Abstract
Background
Cancer screening is a complex process encompassing risk assessment, the initial screening examination, diagnostic evaluation, and treatment of cancer precursors or early cancers. Metrics that enable comparisons across different screening targets are needed. We present population-based screening metrics for breast, cervical, and colorectal cancers for nine sites participating in the Population-based Research Optimizing Screening through Personalized Regimens consortium.
Methods
We describe how selected metrics map to a trans-organ conceptual model of the screening process. For each cancer type, we calculated calendar year 2013 metrics for the screen-eligible target population (breast: ages 40–74 years; cervical: ages 21–64 years; colorectal: ages 50–75 years). Metrics for screening participation, timely diagnostic evaluation, and diagnosed cancers in the screened and total populations are presented for the total eligible population and stratified by age group and cancer type.
Results
The overall screening-eligible populations in 2013 were 305 568 participants for breast, 3 160 128 for cervical, and 2 363 922 for colorectal cancer screening. Being up-to-date for testing was common for all three cancer types: breast (63.5%), cervical (84.6%), and colorectal (77.5%). The percentage of abnormal screens ranged from 10.7% for breast, 4.4% for cervical, and 4.5% for colorectal cancer screening. Abnormal breast screens were followed up diagnostically in almost all (96.8%) cases, and cervical and colorectal were similar (76.2% and 76.3%, respectively). Cancer rates per 1000 screens were 5.66, 0.17, and 1.46 for breast, cervical, and colorectal cancer, respectively.
Conclusions
Comprehensive assessment of metrics by the Population-based Research Optimizing Screening through Personalized Regimens consortium enabled systematic identification of screening process steps in need of improvement. We encourage widespread use of common metrics to allow interventions to be tested across cancer types and health-care settings.
Effective population-based cancer screening can be achieved only if there is high participation by screen-eligible individuals coupled with appropriate and timely follow-up of abnormal findings. Organized screening programs typically report the percentage of the population accepting an invitation to undergo screening (1,2) and additionally within groups targeted for outreach (3,4). In the United States, opportunistic screening (initiated by the patient or provider during a clinical visit) is common, and organized screening outreach programs are largely limited to integrated health-care systems (5). National estimates of screening participation are obtained from self-reported data such as the National Health Interview Survey (NHIS) (6,7) and the Behavioral Risk Factor Surveillance System survey (BRFSS) (8). Other sources of information on screening participation come from claims data (9) and electronic health records (10,11).
Participation is the first step in the complex process of screening that begins with the screening step, followed by testing of those screened positive, and then follow-up for a definitive diagnosis and initiation of therapy in those with abnormalities. Conceptual models of screening reflect this complex process that differs across cancer types (12–15). The screening process begins with a population at risk initially participating in a given modality and is followed by further testing, diagnostic procedures, and treatment if warranted. Most organized screening programs, national surveys, and quality metrics (eg, Healthcare Effectiveness Data and Information Set [HEDIS]) assess screening participation; fewer assess downstream steps in the screening process. Completion of the entire screening process is dependent on a hierarchy of individual factors, providers, health-care systems, and policies that influence its success (16–18). To investigate these factors, the National Cancer Institute funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR), a consortium comprised of 10 research centers across the United States (19). We describe population-based metrics to accompany our trans-organ conceptual model (15) and provide an example of how the metrics can assess key steps in the screening continuum. We report data from PROSPR allowing comparison with international data of screening participation, timely follow-up of abnormal examinations by age groups, and cancer outcomes following screening (20,21). Our operationalization of the metrics across cancer types can serve as a resource for others seeking to move beyond evaluating screening uptake and to comprehensively measure the entire screening continuum (22,23).
Methods
Study Setting
The overall aim of PROSPR is to conduct multi-site, coordinated, transdisciplinary research to evaluate and improve breast, cervical, and colorectal cancer screening. The PROSPR Research Centers reflect the diversity of US health-care delivery models. Breast data were derived from four sites: the University of Vermont, capturing data from all women receiving breast imaging at radiology facilities in the state of Vermont; the University of Pennsylvania, collecting data from an integrated health-care delivery system; and Dartmouth-Hitchcock Health System in New Hampshire and Brigham and Women’s Hospital in Massachusetts, capturing data within their primary care practice networks. Cervical data were obtained from five sites: Kaiser Permanente Washington (formerly Group Health), a mixed-model health-care system in Washington state; Kaiser Permanente Northern California and Kaiser Permanente Southern California, integrated health-care systems in California; Parkland-University of Texas Southwestern, which is the sole safety-net provider for underinsured and uninsured Dallas County residents; and the New Mexico HPV Pap Registry located at the University of New Mexico, gathering data from all women in New Mexico undergoing cervical cancer screening, diagnosis, and treatment. All cervical sites except New Mexico also collected colorectal cancer screening data. Additional site details have been published previously (13,14,24).
Screening data were extracted from health information systems within the period 2010–2014. Cancer outcome data were available from Surveillance, Epidemiology, and End Results registries; statewide cancer registries; local tumor registries; and pathology records. All sites submitted common data elements to a statistical center that harmonized data, conducted data quality assurance, and completed pooled analyses.
