.04) | 0.9 (0.3) | 1.00 (Referent) | — | — | — |
| <4 | Moderate | 63 | 13 445 | 0.4 (0.07) | 2.6 (0.6) | 3.14 (1.75 to 5.66) | <.001 | 1.00 (Referent) | — | |
| ≥4 | High | 90 | 1680 | 4.1 (0.6) | 18.3 (3.7) | 31.31 (17.73 to 55.27) | <.001 | 9.96 (7.20 to 13.77) | <.001 | |
| Age 25 y | — | Sibling | 12 | 3796 | 0.3 (0.1) | 0.9 (0.3) | 1.00 (Referent) | — | — | — |
| <4 | Moderate | 80 | 11 825 | 0.7 (0.1) | 2.7 (0.4) | 3.36 (1.83 to 6.16) | <.001 | 1.00 (Referent) | — | |
| ≥4 | High | 65 | 1439 | 4.8 (0.8) | 15.0 (2.8) | 24.46 (13.19 to 45.35) | <.001 | 7.28 (5.24 to 10.10) | <.001 | |
| Age 30 y | — | Sibling | 11 | 2923 | 0.3 (0.1) | 0.9 (0.3) | 1.00 (Referent) | — | — | — |
| <4 | Moderate | 62 | 7369 | 0.8 (0.1) | 2.8 (0.5) | 2.98 (1.57 to 5.68) | <.001 | 1.00 (Referent) | — | |
| ≥4 | High | 48 | 1105 | 5.9 (1.0) | 12.1 (2.3) | 18.01 (9.30 to 34.86) | <.001 | 6.04 (4.14 to 8.81) | <.001 | |
| Age 35 y | — | Sibling | 5 | 2077 | 0.3 (0.2) | 0.6 (0.3) | 1.00 (Referent) | — | — | — |
| <5 | Moderate | 31 | 3956 | 1.3 (0.3) | 2.2 (0.5) | 4.09 (1.60 to 10.48) | .003 | 1.00 (Referent) | — | |
| ≥5 | High | 29 | 877 | 6.0 (1.3) | 10.2 (2.5) | 22.54 (8.75 to 58.05) | <.001 | 5.51 (3.32 to 9.17) | <.001 | |
Number at risk varies by outcome and model as they exclude individuals with missing data and account for the sampling weights of survivors of acute lymphoblastic leukemia. CI = confidence interval.
Comparisons are vs the immediate preceding group (eg, high vs moderate).
Two-sided Wald test.
Figure 2.
Model calibration. The predicted and observed incidence rates for heart failure (A), ischemic heart disease (B), and stroke (C) are shown according to moderate- and high-risk status across each 5-year age time point.
Discussion
Our results showed that subsets of childhood cancer survivors are at very high risk of serious cardiovascular disease, reaching or exceeding 10% by age 50 years. In contrast, commonly used general population risk estimators are not designed for younger adults (eg, age <30 years for Framingham, age <40 years for the pooled risk estimator) (10,31). Compared with our models, general population risk estimators may greatly underestimate cardiovascular risk, particularly when known cardiotoxic cancer treatment exposures are not accounted for (examples of patient scenarios, Table 5). As expected, anthracyclines and chest radiation remained influential predictors for heart failure, chest radiation remained an influential predictor for ischemic heart disease, and cranial radiation remained an influential predictor for stroke. Although the prevalence of potentially modifiable conditions such as diabetes, dyslipidemia, or hypertension was relatively low (<10%), even by age 35 years, these conditions often had as much predictive weight as moderate doses of anthracyclines and radiation in many of our models, and therefore, would be important to factor in among affected survivors (32). To facilitate the use of our risk estimators, we have created an online risk calculator (available at ccss.stjude.org/cvcalc).
Table 5.