Approach
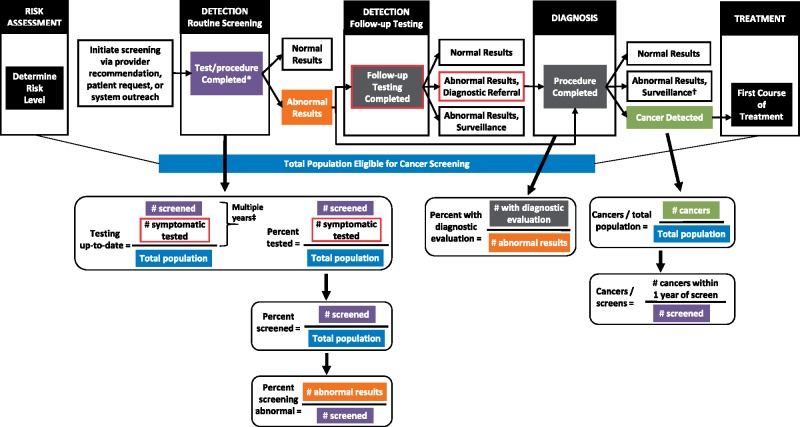
Figure 1 illustrates metrics mapped across the cancer screening continuum. Although screening modalities differ across cancer types, unifying measures can assess how each goal of the model (eg, detection to follow-up testing) track with multiple steps (clinical care activities) and describe if those needing downstream steps in the continuum received appropriate and timely care. We assess major components in the continuum such as routine screening, detection, follow-up, and diagnosis. This includes quantifying screening participation during a calendar year and identifying those who screen abnormal and thus need further follow-up testing and/or diagnostic assessment. We then assess whether abnormal screens are evaluated with the appropriate follow-up test or diagnostic evaluation procedure in a timely manner according to management guidelines and whether cancer is diagnosed in the screened population and the larger screening-eligible population.
Figure 1.
Populations for calculating 2013 cancer screening metrics. The figure shows the Population-based Research Optimizing Screening through Personalized Regimens conceptual model adapted from Beaber et al. (15). The operational definition for each metric is illustrated based on the relevant step of the screening process. *Screening tests include mammography (breast), Papanicolaou (Pap) or Pap/human papillomavirus (cervical), and fecal occult blood tests or fecal immunochemical tests, sigmoidoscopy, or colonoscopy (colorectal).
†For cervical and colorectal detected abnormalities, excisional treatment may precede surveillance.
‡Duration depends on cancer type and screening modality. Specific components for each metric are detailed in Table 1.
We include only some prospective measures of the screening process. Each cancer type has its own specific complexities including multiple approved screening modalities and distinct recommendations for follow-up tests or diagnostic procedures based on severity of screening results. We developed simple definitions (Figure 1; Table 1) as a common framework for describing screening performance across cancer types, acknowledging the availability of more detailed cancer-specific metrics.
Table 1.
Definitions of cancer screening process metrics in PROSPR
| Screening metrics | Breast | Cervical | Colorectal |
|---|---|---|---|
| Population* | Women ages 40–74 y with no known history of breast cancer and ≥30 d in PROSPR cohort | Women ages 21–64 y with no known history of invasive cervical cancer or removal of cervix and ≥30 d in PROSPR cohort | Men and women ages 50–75 y with no known history of invasive colorectal cancer, colectomy, or proctectomy, and ≥30 d in PROSPR cohort |
| Screening participation | |||
| Tested in a calendar year | Receipt of breast imaging | Receipt of Pap test or co-testing | Receipt of gFOBT/FIT that is not in-office, flexible sigmoidoscopy, or colonoscopy |
| Screened in a calendar year | Receipt of breast imaging with indication of screening and no other breast imaging within 3 mo prior | Receipt of a Pap test with no other Pap test within 300 d prior | Receipt of gFOBT/FIT that is not in-office, flexible sigmoidoscopy, or colonoscopy with an indication of screening† |
| Up-to-date on testing in 2013‡ | Receipt of breast imaging in 2012 or 2013 | Receipt of a Pap test during 2010–2013 | Receipt of gFOBT/FIT that is not in-office during 2012–2013, flexible sigmoidoscopy during 2008–2013, or colonoscopy during 2003–2013 |
| Initial screening outcomes and diagnostic evaluation | |||
| Percent abnormal on screening test§ | BI-RADS 0, 3, 4, or 5 result among those who received screening‖ | The following results among those who received a screening Pap: If Pap only: LSIL or higher grade result (LSIL, ASC-H, HSIL, squamous cell carcinoma, AGC, AIS, suspicious for malignancy, adenocarcinoma, carcinoma NOS); | Abnormal gFOBT/FIT result among those who received gFOBT/FIT that was not in-office; only gFOBT/FIT screens included because colonoscopy and sigmoidoscopy may lead to removal of cancer precursors and invasive cancer, complicating the definition of an abnormal screen |
| If Pap and high-risk HPV results: ASC-US/high-risk HPV+, LSIL or higher grade result/any HPV result; | |||
| If Pap and genotype HPV results: any HPV 16+ and/or HPV 18+ | |||
| Percent with timely diagnostic evaluation¶ | Among those with abnormal screening results, additional breast imaging or biopsy within 3 mo (9 mo for BI-RADS 3) of abnormal result | Among those with abnormal screening results, any colposcopy or cervical biopsy within 15 mo of abnormal result | Among those with abnormal gFOBT/FIT results, colonoscopy within 6 mo of abnormal result |
| Cancer incidence in screened population#,** | Invasive breast cancer or DCIS diagnoses within 1 y of screening test among those who received screening | Invasive cervical cancer diagnoses within 1 y of screening test among those who received screening | Invasive colorectal cancer diagnoses within 1 y of screening test among those who received screening |
| Cancer incidence in overall population** | Invasive breast cancer or DCIS diagnoses among entire eligible population | Invasive cervical cancer diagnoses among entire eligible population | Invasive colorectal cancer diagnoses among entire eligible population |
Age is calculated based on age at the end of the calendar year. US Census and American Community Survey data are used to estimate the population for statewide registry sites (Vermont and New Mexico). AGC = atypical glandular cells; AIS = adenocarcinoma in situ; ASC-H = high-grade squamous intraepithelial lesions; ASC-US = atypical squamous cells of undetermined significance; BI-RADS = Breast Imaging Reporting and Data System; DCIS = ductal carcinoma in situ; gFOBT/FIT= guaiac fecal occult blood test or fecal immunochemical test; HPV = human papillomavirus; HSIL = high-grade squamous intraepithelial lesions; LSIL = low-grade squamous intraepithelial lesions; NOS = not otherwise specified; Pap = Papanicolaou test; PROSPR = Population-based Research Optimizing Screening through Personalized Regimens.
Colonoscopy indication was assigned using the Kaiser Permanente standardized algorithm.
Calculated among individuals who did not exit the cohort from 2010 to 2013 for Kaiser Permanente Washington, Kaiser Permanente Northern California, Kaiser Permanente Southern California, and Parklad–University of Texas Southwestern. Calculated only for years 2012–2013 for University of New Mexico cervical data.
The initial BI-RADS result was used if there were multiple exams on the same day. Sometimes a BI-RADS 0 result was inferred based on other imaging or procedures performed on the same date.
For cervical, the first screening Pap in the calendar year was used if there were multiple screening Pap tests in a calendar year. Due to screening management guidelines, women younger than 25 years of age with LSIL results were not classified as abnormal.
Limited to those with sufficient observation follow-up.
Limited to those with sufficient observation follow-up. If there were multiple screens in the calendar year, the first screen was used.
Kaiser Permanente Washington cervical and colorectal calculations were restricted to individuals residing in an area covered by the Seattle-Puget Sound Surveillance, Epidemiology, and End Results registry.
Computation of Screening Metrics
We calculated metrics in calendar year 2013 among individuals who were age-eligible for breast, cervical, or colorectal cancer screening according to United States Preventive Services Task Force recommendations and were at risk for the cancer of interest. Some cancer rates were based on 2012 data instead due to incompleteness of 2013 data. Table 1 summarizes the study populations and the inclusion criteria. Briefly, we focused on three screening-eligible populations: breast: women ages 40–74 years with no known history of breast cancer; cervical: women ages 21–64 years with no known history of invasive cervical cancer or removal of the cervix; and colorectal: women and men ages 50–75 years with no known history of invasive colorectal cancer, colectomy, or proctectomy. Additionally, we calculated all metrics stratified by age groups (breast: ages 40–49, 50–64, and 65–74 years; cervical: ages 21–29, 30–49, and 50–64 years; colorectal: ages 50–64 and 65–75 years). The Vermont and New Mexico sites were statewide registries so we used US Census and American Community Survey data to estimate the population size for each age group.
Screening Participation
For each cancer-specific study population, we computed the percentage tested or screened in calendar year 2013. The percentage tested included all tests regardless of the indication, whereas the percentage screened was restricted to tests with an indication of screening as recorded or estimated by an algorithm. For breast cancer screening, exams were limited to those with no other breast imaging within the prior 3 months and were identified via structured codes and natural language processing (25). Cervical cancer screening was defined as Papanicolaou (Pap) tests with no other Pap tests in the prior 300 days (18). Colorectal cancer screening tests included fecal occult blood tests or fecal immunochemical tests (gFOBT/FIT) that were not in-office, flexible sigmoidoscopies, and colonoscopies with an indication of screening assigned by an algorithm (26). Age-stratified population metrics use age on the last day of the calendar year for computation.
To better estimate the prevalence of screening coverage in the population, we calculated the percentage up-to-date with breast, cervical, and colorectal testing in 2013. This metric included those receiving testing regardless of indication in 2013 and those not in need of screening based on testing in prior years (27). We restricted the population to individuals who were part of the cohort long enough to assess testing before 2013. For colorectal cancer screening, the duration of follow-up was not long enough to guarantee that all previous testing was observed because colonoscopy during the prior 10 years might not be ascertained.
Initial Screening Outcomes and Diagnostic Evaluation
We calculated the percentage of abnormal screening exams stratified by age at the time of the screen. We defined an abnormal breast cancer screen as Breast Imaging-Reporting and Data System 0, 3, 4, or 5. For cervical cancer screening, we limited abnormal results (atypical squamous cells of undetermined significance/human papillomavirus (HPV) + or higher) to those requiring a follow-up colposcopy or cervical biopsy (28). For colorectal cancer screening, we included only abnormal gFOBT/FIT results, which are well defined and require definitive action with a diagnostic colonoscopy. We computed diagnostic evaluation among those with abnormal screening results using cancer-specific follow-up intervals—3 months for additional breast imaging or biopsy (9 months for short-term follow-up assessments), 15 months for colposcopy with or without biopsy, and 6 months for diagnostic colonoscopy.
Cancer Diagnosis
A common measure of screening benefit is the yield of cancers diagnosed among the screened population. For each cancer type, we calculated the percentage of patients with incident cancers detected within 1 year of a screening test among those who received screening and had at least 1 year of follow-up. Additionally, we computed the incidence of cancers in the entire screening-eligible population regardless of whether screened.
Because we report descriptive statistics only, we did not compute P values. We also calculated all screening process metrics by individual site to assess variability in the metrics across health-care systems. All analyses were performed using SAS Version 9.4 (SAS Institute). All activities were approved by the institutional review boards of the research centers and included waivers of informed consent for participants.
Results
Study population characteristics are shown in Table 2 for each target screening population. The estimated screening-eligible populations in 2013 were 305 568 participants for breast, 3 160 128 for cervical, and 2 363 922 for colorectal cancer screening. The age range 50–64 years is included in screening recommendations for all cancer types and comprises more than two-thirds of those recommended for colorectal cancer screening, one-half of the target for breast screening, and one-third of those recommended for cervical screening. The breast population resides in the northeast United States and is largely non-Hispanic white and black. Both the cervical and colorectal cancer populations are more racially diverse and include a large Hispanic population. Most participants had health insurance because they were identified through their health plan.
Table 2.