Illustration of differences between cancer survivor-specific and commonly used general population cardiovascular risk estimators*
| Patient scenario | Childhood Cancer Survivor Study | 2013 ACC and AHA Pooled Risk Model (10) | General Framingham Model (31) |
|---|---|---|---|
| 35-year-old white non-Hispanic man with history of Hodgkin lymphoma diagnosed at age 13 years, treated with anthracyclines (doxorubicin 300 mg/m2), cyclophosphamide (alkylator), and mediastinal and paraaortic radiation (23 Gy). He is overweight (BMI = 28), has blood pressure 118/75 mmHg (not on treatment), total cholesterol 170 mg/dL, and HDL 36 mg/dL (not on treatment), does not have diabetes, and is a nonsmoker. |
|
|
|
| 30-year-old African American woman with history of Wilms tumor diagnosed at age 6 years, treated with anthracyclines (doxorubicin 100 mg/m2), whole-abdomen radiation (11 Gy; includes dose to lower chest), and unilateral nephrectomy. She has normal weight (BMI = 21) and is being treated for hypertension (blood pressure on treatment 125/82 mmHg), total cholesterol 150 mg/dL and HDL 50 mg/dL (not on treatment), does not have diabetes, and is a nonsmoker. |
|
|
|
| 20-year-old white non-Hispanic woman with history of acute lymphoblastic leukemia diagnosed at age 2 years, treated with anthracyclines (doxorubicin 75 mg/m2) and cyclophosphamide (alkylator). She has normal weight (BMI = 22), is not being treated for any risk condition (blood pressure 115/70 mmHg; total cholesterol 150 mg/dL, HDL 45 mg/dL), but is a current smoker. |
|
|
|
*ACC = American College of Cardiology; AHA = American Heart Association; BMI = body mass index; Gy = Gray; HDL = high-density lipoprotein; mmHg = millimeters of mercury.
Most national societies and professional organizations, including the American Heart Association, the American Academy of Pediatrics, and the COG recommend routine screening of blood pressure at clinical encounters for childhood cancer survivors (33,34). The American Heart Association and the American Academy of Pediatrics consider all childhood cancer survivors to be at high risk for accelerated atherosclerosis and recommend a baseline lipid profile and blood glucose in all patients. The COG guidelines recommend repeating the lipid profile and blood glucose (or hemoglobin A1c) every 2 years in those at particularly high risk of dyslipidemia and/or diabetes (ie, those exposed to any form of heart and/or abdominal radiation). Nevertheless, many young adult survivors of childhood cancer may remain underdiagnosed and undertreated (35). Finally, the COG guidelines also contain recommendations for periodic assessment of left ventricular systolic function in patients exposed to anthracyclines and chest radiation and consideration of Doppler ultrasound to assess for early carotid artery disease in those exposed to high doses of neck radiation (≥40 Gy) (34). However, the optimal surveillance frequencies for many of these conditions in cancer survivors remain debatable (13,36–38).
Given these considerations, the development of prediction models with reasonable discrimination offers the possibility of more personalized risk assessment and a more robust platform on which to test the cost-effectiveness of different surveillance strategies. Similar to guidelines established for cardiovascular risk assessment in the general population, our models are primarily based on absolute rather than relative risk estimates, which may be more meaningful when counseling individuals (10). Similarly, our models are by necessity based on group averages and have not been formally evaluated in clinical trials that test whether using these risk estimators will reduce clinical events. Nevertheless, given that well-established treatment options exist for hypertension, dyslipidemia, and diabetes, addressing these conditions at least offers the potential to reduce future cardiovascular risk in high-risk childhood cancer survivors (33). However, it remains to be seen whether the benefits from tight control of cardiovascular risk factors seen in the general population will translate to cancer survivors, in whom the underlying pathophysiology leading to accelerated atherosclerosis following radiation or anthracycline-mediated cardiomyopathy may differ from typical ischemic heart disease, stroke, and heart failure.
Our results have several limitations. Although CCSS conducts periodic linkages with the US National Death Index, it is possible that nonfatal events could be differentially reported and be more or less likely among those lost to follow-up. We have previously shown that the performance of cardiovascular disease prediction models based on CCSS participants’ self-reported outcomes are robust when compared with external cohorts that used prospective clinical ascertainment and/or medical record review (16,17). For the current analysis, the performance of our models over time could be replicated internally only by reserving a random subset of the CCSS data (vs external replication). However, we are unaware of another external cohort that is large enough, with sufficient length of follow-up, and has captured the same breadth of health outcomes that would allow external validation. Nevertheless, as other cohorts mature, attempts at external replication may become possible.
Our models’ performance was only moderate with AUCs and C-statistics around 0.7. Performance may be enhanced if physiologic or laboratory data were available, similar to general population cardiovascular risk estimators (10). We also were unable to incorporate lifestyle factors such as smoking and physical activity into our models, which may also improve model performance. Finally, the proportion of racial and ethnic minorities was relatively low (<20%), and it is possible that our models could perform less well in minority populations (39,40).