Demographics of the Population-based Research Optimizing Screening through Personalized Regimens populations in 2013
| Demographic | Breast* No. (%) | Cervical† No. (%) | Colorectal No. (%) |
|---|---|---|---|
| Estimated population | 305 568 (100.0) | 3 163 677 (100.0) | 2 363 922 (100.0) |
| Age group, y | |||
| 21–29 | — | 692 070 (21.9) | — |
| 30–39 | — | 748 832 (23.7) | — |
| 40–49 | 91 864 (30.1) | 710 092 (22.4) | — |
| 50–64 | 151 425 (49.6) | 1 012 683 (32.0) | 1 647 781 (69.7) |
| 65–75 (colorectal) and 65–74 (breast) | 62 279 (20.3) | — | 716 141 (30.3) |
| Race/ethnicity‡ | |||
| Non-Hispanic white | 246 601 (81.8) | 1 233 177 (41.2) | 1 212 005 (54.8) |
| Non-Hispanic black | 33 198 (11.0) | 240 118 (8.1) | 203 029 (9.2) |
| Hispanic | 9210 (3.1) | 1 025 846 (34.6) | 473 646 (21.4) |
| Asian/Pacific Islander | 6526 (2.2) | 380 503 (12.8) | 288 791 (13.1) |
| Native American/Alaskan Native | 654 (0.2) | 61 740 (2.1) | 7147 (0.3) |
| Other/multiracial | 5102 (1.7) | 34 313 (1.2) | 26 728 (1.2) |
| Unknown/missing§ | 4277 (1.4) | 197 980 (6.3) | 152 576 (6.4) |
| Enumerated population | 250 785 | 2 568 424 | 2 363 922 |
| Sex‡ | |||
| Female | 250 785 (100.0) | 2 568 424 (100.0) | 1 251 460 (52.9) |
| Male | — | — | 1 112 341 (47.1) |
| Unknown/missing§ | (0.1) | ||
| Health insurance‡ | |||
| Medicaid | 11.019 (7.3) | 85 316 (3.4) | 26 859 (1.1) |
| Medicare | 29 239 (19.5) | 72 167 (2.9) | 704 842 (30.0) |
| Commercial/private | 102 994 (68.7) | 2 281 125 (90.6) | 1 587 302 (67.5) |
| Other government | 3782 (2.5) | 23 452 (0.9) | 3878 (0.2) |
| Uninsured/charity | 2882 (1.9) | 55 092 (2.2) | 28 855 (1.2) |
| Unknown/missing§ | (40.2) | 51 272 (2.0) | 12 186 (0.5) |
| Charlson comorbidity‡ | |||
| 0 | 93 730 (72.3) | 1 688 705 (82.3) | 1 253 816 (62.4) |
| 1–3 | 32 034 (24.7) | 334 592 (16.3) | 619 613 (30.8) |
| 4+ | 3895 (3.0) | 27 855 (1.4) | 136 175 (6.8) |
| Unknown/missing§ | 121 126 (48.3) | 517 272 (20.1) | 354 318 (15.0) |
For one site in the breast population, the population is estimated from Census data classified by age and race or ethnicity. For the three remaining sites, the enumerated population is included. Health insurance status and Charlson comorbidity score are not collected at that one site so the percentage of missing data for the combined breast population is high.
For one site in the cervical population, the population is estimated from Census data classified by age and race or ethnicity. For the four remaining sites the enumerated population is included. Charlson comorbidity score is not collected at that one site so the percentage of missing data for the combined cervical population is high.
Percentages are given for nonmissing data for race or ethnicity, sex, health insurance, and Charlson comorbidity score so sum to 100%.
This row gives the percentage of the population sample for whom that variable was not collected.
The metrics are provided for breast, cervical, and colorectal (Table 3) screening by age group. Approximately 35–48% of women were screened in 2013 for breast cancer with the percentage increasing by age. Because screening is recommended every 2 years, the percentage of women classified as up-to-date on testing was much higher because it reflects testing in 2012–2013. The percentage of abnormal screening examinations decreased with age and ranged from 8.2 to 14.6%. Overall, 41.9% were screened in 2013, with 63.5% considered up-to-date on testing and 10.7% of those screened had an abnormal finding. Almost all women (96.8%) with an abnormal result received timely follow-up. The overall cancer incidence among those screened was 5.66 per 1000 in 2013, and population incidence overall was 3.18 per 1000 women. The pattern for percentage screened by age was similar for all sites, and absolute differences across sites were small (Figure 2A). Supplementary Table 1 (available online) shows the metrics by sites collapsed across age groups and Supplementary Table 2 (available online) shows age-specific metrics for all four sites.
Table 3.
Cancer screening process metrics by age group
| Screening metric | Panel A: breast cancer screening Age group, y |
Panel B: cervical cancer screening Age group, y |
Panel C: colorectal cancer screening Age group, y |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 40–74 | 40–49 | 50–64 | 65–74 | 21–64 | 21–29 | 30–49 | 50–64 | 50–75 | 50–64 | 65–75 | |
| N | 305 568 | 91 864 | 151 425 | 62 279 | 3 160 128 | 689 945 | 1 458 783 | 1 011 400 | 2 363 922 | 1 647 781 | 716 141 |
| Percent tested | 44.6 | 38.0 | 46.1 | 50.8 | 25.7 | 27.3 | 27.1 | 22.6 | 40.3 | 39.5 | 42.3 |
| Percent screened | 41.9 | 35.2 | 43.5 | 47.7 | 25.0 | 26.3 | 26.4 | 22.2 | 37.7 | 37.4 | 38.67 |
| Percent testing up-to-date | 63.5 | 53.7 | 66.0 | 71.1 | 84.6 | 72.1 | 89.5 | 84.4 | 77.5 | 73.7 | 85.9 |
| Percent screening abnormal | 10.7 | 14.6 | 9.8 | 8.2 | 4.4 | 6.2 | 4.7 | 2.4 | 4.5 | 4.1 | 5.4 |
| Percent with diagnostic evaluation* | 96.8 | 97.0 | 96.7 | 96.8 | 76.22 | 69.4 | 80.7 | 76.2 | 76.3 | 77.6 | 74.2 |
| Cancers per 1000 screens* | 5.66 | 3.73 | 5.41 | 8.37 | 0.17 | 0.05 | 0.19 | 0.22 | 1.46 | 1.29 | 1.84 |
| Cancers per 1000 persons in the population* | 3.18 | 2.07 | 3.12 | 4.96 | 0.07 | 0.02 | 0.09 | 0.08 | 0.77 | 0.60 | 1.19 |
In the year 2013 for panel A. In the year 2012 for panels B and C.
Figure 2.
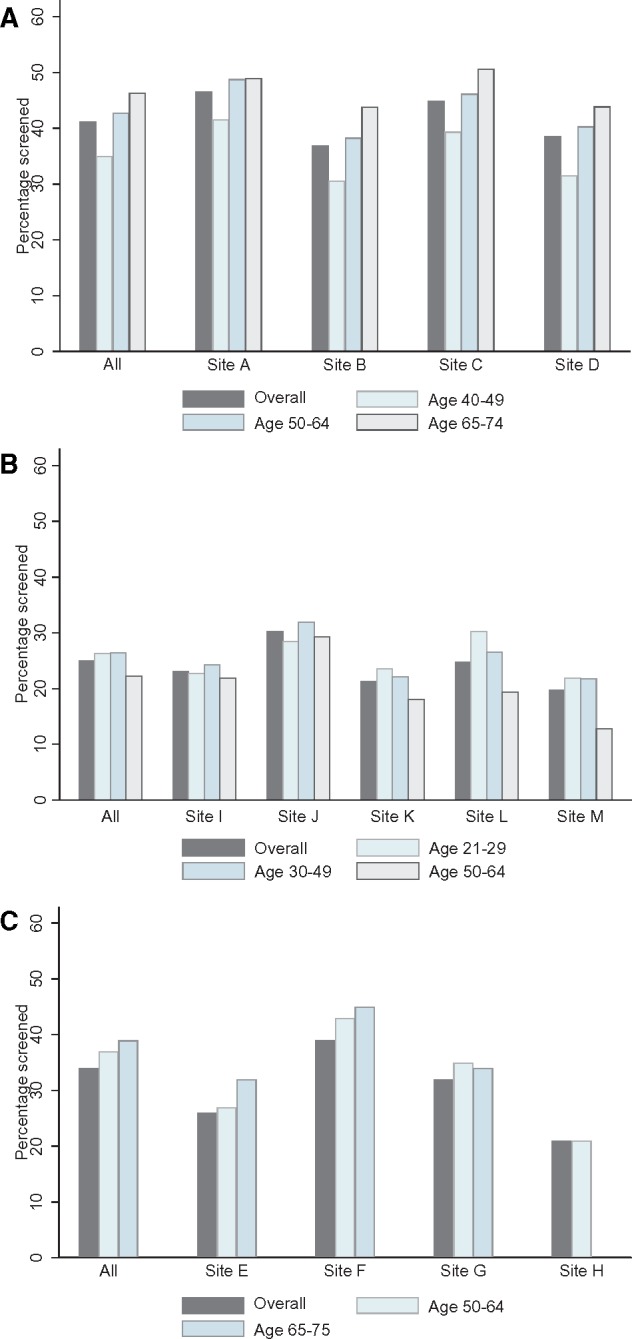
Percentage of the Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) population screened in 2013 by cancer type. The figure shows one metric, percentage of the population screened in the calendar year 2013, for the three types of cancer screening: A) breast, B) cervical, and C) colorectal. Within each cluster, the percentage screened is given over all age groups and then by each age group. The clusters are all sites combined, followed by each PROSPR research site.
Only one-quarter (25.0%) of participants received cervical cancer screening in 2013, but the percentage who were up-to-date with testing was very high (84.6%) because cervical screening affords several years of coverage (Table 3). The percentage of women with abnormal cervical screens was 4.4% and of those 76.2% had timely follow-up after the abnormal screen. The cancer incidence per screen overall was low and increased with age. The cancer incidence in the population was lowest in women aged 21–29 years, but similar in the two upper age groups. Overall cervical cancer rates were 0.17 per 1000 women screened. Cervical cancer screening metrics varied moderately across sites (Figure 2B; Supplementary Tables 1 and 3, available online).
Colorectal cancer testing and screening were similar between the two age groups in 2013, and being up-to-date for colorectal cancer testing increased with age though the overall percentage was high (77.5%). Of those screened with gFOBT/FIT, 4.5% had abnormal findings and of those 76.3% had subsequent timely follow-up (Table 3). Diagnostic evaluation was similar by age, but cancer incidence for the entire population and those screened increased with age. For every 1000 individuals screened, 1.46 cancers were found within 1 year. Colorectal cancer screening demonstrated greater between-site variation in follow-up than breast or cervical screening (Figure 2C;Supplementary Table 4, available online).
Figure 3 shows how the PROSPR measures of being up-to-date on testing compare with self-reported survey results from the 2015 NHIS and from the 2013–2014 BRFSS survey using the median of the individual states’ results (7,29). For breast screening, we restricted the comparison to women aged 50–74 years and observed that self-reported results from NHIS were only 4% higher than the observed results for women undergoing mammography in the last 2 years within the PROSPR consortium. For cervical cancer screening, all three results were similar with a range of only 2%. For colorectal cancer screening, the differences were much larger with the percentage up-to-date much higher in PROSPR than either the BRFSS or NHIS.
Figure 3.