Nevertheless, we believe that our relatively simple models can still be useful, because the parameters employed are readily available from most contemporary cancer survivorship treatment summaries and care plans (41). Importantly, the cancer treatment exposures and dose categories featured in these models continue to be used commonly in current oncology treatment protocols (42,43). Although the models we presented require knowledge of anthracycline and radiation doses, our prior research suggests that models with dose information tend to perform better than simpler models that account for exposure alone (without dose information) (16,17). However, given our relatively broad dose categories, it should be possible in many cases to extrapolate an individual’s likely dose given his or her cancer diagnosis and treatment era (42,43).
In summary, general population risk estimators may greatly underestimate the risk of serious cardiovascular disease in survivors of childhood cancer. Therefore, prediction models designed specifically for childhood cancer survivors across a range of young adult ages that account both for cancer treatments and traditional cardiovascular risk factors may play an important role in increasing awareness among survivors as well as their health-care providers of the importance of controlling comorbid cardiovascular conditions. These models may also help improve the rigor of future studies that attempt to refine survivor-specific cardiovascular health surveillance strategies.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers CA21765, CA55727, CA151775, CA204378, CA211996), the American Lebanese Syrian Associated Charities, and the Leukemia and Lymphoma Society.
Notes
None of the funders had any role in the design, conduct, data collection, analysis, interpretation, manuscript preparation, or submission of this study.
Supplementary Material
References
- 1. Robison LL, Hudson MM.. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lipshultz SE, Adams MJ, Colan SD.. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–1995. [DOI] [PubMed] [Google Scholar]
- 3. Mulrooney DA, Armstrong GT, Huang S, et al. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study. Ann Intern Med. 2016;164(2):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Haas EC, Oosting SF, Lefrandt JD, Wolffenbuttel BH, Sleijfer DT, Gietema JA.. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010;11(2):193–203. [DOI] [PubMed] [Google Scholar]
- 7. Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer—a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB.. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. [DOI] [PubMed] [Google Scholar]
- 9. Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. [DOI] [PubMed] [Google Scholar]
- 10. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 11.American College of Cardiology. ASCVD risk estimator plus. http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/. Accessed March 1, 2019.
- 12. Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–4990. [DOI] [PubMed] [Google Scholar]
- 13. Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893–911. [DOI] [PubMed] [Google Scholar]
- 15. Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chow EJ, Chen Y, Kremer LC, et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33(5):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chow EJ, Chen Y, Hudson MM, et al. Prediction of ischemic heart disease and stroke in survivors of childhood cancer. J Clin Oncol. 2018;36(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1 pt 2):141–157. [DOI] [PubMed] [Google Scholar]
- 20. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32(12):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed March 1, 2019.
- 23. Emmons K, Li FP, Whitton J, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2002;20(6):1608–1616. [DOI] [PubMed] [Google Scholar]
- 24. Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2014;32(32):3643–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103(8):1730–1739. [DOI] [PubMed] [Google Scholar]
- 26. Feijen EA, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity [published online ahead of print January 31, 2019]. JAMA Oncol. doi:10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heagerty PJ, Zheng Y.. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61(1):92–105. [DOI] [PubMed] [Google Scholar]
- 28. Harrell FE Jr, Lee KL, Mark DB.. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 29. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed New York: Wiley; 2002. [Google Scholar]
- 30. Zeger SL, Liang KY.. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 31. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. [DOI] [PubMed] [Google Scholar]
- 32. Pepe MS, Kerr KF, Longton G, Wang Z.. Testing for improvement in prediction model performance. Stat Med. 2013;32(9):1467–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: Endorsed by the American Academy of Pediatrics. Circulation. 2006;114(24):2710–2738. [DOI] [PubMed] [Google Scholar]
- 34. Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121(2):e387–e396. [DOI] [PubMed] [Google Scholar]
- 35. Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen AB, Punglia RS, Kuntz KM, Mauch PM, Ng AK.. Cost effectiveness and screening interval of lipid screening in Hodgkin’s lymphoma survivors. J Clin Oncol. 2009;27(32):5383–5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeh JM, Nohria A, Diller L.. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Ann Intern Med. 2014;160(10):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wong FL, Bhatia S, Landier W, et al. Cost-effectiveness of the Children’s Oncology Group Long-Term Follow-Up Screening Guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 2014;160(10):672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Q, Leisenring WM, Ness KK, et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(14):1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fox ER, Samdarshi TE, Musani SK, et al. Development and validation of risk prediction models for cardiovascular events in black adults: the Jackson Heart Study cohort. JAMA Cardiol. 2016;1(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hewitt M, Greenfield S, Stovall E; National Cancer Policy Board Committee on Cancer Survivorship. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: Institute of Medicine and National Research Council, National Academies Press; 2006. [Google Scholar]
- 42. Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60(7):1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.