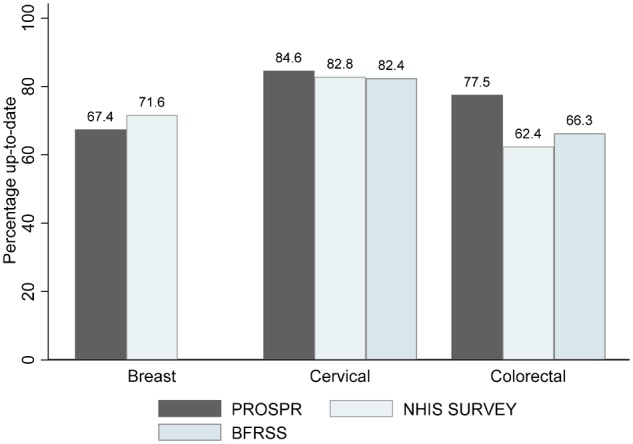
Percentage of the age-eligible Population-based Research Optimizing Screening through Personalized Regimens population who was up-to-date with breast, cervical, or colorectal cancer testing compared with data from self-report. The figure compares the percentage of the population up-to-date on testing compared with survey-based derived results from the 2015 National Health Interview Survey (NHIS) (7) and from the 2015 Behavioral Risk Factor Surveillance System (BRFSS) survey (29).
Discussion
Despite widely different screening strategies and modalities for each cancer type, we provide a general comprehensive framework of screening metrics overlaid on a conceptual model of the entire screening process. We provide comparable metrics for breast, cervical, and colorectal cancer screening, describing participation, short-term outcomes, and cancer incidence in a unified manner that allows comparison across cancer types and diverse health-care systems.
The percentage up-to-date on testing for each cancer is an appropriate gauge of screening participation as it depends on screening interval. We found most participants were up-to-date for testing with estimates of 63.5%, 84.6%, and 77.5% for breast, cervical, and colorectal cancer, respectively. We compared these estimates with those based on survey data where one might expect some overreporting of screening participation or recency (30). PROSPR’s estimate for being up-to-date on breast cancer testing was slightly less than that reported in the most recent NHIS survey (7). Our estimate for colorectal cancer testing exceeded both the BRFSS (29) and NHIS (7) survey estimates considerably, whereas our estimate for cervical cancer testing only slightly exceeded the survey results. The PROSPR cervical and colorectal cancer screening populations are largely from integrated care organizations that focus on delivering preventive care services and thus induce high screening participation, so these may be overestimates of screening in more general populations.
The probability of a screen being abnormal depends on many factors, including the definition of “abnormal,” but on average is 4–5% for cervical and colorectal cancer and 11% for breast cancer screening. The Mammography Quality Standards Act provided strong guidelines about accreditation of radiologists, performance of the examination, and reporting of results to patients (31). Due to these strict standards, abnormal exams in breast cancer screening are almost uniformly followed by diagnostic action. However, for cervical and colorectal cancer screening, only 76% of abnormal screens have timely diagnostic evaluation recorded in PROSPR. Research is ongoing within PROSPR to determine why subsequent diagnostic work-up does not occur. Finally, we report the cancer rates for both the screened and screening-eligible population. Incidence is greatest for breast cancer, then colorectal cancer, followed by cervical cancer.
In the United States there are few reports of metrics for breast, cervical, and colorectal cancer screening that follow a target population from risk assessment through cancer diagnosis. Although many organizations calculate HEDIS screening participation measures, there has been little direct examination or comparison of HEDIS measures to date (32). The comprehensive Ontario Cancer Screening Performance Report 2016 includes performance metrics for all three cancer types (3). Similar to our findings, they report that about 64.8% of women in the age range 50–74 years are up-to-date on breast cancer screening. Of those, about 8.6% are abnormal with 98.4% receiving follow-up within 6 months. They also report 63.4% of women aged 21–69 years are up-to-date on cervical screening. Approximately 5.2% of Ontario women had an abnormal cervical screen and 84.0% had follow-up after receiving notification of a high-grade abnormal screen, similar to our PROSPR results. For those aged 50–74 years, 39.9% needed colorectal cancer screening in 2014, suggesting 60.1% were up-to-date. Of those screened with gFOBT/FIT, 4.0% were abnormal and 77.1% had colonoscopy within 6 months. We found higher participation for cervical and colorectal cancer screening, but not breast, in the PROSPR vs the Ontario population. However, once an individual was tested, the outcomes for colorectal cancer were comparable between the Ontario and PROSPR populations. This increases confidence in the PROSPR outcomes as being representative of the underlying population.
Organized screening programs in Europe are supported through government-funded health plans with guidelines for performance. The breast screening guidelines (4th edition) have many indicators of quality that specify both acceptable and desirable targets of performance (33,34). For example, the target participation percentages for women invited to screening are 70% (acceptable) and 75% (desirable). Cervical quality assurance guidelines have also been established (35). Guidelines for colorectal cancer screening are recent and specify acceptable (45%) and desirable (65%) levels for colorectal cancer screening participation (36,37).
We presented a limited set of metrics, though others can be calculated (eg, positive and negative predictive value, cancer detection rate, sensitivity, specificity, advanced cancer rate, and overdiagnosis). We chose to focus on metrics related to health-care delivery rather than characteristics of the screening tools. For breast cancer screening with digital mammography, there are excellent guidelines about the performance of the screening test that can be used for comparison with an individual radiologist’s performance (38). However, the performance of screening in population-based settings depends not only on screening test performance, but also on whether it is used appropriately in the population. PROSPR plans to extensively evaluate multilevel influences (eg, provider, facility, health system) as well as patient factors on these outcomes to improve the impact of screening. We intentionally considered only general trends collapsed across health-care systems, though differences between systems and individual groups have been a focus of other PROSPR publications (39–53). Our primary goal was to provide performance metrics across different cancer types. This encourages standardization across cancers and enables testing of interventions across cancer types. We calculated these metrics for different sites in the PROSPR network to showcase how assessment of cancer screening could be a unified activity rather than specific to a cancer type.
It is uncertain whether our findings can be generalized beyond the nine health systems we examined because there are limitations by geography, type of health-care systems, and restriction to a mostly insured population. We provide a framework for calculating metrics that we hope other health-care systems can use to derive their own screening metrics. In these analyses it was necessary to combine a variety of cancer-specific tests. Each screening type may have multiple definitions of an “abnormal” screen and appropriate action. Colonoscopy and colposcopy can directly eliminate cancer precursors though most screening tests lead to more diagnostic work-up and biopsies. The outcome of one screen may influence the follow-up schedule for the next screen. The ability to assess being up-to-date depends on availability of past screening history, and assessment of long-term outcomes depends on adequate follow-up after a screening examination. These differences will be covered in detail in future PROSPR publications.
Despite these limitations, PROSPR allows a comprehensive view of the screening process across cancer types. Metrics are proposed for systematic assessments of steps in the cancer screening process. These metrics provide a standardized method for comparing different settings, and the examples provided allow comparison with organized screening programs outside the United States. PROSPR is establishing uniform definitions that will allow prospective collection and comparison across both sites and cancer types. With longer follow-up, we will characterize screening cohorts and follow individuals through each screening cycle to characterize cancer detection in each round of screening as well as subsequent survival and possible overdiagnosis. Importantly, we will learn not only how to enhance appropriate uptake and repeat screening but also improve follow-up and diagnosis, thereby reducing mortality and morbidity.
Funding
This work was supported by the National Cancer Institute (NCI)-funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium (grant numbers U54CA163262, U54CA163262-04S1, U54CA163261, U54CA163261-04S1, U54CA163308, U54CA163308-04S1, U54CA163313, U54CA163303, U54CA163307, U54CA164336, and U01CA163304).
Notes
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the United States Government.
Potential conflicts of interest: None.
The authors thank the participating PROSPR Research Centers for the data they have provided for this study. A listing of PROSPR investigators and staff is provided on our website: http://healthcaredelivery.cancer.gov/prospr/.
Supplementary Material
References
- 1. Bulliard JL, Garcia M, Blom J, Senore C, Mai V, Klabunde C.. Sorting out measures and definitions of screening participation to improve comparability: the example of colorectal cancer. Eur J Cancer. 2014;50(2):434–446. [DOI] [PubMed] [Google Scholar]
- 2. Klabunde C, Blom J, Bulliard JL, et al. Participation rates for organized colorectal cancer screening programmes: an international comparison. J Med Screen. 2015;22(3):119–126. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Care Ontario. Ontario Cancer Screening Performance Report 2016. Toronto: Cancer Care Ontario; 2016. [Google Scholar]
- 4. Guiriguet C, Pera G, Castells A, et al. Impact of comorbid conditions on participation in an organised colorectal cancer screening programme: a cross-sectional study. BMC Cancer. 2017;17(1):524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gould MK, Sakoda LC, Ritzwoller DP, et al. Monitoring lung cancer screening use and outcomes at four cancer research network sites. Ann Am Thorac Soc. 2017;14(12):1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watson M, Benard V, King J, Crawford A, Saraiya M.. National assessment of HPV and Pap tests: changes in cervical cancer screening, National Health Interview Survey . Prev Med. 2017;100:243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chowdhury PP, Mawokomatanda T, Xu F, et al. Surveillance for certain health behaviors, chronic diseases, and conditions, access to health care, and use of preventive health services among states and selected local areas–behavioral risk factor surveillance system, United States, 2012. MMWR Surveill Summ. 2016;65(4):1–142. [DOI] [PubMed] [Google Scholar]
- 9. Naimer MS, Kwong JC, Bhatia D, et al. The effect of changes in cervical cancer screening guidelines on chlamydia testing. Ann Fam Med. 2017;15(4):329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bailey SR, Heintzman JD, Marino M, et al. Measuring preventive care delivery: comparing rates across three data sources. Am J Prev Med. 2016;51(5):752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan KS, Fowles JB, Weiner JP.. Review: electronic health records and the reliability and validity of quality measures: a review of the literature. Med Care Res Rev. 2010;67(5):503–527. [DOI] [PubMed] [Google Scholar]
- 12. Taplin SH, Rodgers AB.. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010(40):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Onega T, Beaber EF, Sprague BL, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer. 2014;120(19):2955–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tiro JA, Kamineni A, Levin TR, et al. The colorectal cancer screening process in community settings: a conceptual model for the population-based research optimizing screening through personalized regimens consortium. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beaber EF, Kim JJ, Schapira MM, et al. Unifying screening processes within the PROSPR consortium: a conceptual model for breast, cervical, and colorectal cancer screening. J Natl Cancer Inst. 2015;107(6):djv120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taplin SH, Anhang Price R, Edwards HM, et al. Introduction: understanding and influencing multilevel factors across the cancer care continuum. J Natl Cancer Inst Monogr. 2012;2012(44):2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anhang Price R, Zapka J, Edwards H, Taplin SH.. Organizational factors and the cancer screening process. J Natl Cancer Inst Monogr. 2010;2010(40):38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zapka J, Taplin SH, Price RA, et al. Factors in quality care—the case of follow-up to abnormal cancer screening tests—problems in the steps and interfaces of care. J Natl Cancer Inst Monogr. 2010;2010(40):58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) http://healthcaredelivery.cancer.gov/prospr/. Accessed July 26, 2017.
- 20. Tosteson AN, Beaber EF, Tiro J, et al. Variation in screening abnormality rates and follow-up of breast, cervical and colorectal cancer screening within the PROSPR consortium. J Gen Intern Med. 2016;31(4):372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doubeni CA, Gabler NB, Wheeler CM, et al. Timely follow-up of positive cancer screening results: a systematic review and recommendations from the PROSPR Consortium. CA Cancer J Clin. 2018;68(3):199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NordScreen http://nordscreen.org/. Accessed February 7, 2018.
- 23. EU-topia http://eu-topia.org/. Accessed February 7, 2018.
- 24. Kamineni A, Tiro JA, Beaber EF, et al. ; PROSPR Consortium. Cervical cancer screening research in the PROSPR I consortium: rationale, methods and baseline findings from a US cohort. Int J Cancer. 2019;144(6):1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiss JE, Goodrich M, Harris KA, et al. Challenges with identifying indication for examination in breast imaging as a key clinical attribute in practice, research, and policy. J Am Coll Radiol JACR. 2016;14(2):198–207.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee JK, Jensen CD, Lee A, et al. Development and validation of an algorithm for classifying colonoscopy indication. Gastrointest Endosc. 2015;81(3):575–582.e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chubak J, Hubbard R.. Defining and measuring adherence to cancer screening. J Med Screen. 2016;23(4):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Massad LS, Einstein MH, Huh WK, et al. ; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121(4):829–846. [DOI] [PubMed] [Google Scholar]
- 29. Gamble S, Mawokomatanda T, Xu F, et al. Surveillance for certain health behaviors and conditions among states and selected local areas—behavioral risk factor surveillance system, United States, 2013 and 2014. MMWR Surveill Summ. 2017;66(16):1–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rauscher GH, Johnson TP, Cho YI, et al. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17(4):748–757. [DOI] [PubMed] [Google Scholar]
- 31. Butler PF. MQSA (Mammography Quality Standards Act) update—focusing on quality assurance. Radiol Manage. 1998;20(4):40–50. [PubMed] [Google Scholar]
- 32.National Committee for Quality Assurance. http://www.ncqa.org/hedis-quality-measurement/hedis-measures. Accessed July 5, 2018.
- 33. von Karsa L, Arrossi S.. Development and implementation of guidelines for quality assurance in breast cancer screening: the European experience. Salud Publica Mex. 2013;55(3):318–328. [DOI] [PubMed] [Google Scholar]
- 34. Perry N, Broeders M, de Wolf C, et al. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—summary document. Ann Oncol. 2007;19(4):614–622. [DOI] [PubMed] [Google Scholar]
- 35. Arbyn M, Anttila A, Jordan J, et al. European guidelines for quality assurance in cervical cancer screening. Second edition—summary document. Ann Oncol. 2010;21(3):448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. von Karsa L, Patnick J, Segnan N.. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—executive summary. Endoscopy. 2012;44(Suppl 3):SE1–SE8. [DOI] [PubMed] [Google Scholar]
- 37. Moss S, Ancelle-Park R, Brenner H; International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition--evaluation and interpretation of screening outcomes. Endoscopy. 2012;44(Suppl 3):SE49–SE64. [DOI] [PubMed] [Google Scholar]
- 38. Lehman CD, Arao RF, Sprague BL, et al. National performance benchmarks for modern screening digital mammography: update from the breast cancer surveillance consortium. Radiology. 2017;283(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beaber EF, Tosteson AN, Haas JS, et al. Breast cancer screening initiation after turning 40 years of age within the PROSPR consortium. Breast Cancer Res Treat. 2016;160(2):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sprague BL, Conant EF, Onega T, et al. Variation in mammographic breast density assessments among radiologists in clinical practice. Ann Intern Med. 2016;165(7):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klabunde CN, Zheng Y, Quinn VP, et al. Influence of age and comorbidity on colorectal cancer screening in the elderly. Am J Prev Med. 2016;51(3):e67–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Halm EA, Beaber EF, McLerran D, et al. Association between primary care visits and colorectal cancer screening outcomes in the era of population health outreach. J Gen Intern Med. 2016;31(10):1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schapira MM, Sprague BL, Klabunde CN, et al. Inadequate systems to support breast and cervical cancer screening in primary care practice. J Gen Intern Med. 2016;31(10):1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McCarthy AM, Kim JJ, Beaber EF, et al. Follow-up of abnormal breast and colorectal cancer screening by race/ethnicity. Am J Prev Med. 2016;51(4):507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burnett-Hartman AN, Mehta SJ, Zheng Y, et al. Racial/ethnic disparities in colorectal cancer screening across healthcare systems. Am J Prev Med. 2016;51(4):e107–e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conant EF, Beaber EF, Sprague BL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: a cohort study within the PROSPR consortium. Breast Cancer Res Treat. 2016;156(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to colonoscopy after positive fecal blood test in four U.S. health care systems. Cancer Epidemiol Biomarkers Prev. 2016;25(2):344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haas JS, Sprague BL, Klabunde CN, et al. Provider attitudes and screening practices following changes in breast and cervical cancer screening guidelines. J Gen Intern Med. 2016;31(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clark CR, Tosteson TD, Tosteson ANA, et al. Diffusion of digital breast tomosynthesis among women in primary care: associations with insurance type. Cancer Med. 2017;6(5):1102–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Haas JS, Barlow WE, Schapira MM, et al. Primary care providers' beliefs and recommendations and use of screening mammography by their patients. J Gen Intern Med. 2017;32(4):449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McCarthy AM, Barlow WE, Conant EF, et al. Breast cancer with a poor prognosis diagnosed after screening mammography with negative results. JAMA Oncol. 2018;4(7):998–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schapira MM, Barlow WE, Conant EF, et al. Communication practices of mammography facilities and timely follow-up of a screening mammogram with a BI-RADS 0 assessment. Acad Radiol. 2018;25(9):1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kamineni A, Tiro JA, Beaber EF, et al. Cervical cancer screening research in the PROSPR I consortium: rationale, methods, and baseline findings from a U.S. cohort. Int J Cancer. 2018;144(6):1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.